User login
Topical Cannabinoids in Dermatology
The prevalence of topical cannabinoids has risen sharply in recent years. Commercial advertisers promote their usage as a safe means to treat a multitude of skin disorders, including atopic dermatitis (AD), psoriasis, and acne. Topical compounds have garnered interest in laboratory studies, but the purchase of commercial formulations is limited to over-the-counter products from unregulated suppliers. In this article, we review the scientific evidence behind topical cannabinoids and evaluate their role in clinical dermatology.
Background
Cannabis is designated as a Schedule I drug, according to the Controlled Substances Act of 1970. This listing is given to substances with no therapeutic value and a high potential for abuse. However, as of 2017, 29 states and the District of Columbia have laws legalizing cannabis in some capacity. These regulations typically apply to medicinal use, though several states have now legalized recreational use.
Cannabinoids represent a broad class of chemical compounds derived from the cannabis plant. Originally, this class only comprised phytocannabinoids, cannabinoids produced by the cannabis plant. Tetrahydrocannabinol (THC) is the most well-known phytocannabinoid and leads to the psychoactive effects typically associated with cannabis use. Later investigation led to the discovery of endocannabinoids, cannabinoids that are naturally produced by human and animal bodies, as well as synthetic cannabinoids.1 Cannabidiol is a phytocannabinoid that has been investigated in neurologic and anti-inflammatory conditions.2-4
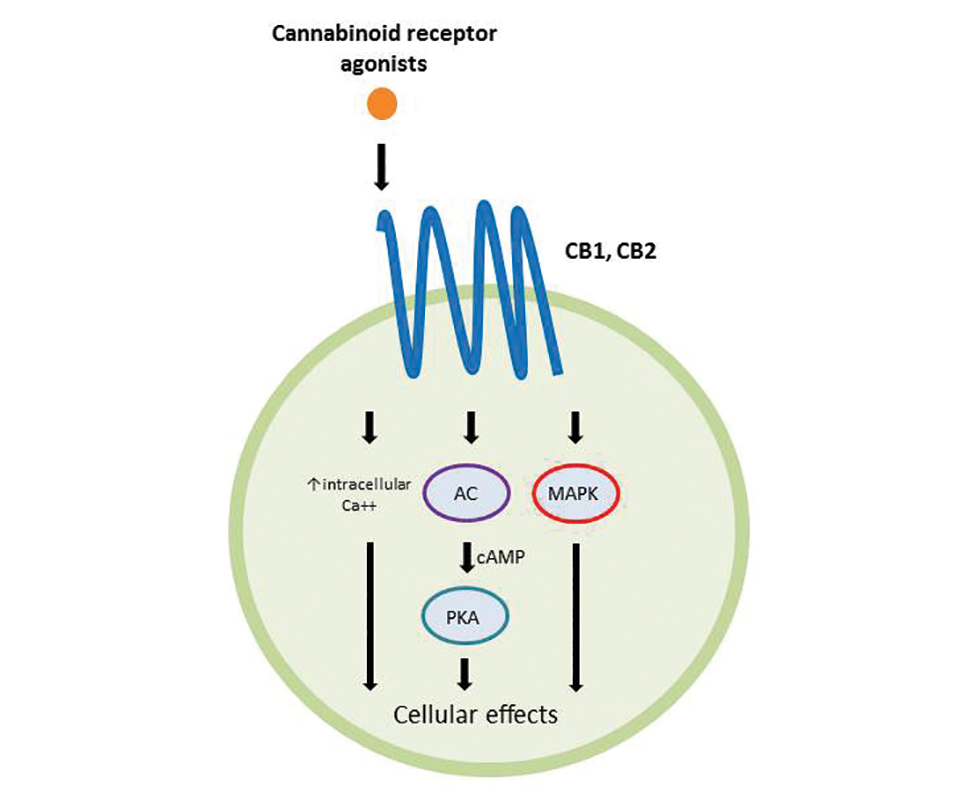
Cannabinoids act as agonists on 2 principal receptors— cannabinoid receptor type 1 (CB1) and cannabinoid receptor type 2 (CB2)—which are both G protein–coupled receptors (Figure).5 Both have distinct distributions throughout different organ systems, to which cannabinoids (eg, THC, cannabidiol, endocannabinoids) show differential binding.6,7 Importantly, the expression of CB1 and CB2 has been identified on sensory nerve fibers, inflammatory cells, and adnexal structures of human skin.8 Based on these associations, topical application of cannabinoids has become a modality of interest for dermatological disorders. These formulations aim to influence cutaneous morphology without producing psychoactive effects.
Topical Cannabinoids in Inflammatory Disorders
Atopic dermatitis has emerged as an active area of investigation for cannabinoid receptors and topical agonists (Table 1). In an animal model, Kim et al9 examined the effects of CB1 agonism on skin inflammation. Mice treated with topical CB1 agonists showed greater recovery of epidermal barrier function in acutely abrogated skin relative to those treated with a vehicle preparation. In addition, agonism of CB1 led to significant (P<.001) decreases in skin fold thickness among models of acute and chronic skin inflammation.9
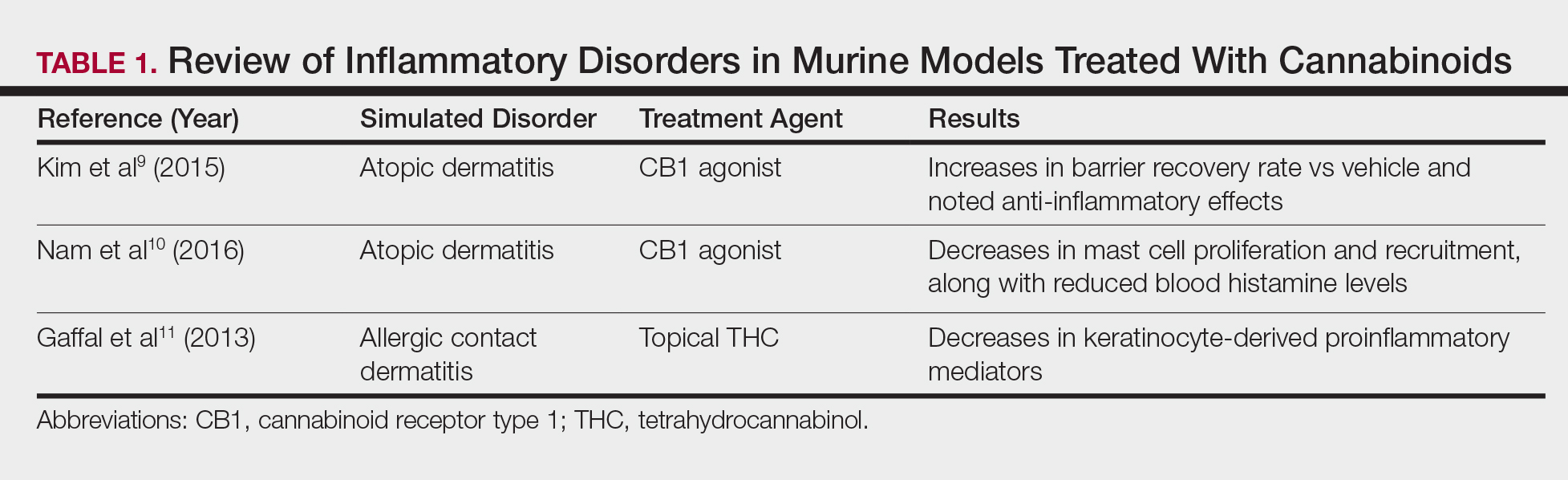
Nam et al10 also examined the role of topical CB1 agonists in mice with induced AD-like symptoms. Relative to treatment with vehicle, CB1 agonists significantly reduced the recruitment of mast cells (P<.01) and lowered the blood concentration of histamine (P<.05). Given the noted decrease in the release of inflammatory mediators, the authors speculated that topical agonsim of CB1 may prove useful in several conditions related to mast cell activation, such as AD, contact dermatitis, and psoriasis.10
The anti-inflammatory properties of topical THC were evaluated by Gaffal et al.11 In a mouse model of allergic contact dermatitis, mice treated with topical THC showed decreases in myeloid immune cell infiltration, with these beneficial effects existing even in mice with deficient CB1 and CB2 receptors. These results support a potentially wide anti-inflammatory activity of topical THC.11
Topical Cannabinoids in Pain Management
The effects of smoked cannabis in treating pain have undergone thorough investigation over recent years. Benefits have been noted in treating neuropathic pain, particularly in human immunodeficiency virus–associated sensory neuropathy.12-15 Smoked cannabis also may provide value as a synergistic therapy with opioids, thereby allowing for lower opioid doses.16
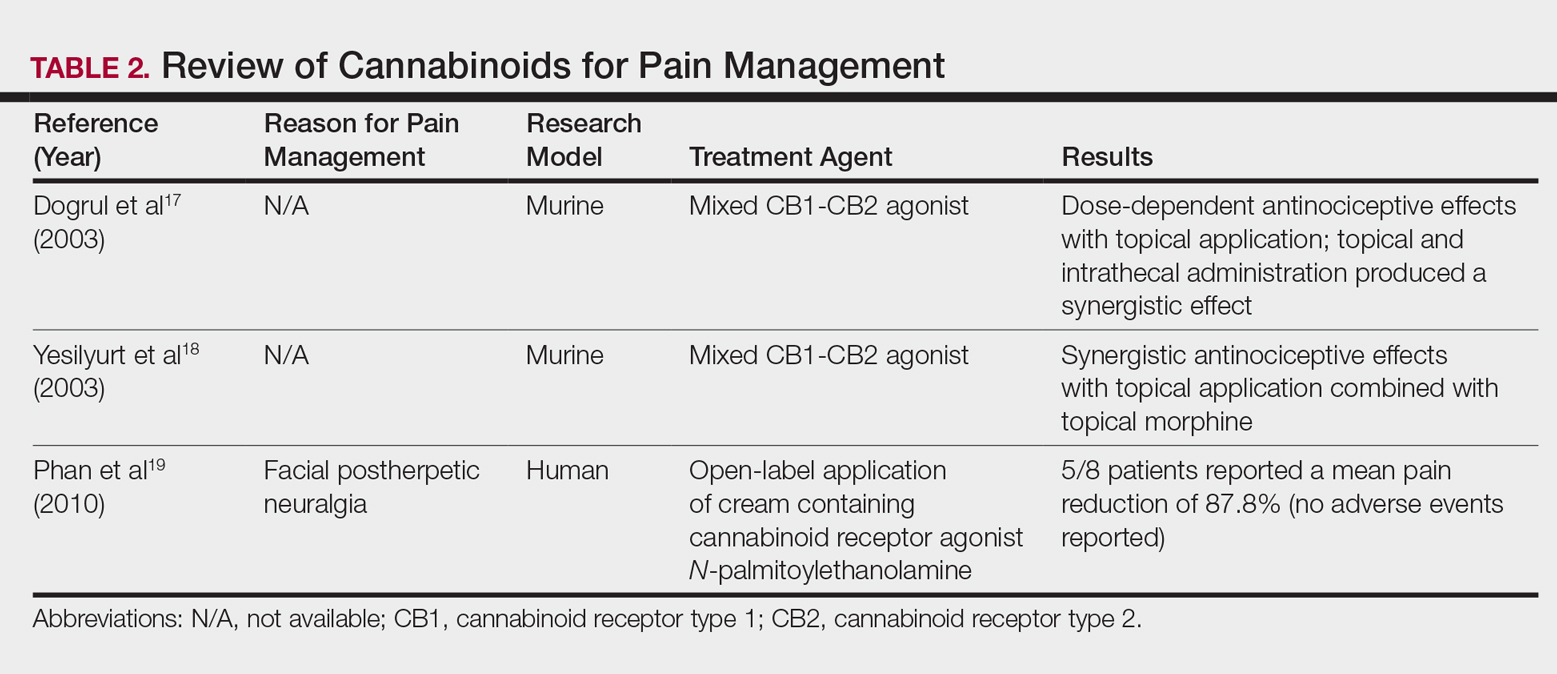
In contrast, research into the relationship between topical application of cannabinoids and nociception remains in preliminary stages (Table 2). In a mouse model, Dogrul et al17 assessed the topical antinociceptive potential of a mixed CB1-CB2 agonist. Results showed significant (P<.01) and dose-dependent antinociceptive effects relative to treatment with a vehicle.17 In a related study, Yesilyurt et al18 evaluated whether a mixed CB1-CB2 agonist could enhance the antinociceptive effects of topical opioids. Among mice treated with the combination of a cannabinoid agonist and topical morphine, a significantly (P<.05) greater analgesic effect was demonstrated relative to topical morphine alone.18
Studies in humans have been far more limited. Phan et al19 conducted a small, nonrandomized, open-label trial of a topical cannabinoid cream in patients with facial postherpetic neuralgia. Of 8 patients treated, 5 noted a mean pain reduction of 87.8%. No comparison vehicle was used. Based on this narrow study design, it is difficult to extrapolate these positive results to a broader patient population.19
Commercial Products
Although preliminary models with topical cannabinoids have shown potential, large-scale clinical trials in humans have yet to be performed. Despite this lack of investigation, commercial formulations of topical cannabinoids are available to dermatology patients. These formulations are nonstandardized, and no safety data exists regarding their use. Topical cannabinoids on the market may contain various amounts of active ingredient and may be combined with a range of other compounds.
In dermatology offices, it is not uncommon for patients to express an intention to use topical cannabinoid products following their planned treatment or procedure. Patients also have been known to use topical cannabinoid products prior to dermatologic procedures, sometimes in place of an approved topical anesthetic, without consulting the physician performing the procedure. With interventions that lead to active areas of wound healing, the application of such products may increase the risk for contamination and infection. Therefore, patients should be counseled that the use of commercial topical cannabinoids could jeopardize the success of their planned procedure, put them at risk for infection, and possibly lead to systemic absorption and/or changes in wound-healing capacities.
Conclusion
Based on the results from recent animal models, cannabinoids may have a role in future treatment algorithms for several inflammatory conditions. However, current efficacy and safety data are almost entirely limited to preliminary animal studies in rodents. In addition, the formulation of topical cannabinoid products is nonstandardized and poorly regulated. As such, the present evidence does not support the use of topical cannabinoids in dermatology practices. Dermatologists should ask patients about the use of any cannabinoid products as part of a treatment program, especially given the unsubstantiated claims often made by unscrupulous advertisers. This issue highlights the need for further research and regulation.
- Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389-462.
- Giacoppo S, Galuppo M, Pollastro F, et al. A new formulation of cannabidiol in cream shows therapeutic effects in a mouse model of experimental autoimmune encephalomyelitis. Daru. 2015;23:48.
- Hammell DC, Zhang LP, Ma F, et al. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur J Pain. 2016;20:936-948.
- Schicho R, Storr M. Topical and systemic cannabidiol improves trinitrobenzene sulfonic acid colitis in mice. Pharmacology. 2012;89:149-155.
- Howlett AC, Barth F, Bonner TI, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161-202.
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199-215.
- Svizenska I, Dubovy P, Sulcova A. Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures—a short review. Pharmacol Biochem Behav. 2008;90:501-511.
- Stander S, Schmelz M, Metze D, et al. Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. J Dermatol Sci. 2005;38:177-188.
- Kim HJ, Kim B, Park BM, et al. Topical cannabinoid receptor 1 agonist attenuates the cutaneous inflammatory responses in oxazolone-induced atopic dermatitis model. Int J Dermatol. 2015;54:E401-E408.
- Nam G, Jeong SK, Park BM, et al. Selective cannabinoid receptor-1 agonists regulate mast cell activation in an oxazolone-induced atopic dermatitis model. Ann Dermatol. 2016;28:22-29.
- Gaffal E, Cron M, Glodde N, et al. Anti-inflammatory activity of topical THC in DNFB-mediated mouse allergic contact dermatitis independent of CB1 and CB2 receptors. Allergy. 2013;68:994-1000.
- Abrams DI, Jay CA, Shade SB, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68:515-521.
- Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2009;34:672-680.
- Wilsey B, Marcotte T, Deutsch R, et al. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain. 2013;14:136-148.
- Wilsey B, Marcotte T, Tsodikov A, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain. 2008;9:506-521.
- Abrams DI, Couey P, Shade SB, et al. Cannabinoid-opioid interaction in chronic pain. Clin Pharmacol Ther. 2011;90:844-851.
- Dogrul A, Gul H, Akar A, et al. Topical cannabinoid antinociception: synergy with spinal sites. Pain. 2003;105:11-16.
- Yesilyurt O, Dogrul A, Gul H, et al. Topical cannabinoid enhances topical morphine antinociception. Pain. 2003;105:303-308.
- Phan NQ, Siepmann D, Gralow I, et al. Adjuvant topical therapy with a cannabinoid receptor agonist in facial postherpetic neuralgia. J Dtsch Dermatol Ges. 2010;8:88-91.
The prevalence of topical cannabinoids has risen sharply in recent years. Commercial advertisers promote their usage as a safe means to treat a multitude of skin disorders, including atopic dermatitis (AD), psoriasis, and acne. Topical compounds have garnered interest in laboratory studies, but the purchase of commercial formulations is limited to over-the-counter products from unregulated suppliers. In this article, we review the scientific evidence behind topical cannabinoids and evaluate their role in clinical dermatology.
Background
Cannabis is designated as a Schedule I drug, according to the Controlled Substances Act of 1970. This listing is given to substances with no therapeutic value and a high potential for abuse. However, as of 2017, 29 states and the District of Columbia have laws legalizing cannabis in some capacity. These regulations typically apply to medicinal use, though several states have now legalized recreational use.
Cannabinoids represent a broad class of chemical compounds derived from the cannabis plant. Originally, this class only comprised phytocannabinoids, cannabinoids produced by the cannabis plant. Tetrahydrocannabinol (THC) is the most well-known phytocannabinoid and leads to the psychoactive effects typically associated with cannabis use. Later investigation led to the discovery of endocannabinoids, cannabinoids that are naturally produced by human and animal bodies, as well as synthetic cannabinoids.1 Cannabidiol is a phytocannabinoid that has been investigated in neurologic and anti-inflammatory conditions.2-4
Cannabinoids act as agonists on 2 principal receptors— cannabinoid receptor type 1 (CB1) and cannabinoid receptor type 2 (CB2)—which are both G protein–coupled receptors (Figure).5 Both have distinct distributions throughout different organ systems, to which cannabinoids (eg, THC, cannabidiol, endocannabinoids) show differential binding.6,7 Importantly, the expression of CB1 and CB2 has been identified on sensory nerve fibers, inflammatory cells, and adnexal structures of human skin.8 Based on these associations, topical application of cannabinoids has become a modality of interest for dermatological disorders. These formulations aim to influence cutaneous morphology without producing psychoactive effects.

Topical Cannabinoids in Inflammatory Disorders
Atopic dermatitis has emerged as an active area of investigation for cannabinoid receptors and topical agonists (Table 1). In an animal model, Kim et al9 examined the effects of CB1 agonism on skin inflammation. Mice treated with topical CB1 agonists showed greater recovery of epidermal barrier function in acutely abrogated skin relative to those treated with a vehicle preparation. In addition, agonism of CB1 led to significant (P<.001) decreases in skin fold thickness among models of acute and chronic skin inflammation.9

Nam et al10 also examined the role of topical CB1 agonists in mice with induced AD-like symptoms. Relative to treatment with vehicle, CB1 agonists significantly reduced the recruitment of mast cells (P<.01) and lowered the blood concentration of histamine (P<.05). Given the noted decrease in the release of inflammatory mediators, the authors speculated that topical agonsim of CB1 may prove useful in several conditions related to mast cell activation, such as AD, contact dermatitis, and psoriasis.10
The anti-inflammatory properties of topical THC were evaluated by Gaffal et al.11 In a mouse model of allergic contact dermatitis, mice treated with topical THC showed decreases in myeloid immune cell infiltration, with these beneficial effects existing even in mice with deficient CB1 and CB2 receptors. These results support a potentially wide anti-inflammatory activity of topical THC.11
Topical Cannabinoids in Pain Management
The effects of smoked cannabis in treating pain have undergone thorough investigation over recent years. Benefits have been noted in treating neuropathic pain, particularly in human immunodeficiency virus–associated sensory neuropathy.12-15 Smoked cannabis also may provide value as a synergistic therapy with opioids, thereby allowing for lower opioid doses.16
In contrast, research into the relationship between topical application of cannabinoids and nociception remains in preliminary stages (Table 2). In a mouse model, Dogrul et al17 assessed the topical antinociceptive potential of a mixed CB1-CB2 agonist. Results showed significant (P<.01) and dose-dependent antinociceptive effects relative to treatment with a vehicle.17 In a related study, Yesilyurt et al18 evaluated whether a mixed CB1-CB2 agonist could enhance the antinociceptive effects of topical opioids. Among mice treated with the combination of a cannabinoid agonist and topical morphine, a significantly (P<.05) greater analgesic effect was demonstrated relative to topical morphine alone.18
Studies in humans have been far more limited. Phan et al19 conducted a small, nonrandomized, open-label trial of a topical cannabinoid cream in patients with facial postherpetic neuralgia. Of 8 patients treated, 5 noted a mean pain reduction of 87.8%. No comparison vehicle was used. Based on this narrow study design, it is difficult to extrapolate these positive results to a broader patient population.19

Commercial Products
Although preliminary models with topical cannabinoids have shown potential, large-scale clinical trials in humans have yet to be performed. Despite this lack of investigation, commercial formulations of topical cannabinoids are available to dermatology patients. These formulations are nonstandardized, and no safety data exists regarding their use. Topical cannabinoids on the market may contain various amounts of active ingredient and may be combined with a range of other compounds.
In dermatology offices, it is not uncommon for patients to express an intention to use topical cannabinoid products following their planned treatment or procedure. Patients also have been known to use topical cannabinoid products prior to dermatologic procedures, sometimes in place of an approved topical anesthetic, without consulting the physician performing the procedure. With interventions that lead to active areas of wound healing, the application of such products may increase the risk for contamination and infection. Therefore, patients should be counseled that the use of commercial topical cannabinoids could jeopardize the success of their planned procedure, put them at risk for infection, and possibly lead to systemic absorption and/or changes in wound-healing capacities.
Conclusion
Based on the results from recent animal models, cannabinoids may have a role in future treatment algorithms for several inflammatory conditions. However, current efficacy and safety data are almost entirely limited to preliminary animal studies in rodents. In addition, the formulation of topical cannabinoid products is nonstandardized and poorly regulated. As such, the present evidence does not support the use of topical cannabinoids in dermatology practices. Dermatologists should ask patients about the use of any cannabinoid products as part of a treatment program, especially given the unsubstantiated claims often made by unscrupulous advertisers. This issue highlights the need for further research and regulation.
The prevalence of topical cannabinoids has risen sharply in recent years. Commercial advertisers promote their usage as a safe means to treat a multitude of skin disorders, including atopic dermatitis (AD), psoriasis, and acne. Topical compounds have garnered interest in laboratory studies, but the purchase of commercial formulations is limited to over-the-counter products from unregulated suppliers. In this article, we review the scientific evidence behind topical cannabinoids and evaluate their role in clinical dermatology.
Background
Cannabis is designated as a Schedule I drug, according to the Controlled Substances Act of 1970. This listing is given to substances with no therapeutic value and a high potential for abuse. However, as of 2017, 29 states and the District of Columbia have laws legalizing cannabis in some capacity. These regulations typically apply to medicinal use, though several states have now legalized recreational use.
Cannabinoids represent a broad class of chemical compounds derived from the cannabis plant. Originally, this class only comprised phytocannabinoids, cannabinoids produced by the cannabis plant. Tetrahydrocannabinol (THC) is the most well-known phytocannabinoid and leads to the psychoactive effects typically associated with cannabis use. Later investigation led to the discovery of endocannabinoids, cannabinoids that are naturally produced by human and animal bodies, as well as synthetic cannabinoids.1 Cannabidiol is a phytocannabinoid that has been investigated in neurologic and anti-inflammatory conditions.2-4
Cannabinoids act as agonists on 2 principal receptors— cannabinoid receptor type 1 (CB1) and cannabinoid receptor type 2 (CB2)—which are both G protein–coupled receptors (Figure).5 Both have distinct distributions throughout different organ systems, to which cannabinoids (eg, THC, cannabidiol, endocannabinoids) show differential binding.6,7 Importantly, the expression of CB1 and CB2 has been identified on sensory nerve fibers, inflammatory cells, and adnexal structures of human skin.8 Based on these associations, topical application of cannabinoids has become a modality of interest for dermatological disorders. These formulations aim to influence cutaneous morphology without producing psychoactive effects.

Topical Cannabinoids in Inflammatory Disorders
Atopic dermatitis has emerged as an active area of investigation for cannabinoid receptors and topical agonists (Table 1). In an animal model, Kim et al9 examined the effects of CB1 agonism on skin inflammation. Mice treated with topical CB1 agonists showed greater recovery of epidermal barrier function in acutely abrogated skin relative to those treated with a vehicle preparation. In addition, agonism of CB1 led to significant (P<.001) decreases in skin fold thickness among models of acute and chronic skin inflammation.9

Nam et al10 also examined the role of topical CB1 agonists in mice with induced AD-like symptoms. Relative to treatment with vehicle, CB1 agonists significantly reduced the recruitment of mast cells (P<.01) and lowered the blood concentration of histamine (P<.05). Given the noted decrease in the release of inflammatory mediators, the authors speculated that topical agonsim of CB1 may prove useful in several conditions related to mast cell activation, such as AD, contact dermatitis, and psoriasis.10
The anti-inflammatory properties of topical THC were evaluated by Gaffal et al.11 In a mouse model of allergic contact dermatitis, mice treated with topical THC showed decreases in myeloid immune cell infiltration, with these beneficial effects existing even in mice with deficient CB1 and CB2 receptors. These results support a potentially wide anti-inflammatory activity of topical THC.11
Topical Cannabinoids in Pain Management
The effects of smoked cannabis in treating pain have undergone thorough investigation over recent years. Benefits have been noted in treating neuropathic pain, particularly in human immunodeficiency virus–associated sensory neuropathy.12-15 Smoked cannabis also may provide value as a synergistic therapy with opioids, thereby allowing for lower opioid doses.16
In contrast, research into the relationship between topical application of cannabinoids and nociception remains in preliminary stages (Table 2). In a mouse model, Dogrul et al17 assessed the topical antinociceptive potential of a mixed CB1-CB2 agonist. Results showed significant (P<.01) and dose-dependent antinociceptive effects relative to treatment with a vehicle.17 In a related study, Yesilyurt et al18 evaluated whether a mixed CB1-CB2 agonist could enhance the antinociceptive effects of topical opioids. Among mice treated with the combination of a cannabinoid agonist and topical morphine, a significantly (P<.05) greater analgesic effect was demonstrated relative to topical morphine alone.18
Studies in humans have been far more limited. Phan et al19 conducted a small, nonrandomized, open-label trial of a topical cannabinoid cream in patients with facial postherpetic neuralgia. Of 8 patients treated, 5 noted a mean pain reduction of 87.8%. No comparison vehicle was used. Based on this narrow study design, it is difficult to extrapolate these positive results to a broader patient population.19

Commercial Products
Although preliminary models with topical cannabinoids have shown potential, large-scale clinical trials in humans have yet to be performed. Despite this lack of investigation, commercial formulations of topical cannabinoids are available to dermatology patients. These formulations are nonstandardized, and no safety data exists regarding their use. Topical cannabinoids on the market may contain various amounts of active ingredient and may be combined with a range of other compounds.
In dermatology offices, it is not uncommon for patients to express an intention to use topical cannabinoid products following their planned treatment or procedure. Patients also have been known to use topical cannabinoid products prior to dermatologic procedures, sometimes in place of an approved topical anesthetic, without consulting the physician performing the procedure. With interventions that lead to active areas of wound healing, the application of such products may increase the risk for contamination and infection. Therefore, patients should be counseled that the use of commercial topical cannabinoids could jeopardize the success of their planned procedure, put them at risk for infection, and possibly lead to systemic absorption and/or changes in wound-healing capacities.
Conclusion
Based on the results from recent animal models, cannabinoids may have a role in future treatment algorithms for several inflammatory conditions. However, current efficacy and safety data are almost entirely limited to preliminary animal studies in rodents. In addition, the formulation of topical cannabinoid products is nonstandardized and poorly regulated. As such, the present evidence does not support the use of topical cannabinoids in dermatology practices. Dermatologists should ask patients about the use of any cannabinoid products as part of a treatment program, especially given the unsubstantiated claims often made by unscrupulous advertisers. This issue highlights the need for further research and regulation.
- Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389-462.
- Giacoppo S, Galuppo M, Pollastro F, et al. A new formulation of cannabidiol in cream shows therapeutic effects in a mouse model of experimental autoimmune encephalomyelitis. Daru. 2015;23:48.
- Hammell DC, Zhang LP, Ma F, et al. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur J Pain. 2016;20:936-948.
- Schicho R, Storr M. Topical and systemic cannabidiol improves trinitrobenzene sulfonic acid colitis in mice. Pharmacology. 2012;89:149-155.
- Howlett AC, Barth F, Bonner TI, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161-202.
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199-215.
- Svizenska I, Dubovy P, Sulcova A. Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures—a short review. Pharmacol Biochem Behav. 2008;90:501-511.
- Stander S, Schmelz M, Metze D, et al. Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. J Dermatol Sci. 2005;38:177-188.
- Kim HJ, Kim B, Park BM, et al. Topical cannabinoid receptor 1 agonist attenuates the cutaneous inflammatory responses in oxazolone-induced atopic dermatitis model. Int J Dermatol. 2015;54:E401-E408.
- Nam G, Jeong SK, Park BM, et al. Selective cannabinoid receptor-1 agonists regulate mast cell activation in an oxazolone-induced atopic dermatitis model. Ann Dermatol. 2016;28:22-29.
- Gaffal E, Cron M, Glodde N, et al. Anti-inflammatory activity of topical THC in DNFB-mediated mouse allergic contact dermatitis independent of CB1 and CB2 receptors. Allergy. 2013;68:994-1000.
- Abrams DI, Jay CA, Shade SB, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68:515-521.
- Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2009;34:672-680.
- Wilsey B, Marcotte T, Deutsch R, et al. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain. 2013;14:136-148.
- Wilsey B, Marcotte T, Tsodikov A, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain. 2008;9:506-521.
- Abrams DI, Couey P, Shade SB, et al. Cannabinoid-opioid interaction in chronic pain. Clin Pharmacol Ther. 2011;90:844-851.
- Dogrul A, Gul H, Akar A, et al. Topical cannabinoid antinociception: synergy with spinal sites. Pain. 2003;105:11-16.
- Yesilyurt O, Dogrul A, Gul H, et al. Topical cannabinoid enhances topical morphine antinociception. Pain. 2003;105:303-308.
- Phan NQ, Siepmann D, Gralow I, et al. Adjuvant topical therapy with a cannabinoid receptor agonist in facial postherpetic neuralgia. J Dtsch Dermatol Ges. 2010;8:88-91.
- Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389-462.
- Giacoppo S, Galuppo M, Pollastro F, et al. A new formulation of cannabidiol in cream shows therapeutic effects in a mouse model of experimental autoimmune encephalomyelitis. Daru. 2015;23:48.
- Hammell DC, Zhang LP, Ma F, et al. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur J Pain. 2016;20:936-948.
- Schicho R, Storr M. Topical and systemic cannabidiol improves trinitrobenzene sulfonic acid colitis in mice. Pharmacology. 2012;89:149-155.
- Howlett AC, Barth F, Bonner TI, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161-202.
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199-215.
- Svizenska I, Dubovy P, Sulcova A. Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures—a short review. Pharmacol Biochem Behav. 2008;90:501-511.
- Stander S, Schmelz M, Metze D, et al. Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. J Dermatol Sci. 2005;38:177-188.
- Kim HJ, Kim B, Park BM, et al. Topical cannabinoid receptor 1 agonist attenuates the cutaneous inflammatory responses in oxazolone-induced atopic dermatitis model. Int J Dermatol. 2015;54:E401-E408.
- Nam G, Jeong SK, Park BM, et al. Selective cannabinoid receptor-1 agonists regulate mast cell activation in an oxazolone-induced atopic dermatitis model. Ann Dermatol. 2016;28:22-29.
- Gaffal E, Cron M, Glodde N, et al. Anti-inflammatory activity of topical THC in DNFB-mediated mouse allergic contact dermatitis independent of CB1 and CB2 receptors. Allergy. 2013;68:994-1000.
- Abrams DI, Jay CA, Shade SB, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68:515-521.
- Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2009;34:672-680.
- Wilsey B, Marcotte T, Deutsch R, et al. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain. 2013;14:136-148.
- Wilsey B, Marcotte T, Tsodikov A, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain. 2008;9:506-521.
- Abrams DI, Couey P, Shade SB, et al. Cannabinoid-opioid interaction in chronic pain. Clin Pharmacol Ther. 2011;90:844-851.
- Dogrul A, Gul H, Akar A, et al. Topical cannabinoid antinociception: synergy with spinal sites. Pain. 2003;105:11-16.
- Yesilyurt O, Dogrul A, Gul H, et al. Topical cannabinoid enhances topical morphine antinociception. Pain. 2003;105:303-308.
- Phan NQ, Siepmann D, Gralow I, et al. Adjuvant topical therapy with a cannabinoid receptor agonist in facial postherpetic neuralgia. J Dtsch Dermatol Ges. 2010;8:88-91.
Practice Points
- Topical cannabinoids are advertised by companies as treatment options for numerous dermatologic conditions.
- Despite promising data in rodent models, there have been no rigorous studies to date confirming efficacy or safety in humans.
- Dermatologists should therefore inquire with patients about the use of any topical cannabinoid products, especially around the time of planned procedures, as they may affect treatment outcomes.
Microneedling Therapy With and Without Platelet-Rich Plasma
Microneedling therapy, also known as collagen induction therapy or percutaneous collagen induction, is an increasingly popular treatment modality for skin rejuvenation. The approach employs small needles to puncture the skin and stimulate local collagen production in a minimally invasive manner. Recently, clinicians have incorporated the use of platelet-rich plasma (PRP) with the aim of augmenting cosmetic outcomes. In this article, we examine the utility of this approach by reviewing comparison studies of microneedling therapy with and without the application of PRP.
Dr. Gary Goldenberg demonstrates microneedling with platelet-rich plasma in a procedural video available here.
Microneedling Therapy
The use of microneedling first gained attention in the 1990s. Initially, Camirand and Doucet1 described tattooing without pigment for the treatment of achromatic and hypertrophic scars. Fernandes2 evolved this concept and developed a drum-shaped device with fine protruding needles to puncture the skin. Microneedling devices have expanded in recent years and now include both cord- and battery-powered pens and rollers, with needles ranging in length from 0.25 to 3.0 mm.
Treatment with microneedling promotes skin rejuvenation by creating small puncture wounds in the epidermis and dermis. This injury triggers the wound healing cascade and alters the modulation of growth factors to promote regenerative effects.3,4 Following microneedling therapy, increases occur in elastic fiber formation, collagen deposition, and dermal thickness (Figure).5 Of interesting histologic note, collagen is deposited in the normal lattice pattern following this treatment rather than in the parallel bundles typical of scars.6 Microneedling preserves the overall integrity of the epidermal layers and basement membrane, allowing the epidermis to heal without abnormality, verified on histology by a normal stratum corneum, enhanced stratum granulosum, and normal rete ridges.7
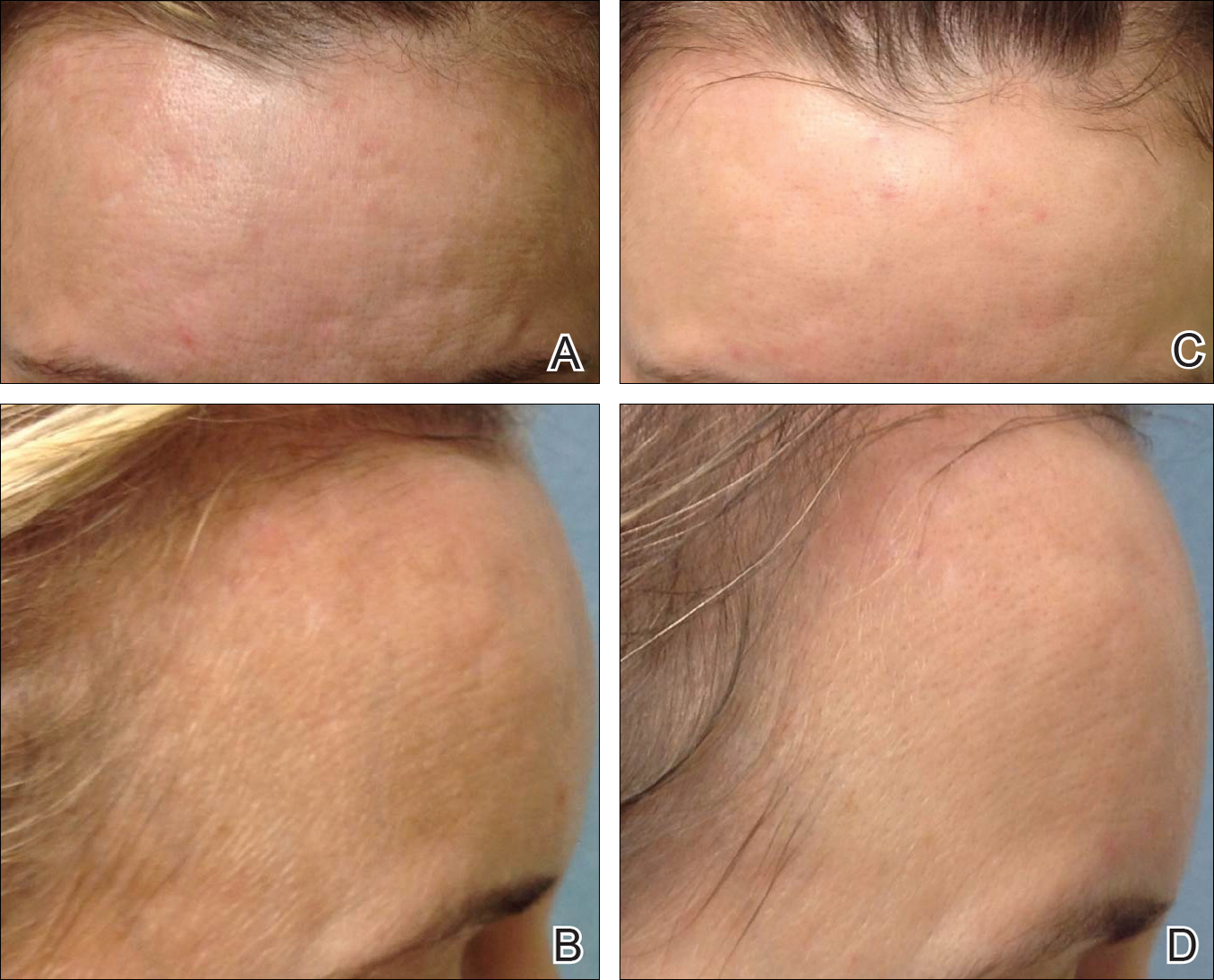
Microneedling has demonstrated several uses beyond general skin rejuvenation. In patients with atrophic acne scars, therapy can lead to improved scar appearance, skin texture, and patient satisfaction.8,9 Hypertrophic and dyspigmented burn scars on the body, face, arms, and legs have shown to be receptive to repeated treatments.10 Microneedling also has shown promise in treating androgenic alopecia, increasing hair regrowth in patients who previously showed poor response to conventional therapy with minoxidil and finasteride.11,12
Platelet-Rich Plasma
Platelet-rich plasma is developed by enriching blood with an autologous concentration of platelets. The preparation of PRP begins with whole blood, commonly obtained peripherally by venipuncture. Samples undergo centrifugation to allow separation of the blood into 3 layers: platelet-poor plasma, PRP, and erythrocytes.13 The typical platelet count of whole blood is approximately 200,000/µL; PRP aims to prepare a platelet count of at least 1,000,000/µL in a 5-mL volume.14
An attractive component of PRP is its high concentration of growth factors, including platelet-derived growth factor, transforming growth factor, vascular endothelial growth factor, and epithelial growth factor.15 Because of the regenerative effects of these proteins, PRP has been investigated as a modality to augment wound healing in a variety of clinical areas, such as maxillofacial surgery, orthopedics, cardiovascular surgery, and treatment of soft tissue ulcers.16
Combination Use of Microneedling and PRP
Several studies have compared the effects of microneedling with and without the application of PRP (Table).17-20 In an animal model, Akcal et al17 examined the effects of microneedling and PRP on skin flap survival. Eight rats were randomly divided into 5 groups: sham, control, microneedling alone, microneedling plus PRP, and microneedling plus platelet-poor plasma. Treatments were applied to skin flaps after 4 hours of induced ischemia. The surviving flap area was measured, with results demonstrating significantly higher viable areas in the microneedling plus PRP group relative to all other groups (P<.01). On histologic examination, the microneedling plus PRP group showed well-organized epidermal layers and a dermal integrity that matched the dermis of the sham group.17
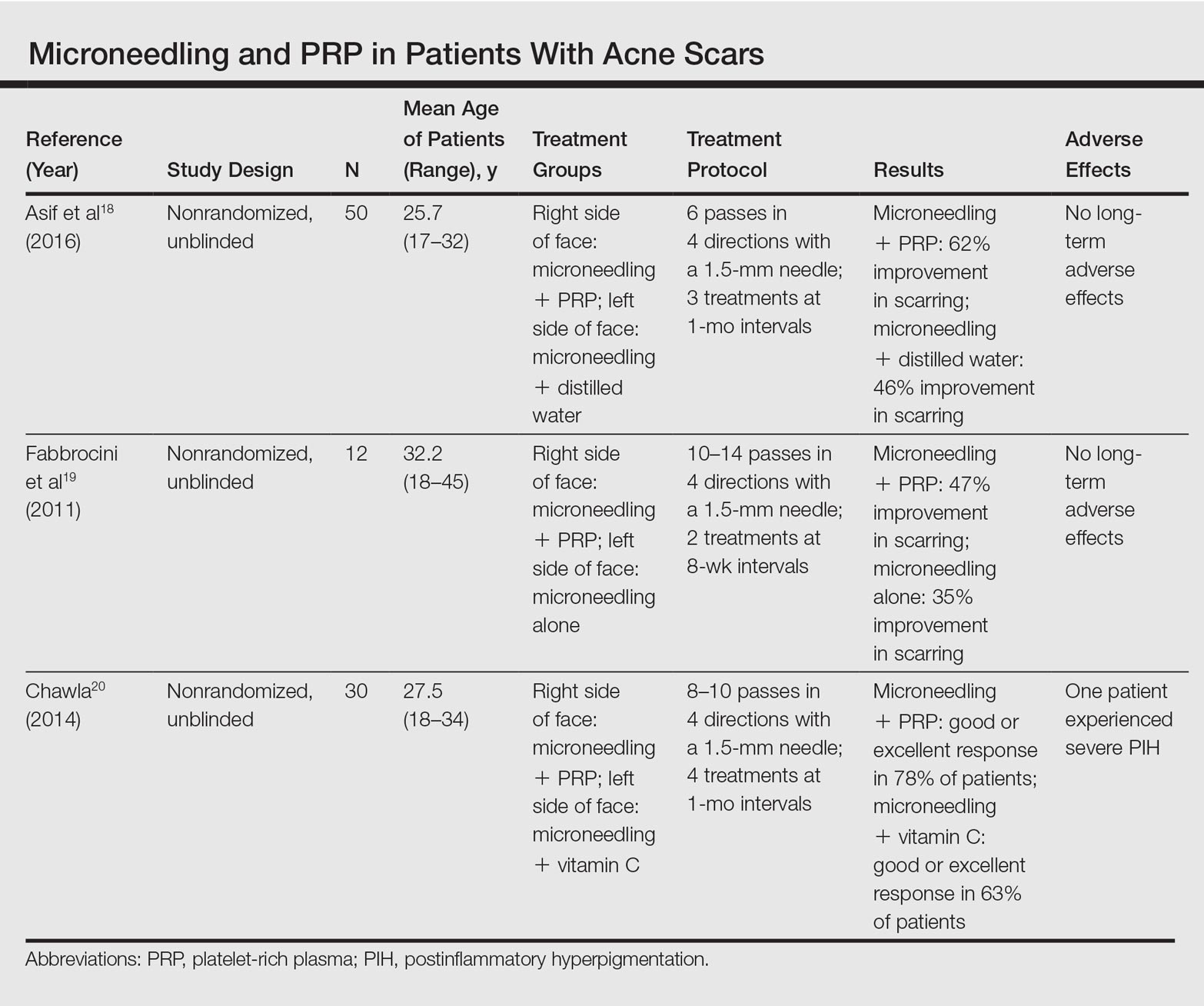
Asif et al18 performed a split-face comparison study of 50 patients with atrophic acne scars. On the right side, microneedling was performed followed by intradermal injections and topical application of PRP. On the left side, microneedling was performed followed by intradermal injections of distilled water. The study included 3 treatment sessions with 1 month between each session. Scars were assessed using the Goodman and Baron scale,21 which is designed to grade the morphology of postacne scarring. Scars on the right side improved by 62.2% and scars on the left side improved by 45.8%; prior to treatment, both sides demonstrated similar severity scores, but final severity scores were significantly reduced in the microneedling plus PRP group relative to the microneedling plus distilled water group (P<.00001). No residual side effects from treatment were reported.18
Examining the degree of improvement more carefully, microneedling plus PRP yielded excellent improvement in 40% (20/50) of patients and good improvement in 60% (30/50).18 Microneedling plus distilled water led to excellent improvement in 10% (5/50) and good improvement in 84% (42/50). Given that microneedling plus distilled water still provided good to excellent results in 94% of patients, the addition of PRP was helpful though not necessary in achieving meaningful benefit.18
In another split-face study, Fabbrocini et al19 evaluated 12 adult patients with acne scars. The right side of the face received microneedling plus PRP, while the left side received microneedling alone. Two treatments were performed 8 weeks apart. Severity scores (0=no lesions; 10=maximum severity) were used to assess patient outcomes throughout the study. Acne scars improved on both sides of the face following the treatment period, but the reduction in scar severity with microneedling plus PRP (3.5 points) was significantly greater than with microneedling alone (2.6 points)(P<.05). Patients tended to experience2 to 3 days of mild swelling and erythema after treatment regardless of PRP addition. With only 12 patients, the study was limited by a small sample size. The 10-point grading system differed from the Goodman and Baron scale in that it lacked corresponding qualitative markers, likely decreasing reproducibility.19
Chawla20 compared the effectiveness of combination therapy with microneedling plus PRP versus microneedling and vitamin C application. In a split-face study of 30 patients with atrophic acne scars, the right side of the face was treated with microneedling plus PRP and the left side was treated with microneedling plus vitamin C. Four sessions were performed with an interval of 1 month in between treatments. The Goodman and Baron Scale was used to assess treatment efficacy. Overall, both treatments led to improved outcomes, but in categorizing patients who demonstrated poor responses, a significantly larger percentage existed in the microneedling plus vitamin C group (37% [10/27]) versus the microneedling plus PRP group (22% [6/27])(P=.021). Additionally, aggregate patient satisfaction scores were higher with microneedling plus PRP relative to microneedling plus vitamin C (P=.01). Of note, assessments of improvement were performed by the treating physician and patient satisfaction reports were completed with knowledge of the therapies and cost factor, which may have influenced results.20
Conclusion
Microneedling therapy continues to evolve with a range of applications now emerging in dermatology. As PRP has gained popularity, there has been increased interest in its utilization to amplify the regenerative effects of microneedling. Although the number of direct comparisons examining microneedling with and without PRP is limited, the available evidence indicates that the addition of PRP may improve cosmetic outcomes. These results have been demonstrated primarily in the management of acne scars, but favorable effects may extend to other indications. Continued study is warranted to further quantify the degree of these benefits and to elucidate optimal treatment schedules.
In addition, it is important to consider a cost-benefit analysis of PRP. The price of PRP varies depending on the clinical site but in certain cases may double the cost of a microneedling treatment session. Although studies have demonstrated a statistically significant benefit to PRP, the clinical significance of this supplementary treatment must be weighed against the increased expense. A discussion should take place with the consideration that microneedling alone can provide a satisfactory result for some patients.
- Camirand A, Doucet J. Needle dermabrasion. Aesthetic Plast Surg. 1997;21:48-51.
- Fernandes D. Percutaneous collagen induction: an alternative to laser resurfacing. Aesthet Surg J. 2002;22:307-309.
- Fabbrocini G, Fardella N, Monfrecola A, et al. Acne scarring treatment using skin needling. Clin Exp Dermatol. 2009;34:874-879.
- Zeitter S, Sikora Z, Jahn S, et al. Microneedling: matching the results of medical needling and repetitive treatments to maximize potential for skin regeneration [published online February 7, 2014]. Burns. 2014;40:966-973.
- Schwarz M, Laaff H. A prospective controlled assessment of microneedling with the Dermaroller device. Plast Reconstr Surg. 2011;127:E146-E148.
- Fernandes D, Signorini M. Combating photoaging with percutaneous collagen induction. Clin Dermatol. 2008;26:192-199.
- Aust MC, Fernandes D, Kolokythas P, et al. Percutaneous collagen induction therapy: an alternative treatment for scars, wrinkles, and skin laxity. Plast Reconstr Surg. 2008;121:1421-1429.
- El-Domyati M, Barakat M, Awad S, et al. Microneedling therapy for atrophic acne scars: an objective evaluation. J Clin Aesthet Dermatol. 2015;8:36-42.
- Leheta T, El Tawdy A, Abdel Hay R, et al. Percutaneous collagen induction versus full-concentration trichloroacetic acid in the treatment of atrophic acne scars. Dermatol Surg. 2011;37:207-216.
- Aust MC, Knobloch K, Reimers K, et al. Percutaneous collagen induction therapy: an alternative treatment for burn scars. Burns. 2010;36:836-843.
- Dhurat R, Mathapati S. Response to microneedling treatment in men with androgenetic alopecia who failed to respond to conventional therapy. Indian J Dermatol. 2015;60:260-263.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichology. 2013;5:6-11.
- Wang HL, Avila G. Platelet rich plasma: myth or reality? Eur J Dent. 2007;1:192-194.
- Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10:225-228.
- Lubkowska A, Dolegowska B, Banfi G. Growth factor content in PRP and their applicability in medicine. J Biol Regul Homeost Agents. 2012;26(2 suppl 1):3S-22S.
- Pietrzak WS, Eppley BL. Platelet rich plasma: biology and new technology. J Craniofac Surg. 2005;16:1043-1054.
- Akcal A, Savas SA, Gorgulu T, et al. The effect of platelete rich plasma combined with microneedling on full venous outflow compromise in a rat skin flap model. Plast Reconstr Surg. 2015;136(4 suppl):71-72.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study [published online January 8, 2016]. J Cosmet Dermatol. 2016;15:434-443.
- Fabbrocini G, De Vita V, Pastore F, et al. Combined use of skin needling and platelet-rich plasma in acne scarring treatment. Cosmet Dermatol. 2011;24:177-183.
- Chawla S. Split face comparative study of microneedling with PRP versus microneedling with vitamin C in treating atrophic post acne scars. J Cutan Aesthet Surg. 2014;7:209-212.
- Goodman GJ, Baron JA. Postacne scarring: a qualitative global scarring grading system. Dermatol Surg. 2006;32:1458-1466.
Microneedling therapy, also known as collagen induction therapy or percutaneous collagen induction, is an increasingly popular treatment modality for skin rejuvenation. The approach employs small needles to puncture the skin and stimulate local collagen production in a minimally invasive manner. Recently, clinicians have incorporated the use of platelet-rich plasma (PRP) with the aim of augmenting cosmetic outcomes. In this article, we examine the utility of this approach by reviewing comparison studies of microneedling therapy with and without the application of PRP.
Dr. Gary Goldenberg demonstrates microneedling with platelet-rich plasma in a procedural video available here.
Microneedling Therapy
The use of microneedling first gained attention in the 1990s. Initially, Camirand and Doucet1 described tattooing without pigment for the treatment of achromatic and hypertrophic scars. Fernandes2 evolved this concept and developed a drum-shaped device with fine protruding needles to puncture the skin. Microneedling devices have expanded in recent years and now include both cord- and battery-powered pens and rollers, with needles ranging in length from 0.25 to 3.0 mm.
Treatment with microneedling promotes skin rejuvenation by creating small puncture wounds in the epidermis and dermis. This injury triggers the wound healing cascade and alters the modulation of growth factors to promote regenerative effects.3,4 Following microneedling therapy, increases occur in elastic fiber formation, collagen deposition, and dermal thickness (Figure).5 Of interesting histologic note, collagen is deposited in the normal lattice pattern following this treatment rather than in the parallel bundles typical of scars.6 Microneedling preserves the overall integrity of the epidermal layers and basement membrane, allowing the epidermis to heal without abnormality, verified on histology by a normal stratum corneum, enhanced stratum granulosum, and normal rete ridges.7

Microneedling has demonstrated several uses beyond general skin rejuvenation. In patients with atrophic acne scars, therapy can lead to improved scar appearance, skin texture, and patient satisfaction.8,9 Hypertrophic and dyspigmented burn scars on the body, face, arms, and legs have shown to be receptive to repeated treatments.10 Microneedling also has shown promise in treating androgenic alopecia, increasing hair regrowth in patients who previously showed poor response to conventional therapy with minoxidil and finasteride.11,12
Platelet-Rich Plasma
Platelet-rich plasma is developed by enriching blood with an autologous concentration of platelets. The preparation of PRP begins with whole blood, commonly obtained peripherally by venipuncture. Samples undergo centrifugation to allow separation of the blood into 3 layers: platelet-poor plasma, PRP, and erythrocytes.13 The typical platelet count of whole blood is approximately 200,000/µL; PRP aims to prepare a platelet count of at least 1,000,000/µL in a 5-mL volume.14
An attractive component of PRP is its high concentration of growth factors, including platelet-derived growth factor, transforming growth factor, vascular endothelial growth factor, and epithelial growth factor.15 Because of the regenerative effects of these proteins, PRP has been investigated as a modality to augment wound healing in a variety of clinical areas, such as maxillofacial surgery, orthopedics, cardiovascular surgery, and treatment of soft tissue ulcers.16
Combination Use of Microneedling and PRP
Several studies have compared the effects of microneedling with and without the application of PRP (Table).17-20 In an animal model, Akcal et al17 examined the effects of microneedling and PRP on skin flap survival. Eight rats were randomly divided into 5 groups: sham, control, microneedling alone, microneedling plus PRP, and microneedling plus platelet-poor plasma. Treatments were applied to skin flaps after 4 hours of induced ischemia. The surviving flap area was measured, with results demonstrating significantly higher viable areas in the microneedling plus PRP group relative to all other groups (P<.01). On histologic examination, the microneedling plus PRP group showed well-organized epidermal layers and a dermal integrity that matched the dermis of the sham group.17

Asif et al18 performed a split-face comparison study of 50 patients with atrophic acne scars. On the right side, microneedling was performed followed by intradermal injections and topical application of PRP. On the left side, microneedling was performed followed by intradermal injections of distilled water. The study included 3 treatment sessions with 1 month between each session. Scars were assessed using the Goodman and Baron scale,21 which is designed to grade the morphology of postacne scarring. Scars on the right side improved by 62.2% and scars on the left side improved by 45.8%; prior to treatment, both sides demonstrated similar severity scores, but final severity scores were significantly reduced in the microneedling plus PRP group relative to the microneedling plus distilled water group (P<.00001). No residual side effects from treatment were reported.18
Examining the degree of improvement more carefully, microneedling plus PRP yielded excellent improvement in 40% (20/50) of patients and good improvement in 60% (30/50).18 Microneedling plus distilled water led to excellent improvement in 10% (5/50) and good improvement in 84% (42/50). Given that microneedling plus distilled water still provided good to excellent results in 94% of patients, the addition of PRP was helpful though not necessary in achieving meaningful benefit.18
In another split-face study, Fabbrocini et al19 evaluated 12 adult patients with acne scars. The right side of the face received microneedling plus PRP, while the left side received microneedling alone. Two treatments were performed 8 weeks apart. Severity scores (0=no lesions; 10=maximum severity) were used to assess patient outcomes throughout the study. Acne scars improved on both sides of the face following the treatment period, but the reduction in scar severity with microneedling plus PRP (3.5 points) was significantly greater than with microneedling alone (2.6 points)(P<.05). Patients tended to experience2 to 3 days of mild swelling and erythema after treatment regardless of PRP addition. With only 12 patients, the study was limited by a small sample size. The 10-point grading system differed from the Goodman and Baron scale in that it lacked corresponding qualitative markers, likely decreasing reproducibility.19
Chawla20 compared the effectiveness of combination therapy with microneedling plus PRP versus microneedling and vitamin C application. In a split-face study of 30 patients with atrophic acne scars, the right side of the face was treated with microneedling plus PRP and the left side was treated with microneedling plus vitamin C. Four sessions were performed with an interval of 1 month in between treatments. The Goodman and Baron Scale was used to assess treatment efficacy. Overall, both treatments led to improved outcomes, but in categorizing patients who demonstrated poor responses, a significantly larger percentage existed in the microneedling plus vitamin C group (37% [10/27]) versus the microneedling plus PRP group (22% [6/27])(P=.021). Additionally, aggregate patient satisfaction scores were higher with microneedling plus PRP relative to microneedling plus vitamin C (P=.01). Of note, assessments of improvement were performed by the treating physician and patient satisfaction reports were completed with knowledge of the therapies and cost factor, which may have influenced results.20
Conclusion
Microneedling therapy continues to evolve with a range of applications now emerging in dermatology. As PRP has gained popularity, there has been increased interest in its utilization to amplify the regenerative effects of microneedling. Although the number of direct comparisons examining microneedling with and without PRP is limited, the available evidence indicates that the addition of PRP may improve cosmetic outcomes. These results have been demonstrated primarily in the management of acne scars, but favorable effects may extend to other indications. Continued study is warranted to further quantify the degree of these benefits and to elucidate optimal treatment schedules.
In addition, it is important to consider a cost-benefit analysis of PRP. The price of PRP varies depending on the clinical site but in certain cases may double the cost of a microneedling treatment session. Although studies have demonstrated a statistically significant benefit to PRP, the clinical significance of this supplementary treatment must be weighed against the increased expense. A discussion should take place with the consideration that microneedling alone can provide a satisfactory result for some patients.
Microneedling therapy, also known as collagen induction therapy or percutaneous collagen induction, is an increasingly popular treatment modality for skin rejuvenation. The approach employs small needles to puncture the skin and stimulate local collagen production in a minimally invasive manner. Recently, clinicians have incorporated the use of platelet-rich plasma (PRP) with the aim of augmenting cosmetic outcomes. In this article, we examine the utility of this approach by reviewing comparison studies of microneedling therapy with and without the application of PRP.
Dr. Gary Goldenberg demonstrates microneedling with platelet-rich plasma in a procedural video available here.
Microneedling Therapy
The use of microneedling first gained attention in the 1990s. Initially, Camirand and Doucet1 described tattooing without pigment for the treatment of achromatic and hypertrophic scars. Fernandes2 evolved this concept and developed a drum-shaped device with fine protruding needles to puncture the skin. Microneedling devices have expanded in recent years and now include both cord- and battery-powered pens and rollers, with needles ranging in length from 0.25 to 3.0 mm.
Treatment with microneedling promotes skin rejuvenation by creating small puncture wounds in the epidermis and dermis. This injury triggers the wound healing cascade and alters the modulation of growth factors to promote regenerative effects.3,4 Following microneedling therapy, increases occur in elastic fiber formation, collagen deposition, and dermal thickness (Figure).5 Of interesting histologic note, collagen is deposited in the normal lattice pattern following this treatment rather than in the parallel bundles typical of scars.6 Microneedling preserves the overall integrity of the epidermal layers and basement membrane, allowing the epidermis to heal without abnormality, verified on histology by a normal stratum corneum, enhanced stratum granulosum, and normal rete ridges.7

Microneedling has demonstrated several uses beyond general skin rejuvenation. In patients with atrophic acne scars, therapy can lead to improved scar appearance, skin texture, and patient satisfaction.8,9 Hypertrophic and dyspigmented burn scars on the body, face, arms, and legs have shown to be receptive to repeated treatments.10 Microneedling also has shown promise in treating androgenic alopecia, increasing hair regrowth in patients who previously showed poor response to conventional therapy with minoxidil and finasteride.11,12
Platelet-Rich Plasma
Platelet-rich plasma is developed by enriching blood with an autologous concentration of platelets. The preparation of PRP begins with whole blood, commonly obtained peripherally by venipuncture. Samples undergo centrifugation to allow separation of the blood into 3 layers: platelet-poor plasma, PRP, and erythrocytes.13 The typical platelet count of whole blood is approximately 200,000/µL; PRP aims to prepare a platelet count of at least 1,000,000/µL in a 5-mL volume.14
An attractive component of PRP is its high concentration of growth factors, including platelet-derived growth factor, transforming growth factor, vascular endothelial growth factor, and epithelial growth factor.15 Because of the regenerative effects of these proteins, PRP has been investigated as a modality to augment wound healing in a variety of clinical areas, such as maxillofacial surgery, orthopedics, cardiovascular surgery, and treatment of soft tissue ulcers.16
Combination Use of Microneedling and PRP
Several studies have compared the effects of microneedling with and without the application of PRP (Table).17-20 In an animal model, Akcal et al17 examined the effects of microneedling and PRP on skin flap survival. Eight rats were randomly divided into 5 groups: sham, control, microneedling alone, microneedling plus PRP, and microneedling plus platelet-poor plasma. Treatments were applied to skin flaps after 4 hours of induced ischemia. The surviving flap area was measured, with results demonstrating significantly higher viable areas in the microneedling plus PRP group relative to all other groups (P<.01). On histologic examination, the microneedling plus PRP group showed well-organized epidermal layers and a dermal integrity that matched the dermis of the sham group.17

Asif et al18 performed a split-face comparison study of 50 patients with atrophic acne scars. On the right side, microneedling was performed followed by intradermal injections and topical application of PRP. On the left side, microneedling was performed followed by intradermal injections of distilled water. The study included 3 treatment sessions with 1 month between each session. Scars were assessed using the Goodman and Baron scale,21 which is designed to grade the morphology of postacne scarring. Scars on the right side improved by 62.2% and scars on the left side improved by 45.8%; prior to treatment, both sides demonstrated similar severity scores, but final severity scores were significantly reduced in the microneedling plus PRP group relative to the microneedling plus distilled water group (P<.00001). No residual side effects from treatment were reported.18
Examining the degree of improvement more carefully, microneedling plus PRP yielded excellent improvement in 40% (20/50) of patients and good improvement in 60% (30/50).18 Microneedling plus distilled water led to excellent improvement in 10% (5/50) and good improvement in 84% (42/50). Given that microneedling plus distilled water still provided good to excellent results in 94% of patients, the addition of PRP was helpful though not necessary in achieving meaningful benefit.18
In another split-face study, Fabbrocini et al19 evaluated 12 adult patients with acne scars. The right side of the face received microneedling plus PRP, while the left side received microneedling alone. Two treatments were performed 8 weeks apart. Severity scores (0=no lesions; 10=maximum severity) were used to assess patient outcomes throughout the study. Acne scars improved on both sides of the face following the treatment period, but the reduction in scar severity with microneedling plus PRP (3.5 points) was significantly greater than with microneedling alone (2.6 points)(P<.05). Patients tended to experience2 to 3 days of mild swelling and erythema after treatment regardless of PRP addition. With only 12 patients, the study was limited by a small sample size. The 10-point grading system differed from the Goodman and Baron scale in that it lacked corresponding qualitative markers, likely decreasing reproducibility.19
Chawla20 compared the effectiveness of combination therapy with microneedling plus PRP versus microneedling and vitamin C application. In a split-face study of 30 patients with atrophic acne scars, the right side of the face was treated with microneedling plus PRP and the left side was treated with microneedling plus vitamin C. Four sessions were performed with an interval of 1 month in between treatments. The Goodman and Baron Scale was used to assess treatment efficacy. Overall, both treatments led to improved outcomes, but in categorizing patients who demonstrated poor responses, a significantly larger percentage existed in the microneedling plus vitamin C group (37% [10/27]) versus the microneedling plus PRP group (22% [6/27])(P=.021). Additionally, aggregate patient satisfaction scores were higher with microneedling plus PRP relative to microneedling plus vitamin C (P=.01). Of note, assessments of improvement were performed by the treating physician and patient satisfaction reports were completed with knowledge of the therapies and cost factor, which may have influenced results.20
Conclusion
Microneedling therapy continues to evolve with a range of applications now emerging in dermatology. As PRP has gained popularity, there has been increased interest in its utilization to amplify the regenerative effects of microneedling. Although the number of direct comparisons examining microneedling with and without PRP is limited, the available evidence indicates that the addition of PRP may improve cosmetic outcomes. These results have been demonstrated primarily in the management of acne scars, but favorable effects may extend to other indications. Continued study is warranted to further quantify the degree of these benefits and to elucidate optimal treatment schedules.
In addition, it is important to consider a cost-benefit analysis of PRP. The price of PRP varies depending on the clinical site but in certain cases may double the cost of a microneedling treatment session. Although studies have demonstrated a statistically significant benefit to PRP, the clinical significance of this supplementary treatment must be weighed against the increased expense. A discussion should take place with the consideration that microneedling alone can provide a satisfactory result for some patients.
- Camirand A, Doucet J. Needle dermabrasion. Aesthetic Plast Surg. 1997;21:48-51.
- Fernandes D. Percutaneous collagen induction: an alternative to laser resurfacing. Aesthet Surg J. 2002;22:307-309.
- Fabbrocini G, Fardella N, Monfrecola A, et al. Acne scarring treatment using skin needling. Clin Exp Dermatol. 2009;34:874-879.
- Zeitter S, Sikora Z, Jahn S, et al. Microneedling: matching the results of medical needling and repetitive treatments to maximize potential for skin regeneration [published online February 7, 2014]. Burns. 2014;40:966-973.
- Schwarz M, Laaff H. A prospective controlled assessment of microneedling with the Dermaroller device. Plast Reconstr Surg. 2011;127:E146-E148.
- Fernandes D, Signorini M. Combating photoaging with percutaneous collagen induction. Clin Dermatol. 2008;26:192-199.
- Aust MC, Fernandes D, Kolokythas P, et al. Percutaneous collagen induction therapy: an alternative treatment for scars, wrinkles, and skin laxity. Plast Reconstr Surg. 2008;121:1421-1429.
- El-Domyati M, Barakat M, Awad S, et al. Microneedling therapy for atrophic acne scars: an objective evaluation. J Clin Aesthet Dermatol. 2015;8:36-42.
- Leheta T, El Tawdy A, Abdel Hay R, et al. Percutaneous collagen induction versus full-concentration trichloroacetic acid in the treatment of atrophic acne scars. Dermatol Surg. 2011;37:207-216.
- Aust MC, Knobloch K, Reimers K, et al. Percutaneous collagen induction therapy: an alternative treatment for burn scars. Burns. 2010;36:836-843.
- Dhurat R, Mathapati S. Response to microneedling treatment in men with androgenetic alopecia who failed to respond to conventional therapy. Indian J Dermatol. 2015;60:260-263.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichology. 2013;5:6-11.
- Wang HL, Avila G. Platelet rich plasma: myth or reality? Eur J Dent. 2007;1:192-194.
- Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10:225-228.
- Lubkowska A, Dolegowska B, Banfi G. Growth factor content in PRP and their applicability in medicine. J Biol Regul Homeost Agents. 2012;26(2 suppl 1):3S-22S.
- Pietrzak WS, Eppley BL. Platelet rich plasma: biology and new technology. J Craniofac Surg. 2005;16:1043-1054.
- Akcal A, Savas SA, Gorgulu T, et al. The effect of platelete rich plasma combined with microneedling on full venous outflow compromise in a rat skin flap model. Plast Reconstr Surg. 2015;136(4 suppl):71-72.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study [published online January 8, 2016]. J Cosmet Dermatol. 2016;15:434-443.
- Fabbrocini G, De Vita V, Pastore F, et al. Combined use of skin needling and platelet-rich plasma in acne scarring treatment. Cosmet Dermatol. 2011;24:177-183.
- Chawla S. Split face comparative study of microneedling with PRP versus microneedling with vitamin C in treating atrophic post acne scars. J Cutan Aesthet Surg. 2014;7:209-212.
- Goodman GJ, Baron JA. Postacne scarring: a qualitative global scarring grading system. Dermatol Surg. 2006;32:1458-1466.
- Camirand A, Doucet J. Needle dermabrasion. Aesthetic Plast Surg. 1997;21:48-51.
- Fernandes D. Percutaneous collagen induction: an alternative to laser resurfacing. Aesthet Surg J. 2002;22:307-309.
- Fabbrocini G, Fardella N, Monfrecola A, et al. Acne scarring treatment using skin needling. Clin Exp Dermatol. 2009;34:874-879.
- Zeitter S, Sikora Z, Jahn S, et al. Microneedling: matching the results of medical needling and repetitive treatments to maximize potential for skin regeneration [published online February 7, 2014]. Burns. 2014;40:966-973.
- Schwarz M, Laaff H. A prospective controlled assessment of microneedling with the Dermaroller device. Plast Reconstr Surg. 2011;127:E146-E148.
- Fernandes D, Signorini M. Combating photoaging with percutaneous collagen induction. Clin Dermatol. 2008;26:192-199.
- Aust MC, Fernandes D, Kolokythas P, et al. Percutaneous collagen induction therapy: an alternative treatment for scars, wrinkles, and skin laxity. Plast Reconstr Surg. 2008;121:1421-1429.
- El-Domyati M, Barakat M, Awad S, et al. Microneedling therapy for atrophic acne scars: an objective evaluation. J Clin Aesthet Dermatol. 2015;8:36-42.
- Leheta T, El Tawdy A, Abdel Hay R, et al. Percutaneous collagen induction versus full-concentration trichloroacetic acid in the treatment of atrophic acne scars. Dermatol Surg. 2011;37:207-216.
- Aust MC, Knobloch K, Reimers K, et al. Percutaneous collagen induction therapy: an alternative treatment for burn scars. Burns. 2010;36:836-843.
- Dhurat R, Mathapati S. Response to microneedling treatment in men with androgenetic alopecia who failed to respond to conventional therapy. Indian J Dermatol. 2015;60:260-263.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichology. 2013;5:6-11.
- Wang HL, Avila G. Platelet rich plasma: myth or reality? Eur J Dent. 2007;1:192-194.
- Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10:225-228.
- Lubkowska A, Dolegowska B, Banfi G. Growth factor content in PRP and their applicability in medicine. J Biol Regul Homeost Agents. 2012;26(2 suppl 1):3S-22S.
- Pietrzak WS, Eppley BL. Platelet rich plasma: biology and new technology. J Craniofac Surg. 2005;16:1043-1054.
- Akcal A, Savas SA, Gorgulu T, et al. The effect of platelete rich plasma combined with microneedling on full venous outflow compromise in a rat skin flap model. Plast Reconstr Surg. 2015;136(4 suppl):71-72.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study [published online January 8, 2016]. J Cosmet Dermatol. 2016;15:434-443.
- Fabbrocini G, De Vita V, Pastore F, et al. Combined use of skin needling and platelet-rich plasma in acne scarring treatment. Cosmet Dermatol. 2011;24:177-183.
- Chawla S. Split face comparative study of microneedling with PRP versus microneedling with vitamin C in treating atrophic post acne scars. J Cutan Aesthet Surg. 2014;7:209-212.
- Goodman GJ, Baron JA. Postacne scarring: a qualitative global scarring grading system. Dermatol Surg. 2006;32:1458-1466.
Practice Points
- Microneedling is an effective therapy for skin rejuvenation.
- Preliminary evidence indicates that the addition of platelet-rich plasma to microneedling improves cosmetic outcomes.