User login
From neuroplasticity to psychoplasticity: Psilocybin may reverse personality disorders and political fanaticism
One of psychiatry’s long-standing dogmas is that personality disorders are enduring, unchangeable, and not amenable to treatment with potent psychotropics or intensive psychotherapy. I propose that this dogma may soon be shattered.
Several other dogmas in psychiatry have been demolished over the past several decades:
- that “insanity” is completely irreversible and requires lifetime institutionalization. The serendipitous discovery of chlorpromazine1 annihilated this centuries-old dogma
- that chronic, severe, refractory depression (with ongoing suicidal urges) that fails to improve with pharmacotherapy or electroconvulsive therapy (ECT) is hopeless and untreatable, until ketamine not only pulverized this dogma, but did it with lightning speed, dazzling us all2
- that dissociative agents such as ketamine are dangerous and condemnable drugs of abuse, until the therapeutic effect of ketamine slayed that dragon3
- that ECT “fries” the brain (as malevolently propagated by antipsychiatry cults), which was completely disproven by neuroimaging studies that show the hippocampus (which shrinks during depression) actually grows by >10% after a few ECT sessions4
- that psychotherapy is not a “real” treatment because talking cannot reverse a psychiatric brain disorder, until studies showed significant neuroplasticity with psychotherapy and decrease in inflammatory biomarkers with cognitive-behavioral therapy (CBT)5
- that persons with refractory hallucinations and delusions are doomed to a life of disability, until clozapine torpedoed that pessimistic dogma6
- that hallucinogens/psychedelics are dangerous and should be banned, until a jarring paradigm shift occurred with the discovery of psilocybin’s transformative effects, and the remarkable therapeutic effects of its mystical trips.7
Psilocybin’s therapeutic effects
Psilocybin has already proved to have a strong and lasting effect on depression and promises to have therapeutic benefits for patients with substance use disorders, posttraumatic stress disorder (PTSD), and anxiety.8 In addition, when the multiple psychological and neurobiological effects of psilocybin (and of other psychedelics) are examined, I see a very promising path to amelioration of severe personality disorders such as psychopathy, antisocial behavior, and narcissism. The mechanism(s) of action of psilocybin on the human brain are drastically different from any man-made psychotropic agent. As a psychiatric neuroscientist, I envision the neurologic impact of psilocybin to be conducive to a complete transformation of a patient’s view of themself, other people, and the meaning of life. It is reminiscent of religious conversion.
The psychological effects of psilocybin in humans have been described as follows:
- emotional breakthrough9
- increased psychological flexibility,10,11 a very cortical effect
- mystical experience,12 which results in sudden and significant changes in behavior and perception and includes the following dimensions: sacredness, noetic quality, deeply felt positive mood, ineffability, paradoxicality, and transcendence of time and space13
- oceanic boundlessness, feeling “one with the universe”14
- universal interconnectedness, insightfulness, blissful state, spiritual experience14
- ego dissolution,15 with loss of one’s personal identity
- increased neuroplasticity16
- changes in cognition and increase in insight.17
The neurobiological effects of psilocybin are mediated by serotonin 5HT2A agonism and include the following18:
- reduction in the activity of the medial prefrontal cortex, which regulates memory, attention, inhibitory control, and habit
- a decrease in the connectivity between the medial prefrontal cortex and the posterior cingulate cortex, which regulates memory and emotions
- reducing the default mode network, which is active during rest, stimulating internal thoughts and reminiscing about previous feelings and events, sometimes including ruminations. Psilocybin reverses those processes to thinking about others, not just the self, and becoming more open-minded about the world and other people. This can be therapeutic for depression, which is often associated with negative ruminations but also with entrenched habits (addictive behaviors), anxiety, PTSD, and obsessive-compulsive disorders
- increased global functional connectivity among various brain networks, leading to stronger functional integration of behavior
- collapse of major cortical oscillatory rhythms such as alpha and others that perpetuate “prior” beliefs
- extensive neuroplasticity and recalibration of thought processes and decomposition of pathological beliefs, referred to as REBUS (relaxed beliefs under psychedelics).
The bottom line is psilocybin and other psychedelics can dramatically alter, reshape, and relax rigid beliefs and personality traits by decreasing “neuroticism” and increasing “extraversion,” insightfulness, openness, and possibly conscientiousness.19 Although no studies of psychedelics in psychopathic, antisocial, or narcissistic personality disorders have been conducted, it is very reasonable to speculate that psilocybin may reverse traits of these disorders such as callousness, lack of empathy, and pathological self-centeredness.
Going further, a preliminary report suggests psilocybin can modify political views by decreasing authoritarianism and increasing libertarianism.20,21 In the current political zeitgeist, could psychedelics such as psilocybin reduce or even eliminate political extremism and visceral hatred on all sides? It would be remarkable research to carry out to heal a politically divided populace.The dogma of untreatable personality disorders or hopelessly entrenched political extremism is on the chopping block, and psychedelics offer hope to splinter those beliefs by concurrently remodeling brain tissue (neuroplasticity) and rectifying the mindset (psychoplasticity).
1. Delay J, Deniker P. Neuroleptic effects of chlorpromazine in therapeutics of neuropsychiatry. J Clin Exp Psychopathol. 1955;16(2):104-112.
2. Walsh Z, Mollaahmetoglu OM, Rootman, J, et al. Ketamine for the treatment of mental health and substance use disorders: comprehensive systematic review. BJPsych Open. 2021;8(1):e19. doi:10.1192/bjo.2021.1061
3. Lener MS, Kadriu B, Zarate CA Jr. Ketamine and beyond: investigations into the potential of glutamatergic agents to treat depression. Drugs. 2017;77(4):381-401.
4. Ayers B, Leaver A, Woods RP, et al. Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry. 2016;79(4):282-292.
5. Cao B, Li R, Ding L, Xu J, et al. Does cognitive behaviour therapy affect peripheral inflammation of depression? A protocol for the systematic review and meta-analysis. BMJ Open. 2021;11(12):e048162. doi:10.1136/bmjopen-2020-048162
6. Wagner E, Siafis S, Fernando P, et al. Efficacy and safety of clozapine in psychotic disorders—a systematic quantitative meta-review. Transl Psychiatry. 2021;11(1):487.
7. Daws RE, Timmermann C, Giribaldi B, et al. Increased global integration in the brain after psilocybin therapy for depression. Nat Med. 2022;28(4):844-851.
8. Pearson C, Siegel J, Gold JA. Psilocybin-assisted psychotherapy for depression: emerging research on a psychedelic compound with a rich history. J Neurol Sci. 2022;434:120096. doi:10.1016/j.jns.2021.120096
9. Roseman L, Haijen E, Idialu-Ikato K, et al. Emotional breakthrough and psychedelics: validation of the Emotional Breakthrough Inventory. J Psychopharmacol. 2019;33(9):1076-1087.
10. Davis AK, Barrett FS, Griffiths RR. Psychological flexibility mediates the relations between acute psychedelic effects and subjective decreases in depression and anxiety. J Contextual Behav Sci. 2020;15:39-45.
11. Hayes SC, Luoma JB, Bond FW, et al. Acceptance and commitment therapy: model, processes and outcomes. Behav Res Ther. 2006;44(1):1-25.
12. Ross S, Bossis A, Guss J, et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol. 2016;30(12):1165-1180.
13. Stace WT. Mysticism and Philosophy. Macmillan Pub Ltd; 1960:37.
14. Barrett FS, Griffiths RR. Classic hallucinogens and mystical experiences: phenomenology and neural correlates. Curr Top Behav Neurosci. 2018;36:393-430.
15. Nour MM, Evans L, Nutt D, et al. Ego-dissolution and psychedelics: validation of the Ego-Dissolution Inventory (EDI). Front Hum Neurosci. 2016;10:269. doi:10.3389/fnhum.2016.00269
16. Olson DE. The subjective effects of psychedelics may not be necessary for their enduring therapeutic effects. ACS Pharmacol Transl Sci. 2020;4(2):563-567.
17. Carhart-Harris RL, Bolstridge M, Day CMJ, et al. Psilocybin with psychological support for treatment-resistant depression: six-month follow-up. Psychopharmacology (Berl). 2018;235(2):399-408.
18. Carhart-Harris RL. How do psychedelics work? Curr Opin Psychiatry. 2019;32(1):16-21.
19. Erritzoe D, Roseman L, Nour MM, et al. Effects of psilocybin therapy on personality structure. Acta Psychiatr Scand. 2018;138(5):368-378.
20. Lyons T, Carhart-Harris RL. Increased nature relatedness and decreased authoritarian political views after psilocybin for treatment-resistant depression. J Psychopharmacol. 2018;32(7):811-819.
21. Nour MM, Evans L, Carhart-Harris RL. Psychedelics, personality and political perspectives. J Psychoactive Drugs. 2017;49(3):182-191.
One of psychiatry’s long-standing dogmas is that personality disorders are enduring, unchangeable, and not amenable to treatment with potent psychotropics or intensive psychotherapy. I propose that this dogma may soon be shattered.
Several other dogmas in psychiatry have been demolished over the past several decades:
- that “insanity” is completely irreversible and requires lifetime institutionalization. The serendipitous discovery of chlorpromazine1 annihilated this centuries-old dogma
- that chronic, severe, refractory depression (with ongoing suicidal urges) that fails to improve with pharmacotherapy or electroconvulsive therapy (ECT) is hopeless and untreatable, until ketamine not only pulverized this dogma, but did it with lightning speed, dazzling us all2
- that dissociative agents such as ketamine are dangerous and condemnable drugs of abuse, until the therapeutic effect of ketamine slayed that dragon3
- that ECT “fries” the brain (as malevolently propagated by antipsychiatry cults), which was completely disproven by neuroimaging studies that show the hippocampus (which shrinks during depression) actually grows by >10% after a few ECT sessions4
- that psychotherapy is not a “real” treatment because talking cannot reverse a psychiatric brain disorder, until studies showed significant neuroplasticity with psychotherapy and decrease in inflammatory biomarkers with cognitive-behavioral therapy (CBT)5
- that persons with refractory hallucinations and delusions are doomed to a life of disability, until clozapine torpedoed that pessimistic dogma6
- that hallucinogens/psychedelics are dangerous and should be banned, until a jarring paradigm shift occurred with the discovery of psilocybin’s transformative effects, and the remarkable therapeutic effects of its mystical trips.7
Psilocybin’s therapeutic effects
Psilocybin has already proved to have a strong and lasting effect on depression and promises to have therapeutic benefits for patients with substance use disorders, posttraumatic stress disorder (PTSD), and anxiety.8 In addition, when the multiple psychological and neurobiological effects of psilocybin (and of other psychedelics) are examined, I see a very promising path to amelioration of severe personality disorders such as psychopathy, antisocial behavior, and narcissism. The mechanism(s) of action of psilocybin on the human brain are drastically different from any man-made psychotropic agent. As a psychiatric neuroscientist, I envision the neurologic impact of psilocybin to be conducive to a complete transformation of a patient’s view of themself, other people, and the meaning of life. It is reminiscent of religious conversion.
The psychological effects of psilocybin in humans have been described as follows:
- emotional breakthrough9
- increased psychological flexibility,10,11 a very cortical effect
- mystical experience,12 which results in sudden and significant changes in behavior and perception and includes the following dimensions: sacredness, noetic quality, deeply felt positive mood, ineffability, paradoxicality, and transcendence of time and space13
- oceanic boundlessness, feeling “one with the universe”14
- universal interconnectedness, insightfulness, blissful state, spiritual experience14
- ego dissolution,15 with loss of one’s personal identity
- increased neuroplasticity16
- changes in cognition and increase in insight.17
The neurobiological effects of psilocybin are mediated by serotonin 5HT2A agonism and include the following18:
- reduction in the activity of the medial prefrontal cortex, which regulates memory, attention, inhibitory control, and habit
- a decrease in the connectivity between the medial prefrontal cortex and the posterior cingulate cortex, which regulates memory and emotions
- reducing the default mode network, which is active during rest, stimulating internal thoughts and reminiscing about previous feelings and events, sometimes including ruminations. Psilocybin reverses those processes to thinking about others, not just the self, and becoming more open-minded about the world and other people. This can be therapeutic for depression, which is often associated with negative ruminations but also with entrenched habits (addictive behaviors), anxiety, PTSD, and obsessive-compulsive disorders
- increased global functional connectivity among various brain networks, leading to stronger functional integration of behavior
- collapse of major cortical oscillatory rhythms such as alpha and others that perpetuate “prior” beliefs
- extensive neuroplasticity and recalibration of thought processes and decomposition of pathological beliefs, referred to as REBUS (relaxed beliefs under psychedelics).
The bottom line is psilocybin and other psychedelics can dramatically alter, reshape, and relax rigid beliefs and personality traits by decreasing “neuroticism” and increasing “extraversion,” insightfulness, openness, and possibly conscientiousness.19 Although no studies of psychedelics in psychopathic, antisocial, or narcissistic personality disorders have been conducted, it is very reasonable to speculate that psilocybin may reverse traits of these disorders such as callousness, lack of empathy, and pathological self-centeredness.
Going further, a preliminary report suggests psilocybin can modify political views by decreasing authoritarianism and increasing libertarianism.20,21 In the current political zeitgeist, could psychedelics such as psilocybin reduce or even eliminate political extremism and visceral hatred on all sides? It would be remarkable research to carry out to heal a politically divided populace.The dogma of untreatable personality disorders or hopelessly entrenched political extremism is on the chopping block, and psychedelics offer hope to splinter those beliefs by concurrently remodeling brain tissue (neuroplasticity) and rectifying the mindset (psychoplasticity).
One of psychiatry’s long-standing dogmas is that personality disorders are enduring, unchangeable, and not amenable to treatment with potent psychotropics or intensive psychotherapy. I propose that this dogma may soon be shattered.
Several other dogmas in psychiatry have been demolished over the past several decades:
- that “insanity” is completely irreversible and requires lifetime institutionalization. The serendipitous discovery of chlorpromazine1 annihilated this centuries-old dogma
- that chronic, severe, refractory depression (with ongoing suicidal urges) that fails to improve with pharmacotherapy or electroconvulsive therapy (ECT) is hopeless and untreatable, until ketamine not only pulverized this dogma, but did it with lightning speed, dazzling us all2
- that dissociative agents such as ketamine are dangerous and condemnable drugs of abuse, until the therapeutic effect of ketamine slayed that dragon3
- that ECT “fries” the brain (as malevolently propagated by antipsychiatry cults), which was completely disproven by neuroimaging studies that show the hippocampus (which shrinks during depression) actually grows by >10% after a few ECT sessions4
- that psychotherapy is not a “real” treatment because talking cannot reverse a psychiatric brain disorder, until studies showed significant neuroplasticity with psychotherapy and decrease in inflammatory biomarkers with cognitive-behavioral therapy (CBT)5
- that persons with refractory hallucinations and delusions are doomed to a life of disability, until clozapine torpedoed that pessimistic dogma6
- that hallucinogens/psychedelics are dangerous and should be banned, until a jarring paradigm shift occurred with the discovery of psilocybin’s transformative effects, and the remarkable therapeutic effects of its mystical trips.7
Psilocybin’s therapeutic effects
Psilocybin has already proved to have a strong and lasting effect on depression and promises to have therapeutic benefits for patients with substance use disorders, posttraumatic stress disorder (PTSD), and anxiety.8 In addition, when the multiple psychological and neurobiological effects of psilocybin (and of other psychedelics) are examined, I see a very promising path to amelioration of severe personality disorders such as psychopathy, antisocial behavior, and narcissism. The mechanism(s) of action of psilocybin on the human brain are drastically different from any man-made psychotropic agent. As a psychiatric neuroscientist, I envision the neurologic impact of psilocybin to be conducive to a complete transformation of a patient’s view of themself, other people, and the meaning of life. It is reminiscent of religious conversion.
The psychological effects of psilocybin in humans have been described as follows:
- emotional breakthrough9
- increased psychological flexibility,10,11 a very cortical effect
- mystical experience,12 which results in sudden and significant changes in behavior and perception and includes the following dimensions: sacredness, noetic quality, deeply felt positive mood, ineffability, paradoxicality, and transcendence of time and space13
- oceanic boundlessness, feeling “one with the universe”14
- universal interconnectedness, insightfulness, blissful state, spiritual experience14
- ego dissolution,15 with loss of one’s personal identity
- increased neuroplasticity16
- changes in cognition and increase in insight.17
The neurobiological effects of psilocybin are mediated by serotonin 5HT2A agonism and include the following18:
- reduction in the activity of the medial prefrontal cortex, which regulates memory, attention, inhibitory control, and habit
- a decrease in the connectivity between the medial prefrontal cortex and the posterior cingulate cortex, which regulates memory and emotions
- reducing the default mode network, which is active during rest, stimulating internal thoughts and reminiscing about previous feelings and events, sometimes including ruminations. Psilocybin reverses those processes to thinking about others, not just the self, and becoming more open-minded about the world and other people. This can be therapeutic for depression, which is often associated with negative ruminations but also with entrenched habits (addictive behaviors), anxiety, PTSD, and obsessive-compulsive disorders
- increased global functional connectivity among various brain networks, leading to stronger functional integration of behavior
- collapse of major cortical oscillatory rhythms such as alpha and others that perpetuate “prior” beliefs
- extensive neuroplasticity and recalibration of thought processes and decomposition of pathological beliefs, referred to as REBUS (relaxed beliefs under psychedelics).
The bottom line is psilocybin and other psychedelics can dramatically alter, reshape, and relax rigid beliefs and personality traits by decreasing “neuroticism” and increasing “extraversion,” insightfulness, openness, and possibly conscientiousness.19 Although no studies of psychedelics in psychopathic, antisocial, or narcissistic personality disorders have been conducted, it is very reasonable to speculate that psilocybin may reverse traits of these disorders such as callousness, lack of empathy, and pathological self-centeredness.
Going further, a preliminary report suggests psilocybin can modify political views by decreasing authoritarianism and increasing libertarianism.20,21 In the current political zeitgeist, could psychedelics such as psilocybin reduce or even eliminate political extremism and visceral hatred on all sides? It would be remarkable research to carry out to heal a politically divided populace.The dogma of untreatable personality disorders or hopelessly entrenched political extremism is on the chopping block, and psychedelics offer hope to splinter those beliefs by concurrently remodeling brain tissue (neuroplasticity) and rectifying the mindset (psychoplasticity).
1. Delay J, Deniker P. Neuroleptic effects of chlorpromazine in therapeutics of neuropsychiatry. J Clin Exp Psychopathol. 1955;16(2):104-112.
2. Walsh Z, Mollaahmetoglu OM, Rootman, J, et al. Ketamine for the treatment of mental health and substance use disorders: comprehensive systematic review. BJPsych Open. 2021;8(1):e19. doi:10.1192/bjo.2021.1061
3. Lener MS, Kadriu B, Zarate CA Jr. Ketamine and beyond: investigations into the potential of glutamatergic agents to treat depression. Drugs. 2017;77(4):381-401.
4. Ayers B, Leaver A, Woods RP, et al. Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry. 2016;79(4):282-292.
5. Cao B, Li R, Ding L, Xu J, et al. Does cognitive behaviour therapy affect peripheral inflammation of depression? A protocol for the systematic review and meta-analysis. BMJ Open. 2021;11(12):e048162. doi:10.1136/bmjopen-2020-048162
6. Wagner E, Siafis S, Fernando P, et al. Efficacy and safety of clozapine in psychotic disorders—a systematic quantitative meta-review. Transl Psychiatry. 2021;11(1):487.
7. Daws RE, Timmermann C, Giribaldi B, et al. Increased global integration in the brain after psilocybin therapy for depression. Nat Med. 2022;28(4):844-851.
8. Pearson C, Siegel J, Gold JA. Psilocybin-assisted psychotherapy for depression: emerging research on a psychedelic compound with a rich history. J Neurol Sci. 2022;434:120096. doi:10.1016/j.jns.2021.120096
9. Roseman L, Haijen E, Idialu-Ikato K, et al. Emotional breakthrough and psychedelics: validation of the Emotional Breakthrough Inventory. J Psychopharmacol. 2019;33(9):1076-1087.
10. Davis AK, Barrett FS, Griffiths RR. Psychological flexibility mediates the relations between acute psychedelic effects and subjective decreases in depression and anxiety. J Contextual Behav Sci. 2020;15:39-45.
11. Hayes SC, Luoma JB, Bond FW, et al. Acceptance and commitment therapy: model, processes and outcomes. Behav Res Ther. 2006;44(1):1-25.
12. Ross S, Bossis A, Guss J, et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol. 2016;30(12):1165-1180.
13. Stace WT. Mysticism and Philosophy. Macmillan Pub Ltd; 1960:37.
14. Barrett FS, Griffiths RR. Classic hallucinogens and mystical experiences: phenomenology and neural correlates. Curr Top Behav Neurosci. 2018;36:393-430.
15. Nour MM, Evans L, Nutt D, et al. Ego-dissolution and psychedelics: validation of the Ego-Dissolution Inventory (EDI). Front Hum Neurosci. 2016;10:269. doi:10.3389/fnhum.2016.00269
16. Olson DE. The subjective effects of psychedelics may not be necessary for their enduring therapeutic effects. ACS Pharmacol Transl Sci. 2020;4(2):563-567.
17. Carhart-Harris RL, Bolstridge M, Day CMJ, et al. Psilocybin with psychological support for treatment-resistant depression: six-month follow-up. Psychopharmacology (Berl). 2018;235(2):399-408.
18. Carhart-Harris RL. How do psychedelics work? Curr Opin Psychiatry. 2019;32(1):16-21.
19. Erritzoe D, Roseman L, Nour MM, et al. Effects of psilocybin therapy on personality structure. Acta Psychiatr Scand. 2018;138(5):368-378.
20. Lyons T, Carhart-Harris RL. Increased nature relatedness and decreased authoritarian political views after psilocybin for treatment-resistant depression. J Psychopharmacol. 2018;32(7):811-819.
21. Nour MM, Evans L, Carhart-Harris RL. Psychedelics, personality and political perspectives. J Psychoactive Drugs. 2017;49(3):182-191.
1. Delay J, Deniker P. Neuroleptic effects of chlorpromazine in therapeutics of neuropsychiatry. J Clin Exp Psychopathol. 1955;16(2):104-112.
2. Walsh Z, Mollaahmetoglu OM, Rootman, J, et al. Ketamine for the treatment of mental health and substance use disorders: comprehensive systematic review. BJPsych Open. 2021;8(1):e19. doi:10.1192/bjo.2021.1061
3. Lener MS, Kadriu B, Zarate CA Jr. Ketamine and beyond: investigations into the potential of glutamatergic agents to treat depression. Drugs. 2017;77(4):381-401.
4. Ayers B, Leaver A, Woods RP, et al. Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry. 2016;79(4):282-292.
5. Cao B, Li R, Ding L, Xu J, et al. Does cognitive behaviour therapy affect peripheral inflammation of depression? A protocol for the systematic review and meta-analysis. BMJ Open. 2021;11(12):e048162. doi:10.1136/bmjopen-2020-048162
6. Wagner E, Siafis S, Fernando P, et al. Efficacy and safety of clozapine in psychotic disorders—a systematic quantitative meta-review. Transl Psychiatry. 2021;11(1):487.
7. Daws RE, Timmermann C, Giribaldi B, et al. Increased global integration in the brain after psilocybin therapy for depression. Nat Med. 2022;28(4):844-851.
8. Pearson C, Siegel J, Gold JA. Psilocybin-assisted psychotherapy for depression: emerging research on a psychedelic compound with a rich history. J Neurol Sci. 2022;434:120096. doi:10.1016/j.jns.2021.120096
9. Roseman L, Haijen E, Idialu-Ikato K, et al. Emotional breakthrough and psychedelics: validation of the Emotional Breakthrough Inventory. J Psychopharmacol. 2019;33(9):1076-1087.
10. Davis AK, Barrett FS, Griffiths RR. Psychological flexibility mediates the relations between acute psychedelic effects and subjective decreases in depression and anxiety. J Contextual Behav Sci. 2020;15:39-45.
11. Hayes SC, Luoma JB, Bond FW, et al. Acceptance and commitment therapy: model, processes and outcomes. Behav Res Ther. 2006;44(1):1-25.
12. Ross S, Bossis A, Guss J, et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol. 2016;30(12):1165-1180.
13. Stace WT. Mysticism and Philosophy. Macmillan Pub Ltd; 1960:37.
14. Barrett FS, Griffiths RR. Classic hallucinogens and mystical experiences: phenomenology and neural correlates. Curr Top Behav Neurosci. 2018;36:393-430.
15. Nour MM, Evans L, Nutt D, et al. Ego-dissolution and psychedelics: validation of the Ego-Dissolution Inventory (EDI). Front Hum Neurosci. 2016;10:269. doi:10.3389/fnhum.2016.00269
16. Olson DE. The subjective effects of psychedelics may not be necessary for their enduring therapeutic effects. ACS Pharmacol Transl Sci. 2020;4(2):563-567.
17. Carhart-Harris RL, Bolstridge M, Day CMJ, et al. Psilocybin with psychological support for treatment-resistant depression: six-month follow-up. Psychopharmacology (Berl). 2018;235(2):399-408.
18. Carhart-Harris RL. How do psychedelics work? Curr Opin Psychiatry. 2019;32(1):16-21.
19. Erritzoe D, Roseman L, Nour MM, et al. Effects of psilocybin therapy on personality structure. Acta Psychiatr Scand. 2018;138(5):368-378.
20. Lyons T, Carhart-Harris RL. Increased nature relatedness and decreased authoritarian political views after psilocybin for treatment-resistant depression. J Psychopharmacol. 2018;32(7):811-819.
21. Nour MM, Evans L, Carhart-Harris RL. Psychedelics, personality and political perspectives. J Psychoactive Drugs. 2017;49(3):182-191.
Reversing depression: A plethora of therapeutic strategies and mechanisms
Despite much progress, major depressive disorder (MDD) continues to be a challenging and life-threatening neuropsychiatric disorder. It is highly prevalent and afflicts tens of millions of Americans.
It is also ranked as the No. 1 disabling medical (not just psychiatric) condition by the World Health Organization.1 A significant proportion of patients with MDD do not respond adequately to several rounds of antidepressant medications,2 and many are labeled as having “treatment-resistant depression” (TRD).
In a previous article, I provocatively proposed that TRD is a myth.3 What I meant is that in a heterogeneous syndrome such as depression, failure to respond to 1, 2, or even 3 antidepressants should not imply TRD, because there is a “right treatment” that has not yet been identified for a given depressed patient. Most of those labeled as TRD have simply not yet received the pharmacotherapy or somatic therapy with the requisite mechanism of action for their variant of depression within a heterogeneous syndrome. IV ketamine, which, astonishingly, often reverses severe TRD of chronic duration within a few hours, is a prime example of why the term TRD is often used prematurely. Ketamine’s mechanism of action (immediate neuroplasticity via glutamate N-methyl-
Some clinicians may not be aware of the abundance of mechanisms of action currently available for the treatment of MDD as well as bipolar depression. Many practitioners, in both psychiatry and primary care, usually start the treatment of depression with a selective serotonin reuptake inhibitor, and if that does not produce a response or remission, they might switch to a serotonin-norepinephrine reuptake inhibitor. If that does not control the patient’s depressive symptoms, they start entertaining the notion that the patient may have TRD, not realizing that they have barely scratched the surface of the many therapeutic options and mechanisms of action, one of which could be the “best match” for a given patient.4
There will come a day when “precision psychiatry” finally arrives, and specific biomarkers will be developed to identify the “right” treatment for each patient within the heterogenous syndrome of depression.5 Until that day arrives, the treatment of depression will continue to be a process of trial and error, and hit or miss. But research will eventually discover genetic, neurochemical, neurophysiological, neuroimaging, or neuroimmune biomarkers that will rapidly guide clinicians to the correct treatment. This is critical to avoid inordinate delays in achieving remission and avert the ever-present risk of suicidal behavior.
The Table6 provides an overview of the numerous treatments currently available to manage depression. All increase brain-derived neurotrophic factor and restore healthy neuroplasticity and neurogenesis, which are impaired in MDD and currently believed to be a final common pathway for all depression treatments.7
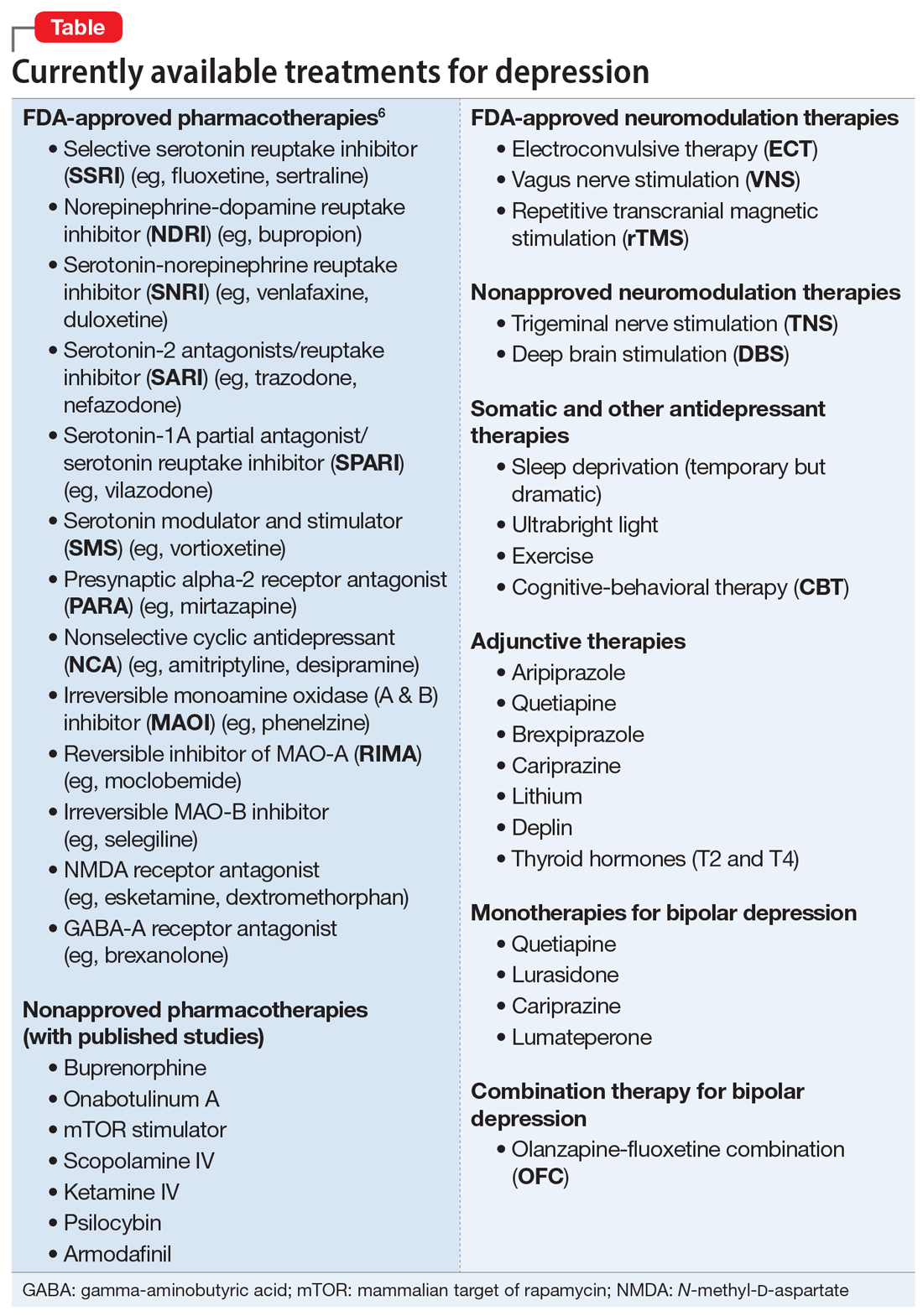
These 41 therapeutic approaches to treating MDD or bipolar depression reflect the heterogeneity of mechanisms of action to address an equally heterogeneous syndrome. This implies that clinicians have a wide array of on-label options to manage patients with depression, aiming for remission, not just a good response, which typically is defined as a ≥50% reduction in total score on one of the validated rating scales used to quantify depression severity, such as the Montgomery-Åsberg Depression Rating Scale, Hamilton Depression Rating Scale, or Calgary Depression Scale for Schizophrenia.
Continue to: When several FDA-approved pharmacotherapies...
When several FDA-approved pharmacotherapies fall short and produce a suboptimal response, clinicians can resort to other treatment options known to have a higher efficacy than oral antidepressants. These include electroconvulsive therapy, repetitive transcranial magnetic stimulation, and vagus nerve stimulation. Other on-label options include adjunctive therapy with one of the approved second-generation antipsychotic agents or with adjunctive esketamine.
But if the patient still does not improve, one of many emerging off-label treatment options may work. One of the exciting new discoveries is the hallucinogen psilocybin, whose mechanism of action is truly unique. Unlike standard antidepressant medications, which modulate neurotransmitters, psilocybin increases the brain’s network flexibility, decreases the modularity of several key brain networks (especially the default-brain network, or DMN), and alters the dark and distorted mental perspective of depression to a much healthier and optimistic outlook about the self and the world.8 Such novel breakthroughs in the treatment of severe depression will shed some unprecedented insights into the core neurobiology of depression, and may lead to early intervention and prevention.
As the saying goes, all roads lead to Rome. Psychiatric clinicians should rejoice that there are abundant approaches and therapeutic mechanisms to relieve their severely melancholic (and often suicidal) patients from the grips of this disabling and life-altering brain syndrome.
1. World Health Organization. Depression: let’s talk says WHO, as depression tops list of causes of ill health. March 30, 2017. Accessed July 5, 2022. www.who.int/news/item/30-03-2017--depression-let-s-talk-says-who-as-depression-tops-list-of-causes-of-ill-health
2. Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Eng J Med. 2006;354(12)1243-1252.
3. Nasrallah HA. Treatment resistance is a myth! Current Psychiatry. 2021;20(3):14-16,28.
4. Nasrallah HA. 10 Recent paradigm shifts in the neurobiology and treatment of depression. Current Psychiatry. 2015;14(2):10-13.
5. Nasrallah HA. Biomarkers in neuropsychiatric disorders: translating research to clinical applications. Biomarkers in Neuropsychiatry. 2019;1:100001. doi:10.1016/j.bionps.2019.100001
6. Procyshyn RM, Bezchlibnyk-Butler KZ, Jeffries JJ. Clinical Handbook of Psychotropic Drugs. 23rd ed. Hogrefe; 2019.
7. Tartt AN, Mariani, MB, Hen R, et al. Dysregulation of adult hippocampal neuroplasticity in major depression: pathogenesis and therapeutic implications. Mol Psychiatry. 2022;27(6):2689-2699.
8. Lowe H, Toyang N, Steele B, et al. The therapeutic potential of psilocybin. Molecules. 2021;26(10):2948. doi: 10.3390/molecules26102948
Despite much progress, major depressive disorder (MDD) continues to be a challenging and life-threatening neuropsychiatric disorder. It is highly prevalent and afflicts tens of millions of Americans.
It is also ranked as the No. 1 disabling medical (not just psychiatric) condition by the World Health Organization.1 A significant proportion of patients with MDD do not respond adequately to several rounds of antidepressant medications,2 and many are labeled as having “treatment-resistant depression” (TRD).
In a previous article, I provocatively proposed that TRD is a myth.3 What I meant is that in a heterogeneous syndrome such as depression, failure to respond to 1, 2, or even 3 antidepressants should not imply TRD, because there is a “right treatment” that has not yet been identified for a given depressed patient. Most of those labeled as TRD have simply not yet received the pharmacotherapy or somatic therapy with the requisite mechanism of action for their variant of depression within a heterogeneous syndrome. IV ketamine, which, astonishingly, often reverses severe TRD of chronic duration within a few hours, is a prime example of why the term TRD is often used prematurely. Ketamine’s mechanism of action (immediate neuroplasticity via glutamate N-methyl-
Some clinicians may not be aware of the abundance of mechanisms of action currently available for the treatment of MDD as well as bipolar depression. Many practitioners, in both psychiatry and primary care, usually start the treatment of depression with a selective serotonin reuptake inhibitor, and if that does not produce a response or remission, they might switch to a serotonin-norepinephrine reuptake inhibitor. If that does not control the patient’s depressive symptoms, they start entertaining the notion that the patient may have TRD, not realizing that they have barely scratched the surface of the many therapeutic options and mechanisms of action, one of which could be the “best match” for a given patient.4
There will come a day when “precision psychiatry” finally arrives, and specific biomarkers will be developed to identify the “right” treatment for each patient within the heterogenous syndrome of depression.5 Until that day arrives, the treatment of depression will continue to be a process of trial and error, and hit or miss. But research will eventually discover genetic, neurochemical, neurophysiological, neuroimaging, or neuroimmune biomarkers that will rapidly guide clinicians to the correct treatment. This is critical to avoid inordinate delays in achieving remission and avert the ever-present risk of suicidal behavior.
The Table6 provides an overview of the numerous treatments currently available to manage depression. All increase brain-derived neurotrophic factor and restore healthy neuroplasticity and neurogenesis, which are impaired in MDD and currently believed to be a final common pathway for all depression treatments.7

These 41 therapeutic approaches to treating MDD or bipolar depression reflect the heterogeneity of mechanisms of action to address an equally heterogeneous syndrome. This implies that clinicians have a wide array of on-label options to manage patients with depression, aiming for remission, not just a good response, which typically is defined as a ≥50% reduction in total score on one of the validated rating scales used to quantify depression severity, such as the Montgomery-Åsberg Depression Rating Scale, Hamilton Depression Rating Scale, or Calgary Depression Scale for Schizophrenia.
Continue to: When several FDA-approved pharmacotherapies...
When several FDA-approved pharmacotherapies fall short and produce a suboptimal response, clinicians can resort to other treatment options known to have a higher efficacy than oral antidepressants. These include electroconvulsive therapy, repetitive transcranial magnetic stimulation, and vagus nerve stimulation. Other on-label options include adjunctive therapy with one of the approved second-generation antipsychotic agents or with adjunctive esketamine.
But if the patient still does not improve, one of many emerging off-label treatment options may work. One of the exciting new discoveries is the hallucinogen psilocybin, whose mechanism of action is truly unique. Unlike standard antidepressant medications, which modulate neurotransmitters, psilocybin increases the brain’s network flexibility, decreases the modularity of several key brain networks (especially the default-brain network, or DMN), and alters the dark and distorted mental perspective of depression to a much healthier and optimistic outlook about the self and the world.8 Such novel breakthroughs in the treatment of severe depression will shed some unprecedented insights into the core neurobiology of depression, and may lead to early intervention and prevention.
As the saying goes, all roads lead to Rome. Psychiatric clinicians should rejoice that there are abundant approaches and therapeutic mechanisms to relieve their severely melancholic (and often suicidal) patients from the grips of this disabling and life-altering brain syndrome.
Despite much progress, major depressive disorder (MDD) continues to be a challenging and life-threatening neuropsychiatric disorder. It is highly prevalent and afflicts tens of millions of Americans.
It is also ranked as the No. 1 disabling medical (not just psychiatric) condition by the World Health Organization.1 A significant proportion of patients with MDD do not respond adequately to several rounds of antidepressant medications,2 and many are labeled as having “treatment-resistant depression” (TRD).
In a previous article, I provocatively proposed that TRD is a myth.3 What I meant is that in a heterogeneous syndrome such as depression, failure to respond to 1, 2, or even 3 antidepressants should not imply TRD, because there is a “right treatment” that has not yet been identified for a given depressed patient. Most of those labeled as TRD have simply not yet received the pharmacotherapy or somatic therapy with the requisite mechanism of action for their variant of depression within a heterogeneous syndrome. IV ketamine, which, astonishingly, often reverses severe TRD of chronic duration within a few hours, is a prime example of why the term TRD is often used prematurely. Ketamine’s mechanism of action (immediate neuroplasticity via glutamate N-methyl-
Some clinicians may not be aware of the abundance of mechanisms of action currently available for the treatment of MDD as well as bipolar depression. Many practitioners, in both psychiatry and primary care, usually start the treatment of depression with a selective serotonin reuptake inhibitor, and if that does not produce a response or remission, they might switch to a serotonin-norepinephrine reuptake inhibitor. If that does not control the patient’s depressive symptoms, they start entertaining the notion that the patient may have TRD, not realizing that they have barely scratched the surface of the many therapeutic options and mechanisms of action, one of which could be the “best match” for a given patient.4
There will come a day when “precision psychiatry” finally arrives, and specific biomarkers will be developed to identify the “right” treatment for each patient within the heterogenous syndrome of depression.5 Until that day arrives, the treatment of depression will continue to be a process of trial and error, and hit or miss. But research will eventually discover genetic, neurochemical, neurophysiological, neuroimaging, or neuroimmune biomarkers that will rapidly guide clinicians to the correct treatment. This is critical to avoid inordinate delays in achieving remission and avert the ever-present risk of suicidal behavior.
The Table6 provides an overview of the numerous treatments currently available to manage depression. All increase brain-derived neurotrophic factor and restore healthy neuroplasticity and neurogenesis, which are impaired in MDD and currently believed to be a final common pathway for all depression treatments.7

These 41 therapeutic approaches to treating MDD or bipolar depression reflect the heterogeneity of mechanisms of action to address an equally heterogeneous syndrome. This implies that clinicians have a wide array of on-label options to manage patients with depression, aiming for remission, not just a good response, which typically is defined as a ≥50% reduction in total score on one of the validated rating scales used to quantify depression severity, such as the Montgomery-Åsberg Depression Rating Scale, Hamilton Depression Rating Scale, or Calgary Depression Scale for Schizophrenia.
Continue to: When several FDA-approved pharmacotherapies...
When several FDA-approved pharmacotherapies fall short and produce a suboptimal response, clinicians can resort to other treatment options known to have a higher efficacy than oral antidepressants. These include electroconvulsive therapy, repetitive transcranial magnetic stimulation, and vagus nerve stimulation. Other on-label options include adjunctive therapy with one of the approved second-generation antipsychotic agents or with adjunctive esketamine.
But if the patient still does not improve, one of many emerging off-label treatment options may work. One of the exciting new discoveries is the hallucinogen psilocybin, whose mechanism of action is truly unique. Unlike standard antidepressant medications, which modulate neurotransmitters, psilocybin increases the brain’s network flexibility, decreases the modularity of several key brain networks (especially the default-brain network, or DMN), and alters the dark and distorted mental perspective of depression to a much healthier and optimistic outlook about the self and the world.8 Such novel breakthroughs in the treatment of severe depression will shed some unprecedented insights into the core neurobiology of depression, and may lead to early intervention and prevention.
As the saying goes, all roads lead to Rome. Psychiatric clinicians should rejoice that there are abundant approaches and therapeutic mechanisms to relieve their severely melancholic (and often suicidal) patients from the grips of this disabling and life-altering brain syndrome.
1. World Health Organization. Depression: let’s talk says WHO, as depression tops list of causes of ill health. March 30, 2017. Accessed July 5, 2022. www.who.int/news/item/30-03-2017--depression-let-s-talk-says-who-as-depression-tops-list-of-causes-of-ill-health
2. Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Eng J Med. 2006;354(12)1243-1252.
3. Nasrallah HA. Treatment resistance is a myth! Current Psychiatry. 2021;20(3):14-16,28.
4. Nasrallah HA. 10 Recent paradigm shifts in the neurobiology and treatment of depression. Current Psychiatry. 2015;14(2):10-13.
5. Nasrallah HA. Biomarkers in neuropsychiatric disorders: translating research to clinical applications. Biomarkers in Neuropsychiatry. 2019;1:100001. doi:10.1016/j.bionps.2019.100001
6. Procyshyn RM, Bezchlibnyk-Butler KZ, Jeffries JJ. Clinical Handbook of Psychotropic Drugs. 23rd ed. Hogrefe; 2019.
7. Tartt AN, Mariani, MB, Hen R, et al. Dysregulation of adult hippocampal neuroplasticity in major depression: pathogenesis and therapeutic implications. Mol Psychiatry. 2022;27(6):2689-2699.
8. Lowe H, Toyang N, Steele B, et al. The therapeutic potential of psilocybin. Molecules. 2021;26(10):2948. doi: 10.3390/molecules26102948
1. World Health Organization. Depression: let’s talk says WHO, as depression tops list of causes of ill health. March 30, 2017. Accessed July 5, 2022. www.who.int/news/item/30-03-2017--depression-let-s-talk-says-who-as-depression-tops-list-of-causes-of-ill-health
2. Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Eng J Med. 2006;354(12)1243-1252.
3. Nasrallah HA. Treatment resistance is a myth! Current Psychiatry. 2021;20(3):14-16,28.
4. Nasrallah HA. 10 Recent paradigm shifts in the neurobiology and treatment of depression. Current Psychiatry. 2015;14(2):10-13.
5. Nasrallah HA. Biomarkers in neuropsychiatric disorders: translating research to clinical applications. Biomarkers in Neuropsychiatry. 2019;1:100001. doi:10.1016/j.bionps.2019.100001
6. Procyshyn RM, Bezchlibnyk-Butler KZ, Jeffries JJ. Clinical Handbook of Psychotropic Drugs. 23rd ed. Hogrefe; 2019.
7. Tartt AN, Mariani, MB, Hen R, et al. Dysregulation of adult hippocampal neuroplasticity in major depression: pathogenesis and therapeutic implications. Mol Psychiatry. 2022;27(6):2689-2699.
8. Lowe H, Toyang N, Steele B, et al. The therapeutic potential of psilocybin. Molecules. 2021;26(10):2948. doi: 10.3390/molecules26102948
3 steps to bend the curve of schizophrenia
Schizophrenia is arguably the most serious psychiatric brain syndrome. It disables teens and young adults and robs them of their potential and life dreams. It is widely regarded as a hopeless illness.
But it does not have to be. The reason most patients with schizophrenia do not return to their baseline is because obsolete clinical management approaches, a carryover from the last century, continue to be used.
Approximately 20 years ago, psychiatric researchers made a major discovery: psychosis is a neurotoxic state, and each psychotic episode is associated with significant brain damage in both gray and white matter.1 Based on that discovery, a more rational management of schizophrenia has emerged, focused on protecting patients from experiencing psychotic recurrence after the first-episode psychosis (FEP). In the past century, this strategy did not exist because psychiatrists were in a state of scientific ignorance, completely unaware that the malignant component of schizophrenia that leads to disability is psychotic relapses, the primary cause of which is very poor medication adherence after hospital discharge following the FEP.
Based on the emerging scientific evidence, here are 3 essential principles to halt the deterioration and bend the curve of outcomes in schizophrenia:
1. Minimize the duration of untreated psychosis (DUP)
Numerous studies have shown that the longer the DUP, the worse the outcome in schizophrenia.2,3 It is therefore vital to shorten the DUP spanning the emergence of psychotic symptoms at home, prior to the first hospital admission.4 The DUP is often prolonged from weeks to months by a combination of anosognosia by the patient, who fails to recognize how pathological their hallucinations and delusions are, plus the stigma of mental illness, which leads parents to delay bringing their son or daughter for psychiatric evaluation and treatment.
Another reason for a prolonged DUP is the legal system’s governing of the initiation of antipsychotic medications for an acutely psychotic patient who does not believe he/she is sick, and who adamantly refuses to receive medications. Laws passed decades ago have not kept up with scientific advances about brain damage during the DUP. Instead of delegating the rapid administration of an antipsychotic medication to the psychiatric physician who evaluated and diagnosed a patient with acute psychosis, the legal system further prolongs the DUP by requiring the psychiatrist to go to court and have a judge order the administration of antipsychotic medications. Such a legal requirement that delays urgently needed treatment has never been imposed on neurologists when administering medication to an obtunded stroke patient. Yet psychosis damages brain tissue and must be treated as urgently as stroke.5
Perhaps the most common reason for a long DUP is the recurrent relapses of psychosis, almost always caused by the high nonadherence rate among patients with schizophrenia due to multiple factors related to the illness itself.6 Ensuring uninterrupted delivery of an antipsychotic to a patient’s brain is as important to maintaining remission in schizophrenia as uninterrupted insulin treatment is for an individual with diabetes. The only way to guarantee ongoing daily pharmacotherapy in schizophrenia and avoid a longer DUP and more brain damage is to use long-acting injectable (LAI) formulations of antipsychotic medications, which are infrequently used despite making eminent sense to protect patients from the tragic consequences of psychotic relapse.7
Continue to: Start very early use of LAIs
2. Start very early use of LAIs
There is no doubt that switching from an oral to an LAI antipsychotic immediately after hospital discharge for the FEP is the single most important medical decision psychiatrists can make for patients with schizophrenia.8 This is because disability in schizophrenia begins after the second episode, not the first.9-11 Therefore, psychiatrists must behave like cardiologists,12 who strive to prevent a second destructive myocardial infarction. Regrettably, 99.9% of psychiatric practitioners never start an LAI after the FEP, and usually wait until the patient experiences multiple relapses, after extensive gray matter atrophy and white matter disintegration have occurred due to the neuroinflammation and oxidative stress (free radicals) that occur with every psychotic episode.13,14 This clearly does not make clinical sense, but remains the standard current practice.
In oncology, chemotherapy is far more effective in Stage 1 cancer, immediately after the diagnosis is made, rather than in Stage 4, when the prognosis is very poor. Similarly, LAIs are best used in Stage 1 schizophrenia, which is the first episode (schizophrenia researchers now regard the illness as having stages).15 Unfortunately, it is now rare for patients with schizophrenia to be switched to LAI pharmacotherapy right after recovery from the FEP. Instead, LAIs are more commonly used in Stage 3 or Stage 4, when the brains of patients with chronic schizophrenia have been already structurally damaged, and functional disability had set in. Bending the cure of outcome in schizophrenia is only possible when LAIs are used very early to prevent the second episode.
The prevention of relapse by using LAIs in FEP is truly remarkable. Subotnik et al16 reported that only 5% of FEP patients who received an LAI antipsychotic relapsed, compared to 33% of those who received an oral formulation of the same antipsychotic (a 650% difference). It is frankly inexplicable why psychiatrists do not exploit the relapse-preventing properties of LAIs at the time of discharge after the FEP, and instead continue to perpetuate the use of prescribing oral tablets to patients who are incapable of full adherence and doomed to “self-destruct.” This was the practice model in the previous century, when there was total ignorance about the brain-damaging effects of psychosis, and no sense of urgency about preventing psychotic relapses and DUP. Psychiatrists regarded LAIs as a last resort instead of a life-saving first resort.
In addition to relapse prevention,17 the benefits of second-generation LAIs include neuroprotection18 and lower all-cause mortality,19 a remarkable triad of benefits for patients with schizophrenia.20
3. Implement comprehensive psychosocial treatment
Most patients with schizophrenia do not have access to the array of psychosocial treatments that have been shown to be vital for rehabilitation following the FEP, just as physical rehabilitation is indispensable after the first stroke. Studies such as RAISE,21 which was funded by the National Institute of Mental Health, have demonstrated the value of psychosocial therapies (Table21-23). Collaborative care with primary care physicians is also essential due to the high prevalence of metabolic disorders (obesity, diabetics, dyslipidemia, hypertension), which tend to be undertreated in patients with schizophrenia.24

Finally, when patients continue to experience delusions and hallucinations despite full adherence (with LAIs), clozapine must be used. Like LAIs, clozapine is woefully underutilized25 despite having been shown to restore mental health and full recovery to many (but not all) patients written off as hopeless due to persistent and refractory psychotic symptoms.26
If clinicians who treat schizophrenia implement these 3 steps in their FEP patients, they will be gratified to witness a more benign trajectory of schizophrenia, which I have personally seen. The curve can indeed be bent in favor of better outcomes. By using the 3 evidence-based steps described here, clinicians will realize that schizophrenia does not have to carry the label of “the worst disease affecting mankind,” as an editorial in a top-tier journal pessimistically stated over 3 decades ago.27
1. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
2. Howes OD, Whitehurst T, Shatalina E, et al. The clinical significance of duration of untreated psychosis: an umbrella review and random-effects meta-analysis. World Psychiatry. 2021;20(1):75-95.
3. Oliver D, Davies C, Crossland G, et al. Can we reduce the duration of untreated psychosis? A systematic review and meta-analysis of controlled interventional studies. Schizophr Bull. 2018;44(6):1362-1372.
4. Srihari VH, Ferrara M, Li F, et al. Reducing the duration of untreated psychosis (DUP) in a US community: a quasi-experimental trial. Schizophr Bull Open. 2022;3(1):sgab057. doi:10.1093/schizbullopen/sgab057
5. Nasrallah HA, Roque A. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
6. Lieslehto J, Tiihonen J, Lähteenvuo M, et al. Primary nonadherence to antipsychotic treatment among persons with schizophrenia. Schizophr Bull. 2022;48(3):665-663.
7. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
8. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
9. Alvarez-Jiménez M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
10. Taipale H, Tanskanen A, Correll CU, et al. Real-world effectiveness of antipsychotic doses for relapse prevention in patients with first-episode schizophrenia in Finland: a nationwide, register-based cohort study. Lancet Psychiatry. 2022;9(4):271-279.
11. Gardner KN, Nasrallah HA. Managing first-episode psychosis: rationale and evidence for nonstandard first-line treatments for schizophrenia. Current Psychiatry. 2015;14(7):38-45,e3.
12. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
13. Feigenson KA, Kusnecov AW, Silverstein SM. Inflammation and the two-hit hypothesis of schizophrenia. Neurosci Biobehav Rev. 2014;38:72-93.
14. Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry. 2013;74(6):400-409.
15. Lavoie S, Polari AR, Goldstone S, et al. Staging model in psychiatry: review of the evolution of electroencephalography abnormalities in major psychiatric disorders. Early Interv Psychiatry. 2019;13(6):1319-1328.
16. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
17. Lin YH, Wu CS, Liu CC, et al. Comparative effectiveness of antipsychotics in preventing readmission for first-admission schizophrenia patients in national cohorts from 2001 to 2017 in Taiwan. Schizophr Bull. 2022;sbac046. doi:10.1093/schbul/sbac046
18. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7.
19. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280.
20. Nasrallah HA. Triple advantages of injectable long acting second generation antipsychotics: relapse prevention, neuroprotection, and lower mortality. Schizophr Res. 2018;197:69-70.
21. Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE Early Treatment Program. Am J Psychiatry. 2016;173(4):362-372.
22. Keshavan MS, Ongur D, Srihari VH. Toward an expanded and personalized approach to coordinated specialty care in early course psychoses. Schizophr Res. 2022;241:119-121.
23. Srihari VH, Keshavan MS. Early intervention services for schizophrenia: looking back and looking ahead. Schizophr Bull. 2022;48(3):544-550.
24. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
25. Nasrallah HA. Clozapine is a vastly underutilized, unique agent with multiple applications. Current Psychiatry. 2014;13(10):21,24-25.
26. CureSZ Foundation. Clozapine success stories. Accessed June 1, 2022. https://curesz.org/clozapine-success-stories/
27. Where next with psychiatric illness? Nature. 1988;336(6195):95-96.
Schizophrenia is arguably the most serious psychiatric brain syndrome. It disables teens and young adults and robs them of their potential and life dreams. It is widely regarded as a hopeless illness.
But it does not have to be. The reason most patients with schizophrenia do not return to their baseline is because obsolete clinical management approaches, a carryover from the last century, continue to be used.
Approximately 20 years ago, psychiatric researchers made a major discovery: psychosis is a neurotoxic state, and each psychotic episode is associated with significant brain damage in both gray and white matter.1 Based on that discovery, a more rational management of schizophrenia has emerged, focused on protecting patients from experiencing psychotic recurrence after the first-episode psychosis (FEP). In the past century, this strategy did not exist because psychiatrists were in a state of scientific ignorance, completely unaware that the malignant component of schizophrenia that leads to disability is psychotic relapses, the primary cause of which is very poor medication adherence after hospital discharge following the FEP.
Based on the emerging scientific evidence, here are 3 essential principles to halt the deterioration and bend the curve of outcomes in schizophrenia:
1. Minimize the duration of untreated psychosis (DUP)
Numerous studies have shown that the longer the DUP, the worse the outcome in schizophrenia.2,3 It is therefore vital to shorten the DUP spanning the emergence of psychotic symptoms at home, prior to the first hospital admission.4 The DUP is often prolonged from weeks to months by a combination of anosognosia by the patient, who fails to recognize how pathological their hallucinations and delusions are, plus the stigma of mental illness, which leads parents to delay bringing their son or daughter for psychiatric evaluation and treatment.
Another reason for a prolonged DUP is the legal system’s governing of the initiation of antipsychotic medications for an acutely psychotic patient who does not believe he/she is sick, and who adamantly refuses to receive medications. Laws passed decades ago have not kept up with scientific advances about brain damage during the DUP. Instead of delegating the rapid administration of an antipsychotic medication to the psychiatric physician who evaluated and diagnosed a patient with acute psychosis, the legal system further prolongs the DUP by requiring the psychiatrist to go to court and have a judge order the administration of antipsychotic medications. Such a legal requirement that delays urgently needed treatment has never been imposed on neurologists when administering medication to an obtunded stroke patient. Yet psychosis damages brain tissue and must be treated as urgently as stroke.5
Perhaps the most common reason for a long DUP is the recurrent relapses of psychosis, almost always caused by the high nonadherence rate among patients with schizophrenia due to multiple factors related to the illness itself.6 Ensuring uninterrupted delivery of an antipsychotic to a patient’s brain is as important to maintaining remission in schizophrenia as uninterrupted insulin treatment is for an individual with diabetes. The only way to guarantee ongoing daily pharmacotherapy in schizophrenia and avoid a longer DUP and more brain damage is to use long-acting injectable (LAI) formulations of antipsychotic medications, which are infrequently used despite making eminent sense to protect patients from the tragic consequences of psychotic relapse.7
Continue to: Start very early use of LAIs
2. Start very early use of LAIs
There is no doubt that switching from an oral to an LAI antipsychotic immediately after hospital discharge for the FEP is the single most important medical decision psychiatrists can make for patients with schizophrenia.8 This is because disability in schizophrenia begins after the second episode, not the first.9-11 Therefore, psychiatrists must behave like cardiologists,12 who strive to prevent a second destructive myocardial infarction. Regrettably, 99.9% of psychiatric practitioners never start an LAI after the FEP, and usually wait until the patient experiences multiple relapses, after extensive gray matter atrophy and white matter disintegration have occurred due to the neuroinflammation and oxidative stress (free radicals) that occur with every psychotic episode.13,14 This clearly does not make clinical sense, but remains the standard current practice.
In oncology, chemotherapy is far more effective in Stage 1 cancer, immediately after the diagnosis is made, rather than in Stage 4, when the prognosis is very poor. Similarly, LAIs are best used in Stage 1 schizophrenia, which is the first episode (schizophrenia researchers now regard the illness as having stages).15 Unfortunately, it is now rare for patients with schizophrenia to be switched to LAI pharmacotherapy right after recovery from the FEP. Instead, LAIs are more commonly used in Stage 3 or Stage 4, when the brains of patients with chronic schizophrenia have been already structurally damaged, and functional disability had set in. Bending the cure of outcome in schizophrenia is only possible when LAIs are used very early to prevent the second episode.
The prevention of relapse by using LAIs in FEP is truly remarkable. Subotnik et al16 reported that only 5% of FEP patients who received an LAI antipsychotic relapsed, compared to 33% of those who received an oral formulation of the same antipsychotic (a 650% difference). It is frankly inexplicable why psychiatrists do not exploit the relapse-preventing properties of LAIs at the time of discharge after the FEP, and instead continue to perpetuate the use of prescribing oral tablets to patients who are incapable of full adherence and doomed to “self-destruct.” This was the practice model in the previous century, when there was total ignorance about the brain-damaging effects of psychosis, and no sense of urgency about preventing psychotic relapses and DUP. Psychiatrists regarded LAIs as a last resort instead of a life-saving first resort.
In addition to relapse prevention,17 the benefits of second-generation LAIs include neuroprotection18 and lower all-cause mortality,19 a remarkable triad of benefits for patients with schizophrenia.20
3. Implement comprehensive psychosocial treatment
Most patients with schizophrenia do not have access to the array of psychosocial treatments that have been shown to be vital for rehabilitation following the FEP, just as physical rehabilitation is indispensable after the first stroke. Studies such as RAISE,21 which was funded by the National Institute of Mental Health, have demonstrated the value of psychosocial therapies (Table21-23). Collaborative care with primary care physicians is also essential due to the high prevalence of metabolic disorders (obesity, diabetics, dyslipidemia, hypertension), which tend to be undertreated in patients with schizophrenia.24

Finally, when patients continue to experience delusions and hallucinations despite full adherence (with LAIs), clozapine must be used. Like LAIs, clozapine is woefully underutilized25 despite having been shown to restore mental health and full recovery to many (but not all) patients written off as hopeless due to persistent and refractory psychotic symptoms.26
If clinicians who treat schizophrenia implement these 3 steps in their FEP patients, they will be gratified to witness a more benign trajectory of schizophrenia, which I have personally seen. The curve can indeed be bent in favor of better outcomes. By using the 3 evidence-based steps described here, clinicians will realize that schizophrenia does not have to carry the label of “the worst disease affecting mankind,” as an editorial in a top-tier journal pessimistically stated over 3 decades ago.27
Schizophrenia is arguably the most serious psychiatric brain syndrome. It disables teens and young adults and robs them of their potential and life dreams. It is widely regarded as a hopeless illness.
But it does not have to be. The reason most patients with schizophrenia do not return to their baseline is because obsolete clinical management approaches, a carryover from the last century, continue to be used.
Approximately 20 years ago, psychiatric researchers made a major discovery: psychosis is a neurotoxic state, and each psychotic episode is associated with significant brain damage in both gray and white matter.1 Based on that discovery, a more rational management of schizophrenia has emerged, focused on protecting patients from experiencing psychotic recurrence after the first-episode psychosis (FEP). In the past century, this strategy did not exist because psychiatrists were in a state of scientific ignorance, completely unaware that the malignant component of schizophrenia that leads to disability is psychotic relapses, the primary cause of which is very poor medication adherence after hospital discharge following the FEP.
Based on the emerging scientific evidence, here are 3 essential principles to halt the deterioration and bend the curve of outcomes in schizophrenia:
1. Minimize the duration of untreated psychosis (DUP)
Numerous studies have shown that the longer the DUP, the worse the outcome in schizophrenia.2,3 It is therefore vital to shorten the DUP spanning the emergence of psychotic symptoms at home, prior to the first hospital admission.4 The DUP is often prolonged from weeks to months by a combination of anosognosia by the patient, who fails to recognize how pathological their hallucinations and delusions are, plus the stigma of mental illness, which leads parents to delay bringing their son or daughter for psychiatric evaluation and treatment.
Another reason for a prolonged DUP is the legal system’s governing of the initiation of antipsychotic medications for an acutely psychotic patient who does not believe he/she is sick, and who adamantly refuses to receive medications. Laws passed decades ago have not kept up with scientific advances about brain damage during the DUP. Instead of delegating the rapid administration of an antipsychotic medication to the psychiatric physician who evaluated and diagnosed a patient with acute psychosis, the legal system further prolongs the DUP by requiring the psychiatrist to go to court and have a judge order the administration of antipsychotic medications. Such a legal requirement that delays urgently needed treatment has never been imposed on neurologists when administering medication to an obtunded stroke patient. Yet psychosis damages brain tissue and must be treated as urgently as stroke.5
Perhaps the most common reason for a long DUP is the recurrent relapses of psychosis, almost always caused by the high nonadherence rate among patients with schizophrenia due to multiple factors related to the illness itself.6 Ensuring uninterrupted delivery of an antipsychotic to a patient’s brain is as important to maintaining remission in schizophrenia as uninterrupted insulin treatment is for an individual with diabetes. The only way to guarantee ongoing daily pharmacotherapy in schizophrenia and avoid a longer DUP and more brain damage is to use long-acting injectable (LAI) formulations of antipsychotic medications, which are infrequently used despite making eminent sense to protect patients from the tragic consequences of psychotic relapse.7
Continue to: Start very early use of LAIs
2. Start very early use of LAIs
There is no doubt that switching from an oral to an LAI antipsychotic immediately after hospital discharge for the FEP is the single most important medical decision psychiatrists can make for patients with schizophrenia.8 This is because disability in schizophrenia begins after the second episode, not the first.9-11 Therefore, psychiatrists must behave like cardiologists,12 who strive to prevent a second destructive myocardial infarction. Regrettably, 99.9% of psychiatric practitioners never start an LAI after the FEP, and usually wait until the patient experiences multiple relapses, after extensive gray matter atrophy and white matter disintegration have occurred due to the neuroinflammation and oxidative stress (free radicals) that occur with every psychotic episode.13,14 This clearly does not make clinical sense, but remains the standard current practice.
In oncology, chemotherapy is far more effective in Stage 1 cancer, immediately after the diagnosis is made, rather than in Stage 4, when the prognosis is very poor. Similarly, LAIs are best used in Stage 1 schizophrenia, which is the first episode (schizophrenia researchers now regard the illness as having stages).15 Unfortunately, it is now rare for patients with schizophrenia to be switched to LAI pharmacotherapy right after recovery from the FEP. Instead, LAIs are more commonly used in Stage 3 or Stage 4, when the brains of patients with chronic schizophrenia have been already structurally damaged, and functional disability had set in. Bending the cure of outcome in schizophrenia is only possible when LAIs are used very early to prevent the second episode.
The prevention of relapse by using LAIs in FEP is truly remarkable. Subotnik et al16 reported that only 5% of FEP patients who received an LAI antipsychotic relapsed, compared to 33% of those who received an oral formulation of the same antipsychotic (a 650% difference). It is frankly inexplicable why psychiatrists do not exploit the relapse-preventing properties of LAIs at the time of discharge after the FEP, and instead continue to perpetuate the use of prescribing oral tablets to patients who are incapable of full adherence and doomed to “self-destruct.” This was the practice model in the previous century, when there was total ignorance about the brain-damaging effects of psychosis, and no sense of urgency about preventing psychotic relapses and DUP. Psychiatrists regarded LAIs as a last resort instead of a life-saving first resort.
In addition to relapse prevention,17 the benefits of second-generation LAIs include neuroprotection18 and lower all-cause mortality,19 a remarkable triad of benefits for patients with schizophrenia.20
3. Implement comprehensive psychosocial treatment
Most patients with schizophrenia do not have access to the array of psychosocial treatments that have been shown to be vital for rehabilitation following the FEP, just as physical rehabilitation is indispensable after the first stroke. Studies such as RAISE,21 which was funded by the National Institute of Mental Health, have demonstrated the value of psychosocial therapies (Table21-23). Collaborative care with primary care physicians is also essential due to the high prevalence of metabolic disorders (obesity, diabetics, dyslipidemia, hypertension), which tend to be undertreated in patients with schizophrenia.24

Finally, when patients continue to experience delusions and hallucinations despite full adherence (with LAIs), clozapine must be used. Like LAIs, clozapine is woefully underutilized25 despite having been shown to restore mental health and full recovery to many (but not all) patients written off as hopeless due to persistent and refractory psychotic symptoms.26
If clinicians who treat schizophrenia implement these 3 steps in their FEP patients, they will be gratified to witness a more benign trajectory of schizophrenia, which I have personally seen. The curve can indeed be bent in favor of better outcomes. By using the 3 evidence-based steps described here, clinicians will realize that schizophrenia does not have to carry the label of “the worst disease affecting mankind,” as an editorial in a top-tier journal pessimistically stated over 3 decades ago.27
1. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
2. Howes OD, Whitehurst T, Shatalina E, et al. The clinical significance of duration of untreated psychosis: an umbrella review and random-effects meta-analysis. World Psychiatry. 2021;20(1):75-95.
3. Oliver D, Davies C, Crossland G, et al. Can we reduce the duration of untreated psychosis? A systematic review and meta-analysis of controlled interventional studies. Schizophr Bull. 2018;44(6):1362-1372.
4. Srihari VH, Ferrara M, Li F, et al. Reducing the duration of untreated psychosis (DUP) in a US community: a quasi-experimental trial. Schizophr Bull Open. 2022;3(1):sgab057. doi:10.1093/schizbullopen/sgab057
5. Nasrallah HA, Roque A. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
6. Lieslehto J, Tiihonen J, Lähteenvuo M, et al. Primary nonadherence to antipsychotic treatment among persons with schizophrenia. Schizophr Bull. 2022;48(3):665-663.
7. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
8. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
9. Alvarez-Jiménez M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
10. Taipale H, Tanskanen A, Correll CU, et al. Real-world effectiveness of antipsychotic doses for relapse prevention in patients with first-episode schizophrenia in Finland: a nationwide, register-based cohort study. Lancet Psychiatry. 2022;9(4):271-279.
11. Gardner KN, Nasrallah HA. Managing first-episode psychosis: rationale and evidence for nonstandard first-line treatments for schizophrenia. Current Psychiatry. 2015;14(7):38-45,e3.
12. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
13. Feigenson KA, Kusnecov AW, Silverstein SM. Inflammation and the two-hit hypothesis of schizophrenia. Neurosci Biobehav Rev. 2014;38:72-93.
14. Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry. 2013;74(6):400-409.
15. Lavoie S, Polari AR, Goldstone S, et al. Staging model in psychiatry: review of the evolution of electroencephalography abnormalities in major psychiatric disorders. Early Interv Psychiatry. 2019;13(6):1319-1328.
16. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
17. Lin YH, Wu CS, Liu CC, et al. Comparative effectiveness of antipsychotics in preventing readmission for first-admission schizophrenia patients in national cohorts from 2001 to 2017 in Taiwan. Schizophr Bull. 2022;sbac046. doi:10.1093/schbul/sbac046
18. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7.
19. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280.
20. Nasrallah HA. Triple advantages of injectable long acting second generation antipsychotics: relapse prevention, neuroprotection, and lower mortality. Schizophr Res. 2018;197:69-70.
21. Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE Early Treatment Program. Am J Psychiatry. 2016;173(4):362-372.
22. Keshavan MS, Ongur D, Srihari VH. Toward an expanded and personalized approach to coordinated specialty care in early course psychoses. Schizophr Res. 2022;241:119-121.
23. Srihari VH, Keshavan MS. Early intervention services for schizophrenia: looking back and looking ahead. Schizophr Bull. 2022;48(3):544-550.
24. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
25. Nasrallah HA. Clozapine is a vastly underutilized, unique agent with multiple applications. Current Psychiatry. 2014;13(10):21,24-25.
26. CureSZ Foundation. Clozapine success stories. Accessed June 1, 2022. https://curesz.org/clozapine-success-stories/
27. Where next with psychiatric illness? Nature. 1988;336(6195):95-96.
1. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
2. Howes OD, Whitehurst T, Shatalina E, et al. The clinical significance of duration of untreated psychosis: an umbrella review and random-effects meta-analysis. World Psychiatry. 2021;20(1):75-95.
3. Oliver D, Davies C, Crossland G, et al. Can we reduce the duration of untreated psychosis? A systematic review and meta-analysis of controlled interventional studies. Schizophr Bull. 2018;44(6):1362-1372.
4. Srihari VH, Ferrara M, Li F, et al. Reducing the duration of untreated psychosis (DUP) in a US community: a quasi-experimental trial. Schizophr Bull Open. 2022;3(1):sgab057. doi:10.1093/schizbullopen/sgab057
5. Nasrallah HA, Roque A. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
6. Lieslehto J, Tiihonen J, Lähteenvuo M, et al. Primary nonadherence to antipsychotic treatment among persons with schizophrenia. Schizophr Bull. 2022;48(3):665-663.
7. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
8. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
9. Alvarez-Jiménez M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
10. Taipale H, Tanskanen A, Correll CU, et al. Real-world effectiveness of antipsychotic doses for relapse prevention in patients with first-episode schizophrenia in Finland: a nationwide, register-based cohort study. Lancet Psychiatry. 2022;9(4):271-279.
11. Gardner KN, Nasrallah HA. Managing first-episode psychosis: rationale and evidence for nonstandard first-line treatments for schizophrenia. Current Psychiatry. 2015;14(7):38-45,e3.
12. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
13. Feigenson KA, Kusnecov AW, Silverstein SM. Inflammation and the two-hit hypothesis of schizophrenia. Neurosci Biobehav Rev. 2014;38:72-93.
14. Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry. 2013;74(6):400-409.
15. Lavoie S, Polari AR, Goldstone S, et al. Staging model in psychiatry: review of the evolution of electroencephalography abnormalities in major psychiatric disorders. Early Interv Psychiatry. 2019;13(6):1319-1328.
16. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
17. Lin YH, Wu CS, Liu CC, et al. Comparative effectiveness of antipsychotics in preventing readmission for first-admission schizophrenia patients in national cohorts from 2001 to 2017 in Taiwan. Schizophr Bull. 2022;sbac046. doi:10.1093/schbul/sbac046
18. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7.
19. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280.
20. Nasrallah HA. Triple advantages of injectable long acting second generation antipsychotics: relapse prevention, neuroprotection, and lower mortality. Schizophr Res. 2018;197:69-70.
21. Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE Early Treatment Program. Am J Psychiatry. 2016;173(4):362-372.
22. Keshavan MS, Ongur D, Srihari VH. Toward an expanded and personalized approach to coordinated specialty care in early course psychoses. Schizophr Res. 2022;241:119-121.
23. Srihari VH, Keshavan MS. Early intervention services for schizophrenia: looking back and looking ahead. Schizophr Bull. 2022;48(3):544-550.
24. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
25. Nasrallah HA. Clozapine is a vastly underutilized, unique agent with multiple applications. Current Psychiatry. 2014;13(10):21,24-25.
26. CureSZ Foundation. Clozapine success stories. Accessed June 1, 2022. https://curesz.org/clozapine-success-stories/
27. Where next with psychiatric illness? Nature. 1988;336(6195):95-96.
A PSYCHIATRIC MANIFESTO: Stigma is hate speech and a hate crime
Having witnessed the devastating impact of stigma on patients with mental illness throughout my psychiatric career, I am fed up and disgusted with this malevolent scourge.
I regard the stigma that engulfs neuropsychiatric disorders as a malignancy that mutilates patients’ souls and hastens their mortality.
Stigma is hate speech
How would you feel if you had a serious medical illness, a disabling brain disorder such as schizophrenia, depression, or anxiety, and people refer to you with pejorative and insulting terms such as crazy, deranged, lunatic, unhinged, nutty, insane, wacky, berserk, cuckoo, bonkers, flaky, screwball, or unglued? This is hate speech generated by stigma against people with mental illness. Individuals with heart disease, cancer, or diabetes never get called such disgraceful and stigmatizing terms that shame, stain, besmirch, and scar them, which happens daily to persons with psychiatric brain disorders.
The damage and harm of the discriminatory stigma on our patients is multifaceted. It is painful, detrimental, pernicious, and deleterious. It is corrosive to their spirits, crippling to their self-image, and subversive to their self-confidence. Hate speech is not simply words, but a menacing weapon that assaults the core humanity of medically ill psychiatric patients.
Although hate speech is punishable by law, there are rarely any legal actions against those who hurl hate speech at psychiatric patients every day. Society has institutionalized the stigma of mental illness and takes it in stride instead of recognizing it as an illegal, harmful act.
Long before the stresses of the COVID-19 pandemic, 43% of the population had been shown to experience a diagnosable psychiatric disorder over the course of their life.1 Thus, tens of millions of people are burdened by stigma and the hate speech associated with it. This is directly related to massive ignorance about mental illness being the result of a neurobiological condition due to either genetic or intrauterine adverse events that disrupt brain development. Delusions and hallucinations are symptoms of a malfunctioning brain, depression is not a sign of personal weakness, anxiety is the most prevalent mental disorder in the world, and obsessive-compulsive disorder (OCD) is not odd behavior but the result of dysfunction of neural circuits. Correcting public misperceptions about psychiatric brain disorders can mitigate stigma, but it has yet to happen.
Stigma is a hate crime
Stigma can accelerate physical death and premature mortality. Many studies have confirmed that persons with schizophrenia do not receive basic primary care treatments for the life-shortening medical conditions that often afflict them, such as diabetes, dyslipidemia, and hypertension.2 Stigma is responsible for a significant disparity of medical3-5 and intensive care6 among individuals with mental illness compared to the general population. It’s no wonder most psychiatric disorders are associated with accelerated mortality.7 A recent study during the pandemic by Balasuriya et al8 reported that patients with depression had poor access to care. Stigma interferes with or delays necessary medical care, leading to clinical deterioration and unnecessary, preventable death. Stigma shortens life and is a hate crime.
Continue to: The extremely high suicide rates...
The extremely high suicide rates among individuals with serious mental illness, who live under the oppressiveness of stigma, is another example of how stigma is a hate crime that can cause patients with psychiatric disorders to give up and end their lives. Zaheer et al9 found that young patients with schizophrenia had an astronomical suicide rate compared to the general population (1 in 52 in individuals with schizophrenia, compared to 12 in 100,000 in the general population, roughly a 200-fold increase!). This is clearly a consequence of stigma and discrimination,10 which leads to demoralization, shame, loneliness, distress, and hopelessness. Stigma can be fatal, and that makes it a hate crime.
Stigma also limits vocational opportunities for individuals with mental illness. They are either not hired, or quickly fired. Even highly educated professionals such as physicians, nurses, lawyers, or teachers can lose their jobs if they divulge a history of a psychiatric disorder or alcohol or substance abuse, regardless of whether they are receiving treatment and are medically in remission. Even highly qualified politicians have been deemed “ineligible” for higher office if they disclose a history of psychiatric treatment. Stigma is loaded with outrageous discrimination that deprives our patients of “the pursuit of happiness,” a fundamental constitutional right.
Stigma surrounding the mental health professions
Stigma also engulfs mental health professionals, simply because they deal with psychiatric patients every day. In a classic article titled “The Enigma of Stigma,”11 Dr. Paul Fink, past president of the American Psychiatric Association (1988-1989), described how psychiatrists are perceived as “different” from other physicians by the public and by the media. He said psychiatrists are tarred by the same brush as their patients as “undesirables” in society. And movies such as Psycho and One Flew Over the Cuckoo’s Nest reinforce the stigma against both psychiatric patients and the psychiatrists and nurses who treat them. The health care system that carves out “behavioral health” from the umbrella of “medical care” further accentuates the stigma by portraying the “separateness” of psychiatry, a genuine medical specialty, from its fellow medical disciplines. This becomes fodder for the antipsychiatry movement at every turn and can even lead to questioning the existence of mental illness, as Thomas Szasz12 did by declaring that mental illness is a myth and describing psychiatry as “the science of lies.” No other medical specialty endures abuse and insults like psychiatry, and that’s a direct result of stigma.
Extinguishing stigma is a societal imperative
So what can be done to squelch stigma and defeat it once and for all, so that psychiatric patients can be treated with dignity and compassion, like people with cancer, heart attacks, diabetes, or brain tumors? The pandemic, terrible as it has been for the entire world, did have the silver lining of raising awareness about the ubiquity of psychiatric symptoms, such as anxiety and depression, across all ages, genders, educational and religious backgrounds, and socioeconomic classes. But there should also be a robust legal battle against the damaging effects of stigma. There are laws to sanction and penalize hate speech and hate crimes that must be implemented when stigma is documented. There are also parity laws, but they have no teeth and have not ameliorated the insurance discrepancies and economic burden of psychiatric disorders. A bold step would be to reclassify serious psychiatric brain disorders (schizophrenia, bipolar disorder, major depressive disorder, OCD, attention-deficit/hyperactivity disorder, generalized anxiety disorder/panic attacks, and borderline personality disorder) as neurologic disorders, which would automatically give patients with these disorders broad access to medical care, which happened when autism was reclassified as a neurologic disorder. Finally, a much more intensive public education must be disseminated about the neurobiological etiologies, brain structure, and function in psychiatric disorders, and the psychiatric symptoms associated with all neurologic disorders. Regrettably, empathy can be difficult to teach.
Stigma is hate speech and a hate crime. It must be permanently eliminated by effective laws and by erasing the widespread ignorance about the medical and neurologic roots of mental disorders, and by emphasizing the fact that they are as treatable as other general medical conditions.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
3. Druss BG, Rosenheck RA. Use of medical services by veterans with mental disorders. Psychosomatics. 1997;38(5):451-458.
4. Druss BG, Rosenheck RA. Mental disorders and access to medical care in the United States. Am J Psychiatry. 1998;155(12):1775-1777.
5. Druss BG, Bradford WD, Rosenheck RA, et al. Quality of medical care and excess mortality in older patients with mental disorders. Arch Gen Psychiatry. 2001;58(6):565-572.
6. Druss BG, Bradford DW, Rosenheck RA, et al. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;283(4):506-511.
7. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
8. Balasuriya L, Quinton JK, Canavan ME, et al. The association between history of depression and access to care among Medicare beneficiaries during the COVID-19 pandemic. J Gen Intern Med. 2021;36(12):3778-3785.
9. Zaheer J, Olfson M, Mallia E, et al. Predictors of suicide at time of diagnosis in schizophrenia spectrum disorder: a 20-year total population study in Ontario, Canada. Schizophr Res. 2020;222:382-388.
10. Brohan E, Thornicroft G, Rüsch N, et al. Measuring discrimination experienced by people with a mental illness: replication of the short-form DISCUS in six world regions. Psychol Med. 2022:1-11. doi:10.1017/S0033291722000630
11. Fink P. The enigma of stigma and its relation to psychiatric education. Psychiatric Annals. 1983;13(9):669-690.
12. Szasz T. The Myth of Mental Illness. Harper Collins; 1960.
Having witnessed the devastating impact of stigma on patients with mental illness throughout my psychiatric career, I am fed up and disgusted with this malevolent scourge.
I regard the stigma that engulfs neuropsychiatric disorders as a malignancy that mutilates patients’ souls and hastens their mortality.
Stigma is hate speech
How would you feel if you had a serious medical illness, a disabling brain disorder such as schizophrenia, depression, or anxiety, and people refer to you with pejorative and insulting terms such as crazy, deranged, lunatic, unhinged, nutty, insane, wacky, berserk, cuckoo, bonkers, flaky, screwball, or unglued? This is hate speech generated by stigma against people with mental illness. Individuals with heart disease, cancer, or diabetes never get called such disgraceful and stigmatizing terms that shame, stain, besmirch, and scar them, which happens daily to persons with psychiatric brain disorders.
The damage and harm of the discriminatory stigma on our patients is multifaceted. It is painful, detrimental, pernicious, and deleterious. It is corrosive to their spirits, crippling to their self-image, and subversive to their self-confidence. Hate speech is not simply words, but a menacing weapon that assaults the core humanity of medically ill psychiatric patients.
Although hate speech is punishable by law, there are rarely any legal actions against those who hurl hate speech at psychiatric patients every day. Society has institutionalized the stigma of mental illness and takes it in stride instead of recognizing it as an illegal, harmful act.
Long before the stresses of the COVID-19 pandemic, 43% of the population had been shown to experience a diagnosable psychiatric disorder over the course of their life.1 Thus, tens of millions of people are burdened by stigma and the hate speech associated with it. This is directly related to massive ignorance about mental illness being the result of a neurobiological condition due to either genetic or intrauterine adverse events that disrupt brain development. Delusions and hallucinations are symptoms of a malfunctioning brain, depression is not a sign of personal weakness, anxiety is the most prevalent mental disorder in the world, and obsessive-compulsive disorder (OCD) is not odd behavior but the result of dysfunction of neural circuits. Correcting public misperceptions about psychiatric brain disorders can mitigate stigma, but it has yet to happen.
Stigma is a hate crime
Stigma can accelerate physical death and premature mortality. Many studies have confirmed that persons with schizophrenia do not receive basic primary care treatments for the life-shortening medical conditions that often afflict them, such as diabetes, dyslipidemia, and hypertension.2 Stigma is responsible for a significant disparity of medical3-5 and intensive care6 among individuals with mental illness compared to the general population. It’s no wonder most psychiatric disorders are associated with accelerated mortality.7 A recent study during the pandemic by Balasuriya et al8 reported that patients with depression had poor access to care. Stigma interferes with or delays necessary medical care, leading to clinical deterioration and unnecessary, preventable death. Stigma shortens life and is a hate crime.
Continue to: The extremely high suicide rates...
The extremely high suicide rates among individuals with serious mental illness, who live under the oppressiveness of stigma, is another example of how stigma is a hate crime that can cause patients with psychiatric disorders to give up and end their lives. Zaheer et al9 found that young patients with schizophrenia had an astronomical suicide rate compared to the general population (1 in 52 in individuals with schizophrenia, compared to 12 in 100,000 in the general population, roughly a 200-fold increase!). This is clearly a consequence of stigma and discrimination,10 which leads to demoralization, shame, loneliness, distress, and hopelessness. Stigma can be fatal, and that makes it a hate crime.
Stigma also limits vocational opportunities for individuals with mental illness. They are either not hired, or quickly fired. Even highly educated professionals such as physicians, nurses, lawyers, or teachers can lose their jobs if they divulge a history of a psychiatric disorder or alcohol or substance abuse, regardless of whether they are receiving treatment and are medically in remission. Even highly qualified politicians have been deemed “ineligible” for higher office if they disclose a history of psychiatric treatment. Stigma is loaded with outrageous discrimination that deprives our patients of “the pursuit of happiness,” a fundamental constitutional right.
Stigma surrounding the mental health professions
Stigma also engulfs mental health professionals, simply because they deal with psychiatric patients every day. In a classic article titled “The Enigma of Stigma,”11 Dr. Paul Fink, past president of the American Psychiatric Association (1988-1989), described how psychiatrists are perceived as “different” from other physicians by the public and by the media. He said psychiatrists are tarred by the same brush as their patients as “undesirables” in society. And movies such as Psycho and One Flew Over the Cuckoo’s Nest reinforce the stigma against both psychiatric patients and the psychiatrists and nurses who treat them. The health care system that carves out “behavioral health” from the umbrella of “medical care” further accentuates the stigma by portraying the “separateness” of psychiatry, a genuine medical specialty, from its fellow medical disciplines. This becomes fodder for the antipsychiatry movement at every turn and can even lead to questioning the existence of mental illness, as Thomas Szasz12 did by declaring that mental illness is a myth and describing psychiatry as “the science of lies.” No other medical specialty endures abuse and insults like psychiatry, and that’s a direct result of stigma.
Extinguishing stigma is a societal imperative
So what can be done to squelch stigma and defeat it once and for all, so that psychiatric patients can be treated with dignity and compassion, like people with cancer, heart attacks, diabetes, or brain tumors? The pandemic, terrible as it has been for the entire world, did have the silver lining of raising awareness about the ubiquity of psychiatric symptoms, such as anxiety and depression, across all ages, genders, educational and religious backgrounds, and socioeconomic classes. But there should also be a robust legal battle against the damaging effects of stigma. There are laws to sanction and penalize hate speech and hate crimes that must be implemented when stigma is documented. There are also parity laws, but they have no teeth and have not ameliorated the insurance discrepancies and economic burden of psychiatric disorders. A bold step would be to reclassify serious psychiatric brain disorders (schizophrenia, bipolar disorder, major depressive disorder, OCD, attention-deficit/hyperactivity disorder, generalized anxiety disorder/panic attacks, and borderline personality disorder) as neurologic disorders, which would automatically give patients with these disorders broad access to medical care, which happened when autism was reclassified as a neurologic disorder. Finally, a much more intensive public education must be disseminated about the neurobiological etiologies, brain structure, and function in psychiatric disorders, and the psychiatric symptoms associated with all neurologic disorders. Regrettably, empathy can be difficult to teach.
Stigma is hate speech and a hate crime. It must be permanently eliminated by effective laws and by erasing the widespread ignorance about the medical and neurologic roots of mental disorders, and by emphasizing the fact that they are as treatable as other general medical conditions.
Having witnessed the devastating impact of stigma on patients with mental illness throughout my psychiatric career, I am fed up and disgusted with this malevolent scourge.
I regard the stigma that engulfs neuropsychiatric disorders as a malignancy that mutilates patients’ souls and hastens their mortality.
Stigma is hate speech
How would you feel if you had a serious medical illness, a disabling brain disorder such as schizophrenia, depression, or anxiety, and people refer to you with pejorative and insulting terms such as crazy, deranged, lunatic, unhinged, nutty, insane, wacky, berserk, cuckoo, bonkers, flaky, screwball, or unglued? This is hate speech generated by stigma against people with mental illness. Individuals with heart disease, cancer, or diabetes never get called such disgraceful and stigmatizing terms that shame, stain, besmirch, and scar them, which happens daily to persons with psychiatric brain disorders.
The damage and harm of the discriminatory stigma on our patients is multifaceted. It is painful, detrimental, pernicious, and deleterious. It is corrosive to their spirits, crippling to their self-image, and subversive to their self-confidence. Hate speech is not simply words, but a menacing weapon that assaults the core humanity of medically ill psychiatric patients.
Although hate speech is punishable by law, there are rarely any legal actions against those who hurl hate speech at psychiatric patients every day. Society has institutionalized the stigma of mental illness and takes it in stride instead of recognizing it as an illegal, harmful act.
Long before the stresses of the COVID-19 pandemic, 43% of the population had been shown to experience a diagnosable psychiatric disorder over the course of their life.1 Thus, tens of millions of people are burdened by stigma and the hate speech associated with it. This is directly related to massive ignorance about mental illness being the result of a neurobiological condition due to either genetic or intrauterine adverse events that disrupt brain development. Delusions and hallucinations are symptoms of a malfunctioning brain, depression is not a sign of personal weakness, anxiety is the most prevalent mental disorder in the world, and obsessive-compulsive disorder (OCD) is not odd behavior but the result of dysfunction of neural circuits. Correcting public misperceptions about psychiatric brain disorders can mitigate stigma, but it has yet to happen.
Stigma is a hate crime
Stigma can accelerate physical death and premature mortality. Many studies have confirmed that persons with schizophrenia do not receive basic primary care treatments for the life-shortening medical conditions that often afflict them, such as diabetes, dyslipidemia, and hypertension.2 Stigma is responsible for a significant disparity of medical3-5 and intensive care6 among individuals with mental illness compared to the general population. It’s no wonder most psychiatric disorders are associated with accelerated mortality.7 A recent study during the pandemic by Balasuriya et al8 reported that patients with depression had poor access to care. Stigma interferes with or delays necessary medical care, leading to clinical deterioration and unnecessary, preventable death. Stigma shortens life and is a hate crime.
Continue to: The extremely high suicide rates...
The extremely high suicide rates among individuals with serious mental illness, who live under the oppressiveness of stigma, is another example of how stigma is a hate crime that can cause patients with psychiatric disorders to give up and end their lives. Zaheer et al9 found that young patients with schizophrenia had an astronomical suicide rate compared to the general population (1 in 52 in individuals with schizophrenia, compared to 12 in 100,000 in the general population, roughly a 200-fold increase!). This is clearly a consequence of stigma and discrimination,10 which leads to demoralization, shame, loneliness, distress, and hopelessness. Stigma can be fatal, and that makes it a hate crime.
Stigma also limits vocational opportunities for individuals with mental illness. They are either not hired, or quickly fired. Even highly educated professionals such as physicians, nurses, lawyers, or teachers can lose their jobs if they divulge a history of a psychiatric disorder or alcohol or substance abuse, regardless of whether they are receiving treatment and are medically in remission. Even highly qualified politicians have been deemed “ineligible” for higher office if they disclose a history of psychiatric treatment. Stigma is loaded with outrageous discrimination that deprives our patients of “the pursuit of happiness,” a fundamental constitutional right.
Stigma surrounding the mental health professions
Stigma also engulfs mental health professionals, simply because they deal with psychiatric patients every day. In a classic article titled “The Enigma of Stigma,”11 Dr. Paul Fink, past president of the American Psychiatric Association (1988-1989), described how psychiatrists are perceived as “different” from other physicians by the public and by the media. He said psychiatrists are tarred by the same brush as their patients as “undesirables” in society. And movies such as Psycho and One Flew Over the Cuckoo’s Nest reinforce the stigma against both psychiatric patients and the psychiatrists and nurses who treat them. The health care system that carves out “behavioral health” from the umbrella of “medical care” further accentuates the stigma by portraying the “separateness” of psychiatry, a genuine medical specialty, from its fellow medical disciplines. This becomes fodder for the antipsychiatry movement at every turn and can even lead to questioning the existence of mental illness, as Thomas Szasz12 did by declaring that mental illness is a myth and describing psychiatry as “the science of lies.” No other medical specialty endures abuse and insults like psychiatry, and that’s a direct result of stigma.
Extinguishing stigma is a societal imperative
So what can be done to squelch stigma and defeat it once and for all, so that psychiatric patients can be treated with dignity and compassion, like people with cancer, heart attacks, diabetes, or brain tumors? The pandemic, terrible as it has been for the entire world, did have the silver lining of raising awareness about the ubiquity of psychiatric symptoms, such as anxiety and depression, across all ages, genders, educational and religious backgrounds, and socioeconomic classes. But there should also be a robust legal battle against the damaging effects of stigma. There are laws to sanction and penalize hate speech and hate crimes that must be implemented when stigma is documented. There are also parity laws, but they have no teeth and have not ameliorated the insurance discrepancies and economic burden of psychiatric disorders. A bold step would be to reclassify serious psychiatric brain disorders (schizophrenia, bipolar disorder, major depressive disorder, OCD, attention-deficit/hyperactivity disorder, generalized anxiety disorder/panic attacks, and borderline personality disorder) as neurologic disorders, which would automatically give patients with these disorders broad access to medical care, which happened when autism was reclassified as a neurologic disorder. Finally, a much more intensive public education must be disseminated about the neurobiological etiologies, brain structure, and function in psychiatric disorders, and the psychiatric symptoms associated with all neurologic disorders. Regrettably, empathy can be difficult to teach.
Stigma is hate speech and a hate crime. It must be permanently eliminated by effective laws and by erasing the widespread ignorance about the medical and neurologic roots of mental disorders, and by emphasizing the fact that they are as treatable as other general medical conditions.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
3. Druss BG, Rosenheck RA. Use of medical services by veterans with mental disorders. Psychosomatics. 1997;38(5):451-458.
4. Druss BG, Rosenheck RA. Mental disorders and access to medical care in the United States. Am J Psychiatry. 1998;155(12):1775-1777.
5. Druss BG, Bradford WD, Rosenheck RA, et al. Quality of medical care and excess mortality in older patients with mental disorders. Arch Gen Psychiatry. 2001;58(6):565-572.
6. Druss BG, Bradford DW, Rosenheck RA, et al. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;283(4):506-511.
7. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
8. Balasuriya L, Quinton JK, Canavan ME, et al. The association between history of depression and access to care among Medicare beneficiaries during the COVID-19 pandemic. J Gen Intern Med. 2021;36(12):3778-3785.
9. Zaheer J, Olfson M, Mallia E, et al. Predictors of suicide at time of diagnosis in schizophrenia spectrum disorder: a 20-year total population study in Ontario, Canada. Schizophr Res. 2020;222:382-388.
10. Brohan E, Thornicroft G, Rüsch N, et al. Measuring discrimination experienced by people with a mental illness: replication of the short-form DISCUS in six world regions. Psychol Med. 2022:1-11. doi:10.1017/S0033291722000630
11. Fink P. The enigma of stigma and its relation to psychiatric education. Psychiatric Annals. 1983;13(9):669-690.
12. Szasz T. The Myth of Mental Illness. Harper Collins; 1960.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
3. Druss BG, Rosenheck RA. Use of medical services by veterans with mental disorders. Psychosomatics. 1997;38(5):451-458.
4. Druss BG, Rosenheck RA. Mental disorders and access to medical care in the United States. Am J Psychiatry. 1998;155(12):1775-1777.
5. Druss BG, Bradford WD, Rosenheck RA, et al. Quality of medical care and excess mortality in older patients with mental disorders. Arch Gen Psychiatry. 2001;58(6):565-572.
6. Druss BG, Bradford DW, Rosenheck RA, et al. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;283(4):506-511.
7. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
8. Balasuriya L, Quinton JK, Canavan ME, et al. The association between history of depression and access to care among Medicare beneficiaries during the COVID-19 pandemic. J Gen Intern Med. 2021;36(12):3778-3785.
9. Zaheer J, Olfson M, Mallia E, et al. Predictors of suicide at time of diagnosis in schizophrenia spectrum disorder: a 20-year total population study in Ontario, Canada. Schizophr Res. 2020;222:382-388.
10. Brohan E, Thornicroft G, Rüsch N, et al. Measuring discrimination experienced by people with a mental illness: replication of the short-form DISCUS in six world regions. Psychol Med. 2022:1-11. doi:10.1017/S0033291722000630
11. Fink P. The enigma of stigma and its relation to psychiatric education. Psychiatric Annals. 1983;13(9):669-690.
12. Szasz T. The Myth of Mental Illness. Harper Collins; 1960.
The neurobiology of Jeopardy! champions
As a regular viewer of Jeopardy! I find it both interesting and educational. But the psychiatric neuroscientist in me marvels at the splendid cerebral attributes embedded in the brains of Jeopardy! champions.
Back in my college days, I participated in what were then called “general knowledge contests” and won a couple of trophies, the most gratifying of which was when our team of medical students beat the faculty team! Later, when my wife and I had children, Trivial Pursuit was a game frequently played in our household. So it is no wonder I have often thought of the remarkable, sometimes stunning intellectual performances of Jeopardy! champions.
What does it take to excel at Jeopardy!?
Watching contestants successfully answer a bewildering array of questions across an extensive spectrum of topics is simply dazzling and prompts me to ask: Which neurologic structures play a central role in the brains of Jeopardy! champions? So I channeled my inner neurobiologist and came up with the following prerequisites to excel at Jeopardy!:
- A hippocampus on steroids! Memory is obviously a core ingredient for responding to Jeopardy! questions. Unlike ordinary mortals, Jeopardy! champions appear to retain and instantaneously, accurately recall everything they have read, saw, or heard.
- A sublime network of dendritic spines, where learning is immediately transduced to biological memories, thanks to the wonders of experiential neuroplasticity in homo sapiens.
- A superlative frontal lobe, which provides the champion with an ultra-rapid abstraction ability in the dorsolateral prefrontal cortex, along with razor-sharp concentration and attention.
- An extremely well-myelinated network of the 137,000 miles of white matter fibers in the human brain. This is what leads to fabulous processing speed. Rapid neurotransmission is impossible without very well-myelinated axons and dendrites. It is not enough for a Jeopardy! champion to know the answer and retrieve it from the hippocampus—they also must transmit the answer at lightning speed to the speech area, and then activate the motor area to enunciate the answer. Processing speed is the foundation of overall cognitive functioning.
- A first-rate Broca’s area, referred to as “the brain’s scriptwriter,” which shapes human speech. It receives the flow of sensory information from the temporal cortex, devises a plan for speaking, and passes that plan seamlessly to the motor cortex, which controls the movements of the mouth.
- Blistering speed reflexes to click the handheld response buzzer within a fraction of a millisecond after the host finishes reading the clue (not before, or a penalty is incurred). Jeopardy! champions always click the buzzer faster than their competitors, who may know the answer but have ordinary motor reflexes (also related to the degree of myelination and a motoric component of processing speed).
- A thick corpus callosum, the largest interhemispheric commissure, a bundle of 200 million white matter fibers connecting analogous regions in the right and left hemispheres, is vital for the rapid bidirectional transfer of bits of information from the intuitive/nonverbal right hemisphere to the mathematical/verbal left hemisphere, when the answer requires right hemispheric input.
- A bright occipital cortex and exceptional optic nerve and retina, so that champions can recognize faces or locations and read the questions before the host finishes reading them, which gives them an awesome edge on other contestants.
Obviously, the brains of Jeopardy! champions are a breed of their own, with exceptional performances by multiple regions converging to produce a winning performance. But during their childhood and youthful years, such brains also generate motivation, curiosity, and interest in a wide range of topics, from cultures, regions, music genres, and word games to history, geography, sports, science, medicine, astronomy, and Greek mythology.
Jeopardy! champions may appear to have regular jobs and ordinary lives, but they have resplendent “renaissance” brains. I wonder how they spent their childhood, who mentored them, what type of family lives they had, and what they dream of accomplishing other than winning on Jeopardy!. Will their awe-inspiring performance in Jeopardy! translate to overall success in life? That’s a story that remains to be told.
As a regular viewer of Jeopardy! I find it both interesting and educational. But the psychiatric neuroscientist in me marvels at the splendid cerebral attributes embedded in the brains of Jeopardy! champions.
Back in my college days, I participated in what were then called “general knowledge contests” and won a couple of trophies, the most gratifying of which was when our team of medical students beat the faculty team! Later, when my wife and I had children, Trivial Pursuit was a game frequently played in our household. So it is no wonder I have often thought of the remarkable, sometimes stunning intellectual performances of Jeopardy! champions.
What does it take to excel at Jeopardy!?
Watching contestants successfully answer a bewildering array of questions across an extensive spectrum of topics is simply dazzling and prompts me to ask: Which neurologic structures play a central role in the brains of Jeopardy! champions? So I channeled my inner neurobiologist and came up with the following prerequisites to excel at Jeopardy!:
- A hippocampus on steroids! Memory is obviously a core ingredient for responding to Jeopardy! questions. Unlike ordinary mortals, Jeopardy! champions appear to retain and instantaneously, accurately recall everything they have read, saw, or heard.
- A sublime network of dendritic spines, where learning is immediately transduced to biological memories, thanks to the wonders of experiential neuroplasticity in homo sapiens.
- A superlative frontal lobe, which provides the champion with an ultra-rapid abstraction ability in the dorsolateral prefrontal cortex, along with razor-sharp concentration and attention.
- An extremely well-myelinated network of the 137,000 miles of white matter fibers in the human brain. This is what leads to fabulous processing speed. Rapid neurotransmission is impossible without very well-myelinated axons and dendrites. It is not enough for a Jeopardy! champion to know the answer and retrieve it from the hippocampus—they also must transmit the answer at lightning speed to the speech area, and then activate the motor area to enunciate the answer. Processing speed is the foundation of overall cognitive functioning.
- A first-rate Broca’s area, referred to as “the brain’s scriptwriter,” which shapes human speech. It receives the flow of sensory information from the temporal cortex, devises a plan for speaking, and passes that plan seamlessly to the motor cortex, which controls the movements of the mouth.
- Blistering speed reflexes to click the handheld response buzzer within a fraction of a millisecond after the host finishes reading the clue (not before, or a penalty is incurred). Jeopardy! champions always click the buzzer faster than their competitors, who may know the answer but have ordinary motor reflexes (also related to the degree of myelination and a motoric component of processing speed).
- A thick corpus callosum, the largest interhemispheric commissure, a bundle of 200 million white matter fibers connecting analogous regions in the right and left hemispheres, is vital for the rapid bidirectional transfer of bits of information from the intuitive/nonverbal right hemisphere to the mathematical/verbal left hemisphere, when the answer requires right hemispheric input.
- A bright occipital cortex and exceptional optic nerve and retina, so that champions can recognize faces or locations and read the questions before the host finishes reading them, which gives them an awesome edge on other contestants.
Obviously, the brains of Jeopardy! champions are a breed of their own, with exceptional performances by multiple regions converging to produce a winning performance. But during their childhood and youthful years, such brains also generate motivation, curiosity, and interest in a wide range of topics, from cultures, regions, music genres, and word games to history, geography, sports, science, medicine, astronomy, and Greek mythology.
Jeopardy! champions may appear to have regular jobs and ordinary lives, but they have resplendent “renaissance” brains. I wonder how they spent their childhood, who mentored them, what type of family lives they had, and what they dream of accomplishing other than winning on Jeopardy!. Will their awe-inspiring performance in Jeopardy! translate to overall success in life? That’s a story that remains to be told.
As a regular viewer of Jeopardy! I find it both interesting and educational. But the psychiatric neuroscientist in me marvels at the splendid cerebral attributes embedded in the brains of Jeopardy! champions.
Back in my college days, I participated in what were then called “general knowledge contests” and won a couple of trophies, the most gratifying of which was when our team of medical students beat the faculty team! Later, when my wife and I had children, Trivial Pursuit was a game frequently played in our household. So it is no wonder I have often thought of the remarkable, sometimes stunning intellectual performances of Jeopardy! champions.
What does it take to excel at Jeopardy!?
Watching contestants successfully answer a bewildering array of questions across an extensive spectrum of topics is simply dazzling and prompts me to ask: Which neurologic structures play a central role in the brains of Jeopardy! champions? So I channeled my inner neurobiologist and came up with the following prerequisites to excel at Jeopardy!:
- A hippocampus on steroids! Memory is obviously a core ingredient for responding to Jeopardy! questions. Unlike ordinary mortals, Jeopardy! champions appear to retain and instantaneously, accurately recall everything they have read, saw, or heard.
- A sublime network of dendritic spines, where learning is immediately transduced to biological memories, thanks to the wonders of experiential neuroplasticity in homo sapiens.
- A superlative frontal lobe, which provides the champion with an ultra-rapid abstraction ability in the dorsolateral prefrontal cortex, along with razor-sharp concentration and attention.
- An extremely well-myelinated network of the 137,000 miles of white matter fibers in the human brain. This is what leads to fabulous processing speed. Rapid neurotransmission is impossible without very well-myelinated axons and dendrites. It is not enough for a Jeopardy! champion to know the answer and retrieve it from the hippocampus—they also must transmit the answer at lightning speed to the speech area, and then activate the motor area to enunciate the answer. Processing speed is the foundation of overall cognitive functioning.
- A first-rate Broca’s area, referred to as “the brain’s scriptwriter,” which shapes human speech. It receives the flow of sensory information from the temporal cortex, devises a plan for speaking, and passes that plan seamlessly to the motor cortex, which controls the movements of the mouth.
- Blistering speed reflexes to click the handheld response buzzer within a fraction of a millisecond after the host finishes reading the clue (not before, or a penalty is incurred). Jeopardy! champions always click the buzzer faster than their competitors, who may know the answer but have ordinary motor reflexes (also related to the degree of myelination and a motoric component of processing speed).
- A thick corpus callosum, the largest interhemispheric commissure, a bundle of 200 million white matter fibers connecting analogous regions in the right and left hemispheres, is vital for the rapid bidirectional transfer of bits of information from the intuitive/nonverbal right hemisphere to the mathematical/verbal left hemisphere, when the answer requires right hemispheric input.
- A bright occipital cortex and exceptional optic nerve and retina, so that champions can recognize faces or locations and read the questions before the host finishes reading them, which gives them an awesome edge on other contestants.
Obviously, the brains of Jeopardy! champions are a breed of their own, with exceptional performances by multiple regions converging to produce a winning performance. But during their childhood and youthful years, such brains also generate motivation, curiosity, and interest in a wide range of topics, from cultures, regions, music genres, and word games to history, geography, sports, science, medicine, astronomy, and Greek mythology.
Jeopardy! champions may appear to have regular jobs and ordinary lives, but they have resplendent “renaissance” brains. I wonder how they spent their childhood, who mentored them, what type of family lives they had, and what they dream of accomplishing other than winning on Jeopardy!. Will their awe-inspiring performance in Jeopardy! translate to overall success in life? That’s a story that remains to be told.
Sexual activity alters the microbiome, with potential psychiatric implications
Evidence is strong that sexual partners transmit microbiota (bacteria, viruses, fungi, protozoa, and archaea) to each other. While microbial flora are abundant in the gastrointestinal tract, they are also present in the vagina, penis, urethra, mouth, and skin.1 For better or worse, sexual contact of all types means that participants will acquire each other’s microbiota.
The 39 trillion microbiota in the body (which exceed the 30 trillion cells in the body) are commensal and influence both the larger brain in the skull and the smaller enteric brain in the gut. The microbiota and their microbiome genes (1,000 times larger than the human genome) have been linked to depression, anxiety, psychosis, and autism.2-4 They produce 90% of the body’s serotonin, as well as catecholamines (norepinephrine, epinephrine, dopamine), make hormones (eg, cortisol), and modulate the immune system. Microbiota have several important functions, including food digestion, synthesis of vitamins, autoimmunity, hypothalamic-pituitary-adrenal axis regulation, and CNS modulation.
Consequences of dysbiosis
Everyone should be concerned about maintaining a healthy diversity of microbiota in their body, with a predominance of beneficial bacteria such as Lactobacillus and Bacteroides, and avoiding acquiring pathogenic bacteria such as Gardnerella, Prevotella, and Atopobium. Sexual activity involving a partner with unhealthy microbiota may increase the risk of dysbiosis, defined as a reduction in microbiota diversity, including a loss of beneficial bacteria and a rise in harmful bacteria.
Dysbiosis is associated with multiple symptoms, including5:
- brain “fog,” irritability, mood changes, and anxiety
- bloating, loss of intestinal permeability, and insufficient reclamation of nutrients
- congestion of certain organs, such as the liver, gallbladder, and pancreas
- production of antigen-antibody complexes in response to chemicals in partially digested food
- aggravation of inflammatory disorders such as migraine, arthritis, and autoimmune disorders.
Apart from intimate sexual contact, simply sharing a household with someone leads to sharing of gut microflora. Persons who live together, whether genetically related or not, have similar microbiota. Compared with people living in separate households, cohabiting human pairs, dog pairs, and human-dog pairs share most of their microbiota (especially in the skin).
A consequence of acquiring pathogenic microbiota in the vagina is bacterial vaginosis (BV), which is not an infection but an ecologic imbalance in the composition of the vaginal microbiota. BV is caused by a significant decline in the beneficial vaginal Lactobacillus and a marked increase in the non-Lactobacillus taxa (especially Gardnerella and Atopobium).6 It can last for a least 1 week after sexual intercourse. BV is rare or absent among virgins. For a male partner, penile microbiota changes significantly after unprotected sex.6
Pathogenic bacteria can be cultivated from the glans, the coronal sulcus, and the prepuce, as well as from the penile skin, semen, urethra, and urine.6 Diverse bacteria exist in human semen, regardless if the male is fertile or infertile.7Anaerococcus is a biomarker for low sperm quality. Many of the semen bacteria are also found in the vagina of women with BV.7 Semen is a medium for the transmission of bacteria and viruses between men and women, and can contribute to sexually transmitted diseases.8
There are approximately 21 million cases of BV in the United States each year, and BV can also increase the risk of HIV and poor obstetric outcomes.9 The microbiota in the penile skin and urethra in males who have monogamous relationships with females are very similar to the vaginal microbiota of their female partner.
Consequences of BV include:
- decrease in hydrogen peroxide–producing bacilli
- prevalence of anaerobic bacteria (Prevotella, Gardnerella, and Atopobium)
- alkalinization, fishy odor, and gray-white vaginal discharge
- increase in the rate of pelvic inflammatory disease, ectopic pregnancy, endometriosis, preterm birth, and tubal factor infertility.9
Circumcision decreases the risk of BV. There is an increased rate of BV bacterial taxa in men with extramarital affairs and in women with multiple partners. Both oral and vaginal sex increase the abundance of Lactobacillus in the male oral and penile microbiota. Gingivitis has also been reported after oral sex.10
A link to psychiatric disorders
Given that all forms of sexual contact (vaginal, oral, anal, or skin) can transmit microbiota bidirectionally between partners, it is vital to practice safe sex and consider a monogamous relationship rather than indiscriminate promiscuity. Unfortunately, certain psychiatric disorders, such as bipolar disorder, are associated with hypersexuality and multiple partners, which may disrupt the microbiota. This can further disrupt the diversity of an individual’s microbiome and may put them at risk for mood, anxiety, and other psychiatric disorders. Another problem is sexually transmitted infections such as gonorrhea or syphilis require antibiotic therapy. It is well established that antibiotics kill both the bad pathogenic and the good nonpathogenic microbiota, further exacerbating dysbiosis and leading to disruptions in the microbiota-gut-brain (MGB) axis, which then results in psychiatric disorders.
The MGB axis modulates neurological processes via the vagus nerve, the major “highway” connecting the gut and brain for bidirectional traffic. The MGB axis produces microbial metabolites and immune factors that can lead to changes in brain neurotransmitters as well as neuroinflammation and psychiatric symptoms such as depression and anxiety.5
Many researchers are focusing on how to exploit the microbiome to develop novel therapeutic strategies, and encouraging advances are emerging.5 But the exact mechanisms by which the gut microbiome can impact mental health is still a work in progress. It is highly likely that dysbiosis is associated with mood and anxiety symptoms.
The bottom line: Sexual activity—whether it is heavy kissing, vaginal intercourse, oral sex, anal sex, or extensive skin contact—can lead to the exchange of microbiota. If an individual has dysbiosis, that could impact the mental health of their sexual partner(s). This raises the question of whether counseling patients about avoiding indiscriminate sex and practicing safe sex is as important for mental health as diet and exercise counseling is for physical health.
1. Reid G, Younes JA, Van der Mei HC, et al. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol. 2011;9(1):27-38.
2. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701-712.
3. Peirce JM, Alviña K. The role of inflammation and the gut microbiome in depression and anxiety. J Neurosci Res. 2019;97(10):1223-1241.
4. Yolken R, Prandovszky E, Severance EG, et al. The oropharyngeal microbiome is altered in individuals with schizophrenia and mania. Schizophr Res. 2021;234:51-57.
5. Capuco A, Urits I, Hasoon J, et al. Current perspectives on gut microbiome dysbiosis and depression. Adv Ther. 2020;37(4):1328-1346.
6. Zozaya M, Ferris MJ, Siren JD, et al. Bacterial communities in penile skin, male urethra, and vagina of heterosexual couples with and without bacterial vaginosis. Microbiome. 2016;4:16. doi:10.1186/s40168-016-0161-6
7. Hou D, Zhou X, Zhong X, et al. Microbiota of the seminal fluid from healthy and infertile men. Fertil Steril. 2013;100(5):1261-1269.
8. Gallo MF, Warner L, King CC, et al. Association between semen exposure and incident bacterial vaginosis. Infect Dis Obstet Gynecol. 2011;2011:842652.
9. Liu CM, Hungate BA, Tobian AA, et al. Penile microbiota and female partner bacterial vaginosis in Rakai, Uganda. mBio. 2015;6(3):e00589. doi:10.1128/mBio.00589-15
10. Carda-Diéguez M, Cárdenas N, Aparicio M, et al. Variations in vaginal, penile, and oral microbiota after sexual intercourse: a case report. Front Med. 2019;6:178. doi:10.3389/fmed.2019.00178
Evidence is strong that sexual partners transmit microbiota (bacteria, viruses, fungi, protozoa, and archaea) to each other. While microbial flora are abundant in the gastrointestinal tract, they are also present in the vagina, penis, urethra, mouth, and skin.1 For better or worse, sexual contact of all types means that participants will acquire each other’s microbiota.
The 39 trillion microbiota in the body (which exceed the 30 trillion cells in the body) are commensal and influence both the larger brain in the skull and the smaller enteric brain in the gut. The microbiota and their microbiome genes (1,000 times larger than the human genome) have been linked to depression, anxiety, psychosis, and autism.2-4 They produce 90% of the body’s serotonin, as well as catecholamines (norepinephrine, epinephrine, dopamine), make hormones (eg, cortisol), and modulate the immune system. Microbiota have several important functions, including food digestion, synthesis of vitamins, autoimmunity, hypothalamic-pituitary-adrenal axis regulation, and CNS modulation.
Consequences of dysbiosis
Everyone should be concerned about maintaining a healthy diversity of microbiota in their body, with a predominance of beneficial bacteria such as Lactobacillus and Bacteroides, and avoiding acquiring pathogenic bacteria such as Gardnerella, Prevotella, and Atopobium. Sexual activity involving a partner with unhealthy microbiota may increase the risk of dysbiosis, defined as a reduction in microbiota diversity, including a loss of beneficial bacteria and a rise in harmful bacteria.
Dysbiosis is associated with multiple symptoms, including5:
- brain “fog,” irritability, mood changes, and anxiety
- bloating, loss of intestinal permeability, and insufficient reclamation of nutrients
- congestion of certain organs, such as the liver, gallbladder, and pancreas
- production of antigen-antibody complexes in response to chemicals in partially digested food
- aggravation of inflammatory disorders such as migraine, arthritis, and autoimmune disorders.
Apart from intimate sexual contact, simply sharing a household with someone leads to sharing of gut microflora. Persons who live together, whether genetically related or not, have similar microbiota. Compared with people living in separate households, cohabiting human pairs, dog pairs, and human-dog pairs share most of their microbiota (especially in the skin).
A consequence of acquiring pathogenic microbiota in the vagina is bacterial vaginosis (BV), which is not an infection but an ecologic imbalance in the composition of the vaginal microbiota. BV is caused by a significant decline in the beneficial vaginal Lactobacillus and a marked increase in the non-Lactobacillus taxa (especially Gardnerella and Atopobium).6 It can last for a least 1 week after sexual intercourse. BV is rare or absent among virgins. For a male partner, penile microbiota changes significantly after unprotected sex.6
Pathogenic bacteria can be cultivated from the glans, the coronal sulcus, and the prepuce, as well as from the penile skin, semen, urethra, and urine.6 Diverse bacteria exist in human semen, regardless if the male is fertile or infertile.7Anaerococcus is a biomarker for low sperm quality. Many of the semen bacteria are also found in the vagina of women with BV.7 Semen is a medium for the transmission of bacteria and viruses between men and women, and can contribute to sexually transmitted diseases.8
There are approximately 21 million cases of BV in the United States each year, and BV can also increase the risk of HIV and poor obstetric outcomes.9 The microbiota in the penile skin and urethra in males who have monogamous relationships with females are very similar to the vaginal microbiota of their female partner.
Consequences of BV include:
- decrease in hydrogen peroxide–producing bacilli
- prevalence of anaerobic bacteria (Prevotella, Gardnerella, and Atopobium)
- alkalinization, fishy odor, and gray-white vaginal discharge
- increase in the rate of pelvic inflammatory disease, ectopic pregnancy, endometriosis, preterm birth, and tubal factor infertility.9
Circumcision decreases the risk of BV. There is an increased rate of BV bacterial taxa in men with extramarital affairs and in women with multiple partners. Both oral and vaginal sex increase the abundance of Lactobacillus in the male oral and penile microbiota. Gingivitis has also been reported after oral sex.10
A link to psychiatric disorders
Given that all forms of sexual contact (vaginal, oral, anal, or skin) can transmit microbiota bidirectionally between partners, it is vital to practice safe sex and consider a monogamous relationship rather than indiscriminate promiscuity. Unfortunately, certain psychiatric disorders, such as bipolar disorder, are associated with hypersexuality and multiple partners, which may disrupt the microbiota. This can further disrupt the diversity of an individual’s microbiome and may put them at risk for mood, anxiety, and other psychiatric disorders. Another problem is sexually transmitted infections such as gonorrhea or syphilis require antibiotic therapy. It is well established that antibiotics kill both the bad pathogenic and the good nonpathogenic microbiota, further exacerbating dysbiosis and leading to disruptions in the microbiota-gut-brain (MGB) axis, which then results in psychiatric disorders.
The MGB axis modulates neurological processes via the vagus nerve, the major “highway” connecting the gut and brain for bidirectional traffic. The MGB axis produces microbial metabolites and immune factors that can lead to changes in brain neurotransmitters as well as neuroinflammation and psychiatric symptoms such as depression and anxiety.5
Many researchers are focusing on how to exploit the microbiome to develop novel therapeutic strategies, and encouraging advances are emerging.5 But the exact mechanisms by which the gut microbiome can impact mental health is still a work in progress. It is highly likely that dysbiosis is associated with mood and anxiety symptoms.
The bottom line: Sexual activity—whether it is heavy kissing, vaginal intercourse, oral sex, anal sex, or extensive skin contact—can lead to the exchange of microbiota. If an individual has dysbiosis, that could impact the mental health of their sexual partner(s). This raises the question of whether counseling patients about avoiding indiscriminate sex and practicing safe sex is as important for mental health as diet and exercise counseling is for physical health.
Evidence is strong that sexual partners transmit microbiota (bacteria, viruses, fungi, protozoa, and archaea) to each other. While microbial flora are abundant in the gastrointestinal tract, they are also present in the vagina, penis, urethra, mouth, and skin.1 For better or worse, sexual contact of all types means that participants will acquire each other’s microbiota.
The 39 trillion microbiota in the body (which exceed the 30 trillion cells in the body) are commensal and influence both the larger brain in the skull and the smaller enteric brain in the gut. The microbiota and their microbiome genes (1,000 times larger than the human genome) have been linked to depression, anxiety, psychosis, and autism.2-4 They produce 90% of the body’s serotonin, as well as catecholamines (norepinephrine, epinephrine, dopamine), make hormones (eg, cortisol), and modulate the immune system. Microbiota have several important functions, including food digestion, synthesis of vitamins, autoimmunity, hypothalamic-pituitary-adrenal axis regulation, and CNS modulation.
Consequences of dysbiosis
Everyone should be concerned about maintaining a healthy diversity of microbiota in their body, with a predominance of beneficial bacteria such as Lactobacillus and Bacteroides, and avoiding acquiring pathogenic bacteria such as Gardnerella, Prevotella, and Atopobium. Sexual activity involving a partner with unhealthy microbiota may increase the risk of dysbiosis, defined as a reduction in microbiota diversity, including a loss of beneficial bacteria and a rise in harmful bacteria.
Dysbiosis is associated with multiple symptoms, including5:
- brain “fog,” irritability, mood changes, and anxiety
- bloating, loss of intestinal permeability, and insufficient reclamation of nutrients
- congestion of certain organs, such as the liver, gallbladder, and pancreas
- production of antigen-antibody complexes in response to chemicals in partially digested food
- aggravation of inflammatory disorders such as migraine, arthritis, and autoimmune disorders.
Apart from intimate sexual contact, simply sharing a household with someone leads to sharing of gut microflora. Persons who live together, whether genetically related or not, have similar microbiota. Compared with people living in separate households, cohabiting human pairs, dog pairs, and human-dog pairs share most of their microbiota (especially in the skin).
A consequence of acquiring pathogenic microbiota in the vagina is bacterial vaginosis (BV), which is not an infection but an ecologic imbalance in the composition of the vaginal microbiota. BV is caused by a significant decline in the beneficial vaginal Lactobacillus and a marked increase in the non-Lactobacillus taxa (especially Gardnerella and Atopobium).6 It can last for a least 1 week after sexual intercourse. BV is rare or absent among virgins. For a male partner, penile microbiota changes significantly after unprotected sex.6
Pathogenic bacteria can be cultivated from the glans, the coronal sulcus, and the prepuce, as well as from the penile skin, semen, urethra, and urine.6 Diverse bacteria exist in human semen, regardless if the male is fertile or infertile.7Anaerococcus is a biomarker for low sperm quality. Many of the semen bacteria are also found in the vagina of women with BV.7 Semen is a medium for the transmission of bacteria and viruses between men and women, and can contribute to sexually transmitted diseases.8
There are approximately 21 million cases of BV in the United States each year, and BV can also increase the risk of HIV and poor obstetric outcomes.9 The microbiota in the penile skin and urethra in males who have monogamous relationships with females are very similar to the vaginal microbiota of their female partner.
Consequences of BV include:
- decrease in hydrogen peroxide–producing bacilli
- prevalence of anaerobic bacteria (Prevotella, Gardnerella, and Atopobium)
- alkalinization, fishy odor, and gray-white vaginal discharge
- increase in the rate of pelvic inflammatory disease, ectopic pregnancy, endometriosis, preterm birth, and tubal factor infertility.9
Circumcision decreases the risk of BV. There is an increased rate of BV bacterial taxa in men with extramarital affairs and in women with multiple partners. Both oral and vaginal sex increase the abundance of Lactobacillus in the male oral and penile microbiota. Gingivitis has also been reported after oral sex.10
A link to psychiatric disorders
Given that all forms of sexual contact (vaginal, oral, anal, or skin) can transmit microbiota bidirectionally between partners, it is vital to practice safe sex and consider a monogamous relationship rather than indiscriminate promiscuity. Unfortunately, certain psychiatric disorders, such as bipolar disorder, are associated with hypersexuality and multiple partners, which may disrupt the microbiota. This can further disrupt the diversity of an individual’s microbiome and may put them at risk for mood, anxiety, and other psychiatric disorders. Another problem is sexually transmitted infections such as gonorrhea or syphilis require antibiotic therapy. It is well established that antibiotics kill both the bad pathogenic and the good nonpathogenic microbiota, further exacerbating dysbiosis and leading to disruptions in the microbiota-gut-brain (MGB) axis, which then results in psychiatric disorders.
The MGB axis modulates neurological processes via the vagus nerve, the major “highway” connecting the gut and brain for bidirectional traffic. The MGB axis produces microbial metabolites and immune factors that can lead to changes in brain neurotransmitters as well as neuroinflammation and psychiatric symptoms such as depression and anxiety.5
Many researchers are focusing on how to exploit the microbiome to develop novel therapeutic strategies, and encouraging advances are emerging.5 But the exact mechanisms by which the gut microbiome can impact mental health is still a work in progress. It is highly likely that dysbiosis is associated with mood and anxiety symptoms.
The bottom line: Sexual activity—whether it is heavy kissing, vaginal intercourse, oral sex, anal sex, or extensive skin contact—can lead to the exchange of microbiota. If an individual has dysbiosis, that could impact the mental health of their sexual partner(s). This raises the question of whether counseling patients about avoiding indiscriminate sex and practicing safe sex is as important for mental health as diet and exercise counseling is for physical health.
1. Reid G, Younes JA, Van der Mei HC, et al. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol. 2011;9(1):27-38.
2. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701-712.
3. Peirce JM, Alviña K. The role of inflammation and the gut microbiome in depression and anxiety. J Neurosci Res. 2019;97(10):1223-1241.
4. Yolken R, Prandovszky E, Severance EG, et al. The oropharyngeal microbiome is altered in individuals with schizophrenia and mania. Schizophr Res. 2021;234:51-57.
5. Capuco A, Urits I, Hasoon J, et al. Current perspectives on gut microbiome dysbiosis and depression. Adv Ther. 2020;37(4):1328-1346.
6. Zozaya M, Ferris MJ, Siren JD, et al. Bacterial communities in penile skin, male urethra, and vagina of heterosexual couples with and without bacterial vaginosis. Microbiome. 2016;4:16. doi:10.1186/s40168-016-0161-6
7. Hou D, Zhou X, Zhong X, et al. Microbiota of the seminal fluid from healthy and infertile men. Fertil Steril. 2013;100(5):1261-1269.
8. Gallo MF, Warner L, King CC, et al. Association between semen exposure and incident bacterial vaginosis. Infect Dis Obstet Gynecol. 2011;2011:842652.
9. Liu CM, Hungate BA, Tobian AA, et al. Penile microbiota and female partner bacterial vaginosis in Rakai, Uganda. mBio. 2015;6(3):e00589. doi:10.1128/mBio.00589-15
10. Carda-Diéguez M, Cárdenas N, Aparicio M, et al. Variations in vaginal, penile, and oral microbiota after sexual intercourse: a case report. Front Med. 2019;6:178. doi:10.3389/fmed.2019.00178
1. Reid G, Younes JA, Van der Mei HC, et al. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol. 2011;9(1):27-38.
2. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701-712.
3. Peirce JM, Alviña K. The role of inflammation and the gut microbiome in depression and anxiety. J Neurosci Res. 2019;97(10):1223-1241.
4. Yolken R, Prandovszky E, Severance EG, et al. The oropharyngeal microbiome is altered in individuals with schizophrenia and mania. Schizophr Res. 2021;234:51-57.
5. Capuco A, Urits I, Hasoon J, et al. Current perspectives on gut microbiome dysbiosis and depression. Adv Ther. 2020;37(4):1328-1346.
6. Zozaya M, Ferris MJ, Siren JD, et al. Bacterial communities in penile skin, male urethra, and vagina of heterosexual couples with and without bacterial vaginosis. Microbiome. 2016;4:16. doi:10.1186/s40168-016-0161-6
7. Hou D, Zhou X, Zhong X, et al. Microbiota of the seminal fluid from healthy and infertile men. Fertil Steril. 2013;100(5):1261-1269.
8. Gallo MF, Warner L, King CC, et al. Association between semen exposure and incident bacterial vaginosis. Infect Dis Obstet Gynecol. 2011;2011:842652.
9. Liu CM, Hungate BA, Tobian AA, et al. Penile microbiota and female partner bacterial vaginosis in Rakai, Uganda. mBio. 2015;6(3):e00589. doi:10.1128/mBio.00589-15
10. Carda-Diéguez M, Cárdenas N, Aparicio M, et al. Variations in vaginal, penile, and oral microbiota after sexual intercourse: a case report. Front Med. 2019;6:178. doi:10.3389/fmed.2019.00178
Beyond diabetes: The beneficial uses of metformin in psychiatry
Metabolic dysregulation is quite common among psychiatric patients, especially those with psychotic or mood disorders. Obesity, diabetes, and dyslipidemia can be present at the onset of the illness, or as an iatrogenic complication. This often leads to premature mortality due to elevated cardiovascular and cerebrovascular risks.
Enter metformin. It is the most widely used hypoglycemic agent for type 2 diabetes (T2D), and it is frequently used by psychiatric clinicians. Discovered in 1922 and developed in France in the 1950s, metformin was approved for use in the United States in 1995, 3 decades after its launch in Europe. Its original trade name in the United States was Glucophage, and it is currently available from several companies in generic form. It is included on the World Health Organization list of essential medications.
T2D is currently an epidemic across the general populations globally, especially in the United States, where approximately 95% of the 37 million individuals with diabetes have been diagnosed with T2D.1 This is 300% higher than the prevalence in the 1970s. No wonder metformin is one of the most often-used drugs in all of medicine, and a staple in primary care and psychiatry. It has helped countless patients avoid the multisystem hazards of insulin resistance, which is the root cause of T2D.
Metformin exerts its hypoglycemic effects by:
- decreasing glucose production from the liver
- increasing insulin receptors’ sensitivity in various body tissues
- increasing secretion of growth differentiating factor, which reduces appetite and calorie intake.
In 2017, the American College of Physicians updated its guidelines to adopt metformin as the first-line treatment for T2D, especially because the class of sulfonylureas were associated with a more than 5-fold higher risk of severe low blood sugar events compared with metformin.2 In addition, metformin causes weight loss, while sulfonylureas are associated with weight gain. Metformin is particularly useful in gestational diabetes, where babies are born with less visceral fat and are less prone to insulin resistance later in life as adults.
The adverse effects of metformin are dose-related and mostly gastrointestinal (GI), including nausea, vomiting, cramps, diarrhea, and flatulence. Gradual titration or using the extended-release formulation can lower or avert GI discomfort. Metformin should not be used in patients with severe kidney or liver disease. With long-term use, metformin can cause malabsorption and eventual deficiency of vitamin B12.
The metabolic benefits of metformin listed below are why psychiatrists use it in clinical practice. However, this medication has several benefits that go beyond metabolic disorders. Clinicians should be aware of all of the following salutary physical and mental effects of metformin.
Metabolic benefits
- Decreasing glucose dysregulation with the use of clozapine and other antipsychotics.3
- Decreasing weight, body mass index, and waist circumference with the use of clozapine.4
- Decreasing triglycerides and total cholesterol.5
- Mitigating clozapine-induced obesity, especially if used prophylactically.6
- Lowering antipsychotic-induced weight gain.7
Continue on to: Nonmetabolic benefits...
Nonmetabolic benefits
- Lowering elevated serum prolactin levels to avert sexual dysfunction.8-10
- Increasing the production of neurons by inducing neurogenesis.11,12
- Activating the cerebral cortex to blunt the adverse effects of clozapine (such as deterioration of motivation, attention, cognition, and behavior) and increasing the activity of the dopamine D1 receptor, which is believed to be involved with cognition in schizophrenia.13
- Reducing the symptoms of anxiety and depression by increasing serotonin activity and hippocampal concentration of serotonin.14
- Decreasing the depressive symptoms known to be associated with uncontrolled diabetes.15
- Improving insulin resistance associated with polycystic ovary syndrome and helping with infertility.16
- Exerting multiple anti-aging effects (Table17). Metformin reduces several hallmarks of aging and may increase longevity.17
- Lowering the risks of cancer, dementia, and mortality in patients with and without diabetes18 due to its anti-aging effects. Scientists are actively studying metformin’s anti-aging effects and trying to develop drugs with similar effects.
- Counteracting inflammatory bowel disease, osteoporosis, neurodegeneration, inflammation, frailty, and senescence.19
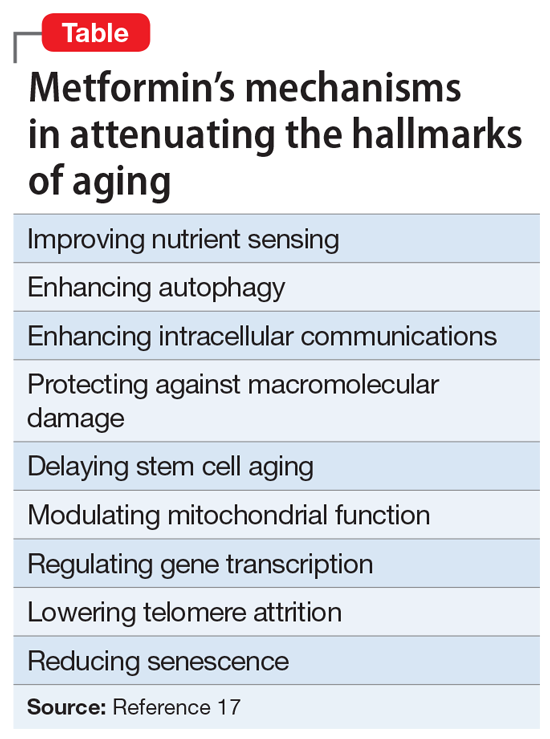
Metformin may sound like a wonder drug or panacea, but most of its multiple beneficial effects have been reported and replicated. Its therapeutic effects on obesity, diabetes, and dyslipidemia can prevent early mortality, but its anti-aging effects are also important and may help reduce premature mortality, which is common in psychiatric patients.20 So, the question arises: At some point, will metformin be used for persons not afflicted by diabetes or metabolic syndrome? For now, psychiatrists should continue to use it on label, but in the future, our patients may benefit from its “fringe benefits.”
1. Centers for Disease Control and Prevention. Type 2 diabetes. Accessed January 28, 2022. https://www.cdc.gov/diabetes/basics/type2.html
2. Qaseem A, Barry MJ, Humphrey LL, et al; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
3. Agarwal SM, Panda R, Costa-Dookhan KA, et al. Metformin for early comorbid glucose dysregulation and schizophrenia spectrum disorders: a pilot double-blind randomized clinical trial. Transl Psychiatry. 2021;11(1):219.
4. Hebrani P, Manteghi AA, Behdani F, et al. Double-blind, randomized, clinical trial of metformin as add-on treatment with clozapine in treatment of schizophrenia disorder. J Res Med Sci. 2015;20(4):364-371.
5. Jiang WL, Cai DB, Yin F, et al. Adjunctive metformin for antipsychotic-induced dyslipidemia: a meta-analysis of randomized, double-blind, placebo-controlled trials. Transl Psychiatry. 2020;10(1):117.
6. Siskind DJ, Leung J, Russell AW, et al. Metformin for clozapine associated obesity: a systematic review and meta-analysis. PLoS One. 2016;11(6):e0156208
7. de Silva VA, Suraweera C, Ratnatunga SS, et al. Metformin in prevention and treatment of antipsychotic induced weight gain: a systematic review and meta-analysis. BMC Psychiatry. 2016;16(1):341.
8. Zheng W, Yang XH, Cai DB, et al. Adjunctive metformin for antipsychotic-related hyperprolactinemia: a meta-analysis of randomized controlled trials. J Psychopharmacol. 2017;31(5):625-631.
9. Krysiak R, Kowalcze K, Szkrobka W, et al. The effect of metformin on prolactin levels in patients with drug-induced hyperprolactinemia. Eur J Intern Med. 2016;30:94-98.
10. Bo QJ, Wang ZM, Li XB, et al. Adjunctive metformin for antipsychotic-induced hyperprolactinemia: a systematic review. Psychiatry Res. 2016;237:257-263.
11. Wang J, Gallagher D, DeVito LM, et al. Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell. 2012;11(1):23-35.
12. Fatt M, Hsu K, He L, et al. Metformin acts on two different molecular pathways to enhance adult neural precursor proliferation/self-renewal and differentiation. Stem Cell Reports. 2015;5(6):988-995.
13. Horvath G, Kis G, Kekesi G, et al. Interaction of clozapine with metformin in a schizophrenia rat model. Sci Rep. 2021;11(1):16862.
14. Zemdegs J, Martin H, Pintana H, et al. Metformin promotes anxiolytic and antidepressant-like responses in insulin-resistant mice by decreasing circulating branched-chain amino acids. J Neurosci. 2019;39(30):5935-5948.
15. B˘adescu SV, T˘ataru C, Kobylinska L, et al. The association between diabetes mellitus and depression. J Med Life. 2016;9(2):120-125.
16. Erensoy H, Niafar M, Ghafarzadeh S, et al. A pilot trial of metformin for insulin resistance and mood disturbances in adolescent and adult women with polycystic ovary syndrome. Gynecol Endocrinol. 2019;35(1):72-75.
17. Kulkarni AS, Gubbi S, Barzilai N. Benefits of metformin in attenuating the hallmarks of aging. Cell Metab. 2020;32(1):15-30.
18. Campbell JM, Bellman SM, Stephenson MD, et al. Metformin reduces all-cause mortality and diseases of ageing independent of its effect on diabetes control: a systematic review and meta-analysis. Ageing Res Rev. 2017;40:31-44.
19. Ala M, Ala M. Metformin for cardiovascular protection, inflammatory bowel disease, osteoporosis, periodontitis, polycystic ovarian syndrome, neurodegeneration, cancer, inflammation and senescence: what is next? ACS Pharmacol Transl Sci. 2021;4(6):1747-1770.
20. Nasrallah HA. Premature mortality across most psychiatric disorders. Current Psychiatry. 2019;8(10):9-10,12,34.
Metabolic dysregulation is quite common among psychiatric patients, especially those with psychotic or mood disorders. Obesity, diabetes, and dyslipidemia can be present at the onset of the illness, or as an iatrogenic complication. This often leads to premature mortality due to elevated cardiovascular and cerebrovascular risks.
Enter metformin. It is the most widely used hypoglycemic agent for type 2 diabetes (T2D), and it is frequently used by psychiatric clinicians. Discovered in 1922 and developed in France in the 1950s, metformin was approved for use in the United States in 1995, 3 decades after its launch in Europe. Its original trade name in the United States was Glucophage, and it is currently available from several companies in generic form. It is included on the World Health Organization list of essential medications.
T2D is currently an epidemic across the general populations globally, especially in the United States, where approximately 95% of the 37 million individuals with diabetes have been diagnosed with T2D.1 This is 300% higher than the prevalence in the 1970s. No wonder metformin is one of the most often-used drugs in all of medicine, and a staple in primary care and psychiatry. It has helped countless patients avoid the multisystem hazards of insulin resistance, which is the root cause of T2D.
Metformin exerts its hypoglycemic effects by:
- decreasing glucose production from the liver
- increasing insulin receptors’ sensitivity in various body tissues
- increasing secretion of growth differentiating factor, which reduces appetite and calorie intake.
In 2017, the American College of Physicians updated its guidelines to adopt metformin as the first-line treatment for T2D, especially because the class of sulfonylureas were associated with a more than 5-fold higher risk of severe low blood sugar events compared with metformin.2 In addition, metformin causes weight loss, while sulfonylureas are associated with weight gain. Metformin is particularly useful in gestational diabetes, where babies are born with less visceral fat and are less prone to insulin resistance later in life as adults.
The adverse effects of metformin are dose-related and mostly gastrointestinal (GI), including nausea, vomiting, cramps, diarrhea, and flatulence. Gradual titration or using the extended-release formulation can lower or avert GI discomfort. Metformin should not be used in patients with severe kidney or liver disease. With long-term use, metformin can cause malabsorption and eventual deficiency of vitamin B12.
The metabolic benefits of metformin listed below are why psychiatrists use it in clinical practice. However, this medication has several benefits that go beyond metabolic disorders. Clinicians should be aware of all of the following salutary physical and mental effects of metformin.
Metabolic benefits
- Decreasing glucose dysregulation with the use of clozapine and other antipsychotics.3
- Decreasing weight, body mass index, and waist circumference with the use of clozapine.4
- Decreasing triglycerides and total cholesterol.5
- Mitigating clozapine-induced obesity, especially if used prophylactically.6
- Lowering antipsychotic-induced weight gain.7
Continue on to: Nonmetabolic benefits...
Nonmetabolic benefits
- Lowering elevated serum prolactin levels to avert sexual dysfunction.8-10
- Increasing the production of neurons by inducing neurogenesis.11,12
- Activating the cerebral cortex to blunt the adverse effects of clozapine (such as deterioration of motivation, attention, cognition, and behavior) and increasing the activity of the dopamine D1 receptor, which is believed to be involved with cognition in schizophrenia.13
- Reducing the symptoms of anxiety and depression by increasing serotonin activity and hippocampal concentration of serotonin.14
- Decreasing the depressive symptoms known to be associated with uncontrolled diabetes.15
- Improving insulin resistance associated with polycystic ovary syndrome and helping with infertility.16
- Exerting multiple anti-aging effects (Table17). Metformin reduces several hallmarks of aging and may increase longevity.17
- Lowering the risks of cancer, dementia, and mortality in patients with and without diabetes18 due to its anti-aging effects. Scientists are actively studying metformin’s anti-aging effects and trying to develop drugs with similar effects.
- Counteracting inflammatory bowel disease, osteoporosis, neurodegeneration, inflammation, frailty, and senescence.19

Metformin may sound like a wonder drug or panacea, but most of its multiple beneficial effects have been reported and replicated. Its therapeutic effects on obesity, diabetes, and dyslipidemia can prevent early mortality, but its anti-aging effects are also important and may help reduce premature mortality, which is common in psychiatric patients.20 So, the question arises: At some point, will metformin be used for persons not afflicted by diabetes or metabolic syndrome? For now, psychiatrists should continue to use it on label, but in the future, our patients may benefit from its “fringe benefits.”
Metabolic dysregulation is quite common among psychiatric patients, especially those with psychotic or mood disorders. Obesity, diabetes, and dyslipidemia can be present at the onset of the illness, or as an iatrogenic complication. This often leads to premature mortality due to elevated cardiovascular and cerebrovascular risks.
Enter metformin. It is the most widely used hypoglycemic agent for type 2 diabetes (T2D), and it is frequently used by psychiatric clinicians. Discovered in 1922 and developed in France in the 1950s, metformin was approved for use in the United States in 1995, 3 decades after its launch in Europe. Its original trade name in the United States was Glucophage, and it is currently available from several companies in generic form. It is included on the World Health Organization list of essential medications.
T2D is currently an epidemic across the general populations globally, especially in the United States, where approximately 95% of the 37 million individuals with diabetes have been diagnosed with T2D.1 This is 300% higher than the prevalence in the 1970s. No wonder metformin is one of the most often-used drugs in all of medicine, and a staple in primary care and psychiatry. It has helped countless patients avoid the multisystem hazards of insulin resistance, which is the root cause of T2D.
Metformin exerts its hypoglycemic effects by:
- decreasing glucose production from the liver
- increasing insulin receptors’ sensitivity in various body tissues
- increasing secretion of growth differentiating factor, which reduces appetite and calorie intake.
In 2017, the American College of Physicians updated its guidelines to adopt metformin as the first-line treatment for T2D, especially because the class of sulfonylureas were associated with a more than 5-fold higher risk of severe low blood sugar events compared with metformin.2 In addition, metformin causes weight loss, while sulfonylureas are associated with weight gain. Metformin is particularly useful in gestational diabetes, where babies are born with less visceral fat and are less prone to insulin resistance later in life as adults.
The adverse effects of metformin are dose-related and mostly gastrointestinal (GI), including nausea, vomiting, cramps, diarrhea, and flatulence. Gradual titration or using the extended-release formulation can lower or avert GI discomfort. Metformin should not be used in patients with severe kidney or liver disease. With long-term use, metformin can cause malabsorption and eventual deficiency of vitamin B12.
The metabolic benefits of metformin listed below are why psychiatrists use it in clinical practice. However, this medication has several benefits that go beyond metabolic disorders. Clinicians should be aware of all of the following salutary physical and mental effects of metformin.
Metabolic benefits
- Decreasing glucose dysregulation with the use of clozapine and other antipsychotics.3
- Decreasing weight, body mass index, and waist circumference with the use of clozapine.4
- Decreasing triglycerides and total cholesterol.5
- Mitigating clozapine-induced obesity, especially if used prophylactically.6
- Lowering antipsychotic-induced weight gain.7
Continue on to: Nonmetabolic benefits...
Nonmetabolic benefits
- Lowering elevated serum prolactin levels to avert sexual dysfunction.8-10
- Increasing the production of neurons by inducing neurogenesis.11,12
- Activating the cerebral cortex to blunt the adverse effects of clozapine (such as deterioration of motivation, attention, cognition, and behavior) and increasing the activity of the dopamine D1 receptor, which is believed to be involved with cognition in schizophrenia.13
- Reducing the symptoms of anxiety and depression by increasing serotonin activity and hippocampal concentration of serotonin.14
- Decreasing the depressive symptoms known to be associated with uncontrolled diabetes.15
- Improving insulin resistance associated with polycystic ovary syndrome and helping with infertility.16
- Exerting multiple anti-aging effects (Table17). Metformin reduces several hallmarks of aging and may increase longevity.17
- Lowering the risks of cancer, dementia, and mortality in patients with and without diabetes18 due to its anti-aging effects. Scientists are actively studying metformin’s anti-aging effects and trying to develop drugs with similar effects.
- Counteracting inflammatory bowel disease, osteoporosis, neurodegeneration, inflammation, frailty, and senescence.19

Metformin may sound like a wonder drug or panacea, but most of its multiple beneficial effects have been reported and replicated. Its therapeutic effects on obesity, diabetes, and dyslipidemia can prevent early mortality, but its anti-aging effects are also important and may help reduce premature mortality, which is common in psychiatric patients.20 So, the question arises: At some point, will metformin be used for persons not afflicted by diabetes or metabolic syndrome? For now, psychiatrists should continue to use it on label, but in the future, our patients may benefit from its “fringe benefits.”
1. Centers for Disease Control and Prevention. Type 2 diabetes. Accessed January 28, 2022. https://www.cdc.gov/diabetes/basics/type2.html
2. Qaseem A, Barry MJ, Humphrey LL, et al; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
3. Agarwal SM, Panda R, Costa-Dookhan KA, et al. Metformin for early comorbid glucose dysregulation and schizophrenia spectrum disorders: a pilot double-blind randomized clinical trial. Transl Psychiatry. 2021;11(1):219.
4. Hebrani P, Manteghi AA, Behdani F, et al. Double-blind, randomized, clinical trial of metformin as add-on treatment with clozapine in treatment of schizophrenia disorder. J Res Med Sci. 2015;20(4):364-371.
5. Jiang WL, Cai DB, Yin F, et al. Adjunctive metformin for antipsychotic-induced dyslipidemia: a meta-analysis of randomized, double-blind, placebo-controlled trials. Transl Psychiatry. 2020;10(1):117.
6. Siskind DJ, Leung J, Russell AW, et al. Metformin for clozapine associated obesity: a systematic review and meta-analysis. PLoS One. 2016;11(6):e0156208
7. de Silva VA, Suraweera C, Ratnatunga SS, et al. Metformin in prevention and treatment of antipsychotic induced weight gain: a systematic review and meta-analysis. BMC Psychiatry. 2016;16(1):341.
8. Zheng W, Yang XH, Cai DB, et al. Adjunctive metformin for antipsychotic-related hyperprolactinemia: a meta-analysis of randomized controlled trials. J Psychopharmacol. 2017;31(5):625-631.
9. Krysiak R, Kowalcze K, Szkrobka W, et al. The effect of metformin on prolactin levels in patients with drug-induced hyperprolactinemia. Eur J Intern Med. 2016;30:94-98.
10. Bo QJ, Wang ZM, Li XB, et al. Adjunctive metformin for antipsychotic-induced hyperprolactinemia: a systematic review. Psychiatry Res. 2016;237:257-263.
11. Wang J, Gallagher D, DeVito LM, et al. Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell. 2012;11(1):23-35.
12. Fatt M, Hsu K, He L, et al. Metformin acts on two different molecular pathways to enhance adult neural precursor proliferation/self-renewal and differentiation. Stem Cell Reports. 2015;5(6):988-995.
13. Horvath G, Kis G, Kekesi G, et al. Interaction of clozapine with metformin in a schizophrenia rat model. Sci Rep. 2021;11(1):16862.
14. Zemdegs J, Martin H, Pintana H, et al. Metformin promotes anxiolytic and antidepressant-like responses in insulin-resistant mice by decreasing circulating branched-chain amino acids. J Neurosci. 2019;39(30):5935-5948.
15. B˘adescu SV, T˘ataru C, Kobylinska L, et al. The association between diabetes mellitus and depression. J Med Life. 2016;9(2):120-125.
16. Erensoy H, Niafar M, Ghafarzadeh S, et al. A pilot trial of metformin for insulin resistance and mood disturbances in adolescent and adult women with polycystic ovary syndrome. Gynecol Endocrinol. 2019;35(1):72-75.
17. Kulkarni AS, Gubbi S, Barzilai N. Benefits of metformin in attenuating the hallmarks of aging. Cell Metab. 2020;32(1):15-30.
18. Campbell JM, Bellman SM, Stephenson MD, et al. Metformin reduces all-cause mortality and diseases of ageing independent of its effect on diabetes control: a systematic review and meta-analysis. Ageing Res Rev. 2017;40:31-44.
19. Ala M, Ala M. Metformin for cardiovascular protection, inflammatory bowel disease, osteoporosis, periodontitis, polycystic ovarian syndrome, neurodegeneration, cancer, inflammation and senescence: what is next? ACS Pharmacol Transl Sci. 2021;4(6):1747-1770.
20. Nasrallah HA. Premature mortality across most psychiatric disorders. Current Psychiatry. 2019;8(10):9-10,12,34.
1. Centers for Disease Control and Prevention. Type 2 diabetes. Accessed January 28, 2022. https://www.cdc.gov/diabetes/basics/type2.html
2. Qaseem A, Barry MJ, Humphrey LL, et al; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
3. Agarwal SM, Panda R, Costa-Dookhan KA, et al. Metformin for early comorbid glucose dysregulation and schizophrenia spectrum disorders: a pilot double-blind randomized clinical trial. Transl Psychiatry. 2021;11(1):219.
4. Hebrani P, Manteghi AA, Behdani F, et al. Double-blind, randomized, clinical trial of metformin as add-on treatment with clozapine in treatment of schizophrenia disorder. J Res Med Sci. 2015;20(4):364-371.
5. Jiang WL, Cai DB, Yin F, et al. Adjunctive metformin for antipsychotic-induced dyslipidemia: a meta-analysis of randomized, double-blind, placebo-controlled trials. Transl Psychiatry. 2020;10(1):117.
6. Siskind DJ, Leung J, Russell AW, et al. Metformin for clozapine associated obesity: a systematic review and meta-analysis. PLoS One. 2016;11(6):e0156208
7. de Silva VA, Suraweera C, Ratnatunga SS, et al. Metformin in prevention and treatment of antipsychotic induced weight gain: a systematic review and meta-analysis. BMC Psychiatry. 2016;16(1):341.
8. Zheng W, Yang XH, Cai DB, et al. Adjunctive metformin for antipsychotic-related hyperprolactinemia: a meta-analysis of randomized controlled trials. J Psychopharmacol. 2017;31(5):625-631.
9. Krysiak R, Kowalcze K, Szkrobka W, et al. The effect of metformin on prolactin levels in patients with drug-induced hyperprolactinemia. Eur J Intern Med. 2016;30:94-98.
10. Bo QJ, Wang ZM, Li XB, et al. Adjunctive metformin for antipsychotic-induced hyperprolactinemia: a systematic review. Psychiatry Res. 2016;237:257-263.
11. Wang J, Gallagher D, DeVito LM, et al. Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell. 2012;11(1):23-35.
12. Fatt M, Hsu K, He L, et al. Metformin acts on two different molecular pathways to enhance adult neural precursor proliferation/self-renewal and differentiation. Stem Cell Reports. 2015;5(6):988-995.
13. Horvath G, Kis G, Kekesi G, et al. Interaction of clozapine with metformin in a schizophrenia rat model. Sci Rep. 2021;11(1):16862.
14. Zemdegs J, Martin H, Pintana H, et al. Metformin promotes anxiolytic and antidepressant-like responses in insulin-resistant mice by decreasing circulating branched-chain amino acids. J Neurosci. 2019;39(30):5935-5948.
15. B˘adescu SV, T˘ataru C, Kobylinska L, et al. The association between diabetes mellitus and depression. J Med Life. 2016;9(2):120-125.
16. Erensoy H, Niafar M, Ghafarzadeh S, et al. A pilot trial of metformin for insulin resistance and mood disturbances in adolescent and adult women with polycystic ovary syndrome. Gynecol Endocrinol. 2019;35(1):72-75.
17. Kulkarni AS, Gubbi S, Barzilai N. Benefits of metformin in attenuating the hallmarks of aging. Cell Metab. 2020;32(1):15-30.
18. Campbell JM, Bellman SM, Stephenson MD, et al. Metformin reduces all-cause mortality and diseases of ageing independent of its effect on diabetes control: a systematic review and meta-analysis. Ageing Res Rev. 2017;40:31-44.
19. Ala M, Ala M. Metformin for cardiovascular protection, inflammatory bowel disease, osteoporosis, periodontitis, polycystic ovarian syndrome, neurodegeneration, cancer, inflammation and senescence: what is next? ACS Pharmacol Transl Sci. 2021;4(6):1747-1770.
20. Nasrallah HA. Premature mortality across most psychiatric disorders. Current Psychiatry. 2019;8(10):9-10,12,34.
Comments & Controversies
A broken system
I was relieved to see your article “I have a dream … for psychiatry” (From the Editor,
Psychiatry does need better treatments. On the other hand, we do have many effective treatments that simply are not available to many.
This brings me to ask, how is it that overall psychiatric care is actually worse now than in, say, the late 20th century? There might have been fewer psychopharmacologic treatments available back then, but there was overall better access to care, and a largely intact system. For lower-functioning patients, such as those who are homeless or in jail, I do believe this is the case, as I will explain. But even higher-functioning private practice patients are affected by the shortage of psychiatrists.
In 2022, the system is broken. Funding is abysmal, and numerous psychiatric hospital closures across the United States have led to simply no reasonable local access for many.
Prisons and jails are the new treatment centers! As you have rightly pointed out, by being housed in prisons with violent criminals, severely mentally ill patients are subjected to physical and sexual abuse daily.
Laws meant to protect mentally ill individuals, such as psychiatric holds, are often not implemented. Severely mentally ill patients can meet the criteria to be categorized as a danger to self, danger to others, or gravely disabled, but can’t get crisis intervention. Abandoning these patients to the streets is, in part, fueling homelessness and drug addiction.
In my opinion, the broken system is the fundamental problem that needs to be solved. Although I long for novel treatments, if there were such breakthrough treatments available—as exciting as that may be—how could they be delivered effectively in our current broken system? In other words, how can these patients be treated with neuroscientific breakthrough treatments without the necessary psychiatric infrastructure? We are at such an extreme, I worry for our specialty.
In Karl Menninger’s The Crime of Punishment, one passage stuck with me: “I suspect that all the crimes committed by all the jailed criminals do not equal in total social damage that of the crimes committed against them.”1 I have often wondered how that relates to the criminalized mentally ill, who are punished daily by being in horrific, abusive, unsafe settings. What truly is their crime? Being mentally ill?
Given how the system is now engineered to throw these patients in prison and allow them to be abused instead of admitting them to a psychiatric hospital, one must wonder: How did this come to be? Could it go beyond stigma to actual hatred and contempt for these people? After all, as psychiatrists, the abuse is in plain sight.
Finally, I have often wondered why there has not been a robust psychiatric organizational response to the breakdown in access to patient care. I can only hope that one day there can be.
Dr. Nasrallah responds
Thank you for your comments on my editorial. I sense that you are quite frustrated with the current status of psychiatry, and are longing for improvements.
I do share some of your concerns about: 1) society turning a blind eye to the mentally ill (and I have written about that from the angle of tragically high suicide rate1); 2) the hatred and contempt embedded within stigma of serious mental disorders; 3) the deplorable criminalization and trans-institutionalization of our patients from state hospitals to jails and prisons; 4) the shortage of acute psychiatric beds in many communities because the wards were converted to highly lucrative, procedure-oriented programs; 5) the dysfunctional public mental health system; and 6) the need for new and novel treatments.
However, despite those challenges, I remain optimistic that the future of psychiatry is bright because I keep abreast of the stunning neuroscience advances every day that will be translated into psychiatric treatments in the future. I envision a time when these brain research breakthroughs will lead to important clinical applications, such as a better diagnostic system using biomarkers (precision psychiatry), not just a cluster of clinical symptoms, and to brave new therapeutic interventions with superior efficacy and better safety. I would not be surprised if psychiatry and neurology will again merge after decades of separation, and that will certainly erase much of the stigma of disorders of the mind, which is the virtual brain.
Please hang in there, and do not let your patients perceive a sense of resignation and pessimism about psychiatry. Both our patients and psychiatrists need to be uplifted by hope for a better future.
1. Menninger K. The Crime of Punishment. Viking Adult; 1968.
2. Nasrallah HA. The scourge of societal anosognosia about the mentally ill. Current Psychiatry. 2016;15(6):19,23-24.
A broken system
I was relieved to see your article “I have a dream … for psychiatry” (From the Editor,
Psychiatry does need better treatments. On the other hand, we do have many effective treatments that simply are not available to many.
This brings me to ask, how is it that overall psychiatric care is actually worse now than in, say, the late 20th century? There might have been fewer psychopharmacologic treatments available back then, but there was overall better access to care, and a largely intact system. For lower-functioning patients, such as those who are homeless or in jail, I do believe this is the case, as I will explain. But even higher-functioning private practice patients are affected by the shortage of psychiatrists.
In 2022, the system is broken. Funding is abysmal, and numerous psychiatric hospital closures across the United States have led to simply no reasonable local access for many.
Prisons and jails are the new treatment centers! As you have rightly pointed out, by being housed in prisons with violent criminals, severely mentally ill patients are subjected to physical and sexual abuse daily.
Laws meant to protect mentally ill individuals, such as psychiatric holds, are often not implemented. Severely mentally ill patients can meet the criteria to be categorized as a danger to self, danger to others, or gravely disabled, but can’t get crisis intervention. Abandoning these patients to the streets is, in part, fueling homelessness and drug addiction.
In my opinion, the broken system is the fundamental problem that needs to be solved. Although I long for novel treatments, if there were such breakthrough treatments available—as exciting as that may be—how could they be delivered effectively in our current broken system? In other words, how can these patients be treated with neuroscientific breakthrough treatments without the necessary psychiatric infrastructure? We are at such an extreme, I worry for our specialty.
In Karl Menninger’s The Crime of Punishment, one passage stuck with me: “I suspect that all the crimes committed by all the jailed criminals do not equal in total social damage that of the crimes committed against them.”1 I have often wondered how that relates to the criminalized mentally ill, who are punished daily by being in horrific, abusive, unsafe settings. What truly is their crime? Being mentally ill?
Given how the system is now engineered to throw these patients in prison and allow them to be abused instead of admitting them to a psychiatric hospital, one must wonder: How did this come to be? Could it go beyond stigma to actual hatred and contempt for these people? After all, as psychiatrists, the abuse is in plain sight.
Finally, I have often wondered why there has not been a robust psychiatric organizational response to the breakdown in access to patient care. I can only hope that one day there can be.
Dr. Nasrallah responds
Thank you for your comments on my editorial. I sense that you are quite frustrated with the current status of psychiatry, and are longing for improvements.
I do share some of your concerns about: 1) society turning a blind eye to the mentally ill (and I have written about that from the angle of tragically high suicide rate1); 2) the hatred and contempt embedded within stigma of serious mental disorders; 3) the deplorable criminalization and trans-institutionalization of our patients from state hospitals to jails and prisons; 4) the shortage of acute psychiatric beds in many communities because the wards were converted to highly lucrative, procedure-oriented programs; 5) the dysfunctional public mental health system; and 6) the need for new and novel treatments.
However, despite those challenges, I remain optimistic that the future of psychiatry is bright because I keep abreast of the stunning neuroscience advances every day that will be translated into psychiatric treatments in the future. I envision a time when these brain research breakthroughs will lead to important clinical applications, such as a better diagnostic system using biomarkers (precision psychiatry), not just a cluster of clinical symptoms, and to brave new therapeutic interventions with superior efficacy and better safety. I would not be surprised if psychiatry and neurology will again merge after decades of separation, and that will certainly erase much of the stigma of disorders of the mind, which is the virtual brain.
Please hang in there, and do not let your patients perceive a sense of resignation and pessimism about psychiatry. Both our patients and psychiatrists need to be uplifted by hope for a better future.
A broken system
I was relieved to see your article “I have a dream … for psychiatry” (From the Editor,
Psychiatry does need better treatments. On the other hand, we do have many effective treatments that simply are not available to many.
This brings me to ask, how is it that overall psychiatric care is actually worse now than in, say, the late 20th century? There might have been fewer psychopharmacologic treatments available back then, but there was overall better access to care, and a largely intact system. For lower-functioning patients, such as those who are homeless or in jail, I do believe this is the case, as I will explain. But even higher-functioning private practice patients are affected by the shortage of psychiatrists.
In 2022, the system is broken. Funding is abysmal, and numerous psychiatric hospital closures across the United States have led to simply no reasonable local access for many.
Prisons and jails are the new treatment centers! As you have rightly pointed out, by being housed in prisons with violent criminals, severely mentally ill patients are subjected to physical and sexual abuse daily.
Laws meant to protect mentally ill individuals, such as psychiatric holds, are often not implemented. Severely mentally ill patients can meet the criteria to be categorized as a danger to self, danger to others, or gravely disabled, but can’t get crisis intervention. Abandoning these patients to the streets is, in part, fueling homelessness and drug addiction.
In my opinion, the broken system is the fundamental problem that needs to be solved. Although I long for novel treatments, if there were such breakthrough treatments available—as exciting as that may be—how could they be delivered effectively in our current broken system? In other words, how can these patients be treated with neuroscientific breakthrough treatments without the necessary psychiatric infrastructure? We are at such an extreme, I worry for our specialty.
In Karl Menninger’s The Crime of Punishment, one passage stuck with me: “I suspect that all the crimes committed by all the jailed criminals do not equal in total social damage that of the crimes committed against them.”1 I have often wondered how that relates to the criminalized mentally ill, who are punished daily by being in horrific, abusive, unsafe settings. What truly is their crime? Being mentally ill?
Given how the system is now engineered to throw these patients in prison and allow them to be abused instead of admitting them to a psychiatric hospital, one must wonder: How did this come to be? Could it go beyond stigma to actual hatred and contempt for these people? After all, as psychiatrists, the abuse is in plain sight.
Finally, I have often wondered why there has not been a robust psychiatric organizational response to the breakdown in access to patient care. I can only hope that one day there can be.
Dr. Nasrallah responds
Thank you for your comments on my editorial. I sense that you are quite frustrated with the current status of psychiatry, and are longing for improvements.
I do share some of your concerns about: 1) society turning a blind eye to the mentally ill (and I have written about that from the angle of tragically high suicide rate1); 2) the hatred and contempt embedded within stigma of serious mental disorders; 3) the deplorable criminalization and trans-institutionalization of our patients from state hospitals to jails and prisons; 4) the shortage of acute psychiatric beds in many communities because the wards were converted to highly lucrative, procedure-oriented programs; 5) the dysfunctional public mental health system; and 6) the need for new and novel treatments.
However, despite those challenges, I remain optimistic that the future of psychiatry is bright because I keep abreast of the stunning neuroscience advances every day that will be translated into psychiatric treatments in the future. I envision a time when these brain research breakthroughs will lead to important clinical applications, such as a better diagnostic system using biomarkers (precision psychiatry), not just a cluster of clinical symptoms, and to brave new therapeutic interventions with superior efficacy and better safety. I would not be surprised if psychiatry and neurology will again merge after decades of separation, and that will certainly erase much of the stigma of disorders of the mind, which is the virtual brain.
Please hang in there, and do not let your patients perceive a sense of resignation and pessimism about psychiatry. Both our patients and psychiatrists need to be uplifted by hope for a better future.
1. Menninger K. The Crime of Punishment. Viking Adult; 1968.
2. Nasrallah HA. The scourge of societal anosognosia about the mentally ill. Current Psychiatry. 2016;15(6):19,23-24.
1. Menninger K. The Crime of Punishment. Viking Adult; 1968.
2. Nasrallah HA. The scourge of societal anosognosia about the mentally ill. Current Psychiatry. 2016;15(6):19,23-24.
It’s time for moonshot thinking in psychiatry
“I believe that this nation should commit itself to achieving the goal, before the decade is out, of landing a man on the Moon and returning him safely to Earth.”
President John F. Kennedy, May 25, 1961
Despite significant progress, there remain many unmet needs in psychiatry. These include a granular understanding of the neurobiology of various psychopathologies, an objective and valid diagnostic schema, and disease-modifying treatments for chronic and disabling psychiatric disorders. Several moonshots are needed to address those festering needs.
A “moonshot” is an extremely ambitious, dramatic, imaginative, and inspiring goal. Landing on the Moon was generally believed to be impossible when President Kennedy boldly set that as a goal for the United States in 1961. Yet, 8 short years later, on July 20, 1969, Neil Armstrong stepped off the lunar module ladder onto the Moon’s surface, a feat that captured the imagination of the nation and the world. I distinctly remember watching it on television with amazement as a young boy. It was a surreal experience. That’s what achieving a moonshot feels like.
Successful organizations should always have 1 or more moonshots (American Psychiatric Association and National Institute of Mental Health [NIMH], are you listening?). Setting lofty goals that require monumental determination and effort to accomplish will have a transformative, long-lasting impact. The construction of the Panama Canal to connect 2 oceans and the Manhattan Project to develop the first nuclear bomb, which ended World War II, are examples of moonshots that continue to reverberate. A more recent moonshot is the driverless car, which in the past was a laughable idea but is now a reality that will change society and the world in many ways. Innovative billionaire moguls now speak loudly about colonizing Mars, which sounds improbable and highly risky, but it’s a moonshot that may be achieved within a few years. Establishing world peace is a moonshot that requires collective Kennedy-esque vision and motivation among world leaders, which currently is sadly lacking.
So, for contemporary psychiatry, what is the equivalent of landing on the Moon? Here is the list that pops in my brain’s mind (let us know which of these would be your top 3 moonshots by taking our survey at https://bit.ly/3qkKqTa):
- A cure for schizophrenia (across positive, negative, and cognitive symptom domains)
- A cure for mood disorders, unipolar and bipolar (including suicide)
- A cure for anxiety disorders
- A cure for obsessive-compulsive disorder
- A cure for posttraumatic stress disorder
- A cure for alcoholism/addiction
- A cure for autism
- A cure for Alzheimer’s disease and other dementias
- A cure for personality disorders, especially antisocial and borderline
- A cure for the visceral hatred across political parties that permeates our society (obviously not a psychiatric category, but perhaps it should be added to DSM because it is so destructive).
Those moonshots may be regarded as absurd, and totally unachievable, but so was landing on the Moon, until it was accomplished. Psychiatry must stop thinking small and being content with tiny advances (which is like changing the chairs to more comfortable sofas on the deck of the Titanic and calling it “progress…”). Psychiatry needs to be unified under the flag of “moonshot thinking” by several visionary and transformative leaders to start believing in a miraculously better future for our patients. But to pave the way for moonshots in psychiatry, the leading organizations must collaborate closely to open the door for unprecedented scientific and medical breakthroughs of a moonshot by:
- Lobbying effectively to secure massive funding for research from federal, state, corporate, and foundation sources (perhaps convincing the Gates Foundation that schizophrenia is as devastating worldwide as malaria may bring a few badly needed billions into psychiatric brain research).
- Reminding members of Congress that in the United States, costs associated with psychiatric brain disorders total an estimated $700 billion annually,1 and that this must be addressed by boosting the meager NIMH budget by at least an order of magnitude. The NIMH should disproportionately invest its resources on severe brain disorders such as schizophrenia because breakthrough advances in its neurobiology will provide unprecedented insights to the pathophysiology of other severe psychiatric brain disorders.
- Partnering intimately with the pharmaceutical industry in a powerful public-private coalition to exploit the extensive research infrastructure of this industry.
- Creating the necessary army of researchers (physician-scientists) by providing huge incentives to medical students and psychiatric residents to pursue careers in neuroscience research. Incentives can include paying for an individual’s entire medical education and research training, and providing generous salaries that match or exceed the income of a very successful clinical practice.
- Convincing all psychiatric clinicians to support research by referring patients to research projects. Clinical psychiatrists are badly needed to care for the population, but they must be reminded that every treatment they are using today was a research project in the past, and that the research of today will evolve into the treatments (or cures) of tomorrow.
Pursuing lofty moonshots via innovative research is very likely to enhance serendipity and lead to unexpected discoveries along the way. As Louis Pasteur said, “chance only favors the prepared mind.”2 Moonshot thinking in psychiatry today is more feasible than ever before because of the many advances in research methods (neuroimaging, pluripotent cells, optogenetics, CRISPR, etc) and complex data management technologies (big data, machine learning, artificial intelligence), each of which qualifies as a preparatory moonshot in its own right.
Given the tragic consequences of psychiatric brain disorders, it is imperative that we “think big.” Humanity expects us to do that. We must envision the future of psychiatry as dramatically different from the present. Moonshot thinking is the indispensable vehicle to take us there.
1. Discovery Mood and Anxiety Program. The rising cost of mental health and substance abuse in the United States. Accessed January 13, 2022. https://discoverymood.com/blog/cost-of-mental-health-increase/
2. Wikiquote. Louis Pasteur. Accessed January 10, 2022. https://en.wikiquote.org/wiki/Louis_Pasteur
“I believe that this nation should commit itself to achieving the goal, before the decade is out, of landing a man on the Moon and returning him safely to Earth.”
President John F. Kennedy, May 25, 1961
Despite significant progress, there remain many unmet needs in psychiatry. These include a granular understanding of the neurobiology of various psychopathologies, an objective and valid diagnostic schema, and disease-modifying treatments for chronic and disabling psychiatric disorders. Several moonshots are needed to address those festering needs.
A “moonshot” is an extremely ambitious, dramatic, imaginative, and inspiring goal. Landing on the Moon was generally believed to be impossible when President Kennedy boldly set that as a goal for the United States in 1961. Yet, 8 short years later, on July 20, 1969, Neil Armstrong stepped off the lunar module ladder onto the Moon’s surface, a feat that captured the imagination of the nation and the world. I distinctly remember watching it on television with amazement as a young boy. It was a surreal experience. That’s what achieving a moonshot feels like.
Successful organizations should always have 1 or more moonshots (American Psychiatric Association and National Institute of Mental Health [NIMH], are you listening?). Setting lofty goals that require monumental determination and effort to accomplish will have a transformative, long-lasting impact. The construction of the Panama Canal to connect 2 oceans and the Manhattan Project to develop the first nuclear bomb, which ended World War II, are examples of moonshots that continue to reverberate. A more recent moonshot is the driverless car, which in the past was a laughable idea but is now a reality that will change society and the world in many ways. Innovative billionaire moguls now speak loudly about colonizing Mars, which sounds improbable and highly risky, but it’s a moonshot that may be achieved within a few years. Establishing world peace is a moonshot that requires collective Kennedy-esque vision and motivation among world leaders, which currently is sadly lacking.
So, for contemporary psychiatry, what is the equivalent of landing on the Moon? Here is the list that pops in my brain’s mind (let us know which of these would be your top 3 moonshots by taking our survey at https://bit.ly/3qkKqTa):
- A cure for schizophrenia (across positive, negative, and cognitive symptom domains)
- A cure for mood disorders, unipolar and bipolar (including suicide)
- A cure for anxiety disorders
- A cure for obsessive-compulsive disorder
- A cure for posttraumatic stress disorder
- A cure for alcoholism/addiction
- A cure for autism
- A cure for Alzheimer’s disease and other dementias
- A cure for personality disorders, especially antisocial and borderline
- A cure for the visceral hatred across political parties that permeates our society (obviously not a psychiatric category, but perhaps it should be added to DSM because it is so destructive).
Those moonshots may be regarded as absurd, and totally unachievable, but so was landing on the Moon, until it was accomplished. Psychiatry must stop thinking small and being content with tiny advances (which is like changing the chairs to more comfortable sofas on the deck of the Titanic and calling it “progress…”). Psychiatry needs to be unified under the flag of “moonshot thinking” by several visionary and transformative leaders to start believing in a miraculously better future for our patients. But to pave the way for moonshots in psychiatry, the leading organizations must collaborate closely to open the door for unprecedented scientific and medical breakthroughs of a moonshot by:
- Lobbying effectively to secure massive funding for research from federal, state, corporate, and foundation sources (perhaps convincing the Gates Foundation that schizophrenia is as devastating worldwide as malaria may bring a few badly needed billions into psychiatric brain research).
- Reminding members of Congress that in the United States, costs associated with psychiatric brain disorders total an estimated $700 billion annually,1 and that this must be addressed by boosting the meager NIMH budget by at least an order of magnitude. The NIMH should disproportionately invest its resources on severe brain disorders such as schizophrenia because breakthrough advances in its neurobiology will provide unprecedented insights to the pathophysiology of other severe psychiatric brain disorders.
- Partnering intimately with the pharmaceutical industry in a powerful public-private coalition to exploit the extensive research infrastructure of this industry.
- Creating the necessary army of researchers (physician-scientists) by providing huge incentives to medical students and psychiatric residents to pursue careers in neuroscience research. Incentives can include paying for an individual’s entire medical education and research training, and providing generous salaries that match or exceed the income of a very successful clinical practice.
- Convincing all psychiatric clinicians to support research by referring patients to research projects. Clinical psychiatrists are badly needed to care for the population, but they must be reminded that every treatment they are using today was a research project in the past, and that the research of today will evolve into the treatments (or cures) of tomorrow.
Pursuing lofty moonshots via innovative research is very likely to enhance serendipity and lead to unexpected discoveries along the way. As Louis Pasteur said, “chance only favors the prepared mind.”2 Moonshot thinking in psychiatry today is more feasible than ever before because of the many advances in research methods (neuroimaging, pluripotent cells, optogenetics, CRISPR, etc) and complex data management technologies (big data, machine learning, artificial intelligence), each of which qualifies as a preparatory moonshot in its own right.
Given the tragic consequences of psychiatric brain disorders, it is imperative that we “think big.” Humanity expects us to do that. We must envision the future of psychiatry as dramatically different from the present. Moonshot thinking is the indispensable vehicle to take us there.
“I believe that this nation should commit itself to achieving the goal, before the decade is out, of landing a man on the Moon and returning him safely to Earth.”
President John F. Kennedy, May 25, 1961
Despite significant progress, there remain many unmet needs in psychiatry. These include a granular understanding of the neurobiology of various psychopathologies, an objective and valid diagnostic schema, and disease-modifying treatments for chronic and disabling psychiatric disorders. Several moonshots are needed to address those festering needs.
A “moonshot” is an extremely ambitious, dramatic, imaginative, and inspiring goal. Landing on the Moon was generally believed to be impossible when President Kennedy boldly set that as a goal for the United States in 1961. Yet, 8 short years later, on July 20, 1969, Neil Armstrong stepped off the lunar module ladder onto the Moon’s surface, a feat that captured the imagination of the nation and the world. I distinctly remember watching it on television with amazement as a young boy. It was a surreal experience. That’s what achieving a moonshot feels like.
Successful organizations should always have 1 or more moonshots (American Psychiatric Association and National Institute of Mental Health [NIMH], are you listening?). Setting lofty goals that require monumental determination and effort to accomplish will have a transformative, long-lasting impact. The construction of the Panama Canal to connect 2 oceans and the Manhattan Project to develop the first nuclear bomb, which ended World War II, are examples of moonshots that continue to reverberate. A more recent moonshot is the driverless car, which in the past was a laughable idea but is now a reality that will change society and the world in many ways. Innovative billionaire moguls now speak loudly about colonizing Mars, which sounds improbable and highly risky, but it’s a moonshot that may be achieved within a few years. Establishing world peace is a moonshot that requires collective Kennedy-esque vision and motivation among world leaders, which currently is sadly lacking.
So, for contemporary psychiatry, what is the equivalent of landing on the Moon? Here is the list that pops in my brain’s mind (let us know which of these would be your top 3 moonshots by taking our survey at https://bit.ly/3qkKqTa):
- A cure for schizophrenia (across positive, negative, and cognitive symptom domains)
- A cure for mood disorders, unipolar and bipolar (including suicide)
- A cure for anxiety disorders
- A cure for obsessive-compulsive disorder
- A cure for posttraumatic stress disorder
- A cure for alcoholism/addiction
- A cure for autism
- A cure for Alzheimer’s disease and other dementias
- A cure for personality disorders, especially antisocial and borderline
- A cure for the visceral hatred across political parties that permeates our society (obviously not a psychiatric category, but perhaps it should be added to DSM because it is so destructive).
Those moonshots may be regarded as absurd, and totally unachievable, but so was landing on the Moon, until it was accomplished. Psychiatry must stop thinking small and being content with tiny advances (which is like changing the chairs to more comfortable sofas on the deck of the Titanic and calling it “progress…”). Psychiatry needs to be unified under the flag of “moonshot thinking” by several visionary and transformative leaders to start believing in a miraculously better future for our patients. But to pave the way for moonshots in psychiatry, the leading organizations must collaborate closely to open the door for unprecedented scientific and medical breakthroughs of a moonshot by:
- Lobbying effectively to secure massive funding for research from federal, state, corporate, and foundation sources (perhaps convincing the Gates Foundation that schizophrenia is as devastating worldwide as malaria may bring a few badly needed billions into psychiatric brain research).
- Reminding members of Congress that in the United States, costs associated with psychiatric brain disorders total an estimated $700 billion annually,1 and that this must be addressed by boosting the meager NIMH budget by at least an order of magnitude. The NIMH should disproportionately invest its resources on severe brain disorders such as schizophrenia because breakthrough advances in its neurobiology will provide unprecedented insights to the pathophysiology of other severe psychiatric brain disorders.
- Partnering intimately with the pharmaceutical industry in a powerful public-private coalition to exploit the extensive research infrastructure of this industry.
- Creating the necessary army of researchers (physician-scientists) by providing huge incentives to medical students and psychiatric residents to pursue careers in neuroscience research. Incentives can include paying for an individual’s entire medical education and research training, and providing generous salaries that match or exceed the income of a very successful clinical practice.
- Convincing all psychiatric clinicians to support research by referring patients to research projects. Clinical psychiatrists are badly needed to care for the population, but they must be reminded that every treatment they are using today was a research project in the past, and that the research of today will evolve into the treatments (or cures) of tomorrow.
Pursuing lofty moonshots via innovative research is very likely to enhance serendipity and lead to unexpected discoveries along the way. As Louis Pasteur said, “chance only favors the prepared mind.”2 Moonshot thinking in psychiatry today is more feasible than ever before because of the many advances in research methods (neuroimaging, pluripotent cells, optogenetics, CRISPR, etc) and complex data management technologies (big data, machine learning, artificial intelligence), each of which qualifies as a preparatory moonshot in its own right.
Given the tragic consequences of psychiatric brain disorders, it is imperative that we “think big.” Humanity expects us to do that. We must envision the future of psychiatry as dramatically different from the present. Moonshot thinking is the indispensable vehicle to take us there.
1. Discovery Mood and Anxiety Program. The rising cost of mental health and substance abuse in the United States. Accessed January 13, 2022. https://discoverymood.com/blog/cost-of-mental-health-increase/
2. Wikiquote. Louis Pasteur. Accessed January 10, 2022. https://en.wikiquote.org/wiki/Louis_Pasteur
1. Discovery Mood and Anxiety Program. The rising cost of mental health and substance abuse in the United States. Accessed January 13, 2022. https://discoverymood.com/blog/cost-of-mental-health-increase/
2. Wikiquote. Louis Pasteur. Accessed January 10, 2022. https://en.wikiquote.org/wiki/Louis_Pasteur
Is anosognosia a delusion, a negative symptom, or a cognitive deficit?
Anosognosia is the lack of awareness of a disabling physical or mental illness. The term was coined by Joseph Babinski in 1914 following his observations that patients with left-side paralysis due to right hemisphere stroke do not recognize their hemiplegia and strongly deny that there is anything physically wrong with their body, or that they need treatment or rehabilitation.
Psychiatrists have long observed anosognosia in patients with acute psychoses such as schizophrenia or mania who vehemently deny that there is anything wrong with them, despite experiencing hallucinations, delusions, and/or bizarre behavior. They adamantly refuse medical care and often have to be involuntarily hospitalized to receive urgently needed medications they don’t believe they need.
So is anosognosia in schizophrenia a fixed false belief (delusion), a negative symptom, or a cognitive deficit? Arguments can be made for any of those 3 options, but the evidence suggests that anosognosia is a disorder of consciousness, a “meta-cognitive” deficit, or, as I referred to it in a previous publication, the loss of self-proprioception.1
Anosognosia in neurologic brain disorders
Although right hemispheric stroke is the most common disease state associated with anosognosia,2 other neurologic disorders can be associated with anosognosia, including Anton’s syndrome of cortical blindness,3 traumatic brain injury,4 Wernicke’s aphasia,5 mild cognitive impairment,6 and Alzheimer’s disease.7 In addition to anosognosia, those disorders can be accompanied by indifference to the deficit, which is referred to as “anosodiaphoria.”
The neuroanatomy of anosognosia generally implicates right hemisphere deficits, especially the frontal cortex, the right parietal lobe, the temporoparietal cortex, and the thalamus. It can be conceptualized as a disturbance of “body schema” because all motor and sensory functions of the body have a “representation” in brain structure.
Anosognosia in psychiatric brain disorders
Although schizophrenia is most frequently associated with anosognosia, other psychiatric disorders also exhibit this absence of insight. They include delusional disorder,8 bipolar disorder,9 intellectual disability,10 and personality disorders.11 In all those psychiatric disorders, there is a lack of self-reflection (metacognition). At the neuroanatomical level, most studies have focused on schizophrenia, and abnormalities have been described in the frontal and parietal regions. Significant pathology in the inferior parietal lobe has been identified in schizophrenia.12 However, the right insula, which is connected to multiple neural circuits,13 appears to be intimately associated with anosognosia when impaired. The insula also regulates interoception and a “sense of self.”14 The loss of cortical gray matter in schizophrenia is most pronounced in the insula bilaterally. Another neurologic mechanism associated with anosognosia in schizophrenia is the default mode network (DMN). The DMN, which usually is overactive at rest and is deactivated during a focused activity, is involved in both insight and social cognition.15
Measurement of anosognosia
Several rating scales are used to measure the severity of anosognosia and the loss of insight. They include:
- The Insight and Treatment Attitude Questionnaire16
- The Scale to Assess Unawareness of Mental Disorder17
- The Beck Cognitive Insight Scale,18 the only self-administered scale that measures a patient’s ability to evaluate their psychiatric beliefs and possibly modify them
- The Positive and Negative Syndrome Scale,19 which is the gold standard for measuring the overall severity of schizophrenia, has only 1 item related to insight within the 16-item General Subscale (G12: Lack of judgement and insight).
Continue to: Consequences of anosognosia...
Consequences of anosognosia
Patients with anosognosia neglect themselves both mentally and physically and fail to seek or accept medical attention. Thus, schizophrenia is associated with many serious and damaging consequences due to the lack of self-monitoring or appraising their health needs. The Table summarizes the multiple consequences of anosognosia.
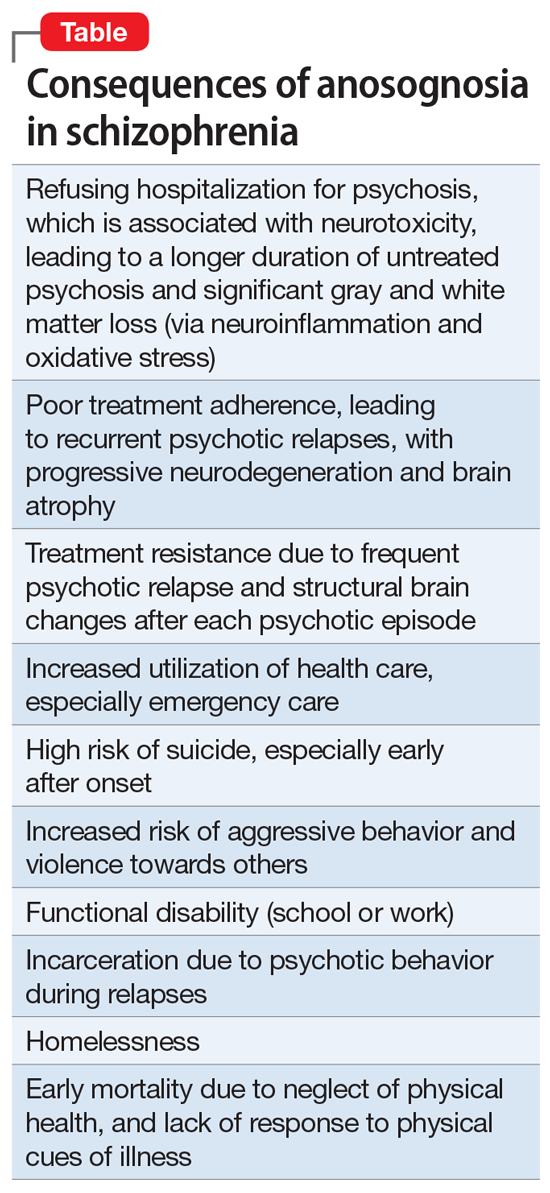
Is anosognosia treatable or irreversible?
Schizophrenia is well established to be a heterogeneous syndrome with hundreds of biotypes that share a similar phenotype of positive, negative, cognitive, mood, and neuromotor symptoms of variable severities.20 This includes anosognosia, which has been reported in 57% to 98% of patients in various studies.21,22
So what happens to anosognosia with antipsychotic therapy? In the first study that used a long-acting injectable (LAI) second-generation antipsychotic (SGA) in first-episode psychosis to ensure full adherence, Emsley et al23 reported a 64% remission rate after 2 years of treatment, and observed that many patients regained their insight after several months of uninterrupted antipsychotic pharmacotherapy. This suggests that avoiding psychotic relapse with uninterrupted antipsychotic therapy with LAIs may help restore insight. I have personally witnessed reversal of anosognosia in patients with first-episode schizophrenia whom I treated with LAI SGAs continuously for several years; these patients not only regained insight into their illness but were able to return to college or to work.
There is also evidence that stroke patients with left-side hemiplegia, or patients with cortical blindness (due to calcarine cortex damage secondary to posterior cerebral artery infarct), who paradoxically deny being blind due to anosognosia, do regain their insight after several months. Cognitive-behavioral therapy (CBT) and adherence therapy, as well as psychoeducation, can help in reversing anosognosia. Bilateral electroconvulsive therapy has been reported to improve insight in schizophrenia. Transcranial magnetic stimulation over the posterior parietal cortex has been reported to restore insight in patients with visuospatial neglect due to a stroke. However, more research targeting anosognosia along with psychotic symptoms is needed. It should be noted that patients with bipolar disorder who have anosognosia during the manic phase of their illness do have insight when they switch to a depressed phase,9 which suggests that anosognosia is reversible in bipolar disorder and is phase-dependent (ie, a state, not a trait, variable).
A symptom of impaired consciousness
A large body of evidence links lesions in the right hemisphere to delusion and to anosognosia.24 Gazzaniga and Miller25 published a book chapter with the provocative title “the left hemisphere does not miss the right hemisphere.” Such right-hemisphere lesions can lead to a disruption of consciousness, leading to anosognosia. Schizophrenia is a pervasive brain syndrome involving multiple brain regions and a wide range of clinical symptoms ranging across psychotic as well as negative and cognitive domains. Anosognosia can be conceptualized as a psychotic symptom (delusion), a negative symptom (self-monitoring deficit), or a cognitive failure. However, anosognosia in schizophrenia can be best understood as a symptom of impaired consciousness and self-pathology,26 where the brain fails to process and recognize one’s mental function, which culminates in faulty reality testing.
Schizophrenia is a neurologic syndrome associated with numerous psychiatric manifestations, and anosognosia is one of its fundamental initial symptoms.
1. Nasrallah HA. Impaired mental proprioception in schizophrenia. Current Psychiatry. 2012;11(8):4-5.
2. Kirsch LP, Mathys C, Papadaki C, et al. Updating beliefs beyond the here-and-now: the counter-factual self in anosognosia for hemiplegia. Brain Commun. 2021;3(2):fcab098. doi: 10.1093/braincomms/fcab098
3. Das JM, Nagvi IA. Anton syndrome. StatPearls Publishing. Updated April 10, 2021. Accessed December 13, 2021. https://www.ncbi.nlm.nih.gov/books/NBK538155/
4. Steward KA, Kretzmer T. Anosognosia in moderate-to-severe traumatic brain injury: a review of prevalence, clinical correlates, and diversity considerations. Clin Neuropsychol. 2021:1-20.
5. Klarendié M, Gorišek VR, Granda G, et al. Auditory agnosia with anosognosia. Cortex. 2021;137:255-270.
6. Bastin C, Giacomelli F, Miévis F, et al. Anosognosia in mild cognitive impairment: lack of awareness of memory difficulties characterizes prodromal Alzheimer’s disease. Front Psychiatry. 202;12:631518.
7. Chen S, Song Y, Xu W, et al; Alzheimer’s Disease Neuroimaging Initiative. Impaired memory awareness and loss integration in self-referential network across the progression of Alzheimer’s disease spectrum. J Alzheimers Dis. 2021;83(1):111-126.
8. Turnbull OH, Fotopoulou A, Solms M. Anosognosia as motivated unawareness: the ‘defence’ hypothesis revisited. Cortex. 2014;61:18-29.
9. Ibrahim SU, Kalyanasundaram VB, Ramanathan SA, et al. Trajectory of insight on various dimensions among bipolar disorder in-patients. Ind Psychiatry J. 2020;29(2):285-292.
10. Levine DN. Unawareness of visual and sensorimotor defects: a hypothesis. Brain Cogn. 1990;13(2):233-281.
11. Pourmohammad P, Imani M, Goodarzi MA, et al. Impaired complex theory of mind and low emotional self-awareness in outpatients with borderline personality disorder compared to healthy controls: a cross-sectional study. J Psychiatr Res. 2021;143:445-450.
12. Torrey EF. Schizophrenia and the inferior parietal lobule. Schizophr Res. 2007;97(1-3):215-225.
13. Dionisio S, Mayoglou L, Cho SM, et al. Connectivity of the human insula: a cortico-cortical evoked potential (CCEP) study. Cortex. 2019;120:419-442.
14. Nord CL, Lawson RP, Dalgleish T. Disrupted dorsal mid-insula activation during interoception across psychiatric disorders. Am J Psychiatry. 2021;178(8):761-770.
15. Glahn DC, Laird AR, Ellison-Wright I, et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64(9):774-781.
16. McEvoy JP, Freter S, Everett G, et al. Insight and the clinical outcome of schizophrenic patients. J Nerv Ment Dis. 1989;177(1):48-51.
17. Amador XF, Strauss DH, Yale SA, et al. Assessment of insight in psychosis. Am J Psychiatry. 1993;150(6):873-879.
18. Beck AT, Baruch E, Balter JM, et al. A new instrument for measuring insight: the Beck Cognitive Insight Scale. Schizophr Res. 2004;68(2-3):319-329.
19. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
20. Nasrallah HA. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
21. Buckley PF, Wirshing DA, Bhushan P, et al. Lack of insight in schizophrenia: impact on treatment adherence. CNS Drugs. 2007;21(2):129-141.
22. Lehrer DS, Lorenz J. Anosognosia in schizophrenia: hidden in plain sight. Innov Clin Neurosci. 2014;11(5-6):101-107.
23. Emsley R, Medori R, Koen L, et al. Long-acting injectable risperidone in the treatment of subjects with recent-onset psychosis: a preliminary study. J Clin Psychopharmacol. 2008;28(2):210-213.
24. Gurin L, Blum S. Delusions and the right hemisphere: a review of the case for the right hemisphere as a mediator of reality-based belief. J Neuropsychiatry Clin Neurosci. 2017;29(3):225-235.
25. Gazzaniga MS, Miller M. The left hemisphere does not miss the right hemisphere. In: Laureys S, Tononi G (eds). The Neurology of Consciousness. Cognitive Neuroscience and Neuropathology. Academic Press; 2008:261-270.
26. Cooney JW, Gazzaniga MS. Neurological disorders and the structure of human consciousness. Trends Cogn Sci. 2003;7(4):161-165.
Anosognosia is the lack of awareness of a disabling physical or mental illness. The term was coined by Joseph Babinski in 1914 following his observations that patients with left-side paralysis due to right hemisphere stroke do not recognize their hemiplegia and strongly deny that there is anything physically wrong with their body, or that they need treatment or rehabilitation.
Psychiatrists have long observed anosognosia in patients with acute psychoses such as schizophrenia or mania who vehemently deny that there is anything wrong with them, despite experiencing hallucinations, delusions, and/or bizarre behavior. They adamantly refuse medical care and often have to be involuntarily hospitalized to receive urgently needed medications they don’t believe they need.
So is anosognosia in schizophrenia a fixed false belief (delusion), a negative symptom, or a cognitive deficit? Arguments can be made for any of those 3 options, but the evidence suggests that anosognosia is a disorder of consciousness, a “meta-cognitive” deficit, or, as I referred to it in a previous publication, the loss of self-proprioception.1
Anosognosia in neurologic brain disorders
Although right hemispheric stroke is the most common disease state associated with anosognosia,2 other neurologic disorders can be associated with anosognosia, including Anton’s syndrome of cortical blindness,3 traumatic brain injury,4 Wernicke’s aphasia,5 mild cognitive impairment,6 and Alzheimer’s disease.7 In addition to anosognosia, those disorders can be accompanied by indifference to the deficit, which is referred to as “anosodiaphoria.”
The neuroanatomy of anosognosia generally implicates right hemisphere deficits, especially the frontal cortex, the right parietal lobe, the temporoparietal cortex, and the thalamus. It can be conceptualized as a disturbance of “body schema” because all motor and sensory functions of the body have a “representation” in brain structure.
Anosognosia in psychiatric brain disorders
Although schizophrenia is most frequently associated with anosognosia, other psychiatric disorders also exhibit this absence of insight. They include delusional disorder,8 bipolar disorder,9 intellectual disability,10 and personality disorders.11 In all those psychiatric disorders, there is a lack of self-reflection (metacognition). At the neuroanatomical level, most studies have focused on schizophrenia, and abnormalities have been described in the frontal and parietal regions. Significant pathology in the inferior parietal lobe has been identified in schizophrenia.12 However, the right insula, which is connected to multiple neural circuits,13 appears to be intimately associated with anosognosia when impaired. The insula also regulates interoception and a “sense of self.”14 The loss of cortical gray matter in schizophrenia is most pronounced in the insula bilaterally. Another neurologic mechanism associated with anosognosia in schizophrenia is the default mode network (DMN). The DMN, which usually is overactive at rest and is deactivated during a focused activity, is involved in both insight and social cognition.15
Measurement of anosognosia
Several rating scales are used to measure the severity of anosognosia and the loss of insight. They include:
- The Insight and Treatment Attitude Questionnaire16
- The Scale to Assess Unawareness of Mental Disorder17
- The Beck Cognitive Insight Scale,18 the only self-administered scale that measures a patient’s ability to evaluate their psychiatric beliefs and possibly modify them
- The Positive and Negative Syndrome Scale,19 which is the gold standard for measuring the overall severity of schizophrenia, has only 1 item related to insight within the 16-item General Subscale (G12: Lack of judgement and insight).
Continue to: Consequences of anosognosia...
Consequences of anosognosia
Patients with anosognosia neglect themselves both mentally and physically and fail to seek or accept medical attention. Thus, schizophrenia is associated with many serious and damaging consequences due to the lack of self-monitoring or appraising their health needs. The Table summarizes the multiple consequences of anosognosia.

Is anosognosia treatable or irreversible?
Schizophrenia is well established to be a heterogeneous syndrome with hundreds of biotypes that share a similar phenotype of positive, negative, cognitive, mood, and neuromotor symptoms of variable severities.20 This includes anosognosia, which has been reported in 57% to 98% of patients in various studies.21,22
So what happens to anosognosia with antipsychotic therapy? In the first study that used a long-acting injectable (LAI) second-generation antipsychotic (SGA) in first-episode psychosis to ensure full adherence, Emsley et al23 reported a 64% remission rate after 2 years of treatment, and observed that many patients regained their insight after several months of uninterrupted antipsychotic pharmacotherapy. This suggests that avoiding psychotic relapse with uninterrupted antipsychotic therapy with LAIs may help restore insight. I have personally witnessed reversal of anosognosia in patients with first-episode schizophrenia whom I treated with LAI SGAs continuously for several years; these patients not only regained insight into their illness but were able to return to college or to work.
There is also evidence that stroke patients with left-side hemiplegia, or patients with cortical blindness (due to calcarine cortex damage secondary to posterior cerebral artery infarct), who paradoxically deny being blind due to anosognosia, do regain their insight after several months. Cognitive-behavioral therapy (CBT) and adherence therapy, as well as psychoeducation, can help in reversing anosognosia. Bilateral electroconvulsive therapy has been reported to improve insight in schizophrenia. Transcranial magnetic stimulation over the posterior parietal cortex has been reported to restore insight in patients with visuospatial neglect due to a stroke. However, more research targeting anosognosia along with psychotic symptoms is needed. It should be noted that patients with bipolar disorder who have anosognosia during the manic phase of their illness do have insight when they switch to a depressed phase,9 which suggests that anosognosia is reversible in bipolar disorder and is phase-dependent (ie, a state, not a trait, variable).
A symptom of impaired consciousness
A large body of evidence links lesions in the right hemisphere to delusion and to anosognosia.24 Gazzaniga and Miller25 published a book chapter with the provocative title “the left hemisphere does not miss the right hemisphere.” Such right-hemisphere lesions can lead to a disruption of consciousness, leading to anosognosia. Schizophrenia is a pervasive brain syndrome involving multiple brain regions and a wide range of clinical symptoms ranging across psychotic as well as negative and cognitive domains. Anosognosia can be conceptualized as a psychotic symptom (delusion), a negative symptom (self-monitoring deficit), or a cognitive failure. However, anosognosia in schizophrenia can be best understood as a symptom of impaired consciousness and self-pathology,26 where the brain fails to process and recognize one’s mental function, which culminates in faulty reality testing.
Schizophrenia is a neurologic syndrome associated with numerous psychiatric manifestations, and anosognosia is one of its fundamental initial symptoms.
Anosognosia is the lack of awareness of a disabling physical or mental illness. The term was coined by Joseph Babinski in 1914 following his observations that patients with left-side paralysis due to right hemisphere stroke do not recognize their hemiplegia and strongly deny that there is anything physically wrong with their body, or that they need treatment or rehabilitation.
Psychiatrists have long observed anosognosia in patients with acute psychoses such as schizophrenia or mania who vehemently deny that there is anything wrong with them, despite experiencing hallucinations, delusions, and/or bizarre behavior. They adamantly refuse medical care and often have to be involuntarily hospitalized to receive urgently needed medications they don’t believe they need.
So is anosognosia in schizophrenia a fixed false belief (delusion), a negative symptom, or a cognitive deficit? Arguments can be made for any of those 3 options, but the evidence suggests that anosognosia is a disorder of consciousness, a “meta-cognitive” deficit, or, as I referred to it in a previous publication, the loss of self-proprioception.1
Anosognosia in neurologic brain disorders
Although right hemispheric stroke is the most common disease state associated with anosognosia,2 other neurologic disorders can be associated with anosognosia, including Anton’s syndrome of cortical blindness,3 traumatic brain injury,4 Wernicke’s aphasia,5 mild cognitive impairment,6 and Alzheimer’s disease.7 In addition to anosognosia, those disorders can be accompanied by indifference to the deficit, which is referred to as “anosodiaphoria.”
The neuroanatomy of anosognosia generally implicates right hemisphere deficits, especially the frontal cortex, the right parietal lobe, the temporoparietal cortex, and the thalamus. It can be conceptualized as a disturbance of “body schema” because all motor and sensory functions of the body have a “representation” in brain structure.
Anosognosia in psychiatric brain disorders
Although schizophrenia is most frequently associated with anosognosia, other psychiatric disorders also exhibit this absence of insight. They include delusional disorder,8 bipolar disorder,9 intellectual disability,10 and personality disorders.11 In all those psychiatric disorders, there is a lack of self-reflection (metacognition). At the neuroanatomical level, most studies have focused on schizophrenia, and abnormalities have been described in the frontal and parietal regions. Significant pathology in the inferior parietal lobe has been identified in schizophrenia.12 However, the right insula, which is connected to multiple neural circuits,13 appears to be intimately associated with anosognosia when impaired. The insula also regulates interoception and a “sense of self.”14 The loss of cortical gray matter in schizophrenia is most pronounced in the insula bilaterally. Another neurologic mechanism associated with anosognosia in schizophrenia is the default mode network (DMN). The DMN, which usually is overactive at rest and is deactivated during a focused activity, is involved in both insight and social cognition.15
Measurement of anosognosia
Several rating scales are used to measure the severity of anosognosia and the loss of insight. They include:
- The Insight and Treatment Attitude Questionnaire16
- The Scale to Assess Unawareness of Mental Disorder17
- The Beck Cognitive Insight Scale,18 the only self-administered scale that measures a patient’s ability to evaluate their psychiatric beliefs and possibly modify them
- The Positive and Negative Syndrome Scale,19 which is the gold standard for measuring the overall severity of schizophrenia, has only 1 item related to insight within the 16-item General Subscale (G12: Lack of judgement and insight).
Continue to: Consequences of anosognosia...
Consequences of anosognosia
Patients with anosognosia neglect themselves both mentally and physically and fail to seek or accept medical attention. Thus, schizophrenia is associated with many serious and damaging consequences due to the lack of self-monitoring or appraising their health needs. The Table summarizes the multiple consequences of anosognosia.

Is anosognosia treatable or irreversible?
Schizophrenia is well established to be a heterogeneous syndrome with hundreds of biotypes that share a similar phenotype of positive, negative, cognitive, mood, and neuromotor symptoms of variable severities.20 This includes anosognosia, which has been reported in 57% to 98% of patients in various studies.21,22
So what happens to anosognosia with antipsychotic therapy? In the first study that used a long-acting injectable (LAI) second-generation antipsychotic (SGA) in first-episode psychosis to ensure full adherence, Emsley et al23 reported a 64% remission rate after 2 years of treatment, and observed that many patients regained their insight after several months of uninterrupted antipsychotic pharmacotherapy. This suggests that avoiding psychotic relapse with uninterrupted antipsychotic therapy with LAIs may help restore insight. I have personally witnessed reversal of anosognosia in patients with first-episode schizophrenia whom I treated with LAI SGAs continuously for several years; these patients not only regained insight into their illness but were able to return to college or to work.
There is also evidence that stroke patients with left-side hemiplegia, or patients with cortical blindness (due to calcarine cortex damage secondary to posterior cerebral artery infarct), who paradoxically deny being blind due to anosognosia, do regain their insight after several months. Cognitive-behavioral therapy (CBT) and adherence therapy, as well as psychoeducation, can help in reversing anosognosia. Bilateral electroconvulsive therapy has been reported to improve insight in schizophrenia. Transcranial magnetic stimulation over the posterior parietal cortex has been reported to restore insight in patients with visuospatial neglect due to a stroke. However, more research targeting anosognosia along with psychotic symptoms is needed. It should be noted that patients with bipolar disorder who have anosognosia during the manic phase of their illness do have insight when they switch to a depressed phase,9 which suggests that anosognosia is reversible in bipolar disorder and is phase-dependent (ie, a state, not a trait, variable).
A symptom of impaired consciousness
A large body of evidence links lesions in the right hemisphere to delusion and to anosognosia.24 Gazzaniga and Miller25 published a book chapter with the provocative title “the left hemisphere does not miss the right hemisphere.” Such right-hemisphere lesions can lead to a disruption of consciousness, leading to anosognosia. Schizophrenia is a pervasive brain syndrome involving multiple brain regions and a wide range of clinical symptoms ranging across psychotic as well as negative and cognitive domains. Anosognosia can be conceptualized as a psychotic symptom (delusion), a negative symptom (self-monitoring deficit), or a cognitive failure. However, anosognosia in schizophrenia can be best understood as a symptom of impaired consciousness and self-pathology,26 where the brain fails to process and recognize one’s mental function, which culminates in faulty reality testing.
Schizophrenia is a neurologic syndrome associated with numerous psychiatric manifestations, and anosognosia is one of its fundamental initial symptoms.
1. Nasrallah HA. Impaired mental proprioception in schizophrenia. Current Psychiatry. 2012;11(8):4-5.
2. Kirsch LP, Mathys C, Papadaki C, et al. Updating beliefs beyond the here-and-now: the counter-factual self in anosognosia for hemiplegia. Brain Commun. 2021;3(2):fcab098. doi: 10.1093/braincomms/fcab098
3. Das JM, Nagvi IA. Anton syndrome. StatPearls Publishing. Updated April 10, 2021. Accessed December 13, 2021. https://www.ncbi.nlm.nih.gov/books/NBK538155/
4. Steward KA, Kretzmer T. Anosognosia in moderate-to-severe traumatic brain injury: a review of prevalence, clinical correlates, and diversity considerations. Clin Neuropsychol. 2021:1-20.
5. Klarendié M, Gorišek VR, Granda G, et al. Auditory agnosia with anosognosia. Cortex. 2021;137:255-270.
6. Bastin C, Giacomelli F, Miévis F, et al. Anosognosia in mild cognitive impairment: lack of awareness of memory difficulties characterizes prodromal Alzheimer’s disease. Front Psychiatry. 202;12:631518.
7. Chen S, Song Y, Xu W, et al; Alzheimer’s Disease Neuroimaging Initiative. Impaired memory awareness and loss integration in self-referential network across the progression of Alzheimer’s disease spectrum. J Alzheimers Dis. 2021;83(1):111-126.
8. Turnbull OH, Fotopoulou A, Solms M. Anosognosia as motivated unawareness: the ‘defence’ hypothesis revisited. Cortex. 2014;61:18-29.
9. Ibrahim SU, Kalyanasundaram VB, Ramanathan SA, et al. Trajectory of insight on various dimensions among bipolar disorder in-patients. Ind Psychiatry J. 2020;29(2):285-292.
10. Levine DN. Unawareness of visual and sensorimotor defects: a hypothesis. Brain Cogn. 1990;13(2):233-281.
11. Pourmohammad P, Imani M, Goodarzi MA, et al. Impaired complex theory of mind and low emotional self-awareness in outpatients with borderline personality disorder compared to healthy controls: a cross-sectional study. J Psychiatr Res. 2021;143:445-450.
12. Torrey EF. Schizophrenia and the inferior parietal lobule. Schizophr Res. 2007;97(1-3):215-225.
13. Dionisio S, Mayoglou L, Cho SM, et al. Connectivity of the human insula: a cortico-cortical evoked potential (CCEP) study. Cortex. 2019;120:419-442.
14. Nord CL, Lawson RP, Dalgleish T. Disrupted dorsal mid-insula activation during interoception across psychiatric disorders. Am J Psychiatry. 2021;178(8):761-770.
15. Glahn DC, Laird AR, Ellison-Wright I, et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64(9):774-781.
16. McEvoy JP, Freter S, Everett G, et al. Insight and the clinical outcome of schizophrenic patients. J Nerv Ment Dis. 1989;177(1):48-51.
17. Amador XF, Strauss DH, Yale SA, et al. Assessment of insight in psychosis. Am J Psychiatry. 1993;150(6):873-879.
18. Beck AT, Baruch E, Balter JM, et al. A new instrument for measuring insight: the Beck Cognitive Insight Scale. Schizophr Res. 2004;68(2-3):319-329.
19. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
20. Nasrallah HA. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
21. Buckley PF, Wirshing DA, Bhushan P, et al. Lack of insight in schizophrenia: impact on treatment adherence. CNS Drugs. 2007;21(2):129-141.
22. Lehrer DS, Lorenz J. Anosognosia in schizophrenia: hidden in plain sight. Innov Clin Neurosci. 2014;11(5-6):101-107.
23. Emsley R, Medori R, Koen L, et al. Long-acting injectable risperidone in the treatment of subjects with recent-onset psychosis: a preliminary study. J Clin Psychopharmacol. 2008;28(2):210-213.
24. Gurin L, Blum S. Delusions and the right hemisphere: a review of the case for the right hemisphere as a mediator of reality-based belief. J Neuropsychiatry Clin Neurosci. 2017;29(3):225-235.
25. Gazzaniga MS, Miller M. The left hemisphere does not miss the right hemisphere. In: Laureys S, Tononi G (eds). The Neurology of Consciousness. Cognitive Neuroscience and Neuropathology. Academic Press; 2008:261-270.
26. Cooney JW, Gazzaniga MS. Neurological disorders and the structure of human consciousness. Trends Cogn Sci. 2003;7(4):161-165.
1. Nasrallah HA. Impaired mental proprioception in schizophrenia. Current Psychiatry. 2012;11(8):4-5.
2. Kirsch LP, Mathys C, Papadaki C, et al. Updating beliefs beyond the here-and-now: the counter-factual self in anosognosia for hemiplegia. Brain Commun. 2021;3(2):fcab098. doi: 10.1093/braincomms/fcab098
3. Das JM, Nagvi IA. Anton syndrome. StatPearls Publishing. Updated April 10, 2021. Accessed December 13, 2021. https://www.ncbi.nlm.nih.gov/books/NBK538155/
4. Steward KA, Kretzmer T. Anosognosia in moderate-to-severe traumatic brain injury: a review of prevalence, clinical correlates, and diversity considerations. Clin Neuropsychol. 2021:1-20.
5. Klarendié M, Gorišek VR, Granda G, et al. Auditory agnosia with anosognosia. Cortex. 2021;137:255-270.
6. Bastin C, Giacomelli F, Miévis F, et al. Anosognosia in mild cognitive impairment: lack of awareness of memory difficulties characterizes prodromal Alzheimer’s disease. Front Psychiatry. 202;12:631518.
7. Chen S, Song Y, Xu W, et al; Alzheimer’s Disease Neuroimaging Initiative. Impaired memory awareness and loss integration in self-referential network across the progression of Alzheimer’s disease spectrum. J Alzheimers Dis. 2021;83(1):111-126.
8. Turnbull OH, Fotopoulou A, Solms M. Anosognosia as motivated unawareness: the ‘defence’ hypothesis revisited. Cortex. 2014;61:18-29.
9. Ibrahim SU, Kalyanasundaram VB, Ramanathan SA, et al. Trajectory of insight on various dimensions among bipolar disorder in-patients. Ind Psychiatry J. 2020;29(2):285-292.
10. Levine DN. Unawareness of visual and sensorimotor defects: a hypothesis. Brain Cogn. 1990;13(2):233-281.
11. Pourmohammad P, Imani M, Goodarzi MA, et al. Impaired complex theory of mind and low emotional self-awareness in outpatients with borderline personality disorder compared to healthy controls: a cross-sectional study. J Psychiatr Res. 2021;143:445-450.
12. Torrey EF. Schizophrenia and the inferior parietal lobule. Schizophr Res. 2007;97(1-3):215-225.
13. Dionisio S, Mayoglou L, Cho SM, et al. Connectivity of the human insula: a cortico-cortical evoked potential (CCEP) study. Cortex. 2019;120:419-442.
14. Nord CL, Lawson RP, Dalgleish T. Disrupted dorsal mid-insula activation during interoception across psychiatric disorders. Am J Psychiatry. 2021;178(8):761-770.
15. Glahn DC, Laird AR, Ellison-Wright I, et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64(9):774-781.
16. McEvoy JP, Freter S, Everett G, et al. Insight and the clinical outcome of schizophrenic patients. J Nerv Ment Dis. 1989;177(1):48-51.
17. Amador XF, Strauss DH, Yale SA, et al. Assessment of insight in psychosis. Am J Psychiatry. 1993;150(6):873-879.
18. Beck AT, Baruch E, Balter JM, et al. A new instrument for measuring insight: the Beck Cognitive Insight Scale. Schizophr Res. 2004;68(2-3):319-329.
19. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
20. Nasrallah HA. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
21. Buckley PF, Wirshing DA, Bhushan P, et al. Lack of insight in schizophrenia: impact on treatment adherence. CNS Drugs. 2007;21(2):129-141.
22. Lehrer DS, Lorenz J. Anosognosia in schizophrenia: hidden in plain sight. Innov Clin Neurosci. 2014;11(5-6):101-107.
23. Emsley R, Medori R, Koen L, et al. Long-acting injectable risperidone in the treatment of subjects with recent-onset psychosis: a preliminary study. J Clin Psychopharmacol. 2008;28(2):210-213.
24. Gurin L, Blum S. Delusions and the right hemisphere: a review of the case for the right hemisphere as a mediator of reality-based belief. J Neuropsychiatry Clin Neurosci. 2017;29(3):225-235.
25. Gazzaniga MS, Miller M. The left hemisphere does not miss the right hemisphere. In: Laureys S, Tononi G (eds). The Neurology of Consciousness. Cognitive Neuroscience and Neuropathology. Academic Press; 2008:261-270.
26. Cooney JW, Gazzaniga MS. Neurological disorders and the structure of human consciousness. Trends Cogn Sci. 2003;7(4):161-165.
