User login
Adjuvant oxaliplatin boosts colon cancer survival
After curative-intent surgery, patients with stage III colon cancer who received oxaliplatin plus capecitabine (XELOX) had improved disease-free survival and overall survival, compared with those who received fluorouracil/folinic acid (FU/FA), according to secondary analysis of the NO16968 study.
After a median 74-month follow-up, 320 of 944 patients in the XELOX group (34%) experienced relapse, developed a new colon cancer, or died, compared with 379 of 942 patients in the FU/FA group (40%), for a hazard ratio of 0.80 (95% confidence interval, 0.69-0.93; P = .004). After a median follow-up of 83 months, 242 (26%) in the XELOX group died, compared with 286 (30%) in the FU/FA group, for a HR of 0.83 (95% CI, 0.70-0.99; P = .04).
Disease-free survival (DFS) and overall survival (OS) rates were consistently higher for XELOX after 3-7 years of follow-up, and the differences between the treatments increased after the primary analysis. Survival rates for XELOX and FU/FA were 86% vs. 84%, respectively, in year 3; 80% vs. 78% in year 4; 77% vs. 74% in year 5; 76% vs. 71% in year 6; and 73% vs. 67% in year 7.
“Multiple incremental advances in the treatment and detection of recurrent colon cancer mean that a longer follow-up of 6 or 7 years may be required to assess survival benefit in modern adjuvant trials reliably,” wrote Dr. Hans-Joachim Schmoll of the department of oncology/hematology at Martin Luther University in Halle, Germany, and his colleagues (J Clin Oncol. 2015 Aug 31. doi: 10.1200/JCO.2014.60.9107).
The survival benefits observed in this trial are in line with those of the previous MOSAIC trial, which used a similar cumulative dose of oxaliplatin (1,040 mg/m2 and 1,020 mg/m2, respectively).
“Together, these trials show that oxaliplatin improves OS when added to a fluoropyrimidine as adjuvant therapy in patients with stage III colon cancer, and that the fluoropyrimidine may be delivered intravenously by infusion or orally for the same result,” the investigators observed.
The multinational, open-label, parallel, randomized phase III study included 1,886 patients who received XELOX or FU/FA from 2003 to 2004.
Subset analysis for patients older than 70 years showed an increased survival benefit for XELOX over FU/FA, but the effect size was smaller than in younger patients and the conclusions were not definitive because of the small subgroup sizes.
A biomarker analysis included 26% of the trial participants: 242 in the XELOX group and 256 in the FU/FA group. Patients in the XELOX group who had lower expression of dihydropyrimidine dehydrogenase had better outcomes (P less than 001). Patients in the FU/FA group showed no significant associations between tumor biomarkers and outcomes. The study was supported by F. Hoffmann-La Roche. Dr. Schmoll reported consulting or advisory roles with Genentech, Taiho Pharmaceutical, and Bayer Schering Pharma. Several of his coauthors reported ties to industry sources.
After curative-intent surgery, patients with stage III colon cancer who received oxaliplatin plus capecitabine (XELOX) had improved disease-free survival and overall survival, compared with those who received fluorouracil/folinic acid (FU/FA), according to secondary analysis of the NO16968 study.
After a median 74-month follow-up, 320 of 944 patients in the XELOX group (34%) experienced relapse, developed a new colon cancer, or died, compared with 379 of 942 patients in the FU/FA group (40%), for a hazard ratio of 0.80 (95% confidence interval, 0.69-0.93; P = .004). After a median follow-up of 83 months, 242 (26%) in the XELOX group died, compared with 286 (30%) in the FU/FA group, for a HR of 0.83 (95% CI, 0.70-0.99; P = .04).
Disease-free survival (DFS) and overall survival (OS) rates were consistently higher for XELOX after 3-7 years of follow-up, and the differences between the treatments increased after the primary analysis. Survival rates for XELOX and FU/FA were 86% vs. 84%, respectively, in year 3; 80% vs. 78% in year 4; 77% vs. 74% in year 5; 76% vs. 71% in year 6; and 73% vs. 67% in year 7.
“Multiple incremental advances in the treatment and detection of recurrent colon cancer mean that a longer follow-up of 6 or 7 years may be required to assess survival benefit in modern adjuvant trials reliably,” wrote Dr. Hans-Joachim Schmoll of the department of oncology/hematology at Martin Luther University in Halle, Germany, and his colleagues (J Clin Oncol. 2015 Aug 31. doi: 10.1200/JCO.2014.60.9107).
The survival benefits observed in this trial are in line with those of the previous MOSAIC trial, which used a similar cumulative dose of oxaliplatin (1,040 mg/m2 and 1,020 mg/m2, respectively).
“Together, these trials show that oxaliplatin improves OS when added to a fluoropyrimidine as adjuvant therapy in patients with stage III colon cancer, and that the fluoropyrimidine may be delivered intravenously by infusion or orally for the same result,” the investigators observed.
The multinational, open-label, parallel, randomized phase III study included 1,886 patients who received XELOX or FU/FA from 2003 to 2004.
Subset analysis for patients older than 70 years showed an increased survival benefit for XELOX over FU/FA, but the effect size was smaller than in younger patients and the conclusions were not definitive because of the small subgroup sizes.
A biomarker analysis included 26% of the trial participants: 242 in the XELOX group and 256 in the FU/FA group. Patients in the XELOX group who had lower expression of dihydropyrimidine dehydrogenase had better outcomes (P less than 001). Patients in the FU/FA group showed no significant associations between tumor biomarkers and outcomes. The study was supported by F. Hoffmann-La Roche. Dr. Schmoll reported consulting or advisory roles with Genentech, Taiho Pharmaceutical, and Bayer Schering Pharma. Several of his coauthors reported ties to industry sources.
After curative-intent surgery, patients with stage III colon cancer who received oxaliplatin plus capecitabine (XELOX) had improved disease-free survival and overall survival, compared with those who received fluorouracil/folinic acid (FU/FA), according to secondary analysis of the NO16968 study.
After a median 74-month follow-up, 320 of 944 patients in the XELOX group (34%) experienced relapse, developed a new colon cancer, or died, compared with 379 of 942 patients in the FU/FA group (40%), for a hazard ratio of 0.80 (95% confidence interval, 0.69-0.93; P = .004). After a median follow-up of 83 months, 242 (26%) in the XELOX group died, compared with 286 (30%) in the FU/FA group, for a HR of 0.83 (95% CI, 0.70-0.99; P = .04).
Disease-free survival (DFS) and overall survival (OS) rates were consistently higher for XELOX after 3-7 years of follow-up, and the differences between the treatments increased after the primary analysis. Survival rates for XELOX and FU/FA were 86% vs. 84%, respectively, in year 3; 80% vs. 78% in year 4; 77% vs. 74% in year 5; 76% vs. 71% in year 6; and 73% vs. 67% in year 7.
“Multiple incremental advances in the treatment and detection of recurrent colon cancer mean that a longer follow-up of 6 or 7 years may be required to assess survival benefit in modern adjuvant trials reliably,” wrote Dr. Hans-Joachim Schmoll of the department of oncology/hematology at Martin Luther University in Halle, Germany, and his colleagues (J Clin Oncol. 2015 Aug 31. doi: 10.1200/JCO.2014.60.9107).
The survival benefits observed in this trial are in line with those of the previous MOSAIC trial, which used a similar cumulative dose of oxaliplatin (1,040 mg/m2 and 1,020 mg/m2, respectively).
“Together, these trials show that oxaliplatin improves OS when added to a fluoropyrimidine as adjuvant therapy in patients with stage III colon cancer, and that the fluoropyrimidine may be delivered intravenously by infusion or orally for the same result,” the investigators observed.
The multinational, open-label, parallel, randomized phase III study included 1,886 patients who received XELOX or FU/FA from 2003 to 2004.
Subset analysis for patients older than 70 years showed an increased survival benefit for XELOX over FU/FA, but the effect size was smaller than in younger patients and the conclusions were not definitive because of the small subgroup sizes.
A biomarker analysis included 26% of the trial participants: 242 in the XELOX group and 256 in the FU/FA group. Patients in the XELOX group who had lower expression of dihydropyrimidine dehydrogenase had better outcomes (P less than 001). Patients in the FU/FA group showed no significant associations between tumor biomarkers and outcomes. The study was supported by F. Hoffmann-La Roche. Dr. Schmoll reported consulting or advisory roles with Genentech, Taiho Pharmaceutical, and Bayer Schering Pharma. Several of his coauthors reported ties to industry sources.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: After a median 7-year follow-up, oxaliplatin plus capecitabine (XELOX) was associated with significantly improved DFS and OS, compared with bolus fluorouracil/folinic acid (FU/FA).
Major finding: Overall, 34% of the XELOX group experienced relapse, developed a new colon cancer, or died, compared with 40% in the FU/FA group (HR, 0.80; P = .004).
Data source: The multinational, open-label, randomized NO16968 study included 1,886 patients who received XELOX or FU/FA from 2003 to 2004.
Disclosures: The study was supported by F. Hoffmann-La Roche. Dr. Schmoll reported consulting or advisory roles with Genentech, Taiho Pharmaceutical, and Bayer Schering Pharma. Several of his coauthors reported ties to industry sources.
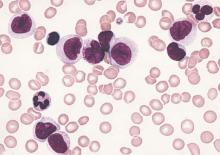
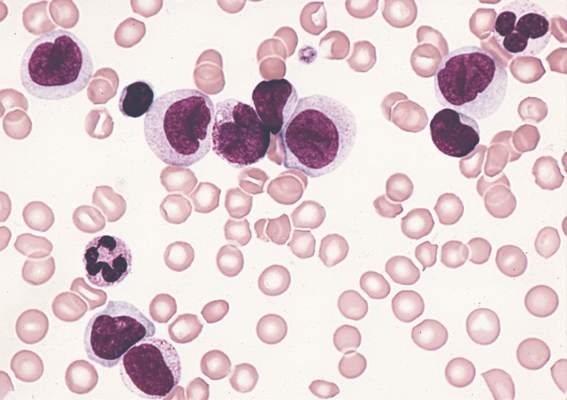
Outcomes worse with secondary and therapy-related AML compared with de novo AML
Clinical outcomes were significantly worse among younger patients with secondary and therapy-related acute myeloid leukemia (sAML and tAML, respectively) compared with de novo disease, suggesting the presence of distinct AML subtypes, in a study published online Aug. 24 in Journal of Clinical Oncology.
In the Danish population-based study, patients under age 60 with de novo AML had a 3-year survival rate of 52%, compared with 35% for AML secondary to myelodysplastic syndrome (MDS-sAML), 19% for AML secondary to chronic myelomonocytic leukemia or myeloproliferative neoplasia (non-MDS-sAML), and 27% for tAML.
Non-MDS-sAML was associated with poorer outcomes at any age, but patients older than 60 years with MDS-sAML and tAML had outcomes similar to those of de novo AML, Dr. Lene Sofie Granfeldt Ostgard and associates reported (J Clin Oncol. 2015 Aug 24. doi:10.1200/JCO.2014.60.0890).
The cohort study evaluated records of 3,055 patients diagnosed with AML from 2000 to 2013. Overall, 73.6% had de novo AML, 19.8% had sAML (11.5% MDS, 8.3% non-MDS), and 6.6% had tAML. The researchers focused on the 1,567 patients (51.3%) who underwent curative intent therapy. Patients with sAML were less likely to receive intensive therapy than were patients with tAML or de novo AML. Patients who underwent intensive treatment had superior survival rates compared with patients within the same subgroup who did not undergo treatment.
Contrary to previous reports, the investigators did not observe an increase in the incidence of AML, sAML, or tAML over the 14-year study period. They did, however, find that clinical trial accrual rates for patients with sAML and tAML increased significantly over that period, which may account for reports of a temporal increase in tAML.
Adverse-risk cytogenetics were most prevalent in patients with tAML, likely because of clonal selection of chemotherapy-resistant p53-mutated cells. Patients with non-MDS-sAML had a higher frequency of aberrant karyotypes, though not classified as adverse, which the authors speculate may explain the worse outcomes observed in this group.
“Given the extremely poor prognosis associated with an antecedent diagnosis of CMML [chronic myelomonocytic leukemia] or MPN [myeloproliferative neoplasia], these entities should be considered separately from prior MDS when investigating outcomes and new treatment strategies in patients with sAML,” wrote Dr. Ostgard of Aarhus University Hospital, Denmark, and colleagues.
The presence of MDS-sAML and tAML was associated with worse survival, but the association was weaker in older patients and those with adverse cytogenetics, suggesting the relative effect of MDS or prior chemotherapy is small in patients with an already poor prognosis.
Dr. Ostgard reported consulting or advisory roles with Bristol-Myers Squibb and Abbvie. Several of her coauthors reported ties to industry sources.
Clinical outcomes were significantly worse among younger patients with secondary and therapy-related acute myeloid leukemia (sAML and tAML, respectively) compared with de novo disease, suggesting the presence of distinct AML subtypes, in a study published online Aug. 24 in Journal of Clinical Oncology.
In the Danish population-based study, patients under age 60 with de novo AML had a 3-year survival rate of 52%, compared with 35% for AML secondary to myelodysplastic syndrome (MDS-sAML), 19% for AML secondary to chronic myelomonocytic leukemia or myeloproliferative neoplasia (non-MDS-sAML), and 27% for tAML.
Non-MDS-sAML was associated with poorer outcomes at any age, but patients older than 60 years with MDS-sAML and tAML had outcomes similar to those of de novo AML, Dr. Lene Sofie Granfeldt Ostgard and associates reported (J Clin Oncol. 2015 Aug 24. doi:10.1200/JCO.2014.60.0890).
The cohort study evaluated records of 3,055 patients diagnosed with AML from 2000 to 2013. Overall, 73.6% had de novo AML, 19.8% had sAML (11.5% MDS, 8.3% non-MDS), and 6.6% had tAML. The researchers focused on the 1,567 patients (51.3%) who underwent curative intent therapy. Patients with sAML were less likely to receive intensive therapy than were patients with tAML or de novo AML. Patients who underwent intensive treatment had superior survival rates compared with patients within the same subgroup who did not undergo treatment.
Contrary to previous reports, the investigators did not observe an increase in the incidence of AML, sAML, or tAML over the 14-year study period. They did, however, find that clinical trial accrual rates for patients with sAML and tAML increased significantly over that period, which may account for reports of a temporal increase in tAML.
Adverse-risk cytogenetics were most prevalent in patients with tAML, likely because of clonal selection of chemotherapy-resistant p53-mutated cells. Patients with non-MDS-sAML had a higher frequency of aberrant karyotypes, though not classified as adverse, which the authors speculate may explain the worse outcomes observed in this group.
“Given the extremely poor prognosis associated with an antecedent diagnosis of CMML [chronic myelomonocytic leukemia] or MPN [myeloproliferative neoplasia], these entities should be considered separately from prior MDS when investigating outcomes and new treatment strategies in patients with sAML,” wrote Dr. Ostgard of Aarhus University Hospital, Denmark, and colleagues.
The presence of MDS-sAML and tAML was associated with worse survival, but the association was weaker in older patients and those with adverse cytogenetics, suggesting the relative effect of MDS or prior chemotherapy is small in patients with an already poor prognosis.
Dr. Ostgard reported consulting or advisory roles with Bristol-Myers Squibb and Abbvie. Several of her coauthors reported ties to industry sources.
Clinical outcomes were significantly worse among younger patients with secondary and therapy-related acute myeloid leukemia (sAML and tAML, respectively) compared with de novo disease, suggesting the presence of distinct AML subtypes, in a study published online Aug. 24 in Journal of Clinical Oncology.
In the Danish population-based study, patients under age 60 with de novo AML had a 3-year survival rate of 52%, compared with 35% for AML secondary to myelodysplastic syndrome (MDS-sAML), 19% for AML secondary to chronic myelomonocytic leukemia or myeloproliferative neoplasia (non-MDS-sAML), and 27% for tAML.
Non-MDS-sAML was associated with poorer outcomes at any age, but patients older than 60 years with MDS-sAML and tAML had outcomes similar to those of de novo AML, Dr. Lene Sofie Granfeldt Ostgard and associates reported (J Clin Oncol. 2015 Aug 24. doi:10.1200/JCO.2014.60.0890).
The cohort study evaluated records of 3,055 patients diagnosed with AML from 2000 to 2013. Overall, 73.6% had de novo AML, 19.8% had sAML (11.5% MDS, 8.3% non-MDS), and 6.6% had tAML. The researchers focused on the 1,567 patients (51.3%) who underwent curative intent therapy. Patients with sAML were less likely to receive intensive therapy than were patients with tAML or de novo AML. Patients who underwent intensive treatment had superior survival rates compared with patients within the same subgroup who did not undergo treatment.
Contrary to previous reports, the investigators did not observe an increase in the incidence of AML, sAML, or tAML over the 14-year study period. They did, however, find that clinical trial accrual rates for patients with sAML and tAML increased significantly over that period, which may account for reports of a temporal increase in tAML.
Adverse-risk cytogenetics were most prevalent in patients with tAML, likely because of clonal selection of chemotherapy-resistant p53-mutated cells. Patients with non-MDS-sAML had a higher frequency of aberrant karyotypes, though not classified as adverse, which the authors speculate may explain the worse outcomes observed in this group.
“Given the extremely poor prognosis associated with an antecedent diagnosis of CMML [chronic myelomonocytic leukemia] or MPN [myeloproliferative neoplasia], these entities should be considered separately from prior MDS when investigating outcomes and new treatment strategies in patients with sAML,” wrote Dr. Ostgard of Aarhus University Hospital, Denmark, and colleagues.
The presence of MDS-sAML and tAML was associated with worse survival, but the association was weaker in older patients and those with adverse cytogenetics, suggesting the relative effect of MDS or prior chemotherapy is small in patients with an already poor prognosis.
Dr. Ostgard reported consulting or advisory roles with Bristol-Myers Squibb and Abbvie. Several of her coauthors reported ties to industry sources.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Younger patients with secondary and therapy-related acute myeloid leukemia (sAML and tAML, respectively) have worse outcomes than do patients with de novo AML.
Major finding: Three-year survival for patients under 60 years of age with de novo AML was 52%, compared with 35% for sAML (myelodysplastic syndrome), 19% for sAML (nonmyelodysplastic syndrome), and 27% for tAML.
Data source: A population-based Danish cohort study of 3,055 patients with AML diagnosed from 2000 to 2013.
Disclosures: Dr. Ostgard reported consulting or advisory roles with Bristol-Myers Squibb and Abbvie. Several coauthors reported ties to industry sources.
Regorafenib provides low value in metastatic colorectal cancer
Regorafenib carries high incremental cost and delivers small incremental benefit, providing low value for patients with metastatic colorectal cancer, researchers reported online Aug. 24 in the Journal of Clinical Oncology.
Compared with best supportive care, use of regorafenib resulted in a gain of 6 weeks of life, which dropped to 2 quality-adjusted weeks of life (0.04 quality-adjusted life years [QALYs]) in light of its significant toxicity profile. With the cost of treatment estimated between $32,000 and $43,000, depending on the dose, the incremental cost-effectiveness ratio (ICER) was $730,000-$980,000 per QALY, Dr. Daniel Goldstein and his associates reported (J Clin Oncol. 2015 Aug 24. doi: 10.1200/JCO.2014.61.9569).
“With increasing deductibles and copays, our patients are now bearing a significant burden of the cost of drugs,” wrote Dr. Goldstein of the department of hematology and medical oncology at Emory University, Atlanta, and his colleagues. There is a need, they wrote, for new strategies “in patient selection for clinical trials, guideline development, and payer coverage determination that address clinical value in addition to statistically significant clinical benefit.”
The CORRECT (Colorectal Cancer Treated With Regorafenib or Placebo After Failure of Standard Therapy) trial indicated an overall survival benefit of 1.4 months for regorafenib, compared with placebo, in patients with colorectal cancer who progressed after standard regimens. Adverse events of grade 3 or 4 occurred in 54% of the regorafenib arm, compared with 14% in the placebo arm.
The analysis based on the CORRECT data indicated a low likelihood that regorafenib would be considered cost effective at a willingness to pay threshold less than $600,000 per QALY. Regorafenib exceeds typical accepted values for cost-effectiveness.
Model parameters that most influenced ICER were regorafenib cost, probability of stopping treatment due to an adverse event, and baseline utility value. The ICER remained greater than $550,000 per QALY across a broad range of each of these variables.
Given the increasing amount of out-of-pocket costs for patients, the investigators recommend “a careful discussion between physicians and patients regarding the additional benefit and potential total drug cost before starting regorafenib.”
The study was supported by internal funds and by a grant from the National Institutes of Health. Dr. Goldstein reported having no disclosures. Three of his coauthors reported ties to industry.
Regorafenib carries high incremental cost and delivers small incremental benefit, providing low value for patients with metastatic colorectal cancer, researchers reported online Aug. 24 in the Journal of Clinical Oncology.
Compared with best supportive care, use of regorafenib resulted in a gain of 6 weeks of life, which dropped to 2 quality-adjusted weeks of life (0.04 quality-adjusted life years [QALYs]) in light of its significant toxicity profile. With the cost of treatment estimated between $32,000 and $43,000, depending on the dose, the incremental cost-effectiveness ratio (ICER) was $730,000-$980,000 per QALY, Dr. Daniel Goldstein and his associates reported (J Clin Oncol. 2015 Aug 24. doi: 10.1200/JCO.2014.61.9569).
“With increasing deductibles and copays, our patients are now bearing a significant burden of the cost of drugs,” wrote Dr. Goldstein of the department of hematology and medical oncology at Emory University, Atlanta, and his colleagues. There is a need, they wrote, for new strategies “in patient selection for clinical trials, guideline development, and payer coverage determination that address clinical value in addition to statistically significant clinical benefit.”
The CORRECT (Colorectal Cancer Treated With Regorafenib or Placebo After Failure of Standard Therapy) trial indicated an overall survival benefit of 1.4 months for regorafenib, compared with placebo, in patients with colorectal cancer who progressed after standard regimens. Adverse events of grade 3 or 4 occurred in 54% of the regorafenib arm, compared with 14% in the placebo arm.
The analysis based on the CORRECT data indicated a low likelihood that regorafenib would be considered cost effective at a willingness to pay threshold less than $600,000 per QALY. Regorafenib exceeds typical accepted values for cost-effectiveness.
Model parameters that most influenced ICER were regorafenib cost, probability of stopping treatment due to an adverse event, and baseline utility value. The ICER remained greater than $550,000 per QALY across a broad range of each of these variables.
Given the increasing amount of out-of-pocket costs for patients, the investigators recommend “a careful discussion between physicians and patients regarding the additional benefit and potential total drug cost before starting regorafenib.”
The study was supported by internal funds and by a grant from the National Institutes of Health. Dr. Goldstein reported having no disclosures. Three of his coauthors reported ties to industry.
Regorafenib carries high incremental cost and delivers small incremental benefit, providing low value for patients with metastatic colorectal cancer, researchers reported online Aug. 24 in the Journal of Clinical Oncology.
Compared with best supportive care, use of regorafenib resulted in a gain of 6 weeks of life, which dropped to 2 quality-adjusted weeks of life (0.04 quality-adjusted life years [QALYs]) in light of its significant toxicity profile. With the cost of treatment estimated between $32,000 and $43,000, depending on the dose, the incremental cost-effectiveness ratio (ICER) was $730,000-$980,000 per QALY, Dr. Daniel Goldstein and his associates reported (J Clin Oncol. 2015 Aug 24. doi: 10.1200/JCO.2014.61.9569).
“With increasing deductibles and copays, our patients are now bearing a significant burden of the cost of drugs,” wrote Dr. Goldstein of the department of hematology and medical oncology at Emory University, Atlanta, and his colleagues. There is a need, they wrote, for new strategies “in patient selection for clinical trials, guideline development, and payer coverage determination that address clinical value in addition to statistically significant clinical benefit.”
The CORRECT (Colorectal Cancer Treated With Regorafenib or Placebo After Failure of Standard Therapy) trial indicated an overall survival benefit of 1.4 months for regorafenib, compared with placebo, in patients with colorectal cancer who progressed after standard regimens. Adverse events of grade 3 or 4 occurred in 54% of the regorafenib arm, compared with 14% in the placebo arm.
The analysis based on the CORRECT data indicated a low likelihood that regorafenib would be considered cost effective at a willingness to pay threshold less than $600,000 per QALY. Regorafenib exceeds typical accepted values for cost-effectiveness.
Model parameters that most influenced ICER were regorafenib cost, probability of stopping treatment due to an adverse event, and baseline utility value. The ICER remained greater than $550,000 per QALY across a broad range of each of these variables.
Given the increasing amount of out-of-pocket costs for patients, the investigators recommend “a careful discussion between physicians and patients regarding the additional benefit and potential total drug cost before starting regorafenib.”
The study was supported by internal funds and by a grant from the National Institutes of Health. Dr. Goldstein reported having no disclosures. Three of his coauthors reported ties to industry.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: For treatment-refractory metastatic colorectal cancer, regorafenib provides low value at its current cost.
Major finding: Compared with supportive care, regorafenib produced a gain of 2 quality-adjusted life weeks (0.04 quality-adjusted life years), for an incremental cost-effectiveness ratio estimated at $730,000-$980,000 per QALY.
Data source: Analysis based on results from the CORRECT trial.
Disclosures: The study was supported by internal funds and by a grant from the National Institutes of Health. Dr. Goldstein reported having no disclosures. Three of his coauthors reported ties to industry.
Persistent HPV16 DNA in oral rinse signaled oropharyngeal cancer’s return
Patients with human papillomavirus–related oropharyngeal carcinoma (HPV-OPC) who had human papillomavirus type 16 (HPV16) detected in oral rinses both at diagnosis and at any time after treatment were more likely to have OPC recurrence, according to a report published online in JAMA Oncology.
Detection of HPV16 DNA was frequent among patients at diagnosis (67 of 124 patients), but rare posttreatment (6 of 124 patients). Persistent HPV16 detection (at and after diagnosis) was associated with greater risk of disease recurrence (hazard ratio, 29.7) and death (HR, 23.5).
Approximately 10%-25% of patients with HPV-OPC experience progression after treatment, and surgical salvage is the most favorable treatment in these cases. However, surgery is not feasible for the significant proportion of HPV-OPC cases that have already spread to distant sites at the time of diagnosis.
“There is a need for clinically relevant biomarkers of disease recurrence to facilitate timely initiation of aggressive diagnostic investigation and subsequent salvage treatment to potentially improve outcomes for the growing population of HPV-OPC survivors,” wrote Dr. Eleni M. Rettig of the department of otolaryngology–head and neck surgery, Johns Hopkins University, Baltimore, and her colleagues (JAMA Oncol. 2015 July 30 [doi:10.1001/jamaoncol.2015.2524]).
“Detection of recurrent local or locoregional disease prior to distant spread is particularly desirable given the favorable response of HPV-OPC to surgical salvage,” the study authors observed.
The multisite, prospective cohort study evaluated 124 patients with HPV-related oropharyngeal carcinoma who had at least one posttreatment oral rinse sample. Most patients with HPV16 DNA detected at diagnosis had none detected after treatment (62 of 67 patients). Contrary to persistent HPV16 DNA detection, HPV16 detected at diagnosis was not significantly associated with disease-free or overall survival.
Based on the six patients who had posttreatment detection of HPV16 DNA in oral rinses, the sensitivity and specificity of predicting recurrence at 9-12 months were 43% and 100%, respectively. Considering only local disease recurrence, the sensitivity and specificity were 100% and 100%, respectively.
The median time from the first posttreatment detection of HPV16 DNA to recurrence was 7.0 months. That “clinically meaningful lead time” is important in evaluating HPV16 DNA detection in oral rinses as “a valuable tool for long-term posttreatment surveillance of HPV-OPC for local recurrence,” the investigators concluded.
Dr. Rettig reported having no disclosures. Several coauthors reported ties to industry sources.
HPV16 DNA was detected in oral rinses in just 54% of patients with HPV-OPC at diagnosis. This low sensitivity in the presence of gross disease calls into question the test’s utility as a biomarker for subclinical disease. The positive predictive value (PPV) of 83%, calculated based on five of six patients who had persistent HPV16 DNA detection and disease recurrence, requires further analysis.
First, the persistence criteria immediately eliminates the 46% of patients who had HPV-OPC but tested negative for HPV16 DNA at diagnosis. Second, the intent of the biomarker is early detection of local or locoregional recurrence, but only two of the patients had local or locoregional recurrence prompting surgical salvage.
Detection of HPV16 DNA had a median lead time of 7 months, but clinical utility of the test requires some assumptions about the natural course of HPV-OPC. First, local or locoregional recurrence must precede the spread of disease to distant sites; second, earlier surgical salvage must prevent systemic reseeding; and finally, the oral rinse test must be robust enough to warrant salvage surgery in the absence of measurable disease.
The operating characteristics of the HPV16 DNA oral rinse test (e.g., low sensitivity and low confidence in PPV) preclude clinical adoption. However, the high negative predictive value of the test may be useful as criteria to scale back surveillance visits and/or costly imaging, particularly during prospective trials.
Dr. Julie E. Bauman is director of the head and neck cancer section in the division of hematology-oncology at the University of Pittsburgh. Dr. Robert L. Ferris is chief, division of head and neck surgery at the University of Pittsburgh. Dr. Bauman and Dr. Ferris reported no conflicts of interest. These comments were taken from their editorial accompanying the study (JAMA Oncol. 2015 July 30 [doi:10.1001/jamaoncol.2015.2606]).
HPV16 DNA was detected in oral rinses in just 54% of patients with HPV-OPC at diagnosis. This low sensitivity in the presence of gross disease calls into question the test’s utility as a biomarker for subclinical disease. The positive predictive value (PPV) of 83%, calculated based on five of six patients who had persistent HPV16 DNA detection and disease recurrence, requires further analysis.
First, the persistence criteria immediately eliminates the 46% of patients who had HPV-OPC but tested negative for HPV16 DNA at diagnosis. Second, the intent of the biomarker is early detection of local or locoregional recurrence, but only two of the patients had local or locoregional recurrence prompting surgical salvage.
Detection of HPV16 DNA had a median lead time of 7 months, but clinical utility of the test requires some assumptions about the natural course of HPV-OPC. First, local or locoregional recurrence must precede the spread of disease to distant sites; second, earlier surgical salvage must prevent systemic reseeding; and finally, the oral rinse test must be robust enough to warrant salvage surgery in the absence of measurable disease.
The operating characteristics of the HPV16 DNA oral rinse test (e.g., low sensitivity and low confidence in PPV) preclude clinical adoption. However, the high negative predictive value of the test may be useful as criteria to scale back surveillance visits and/or costly imaging, particularly during prospective trials.
Dr. Julie E. Bauman is director of the head and neck cancer section in the division of hematology-oncology at the University of Pittsburgh. Dr. Robert L. Ferris is chief, division of head and neck surgery at the University of Pittsburgh. Dr. Bauman and Dr. Ferris reported no conflicts of interest. These comments were taken from their editorial accompanying the study (JAMA Oncol. 2015 July 30 [doi:10.1001/jamaoncol.2015.2606]).
HPV16 DNA was detected in oral rinses in just 54% of patients with HPV-OPC at diagnosis. This low sensitivity in the presence of gross disease calls into question the test’s utility as a biomarker for subclinical disease. The positive predictive value (PPV) of 83%, calculated based on five of six patients who had persistent HPV16 DNA detection and disease recurrence, requires further analysis.
First, the persistence criteria immediately eliminates the 46% of patients who had HPV-OPC but tested negative for HPV16 DNA at diagnosis. Second, the intent of the biomarker is early detection of local or locoregional recurrence, but only two of the patients had local or locoregional recurrence prompting surgical salvage.
Detection of HPV16 DNA had a median lead time of 7 months, but clinical utility of the test requires some assumptions about the natural course of HPV-OPC. First, local or locoregional recurrence must precede the spread of disease to distant sites; second, earlier surgical salvage must prevent systemic reseeding; and finally, the oral rinse test must be robust enough to warrant salvage surgery in the absence of measurable disease.
The operating characteristics of the HPV16 DNA oral rinse test (e.g., low sensitivity and low confidence in PPV) preclude clinical adoption. However, the high negative predictive value of the test may be useful as criteria to scale back surveillance visits and/or costly imaging, particularly during prospective trials.
Dr. Julie E. Bauman is director of the head and neck cancer section in the division of hematology-oncology at the University of Pittsburgh. Dr. Robert L. Ferris is chief, division of head and neck surgery at the University of Pittsburgh. Dr. Bauman and Dr. Ferris reported no conflicts of interest. These comments were taken from their editorial accompanying the study (JAMA Oncol. 2015 July 30 [doi:10.1001/jamaoncol.2015.2606]).
Patients with human papillomavirus–related oropharyngeal carcinoma (HPV-OPC) who had human papillomavirus type 16 (HPV16) detected in oral rinses both at diagnosis and at any time after treatment were more likely to have OPC recurrence, according to a report published online in JAMA Oncology.
Detection of HPV16 DNA was frequent among patients at diagnosis (67 of 124 patients), but rare posttreatment (6 of 124 patients). Persistent HPV16 detection (at and after diagnosis) was associated with greater risk of disease recurrence (hazard ratio, 29.7) and death (HR, 23.5).
Approximately 10%-25% of patients with HPV-OPC experience progression after treatment, and surgical salvage is the most favorable treatment in these cases. However, surgery is not feasible for the significant proportion of HPV-OPC cases that have already spread to distant sites at the time of diagnosis.
“There is a need for clinically relevant biomarkers of disease recurrence to facilitate timely initiation of aggressive diagnostic investigation and subsequent salvage treatment to potentially improve outcomes for the growing population of HPV-OPC survivors,” wrote Dr. Eleni M. Rettig of the department of otolaryngology–head and neck surgery, Johns Hopkins University, Baltimore, and her colleagues (JAMA Oncol. 2015 July 30 [doi:10.1001/jamaoncol.2015.2524]).
“Detection of recurrent local or locoregional disease prior to distant spread is particularly desirable given the favorable response of HPV-OPC to surgical salvage,” the study authors observed.
The multisite, prospective cohort study evaluated 124 patients with HPV-related oropharyngeal carcinoma who had at least one posttreatment oral rinse sample. Most patients with HPV16 DNA detected at diagnosis had none detected after treatment (62 of 67 patients). Contrary to persistent HPV16 DNA detection, HPV16 detected at diagnosis was not significantly associated with disease-free or overall survival.
Based on the six patients who had posttreatment detection of HPV16 DNA in oral rinses, the sensitivity and specificity of predicting recurrence at 9-12 months were 43% and 100%, respectively. Considering only local disease recurrence, the sensitivity and specificity were 100% and 100%, respectively.
The median time from the first posttreatment detection of HPV16 DNA to recurrence was 7.0 months. That “clinically meaningful lead time” is important in evaluating HPV16 DNA detection in oral rinses as “a valuable tool for long-term posttreatment surveillance of HPV-OPC for local recurrence,” the investigators concluded.
Dr. Rettig reported having no disclosures. Several coauthors reported ties to industry sources.
Patients with human papillomavirus–related oropharyngeal carcinoma (HPV-OPC) who had human papillomavirus type 16 (HPV16) detected in oral rinses both at diagnosis and at any time after treatment were more likely to have OPC recurrence, according to a report published online in JAMA Oncology.
Detection of HPV16 DNA was frequent among patients at diagnosis (67 of 124 patients), but rare posttreatment (6 of 124 patients). Persistent HPV16 detection (at and after diagnosis) was associated with greater risk of disease recurrence (hazard ratio, 29.7) and death (HR, 23.5).
Approximately 10%-25% of patients with HPV-OPC experience progression after treatment, and surgical salvage is the most favorable treatment in these cases. However, surgery is not feasible for the significant proportion of HPV-OPC cases that have already spread to distant sites at the time of diagnosis.
“There is a need for clinically relevant biomarkers of disease recurrence to facilitate timely initiation of aggressive diagnostic investigation and subsequent salvage treatment to potentially improve outcomes for the growing population of HPV-OPC survivors,” wrote Dr. Eleni M. Rettig of the department of otolaryngology–head and neck surgery, Johns Hopkins University, Baltimore, and her colleagues (JAMA Oncol. 2015 July 30 [doi:10.1001/jamaoncol.2015.2524]).
“Detection of recurrent local or locoregional disease prior to distant spread is particularly desirable given the favorable response of HPV-OPC to surgical salvage,” the study authors observed.
The multisite, prospective cohort study evaluated 124 patients with HPV-related oropharyngeal carcinoma who had at least one posttreatment oral rinse sample. Most patients with HPV16 DNA detected at diagnosis had none detected after treatment (62 of 67 patients). Contrary to persistent HPV16 DNA detection, HPV16 detected at diagnosis was not significantly associated with disease-free or overall survival.
Based on the six patients who had posttreatment detection of HPV16 DNA in oral rinses, the sensitivity and specificity of predicting recurrence at 9-12 months were 43% and 100%, respectively. Considering only local disease recurrence, the sensitivity and specificity were 100% and 100%, respectively.
The median time from the first posttreatment detection of HPV16 DNA to recurrence was 7.0 months. That “clinically meaningful lead time” is important in evaluating HPV16 DNA detection in oral rinses as “a valuable tool for long-term posttreatment surveillance of HPV-OPC for local recurrence,” the investigators concluded.
Dr. Rettig reported having no disclosures. Several coauthors reported ties to industry sources.
FROM JAMA ONCOLOGY
Key clinical point: Persistent human papillomavirus type 16 (HPV16) DNA detected in posttreatment oral rinses, although infrequent, was associated with HPV-related oropharyngeal carcinoma.
Major finding: Detection of HPV16 DNA in oral rinses both at diagnosis and at any time after treatment was associated with increased risk of recurrence (hazard ratio, 29.7) and death (HR, 23.5).
Data source: The multisite, prospective cohort study evaluated 124 patients with HPV-related oropharyngeal carcinoma who had at least one posttreatment oral rinse sample.
Disclosures: Dr. Rettig reported having no disclosures. Several coauthors reported ties to industry sources.
Similar outcomes for salvage vs. planned surgery after chemoradiotherapy in esophageal cancer
For patients with esophageal cancer, salvage surgery after definitive chemoradiotherapy had similar mortality and morbidity rates, and similar survival outcomes, to the combination of neoadjuvant chemoradiation and planned surgery, according to a study published online in the Journal of Clinical Oncology.
Definitive chemoradiotherapy (dCRT) is an alternative to highly invasive surgical resection, which carries a significant rate of morbidity and mortality; however, recent data indicate that 50% of patients with complete response to dCRT experience tumor recurrence.
“Our study demonstrated a similar survival and recurrence pattern for the SALV [salvage surgery after definitive chemoradiotherapy] and NCRS [neoadjuvant chemoradiation and planned surgery] groups, potentially validating an approach of dCRT with reserved SALV for persistent or recurrent disease. Importantly, there were no differences in oncologic safety of surgery, including extent of nodal dissection, between the SALV and NCRS groups,” wrote Dr. Sheraz Markar, a clinical research fellow from Imperial College, London, and colleagues (Journ. Clin. Onc. 2015 July 20 [doi:10.1200/JCO.2014.59.9092]).
The retrospective study compared 308 patients with esophageal cancer who underwent SALV with 540 patients who received NCRS at European centers from 2000 to 2010. After a median follow up of 54 months, the SALV and NCRS groups had similar rates of 3-year overall survival (43.3% vs. 40.1% ) and disease-free survival (39.2% vs. 32.8%). The two groups also had similar rates tumor recurrence: overall (46.8% vs. 47.9%), locoregional (18.8% vs. 15.9%), distant (24.3% vs. 28.1%) and mixed (13.0% vs. 13.5%).
The SALV and NCRS groups had similar rates of in-hospital mortality (8.4% vs. 9.3%) and morbidity (63.6% vs. 58.9%), but SALV patients had significantly more complications from anastomotic leak (17.2% vs. 10.7%) and surgical site infection (18.5% vs. 12.2%).
Subset analysis of the SALV group showed that patients who received a total radiation dose ≥ 55 Gy (compared with SALV patients who received a lower dose) had significantly increased in-hospital mortality (27.8% vs. 4.3%; P < .001), overall morbidity (75.9% vs. 61%; P = .039), anastomotic leak (27.8% vs. 15%; P = .023), surgical site infection (29.6% vs. 16.1%; P = .02), and pulmonary complications (55.6% vs. 40.2%; P = .038).
“Currently, there is no evidence in terms of locoregional control or survival benefit to support a high total radiation dose (> 50 Gy) in patients receiving dCRT,” according to the researchers, who noted that the findings suggest, “an upper threshold of 50 Gy should be used in these patients to optimize the benefits of dCRT without compromising the safety of SALV, if required.”
Patients who underwent SALV at high-volume centers had significantly lower rates of in-hospital mortality (6.3% vs. 16.2%; P = .009) and overall morbidity (58.8% vs. 80.9%; P = .001) compared with procedures done at low-volume centers.
Compared with recurrent disease, patients with persistent disease after dCRT had poorer long-term prognoses, suggesting a more aggressive tumor biology. Early identification of CRT-resistant tumors to allow early surgical treatment is an important area for future investigation, the investigators said.
For patients with esophageal cancer, salvage surgery after definitive chemoradiotherapy had similar mortality and morbidity rates, and similar survival outcomes, to the combination of neoadjuvant chemoradiation and planned surgery, according to a study published online in the Journal of Clinical Oncology.
Definitive chemoradiotherapy (dCRT) is an alternative to highly invasive surgical resection, which carries a significant rate of morbidity and mortality; however, recent data indicate that 50% of patients with complete response to dCRT experience tumor recurrence.
“Our study demonstrated a similar survival and recurrence pattern for the SALV [salvage surgery after definitive chemoradiotherapy] and NCRS [neoadjuvant chemoradiation and planned surgery] groups, potentially validating an approach of dCRT with reserved SALV for persistent or recurrent disease. Importantly, there were no differences in oncologic safety of surgery, including extent of nodal dissection, between the SALV and NCRS groups,” wrote Dr. Sheraz Markar, a clinical research fellow from Imperial College, London, and colleagues (Journ. Clin. Onc. 2015 July 20 [doi:10.1200/JCO.2014.59.9092]).
The retrospective study compared 308 patients with esophageal cancer who underwent SALV with 540 patients who received NCRS at European centers from 2000 to 2010. After a median follow up of 54 months, the SALV and NCRS groups had similar rates of 3-year overall survival (43.3% vs. 40.1% ) and disease-free survival (39.2% vs. 32.8%). The two groups also had similar rates tumor recurrence: overall (46.8% vs. 47.9%), locoregional (18.8% vs. 15.9%), distant (24.3% vs. 28.1%) and mixed (13.0% vs. 13.5%).
The SALV and NCRS groups had similar rates of in-hospital mortality (8.4% vs. 9.3%) and morbidity (63.6% vs. 58.9%), but SALV patients had significantly more complications from anastomotic leak (17.2% vs. 10.7%) and surgical site infection (18.5% vs. 12.2%).
Subset analysis of the SALV group showed that patients who received a total radiation dose ≥ 55 Gy (compared with SALV patients who received a lower dose) had significantly increased in-hospital mortality (27.8% vs. 4.3%; P < .001), overall morbidity (75.9% vs. 61%; P = .039), anastomotic leak (27.8% vs. 15%; P = .023), surgical site infection (29.6% vs. 16.1%; P = .02), and pulmonary complications (55.6% vs. 40.2%; P = .038).
“Currently, there is no evidence in terms of locoregional control or survival benefit to support a high total radiation dose (> 50 Gy) in patients receiving dCRT,” according to the researchers, who noted that the findings suggest, “an upper threshold of 50 Gy should be used in these patients to optimize the benefits of dCRT without compromising the safety of SALV, if required.”
Patients who underwent SALV at high-volume centers had significantly lower rates of in-hospital mortality (6.3% vs. 16.2%; P = .009) and overall morbidity (58.8% vs. 80.9%; P = .001) compared with procedures done at low-volume centers.
Compared with recurrent disease, patients with persistent disease after dCRT had poorer long-term prognoses, suggesting a more aggressive tumor biology. Early identification of CRT-resistant tumors to allow early surgical treatment is an important area for future investigation, the investigators said.
For patients with esophageal cancer, salvage surgery after definitive chemoradiotherapy had similar mortality and morbidity rates, and similar survival outcomes, to the combination of neoadjuvant chemoradiation and planned surgery, according to a study published online in the Journal of Clinical Oncology.
Definitive chemoradiotherapy (dCRT) is an alternative to highly invasive surgical resection, which carries a significant rate of morbidity and mortality; however, recent data indicate that 50% of patients with complete response to dCRT experience tumor recurrence.
“Our study demonstrated a similar survival and recurrence pattern for the SALV [salvage surgery after definitive chemoradiotherapy] and NCRS [neoadjuvant chemoradiation and planned surgery] groups, potentially validating an approach of dCRT with reserved SALV for persistent or recurrent disease. Importantly, there were no differences in oncologic safety of surgery, including extent of nodal dissection, between the SALV and NCRS groups,” wrote Dr. Sheraz Markar, a clinical research fellow from Imperial College, London, and colleagues (Journ. Clin. Onc. 2015 July 20 [doi:10.1200/JCO.2014.59.9092]).
The retrospective study compared 308 patients with esophageal cancer who underwent SALV with 540 patients who received NCRS at European centers from 2000 to 2010. After a median follow up of 54 months, the SALV and NCRS groups had similar rates of 3-year overall survival (43.3% vs. 40.1% ) and disease-free survival (39.2% vs. 32.8%). The two groups also had similar rates tumor recurrence: overall (46.8% vs. 47.9%), locoregional (18.8% vs. 15.9%), distant (24.3% vs. 28.1%) and mixed (13.0% vs. 13.5%).
The SALV and NCRS groups had similar rates of in-hospital mortality (8.4% vs. 9.3%) and morbidity (63.6% vs. 58.9%), but SALV patients had significantly more complications from anastomotic leak (17.2% vs. 10.7%) and surgical site infection (18.5% vs. 12.2%).
Subset analysis of the SALV group showed that patients who received a total radiation dose ≥ 55 Gy (compared with SALV patients who received a lower dose) had significantly increased in-hospital mortality (27.8% vs. 4.3%; P < .001), overall morbidity (75.9% vs. 61%; P = .039), anastomotic leak (27.8% vs. 15%; P = .023), surgical site infection (29.6% vs. 16.1%; P = .02), and pulmonary complications (55.6% vs. 40.2%; P = .038).
“Currently, there is no evidence in terms of locoregional control or survival benefit to support a high total radiation dose (> 50 Gy) in patients receiving dCRT,” according to the researchers, who noted that the findings suggest, “an upper threshold of 50 Gy should be used in these patients to optimize the benefits of dCRT without compromising the safety of SALV, if required.”
Patients who underwent SALV at high-volume centers had significantly lower rates of in-hospital mortality (6.3% vs. 16.2%; P = .009) and overall morbidity (58.8% vs. 80.9%; P = .001) compared with procedures done at low-volume centers.
Compared with recurrent disease, patients with persistent disease after dCRT had poorer long-term prognoses, suggesting a more aggressive tumor biology. Early identification of CRT-resistant tumors to allow early surgical treatment is an important area for future investigation, the investigators said.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: As management for esophageal cancer, salvage esophagectomy after definitive chemoradiotherapy (SALV) produced similar outcomes to the combination of neoadjuvant chemoradiation and planned surgery (NCRS).
Major finding: The SALV and NCRS groups had similar rates of 3-year overall survival (43.3% vs. 40.1% ) and disease-free survival (39.2% vs. 32.8%), tumor recurrence (46.8% vs. 47.9%), and in-hospital mortality (8.4% vs. 9.3%) and morbidity (63.6% vs. 58.9%).
Data source: Retrospective analysis of 848 patients (308 SALV, 540 NCRS) who underwent surgical resection for esophageal cancer in French-speaking European centers from 2000 to 2010.
Disclosures: Dr. Markar reported having no disclosures. Two of his coauthors reported ties to industry.
Hereditary cancer syndromes highly prevalent in young adults with colorectal cancer
Patients diagnosed with colorectal cancer at a younger age are more likely to have hereditary cancer syndromes, according to a study published online in the Journal of Clinical Oncology.
About one-third of patients who were diagnosed with colorectal cancer (CRC) at age 35 years or younger had a hereditary cancer syndrome, which is much higher than the 2%-5% rate seen in the general CRC population.
“Our results support a referral to genetic counseling for hereditary cancer syndromes for all patients diagnosed with CRC at 35 years or younger, regardless of family history of CRC, even when patients have normal MSI [microsatellite instability] and IHC [immunohistochemistry] tumor study results,” wrote Maureen Mork and colleagues from the University of Texas MD Anderson Cancer, Houston (J. Clin. Oncol. 2015 July 20).
Many of the patients with hereditary syndromes had no family history of CRC, and they represented both de novo mutations and incomplete penetrance. Among 67 patients in the hereditary group, about 23 were diagnosed with Lynch syndrome (LS), 22 with mutation-negative LS, and 20 presented with a familial adenomatous polyposis (FAP) phenotype.
Patients without a hereditary syndrome had more frequent left-sided colon tumors, metastatic disease, and poor differentiation, compared with the hereditary group. Differences in disease stage at diagnosis may have resulted from increased surveillance among those with family history of disease.
Revised Bethesda guidelines recommend testing patients for LS, but the authors support broader evaluation because the hereditary syndromes observed in the study were not limited to LS. Adolescent and young adult patients with CRC may benefit from having “comprehensive hereditary CRC genetic testing panels, especially in the absence of a clinical phenotype or with normal MSI and IHC tumor studies,” they wrote.

|
| Dr. Barbara Jung |
Early-age colorectal cancer is on the rise and, commonly, no genetic syndrome can be found. However, this study is a reminder that comprehensive genetic testing and counseling should be undertaken before coming to that conclusion. While Lynch syndrome is rare in MSI-negative tumors, it can occur and when missed not only dramatically affects the management of the patient, but also presents a missed opportunity to detect affected relatives.
The field of cancer genetics is evolving rapidly, and diagnostic tests are vastly expanding. When to order what test and, most importantly, how to interpret results as complex as genetic panels need expert involvement more than ever. It needs to be emphasized, however, that the majority of early cancers that do not meet criteria for a hereditary syndrome will not fit into any known genetic category. Nevertheless, this paper reinforces the notion that if a genetic cancer syndrome is suspected despite the lack of family history or other typical risk stratification, refer or seek advice early. This will also allow for us to better understand young patients with aggressive colon cancer outside of genetic syndromes.
Dr. Barbara Jung is associate professor of medicine, chief of the division of gastroenterology and hepatology, University of Illinois at Chicago. She has no conflicts of interest.

|
| Dr. Barbara Jung |
Early-age colorectal cancer is on the rise and, commonly, no genetic syndrome can be found. However, this study is a reminder that comprehensive genetic testing and counseling should be undertaken before coming to that conclusion. While Lynch syndrome is rare in MSI-negative tumors, it can occur and when missed not only dramatically affects the management of the patient, but also presents a missed opportunity to detect affected relatives.
The field of cancer genetics is evolving rapidly, and diagnostic tests are vastly expanding. When to order what test and, most importantly, how to interpret results as complex as genetic panels need expert involvement more than ever. It needs to be emphasized, however, that the majority of early cancers that do not meet criteria for a hereditary syndrome will not fit into any known genetic category. Nevertheless, this paper reinforces the notion that if a genetic cancer syndrome is suspected despite the lack of family history or other typical risk stratification, refer or seek advice early. This will also allow for us to better understand young patients with aggressive colon cancer outside of genetic syndromes.
Dr. Barbara Jung is associate professor of medicine, chief of the division of gastroenterology and hepatology, University of Illinois at Chicago. She has no conflicts of interest.

|
| Dr. Barbara Jung |
Early-age colorectal cancer is on the rise and, commonly, no genetic syndrome can be found. However, this study is a reminder that comprehensive genetic testing and counseling should be undertaken before coming to that conclusion. While Lynch syndrome is rare in MSI-negative tumors, it can occur and when missed not only dramatically affects the management of the patient, but also presents a missed opportunity to detect affected relatives.
The field of cancer genetics is evolving rapidly, and diagnostic tests are vastly expanding. When to order what test and, most importantly, how to interpret results as complex as genetic panels need expert involvement more than ever. It needs to be emphasized, however, that the majority of early cancers that do not meet criteria for a hereditary syndrome will not fit into any known genetic category. Nevertheless, this paper reinforces the notion that if a genetic cancer syndrome is suspected despite the lack of family history or other typical risk stratification, refer or seek advice early. This will also allow for us to better understand young patients with aggressive colon cancer outside of genetic syndromes.
Dr. Barbara Jung is associate professor of medicine, chief of the division of gastroenterology and hepatology, University of Illinois at Chicago. She has no conflicts of interest.
Patients diagnosed with colorectal cancer at a younger age are more likely to have hereditary cancer syndromes, according to a study published online in the Journal of Clinical Oncology.
About one-third of patients who were diagnosed with colorectal cancer (CRC) at age 35 years or younger had a hereditary cancer syndrome, which is much higher than the 2%-5% rate seen in the general CRC population.
“Our results support a referral to genetic counseling for hereditary cancer syndromes for all patients diagnosed with CRC at 35 years or younger, regardless of family history of CRC, even when patients have normal MSI [microsatellite instability] and IHC [immunohistochemistry] tumor study results,” wrote Maureen Mork and colleagues from the University of Texas MD Anderson Cancer, Houston (J. Clin. Oncol. 2015 July 20).
Many of the patients with hereditary syndromes had no family history of CRC, and they represented both de novo mutations and incomplete penetrance. Among 67 patients in the hereditary group, about 23 were diagnosed with Lynch syndrome (LS), 22 with mutation-negative LS, and 20 presented with a familial adenomatous polyposis (FAP) phenotype.
Patients without a hereditary syndrome had more frequent left-sided colon tumors, metastatic disease, and poor differentiation, compared with the hereditary group. Differences in disease stage at diagnosis may have resulted from increased surveillance among those with family history of disease.
Revised Bethesda guidelines recommend testing patients for LS, but the authors support broader evaluation because the hereditary syndromes observed in the study were not limited to LS. Adolescent and young adult patients with CRC may benefit from having “comprehensive hereditary CRC genetic testing panels, especially in the absence of a clinical phenotype or with normal MSI and IHC tumor studies,” they wrote.
Patients diagnosed with colorectal cancer at a younger age are more likely to have hereditary cancer syndromes, according to a study published online in the Journal of Clinical Oncology.
About one-third of patients who were diagnosed with colorectal cancer (CRC) at age 35 years or younger had a hereditary cancer syndrome, which is much higher than the 2%-5% rate seen in the general CRC population.
“Our results support a referral to genetic counseling for hereditary cancer syndromes for all patients diagnosed with CRC at 35 years or younger, regardless of family history of CRC, even when patients have normal MSI [microsatellite instability] and IHC [immunohistochemistry] tumor study results,” wrote Maureen Mork and colleagues from the University of Texas MD Anderson Cancer, Houston (J. Clin. Oncol. 2015 July 20).
Many of the patients with hereditary syndromes had no family history of CRC, and they represented both de novo mutations and incomplete penetrance. Among 67 patients in the hereditary group, about 23 were diagnosed with Lynch syndrome (LS), 22 with mutation-negative LS, and 20 presented with a familial adenomatous polyposis (FAP) phenotype.
Patients without a hereditary syndrome had more frequent left-sided colon tumors, metastatic disease, and poor differentiation, compared with the hereditary group. Differences in disease stage at diagnosis may have resulted from increased surveillance among those with family history of disease.
Revised Bethesda guidelines recommend testing patients for LS, but the authors support broader evaluation because the hereditary syndromes observed in the study were not limited to LS. Adolescent and young adult patients with CRC may benefit from having “comprehensive hereditary CRC genetic testing panels, especially in the absence of a clinical phenotype or with normal MSI and IHC tumor studies,” they wrote.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Patients age 35 years and younger who have colorectal cancer have a high frequency of hereditary cancer syndromes.
Major finding: In total, 34.7% of the adolescent and young adult population had a hereditary cancer syndrome, compared with 2%-5% of the general colorectal cancer population.
Data source: From 2009 to 2013, data from 193 adolescents and young adults with colorectal cancer were collected, including clinicopathologic information, tumor and genetic testing, and family history.
Disclosures: The authors disclosed no potential conflicts of interest.
Pomalidomide increases overall survival in multiple myeloma
In patients with multiple myeloma refractory to lenalidomide, pomalidomide administered in a lower-dose, continuous schedule resulted in overall and event-free survival similar to that of intermittent dosing, which is approved by the Food and Drug Administration, according to a report published in Blood.
Both regimens led to rapid activation of innate and adaptive immunity and benefitted expected survival in the heavily pretreated patient population. The cohort given intermittent dosing had a mean 54% reduction in measurable disease, compared with 28% for the cohort given continuous dosing (P = .02); both cohorts had similar event-free survival (4.3 vs. 5.3 months) and overall survival (21.7 vs. 17.7 months).
“In our study, although the 21/28-day schedule led to a greater reduction in measurable disease (although the differences in overall response rate did not reach statistical significance), it also led to a greater incidence of grade 3/4 treatment-related adverse events. Taken together, in our view, these data do not suggest a clear advantage for either regimen based on clinical parameters alone,” wrote Dr. Kartik Sehgal of Yale University, New Haven, Conn., and his colleagues (Blood 2015 June 25 [doi:10.1001/jamaoncol.2015.2010]).
The prospective phase II trial randomized 39 patients, who had received at least two (median four) prior therapies including lenalidomide, to receive pomalidomide in continuous (2 mg/day for 28/28 days) or intermittent (4 mg/day for 21/28 days) dosing schedules. All patients received dexamethasone at 40 mg weekly, starting with cycle two.
The most common adverse effect was myelosuppression, and the pattern of specific toxicities was similar in the two groups. Patients in the intermittent dosing cohort experienced more grade 3/4 adverse effects than the continuous dosing cohort (90% vs. 58%; P = .03).
Both pomalidomide and lenalidomide share the same cellular target (cereblon), and pharmacodynamic studies of the two pomalidomide regimens sought to clarify the mechanism of pomalidomide activity in lenalidomide-refractory multiple myeloma. The data suggest that pomalidomide affects the tumor microenvironment and immune cells. These effects may be amplified by blockade of immune checkpoints, the authors noted.
“Optimizing the immune effects of pomalidomide/lenalidomide on the tumor microenvironment with combination therapies may enhance their therapeutic potential in MM [multiple myeloma] and other cancers,” the authors wrote.
In patients with multiple myeloma refractory to lenalidomide, pomalidomide administered in a lower-dose, continuous schedule resulted in overall and event-free survival similar to that of intermittent dosing, which is approved by the Food and Drug Administration, according to a report published in Blood.
Both regimens led to rapid activation of innate and adaptive immunity and benefitted expected survival in the heavily pretreated patient population. The cohort given intermittent dosing had a mean 54% reduction in measurable disease, compared with 28% for the cohort given continuous dosing (P = .02); both cohorts had similar event-free survival (4.3 vs. 5.3 months) and overall survival (21.7 vs. 17.7 months).
“In our study, although the 21/28-day schedule led to a greater reduction in measurable disease (although the differences in overall response rate did not reach statistical significance), it also led to a greater incidence of grade 3/4 treatment-related adverse events. Taken together, in our view, these data do not suggest a clear advantage for either regimen based on clinical parameters alone,” wrote Dr. Kartik Sehgal of Yale University, New Haven, Conn., and his colleagues (Blood 2015 June 25 [doi:10.1001/jamaoncol.2015.2010]).
The prospective phase II trial randomized 39 patients, who had received at least two (median four) prior therapies including lenalidomide, to receive pomalidomide in continuous (2 mg/day for 28/28 days) or intermittent (4 mg/day for 21/28 days) dosing schedules. All patients received dexamethasone at 40 mg weekly, starting with cycle two.
The most common adverse effect was myelosuppression, and the pattern of specific toxicities was similar in the two groups. Patients in the intermittent dosing cohort experienced more grade 3/4 adverse effects than the continuous dosing cohort (90% vs. 58%; P = .03).
Both pomalidomide and lenalidomide share the same cellular target (cereblon), and pharmacodynamic studies of the two pomalidomide regimens sought to clarify the mechanism of pomalidomide activity in lenalidomide-refractory multiple myeloma. The data suggest that pomalidomide affects the tumor microenvironment and immune cells. These effects may be amplified by blockade of immune checkpoints, the authors noted.
“Optimizing the immune effects of pomalidomide/lenalidomide on the tumor microenvironment with combination therapies may enhance their therapeutic potential in MM [multiple myeloma] and other cancers,” the authors wrote.
In patients with multiple myeloma refractory to lenalidomide, pomalidomide administered in a lower-dose, continuous schedule resulted in overall and event-free survival similar to that of intermittent dosing, which is approved by the Food and Drug Administration, according to a report published in Blood.
Both regimens led to rapid activation of innate and adaptive immunity and benefitted expected survival in the heavily pretreated patient population. The cohort given intermittent dosing had a mean 54% reduction in measurable disease, compared with 28% for the cohort given continuous dosing (P = .02); both cohorts had similar event-free survival (4.3 vs. 5.3 months) and overall survival (21.7 vs. 17.7 months).
“In our study, although the 21/28-day schedule led to a greater reduction in measurable disease (although the differences in overall response rate did not reach statistical significance), it also led to a greater incidence of grade 3/4 treatment-related adverse events. Taken together, in our view, these data do not suggest a clear advantage for either regimen based on clinical parameters alone,” wrote Dr. Kartik Sehgal of Yale University, New Haven, Conn., and his colleagues (Blood 2015 June 25 [doi:10.1001/jamaoncol.2015.2010]).
The prospective phase II trial randomized 39 patients, who had received at least two (median four) prior therapies including lenalidomide, to receive pomalidomide in continuous (2 mg/day for 28/28 days) or intermittent (4 mg/day for 21/28 days) dosing schedules. All patients received dexamethasone at 40 mg weekly, starting with cycle two.
The most common adverse effect was myelosuppression, and the pattern of specific toxicities was similar in the two groups. Patients in the intermittent dosing cohort experienced more grade 3/4 adverse effects than the continuous dosing cohort (90% vs. 58%; P = .03).
Both pomalidomide and lenalidomide share the same cellular target (cereblon), and pharmacodynamic studies of the two pomalidomide regimens sought to clarify the mechanism of pomalidomide activity in lenalidomide-refractory multiple myeloma. The data suggest that pomalidomide affects the tumor microenvironment and immune cells. These effects may be amplified by blockade of immune checkpoints, the authors noted.
“Optimizing the immune effects of pomalidomide/lenalidomide on the tumor microenvironment with combination therapies may enhance their therapeutic potential in MM [multiple myeloma] and other cancers,” the authors wrote.
FROM BLOOD
Key clinical point: In patients with multiple myeloma, both continuous and intermittent dosing regimens of pomalidomide had similar favorable impacts on overall and event-free survival, and led to rapid activation of innate and adaptive immunity.
Major finding: The intermittent dosing cohort had a mean 54% reduction in measurable disease, compared with 28% for the continuous dosing cohort (P = .02); both cohorts had similar event-free (4.3 vs. 5.3 months) and overall (21.7 vs. 17.7 months) survival.
Data source: This prospective randomized phase II trial evaluated 39 patients who had received at least two (median four) prior therapies including lenalidomide.
Disclosures: Dr. Seghal reported having no disclosures. Some of his coauthors are employed by or consult for Celgene, the makers of pomalidomide (Pomalyst).
Doxorubicin, radiation doses predict heart risk in lymphoma survivors
Adult lymphoma survivors who were treated with autologous hematopoietic stem-cell transplantation had a greater than sixfold increased risk of left ventricular systolic dysfunction compared with controls, according to a study published online in the Journal of Clinical Oncology.
Among 274 adult survivors of Hodgkin or non-Hodgkin lymphoma, 16% had left ventricular systolic dysfunction (LVSD): 11% had overt heart failure (HF) and 5% had asymptomatic LVSD, defined as a left ventricular ejection fraction of less than 50%.Heart symptoms were significantly associated with exposure to doxorubicin at a cumulative dose of 300 mg/m2 or more and with cardiac radiation therapy of more than 30 Gy. Recognizing these patient risk factors allows for more intensive follow-up with the goal of “identification and early treatment of asymptomatic LVSD [which] may prevent the development of HF,” wrote Dr. Klaus Murbraech of Oslo University Hospital and his colleagues (J. Clin. Oncol. 2015 July 13 [doi:10.1200/JCO.2015.60.8125]).
The investigators observed no association between lower-dose cardiac radiation therapy and LVSD. There was only a marginally significant association between the presence of two or more traditional cardiovascular disease risk factors and LVSD.
The cross-sectional multicenter cohort study is the first to assess the prevalence of LVSD, according to Dr. Murbraech and his colleagues. The study included adult survivors of Hodgkin or non-Hodgkin lymphoma, median age 56 years, who underwent autologous stem-cell transplants in Norway from 1987 to 2008. The median observation time was 13 years (range, 4-34 years). The control group consisted of initially healthy patients in an echocardiographic follow-up study. Controls were matched to patients based on age, sex, systolic blood pressure, and body mass index.
The study was supported by the South-Eastern Norway Regional Health Authority and Extrastiftelsen. Dr. Murbraech reported having no disclosures.
Adult lymphoma survivors who were treated with autologous hematopoietic stem-cell transplantation had a greater than sixfold increased risk of left ventricular systolic dysfunction compared with controls, according to a study published online in the Journal of Clinical Oncology.
Among 274 adult survivors of Hodgkin or non-Hodgkin lymphoma, 16% had left ventricular systolic dysfunction (LVSD): 11% had overt heart failure (HF) and 5% had asymptomatic LVSD, defined as a left ventricular ejection fraction of less than 50%.Heart symptoms were significantly associated with exposure to doxorubicin at a cumulative dose of 300 mg/m2 or more and with cardiac radiation therapy of more than 30 Gy. Recognizing these patient risk factors allows for more intensive follow-up with the goal of “identification and early treatment of asymptomatic LVSD [which] may prevent the development of HF,” wrote Dr. Klaus Murbraech of Oslo University Hospital and his colleagues (J. Clin. Oncol. 2015 July 13 [doi:10.1200/JCO.2015.60.8125]).
The investigators observed no association between lower-dose cardiac radiation therapy and LVSD. There was only a marginally significant association between the presence of two or more traditional cardiovascular disease risk factors and LVSD.
The cross-sectional multicenter cohort study is the first to assess the prevalence of LVSD, according to Dr. Murbraech and his colleagues. The study included adult survivors of Hodgkin or non-Hodgkin lymphoma, median age 56 years, who underwent autologous stem-cell transplants in Norway from 1987 to 2008. The median observation time was 13 years (range, 4-34 years). The control group consisted of initially healthy patients in an echocardiographic follow-up study. Controls were matched to patients based on age, sex, systolic blood pressure, and body mass index.
The study was supported by the South-Eastern Norway Regional Health Authority and Extrastiftelsen. Dr. Murbraech reported having no disclosures.
Adult lymphoma survivors who were treated with autologous hematopoietic stem-cell transplantation had a greater than sixfold increased risk of left ventricular systolic dysfunction compared with controls, according to a study published online in the Journal of Clinical Oncology.
Among 274 adult survivors of Hodgkin or non-Hodgkin lymphoma, 16% had left ventricular systolic dysfunction (LVSD): 11% had overt heart failure (HF) and 5% had asymptomatic LVSD, defined as a left ventricular ejection fraction of less than 50%.Heart symptoms were significantly associated with exposure to doxorubicin at a cumulative dose of 300 mg/m2 or more and with cardiac radiation therapy of more than 30 Gy. Recognizing these patient risk factors allows for more intensive follow-up with the goal of “identification and early treatment of asymptomatic LVSD [which] may prevent the development of HF,” wrote Dr. Klaus Murbraech of Oslo University Hospital and his colleagues (J. Clin. Oncol. 2015 July 13 [doi:10.1200/JCO.2015.60.8125]).
The investigators observed no association between lower-dose cardiac radiation therapy and LVSD. There was only a marginally significant association between the presence of two or more traditional cardiovascular disease risk factors and LVSD.
The cross-sectional multicenter cohort study is the first to assess the prevalence of LVSD, according to Dr. Murbraech and his colleagues. The study included adult survivors of Hodgkin or non-Hodgkin lymphoma, median age 56 years, who underwent autologous stem-cell transplants in Norway from 1987 to 2008. The median observation time was 13 years (range, 4-34 years). The control group consisted of initially healthy patients in an echocardiographic follow-up study. Controls were matched to patients based on age, sex, systolic blood pressure, and body mass index.
The study was supported by the South-Eastern Norway Regional Health Authority and Extrastiftelsen. Dr. Murbraech reported having no disclosures.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Lymphoma survivors treated with autologous hematopoietic stem-cell transplantation (auto-HSC) had a significantly higher risk of left ventricular systolic dysfunction than did controls.
Major finding: Treatment with at least 300 mg/m2 cumulative of doxorubicin and with over 30 Gy of cardiac radiation therapy were independent risk factors for LVSD.
Data source: A cross-sectional multicenter cohort study of 274 Hodgkin or non-Hodgkin lymphoma survivors.
Disclosures: Supported by the South-Eastern Norway Regional Health Authority and Extrastiftelsen. Dr. Murbraech reported having no disclosures.
Gene expression profiles help identify metastasis in primary cutaneous melanoma
In patients presenting with cutaneous melanoma, use of molecular data can help determine whether there is a high likelihood of nodal metastasis that warrants sentinel lymph node biopsy, according to a report published online in the Journal of Clinical Oncology.
A large number of sentinel lymph node (SLN) biopsies could be avoided if nodal metastasis in patients with primary cutaneous melanoma were better identified at the time of diagnosis, according to Dr. Alexander Meves of the department of dermatology at the Mayo Clinic in Rochester, Minn., and colleagues.
“In this study, we found that molecular data in combination with Breslow depth, tumor ulceration, and patient age were useful for discriminating between primary cutaneous melanomas that had or had not metastasized to SLN,” they wrote (J. Clin. Oncol. 2015 July 6 [doi:10.1200/JCO.2014.60.7002]).
Based on samples from 160 patients that included benign nevi and primary skin melanomas with and without SLN metastasis, investigators identified genes differentially expressed between metastatic and nonmetastatic pigmented skin lesions, and found a cluster of genes associated with integrin cell adhesion. Of particular interest, the integrin cell adhesion receptor, beta-3 integrin (ITGB3), was upregulated in regionally metastatic melanoma.
Information from a cohort of 360 patients, including 74 (20.6%) with biopsy-confirmed nodal metastasis, was used to derive prediction models for SLN metastasis based on clinicopathologic factors alone and in combination with molecular data. Younger age, tumor ulceration, and greater Breslow depth contributed to the clinicopathologic model. The combined model added expression data from four genes: ITGB3, cellular tumor antigen p53 (TP53), laminin B1 chain (LAMB1), and tissue-type plasminogen activator (PLAT; protein name, t-PA). Area under the receiver operating characteristic (ROC) curve for the combined clinicopathologic plus molecular model was 0.89, compared with 0.78 for the clinicopathologic model alone (P < .001). Performance of the models on the validation cohort (n = 146) was similar, with the area under the ROC curve at 0.93. Using a 10% cutoff, the false-positive rate was 22% and the false-negative rate was 0%.
Expression data combined with clinicopathologic features can be used to calculate the predicted probability of SLN positivity at the time of primary diagnosis, which has “the potential to improve patient care by avoiding unnecessary SLN procedures,” the authors wrote.
In patients presenting with cutaneous melanoma, use of molecular data can help determine whether there is a high likelihood of nodal metastasis that warrants sentinel lymph node biopsy, according to a report published online in the Journal of Clinical Oncology.
A large number of sentinel lymph node (SLN) biopsies could be avoided if nodal metastasis in patients with primary cutaneous melanoma were better identified at the time of diagnosis, according to Dr. Alexander Meves of the department of dermatology at the Mayo Clinic in Rochester, Minn., and colleagues.
“In this study, we found that molecular data in combination with Breslow depth, tumor ulceration, and patient age were useful for discriminating between primary cutaneous melanomas that had or had not metastasized to SLN,” they wrote (J. Clin. Oncol. 2015 July 6 [doi:10.1200/JCO.2014.60.7002]).
Based on samples from 160 patients that included benign nevi and primary skin melanomas with and without SLN metastasis, investigators identified genes differentially expressed between metastatic and nonmetastatic pigmented skin lesions, and found a cluster of genes associated with integrin cell adhesion. Of particular interest, the integrin cell adhesion receptor, beta-3 integrin (ITGB3), was upregulated in regionally metastatic melanoma.
Information from a cohort of 360 patients, including 74 (20.6%) with biopsy-confirmed nodal metastasis, was used to derive prediction models for SLN metastasis based on clinicopathologic factors alone and in combination with molecular data. Younger age, tumor ulceration, and greater Breslow depth contributed to the clinicopathologic model. The combined model added expression data from four genes: ITGB3, cellular tumor antigen p53 (TP53), laminin B1 chain (LAMB1), and tissue-type plasminogen activator (PLAT; protein name, t-PA). Area under the receiver operating characteristic (ROC) curve for the combined clinicopathologic plus molecular model was 0.89, compared with 0.78 for the clinicopathologic model alone (P < .001). Performance of the models on the validation cohort (n = 146) was similar, with the area under the ROC curve at 0.93. Using a 10% cutoff, the false-positive rate was 22% and the false-negative rate was 0%.
Expression data combined with clinicopathologic features can be used to calculate the predicted probability of SLN positivity at the time of primary diagnosis, which has “the potential to improve patient care by avoiding unnecessary SLN procedures,” the authors wrote.
In patients presenting with cutaneous melanoma, use of molecular data can help determine whether there is a high likelihood of nodal metastasis that warrants sentinel lymph node biopsy, according to a report published online in the Journal of Clinical Oncology.
A large number of sentinel lymph node (SLN) biopsies could be avoided if nodal metastasis in patients with primary cutaneous melanoma were better identified at the time of diagnosis, according to Dr. Alexander Meves of the department of dermatology at the Mayo Clinic in Rochester, Minn., and colleagues.
“In this study, we found that molecular data in combination with Breslow depth, tumor ulceration, and patient age were useful for discriminating between primary cutaneous melanomas that had or had not metastasized to SLN,” they wrote (J. Clin. Oncol. 2015 July 6 [doi:10.1200/JCO.2014.60.7002]).
Based on samples from 160 patients that included benign nevi and primary skin melanomas with and without SLN metastasis, investigators identified genes differentially expressed between metastatic and nonmetastatic pigmented skin lesions, and found a cluster of genes associated with integrin cell adhesion. Of particular interest, the integrin cell adhesion receptor, beta-3 integrin (ITGB3), was upregulated in regionally metastatic melanoma.
Information from a cohort of 360 patients, including 74 (20.6%) with biopsy-confirmed nodal metastasis, was used to derive prediction models for SLN metastasis based on clinicopathologic factors alone and in combination with molecular data. Younger age, tumor ulceration, and greater Breslow depth contributed to the clinicopathologic model. The combined model added expression data from four genes: ITGB3, cellular tumor antigen p53 (TP53), laminin B1 chain (LAMB1), and tissue-type plasminogen activator (PLAT; protein name, t-PA). Area under the receiver operating characteristic (ROC) curve for the combined clinicopathologic plus molecular model was 0.89, compared with 0.78 for the clinicopathologic model alone (P < .001). Performance of the models on the validation cohort (n = 146) was similar, with the area under the ROC curve at 0.93. Using a 10% cutoff, the false-positive rate was 22% and the false-negative rate was 0%.
Expression data combined with clinicopathologic features can be used to calculate the predicted probability of SLN positivity at the time of primary diagnosis, which has “the potential to improve patient care by avoiding unnecessary SLN procedures,” the authors wrote.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Molecular data combined with clinicopathologic variables better discriminated between primary cutaneous melanomas that had or had not metastasized to sentinel lymph nodes than clinicopathologic features alone.
Major finding: Area under the ROC curve for the combined clinicopathologic plus molecular model was 0.89, compared with 0.78 for the clinicopathologic model alone (P < .001).
Data source: Differential gene expression analysis by next-generation sequencing was carried out on 160 patient samples that included benign nevi and primary skin melanomas with and without SLN metastasis. Model generation included data from 360 melanomas and validation from 146 melanomas.
Disclosures: Dr. Meves reported having no disclosures. Two of his coauthors reported ties to industry sources.
Adolescent cancer survivors report more emotional, neurocognitive impairment than do siblings
Adult survivors of cancer who were diagnosed between the ages of 11 and 21 years self-reported higher rates of impairment in emotional and neurocognitive outcomes compared with their sibling counterparts, according to researchers.
Compared with siblings, survivors reported greater anxiety (odds ratio [OR], 2.0; 95% CI, 1.17-3.43), somatization (2.36; 1.55-3.60), and depression (1.55; 1.04-2.30). Higher rates of neurocognitive problems included task efficiency (1.72; 1.21-2.43), emotional regulation (1.74; 1.26-2.40), and memory (1.44; 1.09-1.89). Survivors were significantly less likely to be employed (P < .001).
Previous reports have shown that survivors of childhood cancers have increased risk for impaired neurocognitive functioning, but this is the first study to examine outcomes in survivors who were diagnosed during adolescence and early young adulthood.
“Cancer treatment during this time has the potential to interfere with adolescents’ separation from caregivers, autonomy with regard to planning social and academic schedules, participation in social activities, and maintaining privacy, particularly of their bodies,” wrote Dr. Pinki Prasad, assistant professor of pediatrics at Louisiana State University, New Orleans.
Survivors who were diagnosed with CNS tumors or leukemia during adolescence reported rates of emotional distress and neurocognitive dysfunction similar to rates of those diagnosed during early childhood, whereas diagnoses of lymphoma/sarcoma during adolescence resulted in lower risk of impairment compared with early childhood diagnoses. This may be due to the fact that the leukemia/CNS tumor group was more likely to receive cranial radiation therapy, a predictor of neurocognitive late effects, Dr. Prasad and colleagues wrote (J. Clin. Oncol. 2015 July 6 [doi:10.1200/JCO.2014.57.7528]).
Among those diagnosed with lymphomas or sarcomas during adolescence, treatment with corticosteroids was associated with greater risk of self-reported difficulties with somatization, anxiety, task efficiency, and memory.
The Childhood Cancer Survivor Study (CCSS) is a multicenter, retrospective cohort study that comprised 2,589 survivors who were diagnosed from 1970 through 1986 when they were between the ages 11 and 21 years, and 360 sibling counterparts. Participants completed the Brief Symptom Inventory–18, which measures symptoms of emotional distress, and the CCSS Neurocognitive Questionnaire.
The authors noted that the results indicate “high rates of self-reported impairment in neurocognitive function and psychological distress that are associated with limitation in development of adult social milestones,” and that further follow-up with survivors of adolescent and early young adult cancers may be necessary.
Adult survivors of cancer who were diagnosed between the ages of 11 and 21 years self-reported higher rates of impairment in emotional and neurocognitive outcomes compared with their sibling counterparts, according to researchers.
Compared with siblings, survivors reported greater anxiety (odds ratio [OR], 2.0; 95% CI, 1.17-3.43), somatization (2.36; 1.55-3.60), and depression (1.55; 1.04-2.30). Higher rates of neurocognitive problems included task efficiency (1.72; 1.21-2.43), emotional regulation (1.74; 1.26-2.40), and memory (1.44; 1.09-1.89). Survivors were significantly less likely to be employed (P < .001).
Previous reports have shown that survivors of childhood cancers have increased risk for impaired neurocognitive functioning, but this is the first study to examine outcomes in survivors who were diagnosed during adolescence and early young adulthood.
“Cancer treatment during this time has the potential to interfere with adolescents’ separation from caregivers, autonomy with regard to planning social and academic schedules, participation in social activities, and maintaining privacy, particularly of their bodies,” wrote Dr. Pinki Prasad, assistant professor of pediatrics at Louisiana State University, New Orleans.
Survivors who were diagnosed with CNS tumors or leukemia during adolescence reported rates of emotional distress and neurocognitive dysfunction similar to rates of those diagnosed during early childhood, whereas diagnoses of lymphoma/sarcoma during adolescence resulted in lower risk of impairment compared with early childhood diagnoses. This may be due to the fact that the leukemia/CNS tumor group was more likely to receive cranial radiation therapy, a predictor of neurocognitive late effects, Dr. Prasad and colleagues wrote (J. Clin. Oncol. 2015 July 6 [doi:10.1200/JCO.2014.57.7528]).
Among those diagnosed with lymphomas or sarcomas during adolescence, treatment with corticosteroids was associated with greater risk of self-reported difficulties with somatization, anxiety, task efficiency, and memory.
The Childhood Cancer Survivor Study (CCSS) is a multicenter, retrospective cohort study that comprised 2,589 survivors who were diagnosed from 1970 through 1986 when they were between the ages 11 and 21 years, and 360 sibling counterparts. Participants completed the Brief Symptom Inventory–18, which measures symptoms of emotional distress, and the CCSS Neurocognitive Questionnaire.
The authors noted that the results indicate “high rates of self-reported impairment in neurocognitive function and psychological distress that are associated with limitation in development of adult social milestones,” and that further follow-up with survivors of adolescent and early young adult cancers may be necessary.
Adult survivors of cancer who were diagnosed between the ages of 11 and 21 years self-reported higher rates of impairment in emotional and neurocognitive outcomes compared with their sibling counterparts, according to researchers.
Compared with siblings, survivors reported greater anxiety (odds ratio [OR], 2.0; 95% CI, 1.17-3.43), somatization (2.36; 1.55-3.60), and depression (1.55; 1.04-2.30). Higher rates of neurocognitive problems included task efficiency (1.72; 1.21-2.43), emotional regulation (1.74; 1.26-2.40), and memory (1.44; 1.09-1.89). Survivors were significantly less likely to be employed (P < .001).
Previous reports have shown that survivors of childhood cancers have increased risk for impaired neurocognitive functioning, but this is the first study to examine outcomes in survivors who were diagnosed during adolescence and early young adulthood.
“Cancer treatment during this time has the potential to interfere with adolescents’ separation from caregivers, autonomy with regard to planning social and academic schedules, participation in social activities, and maintaining privacy, particularly of their bodies,” wrote Dr. Pinki Prasad, assistant professor of pediatrics at Louisiana State University, New Orleans.
Survivors who were diagnosed with CNS tumors or leukemia during adolescence reported rates of emotional distress and neurocognitive dysfunction similar to rates of those diagnosed during early childhood, whereas diagnoses of lymphoma/sarcoma during adolescence resulted in lower risk of impairment compared with early childhood diagnoses. This may be due to the fact that the leukemia/CNS tumor group was more likely to receive cranial radiation therapy, a predictor of neurocognitive late effects, Dr. Prasad and colleagues wrote (J. Clin. Oncol. 2015 July 6 [doi:10.1200/JCO.2014.57.7528]).
Among those diagnosed with lymphomas or sarcomas during adolescence, treatment with corticosteroids was associated with greater risk of self-reported difficulties with somatization, anxiety, task efficiency, and memory.
The Childhood Cancer Survivor Study (CCSS) is a multicenter, retrospective cohort study that comprised 2,589 survivors who were diagnosed from 1970 through 1986 when they were between the ages 11 and 21 years, and 360 sibling counterparts. Participants completed the Brief Symptom Inventory–18, which measures symptoms of emotional distress, and the CCSS Neurocognitive Questionnaire.
The authors noted that the results indicate “high rates of self-reported impairment in neurocognitive function and psychological distress that are associated with limitation in development of adult social milestones,” and that further follow-up with survivors of adolescent and early young adult cancers may be necessary.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Adult cancer survivors diagnosed between ages 11 and 21 years self-reported higher rates of emotional distress and neurocognitive dysfunction compared with sibling counterparts.
Major finding: Compared with siblings, survivors reported greater anxiety (odds ratio, 2.0; 95% CI, 1.17-3.43), somatization (2.36; 1.55-3.60), and depression (1.55; 1.04-2.30). Higher rates of neurocognitive problems included task efficiency (1.72; 1.21-2.43), emotional regulation (1.74; 1.26-2.40), and memory (1.44; 1.09-1.89).
Data source: The Childhood Cancer Survivor Study, a multicenter, retrospective cohort study of 2,589 survivors diagnosed from 1970 through 1986 when they were between the ages 11 and 21 years, and 360 sibling counterparts.
Disclosures: The National Cancer Institute supported the study. Dr. Prasad reported having no disclosures.