User login
Pregnancy-Induced Hypertension
Hypertensive disorders represent one of the most common medical complications of pregnancy.1,2 Based on a nationwide inpatient sample examining more than 36 million deliveries in the United States, the prevalence of associated hypertensive disorders increased from 67.2 per 1,000 deliveries in 1998 to 83.4 per 1,000 deliveries in 2006.3Pregnancy-induced hypertension (also referred to as gestational hypertension or hypertensive disorder of pregnancy)4-6 is estimated to affect 6% to 8% of US pregnancies.1,2
Women who develop severe hypertension during pregnancy may experience adverse effects similar to those associated with mild preeclampsia.2,7,8 In the mother, these may range from elevated liver enzymes to renal dysfunction; and in the fetus, from preterm delivery to intrauterine restriction of fetal growth.7,8
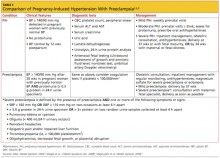
This article will review the risk factors, clinical presentation, diagnosis, and management of pregnancy-induced hypertension. A brief discussion of preeclampsia as it relates to gestational hypertension will be included (see Table 12,6,9).
Classification, Definitions
Pregnancy-induced hypertension (PIH) is classified as mild or severe. Mild PIH is defined as new-onset hypertension (systolic blood pressure ≥ 140 mm Hg and/or diastolic blood pressure ≥ 90 mm Hg), occurring after 20 weeks’ gestation. The majority of cases of mild PIH develop beyond 37 weeks’ gestation, and in these cases, pregnancy outcomes are comparable to those of normotensive pregnancies.2,7,8
Severe PIH is defined as sustained elevated blood pressures of ≥ 160 mm Hg systolic and ≥ 110 mm Hg diastolic. In prospective cohort studies in which calcium supplementation and low-dose aspirin use were being investigated for prevention of preeclampsia in healthy pregnant women, those who were severely hypertensive were found to be at increased risk for certain maternal comorbidities (eg, cesarean delivery, renal dysfunction, elevated liver enzymes, placental abruption) and perinatal morbidities (delivery before 37 weeks’ gestation, low birth weight, fetal growth restriction, and neonatal ICU admission), compared with patients who were normotensive or mildly hypertensive.7,8
The diagnosis of PIH may later be amended or replaced by one of the following diagnoses: preeclampsia, if proteinuria (to be defined and discussed later) develops; chronic hypertension, if blood pressure remains elevated past 12 weeks postpartum; or transient hypertension of pregnancy, if blood pressure normalizes by 12 weeks postpartum.5,6,10
Pathophysiology and Risk Factors
Although the pathophysiology of PIH is not well understood, the pathogenesis of preeclampsia likely involves abnormalities in the development, implantation, or perfusion of the placenta, and often leads to impaired maternal organ function.6,11 It is not clear whether PIH and preeclampsia are two different diseases that share a manifestation of elevated blood pressure or whether PIH represents an early stage of preeclampsia.4,12 However, women with preexisting hypertension, especially severe hypertension, are at increased risk for preeclampsia, placental abruption, and fetal growth restriction.2
There are some similarities and some distinct differences among the clinical features and risk factors associated with PIH, compared with those of preeclampsia. Risk factors for PIH include a pre-pregnancy BMI of 25 or greater, PIH and/or preeclampsia in previous pregnancies, and history of renal disease, cardiac disease, or diabetes. The most important risk factors for preeclampsia include preexisting diabetes or nephropathy, chronic hypertension, PIH or preeclampsia in a previous pregnancy, maternal age younger than 18 or older than 34, African-American ethnicity, first pregnancy, multiple pregnancy, history of preeclampsia in the patient’s mother or sister, obesity, autoimmune disease, and an interval between pregnancies longer than 10 years.4-6,13-15
The risk for preeclampsia in patients with PIH is approximately 15% to 25%12,16; according to Magee et al,6 35% of women with PIH onset before 37 weeks’ gestation develop preeclampsia.6,12,17 The risk for recurrence of PIH in subsequent pregnancies is about 26%, whereas women who experience preeclampsia in one pregnancy have a comparable risk for PIH or preeclampsia (about 14% each) in subsequent pregnancies.18
Clinical Presentation and Diagnostic Evaluation
Blood pressure should be measured and recorded at every prenatal visit, using the correct-sized cuff, with the patient in a seated position.5 Gestational hypertension is a clinical diagnosis confirmed by at least two accurate blood pressure measurements in the same arm in women without proteinuria, with readings of ≥ 140 mm Hg systolic and/or ≥ 90 mm Hg diastolic. It should then be determined whether the patient’s hypertension is mild or severe (ie, blood pressure > 160/110 mm Hg). The patient with severe PIH should be evaluated for signs of preeclampsia, as discussed below.
Patients with mild PIH are often asymptomatic, and the diagnosis is made at a prenatal visit as a result of routine blood pressure monitoring; this is one of many reasons to encourage early and regular prenatal care. Blood pressure may be higher at night in hypertensive disorders of pregnancy.10
In contrast to patients with mild PIH, the clinical presentation of those with severe PIH or preeclampsia (and the potential for impending eclampsia) may include the following symptoms and signs:
- Generalized edema, including that of the face and hands
- Rapid weight gain
- Blurred vision or scotomata (ie, areas of diminished vision in the visual field)
- Severe, throbbing or pounding headaches
- Epigastric or right upper quadrant pain
- Oliguria (urinary output < 500 mL/d)
- Nausea, with or without vomiting
- Hyperactive reflexes
- Chest pain or tightness
- Shortness of breath.2,6,14
Medical History
Important questions to address in the patient’s medical history relate to risk factors for PIH, such as a history of renal disease, cardiac disease, or diabetes, previous history of PIH and/or preeclampsia, and abuse of cocaine or amphetamines—in addition to the specific aforementioned symptoms and signs of severe preeclampsia.5,6
Physical Examination
The clinician performing the physical exam should be attentive to accurate blood pressure measurements and any signs that suggest preeclampsia. Weight should be measured and BMI calculated at each prenatal visit.
If the patient’s blood pressure is markedly elevated, the focused physical examination should include an ophthalmologic examination for jaundice and for evidence of hypertensive retinopathy or papilledema; pulmonary and cardiac examination; abdominal examination, including palpation of the liver; examination of the face and extremities for edema; and a complete neurologic examination, including assessment of deep tendon reflexes and examination for clonus.
Laboratory Testing
In patients with PIH, laboratory evaluation should be focused to rule out preeclampsia. The potential for proteinuria (defined as ≥ 0.3 g/d in a 24-hour urine sample1,14) must be investigated at diagnosis and at regular visits during the pregnancy.1 At least two random urine samples, collected at least 6 hours apart, should be evaluated for protein. A spot (random) urine sample with a result of 2+ protein or greater is highly suggestive of proteinuria; a 24-hour urine collection is the gold standard by which such findings should be confirmed and protein levels in the urine quantified.1,14
Elevated blood pressure and proteinuria are the hallmarks of preeclampsia.6 Patients affected by these developments must be evaluated for signs and symptoms of severe preeclampsia. However, those with only mild elevations in blood pressure and little or no proteinuria may complain of sudden-onset throbbing or pounding headache, blurry vision, and severe epigastric pain—possibly indicating severe preeclampsia.5,10
In addition to laboratory evaluation for urinary protein excretion, the following tests are recommended by the American College of Obstetricians and Gynecologists (ACOG)14 to assess for end organ involvement, which is consistent with severe preeclampsia:
- Hematocrit, which may be either high, to suggest hemoconcentration; or low, indicating hemolysis
- Platelet count, which is normal in women with PIH and low in those with severe preeclampsia; if results are abnormal, this test should be followed by coagulation testing (international normalized ratio, activated partial thromboplastin time, fibrinogen)
- Renal function testing (blood urea nitrogen and creatinine may be elevated in severe preeclampsia), and random urine testing for proteinuria, as explained earlier
- Liver enzymes (which are elevated in severe preeclampsia), and
- Lactate dehydrogenase (which is elevated in severe preeclampsia).1,14
Additionally, researchers conducting a small cohort study (n = 163) reported in 2009 that in women with PIH, serum uric acid levels exceeding 309 µmol/L were predictive of preeclampsia, with 87.7% sensitivity and 93.3% specificity.19 An increase from first-trimester serum uric acid levels was also a strong prognostic factor for preeclampsia. Earlier this year, a Canadian investigative team reported an increased risk for premature birth (odds ratio, 3.2) and small infant size for gestational age (odds ratio, 2.5) in women with PIH and hyperuricemia.20 While the predictive value of uric acid has been debated to some extent,14 measurement is often included in the workup of patients with hypertensive pregnancies.5,6
The frequency of prenatal visits, laboratory testing, and fetal monitoring should be adjusted according to the severity of PIH. In mildly hypertensive patients, the general recommendation is urine and blood testing at weekly prenatal visits.14 Fetal well-being must be monitored regularly, although neither the type nor frequency of such testing has been well established. Generally, patients should be advised to count daily fetal movements, and they should be scheduled for either a nonstress test (NST) or a biophysical profile as soon as a diagnosis of PIH is made.1,2,6,14
According to a 2010 guidance from the United Kingdom’s National Institute for Health and Clinical Excellence (NICE),13,21 pregnant women with mild to moderate hypertension should undergo an initial ultrasonographic assessment of fetal growth and amniotic fluid volume at the time of diagnosis, then serially every 3 to 4 weeks. If results from initial fetal testing are normal, patients with mild PIH do not require repeat testing after 34 weeks’ gestation, unless conditions change (eg, preeclampsia, worsening hypertension, and/or change in fetal movements).1,2,14 The NICE guidelines also recommend umbilical artery Doppler velocimetry.13,21
In patients with severe PIH, an NST ultrasound assessment of fetal growth and amniotic fluid volume and umbilical artery Doppler velocimetry should be performed at diagnosis to evaluate for placental dysfunction.6,21 If all test results are normal, the ultrasound and umbilical artery Doppler velocimetry need not be repeated more frequently than every two weeks, and the NST no more than once per week.13
Treatment/Management and Follow-Up
Regular prenatal monitoring to assess for worsening of PIH and/or development of preeclampsia is key to management. Figure 12 outlines an algorithm for managing PIH, which is guided by the severity of the condition. Patients with mild PIH can be managed with weekly outpatient visits and assessed for signs and symptoms of preeclampsia, monitoring of fetal movements, weight, blood pressure measurements, and urine and blood tests.2
At each visit, it is important to instruct patients to report immediately any of the following symptoms: new-onset severe headache, visual changes, epigastric or right upper quadrant pain, nausea or vomiting, difficulty breathing or chest tightness, as well as vaginal bleeding, decreased fetal movements, or uterine contractions.6,14
Generally, expectant management with delivery at term is recommended for women with mild PIH.2 Vaginal delivery (or cesarean delivery, if indicated) is recommended at 37 weeks or when fetal maturity is confirmed; and at 34 weeks if fetal or maternal distress is evident.2,6
Findings from the Hypertension and Preeclampsia Intervention Trial At Term (HYPITAT),22 an open-label, randomized clinical trial in women with PIH or mild preeclampsia, suggested an association between induction of labor between 36 and 41 weeks’ gestation and improved maternal outcomes (specifically, reduced risk for severe hypertension), compared with expectant management. Similarly, in a literature review by Caughey et al,23 results from nine randomized controlled trials indicated a reduced risk for cesarean delivery in women who underwent induction of labor, compared with expectant management. Rates of “successful” induction of labor (ie, procedures resulting in vaginal rather than cesarean delivery) were greater in women with higher parity, a favorable cervix, and earlier gestational age.
Based on data from the HYPITAT trial,22 a cost-effectiveness analysis of induction of labor compared with expectant management revealed an 11% reduction in the average cost in delivery that followed induction of labor, compared with expectant management, in women with PIH or mild preeclampsia.24 Caughey et al23 reported similar savings, particularly when induction of labor was performed at 41 weeks’ gestation.
If induction of labor is being considered in a woman with an unfavorable cervix, administration of prostaglandins is recommended to enhance cervical ripening.6
Medication
Pregnant women should be advised to discontinue previously prescribed ACE inhibitors, angiotensin receptor blockers, or thiazide diuretics, which are associated with congenital abnormalities, intrauterine growth restriction, and/or neonatal nephropathy.5,6,13,21
Antihypertensive medication is not recommended for women with mild to moderate PIH, as it does not appear to improve outcomes. Evidence was found insufficient in a 2007 Cochrane review to determine the potential impact of antihypertensive medications for treatment of mild to moderate PIH on clinical outcomes such as preterm birth, infant mortality, and infant size relative to gestational age.25 A similar review conducted in 2011, with primary outcomes that included severe preeclampsia, eclampsia, and maternal death or perinatal death, concluded only that further study was needed to determine how tightly blood pressure must be controlled to improve maternal and fetal outcomes in patients with PIH.26
ACOG14 recommends antihypertensive therapy (eg, hydralazine, labetalol) only for women with diastolic blood pressure of 105 to 110 mm Hg or higher.1,2 There are several recommendations from different organizations regarding the choice of antihypertensive medications for PIH. In the UK’s NICE guidance,21 it is recommended that patients with moderate to severe PIH take oral labetalol as first-line treatment to keep systolic blood pressure below 150 mm Hg and diastolic blood pressure between 80 and 100 mm Hg.
Like severe preeclampsia, severe PIH should be managed in an inpatient setting.6,14 IV labetalol or hydralazine is recommended to lower the blood pressure to less than 160/110 mm Hg, although current evidence is insufficient to identify a target blood pressure.6,26 A 2002 ACOG practice bulletin recommends one of the following:
- Hydralazine 5 to 10 mg IV every 15 to 20 minutes until the desired response is achieved; or
- Labetalol 20 mg IV bolus, followed by 40 mg if not effective within 10 minutes, then 80 mg every 10 minutes with maximum total dose of 220 mg.1,14
In women who have severe PIH or who develop severe preeclampsia or eclampsia, magnesium sulfate is administered to prevent or treat seizures.14 This agent should be used during labor and for at least 24 hours postpartum.2 Dosing of magnesium sulfate for this indication is 4 g IV bolus, followed by infusion of 1 g/h. It is important to monitor treated patients for signs of toxicity, including muscle weakness, loss of patellar reflexes, hypoventilation, pulmonary edema, hypotension, and bradycardia. IV calcium gluconate should be readily available for use as an antidote to life-threatening hypermagnesemia.27,28
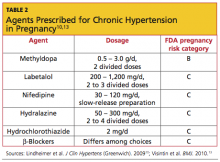
For chronic hypertension in pregnancy, the American Society of Hypertension10 has recommended several agents. There is no consensus on which medication is most appropriate (see Table 210,13).
Patient Education
Patient education is an important aspect of caring for women with PIH. The American Academy of Family Physicians29,30 provides a comprehensive patient education resource that defines the hypertensive disorders in pregnancy, explains the symptoms and signs of severe hypertension or preeclampsia, and describes appropriate diagnostic tests, monitoring, and treatment options. (See http://familydoctor.org/familydoctor/en/diseases-conditions/pregnancy-induced-hypertension.printerview.all.html.)
Patients who are considering pregnancy should be counseled to maintain a healthy weight prior to and during pregnancy. Adequate dietary calcium can reduce the risk for PIH, and calcium supplementation has been shown to reduce the risk for preeclampsia, especially in women at high risk.31 The Society of Obstetricians and Gynaecologists of Canada recommends low-dose aspirin (75 mg/d) at bedtime for high-risk women, starting before pregnancy, or upon diagnosis of pregnancy to prevent preeclampsia.6 Although there is insufficient evidence to recommend dietary salt restriction, excess salt can increase fluid retention and possibly blood pressure. Patients should be urged to attend all scheduled prenatal visits and to review the warning signs and symptoms of severe hypertension and preeclampsia at each visit.
Mode of delivery may be discussed and will depend on the severity of hypertension, presence of preeclampsia, and fetal well-being. Vaginal delivery at term is considered optimal unless there are indications for cesarean delivery. Induction of labor may be considered at term in patients with PIH. Those with severe preeclampsia who may require preterm delivery must be prepared for potential issues associated with prematurity.
Follow-Up and Prognosis
Patients with PIH should be evaluated postpartum for persistent hypertension. Blood pressure in patients with PIH usually normalizes by day 7 postpartum.32 If blood pressure elevation persists past 12 weeks postpartum, the patient’s diagnosis is revised to chronic hypertension and managed accordingly.
In the patient with persistent hypertension who chooses breastfeeding, it is important to select an antihypertensive medication with low transfer into breast milk. Many β-adrenergic antagonists and calcium channel antagonists are considered “compatible” with breastfeeding by the American Academy of Pediatrics.33
In addition to the potential for recurrent PIH in subsequent pregnancies, women with PIH are at increased risk for hypertension later in life, and findings from several large cohort studies suggest increased cardiovascular risk in patients with hypertensive pregnancies.16,34 Magnussen et al,9 who followed more than 15,000 mothers of singleton infants for several years postpartum, found that those who experienced hypertensive disorders (particularly recurrent hypertensive disorders) during pregnancy were more likely than normotensive women to subsequently develop diabetes, dyslipidemia, and hypertension. Women who remained normotensive while pregnant generally had lower BMI measurements than those who experienced PIH or preeclampsia.
Conclusion
Hypertensive disorders commonly develop during pregnancy. It is important to diagnose and classify PIH during routine prenatal visits. Once the diagnosis is made, patients must be monitored closely for increasing blood pressure or development of preeclampsia. Urine protein testing is a key clinical test to detect preeclampsia, and positive findings on a random urine protein dipstick should be confirmed and quantified with a 24-hour urine collection.
In addition to undergoing frequent blood pressure measurements and urine protein tests, patients should be asked about signs and symptoms that suggest preeclampsia. Women with mild PIH can be managed as outpatients with prenatal visits at least weekly, followed by delivery at term. Severely hypertensive patients are managed in the hospital with antihypertensive medications and prompt delivery at 34 weeks’ gestation or beyond, should maternal or fetal distress become evident.
1. Report of the National High Blood Pressure Education Program Working Group on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183(1):S1-S22.
2. Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol. 2003;102(1):181-192.
3. Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009; 113(6):1299-1306.
4. Villar J, Carroli, G, Wojdyla D, et al; World Health Organization Antenatal Care Trial Research Group. Preeclampsia, gestational hypertension and intrauterine growth restriction, related or independent conditions. Am J Obstet Gynecol. 2006;194(4):921-931.
5. Leeman L, Fontaine P. Hypertensive disorders of pregnancy. Am Fam Physician. 2008;78(1):
93-100.
6. Magee LA, Helewa M, Moutquin JM, von Dadelszen P; Hypertension Guideline Committee; Strategic Training Initiative in Research in the Reproductive Health Sciences (STIRRHS) Scholars. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. J Obstet Gynaecol Can. 2008;30(3 suppl):S1-S48.
7. Buchbinder A, Sibai BM, Caritis S, et al. Adverse perinatal outcomes are significantly higher in severe gestational hypertension than in mild preeclampsia. Am J Obstet Gynecol. 2002;186(1):66-71.
8. Hauth JC, Ewell MG, Levine RJ, et al; Calcium for Preeclampsia Prevention Study Group. Pregnancy outcomes in healthy nulliparas who developed hypertension. Obstet Gynecol. 2000;95(1):24-28.
9. Magnussen EB, Vatten LJ, Smith GD, Romundstad PR. Hypertensive disorders in pregnancy and subsequently measured cardiovascular risk factors. Obstet Gynecol. 2009; 114(5):961-970.
10. Lindheimer MD, Taler SJ, Cunningham FG; American Society of Hypertension (ASH). ASH position paper: hypertension in pregnancy. J Clin Hypertens (Greenwich). 2009;11(4):214-225.
11. Roberts JM, Gammill HS. Preeclampsia: recent insights. Hypertension. 2005;46(6):
1243-1249.
12. Saudan P, Brown MA, Buddle ML, Jones M. Does gestational hypertension become pre-eclampsia? Br J Obstet Gynaecol. 1998;105 (11):1177-1184.
13. Visintin C, Mugglestone MA, Almerie MQ, et al; Guideline Development Group. Management of hypertensive disorders during pregnancy: summary of NICE guidance. BMJ. 2010;341:c2207.
14. ACOG Committee on Practice Bulletins—Obstetrics. Clinical Management Guidelines for Obstetrician–Gynecologists. Diagnosis and management of preeclampsia and eclampsia: ACOG practice bulletin No. 33. Obstet Gynecol. 2002;99:159-167.
15. Parazzini F, Bortolus R, Chatenoud L, et al; Italian Study of Aspirin in Pregnancy Group. Risk factors for pregnancy-induced hypertension in women at high risk for the condition. Epidemiology. 1996;7(3):306-308.
16. Hjartardottir S, Leifsson BG, Geirsson RT, Steinthorsdottir V. Recurrence of hypertensive disorder in second pregnancy. Am J Obstet Gynecol. 2006;194(4):916-920.
17. Barton JR, O’Brien JM, Bergauer NK, et al. Mild gestational hypertension remote from term: progression and outcome. Am J Obstet Gynecol. 2001;184(5):979-983.
18. Brown MA, Mackenzie C, Dunsmuir W, et al. Can we predict recurrence of pre-eclampsia or gestational hypertension? BJOG. 2007; 114(8):984-993.
19. Bellomo G, Venanzi S, Saronio P, et al. Prognostic significance of serum uric acid in women with gestational hypertension. Hypertension. 2011;58(4):704-708.
20. Hawkins TL, Roberts JM, Mangos GJ, et al. Plasma uric acid remains a marker of poor outcome in hypertensive pregnancy: a retrospective cohort study. BJOG. 2012;119(4):484-492.
21. National Institute for Health and Clinical Excellence. Hypertension in pregnancy: the management of hypertensive disorders during pregnancy. www.nice.org.uk/nicemedia/live/13098/50418/50418.pdf. Accessed April 16, 2012.
22. Koopmans CM, Bijlenga D, Groen H, et al. Induction of labour versus expectant monitoring for gestational hypertension or mild pre-eclampsia after 36 weeks’ gestation (HYPITAT): a multicentre, open-label randomised controlled trial. Lancet. 2009;374(9694):979-988.
23. Caughey AB, Sundaram V, Kaimal AJ, et al. Maternal and neonatal outcomes of elective induction of labor. Evid Rep Technol Assess (Full Rep). 2009;(176):1-257.
24. Shennan A, Hezelgrave N. An economic analysis of induction of labour and expectant monitoring in women with gestational hypertension or pre-eclampsia at term (HYPITAT trial). BJOG. 2010;117(13):1575-1576.
25. Abalos E, Duley L, Steyn DW, Henderson-Smart DJ. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2007 Jan 24;(1):CD002252.
26. Nabhan AF, Elsedawy MM. Tight control of mild-moderate pre-existing or non-proteinuric gestational hypertension. Cochrane Database Syst Rev. 2011 Jul 6;(7):CD006907.
27. Duley L, Gülmezoglu AM, Henderson-Smart DJ. Magnesium sulphate and other anticonvulsants for women with preeclampsia. Cochrane Database Syst Rev. 2003;(2):CD000025.
28. Kraft MD, Btaiche IF, Sacks GS, Kudsk KA. Treatment of electrolyte disorders in adult patients in the intensive care unit. Am J Health Syst Pharm. 2005;62(16):1663-1682.
29. FamilyDoctor.org. Pregnancy-induced hypertension (updated 2010). http://familydoctor.org/familydoctor/en/diseases-conditions/pregnancy-induced-hypertension.printerview
.all.html. Accessed April 16, 2012.
30. Zamorski MA, Green LA. NHBPEP report on high blood pressure in pregnancy: a summary for family physicians. Am Fam Physician. 2001; 64(2):263-270.
31. Hofmeyr GJ, Duley L, Atallah A. Dietary calcium supplementation for prevention of pre-eclampsia and related problems: a systematic review and commentary. BJOG. 2007;114(8): 933-943.
32. Ferrazzani S, De Carolis S, Pomini F, et al. The duration of hypertension in the puerperium of preeclamptic women: relationship with renal impairment and week of delivery. Am J Obstet Gynecol. 1994;171(2):506-512.
33. Beardmore KS, Morris JM, Gallery ED. Excretion of antihypertensive medication into human breast milk: a systematic review. Hypertens Pregnancy. 2002;21(1):85-95.
34. Wilson BJ, Watson MS, Prescott GJ, et al. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. BMJ. 2003;326(7394):845.
Hypertensive disorders represent one of the most common medical complications of pregnancy.1,2 Based on a nationwide inpatient sample examining more than 36 million deliveries in the United States, the prevalence of associated hypertensive disorders increased from 67.2 per 1,000 deliveries in 1998 to 83.4 per 1,000 deliveries in 2006.3Pregnancy-induced hypertension (also referred to as gestational hypertension or hypertensive disorder of pregnancy)4-6 is estimated to affect 6% to 8% of US pregnancies.1,2
Women who develop severe hypertension during pregnancy may experience adverse effects similar to those associated with mild preeclampsia.2,7,8 In the mother, these may range from elevated liver enzymes to renal dysfunction; and in the fetus, from preterm delivery to intrauterine restriction of fetal growth.7,8
This article will review the risk factors, clinical presentation, diagnosis, and management of pregnancy-induced hypertension. A brief discussion of preeclampsia as it relates to gestational hypertension will be included (see Table 12,6,9).
Classification, Definitions
Pregnancy-induced hypertension (PIH) is classified as mild or severe. Mild PIH is defined as new-onset hypertension (systolic blood pressure ≥ 140 mm Hg and/or diastolic blood pressure ≥ 90 mm Hg), occurring after 20 weeks’ gestation. The majority of cases of mild PIH develop beyond 37 weeks’ gestation, and in these cases, pregnancy outcomes are comparable to those of normotensive pregnancies.2,7,8
Severe PIH is defined as sustained elevated blood pressures of ≥ 160 mm Hg systolic and ≥ 110 mm Hg diastolic. In prospective cohort studies in which calcium supplementation and low-dose aspirin use were being investigated for prevention of preeclampsia in healthy pregnant women, those who were severely hypertensive were found to be at increased risk for certain maternal comorbidities (eg, cesarean delivery, renal dysfunction, elevated liver enzymes, placental abruption) and perinatal morbidities (delivery before 37 weeks’ gestation, low birth weight, fetal growth restriction, and neonatal ICU admission), compared with patients who were normotensive or mildly hypertensive.7,8
The diagnosis of PIH may later be amended or replaced by one of the following diagnoses: preeclampsia, if proteinuria (to be defined and discussed later) develops; chronic hypertension, if blood pressure remains elevated past 12 weeks postpartum; or transient hypertension of pregnancy, if blood pressure normalizes by 12 weeks postpartum.5,6,10
Pathophysiology and Risk Factors
Although the pathophysiology of PIH is not well understood, the pathogenesis of preeclampsia likely involves abnormalities in the development, implantation, or perfusion of the placenta, and often leads to impaired maternal organ function.6,11 It is not clear whether PIH and preeclampsia are two different diseases that share a manifestation of elevated blood pressure or whether PIH represents an early stage of preeclampsia.4,12 However, women with preexisting hypertension, especially severe hypertension, are at increased risk for preeclampsia, placental abruption, and fetal growth restriction.2
There are some similarities and some distinct differences among the clinical features and risk factors associated with PIH, compared with those of preeclampsia. Risk factors for PIH include a pre-pregnancy BMI of 25 or greater, PIH and/or preeclampsia in previous pregnancies, and history of renal disease, cardiac disease, or diabetes. The most important risk factors for preeclampsia include preexisting diabetes or nephropathy, chronic hypertension, PIH or preeclampsia in a previous pregnancy, maternal age younger than 18 or older than 34, African-American ethnicity, first pregnancy, multiple pregnancy, history of preeclampsia in the patient’s mother or sister, obesity, autoimmune disease, and an interval between pregnancies longer than 10 years.4-6,13-15
The risk for preeclampsia in patients with PIH is approximately 15% to 25%12,16; according to Magee et al,6 35% of women with PIH onset before 37 weeks’ gestation develop preeclampsia.6,12,17 The risk for recurrence of PIH in subsequent pregnancies is about 26%, whereas women who experience preeclampsia in one pregnancy have a comparable risk for PIH or preeclampsia (about 14% each) in subsequent pregnancies.18
Clinical Presentation and Diagnostic Evaluation
Blood pressure should be measured and recorded at every prenatal visit, using the correct-sized cuff, with the patient in a seated position.5 Gestational hypertension is a clinical diagnosis confirmed by at least two accurate blood pressure measurements in the same arm in women without proteinuria, with readings of ≥ 140 mm Hg systolic and/or ≥ 90 mm Hg diastolic. It should then be determined whether the patient’s hypertension is mild or severe (ie, blood pressure > 160/110 mm Hg). The patient with severe PIH should be evaluated for signs of preeclampsia, as discussed below.
Patients with mild PIH are often asymptomatic, and the diagnosis is made at a prenatal visit as a result of routine blood pressure monitoring; this is one of many reasons to encourage early and regular prenatal care. Blood pressure may be higher at night in hypertensive disorders of pregnancy.10
In contrast to patients with mild PIH, the clinical presentation of those with severe PIH or preeclampsia (and the potential for impending eclampsia) may include the following symptoms and signs:
- Generalized edema, including that of the face and hands
- Rapid weight gain
- Blurred vision or scotomata (ie, areas of diminished vision in the visual field)
- Severe, throbbing or pounding headaches
- Epigastric or right upper quadrant pain
- Oliguria (urinary output < 500 mL/d)
- Nausea, with or without vomiting
- Hyperactive reflexes
- Chest pain or tightness
- Shortness of breath.2,6,14
Medical History
Important questions to address in the patient’s medical history relate to risk factors for PIH, such as a history of renal disease, cardiac disease, or diabetes, previous history of PIH and/or preeclampsia, and abuse of cocaine or amphetamines—in addition to the specific aforementioned symptoms and signs of severe preeclampsia.5,6
Physical Examination
The clinician performing the physical exam should be attentive to accurate blood pressure measurements and any signs that suggest preeclampsia. Weight should be measured and BMI calculated at each prenatal visit.
If the patient’s blood pressure is markedly elevated, the focused physical examination should include an ophthalmologic examination for jaundice and for evidence of hypertensive retinopathy or papilledema; pulmonary and cardiac examination; abdominal examination, including palpation of the liver; examination of the face and extremities for edema; and a complete neurologic examination, including assessment of deep tendon reflexes and examination for clonus.
Laboratory Testing
In patients with PIH, laboratory evaluation should be focused to rule out preeclampsia. The potential for proteinuria (defined as ≥ 0.3 g/d in a 24-hour urine sample1,14) must be investigated at diagnosis and at regular visits during the pregnancy.1 At least two random urine samples, collected at least 6 hours apart, should be evaluated for protein. A spot (random) urine sample with a result of 2+ protein or greater is highly suggestive of proteinuria; a 24-hour urine collection is the gold standard by which such findings should be confirmed and protein levels in the urine quantified.1,14
Elevated blood pressure and proteinuria are the hallmarks of preeclampsia.6 Patients affected by these developments must be evaluated for signs and symptoms of severe preeclampsia. However, those with only mild elevations in blood pressure and little or no proteinuria may complain of sudden-onset throbbing or pounding headache, blurry vision, and severe epigastric pain—possibly indicating severe preeclampsia.5,10
In addition to laboratory evaluation for urinary protein excretion, the following tests are recommended by the American College of Obstetricians and Gynecologists (ACOG)14 to assess for end organ involvement, which is consistent with severe preeclampsia:
- Hematocrit, which may be either high, to suggest hemoconcentration; or low, indicating hemolysis
- Platelet count, which is normal in women with PIH and low in those with severe preeclampsia; if results are abnormal, this test should be followed by coagulation testing (international normalized ratio, activated partial thromboplastin time, fibrinogen)
- Renal function testing (blood urea nitrogen and creatinine may be elevated in severe preeclampsia), and random urine testing for proteinuria, as explained earlier
- Liver enzymes (which are elevated in severe preeclampsia), and
- Lactate dehydrogenase (which is elevated in severe preeclampsia).1,14
Additionally, researchers conducting a small cohort study (n = 163) reported in 2009 that in women with PIH, serum uric acid levels exceeding 309 µmol/L were predictive of preeclampsia, with 87.7% sensitivity and 93.3% specificity.19 An increase from first-trimester serum uric acid levels was also a strong prognostic factor for preeclampsia. Earlier this year, a Canadian investigative team reported an increased risk for premature birth (odds ratio, 3.2) and small infant size for gestational age (odds ratio, 2.5) in women with PIH and hyperuricemia.20 While the predictive value of uric acid has been debated to some extent,14 measurement is often included in the workup of patients with hypertensive pregnancies.5,6
The frequency of prenatal visits, laboratory testing, and fetal monitoring should be adjusted according to the severity of PIH. In mildly hypertensive patients, the general recommendation is urine and blood testing at weekly prenatal visits.14 Fetal well-being must be monitored regularly, although neither the type nor frequency of such testing has been well established. Generally, patients should be advised to count daily fetal movements, and they should be scheduled for either a nonstress test (NST) or a biophysical profile as soon as a diagnosis of PIH is made.1,2,6,14
According to a 2010 guidance from the United Kingdom’s National Institute for Health and Clinical Excellence (NICE),13,21 pregnant women with mild to moderate hypertension should undergo an initial ultrasonographic assessment of fetal growth and amniotic fluid volume at the time of diagnosis, then serially every 3 to 4 weeks. If results from initial fetal testing are normal, patients with mild PIH do not require repeat testing after 34 weeks’ gestation, unless conditions change (eg, preeclampsia, worsening hypertension, and/or change in fetal movements).1,2,14 The NICE guidelines also recommend umbilical artery Doppler velocimetry.13,21
In patients with severe PIH, an NST ultrasound assessment of fetal growth and amniotic fluid volume and umbilical artery Doppler velocimetry should be performed at diagnosis to evaluate for placental dysfunction.6,21 If all test results are normal, the ultrasound and umbilical artery Doppler velocimetry need not be repeated more frequently than every two weeks, and the NST no more than once per week.13
Treatment/Management and Follow-Up
Regular prenatal monitoring to assess for worsening of PIH and/or development of preeclampsia is key to management. Figure 12 outlines an algorithm for managing PIH, which is guided by the severity of the condition. Patients with mild PIH can be managed with weekly outpatient visits and assessed for signs and symptoms of preeclampsia, monitoring of fetal movements, weight, blood pressure measurements, and urine and blood tests.2
At each visit, it is important to instruct patients to report immediately any of the following symptoms: new-onset severe headache, visual changes, epigastric or right upper quadrant pain, nausea or vomiting, difficulty breathing or chest tightness, as well as vaginal bleeding, decreased fetal movements, or uterine contractions.6,14
Generally, expectant management with delivery at term is recommended for women with mild PIH.2 Vaginal delivery (or cesarean delivery, if indicated) is recommended at 37 weeks or when fetal maturity is confirmed; and at 34 weeks if fetal or maternal distress is evident.2,6
Findings from the Hypertension and Preeclampsia Intervention Trial At Term (HYPITAT),22 an open-label, randomized clinical trial in women with PIH or mild preeclampsia, suggested an association between induction of labor between 36 and 41 weeks’ gestation and improved maternal outcomes (specifically, reduced risk for severe hypertension), compared with expectant management. Similarly, in a literature review by Caughey et al,23 results from nine randomized controlled trials indicated a reduced risk for cesarean delivery in women who underwent induction of labor, compared with expectant management. Rates of “successful” induction of labor (ie, procedures resulting in vaginal rather than cesarean delivery) were greater in women with higher parity, a favorable cervix, and earlier gestational age.
Based on data from the HYPITAT trial,22 a cost-effectiveness analysis of induction of labor compared with expectant management revealed an 11% reduction in the average cost in delivery that followed induction of labor, compared with expectant management, in women with PIH or mild preeclampsia.24 Caughey et al23 reported similar savings, particularly when induction of labor was performed at 41 weeks’ gestation.
If induction of labor is being considered in a woman with an unfavorable cervix, administration of prostaglandins is recommended to enhance cervical ripening.6
Medication
Pregnant women should be advised to discontinue previously prescribed ACE inhibitors, angiotensin receptor blockers, or thiazide diuretics, which are associated with congenital abnormalities, intrauterine growth restriction, and/or neonatal nephropathy.5,6,13,21
Antihypertensive medication is not recommended for women with mild to moderate PIH, as it does not appear to improve outcomes. Evidence was found insufficient in a 2007 Cochrane review to determine the potential impact of antihypertensive medications for treatment of mild to moderate PIH on clinical outcomes such as preterm birth, infant mortality, and infant size relative to gestational age.25 A similar review conducted in 2011, with primary outcomes that included severe preeclampsia, eclampsia, and maternal death or perinatal death, concluded only that further study was needed to determine how tightly blood pressure must be controlled to improve maternal and fetal outcomes in patients with PIH.26
ACOG14 recommends antihypertensive therapy (eg, hydralazine, labetalol) only for women with diastolic blood pressure of 105 to 110 mm Hg or higher.1,2 There are several recommendations from different organizations regarding the choice of antihypertensive medications for PIH. In the UK’s NICE guidance,21 it is recommended that patients with moderate to severe PIH take oral labetalol as first-line treatment to keep systolic blood pressure below 150 mm Hg and diastolic blood pressure between 80 and 100 mm Hg.
Like severe preeclampsia, severe PIH should be managed in an inpatient setting.6,14 IV labetalol or hydralazine is recommended to lower the blood pressure to less than 160/110 mm Hg, although current evidence is insufficient to identify a target blood pressure.6,26 A 2002 ACOG practice bulletin recommends one of the following:
- Hydralazine 5 to 10 mg IV every 15 to 20 minutes until the desired response is achieved; or
- Labetalol 20 mg IV bolus, followed by 40 mg if not effective within 10 minutes, then 80 mg every 10 minutes with maximum total dose of 220 mg.1,14
In women who have severe PIH or who develop severe preeclampsia or eclampsia, magnesium sulfate is administered to prevent or treat seizures.14 This agent should be used during labor and for at least 24 hours postpartum.2 Dosing of magnesium sulfate for this indication is 4 g IV bolus, followed by infusion of 1 g/h. It is important to monitor treated patients for signs of toxicity, including muscle weakness, loss of patellar reflexes, hypoventilation, pulmonary edema, hypotension, and bradycardia. IV calcium gluconate should be readily available for use as an antidote to life-threatening hypermagnesemia.27,28
For chronic hypertension in pregnancy, the American Society of Hypertension10 has recommended several agents. There is no consensus on which medication is most appropriate (see Table 210,13).
Patient Education
Patient education is an important aspect of caring for women with PIH. The American Academy of Family Physicians29,30 provides a comprehensive patient education resource that defines the hypertensive disorders in pregnancy, explains the symptoms and signs of severe hypertension or preeclampsia, and describes appropriate diagnostic tests, monitoring, and treatment options. (See http://familydoctor.org/familydoctor/en/diseases-conditions/pregnancy-induced-hypertension.printerview.all.html.)
Patients who are considering pregnancy should be counseled to maintain a healthy weight prior to and during pregnancy. Adequate dietary calcium can reduce the risk for PIH, and calcium supplementation has been shown to reduce the risk for preeclampsia, especially in women at high risk.31 The Society of Obstetricians and Gynaecologists of Canada recommends low-dose aspirin (75 mg/d) at bedtime for high-risk women, starting before pregnancy, or upon diagnosis of pregnancy to prevent preeclampsia.6 Although there is insufficient evidence to recommend dietary salt restriction, excess salt can increase fluid retention and possibly blood pressure. Patients should be urged to attend all scheduled prenatal visits and to review the warning signs and symptoms of severe hypertension and preeclampsia at each visit.
Mode of delivery may be discussed and will depend on the severity of hypertension, presence of preeclampsia, and fetal well-being. Vaginal delivery at term is considered optimal unless there are indications for cesarean delivery. Induction of labor may be considered at term in patients with PIH. Those with severe preeclampsia who may require preterm delivery must be prepared for potential issues associated with prematurity.
Follow-Up and Prognosis
Patients with PIH should be evaluated postpartum for persistent hypertension. Blood pressure in patients with PIH usually normalizes by day 7 postpartum.32 If blood pressure elevation persists past 12 weeks postpartum, the patient’s diagnosis is revised to chronic hypertension and managed accordingly.
In the patient with persistent hypertension who chooses breastfeeding, it is important to select an antihypertensive medication with low transfer into breast milk. Many β-adrenergic antagonists and calcium channel antagonists are considered “compatible” with breastfeeding by the American Academy of Pediatrics.33
In addition to the potential for recurrent PIH in subsequent pregnancies, women with PIH are at increased risk for hypertension later in life, and findings from several large cohort studies suggest increased cardiovascular risk in patients with hypertensive pregnancies.16,34 Magnussen et al,9 who followed more than 15,000 mothers of singleton infants for several years postpartum, found that those who experienced hypertensive disorders (particularly recurrent hypertensive disorders) during pregnancy were more likely than normotensive women to subsequently develop diabetes, dyslipidemia, and hypertension. Women who remained normotensive while pregnant generally had lower BMI measurements than those who experienced PIH or preeclampsia.
Conclusion
Hypertensive disorders commonly develop during pregnancy. It is important to diagnose and classify PIH during routine prenatal visits. Once the diagnosis is made, patients must be monitored closely for increasing blood pressure or development of preeclampsia. Urine protein testing is a key clinical test to detect preeclampsia, and positive findings on a random urine protein dipstick should be confirmed and quantified with a 24-hour urine collection.
In addition to undergoing frequent blood pressure measurements and urine protein tests, patients should be asked about signs and symptoms that suggest preeclampsia. Women with mild PIH can be managed as outpatients with prenatal visits at least weekly, followed by delivery at term. Severely hypertensive patients are managed in the hospital with antihypertensive medications and prompt delivery at 34 weeks’ gestation or beyond, should maternal or fetal distress become evident.
Hypertensive disorders represent one of the most common medical complications of pregnancy.1,2 Based on a nationwide inpatient sample examining more than 36 million deliveries in the United States, the prevalence of associated hypertensive disorders increased from 67.2 per 1,000 deliveries in 1998 to 83.4 per 1,000 deliveries in 2006.3Pregnancy-induced hypertension (also referred to as gestational hypertension or hypertensive disorder of pregnancy)4-6 is estimated to affect 6% to 8% of US pregnancies.1,2
Women who develop severe hypertension during pregnancy may experience adverse effects similar to those associated with mild preeclampsia.2,7,8 In the mother, these may range from elevated liver enzymes to renal dysfunction; and in the fetus, from preterm delivery to intrauterine restriction of fetal growth.7,8
This article will review the risk factors, clinical presentation, diagnosis, and management of pregnancy-induced hypertension. A brief discussion of preeclampsia as it relates to gestational hypertension will be included (see Table 12,6,9).
Classification, Definitions
Pregnancy-induced hypertension (PIH) is classified as mild or severe. Mild PIH is defined as new-onset hypertension (systolic blood pressure ≥ 140 mm Hg and/or diastolic blood pressure ≥ 90 mm Hg), occurring after 20 weeks’ gestation. The majority of cases of mild PIH develop beyond 37 weeks’ gestation, and in these cases, pregnancy outcomes are comparable to those of normotensive pregnancies.2,7,8
Severe PIH is defined as sustained elevated blood pressures of ≥ 160 mm Hg systolic and ≥ 110 mm Hg diastolic. In prospective cohort studies in which calcium supplementation and low-dose aspirin use were being investigated for prevention of preeclampsia in healthy pregnant women, those who were severely hypertensive were found to be at increased risk for certain maternal comorbidities (eg, cesarean delivery, renal dysfunction, elevated liver enzymes, placental abruption) and perinatal morbidities (delivery before 37 weeks’ gestation, low birth weight, fetal growth restriction, and neonatal ICU admission), compared with patients who were normotensive or mildly hypertensive.7,8
The diagnosis of PIH may later be amended or replaced by one of the following diagnoses: preeclampsia, if proteinuria (to be defined and discussed later) develops; chronic hypertension, if blood pressure remains elevated past 12 weeks postpartum; or transient hypertension of pregnancy, if blood pressure normalizes by 12 weeks postpartum.5,6,10
Pathophysiology and Risk Factors
Although the pathophysiology of PIH is not well understood, the pathogenesis of preeclampsia likely involves abnormalities in the development, implantation, or perfusion of the placenta, and often leads to impaired maternal organ function.6,11 It is not clear whether PIH and preeclampsia are two different diseases that share a manifestation of elevated blood pressure or whether PIH represents an early stage of preeclampsia.4,12 However, women with preexisting hypertension, especially severe hypertension, are at increased risk for preeclampsia, placental abruption, and fetal growth restriction.2
There are some similarities and some distinct differences among the clinical features and risk factors associated with PIH, compared with those of preeclampsia. Risk factors for PIH include a pre-pregnancy BMI of 25 or greater, PIH and/or preeclampsia in previous pregnancies, and history of renal disease, cardiac disease, or diabetes. The most important risk factors for preeclampsia include preexisting diabetes or nephropathy, chronic hypertension, PIH or preeclampsia in a previous pregnancy, maternal age younger than 18 or older than 34, African-American ethnicity, first pregnancy, multiple pregnancy, history of preeclampsia in the patient’s mother or sister, obesity, autoimmune disease, and an interval between pregnancies longer than 10 years.4-6,13-15
The risk for preeclampsia in patients with PIH is approximately 15% to 25%12,16; according to Magee et al,6 35% of women with PIH onset before 37 weeks’ gestation develop preeclampsia.6,12,17 The risk for recurrence of PIH in subsequent pregnancies is about 26%, whereas women who experience preeclampsia in one pregnancy have a comparable risk for PIH or preeclampsia (about 14% each) in subsequent pregnancies.18
Clinical Presentation and Diagnostic Evaluation
Blood pressure should be measured and recorded at every prenatal visit, using the correct-sized cuff, with the patient in a seated position.5 Gestational hypertension is a clinical diagnosis confirmed by at least two accurate blood pressure measurements in the same arm in women without proteinuria, with readings of ≥ 140 mm Hg systolic and/or ≥ 90 mm Hg diastolic. It should then be determined whether the patient’s hypertension is mild or severe (ie, blood pressure > 160/110 mm Hg). The patient with severe PIH should be evaluated for signs of preeclampsia, as discussed below.
Patients with mild PIH are often asymptomatic, and the diagnosis is made at a prenatal visit as a result of routine blood pressure monitoring; this is one of many reasons to encourage early and regular prenatal care. Blood pressure may be higher at night in hypertensive disorders of pregnancy.10
In contrast to patients with mild PIH, the clinical presentation of those with severe PIH or preeclampsia (and the potential for impending eclampsia) may include the following symptoms and signs:
- Generalized edema, including that of the face and hands
- Rapid weight gain
- Blurred vision or scotomata (ie, areas of diminished vision in the visual field)
- Severe, throbbing or pounding headaches
- Epigastric or right upper quadrant pain
- Oliguria (urinary output < 500 mL/d)
- Nausea, with or without vomiting
- Hyperactive reflexes
- Chest pain or tightness
- Shortness of breath.2,6,14
Medical History
Important questions to address in the patient’s medical history relate to risk factors for PIH, such as a history of renal disease, cardiac disease, or diabetes, previous history of PIH and/or preeclampsia, and abuse of cocaine or amphetamines—in addition to the specific aforementioned symptoms and signs of severe preeclampsia.5,6
Physical Examination
The clinician performing the physical exam should be attentive to accurate blood pressure measurements and any signs that suggest preeclampsia. Weight should be measured and BMI calculated at each prenatal visit.
If the patient’s blood pressure is markedly elevated, the focused physical examination should include an ophthalmologic examination for jaundice and for evidence of hypertensive retinopathy or papilledema; pulmonary and cardiac examination; abdominal examination, including palpation of the liver; examination of the face and extremities for edema; and a complete neurologic examination, including assessment of deep tendon reflexes and examination for clonus.
Laboratory Testing
In patients with PIH, laboratory evaluation should be focused to rule out preeclampsia. The potential for proteinuria (defined as ≥ 0.3 g/d in a 24-hour urine sample1,14) must be investigated at diagnosis and at regular visits during the pregnancy.1 At least two random urine samples, collected at least 6 hours apart, should be evaluated for protein. A spot (random) urine sample with a result of 2+ protein or greater is highly suggestive of proteinuria; a 24-hour urine collection is the gold standard by which such findings should be confirmed and protein levels in the urine quantified.1,14
Elevated blood pressure and proteinuria are the hallmarks of preeclampsia.6 Patients affected by these developments must be evaluated for signs and symptoms of severe preeclampsia. However, those with only mild elevations in blood pressure and little or no proteinuria may complain of sudden-onset throbbing or pounding headache, blurry vision, and severe epigastric pain—possibly indicating severe preeclampsia.5,10
In addition to laboratory evaluation for urinary protein excretion, the following tests are recommended by the American College of Obstetricians and Gynecologists (ACOG)14 to assess for end organ involvement, which is consistent with severe preeclampsia:
- Hematocrit, which may be either high, to suggest hemoconcentration; or low, indicating hemolysis
- Platelet count, which is normal in women with PIH and low in those with severe preeclampsia; if results are abnormal, this test should be followed by coagulation testing (international normalized ratio, activated partial thromboplastin time, fibrinogen)
- Renal function testing (blood urea nitrogen and creatinine may be elevated in severe preeclampsia), and random urine testing for proteinuria, as explained earlier
- Liver enzymes (which are elevated in severe preeclampsia), and
- Lactate dehydrogenase (which is elevated in severe preeclampsia).1,14
Additionally, researchers conducting a small cohort study (n = 163) reported in 2009 that in women with PIH, serum uric acid levels exceeding 309 µmol/L were predictive of preeclampsia, with 87.7% sensitivity and 93.3% specificity.19 An increase from first-trimester serum uric acid levels was also a strong prognostic factor for preeclampsia. Earlier this year, a Canadian investigative team reported an increased risk for premature birth (odds ratio, 3.2) and small infant size for gestational age (odds ratio, 2.5) in women with PIH and hyperuricemia.20 While the predictive value of uric acid has been debated to some extent,14 measurement is often included in the workup of patients with hypertensive pregnancies.5,6
The frequency of prenatal visits, laboratory testing, and fetal monitoring should be adjusted according to the severity of PIH. In mildly hypertensive patients, the general recommendation is urine and blood testing at weekly prenatal visits.14 Fetal well-being must be monitored regularly, although neither the type nor frequency of such testing has been well established. Generally, patients should be advised to count daily fetal movements, and they should be scheduled for either a nonstress test (NST) or a biophysical profile as soon as a diagnosis of PIH is made.1,2,6,14
According to a 2010 guidance from the United Kingdom’s National Institute for Health and Clinical Excellence (NICE),13,21 pregnant women with mild to moderate hypertension should undergo an initial ultrasonographic assessment of fetal growth and amniotic fluid volume at the time of diagnosis, then serially every 3 to 4 weeks. If results from initial fetal testing are normal, patients with mild PIH do not require repeat testing after 34 weeks’ gestation, unless conditions change (eg, preeclampsia, worsening hypertension, and/or change in fetal movements).1,2,14 The NICE guidelines also recommend umbilical artery Doppler velocimetry.13,21
In patients with severe PIH, an NST ultrasound assessment of fetal growth and amniotic fluid volume and umbilical artery Doppler velocimetry should be performed at diagnosis to evaluate for placental dysfunction.6,21 If all test results are normal, the ultrasound and umbilical artery Doppler velocimetry need not be repeated more frequently than every two weeks, and the NST no more than once per week.13
Treatment/Management and Follow-Up
Regular prenatal monitoring to assess for worsening of PIH and/or development of preeclampsia is key to management. Figure 12 outlines an algorithm for managing PIH, which is guided by the severity of the condition. Patients with mild PIH can be managed with weekly outpatient visits and assessed for signs and symptoms of preeclampsia, monitoring of fetal movements, weight, blood pressure measurements, and urine and blood tests.2
At each visit, it is important to instruct patients to report immediately any of the following symptoms: new-onset severe headache, visual changes, epigastric or right upper quadrant pain, nausea or vomiting, difficulty breathing or chest tightness, as well as vaginal bleeding, decreased fetal movements, or uterine contractions.6,14
Generally, expectant management with delivery at term is recommended for women with mild PIH.2 Vaginal delivery (or cesarean delivery, if indicated) is recommended at 37 weeks or when fetal maturity is confirmed; and at 34 weeks if fetal or maternal distress is evident.2,6
Findings from the Hypertension and Preeclampsia Intervention Trial At Term (HYPITAT),22 an open-label, randomized clinical trial in women with PIH or mild preeclampsia, suggested an association between induction of labor between 36 and 41 weeks’ gestation and improved maternal outcomes (specifically, reduced risk for severe hypertension), compared with expectant management. Similarly, in a literature review by Caughey et al,23 results from nine randomized controlled trials indicated a reduced risk for cesarean delivery in women who underwent induction of labor, compared with expectant management. Rates of “successful” induction of labor (ie, procedures resulting in vaginal rather than cesarean delivery) were greater in women with higher parity, a favorable cervix, and earlier gestational age.
Based on data from the HYPITAT trial,22 a cost-effectiveness analysis of induction of labor compared with expectant management revealed an 11% reduction in the average cost in delivery that followed induction of labor, compared with expectant management, in women with PIH or mild preeclampsia.24 Caughey et al23 reported similar savings, particularly when induction of labor was performed at 41 weeks’ gestation.
If induction of labor is being considered in a woman with an unfavorable cervix, administration of prostaglandins is recommended to enhance cervical ripening.6
Medication
Pregnant women should be advised to discontinue previously prescribed ACE inhibitors, angiotensin receptor blockers, or thiazide diuretics, which are associated with congenital abnormalities, intrauterine growth restriction, and/or neonatal nephropathy.5,6,13,21
Antihypertensive medication is not recommended for women with mild to moderate PIH, as it does not appear to improve outcomes. Evidence was found insufficient in a 2007 Cochrane review to determine the potential impact of antihypertensive medications for treatment of mild to moderate PIH on clinical outcomes such as preterm birth, infant mortality, and infant size relative to gestational age.25 A similar review conducted in 2011, with primary outcomes that included severe preeclampsia, eclampsia, and maternal death or perinatal death, concluded only that further study was needed to determine how tightly blood pressure must be controlled to improve maternal and fetal outcomes in patients with PIH.26
ACOG14 recommends antihypertensive therapy (eg, hydralazine, labetalol) only for women with diastolic blood pressure of 105 to 110 mm Hg or higher.1,2 There are several recommendations from different organizations regarding the choice of antihypertensive medications for PIH. In the UK’s NICE guidance,21 it is recommended that patients with moderate to severe PIH take oral labetalol as first-line treatment to keep systolic blood pressure below 150 mm Hg and diastolic blood pressure between 80 and 100 mm Hg.
Like severe preeclampsia, severe PIH should be managed in an inpatient setting.6,14 IV labetalol or hydralazine is recommended to lower the blood pressure to less than 160/110 mm Hg, although current evidence is insufficient to identify a target blood pressure.6,26 A 2002 ACOG practice bulletin recommends one of the following:
- Hydralazine 5 to 10 mg IV every 15 to 20 minutes until the desired response is achieved; or
- Labetalol 20 mg IV bolus, followed by 40 mg if not effective within 10 minutes, then 80 mg every 10 minutes with maximum total dose of 220 mg.1,14
In women who have severe PIH or who develop severe preeclampsia or eclampsia, magnesium sulfate is administered to prevent or treat seizures.14 This agent should be used during labor and for at least 24 hours postpartum.2 Dosing of magnesium sulfate for this indication is 4 g IV bolus, followed by infusion of 1 g/h. It is important to monitor treated patients for signs of toxicity, including muscle weakness, loss of patellar reflexes, hypoventilation, pulmonary edema, hypotension, and bradycardia. IV calcium gluconate should be readily available for use as an antidote to life-threatening hypermagnesemia.27,28
For chronic hypertension in pregnancy, the American Society of Hypertension10 has recommended several agents. There is no consensus on which medication is most appropriate (see Table 210,13).
Patient Education
Patient education is an important aspect of caring for women with PIH. The American Academy of Family Physicians29,30 provides a comprehensive patient education resource that defines the hypertensive disorders in pregnancy, explains the symptoms and signs of severe hypertension or preeclampsia, and describes appropriate diagnostic tests, monitoring, and treatment options. (See http://familydoctor.org/familydoctor/en/diseases-conditions/pregnancy-induced-hypertension.printerview.all.html.)
Patients who are considering pregnancy should be counseled to maintain a healthy weight prior to and during pregnancy. Adequate dietary calcium can reduce the risk for PIH, and calcium supplementation has been shown to reduce the risk for preeclampsia, especially in women at high risk.31 The Society of Obstetricians and Gynaecologists of Canada recommends low-dose aspirin (75 mg/d) at bedtime for high-risk women, starting before pregnancy, or upon diagnosis of pregnancy to prevent preeclampsia.6 Although there is insufficient evidence to recommend dietary salt restriction, excess salt can increase fluid retention and possibly blood pressure. Patients should be urged to attend all scheduled prenatal visits and to review the warning signs and symptoms of severe hypertension and preeclampsia at each visit.
Mode of delivery may be discussed and will depend on the severity of hypertension, presence of preeclampsia, and fetal well-being. Vaginal delivery at term is considered optimal unless there are indications for cesarean delivery. Induction of labor may be considered at term in patients with PIH. Those with severe preeclampsia who may require preterm delivery must be prepared for potential issues associated with prematurity.
Follow-Up and Prognosis
Patients with PIH should be evaluated postpartum for persistent hypertension. Blood pressure in patients with PIH usually normalizes by day 7 postpartum.32 If blood pressure elevation persists past 12 weeks postpartum, the patient’s diagnosis is revised to chronic hypertension and managed accordingly.
In the patient with persistent hypertension who chooses breastfeeding, it is important to select an antihypertensive medication with low transfer into breast milk. Many β-adrenergic antagonists and calcium channel antagonists are considered “compatible” with breastfeeding by the American Academy of Pediatrics.33
In addition to the potential for recurrent PIH in subsequent pregnancies, women with PIH are at increased risk for hypertension later in life, and findings from several large cohort studies suggest increased cardiovascular risk in patients with hypertensive pregnancies.16,34 Magnussen et al,9 who followed more than 15,000 mothers of singleton infants for several years postpartum, found that those who experienced hypertensive disorders (particularly recurrent hypertensive disorders) during pregnancy were more likely than normotensive women to subsequently develop diabetes, dyslipidemia, and hypertension. Women who remained normotensive while pregnant generally had lower BMI measurements than those who experienced PIH or preeclampsia.
Conclusion
Hypertensive disorders commonly develop during pregnancy. It is important to diagnose and classify PIH during routine prenatal visits. Once the diagnosis is made, patients must be monitored closely for increasing blood pressure or development of preeclampsia. Urine protein testing is a key clinical test to detect preeclampsia, and positive findings on a random urine protein dipstick should be confirmed and quantified with a 24-hour urine collection.
In addition to undergoing frequent blood pressure measurements and urine protein tests, patients should be asked about signs and symptoms that suggest preeclampsia. Women with mild PIH can be managed as outpatients with prenatal visits at least weekly, followed by delivery at term. Severely hypertensive patients are managed in the hospital with antihypertensive medications and prompt delivery at 34 weeks’ gestation or beyond, should maternal or fetal distress become evident.
1. Report of the National High Blood Pressure Education Program Working Group on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183(1):S1-S22.
2. Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol. 2003;102(1):181-192.
3. Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009; 113(6):1299-1306.
4. Villar J, Carroli, G, Wojdyla D, et al; World Health Organization Antenatal Care Trial Research Group. Preeclampsia, gestational hypertension and intrauterine growth restriction, related or independent conditions. Am J Obstet Gynecol. 2006;194(4):921-931.
5. Leeman L, Fontaine P. Hypertensive disorders of pregnancy. Am Fam Physician. 2008;78(1):
93-100.
6. Magee LA, Helewa M, Moutquin JM, von Dadelszen P; Hypertension Guideline Committee; Strategic Training Initiative in Research in the Reproductive Health Sciences (STIRRHS) Scholars. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. J Obstet Gynaecol Can. 2008;30(3 suppl):S1-S48.
7. Buchbinder A, Sibai BM, Caritis S, et al. Adverse perinatal outcomes are significantly higher in severe gestational hypertension than in mild preeclampsia. Am J Obstet Gynecol. 2002;186(1):66-71.
8. Hauth JC, Ewell MG, Levine RJ, et al; Calcium for Preeclampsia Prevention Study Group. Pregnancy outcomes in healthy nulliparas who developed hypertension. Obstet Gynecol. 2000;95(1):24-28.
9. Magnussen EB, Vatten LJ, Smith GD, Romundstad PR. Hypertensive disorders in pregnancy and subsequently measured cardiovascular risk factors. Obstet Gynecol. 2009; 114(5):961-970.
10. Lindheimer MD, Taler SJ, Cunningham FG; American Society of Hypertension (ASH). ASH position paper: hypertension in pregnancy. J Clin Hypertens (Greenwich). 2009;11(4):214-225.
11. Roberts JM, Gammill HS. Preeclampsia: recent insights. Hypertension. 2005;46(6):
1243-1249.
12. Saudan P, Brown MA, Buddle ML, Jones M. Does gestational hypertension become pre-eclampsia? Br J Obstet Gynaecol. 1998;105 (11):1177-1184.
13. Visintin C, Mugglestone MA, Almerie MQ, et al; Guideline Development Group. Management of hypertensive disorders during pregnancy: summary of NICE guidance. BMJ. 2010;341:c2207.
14. ACOG Committee on Practice Bulletins—Obstetrics. Clinical Management Guidelines for Obstetrician–Gynecologists. Diagnosis and management of preeclampsia and eclampsia: ACOG practice bulletin No. 33. Obstet Gynecol. 2002;99:159-167.
15. Parazzini F, Bortolus R, Chatenoud L, et al; Italian Study of Aspirin in Pregnancy Group. Risk factors for pregnancy-induced hypertension in women at high risk for the condition. Epidemiology. 1996;7(3):306-308.
16. Hjartardottir S, Leifsson BG, Geirsson RT, Steinthorsdottir V. Recurrence of hypertensive disorder in second pregnancy. Am J Obstet Gynecol. 2006;194(4):916-920.
17. Barton JR, O’Brien JM, Bergauer NK, et al. Mild gestational hypertension remote from term: progression and outcome. Am J Obstet Gynecol. 2001;184(5):979-983.
18. Brown MA, Mackenzie C, Dunsmuir W, et al. Can we predict recurrence of pre-eclampsia or gestational hypertension? BJOG. 2007; 114(8):984-993.
19. Bellomo G, Venanzi S, Saronio P, et al. Prognostic significance of serum uric acid in women with gestational hypertension. Hypertension. 2011;58(4):704-708.
20. Hawkins TL, Roberts JM, Mangos GJ, et al. Plasma uric acid remains a marker of poor outcome in hypertensive pregnancy: a retrospective cohort study. BJOG. 2012;119(4):484-492.
21. National Institute for Health and Clinical Excellence. Hypertension in pregnancy: the management of hypertensive disorders during pregnancy. www.nice.org.uk/nicemedia/live/13098/50418/50418.pdf. Accessed April 16, 2012.
22. Koopmans CM, Bijlenga D, Groen H, et al. Induction of labour versus expectant monitoring for gestational hypertension or mild pre-eclampsia after 36 weeks’ gestation (HYPITAT): a multicentre, open-label randomised controlled trial. Lancet. 2009;374(9694):979-988.
23. Caughey AB, Sundaram V, Kaimal AJ, et al. Maternal and neonatal outcomes of elective induction of labor. Evid Rep Technol Assess (Full Rep). 2009;(176):1-257.
24. Shennan A, Hezelgrave N. An economic analysis of induction of labour and expectant monitoring in women with gestational hypertension or pre-eclampsia at term (HYPITAT trial). BJOG. 2010;117(13):1575-1576.
25. Abalos E, Duley L, Steyn DW, Henderson-Smart DJ. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2007 Jan 24;(1):CD002252.
26. Nabhan AF, Elsedawy MM. Tight control of mild-moderate pre-existing or non-proteinuric gestational hypertension. Cochrane Database Syst Rev. 2011 Jul 6;(7):CD006907.
27. Duley L, Gülmezoglu AM, Henderson-Smart DJ. Magnesium sulphate and other anticonvulsants for women with preeclampsia. Cochrane Database Syst Rev. 2003;(2):CD000025.
28. Kraft MD, Btaiche IF, Sacks GS, Kudsk KA. Treatment of electrolyte disorders in adult patients in the intensive care unit. Am J Health Syst Pharm. 2005;62(16):1663-1682.
29. FamilyDoctor.org. Pregnancy-induced hypertension (updated 2010). http://familydoctor.org/familydoctor/en/diseases-conditions/pregnancy-induced-hypertension.printerview
.all.html. Accessed April 16, 2012.
30. Zamorski MA, Green LA. NHBPEP report on high blood pressure in pregnancy: a summary for family physicians. Am Fam Physician. 2001; 64(2):263-270.
31. Hofmeyr GJ, Duley L, Atallah A. Dietary calcium supplementation for prevention of pre-eclampsia and related problems: a systematic review and commentary. BJOG. 2007;114(8): 933-943.
32. Ferrazzani S, De Carolis S, Pomini F, et al. The duration of hypertension in the puerperium of preeclamptic women: relationship with renal impairment and week of delivery. Am J Obstet Gynecol. 1994;171(2):506-512.
33. Beardmore KS, Morris JM, Gallery ED. Excretion of antihypertensive medication into human breast milk: a systematic review. Hypertens Pregnancy. 2002;21(1):85-95.
34. Wilson BJ, Watson MS, Prescott GJ, et al. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. BMJ. 2003;326(7394):845.
1. Report of the National High Blood Pressure Education Program Working Group on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183(1):S1-S22.
2. Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol. 2003;102(1):181-192.
3. Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009; 113(6):1299-1306.
4. Villar J, Carroli, G, Wojdyla D, et al; World Health Organization Antenatal Care Trial Research Group. Preeclampsia, gestational hypertension and intrauterine growth restriction, related or independent conditions. Am J Obstet Gynecol. 2006;194(4):921-931.
5. Leeman L, Fontaine P. Hypertensive disorders of pregnancy. Am Fam Physician. 2008;78(1):
93-100.
6. Magee LA, Helewa M, Moutquin JM, von Dadelszen P; Hypertension Guideline Committee; Strategic Training Initiative in Research in the Reproductive Health Sciences (STIRRHS) Scholars. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. J Obstet Gynaecol Can. 2008;30(3 suppl):S1-S48.
7. Buchbinder A, Sibai BM, Caritis S, et al. Adverse perinatal outcomes are significantly higher in severe gestational hypertension than in mild preeclampsia. Am J Obstet Gynecol. 2002;186(1):66-71.
8. Hauth JC, Ewell MG, Levine RJ, et al; Calcium for Preeclampsia Prevention Study Group. Pregnancy outcomes in healthy nulliparas who developed hypertension. Obstet Gynecol. 2000;95(1):24-28.
9. Magnussen EB, Vatten LJ, Smith GD, Romundstad PR. Hypertensive disorders in pregnancy and subsequently measured cardiovascular risk factors. Obstet Gynecol. 2009; 114(5):961-970.
10. Lindheimer MD, Taler SJ, Cunningham FG; American Society of Hypertension (ASH). ASH position paper: hypertension in pregnancy. J Clin Hypertens (Greenwich). 2009;11(4):214-225.
11. Roberts JM, Gammill HS. Preeclampsia: recent insights. Hypertension. 2005;46(6):
1243-1249.
12. Saudan P, Brown MA, Buddle ML, Jones M. Does gestational hypertension become pre-eclampsia? Br J Obstet Gynaecol. 1998;105 (11):1177-1184.
13. Visintin C, Mugglestone MA, Almerie MQ, et al; Guideline Development Group. Management of hypertensive disorders during pregnancy: summary of NICE guidance. BMJ. 2010;341:c2207.
14. ACOG Committee on Practice Bulletins—Obstetrics. Clinical Management Guidelines for Obstetrician–Gynecologists. Diagnosis and management of preeclampsia and eclampsia: ACOG practice bulletin No. 33. Obstet Gynecol. 2002;99:159-167.
15. Parazzini F, Bortolus R, Chatenoud L, et al; Italian Study of Aspirin in Pregnancy Group. Risk factors for pregnancy-induced hypertension in women at high risk for the condition. Epidemiology. 1996;7(3):306-308.
16. Hjartardottir S, Leifsson BG, Geirsson RT, Steinthorsdottir V. Recurrence of hypertensive disorder in second pregnancy. Am J Obstet Gynecol. 2006;194(4):916-920.
17. Barton JR, O’Brien JM, Bergauer NK, et al. Mild gestational hypertension remote from term: progression and outcome. Am J Obstet Gynecol. 2001;184(5):979-983.
18. Brown MA, Mackenzie C, Dunsmuir W, et al. Can we predict recurrence of pre-eclampsia or gestational hypertension? BJOG. 2007; 114(8):984-993.
19. Bellomo G, Venanzi S, Saronio P, et al. Prognostic significance of serum uric acid in women with gestational hypertension. Hypertension. 2011;58(4):704-708.
20. Hawkins TL, Roberts JM, Mangos GJ, et al. Plasma uric acid remains a marker of poor outcome in hypertensive pregnancy: a retrospective cohort study. BJOG. 2012;119(4):484-492.
21. National Institute for Health and Clinical Excellence. Hypertension in pregnancy: the management of hypertensive disorders during pregnancy. www.nice.org.uk/nicemedia/live/13098/50418/50418.pdf. Accessed April 16, 2012.
22. Koopmans CM, Bijlenga D, Groen H, et al. Induction of labour versus expectant monitoring for gestational hypertension or mild pre-eclampsia after 36 weeks’ gestation (HYPITAT): a multicentre, open-label randomised controlled trial. Lancet. 2009;374(9694):979-988.
23. Caughey AB, Sundaram V, Kaimal AJ, et al. Maternal and neonatal outcomes of elective induction of labor. Evid Rep Technol Assess (Full Rep). 2009;(176):1-257.
24. Shennan A, Hezelgrave N. An economic analysis of induction of labour and expectant monitoring in women with gestational hypertension or pre-eclampsia at term (HYPITAT trial). BJOG. 2010;117(13):1575-1576.
25. Abalos E, Duley L, Steyn DW, Henderson-Smart DJ. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2007 Jan 24;(1):CD002252.
26. Nabhan AF, Elsedawy MM. Tight control of mild-moderate pre-existing or non-proteinuric gestational hypertension. Cochrane Database Syst Rev. 2011 Jul 6;(7):CD006907.
27. Duley L, Gülmezoglu AM, Henderson-Smart DJ. Magnesium sulphate and other anticonvulsants for women with preeclampsia. Cochrane Database Syst Rev. 2003;(2):CD000025.
28. Kraft MD, Btaiche IF, Sacks GS, Kudsk KA. Treatment of electrolyte disorders in adult patients in the intensive care unit. Am J Health Syst Pharm. 2005;62(16):1663-1682.
29. FamilyDoctor.org. Pregnancy-induced hypertension (updated 2010). http://familydoctor.org/familydoctor/en/diseases-conditions/pregnancy-induced-hypertension.printerview
.all.html. Accessed April 16, 2012.
30. Zamorski MA, Green LA. NHBPEP report on high blood pressure in pregnancy: a summary for family physicians. Am Fam Physician. 2001; 64(2):263-270.
31. Hofmeyr GJ, Duley L, Atallah A. Dietary calcium supplementation for prevention of pre-eclampsia and related problems: a systematic review and commentary. BJOG. 2007;114(8): 933-943.
32. Ferrazzani S, De Carolis S, Pomini F, et al. The duration of hypertension in the puerperium of preeclamptic women: relationship with renal impairment and week of delivery. Am J Obstet Gynecol. 1994;171(2):506-512.
33. Beardmore KS, Morris JM, Gallery ED. Excretion of antihypertensive medication into human breast milk: a systematic review. Hypertens Pregnancy. 2002;21(1):85-95.
34. Wilson BJ, Watson MS, Prescott GJ, et al. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. BMJ. 2003;326(7394):845.
Probing Postpartum Depression
Care of the postpartum patient is an important component of primary care practice—particularly for clinicians who provide women’s health care and are called upon to effectively screen, identify, and manage patients who may be at risk for postpartum depression (PPD). Effective management of the postpartum patient also extends to care of her infant(s) and ideally should involve a multidisciplinary team, including the primary care provider, the pediatrician, and a psychologist or a psychiatrist.1
PPD is commonly described as depression that begins within the first month after delivery.2 It is diagnosed using the same criteria from the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)3 as are used to identify major depressive disorder. According to the DSM-IV, PPD begins within the first four weeks after the infant’s birth.2,3 Although onset can occur at any time between 24 hours after a woman gives birth to several months later, PPD most commonly occurs within the first six weeks after delivery.1
The woman with PPD may be reluctant to report her symptoms for a number of reasons, including lack of motivation or energy, fatigue, embarrassment that she is experiencing such symptoms during a presumably happy time in her life—even fear that her child may be removed from her care.4 In addition to depressive symptoms (eg, sleeping difficulty, appetite changes, anhedonia, and guilt), women affected by PPD often experience anxiety and may become obsessed with the health, feeding, and sleeping behaviors of their infants.1,5
Postpartum mood disorders occur in a spectrum, ranging from postpartum baby blues to postpartum psychosis. By DSM-IV definition, a diagnosis of major or minor PPD requires that depressed mood, loss of interest or pleasure, or other characteristic symptoms be present most of the day, nearly every day, for at least two weeks.3,6 PPD must be differentiated from the more common and less severe baby blues, which usually start two to three days after delivery and last less than two weeks.2,7 Between 40% and 80% of women who have given birth experience baby blues, which are characterized by transient mood swings, irritability, crying spells, difficulty sleeping, and difficulty concentrating.8 Experiencing baby blues may place women at increased risk for PPD.9
Postpartum, or puerperal, psychosis occurs in approximately 0.2% of women,10 with an early and sudden onset—that is, within the first week to four weeks postpartum. This severe condition is characterized by hallucinations and delusions often centered on the infant, in addition to insomnia, agitation, and extreme behavioral changes. Postpartum psychosis, which can recur in subsequent pregnancies, may be a manifestation of a preexisting affective disorder, such as bipolar disorder.10 Postpartum psychosis is considered an emergent condition because the safety and well-being of both mother and infant may be at serious risk.2,10
EPIDEMIOLOGY AND RISK FACTORS
PPD is often cited as affecting 10% to 15% of women within the first year after childbirth.11,12 Currently, reported prevalence rates of PPD range from 5% to 20% of women who have recently given birth, depending on the source of information. Among 17 US states participating in the Pregnancy Risk Assessment Monitoring System, a CDC surveillance project, prevalence of self-reported PPD symptoms ranged from 11.7% in Maine to 20.4% in New Mexico.11,13 According to the Agency for Healthcare Research and Quality (AHRQ),14 the prevalence of major or minor depression ranges from 6.5% to 12.9% at different times during the year following delivery, although study design varied throughout the research used, and confidence intervals were deemed wide.14,15
Nevertheless, according to results from a systematic review of 28 studies, the prevalence of minor or major depression is estimated at up to 19.2% of women during the first three months postpartum, and major depression in up to 7% during that time.16 A 2007 chart review of 4,398 US women experiencing live births identified PPD in 10.4%.17
Incidence of PPD is “much higher than the quoted rate of 10% to 15%,” concludes Almond18 after a comprehensive literature review. The condition also affects women globally, the British researcher reports: Not only did she find numerous data on the incidence of PPD in high-income countries, including the US, the United Kingdom, and Australia, but she concluded that incidence rates of PPD in developing countries are grossly underestimated, according to epidemiologic studies in low- and middle-income countries (eg, Pakistan, Indonesia, Vietnam). Additionally, the risk factors for PPD are likely to be influenced by cultural differences, and attempts to identify PPD must be culturally sensitive.18
Several risk factors have been associated with PPD. Perhaps the most significant risk factor is a personal history of depression (prior to pregnancy or postpartum); at least one-half of women with PPD experience onset of depressive symptoms before or during their pregnancies,19,20 and one research group reported a relative risk (RR) of 1.87 for PPD in women with a history of depression, compared with those without such a history.21 Thus, women previously affected by depression should be carefully monitored in the immediate postpartum period for any signs of depressed mood, anxiety, sleep difficulties, loss of appetite or energy, and psychomotor changes.
According to McCoy et al,21 neither patient age nor marital status nor method of delivery appeared to be associated with PPD at four weeks postpartum. Women who were feeding by formula alone were more likely to experience PPD (RR, 2.04) than were those who breastfed their infants. Women who smoked were more likely to be affected by PPD (RR, 1.58) than were nonsmokers.
Additional risk factors for PPD identified by Dennis et al22 included pregnancy-induced hypertension and immigration within the previous five years. Various psychosocial stressors may also represent risk factors for PPD, including lack of social support, financial concerns, miscarriage or fetal demise, limited partner support, physical abuse before or during pregnancy, lack of readiness for hospital discharge, and complications during pregnancy and delivery (eg, low birth weight, premature birth, admission of the infant to the neonatal ICU).11,22,23
It is important to identify women who are at highest risk for PPD as soon as possible. High-risk patients should be screened upon discharge from the hospital and certainly on or before the first postpartum visit.
CLINICAL MANIFESTATIONS
Clinical manifestations of PPD include depressed mood for at least two weeks with changes in somatic functions, such as sleep, energy level, appetite, weight, gastrointestinal functioning, and decrease in libido.3,24 These manifestations are more severe and prolonged than those associated with baby blues (which almost always resolve within two weeks postpartum).2,7
On physical examination, the patient with PPD may appear tearful and disheveled, with psychomotor retardation. She may report that she is unable to sleep even when her infant is sleeping, or that she has a significant lack of energy despite sufficient sleep; she may admit being unable to get out of bed for hours.2 The patient may report a significant decrease in appetite and little enjoyment in eating, which may lead to rapid weight loss.
Other symptoms may include obsessive thoughts about the infant and his or her care, significant anxiety (possibly manifested in panic attacks), uncontrollable crying, guilt, feelings of being overwhelmed or unable to care for the infant, mood swings, and severe irritability or even anger.2
Severe fatigue may warrant hemoglobin/hematocrit evaluation and possibly measurement of serum thyroid-stimulating hormone (TSH).25,26
It is essential to rule out postpartum psychosis, which is associated with prolonged lack of sleep, confusion, lapsed insight, cognitive impairment, “grossly disorganized behavior,”10 and delusions or hallucinations.5,10 The patient should be asked specifically about unusual or bizarre thoughts or beliefs concerning the infant, in addition to thoughts of harming herself or others, particularly the infant.10
SCREENING
Numerous researchers have suggested that PPD is underrecognized and undertreated.5,6,18,27,28 Screening for PPD in the United States is not standardized and is highly variable.29 The American Academy of Family Physicians supports universal screening for PPD at the first postpartum visit, between two and six weeks.30,31 According to a 2010 Committee Opinion from the American College of Obstetricians and Gynecologists, “at this time, there is insufficient evidence to support a firm recommendation for universal antepartum or postpartum screening; however, screening for depression has the potential to benefit a woman and her family and should be strongly considered.”32
As the AHRQ14 notes, symptoms of PPD may not peak in some women until after their first postpartum visit, and providers of family medicine, internal medicine, and pediatric care may also be in a position to provide screening. The initial well-baby examination by the pediatric primary care provider, for example, presents an important opportunity to screen new mothers for PPD. According to Chaudron et al,33 the well-being of the infant should outweigh any scope-of-practice concerns, practitioner time limitations, or reimbursement issues; rather, screening efforts can be considered “tools to enhance [mothers’] ability to care for their children in a way that is supportive and not punitive.”33
In 2009, Sheeder and colleagues28 reported on a prospective study of 199 mothers in an adolescent maternity clinic who were screened using the Edinburgh Postnatal Depression Scale (EPDS)34 at each well-baby visit during the first six months postpartum. The authors concluded that the optimal time for screening for PPD in this setting is two months after delivery, although repeated screening may identify worsening of depressive symptoms.28
Several screening tools are available for the detection of PPD, particularly the EPDS,34 the Postpartum Depression Screening Scale,35 and the Patient Health Questionnaire–936-38 (see table,11,13,34-37 ). The EPDS, a widely used and well-validated formal screening tool,27,37 is a 10-item self-report questionnaire designed to detect depression in the postpartum period. Cox et al,34 who developed the scale, initially reported its sensitivity at 86% and specificity at 78%21; since then, the tool’s reported sensitivity for detecting major depression in the postpartum period has ranged from 60% to 96%, and specificity from 45% to 97%.6,39 The EPDS has been shown to result in a diagnosis of PPD in significantly more women than routine clinical evaluation (35.4% vs 6.3%, respectively).40 It is possible to administer the EPDS by telephone.41
The validity of the EPDS tool in detecting PPD was recently examined in a systematic review of 37 studies. Gibson et al42 concluded that the heterogeneity of these studies (ie, differences in study methodology, language used, and diagnostic criteria) precluded meta-analysis and did not provide clear support of EPDS as an accurate screening tool for PPD, especially across diverse cultures. In a similar review, Hewitt and colleagues6 sought to “provide an overview of all available methods to identify postnatal depression in primary care and to assess their validity.” They concluded that the EPDS is the most frequently reported screening tool and, with an overall sensitivity of 86% and overall specificity of 87%, its diagnostic performance seems “reasonably good.”6 Of note, fewer data have been collected to demonstrate the effectiveness of other screening tools.35,36,38,41
Cost-effectiveness is a consideration in the use of screening programs for PPD. According to a hypothetical cohort analysis conducted in the UK, the costs of treating women with false-positive screening results made implementation of a formal screening strategy for PPD not cost-effective, compared with usual care only, for use by the British National Health Service.43
MANAGEMENT
Once PPD is identified, treatment should be initiated as quickly as possible; referral for psychological counseling is an appropriate initial strategy for mild to moderate symptoms of PPD.5 A clinical care manager can be a valuable resource to provide education and coordination of care for women affected by PPD. NPs and PAs in primary care, obstetrics/gynecology, women’s health, and psychiatry or psychology can play an important role in the identification and management of PPD.
Treatment of PPD involves combination therapy—short-term psychological therapy combined with pharmacotherapy. According to investigators in a Cochrane Review of nine trials reporting short-term outcomes for 956 women with PPD, their findings suggest that psychosocial and psychological interventions are an effective option for reducing symptoms of PPD.44 Compared with usual care, the types of psychological therapy that were found most effective included cognitive behavioral therapy, interpersonal therapy, and psychodynamic therapy. As most trials’ follow-up periods were limited to six months, however, neither the long-term effects of psychological therapy nor the relative effectiveness of each type of therapy was made clear by these studies.44,45
Pharmacotherapy
Although antidepressant drugs are known to be effective for the treatment of major depressive disorder, well-designed clinical trials demonstrating the overall effectiveness of antidepressants in treatment of PPD have been limited.46 According to Ng et al,46 who in 2010 performed a systematic review of studies examining pharmacologic interventions for PPD, preliminary evidence showing the effectiveness of antidepressants and hormone therapy should prompt the initiation of larger, more rigorous randomized and controlled trials.
The choice of antidepressants will be influenced by the mother’s breastfeeding status and whether PPD represents her first episode of depression or a recurrence of previous major depression. If the patient is not breastfeeding, the choice among antidepressants is similar to those used for treatment of nonpuerperal major depression. If PPD is a relapse of a prior depression, the therapeutic agent that was most effective and best-tolerated for previous depression should be prescribed.47
Generally, the SSRIs are considered first-line agents because of their superior safety profile.47 Fluoxetine has been shown in a small randomized trial (n = 87) to be significantly more effective than placebo and as effective as a full course of cognitive-behavioral counseling.45 In non–placebo-controlled studies, sertraline, fluvoxamine, and venlafaxine all produced improvement in PPD symptoms.48,49
Whether the mother is breastfeeding her infant will influence the use and choice of antidepressants for PPD. Although barely detectable levels of certain antidepressant medications (including the SSRIs sertraline and paroxetine, and the tricyclic antidepressant nortriptyline) have been reported in breast milk or in infant serum,50,51 it is recommended that the lowest possible therapeutic dose be prescribed, and that infants be carefully monitored for adverse effects.51
Fluoxetine, it should be noted, has been found to be transmitted through breast milk and was associated with reduced infant weight gain (specifically, by 392 g over six months) in a comparison between 64 fluoxetine-treated mothers and 38 non-treated mother-infant pairs.52 Thus, fluoxetine use should be avoided in women who are breastfeeding.
In small studies, paroxetine and fluvoxamine were not detected in infant serum, and although low levels of sertraline were detected in one-fourth of infants whose mothers received doses exceeding 100 mg/d, no adverse infant outcomes were noted.52-54 Paroxetine, nortriptyline, and sertraline appear to be relatively safer antidepressant choices in breastfeeding women with PPD.50,52,53
Antidepressants should be continued for six months after full remission of depressive symptoms. Longer courses of therapy may be necessary in patients who experience recurrent major depressive episodes.55
Few researchers have reported on the use of hormonal therapy for PPD. Yet significant hormonal fluctuations,56,57 including “estrogen withdrawal at parturition,”56 are known to occur after childbirth; in their study of women with severe PPD, Ahokas et al57 found that two-thirds of participants had serum estradiol concentrations below the cutoff for gonadal failure. Such a deficiency is likely to contribute to mood disturbances.56,57
In a small, double-blind, placebo-controlled study that enrolled women with severe, persistent PPD (mean EPDS score, 21.8), six months’ treatment with transdermally administered estradiol was associated with significantly greater relief of depressive symptoms than was found in controls (mean EPDS scores at one month, 13.3 vs 16.5, respectively). Of note, more than half of the women studied were concurrently receiving antidepressants.58
Additional studies may elucidate the role of estrogen therapy in the treatment of PPD.
Nonpharmacologic Options
An effective nonpharmacologic option for rapid resolution of severe symptoms of PPD is electroconvulsive therapy (ECT).59-61 Its use is safe in nursing mothers because it does not affect breast milk. ECT is considered especially useful for women who have not responded to pharmacotherapy, those experiencing severe psychotic depression, and those who are considered at high risk for suicide or infanticide.
Patients typically receive three treatments per week; three to six treatments often produce an effective response.59 Anesthesia administered to women who undergo ECT has not been shown to have a negative effect on infants who are being breastfed.60,61
Interpersonal strategies to address PPD should not be overlooked. A pilot study conducted in Canada showed promising results when women who had previously experienced PPD were trained to provide peer support by telephone to mothers who were deemed at high risk for PPD (ie, those with EPDS scores > 12). At four weeks and eight weeks postpartum, follow-up EPDS scores exceeded 12 in 10% and 15%, respectively, of women receiving peer support, compared with 41% and 52%, respectively, of controls.62
Participation in one of numerous support groups that exist for women with PPD (see box, for online information) may reduce isolation in these women and possibly offer additional benefits.
COMPLICATIONS
PPD may be associated with significant complications, underscoring the importance of prompt identification and treatment.5 Maternal depressive symptoms in the critical postnatal period, for example, have been associated with long-term impairment of mother-child bonding. In one study of 101 women, lower-quality maternal bonding was found in women who had symptoms of depression at two weeks, six weeks, and four months postpartum—but not in those with depression at 14 months.63 Additionally, it was found in a systematic review of 49 studies that women with PPD were likely to discontinue breastfeeding earlier than women not affected.64
Delayed growth and development has been reported in infants of mothers with untreated or inadequately treated PPD.65 It has also been suggested that children of depressed mothers may have an increased risk for anxiety, depression, hyperactivity, and other behavioral disorders later in childhood.65-67
PROGNOSIS
Untreated PPD may resolve spontaneously within three to six months, but in about one-quarter of PPD patients, depressive symptoms persist one year after delivery.24,68,69 PPD increases a woman’s risk for future episodes of major depression.2,5
PREVENTION
As previously discussed, the risk for PPD is greatest in women with a history of mood disorders (25%) and PPD (50%).2 Although several approaches have been studied to prevent PPD, no clear optimal strategy has been revealed. In one Cochrane review, insufficient evidence was found to justify prophylactic use of antidepressants.70 Similarly, findings in a second review fell short of confirming the effectiveness of prenatal psychosocial or psychological interventions to prevent antenatal depression.71
Additional studies are needed to make recommendations on prevention of PPD in high-risk patients. Until such recommendations emerge, close monitoring, screening, and follow-up are essential for these women.
CONCLUSION
Postpartum depression is an important concern among childbearing women, as it is associated with adverse maternal and infant outcomes. A personal history of depression is a major risk factor for PPD. It is imperative to question women about signs and symptoms of depression during the immediate postpartum period; it is particularly important to inquire about thoughts of harm to self or to the infant.
Pharmacotherapy combined with adjunctive psychological therapy is indicated for new mothers with significant depressive symptoms. The choice of antidepressants is based on previous response to antidepressants and the woman’s breastfeeding status. Generally, SSRIs are effective and well tolerated for major depression; based on results from small studies, they appear to be safe for breastfeeding mothers.
Electroconvulsive therapy is considered a safe and effective option for women with severe symptoms of PPD.
REFERENCES
1. Muzik M, Marcus SM, Heringhausen JE, Flynn H. When depression complicates childbearing: guidelines for screening and treatment during antenatal and postpartum obstetric care. Obstet Gynecol Clin North Am. 2009;36(4):771-788.
2. Wisner KL, Parry BL, Piontek CM. Clinical practice: postpartum depression. N Engl J Med. 2002;347(3):194-199.
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
4. Whitton A, Warner R, Appleby L. The pathway to care in post-natal depression: women’s attitudes to post-natal depression and its treatment. Br J Gen Pract. 1996;46(408):427-428.
5. Sit DK, Wisner KL. Identification of postpartum depression. Clin Obstet Gynecol. 2009; 52(3):456-468.
6. Hewitt C, Gilbody S, Brealey S, et al. Methods to identify postnatal depression in primary care: an integrated evidence synthesis and value of information analysis. Health Technol Assess. 2009;13(36):1-145, 147-230.
7. Epperson CN. Postpartum major depression: detection and treatment. Am Fam Physician. 1999;59(8):2247-2254, 2259-2260.
8. O’Hara MW, Schlechte JA, Lewis DA, Wright EJ. Prospective study of postpartum blues: biologic and psychosocial factors. Arch Gen Psychiatry. 1991;48(9):801-806.
9. Hannah P, Adams D, Lee A, et al. Links between early post-partum mood and post-natal depression. Br J Psychiatry. 1992;160:777-780.
10. Sit D, Rothschild AJ, Wisner KL. A review of postpartum psychosis. J Womens Health (Larchmt). 2006;15(4):352-368.
11. CDC. Prevalence of self-reported postpartum depressive symptoms—17 states, 2004–2005. MMWR Weekly. 2008;57(14):361-366.
12. O’Hara MW, Swain AM. Rates and risk of postpartum depression: a meta-analysis. Int Rev Psychiatry. 1996;8:37-54.
13. Whooley MA, Avins AL, Miranda J, Browner WS. Case-finding instruments for depression: two questions are as good as many. J Gen Intern Med. 1997;12(7):439-445.
14. Agency for Healthcare Research and Quality. Perinatal Depression: Prevalence, Screening Accuracy, and Screening Outcomes: Summary. Evidence Report/Technology Assessment No. 119. www.ahrq.gov/clinic/epcsums/peridep sum.htm. Accessed January 24, 2011.
15. Gaynes BN, Gavin N, Meltzer-Brody S, et al. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evid Rep Technol Assess (Summ). 2005 Feb;(119):1-8.
16. Gavin NI, Gaynes BN, Lohr KN, et al. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005; 106(5 pt 1):1071-1083.
17. Dietz PM, Williams SB, Callaghan WM, et al. Clinically identified maternal depression before, during, and after pregnancies ending in live births. Am J Psychiatry. 2007;164(10): 1515-1520.
18. Almond P. Postnatal depression a global public health perspective. Perspect Public Health. 2009;129(5):221-227.
19. Lee D, Yip A, Chiu H, et al. A psychiatric epidemiological study of postpartum Chinese women. Am J Psychiatry. 2001;158(2):220-226.
20. Cooper PJ, Tomlinson M, Swartz L, et al. Post-partum depression and the mother-infant relationship in a South African peri-urban settlement. Br J Psychiatry. 1999;175:554-558.
21. McCoy SJB, Beal JM, Miller SBM, et al. Risk factors for postpartum depression: a retrospective investigation at 4-weeks postnatal and a review of the literature. J Am Osteopath Assoc. 2006;106(4):193-198.
22. Dennis CL, Janssen PA, Singer J. Identifying women at-risk for postpartum depression in the immediate postpartum period. Acta Psychiatr Scand. 2004;110(5):338-346.
23. Milgrom J, Gemmill AW, Bilszta JL, et al. Antenatal risk factors for postnatal depression: a large prospective study. J Affect Disord. 2008;108(1-2):147-157.
24. Nonacs R, Cohen LS. Postpartum mood disorders: diagnosis and treatment guidelines. J Clin Psychiatry. 1998;59 suppl 2:34-40.
25. Corwin EJ, Murray-Kolb LE, Beard JL. Low hemoglobin level is a risk factor for postpartum depression. J Nutr. 2003;133(12):4139-4142.
26. McCoy SJ, Beal JM, Payton ME, et al. Postpartum thyroid measures and depressive symptomology: a pilot study. J Am Osteopath Assoc. 2008;108(9):503-507.
27. Seehusen DA, Baldwin LM, Runkle GP, Clark G. Are family physicians appropriately screening for postpartum depression? J Am Board Fam Pract. 2005;18(2):104-112.
28. Sheeder J, Kabir K, Stafford B. Screening for postpartum depression at well-child visits: is once enough during the first 6 months of life? Pediatrics. 2009;123(6):e982-e988.
29. Pearlstein T, Howard M, Salisbury A, Zlotnick C. Postpartum depression. Am J Obstet Gynecol. 2009;200(4):357-364.
30. Shaver K. Treating postpartum depression during lactation. Prescriber’s Letter. 2001 Aug;8(8):47.
31. Georgiopoulos AM, Bryan TL, Wollan P, Yawn BP. Routine screening for postpartum depression. J Fam Pract. 2001;50(20):117-122.
32. American College of Obstetricians and Gynecologists, Committee on Obstetric Practice. Committee Opinion No. 453: Screening for depression during and after pregnancy. Obstet Gynecol. 2010;115(2 pt 1):394-395.
33. Chaudron LH, Szilagyi PG, Campbell AT, et al. Legal and ethical considerations: risks and benefits of postpartum depression screening at well-child visits. Pediatrics. 2007;119(1):123-128.
34. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782-786.
35. Beck CT, Gable RK. Postpartum Depression Screening Scale: development and psychometric testing. Nurs Res. 2000;49(5):272-282.
36. Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders, Patient Health Questionnaire. JAMA. 1999; 282(18):1737-44.
37. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
38. Yawn BP, Pace W, Wollan PC, et al. Concordance of Edinburgh Postnatal Depression Scale (EPDS) and Patient Health Questionnaire (PHQ-9) to assess increased risk of depression among postpartum women. J Am Board Fam Med. 2009;22(5):483-491.
39. Murray L, Carothers AD. The validation of the Edinburgh Post-natal Depression Scale on a community sample. Br J Psychiatry. 1990;157: 288-290.
40. Evins GG, Theofrastous JP, Galvin SL. Postpartum depression: a comparison of screening and routine clinical evaluation. Am J Obstet Gynecol. 2000;182(5):1080-1082.
41. Hanusa BH, Scholle SH, Haskett RF, et al. Screening for depression in the postpartum period: a comparison of three instruments. J Womens Health (Larchmt). 2008;17(4):585-596.
42. Gibson J, McKenzie-McHarg K, Shakespeare J, et al. A systematic review of studies validating the Edinburgh postnatal depression scale in antepartum and postpartum women. Acta Psychiatr Scand. 2009;119(5):350-364.
43. Paulden M, Palmer S, Hewitt C, Gilbody S. Screening for postnatal depression in primary care: cost effectiveness analysis. BMJ. 2009; 339:b5203.
44. Dennis CL,Hodnett ED. Psychosocial and psychological interventions for treating postpartum depression. Cochrane Database Syst Rev. 2007;(4):CD006116.
45. Appleby L, Warner R, Whitton A, Faragher B. A controlled study of fluoxetine and cognitive-behavioural counselling in the treatment of postnatal depression. BMJ. 1997;314(7085): 932-936.
46. Ng RC, Hirata CK, Yeung W, et al. Pharmacologic treatment for postpartum depression: a systematic review. Pharmacotherapy. 2010; 30(9):928-941.
47. Whitby DH, Smith KM. The use of tricyclic antidepressants and selective serotonin reuptake inhibitors in women who are breastfeeding. Phamacotherapy. 2005;25(3):411-425.
48. Suri R, Burt VK, Altshuler LL, et al. Fluvoxamine for postpartum depression. Am J Psychiatry. 2001;158(10):1739-1740.
49. Cohen LS, Viguera AC, Bouffard SM, et al. Venlafaxine in the treatment of postpartum depression. J Clin Psychiatry. 2001;62(8):592-596.
50. Wisner KL, Hanusa BH, Perel JM, et al. Postpartum depression: a randomized trial of sertraline versus nortriptyline. J Clin Psychopharmacol. 2006;26(4):353-360.
51. Freeman MP. Postpartum depression treatment and breastfeeding. J Clin Psychiatry. 2009;79(9):e35.
52. Gjerdingen D. The effectiveness of various postpartum depression treatments and the impact of antidepressant drugs on nursing infants. J Am Board Fam Pract. 2003;16(5): 372-382.
53. Wisner KL, Perel JM, Findling RL. Antidepressant treatment during breast-feeding. Am J Psychiatry. 1996;153(9):1132-1137.
54. Hendrick V, Fukuchi A, Altshuler L, et al. Use of sertraline, paroxetine and fluvoxamine by nursing women. Br J Psychiatry. 2001;179: 163-166.
55. Davidson JR. Major depressive disorder treatment guidelines in America and Europe.
J Clin Psychiatry. 2010;71 suppl E1:e04.
56. Moses-Kolko EL, Berga SL, Kalro B, et al. Transdermal estradiol for postpartum depression: a promising treatment option. Clin Obstet Gynecol. 2009;52(3):516-529.
57. Ahokas A, Kaukoranta J, Wahlbeck K, Aito M. Estrogen deficiency in severe postpartum depression: successful treatment with sublingual physiologic 17beta-estradiol: a preliminary study. J Clin Psychiatry. 2001;62(5):
332-336.
58. Gregoire AJ, Kumar R, Everitt B, et al. Transdermal oestrogen for treatment of severe postnatal depression. Lancet. 1996;347(9006): 930-933.
59. Forray A, Ostroff RB. The use of electroconvulsive therapy in postpartum affective disorders. J ECT. 2007;23(3):188-193.
60. Altshuler LL, Cohen L, Szuba MP, et al. Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry. 1996;153(5):592-606.
61. Rabheru K. The use of electroconvulsive therapy in special patient populations. Can J Psychiatry. 2001;46(8):710-719.
62. Dennis CL. The effect of peer support on postpartum depression: a pilot randomized controlled trial. Can J Psychiatry. 2003;48(2): 115-124.
63. Moehler E, Brunner R, Wiebel A, et al. Maternal depressive symptoms in the postnatal period are associated with long-term impairment of mother-child bonding. Arch Womens Ment Health. 2006;9(5):273-278.
64. Dennis CL, McQueen K. The relationship between infant-feeding outcomes and postpartum depression: a qualitative systematic review. Pediatrics. 2009;123(4):e736-e751.
65. Hirst KP, Moutier CY. Postpartum major depression. Am Fam Physician. 2010;82(8): 926-933.
66. Beck CT. The effects of postpartum depression on child development: a meta-analysis. Arch Psychiatr Nurs. 1998;12(1):12-20.
67. Weissman MM, Pilowsky DJ, Wickramaratne PJ, et al. Remissions in maternal depression and child psychopathology: a STAR*D-child report. JAMA. 2006;295(12):1389-1398.
68. Cooper PJ, Murray L. Course and recurrence of postnatal depression: evidence for the specificity of the diagnostic concept. Br J Psychiatry. 1995;166(2):191-195.
69. Kumar R, Robson KM. A prospective study of emotional disorders in childbearing women. Br J Psychiatry. 1984;144:35-47.
70. Howard LM, Hoffbrand S, Henshaw C, et al. Antidepressant prevention of postnatal depression. Cochrane Database Syst Rev. 2005;(2):CD00436.
71. Dennis CL, Ross LE, Grigoriadis S. Psychosocial and psychological interventions for treating antenatal depression. Cochrane Database Syst Rev. 2007;(3):CD006309.
Care of the postpartum patient is an important component of primary care practice—particularly for clinicians who provide women’s health care and are called upon to effectively screen, identify, and manage patients who may be at risk for postpartum depression (PPD). Effective management of the postpartum patient also extends to care of her infant(s) and ideally should involve a multidisciplinary team, including the primary care provider, the pediatrician, and a psychologist or a psychiatrist.1
PPD is commonly described as depression that begins within the first month after delivery.2 It is diagnosed using the same criteria from the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)3 as are used to identify major depressive disorder. According to the DSM-IV, PPD begins within the first four weeks after the infant’s birth.2,3 Although onset can occur at any time between 24 hours after a woman gives birth to several months later, PPD most commonly occurs within the first six weeks after delivery.1
The woman with PPD may be reluctant to report her symptoms for a number of reasons, including lack of motivation or energy, fatigue, embarrassment that she is experiencing such symptoms during a presumably happy time in her life—even fear that her child may be removed from her care.4 In addition to depressive symptoms (eg, sleeping difficulty, appetite changes, anhedonia, and guilt), women affected by PPD often experience anxiety and may become obsessed with the health, feeding, and sleeping behaviors of their infants.1,5
Postpartum mood disorders occur in a spectrum, ranging from postpartum baby blues to postpartum psychosis. By DSM-IV definition, a diagnosis of major or minor PPD requires that depressed mood, loss of interest or pleasure, or other characteristic symptoms be present most of the day, nearly every day, for at least two weeks.3,6 PPD must be differentiated from the more common and less severe baby blues, which usually start two to three days after delivery and last less than two weeks.2,7 Between 40% and 80% of women who have given birth experience baby blues, which are characterized by transient mood swings, irritability, crying spells, difficulty sleeping, and difficulty concentrating.8 Experiencing baby blues may place women at increased risk for PPD.9
Postpartum, or puerperal, psychosis occurs in approximately 0.2% of women,10 with an early and sudden onset—that is, within the first week to four weeks postpartum. This severe condition is characterized by hallucinations and delusions often centered on the infant, in addition to insomnia, agitation, and extreme behavioral changes. Postpartum psychosis, which can recur in subsequent pregnancies, may be a manifestation of a preexisting affective disorder, such as bipolar disorder.10 Postpartum psychosis is considered an emergent condition because the safety and well-being of both mother and infant may be at serious risk.2,10
EPIDEMIOLOGY AND RISK FACTORS
PPD is often cited as affecting 10% to 15% of women within the first year after childbirth.11,12 Currently, reported prevalence rates of PPD range from 5% to 20% of women who have recently given birth, depending on the source of information. Among 17 US states participating in the Pregnancy Risk Assessment Monitoring System, a CDC surveillance project, prevalence of self-reported PPD symptoms ranged from 11.7% in Maine to 20.4% in New Mexico.11,13 According to the Agency for Healthcare Research and Quality (AHRQ),14 the prevalence of major or minor depression ranges from 6.5% to 12.9% at different times during the year following delivery, although study design varied throughout the research used, and confidence intervals were deemed wide.14,15
Nevertheless, according to results from a systematic review of 28 studies, the prevalence of minor or major depression is estimated at up to 19.2% of women during the first three months postpartum, and major depression in up to 7% during that time.16 A 2007 chart review of 4,398 US women experiencing live births identified PPD in 10.4%.17
Incidence of PPD is “much higher than the quoted rate of 10% to 15%,” concludes Almond18 after a comprehensive literature review. The condition also affects women globally, the British researcher reports: Not only did she find numerous data on the incidence of PPD in high-income countries, including the US, the United Kingdom, and Australia, but she concluded that incidence rates of PPD in developing countries are grossly underestimated, according to epidemiologic studies in low- and middle-income countries (eg, Pakistan, Indonesia, Vietnam). Additionally, the risk factors for PPD are likely to be influenced by cultural differences, and attempts to identify PPD must be culturally sensitive.18
Several risk factors have been associated with PPD. Perhaps the most significant risk factor is a personal history of depression (prior to pregnancy or postpartum); at least one-half of women with PPD experience onset of depressive symptoms before or during their pregnancies,19,20 and one research group reported a relative risk (RR) of 1.87 for PPD in women with a history of depression, compared with those without such a history.21 Thus, women previously affected by depression should be carefully monitored in the immediate postpartum period for any signs of depressed mood, anxiety, sleep difficulties, loss of appetite or energy, and psychomotor changes.
According to McCoy et al,21 neither patient age nor marital status nor method of delivery appeared to be associated with PPD at four weeks postpartum. Women who were feeding by formula alone were more likely to experience PPD (RR, 2.04) than were those who breastfed their infants. Women who smoked were more likely to be affected by PPD (RR, 1.58) than were nonsmokers.
Additional risk factors for PPD identified by Dennis et al22 included pregnancy-induced hypertension and immigration within the previous five years. Various psychosocial stressors may also represent risk factors for PPD, including lack of social support, financial concerns, miscarriage or fetal demise, limited partner support, physical abuse before or during pregnancy, lack of readiness for hospital discharge, and complications during pregnancy and delivery (eg, low birth weight, premature birth, admission of the infant to the neonatal ICU).11,22,23
It is important to identify women who are at highest risk for PPD as soon as possible. High-risk patients should be screened upon discharge from the hospital and certainly on or before the first postpartum visit.
CLINICAL MANIFESTATIONS
Clinical manifestations of PPD include depressed mood for at least two weeks with changes in somatic functions, such as sleep, energy level, appetite, weight, gastrointestinal functioning, and decrease in libido.3,24 These manifestations are more severe and prolonged than those associated with baby blues (which almost always resolve within two weeks postpartum).2,7
On physical examination, the patient with PPD may appear tearful and disheveled, with psychomotor retardation. She may report that she is unable to sleep even when her infant is sleeping, or that she has a significant lack of energy despite sufficient sleep; she may admit being unable to get out of bed for hours.2 The patient may report a significant decrease in appetite and little enjoyment in eating, which may lead to rapid weight loss.
Other symptoms may include obsessive thoughts about the infant and his or her care, significant anxiety (possibly manifested in panic attacks), uncontrollable crying, guilt, feelings of being overwhelmed or unable to care for the infant, mood swings, and severe irritability or even anger.2
Severe fatigue may warrant hemoglobin/hematocrit evaluation and possibly measurement of serum thyroid-stimulating hormone (TSH).25,26
It is essential to rule out postpartum psychosis, which is associated with prolonged lack of sleep, confusion, lapsed insight, cognitive impairment, “grossly disorganized behavior,”10 and delusions or hallucinations.5,10 The patient should be asked specifically about unusual or bizarre thoughts or beliefs concerning the infant, in addition to thoughts of harming herself or others, particularly the infant.10
SCREENING
Numerous researchers have suggested that PPD is underrecognized and undertreated.5,6,18,27,28 Screening for PPD in the United States is not standardized and is highly variable.29 The American Academy of Family Physicians supports universal screening for PPD at the first postpartum visit, between two and six weeks.30,31 According to a 2010 Committee Opinion from the American College of Obstetricians and Gynecologists, “at this time, there is insufficient evidence to support a firm recommendation for universal antepartum or postpartum screening; however, screening for depression has the potential to benefit a woman and her family and should be strongly considered.”32
As the AHRQ14 notes, symptoms of PPD may not peak in some women until after their first postpartum visit, and providers of family medicine, internal medicine, and pediatric care may also be in a position to provide screening. The initial well-baby examination by the pediatric primary care provider, for example, presents an important opportunity to screen new mothers for PPD. According to Chaudron et al,33 the well-being of the infant should outweigh any scope-of-practice concerns, practitioner time limitations, or reimbursement issues; rather, screening efforts can be considered “tools to enhance [mothers’] ability to care for their children in a way that is supportive and not punitive.”33
In 2009, Sheeder and colleagues28 reported on a prospective study of 199 mothers in an adolescent maternity clinic who were screened using the Edinburgh Postnatal Depression Scale (EPDS)34 at each well-baby visit during the first six months postpartum. The authors concluded that the optimal time for screening for PPD in this setting is two months after delivery, although repeated screening may identify worsening of depressive symptoms.28
Several screening tools are available for the detection of PPD, particularly the EPDS,34 the Postpartum Depression Screening Scale,35 and the Patient Health Questionnaire–936-38 (see table,11,13,34-37 ). The EPDS, a widely used and well-validated formal screening tool,27,37 is a 10-item self-report questionnaire designed to detect depression in the postpartum period. Cox et al,34 who developed the scale, initially reported its sensitivity at 86% and specificity at 78%21; since then, the tool’s reported sensitivity for detecting major depression in the postpartum period has ranged from 60% to 96%, and specificity from 45% to 97%.6,39 The EPDS has been shown to result in a diagnosis of PPD in significantly more women than routine clinical evaluation (35.4% vs 6.3%, respectively).40 It is possible to administer the EPDS by telephone.41
The validity of the EPDS tool in detecting PPD was recently examined in a systematic review of 37 studies. Gibson et al42 concluded that the heterogeneity of these studies (ie, differences in study methodology, language used, and diagnostic criteria) precluded meta-analysis and did not provide clear support of EPDS as an accurate screening tool for PPD, especially across diverse cultures. In a similar review, Hewitt and colleagues6 sought to “provide an overview of all available methods to identify postnatal depression in primary care and to assess their validity.” They concluded that the EPDS is the most frequently reported screening tool and, with an overall sensitivity of 86% and overall specificity of 87%, its diagnostic performance seems “reasonably good.”6 Of note, fewer data have been collected to demonstrate the effectiveness of other screening tools.35,36,38,41
Cost-effectiveness is a consideration in the use of screening programs for PPD. According to a hypothetical cohort analysis conducted in the UK, the costs of treating women with false-positive screening results made implementation of a formal screening strategy for PPD not cost-effective, compared with usual care only, for use by the British National Health Service.43
MANAGEMENT
Once PPD is identified, treatment should be initiated as quickly as possible; referral for psychological counseling is an appropriate initial strategy for mild to moderate symptoms of PPD.5 A clinical care manager can be a valuable resource to provide education and coordination of care for women affected by PPD. NPs and PAs in primary care, obstetrics/gynecology, women’s health, and psychiatry or psychology can play an important role in the identification and management of PPD.
Treatment of PPD involves combination therapy—short-term psychological therapy combined with pharmacotherapy. According to investigators in a Cochrane Review of nine trials reporting short-term outcomes for 956 women with PPD, their findings suggest that psychosocial and psychological interventions are an effective option for reducing symptoms of PPD.44 Compared with usual care, the types of psychological therapy that were found most effective included cognitive behavioral therapy, interpersonal therapy, and psychodynamic therapy. As most trials’ follow-up periods were limited to six months, however, neither the long-term effects of psychological therapy nor the relative effectiveness of each type of therapy was made clear by these studies.44,45
Pharmacotherapy
Although antidepressant drugs are known to be effective for the treatment of major depressive disorder, well-designed clinical trials demonstrating the overall effectiveness of antidepressants in treatment of PPD have been limited.46 According to Ng et al,46 who in 2010 performed a systematic review of studies examining pharmacologic interventions for PPD, preliminary evidence showing the effectiveness of antidepressants and hormone therapy should prompt the initiation of larger, more rigorous randomized and controlled trials.
The choice of antidepressants will be influenced by the mother’s breastfeeding status and whether PPD represents her first episode of depression or a recurrence of previous major depression. If the patient is not breastfeeding, the choice among antidepressants is similar to those used for treatment of nonpuerperal major depression. If PPD is a relapse of a prior depression, the therapeutic agent that was most effective and best-tolerated for previous depression should be prescribed.47
Generally, the SSRIs are considered first-line agents because of their superior safety profile.47 Fluoxetine has been shown in a small randomized trial (n = 87) to be significantly more effective than placebo and as effective as a full course of cognitive-behavioral counseling.45 In non–placebo-controlled studies, sertraline, fluvoxamine, and venlafaxine all produced improvement in PPD symptoms.48,49
Whether the mother is breastfeeding her infant will influence the use and choice of antidepressants for PPD. Although barely detectable levels of certain antidepressant medications (including the SSRIs sertraline and paroxetine, and the tricyclic antidepressant nortriptyline) have been reported in breast milk or in infant serum,50,51 it is recommended that the lowest possible therapeutic dose be prescribed, and that infants be carefully monitored for adverse effects.51
Fluoxetine, it should be noted, has been found to be transmitted through breast milk and was associated with reduced infant weight gain (specifically, by 392 g over six months) in a comparison between 64 fluoxetine-treated mothers and 38 non-treated mother-infant pairs.52 Thus, fluoxetine use should be avoided in women who are breastfeeding.
In small studies, paroxetine and fluvoxamine were not detected in infant serum, and although low levels of sertraline were detected in one-fourth of infants whose mothers received doses exceeding 100 mg/d, no adverse infant outcomes were noted.52-54 Paroxetine, nortriptyline, and sertraline appear to be relatively safer antidepressant choices in breastfeeding women with PPD.50,52,53
Antidepressants should be continued for six months after full remission of depressive symptoms. Longer courses of therapy may be necessary in patients who experience recurrent major depressive episodes.55
Few researchers have reported on the use of hormonal therapy for PPD. Yet significant hormonal fluctuations,56,57 including “estrogen withdrawal at parturition,”56 are known to occur after childbirth; in their study of women with severe PPD, Ahokas et al57 found that two-thirds of participants had serum estradiol concentrations below the cutoff for gonadal failure. Such a deficiency is likely to contribute to mood disturbances.56,57
In a small, double-blind, placebo-controlled study that enrolled women with severe, persistent PPD (mean EPDS score, 21.8), six months’ treatment with transdermally administered estradiol was associated with significantly greater relief of depressive symptoms than was found in controls (mean EPDS scores at one month, 13.3 vs 16.5, respectively). Of note, more than half of the women studied were concurrently receiving antidepressants.58
Additional studies may elucidate the role of estrogen therapy in the treatment of PPD.
Nonpharmacologic Options
An effective nonpharmacologic option for rapid resolution of severe symptoms of PPD is electroconvulsive therapy (ECT).59-61 Its use is safe in nursing mothers because it does not affect breast milk. ECT is considered especially useful for women who have not responded to pharmacotherapy, those experiencing severe psychotic depression, and those who are considered at high risk for suicide or infanticide.
Patients typically receive three treatments per week; three to six treatments often produce an effective response.59 Anesthesia administered to women who undergo ECT has not been shown to have a negative effect on infants who are being breastfed.60,61
Interpersonal strategies to address PPD should not be overlooked. A pilot study conducted in Canada showed promising results when women who had previously experienced PPD were trained to provide peer support by telephone to mothers who were deemed at high risk for PPD (ie, those with EPDS scores > 12). At four weeks and eight weeks postpartum, follow-up EPDS scores exceeded 12 in 10% and 15%, respectively, of women receiving peer support, compared with 41% and 52%, respectively, of controls.62
Participation in one of numerous support groups that exist for women with PPD (see box, for online information) may reduce isolation in these women and possibly offer additional benefits.
COMPLICATIONS
PPD may be associated with significant complications, underscoring the importance of prompt identification and treatment.5 Maternal depressive symptoms in the critical postnatal period, for example, have been associated with long-term impairment of mother-child bonding. In one study of 101 women, lower-quality maternal bonding was found in women who had symptoms of depression at two weeks, six weeks, and four months postpartum—but not in those with depression at 14 months.63 Additionally, it was found in a systematic review of 49 studies that women with PPD were likely to discontinue breastfeeding earlier than women not affected.64
Delayed growth and development has been reported in infants of mothers with untreated or inadequately treated PPD.65 It has also been suggested that children of depressed mothers may have an increased risk for anxiety, depression, hyperactivity, and other behavioral disorders later in childhood.65-67
PROGNOSIS
Untreated PPD may resolve spontaneously within three to six months, but in about one-quarter of PPD patients, depressive symptoms persist one year after delivery.24,68,69 PPD increases a woman’s risk for future episodes of major depression.2,5
PREVENTION
As previously discussed, the risk for PPD is greatest in women with a history of mood disorders (25%) and PPD (50%).2 Although several approaches have been studied to prevent PPD, no clear optimal strategy has been revealed. In one Cochrane review, insufficient evidence was found to justify prophylactic use of antidepressants.70 Similarly, findings in a second review fell short of confirming the effectiveness of prenatal psychosocial or psychological interventions to prevent antenatal depression.71
Additional studies are needed to make recommendations on prevention of PPD in high-risk patients. Until such recommendations emerge, close monitoring, screening, and follow-up are essential for these women.
CONCLUSION
Postpartum depression is an important concern among childbearing women, as it is associated with adverse maternal and infant outcomes. A personal history of depression is a major risk factor for PPD. It is imperative to question women about signs and symptoms of depression during the immediate postpartum period; it is particularly important to inquire about thoughts of harm to self or to the infant.
Pharmacotherapy combined with adjunctive psychological therapy is indicated for new mothers with significant depressive symptoms. The choice of antidepressants is based on previous response to antidepressants and the woman’s breastfeeding status. Generally, SSRIs are effective and well tolerated for major depression; based on results from small studies, they appear to be safe for breastfeeding mothers.
Electroconvulsive therapy is considered a safe and effective option for women with severe symptoms of PPD.
REFERENCES
1. Muzik M, Marcus SM, Heringhausen JE, Flynn H. When depression complicates childbearing: guidelines for screening and treatment during antenatal and postpartum obstetric care. Obstet Gynecol Clin North Am. 2009;36(4):771-788.
2. Wisner KL, Parry BL, Piontek CM. Clinical practice: postpartum depression. N Engl J Med. 2002;347(3):194-199.
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
4. Whitton A, Warner R, Appleby L. The pathway to care in post-natal depression: women’s attitudes to post-natal depression and its treatment. Br J Gen Pract. 1996;46(408):427-428.
5. Sit DK, Wisner KL. Identification of postpartum depression. Clin Obstet Gynecol. 2009; 52(3):456-468.
6. Hewitt C, Gilbody S, Brealey S, et al. Methods to identify postnatal depression in primary care: an integrated evidence synthesis and value of information analysis. Health Technol Assess. 2009;13(36):1-145, 147-230.
7. Epperson CN. Postpartum major depression: detection and treatment. Am Fam Physician. 1999;59(8):2247-2254, 2259-2260.
8. O’Hara MW, Schlechte JA, Lewis DA, Wright EJ. Prospective study of postpartum blues: biologic and psychosocial factors. Arch Gen Psychiatry. 1991;48(9):801-806.
9. Hannah P, Adams D, Lee A, et al. Links between early post-partum mood and post-natal depression. Br J Psychiatry. 1992;160:777-780.
10. Sit D, Rothschild AJ, Wisner KL. A review of postpartum psychosis. J Womens Health (Larchmt). 2006;15(4):352-368.
11. CDC. Prevalence of self-reported postpartum depressive symptoms—17 states, 2004–2005. MMWR Weekly. 2008;57(14):361-366.
12. O’Hara MW, Swain AM. Rates and risk of postpartum depression: a meta-analysis. Int Rev Psychiatry. 1996;8:37-54.
13. Whooley MA, Avins AL, Miranda J, Browner WS. Case-finding instruments for depression: two questions are as good as many. J Gen Intern Med. 1997;12(7):439-445.
14. Agency for Healthcare Research and Quality. Perinatal Depression: Prevalence, Screening Accuracy, and Screening Outcomes: Summary. Evidence Report/Technology Assessment No. 119. www.ahrq.gov/clinic/epcsums/peridep sum.htm. Accessed January 24, 2011.
15. Gaynes BN, Gavin N, Meltzer-Brody S, et al. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evid Rep Technol Assess (Summ). 2005 Feb;(119):1-8.
16. Gavin NI, Gaynes BN, Lohr KN, et al. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005; 106(5 pt 1):1071-1083.
17. Dietz PM, Williams SB, Callaghan WM, et al. Clinically identified maternal depression before, during, and after pregnancies ending in live births. Am J Psychiatry. 2007;164(10): 1515-1520.
18. Almond P. Postnatal depression a global public health perspective. Perspect Public Health. 2009;129(5):221-227.
19. Lee D, Yip A, Chiu H, et al. A psychiatric epidemiological study of postpartum Chinese women. Am J Psychiatry. 2001;158(2):220-226.
20. Cooper PJ, Tomlinson M, Swartz L, et al. Post-partum depression and the mother-infant relationship in a South African peri-urban settlement. Br J Psychiatry. 1999;175:554-558.
21. McCoy SJB, Beal JM, Miller SBM, et al. Risk factors for postpartum depression: a retrospective investigation at 4-weeks postnatal and a review of the literature. J Am Osteopath Assoc. 2006;106(4):193-198.
22. Dennis CL, Janssen PA, Singer J. Identifying women at-risk for postpartum depression in the immediate postpartum period. Acta Psychiatr Scand. 2004;110(5):338-346.
23. Milgrom J, Gemmill AW, Bilszta JL, et al. Antenatal risk factors for postnatal depression: a large prospective study. J Affect Disord. 2008;108(1-2):147-157.
24. Nonacs R, Cohen LS. Postpartum mood disorders: diagnosis and treatment guidelines. J Clin Psychiatry. 1998;59 suppl 2:34-40.
25. Corwin EJ, Murray-Kolb LE, Beard JL. Low hemoglobin level is a risk factor for postpartum depression. J Nutr. 2003;133(12):4139-4142.
26. McCoy SJ, Beal JM, Payton ME, et al. Postpartum thyroid measures and depressive symptomology: a pilot study. J Am Osteopath Assoc. 2008;108(9):503-507.
27. Seehusen DA, Baldwin LM, Runkle GP, Clark G. Are family physicians appropriately screening for postpartum depression? J Am Board Fam Pract. 2005;18(2):104-112.
28. Sheeder J, Kabir K, Stafford B. Screening for postpartum depression at well-child visits: is once enough during the first 6 months of life? Pediatrics. 2009;123(6):e982-e988.
29. Pearlstein T, Howard M, Salisbury A, Zlotnick C. Postpartum depression. Am J Obstet Gynecol. 2009;200(4):357-364.
30. Shaver K. Treating postpartum depression during lactation. Prescriber’s Letter. 2001 Aug;8(8):47.
31. Georgiopoulos AM, Bryan TL, Wollan P, Yawn BP. Routine screening for postpartum depression. J Fam Pract. 2001;50(20):117-122.
32. American College of Obstetricians and Gynecologists, Committee on Obstetric Practice. Committee Opinion No. 453: Screening for depression during and after pregnancy. Obstet Gynecol. 2010;115(2 pt 1):394-395.
33. Chaudron LH, Szilagyi PG, Campbell AT, et al. Legal and ethical considerations: risks and benefits of postpartum depression screening at well-child visits. Pediatrics. 2007;119(1):123-128.
34. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782-786.
35. Beck CT, Gable RK. Postpartum Depression Screening Scale: development and psychometric testing. Nurs Res. 2000;49(5):272-282.
36. Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders, Patient Health Questionnaire. JAMA. 1999; 282(18):1737-44.
37. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
38. Yawn BP, Pace W, Wollan PC, et al. Concordance of Edinburgh Postnatal Depression Scale (EPDS) and Patient Health Questionnaire (PHQ-9) to assess increased risk of depression among postpartum women. J Am Board Fam Med. 2009;22(5):483-491.
39. Murray L, Carothers AD. The validation of the Edinburgh Post-natal Depression Scale on a community sample. Br J Psychiatry. 1990;157: 288-290.
40. Evins GG, Theofrastous JP, Galvin SL. Postpartum depression: a comparison of screening and routine clinical evaluation. Am J Obstet Gynecol. 2000;182(5):1080-1082.
41. Hanusa BH, Scholle SH, Haskett RF, et al. Screening for depression in the postpartum period: a comparison of three instruments. J Womens Health (Larchmt). 2008;17(4):585-596.
42. Gibson J, McKenzie-McHarg K, Shakespeare J, et al. A systematic review of studies validating the Edinburgh postnatal depression scale in antepartum and postpartum women. Acta Psychiatr Scand. 2009;119(5):350-364.
43. Paulden M, Palmer S, Hewitt C, Gilbody S. Screening for postnatal depression in primary care: cost effectiveness analysis. BMJ. 2009; 339:b5203.
44. Dennis CL,Hodnett ED. Psychosocial and psychological interventions for treating postpartum depression. Cochrane Database Syst Rev. 2007;(4):CD006116.
45. Appleby L, Warner R, Whitton A, Faragher B. A controlled study of fluoxetine and cognitive-behavioural counselling in the treatment of postnatal depression. BMJ. 1997;314(7085): 932-936.
46. Ng RC, Hirata CK, Yeung W, et al. Pharmacologic treatment for postpartum depression: a systematic review. Pharmacotherapy. 2010; 30(9):928-941.
47. Whitby DH, Smith KM. The use of tricyclic antidepressants and selective serotonin reuptake inhibitors in women who are breastfeeding. Phamacotherapy. 2005;25(3):411-425.
48. Suri R, Burt VK, Altshuler LL, et al. Fluvoxamine for postpartum depression. Am J Psychiatry. 2001;158(10):1739-1740.
49. Cohen LS, Viguera AC, Bouffard SM, et al. Venlafaxine in the treatment of postpartum depression. J Clin Psychiatry. 2001;62(8):592-596.
50. Wisner KL, Hanusa BH, Perel JM, et al. Postpartum depression: a randomized trial of sertraline versus nortriptyline. J Clin Psychopharmacol. 2006;26(4):353-360.
51. Freeman MP. Postpartum depression treatment and breastfeeding. J Clin Psychiatry. 2009;79(9):e35.
52. Gjerdingen D. The effectiveness of various postpartum depression treatments and the impact of antidepressant drugs on nursing infants. J Am Board Fam Pract. 2003;16(5): 372-382.
53. Wisner KL, Perel JM, Findling RL. Antidepressant treatment during breast-feeding. Am J Psychiatry. 1996;153(9):1132-1137.
54. Hendrick V, Fukuchi A, Altshuler L, et al. Use of sertraline, paroxetine and fluvoxamine by nursing women. Br J Psychiatry. 2001;179: 163-166.
55. Davidson JR. Major depressive disorder treatment guidelines in America and Europe.
J Clin Psychiatry. 2010;71 suppl E1:e04.
56. Moses-Kolko EL, Berga SL, Kalro B, et al. Transdermal estradiol for postpartum depression: a promising treatment option. Clin Obstet Gynecol. 2009;52(3):516-529.
57. Ahokas A, Kaukoranta J, Wahlbeck K, Aito M. Estrogen deficiency in severe postpartum depression: successful treatment with sublingual physiologic 17beta-estradiol: a preliminary study. J Clin Psychiatry. 2001;62(5):
332-336.
58. Gregoire AJ, Kumar R, Everitt B, et al. Transdermal oestrogen for treatment of severe postnatal depression. Lancet. 1996;347(9006): 930-933.
59. Forray A, Ostroff RB. The use of electroconvulsive therapy in postpartum affective disorders. J ECT. 2007;23(3):188-193.
60. Altshuler LL, Cohen L, Szuba MP, et al. Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry. 1996;153(5):592-606.
61. Rabheru K. The use of electroconvulsive therapy in special patient populations. Can J Psychiatry. 2001;46(8):710-719.
62. Dennis CL. The effect of peer support on postpartum depression: a pilot randomized controlled trial. Can J Psychiatry. 2003;48(2): 115-124.
63. Moehler E, Brunner R, Wiebel A, et al. Maternal depressive symptoms in the postnatal period are associated with long-term impairment of mother-child bonding. Arch Womens Ment Health. 2006;9(5):273-278.
64. Dennis CL, McQueen K. The relationship between infant-feeding outcomes and postpartum depression: a qualitative systematic review. Pediatrics. 2009;123(4):e736-e751.
65. Hirst KP, Moutier CY. Postpartum major depression. Am Fam Physician. 2010;82(8): 926-933.
66. Beck CT. The effects of postpartum depression on child development: a meta-analysis. Arch Psychiatr Nurs. 1998;12(1):12-20.
67. Weissman MM, Pilowsky DJ, Wickramaratne PJ, et al. Remissions in maternal depression and child psychopathology: a STAR*D-child report. JAMA. 2006;295(12):1389-1398.
68. Cooper PJ, Murray L. Course and recurrence of postnatal depression: evidence for the specificity of the diagnostic concept. Br J Psychiatry. 1995;166(2):191-195.
69. Kumar R, Robson KM. A prospective study of emotional disorders in childbearing women. Br J Psychiatry. 1984;144:35-47.
70. Howard LM, Hoffbrand S, Henshaw C, et al. Antidepressant prevention of postnatal depression. Cochrane Database Syst Rev. 2005;(2):CD00436.
71. Dennis CL, Ross LE, Grigoriadis S. Psychosocial and psychological interventions for treating antenatal depression. Cochrane Database Syst Rev. 2007;(3):CD006309.
Care of the postpartum patient is an important component of primary care practice—particularly for clinicians who provide women’s health care and are called upon to effectively screen, identify, and manage patients who may be at risk for postpartum depression (PPD). Effective management of the postpartum patient also extends to care of her infant(s) and ideally should involve a multidisciplinary team, including the primary care provider, the pediatrician, and a psychologist or a psychiatrist.1
PPD is commonly described as depression that begins within the first month after delivery.2 It is diagnosed using the same criteria from the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)3 as are used to identify major depressive disorder. According to the DSM-IV, PPD begins within the first four weeks after the infant’s birth.2,3 Although onset can occur at any time between 24 hours after a woman gives birth to several months later, PPD most commonly occurs within the first six weeks after delivery.1
The woman with PPD may be reluctant to report her symptoms for a number of reasons, including lack of motivation or energy, fatigue, embarrassment that she is experiencing such symptoms during a presumably happy time in her life—even fear that her child may be removed from her care.4 In addition to depressive symptoms (eg, sleeping difficulty, appetite changes, anhedonia, and guilt), women affected by PPD often experience anxiety and may become obsessed with the health, feeding, and sleeping behaviors of their infants.1,5
Postpartum mood disorders occur in a spectrum, ranging from postpartum baby blues to postpartum psychosis. By DSM-IV definition, a diagnosis of major or minor PPD requires that depressed mood, loss of interest or pleasure, or other characteristic symptoms be present most of the day, nearly every day, for at least two weeks.3,6 PPD must be differentiated from the more common and less severe baby blues, which usually start two to three days after delivery and last less than two weeks.2,7 Between 40% and 80% of women who have given birth experience baby blues, which are characterized by transient mood swings, irritability, crying spells, difficulty sleeping, and difficulty concentrating.8 Experiencing baby blues may place women at increased risk for PPD.9
Postpartum, or puerperal, psychosis occurs in approximately 0.2% of women,10 with an early and sudden onset—that is, within the first week to four weeks postpartum. This severe condition is characterized by hallucinations and delusions often centered on the infant, in addition to insomnia, agitation, and extreme behavioral changes. Postpartum psychosis, which can recur in subsequent pregnancies, may be a manifestation of a preexisting affective disorder, such as bipolar disorder.10 Postpartum psychosis is considered an emergent condition because the safety and well-being of both mother and infant may be at serious risk.2,10
EPIDEMIOLOGY AND RISK FACTORS
PPD is often cited as affecting 10% to 15% of women within the first year after childbirth.11,12 Currently, reported prevalence rates of PPD range from 5% to 20% of women who have recently given birth, depending on the source of information. Among 17 US states participating in the Pregnancy Risk Assessment Monitoring System, a CDC surveillance project, prevalence of self-reported PPD symptoms ranged from 11.7% in Maine to 20.4% in New Mexico.11,13 According to the Agency for Healthcare Research and Quality (AHRQ),14 the prevalence of major or minor depression ranges from 6.5% to 12.9% at different times during the year following delivery, although study design varied throughout the research used, and confidence intervals were deemed wide.14,15
Nevertheless, according to results from a systematic review of 28 studies, the prevalence of minor or major depression is estimated at up to 19.2% of women during the first three months postpartum, and major depression in up to 7% during that time.16 A 2007 chart review of 4,398 US women experiencing live births identified PPD in 10.4%.17
Incidence of PPD is “much higher than the quoted rate of 10% to 15%,” concludes Almond18 after a comprehensive literature review. The condition also affects women globally, the British researcher reports: Not only did she find numerous data on the incidence of PPD in high-income countries, including the US, the United Kingdom, and Australia, but she concluded that incidence rates of PPD in developing countries are grossly underestimated, according to epidemiologic studies in low- and middle-income countries (eg, Pakistan, Indonesia, Vietnam). Additionally, the risk factors for PPD are likely to be influenced by cultural differences, and attempts to identify PPD must be culturally sensitive.18
Several risk factors have been associated with PPD. Perhaps the most significant risk factor is a personal history of depression (prior to pregnancy or postpartum); at least one-half of women with PPD experience onset of depressive symptoms before or during their pregnancies,19,20 and one research group reported a relative risk (RR) of 1.87 for PPD in women with a history of depression, compared with those without such a history.21 Thus, women previously affected by depression should be carefully monitored in the immediate postpartum period for any signs of depressed mood, anxiety, sleep difficulties, loss of appetite or energy, and psychomotor changes.
According to McCoy et al,21 neither patient age nor marital status nor method of delivery appeared to be associated with PPD at four weeks postpartum. Women who were feeding by formula alone were more likely to experience PPD (RR, 2.04) than were those who breastfed their infants. Women who smoked were more likely to be affected by PPD (RR, 1.58) than were nonsmokers.
Additional risk factors for PPD identified by Dennis et al22 included pregnancy-induced hypertension and immigration within the previous five years. Various psychosocial stressors may also represent risk factors for PPD, including lack of social support, financial concerns, miscarriage or fetal demise, limited partner support, physical abuse before or during pregnancy, lack of readiness for hospital discharge, and complications during pregnancy and delivery (eg, low birth weight, premature birth, admission of the infant to the neonatal ICU).11,22,23
It is important to identify women who are at highest risk for PPD as soon as possible. High-risk patients should be screened upon discharge from the hospital and certainly on or before the first postpartum visit.
CLINICAL MANIFESTATIONS
Clinical manifestations of PPD include depressed mood for at least two weeks with changes in somatic functions, such as sleep, energy level, appetite, weight, gastrointestinal functioning, and decrease in libido.3,24 These manifestations are more severe and prolonged than those associated with baby blues (which almost always resolve within two weeks postpartum).2,7
On physical examination, the patient with PPD may appear tearful and disheveled, with psychomotor retardation. She may report that she is unable to sleep even when her infant is sleeping, or that she has a significant lack of energy despite sufficient sleep; she may admit being unable to get out of bed for hours.2 The patient may report a significant decrease in appetite and little enjoyment in eating, which may lead to rapid weight loss.
Other symptoms may include obsessive thoughts about the infant and his or her care, significant anxiety (possibly manifested in panic attacks), uncontrollable crying, guilt, feelings of being overwhelmed or unable to care for the infant, mood swings, and severe irritability or even anger.2
Severe fatigue may warrant hemoglobin/hematocrit evaluation and possibly measurement of serum thyroid-stimulating hormone (TSH).25,26
It is essential to rule out postpartum psychosis, which is associated with prolonged lack of sleep, confusion, lapsed insight, cognitive impairment, “grossly disorganized behavior,”10 and delusions or hallucinations.5,10 The patient should be asked specifically about unusual or bizarre thoughts or beliefs concerning the infant, in addition to thoughts of harming herself or others, particularly the infant.10
SCREENING
Numerous researchers have suggested that PPD is underrecognized and undertreated.5,6,18,27,28 Screening for PPD in the United States is not standardized and is highly variable.29 The American Academy of Family Physicians supports universal screening for PPD at the first postpartum visit, between two and six weeks.30,31 According to a 2010 Committee Opinion from the American College of Obstetricians and Gynecologists, “at this time, there is insufficient evidence to support a firm recommendation for universal antepartum or postpartum screening; however, screening for depression has the potential to benefit a woman and her family and should be strongly considered.”32
As the AHRQ14 notes, symptoms of PPD may not peak in some women until after their first postpartum visit, and providers of family medicine, internal medicine, and pediatric care may also be in a position to provide screening. The initial well-baby examination by the pediatric primary care provider, for example, presents an important opportunity to screen new mothers for PPD. According to Chaudron et al,33 the well-being of the infant should outweigh any scope-of-practice concerns, practitioner time limitations, or reimbursement issues; rather, screening efforts can be considered “tools to enhance [mothers’] ability to care for their children in a way that is supportive and not punitive.”33
In 2009, Sheeder and colleagues28 reported on a prospective study of 199 mothers in an adolescent maternity clinic who were screened using the Edinburgh Postnatal Depression Scale (EPDS)34 at each well-baby visit during the first six months postpartum. The authors concluded that the optimal time for screening for PPD in this setting is two months after delivery, although repeated screening may identify worsening of depressive symptoms.28
Several screening tools are available for the detection of PPD, particularly the EPDS,34 the Postpartum Depression Screening Scale,35 and the Patient Health Questionnaire–936-38 (see table,11,13,34-37 ). The EPDS, a widely used and well-validated formal screening tool,27,37 is a 10-item self-report questionnaire designed to detect depression in the postpartum period. Cox et al,34 who developed the scale, initially reported its sensitivity at 86% and specificity at 78%21; since then, the tool’s reported sensitivity for detecting major depression in the postpartum period has ranged from 60% to 96%, and specificity from 45% to 97%.6,39 The EPDS has been shown to result in a diagnosis of PPD in significantly more women than routine clinical evaluation (35.4% vs 6.3%, respectively).40 It is possible to administer the EPDS by telephone.41
The validity of the EPDS tool in detecting PPD was recently examined in a systematic review of 37 studies. Gibson et al42 concluded that the heterogeneity of these studies (ie, differences in study methodology, language used, and diagnostic criteria) precluded meta-analysis and did not provide clear support of EPDS as an accurate screening tool for PPD, especially across diverse cultures. In a similar review, Hewitt and colleagues6 sought to “provide an overview of all available methods to identify postnatal depression in primary care and to assess their validity.” They concluded that the EPDS is the most frequently reported screening tool and, with an overall sensitivity of 86% and overall specificity of 87%, its diagnostic performance seems “reasonably good.”6 Of note, fewer data have been collected to demonstrate the effectiveness of other screening tools.35,36,38,41
Cost-effectiveness is a consideration in the use of screening programs for PPD. According to a hypothetical cohort analysis conducted in the UK, the costs of treating women with false-positive screening results made implementation of a formal screening strategy for PPD not cost-effective, compared with usual care only, for use by the British National Health Service.43
MANAGEMENT
Once PPD is identified, treatment should be initiated as quickly as possible; referral for psychological counseling is an appropriate initial strategy for mild to moderate symptoms of PPD.5 A clinical care manager can be a valuable resource to provide education and coordination of care for women affected by PPD. NPs and PAs in primary care, obstetrics/gynecology, women’s health, and psychiatry or psychology can play an important role in the identification and management of PPD.
Treatment of PPD involves combination therapy—short-term psychological therapy combined with pharmacotherapy. According to investigators in a Cochrane Review of nine trials reporting short-term outcomes for 956 women with PPD, their findings suggest that psychosocial and psychological interventions are an effective option for reducing symptoms of PPD.44 Compared with usual care, the types of psychological therapy that were found most effective included cognitive behavioral therapy, interpersonal therapy, and psychodynamic therapy. As most trials’ follow-up periods were limited to six months, however, neither the long-term effects of psychological therapy nor the relative effectiveness of each type of therapy was made clear by these studies.44,45
Pharmacotherapy
Although antidepressant drugs are known to be effective for the treatment of major depressive disorder, well-designed clinical trials demonstrating the overall effectiveness of antidepressants in treatment of PPD have been limited.46 According to Ng et al,46 who in 2010 performed a systematic review of studies examining pharmacologic interventions for PPD, preliminary evidence showing the effectiveness of antidepressants and hormone therapy should prompt the initiation of larger, more rigorous randomized and controlled trials.
The choice of antidepressants will be influenced by the mother’s breastfeeding status and whether PPD represents her first episode of depression or a recurrence of previous major depression. If the patient is not breastfeeding, the choice among antidepressants is similar to those used for treatment of nonpuerperal major depression. If PPD is a relapse of a prior depression, the therapeutic agent that was most effective and best-tolerated for previous depression should be prescribed.47
Generally, the SSRIs are considered first-line agents because of their superior safety profile.47 Fluoxetine has been shown in a small randomized trial (n = 87) to be significantly more effective than placebo and as effective as a full course of cognitive-behavioral counseling.45 In non–placebo-controlled studies, sertraline, fluvoxamine, and venlafaxine all produced improvement in PPD symptoms.48,49
Whether the mother is breastfeeding her infant will influence the use and choice of antidepressants for PPD. Although barely detectable levels of certain antidepressant medications (including the SSRIs sertraline and paroxetine, and the tricyclic antidepressant nortriptyline) have been reported in breast milk or in infant serum,50,51 it is recommended that the lowest possible therapeutic dose be prescribed, and that infants be carefully monitored for adverse effects.51
Fluoxetine, it should be noted, has been found to be transmitted through breast milk and was associated with reduced infant weight gain (specifically, by 392 g over six months) in a comparison between 64 fluoxetine-treated mothers and 38 non-treated mother-infant pairs.52 Thus, fluoxetine use should be avoided in women who are breastfeeding.
In small studies, paroxetine and fluvoxamine were not detected in infant serum, and although low levels of sertraline were detected in one-fourth of infants whose mothers received doses exceeding 100 mg/d, no adverse infant outcomes were noted.52-54 Paroxetine, nortriptyline, and sertraline appear to be relatively safer antidepressant choices in breastfeeding women with PPD.50,52,53
Antidepressants should be continued for six months after full remission of depressive symptoms. Longer courses of therapy may be necessary in patients who experience recurrent major depressive episodes.55
Few researchers have reported on the use of hormonal therapy for PPD. Yet significant hormonal fluctuations,56,57 including “estrogen withdrawal at parturition,”56 are known to occur after childbirth; in their study of women with severe PPD, Ahokas et al57 found that two-thirds of participants had serum estradiol concentrations below the cutoff for gonadal failure. Such a deficiency is likely to contribute to mood disturbances.56,57
In a small, double-blind, placebo-controlled study that enrolled women with severe, persistent PPD (mean EPDS score, 21.8), six months’ treatment with transdermally administered estradiol was associated with significantly greater relief of depressive symptoms than was found in controls (mean EPDS scores at one month, 13.3 vs 16.5, respectively). Of note, more than half of the women studied were concurrently receiving antidepressants.58
Additional studies may elucidate the role of estrogen therapy in the treatment of PPD.
Nonpharmacologic Options
An effective nonpharmacologic option for rapid resolution of severe symptoms of PPD is electroconvulsive therapy (ECT).59-61 Its use is safe in nursing mothers because it does not affect breast milk. ECT is considered especially useful for women who have not responded to pharmacotherapy, those experiencing severe psychotic depression, and those who are considered at high risk for suicide or infanticide.
Patients typically receive three treatments per week; three to six treatments often produce an effective response.59 Anesthesia administered to women who undergo ECT has not been shown to have a negative effect on infants who are being breastfed.60,61
Interpersonal strategies to address PPD should not be overlooked. A pilot study conducted in Canada showed promising results when women who had previously experienced PPD were trained to provide peer support by telephone to mothers who were deemed at high risk for PPD (ie, those with EPDS scores > 12). At four weeks and eight weeks postpartum, follow-up EPDS scores exceeded 12 in 10% and 15%, respectively, of women receiving peer support, compared with 41% and 52%, respectively, of controls.62
Participation in one of numerous support groups that exist for women with PPD (see box, for online information) may reduce isolation in these women and possibly offer additional benefits.
COMPLICATIONS
PPD may be associated with significant complications, underscoring the importance of prompt identification and treatment.5 Maternal depressive symptoms in the critical postnatal period, for example, have been associated with long-term impairment of mother-child bonding. In one study of 101 women, lower-quality maternal bonding was found in women who had symptoms of depression at two weeks, six weeks, and four months postpartum—but not in those with depression at 14 months.63 Additionally, it was found in a systematic review of 49 studies that women with PPD were likely to discontinue breastfeeding earlier than women not affected.64
Delayed growth and development has been reported in infants of mothers with untreated or inadequately treated PPD.65 It has also been suggested that children of depressed mothers may have an increased risk for anxiety, depression, hyperactivity, and other behavioral disorders later in childhood.65-67
PROGNOSIS
Untreated PPD may resolve spontaneously within three to six months, but in about one-quarter of PPD patients, depressive symptoms persist one year after delivery.24,68,69 PPD increases a woman’s risk for future episodes of major depression.2,5
PREVENTION
As previously discussed, the risk for PPD is greatest in women with a history of mood disorders (25%) and PPD (50%).2 Although several approaches have been studied to prevent PPD, no clear optimal strategy has been revealed. In one Cochrane review, insufficient evidence was found to justify prophylactic use of antidepressants.70 Similarly, findings in a second review fell short of confirming the effectiveness of prenatal psychosocial or psychological interventions to prevent antenatal depression.71
Additional studies are needed to make recommendations on prevention of PPD in high-risk patients. Until such recommendations emerge, close monitoring, screening, and follow-up are essential for these women.
CONCLUSION
Postpartum depression is an important concern among childbearing women, as it is associated with adverse maternal and infant outcomes. A personal history of depression is a major risk factor for PPD. It is imperative to question women about signs and symptoms of depression during the immediate postpartum period; it is particularly important to inquire about thoughts of harm to self or to the infant.
Pharmacotherapy combined with adjunctive psychological therapy is indicated for new mothers with significant depressive symptoms. The choice of antidepressants is based on previous response to antidepressants and the woman’s breastfeeding status. Generally, SSRIs are effective and well tolerated for major depression; based on results from small studies, they appear to be safe for breastfeeding mothers.
Electroconvulsive therapy is considered a safe and effective option for women with severe symptoms of PPD.
REFERENCES
1. Muzik M, Marcus SM, Heringhausen JE, Flynn H. When depression complicates childbearing: guidelines for screening and treatment during antenatal and postpartum obstetric care. Obstet Gynecol Clin North Am. 2009;36(4):771-788.
2. Wisner KL, Parry BL, Piontek CM. Clinical practice: postpartum depression. N Engl J Med. 2002;347(3):194-199.
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
4. Whitton A, Warner R, Appleby L. The pathway to care in post-natal depression: women’s attitudes to post-natal depression and its treatment. Br J Gen Pract. 1996;46(408):427-428.
5. Sit DK, Wisner KL. Identification of postpartum depression. Clin Obstet Gynecol. 2009; 52(3):456-468.
6. Hewitt C, Gilbody S, Brealey S, et al. Methods to identify postnatal depression in primary care: an integrated evidence synthesis and value of information analysis. Health Technol Assess. 2009;13(36):1-145, 147-230.
7. Epperson CN. Postpartum major depression: detection and treatment. Am Fam Physician. 1999;59(8):2247-2254, 2259-2260.
8. O’Hara MW, Schlechte JA, Lewis DA, Wright EJ. Prospective study of postpartum blues: biologic and psychosocial factors. Arch Gen Psychiatry. 1991;48(9):801-806.
9. Hannah P, Adams D, Lee A, et al. Links between early post-partum mood and post-natal depression. Br J Psychiatry. 1992;160:777-780.
10. Sit D, Rothschild AJ, Wisner KL. A review of postpartum psychosis. J Womens Health (Larchmt). 2006;15(4):352-368.
11. CDC. Prevalence of self-reported postpartum depressive symptoms—17 states, 2004–2005. MMWR Weekly. 2008;57(14):361-366.
12. O’Hara MW, Swain AM. Rates and risk of postpartum depression: a meta-analysis. Int Rev Psychiatry. 1996;8:37-54.
13. Whooley MA, Avins AL, Miranda J, Browner WS. Case-finding instruments for depression: two questions are as good as many. J Gen Intern Med. 1997;12(7):439-445.
14. Agency for Healthcare Research and Quality. Perinatal Depression: Prevalence, Screening Accuracy, and Screening Outcomes: Summary. Evidence Report/Technology Assessment No. 119. www.ahrq.gov/clinic/epcsums/peridep sum.htm. Accessed January 24, 2011.
15. Gaynes BN, Gavin N, Meltzer-Brody S, et al. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evid Rep Technol Assess (Summ). 2005 Feb;(119):1-8.
16. Gavin NI, Gaynes BN, Lohr KN, et al. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005; 106(5 pt 1):1071-1083.
17. Dietz PM, Williams SB, Callaghan WM, et al. Clinically identified maternal depression before, during, and after pregnancies ending in live births. Am J Psychiatry. 2007;164(10): 1515-1520.
18. Almond P. Postnatal depression a global public health perspective. Perspect Public Health. 2009;129(5):221-227.
19. Lee D, Yip A, Chiu H, et al. A psychiatric epidemiological study of postpartum Chinese women. Am J Psychiatry. 2001;158(2):220-226.
20. Cooper PJ, Tomlinson M, Swartz L, et al. Post-partum depression and the mother-infant relationship in a South African peri-urban settlement. Br J Psychiatry. 1999;175:554-558.
21. McCoy SJB, Beal JM, Miller SBM, et al. Risk factors for postpartum depression: a retrospective investigation at 4-weeks postnatal and a review of the literature. J Am Osteopath Assoc. 2006;106(4):193-198.
22. Dennis CL, Janssen PA, Singer J. Identifying women at-risk for postpartum depression in the immediate postpartum period. Acta Psychiatr Scand. 2004;110(5):338-346.
23. Milgrom J, Gemmill AW, Bilszta JL, et al. Antenatal risk factors for postnatal depression: a large prospective study. J Affect Disord. 2008;108(1-2):147-157.
24. Nonacs R, Cohen LS. Postpartum mood disorders: diagnosis and treatment guidelines. J Clin Psychiatry. 1998;59 suppl 2:34-40.
25. Corwin EJ, Murray-Kolb LE, Beard JL. Low hemoglobin level is a risk factor for postpartum depression. J Nutr. 2003;133(12):4139-4142.
26. McCoy SJ, Beal JM, Payton ME, et al. Postpartum thyroid measures and depressive symptomology: a pilot study. J Am Osteopath Assoc. 2008;108(9):503-507.
27. Seehusen DA, Baldwin LM, Runkle GP, Clark G. Are family physicians appropriately screening for postpartum depression? J Am Board Fam Pract. 2005;18(2):104-112.
28. Sheeder J, Kabir K, Stafford B. Screening for postpartum depression at well-child visits: is once enough during the first 6 months of life? Pediatrics. 2009;123(6):e982-e988.
29. Pearlstein T, Howard M, Salisbury A, Zlotnick C. Postpartum depression. Am J Obstet Gynecol. 2009;200(4):357-364.
30. Shaver K. Treating postpartum depression during lactation. Prescriber’s Letter. 2001 Aug;8(8):47.
31. Georgiopoulos AM, Bryan TL, Wollan P, Yawn BP. Routine screening for postpartum depression. J Fam Pract. 2001;50(20):117-122.
32. American College of Obstetricians and Gynecologists, Committee on Obstetric Practice. Committee Opinion No. 453: Screening for depression during and after pregnancy. Obstet Gynecol. 2010;115(2 pt 1):394-395.
33. Chaudron LH, Szilagyi PG, Campbell AT, et al. Legal and ethical considerations: risks and benefits of postpartum depression screening at well-child visits. Pediatrics. 2007;119(1):123-128.
34. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782-786.
35. Beck CT, Gable RK. Postpartum Depression Screening Scale: development and psychometric testing. Nurs Res. 2000;49(5):272-282.
36. Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders, Patient Health Questionnaire. JAMA. 1999; 282(18):1737-44.
37. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
38. Yawn BP, Pace W, Wollan PC, et al. Concordance of Edinburgh Postnatal Depression Scale (EPDS) and Patient Health Questionnaire (PHQ-9) to assess increased risk of depression among postpartum women. J Am Board Fam Med. 2009;22(5):483-491.
39. Murray L, Carothers AD. The validation of the Edinburgh Post-natal Depression Scale on a community sample. Br J Psychiatry. 1990;157: 288-290.
40. Evins GG, Theofrastous JP, Galvin SL. Postpartum depression: a comparison of screening and routine clinical evaluation. Am J Obstet Gynecol. 2000;182(5):1080-1082.
41. Hanusa BH, Scholle SH, Haskett RF, et al. Screening for depression in the postpartum period: a comparison of three instruments. J Womens Health (Larchmt). 2008;17(4):585-596.
42. Gibson J, McKenzie-McHarg K, Shakespeare J, et al. A systematic review of studies validating the Edinburgh postnatal depression scale in antepartum and postpartum women. Acta Psychiatr Scand. 2009;119(5):350-364.
43. Paulden M, Palmer S, Hewitt C, Gilbody S. Screening for postnatal depression in primary care: cost effectiveness analysis. BMJ. 2009; 339:b5203.
44. Dennis CL,Hodnett ED. Psychosocial and psychological interventions for treating postpartum depression. Cochrane Database Syst Rev. 2007;(4):CD006116.
45. Appleby L, Warner R, Whitton A, Faragher B. A controlled study of fluoxetine and cognitive-behavioural counselling in the treatment of postnatal depression. BMJ. 1997;314(7085): 932-936.
46. Ng RC, Hirata CK, Yeung W, et al. Pharmacologic treatment for postpartum depression: a systematic review. Pharmacotherapy. 2010; 30(9):928-941.
47. Whitby DH, Smith KM. The use of tricyclic antidepressants and selective serotonin reuptake inhibitors in women who are breastfeeding. Phamacotherapy. 2005;25(3):411-425.
48. Suri R, Burt VK, Altshuler LL, et al. Fluvoxamine for postpartum depression. Am J Psychiatry. 2001;158(10):1739-1740.
49. Cohen LS, Viguera AC, Bouffard SM, et al. Venlafaxine in the treatment of postpartum depression. J Clin Psychiatry. 2001;62(8):592-596.
50. Wisner KL, Hanusa BH, Perel JM, et al. Postpartum depression: a randomized trial of sertraline versus nortriptyline. J Clin Psychopharmacol. 2006;26(4):353-360.
51. Freeman MP. Postpartum depression treatment and breastfeeding. J Clin Psychiatry. 2009;79(9):e35.
52. Gjerdingen D. The effectiveness of various postpartum depression treatments and the impact of antidepressant drugs on nursing infants. J Am Board Fam Pract. 2003;16(5): 372-382.
53. Wisner KL, Perel JM, Findling RL. Antidepressant treatment during breast-feeding. Am J Psychiatry. 1996;153(9):1132-1137.
54. Hendrick V, Fukuchi A, Altshuler L, et al. Use of sertraline, paroxetine and fluvoxamine by nursing women. Br J Psychiatry. 2001;179: 163-166.
55. Davidson JR. Major depressive disorder treatment guidelines in America and Europe.
J Clin Psychiatry. 2010;71 suppl E1:e04.
56. Moses-Kolko EL, Berga SL, Kalro B, et al. Transdermal estradiol for postpartum depression: a promising treatment option. Clin Obstet Gynecol. 2009;52(3):516-529.
57. Ahokas A, Kaukoranta J, Wahlbeck K, Aito M. Estrogen deficiency in severe postpartum depression: successful treatment with sublingual physiologic 17beta-estradiol: a preliminary study. J Clin Psychiatry. 2001;62(5):
332-336.
58. Gregoire AJ, Kumar R, Everitt B, et al. Transdermal oestrogen for treatment of severe postnatal depression. Lancet. 1996;347(9006): 930-933.
59. Forray A, Ostroff RB. The use of electroconvulsive therapy in postpartum affective disorders. J ECT. 2007;23(3):188-193.
60. Altshuler LL, Cohen L, Szuba MP, et al. Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry. 1996;153(5):592-606.
61. Rabheru K. The use of electroconvulsive therapy in special patient populations. Can J Psychiatry. 2001;46(8):710-719.
62. Dennis CL. The effect of peer support on postpartum depression: a pilot randomized controlled trial. Can J Psychiatry. 2003;48(2): 115-124.
63. Moehler E, Brunner R, Wiebel A, et al. Maternal depressive symptoms in the postnatal period are associated with long-term impairment of mother-child bonding. Arch Womens Ment Health. 2006;9(5):273-278.
64. Dennis CL, McQueen K. The relationship between infant-feeding outcomes and postpartum depression: a qualitative systematic review. Pediatrics. 2009;123(4):e736-e751.
65. Hirst KP, Moutier CY. Postpartum major depression. Am Fam Physician. 2010;82(8): 926-933.
66. Beck CT. The effects of postpartum depression on child development: a meta-analysis. Arch Psychiatr Nurs. 1998;12(1):12-20.
67. Weissman MM, Pilowsky DJ, Wickramaratne PJ, et al. Remissions in maternal depression and child psychopathology: a STAR*D-child report. JAMA. 2006;295(12):1389-1398.
68. Cooper PJ, Murray L. Course and recurrence of postnatal depression: evidence for the specificity of the diagnostic concept. Br J Psychiatry. 1995;166(2):191-195.
69. Kumar R, Robson KM. A prospective study of emotional disorders in childbearing women. Br J Psychiatry. 1984;144:35-47.
70. Howard LM, Hoffbrand S, Henshaw C, et al. Antidepressant prevention of postnatal depression. Cochrane Database Syst Rev. 2005;(2):CD00436.
71. Dennis CL, Ross LE, Grigoriadis S. Psychosocial and psychological interventions for treating antenatal depression. Cochrane Database Syst Rev. 2007;(3):CD006309.