User login
Management of adults with syncope
Syncope is characterized by sudden transient loss of consciousness due to cerebral hypoperfusion and is typically associated with an inability to maintain postural tone. There are many different causes and clinical presentations of syncope and the incidence varies depending on the population. Estimated lifetime prevalence rates are as high as 41% for a single episode of syncope, with recurrent syncope occurring in 13.5% of the general population. Incidence follows a trimodal distribution with peaks at age 20, 60, and 80 years for both men and women. The National Hospital Ambulatory Medical Care Survey reported 6.7 million episodes of syncope in the emergency department, which is where most patients with syncope initially present. However, patients may also present to the primary care outpatient setting, and providers should be equipped for initial evaluation and management.
Previous and current treatment guidelines
Although there have been general reviews published by general and specialty societies, there were no comprehensive guidelines on the evaluation and management of syncope until recently. The 2017 guideline from the American College of Cardiology, American Heart Association, and Heart Rhythm Society is intended to provide guidance on evaluation and management of syncope, specifically in the context of different clinical settings, specific causes, or selected circumstances.1
What primary care providers should know
A detailed history and physical exam should be performed in all patients with syncope. Useful details include the setting in which syncope occurs, prodromal symptoms, witness reports, postevent symptoms, comorbidities, medication use, past medical history, and family history. The physical exam should include orthostatic vital signs, cardiac exam, neurologic exam, and any other relevant systems. A resting 12-lead ECG in the initial evaluation is recommended to detect underlying arrhythmia or structural heart disease (Class I recommendation – strong).
There are many different causes of syncope (see Table 1). Vasovagal syncope, a form of reflex syncope mediated by the vasovagal reflex, is the most common cause of syncope and a frequent reason for emergency department visits. There is often a prodrome of diaphoresis, warmth, nausea, and/or pallor, often followed by fatigue. The diagnosis can be made by the history, physical exam, and eyewitness observation.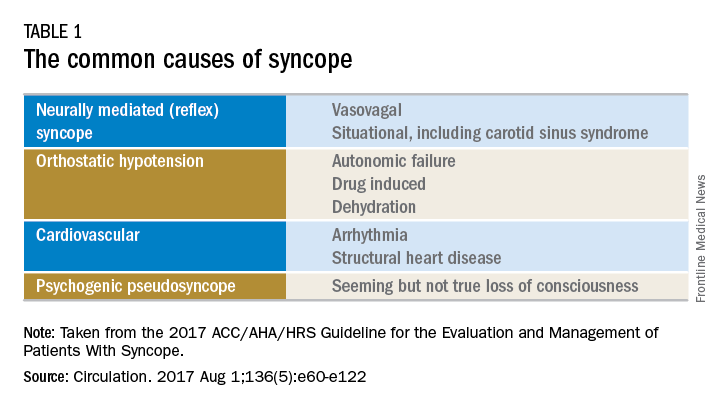
Once the initial evaluation is complete, further evaluation and management depends on the presence of risk factors presented in Table 2. Outpatient management is reasonable for patients with presumptive reflex-mediated syncope when there is an absence of serious medical conditions such as cardiac disease or comorbid neurologic disease. While hospital-based evaluation has not been shown to improve outcomes in patients with a low risk profile, hospital-based evaluation and treatment are recommended for patients presenting with syncope who have a serious medical condition potentially relevant to the cause of syncope.2 Serious medical conditions that require hospital management include arrhythmia, cardiac ischemia, severe aortic stenosis, hypertrophic cardiomyopathy, aortic dissection, acute heart failure, severe anemia, or major traumatic injury. Finally, patients with intermediate risk may benefit from an observational protocol in the emergency department.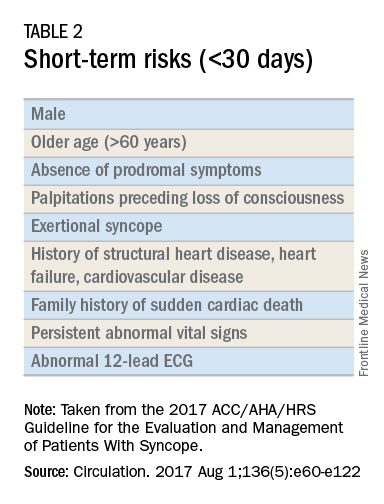
Routine and comprehensive laboratory testing is not useful in syncope work-up (Class III recommendation – no benefit). Routine cardiac imaging is not recommended unless a cardiac etiology is suspected and routine neurological imaging and EEG are not recommended in the absence of focal neurologic findings. Additional work-up may be indicated if initial evaluation suggests a more specific etiology. If the initial evaluation suggests neurogenic orthostatic hypotension but the diagnosis is not clear, then referral for an autonomic evaluation is reasonable. If reflex syncope is suspected, tilt-table testing may be helpful to confirm the diagnosis. Lastly, if a cardiovascular etiology is suspected, it is recommended that the patient have cardiac monitoring in the acute care setting. In this later group, stress testing, transthoracic echocardiogram, electrophysiology study, and/or MRI or CT may be useful. Electrophysiologic testing is reasonable in patients with suspected arrhythmia as the etiology for syncope (Class IIa recommendation – moderate strength). The guideline provides a convenient summary algorithm to approach the initial and subsequent evaluations for syncope based on the initial evaluation and presenting symptoms.
Special populations
There are specific considerations for certain populations. In the pediatric population, the vast majority of syncopal episodes are reflex syncope but breath-holding spells should also be considered. In the geriatric population, particularly individuals older than 75 years, the incidence of syncope is high, the differential diagnosis is broad, and the diagnosis may be imprecise given amnesia, falls, lack of witnesses, and polypharmacy. In this group, morbidity is high because of multimorbidity and frailty. A careful history and physical exam with orthostatic vital signs is important, as is a multidisciplinary approach with geriatric consultation when needed.
Summary
Syncope is a common clinical syndrome often presenting to the emergency department or primary care setting. There are many causes, the most common being vasovagal syncope. In the initial evaluation, providers should perform a detailed history and physical exam, check orthostatic signs and perform a 12-lead ECG. Patients can be evaluated and managed safely in the outpatient setting in the absence of risk factors. Routine comprehensive laboratory testing and cardiac imaging are often not needed. For patients with defined risk factors, a more detailed evaluation in the hospital is recommended.
Dr. Li is a second-year resident in the family medicine residency program in the department of family and community medicine at the Sidney Kimmel Medical College at Thomas Jefferson University, Philadelphia. Dr. Mills is assistant residency program director and assistant professor in the departments of family and community medicine and physiology at the Sidney Kimmel Medical College. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
References
1. Shen W, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope. Circulation. 2017 Aug 1;136(5):e60-e122. doi: 10.1161/CIR.0000000000000499. Epub 2017 Mar 9.
2. Soteriades ES, Evans JC, Larson MG, et al. Incidence and prognosis of syncope. N Engl J Med. 2002;347(12):878-85.
Syncope is characterized by sudden transient loss of consciousness due to cerebral hypoperfusion and is typically associated with an inability to maintain postural tone. There are many different causes and clinical presentations of syncope and the incidence varies depending on the population. Estimated lifetime prevalence rates are as high as 41% for a single episode of syncope, with recurrent syncope occurring in 13.5% of the general population. Incidence follows a trimodal distribution with peaks at age 20, 60, and 80 years for both men and women. The National Hospital Ambulatory Medical Care Survey reported 6.7 million episodes of syncope in the emergency department, which is where most patients with syncope initially present. However, patients may also present to the primary care outpatient setting, and providers should be equipped for initial evaluation and management.
Previous and current treatment guidelines
Although there have been general reviews published by general and specialty societies, there were no comprehensive guidelines on the evaluation and management of syncope until recently. The 2017 guideline from the American College of Cardiology, American Heart Association, and Heart Rhythm Society is intended to provide guidance on evaluation and management of syncope, specifically in the context of different clinical settings, specific causes, or selected circumstances.1
What primary care providers should know
A detailed history and physical exam should be performed in all patients with syncope. Useful details include the setting in which syncope occurs, prodromal symptoms, witness reports, postevent symptoms, comorbidities, medication use, past medical history, and family history. The physical exam should include orthostatic vital signs, cardiac exam, neurologic exam, and any other relevant systems. A resting 12-lead ECG in the initial evaluation is recommended to detect underlying arrhythmia or structural heart disease (Class I recommendation – strong).
There are many different causes of syncope (see Table 1). Vasovagal syncope, a form of reflex syncope mediated by the vasovagal reflex, is the most common cause of syncope and a frequent reason for emergency department visits. There is often a prodrome of diaphoresis, warmth, nausea, and/or pallor, often followed by fatigue. The diagnosis can be made by the history, physical exam, and eyewitness observation.
Once the initial evaluation is complete, further evaluation and management depends on the presence of risk factors presented in Table 2. Outpatient management is reasonable for patients with presumptive reflex-mediated syncope when there is an absence of serious medical conditions such as cardiac disease or comorbid neurologic disease. While hospital-based evaluation has not been shown to improve outcomes in patients with a low risk profile, hospital-based evaluation and treatment are recommended for patients presenting with syncope who have a serious medical condition potentially relevant to the cause of syncope.2 Serious medical conditions that require hospital management include arrhythmia, cardiac ischemia, severe aortic stenosis, hypertrophic cardiomyopathy, aortic dissection, acute heart failure, severe anemia, or major traumatic injury. Finally, patients with intermediate risk may benefit from an observational protocol in the emergency department.
Routine and comprehensive laboratory testing is not useful in syncope work-up (Class III recommendation – no benefit). Routine cardiac imaging is not recommended unless a cardiac etiology is suspected and routine neurological imaging and EEG are not recommended in the absence of focal neurologic findings. Additional work-up may be indicated if initial evaluation suggests a more specific etiology. If the initial evaluation suggests neurogenic orthostatic hypotension but the diagnosis is not clear, then referral for an autonomic evaluation is reasonable. If reflex syncope is suspected, tilt-table testing may be helpful to confirm the diagnosis. Lastly, if a cardiovascular etiology is suspected, it is recommended that the patient have cardiac monitoring in the acute care setting. In this later group, stress testing, transthoracic echocardiogram, electrophysiology study, and/or MRI or CT may be useful. Electrophysiologic testing is reasonable in patients with suspected arrhythmia as the etiology for syncope (Class IIa recommendation – moderate strength). The guideline provides a convenient summary algorithm to approach the initial and subsequent evaluations for syncope based on the initial evaluation and presenting symptoms.
Special populations
There are specific considerations for certain populations. In the pediatric population, the vast majority of syncopal episodes are reflex syncope but breath-holding spells should also be considered. In the geriatric population, particularly individuals older than 75 years, the incidence of syncope is high, the differential diagnosis is broad, and the diagnosis may be imprecise given amnesia, falls, lack of witnesses, and polypharmacy. In this group, morbidity is high because of multimorbidity and frailty. A careful history and physical exam with orthostatic vital signs is important, as is a multidisciplinary approach with geriatric consultation when needed.
Summary
Syncope is a common clinical syndrome often presenting to the emergency department or primary care setting. There are many causes, the most common being vasovagal syncope. In the initial evaluation, providers should perform a detailed history and physical exam, check orthostatic signs and perform a 12-lead ECG. Patients can be evaluated and managed safely in the outpatient setting in the absence of risk factors. Routine comprehensive laboratory testing and cardiac imaging are often not needed. For patients with defined risk factors, a more detailed evaluation in the hospital is recommended.
Dr. Li is a second-year resident in the family medicine residency program in the department of family and community medicine at the Sidney Kimmel Medical College at Thomas Jefferson University, Philadelphia. Dr. Mills is assistant residency program director and assistant professor in the departments of family and community medicine and physiology at the Sidney Kimmel Medical College. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
References
1. Shen W, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope. Circulation. 2017 Aug 1;136(5):e60-e122. doi: 10.1161/CIR.0000000000000499. Epub 2017 Mar 9.
2. Soteriades ES, Evans JC, Larson MG, et al. Incidence and prognosis of syncope. N Engl J Med. 2002;347(12):878-85.
Syncope is characterized by sudden transient loss of consciousness due to cerebral hypoperfusion and is typically associated with an inability to maintain postural tone. There are many different causes and clinical presentations of syncope and the incidence varies depending on the population. Estimated lifetime prevalence rates are as high as 41% for a single episode of syncope, with recurrent syncope occurring in 13.5% of the general population. Incidence follows a trimodal distribution with peaks at age 20, 60, and 80 years for both men and women. The National Hospital Ambulatory Medical Care Survey reported 6.7 million episodes of syncope in the emergency department, which is where most patients with syncope initially present. However, patients may also present to the primary care outpatient setting, and providers should be equipped for initial evaluation and management.
Previous and current treatment guidelines
Although there have been general reviews published by general and specialty societies, there were no comprehensive guidelines on the evaluation and management of syncope until recently. The 2017 guideline from the American College of Cardiology, American Heart Association, and Heart Rhythm Society is intended to provide guidance on evaluation and management of syncope, specifically in the context of different clinical settings, specific causes, or selected circumstances.1
What primary care providers should know
A detailed history and physical exam should be performed in all patients with syncope. Useful details include the setting in which syncope occurs, prodromal symptoms, witness reports, postevent symptoms, comorbidities, medication use, past medical history, and family history. The physical exam should include orthostatic vital signs, cardiac exam, neurologic exam, and any other relevant systems. A resting 12-lead ECG in the initial evaluation is recommended to detect underlying arrhythmia or structural heart disease (Class I recommendation – strong).
There are many different causes of syncope (see Table 1). Vasovagal syncope, a form of reflex syncope mediated by the vasovagal reflex, is the most common cause of syncope and a frequent reason for emergency department visits. There is often a prodrome of diaphoresis, warmth, nausea, and/or pallor, often followed by fatigue. The diagnosis can be made by the history, physical exam, and eyewitness observation.
Once the initial evaluation is complete, further evaluation and management depends on the presence of risk factors presented in Table 2. Outpatient management is reasonable for patients with presumptive reflex-mediated syncope when there is an absence of serious medical conditions such as cardiac disease or comorbid neurologic disease. While hospital-based evaluation has not been shown to improve outcomes in patients with a low risk profile, hospital-based evaluation and treatment are recommended for patients presenting with syncope who have a serious medical condition potentially relevant to the cause of syncope.2 Serious medical conditions that require hospital management include arrhythmia, cardiac ischemia, severe aortic stenosis, hypertrophic cardiomyopathy, aortic dissection, acute heart failure, severe anemia, or major traumatic injury. Finally, patients with intermediate risk may benefit from an observational protocol in the emergency department.
Routine and comprehensive laboratory testing is not useful in syncope work-up (Class III recommendation – no benefit). Routine cardiac imaging is not recommended unless a cardiac etiology is suspected and routine neurological imaging and EEG are not recommended in the absence of focal neurologic findings. Additional work-up may be indicated if initial evaluation suggests a more specific etiology. If the initial evaluation suggests neurogenic orthostatic hypotension but the diagnosis is not clear, then referral for an autonomic evaluation is reasonable. If reflex syncope is suspected, tilt-table testing may be helpful to confirm the diagnosis. Lastly, if a cardiovascular etiology is suspected, it is recommended that the patient have cardiac monitoring in the acute care setting. In this later group, stress testing, transthoracic echocardiogram, electrophysiology study, and/or MRI or CT may be useful. Electrophysiologic testing is reasonable in patients with suspected arrhythmia as the etiology for syncope (Class IIa recommendation – moderate strength). The guideline provides a convenient summary algorithm to approach the initial and subsequent evaluations for syncope based on the initial evaluation and presenting symptoms.
Special populations
There are specific considerations for certain populations. In the pediatric population, the vast majority of syncopal episodes are reflex syncope but breath-holding spells should also be considered. In the geriatric population, particularly individuals older than 75 years, the incidence of syncope is high, the differential diagnosis is broad, and the diagnosis may be imprecise given amnesia, falls, lack of witnesses, and polypharmacy. In this group, morbidity is high because of multimorbidity and frailty. A careful history and physical exam with orthostatic vital signs is important, as is a multidisciplinary approach with geriatric consultation when needed.
Summary
Syncope is a common clinical syndrome often presenting to the emergency department or primary care setting. There are many causes, the most common being vasovagal syncope. In the initial evaluation, providers should perform a detailed history and physical exam, check orthostatic signs and perform a 12-lead ECG. Patients can be evaluated and managed safely in the outpatient setting in the absence of risk factors. Routine comprehensive laboratory testing and cardiac imaging are often not needed. For patients with defined risk factors, a more detailed evaluation in the hospital is recommended.
Dr. Li is a second-year resident in the family medicine residency program in the department of family and community medicine at the Sidney Kimmel Medical College at Thomas Jefferson University, Philadelphia. Dr. Mills is assistant residency program director and assistant professor in the departments of family and community medicine and physiology at the Sidney Kimmel Medical College. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
References
1. Shen W, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope. Circulation. 2017 Aug 1;136(5):e60-e122. doi: 10.1161/CIR.0000000000000499. Epub 2017 Mar 9.
2. Soteriades ES, Evans JC, Larson MG, et al. Incidence and prognosis of syncope. N Engl J Med. 2002;347(12):878-85.
Clinical Guidelines: Hospital-acquired and ventilator-associated pneumonia
Hospital-acquired pneumonia (HAP) is pneumonia that presents at least 48 hours after admission to the hospital. In contrast, ventilator-associated pneumonia (VAP), is pneumonia that clinically presents 48 hours after endotracheal intubation. Together, these are some of the most common hospital-acquired infections in the United States and pose a considerable burden on hospitals nationwide.
The Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS) recently updated their management guidelines for HAP and VAP with a goal of striking a balance between providing appropriate early antibiotic coverage and avoiding unnecessary treatment that can lead to adverse effects such as Clostridium difficile infections and development of antibiotic resistance.1 This update eliminated the concept of Healthcare Associated Pneumonia (HCAP), often used for patients in skilled care facilities, because newer evidence has shown that patients who had met these criteria did not have a higher incidence of multidrug resistant pathogens; rather, they have microbial etiologies and sensitivities that are similar to adults with community acquired pneumonia (CAP).
Hospital-acquired pneumonia
Reasons to cover for MRSA in HAP:
Risk factors:
• IV antibiotic treatment within 90 days
• Treatment in a unit where the prevalence of MRSA is greater than 20% or unknown
• Prior detection of MRSA by culture or nonculture screening (weaker risk factor)
High risk of mortality: • Septic shock
• Need for ventilator support
MRSA should be covered with use of either vancomycin or linezolid in these cases.
In addition, patients with HAP should be covered for Pseudomonas aeruginosa and other gram-negative bacilli. For patients with risk factors for pseudomonas or other gram-negative infection or a high risk for mortality, then two antipseudomonal antibiotics from different classes are recommended, such as piperacillin-tazobactam/tobramycin or cefepime/amikacin.
Use two antipseudomonal antibiotics in HAP if the patient has these risk factors:
Pseudomonas risk factors:
• IV antibiotic treatment within 90 days
• Structural lung disease increasing the risk of gram-negative infection (bronchiectasis, cystic fibrosis)
• High-quality gram stain from respiratory specimen showing predominant and numerous gram-negative bacilli
High risk of mortality:
• Septic shock
• Need for ventilator support
Ventilator-associated pneumonia
General management of VAP is similar to HAP in that empiric treatment should be tailored to the local distribution and susceptibilities of pathogens based on each hospital’s antibiogram. All regimens should cover for S. aureus, P. aeruginosa, and other gram-negative bacilli based on the risk of mortality associated with the need for ventilator support. MSSA should be covered for VAP unless the patient has methicillin-resistant risk factors (see below).
MRSA should be covered for VAP if:
• Patient has had IV antibiotic use within past 90 days
• Hospital unit has greater than 10%-20% of S. aureus isolates are MRSA or MRSA prevalence unknown
Only one antipseudomonal agent should be used unless there are one of the following characteristics present, as described below.
Use two antipseudomonal agents in VAP if:
• Prior IV antibiotic use within 90 days
• Septic shock at time of VAP
• Acute respiratory distress syndrome preceding VAP
• 5 or more days of hospitalization prior to the occurrence of VAP
• Acute renal replacement therapy prior to VAP onset
• Greater than 10% of gram-negative isolates are resistant to an agent being considered for monotherapy
• Local antibiotic susceptibility rates unknown
In both HAP and VAP, antibiotics should be de-escalated to those with a narrower spectrum after initial empiric therapy, ideally within 72 hours and based on sputum or blood culture results. The guidelines support obtaining noninvasive sputum cultures in patients with VAP (endotracheal aspirates) and HAP (spontaneous expectoration, induced sputum, or nasotracheal suctioning in a patient who is unable to cooperate to produce a sputum sample). Patients who are improving clinically may be switched to appropriate oral therapy based on the susceptibility of an identified organism. Another key change is that of the standard duration of therapy. Previously, patients were treated for up to 2-3 weeks with antibiotics. The new IDSA/ATS guidelines recommend that patients should be treated with 7 days of antibiotics rather than a longer course.
The bottom line
Empiric therapy for HAP and VAP should be tailored to each hospital’s local pathogen distribution and antimicrobial susceptibilities, as detailed in an antibiogram. In HAP and VAP, empiric antibiotics should cover for S. aureus, but it only needs to target MRSA if risk factors are present, prevalence is greater than 20% or unknown, and – if HAP – a high risk of mortality. P. aeruginosa and other gram-negative bacilli should also be covered in empiric regimens. Dual antipseudomonal antibiotics is only recommended to be used in HAP if there are specific pseudomonal risk factors or a high risk of mortality. They should be used in VAP if there are multidrug-resistant risk factors present or there is a high/unknown prevalence of resistant organisms. All antibiotic regimens should be deescalated rather than maintained, and both HAP and VAP patients ought to be treated for 7 days.
References
1. Kalil AC, Metersky ML, Klompas M, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016 Sep 1;63(5):557-82.
2. Beardsley JR, Williamson JC, Johnson JW, Ohl CA, Karchmer TB, Bowton DL. Using local microbiologic data to develop institution-specific guidelines for the treatment of hospital-acquired pneumonia. Chest. 2006 Sep;130(3):787-93.
Dr. Botti is a second-year resident in the family medicine residency program department of family and community medicine at Jefferson Medical College, Philadelphia. Dr. Mills is assistant residency program director and assistant professor in the department of family and community medicine and department of physiology at Jefferson Medical College, Philadelphia. Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia.
Hospital-acquired pneumonia (HAP) is pneumonia that presents at least 48 hours after admission to the hospital. In contrast, ventilator-associated pneumonia (VAP), is pneumonia that clinically presents 48 hours after endotracheal intubation. Together, these are some of the most common hospital-acquired infections in the United States and pose a considerable burden on hospitals nationwide.
The Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS) recently updated their management guidelines for HAP and VAP with a goal of striking a balance between providing appropriate early antibiotic coverage and avoiding unnecessary treatment that can lead to adverse effects such as Clostridium difficile infections and development of antibiotic resistance.1 This update eliminated the concept of Healthcare Associated Pneumonia (HCAP), often used for patients in skilled care facilities, because newer evidence has shown that patients who had met these criteria did not have a higher incidence of multidrug resistant pathogens; rather, they have microbial etiologies and sensitivities that are similar to adults with community acquired pneumonia (CAP).
Hospital-acquired pneumonia
Reasons to cover for MRSA in HAP:
Risk factors:
• IV antibiotic treatment within 90 days
• Treatment in a unit where the prevalence of MRSA is greater than 20% or unknown
• Prior detection of MRSA by culture or nonculture screening (weaker risk factor)
High risk of mortality: • Septic shock
• Need for ventilator support
MRSA should be covered with use of either vancomycin or linezolid in these cases.
In addition, patients with HAP should be covered for Pseudomonas aeruginosa and other gram-negative bacilli. For patients with risk factors for pseudomonas or other gram-negative infection or a high risk for mortality, then two antipseudomonal antibiotics from different classes are recommended, such as piperacillin-tazobactam/tobramycin or cefepime/amikacin.
Use two antipseudomonal antibiotics in HAP if the patient has these risk factors:
Pseudomonas risk factors:
• IV antibiotic treatment within 90 days
• Structural lung disease increasing the risk of gram-negative infection (bronchiectasis, cystic fibrosis)
• High-quality gram stain from respiratory specimen showing predominant and numerous gram-negative bacilli
High risk of mortality:
• Septic shock
• Need for ventilator support
Ventilator-associated pneumonia
General management of VAP is similar to HAP in that empiric treatment should be tailored to the local distribution and susceptibilities of pathogens based on each hospital’s antibiogram. All regimens should cover for S. aureus, P. aeruginosa, and other gram-negative bacilli based on the risk of mortality associated with the need for ventilator support. MSSA should be covered for VAP unless the patient has methicillin-resistant risk factors (see below).
MRSA should be covered for VAP if:
• Patient has had IV antibiotic use within past 90 days
• Hospital unit has greater than 10%-20% of S. aureus isolates are MRSA or MRSA prevalence unknown
Only one antipseudomonal agent should be used unless there are one of the following characteristics present, as described below.
Use two antipseudomonal agents in VAP if:
• Prior IV antibiotic use within 90 days
• Septic shock at time of VAP
• Acute respiratory distress syndrome preceding VAP
• 5 or more days of hospitalization prior to the occurrence of VAP
• Acute renal replacement therapy prior to VAP onset
• Greater than 10% of gram-negative isolates are resistant to an agent being considered for monotherapy
• Local antibiotic susceptibility rates unknown
In both HAP and VAP, antibiotics should be de-escalated to those with a narrower spectrum after initial empiric therapy, ideally within 72 hours and based on sputum or blood culture results. The guidelines support obtaining noninvasive sputum cultures in patients with VAP (endotracheal aspirates) and HAP (spontaneous expectoration, induced sputum, or nasotracheal suctioning in a patient who is unable to cooperate to produce a sputum sample). Patients who are improving clinically may be switched to appropriate oral therapy based on the susceptibility of an identified organism. Another key change is that of the standard duration of therapy. Previously, patients were treated for up to 2-3 weeks with antibiotics. The new IDSA/ATS guidelines recommend that patients should be treated with 7 days of antibiotics rather than a longer course.
The bottom line
Empiric therapy for HAP and VAP should be tailored to each hospital’s local pathogen distribution and antimicrobial susceptibilities, as detailed in an antibiogram. In HAP and VAP, empiric antibiotics should cover for S. aureus, but it only needs to target MRSA if risk factors are present, prevalence is greater than 20% or unknown, and – if HAP – a high risk of mortality. P. aeruginosa and other gram-negative bacilli should also be covered in empiric regimens. Dual antipseudomonal antibiotics is only recommended to be used in HAP if there are specific pseudomonal risk factors or a high risk of mortality. They should be used in VAP if there are multidrug-resistant risk factors present or there is a high/unknown prevalence of resistant organisms. All antibiotic regimens should be deescalated rather than maintained, and both HAP and VAP patients ought to be treated for 7 days.
References
1. Kalil AC, Metersky ML, Klompas M, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016 Sep 1;63(5):557-82.
2. Beardsley JR, Williamson JC, Johnson JW, Ohl CA, Karchmer TB, Bowton DL. Using local microbiologic data to develop institution-specific guidelines for the treatment of hospital-acquired pneumonia. Chest. 2006 Sep;130(3):787-93.
Dr. Botti is a second-year resident in the family medicine residency program department of family and community medicine at Jefferson Medical College, Philadelphia. Dr. Mills is assistant residency program director and assistant professor in the department of family and community medicine and department of physiology at Jefferson Medical College, Philadelphia. Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia.
Hospital-acquired pneumonia (HAP) is pneumonia that presents at least 48 hours after admission to the hospital. In contrast, ventilator-associated pneumonia (VAP), is pneumonia that clinically presents 48 hours after endotracheal intubation. Together, these are some of the most common hospital-acquired infections in the United States and pose a considerable burden on hospitals nationwide.
The Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS) recently updated their management guidelines for HAP and VAP with a goal of striking a balance between providing appropriate early antibiotic coverage and avoiding unnecessary treatment that can lead to adverse effects such as Clostridium difficile infections and development of antibiotic resistance.1 This update eliminated the concept of Healthcare Associated Pneumonia (HCAP), often used for patients in skilled care facilities, because newer evidence has shown that patients who had met these criteria did not have a higher incidence of multidrug resistant pathogens; rather, they have microbial etiologies and sensitivities that are similar to adults with community acquired pneumonia (CAP).
Hospital-acquired pneumonia
Reasons to cover for MRSA in HAP:
Risk factors:
• IV antibiotic treatment within 90 days
• Treatment in a unit where the prevalence of MRSA is greater than 20% or unknown
• Prior detection of MRSA by culture or nonculture screening (weaker risk factor)
High risk of mortality: • Septic shock
• Need for ventilator support
MRSA should be covered with use of either vancomycin or linezolid in these cases.
In addition, patients with HAP should be covered for Pseudomonas aeruginosa and other gram-negative bacilli. For patients with risk factors for pseudomonas or other gram-negative infection or a high risk for mortality, then two antipseudomonal antibiotics from different classes are recommended, such as piperacillin-tazobactam/tobramycin or cefepime/amikacin.
Use two antipseudomonal antibiotics in HAP if the patient has these risk factors:
Pseudomonas risk factors:
• IV antibiotic treatment within 90 days
• Structural lung disease increasing the risk of gram-negative infection (bronchiectasis, cystic fibrosis)
• High-quality gram stain from respiratory specimen showing predominant and numerous gram-negative bacilli
High risk of mortality:
• Septic shock
• Need for ventilator support
Ventilator-associated pneumonia
General management of VAP is similar to HAP in that empiric treatment should be tailored to the local distribution and susceptibilities of pathogens based on each hospital’s antibiogram. All regimens should cover for S. aureus, P. aeruginosa, and other gram-negative bacilli based on the risk of mortality associated with the need for ventilator support. MSSA should be covered for VAP unless the patient has methicillin-resistant risk factors (see below).
MRSA should be covered for VAP if:
• Patient has had IV antibiotic use within past 90 days
• Hospital unit has greater than 10%-20% of S. aureus isolates are MRSA or MRSA prevalence unknown
Only one antipseudomonal agent should be used unless there are one of the following characteristics present, as described below.
Use two antipseudomonal agents in VAP if:
• Prior IV antibiotic use within 90 days
• Septic shock at time of VAP
• Acute respiratory distress syndrome preceding VAP
• 5 or more days of hospitalization prior to the occurrence of VAP
• Acute renal replacement therapy prior to VAP onset
• Greater than 10% of gram-negative isolates are resistant to an agent being considered for monotherapy
• Local antibiotic susceptibility rates unknown
In both HAP and VAP, antibiotics should be de-escalated to those with a narrower spectrum after initial empiric therapy, ideally within 72 hours and based on sputum or blood culture results. The guidelines support obtaining noninvasive sputum cultures in patients with VAP (endotracheal aspirates) and HAP (spontaneous expectoration, induced sputum, or nasotracheal suctioning in a patient who is unable to cooperate to produce a sputum sample). Patients who are improving clinically may be switched to appropriate oral therapy based on the susceptibility of an identified organism. Another key change is that of the standard duration of therapy. Previously, patients were treated for up to 2-3 weeks with antibiotics. The new IDSA/ATS guidelines recommend that patients should be treated with 7 days of antibiotics rather than a longer course.
The bottom line
Empiric therapy for HAP and VAP should be tailored to each hospital’s local pathogen distribution and antimicrobial susceptibilities, as detailed in an antibiogram. In HAP and VAP, empiric antibiotics should cover for S. aureus, but it only needs to target MRSA if risk factors are present, prevalence is greater than 20% or unknown, and – if HAP – a high risk of mortality. P. aeruginosa and other gram-negative bacilli should also be covered in empiric regimens. Dual antipseudomonal antibiotics is only recommended to be used in HAP if there are specific pseudomonal risk factors or a high risk of mortality. They should be used in VAP if there are multidrug-resistant risk factors present or there is a high/unknown prevalence of resistant organisms. All antibiotic regimens should be deescalated rather than maintained, and both HAP and VAP patients ought to be treated for 7 days.
References
1. Kalil AC, Metersky ML, Klompas M, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016 Sep 1;63(5):557-82.
2. Beardsley JR, Williamson JC, Johnson JW, Ohl CA, Karchmer TB, Bowton DL. Using local microbiologic data to develop institution-specific guidelines for the treatment of hospital-acquired pneumonia. Chest. 2006 Sep;130(3):787-93.
Dr. Botti is a second-year resident in the family medicine residency program department of family and community medicine at Jefferson Medical College, Philadelphia. Dr. Mills is assistant residency program director and assistant professor in the department of family and community medicine and department of physiology at Jefferson Medical College, Philadelphia. Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia.
Update on the third international consensus definitions for sepsis and septic shock
Sepsis is the primary cause of death from infection. Early identification and treatment of sepsis is important in improving patient outcomes. The consensus conference sought to differentiate sepsis, which is defined as “life-threatening organ dysfunction caused by a dysregulated host response to infection” from uncomplicated infection.
Sepsis was last classified in a 2001 guideline that based its definition on the presence of two or more systemic inflammatory response syndrome (SIRS) criteria, which included an elevated temperature, heart rate higher than 90 bpm, respiratory rate higher than 20 breaths per minute, and a white blood cell count greater than greater than 12,000 mcL or less than 4,000 mcL or greater than 10% immature bands.
The problem with the SIRS definition of sepsis is that while it reflects a response to infection, it does not sufficiently distinguish between individuals with infections and those with a dysregulated response that leads to a poor prognosis, which is the definition of sepsis. The current consensus conference redefines sepsis with a more direct emphasis on organ dysfunction, as this is the aspect of sepsis that is most clearly linked to patient outcomes.
In the consensus conference document, sepsis is defined as a “life-threatening organ dysfunction caused by a dysregulated host response to infection.” The guidelines recommend using the quick version of the sequential (sepsis-related) organ failure assessment score (qSOFA) to identify patients with sepsis. In its long form, the SOFA used seven clinical and laboratory data points for completion, and is best suited to use in an intensive care setting where detailed data are available. The qSOFA score has only three criteria and by being easier to use can aid in rapid identification of sepsis and the patients most likely to deteriorate from sepsis.
The qSOFA criteria predict poor outcome in patients with infection who have two or more of the following: respiratory rate greater than or equal to 22 breaths/min, new or worsened altered mentation, or systolic blood pressure less than or equal to 100 mm Hg. Unlike the full SOFA score, the qSOFA does not require any laboratory testing and so can be performed in the office or bedside on a hospital floor. The qSOFA does not necessarily define sepsis, rather it identifies patients at a higher risk of hospital death or prolonged ICU stay. The consensus conference suggests that “qSOFA criteria be used to prompt clinicians to further investigate for organ dysfunction, initiate or escalate therapy as appropriate, and consider referral to critical care or increase the frequency of monitoring, if such actions have not already been undertaken.” The task force suggested that the qSOFA score may be a helpful adjunct to best clinical judgment for identifying patients who might benefit from a higher level of care.
Septic shock is defined as a subset of sepsis in which profound circulatory, cellular, and metabolic abnormalities are associated with a greater risk for death than sepsis alone. Septic shock can be identified when, after adequate fluid resuscitation, the patient requires vasopressor therapy to maintain mean arterial pressure of at least 65 mm Hg and has a serum lactate level greater than 2 mmol/L.
Once sepsis is suspected, prompt therapy needs to be started as per the Surviving Sepsis Campaign Guidelines. The qSOFA criteria can be used to identify patients at high risk for morbidity and mortality. Within 3 hours, a lactate level should be obtained as well as blood cultures from two separate sites drawn prior to administration of antibiotics (but do not delay antibiotic administration). Empiric broad-spectrum antibiotics should be given within 45 minutes of the identification of sepsis. Antibiotic choice will vary per clinician/institution preference, but should likely include coverage for Pseudomonas and MRSA (piperacillin/tazobactam and vancomycin, for example). Antibiotics should be reassessed daily for de-escalation. Administer 30 mL/kg crystalloid for hypotension or lactate greater than or equal to 4 mmol/L. Within 6 hours, vasopressors should be given for hypotension that does not respond to initial fluid resuscitation to maintain a mean arterial pressure (MAP) of at least 65mm Hg. In the event of persistent hypotension after initial fluid administration (MAP under 65 mm Hg) or if initial lactate was greater than or equal to 4 mmol/L, volume status and tissue perfusion should be reassessed and lactate should be rechecked if it was initially elevated.
The bottom line
A 2016 international task force recommended that the definition of sepsis should be changed to emphasize organ dysfunction rather than a systemic inflammatory response. Use of the qSOFA score, which relies only on clinically observable data rather than laboratory evaluation, is recommended to identify patients at high risk for morbidity and mortality. Early recognition of sepsis and evaluation with qSOFT should facilitate early treatment and improve survival.
References
Singer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) FRCP; JAMA. 2016;315[8]:801-10. doi: 10.1001/jama.2016.0287.
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003 Apr;31(4):1250-6.
Singer M, Deutschman CS, Seymour C, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016 Feb 23;315(8):801-10.
Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004 Mar;32(3):858-73.
Dr. Mills is assistant residency program director and assistant professor in the department of family and community medicine and department of physiology at Sidney Kimmel Medical College at Thomas Jefferson University. Dr. Botti is a second-year resident in the family medicine residency program department of family and community medicine at Sidney Kimmel Medical College at Thomas Jefferson University. Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia.
Sepsis is the primary cause of death from infection. Early identification and treatment of sepsis is important in improving patient outcomes. The consensus conference sought to differentiate sepsis, which is defined as “life-threatening organ dysfunction caused by a dysregulated host response to infection” from uncomplicated infection.
Sepsis was last classified in a 2001 guideline that based its definition on the presence of two or more systemic inflammatory response syndrome (SIRS) criteria, which included an elevated temperature, heart rate higher than 90 bpm, respiratory rate higher than 20 breaths per minute, and a white blood cell count greater than greater than 12,000 mcL or less than 4,000 mcL or greater than 10% immature bands.
The problem with the SIRS definition of sepsis is that while it reflects a response to infection, it does not sufficiently distinguish between individuals with infections and those with a dysregulated response that leads to a poor prognosis, which is the definition of sepsis. The current consensus conference redefines sepsis with a more direct emphasis on organ dysfunction, as this is the aspect of sepsis that is most clearly linked to patient outcomes.
In the consensus conference document, sepsis is defined as a “life-threatening organ dysfunction caused by a dysregulated host response to infection.” The guidelines recommend using the quick version of the sequential (sepsis-related) organ failure assessment score (qSOFA) to identify patients with sepsis. In its long form, the SOFA used seven clinical and laboratory data points for completion, and is best suited to use in an intensive care setting where detailed data are available. The qSOFA score has only three criteria and by being easier to use can aid in rapid identification of sepsis and the patients most likely to deteriorate from sepsis.
The qSOFA criteria predict poor outcome in patients with infection who have two or more of the following: respiratory rate greater than or equal to 22 breaths/min, new or worsened altered mentation, or systolic blood pressure less than or equal to 100 mm Hg. Unlike the full SOFA score, the qSOFA does not require any laboratory testing and so can be performed in the office or bedside on a hospital floor. The qSOFA does not necessarily define sepsis, rather it identifies patients at a higher risk of hospital death or prolonged ICU stay. The consensus conference suggests that “qSOFA criteria be used to prompt clinicians to further investigate for organ dysfunction, initiate or escalate therapy as appropriate, and consider referral to critical care or increase the frequency of monitoring, if such actions have not already been undertaken.” The task force suggested that the qSOFA score may be a helpful adjunct to best clinical judgment for identifying patients who might benefit from a higher level of care.
Septic shock is defined as a subset of sepsis in which profound circulatory, cellular, and metabolic abnormalities are associated with a greater risk for death than sepsis alone. Septic shock can be identified when, after adequate fluid resuscitation, the patient requires vasopressor therapy to maintain mean arterial pressure of at least 65 mm Hg and has a serum lactate level greater than 2 mmol/L.
Once sepsis is suspected, prompt therapy needs to be started as per the Surviving Sepsis Campaign Guidelines. The qSOFA criteria can be used to identify patients at high risk for morbidity and mortality. Within 3 hours, a lactate level should be obtained as well as blood cultures from two separate sites drawn prior to administration of antibiotics (but do not delay antibiotic administration). Empiric broad-spectrum antibiotics should be given within 45 minutes of the identification of sepsis. Antibiotic choice will vary per clinician/institution preference, but should likely include coverage for Pseudomonas and MRSA (piperacillin/tazobactam and vancomycin, for example). Antibiotics should be reassessed daily for de-escalation. Administer 30 mL/kg crystalloid for hypotension or lactate greater than or equal to 4 mmol/L. Within 6 hours, vasopressors should be given for hypotension that does not respond to initial fluid resuscitation to maintain a mean arterial pressure (MAP) of at least 65mm Hg. In the event of persistent hypotension after initial fluid administration (MAP under 65 mm Hg) or if initial lactate was greater than or equal to 4 mmol/L, volume status and tissue perfusion should be reassessed and lactate should be rechecked if it was initially elevated.
The bottom line
A 2016 international task force recommended that the definition of sepsis should be changed to emphasize organ dysfunction rather than a systemic inflammatory response. Use of the qSOFA score, which relies only on clinically observable data rather than laboratory evaluation, is recommended to identify patients at high risk for morbidity and mortality. Early recognition of sepsis and evaluation with qSOFT should facilitate early treatment and improve survival.
References
Singer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) FRCP; JAMA. 2016;315[8]:801-10. doi: 10.1001/jama.2016.0287.
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003 Apr;31(4):1250-6.
Singer M, Deutschman CS, Seymour C, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016 Feb 23;315(8):801-10.
Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004 Mar;32(3):858-73.
Dr. Mills is assistant residency program director and assistant professor in the department of family and community medicine and department of physiology at Sidney Kimmel Medical College at Thomas Jefferson University. Dr. Botti is a second-year resident in the family medicine residency program department of family and community medicine at Sidney Kimmel Medical College at Thomas Jefferson University. Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia.
Sepsis is the primary cause of death from infection. Early identification and treatment of sepsis is important in improving patient outcomes. The consensus conference sought to differentiate sepsis, which is defined as “life-threatening organ dysfunction caused by a dysregulated host response to infection” from uncomplicated infection.
Sepsis was last classified in a 2001 guideline that based its definition on the presence of two or more systemic inflammatory response syndrome (SIRS) criteria, which included an elevated temperature, heart rate higher than 90 bpm, respiratory rate higher than 20 breaths per minute, and a white blood cell count greater than greater than 12,000 mcL or less than 4,000 mcL or greater than 10% immature bands.
The problem with the SIRS definition of sepsis is that while it reflects a response to infection, it does not sufficiently distinguish between individuals with infections and those with a dysregulated response that leads to a poor prognosis, which is the definition of sepsis. The current consensus conference redefines sepsis with a more direct emphasis on organ dysfunction, as this is the aspect of sepsis that is most clearly linked to patient outcomes.
In the consensus conference document, sepsis is defined as a “life-threatening organ dysfunction caused by a dysregulated host response to infection.” The guidelines recommend using the quick version of the sequential (sepsis-related) organ failure assessment score (qSOFA) to identify patients with sepsis. In its long form, the SOFA used seven clinical and laboratory data points for completion, and is best suited to use in an intensive care setting where detailed data are available. The qSOFA score has only three criteria and by being easier to use can aid in rapid identification of sepsis and the patients most likely to deteriorate from sepsis.
The qSOFA criteria predict poor outcome in patients with infection who have two or more of the following: respiratory rate greater than or equal to 22 breaths/min, new or worsened altered mentation, or systolic blood pressure less than or equal to 100 mm Hg. Unlike the full SOFA score, the qSOFA does not require any laboratory testing and so can be performed in the office or bedside on a hospital floor. The qSOFA does not necessarily define sepsis, rather it identifies patients at a higher risk of hospital death or prolonged ICU stay. The consensus conference suggests that “qSOFA criteria be used to prompt clinicians to further investigate for organ dysfunction, initiate or escalate therapy as appropriate, and consider referral to critical care or increase the frequency of monitoring, if such actions have not already been undertaken.” The task force suggested that the qSOFA score may be a helpful adjunct to best clinical judgment for identifying patients who might benefit from a higher level of care.
Septic shock is defined as a subset of sepsis in which profound circulatory, cellular, and metabolic abnormalities are associated with a greater risk for death than sepsis alone. Septic shock can be identified when, after adequate fluid resuscitation, the patient requires vasopressor therapy to maintain mean arterial pressure of at least 65 mm Hg and has a serum lactate level greater than 2 mmol/L.
Once sepsis is suspected, prompt therapy needs to be started as per the Surviving Sepsis Campaign Guidelines. The qSOFA criteria can be used to identify patients at high risk for morbidity and mortality. Within 3 hours, a lactate level should be obtained as well as blood cultures from two separate sites drawn prior to administration of antibiotics (but do not delay antibiotic administration). Empiric broad-spectrum antibiotics should be given within 45 minutes of the identification of sepsis. Antibiotic choice will vary per clinician/institution preference, but should likely include coverage for Pseudomonas and MRSA (piperacillin/tazobactam and vancomycin, for example). Antibiotics should be reassessed daily for de-escalation. Administer 30 mL/kg crystalloid for hypotension or lactate greater than or equal to 4 mmol/L. Within 6 hours, vasopressors should be given for hypotension that does not respond to initial fluid resuscitation to maintain a mean arterial pressure (MAP) of at least 65mm Hg. In the event of persistent hypotension after initial fluid administration (MAP under 65 mm Hg) or if initial lactate was greater than or equal to 4 mmol/L, volume status and tissue perfusion should be reassessed and lactate should be rechecked if it was initially elevated.
The bottom line
A 2016 international task force recommended that the definition of sepsis should be changed to emphasize organ dysfunction rather than a systemic inflammatory response. Use of the qSOFA score, which relies only on clinically observable data rather than laboratory evaluation, is recommended to identify patients at high risk for morbidity and mortality. Early recognition of sepsis and evaluation with qSOFT should facilitate early treatment and improve survival.
References
Singer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) FRCP; JAMA. 2016;315[8]:801-10. doi: 10.1001/jama.2016.0287.
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003 Apr;31(4):1250-6.
Singer M, Deutschman CS, Seymour C, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016 Feb 23;315(8):801-10.
Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004 Mar;32(3):858-73.
Dr. Mills is assistant residency program director and assistant professor in the department of family and community medicine and department of physiology at Sidney Kimmel Medical College at Thomas Jefferson University. Dr. Botti is a second-year resident in the family medicine residency program department of family and community medicine at Sidney Kimmel Medical College at Thomas Jefferson University. Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia.
Strategies to help reduce hospital readmissions
› Use risk stratification methods such as the Probability of Repeated Admission (Pra) or the LACE index to identify patients at high risk for readmission. B
› Take steps to ensure that follow-up appointments are made within the first one to 2 weeks of discharge, depending on the patient’s risk of readmission. C
› Reconcile preadmission and postdischarge medications to identify discrepancies and possible interactions. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Charles T, age 74, has a 3-year history of myocardial infarction (MI) and congestive heart failure (CHF) and a 10-year his-tory of type 2 diabetes with retinopathy. You have cared for him in the outpatient setting for 8 years. You are notified that he is in the emergency department (ED) and being admitted to the hospital, again. This is his third ED visit in the past 3 months; he was hospitalized for 6 days during his last admission 3 weeks ago.
What should you do with this information? How can you best communicate with the admitting team?
Hospital readmissions are widespread, costly, and often avoidable. Nearly 20% of Medicare beneficiaries discharged from hospitals are rehospitalized within 30 days, and 34% are rehospitalized within 90 days.1 For patients with conditions like CHF, the rate of readmission within 30 days approaches 25%.2 The estimated cost to Medicare for unplanned rehospitalizations in 2004 was $17.4 billion.1 The Centers for Medicare and Medicaid Services penalizes hospitals for high rates of readmission within 30 days of discharge for patients with CHF, MI, and pneumonia.
“Avoidable” hospitalizations are those that may be prevented by effective outpatient management and improved care coordination. Although efforts to reduce readmissions have focused on improving the discharge process, family physicians (FPs) can play a central role in reducing readmissions. This article describes key approaches that FPs can take to address this important issue. Because patients ages ≥65 years consistently have the highest rate of hospital readmissions,1 we will focus on this population.
Multiple complex factors are associated with hospital readmissions
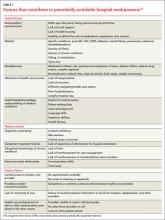
Characteristics of the patient, physician, and health care setting contribute to potentially avoidable readmissions (TABLE 1).3,4
Medical conditions and comorbidities associated with high rates of rehospitalization include CHF, acute MI, pneumonia, diabetes, and chronic obstructive pulmonary disease. However, a recent study found that a diverse range of conditions, frequently differing from the index cause of hospitalization, were responsible for 30-day readmissions of Medicare patients.5
Identifying those at high risk: Why and how
Determining which patients are at highest risk for readmission enables health care teams to match the intensity of interventions to the individual’s likelihood of readmission. However, current readmission risk prediction models remain a work in progress6 and few models have been tested in the outpatient setting. Despite numerous limitations, it’s still important to focus resources more efficiently. Thus, we recommend using risk stratification tools to identify patients at high risk for readmission.
Many risk stratification methods use data from electronic medical records (EMRs) and administrative databases or self-reported data from patients.7 Risk prediction tools that are relatively simple and easy to administer or generate through EMRs—such as the Probability of Repeated Admission (Pra),8 the LACE (Length of stay, acuity of the admission, comorbidities, ED visits in the previous 6 months) index,9 or the Community Assessment Risk Screen (CARS)10—may be best for use in the primary care setting. These tools generally identify key risk factors, such as prior health care utilization, presence of specific conditions such as heart disease or cognitive impairment, self-reported health status, absence of a caregiver, and/or need for assistance with daily routines.
Many of these tools have been used to identify high-risk older adults and may not be appropriate for patients who are likely to be readmitted for different reasons, such as mental illness, substance abuse, or chronic pain. Therefore, it is important to use a risk stratification method that captures the issues most likely to cause readmissions in your patient population, or to consider using a variety of methods.
The American Academy of Family Physicians (AAFP) offers resources to help FPs design methods for determining a patient’s health risk status and linking higher levels of risk to increasing care management at http://www.aafp.org/practice-management/pcmh/initiatives/cpci/rscm.html.
CASE › Mr. T has been admitted to the hospital 3 times in the past 3 months, so you use the lace index to evaluate his risk. You determine that Mr. T’s score is 15, which means his expected risk of death or unplanned readmission is 26.6% (TABLE 2).8,11 What are your next steps?
Foster communication between the hospital and outpatient office
Patients are particularly vulnerable during the transition from hospital to home. Delayed or inaccurate information adversely affects continuity of care, patient safety and satisfaction, and efficient use of resources.12 Discharge summaries are the main method of communication between providers, but their content, timeliness, availability, and quality frequently are lacking.13 Discharge summaries are available at only 12% to 34% of first postdischarge visits, and these summaries often lack important information such as diagnostic test results (33%-63%) or discharge medications (2%-40%).12 Although researchers have not consistently found that transferring a discharge summary to an outpatient physician reduces readmission rates, it is likely that direct communication can improve the handoff process independent of its effects on readmissions.12,14
Timely follow-up appointments are essential
Many factors influence the need for rapid follow-up, including disease severity, management complexity, ability of the patient to provide sufficient self-care, and adequacy of social supports.15,16 Studies have found that discharged patients who receive timely outpatient follow-up are less likely to be readmitted.1,17 While the optimal time interval between discharge and the first follow-up appointment is unknown, some literature supports follow-up within 4 weeks.15,18 However, because readmissions often cluster in the first several days or week following discharge,18 follow-up within the first 2 weeks (and within the first week for higher-risk patients) may be appropriate.19 Ideally, follow-up appointments should be scheduled before the patient is discharged. Patients who schedule a follow-up appointment before they are discharged are more likely to make their follow-up visit than those who are asked to call after discharge and schedule their own appointment.12
Employ outpatient follow-up alternatives
Follow-up telephone calls to patients after discharge help patients understand and adhere to discharge instructions and troubleshoot problems. Clinicians who use scripted telephone calls can evaluate symptoms related to the index hospitalization, provide patient education, schedule relevant appointments or testing, and, most importantly, initiate medication reconciliation, which is described at right.20 The FIGURE includes the script we use at our practice.
Home visits may be appropriate for certain patients, including the frail elderly. Home visits allow clinicians to evaluate the patient’s environmental safety, social sup port, and medication adherence.12 Preventive home visits generally have not been found to reduce hospital readmissions, but do enhance patient satisfaction with care.21
Bundled interventions, such as alternating home visits and follow-up telephone calls, may be more effective than individual interventions in reducing readmission.22
Reconciling medications may have far-reaching benefits
Medication discrepancies are observed in up to 70% of all patients at admission or discharge and are associated with adverse drug events (ADEs).23 To prevent ADEs and possibly readmission, take the following steps to reconcile a patient’s medications23:
Obtain a complete list of current medications. Information on all of the patient’s prescription and nonprescription medications should be collected from the patient/caregiver, the discharge summary, prescription bottles, home visits, and pharmacies.12,24
Reconcile preadmission and postdischarge medications. Clarify any discrepancies, review all medications for safety and appropriateness, and, when appropriate, resume any held medications and/or discontinue unnecessary ones.
Research shows that patients who received a phone call from a pharmacist within 3 to 7 days of discharge had lower readmission rates.Enlist pharmacy support. Pharmacists are uniquely positioned to review indications as well as potential duplication and interactions of a patient’s medications. Inpatient studies have demonstrated that partnering with pharmacists results in fewer ADEs.12,25 One study showed that patients at high risk for readmission who received a phone call from a pharmacist 3 to 7 days after discharge had lower readmission rates.26 The pharmacist reconciled the patients’ medications and ensured that patients had a clear understanding of each medication, its common safety concerns, and how often they were supposed to take it.26
Make medication adherence as easy as possible
As many as half of all patients don’t take their medications as prescribed.27 There is limited data on health outcomes associated with medication nonadherence, and existing data frequently are contradictory—some studies have found that as many as 11% of hospital admissions are attributed to nonadherence, while others show no association.28
Factors that affect adherence include psychiatric or cognitive impairment, limited insight into disease process or lack of belief in benefit of treatment, medication cost or adverse effect profile, poor provider-patient relationship, limited access to care or medication, or complexity of treatment.29 To promote medication adherence, consider the following educational and behavioral strategies30:
Identify patients at risk for nonadherence. This includes those with complex regimens and/or uncontrolled disease states or symptoms.
Increase patient communication and counseling. Patient education, particularly on the importance of adherence, is one of the few solo interventions that can improve compliance.31 Involving caregivers and using both verbal and written materials provides additional benefit.31,32
Simplify dosing schedules. Simple, convenient medication regimens may im- prove adherence. For example, adjusting dosing from 3 times a day to once a day can increase adherence from 59% to 83%.33 Aids such as pillboxes to organize medications may be of benefit.29,32
Ensure consistent follow-up. Patients who miss appointments are more likely to be nonadherent. They may benefit from easy access, help with scheduling, and frequent visits.32
Be mindful of patients’ out-of-pocket expenses. Reducing copayments improves adherence rates.30
Minimize polypharmacy. Polypharmacy has been independently associated with nonadherence and increased risk for ADEs.34
Identify patients who have limited health literacy. Limited health literacy may be linked to increased medication errors and nonadherence.12,35 Patients with low health literacy may be unable to identify medications recorded in their medical record. TABLE W336-41 outlines strategies for identifying patients with low health literacy and improving communication with them.
CASE › By speaking with hospital staff before Mr. T is discharged, you are able to confirm that he has scheduled a follow-up visit with you for one week after discharge, and that a discharge summary will be available for him to bring to that visit. Mr. T brings his discharge summary with him to your office, and you reconcile his medication list. Because he is your last patient of the day, you have some time to sit with him and his wife to explore his goals of care.
Improve care—and possibly reduce readmissions—through goal setting
Goal setting is an important element of postdischarge follow-up, particularly for elderly patients and those with progressive or end-stage diseases. Goal setting can improve patient care by linking care plans with desired outcomes and keeping diagnostic and therapeutic interventions relevant to the patient.42 A patient who understands the purpose of a recommendation—especially when directly linked to a patient-derived goal—may be more likely to adhere to the plan of care.
Asking patients to articulate their goals of care using “Ask-Tell-Ask” framework described in TABLE W336-41 will allow you to deliver the prognosis, reinforce treatment options to achieve patient-specific goals, empower patients to assert their preferences, and develop a follow-up plan to see if treatment is successful.
Empowering patients
Consider using both verbal and written approaches when educating patients about self-care behaviors such as monitoring symptoms and adhering to dietary/behavior restrictions and medication instructions. One study showed that a brief one-on-one patient education session decreased readmissions in patients with heart failure,43 although another study found that patient education alone yielded a nonsignificant decrease.44
Providing caregivers with education and support is a critical and perhaps overlooked opportunity to reduce readmissions.45 Involving key family members in discharge planning, preparation, follow-up, and ongoing management is essential in caring for patients with functional deficits and/or complex care needs. Educating caregivers can help them feel more prepared and effective in their roles.
Establish an “action plan.” For patients with chronic, periodically symptomatic diseases such as asthma and heart failure, action planning can be useful. Action plans should include information that reinforces patients’ daily self-care behaviors and instructions for what to do if symptoms get worse. Action planning also might include simple if-then plans (“if x happens, then I will do y”), which can help with problem solving for common scenarios. Action plans have been shown to reduce admissions for children with asthma46 and adults with heart failure when coupled with home monitoring or telephone support from a registered nurse.16,47
Generate an individualized care plan for each patient, taking into account your patient’s health literacy, goals of care, and level of social support. This care plan may include educational and behavioral interventions, action planning, and follow-up plans. Most successful approaches to reducing readmissions have included both system-level and patient-level interventions that use an interdisciplinary team of providers.48
Make the most of follow-up visits. The traditional 15-minute FP visit can make it challenging to provide the level of care necessary for recently discharged patients. Multiple models of team-based care have been proposed to improve this situation, including using the “teamlet” model, which may include a clinician and one or 2 health coaches.49 During each visit, the health coaches—often medical assistants trained in chronic disease self-management skills—see patients before and after the physician. They also contact patients be- tween visits to facilitate action planning and to promote self-management.
Palliative care programs: A resource for FPs
The growth of palliative care programs in US hospitals has helped increase the emphasis on establishing goals of care. Inpatient-based palliative care consultation programs work with patients and families to establish goals. However, after discharge, many of these goals and plans begin to unravel due to gaps in the current health care model, including lack of follow-up and support.50 Outpatient palliative care programs have begun to address these gaps in care.50 Comprehensive palliative care programs are quickly becoming an important resource for FPs to help address transitional care issues.
CASE › When you ask Mr. and Mrs. T about his goals for treatment, they say are getting tired of the “back and forth” to the hospital. After discussing his lengthy history of worsening CHF and diabetes, you raise the idea of palliative care, including hospice, with the couple. They acknowledge that they have had family members get hospice care, and they are open to it—just not yet. The 3 of you craft an “if-then” plan of care to use at home. You schedule a 2-week follow-up visit and remind Mr. T and his wife of your office’s 24-hour on-call service.
CORRESPONDENCE
Danielle Snyderman, MD, Department of Family and Community Medicine, Jefferson University, 1015 Walnut Street, Suite 401, Philadelphia, Pa 19107; [email protected]
1. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418-1428
2. O’Connor CM, Miller AB, Blair JE, et al; Efficacy of Vasopressin Antagonism in heart Failure Outcome Study with Tolvaptan (EVEREST) investigators. Causes of death and rehospitalization in patients hospitalized with worsening heart failure and reduce left ventricular ejection fraction; results from EVEREST program. Am Heart J. 2010;159:841-849.e1.
3. Garrison GM, Mansukhani MP, Bohn B. Predictors of thirty-day readmission among hospitalized family medicine patients. J Am Board Fam Med. 2013;26:71-77.
4. Boult C, Dowd B, McCaffrey D, et al. Screening elders for risk of hospital admission. J Am Geriatr Soc. 1993;41:811-817.
5. Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355-363.
6. Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306:1688-1698.
7. Haas LR, Takahashi PY, Shah ND, et al. Risk-stratification methods for identifying patients for care coordination. Am J Manag Care. 2013;19:725-732.
8. Wallace E, Hinchey T, Dimitrov BD, et al. A systematic review of the probability of repeated admission score in community-dwelling adults. J Am Geriatr Soc. 2013;61:357-364.
9. Cotter PE, Bhalla VK, Wallis SJ, et al. Predicting readmissions: poor performance of the LACE index in an older UK population. Age Ageing. 2012;41:784-789.
10. Shelton P, Sager MA, Schraeder C. The community assessment risk screen (CARS): identifying elderly persons at risk for hospitalization or emergency department visit. Am J Manag Care. 2000;6:925-933.
11. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373-383.
12. Kripalani S, Jackson AT, Schnipper JL, et al. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2:314-323.
13. Kim CS, Flanders SA. In the clinic. Transitions of care. Ann Intern Med. 2013;158(5 pt 1):ITC3-1.
14. Hansen LO, Strater A, Smith L, et al. Hospital discharge documentation and risk of rehospitalisation. BMJ Qual Saf. 2011;20:773-778.
15. Vaduganathan M, Bonow RO, Gheorghiade M. Thirty-day readmissions: the clock is ticking. JAMA. 2013;309:345-346.
16. Hansen LO, Young RS, Hinami K, et al. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155:520-528.
17. Misky GJ, Wald HL, Coleman EA. Post-hospitalization transitions: Examining the effects of timing of primary care provider follow-up. J Hosp Med. 2010;5:392-397.
18. van Walraven C, Jennings A, Taljaard M, et al. Incidence of potentially avoidable urgent readmissions and their relation to all-cause urgent readmissions. CMAJ. 2011;183:E1067-E1072.
19. Tang, N. A primary care physician’s ideal transitions of care—where’s the evidence? J Hosp Med. 2013;8:472-477.
20. Crocker JB, Crocker JT, Greenwald JL. Telephone follow-up as a primary care intervention for postdischarge outcomes improvement: a systematic review. Am J Med. 2012;125:915-921.
21. Wong FK, Chow S, Chung L, et al. Can home visits help reduce hospital readmissions? Randomized controlled trial. J Adv Nurs. 2008;62:585-595.
22. Wong FK, Chow SK, Chan TM, et al. Comparison of effects between home visits with telephone calls and telephone calls only for transitional discharge support: a randomised controlled trial. Age Ageing. 2014;43:91-97.
23. Mueller SK, Sponsler KC, Kripalani S, et al. Hospital-based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172:1057-1069.
24. Glintborg B, Andersen SE, Dalhoff K. Insufficient communication about medication use at the interface between hospital and primary care. Qual Saf Health Care. 2007;16:34-39.
25. Schnipper JL, Kirwin JL, Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166:565-571.
26. Kilcup M, Schultz D, Carlson J, et al. Postdischarge pharmacist medication reconciliation: impact on readmission rates and financial savings. J Am Pharm Assoc (2003). 2013;53:78-84.
27. Vermeire E, Hearnshaw H, Van Royen P, et al. Patient adherence to treatment: three decades of research. A comprehensive review. J Clin Pharm Ther. 2001;26:331-342.
28. Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors. Ann Pharmacother. 2004;38:303-312.
29. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487-497.
30. Viswanathan M, Golin CE, Jones CD, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med. 2012;157:785-795.
31. McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: scientific review. JAMA. 2002;288:2868-2879.
32. Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions: a systematic review. Arch Intern Med. 2007;167:540-550.
33. Eisen SA, Miller DK, Woodward RS, et al. The effect of prescribed daily dose frequency on patient medication compliance. Arch Intern Med. 1990;150:1881-1884.
34. Field TS, Gurwitz JH, Avorn J, et al. Risk factors for adverse drug events among nursing home residents. Arch Intern Med. 2001;161:1629-1634.
35. Persell SD, Osborn CY, Richard R, et al. Limited health literacy is a barrier to medication reconciliation in ambulatory care. J Gen Intern Med. 2007;22:1523-1526.
36. Weiss BD. Health Literacy and Patient Safety: Help Patients Understand. Manual for Clinicians. Chicago, IL: American Medical Association Foundation; 2007.
37. Chew LD, Bradley KA, Bokyo EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36:588-594.
38. Wallace LS, Rogers ES, Roskos SE, et al. Brief report: screening items to identify patients with limited health literacy skills. J Gen Intern Med. 2006;21:874-877.
39. Doak CC, Doak LG, Root JH. Teaching Patients with Low Literacy Skills. 2nd ed. Philadelphia, PA: JB Lippincott Company; 1996.
40. Back AL, Arnold RM, Baile WF, et al. Approaching difficult communication tasks in oncology. CA Cancer J Clin. 2005;55: 164-177.
41. Doak LG, Doak CC, eds. Pfizer Principles for Clear Health Communication: A Handbook for Creating Patient Education Materials that Enhance Understanding and Promote Health Outcomes. 2nd ed. New York, NY: Pfizer; 2004.
42. Bradley EH, Bogardus ST Jr, Tinetti M, et al. Goal-setting in clinical medicine. Soc Sci Med. 1999;49:267-278.
43. Koelling TM, Johnson ML, Cody RJ, et al. Discharge education improves clinical outcomes in patients with chronic heart failure. Circulation. 2005;111:179-185.
44. Krumholz HM, Amatruda J, Smith GL, et al. Randomized trial of an education and support intervention to prevent readmission of patients with heart failure. J Am Coll Cardiol. 2002;39:83-89.
45. Burke RE, Coleman EA. Interventions to decrease hospital readmissions: keys for cost-effectiveness. JAMA Intern Med. 2013;173:695-698.
46. Kessler KR. Relationship between the use of asthma action plans and asthma exacerbations in children with asthma: A systematic review. J Asthma Allergy Educators. 2011;2:11-21.
47. Maric B, Kaan A, Ignaszewski A, et al. A systematic review of telemonitoring technologies in heart failure. Eur J Heart Fail. 2009;11:506-517.
48. Boutwell A, Hwu S. Effective Interventions to Reduce Rehospitalizations: A Survey of the Published Evidence. Cambridge, MA: Institute for Healthcare Improvement; 2009.
49. Bodenheimer T, Laing BY. The teamlet model of primary care. Ann Fam Med. 2007;5:457-461.
50. Meier D, Beresford L. Outpatient clinics are a new frontier for palliative care. J Pall Med. 2008;11:823-828.
› Use risk stratification methods such as the Probability of Repeated Admission (Pra) or the LACE index to identify patients at high risk for readmission. B
› Take steps to ensure that follow-up appointments are made within the first one to 2 weeks of discharge, depending on the patient’s risk of readmission. C
› Reconcile preadmission and postdischarge medications to identify discrepancies and possible interactions. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Charles T, age 74, has a 3-year history of myocardial infarction (MI) and congestive heart failure (CHF) and a 10-year his-tory of type 2 diabetes with retinopathy. You have cared for him in the outpatient setting for 8 years. You are notified that he is in the emergency department (ED) and being admitted to the hospital, again. This is his third ED visit in the past 3 months; he was hospitalized for 6 days during his last admission 3 weeks ago.
What should you do with this information? How can you best communicate with the admitting team?
Hospital readmissions are widespread, costly, and often avoidable. Nearly 20% of Medicare beneficiaries discharged from hospitals are rehospitalized within 30 days, and 34% are rehospitalized within 90 days.1 For patients with conditions like CHF, the rate of readmission within 30 days approaches 25%.2 The estimated cost to Medicare for unplanned rehospitalizations in 2004 was $17.4 billion.1 The Centers for Medicare and Medicaid Services penalizes hospitals for high rates of readmission within 30 days of discharge for patients with CHF, MI, and pneumonia.
“Avoidable” hospitalizations are those that may be prevented by effective outpatient management and improved care coordination. Although efforts to reduce readmissions have focused on improving the discharge process, family physicians (FPs) can play a central role in reducing readmissions. This article describes key approaches that FPs can take to address this important issue. Because patients ages ≥65 years consistently have the highest rate of hospital readmissions,1 we will focus on this population.
Multiple complex factors are associated with hospital readmissions
Characteristics of the patient, physician, and health care setting contribute to potentially avoidable readmissions (TABLE 1).3,4
Medical conditions and comorbidities associated with high rates of rehospitalization include CHF, acute MI, pneumonia, diabetes, and chronic obstructive pulmonary disease. However, a recent study found that a diverse range of conditions, frequently differing from the index cause of hospitalization, were responsible for 30-day readmissions of Medicare patients.5
Identifying those at high risk: Why and how
Determining which patients are at highest risk for readmission enables health care teams to match the intensity of interventions to the individual’s likelihood of readmission. However, current readmission risk prediction models remain a work in progress6 and few models have been tested in the outpatient setting. Despite numerous limitations, it’s still important to focus resources more efficiently. Thus, we recommend using risk stratification tools to identify patients at high risk for readmission.
Many risk stratification methods use data from electronic medical records (EMRs) and administrative databases or self-reported data from patients.7 Risk prediction tools that are relatively simple and easy to administer or generate through EMRs—such as the Probability of Repeated Admission (Pra),8 the LACE (Length of stay, acuity of the admission, comorbidities, ED visits in the previous 6 months) index,9 or the Community Assessment Risk Screen (CARS)10—may be best for use in the primary care setting. These tools generally identify key risk factors, such as prior health care utilization, presence of specific conditions such as heart disease or cognitive impairment, self-reported health status, absence of a caregiver, and/or need for assistance with daily routines.
Many of these tools have been used to identify high-risk older adults and may not be appropriate for patients who are likely to be readmitted for different reasons, such as mental illness, substance abuse, or chronic pain. Therefore, it is important to use a risk stratification method that captures the issues most likely to cause readmissions in your patient population, or to consider using a variety of methods.
The American Academy of Family Physicians (AAFP) offers resources to help FPs design methods for determining a patient’s health risk status and linking higher levels of risk to increasing care management at http://www.aafp.org/practice-management/pcmh/initiatives/cpci/rscm.html.
CASE › Mr. T has been admitted to the hospital 3 times in the past 3 months, so you use the lace index to evaluate his risk. You determine that Mr. T’s score is 15, which means his expected risk of death or unplanned readmission is 26.6% (TABLE 2).8,11 What are your next steps?
Foster communication between the hospital and outpatient office
Patients are particularly vulnerable during the transition from hospital to home. Delayed or inaccurate information adversely affects continuity of care, patient safety and satisfaction, and efficient use of resources.12 Discharge summaries are the main method of communication between providers, but their content, timeliness, availability, and quality frequently are lacking.13 Discharge summaries are available at only 12% to 34% of first postdischarge visits, and these summaries often lack important information such as diagnostic test results (33%-63%) or discharge medications (2%-40%).12 Although researchers have not consistently found that transferring a discharge summary to an outpatient physician reduces readmission rates, it is likely that direct communication can improve the handoff process independent of its effects on readmissions.12,14
Timely follow-up appointments are essential
Many factors influence the need for rapid follow-up, including disease severity, management complexity, ability of the patient to provide sufficient self-care, and adequacy of social supports.15,16 Studies have found that discharged patients who receive timely outpatient follow-up are less likely to be readmitted.1,17 While the optimal time interval between discharge and the first follow-up appointment is unknown, some literature supports follow-up within 4 weeks.15,18 However, because readmissions often cluster in the first several days or week following discharge,18 follow-up within the first 2 weeks (and within the first week for higher-risk patients) may be appropriate.19 Ideally, follow-up appointments should be scheduled before the patient is discharged. Patients who schedule a follow-up appointment before they are discharged are more likely to make their follow-up visit than those who are asked to call after discharge and schedule their own appointment.12
Employ outpatient follow-up alternatives
Follow-up telephone calls to patients after discharge help patients understand and adhere to discharge instructions and troubleshoot problems. Clinicians who use scripted telephone calls can evaluate symptoms related to the index hospitalization, provide patient education, schedule relevant appointments or testing, and, most importantly, initiate medication reconciliation, which is described at right.20 The FIGURE includes the script we use at our practice.
Home visits may be appropriate for certain patients, including the frail elderly. Home visits allow clinicians to evaluate the patient’s environmental safety, social sup port, and medication adherence.12 Preventive home visits generally have not been found to reduce hospital readmissions, but do enhance patient satisfaction with care.21
Bundled interventions, such as alternating home visits and follow-up telephone calls, may be more effective than individual interventions in reducing readmission.22
Reconciling medications may have far-reaching benefits
Medication discrepancies are observed in up to 70% of all patients at admission or discharge and are associated with adverse drug events (ADEs).23 To prevent ADEs and possibly readmission, take the following steps to reconcile a patient’s medications23:
Obtain a complete list of current medications. Information on all of the patient’s prescription and nonprescription medications should be collected from the patient/caregiver, the discharge summary, prescription bottles, home visits, and pharmacies.12,24
Reconcile preadmission and postdischarge medications. Clarify any discrepancies, review all medications for safety and appropriateness, and, when appropriate, resume any held medications and/or discontinue unnecessary ones.
Research shows that patients who received a phone call from a pharmacist within 3 to 7 days of discharge had lower readmission rates.Enlist pharmacy support. Pharmacists are uniquely positioned to review indications as well as potential duplication and interactions of a patient’s medications. Inpatient studies have demonstrated that partnering with pharmacists results in fewer ADEs.12,25 One study showed that patients at high risk for readmission who received a phone call from a pharmacist 3 to 7 days after discharge had lower readmission rates.26 The pharmacist reconciled the patients’ medications and ensured that patients had a clear understanding of each medication, its common safety concerns, and how often they were supposed to take it.26
Make medication adherence as easy as possible
As many as half of all patients don’t take their medications as prescribed.27 There is limited data on health outcomes associated with medication nonadherence, and existing data frequently are contradictory—some studies have found that as many as 11% of hospital admissions are attributed to nonadherence, while others show no association.28
Factors that affect adherence include psychiatric or cognitive impairment, limited insight into disease process or lack of belief in benefit of treatment, medication cost or adverse effect profile, poor provider-patient relationship, limited access to care or medication, or complexity of treatment.29 To promote medication adherence, consider the following educational and behavioral strategies30:
Identify patients at risk for nonadherence. This includes those with complex regimens and/or uncontrolled disease states or symptoms.
Increase patient communication and counseling. Patient education, particularly on the importance of adherence, is one of the few solo interventions that can improve compliance.31 Involving caregivers and using both verbal and written materials provides additional benefit.31,32
Simplify dosing schedules. Simple, convenient medication regimens may im- prove adherence. For example, adjusting dosing from 3 times a day to once a day can increase adherence from 59% to 83%.33 Aids such as pillboxes to organize medications may be of benefit.29,32
Ensure consistent follow-up. Patients who miss appointments are more likely to be nonadherent. They may benefit from easy access, help with scheduling, and frequent visits.32
Be mindful of patients’ out-of-pocket expenses. Reducing copayments improves adherence rates.30
Minimize polypharmacy. Polypharmacy has been independently associated with nonadherence and increased risk for ADEs.34
Identify patients who have limited health literacy. Limited health literacy may be linked to increased medication errors and nonadherence.12,35 Patients with low health literacy may be unable to identify medications recorded in their medical record. TABLE W336-41 outlines strategies for identifying patients with low health literacy and improving communication with them.
CASE › By speaking with hospital staff before Mr. T is discharged, you are able to confirm that he has scheduled a follow-up visit with you for one week after discharge, and that a discharge summary will be available for him to bring to that visit. Mr. T brings his discharge summary with him to your office, and you reconcile his medication list. Because he is your last patient of the day, you have some time to sit with him and his wife to explore his goals of care.
Improve care—and possibly reduce readmissions—through goal setting
Goal setting is an important element of postdischarge follow-up, particularly for elderly patients and those with progressive or end-stage diseases. Goal setting can improve patient care by linking care plans with desired outcomes and keeping diagnostic and therapeutic interventions relevant to the patient.42 A patient who understands the purpose of a recommendation—especially when directly linked to a patient-derived goal—may be more likely to adhere to the plan of care.
Asking patients to articulate their goals of care using “Ask-Tell-Ask” framework described in TABLE W336-41 will allow you to deliver the prognosis, reinforce treatment options to achieve patient-specific goals, empower patients to assert their preferences, and develop a follow-up plan to see if treatment is successful.
Empowering patients
Consider using both verbal and written approaches when educating patients about self-care behaviors such as monitoring symptoms and adhering to dietary/behavior restrictions and medication instructions. One study showed that a brief one-on-one patient education session decreased readmissions in patients with heart failure,43 although another study found that patient education alone yielded a nonsignificant decrease.44
Providing caregivers with education and support is a critical and perhaps overlooked opportunity to reduce readmissions.45 Involving key family members in discharge planning, preparation, follow-up, and ongoing management is essential in caring for patients with functional deficits and/or complex care needs. Educating caregivers can help them feel more prepared and effective in their roles.
Establish an “action plan.” For patients with chronic, periodically symptomatic diseases such as asthma and heart failure, action planning can be useful. Action plans should include information that reinforces patients’ daily self-care behaviors and instructions for what to do if symptoms get worse. Action planning also might include simple if-then plans (“if x happens, then I will do y”), which can help with problem solving for common scenarios. Action plans have been shown to reduce admissions for children with asthma46 and adults with heart failure when coupled with home monitoring or telephone support from a registered nurse.16,47
Generate an individualized care plan for each patient, taking into account your patient’s health literacy, goals of care, and level of social support. This care plan may include educational and behavioral interventions, action planning, and follow-up plans. Most successful approaches to reducing readmissions have included both system-level and patient-level interventions that use an interdisciplinary team of providers.48
Make the most of follow-up visits. The traditional 15-minute FP visit can make it challenging to provide the level of care necessary for recently discharged patients. Multiple models of team-based care have been proposed to improve this situation, including using the “teamlet” model, which may include a clinician and one or 2 health coaches.49 During each visit, the health coaches—often medical assistants trained in chronic disease self-management skills—see patients before and after the physician. They also contact patients be- tween visits to facilitate action planning and to promote self-management.
Palliative care programs: A resource for FPs
The growth of palliative care programs in US hospitals has helped increase the emphasis on establishing goals of care. Inpatient-based palliative care consultation programs work with patients and families to establish goals. However, after discharge, many of these goals and plans begin to unravel due to gaps in the current health care model, including lack of follow-up and support.50 Outpatient palliative care programs have begun to address these gaps in care.50 Comprehensive palliative care programs are quickly becoming an important resource for FPs to help address transitional care issues.
CASE › When you ask Mr. and Mrs. T about his goals for treatment, they say are getting tired of the “back and forth” to the hospital. After discussing his lengthy history of worsening CHF and diabetes, you raise the idea of palliative care, including hospice, with the couple. They acknowledge that they have had family members get hospice care, and they are open to it—just not yet. The 3 of you craft an “if-then” plan of care to use at home. You schedule a 2-week follow-up visit and remind Mr. T and his wife of your office’s 24-hour on-call service.
CORRESPONDENCE
Danielle Snyderman, MD, Department of Family and Community Medicine, Jefferson University, 1015 Walnut Street, Suite 401, Philadelphia, Pa 19107; [email protected]
› Use risk stratification methods such as the Probability of Repeated Admission (Pra) or the LACE index to identify patients at high risk for readmission. B
› Take steps to ensure that follow-up appointments are made within the first one to 2 weeks of discharge, depending on the patient’s risk of readmission. C
› Reconcile preadmission and postdischarge medications to identify discrepancies and possible interactions. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Charles T, age 74, has a 3-year history of myocardial infarction (MI) and congestive heart failure (CHF) and a 10-year his-tory of type 2 diabetes with retinopathy. You have cared for him in the outpatient setting for 8 years. You are notified that he is in the emergency department (ED) and being admitted to the hospital, again. This is his third ED visit in the past 3 months; he was hospitalized for 6 days during his last admission 3 weeks ago.
What should you do with this information? How can you best communicate with the admitting team?
Hospital readmissions are widespread, costly, and often avoidable. Nearly 20% of Medicare beneficiaries discharged from hospitals are rehospitalized within 30 days, and 34% are rehospitalized within 90 days.1 For patients with conditions like CHF, the rate of readmission within 30 days approaches 25%.2 The estimated cost to Medicare for unplanned rehospitalizations in 2004 was $17.4 billion.1 The Centers for Medicare and Medicaid Services penalizes hospitals for high rates of readmission within 30 days of discharge for patients with CHF, MI, and pneumonia.
“Avoidable” hospitalizations are those that may be prevented by effective outpatient management and improved care coordination. Although efforts to reduce readmissions have focused on improving the discharge process, family physicians (FPs) can play a central role in reducing readmissions. This article describes key approaches that FPs can take to address this important issue. Because patients ages ≥65 years consistently have the highest rate of hospital readmissions,1 we will focus on this population.
Multiple complex factors are associated with hospital readmissions
Characteristics of the patient, physician, and health care setting contribute to potentially avoidable readmissions (TABLE 1).3,4
Medical conditions and comorbidities associated with high rates of rehospitalization include CHF, acute MI, pneumonia, diabetes, and chronic obstructive pulmonary disease. However, a recent study found that a diverse range of conditions, frequently differing from the index cause of hospitalization, were responsible for 30-day readmissions of Medicare patients.5
Identifying those at high risk: Why and how
Determining which patients are at highest risk for readmission enables health care teams to match the intensity of interventions to the individual’s likelihood of readmission. However, current readmission risk prediction models remain a work in progress6 and few models have been tested in the outpatient setting. Despite numerous limitations, it’s still important to focus resources more efficiently. Thus, we recommend using risk stratification tools to identify patients at high risk for readmission.
Many risk stratification methods use data from electronic medical records (EMRs) and administrative databases or self-reported data from patients.7 Risk prediction tools that are relatively simple and easy to administer or generate through EMRs—such as the Probability of Repeated Admission (Pra),8 the LACE (Length of stay, acuity of the admission, comorbidities, ED visits in the previous 6 months) index,9 or the Community Assessment Risk Screen (CARS)10—may be best for use in the primary care setting. These tools generally identify key risk factors, such as prior health care utilization, presence of specific conditions such as heart disease or cognitive impairment, self-reported health status, absence of a caregiver, and/or need for assistance with daily routines.
Many of these tools have been used to identify high-risk older adults and may not be appropriate for patients who are likely to be readmitted for different reasons, such as mental illness, substance abuse, or chronic pain. Therefore, it is important to use a risk stratification method that captures the issues most likely to cause readmissions in your patient population, or to consider using a variety of methods.
The American Academy of Family Physicians (AAFP) offers resources to help FPs design methods for determining a patient’s health risk status and linking higher levels of risk to increasing care management at http://www.aafp.org/practice-management/pcmh/initiatives/cpci/rscm.html.
CASE › Mr. T has been admitted to the hospital 3 times in the past 3 months, so you use the lace index to evaluate his risk. You determine that Mr. T’s score is 15, which means his expected risk of death or unplanned readmission is 26.6% (TABLE 2).8,11 What are your next steps?
Foster communication between the hospital and outpatient office
Patients are particularly vulnerable during the transition from hospital to home. Delayed or inaccurate information adversely affects continuity of care, patient safety and satisfaction, and efficient use of resources.12 Discharge summaries are the main method of communication between providers, but their content, timeliness, availability, and quality frequently are lacking.13 Discharge summaries are available at only 12% to 34% of first postdischarge visits, and these summaries often lack important information such as diagnostic test results (33%-63%) or discharge medications (2%-40%).12 Although researchers have not consistently found that transferring a discharge summary to an outpatient physician reduces readmission rates, it is likely that direct communication can improve the handoff process independent of its effects on readmissions.12,14
Timely follow-up appointments are essential
Many factors influence the need for rapid follow-up, including disease severity, management complexity, ability of the patient to provide sufficient self-care, and adequacy of social supports.15,16 Studies have found that discharged patients who receive timely outpatient follow-up are less likely to be readmitted.1,17 While the optimal time interval between discharge and the first follow-up appointment is unknown, some literature supports follow-up within 4 weeks.15,18 However, because readmissions often cluster in the first several days or week following discharge,18 follow-up within the first 2 weeks (and within the first week for higher-risk patients) may be appropriate.19 Ideally, follow-up appointments should be scheduled before the patient is discharged. Patients who schedule a follow-up appointment before they are discharged are more likely to make their follow-up visit than those who are asked to call after discharge and schedule their own appointment.12
Employ outpatient follow-up alternatives
Follow-up telephone calls to patients after discharge help patients understand and adhere to discharge instructions and troubleshoot problems. Clinicians who use scripted telephone calls can evaluate symptoms related to the index hospitalization, provide patient education, schedule relevant appointments or testing, and, most importantly, initiate medication reconciliation, which is described at right.20 The FIGURE includes the script we use at our practice.
Home visits may be appropriate for certain patients, including the frail elderly. Home visits allow clinicians to evaluate the patient’s environmental safety, social sup port, and medication adherence.12 Preventive home visits generally have not been found to reduce hospital readmissions, but do enhance patient satisfaction with care.21
Bundled interventions, such as alternating home visits and follow-up telephone calls, may be more effective than individual interventions in reducing readmission.22
Reconciling medications may have far-reaching benefits
Medication discrepancies are observed in up to 70% of all patients at admission or discharge and are associated with adverse drug events (ADEs).23 To prevent ADEs and possibly readmission, take the following steps to reconcile a patient’s medications23:
Obtain a complete list of current medications. Information on all of the patient’s prescription and nonprescription medications should be collected from the patient/caregiver, the discharge summary, prescription bottles, home visits, and pharmacies.12,24
Reconcile preadmission and postdischarge medications. Clarify any discrepancies, review all medications for safety and appropriateness, and, when appropriate, resume any held medications and/or discontinue unnecessary ones.
Research shows that patients who received a phone call from a pharmacist within 3 to 7 days of discharge had lower readmission rates.Enlist pharmacy support. Pharmacists are uniquely positioned to review indications as well as potential duplication and interactions of a patient’s medications. Inpatient studies have demonstrated that partnering with pharmacists results in fewer ADEs.12,25 One study showed that patients at high risk for readmission who received a phone call from a pharmacist 3 to 7 days after discharge had lower readmission rates.26 The pharmacist reconciled the patients’ medications and ensured that patients had a clear understanding of each medication, its common safety concerns, and how often they were supposed to take it.26
Make medication adherence as easy as possible
As many as half of all patients don’t take their medications as prescribed.27 There is limited data on health outcomes associated with medication nonadherence, and existing data frequently are contradictory—some studies have found that as many as 11% of hospital admissions are attributed to nonadherence, while others show no association.28
Factors that affect adherence include psychiatric or cognitive impairment, limited insight into disease process or lack of belief in benefit of treatment, medication cost or adverse effect profile, poor provider-patient relationship, limited access to care or medication, or complexity of treatment.29 To promote medication adherence, consider the following educational and behavioral strategies30:
Identify patients at risk for nonadherence. This includes those with complex regimens and/or uncontrolled disease states or symptoms.
Increase patient communication and counseling. Patient education, particularly on the importance of adherence, is one of the few solo interventions that can improve compliance.31 Involving caregivers and using both verbal and written materials provides additional benefit.31,32
Simplify dosing schedules. Simple, convenient medication regimens may im- prove adherence. For example, adjusting dosing from 3 times a day to once a day can increase adherence from 59% to 83%.33 Aids such as pillboxes to organize medications may be of benefit.29,32
Ensure consistent follow-up. Patients who miss appointments are more likely to be nonadherent. They may benefit from easy access, help with scheduling, and frequent visits.32
Be mindful of patients’ out-of-pocket expenses. Reducing copayments improves adherence rates.30
Minimize polypharmacy. Polypharmacy has been independently associated with nonadherence and increased risk for ADEs.34
Identify patients who have limited health literacy. Limited health literacy may be linked to increased medication errors and nonadherence.12,35 Patients with low health literacy may be unable to identify medications recorded in their medical record. TABLE W336-41 outlines strategies for identifying patients with low health literacy and improving communication with them.
CASE › By speaking with hospital staff before Mr. T is discharged, you are able to confirm that he has scheduled a follow-up visit with you for one week after discharge, and that a discharge summary will be available for him to bring to that visit. Mr. T brings his discharge summary with him to your office, and you reconcile his medication list. Because he is your last patient of the day, you have some time to sit with him and his wife to explore his goals of care.
Improve care—and possibly reduce readmissions—through goal setting
Goal setting is an important element of postdischarge follow-up, particularly for elderly patients and those with progressive or end-stage diseases. Goal setting can improve patient care by linking care plans with desired outcomes and keeping diagnostic and therapeutic interventions relevant to the patient.42 A patient who understands the purpose of a recommendation—especially when directly linked to a patient-derived goal—may be more likely to adhere to the plan of care.
Asking patients to articulate their goals of care using “Ask-Tell-Ask” framework described in TABLE W336-41 will allow you to deliver the prognosis, reinforce treatment options to achieve patient-specific goals, empower patients to assert their preferences, and develop a follow-up plan to see if treatment is successful.
Empowering patients
Consider using both verbal and written approaches when educating patients about self-care behaviors such as monitoring symptoms and adhering to dietary/behavior restrictions and medication instructions. One study showed that a brief one-on-one patient education session decreased readmissions in patients with heart failure,43 although another study found that patient education alone yielded a nonsignificant decrease.44
Providing caregivers with education and support is a critical and perhaps overlooked opportunity to reduce readmissions.45 Involving key family members in discharge planning, preparation, follow-up, and ongoing management is essential in caring for patients with functional deficits and/or complex care needs. Educating caregivers can help them feel more prepared and effective in their roles.
Establish an “action plan.” For patients with chronic, periodically symptomatic diseases such as asthma and heart failure, action planning can be useful. Action plans should include information that reinforces patients’ daily self-care behaviors and instructions for what to do if symptoms get worse. Action planning also might include simple if-then plans (“if x happens, then I will do y”), which can help with problem solving for common scenarios. Action plans have been shown to reduce admissions for children with asthma46 and adults with heart failure when coupled with home monitoring or telephone support from a registered nurse.16,47
Generate an individualized care plan for each patient, taking into account your patient’s health literacy, goals of care, and level of social support. This care plan may include educational and behavioral interventions, action planning, and follow-up plans. Most successful approaches to reducing readmissions have included both system-level and patient-level interventions that use an interdisciplinary team of providers.48
Make the most of follow-up visits. The traditional 15-minute FP visit can make it challenging to provide the level of care necessary for recently discharged patients. Multiple models of team-based care have been proposed to improve this situation, including using the “teamlet” model, which may include a clinician and one or 2 health coaches.49 During each visit, the health coaches—often medical assistants trained in chronic disease self-management skills—see patients before and after the physician. They also contact patients be- tween visits to facilitate action planning and to promote self-management.
Palliative care programs: A resource for FPs
The growth of palliative care programs in US hospitals has helped increase the emphasis on establishing goals of care. Inpatient-based palliative care consultation programs work with patients and families to establish goals. However, after discharge, many of these goals and plans begin to unravel due to gaps in the current health care model, including lack of follow-up and support.50 Outpatient palliative care programs have begun to address these gaps in care.50 Comprehensive palliative care programs are quickly becoming an important resource for FPs to help address transitional care issues.
CASE › When you ask Mr. and Mrs. T about his goals for treatment, they say are getting tired of the “back and forth” to the hospital. After discussing his lengthy history of worsening CHF and diabetes, you raise the idea of palliative care, including hospice, with the couple. They acknowledge that they have had family members get hospice care, and they are open to it—just not yet. The 3 of you craft an “if-then” plan of care to use at home. You schedule a 2-week follow-up visit and remind Mr. T and his wife of your office’s 24-hour on-call service.
CORRESPONDENCE
Danielle Snyderman, MD, Department of Family and Community Medicine, Jefferson University, 1015 Walnut Street, Suite 401, Philadelphia, Pa 19107; [email protected]
1. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418-1428
2. O’Connor CM, Miller AB, Blair JE, et al; Efficacy of Vasopressin Antagonism in heart Failure Outcome Study with Tolvaptan (EVEREST) investigators. Causes of death and rehospitalization in patients hospitalized with worsening heart failure and reduce left ventricular ejection fraction; results from EVEREST program. Am Heart J. 2010;159:841-849.e1.
3. Garrison GM, Mansukhani MP, Bohn B. Predictors of thirty-day readmission among hospitalized family medicine patients. J Am Board Fam Med. 2013;26:71-77.
4. Boult C, Dowd B, McCaffrey D, et al. Screening elders for risk of hospital admission. J Am Geriatr Soc. 1993;41:811-817.
5. Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355-363.
6. Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306:1688-1698.
7. Haas LR, Takahashi PY, Shah ND, et al. Risk-stratification methods for identifying patients for care coordination. Am J Manag Care. 2013;19:725-732.
8. Wallace E, Hinchey T, Dimitrov BD, et al. A systematic review of the probability of repeated admission score in community-dwelling adults. J Am Geriatr Soc. 2013;61:357-364.
9. Cotter PE, Bhalla VK, Wallis SJ, et al. Predicting readmissions: poor performance of the LACE index in an older UK population. Age Ageing. 2012;41:784-789.
10. Shelton P, Sager MA, Schraeder C. The community assessment risk screen (CARS): identifying elderly persons at risk for hospitalization or emergency department visit. Am J Manag Care. 2000;6:925-933.
11. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373-383.
12. Kripalani S, Jackson AT, Schnipper JL, et al. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2:314-323.
13. Kim CS, Flanders SA. In the clinic. Transitions of care. Ann Intern Med. 2013;158(5 pt 1):ITC3-1.
14. Hansen LO, Strater A, Smith L, et al. Hospital discharge documentation and risk of rehospitalisation. BMJ Qual Saf. 2011;20:773-778.
15. Vaduganathan M, Bonow RO, Gheorghiade M. Thirty-day readmissions: the clock is ticking. JAMA. 2013;309:345-346.
16. Hansen LO, Young RS, Hinami K, et al. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155:520-528.
17. Misky GJ, Wald HL, Coleman EA. Post-hospitalization transitions: Examining the effects of timing of primary care provider follow-up. J Hosp Med. 2010;5:392-397.
18. van Walraven C, Jennings A, Taljaard M, et al. Incidence of potentially avoidable urgent readmissions and their relation to all-cause urgent readmissions. CMAJ. 2011;183:E1067-E1072.
19. Tang, N. A primary care physician’s ideal transitions of care—where’s the evidence? J Hosp Med. 2013;8:472-477.
20. Crocker JB, Crocker JT, Greenwald JL. Telephone follow-up as a primary care intervention for postdischarge outcomes improvement: a systematic review. Am J Med. 2012;125:915-921.
21. Wong FK, Chow S, Chung L, et al. Can home visits help reduce hospital readmissions? Randomized controlled trial. J Adv Nurs. 2008;62:585-595.
22. Wong FK, Chow SK, Chan TM, et al. Comparison of effects between home visits with telephone calls and telephone calls only for transitional discharge support: a randomised controlled trial. Age Ageing. 2014;43:91-97.
23. Mueller SK, Sponsler KC, Kripalani S, et al. Hospital-based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172:1057-1069.
24. Glintborg B, Andersen SE, Dalhoff K. Insufficient communication about medication use at the interface between hospital and primary care. Qual Saf Health Care. 2007;16:34-39.
25. Schnipper JL, Kirwin JL, Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166:565-571.
26. Kilcup M, Schultz D, Carlson J, et al. Postdischarge pharmacist medication reconciliation: impact on readmission rates and financial savings. J Am Pharm Assoc (2003). 2013;53:78-84.
27. Vermeire E, Hearnshaw H, Van Royen P, et al. Patient adherence to treatment: three decades of research. A comprehensive review. J Clin Pharm Ther. 2001;26:331-342.
28. Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors. Ann Pharmacother. 2004;38:303-312.
29. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487-497.
30. Viswanathan M, Golin CE, Jones CD, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med. 2012;157:785-795.
31. McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: scientific review. JAMA. 2002;288:2868-2879.
32. Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions: a systematic review. Arch Intern Med. 2007;167:540-550.
33. Eisen SA, Miller DK, Woodward RS, et al. The effect of prescribed daily dose frequency on patient medication compliance. Arch Intern Med. 1990;150:1881-1884.
34. Field TS, Gurwitz JH, Avorn J, et al. Risk factors for adverse drug events among nursing home residents. Arch Intern Med. 2001;161:1629-1634.
35. Persell SD, Osborn CY, Richard R, et al. Limited health literacy is a barrier to medication reconciliation in ambulatory care. J Gen Intern Med. 2007;22:1523-1526.
36. Weiss BD. Health Literacy and Patient Safety: Help Patients Understand. Manual for Clinicians. Chicago, IL: American Medical Association Foundation; 2007.
37. Chew LD, Bradley KA, Bokyo EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36:588-594.
38. Wallace LS, Rogers ES, Roskos SE, et al. Brief report: screening items to identify patients with limited health literacy skills. J Gen Intern Med. 2006;21:874-877.
39. Doak CC, Doak LG, Root JH. Teaching Patients with Low Literacy Skills. 2nd ed. Philadelphia, PA: JB Lippincott Company; 1996.
40. Back AL, Arnold RM, Baile WF, et al. Approaching difficult communication tasks in oncology. CA Cancer J Clin. 2005;55: 164-177.
41. Doak LG, Doak CC, eds. Pfizer Principles for Clear Health Communication: A Handbook for Creating Patient Education Materials that Enhance Understanding and Promote Health Outcomes. 2nd ed. New York, NY: Pfizer; 2004.
42. Bradley EH, Bogardus ST Jr, Tinetti M, et al. Goal-setting in clinical medicine. Soc Sci Med. 1999;49:267-278.
43. Koelling TM, Johnson ML, Cody RJ, et al. Discharge education improves clinical outcomes in patients with chronic heart failure. Circulation. 2005;111:179-185.
44. Krumholz HM, Amatruda J, Smith GL, et al. Randomized trial of an education and support intervention to prevent readmission of patients with heart failure. J Am Coll Cardiol. 2002;39:83-89.
45. Burke RE, Coleman EA. Interventions to decrease hospital readmissions: keys for cost-effectiveness. JAMA Intern Med. 2013;173:695-698.
46. Kessler KR. Relationship between the use of asthma action plans and asthma exacerbations in children with asthma: A systematic review. J Asthma Allergy Educators. 2011;2:11-21.
47. Maric B, Kaan A, Ignaszewski A, et al. A systematic review of telemonitoring technologies in heart failure. Eur J Heart Fail. 2009;11:506-517.
48. Boutwell A, Hwu S. Effective Interventions to Reduce Rehospitalizations: A Survey of the Published Evidence. Cambridge, MA: Institute for Healthcare Improvement; 2009.
49. Bodenheimer T, Laing BY. The teamlet model of primary care. Ann Fam Med. 2007;5:457-461.
50. Meier D, Beresford L. Outpatient clinics are a new frontier for palliative care. J Pall Med. 2008;11:823-828.
1. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418-1428
2. O’Connor CM, Miller AB, Blair JE, et al; Efficacy of Vasopressin Antagonism in heart Failure Outcome Study with Tolvaptan (EVEREST) investigators. Causes of death and rehospitalization in patients hospitalized with worsening heart failure and reduce left ventricular ejection fraction; results from EVEREST program. Am Heart J. 2010;159:841-849.e1.
3. Garrison GM, Mansukhani MP, Bohn B. Predictors of thirty-day readmission among hospitalized family medicine patients. J Am Board Fam Med. 2013;26:71-77.
4. Boult C, Dowd B, McCaffrey D, et al. Screening elders for risk of hospital admission. J Am Geriatr Soc. 1993;41:811-817.
5. Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355-363.
6. Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306:1688-1698.
7. Haas LR, Takahashi PY, Shah ND, et al. Risk-stratification methods for identifying patients for care coordination. Am J Manag Care. 2013;19:725-732.
8. Wallace E, Hinchey T, Dimitrov BD, et al. A systematic review of the probability of repeated admission score in community-dwelling adults. J Am Geriatr Soc. 2013;61:357-364.
9. Cotter PE, Bhalla VK, Wallis SJ, et al. Predicting readmissions: poor performance of the LACE index in an older UK population. Age Ageing. 2012;41:784-789.
10. Shelton P, Sager MA, Schraeder C. The community assessment risk screen (CARS): identifying elderly persons at risk for hospitalization or emergency department visit. Am J Manag Care. 2000;6:925-933.
11. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373-383.
12. Kripalani S, Jackson AT, Schnipper JL, et al. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2:314-323.
13. Kim CS, Flanders SA. In the clinic. Transitions of care. Ann Intern Med. 2013;158(5 pt 1):ITC3-1.
14. Hansen LO, Strater A, Smith L, et al. Hospital discharge documentation and risk of rehospitalisation. BMJ Qual Saf. 2011;20:773-778.
15. Vaduganathan M, Bonow RO, Gheorghiade M. Thirty-day readmissions: the clock is ticking. JAMA. 2013;309:345-346.
16. Hansen LO, Young RS, Hinami K, et al. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155:520-528.
17. Misky GJ, Wald HL, Coleman EA. Post-hospitalization transitions: Examining the effects of timing of primary care provider follow-up. J Hosp Med. 2010;5:392-397.
18. van Walraven C, Jennings A, Taljaard M, et al. Incidence of potentially avoidable urgent readmissions and their relation to all-cause urgent readmissions. CMAJ. 2011;183:E1067-E1072.
19. Tang, N. A primary care physician’s ideal transitions of care—where’s the evidence? J Hosp Med. 2013;8:472-477.
20. Crocker JB, Crocker JT, Greenwald JL. Telephone follow-up as a primary care intervention for postdischarge outcomes improvement: a systematic review. Am J Med. 2012;125:915-921.
21. Wong FK, Chow S, Chung L, et al. Can home visits help reduce hospital readmissions? Randomized controlled trial. J Adv Nurs. 2008;62:585-595.
22. Wong FK, Chow SK, Chan TM, et al. Comparison of effects between home visits with telephone calls and telephone calls only for transitional discharge support: a randomised controlled trial. Age Ageing. 2014;43:91-97.
23. Mueller SK, Sponsler KC, Kripalani S, et al. Hospital-based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172:1057-1069.
24. Glintborg B, Andersen SE, Dalhoff K. Insufficient communication about medication use at the interface between hospital and primary care. Qual Saf Health Care. 2007;16:34-39.
25. Schnipper JL, Kirwin JL, Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166:565-571.
26. Kilcup M, Schultz D, Carlson J, et al. Postdischarge pharmacist medication reconciliation: impact on readmission rates and financial savings. J Am Pharm Assoc (2003). 2013;53:78-84.
27. Vermeire E, Hearnshaw H, Van Royen P, et al. Patient adherence to treatment: three decades of research. A comprehensive review. J Clin Pharm Ther. 2001;26:331-342.
28. Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors. Ann Pharmacother. 2004;38:303-312.
29. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487-497.
30. Viswanathan M, Golin CE, Jones CD, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med. 2012;157:785-795.
31. McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: scientific review. JAMA. 2002;288:2868-2879.
32. Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions: a systematic review. Arch Intern Med. 2007;167:540-550.
33. Eisen SA, Miller DK, Woodward RS, et al. The effect of prescribed daily dose frequency on patient medication compliance. Arch Intern Med. 1990;150:1881-1884.
34. Field TS, Gurwitz JH, Avorn J, et al. Risk factors for adverse drug events among nursing home residents. Arch Intern Med. 2001;161:1629-1634.
35. Persell SD, Osborn CY, Richard R, et al. Limited health literacy is a barrier to medication reconciliation in ambulatory care. J Gen Intern Med. 2007;22:1523-1526.
36. Weiss BD. Health Literacy and Patient Safety: Help Patients Understand. Manual for Clinicians. Chicago, IL: American Medical Association Foundation; 2007.
37. Chew LD, Bradley KA, Bokyo EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36:588-594.
38. Wallace LS, Rogers ES, Roskos SE, et al. Brief report: screening items to identify patients with limited health literacy skills. J Gen Intern Med. 2006;21:874-877.
39. Doak CC, Doak LG, Root JH. Teaching Patients with Low Literacy Skills. 2nd ed. Philadelphia, PA: JB Lippincott Company; 1996.
40. Back AL, Arnold RM, Baile WF, et al. Approaching difficult communication tasks in oncology. CA Cancer J Clin. 2005;55: 164-177.
41. Doak LG, Doak CC, eds. Pfizer Principles for Clear Health Communication: A Handbook for Creating Patient Education Materials that Enhance Understanding and Promote Health Outcomes. 2nd ed. New York, NY: Pfizer; 2004.
42. Bradley EH, Bogardus ST Jr, Tinetti M, et al. Goal-setting in clinical medicine. Soc Sci Med. 1999;49:267-278.
43. Koelling TM, Johnson ML, Cody RJ, et al. Discharge education improves clinical outcomes in patients with chronic heart failure. Circulation. 2005;111:179-185.
44. Krumholz HM, Amatruda J, Smith GL, et al. Randomized trial of an education and support intervention to prevent readmission of patients with heart failure. J Am Coll Cardiol. 2002;39:83-89.
45. Burke RE, Coleman EA. Interventions to decrease hospital readmissions: keys for cost-effectiveness. JAMA Intern Med. 2013;173:695-698.
46. Kessler KR. Relationship between the use of asthma action plans and asthma exacerbations in children with asthma: A systematic review. J Asthma Allergy Educators. 2011;2:11-21.
47. Maric B, Kaan A, Ignaszewski A, et al. A systematic review of telemonitoring technologies in heart failure. Eur J Heart Fail. 2009;11:506-517.
48. Boutwell A, Hwu S. Effective Interventions to Reduce Rehospitalizations: A Survey of the Published Evidence. Cambridge, MA: Institute for Healthcare Improvement; 2009.
49. Bodenheimer T, Laing BY. The teamlet model of primary care. Ann Fam Med. 2007;5:457-461.
50. Meier D, Beresford L. Outpatient clinics are a new frontier for palliative care. J Pall Med. 2008;11:823-828.
Learn how to get reimbursed for postdischarge care
The refugee medical exam: What you need to do
• Use tuberculin skin testing alone or in conjunction with interferon-gamma release assay to screen children younger than 5 years for tuberculosis. A
• Include 2 evaluations for ova and parasites plus a complete blood count with differential when screening refugees for parasitic infections. B
• Screen all adolescent and adult refugees for human immunodeficiency virus infection. A
• Check blood lead levels in all children 6 months to 16 years of age on arrival in the United States B and 6 months later. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
In 2011, 56,384 refugees fleeing persecution in their native countries were admitted to the United States. The largest numbers came from Burma (30.1%), Bhutan (26.6%), and Iraq (16.7%).1 They joined the more than 3 million refugees from all over the world who have resettled in this country since 1975. 1
Refugees arrive in the United States with complex medical issues, including illnesses rarely seen here, mental health concerns, and chronic conditions such as diabetes and hypertension. After arrival, they undergo a domestic refugee medical examination (DRME). This DRME, along with well-planned follow-up, can go a long way toward helping refugees show the proof of vaccination and control of chronic health conditions that are required when they apply for lawful permanent resident status.
The Centers for Disease Control and Prevention (CDC) has published guidelines to help with medical decision making and screening of refugees, but limited information is available on the necessary strategies to address chronic health conditions within the context of the DRME.2 Moreover, differences in refugee experience and health status based on country of origin may demand more detailed, region-specific guidelines.3-9 No standard recommendations address the importance of providing not just initial screening, but comprehensive longitudinal care, as well.
Since 2007, our outpatient practice (MA, KS, GM, PM) has performed the DRME and provided ongoing care for more than 900 refugees resettled in Philadelphia. The practice, which is associated with an urban academic medical center and closely coordinates refugee care with a local resettlement agency, has earned recognition as a Level 3 (top-level certification) patient-centered medical home by the National Committee on Quality Assurance. We offer here a framework for providing comprehensive care to refugees, based on CDC guidelines, available evidence, and our experience.
Prelude: The overseas medical exam
All refugees must undergo an overseas medical examination (OME) no longer than 12 months before resettlement in the United States. Physicians selected by US Department of State consular officials perform the examinations.
The OME includes a medical history, physical examination, and testing to screen for mental illness, drug abuse, syphilis, leprosy, and tuberculosis (TB). Some vaccinations and empiric treatment for parasites also may be provided at the time of the examination.10-12
The OME screens for Class A disorders, which render a refugee ineligible for admission to the United States until treated or stabilized, and Class B conditions, which require close follow-up on arrival (TABLE 1).12 Despite recent steps toward standardization, the quality and thoroughness of OMEs completed at different examination sites still vary substantially.
TABLE 1
Overseas medical examination: Class A and B conditions12
| Class A* | Class B† |
|---|---|
| Active or infectious tuberculosis Untreated STI: syphilis, gonorrhea, chancroid, granuloma inguinale, or lymphogranuloma venereum Hansen’s disease (leprosy) Drug or alcohol addiction/abuse Mental illness with harmful behavior | Inactive or noninfectious tuberculosis Treated STI Treated or paucibacillary Hansen’s disease Sustained remission from drug or alcohol addiction or abuse Well-controlled mental illness Pregnancy |
| STI, sexually transmitted infection. *Class A disorders render a refugee ineligible for admission to the United States until he or she is treated or stabilized. †Class B disorders require close follow-up upon the refugee’s arrival in the United States | |
Arrival in United States is followed by DRME
When refugees arrive in the United States, they are advised to undergo a DRME, which any licensed practitioner may perform, preferably within 90 days. More rapid evaluation is encouraged for medically complex refugees or refugees arriving with Class A or B conditions. Because refugees are eligible for only 8 months of medical assistance, we strongly recommend that the DRME be done promptly.
The CDC publishes guidelines for components of the initial DRME, but state requirements and individual examinations vary widely.2,10,13,14 We outline here the elements of the exam identified by the CDC, supplemented with recommendations based on published evidence and our experiences in caring for refugees.
Screen for tuberculosis
Refugees have a higher prevalence of latent tuberculosis infection (LTBI) and active TB than the general US population. An estimated one-third of the world’s population has LTBI.15 Since 2002, more than 50% of all people diagnosed with TB in the United States have been born outside the country.16
Although otherwise healthy adults with LTBI have a lifetime risk of approximately 10% that it will progress to active TB,17 infants, young children, and people coinfected with HIV have a rate of progression of around 10% per year. It is imperative, therefore, that all refugees be screened for TB and treated appropriately.8,18,19
Refugees are screened for active TB with a chest radiograph and possibly a sputum analysis during the OME. Because screening may take place as long as 12 months before arrival in the United States, refugees may be re-exposed to TB in the refugee camp before departure. They are not screened for LTBI before coming to the United States.11,12
Domestic screening for LTBI is complicated by routine use in many foreign countries of the Bacille Calmette-Guérin (BCG) vaccine, which can reduce the incidence of TB meningitis and disseminated TB in children, but does not protect adults against primary infection or reactivation of TB. Tuberculin skin testing using purified protein derivative, which has typically been used for screening, can render false-positive results, particularly in the context of previous BCG vaccination.
Interferon-gamma release assay (IGRA) is an alternative screening option that has been approved for use in the United States.15,20 Because the IGRA is a blood test, it eliminates interpretation errors associated with tuberculin skin testing and is not affected by BCG vaccination. IGRA testing also does not require an additional office visit.
For these reasons, we recommend screening all refugees older than 5 years with IGRAs, where available. In light of scant data and apparent differences in immune response in young children, the CDC recommends using tuberculin skin testing either alone or in conjunction with IGRA testing for all children younger than 5 years.20,21
Positive screening tests must be followed up with a chest radiograph. Perform serial sputum evaluation whenever the chest radiograph indicates potential active TB.
Everyone with latent or active TB must be treated according to CDC recommendations adapted from guidelines established by the American Thoracic Society and Infectious Diseases Society of America.22,23 For latent TB, the CDC calls for treatment with isoniazid for 9 months or rifampin for 4 months.
- Patients older than 18 years should receive the adult dose of isoniazid: 5 mg/kg per day orally to a maximum daily dose of 300 mg. Children should receive 10 to 20 mg/kg per day orally to a maximum daily dose of 300 mg. Twice weekly therapy schedules are also available and commonly used for children who receive directly observed treatment in school.
- The adult dosage of rifampin (for patients >15 years) is 10 mg/kg per day orally to a maximum daily dose of 600 mg; the pediatric dose is 10 to 20 mg/kg per day orally, also to a maximum daily dose of 600 mg.
Patients taking isoniazid who are pregnant or breastfeeding or have diabetes, renal failure, alcoholism, malnutrition, HIV, or a seizure disorder should receive pyridoxine (vitamin B6) supplementation to aid in preventing peripheral neuropathy, in an adult oral dose of 25 to 50 mg/d or a pediatric oral dose of 6.25 mg/d. Additional information on treating latent TB is available at http://www.cdc.gov/tb/topic/treatment/ltbi.htm.
For patients with active TB, treatment is more complex, based on the patient’s overall health. Please refer to the CDC recommendation for the treatment of active TB (http://www.cdc.gov/tb/topic/treatment/tbdisease.htm) or contact your local TB control division.
Patients may receive TB treatment from either individual medical providers or city or state health departments, depending on local capacity. In our practice, we treat LTBI in adults. The Philadelphia Department of Public Health’s TB Control Program manages LTBI in children and all suspected cases of active TB. We recommend providing everyone treated for latent or active TB with documentation of treatment completion.
Diagnose and treat problematic parasites
Intestinal parasites are among the infections most often found in refugee populations.7,8,24-29 Common pathogens in untreated refugees are Ascaris lumbricoides, hookworm (Ancylostoma duodenale and Necator americanus), Schistosoma species, Strongyloides stercoralis, Trichuris trichiura, and Giardia lamblia.
Although sustained domestic transmission is unlikely, these parasites may cause growth delay, anemia, hyperinfestation syndrome and disseminated infection (A lumbricoides and S stercoralis), and increased cancer risk (Schistosoma hematobium).7 In the late 1990s, the CDC initiated empiric treatment before departure for the United States for A lumbricoides (albendazole), S stercoralis (ivermectin), Schistosoma species (praziquantel), and other parasites in certain refugee populations, which has decreased but not eliminated the threat.7
All refugees should be receiving appropriate predeparture treatment for parasitic infections. For newly arrived refugees who have received no predeparture therapy or incomplete therapy, the CDC recommends screening for parasites or providing presumptive treatment (TABLE 2).
TABLE 2
Empiric treatment of parasites
| Refugee region of origin | Organism | Adult therapy |
|---|---|---|
| Middle East, South Asia, Southeast Asia | Strongyloides stercoralis Other roundworms | Ivermectin 200 μg/kg/d orally for 2 days Albendazole 400 mg orally, 1 dose |
| Africa | Schistosoma species S stercoralis Other roundworms | Praziquantel 20 mg/kg orally, 2 doses Ivermectin 200 μg/kg/d orally for 2 days Albendazole 400 mg orally, 1 dose |
| Source: CDC. Immigrant and Refugee Health: Domestic Intestinal Parasite Guidelines. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/intestinal-parasites-domestic.html. Accessed November 19, 2012. | ||
The optimal screening regimen for parasites in refugee populations is controversial. Although most screening programs rely on one or more microscopic examinations of stool for ova and parasites, this test is expensive, requires special handling, depends on the reviewer’s expertise, and remains relatively insensitive. A comprehensive review of stool ova and parasites in high-risk populations concluded that the use of 2 independently collected stool samples improved sensitivity at acceptable cost.30
New, more sensitive and specific assays have been developed for many parasites, including Cryptosporidium parvum, Entamoeba histolytica, G lamblia, S stercoralis, and Schistosoma species, but we do not recommend these specialized tests unless the provider strongly suspects a specific parasite based on history and physical exam or persistent eosinophilia.
All refugees should have a complete blood count with differential to help identify occult parasitemia. Although a finding of eosinophilia may result from successful empiric therapy for an already-treated parasite, it must be followed up with more specific testing for S stercoralis, even in otherwise asymptomatic patients. African refugees with eosinophilia also should be tested for Schistosoma, and Somali Bantu should be treated empirically for both S stercoralis and Schistosoma.31 In line with CDC guidelines, ongoing failure to identify the cause of eosinophilia in a refugee should prompt referral to an infectious disease specialist and further work-up.
Three to 6 months after antibiotic treatment of any parasite, immunocompromised patients and those with suspected treatment failure should undergo a test of cure comprised of 2 stool ova and parasite studies and a follow-up CBC with differential.32
Screen for HIV
Since January 4, 2010, after HIV was removed from the Class A diagnosis list, refugees are no longer tested for HIV before arrival in the United States.11 Nevertheless, we recommend screening all refugees on arrival, regardless of age, for HIV types 1 and 2, unless they opt out, for the following reasons:
- approximately 14% of incoming refugees arrive from countries with an HIV prevalence of more than 5%33
- the increasing use of rape as a tool of torture and repression puts refugees at particular risk for HIV
- current CDC guidelines recommend HIV screening at the time of first encounter in all health care settings for everyone from 13 to 64 years of age and any patient who requests it.34
We also strongly recommend repeat screening 3 to 6 months after resettlement for refugees with recent potential exposure or who engage in high-risk activity.
Watch for ubiquitous hepatitis infection
In accordance with CDC vaccination guidelines and American Association of Pediatrics (AAP) Bright Futures recommendations, we endorse hepatitis A serology testing with reflex vaccination unless immunity is documented for refugees 1 to 18 years of age.35,36
A third of the world’s population shows serologic evidence of past infection with hepatitis B virus (HBV); high rates occur in Southeast Asia and sub-Saharan Africa, where most infections are transmitted perinatally.37,38 A study of Minnesota refugees found 7% to be positive for hepatitis B surface antigen (HBsAg), with a higher prevalence among refugees from sub-Saharan Africa.8
Most screening protocols for refugees test for HBsAg and antibody to hepatitis B surface antigen (HBsAb); it is reasonable to add a screen for antibody to hepatitis B core antigen (HBcAb). We recommend screening for HBV infection using HBsAg, HBsAb, and HBcAb to minimize underdiagnosis in this high-risk population. Refugees without immunity to HBV should be offered vaccination.18 Encourage immunization, especially for patients with hepatitis or cirrhosis from any cause.
Hepatitis C screening should follow CDC guidelines for the general population, focusing on high-risk groups such as injection drug users, victims of sexual violence, people with multiple sexual partners, recipients of blood transfusions, people with any other type of hepatitis, and one-time screening for individuals born between 1945 and 1965.39,40
Monitor for malaria
Many refugees come to the United States from areas where malaria is endemic.41 In 2007, the CDC instituted empiric treatment before arrival in the United States for all refugees from sub-Saharan Africa because the rapid test for malaria approved by the US Food and Drug Administration has low sensitivity and specificity,2 malarial vectors are present throughout much of the United States, and malaria (specifically Plasmodium falciparum) causes significant morbidity and mortality. If written confirmation of predeparture treatment is not available, refugees from sub-Saharan Africa should receive presumptive treatment, outlined in TABLE 3,42 as part of the initial DRME.
TABLE 3
Presumptive postarrival malaria treatment for refugees from sub-Saharan Africa42
| Directly observed treatment received in country of origin? | Recommended treatment* | |
|---|---|---|
| Children | Adults | |
| Yes | None | None |
| No | Atovaquone-proguanil (62.5/25 mg): 5-8 kg: 2 tablets per day for 3 days 9-10 kg: 3 tablets per day for 3 days Atovaquone-proguanil (250/100 mg): 11-20 kg: 1 tablet per day for 3 days 21-30 kg: 2 tablets per day for 3 days 31-40 kg: 3 tablets per day for 3 days >40 kg: 4 tablets per day for 3 days | Atovaquone-proguanil (250/100 mg): 4 tablets per day for 3 days |
| *Do not presumptively treat pregnant or lactating women or children weighing <5 kg. An infectious disease consult is recommended for these patients. | ||
Based on our experience and expert opinion, we recommend routinely monitoring all refugees from endemic areas for symptoms of malarial disease during the initial 3 months after resettlement. Relapsing fevers, unexplained malaise or fatigue, pallor, thrombocytopenia, or splenomegaly should trigger additional testing with thick- and thin-blood smears for trophozoites (3 separate samples drawn at 12- to 24-hour intervals).
Be alert for malnutrition
Acute and chronic malnutrition, as well as micronutrient deficiencies, have been noted in refugees coming from refugee camps. A survey of Bhutanese refugees in a camp in Nepal found that 25.1% of children were underweight and 4.8% of them were severely underweight. Moreover, 43.3% of children had anemia.43 Recognizing that refugees may be at high risk for iron deficiency, we recommend evaluating children and adolescents for this deficit according to AAP guidelines.44
We also recommend screening body mass index (BMI) to identify refugees at risk. Height, weight, and BMI must be followed over time to ensure appropriate acclimation to the US diet.
Also consider vitamin D deficiency and rickets in refugee populations, particularly people with darker skin and women who wear veils.45,46 Based on our experiences and CDC guidelines, we recommend a multivitamin with iron for children 6 to 59 months of age.12
Check lead levels in children
Refugee children are at risk of elevated blood lead levels (>10 ’g/dL) resulting from pre-departure environmental exposure and iron deficiency anemia, which can enhance absorption of lead. Refugees also are more likely to resettle in poor neighborhoods with substandard housing, increasing their risk of domestic lead exposure.
Studies of refugee children at initial screening have shown prevalences of elevated blood lead levels of 6.3% in a Cuban refugee population in Miami and higher rates (11%-22%) in mixed refugee populations in Massachusetts.6,47 A study in New Hampshire found that approximately 30% of refugee children with normal lead levels on initial screen had elevated levels when checked several months later.48
Consistent with CDC guidelines,49 our experience, and the findings of the State of Minnesota,50 we recommend checking blood lead levels in all children 6 months to 16 years of age upon arrival in the United States and repeat lead testing 3 to 6 months after placement in a permanent residence.
Bring vaccinations up to date
US law requires anyone seeking an immigrant visa to show proof of vaccination against vaccine-preventable diseases, as recommended by the US Advisory Committee on Immunization Practices.51 Vaccination requirements that apply to other immigrant groups do not apply to refugees at the time of their initial admission to the United States, but refugees must be vaccinated when they seek a green card or permanent US residence.
All refugees are eligible for adjustment of status after they have lived in the United States for a year and need proof of vaccination to apply.51 Moreover, schools may bar refugee children from attending if their vaccinations are not up-to-date, which, in turn, may hinder their parents’ ability to find employment. CDC guidelines for vaccinating immigrants and refugees applying for permanent residence are available at http://www.cdc.gov/immigrantrefugeehealth/pdf/2009-vaccination-technical-instructions.pdf (see the table on page 12).52 Because of the large number of vaccinations required for children and even many adults, health care providers should be familiar with the CDC’s recommended immunization and catch-up schedules.35
Vaccinations given in other countries are acceptable if appropriately recorded in Institute of Medicine documentation, or if original vaccination records are available and the vaccinations conform to appropriate intervals and age guidelines. Refugees must bring their records with them to medical appointments. Laboratory evidence of immunity is acceptable for measles, mumps, rubella (MMR), hepatitis A, hepatitis B, polio, and varicella, but there is debate about whether such testing should be performed before immunization.18,53 Health care providers need to assess each patient based on age and risk factors to decide whether immunity testing is appropriate.
In our practice, we routinely test all adults for immunity to varicella, hepatitis A, hepatitis B, and MMR. For children, we rely on documented immunization records, not antibody titers, for evidence of previous vaccination.
Pay attention to mental health issues
Many refugees have been exposed to trauma, often including war and torture, increasing their risk for mental illness. A large 2005 review found that serious mental disorders, including post-traumatic stress disorder (PTSD), major depressive disorder, and generalized anxiety disorder are significantly more prevalent among refugees than the general population.5 Many screening tests for PTSD have been proposed54 but have not been validated in all immigrant or refugee populations.55
Mental health care for refugees is complicated by language and cultural barriers, adjustment disorders, access to psychiatric services, and uncertainty about effective treatments in refugee populations. Despite the higher prevalence of mental illness among refugees, many in the mental health field have raised concerns about the applicability of Western concepts of mental health, including PTSD, in this group.56
Refugees who are victims of torture should be referred to experienced mental health practitioners. After ruling out acute psychosis and destructive behaviors, we recommend postponing an exhaustive mental health screening until several months after arrival. In our medical home model, we evaluate patients on an ongoing basis, giving us an opportunity to identify emerging or worsening mental health conditions.
Evaluate dental health
The incidence of dental caries and periodontal disease among refugees varies widely among different groups of refugees. Data on pediatric refugees in the United States have shown dental caries to be common, with prevalences between 16.7% and 42%, with marked differences based on region of origin.3,57,58 In our practice, we also have noted heavy use of betel nut in the Southeast Asian community, leading to significant dental disease.
All refugees should have their dentition evaluated at the initial DRME. We recommend subsequent formal dental examination for all patients, giving priority to those with clear evidence of active disease.
Identify and address chronic disease
Refugees carry a substantial burden of chronic disease, although marked regional variation has been noted.4 A study of Massachusetts refugees from 2001 through 2005 demonstrated that 46.8% were overweight or obese, 22.6% had hypertension, and 3.1% had diabetes. Smoking is also highly prevalent in refugee populations.59
Our findings confirm high rates of chronic disease, particularly among Iraqi and geriatric refugees. These patients require close follow-up after the DRME to minimize sequelae from chronic conditions. Multi-disciplinary teams in the patient-centered medical home may provide an opportunity to promptly address chronic health conditions that can have severe short-term consequences if not adequately managed (eg, insulin dosage adjustment based on diet in patients with diabetes).
We recommend a comprehensive medical history and evaluation for chronic disease, including diabetes and hypertension, at the DRME and on an ongoing basis. Although many refugees have never had any health screening and substantial cultural barriers may exist, especially with regard to women’s health and age-based cancer screening, refugees generally should receive the same preventive care as the rest of the US population until further research has been done in this area.
We recommend introducing age-based cancer screening and other preventive care for refugees within 2 months of their initial visit. This model of care has already been endorsed by the Minnesota Department of Health’s Refugee Health Program, one of the leading health care providers for refugees in the United States.60
Toward better care models
The medical care of refugees is complex, but the prepared primary care provider can manage it effectively. TABLE 4 summarizes our recommendations for the DRME based on our experiences and the available literature. Standardized screening guidelines and comprehensive programs, perhaps incorporating the concept of the patient-centered medical home, will likely improve both the initial and continuing care of this population.
TABLE 4
Summary recommendations for the domestic refugee medical exam
History
|
| Physical exam In addition to the essential components of the physical exam, pay attention to:
|
Initial laboratory evaluation
|
| Ongoing care Include:
|
| CBC, complete blood count; HIV, human immunode"ciency virus; IGRA, interferon-gamma release assay; MMR, measles, mumps, rubella; TST, tuberculin skin testing. Adapted from: Centers for Disease Control and Prevention. Immigrant and Refugee Health: Guidelines for the US Domestic Medical Examination for Newly Arriving Refugees. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/domestic-guidelines.html. Accessed November 19, 2012. |
Ongoing study is essential to better address the health care needs of refugees. Although they comprise only a small segment of immigrants living in the United States, the experience of caring for them may help develop models to provide better care to other foreign-born patients.
CORRESPONDENCE Marc Altshuler, MD, Department of Family and Community Medicine, Jefferson Medical College, Thomas Jefferson University, 833 Chestnut Street, Suite 301, Philadelphia, PA 19107; [email protected]
1. Martin D, Yankay J. Refugees and Asylees: 2011. Annual Flow Report. Offce of Immigration Statistics, US Department of Home-land Security. May 2012. Available at: http://www.dhs.gov/xlibrary/assets/statistics/publications/ois_rfa_fr_2011.pdf. Accessed October 29, 2012.
2. Stauffer WM, Kamat D, Walker PF. Screening of international immigrants, refugees and adoptees. Prim Care. 2002;29:879-905.
3. Cote S, Geltman P, Nunn M, et al. Dental caries of refugee children compared with US children. Pediatrics. 2004;114:e733-e740.
4. Geltman PL, Dookeran NM, Battaglia T, et al. Chronic disease and its risk factors among refugees and asylees in Massachusetts, 2001-2005. Prev Chronic Dis. 2010;7:A51.-
5. Fazel M, Wheeler J, Danesh J. Prevalence of serious mental disorder in 7000 refugees resettled in western countries: a systematic review. Lancet. 2005;365:1309-1314.
6. Geltman PL, Brown MJ, Cochran J. Lead poisoning among refugee children resettled in Massachusetts, 1995 to 1999. Pediatrics. 2001;108:158-162.
7. Geltman PL, Cochran J, Hedgecock C. Intestinal parasites among African refugees resettled in Massachusetts and the impact of an overseas pre-departure treatment program. Am J Trop Med Hyg. 2003;69:657.-
8. Lifson AR, Thai D, O’Fallon A, et al. Prevalence of tuberculosis, hepatitis B virus, and intestinal parasitic infections among refugees to Minnesota. Public Health Rep. 2002;117:69-77.
9. Power DV, Moody E, Trussell K, et al. Caring for the Karen. A newly arrived refugee group. Minn Med. 2010;93:49-53.
10. Centers for Disease Control and Prevention. Health considerations of newly arrived immigrants and refugees. In: Centers for Disease Control and Prevention. Travelers’ Health—Yellow Book. Chapt. 9. Available at: http://wwwnc.cdc.gov/travel/yellow-book/2010/table-of-contents.aspx#20. Accessed November 15, 2012.
11. Centers for Disease Control and Prevention. Medical Examination of Immigrants and Refugees. Available at: http://www.cdc.gov/immigrantrefugeehealth/exams/medical-examination.html. Accessed November 15, 2012.
12. Centers for Disease Control and Prevention. Technical Instructions For The Medical History and Physical Examination of Aliens in the United States. Available at: http://www.cdc.gov/immigrantrefugeehealth/exams/ti/civil/technical-instructions/civil-surgeons/medical-history-physical-examination.html. Accessed November 15, 2010.
13. Seybolt L, Barnett E, Stauffer W. US medical screening for immigrants and refugees: clinical issues. In: Walker P, Barnett E, eds. Immigrant Medicine. Phildelphia, PA: Saunders Elsevier; 2007:135-150.
14. United States Department of Health and Human Services, Offce of Refugee Resettlement. ORR State Letter: Revised Medical Screening Guidelines for Newly Arrived Refugees. Available at: http://www.acf.hhs.gov/sites/default/files/orr/state_letter_12_09_revised_medical_screening_guidelines_for_newly.pdf. Accessed October 29, 2012.
15. Centers for Disease Control and Prevention. Guidelines for the investigation of contacts of persons with infectious tuberculosis— guidelines for using the QuantiFERON-TB gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep. 2005;54(RR-15):1-55.
16. Centers for Disease Control and Prevention. Executive Commentary: Highlights of 2011 Report. Available at: http://www.cdc.gov/tb/statistics/reports/2011/pdf/ExecutiveCommentary.pdf. Accessed November 19, 2012.
17. Kuma V, Abbas AK, Fausto N, et al. Robbins Basic Pathology. 8th ed. Philadelphia, Pa: Saunders Elsevier; 2007:516–522.
18. Barnett ED. Infectious disease screening for refugees resettled in the United States. Clin Infect Dis. 2004;39:833-841.
19. DeRiemer K, Chin DP, Schecter DF, et al. Tuberculosis among immigrants and refugees. Arch Intern Med. 1998;158:753-760.
20. Centers for Disease Control and Prevention. Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recomm Rec. 2010;59(RR-5):1-25.
21. Bright Futures at Georgetown University. Health Supervision— Laboratory Tests: Tuberculosis (TB) Screening. Available at: http://www.brightfutures.org/pocket/pdf/30_37.pdf. Accessed November 19, 2012.
22. Centers for Disease Control and Prevention. Treatment of tuberculosis. American Thoracic Society, CDC, Infectious Diseases Society of America. MMWR Recomm Rep 2003;52(RR-11):1-77.
23. Centers for Disease Control and Prevention. Update: adverse event data and revised American Thoracic Society/CDC recommendations against the use of rifampin and pyrazinamide for treament of latent tuberculosis infection—United States. MMWR Morb Mortal Wkly Rep. 2003;52:735-739.
24. Caruana SR, Kelly HA, Ngeow JY, et al. Undiagnosed and potentially lethal parasite infections among immigrants and refugees in Australia. J Travel Med. 2006;13:233-239.
25. Dawson-Hahn EE, Greenberg SL, Domachowske JB, et al. Eosinophilia and the seroprevalence of schistosomiasis and strongyloidiasis in newly arrived pediatric refugees: an examination of Centers for Disease Control and Prevention screening guidelines. J Pediatr. 2010;156:1016-1018.
26. Garg PK, Perry S, Dorn M, et al. Risk of intestinal helminth and protozoan infection in a refugee population. Am J Trop Med Hyg. 2005;73:386-391.
27. Parenti DM, Lucas D, Lee A, et al. Health status of Ethiopian refugees in the United States. Am J Public Health. 1987;77:1542-1543.
28. Parish R. Intestinal parasites in Southeast Asian refugee children. West J Med. 1985;143:47-49.
29. Sutherland JE, Avant RF, Franz WB, 3rd, et al. Indochinese refugee health assessment and treatment. J Fam Pract. 1983;16:61-67.
30. Cartwright C. Utility of multiple-stool-specimen ova and parasite examinations in a high-prevalence setting. J Clin Microbiol. 1999;37:2408-2411.
31. Centers for Disease Control and Prevention. Recommendations for Presumptive Treatment of Schistosomiasis and Strongyloidiasis Among the Somali Bantu Refugees. June 13, 2005. Available at: http://archive.acf.hhs.gov/programs/orr/policy/sl05-18attach-ment2.pdf. Accessed November 19, 2012.
32. Centers for Disease Control and Prevention. Division of Global Migration and Quarantine. Guidelines for Evaluation of Refugees for Intestinal and Tissue-Invasive Parasitic Infections during Domestic Medical Examination. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/intestinal-parasites-domestic.html. Accessed October 30, 2012.
33. Centers for Disease Control and Prevention. Screening for HIV Infection During the Refugee Domestic Medical Examination. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/screening-hiv-infection-domestic.html. Accessed November 15, 2012.
34. Centers for Disease Control and Prevention. Revised recommendations for HIV testing of adults, adolescents and pregnant women in healthcare settings. MMWR Recomm Rep. 2006;55(RR-14):1-17.
35. Centers for Disease Control and Prevention National Immunization Program. Available at: http://www.cdc.gov/vaccines/vpdvac/hepa/default.htm. Accessed November 19, 2012.
36. American Academy of Pediatrics. Red Book. Available at: http://www2.aap.org/immunization/illnesses/hepb/hepa.html. Accessed November 19, 2012.
37. Lai CL, Ratziu V, Yuen MF, et al. Viral hepatitis B. Lancet. 2003;362:2089-2094.
38. Lin K, Kirchner J. Hepatitis B. Am Fam Phyician. 2004;69:75-82.
39. Ghany MG, Strader DB, Thomas DL, et al. American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374.
40. Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Morb Mortal Wkly Rep. 2012;61(RR-4):1-31.
41. Rowland M, Nosten F. Malaria epidemiology and control in refugee camps and complex emergencies. Ann Trop Med Parasitol. 2001;95:741-754.
42. Centers for Disease Control and Prevention, Malaria Branch, Division of Parasitic Diseases, Division of Global Migration and Quarantine and Malaria Branch. Presumptive Treatment of P falciparum Malaria in Refugees Relocating from sub-Saharan Africa to the United States. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/malaria-guidelines-domestic.html. Accessed November 15, 2012.
43. Centers for Disease Control and Prevention. Malnutrition and micronutrient deficiencies among Bhutanese refugee children— Nepal, 2007. MMWR Morb Mortal Wkly Rep. 2008;57:370-373.
44. American Academy of Pediatrics Bright Futures. Guidelines for Health Supervision of Infants, Children, and Adolescents— Theme 5: Promoting Healthy Nutrition. Available at: http://brightfutures.aap.org/pdfs/Guidelines_PDF/6-Promoting_Healthy_Nutrition.pdf. Accessed November 15, 2012.
45. Benson J, Skull S. Hiding from the sun: vitamin D deficiency in refugees. Aust Fam Physician. 2007;36:355-357.
46. Stellinga-Boelen A. Vitamin D levels in children of asylum seekers in The Netherlands in relation to season and dietary intake. Eur J Pediatr. 2007;166:201-206.
47. Trepka MJ, Pekovic V, Santana JC, et al. Risk factors for lead poisoning among Cuban refugee children. Public Health Rep. 2005;120:179-185.
48. Centers for Disease Control and Prevention. Elevated blood lead levels in refugee children, New Hampshire, 2003-2004. MMWR Morb Mortal Wkly Rep. 2005;54:42-46.
49. Centers for Disease Control and Prevention. Screening for Lead at the Domestic Refugee Medical Exam. Available at: http://www.cdc.gov/immigrantrefugeehealth/pdf/lead.pdf. Accessed November 15, 2012.
50. Zabel EW, Smith ME, O’Fallon A. Implementation of CDC refugee blood testing guidelines in Minnesota. Public Health Rep. 2008;123:111-125.
51. United States Citizenship and Immigration Services. Available at: http://www.uscis.gov/portal/site/uscis/menuitem.5af9bb95919f35e66f614176543f6d1a/?vgnextoid=3384cc5222$5210VgnVCM100000082ca60aRCRD&vgnextchannel=6abe6d26d17df110VgnVCM1000004718190aRCRD. Accessed November 15, 2012.
52. Centers for Disease Control and Prevention. Vaccination Requirements for Adjustment of Status for US Permanent Residence: Technical Instructions for Civil Surgeons. December 14, 2009. Available at: http://www.cdc.gov/immigrantrefugeehealth/pdf/2009-vaccination-technical-instructions.pdf. Accessed November 15, 2012
53. Phillips C. Better primary healthcare for refugees: catch up immunisation. Aust Fam Physician. 2007;36:440-443.
54. Brewin C. Systematic review of screening instruments for adults at risk of PTSD. J Trauma Stress. 2005;18:53-62.
55. Crumlish N, O’Rourke K. A systematic review of treatments for post-traumatic stress disorder among refugees and asylum-seekers. J Nerv Ment Dis. 2010;198:237-251.
56. Watters C. Emerging paradigms in the mental health care of refugees. Soc Sci Med. 2001;53:1709-1718.
57. Hayes EB, Talbot SB, Matheson ES, et al. Health status of pediatric refugees in Portland, ME. Arch Pediatr Adolesc Med. 1998;152:564-568.
58. Meropol S. Health status of pediatric refugees in Buffalo, NY. Arch Pediatr Adolesc Med. 1995;149:887-892.
59. Barnes DM, Harrison C, Heneghan R. Health risk and promotion behaviors in refugee populations. J Health Care Poor Underserved. 2004;15:347-356.
60. Dicker S, Stauffer WM, Mamo B, et al. Initial refugee health assessments: new recommendations for Minnesota. Minn Med. 2010;93:45-48.
• Use tuberculin skin testing alone or in conjunction with interferon-gamma release assay to screen children younger than 5 years for tuberculosis. A
• Include 2 evaluations for ova and parasites plus a complete blood count with differential when screening refugees for parasitic infections. B
• Screen all adolescent and adult refugees for human immunodeficiency virus infection. A
• Check blood lead levels in all children 6 months to 16 years of age on arrival in the United States B and 6 months later. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
In 2011, 56,384 refugees fleeing persecution in their native countries were admitted to the United States. The largest numbers came from Burma (30.1%), Bhutan (26.6%), and Iraq (16.7%).1 They joined the more than 3 million refugees from all over the world who have resettled in this country since 1975. 1
Refugees arrive in the United States with complex medical issues, including illnesses rarely seen here, mental health concerns, and chronic conditions such as diabetes and hypertension. After arrival, they undergo a domestic refugee medical examination (DRME). This DRME, along with well-planned follow-up, can go a long way toward helping refugees show the proof of vaccination and control of chronic health conditions that are required when they apply for lawful permanent resident status.
The Centers for Disease Control and Prevention (CDC) has published guidelines to help with medical decision making and screening of refugees, but limited information is available on the necessary strategies to address chronic health conditions within the context of the DRME.2 Moreover, differences in refugee experience and health status based on country of origin may demand more detailed, region-specific guidelines.3-9 No standard recommendations address the importance of providing not just initial screening, but comprehensive longitudinal care, as well.
Since 2007, our outpatient practice (MA, KS, GM, PM) has performed the DRME and provided ongoing care for more than 900 refugees resettled in Philadelphia. The practice, which is associated with an urban academic medical center and closely coordinates refugee care with a local resettlement agency, has earned recognition as a Level 3 (top-level certification) patient-centered medical home by the National Committee on Quality Assurance. We offer here a framework for providing comprehensive care to refugees, based on CDC guidelines, available evidence, and our experience.
Prelude: The overseas medical exam
All refugees must undergo an overseas medical examination (OME) no longer than 12 months before resettlement in the United States. Physicians selected by US Department of State consular officials perform the examinations.
The OME includes a medical history, physical examination, and testing to screen for mental illness, drug abuse, syphilis, leprosy, and tuberculosis (TB). Some vaccinations and empiric treatment for parasites also may be provided at the time of the examination.10-12
The OME screens for Class A disorders, which render a refugee ineligible for admission to the United States until treated or stabilized, and Class B conditions, which require close follow-up on arrival (TABLE 1).12 Despite recent steps toward standardization, the quality and thoroughness of OMEs completed at different examination sites still vary substantially.
TABLE 1
Overseas medical examination: Class A and B conditions12
| Class A* | Class B† |
|---|---|
| Active or infectious tuberculosis Untreated STI: syphilis, gonorrhea, chancroid, granuloma inguinale, or lymphogranuloma venereum Hansen’s disease (leprosy) Drug or alcohol addiction/abuse Mental illness with harmful behavior | Inactive or noninfectious tuberculosis Treated STI Treated or paucibacillary Hansen’s disease Sustained remission from drug or alcohol addiction or abuse Well-controlled mental illness Pregnancy |
| STI, sexually transmitted infection. *Class A disorders render a refugee ineligible for admission to the United States until he or she is treated or stabilized. †Class B disorders require close follow-up upon the refugee’s arrival in the United States | |
Arrival in United States is followed by DRME
When refugees arrive in the United States, they are advised to undergo a DRME, which any licensed practitioner may perform, preferably within 90 days. More rapid evaluation is encouraged for medically complex refugees or refugees arriving with Class A or B conditions. Because refugees are eligible for only 8 months of medical assistance, we strongly recommend that the DRME be done promptly.
The CDC publishes guidelines for components of the initial DRME, but state requirements and individual examinations vary widely.2,10,13,14 We outline here the elements of the exam identified by the CDC, supplemented with recommendations based on published evidence and our experiences in caring for refugees.
Screen for tuberculosis
Refugees have a higher prevalence of latent tuberculosis infection (LTBI) and active TB than the general US population. An estimated one-third of the world’s population has LTBI.15 Since 2002, more than 50% of all people diagnosed with TB in the United States have been born outside the country.16
Although otherwise healthy adults with LTBI have a lifetime risk of approximately 10% that it will progress to active TB,17 infants, young children, and people coinfected with HIV have a rate of progression of around 10% per year. It is imperative, therefore, that all refugees be screened for TB and treated appropriately.8,18,19
Refugees are screened for active TB with a chest radiograph and possibly a sputum analysis during the OME. Because screening may take place as long as 12 months before arrival in the United States, refugees may be re-exposed to TB in the refugee camp before departure. They are not screened for LTBI before coming to the United States.11,12
Domestic screening for LTBI is complicated by routine use in many foreign countries of the Bacille Calmette-Guérin (BCG) vaccine, which can reduce the incidence of TB meningitis and disseminated TB in children, but does not protect adults against primary infection or reactivation of TB. Tuberculin skin testing using purified protein derivative, which has typically been used for screening, can render false-positive results, particularly in the context of previous BCG vaccination.
Interferon-gamma release assay (IGRA) is an alternative screening option that has been approved for use in the United States.15,20 Because the IGRA is a blood test, it eliminates interpretation errors associated with tuberculin skin testing and is not affected by BCG vaccination. IGRA testing also does not require an additional office visit.
For these reasons, we recommend screening all refugees older than 5 years with IGRAs, where available. In light of scant data and apparent differences in immune response in young children, the CDC recommends using tuberculin skin testing either alone or in conjunction with IGRA testing for all children younger than 5 years.20,21
Positive screening tests must be followed up with a chest radiograph. Perform serial sputum evaluation whenever the chest radiograph indicates potential active TB.
Everyone with latent or active TB must be treated according to CDC recommendations adapted from guidelines established by the American Thoracic Society and Infectious Diseases Society of America.22,23 For latent TB, the CDC calls for treatment with isoniazid for 9 months or rifampin for 4 months.
- Patients older than 18 years should receive the adult dose of isoniazid: 5 mg/kg per day orally to a maximum daily dose of 300 mg. Children should receive 10 to 20 mg/kg per day orally to a maximum daily dose of 300 mg. Twice weekly therapy schedules are also available and commonly used for children who receive directly observed treatment in school.
- The adult dosage of rifampin (for patients >15 years) is 10 mg/kg per day orally to a maximum daily dose of 600 mg; the pediatric dose is 10 to 20 mg/kg per day orally, also to a maximum daily dose of 600 mg.
Patients taking isoniazid who are pregnant or breastfeeding or have diabetes, renal failure, alcoholism, malnutrition, HIV, or a seizure disorder should receive pyridoxine (vitamin B6) supplementation to aid in preventing peripheral neuropathy, in an adult oral dose of 25 to 50 mg/d or a pediatric oral dose of 6.25 mg/d. Additional information on treating latent TB is available at http://www.cdc.gov/tb/topic/treatment/ltbi.htm.
For patients with active TB, treatment is more complex, based on the patient’s overall health. Please refer to the CDC recommendation for the treatment of active TB (http://www.cdc.gov/tb/topic/treatment/tbdisease.htm) or contact your local TB control division.
Patients may receive TB treatment from either individual medical providers or city or state health departments, depending on local capacity. In our practice, we treat LTBI in adults. The Philadelphia Department of Public Health’s TB Control Program manages LTBI in children and all suspected cases of active TB. We recommend providing everyone treated for latent or active TB with documentation of treatment completion.
Diagnose and treat problematic parasites
Intestinal parasites are among the infections most often found in refugee populations.7,8,24-29 Common pathogens in untreated refugees are Ascaris lumbricoides, hookworm (Ancylostoma duodenale and Necator americanus), Schistosoma species, Strongyloides stercoralis, Trichuris trichiura, and Giardia lamblia.
Although sustained domestic transmission is unlikely, these parasites may cause growth delay, anemia, hyperinfestation syndrome and disseminated infection (A lumbricoides and S stercoralis), and increased cancer risk (Schistosoma hematobium).7 In the late 1990s, the CDC initiated empiric treatment before departure for the United States for A lumbricoides (albendazole), S stercoralis (ivermectin), Schistosoma species (praziquantel), and other parasites in certain refugee populations, which has decreased but not eliminated the threat.7
All refugees should be receiving appropriate predeparture treatment for parasitic infections. For newly arrived refugees who have received no predeparture therapy or incomplete therapy, the CDC recommends screening for parasites or providing presumptive treatment (TABLE 2).
TABLE 2
Empiric treatment of parasites
| Refugee region of origin | Organism | Adult therapy |
|---|---|---|
| Middle East, South Asia, Southeast Asia | Strongyloides stercoralis Other roundworms | Ivermectin 200 μg/kg/d orally for 2 days Albendazole 400 mg orally, 1 dose |
| Africa | Schistosoma species S stercoralis Other roundworms | Praziquantel 20 mg/kg orally, 2 doses Ivermectin 200 μg/kg/d orally for 2 days Albendazole 400 mg orally, 1 dose |
| Source: CDC. Immigrant and Refugee Health: Domestic Intestinal Parasite Guidelines. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/intestinal-parasites-domestic.html. Accessed November 19, 2012. | ||
The optimal screening regimen for parasites in refugee populations is controversial. Although most screening programs rely on one or more microscopic examinations of stool for ova and parasites, this test is expensive, requires special handling, depends on the reviewer’s expertise, and remains relatively insensitive. A comprehensive review of stool ova and parasites in high-risk populations concluded that the use of 2 independently collected stool samples improved sensitivity at acceptable cost.30
New, more sensitive and specific assays have been developed for many parasites, including Cryptosporidium parvum, Entamoeba histolytica, G lamblia, S stercoralis, and Schistosoma species, but we do not recommend these specialized tests unless the provider strongly suspects a specific parasite based on history and physical exam or persistent eosinophilia.
All refugees should have a complete blood count with differential to help identify occult parasitemia. Although a finding of eosinophilia may result from successful empiric therapy for an already-treated parasite, it must be followed up with more specific testing for S stercoralis, even in otherwise asymptomatic patients. African refugees with eosinophilia also should be tested for Schistosoma, and Somali Bantu should be treated empirically for both S stercoralis and Schistosoma.31 In line with CDC guidelines, ongoing failure to identify the cause of eosinophilia in a refugee should prompt referral to an infectious disease specialist and further work-up.
Three to 6 months after antibiotic treatment of any parasite, immunocompromised patients and those with suspected treatment failure should undergo a test of cure comprised of 2 stool ova and parasite studies and a follow-up CBC with differential.32
Screen for HIV
Since January 4, 2010, after HIV was removed from the Class A diagnosis list, refugees are no longer tested for HIV before arrival in the United States.11 Nevertheless, we recommend screening all refugees on arrival, regardless of age, for HIV types 1 and 2, unless they opt out, for the following reasons:
- approximately 14% of incoming refugees arrive from countries with an HIV prevalence of more than 5%33
- the increasing use of rape as a tool of torture and repression puts refugees at particular risk for HIV
- current CDC guidelines recommend HIV screening at the time of first encounter in all health care settings for everyone from 13 to 64 years of age and any patient who requests it.34
We also strongly recommend repeat screening 3 to 6 months after resettlement for refugees with recent potential exposure or who engage in high-risk activity.
Watch for ubiquitous hepatitis infection
In accordance with CDC vaccination guidelines and American Association of Pediatrics (AAP) Bright Futures recommendations, we endorse hepatitis A serology testing with reflex vaccination unless immunity is documented for refugees 1 to 18 years of age.35,36
A third of the world’s population shows serologic evidence of past infection with hepatitis B virus (HBV); high rates occur in Southeast Asia and sub-Saharan Africa, where most infections are transmitted perinatally.37,38 A study of Minnesota refugees found 7% to be positive for hepatitis B surface antigen (HBsAg), with a higher prevalence among refugees from sub-Saharan Africa.8
Most screening protocols for refugees test for HBsAg and antibody to hepatitis B surface antigen (HBsAb); it is reasonable to add a screen for antibody to hepatitis B core antigen (HBcAb). We recommend screening for HBV infection using HBsAg, HBsAb, and HBcAb to minimize underdiagnosis in this high-risk population. Refugees without immunity to HBV should be offered vaccination.18 Encourage immunization, especially for patients with hepatitis or cirrhosis from any cause.
Hepatitis C screening should follow CDC guidelines for the general population, focusing on high-risk groups such as injection drug users, victims of sexual violence, people with multiple sexual partners, recipients of blood transfusions, people with any other type of hepatitis, and one-time screening for individuals born between 1945 and 1965.39,40
Monitor for malaria
Many refugees come to the United States from areas where malaria is endemic.41 In 2007, the CDC instituted empiric treatment before arrival in the United States for all refugees from sub-Saharan Africa because the rapid test for malaria approved by the US Food and Drug Administration has low sensitivity and specificity,2 malarial vectors are present throughout much of the United States, and malaria (specifically Plasmodium falciparum) causes significant morbidity and mortality. If written confirmation of predeparture treatment is not available, refugees from sub-Saharan Africa should receive presumptive treatment, outlined in TABLE 3,42 as part of the initial DRME.
TABLE 3
Presumptive postarrival malaria treatment for refugees from sub-Saharan Africa42
| Directly observed treatment received in country of origin? | Recommended treatment* | |
|---|---|---|
| Children | Adults | |
| Yes | None | None |
| No | Atovaquone-proguanil (62.5/25 mg): 5-8 kg: 2 tablets per day for 3 days 9-10 kg: 3 tablets per day for 3 days Atovaquone-proguanil (250/100 mg): 11-20 kg: 1 tablet per day for 3 days 21-30 kg: 2 tablets per day for 3 days 31-40 kg: 3 tablets per day for 3 days >40 kg: 4 tablets per day for 3 days | Atovaquone-proguanil (250/100 mg): 4 tablets per day for 3 days |
| *Do not presumptively treat pregnant or lactating women or children weighing <5 kg. An infectious disease consult is recommended for these patients. | ||
Based on our experience and expert opinion, we recommend routinely monitoring all refugees from endemic areas for symptoms of malarial disease during the initial 3 months after resettlement. Relapsing fevers, unexplained malaise or fatigue, pallor, thrombocytopenia, or splenomegaly should trigger additional testing with thick- and thin-blood smears for trophozoites (3 separate samples drawn at 12- to 24-hour intervals).
Be alert for malnutrition
Acute and chronic malnutrition, as well as micronutrient deficiencies, have been noted in refugees coming from refugee camps. A survey of Bhutanese refugees in a camp in Nepal found that 25.1% of children were underweight and 4.8% of them were severely underweight. Moreover, 43.3% of children had anemia.43 Recognizing that refugees may be at high risk for iron deficiency, we recommend evaluating children and adolescents for this deficit according to AAP guidelines.44
We also recommend screening body mass index (BMI) to identify refugees at risk. Height, weight, and BMI must be followed over time to ensure appropriate acclimation to the US diet.
Also consider vitamin D deficiency and rickets in refugee populations, particularly people with darker skin and women who wear veils.45,46 Based on our experiences and CDC guidelines, we recommend a multivitamin with iron for children 6 to 59 months of age.12
Check lead levels in children
Refugee children are at risk of elevated blood lead levels (>10 ’g/dL) resulting from pre-departure environmental exposure and iron deficiency anemia, which can enhance absorption of lead. Refugees also are more likely to resettle in poor neighborhoods with substandard housing, increasing their risk of domestic lead exposure.
Studies of refugee children at initial screening have shown prevalences of elevated blood lead levels of 6.3% in a Cuban refugee population in Miami and higher rates (11%-22%) in mixed refugee populations in Massachusetts.6,47 A study in New Hampshire found that approximately 30% of refugee children with normal lead levels on initial screen had elevated levels when checked several months later.48
Consistent with CDC guidelines,49 our experience, and the findings of the State of Minnesota,50 we recommend checking blood lead levels in all children 6 months to 16 years of age upon arrival in the United States and repeat lead testing 3 to 6 months after placement in a permanent residence.
Bring vaccinations up to date
US law requires anyone seeking an immigrant visa to show proof of vaccination against vaccine-preventable diseases, as recommended by the US Advisory Committee on Immunization Practices.51 Vaccination requirements that apply to other immigrant groups do not apply to refugees at the time of their initial admission to the United States, but refugees must be vaccinated when they seek a green card or permanent US residence.
All refugees are eligible for adjustment of status after they have lived in the United States for a year and need proof of vaccination to apply.51 Moreover, schools may bar refugee children from attending if their vaccinations are not up-to-date, which, in turn, may hinder their parents’ ability to find employment. CDC guidelines for vaccinating immigrants and refugees applying for permanent residence are available at http://www.cdc.gov/immigrantrefugeehealth/pdf/2009-vaccination-technical-instructions.pdf (see the table on page 12).52 Because of the large number of vaccinations required for children and even many adults, health care providers should be familiar with the CDC’s recommended immunization and catch-up schedules.35
Vaccinations given in other countries are acceptable if appropriately recorded in Institute of Medicine documentation, or if original vaccination records are available and the vaccinations conform to appropriate intervals and age guidelines. Refugees must bring their records with them to medical appointments. Laboratory evidence of immunity is acceptable for measles, mumps, rubella (MMR), hepatitis A, hepatitis B, polio, and varicella, but there is debate about whether such testing should be performed before immunization.18,53 Health care providers need to assess each patient based on age and risk factors to decide whether immunity testing is appropriate.
In our practice, we routinely test all adults for immunity to varicella, hepatitis A, hepatitis B, and MMR. For children, we rely on documented immunization records, not antibody titers, for evidence of previous vaccination.
Pay attention to mental health issues
Many refugees have been exposed to trauma, often including war and torture, increasing their risk for mental illness. A large 2005 review found that serious mental disorders, including post-traumatic stress disorder (PTSD), major depressive disorder, and generalized anxiety disorder are significantly more prevalent among refugees than the general population.5 Many screening tests for PTSD have been proposed54 but have not been validated in all immigrant or refugee populations.55
Mental health care for refugees is complicated by language and cultural barriers, adjustment disorders, access to psychiatric services, and uncertainty about effective treatments in refugee populations. Despite the higher prevalence of mental illness among refugees, many in the mental health field have raised concerns about the applicability of Western concepts of mental health, including PTSD, in this group.56
Refugees who are victims of torture should be referred to experienced mental health practitioners. After ruling out acute psychosis and destructive behaviors, we recommend postponing an exhaustive mental health screening until several months after arrival. In our medical home model, we evaluate patients on an ongoing basis, giving us an opportunity to identify emerging or worsening mental health conditions.
Evaluate dental health
The incidence of dental caries and periodontal disease among refugees varies widely among different groups of refugees. Data on pediatric refugees in the United States have shown dental caries to be common, with prevalences between 16.7% and 42%, with marked differences based on region of origin.3,57,58 In our practice, we also have noted heavy use of betel nut in the Southeast Asian community, leading to significant dental disease.
All refugees should have their dentition evaluated at the initial DRME. We recommend subsequent formal dental examination for all patients, giving priority to those with clear evidence of active disease.
Identify and address chronic disease
Refugees carry a substantial burden of chronic disease, although marked regional variation has been noted.4 A study of Massachusetts refugees from 2001 through 2005 demonstrated that 46.8% were overweight or obese, 22.6% had hypertension, and 3.1% had diabetes. Smoking is also highly prevalent in refugee populations.59
Our findings confirm high rates of chronic disease, particularly among Iraqi and geriatric refugees. These patients require close follow-up after the DRME to minimize sequelae from chronic conditions. Multi-disciplinary teams in the patient-centered medical home may provide an opportunity to promptly address chronic health conditions that can have severe short-term consequences if not adequately managed (eg, insulin dosage adjustment based on diet in patients with diabetes).
We recommend a comprehensive medical history and evaluation for chronic disease, including diabetes and hypertension, at the DRME and on an ongoing basis. Although many refugees have never had any health screening and substantial cultural barriers may exist, especially with regard to women’s health and age-based cancer screening, refugees generally should receive the same preventive care as the rest of the US population until further research has been done in this area.
We recommend introducing age-based cancer screening and other preventive care for refugees within 2 months of their initial visit. This model of care has already been endorsed by the Minnesota Department of Health’s Refugee Health Program, one of the leading health care providers for refugees in the United States.60
Toward better care models
The medical care of refugees is complex, but the prepared primary care provider can manage it effectively. TABLE 4 summarizes our recommendations for the DRME based on our experiences and the available literature. Standardized screening guidelines and comprehensive programs, perhaps incorporating the concept of the patient-centered medical home, will likely improve both the initial and continuing care of this population.
TABLE 4
Summary recommendations for the domestic refugee medical exam
History
|
| Physical exam In addition to the essential components of the physical exam, pay attention to:
|
Initial laboratory evaluation
|
| Ongoing care Include:
|
| CBC, complete blood count; HIV, human immunode"ciency virus; IGRA, interferon-gamma release assay; MMR, measles, mumps, rubella; TST, tuberculin skin testing. Adapted from: Centers for Disease Control and Prevention. Immigrant and Refugee Health: Guidelines for the US Domestic Medical Examination for Newly Arriving Refugees. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/domestic-guidelines.html. Accessed November 19, 2012. |
Ongoing study is essential to better address the health care needs of refugees. Although they comprise only a small segment of immigrants living in the United States, the experience of caring for them may help develop models to provide better care to other foreign-born patients.
CORRESPONDENCE Marc Altshuler, MD, Department of Family and Community Medicine, Jefferson Medical College, Thomas Jefferson University, 833 Chestnut Street, Suite 301, Philadelphia, PA 19107; [email protected]
• Use tuberculin skin testing alone or in conjunction with interferon-gamma release assay to screen children younger than 5 years for tuberculosis. A
• Include 2 evaluations for ova and parasites plus a complete blood count with differential when screening refugees for parasitic infections. B
• Screen all adolescent and adult refugees for human immunodeficiency virus infection. A
• Check blood lead levels in all children 6 months to 16 years of age on arrival in the United States B and 6 months later. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
In 2011, 56,384 refugees fleeing persecution in their native countries were admitted to the United States. The largest numbers came from Burma (30.1%), Bhutan (26.6%), and Iraq (16.7%).1 They joined the more than 3 million refugees from all over the world who have resettled in this country since 1975. 1
Refugees arrive in the United States with complex medical issues, including illnesses rarely seen here, mental health concerns, and chronic conditions such as diabetes and hypertension. After arrival, they undergo a domestic refugee medical examination (DRME). This DRME, along with well-planned follow-up, can go a long way toward helping refugees show the proof of vaccination and control of chronic health conditions that are required when they apply for lawful permanent resident status.
The Centers for Disease Control and Prevention (CDC) has published guidelines to help with medical decision making and screening of refugees, but limited information is available on the necessary strategies to address chronic health conditions within the context of the DRME.2 Moreover, differences in refugee experience and health status based on country of origin may demand more detailed, region-specific guidelines.3-9 No standard recommendations address the importance of providing not just initial screening, but comprehensive longitudinal care, as well.
Since 2007, our outpatient practice (MA, KS, GM, PM) has performed the DRME and provided ongoing care for more than 900 refugees resettled in Philadelphia. The practice, which is associated with an urban academic medical center and closely coordinates refugee care with a local resettlement agency, has earned recognition as a Level 3 (top-level certification) patient-centered medical home by the National Committee on Quality Assurance. We offer here a framework for providing comprehensive care to refugees, based on CDC guidelines, available evidence, and our experience.
Prelude: The overseas medical exam
All refugees must undergo an overseas medical examination (OME) no longer than 12 months before resettlement in the United States. Physicians selected by US Department of State consular officials perform the examinations.
The OME includes a medical history, physical examination, and testing to screen for mental illness, drug abuse, syphilis, leprosy, and tuberculosis (TB). Some vaccinations and empiric treatment for parasites also may be provided at the time of the examination.10-12
The OME screens for Class A disorders, which render a refugee ineligible for admission to the United States until treated or stabilized, and Class B conditions, which require close follow-up on arrival (TABLE 1).12 Despite recent steps toward standardization, the quality and thoroughness of OMEs completed at different examination sites still vary substantially.
TABLE 1
Overseas medical examination: Class A and B conditions12
| Class A* | Class B† |
|---|---|
| Active or infectious tuberculosis Untreated STI: syphilis, gonorrhea, chancroid, granuloma inguinale, or lymphogranuloma venereum Hansen’s disease (leprosy) Drug or alcohol addiction/abuse Mental illness with harmful behavior | Inactive or noninfectious tuberculosis Treated STI Treated or paucibacillary Hansen’s disease Sustained remission from drug or alcohol addiction or abuse Well-controlled mental illness Pregnancy |
| STI, sexually transmitted infection. *Class A disorders render a refugee ineligible for admission to the United States until he or she is treated or stabilized. †Class B disorders require close follow-up upon the refugee’s arrival in the United States | |
Arrival in United States is followed by DRME
When refugees arrive in the United States, they are advised to undergo a DRME, which any licensed practitioner may perform, preferably within 90 days. More rapid evaluation is encouraged for medically complex refugees or refugees arriving with Class A or B conditions. Because refugees are eligible for only 8 months of medical assistance, we strongly recommend that the DRME be done promptly.
The CDC publishes guidelines for components of the initial DRME, but state requirements and individual examinations vary widely.2,10,13,14 We outline here the elements of the exam identified by the CDC, supplemented with recommendations based on published evidence and our experiences in caring for refugees.
Screen for tuberculosis
Refugees have a higher prevalence of latent tuberculosis infection (LTBI) and active TB than the general US population. An estimated one-third of the world’s population has LTBI.15 Since 2002, more than 50% of all people diagnosed with TB in the United States have been born outside the country.16
Although otherwise healthy adults with LTBI have a lifetime risk of approximately 10% that it will progress to active TB,17 infants, young children, and people coinfected with HIV have a rate of progression of around 10% per year. It is imperative, therefore, that all refugees be screened for TB and treated appropriately.8,18,19
Refugees are screened for active TB with a chest radiograph and possibly a sputum analysis during the OME. Because screening may take place as long as 12 months before arrival in the United States, refugees may be re-exposed to TB in the refugee camp before departure. They are not screened for LTBI before coming to the United States.11,12
Domestic screening for LTBI is complicated by routine use in many foreign countries of the Bacille Calmette-Guérin (BCG) vaccine, which can reduce the incidence of TB meningitis and disseminated TB in children, but does not protect adults against primary infection or reactivation of TB. Tuberculin skin testing using purified protein derivative, which has typically been used for screening, can render false-positive results, particularly in the context of previous BCG vaccination.
Interferon-gamma release assay (IGRA) is an alternative screening option that has been approved for use in the United States.15,20 Because the IGRA is a blood test, it eliminates interpretation errors associated with tuberculin skin testing and is not affected by BCG vaccination. IGRA testing also does not require an additional office visit.
For these reasons, we recommend screening all refugees older than 5 years with IGRAs, where available. In light of scant data and apparent differences in immune response in young children, the CDC recommends using tuberculin skin testing either alone or in conjunction with IGRA testing for all children younger than 5 years.20,21
Positive screening tests must be followed up with a chest radiograph. Perform serial sputum evaluation whenever the chest radiograph indicates potential active TB.
Everyone with latent or active TB must be treated according to CDC recommendations adapted from guidelines established by the American Thoracic Society and Infectious Diseases Society of America.22,23 For latent TB, the CDC calls for treatment with isoniazid for 9 months or rifampin for 4 months.
- Patients older than 18 years should receive the adult dose of isoniazid: 5 mg/kg per day orally to a maximum daily dose of 300 mg. Children should receive 10 to 20 mg/kg per day orally to a maximum daily dose of 300 mg. Twice weekly therapy schedules are also available and commonly used for children who receive directly observed treatment in school.
- The adult dosage of rifampin (for patients >15 years) is 10 mg/kg per day orally to a maximum daily dose of 600 mg; the pediatric dose is 10 to 20 mg/kg per day orally, also to a maximum daily dose of 600 mg.
Patients taking isoniazid who are pregnant or breastfeeding or have diabetes, renal failure, alcoholism, malnutrition, HIV, or a seizure disorder should receive pyridoxine (vitamin B6) supplementation to aid in preventing peripheral neuropathy, in an adult oral dose of 25 to 50 mg/d or a pediatric oral dose of 6.25 mg/d. Additional information on treating latent TB is available at http://www.cdc.gov/tb/topic/treatment/ltbi.htm.
For patients with active TB, treatment is more complex, based on the patient’s overall health. Please refer to the CDC recommendation for the treatment of active TB (http://www.cdc.gov/tb/topic/treatment/tbdisease.htm) or contact your local TB control division.
Patients may receive TB treatment from either individual medical providers or city or state health departments, depending on local capacity. In our practice, we treat LTBI in adults. The Philadelphia Department of Public Health’s TB Control Program manages LTBI in children and all suspected cases of active TB. We recommend providing everyone treated for latent or active TB with documentation of treatment completion.
Diagnose and treat problematic parasites
Intestinal parasites are among the infections most often found in refugee populations.7,8,24-29 Common pathogens in untreated refugees are Ascaris lumbricoides, hookworm (Ancylostoma duodenale and Necator americanus), Schistosoma species, Strongyloides stercoralis, Trichuris trichiura, and Giardia lamblia.
Although sustained domestic transmission is unlikely, these parasites may cause growth delay, anemia, hyperinfestation syndrome and disseminated infection (A lumbricoides and S stercoralis), and increased cancer risk (Schistosoma hematobium).7 In the late 1990s, the CDC initiated empiric treatment before departure for the United States for A lumbricoides (albendazole), S stercoralis (ivermectin), Schistosoma species (praziquantel), and other parasites in certain refugee populations, which has decreased but not eliminated the threat.7
All refugees should be receiving appropriate predeparture treatment for parasitic infections. For newly arrived refugees who have received no predeparture therapy or incomplete therapy, the CDC recommends screening for parasites or providing presumptive treatment (TABLE 2).
TABLE 2
Empiric treatment of parasites
| Refugee region of origin | Organism | Adult therapy |
|---|---|---|
| Middle East, South Asia, Southeast Asia | Strongyloides stercoralis Other roundworms | Ivermectin 200 μg/kg/d orally for 2 days Albendazole 400 mg orally, 1 dose |
| Africa | Schistosoma species S stercoralis Other roundworms | Praziquantel 20 mg/kg orally, 2 doses Ivermectin 200 μg/kg/d orally for 2 days Albendazole 400 mg orally, 1 dose |
| Source: CDC. Immigrant and Refugee Health: Domestic Intestinal Parasite Guidelines. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/intestinal-parasites-domestic.html. Accessed November 19, 2012. | ||
The optimal screening regimen for parasites in refugee populations is controversial. Although most screening programs rely on one or more microscopic examinations of stool for ova and parasites, this test is expensive, requires special handling, depends on the reviewer’s expertise, and remains relatively insensitive. A comprehensive review of stool ova and parasites in high-risk populations concluded that the use of 2 independently collected stool samples improved sensitivity at acceptable cost.30
New, more sensitive and specific assays have been developed for many parasites, including Cryptosporidium parvum, Entamoeba histolytica, G lamblia, S stercoralis, and Schistosoma species, but we do not recommend these specialized tests unless the provider strongly suspects a specific parasite based on history and physical exam or persistent eosinophilia.
All refugees should have a complete blood count with differential to help identify occult parasitemia. Although a finding of eosinophilia may result from successful empiric therapy for an already-treated parasite, it must be followed up with more specific testing for S stercoralis, even in otherwise asymptomatic patients. African refugees with eosinophilia also should be tested for Schistosoma, and Somali Bantu should be treated empirically for both S stercoralis and Schistosoma.31 In line with CDC guidelines, ongoing failure to identify the cause of eosinophilia in a refugee should prompt referral to an infectious disease specialist and further work-up.
Three to 6 months after antibiotic treatment of any parasite, immunocompromised patients and those with suspected treatment failure should undergo a test of cure comprised of 2 stool ova and parasite studies and a follow-up CBC with differential.32
Screen for HIV
Since January 4, 2010, after HIV was removed from the Class A diagnosis list, refugees are no longer tested for HIV before arrival in the United States.11 Nevertheless, we recommend screening all refugees on arrival, regardless of age, for HIV types 1 and 2, unless they opt out, for the following reasons:
- approximately 14% of incoming refugees arrive from countries with an HIV prevalence of more than 5%33
- the increasing use of rape as a tool of torture and repression puts refugees at particular risk for HIV
- current CDC guidelines recommend HIV screening at the time of first encounter in all health care settings for everyone from 13 to 64 years of age and any patient who requests it.34
We also strongly recommend repeat screening 3 to 6 months after resettlement for refugees with recent potential exposure or who engage in high-risk activity.
Watch for ubiquitous hepatitis infection
In accordance with CDC vaccination guidelines and American Association of Pediatrics (AAP) Bright Futures recommendations, we endorse hepatitis A serology testing with reflex vaccination unless immunity is documented for refugees 1 to 18 years of age.35,36
A third of the world’s population shows serologic evidence of past infection with hepatitis B virus (HBV); high rates occur in Southeast Asia and sub-Saharan Africa, where most infections are transmitted perinatally.37,38 A study of Minnesota refugees found 7% to be positive for hepatitis B surface antigen (HBsAg), with a higher prevalence among refugees from sub-Saharan Africa.8
Most screening protocols for refugees test for HBsAg and antibody to hepatitis B surface antigen (HBsAb); it is reasonable to add a screen for antibody to hepatitis B core antigen (HBcAb). We recommend screening for HBV infection using HBsAg, HBsAb, and HBcAb to minimize underdiagnosis in this high-risk population. Refugees without immunity to HBV should be offered vaccination.18 Encourage immunization, especially for patients with hepatitis or cirrhosis from any cause.
Hepatitis C screening should follow CDC guidelines for the general population, focusing on high-risk groups such as injection drug users, victims of sexual violence, people with multiple sexual partners, recipients of blood transfusions, people with any other type of hepatitis, and one-time screening for individuals born between 1945 and 1965.39,40
Monitor for malaria
Many refugees come to the United States from areas where malaria is endemic.41 In 2007, the CDC instituted empiric treatment before arrival in the United States for all refugees from sub-Saharan Africa because the rapid test for malaria approved by the US Food and Drug Administration has low sensitivity and specificity,2 malarial vectors are present throughout much of the United States, and malaria (specifically Plasmodium falciparum) causes significant morbidity and mortality. If written confirmation of predeparture treatment is not available, refugees from sub-Saharan Africa should receive presumptive treatment, outlined in TABLE 3,42 as part of the initial DRME.
TABLE 3
Presumptive postarrival malaria treatment for refugees from sub-Saharan Africa42
| Directly observed treatment received in country of origin? | Recommended treatment* | |
|---|---|---|
| Children | Adults | |
| Yes | None | None |
| No | Atovaquone-proguanil (62.5/25 mg): 5-8 kg: 2 tablets per day for 3 days 9-10 kg: 3 tablets per day for 3 days Atovaquone-proguanil (250/100 mg): 11-20 kg: 1 tablet per day for 3 days 21-30 kg: 2 tablets per day for 3 days 31-40 kg: 3 tablets per day for 3 days >40 kg: 4 tablets per day for 3 days | Atovaquone-proguanil (250/100 mg): 4 tablets per day for 3 days |
| *Do not presumptively treat pregnant or lactating women or children weighing <5 kg. An infectious disease consult is recommended for these patients. | ||
Based on our experience and expert opinion, we recommend routinely monitoring all refugees from endemic areas for symptoms of malarial disease during the initial 3 months after resettlement. Relapsing fevers, unexplained malaise or fatigue, pallor, thrombocytopenia, or splenomegaly should trigger additional testing with thick- and thin-blood smears for trophozoites (3 separate samples drawn at 12- to 24-hour intervals).
Be alert for malnutrition
Acute and chronic malnutrition, as well as micronutrient deficiencies, have been noted in refugees coming from refugee camps. A survey of Bhutanese refugees in a camp in Nepal found that 25.1% of children were underweight and 4.8% of them were severely underweight. Moreover, 43.3% of children had anemia.43 Recognizing that refugees may be at high risk for iron deficiency, we recommend evaluating children and adolescents for this deficit according to AAP guidelines.44
We also recommend screening body mass index (BMI) to identify refugees at risk. Height, weight, and BMI must be followed over time to ensure appropriate acclimation to the US diet.
Also consider vitamin D deficiency and rickets in refugee populations, particularly people with darker skin and women who wear veils.45,46 Based on our experiences and CDC guidelines, we recommend a multivitamin with iron for children 6 to 59 months of age.12
Check lead levels in children
Refugee children are at risk of elevated blood lead levels (>10 ’g/dL) resulting from pre-departure environmental exposure and iron deficiency anemia, which can enhance absorption of lead. Refugees also are more likely to resettle in poor neighborhoods with substandard housing, increasing their risk of domestic lead exposure.
Studies of refugee children at initial screening have shown prevalences of elevated blood lead levels of 6.3% in a Cuban refugee population in Miami and higher rates (11%-22%) in mixed refugee populations in Massachusetts.6,47 A study in New Hampshire found that approximately 30% of refugee children with normal lead levels on initial screen had elevated levels when checked several months later.48
Consistent with CDC guidelines,49 our experience, and the findings of the State of Minnesota,50 we recommend checking blood lead levels in all children 6 months to 16 years of age upon arrival in the United States and repeat lead testing 3 to 6 months after placement in a permanent residence.
Bring vaccinations up to date
US law requires anyone seeking an immigrant visa to show proof of vaccination against vaccine-preventable diseases, as recommended by the US Advisory Committee on Immunization Practices.51 Vaccination requirements that apply to other immigrant groups do not apply to refugees at the time of their initial admission to the United States, but refugees must be vaccinated when they seek a green card or permanent US residence.
All refugees are eligible for adjustment of status after they have lived in the United States for a year and need proof of vaccination to apply.51 Moreover, schools may bar refugee children from attending if their vaccinations are not up-to-date, which, in turn, may hinder their parents’ ability to find employment. CDC guidelines for vaccinating immigrants and refugees applying for permanent residence are available at http://www.cdc.gov/immigrantrefugeehealth/pdf/2009-vaccination-technical-instructions.pdf (see the table on page 12).52 Because of the large number of vaccinations required for children and even many adults, health care providers should be familiar with the CDC’s recommended immunization and catch-up schedules.35
Vaccinations given in other countries are acceptable if appropriately recorded in Institute of Medicine documentation, or if original vaccination records are available and the vaccinations conform to appropriate intervals and age guidelines. Refugees must bring their records with them to medical appointments. Laboratory evidence of immunity is acceptable for measles, mumps, rubella (MMR), hepatitis A, hepatitis B, polio, and varicella, but there is debate about whether such testing should be performed before immunization.18,53 Health care providers need to assess each patient based on age and risk factors to decide whether immunity testing is appropriate.
In our practice, we routinely test all adults for immunity to varicella, hepatitis A, hepatitis B, and MMR. For children, we rely on documented immunization records, not antibody titers, for evidence of previous vaccination.
Pay attention to mental health issues
Many refugees have been exposed to trauma, often including war and torture, increasing their risk for mental illness. A large 2005 review found that serious mental disorders, including post-traumatic stress disorder (PTSD), major depressive disorder, and generalized anxiety disorder are significantly more prevalent among refugees than the general population.5 Many screening tests for PTSD have been proposed54 but have not been validated in all immigrant or refugee populations.55
Mental health care for refugees is complicated by language and cultural barriers, adjustment disorders, access to psychiatric services, and uncertainty about effective treatments in refugee populations. Despite the higher prevalence of mental illness among refugees, many in the mental health field have raised concerns about the applicability of Western concepts of mental health, including PTSD, in this group.56
Refugees who are victims of torture should be referred to experienced mental health practitioners. After ruling out acute psychosis and destructive behaviors, we recommend postponing an exhaustive mental health screening until several months after arrival. In our medical home model, we evaluate patients on an ongoing basis, giving us an opportunity to identify emerging or worsening mental health conditions.
Evaluate dental health
The incidence of dental caries and periodontal disease among refugees varies widely among different groups of refugees. Data on pediatric refugees in the United States have shown dental caries to be common, with prevalences between 16.7% and 42%, with marked differences based on region of origin.3,57,58 In our practice, we also have noted heavy use of betel nut in the Southeast Asian community, leading to significant dental disease.
All refugees should have their dentition evaluated at the initial DRME. We recommend subsequent formal dental examination for all patients, giving priority to those with clear evidence of active disease.
Identify and address chronic disease
Refugees carry a substantial burden of chronic disease, although marked regional variation has been noted.4 A study of Massachusetts refugees from 2001 through 2005 demonstrated that 46.8% were overweight or obese, 22.6% had hypertension, and 3.1% had diabetes. Smoking is also highly prevalent in refugee populations.59
Our findings confirm high rates of chronic disease, particularly among Iraqi and geriatric refugees. These patients require close follow-up after the DRME to minimize sequelae from chronic conditions. Multi-disciplinary teams in the patient-centered medical home may provide an opportunity to promptly address chronic health conditions that can have severe short-term consequences if not adequately managed (eg, insulin dosage adjustment based on diet in patients with diabetes).
We recommend a comprehensive medical history and evaluation for chronic disease, including diabetes and hypertension, at the DRME and on an ongoing basis. Although many refugees have never had any health screening and substantial cultural barriers may exist, especially with regard to women’s health and age-based cancer screening, refugees generally should receive the same preventive care as the rest of the US population until further research has been done in this area.
We recommend introducing age-based cancer screening and other preventive care for refugees within 2 months of their initial visit. This model of care has already been endorsed by the Minnesota Department of Health’s Refugee Health Program, one of the leading health care providers for refugees in the United States.60
Toward better care models
The medical care of refugees is complex, but the prepared primary care provider can manage it effectively. TABLE 4 summarizes our recommendations for the DRME based on our experiences and the available literature. Standardized screening guidelines and comprehensive programs, perhaps incorporating the concept of the patient-centered medical home, will likely improve both the initial and continuing care of this population.
TABLE 4
Summary recommendations for the domestic refugee medical exam
History
|
| Physical exam In addition to the essential components of the physical exam, pay attention to:
|
Initial laboratory evaluation
|
| Ongoing care Include:
|
| CBC, complete blood count; HIV, human immunode"ciency virus; IGRA, interferon-gamma release assay; MMR, measles, mumps, rubella; TST, tuberculin skin testing. Adapted from: Centers for Disease Control and Prevention. Immigrant and Refugee Health: Guidelines for the US Domestic Medical Examination for Newly Arriving Refugees. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/domestic-guidelines.html. Accessed November 19, 2012. |
Ongoing study is essential to better address the health care needs of refugees. Although they comprise only a small segment of immigrants living in the United States, the experience of caring for them may help develop models to provide better care to other foreign-born patients.
CORRESPONDENCE Marc Altshuler, MD, Department of Family and Community Medicine, Jefferson Medical College, Thomas Jefferson University, 833 Chestnut Street, Suite 301, Philadelphia, PA 19107; [email protected]
1. Martin D, Yankay J. Refugees and Asylees: 2011. Annual Flow Report. Offce of Immigration Statistics, US Department of Home-land Security. May 2012. Available at: http://www.dhs.gov/xlibrary/assets/statistics/publications/ois_rfa_fr_2011.pdf. Accessed October 29, 2012.
2. Stauffer WM, Kamat D, Walker PF. Screening of international immigrants, refugees and adoptees. Prim Care. 2002;29:879-905.
3. Cote S, Geltman P, Nunn M, et al. Dental caries of refugee children compared with US children. Pediatrics. 2004;114:e733-e740.
4. Geltman PL, Dookeran NM, Battaglia T, et al. Chronic disease and its risk factors among refugees and asylees in Massachusetts, 2001-2005. Prev Chronic Dis. 2010;7:A51.-
5. Fazel M, Wheeler J, Danesh J. Prevalence of serious mental disorder in 7000 refugees resettled in western countries: a systematic review. Lancet. 2005;365:1309-1314.
6. Geltman PL, Brown MJ, Cochran J. Lead poisoning among refugee children resettled in Massachusetts, 1995 to 1999. Pediatrics. 2001;108:158-162.
7. Geltman PL, Cochran J, Hedgecock C. Intestinal parasites among African refugees resettled in Massachusetts and the impact of an overseas pre-departure treatment program. Am J Trop Med Hyg. 2003;69:657.-
8. Lifson AR, Thai D, O’Fallon A, et al. Prevalence of tuberculosis, hepatitis B virus, and intestinal parasitic infections among refugees to Minnesota. Public Health Rep. 2002;117:69-77.
9. Power DV, Moody E, Trussell K, et al. Caring for the Karen. A newly arrived refugee group. Minn Med. 2010;93:49-53.
10. Centers for Disease Control and Prevention. Health considerations of newly arrived immigrants and refugees. In: Centers for Disease Control and Prevention. Travelers’ Health—Yellow Book. Chapt. 9. Available at: http://wwwnc.cdc.gov/travel/yellow-book/2010/table-of-contents.aspx#20. Accessed November 15, 2012.
11. Centers for Disease Control and Prevention. Medical Examination of Immigrants and Refugees. Available at: http://www.cdc.gov/immigrantrefugeehealth/exams/medical-examination.html. Accessed November 15, 2012.
12. Centers for Disease Control and Prevention. Technical Instructions For The Medical History and Physical Examination of Aliens in the United States. Available at: http://www.cdc.gov/immigrantrefugeehealth/exams/ti/civil/technical-instructions/civil-surgeons/medical-history-physical-examination.html. Accessed November 15, 2010.
13. Seybolt L, Barnett E, Stauffer W. US medical screening for immigrants and refugees: clinical issues. In: Walker P, Barnett E, eds. Immigrant Medicine. Phildelphia, PA: Saunders Elsevier; 2007:135-150.
14. United States Department of Health and Human Services, Offce of Refugee Resettlement. ORR State Letter: Revised Medical Screening Guidelines for Newly Arrived Refugees. Available at: http://www.acf.hhs.gov/sites/default/files/orr/state_letter_12_09_revised_medical_screening_guidelines_for_newly.pdf. Accessed October 29, 2012.
15. Centers for Disease Control and Prevention. Guidelines for the investigation of contacts of persons with infectious tuberculosis— guidelines for using the QuantiFERON-TB gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep. 2005;54(RR-15):1-55.
16. Centers for Disease Control and Prevention. Executive Commentary: Highlights of 2011 Report. Available at: http://www.cdc.gov/tb/statistics/reports/2011/pdf/ExecutiveCommentary.pdf. Accessed November 19, 2012.
17. Kuma V, Abbas AK, Fausto N, et al. Robbins Basic Pathology. 8th ed. Philadelphia, Pa: Saunders Elsevier; 2007:516–522.
18. Barnett ED. Infectious disease screening for refugees resettled in the United States. Clin Infect Dis. 2004;39:833-841.
19. DeRiemer K, Chin DP, Schecter DF, et al. Tuberculosis among immigrants and refugees. Arch Intern Med. 1998;158:753-760.
20. Centers for Disease Control and Prevention. Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recomm Rec. 2010;59(RR-5):1-25.
21. Bright Futures at Georgetown University. Health Supervision— Laboratory Tests: Tuberculosis (TB) Screening. Available at: http://www.brightfutures.org/pocket/pdf/30_37.pdf. Accessed November 19, 2012.
22. Centers for Disease Control and Prevention. Treatment of tuberculosis. American Thoracic Society, CDC, Infectious Diseases Society of America. MMWR Recomm Rep 2003;52(RR-11):1-77.
23. Centers for Disease Control and Prevention. Update: adverse event data and revised American Thoracic Society/CDC recommendations against the use of rifampin and pyrazinamide for treament of latent tuberculosis infection—United States. MMWR Morb Mortal Wkly Rep. 2003;52:735-739.
24. Caruana SR, Kelly HA, Ngeow JY, et al. Undiagnosed and potentially lethal parasite infections among immigrants and refugees in Australia. J Travel Med. 2006;13:233-239.
25. Dawson-Hahn EE, Greenberg SL, Domachowske JB, et al. Eosinophilia and the seroprevalence of schistosomiasis and strongyloidiasis in newly arrived pediatric refugees: an examination of Centers for Disease Control and Prevention screening guidelines. J Pediatr. 2010;156:1016-1018.
26. Garg PK, Perry S, Dorn M, et al. Risk of intestinal helminth and protozoan infection in a refugee population. Am J Trop Med Hyg. 2005;73:386-391.
27. Parenti DM, Lucas D, Lee A, et al. Health status of Ethiopian refugees in the United States. Am J Public Health. 1987;77:1542-1543.
28. Parish R. Intestinal parasites in Southeast Asian refugee children. West J Med. 1985;143:47-49.
29. Sutherland JE, Avant RF, Franz WB, 3rd, et al. Indochinese refugee health assessment and treatment. J Fam Pract. 1983;16:61-67.
30. Cartwright C. Utility of multiple-stool-specimen ova and parasite examinations in a high-prevalence setting. J Clin Microbiol. 1999;37:2408-2411.
31. Centers for Disease Control and Prevention. Recommendations for Presumptive Treatment of Schistosomiasis and Strongyloidiasis Among the Somali Bantu Refugees. June 13, 2005. Available at: http://archive.acf.hhs.gov/programs/orr/policy/sl05-18attach-ment2.pdf. Accessed November 19, 2012.
32. Centers for Disease Control and Prevention. Division of Global Migration and Quarantine. Guidelines for Evaluation of Refugees for Intestinal and Tissue-Invasive Parasitic Infections during Domestic Medical Examination. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/intestinal-parasites-domestic.html. Accessed October 30, 2012.
33. Centers for Disease Control and Prevention. Screening for HIV Infection During the Refugee Domestic Medical Examination. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/screening-hiv-infection-domestic.html. Accessed November 15, 2012.
34. Centers for Disease Control and Prevention. Revised recommendations for HIV testing of adults, adolescents and pregnant women in healthcare settings. MMWR Recomm Rep. 2006;55(RR-14):1-17.
35. Centers for Disease Control and Prevention National Immunization Program. Available at: http://www.cdc.gov/vaccines/vpdvac/hepa/default.htm. Accessed November 19, 2012.
36. American Academy of Pediatrics. Red Book. Available at: http://www2.aap.org/immunization/illnesses/hepb/hepa.html. Accessed November 19, 2012.
37. Lai CL, Ratziu V, Yuen MF, et al. Viral hepatitis B. Lancet. 2003;362:2089-2094.
38. Lin K, Kirchner J. Hepatitis B. Am Fam Phyician. 2004;69:75-82.
39. Ghany MG, Strader DB, Thomas DL, et al. American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374.
40. Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Morb Mortal Wkly Rep. 2012;61(RR-4):1-31.
41. Rowland M, Nosten F. Malaria epidemiology and control in refugee camps and complex emergencies. Ann Trop Med Parasitol. 2001;95:741-754.
42. Centers for Disease Control and Prevention, Malaria Branch, Division of Parasitic Diseases, Division of Global Migration and Quarantine and Malaria Branch. Presumptive Treatment of P falciparum Malaria in Refugees Relocating from sub-Saharan Africa to the United States. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/malaria-guidelines-domestic.html. Accessed November 15, 2012.
43. Centers for Disease Control and Prevention. Malnutrition and micronutrient deficiencies among Bhutanese refugee children— Nepal, 2007. MMWR Morb Mortal Wkly Rep. 2008;57:370-373.
44. American Academy of Pediatrics Bright Futures. Guidelines for Health Supervision of Infants, Children, and Adolescents— Theme 5: Promoting Healthy Nutrition. Available at: http://brightfutures.aap.org/pdfs/Guidelines_PDF/6-Promoting_Healthy_Nutrition.pdf. Accessed November 15, 2012.
45. Benson J, Skull S. Hiding from the sun: vitamin D deficiency in refugees. Aust Fam Physician. 2007;36:355-357.
46. Stellinga-Boelen A. Vitamin D levels in children of asylum seekers in The Netherlands in relation to season and dietary intake. Eur J Pediatr. 2007;166:201-206.
47. Trepka MJ, Pekovic V, Santana JC, et al. Risk factors for lead poisoning among Cuban refugee children. Public Health Rep. 2005;120:179-185.
48. Centers for Disease Control and Prevention. Elevated blood lead levels in refugee children, New Hampshire, 2003-2004. MMWR Morb Mortal Wkly Rep. 2005;54:42-46.
49. Centers for Disease Control and Prevention. Screening for Lead at the Domestic Refugee Medical Exam. Available at: http://www.cdc.gov/immigrantrefugeehealth/pdf/lead.pdf. Accessed November 15, 2012.
50. Zabel EW, Smith ME, O’Fallon A. Implementation of CDC refugee blood testing guidelines in Minnesota. Public Health Rep. 2008;123:111-125.
51. United States Citizenship and Immigration Services. Available at: http://www.uscis.gov/portal/site/uscis/menuitem.5af9bb95919f35e66f614176543f6d1a/?vgnextoid=3384cc5222$5210VgnVCM100000082ca60aRCRD&vgnextchannel=6abe6d26d17df110VgnVCM1000004718190aRCRD. Accessed November 15, 2012.
52. Centers for Disease Control and Prevention. Vaccination Requirements for Adjustment of Status for US Permanent Residence: Technical Instructions for Civil Surgeons. December 14, 2009. Available at: http://www.cdc.gov/immigrantrefugeehealth/pdf/2009-vaccination-technical-instructions.pdf. Accessed November 15, 2012
53. Phillips C. Better primary healthcare for refugees: catch up immunisation. Aust Fam Physician. 2007;36:440-443.
54. Brewin C. Systematic review of screening instruments for adults at risk of PTSD. J Trauma Stress. 2005;18:53-62.
55. Crumlish N, O’Rourke K. A systematic review of treatments for post-traumatic stress disorder among refugees and asylum-seekers. J Nerv Ment Dis. 2010;198:237-251.
56. Watters C. Emerging paradigms in the mental health care of refugees. Soc Sci Med. 2001;53:1709-1718.
57. Hayes EB, Talbot SB, Matheson ES, et al. Health status of pediatric refugees in Portland, ME. Arch Pediatr Adolesc Med. 1998;152:564-568.
58. Meropol S. Health status of pediatric refugees in Buffalo, NY. Arch Pediatr Adolesc Med. 1995;149:887-892.
59. Barnes DM, Harrison C, Heneghan R. Health risk and promotion behaviors in refugee populations. J Health Care Poor Underserved. 2004;15:347-356.
60. Dicker S, Stauffer WM, Mamo B, et al. Initial refugee health assessments: new recommendations for Minnesota. Minn Med. 2010;93:45-48.
1. Martin D, Yankay J. Refugees and Asylees: 2011. Annual Flow Report. Offce of Immigration Statistics, US Department of Home-land Security. May 2012. Available at: http://www.dhs.gov/xlibrary/assets/statistics/publications/ois_rfa_fr_2011.pdf. Accessed October 29, 2012.
2. Stauffer WM, Kamat D, Walker PF. Screening of international immigrants, refugees and adoptees. Prim Care. 2002;29:879-905.
3. Cote S, Geltman P, Nunn M, et al. Dental caries of refugee children compared with US children. Pediatrics. 2004;114:e733-e740.
4. Geltman PL, Dookeran NM, Battaglia T, et al. Chronic disease and its risk factors among refugees and asylees in Massachusetts, 2001-2005. Prev Chronic Dis. 2010;7:A51.-
5. Fazel M, Wheeler J, Danesh J. Prevalence of serious mental disorder in 7000 refugees resettled in western countries: a systematic review. Lancet. 2005;365:1309-1314.
6. Geltman PL, Brown MJ, Cochran J. Lead poisoning among refugee children resettled in Massachusetts, 1995 to 1999. Pediatrics. 2001;108:158-162.
7. Geltman PL, Cochran J, Hedgecock C. Intestinal parasites among African refugees resettled in Massachusetts and the impact of an overseas pre-departure treatment program. Am J Trop Med Hyg. 2003;69:657.-
8. Lifson AR, Thai D, O’Fallon A, et al. Prevalence of tuberculosis, hepatitis B virus, and intestinal parasitic infections among refugees to Minnesota. Public Health Rep. 2002;117:69-77.
9. Power DV, Moody E, Trussell K, et al. Caring for the Karen. A newly arrived refugee group. Minn Med. 2010;93:49-53.
10. Centers for Disease Control and Prevention. Health considerations of newly arrived immigrants and refugees. In: Centers for Disease Control and Prevention. Travelers’ Health—Yellow Book. Chapt. 9. Available at: http://wwwnc.cdc.gov/travel/yellow-book/2010/table-of-contents.aspx#20. Accessed November 15, 2012.
11. Centers for Disease Control and Prevention. Medical Examination of Immigrants and Refugees. Available at: http://www.cdc.gov/immigrantrefugeehealth/exams/medical-examination.html. Accessed November 15, 2012.
12. Centers for Disease Control and Prevention. Technical Instructions For The Medical History and Physical Examination of Aliens in the United States. Available at: http://www.cdc.gov/immigrantrefugeehealth/exams/ti/civil/technical-instructions/civil-surgeons/medical-history-physical-examination.html. Accessed November 15, 2010.
13. Seybolt L, Barnett E, Stauffer W. US medical screening for immigrants and refugees: clinical issues. In: Walker P, Barnett E, eds. Immigrant Medicine. Phildelphia, PA: Saunders Elsevier; 2007:135-150.
14. United States Department of Health and Human Services, Offce of Refugee Resettlement. ORR State Letter: Revised Medical Screening Guidelines for Newly Arrived Refugees. Available at: http://www.acf.hhs.gov/sites/default/files/orr/state_letter_12_09_revised_medical_screening_guidelines_for_newly.pdf. Accessed October 29, 2012.
15. Centers for Disease Control and Prevention. Guidelines for the investigation of contacts of persons with infectious tuberculosis— guidelines for using the QuantiFERON-TB gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep. 2005;54(RR-15):1-55.
16. Centers for Disease Control and Prevention. Executive Commentary: Highlights of 2011 Report. Available at: http://www.cdc.gov/tb/statistics/reports/2011/pdf/ExecutiveCommentary.pdf. Accessed November 19, 2012.
17. Kuma V, Abbas AK, Fausto N, et al. Robbins Basic Pathology. 8th ed. Philadelphia, Pa: Saunders Elsevier; 2007:516–522.
18. Barnett ED. Infectious disease screening for refugees resettled in the United States. Clin Infect Dis. 2004;39:833-841.
19. DeRiemer K, Chin DP, Schecter DF, et al. Tuberculosis among immigrants and refugees. Arch Intern Med. 1998;158:753-760.
20. Centers for Disease Control and Prevention. Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recomm Rec. 2010;59(RR-5):1-25.
21. Bright Futures at Georgetown University. Health Supervision— Laboratory Tests: Tuberculosis (TB) Screening. Available at: http://www.brightfutures.org/pocket/pdf/30_37.pdf. Accessed November 19, 2012.
22. Centers for Disease Control and Prevention. Treatment of tuberculosis. American Thoracic Society, CDC, Infectious Diseases Society of America. MMWR Recomm Rep 2003;52(RR-11):1-77.
23. Centers for Disease Control and Prevention. Update: adverse event data and revised American Thoracic Society/CDC recommendations against the use of rifampin and pyrazinamide for treament of latent tuberculosis infection—United States. MMWR Morb Mortal Wkly Rep. 2003;52:735-739.
24. Caruana SR, Kelly HA, Ngeow JY, et al. Undiagnosed and potentially lethal parasite infections among immigrants and refugees in Australia. J Travel Med. 2006;13:233-239.
25. Dawson-Hahn EE, Greenberg SL, Domachowske JB, et al. Eosinophilia and the seroprevalence of schistosomiasis and strongyloidiasis in newly arrived pediatric refugees: an examination of Centers for Disease Control and Prevention screening guidelines. J Pediatr. 2010;156:1016-1018.
26. Garg PK, Perry S, Dorn M, et al. Risk of intestinal helminth and protozoan infection in a refugee population. Am J Trop Med Hyg. 2005;73:386-391.
27. Parenti DM, Lucas D, Lee A, et al. Health status of Ethiopian refugees in the United States. Am J Public Health. 1987;77:1542-1543.
28. Parish R. Intestinal parasites in Southeast Asian refugee children. West J Med. 1985;143:47-49.
29. Sutherland JE, Avant RF, Franz WB, 3rd, et al. Indochinese refugee health assessment and treatment. J Fam Pract. 1983;16:61-67.
30. Cartwright C. Utility of multiple-stool-specimen ova and parasite examinations in a high-prevalence setting. J Clin Microbiol. 1999;37:2408-2411.
31. Centers for Disease Control and Prevention. Recommendations for Presumptive Treatment of Schistosomiasis and Strongyloidiasis Among the Somali Bantu Refugees. June 13, 2005. Available at: http://archive.acf.hhs.gov/programs/orr/policy/sl05-18attach-ment2.pdf. Accessed November 19, 2012.
32. Centers for Disease Control and Prevention. Division of Global Migration and Quarantine. Guidelines for Evaluation of Refugees for Intestinal and Tissue-Invasive Parasitic Infections during Domestic Medical Examination. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/intestinal-parasites-domestic.html. Accessed October 30, 2012.
33. Centers for Disease Control and Prevention. Screening for HIV Infection During the Refugee Domestic Medical Examination. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/screening-hiv-infection-domestic.html. Accessed November 15, 2012.
34. Centers for Disease Control and Prevention. Revised recommendations for HIV testing of adults, adolescents and pregnant women in healthcare settings. MMWR Recomm Rep. 2006;55(RR-14):1-17.
35. Centers for Disease Control and Prevention National Immunization Program. Available at: http://www.cdc.gov/vaccines/vpdvac/hepa/default.htm. Accessed November 19, 2012.
36. American Academy of Pediatrics. Red Book. Available at: http://www2.aap.org/immunization/illnesses/hepb/hepa.html. Accessed November 19, 2012.
37. Lai CL, Ratziu V, Yuen MF, et al. Viral hepatitis B. Lancet. 2003;362:2089-2094.
38. Lin K, Kirchner J. Hepatitis B. Am Fam Phyician. 2004;69:75-82.
39. Ghany MG, Strader DB, Thomas DL, et al. American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374.
40. Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Morb Mortal Wkly Rep. 2012;61(RR-4):1-31.
41. Rowland M, Nosten F. Malaria epidemiology and control in refugee camps and complex emergencies. Ann Trop Med Parasitol. 2001;95:741-754.
42. Centers for Disease Control and Prevention, Malaria Branch, Division of Parasitic Diseases, Division of Global Migration and Quarantine and Malaria Branch. Presumptive Treatment of P falciparum Malaria in Refugees Relocating from sub-Saharan Africa to the United States. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/malaria-guidelines-domestic.html. Accessed November 15, 2012.
43. Centers for Disease Control and Prevention. Malnutrition and micronutrient deficiencies among Bhutanese refugee children— Nepal, 2007. MMWR Morb Mortal Wkly Rep. 2008;57:370-373.
44. American Academy of Pediatrics Bright Futures. Guidelines for Health Supervision of Infants, Children, and Adolescents— Theme 5: Promoting Healthy Nutrition. Available at: http://brightfutures.aap.org/pdfs/Guidelines_PDF/6-Promoting_Healthy_Nutrition.pdf. Accessed November 15, 2012.
45. Benson J, Skull S. Hiding from the sun: vitamin D deficiency in refugees. Aust Fam Physician. 2007;36:355-357.
46. Stellinga-Boelen A. Vitamin D levels in children of asylum seekers in The Netherlands in relation to season and dietary intake. Eur J Pediatr. 2007;166:201-206.
47. Trepka MJ, Pekovic V, Santana JC, et al. Risk factors for lead poisoning among Cuban refugee children. Public Health Rep. 2005;120:179-185.
48. Centers for Disease Control and Prevention. Elevated blood lead levels in refugee children, New Hampshire, 2003-2004. MMWR Morb Mortal Wkly Rep. 2005;54:42-46.
49. Centers for Disease Control and Prevention. Screening for Lead at the Domestic Refugee Medical Exam. Available at: http://www.cdc.gov/immigrantrefugeehealth/pdf/lead.pdf. Accessed November 15, 2012.
50. Zabel EW, Smith ME, O’Fallon A. Implementation of CDC refugee blood testing guidelines in Minnesota. Public Health Rep. 2008;123:111-125.
51. United States Citizenship and Immigration Services. Available at: http://www.uscis.gov/portal/site/uscis/menuitem.5af9bb95919f35e66f614176543f6d1a/?vgnextoid=3384cc5222$5210VgnVCM100000082ca60aRCRD&vgnextchannel=6abe6d26d17df110VgnVCM1000004718190aRCRD. Accessed November 15, 2012.
52. Centers for Disease Control and Prevention. Vaccination Requirements for Adjustment of Status for US Permanent Residence: Technical Instructions for Civil Surgeons. December 14, 2009. Available at: http://www.cdc.gov/immigrantrefugeehealth/pdf/2009-vaccination-technical-instructions.pdf. Accessed November 15, 2012
53. Phillips C. Better primary healthcare for refugees: catch up immunisation. Aust Fam Physician. 2007;36:440-443.
54. Brewin C. Systematic review of screening instruments for adults at risk of PTSD. J Trauma Stress. 2005;18:53-62.
55. Crumlish N, O’Rourke K. A systematic review of treatments for post-traumatic stress disorder among refugees and asylum-seekers. J Nerv Ment Dis. 2010;198:237-251.
56. Watters C. Emerging paradigms in the mental health care of refugees. Soc Sci Med. 2001;53:1709-1718.
57. Hayes EB, Talbot SB, Matheson ES, et al. Health status of pediatric refugees in Portland, ME. Arch Pediatr Adolesc Med. 1998;152:564-568.
58. Meropol S. Health status of pediatric refugees in Buffalo, NY. Arch Pediatr Adolesc Med. 1995;149:887-892.
59. Barnes DM, Harrison C, Heneghan R. Health risk and promotion behaviors in refugee populations. J Health Care Poor Underserved. 2004;15:347-356.
60. Dicker S, Stauffer WM, Mamo B, et al. Initial refugee health assessments: new recommendations for Minnesota. Minn Med. 2010;93:45-48.