User login
Tension Pneumothorax After Ultrasound-Guided Interscalene Block and Shoulder Arthroscopy
Interscalene brachial plexus anesthesia is commonly used for arthroscopic and open procedures of the shoulder. This regional anesthetic targets the trunks of the brachial plexus and anesthetizes the area about the shoulder and proximal arm. Its use may obviate the need for concomitant general anesthesia, potentially reducing the use of postoperative intravenous and oral pain medication. Furthermore, patients often bypass the acute postoperative anesthesia care unit and proceed directly to the ambulatory unit, permitting earlier hospital discharge. Previous reports in the literature have demonstrated higher rates of neurologic, cardiac, and pulmonary complications from this procedure; in particular, the incidence of pneumothorax was reported as high as 3%.1 Techniques to localize the nerves, such as electrical nerve stimulation and, more recently, ultrasound guidance, have reduced these complication rates.2,3 Successful administration of the block has been shown to result in satisfactory postoperative pain relief.2 However, ultrasound-guided interscalene nerve blocks remain operator-dependent and complications may still occur.
We report a case of tension pneumothorax after arthroscopic rotator cuff repair and subacromial decompression with an ultrasound-guided interscalene block. Immediate recognition and treatment of this complication resulted in a good clinical outcome. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old woman presented with 3 months of right shoulder pain after a fall. Examination was pertinent for weakness in forward elevation and positive rotator cuff impingement signs. She remained symptomatic despite a course of nonsurgical management that included cortisone injections and physical therapy. Magnetic resonance imaging of the shoulder showed a full-thickness supraspinatus tear with minimal fatty atrophy. After a discussion of her treatment options, she elected to undergo an arthroscopic rotator cuff repair with subacromial decompression. An evaluation by her internist revealed no pertinent medical history apart from obesity (body mass index, 36). Specifically, there was no reported history of chronic obstructive pulmonary disease or asthma. She denied any prior cigarette smoking.
The patient was evaluated by the regional anesthesia team and was classified as a class 2 airway. An interscalene brachial plexus block was performed using a 2-inch, 22-gauge needle inserted into the interscalene groove. Using an out-of-plane technique under direct ultrasound guidance, 30 mL of 0.52% ropivacaine was injected. The block was considered successful, and no complications, such as resistance, paresthesias, pain, or blood on aspiration, were noted during injection. The patient had no complaints of chest pain or shortness of breath immediately afterward, and all vital signs were stable throughout the procedure.
The patient was brought to the operating room and placed in the beach-chair position. Induction for general anesthesia was started 15 minutes after the regional anesthetic, with 2 intubation attempts necessary because of poor airway visualization. After placement of the endotracheal tube, breath sounds were noted to be equal bilaterally. The arthroscopic procedure consisted of double-row rotator cuff repair, subacromial decompression, and débridement of the glenohumeral joint for synovitis, using standard arthroscopic portals. There were no difficulties with trocar placement, and bleeding was minimal throughout the case. The total surgical time was 150 minutes and a pump pressure of 30 mm Hg was maintained during the arthroscopy.
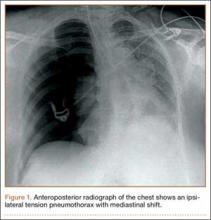
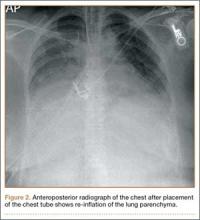
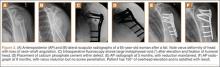
Within the first 60 minutes of the start of the arthroscopic procedure, the patient was noted to be intermittently hypotensive with mean arterial pressure (MAP) ranging from the 30s to 130s mm Hg and pulse in the 70 to 80 beats/min range. FiO2 in the 85% to 95% range was maintained throughout the procedure. During that time, 50 μg phenylephrine was administered on 4 separate occasions to maintain her blood pressure. The labile blood pressure was attributed by the anesthesiologist to the beach-chair position. During an attempted extubation upon conclusion of the surgery, the patient became hypotensive with MAP that ranged from the 40s to 60s mm Hg and tachycardic to 90 beats/min. The oxygen saturation was in the low 90s and tidal volume was poor. Absent lung sounds were noted on the right chest. An urgent portable chest radiograph showed a large right-sided tension pneumothorax with mediastinal shift (Figure 1). After an immediate general surgery consultation, a chest tube was placed in the operating room. The patient’s vital signs improved and a repeat chest radiograph revealed successful re-expansion of the lung (Figure 2). She was transferred to the acute postoperative anesthesia care unit and extubated in the intensive care unit later that day.
The patient’s chest tube was removed 2 days later and she was discharged home on hospital day 5 with a completely resolved pneumothorax. She was seen 1 week later in the office for a postoperative visit and reported feeling well without chest pain or shortness of breath.
Discussion
Interscalene brachial plexus anesthesia was first described by Winnie4 in 1970. This block targets the trunks of the brachial plexus, which are enclosed in a fascial sheath between the anterior and middle scalene muscles. In this region lie several structures at risk: the phrenic nerve superficially and inferiorly; the carotid sheath located superficially and medially; the subclavian artery parallel to the trunks; and the cupula of the lung that lies deep and inferior to the anterior scalene muscle. Recognized complications of the block include vocal hoarseness, Horner syndrome, and hemidiaphragmatic paresis caused by the temporary blockade of the ipsilateral recurrent laryngeal nerve, stellate ganglion, and phrenic nerve, in that order.5 Use of the interscalene block has been associated with minimal risk for pneumothorax, because the needle entry point is superior and directed away from the lung pleura.6 This is in contrast to the more inferiorly placed supraclavicular block, located in closer proximity to the lung cupula.5
Two different approaches are commonly used during ultrasound-guided nerve blocks. The in-plane approach generates a long-axis view of the needle by advancing the needle parallel with the long axis of the ultrasound probe. While this allows direct visualization of the needle tip, it requires deeper needle insertion from lateral to medial, causing puncture of the middle scalene muscle that may increase patient discomfort and risk nerve injury within the muscle.7 The out-of-plane approach used on our patient involves needle insertion parallel to the brachial plexus, but along the short axis of the ultrasound probe. Although this permits the operator to assess the periphery of the nerve, it may lead to poor needle-tip visualization during the procedure. As a result, operators often use a combination of tissue disturbance and “hydrolocation,” in which fluid is injected to indicate the needle-tip location.8,9
Tension pneumothorax represents the accumulation of air in the pleural space that leads to impaired pulmonary and cardiac function. It is often caused by disruption or puncture of the parietal or visceral pleura, creating a connection between the alveoli and pleural cavity. The gradual buildup of air in the pleural cavity results in increased intrapleural pressure, which compresses and ultimately collapses the ipsilateral lung. Venous compression restricts blood return to the heart and reduces cardiac output. Clinical manifestations include dyspnea, hypoxemia, tachycardia, and hypotension.10 Multiple techniques were developed to better localize the brachial plexus while reducing injury to nearby structures, including the lung. These include eliciting needle paresthesias, electrical nerve stimulation, and ultrasound guidance. While nerve stimulation was once the gold standard for brachial plexus localization, ultrasound guidance has gained in popularity because of its noninvasive nature and dynamic capability to identify nerves and surrounding structures.11 Perlas and colleagues12 determined the sensitivity of needle paresthesias and nerve stimulation to be 38% and 75%, respectively, in cases in which plexus localization had been confirmed by ultrasound.
Several studies have reported on the efficacy of interscalene nerve block with either nerve stimulation or ultrasound guidance in the setting of shoulder surgery.2,3 Bishop and colleagues3 reviewed 547 patients who underwent interscalene regional anesthesia with nerve stimulation for both arthroscopic and open-shoulder procedures. They reported a 97% success rate and 12 (2.3%) minor complications, including sensory neuropathy and complex regional pain syndrome. There were no cases of pneumothorax, cardiac events, or other major complications.3 In a prospective study of 1319 patients, Singh and colleagues2 reported a 99.6% success rate using ultrasound-guided interscalene blocks for their shoulder surgeries. A total of 38 adverse events (2.88%) were identified: 14 transient neurologic events, including ear numbness, digital numbness, and brachial plexitis; 1 case of intraoperative bradycardia, and 2 cancellations after the block for chest pain and flank pain, which yielded negative cardiac workups. Other complications included postoperative emergency room visits and hospital admissions for reasons unrelated to the block.2 Interscalene regional anesthesia, therefore, provides effective anesthesia for shoulder surgery with low complication rates.
Pneumothorax after ultrasound-guided interscalene block has rarely been reported.13,14 In a review of 144 ultrasound-guided indwelling interscalene catheter placements, a 98% successful block rate with a single complication of small pneumothorax after total shoulder arthroplasty was reported.13 Mandim and colleagues14 reported a case of pneumothorax in a smoker who underwent an ultrasound-guided brachial plexus block prior to open reduction and internal fixation of an ulnar fracture. While the patient was asymptomatic and vital signs remained stable during the procedure, the patient complained postoperatively of chest pain with hypoxia, tachycardia, and hypotension. A chest radiograph confirmed an ipsilateral pneumothorax, and the patient was treated successfully with chest-tube placement. The authors attributed this complication to a higher pleural dome resulting from a hyperinflated lung caused by chronic smoking. Our patient reported no history of smoking and her preoperative chest radiograph had no evidence of lung disease.
In contrast, several cases of pneumothorax after shoulder surgery have been reported in the absence of nerve block. Oldman and Peng1 reported a 41-year-old nonsmoker who underwent arthroscopic labral repair and subacromial decompression. The preoperative nerve block was cancelled, and the patient received general endotracheal anesthesia alone. Fifty minutes after the case, the patient developed chest pain and hypoxia. A chest radiograph showed a small pneumothorax that was managed conservatively. The pneumothorax was attributed to spontaneous rupture of a preexisting lung bulla, suggesting that blocks are not always the cause of this complication. Furthermore, Dietzel and Ciullo15 reported 4 cases of spontaneous pneumothorax within 24 hours of uncomplicated arthroscopic shoulder procedures under general anesthesia in the lateral decubitus position. The patient ages ranged from 22 to 38 years, and medical histories were all significant for preexisting lung disease, remote history of pneumonia, and heavy smoking. Three of the patients experienced symptoms at home the day after surgery. The authors concluded that these cases were likely caused by rupture of blebs or bullae from underlying lung disease; these ruptured blebs or bullae are difficult to detect and usually located in the upper lung. The pressure gradient from the positive pressure of anesthesia and the ipsilateral upper lung is thought to be highest in the lateral decubitus position, increasing their chance of rupture.15
Finally, Lee and colleagues16 described 3 patients aged 40 to 45 years who underwent uncomplicated subacromial decompression in the beach-chair position under general anesthesia. Significant shoulder, neck, and axillary swelling were noted after surgery, and a chest radiograph showed tension pneumothorax, subcutaneous emphysema, and pneumomediastinum. The authors speculated that pressure in the subacromial space may become negative relative to atmospheric pressure when the shaver and suction are running, drawing in air through other portals. When the suction is discontinued, fluid infusion may push air into the surrounding tissue, leading to subcutaneous emphysema, which may spread to the mediastinum.16
Conclusion
Ultrasound-guided interscalene nerve blocks have successfully provided anesthesia for shoulder surgeries with low complication rates. Although the incidence of pneumothorax has decreased significantly with ultrasound guidance, the success of this procedure is highly operator-dependent. We present the case of an otherwise healthy patient without known pulmonary disease who developed a tension pneumothorax after the administration of ultrasound-guided regional and general anesthesia for arthroscopic shoulder surgery. Orthopedic surgeons and anesthesiologists must remain vigilant for pneumothorax during the perioperative period after shoulder surgery performed under interscalene regional aesthesia, particularly in the setting of hypotension, hypoxia, and/or tachycardia. Risk factors, such as history of smoking and preexisting lung disease, may predispose patients to the development of pneumothorax. Timely recognition and placement of a chest tube result in satisfactory clinical outcomes.
1. Oldman M, Peng Pi P. Pneumothorax after shoulder arthroscopy: don’t blame it on regional anesthesia. Reg Anesth Pain Med. 2004;29(4):382-383.
2. Singh A, Kelly C, O’Brien T, Wilson J, Warner JJ. Ultrasound-guided interscalene block anesthesia for shoulder arthroscopy: a prospective study of 1319 patients. J Bone Joint Surg Am. 2012;94(22):2040-2046.
3. Bishop JY, Sprague M, Gelber J, et al. Interscalene regional anesthesia for shoulder surgery. J Bone Joint Surg Am. 2005;87(5):974-979.
4. Winnie AP. Interscalene brachial plexus block. Anesth Analg. 1970;49(3):455-466.
5. Mian A, Chaudhry I, Huang R, Rizk E, Tubbs RS, Loukas M. Brachial plexus anesthesia: a review of the relevant anatomy, complications, and anatomical variations. Clin Anat. 2014;27(2):210-221.
6. Brown AR, Weiss R, Greenberg C, Flatow EL, Bigliani LU. Interscalene block for shoulder arthroscopy: comparison with general anesthesia. Arthroscopy. 1993;9(3):295-300.
7. Marhofer P, Harrop-Griffiths W, Willschke H, Kirchmair L. Fifteen years of ultrasound guidance in regional anaesthesia: Part 2 - recent developments in block techniques. Br J Anaesth. 2010;104(6):673-683.
8. Sites BD, Spence BC, Gallagher J, et al. Regional anesthesia meets ultrasound: a specialty in transition. Acta Anaesthesiol Scand. 2008;52(4):456-466.
9. Ilfeld BM, Fredrickson MJ, Mariano ER. Ultrasound-guided perineural catheter insertion: three approaches but few illuminating data. Reg Anesth Pain Med. 2010;35(2):123-126.
10. Choi WI. Pneumothorax. Tuberc Respir Dis (Seoul). 2014;76(3):99-104.
11. Klaastad O, Sauter AR, Dodgson MS. Brachial plexus block with or without ultrasound guidance. Curr Opin Anaesthesiol. 2009;22(5):655-660.
12. Perlas A, Niazi A, McCartney C, Chan V, Xu D, Abbas S. The sensitivity of motor response to nerve stimulation and paresthesia for nerve localization as evaluated by ultrasound. Reg Anesth Pain Med. 2006;31(5):445-450.
13. Bryan NA, Swenson JD, Greis PE, Burks RT. Indwelling interscalene catheter use in an outpatient setting for shoulder surgery: technique, efficacy, and complications. J Shoulder Elbow Surg. 2007;16(4):388-395.
14. Mandim BL, Alves RR, Almeida R, Pontes JP, Arantes LJ, Morais FP. Pneumothorax post brachial plexus block guided by ultrasound: a case report. Rev Bras Anestesiol. 2012;62(5):741-747.
15. Dietzel DP, Ciullo JV. Spontaneous pneumothorax after shoulder arthroscopy: a report of four cases. Arthroscopy. 1996;12(1):99-102.
16. Lee HC, Dewan N, Crosby L. Subcutaneous emphysema, pneumomediastinum, and potentially life-threatening tension pneumothorax. Pulmonary complications from arthroscopic shoulder decompression. Chest. 1992;101(5):1265-1267.
Interscalene brachial plexus anesthesia is commonly used for arthroscopic and open procedures of the shoulder. This regional anesthetic targets the trunks of the brachial plexus and anesthetizes the area about the shoulder and proximal arm. Its use may obviate the need for concomitant general anesthesia, potentially reducing the use of postoperative intravenous and oral pain medication. Furthermore, patients often bypass the acute postoperative anesthesia care unit and proceed directly to the ambulatory unit, permitting earlier hospital discharge. Previous reports in the literature have demonstrated higher rates of neurologic, cardiac, and pulmonary complications from this procedure; in particular, the incidence of pneumothorax was reported as high as 3%.1 Techniques to localize the nerves, such as electrical nerve stimulation and, more recently, ultrasound guidance, have reduced these complication rates.2,3 Successful administration of the block has been shown to result in satisfactory postoperative pain relief.2 However, ultrasound-guided interscalene nerve blocks remain operator-dependent and complications may still occur.
We report a case of tension pneumothorax after arthroscopic rotator cuff repair and subacromial decompression with an ultrasound-guided interscalene block. Immediate recognition and treatment of this complication resulted in a good clinical outcome. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old woman presented with 3 months of right shoulder pain after a fall. Examination was pertinent for weakness in forward elevation and positive rotator cuff impingement signs. She remained symptomatic despite a course of nonsurgical management that included cortisone injections and physical therapy. Magnetic resonance imaging of the shoulder showed a full-thickness supraspinatus tear with minimal fatty atrophy. After a discussion of her treatment options, she elected to undergo an arthroscopic rotator cuff repair with subacromial decompression. An evaluation by her internist revealed no pertinent medical history apart from obesity (body mass index, 36). Specifically, there was no reported history of chronic obstructive pulmonary disease or asthma. She denied any prior cigarette smoking.
The patient was evaluated by the regional anesthesia team and was classified as a class 2 airway. An interscalene brachial plexus block was performed using a 2-inch, 22-gauge needle inserted into the interscalene groove. Using an out-of-plane technique under direct ultrasound guidance, 30 mL of 0.52% ropivacaine was injected. The block was considered successful, and no complications, such as resistance, paresthesias, pain, or blood on aspiration, were noted during injection. The patient had no complaints of chest pain or shortness of breath immediately afterward, and all vital signs were stable throughout the procedure.
The patient was brought to the operating room and placed in the beach-chair position. Induction for general anesthesia was started 15 minutes after the regional anesthetic, with 2 intubation attempts necessary because of poor airway visualization. After placement of the endotracheal tube, breath sounds were noted to be equal bilaterally. The arthroscopic procedure consisted of double-row rotator cuff repair, subacromial decompression, and débridement of the glenohumeral joint for synovitis, using standard arthroscopic portals. There were no difficulties with trocar placement, and bleeding was minimal throughout the case. The total surgical time was 150 minutes and a pump pressure of 30 mm Hg was maintained during the arthroscopy.
Within the first 60 minutes of the start of the arthroscopic procedure, the patient was noted to be intermittently hypotensive with mean arterial pressure (MAP) ranging from the 30s to 130s mm Hg and pulse in the 70 to 80 beats/min range. FiO2 in the 85% to 95% range was maintained throughout the procedure. During that time, 50 μg phenylephrine was administered on 4 separate occasions to maintain her blood pressure. The labile blood pressure was attributed by the anesthesiologist to the beach-chair position. During an attempted extubation upon conclusion of the surgery, the patient became hypotensive with MAP that ranged from the 40s to 60s mm Hg and tachycardic to 90 beats/min. The oxygen saturation was in the low 90s and tidal volume was poor. Absent lung sounds were noted on the right chest. An urgent portable chest radiograph showed a large right-sided tension pneumothorax with mediastinal shift (Figure 1). After an immediate general surgery consultation, a chest tube was placed in the operating room. The patient’s vital signs improved and a repeat chest radiograph revealed successful re-expansion of the lung (Figure 2). She was transferred to the acute postoperative anesthesia care unit and extubated in the intensive care unit later that day.
The patient’s chest tube was removed 2 days later and she was discharged home on hospital day 5 with a completely resolved pneumothorax. She was seen 1 week later in the office for a postoperative visit and reported feeling well without chest pain or shortness of breath.
Discussion
Interscalene brachial plexus anesthesia was first described by Winnie4 in 1970. This block targets the trunks of the brachial plexus, which are enclosed in a fascial sheath between the anterior and middle scalene muscles. In this region lie several structures at risk: the phrenic nerve superficially and inferiorly; the carotid sheath located superficially and medially; the subclavian artery parallel to the trunks; and the cupula of the lung that lies deep and inferior to the anterior scalene muscle. Recognized complications of the block include vocal hoarseness, Horner syndrome, and hemidiaphragmatic paresis caused by the temporary blockade of the ipsilateral recurrent laryngeal nerve, stellate ganglion, and phrenic nerve, in that order.5 Use of the interscalene block has been associated with minimal risk for pneumothorax, because the needle entry point is superior and directed away from the lung pleura.6 This is in contrast to the more inferiorly placed supraclavicular block, located in closer proximity to the lung cupula.5
Two different approaches are commonly used during ultrasound-guided nerve blocks. The in-plane approach generates a long-axis view of the needle by advancing the needle parallel with the long axis of the ultrasound probe. While this allows direct visualization of the needle tip, it requires deeper needle insertion from lateral to medial, causing puncture of the middle scalene muscle that may increase patient discomfort and risk nerve injury within the muscle.7 The out-of-plane approach used on our patient involves needle insertion parallel to the brachial plexus, but along the short axis of the ultrasound probe. Although this permits the operator to assess the periphery of the nerve, it may lead to poor needle-tip visualization during the procedure. As a result, operators often use a combination of tissue disturbance and “hydrolocation,” in which fluid is injected to indicate the needle-tip location.8,9
Tension pneumothorax represents the accumulation of air in the pleural space that leads to impaired pulmonary and cardiac function. It is often caused by disruption or puncture of the parietal or visceral pleura, creating a connection between the alveoli and pleural cavity. The gradual buildup of air in the pleural cavity results in increased intrapleural pressure, which compresses and ultimately collapses the ipsilateral lung. Venous compression restricts blood return to the heart and reduces cardiac output. Clinical manifestations include dyspnea, hypoxemia, tachycardia, and hypotension.10 Multiple techniques were developed to better localize the brachial plexus while reducing injury to nearby structures, including the lung. These include eliciting needle paresthesias, electrical nerve stimulation, and ultrasound guidance. While nerve stimulation was once the gold standard for brachial plexus localization, ultrasound guidance has gained in popularity because of its noninvasive nature and dynamic capability to identify nerves and surrounding structures.11 Perlas and colleagues12 determined the sensitivity of needle paresthesias and nerve stimulation to be 38% and 75%, respectively, in cases in which plexus localization had been confirmed by ultrasound.
Several studies have reported on the efficacy of interscalene nerve block with either nerve stimulation or ultrasound guidance in the setting of shoulder surgery.2,3 Bishop and colleagues3 reviewed 547 patients who underwent interscalene regional anesthesia with nerve stimulation for both arthroscopic and open-shoulder procedures. They reported a 97% success rate and 12 (2.3%) minor complications, including sensory neuropathy and complex regional pain syndrome. There were no cases of pneumothorax, cardiac events, or other major complications.3 In a prospective study of 1319 patients, Singh and colleagues2 reported a 99.6% success rate using ultrasound-guided interscalene blocks for their shoulder surgeries. A total of 38 adverse events (2.88%) were identified: 14 transient neurologic events, including ear numbness, digital numbness, and brachial plexitis; 1 case of intraoperative bradycardia, and 2 cancellations after the block for chest pain and flank pain, which yielded negative cardiac workups. Other complications included postoperative emergency room visits and hospital admissions for reasons unrelated to the block.2 Interscalene regional anesthesia, therefore, provides effective anesthesia for shoulder surgery with low complication rates.
Pneumothorax after ultrasound-guided interscalene block has rarely been reported.13,14 In a review of 144 ultrasound-guided indwelling interscalene catheter placements, a 98% successful block rate with a single complication of small pneumothorax after total shoulder arthroplasty was reported.13 Mandim and colleagues14 reported a case of pneumothorax in a smoker who underwent an ultrasound-guided brachial plexus block prior to open reduction and internal fixation of an ulnar fracture. While the patient was asymptomatic and vital signs remained stable during the procedure, the patient complained postoperatively of chest pain with hypoxia, tachycardia, and hypotension. A chest radiograph confirmed an ipsilateral pneumothorax, and the patient was treated successfully with chest-tube placement. The authors attributed this complication to a higher pleural dome resulting from a hyperinflated lung caused by chronic smoking. Our patient reported no history of smoking and her preoperative chest radiograph had no evidence of lung disease.
In contrast, several cases of pneumothorax after shoulder surgery have been reported in the absence of nerve block. Oldman and Peng1 reported a 41-year-old nonsmoker who underwent arthroscopic labral repair and subacromial decompression. The preoperative nerve block was cancelled, and the patient received general endotracheal anesthesia alone. Fifty minutes after the case, the patient developed chest pain and hypoxia. A chest radiograph showed a small pneumothorax that was managed conservatively. The pneumothorax was attributed to spontaneous rupture of a preexisting lung bulla, suggesting that blocks are not always the cause of this complication. Furthermore, Dietzel and Ciullo15 reported 4 cases of spontaneous pneumothorax within 24 hours of uncomplicated arthroscopic shoulder procedures under general anesthesia in the lateral decubitus position. The patient ages ranged from 22 to 38 years, and medical histories were all significant for preexisting lung disease, remote history of pneumonia, and heavy smoking. Three of the patients experienced symptoms at home the day after surgery. The authors concluded that these cases were likely caused by rupture of blebs or bullae from underlying lung disease; these ruptured blebs or bullae are difficult to detect and usually located in the upper lung. The pressure gradient from the positive pressure of anesthesia and the ipsilateral upper lung is thought to be highest in the lateral decubitus position, increasing their chance of rupture.15
Finally, Lee and colleagues16 described 3 patients aged 40 to 45 years who underwent uncomplicated subacromial decompression in the beach-chair position under general anesthesia. Significant shoulder, neck, and axillary swelling were noted after surgery, and a chest radiograph showed tension pneumothorax, subcutaneous emphysema, and pneumomediastinum. The authors speculated that pressure in the subacromial space may become negative relative to atmospheric pressure when the shaver and suction are running, drawing in air through other portals. When the suction is discontinued, fluid infusion may push air into the surrounding tissue, leading to subcutaneous emphysema, which may spread to the mediastinum.16
Conclusion
Ultrasound-guided interscalene nerve blocks have successfully provided anesthesia for shoulder surgeries with low complication rates. Although the incidence of pneumothorax has decreased significantly with ultrasound guidance, the success of this procedure is highly operator-dependent. We present the case of an otherwise healthy patient without known pulmonary disease who developed a tension pneumothorax after the administration of ultrasound-guided regional and general anesthesia for arthroscopic shoulder surgery. Orthopedic surgeons and anesthesiologists must remain vigilant for pneumothorax during the perioperative period after shoulder surgery performed under interscalene regional aesthesia, particularly in the setting of hypotension, hypoxia, and/or tachycardia. Risk factors, such as history of smoking and preexisting lung disease, may predispose patients to the development of pneumothorax. Timely recognition and placement of a chest tube result in satisfactory clinical outcomes.
Interscalene brachial plexus anesthesia is commonly used for arthroscopic and open procedures of the shoulder. This regional anesthetic targets the trunks of the brachial plexus and anesthetizes the area about the shoulder and proximal arm. Its use may obviate the need for concomitant general anesthesia, potentially reducing the use of postoperative intravenous and oral pain medication. Furthermore, patients often bypass the acute postoperative anesthesia care unit and proceed directly to the ambulatory unit, permitting earlier hospital discharge. Previous reports in the literature have demonstrated higher rates of neurologic, cardiac, and pulmonary complications from this procedure; in particular, the incidence of pneumothorax was reported as high as 3%.1 Techniques to localize the nerves, such as electrical nerve stimulation and, more recently, ultrasound guidance, have reduced these complication rates.2,3 Successful administration of the block has been shown to result in satisfactory postoperative pain relief.2 However, ultrasound-guided interscalene nerve blocks remain operator-dependent and complications may still occur.
We report a case of tension pneumothorax after arthroscopic rotator cuff repair and subacromial decompression with an ultrasound-guided interscalene block. Immediate recognition and treatment of this complication resulted in a good clinical outcome. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old woman presented with 3 months of right shoulder pain after a fall. Examination was pertinent for weakness in forward elevation and positive rotator cuff impingement signs. She remained symptomatic despite a course of nonsurgical management that included cortisone injections and physical therapy. Magnetic resonance imaging of the shoulder showed a full-thickness supraspinatus tear with minimal fatty atrophy. After a discussion of her treatment options, she elected to undergo an arthroscopic rotator cuff repair with subacromial decompression. An evaluation by her internist revealed no pertinent medical history apart from obesity (body mass index, 36). Specifically, there was no reported history of chronic obstructive pulmonary disease or asthma. She denied any prior cigarette smoking.
The patient was evaluated by the regional anesthesia team and was classified as a class 2 airway. An interscalene brachial plexus block was performed using a 2-inch, 22-gauge needle inserted into the interscalene groove. Using an out-of-plane technique under direct ultrasound guidance, 30 mL of 0.52% ropivacaine was injected. The block was considered successful, and no complications, such as resistance, paresthesias, pain, or blood on aspiration, were noted during injection. The patient had no complaints of chest pain or shortness of breath immediately afterward, and all vital signs were stable throughout the procedure.
The patient was brought to the operating room and placed in the beach-chair position. Induction for general anesthesia was started 15 minutes after the regional anesthetic, with 2 intubation attempts necessary because of poor airway visualization. After placement of the endotracheal tube, breath sounds were noted to be equal bilaterally. The arthroscopic procedure consisted of double-row rotator cuff repair, subacromial decompression, and débridement of the glenohumeral joint for synovitis, using standard arthroscopic portals. There were no difficulties with trocar placement, and bleeding was minimal throughout the case. The total surgical time was 150 minutes and a pump pressure of 30 mm Hg was maintained during the arthroscopy.
Within the first 60 minutes of the start of the arthroscopic procedure, the patient was noted to be intermittently hypotensive with mean arterial pressure (MAP) ranging from the 30s to 130s mm Hg and pulse in the 70 to 80 beats/min range. FiO2 in the 85% to 95% range was maintained throughout the procedure. During that time, 50 μg phenylephrine was administered on 4 separate occasions to maintain her blood pressure. The labile blood pressure was attributed by the anesthesiologist to the beach-chair position. During an attempted extubation upon conclusion of the surgery, the patient became hypotensive with MAP that ranged from the 40s to 60s mm Hg and tachycardic to 90 beats/min. The oxygen saturation was in the low 90s and tidal volume was poor. Absent lung sounds were noted on the right chest. An urgent portable chest radiograph showed a large right-sided tension pneumothorax with mediastinal shift (Figure 1). After an immediate general surgery consultation, a chest tube was placed in the operating room. The patient’s vital signs improved and a repeat chest radiograph revealed successful re-expansion of the lung (Figure 2). She was transferred to the acute postoperative anesthesia care unit and extubated in the intensive care unit later that day.
The patient’s chest tube was removed 2 days later and she was discharged home on hospital day 5 with a completely resolved pneumothorax. She was seen 1 week later in the office for a postoperative visit and reported feeling well without chest pain or shortness of breath.
Discussion
Interscalene brachial plexus anesthesia was first described by Winnie4 in 1970. This block targets the trunks of the brachial plexus, which are enclosed in a fascial sheath between the anterior and middle scalene muscles. In this region lie several structures at risk: the phrenic nerve superficially and inferiorly; the carotid sheath located superficially and medially; the subclavian artery parallel to the trunks; and the cupula of the lung that lies deep and inferior to the anterior scalene muscle. Recognized complications of the block include vocal hoarseness, Horner syndrome, and hemidiaphragmatic paresis caused by the temporary blockade of the ipsilateral recurrent laryngeal nerve, stellate ganglion, and phrenic nerve, in that order.5 Use of the interscalene block has been associated with minimal risk for pneumothorax, because the needle entry point is superior and directed away from the lung pleura.6 This is in contrast to the more inferiorly placed supraclavicular block, located in closer proximity to the lung cupula.5
Two different approaches are commonly used during ultrasound-guided nerve blocks. The in-plane approach generates a long-axis view of the needle by advancing the needle parallel with the long axis of the ultrasound probe. While this allows direct visualization of the needle tip, it requires deeper needle insertion from lateral to medial, causing puncture of the middle scalene muscle that may increase patient discomfort and risk nerve injury within the muscle.7 The out-of-plane approach used on our patient involves needle insertion parallel to the brachial plexus, but along the short axis of the ultrasound probe. Although this permits the operator to assess the periphery of the nerve, it may lead to poor needle-tip visualization during the procedure. As a result, operators often use a combination of tissue disturbance and “hydrolocation,” in which fluid is injected to indicate the needle-tip location.8,9
Tension pneumothorax represents the accumulation of air in the pleural space that leads to impaired pulmonary and cardiac function. It is often caused by disruption or puncture of the parietal or visceral pleura, creating a connection between the alveoli and pleural cavity. The gradual buildup of air in the pleural cavity results in increased intrapleural pressure, which compresses and ultimately collapses the ipsilateral lung. Venous compression restricts blood return to the heart and reduces cardiac output. Clinical manifestations include dyspnea, hypoxemia, tachycardia, and hypotension.10 Multiple techniques were developed to better localize the brachial plexus while reducing injury to nearby structures, including the lung. These include eliciting needle paresthesias, electrical nerve stimulation, and ultrasound guidance. While nerve stimulation was once the gold standard for brachial plexus localization, ultrasound guidance has gained in popularity because of its noninvasive nature and dynamic capability to identify nerves and surrounding structures.11 Perlas and colleagues12 determined the sensitivity of needle paresthesias and nerve stimulation to be 38% and 75%, respectively, in cases in which plexus localization had been confirmed by ultrasound.
Several studies have reported on the efficacy of interscalene nerve block with either nerve stimulation or ultrasound guidance in the setting of shoulder surgery.2,3 Bishop and colleagues3 reviewed 547 patients who underwent interscalene regional anesthesia with nerve stimulation for both arthroscopic and open-shoulder procedures. They reported a 97% success rate and 12 (2.3%) minor complications, including sensory neuropathy and complex regional pain syndrome. There were no cases of pneumothorax, cardiac events, or other major complications.3 In a prospective study of 1319 patients, Singh and colleagues2 reported a 99.6% success rate using ultrasound-guided interscalene blocks for their shoulder surgeries. A total of 38 adverse events (2.88%) were identified: 14 transient neurologic events, including ear numbness, digital numbness, and brachial plexitis; 1 case of intraoperative bradycardia, and 2 cancellations after the block for chest pain and flank pain, which yielded negative cardiac workups. Other complications included postoperative emergency room visits and hospital admissions for reasons unrelated to the block.2 Interscalene regional anesthesia, therefore, provides effective anesthesia for shoulder surgery with low complication rates.
Pneumothorax after ultrasound-guided interscalene block has rarely been reported.13,14 In a review of 144 ultrasound-guided indwelling interscalene catheter placements, a 98% successful block rate with a single complication of small pneumothorax after total shoulder arthroplasty was reported.13 Mandim and colleagues14 reported a case of pneumothorax in a smoker who underwent an ultrasound-guided brachial plexus block prior to open reduction and internal fixation of an ulnar fracture. While the patient was asymptomatic and vital signs remained stable during the procedure, the patient complained postoperatively of chest pain with hypoxia, tachycardia, and hypotension. A chest radiograph confirmed an ipsilateral pneumothorax, and the patient was treated successfully with chest-tube placement. The authors attributed this complication to a higher pleural dome resulting from a hyperinflated lung caused by chronic smoking. Our patient reported no history of smoking and her preoperative chest radiograph had no evidence of lung disease.
In contrast, several cases of pneumothorax after shoulder surgery have been reported in the absence of nerve block. Oldman and Peng1 reported a 41-year-old nonsmoker who underwent arthroscopic labral repair and subacromial decompression. The preoperative nerve block was cancelled, and the patient received general endotracheal anesthesia alone. Fifty minutes after the case, the patient developed chest pain and hypoxia. A chest radiograph showed a small pneumothorax that was managed conservatively. The pneumothorax was attributed to spontaneous rupture of a preexisting lung bulla, suggesting that blocks are not always the cause of this complication. Furthermore, Dietzel and Ciullo15 reported 4 cases of spontaneous pneumothorax within 24 hours of uncomplicated arthroscopic shoulder procedures under general anesthesia in the lateral decubitus position. The patient ages ranged from 22 to 38 years, and medical histories were all significant for preexisting lung disease, remote history of pneumonia, and heavy smoking. Three of the patients experienced symptoms at home the day after surgery. The authors concluded that these cases were likely caused by rupture of blebs or bullae from underlying lung disease; these ruptured blebs or bullae are difficult to detect and usually located in the upper lung. The pressure gradient from the positive pressure of anesthesia and the ipsilateral upper lung is thought to be highest in the lateral decubitus position, increasing their chance of rupture.15
Finally, Lee and colleagues16 described 3 patients aged 40 to 45 years who underwent uncomplicated subacromial decompression in the beach-chair position under general anesthesia. Significant shoulder, neck, and axillary swelling were noted after surgery, and a chest radiograph showed tension pneumothorax, subcutaneous emphysema, and pneumomediastinum. The authors speculated that pressure in the subacromial space may become negative relative to atmospheric pressure when the shaver and suction are running, drawing in air through other portals. When the suction is discontinued, fluid infusion may push air into the surrounding tissue, leading to subcutaneous emphysema, which may spread to the mediastinum.16
Conclusion
Ultrasound-guided interscalene nerve blocks have successfully provided anesthesia for shoulder surgeries with low complication rates. Although the incidence of pneumothorax has decreased significantly with ultrasound guidance, the success of this procedure is highly operator-dependent. We present the case of an otherwise healthy patient without known pulmonary disease who developed a tension pneumothorax after the administration of ultrasound-guided regional and general anesthesia for arthroscopic shoulder surgery. Orthopedic surgeons and anesthesiologists must remain vigilant for pneumothorax during the perioperative period after shoulder surgery performed under interscalene regional aesthesia, particularly in the setting of hypotension, hypoxia, and/or tachycardia. Risk factors, such as history of smoking and preexisting lung disease, may predispose patients to the development of pneumothorax. Timely recognition and placement of a chest tube result in satisfactory clinical outcomes.
1. Oldman M, Peng Pi P. Pneumothorax after shoulder arthroscopy: don’t blame it on regional anesthesia. Reg Anesth Pain Med. 2004;29(4):382-383.
2. Singh A, Kelly C, O’Brien T, Wilson J, Warner JJ. Ultrasound-guided interscalene block anesthesia for shoulder arthroscopy: a prospective study of 1319 patients. J Bone Joint Surg Am. 2012;94(22):2040-2046.
3. Bishop JY, Sprague M, Gelber J, et al. Interscalene regional anesthesia for shoulder surgery. J Bone Joint Surg Am. 2005;87(5):974-979.
4. Winnie AP. Interscalene brachial plexus block. Anesth Analg. 1970;49(3):455-466.
5. Mian A, Chaudhry I, Huang R, Rizk E, Tubbs RS, Loukas M. Brachial plexus anesthesia: a review of the relevant anatomy, complications, and anatomical variations. Clin Anat. 2014;27(2):210-221.
6. Brown AR, Weiss R, Greenberg C, Flatow EL, Bigliani LU. Interscalene block for shoulder arthroscopy: comparison with general anesthesia. Arthroscopy. 1993;9(3):295-300.
7. Marhofer P, Harrop-Griffiths W, Willschke H, Kirchmair L. Fifteen years of ultrasound guidance in regional anaesthesia: Part 2 - recent developments in block techniques. Br J Anaesth. 2010;104(6):673-683.
8. Sites BD, Spence BC, Gallagher J, et al. Regional anesthesia meets ultrasound: a specialty in transition. Acta Anaesthesiol Scand. 2008;52(4):456-466.
9. Ilfeld BM, Fredrickson MJ, Mariano ER. Ultrasound-guided perineural catheter insertion: three approaches but few illuminating data. Reg Anesth Pain Med. 2010;35(2):123-126.
10. Choi WI. Pneumothorax. Tuberc Respir Dis (Seoul). 2014;76(3):99-104.
11. Klaastad O, Sauter AR, Dodgson MS. Brachial plexus block with or without ultrasound guidance. Curr Opin Anaesthesiol. 2009;22(5):655-660.
12. Perlas A, Niazi A, McCartney C, Chan V, Xu D, Abbas S. The sensitivity of motor response to nerve stimulation and paresthesia for nerve localization as evaluated by ultrasound. Reg Anesth Pain Med. 2006;31(5):445-450.
13. Bryan NA, Swenson JD, Greis PE, Burks RT. Indwelling interscalene catheter use in an outpatient setting for shoulder surgery: technique, efficacy, and complications. J Shoulder Elbow Surg. 2007;16(4):388-395.
14. Mandim BL, Alves RR, Almeida R, Pontes JP, Arantes LJ, Morais FP. Pneumothorax post brachial plexus block guided by ultrasound: a case report. Rev Bras Anestesiol. 2012;62(5):741-747.
15. Dietzel DP, Ciullo JV. Spontaneous pneumothorax after shoulder arthroscopy: a report of four cases. Arthroscopy. 1996;12(1):99-102.
16. Lee HC, Dewan N, Crosby L. Subcutaneous emphysema, pneumomediastinum, and potentially life-threatening tension pneumothorax. Pulmonary complications from arthroscopic shoulder decompression. Chest. 1992;101(5):1265-1267.
1. Oldman M, Peng Pi P. Pneumothorax after shoulder arthroscopy: don’t blame it on regional anesthesia. Reg Anesth Pain Med. 2004;29(4):382-383.
2. Singh A, Kelly C, O’Brien T, Wilson J, Warner JJ. Ultrasound-guided interscalene block anesthesia for shoulder arthroscopy: a prospective study of 1319 patients. J Bone Joint Surg Am. 2012;94(22):2040-2046.
3. Bishop JY, Sprague M, Gelber J, et al. Interscalene regional anesthesia for shoulder surgery. J Bone Joint Surg Am. 2005;87(5):974-979.
4. Winnie AP. Interscalene brachial plexus block. Anesth Analg. 1970;49(3):455-466.
5. Mian A, Chaudhry I, Huang R, Rizk E, Tubbs RS, Loukas M. Brachial plexus anesthesia: a review of the relevant anatomy, complications, and anatomical variations. Clin Anat. 2014;27(2):210-221.
6. Brown AR, Weiss R, Greenberg C, Flatow EL, Bigliani LU. Interscalene block for shoulder arthroscopy: comparison with general anesthesia. Arthroscopy. 1993;9(3):295-300.
7. Marhofer P, Harrop-Griffiths W, Willschke H, Kirchmair L. Fifteen years of ultrasound guidance in regional anaesthesia: Part 2 - recent developments in block techniques. Br J Anaesth. 2010;104(6):673-683.
8. Sites BD, Spence BC, Gallagher J, et al. Regional anesthesia meets ultrasound: a specialty in transition. Acta Anaesthesiol Scand. 2008;52(4):456-466.
9. Ilfeld BM, Fredrickson MJ, Mariano ER. Ultrasound-guided perineural catheter insertion: three approaches but few illuminating data. Reg Anesth Pain Med. 2010;35(2):123-126.
10. Choi WI. Pneumothorax. Tuberc Respir Dis (Seoul). 2014;76(3):99-104.
11. Klaastad O, Sauter AR, Dodgson MS. Brachial plexus block with or without ultrasound guidance. Curr Opin Anaesthesiol. 2009;22(5):655-660.
12. Perlas A, Niazi A, McCartney C, Chan V, Xu D, Abbas S. The sensitivity of motor response to nerve stimulation and paresthesia for nerve localization as evaluated by ultrasound. Reg Anesth Pain Med. 2006;31(5):445-450.
13. Bryan NA, Swenson JD, Greis PE, Burks RT. Indwelling interscalene catheter use in an outpatient setting for shoulder surgery: technique, efficacy, and complications. J Shoulder Elbow Surg. 2007;16(4):388-395.
14. Mandim BL, Alves RR, Almeida R, Pontes JP, Arantes LJ, Morais FP. Pneumothorax post brachial plexus block guided by ultrasound: a case report. Rev Bras Anestesiol. 2012;62(5):741-747.
15. Dietzel DP, Ciullo JV. Spontaneous pneumothorax after shoulder arthroscopy: a report of four cases. Arthroscopy. 1996;12(1):99-102.
16. Lee HC, Dewan N, Crosby L. Subcutaneous emphysema, pneumomediastinum, and potentially life-threatening tension pneumothorax. Pulmonary complications from arthroscopic shoulder decompression. Chest. 1992;101(5):1265-1267.
Reducing Postoperative Fracture Displacement After Locked Plating of Proximal Humerus Fractures: Current Concepts
Proximal humerus fractures account for 4% to 5% of all fractures.1 These fractures occur most frequently in the elderly—patients older than 60 years sustain 71% of these injuries2—and in females.1,3 Given an aging population, this incidence is predicted to increase 3-fold over the next 30 years.4 There is much debate regarding management of acute, displaced proximal humerus fractures. A recent Cochrane Review of published outcomes of operative and nonoperative treatment of displaced proximal humerus fractures found insufficient evidence supporting either modality, though surgery was associated with additional procedures.5 A review of 1000 proximal humerus fractures found that 49% had less than 1 cm of displacement of the major fragments or angulation of less than 45°.3 Other authors have reported similar findings.6,7 Although the incidence of proximal humerus fractures has remained stable over the past decade, from 1999 to 2005 there was a 25% relative increase in surgical management, including a relative increase of 29% in open reduction and internal fixation (ORIF) versus a 20% increase in arthroplasty.1
Locking plates have consistently demonstrated biomechanical superiority over other forms of fixation in osteoporotic bone.8-11 Egol and colleagues8 found that osteoporotic bone limited the torque of fixation to values less than what is required for adequate frictional force between the plate and the bone. This problem can be overcome with fixed-angle devices, such as locked plates.9 Compared with locked nail constructs, proximal humerus locking plates have demonstrated superiority in torsion, loading, and varus bending.10,11 Compared with blade plates, proximal humerus locking plates exhibited increased stiffness and torsional fatigue resistance.12 In a randomized clinical trial, Olerud and colleagues13 reported superior functional results with locking plate fixation compared with nonoperative treatment of displaced 3-part fractures in elderly patients with 2-year follow-up, though these clinical results were not supported by others.14 Two recent case–control studies comparing functional outcomes for 3- and 4-part fractures with follow-up of more than 2 years revealed higher Constant scores after locked plating compared with hemiarthroplasty, though complications were higher with locked plates.15,16 Adoption of locked proximal humerus plating has been correlated with good clinical outcomes and union rates, though this has been accompanied by a higher rate of reoperation.7 Reoperation rates from 1999 to 2005 increased both in the immediate postoperative period (odds ratio, 3.36) and at 1 year (odds ratio, 3.90).1
Complications of Locked Plating
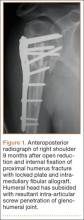
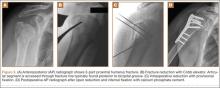
Regardless of fixation type, reduced humeral head bone mass and quality may lead to implant loosening, fracture redisplacement, and, ultimately, poor outcomes. Baseline osteoporosis may predict likelihood of fixation failure.17 Multiple studies have reported on the implant-related complications associated with locking plate fixation—most commonly, intra-articular screw penetration, postoperative fracture displacement, and avascular necrosis (AVN)18-24 (Figure 1). A meta-analysis of 12 studies with a total of 514 proximal humerus fractures treated with locking plate fixation showed an overall complication rate of 49% and a 13.8% reoperation rate.25 The most common indication for reoperation involved intra-articular screw perforation. The most common complications were varus malunion (16%), osteonecrosis (10%), intra-articular screw penetration (8%), subacromial impingement (6%), and infection (4%).
Suboptimal intraoperative fracture reduction, specifically with residual varus, has been correlated with loss of fracture fixation. In a series of 153 fractures, loss of fixation occurred in 13.7% of cases, with the leading risk factor being varus malreduction.19 Failure rates were 30.4% and 11% when the head shaft angle was less than 120° and when it was 120° or more, respectively. Solberg and colleagues16 found that initial postoperative varus angulation of more than 20° resulted in universal loss of fixation. Conversion of these cases to hemiarthroplasty resulted in poor outcomes. Preoperative fracture alignment may also predict fixation failure.22 In one series, initial varus angulation healed with a mean 16° varus and a Constant score of 63, whereas initial valgus alignment healed with 6° varus and a Constant score of 71.22 Complications occurred in fractures that were initially in varus 79% of the time and initially in valgus 19% of the time. Screw perforation has been associated with loss of reduction 44% of the time.20
In an analysis of locking plate constructs revised after early (<4 weeks) failure in 8 patients with osteoporosis, Micic and colleagues21 found implant pullout leading to varus malalignment. All cases lacked medial support and subchondral screw purchase; 3 were initially malreduced. Owsley and Gorczyca23 retrospectively reviewed 53 cases of displaced proximal humerus fractures treated with locked plating. Despite the high rate of radiographic union, 36% developed complications, including screw cutout (23%), varus displacement of more than 10° (25%), and AVN (4%); 13% required revision. These complications disproportionately affected patients older than 60 years (57%) and negatively affected functional outcomes.
Augmentation Techniques
Despite its reported complications, proximal humerus locked plating remains the most widely used type of fixation.1 Advancements in locking plate design, improved understanding of fixation principles, and adoption of techniques augmenting proximal humerus locking plate fixation, particularly in osteoporotic bone, have reduced postoperative complications (Table 1).
Rotator Cuff Sutures
A widely adopted technique for neutralizing rotator cuff–deforming forces, which theoretically can cause fracture displacement, is incorporation of heavy nonabsorbable sutures. These sutures are placed through the rotator cuff–tuberosity junction and tied down after being passed through the plate. Obtaining and maintaining tuberosity reduction are essential in achieving good functional outcomes after fixation. In addition, tension band sutures may be particularly useful in the setting of initial varus deformity.26
Although clinical use of these sutures is common, biomechanical studies of their adjunctive contribution to fracture stability are lacking.27 The rotator cuff musculature has a maximal contractile force of 3.5 kg/cm2.28 Ricchetti and colleagues29 described a technique that involves using a locked plate and tagging the rotator cuff with heavy nonabsorbable sutures. Selective traction on the sutures can help obtain and maintain fracture reduction. Multiple studies have reported on suture use with locked plating for proximal humerus fractures.29-34 Badman and colleagues30 retrospectively reviewed 81 cases of metaphyseal defects or medial comminution treated with locked plating, rotator cuff sutures, and structural allograft. All cases healed within 6 months after surgery. The incidence of screw cutout was 3.7%, the incidence of AVN was 6.2%, and the incidence of varus collapse was 6%. A cadaveric study that used specimens (mean age, 77 years) with a simulated 3-part proximal humerus fracture treated with a locked plate both with and without cerclage sutures found no difference in interfragmentary motion between the groups.27 The authors concluded that additive sutures are not required for anatomically reduced fractures. Multiple sutures may counteract the deforming forces that act on bony segments that cannot be adequately maintained with screws, such as an osteoporotic greater tuberosity.
Medial Column Restoration
The importance of reducing and maintaining the medial calcar to provide biomechanical support for a laterally placed plate has been recognized.26,34-37 Gardner and colleagues26 suggested that medial support was achieved if the medial cortex was anatomically reduced, if the proximal fragment was impacted laterally onto the shaft, or if 1 or more inferomedial screws were placed. Cases that did not achieve medial support developed significantly more humeral head subsidence (5.8 mm vs 1.2 mm) and screw penetration. Krappinger and colleagues36 found that factors leading to fixation failure included age, local bone mineral density, anatomical reduction, and restoration of the medial cortical support. The authors concluded that anatomical reduction and restoration of the medial cortex were important in minimizing mechanical loads at the bone–implant interface. Biomechanically, Lescheid and colleagues37 found that the most stable construct was anatomical reduction with medial cortical contact. In the setting of comminution, however, it may be preferable to intentionally perform varus malreduction to achieve medial contact than to achieve anatomical reduction with a fracture gap. Badman and colleagues30 found that the incidence of screw penetration was 6% in patients with an intact medial calcar versus 29% in patients without medial support. In a retrospective analysis of patients treated with a locking plate and suture augmentation, Jung and colleagues35 concluded that restoring medial support was the most reliable factor in the prevention of loss of reduction with or without screw perforation. Last, Solberg and colleagues16 reported better clinical outcomes when the length of the metaphyseal segment attached to the articular fragment was more than 2 mm. A length of less than 2 mm was predictive of developing AVN.
Use of Bone Void Fillers
Allograft. Allograft is cancellous or corticocancellous chips or tricortical graft used as osteoconductive filler for metaphyseal defects.38 An increasingly popular technique involves using an endosteal fibular allograft strut to indirectly reduce the fracture and help support the medial calcar.39-42 Hettrich and colleagues40 reported on radiographic outcomes of displaced proximal humerus fractures with medial comminution treated with a locked plate and an endosteal fibular allograft or semitubular plate. The reduction was maintained in 96% of cases; there was 1 varus collapse. There were no cases of implant failure, screw perforation, or AVN. Other authors have also reported on successful use of fibular allograft in conjunction with a locked plate; the rate of reduction loss was low, and there were no cases of screw cutout or intra-articular screw penetration.30,41,42 These clinical outcomes are supported by results of biomechanical studies of the added benefit of intramedullary fibular allograft.43-46 Mathison and colleagues43 reported that a construct with fibular allograft and a locking plate increased the failure load by 1.72 times and the stiffness by 3.84 times compared with a control group of locking plate only. Bae and colleagues46 found significantly higher maximum failure load and construct stiffness with no varus collapse in specimens prepared with locked plate and fibular strut augmentation compared with a control group.
Others have successfully used cancellous allograft to fill humeral head bone defects.29,32,47-49 Duralde and Leddy47 reported 100% radiographic union and 81% good to excellent results in cases treated with a locking plate and morselized cancellous allograft to fill bone voids. Varus collapse and screw cutout did not occur, but there were 2 cases of AVN. Ricchetti and colleagues29 reviewed 54 cases treated with a locking plate and rotator cuff suture construct. Allograft cancellous chips and demineralized bone matrix were used in 3- and 4-part fractures (70% of cases) along with shorter screws in the humeral head. Major complications included AVN (1), fixation failure (3), and varus malunion (5). Others investigators have had less favorable results with use of cancellous bone graft. Schliemann and colleagues19 reported on 27 patients who were older than 65 years when they underwent ORIF with rotator cuff sutures to stabilize the tuberosities and either cancellous graft or a synthetic bone substitute in patients with massive metaphyseal defects. Patient-reported outcomes were superior to Constant scores. Complications included screw penetration (22.2%), reduction loss (44.4%), implant failure (3.7%), and AVN (29.6%).
Autograft. Autograft has both osteoconductive and osteoinductive properties and has been successfully used for metaphyseal defects.32,50 Kim and colleagues50 reported on patients with 4-part proximal humerus fractures treated with a locking plate and autologous iliac graft. All cases achieved union and had good or excellent outcomes. There were no cases of AVN, varus collapse, or hardware-related complications.
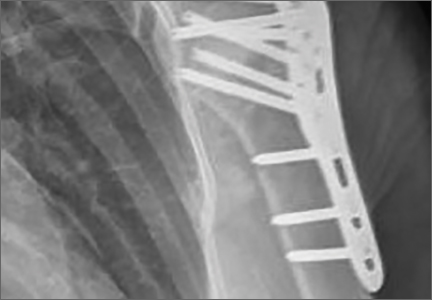
Bone Cement. Calcium phosphate cement has osteoconductive properties and enhances screw purchase in cancellous bone (Figures 2A–2F). It can be injected or molded into bone voids to provide improved compressive strength. It is resorbed through cell-mediated processes resembling bone remodeling and does not disappear until new bone forms. (Calcium sulfate cement, on the other hand, resorbs through a chemical process independent of new bone formation.51) Egol and colleagues52 reviewed the cases of 92 patients (mean age, 61 years) with 2-, 3-, and 4-part proximal humerus fractures treated with locked plate fixation. Metaphyseal defects were treated with no augmentation, augmentation with cancellous chips, or augmentation with calcium phosphate cement. Adding calcium phosphate cement was associated with lower incidence of intra-articular screw penetration and humeral head settling. In a recent cadaveric biomechanical study using 2-part proximal humerus fractures with metaphyseal comminution, the group augmented with calcium phosphate cement had enhanced axial stiffness and load to failure with reduced screw penetration.53 Other biomechanical studies have found increased screw pullout strength54 and decreased interfragmentary motion when specimens were augmented with calcium phosphate cement.55
Similar good clinical and radiographic outcomes have been observed with use of calcium sulfate cement.56,57 Somasundaram and colleagues56 reported good clinical outcomes in 82% of patients treated with locking plates and calcium sulfate cement used to fill metaphyseal voids. All fractures united without infection, fixation failure, subsequent malunion, tuberosity failure, or AVN. Lee and Shin57 compared outcomes of 14 patients who received calcium sulfate augmentation with outcomes of patients who did not receive this augmentation. Overall, 89% of patients had good or excellent results. Calcium sulfate cement did not affect the reduction failure rate or clinical outcomes in cases in which medial cortical reduction was achieved. However, postoperative displacement caused by lack of medial support was associated with poor outcomes.
Screw Placement
Screws optimally should be placed in the posterior-medial-inferior aspect of the humeral head to provide medial support for the fracture and mechanical stability.58 Cadaveric studies have shown the highest cancellous bone density in the proximal, posterior, and medial portions of the humeral head.59-63 Similarly, in a cadaveric study, Liew and colleagues61 found greater screw purchase and higher pullout strength when the screw was placed in the center of the humeral head, within subchondral bone; fixation was poorest when the screw was placed in the anterosuperior region of the humeral head. Tingart and colleagues62 reported that humeral head trabecular density significantly affected pullout strength of cancellous screws. In addition, the most pullout strength was at the center of the head, and the least within the anterosuperior head. Trabecular density was higher in the inferior and posterior regions than in the superior and anterior regions.
Most locking plate designs allow screws to be placed at the level of the medial calcar—the goal being to provide medial column support (Table 2). Zhang and colleagues58 treated 2-, 3-, and 4-part fractures with a locking plate and randomized them into receiving the plate with or without medial support screws. For 3- and 4-part fractures, the group with these screws had a significantly greater final neck-shaft angle and smaller angulation loss compared with the group without screws. No additional benefit was found for 2-part fractures. Erhardt and colleagues63 simulated unstable proximal humerus fractures using cadavers and testing different fixation methods using a polyaxial locking plate. They found that 5 screws in the head fragment and an inferomedial support screw significantly reduced the risk of screw perforation. Other authors have concluded that placing 1 or more inferomedial screws is important in cases of medial comminution or medial column malreduction.26 Interestingly, compared with use of a polyaxial implant, which allows for adjustment of screw direction, use of a monoaxial locking plate did not lead to a clinically different outcome or complication profile.64
Techniques have been used to achieve subchondral purchase of locking screws while reducing iatrogenic articular perforation.65 However, given the incidence of fracture settling and subsequent postoperative screw penetration, many authors currently recommend using shorter divergent screws combined with other augmentation techniques, described previously.17,29,32
Physical Therapy
There is no standardized physiotherapy regimen for postoperative management of proximal humerus fractures treated with locking plates.25 In older patients, immediate active range of motion (ROM) exercises should be delayed until early callus is noted, though there is a risk for stiffness. Lee and Shin57 found that a delay in rehabilitation after ORIF was an independent risk for poor clinical outcome. Namdari and colleagues17 recommended sling use only for comfort and initiated non-load-bearing activities and pendulum exercises immediately after surgery. Patients with adequate reduction at 4 to 6 weeks were advanced to full weight-bearing. Badman and colleagues30 initiated passive-assisted ROM exercises when the wound was healed at 2 weeks in 2-part fractures, whereas patients with 3- and 4-part fractures were immobilized until radiographic healing. Formal therapy was started after 6 weeks. Stiffness was reported in 5% of patients. For patients with stable fixation, Ricchetti and colleagues29 recommended passive shoulder ROM exercises on postoperative day 1; at 4 to 6 weeks, patients should start active shoulder ROM exercises, and then resistance exercises at 10 to 12 weeks. Other authors are more conservative—only sling immobilization and pendulum exercises the first month.66 Barlow and colleagues32 immobilized their patients (age, >75 years) for 6 weeks. No patient developed disabling stiffness. The authors suggested that patients older than 75 years may not be prone to stiffness.
Our Preferred Treatment Method
All proximal humerus fractures are approached anteriorly through the deltopectoral interval (Figure 3A). The long head biceps is identified and truncated for later tenodesis. Multiple No. 5 Ethibond sutures (Ethicon) are placed at the bone–tendon interface. The fracture is reduced with a Cobb elevator (Figure 3B), and provisional Kirschner wires are placed within the head (Figure 3C). The plate is affixed to the humeral head with its anterior border paralleling the posterior aspect of the bicipital groove. Multiple locking screws are placed within the superior and posterior humeral head. Nonlocking screws are then used to fix the plate to the shaft to reduce the specific deformity. Under fluoroscopy, any metaphyseal void is filled with calcium phosphate cement (Figure 3D). The remaining inferior screws are placed within the humeral head. Dr. Gruson uses screws 4 to 6 mm short of subchondral bone to reduce the risk for joint penetration. The rotator cuff sutures are tied down through the plate. Patients are started on progressive supine passive ROM exercises at 7 days, followed by supine active-assisted ROM exercises 6 weeks after fracture healing is confirmed radiographically.
Conclusion
Use of locked plating for proximal humerus fractures has increased, particularly in the elderly. Resulting complications include intra-articular screw penetration, postoperative fracture displacement, and AVN. Recognition of the importance of reducing and supporting the medial calcar, filling any metaphyseal defects, and selectively placing screws within the humeral head has lowered the incidence of these complications. Further comparative studies evaluating the efficacy of individual augmentation techniques are needed to determine their contribution to successful fracture healing and their cost-effectiveness. Results of such studies may help in the development of protocols for more standardized implementation of these techniques and in understanding which specific fracture patterns and patients would benefit from their use.
1. Bell JE, Leung BC, Spratt KF, et al. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am. 2011;93(2):121-131.
2. Aaron D, Shatsky J, Paredes JC, Jiang C, Parsons BO, Flatow EL. Proximal humeral fractures: internal fixation. J Bone Joint Surg Am. 2012;94(24):2280-2288.
3. Court-Brown CM, Garg A, McQueen MM. The epidemiology of proximal humeral fractures. Acta Orthop Scand. 2001;72(4):365-371.
4. Kannus P, Palvanen M, Niemi S, Parkkari J, Jarvinen M, Vuori I. Increasing number and incidence of osteoporotic fractures of the proximal humerus in elderly people. BMJ. 1996;313(7064):1051-1052.
5. Handoll HH, Ollivere BJ, Rollins KE. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2012;12:CD000434.
6. Tamai K, Ishige N, Kuroda S, et al. Four-segment classification of proximal humeral fractures revisited: a multicenter study on 509 cases. J Shoulder Elbow Surg. 2009;18(6):845-850.
7. Rothberg D, Higgins T. Fractures of the proximal humerus. Orthop Clin North Am. 2013;44(1):9-19.
8. Egol KA, Kubiak EN, Fulkerson E, Kummer FJ, Koval KJ. Biomechanics of locked plates and screws. J Orthop Trauma. 2004;18(8):488-493.
9. Miranda MA. Locking plate technology and its role in osteoporotic fractures. Injury. 2007;38(suppl 3):35-39.
10. Foruria AM, Carrascal MT, Revilla C, Munuera L, Sanchez-Sotelo J. Proximal humerus fracture rotational stability after fixation using a locking plate or a fixed-angle locked nail: the role of implant stiffness. Clin Biomech. 2010;25(4):307-311.
11. Weinstein DM, Bratton DR, Ciccone WJ 2nd, Elias JJ. Locking plates improve torsional resistance in the stabilization of three-part proximal humeral fractures. J Shoulder Elbow Surg. 2006;15(2):239-243.
12. Siffri PC, Peindl RD, Coley ER, Norton J, Connor PM, Kellam JF. Biomechanical analysis of blade plate versus locking plate fixation for a proximal humerus fracture: comparison using cadaveric and synthetic humeri. J Orthop Trauma. 2006;20(8):547-554.
13. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Internal fixation versus nonoperative treatment of displaced 3-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(5):747-755.
14. Fjalestad T, Hole MO, Hovden IA, Blucher J, Stromsoe K. Surgical treatment with an angular stable plate for complex displaced proximal humeral fractures in elderly patients: a randomized controlled trial. J Orthop Trauma. 2012;26(2):98-106.
15. Wild JR, DeMers A, French R, et al. Functional outcomes for surgically treated 3- and 4-part proximal humerus fractures. Orthopedics. 2011;34(10):e629-e633.
16. Solberg BD, Moon CN, Franco DP, Paiement GD. Surgical treatment of three and four-part proximal humeral fractures. J Bone Joint Surg Am. 2009;91(7):1689-1697.
17. Namdari S, Voleti PB, Mehta S. Evaluation of the osteoporotic proximal humeral fracture and strategies for structural augmentation during surgical treatment. J Shoulder Elbow Surg. 2012;21(12):1787-1795.
18. Agudelo J, Schurmann M, Stahel P, et al. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma. 2007;21(10):676-681.
19. Schliemann B, Siemoneit J, Theisen C, Kosters C, Weimann A, Raschke MJ. Complex fractures of the proximal humerus in the elderly—outcome and complications after locking plate fixation. Musculoskelet Surg. 2012;96(suppl 1):S3-S11.
20. Thanasas C, Kontakis G, Angoules A, Limb D, Giannoudis P. Treatment of proximal humerus fractures with locking plates: a systematic review. J Shoulder Elbow Surg. 2009;18(6):837-844.
21. Micic ID, Kim KC, Shin DJ, et al. Analysis of early failure of the locking compression plate in osteoporotic proximal humerus fractures. J Orthop Sci. 2009;14(5):596-601.
22. Solberg BD, Moon CN, Franco DP, Paiement GD. Locked plating of 3- and 4-part proximal humerus fractures in older patients: the effect of initial fracture pattern on outcome. J Orthop Trauma. 2009;23(2):113-119.
23. Owsley KC, Gorczyca JT. Fracture displacement and screw cutout after open reduction and locked plate fixation of proximal humeral fractures [corrected]. J Bone Joint Surg Am. 2008;90(2):233-240.
24. Fankhauser F, Boldin C, Schippinger G, Haunschmid C, Szyszkowitz R. A new locking plate for unstable fractures of the proximal humerus. Clin Orthop. 2005;(430):176-181.
25. Sproul RC, Iyengar JJ, Devcic Z, Feeley BT. A systematic review of locking plate fixation of proximal humerus fractures. Injury. 2011;42(4):408-413.
26. Gardner MJ, Weil Y, Barker JU, Kelly BT, Helfet DL, Lorich DG. The importance of medial support in locked plating of proximal humerus fractures. J Orthop Trauma. 2007;21(3):185-191.
27. Voigt C, Hurschler C, Rech L, Vossenrich R, Lill H. Additive fiber-cerclages in proximal humeral fractures stabilized by locking plates. No effect on fracture stabilization and rotator cuff function in human shoulder specimens. Acta Orthop. 2009;80(4):465-471.
28. Lo IK, Burkhart SS. Biomechanical principles of arthroscopic repair of the rotator cuff. Oper Tech Orthop. 2002;12(3):140-155.
29. Ricchetti ET, Warrender WJ, Abboud JA. Use of locking plates in the treatment of proximal humerus fractures. J Shoulder Elbow Surg. 2010;19(2 suppl):66-75.
30. Badman B, Frankle M, Keating C, Henderson L, Brooks J, Mighell M. Results of proximal humeral locked plating with supplemental suture fixation of rotator cuff. J Shoulder Elbow Surg. 2011;20(4):616-624.
31. Nho SJ, Brophy RH, Barker JU, Cornell CN, MacGillivray JD. Management of proximal humeral fractures based on current literature. J Bone Joint Surg Am. 2007;89(suppl 3):44-58.
32. Barlow JD, Sanchez-Sotelo J, Torchia M. Proximal humerus fractures in the elderly can be reliably fixed with a “hybrid” locked-plating technique. Clin Orthop. 2011;469(12):3281-3291.
33. Cho CH, Jung GH, Song KS. Tension suture fixation using 2 washers for proximal humeral fractures. Orthopedics. 2012;35(3):202-205.
34. Brunner F, Sommer C, Bahrs C, et al. Open reduction and internal fixation of proximal humerus fractures using a proximal humeral locked plate: a prospective multicenter analysis. J Orthop Trauma. 2009;23(3):163-172.
35. Jung WB, Moon ES, Kim SK, Kovacevic D, Kim MS. Does medial support decrease major complications of unstable proximal humerus fractures treated with locking plate? BMC Musculoskelet Disord. 2013;14:102.
36. Krappinger D, Bizzotto N, Riedmann S, Kammerlander C, Hengg C, Kralinger FS. Predicting failure after surgical fixation of proximal humerus fractures. Injury. 2011;42(11):1283-1288.
37. Lescheid J, Zdero R, Shah S, Kuzyk PR, Schemitsch EH. The biomechanics of locked plating for repairing proximal humerus fractures with or without medial cortical support. J Trauma. 2010;69(5):1235-1242.
38. De Long WG Jr, Einhorn TA, Koval K, et al. Bone grafts and bone graft substitutes in orthopaedic trauma surgery. A critical analysis. J Bone Joint Surg Am. 2007;89(3):649-658.
39. Gardner MJ, Boraiah S, Helfet DL, Lorich DG. Indirect medial reduction and strut support of proximal humerus fractures using an endosteal implant. J Orthop Trauma. 2008;22(3):195-200.
40. Hettrich CM, Neviaser A, Beamer BS, Paul O, Helfet DL, Lorich DG. Locked plating of the proximal humerus using an endosteal implant. J Orthop Trauma. 2012;26(4):212-215.
41. Matassi F, Angeloni R, Carulli C, et al. Locking plate and fibular allograft augmentation in unstable fractures of proximal humerus. Injury. 2012;43(11):1939-1942.
42. Neviaser AS, Hettrich CM, Beamer BS, Dines JS, Lorich DG. Endosteal strut augment reduces complications associated with proximal humeral locking plates. Clin Orthop. 2011;469(12):3300-3306.
43. Mathison C, Chaudhary R, Beaupre L, Reynolds M, Adeeb S, Bouliane M. Biomechanical analysis of proximal humeral fixation using locking plate fixation with an intramedullary fibular allograft. Clin Biomech. 2010;25(7):642-646.
44. Osterhoff G, Baumgartner D, Favre P, et al. Medial support by fibula bone graft in angular stable plate fixation of proximal humeral fractures: an in vitro study with synthetic bone. J Shoulder Elbow Surg. 2011;20(5):740-746.
45. Chow RM, Begum F, Beaupre LA, Carey JP, Adeeb S, Bouliane MJ. Proximal humeral fracture fixation: locking plate construct +/- intramedullary fibular allograft. J Shoulder Elbow Surg. 2012;21(7):894-901.
46. Bae JH, Oh JK, Chon CS, Oh CW, Hwang JH, Yoon YC. The biomechanical performance of locking plate fixation with intramedullary fibular strut graft augmentation in the treatment of unstable fractures of the proximal humerus. J Bone Joint Surg Br. 2011;93(7):937-941.
47. Duralde XA, Leddy LR. The results of ORIF of displaced unstable proximal humeral fractures using a locking plate. J Shoulder Elbow Surg. 2010;19(4):480-488.
48. Robinson CM, Wylie JR, Ray AG, et al. Proximal humeral fractures with a severe varus deformity treated by fixation with a locking plate. J Bone Joint Surg Br. 2010;92(5):672-678.
49. Ong C, Bechtel C, Walsh M, Zuckerman JD, Egol KA. Three- and four-part fractures have poorer function than one-part proximal humerus fractures. Clin Orthop. 2011;469(12):3292-3299.
50. Kim SH, Lee YH, Chung SW, et al. Outcomes for four-part proximal humerus fractures treated with a locking compression plate and an autologous iliac bone impaction graft. Injury. 2012;43(10):1724-1731.
51. Larsson S. Calcium phosphates: what is the evidence? J Orthop Trauma. 2010;24(suppl 1):S41-S45.
52. Egol KA, Sugi MT, Ong CC, Montero N, Davidovitch R, Zuckerman JD. Fracture site augmentation with calcium phosphate cement reduces screw penetration after open reduction–internal fixation of proximal humeral fractures. J Shoulder Elbow Surg. 2012;21(6):741-748.
53. Gradl G, Knobe M, Stoffel M, Prescher A, Dirrichs T, Pape HC. Biomechanical evaluation of locking plate fixation of proximal humeral fractures augmented with calcium phosphate cement. J Orthop Trauma. 2013;27(7):399-404.
54. Collinge C, Merk B, Lautenschlager EP. Mechanical evaluation of fracture fixation augmented with tricalcium phosphate bone cement in a porous osteoporotic cancellous bone model. J Orthop Trauma. 2007;21(2):124-128.
55. Kwon BK, Goertzen DJ, O’Brien PJ, Broekhuyse HM, Oxland TR. Biomechanical evaluation of proximal humeral fracture fixation supplemented with calcium phosphate cement. J Bone Joint Surg Am. 2002;84(6):951-961.
56. Somasundaram K, Huber CP, Babu V, Zadeh H. Proximal humeral fractures: the role of calcium sulphate augmentation and extended deltoid splitting approach in internal fixation using locking plates. Injury. 2013;44(4):481-487.
57. Lee CW, Shin SJ. Prognostic factors for unstable proximal humeral fractures treated with locking-plate fixation. J Shoulder Elbow Surg. 2009;18(1):83-88.
58. Zhang L, Zheng J, Wang W, et al. The clinical benefit of medial support screws in locking plating of proximal humerus fractures: a prospective randomized study. Int Orthop. 2011;35(11):1655-1661.
59. Brianza S, Roderer G, Schiuma D, et al. Where do locking screws purchase in the humeral head? Injury. 2012;43(6):850-855.
60. Hepp P, Lill H, Bail H, et al. Where should implants be anchored in the humeral head? Clin Orthop. 2003;(415):139-147.
61. Liew AS, Johnson JA, Patterson SD, King GJ, Chess DG. Effect of screw placement on fixation in the humeral head. J Shoulder Elbow Surg. 2000;9(5):423-426.
62. Tingart MJ, Lehtinen J, Zurakowski D, Warner JJ, Apreleva M. Proximal humeral fractures: regional differences in bone mineral density of the humeral head affect the fixation strength of cancellous screws. J Shoulder Elbow Surg. 2006;15(5):620-624.
63. Erhardt JB, Stoffel K, Kampshoff J, Badur N, Yates P, Kuster MS. The position and number of screws influence screw perforation of the humeral head in modern locking plates: a cadaver study. J Orthop Trauma. 2012;26(10):e188-e192.
64. Konigshausen M, Kubler L, Godry H, Citak M, Schildhauer TA, Seybold D. Clinical outcome and complications using a polyaxial locking plate in the treatment of displaced proximal humerus fractures. A reliable system? Injury. 2012;43(2):223-231.
65. Bengard MJ, Gardner MJ. Screw depth sounding in proximal humerus fractures to avoid iatrogenic intra-articular penetration. J Orthop Trauma. 2011;25(10):630-633.
66. Ring D. Current concepts in plate and screw fixation of osteoporotic proximal humerus fractures. Injury. 2007;38(3):S59-S68.
Proximal humerus fractures account for 4% to 5% of all fractures.1 These fractures occur most frequently in the elderly—patients older than 60 years sustain 71% of these injuries2—and in females.1,3 Given an aging population, this incidence is predicted to increase 3-fold over the next 30 years.4 There is much debate regarding management of acute, displaced proximal humerus fractures. A recent Cochrane Review of published outcomes of operative and nonoperative treatment of displaced proximal humerus fractures found insufficient evidence supporting either modality, though surgery was associated with additional procedures.5 A review of 1000 proximal humerus fractures found that 49% had less than 1 cm of displacement of the major fragments or angulation of less than 45°.3 Other authors have reported similar findings.6,7 Although the incidence of proximal humerus fractures has remained stable over the past decade, from 1999 to 2005 there was a 25% relative increase in surgical management, including a relative increase of 29% in open reduction and internal fixation (ORIF) versus a 20% increase in arthroplasty.1
Locking plates have consistently demonstrated biomechanical superiority over other forms of fixation in osteoporotic bone.8-11 Egol and colleagues8 found that osteoporotic bone limited the torque of fixation to values less than what is required for adequate frictional force between the plate and the bone. This problem can be overcome with fixed-angle devices, such as locked plates.9 Compared with locked nail constructs, proximal humerus locking plates have demonstrated superiority in torsion, loading, and varus bending.10,11 Compared with blade plates, proximal humerus locking plates exhibited increased stiffness and torsional fatigue resistance.12 In a randomized clinical trial, Olerud and colleagues13 reported superior functional results with locking plate fixation compared with nonoperative treatment of displaced 3-part fractures in elderly patients with 2-year follow-up, though these clinical results were not supported by others.14 Two recent case–control studies comparing functional outcomes for 3- and 4-part fractures with follow-up of more than 2 years revealed higher Constant scores after locked plating compared with hemiarthroplasty, though complications were higher with locked plates.15,16 Adoption of locked proximal humerus plating has been correlated with good clinical outcomes and union rates, though this has been accompanied by a higher rate of reoperation.7 Reoperation rates from 1999 to 2005 increased both in the immediate postoperative period (odds ratio, 3.36) and at 1 year (odds ratio, 3.90).1
Complications of Locked Plating
Regardless of fixation type, reduced humeral head bone mass and quality may lead to implant loosening, fracture redisplacement, and, ultimately, poor outcomes. Baseline osteoporosis may predict likelihood of fixation failure.17 Multiple studies have reported on the implant-related complications associated with locking plate fixation—most commonly, intra-articular screw penetration, postoperative fracture displacement, and avascular necrosis (AVN)18-24 (Figure 1). A meta-analysis of 12 studies with a total of 514 proximal humerus fractures treated with locking plate fixation showed an overall complication rate of 49% and a 13.8% reoperation rate.25 The most common indication for reoperation involved intra-articular screw perforation. The most common complications were varus malunion (16%), osteonecrosis (10%), intra-articular screw penetration (8%), subacromial impingement (6%), and infection (4%).
Suboptimal intraoperative fracture reduction, specifically with residual varus, has been correlated with loss of fracture fixation. In a series of 153 fractures, loss of fixation occurred in 13.7% of cases, with the leading risk factor being varus malreduction.19 Failure rates were 30.4% and 11% when the head shaft angle was less than 120° and when it was 120° or more, respectively. Solberg and colleagues16 found that initial postoperative varus angulation of more than 20° resulted in universal loss of fixation. Conversion of these cases to hemiarthroplasty resulted in poor outcomes. Preoperative fracture alignment may also predict fixation failure.22 In one series, initial varus angulation healed with a mean 16° varus and a Constant score of 63, whereas initial valgus alignment healed with 6° varus and a Constant score of 71.22 Complications occurred in fractures that were initially in varus 79% of the time and initially in valgus 19% of the time. Screw perforation has been associated with loss of reduction 44% of the time.20
In an analysis of locking plate constructs revised after early (<4 weeks) failure in 8 patients with osteoporosis, Micic and colleagues21 found implant pullout leading to varus malalignment. All cases lacked medial support and subchondral screw purchase; 3 were initially malreduced. Owsley and Gorczyca23 retrospectively reviewed 53 cases of displaced proximal humerus fractures treated with locked plating. Despite the high rate of radiographic union, 36% developed complications, including screw cutout (23%), varus displacement of more than 10° (25%), and AVN (4%); 13% required revision. These complications disproportionately affected patients older than 60 years (57%) and negatively affected functional outcomes.
Augmentation Techniques
Despite its reported complications, proximal humerus locked plating remains the most widely used type of fixation.1 Advancements in locking plate design, improved understanding of fixation principles, and adoption of techniques augmenting proximal humerus locking plate fixation, particularly in osteoporotic bone, have reduced postoperative complications (Table 1).
Rotator Cuff Sutures
A widely adopted technique for neutralizing rotator cuff–deforming forces, which theoretically can cause fracture displacement, is incorporation of heavy nonabsorbable sutures. These sutures are placed through the rotator cuff–tuberosity junction and tied down after being passed through the plate. Obtaining and maintaining tuberosity reduction are essential in achieving good functional outcomes after fixation. In addition, tension band sutures may be particularly useful in the setting of initial varus deformity.26
Although clinical use of these sutures is common, biomechanical studies of their adjunctive contribution to fracture stability are lacking.27 The rotator cuff musculature has a maximal contractile force of 3.5 kg/cm2.28 Ricchetti and colleagues29 described a technique that involves using a locked plate and tagging the rotator cuff with heavy nonabsorbable sutures. Selective traction on the sutures can help obtain and maintain fracture reduction. Multiple studies have reported on suture use with locked plating for proximal humerus fractures.29-34 Badman and colleagues30 retrospectively reviewed 81 cases of metaphyseal defects or medial comminution treated with locked plating, rotator cuff sutures, and structural allograft. All cases healed within 6 months after surgery. The incidence of screw cutout was 3.7%, the incidence of AVN was 6.2%, and the incidence of varus collapse was 6%. A cadaveric study that used specimens (mean age, 77 years) with a simulated 3-part proximal humerus fracture treated with a locked plate both with and without cerclage sutures found no difference in interfragmentary motion between the groups.27 The authors concluded that additive sutures are not required for anatomically reduced fractures. Multiple sutures may counteract the deforming forces that act on bony segments that cannot be adequately maintained with screws, such as an osteoporotic greater tuberosity.
Medial Column Restoration
The importance of reducing and maintaining the medial calcar to provide biomechanical support for a laterally placed plate has been recognized.26,34-37 Gardner and colleagues26 suggested that medial support was achieved if the medial cortex was anatomically reduced, if the proximal fragment was impacted laterally onto the shaft, or if 1 or more inferomedial screws were placed. Cases that did not achieve medial support developed significantly more humeral head subsidence (5.8 mm vs 1.2 mm) and screw penetration. Krappinger and colleagues36 found that factors leading to fixation failure included age, local bone mineral density, anatomical reduction, and restoration of the medial cortical support. The authors concluded that anatomical reduction and restoration of the medial cortex were important in minimizing mechanical loads at the bone–implant interface. Biomechanically, Lescheid and colleagues37 found that the most stable construct was anatomical reduction with medial cortical contact. In the setting of comminution, however, it may be preferable to intentionally perform varus malreduction to achieve medial contact than to achieve anatomical reduction with a fracture gap. Badman and colleagues30 found that the incidence of screw penetration was 6% in patients with an intact medial calcar versus 29% in patients without medial support. In a retrospective analysis of patients treated with a locking plate and suture augmentation, Jung and colleagues35 concluded that restoring medial support was the most reliable factor in the prevention of loss of reduction with or without screw perforation. Last, Solberg and colleagues16 reported better clinical outcomes when the length of the metaphyseal segment attached to the articular fragment was more than 2 mm. A length of less than 2 mm was predictive of developing AVN.
Use of Bone Void Fillers
Allograft. Allograft is cancellous or corticocancellous chips or tricortical graft used as osteoconductive filler for metaphyseal defects.38 An increasingly popular technique involves using an endosteal fibular allograft strut to indirectly reduce the fracture and help support the medial calcar.39-42 Hettrich and colleagues40 reported on radiographic outcomes of displaced proximal humerus fractures with medial comminution treated with a locked plate and an endosteal fibular allograft or semitubular plate. The reduction was maintained in 96% of cases; there was 1 varus collapse. There were no cases of implant failure, screw perforation, or AVN. Other authors have also reported on successful use of fibular allograft in conjunction with a locked plate; the rate of reduction loss was low, and there were no cases of screw cutout or intra-articular screw penetration.30,41,42 These clinical outcomes are supported by results of biomechanical studies of the added benefit of intramedullary fibular allograft.43-46 Mathison and colleagues43 reported that a construct with fibular allograft and a locking plate increased the failure load by 1.72 times and the stiffness by 3.84 times compared with a control group of locking plate only. Bae and colleagues46 found significantly higher maximum failure load and construct stiffness with no varus collapse in specimens prepared with locked plate and fibular strut augmentation compared with a control group.
Others have successfully used cancellous allograft to fill humeral head bone defects.29,32,47-49 Duralde and Leddy47 reported 100% radiographic union and 81% good to excellent results in cases treated with a locking plate and morselized cancellous allograft to fill bone voids. Varus collapse and screw cutout did not occur, but there were 2 cases of AVN. Ricchetti and colleagues29 reviewed 54 cases treated with a locking plate and rotator cuff suture construct. Allograft cancellous chips and demineralized bone matrix were used in 3- and 4-part fractures (70% of cases) along with shorter screws in the humeral head. Major complications included AVN (1), fixation failure (3), and varus malunion (5). Others investigators have had less favorable results with use of cancellous bone graft. Schliemann and colleagues19 reported on 27 patients who were older than 65 years when they underwent ORIF with rotator cuff sutures to stabilize the tuberosities and either cancellous graft or a synthetic bone substitute in patients with massive metaphyseal defects. Patient-reported outcomes were superior to Constant scores. Complications included screw penetration (22.2%), reduction loss (44.4%), implant failure (3.7%), and AVN (29.6%).
Autograft. Autograft has both osteoconductive and osteoinductive properties and has been successfully used for metaphyseal defects.32,50 Kim and colleagues50 reported on patients with 4-part proximal humerus fractures treated with a locking plate and autologous iliac graft. All cases achieved union and had good or excellent outcomes. There were no cases of AVN, varus collapse, or hardware-related complications.
Bone Cement. Calcium phosphate cement has osteoconductive properties and enhances screw purchase in cancellous bone (Figures 2A–2F). It can be injected or molded into bone voids to provide improved compressive strength. It is resorbed through cell-mediated processes resembling bone remodeling and does not disappear until new bone forms. (Calcium sulfate cement, on the other hand, resorbs through a chemical process independent of new bone formation.51) Egol and colleagues52 reviewed the cases of 92 patients (mean age, 61 years) with 2-, 3-, and 4-part proximal humerus fractures treated with locked plate fixation. Metaphyseal defects were treated with no augmentation, augmentation with cancellous chips, or augmentation with calcium phosphate cement. Adding calcium phosphate cement was associated with lower incidence of intra-articular screw penetration and humeral head settling. In a recent cadaveric biomechanical study using 2-part proximal humerus fractures with metaphyseal comminution, the group augmented with calcium phosphate cement had enhanced axial stiffness and load to failure with reduced screw penetration.53 Other biomechanical studies have found increased screw pullout strength54 and decreased interfragmentary motion when specimens were augmented with calcium phosphate cement.55
Similar good clinical and radiographic outcomes have been observed with use of calcium sulfate cement.56,57 Somasundaram and colleagues56 reported good clinical outcomes in 82% of patients treated with locking plates and calcium sulfate cement used to fill metaphyseal voids. All fractures united without infection, fixation failure, subsequent malunion, tuberosity failure, or AVN. Lee and Shin57 compared outcomes of 14 patients who received calcium sulfate augmentation with outcomes of patients who did not receive this augmentation. Overall, 89% of patients had good or excellent results. Calcium sulfate cement did not affect the reduction failure rate or clinical outcomes in cases in which medial cortical reduction was achieved. However, postoperative displacement caused by lack of medial support was associated with poor outcomes.
Screw Placement
Screws optimally should be placed in the posterior-medial-inferior aspect of the humeral head to provide medial support for the fracture and mechanical stability.58 Cadaveric studies have shown the highest cancellous bone density in the proximal, posterior, and medial portions of the humeral head.59-63 Similarly, in a cadaveric study, Liew and colleagues61 found greater screw purchase and higher pullout strength when the screw was placed in the center of the humeral head, within subchondral bone; fixation was poorest when the screw was placed in the anterosuperior region of the humeral head. Tingart and colleagues62 reported that humeral head trabecular density significantly affected pullout strength of cancellous screws. In addition, the most pullout strength was at the center of the head, and the least within the anterosuperior head. Trabecular density was higher in the inferior and posterior regions than in the superior and anterior regions.
Most locking plate designs allow screws to be placed at the level of the medial calcar—the goal being to provide medial column support (Table 2). Zhang and colleagues58 treated 2-, 3-, and 4-part fractures with a locking plate and randomized them into receiving the plate with or without medial support screws. For 3- and 4-part fractures, the group with these screws had a significantly greater final neck-shaft angle and smaller angulation loss compared with the group without screws. No additional benefit was found for 2-part fractures. Erhardt and colleagues63 simulated unstable proximal humerus fractures using cadavers and testing different fixation methods using a polyaxial locking plate. They found that 5 screws in the head fragment and an inferomedial support screw significantly reduced the risk of screw perforation. Other authors have concluded that placing 1 or more inferomedial screws is important in cases of medial comminution or medial column malreduction.26 Interestingly, compared with use of a polyaxial implant, which allows for adjustment of screw direction, use of a monoaxial locking plate did not lead to a clinically different outcome or complication profile.64
Techniques have been used to achieve subchondral purchase of locking screws while reducing iatrogenic articular perforation.65 However, given the incidence of fracture settling and subsequent postoperative screw penetration, many authors currently recommend using shorter divergent screws combined with other augmentation techniques, described previously.17,29,32
Physical Therapy
There is no standardized physiotherapy regimen for postoperative management of proximal humerus fractures treated with locking plates.25 In older patients, immediate active range of motion (ROM) exercises should be delayed until early callus is noted, though there is a risk for stiffness. Lee and Shin57 found that a delay in rehabilitation after ORIF was an independent risk for poor clinical outcome. Namdari and colleagues17 recommended sling use only for comfort and initiated non-load-bearing activities and pendulum exercises immediately after surgery. Patients with adequate reduction at 4 to 6 weeks were advanced to full weight-bearing. Badman and colleagues30 initiated passive-assisted ROM exercises when the wound was healed at 2 weeks in 2-part fractures, whereas patients with 3- and 4-part fractures were immobilized until radiographic healing. Formal therapy was started after 6 weeks. Stiffness was reported in 5% of patients. For patients with stable fixation, Ricchetti and colleagues29 recommended passive shoulder ROM exercises on postoperative day 1; at 4 to 6 weeks, patients should start active shoulder ROM exercises, and then resistance exercises at 10 to 12 weeks. Other authors are more conservative—only sling immobilization and pendulum exercises the first month.66 Barlow and colleagues32 immobilized their patients (age, >75 years) for 6 weeks. No patient developed disabling stiffness. The authors suggested that patients older than 75 years may not be prone to stiffness.
Our Preferred Treatment Method
All proximal humerus fractures are approached anteriorly through the deltopectoral interval (Figure 3A). The long head biceps is identified and truncated for later tenodesis. Multiple No. 5 Ethibond sutures (Ethicon) are placed at the bone–tendon interface. The fracture is reduced with a Cobb elevator (Figure 3B), and provisional Kirschner wires are placed within the head (Figure 3C). The plate is affixed to the humeral head with its anterior border paralleling the posterior aspect of the bicipital groove. Multiple locking screws are placed within the superior and posterior humeral head. Nonlocking screws are then used to fix the plate to the shaft to reduce the specific deformity. Under fluoroscopy, any metaphyseal void is filled with calcium phosphate cement (Figure 3D). The remaining inferior screws are placed within the humeral head. Dr. Gruson uses screws 4 to 6 mm short of subchondral bone to reduce the risk for joint penetration. The rotator cuff sutures are tied down through the plate. Patients are started on progressive supine passive ROM exercises at 7 days, followed by supine active-assisted ROM exercises 6 weeks after fracture healing is confirmed radiographically.
Conclusion
Use of locked plating for proximal humerus fractures has increased, particularly in the elderly. Resulting complications include intra-articular screw penetration, postoperative fracture displacement, and AVN. Recognition of the importance of reducing and supporting the medial calcar, filling any metaphyseal defects, and selectively placing screws within the humeral head has lowered the incidence of these complications. Further comparative studies evaluating the efficacy of individual augmentation techniques are needed to determine their contribution to successful fracture healing and their cost-effectiveness. Results of such studies may help in the development of protocols for more standardized implementation of these techniques and in understanding which specific fracture patterns and patients would benefit from their use.
Proximal humerus fractures account for 4% to 5% of all fractures.1 These fractures occur most frequently in the elderly—patients older than 60 years sustain 71% of these injuries2—and in females.1,3 Given an aging population, this incidence is predicted to increase 3-fold over the next 30 years.4 There is much debate regarding management of acute, displaced proximal humerus fractures. A recent Cochrane Review of published outcomes of operative and nonoperative treatment of displaced proximal humerus fractures found insufficient evidence supporting either modality, though surgery was associated with additional procedures.5 A review of 1000 proximal humerus fractures found that 49% had less than 1 cm of displacement of the major fragments or angulation of less than 45°.3 Other authors have reported similar findings.6,7 Although the incidence of proximal humerus fractures has remained stable over the past decade, from 1999 to 2005 there was a 25% relative increase in surgical management, including a relative increase of 29% in open reduction and internal fixation (ORIF) versus a 20% increase in arthroplasty.1
Locking plates have consistently demonstrated biomechanical superiority over other forms of fixation in osteoporotic bone.8-11 Egol and colleagues8 found that osteoporotic bone limited the torque of fixation to values less than what is required for adequate frictional force between the plate and the bone. This problem can be overcome with fixed-angle devices, such as locked plates.9 Compared with locked nail constructs, proximal humerus locking plates have demonstrated superiority in torsion, loading, and varus bending.10,11 Compared with blade plates, proximal humerus locking plates exhibited increased stiffness and torsional fatigue resistance.12 In a randomized clinical trial, Olerud and colleagues13 reported superior functional results with locking plate fixation compared with nonoperative treatment of displaced 3-part fractures in elderly patients with 2-year follow-up, though these clinical results were not supported by others.14 Two recent case–control studies comparing functional outcomes for 3- and 4-part fractures with follow-up of more than 2 years revealed higher Constant scores after locked plating compared with hemiarthroplasty, though complications were higher with locked plates.15,16 Adoption of locked proximal humerus plating has been correlated with good clinical outcomes and union rates, though this has been accompanied by a higher rate of reoperation.7 Reoperation rates from 1999 to 2005 increased both in the immediate postoperative period (odds ratio, 3.36) and at 1 year (odds ratio, 3.90).1
Complications of Locked Plating
Regardless of fixation type, reduced humeral head bone mass and quality may lead to implant loosening, fracture redisplacement, and, ultimately, poor outcomes. Baseline osteoporosis may predict likelihood of fixation failure.17 Multiple studies have reported on the implant-related complications associated with locking plate fixation—most commonly, intra-articular screw penetration, postoperative fracture displacement, and avascular necrosis (AVN)18-24 (Figure 1). A meta-analysis of 12 studies with a total of 514 proximal humerus fractures treated with locking plate fixation showed an overall complication rate of 49% and a 13.8% reoperation rate.25 The most common indication for reoperation involved intra-articular screw perforation. The most common complications were varus malunion (16%), osteonecrosis (10%), intra-articular screw penetration (8%), subacromial impingement (6%), and infection (4%).
Suboptimal intraoperative fracture reduction, specifically with residual varus, has been correlated with loss of fracture fixation. In a series of 153 fractures, loss of fixation occurred in 13.7% of cases, with the leading risk factor being varus malreduction.19 Failure rates were 30.4% and 11% when the head shaft angle was less than 120° and when it was 120° or more, respectively. Solberg and colleagues16 found that initial postoperative varus angulation of more than 20° resulted in universal loss of fixation. Conversion of these cases to hemiarthroplasty resulted in poor outcomes. Preoperative fracture alignment may also predict fixation failure.22 In one series, initial varus angulation healed with a mean 16° varus and a Constant score of 63, whereas initial valgus alignment healed with 6° varus and a Constant score of 71.22 Complications occurred in fractures that were initially in varus 79% of the time and initially in valgus 19% of the time. Screw perforation has been associated with loss of reduction 44% of the time.20
In an analysis of locking plate constructs revised after early (<4 weeks) failure in 8 patients with osteoporosis, Micic and colleagues21 found implant pullout leading to varus malalignment. All cases lacked medial support and subchondral screw purchase; 3 were initially malreduced. Owsley and Gorczyca23 retrospectively reviewed 53 cases of displaced proximal humerus fractures treated with locked plating. Despite the high rate of radiographic union, 36% developed complications, including screw cutout (23%), varus displacement of more than 10° (25%), and AVN (4%); 13% required revision. These complications disproportionately affected patients older than 60 years (57%) and negatively affected functional outcomes.
Augmentation Techniques
Despite its reported complications, proximal humerus locked plating remains the most widely used type of fixation.1 Advancements in locking plate design, improved understanding of fixation principles, and adoption of techniques augmenting proximal humerus locking plate fixation, particularly in osteoporotic bone, have reduced postoperative complications (Table 1).
Rotator Cuff Sutures
A widely adopted technique for neutralizing rotator cuff–deforming forces, which theoretically can cause fracture displacement, is incorporation of heavy nonabsorbable sutures. These sutures are placed through the rotator cuff–tuberosity junction and tied down after being passed through the plate. Obtaining and maintaining tuberosity reduction are essential in achieving good functional outcomes after fixation. In addition, tension band sutures may be particularly useful in the setting of initial varus deformity.26
Although clinical use of these sutures is common, biomechanical studies of their adjunctive contribution to fracture stability are lacking.27 The rotator cuff musculature has a maximal contractile force of 3.5 kg/cm2.28 Ricchetti and colleagues29 described a technique that involves using a locked plate and tagging the rotator cuff with heavy nonabsorbable sutures. Selective traction on the sutures can help obtain and maintain fracture reduction. Multiple studies have reported on suture use with locked plating for proximal humerus fractures.29-34 Badman and colleagues30 retrospectively reviewed 81 cases of metaphyseal defects or medial comminution treated with locked plating, rotator cuff sutures, and structural allograft. All cases healed within 6 months after surgery. The incidence of screw cutout was 3.7%, the incidence of AVN was 6.2%, and the incidence of varus collapse was 6%. A cadaveric study that used specimens (mean age, 77 years) with a simulated 3-part proximal humerus fracture treated with a locked plate both with and without cerclage sutures found no difference in interfragmentary motion between the groups.27 The authors concluded that additive sutures are not required for anatomically reduced fractures. Multiple sutures may counteract the deforming forces that act on bony segments that cannot be adequately maintained with screws, such as an osteoporotic greater tuberosity.
Medial Column Restoration
The importance of reducing and maintaining the medial calcar to provide biomechanical support for a laterally placed plate has been recognized.26,34-37 Gardner and colleagues26 suggested that medial support was achieved if the medial cortex was anatomically reduced, if the proximal fragment was impacted laterally onto the shaft, or if 1 or more inferomedial screws were placed. Cases that did not achieve medial support developed significantly more humeral head subsidence (5.8 mm vs 1.2 mm) and screw penetration. Krappinger and colleagues36 found that factors leading to fixation failure included age, local bone mineral density, anatomical reduction, and restoration of the medial cortical support. The authors concluded that anatomical reduction and restoration of the medial cortex were important in minimizing mechanical loads at the bone–implant interface. Biomechanically, Lescheid and colleagues37 found that the most stable construct was anatomical reduction with medial cortical contact. In the setting of comminution, however, it may be preferable to intentionally perform varus malreduction to achieve medial contact than to achieve anatomical reduction with a fracture gap. Badman and colleagues30 found that the incidence of screw penetration was 6% in patients with an intact medial calcar versus 29% in patients without medial support. In a retrospective analysis of patients treated with a locking plate and suture augmentation, Jung and colleagues35 concluded that restoring medial support was the most reliable factor in the prevention of loss of reduction with or without screw perforation. Last, Solberg and colleagues16 reported better clinical outcomes when the length of the metaphyseal segment attached to the articular fragment was more than 2 mm. A length of less than 2 mm was predictive of developing AVN.
Use of Bone Void Fillers
Allograft. Allograft is cancellous or corticocancellous chips or tricortical graft used as osteoconductive filler for metaphyseal defects.38 An increasingly popular technique involves using an endosteal fibular allograft strut to indirectly reduce the fracture and help support the medial calcar.39-42 Hettrich and colleagues40 reported on radiographic outcomes of displaced proximal humerus fractures with medial comminution treated with a locked plate and an endosteal fibular allograft or semitubular plate. The reduction was maintained in 96% of cases; there was 1 varus collapse. There were no cases of implant failure, screw perforation, or AVN. Other authors have also reported on successful use of fibular allograft in conjunction with a locked plate; the rate of reduction loss was low, and there were no cases of screw cutout or intra-articular screw penetration.30,41,42 These clinical outcomes are supported by results of biomechanical studies of the added benefit of intramedullary fibular allograft.43-46 Mathison and colleagues43 reported that a construct with fibular allograft and a locking plate increased the failure load by 1.72 times and the stiffness by 3.84 times compared with a control group of locking plate only. Bae and colleagues46 found significantly higher maximum failure load and construct stiffness with no varus collapse in specimens prepared with locked plate and fibular strut augmentation compared with a control group.
Others have successfully used cancellous allograft to fill humeral head bone defects.29,32,47-49 Duralde and Leddy47 reported 100% radiographic union and 81% good to excellent results in cases treated with a locking plate and morselized cancellous allograft to fill bone voids. Varus collapse and screw cutout did not occur, but there were 2 cases of AVN. Ricchetti and colleagues29 reviewed 54 cases treated with a locking plate and rotator cuff suture construct. Allograft cancellous chips and demineralized bone matrix were used in 3- and 4-part fractures (70% of cases) along with shorter screws in the humeral head. Major complications included AVN (1), fixation failure (3), and varus malunion (5). Others investigators have had less favorable results with use of cancellous bone graft. Schliemann and colleagues19 reported on 27 patients who were older than 65 years when they underwent ORIF with rotator cuff sutures to stabilize the tuberosities and either cancellous graft or a synthetic bone substitute in patients with massive metaphyseal defects. Patient-reported outcomes were superior to Constant scores. Complications included screw penetration (22.2%), reduction loss (44.4%), implant failure (3.7%), and AVN (29.6%).
Autograft. Autograft has both osteoconductive and osteoinductive properties and has been successfully used for metaphyseal defects.32,50 Kim and colleagues50 reported on patients with 4-part proximal humerus fractures treated with a locking plate and autologous iliac graft. All cases achieved union and had good or excellent outcomes. There were no cases of AVN, varus collapse, or hardware-related complications.
Bone Cement. Calcium phosphate cement has osteoconductive properties and enhances screw purchase in cancellous bone (Figures 2A–2F). It can be injected or molded into bone voids to provide improved compressive strength. It is resorbed through cell-mediated processes resembling bone remodeling and does not disappear until new bone forms. (Calcium sulfate cement, on the other hand, resorbs through a chemical process independent of new bone formation.51) Egol and colleagues52 reviewed the cases of 92 patients (mean age, 61 years) with 2-, 3-, and 4-part proximal humerus fractures treated with locked plate fixation. Metaphyseal defects were treated with no augmentation, augmentation with cancellous chips, or augmentation with calcium phosphate cement. Adding calcium phosphate cement was associated with lower incidence of intra-articular screw penetration and humeral head settling. In a recent cadaveric biomechanical study using 2-part proximal humerus fractures with metaphyseal comminution, the group augmented with calcium phosphate cement had enhanced axial stiffness and load to failure with reduced screw penetration.53 Other biomechanical studies have found increased screw pullout strength54 and decreased interfragmentary motion when specimens were augmented with calcium phosphate cement.55
Similar good clinical and radiographic outcomes have been observed with use of calcium sulfate cement.56,57 Somasundaram and colleagues56 reported good clinical outcomes in 82% of patients treated with locking plates and calcium sulfate cement used to fill metaphyseal voids. All fractures united without infection, fixation failure, subsequent malunion, tuberosity failure, or AVN. Lee and Shin57 compared outcomes of 14 patients who received calcium sulfate augmentation with outcomes of patients who did not receive this augmentation. Overall, 89% of patients had good or excellent results. Calcium sulfate cement did not affect the reduction failure rate or clinical outcomes in cases in which medial cortical reduction was achieved. However, postoperative displacement caused by lack of medial support was associated with poor outcomes.
Screw Placement
Screws optimally should be placed in the posterior-medial-inferior aspect of the humeral head to provide medial support for the fracture and mechanical stability.58 Cadaveric studies have shown the highest cancellous bone density in the proximal, posterior, and medial portions of the humeral head.59-63 Similarly, in a cadaveric study, Liew and colleagues61 found greater screw purchase and higher pullout strength when the screw was placed in the center of the humeral head, within subchondral bone; fixation was poorest when the screw was placed in the anterosuperior region of the humeral head. Tingart and colleagues62 reported that humeral head trabecular density significantly affected pullout strength of cancellous screws. In addition, the most pullout strength was at the center of the head, and the least within the anterosuperior head. Trabecular density was higher in the inferior and posterior regions than in the superior and anterior regions.
Most locking plate designs allow screws to be placed at the level of the medial calcar—the goal being to provide medial column support (Table 2). Zhang and colleagues58 treated 2-, 3-, and 4-part fractures with a locking plate and randomized them into receiving the plate with or without medial support screws. For 3- and 4-part fractures, the group with these screws had a significantly greater final neck-shaft angle and smaller angulation loss compared with the group without screws. No additional benefit was found for 2-part fractures. Erhardt and colleagues63 simulated unstable proximal humerus fractures using cadavers and testing different fixation methods using a polyaxial locking plate. They found that 5 screws in the head fragment and an inferomedial support screw significantly reduced the risk of screw perforation. Other authors have concluded that placing 1 or more inferomedial screws is important in cases of medial comminution or medial column malreduction.26 Interestingly, compared with use of a polyaxial implant, which allows for adjustment of screw direction, use of a monoaxial locking plate did not lead to a clinically different outcome or complication profile.64
Techniques have been used to achieve subchondral purchase of locking screws while reducing iatrogenic articular perforation.65 However, given the incidence of fracture settling and subsequent postoperative screw penetration, many authors currently recommend using shorter divergent screws combined with other augmentation techniques, described previously.17,29,32
Physical Therapy
There is no standardized physiotherapy regimen for postoperative management of proximal humerus fractures treated with locking plates.25 In older patients, immediate active range of motion (ROM) exercises should be delayed until early callus is noted, though there is a risk for stiffness. Lee and Shin57 found that a delay in rehabilitation after ORIF was an independent risk for poor clinical outcome. Namdari and colleagues17 recommended sling use only for comfort and initiated non-load-bearing activities and pendulum exercises immediately after surgery. Patients with adequate reduction at 4 to 6 weeks were advanced to full weight-bearing. Badman and colleagues30 initiated passive-assisted ROM exercises when the wound was healed at 2 weeks in 2-part fractures, whereas patients with 3- and 4-part fractures were immobilized until radiographic healing. Formal therapy was started after 6 weeks. Stiffness was reported in 5% of patients. For patients with stable fixation, Ricchetti and colleagues29 recommended passive shoulder ROM exercises on postoperative day 1; at 4 to 6 weeks, patients should start active shoulder ROM exercises, and then resistance exercises at 10 to 12 weeks. Other authors are more conservative—only sling immobilization and pendulum exercises the first month.66 Barlow and colleagues32 immobilized their patients (age, >75 years) for 6 weeks. No patient developed disabling stiffness. The authors suggested that patients older than 75 years may not be prone to stiffness.
Our Preferred Treatment Method
All proximal humerus fractures are approached anteriorly through the deltopectoral interval (Figure 3A). The long head biceps is identified and truncated for later tenodesis. Multiple No. 5 Ethibond sutures (Ethicon) are placed at the bone–tendon interface. The fracture is reduced with a Cobb elevator (Figure 3B), and provisional Kirschner wires are placed within the head (Figure 3C). The plate is affixed to the humeral head with its anterior border paralleling the posterior aspect of the bicipital groove. Multiple locking screws are placed within the superior and posterior humeral head. Nonlocking screws are then used to fix the plate to the shaft to reduce the specific deformity. Under fluoroscopy, any metaphyseal void is filled with calcium phosphate cement (Figure 3D). The remaining inferior screws are placed within the humeral head. Dr. Gruson uses screws 4 to 6 mm short of subchondral bone to reduce the risk for joint penetration. The rotator cuff sutures are tied down through the plate. Patients are started on progressive supine passive ROM exercises at 7 days, followed by supine active-assisted ROM exercises 6 weeks after fracture healing is confirmed radiographically.
Conclusion
Use of locked plating for proximal humerus fractures has increased, particularly in the elderly. Resulting complications include intra-articular screw penetration, postoperative fracture displacement, and AVN. Recognition of the importance of reducing and supporting the medial calcar, filling any metaphyseal defects, and selectively placing screws within the humeral head has lowered the incidence of these complications. Further comparative studies evaluating the efficacy of individual augmentation techniques are needed to determine their contribution to successful fracture healing and their cost-effectiveness. Results of such studies may help in the development of protocols for more standardized implementation of these techniques and in understanding which specific fracture patterns and patients would benefit from their use.
1. Bell JE, Leung BC, Spratt KF, et al. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am. 2011;93(2):121-131.
2. Aaron D, Shatsky J, Paredes JC, Jiang C, Parsons BO, Flatow EL. Proximal humeral fractures: internal fixation. J Bone Joint Surg Am. 2012;94(24):2280-2288.
3. Court-Brown CM, Garg A, McQueen MM. The epidemiology of proximal humeral fractures. Acta Orthop Scand. 2001;72(4):365-371.
4. Kannus P, Palvanen M, Niemi S, Parkkari J, Jarvinen M, Vuori I. Increasing number and incidence of osteoporotic fractures of the proximal humerus in elderly people. BMJ. 1996;313(7064):1051-1052.
5. Handoll HH, Ollivere BJ, Rollins KE. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2012;12:CD000434.
6. Tamai K, Ishige N, Kuroda S, et al. Four-segment classification of proximal humeral fractures revisited: a multicenter study on 509 cases. J Shoulder Elbow Surg. 2009;18(6):845-850.
7. Rothberg D, Higgins T. Fractures of the proximal humerus. Orthop Clin North Am. 2013;44(1):9-19.
8. Egol KA, Kubiak EN, Fulkerson E, Kummer FJ, Koval KJ. Biomechanics of locked plates and screws. J Orthop Trauma. 2004;18(8):488-493.
9. Miranda MA. Locking plate technology and its role in osteoporotic fractures. Injury. 2007;38(suppl 3):35-39.
10. Foruria AM, Carrascal MT, Revilla C, Munuera L, Sanchez-Sotelo J. Proximal humerus fracture rotational stability after fixation using a locking plate or a fixed-angle locked nail: the role of implant stiffness. Clin Biomech. 2010;25(4):307-311.
11. Weinstein DM, Bratton DR, Ciccone WJ 2nd, Elias JJ. Locking plates improve torsional resistance in the stabilization of three-part proximal humeral fractures. J Shoulder Elbow Surg. 2006;15(2):239-243.
12. Siffri PC, Peindl RD, Coley ER, Norton J, Connor PM, Kellam JF. Biomechanical analysis of blade plate versus locking plate fixation for a proximal humerus fracture: comparison using cadaveric and synthetic humeri. J Orthop Trauma. 2006;20(8):547-554.
13. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Internal fixation versus nonoperative treatment of displaced 3-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(5):747-755.
14. Fjalestad T, Hole MO, Hovden IA, Blucher J, Stromsoe K. Surgical treatment with an angular stable plate for complex displaced proximal humeral fractures in elderly patients: a randomized controlled trial. J Orthop Trauma. 2012;26(2):98-106.
15. Wild JR, DeMers A, French R, et al. Functional outcomes for surgically treated 3- and 4-part proximal humerus fractures. Orthopedics. 2011;34(10):e629-e633.
16. Solberg BD, Moon CN, Franco DP, Paiement GD. Surgical treatment of three and four-part proximal humeral fractures. J Bone Joint Surg Am. 2009;91(7):1689-1697.
17. Namdari S, Voleti PB, Mehta S. Evaluation of the osteoporotic proximal humeral fracture and strategies for structural augmentation during surgical treatment. J Shoulder Elbow Surg. 2012;21(12):1787-1795.
18. Agudelo J, Schurmann M, Stahel P, et al. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma. 2007;21(10):676-681.
19. Schliemann B, Siemoneit J, Theisen C, Kosters C, Weimann A, Raschke MJ. Complex fractures of the proximal humerus in the elderly—outcome and complications after locking plate fixation. Musculoskelet Surg. 2012;96(suppl 1):S3-S11.
20. Thanasas C, Kontakis G, Angoules A, Limb D, Giannoudis P. Treatment of proximal humerus fractures with locking plates: a systematic review. J Shoulder Elbow Surg. 2009;18(6):837-844.
21. Micic ID, Kim KC, Shin DJ, et al. Analysis of early failure of the locking compression plate in osteoporotic proximal humerus fractures. J Orthop Sci. 2009;14(5):596-601.
22. Solberg BD, Moon CN, Franco DP, Paiement GD. Locked plating of 3- and 4-part proximal humerus fractures in older patients: the effect of initial fracture pattern on outcome. J Orthop Trauma. 2009;23(2):113-119.
23. Owsley KC, Gorczyca JT. Fracture displacement and screw cutout after open reduction and locked plate fixation of proximal humeral fractures [corrected]. J Bone Joint Surg Am. 2008;90(2):233-240.
24. Fankhauser F, Boldin C, Schippinger G, Haunschmid C, Szyszkowitz R. A new locking plate for unstable fractures of the proximal humerus. Clin Orthop. 2005;(430):176-181.
25. Sproul RC, Iyengar JJ, Devcic Z, Feeley BT. A systematic review of locking plate fixation of proximal humerus fractures. Injury. 2011;42(4):408-413.
26. Gardner MJ, Weil Y, Barker JU, Kelly BT, Helfet DL, Lorich DG. The importance of medial support in locked plating of proximal humerus fractures. J Orthop Trauma. 2007;21(3):185-191.
27. Voigt C, Hurschler C, Rech L, Vossenrich R, Lill H. Additive fiber-cerclages in proximal humeral fractures stabilized by locking plates. No effect on fracture stabilization and rotator cuff function in human shoulder specimens. Acta Orthop. 2009;80(4):465-471.
28. Lo IK, Burkhart SS. Biomechanical principles of arthroscopic repair of the rotator cuff. Oper Tech Orthop. 2002;12(3):140-155.
29. Ricchetti ET, Warrender WJ, Abboud JA. Use of locking plates in the treatment of proximal humerus fractures. J Shoulder Elbow Surg. 2010;19(2 suppl):66-75.
30. Badman B, Frankle M, Keating C, Henderson L, Brooks J, Mighell M. Results of proximal humeral locked plating with supplemental suture fixation of rotator cuff. J Shoulder Elbow Surg. 2011;20(4):616-624.
31. Nho SJ, Brophy RH, Barker JU, Cornell CN, MacGillivray JD. Management of proximal humeral fractures based on current literature. J Bone Joint Surg Am. 2007;89(suppl 3):44-58.
32. Barlow JD, Sanchez-Sotelo J, Torchia M. Proximal humerus fractures in the elderly can be reliably fixed with a “hybrid” locked-plating technique. Clin Orthop. 2011;469(12):3281-3291.
33. Cho CH, Jung GH, Song KS. Tension suture fixation using 2 washers for proximal humeral fractures. Orthopedics. 2012;35(3):202-205.
34. Brunner F, Sommer C, Bahrs C, et al. Open reduction and internal fixation of proximal humerus fractures using a proximal humeral locked plate: a prospective multicenter analysis. J Orthop Trauma. 2009;23(3):163-172.
35. Jung WB, Moon ES, Kim SK, Kovacevic D, Kim MS. Does medial support decrease major complications of unstable proximal humerus fractures treated with locking plate? BMC Musculoskelet Disord. 2013;14:102.
36. Krappinger D, Bizzotto N, Riedmann S, Kammerlander C, Hengg C, Kralinger FS. Predicting failure after surgical fixation of proximal humerus fractures. Injury. 2011;42(11):1283-1288.
37. Lescheid J, Zdero R, Shah S, Kuzyk PR, Schemitsch EH. The biomechanics of locked plating for repairing proximal humerus fractures with or without medial cortical support. J Trauma. 2010;69(5):1235-1242.
38. De Long WG Jr, Einhorn TA, Koval K, et al. Bone grafts and bone graft substitutes in orthopaedic trauma surgery. A critical analysis. J Bone Joint Surg Am. 2007;89(3):649-658.
39. Gardner MJ, Boraiah S, Helfet DL, Lorich DG. Indirect medial reduction and strut support of proximal humerus fractures using an endosteal implant. J Orthop Trauma. 2008;22(3):195-200.
40. Hettrich CM, Neviaser A, Beamer BS, Paul O, Helfet DL, Lorich DG. Locked plating of the proximal humerus using an endosteal implant. J Orthop Trauma. 2012;26(4):212-215.
41. Matassi F, Angeloni R, Carulli C, et al. Locking plate and fibular allograft augmentation in unstable fractures of proximal humerus. Injury. 2012;43(11):1939-1942.
42. Neviaser AS, Hettrich CM, Beamer BS, Dines JS, Lorich DG. Endosteal strut augment reduces complications associated with proximal humeral locking plates. Clin Orthop. 2011;469(12):3300-3306.
43. Mathison C, Chaudhary R, Beaupre L, Reynolds M, Adeeb S, Bouliane M. Biomechanical analysis of proximal humeral fixation using locking plate fixation with an intramedullary fibular allograft. Clin Biomech. 2010;25(7):642-646.
44. Osterhoff G, Baumgartner D, Favre P, et al. Medial support by fibula bone graft in angular stable plate fixation of proximal humeral fractures: an in vitro study with synthetic bone. J Shoulder Elbow Surg. 2011;20(5):740-746.
45. Chow RM, Begum F, Beaupre LA, Carey JP, Adeeb S, Bouliane MJ. Proximal humeral fracture fixation: locking plate construct +/- intramedullary fibular allograft. J Shoulder Elbow Surg. 2012;21(7):894-901.
46. Bae JH, Oh JK, Chon CS, Oh CW, Hwang JH, Yoon YC. The biomechanical performance of locking plate fixation with intramedullary fibular strut graft augmentation in the treatment of unstable fractures of the proximal humerus. J Bone Joint Surg Br. 2011;93(7):937-941.
47. Duralde XA, Leddy LR. The results of ORIF of displaced unstable proximal humeral fractures using a locking plate. J Shoulder Elbow Surg. 2010;19(4):480-488.
48. Robinson CM, Wylie JR, Ray AG, et al. Proximal humeral fractures with a severe varus deformity treated by fixation with a locking plate. J Bone Joint Surg Br. 2010;92(5):672-678.
49. Ong C, Bechtel C, Walsh M, Zuckerman JD, Egol KA. Three- and four-part fractures have poorer function than one-part proximal humerus fractures. Clin Orthop. 2011;469(12):3292-3299.
50. Kim SH, Lee YH, Chung SW, et al. Outcomes for four-part proximal humerus fractures treated with a locking compression plate and an autologous iliac bone impaction graft. Injury. 2012;43(10):1724-1731.
51. Larsson S. Calcium phosphates: what is the evidence? J Orthop Trauma. 2010;24(suppl 1):S41-S45.
52. Egol KA, Sugi MT, Ong CC, Montero N, Davidovitch R, Zuckerman JD. Fracture site augmentation with calcium phosphate cement reduces screw penetration after open reduction–internal fixation of proximal humeral fractures. J Shoulder Elbow Surg. 2012;21(6):741-748.
53. Gradl G, Knobe M, Stoffel M, Prescher A, Dirrichs T, Pape HC. Biomechanical evaluation of locking plate fixation of proximal humeral fractures augmented with calcium phosphate cement. J Orthop Trauma. 2013;27(7):399-404.
54. Collinge C, Merk B, Lautenschlager EP. Mechanical evaluation of fracture fixation augmented with tricalcium phosphate bone cement in a porous osteoporotic cancellous bone model. J Orthop Trauma. 2007;21(2):124-128.
55. Kwon BK, Goertzen DJ, O’Brien PJ, Broekhuyse HM, Oxland TR. Biomechanical evaluation of proximal humeral fracture fixation supplemented with calcium phosphate cement. J Bone Joint Surg Am. 2002;84(6):951-961.
56. Somasundaram K, Huber CP, Babu V, Zadeh H. Proximal humeral fractures: the role of calcium sulphate augmentation and extended deltoid splitting approach in internal fixation using locking plates. Injury. 2013;44(4):481-487.
57. Lee CW, Shin SJ. Prognostic factors for unstable proximal humeral fractures treated with locking-plate fixation. J Shoulder Elbow Surg. 2009;18(1):83-88.
58. Zhang L, Zheng J, Wang W, et al. The clinical benefit of medial support screws in locking plating of proximal humerus fractures: a prospective randomized study. Int Orthop. 2011;35(11):1655-1661.
59. Brianza S, Roderer G, Schiuma D, et al. Where do locking screws purchase in the humeral head? Injury. 2012;43(6):850-855.
60. Hepp P, Lill H, Bail H, et al. Where should implants be anchored in the humeral head? Clin Orthop. 2003;(415):139-147.
61. Liew AS, Johnson JA, Patterson SD, King GJ, Chess DG. Effect of screw placement on fixation in the humeral head. J Shoulder Elbow Surg. 2000;9(5):423-426.
62. Tingart MJ, Lehtinen J, Zurakowski D, Warner JJ, Apreleva M. Proximal humeral fractures: regional differences in bone mineral density of the humeral head affect the fixation strength of cancellous screws. J Shoulder Elbow Surg. 2006;15(5):620-624.
63. Erhardt JB, Stoffel K, Kampshoff J, Badur N, Yates P, Kuster MS. The position and number of screws influence screw perforation of the humeral head in modern locking plates: a cadaver study. J Orthop Trauma. 2012;26(10):e188-e192.
64. Konigshausen M, Kubler L, Godry H, Citak M, Schildhauer TA, Seybold D. Clinical outcome and complications using a polyaxial locking plate in the treatment of displaced proximal humerus fractures. A reliable system? Injury. 2012;43(2):223-231.
65. Bengard MJ, Gardner MJ. Screw depth sounding in proximal humerus fractures to avoid iatrogenic intra-articular penetration. J Orthop Trauma. 2011;25(10):630-633.
66. Ring D. Current concepts in plate and screw fixation of osteoporotic proximal humerus fractures. Injury. 2007;38(3):S59-S68.
1. Bell JE, Leung BC, Spratt KF, et al. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am. 2011;93(2):121-131.
2. Aaron D, Shatsky J, Paredes JC, Jiang C, Parsons BO, Flatow EL. Proximal humeral fractures: internal fixation. J Bone Joint Surg Am. 2012;94(24):2280-2288.
3. Court-Brown CM, Garg A, McQueen MM. The epidemiology of proximal humeral fractures. Acta Orthop Scand. 2001;72(4):365-371.
4. Kannus P, Palvanen M, Niemi S, Parkkari J, Jarvinen M, Vuori I. Increasing number and incidence of osteoporotic fractures of the proximal humerus in elderly people. BMJ. 1996;313(7064):1051-1052.
5. Handoll HH, Ollivere BJ, Rollins KE. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2012;12:CD000434.
6. Tamai K, Ishige N, Kuroda S, et al. Four-segment classification of proximal humeral fractures revisited: a multicenter study on 509 cases. J Shoulder Elbow Surg. 2009;18(6):845-850.
7. Rothberg D, Higgins T. Fractures of the proximal humerus. Orthop Clin North Am. 2013;44(1):9-19.
8. Egol KA, Kubiak EN, Fulkerson E, Kummer FJ, Koval KJ. Biomechanics of locked plates and screws. J Orthop Trauma. 2004;18(8):488-493.
9. Miranda MA. Locking plate technology and its role in osteoporotic fractures. Injury. 2007;38(suppl 3):35-39.
10. Foruria AM, Carrascal MT, Revilla C, Munuera L, Sanchez-Sotelo J. Proximal humerus fracture rotational stability after fixation using a locking plate or a fixed-angle locked nail: the role of implant stiffness. Clin Biomech. 2010;25(4):307-311.
11. Weinstein DM, Bratton DR, Ciccone WJ 2nd, Elias JJ. Locking plates improve torsional resistance in the stabilization of three-part proximal humeral fractures. J Shoulder Elbow Surg. 2006;15(2):239-243.
12. Siffri PC, Peindl RD, Coley ER, Norton J, Connor PM, Kellam JF. Biomechanical analysis of blade plate versus locking plate fixation for a proximal humerus fracture: comparison using cadaveric and synthetic humeri. J Orthop Trauma. 2006;20(8):547-554.
13. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Internal fixation versus nonoperative treatment of displaced 3-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(5):747-755.
14. Fjalestad T, Hole MO, Hovden IA, Blucher J, Stromsoe K. Surgical treatment with an angular stable plate for complex displaced proximal humeral fractures in elderly patients: a randomized controlled trial. J Orthop Trauma. 2012;26(2):98-106.
15. Wild JR, DeMers A, French R, et al. Functional outcomes for surgically treated 3- and 4-part proximal humerus fractures. Orthopedics. 2011;34(10):e629-e633.
16. Solberg BD, Moon CN, Franco DP, Paiement GD. Surgical treatment of three and four-part proximal humeral fractures. J Bone Joint Surg Am. 2009;91(7):1689-1697.
17. Namdari S, Voleti PB, Mehta S. Evaluation of the osteoporotic proximal humeral fracture and strategies for structural augmentation during surgical treatment. J Shoulder Elbow Surg. 2012;21(12):1787-1795.
18. Agudelo J, Schurmann M, Stahel P, et al. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma. 2007;21(10):676-681.
19. Schliemann B, Siemoneit J, Theisen C, Kosters C, Weimann A, Raschke MJ. Complex fractures of the proximal humerus in the elderly—outcome and complications after locking plate fixation. Musculoskelet Surg. 2012;96(suppl 1):S3-S11.
20. Thanasas C, Kontakis G, Angoules A, Limb D, Giannoudis P. Treatment of proximal humerus fractures with locking plates: a systematic review. J Shoulder Elbow Surg. 2009;18(6):837-844.
21. Micic ID, Kim KC, Shin DJ, et al. Analysis of early failure of the locking compression plate in osteoporotic proximal humerus fractures. J Orthop Sci. 2009;14(5):596-601.
22. Solberg BD, Moon CN, Franco DP, Paiement GD. Locked plating of 3- and 4-part proximal humerus fractures in older patients: the effect of initial fracture pattern on outcome. J Orthop Trauma. 2009;23(2):113-119.
23. Owsley KC, Gorczyca JT. Fracture displacement and screw cutout after open reduction and locked plate fixation of proximal humeral fractures [corrected]. J Bone Joint Surg Am. 2008;90(2):233-240.
24. Fankhauser F, Boldin C, Schippinger G, Haunschmid C, Szyszkowitz R. A new locking plate for unstable fractures of the proximal humerus. Clin Orthop. 2005;(430):176-181.
25. Sproul RC, Iyengar JJ, Devcic Z, Feeley BT. A systematic review of locking plate fixation of proximal humerus fractures. Injury. 2011;42(4):408-413.
26. Gardner MJ, Weil Y, Barker JU, Kelly BT, Helfet DL, Lorich DG. The importance of medial support in locked plating of proximal humerus fractures. J Orthop Trauma. 2007;21(3):185-191.
27. Voigt C, Hurschler C, Rech L, Vossenrich R, Lill H. Additive fiber-cerclages in proximal humeral fractures stabilized by locking plates. No effect on fracture stabilization and rotator cuff function in human shoulder specimens. Acta Orthop. 2009;80(4):465-471.
28. Lo IK, Burkhart SS. Biomechanical principles of arthroscopic repair of the rotator cuff. Oper Tech Orthop. 2002;12(3):140-155.
29. Ricchetti ET, Warrender WJ, Abboud JA. Use of locking plates in the treatment of proximal humerus fractures. J Shoulder Elbow Surg. 2010;19(2 suppl):66-75.
30. Badman B, Frankle M, Keating C, Henderson L, Brooks J, Mighell M. Results of proximal humeral locked plating with supplemental suture fixation of rotator cuff. J Shoulder Elbow Surg. 2011;20(4):616-624.
31. Nho SJ, Brophy RH, Barker JU, Cornell CN, MacGillivray JD. Management of proximal humeral fractures based on current literature. J Bone Joint Surg Am. 2007;89(suppl 3):44-58.
32. Barlow JD, Sanchez-Sotelo J, Torchia M. Proximal humerus fractures in the elderly can be reliably fixed with a “hybrid” locked-plating technique. Clin Orthop. 2011;469(12):3281-3291.
33. Cho CH, Jung GH, Song KS. Tension suture fixation using 2 washers for proximal humeral fractures. Orthopedics. 2012;35(3):202-205.
34. Brunner F, Sommer C, Bahrs C, et al. Open reduction and internal fixation of proximal humerus fractures using a proximal humeral locked plate: a prospective multicenter analysis. J Orthop Trauma. 2009;23(3):163-172.
35. Jung WB, Moon ES, Kim SK, Kovacevic D, Kim MS. Does medial support decrease major complications of unstable proximal humerus fractures treated with locking plate? BMC Musculoskelet Disord. 2013;14:102.
36. Krappinger D, Bizzotto N, Riedmann S, Kammerlander C, Hengg C, Kralinger FS. Predicting failure after surgical fixation of proximal humerus fractures. Injury. 2011;42(11):1283-1288.
37. Lescheid J, Zdero R, Shah S, Kuzyk PR, Schemitsch EH. The biomechanics of locked plating for repairing proximal humerus fractures with or without medial cortical support. J Trauma. 2010;69(5):1235-1242.
38. De Long WG Jr, Einhorn TA, Koval K, et al. Bone grafts and bone graft substitutes in orthopaedic trauma surgery. A critical analysis. J Bone Joint Surg Am. 2007;89(3):649-658.
39. Gardner MJ, Boraiah S, Helfet DL, Lorich DG. Indirect medial reduction and strut support of proximal humerus fractures using an endosteal implant. J Orthop Trauma. 2008;22(3):195-200.
40. Hettrich CM, Neviaser A, Beamer BS, Paul O, Helfet DL, Lorich DG. Locked plating of the proximal humerus using an endosteal implant. J Orthop Trauma. 2012;26(4):212-215.
41. Matassi F, Angeloni R, Carulli C, et al. Locking plate and fibular allograft augmentation in unstable fractures of proximal humerus. Injury. 2012;43(11):1939-1942.
42. Neviaser AS, Hettrich CM, Beamer BS, Dines JS, Lorich DG. Endosteal strut augment reduces complications associated with proximal humeral locking plates. Clin Orthop. 2011;469(12):3300-3306.
43. Mathison C, Chaudhary R, Beaupre L, Reynolds M, Adeeb S, Bouliane M. Biomechanical analysis of proximal humeral fixation using locking plate fixation with an intramedullary fibular allograft. Clin Biomech. 2010;25(7):642-646.
44. Osterhoff G, Baumgartner D, Favre P, et al. Medial support by fibula bone graft in angular stable plate fixation of proximal humeral fractures: an in vitro study with synthetic bone. J Shoulder Elbow Surg. 2011;20(5):740-746.
45. Chow RM, Begum F, Beaupre LA, Carey JP, Adeeb S, Bouliane MJ. Proximal humeral fracture fixation: locking plate construct +/- intramedullary fibular allograft. J Shoulder Elbow Surg. 2012;21(7):894-901.
46. Bae JH, Oh JK, Chon CS, Oh CW, Hwang JH, Yoon YC. The biomechanical performance of locking plate fixation with intramedullary fibular strut graft augmentation in the treatment of unstable fractures of the proximal humerus. J Bone Joint Surg Br. 2011;93(7):937-941.
47. Duralde XA, Leddy LR. The results of ORIF of displaced unstable proximal humeral fractures using a locking plate. J Shoulder Elbow Surg. 2010;19(4):480-488.
48. Robinson CM, Wylie JR, Ray AG, et al. Proximal humeral fractures with a severe varus deformity treated by fixation with a locking plate. J Bone Joint Surg Br. 2010;92(5):672-678.
49. Ong C, Bechtel C, Walsh M, Zuckerman JD, Egol KA. Three- and four-part fractures have poorer function than one-part proximal humerus fractures. Clin Orthop. 2011;469(12):3292-3299.
50. Kim SH, Lee YH, Chung SW, et al. Outcomes for four-part proximal humerus fractures treated with a locking compression plate and an autologous iliac bone impaction graft. Injury. 2012;43(10):1724-1731.
51. Larsson S. Calcium phosphates: what is the evidence? J Orthop Trauma. 2010;24(suppl 1):S41-S45.
52. Egol KA, Sugi MT, Ong CC, Montero N, Davidovitch R, Zuckerman JD. Fracture site augmentation with calcium phosphate cement reduces screw penetration after open reduction–internal fixation of proximal humeral fractures. J Shoulder Elbow Surg. 2012;21(6):741-748.
53. Gradl G, Knobe M, Stoffel M, Prescher A, Dirrichs T, Pape HC. Biomechanical evaluation of locking plate fixation of proximal humeral fractures augmented with calcium phosphate cement. J Orthop Trauma. 2013;27(7):399-404.
54. Collinge C, Merk B, Lautenschlager EP. Mechanical evaluation of fracture fixation augmented with tricalcium phosphate bone cement in a porous osteoporotic cancellous bone model. J Orthop Trauma. 2007;21(2):124-128.
55. Kwon BK, Goertzen DJ, O’Brien PJ, Broekhuyse HM, Oxland TR. Biomechanical evaluation of proximal humeral fracture fixation supplemented with calcium phosphate cement. J Bone Joint Surg Am. 2002;84(6):951-961.
56. Somasundaram K, Huber CP, Babu V, Zadeh H. Proximal humeral fractures: the role of calcium sulphate augmentation and extended deltoid splitting approach in internal fixation using locking plates. Injury. 2013;44(4):481-487.
57. Lee CW, Shin SJ. Prognostic factors for unstable proximal humeral fractures treated with locking-plate fixation. J Shoulder Elbow Surg. 2009;18(1):83-88.
58. Zhang L, Zheng J, Wang W, et al. The clinical benefit of medial support screws in locking plating of proximal humerus fractures: a prospective randomized study. Int Orthop. 2011;35(11):1655-1661.
59. Brianza S, Roderer G, Schiuma D, et al. Where do locking screws purchase in the humeral head? Injury. 2012;43(6):850-855.
60. Hepp P, Lill H, Bail H, et al. Where should implants be anchored in the humeral head? Clin Orthop. 2003;(415):139-147.
61. Liew AS, Johnson JA, Patterson SD, King GJ, Chess DG. Effect of screw placement on fixation in the humeral head. J Shoulder Elbow Surg. 2000;9(5):423-426.
62. Tingart MJ, Lehtinen J, Zurakowski D, Warner JJ, Apreleva M. Proximal humeral fractures: regional differences in bone mineral density of the humeral head affect the fixation strength of cancellous screws. J Shoulder Elbow Surg. 2006;15(5):620-624.
63. Erhardt JB, Stoffel K, Kampshoff J, Badur N, Yates P, Kuster MS. The position and number of screws influence screw perforation of the humeral head in modern locking plates: a cadaver study. J Orthop Trauma. 2012;26(10):e188-e192.
64. Konigshausen M, Kubler L, Godry H, Citak M, Schildhauer TA, Seybold D. Clinical outcome and complications using a polyaxial locking plate in the treatment of displaced proximal humerus fractures. A reliable system? Injury. 2012;43(2):223-231.
65. Bengard MJ, Gardner MJ. Screw depth sounding in proximal humerus fractures to avoid iatrogenic intra-articular penetration. J Orthop Trauma. 2011;25(10):630-633.
66. Ring D. Current concepts in plate and screw fixation of osteoporotic proximal humerus fractures. Injury. 2007;38(3):S59-S68.