User login
Role of Psoriasis in the Development of Merkel Cell Carcinoma
1. O’Brien T, Power DG. Metastatic Merkel-cell carcinoma: the dawn of a new era. BMJ Case Rep. 2018;11:2018. doi:10.1136/bcr-2018-224924.
2. Del Marmol V, Lebbé C. New perspectives in Merkel cell carcinoma. Curr Opin Oncol. 2019;31:72-83.
3. Garcia-Carbonero R, Marquez-Rodas I, de la Cruz-Merino L, et al. Recent therapeutic advances and change in treatment paradigm of patients with Merkel cell carcinoma [published online April 8, 2019]. Oncologist. doi:10.1634/theoncologist.2018-0718.
4. Samimi M, Gardair C, Nicol JT, et al. Merkel cell polyomavirus in Merkel cell carcinoma: clinical and therapeutic perspectives. Semin Oncol. 2015;42:347-358.
5. Kitamura N, Tomita R, Yamamoto M, et al. Complete remission of Merkel cell carcinoma on the upper lip treated with radiation monotherapy and a literature review of Japanese cases. World J Surg Oncol. 2015;13:152.
6. Timmer FC, Klop WM, Relyveld GN, et al. Merkel cell carcinoma of the head and neck: emphasizing the risk of undertreatment. Eur Arch Otorhinolaryngol. 2016;273:1243-1252.
7. Açıkalın A, Paydas¸ S, Güleç ÜK, et al. A unique case of Merkel cell carcinoma with ovarian metastasis. Balkan Med J. 2014;31:356-359.
8. Yousif J, Yousif B, Kuriata MA. Complete remission of metastatic Merkel cell carcinoma in a patient with severe psoriasis. Cutis. 2018;101:E24-E27.
9. Grandhaye M, Teixeira PG, Henrot P, et al. Focus on Merkel cell carcinoma: diagnosis and staging. Skeletal Radiol. 2015;44:777-786.
10. Chatzinasiou F, Papadavid E, Korkolopoulou P, et al. An unusual case of diffuse Merkel cell carcinoma successfully treated with low dose radiotherapy. Dermatol Ther. 2015;28:282-286.
11. Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature. Int J Surg Case Rep. 2015;7C:104-108.
12. National Comprehensive Cancer Network. Merkel cell carcinoma. Published October 3, 2016. http://merkelcell.org/wp-content/uploads/2015/10/MccNccn.pdf. Accessed September 10, 2019.
13. Coggshall K, Tello TL, North JP, Yu SS. Merkel cell carcinoma: an update and review: pathogenesis, diagnosis, and staging. J Am Acad Dermatol. 2018;78:433-442.
14. Lanoy E, Engels EA. Skin cancers associated with autoimmune conditions among elderly adults. Br J Cancer. 2010;103:112-114.
15. Mertz KD, Junt T, Schmid M, et al. Inflammatory monocytes are a reservoir for Merkel cell polyomavirus. J Invest Dermatol. 2009;130:1146-1151.
1. O’Brien T, Power DG. Metastatic Merkel-cell carcinoma: the dawn of a new era. BMJ Case Rep. 2018;11:2018. doi:10.1136/bcr-2018-224924.
2. Del Marmol V, Lebbé C. New perspectives in Merkel cell carcinoma. Curr Opin Oncol. 2019;31:72-83.
3. Garcia-Carbonero R, Marquez-Rodas I, de la Cruz-Merino L, et al. Recent therapeutic advances and change in treatment paradigm of patients with Merkel cell carcinoma [published online April 8, 2019]. Oncologist. doi:10.1634/theoncologist.2018-0718.
4. Samimi M, Gardair C, Nicol JT, et al. Merkel cell polyomavirus in Merkel cell carcinoma: clinical and therapeutic perspectives. Semin Oncol. 2015;42:347-358.
5. Kitamura N, Tomita R, Yamamoto M, et al. Complete remission of Merkel cell carcinoma on the upper lip treated with radiation monotherapy and a literature review of Japanese cases. World J Surg Oncol. 2015;13:152.
6. Timmer FC, Klop WM, Relyveld GN, et al. Merkel cell carcinoma of the head and neck: emphasizing the risk of undertreatment. Eur Arch Otorhinolaryngol. 2016;273:1243-1252.
7. Açıkalın A, Paydas¸ S, Güleç ÜK, et al. A unique case of Merkel cell carcinoma with ovarian metastasis. Balkan Med J. 2014;31:356-359.
8. Yousif J, Yousif B, Kuriata MA. Complete remission of metastatic Merkel cell carcinoma in a patient with severe psoriasis. Cutis. 2018;101:E24-E27.
9. Grandhaye M, Teixeira PG, Henrot P, et al. Focus on Merkel cell carcinoma: diagnosis and staging. Skeletal Radiol. 2015;44:777-786.
10. Chatzinasiou F, Papadavid E, Korkolopoulou P, et al. An unusual case of diffuse Merkel cell carcinoma successfully treated with low dose radiotherapy. Dermatol Ther. 2015;28:282-286.
11. Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature. Int J Surg Case Rep. 2015;7C:104-108.
12. National Comprehensive Cancer Network. Merkel cell carcinoma. Published October 3, 2016. http://merkelcell.org/wp-content/uploads/2015/10/MccNccn.pdf. Accessed September 10, 2019.
13. Coggshall K, Tello TL, North JP, Yu SS. Merkel cell carcinoma: an update and review: pathogenesis, diagnosis, and staging. J Am Acad Dermatol. 2018;78:433-442.
14. Lanoy E, Engels EA. Skin cancers associated with autoimmune conditions among elderly adults. Br J Cancer. 2010;103:112-114.
15. Mertz KD, Junt T, Schmid M, et al. Inflammatory monocytes are a reservoir for Merkel cell polyomavirus. J Invest Dermatol. 2009;130:1146-1151.
1. O’Brien T, Power DG. Metastatic Merkel-cell carcinoma: the dawn of a new era. BMJ Case Rep. 2018;11:2018. doi:10.1136/bcr-2018-224924.
2. Del Marmol V, Lebbé C. New perspectives in Merkel cell carcinoma. Curr Opin Oncol. 2019;31:72-83.
3. Garcia-Carbonero R, Marquez-Rodas I, de la Cruz-Merino L, et al. Recent therapeutic advances and change in treatment paradigm of patients with Merkel cell carcinoma [published online April 8, 2019]. Oncologist. doi:10.1634/theoncologist.2018-0718.
4. Samimi M, Gardair C, Nicol JT, et al. Merkel cell polyomavirus in Merkel cell carcinoma: clinical and therapeutic perspectives. Semin Oncol. 2015;42:347-358.
5. Kitamura N, Tomita R, Yamamoto M, et al. Complete remission of Merkel cell carcinoma on the upper lip treated with radiation monotherapy and a literature review of Japanese cases. World J Surg Oncol. 2015;13:152.
6. Timmer FC, Klop WM, Relyveld GN, et al. Merkel cell carcinoma of the head and neck: emphasizing the risk of undertreatment. Eur Arch Otorhinolaryngol. 2016;273:1243-1252.
7. Açıkalın A, Paydas¸ S, Güleç ÜK, et al. A unique case of Merkel cell carcinoma with ovarian metastasis. Balkan Med J. 2014;31:356-359.
8. Yousif J, Yousif B, Kuriata MA. Complete remission of metastatic Merkel cell carcinoma in a patient with severe psoriasis. Cutis. 2018;101:E24-E27.
9. Grandhaye M, Teixeira PG, Henrot P, et al. Focus on Merkel cell carcinoma: diagnosis and staging. Skeletal Radiol. 2015;44:777-786.
10. Chatzinasiou F, Papadavid E, Korkolopoulou P, et al. An unusual case of diffuse Merkel cell carcinoma successfully treated with low dose radiotherapy. Dermatol Ther. 2015;28:282-286.
11. Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature. Int J Surg Case Rep. 2015;7C:104-108.
12. National Comprehensive Cancer Network. Merkel cell carcinoma. Published October 3, 2016. http://merkelcell.org/wp-content/uploads/2015/10/MccNccn.pdf. Accessed September 10, 2019.
13. Coggshall K, Tello TL, North JP, Yu SS. Merkel cell carcinoma: an update and review: pathogenesis, diagnosis, and staging. J Am Acad Dermatol. 2018;78:433-442.
14. Lanoy E, Engels EA. Skin cancers associated with autoimmune conditions among elderly adults. Br J Cancer. 2010;103:112-114.
15. Mertz KD, Junt T, Schmid M, et al. Inflammatory monocytes are a reservoir for Merkel cell polyomavirus. J Invest Dermatol. 2009;130:1146-1151.
Merkel Cell Carcinoma in a Patient With a History of Psoriasis
Background
Merkel cell carcinoma (MCC) is a rare, aggressive cutaneous malignancy.1 Immunosuppression, advanced age, and UV light exposure of fair skin are major risk factors; additionally, polyomavirus infection is detected in as many as 80% of cases.2-5 Merkel cells are slow-responding mechanoreceptors located within the basal layer of the epidermis.6 Approximately half of patients present with localized disease, in which surgical resection with or without adjuvant radiotherapy is usually indicated. However, overall prognosis is poor with MCC due to high recurrence rates.3
This neuroendocrine carcinoma has remarkable metastatic potential (34%–75%) and can invade regional lymph nodes; distant metastasis also can occur, most commonly to the liver, lungs, bones, and brain.7,8 Approximately 25% of patients present with palpable lymphadenopathy and 5% with distant metastasis at the time of diagnosis. The frequency of metastasis at diagnosis as well as high recurrence rates after treatment contribute to the overall poor prognosis of MCC. Local recurrence rates have been reported at 25%, with lymph node involvement in 52% and metastasis in 34% of cases; most recurrences occur within 2 years of diagnosis.8 The aggressiveness of the tumor determines patient mortality; in cases without lymph node involvement, the 5-year survival rate is 83.3%. However, the 5-year survival rate drops to 58.3% with lymph node involvement; in patients with metastatic disease, it is just 31.3%.8,9 Although MCC is a chemosensitive disease, durable responses are rarely seen in the advanced setting.3 Most patients with metastatic disease have a median progression-free survival of only 3 months.1
Presentation and Diagnosis
An MCC tumor classically presents as a red to violaceous, painless nodule with a smooth shiny surface, most often on the sun-exposed head and neck region.3,9-11 Approximately 50% of MCC cases present in the head and neck region, 32% to 38% present on the extremities, and 12% to 14% on the trunk.6 Unfortunately, this nonspecific presentation may lead to diagnostic uncertainty and a consequent delay in treatment. Definitive diagnosis of MCC can only be achieved with a skin biopsy, which allows for distinction from other clinically similar-appearing neoplasms.
Specific guidelines for an MCC diagnostic evaluation have been proposed by the National Comprehensive Cancer Network (NCCN) based on a framework of clinical presentation, preliminary workup, diagnosis, and additional workup.12 The simplified algorithm for evaluation includes the following12,13:
- Examine skin and lymph nodes
- Obtain a biopsy specimen stained with hematoxylin and eosin (including Breslow thickness and evidence of lymphovascular invasion) and immunostaining (including but not limited to cytokeratin (CK) 20 and thyroid transcription factor 1)
- Obtain a sentinel lymph node biopsy specimen from patients with negative clinical nodes, prior to excision when possible
- Perform fine-needle aspiration or core biopsy first for patients with positive clinical nodes; if negative, consider open biopsy, but if positive, proceed to next step
- Perform imaging as clinically indicated with magnetic resonance imaging, computed tomography, or positron emission tomography–computed tomography
- Consider consultation with a multidisciplinary tumor board
Histologic Features of MCC
Merkel cell carcinoma presents histologically as small round basophilic cells penetrating through the dermis in 3 histologic patterns: the trabecular, intermediate (80% of cases), and small cell type.8,10 Immunohistochemical features include CK20 positivity (showing paranuclear dotlike depositions in the cytoplasm or cell membrane) and CK7 negativity, which allows it to be differentiated from other neoplasms. Chromogranin and synaptophysin positivity provide further histologic confirmation. The absence of peripheral palisading, retraction artifact, and a fibromyxoid stroma allow for distinction from cutaneous basal cell carcinoma, which may display these features histologically. Other immunohistochemical markers that may be of value include thyroid transcription factor 1, which is typically positive in cutaneous metastasis of neuroendocrine carcinoma of the lung; S-100 and human melanoma black 45, which are positive in melanoma; and leukocyte common antigen (CD45), which can be positive in lymphoma. These stains are classically negative in MCC.4,8
Merkel Cell Polyomavirus and Other Risk Factors
Merkel cell carcinoma is frequently associated with the presence of Merkel cell polyomavirus (MCPyV) in tumor specimens, with a prevalence of 70% to 80% in all cases.8 Merkel cell polyomavirus is a class 2A carcinogen (ie, a probable carcinogen to humans) and is classified among a group of viruses that encode T antigens (ie, an antigen coded by a viral genome associated with transformation of infected cells by tumor viruses), which can lead to initiation of tumorigenesis through interference with cellular tumor suppressing proteins such as p53.10
Immunosuppression and UV-exposed fair skin also are considered major risk factors, which may be explained by the increase in MCPyV small T-antigen transcripts induced by UV irradiation.10 Moreover, as is seen in other cancers induced by viruses, host immunity can hinder tumor progression and development. Therefore, impairment of normal immune function likely creates a higher risk for MCC development as well as the potential for a worse prognosis.4,8 The precise incidence of MCC in immunosuppressed patients appears unclear; it is possible that chronic immunosuppressive therapy may play a notable role in the pathogenesis of the tumor.4,8
Impact of Comorbidities on MCC
These risk factors were all observed in the case patient. However, it also is possible that his associated comorbidities also had an impact on the presentation of his disease.8 For example, patients with rheumatoid arthritis have been shown to have an elevated risk for the development of MCC.14 Moreover, inflammatory monocytes infected with MCPyV, as would be expected in a patient with a history of chronic psoriasis prior to diagnosis of MCC, also may contribute to the pathogenesis of MCC by migrating to inflammatory skin lesions, such as those seen in psoriasis, releasing MCPyV locally and infecting Merkel cells.8,15 Testing for MCPyV was never performed in the case patient; however, it certainly would be prudent to do so in such a presentation. Additionally, further studies to determine the correlation of MCC to these disease processes are warranted.8
Given that MCC tends to affect an older population in which other notable comorbidities are not uncommon, the cost, side effects, and convenience for patients of any treatment plan are important considerations.8 In the case study, a combined regimen of carboplatin and VP-16 (etoposide) was utilized. This regimen was well tolerated by the patient and successful in achieving complete radiologic and clinical remission of his metastatic disease. This combination has been shown to prolong survival in patients with distant metastasis compared to those patients not receiving chemotherapy.6 In all high-risk patients, close clinical monitoring is essential to help optimize outcomes.
Bottom Line
Merkel cell carcinoma is a rare but aggressive cutaneous neoplasm that most frequently affects elderly patients, immunosuppressed patients, and individuals with chronic UV sun damage. An association between the oncogenesis of MCC and infection with MCPyV has been documented, but other underlying diseases such as psoriasis and rheumatoid arthritis also may play a role. In this case, the patient’s history of chronic immunosuppressive therapy for treatment of psoriasis and inflammatory joint disease likely played a role in the pathogenesis of the tumor. This potential risk should be an important point of discussion with any patient requiring this type of long-term management for disease control. This unique clinical case highlights a patient with substantial comorbidities who developed metastatic MCC and achieved complete clinical and radiologic remission following treatment with surgery and chemotherapy.
- O’Brien T, Power DG. Metastatic Merkel-cell carcinoma: the dawn of a new era. BMJ Case Rep. 2018;11:2018. doi:10.1136/bcr-2018-224924.
- Del Marmol V, Lebbé C. New perspectives in Merkel cell carcinoma. Curr Opin Oncol. 2019;31:72-83.
- Garcia-Carbonero R, Marquez-Rodas I, de la Cruz-Merino L, et al. Recent therapeutic advances and change in treatment paradigm of patients with Merkel cell carcinoma [published online April 8, 2019]. Oncologist. doi:10.1634/theoncologist.2018-0718.
- Samimi M, Gardair C, Nicol JT, et al. Merkel cell polyomavirus in Merkel cell carcinoma: clinical and therapeutic perspectives. Semin Oncol. 2015;42:347-358.
- Kitamura N, Tomita R, Yamamoto M, et al. Complete remission of Merkel cell carcinoma on the upper lip treated with radiation monotherapy and a literature review of Japanese cases. World J Surg Oncol. 2015;13:152.
- Timmer FC, Klop WM, Relyveld GN, et al. Merkel cell carcinoma of the head and neck: emphasizing the risk of undertreatment. Eur Arch Otorhinolaryngol. 2016;273:1243-1252.
- Açıkalın A, Paydas¸ S, Güleç ÜK, et al. A unique case of Merkel cell carcinoma with ovarian metastasis. Balkan Med J. 2014;31:356-359.
- Yousif J, Yousif B, Kuriata MA. Complete remission of metastatic Merkel cell carcinoma in a patient with severe psoriasis. Cutis. 2018;101:E24-E27.
- Grandhaye M, Teixeira PG, Henrot P, et al. Focus on Merkel cell carcinoma: diagnosis and staging. Skeletal Radiol. 2015;44:777-786.
- Chatzinasiou F, Papadavid E, Korkolopoulou P, et al. An unusual case of diffuse Merkel cell carcinoma successfully treated with low dose radiotherapy. Dermatol Ther. 2015;28:282-286.
- Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature. Int J Surg Case Rep. 2015;7C:104-108.
- National Comprehensive Cancer Network. Merkel cell carcinoma. Published October 3, 2016. http://merkelcell.org/wp-content/uploads/2015/10/MccNccn.pdf. Accessed September 10, 2019.
- Coggshall K, Tello TL, North JP, Yu SS. Merkel cell carcinoma: an update and review: pathogenesis, diagnosis, and staging. J Am Acad Dermatol. 2018;78:433-442.
- Lanoy E, Engels EA. Skin cancers associated with autoimmune conditions among elderly adults. Br J Cancer. 2010;103:112-114.
- Mertz KD, Junt T, Schmid M, et al. Inflammatory monocytes are a reservoir for Merkel cell polyomavirus. J Invest Dermatol. 2009;130:1146-1151.
Background
Merkel cell carcinoma (MCC) is a rare, aggressive cutaneous malignancy.1 Immunosuppression, advanced age, and UV light exposure of fair skin are major risk factors; additionally, polyomavirus infection is detected in as many as 80% of cases.2-5 Merkel cells are slow-responding mechanoreceptors located within the basal layer of the epidermis.6 Approximately half of patients present with localized disease, in which surgical resection with or without adjuvant radiotherapy is usually indicated. However, overall prognosis is poor with MCC due to high recurrence rates.3
This neuroendocrine carcinoma has remarkable metastatic potential (34%–75%) and can invade regional lymph nodes; distant metastasis also can occur, most commonly to the liver, lungs, bones, and brain.7,8 Approximately 25% of patients present with palpable lymphadenopathy and 5% with distant metastasis at the time of diagnosis. The frequency of metastasis at diagnosis as well as high recurrence rates after treatment contribute to the overall poor prognosis of MCC. Local recurrence rates have been reported at 25%, with lymph node involvement in 52% and metastasis in 34% of cases; most recurrences occur within 2 years of diagnosis.8 The aggressiveness of the tumor determines patient mortality; in cases without lymph node involvement, the 5-year survival rate is 83.3%. However, the 5-year survival rate drops to 58.3% with lymph node involvement; in patients with metastatic disease, it is just 31.3%.8,9 Although MCC is a chemosensitive disease, durable responses are rarely seen in the advanced setting.3 Most patients with metastatic disease have a median progression-free survival of only 3 months.1
Presentation and Diagnosis
An MCC tumor classically presents as a red to violaceous, painless nodule with a smooth shiny surface, most often on the sun-exposed head and neck region.3,9-11 Approximately 50% of MCC cases present in the head and neck region, 32% to 38% present on the extremities, and 12% to 14% on the trunk.6 Unfortunately, this nonspecific presentation may lead to diagnostic uncertainty and a consequent delay in treatment. Definitive diagnosis of MCC can only be achieved with a skin biopsy, which allows for distinction from other clinically similar-appearing neoplasms.
Specific guidelines for an MCC diagnostic evaluation have been proposed by the National Comprehensive Cancer Network (NCCN) based on a framework of clinical presentation, preliminary workup, diagnosis, and additional workup.12 The simplified algorithm for evaluation includes the following12,13:
- Examine skin and lymph nodes
- Obtain a biopsy specimen stained with hematoxylin and eosin (including Breslow thickness and evidence of lymphovascular invasion) and immunostaining (including but not limited to cytokeratin (CK) 20 and thyroid transcription factor 1)
- Obtain a sentinel lymph node biopsy specimen from patients with negative clinical nodes, prior to excision when possible
- Perform fine-needle aspiration or core biopsy first for patients with positive clinical nodes; if negative, consider open biopsy, but if positive, proceed to next step
- Perform imaging as clinically indicated with magnetic resonance imaging, computed tomography, or positron emission tomography–computed tomography
- Consider consultation with a multidisciplinary tumor board
Histologic Features of MCC
Merkel cell carcinoma presents histologically as small round basophilic cells penetrating through the dermis in 3 histologic patterns: the trabecular, intermediate (80% of cases), and small cell type.8,10 Immunohistochemical features include CK20 positivity (showing paranuclear dotlike depositions in the cytoplasm or cell membrane) and CK7 negativity, which allows it to be differentiated from other neoplasms. Chromogranin and synaptophysin positivity provide further histologic confirmation. The absence of peripheral palisading, retraction artifact, and a fibromyxoid stroma allow for distinction from cutaneous basal cell carcinoma, which may display these features histologically. Other immunohistochemical markers that may be of value include thyroid transcription factor 1, which is typically positive in cutaneous metastasis of neuroendocrine carcinoma of the lung; S-100 and human melanoma black 45, which are positive in melanoma; and leukocyte common antigen (CD45), which can be positive in lymphoma. These stains are classically negative in MCC.4,8
Merkel Cell Polyomavirus and Other Risk Factors
Merkel cell carcinoma is frequently associated with the presence of Merkel cell polyomavirus (MCPyV) in tumor specimens, with a prevalence of 70% to 80% in all cases.8 Merkel cell polyomavirus is a class 2A carcinogen (ie, a probable carcinogen to humans) and is classified among a group of viruses that encode T antigens (ie, an antigen coded by a viral genome associated with transformation of infected cells by tumor viruses), which can lead to initiation of tumorigenesis through interference with cellular tumor suppressing proteins such as p53.10
Immunosuppression and UV-exposed fair skin also are considered major risk factors, which may be explained by the increase in MCPyV small T-antigen transcripts induced by UV irradiation.10 Moreover, as is seen in other cancers induced by viruses, host immunity can hinder tumor progression and development. Therefore, impairment of normal immune function likely creates a higher risk for MCC development as well as the potential for a worse prognosis.4,8 The precise incidence of MCC in immunosuppressed patients appears unclear; it is possible that chronic immunosuppressive therapy may play a notable role in the pathogenesis of the tumor.4,8
Impact of Comorbidities on MCC
These risk factors were all observed in the case patient. However, it also is possible that his associated comorbidities also had an impact on the presentation of his disease.8 For example, patients with rheumatoid arthritis have been shown to have an elevated risk for the development of MCC.14 Moreover, inflammatory monocytes infected with MCPyV, as would be expected in a patient with a history of chronic psoriasis prior to diagnosis of MCC, also may contribute to the pathogenesis of MCC by migrating to inflammatory skin lesions, such as those seen in psoriasis, releasing MCPyV locally and infecting Merkel cells.8,15 Testing for MCPyV was never performed in the case patient; however, it certainly would be prudent to do so in such a presentation. Additionally, further studies to determine the correlation of MCC to these disease processes are warranted.8
Given that MCC tends to affect an older population in which other notable comorbidities are not uncommon, the cost, side effects, and convenience for patients of any treatment plan are important considerations.8 In the case study, a combined regimen of carboplatin and VP-16 (etoposide) was utilized. This regimen was well tolerated by the patient and successful in achieving complete radiologic and clinical remission of his metastatic disease. This combination has been shown to prolong survival in patients with distant metastasis compared to those patients not receiving chemotherapy.6 In all high-risk patients, close clinical monitoring is essential to help optimize outcomes.
Bottom Line
Merkel cell carcinoma is a rare but aggressive cutaneous neoplasm that most frequently affects elderly patients, immunosuppressed patients, and individuals with chronic UV sun damage. An association between the oncogenesis of MCC and infection with MCPyV has been documented, but other underlying diseases such as psoriasis and rheumatoid arthritis also may play a role. In this case, the patient’s history of chronic immunosuppressive therapy for treatment of psoriasis and inflammatory joint disease likely played a role in the pathogenesis of the tumor. This potential risk should be an important point of discussion with any patient requiring this type of long-term management for disease control. This unique clinical case highlights a patient with substantial comorbidities who developed metastatic MCC and achieved complete clinical and radiologic remission following treatment with surgery and chemotherapy.
Background
Merkel cell carcinoma (MCC) is a rare, aggressive cutaneous malignancy.1 Immunosuppression, advanced age, and UV light exposure of fair skin are major risk factors; additionally, polyomavirus infection is detected in as many as 80% of cases.2-5 Merkel cells are slow-responding mechanoreceptors located within the basal layer of the epidermis.6 Approximately half of patients present with localized disease, in which surgical resection with or without adjuvant radiotherapy is usually indicated. However, overall prognosis is poor with MCC due to high recurrence rates.3
This neuroendocrine carcinoma has remarkable metastatic potential (34%–75%) and can invade regional lymph nodes; distant metastasis also can occur, most commonly to the liver, lungs, bones, and brain.7,8 Approximately 25% of patients present with palpable lymphadenopathy and 5% with distant metastasis at the time of diagnosis. The frequency of metastasis at diagnosis as well as high recurrence rates after treatment contribute to the overall poor prognosis of MCC. Local recurrence rates have been reported at 25%, with lymph node involvement in 52% and metastasis in 34% of cases; most recurrences occur within 2 years of diagnosis.8 The aggressiveness of the tumor determines patient mortality; in cases without lymph node involvement, the 5-year survival rate is 83.3%. However, the 5-year survival rate drops to 58.3% with lymph node involvement; in patients with metastatic disease, it is just 31.3%.8,9 Although MCC is a chemosensitive disease, durable responses are rarely seen in the advanced setting.3 Most patients with metastatic disease have a median progression-free survival of only 3 months.1
Presentation and Diagnosis
An MCC tumor classically presents as a red to violaceous, painless nodule with a smooth shiny surface, most often on the sun-exposed head and neck region.3,9-11 Approximately 50% of MCC cases present in the head and neck region, 32% to 38% present on the extremities, and 12% to 14% on the trunk.6 Unfortunately, this nonspecific presentation may lead to diagnostic uncertainty and a consequent delay in treatment. Definitive diagnosis of MCC can only be achieved with a skin biopsy, which allows for distinction from other clinically similar-appearing neoplasms.
Specific guidelines for an MCC diagnostic evaluation have been proposed by the National Comprehensive Cancer Network (NCCN) based on a framework of clinical presentation, preliminary workup, diagnosis, and additional workup.12 The simplified algorithm for evaluation includes the following12,13:
- Examine skin and lymph nodes
- Obtain a biopsy specimen stained with hematoxylin and eosin (including Breslow thickness and evidence of lymphovascular invasion) and immunostaining (including but not limited to cytokeratin (CK) 20 and thyroid transcription factor 1)
- Obtain a sentinel lymph node biopsy specimen from patients with negative clinical nodes, prior to excision when possible
- Perform fine-needle aspiration or core biopsy first for patients with positive clinical nodes; if negative, consider open biopsy, but if positive, proceed to next step
- Perform imaging as clinically indicated with magnetic resonance imaging, computed tomography, or positron emission tomography–computed tomography
- Consider consultation with a multidisciplinary tumor board
Histologic Features of MCC
Merkel cell carcinoma presents histologically as small round basophilic cells penetrating through the dermis in 3 histologic patterns: the trabecular, intermediate (80% of cases), and small cell type.8,10 Immunohistochemical features include CK20 positivity (showing paranuclear dotlike depositions in the cytoplasm or cell membrane) and CK7 negativity, which allows it to be differentiated from other neoplasms. Chromogranin and synaptophysin positivity provide further histologic confirmation. The absence of peripheral palisading, retraction artifact, and a fibromyxoid stroma allow for distinction from cutaneous basal cell carcinoma, which may display these features histologically. Other immunohistochemical markers that may be of value include thyroid transcription factor 1, which is typically positive in cutaneous metastasis of neuroendocrine carcinoma of the lung; S-100 and human melanoma black 45, which are positive in melanoma; and leukocyte common antigen (CD45), which can be positive in lymphoma. These stains are classically negative in MCC.4,8
Merkel Cell Polyomavirus and Other Risk Factors
Merkel cell carcinoma is frequently associated with the presence of Merkel cell polyomavirus (MCPyV) in tumor specimens, with a prevalence of 70% to 80% in all cases.8 Merkel cell polyomavirus is a class 2A carcinogen (ie, a probable carcinogen to humans) and is classified among a group of viruses that encode T antigens (ie, an antigen coded by a viral genome associated with transformation of infected cells by tumor viruses), which can lead to initiation of tumorigenesis through interference with cellular tumor suppressing proteins such as p53.10
Immunosuppression and UV-exposed fair skin also are considered major risk factors, which may be explained by the increase in MCPyV small T-antigen transcripts induced by UV irradiation.10 Moreover, as is seen in other cancers induced by viruses, host immunity can hinder tumor progression and development. Therefore, impairment of normal immune function likely creates a higher risk for MCC development as well as the potential for a worse prognosis.4,8 The precise incidence of MCC in immunosuppressed patients appears unclear; it is possible that chronic immunosuppressive therapy may play a notable role in the pathogenesis of the tumor.4,8
Impact of Comorbidities on MCC
These risk factors were all observed in the case patient. However, it also is possible that his associated comorbidities also had an impact on the presentation of his disease.8 For example, patients with rheumatoid arthritis have been shown to have an elevated risk for the development of MCC.14 Moreover, inflammatory monocytes infected with MCPyV, as would be expected in a patient with a history of chronic psoriasis prior to diagnosis of MCC, also may contribute to the pathogenesis of MCC by migrating to inflammatory skin lesions, such as those seen in psoriasis, releasing MCPyV locally and infecting Merkel cells.8,15 Testing for MCPyV was never performed in the case patient; however, it certainly would be prudent to do so in such a presentation. Additionally, further studies to determine the correlation of MCC to these disease processes are warranted.8
Given that MCC tends to affect an older population in which other notable comorbidities are not uncommon, the cost, side effects, and convenience for patients of any treatment plan are important considerations.8 In the case study, a combined regimen of carboplatin and VP-16 (etoposide) was utilized. This regimen was well tolerated by the patient and successful in achieving complete radiologic and clinical remission of his metastatic disease. This combination has been shown to prolong survival in patients with distant metastasis compared to those patients not receiving chemotherapy.6 In all high-risk patients, close clinical monitoring is essential to help optimize outcomes.
Bottom Line
Merkel cell carcinoma is a rare but aggressive cutaneous neoplasm that most frequently affects elderly patients, immunosuppressed patients, and individuals with chronic UV sun damage. An association between the oncogenesis of MCC and infection with MCPyV has been documented, but other underlying diseases such as psoriasis and rheumatoid arthritis also may play a role. In this case, the patient’s history of chronic immunosuppressive therapy for treatment of psoriasis and inflammatory joint disease likely played a role in the pathogenesis of the tumor. This potential risk should be an important point of discussion with any patient requiring this type of long-term management for disease control. This unique clinical case highlights a patient with substantial comorbidities who developed metastatic MCC and achieved complete clinical and radiologic remission following treatment with surgery and chemotherapy.
- O’Brien T, Power DG. Metastatic Merkel-cell carcinoma: the dawn of a new era. BMJ Case Rep. 2018;11:2018. doi:10.1136/bcr-2018-224924.
- Del Marmol V, Lebbé C. New perspectives in Merkel cell carcinoma. Curr Opin Oncol. 2019;31:72-83.
- Garcia-Carbonero R, Marquez-Rodas I, de la Cruz-Merino L, et al. Recent therapeutic advances and change in treatment paradigm of patients with Merkel cell carcinoma [published online April 8, 2019]. Oncologist. doi:10.1634/theoncologist.2018-0718.
- Samimi M, Gardair C, Nicol JT, et al. Merkel cell polyomavirus in Merkel cell carcinoma: clinical and therapeutic perspectives. Semin Oncol. 2015;42:347-358.
- Kitamura N, Tomita R, Yamamoto M, et al. Complete remission of Merkel cell carcinoma on the upper lip treated with radiation monotherapy and a literature review of Japanese cases. World J Surg Oncol. 2015;13:152.
- Timmer FC, Klop WM, Relyveld GN, et al. Merkel cell carcinoma of the head and neck: emphasizing the risk of undertreatment. Eur Arch Otorhinolaryngol. 2016;273:1243-1252.
- Açıkalın A, Paydas¸ S, Güleç ÜK, et al. A unique case of Merkel cell carcinoma with ovarian metastasis. Balkan Med J. 2014;31:356-359.
- Yousif J, Yousif B, Kuriata MA. Complete remission of metastatic Merkel cell carcinoma in a patient with severe psoriasis. Cutis. 2018;101:E24-E27.
- Grandhaye M, Teixeira PG, Henrot P, et al. Focus on Merkel cell carcinoma: diagnosis and staging. Skeletal Radiol. 2015;44:777-786.
- Chatzinasiou F, Papadavid E, Korkolopoulou P, et al. An unusual case of diffuse Merkel cell carcinoma successfully treated with low dose radiotherapy. Dermatol Ther. 2015;28:282-286.
- Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature. Int J Surg Case Rep. 2015;7C:104-108.
- National Comprehensive Cancer Network. Merkel cell carcinoma. Published October 3, 2016. http://merkelcell.org/wp-content/uploads/2015/10/MccNccn.pdf. Accessed September 10, 2019.
- Coggshall K, Tello TL, North JP, Yu SS. Merkel cell carcinoma: an update and review: pathogenesis, diagnosis, and staging. J Am Acad Dermatol. 2018;78:433-442.
- Lanoy E, Engels EA. Skin cancers associated with autoimmune conditions among elderly adults. Br J Cancer. 2010;103:112-114.
- Mertz KD, Junt T, Schmid M, et al. Inflammatory monocytes are a reservoir for Merkel cell polyomavirus. J Invest Dermatol. 2009;130:1146-1151.
- O’Brien T, Power DG. Metastatic Merkel-cell carcinoma: the dawn of a new era. BMJ Case Rep. 2018;11:2018. doi:10.1136/bcr-2018-224924.
- Del Marmol V, Lebbé C. New perspectives in Merkel cell carcinoma. Curr Opin Oncol. 2019;31:72-83.
- Garcia-Carbonero R, Marquez-Rodas I, de la Cruz-Merino L, et al. Recent therapeutic advances and change in treatment paradigm of patients with Merkel cell carcinoma [published online April 8, 2019]. Oncologist. doi:10.1634/theoncologist.2018-0718.
- Samimi M, Gardair C, Nicol JT, et al. Merkel cell polyomavirus in Merkel cell carcinoma: clinical and therapeutic perspectives. Semin Oncol. 2015;42:347-358.
- Kitamura N, Tomita R, Yamamoto M, et al. Complete remission of Merkel cell carcinoma on the upper lip treated with radiation monotherapy and a literature review of Japanese cases. World J Surg Oncol. 2015;13:152.
- Timmer FC, Klop WM, Relyveld GN, et al. Merkel cell carcinoma of the head and neck: emphasizing the risk of undertreatment. Eur Arch Otorhinolaryngol. 2016;273:1243-1252.
- Açıkalın A, Paydas¸ S, Güleç ÜK, et al. A unique case of Merkel cell carcinoma with ovarian metastasis. Balkan Med J. 2014;31:356-359.
- Yousif J, Yousif B, Kuriata MA. Complete remission of metastatic Merkel cell carcinoma in a patient with severe psoriasis. Cutis. 2018;101:E24-E27.
- Grandhaye M, Teixeira PG, Henrot P, et al. Focus on Merkel cell carcinoma: diagnosis and staging. Skeletal Radiol. 2015;44:777-786.
- Chatzinasiou F, Papadavid E, Korkolopoulou P, et al. An unusual case of diffuse Merkel cell carcinoma successfully treated with low dose radiotherapy. Dermatol Ther. 2015;28:282-286.
- Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature. Int J Surg Case Rep. 2015;7C:104-108.
- National Comprehensive Cancer Network. Merkel cell carcinoma. Published October 3, 2016. http://merkelcell.org/wp-content/uploads/2015/10/MccNccn.pdf. Accessed September 10, 2019.
- Coggshall K, Tello TL, North JP, Yu SS. Merkel cell carcinoma: an update and review: pathogenesis, diagnosis, and staging. J Am Acad Dermatol. 2018;78:433-442.
- Lanoy E, Engels EA. Skin cancers associated with autoimmune conditions among elderly adults. Br J Cancer. 2010;103:112-114.
- Mertz KD, Junt T, Schmid M, et al. Inflammatory monocytes are a reservoir for Merkel cell polyomavirus. J Invest Dermatol. 2009;130:1146-1151.
The Case
A 69-year-old white man presented with a skin lesion on the back of 1 to 2 weeks’ duration. The patient stated he was unaware of the lesion, but his wife had recently noticed it; he denied any bleeding, pain, pruritus, or other associated symptoms with the lesion. He also denied any prior treatment to the area. The patient’s medical history was remarkable for severe psoriasis involving more than 80% body surface area, psoriatic arthritis, rheumatoid arthritis, ankylosing spondylitis, coronary artery disease, squamous cell carcinoma, and actinic keratoses. The patient had been on numerous treatment regimens for the control of psoriasis over the last 20 years, including topical corticosteroids, psoralen plus UVA and UVB phototherapy, gold injections, acitretin, prednisone, efalizumab, ustekinumab, and alefacept at presentation of this new skin lesion. The use of immunosuppressive agents for his psoriasis provided an additional benefit of controlling the inflammatory arthritic disease.
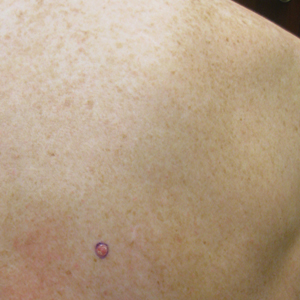
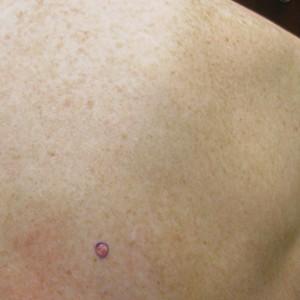
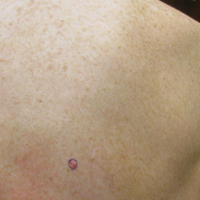
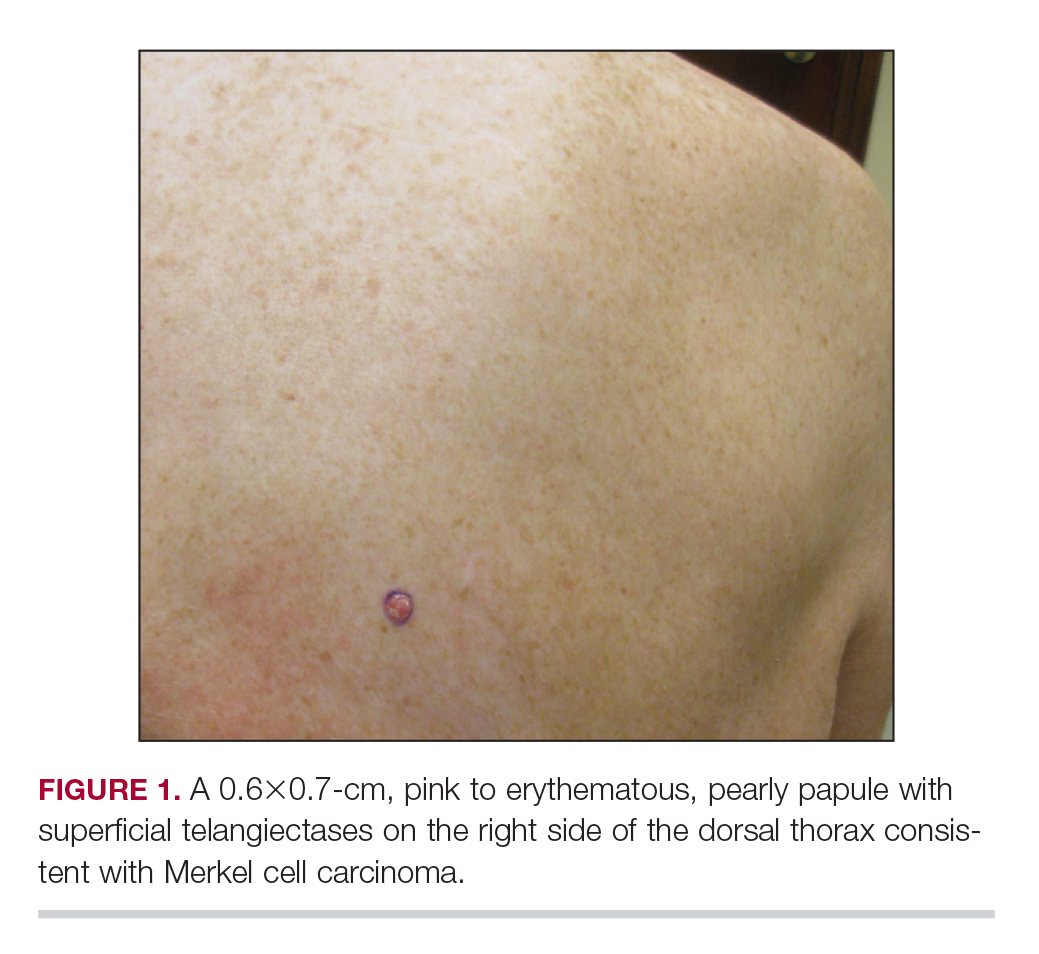
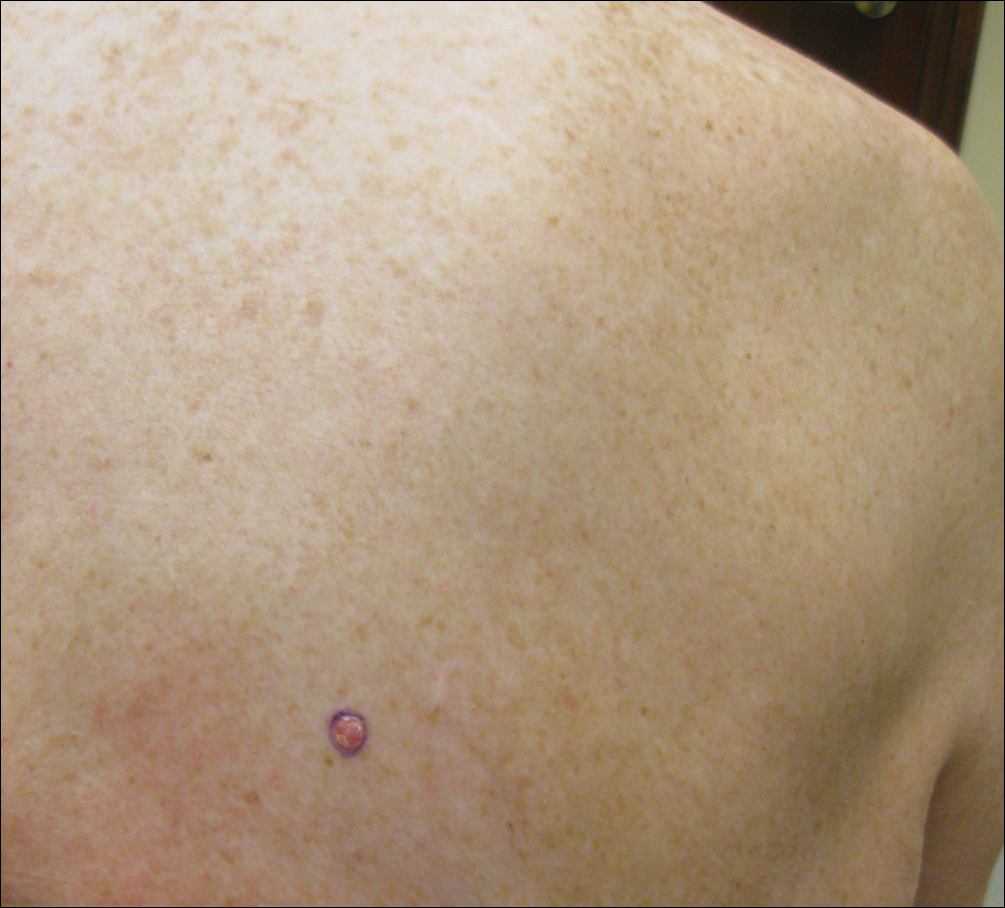
Physical examination revealed a 0.6×0.7-cm, pink to erythematous, pearly papule with superficial telangiectases on the right side of the dorsal thorax (Figure 1). Multiple well-demarcated erythematous plaques with silvery scale and areas of secondary excoriation were noted on the trunk and both legs consistent with the patient’s history of psoriasis.
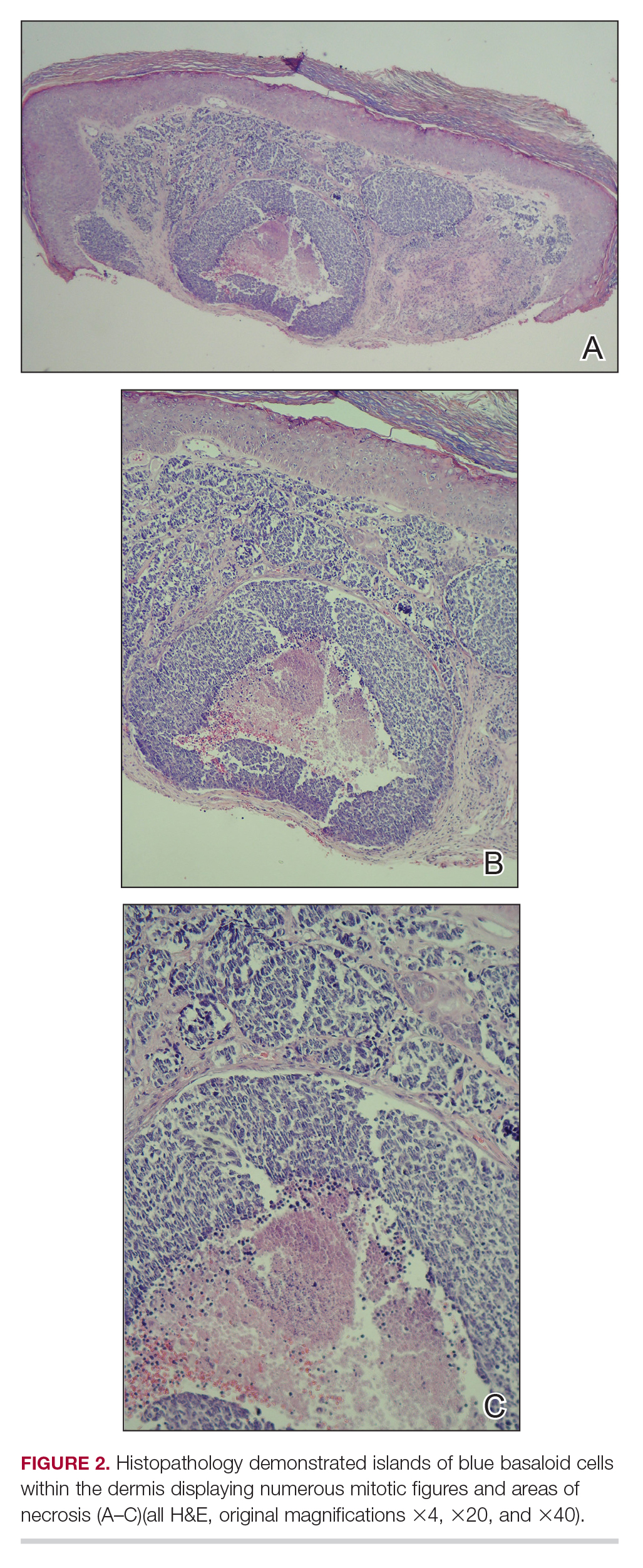
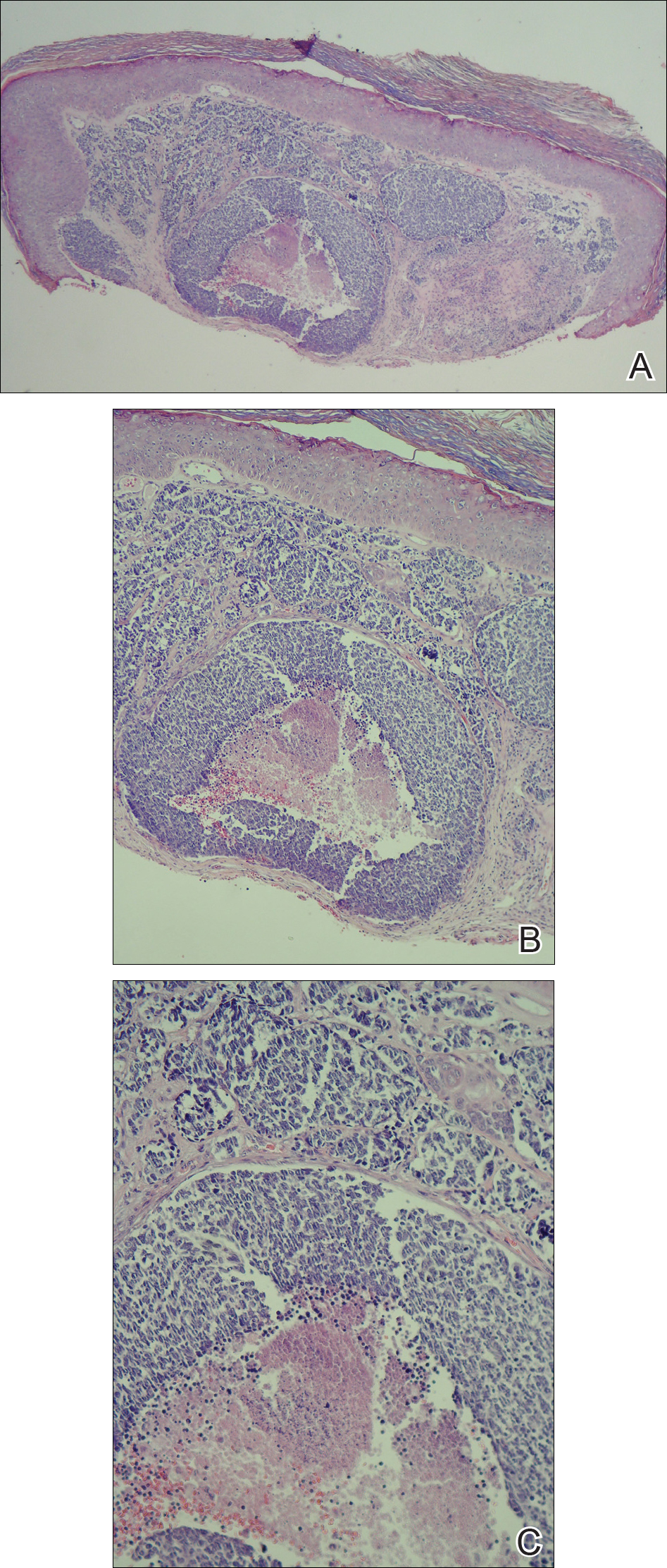
A shave biopsy was performed on the skin lesion on the right side of the dorsal thorax with a suspected clinical diagnosis of basal cell carcinoma. Two weeks later, the patient returned to discuss the pathology report, which revealed nodules of basaloid cells with tightly packed vesicular nuclei and scant cytoplasm in sheets within the superficial dermis, as well as areas of nuclear molding, numerous mitotic figures, and areas of focal necrosis (Figure 2). In addition, immunostaining was positive for cytokeratin 20 antibodies with a characteristic paranuclear dot uptake of the antibody. These findings were consistent with a diagnosis of Merkel cell carcinoma (MCC). At that time, alefacept was discontinued and the patient was referred to a tertiary referral center for further evaluation and treatment.
Treatment
He subsequently underwent wide excision with 1-cm margins of the MCC, with intraoperative lymphatic mapping/sentinel lymph node biopsy of the right axillary nodal basin 1 month later, which he tolerated well without any associated complications. Further histopathologic examination revealed the deep, medial, and lateral surgical margins to be negative of residual neoplasm. However, one sentinel lymph node indicated positivity for micrometastatic MCC, consistent with stage IIIA disease progression. The patient underwent a second procedure the following month for complete right axillary lymph node dissection. Histopathologic examination of the right axillary contents included 28 lymph nodes, which were negative for carcinoma. He continued to do well without any signs of clinical recurrence or distant metastasis at subsequent follow-up visits.
Patient Outcome
Approximately 2.5 years after the second procedure, the patient began to develop right upper quadrant abdominal pain of an unclear etiology. Computed tomography of the abdomen and pelvis was performed, revealing areas of calcification and findings consistent with malignant lymphadenopathy. Multiple hepatic lesions also were noted, including a 9-cm lesion in the posterior right hepatic lobe. Computed tomography–guided biopsy of the liver lesion was performed, and the findings were consistent with metastatic MCC, indicating progression to stage IV disease. The patient was subsequently started on combination chemotherapeutic treatment with carboplatin and VP-16, with a planned treatment course of 4 to 6 cycles. He was able to complete a total of 6 cycles over a 4-month period and tolerated the treatment regimen fairly well. Follow-up positron emission tomography–computed tomography was within normal limits with no evidence of any hypermetabolic activity noted, indicating a complete radiographic remission of MCC. He was seen approximately 1 month after completion of treatment for clinical follow-up and monthly thereafter.
While on chemotherapy, the patient experienced a notable improvement in the psoriasis and psoriatic joint disease. Upon completion of chemotherapy, he was restarted on the same treatment plan that was utilized prior to surgery including topical corticosteroids, calcitriol, intramuscular steroid injections, and UVB phototherapy, which provided substantial control of psoriasis and arthritic joint disease. The patient later died, likely due to his multiple comorbidities.
This case was adapted from Yousif J, Yousif B, Kuriata MA. Complete remission of metastatic Merkel cell carcinoma in a patient with severe psoriasis. Cutis. 2018;101:E24-E27.
Complete Remission of Metastatic Merkel Cell Carcinoma in a Patient With Severe Psoriasis
To the Editor:
A 69-year-old white man presented with a skin lesion on the back of 1 to 2 weeks’ duration. The patient stated he was unaware of it, but his wife had recently noticed the new spot. He denied any bleeding, pain, pruritus, or other associated symptoms with the lesion. He also denied any prior treatment to the area. The patient’s medical history was remarkable for severe psoriasis involving more than 80% body surface area, psoriatic arthritis, rheumatoid arthritis, ankylosing spondylitis, coronary artery disease, squamous cell carcinoma, and actinic keratoses. He had been on multiple treatment regimens over the last 20 years for control of psoriasis including topical corticosteroids, psoralen plus UVA and UVB phototherapy, gold injections, acitretin, prednisone, efalizumab, ustekinumab, and alefacept upon evaluation of this new skin lesion. Utilization of immunosuppressive agents also provided an additional benefit of controlling the patient’s inflammatory arthritic disease.
On physical examination a 0.6×0.7-cm, pink to erythematous, pearly papule with superficial telangiectases was noted on the right side of the dorsal thorax (Figure 1). Multiple well-demarcated erythematous plaques with silvery scale and areas of secondary excoriation were noted on the trunk and both legs consistent with the patient’s history of psoriasis.
A shave biopsy was performed on the skin lesion on the right side of the dorsal thorax with a suspected clinical diagnosis of basal cell carcinoma. Two weeks later the patient returned for a discussion of the pathology report, which revealed nodules of basaloid cells with tightly packed vesicular nuclei and scant cytoplasm in sheets within the superficial dermis, as well as areas of nuclear molding, numerous mitotic figures, and areas of focal necrosis (Figure 2). In addition, immunostaining was positive for cytokeratin (CK) 20 antibodies with a characteristic paranuclear dot uptake of the antibody. These findings were consistent with a diagnosis of Merkel cell carcinoma (MCC). At that time, alefacept was discontinued and he was referred to a tertiary referral center for further evaluation and treatment.
The patient subsequently underwent wide excision with 1-cm margins of the MCC, with intraoperative lymphatic mapping/sentinel lymph node biopsy (SLNB) of the right axillary nodal basin 1 month later, which he tolerated well without any associated complications. Further histopathologic examination revealed the deep, medial, and lateral surgical margins to be negative of residual neoplasm. However, one sentinel lymph node indicated positivity for micrometastatic MCC, consistent with stage IIIA disease progression.
He underwent a second procedure the following month for complete right axillary lymph node dissection. Histopathologic examination of the right axillary contents included 28 lymph nodes, which were negative for carcinoma. He continued to do well without any signs of clinical recurrence or distant metastasis at subsequent follow-up visits.
Approximately 2.5 years after the second procedure, the patient began to develop right upper quadrant abdominal pain of an unclear etiology. Computed tomography of the abdomen and pelvis was performed, revealing areas of calcification and findings consistent with malignant lymphadenopathy. Multiple hepatic lesions also were noted including a 9-cm lesion in the posterior right hepatic lobe. Computed tomography–guided biopsy of the liver lesion was performed and the findings were consistent with metastatic MCC, indicating progression to stage IV disease.
The patient was subsequently started on combination chemotherapeutic treatment with carboplatin and VP-16, with a planned treatment course of 4 to 6 cycles. He was able to complete a total of 6 cycles over a 4-month period, tolerating the treatment regimen fairly well. Follow-up positron emission tomography–computed tomography was within normal limits with no evidence of any hypermetabolic activity noted, indicating a complete radiographic remission of MCC. He was seen approximately 1 month after completion of treatment for clinical follow-up and monthly thereafter.
While on chemotherapy, the patient experienced a notable improvement in the psoriasis and psoriatic joint disease. Upon completion of chemotherapy, he was restarted on the same treatment plan that was utilized prior to surgery including topical corticosteroids, calcitriol, intramuscular steroid injections, and UVB phototherapy, which provided substantial control of psoriasis and arthritic joint disease. The patient later died, likely due to his multiple comorbidities.
Merkel cells are slow-responding mechanoreceptors located within the basal layer of the epidermis and are the source of a rare aggressive cutaneous malignancy.1 Merkel cell carcinoma was first noted in 1972 and termed trabecular carcinoma of the skin, and it accounts for less than 1% of all nonmelanoma skin cancer.2,3 This primary neuroendocrine carcinoma has remarkable metastatic potential (34%–75%) and can invade regional lymph nodes, as well as distant metastasis most commonly to the liver, lungs, bones, and brain.2 Approximately 25% of patients present with palpable lymphadenopathy and 5% with distant metastasis at the time of diagnosis. This frequency of metastasis at diagnosis as well as the recurrence after treatment contributes to the poor prognosis of MCC. Local recurrence rates have been reported at 25% with lymph node involvement in 52% and metastasis in 34%, with most recurrences occurring within 2 years of diagnosis. Patient mortality is dependent on the aggressiveness of the tumor, with 5-year survival rates of 83.3% without lymph node involvement, 58.3% with lymph node involvement, and 31.3% in those with metastatic disease.4
The tumor classically presents as a red to violaceous, painless nodule with a smooth shiny surface most often on the head and neck region.4-6 Approximately 50% of MCC cases present in the head and neck region, 32% to 38% on the extremities, and 12% to 14% on the trunk.1 This nonspecific presentation may lead to diagnostic uncertainty and a consequent delay in treatment. Definitive diagnosis of MCC is achieved with a skin biopsy and allows for distinction from other clinically similar–appearing neoplasms. Merkel cell carcinoma presents histologically as small round basophilic cells penetrating through the dermis in 3 histologic patterns: the trabecular, intermediate (80% of cases), and small cell type.5 It may be differentiated immunohistochemically from other neoplasms, as it displays CK20 positivity (showing paranuclear dotlike depositions in the cytoplasm or cell membrane) and is negative for CK7. Chromagranin and synaptophysin positivity also may provide further histologic confirmation. In addition, absence of peripheral palisading, retraction artifact, and a fibromyxoid stroma allow for distinction from cutaneous basal cell carcinoma, which may display these features histologically. Other immunohistochemical markers that may be of value include thyroid transcription factor 1, which is typically positive in cutaneous metastasis of neuroendocrine carcinoma of the lung; S-100 and human melanoma black 45, which are positive in melanoma; and leukocyte common antigen (CD45), which can be positive in lymphoma. These stains are classically negative in MCC.3
Merkel cell carcinoma is commonly associated with the presence of Merkel cell polyomavirus (MCPyV) in tumor specimens, with a prevalence of 70% to 80% in all cases. Merkel cell polyomavirus is a class 2A carcinogen (ie, a probable carcinogen to humans) and is classified among a group of viruses that encode T antigens (ie, an antigen coded by a viral genome associated with transformation of infected cells by tumor viruses), which can lead to initiation of tumorigenesis through interference with cellular tumor suppressing proteins such as p53.5 In addition, several risk factors have been associated with the development of MCC including immunosuppression, older age (>50 years), and UV-exposed fair skin.7 One explanation for this phenomenon is the increase in MCPyV small T antigen transcripts induced by UV irradiation.5 In addition, as with other cancers induced by viruses, host immunity can impede tumor progression and development. Therefore, impairment of normal immune function likely creates a higher risk for MCC development and potential for a worse prognosis.3Although the exact incidence of MCC in immunosuppressed patients appears unclear, chronic immunosuppressive therapy may play a notable role in the pathogenesis of the tumor.3
Although each of these factors was observed in our patient, it also was possible that his associated comorbidities further contributed to disease presentation. In particular, rheumatoid arthritis has been shown to carry an increased risk for the development of MCC.8 In addition, inflammatory monocytes infected with MCPyV, as evidenced in a patient with a history of chronic psoriasis prior to diagnosis of MCC, also may contribute to the pathogenesis of MCC by traveling to inflammatory skin lesions, such as those seen in psoriasis, releasing MCPyV locally and infecting Merkel cells.9 Although MCPyV testing was never performed in our patient, it certainly would be prudent as well as further studies determining the correlation of MCC to these disease processes.
Although regression is rare, multiple cases have documented spontaneous regression of MCC after biopsy of these lesions.4,6,10 The exact mechanism is unclear, but apoptosis induced by T-cell immunity is suspected to play a role. Programmed cell death 1 protein (PD-1)–positive cells play a role. The PD-1 receptor is an inhibitory receptor expressed by T cells and in approximately half of tumor-infiltrating cells in MCC. It was found that in a regressed case of MCC there was a notably lower percentage of PD-1 positivity compared to cases with no apparent regression, suggesting that PD-1–positive cells suppress tumor immunity to MCC and that significant reduction in these cells may induce clinical regression.10 Additional investigation would be beneficial to examine the relationship of this phenomenon to tumor regression.
Initial evaluation of these patients should include a meticulous clinical examination with an emphasis on detection of cutaneous, lymph node, and distant metastasis. Due to the risk of metastatic potential, regional lymph node ultrasonography and computed tomography of the chest, abdomen, and pelvis typically are recommended at baseline. Other imaging modalities may be warranted based on clinical findings.3 Treatment modalities include various approaches, with surgical excision of the primary tumor with more than 1-cm margin to the fascial plane being the primary modality for uncomplicated cases.1,3,7 In addition, SLNB also should be performed at the time of the procedure. In the case of a positive SLNB or suspected regional lymph node involvement upon initial examination, radical regional lymph node dissection also is recommended.3 Although some authorities advocate postsurgical radiation therapy to minimize the risk of local recurrence, there does not appear to be a clear benefit in survival rate.3,5 However, radiation treatment as monotherapy has been advocated in certain instances, particularly in cases of unresectable tumors or patients who are poor surgical candidates.5,7 Cases of distant metastasis (stage IV disease) may include management with surgery, radiation, and/or chemotherapy. Although none of these modalities have consistently shown to improve survival, there appears to be up to a 60% response with chemotherapy in these patients.3
Because MCC tends to affect an older population, often with other notable comorbidities, important considerations involving a treatment plan include the cost, side effects, and convenience for patients. The combination of carboplatin and VP-16 (etoposide) was utilized and tolerated well in our patient, and it has been successful in achieving complete radiologic and clinical remission of his metastatic disease. This combination appears to prolong survival in patients with distant metastasis, as compared to those patients not receiving chemotherapy.1 Our patient has since died, but in these high-risk patients, close clinical monitoring is essential to help optimize their prognosis.
Merkel cell carcinoma is a rare aggressive cutaneous neoplasm that most commonly affects the elderly, immunosuppressed, and those with chronic UV sun damage. An association between the oncogenesis of MCC and infection with MCPyV has been documented, but other underlying diseases also may play a role in this process including rheumatoid arthritis and psoriasis. Although these risk factors were associated with our patient, his history of chronic immunosuppressive therapy for treatment of his psoriasis and inflammatory joint disease likely played a role in the pathogenesis of the tumor and should be an important point of discussion with any patient requiring this type of long-term management for disease control. Our unique clinical case highlights a patient with substantial comorbidities who developed metastatic MCC and achieved complete clinical and radiologic remission after treatment with surgery and chemotherapy.
- Timmer FC, Klop WM, Relyveld GN, et al. Merkel cell carcinoma of the head and neck: emphasizing the risk of undertreatment [published online March 11, 2015]. Eur Arch Otorhinolaryngol. 2016;273:1243-1252.
- Açıkalın A, Paydas¸ S, Güleç ÜK, et al. A unique case of Merkel cell carcinoma with ovarian metastasis [published online December 1, 2014]. Balkan Med J. 2014;31:356-359.
- Samimi M, Gardair C, Nicol JT, et al. Merkel cell polyomavirus in Merkel cell carcinoma: clinical and therapeutic perspectives [published online Dec 31, 2014]. Semin Oncol. 2015;42:347-358.
- Grandhaye M, Teixeira PG, Henrot P, et al. Focus on Merkel cell carcinoma: diagnosis and staging [published online January 30, 2015]. Skeletal Radiol. 2015;44:777-786.
- Chatzinasiou F, Papadavid E, Korkolopoulou P, et al. An unusual case of diffuse Merkel cell carcinoma successfully treated with low dose radiotherapy [published online May 14, 2015]. Dermatol Ther. 2015;28:282-286.
- Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature [published online November 13, 2014]. Int J Surg Case Rep. 2015;7C:104-108.
- Kitamura N, Tomita R, Yamamoto M, et al. Complete remission of Merkel cell carcinoma on the upper lip treated with radiation monotherapy and a literature review of Japanese cases. World J Surg Oncol. 2015;13:152.
- Lanoy E, Engels EA. Skin cancers associated with autoimmune conditions among elderly adults [published online June 15, 2010]. Br J Cancer. 2010;103:112-114.
- Mertz KD, Junt T, Schmid M, et al. Inflammatory monocytes are a reservoir for Merkel cell polyomavirus [published online December 17, 2009]. J Invest Dermatol. 2009;130:1146-1151.
- Fujimoto N, Nakanishi G, Kabuto M, et al. Merkel cell carcinoma showing regression after biopsy: evaluation of programmed cell death 1-positive cells [published online February 24, 2015]. J Dermatol. 2015;42:496-499.
To the Editor:
A 69-year-old white man presented with a skin lesion on the back of 1 to 2 weeks’ duration. The patient stated he was unaware of it, but his wife had recently noticed the new spot. He denied any bleeding, pain, pruritus, or other associated symptoms with the lesion. He also denied any prior treatment to the area. The patient’s medical history was remarkable for severe psoriasis involving more than 80% body surface area, psoriatic arthritis, rheumatoid arthritis, ankylosing spondylitis, coronary artery disease, squamous cell carcinoma, and actinic keratoses. He had been on multiple treatment regimens over the last 20 years for control of psoriasis including topical corticosteroids, psoralen plus UVA and UVB phototherapy, gold injections, acitretin, prednisone, efalizumab, ustekinumab, and alefacept upon evaluation of this new skin lesion. Utilization of immunosuppressive agents also provided an additional benefit of controlling the patient’s inflammatory arthritic disease.
On physical examination a 0.6×0.7-cm, pink to erythematous, pearly papule with superficial telangiectases was noted on the right side of the dorsal thorax (Figure 1). Multiple well-demarcated erythematous plaques with silvery scale and areas of secondary excoriation were noted on the trunk and both legs consistent with the patient’s history of psoriasis.

A shave biopsy was performed on the skin lesion on the right side of the dorsal thorax with a suspected clinical diagnosis of basal cell carcinoma. Two weeks later the patient returned for a discussion of the pathology report, which revealed nodules of basaloid cells with tightly packed vesicular nuclei and scant cytoplasm in sheets within the superficial dermis, as well as areas of nuclear molding, numerous mitotic figures, and areas of focal necrosis (Figure 2). In addition, immunostaining was positive for cytokeratin (CK) 20 antibodies with a characteristic paranuclear dot uptake of the antibody. These findings were consistent with a diagnosis of Merkel cell carcinoma (MCC). At that time, alefacept was discontinued and he was referred to a tertiary referral center for further evaluation and treatment.

The patient subsequently underwent wide excision with 1-cm margins of the MCC, with intraoperative lymphatic mapping/sentinel lymph node biopsy (SLNB) of the right axillary nodal basin 1 month later, which he tolerated well without any associated complications. Further histopathologic examination revealed the deep, medial, and lateral surgical margins to be negative of residual neoplasm. However, one sentinel lymph node indicated positivity for micrometastatic MCC, consistent with stage IIIA disease progression.
He underwent a second procedure the following month for complete right axillary lymph node dissection. Histopathologic examination of the right axillary contents included 28 lymph nodes, which were negative for carcinoma. He continued to do well without any signs of clinical recurrence or distant metastasis at subsequent follow-up visits.
Approximately 2.5 years after the second procedure, the patient began to develop right upper quadrant abdominal pain of an unclear etiology. Computed tomography of the abdomen and pelvis was performed, revealing areas of calcification and findings consistent with malignant lymphadenopathy. Multiple hepatic lesions also were noted including a 9-cm lesion in the posterior right hepatic lobe. Computed tomography–guided biopsy of the liver lesion was performed and the findings were consistent with metastatic MCC, indicating progression to stage IV disease.
The patient was subsequently started on combination chemotherapeutic treatment with carboplatin and VP-16, with a planned treatment course of 4 to 6 cycles. He was able to complete a total of 6 cycles over a 4-month period, tolerating the treatment regimen fairly well. Follow-up positron emission tomography–computed tomography was within normal limits with no evidence of any hypermetabolic activity noted, indicating a complete radiographic remission of MCC. He was seen approximately 1 month after completion of treatment for clinical follow-up and monthly thereafter.
While on chemotherapy, the patient experienced a notable improvement in the psoriasis and psoriatic joint disease. Upon completion of chemotherapy, he was restarted on the same treatment plan that was utilized prior to surgery including topical corticosteroids, calcitriol, intramuscular steroid injections, and UVB phototherapy, which provided substantial control of psoriasis and arthritic joint disease. The patient later died, likely due to his multiple comorbidities.
Merkel cells are slow-responding mechanoreceptors located within the basal layer of the epidermis and are the source of a rare aggressive cutaneous malignancy.1 Merkel cell carcinoma was first noted in 1972 and termed trabecular carcinoma of the skin, and it accounts for less than 1% of all nonmelanoma skin cancer.2,3 This primary neuroendocrine carcinoma has remarkable metastatic potential (34%–75%) and can invade regional lymph nodes, as well as distant metastasis most commonly to the liver, lungs, bones, and brain.2 Approximately 25% of patients present with palpable lymphadenopathy and 5% with distant metastasis at the time of diagnosis. This frequency of metastasis at diagnosis as well as the recurrence after treatment contributes to the poor prognosis of MCC. Local recurrence rates have been reported at 25% with lymph node involvement in 52% and metastasis in 34%, with most recurrences occurring within 2 years of diagnosis. Patient mortality is dependent on the aggressiveness of the tumor, with 5-year survival rates of 83.3% without lymph node involvement, 58.3% with lymph node involvement, and 31.3% in those with metastatic disease.4
The tumor classically presents as a red to violaceous, painless nodule with a smooth shiny surface most often on the head and neck region.4-6 Approximately 50% of MCC cases present in the head and neck region, 32% to 38% on the extremities, and 12% to 14% on the trunk.1 This nonspecific presentation may lead to diagnostic uncertainty and a consequent delay in treatment. Definitive diagnosis of MCC is achieved with a skin biopsy and allows for distinction from other clinically similar–appearing neoplasms. Merkel cell carcinoma presents histologically as small round basophilic cells penetrating through the dermis in 3 histologic patterns: the trabecular, intermediate (80% of cases), and small cell type.5 It may be differentiated immunohistochemically from other neoplasms, as it displays CK20 positivity (showing paranuclear dotlike depositions in the cytoplasm or cell membrane) and is negative for CK7. Chromagranin and synaptophysin positivity also may provide further histologic confirmation. In addition, absence of peripheral palisading, retraction artifact, and a fibromyxoid stroma allow for distinction from cutaneous basal cell carcinoma, which may display these features histologically. Other immunohistochemical markers that may be of value include thyroid transcription factor 1, which is typically positive in cutaneous metastasis of neuroendocrine carcinoma of the lung; S-100 and human melanoma black 45, which are positive in melanoma; and leukocyte common antigen (CD45), which can be positive in lymphoma. These stains are classically negative in MCC.3
Merkel cell carcinoma is commonly associated with the presence of Merkel cell polyomavirus (MCPyV) in tumor specimens, with a prevalence of 70% to 80% in all cases. Merkel cell polyomavirus is a class 2A carcinogen (ie, a probable carcinogen to humans) and is classified among a group of viruses that encode T antigens (ie, an antigen coded by a viral genome associated with transformation of infected cells by tumor viruses), which can lead to initiation of tumorigenesis through interference with cellular tumor suppressing proteins such as p53.5 In addition, several risk factors have been associated with the development of MCC including immunosuppression, older age (>50 years), and UV-exposed fair skin.7 One explanation for this phenomenon is the increase in MCPyV small T antigen transcripts induced by UV irradiation.5 In addition, as with other cancers induced by viruses, host immunity can impede tumor progression and development. Therefore, impairment of normal immune function likely creates a higher risk for MCC development and potential for a worse prognosis.3Although the exact incidence of MCC in immunosuppressed patients appears unclear, chronic immunosuppressive therapy may play a notable role in the pathogenesis of the tumor.3
Although each of these factors was observed in our patient, it also was possible that his associated comorbidities further contributed to disease presentation. In particular, rheumatoid arthritis has been shown to carry an increased risk for the development of MCC.8 In addition, inflammatory monocytes infected with MCPyV, as evidenced in a patient with a history of chronic psoriasis prior to diagnosis of MCC, also may contribute to the pathogenesis of MCC by traveling to inflammatory skin lesions, such as those seen in psoriasis, releasing MCPyV locally and infecting Merkel cells.9 Although MCPyV testing was never performed in our patient, it certainly would be prudent as well as further studies determining the correlation of MCC to these disease processes.
Although regression is rare, multiple cases have documented spontaneous regression of MCC after biopsy of these lesions.4,6,10 The exact mechanism is unclear, but apoptosis induced by T-cell immunity is suspected to play a role. Programmed cell death 1 protein (PD-1)–positive cells play a role. The PD-1 receptor is an inhibitory receptor expressed by T cells and in approximately half of tumor-infiltrating cells in MCC. It was found that in a regressed case of MCC there was a notably lower percentage of PD-1 positivity compared to cases with no apparent regression, suggesting that PD-1–positive cells suppress tumor immunity to MCC and that significant reduction in these cells may induce clinical regression.10 Additional investigation would be beneficial to examine the relationship of this phenomenon to tumor regression.
Initial evaluation of these patients should include a meticulous clinical examination with an emphasis on detection of cutaneous, lymph node, and distant metastasis. Due to the risk of metastatic potential, regional lymph node ultrasonography and computed tomography of the chest, abdomen, and pelvis typically are recommended at baseline. Other imaging modalities may be warranted based on clinical findings.3 Treatment modalities include various approaches, with surgical excision of the primary tumor with more than 1-cm margin to the fascial plane being the primary modality for uncomplicated cases.1,3,7 In addition, SLNB also should be performed at the time of the procedure. In the case of a positive SLNB or suspected regional lymph node involvement upon initial examination, radical regional lymph node dissection also is recommended.3 Although some authorities advocate postsurgical radiation therapy to minimize the risk of local recurrence, there does not appear to be a clear benefit in survival rate.3,5 However, radiation treatment as monotherapy has been advocated in certain instances, particularly in cases of unresectable tumors or patients who are poor surgical candidates.5,7 Cases of distant metastasis (stage IV disease) may include management with surgery, radiation, and/or chemotherapy. Although none of these modalities have consistently shown to improve survival, there appears to be up to a 60% response with chemotherapy in these patients.3
Because MCC tends to affect an older population, often with other notable comorbidities, important considerations involving a treatment plan include the cost, side effects, and convenience for patients. The combination of carboplatin and VP-16 (etoposide) was utilized and tolerated well in our patient, and it has been successful in achieving complete radiologic and clinical remission of his metastatic disease. This combination appears to prolong survival in patients with distant metastasis, as compared to those patients not receiving chemotherapy.1 Our patient has since died, but in these high-risk patients, close clinical monitoring is essential to help optimize their prognosis.
Merkel cell carcinoma is a rare aggressive cutaneous neoplasm that most commonly affects the elderly, immunosuppressed, and those with chronic UV sun damage. An association between the oncogenesis of MCC and infection with MCPyV has been documented, but other underlying diseases also may play a role in this process including rheumatoid arthritis and psoriasis. Although these risk factors were associated with our patient, his history of chronic immunosuppressive therapy for treatment of his psoriasis and inflammatory joint disease likely played a role in the pathogenesis of the tumor and should be an important point of discussion with any patient requiring this type of long-term management for disease control. Our unique clinical case highlights a patient with substantial comorbidities who developed metastatic MCC and achieved complete clinical and radiologic remission after treatment with surgery and chemotherapy.
To the Editor:
A 69-year-old white man presented with a skin lesion on the back of 1 to 2 weeks’ duration. The patient stated he was unaware of it, but his wife had recently noticed the new spot. He denied any bleeding, pain, pruritus, or other associated symptoms with the lesion. He also denied any prior treatment to the area. The patient’s medical history was remarkable for severe psoriasis involving more than 80% body surface area, psoriatic arthritis, rheumatoid arthritis, ankylosing spondylitis, coronary artery disease, squamous cell carcinoma, and actinic keratoses. He had been on multiple treatment regimens over the last 20 years for control of psoriasis including topical corticosteroids, psoralen plus UVA and UVB phototherapy, gold injections, acitretin, prednisone, efalizumab, ustekinumab, and alefacept upon evaluation of this new skin lesion. Utilization of immunosuppressive agents also provided an additional benefit of controlling the patient’s inflammatory arthritic disease.
On physical examination a 0.6×0.7-cm, pink to erythematous, pearly papule with superficial telangiectases was noted on the right side of the dorsal thorax (Figure 1). Multiple well-demarcated erythematous plaques with silvery scale and areas of secondary excoriation were noted on the trunk and both legs consistent with the patient’s history of psoriasis.

A shave biopsy was performed on the skin lesion on the right side of the dorsal thorax with a suspected clinical diagnosis of basal cell carcinoma. Two weeks later the patient returned for a discussion of the pathology report, which revealed nodules of basaloid cells with tightly packed vesicular nuclei and scant cytoplasm in sheets within the superficial dermis, as well as areas of nuclear molding, numerous mitotic figures, and areas of focal necrosis (Figure 2). In addition, immunostaining was positive for cytokeratin (CK) 20 antibodies with a characteristic paranuclear dot uptake of the antibody. These findings were consistent with a diagnosis of Merkel cell carcinoma (MCC). At that time, alefacept was discontinued and he was referred to a tertiary referral center for further evaluation and treatment.

The patient subsequently underwent wide excision with 1-cm margins of the MCC, with intraoperative lymphatic mapping/sentinel lymph node biopsy (SLNB) of the right axillary nodal basin 1 month later, which he tolerated well without any associated complications. Further histopathologic examination revealed the deep, medial, and lateral surgical margins to be negative of residual neoplasm. However, one sentinel lymph node indicated positivity for micrometastatic MCC, consistent with stage IIIA disease progression.
He underwent a second procedure the following month for complete right axillary lymph node dissection. Histopathologic examination of the right axillary contents included 28 lymph nodes, which were negative for carcinoma. He continued to do well without any signs of clinical recurrence or distant metastasis at subsequent follow-up visits.
Approximately 2.5 years after the second procedure, the patient began to develop right upper quadrant abdominal pain of an unclear etiology. Computed tomography of the abdomen and pelvis was performed, revealing areas of calcification and findings consistent with malignant lymphadenopathy. Multiple hepatic lesions also were noted including a 9-cm lesion in the posterior right hepatic lobe. Computed tomography–guided biopsy of the liver lesion was performed and the findings were consistent with metastatic MCC, indicating progression to stage IV disease.
The patient was subsequently started on combination chemotherapeutic treatment with carboplatin and VP-16, with a planned treatment course of 4 to 6 cycles. He was able to complete a total of 6 cycles over a 4-month period, tolerating the treatment regimen fairly well. Follow-up positron emission tomography–computed tomography was within normal limits with no evidence of any hypermetabolic activity noted, indicating a complete radiographic remission of MCC. He was seen approximately 1 month after completion of treatment for clinical follow-up and monthly thereafter.
While on chemotherapy, the patient experienced a notable improvement in the psoriasis and psoriatic joint disease. Upon completion of chemotherapy, he was restarted on the same treatment plan that was utilized prior to surgery including topical corticosteroids, calcitriol, intramuscular steroid injections, and UVB phototherapy, which provided substantial control of psoriasis and arthritic joint disease. The patient later died, likely due to his multiple comorbidities.
Merkel cells are slow-responding mechanoreceptors located within the basal layer of the epidermis and are the source of a rare aggressive cutaneous malignancy.1 Merkel cell carcinoma was first noted in 1972 and termed trabecular carcinoma of the skin, and it accounts for less than 1% of all nonmelanoma skin cancer.2,3 This primary neuroendocrine carcinoma has remarkable metastatic potential (34%–75%) and can invade regional lymph nodes, as well as distant metastasis most commonly to the liver, lungs, bones, and brain.2 Approximately 25% of patients present with palpable lymphadenopathy and 5% with distant metastasis at the time of diagnosis. This frequency of metastasis at diagnosis as well as the recurrence after treatment contributes to the poor prognosis of MCC. Local recurrence rates have been reported at 25% with lymph node involvement in 52% and metastasis in 34%, with most recurrences occurring within 2 years of diagnosis. Patient mortality is dependent on the aggressiveness of the tumor, with 5-year survival rates of 83.3% without lymph node involvement, 58.3% with lymph node involvement, and 31.3% in those with metastatic disease.4
The tumor classically presents as a red to violaceous, painless nodule with a smooth shiny surface most often on the head and neck region.4-6 Approximately 50% of MCC cases present in the head and neck region, 32% to 38% on the extremities, and 12% to 14% on the trunk.1 This nonspecific presentation may lead to diagnostic uncertainty and a consequent delay in treatment. Definitive diagnosis of MCC is achieved with a skin biopsy and allows for distinction from other clinically similar–appearing neoplasms. Merkel cell carcinoma presents histologically as small round basophilic cells penetrating through the dermis in 3 histologic patterns: the trabecular, intermediate (80% of cases), and small cell type.5 It may be differentiated immunohistochemically from other neoplasms, as it displays CK20 positivity (showing paranuclear dotlike depositions in the cytoplasm or cell membrane) and is negative for CK7. Chromagranin and synaptophysin positivity also may provide further histologic confirmation. In addition, absence of peripheral palisading, retraction artifact, and a fibromyxoid stroma allow for distinction from cutaneous basal cell carcinoma, which may display these features histologically. Other immunohistochemical markers that may be of value include thyroid transcription factor 1, which is typically positive in cutaneous metastasis of neuroendocrine carcinoma of the lung; S-100 and human melanoma black 45, which are positive in melanoma; and leukocyte common antigen (CD45), which can be positive in lymphoma. These stains are classically negative in MCC.3
Merkel cell carcinoma is commonly associated with the presence of Merkel cell polyomavirus (MCPyV) in tumor specimens, with a prevalence of 70% to 80% in all cases. Merkel cell polyomavirus is a class 2A carcinogen (ie, a probable carcinogen to humans) and is classified among a group of viruses that encode T antigens (ie, an antigen coded by a viral genome associated with transformation of infected cells by tumor viruses), which can lead to initiation of tumorigenesis through interference with cellular tumor suppressing proteins such as p53.5 In addition, several risk factors have been associated with the development of MCC including immunosuppression, older age (>50 years), and UV-exposed fair skin.7 One explanation for this phenomenon is the increase in MCPyV small T antigen transcripts induced by UV irradiation.5 In addition, as with other cancers induced by viruses, host immunity can impede tumor progression and development. Therefore, impairment of normal immune function likely creates a higher risk for MCC development and potential for a worse prognosis.3Although the exact incidence of MCC in immunosuppressed patients appears unclear, chronic immunosuppressive therapy may play a notable role in the pathogenesis of the tumor.3
Although each of these factors was observed in our patient, it also was possible that his associated comorbidities further contributed to disease presentation. In particular, rheumatoid arthritis has been shown to carry an increased risk for the development of MCC.8 In addition, inflammatory monocytes infected with MCPyV, as evidenced in a patient with a history of chronic psoriasis prior to diagnosis of MCC, also may contribute to the pathogenesis of MCC by traveling to inflammatory skin lesions, such as those seen in psoriasis, releasing MCPyV locally and infecting Merkel cells.9 Although MCPyV testing was never performed in our patient, it certainly would be prudent as well as further studies determining the correlation of MCC to these disease processes.
Although regression is rare, multiple cases have documented spontaneous regression of MCC after biopsy of these lesions.4,6,10 The exact mechanism is unclear, but apoptosis induced by T-cell immunity is suspected to play a role. Programmed cell death 1 protein (PD-1)–positive cells play a role. The PD-1 receptor is an inhibitory receptor expressed by T cells and in approximately half of tumor-infiltrating cells in MCC. It was found that in a regressed case of MCC there was a notably lower percentage of PD-1 positivity compared to cases with no apparent regression, suggesting that PD-1–positive cells suppress tumor immunity to MCC and that significant reduction in these cells may induce clinical regression.10 Additional investigation would be beneficial to examine the relationship of this phenomenon to tumor regression.
Initial evaluation of these patients should include a meticulous clinical examination with an emphasis on detection of cutaneous, lymph node, and distant metastasis. Due to the risk of metastatic potential, regional lymph node ultrasonography and computed tomography of the chest, abdomen, and pelvis typically are recommended at baseline. Other imaging modalities may be warranted based on clinical findings.3 Treatment modalities include various approaches, with surgical excision of the primary tumor with more than 1-cm margin to the fascial plane being the primary modality for uncomplicated cases.1,3,7 In addition, SLNB also should be performed at the time of the procedure. In the case of a positive SLNB or suspected regional lymph node involvement upon initial examination, radical regional lymph node dissection also is recommended.3 Although some authorities advocate postsurgical radiation therapy to minimize the risk of local recurrence, there does not appear to be a clear benefit in survival rate.3,5 However, radiation treatment as monotherapy has been advocated in certain instances, particularly in cases of unresectable tumors or patients who are poor surgical candidates.5,7 Cases of distant metastasis (stage IV disease) may include management with surgery, radiation, and/or chemotherapy. Although none of these modalities have consistently shown to improve survival, there appears to be up to a 60% response with chemotherapy in these patients.3
Because MCC tends to affect an older population, often with other notable comorbidities, important considerations involving a treatment plan include the cost, side effects, and convenience for patients. The combination of carboplatin and VP-16 (etoposide) was utilized and tolerated well in our patient, and it has been successful in achieving complete radiologic and clinical remission of his metastatic disease. This combination appears to prolong survival in patients with distant metastasis, as compared to those patients not receiving chemotherapy.1 Our patient has since died, but in these high-risk patients, close clinical monitoring is essential to help optimize their prognosis.
Merkel cell carcinoma is a rare aggressive cutaneous neoplasm that most commonly affects the elderly, immunosuppressed, and those with chronic UV sun damage. An association between the oncogenesis of MCC and infection with MCPyV has been documented, but other underlying diseases also may play a role in this process including rheumatoid arthritis and psoriasis. Although these risk factors were associated with our patient, his history of chronic immunosuppressive therapy for treatment of his psoriasis and inflammatory joint disease likely played a role in the pathogenesis of the tumor and should be an important point of discussion with any patient requiring this type of long-term management for disease control. Our unique clinical case highlights a patient with substantial comorbidities who developed metastatic MCC and achieved complete clinical and radiologic remission after treatment with surgery and chemotherapy.
- Timmer FC, Klop WM, Relyveld GN, et al. Merkel cell carcinoma of the head and neck: emphasizing the risk of undertreatment [published online March 11, 2015]. Eur Arch Otorhinolaryngol. 2016;273:1243-1252.
- Açıkalın A, Paydas¸ S, Güleç ÜK, et al. A unique case of Merkel cell carcinoma with ovarian metastasis [published online December 1, 2014]. Balkan Med J. 2014;31:356-359.
- Samimi M, Gardair C, Nicol JT, et al. Merkel cell polyomavirus in Merkel cell carcinoma: clinical and therapeutic perspectives [published online Dec 31, 2014]. Semin Oncol. 2015;42:347-358.
- Grandhaye M, Teixeira PG, Henrot P, et al. Focus on Merkel cell carcinoma: diagnosis and staging [published online January 30, 2015]. Skeletal Radiol. 2015;44:777-786.
- Chatzinasiou F, Papadavid E, Korkolopoulou P, et al. An unusual case of diffuse Merkel cell carcinoma successfully treated with low dose radiotherapy [published online May 14, 2015]. Dermatol Ther. 2015;28:282-286.
- Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature [published online November 13, 2014]. Int J Surg Case Rep. 2015;7C:104-108.
- Kitamura N, Tomita R, Yamamoto M, et al. Complete remission of Merkel cell carcinoma on the upper lip treated with radiation monotherapy and a literature review of Japanese cases. World J Surg Oncol. 2015;13:152.
- Lanoy E, Engels EA. Skin cancers associated with autoimmune conditions among elderly adults [published online June 15, 2010]. Br J Cancer. 2010;103:112-114.
- Mertz KD, Junt T, Schmid M, et al. Inflammatory monocytes are a reservoir for Merkel cell polyomavirus [published online December 17, 2009]. J Invest Dermatol. 2009;130:1146-1151.
- Fujimoto N, Nakanishi G, Kabuto M, et al. Merkel cell carcinoma showing regression after biopsy: evaluation of programmed cell death 1-positive cells [published online February 24, 2015]. J Dermatol. 2015;42:496-499.
- Timmer FC, Klop WM, Relyveld GN, et al. Merkel cell carcinoma of the head and neck: emphasizing the risk of undertreatment [published online March 11, 2015]. Eur Arch Otorhinolaryngol. 2016;273:1243-1252.
- Açıkalın A, Paydas¸ S, Güleç ÜK, et al. A unique case of Merkel cell carcinoma with ovarian metastasis [published online December 1, 2014]. Balkan Med J. 2014;31:356-359.
- Samimi M, Gardair C, Nicol JT, et al. Merkel cell polyomavirus in Merkel cell carcinoma: clinical and therapeutic perspectives [published online Dec 31, 2014]. Semin Oncol. 2015;42:347-358.
- Grandhaye M, Teixeira PG, Henrot P, et al. Focus on Merkel cell carcinoma: diagnosis and staging [published online January 30, 2015]. Skeletal Radiol. 2015;44:777-786.
- Chatzinasiou F, Papadavid E, Korkolopoulou P, et al. An unusual case of diffuse Merkel cell carcinoma successfully treated with low dose radiotherapy [published online May 14, 2015]. Dermatol Ther. 2015;28:282-286.
- Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature [published online November 13, 2014]. Int J Surg Case Rep. 2015;7C:104-108.
- Kitamura N, Tomita R, Yamamoto M, et al. Complete remission of Merkel cell carcinoma on the upper lip treated with radiation monotherapy and a literature review of Japanese cases. World J Surg Oncol. 2015;13:152.
- Lanoy E, Engels EA. Skin cancers associated with autoimmune conditions among elderly adults [published online June 15, 2010]. Br J Cancer. 2010;103:112-114.
- Mertz KD, Junt T, Schmid M, et al. Inflammatory monocytes are a reservoir for Merkel cell polyomavirus [published online December 17, 2009]. J Invest Dermatol. 2009;130:1146-1151.
- Fujimoto N, Nakanishi G, Kabuto M, et al. Merkel cell carcinoma showing regression after biopsy: evaluation of programmed cell death 1-positive cells [published online February 24, 2015]. J Dermatol. 2015;42:496-499.
Practice Points
- Merkel cell carcinoma (MCC) has remarkable metastatic potential.
- Initial evaluation of patients with MCC should include clinical examination to detect cutaneous, lymph node, and distant metastasis.
- Risk factors associated with the development of MCC include immunosuppression, older age, and UV-exposed fair skin.
Frostbite on the Hand of a Homeless Man
To the Editor:
A 58-year-old homeless man presented to the emergency department after being found wandering in the middle of winter in Detroit, Michigan, with altered mental status. A workup for his mental incapacitation uncovered severe electrolyte disturbances, hyperglycemia, and acute renal failure, as well as both alcohol and drug intoxication. After 1 day of admission the patient reported progressive swelling, blistering, and pain in the right hand. The pain was stabbing in nature, worse with movement, and graded 10 of 10 (1=minimal; 10=severe). His medical history was notable for diabetes mellitus with peripheral neuropathy, hypertension, hyperlipidemia, and alcohol and drug abuse. The patient was not taking any medications for these conditions.
Physical examination revealed 2+ moderate pitting edema in all distal extremities, with increased edema of the dorsal aspect of the right hand. The right hand also demonstrated patchy erythema and was warm to touch. The dorsal aspect of the right ring finger had a dusky tip and was studded with several tense blisters (Figure). Vital signs were stable. Based on the patient’s history and physical examination findings, a diagnosis of frostbite was made. Our treatment process involved several modalities including immersion of the affected site in a warm water bath, surgical debridement of blistered sites, tetanus toxoid, penicillin to prevent infection, and oral ibuprofen for pain management. At 3-day follow-up, the patient’s condition substantially improved with a decreased amount of erythema, edema, and pain. All affected sites were successfully preserved with no evidence of focal, motor, or sensory impairment.

Frostbite is a form of localized tissue injury due to extreme cold that most commonly affects the hands and feet, with the greatest incidence occurring in adults aged 30 to 49 years.1,2 Other sites commonly affected include the ears, nose, cheeks, and penis. Frostbite injuries can be categorized into 4 degrees of severity that correlate with the clinical presentation.1,3 Rewarming the affected site is necessary to properly classify the injury, as the initial appearance may be similar among the different degrees of injury. A first-degree injury classically shows a central white plaque with peripheral erythema and is extremely cold to touch. Second-degree injuries display tense blisters filled with clear or milky fluid surrounded by erythema and edema within the first 24 hours. Third-degree injuries are associated with hemorrhagic blisters. Fourth-degree injuries involve complete tissue loss and necrosis.1 Frostbite injuries also may be classified as superficial or deep; the former affects skin and subcutaneous tissue, while the latter affects bones, joints, and tendons.3,4 The superficial form exhibits clear blisters, whereas hemorrhagic blisters demonstrate deep frostbite.
Factors such as the surrounding temperature, length of exposure, and alcohol consumption may exacerbate frostbite injuries.1 Conditions such as atherosclerosis and diabetes mellitus, which can cause neuropathy and peripheral vascular disease, also are potential risks. Psychiatric patients also are at risk for frostbite given the propensity for eccentric behavior as well as the homeless due to inadequate clothing or shelter. Diagnosis often can be made based on medical history and physical examination, though techniques such as radiography, angiography, digital plethysmography, Doppler ultrasonography, and bone scintigraphy (technetium-99) also have been utilized to determine severity and prognosis.2 Differential diagnoses of frostbite are listed in the Table.
Frostbite treatment begins with removal of wet clothing and region protection. Rewarming the site should not begin until refreezing is unlikely to occur and involves placing the injured area in water (temperature, 40°C–42°C) for 15 to 30 minutes to minimize tissue loss.1,2 Analgesics, tetanus toxoid, oral ibuprofen, and benzylpenicillin also are indicated, along with daily hydrotherapy.1,2 White blisters should be debrided, while hemorrhagic blisters should be left intact. Amputation and aggressive debridement typically are delayed until complete ischemia occurs and final demarcation is determined, usually over 1 to 3 months.1 Combination therapy allowed for a positive outcome in our patient.
Frostnip is a mild form of cold injury characterized by localized pain, pallor, and possible numbness.3 Warming the cold area restores the function and sensation with no loss of tissue. Chilblain or pernio refers to a localized cold injury that typically presents as pruritic red-purple papules, macules, plaques, or nodules on the face, anterior tibial surface, or dorsum and tips of the hands and feet.3 The primary cause is repeated exposure to cold, not freezing, temperatures.
Trench foot or cold immersion foot (or hand) is a nonfreezing injury to the hands or feet caused by chronic exposure to wet conditions and temperatures above freezing.3 Painful burning and dysesthesia as well as tissue damage involving edema, blistering, redness, ecchymosis, and ulceration are common. Cellulitis is a bacterial infection of the skin and underlying tissues that can occur anywhere on the body, but the legs are most commonly affected. Typical presentation involves erythema, warmth, swelling, and pain in the infected area.
Although the conditions described above may be considered in the differential diagnosis, physical examination and the patient’s clinical history typically will allow for the distinction of frostbite from these other disease processes.
- Petrone P, Kuncir EJ, Asensio JA. Surgical management and strategies in the treatment of hypothermia and cold injury. Emerg Med Clin North Am. 2003;21:1165-1178.
- Reamy BV. Frostbite: review and current concepts. J Am Board Fam Pract. 1998;11:34-40.
- Jurkovich GJ. Environmental cold-induced injury. Surg Clin North Am. 2007;87:247-267, viii.
- Biem J, Koehncke N, Classen D, et al. Out of the cold: management of hypothermia and frostbite. CMAJ. 2003;168:305-311.
To the Editor:
A 58-year-old homeless man presented to the emergency department after being found wandering in the middle of winter in Detroit, Michigan, with altered mental status. A workup for his mental incapacitation uncovered severe electrolyte disturbances, hyperglycemia, and acute renal failure, as well as both alcohol and drug intoxication. After 1 day of admission the patient reported progressive swelling, blistering, and pain in the right hand. The pain was stabbing in nature, worse with movement, and graded 10 of 10 (1=minimal; 10=severe). His medical history was notable for diabetes mellitus with peripheral neuropathy, hypertension, hyperlipidemia, and alcohol and drug abuse. The patient was not taking any medications for these conditions.
Physical examination revealed 2+ moderate pitting edema in all distal extremities, with increased edema of the dorsal aspect of the right hand. The right hand also demonstrated patchy erythema and was warm to touch. The dorsal aspect of the right ring finger had a dusky tip and was studded with several tense blisters (Figure). Vital signs were stable. Based on the patient’s history and physical examination findings, a diagnosis of frostbite was made. Our treatment process involved several modalities including immersion of the affected site in a warm water bath, surgical debridement of blistered sites, tetanus toxoid, penicillin to prevent infection, and oral ibuprofen for pain management. At 3-day follow-up, the patient’s condition substantially improved with a decreased amount of erythema, edema, and pain. All affected sites were successfully preserved with no evidence of focal, motor, or sensory impairment.

Frostbite is a form of localized tissue injury due to extreme cold that most commonly affects the hands and feet, with the greatest incidence occurring in adults aged 30 to 49 years.1,2 Other sites commonly affected include the ears, nose, cheeks, and penis. Frostbite injuries can be categorized into 4 degrees of severity that correlate with the clinical presentation.1,3 Rewarming the affected site is necessary to properly classify the injury, as the initial appearance may be similar among the different degrees of injury. A first-degree injury classically shows a central white plaque with peripheral erythema and is extremely cold to touch. Second-degree injuries display tense blisters filled with clear or milky fluid surrounded by erythema and edema within the first 24 hours. Third-degree injuries are associated with hemorrhagic blisters. Fourth-degree injuries involve complete tissue loss and necrosis.1 Frostbite injuries also may be classified as superficial or deep; the former affects skin and subcutaneous tissue, while the latter affects bones, joints, and tendons.3,4 The superficial form exhibits clear blisters, whereas hemorrhagic blisters demonstrate deep frostbite.
Factors such as the surrounding temperature, length of exposure, and alcohol consumption may exacerbate frostbite injuries.1 Conditions such as atherosclerosis and diabetes mellitus, which can cause neuropathy and peripheral vascular disease, also are potential risks. Psychiatric patients also are at risk for frostbite given the propensity for eccentric behavior as well as the homeless due to inadequate clothing or shelter. Diagnosis often can be made based on medical history and physical examination, though techniques such as radiography, angiography, digital plethysmography, Doppler ultrasonography, and bone scintigraphy (technetium-99) also have been utilized to determine severity and prognosis.2 Differential diagnoses of frostbite are listed in the Table.

Frostbite treatment begins with removal of wet clothing and region protection. Rewarming the site should not begin until refreezing is unlikely to occur and involves placing the injured area in water (temperature, 40°C–42°C) for 15 to 30 minutes to minimize tissue loss.1,2 Analgesics, tetanus toxoid, oral ibuprofen, and benzylpenicillin also are indicated, along with daily hydrotherapy.1,2 White blisters should be debrided, while hemorrhagic blisters should be left intact. Amputation and aggressive debridement typically are delayed until complete ischemia occurs and final demarcation is determined, usually over 1 to 3 months.1 Combination therapy allowed for a positive outcome in our patient.
Frostnip is a mild form of cold injury characterized by localized pain, pallor, and possible numbness.3 Warming the cold area restores the function and sensation with no loss of tissue. Chilblain or pernio refers to a localized cold injury that typically presents as pruritic red-purple papules, macules, plaques, or nodules on the face, anterior tibial surface, or dorsum and tips of the hands and feet.3 The primary cause is repeated exposure to cold, not freezing, temperatures.
Trench foot or cold immersion foot (or hand) is a nonfreezing injury to the hands or feet caused by chronic exposure to wet conditions and temperatures above freezing.3 Painful burning and dysesthesia as well as tissue damage involving edema, blistering, redness, ecchymosis, and ulceration are common. Cellulitis is a bacterial infection of the skin and underlying tissues that can occur anywhere on the body, but the legs are most commonly affected. Typical presentation involves erythema, warmth, swelling, and pain in the infected area.
Although the conditions described above may be considered in the differential diagnosis, physical examination and the patient’s clinical history typically will allow for the distinction of frostbite from these other disease processes.
To the Editor:
A 58-year-old homeless man presented to the emergency department after being found wandering in the middle of winter in Detroit, Michigan, with altered mental status. A workup for his mental incapacitation uncovered severe electrolyte disturbances, hyperglycemia, and acute renal failure, as well as both alcohol and drug intoxication. After 1 day of admission the patient reported progressive swelling, blistering, and pain in the right hand. The pain was stabbing in nature, worse with movement, and graded 10 of 10 (1=minimal; 10=severe). His medical history was notable for diabetes mellitus with peripheral neuropathy, hypertension, hyperlipidemia, and alcohol and drug abuse. The patient was not taking any medications for these conditions.
Physical examination revealed 2+ moderate pitting edema in all distal extremities, with increased edema of the dorsal aspect of the right hand. The right hand also demonstrated patchy erythema and was warm to touch. The dorsal aspect of the right ring finger had a dusky tip and was studded with several tense blisters (Figure). Vital signs were stable. Based on the patient’s history and physical examination findings, a diagnosis of frostbite was made. Our treatment process involved several modalities including immersion of the affected site in a warm water bath, surgical debridement of blistered sites, tetanus toxoid, penicillin to prevent infection, and oral ibuprofen for pain management. At 3-day follow-up, the patient’s condition substantially improved with a decreased amount of erythema, edema, and pain. All affected sites were successfully preserved with no evidence of focal, motor, or sensory impairment.

Frostbite is a form of localized tissue injury due to extreme cold that most commonly affects the hands and feet, with the greatest incidence occurring in adults aged 30 to 49 years.1,2 Other sites commonly affected include the ears, nose, cheeks, and penis. Frostbite injuries can be categorized into 4 degrees of severity that correlate with the clinical presentation.1,3 Rewarming the affected site is necessary to properly classify the injury, as the initial appearance may be similar among the different degrees of injury. A first-degree injury classically shows a central white plaque with peripheral erythema and is extremely cold to touch. Second-degree injuries display tense blisters filled with clear or milky fluid surrounded by erythema and edema within the first 24 hours. Third-degree injuries are associated with hemorrhagic blisters. Fourth-degree injuries involve complete tissue loss and necrosis.1 Frostbite injuries also may be classified as superficial or deep; the former affects skin and subcutaneous tissue, while the latter affects bones, joints, and tendons.3,4 The superficial form exhibits clear blisters, whereas hemorrhagic blisters demonstrate deep frostbite.
Factors such as the surrounding temperature, length of exposure, and alcohol consumption may exacerbate frostbite injuries.1 Conditions such as atherosclerosis and diabetes mellitus, which can cause neuropathy and peripheral vascular disease, also are potential risks. Psychiatric patients also are at risk for frostbite given the propensity for eccentric behavior as well as the homeless due to inadequate clothing or shelter. Diagnosis often can be made based on medical history and physical examination, though techniques such as radiography, angiography, digital plethysmography, Doppler ultrasonography, and bone scintigraphy (technetium-99) also have been utilized to determine severity and prognosis.2 Differential diagnoses of frostbite are listed in the Table.

Frostbite treatment begins with removal of wet clothing and region protection. Rewarming the site should not begin until refreezing is unlikely to occur and involves placing the injured area in water (temperature, 40°C–42°C) for 15 to 30 minutes to minimize tissue loss.1,2 Analgesics, tetanus toxoid, oral ibuprofen, and benzylpenicillin also are indicated, along with daily hydrotherapy.1,2 White blisters should be debrided, while hemorrhagic blisters should be left intact. Amputation and aggressive debridement typically are delayed until complete ischemia occurs and final demarcation is determined, usually over 1 to 3 months.1 Combination therapy allowed for a positive outcome in our patient.
Frostnip is a mild form of cold injury characterized by localized pain, pallor, and possible numbness.3 Warming the cold area restores the function and sensation with no loss of tissue. Chilblain or pernio refers to a localized cold injury that typically presents as pruritic red-purple papules, macules, plaques, or nodules on the face, anterior tibial surface, or dorsum and tips of the hands and feet.3 The primary cause is repeated exposure to cold, not freezing, temperatures.
Trench foot or cold immersion foot (or hand) is a nonfreezing injury to the hands or feet caused by chronic exposure to wet conditions and temperatures above freezing.3 Painful burning and dysesthesia as well as tissue damage involving edema, blistering, redness, ecchymosis, and ulceration are common. Cellulitis is a bacterial infection of the skin and underlying tissues that can occur anywhere on the body, but the legs are most commonly affected. Typical presentation involves erythema, warmth, swelling, and pain in the infected area.
Although the conditions described above may be considered in the differential diagnosis, physical examination and the patient’s clinical history typically will allow for the distinction of frostbite from these other disease processes.
- Petrone P, Kuncir EJ, Asensio JA. Surgical management and strategies in the treatment of hypothermia and cold injury. Emerg Med Clin North Am. 2003;21:1165-1178.
- Reamy BV. Frostbite: review and current concepts. J Am Board Fam Pract. 1998;11:34-40.
- Jurkovich GJ. Environmental cold-induced injury. Surg Clin North Am. 2007;87:247-267, viii.
- Biem J, Koehncke N, Classen D, et al. Out of the cold: management of hypothermia and frostbite. CMAJ. 2003;168:305-311.
- Petrone P, Kuncir EJ, Asensio JA. Surgical management and strategies in the treatment of hypothermia and cold injury. Emerg Med Clin North Am. 2003;21:1165-1178.
- Reamy BV. Frostbite: review and current concepts. J Am Board Fam Pract. 1998;11:34-40.
- Jurkovich GJ. Environmental cold-induced injury. Surg Clin North Am. 2007;87:247-267, viii.
- Biem J, Koehncke N, Classen D, et al. Out of the cold: management of hypothermia and frostbite. CMAJ. 2003;168:305-311.