User login
Vedolizumab-Induced Acne Fulminans: An Uncommon and Severe Adverse Effect
To the Editor:
Vedolizumab is an innovative monoclonal antibody targeted against the α4β7 integrin that is approved for treatment of moderate to severe ulcerative colitis and Crohn disease refractory to standard treatment.1 Vedolizumab is thought to be gut specific, blocking integrins specific to T lymphocytes destined for the gastrointestinal tract and their interaction with endothelial cells, thereby modulating the adaptive immune system in the gut without systemic immunosuppression.2 It generally is well tolerated, and acne rarely has been reported as an adverse event.3,4 We present a case of acne fulminans without systemic symptoms (AF-WOSS) as a severe side effect of vedolizumab that responded very well to systemic steroids and oral isotretinoin in addition to the discontinuation of treatment.
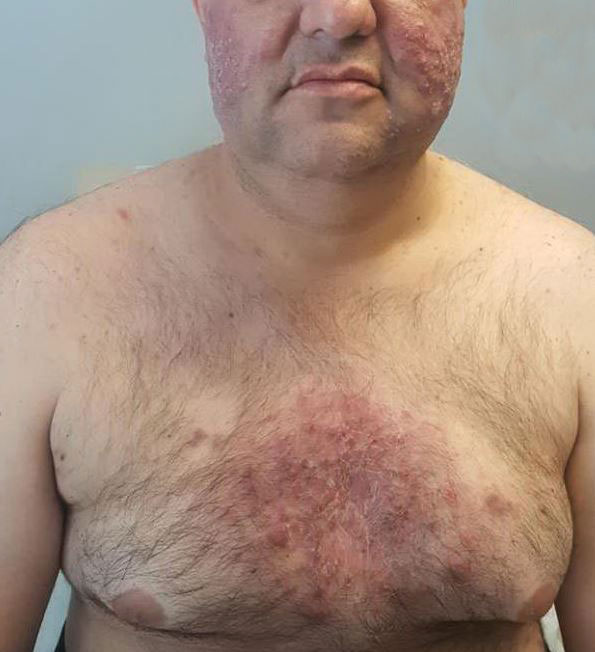
A 46-year-old obese man presented to our dermatology clinic with a chief complaint of rapidly progressive tender skin lesions. The patient had a long-standing history of severe fistulating and stricturing Crohn disease status post–bowel resection with ileostomy and had recently started treatment with vedolizumab after failing treatment with infliximab, adalimumab, certolizumab pegol, ustekinumab, and methotrexate. Several weeks after beginning infusions of vedolizumab, the patient began to develop many erythematous papules and pustules on the face, chest (Figure 1), and buttocks that rapidly progressed into painful and coalescing nodules and cysts over the next several months. He was prescribed benzoyl peroxide wash 10% as well as several weeks of oral doxycycline 100 mg twice daily with no improvement. The patient denied any other new medications or triggers, fever, chills, bone pain, headache, fatigue, or myalgia. The skin involvement continued to worsen with successive vedolizumab infusions over a period of 8 weeks, which ultimately resulted in cessation of vedolizumab.
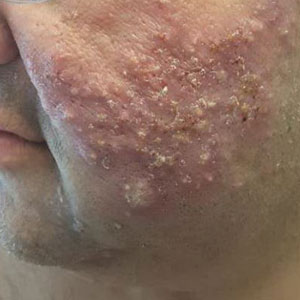
Physical examination revealed large, tender, pink, erythematous, and indurated plaques that were heavily studded with pink papules, pustules, and nodules on the cheeks (Figure 2), central chest, and buttocks. A punch biopsy of a pustule on the cheek showed ruptured suppurative folliculitis. The patient subsequently was diagnosed with AF-WOSS.
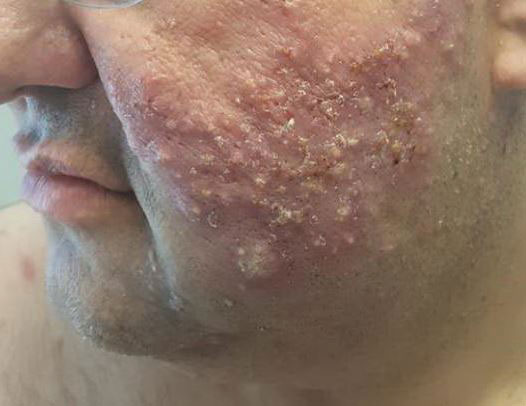
The patient then completed a 7-day course of sulfamethoxazole-trimethoprim followed by a 10-day course of amoxicillin-clavulanic acid, neither of which led to improvement of the lesions. He then was started on an oral prednisone taper (1 mg/kg starting dose) that ultimately totaled 14 weeks in length due to his frequent flares any time prednisone was decreased below 40 mg daily. After 3 weeks on the oral prednisone, the patient was started on 0.3 mg/kg of concomitant oral isotretinoin every other day, which slowly was increased as tolerated until he reached a goal dose of roughly 150 mg/kg, which resolved the acneform papules and pustules and allowed for successful tapering off the prednisone.
Many studies have been published regarding the safety and side-effect profile of vedolizumab, but most do not report acne as an adverse event.3-5 A German cohort study by Baumgart et al3 reported acne as a side effect in 15 of 212 (7.1%) patients but did not classify the severity. Another case report noted nodulocystic acne in a patient receiving vedolizumab for treatment of inflammatory bowel disease; however, this patient responded well to the use of a tetracycline antibiotic and was able to continue therapy with vedolizumab.5 Our patient demonstrated a severe and uncommon case of acne classified as AF-WOSS following initiation of therapy with vedolizumab, which required treatment with systemic steroids plus oral isotretinoin and resulted in cessation of vedolizumab.
As new therapies emerge, it is important to document new or severe adverse effects so providers can choose an appropriate therapy and adequately counsel patients regarding the side effects. Although vedolizumab was thought to have gut-specific action, there is new evidence to suggest that the principal ligand of the α4β7 integrin, mucosal addressin cell adhesion molecule-1, is not only expressed on gut endothelial cells but also on fibroblasts and melanomas, which may provide insight into the observed extraintestinal side effects of vedolizumab.6
- Smith MA, Mohammad RA. Vedolizumab: an α4β7 integrin inhibitor for inflammatory bowel diseases. Ann Pharmacother. 2014;48:1629-1635.
- Singh H, Grewal N, Arora E, et al. Vedolizumab: a novel anti-integrin drug for treatment of inflammatory bowel disease. J Nat Sci Bio Med. 2016;7:4-9.
- Baumgart DC, Bokemeyer B, Drabik A, et al. Vedolizumab induction therapy for inflammatory bowel disease in clinical practice: a nationwide consecutive German cohort study. Aliment Pharmacol Ther. 2016;43:1090-1102.
- Bye WA, Jairath V, Travis SPL. Systematic review: the safety of vedolizumab for the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:3-15.
- Gilhooley E, Doherty G, Lally A. Vedolizumab-induced acne in inflammatory bowel disease. Int J Dermatol. 2018;57:752-753.
- Leung E, Kanwar RK, Kanwar JR, et al. Mucosal vascular addressin cell adhesion molecule-1 is expressed outside the endothelial lineage on fibroblasts and melanoma cells. Immunol Cell Biol. 2003;81:320-327.
To the Editor:
Vedolizumab is an innovative monoclonal antibody targeted against the α4β7 integrin that is approved for treatment of moderate to severe ulcerative colitis and Crohn disease refractory to standard treatment.1 Vedolizumab is thought to be gut specific, blocking integrins specific to T lymphocytes destined for the gastrointestinal tract and their interaction with endothelial cells, thereby modulating the adaptive immune system in the gut without systemic immunosuppression.2 It generally is well tolerated, and acne rarely has been reported as an adverse event.3,4 We present a case of acne fulminans without systemic symptoms (AF-WOSS) as a severe side effect of vedolizumab that responded very well to systemic steroids and oral isotretinoin in addition to the discontinuation of treatment.
A 46-year-old obese man presented to our dermatology clinic with a chief complaint of rapidly progressive tender skin lesions. The patient had a long-standing history of severe fistulating and stricturing Crohn disease status post–bowel resection with ileostomy and had recently started treatment with vedolizumab after failing treatment with infliximab, adalimumab, certolizumab pegol, ustekinumab, and methotrexate. Several weeks after beginning infusions of vedolizumab, the patient began to develop many erythematous papules and pustules on the face, chest (Figure 1), and buttocks that rapidly progressed into painful and coalescing nodules and cysts over the next several months. He was prescribed benzoyl peroxide wash 10% as well as several weeks of oral doxycycline 100 mg twice daily with no improvement. The patient denied any other new medications or triggers, fever, chills, bone pain, headache, fatigue, or myalgia. The skin involvement continued to worsen with successive vedolizumab infusions over a period of 8 weeks, which ultimately resulted in cessation of vedolizumab.

Physical examination revealed large, tender, pink, erythematous, and indurated plaques that were heavily studded with pink papules, pustules, and nodules on the cheeks (Figure 2), central chest, and buttocks. A punch biopsy of a pustule on the cheek showed ruptured suppurative folliculitis. The patient subsequently was diagnosed with AF-WOSS.

The patient then completed a 7-day course of sulfamethoxazole-trimethoprim followed by a 10-day course of amoxicillin-clavulanic acid, neither of which led to improvement of the lesions. He then was started on an oral prednisone taper (1 mg/kg starting dose) that ultimately totaled 14 weeks in length due to his frequent flares any time prednisone was decreased below 40 mg daily. After 3 weeks on the oral prednisone, the patient was started on 0.3 mg/kg of concomitant oral isotretinoin every other day, which slowly was increased as tolerated until he reached a goal dose of roughly 150 mg/kg, which resolved the acneform papules and pustules and allowed for successful tapering off the prednisone.
Many studies have been published regarding the safety and side-effect profile of vedolizumab, but most do not report acne as an adverse event.3-5 A German cohort study by Baumgart et al3 reported acne as a side effect in 15 of 212 (7.1%) patients but did not classify the severity. Another case report noted nodulocystic acne in a patient receiving vedolizumab for treatment of inflammatory bowel disease; however, this patient responded well to the use of a tetracycline antibiotic and was able to continue therapy with vedolizumab.5 Our patient demonstrated a severe and uncommon case of acne classified as AF-WOSS following initiation of therapy with vedolizumab, which required treatment with systemic steroids plus oral isotretinoin and resulted in cessation of vedolizumab.
As new therapies emerge, it is important to document new or severe adverse effects so providers can choose an appropriate therapy and adequately counsel patients regarding the side effects. Although vedolizumab was thought to have gut-specific action, there is new evidence to suggest that the principal ligand of the α4β7 integrin, mucosal addressin cell adhesion molecule-1, is not only expressed on gut endothelial cells but also on fibroblasts and melanomas, which may provide insight into the observed extraintestinal side effects of vedolizumab.6
To the Editor:
Vedolizumab is an innovative monoclonal antibody targeted against the α4β7 integrin that is approved for treatment of moderate to severe ulcerative colitis and Crohn disease refractory to standard treatment.1 Vedolizumab is thought to be gut specific, blocking integrins specific to T lymphocytes destined for the gastrointestinal tract and their interaction with endothelial cells, thereby modulating the adaptive immune system in the gut without systemic immunosuppression.2 It generally is well tolerated, and acne rarely has been reported as an adverse event.3,4 We present a case of acne fulminans without systemic symptoms (AF-WOSS) as a severe side effect of vedolizumab that responded very well to systemic steroids and oral isotretinoin in addition to the discontinuation of treatment.
A 46-year-old obese man presented to our dermatology clinic with a chief complaint of rapidly progressive tender skin lesions. The patient had a long-standing history of severe fistulating and stricturing Crohn disease status post–bowel resection with ileostomy and had recently started treatment with vedolizumab after failing treatment with infliximab, adalimumab, certolizumab pegol, ustekinumab, and methotrexate. Several weeks after beginning infusions of vedolizumab, the patient began to develop many erythematous papules and pustules on the face, chest (Figure 1), and buttocks that rapidly progressed into painful and coalescing nodules and cysts over the next several months. He was prescribed benzoyl peroxide wash 10% as well as several weeks of oral doxycycline 100 mg twice daily with no improvement. The patient denied any other new medications or triggers, fever, chills, bone pain, headache, fatigue, or myalgia. The skin involvement continued to worsen with successive vedolizumab infusions over a period of 8 weeks, which ultimately resulted in cessation of vedolizumab.

Physical examination revealed large, tender, pink, erythematous, and indurated plaques that were heavily studded with pink papules, pustules, and nodules on the cheeks (Figure 2), central chest, and buttocks. A punch biopsy of a pustule on the cheek showed ruptured suppurative folliculitis. The patient subsequently was diagnosed with AF-WOSS.

The patient then completed a 7-day course of sulfamethoxazole-trimethoprim followed by a 10-day course of amoxicillin-clavulanic acid, neither of which led to improvement of the lesions. He then was started on an oral prednisone taper (1 mg/kg starting dose) that ultimately totaled 14 weeks in length due to his frequent flares any time prednisone was decreased below 40 mg daily. After 3 weeks on the oral prednisone, the patient was started on 0.3 mg/kg of concomitant oral isotretinoin every other day, which slowly was increased as tolerated until he reached a goal dose of roughly 150 mg/kg, which resolved the acneform papules and pustules and allowed for successful tapering off the prednisone.
Many studies have been published regarding the safety and side-effect profile of vedolizumab, but most do not report acne as an adverse event.3-5 A German cohort study by Baumgart et al3 reported acne as a side effect in 15 of 212 (7.1%) patients but did not classify the severity. Another case report noted nodulocystic acne in a patient receiving vedolizumab for treatment of inflammatory bowel disease; however, this patient responded well to the use of a tetracycline antibiotic and was able to continue therapy with vedolizumab.5 Our patient demonstrated a severe and uncommon case of acne classified as AF-WOSS following initiation of therapy with vedolizumab, which required treatment with systemic steroids plus oral isotretinoin and resulted in cessation of vedolizumab.
As new therapies emerge, it is important to document new or severe adverse effects so providers can choose an appropriate therapy and adequately counsel patients regarding the side effects. Although vedolizumab was thought to have gut-specific action, there is new evidence to suggest that the principal ligand of the α4β7 integrin, mucosal addressin cell adhesion molecule-1, is not only expressed on gut endothelial cells but also on fibroblasts and melanomas, which may provide insight into the observed extraintestinal side effects of vedolizumab.6
- Smith MA, Mohammad RA. Vedolizumab: an α4β7 integrin inhibitor for inflammatory bowel diseases. Ann Pharmacother. 2014;48:1629-1635.
- Singh H, Grewal N, Arora E, et al. Vedolizumab: a novel anti-integrin drug for treatment of inflammatory bowel disease. J Nat Sci Bio Med. 2016;7:4-9.
- Baumgart DC, Bokemeyer B, Drabik A, et al. Vedolizumab induction therapy for inflammatory bowel disease in clinical practice: a nationwide consecutive German cohort study. Aliment Pharmacol Ther. 2016;43:1090-1102.
- Bye WA, Jairath V, Travis SPL. Systematic review: the safety of vedolizumab for the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:3-15.
- Gilhooley E, Doherty G, Lally A. Vedolizumab-induced acne in inflammatory bowel disease. Int J Dermatol. 2018;57:752-753.
- Leung E, Kanwar RK, Kanwar JR, et al. Mucosal vascular addressin cell adhesion molecule-1 is expressed outside the endothelial lineage on fibroblasts and melanoma cells. Immunol Cell Biol. 2003;81:320-327.
- Smith MA, Mohammad RA. Vedolizumab: an α4β7 integrin inhibitor for inflammatory bowel diseases. Ann Pharmacother. 2014;48:1629-1635.
- Singh H, Grewal N, Arora E, et al. Vedolizumab: a novel anti-integrin drug for treatment of inflammatory bowel disease. J Nat Sci Bio Med. 2016;7:4-9.
- Baumgart DC, Bokemeyer B, Drabik A, et al. Vedolizumab induction therapy for inflammatory bowel disease in clinical practice: a nationwide consecutive German cohort study. Aliment Pharmacol Ther. 2016;43:1090-1102.
- Bye WA, Jairath V, Travis SPL. Systematic review: the safety of vedolizumab for the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:3-15.
- Gilhooley E, Doherty G, Lally A. Vedolizumab-induced acne in inflammatory bowel disease. Int J Dermatol. 2018;57:752-753.
- Leung E, Kanwar RK, Kanwar JR, et al. Mucosal vascular addressin cell adhesion molecule-1 is expressed outside the endothelial lineage on fibroblasts and melanoma cells. Immunol Cell Biol. 2003;81:320-327.
Practice Points
- Vedolizumab, a monoclonal antibody for the treatment of refractory inflammatory bowel disease, was found to cause acne fulminans without systemic symptoms.
- Vedolizumab previously was believed to be a gut-limited immune modulator.
- Off-target cutaneous effects may indicate wider expression of the target integrin of vedolizumab and should be recognized as the drug becomes more widely used.
Botanical Briefs: Phytophotodermatitis Caused by Giant Hogweed (Heracleum mantegazzianum)
Giant hogweed (Heracleum mantegazzianum) is an invasive flowering weed of the family Apiaceae that typically reaches a height of 13 feet, with thick stems; large green leaves; and umbrella-shaped, flat-topped, radial clusters (umbels) of small individual white flowers1 (Figure 1). Because of the size and beauty of giant hogweed, it was widely planted in 19th century ornamental gardens in the United Kingdom and has since naturalized and spread throughout central Europe, Canada, and the United States.1,2 The plant most commonly is found in shady areas near rivers and woodlands.1

Due to the invasive nature of the giant hogweed, its prevalence continues to grow, its eradication remains difficult, and reports of phytophotodermatitis are increasing in number and distribution. In fact, there has been widespread media coverage of the dangers of giant hogweed in the United Kingdom since 20161 and in the United States in 2018 and 2019.3-6
Transmission
Phytophotodermatitis is a type of nonimmunologic dermatitis caused by UV light reacting with a plant-based photosensitizing agent. In the case of giant hogweed, sap from the plant’s fruits, leaves, and stem contain furocoumarins or psoralens.7 Upon activation by UVA radiation, furan rings of these compounds create reactive oxygen species and intercalate with DNA pyrimidine bases, which results in cellular death, damage to successive skin layers, and reduced wound healing at the cellular level.8 This effect is intensified with increased percutaneous absorption of furocoumarin, which can result from high temperature, humidity, skin infection, lack of protective clothing, and moist conditions.9
The highest concentration of phototoxic compounds is found in giant hogweed from June through August,7 which, in combination with people increasing their outdoor activity in the summer, results in a greater prevalence and severity of H mantegazzianum phytophotodermatitis during summer months.
Presentation
Phytophotodermatitis caused by giant hogweed can range from burning and erythema to full-thickness chemical burns that require surgical debridement and skin grafting.8 After exposure to the offending agent, a harmful skin reaction can start within 15 minutes. After a latent period of approximately 24 hours, erythema, edema, and bullae can appear and generally peak by 72 hours.10 In addition to cutaneous injury, inhalation of giant hogweed traces can result in obstructive pulmonary symptoms. Eye contact can result in blindness.9
In addition to the rash caused by giant hogweed, a “weed-wacker dermatitis” or “strimmer rash” can be caused by the similar-appearing but smaller common hogweed (Heracleum sphondylium). Common hogweed is highly prevalent in the United States and often is confused with the larger giant hogweed because of tall stems and white, flat-topped flowers.
Management
Following contact with giant hogweed, a person should immediately avoid UV exposure and rinse the area with soap and water. UV radiation must be avoided for at least 48 hours. If erythema occurs, a topical steroid can be applied to the affected area; pain can be alleviated by a nonsteroidal anti-inflammatory drug.9
Further treatment might be required if bullous lesions are present. Small blisters can be punctured and drained; however, large blisters, extensive epidermal-dermal separation, and large areas of detached epidermis should simply be cleansed and dressed. An oral steroid also can be used to reduce inflammation in moderate and severe cases. Full-thickness injury might require surgical intervention.8
Clinical Case
A 27-year-old male landscaper presented to the emergency department with an increasingly painful blistering rash on the arms and neck of 1 day’s duration. He noticed bright red skin and blisters 18 to 24 hours after trimming what he identified as shoulder-high giant hogweed plants. Neither he nor his coworkers were wearing sunscreen or protective clothing as they cleared the plants for several hours. His coworkers developed similar rashes, but his rash was the most severe, requiring treatment in the emergency department.
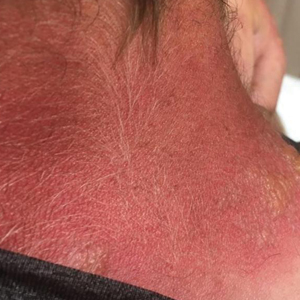
Physical examination showed innumerable 2- to 10-mm, tense vesicles and bullae on a background of blanching erythema in a striking photodistribution along the neck (Figure 2) and arms (Figure 3). He had notable edema of both arms and several large 3- to 4-cm bullae on the ventral aspects of the forearms.
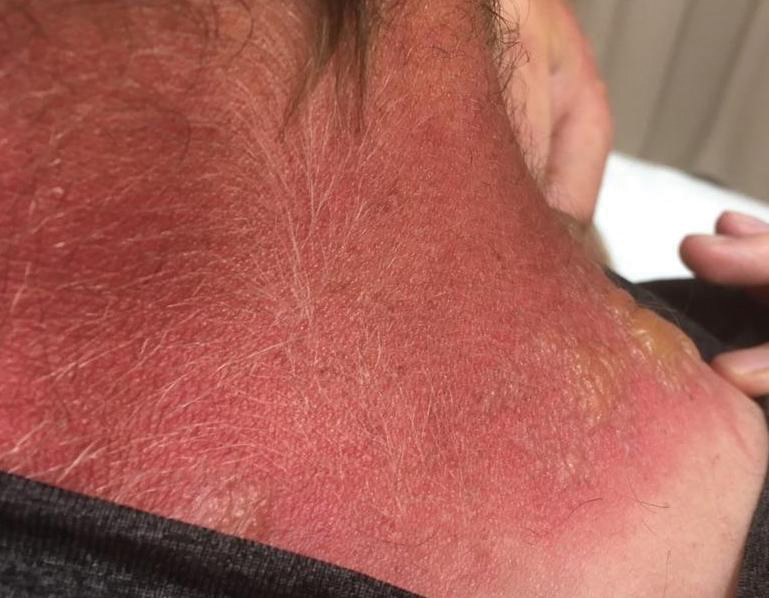
The patient was diagnosed with severe phytophotodermatitis secondary to contact with H mantegazzianum and was started on oral prednisone 70 mg daily (1 mg/kg/d), which was decreased by 10 mg every 3 days until the course of treatment was complete. He also was instructed to apply mupirocin ointment to open areas and petroleum jelly to intact skin. Additionally, he was advised to practice strict photoprotection for the near and distant future.
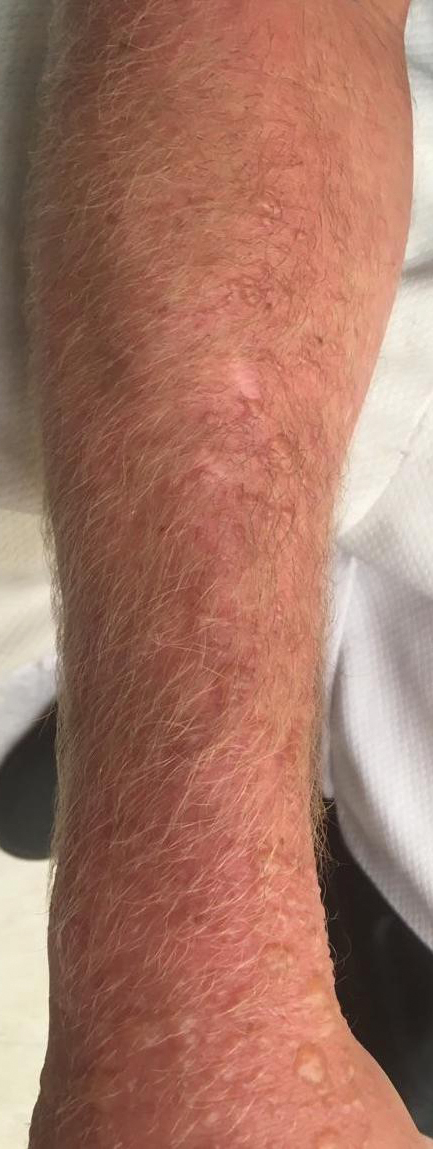
Within several days after treatment began, the phytophotodermatitis dramatically improved, with complete resolution in 1 week. Postinflammatory hyperpigmentation resolved after several weeks.
- Baker B, Bedford J, Kanitkar S. Keeping pace with the media; giant hogweed burns—a case series and comprehensive review [published online December 26, 2016]. Burns. 2017;13:933-938. doi:10.1016/j.burns.2016.10.018
- Klimaszyk P, Klimaszyk D, Piotrowiak M, et al. Unusual complications after occupational exposure to giant hogweed (Heracleum mantegazzianum): a case report. Int J Occup Med Environ Health. 2014;27:141-144. doi:10.2478/s13382-014-0238-z
- Zaveria M, Hauser C. Giant hogweed: a plant that can burn and blind you. but don’t panic. New York Times. July 2, 2018. Accessed October 18, 2021. https://www.nytimes.com/2018/07/02/us/giant-hogweed-nyt.html
- Hignett K. Giant hogweed: New York officials warn residents about dangerous plant that causes serious burns, blisters and scars. Newsweek. June 25, 2019. Accessed October 18, 2021. https://www.newsweek.com/giant-hogweed-new-york-dangerous-plant-burns-skin-sunlight-1445785
- Eastman J. Toxic giant hogweed sap that burns, blisters skin found in Clark County. The Oregonian. Updated July 16, 2019. Accessed October 18, 2021. https://www.oregonlive.com/news/2019/07/toxic-giant-hogweed-plant-that-burns-blisters-skin-found-in-clark-county.html
- O’Kane C. Giant hogweed, plant that causes blindness and third-degree burns, discovered in Virginia. CBS News. June 18, 2018. Accessed October 18, 2021. https://www.cbsnews.com/news/giant-hogweed-plant-causes-blindness-third-degree-burns-discovered-in-virginia-otherstates/
- Pira E, Romano C, Sulotto F, et al. Heracleum mantegazzianum growth phases and furocoumarin content. Contact Dermatitis. 1989;21:300-303. doi:10.1111/j.1600-0536.1989.tb04747.x
- Chan JCY, Sullivan PJ, O’Sullivan MJ, et al. Full thickness burn caused by exposure to giant hogweed: delayed presentation, histological features and surgical management. J Plast Reconstr Aesthet Surg. 2011;64:128-130. doi:10.1016/j.bjps.2010.03.030
- Pfurtscheller K, Trop M. Phototoxic plant burns: report of a case and review of topical wound treatment in children. Pediatr Dermatol. 2014;31:E156-E159. doi:10.1111/pde.12396
- Kavli G, Volden G: Phytophotodermatitis. Photodermatol. 1984;1:65-75.
Giant hogweed (Heracleum mantegazzianum) is an invasive flowering weed of the family Apiaceae that typically reaches a height of 13 feet, with thick stems; large green leaves; and umbrella-shaped, flat-topped, radial clusters (umbels) of small individual white flowers1 (Figure 1). Because of the size and beauty of giant hogweed, it was widely planted in 19th century ornamental gardens in the United Kingdom and has since naturalized and spread throughout central Europe, Canada, and the United States.1,2 The plant most commonly is found in shady areas near rivers and woodlands.1

Due to the invasive nature of the giant hogweed, its prevalence continues to grow, its eradication remains difficult, and reports of phytophotodermatitis are increasing in number and distribution. In fact, there has been widespread media coverage of the dangers of giant hogweed in the United Kingdom since 20161 and in the United States in 2018 and 2019.3-6
Transmission
Phytophotodermatitis is a type of nonimmunologic dermatitis caused by UV light reacting with a plant-based photosensitizing agent. In the case of giant hogweed, sap from the plant’s fruits, leaves, and stem contain furocoumarins or psoralens.7 Upon activation by UVA radiation, furan rings of these compounds create reactive oxygen species and intercalate with DNA pyrimidine bases, which results in cellular death, damage to successive skin layers, and reduced wound healing at the cellular level.8 This effect is intensified with increased percutaneous absorption of furocoumarin, which can result from high temperature, humidity, skin infection, lack of protective clothing, and moist conditions.9
The highest concentration of phototoxic compounds is found in giant hogweed from June through August,7 which, in combination with people increasing their outdoor activity in the summer, results in a greater prevalence and severity of H mantegazzianum phytophotodermatitis during summer months.
Presentation
Phytophotodermatitis caused by giant hogweed can range from burning and erythema to full-thickness chemical burns that require surgical debridement and skin grafting.8 After exposure to the offending agent, a harmful skin reaction can start within 15 minutes. After a latent period of approximately 24 hours, erythema, edema, and bullae can appear and generally peak by 72 hours.10 In addition to cutaneous injury, inhalation of giant hogweed traces can result in obstructive pulmonary symptoms. Eye contact can result in blindness.9
In addition to the rash caused by giant hogweed, a “weed-wacker dermatitis” or “strimmer rash” can be caused by the similar-appearing but smaller common hogweed (Heracleum sphondylium). Common hogweed is highly prevalent in the United States and often is confused with the larger giant hogweed because of tall stems and white, flat-topped flowers.
Management
Following contact with giant hogweed, a person should immediately avoid UV exposure and rinse the area with soap and water. UV radiation must be avoided for at least 48 hours. If erythema occurs, a topical steroid can be applied to the affected area; pain can be alleviated by a nonsteroidal anti-inflammatory drug.9
Further treatment might be required if bullous lesions are present. Small blisters can be punctured and drained; however, large blisters, extensive epidermal-dermal separation, and large areas of detached epidermis should simply be cleansed and dressed. An oral steroid also can be used to reduce inflammation in moderate and severe cases. Full-thickness injury might require surgical intervention.8
Clinical Case
A 27-year-old male landscaper presented to the emergency department with an increasingly painful blistering rash on the arms and neck of 1 day’s duration. He noticed bright red skin and blisters 18 to 24 hours after trimming what he identified as shoulder-high giant hogweed plants. Neither he nor his coworkers were wearing sunscreen or protective clothing as they cleared the plants for several hours. His coworkers developed similar rashes, but his rash was the most severe, requiring treatment in the emergency department.
Physical examination showed innumerable 2- to 10-mm, tense vesicles and bullae on a background of blanching erythema in a striking photodistribution along the neck (Figure 2) and arms (Figure 3). He had notable edema of both arms and several large 3- to 4-cm bullae on the ventral aspects of the forearms.

The patient was diagnosed with severe phytophotodermatitis secondary to contact with H mantegazzianum and was started on oral prednisone 70 mg daily (1 mg/kg/d), which was decreased by 10 mg every 3 days until the course of treatment was complete. He also was instructed to apply mupirocin ointment to open areas and petroleum jelly to intact skin. Additionally, he was advised to practice strict photoprotection for the near and distant future.

Within several days after treatment began, the phytophotodermatitis dramatically improved, with complete resolution in 1 week. Postinflammatory hyperpigmentation resolved after several weeks.
Giant hogweed (Heracleum mantegazzianum) is an invasive flowering weed of the family Apiaceae that typically reaches a height of 13 feet, with thick stems; large green leaves; and umbrella-shaped, flat-topped, radial clusters (umbels) of small individual white flowers1 (Figure 1). Because of the size and beauty of giant hogweed, it was widely planted in 19th century ornamental gardens in the United Kingdom and has since naturalized and spread throughout central Europe, Canada, and the United States.1,2 The plant most commonly is found in shady areas near rivers and woodlands.1

Due to the invasive nature of the giant hogweed, its prevalence continues to grow, its eradication remains difficult, and reports of phytophotodermatitis are increasing in number and distribution. In fact, there has been widespread media coverage of the dangers of giant hogweed in the United Kingdom since 20161 and in the United States in 2018 and 2019.3-6
Transmission
Phytophotodermatitis is a type of nonimmunologic dermatitis caused by UV light reacting with a plant-based photosensitizing agent. In the case of giant hogweed, sap from the plant’s fruits, leaves, and stem contain furocoumarins or psoralens.7 Upon activation by UVA radiation, furan rings of these compounds create reactive oxygen species and intercalate with DNA pyrimidine bases, which results in cellular death, damage to successive skin layers, and reduced wound healing at the cellular level.8 This effect is intensified with increased percutaneous absorption of furocoumarin, which can result from high temperature, humidity, skin infection, lack of protective clothing, and moist conditions.9
The highest concentration of phototoxic compounds is found in giant hogweed from June through August,7 which, in combination with people increasing their outdoor activity in the summer, results in a greater prevalence and severity of H mantegazzianum phytophotodermatitis during summer months.
Presentation
Phytophotodermatitis caused by giant hogweed can range from burning and erythema to full-thickness chemical burns that require surgical debridement and skin grafting.8 After exposure to the offending agent, a harmful skin reaction can start within 15 minutes. After a latent period of approximately 24 hours, erythema, edema, and bullae can appear and generally peak by 72 hours.10 In addition to cutaneous injury, inhalation of giant hogweed traces can result in obstructive pulmonary symptoms. Eye contact can result in blindness.9
In addition to the rash caused by giant hogweed, a “weed-wacker dermatitis” or “strimmer rash” can be caused by the similar-appearing but smaller common hogweed (Heracleum sphondylium). Common hogweed is highly prevalent in the United States and often is confused with the larger giant hogweed because of tall stems and white, flat-topped flowers.
Management
Following contact with giant hogweed, a person should immediately avoid UV exposure and rinse the area with soap and water. UV radiation must be avoided for at least 48 hours. If erythema occurs, a topical steroid can be applied to the affected area; pain can be alleviated by a nonsteroidal anti-inflammatory drug.9
Further treatment might be required if bullous lesions are present. Small blisters can be punctured and drained; however, large blisters, extensive epidermal-dermal separation, and large areas of detached epidermis should simply be cleansed and dressed. An oral steroid also can be used to reduce inflammation in moderate and severe cases. Full-thickness injury might require surgical intervention.8
Clinical Case
A 27-year-old male landscaper presented to the emergency department with an increasingly painful blistering rash on the arms and neck of 1 day’s duration. He noticed bright red skin and blisters 18 to 24 hours after trimming what he identified as shoulder-high giant hogweed plants. Neither he nor his coworkers were wearing sunscreen or protective clothing as they cleared the plants for several hours. His coworkers developed similar rashes, but his rash was the most severe, requiring treatment in the emergency department.
Physical examination showed innumerable 2- to 10-mm, tense vesicles and bullae on a background of blanching erythema in a striking photodistribution along the neck (Figure 2) and arms (Figure 3). He had notable edema of both arms and several large 3- to 4-cm bullae on the ventral aspects of the forearms.

The patient was diagnosed with severe phytophotodermatitis secondary to contact with H mantegazzianum and was started on oral prednisone 70 mg daily (1 mg/kg/d), which was decreased by 10 mg every 3 days until the course of treatment was complete. He also was instructed to apply mupirocin ointment to open areas and petroleum jelly to intact skin. Additionally, he was advised to practice strict photoprotection for the near and distant future.

Within several days after treatment began, the phytophotodermatitis dramatically improved, with complete resolution in 1 week. Postinflammatory hyperpigmentation resolved after several weeks.
- Baker B, Bedford J, Kanitkar S. Keeping pace with the media; giant hogweed burns—a case series and comprehensive review [published online December 26, 2016]. Burns. 2017;13:933-938. doi:10.1016/j.burns.2016.10.018
- Klimaszyk P, Klimaszyk D, Piotrowiak M, et al. Unusual complications after occupational exposure to giant hogweed (Heracleum mantegazzianum): a case report. Int J Occup Med Environ Health. 2014;27:141-144. doi:10.2478/s13382-014-0238-z
- Zaveria M, Hauser C. Giant hogweed: a plant that can burn and blind you. but don’t panic. New York Times. July 2, 2018. Accessed October 18, 2021. https://www.nytimes.com/2018/07/02/us/giant-hogweed-nyt.html
- Hignett K. Giant hogweed: New York officials warn residents about dangerous plant that causes serious burns, blisters and scars. Newsweek. June 25, 2019. Accessed October 18, 2021. https://www.newsweek.com/giant-hogweed-new-york-dangerous-plant-burns-skin-sunlight-1445785
- Eastman J. Toxic giant hogweed sap that burns, blisters skin found in Clark County. The Oregonian. Updated July 16, 2019. Accessed October 18, 2021. https://www.oregonlive.com/news/2019/07/toxic-giant-hogweed-plant-that-burns-blisters-skin-found-in-clark-county.html
- O’Kane C. Giant hogweed, plant that causes blindness and third-degree burns, discovered in Virginia. CBS News. June 18, 2018. Accessed October 18, 2021. https://www.cbsnews.com/news/giant-hogweed-plant-causes-blindness-third-degree-burns-discovered-in-virginia-otherstates/
- Pira E, Romano C, Sulotto F, et al. Heracleum mantegazzianum growth phases and furocoumarin content. Contact Dermatitis. 1989;21:300-303. doi:10.1111/j.1600-0536.1989.tb04747.x
- Chan JCY, Sullivan PJ, O’Sullivan MJ, et al. Full thickness burn caused by exposure to giant hogweed: delayed presentation, histological features and surgical management. J Plast Reconstr Aesthet Surg. 2011;64:128-130. doi:10.1016/j.bjps.2010.03.030
- Pfurtscheller K, Trop M. Phototoxic plant burns: report of a case and review of topical wound treatment in children. Pediatr Dermatol. 2014;31:E156-E159. doi:10.1111/pde.12396
- Kavli G, Volden G: Phytophotodermatitis. Photodermatol. 1984;1:65-75.
- Baker B, Bedford J, Kanitkar S. Keeping pace with the media; giant hogweed burns—a case series and comprehensive review [published online December 26, 2016]. Burns. 2017;13:933-938. doi:10.1016/j.burns.2016.10.018
- Klimaszyk P, Klimaszyk D, Piotrowiak M, et al. Unusual complications after occupational exposure to giant hogweed (Heracleum mantegazzianum): a case report. Int J Occup Med Environ Health. 2014;27:141-144. doi:10.2478/s13382-014-0238-z
- Zaveria M, Hauser C. Giant hogweed: a plant that can burn and blind you. but don’t panic. New York Times. July 2, 2018. Accessed October 18, 2021. https://www.nytimes.com/2018/07/02/us/giant-hogweed-nyt.html
- Hignett K. Giant hogweed: New York officials warn residents about dangerous plant that causes serious burns, blisters and scars. Newsweek. June 25, 2019. Accessed October 18, 2021. https://www.newsweek.com/giant-hogweed-new-york-dangerous-plant-burns-skin-sunlight-1445785
- Eastman J. Toxic giant hogweed sap that burns, blisters skin found in Clark County. The Oregonian. Updated July 16, 2019. Accessed October 18, 2021. https://www.oregonlive.com/news/2019/07/toxic-giant-hogweed-plant-that-burns-blisters-skin-found-in-clark-county.html
- O’Kane C. Giant hogweed, plant that causes blindness and third-degree burns, discovered in Virginia. CBS News. June 18, 2018. Accessed October 18, 2021. https://www.cbsnews.com/news/giant-hogweed-plant-causes-blindness-third-degree-burns-discovered-in-virginia-otherstates/
- Pira E, Romano C, Sulotto F, et al. Heracleum mantegazzianum growth phases and furocoumarin content. Contact Dermatitis. 1989;21:300-303. doi:10.1111/j.1600-0536.1989.tb04747.x
- Chan JCY, Sullivan PJ, O’Sullivan MJ, et al. Full thickness burn caused by exposure to giant hogweed: delayed presentation, histological features and surgical management. J Plast Reconstr Aesthet Surg. 2011;64:128-130. doi:10.1016/j.bjps.2010.03.030
- Pfurtscheller K, Trop M. Phototoxic plant burns: report of a case and review of topical wound treatment in children. Pediatr Dermatol. 2014;31:E156-E159. doi:10.1111/pde.12396
- Kavli G, Volden G: Phytophotodermatitis. Photodermatol. 1984;1:65-75.
PRACTICE POINTS
- The public should be educated, especially during summer months, about how to identify giant hogweed, reduce exposure to the plant’s phototoxin, and thus reduce the risk for severe phytophotodermatitis.
- Phytophotodermatitis should be included in the differential diagnosis when a patient presents with acute erythema and bullae in sun-exposed areas.
- Phytophotodermatitis can be treated by promptly washing the skin with soap and water, protecting the skin from exposure to UV light, and utilizing topical and oral steroids.