User login
Penile Herpes Vegetans in a Patient With Well-controlled HIV
To the Editor:
Herpes vegetans (HV) is an uncommon infection caused by human herpesvirus (HHV) in patients who are immunocompromised, such as those who are HIV positive.1 Unlike typical HHV infection, HV can present with exophytic exudative ulcers and papillomatous vegetations. The presentation of ulcerated genital nodules, especially in an immunocompromised patient, yields an array of disorders in the differential diagnosis, including condyloma latum, condyloma acuminatum, pyogenic granuloma (PG), and verrucous carcinoma.2,3 Histopathology of HV reveals pseudoepitheliomatous hyperplasia, plasma cell infiltration, and positivity for HHV type 1 (HHV-1) and/or HHV type 2 (HHV-2). Herpes vegetans lesions typically require a multimodal treatment approach because many cases are resistant to acyclovir. Treatment options include the nucleoside analogues foscarnet and cidofovir; immunomodulators such as topical imiquimod; and the topical antiviral trifluridine.1,4-6 We describe a case of HV in a patient with a history of well-controlled HIV infection who presented with a painful fungating penile lesion.
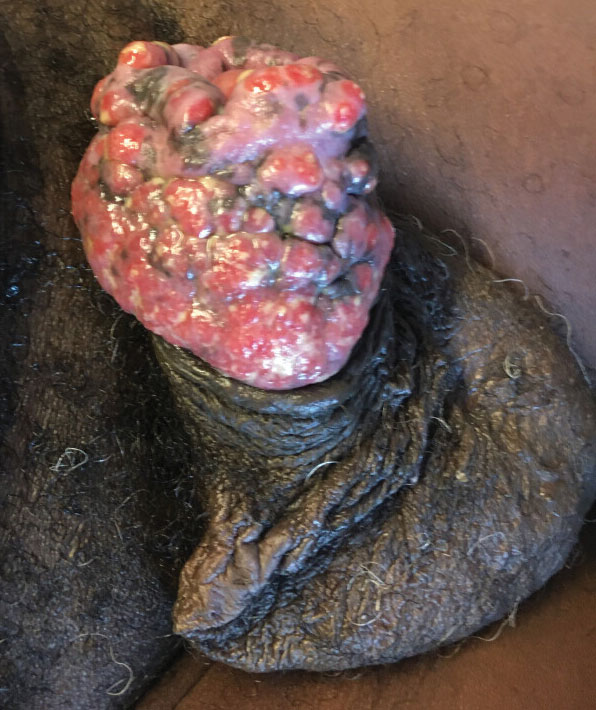
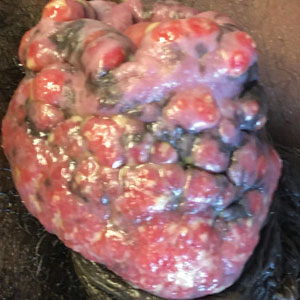
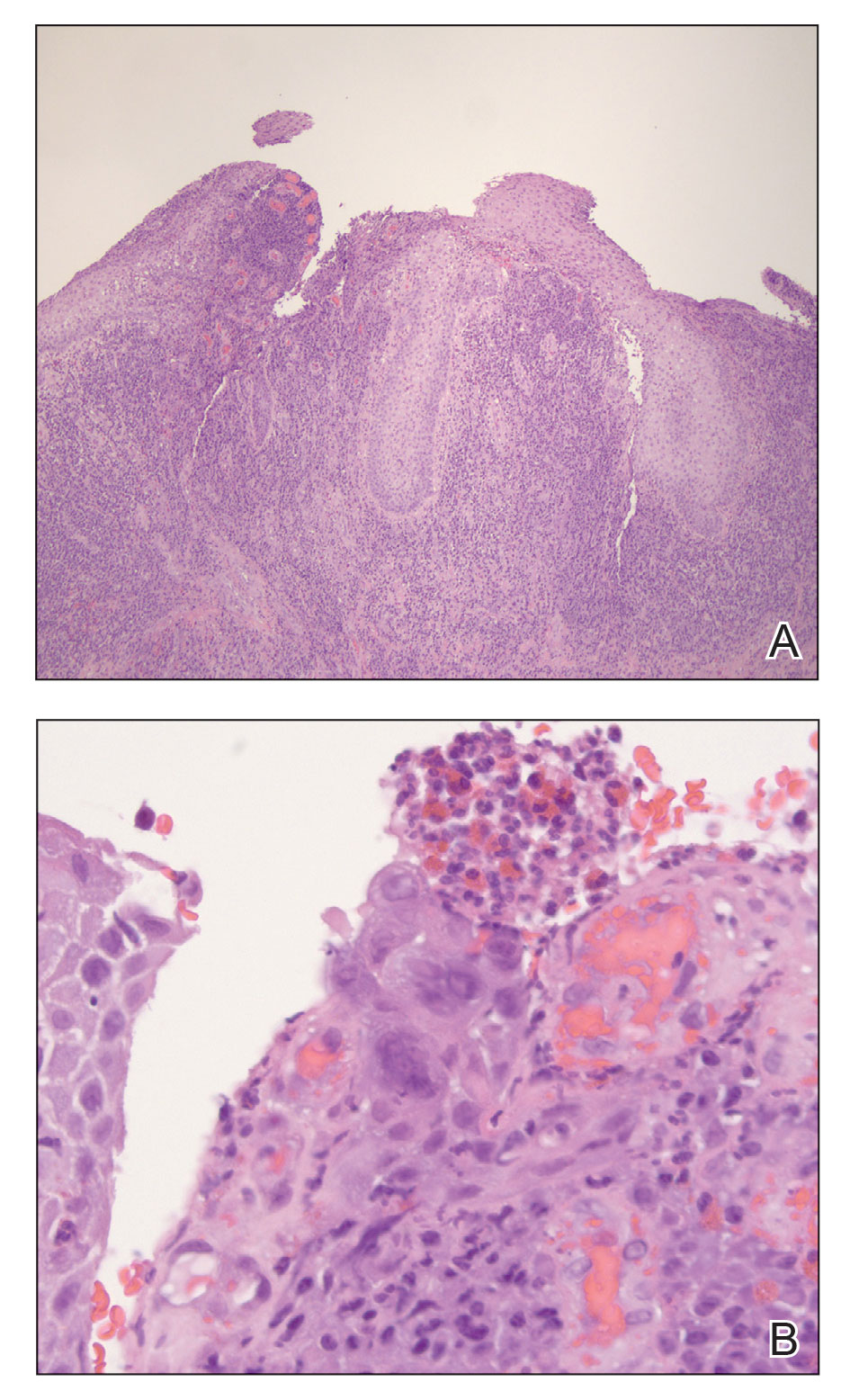
A 55-year-old man presented to the hospital with a painful expanding mass on the distal aspect of the penis of 3 months’ duration. He had a history of HIV infection that was well-controlled by antiretroviral therapy, prior hepatitis B virus infection and acyclovir-resistant genital HHV-2 infection. Physical examination revealed a large, firm, circumferential, exophytic, verrucous plaque with various areas of ulceration and purulent drainage on the distal shaft and glans of the penis (Figure 1). The patient’s most recent absolute CD4 count was 425 cells/mm3 (reference range, 500–1500 cells/mm3). His HIV viral load was undetectable at less than 30 copies/mL. Histopathology with hematoxylin and eosin staining of biopsy material from the penile lesion demonstrated pseudoepitheliomatous epidermal hyperplasia with focal ulceration and a mixed inflammatory infiltrate (Figure 2A). At higher magnification, clear viral cytopathic changes of HHV were noted, including multinucleation, nuclear molding, and homogenous gray nuclei (Figure 2B). Additional staining for fungi, mycobacteria, and spirochetes was negative. In-situ hybridization was negative for human papillomavirus subtypes. A bacterial culture of swabs of the purulent drainage was positive for Staphylococcus aureus and Proteus mirabilis.
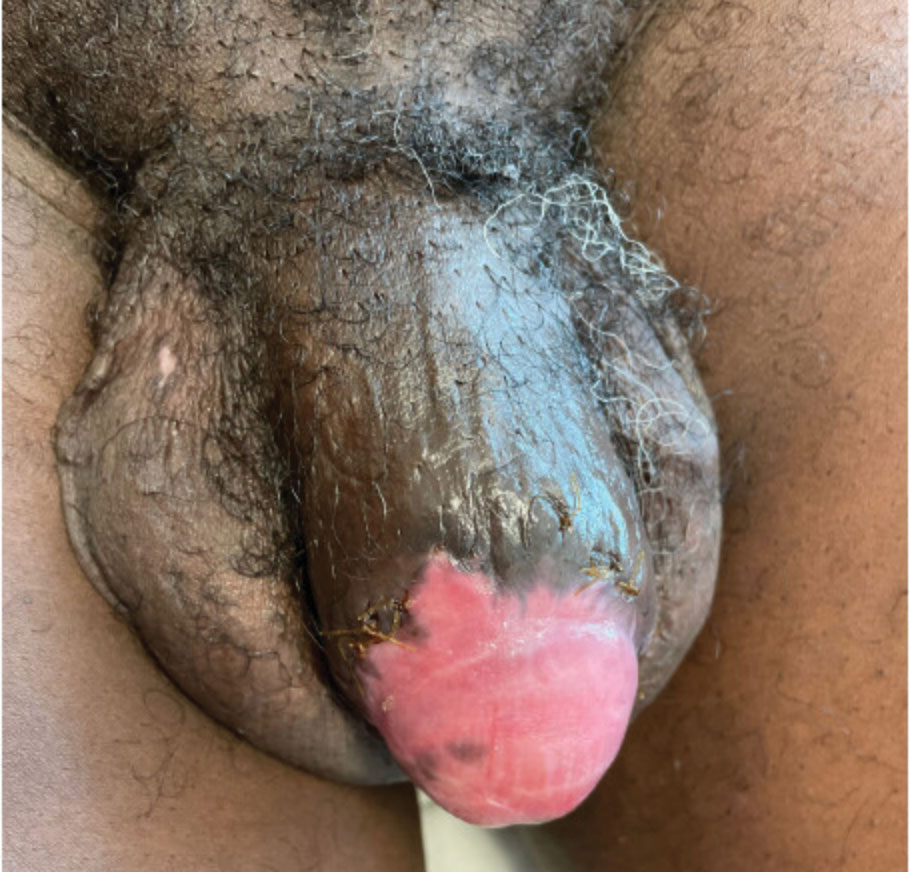
Given the patient’s known history of acyclovir-resistant HHV-2 infection, he received a 28-day course of intravenous foscarnet 40 mg/kg every 12 hours. He also was given a 14-day course of intravenous ampicillin-sulbactam 3 g every 6 hours. The patient gradually improved during a 35-day hospital stay. He was discharged with cidofovir cream 1% and oral valacyclovir; the latter was subsequently discontinued by dermatology because of his known history of acyclovir resistance. Four months after discharge, the patient underwent a circumcision performed by urology to decrease the risk for recurrence and achieve the best cosmetic outcome. At the 6-month follow-up visit, dramatic clinical improvement was evident, with complete resolution of the plaque and only isolated areas of scarring (Figure 3). The patient reported that penile function was preserved.
Herpes vegetans represents a rare infection with HHV-1 or HHV-2, typically in patients who are considerably immunosuppressed, such as those with cancer, those undergoing transplantation, and those with uncontrolled HIV infection.1 Few cases of HV have been described in an immunocompetent patient.2 Our case is unique because the patient’s HIV infection was well controlled at the time HV was diagnosed, demonstrated by his modestly low CD4 count and undetectable HIV viral load.
Patients with HV can present diagnostic and therapeutic challenges. Typically, a diagnosis of cutaneous HHV infection does not require a biopsy; most cases appear as clustered vesicular lesions, making the disease easy to diagnose clinically. However, biopsies and cultures are necessary to identify the underlying cause of atypical verrucous exophytic lesions. Other conditions with clinical features similar to HV include squamous cell carcinoma, condyloma acuminatum, and deep fungal and mycobacterial infections.2,3 A tissue biopsy, histologic staining, and tissue culture should be performed to identify the causative pathogen and potential targets for treatment. Definitive diagnosis is vital to deliver proper treatment modalities, which often involve a multimodal multidisciplinary approach.
Several pathogenic mechanisms of HV have been proposed. One theory suggests that in an immunocompetent patient, HHV typically triggers a lymphocytic response, which leads to activation of interferon alpha. However, in an immunocompromised patient, such as an individual with AIDS, this interferon response is diminished, which explains why these patients typically have a chronic and resistant HHV infection. HIV has an affinity for infecting dermal dendritic cells, which signals activation of tumor necrosis factor and interleukin.6 Both cytokines contribute to an antiapoptotic environment that promotes continued proliferation of these viral cells in the epidermis. Over time, propagation of disinhibited cells can lead to the verrucous and hyperkeratotic-appearing skin that is common in patients with HV.7
Another theorized mechanism underlying hypertrophic herpetic lesions was described in the context of HHV-1 infection and subsequent PG. El Hayderi et al8 reported that histologic and immunohistochemical examination of a patient’s lesion revealed sparse epithelial cell aggregates within PG as well as HHV-1 antigens in the nuclei and cytoplasm of normal-appearing and cytopathic epithelial cells. Immunohistochemical examination also revealed vascular endothelial growth factor within HHV-1–infected epithelial cells and PG endothelial cells, suggesting that PG formation may be indirectly driven by vascular endothelial growth factor and its proangiogenic properties. The pathogenesis of PG in the setting of HHV-1 infection displays many similarities to hyperkeratotic lesions observed in atypical cutaneous manifestations of HHV-2.8
The management of patients with HV continues to be complex, often requiring a multimodal regimen. Although acyclovir has been shown to be highly effective for treating and preventing most HHV infections, acyclovir resistance frequently has been reported in immunocompromised populations.5 Acyclovir resistance can be correlated with the severity of immunodeficiency as well as the duration of acyclovir exposure. Resistance to acyclovir often results from deficient intracellular phosphorylation, which is required for activation of the drug. If patients show resistance to acyclovir and its derivatives, alternate drug classes that do not depend on thymidine kinase phosphorylation should be considered.
Our patient received a combination of intravenous foscarnet and a course of ampicillin-sulbactam while an inpatient due to his documented history of acyclovir-resistant HHV-2 infection, and he was discharged on cidofovir cream 1%. Cidofovir is US Food and Drug Administration approved for treating cytomegalovirus retinitis in patients with AIDS. Although data are limited, topical and intralesional cidofovir have been used to treat acyclovir-resistant cases of HV with documented success.1,9 In refractory HV or when the disease is slow to resolve, intralesional cidofovir has been documented to be an additional treatment option. Intralesional and topical cidofovir carry a much lower risk for adverse effects such as kidney dysfunction compared to intravenous cidofovir1 and can be considered in patients with minimal clinical improvement and those at increased risk for side effects.
Our case demonstrated how a patient with HV may require a complex and prolonged hospital course for appropriate treatment. Our patient required an array of both medical and surgical modalities to reach the desired outcome. Here, a multitude of specialties including infectious disease, dermatology, and urology worked together to reach a positive clinical and cosmetic outcome for this patient.
- Castelo-Soccio L, Bernardin R, Stern J, et al. Successful treatment of acyclovir-resistant herpes simplex virus with intralesional cidofovir. Arch Dermatol. 2010;146:124-126. doi:10.1001/archdermatol.2009.363
- Bae-Harboe Y-SC, Khachemoune A. Verrucous herpetic infection of the scrotum and the groin in an immuno-competent patient: case report and review of the literature. Dermatol Online J. 2012;18. https://doi.org/10.5070/D30sv058j6
- Elosiebo RI, Koubek VA, Patel TS, et al. Vegetative sacral plaque in a patient with human immunodeficiency virus. Cutis. 2015;96:E7-E9.
- Saling C, Slim J, Szabela ME. A case of an atypical resistant granulomatous HHV-1 and HHV-2 ulceration in an AIDS patient treated with intralesional cidofovir. SAGE Open Med Case Rep. 2019;7:2050313X19847029. doi:10.1177/2050313X19847029
- Martinez V, Molina J-M, Scieux C, et al. Topical imiquimod for recurrent acyclovir-resistant HHV infection. Am J Med. 2006 May;119:E9-E11. doi:10.1016/j.amjmed.2005.06.037
- Ronkainen SD, Rothenberger M. Herpes vegetans: an unusual and acyclovir-resistant form of HHV. J Gen Intern Med. 2018;33:393. doi:10.1007/s11606-017-4256-y
- Quesada AE, Galfione S, Colome M, et al. Verrucous herpes of the scrotum presenting clinically as verrucous squamous cell carcinoma: case report and review of the literature. Ann Clin Lab Sci. 2014;44:208-212.
- El Hayderi L, Paurobally D, Fassotte MF, et al. Herpes simplex virus type-I and pyogenic granuloma: a vascular endothelial growth factor-mediated association? Case Rep Dermatol. 2013;5:236-243. doi:10.1159/000354570
- Toro JR, Sanchez S, Turiansky G, et al. Topical cidofovir for the treatment of dermatologic conditions: verruca, condyloma, intraepithelial neoplasia, herpes simplex and its potential use in smallpox. Dermatol Clin. 2003;21:301-319. doi:10.1016/s0733-8635(02)00116-x
To the Editor:
Herpes vegetans (HV) is an uncommon infection caused by human herpesvirus (HHV) in patients who are immunocompromised, such as those who are HIV positive.1 Unlike typical HHV infection, HV can present with exophytic exudative ulcers and papillomatous vegetations. The presentation of ulcerated genital nodules, especially in an immunocompromised patient, yields an array of disorders in the differential diagnosis, including condyloma latum, condyloma acuminatum, pyogenic granuloma (PG), and verrucous carcinoma.2,3 Histopathology of HV reveals pseudoepitheliomatous hyperplasia, plasma cell infiltration, and positivity for HHV type 1 (HHV-1) and/or HHV type 2 (HHV-2). Herpes vegetans lesions typically require a multimodal treatment approach because many cases are resistant to acyclovir. Treatment options include the nucleoside analogues foscarnet and cidofovir; immunomodulators such as topical imiquimod; and the topical antiviral trifluridine.1,4-6 We describe a case of HV in a patient with a history of well-controlled HIV infection who presented with a painful fungating penile lesion.

A 55-year-old man presented to the hospital with a painful expanding mass on the distal aspect of the penis of 3 months’ duration. He had a history of HIV infection that was well-controlled by antiretroviral therapy, prior hepatitis B virus infection and acyclovir-resistant genital HHV-2 infection. Physical examination revealed a large, firm, circumferential, exophytic, verrucous plaque with various areas of ulceration and purulent drainage on the distal shaft and glans of the penis (Figure 1). The patient’s most recent absolute CD4 count was 425 cells/mm3 (reference range, 500–1500 cells/mm3). His HIV viral load was undetectable at less than 30 copies/mL. Histopathology with hematoxylin and eosin staining of biopsy material from the penile lesion demonstrated pseudoepitheliomatous epidermal hyperplasia with focal ulceration and a mixed inflammatory infiltrate (Figure 2A). At higher magnification, clear viral cytopathic changes of HHV were noted, including multinucleation, nuclear molding, and homogenous gray nuclei (Figure 2B). Additional staining for fungi, mycobacteria, and spirochetes was negative. In-situ hybridization was negative for human papillomavirus subtypes. A bacterial culture of swabs of the purulent drainage was positive for Staphylococcus aureus and Proteus mirabilis.

Given the patient’s known history of acyclovir-resistant HHV-2 infection, he received a 28-day course of intravenous foscarnet 40 mg/kg every 12 hours. He also was given a 14-day course of intravenous ampicillin-sulbactam 3 g every 6 hours. The patient gradually improved during a 35-day hospital stay. He was discharged with cidofovir cream 1% and oral valacyclovir; the latter was subsequently discontinued by dermatology because of his known history of acyclovir resistance. Four months after discharge, the patient underwent a circumcision performed by urology to decrease the risk for recurrence and achieve the best cosmetic outcome. At the 6-month follow-up visit, dramatic clinical improvement was evident, with complete resolution of the plaque and only isolated areas of scarring (Figure 3). The patient reported that penile function was preserved.

Herpes vegetans represents a rare infection with HHV-1 or HHV-2, typically in patients who are considerably immunosuppressed, such as those with cancer, those undergoing transplantation, and those with uncontrolled HIV infection.1 Few cases of HV have been described in an immunocompetent patient.2 Our case is unique because the patient’s HIV infection was well controlled at the time HV was diagnosed, demonstrated by his modestly low CD4 count and undetectable HIV viral load.
Patients with HV can present diagnostic and therapeutic challenges. Typically, a diagnosis of cutaneous HHV infection does not require a biopsy; most cases appear as clustered vesicular lesions, making the disease easy to diagnose clinically. However, biopsies and cultures are necessary to identify the underlying cause of atypical verrucous exophytic lesions. Other conditions with clinical features similar to HV include squamous cell carcinoma, condyloma acuminatum, and deep fungal and mycobacterial infections.2,3 A tissue biopsy, histologic staining, and tissue culture should be performed to identify the causative pathogen and potential targets for treatment. Definitive diagnosis is vital to deliver proper treatment modalities, which often involve a multimodal multidisciplinary approach.
Several pathogenic mechanisms of HV have been proposed. One theory suggests that in an immunocompetent patient, HHV typically triggers a lymphocytic response, which leads to activation of interferon alpha. However, in an immunocompromised patient, such as an individual with AIDS, this interferon response is diminished, which explains why these patients typically have a chronic and resistant HHV infection. HIV has an affinity for infecting dermal dendritic cells, which signals activation of tumor necrosis factor and interleukin.6 Both cytokines contribute to an antiapoptotic environment that promotes continued proliferation of these viral cells in the epidermis. Over time, propagation of disinhibited cells can lead to the verrucous and hyperkeratotic-appearing skin that is common in patients with HV.7
Another theorized mechanism underlying hypertrophic herpetic lesions was described in the context of HHV-1 infection and subsequent PG. El Hayderi et al8 reported that histologic and immunohistochemical examination of a patient’s lesion revealed sparse epithelial cell aggregates within PG as well as HHV-1 antigens in the nuclei and cytoplasm of normal-appearing and cytopathic epithelial cells. Immunohistochemical examination also revealed vascular endothelial growth factor within HHV-1–infected epithelial cells and PG endothelial cells, suggesting that PG formation may be indirectly driven by vascular endothelial growth factor and its proangiogenic properties. The pathogenesis of PG in the setting of HHV-1 infection displays many similarities to hyperkeratotic lesions observed in atypical cutaneous manifestations of HHV-2.8
The management of patients with HV continues to be complex, often requiring a multimodal regimen. Although acyclovir has been shown to be highly effective for treating and preventing most HHV infections, acyclovir resistance frequently has been reported in immunocompromised populations.5 Acyclovir resistance can be correlated with the severity of immunodeficiency as well as the duration of acyclovir exposure. Resistance to acyclovir often results from deficient intracellular phosphorylation, which is required for activation of the drug. If patients show resistance to acyclovir and its derivatives, alternate drug classes that do not depend on thymidine kinase phosphorylation should be considered.
Our patient received a combination of intravenous foscarnet and a course of ampicillin-sulbactam while an inpatient due to his documented history of acyclovir-resistant HHV-2 infection, and he was discharged on cidofovir cream 1%. Cidofovir is US Food and Drug Administration approved for treating cytomegalovirus retinitis in patients with AIDS. Although data are limited, topical and intralesional cidofovir have been used to treat acyclovir-resistant cases of HV with documented success.1,9 In refractory HV or when the disease is slow to resolve, intralesional cidofovir has been documented to be an additional treatment option. Intralesional and topical cidofovir carry a much lower risk for adverse effects such as kidney dysfunction compared to intravenous cidofovir1 and can be considered in patients with minimal clinical improvement and those at increased risk for side effects.
Our case demonstrated how a patient with HV may require a complex and prolonged hospital course for appropriate treatment. Our patient required an array of both medical and surgical modalities to reach the desired outcome. Here, a multitude of specialties including infectious disease, dermatology, and urology worked together to reach a positive clinical and cosmetic outcome for this patient.
To the Editor:
Herpes vegetans (HV) is an uncommon infection caused by human herpesvirus (HHV) in patients who are immunocompromised, such as those who are HIV positive.1 Unlike typical HHV infection, HV can present with exophytic exudative ulcers and papillomatous vegetations. The presentation of ulcerated genital nodules, especially in an immunocompromised patient, yields an array of disorders in the differential diagnosis, including condyloma latum, condyloma acuminatum, pyogenic granuloma (PG), and verrucous carcinoma.2,3 Histopathology of HV reveals pseudoepitheliomatous hyperplasia, plasma cell infiltration, and positivity for HHV type 1 (HHV-1) and/or HHV type 2 (HHV-2). Herpes vegetans lesions typically require a multimodal treatment approach because many cases are resistant to acyclovir. Treatment options include the nucleoside analogues foscarnet and cidofovir; immunomodulators such as topical imiquimod; and the topical antiviral trifluridine.1,4-6 We describe a case of HV in a patient with a history of well-controlled HIV infection who presented with a painful fungating penile lesion.

A 55-year-old man presented to the hospital with a painful expanding mass on the distal aspect of the penis of 3 months’ duration. He had a history of HIV infection that was well-controlled by antiretroviral therapy, prior hepatitis B virus infection and acyclovir-resistant genital HHV-2 infection. Physical examination revealed a large, firm, circumferential, exophytic, verrucous plaque with various areas of ulceration and purulent drainage on the distal shaft and glans of the penis (Figure 1). The patient’s most recent absolute CD4 count was 425 cells/mm3 (reference range, 500–1500 cells/mm3). His HIV viral load was undetectable at less than 30 copies/mL. Histopathology with hematoxylin and eosin staining of biopsy material from the penile lesion demonstrated pseudoepitheliomatous epidermal hyperplasia with focal ulceration and a mixed inflammatory infiltrate (Figure 2A). At higher magnification, clear viral cytopathic changes of HHV were noted, including multinucleation, nuclear molding, and homogenous gray nuclei (Figure 2B). Additional staining for fungi, mycobacteria, and spirochetes was negative. In-situ hybridization was negative for human papillomavirus subtypes. A bacterial culture of swabs of the purulent drainage was positive for Staphylococcus aureus and Proteus mirabilis.

Given the patient’s known history of acyclovir-resistant HHV-2 infection, he received a 28-day course of intravenous foscarnet 40 mg/kg every 12 hours. He also was given a 14-day course of intravenous ampicillin-sulbactam 3 g every 6 hours. The patient gradually improved during a 35-day hospital stay. He was discharged with cidofovir cream 1% and oral valacyclovir; the latter was subsequently discontinued by dermatology because of his known history of acyclovir resistance. Four months after discharge, the patient underwent a circumcision performed by urology to decrease the risk for recurrence and achieve the best cosmetic outcome. At the 6-month follow-up visit, dramatic clinical improvement was evident, with complete resolution of the plaque and only isolated areas of scarring (Figure 3). The patient reported that penile function was preserved.

Herpes vegetans represents a rare infection with HHV-1 or HHV-2, typically in patients who are considerably immunosuppressed, such as those with cancer, those undergoing transplantation, and those with uncontrolled HIV infection.1 Few cases of HV have been described in an immunocompetent patient.2 Our case is unique because the patient’s HIV infection was well controlled at the time HV was diagnosed, demonstrated by his modestly low CD4 count and undetectable HIV viral load.
Patients with HV can present diagnostic and therapeutic challenges. Typically, a diagnosis of cutaneous HHV infection does not require a biopsy; most cases appear as clustered vesicular lesions, making the disease easy to diagnose clinically. However, biopsies and cultures are necessary to identify the underlying cause of atypical verrucous exophytic lesions. Other conditions with clinical features similar to HV include squamous cell carcinoma, condyloma acuminatum, and deep fungal and mycobacterial infections.2,3 A tissue biopsy, histologic staining, and tissue culture should be performed to identify the causative pathogen and potential targets for treatment. Definitive diagnosis is vital to deliver proper treatment modalities, which often involve a multimodal multidisciplinary approach.
Several pathogenic mechanisms of HV have been proposed. One theory suggests that in an immunocompetent patient, HHV typically triggers a lymphocytic response, which leads to activation of interferon alpha. However, in an immunocompromised patient, such as an individual with AIDS, this interferon response is diminished, which explains why these patients typically have a chronic and resistant HHV infection. HIV has an affinity for infecting dermal dendritic cells, which signals activation of tumor necrosis factor and interleukin.6 Both cytokines contribute to an antiapoptotic environment that promotes continued proliferation of these viral cells in the epidermis. Over time, propagation of disinhibited cells can lead to the verrucous and hyperkeratotic-appearing skin that is common in patients with HV.7
Another theorized mechanism underlying hypertrophic herpetic lesions was described in the context of HHV-1 infection and subsequent PG. El Hayderi et al8 reported that histologic and immunohistochemical examination of a patient’s lesion revealed sparse epithelial cell aggregates within PG as well as HHV-1 antigens in the nuclei and cytoplasm of normal-appearing and cytopathic epithelial cells. Immunohistochemical examination also revealed vascular endothelial growth factor within HHV-1–infected epithelial cells and PG endothelial cells, suggesting that PG formation may be indirectly driven by vascular endothelial growth factor and its proangiogenic properties. The pathogenesis of PG in the setting of HHV-1 infection displays many similarities to hyperkeratotic lesions observed in atypical cutaneous manifestations of HHV-2.8
The management of patients with HV continues to be complex, often requiring a multimodal regimen. Although acyclovir has been shown to be highly effective for treating and preventing most HHV infections, acyclovir resistance frequently has been reported in immunocompromised populations.5 Acyclovir resistance can be correlated with the severity of immunodeficiency as well as the duration of acyclovir exposure. Resistance to acyclovir often results from deficient intracellular phosphorylation, which is required for activation of the drug. If patients show resistance to acyclovir and its derivatives, alternate drug classes that do not depend on thymidine kinase phosphorylation should be considered.
Our patient received a combination of intravenous foscarnet and a course of ampicillin-sulbactam while an inpatient due to his documented history of acyclovir-resistant HHV-2 infection, and he was discharged on cidofovir cream 1%. Cidofovir is US Food and Drug Administration approved for treating cytomegalovirus retinitis in patients with AIDS. Although data are limited, topical and intralesional cidofovir have been used to treat acyclovir-resistant cases of HV with documented success.1,9 In refractory HV or when the disease is slow to resolve, intralesional cidofovir has been documented to be an additional treatment option. Intralesional and topical cidofovir carry a much lower risk for adverse effects such as kidney dysfunction compared to intravenous cidofovir1 and can be considered in patients with minimal clinical improvement and those at increased risk for side effects.
Our case demonstrated how a patient with HV may require a complex and prolonged hospital course for appropriate treatment. Our patient required an array of both medical and surgical modalities to reach the desired outcome. Here, a multitude of specialties including infectious disease, dermatology, and urology worked together to reach a positive clinical and cosmetic outcome for this patient.
- Castelo-Soccio L, Bernardin R, Stern J, et al. Successful treatment of acyclovir-resistant herpes simplex virus with intralesional cidofovir. Arch Dermatol. 2010;146:124-126. doi:10.1001/archdermatol.2009.363
- Bae-Harboe Y-SC, Khachemoune A. Verrucous herpetic infection of the scrotum and the groin in an immuno-competent patient: case report and review of the literature. Dermatol Online J. 2012;18. https://doi.org/10.5070/D30sv058j6
- Elosiebo RI, Koubek VA, Patel TS, et al. Vegetative sacral plaque in a patient with human immunodeficiency virus. Cutis. 2015;96:E7-E9.
- Saling C, Slim J, Szabela ME. A case of an atypical resistant granulomatous HHV-1 and HHV-2 ulceration in an AIDS patient treated with intralesional cidofovir. SAGE Open Med Case Rep. 2019;7:2050313X19847029. doi:10.1177/2050313X19847029
- Martinez V, Molina J-M, Scieux C, et al. Topical imiquimod for recurrent acyclovir-resistant HHV infection. Am J Med. 2006 May;119:E9-E11. doi:10.1016/j.amjmed.2005.06.037
- Ronkainen SD, Rothenberger M. Herpes vegetans: an unusual and acyclovir-resistant form of HHV. J Gen Intern Med. 2018;33:393. doi:10.1007/s11606-017-4256-y
- Quesada AE, Galfione S, Colome M, et al. Verrucous herpes of the scrotum presenting clinically as verrucous squamous cell carcinoma: case report and review of the literature. Ann Clin Lab Sci. 2014;44:208-212.
- El Hayderi L, Paurobally D, Fassotte MF, et al. Herpes simplex virus type-I and pyogenic granuloma: a vascular endothelial growth factor-mediated association? Case Rep Dermatol. 2013;5:236-243. doi:10.1159/000354570
- Toro JR, Sanchez S, Turiansky G, et al. Topical cidofovir for the treatment of dermatologic conditions: verruca, condyloma, intraepithelial neoplasia, herpes simplex and its potential use in smallpox. Dermatol Clin. 2003;21:301-319. doi:10.1016/s0733-8635(02)00116-x
- Castelo-Soccio L, Bernardin R, Stern J, et al. Successful treatment of acyclovir-resistant herpes simplex virus with intralesional cidofovir. Arch Dermatol. 2010;146:124-126. doi:10.1001/archdermatol.2009.363
- Bae-Harboe Y-SC, Khachemoune A. Verrucous herpetic infection of the scrotum and the groin in an immuno-competent patient: case report and review of the literature. Dermatol Online J. 2012;18. https://doi.org/10.5070/D30sv058j6
- Elosiebo RI, Koubek VA, Patel TS, et al. Vegetative sacral plaque in a patient with human immunodeficiency virus. Cutis. 2015;96:E7-E9.
- Saling C, Slim J, Szabela ME. A case of an atypical resistant granulomatous HHV-1 and HHV-2 ulceration in an AIDS patient treated with intralesional cidofovir. SAGE Open Med Case Rep. 2019;7:2050313X19847029. doi:10.1177/2050313X19847029
- Martinez V, Molina J-M, Scieux C, et al. Topical imiquimod for recurrent acyclovir-resistant HHV infection. Am J Med. 2006 May;119:E9-E11. doi:10.1016/j.amjmed.2005.06.037
- Ronkainen SD, Rothenberger M. Herpes vegetans: an unusual and acyclovir-resistant form of HHV. J Gen Intern Med. 2018;33:393. doi:10.1007/s11606-017-4256-y
- Quesada AE, Galfione S, Colome M, et al. Verrucous herpes of the scrotum presenting clinically as verrucous squamous cell carcinoma: case report and review of the literature. Ann Clin Lab Sci. 2014;44:208-212.
- El Hayderi L, Paurobally D, Fassotte MF, et al. Herpes simplex virus type-I and pyogenic granuloma: a vascular endothelial growth factor-mediated association? Case Rep Dermatol. 2013;5:236-243. doi:10.1159/000354570
- Toro JR, Sanchez S, Turiansky G, et al. Topical cidofovir for the treatment of dermatologic conditions: verruca, condyloma, intraepithelial neoplasia, herpes simplex and its potential use in smallpox. Dermatol Clin. 2003;21:301-319. doi:10.1016/s0733-8635(02)00116-x
Practice Points
- Maintain a high clinical suspicion for herpes vegetans (HV) in a patient who has a history of immunosuppression and presents with exophytic genital lesions.
- A history of resistance to acyclovir requires a multimodal approach to treatment of HV lesions, including medical and surgical therapies.
Review of Ethnoracial Representation in Clinical Trials (Phases 1 Through 4) of Atopic Dermatitis Therapies
To the Editor:
Atopic dermatitis (AD) affects an estimated 7.2% of adults and 10.7% of children in the United States; however, AD might affect different races at a varying rate.1 Compared to their European American counterparts, Asian/Pacific Islanders and African Americans are 7 and 3 times more likely, respectively, to be given a diagnosis of AD.2
Despite being disproportionately affected by AD, minority groups might be underrepresented in clinical trials of AD treatments.3 One explanation for this imbalance might be that ethnoracial representation differs across regions in the United States, perhaps in regions where clinical trials are conducted. Price et al3 investigated racial representation in clinical trials of AD globally and found that patients of color are consistently underrepresented.
Research on racial representation in clinical trials within the United States—on national and regional scales—is lacking from the current AD literature. We conducted a study to compare racial and ethnic disparities in AD clinical trials across regions of the United States.
Using the ClinicalTrials.gov database (www.clinicaltrials.gov) of the National Library of Medicine, we identified clinical trials of AD treatments (encompassing phases 1 through 4) in the United States that were completed before March 14, 2021, with the earliest data from 2013. Search terms included atopic dermatitis, with an advanced search for interventional (clinical trials) and with results.
In total, 95 completed clinical trials were identified, of which 26 (27.4%) reported ethnoracial demographic data. One trial was excluded due to misrepresentation regarding the classification of individuals who identified as more than 1 racial category. Clinical trials for systemic treatments (7 [28%]) and topical treatments (18 [72%]) were identified.
All ethnoracial data were self-reported by trial participants based on US Food and Drug Administration guidelines for racial and ethnic categorization.4 Trial participants who identified ethnically as Hispanic or Latino might have been a part of any racial group. Only 7 of the 25 included clinical trials (28%) provided ethnic demographic data (Hispanic [Latino] or non-Hispanic); 72% of trials failed to report ethnicity. Ethnic data included in our analysis came from only the 7 clinical trials that included these data. International multicenter trials that included a US site were excluded.
Ultimately, the number of trials included in our analysis was 25, comprised of 2443 participants. Data were further organized by US geographic region (Northeast, Midwest, South, West, and multiregion trials [ie, conducted in ≥2 regions]). No AD clinical trials were conducted solely in the Midwest; it was only included within multiregion trials.
Compared to their representation in the 2019 US Census, most minority groups were overrepresented in clinical trials, while White individuals were underrepresented (eTable). The percentages of our findings on representation for race are as follows (US Census data are listed in parentheses for comparison5):
- White: 56.8% (72.5%)
- Black/African American: 28.3% (12.7%)
- Asian: 10.3% (5.5%)
- Multiracial: 1.1% (3.3%)
- Native Hawaiian or other Pacific Islander: 0.3% (0.2%)
- American Indian or Alaska Native: 0.2% (0.8%)
- Other: 0.5% (4.9%).
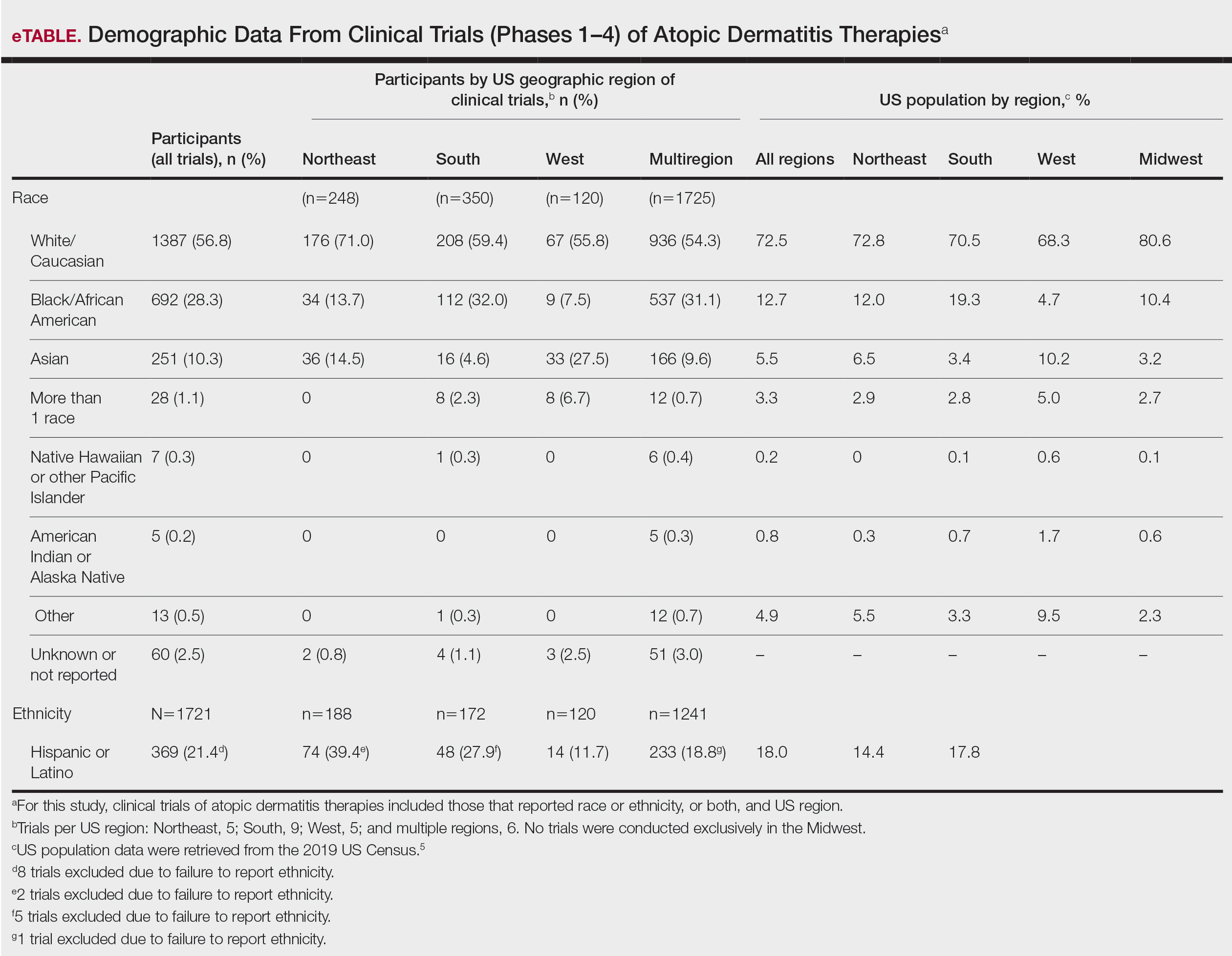
Our findings on representation for ethnicity are as follows (US Census data is listed in parentheses for comparison5):
- Hispanic or Latino: 21.4% (18.0%)
Although representation of Black/African American and Asian participants in clinical trials was higher than their representation in US Census data and representation of White participants was lower in clinical trials than their representation in census data, equal representation among all racial and ethnic groups is still lacking. A potential explanation for this finding might be that requirements for trial inclusion selected for more minority patients, given the propensity for greater severity of AD among those racial groups.2 Another explanation might be that efforts to include minority patients in clinical trials are improving.
There were great differences in ethnoracial representation in clinical trials when regions within the United States were compared. Based on census population data by region, the West had the highest percentage (29.9%) of Hispanic or Latino residents; however, this group represented only 11.7% of participants in AD clinical trials in that region.5
The South had the greatest number of participants in AD clinical trials of any region, which was consistent with research findings on an association between severity of AD and heat.6 With a warmer climate correlating with an increased incidence of AD, it is possible that more people are willing to participate in clinical trials in the South.
The Midwest was the only region in which region-specific clinical trials were not conducted. Recent studies have shown that individuals with AD who live in the Midwest have comparatively less access to health care associated with AD treatment and are more likely to visit an emergency department because of AD than individuals in any other US region.7 This discrepancy highlights the need for increased access to resources and clinical trials focused on the treatment of AD in the Midwest.
In 1993, the National Institutes of Health Revitalization Act established a federal legislative mandate to encourage inclusion of women and people of color in clinical trials.8 During the last 2 decades, there have been improvements in ethnoracial reporting. A 2020 global study found that 81.1% of randomized controlled trials (phases 2 and 3) of AD treatments reported ethnoracial data.3
Equal representation in clinical trials allows for further investigation of the connection between race, AD severity, and treatment efficacy. Clinical trials need to have equal representation of ethnoracial categories across all regions of the United States. If one group is notably overrepresented, ethnoracial associations related to the treatment of AD might go undetected.9 Similarly, if representation is unequal, relationships of treatment efficacy within ethnoracial groups also might go undetected. None of the clinical trials that we analyzed investigated treatment efficacy by race, suggesting that there is a need for future research in this area.
It also is important to note that broad classifications of race and ethnicity are limiting and therefore overlook differences within ethnoracial categories. Although representation of minority patients in clinical trials for AD treatments is improving, we conclude that there remains a need for greater and equal representation of minority groups in clinical trials of AD treatments in the United States.
- Avena-Woods C. Overview of atopic dermatitis. Am J Manag Care. 2017;23(8 suppl):S115-S123.
- Kaufman BP, Guttman‐Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups—variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357. doi:10.1111/exd.13514
- Price KN, Krase JM, Loh TY, et al. Racial and ethnic disparities in global atopic dermatitis clinical trials. Br J Dermatol. 2020;183:378-380. doi:10.1111/bjd.18938
- Collection of race and ethnicity data in clinical trials: guidance for industry and Food and Drug Administration staff. US Food and Drug Administration; October 26, 2016. Accessed February 20, 2022. https://www.fda.gov/media/75453/download
- United States Census Bureau. 2019 Population estimates by age, sex, race and Hispanic origin. Published June 25, 2020. Accessed March 22, 2022. https://www.census.gov/newsroom/press-kits/2020/population-estimates-detailed.html
- Fleischer AB Jr. Atopic dermatitis: the relationship to temperature and seasonality in the United States. Int J Dermatol. 2019;58:465-471. doi:10.1111/ijd.14289
- Wu KK, Nguyen KB, Sandhu JK, et al. Does location matter? geographic variations in healthcare resource use for atopic dermatitis in the United States. J Dermatolog Treat. 2021;32:314-320. doi:10.1080/09546634.2019.1656796
- National Institutes of Health Revitalization Act of 1993, 42 USC 201 (1993). Accessed February 20, 2022. https://www.govinfo.gov/content/pkg/STATUTE-107/pdf/STATUTE-107-Pg122.pdf
- Hirano SA, Murray SB, Harvey VM. Reporting, representation, and subgroup analysis of race and ethnicity in published clinical trials of atopic dermatitis in the United States between 2000 and 2009. Pediatr Dermatol. 2012;29:749-755. doi:10.1111/j.1525-1470.2012.01797.x
To the Editor:
Atopic dermatitis (AD) affects an estimated 7.2% of adults and 10.7% of children in the United States; however, AD might affect different races at a varying rate.1 Compared to their European American counterparts, Asian/Pacific Islanders and African Americans are 7 and 3 times more likely, respectively, to be given a diagnosis of AD.2
Despite being disproportionately affected by AD, minority groups might be underrepresented in clinical trials of AD treatments.3 One explanation for this imbalance might be that ethnoracial representation differs across regions in the United States, perhaps in regions where clinical trials are conducted. Price et al3 investigated racial representation in clinical trials of AD globally and found that patients of color are consistently underrepresented.
Research on racial representation in clinical trials within the United States—on national and regional scales—is lacking from the current AD literature. We conducted a study to compare racial and ethnic disparities in AD clinical trials across regions of the United States.
Using the ClinicalTrials.gov database (www.clinicaltrials.gov) of the National Library of Medicine, we identified clinical trials of AD treatments (encompassing phases 1 through 4) in the United States that were completed before March 14, 2021, with the earliest data from 2013. Search terms included atopic dermatitis, with an advanced search for interventional (clinical trials) and with results.
In total, 95 completed clinical trials were identified, of which 26 (27.4%) reported ethnoracial demographic data. One trial was excluded due to misrepresentation regarding the classification of individuals who identified as more than 1 racial category. Clinical trials for systemic treatments (7 [28%]) and topical treatments (18 [72%]) were identified.
All ethnoracial data were self-reported by trial participants based on US Food and Drug Administration guidelines for racial and ethnic categorization.4 Trial participants who identified ethnically as Hispanic or Latino might have been a part of any racial group. Only 7 of the 25 included clinical trials (28%) provided ethnic demographic data (Hispanic [Latino] or non-Hispanic); 72% of trials failed to report ethnicity. Ethnic data included in our analysis came from only the 7 clinical trials that included these data. International multicenter trials that included a US site were excluded.
Ultimately, the number of trials included in our analysis was 25, comprised of 2443 participants. Data were further organized by US geographic region (Northeast, Midwest, South, West, and multiregion trials [ie, conducted in ≥2 regions]). No AD clinical trials were conducted solely in the Midwest; it was only included within multiregion trials.
Compared to their representation in the 2019 US Census, most minority groups were overrepresented in clinical trials, while White individuals were underrepresented (eTable). The percentages of our findings on representation for race are as follows (US Census data are listed in parentheses for comparison5):
- White: 56.8% (72.5%)
- Black/African American: 28.3% (12.7%)
- Asian: 10.3% (5.5%)
- Multiracial: 1.1% (3.3%)
- Native Hawaiian or other Pacific Islander: 0.3% (0.2%)
- American Indian or Alaska Native: 0.2% (0.8%)
- Other: 0.5% (4.9%).

Our findings on representation for ethnicity are as follows (US Census data is listed in parentheses for comparison5):
- Hispanic or Latino: 21.4% (18.0%)
Although representation of Black/African American and Asian participants in clinical trials was higher than their representation in US Census data and representation of White participants was lower in clinical trials than their representation in census data, equal representation among all racial and ethnic groups is still lacking. A potential explanation for this finding might be that requirements for trial inclusion selected for more minority patients, given the propensity for greater severity of AD among those racial groups.2 Another explanation might be that efforts to include minority patients in clinical trials are improving.
There were great differences in ethnoracial representation in clinical trials when regions within the United States were compared. Based on census population data by region, the West had the highest percentage (29.9%) of Hispanic or Latino residents; however, this group represented only 11.7% of participants in AD clinical trials in that region.5
The South had the greatest number of participants in AD clinical trials of any region, which was consistent with research findings on an association between severity of AD and heat.6 With a warmer climate correlating with an increased incidence of AD, it is possible that more people are willing to participate in clinical trials in the South.
The Midwest was the only region in which region-specific clinical trials were not conducted. Recent studies have shown that individuals with AD who live in the Midwest have comparatively less access to health care associated with AD treatment and are more likely to visit an emergency department because of AD than individuals in any other US region.7 This discrepancy highlights the need for increased access to resources and clinical trials focused on the treatment of AD in the Midwest.
In 1993, the National Institutes of Health Revitalization Act established a federal legislative mandate to encourage inclusion of women and people of color in clinical trials.8 During the last 2 decades, there have been improvements in ethnoracial reporting. A 2020 global study found that 81.1% of randomized controlled trials (phases 2 and 3) of AD treatments reported ethnoracial data.3
Equal representation in clinical trials allows for further investigation of the connection between race, AD severity, and treatment efficacy. Clinical trials need to have equal representation of ethnoracial categories across all regions of the United States. If one group is notably overrepresented, ethnoracial associations related to the treatment of AD might go undetected.9 Similarly, if representation is unequal, relationships of treatment efficacy within ethnoracial groups also might go undetected. None of the clinical trials that we analyzed investigated treatment efficacy by race, suggesting that there is a need for future research in this area.
It also is important to note that broad classifications of race and ethnicity are limiting and therefore overlook differences within ethnoracial categories. Although representation of minority patients in clinical trials for AD treatments is improving, we conclude that there remains a need for greater and equal representation of minority groups in clinical trials of AD treatments in the United States.
To the Editor:
Atopic dermatitis (AD) affects an estimated 7.2% of adults and 10.7% of children in the United States; however, AD might affect different races at a varying rate.1 Compared to their European American counterparts, Asian/Pacific Islanders and African Americans are 7 and 3 times more likely, respectively, to be given a diagnosis of AD.2
Despite being disproportionately affected by AD, minority groups might be underrepresented in clinical trials of AD treatments.3 One explanation for this imbalance might be that ethnoracial representation differs across regions in the United States, perhaps in regions where clinical trials are conducted. Price et al3 investigated racial representation in clinical trials of AD globally and found that patients of color are consistently underrepresented.
Research on racial representation in clinical trials within the United States—on national and regional scales—is lacking from the current AD literature. We conducted a study to compare racial and ethnic disparities in AD clinical trials across regions of the United States.
Using the ClinicalTrials.gov database (www.clinicaltrials.gov) of the National Library of Medicine, we identified clinical trials of AD treatments (encompassing phases 1 through 4) in the United States that were completed before March 14, 2021, with the earliest data from 2013. Search terms included atopic dermatitis, with an advanced search for interventional (clinical trials) and with results.
In total, 95 completed clinical trials were identified, of which 26 (27.4%) reported ethnoracial demographic data. One trial was excluded due to misrepresentation regarding the classification of individuals who identified as more than 1 racial category. Clinical trials for systemic treatments (7 [28%]) and topical treatments (18 [72%]) were identified.
All ethnoracial data were self-reported by trial participants based on US Food and Drug Administration guidelines for racial and ethnic categorization.4 Trial participants who identified ethnically as Hispanic or Latino might have been a part of any racial group. Only 7 of the 25 included clinical trials (28%) provided ethnic demographic data (Hispanic [Latino] or non-Hispanic); 72% of trials failed to report ethnicity. Ethnic data included in our analysis came from only the 7 clinical trials that included these data. International multicenter trials that included a US site were excluded.
Ultimately, the number of trials included in our analysis was 25, comprised of 2443 participants. Data were further organized by US geographic region (Northeast, Midwest, South, West, and multiregion trials [ie, conducted in ≥2 regions]). No AD clinical trials were conducted solely in the Midwest; it was only included within multiregion trials.
Compared to their representation in the 2019 US Census, most minority groups were overrepresented in clinical trials, while White individuals were underrepresented (eTable). The percentages of our findings on representation for race are as follows (US Census data are listed in parentheses for comparison5):
- White: 56.8% (72.5%)
- Black/African American: 28.3% (12.7%)
- Asian: 10.3% (5.5%)
- Multiracial: 1.1% (3.3%)
- Native Hawaiian or other Pacific Islander: 0.3% (0.2%)
- American Indian or Alaska Native: 0.2% (0.8%)
- Other: 0.5% (4.9%).

Our findings on representation for ethnicity are as follows (US Census data is listed in parentheses for comparison5):
- Hispanic or Latino: 21.4% (18.0%)
Although representation of Black/African American and Asian participants in clinical trials was higher than their representation in US Census data and representation of White participants was lower in clinical trials than their representation in census data, equal representation among all racial and ethnic groups is still lacking. A potential explanation for this finding might be that requirements for trial inclusion selected for more minority patients, given the propensity for greater severity of AD among those racial groups.2 Another explanation might be that efforts to include minority patients in clinical trials are improving.
There were great differences in ethnoracial representation in clinical trials when regions within the United States were compared. Based on census population data by region, the West had the highest percentage (29.9%) of Hispanic or Latino residents; however, this group represented only 11.7% of participants in AD clinical trials in that region.5
The South had the greatest number of participants in AD clinical trials of any region, which was consistent with research findings on an association between severity of AD and heat.6 With a warmer climate correlating with an increased incidence of AD, it is possible that more people are willing to participate in clinical trials in the South.
The Midwest was the only region in which region-specific clinical trials were not conducted. Recent studies have shown that individuals with AD who live in the Midwest have comparatively less access to health care associated with AD treatment and are more likely to visit an emergency department because of AD than individuals in any other US region.7 This discrepancy highlights the need for increased access to resources and clinical trials focused on the treatment of AD in the Midwest.
In 1993, the National Institutes of Health Revitalization Act established a federal legislative mandate to encourage inclusion of women and people of color in clinical trials.8 During the last 2 decades, there have been improvements in ethnoracial reporting. A 2020 global study found that 81.1% of randomized controlled trials (phases 2 and 3) of AD treatments reported ethnoracial data.3
Equal representation in clinical trials allows for further investigation of the connection between race, AD severity, and treatment efficacy. Clinical trials need to have equal representation of ethnoracial categories across all regions of the United States. If one group is notably overrepresented, ethnoracial associations related to the treatment of AD might go undetected.9 Similarly, if representation is unequal, relationships of treatment efficacy within ethnoracial groups also might go undetected. None of the clinical trials that we analyzed investigated treatment efficacy by race, suggesting that there is a need for future research in this area.
It also is important to note that broad classifications of race and ethnicity are limiting and therefore overlook differences within ethnoracial categories. Although representation of minority patients in clinical trials for AD treatments is improving, we conclude that there remains a need for greater and equal representation of minority groups in clinical trials of AD treatments in the United States.
- Avena-Woods C. Overview of atopic dermatitis. Am J Manag Care. 2017;23(8 suppl):S115-S123.
- Kaufman BP, Guttman‐Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups—variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357. doi:10.1111/exd.13514
- Price KN, Krase JM, Loh TY, et al. Racial and ethnic disparities in global atopic dermatitis clinical trials. Br J Dermatol. 2020;183:378-380. doi:10.1111/bjd.18938
- Collection of race and ethnicity data in clinical trials: guidance for industry and Food and Drug Administration staff. US Food and Drug Administration; October 26, 2016. Accessed February 20, 2022. https://www.fda.gov/media/75453/download
- United States Census Bureau. 2019 Population estimates by age, sex, race and Hispanic origin. Published June 25, 2020. Accessed March 22, 2022. https://www.census.gov/newsroom/press-kits/2020/population-estimates-detailed.html
- Fleischer AB Jr. Atopic dermatitis: the relationship to temperature and seasonality in the United States. Int J Dermatol. 2019;58:465-471. doi:10.1111/ijd.14289
- Wu KK, Nguyen KB, Sandhu JK, et al. Does location matter? geographic variations in healthcare resource use for atopic dermatitis in the United States. J Dermatolog Treat. 2021;32:314-320. doi:10.1080/09546634.2019.1656796
- National Institutes of Health Revitalization Act of 1993, 42 USC 201 (1993). Accessed February 20, 2022. https://www.govinfo.gov/content/pkg/STATUTE-107/pdf/STATUTE-107-Pg122.pdf
- Hirano SA, Murray SB, Harvey VM. Reporting, representation, and subgroup analysis of race and ethnicity in published clinical trials of atopic dermatitis in the United States between 2000 and 2009. Pediatr Dermatol. 2012;29:749-755. doi:10.1111/j.1525-1470.2012.01797.x
- Avena-Woods C. Overview of atopic dermatitis. Am J Manag Care. 2017;23(8 suppl):S115-S123.
- Kaufman BP, Guttman‐Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups—variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357. doi:10.1111/exd.13514
- Price KN, Krase JM, Loh TY, et al. Racial and ethnic disparities in global atopic dermatitis clinical trials. Br J Dermatol. 2020;183:378-380. doi:10.1111/bjd.18938
- Collection of race and ethnicity data in clinical trials: guidance for industry and Food and Drug Administration staff. US Food and Drug Administration; October 26, 2016. Accessed February 20, 2022. https://www.fda.gov/media/75453/download
- United States Census Bureau. 2019 Population estimates by age, sex, race and Hispanic origin. Published June 25, 2020. Accessed March 22, 2022. https://www.census.gov/newsroom/press-kits/2020/population-estimates-detailed.html
- Fleischer AB Jr. Atopic dermatitis: the relationship to temperature and seasonality in the United States. Int J Dermatol. 2019;58:465-471. doi:10.1111/ijd.14289
- Wu KK, Nguyen KB, Sandhu JK, et al. Does location matter? geographic variations in healthcare resource use for atopic dermatitis in the United States. J Dermatolog Treat. 2021;32:314-320. doi:10.1080/09546634.2019.1656796
- National Institutes of Health Revitalization Act of 1993, 42 USC 201 (1993). Accessed February 20, 2022. https://www.govinfo.gov/content/pkg/STATUTE-107/pdf/STATUTE-107-Pg122.pdf
- Hirano SA, Murray SB, Harvey VM. Reporting, representation, and subgroup analysis of race and ethnicity in published clinical trials of atopic dermatitis in the United States between 2000 and 2009. Pediatr Dermatol. 2012;29:749-755. doi:10.1111/j.1525-1470.2012.01797.x
Practice Points
- Although minority groups are disproportionally affected by atopic dermatitis (AD), they may be underrepresented in clinical trials for AD in the United States.
- Equal representation among ethnoracial groups in clinical trials is important to allow for a more thorough investigation of the efficacy of treatments for AD.