User login
Jeff Evans has been editor of Rheumatology News/MDedge Rheumatology and the EULAR Congress News since 2013. He started at Frontline Medical Communications in 2001 and was a reporter for 8 years before serving as editor of Clinical Neurology News and World Neurology, and briefly as editor of GI & Hepatology News. He graduated cum laude from Cornell University (New York) with a BA in biological sciences, concentrating in neurobiology and behavior.
MS drugs in Medicare Part D: Higher tiers, less coverage, more prior authorizations
LOS ANGELES – Under Medicare Part D, private prescription drug plans and those bundled with Medicare Advantage plans have steadily raised injectable and oral disease-modifying therapies for multiple sclerosis to higher tiers with higher cost sharing, reduced coverage of particular drugs, and increased prior authorizations during the 10-year period of 2007-2016, according to an analysis conducted by Oregon State University researchers.
At the annual meeting of the American Academy of Neurology, Daniel Hartung, PharmD, of Oregon Health and Science University, Portland, and his colleagues reported that the proportion of plans with disease-modifying therapies (DMTs) in the highest tiers, generally tier 5 and above, rose from 11% in 2007 to 95% in 2016.
The scope of drugs covered by the plans declined marginally. Over the 10-year period, plans covering at least three DMTs declined from 98% to 95%, but plans that covered interferon beta-1b, intramuscular or subcutaneous interferon beta-1a, or glatiramer acetate declined from 85%-100% of plans to 60%-81%.
Plans with prior authorizations for DMTs rose across the board. The percentage of plans with at least one DMT not needing prior authorization dropped from 40% to 27%, while plans that covered interferon beta-1b, intramuscular or subcutaneous interferon beta-1a, or glatiramer acetate rose from 62%-65% to 77%-80%.
Based on 2016 coverage characteristics, Dr. Hartung and his associates estimated that the expected annual out-of-pocket costs for patients in 2019 would be over $5,000 for all drugs in the analysis, after accounting for the Bipartisan Budget Act’s closing the Part D coverage gap in 2019.
Part D covers noninfusible DMTs, which in this analysis included glatiramer acetate 20 and 40 mg (Copaxone 20 and Copaxone 40), generic glatiramer acetate 20 mg (Glatopa), interferon beta-1a intramuscular (Avonex), interferon beta-1a subcutaneous (Rebif), interferon beta-1b (Extavia and Betaseron), peginterferon beta-1a (Plegridy), fingolimod (Gilenya), teriflunomide (Aubagio), and dimethyl fumarate (Tecfidera). The infusible drugs natalizumab (Tysabri), alemtuzumab (Lemtrada), and ocrelizumab (Ocrevus) fall under Medicare Part B.
The study was supported by the National Multiple Sclerosis Society. None of the authors had anything to disclose.
SOURCE: Hartung D et al. AAN 2018. Abstract P3.161
LOS ANGELES – Under Medicare Part D, private prescription drug plans and those bundled with Medicare Advantage plans have steadily raised injectable and oral disease-modifying therapies for multiple sclerosis to higher tiers with higher cost sharing, reduced coverage of particular drugs, and increased prior authorizations during the 10-year period of 2007-2016, according to an analysis conducted by Oregon State University researchers.
At the annual meeting of the American Academy of Neurology, Daniel Hartung, PharmD, of Oregon Health and Science University, Portland, and his colleagues reported that the proportion of plans with disease-modifying therapies (DMTs) in the highest tiers, generally tier 5 and above, rose from 11% in 2007 to 95% in 2016.
The scope of drugs covered by the plans declined marginally. Over the 10-year period, plans covering at least three DMTs declined from 98% to 95%, but plans that covered interferon beta-1b, intramuscular or subcutaneous interferon beta-1a, or glatiramer acetate declined from 85%-100% of plans to 60%-81%.
Plans with prior authorizations for DMTs rose across the board. The percentage of plans with at least one DMT not needing prior authorization dropped from 40% to 27%, while plans that covered interferon beta-1b, intramuscular or subcutaneous interferon beta-1a, or glatiramer acetate rose from 62%-65% to 77%-80%.
Based on 2016 coverage characteristics, Dr. Hartung and his associates estimated that the expected annual out-of-pocket costs for patients in 2019 would be over $5,000 for all drugs in the analysis, after accounting for the Bipartisan Budget Act’s closing the Part D coverage gap in 2019.
Part D covers noninfusible DMTs, which in this analysis included glatiramer acetate 20 and 40 mg (Copaxone 20 and Copaxone 40), generic glatiramer acetate 20 mg (Glatopa), interferon beta-1a intramuscular (Avonex), interferon beta-1a subcutaneous (Rebif), interferon beta-1b (Extavia and Betaseron), peginterferon beta-1a (Plegridy), fingolimod (Gilenya), teriflunomide (Aubagio), and dimethyl fumarate (Tecfidera). The infusible drugs natalizumab (Tysabri), alemtuzumab (Lemtrada), and ocrelizumab (Ocrevus) fall under Medicare Part B.
The study was supported by the National Multiple Sclerosis Society. None of the authors had anything to disclose.
SOURCE: Hartung D et al. AAN 2018. Abstract P3.161
LOS ANGELES – Under Medicare Part D, private prescription drug plans and those bundled with Medicare Advantage plans have steadily raised injectable and oral disease-modifying therapies for multiple sclerosis to higher tiers with higher cost sharing, reduced coverage of particular drugs, and increased prior authorizations during the 10-year period of 2007-2016, according to an analysis conducted by Oregon State University researchers.
At the annual meeting of the American Academy of Neurology, Daniel Hartung, PharmD, of Oregon Health and Science University, Portland, and his colleagues reported that the proportion of plans with disease-modifying therapies (DMTs) in the highest tiers, generally tier 5 and above, rose from 11% in 2007 to 95% in 2016.
The scope of drugs covered by the plans declined marginally. Over the 10-year period, plans covering at least three DMTs declined from 98% to 95%, but plans that covered interferon beta-1b, intramuscular or subcutaneous interferon beta-1a, or glatiramer acetate declined from 85%-100% of plans to 60%-81%.
Plans with prior authorizations for DMTs rose across the board. The percentage of plans with at least one DMT not needing prior authorization dropped from 40% to 27%, while plans that covered interferon beta-1b, intramuscular or subcutaneous interferon beta-1a, or glatiramer acetate rose from 62%-65% to 77%-80%.
Based on 2016 coverage characteristics, Dr. Hartung and his associates estimated that the expected annual out-of-pocket costs for patients in 2019 would be over $5,000 for all drugs in the analysis, after accounting for the Bipartisan Budget Act’s closing the Part D coverage gap in 2019.
Part D covers noninfusible DMTs, which in this analysis included glatiramer acetate 20 and 40 mg (Copaxone 20 and Copaxone 40), generic glatiramer acetate 20 mg (Glatopa), interferon beta-1a intramuscular (Avonex), interferon beta-1a subcutaneous (Rebif), interferon beta-1b (Extavia and Betaseron), peginterferon beta-1a (Plegridy), fingolimod (Gilenya), teriflunomide (Aubagio), and dimethyl fumarate (Tecfidera). The infusible drugs natalizumab (Tysabri), alemtuzumab (Lemtrada), and ocrelizumab (Ocrevus) fall under Medicare Part B.
The study was supported by the National Multiple Sclerosis Society. None of the authors had anything to disclose.
SOURCE: Hartung D et al. AAN 2018. Abstract P3.161
REPORTING FROM AAN 2018
VIDEO: Meeting stroke screening demand will require systems’ reorganization
In a video interview at the annual meeting of the American Academy of Neurology, Dr. Wechsler described steps being taken at the University of Pittsburgh Medical Center’s comprehensive stroke center to handle the additional workload.
UPMC conducts telemedicine acute stroke evaluations of patients at community hospitals’ primary stroke centers in the greater Pittsburgh area to make sure that only the cases that require mechanical thrombectomy are transferred to them for specialized care, while also continuing to see nontransferred patients via telemedicine for follow-up, said Dr. Wechsler, chair of the department of neurology at UPMC and founder of its Stroke Institute and telestroke network.
This sort of solution may be more feasible and practical for comprehensive stroke centers to implement in order to manage the number of cases, instead of expanding neurology residencies, capping stroke services, adding a nonteaching service, adding advanced practice providers, or increasing the case loads of vascular neurology fellows and attending neurologists, he said.
In just the short time since the DAWN trial results were released in November 2017 and set the new standard for treating eligible patients with large-vessel occlusions with mechanical thrombectomy within 6-24 hours, stroke admissions and transfers to the comprehensive stroke center at UPMC from November 2017 to February 2018 rose 18% from the same time period a year before, including a 5% rise in telemedicine transfers, Dr. Wechsler said in a presentation at the meeting. These additional cases led to a 35% increase in thrombectomy cases.
Putting the matter into additional perspective, in the time period from November 2014 to February 2017, 30% of all 2,667 acute ischemic stroke patients seen at UPMC would have met DAWN trial inclusion criteria with a 6- to 24-hour window, but less than 3% of all the strokes seen at UPMC would have qualified for thrombectomy under criteria from the DAWN and DEFUSE-3 trials. That makes it imperative for comprehensive stroke centers to triage cases and receive only those that require endovascular treatment, he said.
Meeting the already-rising needs for triaging acute ischemic stroke patients arriving in the window of 6-24 hours will be difficult, considering that there are about 800,000 new strokes per year in the United States but only 1,100 vascular neurologists, nearly 1,100 primary stroke centers, and only 110 comprehensive stroke centers at which endovascular thrombectomy treatment may be offered. As of 2016, he noted that there also were only 74 U.S. stroke fellowship programs with 123 positions offered, of which 34% went unfilled.
In a video interview at the annual meeting of the American Academy of Neurology, Dr. Wechsler described steps being taken at the University of Pittsburgh Medical Center’s comprehensive stroke center to handle the additional workload.
UPMC conducts telemedicine acute stroke evaluations of patients at community hospitals’ primary stroke centers in the greater Pittsburgh area to make sure that only the cases that require mechanical thrombectomy are transferred to them for specialized care, while also continuing to see nontransferred patients via telemedicine for follow-up, said Dr. Wechsler, chair of the department of neurology at UPMC and founder of its Stroke Institute and telestroke network.
This sort of solution may be more feasible and practical for comprehensive stroke centers to implement in order to manage the number of cases, instead of expanding neurology residencies, capping stroke services, adding a nonteaching service, adding advanced practice providers, or increasing the case loads of vascular neurology fellows and attending neurologists, he said.
In just the short time since the DAWN trial results were released in November 2017 and set the new standard for treating eligible patients with large-vessel occlusions with mechanical thrombectomy within 6-24 hours, stroke admissions and transfers to the comprehensive stroke center at UPMC from November 2017 to February 2018 rose 18% from the same time period a year before, including a 5% rise in telemedicine transfers, Dr. Wechsler said in a presentation at the meeting. These additional cases led to a 35% increase in thrombectomy cases.
Putting the matter into additional perspective, in the time period from November 2014 to February 2017, 30% of all 2,667 acute ischemic stroke patients seen at UPMC would have met DAWN trial inclusion criteria with a 6- to 24-hour window, but less than 3% of all the strokes seen at UPMC would have qualified for thrombectomy under criteria from the DAWN and DEFUSE-3 trials. That makes it imperative for comprehensive stroke centers to triage cases and receive only those that require endovascular treatment, he said.
Meeting the already-rising needs for triaging acute ischemic stroke patients arriving in the window of 6-24 hours will be difficult, considering that there are about 800,000 new strokes per year in the United States but only 1,100 vascular neurologists, nearly 1,100 primary stroke centers, and only 110 comprehensive stroke centers at which endovascular thrombectomy treatment may be offered. As of 2016, he noted that there also were only 74 U.S. stroke fellowship programs with 123 positions offered, of which 34% went unfilled.
In a video interview at the annual meeting of the American Academy of Neurology, Dr. Wechsler described steps being taken at the University of Pittsburgh Medical Center’s comprehensive stroke center to handle the additional workload.
UPMC conducts telemedicine acute stroke evaluations of patients at community hospitals’ primary stroke centers in the greater Pittsburgh area to make sure that only the cases that require mechanical thrombectomy are transferred to them for specialized care, while also continuing to see nontransferred patients via telemedicine for follow-up, said Dr. Wechsler, chair of the department of neurology at UPMC and founder of its Stroke Institute and telestroke network.
This sort of solution may be more feasible and practical for comprehensive stroke centers to implement in order to manage the number of cases, instead of expanding neurology residencies, capping stroke services, adding a nonteaching service, adding advanced practice providers, or increasing the case loads of vascular neurology fellows and attending neurologists, he said.
In just the short time since the DAWN trial results were released in November 2017 and set the new standard for treating eligible patients with large-vessel occlusions with mechanical thrombectomy within 6-24 hours, stroke admissions and transfers to the comprehensive stroke center at UPMC from November 2017 to February 2018 rose 18% from the same time period a year before, including a 5% rise in telemedicine transfers, Dr. Wechsler said in a presentation at the meeting. These additional cases led to a 35% increase in thrombectomy cases.
Putting the matter into additional perspective, in the time period from November 2014 to February 2017, 30% of all 2,667 acute ischemic stroke patients seen at UPMC would have met DAWN trial inclusion criteria with a 6- to 24-hour window, but less than 3% of all the strokes seen at UPMC would have qualified for thrombectomy under criteria from the DAWN and DEFUSE-3 trials. That makes it imperative for comprehensive stroke centers to triage cases and receive only those that require endovascular treatment, he said.
Meeting the already-rising needs for triaging acute ischemic stroke patients arriving in the window of 6-24 hours will be difficult, considering that there are about 800,000 new strokes per year in the United States but only 1,100 vascular neurologists, nearly 1,100 primary stroke centers, and only 110 comprehensive stroke centers at which endovascular thrombectomy treatment may be offered. As of 2016, he noted that there also were only 74 U.S. stroke fellowship programs with 123 positions offered, of which 34% went unfilled.
REPORTING FROM AAN 2018
VIDEOS: High-priced drugs, out-of-pocket costs raise challenges for neurologists
LOS ANGELES – Neurologists can play an important role in helping patients gain access to high-cost, breakthrough drugs, while at the same time guiding patients to lower-cost options whenever possible, speakers said at the annual meeting of the American Academy of Neurology.
The use of the Orphan Drug approval pathway established in 1983 has gained a great deal of steam for rare neurologic diseases in recent years with the approval of a number of drugs, such as nusinersen (Spinraza) for spinal muscular atrophy, eteplirsen (Exondys 51) for Duchenne muscular dystrophy, and edaravone (Radicava) for amyotrophic lateral sclerosis, said Nicholas Johnson, MD, a pediatric neuromuscular disease specialist at the University of Utah, Salt Lake City.
But given that only 2% of U.S. physicians are neurologists, yet 18% of rare diseases are neurologic and 11% of drugs in development overall are for neurologic diseases, there are a great deal of challenges arising for neurologists in getting access to these new high-priced drugs for their patients, said Dr. Johnson, who leads the AAN’s Neurology Drug Pricing Task Force and is also chair of the AAN Government Relations Committee.
These challenges range from increased administrative burden on staff, getting insurance approval, finding administration sites, and the ability to perform special patient assessments, he said in an interview.
Dr. Nicholas Johnson’s interview:
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Brian Callaghan’s interview:
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Many high-priced drugs commonly prescribed for chronic neurologic conditions and diagnostic tests also have high out-of-pocket costs for patients, but it is remarkably hard even for well-informed experts to find the actual costs that patients will pay out of pocket for such drugs and tests, according to Brian Callaghan, MD, a neuromuscular disease specialist at the University of Michigan, Ann Arbor.
Neurologist can seek to find more affordable alternatives to drugs when the out-of-pocket expenses are too great, said Dr. Callaghan, who also serves on the Neurology Drug Pricing Task Force. It may be advisable to put certain drugs lower on a list of potential treatment options than others for a chronic condition such as epilepsy because of their out-of-pocket costs, but it can be frustratingly hard to determine these costs in advance.
The University of Michigan Health System is unique in having drug cost data provided as part of information presented to physicians in electronic health records, but this is not the case in most other clinics. Until doctors can regularly access patient-specific drug and diagnostic testing out-of-pocket costs through EHRs, finding the best affordable medications for patients will remain a costly and time-consuming process, he said in an interview.
LOS ANGELES – Neurologists can play an important role in helping patients gain access to high-cost, breakthrough drugs, while at the same time guiding patients to lower-cost options whenever possible, speakers said at the annual meeting of the American Academy of Neurology.
The use of the Orphan Drug approval pathway established in 1983 has gained a great deal of steam for rare neurologic diseases in recent years with the approval of a number of drugs, such as nusinersen (Spinraza) for spinal muscular atrophy, eteplirsen (Exondys 51) for Duchenne muscular dystrophy, and edaravone (Radicava) for amyotrophic lateral sclerosis, said Nicholas Johnson, MD, a pediatric neuromuscular disease specialist at the University of Utah, Salt Lake City.
But given that only 2% of U.S. physicians are neurologists, yet 18% of rare diseases are neurologic and 11% of drugs in development overall are for neurologic diseases, there are a great deal of challenges arising for neurologists in getting access to these new high-priced drugs for their patients, said Dr. Johnson, who leads the AAN’s Neurology Drug Pricing Task Force and is also chair of the AAN Government Relations Committee.
These challenges range from increased administrative burden on staff, getting insurance approval, finding administration sites, and the ability to perform special patient assessments, he said in an interview.
Dr. Nicholas Johnson’s interview:
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Brian Callaghan’s interview:
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Many high-priced drugs commonly prescribed for chronic neurologic conditions and diagnostic tests also have high out-of-pocket costs for patients, but it is remarkably hard even for well-informed experts to find the actual costs that patients will pay out of pocket for such drugs and tests, according to Brian Callaghan, MD, a neuromuscular disease specialist at the University of Michigan, Ann Arbor.
Neurologist can seek to find more affordable alternatives to drugs when the out-of-pocket expenses are too great, said Dr. Callaghan, who also serves on the Neurology Drug Pricing Task Force. It may be advisable to put certain drugs lower on a list of potential treatment options than others for a chronic condition such as epilepsy because of their out-of-pocket costs, but it can be frustratingly hard to determine these costs in advance.
The University of Michigan Health System is unique in having drug cost data provided as part of information presented to physicians in electronic health records, but this is not the case in most other clinics. Until doctors can regularly access patient-specific drug and diagnostic testing out-of-pocket costs through EHRs, finding the best affordable medications for patients will remain a costly and time-consuming process, he said in an interview.
LOS ANGELES – Neurologists can play an important role in helping patients gain access to high-cost, breakthrough drugs, while at the same time guiding patients to lower-cost options whenever possible, speakers said at the annual meeting of the American Academy of Neurology.
The use of the Orphan Drug approval pathway established in 1983 has gained a great deal of steam for rare neurologic diseases in recent years with the approval of a number of drugs, such as nusinersen (Spinraza) for spinal muscular atrophy, eteplirsen (Exondys 51) for Duchenne muscular dystrophy, and edaravone (Radicava) for amyotrophic lateral sclerosis, said Nicholas Johnson, MD, a pediatric neuromuscular disease specialist at the University of Utah, Salt Lake City.
But given that only 2% of U.S. physicians are neurologists, yet 18% of rare diseases are neurologic and 11% of drugs in development overall are for neurologic diseases, there are a great deal of challenges arising for neurologists in getting access to these new high-priced drugs for their patients, said Dr. Johnson, who leads the AAN’s Neurology Drug Pricing Task Force and is also chair of the AAN Government Relations Committee.
These challenges range from increased administrative burden on staff, getting insurance approval, finding administration sites, and the ability to perform special patient assessments, he said in an interview.
Dr. Nicholas Johnson’s interview:
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Brian Callaghan’s interview:
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Many high-priced drugs commonly prescribed for chronic neurologic conditions and diagnostic tests also have high out-of-pocket costs for patients, but it is remarkably hard even for well-informed experts to find the actual costs that patients will pay out of pocket for such drugs and tests, according to Brian Callaghan, MD, a neuromuscular disease specialist at the University of Michigan, Ann Arbor.
Neurologist can seek to find more affordable alternatives to drugs when the out-of-pocket expenses are too great, said Dr. Callaghan, who also serves on the Neurology Drug Pricing Task Force. It may be advisable to put certain drugs lower on a list of potential treatment options than others for a chronic condition such as epilepsy because of their out-of-pocket costs, but it can be frustratingly hard to determine these costs in advance.
The University of Michigan Health System is unique in having drug cost data provided as part of information presented to physicians in electronic health records, but this is not the case in most other clinics. Until doctors can regularly access patient-specific drug and diagnostic testing out-of-pocket costs through EHRs, finding the best affordable medications for patients will remain a costly and time-consuming process, he said in an interview.
REPORTING FROM AAN 2018
Real-world data so far support pimavanserin trial results
LOS ANGELES – Two studies of real-world use of pimavanserin after its approval in March 2016 for the treatment of Parkinson’s disease–associated psychosis indicate its effectiveness, tolerability, and safety in line with clinical trial results, according to reports presented at the annual meeting of the American Academy of Neurology.
In the first study, 102 patients were prescribed pimavanserin (Nuplazid) during May 2016-March 2018. Of the 88 patients who actually took the drug, 83% had Parkinson’s, and 78% were men. Participants’ mean age was 79 years. Nearly a third had deep brain stimulation.
About two-thirds of the patients started taking pimavanserin after failing prior antipsychotic therapy, whereas the other third had not previously taken an antipsychotic. Quetiapine had been used by 91% of previous antipsychotic users, and another 20% had taken clozapine. Other antipsychotics were less commonly used.
There was no consistent strategy implemented for starting pimavanserin: 17 stopped or were advised to stop their other antipsychotic before starting pimavanserin, 15 were told to taper off their other antipsychotic for 1 week or for up to 3 months, and 22 were told to keep taking their other antipsychotic after starting pimavanserin.
The mean treatment duration has been nearly 11 months for the two-thirds of patients who remain on pimavanserin, including 38 who take it alone and another 20 who take it with another antipsychotic.
A total of 10 patients were unable to tolerate pimavanserin because of adverse events, 5 of whom had generalized weakness/gait instability. This adverse event was the only difference in adverse events that was seen from the clinical trials, said lead author Jessie Sellers, a nurse practitioner at Vanderbilt University Medical Center in Nashville, Tenn.
“But overall the drug was really well tolerated and was effective,” she said.
There also was no increase in mortality detected in users, providing evidence against an association with mortality in older people with dementia-related psychosis that previously led to a boxed warning for atypical antipsychotics, noted senior author Daniel O. Claassen, MD. A total of 6 of the 88 patients died, compared with 5 of the 14 patients who never started the drug.
Patients who stopped pimavanserin and started another antipsychotic had only limited success. Of 11 patients who stopped pimavanserin and started another antipsychotic, only 5 were successful. Another six who stopped pimavanserin did not take another antipsychotic drug, primarily because of the resolution of their symptoms.
The pimavanserin status was unknown for four patients, and another three patients who stopped the drug had not returned for follow-up.
Abhimanyu Mahajan, MD, and other researchers from Henry Ford Hospital in Detroit reported similar results with pimavanserin at the AAN annual meeting in a separate, smaller, retrospective chart review of 16 patients with Parkinson’s disease–associated psychosis and 1 with Lewy body dementia.
These patients had a mean duration of parkinsonism of nearly 12 years and more than 2 years of psychotic symptoms, which consisted of daily or continuous hallucinations in all but one patient.
Telephone interviews with patients and caregivers revealed that 10 of 14 had improvement in hallucinations, and 3 had stopped taking it because of either no benefit or remission. Of six patients who took pimavanserin monotherapy, half improved from severe to mild hallucination severity (less than one episode per week), two had no change, and one improved from severe to moderate. For the other eight patients who took pimavanserin with another antipsychotic, two had no change in hallucination severity, two went from severe to mild, three improved from severe to moderate, and one went from moderate to mild. The patients reported no major adverse events.
Ms. Sellers and another author had no disclosures. Dr. Claassen disclosed personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with several companies, including Acadia, which markets pimavanserin.
Two of the authors of Dr. Mahajan’s study reported financial ties to Acadia and other companies.
SOURCE: Sellers J et al. AAN 2018, abstract P1.040 and Mahajan A et al. AAN 2018, abstract P5.065
LOS ANGELES – Two studies of real-world use of pimavanserin after its approval in March 2016 for the treatment of Parkinson’s disease–associated psychosis indicate its effectiveness, tolerability, and safety in line with clinical trial results, according to reports presented at the annual meeting of the American Academy of Neurology.
In the first study, 102 patients were prescribed pimavanserin (Nuplazid) during May 2016-March 2018. Of the 88 patients who actually took the drug, 83% had Parkinson’s, and 78% were men. Participants’ mean age was 79 years. Nearly a third had deep brain stimulation.
About two-thirds of the patients started taking pimavanserin after failing prior antipsychotic therapy, whereas the other third had not previously taken an antipsychotic. Quetiapine had been used by 91% of previous antipsychotic users, and another 20% had taken clozapine. Other antipsychotics were less commonly used.
There was no consistent strategy implemented for starting pimavanserin: 17 stopped or were advised to stop their other antipsychotic before starting pimavanserin, 15 were told to taper off their other antipsychotic for 1 week or for up to 3 months, and 22 were told to keep taking their other antipsychotic after starting pimavanserin.
The mean treatment duration has been nearly 11 months for the two-thirds of patients who remain on pimavanserin, including 38 who take it alone and another 20 who take it with another antipsychotic.
A total of 10 patients were unable to tolerate pimavanserin because of adverse events, 5 of whom had generalized weakness/gait instability. This adverse event was the only difference in adverse events that was seen from the clinical trials, said lead author Jessie Sellers, a nurse practitioner at Vanderbilt University Medical Center in Nashville, Tenn.
“But overall the drug was really well tolerated and was effective,” she said.
There also was no increase in mortality detected in users, providing evidence against an association with mortality in older people with dementia-related psychosis that previously led to a boxed warning for atypical antipsychotics, noted senior author Daniel O. Claassen, MD. A total of 6 of the 88 patients died, compared with 5 of the 14 patients who never started the drug.
Patients who stopped pimavanserin and started another antipsychotic had only limited success. Of 11 patients who stopped pimavanserin and started another antipsychotic, only 5 were successful. Another six who stopped pimavanserin did not take another antipsychotic drug, primarily because of the resolution of their symptoms.
The pimavanserin status was unknown for four patients, and another three patients who stopped the drug had not returned for follow-up.
Abhimanyu Mahajan, MD, and other researchers from Henry Ford Hospital in Detroit reported similar results with pimavanserin at the AAN annual meeting in a separate, smaller, retrospective chart review of 16 patients with Parkinson’s disease–associated psychosis and 1 with Lewy body dementia.
These patients had a mean duration of parkinsonism of nearly 12 years and more than 2 years of psychotic symptoms, which consisted of daily or continuous hallucinations in all but one patient.
Telephone interviews with patients and caregivers revealed that 10 of 14 had improvement in hallucinations, and 3 had stopped taking it because of either no benefit or remission. Of six patients who took pimavanserin monotherapy, half improved from severe to mild hallucination severity (less than one episode per week), two had no change, and one improved from severe to moderate. For the other eight patients who took pimavanserin with another antipsychotic, two had no change in hallucination severity, two went from severe to mild, three improved from severe to moderate, and one went from moderate to mild. The patients reported no major adverse events.
Ms. Sellers and another author had no disclosures. Dr. Claassen disclosed personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with several companies, including Acadia, which markets pimavanserin.
Two of the authors of Dr. Mahajan’s study reported financial ties to Acadia and other companies.
SOURCE: Sellers J et al. AAN 2018, abstract P1.040 and Mahajan A et al. AAN 2018, abstract P5.065
LOS ANGELES – Two studies of real-world use of pimavanserin after its approval in March 2016 for the treatment of Parkinson’s disease–associated psychosis indicate its effectiveness, tolerability, and safety in line with clinical trial results, according to reports presented at the annual meeting of the American Academy of Neurology.
In the first study, 102 patients were prescribed pimavanserin (Nuplazid) during May 2016-March 2018. Of the 88 patients who actually took the drug, 83% had Parkinson’s, and 78% were men. Participants’ mean age was 79 years. Nearly a third had deep brain stimulation.
About two-thirds of the patients started taking pimavanserin after failing prior antipsychotic therapy, whereas the other third had not previously taken an antipsychotic. Quetiapine had been used by 91% of previous antipsychotic users, and another 20% had taken clozapine. Other antipsychotics were less commonly used.
There was no consistent strategy implemented for starting pimavanserin: 17 stopped or were advised to stop their other antipsychotic before starting pimavanserin, 15 were told to taper off their other antipsychotic for 1 week or for up to 3 months, and 22 were told to keep taking their other antipsychotic after starting pimavanserin.
The mean treatment duration has been nearly 11 months for the two-thirds of patients who remain on pimavanserin, including 38 who take it alone and another 20 who take it with another antipsychotic.
A total of 10 patients were unable to tolerate pimavanserin because of adverse events, 5 of whom had generalized weakness/gait instability. This adverse event was the only difference in adverse events that was seen from the clinical trials, said lead author Jessie Sellers, a nurse practitioner at Vanderbilt University Medical Center in Nashville, Tenn.
“But overall the drug was really well tolerated and was effective,” she said.
There also was no increase in mortality detected in users, providing evidence against an association with mortality in older people with dementia-related psychosis that previously led to a boxed warning for atypical antipsychotics, noted senior author Daniel O. Claassen, MD. A total of 6 of the 88 patients died, compared with 5 of the 14 patients who never started the drug.
Patients who stopped pimavanserin and started another antipsychotic had only limited success. Of 11 patients who stopped pimavanserin and started another antipsychotic, only 5 were successful. Another six who stopped pimavanserin did not take another antipsychotic drug, primarily because of the resolution of their symptoms.
The pimavanserin status was unknown for four patients, and another three patients who stopped the drug had not returned for follow-up.
Abhimanyu Mahajan, MD, and other researchers from Henry Ford Hospital in Detroit reported similar results with pimavanserin at the AAN annual meeting in a separate, smaller, retrospective chart review of 16 patients with Parkinson’s disease–associated psychosis and 1 with Lewy body dementia.
These patients had a mean duration of parkinsonism of nearly 12 years and more than 2 years of psychotic symptoms, which consisted of daily or continuous hallucinations in all but one patient.
Telephone interviews with patients and caregivers revealed that 10 of 14 had improvement in hallucinations, and 3 had stopped taking it because of either no benefit or remission. Of six patients who took pimavanserin monotherapy, half improved from severe to mild hallucination severity (less than one episode per week), two had no change, and one improved from severe to moderate. For the other eight patients who took pimavanserin with another antipsychotic, two had no change in hallucination severity, two went from severe to mild, three improved from severe to moderate, and one went from moderate to mild. The patients reported no major adverse events.
Ms. Sellers and another author had no disclosures. Dr. Claassen disclosed personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with several companies, including Acadia, which markets pimavanserin.
Two of the authors of Dr. Mahajan’s study reported financial ties to Acadia and other companies.
SOURCE: Sellers J et al. AAN 2018, abstract P1.040 and Mahajan A et al. AAN 2018, abstract P5.065
REPORTING FROM AAN 2018
Key clinical point:
Major finding: Psychotic symptoms improved in 71%-88% of patients taking pimavanserin.
Study details: Two retrospective chart reviews totaling 105 patients who took pimavanserin.
Disclosures: One or more authors in each study reported financial ties to Acadia, which markets pimavanserin.
Source: Sellers J et al. AAN 2018, abstract P1.040, and Mahajan A et al. AAN 2018, abstract P5.065.
Blood-brain barrier health may signal early loss of MS treatment response
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) of blood-brain barrier permeability in patients with relapsing-remitting multiple sclerosis may serve as an early marker of suboptimal treatment response to natalizumab or fingolimod, Danish researchers reported in a prospective, observational study.
The imaging method, when applied at baseline and at 3 months and 6 months after starting treatment with either drug in 35 patients, yielded a predictive threshold for determining if patients at 2 years of treatment would fail to meet criteria used for no evidence of disease activity (NEDA). The method is believed to work for natalizumab (Tysabri) and fingolimod (Gilenya) by measuring their effect on the passage of lymphocytes through the blood-brain barrier (BBB) because even though the two drugs have different mechanisms of action, “their final effect is the same, i.e., a reduction of the absolute number of lymphocytes trafficking across the BBB,” wrote Stig P. Cramer, MD, PhD, of the Rigshospitalet, Copenhagen, and his colleagues. The report was published in Annals of Neurology.
The investigators used three disease activity domains to define NEDA status: no new neurologic symptoms or signs that lasted more than 24 hours in the absence of concurrent fever or illness; no sustained Expanded Disability Status Scale score increase of one or more points for 6 or more months; and no new T2 hyperintense lesions and T1 gadolinium-enhancing lesions. They disregarded disease activity occurring within the first 3 months after initiation of natalizumab or fingolimod when assessing NEDA status to allow for development of a full treatment effect.
“In summary, we find that a single DCE-MRI at 6 months after initiation of natalizumab or fingolimod treatment provides information on the state of health of the BBB that enables reliable stratification of treatment response. Thus, DCE-MRI can enable early detection of long-term suboptimal treatment response in [relapsing-remitting multiple sclerosis], and a personalized medicine approach to treatment, limitations being the long scan time (15 minutes). These results and the proposed thresholds require validation in larger studies,” the researchers said.
The research was supported by grants from the Research Foundation of the Capital Region of Denmark, the Foundation for Health Research, the Danish Council for Independent Research, Rigshospitalets forskningspuljer, the Danish Multiple Sclerosis Society, and Biogen. Several authors reported financial relationships with Biogen, which sells natalizumab. But the company had no influence on study setup, subject inclusion, data analysis, interpretation of results, or publishing decisions, and any intellectual rights belong to the authors alone, they said.
SOURCE: Cramer S et al. Ann Neurol. 2018 Mar 31. doi: 10.1002/ana.25219.
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) of blood-brain barrier permeability in patients with relapsing-remitting multiple sclerosis may serve as an early marker of suboptimal treatment response to natalizumab or fingolimod, Danish researchers reported in a prospective, observational study.
The imaging method, when applied at baseline and at 3 months and 6 months after starting treatment with either drug in 35 patients, yielded a predictive threshold for determining if patients at 2 years of treatment would fail to meet criteria used for no evidence of disease activity (NEDA). The method is believed to work for natalizumab (Tysabri) and fingolimod (Gilenya) by measuring their effect on the passage of lymphocytes through the blood-brain barrier (BBB) because even though the two drugs have different mechanisms of action, “their final effect is the same, i.e., a reduction of the absolute number of lymphocytes trafficking across the BBB,” wrote Stig P. Cramer, MD, PhD, of the Rigshospitalet, Copenhagen, and his colleagues. The report was published in Annals of Neurology.
The investigators used three disease activity domains to define NEDA status: no new neurologic symptoms or signs that lasted more than 24 hours in the absence of concurrent fever or illness; no sustained Expanded Disability Status Scale score increase of one or more points for 6 or more months; and no new T2 hyperintense lesions and T1 gadolinium-enhancing lesions. They disregarded disease activity occurring within the first 3 months after initiation of natalizumab or fingolimod when assessing NEDA status to allow for development of a full treatment effect.
“In summary, we find that a single DCE-MRI at 6 months after initiation of natalizumab or fingolimod treatment provides information on the state of health of the BBB that enables reliable stratification of treatment response. Thus, DCE-MRI can enable early detection of long-term suboptimal treatment response in [relapsing-remitting multiple sclerosis], and a personalized medicine approach to treatment, limitations being the long scan time (15 minutes). These results and the proposed thresholds require validation in larger studies,” the researchers said.
The research was supported by grants from the Research Foundation of the Capital Region of Denmark, the Foundation for Health Research, the Danish Council for Independent Research, Rigshospitalets forskningspuljer, the Danish Multiple Sclerosis Society, and Biogen. Several authors reported financial relationships with Biogen, which sells natalizumab. But the company had no influence on study setup, subject inclusion, data analysis, interpretation of results, or publishing decisions, and any intellectual rights belong to the authors alone, they said.
SOURCE: Cramer S et al. Ann Neurol. 2018 Mar 31. doi: 10.1002/ana.25219.
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) of blood-brain barrier permeability in patients with relapsing-remitting multiple sclerosis may serve as an early marker of suboptimal treatment response to natalizumab or fingolimod, Danish researchers reported in a prospective, observational study.
The imaging method, when applied at baseline and at 3 months and 6 months after starting treatment with either drug in 35 patients, yielded a predictive threshold for determining if patients at 2 years of treatment would fail to meet criteria used for no evidence of disease activity (NEDA). The method is believed to work for natalizumab (Tysabri) and fingolimod (Gilenya) by measuring their effect on the passage of lymphocytes through the blood-brain barrier (BBB) because even though the two drugs have different mechanisms of action, “their final effect is the same, i.e., a reduction of the absolute number of lymphocytes trafficking across the BBB,” wrote Stig P. Cramer, MD, PhD, of the Rigshospitalet, Copenhagen, and his colleagues. The report was published in Annals of Neurology.
The investigators used three disease activity domains to define NEDA status: no new neurologic symptoms or signs that lasted more than 24 hours in the absence of concurrent fever or illness; no sustained Expanded Disability Status Scale score increase of one or more points for 6 or more months; and no new T2 hyperintense lesions and T1 gadolinium-enhancing lesions. They disregarded disease activity occurring within the first 3 months after initiation of natalizumab or fingolimod when assessing NEDA status to allow for development of a full treatment effect.
“In summary, we find that a single DCE-MRI at 6 months after initiation of natalizumab or fingolimod treatment provides information on the state of health of the BBB that enables reliable stratification of treatment response. Thus, DCE-MRI can enable early detection of long-term suboptimal treatment response in [relapsing-remitting multiple sclerosis], and a personalized medicine approach to treatment, limitations being the long scan time (15 minutes). These results and the proposed thresholds require validation in larger studies,” the researchers said.
The research was supported by grants from the Research Foundation of the Capital Region of Denmark, the Foundation for Health Research, the Danish Council for Independent Research, Rigshospitalets forskningspuljer, the Danish Multiple Sclerosis Society, and Biogen. Several authors reported financial relationships with Biogen, which sells natalizumab. But the company had no influence on study setup, subject inclusion, data analysis, interpretation of results, or publishing decisions, and any intellectual rights belong to the authors alone, they said.
SOURCE: Cramer S et al. Ann Neurol. 2018 Mar 31. doi: 10.1002/ana.25219.
FROM ANNALS OF NEUROLOGY
Key clinical point:
Major finding: A DCE-MRI measure of blood-brain permeability 6 months after starting natalizumab or fingolimod could predict the loss of NEDA at 2 years with an area under the curve value of 0.84.
Study details: A prospective, observational study of 35 patients with relapsing-remitting MS who started taking natalizumab or fingolimod.
Disclosures: The research was supported by grants from the Research Foundation of the Capital Region of Denmark, the Foundation for Health Research, the Danish Council for Independent Research, Rigshospitalets forskningspuljer, the Danish Multiple Sclerosis Society, and Biogen. Several authors reported financial relationships with Biogen, which sells natalizumab. Biogen had no influence on study setup, subject inclusion, data analysis, interpretation of results, or publishing decisions, and any intellectual rights belong to the authors alone, they said.
Source: Cramer S et al. Ann Neurol. 2018 Mar 31. doi: 10.1002/ana.25219.
Erenumab found beneficial to migraine patients with unsuccessful preventive treatment history
Results from the phase 3b LIBERTY trial of the investigational migraine-prevention drug erenumab indicate its potential effectiveness in patients with episodic migraine attacks who have unsuccessfully tried other migraine-prevention drugs to reduce the frequency of migraine days.
Erenumab, a fully human monoclonal antibody that is designed to block the calcitonin gene-related peptide (CGRP) receptor, reduced the average number of monthly migraine headaches by half or more for 30% of study participants, which is a level of improvement that “can greatly improve a person’s quality of life,” first author Uwe Reuter, MD, of Charité–University Medicine Berlin said in a press release. Dr. Reuter will present the full results of the study during the Emerging Science Platform Session at the annual meeting of the American Academy of Neurology in Los Angeles on April 24.
At 3 months, patients treated with erenumab significantly more often met the study’s primary endpoint of the proportion of patients achieving a 50% or greater reduction in mean monthly migraine days (MMDs) during weeks 9-12: At week 12, 30% on erenumab vs. 14% on placebo (odds ratio, 2.73; 95% confidence interval, 1.43-5.19) met the endpoint.
Those treated with erenumab also had a greater average reduction in MMDs in several secondary endpoints. For those on erenumab, there was an overall mean difference of –1.61 MMDs, compared with placebo. Erenumab-treated patients also had an overall mean difference of –1.73 acute medication days, compared with placebo.
The authors reported that erenumab had safety and tolerability comparable to placebo, and none of the patients taking erenumab discontinued because of adverse events.
Dr. Reuter cautioned that additional studies will need to be conducted to determine if the effects last longer than 3 months and to identify patients most likely to respond.
The study was funded by Novartis, which is developing erenumab.
SOURCE: Reuter E et al. AAN 2018, Emerging Science Abstract 009.
Results from the phase 3b LIBERTY trial of the investigational migraine-prevention drug erenumab indicate its potential effectiveness in patients with episodic migraine attacks who have unsuccessfully tried other migraine-prevention drugs to reduce the frequency of migraine days.
Erenumab, a fully human monoclonal antibody that is designed to block the calcitonin gene-related peptide (CGRP) receptor, reduced the average number of monthly migraine headaches by half or more for 30% of study participants, which is a level of improvement that “can greatly improve a person’s quality of life,” first author Uwe Reuter, MD, of Charité–University Medicine Berlin said in a press release. Dr. Reuter will present the full results of the study during the Emerging Science Platform Session at the annual meeting of the American Academy of Neurology in Los Angeles on April 24.
At 3 months, patients treated with erenumab significantly more often met the study’s primary endpoint of the proportion of patients achieving a 50% or greater reduction in mean monthly migraine days (MMDs) during weeks 9-12: At week 12, 30% on erenumab vs. 14% on placebo (odds ratio, 2.73; 95% confidence interval, 1.43-5.19) met the endpoint.
Those treated with erenumab also had a greater average reduction in MMDs in several secondary endpoints. For those on erenumab, there was an overall mean difference of –1.61 MMDs, compared with placebo. Erenumab-treated patients also had an overall mean difference of –1.73 acute medication days, compared with placebo.
The authors reported that erenumab had safety and tolerability comparable to placebo, and none of the patients taking erenumab discontinued because of adverse events.
Dr. Reuter cautioned that additional studies will need to be conducted to determine if the effects last longer than 3 months and to identify patients most likely to respond.
The study was funded by Novartis, which is developing erenumab.
SOURCE: Reuter E et al. AAN 2018, Emerging Science Abstract 009.
Results from the phase 3b LIBERTY trial of the investigational migraine-prevention drug erenumab indicate its potential effectiveness in patients with episodic migraine attacks who have unsuccessfully tried other migraine-prevention drugs to reduce the frequency of migraine days.
Erenumab, a fully human monoclonal antibody that is designed to block the calcitonin gene-related peptide (CGRP) receptor, reduced the average number of monthly migraine headaches by half or more for 30% of study participants, which is a level of improvement that “can greatly improve a person’s quality of life,” first author Uwe Reuter, MD, of Charité–University Medicine Berlin said in a press release. Dr. Reuter will present the full results of the study during the Emerging Science Platform Session at the annual meeting of the American Academy of Neurology in Los Angeles on April 24.
At 3 months, patients treated with erenumab significantly more often met the study’s primary endpoint of the proportion of patients achieving a 50% or greater reduction in mean monthly migraine days (MMDs) during weeks 9-12: At week 12, 30% on erenumab vs. 14% on placebo (odds ratio, 2.73; 95% confidence interval, 1.43-5.19) met the endpoint.
Those treated with erenumab also had a greater average reduction in MMDs in several secondary endpoints. For those on erenumab, there was an overall mean difference of –1.61 MMDs, compared with placebo. Erenumab-treated patients also had an overall mean difference of –1.73 acute medication days, compared with placebo.
The authors reported that erenumab had safety and tolerability comparable to placebo, and none of the patients taking erenumab discontinued because of adverse events.
Dr. Reuter cautioned that additional studies will need to be conducted to determine if the effects last longer than 3 months and to identify patients most likely to respond.
The study was funded by Novartis, which is developing erenumab.
SOURCE: Reuter E et al. AAN 2018, Emerging Science Abstract 009.
FROM AAN 2018
Key clinical point:
Major finding: Patients treated with erenumab significantly more often achieved a 50% or greater reduction in mean monthly migraine days during weeks 9-12: At week 12, 30% with erenumab vs. 14% with placebo met the endpoint.
Study details: A 12-week, double-blind study of 246 patients with episodic migraine randomized to receive erenumab 140 mg or placebo.
Disclosures: The study was funded by Novartis, which is developing erenumab.
Source: Reuter E et al. AAN 2018, Emerging Science Abstract 009.
Top AAN picks from Clinical Neurology News’ medical editor
Standout presentations at this year’s American Academy of Neurology annual meeting range from targeting tau in Alzheimer’s disease, to new treatments for spinal muscular atrophy, to the controversial topic of allowing your child to play contact sports, but all are sure to have an impact, according to Clinical Neurology News Medical Editor Richard J. Caselli, MD.
“There are a lot of good talks and papers being presented, and it is impossible without having seen and heard them all to accurately predict what will be the real standouts, but from a purely personal perspective, and with all due apologies to any others not mentioned below, these are some of the ones I think could have large and, in some cases, almost immediate impact or potential impact,” said Dr. Caselli, professor of neurology at the Mayo Clinic Arizona in Scottsdale and also associate director and clinical core director of the Arizona Alzheimer’s Disease Center.
Targeting tau in Alzheimer’s
The measuring of plasma tau to detect preclinical Alzheimer’s, as is described in the abstract from Pase and colleagues, is an “intriguing” approach, Dr. Caselli said. In that study, higher plasma tau levels were observed across correlates of preclinical Alzheimer’s: poorer cognitive function and smaller hippocampal volumes on MRI. Plasma tau level was also a strong predictor of future dementia. It will be presented Friday, April 27, 1:00-3:00 in S48, “Novel Biomarkers in Aging and Dementia.”
Focus continues on SMA
More advancements continue to be made in the treatment of various forms of spinal muscular trophy. In Monday morning’s Presidential Plenary Session, Richard Finkel’s presentation in receipt of the Sidney Carter Award in Child Neurology, should chart the development, current state, and future of antisense oligonucleotide therapy for SMA.
In the Emerging Science poster program on Wednesday, April 25, attendees will get an update on trial results for a different approach to the treatment of SMA using AVXS-101 gene replacement therapy for SMA type 1. John W. Day, MD, PhD, will provide longer-term outcomes after last year’s presentation of results in 15 patients.
Big news in stroke
Gregory Albers, MD, will describe in the Clinical Trials Plenary Session how new evidence from stroke thrombectomy trials such as DEFUSE 3 have led to new recommendations for extending the time window for thrombectomy. The results of DEFUSE 3 were first reported in January at the International Stroke Conference.
Other plenary presentations
In Wednesday’s Frontiers in Neuroscience Session, Dr. Caselli recommended Alan Evans’ discussion of the development and current and upcoming work to use and update the giant, freely accessible “BigBrain” High Resolution 3D Digital Human Brain Atlas.
In the always “fun and interesting” Controversies in Neurology on the morning of Thursday, April 26, the debate on “Should We Use Biomarkers Alone For Diagnosis of Alzheimer’s?” takes on greater interest now that the National Institute on Aging and the Alzheimer’s Association have defined Alzheimer’s disease as a diagnosis based on biomarkers. The separate debate of “Would You Let Your Child Play Contact Sports?” should also bring lots of interesting questions to the forefront of attendees’ minds.
Dr. Steven R. Messé’s talk, “Finally, Some Closure on PFO Closure,” at the Neurology Year in Review on Friday morning, April 27, is “of immediate relevance” as recent clinical trials have begun to determine patient groups for whom PFO closure appears worthwhile, Dr. Caselli said.
He has no relevant disclosures.
Standout presentations at this year’s American Academy of Neurology annual meeting range from targeting tau in Alzheimer’s disease, to new treatments for spinal muscular atrophy, to the controversial topic of allowing your child to play contact sports, but all are sure to have an impact, according to Clinical Neurology News Medical Editor Richard J. Caselli, MD.
“There are a lot of good talks and papers being presented, and it is impossible without having seen and heard them all to accurately predict what will be the real standouts, but from a purely personal perspective, and with all due apologies to any others not mentioned below, these are some of the ones I think could have large and, in some cases, almost immediate impact or potential impact,” said Dr. Caselli, professor of neurology at the Mayo Clinic Arizona in Scottsdale and also associate director and clinical core director of the Arizona Alzheimer’s Disease Center.
Targeting tau in Alzheimer’s
The measuring of plasma tau to detect preclinical Alzheimer’s, as is described in the abstract from Pase and colleagues, is an “intriguing” approach, Dr. Caselli said. In that study, higher plasma tau levels were observed across correlates of preclinical Alzheimer’s: poorer cognitive function and smaller hippocampal volumes on MRI. Plasma tau level was also a strong predictor of future dementia. It will be presented Friday, April 27, 1:00-3:00 in S48, “Novel Biomarkers in Aging and Dementia.”
Focus continues on SMA
More advancements continue to be made in the treatment of various forms of spinal muscular trophy. In Monday morning’s Presidential Plenary Session, Richard Finkel’s presentation in receipt of the Sidney Carter Award in Child Neurology, should chart the development, current state, and future of antisense oligonucleotide therapy for SMA.
In the Emerging Science poster program on Wednesday, April 25, attendees will get an update on trial results for a different approach to the treatment of SMA using AVXS-101 gene replacement therapy for SMA type 1. John W. Day, MD, PhD, will provide longer-term outcomes after last year’s presentation of results in 15 patients.
Big news in stroke
Gregory Albers, MD, will describe in the Clinical Trials Plenary Session how new evidence from stroke thrombectomy trials such as DEFUSE 3 have led to new recommendations for extending the time window for thrombectomy. The results of DEFUSE 3 were first reported in January at the International Stroke Conference.
Other plenary presentations
In Wednesday’s Frontiers in Neuroscience Session, Dr. Caselli recommended Alan Evans’ discussion of the development and current and upcoming work to use and update the giant, freely accessible “BigBrain” High Resolution 3D Digital Human Brain Atlas.
In the always “fun and interesting” Controversies in Neurology on the morning of Thursday, April 26, the debate on “Should We Use Biomarkers Alone For Diagnosis of Alzheimer’s?” takes on greater interest now that the National Institute on Aging and the Alzheimer’s Association have defined Alzheimer’s disease as a diagnosis based on biomarkers. The separate debate of “Would You Let Your Child Play Contact Sports?” should also bring lots of interesting questions to the forefront of attendees’ minds.
Dr. Steven R. Messé’s talk, “Finally, Some Closure on PFO Closure,” at the Neurology Year in Review on Friday morning, April 27, is “of immediate relevance” as recent clinical trials have begun to determine patient groups for whom PFO closure appears worthwhile, Dr. Caselli said.
He has no relevant disclosures.
Standout presentations at this year’s American Academy of Neurology annual meeting range from targeting tau in Alzheimer’s disease, to new treatments for spinal muscular atrophy, to the controversial topic of allowing your child to play contact sports, but all are sure to have an impact, according to Clinical Neurology News Medical Editor Richard J. Caselli, MD.
“There are a lot of good talks and papers being presented, and it is impossible without having seen and heard them all to accurately predict what will be the real standouts, but from a purely personal perspective, and with all due apologies to any others not mentioned below, these are some of the ones I think could have large and, in some cases, almost immediate impact or potential impact,” said Dr. Caselli, professor of neurology at the Mayo Clinic Arizona in Scottsdale and also associate director and clinical core director of the Arizona Alzheimer’s Disease Center.
Targeting tau in Alzheimer’s
The measuring of plasma tau to detect preclinical Alzheimer’s, as is described in the abstract from Pase and colleagues, is an “intriguing” approach, Dr. Caselli said. In that study, higher plasma tau levels were observed across correlates of preclinical Alzheimer’s: poorer cognitive function and smaller hippocampal volumes on MRI. Plasma tau level was also a strong predictor of future dementia. It will be presented Friday, April 27, 1:00-3:00 in S48, “Novel Biomarkers in Aging and Dementia.”
Focus continues on SMA
More advancements continue to be made in the treatment of various forms of spinal muscular trophy. In Monday morning’s Presidential Plenary Session, Richard Finkel’s presentation in receipt of the Sidney Carter Award in Child Neurology, should chart the development, current state, and future of antisense oligonucleotide therapy for SMA.
In the Emerging Science poster program on Wednesday, April 25, attendees will get an update on trial results for a different approach to the treatment of SMA using AVXS-101 gene replacement therapy for SMA type 1. John W. Day, MD, PhD, will provide longer-term outcomes after last year’s presentation of results in 15 patients.
Big news in stroke
Gregory Albers, MD, will describe in the Clinical Trials Plenary Session how new evidence from stroke thrombectomy trials such as DEFUSE 3 have led to new recommendations for extending the time window for thrombectomy. The results of DEFUSE 3 were first reported in January at the International Stroke Conference.
Other plenary presentations
In Wednesday’s Frontiers in Neuroscience Session, Dr. Caselli recommended Alan Evans’ discussion of the development and current and upcoming work to use and update the giant, freely accessible “BigBrain” High Resolution 3D Digital Human Brain Atlas.
In the always “fun and interesting” Controversies in Neurology on the morning of Thursday, April 26, the debate on “Should We Use Biomarkers Alone For Diagnosis of Alzheimer’s?” takes on greater interest now that the National Institute on Aging and the Alzheimer’s Association have defined Alzheimer’s disease as a diagnosis based on biomarkers. The separate debate of “Would You Let Your Child Play Contact Sports?” should also bring lots of interesting questions to the forefront of attendees’ minds.
Dr. Steven R. Messé’s talk, “Finally, Some Closure on PFO Closure,” at the Neurology Year in Review on Friday morning, April 27, is “of immediate relevance” as recent clinical trials have begun to determine patient groups for whom PFO closure appears worthwhile, Dr. Caselli said.
He has no relevant disclosures.
Gait freezing relieved by spinal cord, transcranial direct-current stimulation
The application of transcranial direct-current stimulation and spinal cord stimulation alleviated freezing of gait in two separate studies of patients with idiopathic Parkinson’s disease published online in Movement Disorders.
In the first study, Moria Dagan of Tel Aviv University and her colleagues reported that transcranial direct-current stimulation (tDCS) applied simultaneously to the primary motor cortex (M1) and the left dorsolateral prefrontal cortex (DLPFC) improved freezing of gait (FOG) more than M1 stimulation alone or sham stimulation in a double-blind, randomized trial of 20 patients. The second study, reported by Olivia Samotus and her associates at the London (Ont.) Health Sciences Centre, found that open-label, midthoracic epidural spinal cord stimulation (SCS) in five patients produced significantly reduced FOG episodes and also provided sustained improvement in gait measurements.
The tDCS study used a crossover design to show that, over the course of four separate, 20-minute tDCS sessions, FOG-provoking test scores improved in 15 of 17 patients who received multitarget stimulation and were significantly improved on average, whereas the scores of patients who had M1 stimulation alone or sham stimulation did not improve. Improvement in FOG severity nearly reached statistical significance when compared with M1 only and sham stimulation (P = .06). Three patients were unable to complete the FOG-provoking test and were excluded from the analysis.
The investigators tested simultaneous tDCS of the M1 and left DLPFC because a previous study showed improvement in FOG after M1 stimulation, and other evidence suggests that FOG might also arise through deficits in executive function mediated by the DLPFC. Previous research in other patient populations has also shown that tDCS improves cognition, gait, and postural control, and a prior study using transcranial magnetic stimulation of the M1 and left DLPFC improved gait and cognition in patients with FOG.
“We suggest that the results move this emerging approach a key step forward, as they support the idea that the cognitive executive circuit plays a role in FOG and the possibility that multitarget stimulation may have value as an intervention for ameliorating FOG,” Ms. Dagan and her colleagues wrote.
In a separate, nonrandomized pilot trial, SCS produced improvements in stride velocity, step length, and single- and double-support phases in follow-up visits out to 6 months post surgery, in addition to significantly decreasing FOG episodes. The study is the first to use objective gait technology to assess the efficacy of SCS for gait in advanced Parkinson’s disease patients. Ms. Samotus and her associates observed these changes after testing a range of pulse width and frequency combinations in each of the five participants to determine which settings best improved gait for each participant. Overall, the results suggest that pulse widths of “300-400 microseconds and frequencies of 30-130 Hz may be safe and possibly effective for reducing FOG frequency and improving gait dysfunction in advanced Parkinson’s disease patients.”
The tDCS trial was supported by the Michael J. Fox Foundation for Parkinson’s Research. One investigator disclosed that he is cofounder and shareholder of Neuroelectrics, which makes brain stimulation technologies such as the ones used in the study. No outside funding was reported for the SCS study. One investigator in the SCS study reported ties to pharmaceutical companies and device manufacturers.
SOURCES: Dagan M et al. Mov Disord. 2018 Feb 13. doi: 10.1002/mds.27300; Samotus O et al. Mov Disord. 2018 Feb 14. doi: 10.1002/mds.27299.
The application of transcranial direct-current stimulation and spinal cord stimulation alleviated freezing of gait in two separate studies of patients with idiopathic Parkinson’s disease published online in Movement Disorders.
In the first study, Moria Dagan of Tel Aviv University and her colleagues reported that transcranial direct-current stimulation (tDCS) applied simultaneously to the primary motor cortex (M1) and the left dorsolateral prefrontal cortex (DLPFC) improved freezing of gait (FOG) more than M1 stimulation alone or sham stimulation in a double-blind, randomized trial of 20 patients. The second study, reported by Olivia Samotus and her associates at the London (Ont.) Health Sciences Centre, found that open-label, midthoracic epidural spinal cord stimulation (SCS) in five patients produced significantly reduced FOG episodes and also provided sustained improvement in gait measurements.
The tDCS study used a crossover design to show that, over the course of four separate, 20-minute tDCS sessions, FOG-provoking test scores improved in 15 of 17 patients who received multitarget stimulation and were significantly improved on average, whereas the scores of patients who had M1 stimulation alone or sham stimulation did not improve. Improvement in FOG severity nearly reached statistical significance when compared with M1 only and sham stimulation (P = .06). Three patients were unable to complete the FOG-provoking test and were excluded from the analysis.
The investigators tested simultaneous tDCS of the M1 and left DLPFC because a previous study showed improvement in FOG after M1 stimulation, and other evidence suggests that FOG might also arise through deficits in executive function mediated by the DLPFC. Previous research in other patient populations has also shown that tDCS improves cognition, gait, and postural control, and a prior study using transcranial magnetic stimulation of the M1 and left DLPFC improved gait and cognition in patients with FOG.
“We suggest that the results move this emerging approach a key step forward, as they support the idea that the cognitive executive circuit plays a role in FOG and the possibility that multitarget stimulation may have value as an intervention for ameliorating FOG,” Ms. Dagan and her colleagues wrote.
In a separate, nonrandomized pilot trial, SCS produced improvements in stride velocity, step length, and single- and double-support phases in follow-up visits out to 6 months post surgery, in addition to significantly decreasing FOG episodes. The study is the first to use objective gait technology to assess the efficacy of SCS for gait in advanced Parkinson’s disease patients. Ms. Samotus and her associates observed these changes after testing a range of pulse width and frequency combinations in each of the five participants to determine which settings best improved gait for each participant. Overall, the results suggest that pulse widths of “300-400 microseconds and frequencies of 30-130 Hz may be safe and possibly effective for reducing FOG frequency and improving gait dysfunction in advanced Parkinson’s disease patients.”
The tDCS trial was supported by the Michael J. Fox Foundation for Parkinson’s Research. One investigator disclosed that he is cofounder and shareholder of Neuroelectrics, which makes brain stimulation technologies such as the ones used in the study. No outside funding was reported for the SCS study. One investigator in the SCS study reported ties to pharmaceutical companies and device manufacturers.
SOURCES: Dagan M et al. Mov Disord. 2018 Feb 13. doi: 10.1002/mds.27300; Samotus O et al. Mov Disord. 2018 Feb 14. doi: 10.1002/mds.27299.
The application of transcranial direct-current stimulation and spinal cord stimulation alleviated freezing of gait in two separate studies of patients with idiopathic Parkinson’s disease published online in Movement Disorders.
In the first study, Moria Dagan of Tel Aviv University and her colleagues reported that transcranial direct-current stimulation (tDCS) applied simultaneously to the primary motor cortex (M1) and the left dorsolateral prefrontal cortex (DLPFC) improved freezing of gait (FOG) more than M1 stimulation alone or sham stimulation in a double-blind, randomized trial of 20 patients. The second study, reported by Olivia Samotus and her associates at the London (Ont.) Health Sciences Centre, found that open-label, midthoracic epidural spinal cord stimulation (SCS) in five patients produced significantly reduced FOG episodes and also provided sustained improvement in gait measurements.
The tDCS study used a crossover design to show that, over the course of four separate, 20-minute tDCS sessions, FOG-provoking test scores improved in 15 of 17 patients who received multitarget stimulation and were significantly improved on average, whereas the scores of patients who had M1 stimulation alone or sham stimulation did not improve. Improvement in FOG severity nearly reached statistical significance when compared with M1 only and sham stimulation (P = .06). Three patients were unable to complete the FOG-provoking test and were excluded from the analysis.
The investigators tested simultaneous tDCS of the M1 and left DLPFC because a previous study showed improvement in FOG after M1 stimulation, and other evidence suggests that FOG might also arise through deficits in executive function mediated by the DLPFC. Previous research in other patient populations has also shown that tDCS improves cognition, gait, and postural control, and a prior study using transcranial magnetic stimulation of the M1 and left DLPFC improved gait and cognition in patients with FOG.
“We suggest that the results move this emerging approach a key step forward, as they support the idea that the cognitive executive circuit plays a role in FOG and the possibility that multitarget stimulation may have value as an intervention for ameliorating FOG,” Ms. Dagan and her colleagues wrote.
In a separate, nonrandomized pilot trial, SCS produced improvements in stride velocity, step length, and single- and double-support phases in follow-up visits out to 6 months post surgery, in addition to significantly decreasing FOG episodes. The study is the first to use objective gait technology to assess the efficacy of SCS for gait in advanced Parkinson’s disease patients. Ms. Samotus and her associates observed these changes after testing a range of pulse width and frequency combinations in each of the five participants to determine which settings best improved gait for each participant. Overall, the results suggest that pulse widths of “300-400 microseconds and frequencies of 30-130 Hz may be safe and possibly effective for reducing FOG frequency and improving gait dysfunction in advanced Parkinson’s disease patients.”
The tDCS trial was supported by the Michael J. Fox Foundation for Parkinson’s Research. One investigator disclosed that he is cofounder and shareholder of Neuroelectrics, which makes brain stimulation technologies such as the ones used in the study. No outside funding was reported for the SCS study. One investigator in the SCS study reported ties to pharmaceutical companies and device manufacturers.
SOURCES: Dagan M et al. Mov Disord. 2018 Feb 13. doi: 10.1002/mds.27300; Samotus O et al. Mov Disord. 2018 Feb 14. doi: 10.1002/mds.27299.
FROM MOVEMENT DISORDERS
Key clinical point:
Major finding: Freezing of gait–provoking test scores improved in 15 of 17 patients who received simultaneous tDCS to the primary motor cortex and the left dorsolateral prefrontal cortex.
Study details: A double-blind, randomized trial of tDCS in 20 Parkinson’s disease patients and a nonrandomized, open-label study of spinal cord stimulation in 5 Parkinson’s patients.
Disclosures: The tDCS trial was supported by the Michael J. Fox Foundation for Parkinson’s Research. One investigator disclosed that he is cofounder and shareholder of Neuroelectrics, which makes brain stimulation technologies such as the ones used in the study. No outside funding was reported for the SCS study. One investigator in the SCS study reported ties to pharmaceutical companies and device manufacturers.
Sources: Dagan M et al. Mov Disord. 2018 Feb 13. doi: 10.1002/mds.27300; Samotus O et al. Mov Disord. 2018 Feb 14. doi: 10.1002/mds.27299.
‘Real-world’ study finds treat-to-target benefits out to 5 years
A treat-to-target (T2T) strategy in daily clinical practice for patients with early rheumatoid arthritis proved successful in maintaining good disease- and patient-related outcomes over a 5-year period at two rheumatology clinics in the Netherlands.
The observational study builds on previous research on the long-term results of continuous application of T2T strategies in rheumatoid arthritis, for which there have been few published studies. “Long-term data from more recent randomized controlled clinical trials, using a T2T approach and biologicals, have shown good clinical outcomes. However, the generalizability of these results is hampered by the selection of specific patient groups in clinical trials and strict exclusion criteria. Patients seen in real-life practice may differ substantially from those in randomized clinical trials,” first author Letty G.A. Versteeg of Medisch Spectrum Twente, Enschede, the Netherlands, and her colleagues wrote in Clinical Rheumatology.
The investigators examined outcomes for 229 patients with very early RA who enrolled in the Dutch Rheumatoid Arthritis Monitoring (DREAM) remission induction cohort during 2006-2009, which included 5 years of follow-up for 171 of the patients. These patients underwent a protocoled T2T strategy aimed at remission, defined as a 28-joint Disease Activity Score (DAS28) of less than 2.6.
“In previous publications on the [DREAM] remission induction cohort, successful implementation of T2T in daily clinical practice was demonstrated. Achieving remission within the first year of treatment was shown to be a realistic goal for an important proportion of patients,” the authors wrote.
All patients started methotrexate monotherapy at an initial dosage of 15 mg/week that could be increased to a maximum dosage of 25 mg/week in week 8. Patients took folic acid on the second day after methotrexate. By week 12, those with persistent disease activity added sulfasalazine, starting at 2,000 mg/day and increasing if necessary to a maximum of 3,000 mg/day at week 20. Patients whose DAS28 remained at 3.2 or greater at week 24 received a tumor necrosis factor inhibitor. Those who reached remission had no change in medication, and when remission lasted for at least 6 months, medication was gradually tapered and eventually discontinued. Patients who had flares in which disease activity increased to a DAS28 of 2.6 and higher restarted their last effective medication or dosage, which could subsequently be intensified if necessary. Patients with comorbidities and contraindications for medication were not excluded because deviations from the protocol were allowed. The protocol also allowed concomitant treatment with NSAIDs, prednisolone at a dosage of less than 10 mg/day, and intra-articular corticosteroid injections.
The rate of DAS28-defined remission rose to 63% (126 of 199 patients) by the end of the first year, and only 5% had high disease activity at 24 weeks. The rate of remission remained stable over the next 4 years. This rate of remission was reflected as a drop from an overall mean DAS28 of 4.93 at baseline to 2.49 at 5 years. The majority of the drop in DAS28 occurred during the first 3 months (–1.63 points), and by the end of the first year of treatment, mean disease activity stayed below 2.6 on the DAS28.
The investigators saw a sustained remission at least once in 144 of the 171 patients with 5-year outcome data available, including sustained remission for 1 year or longer in 115. Median time to the first sustained remission proved to be 50 weeks, and half had this last less than 97 weeks and half more than 97 weeks.
During the 5-year follow-up, 17% of patients received treatment with biologics, with a median start of their first biologic at about 54 weeks after baseline. This first biologic was used continuously for a median of 29 weeks, and close to one-third of patients who started a biologic switched to a second biologic after a median duration of 41 weeks on the first. About two-thirds did not need a second biologic. A total of 66% of patients who took a biologic had at least one period of sustained remission.
Functional disability improved overall at 5 years as determined by Health Assessment Questionnaire (HAQ) scores that were available for 107 patients. HAQ scores decreased from a median of 1.125 at baseline to 0.375 after 24 weeks (P less than .001), where they remained stable throughout the rest of follow-up. Overall, nearly 70% of the patients with available 5-year data had a change in their individual HAQ score that was clinically meaningful from baseline to 24 weeks.
“Our study describes long-term outcome of implementation and continuous application of T2T to RA patients in daily clinical practice. The outcomes are similar to or even better than the results of T2T randomized clinical trials, in which strict selection of patients and controlled conditions were followed. These ‘real-life data’ are of important additional value in the evidence for the effectiveness of a T2T approach in RA patients,” the investigators concluded.
They had no disclosures to report.
SOURCE: Versteeg G et al. Clin Rheumatol. 2018 Feb 1. doi: 10.1007/s10067-017-3962-5.
A treat-to-target (T2T) strategy in daily clinical practice for patients with early rheumatoid arthritis proved successful in maintaining good disease- and patient-related outcomes over a 5-year period at two rheumatology clinics in the Netherlands.
The observational study builds on previous research on the long-term results of continuous application of T2T strategies in rheumatoid arthritis, for which there have been few published studies. “Long-term data from more recent randomized controlled clinical trials, using a T2T approach and biologicals, have shown good clinical outcomes. However, the generalizability of these results is hampered by the selection of specific patient groups in clinical trials and strict exclusion criteria. Patients seen in real-life practice may differ substantially from those in randomized clinical trials,” first author Letty G.A. Versteeg of Medisch Spectrum Twente, Enschede, the Netherlands, and her colleagues wrote in Clinical Rheumatology.
The investigators examined outcomes for 229 patients with very early RA who enrolled in the Dutch Rheumatoid Arthritis Monitoring (DREAM) remission induction cohort during 2006-2009, which included 5 years of follow-up for 171 of the patients. These patients underwent a protocoled T2T strategy aimed at remission, defined as a 28-joint Disease Activity Score (DAS28) of less than 2.6.
“In previous publications on the [DREAM] remission induction cohort, successful implementation of T2T in daily clinical practice was demonstrated. Achieving remission within the first year of treatment was shown to be a realistic goal for an important proportion of patients,” the authors wrote.
All patients started methotrexate monotherapy at an initial dosage of 15 mg/week that could be increased to a maximum dosage of 25 mg/week in week 8. Patients took folic acid on the second day after methotrexate. By week 12, those with persistent disease activity added sulfasalazine, starting at 2,000 mg/day and increasing if necessary to a maximum of 3,000 mg/day at week 20. Patients whose DAS28 remained at 3.2 or greater at week 24 received a tumor necrosis factor inhibitor. Those who reached remission had no change in medication, and when remission lasted for at least 6 months, medication was gradually tapered and eventually discontinued. Patients who had flares in which disease activity increased to a DAS28 of 2.6 and higher restarted their last effective medication or dosage, which could subsequently be intensified if necessary. Patients with comorbidities and contraindications for medication were not excluded because deviations from the protocol were allowed. The protocol also allowed concomitant treatment with NSAIDs, prednisolone at a dosage of less than 10 mg/day, and intra-articular corticosteroid injections.
The rate of DAS28-defined remission rose to 63% (126 of 199 patients) by the end of the first year, and only 5% had high disease activity at 24 weeks. The rate of remission remained stable over the next 4 years. This rate of remission was reflected as a drop from an overall mean DAS28 of 4.93 at baseline to 2.49 at 5 years. The majority of the drop in DAS28 occurred during the first 3 months (–1.63 points), and by the end of the first year of treatment, mean disease activity stayed below 2.6 on the DAS28.
The investigators saw a sustained remission at least once in 144 of the 171 patients with 5-year outcome data available, including sustained remission for 1 year or longer in 115. Median time to the first sustained remission proved to be 50 weeks, and half had this last less than 97 weeks and half more than 97 weeks.
During the 5-year follow-up, 17% of patients received treatment with biologics, with a median start of their first biologic at about 54 weeks after baseline. This first biologic was used continuously for a median of 29 weeks, and close to one-third of patients who started a biologic switched to a second biologic after a median duration of 41 weeks on the first. About two-thirds did not need a second biologic. A total of 66% of patients who took a biologic had at least one period of sustained remission.
Functional disability improved overall at 5 years as determined by Health Assessment Questionnaire (HAQ) scores that were available for 107 patients. HAQ scores decreased from a median of 1.125 at baseline to 0.375 after 24 weeks (P less than .001), where they remained stable throughout the rest of follow-up. Overall, nearly 70% of the patients with available 5-year data had a change in their individual HAQ score that was clinically meaningful from baseline to 24 weeks.
“Our study describes long-term outcome of implementation and continuous application of T2T to RA patients in daily clinical practice. The outcomes are similar to or even better than the results of T2T randomized clinical trials, in which strict selection of patients and controlled conditions were followed. These ‘real-life data’ are of important additional value in the evidence for the effectiveness of a T2T approach in RA patients,” the investigators concluded.
They had no disclosures to report.
SOURCE: Versteeg G et al. Clin Rheumatol. 2018 Feb 1. doi: 10.1007/s10067-017-3962-5.
A treat-to-target (T2T) strategy in daily clinical practice for patients with early rheumatoid arthritis proved successful in maintaining good disease- and patient-related outcomes over a 5-year period at two rheumatology clinics in the Netherlands.
The observational study builds on previous research on the long-term results of continuous application of T2T strategies in rheumatoid arthritis, for which there have been few published studies. “Long-term data from more recent randomized controlled clinical trials, using a T2T approach and biologicals, have shown good clinical outcomes. However, the generalizability of these results is hampered by the selection of specific patient groups in clinical trials and strict exclusion criteria. Patients seen in real-life practice may differ substantially from those in randomized clinical trials,” first author Letty G.A. Versteeg of Medisch Spectrum Twente, Enschede, the Netherlands, and her colleagues wrote in Clinical Rheumatology.
The investigators examined outcomes for 229 patients with very early RA who enrolled in the Dutch Rheumatoid Arthritis Monitoring (DREAM) remission induction cohort during 2006-2009, which included 5 years of follow-up for 171 of the patients. These patients underwent a protocoled T2T strategy aimed at remission, defined as a 28-joint Disease Activity Score (DAS28) of less than 2.6.
“In previous publications on the [DREAM] remission induction cohort, successful implementation of T2T in daily clinical practice was demonstrated. Achieving remission within the first year of treatment was shown to be a realistic goal for an important proportion of patients,” the authors wrote.
All patients started methotrexate monotherapy at an initial dosage of 15 mg/week that could be increased to a maximum dosage of 25 mg/week in week 8. Patients took folic acid on the second day after methotrexate. By week 12, those with persistent disease activity added sulfasalazine, starting at 2,000 mg/day and increasing if necessary to a maximum of 3,000 mg/day at week 20. Patients whose DAS28 remained at 3.2 or greater at week 24 received a tumor necrosis factor inhibitor. Those who reached remission had no change in medication, and when remission lasted for at least 6 months, medication was gradually tapered and eventually discontinued. Patients who had flares in which disease activity increased to a DAS28 of 2.6 and higher restarted their last effective medication or dosage, which could subsequently be intensified if necessary. Patients with comorbidities and contraindications for medication were not excluded because deviations from the protocol were allowed. The protocol also allowed concomitant treatment with NSAIDs, prednisolone at a dosage of less than 10 mg/day, and intra-articular corticosteroid injections.
The rate of DAS28-defined remission rose to 63% (126 of 199 patients) by the end of the first year, and only 5% had high disease activity at 24 weeks. The rate of remission remained stable over the next 4 years. This rate of remission was reflected as a drop from an overall mean DAS28 of 4.93 at baseline to 2.49 at 5 years. The majority of the drop in DAS28 occurred during the first 3 months (–1.63 points), and by the end of the first year of treatment, mean disease activity stayed below 2.6 on the DAS28.
The investigators saw a sustained remission at least once in 144 of the 171 patients with 5-year outcome data available, including sustained remission for 1 year or longer in 115. Median time to the first sustained remission proved to be 50 weeks, and half had this last less than 97 weeks and half more than 97 weeks.
During the 5-year follow-up, 17% of patients received treatment with biologics, with a median start of their first biologic at about 54 weeks after baseline. This first biologic was used continuously for a median of 29 weeks, and close to one-third of patients who started a biologic switched to a second biologic after a median duration of 41 weeks on the first. About two-thirds did not need a second biologic. A total of 66% of patients who took a biologic had at least one period of sustained remission.
Functional disability improved overall at 5 years as determined by Health Assessment Questionnaire (HAQ) scores that were available for 107 patients. HAQ scores decreased from a median of 1.125 at baseline to 0.375 after 24 weeks (P less than .001), where they remained stable throughout the rest of follow-up. Overall, nearly 70% of the patients with available 5-year data had a change in their individual HAQ score that was clinically meaningful from baseline to 24 weeks.
“Our study describes long-term outcome of implementation and continuous application of T2T to RA patients in daily clinical practice. The outcomes are similar to or even better than the results of T2T randomized clinical trials, in which strict selection of patients and controlled conditions were followed. These ‘real-life data’ are of important additional value in the evidence for the effectiveness of a T2T approach in RA patients,” the investigators concluded.
They had no disclosures to report.
SOURCE: Versteeg G et al. Clin Rheumatol. 2018 Feb 1. doi: 10.1007/s10067-017-3962-5.
FROM CLINICAL RHEUMATOLOGY
Key clinical point:
Major finding: The rate of DAS28 remission rose to 63% by the end of the first year and remained stable over the next 4 years.
Study details: An observational cohort study of 171 patients with 5 years of follow-up data.
Disclosures: The investigators had no disclosures to report.
Source: Versteeg G et al. Clin Rheumatol. 2018 Feb 1. doi: 10.1007/s10067-017-3962-5.
VIDEO: Advanced alternative payment model for RA set to undergo testing
SAN DIEGO – for evaluation, in order to provide rheumatologists with another payment pathway under Medicare’s new Quality Payment Program.
The draft version of the rheumatoid arthritis advanced alternative payment model (APM), prepared by the American College of Rheumatology and unveiled at its annual meeting, aims to give rheumatologists a more focused opportunity to participate in value-based care and potentially earn greater incentive payments. The model is geared not only to private practice rheumatologists, but also to those in academia.
The Quality Payment Program, including its advanced APM track, was established by the Medicare and CHIP Reauthorization Act of 2015 (MACRA).
Other societies are working on or have submitted APMs for approval, Timothy Laing, MD, a member of the RA APM working group and also the ACR representative to the American Medical Association’s Relative Value Scale Update Committee and CPT Advisory Committee, said at the ACR meeting.
After presenting a draft of the RA APM at an AMA workshop in October, Dr. Laing came away encouraged by the attendees’ response to the usefulness and flexibility of the model to pay for services that rheumatologists are currently frustrated by in a fee-for-services system. “It’s a big if, but I think if we can make the money work, this will be a shift and it will have a lot of impact on how we practice, and I think it will be generalizable to more than one condition.”
The cochair of the RA APM working group, Kwas Huston, MD, presented the draft at the meeting. He noted that advanced APMs could be developed just for specific diseases, for all inflammatory arthritis, all types of vasculitis, or for all rheumatic diseases. RA was chosen first because the ACR is new to the development of APMs and “needed to start somewhere.”
“This model allows us to improve our ability to care for patients and is more sustainable over time for rheumatologists from a revenue standpoint,” Dr. Huston, a rheumatologist with Kansas City (Mo.) Physician Partners, said in an interview.
The RA APM helps to reduce barriers to good care by providing adequate reimbursement for cognitive services through monthly payments rather than relying on payment for separate office visits, Dr. Huston said. The model allows for more time spent in shared decision making, educational activities, and improving treatment adherence. It also builds in payment for non–face-to-face communication between rheumatologists, primary care physicians, and other specialists; interaction with patients via phone calls, email, and telemedicine; and using nurses or other staff to help with chronic disease management. It’s meant to be flexible for use in diverse settings by allowing comanagement of patients in rural areas and places where there is a shortage of rheumatologists or travel is difficult.
Why join an advanced APM?
Rheumatologists may want to join an advanced APM because of the potentially unsustainable, “zero-sum” nature of MACRA’s Merit-based Incentive Payment System (MIPS), in which the losers pay for the winners, said Angus Worthing, MD, chair of the ACR’s Government Affairs Committee and a member of the RA APM working group. In MIPS, there are expected over time to be fewer and fewer “losers” in the program, either because participants perform better or the losers drop out. In addition, advanced APM participants will receive a 5% bonus in 2019-2024 and Medicare payment updates will be higher for advanced APMs in 2026 and beyond than for MIPS (0.75% vs. 0.25%).
Beyond the financial practicalities of MACRA, it’s beneficial for rheumatologists to have their own advanced APM because it’s better than being stuck in one that’s “written by the government and doesn’t cater to other specialties; it’s specific to rheumatology, our patients, our work flow, and what we think is valuable,” said Dr. Worthing, a practicing rheumatologist in the Washington area.
To participate in an advanced APM, clinicians will need to have 25% of payments for Part B fall under professional services in the advanced APM or have 20% of their patients receive Part B professional services through the advanced APM. However, Dr. Worthing advised keeping an eye out for new thresholds for participating in the APM track because “it will probably be hard to get 25% of your Medicare reimbursements or 20% of your patients in the first year you’re in it.”
The RA APM’s treatment pathway
Undergirding the whole model is a treatment pathway that takes a standardized approach to RA care, based on ACR 2015 guidelines, and will be updated regularly by the ACR, Dr. Huston said. Following the guidelines gives an opportunity to lower spending but increase the percentage that’s going to rheumatologists by reducing the variability in initiation of expensive medications. “Currently, we get about 2.5 cents on the dollar for every dollar that’s spent on rheumatoid arthritis care, and we want to increase the part that’s going to the rheumatologist to provide more services but decrease total spending,” he said.
The pathway requires the use of methotrexate and/or another disease-modifying antirheumatic drug (DMARD) before targeted therapy. However, the model also allows for treating unique patients by requiring only 75% adherence to the guidelines. Deviation from the guidelines is allowed if a patient has a contraindication, intolerance, or inadequate response to a DMARD, or if there are barriers outside of the rheumatologist’s control, such as insurance coverage. The ACR guidelines also specify the frequency and type of monitoring that’s needed for treatment.
Because the ACR guidelines will be followed, the model asks that payers make patients eligible for lower out-of-pocket costs for medications. Following the guidelines should also reduce the need for prior authorizations. Providers in the advanced APM would attest to 75% adherence to the pathway, which would be subject to audit. “We want to reduce the reporting burden. In MIPS, it’s very complicated. It’s hard to know how to report all of this. In the APM pathway, we’re trying to simplify reporting. You only have to report two things; one of them is following the treatment pathway 75% of the time” and the other is an outcome measure, he said.
The payments made under this RA treatment pathway are divided into four areas: diagnosis and treatment planning, support for primary care physicians in diagnosing joint symptoms, the initial treatment of RA patients, and continued care for RA.
Diagnosis and treatment planning
This step offers a one-time payment to support all the costs of evaluation, testing, diagnosis, and treatment planning for a patient who has symptoms that potentially indicate RA, has not been previously treated or diagnosed with RA, or has been treated unsuccessfully for RA by other physicians. It is not dependent on the number of visits.
This phase also covers basic lab testing and imaging, which if not done by the rheumatology practice, would then have a standardized amount deducted from the payment and be paid separately. Lab tests and imaging performed for other conditions would be paid separately as well.
The payment covers communication with other physicians, spending more time with patients in a shared decision-making process regarding treatment options, and developing a RA treatment plan.
“If you don’t end up diagnosing RA, you still get the payment. But there will be two different payments; one is a little lower if they don’t have RA. If they do have RA, then you spend more time with them developing this treatment plan, so that would be a higher payment,” Dr. Huston said.
Support for primary care physicians in diagnosing joint symptoms
This payment goes to a rheumatologist or a nurse practitioner or physician assistant who is working under the supervision of a rheumatologist for a patient who is under the care of a primary care physician who has an agreement to work collaboratively with the rheumatology practice. The payment, which is limited to one bill for one patient in a 1-year period, is for communication between the rheumatologist and the primary care physician about patients with symptoms that might indicate RA to determine the need for referral.
“This communication could be a phone call, an email, face-to-face, or some other form of communication ... to discuss how fast the patient may need to be seen or if there are other tests that need to be done before expediting referrals for patients who are higher risk,” Dr. Huston explained.
The payment would still be made if the patient does not require referral to a rheumatologist.
Initial treatment of RA patients
Payment for initial treatment can be made to a rheumatologist, a nurse practitioner or physician assistant under the supervision of a rheumatologist, or a team comprising the rheumatologist and a primary care physician who have a formal arrangement to support the early treatment of RA.
The latter scenario is intended for rural areas and other areas where there is a shortage of rheumatologists. The formal arrangement would specify how payments are shared and who is responsible for each of the accountability requirements and for treatment pathway, “but there is a lot of flexibility, and this can vary quite a bit, so what happens in rural Alaska where the primary care doctors might be more involved is not going to be the same as in a big city where the primary care doctors may not want to be involved at all. So there is no requirement for primary care doctors to be involved, but it just provides the resources in areas where that might make sense,” Dr. Huston noted.
The initial treatment payment would be made monthly for 6 months, replacing evaluation and management billing for office visits related to RA. It pays for typical lab tests and imaging and allows flexibility for non–face-to-face communications, and enhanced services to patients who need them. This payment is also stratified to adjust for sicker patients who have more comorbidities, he said.
Continued care for RA
This component of the payment structure also can be made to a rheumatologist, a nurse practitioner or physician assistant under the supervision of a rheumatologist, or the rheumatologist–primary care physician team. Continued care payments are made monthly and, just as with initial treatment payments, they are meant to replace E&M billing for office visits and pay for the same kinds of resources used in initial treatment, including stratified payments to adjust for patient characteristics.
Patients who come to a rheumatologist with established RA would enter this treatment pathway under this kind of payment.
RA APM’s accountability requirements
Participants in this model would be required to see a patient face-to-face at least every 6 months and to document their disease activity using a validated scale approved by the ACR for use in the RA APM, such as the RAPID-3 (Routine Assessment of Patient Index Data–3), the CDAI (Clinical Disease Activity Index), the SDAI (Simple Disease Activity Index), or the DAS28 (28-joint Disease Activity Score). Payment would also require keeping a written treatment plan that’s consistent with the ACR’s approved treatment pathway.
Changes in medication require communication with the patient within 2 weeks to help improve treatment adherence. Quality measures will still need to be recorded, such as a functional assessment, tuberculosis screening prior to starting biologics, and having a plan for steroid use, but they are not required to be reported. “You attest to that,” he said.
However, participants will need to report that they are following the treatment pathway for patients and an outcome measure for continued care of RA. These are necessary, Dr. Huston said, because “we are asking that we increase the money that’s going to the rheumatologist for managing patients with RA, so we have to show that we’re accountable and doing good with that money and that we’re taking care of our patients. ... and if we have an outcome measure, then we don’t have to report all those process measures that we do in MIPS.”
The outcome measure would be reporting:
• At least (some %) of patients with low disease activity remained in low disease activity.
• At least (some %) of patients with moderate disease activity stayed in the same or a lower disease activity category.
• At least (some %) of patients with a high disease activity had a lower disease activity category.
It’s unknown yet what the threshold percentage for each disease activity level would be, but it will be obtained from the ACR’s Rheumatology Informatics System for Effectiveness (RISE) Registry and will be refined over time from there. The outcome measure is not validated yet – none exists for RA for use in clinical practice – because it’s not yet known how to risk stratify patients in these disease activity levels for their comorbidities and socioeconomic factors. “Those are all things we need to learn over time,” Dr. Huston said, “but this is our good-faith effort at developing an outcome measure which we think will become more robust as we gather more and more data.”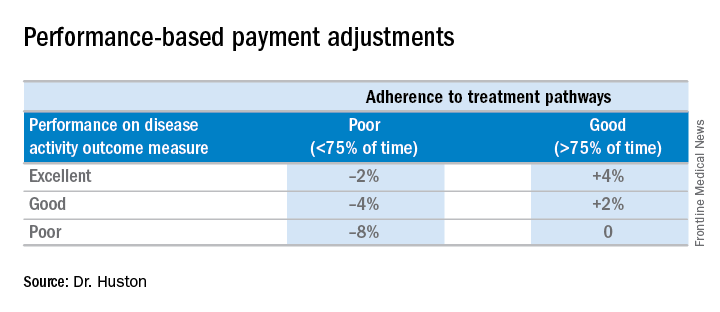
Performing all of these requirements would likely require more staff, and so the model will be built to account for these higher costs, Dr. Huston said.
Advantages of the RA APM
The RA APM’s advantages, according to Dr. Huston, stem from its payment for high-value services; the avoidance of the penalties and reporting burdens imposed by MIPS; a reduction in documentation requirements, allowing clinicians to take notes on history of present illness and review of systems however they want; a reduction in prior authorizations; and more control over performance measures.
Another big advantage of the RA APM is that participants “are not responsible for the price of drugs, whereas in MIPS you are responsible. When [the Centers for Medicare & Medicaid Services] calculates your cost category [in MIPS], that includes Part B drugs, and when the MIPS adjustment factor is applied to your revenue, that includes revenue from Part B drugs,” Dr. Huston said.
In addition, he noted that it will be possible for a rheumatologist to be a participant in just one or two APMs and still have the benefit of being out of MIPS.
Next steps
The next steps for the development of the RA APM include refining the treatment pathway, analyzing RISE data outcome thresholds, and modeling the financial impact on practices by running data from three to five practices across the country through the model to determine what the payment levels should be. Once those steps are completed, the RA APM can be submitted to the Physician-Focused Payment Model Technical Advisory Committee for approval, which will then send it to the CMS to run it through its Innovation Center to test the model in several practices to gather more data and refine the payment rates until it is ready to be expanded and implemented.
Dr. Huston, Dr. Worthing, and Dr. Laing had no relevant conflicts of interest to disclose.
SAN DIEGO – for evaluation, in order to provide rheumatologists with another payment pathway under Medicare’s new Quality Payment Program.
The draft version of the rheumatoid arthritis advanced alternative payment model (APM), prepared by the American College of Rheumatology and unveiled at its annual meeting, aims to give rheumatologists a more focused opportunity to participate in value-based care and potentially earn greater incentive payments. The model is geared not only to private practice rheumatologists, but also to those in academia.
The Quality Payment Program, including its advanced APM track, was established by the Medicare and CHIP Reauthorization Act of 2015 (MACRA).
Other societies are working on or have submitted APMs for approval, Timothy Laing, MD, a member of the RA APM working group and also the ACR representative to the American Medical Association’s Relative Value Scale Update Committee and CPT Advisory Committee, said at the ACR meeting.
After presenting a draft of the RA APM at an AMA workshop in October, Dr. Laing came away encouraged by the attendees’ response to the usefulness and flexibility of the model to pay for services that rheumatologists are currently frustrated by in a fee-for-services system. “It’s a big if, but I think if we can make the money work, this will be a shift and it will have a lot of impact on how we practice, and I think it will be generalizable to more than one condition.”
The cochair of the RA APM working group, Kwas Huston, MD, presented the draft at the meeting. He noted that advanced APMs could be developed just for specific diseases, for all inflammatory arthritis, all types of vasculitis, or for all rheumatic diseases. RA was chosen first because the ACR is new to the development of APMs and “needed to start somewhere.”
“This model allows us to improve our ability to care for patients and is more sustainable over time for rheumatologists from a revenue standpoint,” Dr. Huston, a rheumatologist with Kansas City (Mo.) Physician Partners, said in an interview.
The RA APM helps to reduce barriers to good care by providing adequate reimbursement for cognitive services through monthly payments rather than relying on payment for separate office visits, Dr. Huston said. The model allows for more time spent in shared decision making, educational activities, and improving treatment adherence. It also builds in payment for non–face-to-face communication between rheumatologists, primary care physicians, and other specialists; interaction with patients via phone calls, email, and telemedicine; and using nurses or other staff to help with chronic disease management. It’s meant to be flexible for use in diverse settings by allowing comanagement of patients in rural areas and places where there is a shortage of rheumatologists or travel is difficult.
Why join an advanced APM?
Rheumatologists may want to join an advanced APM because of the potentially unsustainable, “zero-sum” nature of MACRA’s Merit-based Incentive Payment System (MIPS), in which the losers pay for the winners, said Angus Worthing, MD, chair of the ACR’s Government Affairs Committee and a member of the RA APM working group. In MIPS, there are expected over time to be fewer and fewer “losers” in the program, either because participants perform better or the losers drop out. In addition, advanced APM participants will receive a 5% bonus in 2019-2024 and Medicare payment updates will be higher for advanced APMs in 2026 and beyond than for MIPS (0.75% vs. 0.25%).
Beyond the financial practicalities of MACRA, it’s beneficial for rheumatologists to have their own advanced APM because it’s better than being stuck in one that’s “written by the government and doesn’t cater to other specialties; it’s specific to rheumatology, our patients, our work flow, and what we think is valuable,” said Dr. Worthing, a practicing rheumatologist in the Washington area.
To participate in an advanced APM, clinicians will need to have 25% of payments for Part B fall under professional services in the advanced APM or have 20% of their patients receive Part B professional services through the advanced APM. However, Dr. Worthing advised keeping an eye out for new thresholds for participating in the APM track because “it will probably be hard to get 25% of your Medicare reimbursements or 20% of your patients in the first year you’re in it.”
The RA APM’s treatment pathway
Undergirding the whole model is a treatment pathway that takes a standardized approach to RA care, based on ACR 2015 guidelines, and will be updated regularly by the ACR, Dr. Huston said. Following the guidelines gives an opportunity to lower spending but increase the percentage that’s going to rheumatologists by reducing the variability in initiation of expensive medications. “Currently, we get about 2.5 cents on the dollar for every dollar that’s spent on rheumatoid arthritis care, and we want to increase the part that’s going to the rheumatologist to provide more services but decrease total spending,” he said.
The pathway requires the use of methotrexate and/or another disease-modifying antirheumatic drug (DMARD) before targeted therapy. However, the model also allows for treating unique patients by requiring only 75% adherence to the guidelines. Deviation from the guidelines is allowed if a patient has a contraindication, intolerance, or inadequate response to a DMARD, or if there are barriers outside of the rheumatologist’s control, such as insurance coverage. The ACR guidelines also specify the frequency and type of monitoring that’s needed for treatment.
Because the ACR guidelines will be followed, the model asks that payers make patients eligible for lower out-of-pocket costs for medications. Following the guidelines should also reduce the need for prior authorizations. Providers in the advanced APM would attest to 75% adherence to the pathway, which would be subject to audit. “We want to reduce the reporting burden. In MIPS, it’s very complicated. It’s hard to know how to report all of this. In the APM pathway, we’re trying to simplify reporting. You only have to report two things; one of them is following the treatment pathway 75% of the time” and the other is an outcome measure, he said.
The payments made under this RA treatment pathway are divided into four areas: diagnosis and treatment planning, support for primary care physicians in diagnosing joint symptoms, the initial treatment of RA patients, and continued care for RA.
Diagnosis and treatment planning
This step offers a one-time payment to support all the costs of evaluation, testing, diagnosis, and treatment planning for a patient who has symptoms that potentially indicate RA, has not been previously treated or diagnosed with RA, or has been treated unsuccessfully for RA by other physicians. It is not dependent on the number of visits.
This phase also covers basic lab testing and imaging, which if not done by the rheumatology practice, would then have a standardized amount deducted from the payment and be paid separately. Lab tests and imaging performed for other conditions would be paid separately as well.
The payment covers communication with other physicians, spending more time with patients in a shared decision-making process regarding treatment options, and developing a RA treatment plan.
“If you don’t end up diagnosing RA, you still get the payment. But there will be two different payments; one is a little lower if they don’t have RA. If they do have RA, then you spend more time with them developing this treatment plan, so that would be a higher payment,” Dr. Huston said.
Support for primary care physicians in diagnosing joint symptoms
This payment goes to a rheumatologist or a nurse practitioner or physician assistant who is working under the supervision of a rheumatologist for a patient who is under the care of a primary care physician who has an agreement to work collaboratively with the rheumatology practice. The payment, which is limited to one bill for one patient in a 1-year period, is for communication between the rheumatologist and the primary care physician about patients with symptoms that might indicate RA to determine the need for referral.
“This communication could be a phone call, an email, face-to-face, or some other form of communication ... to discuss how fast the patient may need to be seen or if there are other tests that need to be done before expediting referrals for patients who are higher risk,” Dr. Huston explained.
The payment would still be made if the patient does not require referral to a rheumatologist.
Initial treatment of RA patients
Payment for initial treatment can be made to a rheumatologist, a nurse practitioner or physician assistant under the supervision of a rheumatologist, or a team comprising the rheumatologist and a primary care physician who have a formal arrangement to support the early treatment of RA.
The latter scenario is intended for rural areas and other areas where there is a shortage of rheumatologists. The formal arrangement would specify how payments are shared and who is responsible for each of the accountability requirements and for treatment pathway, “but there is a lot of flexibility, and this can vary quite a bit, so what happens in rural Alaska where the primary care doctors might be more involved is not going to be the same as in a big city where the primary care doctors may not want to be involved at all. So there is no requirement for primary care doctors to be involved, but it just provides the resources in areas where that might make sense,” Dr. Huston noted.
The initial treatment payment would be made monthly for 6 months, replacing evaluation and management billing for office visits related to RA. It pays for typical lab tests and imaging and allows flexibility for non–face-to-face communications, and enhanced services to patients who need them. This payment is also stratified to adjust for sicker patients who have more comorbidities, he said.
Continued care for RA
This component of the payment structure also can be made to a rheumatologist, a nurse practitioner or physician assistant under the supervision of a rheumatologist, or the rheumatologist–primary care physician team. Continued care payments are made monthly and, just as with initial treatment payments, they are meant to replace E&M billing for office visits and pay for the same kinds of resources used in initial treatment, including stratified payments to adjust for patient characteristics.
Patients who come to a rheumatologist with established RA would enter this treatment pathway under this kind of payment.
RA APM’s accountability requirements
Participants in this model would be required to see a patient face-to-face at least every 6 months and to document their disease activity using a validated scale approved by the ACR for use in the RA APM, such as the RAPID-3 (Routine Assessment of Patient Index Data–3), the CDAI (Clinical Disease Activity Index), the SDAI (Simple Disease Activity Index), or the DAS28 (28-joint Disease Activity Score). Payment would also require keeping a written treatment plan that’s consistent with the ACR’s approved treatment pathway.
Changes in medication require communication with the patient within 2 weeks to help improve treatment adherence. Quality measures will still need to be recorded, such as a functional assessment, tuberculosis screening prior to starting biologics, and having a plan for steroid use, but they are not required to be reported. “You attest to that,” he said.
However, participants will need to report that they are following the treatment pathway for patients and an outcome measure for continued care of RA. These are necessary, Dr. Huston said, because “we are asking that we increase the money that’s going to the rheumatologist for managing patients with RA, so we have to show that we’re accountable and doing good with that money and that we’re taking care of our patients. ... and if we have an outcome measure, then we don’t have to report all those process measures that we do in MIPS.”
The outcome measure would be reporting:
• At least (some %) of patients with low disease activity remained in low disease activity.
• At least (some %) of patients with moderate disease activity stayed in the same or a lower disease activity category.
• At least (some %) of patients with a high disease activity had a lower disease activity category.
It’s unknown yet what the threshold percentage for each disease activity level would be, but it will be obtained from the ACR’s Rheumatology Informatics System for Effectiveness (RISE) Registry and will be refined over time from there. The outcome measure is not validated yet – none exists for RA for use in clinical practice – because it’s not yet known how to risk stratify patients in these disease activity levels for their comorbidities and socioeconomic factors. “Those are all things we need to learn over time,” Dr. Huston said, “but this is our good-faith effort at developing an outcome measure which we think will become more robust as we gather more and more data.”
Performing all of these requirements would likely require more staff, and so the model will be built to account for these higher costs, Dr. Huston said.
Advantages of the RA APM
The RA APM’s advantages, according to Dr. Huston, stem from its payment for high-value services; the avoidance of the penalties and reporting burdens imposed by MIPS; a reduction in documentation requirements, allowing clinicians to take notes on history of present illness and review of systems however they want; a reduction in prior authorizations; and more control over performance measures.
Another big advantage of the RA APM is that participants “are not responsible for the price of drugs, whereas in MIPS you are responsible. When [the Centers for Medicare & Medicaid Services] calculates your cost category [in MIPS], that includes Part B drugs, and when the MIPS adjustment factor is applied to your revenue, that includes revenue from Part B drugs,” Dr. Huston said.
In addition, he noted that it will be possible for a rheumatologist to be a participant in just one or two APMs and still have the benefit of being out of MIPS.
Next steps
The next steps for the development of the RA APM include refining the treatment pathway, analyzing RISE data outcome thresholds, and modeling the financial impact on practices by running data from three to five practices across the country through the model to determine what the payment levels should be. Once those steps are completed, the RA APM can be submitted to the Physician-Focused Payment Model Technical Advisory Committee for approval, which will then send it to the CMS to run it through its Innovation Center to test the model in several practices to gather more data and refine the payment rates until it is ready to be expanded and implemented.
Dr. Huston, Dr. Worthing, and Dr. Laing had no relevant conflicts of interest to disclose.
SAN DIEGO – for evaluation, in order to provide rheumatologists with another payment pathway under Medicare’s new Quality Payment Program.
The draft version of the rheumatoid arthritis advanced alternative payment model (APM), prepared by the American College of Rheumatology and unveiled at its annual meeting, aims to give rheumatologists a more focused opportunity to participate in value-based care and potentially earn greater incentive payments. The model is geared not only to private practice rheumatologists, but also to those in academia.
The Quality Payment Program, including its advanced APM track, was established by the Medicare and CHIP Reauthorization Act of 2015 (MACRA).
Other societies are working on or have submitted APMs for approval, Timothy Laing, MD, a member of the RA APM working group and also the ACR representative to the American Medical Association’s Relative Value Scale Update Committee and CPT Advisory Committee, said at the ACR meeting.
After presenting a draft of the RA APM at an AMA workshop in October, Dr. Laing came away encouraged by the attendees’ response to the usefulness and flexibility of the model to pay for services that rheumatologists are currently frustrated by in a fee-for-services system. “It’s a big if, but I think if we can make the money work, this will be a shift and it will have a lot of impact on how we practice, and I think it will be generalizable to more than one condition.”
The cochair of the RA APM working group, Kwas Huston, MD, presented the draft at the meeting. He noted that advanced APMs could be developed just for specific diseases, for all inflammatory arthritis, all types of vasculitis, or for all rheumatic diseases. RA was chosen first because the ACR is new to the development of APMs and “needed to start somewhere.”
“This model allows us to improve our ability to care for patients and is more sustainable over time for rheumatologists from a revenue standpoint,” Dr. Huston, a rheumatologist with Kansas City (Mo.) Physician Partners, said in an interview.
The RA APM helps to reduce barriers to good care by providing adequate reimbursement for cognitive services through monthly payments rather than relying on payment for separate office visits, Dr. Huston said. The model allows for more time spent in shared decision making, educational activities, and improving treatment adherence. It also builds in payment for non–face-to-face communication between rheumatologists, primary care physicians, and other specialists; interaction with patients via phone calls, email, and telemedicine; and using nurses or other staff to help with chronic disease management. It’s meant to be flexible for use in diverse settings by allowing comanagement of patients in rural areas and places where there is a shortage of rheumatologists or travel is difficult.
Why join an advanced APM?
Rheumatologists may want to join an advanced APM because of the potentially unsustainable, “zero-sum” nature of MACRA’s Merit-based Incentive Payment System (MIPS), in which the losers pay for the winners, said Angus Worthing, MD, chair of the ACR’s Government Affairs Committee and a member of the RA APM working group. In MIPS, there are expected over time to be fewer and fewer “losers” in the program, either because participants perform better or the losers drop out. In addition, advanced APM participants will receive a 5% bonus in 2019-2024 and Medicare payment updates will be higher for advanced APMs in 2026 and beyond than for MIPS (0.75% vs. 0.25%).
Beyond the financial practicalities of MACRA, it’s beneficial for rheumatologists to have their own advanced APM because it’s better than being stuck in one that’s “written by the government and doesn’t cater to other specialties; it’s specific to rheumatology, our patients, our work flow, and what we think is valuable,” said Dr. Worthing, a practicing rheumatologist in the Washington area.
To participate in an advanced APM, clinicians will need to have 25% of payments for Part B fall under professional services in the advanced APM or have 20% of their patients receive Part B professional services through the advanced APM. However, Dr. Worthing advised keeping an eye out for new thresholds for participating in the APM track because “it will probably be hard to get 25% of your Medicare reimbursements or 20% of your patients in the first year you’re in it.”
The RA APM’s treatment pathway
Undergirding the whole model is a treatment pathway that takes a standardized approach to RA care, based on ACR 2015 guidelines, and will be updated regularly by the ACR, Dr. Huston said. Following the guidelines gives an opportunity to lower spending but increase the percentage that’s going to rheumatologists by reducing the variability in initiation of expensive medications. “Currently, we get about 2.5 cents on the dollar for every dollar that’s spent on rheumatoid arthritis care, and we want to increase the part that’s going to the rheumatologist to provide more services but decrease total spending,” he said.
The pathway requires the use of methotrexate and/or another disease-modifying antirheumatic drug (DMARD) before targeted therapy. However, the model also allows for treating unique patients by requiring only 75% adherence to the guidelines. Deviation from the guidelines is allowed if a patient has a contraindication, intolerance, or inadequate response to a DMARD, or if there are barriers outside of the rheumatologist’s control, such as insurance coverage. The ACR guidelines also specify the frequency and type of monitoring that’s needed for treatment.
Because the ACR guidelines will be followed, the model asks that payers make patients eligible for lower out-of-pocket costs for medications. Following the guidelines should also reduce the need for prior authorizations. Providers in the advanced APM would attest to 75% adherence to the pathway, which would be subject to audit. “We want to reduce the reporting burden. In MIPS, it’s very complicated. It’s hard to know how to report all of this. In the APM pathway, we’re trying to simplify reporting. You only have to report two things; one of them is following the treatment pathway 75% of the time” and the other is an outcome measure, he said.
The payments made under this RA treatment pathway are divided into four areas: diagnosis and treatment planning, support for primary care physicians in diagnosing joint symptoms, the initial treatment of RA patients, and continued care for RA.
Diagnosis and treatment planning
This step offers a one-time payment to support all the costs of evaluation, testing, diagnosis, and treatment planning for a patient who has symptoms that potentially indicate RA, has not been previously treated or diagnosed with RA, or has been treated unsuccessfully for RA by other physicians. It is not dependent on the number of visits.
This phase also covers basic lab testing and imaging, which if not done by the rheumatology practice, would then have a standardized amount deducted from the payment and be paid separately. Lab tests and imaging performed for other conditions would be paid separately as well.
The payment covers communication with other physicians, spending more time with patients in a shared decision-making process regarding treatment options, and developing a RA treatment plan.
“If you don’t end up diagnosing RA, you still get the payment. But there will be two different payments; one is a little lower if they don’t have RA. If they do have RA, then you spend more time with them developing this treatment plan, so that would be a higher payment,” Dr. Huston said.
Support for primary care physicians in diagnosing joint symptoms
This payment goes to a rheumatologist or a nurse practitioner or physician assistant who is working under the supervision of a rheumatologist for a patient who is under the care of a primary care physician who has an agreement to work collaboratively with the rheumatology practice. The payment, which is limited to one bill for one patient in a 1-year period, is for communication between the rheumatologist and the primary care physician about patients with symptoms that might indicate RA to determine the need for referral.
“This communication could be a phone call, an email, face-to-face, or some other form of communication ... to discuss how fast the patient may need to be seen or if there are other tests that need to be done before expediting referrals for patients who are higher risk,” Dr. Huston explained.
The payment would still be made if the patient does not require referral to a rheumatologist.
Initial treatment of RA patients
Payment for initial treatment can be made to a rheumatologist, a nurse practitioner or physician assistant under the supervision of a rheumatologist, or a team comprising the rheumatologist and a primary care physician who have a formal arrangement to support the early treatment of RA.
The latter scenario is intended for rural areas and other areas where there is a shortage of rheumatologists. The formal arrangement would specify how payments are shared and who is responsible for each of the accountability requirements and for treatment pathway, “but there is a lot of flexibility, and this can vary quite a bit, so what happens in rural Alaska where the primary care doctors might be more involved is not going to be the same as in a big city where the primary care doctors may not want to be involved at all. So there is no requirement for primary care doctors to be involved, but it just provides the resources in areas where that might make sense,” Dr. Huston noted.
The initial treatment payment would be made monthly for 6 months, replacing evaluation and management billing for office visits related to RA. It pays for typical lab tests and imaging and allows flexibility for non–face-to-face communications, and enhanced services to patients who need them. This payment is also stratified to adjust for sicker patients who have more comorbidities, he said.
Continued care for RA
This component of the payment structure also can be made to a rheumatologist, a nurse practitioner or physician assistant under the supervision of a rheumatologist, or the rheumatologist–primary care physician team. Continued care payments are made monthly and, just as with initial treatment payments, they are meant to replace E&M billing for office visits and pay for the same kinds of resources used in initial treatment, including stratified payments to adjust for patient characteristics.
Patients who come to a rheumatologist with established RA would enter this treatment pathway under this kind of payment.
RA APM’s accountability requirements
Participants in this model would be required to see a patient face-to-face at least every 6 months and to document their disease activity using a validated scale approved by the ACR for use in the RA APM, such as the RAPID-3 (Routine Assessment of Patient Index Data–3), the CDAI (Clinical Disease Activity Index), the SDAI (Simple Disease Activity Index), or the DAS28 (28-joint Disease Activity Score). Payment would also require keeping a written treatment plan that’s consistent with the ACR’s approved treatment pathway.
Changes in medication require communication with the patient within 2 weeks to help improve treatment adherence. Quality measures will still need to be recorded, such as a functional assessment, tuberculosis screening prior to starting biologics, and having a plan for steroid use, but they are not required to be reported. “You attest to that,” he said.
However, participants will need to report that they are following the treatment pathway for patients and an outcome measure for continued care of RA. These are necessary, Dr. Huston said, because “we are asking that we increase the money that’s going to the rheumatologist for managing patients with RA, so we have to show that we’re accountable and doing good with that money and that we’re taking care of our patients. ... and if we have an outcome measure, then we don’t have to report all those process measures that we do in MIPS.”
The outcome measure would be reporting:
• At least (some %) of patients with low disease activity remained in low disease activity.
• At least (some %) of patients with moderate disease activity stayed in the same or a lower disease activity category.
• At least (some %) of patients with a high disease activity had a lower disease activity category.
It’s unknown yet what the threshold percentage for each disease activity level would be, but it will be obtained from the ACR’s Rheumatology Informatics System for Effectiveness (RISE) Registry and will be refined over time from there. The outcome measure is not validated yet – none exists for RA for use in clinical practice – because it’s not yet known how to risk stratify patients in these disease activity levels for their comorbidities and socioeconomic factors. “Those are all things we need to learn over time,” Dr. Huston said, “but this is our good-faith effort at developing an outcome measure which we think will become more robust as we gather more and more data.”
Performing all of these requirements would likely require more staff, and so the model will be built to account for these higher costs, Dr. Huston said.
Advantages of the RA APM
The RA APM’s advantages, according to Dr. Huston, stem from its payment for high-value services; the avoidance of the penalties and reporting burdens imposed by MIPS; a reduction in documentation requirements, allowing clinicians to take notes on history of present illness and review of systems however they want; a reduction in prior authorizations; and more control over performance measures.
Another big advantage of the RA APM is that participants “are not responsible for the price of drugs, whereas in MIPS you are responsible. When [the Centers for Medicare & Medicaid Services] calculates your cost category [in MIPS], that includes Part B drugs, and when the MIPS adjustment factor is applied to your revenue, that includes revenue from Part B drugs,” Dr. Huston said.
In addition, he noted that it will be possible for a rheumatologist to be a participant in just one or two APMs and still have the benefit of being out of MIPS.
Next steps
The next steps for the development of the RA APM include refining the treatment pathway, analyzing RISE data outcome thresholds, and modeling the financial impact on practices by running data from three to five practices across the country through the model to determine what the payment levels should be. Once those steps are completed, the RA APM can be submitted to the Physician-Focused Payment Model Technical Advisory Committee for approval, which will then send it to the CMS to run it through its Innovation Center to test the model in several practices to gather more data and refine the payment rates until it is ready to be expanded and implemented.
Dr. Huston, Dr. Worthing, and Dr. Laing had no relevant conflicts of interest to disclose.
AT ACR 2017