User login
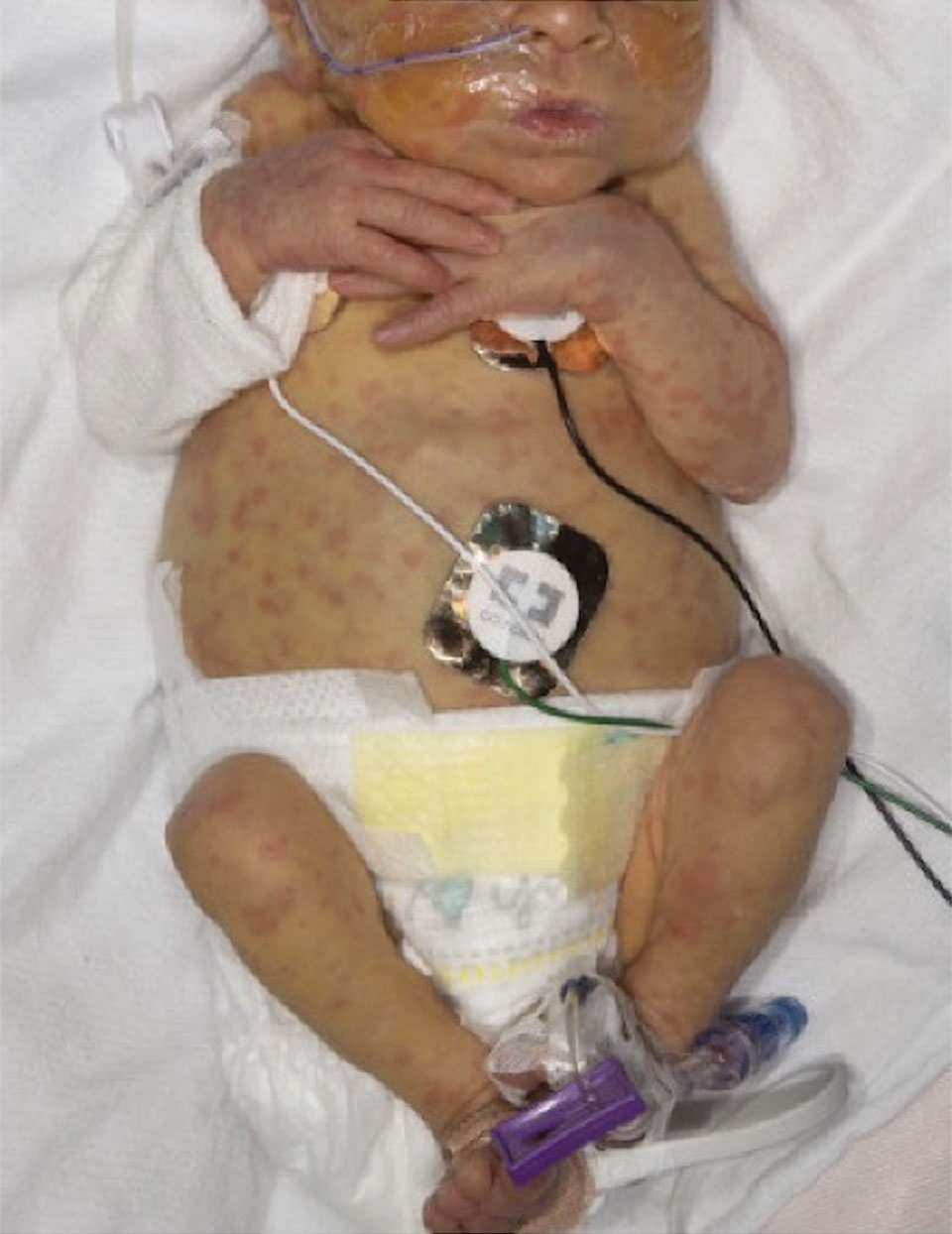
Scattered Red-Brown, Centrally Violaceous, Blanching Papules on an Infant
The Diagnosis: Neonatal-Onset Multisystem Inflammatory Disorder (NOMID)
The punch biopsy demonstrated a predominantly deep but somewhat superficial, periadnexal, neutrophilic and eosinophilic infiltrate (Figure). The eruption resolved 3 days later with supportive treatment, including appropriate wound care. Genetic analysis revealed an autosomal-dominant NLR family pyrin domain containing 3 gene, NLRP3, de novo variant associated with neonatal-onset multisystem inflammatory disorder (NOMID). Additional workup to characterize our patient’s inflammatory profile revealed elevated IL-18, CD3, CD4, S100A12, and S100A8/A9 levels. On day 48 of life, she was started on anakinra, an IL-1 inhibitor, at a dose of 1 mg/kg subcutaneously, which eventually was titrated to 10 mg/kg at hospital discharge. Hearing screenings were within normal limits.

Cryopyrin-associated periodic syndromes (CAPS) consist of 3 rare, IL-1–associated, autoinflammatory disorders, including familial cold autoinflammatory syndrome (FCAS), Muckle-Wells syndrome (MWS), and NOMID (also known as chronic infantile neurologic cutaneous and articular syndrome). These conditions result from a sporadic or autosomal-dominant gain-of-function mutations in a single gene, NLRP3, on chromosome 1q44. NLRP3 encodes for cryopyrin, an important component of an IL-1 and IL-18 activating inflammasome.1 The most severe manifestation of CAPS is NOMID, which typically presents at birth as a migratory urticarial eruption, growth failure, myalgia, fever, and abnormal facial features, including frontal bossing, saddle-shaped nose, and protruding eyes.2 The illness also can manifest with hepatosplenomegaly, lymphadenopathy, uveitis, sensorineural hearing loss, cerebral atrophy, and other neurologic manifestations.3 A diagnosis of chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE) syndrome was less likely given that our patient remained afebrile and did not show signs of lipodystrophy and persistent violaceous eyelid swelling. Both FCAS and MWS are less severe forms of CAPS when compared to NOMID. Familial cold autoinflammatory syndrome was less likely given the absence of the typical periodic fever pattern associated with the condition and severity of our patient’s symptoms. Muckle-Wells syndrome typically presents in adolescence with symptoms of FCAS, painful urticarial plaques, and progressive sensorinueral hearing loss. Tumor necrosis factor receptor–associated periodic fever (TRAPS) usually is associated with episodic fevers, abdominal pain, periorbital edema, migratory erythema, and arthralgia.1,3,4
Diagnostic criteria for CAPS include elevated inflammatory markers and serum amyloid, plus at least 2 of the typical CAPS symptoms: urticarial rash, cold-triggered episodes, sensorineural hearing loss, musculoskeletal symptoms, chronic aseptic meningitis, and skeletal abnormalities.4 The sensitivity and specificity of these diagnostic criteria are 84% and 91%, respectively. Additional findings that can be seen but are not part of the diagnostic criteria include intermittent fever, transient joint swelling, bony overgrowths, uveitis, optic disc edema, impaired growth, and hepatosplenomegaly.5 Laboratory findings may reveal leukocytosis, eosinophilia, anemia, and/or thrombocytopenia.3,5
Genetic testing, skin biopsies, ophthalmic examinations, neuroimaging, joint radiography, cerebrospinal fluid tests, and hearing examinations can be performed for confirmation of diagnosis and evaluation of systemic complications.4 A skin biopsy may reveal a neutrophilic infiltrate. Ophthalmic examination can demonstrate uveitis and optic disk edema. Neuroimaging may reveal cerebral atrophy or ventricular dilation. Lastly, joint radiography can be used to evaluate for the presence of premature long bone ossification or osseous overgrowth.4
In summary, NOMID is a multisystemic disorder with cutaneous manifestations. Early recognition of this entity is important given the severe sequelae and available efficacious therapy. Dermatologists should be aware of these manifestations, as dermatologic consultation and a skin biopsy may aid in diagnosis.
- Lachmann HJ. Periodic fever syndromes. Best Pract Res Clin Rheumatol. 2017;31:596-609. doi:10.1016/j.berh.2017.12.001
- Hull KM, Shoham N, Jin Chae J, et al. The expanding spectrum of systemic autoinflammatory disorders and their rheumatic manifestations. Curr Opin Rheumatol. 2003;15:61-69. doi:10.1097/00002281-200301000-00011
- Ahmadi N, Brewer CC, Zalewski C, et al. Cryopyrin-associated periodic syndromes: otolaryngologic and audiologic manifestations. Otolaryngol Head Neck Surg. 2011;145:295-302. doi:10.1177/0194599811402296
- Kuemmerle-Deschner JB, Ozen S, Tyrrell PN, et al. Diagnostic criteria for cryopyrin-associated periodic syndrome (CAPS). Ann Rheum Dis. 2017;76:942-947. doi:10.1136/annrheumdis-2016-209686
- Aksentijevich I, Nowak M, Mallah M, et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrinassociated autoinflammatory diseases. Arthritis Rheum. 2002; 46:3340-3348. doi:10.1002/art.10688
The Diagnosis: Neonatal-Onset Multisystem Inflammatory Disorder (NOMID)
The punch biopsy demonstrated a predominantly deep but somewhat superficial, periadnexal, neutrophilic and eosinophilic infiltrate (Figure). The eruption resolved 3 days later with supportive treatment, including appropriate wound care. Genetic analysis revealed an autosomal-dominant NLR family pyrin domain containing 3 gene, NLRP3, de novo variant associated with neonatal-onset multisystem inflammatory disorder (NOMID). Additional workup to characterize our patient’s inflammatory profile revealed elevated IL-18, CD3, CD4, S100A12, and S100A8/A9 levels. On day 48 of life, she was started on anakinra, an IL-1 inhibitor, at a dose of 1 mg/kg subcutaneously, which eventually was titrated to 10 mg/kg at hospital discharge. Hearing screenings were within normal limits.

Cryopyrin-associated periodic syndromes (CAPS) consist of 3 rare, IL-1–associated, autoinflammatory disorders, including familial cold autoinflammatory syndrome (FCAS), Muckle-Wells syndrome (MWS), and NOMID (also known as chronic infantile neurologic cutaneous and articular syndrome). These conditions result from a sporadic or autosomal-dominant gain-of-function mutations in a single gene, NLRP3, on chromosome 1q44. NLRP3 encodes for cryopyrin, an important component of an IL-1 and IL-18 activating inflammasome.1 The most severe manifestation of CAPS is NOMID, which typically presents at birth as a migratory urticarial eruption, growth failure, myalgia, fever, and abnormal facial features, including frontal bossing, saddle-shaped nose, and protruding eyes.2 The illness also can manifest with hepatosplenomegaly, lymphadenopathy, uveitis, sensorineural hearing loss, cerebral atrophy, and other neurologic manifestations.3 A diagnosis of chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE) syndrome was less likely given that our patient remained afebrile and did not show signs of lipodystrophy and persistent violaceous eyelid swelling. Both FCAS and MWS are less severe forms of CAPS when compared to NOMID. Familial cold autoinflammatory syndrome was less likely given the absence of the typical periodic fever pattern associated with the condition and severity of our patient’s symptoms. Muckle-Wells syndrome typically presents in adolescence with symptoms of FCAS, painful urticarial plaques, and progressive sensorinueral hearing loss. Tumor necrosis factor receptor–associated periodic fever (TRAPS) usually is associated with episodic fevers, abdominal pain, periorbital edema, migratory erythema, and arthralgia.1,3,4
Diagnostic criteria for CAPS include elevated inflammatory markers and serum amyloid, plus at least 2 of the typical CAPS symptoms: urticarial rash, cold-triggered episodes, sensorineural hearing loss, musculoskeletal symptoms, chronic aseptic meningitis, and skeletal abnormalities.4 The sensitivity and specificity of these diagnostic criteria are 84% and 91%, respectively. Additional findings that can be seen but are not part of the diagnostic criteria include intermittent fever, transient joint swelling, bony overgrowths, uveitis, optic disc edema, impaired growth, and hepatosplenomegaly.5 Laboratory findings may reveal leukocytosis, eosinophilia, anemia, and/or thrombocytopenia.3,5
Genetic testing, skin biopsies, ophthalmic examinations, neuroimaging, joint radiography, cerebrospinal fluid tests, and hearing examinations can be performed for confirmation of diagnosis and evaluation of systemic complications.4 A skin biopsy may reveal a neutrophilic infiltrate. Ophthalmic examination can demonstrate uveitis and optic disk edema. Neuroimaging may reveal cerebral atrophy or ventricular dilation. Lastly, joint radiography can be used to evaluate for the presence of premature long bone ossification or osseous overgrowth.4
In summary, NOMID is a multisystemic disorder with cutaneous manifestations. Early recognition of this entity is important given the severe sequelae and available efficacious therapy. Dermatologists should be aware of these manifestations, as dermatologic consultation and a skin biopsy may aid in diagnosis.
The Diagnosis: Neonatal-Onset Multisystem Inflammatory Disorder (NOMID)
The punch biopsy demonstrated a predominantly deep but somewhat superficial, periadnexal, neutrophilic and eosinophilic infiltrate (Figure). The eruption resolved 3 days later with supportive treatment, including appropriate wound care. Genetic analysis revealed an autosomal-dominant NLR family pyrin domain containing 3 gene, NLRP3, de novo variant associated with neonatal-onset multisystem inflammatory disorder (NOMID). Additional workup to characterize our patient’s inflammatory profile revealed elevated IL-18, CD3, CD4, S100A12, and S100A8/A9 levels. On day 48 of life, she was started on anakinra, an IL-1 inhibitor, at a dose of 1 mg/kg subcutaneously, which eventually was titrated to 10 mg/kg at hospital discharge. Hearing screenings were within normal limits.

Cryopyrin-associated periodic syndromes (CAPS) consist of 3 rare, IL-1–associated, autoinflammatory disorders, including familial cold autoinflammatory syndrome (FCAS), Muckle-Wells syndrome (MWS), and NOMID (also known as chronic infantile neurologic cutaneous and articular syndrome). These conditions result from a sporadic or autosomal-dominant gain-of-function mutations in a single gene, NLRP3, on chromosome 1q44. NLRP3 encodes for cryopyrin, an important component of an IL-1 and IL-18 activating inflammasome.1 The most severe manifestation of CAPS is NOMID, which typically presents at birth as a migratory urticarial eruption, growth failure, myalgia, fever, and abnormal facial features, including frontal bossing, saddle-shaped nose, and protruding eyes.2 The illness also can manifest with hepatosplenomegaly, lymphadenopathy, uveitis, sensorineural hearing loss, cerebral atrophy, and other neurologic manifestations.3 A diagnosis of chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE) syndrome was less likely given that our patient remained afebrile and did not show signs of lipodystrophy and persistent violaceous eyelid swelling. Both FCAS and MWS are less severe forms of CAPS when compared to NOMID. Familial cold autoinflammatory syndrome was less likely given the absence of the typical periodic fever pattern associated with the condition and severity of our patient’s symptoms. Muckle-Wells syndrome typically presents in adolescence with symptoms of FCAS, painful urticarial plaques, and progressive sensorinueral hearing loss. Tumor necrosis factor receptor–associated periodic fever (TRAPS) usually is associated with episodic fevers, abdominal pain, periorbital edema, migratory erythema, and arthralgia.1,3,4
Diagnostic criteria for CAPS include elevated inflammatory markers and serum amyloid, plus at least 2 of the typical CAPS symptoms: urticarial rash, cold-triggered episodes, sensorineural hearing loss, musculoskeletal symptoms, chronic aseptic meningitis, and skeletal abnormalities.4 The sensitivity and specificity of these diagnostic criteria are 84% and 91%, respectively. Additional findings that can be seen but are not part of the diagnostic criteria include intermittent fever, transient joint swelling, bony overgrowths, uveitis, optic disc edema, impaired growth, and hepatosplenomegaly.5 Laboratory findings may reveal leukocytosis, eosinophilia, anemia, and/or thrombocytopenia.3,5
Genetic testing, skin biopsies, ophthalmic examinations, neuroimaging, joint radiography, cerebrospinal fluid tests, and hearing examinations can be performed for confirmation of diagnosis and evaluation of systemic complications.4 A skin biopsy may reveal a neutrophilic infiltrate. Ophthalmic examination can demonstrate uveitis and optic disk edema. Neuroimaging may reveal cerebral atrophy or ventricular dilation. Lastly, joint radiography can be used to evaluate for the presence of premature long bone ossification or osseous overgrowth.4
In summary, NOMID is a multisystemic disorder with cutaneous manifestations. Early recognition of this entity is important given the severe sequelae and available efficacious therapy. Dermatologists should be aware of these manifestations, as dermatologic consultation and a skin biopsy may aid in diagnosis.
- Lachmann HJ. Periodic fever syndromes. Best Pract Res Clin Rheumatol. 2017;31:596-609. doi:10.1016/j.berh.2017.12.001
- Hull KM, Shoham N, Jin Chae J, et al. The expanding spectrum of systemic autoinflammatory disorders and their rheumatic manifestations. Curr Opin Rheumatol. 2003;15:61-69. doi:10.1097/00002281-200301000-00011
- Ahmadi N, Brewer CC, Zalewski C, et al. Cryopyrin-associated periodic syndromes: otolaryngologic and audiologic manifestations. Otolaryngol Head Neck Surg. 2011;145:295-302. doi:10.1177/0194599811402296
- Kuemmerle-Deschner JB, Ozen S, Tyrrell PN, et al. Diagnostic criteria for cryopyrin-associated periodic syndrome (CAPS). Ann Rheum Dis. 2017;76:942-947. doi:10.1136/annrheumdis-2016-209686
- Aksentijevich I, Nowak M, Mallah M, et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrinassociated autoinflammatory diseases. Arthritis Rheum. 2002; 46:3340-3348. doi:10.1002/art.10688
- Lachmann HJ. Periodic fever syndromes. Best Pract Res Clin Rheumatol. 2017;31:596-609. doi:10.1016/j.berh.2017.12.001
- Hull KM, Shoham N, Jin Chae J, et al. The expanding spectrum of systemic autoinflammatory disorders and their rheumatic manifestations. Curr Opin Rheumatol. 2003;15:61-69. doi:10.1097/00002281-200301000-00011
- Ahmadi N, Brewer CC, Zalewski C, et al. Cryopyrin-associated periodic syndromes: otolaryngologic and audiologic manifestations. Otolaryngol Head Neck Surg. 2011;145:295-302. doi:10.1177/0194599811402296
- Kuemmerle-Deschner JB, Ozen S, Tyrrell PN, et al. Diagnostic criteria for cryopyrin-associated periodic syndrome (CAPS). Ann Rheum Dis. 2017;76:942-947. doi:10.1136/annrheumdis-2016-209686
- Aksentijevich I, Nowak M, Mallah M, et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrinassociated autoinflammatory diseases. Arthritis Rheum. 2002; 46:3340-3348. doi:10.1002/art.10688
A 2-week-old infant girl was transferred to a specialty pediatric hospital where dermatology was consulted for evaluation of a diffuse eruption triggered by cold that was similar to an eruption present at birth. She was born at 31 weeks and 2 days’ gestation at an outside hospital via caesarean delivery. Early delivery was prompted by superimposed pre-eclampsia with severe hypertension after administration of antenatal steroids. At birth, the infant was cyanotic and apneic and had a documented skin eruption, according to the medical record. She had thrombocytopenia, elevated C-reactive protein, and an elevated temperature without fever. Extensive septic workup, including blood, urine, and cerebrospinal fluid cultures; herpes simplex virus and cytomegalovirus screening; and Toxoplasma polymerase chain reaction were negative. Magnetic resonance imaging of the brain revealed no evidence of intracranial congenital infection. Ampicillinsulbactam was initiated for presumed culture-negative sepsis. On day 2 of hospitalization, she developed conjunctival icterus, hepatomegaly, and jaundice. Direct hyperbilirubinemia; anemia; and elevated triglycerides, ferritin, and ammonia all were present. Coagulation studies were normal. Subsequent workup, including abdominal ultrasonography and hepatobiliary iminodiacetic acid scan, was concerning for biliary atresia. Despite appropriate treatment, her condition did not improve and she was transferred. Repeat abdominal ultrasonography on day 24 of life confirmed hepatomegaly but did not demonstrate other findings of biliary atresia. At the current presentation, physical examination revealed many scattered, redbrown and centrally violaceous, blanching papules measuring a few millimeters involving the trunk, arms, buttocks, and legs. A punch biopsy was obtained.