User login
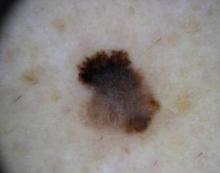
Dermoscopy Underutilized by Dermatologists
While dermoscopy can enhance the diagnosis of a number of skin conditions, it is especially useful in determining whether neoplasms should undergo biopsy.
"Patients are becoming aware of the technique and more and more are expecting their dermatologists to be skilled in its application," Dr. David L. Swanson said in an interview.
However, fewer than half of academic dermatologists and less than a quarter of practicing dermatologists use dermoscopy in the United States, according to Dr. Swanson, chief of medical dermatology at the Mayo Clinic in Scottsdale, Ariz. In comparison, dermoscopy is taught to primary care physicians in Europe and Australia.
He discussed methods for assessing whether a lesion should be biopsied.
With the two-step pattern recognition method, the dermoscopist must first determine whether a neoplasm is a melanocytic proliferative one, such as a nevus or melanoma. "The reason that the first step is to consider nevi or melanomas is the importance of identifying the latter – especially melanomas that are not obvious with the naked eye," said Dr. Swanson at a seminar on women's and pediatric dermatology sponsored by Skin Disease Education Foundation.
If the lesion is melanocytic proliferative, the dermoscopist then applies the methods of interpretation for nevi or melanoma – such as pattern recognition or an algorithm. "If it isn't, then there are other diagnostic features that are used to lead to a clinical impression," he said.
The dermoscopy three-point rule has a high sensitivity for melanoma, he said. If a pigmented lesion has any two of the three criteria – atypical asymmetry, atypical pigment network, or blue-white structures – there is a likelihood of melanoma, and the lesion should be biopsied.
"The three-point rule is easier to learn and apply but as with all algorithms, there are a lot of exceptions to the rules. So one has to be willing to learn those exceptions and step out of the algorithm if they aren't certain," said Dr. Swanson. "One specific problem with the three-point method is that it doesn't take into account an atypical vasculature. Any dermoscopist using the three-point rule has to keep that in mind as an additional feature."
The seven-point algorithm – developed by Dr. Giuseppe Argenziano and others – is aimed at helping the novice dermoscopist. "It is a fairly reproducible method with very good specificity and sensitivity for making a decision to biopsy a melanocytic lesion. The three-point algorithm actually was derived from it as a method to simplify," said Dr. Swanson. "Most experienced dermoscopists only use algorithms occasionally, because with experience they become comfortable with pattern recognition."
The seven-point algorithm includes the criteria of the three-point rule, plus four minor criteria: streaks, regression pattern, irregular diffuse pigmentation, and irregular dot and globules.
Disclosures: Dr. Swanson reported having none. SDEF and this news organization are owned by Elsevier.
While dermoscopy can enhance the diagnosis of a number of skin conditions, it is especially useful in determining whether neoplasms should undergo biopsy.
"Patients are becoming aware of the technique and more and more are expecting their dermatologists to be skilled in its application," Dr. David L. Swanson said in an interview.
However, fewer than half of academic dermatologists and less than a quarter of practicing dermatologists use dermoscopy in the United States, according to Dr. Swanson, chief of medical dermatology at the Mayo Clinic in Scottsdale, Ariz. In comparison, dermoscopy is taught to primary care physicians in Europe and Australia.
He discussed methods for assessing whether a lesion should be biopsied.
With the two-step pattern recognition method, the dermoscopist must first determine whether a neoplasm is a melanocytic proliferative one, such as a nevus or melanoma. "The reason that the first step is to consider nevi or melanomas is the importance of identifying the latter – especially melanomas that are not obvious with the naked eye," said Dr. Swanson at a seminar on women's and pediatric dermatology sponsored by Skin Disease Education Foundation.
If the lesion is melanocytic proliferative, the dermoscopist then applies the methods of interpretation for nevi or melanoma – such as pattern recognition or an algorithm. "If it isn't, then there are other diagnostic features that are used to lead to a clinical impression," he said.
The dermoscopy three-point rule has a high sensitivity for melanoma, he said. If a pigmented lesion has any two of the three criteria – atypical asymmetry, atypical pigment network, or blue-white structures – there is a likelihood of melanoma, and the lesion should be biopsied.
"The three-point rule is easier to learn and apply but as with all algorithms, there are a lot of exceptions to the rules. So one has to be willing to learn those exceptions and step out of the algorithm if they aren't certain," said Dr. Swanson. "One specific problem with the three-point method is that it doesn't take into account an atypical vasculature. Any dermoscopist using the three-point rule has to keep that in mind as an additional feature."
The seven-point algorithm – developed by Dr. Giuseppe Argenziano and others – is aimed at helping the novice dermoscopist. "It is a fairly reproducible method with very good specificity and sensitivity for making a decision to biopsy a melanocytic lesion. The three-point algorithm actually was derived from it as a method to simplify," said Dr. Swanson. "Most experienced dermoscopists only use algorithms occasionally, because with experience they become comfortable with pattern recognition."
The seven-point algorithm includes the criteria of the three-point rule, plus four minor criteria: streaks, regression pattern, irregular diffuse pigmentation, and irregular dot and globules.
Disclosures: Dr. Swanson reported having none. SDEF and this news organization are owned by Elsevier.
While dermoscopy can enhance the diagnosis of a number of skin conditions, it is especially useful in determining whether neoplasms should undergo biopsy.
"Patients are becoming aware of the technique and more and more are expecting their dermatologists to be skilled in its application," Dr. David L. Swanson said in an interview.
However, fewer than half of academic dermatologists and less than a quarter of practicing dermatologists use dermoscopy in the United States, according to Dr. Swanson, chief of medical dermatology at the Mayo Clinic in Scottsdale, Ariz. In comparison, dermoscopy is taught to primary care physicians in Europe and Australia.
He discussed methods for assessing whether a lesion should be biopsied.
With the two-step pattern recognition method, the dermoscopist must first determine whether a neoplasm is a melanocytic proliferative one, such as a nevus or melanoma. "The reason that the first step is to consider nevi or melanomas is the importance of identifying the latter – especially melanomas that are not obvious with the naked eye," said Dr. Swanson at a seminar on women's and pediatric dermatology sponsored by Skin Disease Education Foundation.
If the lesion is melanocytic proliferative, the dermoscopist then applies the methods of interpretation for nevi or melanoma – such as pattern recognition or an algorithm. "If it isn't, then there are other diagnostic features that are used to lead to a clinical impression," he said.
The dermoscopy three-point rule has a high sensitivity for melanoma, he said. If a pigmented lesion has any two of the three criteria – atypical asymmetry, atypical pigment network, or blue-white structures – there is a likelihood of melanoma, and the lesion should be biopsied.
"The three-point rule is easier to learn and apply but as with all algorithms, there are a lot of exceptions to the rules. So one has to be willing to learn those exceptions and step out of the algorithm if they aren't certain," said Dr. Swanson. "One specific problem with the three-point method is that it doesn't take into account an atypical vasculature. Any dermoscopist using the three-point rule has to keep that in mind as an additional feature."
The seven-point algorithm – developed by Dr. Giuseppe Argenziano and others – is aimed at helping the novice dermoscopist. "It is a fairly reproducible method with very good specificity and sensitivity for making a decision to biopsy a melanocytic lesion. The three-point algorithm actually was derived from it as a method to simplify," said Dr. Swanson. "Most experienced dermoscopists only use algorithms occasionally, because with experience they become comfortable with pattern recognition."
The seven-point algorithm includes the criteria of the three-point rule, plus four minor criteria: streaks, regression pattern, irregular diffuse pigmentation, and irregular dot and globules.
Disclosures: Dr. Swanson reported having none. SDEF and this news organization are owned by Elsevier.
EXPERT ANALYSIS FROM A SEMINAR ON WOMEN'S AND PEDIATRIC DERMATOLOGY
Daily Fondaparinux Cuts Risk of Complications, Death in Lower-Limb Superficial-Vein Thrombosis
Daily fondaparinux significantly reduced the relative risk of symptomatic thromboembolic complications or death by 85% – without increasing the incidence of bleeding – in a study of some 3,000 patients with acute symptomatic lower-limb superficial-vein thrombosis.
“Fondaparinux also reduced the risk of symptomatic recurrence of superficial-vein thrombosis and, more important, its extension to the saphenofemoral junction – a finding that is clinically relevant because such extension is considered to increase the risk of deep-vein thrombosis and pulmonary embolism, thereby prompting escalation of therapy (for example, to full-dose anticoagulation or surgery),” Dr. Hervé Decousus and his coinvestigators reported Sept. 23 in the New England Journal of Medicine
The results may help fill in knowledge gaps about the appropriate treatment for superficial-vein thrombosis. Currently, therapeutic strategies range from no treatment to the use of anti-inflammatory drugs, anticoagulant agents, or even surgery.
Investigators in the study, known as the Comparison of Arixtra in Lower Limb Superficial Vein Thrombosis with Placebo (CALISTO) trial, compared the efficacy and safety of the specific factor Xa inhibitor fondaparinux with placebo in reducing symptomatic venous thromboembolic complications or death from any cause. The study comprised 3,002 patients with acute, isolated superficial-vein thrombosis of the legs (N. Engl. J. Med. 2010;363:1222-32).
Patients 18 years or older with acute, symptomatic lower-limb superficial-vein thrombosis at least 5-cm long were eligible for the study. They were excluded from the study if they met various specific criteria, such as a documented history of SVT within the past 3 months or DVT or PE within the past 6 months.
Between March 2007 and May 2009, a total of 3,002 patients were enrolled and randomized to receive 2.5 mg subcutaneous fondaparinux per day or daily subcutaneous placebo for 45 days. Overall, 1,481 patients in the fondaparinux group and 1,467 in the placebo group completed the follow-up visit at day 75, wrote Dr. Decousus of the Centre Hôpitalier Universitaire Saint-Étienne in France, and his coinvestigators.
Patients could choose whether to self-administer the injections, were encouraged to use graduated compression stockings, and were allowed to take acetaminophen or topical NSAIDs as needed. Concomitant treatment with other thrombolytic, anticoagulant, or antiplatelet agents was prohibited.
The primary efficacy outcome – the composite of death from any cause, symptomatic confirmed PE or DVT, or confirmed symptomatic extension to the saphenofemoral junction or symptomatic recurrence of superficial-vein thrombosis up to day 47 – occurred in a significantly greater percentage of those in the placebo group (5.9% vs. 0.9%; relative risk with fondaparinux, 0.15). Twenty patients would need to be treated to prevent one death, PE, DVT, extension to the saphenofemoral junction or symptomatic recurrence of superficial-vein thrombosis.
In addition, the incidence of each component of the primary efficacy outcome was significantly reduced in the fondaparinux group, except for the incidence of death, which did not differ significantly. The risk of the composite of DVT or PE was significantly reduced by 85% with fondaparinux, compared with placebo (0.2% vs. 1.3% affected in each group, respectively). To prevent one DVT or PE, 88 patients would need to be treated.
“This benefit was evident within the first days after treatment was initiated, supporting the adequacy of the prophylactic dose of 2.5 mg of fondaparinux and in accord with the substantial efficacy data already available with respect to a dose of 2.5 mg of fondaparinux in various clinical settings,” the investigators wrote.
In addition, more patients in the placebo group underwent surgery for superficial-vein thrombosis than in the fondaparinux group (3.5% vs. 0.5% by day 77), including ligation of the saphenofemoral junction.
Major bleeding had occurred in one patient in each group by day 47. The rates of clinically relevant nonmajor, minor, and total bleeding and arterial thromboembolic complications did not differ significantly between groups.
The study was supported by GlaxoSmithKline, which markets Arixtra (fondaparinux). In addition, 8 of the 11 study authors reported significant financial relationships with several pharmaceutical companies, including GlaxoSmithKline.
While overall efficacy and safety are crucial information for the approval and widespread use of a new drug, so is cost effectiveness, Dr. Lee Goldman and Dr. Jeffrey Ginsberg wrote in an accompanying commentary.
In this study, the researchers determined that 88 patients would need to be treated with fondaparinux to prevent one nonfatal episode of deep-vein thrombosis or pulmonary embolism. “We found that in New York City, the price of a 45-day regimen of 2.5 mg of fondaparinux once daily ranged from $2,124 to $7,380 at four major pharmacies. Even at the lowest quoted price and considering the 98.3% estimated adherence rate, the cost of the treatment for 1,500 patients would be about $3.13 million,” they wrote.
“On the basis of the incremental 1-year costs for the medical care of a patient with a pulmonary embolus or deep-vein thrombosis, an estimated $250,000 or so in medical care costs would be averted, resulting in a net cost of fondaparinux treatment of about $2.88 million, or about $1,900 per treated patient, without any lives saved.”
However, no clinical trial phases currently assess this important information. Likewise, decisions regarding Medicare reimbursement in the United States do not “consider the cost or cost-effectiveness of the agent itself or of the strategy into which it is incorporated,” according to Dr. Goldman and Dr. Ginsberg.
“We recommend that the Food and Drug Administration give serious thought to mandating phase 3.5 trials to document the costs, the effects on quality of life, and the cost-effectiveness of new interventions so as to reach a consensus regarding their worthiness. Until such data are available and consensus is reached, it would seem premature to recommend that fondaparinux be used routinely in the treatment of superficial-vein thrombosis.”
Dr. Lee Goldman is the executive vice president for Health and Biomedical Sciences and dean of the Faculties of Health Sciences and Medicine at Columbia University in New York. Dr. Jeffrey Ginsberg is a professor of hematology and thromboembolism at McMaster University in Hamilton, Ontario. Both Dr. Goldman and Dr. Ginsberg reported that they have no relevant financial relationships.
While overall efficacy and safety are crucial information for the approval and widespread use of a new drug, so is cost effectiveness, Dr. Lee Goldman and Dr. Jeffrey Ginsberg wrote in an accompanying commentary.
In this study, the researchers determined that 88 patients would need to be treated with fondaparinux to prevent one nonfatal episode of deep-vein thrombosis or pulmonary embolism. “We found that in New York City, the price of a 45-day regimen of 2.5 mg of fondaparinux once daily ranged from $2,124 to $7,380 at four major pharmacies. Even at the lowest quoted price and considering the 98.3% estimated adherence rate, the cost of the treatment for 1,500 patients would be about $3.13 million,” they wrote.
“On the basis of the incremental 1-year costs for the medical care of a patient with a pulmonary embolus or deep-vein thrombosis, an estimated $250,000 or so in medical care costs would be averted, resulting in a net cost of fondaparinux treatment of about $2.88 million, or about $1,900 per treated patient, without any lives saved.”
However, no clinical trial phases currently assess this important information. Likewise, decisions regarding Medicare reimbursement in the United States do not “consider the cost or cost-effectiveness of the agent itself or of the strategy into which it is incorporated,” according to Dr. Goldman and Dr. Ginsberg.
“We recommend that the Food and Drug Administration give serious thought to mandating phase 3.5 trials to document the costs, the effects on quality of life, and the cost-effectiveness of new interventions so as to reach a consensus regarding their worthiness. Until such data are available and consensus is reached, it would seem premature to recommend that fondaparinux be used routinely in the treatment of superficial-vein thrombosis.”
Dr. Lee Goldman is the executive vice president for Health and Biomedical Sciences and dean of the Faculties of Health Sciences and Medicine at Columbia University in New York. Dr. Jeffrey Ginsberg is a professor of hematology and thromboembolism at McMaster University in Hamilton, Ontario. Both Dr. Goldman and Dr. Ginsberg reported that they have no relevant financial relationships.
While overall efficacy and safety are crucial information for the approval and widespread use of a new drug, so is cost effectiveness, Dr. Lee Goldman and Dr. Jeffrey Ginsberg wrote in an accompanying commentary.
In this study, the researchers determined that 88 patients would need to be treated with fondaparinux to prevent one nonfatal episode of deep-vein thrombosis or pulmonary embolism. “We found that in New York City, the price of a 45-day regimen of 2.5 mg of fondaparinux once daily ranged from $2,124 to $7,380 at four major pharmacies. Even at the lowest quoted price and considering the 98.3% estimated adherence rate, the cost of the treatment for 1,500 patients would be about $3.13 million,” they wrote.
“On the basis of the incremental 1-year costs for the medical care of a patient with a pulmonary embolus or deep-vein thrombosis, an estimated $250,000 or so in medical care costs would be averted, resulting in a net cost of fondaparinux treatment of about $2.88 million, or about $1,900 per treated patient, without any lives saved.”
However, no clinical trial phases currently assess this important information. Likewise, decisions regarding Medicare reimbursement in the United States do not “consider the cost or cost-effectiveness of the agent itself or of the strategy into which it is incorporated,” according to Dr. Goldman and Dr. Ginsberg.
“We recommend that the Food and Drug Administration give serious thought to mandating phase 3.5 trials to document the costs, the effects on quality of life, and the cost-effectiveness of new interventions so as to reach a consensus regarding their worthiness. Until such data are available and consensus is reached, it would seem premature to recommend that fondaparinux be used routinely in the treatment of superficial-vein thrombosis.”
Dr. Lee Goldman is the executive vice president for Health and Biomedical Sciences and dean of the Faculties of Health Sciences and Medicine at Columbia University in New York. Dr. Jeffrey Ginsberg is a professor of hematology and thromboembolism at McMaster University in Hamilton, Ontario. Both Dr. Goldman and Dr. Ginsberg reported that they have no relevant financial relationships.
Daily fondaparinux significantly reduced the relative risk of symptomatic thromboembolic complications or death by 85% – without increasing the incidence of bleeding – in a study of some 3,000 patients with acute symptomatic lower-limb superficial-vein thrombosis.
“Fondaparinux also reduced the risk of symptomatic recurrence of superficial-vein thrombosis and, more important, its extension to the saphenofemoral junction – a finding that is clinically relevant because such extension is considered to increase the risk of deep-vein thrombosis and pulmonary embolism, thereby prompting escalation of therapy (for example, to full-dose anticoagulation or surgery),” Dr. Hervé Decousus and his coinvestigators reported Sept. 23 in the New England Journal of Medicine
The results may help fill in knowledge gaps about the appropriate treatment for superficial-vein thrombosis. Currently, therapeutic strategies range from no treatment to the use of anti-inflammatory drugs, anticoagulant agents, or even surgery.
Investigators in the study, known as the Comparison of Arixtra in Lower Limb Superficial Vein Thrombosis with Placebo (CALISTO) trial, compared the efficacy and safety of the specific factor Xa inhibitor fondaparinux with placebo in reducing symptomatic venous thromboembolic complications or death from any cause. The study comprised 3,002 patients with acute, isolated superficial-vein thrombosis of the legs (N. Engl. J. Med. 2010;363:1222-32).
Patients 18 years or older with acute, symptomatic lower-limb superficial-vein thrombosis at least 5-cm long were eligible for the study. They were excluded from the study if they met various specific criteria, such as a documented history of SVT within the past 3 months or DVT or PE within the past 6 months.
Between March 2007 and May 2009, a total of 3,002 patients were enrolled and randomized to receive 2.5 mg subcutaneous fondaparinux per day or daily subcutaneous placebo for 45 days. Overall, 1,481 patients in the fondaparinux group and 1,467 in the placebo group completed the follow-up visit at day 75, wrote Dr. Decousus of the Centre Hôpitalier Universitaire Saint-Étienne in France, and his coinvestigators.
Patients could choose whether to self-administer the injections, were encouraged to use graduated compression stockings, and were allowed to take acetaminophen or topical NSAIDs as needed. Concomitant treatment with other thrombolytic, anticoagulant, or antiplatelet agents was prohibited.
The primary efficacy outcome – the composite of death from any cause, symptomatic confirmed PE or DVT, or confirmed symptomatic extension to the saphenofemoral junction or symptomatic recurrence of superficial-vein thrombosis up to day 47 – occurred in a significantly greater percentage of those in the placebo group (5.9% vs. 0.9%; relative risk with fondaparinux, 0.15). Twenty patients would need to be treated to prevent one death, PE, DVT, extension to the saphenofemoral junction or symptomatic recurrence of superficial-vein thrombosis.
In addition, the incidence of each component of the primary efficacy outcome was significantly reduced in the fondaparinux group, except for the incidence of death, which did not differ significantly. The risk of the composite of DVT or PE was significantly reduced by 85% with fondaparinux, compared with placebo (0.2% vs. 1.3% affected in each group, respectively). To prevent one DVT or PE, 88 patients would need to be treated.
“This benefit was evident within the first days after treatment was initiated, supporting the adequacy of the prophylactic dose of 2.5 mg of fondaparinux and in accord with the substantial efficacy data already available with respect to a dose of 2.5 mg of fondaparinux in various clinical settings,” the investigators wrote.
In addition, more patients in the placebo group underwent surgery for superficial-vein thrombosis than in the fondaparinux group (3.5% vs. 0.5% by day 77), including ligation of the saphenofemoral junction.
Major bleeding had occurred in one patient in each group by day 47. The rates of clinically relevant nonmajor, minor, and total bleeding and arterial thromboembolic complications did not differ significantly between groups.
The study was supported by GlaxoSmithKline, which markets Arixtra (fondaparinux). In addition, 8 of the 11 study authors reported significant financial relationships with several pharmaceutical companies, including GlaxoSmithKline.
Daily fondaparinux significantly reduced the relative risk of symptomatic thromboembolic complications or death by 85% – without increasing the incidence of bleeding – in a study of some 3,000 patients with acute symptomatic lower-limb superficial-vein thrombosis.
“Fondaparinux also reduced the risk of symptomatic recurrence of superficial-vein thrombosis and, more important, its extension to the saphenofemoral junction – a finding that is clinically relevant because such extension is considered to increase the risk of deep-vein thrombosis and pulmonary embolism, thereby prompting escalation of therapy (for example, to full-dose anticoagulation or surgery),” Dr. Hervé Decousus and his coinvestigators reported Sept. 23 in the New England Journal of Medicine
The results may help fill in knowledge gaps about the appropriate treatment for superficial-vein thrombosis. Currently, therapeutic strategies range from no treatment to the use of anti-inflammatory drugs, anticoagulant agents, or even surgery.
Investigators in the study, known as the Comparison of Arixtra in Lower Limb Superficial Vein Thrombosis with Placebo (CALISTO) trial, compared the efficacy and safety of the specific factor Xa inhibitor fondaparinux with placebo in reducing symptomatic venous thromboembolic complications or death from any cause. The study comprised 3,002 patients with acute, isolated superficial-vein thrombosis of the legs (N. Engl. J. Med. 2010;363:1222-32).
Patients 18 years or older with acute, symptomatic lower-limb superficial-vein thrombosis at least 5-cm long were eligible for the study. They were excluded from the study if they met various specific criteria, such as a documented history of SVT within the past 3 months or DVT or PE within the past 6 months.
Between March 2007 and May 2009, a total of 3,002 patients were enrolled and randomized to receive 2.5 mg subcutaneous fondaparinux per day or daily subcutaneous placebo for 45 days. Overall, 1,481 patients in the fondaparinux group and 1,467 in the placebo group completed the follow-up visit at day 75, wrote Dr. Decousus of the Centre Hôpitalier Universitaire Saint-Étienne in France, and his coinvestigators.
Patients could choose whether to self-administer the injections, were encouraged to use graduated compression stockings, and were allowed to take acetaminophen or topical NSAIDs as needed. Concomitant treatment with other thrombolytic, anticoagulant, or antiplatelet agents was prohibited.
The primary efficacy outcome – the composite of death from any cause, symptomatic confirmed PE or DVT, or confirmed symptomatic extension to the saphenofemoral junction or symptomatic recurrence of superficial-vein thrombosis up to day 47 – occurred in a significantly greater percentage of those in the placebo group (5.9% vs. 0.9%; relative risk with fondaparinux, 0.15). Twenty patients would need to be treated to prevent one death, PE, DVT, extension to the saphenofemoral junction or symptomatic recurrence of superficial-vein thrombosis.
In addition, the incidence of each component of the primary efficacy outcome was significantly reduced in the fondaparinux group, except for the incidence of death, which did not differ significantly. The risk of the composite of DVT or PE was significantly reduced by 85% with fondaparinux, compared with placebo (0.2% vs. 1.3% affected in each group, respectively). To prevent one DVT or PE, 88 patients would need to be treated.
“This benefit was evident within the first days after treatment was initiated, supporting the adequacy of the prophylactic dose of 2.5 mg of fondaparinux and in accord with the substantial efficacy data already available with respect to a dose of 2.5 mg of fondaparinux in various clinical settings,” the investigators wrote.
In addition, more patients in the placebo group underwent surgery for superficial-vein thrombosis than in the fondaparinux group (3.5% vs. 0.5% by day 77), including ligation of the saphenofemoral junction.
Major bleeding had occurred in one patient in each group by day 47. The rates of clinically relevant nonmajor, minor, and total bleeding and arterial thromboembolic complications did not differ significantly between groups.
The study was supported by GlaxoSmithKline, which markets Arixtra (fondaparinux). In addition, 8 of the 11 study authors reported significant financial relationships with several pharmaceutical companies, including GlaxoSmithKline.
Large Population-Based Study Confirms Increased Risk of VTE With Antipsychotics
Antipsychotic drugs are associated with an almost one-third (32%) greater risk of venous thromboembolism, according to results of a nested case-control study of more than 100,000 primary care patients in the United Kingdom. The study was published online Sept. 21 in the British Medical Journal.
Previous research has suggested that these drugs – also commonly used for nausea, vomiting, and vertigo – might be linked with an increased risk of venous thromboembolism (VTE). However, the results have been inconsistent.
In the current study, the association was even greater for new users of antipsychotics. Those with any antipsychotic use in last 3 months had a 56% increased risk of VTE. Patients who started taking an antipsychotic in the past 3 months had a 97% increased risk. However, the absolute risks were low, with an excess of four extra cases of VTE per 10,000 patients treated over 1 year in patients of all ages and 10 for patients aged 65 years and older.
“Our study adds to the accumulating evidence of adverse health events associated with antipsychotic drugs,” the researchers wrote (BMJ 2010 Sept. 21[doi:10.1136/bmj.c4245]). “If other studies replicate these findings, antipsychotic drugs should be used more cautiously for nausea and agitation, etc., especially among patients at high risk of thromboembolism.”
The researchers used data from the QResearch database, which includes primary care clinical records for more than 11 million people registered in the past 16 years at more than 500 U.K. general practices. The study population was an open cohort of patients aged 16-100 years, who were registered with participating practices between January 1996 and July 2007. Case patients had a first-ever record of VTE – either deep vein thrombosis or pulmonary embolism – during the study period (including postmortem diagnoses).
Incidence density sampling was used to identify up to four control patients for each case patient. Control patients were matched by age, calendar time, sex, and practice. Control patients had no diagnoses of VTE prior to the date of the first recorded diagnosis of VTE in their matched case patient (index date).
Both case and control patients had to have at least 24 months of data before the index date. Control patients were excluded if they had any prescriptions for warfarin prior to the index date. Case patients with any use of warfarin earlier than 6 weeks prior to VTE diagnosis were also excluded, according to Chris Parker, a medical statistician at Hucknall Health Centre in Nottingham, England, and colleagues.
Each patient was classified for exposure to antipsychotics as a current user (one or more prescriptions within 3 months before the index date), a recent user (prescriptions within 4-12 months before the index date), a past user (prescriptions within 13-24 months before the index date), or not exposed within the previous 24 months.
Current users were subclassified as new users (a prescription for antipsychotic within 3 months of the index date, after at least 12 months with no prescription for that antipsychotic) or as continuing users. Exposure during the previous 24 months also was classified according by type (conventional, atypical, or both) and potency (high potency, low potency, or both). High potency was defined as equivalent dose of more than 100 mg chlorpromazine.
Patients with more than one mental health indication were categorized according to a hierarchy: schizophrenia, bipolar disorder without schizophrenia, and dementia without schizophrenia or bipolar disorder.
The analyses were adjusted for socioeconomic status, comorbidities (coronary heart disease, cardiac failure, stroke, cancer, inflammatory bowel disease, liver disease, varicose veins, gastrointestinal bleed, Parkinson’s disease, renal disease, asthma, diabetes, hypertension, hyperlipidemia), drug variables, and the number of complete months of data before the index date.
In addition, data were extracted for 6 months prior to the index date for several events that are associated with increased risk in the short term (hip surgery, hip or lower limb fractures, acute infections, pregnancy). The researchers also looked for whether there was any computer-recorded evidence of a hospital admission in the preceding 31-183 days.
In all, 25,532 eligible case patients – 15,975 with DVT and 9,557 with pulmonary embolism – and 89,491 control patients were included in the study. In terms of mental health disorders, the overall prevalence was 0.4% for schizophrenia, 0.3% for bipolar disorder, and 1.0% for dementia. Eight case patients and 31 control patients had more than one disorder. In all, 8.3% of case patients and 5.3% of control patients had received an antipsychotic in the previous 24 months.
Overall, antipsychotic users had a 32% greater risk of VTE than did nonusers. Among those on antipsychotics, 38% were current users and their increase in risk was 56% compared with 36% for recent users. The risk was not significantly increased for past users. Among current antipsychotic users, 15% had started a new drug within the 3 months prior to the index date. This group of new users showed a greater increase in risk (97%) than did continuing users (29%).
Patients who were prescribed atypical antipsychotic drugs had a greater risk of VTE than did those who were prescribed conventional antipsychotics – 73% compared with 28%. Patients who had received only one prescription in the previous 12 months had a significantly greater risk (32%) than did those receiving none. In addition, those who were prescribed two or more different antipsychotics had a greater risk (99%) than did those who received only one (29%).
The most commonly prescribed drug was prochlorperazine – a high-potency phenothiazine – which also is commonly prescribed for nausea, vomiting, and vertigo. Prochlorperazine was prescribed for 75% of the total number of those on antipsychotics. The other most commonly prescribed drugs were (in order) risperidone, haloperidol, olanzapine, chlorpromazine, trifluoperazine, and quetiapine. Separate odds ratios were estimated for exposure to these drugs, with highest risks for those patients who were prescribed quetiapine, chlorpromazine, and haloperidol, with adjusted odds ratios of 2.81, 1.77, and 2.17, respectively.
Individuals with a diagnosis of dementia were at higher risk than were those with diagnoses of schizophrenia, bipolar disorder, or none of these conditions. There was a more than threefold increase associated with cancer and a roughly 13-fold increase associated with recent surgery or fractures. “Individuals prescribed statins or aspirin in the past 24 months had a lower risk of venous thromboembolism, and those prescribed NSAIDs, oral contraceptives, hormone replacement therapy, tamoxifen, or antimanics had a higher risk,” the authors noted.
The number needed for harm for any antipsychotic use in the past 24 months for patients aged 65 years and older was 1,044; for new users in the past 3 months this number was 344; and for continuing users it was 1,152. The corresponding numbers of excess cases of VTE per 10,000 treated patients were 10, 29, and 9, respectively.
The study authors reported that they have no relevant financial relationships.
The validity of the findings is strengthened by the large sample size and the low potential for exposure and outcome misclassification because of the detailed source of data and adjustment for a large number of confounders, according to Dr. Rosa Liperoti and Dr. Giovanni Gambassi.
It’s been demonstrated that “patients with schizophrenia have an increased risk of venous thromboembolism (VTE), and this might be associated with the use of antipsychotics, especially low-potency drugs such as chlorpromazine and thioridazine. So far, however, the possibility that the underlying psychiatric disorders themselves – and not the antipsychotics – are associated with VTE has never been excluded. This could occur, for example, by the increased concentrations of adrenaline seen during psychotic excitation increasing blood coagulation,” they noted.
In this study, in almost all cases the reason for prescription of antipsychotics could not be ascertained. Most of the antipsychotics used were conventional agents, with prochlorperazine – probably given for nausea and vomiting – accounting for almost 80% of all prescriptions, they wrote. “These findings indicate that VTE is directly linked to the use of an antipsychotic, and that the risk of VTE increases early after starting the drug.”
The implications are potentially far reaching. “Despite their association with serious risks and few data to support their efficacy, antipsychotics are widely used, and in 2008 they became the top selling drug class in the United States. Despite efforts to improve non–drug based interventions, antipsychotics are often used, especially for the treatment of agitation in people with dementia,” they wrote.
The findings suggest that physicians “need to be able to identify the best candidates for antipsychotic treatment, such as those people with the lowest vascular risk profile who may respond to short-term and low-dose treatment with antipsychotics because of individual pharmacogenetic characteristics, and those who may be more susceptible to developing side effects as a result of individual vascular risk factors possibly interacting with antipsychotics.”
Dr. Rosa Liperoti is a specialist in geriatrics and Dr. Giovanni Gambassi is a professor of geriatrics at Universit? Cattolica del Sacro Cuore in Rome. Both Dr. Liperoti and Dr. Gambassi reported that they have no relevant financial relationships. These comments were taken from a commentary that accompanied the study (BMJ 2010 Sept. 21[doi: 10.1136/bmj.c4216]).
The validity of the findings is strengthened by the large sample size and the low potential for exposure and outcome misclassification because of the detailed source of data and adjustment for a large number of confounders, according to Dr. Rosa Liperoti and Dr. Giovanni Gambassi.
It’s been demonstrated that “patients with schizophrenia have an increased risk of venous thromboembolism (VTE), and this might be associated with the use of antipsychotics, especially low-potency drugs such as chlorpromazine and thioridazine. So far, however, the possibility that the underlying psychiatric disorders themselves – and not the antipsychotics – are associated with VTE has never been excluded. This could occur, for example, by the increased concentrations of adrenaline seen during psychotic excitation increasing blood coagulation,” they noted.
In this study, in almost all cases the reason for prescription of antipsychotics could not be ascertained. Most of the antipsychotics used were conventional agents, with prochlorperazine – probably given for nausea and vomiting – accounting for almost 80% of all prescriptions, they wrote. “These findings indicate that VTE is directly linked to the use of an antipsychotic, and that the risk of VTE increases early after starting the drug.”
The implications are potentially far reaching. “Despite their association with serious risks and few data to support their efficacy, antipsychotics are widely used, and in 2008 they became the top selling drug class in the United States. Despite efforts to improve non–drug based interventions, antipsychotics are often used, especially for the treatment of agitation in people with dementia,” they wrote.
The findings suggest that physicians “need to be able to identify the best candidates for antipsychotic treatment, such as those people with the lowest vascular risk profile who may respond to short-term and low-dose treatment with antipsychotics because of individual pharmacogenetic characteristics, and those who may be more susceptible to developing side effects as a result of individual vascular risk factors possibly interacting with antipsychotics.”
Dr. Rosa Liperoti is a specialist in geriatrics and Dr. Giovanni Gambassi is a professor of geriatrics at Universit? Cattolica del Sacro Cuore in Rome. Both Dr. Liperoti and Dr. Gambassi reported that they have no relevant financial relationships. These comments were taken from a commentary that accompanied the study (BMJ 2010 Sept. 21[doi: 10.1136/bmj.c4216]).
The validity of the findings is strengthened by the large sample size and the low potential for exposure and outcome misclassification because of the detailed source of data and adjustment for a large number of confounders, according to Dr. Rosa Liperoti and Dr. Giovanni Gambassi.
It’s been demonstrated that “patients with schizophrenia have an increased risk of venous thromboembolism (VTE), and this might be associated with the use of antipsychotics, especially low-potency drugs such as chlorpromazine and thioridazine. So far, however, the possibility that the underlying psychiatric disorders themselves – and not the antipsychotics – are associated with VTE has never been excluded. This could occur, for example, by the increased concentrations of adrenaline seen during psychotic excitation increasing blood coagulation,” they noted.
In this study, in almost all cases the reason for prescription of antipsychotics could not be ascertained. Most of the antipsychotics used were conventional agents, with prochlorperazine – probably given for nausea and vomiting – accounting for almost 80% of all prescriptions, they wrote. “These findings indicate that VTE is directly linked to the use of an antipsychotic, and that the risk of VTE increases early after starting the drug.”
The implications are potentially far reaching. “Despite their association with serious risks and few data to support their efficacy, antipsychotics are widely used, and in 2008 they became the top selling drug class in the United States. Despite efforts to improve non–drug based interventions, antipsychotics are often used, especially for the treatment of agitation in people with dementia,” they wrote.
The findings suggest that physicians “need to be able to identify the best candidates for antipsychotic treatment, such as those people with the lowest vascular risk profile who may respond to short-term and low-dose treatment with antipsychotics because of individual pharmacogenetic characteristics, and those who may be more susceptible to developing side effects as a result of individual vascular risk factors possibly interacting with antipsychotics.”
Dr. Rosa Liperoti is a specialist in geriatrics and Dr. Giovanni Gambassi is a professor of geriatrics at Universit? Cattolica del Sacro Cuore in Rome. Both Dr. Liperoti and Dr. Gambassi reported that they have no relevant financial relationships. These comments were taken from a commentary that accompanied the study (BMJ 2010 Sept. 21[doi: 10.1136/bmj.c4216]).
Antipsychotic drugs are associated with an almost one-third (32%) greater risk of venous thromboembolism, according to results of a nested case-control study of more than 100,000 primary care patients in the United Kingdom. The study was published online Sept. 21 in the British Medical Journal.
Previous research has suggested that these drugs – also commonly used for nausea, vomiting, and vertigo – might be linked with an increased risk of venous thromboembolism (VTE). However, the results have been inconsistent.
In the current study, the association was even greater for new users of antipsychotics. Those with any antipsychotic use in last 3 months had a 56% increased risk of VTE. Patients who started taking an antipsychotic in the past 3 months had a 97% increased risk. However, the absolute risks were low, with an excess of four extra cases of VTE per 10,000 patients treated over 1 year in patients of all ages and 10 for patients aged 65 years and older.
“Our study adds to the accumulating evidence of adverse health events associated with antipsychotic drugs,” the researchers wrote (BMJ 2010 Sept. 21[doi:10.1136/bmj.c4245]). “If other studies replicate these findings, antipsychotic drugs should be used more cautiously for nausea and agitation, etc., especially among patients at high risk of thromboembolism.”
The researchers used data from the QResearch database, which includes primary care clinical records for more than 11 million people registered in the past 16 years at more than 500 U.K. general practices. The study population was an open cohort of patients aged 16-100 years, who were registered with participating practices between January 1996 and July 2007. Case patients had a first-ever record of VTE – either deep vein thrombosis or pulmonary embolism – during the study period (including postmortem diagnoses).
Incidence density sampling was used to identify up to four control patients for each case patient. Control patients were matched by age, calendar time, sex, and practice. Control patients had no diagnoses of VTE prior to the date of the first recorded diagnosis of VTE in their matched case patient (index date).
Both case and control patients had to have at least 24 months of data before the index date. Control patients were excluded if they had any prescriptions for warfarin prior to the index date. Case patients with any use of warfarin earlier than 6 weeks prior to VTE diagnosis were also excluded, according to Chris Parker, a medical statistician at Hucknall Health Centre in Nottingham, England, and colleagues.
Each patient was classified for exposure to antipsychotics as a current user (one or more prescriptions within 3 months before the index date), a recent user (prescriptions within 4-12 months before the index date), a past user (prescriptions within 13-24 months before the index date), or not exposed within the previous 24 months.
Current users were subclassified as new users (a prescription for antipsychotic within 3 months of the index date, after at least 12 months with no prescription for that antipsychotic) or as continuing users. Exposure during the previous 24 months also was classified according by type (conventional, atypical, or both) and potency (high potency, low potency, or both). High potency was defined as equivalent dose of more than 100 mg chlorpromazine.
Patients with more than one mental health indication were categorized according to a hierarchy: schizophrenia, bipolar disorder without schizophrenia, and dementia without schizophrenia or bipolar disorder.
The analyses were adjusted for socioeconomic status, comorbidities (coronary heart disease, cardiac failure, stroke, cancer, inflammatory bowel disease, liver disease, varicose veins, gastrointestinal bleed, Parkinson’s disease, renal disease, asthma, diabetes, hypertension, hyperlipidemia), drug variables, and the number of complete months of data before the index date.
In addition, data were extracted for 6 months prior to the index date for several events that are associated with increased risk in the short term (hip surgery, hip or lower limb fractures, acute infections, pregnancy). The researchers also looked for whether there was any computer-recorded evidence of a hospital admission in the preceding 31-183 days.
In all, 25,532 eligible case patients – 15,975 with DVT and 9,557 with pulmonary embolism – and 89,491 control patients were included in the study. In terms of mental health disorders, the overall prevalence was 0.4% for schizophrenia, 0.3% for bipolar disorder, and 1.0% for dementia. Eight case patients and 31 control patients had more than one disorder. In all, 8.3% of case patients and 5.3% of control patients had received an antipsychotic in the previous 24 months.
Overall, antipsychotic users had a 32% greater risk of VTE than did nonusers. Among those on antipsychotics, 38% were current users and their increase in risk was 56% compared with 36% for recent users. The risk was not significantly increased for past users. Among current antipsychotic users, 15% had started a new drug within the 3 months prior to the index date. This group of new users showed a greater increase in risk (97%) than did continuing users (29%).
Patients who were prescribed atypical antipsychotic drugs had a greater risk of VTE than did those who were prescribed conventional antipsychotics – 73% compared with 28%. Patients who had received only one prescription in the previous 12 months had a significantly greater risk (32%) than did those receiving none. In addition, those who were prescribed two or more different antipsychotics had a greater risk (99%) than did those who received only one (29%).
The most commonly prescribed drug was prochlorperazine – a high-potency phenothiazine – which also is commonly prescribed for nausea, vomiting, and vertigo. Prochlorperazine was prescribed for 75% of the total number of those on antipsychotics. The other most commonly prescribed drugs were (in order) risperidone, haloperidol, olanzapine, chlorpromazine, trifluoperazine, and quetiapine. Separate odds ratios were estimated for exposure to these drugs, with highest risks for those patients who were prescribed quetiapine, chlorpromazine, and haloperidol, with adjusted odds ratios of 2.81, 1.77, and 2.17, respectively.
Individuals with a diagnosis of dementia were at higher risk than were those with diagnoses of schizophrenia, bipolar disorder, or none of these conditions. There was a more than threefold increase associated with cancer and a roughly 13-fold increase associated with recent surgery or fractures. “Individuals prescribed statins or aspirin in the past 24 months had a lower risk of venous thromboembolism, and those prescribed NSAIDs, oral contraceptives, hormone replacement therapy, tamoxifen, or antimanics had a higher risk,” the authors noted.
The number needed for harm for any antipsychotic use in the past 24 months for patients aged 65 years and older was 1,044; for new users in the past 3 months this number was 344; and for continuing users it was 1,152. The corresponding numbers of excess cases of VTE per 10,000 treated patients were 10, 29, and 9, respectively.
The study authors reported that they have no relevant financial relationships.
Antipsychotic drugs are associated with an almost one-third (32%) greater risk of venous thromboembolism, according to results of a nested case-control study of more than 100,000 primary care patients in the United Kingdom. The study was published online Sept. 21 in the British Medical Journal.
Previous research has suggested that these drugs – also commonly used for nausea, vomiting, and vertigo – might be linked with an increased risk of venous thromboembolism (VTE). However, the results have been inconsistent.
In the current study, the association was even greater for new users of antipsychotics. Those with any antipsychotic use in last 3 months had a 56% increased risk of VTE. Patients who started taking an antipsychotic in the past 3 months had a 97% increased risk. However, the absolute risks were low, with an excess of four extra cases of VTE per 10,000 patients treated over 1 year in patients of all ages and 10 for patients aged 65 years and older.
“Our study adds to the accumulating evidence of adverse health events associated with antipsychotic drugs,” the researchers wrote (BMJ 2010 Sept. 21[doi:10.1136/bmj.c4245]). “If other studies replicate these findings, antipsychotic drugs should be used more cautiously for nausea and agitation, etc., especially among patients at high risk of thromboembolism.”
The researchers used data from the QResearch database, which includes primary care clinical records for more than 11 million people registered in the past 16 years at more than 500 U.K. general practices. The study population was an open cohort of patients aged 16-100 years, who were registered with participating practices between January 1996 and July 2007. Case patients had a first-ever record of VTE – either deep vein thrombosis or pulmonary embolism – during the study period (including postmortem diagnoses).
Incidence density sampling was used to identify up to four control patients for each case patient. Control patients were matched by age, calendar time, sex, and practice. Control patients had no diagnoses of VTE prior to the date of the first recorded diagnosis of VTE in their matched case patient (index date).
Both case and control patients had to have at least 24 months of data before the index date. Control patients were excluded if they had any prescriptions for warfarin prior to the index date. Case patients with any use of warfarin earlier than 6 weeks prior to VTE diagnosis were also excluded, according to Chris Parker, a medical statistician at Hucknall Health Centre in Nottingham, England, and colleagues.
Each patient was classified for exposure to antipsychotics as a current user (one or more prescriptions within 3 months before the index date), a recent user (prescriptions within 4-12 months before the index date), a past user (prescriptions within 13-24 months before the index date), or not exposed within the previous 24 months.
Current users were subclassified as new users (a prescription for antipsychotic within 3 months of the index date, after at least 12 months with no prescription for that antipsychotic) or as continuing users. Exposure during the previous 24 months also was classified according by type (conventional, atypical, or both) and potency (high potency, low potency, or both). High potency was defined as equivalent dose of more than 100 mg chlorpromazine.
Patients with more than one mental health indication were categorized according to a hierarchy: schizophrenia, bipolar disorder without schizophrenia, and dementia without schizophrenia or bipolar disorder.
The analyses were adjusted for socioeconomic status, comorbidities (coronary heart disease, cardiac failure, stroke, cancer, inflammatory bowel disease, liver disease, varicose veins, gastrointestinal bleed, Parkinson’s disease, renal disease, asthma, diabetes, hypertension, hyperlipidemia), drug variables, and the number of complete months of data before the index date.
In addition, data were extracted for 6 months prior to the index date for several events that are associated with increased risk in the short term (hip surgery, hip or lower limb fractures, acute infections, pregnancy). The researchers also looked for whether there was any computer-recorded evidence of a hospital admission in the preceding 31-183 days.
In all, 25,532 eligible case patients – 15,975 with DVT and 9,557 with pulmonary embolism – and 89,491 control patients were included in the study. In terms of mental health disorders, the overall prevalence was 0.4% for schizophrenia, 0.3% for bipolar disorder, and 1.0% for dementia. Eight case patients and 31 control patients had more than one disorder. In all, 8.3% of case patients and 5.3% of control patients had received an antipsychotic in the previous 24 months.
Overall, antipsychotic users had a 32% greater risk of VTE than did nonusers. Among those on antipsychotics, 38% were current users and their increase in risk was 56% compared with 36% for recent users. The risk was not significantly increased for past users. Among current antipsychotic users, 15% had started a new drug within the 3 months prior to the index date. This group of new users showed a greater increase in risk (97%) than did continuing users (29%).
Patients who were prescribed atypical antipsychotic drugs had a greater risk of VTE than did those who were prescribed conventional antipsychotics – 73% compared with 28%. Patients who had received only one prescription in the previous 12 months had a significantly greater risk (32%) than did those receiving none. In addition, those who were prescribed two or more different antipsychotics had a greater risk (99%) than did those who received only one (29%).
The most commonly prescribed drug was prochlorperazine – a high-potency phenothiazine – which also is commonly prescribed for nausea, vomiting, and vertigo. Prochlorperazine was prescribed for 75% of the total number of those on antipsychotics. The other most commonly prescribed drugs were (in order) risperidone, haloperidol, olanzapine, chlorpromazine, trifluoperazine, and quetiapine. Separate odds ratios were estimated for exposure to these drugs, with highest risks for those patients who were prescribed quetiapine, chlorpromazine, and haloperidol, with adjusted odds ratios of 2.81, 1.77, and 2.17, respectively.
Individuals with a diagnosis of dementia were at higher risk than were those with diagnoses of schizophrenia, bipolar disorder, or none of these conditions. There was a more than threefold increase associated with cancer and a roughly 13-fold increase associated with recent surgery or fractures. “Individuals prescribed statins or aspirin in the past 24 months had a lower risk of venous thromboembolism, and those prescribed NSAIDs, oral contraceptives, hormone replacement therapy, tamoxifen, or antimanics had a higher risk,” the authors noted.
The number needed for harm for any antipsychotic use in the past 24 months for patients aged 65 years and older was 1,044; for new users in the past 3 months this number was 344; and for continuing users it was 1,152. The corresponding numbers of excess cases of VTE per 10,000 treated patients were 10, 29, and 9, respectively.
The study authors reported that they have no relevant financial relationships.
FROM THE BRITISH MEDICAL JOURNAL
Large Population-Based Study Confirms Increased Risk of VTE With Antipsychotics
Antipsychotic drugs are associated with an almost one-third (32%) greater risk of venous thromboembolism, according to results of a nested case-control study of more than 100,000 primary care patients in the United Kingdom. The study was published online Sept. 21 in the British Medical Journal.
Previous research has suggested that these drugs – also commonly used for nausea, vomiting, and vertigo – might be linked with an increased risk of venous thromboembolism (VTE). However, the results have been inconsistent.
In the current study, the association was even greater for new users of antipsychotics. Those with any antipsychotic use in last 3 months had a 56% increased risk of VTE. Patients who started taking an antipsychotic in the past 3 months had a 97% increased risk. However, the absolute risks were low, with an excess of four extra cases of VTE per 10,000 patients treated over 1 year in patients of all ages and 10 for patients aged 65 years and older.
“Our study adds to the accumulating evidence of adverse health events associated with antipsychotic drugs,” the researchers wrote (BMJ 2010 Sept. 21[doi:10.1136/bmj.c4245]). “If other studies replicate these findings, antipsychotic drugs should be used more cautiously for nausea and agitation, etc., especially among patients at high risk of thromboembolism.”
The researchers used data from the QResearch database, which includes primary care clinical records for more than 11 million people registered in the past 16 years at more than 500 U.K. general practices. The study population was an open cohort of patients aged 16-100 years, who were registered with participating practices between January 1996 and July 2007. Case patients had a first-ever record of VTE – either deep vein thrombosis or pulmonary embolism – during the study period (including postmortem diagnoses).
Incidence density sampling was used to identify up to four control patients for each case patient. Control patients were matched by age, calendar time, sex, and practice. Control patients had no diagnoses of VTE prior to the date of the first recorded diagnosis of VTE in their matched case patient (index date).
Both case and control patients had to have at least 24 months of data before the index date. Control patients were excluded if they had any prescriptions for warfarin prior to the index date. Case patients with any use of warfarin earlier than 6 weeks prior to VTE diagnosis were also excluded, according to Chris Parker, a medical statistician at Hucknall Health Centre in Nottingham, England, and colleagues.
Each patient was classified for exposure to antipsychotics as a current user (one or more prescriptions within 3 months before the index date), a recent user (prescriptions within 4-12 months before the index date), a past user (prescriptions within 13-24 months before the index date), or not exposed within the previous 24 months.
Current users were subclassified as new users (a prescription for antipsychotic within 3 months of the index date, after at least 12 months with no prescription for that antipsychotic) or as continuing users. Exposure during the previous 24 months also was classified according by type (conventional, atypical, or both) and potency (high potency, low potency, or both). High potency was defined as equivalent dose of more than 100 mg chlorpromazine.
Patients with more than one mental health indication were categorized according to a hierarchy: schizophrenia, bipolar disorder without schizophrenia, and dementia without schizophrenia or bipolar disorder.
The analyses were adjusted for socioeconomic status, comorbidities (coronary heart disease, cardiac failure, stroke, cancer, inflammatory bowel disease, liver disease, varicose veins, gastrointestinal bleed, Parkinson’s disease, renal disease, asthma, diabetes, hypertension, hyperlipidemia), drug variables, and the number of complete months of data before the index date.
In addition, data were extracted for 6 months prior to the index date for several events that are associated with increased risk in the short term (hip surgery, hip or lower limb fractures, acute infections, pregnancy). The researchers also looked for whether there was any computer-recorded evidence of a hospital admission in the preceding 31-183 days.
In all, 25,532 eligible case patients – 15,975 with DVT and 9,557 with pulmonary embolism – and 89,491 control patients were included in the study. In terms of mental health disorders, the overall prevalence was 0.4% for schizophrenia, 0.3% for bipolar disorder, and 1.0% for dementia. Eight case patients and 31 control patients had more than one disorder. In all, 8.3% of case patients and 5.3% of control patients had received an antipsychotic in the previous 24 months.
Overall, antipsychotic users had a 32% greater risk of VTE than did nonusers. Among those on antipsychotics, 38% were current users and their increase in risk was 56% compared with 36% for recent users. The risk was not significantly increased for past users. Among current antipsychotic users, 15% had started a new drug within the 3 months prior to the index date. This group of new users showed a greater increase in risk (97%) than did continuing users (29%).
Patients who were prescribed atypical antipsychotic drugs had a greater risk of VTE than did those who were prescribed conventional antipsychotics – 73% compared with 28%. Patients who had received only one prescription in the previous 12 months had a significantly greater risk (32%) than did those receiving none. In addition, those who were prescribed two or more different antipsychotics had a greater risk (99%) than did those who received only one (29%).
The most commonly prescribed drug was prochlorperazine – a high-potency phenothiazine – which also is commonly prescribed for nausea, vomiting, and vertigo. Prochlorperazine was prescribed for 75% of the total number of those on antipsychotics. The other most commonly prescribed drugs were (in order) risperidone, haloperidol, olanzapine, chlorpromazine, trifluoperazine, and quetiapine. Separate odds ratios were estimated for exposure to these drugs, with highest risks for those patients who were prescribed quetiapine, chlorpromazine, and haloperidol, with adjusted odds ratios of 2.81, 1.77, and 2.17, respectively.
Individuals with a diagnosis of dementia were at higher risk than were those with diagnoses of schizophrenia, bipolar disorder, or none of these conditions. There was a more than threefold increase associated with cancer and a roughly 13-fold increase associated with recent surgery or fractures. “Individuals prescribed statins or aspirin in the past 24 months had a lower risk of venous thromboembolism, and those prescribed NSAIDs, oral contraceptives, hormone replacement therapy, tamoxifen, or antimanics had a higher risk,” the authors noted.
The number needed for harm for any antipsychotic use in the past 24 months for patients aged 65 years and older was 1,044; for new users in the past 3 months this number was 344; and for continuing users it was 1,152. The corresponding numbers of excess cases of VTE per 10,000 treated patients were 10, 29, and 9, respectively.
The study authors reported that they have no relevant financial relationships.
The validity of the findings is strengthened by the large sample size and the low potential for exposure and outcome misclassification because of the detailed source of data and adjustment for a large number of confounders, according to Dr. Rosa Liperoti and Dr. Giovanni Gambassi.
It’s been demonstrated that “patients with schizophrenia have an increased risk of venous thromboembolism (VTE), and this might be associated with the use of antipsychotics, especially low-potency drugs such as chlorpromazine and thioridazine. So far, however, the possibility that the underlying psychiatric disorders themselves – and not the antipsychotics – are associated with VTE has never been excluded. This could occur, for example, by the increased concentrations of adrenaline seen during psychotic excitation increasing blood coagulation,” they noted.
In this study, in almost all cases the reason for prescription of antipsychotics could not be ascertained. Most of the antipsychotics used were conventional agents, with prochlorperazine – probably given for nausea and vomiting – accounting for almost 80% of all prescriptions, they wrote. “These findings indicate that VTE is directly linked to the use of an antipsychotic, and that the risk of VTE increases early after starting the drug.”
The implications are potentially far reaching. “Despite their association with serious risks and few data to support their efficacy, antipsychotics are widely used, and in 2008 they became the top selling drug class in the United States. Despite efforts to improve non–drug based interventions, antipsychotics are often used, especially for the treatment of agitation in people with dementia,” they wrote.
The findings suggest that physicians “need to be able to identify the best candidates for antipsychotic treatment, such as those people with the lowest vascular risk profile who may respond to short-term and low-dose treatment with antipsychotics because of individual pharmacogenetic characteristics, and those who may be more susceptible to developing side effects as a result of individual vascular risk factors possibly interacting with antipsychotics.”
Dr. Rosa Liperoti is a specialist in geriatrics and Dr. Giovanni Gambassi is a professor of geriatrics at Universit? Cattolica del Sacro Cuore in Rome. Both Dr. Liperoti and Dr. Gambassi reported that they have no relevant financial relationships. These comments were taken from a commentary that accompanied the study (BMJ 2010 Sept. 21[doi: 10.1136/bmj.c4216]).
The validity of the findings is strengthened by the large sample size and the low potential for exposure and outcome misclassification because of the detailed source of data and adjustment for a large number of confounders, according to Dr. Rosa Liperoti and Dr. Giovanni Gambassi.
It’s been demonstrated that “patients with schizophrenia have an increased risk of venous thromboembolism (VTE), and this might be associated with the use of antipsychotics, especially low-potency drugs such as chlorpromazine and thioridazine. So far, however, the possibility that the underlying psychiatric disorders themselves – and not the antipsychotics – are associated with VTE has never been excluded. This could occur, for example, by the increased concentrations of adrenaline seen during psychotic excitation increasing blood coagulation,” they noted.
In this study, in almost all cases the reason for prescription of antipsychotics could not be ascertained. Most of the antipsychotics used were conventional agents, with prochlorperazine – probably given for nausea and vomiting – accounting for almost 80% of all prescriptions, they wrote. “These findings indicate that VTE is directly linked to the use of an antipsychotic, and that the risk of VTE increases early after starting the drug.”
The implications are potentially far reaching. “Despite their association with serious risks and few data to support their efficacy, antipsychotics are widely used, and in 2008 they became the top selling drug class in the United States. Despite efforts to improve non–drug based interventions, antipsychotics are often used, especially for the treatment of agitation in people with dementia,” they wrote.
The findings suggest that physicians “need to be able to identify the best candidates for antipsychotic treatment, such as those people with the lowest vascular risk profile who may respond to short-term and low-dose treatment with antipsychotics because of individual pharmacogenetic characteristics, and those who may be more susceptible to developing side effects as a result of individual vascular risk factors possibly interacting with antipsychotics.”
Dr. Rosa Liperoti is a specialist in geriatrics and Dr. Giovanni Gambassi is a professor of geriatrics at Universit? Cattolica del Sacro Cuore in Rome. Both Dr. Liperoti and Dr. Gambassi reported that they have no relevant financial relationships. These comments were taken from a commentary that accompanied the study (BMJ 2010 Sept. 21[doi: 10.1136/bmj.c4216]).
The validity of the findings is strengthened by the large sample size and the low potential for exposure and outcome misclassification because of the detailed source of data and adjustment for a large number of confounders, according to Dr. Rosa Liperoti and Dr. Giovanni Gambassi.
It’s been demonstrated that “patients with schizophrenia have an increased risk of venous thromboembolism (VTE), and this might be associated with the use of antipsychotics, especially low-potency drugs such as chlorpromazine and thioridazine. So far, however, the possibility that the underlying psychiatric disorders themselves – and not the antipsychotics – are associated with VTE has never been excluded. This could occur, for example, by the increased concentrations of adrenaline seen during psychotic excitation increasing blood coagulation,” they noted.
In this study, in almost all cases the reason for prescription of antipsychotics could not be ascertained. Most of the antipsychotics used were conventional agents, with prochlorperazine – probably given for nausea and vomiting – accounting for almost 80% of all prescriptions, they wrote. “These findings indicate that VTE is directly linked to the use of an antipsychotic, and that the risk of VTE increases early after starting the drug.”
The implications are potentially far reaching. “Despite their association with serious risks and few data to support their efficacy, antipsychotics are widely used, and in 2008 they became the top selling drug class in the United States. Despite efforts to improve non–drug based interventions, antipsychotics are often used, especially for the treatment of agitation in people with dementia,” they wrote.
The findings suggest that physicians “need to be able to identify the best candidates for antipsychotic treatment, such as those people with the lowest vascular risk profile who may respond to short-term and low-dose treatment with antipsychotics because of individual pharmacogenetic characteristics, and those who may be more susceptible to developing side effects as a result of individual vascular risk factors possibly interacting with antipsychotics.”
Dr. Rosa Liperoti is a specialist in geriatrics and Dr. Giovanni Gambassi is a professor of geriatrics at Universit? Cattolica del Sacro Cuore in Rome. Both Dr. Liperoti and Dr. Gambassi reported that they have no relevant financial relationships. These comments were taken from a commentary that accompanied the study (BMJ 2010 Sept. 21[doi: 10.1136/bmj.c4216]).
Antipsychotic drugs are associated with an almost one-third (32%) greater risk of venous thromboembolism, according to results of a nested case-control study of more than 100,000 primary care patients in the United Kingdom. The study was published online Sept. 21 in the British Medical Journal.
Previous research has suggested that these drugs – also commonly used for nausea, vomiting, and vertigo – might be linked with an increased risk of venous thromboembolism (VTE). However, the results have been inconsistent.
In the current study, the association was even greater for new users of antipsychotics. Those with any antipsychotic use in last 3 months had a 56% increased risk of VTE. Patients who started taking an antipsychotic in the past 3 months had a 97% increased risk. However, the absolute risks were low, with an excess of four extra cases of VTE per 10,000 patients treated over 1 year in patients of all ages and 10 for patients aged 65 years and older.
“Our study adds to the accumulating evidence of adverse health events associated with antipsychotic drugs,” the researchers wrote (BMJ 2010 Sept. 21[doi:10.1136/bmj.c4245]). “If other studies replicate these findings, antipsychotic drugs should be used more cautiously for nausea and agitation, etc., especially among patients at high risk of thromboembolism.”
The researchers used data from the QResearch database, which includes primary care clinical records for more than 11 million people registered in the past 16 years at more than 500 U.K. general practices. The study population was an open cohort of patients aged 16-100 years, who were registered with participating practices between January 1996 and July 2007. Case patients had a first-ever record of VTE – either deep vein thrombosis or pulmonary embolism – during the study period (including postmortem diagnoses).
Incidence density sampling was used to identify up to four control patients for each case patient. Control patients were matched by age, calendar time, sex, and practice. Control patients had no diagnoses of VTE prior to the date of the first recorded diagnosis of VTE in their matched case patient (index date).
Both case and control patients had to have at least 24 months of data before the index date. Control patients were excluded if they had any prescriptions for warfarin prior to the index date. Case patients with any use of warfarin earlier than 6 weeks prior to VTE diagnosis were also excluded, according to Chris Parker, a medical statistician at Hucknall Health Centre in Nottingham, England, and colleagues.
Each patient was classified for exposure to antipsychotics as a current user (one or more prescriptions within 3 months before the index date), a recent user (prescriptions within 4-12 months before the index date), a past user (prescriptions within 13-24 months before the index date), or not exposed within the previous 24 months.
Current users were subclassified as new users (a prescription for antipsychotic within 3 months of the index date, after at least 12 months with no prescription for that antipsychotic) or as continuing users. Exposure during the previous 24 months also was classified according by type (conventional, atypical, or both) and potency (high potency, low potency, or both). High potency was defined as equivalent dose of more than 100 mg chlorpromazine.
Patients with more than one mental health indication were categorized according to a hierarchy: schizophrenia, bipolar disorder without schizophrenia, and dementia without schizophrenia or bipolar disorder.
The analyses were adjusted for socioeconomic status, comorbidities (coronary heart disease, cardiac failure, stroke, cancer, inflammatory bowel disease, liver disease, varicose veins, gastrointestinal bleed, Parkinson’s disease, renal disease, asthma, diabetes, hypertension, hyperlipidemia), drug variables, and the number of complete months of data before the index date.
In addition, data were extracted for 6 months prior to the index date for several events that are associated with increased risk in the short term (hip surgery, hip or lower limb fractures, acute infections, pregnancy). The researchers also looked for whether there was any computer-recorded evidence of a hospital admission in the preceding 31-183 days.
In all, 25,532 eligible case patients – 15,975 with DVT and 9,557 with pulmonary embolism – and 89,491 control patients were included in the study. In terms of mental health disorders, the overall prevalence was 0.4% for schizophrenia, 0.3% for bipolar disorder, and 1.0% for dementia. Eight case patients and 31 control patients had more than one disorder. In all, 8.3% of case patients and 5.3% of control patients had received an antipsychotic in the previous 24 months.
Overall, antipsychotic users had a 32% greater risk of VTE than did nonusers. Among those on antipsychotics, 38% were current users and their increase in risk was 56% compared with 36% for recent users. The risk was not significantly increased for past users. Among current antipsychotic users, 15% had started a new drug within the 3 months prior to the index date. This group of new users showed a greater increase in risk (97%) than did continuing users (29%).
Patients who were prescribed atypical antipsychotic drugs had a greater risk of VTE than did those who were prescribed conventional antipsychotics – 73% compared with 28%. Patients who had received only one prescription in the previous 12 months had a significantly greater risk (32%) than did those receiving none. In addition, those who were prescribed two or more different antipsychotics had a greater risk (99%) than did those who received only one (29%).
The most commonly prescribed drug was prochlorperazine – a high-potency phenothiazine – which also is commonly prescribed for nausea, vomiting, and vertigo. Prochlorperazine was prescribed for 75% of the total number of those on antipsychotics. The other most commonly prescribed drugs were (in order) risperidone, haloperidol, olanzapine, chlorpromazine, trifluoperazine, and quetiapine. Separate odds ratios were estimated for exposure to these drugs, with highest risks for those patients who were prescribed quetiapine, chlorpromazine, and haloperidol, with adjusted odds ratios of 2.81, 1.77, and 2.17, respectively.
Individuals with a diagnosis of dementia were at higher risk than were those with diagnoses of schizophrenia, bipolar disorder, or none of these conditions. There was a more than threefold increase associated with cancer and a roughly 13-fold increase associated with recent surgery or fractures. “Individuals prescribed statins or aspirin in the past 24 months had a lower risk of venous thromboembolism, and those prescribed NSAIDs, oral contraceptives, hormone replacement therapy, tamoxifen, or antimanics had a higher risk,” the authors noted.
The number needed for harm for any antipsychotic use in the past 24 months for patients aged 65 years and older was 1,044; for new users in the past 3 months this number was 344; and for continuing users it was 1,152. The corresponding numbers of excess cases of VTE per 10,000 treated patients were 10, 29, and 9, respectively.
The study authors reported that they have no relevant financial relationships.
Antipsychotic drugs are associated with an almost one-third (32%) greater risk of venous thromboembolism, according to results of a nested case-control study of more than 100,000 primary care patients in the United Kingdom. The study was published online Sept. 21 in the British Medical Journal.
Previous research has suggested that these drugs – also commonly used for nausea, vomiting, and vertigo – might be linked with an increased risk of venous thromboembolism (VTE). However, the results have been inconsistent.
In the current study, the association was even greater for new users of antipsychotics. Those with any antipsychotic use in last 3 months had a 56% increased risk of VTE. Patients who started taking an antipsychotic in the past 3 months had a 97% increased risk. However, the absolute risks were low, with an excess of four extra cases of VTE per 10,000 patients treated over 1 year in patients of all ages and 10 for patients aged 65 years and older.
“Our study adds to the accumulating evidence of adverse health events associated with antipsychotic drugs,” the researchers wrote (BMJ 2010 Sept. 21[doi:10.1136/bmj.c4245]). “If other studies replicate these findings, antipsychotic drugs should be used more cautiously for nausea and agitation, etc., especially among patients at high risk of thromboembolism.”
The researchers used data from the QResearch database, which includes primary care clinical records for more than 11 million people registered in the past 16 years at more than 500 U.K. general practices. The study population was an open cohort of patients aged 16-100 years, who were registered with participating practices between January 1996 and July 2007. Case patients had a first-ever record of VTE – either deep vein thrombosis or pulmonary embolism – during the study period (including postmortem diagnoses).
Incidence density sampling was used to identify up to four control patients for each case patient. Control patients were matched by age, calendar time, sex, and practice. Control patients had no diagnoses of VTE prior to the date of the first recorded diagnosis of VTE in their matched case patient (index date).
Both case and control patients had to have at least 24 months of data before the index date. Control patients were excluded if they had any prescriptions for warfarin prior to the index date. Case patients with any use of warfarin earlier than 6 weeks prior to VTE diagnosis were also excluded, according to Chris Parker, a medical statistician at Hucknall Health Centre in Nottingham, England, and colleagues.
Each patient was classified for exposure to antipsychotics as a current user (one or more prescriptions within 3 months before the index date), a recent user (prescriptions within 4-12 months before the index date), a past user (prescriptions within 13-24 months before the index date), or not exposed within the previous 24 months.
Current users were subclassified as new users (a prescription for antipsychotic within 3 months of the index date, after at least 12 months with no prescription for that antipsychotic) or as continuing users. Exposure during the previous 24 months also was classified according by type (conventional, atypical, or both) and potency (high potency, low potency, or both). High potency was defined as equivalent dose of more than 100 mg chlorpromazine.
Patients with more than one mental health indication were categorized according to a hierarchy: schizophrenia, bipolar disorder without schizophrenia, and dementia without schizophrenia or bipolar disorder.
The analyses were adjusted for socioeconomic status, comorbidities (coronary heart disease, cardiac failure, stroke, cancer, inflammatory bowel disease, liver disease, varicose veins, gastrointestinal bleed, Parkinson’s disease, renal disease, asthma, diabetes, hypertension, hyperlipidemia), drug variables, and the number of complete months of data before the index date.
In addition, data were extracted for 6 months prior to the index date for several events that are associated with increased risk in the short term (hip surgery, hip or lower limb fractures, acute infections, pregnancy). The researchers also looked for whether there was any computer-recorded evidence of a hospital admission in the preceding 31-183 days.
In all, 25,532 eligible case patients – 15,975 with DVT and 9,557 with pulmonary embolism – and 89,491 control patients were included in the study. In terms of mental health disorders, the overall prevalence was 0.4% for schizophrenia, 0.3% for bipolar disorder, and 1.0% for dementia. Eight case patients and 31 control patients had more than one disorder. In all, 8.3% of case patients and 5.3% of control patients had received an antipsychotic in the previous 24 months.
Overall, antipsychotic users had a 32% greater risk of VTE than did nonusers. Among those on antipsychotics, 38% were current users and their increase in risk was 56% compared with 36% for recent users. The risk was not significantly increased for past users. Among current antipsychotic users, 15% had started a new drug within the 3 months prior to the index date. This group of new users showed a greater increase in risk (97%) than did continuing users (29%).
Patients who were prescribed atypical antipsychotic drugs had a greater risk of VTE than did those who were prescribed conventional antipsychotics – 73% compared with 28%. Patients who had received only one prescription in the previous 12 months had a significantly greater risk (32%) than did those receiving none. In addition, those who were prescribed two or more different antipsychotics had a greater risk (99%) than did those who received only one (29%).
The most commonly prescribed drug was prochlorperazine – a high-potency phenothiazine – which also is commonly prescribed for nausea, vomiting, and vertigo. Prochlorperazine was prescribed for 75% of the total number of those on antipsychotics. The other most commonly prescribed drugs were (in order) risperidone, haloperidol, olanzapine, chlorpromazine, trifluoperazine, and quetiapine. Separate odds ratios were estimated for exposure to these drugs, with highest risks for those patients who were prescribed quetiapine, chlorpromazine, and haloperidol, with adjusted odds ratios of 2.81, 1.77, and 2.17, respectively.
Individuals with a diagnosis of dementia were at higher risk than were those with diagnoses of schizophrenia, bipolar disorder, or none of these conditions. There was a more than threefold increase associated with cancer and a roughly 13-fold increase associated with recent surgery or fractures. “Individuals prescribed statins or aspirin in the past 24 months had a lower risk of venous thromboembolism, and those prescribed NSAIDs, oral contraceptives, hormone replacement therapy, tamoxifen, or antimanics had a higher risk,” the authors noted.
The number needed for harm for any antipsychotic use in the past 24 months for patients aged 65 years and older was 1,044; for new users in the past 3 months this number was 344; and for continuing users it was 1,152. The corresponding numbers of excess cases of VTE per 10,000 treated patients were 10, 29, and 9, respectively.
The study authors reported that they have no relevant financial relationships.
FROM THE BRITISH MEDICAL JOURNAL
Major Finding: Patients taking antipsychotics had a 32% greater risk of venous thromboembolism, compared with those not on the drugs.
Data Source: A nested case-control study of 115,023 primary care patients in the United Kingdom.
Disclosures: The authors reported that they have no relevant financial relationships.
Teens' Experience With Acne Varies by Ethnicity, Race
Acne severity, frequency, and beliefs about the effect of external factors, such as cleansing habits, all play a part in how likely adolescents are to seek help for their acne.
The findings come from a survey of more than 1,000 middle and high school–aged teens in New Jersey. The study was published online in the journal Pediatric Dermatology (2010 Aug. 26 [doi:10.1111/j.1525-1470.2010.01286.x]).
"Our study demonstrated that grade of acne, knowledge, and beliefs play a role in help-seeking behaviors, which vary to a significant extent by race and ethnicity," wrote Carol E. Cheng and her coinvestigators. Ms. Cheng is a medical student at Boston University. "Better education about how to manage their acne effectively, and guiding appropriate patients to health care providers, may be important ways to decrease the risk of racial disparities in acne morbidity."
Students at five middle schools and three high schools in New Jersey were asked to complete an anonymous survey, in which they were asked questions about their acne severity, frequency, treatment, beliefs about external factors affecting acne, and perception of the psychosocial impact of acne. Students self-reported demographic information on age, gender, grade, ethnicity (Hispanic, not Hispanic, don't know), and race (American Indian, Native Hawaiian, white, black/African American, more than one race, or unknown).
A total of 1,214 students in grades 6-12 completed the survey; their ages ranged from 10 to 18 years. Roughly half (52%) were male. Almost a quarter of the students (24%) were Hispanic, 40% were white, 14% were Asian, and 10% were black.
Students were asked to rate their acne frequency as never, rarely, sometimes, often, or always. Roughly three-quarters (76%) reported that they had acne of some frequency. They were also asked to rate the severity of their acne as none, mild, moderate, or severe. In all, 65% reported that they had acne of some severity.
"The largest differences appeared in the frequency and severity of black respondents, compared with other races, which suggest that this population may be less affected or may perceive themselves to be less affected," the investigators wrote. Black students were 14% less likely than nonblack students to report acne of any frequency. Similarly, black students were 18% less likely than white students to report acne of any severity.
Participants were also asked to report whether they visited a pediatrician, dermatologist, or another type of health professional, or had never seen a health professional for their acne. Most (83%) reported they had not seen a health professional for their acne. This value was similar across ethnicities and races.
Self-reported acne had an impact on the likelihood of respondents having seen a physician and also on the type of physician seen. Students who reported having acne of some severity were more likely to have seen a physician, compared with those who reported not having acne (21% vs. 8%, respectively). Increased acne severity corresponded with increased likelihood of seeing a dermatologist.
Black students with mild or moderate acne were more likely to have seen a health care professional for their acne, compared with white students (relative risk 3.63 and 3.06, respectively). In contrast, Hispanic students with mild or moderate acne were less likely to see a health professional for their acne, compared with non-Hispanic students (RR 0.56 and 0.47, respectively).
Students were also asked if they treated their own acne, followed a health professional's advice, did both, or did something else. There were no significant differences by race or ethnicity with regard to treatment advice.
The researchers asked the students to rate how much they believed that certain factors affected their acne. These included poor cleansing habits, poor dietary habits, decreased sleep, increased stress, increased exercise, increased drinking of water, increased face touching, increased face washing, increased tanning, and pimple popping.
A majority of students believed that the following factors worsened acne: poor cleansing habits (77%), poor dietary habits (56%), stress (60%), increased face touching (70%), and pimple popping (70%).
"Notably, black respondents in 6/10 cases were least likely to believe that a given factor worsened acne relative to other groups, whereas Asian respondents in 7/10 cases were more likely to believe that a given factor worsened acne relative to other groups," the researchers noted.
White students were more likely to have seen a health care professional and follow a treatment suggestion from a physician if they believed external factors affected acne. Black students who believed external factors affected acne were also more likely to follow a treatment suggestion from a physician. "Notably, Asian respondents did not attribute their help-seeking behavior or treatment decisions to any external factors," the investigators reported.
Disclosures: The investigators did not report whether they had any relevant financial relationships.
Acne severity, frequency, and beliefs about the effect of external factors, such as cleansing habits, all play a part in how likely adolescents are to seek help for their acne.
The findings come from a survey of more than 1,000 middle and high school–aged teens in New Jersey. The study was published online in the journal Pediatric Dermatology (2010 Aug. 26 [doi:10.1111/j.1525-1470.2010.01286.x]).
"Our study demonstrated that grade of acne, knowledge, and beliefs play a role in help-seeking behaviors, which vary to a significant extent by race and ethnicity," wrote Carol E. Cheng and her coinvestigators. Ms. Cheng is a medical student at Boston University. "Better education about how to manage their acne effectively, and guiding appropriate patients to health care providers, may be important ways to decrease the risk of racial disparities in acne morbidity."
Students at five middle schools and three high schools in New Jersey were asked to complete an anonymous survey, in which they were asked questions about their acne severity, frequency, treatment, beliefs about external factors affecting acne, and perception of the psychosocial impact of acne. Students self-reported demographic information on age, gender, grade, ethnicity (Hispanic, not Hispanic, don't know), and race (American Indian, Native Hawaiian, white, black/African American, more than one race, or unknown).
A total of 1,214 students in grades 6-12 completed the survey; their ages ranged from 10 to 18 years. Roughly half (52%) were male. Almost a quarter of the students (24%) were Hispanic, 40% were white, 14% were Asian, and 10% were black.
Students were asked to rate their acne frequency as never, rarely, sometimes, often, or always. Roughly three-quarters (76%) reported that they had acne of some frequency. They were also asked to rate the severity of their acne as none, mild, moderate, or severe. In all, 65% reported that they had acne of some severity.
"The largest differences appeared in the frequency and severity of black respondents, compared with other races, which suggest that this population may be less affected or may perceive themselves to be less affected," the investigators wrote. Black students were 14% less likely than nonblack students to report acne of any frequency. Similarly, black students were 18% less likely than white students to report acne of any severity.
Participants were also asked to report whether they visited a pediatrician, dermatologist, or another type of health professional, or had never seen a health professional for their acne. Most (83%) reported they had not seen a health professional for their acne. This value was similar across ethnicities and races.
Self-reported acne had an impact on the likelihood of respondents having seen a physician and also on the type of physician seen. Students who reported having acne of some severity were more likely to have seen a physician, compared with those who reported not having acne (21% vs. 8%, respectively). Increased acne severity corresponded with increased likelihood of seeing a dermatologist.
Black students with mild or moderate acne were more likely to have seen a health care professional for their acne, compared with white students (relative risk 3.63 and 3.06, respectively). In contrast, Hispanic students with mild or moderate acne were less likely to see a health professional for their acne, compared with non-Hispanic students (RR 0.56 and 0.47, respectively).
Students were also asked if they treated their own acne, followed a health professional's advice, did both, or did something else. There were no significant differences by race or ethnicity with regard to treatment advice.
The researchers asked the students to rate how much they believed that certain factors affected their acne. These included poor cleansing habits, poor dietary habits, decreased sleep, increased stress, increased exercise, increased drinking of water, increased face touching, increased face washing, increased tanning, and pimple popping.
A majority of students believed that the following factors worsened acne: poor cleansing habits (77%), poor dietary habits (56%), stress (60%), increased face touching (70%), and pimple popping (70%).
"Notably, black respondents in 6/10 cases were least likely to believe that a given factor worsened acne relative to other groups, whereas Asian respondents in 7/10 cases were more likely to believe that a given factor worsened acne relative to other groups," the researchers noted.
White students were more likely to have seen a health care professional and follow a treatment suggestion from a physician if they believed external factors affected acne. Black students who believed external factors affected acne were also more likely to follow a treatment suggestion from a physician. "Notably, Asian respondents did not attribute their help-seeking behavior or treatment decisions to any external factors," the investigators reported.
Disclosures: The investigators did not report whether they had any relevant financial relationships.
Acne severity, frequency, and beliefs about the effect of external factors, such as cleansing habits, all play a part in how likely adolescents are to seek help for their acne.
The findings come from a survey of more than 1,000 middle and high school–aged teens in New Jersey. The study was published online in the journal Pediatric Dermatology (2010 Aug. 26 [doi:10.1111/j.1525-1470.2010.01286.x]).
"Our study demonstrated that grade of acne, knowledge, and beliefs play a role in help-seeking behaviors, which vary to a significant extent by race and ethnicity," wrote Carol E. Cheng and her coinvestigators. Ms. Cheng is a medical student at Boston University. "Better education about how to manage their acne effectively, and guiding appropriate patients to health care providers, may be important ways to decrease the risk of racial disparities in acne morbidity."
Students at five middle schools and three high schools in New Jersey were asked to complete an anonymous survey, in which they were asked questions about their acne severity, frequency, treatment, beliefs about external factors affecting acne, and perception of the psychosocial impact of acne. Students self-reported demographic information on age, gender, grade, ethnicity (Hispanic, not Hispanic, don't know), and race (American Indian, Native Hawaiian, white, black/African American, more than one race, or unknown).
A total of 1,214 students in grades 6-12 completed the survey; their ages ranged from 10 to 18 years. Roughly half (52%) were male. Almost a quarter of the students (24%) were Hispanic, 40% were white, 14% were Asian, and 10% were black.
Students were asked to rate their acne frequency as never, rarely, sometimes, often, or always. Roughly three-quarters (76%) reported that they had acne of some frequency. They were also asked to rate the severity of their acne as none, mild, moderate, or severe. In all, 65% reported that they had acne of some severity.
"The largest differences appeared in the frequency and severity of black respondents, compared with other races, which suggest that this population may be less affected or may perceive themselves to be less affected," the investigators wrote. Black students were 14% less likely than nonblack students to report acne of any frequency. Similarly, black students were 18% less likely than white students to report acne of any severity.
Participants were also asked to report whether they visited a pediatrician, dermatologist, or another type of health professional, or had never seen a health professional for their acne. Most (83%) reported they had not seen a health professional for their acne. This value was similar across ethnicities and races.
Self-reported acne had an impact on the likelihood of respondents having seen a physician and also on the type of physician seen. Students who reported having acne of some severity were more likely to have seen a physician, compared with those who reported not having acne (21% vs. 8%, respectively). Increased acne severity corresponded with increased likelihood of seeing a dermatologist.
Black students with mild or moderate acne were more likely to have seen a health care professional for their acne, compared with white students (relative risk 3.63 and 3.06, respectively). In contrast, Hispanic students with mild or moderate acne were less likely to see a health professional for their acne, compared with non-Hispanic students (RR 0.56 and 0.47, respectively).
Students were also asked if they treated their own acne, followed a health professional's advice, did both, or did something else. There were no significant differences by race or ethnicity with regard to treatment advice.
The researchers asked the students to rate how much they believed that certain factors affected their acne. These included poor cleansing habits, poor dietary habits, decreased sleep, increased stress, increased exercise, increased drinking of water, increased face touching, increased face washing, increased tanning, and pimple popping.
A majority of students believed that the following factors worsened acne: poor cleansing habits (77%), poor dietary habits (56%), stress (60%), increased face touching (70%), and pimple popping (70%).
"Notably, black respondents in 6/10 cases were least likely to believe that a given factor worsened acne relative to other groups, whereas Asian respondents in 7/10 cases were more likely to believe that a given factor worsened acne relative to other groups," the researchers noted.
White students were more likely to have seen a health care professional and follow a treatment suggestion from a physician if they believed external factors affected acne. Black students who believed external factors affected acne were also more likely to follow a treatment suggestion from a physician. "Notably, Asian respondents did not attribute their help-seeking behavior or treatment decisions to any external factors," the investigators reported.
Disclosures: The investigators did not report whether they had any relevant financial relationships.
Hypofractionated Irradiation Endorsed
A shorter course of hypofractionated whole-breast irradiation may be substituted for treatment with conventional fractions following breast-conserving surgery in selected patients with early-stage breast cancer.
The new recommendation comes from an evidence-based guideline published by the American Society for Radiation Oncology (Int. J. Radiat. Oncol. Biol. Phys. [doi:10.1016/j.ijrobp.2010.04.042]).
The guideline task force concluded that hypofractionated whole-breast irradiation is just as effective as conventional fractions for women who are at least 50 years old at diagnosis and meet all of the following criteria:
▸ The pathologic stage is T1-2N0, and the patient has been treated with breast-conserving surgery.
▸ The patient has not been treated with systemic chemotherapy.
▸ The minimum dose is no less than 93%, and the maximum is no greater than 107% of the prescription dose within the breast along the central axis.
Hypofractionated whole-breast irradiation uses a higher radiation dose for each treatment, but fewer total treatments are necessary. Typically, patients can finish treatment in 4 weeks or less with hypofractionated radiation therapy.
This can help to minimize some of the inconvenience and expense associated with conventionally fractioned whole-breast irradiation, which involves daily treatments for up to 7 weeks.
The guideline authors reported that they have no conflicts of interest.
A shorter course of hypofractionated whole-breast irradiation may be substituted for treatment with conventional fractions following breast-conserving surgery in selected patients with early-stage breast cancer.
The new recommendation comes from an evidence-based guideline published by the American Society for Radiation Oncology (Int. J. Radiat. Oncol. Biol. Phys. [doi:10.1016/j.ijrobp.2010.04.042]).
The guideline task force concluded that hypofractionated whole-breast irradiation is just as effective as conventional fractions for women who are at least 50 years old at diagnosis and meet all of the following criteria:
▸ The pathologic stage is T1-2N0, and the patient has been treated with breast-conserving surgery.
▸ The patient has not been treated with systemic chemotherapy.
▸ The minimum dose is no less than 93%, and the maximum is no greater than 107% of the prescription dose within the breast along the central axis.
Hypofractionated whole-breast irradiation uses a higher radiation dose for each treatment, but fewer total treatments are necessary. Typically, patients can finish treatment in 4 weeks or less with hypofractionated radiation therapy.
This can help to minimize some of the inconvenience and expense associated with conventionally fractioned whole-breast irradiation, which involves daily treatments for up to 7 weeks.
The guideline authors reported that they have no conflicts of interest.
A shorter course of hypofractionated whole-breast irradiation may be substituted for treatment with conventional fractions following breast-conserving surgery in selected patients with early-stage breast cancer.
The new recommendation comes from an evidence-based guideline published by the American Society for Radiation Oncology (Int. J. Radiat. Oncol. Biol. Phys. [doi:10.1016/j.ijrobp.2010.04.042]).
The guideline task force concluded that hypofractionated whole-breast irradiation is just as effective as conventional fractions for women who are at least 50 years old at diagnosis and meet all of the following criteria:
▸ The pathologic stage is T1-2N0, and the patient has been treated with breast-conserving surgery.
▸ The patient has not been treated with systemic chemotherapy.
▸ The minimum dose is no less than 93%, and the maximum is no greater than 107% of the prescription dose within the breast along the central axis.
Hypofractionated whole-breast irradiation uses a higher radiation dose for each treatment, but fewer total treatments are necessary. Typically, patients can finish treatment in 4 weeks or less with hypofractionated radiation therapy.
This can help to minimize some of the inconvenience and expense associated with conventionally fractioned whole-breast irradiation, which involves daily treatments for up to 7 weeks.
The guideline authors reported that they have no conflicts of interest.
Postpartum Thyroiditis Presents Dx Challenges
MINNEAPOLIS — In classic-course postpartum thyroiditis, the hyperthyroid phase is often missed, and it's much more common to identify women in the hypothyroid phase that follows, Dr. Erin Keely said.
“In my experience we miss the hyperthyroid phase clinically all the time because it's a little bit like the army—don't ask, don't tell,” she said. The symptoms—anxiety, insomnia, fatigue, weight loss, and irritability—are normal for most new moms. The classic course of postpartum thyroiditis (PPT) is hyperthyroidism—which starts at about 6 weeks post partum and may last a few months—followed by hypothyroidism and then normalization.
More commonly, women present in the hypothyroid phase, which typically occurs 4-8 months after delivery and may last up to 9–12 months, said Dr. Keely, chief of endocrinology and metabolism at the Ottawa Hospital. Typical symptoms include fatigue, weight gain, constipation, dry skin, depression, and poor exercise tolerance. Women tested in the transition period might appear to have normal thyroid function, however. About 30% of women remain hyperthyroid.
However, because PPT can cause both thyrotoxicosis (high thyroid hormone levels in the blood) and hypothyroidism (low thyroid hormone levels in the blood) in the first year post partum, “you can't make a diagnosis of permanent thyroid disease” at that time, said Dr. Keely.
The exception is for any woman with overt thyroid disease before pregnancy.
To complicate matters, Graves' disease can flare in the postpartum period, making it difficult to tell if a woman is having a flare or PPT. However, Graves' disease is much less common than postpartum thyroiditis, said Dr. Keely. “So, if you were a betting person, you would bet that it's postpartum thyroiditis.”
If the diagnosis is really unclear, radioiodine uptake tests can be performed but most women will likely choose to wait and see, she said.
There are insufficient data currently to recommend screening all women for PPT, according to clinical guidelines from the Endocrine Society (J. Clin. Endocrinol. Metab. 2007;92:s1-s47 [doi:10.1210/jc.2007-0141]). Women who are known to be positive for autoantibodies to thyroid peroxidase (TPO-Ab) should have a thyroid-stimulating hormone (TSH) test performed at 3 and 6 months post partum.
Notably, the prevalence of PPT in women with type I diabetes is threefold greater than in the general population; postpartum screening (TSH test) is recommended for these women at 3 and 6 months post partum.
When it comes to treating PPT, “there is no one answer for all women,” said Dr. Keely. However, in 2002, Dr. Alex S. Stagnaro-Green, now the senior associate dean for education at George Washington University, Washington, proposed a treatment algorithm for PPT (J. Clin. Endocrinol. Metab. 2002;87:4042-7).
In this algorithm, treatment is indicated for a TSH level of 4 mU/mL or greater in the first year post partum.
Dr. Keely added that “one of the most important aspects is to continue the treatment until [the woman] has completed her family.”
If autoantibodies to thyroid peroxidase (TPO-Ab) are found in the first trimester, there is a 30%-55% risk of PPT. “It's interesting though, that 25% of women who are positive in the first trimester become negative by the third trimester,” she said.
Women may become negative for TPO-Ab by term but will rebound in the postpartum period. If TPO-Ab is positive in the third trimester, the risk of PPT is greater than 80%—but if negative the risk is only 0%-5%.
Importantly, “postpartum thyroiditis is a very strong predictor of long-term Hashimoto's thyroiditis,” said Dr. Keely. About 30% of these women develop Hashimoto's thyroiditis within 3 years.
Dr. Keely reported that she had no relevant financial relationships.
MINNEAPOLIS — In classic-course postpartum thyroiditis, the hyperthyroid phase is often missed, and it's much more common to identify women in the hypothyroid phase that follows, Dr. Erin Keely said.
“In my experience we miss the hyperthyroid phase clinically all the time because it's a little bit like the army—don't ask, don't tell,” she said. The symptoms—anxiety, insomnia, fatigue, weight loss, and irritability—are normal for most new moms. The classic course of postpartum thyroiditis (PPT) is hyperthyroidism—which starts at about 6 weeks post partum and may last a few months—followed by hypothyroidism and then normalization.
More commonly, women present in the hypothyroid phase, which typically occurs 4-8 months after delivery and may last up to 9–12 months, said Dr. Keely, chief of endocrinology and metabolism at the Ottawa Hospital. Typical symptoms include fatigue, weight gain, constipation, dry skin, depression, and poor exercise tolerance. Women tested in the transition period might appear to have normal thyroid function, however. About 30% of women remain hyperthyroid.
However, because PPT can cause both thyrotoxicosis (high thyroid hormone levels in the blood) and hypothyroidism (low thyroid hormone levels in the blood) in the first year post partum, “you can't make a diagnosis of permanent thyroid disease” at that time, said Dr. Keely.
The exception is for any woman with overt thyroid disease before pregnancy.
To complicate matters, Graves' disease can flare in the postpartum period, making it difficult to tell if a woman is having a flare or PPT. However, Graves' disease is much less common than postpartum thyroiditis, said Dr. Keely. “So, if you were a betting person, you would bet that it's postpartum thyroiditis.”
If the diagnosis is really unclear, radioiodine uptake tests can be performed but most women will likely choose to wait and see, she said.
There are insufficient data currently to recommend screening all women for PPT, according to clinical guidelines from the Endocrine Society (J. Clin. Endocrinol. Metab. 2007;92:s1-s47 [doi:10.1210/jc.2007-0141]). Women who are known to be positive for autoantibodies to thyroid peroxidase (TPO-Ab) should have a thyroid-stimulating hormone (TSH) test performed at 3 and 6 months post partum.
Notably, the prevalence of PPT in women with type I diabetes is threefold greater than in the general population; postpartum screening (TSH test) is recommended for these women at 3 and 6 months post partum.
When it comes to treating PPT, “there is no one answer for all women,” said Dr. Keely. However, in 2002, Dr. Alex S. Stagnaro-Green, now the senior associate dean for education at George Washington University, Washington, proposed a treatment algorithm for PPT (J. Clin. Endocrinol. Metab. 2002;87:4042-7).
In this algorithm, treatment is indicated for a TSH level of 4 mU/mL or greater in the first year post partum.
Dr. Keely added that “one of the most important aspects is to continue the treatment until [the woman] has completed her family.”
If autoantibodies to thyroid peroxidase (TPO-Ab) are found in the first trimester, there is a 30%-55% risk of PPT. “It's interesting though, that 25% of women who are positive in the first trimester become negative by the third trimester,” she said.
Women may become negative for TPO-Ab by term but will rebound in the postpartum period. If TPO-Ab is positive in the third trimester, the risk of PPT is greater than 80%—but if negative the risk is only 0%-5%.
Importantly, “postpartum thyroiditis is a very strong predictor of long-term Hashimoto's thyroiditis,” said Dr. Keely. About 30% of these women develop Hashimoto's thyroiditis within 3 years.
Dr. Keely reported that she had no relevant financial relationships.
MINNEAPOLIS — In classic-course postpartum thyroiditis, the hyperthyroid phase is often missed, and it's much more common to identify women in the hypothyroid phase that follows, Dr. Erin Keely said.
“In my experience we miss the hyperthyroid phase clinically all the time because it's a little bit like the army—don't ask, don't tell,” she said. The symptoms—anxiety, insomnia, fatigue, weight loss, and irritability—are normal for most new moms. The classic course of postpartum thyroiditis (PPT) is hyperthyroidism—which starts at about 6 weeks post partum and may last a few months—followed by hypothyroidism and then normalization.
More commonly, women present in the hypothyroid phase, which typically occurs 4-8 months after delivery and may last up to 9–12 months, said Dr. Keely, chief of endocrinology and metabolism at the Ottawa Hospital. Typical symptoms include fatigue, weight gain, constipation, dry skin, depression, and poor exercise tolerance. Women tested in the transition period might appear to have normal thyroid function, however. About 30% of women remain hyperthyroid.
However, because PPT can cause both thyrotoxicosis (high thyroid hormone levels in the blood) and hypothyroidism (low thyroid hormone levels in the blood) in the first year post partum, “you can't make a diagnosis of permanent thyroid disease” at that time, said Dr. Keely.
The exception is for any woman with overt thyroid disease before pregnancy.
To complicate matters, Graves' disease can flare in the postpartum period, making it difficult to tell if a woman is having a flare or PPT. However, Graves' disease is much less common than postpartum thyroiditis, said Dr. Keely. “So, if you were a betting person, you would bet that it's postpartum thyroiditis.”
If the diagnosis is really unclear, radioiodine uptake tests can be performed but most women will likely choose to wait and see, she said.
There are insufficient data currently to recommend screening all women for PPT, according to clinical guidelines from the Endocrine Society (J. Clin. Endocrinol. Metab. 2007;92:s1-s47 [doi:10.1210/jc.2007-0141]). Women who are known to be positive for autoantibodies to thyroid peroxidase (TPO-Ab) should have a thyroid-stimulating hormone (TSH) test performed at 3 and 6 months post partum.
Notably, the prevalence of PPT in women with type I diabetes is threefold greater than in the general population; postpartum screening (TSH test) is recommended for these women at 3 and 6 months post partum.
When it comes to treating PPT, “there is no one answer for all women,” said Dr. Keely. However, in 2002, Dr. Alex S. Stagnaro-Green, now the senior associate dean for education at George Washington University, Washington, proposed a treatment algorithm for PPT (J. Clin. Endocrinol. Metab. 2002;87:4042-7).
In this algorithm, treatment is indicated for a TSH level of 4 mU/mL or greater in the first year post partum.
Dr. Keely added that “one of the most important aspects is to continue the treatment until [the woman] has completed her family.”
If autoantibodies to thyroid peroxidase (TPO-Ab) are found in the first trimester, there is a 30%-55% risk of PPT. “It's interesting though, that 25% of women who are positive in the first trimester become negative by the third trimester,” she said.
Women may become negative for TPO-Ab by term but will rebound in the postpartum period. If TPO-Ab is positive in the third trimester, the risk of PPT is greater than 80%—but if negative the risk is only 0%-5%.
Importantly, “postpartum thyroiditis is a very strong predictor of long-term Hashimoto's thyroiditis,” said Dr. Keely. About 30% of these women develop Hashimoto's thyroiditis within 3 years.
Dr. Keely reported that she had no relevant financial relationships.
FDA Panel Recommends That Dextromethorphan Not Be a Scheduled Drug
COLLEGE PARK, Md. – A Food and Drug Administration panel on Sept. 14 voted 15 to 9 against requiring that the cough suppressant dextromethorphan be scheduled under the Controlled Substances Act.
The drug is used in more than 100 over-the-counter cough and cold products available in a wide range of retail outlets. Those voting against scheduling cited concerns about limiting access to the drug, which can be effective for coughs.
The panel was asked to consider the need to schedule dextromethorphan based on a request from the Drug Enforcement Administration for a scientific and medical evaluation and scheduling recommendation. Dextromethorphan abuse has been an ongoing concern for both the FDA and the DEA, starting in the early 1990s. The dextromethrophan-related deaths of five teenagers from the states of Washington, Florida, and Virginia brought the problem to back to national attention in 2005. In each case, death was determined to be the direct result of the toxic effects of dextromethorphan powder. Other case reports of abuse and the effects of overdose of products containing dextromethorphan have been reported in the literature.
The panel also was tasked with determining if the scientific data supports abuse potential for dextromethorphan. In general, panelists agreed that dextromethorphan has abuse potential, but its abuse potential relative to other abused substances is unclear. In 2008, dextromethorphan accounted for almost 8,000 emergency department visits due to nonmedical use (taken as a cough suppressant, alone, or in combination with other medications), based on data from the Drug Abuse Warning Network. That number is up from more than 4,600 in 2004.
The FDA also asked the panel to discuss the effectiveness of current and planned abuse mitigation strategies and to recommend modifications or other measures. Access to bulk dextromethorphan – often used to make capsules used recreationally and available online – was also a significant concern for the panel.
The 2005 deaths prompted the FDA to publish a Talk Paper to warn the public about the risks associated with abuse of the drug later that year. Starting in May 2006, the Consumer Healthcare Products Association – which represents several makers of OTC dextromethorphan–containing products – began to develop voluntary initiatives to reduce teen abuse of dextromethorphan. More information is available at www.stopmedicineabuse.org.
COLLEGE PARK, Md. – A Food and Drug Administration panel on Sept. 14 voted 15 to 9 against requiring that the cough suppressant dextromethorphan be scheduled under the Controlled Substances Act.
The drug is used in more than 100 over-the-counter cough and cold products available in a wide range of retail outlets. Those voting against scheduling cited concerns about limiting access to the drug, which can be effective for coughs.
The panel was asked to consider the need to schedule dextromethorphan based on a request from the Drug Enforcement Administration for a scientific and medical evaluation and scheduling recommendation. Dextromethorphan abuse has been an ongoing concern for both the FDA and the DEA, starting in the early 1990s. The dextromethrophan-related deaths of five teenagers from the states of Washington, Florida, and Virginia brought the problem to back to national attention in 2005. In each case, death was determined to be the direct result of the toxic effects of dextromethorphan powder. Other case reports of abuse and the effects of overdose of products containing dextromethorphan have been reported in the literature.
The panel also was tasked with determining if the scientific data supports abuse potential for dextromethorphan. In general, panelists agreed that dextromethorphan has abuse potential, but its abuse potential relative to other abused substances is unclear. In 2008, dextromethorphan accounted for almost 8,000 emergency department visits due to nonmedical use (taken as a cough suppressant, alone, or in combination with other medications), based on data from the Drug Abuse Warning Network. That number is up from more than 4,600 in 2004.
The FDA also asked the panel to discuss the effectiveness of current and planned abuse mitigation strategies and to recommend modifications or other measures. Access to bulk dextromethorphan – often used to make capsules used recreationally and available online – was also a significant concern for the panel.
The 2005 deaths prompted the FDA to publish a Talk Paper to warn the public about the risks associated with abuse of the drug later that year. Starting in May 2006, the Consumer Healthcare Products Association – which represents several makers of OTC dextromethorphan–containing products – began to develop voluntary initiatives to reduce teen abuse of dextromethorphan. More information is available at www.stopmedicineabuse.org.
COLLEGE PARK, Md. – A Food and Drug Administration panel on Sept. 14 voted 15 to 9 against requiring that the cough suppressant dextromethorphan be scheduled under the Controlled Substances Act.
The drug is used in more than 100 over-the-counter cough and cold products available in a wide range of retail outlets. Those voting against scheduling cited concerns about limiting access to the drug, which can be effective for coughs.
The panel was asked to consider the need to schedule dextromethorphan based on a request from the Drug Enforcement Administration for a scientific and medical evaluation and scheduling recommendation. Dextromethorphan abuse has been an ongoing concern for both the FDA and the DEA, starting in the early 1990s. The dextromethrophan-related deaths of five teenagers from the states of Washington, Florida, and Virginia brought the problem to back to national attention in 2005. In each case, death was determined to be the direct result of the toxic effects of dextromethorphan powder. Other case reports of abuse and the effects of overdose of products containing dextromethorphan have been reported in the literature.
The panel also was tasked with determining if the scientific data supports abuse potential for dextromethorphan. In general, panelists agreed that dextromethorphan has abuse potential, but its abuse potential relative to other abused substances is unclear. In 2008, dextromethorphan accounted for almost 8,000 emergency department visits due to nonmedical use (taken as a cough suppressant, alone, or in combination with other medications), based on data from the Drug Abuse Warning Network. That number is up from more than 4,600 in 2004.
The FDA also asked the panel to discuss the effectiveness of current and planned abuse mitigation strategies and to recommend modifications or other measures. Access to bulk dextromethorphan – often used to make capsules used recreationally and available online – was also a significant concern for the panel.
The 2005 deaths prompted the FDA to publish a Talk Paper to warn the public about the risks associated with abuse of the drug later that year. Starting in May 2006, the Consumer Healthcare Products Association – which represents several makers of OTC dextromethorphan–containing products – began to develop voluntary initiatives to reduce teen abuse of dextromethorphan. More information is available at www.stopmedicineabuse.org.
From a meeting at the Food and Drug Administration
PPT Is Easy to Miss in the Hyperthyroid Phase
MINNEAPOLIS — In classic-course postpartum thyroiditis, the hyperthyroid phase is often missed; it's much more common to identify women in the hypothyroid phase that follows, Dr. Erin Keely said.
“In my experience, we miss the hyperthyroid phase clinically all the time because it's a little bit like the army: Don't ask, don't tell,” she said. The symptoms—anxiety, insomnia, fatigue, weight loss, and irritability—are normal for most new moms.
The classic course of postpartum thyroiditis (PPT) is hyperthyroidism, which starts at about 6 weeks post partum and may last a few months, followed by hypothyroidism and then normalization.
More commonly, women present in the hypothyroid phase, which typically occurs 4-8 months after delivery and may last up to 9-12 months, said Dr. Keely, chief of endocrinology and metabolism at the Ottawa Hospital. Typical symptoms include fatigue, weight gain, constipation, dry skin, depression, and poor exercise tolerance. Women tested in the transition period might appear to have normal thyroid function, however. About 30% of women remain hyperthyroid.
Because PPT can cause both thyrotoxicosis (high serum thyroid hormone levels) and hypothyroidism (low levels) in the first year post partum, “you can't make a diagnosis of permanent thyroid disease,” at that time, said Dr. Keely. The exception is for any woman with overt thyroid disease before pregnancy.
To complicate matters, Graves' disease can flare in the postpartum period, making it difficult to tell if a woman is having a flare or PPT. However, Graves' disease is much less common than PPT, said Dr. Keely.
“So, if you were a betting person, you would bet that it's postpartum thyroiditis.” If the diagnosis is really unclear, radioiodine uptake tests can be performed but most women will likely choose to wait and see, she said.
Current data are insufficient to recommend screening all women for PPT, according to Endocrine Society guidelines (J. Clin. Endocrinol. Metab. 2007;92:s1-47). Women who are known to be positive for autoantibodies to thyroid peroxidase (TPO-Ab) should have a TSH test performed at 3 and 6 months post partum. Notably, the prevalence of PPT in women with type 1 diabetes is threefold greater than in the general population; postpartum TSH screening is recommended at 3 and 6 months.
In PPT treatment, “there is no one answer for all women,” said Dr. Keely. However, in 2002, Dr. Alex S. Stagnaro-Green, now senior associate dean for education at George Washington University, Washington, proposed a PPT treatment algorithm (J. Clin. Endocrinol. Metab. 2002;87:4042-7). In this algorithm, treatment is indicated for a TSH of 4 mU/mL or greater in the first postpartum year. Dr. Keely added that “one of the most important aspects is to continue the treatment until [the woman] has completed her family.”
If TPO-Ab are found in the first trimester, the risk of PPT is 30%-55%. “It's interesting though, that 25% of women who are positive in the first trimester become negative by the third trimester,” she said. Women may become negative by term but rebound in the postpartum period. If TPO-Ab is positive in the third trimester, the risk of PPT is greater than 80%; if negative, the risk is only 0%-5%.
Finally, “postpartum thyroiditis is a very strong predictor of long term Hashimoto's thyroiditis,” said Dr. Keely. About 30% of these women develop Hashimoto's thyroiditis within 3 years.
Disclosures: Dr. Keely reported that she had no relevant financial relationships.
MINNEAPOLIS — In classic-course postpartum thyroiditis, the hyperthyroid phase is often missed; it's much more common to identify women in the hypothyroid phase that follows, Dr. Erin Keely said.
“In my experience, we miss the hyperthyroid phase clinically all the time because it's a little bit like the army: Don't ask, don't tell,” she said. The symptoms—anxiety, insomnia, fatigue, weight loss, and irritability—are normal for most new moms.
The classic course of postpartum thyroiditis (PPT) is hyperthyroidism, which starts at about 6 weeks post partum and may last a few months, followed by hypothyroidism and then normalization.
More commonly, women present in the hypothyroid phase, which typically occurs 4-8 months after delivery and may last up to 9-12 months, said Dr. Keely, chief of endocrinology and metabolism at the Ottawa Hospital. Typical symptoms include fatigue, weight gain, constipation, dry skin, depression, and poor exercise tolerance. Women tested in the transition period might appear to have normal thyroid function, however. About 30% of women remain hyperthyroid.
Because PPT can cause both thyrotoxicosis (high serum thyroid hormone levels) and hypothyroidism (low levels) in the first year post partum, “you can't make a diagnosis of permanent thyroid disease,” at that time, said Dr. Keely. The exception is for any woman with overt thyroid disease before pregnancy.
To complicate matters, Graves' disease can flare in the postpartum period, making it difficult to tell if a woman is having a flare or PPT. However, Graves' disease is much less common than PPT, said Dr. Keely.
“So, if you were a betting person, you would bet that it's postpartum thyroiditis.” If the diagnosis is really unclear, radioiodine uptake tests can be performed but most women will likely choose to wait and see, she said.
Current data are insufficient to recommend screening all women for PPT, according to Endocrine Society guidelines (J. Clin. Endocrinol. Metab. 2007;92:s1-47). Women who are known to be positive for autoantibodies to thyroid peroxidase (TPO-Ab) should have a TSH test performed at 3 and 6 months post partum. Notably, the prevalence of PPT in women with type 1 diabetes is threefold greater than in the general population; postpartum TSH screening is recommended at 3 and 6 months.
In PPT treatment, “there is no one answer for all women,” said Dr. Keely. However, in 2002, Dr. Alex S. Stagnaro-Green, now senior associate dean for education at George Washington University, Washington, proposed a PPT treatment algorithm (J. Clin. Endocrinol. Metab. 2002;87:4042-7). In this algorithm, treatment is indicated for a TSH of 4 mU/mL or greater in the first postpartum year. Dr. Keely added that “one of the most important aspects is to continue the treatment until [the woman] has completed her family.”
If TPO-Ab are found in the first trimester, the risk of PPT is 30%-55%. “It's interesting though, that 25% of women who are positive in the first trimester become negative by the third trimester,” she said. Women may become negative by term but rebound in the postpartum period. If TPO-Ab is positive in the third trimester, the risk of PPT is greater than 80%; if negative, the risk is only 0%-5%.
Finally, “postpartum thyroiditis is a very strong predictor of long term Hashimoto's thyroiditis,” said Dr. Keely. About 30% of these women develop Hashimoto's thyroiditis within 3 years.
Disclosures: Dr. Keely reported that she had no relevant financial relationships.
MINNEAPOLIS — In classic-course postpartum thyroiditis, the hyperthyroid phase is often missed; it's much more common to identify women in the hypothyroid phase that follows, Dr. Erin Keely said.
“In my experience, we miss the hyperthyroid phase clinically all the time because it's a little bit like the army: Don't ask, don't tell,” she said. The symptoms—anxiety, insomnia, fatigue, weight loss, and irritability—are normal for most new moms.
The classic course of postpartum thyroiditis (PPT) is hyperthyroidism, which starts at about 6 weeks post partum and may last a few months, followed by hypothyroidism and then normalization.
More commonly, women present in the hypothyroid phase, which typically occurs 4-8 months after delivery and may last up to 9-12 months, said Dr. Keely, chief of endocrinology and metabolism at the Ottawa Hospital. Typical symptoms include fatigue, weight gain, constipation, dry skin, depression, and poor exercise tolerance. Women tested in the transition period might appear to have normal thyroid function, however. About 30% of women remain hyperthyroid.
Because PPT can cause both thyrotoxicosis (high serum thyroid hormone levels) and hypothyroidism (low levels) in the first year post partum, “you can't make a diagnosis of permanent thyroid disease,” at that time, said Dr. Keely. The exception is for any woman with overt thyroid disease before pregnancy.
To complicate matters, Graves' disease can flare in the postpartum period, making it difficult to tell if a woman is having a flare or PPT. However, Graves' disease is much less common than PPT, said Dr. Keely.
“So, if you were a betting person, you would bet that it's postpartum thyroiditis.” If the diagnosis is really unclear, radioiodine uptake tests can be performed but most women will likely choose to wait and see, she said.
Current data are insufficient to recommend screening all women for PPT, according to Endocrine Society guidelines (J. Clin. Endocrinol. Metab. 2007;92:s1-47). Women who are known to be positive for autoantibodies to thyroid peroxidase (TPO-Ab) should have a TSH test performed at 3 and 6 months post partum. Notably, the prevalence of PPT in women with type 1 diabetes is threefold greater than in the general population; postpartum TSH screening is recommended at 3 and 6 months.
In PPT treatment, “there is no one answer for all women,” said Dr. Keely. However, in 2002, Dr. Alex S. Stagnaro-Green, now senior associate dean for education at George Washington University, Washington, proposed a PPT treatment algorithm (J. Clin. Endocrinol. Metab. 2002;87:4042-7). In this algorithm, treatment is indicated for a TSH of 4 mU/mL or greater in the first postpartum year. Dr. Keely added that “one of the most important aspects is to continue the treatment until [the woman] has completed her family.”
If TPO-Ab are found in the first trimester, the risk of PPT is 30%-55%. “It's interesting though, that 25% of women who are positive in the first trimester become negative by the third trimester,” she said. Women may become negative by term but rebound in the postpartum period. If TPO-Ab is positive in the third trimester, the risk of PPT is greater than 80%; if negative, the risk is only 0%-5%.
Finally, “postpartum thyroiditis is a very strong predictor of long term Hashimoto's thyroiditis,” said Dr. Keely. About 30% of these women develop Hashimoto's thyroiditis within 3 years.
Disclosures: Dr. Keely reported that she had no relevant financial relationships.
Expert opinion from a meeting sponsored by the American Thyroid Association
Start Antibiotics Prior to C-Section Incision : Data show this significantly reduces maternal infections, does not appear to harm newborns.
Antimicrobial prophylaxis now should be given within 60 minutes of the start of a cesarean delivery, rather than after cord clamping,
The recommended change in practice comes from a new opinion by the American College of Obstetricians and Gynecologists' Committee on Obstetric Practice as Committee Opinion No. 465 (Obstet. Gynecol. 2010;116:791-2).
“Based on the latest data, prophylactic antibiotics given to pregnant women before a cesarean significantly reduce maternal infections and do not appear to harm newborns,” Dr. William H. Barth Jr., chair of the committee, said in a statement.
“Anytime you have invasive surgery, you have an increased risk of developing an infection at the incision site,” he said. Infection is the most common complication of cesarean delivery and can occur in an estimated 10%-40% of women who undergo cesarean delivery, compared with 1%-3% of women who deliver vaginally, according to ACOG.
The committee recommends antimicrobial prophylaxis for all cesarean deliveries unless the patient is already receiving appropriate antibiotics. When it is not possible to begin administration within 60 minutes of the first incision—as with emergent delivery—prophylaxis should be administered as soon as possible.
Antimicrobial prophylaxis has been a common practice for cesarean deliveries. However, intraoperative antibiotics have been administered after umbilical clamping due to concerns about neonatal exposure to antibiotics. In particular, it has been theorized that antibiotics in neonatal serum could mask positive bacterial culture results in newborns and that fetal antibiotic exposure could lead to increased newborn colonization or infection with antibiotic-resistant organisms.
Older studies had suggested that when prophylactic antibiotics were given before the cesarean, pediatricians tended to do more invasive neonatal sepsis evaluations and costs were increased, Dr. Barth said in an interview. “This was based on the fear that the antibiotics given to the mother would cross rapidly to the fetus and then mask the signs of infection in the newborn child.” Pediatricians feared that the usual signs of sepsis might be masked by these antibiotics. Given this fear, tests such as blood draws and lumbar punctures that are useful in making a diagnosis of newborn sepsis tended to be used more frequently.
“However, based on recent randomized clinical trials and systematic reviews, giving the mother the antibiotics before the cesarean incision does not appear to increase problems in the newborn. None of the studies were large enough to say that definitively, but given the overall benefit to the mother, our committee—which included pediatricians—felt that this was the right thing to do,” said Dr. Barth, chief of maternal-fetal medicine at Massachusetts General Hospital, Boston.
In fact, preoperative antimicrobial prophylaxis “does not appear to have any deleterious effects on mother or neonate,” the committee wrote. Timing really does make a difference. In the studies reviewed, preoperative administration significantly reduced the rates of endometritis and total maternal infectious morbidity, compared with administration after cord clamping. Just as importantly, preoperative administration was not associated with an increase in neonatal infectious morbidity or the selection of antimicrobial-resistant bacteria causing neonatal sepsis.
The committee recommends that the infusion be timed so that a bactericidal serum level is reached by the time of skin incision. Therapeutic antibiotic levels should be maintained throughout the operation. Readministration is indicated at intervals of one or two times the half-life of the drug during longer procedures. The committee recommends using narrow-spectrum drugs that are effective against gram-positive and gram-negative bacteria and against some anaerobic bacteria – such as first-generation cephalosporins. Notably, obese women may require doses larger than the recommended 1 gram intravenous cefazolin (with a therapeutic dose maintained for 3-4 hours). Clindamycin with gentamicin is an acceptable alternative for women with significant allergies to beta-lactam antibiotics.
Disclosures: Dr. Barth said he had no conflicts of interest to disclose.
Antimicrobial prophylaxis now should be given within 60 minutes of the start of a cesarean delivery, rather than after cord clamping,
The recommended change in practice comes from a new opinion by the American College of Obstetricians and Gynecologists' Committee on Obstetric Practice as Committee Opinion No. 465 (Obstet. Gynecol. 2010;116:791-2).
“Based on the latest data, prophylactic antibiotics given to pregnant women before a cesarean significantly reduce maternal infections and do not appear to harm newborns,” Dr. William H. Barth Jr., chair of the committee, said in a statement.
“Anytime you have invasive surgery, you have an increased risk of developing an infection at the incision site,” he said. Infection is the most common complication of cesarean delivery and can occur in an estimated 10%-40% of women who undergo cesarean delivery, compared with 1%-3% of women who deliver vaginally, according to ACOG.
The committee recommends antimicrobial prophylaxis for all cesarean deliveries unless the patient is already receiving appropriate antibiotics. When it is not possible to begin administration within 60 minutes of the first incision—as with emergent delivery—prophylaxis should be administered as soon as possible.
Antimicrobial prophylaxis has been a common practice for cesarean deliveries. However, intraoperative antibiotics have been administered after umbilical clamping due to concerns about neonatal exposure to antibiotics. In particular, it has been theorized that antibiotics in neonatal serum could mask positive bacterial culture results in newborns and that fetal antibiotic exposure could lead to increased newborn colonization or infection with antibiotic-resistant organisms.
Older studies had suggested that when prophylactic antibiotics were given before the cesarean, pediatricians tended to do more invasive neonatal sepsis evaluations and costs were increased, Dr. Barth said in an interview. “This was based on the fear that the antibiotics given to the mother would cross rapidly to the fetus and then mask the signs of infection in the newborn child.” Pediatricians feared that the usual signs of sepsis might be masked by these antibiotics. Given this fear, tests such as blood draws and lumbar punctures that are useful in making a diagnosis of newborn sepsis tended to be used more frequently.
“However, based on recent randomized clinical trials and systematic reviews, giving the mother the antibiotics before the cesarean incision does not appear to increase problems in the newborn. None of the studies were large enough to say that definitively, but given the overall benefit to the mother, our committee—which included pediatricians—felt that this was the right thing to do,” said Dr. Barth, chief of maternal-fetal medicine at Massachusetts General Hospital, Boston.
In fact, preoperative antimicrobial prophylaxis “does not appear to have any deleterious effects on mother or neonate,” the committee wrote. Timing really does make a difference. In the studies reviewed, preoperative administration significantly reduced the rates of endometritis and total maternal infectious morbidity, compared with administration after cord clamping. Just as importantly, preoperative administration was not associated with an increase in neonatal infectious morbidity or the selection of antimicrobial-resistant bacteria causing neonatal sepsis.
The committee recommends that the infusion be timed so that a bactericidal serum level is reached by the time of skin incision. Therapeutic antibiotic levels should be maintained throughout the operation. Readministration is indicated at intervals of one or two times the half-life of the drug during longer procedures. The committee recommends using narrow-spectrum drugs that are effective against gram-positive and gram-negative bacteria and against some anaerobic bacteria – such as first-generation cephalosporins. Notably, obese women may require doses larger than the recommended 1 gram intravenous cefazolin (with a therapeutic dose maintained for 3-4 hours). Clindamycin with gentamicin is an acceptable alternative for women with significant allergies to beta-lactam antibiotics.
Disclosures: Dr. Barth said he had no conflicts of interest to disclose.
Antimicrobial prophylaxis now should be given within 60 minutes of the start of a cesarean delivery, rather than after cord clamping,
The recommended change in practice comes from a new opinion by the American College of Obstetricians and Gynecologists' Committee on Obstetric Practice as Committee Opinion No. 465 (Obstet. Gynecol. 2010;116:791-2).
“Based on the latest data, prophylactic antibiotics given to pregnant women before a cesarean significantly reduce maternal infections and do not appear to harm newborns,” Dr. William H. Barth Jr., chair of the committee, said in a statement.
“Anytime you have invasive surgery, you have an increased risk of developing an infection at the incision site,” he said. Infection is the most common complication of cesarean delivery and can occur in an estimated 10%-40% of women who undergo cesarean delivery, compared with 1%-3% of women who deliver vaginally, according to ACOG.
The committee recommends antimicrobial prophylaxis for all cesarean deliveries unless the patient is already receiving appropriate antibiotics. When it is not possible to begin administration within 60 minutes of the first incision—as with emergent delivery—prophylaxis should be administered as soon as possible.
Antimicrobial prophylaxis has been a common practice for cesarean deliveries. However, intraoperative antibiotics have been administered after umbilical clamping due to concerns about neonatal exposure to antibiotics. In particular, it has been theorized that antibiotics in neonatal serum could mask positive bacterial culture results in newborns and that fetal antibiotic exposure could lead to increased newborn colonization or infection with antibiotic-resistant organisms.
Older studies had suggested that when prophylactic antibiotics were given before the cesarean, pediatricians tended to do more invasive neonatal sepsis evaluations and costs were increased, Dr. Barth said in an interview. “This was based on the fear that the antibiotics given to the mother would cross rapidly to the fetus and then mask the signs of infection in the newborn child.” Pediatricians feared that the usual signs of sepsis might be masked by these antibiotics. Given this fear, tests such as blood draws and lumbar punctures that are useful in making a diagnosis of newborn sepsis tended to be used more frequently.
“However, based on recent randomized clinical trials and systematic reviews, giving the mother the antibiotics before the cesarean incision does not appear to increase problems in the newborn. None of the studies were large enough to say that definitively, but given the overall benefit to the mother, our committee—which included pediatricians—felt that this was the right thing to do,” said Dr. Barth, chief of maternal-fetal medicine at Massachusetts General Hospital, Boston.
In fact, preoperative antimicrobial prophylaxis “does not appear to have any deleterious effects on mother or neonate,” the committee wrote. Timing really does make a difference. In the studies reviewed, preoperative administration significantly reduced the rates of endometritis and total maternal infectious morbidity, compared with administration after cord clamping. Just as importantly, preoperative administration was not associated with an increase in neonatal infectious morbidity or the selection of antimicrobial-resistant bacteria causing neonatal sepsis.
The committee recommends that the infusion be timed so that a bactericidal serum level is reached by the time of skin incision. Therapeutic antibiotic levels should be maintained throughout the operation. Readministration is indicated at intervals of one or two times the half-life of the drug during longer procedures. The committee recommends using narrow-spectrum drugs that are effective against gram-positive and gram-negative bacteria and against some anaerobic bacteria – such as first-generation cephalosporins. Notably, obese women may require doses larger than the recommended 1 gram intravenous cefazolin (with a therapeutic dose maintained for 3-4 hours). Clindamycin with gentamicin is an acceptable alternative for women with significant allergies to beta-lactam antibiotics.
Disclosures: Dr. Barth said he had no conflicts of interest to disclose.
From Obstetrics & Gynecology