User login
Jennifer Smith is the editor of Oncology Practice, part of MDedge Hematology/Oncology. She was previously the editor of Hematology Times, an editor at Principal Investigators Association, and a reporter at The Oneida Daily Dispatch. She has a BS in journalism.
Investigators target brain metastases in NSCLC
Researchers say they have identified potential prognostic markers and targets for treatment in patients with non–small cell lung cancer (NSCLC) and brain metastases (BM).
There was “substantial” concordance in driver mutations, such as EGFR and KRAS, between BM and primary tumor samples but greater genomic instability in BM samples, reported Hong-Sheng Wang, PhD, of Sun Yat-Sen University in Guangzhou, China, and colleagues.
PI3K signaling was significantly associated with an increased risk of BM, and CDKs and PIK3CA were “potential druggable mutations,” they reported in Cancer.
The researchers conducted a retrospective study of 61 Chinese patients with NSCLC and BM. The patients had adenocarcinoma (n = 50), squamous cell carcinoma (n = 3), and mixed subtypes (n = 8). Less than half of patients (n = 28) had stage IV disease at diagnosis, 36 had metachronous disease, and 25 had synchronous disease. All patients had undergone surgery, 33 had received chemoradiation, 26 had received no systemic treatment, and 5 had been treated with tyrosine kinase inhibitors.
The researchers performed next-generation sequencing on primary tumors and matched BM samples from the 61 patients, targeting 416 cancer-relevant genes.
Results showed high concordance between the primary and BM samples with regard to major driver mutations – 92% for EGFR, 82% for KRAS, and 83% for TP53 mutations.
For nearly half of patients (48%), all mutations found in primary tumor samples were also found in BM samples. In fact, 18% of patients had the same mutational profiles in lung and brain lesions.
Conversely, 30% of patients had more brain-specific mutations, 13% had more lung-specific mutations, 28% had mixed profiles, and 11% had completely unique mutational profiles in lung and brain lesions.
Compared with primary tumor samples, BM samples had a significantly higher frequency of copy number variations (P = .0002); alterations in CDKN2A/2B, CCND1, CDK4, and RB1 (P = .0019); and alterations in PIK3CA, PTEN, STK11, RICTOR, and NF2 (P = .0037).
Patients with activated PI3K signaling in their primary tumors had significantly shorter BM-free survival. The hazard ratio (adjusted for baseline clinicopathologic parameters) was 8.49 (P = .0005).
There was no significant difference in BM-free survival between EGFR-/KRAS-mutated patients and patients with wild-type EGFR/KRAS (P = .29). However, there was a trend toward shorter BM-free survival in patients with TP53 mutations (P = .15).
There was a trend toward shorter BM-free intervals in patients with an activated WNT pathway via CTNNB1 mutations (P = .22) or APC and AXIN2 mutations (P = .015). However, the researchers said these findings should be treated with caution due to a small sample size.
The National Natural Science Foundation of China and the Natural Science Foundation of Guangdong Province supported the research. The researchers disclosed relationships with Geneseeq Technology Inc. in Toronto and Nanjing Geneseeq Technology Inc.
SOURCE: Wang H et al. Cancer. 2019 Jul 9. doi: 10.1002/cncr.32372.
Researchers say they have identified potential prognostic markers and targets for treatment in patients with non–small cell lung cancer (NSCLC) and brain metastases (BM).
There was “substantial” concordance in driver mutations, such as EGFR and KRAS, between BM and primary tumor samples but greater genomic instability in BM samples, reported Hong-Sheng Wang, PhD, of Sun Yat-Sen University in Guangzhou, China, and colleagues.
PI3K signaling was significantly associated with an increased risk of BM, and CDKs and PIK3CA were “potential druggable mutations,” they reported in Cancer.
The researchers conducted a retrospective study of 61 Chinese patients with NSCLC and BM. The patients had adenocarcinoma (n = 50), squamous cell carcinoma (n = 3), and mixed subtypes (n = 8). Less than half of patients (n = 28) had stage IV disease at diagnosis, 36 had metachronous disease, and 25 had synchronous disease. All patients had undergone surgery, 33 had received chemoradiation, 26 had received no systemic treatment, and 5 had been treated with tyrosine kinase inhibitors.
The researchers performed next-generation sequencing on primary tumors and matched BM samples from the 61 patients, targeting 416 cancer-relevant genes.
Results showed high concordance between the primary and BM samples with regard to major driver mutations – 92% for EGFR, 82% for KRAS, and 83% for TP53 mutations.
For nearly half of patients (48%), all mutations found in primary tumor samples were also found in BM samples. In fact, 18% of patients had the same mutational profiles in lung and brain lesions.
Conversely, 30% of patients had more brain-specific mutations, 13% had more lung-specific mutations, 28% had mixed profiles, and 11% had completely unique mutational profiles in lung and brain lesions.
Compared with primary tumor samples, BM samples had a significantly higher frequency of copy number variations (P = .0002); alterations in CDKN2A/2B, CCND1, CDK4, and RB1 (P = .0019); and alterations in PIK3CA, PTEN, STK11, RICTOR, and NF2 (P = .0037).
Patients with activated PI3K signaling in their primary tumors had significantly shorter BM-free survival. The hazard ratio (adjusted for baseline clinicopathologic parameters) was 8.49 (P = .0005).
There was no significant difference in BM-free survival between EGFR-/KRAS-mutated patients and patients with wild-type EGFR/KRAS (P = .29). However, there was a trend toward shorter BM-free survival in patients with TP53 mutations (P = .15).
There was a trend toward shorter BM-free intervals in patients with an activated WNT pathway via CTNNB1 mutations (P = .22) or APC and AXIN2 mutations (P = .015). However, the researchers said these findings should be treated with caution due to a small sample size.
The National Natural Science Foundation of China and the Natural Science Foundation of Guangdong Province supported the research. The researchers disclosed relationships with Geneseeq Technology Inc. in Toronto and Nanjing Geneseeq Technology Inc.
SOURCE: Wang H et al. Cancer. 2019 Jul 9. doi: 10.1002/cncr.32372.
Researchers say they have identified potential prognostic markers and targets for treatment in patients with non–small cell lung cancer (NSCLC) and brain metastases (BM).
There was “substantial” concordance in driver mutations, such as EGFR and KRAS, between BM and primary tumor samples but greater genomic instability in BM samples, reported Hong-Sheng Wang, PhD, of Sun Yat-Sen University in Guangzhou, China, and colleagues.
PI3K signaling was significantly associated with an increased risk of BM, and CDKs and PIK3CA were “potential druggable mutations,” they reported in Cancer.
The researchers conducted a retrospective study of 61 Chinese patients with NSCLC and BM. The patients had adenocarcinoma (n = 50), squamous cell carcinoma (n = 3), and mixed subtypes (n = 8). Less than half of patients (n = 28) had stage IV disease at diagnosis, 36 had metachronous disease, and 25 had synchronous disease. All patients had undergone surgery, 33 had received chemoradiation, 26 had received no systemic treatment, and 5 had been treated with tyrosine kinase inhibitors.
The researchers performed next-generation sequencing on primary tumors and matched BM samples from the 61 patients, targeting 416 cancer-relevant genes.
Results showed high concordance between the primary and BM samples with regard to major driver mutations – 92% for EGFR, 82% for KRAS, and 83% for TP53 mutations.
For nearly half of patients (48%), all mutations found in primary tumor samples were also found in BM samples. In fact, 18% of patients had the same mutational profiles in lung and brain lesions.
Conversely, 30% of patients had more brain-specific mutations, 13% had more lung-specific mutations, 28% had mixed profiles, and 11% had completely unique mutational profiles in lung and brain lesions.
Compared with primary tumor samples, BM samples had a significantly higher frequency of copy number variations (P = .0002); alterations in CDKN2A/2B, CCND1, CDK4, and RB1 (P = .0019); and alterations in PIK3CA, PTEN, STK11, RICTOR, and NF2 (P = .0037).
Patients with activated PI3K signaling in their primary tumors had significantly shorter BM-free survival. The hazard ratio (adjusted for baseline clinicopathologic parameters) was 8.49 (P = .0005).
There was no significant difference in BM-free survival between EGFR-/KRAS-mutated patients and patients with wild-type EGFR/KRAS (P = .29). However, there was a trend toward shorter BM-free survival in patients with TP53 mutations (P = .15).
There was a trend toward shorter BM-free intervals in patients with an activated WNT pathway via CTNNB1 mutations (P = .22) or APC and AXIN2 mutations (P = .015). However, the researchers said these findings should be treated with caution due to a small sample size.
The National Natural Science Foundation of China and the Natural Science Foundation of Guangdong Province supported the research. The researchers disclosed relationships with Geneseeq Technology Inc. in Toronto and Nanjing Geneseeq Technology Inc.
SOURCE: Wang H et al. Cancer. 2019 Jul 9. doi: 10.1002/cncr.32372.
FROM CANCER
Endoscopic treatment effective in T1b esophageal cancer
Findings from a systematic review may help guide treatment of patients with T1b esophageal cancer.
The review suggests that endoscopic submucosal dissection and endoscopic mucosal resection are appropriate for T1b esophageal cancers with a low risk of metastasis. The authors identified several factors associated with a higher risk of lymph node metastasis and said patients with these risk factors may benefit from adjuvant chemotherapy and radiation.
Mohamed O. Othman, MD, of Baylor College of Medicine, Houston, and colleagues conducted the review. Their report is in Clinical Gastroenterology and Hepatology.
The authors cited studies suggesting that survival rates would not be significantly different among early-stage esophageal cancer patients who undergo esophagectomy and those who receive endoscopic treatment, in the absence of lymph node metastasis. The risk of lymph node metastasis is higher in esophageal squamous cell carcinoma (ESCC) than in esophageal adenocarcinoma (EAC), and in those with deeper submucosal invasion (greater than 200 microm for ESCC or greater than 500 microm for EAC). Patients also have a higher risk of lymph node metastasis if they have poorly differentiated tumors, tumors larger than 2 cm, or lymphovascular invasion.
In the studies cited in the review, overall 5-year survival for esophageal squamous cell CA (ESCC) ranged from 68.6% (chemoradiation therapy alone) to 75% (endoscopic resection followed by chemoradiation), compared with 77.7% for surgery. For esophageal adenocarcinoma (EAC), there is less data but greater efficacy, with one study demonstrating 5-year overall survival of 93.9% in the ESD group compared with 97.3% in the surgery group. However, only approximately 15 patients in this study were diagnosed T1b.
The authors concluded that “ESD or EMR can be successfully applied to submucosal invasive cancers that have a low risk of metastatic potential....Future research should focus on novel biological and immunohistochemistry markers which can aid in the prediction of tumor behavior and lymph node metastasis status in T1b esophageal cancer.”
The authors disclosed relationships with Olympus, Boston Scientific, Lumendi, Aries Pharmaceutical, and Fujinon.
SOURCE: Othman MO et al. Clin Gastroenterol Hepatol 2019. doi: 10.1016/j.cgh.2019.05.045.
Findings from a systematic review may help guide treatment of patients with T1b esophageal cancer.
The review suggests that endoscopic submucosal dissection and endoscopic mucosal resection are appropriate for T1b esophageal cancers with a low risk of metastasis. The authors identified several factors associated with a higher risk of lymph node metastasis and said patients with these risk factors may benefit from adjuvant chemotherapy and radiation.
Mohamed O. Othman, MD, of Baylor College of Medicine, Houston, and colleagues conducted the review. Their report is in Clinical Gastroenterology and Hepatology.
The authors cited studies suggesting that survival rates would not be significantly different among early-stage esophageal cancer patients who undergo esophagectomy and those who receive endoscopic treatment, in the absence of lymph node metastasis. The risk of lymph node metastasis is higher in esophageal squamous cell carcinoma (ESCC) than in esophageal adenocarcinoma (EAC), and in those with deeper submucosal invasion (greater than 200 microm for ESCC or greater than 500 microm for EAC). Patients also have a higher risk of lymph node metastasis if they have poorly differentiated tumors, tumors larger than 2 cm, or lymphovascular invasion.
In the studies cited in the review, overall 5-year survival for esophageal squamous cell CA (ESCC) ranged from 68.6% (chemoradiation therapy alone) to 75% (endoscopic resection followed by chemoradiation), compared with 77.7% for surgery. For esophageal adenocarcinoma (EAC), there is less data but greater efficacy, with one study demonstrating 5-year overall survival of 93.9% in the ESD group compared with 97.3% in the surgery group. However, only approximately 15 patients in this study were diagnosed T1b.
The authors concluded that “ESD or EMR can be successfully applied to submucosal invasive cancers that have a low risk of metastatic potential....Future research should focus on novel biological and immunohistochemistry markers which can aid in the prediction of tumor behavior and lymph node metastasis status in T1b esophageal cancer.”
The authors disclosed relationships with Olympus, Boston Scientific, Lumendi, Aries Pharmaceutical, and Fujinon.
SOURCE: Othman MO et al. Clin Gastroenterol Hepatol 2019. doi: 10.1016/j.cgh.2019.05.045.
Findings from a systematic review may help guide treatment of patients with T1b esophageal cancer.
The review suggests that endoscopic submucosal dissection and endoscopic mucosal resection are appropriate for T1b esophageal cancers with a low risk of metastasis. The authors identified several factors associated with a higher risk of lymph node metastasis and said patients with these risk factors may benefit from adjuvant chemotherapy and radiation.
Mohamed O. Othman, MD, of Baylor College of Medicine, Houston, and colleagues conducted the review. Their report is in Clinical Gastroenterology and Hepatology.
The authors cited studies suggesting that survival rates would not be significantly different among early-stage esophageal cancer patients who undergo esophagectomy and those who receive endoscopic treatment, in the absence of lymph node metastasis. The risk of lymph node metastasis is higher in esophageal squamous cell carcinoma (ESCC) than in esophageal adenocarcinoma (EAC), and in those with deeper submucosal invasion (greater than 200 microm for ESCC or greater than 500 microm for EAC). Patients also have a higher risk of lymph node metastasis if they have poorly differentiated tumors, tumors larger than 2 cm, or lymphovascular invasion.
In the studies cited in the review, overall 5-year survival for esophageal squamous cell CA (ESCC) ranged from 68.6% (chemoradiation therapy alone) to 75% (endoscopic resection followed by chemoradiation), compared with 77.7% for surgery. For esophageal adenocarcinoma (EAC), there is less data but greater efficacy, with one study demonstrating 5-year overall survival of 93.9% in the ESD group compared with 97.3% in the surgery group. However, only approximately 15 patients in this study were diagnosed T1b.
The authors concluded that “ESD or EMR can be successfully applied to submucosal invasive cancers that have a low risk of metastatic potential....Future research should focus on novel biological and immunohistochemistry markers which can aid in the prediction of tumor behavior and lymph node metastasis status in T1b esophageal cancer.”
The authors disclosed relationships with Olympus, Boston Scientific, Lumendi, Aries Pharmaceutical, and Fujinon.
SOURCE: Othman MO et al. Clin Gastroenterol Hepatol 2019. doi: 10.1016/j.cgh.2019.05.045.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
New appointments at City of Hope, UA, and Fox Chase
The University of Arizona (UA) Cancer Center has a new interim director, two hematologists have earned new positions at City of Hope, and an assistant professor has joined Fox Chase Cancer Center.
William Cance, MD, has been appointed interim director of the UA Cancer Center. In this role, he will oversee clinical operations and research at the center’s primary locations in Tucson and Phoenix. He will also lead the UA Cancer Center’s efforts to renew its 5-year Cancer Center Support Grant from the National Cancer Institute (NCI).
In addition to his new role, Dr. Cance is a professor at the UA Colleges of Medicine and Pharmacy in Phoenix. He is board certified in general surgery and specializes in thyroid cancer, parathyroid disease, sarcoma, and gastrointestinal cancer. Dr. Cance has received funding from NCI, has served on the NCI Board of Scientific Counselors, and is a member of NCI Subcommittee F.
In other news, Larry Kwak, MD, PhD, has been appointed the deputy director of the Hematologic Malignancies and Stem Cell Transplantation Institute at City of Hope in Duarte, Calif. Dr. Kwak will work with the director of the Institute, Stephen J. Forman, MD, to oversee recruitment, clinical and laboratory research, and faculty development.
Dr. Kwak is also director of the Toni Stephenson Lymphoma Center, vice president and deputy director of the comprehensive cancer center, and the Dr. Michael Friedman Professor in Translational Medicine. Dr. Kwak was named one of TIME magazine’s “100 Most Influential People,” received the Chang-Yul Oh Memorial Award from the Korean Medical Association, and won the Ho-Am Prize in Medicine.
Also at City of Hope, Tanya Siddiqi, MD, has been appointed director of the chronic lymphocytic leukemia (CLL) program within the Toni Stephenson Lymphoma Center of the Hematologic Malignancies and Stem Cell Transplantation Institute. In this role, Dr. Siddiqi will oversee research efforts related to CLL. This includes maintaining the CLL tissue bank she developed, conducting translational studies and clinical trials, and investigating novel therapies for CLL.
Dr. Siddiqi is also an associate clinical professor in the department of hematology & hematopoietic cell transplantation, is the supervising physician of City of Hope’s anticoagulation clinic, and works in the Gehr Family Center for Leukemia Research. Dr. Siddiqi is a member of the National Comprehensive Cancer Network panels for CLL/SLL/hairy cell leukemia and venous thromboembolism.
Lastly, James M. Martin, MD, has been appointed assistant professor in the hematology and bone marrow transplant program within the department of hematology/oncology at Fox Chase Cancer Center in Philadelphia.
Dr. Martin received a medical degree from Ohio State University, Columbus, and completed an internship and residency at Rhode Island Hospital/Brown University, in Providence. He joined Fox Chase Cancer Center/Temple University in 2016 for a 3-year fellowship.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at [email protected], and you could be featured in Movers in Medicine.
The University of Arizona (UA) Cancer Center has a new interim director, two hematologists have earned new positions at City of Hope, and an assistant professor has joined Fox Chase Cancer Center.
William Cance, MD, has been appointed interim director of the UA Cancer Center. In this role, he will oversee clinical operations and research at the center’s primary locations in Tucson and Phoenix. He will also lead the UA Cancer Center’s efforts to renew its 5-year Cancer Center Support Grant from the National Cancer Institute (NCI).
In addition to his new role, Dr. Cance is a professor at the UA Colleges of Medicine and Pharmacy in Phoenix. He is board certified in general surgery and specializes in thyroid cancer, parathyroid disease, sarcoma, and gastrointestinal cancer. Dr. Cance has received funding from NCI, has served on the NCI Board of Scientific Counselors, and is a member of NCI Subcommittee F.
In other news, Larry Kwak, MD, PhD, has been appointed the deputy director of the Hematologic Malignancies and Stem Cell Transplantation Institute at City of Hope in Duarte, Calif. Dr. Kwak will work with the director of the Institute, Stephen J. Forman, MD, to oversee recruitment, clinical and laboratory research, and faculty development.
Dr. Kwak is also director of the Toni Stephenson Lymphoma Center, vice president and deputy director of the comprehensive cancer center, and the Dr. Michael Friedman Professor in Translational Medicine. Dr. Kwak was named one of TIME magazine’s “100 Most Influential People,” received the Chang-Yul Oh Memorial Award from the Korean Medical Association, and won the Ho-Am Prize in Medicine.
Also at City of Hope, Tanya Siddiqi, MD, has been appointed director of the chronic lymphocytic leukemia (CLL) program within the Toni Stephenson Lymphoma Center of the Hematologic Malignancies and Stem Cell Transplantation Institute. In this role, Dr. Siddiqi will oversee research efforts related to CLL. This includes maintaining the CLL tissue bank she developed, conducting translational studies and clinical trials, and investigating novel therapies for CLL.
Dr. Siddiqi is also an associate clinical professor in the department of hematology & hematopoietic cell transplantation, is the supervising physician of City of Hope’s anticoagulation clinic, and works in the Gehr Family Center for Leukemia Research. Dr. Siddiqi is a member of the National Comprehensive Cancer Network panels for CLL/SLL/hairy cell leukemia and venous thromboembolism.
Lastly, James M. Martin, MD, has been appointed assistant professor in the hematology and bone marrow transplant program within the department of hematology/oncology at Fox Chase Cancer Center in Philadelphia.
Dr. Martin received a medical degree from Ohio State University, Columbus, and completed an internship and residency at Rhode Island Hospital/Brown University, in Providence. He joined Fox Chase Cancer Center/Temple University in 2016 for a 3-year fellowship.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at [email protected], and you could be featured in Movers in Medicine.
The University of Arizona (UA) Cancer Center has a new interim director, two hematologists have earned new positions at City of Hope, and an assistant professor has joined Fox Chase Cancer Center.
William Cance, MD, has been appointed interim director of the UA Cancer Center. In this role, he will oversee clinical operations and research at the center’s primary locations in Tucson and Phoenix. He will also lead the UA Cancer Center’s efforts to renew its 5-year Cancer Center Support Grant from the National Cancer Institute (NCI).
In addition to his new role, Dr. Cance is a professor at the UA Colleges of Medicine and Pharmacy in Phoenix. He is board certified in general surgery and specializes in thyroid cancer, parathyroid disease, sarcoma, and gastrointestinal cancer. Dr. Cance has received funding from NCI, has served on the NCI Board of Scientific Counselors, and is a member of NCI Subcommittee F.
In other news, Larry Kwak, MD, PhD, has been appointed the deputy director of the Hematologic Malignancies and Stem Cell Transplantation Institute at City of Hope in Duarte, Calif. Dr. Kwak will work with the director of the Institute, Stephen J. Forman, MD, to oversee recruitment, clinical and laboratory research, and faculty development.
Dr. Kwak is also director of the Toni Stephenson Lymphoma Center, vice president and deputy director of the comprehensive cancer center, and the Dr. Michael Friedman Professor in Translational Medicine. Dr. Kwak was named one of TIME magazine’s “100 Most Influential People,” received the Chang-Yul Oh Memorial Award from the Korean Medical Association, and won the Ho-Am Prize in Medicine.
Also at City of Hope, Tanya Siddiqi, MD, has been appointed director of the chronic lymphocytic leukemia (CLL) program within the Toni Stephenson Lymphoma Center of the Hematologic Malignancies and Stem Cell Transplantation Institute. In this role, Dr. Siddiqi will oversee research efforts related to CLL. This includes maintaining the CLL tissue bank she developed, conducting translational studies and clinical trials, and investigating novel therapies for CLL.
Dr. Siddiqi is also an associate clinical professor in the department of hematology & hematopoietic cell transplantation, is the supervising physician of City of Hope’s anticoagulation clinic, and works in the Gehr Family Center for Leukemia Research. Dr. Siddiqi is a member of the National Comprehensive Cancer Network panels for CLL/SLL/hairy cell leukemia and venous thromboembolism.
Lastly, James M. Martin, MD, has been appointed assistant professor in the hematology and bone marrow transplant program within the department of hematology/oncology at Fox Chase Cancer Center in Philadelphia.
Dr. Martin received a medical degree from Ohio State University, Columbus, and completed an internship and residency at Rhode Island Hospital/Brown University, in Providence. He joined Fox Chase Cancer Center/Temple University in 2016 for a 3-year fellowship.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at [email protected], and you could be featured in Movers in Medicine.
Endoscopic treatment effective in T1b esophageal cancer
Findings from a systematic review may help guide treatment of patients with T1b esophageal cancer.
The review suggests that endoscopic submucosal dissection and endoscopic mucosal resection are appropriate for T1b esophageal cancers with a low risk of metastasis. The authors identified several factors associated with a higher risk of lymph node metastasis and said patients with these risk factors may benefit from adjuvant chemotherapy and radiation.
Mohamed O. Othman, MD, of Baylor College of Medicine, Houston, and colleagues conducted the review. Their report is in Clinical Gastroenterology and Hepatology.
The authors cited studies suggesting that survival rates are not significantly different among early-stage esophageal cancer patients who undergo esophagectomy and those who receive endoscopic treatment (Am J Gastroenterol. 2008;103:1340-5, Gastric Cancer. 2017;20:84-91).
However, studies have indicated that patients with submucosal invasion and an increased risk of metastasis may fare better when endoscopic treatment is combined with chemoradiation (Clin Transl Gastroenterol. 2017;8:e110, Radiat Oncol. 2015;10:31).
With that in mind, the authors described several factors associated with a higher risk of lymph node metastasis in T1b tumors.
First, the risk of lymph node metastasis is higher in esophageal squamous cell carcinoma (ESCC) than in esophageal adenocarcinoma (EAC). The risk is also higher in patients with deeper submucosal invasion (greater than 200 mcm for ESCC or greater than 500 mcm for EAC).
In fact, research suggested the risk of lymph node metastasis is:
- 27% in SM1 ESCC and 6% in SM1 EAC
- 36% in SM2 ESCC and 23% in SM2 EAC
- 55% in SM3 ESCC and 58% in SM3 EAC (Expert Rev Gastroenterol Hepatol. 2011;5:371-84).
Patients also have a higher risk of lymph node metastasis if they have Paris type 0-I protruded lesions or Paris type 0-III excavated lesions. Research suggested that, in ESCC, these lesions confer the highest risk of deep mucosal invasion — 79% for type 0-I protruded lesions and 84% for type 0-III excavated lesions (Surgery 1998;123:432-9).
An additional factor associated with a higher risk of lymph node metastasis in ESCC is type B microvessels. Researchers found that type B vessels could estimate the depth of invasion with 90.5% accuracy (Esophagus 2017;14:105-112).
Patients with poorly differentiated tumors, tumors larger than 2 cm, or lymphovascular invasion have a higher risk of lymph node metastasis as well.
In a study of 782 patients who underwent esophagectomy, those with poorly differentiated tumors and/or tumors larger than 2 cm had a higher rate of lymph node metastasis (Ann Surg Oncol 2018;25:318-25). And in a study of 90 patients with resected T1 EAC, lymphovascular invasion was significantly associated with tumor recurrence and overall survival (Am J Surg Pathol 2005;29:1079-85).
Research has also suggested that immunohistochemistry markers, such as E-cadherin and cyclin D1, are associated with a higher risk of lymph node metastasis (J Surg Oncol 2002;79:166-73).
“Future research should focus on novel biological and immunohistochemistry markers which can aid in the prediction of tumor behavior and lymph node metastasis status in T1b esophageal cancer,” Dr. Othman and colleagues concluded.
The authors disclosed relationships with Olympus, Boston Scientific, Lumendi, Aries Pharmaceutical, and Fujinon.
SOURCE: Othman MO et al. Clin Gastroenterol Hepatol 2019. doi: 10.1016/j.cgh.2019.05.045.
Findings from a systematic review may help guide treatment of patients with T1b esophageal cancer.
The review suggests that endoscopic submucosal dissection and endoscopic mucosal resection are appropriate for T1b esophageal cancers with a low risk of metastasis. The authors identified several factors associated with a higher risk of lymph node metastasis and said patients with these risk factors may benefit from adjuvant chemotherapy and radiation.
Mohamed O. Othman, MD, of Baylor College of Medicine, Houston, and colleagues conducted the review. Their report is in Clinical Gastroenterology and Hepatology.
The authors cited studies suggesting that survival rates are not significantly different among early-stage esophageal cancer patients who undergo esophagectomy and those who receive endoscopic treatment (Am J Gastroenterol. 2008;103:1340-5, Gastric Cancer. 2017;20:84-91).
However, studies have indicated that patients with submucosal invasion and an increased risk of metastasis may fare better when endoscopic treatment is combined with chemoradiation (Clin Transl Gastroenterol. 2017;8:e110, Radiat Oncol. 2015;10:31).
With that in mind, the authors described several factors associated with a higher risk of lymph node metastasis in T1b tumors.
First, the risk of lymph node metastasis is higher in esophageal squamous cell carcinoma (ESCC) than in esophageal adenocarcinoma (EAC). The risk is also higher in patients with deeper submucosal invasion (greater than 200 mcm for ESCC or greater than 500 mcm for EAC).
In fact, research suggested the risk of lymph node metastasis is:
- 27% in SM1 ESCC and 6% in SM1 EAC
- 36% in SM2 ESCC and 23% in SM2 EAC
- 55% in SM3 ESCC and 58% in SM3 EAC (Expert Rev Gastroenterol Hepatol. 2011;5:371-84).
Patients also have a higher risk of lymph node metastasis if they have Paris type 0-I protruded lesions or Paris type 0-III excavated lesions. Research suggested that, in ESCC, these lesions confer the highest risk of deep mucosal invasion — 79% for type 0-I protruded lesions and 84% for type 0-III excavated lesions (Surgery 1998;123:432-9).
An additional factor associated with a higher risk of lymph node metastasis in ESCC is type B microvessels. Researchers found that type B vessels could estimate the depth of invasion with 90.5% accuracy (Esophagus 2017;14:105-112).
Patients with poorly differentiated tumors, tumors larger than 2 cm, or lymphovascular invasion have a higher risk of lymph node metastasis as well.
In a study of 782 patients who underwent esophagectomy, those with poorly differentiated tumors and/or tumors larger than 2 cm had a higher rate of lymph node metastasis (Ann Surg Oncol 2018;25:318-25). And in a study of 90 patients with resected T1 EAC, lymphovascular invasion was significantly associated with tumor recurrence and overall survival (Am J Surg Pathol 2005;29:1079-85).
Research has also suggested that immunohistochemistry markers, such as E-cadherin and cyclin D1, are associated with a higher risk of lymph node metastasis (J Surg Oncol 2002;79:166-73).
“Future research should focus on novel biological and immunohistochemistry markers which can aid in the prediction of tumor behavior and lymph node metastasis status in T1b esophageal cancer,” Dr. Othman and colleagues concluded.
The authors disclosed relationships with Olympus, Boston Scientific, Lumendi, Aries Pharmaceutical, and Fujinon.
SOURCE: Othman MO et al. Clin Gastroenterol Hepatol 2019. doi: 10.1016/j.cgh.2019.05.045.
Findings from a systematic review may help guide treatment of patients with T1b esophageal cancer.
The review suggests that endoscopic submucosal dissection and endoscopic mucosal resection are appropriate for T1b esophageal cancers with a low risk of metastasis. The authors identified several factors associated with a higher risk of lymph node metastasis and said patients with these risk factors may benefit from adjuvant chemotherapy and radiation.
Mohamed O. Othman, MD, of Baylor College of Medicine, Houston, and colleagues conducted the review. Their report is in Clinical Gastroenterology and Hepatology.
The authors cited studies suggesting that survival rates are not significantly different among early-stage esophageal cancer patients who undergo esophagectomy and those who receive endoscopic treatment (Am J Gastroenterol. 2008;103:1340-5, Gastric Cancer. 2017;20:84-91).
However, studies have indicated that patients with submucosal invasion and an increased risk of metastasis may fare better when endoscopic treatment is combined with chemoradiation (Clin Transl Gastroenterol. 2017;8:e110, Radiat Oncol. 2015;10:31).
With that in mind, the authors described several factors associated with a higher risk of lymph node metastasis in T1b tumors.
First, the risk of lymph node metastasis is higher in esophageal squamous cell carcinoma (ESCC) than in esophageal adenocarcinoma (EAC). The risk is also higher in patients with deeper submucosal invasion (greater than 200 mcm for ESCC or greater than 500 mcm for EAC).
In fact, research suggested the risk of lymph node metastasis is:
- 27% in SM1 ESCC and 6% in SM1 EAC
- 36% in SM2 ESCC and 23% in SM2 EAC
- 55% in SM3 ESCC and 58% in SM3 EAC (Expert Rev Gastroenterol Hepatol. 2011;5:371-84).
Patients also have a higher risk of lymph node metastasis if they have Paris type 0-I protruded lesions or Paris type 0-III excavated lesions. Research suggested that, in ESCC, these lesions confer the highest risk of deep mucosal invasion — 79% for type 0-I protruded lesions and 84% for type 0-III excavated lesions (Surgery 1998;123:432-9).
An additional factor associated with a higher risk of lymph node metastasis in ESCC is type B microvessels. Researchers found that type B vessels could estimate the depth of invasion with 90.5% accuracy (Esophagus 2017;14:105-112).
Patients with poorly differentiated tumors, tumors larger than 2 cm, or lymphovascular invasion have a higher risk of lymph node metastasis as well.
In a study of 782 patients who underwent esophagectomy, those with poorly differentiated tumors and/or tumors larger than 2 cm had a higher rate of lymph node metastasis (Ann Surg Oncol 2018;25:318-25). And in a study of 90 patients with resected T1 EAC, lymphovascular invasion was significantly associated with tumor recurrence and overall survival (Am J Surg Pathol 2005;29:1079-85).
Research has also suggested that immunohistochemistry markers, such as E-cadherin and cyclin D1, are associated with a higher risk of lymph node metastasis (J Surg Oncol 2002;79:166-73).
“Future research should focus on novel biological and immunohistochemistry markers which can aid in the prediction of tumor behavior and lymph node metastasis status in T1b esophageal cancer,” Dr. Othman and colleagues concluded.
The authors disclosed relationships with Olympus, Boston Scientific, Lumendi, Aries Pharmaceutical, and Fujinon.
SOURCE: Othman MO et al. Clin Gastroenterol Hepatol 2019. doi: 10.1016/j.cgh.2019.05.045.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
SC daratumumab deemed feasible for every multiple myeloma patient
CHICAGO – Subcutaneous (SC) daratumumab is noninferior to intravenous (IV) daratumumab for patients with relapsed or refractory multiple myeloma (MM), according findings from a phase 3 trial.
In the COLUMBA trial, SC daratumumab proved noninferior to IV daratumumab with regard to overall response rate and maximum trough concentration (Ctrough).
The safety profiles of the two formulations were similar, although patients who received SC daratumumab had a lower rate of infusion-related reactions. SC daratumumab also had a lower treatment burden.
“The COLUMBA study shows that [SC daratumumab] can be used in every myeloma patient [as a] single agent or, maybe in the future, in combination with the different backbones,” said Maria-Victoria Mateos, MD, PhD, of University Hospital of Salamanca (Spain).
Dr. Mateos presented results from the COLUMBA trial at the annual meeting of the American Society of Clinical Oncology.
Dr. Mateos cited a previous phase 1b study that had suggested that SC daratumumab might produce similar results as IV daratumumab (Blood. 2017;130:838) while providing a more convenient delivery method. She pointed out that infusions of IV daratumumab can last hours, while the SC formulation can be delivered in minutes.
The aim of the phase 3 COLUMBA study was to compare the IV and SC formulations head-to-head. The trial enrolled 522 patients with relapsed/refractory multiple myeloma. They were randomized to receive daratumumab SC (n = 263) or IV (n = 259).
The median patient age was 68 years (range, 33-92 years) in the IV arm and 65 years (range, 42-84 years) in the SC arm. Patients had received a median of four prior lines of therapy (range, 1-15 in the IV arm and 2-12 in the SC arm). Most patients were refractory to their last line of therapy – 85% in the IV arm and 80% in the SC arm – and most patients had standard-risk cytogenetics – 83% and 74%, respectively.
Treatment
Patients received SC daratumumab at 1,800 mg and IV daratumumab at 16 mg/kg. Both were given weekly for cycles 1-2, every 2 weeks for cycles 3-6, and every 4 weeks thereafter until disease progression.
The median duration of the first infusion was 421 minutes in the IV arm and 5 minutes in the SC arm. The median duration of the second infusion was 255 minutes and 5 minutes, respectively, and the median duration of subsequent infusions was 205 minutes and 5 minutes, respectively.
At a median follow-up of 7.46 months, 57% of patients in each arm had discontinued the study treatment. The most common reasons for discontinuation were progression – 44% of the IV arm and 43% of the SC arm – and adverse events (AEs) – 8% and 7%, respectively.
Safety
Dr. Mateos said the safety profiles of IV and SC daratumumab were comparable. However, infusion-related reactions were significantly less likely in the SC arm, occurring in 12.7% of those patients and 34.5% of patients in the IV arm (P less than .0001).
Grade 3 or higher treatment-emergent AEs occurred in 49% of patients in the IV arm and 46% of those in the SC arm. Rates of grade 5 AEs were 7% and 5%, respectively. The most common grade 3/4 AEs (in the IV and SC arms, respectively) were anemia (14% and 13%), thrombocytopenia (14% for both), neutropenia (8% and 13%), lymphopenia (6% and 5%), and hypertension (6% and 3%).
Efficacy
One of the study’s primary endpoints was overall response rate, which was 37.1% in the IV arm and 41.1% in the SC arm (relative risk, 1.11; 95% CI, 0.89-1.37; P less than .0001). This met the criteria for noninferiority, and overall response rates were comparable across all patient subgroups, Dr. Mateos noted.
The rates of complete response or stringent complete response were also comparable at 2.7% in the IV arm and 1.9% in the SC arm. Rates of very good partial response were 17.0% and 19.0%, respectively.
The study’s other primary endpoint was maximum Ctrough predose on day 1 of cycle 3. The ratio of maximum Ctrough for daratumumab SC over IV was 107.93% (90% CI, 95.74%-121.67%), which met the noninferiority criterion.
Survival outcomes were also similar between the IV and SC arms. The median progression-free survival was 6.1 months and 5.6 months, respectively (P = .9258). The rate of overall survival at 6 months was 83.0% and 87.5%, respectively (P = .6032).
Considering these results together, Dr. Mateos and colleagues concluded that SC daratumumab is noninferior to IV daratumumab.
“[SC daratumumab] has a reduced treatment burden due to a considerably shorter administration duration, and patients treated with [SC daratumumab] reported higher satisfaction with therapy,” Dr. Mateos said.
The results support the use of flat-dose 1,800-mg SC daratumumab, which is comparable with the IV formulation, she said.
The COLUMBA trial was sponsored by Janssen Research & Development. Dr. Mateos reported relationships with Amgen, Celgene, Janssen-Cilag, and Takeda.
SOURCE: Mateos MV et al. ASCO 2019, Abstract 8005.
CHICAGO – Subcutaneous (SC) daratumumab is noninferior to intravenous (IV) daratumumab for patients with relapsed or refractory multiple myeloma (MM), according findings from a phase 3 trial.
In the COLUMBA trial, SC daratumumab proved noninferior to IV daratumumab with regard to overall response rate and maximum trough concentration (Ctrough).
The safety profiles of the two formulations were similar, although patients who received SC daratumumab had a lower rate of infusion-related reactions. SC daratumumab also had a lower treatment burden.
“The COLUMBA study shows that [SC daratumumab] can be used in every myeloma patient [as a] single agent or, maybe in the future, in combination with the different backbones,” said Maria-Victoria Mateos, MD, PhD, of University Hospital of Salamanca (Spain).
Dr. Mateos presented results from the COLUMBA trial at the annual meeting of the American Society of Clinical Oncology.
Dr. Mateos cited a previous phase 1b study that had suggested that SC daratumumab might produce similar results as IV daratumumab (Blood. 2017;130:838) while providing a more convenient delivery method. She pointed out that infusions of IV daratumumab can last hours, while the SC formulation can be delivered in minutes.
The aim of the phase 3 COLUMBA study was to compare the IV and SC formulations head-to-head. The trial enrolled 522 patients with relapsed/refractory multiple myeloma. They were randomized to receive daratumumab SC (n = 263) or IV (n = 259).
The median patient age was 68 years (range, 33-92 years) in the IV arm and 65 years (range, 42-84 years) in the SC arm. Patients had received a median of four prior lines of therapy (range, 1-15 in the IV arm and 2-12 in the SC arm). Most patients were refractory to their last line of therapy – 85% in the IV arm and 80% in the SC arm – and most patients had standard-risk cytogenetics – 83% and 74%, respectively.
Treatment
Patients received SC daratumumab at 1,800 mg and IV daratumumab at 16 mg/kg. Both were given weekly for cycles 1-2, every 2 weeks for cycles 3-6, and every 4 weeks thereafter until disease progression.
The median duration of the first infusion was 421 minutes in the IV arm and 5 minutes in the SC arm. The median duration of the second infusion was 255 minutes and 5 minutes, respectively, and the median duration of subsequent infusions was 205 minutes and 5 minutes, respectively.
At a median follow-up of 7.46 months, 57% of patients in each arm had discontinued the study treatment. The most common reasons for discontinuation were progression – 44% of the IV arm and 43% of the SC arm – and adverse events (AEs) – 8% and 7%, respectively.
Safety
Dr. Mateos said the safety profiles of IV and SC daratumumab were comparable. However, infusion-related reactions were significantly less likely in the SC arm, occurring in 12.7% of those patients and 34.5% of patients in the IV arm (P less than .0001).
Grade 3 or higher treatment-emergent AEs occurred in 49% of patients in the IV arm and 46% of those in the SC arm. Rates of grade 5 AEs were 7% and 5%, respectively. The most common grade 3/4 AEs (in the IV and SC arms, respectively) were anemia (14% and 13%), thrombocytopenia (14% for both), neutropenia (8% and 13%), lymphopenia (6% and 5%), and hypertension (6% and 3%).
Efficacy
One of the study’s primary endpoints was overall response rate, which was 37.1% in the IV arm and 41.1% in the SC arm (relative risk, 1.11; 95% CI, 0.89-1.37; P less than .0001). This met the criteria for noninferiority, and overall response rates were comparable across all patient subgroups, Dr. Mateos noted.
The rates of complete response or stringent complete response were also comparable at 2.7% in the IV arm and 1.9% in the SC arm. Rates of very good partial response were 17.0% and 19.0%, respectively.
The study’s other primary endpoint was maximum Ctrough predose on day 1 of cycle 3. The ratio of maximum Ctrough for daratumumab SC over IV was 107.93% (90% CI, 95.74%-121.67%), which met the noninferiority criterion.
Survival outcomes were also similar between the IV and SC arms. The median progression-free survival was 6.1 months and 5.6 months, respectively (P = .9258). The rate of overall survival at 6 months was 83.0% and 87.5%, respectively (P = .6032).
Considering these results together, Dr. Mateos and colleagues concluded that SC daratumumab is noninferior to IV daratumumab.
“[SC daratumumab] has a reduced treatment burden due to a considerably shorter administration duration, and patients treated with [SC daratumumab] reported higher satisfaction with therapy,” Dr. Mateos said.
The results support the use of flat-dose 1,800-mg SC daratumumab, which is comparable with the IV formulation, she said.
The COLUMBA trial was sponsored by Janssen Research & Development. Dr. Mateos reported relationships with Amgen, Celgene, Janssen-Cilag, and Takeda.
SOURCE: Mateos MV et al. ASCO 2019, Abstract 8005.
CHICAGO – Subcutaneous (SC) daratumumab is noninferior to intravenous (IV) daratumumab for patients with relapsed or refractory multiple myeloma (MM), according findings from a phase 3 trial.
In the COLUMBA trial, SC daratumumab proved noninferior to IV daratumumab with regard to overall response rate and maximum trough concentration (Ctrough).
The safety profiles of the two formulations were similar, although patients who received SC daratumumab had a lower rate of infusion-related reactions. SC daratumumab also had a lower treatment burden.
“The COLUMBA study shows that [SC daratumumab] can be used in every myeloma patient [as a] single agent or, maybe in the future, in combination with the different backbones,” said Maria-Victoria Mateos, MD, PhD, of University Hospital of Salamanca (Spain).
Dr. Mateos presented results from the COLUMBA trial at the annual meeting of the American Society of Clinical Oncology.
Dr. Mateos cited a previous phase 1b study that had suggested that SC daratumumab might produce similar results as IV daratumumab (Blood. 2017;130:838) while providing a more convenient delivery method. She pointed out that infusions of IV daratumumab can last hours, while the SC formulation can be delivered in minutes.
The aim of the phase 3 COLUMBA study was to compare the IV and SC formulations head-to-head. The trial enrolled 522 patients with relapsed/refractory multiple myeloma. They were randomized to receive daratumumab SC (n = 263) or IV (n = 259).
The median patient age was 68 years (range, 33-92 years) in the IV arm and 65 years (range, 42-84 years) in the SC arm. Patients had received a median of four prior lines of therapy (range, 1-15 in the IV arm and 2-12 in the SC arm). Most patients were refractory to their last line of therapy – 85% in the IV arm and 80% in the SC arm – and most patients had standard-risk cytogenetics – 83% and 74%, respectively.
Treatment
Patients received SC daratumumab at 1,800 mg and IV daratumumab at 16 mg/kg. Both were given weekly for cycles 1-2, every 2 weeks for cycles 3-6, and every 4 weeks thereafter until disease progression.
The median duration of the first infusion was 421 minutes in the IV arm and 5 minutes in the SC arm. The median duration of the second infusion was 255 minutes and 5 minutes, respectively, and the median duration of subsequent infusions was 205 minutes and 5 minutes, respectively.
At a median follow-up of 7.46 months, 57% of patients in each arm had discontinued the study treatment. The most common reasons for discontinuation were progression – 44% of the IV arm and 43% of the SC arm – and adverse events (AEs) – 8% and 7%, respectively.
Safety
Dr. Mateos said the safety profiles of IV and SC daratumumab were comparable. However, infusion-related reactions were significantly less likely in the SC arm, occurring in 12.7% of those patients and 34.5% of patients in the IV arm (P less than .0001).
Grade 3 or higher treatment-emergent AEs occurred in 49% of patients in the IV arm and 46% of those in the SC arm. Rates of grade 5 AEs were 7% and 5%, respectively. The most common grade 3/4 AEs (in the IV and SC arms, respectively) were anemia (14% and 13%), thrombocytopenia (14% for both), neutropenia (8% and 13%), lymphopenia (6% and 5%), and hypertension (6% and 3%).
Efficacy
One of the study’s primary endpoints was overall response rate, which was 37.1% in the IV arm and 41.1% in the SC arm (relative risk, 1.11; 95% CI, 0.89-1.37; P less than .0001). This met the criteria for noninferiority, and overall response rates were comparable across all patient subgroups, Dr. Mateos noted.
The rates of complete response or stringent complete response were also comparable at 2.7% in the IV arm and 1.9% in the SC arm. Rates of very good partial response were 17.0% and 19.0%, respectively.
The study’s other primary endpoint was maximum Ctrough predose on day 1 of cycle 3. The ratio of maximum Ctrough for daratumumab SC over IV was 107.93% (90% CI, 95.74%-121.67%), which met the noninferiority criterion.
Survival outcomes were also similar between the IV and SC arms. The median progression-free survival was 6.1 months and 5.6 months, respectively (P = .9258). The rate of overall survival at 6 months was 83.0% and 87.5%, respectively (P = .6032).
Considering these results together, Dr. Mateos and colleagues concluded that SC daratumumab is noninferior to IV daratumumab.
“[SC daratumumab] has a reduced treatment burden due to a considerably shorter administration duration, and patients treated with [SC daratumumab] reported higher satisfaction with therapy,” Dr. Mateos said.
The results support the use of flat-dose 1,800-mg SC daratumumab, which is comparable with the IV formulation, she said.
The COLUMBA trial was sponsored by Janssen Research & Development. Dr. Mateos reported relationships with Amgen, Celgene, Janssen-Cilag, and Takeda.
SOURCE: Mateos MV et al. ASCO 2019, Abstract 8005.
REPORTING FROM ASCO 2019
CNS-directed therapy appears more effective for synDLBCL
Controlling CNS disease is “paramount” in treating diffuse large B-cell lymphoma with synchronous CNS and systemic disease (synDLBCL), according to researchers.
In a retrospective study, the CNS was the most common site of relapse in patients with synDLBCL, and patients had better outcomes when they received CNS-directed therapy.
The 2-year progression-free survival rate was 50% in patients who received CNS-intensive therapy and 31% in those who received CNS-conservative therapy. The 2-year overall survival rate was 54% and 44%, respectively.
Dr. Joel C. Wight, of Austin Health in Heidelberg, Australia, and colleagues conducted this study and recounted their findings in the British Journal of Haematology.
The researchers retrospectively analyzed 80 patients with synDLBCL treated at 10 centers in Australia and the United Kingdom. Patients had DLBCL not otherwise specified (n = 67); high-grade B-cell lymphoma, including double-hit lymphoma (n = 12); or T-cell histiocyte-rich DLBCL (n = 1).
At baseline, all patients were treatment-naive, they had a median age of 64 years (range, 18-87 years), and 68% were male. Seventy percent of patients had high-risk disease according to the CNS International Prognostic Index (IPI), and 96% had non-CNS extranodal disease. The median number of extranodal sites outside the CNS was 2 (range, 0 to more than 10).
Patients were divided into those who received CNS-intensive therapy (n = 38) and those given CNS-conservative therapy (n = 42). The CNS-conservative group was significantly older (P less than .001), significantly more likely to have high-risk disease according to the National Comprehensive Cancer Network IPI (P = .009) or CNS IPI (P = .01) and significantly more likely to have leptomeningeal disease only (P less than .001).
Treatment
CNS-intensive therapy was defined as any established multiagent IV chemotherapy regimen with two or more CNS-penetrating drugs and cytarabine, with or without intrathecal chemotherapy and/or radiotherapy.
CNS-conservative therapy was defined as one or fewer IV CNS-penetrating chemotherapy agents in induction, with or without intrathecal chemotherapy and/or radiotherapy.
Systemic induction in the CNS-intensive group consisted of R-HyperCVAD (rituximab, hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with IV methotrexate and cytarabine) in 66% of patients and R-CODOX-M/IVAC (rituximab, hyperfractionated cyclophosphamide, vincristine, doxorubicin, methotrexate/ifosfamide, etoposide, cytarabine) in 24% of patients.
The most common systemic induction regimens in the CNS-conservative group were R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone) or CHOP-like regimens, given to 83% of patients.
CNS-directed IV therapy was given to 100% of the CNS-intensive group and 60% of the CNS-conservative group. This consisted of IV methotrexate plus cytarabine (97%) or MATRix (methotrexate, cytarabine, and thiotepa; 3%) in the CNS-intensive group and high-dose methotrexate in the conservative group.
Intrathecal chemotherapy was given to 97% of the CNS-intensive group and 60% of the CNS-conservative group. CNS-directed radiation was given to 32% and 19%, respectively.
Thirteen patients in the CNS-intensive group and one in the CNS-conservative group underwent autologous transplant as consolidation.
Outcomes
Dose reductions were more frequent in the CNS-conservative group than in the CNS-intensive group, at 48% and 18% (P = .009), as was early cessation of chemotherapy, at 52% and 18% (P = .002). Rates of treatment-related mortality were similar, at 13% in the CNS-intensive group and 12% in the CNS-conservative group.
At the end of induction, the complete response rate was 69% in the CNS-intensive group and 51% in the CNS-conservative group (P = .16). Primary refractory disease was observed in 19% and 38% of patients, respectively (P = .07).
The CNS was the most common site of relapse or progression (n = 28). CNS progression or relapse occurred in 25% of the CNS-intensive group and 49% of the CNS-conservative group (P = .03).
The 2-year progression-free survival rate was 50% for the CNS-intensive group and 31% for the CNS-conservative group (P = .006). The 2-year overall survival rate was 54% and 44%, respectively (P = .037).
When patients were matched for induction outcomes, consolidative transplant did not improve survival.
“The most significant factor affecting survival was the ability to control the CNS disease, which was improved by the addition of IV cytarabine to [high-dose methotrexate],” the researchers wrote.
“Whilst the younger age and more intensive systemic treatment of the CNS-intensive group may have contributed to the improved survival, it is clear that CNS disease control was substantially improved by the addition of cytarabine with lower rates of CNS relapse/progression observed.”
The researchers noted that “adequate control of the CNS disease is paramount and is best achieved by intensive CNS-directed induction.”
There was no formal funding for this study, and the researchers did not provide financial disclosures.
SOURCE: Wight JC et al. Br J Haematol. 2019 Jun 24. doi: 10.1111/bjh.16064.
Controlling CNS disease is “paramount” in treating diffuse large B-cell lymphoma with synchronous CNS and systemic disease (synDLBCL), according to researchers.
In a retrospective study, the CNS was the most common site of relapse in patients with synDLBCL, and patients had better outcomes when they received CNS-directed therapy.
The 2-year progression-free survival rate was 50% in patients who received CNS-intensive therapy and 31% in those who received CNS-conservative therapy. The 2-year overall survival rate was 54% and 44%, respectively.
Dr. Joel C. Wight, of Austin Health in Heidelberg, Australia, and colleagues conducted this study and recounted their findings in the British Journal of Haematology.
The researchers retrospectively analyzed 80 patients with synDLBCL treated at 10 centers in Australia and the United Kingdom. Patients had DLBCL not otherwise specified (n = 67); high-grade B-cell lymphoma, including double-hit lymphoma (n = 12); or T-cell histiocyte-rich DLBCL (n = 1).
At baseline, all patients were treatment-naive, they had a median age of 64 years (range, 18-87 years), and 68% were male. Seventy percent of patients had high-risk disease according to the CNS International Prognostic Index (IPI), and 96% had non-CNS extranodal disease. The median number of extranodal sites outside the CNS was 2 (range, 0 to more than 10).
Patients were divided into those who received CNS-intensive therapy (n = 38) and those given CNS-conservative therapy (n = 42). The CNS-conservative group was significantly older (P less than .001), significantly more likely to have high-risk disease according to the National Comprehensive Cancer Network IPI (P = .009) or CNS IPI (P = .01) and significantly more likely to have leptomeningeal disease only (P less than .001).
Treatment
CNS-intensive therapy was defined as any established multiagent IV chemotherapy regimen with two or more CNS-penetrating drugs and cytarabine, with or without intrathecal chemotherapy and/or radiotherapy.
CNS-conservative therapy was defined as one or fewer IV CNS-penetrating chemotherapy agents in induction, with or without intrathecal chemotherapy and/or radiotherapy.
Systemic induction in the CNS-intensive group consisted of R-HyperCVAD (rituximab, hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with IV methotrexate and cytarabine) in 66% of patients and R-CODOX-M/IVAC (rituximab, hyperfractionated cyclophosphamide, vincristine, doxorubicin, methotrexate/ifosfamide, etoposide, cytarabine) in 24% of patients.
The most common systemic induction regimens in the CNS-conservative group were R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone) or CHOP-like regimens, given to 83% of patients.
CNS-directed IV therapy was given to 100% of the CNS-intensive group and 60% of the CNS-conservative group. This consisted of IV methotrexate plus cytarabine (97%) or MATRix (methotrexate, cytarabine, and thiotepa; 3%) in the CNS-intensive group and high-dose methotrexate in the conservative group.
Intrathecal chemotherapy was given to 97% of the CNS-intensive group and 60% of the CNS-conservative group. CNS-directed radiation was given to 32% and 19%, respectively.
Thirteen patients in the CNS-intensive group and one in the CNS-conservative group underwent autologous transplant as consolidation.
Outcomes
Dose reductions were more frequent in the CNS-conservative group than in the CNS-intensive group, at 48% and 18% (P = .009), as was early cessation of chemotherapy, at 52% and 18% (P = .002). Rates of treatment-related mortality were similar, at 13% in the CNS-intensive group and 12% in the CNS-conservative group.
At the end of induction, the complete response rate was 69% in the CNS-intensive group and 51% in the CNS-conservative group (P = .16). Primary refractory disease was observed in 19% and 38% of patients, respectively (P = .07).
The CNS was the most common site of relapse or progression (n = 28). CNS progression or relapse occurred in 25% of the CNS-intensive group and 49% of the CNS-conservative group (P = .03).
The 2-year progression-free survival rate was 50% for the CNS-intensive group and 31% for the CNS-conservative group (P = .006). The 2-year overall survival rate was 54% and 44%, respectively (P = .037).
When patients were matched for induction outcomes, consolidative transplant did not improve survival.
“The most significant factor affecting survival was the ability to control the CNS disease, which was improved by the addition of IV cytarabine to [high-dose methotrexate],” the researchers wrote.
“Whilst the younger age and more intensive systemic treatment of the CNS-intensive group may have contributed to the improved survival, it is clear that CNS disease control was substantially improved by the addition of cytarabine with lower rates of CNS relapse/progression observed.”
The researchers noted that “adequate control of the CNS disease is paramount and is best achieved by intensive CNS-directed induction.”
There was no formal funding for this study, and the researchers did not provide financial disclosures.
SOURCE: Wight JC et al. Br J Haematol. 2019 Jun 24. doi: 10.1111/bjh.16064.
Controlling CNS disease is “paramount” in treating diffuse large B-cell lymphoma with synchronous CNS and systemic disease (synDLBCL), according to researchers.
In a retrospective study, the CNS was the most common site of relapse in patients with synDLBCL, and patients had better outcomes when they received CNS-directed therapy.
The 2-year progression-free survival rate was 50% in patients who received CNS-intensive therapy and 31% in those who received CNS-conservative therapy. The 2-year overall survival rate was 54% and 44%, respectively.
Dr. Joel C. Wight, of Austin Health in Heidelberg, Australia, and colleagues conducted this study and recounted their findings in the British Journal of Haematology.
The researchers retrospectively analyzed 80 patients with synDLBCL treated at 10 centers in Australia and the United Kingdom. Patients had DLBCL not otherwise specified (n = 67); high-grade B-cell lymphoma, including double-hit lymphoma (n = 12); or T-cell histiocyte-rich DLBCL (n = 1).
At baseline, all patients were treatment-naive, they had a median age of 64 years (range, 18-87 years), and 68% were male. Seventy percent of patients had high-risk disease according to the CNS International Prognostic Index (IPI), and 96% had non-CNS extranodal disease. The median number of extranodal sites outside the CNS was 2 (range, 0 to more than 10).
Patients were divided into those who received CNS-intensive therapy (n = 38) and those given CNS-conservative therapy (n = 42). The CNS-conservative group was significantly older (P less than .001), significantly more likely to have high-risk disease according to the National Comprehensive Cancer Network IPI (P = .009) or CNS IPI (P = .01) and significantly more likely to have leptomeningeal disease only (P less than .001).
Treatment
CNS-intensive therapy was defined as any established multiagent IV chemotherapy regimen with two or more CNS-penetrating drugs and cytarabine, with or without intrathecal chemotherapy and/or radiotherapy.
CNS-conservative therapy was defined as one or fewer IV CNS-penetrating chemotherapy agents in induction, with or without intrathecal chemotherapy and/or radiotherapy.
Systemic induction in the CNS-intensive group consisted of R-HyperCVAD (rituximab, hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with IV methotrexate and cytarabine) in 66% of patients and R-CODOX-M/IVAC (rituximab, hyperfractionated cyclophosphamide, vincristine, doxorubicin, methotrexate/ifosfamide, etoposide, cytarabine) in 24% of patients.
The most common systemic induction regimens in the CNS-conservative group were R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone) or CHOP-like regimens, given to 83% of patients.
CNS-directed IV therapy was given to 100% of the CNS-intensive group and 60% of the CNS-conservative group. This consisted of IV methotrexate plus cytarabine (97%) or MATRix (methotrexate, cytarabine, and thiotepa; 3%) in the CNS-intensive group and high-dose methotrexate in the conservative group.
Intrathecal chemotherapy was given to 97% of the CNS-intensive group and 60% of the CNS-conservative group. CNS-directed radiation was given to 32% and 19%, respectively.
Thirteen patients in the CNS-intensive group and one in the CNS-conservative group underwent autologous transplant as consolidation.
Outcomes
Dose reductions were more frequent in the CNS-conservative group than in the CNS-intensive group, at 48% and 18% (P = .009), as was early cessation of chemotherapy, at 52% and 18% (P = .002). Rates of treatment-related mortality were similar, at 13% in the CNS-intensive group and 12% in the CNS-conservative group.
At the end of induction, the complete response rate was 69% in the CNS-intensive group and 51% in the CNS-conservative group (P = .16). Primary refractory disease was observed in 19% and 38% of patients, respectively (P = .07).
The CNS was the most common site of relapse or progression (n = 28). CNS progression or relapse occurred in 25% of the CNS-intensive group and 49% of the CNS-conservative group (P = .03).
The 2-year progression-free survival rate was 50% for the CNS-intensive group and 31% for the CNS-conservative group (P = .006). The 2-year overall survival rate was 54% and 44%, respectively (P = .037).
When patients were matched for induction outcomes, consolidative transplant did not improve survival.
“The most significant factor affecting survival was the ability to control the CNS disease, which was improved by the addition of IV cytarabine to [high-dose methotrexate],” the researchers wrote.
“Whilst the younger age and more intensive systemic treatment of the CNS-intensive group may have contributed to the improved survival, it is clear that CNS disease control was substantially improved by the addition of cytarabine with lower rates of CNS relapse/progression observed.”
The researchers noted that “adequate control of the CNS disease is paramount and is best achieved by intensive CNS-directed induction.”
There was no formal funding for this study, and the researchers did not provide financial disclosures.
SOURCE: Wight JC et al. Br J Haematol. 2019 Jun 24. doi: 10.1111/bjh.16064.
FROM BRITISH JOURNAL OF HAEMATOLOGY
Hemostasis researcher passes away at age 72
George J. Broze Jr., MD, a former professor of medicine at Washington University School of Medicine in St. Louis, died following a heart attack on June 19, 2019, at the age of 72.
Dr. Broze was born in Seattle. He earned a bachelor’s degree from the University of Washington in Seattle and a medical degree from the University of Washington School of Medicine in St. Louis. Dr. Broze completed his internship and residency at North Carolina Memorial Hospital in Chapel Hill.
Dr. Broze became a clinical fellow in hematology at Washington University in 1976 and began teaching there in 1979. Dr. Broze practiced at the Jewish Hospital, Barnes Hospital, and Barnes-Jewish Hospital.
Dr. Broze’s research was focused on hemostasis and the relationship between coagulation and inflammation. He and his colleagues isolated and characterized tissue factor pathway inhibitor, uncovered a pathway for coagulation factor XI activation, and characterized the protein Z-dependent protease inhibitor serpinA10.
Dr. Broze is survived by his wife, two sons, and brother.
In happier news, Thomas J. Smith, MD, of the Johns Hopkins University School of Medicine in Baltimore, has won the Walther Cancer Foundation Palliative and Supportive Care in Oncology Endowed Award and Lecture from the American Society of Clinical Oncology.
The award is given to someone who “has made significant contributions to palliative care practice and research in oncology,” according to ASCO. Dr. Smith and his colleagues are known for their work showing that end-of-life palliative care can improve patient symptoms and quality of life while reducing the cost of care.
Dr. Smith will receive the award and deliver a keynote address at the 2019 Supportive Care in Oncology Symposium, which is set to take place Oct. 25-26 in San Francisco.
Meanwhile, Asya Nina Varshavsky-Yanovsky, MD, PhD, has joined Fox Chase Cancer Center in Philadelphia as an assistant professor in the hematology and bone marrow transplant programs within the department of hematology/oncology.
Dr. Varshavsky-Yanovsky earned her MD and PhD from the Technion-Israel Institute of Technology in Haifa, Israel. She joined Fox Chase Cancer Center/Temple University in 2016 for a 3-year fellowship in hematology/oncology.
Susmitha Apuri, MD, has joined Florida Cancer Specialists & Research Institute and is seeing patients in Inverness. She is board certified in medical oncology, hematology, and internal medicine.
Dr. Apuri earned her medical degree from NTR University of Health Sciences in Vijayawada, India; completed her internship and residency in internal medicine at the University of Miami Miller School of Medicine; and completed her fellowship in hematology/oncology at the University of South Florida/H. Lee Moffitt Cancer and Research Institute in Tampa. Her research and practice interests include malignant and nonmalignant hematology as well as breast, lung, and colorectal cancer.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at [email protected], and you could be featured in Movers in Medicine.
George J. Broze Jr., MD, a former professor of medicine at Washington University School of Medicine in St. Louis, died following a heart attack on June 19, 2019, at the age of 72.
Dr. Broze was born in Seattle. He earned a bachelor’s degree from the University of Washington in Seattle and a medical degree from the University of Washington School of Medicine in St. Louis. Dr. Broze completed his internship and residency at North Carolina Memorial Hospital in Chapel Hill.
Dr. Broze became a clinical fellow in hematology at Washington University in 1976 and began teaching there in 1979. Dr. Broze practiced at the Jewish Hospital, Barnes Hospital, and Barnes-Jewish Hospital.
Dr. Broze’s research was focused on hemostasis and the relationship between coagulation and inflammation. He and his colleagues isolated and characterized tissue factor pathway inhibitor, uncovered a pathway for coagulation factor XI activation, and characterized the protein Z-dependent protease inhibitor serpinA10.
Dr. Broze is survived by his wife, two sons, and brother.
In happier news, Thomas J. Smith, MD, of the Johns Hopkins University School of Medicine in Baltimore, has won the Walther Cancer Foundation Palliative and Supportive Care in Oncology Endowed Award and Lecture from the American Society of Clinical Oncology.
The award is given to someone who “has made significant contributions to palliative care practice and research in oncology,” according to ASCO. Dr. Smith and his colleagues are known for their work showing that end-of-life palliative care can improve patient symptoms and quality of life while reducing the cost of care.
Dr. Smith will receive the award and deliver a keynote address at the 2019 Supportive Care in Oncology Symposium, which is set to take place Oct. 25-26 in San Francisco.
Meanwhile, Asya Nina Varshavsky-Yanovsky, MD, PhD, has joined Fox Chase Cancer Center in Philadelphia as an assistant professor in the hematology and bone marrow transplant programs within the department of hematology/oncology.
Dr. Varshavsky-Yanovsky earned her MD and PhD from the Technion-Israel Institute of Technology in Haifa, Israel. She joined Fox Chase Cancer Center/Temple University in 2016 for a 3-year fellowship in hematology/oncology.
Susmitha Apuri, MD, has joined Florida Cancer Specialists & Research Institute and is seeing patients in Inverness. She is board certified in medical oncology, hematology, and internal medicine.
Dr. Apuri earned her medical degree from NTR University of Health Sciences in Vijayawada, India; completed her internship and residency in internal medicine at the University of Miami Miller School of Medicine; and completed her fellowship in hematology/oncology at the University of South Florida/H. Lee Moffitt Cancer and Research Institute in Tampa. Her research and practice interests include malignant and nonmalignant hematology as well as breast, lung, and colorectal cancer.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at [email protected], and you could be featured in Movers in Medicine.
George J. Broze Jr., MD, a former professor of medicine at Washington University School of Medicine in St. Louis, died following a heart attack on June 19, 2019, at the age of 72.
Dr. Broze was born in Seattle. He earned a bachelor’s degree from the University of Washington in Seattle and a medical degree from the University of Washington School of Medicine in St. Louis. Dr. Broze completed his internship and residency at North Carolina Memorial Hospital in Chapel Hill.
Dr. Broze became a clinical fellow in hematology at Washington University in 1976 and began teaching there in 1979. Dr. Broze practiced at the Jewish Hospital, Barnes Hospital, and Barnes-Jewish Hospital.
Dr. Broze’s research was focused on hemostasis and the relationship between coagulation and inflammation. He and his colleagues isolated and characterized tissue factor pathway inhibitor, uncovered a pathway for coagulation factor XI activation, and characterized the protein Z-dependent protease inhibitor serpinA10.
Dr. Broze is survived by his wife, two sons, and brother.
In happier news, Thomas J. Smith, MD, of the Johns Hopkins University School of Medicine in Baltimore, has won the Walther Cancer Foundation Palliative and Supportive Care in Oncology Endowed Award and Lecture from the American Society of Clinical Oncology.
The award is given to someone who “has made significant contributions to palliative care practice and research in oncology,” according to ASCO. Dr. Smith and his colleagues are known for their work showing that end-of-life palliative care can improve patient symptoms and quality of life while reducing the cost of care.
Dr. Smith will receive the award and deliver a keynote address at the 2019 Supportive Care in Oncology Symposium, which is set to take place Oct. 25-26 in San Francisco.
Meanwhile, Asya Nina Varshavsky-Yanovsky, MD, PhD, has joined Fox Chase Cancer Center in Philadelphia as an assistant professor in the hematology and bone marrow transplant programs within the department of hematology/oncology.
Dr. Varshavsky-Yanovsky earned her MD and PhD from the Technion-Israel Institute of Technology in Haifa, Israel. She joined Fox Chase Cancer Center/Temple University in 2016 for a 3-year fellowship in hematology/oncology.
Susmitha Apuri, MD, has joined Florida Cancer Specialists & Research Institute and is seeing patients in Inverness. She is board certified in medical oncology, hematology, and internal medicine.
Dr. Apuri earned her medical degree from NTR University of Health Sciences in Vijayawada, India; completed her internship and residency in internal medicine at the University of Miami Miller School of Medicine; and completed her fellowship in hematology/oncology at the University of South Florida/H. Lee Moffitt Cancer and Research Institute in Tampa. Her research and practice interests include malignant and nonmalignant hematology as well as breast, lung, and colorectal cancer.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at [email protected], and you could be featured in Movers in Medicine.
MOVERS IN MEDICINE
‘Robust antitumor immune responses’ observed in pediatric ALL
Pediatric acute lymphoblastic leukemia (ALL) may be more vulnerable to immunotherapies than previously thought, according to researchers.
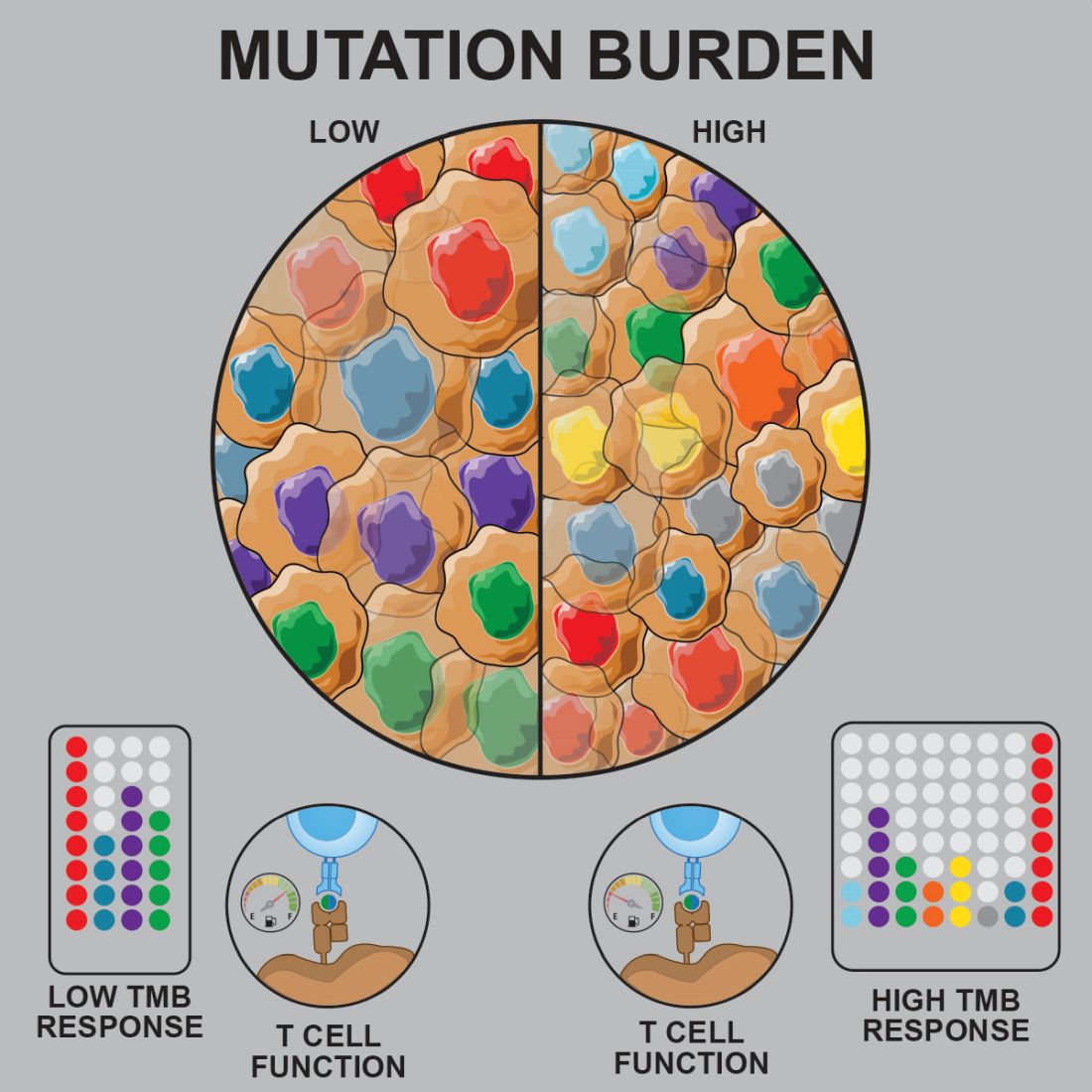
Prior studies suggested that tumors with a low mutational burden don’t elicit strong antitumor responses and therefore aren’t very susceptible to immunotherapy.
Now, researchers have found evidence to suggest that pediatric ALL induces “robust antitumor immune responses” despite a low mutational burden. The investigators identified tumor-associated CD8+ T cells that responded to 86% of neoantigens tested and recognized 68% of neoepitopes tested.
Anthony E. Zamora, PhD, of St. Jude Children’s Research Hospital in Memphis, Tenn., and colleagues recounted these findings in Science Translational Medicine.
The researchers analyzed samples from pediatric patients with ETV-associated ALL (n = 9) or ERG-associated ALL (n = 2) to determine how endogenous CD8+ T cells respond to patient-specific cancer neoantigens.
The investigators first assessed the ability of tumor-specific mutations and gene fusions to generate neoepitopes, or neoantigens predicted to bind patient-specific human leukocyte antigen (HLA) proteins. The team identified 5-28 neoepitopes per patient, including epitopes that spanned the fusion junction in patients with ETV6-RUNX1 fusions.
The researchers then tested whether CD8+ tumor infiltrating lymphocytes (TILs) were directly responsive to mutated neoepitopes. They observed cytokine responses across patient samples, noting that 31 of the 36 putative neoantigens tested (86%) were “immunogenic and capable of inducing robust cytokine responses.”
Next, the investigators mapped TIL responses to specific epitopes using patient-specific tetramers that corresponded to the previously identified neoepitopes. Seventeen of the 25 patient-specific tetramers (68%) bound to TILs above the background set by irrelevant HLA-matched tetramers.
“Within those responses, we observed immunodominance hierarchies among the distinct TIL populations, with a majority of tetramer-bound CD8+ T cells restricted to one or two putative neoepitopes,” the researchers noted.
The team also pointed out that seven of nine patients tested had CD8+ T cells that responded to ETV6-RUNX1.
Finally, the investigators performed transcriptional profiling of ALL-specific CD8+ TILs to assess inter- and intrapatient heterogeneity. The team identified three hierarchical clusters, which were characterized by transcriptional factors and regulators associated with:
- Functional effector CD8+ T cells (TBX21 and EOMES).
- Dysfunctional CD8+ T cells (STAT1/3/4, NR4A2/3, and BCL6).
- Exhausted CD8+ T cells (EOMES, MAF, PRDM1, and BATF).
Considering these findings together, the researchers concluded that “pediatric ALL elicits a potent neoepitope-specific CD8+ T-cell response.” Therefore, adoptive T-cell, monoclonal antibody, and targeted T-cell receptor therapies “should be explored” in pediatric ALL.
This research was supported by the National Institutes of Health, National Cancer Institute, National Institute of General Medical Sciences, Key for a Cure Foundation, and American Lebanese Syrian Associated Charities. The researchers disclosed patent applications and relationships with Pfizer, Amgen, and other companies.
SOURCE: Zamora AE et al. Sci. Transl. Med. 2019 Jun 26. doi: 10.1126/scitranslmed.aat8549.
Pediatric acute lymphoblastic leukemia (ALL) may be more vulnerable to immunotherapies than previously thought, according to researchers.

Prior studies suggested that tumors with a low mutational burden don’t elicit strong antitumor responses and therefore aren’t very susceptible to immunotherapy.
Now, researchers have found evidence to suggest that pediatric ALL induces “robust antitumor immune responses” despite a low mutational burden. The investigators identified tumor-associated CD8+ T cells that responded to 86% of neoantigens tested and recognized 68% of neoepitopes tested.
Anthony E. Zamora, PhD, of St. Jude Children’s Research Hospital in Memphis, Tenn., and colleagues recounted these findings in Science Translational Medicine.
The researchers analyzed samples from pediatric patients with ETV-associated ALL (n = 9) or ERG-associated ALL (n = 2) to determine how endogenous CD8+ T cells respond to patient-specific cancer neoantigens.
The investigators first assessed the ability of tumor-specific mutations and gene fusions to generate neoepitopes, or neoantigens predicted to bind patient-specific human leukocyte antigen (HLA) proteins. The team identified 5-28 neoepitopes per patient, including epitopes that spanned the fusion junction in patients with ETV6-RUNX1 fusions.
The researchers then tested whether CD8+ tumor infiltrating lymphocytes (TILs) were directly responsive to mutated neoepitopes. They observed cytokine responses across patient samples, noting that 31 of the 36 putative neoantigens tested (86%) were “immunogenic and capable of inducing robust cytokine responses.”
Next, the investigators mapped TIL responses to specific epitopes using patient-specific tetramers that corresponded to the previously identified neoepitopes. Seventeen of the 25 patient-specific tetramers (68%) bound to TILs above the background set by irrelevant HLA-matched tetramers.
“Within those responses, we observed immunodominance hierarchies among the distinct TIL populations, with a majority of tetramer-bound CD8+ T cells restricted to one or two putative neoepitopes,” the researchers noted.
The team also pointed out that seven of nine patients tested had CD8+ T cells that responded to ETV6-RUNX1.
Finally, the investigators performed transcriptional profiling of ALL-specific CD8+ TILs to assess inter- and intrapatient heterogeneity. The team identified three hierarchical clusters, which were characterized by transcriptional factors and regulators associated with:
- Functional effector CD8+ T cells (TBX21 and EOMES).
- Dysfunctional CD8+ T cells (STAT1/3/4, NR4A2/3, and BCL6).
- Exhausted CD8+ T cells (EOMES, MAF, PRDM1, and BATF).
Considering these findings together, the researchers concluded that “pediatric ALL elicits a potent neoepitope-specific CD8+ T-cell response.” Therefore, adoptive T-cell, monoclonal antibody, and targeted T-cell receptor therapies “should be explored” in pediatric ALL.
This research was supported by the National Institutes of Health, National Cancer Institute, National Institute of General Medical Sciences, Key for a Cure Foundation, and American Lebanese Syrian Associated Charities. The researchers disclosed patent applications and relationships with Pfizer, Amgen, and other companies.
SOURCE: Zamora AE et al. Sci. Transl. Med. 2019 Jun 26. doi: 10.1126/scitranslmed.aat8549.
Pediatric acute lymphoblastic leukemia (ALL) may be more vulnerable to immunotherapies than previously thought, according to researchers.

Prior studies suggested that tumors with a low mutational burden don’t elicit strong antitumor responses and therefore aren’t very susceptible to immunotherapy.
Now, researchers have found evidence to suggest that pediatric ALL induces “robust antitumor immune responses” despite a low mutational burden. The investigators identified tumor-associated CD8+ T cells that responded to 86% of neoantigens tested and recognized 68% of neoepitopes tested.
Anthony E. Zamora, PhD, of St. Jude Children’s Research Hospital in Memphis, Tenn., and colleagues recounted these findings in Science Translational Medicine.
The researchers analyzed samples from pediatric patients with ETV-associated ALL (n = 9) or ERG-associated ALL (n = 2) to determine how endogenous CD8+ T cells respond to patient-specific cancer neoantigens.
The investigators first assessed the ability of tumor-specific mutations and gene fusions to generate neoepitopes, or neoantigens predicted to bind patient-specific human leukocyte antigen (HLA) proteins. The team identified 5-28 neoepitopes per patient, including epitopes that spanned the fusion junction in patients with ETV6-RUNX1 fusions.
The researchers then tested whether CD8+ tumor infiltrating lymphocytes (TILs) were directly responsive to mutated neoepitopes. They observed cytokine responses across patient samples, noting that 31 of the 36 putative neoantigens tested (86%) were “immunogenic and capable of inducing robust cytokine responses.”
Next, the investigators mapped TIL responses to specific epitopes using patient-specific tetramers that corresponded to the previously identified neoepitopes. Seventeen of the 25 patient-specific tetramers (68%) bound to TILs above the background set by irrelevant HLA-matched tetramers.
“Within those responses, we observed immunodominance hierarchies among the distinct TIL populations, with a majority of tetramer-bound CD8+ T cells restricted to one or two putative neoepitopes,” the researchers noted.
The team also pointed out that seven of nine patients tested had CD8+ T cells that responded to ETV6-RUNX1.
Finally, the investigators performed transcriptional profiling of ALL-specific CD8+ TILs to assess inter- and intrapatient heterogeneity. The team identified three hierarchical clusters, which were characterized by transcriptional factors and regulators associated with:
- Functional effector CD8+ T cells (TBX21 and EOMES).
- Dysfunctional CD8+ T cells (STAT1/3/4, NR4A2/3, and BCL6).
- Exhausted CD8+ T cells (EOMES, MAF, PRDM1, and BATF).
Considering these findings together, the researchers concluded that “pediatric ALL elicits a potent neoepitope-specific CD8+ T-cell response.” Therefore, adoptive T-cell, monoclonal antibody, and targeted T-cell receptor therapies “should be explored” in pediatric ALL.
This research was supported by the National Institutes of Health, National Cancer Institute, National Institute of General Medical Sciences, Key for a Cure Foundation, and American Lebanese Syrian Associated Charities. The researchers disclosed patent applications and relationships with Pfizer, Amgen, and other companies.
SOURCE: Zamora AE et al. Sci. Transl. Med. 2019 Jun 26. doi: 10.1126/scitranslmed.aat8549.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: Preclinical research suggests pediatric acute lymphoblastic leukemia (ALL) induces “robust antitumor immune responses” despite a low mutational burden.
Major finding: Investigators identified tumor-associated CD8+ T cells that responded to 86% of neoantigens tested and recognized 68% of neoepitopes tested.
Study details: Analysis of samples from pediatric patients with ETV-associated ALL (n = 9) or ERG-associated ALL (n = 2).
Disclosures: The research was supported by the National Institutes of Health, National Cancer Institute, National Institute of General Medical Sciences, Key for a Cure Foundation, and American Lebanese Syrian Associated Charities. The researchers disclosed patent applications and relationships with Pfizer, Amgen, and other companies.
Source: Zamora AE et al. Sci. Transl. Med. 2019 Jun 26. doi: 10.1126/scitranslmed.aat8549.
BET inhibitors may target oncogene in ABC-like DLBCL
(ABC-like DLBCL).
Researchers found the TCF4 (E2-2) transcription factor is an oncogene in ABC-like DLBCL, and TCF4 can be targeted via BET inhibition.
The BET protein degrader ARV771 reduced TCF4 expression and exhibited activity against ABC-like DLBCL in vitro and in vivo.
Neeraj Jain, PhD, of the University of Texas MD Anderson Cancer Center in Houston and colleagues reported these findings in Science Translational Medicine.
The researchers performed a genomic analysis of 1,000 DLBCL tumors and discovered that gains of 18q21.2 were the most frequent genetic alteration in ABC-like DLBCL. In analyzing another 249 tumors, the researchers found that TCF4 was the target of the 18q21.2 gains.
Additional experiments with primary DLBCL tumors and DLBCL cell lines indicated that TCF4 regulates IGHM and MYC expression. The researchers also found that the TCF4 gene was “one of the most highly BRD4-loaded genes in DLBCL,” and knocking down BRD4 in DLBCL cell lines reduced TCF4 expression.
These findings prompted the team to test small-molecule BET inhibitors – JQ1 and OTX015 – and a BET protein degrader – ARV771 – in ABC-like DLBCL cell lines with a high TCF4 copy number.
Treatment with JQ1 and OTX015 resulted in upregulation of BRD4, but ARV771 treatment did not. As a result, subsequent experiments were performed with ARV771.
The researchers found that ARV771 induced apoptosis in the cell lines and reduced the expression of TCF4, IgM, and MYC. However, enforced TCF4 expression during ARV771 treatment rescued IgM and MYC expression. This suggests that “reduction of TCF4 is one of the mechanisms by which BET inhibition reduces IgM and MYC expression and induces apoptosis,” according to the researchers.
The team also tested ARV771 in mouse models of ABC-like DLBCL with high TCF4 expression. ARV771 significantly reduced tumor growth and prolonged survival (P less than .05 for both), and there were no signs of toxicity in ARV771-treated mice.
“Our data provide a clear functional rationale for BET inhibition in ABC-like DLBCL,” the researchers wrote. They did note, however, that BCL2 overexpression is associated with resistance to BET inhibitors, and most of the 18q DNA copy number gains the researchers observed in DLBCL encompass TCF4 and BCL2. The team therefore theorized that combining a BET inhibitor with a BCL2 inhibitor could be a promising treatment approach in ABC-like DLBCL and is worthy of further investigation.
This research was supported by the Nebraska Department of Health & Human Services, the Schweitzer Family Fund, the Fred & Pamela Buffet Cancer Center Support Grant, and the MD Anderson Cancer Center NCI CORE Grant. The authors reported having no conflicts of interest related to the study.
SOURCE: Jain N et al. Sci. Transl. Med. 2019 Jun 19. doi: 10.1126/scitranslmed.aav5599.
(ABC-like DLBCL).
Researchers found the TCF4 (E2-2) transcription factor is an oncogene in ABC-like DLBCL, and TCF4 can be targeted via BET inhibition.
The BET protein degrader ARV771 reduced TCF4 expression and exhibited activity against ABC-like DLBCL in vitro and in vivo.
Neeraj Jain, PhD, of the University of Texas MD Anderson Cancer Center in Houston and colleagues reported these findings in Science Translational Medicine.
The researchers performed a genomic analysis of 1,000 DLBCL tumors and discovered that gains of 18q21.2 were the most frequent genetic alteration in ABC-like DLBCL. In analyzing another 249 tumors, the researchers found that TCF4 was the target of the 18q21.2 gains.
Additional experiments with primary DLBCL tumors and DLBCL cell lines indicated that TCF4 regulates IGHM and MYC expression. The researchers also found that the TCF4 gene was “one of the most highly BRD4-loaded genes in DLBCL,” and knocking down BRD4 in DLBCL cell lines reduced TCF4 expression.
These findings prompted the team to test small-molecule BET inhibitors – JQ1 and OTX015 – and a BET protein degrader – ARV771 – in ABC-like DLBCL cell lines with a high TCF4 copy number.
Treatment with JQ1 and OTX015 resulted in upregulation of BRD4, but ARV771 treatment did not. As a result, subsequent experiments were performed with ARV771.
The researchers found that ARV771 induced apoptosis in the cell lines and reduced the expression of TCF4, IgM, and MYC. However, enforced TCF4 expression during ARV771 treatment rescued IgM and MYC expression. This suggests that “reduction of TCF4 is one of the mechanisms by which BET inhibition reduces IgM and MYC expression and induces apoptosis,” according to the researchers.
The team also tested ARV771 in mouse models of ABC-like DLBCL with high TCF4 expression. ARV771 significantly reduced tumor growth and prolonged survival (P less than .05 for both), and there were no signs of toxicity in ARV771-treated mice.
“Our data provide a clear functional rationale for BET inhibition in ABC-like DLBCL,” the researchers wrote. They did note, however, that BCL2 overexpression is associated with resistance to BET inhibitors, and most of the 18q DNA copy number gains the researchers observed in DLBCL encompass TCF4 and BCL2. The team therefore theorized that combining a BET inhibitor with a BCL2 inhibitor could be a promising treatment approach in ABC-like DLBCL and is worthy of further investigation.
This research was supported by the Nebraska Department of Health & Human Services, the Schweitzer Family Fund, the Fred & Pamela Buffet Cancer Center Support Grant, and the MD Anderson Cancer Center NCI CORE Grant. The authors reported having no conflicts of interest related to the study.
SOURCE: Jain N et al. Sci. Transl. Med. 2019 Jun 19. doi: 10.1126/scitranslmed.aav5599.
(ABC-like DLBCL).
Researchers found the TCF4 (E2-2) transcription factor is an oncogene in ABC-like DLBCL, and TCF4 can be targeted via BET inhibition.
The BET protein degrader ARV771 reduced TCF4 expression and exhibited activity against ABC-like DLBCL in vitro and in vivo.
Neeraj Jain, PhD, of the University of Texas MD Anderson Cancer Center in Houston and colleagues reported these findings in Science Translational Medicine.
The researchers performed a genomic analysis of 1,000 DLBCL tumors and discovered that gains of 18q21.2 were the most frequent genetic alteration in ABC-like DLBCL. In analyzing another 249 tumors, the researchers found that TCF4 was the target of the 18q21.2 gains.
Additional experiments with primary DLBCL tumors and DLBCL cell lines indicated that TCF4 regulates IGHM and MYC expression. The researchers also found that the TCF4 gene was “one of the most highly BRD4-loaded genes in DLBCL,” and knocking down BRD4 in DLBCL cell lines reduced TCF4 expression.
These findings prompted the team to test small-molecule BET inhibitors – JQ1 and OTX015 – and a BET protein degrader – ARV771 – in ABC-like DLBCL cell lines with a high TCF4 copy number.
Treatment with JQ1 and OTX015 resulted in upregulation of BRD4, but ARV771 treatment did not. As a result, subsequent experiments were performed with ARV771.
The researchers found that ARV771 induced apoptosis in the cell lines and reduced the expression of TCF4, IgM, and MYC. However, enforced TCF4 expression during ARV771 treatment rescued IgM and MYC expression. This suggests that “reduction of TCF4 is one of the mechanisms by which BET inhibition reduces IgM and MYC expression and induces apoptosis,” according to the researchers.
The team also tested ARV771 in mouse models of ABC-like DLBCL with high TCF4 expression. ARV771 significantly reduced tumor growth and prolonged survival (P less than .05 for both), and there were no signs of toxicity in ARV771-treated mice.
“Our data provide a clear functional rationale for BET inhibition in ABC-like DLBCL,” the researchers wrote. They did note, however, that BCL2 overexpression is associated with resistance to BET inhibitors, and most of the 18q DNA copy number gains the researchers observed in DLBCL encompass TCF4 and BCL2. The team therefore theorized that combining a BET inhibitor with a BCL2 inhibitor could be a promising treatment approach in ABC-like DLBCL and is worthy of further investigation.
This research was supported by the Nebraska Department of Health & Human Services, the Schweitzer Family Fund, the Fred & Pamela Buffet Cancer Center Support Grant, and the MD Anderson Cancer Center NCI CORE Grant. The authors reported having no conflicts of interest related to the study.
SOURCE: Jain N et al. Sci. Transl. Med. 2019 Jun 19. doi: 10.1126/scitranslmed.aav5599.
FROM SCIENCE TRANSLATIONAL MEDICINE
‘Encouraging’ responses seen with durvalumab plus R-CHOP in DLBCL
CHICAGO – A six-drug combination produced complete responses in previously untreated, high-risk diffuse large B-cell lymphoma (DLBCL) patients in a phase 2 trial.
Induction with durvalumab and R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) produced complete response rates of 54% in the entire cohort and 41% in patients with double- or triple-hit lymphoma. Immune-related adverse events (AEs) were common with this regimen, but no unexpected AEs occurred, according to researchers.
Grzegorz S. Nowakowski, MD, of the Mayo Clinic in Rochester, Minn., and colleagues presented these results in a poster at the annual meeting of the American Society of Clinical Oncology.
Treatment
The phase 2 trial (NCT03003520) was designed to assess durvalumab plus R-CHOP as well as durvalumab plus R-CHOP and lenalidomide (R2-CHOP) in patients with previously untreated, high-risk DLBCL. However, the R2-CHOP arm was closed early.
In cycle one, all patients received durvalumab plus R-CHOP. For subsequent cycles, patients with activated B-cell (ABC) DLBCL were assigned to durvalumab plus R2-CHOP, while patients with non-ABC DLBCL continued on durvalumab plus R-CHOP.
The R2-CHOP arm was closed early due to safety issues observed in trials combining checkpoint inhibitors with immunomodulatory agents. A partial clinical hold was placed on the R2-CHOP arm, but patients could continue on the regimen if they experienced a clinical benefit. Any patients with ABC DLBCL who were enrolled after the partial hold received treatment with durvalumab plus R-CHOP.
Induction was given for up to eight cycles and was followed by consolidation with durvalumab alone for up to 12 months from the start of induction.
Patient characteristics
The researchers presented data on 43 patients in the durvalumab plus R-CHOP arm. The patients’ median age was 62 years, and 61% were men.
“I think it’s worth noting that 46% of patients in the durvalumab plus R-CHOP group had very high-risk features, including double-hit or triple-hit genetic features,” said Justin Kline, MD, of the University of Chicago Medicine who reviewed this study in a poster discussion session.
Specifically, 30% of patients had double-hit lymphoma, and 16% had triple-hit lymphoma. Most patients had a high-intermediate-risk (49%) or high-risk (21%) International Prognostic Index score, and 79% of patients had Ann Arbor stage IV disease.
Efficacy
As of Aug. 2, 2018, 70% of patients had completed induction, 2% had completed consolidation, 44% remained on treatment, and 54% had discontinued therapy. The most common reasons for stopping treatment were progression (16%), AEs (14%), and consent withdrawal (12%).
“The combination of durvalumab plus R-CHOP demonstrated encouraging response rates … in subjects with high-risk DLBCL, including double- and triple-hit lymphomas,” Dr. Kline said.
The complete response rate was 54% (20/37) at the end of induction and 68% (n = 25) at the end of consolidation. The partial response rate at the end of consolidation was 30% (n = 11).
In patients with double- or triple-hit lymphoma, the complete response rate at the end of induction was 41% (7/17). The overall response rate in this group was 88% (n = 15).
Safety
“The safety profile was as expected for the components of the combination, and no new safety signals were observed,” Dr. Kline said.
He noted that AEs of special interest, or immune-related AEs, occurred in 61% of patients, but most of these events were grade 1 or 2.
AEs of special interest included diarrhea (28%), rash (23%), infusion-related reactions (16%), dermatitis (12%), hypothyroidism (5%), myocarditis (5%), adrenal insufficiency (2%), and hepatitis (2%).
Grade 3 or 4 AEs of special interest included infusion-related reactions (5%), rash (2%), diarrhea (2%), and hepatitis (2%).
The safety and efficacy results support further evaluation of durvalumab plus R-CHOP, although it will be important to identify DLBCL patients who are more likely to derive a clinical benefit from PD-1 or PD-L1 blockade, Dr. Kline said.
“This early study showed that the combination is feasible,” Dr. Nowakowski added. “I think, down the road, we’ll need to identify patients who can actually benefit from this combination. We definitely have clinical evidence of exceptional responses to PD-1 blockade.”
The trial was sponsored by Celgene. Dr. Nowakowski reported relationships with Celgene, Genentech, MorphoSys, and NanoString Technologies. Dr. Kline reported relationships with Cardinal Health, Merck, Seattle Genetics, Kite/Gilead, ITeos Therapeutics, and Bristol-Myers Squibb.
SOURCE: Nowakowski GS et al. ASCO 2019, Abstract 7520.
CHICAGO – A six-drug combination produced complete responses in previously untreated, high-risk diffuse large B-cell lymphoma (DLBCL) patients in a phase 2 trial.
Induction with durvalumab and R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) produced complete response rates of 54% in the entire cohort and 41% in patients with double- or triple-hit lymphoma. Immune-related adverse events (AEs) were common with this regimen, but no unexpected AEs occurred, according to researchers.
Grzegorz S. Nowakowski, MD, of the Mayo Clinic in Rochester, Minn., and colleagues presented these results in a poster at the annual meeting of the American Society of Clinical Oncology.
Treatment
The phase 2 trial (NCT03003520) was designed to assess durvalumab plus R-CHOP as well as durvalumab plus R-CHOP and lenalidomide (R2-CHOP) in patients with previously untreated, high-risk DLBCL. However, the R2-CHOP arm was closed early.
In cycle one, all patients received durvalumab plus R-CHOP. For subsequent cycles, patients with activated B-cell (ABC) DLBCL were assigned to durvalumab plus R2-CHOP, while patients with non-ABC DLBCL continued on durvalumab plus R-CHOP.
The R2-CHOP arm was closed early due to safety issues observed in trials combining checkpoint inhibitors with immunomodulatory agents. A partial clinical hold was placed on the R2-CHOP arm, but patients could continue on the regimen if they experienced a clinical benefit. Any patients with ABC DLBCL who were enrolled after the partial hold received treatment with durvalumab plus R-CHOP.
Induction was given for up to eight cycles and was followed by consolidation with durvalumab alone for up to 12 months from the start of induction.
Patient characteristics
The researchers presented data on 43 patients in the durvalumab plus R-CHOP arm. The patients’ median age was 62 years, and 61% were men.
“I think it’s worth noting that 46% of patients in the durvalumab plus R-CHOP group had very high-risk features, including double-hit or triple-hit genetic features,” said Justin Kline, MD, of the University of Chicago Medicine who reviewed this study in a poster discussion session.
Specifically, 30% of patients had double-hit lymphoma, and 16% had triple-hit lymphoma. Most patients had a high-intermediate-risk (49%) or high-risk (21%) International Prognostic Index score, and 79% of patients had Ann Arbor stage IV disease.
Efficacy
As of Aug. 2, 2018, 70% of patients had completed induction, 2% had completed consolidation, 44% remained on treatment, and 54% had discontinued therapy. The most common reasons for stopping treatment were progression (16%), AEs (14%), and consent withdrawal (12%).
“The combination of durvalumab plus R-CHOP demonstrated encouraging response rates … in subjects with high-risk DLBCL, including double- and triple-hit lymphomas,” Dr. Kline said.
The complete response rate was 54% (20/37) at the end of induction and 68% (n = 25) at the end of consolidation. The partial response rate at the end of consolidation was 30% (n = 11).
In patients with double- or triple-hit lymphoma, the complete response rate at the end of induction was 41% (7/17). The overall response rate in this group was 88% (n = 15).
Safety
“The safety profile was as expected for the components of the combination, and no new safety signals were observed,” Dr. Kline said.
He noted that AEs of special interest, or immune-related AEs, occurred in 61% of patients, but most of these events were grade 1 or 2.
AEs of special interest included diarrhea (28%), rash (23%), infusion-related reactions (16%), dermatitis (12%), hypothyroidism (5%), myocarditis (5%), adrenal insufficiency (2%), and hepatitis (2%).
Grade 3 or 4 AEs of special interest included infusion-related reactions (5%), rash (2%), diarrhea (2%), and hepatitis (2%).
The safety and efficacy results support further evaluation of durvalumab plus R-CHOP, although it will be important to identify DLBCL patients who are more likely to derive a clinical benefit from PD-1 or PD-L1 blockade, Dr. Kline said.
“This early study showed that the combination is feasible,” Dr. Nowakowski added. “I think, down the road, we’ll need to identify patients who can actually benefit from this combination. We definitely have clinical evidence of exceptional responses to PD-1 blockade.”
The trial was sponsored by Celgene. Dr. Nowakowski reported relationships with Celgene, Genentech, MorphoSys, and NanoString Technologies. Dr. Kline reported relationships with Cardinal Health, Merck, Seattle Genetics, Kite/Gilead, ITeos Therapeutics, and Bristol-Myers Squibb.
SOURCE: Nowakowski GS et al. ASCO 2019, Abstract 7520.
CHICAGO – A six-drug combination produced complete responses in previously untreated, high-risk diffuse large B-cell lymphoma (DLBCL) patients in a phase 2 trial.
Induction with durvalumab and R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) produced complete response rates of 54% in the entire cohort and 41% in patients with double- or triple-hit lymphoma. Immune-related adverse events (AEs) were common with this regimen, but no unexpected AEs occurred, according to researchers.
Grzegorz S. Nowakowski, MD, of the Mayo Clinic in Rochester, Minn., and colleagues presented these results in a poster at the annual meeting of the American Society of Clinical Oncology.
Treatment
The phase 2 trial (NCT03003520) was designed to assess durvalumab plus R-CHOP as well as durvalumab plus R-CHOP and lenalidomide (R2-CHOP) in patients with previously untreated, high-risk DLBCL. However, the R2-CHOP arm was closed early.
In cycle one, all patients received durvalumab plus R-CHOP. For subsequent cycles, patients with activated B-cell (ABC) DLBCL were assigned to durvalumab plus R2-CHOP, while patients with non-ABC DLBCL continued on durvalumab plus R-CHOP.
The R2-CHOP arm was closed early due to safety issues observed in trials combining checkpoint inhibitors with immunomodulatory agents. A partial clinical hold was placed on the R2-CHOP arm, but patients could continue on the regimen if they experienced a clinical benefit. Any patients with ABC DLBCL who were enrolled after the partial hold received treatment with durvalumab plus R-CHOP.
Induction was given for up to eight cycles and was followed by consolidation with durvalumab alone for up to 12 months from the start of induction.
Patient characteristics
The researchers presented data on 43 patients in the durvalumab plus R-CHOP arm. The patients’ median age was 62 years, and 61% were men.
“I think it’s worth noting that 46% of patients in the durvalumab plus R-CHOP group had very high-risk features, including double-hit or triple-hit genetic features,” said Justin Kline, MD, of the University of Chicago Medicine who reviewed this study in a poster discussion session.
Specifically, 30% of patients had double-hit lymphoma, and 16% had triple-hit lymphoma. Most patients had a high-intermediate-risk (49%) or high-risk (21%) International Prognostic Index score, and 79% of patients had Ann Arbor stage IV disease.
Efficacy
As of Aug. 2, 2018, 70% of patients had completed induction, 2% had completed consolidation, 44% remained on treatment, and 54% had discontinued therapy. The most common reasons for stopping treatment were progression (16%), AEs (14%), and consent withdrawal (12%).
“The combination of durvalumab plus R-CHOP demonstrated encouraging response rates … in subjects with high-risk DLBCL, including double- and triple-hit lymphomas,” Dr. Kline said.
The complete response rate was 54% (20/37) at the end of induction and 68% (n = 25) at the end of consolidation. The partial response rate at the end of consolidation was 30% (n = 11).
In patients with double- or triple-hit lymphoma, the complete response rate at the end of induction was 41% (7/17). The overall response rate in this group was 88% (n = 15).
Safety
“The safety profile was as expected for the components of the combination, and no new safety signals were observed,” Dr. Kline said.
He noted that AEs of special interest, or immune-related AEs, occurred in 61% of patients, but most of these events were grade 1 or 2.
AEs of special interest included diarrhea (28%), rash (23%), infusion-related reactions (16%), dermatitis (12%), hypothyroidism (5%), myocarditis (5%), adrenal insufficiency (2%), and hepatitis (2%).
Grade 3 or 4 AEs of special interest included infusion-related reactions (5%), rash (2%), diarrhea (2%), and hepatitis (2%).
The safety and efficacy results support further evaluation of durvalumab plus R-CHOP, although it will be important to identify DLBCL patients who are more likely to derive a clinical benefit from PD-1 or PD-L1 blockade, Dr. Kline said.
“This early study showed that the combination is feasible,” Dr. Nowakowski added. “I think, down the road, we’ll need to identify patients who can actually benefit from this combination. We definitely have clinical evidence of exceptional responses to PD-1 blockade.”
The trial was sponsored by Celgene. Dr. Nowakowski reported relationships with Celgene, Genentech, MorphoSys, and NanoString Technologies. Dr. Kline reported relationships with Cardinal Health, Merck, Seattle Genetics, Kite/Gilead, ITeos Therapeutics, and Bristol-Myers Squibb.
SOURCE: Nowakowski GS et al. ASCO 2019, Abstract 7520.
REPORTING FROM ASCO 2019
















