User login
What family physicians can do to combat bullying
CASE › Stacey, a 12-year-old girl with mild persistent asthma, presents to her family physician (FP) with her mother for her annual well visit. Stacey reports no complaints, but has visited twice recently for acute exacerbations of her asthma, which had previously been well-controlled. When reviewing her social history, Stacey reports that she started her second year of middle school 3 months ago. When asked if she enjoys school, Stacey looks down and says, “School is fine.” Her mother quickly adds that Stacey has quit the school cheerleading team—much to the coach’s dismay—and is having difficulty in her math class, a class in which she normally excels. Stacey appears embarrassed that her mother has brought these things up. Her mother says that at the beginning of the year, 2 girls began picking on Stacey, calling her names and making fun of her on social media and in front of other students.
For many years, bullying was trivialized. Some viewed it as a universal childhood experience; others considered it a rite of passage.1,2 It was not examined as a public health issue until the 1970s. In fact, no legislation addressing bullying or “peer abuse” existed in the United States until the mass shooting at Columbine High School in Littleton, Colo, in 1999. Within 3 years of the Columbine tragedy, the number of state laws that mentioned bullying went from zero to 15; within 10 years of Columbine, 41 states had laws addressing bullying,1 and by 2015, every state, the District of Columbia, and some territories had a bullying law in place.3
As research and advocacy regarding bullying has grown, its impact on the health of children, adolescents, and even adults has become more apparent. In a 2001 study of school-associated violent deaths in the United States between 1994 and 1999, the Centers for Disease Control and Prevention (CDC) found that among students, homicide perpetrators were more than twice as likely as homicide victims to have been bullied by peers.4 Given that homicide is the third leading cause of death in people ages 15 to 24,5 past exposure to bullying may be a significant contributing factor to mortality in this age group.4
In addition to a correlation with homicidal behavior, those involved in bullying—whether as the bully or victim—are at risk for a wide range of symptoms, conditions, and problems including poor psychosocial adjustment, depression, anxiety, suicide (the second leading cause of death in the 10-14 and 15-24 age groups5), academic decline, psychosomatic manifestations, fighting, alcohol use, smoking, and difficulty with the management of chronic diseases.6-10 Not only does being a victim of bullying have a direct impact on a child’s current mental and physical well-being, but it can have lasting psychological and behavioral effects that can follow children well into adulthood.7 The significant impact of bullying on individuals and society as a whole mandates a multifaceted approach that begins in your exam room. What follows is practical advice on screening, counseling, and working with schools and the community at large to curb the bullying epidemic.

Clarifying the problem: The CDC’s definition
Recognizing that varying definitions of bullying were being used in research studies that looked at violent or aggressive behaviors in youth, the CDC published a consensus statement in 2014 that proposed the following definition for bullying:11 any unwanted aggressive behavior by another youth or group of youths who are not siblings or current dating partners that involves an observed or perceived power imbalance and is repeated multiple times or is highly likely to be repeated. This expanded on an earlier definition by Olweus12,13 that also identified a longitudinal nature and power imbalance as key features.
Types of bullying. Direct bullying entails blatant attacks on a targeted young person, while indirect bullying involves communication with others about the targeted individual (eg, spreading harmful rumors). Bullying may be physical, verbal, or relational (eg, excluding someone from their usual social circle, denying friendship, the silent treatment, writing mean letters, eye rolling, etc.) and may involve damage to property. Boys tend toward more direct bullying behaviors, while girls more often engage in indirect bullying, which may be more challenging for both adults and other students to recognize.12,13 With increased use of technology and social media by adolescents, cyberbullying has become increasingly more prevalent, with its effects on adolescent health and academics being every bit as profound as those of traditional bullying.14
About 1 in 4/5 students suffer. The prevalence of bullying ranges by country and culture. The vast majority of early bullying research was conducted in Norway, which found that approximately 15% of students in elementary and secondary schools were involved in bullying in some capacity.12 In a study involving over 200,000 adolescents from 40 European countries, 26% of adolescents reported being involved in bullying, ranging from 8.6% to 45.2% for boys and 4.8% to 35.8% for girls.15 Variations in prevalence may be due to cultural differences in the acts of bullying or differences in interpretation of the term “bullying.”1,15
In the United States, a 2001 survey of more than 15,000 students in public and private schools (grades 6-10) asked the students about their involvement in bullying: 13% said they'd been a bully, 10.6% a victim, and 6.3% said they'd been both.6 There was no significant difference in the frequency of self-reported bullying among urban, suburban, or rural settings.
Despite efforts to educate the public about bullying and work with schools to intervene and prevent bullying, incidence remains largely unchanged. In 2013, the National Crime Victimization Survey reported that approximately 22% of adolescents ages 12 through 18 were victims of bullying.16 Similarly, the CDC's 2015 Youth Risk Behavior Surveillance System reported that 20.2% of high school students experienced bullying on school property.17
Screening: Best practices
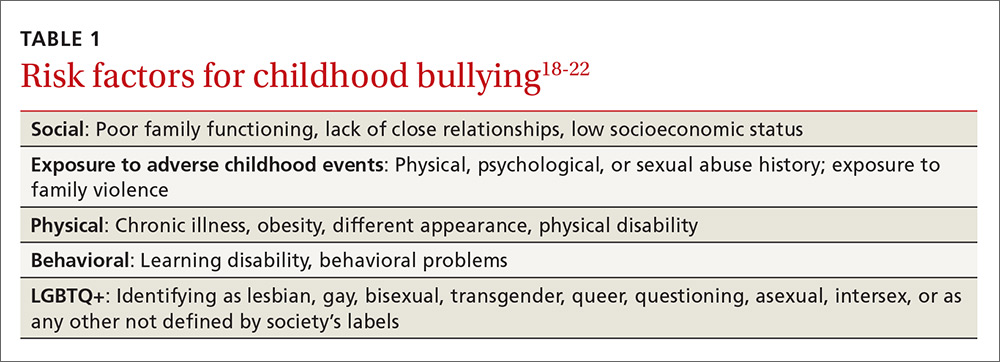
The FP’s role begins with screening children at risk for bullying (TABLE 118-22) or those whose complaints suggest that they may be victims of bullying.
Start screening when children enter elementary school
Given that providers’ time is limited for every patient visit, it is important to address bullying at times that are most likely to yield impactful results. The American Academy of Pediatrics recommends that the topic of bullying be introduced at the 6-year-old well-child visit (a typical age for entry to elementary school).7 Views in the literature are inconsistent regarding when and how to address bullying at other time points. One approach is to pre-screen those with risk factors associated with bullying (TABLE 118-22), and to focus screening on those with warning signs of bullying, which include mood disorders, psychosomatic or behavioral symptoms, substance abuse, self-harm behaviors, suicidal ideation or a suicide attempt, a decline in academic performance, and reports of school truancy. Parental concerns, such as when a child suddenly needs more money for lunch, is having aggressive outbursts, or is exhibiting unexplained physical injuries, should also be regarded as cues to screen.9
Screen patients in high-risk groups

A number of groups of children are at high risk for bullying and warrant targeted screening efforts.
- Children with special health needs. Research has shown that children with special health needs are at increased risk for being bullied.18 In fact, the presence of a chronic disease may increase the risk for bullying, and bullying often negatively impacts chronic disease management. As a result, it’s important to have a high index of suspicion with patients who have a chronic disease and who are not responding as expected to medical management or who experience deterioration after being previously well-controlled.18
- Children who are under- or overweight. Similarly, bullying based on a child's weight is a phenomenon that has been recognized to have a significant impact on children’s emotional health.19
- Youth who identify as lesbian, gay, bisexual, transgender, or queer/questioning (LGBTQ+) are more likely than non-LGBTQ+ peers to attempt suicide when exposed to a hostile social environment, such as that created by bullying.20
Screening need not be complicated

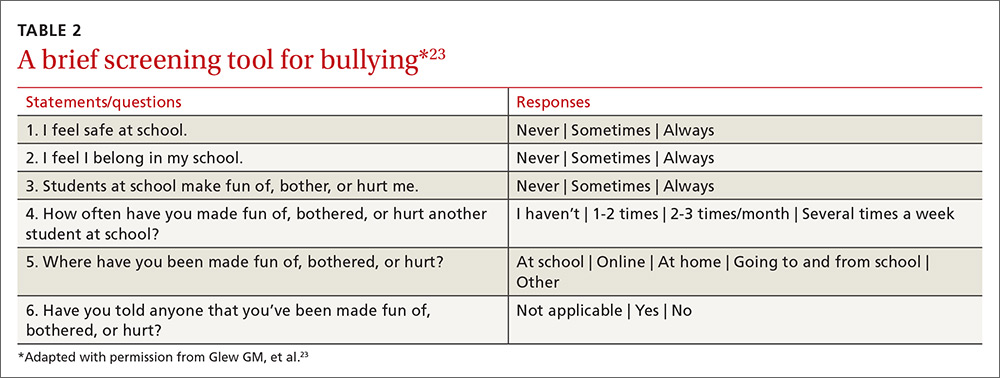
One screening approach is simply to ask patients, “Are you being bullied?” followed by such questions as, “How often are you bullied?” or “How long have you been bullied?” Asking about the setting of the bullying (Does it happen at school? Traveling to/from school? Online?) and other details may help guide interventions and the provision of resources.9 Another approach is to provide patients with some type of written survey (see TABLE 223 for an example) to encourage responses that patients might be reluctant to disclose verbally.23,24 (See “Barriers to screening.")
SIDEBAR
Barriers to screeningScreening for any condition presupposes a response. Ideally, family physicians should be prepared to provide basic counseling, resources, and, if necessary, treatment, if a patient screens positive for bullying. But screening for violence or bullying can be difficult, and evidence-based guidelines for screening and intervention are lacking, leaving many primary care practitioners feeling ill-equipped to meaningfully respond.
One study of the use of a screening tool aimed at intimate partner violence (IPV) showed that even with the availability of a screening tool, health care providers’ use of the tool was inconsistent and referral practices were ineffective.1 Providers cited the following limiting factors in screening for IPV: 1) a lack of immediate referral availability, 2) a lack of time during the office visit, and 3) a lack of confidence in the ability to screen.1 These same issues may be barriers to screening for bullying.
1. Ramachandran DV, Covarrubias L, Watson C, et al. How you screen is as important as whether you screen: a qualitative analysis of violence screening practices in reproductive health clinics. J Community Health. 2013;38:856-863.
Provider and parental interventions
Interventions often entail counseling the patient and the family about bullying and its effects, empowering victims and their caregivers, and screening for bullying comorbidities and correlates.2 Refer patients to behavioral health specialists when there is evidence of pervasive effects on mood, behavior, or social development, but keep in mind that counseling can begin in your own exam room.
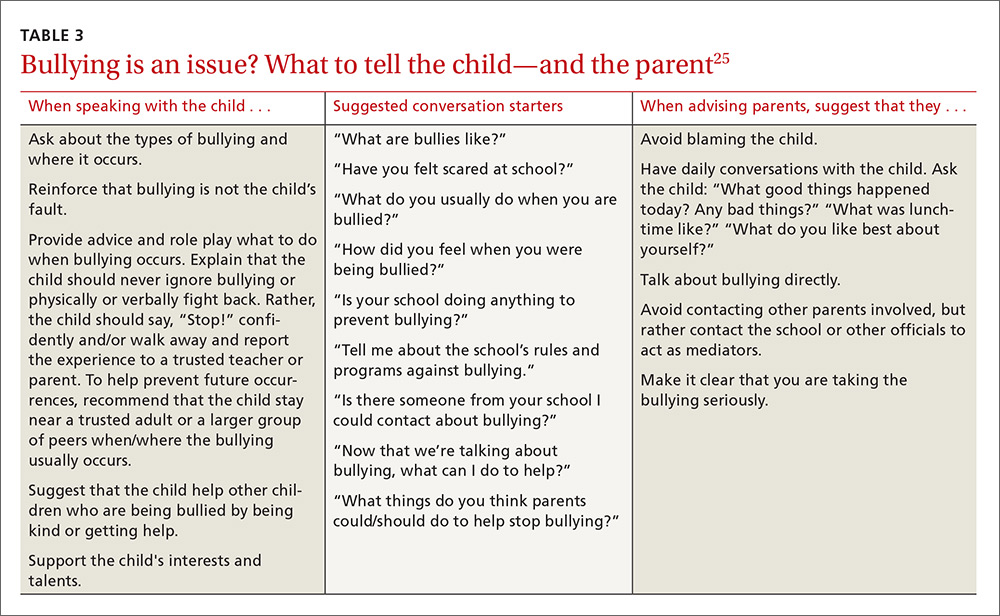
Effective discussion starters. Affirming the problem and its unacceptability, talking about the different types of bullying and where bullying may occur, and asking about patient perceptions of bullying can be effective discussion starters. FPs should help patients identify bullying, open lines of communication between children and their parents and between parents and other caregivers, and demonstrate respect and kindness in their approach to discussing the topic. Encourage children to speak with trusted adults when exposed to bullying. Talk to them about standing up to bullies (saying “stop” confidently or walking away from difficult situations) and staying safe by staying near adults or groups of peers when bullies are present (TABLE 325).
Empowering caregivers. Encourage parents to spend time each day talking with their child about the child’s time away from home (TABLE 325). Counsel parents/caregivers to expand their role. Knowing a child’s friends, encouraging the child academically, and increasing communication are all associated with lower risks of bullying.26 Similarly, parental oversight of Internet and social media use is associated with decreased participation in cyberbullying.27
In addition, the Positive Parenting telephone-based parenting education curriculum has been shown to decrease bullying, physical fighting, physical injuries, and victimization of children.28 The research-based, family strengthening program emphasizes 3 core elements of authoritative parenting: nurturance, discipline, and respect or granting of psychologic autonomy. The program entails 15- to 30-minute weekly phone conversations between parents and educators, as well as videos and a manual.
Are community programs in place—or are they needed?
Many schools have robust, state-mandated programs in place to identify bullying and provide support for students who are victims of bullying. (See “NJ’s harassment and bullying protocol: A case in point.”) Explaining this to victims and their families may help them come forward and seek assistance. FPs who want to advocate for their patients should start with local schools to support such programs and link students at risk with school counselors.
SIDEBAR
NJ's harassment and bullying protocol: A case in pointThere is no federal law that specifically applies to bullying, but all 50 states have some type of anti-bullying legislation on the books, and 40 of those states have additional detailed policies in place addressing the subject.1
New Jersey, for example, began enforcing one of the toughest harassment, intimidation, and bullying (HIB) protocols in the country back in September 20112 in the wake of the death of Rutgers University freshman Tyler Clementi, who committed suicide after his roommate allegedly shot a video of him with another man and posted it to the Internet.3 Among many other things, New Jersey’s legislation stipulates in its Anti-Bullying Bill of Rights4 that:
› Every school/district have plans in place that clearly define, prevent, prohibit, and promptly deal with acts of harassment, intimidation, or bullying, on school grounds, at school-sponsored functions, and on school buses.
› Plans must include a description of the type of behavior expected from each student and the consequences and remedial action for a person who commits an act of harassment, intimidation, or bullying. Student perpetrators may be suspended or expelled if convicted of any type of bullying, whether it be for teasing or something more severe.
› All school employees must act on any incidents of bullying reported to, or witnessed by, them and report such incidents on the same day to the school principal.
› Plans must include provisions and deadlines for investigating and resolving all matters in a timely fashion; investigations into allegations of bullying must be launched within one day.
› Every case of bullying must be reported to the state. Schools are graded by the state on their compliance with anti-bullying standards and policies and their handling of incidents.
› Schools must appoint safety teams made up of parents, teachers, and staff, and school personnel and students must receive extensive anti-bullying training.
1. US Department of Health and Human Services. Stopbullying.gov. Policies and laws. Available at: https://www.stopbullying.gov/laws/index.html.
Accessed January 5, 2017.
2. State of New Jersey Department of Education. An overview of amendments to laws on harassment, intimidation, and bullying. Available at: http://www.state.nj.us/education/students/safety/behavior/hib/overview.pdf. Accessed January 5, 2017.
3. Cohen A. Case study: Why New Jersey’s antibullying law should be a model for other states. Time. September 6, 2011. Available at: http://ideas.time.com/2011/09/06/why-new-jerseys-antibullying-law-should-be-a-model-for-other-states/. Accessed January 5, 2017.
4. New Jersey Legislature. Anti-bullying Bill of Rights Act. Available at: http://www.njleg.state.nj.us/2010/Bills/PL10/122.PDF. Accessed January 5, 2017.
If programs are lacking in your community, there is much you can do to educate yourself about successful programs and advise local community organizations and schools about them. Among the most successful and well-studied interventions for thwarting the bullying epidemic have been school-based community ones. The most studied of these is the Olweus Bullying Prevention Program (OBPP), which is based on 4 principles:1,29
- Adults both at home and at school should take a positive and encouraging interest in students.
- Unacceptable behavior should have strict and well-known limits.
- Sanctions should be applied consistently and should be non-hostile in nature.
- Adults both at home and in the educational environment should act as authorities.
In short, the program focuses on greater awareness and involvement on the part of adults, and employing measures at the school level (eg, surveys, better supervision during break and lunch times), the class level (eg, rules against bullying, regular class meetings with students), and the individual level (eg, serious talks with bullies, victims, parents of involved students).
Research has shown that the OBPP reduces bullying behaviors by as much as 50%, reduces vandalism and truancy, and reduces the number of new victims.12 Limits to the more widespread implementation of the OBPP have consisted mainly of the inability to appropriately train adults, including teachers and other school personnel in educational settings. Despite these limitations, the OBPP has been praised and endorsed by numerous groups, including the US Department of Justice.30
Other non-curricular, school-based programs exist, such as the School-Wide Positive Behavioral Interventions and Supports (SWPBIS). This program is a school-wide prevention strategy aimed at: 1) reducing behavior problems that lead to office discipline referrals and suspensions, and 2) changing perceptions of school safety. (For more information, see https://www.crimesolutions.gov/ProgramDetails.aspx?ID=385 and https://www.pbis.org/school/swpbis-for-beginners.)31
The research-based Second Step: Student Success Through Prevention (SS-SSTP) Middle School Program (http://www.cfchildren.org/second-step/middle-school)32 focuses on the often difficult middle school years. The program helps schools teach and model essential communication, coping, and decision-making skills to help adolescents navigate around common pitfalls such as peer pressure, substance abuse, and bullying (both in-person and online). The program aims to reduce aggression and provide support for a more inclusive environment that helps students stay in school, make good choices, and experience social and academic success.
The Positive Action Program (https://www.positiveaction.net/research/primer),33 which is predicated on the notion that we feel good about ourselves when we do positive things, features scripted lessons and kits of materials (eg, posters, games, worksheets, puzzles) appropriate for each grade level.
CASE › Stacey’s visit to her FP’s office has presented several clues that she may be a victim of bullying. Her mild persistent asthma appears to no longer be as well controlled as it was in the past. Direct questioning has revealed that 2 girls at school have been making fun of Stacey when she uses her inhaled corticosteroid in the morning before class, so she has stopped using it. These same students are on her cheerleading team, so she quit the team to avoid them. Her school-related anxiety is so great that she no longer pays attention in math class and is constantly worried that something is being posted about her online.
Stacy’s FP responds to this information with a multifaceted approach. In the exam room, he screens Stacy for depression. While she is negative and denies any suicidal ideation, Stacy is clearly having anxiety, so the FP refers Stacey to a counselor at a local mental health clinic. With Stacy’s permission, the FP discusses the issue with her mother and they decide together with Stacy that she should talk to a teacher at school about the ongoing bullying. Because this was not the first time that the FP has heard this from a child in the community, the FP plans to attend an upcoming school board meeting to advocate for an evidence-based bullying prevention program to help curb the ongoing problem facing his patients.
CORRESPONDENCE
Robert McClowry, MD, Department of Family and Community Medicine, Jefferson Family Medicine Associates, 833 Chestnut East, 3rd Floor, Suite 301, Philadelphia, PA, 19107-4414; [email protected].
1. Olweus D, Limber SP. Bullying in school: evaluation and dissemination of the Olweus Bullying Prevention Program. Am J Orthopsychiatry. 2010;80:124-134.
2. Lyznicki JM, McCaffree MA, Robinowitz CB, et al. Childhood bullying: implications for physicians. Am Fam Physician. 2004;70:1723-1730.
3. Temkin D. All 50 states now have a bullying law. Now what? The Huffington Post. April 27, 2015. Available at: http://www.huffingtonpost.com/deborah-temkin/all-50-states-now-have-a_b_7153114.html. Accessed January 5, 2017.
4. Anderson M, Kaufman J, Simon TR, et al. School-associated violent deaths in the United States, 1994-1999. JAMA. 2001;286:2695-2702.
5. Centers for Disease Control and Prevention. Injury prevention and control: Data and statistics (WISQARS). Ten leading causes of death and injury. Available at: https://www.cdc.gov/injury/wisqars/leadingcauses.html. Accessed January 5, 2017.
6. Nansel TR, Overpeck M, Pilla RS, et al. Bullying behaviors among US youth: prevalence and association with psychosocial adjustment. JAMA. 2001;285:2094-2100.
7. Committee on Injury, Violence, and Poison Prevention. Policy statement—Role of the pediatrician in youth violence prevention. Pediatrics. 2009;124:393-402.
8. Klein DA, Myhre KK, Ahrendt DM. Bullying among adolescents: a challenge in primary care. Am Fam Physician. 2013;88:87-92.
9. Lamb J, Pepler DJ, Craig W. Approach to bullying and victimization. Can Fam Physician. 2009;55:356-360.
10. Spector ND, Kelly SF. Pediatrician’s role in screening and treatment: bullying, prediabetes, oral health. Curr Opin Pediatr. 2006;18:661-670.
11. Gladden RM, Vivolo-Kantor AM, Hamburger ME, et al. Bullying surveillance among youths: uniform definitions for public health and recommended data elements. National Center for Injury Prevention and Control, Centers for Disease Control and Prevention and US Department of Education; 2014. Available at: https://www.cdc.gov/violenceprevention/pdf/bullying-definitions-final-a.pdf. Accessed June 8, 2016.
12. Olweus D. Bullying at school: basic facts and effects of a school based intervention program. J Child Psychol Psychiatry. 1994;35:1171-1190.
13. Olweus D. Bully/victim problems in school: Facts and intervention. Eur J Psychol Educ. 1997;12:495-510.
14. Kowalski RM, Limber SP. Psychological, physical, and academic correlates of cyberbullying and traditional bullying. J Adolesc Health. 2013;53:S13-S20.
15. Craig W, Harel-Fisch Y, Fogel-Grinvald H, et al. A cross-national profile of bullying and victimization among adolescents in 40 countries. Int J Public Health. 2009;54(Suppl 2):216-224.
16. US Department of Education, National Center for Educational Statistics (2015). Student reports of bullying and cyberbullying: results from the 2013 School Crime Supplement to the National Victimization Survey. Available at: http://nces.ed.gov/pubsearch/pubsinfo.asp?pubid=2015056. Accessed November 9, 2016.
17. Kann L, McManus T, Harris WA, et al. Youth risk behavior surveillance - United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:1-174.
18. Van Cleave J, Davis MM. Bullying and peer victimization among children with special health care needs. Pediatrics. 2006;118:e1212-e1219.
19. Eisenberg ME, Neumark-Sztainer D, Story M. Associations of weight-based teasing and emotional well-being among adolescents. Arch Pediatr Adolesc Med. 2003;157:733-738.
20. Hatzenbuehler ML. The social environment and suicide attempts in lesbian, gay, and bisexual youth. Pediatrics. 2011;127:896-903.
21. Song LY, Singer MI, Anglin TM. Violence exposure and emotional trauma as contributors to adolescents’ violent behaviors. Arch Pediatr Adolesc Med. 1998;152:531-536.
22. Singer MI, Anglin TM, Song LY, et al. Adolescents’ exposure to violence and associated symptoms of psychological trauma. JAMA. 1995;273:477-482.
23. Glew GM, Fan MY, Katon W, et al. Bullying, psychosocial adjustment, and academic performance in elementary school. Arch Pediatr Adolesc Med. 2005;159:1026-1031.
24. Waseem M, Ryan M, Foster CB, et al. Assessment and management of bullied children in the emergency department. Pediatr Emerg Care. 2013;29:389-398.
25. US Department of Health and Human Services. stopbullying.gov. Available at: https//www.stopbullying.gov. Accessed January 5, 2017.
26. Shetgiri R, Lin H, Avila RM, et al. Parental characteristics associated with bullying perpetration in US children aged 10 to 17 years. Am J Public Health. 2012;102:2280-2286.
27. Hinduja S, Patchin JW. Social influences on cyberbullying behaviors among middle and high school students. J Youth Adolesc. 2013;42:711-722.
28. Borowsky IW, Mozayeny S, Stuenkel K, et al. Effects of a primary care-based intervention on violent behavior and injury in children. Pediatrics. 2004;114:e392-e399.
29. Olweus D. Bully/victim problems in school: Facts and intervention. Eur J Psychol Educ. 1997;12:495-510.
30. Mihalic SF. Blueprints for Violence Prevention: Report. US Department of Justice, Office of Justice Programs, Office of Juvenile Justice and Delinquency Prevention; 2004.
31. Waasdorp TE, Bradshaw CP, Leaf PJ. The impact of schoolwide positive behavioral interventions and supports on bullying and peer rejection: a randomized controlled effectiveness trial. Arch Pediatr Adolesc Med. 2012;166:149-156.
32. Espelage DL, Low S, Polanin JR, et al. The impact of a middle school program to reduce aggression, victimization, and sexual violence. J Adolesc Health. 2013;53:180-186.
33. Lewis KM, Schure MB, Bavarian N, et al. Problem behavior and urban, low-income youth: a randomized controlled trial of positive action in Chicago. Am J Prev Med. 2013;44:622-630.
CASE › Stacey, a 12-year-old girl with mild persistent asthma, presents to her family physician (FP) with her mother for her annual well visit. Stacey reports no complaints, but has visited twice recently for acute exacerbations of her asthma, which had previously been well-controlled. When reviewing her social history, Stacey reports that she started her second year of middle school 3 months ago. When asked if she enjoys school, Stacey looks down and says, “School is fine.” Her mother quickly adds that Stacey has quit the school cheerleading team—much to the coach’s dismay—and is having difficulty in her math class, a class in which she normally excels. Stacey appears embarrassed that her mother has brought these things up. Her mother says that at the beginning of the year, 2 girls began picking on Stacey, calling her names and making fun of her on social media and in front of other students.
For many years, bullying was trivialized. Some viewed it as a universal childhood experience; others considered it a rite of passage.1,2 It was not examined as a public health issue until the 1970s. In fact, no legislation addressing bullying or “peer abuse” existed in the United States until the mass shooting at Columbine High School in Littleton, Colo, in 1999. Within 3 years of the Columbine tragedy, the number of state laws that mentioned bullying went from zero to 15; within 10 years of Columbine, 41 states had laws addressing bullying,1 and by 2015, every state, the District of Columbia, and some territories had a bullying law in place.3
As research and advocacy regarding bullying has grown, its impact on the health of children, adolescents, and even adults has become more apparent. In a 2001 study of school-associated violent deaths in the United States between 1994 and 1999, the Centers for Disease Control and Prevention (CDC) found that among students, homicide perpetrators were more than twice as likely as homicide victims to have been bullied by peers.4 Given that homicide is the third leading cause of death in people ages 15 to 24,5 past exposure to bullying may be a significant contributing factor to mortality in this age group.4
In addition to a correlation with homicidal behavior, those involved in bullying—whether as the bully or victim—are at risk for a wide range of symptoms, conditions, and problems including poor psychosocial adjustment, depression, anxiety, suicide (the second leading cause of death in the 10-14 and 15-24 age groups5), academic decline, psychosomatic manifestations, fighting, alcohol use, smoking, and difficulty with the management of chronic diseases.6-10 Not only does being a victim of bullying have a direct impact on a child’s current mental and physical well-being, but it can have lasting psychological and behavioral effects that can follow children well into adulthood.7 The significant impact of bullying on individuals and society as a whole mandates a multifaceted approach that begins in your exam room. What follows is practical advice on screening, counseling, and working with schools and the community at large to curb the bullying epidemic.

Clarifying the problem: The CDC’s definition
Recognizing that varying definitions of bullying were being used in research studies that looked at violent or aggressive behaviors in youth, the CDC published a consensus statement in 2014 that proposed the following definition for bullying:11 any unwanted aggressive behavior by another youth or group of youths who are not siblings or current dating partners that involves an observed or perceived power imbalance and is repeated multiple times or is highly likely to be repeated. This expanded on an earlier definition by Olweus12,13 that also identified a longitudinal nature and power imbalance as key features.
Types of bullying. Direct bullying entails blatant attacks on a targeted young person, while indirect bullying involves communication with others about the targeted individual (eg, spreading harmful rumors). Bullying may be physical, verbal, or relational (eg, excluding someone from their usual social circle, denying friendship, the silent treatment, writing mean letters, eye rolling, etc.) and may involve damage to property. Boys tend toward more direct bullying behaviors, while girls more often engage in indirect bullying, which may be more challenging for both adults and other students to recognize.12,13 With increased use of technology and social media by adolescents, cyberbullying has become increasingly more prevalent, with its effects on adolescent health and academics being every bit as profound as those of traditional bullying.14
About 1 in 4/5 students suffer. The prevalence of bullying ranges by country and culture. The vast majority of early bullying research was conducted in Norway, which found that approximately 15% of students in elementary and secondary schools were involved in bullying in some capacity.12 In a study involving over 200,000 adolescents from 40 European countries, 26% of adolescents reported being involved in bullying, ranging from 8.6% to 45.2% for boys and 4.8% to 35.8% for girls.15 Variations in prevalence may be due to cultural differences in the acts of bullying or differences in interpretation of the term “bullying.”1,15
In the United States, a 2001 survey of more than 15,000 students in public and private schools (grades 6-10) asked the students about their involvement in bullying: 13% said they'd been a bully, 10.6% a victim, and 6.3% said they'd been both.6 There was no significant difference in the frequency of self-reported bullying among urban, suburban, or rural settings.
Despite efforts to educate the public about bullying and work with schools to intervene and prevent bullying, incidence remains largely unchanged. In 2013, the National Crime Victimization Survey reported that approximately 22% of adolescents ages 12 through 18 were victims of bullying.16 Similarly, the CDC's 2015 Youth Risk Behavior Surveillance System reported that 20.2% of high school students experienced bullying on school property.17
Screening: Best practices
The FP’s role begins with screening children at risk for bullying (TABLE 118-22) or those whose complaints suggest that they may be victims of bullying.
Start screening when children enter elementary school
Given that providers’ time is limited for every patient visit, it is important to address bullying at times that are most likely to yield impactful results. The American Academy of Pediatrics recommends that the topic of bullying be introduced at the 6-year-old well-child visit (a typical age for entry to elementary school).7 Views in the literature are inconsistent regarding when and how to address bullying at other time points. One approach is to pre-screen those with risk factors associated with bullying (TABLE 118-22), and to focus screening on those with warning signs of bullying, which include mood disorders, psychosomatic or behavioral symptoms, substance abuse, self-harm behaviors, suicidal ideation or a suicide attempt, a decline in academic performance, and reports of school truancy. Parental concerns, such as when a child suddenly needs more money for lunch, is having aggressive outbursts, or is exhibiting unexplained physical injuries, should also be regarded as cues to screen.9

Screen patients in high-risk groups

A number of groups of children are at high risk for bullying and warrant targeted screening efforts.
- Children with special health needs. Research has shown that children with special health needs are at increased risk for being bullied.18 In fact, the presence of a chronic disease may increase the risk for bullying, and bullying often negatively impacts chronic disease management. As a result, it’s important to have a high index of suspicion with patients who have a chronic disease and who are not responding as expected to medical management or who experience deterioration after being previously well-controlled.18
- Children who are under- or overweight. Similarly, bullying based on a child's weight is a phenomenon that has been recognized to have a significant impact on children’s emotional health.19
- Youth who identify as lesbian, gay, bisexual, transgender, or queer/questioning (LGBTQ+) are more likely than non-LGBTQ+ peers to attempt suicide when exposed to a hostile social environment, such as that created by bullying.20

Screening need not be complicated

One screening approach is simply to ask patients, “Are you being bullied?” followed by such questions as, “How often are you bullied?” or “How long have you been bullied?” Asking about the setting of the bullying (Does it happen at school? Traveling to/from school? Online?) and other details may help guide interventions and the provision of resources.9 Another approach is to provide patients with some type of written survey (see TABLE 223 for an example) to encourage responses that patients might be reluctant to disclose verbally.23,24 (See “Barriers to screening.")
SIDEBAR
Barriers to screeningScreening for any condition presupposes a response. Ideally, family physicians should be prepared to provide basic counseling, resources, and, if necessary, treatment, if a patient screens positive for bullying. But screening for violence or bullying can be difficult, and evidence-based guidelines for screening and intervention are lacking, leaving many primary care practitioners feeling ill-equipped to meaningfully respond.
One study of the use of a screening tool aimed at intimate partner violence (IPV) showed that even with the availability of a screening tool, health care providers’ use of the tool was inconsistent and referral practices were ineffective.1 Providers cited the following limiting factors in screening for IPV: 1) a lack of immediate referral availability, 2) a lack of time during the office visit, and 3) a lack of confidence in the ability to screen.1 These same issues may be barriers to screening for bullying.
1. Ramachandran DV, Covarrubias L, Watson C, et al. How you screen is as important as whether you screen: a qualitative analysis of violence screening practices in reproductive health clinics. J Community Health. 2013;38:856-863.
Provider and parental interventions
Interventions often entail counseling the patient and the family about bullying and its effects, empowering victims and their caregivers, and screening for bullying comorbidities and correlates.2 Refer patients to behavioral health specialists when there is evidence of pervasive effects on mood, behavior, or social development, but keep in mind that counseling can begin in your own exam room.
Effective discussion starters. Affirming the problem and its unacceptability, talking about the different types of bullying and where bullying may occur, and asking about patient perceptions of bullying can be effective discussion starters. FPs should help patients identify bullying, open lines of communication between children and their parents and between parents and other caregivers, and demonstrate respect and kindness in their approach to discussing the topic. Encourage children to speak with trusted adults when exposed to bullying. Talk to them about standing up to bullies (saying “stop” confidently or walking away from difficult situations) and staying safe by staying near adults or groups of peers when bullies are present (TABLE 325).
Empowering caregivers. Encourage parents to spend time each day talking with their child about the child’s time away from home (TABLE 325). Counsel parents/caregivers to expand their role. Knowing a child’s friends, encouraging the child academically, and increasing communication are all associated with lower risks of bullying.26 Similarly, parental oversight of Internet and social media use is associated with decreased participation in cyberbullying.27
In addition, the Positive Parenting telephone-based parenting education curriculum has been shown to decrease bullying, physical fighting, physical injuries, and victimization of children.28 The research-based, family strengthening program emphasizes 3 core elements of authoritative parenting: nurturance, discipline, and respect or granting of psychologic autonomy. The program entails 15- to 30-minute weekly phone conversations between parents and educators, as well as videos and a manual.

Are community programs in place—or are they needed?
Many schools have robust, state-mandated programs in place to identify bullying and provide support for students who are victims of bullying. (See “NJ’s harassment and bullying protocol: A case in point.”) Explaining this to victims and their families may help them come forward and seek assistance. FPs who want to advocate for their patients should start with local schools to support such programs and link students at risk with school counselors.
SIDEBAR
NJ's harassment and bullying protocol: A case in pointThere is no federal law that specifically applies to bullying, but all 50 states have some type of anti-bullying legislation on the books, and 40 of those states have additional detailed policies in place addressing the subject.1
New Jersey, for example, began enforcing one of the toughest harassment, intimidation, and bullying (HIB) protocols in the country back in September 20112 in the wake of the death of Rutgers University freshman Tyler Clementi, who committed suicide after his roommate allegedly shot a video of him with another man and posted it to the Internet.3 Among many other things, New Jersey’s legislation stipulates in its Anti-Bullying Bill of Rights4 that:
› Every school/district have plans in place that clearly define, prevent, prohibit, and promptly deal with acts of harassment, intimidation, or bullying, on school grounds, at school-sponsored functions, and on school buses.
› Plans must include a description of the type of behavior expected from each student and the consequences and remedial action for a person who commits an act of harassment, intimidation, or bullying. Student perpetrators may be suspended or expelled if convicted of any type of bullying, whether it be for teasing or something more severe.
› All school employees must act on any incidents of bullying reported to, or witnessed by, them and report such incidents on the same day to the school principal.
› Plans must include provisions and deadlines for investigating and resolving all matters in a timely fashion; investigations into allegations of bullying must be launched within one day.
› Every case of bullying must be reported to the state. Schools are graded by the state on their compliance with anti-bullying standards and policies and their handling of incidents.
› Schools must appoint safety teams made up of parents, teachers, and staff, and school personnel and students must receive extensive anti-bullying training.
1. US Department of Health and Human Services. Stopbullying.gov. Policies and laws. Available at: https://www.stopbullying.gov/laws/index.html.
Accessed January 5, 2017.
2. State of New Jersey Department of Education. An overview of amendments to laws on harassment, intimidation, and bullying. Available at: http://www.state.nj.us/education/students/safety/behavior/hib/overview.pdf. Accessed January 5, 2017.
3. Cohen A. Case study: Why New Jersey’s antibullying law should be a model for other states. Time. September 6, 2011. Available at: http://ideas.time.com/2011/09/06/why-new-jerseys-antibullying-law-should-be-a-model-for-other-states/. Accessed January 5, 2017.
4. New Jersey Legislature. Anti-bullying Bill of Rights Act. Available at: http://www.njleg.state.nj.us/2010/Bills/PL10/122.PDF. Accessed January 5, 2017.
If programs are lacking in your community, there is much you can do to educate yourself about successful programs and advise local community organizations and schools about them. Among the most successful and well-studied interventions for thwarting the bullying epidemic have been school-based community ones. The most studied of these is the Olweus Bullying Prevention Program (OBPP), which is based on 4 principles:1,29
- Adults both at home and at school should take a positive and encouraging interest in students.
- Unacceptable behavior should have strict and well-known limits.
- Sanctions should be applied consistently and should be non-hostile in nature.
- Adults both at home and in the educational environment should act as authorities.
In short, the program focuses on greater awareness and involvement on the part of adults, and employing measures at the school level (eg, surveys, better supervision during break and lunch times), the class level (eg, rules against bullying, regular class meetings with students), and the individual level (eg, serious talks with bullies, victims, parents of involved students).
Research has shown that the OBPP reduces bullying behaviors by as much as 50%, reduces vandalism and truancy, and reduces the number of new victims.12 Limits to the more widespread implementation of the OBPP have consisted mainly of the inability to appropriately train adults, including teachers and other school personnel in educational settings. Despite these limitations, the OBPP has been praised and endorsed by numerous groups, including the US Department of Justice.30
Other non-curricular, school-based programs exist, such as the School-Wide Positive Behavioral Interventions and Supports (SWPBIS). This program is a school-wide prevention strategy aimed at: 1) reducing behavior problems that lead to office discipline referrals and suspensions, and 2) changing perceptions of school safety. (For more information, see https://www.crimesolutions.gov/ProgramDetails.aspx?ID=385 and https://www.pbis.org/school/swpbis-for-beginners.)31
The research-based Second Step: Student Success Through Prevention (SS-SSTP) Middle School Program (http://www.cfchildren.org/second-step/middle-school)32 focuses on the often difficult middle school years. The program helps schools teach and model essential communication, coping, and decision-making skills to help adolescents navigate around common pitfalls such as peer pressure, substance abuse, and bullying (both in-person and online). The program aims to reduce aggression and provide support for a more inclusive environment that helps students stay in school, make good choices, and experience social and academic success.
The Positive Action Program (https://www.positiveaction.net/research/primer),33 which is predicated on the notion that we feel good about ourselves when we do positive things, features scripted lessons and kits of materials (eg, posters, games, worksheets, puzzles) appropriate for each grade level.
CASE › Stacey’s visit to her FP’s office has presented several clues that she may be a victim of bullying. Her mild persistent asthma appears to no longer be as well controlled as it was in the past. Direct questioning has revealed that 2 girls at school have been making fun of Stacey when she uses her inhaled corticosteroid in the morning before class, so she has stopped using it. These same students are on her cheerleading team, so she quit the team to avoid them. Her school-related anxiety is so great that she no longer pays attention in math class and is constantly worried that something is being posted about her online.
Stacy’s FP responds to this information with a multifaceted approach. In the exam room, he screens Stacy for depression. While she is negative and denies any suicidal ideation, Stacy is clearly having anxiety, so the FP refers Stacey to a counselor at a local mental health clinic. With Stacy’s permission, the FP discusses the issue with her mother and they decide together with Stacy that she should talk to a teacher at school about the ongoing bullying. Because this was not the first time that the FP has heard this from a child in the community, the FP plans to attend an upcoming school board meeting to advocate for an evidence-based bullying prevention program to help curb the ongoing problem facing his patients.
CORRESPONDENCE
Robert McClowry, MD, Department of Family and Community Medicine, Jefferson Family Medicine Associates, 833 Chestnut East, 3rd Floor, Suite 301, Philadelphia, PA, 19107-4414; [email protected].
CASE › Stacey, a 12-year-old girl with mild persistent asthma, presents to her family physician (FP) with her mother for her annual well visit. Stacey reports no complaints, but has visited twice recently for acute exacerbations of her asthma, which had previously been well-controlled. When reviewing her social history, Stacey reports that she started her second year of middle school 3 months ago. When asked if she enjoys school, Stacey looks down and says, “School is fine.” Her mother quickly adds that Stacey has quit the school cheerleading team—much to the coach’s dismay—and is having difficulty in her math class, a class in which she normally excels. Stacey appears embarrassed that her mother has brought these things up. Her mother says that at the beginning of the year, 2 girls began picking on Stacey, calling her names and making fun of her on social media and in front of other students.
For many years, bullying was trivialized. Some viewed it as a universal childhood experience; others considered it a rite of passage.1,2 It was not examined as a public health issue until the 1970s. In fact, no legislation addressing bullying or “peer abuse” existed in the United States until the mass shooting at Columbine High School in Littleton, Colo, in 1999. Within 3 years of the Columbine tragedy, the number of state laws that mentioned bullying went from zero to 15; within 10 years of Columbine, 41 states had laws addressing bullying,1 and by 2015, every state, the District of Columbia, and some territories had a bullying law in place.3
As research and advocacy regarding bullying has grown, its impact on the health of children, adolescents, and even adults has become more apparent. In a 2001 study of school-associated violent deaths in the United States between 1994 and 1999, the Centers for Disease Control and Prevention (CDC) found that among students, homicide perpetrators were more than twice as likely as homicide victims to have been bullied by peers.4 Given that homicide is the third leading cause of death in people ages 15 to 24,5 past exposure to bullying may be a significant contributing factor to mortality in this age group.4
In addition to a correlation with homicidal behavior, those involved in bullying—whether as the bully or victim—are at risk for a wide range of symptoms, conditions, and problems including poor psychosocial adjustment, depression, anxiety, suicide (the second leading cause of death in the 10-14 and 15-24 age groups5), academic decline, psychosomatic manifestations, fighting, alcohol use, smoking, and difficulty with the management of chronic diseases.6-10 Not only does being a victim of bullying have a direct impact on a child’s current mental and physical well-being, but it can have lasting psychological and behavioral effects that can follow children well into adulthood.7 The significant impact of bullying on individuals and society as a whole mandates a multifaceted approach that begins in your exam room. What follows is practical advice on screening, counseling, and working with schools and the community at large to curb the bullying epidemic.

Clarifying the problem: The CDC’s definition
Recognizing that varying definitions of bullying were being used in research studies that looked at violent or aggressive behaviors in youth, the CDC published a consensus statement in 2014 that proposed the following definition for bullying:11 any unwanted aggressive behavior by another youth or group of youths who are not siblings or current dating partners that involves an observed or perceived power imbalance and is repeated multiple times or is highly likely to be repeated. This expanded on an earlier definition by Olweus12,13 that also identified a longitudinal nature and power imbalance as key features.
Types of bullying. Direct bullying entails blatant attacks on a targeted young person, while indirect bullying involves communication with others about the targeted individual (eg, spreading harmful rumors). Bullying may be physical, verbal, or relational (eg, excluding someone from their usual social circle, denying friendship, the silent treatment, writing mean letters, eye rolling, etc.) and may involve damage to property. Boys tend toward more direct bullying behaviors, while girls more often engage in indirect bullying, which may be more challenging for both adults and other students to recognize.12,13 With increased use of technology and social media by adolescents, cyberbullying has become increasingly more prevalent, with its effects on adolescent health and academics being every bit as profound as those of traditional bullying.14
About 1 in 4/5 students suffer. The prevalence of bullying ranges by country and culture. The vast majority of early bullying research was conducted in Norway, which found that approximately 15% of students in elementary and secondary schools were involved in bullying in some capacity.12 In a study involving over 200,000 adolescents from 40 European countries, 26% of adolescents reported being involved in bullying, ranging from 8.6% to 45.2% for boys and 4.8% to 35.8% for girls.15 Variations in prevalence may be due to cultural differences in the acts of bullying or differences in interpretation of the term “bullying.”1,15
In the United States, a 2001 survey of more than 15,000 students in public and private schools (grades 6-10) asked the students about their involvement in bullying: 13% said they'd been a bully, 10.6% a victim, and 6.3% said they'd been both.6 There was no significant difference in the frequency of self-reported bullying among urban, suburban, or rural settings.
Despite efforts to educate the public about bullying and work with schools to intervene and prevent bullying, incidence remains largely unchanged. In 2013, the National Crime Victimization Survey reported that approximately 22% of adolescents ages 12 through 18 were victims of bullying.16 Similarly, the CDC's 2015 Youth Risk Behavior Surveillance System reported that 20.2% of high school students experienced bullying on school property.17
Screening: Best practices
The FP’s role begins with screening children at risk for bullying (TABLE 118-22) or those whose complaints suggest that they may be victims of bullying.
Start screening when children enter elementary school
Given that providers’ time is limited for every patient visit, it is important to address bullying at times that are most likely to yield impactful results. The American Academy of Pediatrics recommends that the topic of bullying be introduced at the 6-year-old well-child visit (a typical age for entry to elementary school).7 Views in the literature are inconsistent regarding when and how to address bullying at other time points. One approach is to pre-screen those with risk factors associated with bullying (TABLE 118-22), and to focus screening on those with warning signs of bullying, which include mood disorders, psychosomatic or behavioral symptoms, substance abuse, self-harm behaviors, suicidal ideation or a suicide attempt, a decline in academic performance, and reports of school truancy. Parental concerns, such as when a child suddenly needs more money for lunch, is having aggressive outbursts, or is exhibiting unexplained physical injuries, should also be regarded as cues to screen.9

Screen patients in high-risk groups

A number of groups of children are at high risk for bullying and warrant targeted screening efforts.
- Children with special health needs. Research has shown that children with special health needs are at increased risk for being bullied.18 In fact, the presence of a chronic disease may increase the risk for bullying, and bullying often negatively impacts chronic disease management. As a result, it’s important to have a high index of suspicion with patients who have a chronic disease and who are not responding as expected to medical management or who experience deterioration after being previously well-controlled.18
- Children who are under- or overweight. Similarly, bullying based on a child's weight is a phenomenon that has been recognized to have a significant impact on children’s emotional health.19
- Youth who identify as lesbian, gay, bisexual, transgender, or queer/questioning (LGBTQ+) are more likely than non-LGBTQ+ peers to attempt suicide when exposed to a hostile social environment, such as that created by bullying.20

Screening need not be complicated

One screening approach is simply to ask patients, “Are you being bullied?” followed by such questions as, “How often are you bullied?” or “How long have you been bullied?” Asking about the setting of the bullying (Does it happen at school? Traveling to/from school? Online?) and other details may help guide interventions and the provision of resources.9 Another approach is to provide patients with some type of written survey (see TABLE 223 for an example) to encourage responses that patients might be reluctant to disclose verbally.23,24 (See “Barriers to screening.")
SIDEBAR
Barriers to screeningScreening for any condition presupposes a response. Ideally, family physicians should be prepared to provide basic counseling, resources, and, if necessary, treatment, if a patient screens positive for bullying. But screening for violence or bullying can be difficult, and evidence-based guidelines for screening and intervention are lacking, leaving many primary care practitioners feeling ill-equipped to meaningfully respond.
One study of the use of a screening tool aimed at intimate partner violence (IPV) showed that even with the availability of a screening tool, health care providers’ use of the tool was inconsistent and referral practices were ineffective.1 Providers cited the following limiting factors in screening for IPV: 1) a lack of immediate referral availability, 2) a lack of time during the office visit, and 3) a lack of confidence in the ability to screen.1 These same issues may be barriers to screening for bullying.
1. Ramachandran DV, Covarrubias L, Watson C, et al. How you screen is as important as whether you screen: a qualitative analysis of violence screening practices in reproductive health clinics. J Community Health. 2013;38:856-863.
Provider and parental interventions
Interventions often entail counseling the patient and the family about bullying and its effects, empowering victims and their caregivers, and screening for bullying comorbidities and correlates.2 Refer patients to behavioral health specialists when there is evidence of pervasive effects on mood, behavior, or social development, but keep in mind that counseling can begin in your own exam room.
Effective discussion starters. Affirming the problem and its unacceptability, talking about the different types of bullying and where bullying may occur, and asking about patient perceptions of bullying can be effective discussion starters. FPs should help patients identify bullying, open lines of communication between children and their parents and between parents and other caregivers, and demonstrate respect and kindness in their approach to discussing the topic. Encourage children to speak with trusted adults when exposed to bullying. Talk to them about standing up to bullies (saying “stop” confidently or walking away from difficult situations) and staying safe by staying near adults or groups of peers when bullies are present (TABLE 325).
Empowering caregivers. Encourage parents to spend time each day talking with their child about the child’s time away from home (TABLE 325). Counsel parents/caregivers to expand their role. Knowing a child’s friends, encouraging the child academically, and increasing communication are all associated with lower risks of bullying.26 Similarly, parental oversight of Internet and social media use is associated with decreased participation in cyberbullying.27
In addition, the Positive Parenting telephone-based parenting education curriculum has been shown to decrease bullying, physical fighting, physical injuries, and victimization of children.28 The research-based, family strengthening program emphasizes 3 core elements of authoritative parenting: nurturance, discipline, and respect or granting of psychologic autonomy. The program entails 15- to 30-minute weekly phone conversations between parents and educators, as well as videos and a manual.

Are community programs in place—or are they needed?
Many schools have robust, state-mandated programs in place to identify bullying and provide support for students who are victims of bullying. (See “NJ’s harassment and bullying protocol: A case in point.”) Explaining this to victims and their families may help them come forward and seek assistance. FPs who want to advocate for their patients should start with local schools to support such programs and link students at risk with school counselors.
SIDEBAR
NJ's harassment and bullying protocol: A case in pointThere is no federal law that specifically applies to bullying, but all 50 states have some type of anti-bullying legislation on the books, and 40 of those states have additional detailed policies in place addressing the subject.1
New Jersey, for example, began enforcing one of the toughest harassment, intimidation, and bullying (HIB) protocols in the country back in September 20112 in the wake of the death of Rutgers University freshman Tyler Clementi, who committed suicide after his roommate allegedly shot a video of him with another man and posted it to the Internet.3 Among many other things, New Jersey’s legislation stipulates in its Anti-Bullying Bill of Rights4 that:
› Every school/district have plans in place that clearly define, prevent, prohibit, and promptly deal with acts of harassment, intimidation, or bullying, on school grounds, at school-sponsored functions, and on school buses.
› Plans must include a description of the type of behavior expected from each student and the consequences and remedial action for a person who commits an act of harassment, intimidation, or bullying. Student perpetrators may be suspended or expelled if convicted of any type of bullying, whether it be for teasing or something more severe.
› All school employees must act on any incidents of bullying reported to, or witnessed by, them and report such incidents on the same day to the school principal.
› Plans must include provisions and deadlines for investigating and resolving all matters in a timely fashion; investigations into allegations of bullying must be launched within one day.
› Every case of bullying must be reported to the state. Schools are graded by the state on their compliance with anti-bullying standards and policies and their handling of incidents.
› Schools must appoint safety teams made up of parents, teachers, and staff, and school personnel and students must receive extensive anti-bullying training.
1. US Department of Health and Human Services. Stopbullying.gov. Policies and laws. Available at: https://www.stopbullying.gov/laws/index.html.
Accessed January 5, 2017.
2. State of New Jersey Department of Education. An overview of amendments to laws on harassment, intimidation, and bullying. Available at: http://www.state.nj.us/education/students/safety/behavior/hib/overview.pdf. Accessed January 5, 2017.
3. Cohen A. Case study: Why New Jersey’s antibullying law should be a model for other states. Time. September 6, 2011. Available at: http://ideas.time.com/2011/09/06/why-new-jerseys-antibullying-law-should-be-a-model-for-other-states/. Accessed January 5, 2017.
4. New Jersey Legislature. Anti-bullying Bill of Rights Act. Available at: http://www.njleg.state.nj.us/2010/Bills/PL10/122.PDF. Accessed January 5, 2017.
If programs are lacking in your community, there is much you can do to educate yourself about successful programs and advise local community organizations and schools about them. Among the most successful and well-studied interventions for thwarting the bullying epidemic have been school-based community ones. The most studied of these is the Olweus Bullying Prevention Program (OBPP), which is based on 4 principles:1,29
- Adults both at home and at school should take a positive and encouraging interest in students.
- Unacceptable behavior should have strict and well-known limits.
- Sanctions should be applied consistently and should be non-hostile in nature.
- Adults both at home and in the educational environment should act as authorities.
In short, the program focuses on greater awareness and involvement on the part of adults, and employing measures at the school level (eg, surveys, better supervision during break and lunch times), the class level (eg, rules against bullying, regular class meetings with students), and the individual level (eg, serious talks with bullies, victims, parents of involved students).
Research has shown that the OBPP reduces bullying behaviors by as much as 50%, reduces vandalism and truancy, and reduces the number of new victims.12 Limits to the more widespread implementation of the OBPP have consisted mainly of the inability to appropriately train adults, including teachers and other school personnel in educational settings. Despite these limitations, the OBPP has been praised and endorsed by numerous groups, including the US Department of Justice.30
Other non-curricular, school-based programs exist, such as the School-Wide Positive Behavioral Interventions and Supports (SWPBIS). This program is a school-wide prevention strategy aimed at: 1) reducing behavior problems that lead to office discipline referrals and suspensions, and 2) changing perceptions of school safety. (For more information, see https://www.crimesolutions.gov/ProgramDetails.aspx?ID=385 and https://www.pbis.org/school/swpbis-for-beginners.)31
The research-based Second Step: Student Success Through Prevention (SS-SSTP) Middle School Program (http://www.cfchildren.org/second-step/middle-school)32 focuses on the often difficult middle school years. The program helps schools teach and model essential communication, coping, and decision-making skills to help adolescents navigate around common pitfalls such as peer pressure, substance abuse, and bullying (both in-person and online). The program aims to reduce aggression and provide support for a more inclusive environment that helps students stay in school, make good choices, and experience social and academic success.
The Positive Action Program (https://www.positiveaction.net/research/primer),33 which is predicated on the notion that we feel good about ourselves when we do positive things, features scripted lessons and kits of materials (eg, posters, games, worksheets, puzzles) appropriate for each grade level.
CASE › Stacey’s visit to her FP’s office has presented several clues that she may be a victim of bullying. Her mild persistent asthma appears to no longer be as well controlled as it was in the past. Direct questioning has revealed that 2 girls at school have been making fun of Stacey when she uses her inhaled corticosteroid in the morning before class, so she has stopped using it. These same students are on her cheerleading team, so she quit the team to avoid them. Her school-related anxiety is so great that she no longer pays attention in math class and is constantly worried that something is being posted about her online.
Stacy’s FP responds to this information with a multifaceted approach. In the exam room, he screens Stacy for depression. While she is negative and denies any suicidal ideation, Stacy is clearly having anxiety, so the FP refers Stacey to a counselor at a local mental health clinic. With Stacy’s permission, the FP discusses the issue with her mother and they decide together with Stacy that she should talk to a teacher at school about the ongoing bullying. Because this was not the first time that the FP has heard this from a child in the community, the FP plans to attend an upcoming school board meeting to advocate for an evidence-based bullying prevention program to help curb the ongoing problem facing his patients.
CORRESPONDENCE
Robert McClowry, MD, Department of Family and Community Medicine, Jefferson Family Medicine Associates, 833 Chestnut East, 3rd Floor, Suite 301, Philadelphia, PA, 19107-4414; [email protected].
1. Olweus D, Limber SP. Bullying in school: evaluation and dissemination of the Olweus Bullying Prevention Program. Am J Orthopsychiatry. 2010;80:124-134.
2. Lyznicki JM, McCaffree MA, Robinowitz CB, et al. Childhood bullying: implications for physicians. Am Fam Physician. 2004;70:1723-1730.
3. Temkin D. All 50 states now have a bullying law. Now what? The Huffington Post. April 27, 2015. Available at: http://www.huffingtonpost.com/deborah-temkin/all-50-states-now-have-a_b_7153114.html. Accessed January 5, 2017.
4. Anderson M, Kaufman J, Simon TR, et al. School-associated violent deaths in the United States, 1994-1999. JAMA. 2001;286:2695-2702.
5. Centers for Disease Control and Prevention. Injury prevention and control: Data and statistics (WISQARS). Ten leading causes of death and injury. Available at: https://www.cdc.gov/injury/wisqars/leadingcauses.html. Accessed January 5, 2017.
6. Nansel TR, Overpeck M, Pilla RS, et al. Bullying behaviors among US youth: prevalence and association with psychosocial adjustment. JAMA. 2001;285:2094-2100.
7. Committee on Injury, Violence, and Poison Prevention. Policy statement—Role of the pediatrician in youth violence prevention. Pediatrics. 2009;124:393-402.
8. Klein DA, Myhre KK, Ahrendt DM. Bullying among adolescents: a challenge in primary care. Am Fam Physician. 2013;88:87-92.
9. Lamb J, Pepler DJ, Craig W. Approach to bullying and victimization. Can Fam Physician. 2009;55:356-360.
10. Spector ND, Kelly SF. Pediatrician’s role in screening and treatment: bullying, prediabetes, oral health. Curr Opin Pediatr. 2006;18:661-670.
11. Gladden RM, Vivolo-Kantor AM, Hamburger ME, et al. Bullying surveillance among youths: uniform definitions for public health and recommended data elements. National Center for Injury Prevention and Control, Centers for Disease Control and Prevention and US Department of Education; 2014. Available at: https://www.cdc.gov/violenceprevention/pdf/bullying-definitions-final-a.pdf. Accessed June 8, 2016.
12. Olweus D. Bullying at school: basic facts and effects of a school based intervention program. J Child Psychol Psychiatry. 1994;35:1171-1190.
13. Olweus D. Bully/victim problems in school: Facts and intervention. Eur J Psychol Educ. 1997;12:495-510.
14. Kowalski RM, Limber SP. Psychological, physical, and academic correlates of cyberbullying and traditional bullying. J Adolesc Health. 2013;53:S13-S20.
15. Craig W, Harel-Fisch Y, Fogel-Grinvald H, et al. A cross-national profile of bullying and victimization among adolescents in 40 countries. Int J Public Health. 2009;54(Suppl 2):216-224.
16. US Department of Education, National Center for Educational Statistics (2015). Student reports of bullying and cyberbullying: results from the 2013 School Crime Supplement to the National Victimization Survey. Available at: http://nces.ed.gov/pubsearch/pubsinfo.asp?pubid=2015056. Accessed November 9, 2016.
17. Kann L, McManus T, Harris WA, et al. Youth risk behavior surveillance - United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:1-174.
18. Van Cleave J, Davis MM. Bullying and peer victimization among children with special health care needs. Pediatrics. 2006;118:e1212-e1219.
19. Eisenberg ME, Neumark-Sztainer D, Story M. Associations of weight-based teasing and emotional well-being among adolescents. Arch Pediatr Adolesc Med. 2003;157:733-738.
20. Hatzenbuehler ML. The social environment and suicide attempts in lesbian, gay, and bisexual youth. Pediatrics. 2011;127:896-903.
21. Song LY, Singer MI, Anglin TM. Violence exposure and emotional trauma as contributors to adolescents’ violent behaviors. Arch Pediatr Adolesc Med. 1998;152:531-536.
22. Singer MI, Anglin TM, Song LY, et al. Adolescents’ exposure to violence and associated symptoms of psychological trauma. JAMA. 1995;273:477-482.
23. Glew GM, Fan MY, Katon W, et al. Bullying, psychosocial adjustment, and academic performance in elementary school. Arch Pediatr Adolesc Med. 2005;159:1026-1031.
24. Waseem M, Ryan M, Foster CB, et al. Assessment and management of bullied children in the emergency department. Pediatr Emerg Care. 2013;29:389-398.
25. US Department of Health and Human Services. stopbullying.gov. Available at: https//www.stopbullying.gov. Accessed January 5, 2017.
26. Shetgiri R, Lin H, Avila RM, et al. Parental characteristics associated with bullying perpetration in US children aged 10 to 17 years. Am J Public Health. 2012;102:2280-2286.
27. Hinduja S, Patchin JW. Social influences on cyberbullying behaviors among middle and high school students. J Youth Adolesc. 2013;42:711-722.
28. Borowsky IW, Mozayeny S, Stuenkel K, et al. Effects of a primary care-based intervention on violent behavior and injury in children. Pediatrics. 2004;114:e392-e399.
29. Olweus D. Bully/victim problems in school: Facts and intervention. Eur J Psychol Educ. 1997;12:495-510.
30. Mihalic SF. Blueprints for Violence Prevention: Report. US Department of Justice, Office of Justice Programs, Office of Juvenile Justice and Delinquency Prevention; 2004.
31. Waasdorp TE, Bradshaw CP, Leaf PJ. The impact of schoolwide positive behavioral interventions and supports on bullying and peer rejection: a randomized controlled effectiveness trial. Arch Pediatr Adolesc Med. 2012;166:149-156.
32. Espelage DL, Low S, Polanin JR, et al. The impact of a middle school program to reduce aggression, victimization, and sexual violence. J Adolesc Health. 2013;53:180-186.
33. Lewis KM, Schure MB, Bavarian N, et al. Problem behavior and urban, low-income youth: a randomized controlled trial of positive action in Chicago. Am J Prev Med. 2013;44:622-630.
1. Olweus D, Limber SP. Bullying in school: evaluation and dissemination of the Olweus Bullying Prevention Program. Am J Orthopsychiatry. 2010;80:124-134.
2. Lyznicki JM, McCaffree MA, Robinowitz CB, et al. Childhood bullying: implications for physicians. Am Fam Physician. 2004;70:1723-1730.
3. Temkin D. All 50 states now have a bullying law. Now what? The Huffington Post. April 27, 2015. Available at: http://www.huffingtonpost.com/deborah-temkin/all-50-states-now-have-a_b_7153114.html. Accessed January 5, 2017.
4. Anderson M, Kaufman J, Simon TR, et al. School-associated violent deaths in the United States, 1994-1999. JAMA. 2001;286:2695-2702.
5. Centers for Disease Control and Prevention. Injury prevention and control: Data and statistics (WISQARS). Ten leading causes of death and injury. Available at: https://www.cdc.gov/injury/wisqars/leadingcauses.html. Accessed January 5, 2017.
6. Nansel TR, Overpeck M, Pilla RS, et al. Bullying behaviors among US youth: prevalence and association with psychosocial adjustment. JAMA. 2001;285:2094-2100.
7. Committee on Injury, Violence, and Poison Prevention. Policy statement—Role of the pediatrician in youth violence prevention. Pediatrics. 2009;124:393-402.
8. Klein DA, Myhre KK, Ahrendt DM. Bullying among adolescents: a challenge in primary care. Am Fam Physician. 2013;88:87-92.
9. Lamb J, Pepler DJ, Craig W. Approach to bullying and victimization. Can Fam Physician. 2009;55:356-360.
10. Spector ND, Kelly SF. Pediatrician’s role in screening and treatment: bullying, prediabetes, oral health. Curr Opin Pediatr. 2006;18:661-670.
11. Gladden RM, Vivolo-Kantor AM, Hamburger ME, et al. Bullying surveillance among youths: uniform definitions for public health and recommended data elements. National Center for Injury Prevention and Control, Centers for Disease Control and Prevention and US Department of Education; 2014. Available at: https://www.cdc.gov/violenceprevention/pdf/bullying-definitions-final-a.pdf. Accessed June 8, 2016.
12. Olweus D. Bullying at school: basic facts and effects of a school based intervention program. J Child Psychol Psychiatry. 1994;35:1171-1190.
13. Olweus D. Bully/victim problems in school: Facts and intervention. Eur J Psychol Educ. 1997;12:495-510.
14. Kowalski RM, Limber SP. Psychological, physical, and academic correlates of cyberbullying and traditional bullying. J Adolesc Health. 2013;53:S13-S20.
15. Craig W, Harel-Fisch Y, Fogel-Grinvald H, et al. A cross-national profile of bullying and victimization among adolescents in 40 countries. Int J Public Health. 2009;54(Suppl 2):216-224.
16. US Department of Education, National Center for Educational Statistics (2015). Student reports of bullying and cyberbullying: results from the 2013 School Crime Supplement to the National Victimization Survey. Available at: http://nces.ed.gov/pubsearch/pubsinfo.asp?pubid=2015056. Accessed November 9, 2016.
17. Kann L, McManus T, Harris WA, et al. Youth risk behavior surveillance - United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:1-174.
18. Van Cleave J, Davis MM. Bullying and peer victimization among children with special health care needs. Pediatrics. 2006;118:e1212-e1219.
19. Eisenberg ME, Neumark-Sztainer D, Story M. Associations of weight-based teasing and emotional well-being among adolescents. Arch Pediatr Adolesc Med. 2003;157:733-738.
20. Hatzenbuehler ML. The social environment and suicide attempts in lesbian, gay, and bisexual youth. Pediatrics. 2011;127:896-903.
21. Song LY, Singer MI, Anglin TM. Violence exposure and emotional trauma as contributors to adolescents’ violent behaviors. Arch Pediatr Adolesc Med. 1998;152:531-536.
22. Singer MI, Anglin TM, Song LY, et al. Adolescents’ exposure to violence and associated symptoms of psychological trauma. JAMA. 1995;273:477-482.
23. Glew GM, Fan MY, Katon W, et al. Bullying, psychosocial adjustment, and academic performance in elementary school. Arch Pediatr Adolesc Med. 2005;159:1026-1031.
24. Waseem M, Ryan M, Foster CB, et al. Assessment and management of bullied children in the emergency department. Pediatr Emerg Care. 2013;29:389-398.
25. US Department of Health and Human Services. stopbullying.gov. Available at: https//www.stopbullying.gov. Accessed January 5, 2017.
26. Shetgiri R, Lin H, Avila RM, et al. Parental characteristics associated with bullying perpetration in US children aged 10 to 17 years. Am J Public Health. 2012;102:2280-2286.
27. Hinduja S, Patchin JW. Social influences on cyberbullying behaviors among middle and high school students. J Youth Adolesc. 2013;42:711-722.
28. Borowsky IW, Mozayeny S, Stuenkel K, et al. Effects of a primary care-based intervention on violent behavior and injury in children. Pediatrics. 2004;114:e392-e399.
29. Olweus D. Bully/victim problems in school: Facts and intervention. Eur J Psychol Educ. 1997;12:495-510.
30. Mihalic SF. Blueprints for Violence Prevention: Report. US Department of Justice, Office of Justice Programs, Office of Juvenile Justice and Delinquency Prevention; 2004.
31. Waasdorp TE, Bradshaw CP, Leaf PJ. The impact of schoolwide positive behavioral interventions and supports on bullying and peer rejection: a randomized controlled effectiveness trial. Arch Pediatr Adolesc Med. 2012;166:149-156.
32. Espelage DL, Low S, Polanin JR, et al. The impact of a middle school program to reduce aggression, victimization, and sexual violence. J Adolesc Health. 2013;53:180-186.
33. Lewis KM, Schure MB, Bavarian N, et al. Problem behavior and urban, low-income youth: a randomized controlled trial of positive action in Chicago. Am J Prev Med. 2013;44:622-630.
PRACTICE RECOMMENDATIONS
› Suspect bullying when children with chronic conditions that were stable begin deteriorating for unexplained reasons or when children become non-adherent to medication regimens. C
› Empower not only patients, but also parents/caregivers, to take action and deter bullying behaviors. B
› Support school-based and community-oriented intervention programs, which have been shown to be among the most effective strategies for curbing bullying. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Heart failure: Best options when ejection fraction is preserved
• Suspect diastolic heart failure in patients who have symptoms of heart failure but a normal ejection fraction, with or without evidence of diastolic abnormalities. B
• Treatment goals for patients who have heart failure with preserved ejection fraction (HFPEF) include normalization of blood pressure, prevention of tachycardia and ischemia, reduction of congestion, and improvement in exercise capacity. B
• Initiate beta-blocker therapy without delay for patients who have acute decompensated HFPEF and tachycardia; consider cardioversion for those with atrial fibrillation. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Most studies of heart failure (HF)—the most common cause of hospitalization in patients older than 65 years1—have focused on patients with reduced ejection fraction (EF). Yet half of those hospitalized for acute decompensated HF have a normal left ventricular EF.2 For these patients, contractility is not the problem—impaired relaxation during diastole is.
Commonly called diastolic HF, heart failure with preserved ejection fraction (HFPEF) is a more precise name for this condition. Patients are usually older than those with a reduced EF.3 Thus, as the US population ages, the prevalence of HFPEF increases, as well.4
Diagnostic criteria have been developed for HFPEF, but there are few large, high-quality studies to guide its treatment. Yet family physicians need to be familiar with HFPEF and know how best to treat it. With extrapolation from studies of patients with reduced EF, as well as expert consensus and our own experience, we offer an evidence-based approach to the management of both stable and acute decompensated HFPEF.
A closer look at diastolic dysfunction
Defined as an abnormality of diastolic compliance, filling, or relaxation of the ventricle, diastolic dysfunction can occur whether EF is normal or abnormal.3 Ventricular diastole includes isovolumic relaxation, early passive filling after mitral valve opening, and active filling during atrial contraction. Transmission of high ventricular pressure to the pulmonary circulation leads to pulmonary edema, dyspnea, and other symptoms of HF. Factors other than abnormal diastolic physiology, such as chronic volume overload, ventricular coupling dyssynchrony, increased autonomic tone leading to reduced venous capacitance, and chronotropic intolerance, may also be involved.5
Patient history: What to look for
A variety of conditions, including ischemia, tachycardia, impaired myocardial relaxation, and age-related loss of myocardial compliance, can contribute to abnormal diastolic function, but the major causes of HFPEF are chronic hypertension, hypertrophic cardiomyopathy, and coronary artery disease (CAD).3 Rarely, infiltrative or restrictive cardiomyopathy (eg, amyloidosis or sarcoidosis) is implicated.6 Noncardiovascular comorbidities such as diabetes, renal impairment, anemia, and chronic lung disease are more prevalent among those with HFPEF, and more women are affected than men.1
Mortality risk. In a study of more than 100,000 hospitalizations for acute decompensated HF, patients with preserved EF had lower in-hospital mortality (3% vs 4% for those with reduced EF).2 Patients with both diabetes and CAD commonly develop HFPEF,7 and the presence of these comorbidities are an independent predictor of 5-year mortality.8
Population studies suggest that 5-year mortality rates for African Americans with HFPEF are higher than for Caucasians with this condition.9 Other predictors of mortality include older age, male sex, lower left ventricular EF, ischemic disease, impaired renal function, and peripheral arterial disease.10-12
Diagnosing HFPEF: What you’ll see, when to test
The presentation of patients with HFPEF is similar to that of individuals with reduced EF. In an outpatient setting, both groups will have reduced exercise capacity; increased neuroendocrine activation, which may cause chronic fluid retention, vasoconstriction, and tachycardia; and a reduced quality of life.5
Neither the American College of Cardiology/American Heart Association (ACC/AHA) nor the Heart Failure Society of America (HFSA)13,14 recommends screening for asymptomatic left ventricular dysfunction. For those with signs and symptoms of HF, however, echocardiography is a key component of the initial evaluation. Echocardiography provides information about left ventricular systolic function, including EF, regional wall motion abnormalities, and wall thickness. Echocardiographic evidence of diastolic abnormalities is found for some patients with HFPEF, while others have no demonstrable diastolic dysfunction.3
While an electrocardiogram (EKG) cannot distinguish between HF with reduced EF and HFPEF, common findings might include signs of ventricular hypertrophy or tachycardia during acute exacerbations. An EKG should be obtained in patients with suspected HF to screen for antecedent causes such as hypertrophy, atrial fibrillation, and ischemia.15
What Doppler echocardiography and the E/A ratio reveal
Doppler echocardiography is used to further evaluate the characteristics of blood flow, showing the relationship among left ventricular (LV) relaxation, atrial pressure, atrial contraction, and blood flow velocity across the mitral valve during diastole. The peak velocity of blood flow during early diastole (called the “E wave”) and late diastole (the atrial contraction, or “A wave”) is measured and the E/A ratio (reflecting the transmitral blood flow pattern) is calculated (FIGURE).3
FIGURE
The E/A ratio* and what it reveals
A, atrial contraction; E, early passive filling; MVC, mitral valve closes; MVO, mitral valve opens.
*E/A ratio represents the relationship between the peak velocity of blood flow during early diastole (E wave) and late diastole (A wave).
Adapted from: Aurigemma GP, Gaasch WH. N Engl J Med. 2004.3
Normally, transmitral flow velocity is greater during early diastole than during atrial contraction, and the E/A ratio is approximately 1.5 (E>A). With early diastolic dysfunction, impaired relaxation prevents blood from flowing passively into the LV during early diastole. This causes reversal of the E/A ratio, which drops to <1 (E<A). As diastolic function worsens, atrial contraction is impaired, and left atrial pressure rises. The result: A reduction in the A wave amplitude and proportionally more blood flow during early diastole and a “pseudonormal” (E>A) ratio, with a greater difference between the E and A than is normally observed. This finding is an independent predictor of all-cause mortality in patients with asymptomatic HF.16
Cardiac catheterization. Invasive measurement of LV filling pressures is the gold standard for diagnosing HFPEF. If echocardiography does not lead to a clear diagnosis, cardiac catheterization can provide information about concomitant pulmonary hypertension and mechanical asynchrony that may contribute to symptomatic HF.1 When the diagnosis is uncertain, additional testing—eg, plasma brain natriuretic peptide (BNP), chest x-ray, or exercise testing—may be necessary to establish a diagnosis of symptomatic HF.
The diagnostic criteria developed by HFSA include clinical evidence of HF and:
- echocardiographic evidence of LV hypertrophy or left atrial enlargement (without atrial fibrillation) or
- evidence of diastolic dysfunction on Doppler echocardiography or cardiac catheterization.14
It is important to note that the diagnostic criteria have not been validated, and the sensitivity and specificity of the various clinical findings are not known.
CASE Carrie W, a 76-year-old woman referred to you by a colleague, presents for follow-up after being hospitalized for HF. She recalls feeling fatigue, chest pain, and out of breath with even minimal exertion before being admitted to the hospital.
You obtain her hospital records, which show that echocardiography found impaired LV relaxation based on a reversed E/A ratio and an EF of 65%. In addition, BNP was elevated, and a chest x-ray showed pulmonary vascular congestion. You note that her blood pressure was 175/103 mm Hg on admission and an EKG showed LV hypertrophy and sinus tachycardia, but no ischemia.
Before being hospitalized, Ms. W was taking extended-release metoprolol, aspirin, and lisinopril. The hospitalist added lovastatin and increased the daily dose of extended-release metoprolol from 25 to 100 mg.
What changes, if any, would you make in her medication regimen?
Diastolic dysfunction as chronic disease
Often asymptomatic, diastolic dysfunction should be thought of as a chronic progressive disease characterized by complex physiologic adaptations that vary over time (See “Staging heart failure: The clinical course of HFPEF”.13) Patients with HFPEF have a difficult time tolerating hemodynamic stress and any perturbation of afterload, heart rate, or ventricular function can precipitate an acute exacerbation.2 Clinical factors that precipitate acute decompensation of HFPEF—which we’ll discuss a bit later—include uncontrolled hypertension; atrial fibrillation; and noncardiovascular comorbidities such as lung disease, renal impairment, or sepsis.2
The ACC/AHA staging system for HF can be applied to patients with HFPEF, both to classify disease severity and to track the progression of the disease. Patients at Stage A are at high risk of developing HF, but early and aggressive treatment of hypertension and other cardiovascular risk factors may delay or potentially prevent the onset of overt disease. Stage B refers to patients with known structural disease, such as a history of myocardial infarction or systolic or diastolic dysfunction, but no symptoms of HF.
Patients at Stage C have evidence of structural disease and symptoms of HF, such as fatigue, shortness of breath, or reduced exercise tolerance. This stage represents the spectrum of patients falling into New York Heart Association (NYHA) Class 1 through 3 categories. Finally, patients at Stage D—analogous to NYHA Class 4—have refractory HF, with marked symptoms even at rest despite maximal medical therapy.
The Acute Decompensated HEart failure national REgistry (ADHERE), in which the records of well over 80,000 Medicare patients were reviewed, found that more than 60% of those hospitalized with HFPEF had uncontrolled hypertension, with a systolic pressure >140 mm Hg; 21% had atrial fibrillation.2 These findings emphasize the importance of aggressive blood pressure (BP) and heart rate control.
Management of HFPEF is goal directed
The aim of pharmacologic treatment of HFPEF is to maintain fluid balance, prevent tachycardia, treat and prevent ischemia, and control hypertension (TABLE).14,17-30 While the use of angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and beta-blockers, among other pharmacologic agents, is well studied for patients with reduced EF, there is limited evidence to guide the treatment of those with HFPEF. Although no single agent or drug class has been shown to be superior for such patients, there are a number of pharmacologic treatments to consider.
TABLE
Management of heart failure with preserved ejection fraction—matching treatment and goals14,17-30
| Treatment goal | Modality |
|---|---|
| Reduce congestion | Diuretics Salt restriction |
| Maintain atrial contraction | A-V pacing Cardioversion |
| Prevent tachycardia | A-V pacing Beta-blockers Calcium channel blockers |
| Prevent/treat ischemia | Antiplatelet therapy Beta-blockers Calcium channel blockers Revascularization Statins |
| Control hypertension | Antihypertensive agents:
|
| Promote regression of LV remodeling | ACE inhibitors ARBs |
| Improve exercise capacity | Supervised exercise program |
| ACE, angiotensin-converting enzyme; ARBs, angiotensin receptor blockers; LV, left ventricle. | |
Inhibition of the renin-angiotensin-aldosterone system
Pathologic activation of the renin-angiotensin-aldosterone system (RAAS) contributes to elevated systolic and diastolic pressure, LV hypertrophy, and LV fibrosis. Inhibition of this system is a promising treatment modality for HFPEF.31
ACE inhibitors. Experimental studies suggest that ACE inhibitors benefit the diastolic properties of the heart, in both short- and long-term use. The PEP-CHF trial found that for older patients with diastolic dysfunction, perindopril led to significant improvements in functional class and exercise capacity but failed to show a statistically significant reduction in all-cause mortality or hospitalization for acute decompensated HF.17
ARBs. There is no evidence to show that ARB therapy improves morbidity or mortality in HFPEF. Using surrogate end points, ARBs have been associated with regression of LV hypertrophy, and losartan was found to improve exercise tolerance and quality of life, compared with hydrochlorothiazide.18,19 In the CHARM-Preserved trial, candesartan showed an insignificant reduction in cardiovascular mortality and hospitalization for HF.
These results must be viewed with caution, however, because adverse effects led to high rates of medication discontinuation.32 In the I-PRESERVE trial, irbesartan conferred no benefit with respect to mortality, hospitalization, or quality of life on patients with HFPEF.33
ACE inhibitor or ARB—not both. ACE inhibitors and ARBs are good choices for BP control in patients with HFPEF, especially if LV hypertrophy is present, but periodic testing of renal function and potassium levels is needed. ACE inhibitors and ARBs should not be used concurrently, as the combination increases the risk of acute renal failure and has no benefit in clinical outcomes.34
BP and rate control
In small trials, beta-blockers have been found to improve diastolic function as seen on echocardiography, but data on morbidity and mortality are lacking.20 A secondary analysis of the OPTIMIZE-HF registry found that beta-blocker therapy was associated with reduced mortality and readmission in patients with reduced EF, but not in those with normal EF.21
Findings from the SENIORS trial were more promising: Treatment with nebivolol reduced both mortality and readmission rates for elderly patients with HF, with similar benefits for those with reduced and preserved EF.22 Overall, beta-blockers appear to be a reasonable choice for heart rate and/or BP control in patients who have HFPEF and atrial fibrillation or hypertension. Carvedilol, long-acting metoprolol, and bisoprolol have been shown to reduce mortality in HF with reduced EF, and it is reasonable to choose one of these agents for patients with preserved EF, as well.23
Calcium channel blockers (CCBs) may be useful in treating patients with HFPEF for both BP and heart rate control, as well. Theoretically, CCBs may also improve the process of relaxation by altering intracellular calcium cycling during the contractile cycle in myocytes. This contrasts with the management of HF patients with reduced EF, for whom the use of nonselective CCBs such as diltiazem and verapamil may adversely affect contractility.
In small RCTs, verapamil has been found to improve HF symptoms and exercise tolerance in patients with HFPEF,24 but no evidence of improved outcomes or mortality rates with CCB use has been found.
Other pharmacologic options to consider
Aldosterone antagonist therapy is an important component of treatment for patients with HF with reduced EF. Data supporting the use of spironolactone use from the RALES trial and eplerenone in the EPHESUS and EMPHASIS-HF trials suggest a reduction in mortality in patients with low (<35%) LVEF.25-27 For patients with preserved EF, however, spironolactone is not generally recommended.
A large National Institutes of Health-sponsored trial is underway to determine if the drug is beneficial for patients with preserved LVEF, and will build on a small study in which 30 patients with HFPEF showed improved myocardial function after treatment with spironolactone.35 Until more data become available, the risks of using aldosterone antagonists outweigh the evidence to support their use in this patient population.
Diuretics are an important component of treatment for all patients with HF and fluid overload. The Antihypertensive and Lipid-Lowering treatment to prevent Heart Attack Trial (ALLHAT) showed a reduced incidence of symptomatic HFPEF in patients taking diuretics.28 As is the case with patients with reduced EF, those with preserved EF should be treated with diuretics if they have symptoms of fluid overload.
Statins. Intensive lipid lowering with statin therapy has been shown in observational studies to benefit patients with HFPEF with respect to mortality, independent of baseline low-density lipoprotein cholesterol.29 RCTs are needed to confirm these observations, but statin therapy is recommended for the secondary prevention of cardiovascular disease, independent of the presence of diastolic dysfunction or HFPEF.
Guard against hypotension. Patients with diastolic dysfunction are susceptible to hypotension if there is a rapid reduction in preload with diuretics, nonselective CCBs, or nitrates, so it is important that doses be titrated slowly.
Nonpharmacologic measures are important, too
In addition to optimizing treatment of comorbid conditions, patients with HFPEF should be advised that lifestyle modifications such as weight loss, smoking cessation, and dietary changes can do much to reduce the risk. You can help by providing an exercise “prescription” (with a specified intensity, frequency, and duration) and dietary guidelines, with emphasis on the importance of a low-sodium diet to prevent fluid overload.14,30 Recommend local programs for patients with HF, which many hospitals and health systems offer as part of their efforts to reduce readmission rates.
Consider cardioversion
Tachycardia shortens the time for filling during diastole; thus, it is poorly tolerated in patients with diastolic dysfunction and could trigger acute decompensation. To avoid the risk, restoration of sinus rhythm should be considered for patients with HFPEF and atrial fibrillation. Patients with known paroxysmal or permanent atrial fibrillation and preserved EF should be seen by a cardiologist to determine whether direct current cardioversion or ablation with a permanent pacemaker is appropriate.11 When cardioversion is contraindicated, a beta-blocker is needed to control heart rate and improve hemodynamics.
Patients with stable angina and HFPEF should be evaluated for revascularization when medical therapy alone is not sufficient for symptom relief.10 Here, too, a cardiology consult is indicated for any patient who has HF and an abnormal noninvasive stress test or persistent symptoms despite optimal drug therapy.
Recognizing and responding to acute decompensated HFPEF
The initial response to acute decompensated HFPEF, like that of HF with reduced EF, should be focused on restoring volume status and providing oxygenation, ventilation, and vasodilator therapy in some cases.11 Unlike those with acute decompensated HF with reduced EF, however, patients with HFPEF can safely tolerate the initiation of beta-blockers in the acute phase, especially when rate control is needed.3 Inotropic agents like digoxin and dobutamine, however, are contraindicated.3
Guidelines recommend hospitalization for patients with abnormal vital signs, arrhythmia, and suspected acute coronary syndromes, and consideration of hospitalization for those with associated comorbid conditions, new HF, or progressive fluid overload.13
CASE Because Ms. W has a normal BP and heart rate and is feeling well, you decline to alter her medication regimen. You do, however, recommend that she begin an exercise program, adopt a low-sodium diet, and maintain regular contact with your office so you can evaluate any changes in status.
You introduce Ms. W to the nurse case manager in your office. The nurse works with the patient to develop an action plan that includes daily tracking of her weight and sodium intake; a progressive walking program, starting with 2-minute sessions and progressing to 15 to 30 minutes 3 to 5 times a week; weekly telephone checkins; and immediate calls to report any weight increase or symptoms of HF.
At follow-up 6 months later, Ms. W has improved BP and reports that she enjoys her new exercise routine. She has more energy and denies any edema or breathing difficulties.
1. Lam CSP, Donal E, Kraigher-Krainer E, et al. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:18-28.
2. Yancy CW, Lopatin M, Stevenson LW, et al. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the acute decompensated heart failure national registry (ADHERE) database. J Am Coll Cardiol. 2006;47:76-84.
3. Aurigemma GP, Gaasch WH. Diastolic heart failure. N Engl J Med. 2004;351:1097-1105.
4. Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251-259.
5. Bench T, Burkhoff D, O’Connell JB, et al. Heart failure with normal ejection fraction: consideration of mechanisms other than diastolic dysfunction. Curr Heart Fail Rep. 2009;6:57-64.
6. Ammash NM, Seward JB, Bailey KR, et al. Clinical profile and outcome of idiopathic restrictive cardiomyopathy. Circulation. 2000;101:2490-2496.
7. Bell DSH. Diabetic cardiomyopathy. Diabetes Care. 2003;26:2949-2951.
8. From AM, Scott CG, Chen HH. The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction: a population-based study. J Am Coll Cardiol. 2010;55:300-305.
9. East MA, Peterson ED, Shaw LK, et al. Racial differences in the outcomes of patients with diastolic heart failure. Am Heart J. 2004;148:151-156.
10. Ahmed A, Aronow WS, Fleg JL. Higher New York Heart Association classes and increased mortality and hospitalization in patients with heart failure and preserved left ventricular function. Am Heart J. 2006;151:444-450.
11. Hillege HL, Nitsch D, Pfeffer MA, et al. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006;113:671-678.
12. Somaratne JB, Berry C, McMurray JJ, et al. The prognostic significance of heart failure with preserved left ventricular ejection fraction: a literature-based meta-analysis. Eur J Heart Fail. 2009;11:855-862.
13. 2005 Writing committee members; Hunt SA, Abraham WT, et al. 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults. Circulation. 2009;119:e391-e479.
14. Heart Failure Society of America. HFSA 2010 comprehensive heart failure practice guideline. J Card Fail. 2010;16:e1-e2.
15. Davie AP, Francis CM, Love MP, et al. Value of the electrocardiogram in identifying heart failure due to left ventricular systolic dysfunction. BMJ. 1996;312:222.-
16. Halley CM, Houghtaling PL, Khalil MK, et al. Mortality rate in patients with diastolic dysfunction and normal systolic function. Arch Intern Med. 2011;171:1082-1087.
17. Cleland JGF, Tendera M, Adamus J, et al. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338-2345.
18. Wachtell K, Bella JN, Rokkedal J, et al. Change in diastolic left ventricular filling after one year of antihypertensive treatment. Circulation. 2002;105:1071-1076.
19. Little WC, Zile MR, Klein A, et al. Effect of losartan and hydrochlorothiazide on exercise tolerance in exertional hypertension and left ventricular diastolic dysfunction. Am J Cardiol. 2006;98:383-385.
20. Bonow RO, Udelson JE. Left ventricular diastolic dysfunction as a cause of congestive heart failure. Mechanisms and management Ann Intern Med. 1992;117:502-510.
21. Hernandez AF, Hammill BG, O’Connor CM, et al. Clinical effectiveness of beta-blockers in heart failure: findings from the OPTIMIZE-HF (organized program to initiate lifesaving treatment in hospitalized patients with heart failure) registry. J Am Coll Cardiol. 2009;53:184-192.
22. Flather MD, Shibata MC, Coats AJS, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J. 2005;26:215-225.
23. Chavey WE, Bleske BE, Van Harrison R, et al. Pharmacologic management of heart failure caused by systolic dysfunction. Am Fam Physician. 2008;77:957-964.
24. Setaro JF, Zaret BL, Schulman DS, et al. Usefulness of verapamil for congestive heart failure associated with abnormal left ventricular diastolic filling and normal left ventricular systolic performance. Am J Cardiol. 1990;66:981-986.
25. Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709-717.
26. Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309-1321.
27. Zannad F, McMurray JJV, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11-21.
28. The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). JAMA. 2002;288:2981-2997.
29. Fukuta H, Sane DC, Brucks S, et al. Statin therapy may be associated with lower mortality in patients with diastolic heart failure. Circulation. 2005;112:357-363.
30. Arcand JAL, Brazel S, Joliffe C, et al. Education by a dietitian in patients with heart failure results in improved adherence with a sodium-restricted diet: a randomized trial. Am Heart J. 2005;150:716.e1-716.e5.
31. Bernal J, Pitta SR, Thatai D. Role of the renin-angiotensin-aldosterone system in diastolic heart failure: potential for pharmacologic intervention. Am J Cardiovasc Drugs. 2006;6:373-381.
32. Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-preserved trial. Lancet. 2003;362:777-781.
33. Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456-2467.
34. Heran BS, Musini VM, Bassett K, et al. Angiotensin receptor blockers for heart failure. Cochrane Database Syst Rev. 2012;(4):CD003040.-
35. Mottram PM, Haluska B, Leano R, et al. Effect of aldosterone antagonism on myocardial dysfunction in hypertensive patients with diastolic heart failure. Circulation. 2004;0110:558-565.
CORRESPONDENCE Geoffrey D. Mills, MD, PhD, Department of Family and Community Medicine, Jefferson Medical College, 833 Chestnut Street, Suite 301, Philadelphia, PA 19107; [email protected]
• Suspect diastolic heart failure in patients who have symptoms of heart failure but a normal ejection fraction, with or without evidence of diastolic abnormalities. B
• Treatment goals for patients who have heart failure with preserved ejection fraction (HFPEF) include normalization of blood pressure, prevention of tachycardia and ischemia, reduction of congestion, and improvement in exercise capacity. B
• Initiate beta-blocker therapy without delay for patients who have acute decompensated HFPEF and tachycardia; consider cardioversion for those with atrial fibrillation. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Most studies of heart failure (HF)—the most common cause of hospitalization in patients older than 65 years1—have focused on patients with reduced ejection fraction (EF). Yet half of those hospitalized for acute decompensated HF have a normal left ventricular EF.2 For these patients, contractility is not the problem—impaired relaxation during diastole is.
Commonly called diastolic HF, heart failure with preserved ejection fraction (HFPEF) is a more precise name for this condition. Patients are usually older than those with a reduced EF.3 Thus, as the US population ages, the prevalence of HFPEF increases, as well.4
Diagnostic criteria have been developed for HFPEF, but there are few large, high-quality studies to guide its treatment. Yet family physicians need to be familiar with HFPEF and know how best to treat it. With extrapolation from studies of patients with reduced EF, as well as expert consensus and our own experience, we offer an evidence-based approach to the management of both stable and acute decompensated HFPEF.
A closer look at diastolic dysfunction
Defined as an abnormality of diastolic compliance, filling, or relaxation of the ventricle, diastolic dysfunction can occur whether EF is normal or abnormal.3 Ventricular diastole includes isovolumic relaxation, early passive filling after mitral valve opening, and active filling during atrial contraction. Transmission of high ventricular pressure to the pulmonary circulation leads to pulmonary edema, dyspnea, and other symptoms of HF. Factors other than abnormal diastolic physiology, such as chronic volume overload, ventricular coupling dyssynchrony, increased autonomic tone leading to reduced venous capacitance, and chronotropic intolerance, may also be involved.5
Patient history: What to look for
A variety of conditions, including ischemia, tachycardia, impaired myocardial relaxation, and age-related loss of myocardial compliance, can contribute to abnormal diastolic function, but the major causes of HFPEF are chronic hypertension, hypertrophic cardiomyopathy, and coronary artery disease (CAD).3 Rarely, infiltrative or restrictive cardiomyopathy (eg, amyloidosis or sarcoidosis) is implicated.6 Noncardiovascular comorbidities such as diabetes, renal impairment, anemia, and chronic lung disease are more prevalent among those with HFPEF, and more women are affected than men.1
Mortality risk. In a study of more than 100,000 hospitalizations for acute decompensated HF, patients with preserved EF had lower in-hospital mortality (3% vs 4% for those with reduced EF).2 Patients with both diabetes and CAD commonly develop HFPEF,7 and the presence of these comorbidities are an independent predictor of 5-year mortality.8
Population studies suggest that 5-year mortality rates for African Americans with HFPEF are higher than for Caucasians with this condition.9 Other predictors of mortality include older age, male sex, lower left ventricular EF, ischemic disease, impaired renal function, and peripheral arterial disease.10-12
Diagnosing HFPEF: What you’ll see, when to test
The presentation of patients with HFPEF is similar to that of individuals with reduced EF. In an outpatient setting, both groups will have reduced exercise capacity; increased neuroendocrine activation, which may cause chronic fluid retention, vasoconstriction, and tachycardia; and a reduced quality of life.5
Neither the American College of Cardiology/American Heart Association (ACC/AHA) nor the Heart Failure Society of America (HFSA)13,14 recommends screening for asymptomatic left ventricular dysfunction. For those with signs and symptoms of HF, however, echocardiography is a key component of the initial evaluation. Echocardiography provides information about left ventricular systolic function, including EF, regional wall motion abnormalities, and wall thickness. Echocardiographic evidence of diastolic abnormalities is found for some patients with HFPEF, while others have no demonstrable diastolic dysfunction.3
While an electrocardiogram (EKG) cannot distinguish between HF with reduced EF and HFPEF, common findings might include signs of ventricular hypertrophy or tachycardia during acute exacerbations. An EKG should be obtained in patients with suspected HF to screen for antecedent causes such as hypertrophy, atrial fibrillation, and ischemia.15
What Doppler echocardiography and the E/A ratio reveal
Doppler echocardiography is used to further evaluate the characteristics of blood flow, showing the relationship among left ventricular (LV) relaxation, atrial pressure, atrial contraction, and blood flow velocity across the mitral valve during diastole. The peak velocity of blood flow during early diastole (called the “E wave”) and late diastole (the atrial contraction, or “A wave”) is measured and the E/A ratio (reflecting the transmitral blood flow pattern) is calculated (FIGURE).3
FIGURE
The E/A ratio* and what it reveals
A, atrial contraction; E, early passive filling; MVC, mitral valve closes; MVO, mitral valve opens.
*E/A ratio represents the relationship between the peak velocity of blood flow during early diastole (E wave) and late diastole (A wave).
Adapted from: Aurigemma GP, Gaasch WH. N Engl J Med. 2004.3
Normally, transmitral flow velocity is greater during early diastole than during atrial contraction, and the E/A ratio is approximately 1.5 (E>A). With early diastolic dysfunction, impaired relaxation prevents blood from flowing passively into the LV during early diastole. This causes reversal of the E/A ratio, which drops to <1 (E<A). As diastolic function worsens, atrial contraction is impaired, and left atrial pressure rises. The result: A reduction in the A wave amplitude and proportionally more blood flow during early diastole and a “pseudonormal” (E>A) ratio, with a greater difference between the E and A than is normally observed. This finding is an independent predictor of all-cause mortality in patients with asymptomatic HF.16
Cardiac catheterization. Invasive measurement of LV filling pressures is the gold standard for diagnosing HFPEF. If echocardiography does not lead to a clear diagnosis, cardiac catheterization can provide information about concomitant pulmonary hypertension and mechanical asynchrony that may contribute to symptomatic HF.1 When the diagnosis is uncertain, additional testing—eg, plasma brain natriuretic peptide (BNP), chest x-ray, or exercise testing—may be necessary to establish a diagnosis of symptomatic HF.
The diagnostic criteria developed by HFSA include clinical evidence of HF and:
- echocardiographic evidence of LV hypertrophy or left atrial enlargement (without atrial fibrillation) or
- evidence of diastolic dysfunction on Doppler echocardiography or cardiac catheterization.14
It is important to note that the diagnostic criteria have not been validated, and the sensitivity and specificity of the various clinical findings are not known.
CASE Carrie W, a 76-year-old woman referred to you by a colleague, presents for follow-up after being hospitalized for HF. She recalls feeling fatigue, chest pain, and out of breath with even minimal exertion before being admitted to the hospital.
You obtain her hospital records, which show that echocardiography found impaired LV relaxation based on a reversed E/A ratio and an EF of 65%. In addition, BNP was elevated, and a chest x-ray showed pulmonary vascular congestion. You note that her blood pressure was 175/103 mm Hg on admission and an EKG showed LV hypertrophy and sinus tachycardia, but no ischemia.
Before being hospitalized, Ms. W was taking extended-release metoprolol, aspirin, and lisinopril. The hospitalist added lovastatin and increased the daily dose of extended-release metoprolol from 25 to 100 mg.
What changes, if any, would you make in her medication regimen?
Diastolic dysfunction as chronic disease
Often asymptomatic, diastolic dysfunction should be thought of as a chronic progressive disease characterized by complex physiologic adaptations that vary over time (See “Staging heart failure: The clinical course of HFPEF”.13) Patients with HFPEF have a difficult time tolerating hemodynamic stress and any perturbation of afterload, heart rate, or ventricular function can precipitate an acute exacerbation.2 Clinical factors that precipitate acute decompensation of HFPEF—which we’ll discuss a bit later—include uncontrolled hypertension; atrial fibrillation; and noncardiovascular comorbidities such as lung disease, renal impairment, or sepsis.2
The ACC/AHA staging system for HF can be applied to patients with HFPEF, both to classify disease severity and to track the progression of the disease. Patients at Stage A are at high risk of developing HF, but early and aggressive treatment of hypertension and other cardiovascular risk factors may delay or potentially prevent the onset of overt disease. Stage B refers to patients with known structural disease, such as a history of myocardial infarction or systolic or diastolic dysfunction, but no symptoms of HF.
Patients at Stage C have evidence of structural disease and symptoms of HF, such as fatigue, shortness of breath, or reduced exercise tolerance. This stage represents the spectrum of patients falling into New York Heart Association (NYHA) Class 1 through 3 categories. Finally, patients at Stage D—analogous to NYHA Class 4—have refractory HF, with marked symptoms even at rest despite maximal medical therapy.
The Acute Decompensated HEart failure national REgistry (ADHERE), in which the records of well over 80,000 Medicare patients were reviewed, found that more than 60% of those hospitalized with HFPEF had uncontrolled hypertension, with a systolic pressure >140 mm Hg; 21% had atrial fibrillation.2 These findings emphasize the importance of aggressive blood pressure (BP) and heart rate control.
Management of HFPEF is goal directed
The aim of pharmacologic treatment of HFPEF is to maintain fluid balance, prevent tachycardia, treat and prevent ischemia, and control hypertension (TABLE).14,17-30 While the use of angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and beta-blockers, among other pharmacologic agents, is well studied for patients with reduced EF, there is limited evidence to guide the treatment of those with HFPEF. Although no single agent or drug class has been shown to be superior for such patients, there are a number of pharmacologic treatments to consider.
TABLE
Management of heart failure with preserved ejection fraction—matching treatment and goals14,17-30
| Treatment goal | Modality |
|---|---|
| Reduce congestion | Diuretics Salt restriction |
| Maintain atrial contraction | A-V pacing Cardioversion |
| Prevent tachycardia | A-V pacing Beta-blockers Calcium channel blockers |
| Prevent/treat ischemia | Antiplatelet therapy Beta-blockers Calcium channel blockers Revascularization Statins |
| Control hypertension | Antihypertensive agents:
|
| Promote regression of LV remodeling | ACE inhibitors ARBs |
| Improve exercise capacity | Supervised exercise program |
| ACE, angiotensin-converting enzyme; ARBs, angiotensin receptor blockers; LV, left ventricle. | |
Inhibition of the renin-angiotensin-aldosterone system
Pathologic activation of the renin-angiotensin-aldosterone system (RAAS) contributes to elevated systolic and diastolic pressure, LV hypertrophy, and LV fibrosis. Inhibition of this system is a promising treatment modality for HFPEF.31
ACE inhibitors. Experimental studies suggest that ACE inhibitors benefit the diastolic properties of the heart, in both short- and long-term use. The PEP-CHF trial found that for older patients with diastolic dysfunction, perindopril led to significant improvements in functional class and exercise capacity but failed to show a statistically significant reduction in all-cause mortality or hospitalization for acute decompensated HF.17
ARBs. There is no evidence to show that ARB therapy improves morbidity or mortality in HFPEF. Using surrogate end points, ARBs have been associated with regression of LV hypertrophy, and losartan was found to improve exercise tolerance and quality of life, compared with hydrochlorothiazide.18,19 In the CHARM-Preserved trial, candesartan showed an insignificant reduction in cardiovascular mortality and hospitalization for HF.
These results must be viewed with caution, however, because adverse effects led to high rates of medication discontinuation.32 In the I-PRESERVE trial, irbesartan conferred no benefit with respect to mortality, hospitalization, or quality of life on patients with HFPEF.33
ACE inhibitor or ARB—not both. ACE inhibitors and ARBs are good choices for BP control in patients with HFPEF, especially if LV hypertrophy is present, but periodic testing of renal function and potassium levels is needed. ACE inhibitors and ARBs should not be used concurrently, as the combination increases the risk of acute renal failure and has no benefit in clinical outcomes.34
BP and rate control
In small trials, beta-blockers have been found to improve diastolic function as seen on echocardiography, but data on morbidity and mortality are lacking.20 A secondary analysis of the OPTIMIZE-HF registry found that beta-blocker therapy was associated with reduced mortality and readmission in patients with reduced EF, but not in those with normal EF.21
Findings from the SENIORS trial were more promising: Treatment with nebivolol reduced both mortality and readmission rates for elderly patients with HF, with similar benefits for those with reduced and preserved EF.22 Overall, beta-blockers appear to be a reasonable choice for heart rate and/or BP control in patients who have HFPEF and atrial fibrillation or hypertension. Carvedilol, long-acting metoprolol, and bisoprolol have been shown to reduce mortality in HF with reduced EF, and it is reasonable to choose one of these agents for patients with preserved EF, as well.23
Calcium channel blockers (CCBs) may be useful in treating patients with HFPEF for both BP and heart rate control, as well. Theoretically, CCBs may also improve the process of relaxation by altering intracellular calcium cycling during the contractile cycle in myocytes. This contrasts with the management of HF patients with reduced EF, for whom the use of nonselective CCBs such as diltiazem and verapamil may adversely affect contractility.
In small RCTs, verapamil has been found to improve HF symptoms and exercise tolerance in patients with HFPEF,24 but no evidence of improved outcomes or mortality rates with CCB use has been found.
Other pharmacologic options to consider
Aldosterone antagonist therapy is an important component of treatment for patients with HF with reduced EF. Data supporting the use of spironolactone use from the RALES trial and eplerenone in the EPHESUS and EMPHASIS-HF trials suggest a reduction in mortality in patients with low (<35%) LVEF.25-27 For patients with preserved EF, however, spironolactone is not generally recommended.
A large National Institutes of Health-sponsored trial is underway to determine if the drug is beneficial for patients with preserved LVEF, and will build on a small study in which 30 patients with HFPEF showed improved myocardial function after treatment with spironolactone.35 Until more data become available, the risks of using aldosterone antagonists outweigh the evidence to support their use in this patient population.
Diuretics are an important component of treatment for all patients with HF and fluid overload. The Antihypertensive and Lipid-Lowering treatment to prevent Heart Attack Trial (ALLHAT) showed a reduced incidence of symptomatic HFPEF in patients taking diuretics.28 As is the case with patients with reduced EF, those with preserved EF should be treated with diuretics if they have symptoms of fluid overload.
Statins. Intensive lipid lowering with statin therapy has been shown in observational studies to benefit patients with HFPEF with respect to mortality, independent of baseline low-density lipoprotein cholesterol.29 RCTs are needed to confirm these observations, but statin therapy is recommended for the secondary prevention of cardiovascular disease, independent of the presence of diastolic dysfunction or HFPEF.
Guard against hypotension. Patients with diastolic dysfunction are susceptible to hypotension if there is a rapid reduction in preload with diuretics, nonselective CCBs, or nitrates, so it is important that doses be titrated slowly.
Nonpharmacologic measures are important, too
In addition to optimizing treatment of comorbid conditions, patients with HFPEF should be advised that lifestyle modifications such as weight loss, smoking cessation, and dietary changes can do much to reduce the risk. You can help by providing an exercise “prescription” (with a specified intensity, frequency, and duration) and dietary guidelines, with emphasis on the importance of a low-sodium diet to prevent fluid overload.14,30 Recommend local programs for patients with HF, which many hospitals and health systems offer as part of their efforts to reduce readmission rates.
Consider cardioversion
Tachycardia shortens the time for filling during diastole; thus, it is poorly tolerated in patients with diastolic dysfunction and could trigger acute decompensation. To avoid the risk, restoration of sinus rhythm should be considered for patients with HFPEF and atrial fibrillation. Patients with known paroxysmal or permanent atrial fibrillation and preserved EF should be seen by a cardiologist to determine whether direct current cardioversion or ablation with a permanent pacemaker is appropriate.11 When cardioversion is contraindicated, a beta-blocker is needed to control heart rate and improve hemodynamics.
Patients with stable angina and HFPEF should be evaluated for revascularization when medical therapy alone is not sufficient for symptom relief.10 Here, too, a cardiology consult is indicated for any patient who has HF and an abnormal noninvasive stress test or persistent symptoms despite optimal drug therapy.
Recognizing and responding to acute decompensated HFPEF
The initial response to acute decompensated HFPEF, like that of HF with reduced EF, should be focused on restoring volume status and providing oxygenation, ventilation, and vasodilator therapy in some cases.11 Unlike those with acute decompensated HF with reduced EF, however, patients with HFPEF can safely tolerate the initiation of beta-blockers in the acute phase, especially when rate control is needed.3 Inotropic agents like digoxin and dobutamine, however, are contraindicated.3
Guidelines recommend hospitalization for patients with abnormal vital signs, arrhythmia, and suspected acute coronary syndromes, and consideration of hospitalization for those with associated comorbid conditions, new HF, or progressive fluid overload.13
CASE Because Ms. W has a normal BP and heart rate and is feeling well, you decline to alter her medication regimen. You do, however, recommend that she begin an exercise program, adopt a low-sodium diet, and maintain regular contact with your office so you can evaluate any changes in status.
You introduce Ms. W to the nurse case manager in your office. The nurse works with the patient to develop an action plan that includes daily tracking of her weight and sodium intake; a progressive walking program, starting with 2-minute sessions and progressing to 15 to 30 minutes 3 to 5 times a week; weekly telephone checkins; and immediate calls to report any weight increase or symptoms of HF.
At follow-up 6 months later, Ms. W has improved BP and reports that she enjoys her new exercise routine. She has more energy and denies any edema or breathing difficulties.
• Suspect diastolic heart failure in patients who have symptoms of heart failure but a normal ejection fraction, with or without evidence of diastolic abnormalities. B
• Treatment goals for patients who have heart failure with preserved ejection fraction (HFPEF) include normalization of blood pressure, prevention of tachycardia and ischemia, reduction of congestion, and improvement in exercise capacity. B
• Initiate beta-blocker therapy without delay for patients who have acute decompensated HFPEF and tachycardia; consider cardioversion for those with atrial fibrillation. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Most studies of heart failure (HF)—the most common cause of hospitalization in patients older than 65 years1—have focused on patients with reduced ejection fraction (EF). Yet half of those hospitalized for acute decompensated HF have a normal left ventricular EF.2 For these patients, contractility is not the problem—impaired relaxation during diastole is.
Commonly called diastolic HF, heart failure with preserved ejection fraction (HFPEF) is a more precise name for this condition. Patients are usually older than those with a reduced EF.3 Thus, as the US population ages, the prevalence of HFPEF increases, as well.4
Diagnostic criteria have been developed for HFPEF, but there are few large, high-quality studies to guide its treatment. Yet family physicians need to be familiar with HFPEF and know how best to treat it. With extrapolation from studies of patients with reduced EF, as well as expert consensus and our own experience, we offer an evidence-based approach to the management of both stable and acute decompensated HFPEF.
A closer look at diastolic dysfunction
Defined as an abnormality of diastolic compliance, filling, or relaxation of the ventricle, diastolic dysfunction can occur whether EF is normal or abnormal.3 Ventricular diastole includes isovolumic relaxation, early passive filling after mitral valve opening, and active filling during atrial contraction. Transmission of high ventricular pressure to the pulmonary circulation leads to pulmonary edema, dyspnea, and other symptoms of HF. Factors other than abnormal diastolic physiology, such as chronic volume overload, ventricular coupling dyssynchrony, increased autonomic tone leading to reduced venous capacitance, and chronotropic intolerance, may also be involved.5
Patient history: What to look for
A variety of conditions, including ischemia, tachycardia, impaired myocardial relaxation, and age-related loss of myocardial compliance, can contribute to abnormal diastolic function, but the major causes of HFPEF are chronic hypertension, hypertrophic cardiomyopathy, and coronary artery disease (CAD).3 Rarely, infiltrative or restrictive cardiomyopathy (eg, amyloidosis or sarcoidosis) is implicated.6 Noncardiovascular comorbidities such as diabetes, renal impairment, anemia, and chronic lung disease are more prevalent among those with HFPEF, and more women are affected than men.1
Mortality risk. In a study of more than 100,000 hospitalizations for acute decompensated HF, patients with preserved EF had lower in-hospital mortality (3% vs 4% for those with reduced EF).2 Patients with both diabetes and CAD commonly develop HFPEF,7 and the presence of these comorbidities are an independent predictor of 5-year mortality.8
Population studies suggest that 5-year mortality rates for African Americans with HFPEF are higher than for Caucasians with this condition.9 Other predictors of mortality include older age, male sex, lower left ventricular EF, ischemic disease, impaired renal function, and peripheral arterial disease.10-12
Diagnosing HFPEF: What you’ll see, when to test
The presentation of patients with HFPEF is similar to that of individuals with reduced EF. In an outpatient setting, both groups will have reduced exercise capacity; increased neuroendocrine activation, which may cause chronic fluid retention, vasoconstriction, and tachycardia; and a reduced quality of life.5
Neither the American College of Cardiology/American Heart Association (ACC/AHA) nor the Heart Failure Society of America (HFSA)13,14 recommends screening for asymptomatic left ventricular dysfunction. For those with signs and symptoms of HF, however, echocardiography is a key component of the initial evaluation. Echocardiography provides information about left ventricular systolic function, including EF, regional wall motion abnormalities, and wall thickness. Echocardiographic evidence of diastolic abnormalities is found for some patients with HFPEF, while others have no demonstrable diastolic dysfunction.3
While an electrocardiogram (EKG) cannot distinguish between HF with reduced EF and HFPEF, common findings might include signs of ventricular hypertrophy or tachycardia during acute exacerbations. An EKG should be obtained in patients with suspected HF to screen for antecedent causes such as hypertrophy, atrial fibrillation, and ischemia.15
What Doppler echocardiography and the E/A ratio reveal
Doppler echocardiography is used to further evaluate the characteristics of blood flow, showing the relationship among left ventricular (LV) relaxation, atrial pressure, atrial contraction, and blood flow velocity across the mitral valve during diastole. The peak velocity of blood flow during early diastole (called the “E wave”) and late diastole (the atrial contraction, or “A wave”) is measured and the E/A ratio (reflecting the transmitral blood flow pattern) is calculated (FIGURE).3
FIGURE
The E/A ratio* and what it reveals
A, atrial contraction; E, early passive filling; MVC, mitral valve closes; MVO, mitral valve opens.
*E/A ratio represents the relationship between the peak velocity of blood flow during early diastole (E wave) and late diastole (A wave).
Adapted from: Aurigemma GP, Gaasch WH. N Engl J Med. 2004.3
Normally, transmitral flow velocity is greater during early diastole than during atrial contraction, and the E/A ratio is approximately 1.5 (E>A). With early diastolic dysfunction, impaired relaxation prevents blood from flowing passively into the LV during early diastole. This causes reversal of the E/A ratio, which drops to <1 (E<A). As diastolic function worsens, atrial contraction is impaired, and left atrial pressure rises. The result: A reduction in the A wave amplitude and proportionally more blood flow during early diastole and a “pseudonormal” (E>A) ratio, with a greater difference between the E and A than is normally observed. This finding is an independent predictor of all-cause mortality in patients with asymptomatic HF.16
Cardiac catheterization. Invasive measurement of LV filling pressures is the gold standard for diagnosing HFPEF. If echocardiography does not lead to a clear diagnosis, cardiac catheterization can provide information about concomitant pulmonary hypertension and mechanical asynchrony that may contribute to symptomatic HF.1 When the diagnosis is uncertain, additional testing—eg, plasma brain natriuretic peptide (BNP), chest x-ray, or exercise testing—may be necessary to establish a diagnosis of symptomatic HF.
The diagnostic criteria developed by HFSA include clinical evidence of HF and:
- echocardiographic evidence of LV hypertrophy or left atrial enlargement (without atrial fibrillation) or
- evidence of diastolic dysfunction on Doppler echocardiography or cardiac catheterization.14
It is important to note that the diagnostic criteria have not been validated, and the sensitivity and specificity of the various clinical findings are not known.
CASE Carrie W, a 76-year-old woman referred to you by a colleague, presents for follow-up after being hospitalized for HF. She recalls feeling fatigue, chest pain, and out of breath with even minimal exertion before being admitted to the hospital.
You obtain her hospital records, which show that echocardiography found impaired LV relaxation based on a reversed E/A ratio and an EF of 65%. In addition, BNP was elevated, and a chest x-ray showed pulmonary vascular congestion. You note that her blood pressure was 175/103 mm Hg on admission and an EKG showed LV hypertrophy and sinus tachycardia, but no ischemia.
Before being hospitalized, Ms. W was taking extended-release metoprolol, aspirin, and lisinopril. The hospitalist added lovastatin and increased the daily dose of extended-release metoprolol from 25 to 100 mg.
What changes, if any, would you make in her medication regimen?
Diastolic dysfunction as chronic disease
Often asymptomatic, diastolic dysfunction should be thought of as a chronic progressive disease characterized by complex physiologic adaptations that vary over time (See “Staging heart failure: The clinical course of HFPEF”.13) Patients with HFPEF have a difficult time tolerating hemodynamic stress and any perturbation of afterload, heart rate, or ventricular function can precipitate an acute exacerbation.2 Clinical factors that precipitate acute decompensation of HFPEF—which we’ll discuss a bit later—include uncontrolled hypertension; atrial fibrillation; and noncardiovascular comorbidities such as lung disease, renal impairment, or sepsis.2
The ACC/AHA staging system for HF can be applied to patients with HFPEF, both to classify disease severity and to track the progression of the disease. Patients at Stage A are at high risk of developing HF, but early and aggressive treatment of hypertension and other cardiovascular risk factors may delay or potentially prevent the onset of overt disease. Stage B refers to patients with known structural disease, such as a history of myocardial infarction or systolic or diastolic dysfunction, but no symptoms of HF.
Patients at Stage C have evidence of structural disease and symptoms of HF, such as fatigue, shortness of breath, or reduced exercise tolerance. This stage represents the spectrum of patients falling into New York Heart Association (NYHA) Class 1 through 3 categories. Finally, patients at Stage D—analogous to NYHA Class 4—have refractory HF, with marked symptoms even at rest despite maximal medical therapy.
The Acute Decompensated HEart failure national REgistry (ADHERE), in which the records of well over 80,000 Medicare patients were reviewed, found that more than 60% of those hospitalized with HFPEF had uncontrolled hypertension, with a systolic pressure >140 mm Hg; 21% had atrial fibrillation.2 These findings emphasize the importance of aggressive blood pressure (BP) and heart rate control.
Management of HFPEF is goal directed
The aim of pharmacologic treatment of HFPEF is to maintain fluid balance, prevent tachycardia, treat and prevent ischemia, and control hypertension (TABLE).14,17-30 While the use of angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and beta-blockers, among other pharmacologic agents, is well studied for patients with reduced EF, there is limited evidence to guide the treatment of those with HFPEF. Although no single agent or drug class has been shown to be superior for such patients, there are a number of pharmacologic treatments to consider.
TABLE
Management of heart failure with preserved ejection fraction—matching treatment and goals14,17-30
| Treatment goal | Modality |
|---|---|
| Reduce congestion | Diuretics Salt restriction |
| Maintain atrial contraction | A-V pacing Cardioversion |
| Prevent tachycardia | A-V pacing Beta-blockers Calcium channel blockers |
| Prevent/treat ischemia | Antiplatelet therapy Beta-blockers Calcium channel blockers Revascularization Statins |
| Control hypertension | Antihypertensive agents:
|
| Promote regression of LV remodeling | ACE inhibitors ARBs |
| Improve exercise capacity | Supervised exercise program |
| ACE, angiotensin-converting enzyme; ARBs, angiotensin receptor blockers; LV, left ventricle. | |
Inhibition of the renin-angiotensin-aldosterone system
Pathologic activation of the renin-angiotensin-aldosterone system (RAAS) contributes to elevated systolic and diastolic pressure, LV hypertrophy, and LV fibrosis. Inhibition of this system is a promising treatment modality for HFPEF.31
ACE inhibitors. Experimental studies suggest that ACE inhibitors benefit the diastolic properties of the heart, in both short- and long-term use. The PEP-CHF trial found that for older patients with diastolic dysfunction, perindopril led to significant improvements in functional class and exercise capacity but failed to show a statistically significant reduction in all-cause mortality or hospitalization for acute decompensated HF.17
ARBs. There is no evidence to show that ARB therapy improves morbidity or mortality in HFPEF. Using surrogate end points, ARBs have been associated with regression of LV hypertrophy, and losartan was found to improve exercise tolerance and quality of life, compared with hydrochlorothiazide.18,19 In the CHARM-Preserved trial, candesartan showed an insignificant reduction in cardiovascular mortality and hospitalization for HF.
These results must be viewed with caution, however, because adverse effects led to high rates of medication discontinuation.32 In the I-PRESERVE trial, irbesartan conferred no benefit with respect to mortality, hospitalization, or quality of life on patients with HFPEF.33
ACE inhibitor or ARB—not both. ACE inhibitors and ARBs are good choices for BP control in patients with HFPEF, especially if LV hypertrophy is present, but periodic testing of renal function and potassium levels is needed. ACE inhibitors and ARBs should not be used concurrently, as the combination increases the risk of acute renal failure and has no benefit in clinical outcomes.34
BP and rate control
In small trials, beta-blockers have been found to improve diastolic function as seen on echocardiography, but data on morbidity and mortality are lacking.20 A secondary analysis of the OPTIMIZE-HF registry found that beta-blocker therapy was associated with reduced mortality and readmission in patients with reduced EF, but not in those with normal EF.21
Findings from the SENIORS trial were more promising: Treatment with nebivolol reduced both mortality and readmission rates for elderly patients with HF, with similar benefits for those with reduced and preserved EF.22 Overall, beta-blockers appear to be a reasonable choice for heart rate and/or BP control in patients who have HFPEF and atrial fibrillation or hypertension. Carvedilol, long-acting metoprolol, and bisoprolol have been shown to reduce mortality in HF with reduced EF, and it is reasonable to choose one of these agents for patients with preserved EF, as well.23
Calcium channel blockers (CCBs) may be useful in treating patients with HFPEF for both BP and heart rate control, as well. Theoretically, CCBs may also improve the process of relaxation by altering intracellular calcium cycling during the contractile cycle in myocytes. This contrasts with the management of HF patients with reduced EF, for whom the use of nonselective CCBs such as diltiazem and verapamil may adversely affect contractility.
In small RCTs, verapamil has been found to improve HF symptoms and exercise tolerance in patients with HFPEF,24 but no evidence of improved outcomes or mortality rates with CCB use has been found.
Other pharmacologic options to consider
Aldosterone antagonist therapy is an important component of treatment for patients with HF with reduced EF. Data supporting the use of spironolactone use from the RALES trial and eplerenone in the EPHESUS and EMPHASIS-HF trials suggest a reduction in mortality in patients with low (<35%) LVEF.25-27 For patients with preserved EF, however, spironolactone is not generally recommended.
A large National Institutes of Health-sponsored trial is underway to determine if the drug is beneficial for patients with preserved LVEF, and will build on a small study in which 30 patients with HFPEF showed improved myocardial function after treatment with spironolactone.35 Until more data become available, the risks of using aldosterone antagonists outweigh the evidence to support their use in this patient population.
Diuretics are an important component of treatment for all patients with HF and fluid overload. The Antihypertensive and Lipid-Lowering treatment to prevent Heart Attack Trial (ALLHAT) showed a reduced incidence of symptomatic HFPEF in patients taking diuretics.28 As is the case with patients with reduced EF, those with preserved EF should be treated with diuretics if they have symptoms of fluid overload.
Statins. Intensive lipid lowering with statin therapy has been shown in observational studies to benefit patients with HFPEF with respect to mortality, independent of baseline low-density lipoprotein cholesterol.29 RCTs are needed to confirm these observations, but statin therapy is recommended for the secondary prevention of cardiovascular disease, independent of the presence of diastolic dysfunction or HFPEF.
Guard against hypotension. Patients with diastolic dysfunction are susceptible to hypotension if there is a rapid reduction in preload with diuretics, nonselective CCBs, or nitrates, so it is important that doses be titrated slowly.
Nonpharmacologic measures are important, too
In addition to optimizing treatment of comorbid conditions, patients with HFPEF should be advised that lifestyle modifications such as weight loss, smoking cessation, and dietary changes can do much to reduce the risk. You can help by providing an exercise “prescription” (with a specified intensity, frequency, and duration) and dietary guidelines, with emphasis on the importance of a low-sodium diet to prevent fluid overload.14,30 Recommend local programs for patients with HF, which many hospitals and health systems offer as part of their efforts to reduce readmission rates.
Consider cardioversion
Tachycardia shortens the time for filling during diastole; thus, it is poorly tolerated in patients with diastolic dysfunction and could trigger acute decompensation. To avoid the risk, restoration of sinus rhythm should be considered for patients with HFPEF and atrial fibrillation. Patients with known paroxysmal or permanent atrial fibrillation and preserved EF should be seen by a cardiologist to determine whether direct current cardioversion or ablation with a permanent pacemaker is appropriate.11 When cardioversion is contraindicated, a beta-blocker is needed to control heart rate and improve hemodynamics.
Patients with stable angina and HFPEF should be evaluated for revascularization when medical therapy alone is not sufficient for symptom relief.10 Here, too, a cardiology consult is indicated for any patient who has HF and an abnormal noninvasive stress test or persistent symptoms despite optimal drug therapy.
Recognizing and responding to acute decompensated HFPEF
The initial response to acute decompensated HFPEF, like that of HF with reduced EF, should be focused on restoring volume status and providing oxygenation, ventilation, and vasodilator therapy in some cases.11 Unlike those with acute decompensated HF with reduced EF, however, patients with HFPEF can safely tolerate the initiation of beta-blockers in the acute phase, especially when rate control is needed.3 Inotropic agents like digoxin and dobutamine, however, are contraindicated.3
Guidelines recommend hospitalization for patients with abnormal vital signs, arrhythmia, and suspected acute coronary syndromes, and consideration of hospitalization for those with associated comorbid conditions, new HF, or progressive fluid overload.13
CASE Because Ms. W has a normal BP and heart rate and is feeling well, you decline to alter her medication regimen. You do, however, recommend that she begin an exercise program, adopt a low-sodium diet, and maintain regular contact with your office so you can evaluate any changes in status.
You introduce Ms. W to the nurse case manager in your office. The nurse works with the patient to develop an action plan that includes daily tracking of her weight and sodium intake; a progressive walking program, starting with 2-minute sessions and progressing to 15 to 30 minutes 3 to 5 times a week; weekly telephone checkins; and immediate calls to report any weight increase or symptoms of HF.
At follow-up 6 months later, Ms. W has improved BP and reports that she enjoys her new exercise routine. She has more energy and denies any edema or breathing difficulties.
1. Lam CSP, Donal E, Kraigher-Krainer E, et al. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:18-28.
2. Yancy CW, Lopatin M, Stevenson LW, et al. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the acute decompensated heart failure national registry (ADHERE) database. J Am Coll Cardiol. 2006;47:76-84.
3. Aurigemma GP, Gaasch WH. Diastolic heart failure. N Engl J Med. 2004;351:1097-1105.
4. Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251-259.
5. Bench T, Burkhoff D, O’Connell JB, et al. Heart failure with normal ejection fraction: consideration of mechanisms other than diastolic dysfunction. Curr Heart Fail Rep. 2009;6:57-64.
6. Ammash NM, Seward JB, Bailey KR, et al. Clinical profile and outcome of idiopathic restrictive cardiomyopathy. Circulation. 2000;101:2490-2496.
7. Bell DSH. Diabetic cardiomyopathy. Diabetes Care. 2003;26:2949-2951.
8. From AM, Scott CG, Chen HH. The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction: a population-based study. J Am Coll Cardiol. 2010;55:300-305.
9. East MA, Peterson ED, Shaw LK, et al. Racial differences in the outcomes of patients with diastolic heart failure. Am Heart J. 2004;148:151-156.
10. Ahmed A, Aronow WS, Fleg JL. Higher New York Heart Association classes and increased mortality and hospitalization in patients with heart failure and preserved left ventricular function. Am Heart J. 2006;151:444-450.
11. Hillege HL, Nitsch D, Pfeffer MA, et al. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006;113:671-678.
12. Somaratne JB, Berry C, McMurray JJ, et al. The prognostic significance of heart failure with preserved left ventricular ejection fraction: a literature-based meta-analysis. Eur J Heart Fail. 2009;11:855-862.
13. 2005 Writing committee members; Hunt SA, Abraham WT, et al. 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults. Circulation. 2009;119:e391-e479.
14. Heart Failure Society of America. HFSA 2010 comprehensive heart failure practice guideline. J Card Fail. 2010;16:e1-e2.
15. Davie AP, Francis CM, Love MP, et al. Value of the electrocardiogram in identifying heart failure due to left ventricular systolic dysfunction. BMJ. 1996;312:222.-
16. Halley CM, Houghtaling PL, Khalil MK, et al. Mortality rate in patients with diastolic dysfunction and normal systolic function. Arch Intern Med. 2011;171:1082-1087.
17. Cleland JGF, Tendera M, Adamus J, et al. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338-2345.
18. Wachtell K, Bella JN, Rokkedal J, et al. Change in diastolic left ventricular filling after one year of antihypertensive treatment. Circulation. 2002;105:1071-1076.
19. Little WC, Zile MR, Klein A, et al. Effect of losartan and hydrochlorothiazide on exercise tolerance in exertional hypertension and left ventricular diastolic dysfunction. Am J Cardiol. 2006;98:383-385.
20. Bonow RO, Udelson JE. Left ventricular diastolic dysfunction as a cause of congestive heart failure. Mechanisms and management Ann Intern Med. 1992;117:502-510.
21. Hernandez AF, Hammill BG, O’Connor CM, et al. Clinical effectiveness of beta-blockers in heart failure: findings from the OPTIMIZE-HF (organized program to initiate lifesaving treatment in hospitalized patients with heart failure) registry. J Am Coll Cardiol. 2009;53:184-192.
22. Flather MD, Shibata MC, Coats AJS, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J. 2005;26:215-225.
23. Chavey WE, Bleske BE, Van Harrison R, et al. Pharmacologic management of heart failure caused by systolic dysfunction. Am Fam Physician. 2008;77:957-964.
24. Setaro JF, Zaret BL, Schulman DS, et al. Usefulness of verapamil for congestive heart failure associated with abnormal left ventricular diastolic filling and normal left ventricular systolic performance. Am J Cardiol. 1990;66:981-986.
25. Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709-717.
26. Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309-1321.
27. Zannad F, McMurray JJV, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11-21.
28. The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). JAMA. 2002;288:2981-2997.
29. Fukuta H, Sane DC, Brucks S, et al. Statin therapy may be associated with lower mortality in patients with diastolic heart failure. Circulation. 2005;112:357-363.
30. Arcand JAL, Brazel S, Joliffe C, et al. Education by a dietitian in patients with heart failure results in improved adherence with a sodium-restricted diet: a randomized trial. Am Heart J. 2005;150:716.e1-716.e5.
31. Bernal J, Pitta SR, Thatai D. Role of the renin-angiotensin-aldosterone system in diastolic heart failure: potential for pharmacologic intervention. Am J Cardiovasc Drugs. 2006;6:373-381.
32. Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-preserved trial. Lancet. 2003;362:777-781.
33. Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456-2467.
34. Heran BS, Musini VM, Bassett K, et al. Angiotensin receptor blockers for heart failure. Cochrane Database Syst Rev. 2012;(4):CD003040.-
35. Mottram PM, Haluska B, Leano R, et al. Effect of aldosterone antagonism on myocardial dysfunction in hypertensive patients with diastolic heart failure. Circulation. 2004;0110:558-565.
CORRESPONDENCE Geoffrey D. Mills, MD, PhD, Department of Family and Community Medicine, Jefferson Medical College, 833 Chestnut Street, Suite 301, Philadelphia, PA 19107; [email protected]
1. Lam CSP, Donal E, Kraigher-Krainer E, et al. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:18-28.
2. Yancy CW, Lopatin M, Stevenson LW, et al. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the acute decompensated heart failure national registry (ADHERE) database. J Am Coll Cardiol. 2006;47:76-84.
3. Aurigemma GP, Gaasch WH. Diastolic heart failure. N Engl J Med. 2004;351:1097-1105.
4. Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251-259.
5. Bench T, Burkhoff D, O’Connell JB, et al. Heart failure with normal ejection fraction: consideration of mechanisms other than diastolic dysfunction. Curr Heart Fail Rep. 2009;6:57-64.
6. Ammash NM, Seward JB, Bailey KR, et al. Clinical profile and outcome of idiopathic restrictive cardiomyopathy. Circulation. 2000;101:2490-2496.
7. Bell DSH. Diabetic cardiomyopathy. Diabetes Care. 2003;26:2949-2951.
8. From AM, Scott CG, Chen HH. The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction: a population-based study. J Am Coll Cardiol. 2010;55:300-305.
9. East MA, Peterson ED, Shaw LK, et al. Racial differences in the outcomes of patients with diastolic heart failure. Am Heart J. 2004;148:151-156.
10. Ahmed A, Aronow WS, Fleg JL. Higher New York Heart Association classes and increased mortality and hospitalization in patients with heart failure and preserved left ventricular function. Am Heart J. 2006;151:444-450.
11. Hillege HL, Nitsch D, Pfeffer MA, et al. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006;113:671-678.
12. Somaratne JB, Berry C, McMurray JJ, et al. The prognostic significance of heart failure with preserved left ventricular ejection fraction: a literature-based meta-analysis. Eur J Heart Fail. 2009;11:855-862.
13. 2005 Writing committee members; Hunt SA, Abraham WT, et al. 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults. Circulation. 2009;119:e391-e479.
14. Heart Failure Society of America. HFSA 2010 comprehensive heart failure practice guideline. J Card Fail. 2010;16:e1-e2.
15. Davie AP, Francis CM, Love MP, et al. Value of the electrocardiogram in identifying heart failure due to left ventricular systolic dysfunction. BMJ. 1996;312:222.-
16. Halley CM, Houghtaling PL, Khalil MK, et al. Mortality rate in patients with diastolic dysfunction and normal systolic function. Arch Intern Med. 2011;171:1082-1087.
17. Cleland JGF, Tendera M, Adamus J, et al. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338-2345.
18. Wachtell K, Bella JN, Rokkedal J, et al. Change in diastolic left ventricular filling after one year of antihypertensive treatment. Circulation. 2002;105:1071-1076.
19. Little WC, Zile MR, Klein A, et al. Effect of losartan and hydrochlorothiazide on exercise tolerance in exertional hypertension and left ventricular diastolic dysfunction. Am J Cardiol. 2006;98:383-385.
20. Bonow RO, Udelson JE. Left ventricular diastolic dysfunction as a cause of congestive heart failure. Mechanisms and management Ann Intern Med. 1992;117:502-510.
21. Hernandez AF, Hammill BG, O’Connor CM, et al. Clinical effectiveness of beta-blockers in heart failure: findings from the OPTIMIZE-HF (organized program to initiate lifesaving treatment in hospitalized patients with heart failure) registry. J Am Coll Cardiol. 2009;53:184-192.
22. Flather MD, Shibata MC, Coats AJS, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J. 2005;26:215-225.
23. Chavey WE, Bleske BE, Van Harrison R, et al. Pharmacologic management of heart failure caused by systolic dysfunction. Am Fam Physician. 2008;77:957-964.
24. Setaro JF, Zaret BL, Schulman DS, et al. Usefulness of verapamil for congestive heart failure associated with abnormal left ventricular diastolic filling and normal left ventricular systolic performance. Am J Cardiol. 1990;66:981-986.
25. Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709-717.
26. Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309-1321.
27. Zannad F, McMurray JJV, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11-21.
28. The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). JAMA. 2002;288:2981-2997.
29. Fukuta H, Sane DC, Brucks S, et al. Statin therapy may be associated with lower mortality in patients with diastolic heart failure. Circulation. 2005;112:357-363.
30. Arcand JAL, Brazel S, Joliffe C, et al. Education by a dietitian in patients with heart failure results in improved adherence with a sodium-restricted diet: a randomized trial. Am Heart J. 2005;150:716.e1-716.e5.
31. Bernal J, Pitta SR, Thatai D. Role of the renin-angiotensin-aldosterone system in diastolic heart failure: potential for pharmacologic intervention. Am J Cardiovasc Drugs. 2006;6:373-381.
32. Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-preserved trial. Lancet. 2003;362:777-781.
33. Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456-2467.
34. Heran BS, Musini VM, Bassett K, et al. Angiotensin receptor blockers for heart failure. Cochrane Database Syst Rev. 2012;(4):CD003040.-
35. Mottram PM, Haluska B, Leano R, et al. Effect of aldosterone antagonism on myocardial dysfunction in hypertensive patients with diastolic heart failure. Circulation. 2004;0110:558-565.
CORRESPONDENCE Geoffrey D. Mills, MD, PhD, Department of Family and Community Medicine, Jefferson Medical College, 833 Chestnut Street, Suite 301, Philadelphia, PA 19107; [email protected]