User login
Long-Term Outcomes of Allograft Reconstruction of the Anterior Cruciate Ligament
Injuries of the anterior cruciate ligament (ACL) are common. Good to excellent long-term results are generally expected in more than 90% of ACL reconstructions.1,2 Although our knowledge of the biomechanics, kinematics, and long-term outcomes of ACL reconstruction is extensive, the ideal graft choice for ACL reconstruction is still up for debate.
Historically, both quadruple-stranded hamstring tendon and bone–patellar tendon–bone (BPTB) autografts have been the most popular graft options for operative reconstruction of the ACL.3 Recently, allograft tissues have become increasingly popular as a graft source. Proponents of allograft ACL reconstruction have cited several advantages over autograft reconstruction, including decreased donor-site morbidity, shorter operative times, and quicker postoperative recovery.4-7 Nevertheless, some authors have recently reported higher rates of both reoperation and graft failure after allograft ACL reconstruction.4,8-11 The 2 senior surgeons in the Sports Medicine Section of the Department of Orthopedic Surgery at the University of Arizona College of Medicine had not recognized such high failure and revision rates in their own clinical practices.
To evaluate the long-term outcomes of allograft ACL reconstruction, we retrospectively reviewed the cases of all patients who underwent allograft or autograft ACL reconstruction by 2 senior surgeons at a single institution over an 8-year period. We hypothesized that the reoperation and revision surgery rates for allograft ACL reconstruction would not be higher than those reported for autograft reconstruction. We also hypothesized that allograft ACL reconstruction failure rates would not be higher for patients younger than 25 years than for patients who are older and less active.
Materials and Methods
This study was approved by the Institutional Review Board at the University of Arizona College of Medicine. We retrospectively reviewed the cases of all patients who underwent primary endoscopic ACL reconstruction at the University of Arizona College of Medicine over an 8-year period (2000–2008). All ACL reconstructions were performed by 2 senior, fellowship-trained sports medicine specialists, including Dr. William A. Grana. Patients were identified from the Current Procedural Terminology (CPT) code for ACL reconstruction. Both autograft and allograft reconstructions were included in the study. Patients undergoing revision ACL reconstruction and patients with multi-ligamentous knee injuries were excluded. All available medical records were reviewed for patient demographics and any concomitant knee pathology. We included patients of all activity levels, patients with acute ACL tears, and patients with chronically ACL-deficient knees. We identified a separate cohort of Division I varsity athletes from the University of Arizona for evaluation. These patients were identified from the injury surveillance system in the athletic training facility of the University of Arizona.
ACL reconstructions at our institution during this 8-year period were performed with both allograft and autograft soft tissue. Allograft tendons were most commonly used. Tibialis anterior allograft was used in the majority of those knees. Tibialis posterior and semitendinosus allografts were used in a small subset of patients. Autograft reconstruction was performed with quadruple-stranded semitendinosus and gracilis tendons. We reviewed operative reports to determine type of graft used for reconstruction.
Patients were assessed clinically by telephone interview and/or mailed survey. They were specifically asked whether there had been any postoperative complications. We reviewed all operative and postoperative follow-up notes for postoperative complications. Objective clinical assessment involved use of the International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, the Tegner-Lysholm Knee Scoring Scale, and the Tegner Activity Scale.
Operative Technique
A standard, transtibial arthroscopically assisted ACL reconstruction was performed in all patients. For autograft reconstruction patients, both the semitendinosus and gracilis tendons were harvested through a small anteromedial incision and prepared to form a quadruple-stranded graft. All allograft tendons were obtained from the Musculoskeletal Transplant Foundation (MTF). Tibialis anterior and tibialis posterior allografts were folded in half to form a double-stranded graft. Alternatively, 2 semitendinosus allografts were prepared in the same fashion as that described for autograft hamstring tendons. The tibial tunnel was placed into the center of the ACL tibial footprint. With use of a transtibial approach, an endoscopic offset guide was used to place the femoral tunnel at the 10- and 2-o’clock positions in the right and left knees, respectively. In almost all cases, the graft was secured on the femoral side with a cortical fixation button. Tibial fixation was obtained with a bioabsorbable interference screw.
After ACL reconstruction, each patient participated in the standard accelerated rehabilitation outlined by Shelbourne and Gray.12 Guided rehabilitation was instituted within 1 week after surgery under the guidance of a physical therapist. Range-of-motion exercises and closed-chain strengthening exercises were begun at this time. The protocol emphasized early return of full terminal extension and normalization of gait patterns. Patients were allowed to return to play only after meeting specific criteria, about 6 months after surgery. Many athletes in our Division I university population are allowed to return to play 5 to 6 months after surgery, after meeting return-to-play criteria.
Statistical Analysis
We used Minitab 14 (Minitab, State College, Pennsylvania) to perform all statistical analyses, unpaired Student t tests to compare IKDC and Tegner-Lysholm results between allograft and autograft groups, and χ2 tests to compare revision and reoperation rates between groups. Significance was set at P = .05.
Results
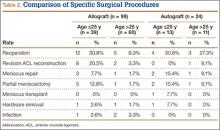
We identified 362 patients who underwent ACL reconstructions at our institution between 2000 and 2008. Of these patients, 302 met the study inclusion criteria. One-hundred twenty-three (40.7%) of the 302 were available for follow-up by telephone interview and/or mailed questionnaire. This follow-up group consisted of 67 males and 56 females. Mean age at surgery was 29 years (range, 17-53 years). Mean follow-up was 50.3 months (range, 11-111 months). Of the 123 patients, 99 underwent allograft ACL reconstruction, and 24 underwent autograft ACL reconstruction. Seventeen (17%) of the 99 allograft cases required additional surgery (Table 1). The reoperation rate for patients under age 25 years (30.8%) was higher than the rate for patients older than 25 years (Table 2). Regarding patients who underwent additional surgeries, mean scores were lower with allograft (Tegner-Lysholm, 59; IKDC, 54) than with autograft (Tegner-Lysholm, 83; IKDC, 79) (Ps = .0025 and .006, respectively).
Revision rates were 10.1% (allograft group) and 4.2% (autograft group) (Table 1). This difference was not statistically significant (P = .18). In the allograft group, the revision rate was higher for patients younger than 25 years (20.5%) than for patients older than 25 years (3.3%) (Table 2). In comparison, in the autograft group, the revision rate was only 4% for patients younger than 25 years. For younger patients, the higher rate of revision with allograft (vs autograft) was statistically significant (P = .038). For older patients, allograft and autograft revision rates did not differ significantly (P = .19). No patient younger than 25 years required revision reconstruction after autograft ACL reconstruction.
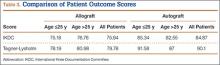
IKDC and Tegner-Lysholm outcome scores for allograft and autograft groups are shown in Table 3. In patients 25 years or younger, IKDC scores were 75.18 after allograft reconstruction and 85.34 after autograft reconstruction—a significant difference (P = .045). In addition, Tegner-Lysholm scores were significantly higher after autograft reconstruction (91.58) than allograft reconstruction (78.19) in these younger patients (P = .003) (Table 3). IKDC and Tegner-Lysholm scores were not significantly different for older patients (Ps = .241 and .211, respectively).
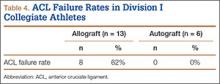
The study also included a subset of 19 primary ACL reconstructions (13 allograft, 6 autograft) performed on Division I athletes from the University of Arizona. (Nineteen [91%] of the 21 athletes in our Division I cohort were available for follow-up.) All these patients were younger than 25 years. All autograft reconstructions were performed with quadruple-stranded gracilis and semitendinosus tendons. ACL graft failure occurred in 8 (62%) of the 13 allograft cases; there were no failures in the autograft group (Table 4). One of the 5 allograft cases that did not fail required multiple surgical débridement procedures for infection, but the graft was ultimately retained. There were no infections among the 6 autograft cases.
Discussion
The ideal graft for ACL reconstruction is still a matter of intense debate. There are many graft options for ACL reconstruction. Both BPTB and hamstring autografts are associated with various graft-specific comorbidities. Anterior knee pain, knee extensor weakness, extension loss, patella fracture, patellofemoral crepitance, and infrapatellar nerve injury have been described with BPTB autografts.13-17 In a meta-analysis of 11 studies comparing BPTB autografts with hamstring autograft, Goldblatt and colleagues17 found more extension loss, kneeling pain, and patellofemoral crepitance in the BPTP group.
Knee flexion weakness, knee flexion loss, increased knee laxity, and saphenous nerve injury have all been described with use of hamstring autografts.16-19 Goldblatt and colleagues17 demonstrated a significant flexion loss in the hamstring group in their meta-analysis as well as increased laxity with both the Lachman test and the pivot shift test. They also found that the hamstring autograft group exhibited side-to-side differences of more than 3 mm on KT-1000 testing when compared with the BPTB autograft group.
Proposed advantages of allograft reconstruction include elimination of donor-site morbidity and/or pain from a less invasive procedure, faster initial recovery, more sizing options, and shorter operative times.4-7 In a 5-year follow-up of patients who had ACL reconstruction with either Achilles allograft or BPTB autograft, Poehling and colleagues7 demonstrated overall similar long-term outcomes between the groups. However, the allograft patients reported less pain 1 and 6 weeks after surgery; better function 1 week, 3 months, and 1 year after surgery; and fewer activity limitations throughout the follow-up period. Lamblin and colleagues20 also found no difference between nonirradiated allograft and autograft tissue in ACL reconstruction in a 2013 meta-analysis of ACL studies published over a 32-year period.
Despite the proposed advantages of allograft ACL reconstruction, several recent studies have demonstrated poorer outcomes in both younger patients and more active patients after allograft reconstruction.8-11,21 In a 2007 meta-analysis, Prodromos and colleagues11 compared a series of allograft reconstructions with previously published data sets of both BPTB and hamstring autografts. They found that allograft reconstructions had significantly lower stability rates than autograft reconstructions. In a case–control study by Borchers and colleagues,10 21 patients with ACL graft failure were identified over a 2-year period, and surgical outcomes were compared with those of 42 age- and sex-matched controls. The authors found higher activity level and allograft use to be risk factors for subsequent graft failure after ACL reconstruction. More important, they showed a multiplicative interaction between higher activity level after ACL reconstruction and allograft use—an interaction that greatly increased the odds for ACL graft failure. Last, in a retrospective review, Singhal and colleagues8 evaluated the outcomes of ACL reconstruction using tibialis anterior tendon allograft and reported a 23.1% revision rate. In addition, 37.7% of patients required repeat surgery. The failure/reoperation rate was 55% for patients 25 years or younger and 24% for patients older than 25 years. The authors recommended not using tibialis anterior allografts in patients 25 years or younger and in patients who frequently engage in level I ACL-dependent sports.
The poor outcomes reported by Singhal and colleagues8 may be related to use of irradiated soft-tissue allografts. In a comparison of nonirradiated BPTB allograft and BPTB autograft in patients 25 years or younger, Barber and colleagues22 found equivalent outcomes at 2-year follow-up. They actually found a higher rate of failure for autograft reconstruction (9.4%) than allograft reconstruction (7.1%). A potential critique of their study is the significant difference between the patient groups’ mean ages: 18.6 years (autograft) versus 20.1 years (allograft). Despite this selection bias, Barber and colleagues22 argued that nonirradiated BPTB allograft is equivalent to BPTB autograft for ACL reconstruction.
Our study is one of the largest allograft studies with a comparison group. The principal findings of this study demonstrate that overall reoperation and revision rates after irradiated soft-tissue allograft ACL reconstruction are higher than those historically quoted for autograft ACL reconstruction. Specifically, allograft patients younger than 25 years had a reoperation rate of 30.8% and a revision rate of 20.5%. (Allograft patients older than 25 years had lower rates of reoperation, 8.3%, and revision, 3.3%.) After revision surgery, autograft patients’ subjective outcomes (IKDC and Tegner-Lysholm scores) were significantly improved compared with those of allograft patients (Ps = .0017 and .0031, respectively). Most compelling, however, is the unexpected and quite concerning 62% failure rate in our high-level Division I intercollegiate athletes.
There are multiple hypotheses regarding the higher failure rates of allograft tissues versus autograft tissues in ACL reconstruction. Processing methods, exposure to ionizing radiation, and the incorporation/ligamentization process have all been cited as possible reasons for allograft failure. All the allograft tendons used in the present study were obtained from MTF, which uses a proprietary “aseptic” processing system that includes washing in buffered saline impregnated with antibiotics (imipenem/cilastatin, amphotericin B, gentamicin) followed by final rinsing in phosphate-buffered saline. The majority of grafts are subjected to low-level irradiation (<2 Mrad/20 kGy) based on the outcomes of MTF’s stringent donor-selection process. Although the washing process has not been shown to alter the structural integrity of donor grafts, multiple studies have outlined the detrimental effects of higher levels of gamma radiation on allograft tissues. Although lower levels are effective against potential bacterial contaminants, a radiation level of 4 Mrad is necessary to kill the human immunodeficiency virus (HIV). Thus, a dose of 4 Mrad or higher is needed to truly “sterilize” a graft. This higher dose is an issue, as it has been known for some time that higher levels of ionizing radiation can have adverse effects on the biomechanical strength of soft-tissue allografts. In fact, ionizing radiation has dose-dependent effects.23-26 Schwartz and colleagues27 showed in a caprine model that radiation exposure at 4 Mrad significantly decreased the biomechanical strength of ACL allografts at 6 months. Balsly and colleagues28 found in a biomechanical study that radiation doses of 18 to 22 Mrad did not significantly affect the mechanical integrity of soft-tissue allografts. Conversely, in an in vivo study, Rappe and colleagues29 showed that Achilles allografts irradiated at a dose of 2.0 to 2.5 Mrad had a failure rate (33%) much higher than that of nonirradiated allografts (2.4%). The radiation dose used by MTF is less than 2 Mrad. Although more than needed to kill bacterial contaminants, this dose is considered by MTF to be below the threshold for biomechanical alterations. Only a minority of grafts is treated without irradiation.
It is possible that any level of radiation affects ligamentization of allograft tissues. Multiple studies have outlined the ligamentization process of autograft tendons in vivo. Patellar tendon autografts undergo central degeneration 2 to 6 weeks after reconstruction, but, by 6 to 12 months, these tendons have structural properties similar to those of the native ACL.30-34 Findings are similar for hamstring autografts.35,36 Goradia and colleagues36 found that, by 52 weeks, semitendinosus autografts transform into a histologic structure similar to that of the normal ACL. Remodeling of allograft tendons has been described as occurring at a much slower rate.27,37-40 Bhatia and colleagues37 demonstrated faster remodeling in autograft tissues versus allograft tissues at early time points in an in vivo rabbit model. Ultimately, differences in graft incorporation and ligamentization may be a primary factor in the higher failure rates of allograft ACL reconstruction. Current rehabilitation protocols may not take into account the longer ligamentization process for allograft tissues. These protocols are largely based on our current understanding of the ligamentization process after autograft reconstruction. It is possible that the rehabilitation program and return-to-play schedule for allograft reconstruction need to be altered to help avoid higher failure rates. The return-to-play protocol at the authors’ institution scheduled most varsity athletes to return to play 6 months after surgery. In some cases, the timetable was shortened, and some athletes were returned to play 5 months after surgery, after meeting all return-to-play criteria. Based on the findings of the present study, this return-to-play schedule may be much too aggressive for high-level athletes after allograft reconstruction. It is possible these allografts have not reached “maturity,” as their autograft counterparts have, and thus are not ready for unrestricted return to play.
Our study had multiple strengths. All reconstructions were performed by 2 senior surgeons with extensive clinical experience. The autograft and allograft reconstructions used the same techniques and rehabilitation protocols. This is one of the largest studies of outcomes of allograft ACL reconstruction and one of the largest studies that used a comparison group of autograft reconstructions. Having a comparison group effectively allowed us to contrast the differences between allograft and autograft tissues. Last, this study evaluated a subgroup of high-level NCAA Division I athletes. Follow-up in the overall study was 40.7%, but follow-up in this subgroup was 91%. The very high follow-up rate in the university population helped us validate the overall results of the study. Study results reinforced the fact that irradiated soft-tissue allograft may not be indicated for ACL reconstruction in a younger, more active patient population and led to a change in approach to ACL reconstruction for Division I intercollegiate athletes at the University of Arizona. Allograft ACL reconstruction is no longer recommended for the intercollegiate athletes at the University of Arizona.
Our study had its limitations. First, it had the inherent biases of a retrospective study. Second, many patients were lost to follow-up. We contacted and surveyed 40.7% of the patients who met the inclusion criteria. We tried reaching them in multiple ways—through US mail, all listed phone numbers, family members, and so forth. Tucson, Arizona is a college town and has a larger transient population, which may have added to the difficulty in contacting patients.
Conclusion
Given the high rates of reoperation and revision surgery with allograft reconstruction in younger patients in this study, we recommend against routine use of irradiated soft-tissue allograft tissue for ACL reconstruction in patients 25 years or younger. In our clinical practices, we prefer using autograft tissue for ACL reconstruction in younger, more active individuals. Irradiated soft-tissue allograft ACL reconstruction is a viable option in the older, less active patient population. Although the overall reoperation rate in this cohort study is acceptable, the revision rate for patients younger than 25 years is concerning and should be taken into account when considering use of irradiated soft-tissue allograft for ACL reconstruction in these younger patients.
1. Schepsis AA, Busconi BD. Sports Medicine. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
2. Campbell WC, Canale ST, Beaty JH. Campbell’s Operative Orthopaedics. 11th ed. Philadelphia, PA: Mosby/Elsevier; 2008.
3. Sherman OH, Banffy MB. Anterior cruciate ligament reconstruction: which graft is best? Arthroscopy. 2004;20(9):974-980.
4. Lee JH, Bae DK, Song SJ, Cho SM, Yoon KH. Comparison of clinical results and second-look arthroscopy findings after arthroscopic anterior cruciate ligament reconstruction using 3 different types of grafts. Arthroscopy. 2010;26(1):41-49.
5. Sun K, Tian SQ, Zhang JH, Xia CS, Zhang CL, Yu TB. Anterior cruciate ligament reconstruction with bone-patellar tendon-bone autograft versus allograft. Arthroscopy. 2009;25(7):750-759.
6. Kuhn MA, Ross G. Allografts in the treatment of anterior cruciate ligament injuries. Sports Med Arthrosc Rev. 2007;15(3):133-138.
7. Poehling GG, Curl WW, Lee CA, et al. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: allograft versus autograft. Arthroscopy. 2005;21(7):774-785.
8. Singhal MC, Gardiner JR, Johnson DL. Failure of primary anterior cruciate ligament surgery using anterior tibialis allograft. Arthroscopy. 2007;23(5):469-475.
9. Barrett GR, Luber K, Replogle WH, Manley JL. Allograft anterior cruciate ligament reconstruction in the young, active patient: Tegner activity level and failure rate. Arthroscopy. 2010;26(12):1593-1601.
10. Borchers JR, Pedroza A, Kaeding C. Activity level and graft type as risk factors for anterior cruciate ligament graft failure: a case–control study. Am J Sports Med. 2009;37(12):2362-2367.
11. Prodromos C, Joyce B, Shi K. A meta-analysis of stability of autografts compared to allografts after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):851-856.
12. Shelbourne KD, Gray T. Anterior cruciate ligament reconstruction with autogenous patellar tendon graft followed by accelerated rehabilitation. A two- to nine-year followup. Am J Sports Med. 1997;25(6):786-795.
13. Rosenberg TD, Franklin JL, Baldwin GN, Nelson KA. Extensor mechanism function after patellar tendon graft harvest for anterior cruciate ligament reconstruction. Am J Sports Med. 1992;20(5):519-525.
14. Piva SR, Childs JD, Klucinec BM, Irrgang JJ, Almeida GJ, Fitzgerald GK. Patella fracture during rehabilitation after bone–patellar tendon–bone anterior cruciate ligament reconstruction: 2 case reports. J Orthop Sports Phys Ther. 2009;39(4):278-286.
15. Lee GH, McCulloch P, Cole BJ, Bush-Joseph CA, Bach BR Jr. The incidence of acute patellar tendon harvest complications for anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(2):162-166.
16. Kartus J, Movin T, Karlsson J. Donor-site morbidity and anterior knee problems after anterior cruciate ligament reconstruction using autografts. Arthroscopy. 2001;17(9):971-980.
17. Goldblatt JP, Fitzsimmons SE, Balk E, Richmond JC. Reconstruction of the anterior cruciate ligament: meta-analysis of patellar tendon versus hamstring tendon autograft. Arthroscopy. 2005;21(7):791-803.
18. Freedman KB, D’Amato MJ, Nedeff DD, Kaz A, Bach BR Jr. Arthroscopic anterior cruciate ligament reconstruction: a metaanalysis comparing patellar tendon and hamstring tendon autografts. Am J Sports Med. 2003;31(1):2-11.
19. Yunes M, Richmond JC, Engels EA, Pinczewski LA. Patellar versus hamstring tendons in anterior cruciate ligament reconstruction: a meta-analysis. Arthroscopy. 2001;17(3):248-257.
20. Lamblin CJ, Waterman BR, Lubowitz JH. Anterior cruciate ligament reconstruction with autografts compared with non-irradiated, non-chemically treated allografts. Arthroscopy. 2013;29(6):1113-1122.
21. Pallis M, Svoboda SJ, Cameron KL, Owens BD. Survival comparison of allograft and autograft anterior cruciate ligament reconstruction at the United States Military Academy. Am J Sports Med. 2012;40(6):1242-1246.
22. Barber FA, Cowden CH 3rd, Sanders EJ. Revision rates after anterior cruciate ligament reconstruction using bone–patellar tendon–bone allograft or autograft in a population 25 years old and younger. Arthroscopy. 2014;30(4):483-491.
23. Salehpour A, Butler DL, Proch FS, et al. Dose-dependent response of gamma irradiation on mechanical properties and related biochemical composition of goat bone–patellar tendon–bone allografts. J Orthop Res. 1995;13(6):898-906.
24. Gibbons MJ, Butler DL, Grood ES, Bylski-Austrow DI, Levy MS, Noyes FR. Effects of gamma irradiation on the initial mechanical and material properties of goat bone–patellar tendon–bone allografts. J Orthop Res. 1991;9(2):209-218.
25. Fideler BM, Vangsness CT Jr, Lu B, Orlando C, Moore T. Gamma irradiation: effects on biomechanical properties of human bone–patellar tendon–bone allografts. Am J Sports Med. 1995;23(5):643-646.
26. De Deyne P, Haut RC. Some effects of gamma irradiation on patellar tendon allografts. Connect Tissue Res. 1991;27(1):51-62.
27. Schwartz HE, Matava MJ, Proch FS, et al. The effect of gamma irradiation on anterior cruciate ligament allograft biomechanical and biochemical properties in the caprine model at time zero and at 6 months after surgery. Am J Sports Med. 2006;34(11):1747-1755.
28. Balsly CR, Cotter AT, Williams LA, Gaskins BD, Moore MA, Wolfinbarger L Jr. Effect of low dose and moderate dose gamma irradiation on the mechanical properties of bone and soft tissue allografts. Cell Tissue Bank. 2008;9(4):289-298.
29. Rappe M, Horodyski M, Meister K, Indelicato PA. Nonirradiated versus irradiated Achilles allograft: in vivo failure comparison. Am J Sports Med. 2007;35(10):1653-1658.
30. Amiel D, Kleiner JB, Akeson WH. The natural history of the anterior cruciate ligament autograft of patellar tendon origin. Am J Sports Med. 1986;14(6):449-462.
31. Amiel D, Kleiner JB, Roux RD, Harwood FL, Akeson WH. The phenomenon of “ligamentization”: anterior cruciate ligament reconstruction with autogenous patellar tendon. J Orthop Res. 1986;4(2):162-172.
32. Arnoczky SP, Tarvin GB, Marshall JL. Anterior cruciate ligament replacement using patellar tendon. An evaluation of graft revascularization in the dog. J Bone Joint Surg Am. 1982;64(2):217-224.
33. Ballock RT, Woo SL, Lyon RM, Hollis JM, Akeson WH. Use of patellar tendon autograft for anterior cruciate ligament reconstruction in the rabbit: a long-term histologic and biomechanical study. J Orthop Res. 1989;7(4):474-485.
34. Clancy WG Jr, Narechania RG, Rosenberg TD, Gmeiner JG, Wisnefske DD, Lange TA. Anterior and posterior cruciate ligament reconstruction in rhesus monkeys. J Bone Joint Surg Am. 1981;63(8):1270-1284.
35. Blickenstaff KR, Grana WA, Egle D. Analysis of a semitendinosus autograft in a rabbit model. Am J Sports Med. 1997;25(4):554-559.
36. Goradia VK, Rochat MC, Kida M, Grana WA. Natural history of a hamstring tendon autograft used for anterior cruciate ligament reconstruction in a sheep model. Am J Sports Med. 2000;28(1):40-46.
37. Bhatia S, Bell R, Frank RM, et al. Bony incorporation of soft tissue anterior cruciate ligament grafts in an animal model: autograft versus allograft with low-dose gamma irradiation. Am J Sports Med. 2012;40(8):1789-1798.
38. Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21(2):176-185.
39. Goertzen MJ, Clahsen H, Schulitz KP. Anterior cruciate ligament reconstruction using cryopreserved irradiated bone-ACL-bone-allograft transplants. Knee Surg Sports Traumatol Arthrosc. 1994;2(3):150-157.
40. Mae T, Shino K, Maeda A, Toritsuka Y, Horibe S, Ochi T. Effect of gamma irradiation on remodeling process of tendon allograft. Clin Orthop. 2003;(414):305-314.
Injuries of the anterior cruciate ligament (ACL) are common. Good to excellent long-term results are generally expected in more than 90% of ACL reconstructions.1,2 Although our knowledge of the biomechanics, kinematics, and long-term outcomes of ACL reconstruction is extensive, the ideal graft choice for ACL reconstruction is still up for debate.
Historically, both quadruple-stranded hamstring tendon and bone–patellar tendon–bone (BPTB) autografts have been the most popular graft options for operative reconstruction of the ACL.3 Recently, allograft tissues have become increasingly popular as a graft source. Proponents of allograft ACL reconstruction have cited several advantages over autograft reconstruction, including decreased donor-site morbidity, shorter operative times, and quicker postoperative recovery.4-7 Nevertheless, some authors have recently reported higher rates of both reoperation and graft failure after allograft ACL reconstruction.4,8-11 The 2 senior surgeons in the Sports Medicine Section of the Department of Orthopedic Surgery at the University of Arizona College of Medicine had not recognized such high failure and revision rates in their own clinical practices.
To evaluate the long-term outcomes of allograft ACL reconstruction, we retrospectively reviewed the cases of all patients who underwent allograft or autograft ACL reconstruction by 2 senior surgeons at a single institution over an 8-year period. We hypothesized that the reoperation and revision surgery rates for allograft ACL reconstruction would not be higher than those reported for autograft reconstruction. We also hypothesized that allograft ACL reconstruction failure rates would not be higher for patients younger than 25 years than for patients who are older and less active.
Materials and Methods
This study was approved by the Institutional Review Board at the University of Arizona College of Medicine. We retrospectively reviewed the cases of all patients who underwent primary endoscopic ACL reconstruction at the University of Arizona College of Medicine over an 8-year period (2000–2008). All ACL reconstructions were performed by 2 senior, fellowship-trained sports medicine specialists, including Dr. William A. Grana. Patients were identified from the Current Procedural Terminology (CPT) code for ACL reconstruction. Both autograft and allograft reconstructions were included in the study. Patients undergoing revision ACL reconstruction and patients with multi-ligamentous knee injuries were excluded. All available medical records were reviewed for patient demographics and any concomitant knee pathology. We included patients of all activity levels, patients with acute ACL tears, and patients with chronically ACL-deficient knees. We identified a separate cohort of Division I varsity athletes from the University of Arizona for evaluation. These patients were identified from the injury surveillance system in the athletic training facility of the University of Arizona.
ACL reconstructions at our institution during this 8-year period were performed with both allograft and autograft soft tissue. Allograft tendons were most commonly used. Tibialis anterior allograft was used in the majority of those knees. Tibialis posterior and semitendinosus allografts were used in a small subset of patients. Autograft reconstruction was performed with quadruple-stranded semitendinosus and gracilis tendons. We reviewed operative reports to determine type of graft used for reconstruction.
Patients were assessed clinically by telephone interview and/or mailed survey. They were specifically asked whether there had been any postoperative complications. We reviewed all operative and postoperative follow-up notes for postoperative complications. Objective clinical assessment involved use of the International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, the Tegner-Lysholm Knee Scoring Scale, and the Tegner Activity Scale.
Operative Technique
A standard, transtibial arthroscopically assisted ACL reconstruction was performed in all patients. For autograft reconstruction patients, both the semitendinosus and gracilis tendons were harvested through a small anteromedial incision and prepared to form a quadruple-stranded graft. All allograft tendons were obtained from the Musculoskeletal Transplant Foundation (MTF). Tibialis anterior and tibialis posterior allografts were folded in half to form a double-stranded graft. Alternatively, 2 semitendinosus allografts were prepared in the same fashion as that described for autograft hamstring tendons. The tibial tunnel was placed into the center of the ACL tibial footprint. With use of a transtibial approach, an endoscopic offset guide was used to place the femoral tunnel at the 10- and 2-o’clock positions in the right and left knees, respectively. In almost all cases, the graft was secured on the femoral side with a cortical fixation button. Tibial fixation was obtained with a bioabsorbable interference screw.
After ACL reconstruction, each patient participated in the standard accelerated rehabilitation outlined by Shelbourne and Gray.12 Guided rehabilitation was instituted within 1 week after surgery under the guidance of a physical therapist. Range-of-motion exercises and closed-chain strengthening exercises were begun at this time. The protocol emphasized early return of full terminal extension and normalization of gait patterns. Patients were allowed to return to play only after meeting specific criteria, about 6 months after surgery. Many athletes in our Division I university population are allowed to return to play 5 to 6 months after surgery, after meeting return-to-play criteria.
Statistical Analysis
We used Minitab 14 (Minitab, State College, Pennsylvania) to perform all statistical analyses, unpaired Student t tests to compare IKDC and Tegner-Lysholm results between allograft and autograft groups, and χ2 tests to compare revision and reoperation rates between groups. Significance was set at P = .05.
Results
We identified 362 patients who underwent ACL reconstructions at our institution between 2000 and 2008. Of these patients, 302 met the study inclusion criteria. One-hundred twenty-three (40.7%) of the 302 were available for follow-up by telephone interview and/or mailed questionnaire. This follow-up group consisted of 67 males and 56 females. Mean age at surgery was 29 years (range, 17-53 years). Mean follow-up was 50.3 months (range, 11-111 months). Of the 123 patients, 99 underwent allograft ACL reconstruction, and 24 underwent autograft ACL reconstruction. Seventeen (17%) of the 99 allograft cases required additional surgery (Table 1). The reoperation rate for patients under age 25 years (30.8%) was higher than the rate for patients older than 25 years (Table 2). Regarding patients who underwent additional surgeries, mean scores were lower with allograft (Tegner-Lysholm, 59; IKDC, 54) than with autograft (Tegner-Lysholm, 83; IKDC, 79) (Ps = .0025 and .006, respectively).
Revision rates were 10.1% (allograft group) and 4.2% (autograft group) (Table 1). This difference was not statistically significant (P = .18). In the allograft group, the revision rate was higher for patients younger than 25 years (20.5%) than for patients older than 25 years (3.3%) (Table 2). In comparison, in the autograft group, the revision rate was only 4% for patients younger than 25 years. For younger patients, the higher rate of revision with allograft (vs autograft) was statistically significant (P = .038). For older patients, allograft and autograft revision rates did not differ significantly (P = .19). No patient younger than 25 years required revision reconstruction after autograft ACL reconstruction.
IKDC and Tegner-Lysholm outcome scores for allograft and autograft groups are shown in Table 3. In patients 25 years or younger, IKDC scores were 75.18 after allograft reconstruction and 85.34 after autograft reconstruction—a significant difference (P = .045). In addition, Tegner-Lysholm scores were significantly higher after autograft reconstruction (91.58) than allograft reconstruction (78.19) in these younger patients (P = .003) (Table 3). IKDC and Tegner-Lysholm scores were not significantly different for older patients (Ps = .241 and .211, respectively).
The study also included a subset of 19 primary ACL reconstructions (13 allograft, 6 autograft) performed on Division I athletes from the University of Arizona. (Nineteen [91%] of the 21 athletes in our Division I cohort were available for follow-up.) All these patients were younger than 25 years. All autograft reconstructions were performed with quadruple-stranded gracilis and semitendinosus tendons. ACL graft failure occurred in 8 (62%) of the 13 allograft cases; there were no failures in the autograft group (Table 4). One of the 5 allograft cases that did not fail required multiple surgical débridement procedures for infection, but the graft was ultimately retained. There were no infections among the 6 autograft cases.
Discussion
The ideal graft for ACL reconstruction is still a matter of intense debate. There are many graft options for ACL reconstruction. Both BPTB and hamstring autografts are associated with various graft-specific comorbidities. Anterior knee pain, knee extensor weakness, extension loss, patella fracture, patellofemoral crepitance, and infrapatellar nerve injury have been described with BPTB autografts.13-17 In a meta-analysis of 11 studies comparing BPTB autografts with hamstring autograft, Goldblatt and colleagues17 found more extension loss, kneeling pain, and patellofemoral crepitance in the BPTP group.
Knee flexion weakness, knee flexion loss, increased knee laxity, and saphenous nerve injury have all been described with use of hamstring autografts.16-19 Goldblatt and colleagues17 demonstrated a significant flexion loss in the hamstring group in their meta-analysis as well as increased laxity with both the Lachman test and the pivot shift test. They also found that the hamstring autograft group exhibited side-to-side differences of more than 3 mm on KT-1000 testing when compared with the BPTB autograft group.
Proposed advantages of allograft reconstruction include elimination of donor-site morbidity and/or pain from a less invasive procedure, faster initial recovery, more sizing options, and shorter operative times.4-7 In a 5-year follow-up of patients who had ACL reconstruction with either Achilles allograft or BPTB autograft, Poehling and colleagues7 demonstrated overall similar long-term outcomes between the groups. However, the allograft patients reported less pain 1 and 6 weeks after surgery; better function 1 week, 3 months, and 1 year after surgery; and fewer activity limitations throughout the follow-up period. Lamblin and colleagues20 also found no difference between nonirradiated allograft and autograft tissue in ACL reconstruction in a 2013 meta-analysis of ACL studies published over a 32-year period.
Despite the proposed advantages of allograft ACL reconstruction, several recent studies have demonstrated poorer outcomes in both younger patients and more active patients after allograft reconstruction.8-11,21 In a 2007 meta-analysis, Prodromos and colleagues11 compared a series of allograft reconstructions with previously published data sets of both BPTB and hamstring autografts. They found that allograft reconstructions had significantly lower stability rates than autograft reconstructions. In a case–control study by Borchers and colleagues,10 21 patients with ACL graft failure were identified over a 2-year period, and surgical outcomes were compared with those of 42 age- and sex-matched controls. The authors found higher activity level and allograft use to be risk factors for subsequent graft failure after ACL reconstruction. More important, they showed a multiplicative interaction between higher activity level after ACL reconstruction and allograft use—an interaction that greatly increased the odds for ACL graft failure. Last, in a retrospective review, Singhal and colleagues8 evaluated the outcomes of ACL reconstruction using tibialis anterior tendon allograft and reported a 23.1% revision rate. In addition, 37.7% of patients required repeat surgery. The failure/reoperation rate was 55% for patients 25 years or younger and 24% for patients older than 25 years. The authors recommended not using tibialis anterior allografts in patients 25 years or younger and in patients who frequently engage in level I ACL-dependent sports.
The poor outcomes reported by Singhal and colleagues8 may be related to use of irradiated soft-tissue allografts. In a comparison of nonirradiated BPTB allograft and BPTB autograft in patients 25 years or younger, Barber and colleagues22 found equivalent outcomes at 2-year follow-up. They actually found a higher rate of failure for autograft reconstruction (9.4%) than allograft reconstruction (7.1%). A potential critique of their study is the significant difference between the patient groups’ mean ages: 18.6 years (autograft) versus 20.1 years (allograft). Despite this selection bias, Barber and colleagues22 argued that nonirradiated BPTB allograft is equivalent to BPTB autograft for ACL reconstruction.
Our study is one of the largest allograft studies with a comparison group. The principal findings of this study demonstrate that overall reoperation and revision rates after irradiated soft-tissue allograft ACL reconstruction are higher than those historically quoted for autograft ACL reconstruction. Specifically, allograft patients younger than 25 years had a reoperation rate of 30.8% and a revision rate of 20.5%. (Allograft patients older than 25 years had lower rates of reoperation, 8.3%, and revision, 3.3%.) After revision surgery, autograft patients’ subjective outcomes (IKDC and Tegner-Lysholm scores) were significantly improved compared with those of allograft patients (Ps = .0017 and .0031, respectively). Most compelling, however, is the unexpected and quite concerning 62% failure rate in our high-level Division I intercollegiate athletes.
There are multiple hypotheses regarding the higher failure rates of allograft tissues versus autograft tissues in ACL reconstruction. Processing methods, exposure to ionizing radiation, and the incorporation/ligamentization process have all been cited as possible reasons for allograft failure. All the allograft tendons used in the present study were obtained from MTF, which uses a proprietary “aseptic” processing system that includes washing in buffered saline impregnated with antibiotics (imipenem/cilastatin, amphotericin B, gentamicin) followed by final rinsing in phosphate-buffered saline. The majority of grafts are subjected to low-level irradiation (<2 Mrad/20 kGy) based on the outcomes of MTF’s stringent donor-selection process. Although the washing process has not been shown to alter the structural integrity of donor grafts, multiple studies have outlined the detrimental effects of higher levels of gamma radiation on allograft tissues. Although lower levels are effective against potential bacterial contaminants, a radiation level of 4 Mrad is necessary to kill the human immunodeficiency virus (HIV). Thus, a dose of 4 Mrad or higher is needed to truly “sterilize” a graft. This higher dose is an issue, as it has been known for some time that higher levels of ionizing radiation can have adverse effects on the biomechanical strength of soft-tissue allografts. In fact, ionizing radiation has dose-dependent effects.23-26 Schwartz and colleagues27 showed in a caprine model that radiation exposure at 4 Mrad significantly decreased the biomechanical strength of ACL allografts at 6 months. Balsly and colleagues28 found in a biomechanical study that radiation doses of 18 to 22 Mrad did not significantly affect the mechanical integrity of soft-tissue allografts. Conversely, in an in vivo study, Rappe and colleagues29 showed that Achilles allografts irradiated at a dose of 2.0 to 2.5 Mrad had a failure rate (33%) much higher than that of nonirradiated allografts (2.4%). The radiation dose used by MTF is less than 2 Mrad. Although more than needed to kill bacterial contaminants, this dose is considered by MTF to be below the threshold for biomechanical alterations. Only a minority of grafts is treated without irradiation.
It is possible that any level of radiation affects ligamentization of allograft tissues. Multiple studies have outlined the ligamentization process of autograft tendons in vivo. Patellar tendon autografts undergo central degeneration 2 to 6 weeks after reconstruction, but, by 6 to 12 months, these tendons have structural properties similar to those of the native ACL.30-34 Findings are similar for hamstring autografts.35,36 Goradia and colleagues36 found that, by 52 weeks, semitendinosus autografts transform into a histologic structure similar to that of the normal ACL. Remodeling of allograft tendons has been described as occurring at a much slower rate.27,37-40 Bhatia and colleagues37 demonstrated faster remodeling in autograft tissues versus allograft tissues at early time points in an in vivo rabbit model. Ultimately, differences in graft incorporation and ligamentization may be a primary factor in the higher failure rates of allograft ACL reconstruction. Current rehabilitation protocols may not take into account the longer ligamentization process for allograft tissues. These protocols are largely based on our current understanding of the ligamentization process after autograft reconstruction. It is possible that the rehabilitation program and return-to-play schedule for allograft reconstruction need to be altered to help avoid higher failure rates. The return-to-play protocol at the authors’ institution scheduled most varsity athletes to return to play 6 months after surgery. In some cases, the timetable was shortened, and some athletes were returned to play 5 months after surgery, after meeting all return-to-play criteria. Based on the findings of the present study, this return-to-play schedule may be much too aggressive for high-level athletes after allograft reconstruction. It is possible these allografts have not reached “maturity,” as their autograft counterparts have, and thus are not ready for unrestricted return to play.
Our study had multiple strengths. All reconstructions were performed by 2 senior surgeons with extensive clinical experience. The autograft and allograft reconstructions used the same techniques and rehabilitation protocols. This is one of the largest studies of outcomes of allograft ACL reconstruction and one of the largest studies that used a comparison group of autograft reconstructions. Having a comparison group effectively allowed us to contrast the differences between allograft and autograft tissues. Last, this study evaluated a subgroup of high-level NCAA Division I athletes. Follow-up in the overall study was 40.7%, but follow-up in this subgroup was 91%. The very high follow-up rate in the university population helped us validate the overall results of the study. Study results reinforced the fact that irradiated soft-tissue allograft may not be indicated for ACL reconstruction in a younger, more active patient population and led to a change in approach to ACL reconstruction for Division I intercollegiate athletes at the University of Arizona. Allograft ACL reconstruction is no longer recommended for the intercollegiate athletes at the University of Arizona.
Our study had its limitations. First, it had the inherent biases of a retrospective study. Second, many patients were lost to follow-up. We contacted and surveyed 40.7% of the patients who met the inclusion criteria. We tried reaching them in multiple ways—through US mail, all listed phone numbers, family members, and so forth. Tucson, Arizona is a college town and has a larger transient population, which may have added to the difficulty in contacting patients.
Conclusion
Given the high rates of reoperation and revision surgery with allograft reconstruction in younger patients in this study, we recommend against routine use of irradiated soft-tissue allograft tissue for ACL reconstruction in patients 25 years or younger. In our clinical practices, we prefer using autograft tissue for ACL reconstruction in younger, more active individuals. Irradiated soft-tissue allograft ACL reconstruction is a viable option in the older, less active patient population. Although the overall reoperation rate in this cohort study is acceptable, the revision rate for patients younger than 25 years is concerning and should be taken into account when considering use of irradiated soft-tissue allograft for ACL reconstruction in these younger patients.
Injuries of the anterior cruciate ligament (ACL) are common. Good to excellent long-term results are generally expected in more than 90% of ACL reconstructions.1,2 Although our knowledge of the biomechanics, kinematics, and long-term outcomes of ACL reconstruction is extensive, the ideal graft choice for ACL reconstruction is still up for debate.
Historically, both quadruple-stranded hamstring tendon and bone–patellar tendon–bone (BPTB) autografts have been the most popular graft options for operative reconstruction of the ACL.3 Recently, allograft tissues have become increasingly popular as a graft source. Proponents of allograft ACL reconstruction have cited several advantages over autograft reconstruction, including decreased donor-site morbidity, shorter operative times, and quicker postoperative recovery.4-7 Nevertheless, some authors have recently reported higher rates of both reoperation and graft failure after allograft ACL reconstruction.4,8-11 The 2 senior surgeons in the Sports Medicine Section of the Department of Orthopedic Surgery at the University of Arizona College of Medicine had not recognized such high failure and revision rates in their own clinical practices.
To evaluate the long-term outcomes of allograft ACL reconstruction, we retrospectively reviewed the cases of all patients who underwent allograft or autograft ACL reconstruction by 2 senior surgeons at a single institution over an 8-year period. We hypothesized that the reoperation and revision surgery rates for allograft ACL reconstruction would not be higher than those reported for autograft reconstruction. We also hypothesized that allograft ACL reconstruction failure rates would not be higher for patients younger than 25 years than for patients who are older and less active.
Materials and Methods
This study was approved by the Institutional Review Board at the University of Arizona College of Medicine. We retrospectively reviewed the cases of all patients who underwent primary endoscopic ACL reconstruction at the University of Arizona College of Medicine over an 8-year period (2000–2008). All ACL reconstructions were performed by 2 senior, fellowship-trained sports medicine specialists, including Dr. William A. Grana. Patients were identified from the Current Procedural Terminology (CPT) code for ACL reconstruction. Both autograft and allograft reconstructions were included in the study. Patients undergoing revision ACL reconstruction and patients with multi-ligamentous knee injuries were excluded. All available medical records were reviewed for patient demographics and any concomitant knee pathology. We included patients of all activity levels, patients with acute ACL tears, and patients with chronically ACL-deficient knees. We identified a separate cohort of Division I varsity athletes from the University of Arizona for evaluation. These patients were identified from the injury surveillance system in the athletic training facility of the University of Arizona.
ACL reconstructions at our institution during this 8-year period were performed with both allograft and autograft soft tissue. Allograft tendons were most commonly used. Tibialis anterior allograft was used in the majority of those knees. Tibialis posterior and semitendinosus allografts were used in a small subset of patients. Autograft reconstruction was performed with quadruple-stranded semitendinosus and gracilis tendons. We reviewed operative reports to determine type of graft used for reconstruction.
Patients were assessed clinically by telephone interview and/or mailed survey. They were specifically asked whether there had been any postoperative complications. We reviewed all operative and postoperative follow-up notes for postoperative complications. Objective clinical assessment involved use of the International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, the Tegner-Lysholm Knee Scoring Scale, and the Tegner Activity Scale.
Operative Technique
A standard, transtibial arthroscopically assisted ACL reconstruction was performed in all patients. For autograft reconstruction patients, both the semitendinosus and gracilis tendons were harvested through a small anteromedial incision and prepared to form a quadruple-stranded graft. All allograft tendons were obtained from the Musculoskeletal Transplant Foundation (MTF). Tibialis anterior and tibialis posterior allografts were folded in half to form a double-stranded graft. Alternatively, 2 semitendinosus allografts were prepared in the same fashion as that described for autograft hamstring tendons. The tibial tunnel was placed into the center of the ACL tibial footprint. With use of a transtibial approach, an endoscopic offset guide was used to place the femoral tunnel at the 10- and 2-o’clock positions in the right and left knees, respectively. In almost all cases, the graft was secured on the femoral side with a cortical fixation button. Tibial fixation was obtained with a bioabsorbable interference screw.
After ACL reconstruction, each patient participated in the standard accelerated rehabilitation outlined by Shelbourne and Gray.12 Guided rehabilitation was instituted within 1 week after surgery under the guidance of a physical therapist. Range-of-motion exercises and closed-chain strengthening exercises were begun at this time. The protocol emphasized early return of full terminal extension and normalization of gait patterns. Patients were allowed to return to play only after meeting specific criteria, about 6 months after surgery. Many athletes in our Division I university population are allowed to return to play 5 to 6 months after surgery, after meeting return-to-play criteria.
Statistical Analysis
We used Minitab 14 (Minitab, State College, Pennsylvania) to perform all statistical analyses, unpaired Student t tests to compare IKDC and Tegner-Lysholm results between allograft and autograft groups, and χ2 tests to compare revision and reoperation rates between groups. Significance was set at P = .05.
Results
We identified 362 patients who underwent ACL reconstructions at our institution between 2000 and 2008. Of these patients, 302 met the study inclusion criteria. One-hundred twenty-three (40.7%) of the 302 were available for follow-up by telephone interview and/or mailed questionnaire. This follow-up group consisted of 67 males and 56 females. Mean age at surgery was 29 years (range, 17-53 years). Mean follow-up was 50.3 months (range, 11-111 months). Of the 123 patients, 99 underwent allograft ACL reconstruction, and 24 underwent autograft ACL reconstruction. Seventeen (17%) of the 99 allograft cases required additional surgery (Table 1). The reoperation rate for patients under age 25 years (30.8%) was higher than the rate for patients older than 25 years (Table 2). Regarding patients who underwent additional surgeries, mean scores were lower with allograft (Tegner-Lysholm, 59; IKDC, 54) than with autograft (Tegner-Lysholm, 83; IKDC, 79) (Ps = .0025 and .006, respectively).
Revision rates were 10.1% (allograft group) and 4.2% (autograft group) (Table 1). This difference was not statistically significant (P = .18). In the allograft group, the revision rate was higher for patients younger than 25 years (20.5%) than for patients older than 25 years (3.3%) (Table 2). In comparison, in the autograft group, the revision rate was only 4% for patients younger than 25 years. For younger patients, the higher rate of revision with allograft (vs autograft) was statistically significant (P = .038). For older patients, allograft and autograft revision rates did not differ significantly (P = .19). No patient younger than 25 years required revision reconstruction after autograft ACL reconstruction.
IKDC and Tegner-Lysholm outcome scores for allograft and autograft groups are shown in Table 3. In patients 25 years or younger, IKDC scores were 75.18 after allograft reconstruction and 85.34 after autograft reconstruction—a significant difference (P = .045). In addition, Tegner-Lysholm scores were significantly higher after autograft reconstruction (91.58) than allograft reconstruction (78.19) in these younger patients (P = .003) (Table 3). IKDC and Tegner-Lysholm scores were not significantly different for older patients (Ps = .241 and .211, respectively).
The study also included a subset of 19 primary ACL reconstructions (13 allograft, 6 autograft) performed on Division I athletes from the University of Arizona. (Nineteen [91%] of the 21 athletes in our Division I cohort were available for follow-up.) All these patients were younger than 25 years. All autograft reconstructions were performed with quadruple-stranded gracilis and semitendinosus tendons. ACL graft failure occurred in 8 (62%) of the 13 allograft cases; there were no failures in the autograft group (Table 4). One of the 5 allograft cases that did not fail required multiple surgical débridement procedures for infection, but the graft was ultimately retained. There were no infections among the 6 autograft cases.
Discussion
The ideal graft for ACL reconstruction is still a matter of intense debate. There are many graft options for ACL reconstruction. Both BPTB and hamstring autografts are associated with various graft-specific comorbidities. Anterior knee pain, knee extensor weakness, extension loss, patella fracture, patellofemoral crepitance, and infrapatellar nerve injury have been described with BPTB autografts.13-17 In a meta-analysis of 11 studies comparing BPTB autografts with hamstring autograft, Goldblatt and colleagues17 found more extension loss, kneeling pain, and patellofemoral crepitance in the BPTP group.
Knee flexion weakness, knee flexion loss, increased knee laxity, and saphenous nerve injury have all been described with use of hamstring autografts.16-19 Goldblatt and colleagues17 demonstrated a significant flexion loss in the hamstring group in their meta-analysis as well as increased laxity with both the Lachman test and the pivot shift test. They also found that the hamstring autograft group exhibited side-to-side differences of more than 3 mm on KT-1000 testing when compared with the BPTB autograft group.
Proposed advantages of allograft reconstruction include elimination of donor-site morbidity and/or pain from a less invasive procedure, faster initial recovery, more sizing options, and shorter operative times.4-7 In a 5-year follow-up of patients who had ACL reconstruction with either Achilles allograft or BPTB autograft, Poehling and colleagues7 demonstrated overall similar long-term outcomes between the groups. However, the allograft patients reported less pain 1 and 6 weeks after surgery; better function 1 week, 3 months, and 1 year after surgery; and fewer activity limitations throughout the follow-up period. Lamblin and colleagues20 also found no difference between nonirradiated allograft and autograft tissue in ACL reconstruction in a 2013 meta-analysis of ACL studies published over a 32-year period.
Despite the proposed advantages of allograft ACL reconstruction, several recent studies have demonstrated poorer outcomes in both younger patients and more active patients after allograft reconstruction.8-11,21 In a 2007 meta-analysis, Prodromos and colleagues11 compared a series of allograft reconstructions with previously published data sets of both BPTB and hamstring autografts. They found that allograft reconstructions had significantly lower stability rates than autograft reconstructions. In a case–control study by Borchers and colleagues,10 21 patients with ACL graft failure were identified over a 2-year period, and surgical outcomes were compared with those of 42 age- and sex-matched controls. The authors found higher activity level and allograft use to be risk factors for subsequent graft failure after ACL reconstruction. More important, they showed a multiplicative interaction between higher activity level after ACL reconstruction and allograft use—an interaction that greatly increased the odds for ACL graft failure. Last, in a retrospective review, Singhal and colleagues8 evaluated the outcomes of ACL reconstruction using tibialis anterior tendon allograft and reported a 23.1% revision rate. In addition, 37.7% of patients required repeat surgery. The failure/reoperation rate was 55% for patients 25 years or younger and 24% for patients older than 25 years. The authors recommended not using tibialis anterior allografts in patients 25 years or younger and in patients who frequently engage in level I ACL-dependent sports.
The poor outcomes reported by Singhal and colleagues8 may be related to use of irradiated soft-tissue allografts. In a comparison of nonirradiated BPTB allograft and BPTB autograft in patients 25 years or younger, Barber and colleagues22 found equivalent outcomes at 2-year follow-up. They actually found a higher rate of failure for autograft reconstruction (9.4%) than allograft reconstruction (7.1%). A potential critique of their study is the significant difference between the patient groups’ mean ages: 18.6 years (autograft) versus 20.1 years (allograft). Despite this selection bias, Barber and colleagues22 argued that nonirradiated BPTB allograft is equivalent to BPTB autograft for ACL reconstruction.
Our study is one of the largest allograft studies with a comparison group. The principal findings of this study demonstrate that overall reoperation and revision rates after irradiated soft-tissue allograft ACL reconstruction are higher than those historically quoted for autograft ACL reconstruction. Specifically, allograft patients younger than 25 years had a reoperation rate of 30.8% and a revision rate of 20.5%. (Allograft patients older than 25 years had lower rates of reoperation, 8.3%, and revision, 3.3%.) After revision surgery, autograft patients’ subjective outcomes (IKDC and Tegner-Lysholm scores) were significantly improved compared with those of allograft patients (Ps = .0017 and .0031, respectively). Most compelling, however, is the unexpected and quite concerning 62% failure rate in our high-level Division I intercollegiate athletes.
There are multiple hypotheses regarding the higher failure rates of allograft tissues versus autograft tissues in ACL reconstruction. Processing methods, exposure to ionizing radiation, and the incorporation/ligamentization process have all been cited as possible reasons for allograft failure. All the allograft tendons used in the present study were obtained from MTF, which uses a proprietary “aseptic” processing system that includes washing in buffered saline impregnated with antibiotics (imipenem/cilastatin, amphotericin B, gentamicin) followed by final rinsing in phosphate-buffered saline. The majority of grafts are subjected to low-level irradiation (<2 Mrad/20 kGy) based on the outcomes of MTF’s stringent donor-selection process. Although the washing process has not been shown to alter the structural integrity of donor grafts, multiple studies have outlined the detrimental effects of higher levels of gamma radiation on allograft tissues. Although lower levels are effective against potential bacterial contaminants, a radiation level of 4 Mrad is necessary to kill the human immunodeficiency virus (HIV). Thus, a dose of 4 Mrad or higher is needed to truly “sterilize” a graft. This higher dose is an issue, as it has been known for some time that higher levels of ionizing radiation can have adverse effects on the biomechanical strength of soft-tissue allografts. In fact, ionizing radiation has dose-dependent effects.23-26 Schwartz and colleagues27 showed in a caprine model that radiation exposure at 4 Mrad significantly decreased the biomechanical strength of ACL allografts at 6 months. Balsly and colleagues28 found in a biomechanical study that radiation doses of 18 to 22 Mrad did not significantly affect the mechanical integrity of soft-tissue allografts. Conversely, in an in vivo study, Rappe and colleagues29 showed that Achilles allografts irradiated at a dose of 2.0 to 2.5 Mrad had a failure rate (33%) much higher than that of nonirradiated allografts (2.4%). The radiation dose used by MTF is less than 2 Mrad. Although more than needed to kill bacterial contaminants, this dose is considered by MTF to be below the threshold for biomechanical alterations. Only a minority of grafts is treated without irradiation.
It is possible that any level of radiation affects ligamentization of allograft tissues. Multiple studies have outlined the ligamentization process of autograft tendons in vivo. Patellar tendon autografts undergo central degeneration 2 to 6 weeks after reconstruction, but, by 6 to 12 months, these tendons have structural properties similar to those of the native ACL.30-34 Findings are similar for hamstring autografts.35,36 Goradia and colleagues36 found that, by 52 weeks, semitendinosus autografts transform into a histologic structure similar to that of the normal ACL. Remodeling of allograft tendons has been described as occurring at a much slower rate.27,37-40 Bhatia and colleagues37 demonstrated faster remodeling in autograft tissues versus allograft tissues at early time points in an in vivo rabbit model. Ultimately, differences in graft incorporation and ligamentization may be a primary factor in the higher failure rates of allograft ACL reconstruction. Current rehabilitation protocols may not take into account the longer ligamentization process for allograft tissues. These protocols are largely based on our current understanding of the ligamentization process after autograft reconstruction. It is possible that the rehabilitation program and return-to-play schedule for allograft reconstruction need to be altered to help avoid higher failure rates. The return-to-play protocol at the authors’ institution scheduled most varsity athletes to return to play 6 months after surgery. In some cases, the timetable was shortened, and some athletes were returned to play 5 months after surgery, after meeting all return-to-play criteria. Based on the findings of the present study, this return-to-play schedule may be much too aggressive for high-level athletes after allograft reconstruction. It is possible these allografts have not reached “maturity,” as their autograft counterparts have, and thus are not ready for unrestricted return to play.
Our study had multiple strengths. All reconstructions were performed by 2 senior surgeons with extensive clinical experience. The autograft and allograft reconstructions used the same techniques and rehabilitation protocols. This is one of the largest studies of outcomes of allograft ACL reconstruction and one of the largest studies that used a comparison group of autograft reconstructions. Having a comparison group effectively allowed us to contrast the differences between allograft and autograft tissues. Last, this study evaluated a subgroup of high-level NCAA Division I athletes. Follow-up in the overall study was 40.7%, but follow-up in this subgroup was 91%. The very high follow-up rate in the university population helped us validate the overall results of the study. Study results reinforced the fact that irradiated soft-tissue allograft may not be indicated for ACL reconstruction in a younger, more active patient population and led to a change in approach to ACL reconstruction for Division I intercollegiate athletes at the University of Arizona. Allograft ACL reconstruction is no longer recommended for the intercollegiate athletes at the University of Arizona.
Our study had its limitations. First, it had the inherent biases of a retrospective study. Second, many patients were lost to follow-up. We contacted and surveyed 40.7% of the patients who met the inclusion criteria. We tried reaching them in multiple ways—through US mail, all listed phone numbers, family members, and so forth. Tucson, Arizona is a college town and has a larger transient population, which may have added to the difficulty in contacting patients.
Conclusion
Given the high rates of reoperation and revision surgery with allograft reconstruction in younger patients in this study, we recommend against routine use of irradiated soft-tissue allograft tissue for ACL reconstruction in patients 25 years or younger. In our clinical practices, we prefer using autograft tissue for ACL reconstruction in younger, more active individuals. Irradiated soft-tissue allograft ACL reconstruction is a viable option in the older, less active patient population. Although the overall reoperation rate in this cohort study is acceptable, the revision rate for patients younger than 25 years is concerning and should be taken into account when considering use of irradiated soft-tissue allograft for ACL reconstruction in these younger patients.
1. Schepsis AA, Busconi BD. Sports Medicine. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
2. Campbell WC, Canale ST, Beaty JH. Campbell’s Operative Orthopaedics. 11th ed. Philadelphia, PA: Mosby/Elsevier; 2008.
3. Sherman OH, Banffy MB. Anterior cruciate ligament reconstruction: which graft is best? Arthroscopy. 2004;20(9):974-980.
4. Lee JH, Bae DK, Song SJ, Cho SM, Yoon KH. Comparison of clinical results and second-look arthroscopy findings after arthroscopic anterior cruciate ligament reconstruction using 3 different types of grafts. Arthroscopy. 2010;26(1):41-49.
5. Sun K, Tian SQ, Zhang JH, Xia CS, Zhang CL, Yu TB. Anterior cruciate ligament reconstruction with bone-patellar tendon-bone autograft versus allograft. Arthroscopy. 2009;25(7):750-759.
6. Kuhn MA, Ross G. Allografts in the treatment of anterior cruciate ligament injuries. Sports Med Arthrosc Rev. 2007;15(3):133-138.
7. Poehling GG, Curl WW, Lee CA, et al. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: allograft versus autograft. Arthroscopy. 2005;21(7):774-785.
8. Singhal MC, Gardiner JR, Johnson DL. Failure of primary anterior cruciate ligament surgery using anterior tibialis allograft. Arthroscopy. 2007;23(5):469-475.
9. Barrett GR, Luber K, Replogle WH, Manley JL. Allograft anterior cruciate ligament reconstruction in the young, active patient: Tegner activity level and failure rate. Arthroscopy. 2010;26(12):1593-1601.
10. Borchers JR, Pedroza A, Kaeding C. Activity level and graft type as risk factors for anterior cruciate ligament graft failure: a case–control study. Am J Sports Med. 2009;37(12):2362-2367.
11. Prodromos C, Joyce B, Shi K. A meta-analysis of stability of autografts compared to allografts after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):851-856.
12. Shelbourne KD, Gray T. Anterior cruciate ligament reconstruction with autogenous patellar tendon graft followed by accelerated rehabilitation. A two- to nine-year followup. Am J Sports Med. 1997;25(6):786-795.
13. Rosenberg TD, Franklin JL, Baldwin GN, Nelson KA. Extensor mechanism function after patellar tendon graft harvest for anterior cruciate ligament reconstruction. Am J Sports Med. 1992;20(5):519-525.
14. Piva SR, Childs JD, Klucinec BM, Irrgang JJ, Almeida GJ, Fitzgerald GK. Patella fracture during rehabilitation after bone–patellar tendon–bone anterior cruciate ligament reconstruction: 2 case reports. J Orthop Sports Phys Ther. 2009;39(4):278-286.
15. Lee GH, McCulloch P, Cole BJ, Bush-Joseph CA, Bach BR Jr. The incidence of acute patellar tendon harvest complications for anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(2):162-166.
16. Kartus J, Movin T, Karlsson J. Donor-site morbidity and anterior knee problems after anterior cruciate ligament reconstruction using autografts. Arthroscopy. 2001;17(9):971-980.
17. Goldblatt JP, Fitzsimmons SE, Balk E, Richmond JC. Reconstruction of the anterior cruciate ligament: meta-analysis of patellar tendon versus hamstring tendon autograft. Arthroscopy. 2005;21(7):791-803.
18. Freedman KB, D’Amato MJ, Nedeff DD, Kaz A, Bach BR Jr. Arthroscopic anterior cruciate ligament reconstruction: a metaanalysis comparing patellar tendon and hamstring tendon autografts. Am J Sports Med. 2003;31(1):2-11.
19. Yunes M, Richmond JC, Engels EA, Pinczewski LA. Patellar versus hamstring tendons in anterior cruciate ligament reconstruction: a meta-analysis. Arthroscopy. 2001;17(3):248-257.
20. Lamblin CJ, Waterman BR, Lubowitz JH. Anterior cruciate ligament reconstruction with autografts compared with non-irradiated, non-chemically treated allografts. Arthroscopy. 2013;29(6):1113-1122.
21. Pallis M, Svoboda SJ, Cameron KL, Owens BD. Survival comparison of allograft and autograft anterior cruciate ligament reconstruction at the United States Military Academy. Am J Sports Med. 2012;40(6):1242-1246.
22. Barber FA, Cowden CH 3rd, Sanders EJ. Revision rates after anterior cruciate ligament reconstruction using bone–patellar tendon–bone allograft or autograft in a population 25 years old and younger. Arthroscopy. 2014;30(4):483-491.
23. Salehpour A, Butler DL, Proch FS, et al. Dose-dependent response of gamma irradiation on mechanical properties and related biochemical composition of goat bone–patellar tendon–bone allografts. J Orthop Res. 1995;13(6):898-906.
24. Gibbons MJ, Butler DL, Grood ES, Bylski-Austrow DI, Levy MS, Noyes FR. Effects of gamma irradiation on the initial mechanical and material properties of goat bone–patellar tendon–bone allografts. J Orthop Res. 1991;9(2):209-218.
25. Fideler BM, Vangsness CT Jr, Lu B, Orlando C, Moore T. Gamma irradiation: effects on biomechanical properties of human bone–patellar tendon–bone allografts. Am J Sports Med. 1995;23(5):643-646.
26. De Deyne P, Haut RC. Some effects of gamma irradiation on patellar tendon allografts. Connect Tissue Res. 1991;27(1):51-62.
27. Schwartz HE, Matava MJ, Proch FS, et al. The effect of gamma irradiation on anterior cruciate ligament allograft biomechanical and biochemical properties in the caprine model at time zero and at 6 months after surgery. Am J Sports Med. 2006;34(11):1747-1755.
28. Balsly CR, Cotter AT, Williams LA, Gaskins BD, Moore MA, Wolfinbarger L Jr. Effect of low dose and moderate dose gamma irradiation on the mechanical properties of bone and soft tissue allografts. Cell Tissue Bank. 2008;9(4):289-298.
29. Rappe M, Horodyski M, Meister K, Indelicato PA. Nonirradiated versus irradiated Achilles allograft: in vivo failure comparison. Am J Sports Med. 2007;35(10):1653-1658.
30. Amiel D, Kleiner JB, Akeson WH. The natural history of the anterior cruciate ligament autograft of patellar tendon origin. Am J Sports Med. 1986;14(6):449-462.
31. Amiel D, Kleiner JB, Roux RD, Harwood FL, Akeson WH. The phenomenon of “ligamentization”: anterior cruciate ligament reconstruction with autogenous patellar tendon. J Orthop Res. 1986;4(2):162-172.
32. Arnoczky SP, Tarvin GB, Marshall JL. Anterior cruciate ligament replacement using patellar tendon. An evaluation of graft revascularization in the dog. J Bone Joint Surg Am. 1982;64(2):217-224.
33. Ballock RT, Woo SL, Lyon RM, Hollis JM, Akeson WH. Use of patellar tendon autograft for anterior cruciate ligament reconstruction in the rabbit: a long-term histologic and biomechanical study. J Orthop Res. 1989;7(4):474-485.
34. Clancy WG Jr, Narechania RG, Rosenberg TD, Gmeiner JG, Wisnefske DD, Lange TA. Anterior and posterior cruciate ligament reconstruction in rhesus monkeys. J Bone Joint Surg Am. 1981;63(8):1270-1284.
35. Blickenstaff KR, Grana WA, Egle D. Analysis of a semitendinosus autograft in a rabbit model. Am J Sports Med. 1997;25(4):554-559.
36. Goradia VK, Rochat MC, Kida M, Grana WA. Natural history of a hamstring tendon autograft used for anterior cruciate ligament reconstruction in a sheep model. Am J Sports Med. 2000;28(1):40-46.
37. Bhatia S, Bell R, Frank RM, et al. Bony incorporation of soft tissue anterior cruciate ligament grafts in an animal model: autograft versus allograft with low-dose gamma irradiation. Am J Sports Med. 2012;40(8):1789-1798.
38. Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21(2):176-185.
39. Goertzen MJ, Clahsen H, Schulitz KP. Anterior cruciate ligament reconstruction using cryopreserved irradiated bone-ACL-bone-allograft transplants. Knee Surg Sports Traumatol Arthrosc. 1994;2(3):150-157.
40. Mae T, Shino K, Maeda A, Toritsuka Y, Horibe S, Ochi T. Effect of gamma irradiation on remodeling process of tendon allograft. Clin Orthop. 2003;(414):305-314.
1. Schepsis AA, Busconi BD. Sports Medicine. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
2. Campbell WC, Canale ST, Beaty JH. Campbell’s Operative Orthopaedics. 11th ed. Philadelphia, PA: Mosby/Elsevier; 2008.
3. Sherman OH, Banffy MB. Anterior cruciate ligament reconstruction: which graft is best? Arthroscopy. 2004;20(9):974-980.
4. Lee JH, Bae DK, Song SJ, Cho SM, Yoon KH. Comparison of clinical results and second-look arthroscopy findings after arthroscopic anterior cruciate ligament reconstruction using 3 different types of grafts. Arthroscopy. 2010;26(1):41-49.
5. Sun K, Tian SQ, Zhang JH, Xia CS, Zhang CL, Yu TB. Anterior cruciate ligament reconstruction with bone-patellar tendon-bone autograft versus allograft. Arthroscopy. 2009;25(7):750-759.
6. Kuhn MA, Ross G. Allografts in the treatment of anterior cruciate ligament injuries. Sports Med Arthrosc Rev. 2007;15(3):133-138.
7. Poehling GG, Curl WW, Lee CA, et al. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: allograft versus autograft. Arthroscopy. 2005;21(7):774-785.
8. Singhal MC, Gardiner JR, Johnson DL. Failure of primary anterior cruciate ligament surgery using anterior tibialis allograft. Arthroscopy. 2007;23(5):469-475.
9. Barrett GR, Luber K, Replogle WH, Manley JL. Allograft anterior cruciate ligament reconstruction in the young, active patient: Tegner activity level and failure rate. Arthroscopy. 2010;26(12):1593-1601.
10. Borchers JR, Pedroza A, Kaeding C. Activity level and graft type as risk factors for anterior cruciate ligament graft failure: a case–control study. Am J Sports Med. 2009;37(12):2362-2367.
11. Prodromos C, Joyce B, Shi K. A meta-analysis of stability of autografts compared to allografts after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):851-856.
12. Shelbourne KD, Gray T. Anterior cruciate ligament reconstruction with autogenous patellar tendon graft followed by accelerated rehabilitation. A two- to nine-year followup. Am J Sports Med. 1997;25(6):786-795.
13. Rosenberg TD, Franklin JL, Baldwin GN, Nelson KA. Extensor mechanism function after patellar tendon graft harvest for anterior cruciate ligament reconstruction. Am J Sports Med. 1992;20(5):519-525.
14. Piva SR, Childs JD, Klucinec BM, Irrgang JJ, Almeida GJ, Fitzgerald GK. Patella fracture during rehabilitation after bone–patellar tendon–bone anterior cruciate ligament reconstruction: 2 case reports. J Orthop Sports Phys Ther. 2009;39(4):278-286.
15. Lee GH, McCulloch P, Cole BJ, Bush-Joseph CA, Bach BR Jr. The incidence of acute patellar tendon harvest complications for anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(2):162-166.
16. Kartus J, Movin T, Karlsson J. Donor-site morbidity and anterior knee problems after anterior cruciate ligament reconstruction using autografts. Arthroscopy. 2001;17(9):971-980.
17. Goldblatt JP, Fitzsimmons SE, Balk E, Richmond JC. Reconstruction of the anterior cruciate ligament: meta-analysis of patellar tendon versus hamstring tendon autograft. Arthroscopy. 2005;21(7):791-803.
18. Freedman KB, D’Amato MJ, Nedeff DD, Kaz A, Bach BR Jr. Arthroscopic anterior cruciate ligament reconstruction: a metaanalysis comparing patellar tendon and hamstring tendon autografts. Am J Sports Med. 2003;31(1):2-11.
19. Yunes M, Richmond JC, Engels EA, Pinczewski LA. Patellar versus hamstring tendons in anterior cruciate ligament reconstruction: a meta-analysis. Arthroscopy. 2001;17(3):248-257.
20. Lamblin CJ, Waterman BR, Lubowitz JH. Anterior cruciate ligament reconstruction with autografts compared with non-irradiated, non-chemically treated allografts. Arthroscopy. 2013;29(6):1113-1122.
21. Pallis M, Svoboda SJ, Cameron KL, Owens BD. Survival comparison of allograft and autograft anterior cruciate ligament reconstruction at the United States Military Academy. Am J Sports Med. 2012;40(6):1242-1246.
22. Barber FA, Cowden CH 3rd, Sanders EJ. Revision rates after anterior cruciate ligament reconstruction using bone–patellar tendon–bone allograft or autograft in a population 25 years old and younger. Arthroscopy. 2014;30(4):483-491.
23. Salehpour A, Butler DL, Proch FS, et al. Dose-dependent response of gamma irradiation on mechanical properties and related biochemical composition of goat bone–patellar tendon–bone allografts. J Orthop Res. 1995;13(6):898-906.
24. Gibbons MJ, Butler DL, Grood ES, Bylski-Austrow DI, Levy MS, Noyes FR. Effects of gamma irradiation on the initial mechanical and material properties of goat bone–patellar tendon–bone allografts. J Orthop Res. 1991;9(2):209-218.
25. Fideler BM, Vangsness CT Jr, Lu B, Orlando C, Moore T. Gamma irradiation: effects on biomechanical properties of human bone–patellar tendon–bone allografts. Am J Sports Med. 1995;23(5):643-646.
26. De Deyne P, Haut RC. Some effects of gamma irradiation on patellar tendon allografts. Connect Tissue Res. 1991;27(1):51-62.
27. Schwartz HE, Matava MJ, Proch FS, et al. The effect of gamma irradiation on anterior cruciate ligament allograft biomechanical and biochemical properties in the caprine model at time zero and at 6 months after surgery. Am J Sports Med. 2006;34(11):1747-1755.
28. Balsly CR, Cotter AT, Williams LA, Gaskins BD, Moore MA, Wolfinbarger L Jr. Effect of low dose and moderate dose gamma irradiation on the mechanical properties of bone and soft tissue allografts. Cell Tissue Bank. 2008;9(4):289-298.
29. Rappe M, Horodyski M, Meister K, Indelicato PA. Nonirradiated versus irradiated Achilles allograft: in vivo failure comparison. Am J Sports Med. 2007;35(10):1653-1658.
30. Amiel D, Kleiner JB, Akeson WH. The natural history of the anterior cruciate ligament autograft of patellar tendon origin. Am J Sports Med. 1986;14(6):449-462.
31. Amiel D, Kleiner JB, Roux RD, Harwood FL, Akeson WH. The phenomenon of “ligamentization”: anterior cruciate ligament reconstruction with autogenous patellar tendon. J Orthop Res. 1986;4(2):162-172.
32. Arnoczky SP, Tarvin GB, Marshall JL. Anterior cruciate ligament replacement using patellar tendon. An evaluation of graft revascularization in the dog. J Bone Joint Surg Am. 1982;64(2):217-224.
33. Ballock RT, Woo SL, Lyon RM, Hollis JM, Akeson WH. Use of patellar tendon autograft for anterior cruciate ligament reconstruction in the rabbit: a long-term histologic and biomechanical study. J Orthop Res. 1989;7(4):474-485.
34. Clancy WG Jr, Narechania RG, Rosenberg TD, Gmeiner JG, Wisnefske DD, Lange TA. Anterior and posterior cruciate ligament reconstruction in rhesus monkeys. J Bone Joint Surg Am. 1981;63(8):1270-1284.
35. Blickenstaff KR, Grana WA, Egle D. Analysis of a semitendinosus autograft in a rabbit model. Am J Sports Med. 1997;25(4):554-559.
36. Goradia VK, Rochat MC, Kida M, Grana WA. Natural history of a hamstring tendon autograft used for anterior cruciate ligament reconstruction in a sheep model. Am J Sports Med. 2000;28(1):40-46.
37. Bhatia S, Bell R, Frank RM, et al. Bony incorporation of soft tissue anterior cruciate ligament grafts in an animal model: autograft versus allograft with low-dose gamma irradiation. Am J Sports Med. 2012;40(8):1789-1798.
38. Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21(2):176-185.
39. Goertzen MJ, Clahsen H, Schulitz KP. Anterior cruciate ligament reconstruction using cryopreserved irradiated bone-ACL-bone-allograft transplants. Knee Surg Sports Traumatol Arthrosc. 1994;2(3):150-157.
40. Mae T, Shino K, Maeda A, Toritsuka Y, Horibe S, Ochi T. Effect of gamma irradiation on remodeling process of tendon allograft. Clin Orthop. 2003;(414):305-314.
Coding Practices Affect the Cost of Distal Radius Fracture Care
Challenging the Conventional Wisdom of Primary Care Medicine
Recently, I heard someone say we have a severe shortage of primary care physicians in Arizona, in the context of noting 46% of our medical school class is to enter a primary care residency. The implication is that there are not enough students being directed to primary care.
Primary care physicians are certainly the faculty students have the most intimate relationships with on a daily basis and from whom they receive the majority of their mentoring.
However, students recognize that for many large areas of content, such as musculoskeletal medicine, the primary care physician is not the best person to provide information or teach physical examination skills. If students choose a primary care residency, about 30% of the patients they see will have musculoskeletal problems. Physicians will need to know how to develop a plan of management and start treatment before the patient is referred to an orthopedic surgeon. The problem is, a majority of physicians do not know and simply send the patient on without appropriate evaluation or the most basic treatment. The need for more primary care physicians is a myth and this concept increases the cost of healthcare when the primary care physician controls access to the patient. Patients will be served just as well if the initial visit was to a nurse practitioner or physician assistant and then triaged on to the appropriate specialist.
This use of primary care doctors as the access point has occurred over the last 20 years in sports medicine as well. The orthopedic surgeon has become a consultant and not the team doctor as it was in past years. Some of the most useful experiences I had were when I was able to sit down with coaches and athletes to discuss options and the relative value of choices, as well as indications for one treatment over another. This is no longer possible because there are 2 layers of insulation from those relationships produced by the presence of a trainer and a “team physician.”
This commentary is based on 37 years of observation of this evolution in the training room and the office treating musculoskeletal problems, which have had treatment delayed or misdiagnosed without the most basic of care rendered. A stress fracture with a deformity so large on the anterior tibia it was eroding the skin when finally I was asked to see the athlete for this “obvious” abscess. A quick check with an image intensifier and the stress fracture was identified after 3 months of prior “treatment.” A referral of a young woman with an inflamed knee, with no history of injury and no treatment or workup, has no signs of mechanical complaints or laxity. When evaluated in my office, she has inflammatory arthritis with an elevated sedimentation rate, C-reactive protein, anti-cyclic citrullinated peptide, and positive rheumatoid factor. The patient said “I knew this wasn’t a torn meniscus, but it was easier to see you than my primary care physician.” Would the patient have been better served to see a nurse practitioner in an urgent care setting started on an NSAID and icing with a referral to a rheumatologist as a better and more cost effective solution to the problem?
These are everyday examples of the need for more informed first responder care of musculoskeletal problems, but is the solution more primary care physicians, or a better triage and initial management approach which are not currently part of the primary care curriculum? I know primary care physicians are busy dealing with the epidemic of mental health problems, obesity and its complications, and complicated patients who have 2 pages of medications to be juggled, which is a common occurrence in our practice. So, I am not singling out these hard working physicians. I do ask for a more efficient approach to musculoskeletal problems and a better approach to triage of these patients, which will result in fewer complications. I believe the increased numbers of providers should be in physician extenders in the short term and better education of primary care physicians in initial management of musculoskeletal problems in the long term. Orthopedic surgeons can take the lead in creating guidelines for management of common symptom sets and point treatment in a reasonable direction.
If primary care is to be the focal point of our national healthcare system, then there should be a discussion of how this initial care is rendered and what its goal should be. Right now, the initial care leads to other problems which complication the definitive care of the patient and cost the healthcare system more than it should. The current approach has evovled, as primary care physicians have become busier dealing with an increasingly larger number of patients on the Centers for Medicare and Medicaid Service rolls.
Perhaps there is an alternative to more primary care physicians to provide the basic care. The current system and its evolution to the full blown Affordable Health Care Act has significant waste in it which has not been addressed in any way by the President and Congress. This discussion calls attention to a portion of this waste, which is substanital and could be changed with nonphysician providers and better education of students and residents.
The conventional wisdom has been to provide more physicians, but in this instance, that may not be the correct approach. Will Rogers said, “It isn’t what we don’t know that gives us trouble, it’s what we know that ain’t so.” The hole we have dug in this country is one which requires extraordinary thinking to fill. Hopefully in this case it will include a consideration of methods and personnel.
Author's Disclosure Statement. The author reports no actual or potential conflict of interest in relation to this article.
Recently, I heard someone say we have a severe shortage of primary care physicians in Arizona, in the context of noting 46% of our medical school class is to enter a primary care residency. The implication is that there are not enough students being directed to primary care.
Primary care physicians are certainly the faculty students have the most intimate relationships with on a daily basis and from whom they receive the majority of their mentoring.
However, students recognize that for many large areas of content, such as musculoskeletal medicine, the primary care physician is not the best person to provide information or teach physical examination skills. If students choose a primary care residency, about 30% of the patients they see will have musculoskeletal problems. Physicians will need to know how to develop a plan of management and start treatment before the patient is referred to an orthopedic surgeon. The problem is, a majority of physicians do not know and simply send the patient on without appropriate evaluation or the most basic treatment. The need for more primary care physicians is a myth and this concept increases the cost of healthcare when the primary care physician controls access to the patient. Patients will be served just as well if the initial visit was to a nurse practitioner or physician assistant and then triaged on to the appropriate specialist.
This use of primary care doctors as the access point has occurred over the last 20 years in sports medicine as well. The orthopedic surgeon has become a consultant and not the team doctor as it was in past years. Some of the most useful experiences I had were when I was able to sit down with coaches and athletes to discuss options and the relative value of choices, as well as indications for one treatment over another. This is no longer possible because there are 2 layers of insulation from those relationships produced by the presence of a trainer and a “team physician.”
This commentary is based on 37 years of observation of this evolution in the training room and the office treating musculoskeletal problems, which have had treatment delayed or misdiagnosed without the most basic of care rendered. A stress fracture with a deformity so large on the anterior tibia it was eroding the skin when finally I was asked to see the athlete for this “obvious” abscess. A quick check with an image intensifier and the stress fracture was identified after 3 months of prior “treatment.” A referral of a young woman with an inflamed knee, with no history of injury and no treatment or workup, has no signs of mechanical complaints or laxity. When evaluated in my office, she has inflammatory arthritis with an elevated sedimentation rate, C-reactive protein, anti-cyclic citrullinated peptide, and positive rheumatoid factor. The patient said “I knew this wasn’t a torn meniscus, but it was easier to see you than my primary care physician.” Would the patient have been better served to see a nurse practitioner in an urgent care setting started on an NSAID and icing with a referral to a rheumatologist as a better and more cost effective solution to the problem?
These are everyday examples of the need for more informed first responder care of musculoskeletal problems, but is the solution more primary care physicians, or a better triage and initial management approach which are not currently part of the primary care curriculum? I know primary care physicians are busy dealing with the epidemic of mental health problems, obesity and its complications, and complicated patients who have 2 pages of medications to be juggled, which is a common occurrence in our practice. So, I am not singling out these hard working physicians. I do ask for a more efficient approach to musculoskeletal problems and a better approach to triage of these patients, which will result in fewer complications. I believe the increased numbers of providers should be in physician extenders in the short term and better education of primary care physicians in initial management of musculoskeletal problems in the long term. Orthopedic surgeons can take the lead in creating guidelines for management of common symptom sets and point treatment in a reasonable direction.
If primary care is to be the focal point of our national healthcare system, then there should be a discussion of how this initial care is rendered and what its goal should be. Right now, the initial care leads to other problems which complication the definitive care of the patient and cost the healthcare system more than it should. The current approach has evovled, as primary care physicians have become busier dealing with an increasingly larger number of patients on the Centers for Medicare and Medicaid Service rolls.
Perhaps there is an alternative to more primary care physicians to provide the basic care. The current system and its evolution to the full blown Affordable Health Care Act has significant waste in it which has not been addressed in any way by the President and Congress. This discussion calls attention to a portion of this waste, which is substanital and could be changed with nonphysician providers and better education of students and residents.
The conventional wisdom has been to provide more physicians, but in this instance, that may not be the correct approach. Will Rogers said, “It isn’t what we don’t know that gives us trouble, it’s what we know that ain’t so.” The hole we have dug in this country is one which requires extraordinary thinking to fill. Hopefully in this case it will include a consideration of methods and personnel.
Author's Disclosure Statement. The author reports no actual or potential conflict of interest in relation to this article.
Recently, I heard someone say we have a severe shortage of primary care physicians in Arizona, in the context of noting 46% of our medical school class is to enter a primary care residency. The implication is that there are not enough students being directed to primary care.
Primary care physicians are certainly the faculty students have the most intimate relationships with on a daily basis and from whom they receive the majority of their mentoring.
However, students recognize that for many large areas of content, such as musculoskeletal medicine, the primary care physician is not the best person to provide information or teach physical examination skills. If students choose a primary care residency, about 30% of the patients they see will have musculoskeletal problems. Physicians will need to know how to develop a plan of management and start treatment before the patient is referred to an orthopedic surgeon. The problem is, a majority of physicians do not know and simply send the patient on without appropriate evaluation or the most basic treatment. The need for more primary care physicians is a myth and this concept increases the cost of healthcare when the primary care physician controls access to the patient. Patients will be served just as well if the initial visit was to a nurse practitioner or physician assistant and then triaged on to the appropriate specialist.
This use of primary care doctors as the access point has occurred over the last 20 years in sports medicine as well. The orthopedic surgeon has become a consultant and not the team doctor as it was in past years. Some of the most useful experiences I had were when I was able to sit down with coaches and athletes to discuss options and the relative value of choices, as well as indications for one treatment over another. This is no longer possible because there are 2 layers of insulation from those relationships produced by the presence of a trainer and a “team physician.”
This commentary is based on 37 years of observation of this evolution in the training room and the office treating musculoskeletal problems, which have had treatment delayed or misdiagnosed without the most basic of care rendered. A stress fracture with a deformity so large on the anterior tibia it was eroding the skin when finally I was asked to see the athlete for this “obvious” abscess. A quick check with an image intensifier and the stress fracture was identified after 3 months of prior “treatment.” A referral of a young woman with an inflamed knee, with no history of injury and no treatment or workup, has no signs of mechanical complaints or laxity. When evaluated in my office, she has inflammatory arthritis with an elevated sedimentation rate, C-reactive protein, anti-cyclic citrullinated peptide, and positive rheumatoid factor. The patient said “I knew this wasn’t a torn meniscus, but it was easier to see you than my primary care physician.” Would the patient have been better served to see a nurse practitioner in an urgent care setting started on an NSAID and icing with a referral to a rheumatologist as a better and more cost effective solution to the problem?
These are everyday examples of the need for more informed first responder care of musculoskeletal problems, but is the solution more primary care physicians, or a better triage and initial management approach which are not currently part of the primary care curriculum? I know primary care physicians are busy dealing with the epidemic of mental health problems, obesity and its complications, and complicated patients who have 2 pages of medications to be juggled, which is a common occurrence in our practice. So, I am not singling out these hard working physicians. I do ask for a more efficient approach to musculoskeletal problems and a better approach to triage of these patients, which will result in fewer complications. I believe the increased numbers of providers should be in physician extenders in the short term and better education of primary care physicians in initial management of musculoskeletal problems in the long term. Orthopedic surgeons can take the lead in creating guidelines for management of common symptom sets and point treatment in a reasonable direction.
If primary care is to be the focal point of our national healthcare system, then there should be a discussion of how this initial care is rendered and what its goal should be. Right now, the initial care leads to other problems which complication the definitive care of the patient and cost the healthcare system more than it should. The current approach has evovled, as primary care physicians have become busier dealing with an increasingly larger number of patients on the Centers for Medicare and Medicaid Service rolls.
Perhaps there is an alternative to more primary care physicians to provide the basic care. The current system and its evolution to the full blown Affordable Health Care Act has significant waste in it which has not been addressed in any way by the President and Congress. This discussion calls attention to a portion of this waste, which is substanital and could be changed with nonphysician providers and better education of students and residents.
The conventional wisdom has been to provide more physicians, but in this instance, that may not be the correct approach. Will Rogers said, “It isn’t what we don’t know that gives us trouble, it’s what we know that ain’t so.” The hole we have dug in this country is one which requires extraordinary thinking to fill. Hopefully in this case it will include a consideration of methods and personnel.
Author's Disclosure Statement. The author reports no actual or potential conflict of interest in relation to this article.