User login
FDA approves pembrolizumab for advanced NSCLC
The Food and Drug Administration has granted accelerated approval for pembrolizumab to treat patients with metastatic non–small cell lung cancer (NSCLC) who have disease that has progressed after other treatments and tumors that express PD-L1. The anti–PD-L1 drug is approved for use with a companion diagnostic, the PD-L1 IHC 22C3 pharmDx test.
Pembrolizumab was approved to treat patients with advanced melanoma in 2014. The effectiveness of the immunotherapy for treating advanced NSCLC was demonstrated among 61 patients enrolled within a larger multicenter, open-label, multipart study, according to an Oct. 2 statement issued by the FDA. These patients had advanced NSCLC that progressed following platinum-based chemotherapy or, if appropriate, targeted therapy for certain genetic mutations (ALK or EGFR), and they each had PD-L1–positive tumors based on the results of the 22C3 pharmDx diagnostic test.
The overall response rate was 41%, and the effect lasted between 2.1 and 9.1 months, the FDA said.
The most common side effects in 550 patients with advanced NSCLC included fatigue, decreased appetite, dyspnea, and cough. Severe immune-mediated side effects occurred involving the lungs, colon, and hormone-producing glands.
Pembrolizumab is marketed as Keytruda by Merck & Co., and the PD-L1 IHC 22C3 pharmDx diagnostic test is marketed by Dako North America Inc.
On Twitter @NikolaidesLaura
The Food and Drug Administration has granted accelerated approval for pembrolizumab to treat patients with metastatic non–small cell lung cancer (NSCLC) who have disease that has progressed after other treatments and tumors that express PD-L1. The anti–PD-L1 drug is approved for use with a companion diagnostic, the PD-L1 IHC 22C3 pharmDx test.
Pembrolizumab was approved to treat patients with advanced melanoma in 2014. The effectiveness of the immunotherapy for treating advanced NSCLC was demonstrated among 61 patients enrolled within a larger multicenter, open-label, multipart study, according to an Oct. 2 statement issued by the FDA. These patients had advanced NSCLC that progressed following platinum-based chemotherapy or, if appropriate, targeted therapy for certain genetic mutations (ALK or EGFR), and they each had PD-L1–positive tumors based on the results of the 22C3 pharmDx diagnostic test.
The overall response rate was 41%, and the effect lasted between 2.1 and 9.1 months, the FDA said.
The most common side effects in 550 patients with advanced NSCLC included fatigue, decreased appetite, dyspnea, and cough. Severe immune-mediated side effects occurred involving the lungs, colon, and hormone-producing glands.
Pembrolizumab is marketed as Keytruda by Merck & Co., and the PD-L1 IHC 22C3 pharmDx diagnostic test is marketed by Dako North America Inc.
On Twitter @NikolaidesLaura
The Food and Drug Administration has granted accelerated approval for pembrolizumab to treat patients with metastatic non–small cell lung cancer (NSCLC) who have disease that has progressed after other treatments and tumors that express PD-L1. The anti–PD-L1 drug is approved for use with a companion diagnostic, the PD-L1 IHC 22C3 pharmDx test.
Pembrolizumab was approved to treat patients with advanced melanoma in 2014. The effectiveness of the immunotherapy for treating advanced NSCLC was demonstrated among 61 patients enrolled within a larger multicenter, open-label, multipart study, according to an Oct. 2 statement issued by the FDA. These patients had advanced NSCLC that progressed following platinum-based chemotherapy or, if appropriate, targeted therapy for certain genetic mutations (ALK or EGFR), and they each had PD-L1–positive tumors based on the results of the 22C3 pharmDx diagnostic test.
The overall response rate was 41%, and the effect lasted between 2.1 and 9.1 months, the FDA said.
The most common side effects in 550 patients with advanced NSCLC included fatigue, decreased appetite, dyspnea, and cough. Severe immune-mediated side effects occurred involving the lungs, colon, and hormone-producing glands.
Pembrolizumab is marketed as Keytruda by Merck & Co., and the PD-L1 IHC 22C3 pharmDx diagnostic test is marketed by Dako North America Inc.
On Twitter @NikolaidesLaura
Immune-related patterns of response present challenges
With the race to develop cancer immunotherapies escalating and new agents appearing in the clinic, oncologists’ decision-making toolbox will need to evolve.
“As immunotherapeutics become increasingly available to patients, clinicians face a major challenge in the evaluation of these novel drugs – the accurate determination of clinical efficacy,” physician-scientists recently wrote in a commentary published online in the Journal of Clinical Oncology.
Response evaluation criteria in solid tumors (RECIST) have typically driven oncologists’ decision making. Patients undergo scans and radiographic measurements to determine the extent of change in tumor size. Scan, treat, repeat is a mantra for advanced cancer patients, so much so some patients have sought to trademark the phrase for T-shirts. And significant tumor growth has traditionally signaled treatment failure.
But although some patients have responded to immune-targeted treatment with tumor shrinkage or stable disease that would be consistent with existing RECIST criteria, distinct immune-related patterns of response are also emerging, including pseudoprogression, said Dr. Victoria L. Chiou and Dr. Mauricio Burotto, both medical oncology fellows at the National Cancer Institute, in their commentary (Jour Clin Onc. 2015 Aug 10. doi: 10.1200/JCO.2015.61.6870).
Investigators first addressed this issue by proposing immune response criteria in 2009, based on data from ipilimumab phase II trials in patients with advanced melanoma. Dr. Jedd D. Wolchok of Memorial Sloan Kettering Cancer Center, New York, and his associates expanded on RECIST by adding two additional patterns of response: response after an increase in total tumor burden and response in the presence of new lesions. All patterns of response were associated with favorable survival, Dr. Wolchok and associates said (Clin Cancer Res 2009 Dec 1. doi: 10.1158/1078-0432.CCR-09-1624).
Subsequent trials in patients with advanced melanoma have confirmed that a subset of patients who are responders using immune response criteria would have been misclassified using RECIST alone.
In a study of patients with metastatic melanoma treated with nivolumab, 10% experienced distinct immune-related responses, according to Dr. Chiou and Dr. Burotto. In another study of patients with advanced melanoma, this one evaluating anti–PD-1 monoclonal antibody pembrolizumab, 6.7% of patients of the patients experienced pseudoprogression, and overall, 12% of patients were classified as responders or as having stable disease by immune response criteria but would have been classified as having progressive disease by RECIST.
It is time to expand on those first immune response criteria, Dr. Chiou and Dr. Burotto conclude. “Five years after the introduction of the immune response criteria, it is necessary to fully characterize the patterns of immune-related phenomena, to understand these patterns across multiple solid tumor types, and to evaluate how these guidelines are used in current clinical practice,” they wrote.
The Food and Drug Administration agrees. Though guidelines for industry were published in 2011 regarding cancer vaccines, there is no specific guidance on approval criteria for other cancer immunotherapies such as checkpoint inhibitors, Dr. Gideon Blumenthal, team leader in the FDA’s Office of Hematology and Oncology Products, Center for Drug Evaluation and Research, said in an interview.
“However, the FDA is actively looking at other metrics of response beyond conventional RECIST criteria, both to help industry in early ‘go/no-go’ decision making, as well as for helping in the design of later stages of trial design and to help inform approval decisions,” he said.
The FDA is interested in investigating internal data sets of immunotherapy trials, and in collaborating with external stakeholders to determine what the optimal endpoints for cancer immunotherapies will be. Median PFS and PFS hazard ratios do not appear to capture the overall survival benefit that patients derive from immunotherapies, particularly in early lung cancer trials with which he is most familiar, Dr. Blumenthal said.
“Overall survival remains the gold standard endpoint in oncology as it is a direct measure of clinical benefit and less subject to bias, but as more effective therapies come on line and patients live longer, overall survival will become more challenging to detect,” he said.
On Twitter @NikolaidesLaura
With the race to develop cancer immunotherapies escalating and new agents appearing in the clinic, oncologists’ decision-making toolbox will need to evolve.
“As immunotherapeutics become increasingly available to patients, clinicians face a major challenge in the evaluation of these novel drugs – the accurate determination of clinical efficacy,” physician-scientists recently wrote in a commentary published online in the Journal of Clinical Oncology.
Response evaluation criteria in solid tumors (RECIST) have typically driven oncologists’ decision making. Patients undergo scans and radiographic measurements to determine the extent of change in tumor size. Scan, treat, repeat is a mantra for advanced cancer patients, so much so some patients have sought to trademark the phrase for T-shirts. And significant tumor growth has traditionally signaled treatment failure.
But although some patients have responded to immune-targeted treatment with tumor shrinkage or stable disease that would be consistent with existing RECIST criteria, distinct immune-related patterns of response are also emerging, including pseudoprogression, said Dr. Victoria L. Chiou and Dr. Mauricio Burotto, both medical oncology fellows at the National Cancer Institute, in their commentary (Jour Clin Onc. 2015 Aug 10. doi: 10.1200/JCO.2015.61.6870).
Investigators first addressed this issue by proposing immune response criteria in 2009, based on data from ipilimumab phase II trials in patients with advanced melanoma. Dr. Jedd D. Wolchok of Memorial Sloan Kettering Cancer Center, New York, and his associates expanded on RECIST by adding two additional patterns of response: response after an increase in total tumor burden and response in the presence of new lesions. All patterns of response were associated with favorable survival, Dr. Wolchok and associates said (Clin Cancer Res 2009 Dec 1. doi: 10.1158/1078-0432.CCR-09-1624).
Subsequent trials in patients with advanced melanoma have confirmed that a subset of patients who are responders using immune response criteria would have been misclassified using RECIST alone.
In a study of patients with metastatic melanoma treated with nivolumab, 10% experienced distinct immune-related responses, according to Dr. Chiou and Dr. Burotto. In another study of patients with advanced melanoma, this one evaluating anti–PD-1 monoclonal antibody pembrolizumab, 6.7% of patients of the patients experienced pseudoprogression, and overall, 12% of patients were classified as responders or as having stable disease by immune response criteria but would have been classified as having progressive disease by RECIST.
It is time to expand on those first immune response criteria, Dr. Chiou and Dr. Burotto conclude. “Five years after the introduction of the immune response criteria, it is necessary to fully characterize the patterns of immune-related phenomena, to understand these patterns across multiple solid tumor types, and to evaluate how these guidelines are used in current clinical practice,” they wrote.
The Food and Drug Administration agrees. Though guidelines for industry were published in 2011 regarding cancer vaccines, there is no specific guidance on approval criteria for other cancer immunotherapies such as checkpoint inhibitors, Dr. Gideon Blumenthal, team leader in the FDA’s Office of Hematology and Oncology Products, Center for Drug Evaluation and Research, said in an interview.
“However, the FDA is actively looking at other metrics of response beyond conventional RECIST criteria, both to help industry in early ‘go/no-go’ decision making, as well as for helping in the design of later stages of trial design and to help inform approval decisions,” he said.
The FDA is interested in investigating internal data sets of immunotherapy trials, and in collaborating with external stakeholders to determine what the optimal endpoints for cancer immunotherapies will be. Median PFS and PFS hazard ratios do not appear to capture the overall survival benefit that patients derive from immunotherapies, particularly in early lung cancer trials with which he is most familiar, Dr. Blumenthal said.
“Overall survival remains the gold standard endpoint in oncology as it is a direct measure of clinical benefit and less subject to bias, but as more effective therapies come on line and patients live longer, overall survival will become more challenging to detect,” he said.
On Twitter @NikolaidesLaura
With the race to develop cancer immunotherapies escalating and new agents appearing in the clinic, oncologists’ decision-making toolbox will need to evolve.
“As immunotherapeutics become increasingly available to patients, clinicians face a major challenge in the evaluation of these novel drugs – the accurate determination of clinical efficacy,” physician-scientists recently wrote in a commentary published online in the Journal of Clinical Oncology.
Response evaluation criteria in solid tumors (RECIST) have typically driven oncologists’ decision making. Patients undergo scans and radiographic measurements to determine the extent of change in tumor size. Scan, treat, repeat is a mantra for advanced cancer patients, so much so some patients have sought to trademark the phrase for T-shirts. And significant tumor growth has traditionally signaled treatment failure.
But although some patients have responded to immune-targeted treatment with tumor shrinkage or stable disease that would be consistent with existing RECIST criteria, distinct immune-related patterns of response are also emerging, including pseudoprogression, said Dr. Victoria L. Chiou and Dr. Mauricio Burotto, both medical oncology fellows at the National Cancer Institute, in their commentary (Jour Clin Onc. 2015 Aug 10. doi: 10.1200/JCO.2015.61.6870).
Investigators first addressed this issue by proposing immune response criteria in 2009, based on data from ipilimumab phase II trials in patients with advanced melanoma. Dr. Jedd D. Wolchok of Memorial Sloan Kettering Cancer Center, New York, and his associates expanded on RECIST by adding two additional patterns of response: response after an increase in total tumor burden and response in the presence of new lesions. All patterns of response were associated with favorable survival, Dr. Wolchok and associates said (Clin Cancer Res 2009 Dec 1. doi: 10.1158/1078-0432.CCR-09-1624).
Subsequent trials in patients with advanced melanoma have confirmed that a subset of patients who are responders using immune response criteria would have been misclassified using RECIST alone.
In a study of patients with metastatic melanoma treated with nivolumab, 10% experienced distinct immune-related responses, according to Dr. Chiou and Dr. Burotto. In another study of patients with advanced melanoma, this one evaluating anti–PD-1 monoclonal antibody pembrolizumab, 6.7% of patients of the patients experienced pseudoprogression, and overall, 12% of patients were classified as responders or as having stable disease by immune response criteria but would have been classified as having progressive disease by RECIST.
It is time to expand on those first immune response criteria, Dr. Chiou and Dr. Burotto conclude. “Five years after the introduction of the immune response criteria, it is necessary to fully characterize the patterns of immune-related phenomena, to understand these patterns across multiple solid tumor types, and to evaluate how these guidelines are used in current clinical practice,” they wrote.
The Food and Drug Administration agrees. Though guidelines for industry were published in 2011 regarding cancer vaccines, there is no specific guidance on approval criteria for other cancer immunotherapies such as checkpoint inhibitors, Dr. Gideon Blumenthal, team leader in the FDA’s Office of Hematology and Oncology Products, Center for Drug Evaluation and Research, said in an interview.
“However, the FDA is actively looking at other metrics of response beyond conventional RECIST criteria, both to help industry in early ‘go/no-go’ decision making, as well as for helping in the design of later stages of trial design and to help inform approval decisions,” he said.
The FDA is interested in investigating internal data sets of immunotherapy trials, and in collaborating with external stakeholders to determine what the optimal endpoints for cancer immunotherapies will be. Median PFS and PFS hazard ratios do not appear to capture the overall survival benefit that patients derive from immunotherapies, particularly in early lung cancer trials with which he is most familiar, Dr. Blumenthal said.
“Overall survival remains the gold standard endpoint in oncology as it is a direct measure of clinical benefit and less subject to bias, but as more effective therapies come on line and patients live longer, overall survival will become more challenging to detect,” he said.
On Twitter @NikolaidesLaura
VIDEO: Dr. William J. Gradishar provides take-home messages from ASCO 2015
CHICAGO – Dr. William J. Gradishar says bevacizumab may not be dead, T-DM1 is definitely not dead, and the jury is still out on bisphosphonates preventing recurrence, based on presentations at the annual meeting of the American Society of Clinical Oncology.
In a video interview, Dr. Gradishar, the Betsy Bramsen Professor of Breast Oncology at Northwestern University, Chicago, also outlines results presented on newer agents, including the CKD 4/6 inhibitor palbociclib and neratinib, a pan-HER tyrosine kinase inhibitor.
Dr. Gradishar had no financial disclosures related to these studies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @nikolaideslaura
CHICAGO – Dr. William J. Gradishar says bevacizumab may not be dead, T-DM1 is definitely not dead, and the jury is still out on bisphosphonates preventing recurrence, based on presentations at the annual meeting of the American Society of Clinical Oncology.
In a video interview, Dr. Gradishar, the Betsy Bramsen Professor of Breast Oncology at Northwestern University, Chicago, also outlines results presented on newer agents, including the CKD 4/6 inhibitor palbociclib and neratinib, a pan-HER tyrosine kinase inhibitor.
Dr. Gradishar had no financial disclosures related to these studies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @nikolaideslaura
CHICAGO – Dr. William J. Gradishar says bevacizumab may not be dead, T-DM1 is definitely not dead, and the jury is still out on bisphosphonates preventing recurrence, based on presentations at the annual meeting of the American Society of Clinical Oncology.
In a video interview, Dr. Gradishar, the Betsy Bramsen Professor of Breast Oncology at Northwestern University, Chicago, also outlines results presented on newer agents, including the CKD 4/6 inhibitor palbociclib and neratinib, a pan-HER tyrosine kinase inhibitor.
Dr. Gradishar had no financial disclosures related to these studies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @nikolaideslaura
EXPERT ANALYSIS FROM THE 2015 ASCO ANNUAL MEETING
VIDEO: Dr. Walter M. Stadler gives take-home messages from ASCO 2015 GU sessions
CHICAGO – Dr. Walter M. Stadler provides his take on ASCO 2015 presentations and discussions around genitourinary research, including practice-changing results on the use of chemotherapy for treating prostate cancer and the timing of androgen suppression. He also addresses the disappointing results for using precision medicine to treat bladder cancer versus the encouraging results from immunotherapy for both bladder and renal cell cancer. Dr. Stadler is the Fred C. Buffett Professor of Medicine and Surgery; associate dean for clinical research; chief, section of hematology/oncology; and director, genitourinary program, University of Chicago. He had no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @nikolaideslaura
CHICAGO – Dr. Walter M. Stadler provides his take on ASCO 2015 presentations and discussions around genitourinary research, including practice-changing results on the use of chemotherapy for treating prostate cancer and the timing of androgen suppression. He also addresses the disappointing results for using precision medicine to treat bladder cancer versus the encouraging results from immunotherapy for both bladder and renal cell cancer. Dr. Stadler is the Fred C. Buffett Professor of Medicine and Surgery; associate dean for clinical research; chief, section of hematology/oncology; and director, genitourinary program, University of Chicago. He had no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @nikolaideslaura
CHICAGO – Dr. Walter M. Stadler provides his take on ASCO 2015 presentations and discussions around genitourinary research, including practice-changing results on the use of chemotherapy for treating prostate cancer and the timing of androgen suppression. He also addresses the disappointing results for using precision medicine to treat bladder cancer versus the encouraging results from immunotherapy for both bladder and renal cell cancer. Dr. Stadler is the Fred C. Buffett Professor of Medicine and Surgery; associate dean for clinical research; chief, section of hematology/oncology; and director, genitourinary program, University of Chicago. He had no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @nikolaideslaura
AT THE 2015 ASCO ANNUAL MEETING
FDA approves first drug for high-risk neuroblastoma
The Food and Drug Administration has approved dinutuximab as part of a multimodality regimen, including surgery, chemotherapy, and radiation therapy, for pediatric patients with high-risk neuroblastoma.
The drug “marks the first approval for a therapy aimed specifically for the treatment of patients with high-risk neuroblastoma,” Dr. Richard Pazdur, director of the office of hematology and oncology products in the FDA’s Center for Drug Evaluation and Research, said in the March 10 FDA announcement.
Dinutuximab is a chimeric monoclonal antibody that targets GD2, a glycolipid on the surface of tumor cells, according to the manufacturer, United Therapeutics.
The FDA granted approval based on a clinical trial of 226 pediatric participants with high-risk neuroblastoma whose tumors shrunk or disappeared after treatment with multiple-drug chemotherapy and surgery followed by additional intensive chemotherapy and who subsequently received bone marrow transplantation support and radiation therapy. Three years after being randomly assigned to receive either isotretinoin or dinutuximab in combination with interleukin-2 and granulocyte macrophage colony-stimulating factor, 63% of participants receiving the combination were alive and free of tumor growth or recurrence, compared with 46% of participants treated with isotretinoin alone.
In an updated analysis, 73% of participants who received the combination were alive, compared with 58% of those receiving isotretinoin alone, the FDA said in the announcement.
The most common side effects were severe pain, fever, low platelet counts, infusion reactions, low blood pressure, hyponatremia, elevated liver enzymes, anemia, vomiting, diarrhea, low potassium levels in the blood, capillary leak syndrome, neutropenia and lymphopenia, hives, and low blood calcium levels, the statement said.
The drug carries a boxed warning that the drug can irritate nerve cells, causing severe pain that requires treatment with intravenous narcotics, and can cause nerve damage and life-threatening infusion reactions, including upper airway swelling, difficulty breathing, and low blood pressure, during or shortly following completion of the infusion. Dinutuximab may also cause other serious side effects, including infections, eye problems, electrolyte abnormalities, and bone marrow suppression.
The FDA granted approval of dinutuximab, to be marketed as Unituxin, following a priority review and orphan product designation.
[email protected]
On Twitter @nikolaideslaura
The Food and Drug Administration has approved dinutuximab as part of a multimodality regimen, including surgery, chemotherapy, and radiation therapy, for pediatric patients with high-risk neuroblastoma.
The drug “marks the first approval for a therapy aimed specifically for the treatment of patients with high-risk neuroblastoma,” Dr. Richard Pazdur, director of the office of hematology and oncology products in the FDA’s Center for Drug Evaluation and Research, said in the March 10 FDA announcement.
Dinutuximab is a chimeric monoclonal antibody that targets GD2, a glycolipid on the surface of tumor cells, according to the manufacturer, United Therapeutics.
The FDA granted approval based on a clinical trial of 226 pediatric participants with high-risk neuroblastoma whose tumors shrunk or disappeared after treatment with multiple-drug chemotherapy and surgery followed by additional intensive chemotherapy and who subsequently received bone marrow transplantation support and radiation therapy. Three years after being randomly assigned to receive either isotretinoin or dinutuximab in combination with interleukin-2 and granulocyte macrophage colony-stimulating factor, 63% of participants receiving the combination were alive and free of tumor growth or recurrence, compared with 46% of participants treated with isotretinoin alone.
In an updated analysis, 73% of participants who received the combination were alive, compared with 58% of those receiving isotretinoin alone, the FDA said in the announcement.
The most common side effects were severe pain, fever, low platelet counts, infusion reactions, low blood pressure, hyponatremia, elevated liver enzymes, anemia, vomiting, diarrhea, low potassium levels in the blood, capillary leak syndrome, neutropenia and lymphopenia, hives, and low blood calcium levels, the statement said.
The drug carries a boxed warning that the drug can irritate nerve cells, causing severe pain that requires treatment with intravenous narcotics, and can cause nerve damage and life-threatening infusion reactions, including upper airway swelling, difficulty breathing, and low blood pressure, during or shortly following completion of the infusion. Dinutuximab may also cause other serious side effects, including infections, eye problems, electrolyte abnormalities, and bone marrow suppression.
The FDA granted approval of dinutuximab, to be marketed as Unituxin, following a priority review and orphan product designation.
[email protected]
On Twitter @nikolaideslaura
The Food and Drug Administration has approved dinutuximab as part of a multimodality regimen, including surgery, chemotherapy, and radiation therapy, for pediatric patients with high-risk neuroblastoma.
The drug “marks the first approval for a therapy aimed specifically for the treatment of patients with high-risk neuroblastoma,” Dr. Richard Pazdur, director of the office of hematology and oncology products in the FDA’s Center for Drug Evaluation and Research, said in the March 10 FDA announcement.
Dinutuximab is a chimeric monoclonal antibody that targets GD2, a glycolipid on the surface of tumor cells, according to the manufacturer, United Therapeutics.
The FDA granted approval based on a clinical trial of 226 pediatric participants with high-risk neuroblastoma whose tumors shrunk or disappeared after treatment with multiple-drug chemotherapy and surgery followed by additional intensive chemotherapy and who subsequently received bone marrow transplantation support and radiation therapy. Three years after being randomly assigned to receive either isotretinoin or dinutuximab in combination with interleukin-2 and granulocyte macrophage colony-stimulating factor, 63% of participants receiving the combination were alive and free of tumor growth or recurrence, compared with 46% of participants treated with isotretinoin alone.
In an updated analysis, 73% of participants who received the combination were alive, compared with 58% of those receiving isotretinoin alone, the FDA said in the announcement.
The most common side effects were severe pain, fever, low platelet counts, infusion reactions, low blood pressure, hyponatremia, elevated liver enzymes, anemia, vomiting, diarrhea, low potassium levels in the blood, capillary leak syndrome, neutropenia and lymphopenia, hives, and low blood calcium levels, the statement said.
The drug carries a boxed warning that the drug can irritate nerve cells, causing severe pain that requires treatment with intravenous narcotics, and can cause nerve damage and life-threatening infusion reactions, including upper airway swelling, difficulty breathing, and low blood pressure, during or shortly following completion of the infusion. Dinutuximab may also cause other serious side effects, including infections, eye problems, electrolyte abnormalities, and bone marrow suppression.
The FDA granted approval of dinutuximab, to be marketed as Unituxin, following a priority review and orphan product designation.
[email protected]
On Twitter @nikolaideslaura
FDA approves nivolumab for treatment of advanced NSCLC
The Food and Drug Administration has expanded approval of the PD-1 inhibitor nivolumab to include treatment of patients who have metastatic squamous non–small cell lung cancer and progress following platinum-based chemotherapy.
In December, the FDA approved nivolumab to treat patients who have metastatic melanoma and no longer respond to other drugs.
Nivolumab for squamous non–small cell lung cancer (NSCLC) was reviewed under the FDA’s priority review program, and is being approved more than 3 months ahead of schedule, the FDA said in the March 4 announcement.
Efficacy was established in a trial of 272 patients with metastatic squamous NSCLC; median overall survival was increased by 3.2 months in 135 patients who received nivolumab, compared with 137 who received docetaxel.
The safety and efficacy of nivolumab to treat squamous NSCLC was also supported by a single-arm trial of 117 participants who had progressed after receiving a platinum-based therapy and at least one additional systemic regimen. The objective response rate was 15%, of whom 59% had response durations of 6 months or longer, according the the FDA statement.
The most common side effects of nivolumab are fatigue, shortness of breath, musculoskeletal pain, decreased appetite, cough, nausea, and constipation. The most serious side effects are severe immune-mediated side effects involving healthy organs, including the lung, colon, liver, kidneys, and hormone-producing glands, the FDA said.
The drug is marketed as Opdivo by Bristol-Myers Squibb.
On Twitter@NikolaidesLaura
The Food and Drug Administration has expanded approval of the PD-1 inhibitor nivolumab to include treatment of patients who have metastatic squamous non–small cell lung cancer and progress following platinum-based chemotherapy.
In December, the FDA approved nivolumab to treat patients who have metastatic melanoma and no longer respond to other drugs.
Nivolumab for squamous non–small cell lung cancer (NSCLC) was reviewed under the FDA’s priority review program, and is being approved more than 3 months ahead of schedule, the FDA said in the March 4 announcement.
Efficacy was established in a trial of 272 patients with metastatic squamous NSCLC; median overall survival was increased by 3.2 months in 135 patients who received nivolumab, compared with 137 who received docetaxel.
The safety and efficacy of nivolumab to treat squamous NSCLC was also supported by a single-arm trial of 117 participants who had progressed after receiving a platinum-based therapy and at least one additional systemic regimen. The objective response rate was 15%, of whom 59% had response durations of 6 months or longer, according the the FDA statement.
The most common side effects of nivolumab are fatigue, shortness of breath, musculoskeletal pain, decreased appetite, cough, nausea, and constipation. The most serious side effects are severe immune-mediated side effects involving healthy organs, including the lung, colon, liver, kidneys, and hormone-producing glands, the FDA said.
The drug is marketed as Opdivo by Bristol-Myers Squibb.
On Twitter@NikolaidesLaura
The Food and Drug Administration has expanded approval of the PD-1 inhibitor nivolumab to include treatment of patients who have metastatic squamous non–small cell lung cancer and progress following platinum-based chemotherapy.
In December, the FDA approved nivolumab to treat patients who have metastatic melanoma and no longer respond to other drugs.
Nivolumab for squamous non–small cell lung cancer (NSCLC) was reviewed under the FDA’s priority review program, and is being approved more than 3 months ahead of schedule, the FDA said in the March 4 announcement.
Efficacy was established in a trial of 272 patients with metastatic squamous NSCLC; median overall survival was increased by 3.2 months in 135 patients who received nivolumab, compared with 137 who received docetaxel.
The safety and efficacy of nivolumab to treat squamous NSCLC was also supported by a single-arm trial of 117 participants who had progressed after receiving a platinum-based therapy and at least one additional systemic regimen. The objective response rate was 15%, of whom 59% had response durations of 6 months or longer, according the the FDA statement.
The most common side effects of nivolumab are fatigue, shortness of breath, musculoskeletal pain, decreased appetite, cough, nausea, and constipation. The most serious side effects are severe immune-mediated side effects involving healthy organs, including the lung, colon, liver, kidneys, and hormone-producing glands, the FDA said.
The drug is marketed as Opdivo by Bristol-Myers Squibb.
On Twitter@NikolaidesLaura
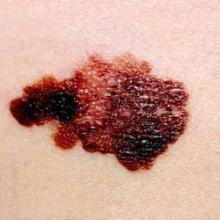
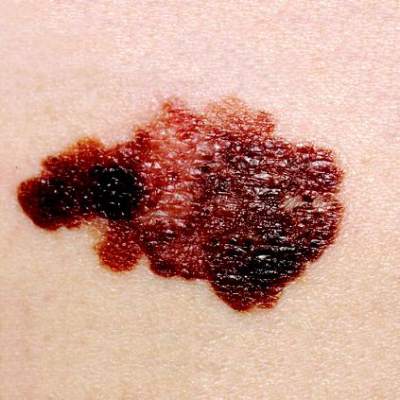
Handheld device illuminates possible routes of melanoma metastases
Investigators using a handheld dermoscopy device that allows visualization of colors, structures, and patterns in skin lesions not evident to the naked eye were able to visualize nonblanching blue and red lines in a branched pattern in two patients with in-transit cutaneous melanoma metastases.
Dr. Michael A. Marchetti and his associates at Memorial Sloan Kettering Cancer Center, New York, reported the “intriguing” visualization of dissemination for cutaneous melanoma metastases in a letter to JAMA Dermatology.
In-transit cutaneous melanoma metastases are those located more than 2 cm from the primary melanoma, but not beyond the regional nodal basin.
The first patient had wide local excision of a primary cutaneous melanoma on the forehead, and a year later, received localized irradiation for satellite skin metastases. A year after that, skin examination revealed six blue macules on the scalp more than 2 cm from the excision scar. Dermoscopy revealed nonblanching bluish lines in a branched pattern. Histopathologic examination of a skin biopsy confirmed in-transit metastatic melanoma with atypical melanocytes present in superficial dermal lymphatics, Dr. Marchetti and his associates reported (JAMA Dermatology 2015;103-5)
The second patient had a history of multiple primary melanomas, the most recent being one on the chest treated with wide local excision. At a follow-up visit 5 years later, skin examination revealed eight blue-gray macules on the chest, all more than 2 cm from the excision scar. Dermoscopy revealed nonblanching, red-bluish, fuzzy, branching lines. Histopathologic examination of a skin biopsy confirmed in-transit metastatic melanoma with atypical melanocytes present in superficial dermal blood vessels, the investigators wrote.
Typical dermoscopic features of cutaneous melanoma metastases include peripheral gray spots, atypical vessels, and a blue nevus-like pattern. The histopathologic findings in these two cases suggest that the dermoscopic color differences correspond to unique microanatomic routes of melanoma dissemination, with blue and red-blue lines corresponding to lymphatic and hematogenous dissemination of tumors, respectively, they said.
“While the factors driving lymphatic vs. hematogenous in-transit dissemination of melanoma remain unknown, as do any differences in their biologic significance, our finding is an intriguing clinical/dermoscopic/histopathologic observation,” the investigators concluded.
On Twitter @nikolaideslaura
Investigators using a handheld dermoscopy device that allows visualization of colors, structures, and patterns in skin lesions not evident to the naked eye were able to visualize nonblanching blue and red lines in a branched pattern in two patients with in-transit cutaneous melanoma metastases.
Dr. Michael A. Marchetti and his associates at Memorial Sloan Kettering Cancer Center, New York, reported the “intriguing” visualization of dissemination for cutaneous melanoma metastases in a letter to JAMA Dermatology.
In-transit cutaneous melanoma metastases are those located more than 2 cm from the primary melanoma, but not beyond the regional nodal basin.
The first patient had wide local excision of a primary cutaneous melanoma on the forehead, and a year later, received localized irradiation for satellite skin metastases. A year after that, skin examination revealed six blue macules on the scalp more than 2 cm from the excision scar. Dermoscopy revealed nonblanching bluish lines in a branched pattern. Histopathologic examination of a skin biopsy confirmed in-transit metastatic melanoma with atypical melanocytes present in superficial dermal lymphatics, Dr. Marchetti and his associates reported (JAMA Dermatology 2015;103-5)
The second patient had a history of multiple primary melanomas, the most recent being one on the chest treated with wide local excision. At a follow-up visit 5 years later, skin examination revealed eight blue-gray macules on the chest, all more than 2 cm from the excision scar. Dermoscopy revealed nonblanching, red-bluish, fuzzy, branching lines. Histopathologic examination of a skin biopsy confirmed in-transit metastatic melanoma with atypical melanocytes present in superficial dermal blood vessels, the investigators wrote.
Typical dermoscopic features of cutaneous melanoma metastases include peripheral gray spots, atypical vessels, and a blue nevus-like pattern. The histopathologic findings in these two cases suggest that the dermoscopic color differences correspond to unique microanatomic routes of melanoma dissemination, with blue and red-blue lines corresponding to lymphatic and hematogenous dissemination of tumors, respectively, they said.
“While the factors driving lymphatic vs. hematogenous in-transit dissemination of melanoma remain unknown, as do any differences in their biologic significance, our finding is an intriguing clinical/dermoscopic/histopathologic observation,” the investigators concluded.
On Twitter @nikolaideslaura
Investigators using a handheld dermoscopy device that allows visualization of colors, structures, and patterns in skin lesions not evident to the naked eye were able to visualize nonblanching blue and red lines in a branched pattern in two patients with in-transit cutaneous melanoma metastases.
Dr. Michael A. Marchetti and his associates at Memorial Sloan Kettering Cancer Center, New York, reported the “intriguing” visualization of dissemination for cutaneous melanoma metastases in a letter to JAMA Dermatology.
In-transit cutaneous melanoma metastases are those located more than 2 cm from the primary melanoma, but not beyond the regional nodal basin.
The first patient had wide local excision of a primary cutaneous melanoma on the forehead, and a year later, received localized irradiation for satellite skin metastases. A year after that, skin examination revealed six blue macules on the scalp more than 2 cm from the excision scar. Dermoscopy revealed nonblanching bluish lines in a branched pattern. Histopathologic examination of a skin biopsy confirmed in-transit metastatic melanoma with atypical melanocytes present in superficial dermal lymphatics, Dr. Marchetti and his associates reported (JAMA Dermatology 2015;103-5)
The second patient had a history of multiple primary melanomas, the most recent being one on the chest treated with wide local excision. At a follow-up visit 5 years later, skin examination revealed eight blue-gray macules on the chest, all more than 2 cm from the excision scar. Dermoscopy revealed nonblanching, red-bluish, fuzzy, branching lines. Histopathologic examination of a skin biopsy confirmed in-transit metastatic melanoma with atypical melanocytes present in superficial dermal blood vessels, the investigators wrote.
Typical dermoscopic features of cutaneous melanoma metastases include peripheral gray spots, atypical vessels, and a blue nevus-like pattern. The histopathologic findings in these two cases suggest that the dermoscopic color differences correspond to unique microanatomic routes of melanoma dissemination, with blue and red-blue lines corresponding to lymphatic and hematogenous dissemination of tumors, respectively, they said.
“While the factors driving lymphatic vs. hematogenous in-transit dissemination of melanoma remain unknown, as do any differences in their biologic significance, our finding is an intriguing clinical/dermoscopic/histopathologic observation,” the investigators concluded.
On Twitter @nikolaideslaura
FROM JAMA DERMATOLOGY
MEK inhibitors can induce skin eruptions with distinctive duskiness
Case reports of unusual drug hypersensitivity to MEK inhibitors, involving skin eruptions with distinctive central duskiness, have been described online in JAMA Dermatology.
Three patients who were receiving different MEK inhibitors (selumetinib, cobimetinib, and trametinib) developed grade 2 or 3 eruptions, all associated with unique duskiness, reported Dr. Urvi Patel and associates at Washington University, St. Louis.
A 60-year-old man with pancreatic cancer who was receiving selumetinib as part of a clinical trial presented with a grade 2 generalized eruption and pruritus 12 days after initiating therapy. He had diffuse targetoid patches with central duskiness. Selumetinib and other study drugs were withheld, the patient was given topical corticosteroid treatment, and the eruption completely resolved after 4 weeks. The patient did not restart the study drugs because of an elevated alkaline phosphatase level and fatigue.
A woman in her 40s who was receiving cobimetinib and other medication for metastatic melanoma developed grade 2 coalescing urticarial patches with surrounding duskiness on day 28 of treatment. Histopathologic examination showed a superficial perivascular lymphocytic infiltrate with rare eosinophils. After treatment was halted for 7 days and a regimen of oral prednisone was started, cobimetinib therapy was reinstituted at a lower dose. There was no recurrence of the eruption 1 year after cobimetinib therapy was restarted, Dr. Patel and associates reported (JAMA Dermatol. 2015 Jan. 14 [doi:10.1001/jamadermatol.2014.3207]).
The third patient, a woman in her 50s with metastatic melanoma, developed a grade 3 eruption 7 weeks into trametinib treatment together with another drug. The worsening urticarial patches and plaques had surrounding diffuse duskiness. After trametinib treatment was withheld for a week, and a regimen of oral prednisone was begun, trametinib therapy was restarted and the eruption did not return.
“As shown in our patients, successful treatment of this MEK inhibitor–associated cutaneous eruption can include a drug holiday and oral corticosteroid therapy, with reinstitution of the drug at a lower dose without recurrence,” Dr. Patel and his associates wrote.
MEK inhibitors target the mitogen-activated protein kinase pathway. Trametinib has been approved for treating advanced melanoma, and more than a dozen other MEK inhibitors are in clinical trials (including selumetinib and cobimetinib) for treatment of melanoma and other solid-organ malignant neoplasms, including pancreatic, hepatocellular, colorectal, and non–small cell lung cancer, the authors noted.
On Twitter @nikolaideslaura
Case reports of unusual drug hypersensitivity to MEK inhibitors, involving skin eruptions with distinctive central duskiness, have been described online in JAMA Dermatology.
Three patients who were receiving different MEK inhibitors (selumetinib, cobimetinib, and trametinib) developed grade 2 or 3 eruptions, all associated with unique duskiness, reported Dr. Urvi Patel and associates at Washington University, St. Louis.
A 60-year-old man with pancreatic cancer who was receiving selumetinib as part of a clinical trial presented with a grade 2 generalized eruption and pruritus 12 days after initiating therapy. He had diffuse targetoid patches with central duskiness. Selumetinib and other study drugs were withheld, the patient was given topical corticosteroid treatment, and the eruption completely resolved after 4 weeks. The patient did not restart the study drugs because of an elevated alkaline phosphatase level and fatigue.
A woman in her 40s who was receiving cobimetinib and other medication for metastatic melanoma developed grade 2 coalescing urticarial patches with surrounding duskiness on day 28 of treatment. Histopathologic examination showed a superficial perivascular lymphocytic infiltrate with rare eosinophils. After treatment was halted for 7 days and a regimen of oral prednisone was started, cobimetinib therapy was reinstituted at a lower dose. There was no recurrence of the eruption 1 year after cobimetinib therapy was restarted, Dr. Patel and associates reported (JAMA Dermatol. 2015 Jan. 14 [doi:10.1001/jamadermatol.2014.3207]).
The third patient, a woman in her 50s with metastatic melanoma, developed a grade 3 eruption 7 weeks into trametinib treatment together with another drug. The worsening urticarial patches and plaques had surrounding diffuse duskiness. After trametinib treatment was withheld for a week, and a regimen of oral prednisone was begun, trametinib therapy was restarted and the eruption did not return.
“As shown in our patients, successful treatment of this MEK inhibitor–associated cutaneous eruption can include a drug holiday and oral corticosteroid therapy, with reinstitution of the drug at a lower dose without recurrence,” Dr. Patel and his associates wrote.
MEK inhibitors target the mitogen-activated protein kinase pathway. Trametinib has been approved for treating advanced melanoma, and more than a dozen other MEK inhibitors are in clinical trials (including selumetinib and cobimetinib) for treatment of melanoma and other solid-organ malignant neoplasms, including pancreatic, hepatocellular, colorectal, and non–small cell lung cancer, the authors noted.
On Twitter @nikolaideslaura
Case reports of unusual drug hypersensitivity to MEK inhibitors, involving skin eruptions with distinctive central duskiness, have been described online in JAMA Dermatology.
Three patients who were receiving different MEK inhibitors (selumetinib, cobimetinib, and trametinib) developed grade 2 or 3 eruptions, all associated with unique duskiness, reported Dr. Urvi Patel and associates at Washington University, St. Louis.
A 60-year-old man with pancreatic cancer who was receiving selumetinib as part of a clinical trial presented with a grade 2 generalized eruption and pruritus 12 days after initiating therapy. He had diffuse targetoid patches with central duskiness. Selumetinib and other study drugs were withheld, the patient was given topical corticosteroid treatment, and the eruption completely resolved after 4 weeks. The patient did not restart the study drugs because of an elevated alkaline phosphatase level and fatigue.
A woman in her 40s who was receiving cobimetinib and other medication for metastatic melanoma developed grade 2 coalescing urticarial patches with surrounding duskiness on day 28 of treatment. Histopathologic examination showed a superficial perivascular lymphocytic infiltrate with rare eosinophils. After treatment was halted for 7 days and a regimen of oral prednisone was started, cobimetinib therapy was reinstituted at a lower dose. There was no recurrence of the eruption 1 year after cobimetinib therapy was restarted, Dr. Patel and associates reported (JAMA Dermatol. 2015 Jan. 14 [doi:10.1001/jamadermatol.2014.3207]).
The third patient, a woman in her 50s with metastatic melanoma, developed a grade 3 eruption 7 weeks into trametinib treatment together with another drug. The worsening urticarial patches and plaques had surrounding diffuse duskiness. After trametinib treatment was withheld for a week, and a regimen of oral prednisone was begun, trametinib therapy was restarted and the eruption did not return.
“As shown in our patients, successful treatment of this MEK inhibitor–associated cutaneous eruption can include a drug holiday and oral corticosteroid therapy, with reinstitution of the drug at a lower dose without recurrence,” Dr. Patel and his associates wrote.
MEK inhibitors target the mitogen-activated protein kinase pathway. Trametinib has been approved for treating advanced melanoma, and more than a dozen other MEK inhibitors are in clinical trials (including selumetinib and cobimetinib) for treatment of melanoma and other solid-organ malignant neoplasms, including pancreatic, hepatocellular, colorectal, and non–small cell lung cancer, the authors noted.
On Twitter @nikolaideslaura
FROM JAMA DERMATOLOGY
Key clinical point: This MEK inhibitor–associated cutaneous eruption can be treated with a drug holiday and oral corticosteroid treatment, restarting the drug at a lower dose without recurrence.
Major finding: Three patients who were receiving different MEK inhibitors (selumetinib, cobimetinib, and trametinib) developed grade 2 or 3 eruptions, all associated with unique duskiness.
Data source: Three case studies of patients receiving different MEK inhibitors.
Disclosures: Dr. Lynn Cornelius has received a research grant from Genentech and is a clinical subinvestigator for GlaxoSmithKline. Dr. Milan J. Anadkat has received honoraria as a speaker and/or consultant from AstraZeneca, Bristol-Myers Squibb, Eisai, ImClone, and Therakos. No other disclosures were reported.
Adding cisplatin to docetaxel monotherapy provides no benefit for elderly with NSCLC
There is no advantage to weekly docetaxel plus cisplatin over docetaxel monotherapy as first-line chemotherapy for elderly patients with advanced non–small cell lung cancer, according to phase III study results published online Jan. 12 in the Journal of Clinical Oncology.
For the study, 276 chemotherapy-naive patients aged 70 years or older with stage III, stage IV, or recurrent non–small cell lung cancer (NSCLC) who were considered unsuitable for bolus cisplatin administration were randomly assigned to receive docetaxel 60 mg/m2 on day 1, every 3 weeks, or docetaxel 20 mg/m2 plus cisplatin 25 mg/m2 on days 1, 8, and 15 every 4 weeks.
At the interim analysis, overall survival was inferior among patients who received docetaxel plus cisplatin, compared with those who received docetaxel only (hazard ratio, 1.56; 95% confidence interval, 0.98 to 2.49), Dr. Tetsuya Abe of Niigata (Japan) Cancer Center Hospital and his associates reported (J. Clin. Onc. 2015 Jan. 12. [doi:10.1200/JCO.2014.55.8627]).
The investigators terminated the study early after finding the predictive probability that the treatment arm would be statistically superior to the monotherapy arm on final analysis was less than 1%. The median survival time as 14.8 months for the monotherapy arm and 13.3 months for the docetaxel plus cisplatin arm (HR, 1.18; 95% CI, 0.83 to 1.69).
Toxicity varied between arms. The rates of neutropenia were higher with docetaxel alone, while rates of grade 3 or greater anemia, anorexia, and hyponatremia were higher in the combination arm, they said.
Docetaxel every 3 weeks remains the standard treatment for elderly patients with advanced NSCLC, Dr. Abe and associates concluded.
Dr. Abe reported having no financial disclosures. Other authors reported honoraria from sanofi-aventis and Bristol-Myers Squibb.
On Twitter @nikolaideslaura
What lesson can we learn from this study, and how should the results influence current management of elderly patients with lung cancer?
It is important to accurately interpret the finding of this study, which is that the combination of cisplatin/docetaxel administered on a weekly schedule is not superior to single-agent docetaxel given every 3 weeks in patients older than 70 years of age with lung cancer. The study does not negate the well-founded recognition that patient age does not preclude clinical benefit of systemic anticancer therapy and that elderly patients with lung cancer should be offered systemic therapy if they are fit enough to tolerate the treatment.
Although the study was designed to evaluate treatment efficacy in elderly patients, only patients who were unsuitable for bolus cisplatin infusion were included. As there is no clear definition for patients unsuitable for bolus cisplatin, we believe that this might have led to the enrollment of a patient subset with unfavorable prognosis. The interpretation, external validity, and generalizability of the result of this study are greatly limited because the unwieldy definition of patients considered unsuitable for cisplatin makes it difficult to establish how well the study population represents the elderly patient population with lung cancer.
There is increasing agreement that treatment decisions for elderly patients should be based on performance status, comorbid conditions, and patient preferences. Treatment decisions based entirely on chronological age and not informed by the tremendous knowledge gained in optimal assessment of older patients in recent years do not serve our patients well. Finally, it is imperative to include functional assessment as an integral component of clinical trials designed for older patients.
Dr. Taofeek K. Owonikoko and Dr. Suresh S. Ramalingam are with Emory University and Winship Cancer Institute, Atlanta. These remarks were extracted from the accompanying editorial (J. Clin. Onc. 2015 Jan. 12 [doi:10.1200/JCO.2014.59.5033]).
What lesson can we learn from this study, and how should the results influence current management of elderly patients with lung cancer?
It is important to accurately interpret the finding of this study, which is that the combination of cisplatin/docetaxel administered on a weekly schedule is not superior to single-agent docetaxel given every 3 weeks in patients older than 70 years of age with lung cancer. The study does not negate the well-founded recognition that patient age does not preclude clinical benefit of systemic anticancer therapy and that elderly patients with lung cancer should be offered systemic therapy if they are fit enough to tolerate the treatment.
Although the study was designed to evaluate treatment efficacy in elderly patients, only patients who were unsuitable for bolus cisplatin infusion were included. As there is no clear definition for patients unsuitable for bolus cisplatin, we believe that this might have led to the enrollment of a patient subset with unfavorable prognosis. The interpretation, external validity, and generalizability of the result of this study are greatly limited because the unwieldy definition of patients considered unsuitable for cisplatin makes it difficult to establish how well the study population represents the elderly patient population with lung cancer.
There is increasing agreement that treatment decisions for elderly patients should be based on performance status, comorbid conditions, and patient preferences. Treatment decisions based entirely on chronological age and not informed by the tremendous knowledge gained in optimal assessment of older patients in recent years do not serve our patients well. Finally, it is imperative to include functional assessment as an integral component of clinical trials designed for older patients.
Dr. Taofeek K. Owonikoko and Dr. Suresh S. Ramalingam are with Emory University and Winship Cancer Institute, Atlanta. These remarks were extracted from the accompanying editorial (J. Clin. Onc. 2015 Jan. 12 [doi:10.1200/JCO.2014.59.5033]).
What lesson can we learn from this study, and how should the results influence current management of elderly patients with lung cancer?
It is important to accurately interpret the finding of this study, which is that the combination of cisplatin/docetaxel administered on a weekly schedule is not superior to single-agent docetaxel given every 3 weeks in patients older than 70 years of age with lung cancer. The study does not negate the well-founded recognition that patient age does not preclude clinical benefit of systemic anticancer therapy and that elderly patients with lung cancer should be offered systemic therapy if they are fit enough to tolerate the treatment.
Although the study was designed to evaluate treatment efficacy in elderly patients, only patients who were unsuitable for bolus cisplatin infusion were included. As there is no clear definition for patients unsuitable for bolus cisplatin, we believe that this might have led to the enrollment of a patient subset with unfavorable prognosis. The interpretation, external validity, and generalizability of the result of this study are greatly limited because the unwieldy definition of patients considered unsuitable for cisplatin makes it difficult to establish how well the study population represents the elderly patient population with lung cancer.
There is increasing agreement that treatment decisions for elderly patients should be based on performance status, comorbid conditions, and patient preferences. Treatment decisions based entirely on chronological age and not informed by the tremendous knowledge gained in optimal assessment of older patients in recent years do not serve our patients well. Finally, it is imperative to include functional assessment as an integral component of clinical trials designed for older patients.
Dr. Taofeek K. Owonikoko and Dr. Suresh S. Ramalingam are with Emory University and Winship Cancer Institute, Atlanta. These remarks were extracted from the accompanying editorial (J. Clin. Onc. 2015 Jan. 12 [doi:10.1200/JCO.2014.59.5033]).
There is no advantage to weekly docetaxel plus cisplatin over docetaxel monotherapy as first-line chemotherapy for elderly patients with advanced non–small cell lung cancer, according to phase III study results published online Jan. 12 in the Journal of Clinical Oncology.
For the study, 276 chemotherapy-naive patients aged 70 years or older with stage III, stage IV, or recurrent non–small cell lung cancer (NSCLC) who were considered unsuitable for bolus cisplatin administration were randomly assigned to receive docetaxel 60 mg/m2 on day 1, every 3 weeks, or docetaxel 20 mg/m2 plus cisplatin 25 mg/m2 on days 1, 8, and 15 every 4 weeks.
At the interim analysis, overall survival was inferior among patients who received docetaxel plus cisplatin, compared with those who received docetaxel only (hazard ratio, 1.56; 95% confidence interval, 0.98 to 2.49), Dr. Tetsuya Abe of Niigata (Japan) Cancer Center Hospital and his associates reported (J. Clin. Onc. 2015 Jan. 12. [doi:10.1200/JCO.2014.55.8627]).
The investigators terminated the study early after finding the predictive probability that the treatment arm would be statistically superior to the monotherapy arm on final analysis was less than 1%. The median survival time as 14.8 months for the monotherapy arm and 13.3 months for the docetaxel plus cisplatin arm (HR, 1.18; 95% CI, 0.83 to 1.69).
Toxicity varied between arms. The rates of neutropenia were higher with docetaxel alone, while rates of grade 3 or greater anemia, anorexia, and hyponatremia were higher in the combination arm, they said.
Docetaxel every 3 weeks remains the standard treatment for elderly patients with advanced NSCLC, Dr. Abe and associates concluded.
Dr. Abe reported having no financial disclosures. Other authors reported honoraria from sanofi-aventis and Bristol-Myers Squibb.
On Twitter @nikolaideslaura
There is no advantage to weekly docetaxel plus cisplatin over docetaxel monotherapy as first-line chemotherapy for elderly patients with advanced non–small cell lung cancer, according to phase III study results published online Jan. 12 in the Journal of Clinical Oncology.
For the study, 276 chemotherapy-naive patients aged 70 years or older with stage III, stage IV, or recurrent non–small cell lung cancer (NSCLC) who were considered unsuitable for bolus cisplatin administration were randomly assigned to receive docetaxel 60 mg/m2 on day 1, every 3 weeks, or docetaxel 20 mg/m2 plus cisplatin 25 mg/m2 on days 1, 8, and 15 every 4 weeks.
At the interim analysis, overall survival was inferior among patients who received docetaxel plus cisplatin, compared with those who received docetaxel only (hazard ratio, 1.56; 95% confidence interval, 0.98 to 2.49), Dr. Tetsuya Abe of Niigata (Japan) Cancer Center Hospital and his associates reported (J. Clin. Onc. 2015 Jan. 12. [doi:10.1200/JCO.2014.55.8627]).
The investigators terminated the study early after finding the predictive probability that the treatment arm would be statistically superior to the monotherapy arm on final analysis was less than 1%. The median survival time as 14.8 months for the monotherapy arm and 13.3 months for the docetaxel plus cisplatin arm (HR, 1.18; 95% CI, 0.83 to 1.69).
Toxicity varied between arms. The rates of neutropenia were higher with docetaxel alone, while rates of grade 3 or greater anemia, anorexia, and hyponatremia were higher in the combination arm, they said.
Docetaxel every 3 weeks remains the standard treatment for elderly patients with advanced NSCLC, Dr. Abe and associates concluded.
Dr. Abe reported having no financial disclosures. Other authors reported honoraria from sanofi-aventis and Bristol-Myers Squibb.
On Twitter @nikolaideslaura
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Docetaxel every 3 weeks remains the standard treatment for elderly patients with advanced non–small cell lung cancer.
Major finding: Overall survival was inferior among patients who received docetaxel plus cisplatin, compared with those who received docetaxel only (hazard ratio, 1.56; 95% confidence interval, 0.98 to 2.49).
Data source: Randomized phase III Japanese trial of 276 elderly patients with stage III, stage IV, or recurrent non–small cell lung cancer (Intergroup Trial JCOG0803/WJOG4307L).
Disclosures: Dr. Abe reported having no financial disclosures. Other authors reported honoraria from sanofi-aventis and Bristol-Myers Squibb.
Postdiagnosis imaging increasing for patients with all stages of thyroid cancer
Diagnosis of low-risk thyroid cancer has increased, yet researchers have unexpectedly found an increase in the use of postdiagnosis imaging among patients with all stages of disease, according to a report published online in Cancer.
“Greater imaging use clearly contributes to increased costs. Specific to thyroid cancer, increased imaging may identify low-volume recurrent disease that is unlikely to be clinically significant, leading to heightened patient anxiety and potentially unnecessary interventions,” Dr. Jaime L. Wiebel and associates at the University of Michigan wrote.
Investigators analyzed the records of 23,669 patients diagnosed with differentiated thyroid cancer from 1991-2009 in the Surveillance, Epidemiology, and End Results-Medicare database, and identified the percentage of patients who underwent a neck ultrasound, I-131 scan, or PET scan during the first 3 years after diagnosis.
“Using the SEER-Medicare database, we unexpectedly found that there was a significant increase in the use of surveillance imaging studies over the past 20 years across all stages of disease,” Dr. Wiebel and her associates wrote.
Patients diagnosed after 2000 were more likely to have localized disease (P < .001) and tumors measuring less than 1 cm (P < .001), compared with those diagnosed between 1991 and 2000, and were more likely to undergo neck ultrasound (odds ratio, 2.15; 95% confidence interval, 2.02 to 2.28) and I-131 scan (odds ratio, 1.44; 95% confidence interval, 1.35 to 1.54).
Compared with 1996 through 2004, PET scan use from 2005 to 2009 increased 32.4-fold (P = .001) in patients with localized disease, 13.1-fold (P < .001) in patients with regional disease, and 33.4-fold (P < .001) in patients with distant differentiated thyroid cancer, Dr. Wiebel and her associates reported (Cancer 2015 Jan. 6 [doi: 10.1002/cncr.29210]).
“We demonstrated the increased use of imaging over time despite the diagnosis of smaller, more limited, low-risk thyroid cancer. Although many of the themes presented in the current study are true for other malignancies, the overuse of imaging is highlighted by the increased use in patients with this relatively indolent malignancy,” the researchers wrote.
On Twitter @nikolaideslaura
Diagnosis of low-risk thyroid cancer has increased, yet researchers have unexpectedly found an increase in the use of postdiagnosis imaging among patients with all stages of disease, according to a report published online in Cancer.
“Greater imaging use clearly contributes to increased costs. Specific to thyroid cancer, increased imaging may identify low-volume recurrent disease that is unlikely to be clinically significant, leading to heightened patient anxiety and potentially unnecessary interventions,” Dr. Jaime L. Wiebel and associates at the University of Michigan wrote.
Investigators analyzed the records of 23,669 patients diagnosed with differentiated thyroid cancer from 1991-2009 in the Surveillance, Epidemiology, and End Results-Medicare database, and identified the percentage of patients who underwent a neck ultrasound, I-131 scan, or PET scan during the first 3 years after diagnosis.
“Using the SEER-Medicare database, we unexpectedly found that there was a significant increase in the use of surveillance imaging studies over the past 20 years across all stages of disease,” Dr. Wiebel and her associates wrote.
Patients diagnosed after 2000 were more likely to have localized disease (P < .001) and tumors measuring less than 1 cm (P < .001), compared with those diagnosed between 1991 and 2000, and were more likely to undergo neck ultrasound (odds ratio, 2.15; 95% confidence interval, 2.02 to 2.28) and I-131 scan (odds ratio, 1.44; 95% confidence interval, 1.35 to 1.54).
Compared with 1996 through 2004, PET scan use from 2005 to 2009 increased 32.4-fold (P = .001) in patients with localized disease, 13.1-fold (P < .001) in patients with regional disease, and 33.4-fold (P < .001) in patients with distant differentiated thyroid cancer, Dr. Wiebel and her associates reported (Cancer 2015 Jan. 6 [doi: 10.1002/cncr.29210]).
“We demonstrated the increased use of imaging over time despite the diagnosis of smaller, more limited, low-risk thyroid cancer. Although many of the themes presented in the current study are true for other malignancies, the overuse of imaging is highlighted by the increased use in patients with this relatively indolent malignancy,” the researchers wrote.
On Twitter @nikolaideslaura
Diagnosis of low-risk thyroid cancer has increased, yet researchers have unexpectedly found an increase in the use of postdiagnosis imaging among patients with all stages of disease, according to a report published online in Cancer.
“Greater imaging use clearly contributes to increased costs. Specific to thyroid cancer, increased imaging may identify low-volume recurrent disease that is unlikely to be clinically significant, leading to heightened patient anxiety and potentially unnecessary interventions,” Dr. Jaime L. Wiebel and associates at the University of Michigan wrote.
Investigators analyzed the records of 23,669 patients diagnosed with differentiated thyroid cancer from 1991-2009 in the Surveillance, Epidemiology, and End Results-Medicare database, and identified the percentage of patients who underwent a neck ultrasound, I-131 scan, or PET scan during the first 3 years after diagnosis.
“Using the SEER-Medicare database, we unexpectedly found that there was a significant increase in the use of surveillance imaging studies over the past 20 years across all stages of disease,” Dr. Wiebel and her associates wrote.
Patients diagnosed after 2000 were more likely to have localized disease (P < .001) and tumors measuring less than 1 cm (P < .001), compared with those diagnosed between 1991 and 2000, and were more likely to undergo neck ultrasound (odds ratio, 2.15; 95% confidence interval, 2.02 to 2.28) and I-131 scan (odds ratio, 1.44; 95% confidence interval, 1.35 to 1.54).
Compared with 1996 through 2004, PET scan use from 2005 to 2009 increased 32.4-fold (P = .001) in patients with localized disease, 13.1-fold (P < .001) in patients with regional disease, and 33.4-fold (P < .001) in patients with distant differentiated thyroid cancer, Dr. Wiebel and her associates reported (Cancer 2015 Jan. 6 [doi: 10.1002/cncr.29210]).
“We demonstrated the increased use of imaging over time despite the diagnosis of smaller, more limited, low-risk thyroid cancer. Although many of the themes presented in the current study are true for other malignancies, the overuse of imaging is highlighted by the increased use in patients with this relatively indolent malignancy,” the researchers wrote.
On Twitter @nikolaideslaura
FROM CANCER
Key clinical point: Increased imaging may identify low-volume recurrent disease that is unlikely to be clinically significant, leading to heightened patient anxiety and potentially unnecessary interventions.
Major finding: Patients diagnosed after 2000 were more likely to have localized disease (P < .001) and tumors measuring less than 1 cm (P < .001) and more likely to undergo neck ultrasound (odds ratio, 2.15; 95% confidence interval, 2.02 to 2.28) and I-131 scan (odds ratio, 1.44; 95% confidence interval, 1.35 to 1.54).
Data source: Records of 23,669 patients diagnosed with differentiated thyroid cancer from January 1, 1991 to December 31, 2009 in the Surveillance, Epidemiology, and End Results-Medicare database.
Disclosures: The study was funded by the University of Michigan MCubed Seed Funding Program and the Punya Foundation for Thyroid Cancer Research. Dr. Wiebel reported no disclosures. Other authors reported financial support from NIH or NCI grants.