User login
Association of Atrial Fibrillation and/or Flutter With Adverse Cardiac Outcomes and Mortality in Patients With Wolff-Parkinson-White Syndrome
Wolff-Parkinson-White (WPW) syndrome is characterized by the presence of ≥ 1 accessory pathways and the development of both recurrent paroxysmal atrial fibrillation (AF) and supraventricular tachycardia that can lead to further malignant arrhythmias resulting in sudden cardiac death (SCD).1-7 Historically, incidental, ventricular pre-excitation on electrocardiogram has conferred a relatively low SCD risk in adults; however, newer WPW syndrome data suggest the endpoint may not be as benign as previously thought.7 The current literature has defined atrioventricular reentrant tachycardia triggering AF, rather than symptoms, as an independent risk factor for malignant arrhythmias. Still, long-term data detailing the association of AF with serious cardiac events and death in patients with WPW syndrome are still limited.1-7
While previous guidelines for the treatment of WPW syndrome only recommended routine electrophysiology testing (EPT) with liberal catheter ablation for symptomatic individuals, the 2015 American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines now suggest its potential benefit for risk stratification in the asymptomatic population.8-12 Given the limited existing data, more long-term studies are needed to corroborate the latest EPT recommendations before routinely applying them in practice. Furthermore, since concomitant AF can lead to adverse cardiac outcomes in patients with WPW syndrome, additional data evaluating this association are also necessary. In this study, we aimed to determine the impact of atrial fibrillation and/or flutter (AF/AFL) on adverse cardiac outcomes and mortality in patients with WPW syndrome.
METHODS
This study used data from the Military Health System (MHS) Database Repository. The MHS is one of the largest health care systems in the country and includes information on about 10 million active duty and retired military service members and their families (51% male; 49% female).13,14 Data were fully anonymized and complied in accordance with federal and state laws, including the Health Insurance Portability and Accountability Act of 1996. The Naval Medical Center Portsmouth Institutional Review Board approved this study.
Study Design
This retrospective, observational cohort study identified MHS patients with WPW syndrome from January 1, 2014, to December 31, 2019. Patients were included if they had ≥ 2 International Classification of Diseases, Ninth Revision (ICD-9) or International Classification of Diseases, Tenth Revision (ICD-10) diagnosis codes for WPW syndrome (ICD-9, 426.7; ICD-10, I45.6) on separate dates; were aged ≥ 18 years at index date; and had ≥ 1 year of continuous eligibility prior to the index date (enrollment gaps ≤ 30 days were considered continuous). Patients were then divided into 2 subgroups by the presence or absence of AF/AFL using diagnostic codes. Patients were excluded if they had evidence of an implantable cardioverter-defibrillator, permanent pacemaker or were missing age or sex data. Patients were followed from index date until the first occurrence of the outcome of interest, MHS disenrollment, or the end of the study period.
Cardiac composite outcomes comprised of sudden cardiac arrest (SCA), ventricular fibrillation (VF), ventricular tachycardia and death, as well as death specifically, were the outcomes of interest and assessed after index date using ICD-9 and ICD-10 codes. Death was defined as all-cause mortality. Time to event was calculated based on the date of the initial component from the composite outcome and date of death specifically for mortality. Those not experiencing an outcome were followed until MHS disenrollment or the end of the study period.
Various patient characteristics were assessed at index including age, sex, military sponsor (the patient’s active or retired duty member through which their dependent receives TRICARE benefits) rank and branch, geographic region, type of US Department of Defense beneficiary, and index year. Clinical characteristics were assessed over a 1-year baseline period prior to index date and included the number of cardiologist and clinical visits for WPW syndrome, Charlson Comorbidity Index (CCI) scores calculated from diagnostic codes outlined in the Quan coding method, and preindex time.15 Comorbidities were assessed at baseline and defined as having ≥ 1 ICD-9 or ICD-10 code for a corresponding condition within 1 year prior to index.
Statistical Analysis
Baseline characteristics were assessed and descriptive statistics for categorical and continuous variables were presented accordingly. To assess bivariate association with exposure, χ2 tests were used to compare categorical variables, while t tests were used to compare continuous variables by exposure status. Incidence proportions and rates were reported for each outcome of interest. Kaplan-Meier curves were constructed to assess the bivariate association between exposure and study outcomes. Cox proportional hazard modeling was performed to estimate the association between AF/AFL and time to each of the outcomes. Multiple models were designed to assess cardiac and metabolic covariates, in addition to baseline characteristics. This included a base model adjusted for age, sex, military sponsor rank and branch, geographic region, and duty status.
Additional models adjusted for cardiac and metabolic confounders and CCI score. A comprehensive model included the base, cardiac, and metabolic covariates. Multicollinearity between covariates was assessed. Variables with a variance inflation factor > 4 or a tolerance level < 0.1 were added to the models. Cox proportional hazard models were used to estimate the unadjusted and adjusted hazard ratios (HRs) and 95% CIs of the association between AF/AFL and the study outcomes. Data were analyzed using SAS, version 9.4 for Windows.
RESULTS
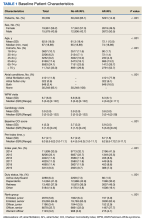
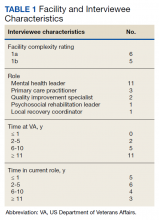
From 2014 through 2019, 35,539 patients with WPW syndrome were identified in the MHS, 5291 had AF/AFL (14.9%); 19,961 were female (56.2%), the mean (SD) age was 62.9 (18.0) years, and 11,742 were aged ≥ 75 years (33.0%) (Table 1).
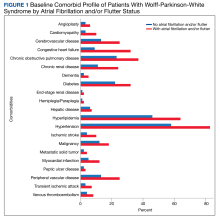
There were 4121 (11.6%), 322 (0.9%), and 848 (2.4%) patients with AF, AFL, and both arrhythmias, respectively. The mean (SD) number of cardiology visits was 3.9 (3.0). The mean (SD) baseline CCI score for the AF/AFL subgroup was 5.9 (3.5) vs 3.7 (2.2) for the non-AF/AFL subgroup (P < .001). The most prevalent comorbid conditions were hypertension, hyperlipidemia, chronic obstructive pulmonary disease, and diabetes (P < .001) (Figure 1).
Composite Outcomes
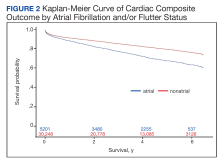
In the overall cohort, during a mean (SD) follow-up time of 3.4 (2.0) years comprising 119,682 total person-years, the components of the composite outcome occurred 6506 times with an incidence rate of 5.44 per 100 person-years. Ventricular tachycardia was the most common event, occurring 3281 times with an incidence rate of 2.74 per 100 person-years. SCA and VF occurred 841 and 135 times with incidence rates of 0.70 and 0.11 per 100 person-years, respectively. Death was the initial event 2249 times with an incidence rate of 1.88 per 100 person-years. Figure 2 shows the Kaplan-Meier curve of cardiac composite outcome by AF/AFL status.
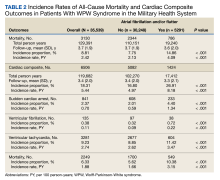
The subgroup with AF/AFL comprised 17,412 total person-years and 1424 cardiac composite incidences compared with 102,270 person years and 5082 incidences in the no AF/AFL group (Table 2). Comparing AF/AFL vs no AF/AFL incidence rates were 8.18 vs 4.97 per 100 person-years, respectively (P < .001). SCA and VF occurred 233 and 38 times and respectively had incidence rates of 1.34 and 0.22 per 100 person-years in the AF/AFL group vs 0.59 and 0.09 per 100 person-years in the no AF/AFL group (P < .001). There were 549 deaths and a 3.15 per 100 person-years incidence rate in the AF/AFL group vs 1700 deaths and a 1.66 incidence rate in the no AF/AFL group (P < .001).
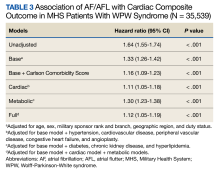
The HR for the composite outcome in the base model was 1.33 (95% CI, 1.26-1.42, P < .001) (Table 3). The association between AF/AFL and the composite outcome remained significant after adjusting for additional metabolic and cardiac covariates. The HRs for the metabolic and cardiac models were 1.30 (95% CI, 1.23-1.38, P < .001) and 1.11 (95% CI, 1.05-1.18, P < .001), respectively. After adjusting for the full model, the HR was 1.12 (95% CI, 1.05-1.19, P < .001).
Mortality
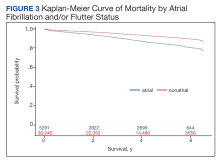
Over the 6-year study period, there was a lower survival probability for patients with AF/AFL. In the overall cohort, during a mean (SD) follow-up time of 3.7 (1.9) years comprising 129,391 total person-years, there were 3130 (8.8%) deaths and an incidence rate of 2.42 per 100 person-years. Death occurred 786 times with a 4.09 incidence rate per 100 person-years in the AF/AFL vs 2344 deaths and a 2.13 incidence rate per 100 person-years in the no AF/AFL group (P < .001). In the non-AF/AFL subgroup, death occurred 2344 times during a mean (SD) follow-up of 3.7 (1.9) years comprising 110,151 total person-years. Figure 3 shows the Kaplan-Meier curve of mortality by AF/AFL status.
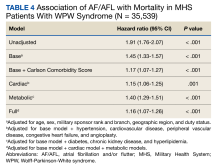
After adjusting for the base, metabolic and cardiac covariates, the HRs for mortality were 1.45 (95% CI, 1.33-1.57, P < .001), 1.40 (95% CI, 1.29-1.51, P < .001) and 1.15 (95% CI, 1.06-1.25, P = .001), respectively (Table 4). The HR after adjusting for the full model was 1.16 (95% CI, 1.07-1.26, P < .001).
DISCUSSION
In this large retrospective cohort study, patients with WPW syndrome and comorbid AF/AFL had a significantly higher association with the cardiac composite outcome and death during a 3-year follow-up period when compared with patients without AF/AFL. After adjusting for confounding variables, the AF/AFL subgroup maintained a 12% and 16% higher association with the composite outcome and mortality, respectively. There was minimal difference in confounding effects between demographic data and metabolic profiles, suggesting one may serve as a proxy for the other.
To our knowledge, this is the largest WPW syndrome cohort study evaluating cardiac outcomes and mortality to date. Although previous research has shown the relatively low and mostly anecdotal SCD incidence within this population, our results demonstrate a higher association of adverse cardiac outcomes and death in an AF/AFL subgroup.16-18 Notably, in this study the AF/AFL cohort was older and had higher CCI scores than their counterparts (P < .001), thus inferring an inherently greater degree of morbidity and 10-year mortality risk. Our study is also unique in that the mean patient age was significantly older than previously reported (63 vs 27 years), which may suggest a longer living history of both ventricular pre-excitation and the comorbidities outlined in Figure 1.19 Given these age discrepancies, it is possible that our overall study population was still relatively low risk and that not all reported deaths were necessarily related to WPW syndrome. Despite these assumptions, when comparing the WPW syndrome subgroups, we still found the AF/AFL cohort maintained a statistically significant higher association with the 2 study outcomes, even after adjusting for the greater presence of comorbidities. This suggests that the presence of AF/AFL may still portend a worse prognosis in patients with WPW syndrome.
Although the association of AF and development of VF in patients with WPW syndrome—due to rapid conduction over the accessory pathway(s)—was first reported > 40 years ago, there has still been few large, long-term data studies exploring mortality in this cohort.19-25 Furthermore, even though the current literature attributes the development of AF with the electrophysiologic properties of the accessory pathway, as well as intrinsic atrial architecture and muscle vulnerability, there is still equivocal consensus regarding EPT screening and ablation indications for asymptomatic patients with WPW syndrome.26-28 Notably, Pappone and colleagues demonstrated the potential benefit of liberal ablation indications for asymptomatic patients, arguing that the intrinsic electrophysiologic properties of the accessory pathway—ie, short accessory-pathway antegrade effective refractory period, inducibility of atrioventricular reentrant tachycardia triggering AF, and multiple accessory pathway—rather than symptoms, are independent predictors of developing malignant arrhythmia.1-5
These findings contradict those reported by Obeyesekere and colleagues, who concluded that the low SCD incidence rates in patients with WPW syndrome precluded routine invasive screening.19,28 They argued that Pappone and colleagues used malignant arrhythmia as a surrogate marker for death, and that the positive predictive value of a short accessory-pathway antegrade effective refractory period for developing malignant arrhythmia was lower than reported (15% vs 82%, respectively) and that its negative predictive value was 100%.1,19,28 Given these conflicting recommendations, we hope our data elucidates the higher association of adverse outcomes and support considerations for more intensive EPT indications in patients with WPW syndrome.
While our study does not report SCD incidence, it does provide robust and reliable mortality data that suggests a greater association of death within an AF/AFL subgroup. Our findings would support more liberal EPT recommendations in patients with WPW syndrome.1-5,8,9 In this study, the SCA incidence rate was more than double the rate in the AF/AFL cohort (P < .001) and is commonly the initial presenting event in WPW syndrome.9 Even though the reported SCD incidence rate is low in WPW syndrome, our data demonstrated an increased association of death within the AF/AFL cohort. Physicians should consider early risk stratification and ablation to prevent potential recurrent malignant arrhythmia leading to death.1-5,8,9,12,19,20
Limitations
As a retrospective study and without access to the National Death Index, we were unable to determine the exact cause or events leading to death and instead utilized all-cause mortality data. Subsequently, our observations may only demonstrate association, rather than causality, between AF/AFL and death in patients with WPW syndrome. Additionally, we could not distinguish between AF and AFL as the arrhythmia leading to death. However, since overall survivability was the outcome of interest, our adjusted HR models were still able to demonstrate the increased association of the composite outcome and death within an AF/AFL cohort.
Although a large cohort was analyzed, due to the constraints of utilizing diagnostic codes to determine study outcomes, we could not distinguish between symptomatic and asymptomatic patients, nor how they were managed prior to the outcome event. However, as recent literature demonstrates, updated predictors of malignant arrhythmia and decisions for early EPT are similar for both symptomatic and asymptomatic patients and should be driven by the intrinsic electrophysiologic properties of the accessory pathway, rather than symptomatology; thus, our inability to discern this should have negligible consequence in determining when to perform risk stratification and ablation.1
MHS eligible patients have direct access to care; the generalizability of our data may not necessarily correspond to a community population with lower socioeconomic status (we did adjust for military sponsor rank which has been used as a proxy), reduced access to care, or uninsured individuals. However, the prevalence of WPW syndrome within our cohort was comparable to the general population, 0.4% vs 0.1%-0.3%, respectively.13,14,19 Similarly, the incidence of AF within our population was comparable to the general population, 15% vs 16%-26%, respectively.23 These similar data points suggest our results may apply beyond MHS patients.
CONCLUSIONS
Patients with WPW syndrome and AF/AFL have a higher association with adverse cardiac outcomes and death. Despite previously reported low SCD incidence rates in this population, our study demonstrates the increased association of mortality in an AF/AFL cohort. The limitations of utilizing all-cause mortality data necessitate further investigation into the etiology behind the deaths in our study population. Since ventricular pre-excitation can predispose patients to AF and potentially lead to malignant arrhythmia and SCD, understanding the cause of mortality will allow physicians to determine the appropriate monitoring and intervention strategies to improve outcomes in this population. Our results suggest consideration for more aggressive EPT screening and ablation recommendations in patients with WPW syndrome may be warranted.
1. Pappone C, Vicedomini G, Manguso F, et al. The natural history of WPW syndrome. Eur Heart J Suppl. 2015; 17 (Supplement A):A8-A11.doi:10.1093/eurheartj/suv004
2. Pappone C, Vicedomini G, Manguso F, et al. Risk of malignant arrhythmias in initially symptomatic patients with Wolff-Parkinson-White syndrome: results of a prospective long-term electrophysiological follow-up study. Circulation. 2012;125(5):661-668. doi:10.1161/CIRCULATIONAHA.111.065722
3. Pappone C, Santinelli V, Rosanio S, et al. Usefulness of invasive electrophysiologic testing to stratify the risk of arrhythmic events in asymptomatic patients with Wolff-Parkinson-White pattern: results from a large prospective long-term follow-up study. J Am Coll Cardiol. 2003;41(2):239-244. doi:10.1016/s0735-1097(02)02706-7
4. Pappone C, Vicedomini G, Manguso F, et al. Wolff-Parkinson-White syndrome in the era of catheter ablation: insights from a registry study of 2169 patients. Circulation. 2014;130(10):811-819. doi:10.1161/CIRCULATIONAHA.114.011154
5. Pappone C, Santinelli V, Manguso F, et al. A randomized study of prophylactic catheter ablation in asymptomatic patients with the Wolff-Parkinson-White syndrome. N Engl J Med. 2003;349(19):1803-1811. doi:10.1056/NEJMoa035345
6. Santinelli V, Radinovic A, Manguso F, et al. Asymptomatic ventricular preexcitation: a long-term prospective follow-up study of 293 adult patients. Circ Arrhythm Electrophysiol. 2009;2(2):102-107. doi:10.1161/CIRCEP.108.827550
7. Santinelli V, Radinovic A, Manguso F, et al. The natural history of asymptomatic ventricular pre-excitation a long-term prospective follow-up study of 184 asymptomatic children. J Am Coll Cardiol. 2009;53(3):275-280. doi:10.1016/j.jacc.2008.09.037
8. Al-Khatib SM, Arshad A, Balk EM, et al. Risk Stratification for Arrhythmic Events in Patients With Asymptomatic Pre-Excitation: A Systematic Review for the 2015 ACC/AHA/HRS Guideline for the Management of Adult Patients With Supraventricular Tachycardia: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2016;67(13):1624-1638. doi:10.1016/j.jacc.2015.09.018
9. Blomström-Lundqvist C, Scheinman MM, Aliot EM, et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Supraventricular Arrhythmias). Circulation. 2003;108(15):1871-1909.doi:10.1161/01.CIR.0000091380.04100.84
10. Pediatric and Congenital Electrophysiology Society (PACES); Heart Rhythm Society (HRS); American College of Cardiology Foundation (ACCF); PACES/HRS expert consensus statement on the management of the asymptomatic young patient with a Wolff-Parkinson-White (WPW, ventricular preexcitation) electrocardiographic pattern: developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology Foundation (ACCF), the American Heart Association (AHA), the American Academy of Pediatrics (AAP), and the Canadian Heart Rhythm Society (CHRS). Heart Rhythm. 2012;9(6):1006-1024. doi:10.1016/j.hrthm.2012.03.050
11. Cohen M, Triedman J. Guidelines for management of asymptomatic ventricular pre-excitation: brave new world or Pandora’s box?. Circ Arrhythm Electrophysiol. 2014;7(2):187-189. doi:10.1161/CIRCEP.114.001528
12. Svendsen JH, Dagres N, Dobreanu D, et al. Current strategy for treatment of patients with Wolff-Parkinson-White syndrome and asymptomatic preexcitation in Europe: European Heart Rhythm Association survey. Europace. 2013;15(5):750-753. doi:10.1093/europace/eut094
13. Gimbel RW, Pangaro L, Barbour G. America’s “undiscovered” laboratory for health services research. Med Care. 2010;48(8):751-756. doi:10.1097/MLR.0b013e3181e35be8
14. Dorrance KA, Ramchandani S, Neil N, Fisher H. Leveraging the military health system as a laboratory for health care reform. Mil Med. 2013;178(2):142-145. doi:10.7205/milmed-d-12-00168
15. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. doi:10.1097/01.mlr.0000182534.19832.83
16. Finocchiaro G, Papadakis M, Behr ER, Sharma S, Sheppard M. Sudden Cardiac Death in Pre-Excitation and Wolff-Parkinson-White: Demographic and Clinical Features. J Am Coll Cardiol. 2017;69(12):1644-1645. doi:10.1016/j.jacc.2017.01.023
17. Munger TM, Packer DL, Hammill SC, et al. A population study of the natural history of Wolff-Parkinson-White syndrome in Olmsted County, Minnesota, 1953-1989. Circulation. 1993;87(3):866-873. doi:10.1161/01.cir.87.3.866
18. Fitzsimmons PJ, McWhirter PD, Peterson DW, Kruyer WB. The natural history of Wolff-Parkinson-White syndrome in 228 military aviators: a long-term follow-up of 22 years. Am Heart J. 2001;142(3):530-536. doi:10.1067/mhj.2001.117779
19. Obeyesekere MN, Leong-Sit P, Massel D, et al. Risk of arrhythmia and sudden death in patients with asymptomatic preexcitation: a meta-analysis. Circulation. 2012;125(19):2308-2315. doi:10.1161/CIRCULATIONAHA.111.055350
20. Waspe LE, Brodman R, Kim SG, Fisher JD. Susceptibility to atrial fibrillation and ventricular tachyarrhythmia in the Wolff-Parkinson-White syndrome: role of the accessory pathway. Am Heart J. 1986;112(6):1141-1152. doi:10.1016/0002-8703(86)90342-x
21. Pietersen AH, Andersen ED, Sandøe E. Atrial fibrillation in the Wolff-Parkinson-White syndrome. Am J Cardiol. 1992;70(5):38A-43A. doi:10.1016/0002-9149(92)91076-g
22. Della Bella P, Brugada P, Talajic M, et al. Atrial fibrillation in patients with an accessory pathway: importance of the conduction properties of the accessory pathway. J Am Coll Cardiol. 1991;17(6):1352-1356. doi:10.1016/s0735-1097(10)80146-9
23. Fujimura O, Klein GJ, Yee R, Sharma AD. Mode of onset of atrial fibrillation in the Wolff-Parkinson-White syndrome: how important is the accessory pathway?. J Am Coll Cardiol. 1990;15(5):1082-1086. doi:10.1016/0735-1097(90)90244-j
24. Montoya PT, Brugada P, Smeets J, et al. Ventricular fibrillation in the Wolff-Parkinson-White syndrome. Eur Heart J. 1991;12(2):144-150. doi:10.1093/oxfordjournals.eurheartj.a059860
25. Klein GJ, Bashore TM, Sellers TD, Pritchett EL, Smith WM, Gallagher JJ. Ventricular fibrillation in the Wolff-Parkinson-White syndrome. N Engl J Med. 1979;301(20):1080-1085. doi:10.1056/NEJM197911153012003
26. Centurion OA. Atrial Fibrillation in the Wolff-Parkinson-White Syndrome. J Atr Fibrillation. 2011;4(1):287. Published 2011 May 4. doi:10.4022/jafib.287
27. Song C, Guo Y, Zheng X, et al. Prognostic Significance and Risk of Atrial Fibrillation of Wolff-Parkinson-White Syndrome in Patients With Hypertrophic Cardiomyopathy. Am J Cardiol. 2018;122(9):1546-1550. doi:10.1016/j.amjcard.2018.07.021
28. Obeyesekere M, Gula LJ, Skanes AC, Leong-Sit P, Klein GJ. Risk of sudden death in Wolff-Parkinson-White syndrome: how high is the risk?. Circulation. 2012;125(5):659-660. doi:10.1161/CIRCULATIONAHA.111.085159
Wolff-Parkinson-White (WPW) syndrome is characterized by the presence of ≥ 1 accessory pathways and the development of both recurrent paroxysmal atrial fibrillation (AF) and supraventricular tachycardia that can lead to further malignant arrhythmias resulting in sudden cardiac death (SCD).1-7 Historically, incidental, ventricular pre-excitation on electrocardiogram has conferred a relatively low SCD risk in adults; however, newer WPW syndrome data suggest the endpoint may not be as benign as previously thought.7 The current literature has defined atrioventricular reentrant tachycardia triggering AF, rather than symptoms, as an independent risk factor for malignant arrhythmias. Still, long-term data detailing the association of AF with serious cardiac events and death in patients with WPW syndrome are still limited.1-7
While previous guidelines for the treatment of WPW syndrome only recommended routine electrophysiology testing (EPT) with liberal catheter ablation for symptomatic individuals, the 2015 American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines now suggest its potential benefit for risk stratification in the asymptomatic population.8-12 Given the limited existing data, more long-term studies are needed to corroborate the latest EPT recommendations before routinely applying them in practice. Furthermore, since concomitant AF can lead to adverse cardiac outcomes in patients with WPW syndrome, additional data evaluating this association are also necessary. In this study, we aimed to determine the impact of atrial fibrillation and/or flutter (AF/AFL) on adverse cardiac outcomes and mortality in patients with WPW syndrome.
METHODS
This study used data from the Military Health System (MHS) Database Repository. The MHS is one of the largest health care systems in the country and includes information on about 10 million active duty and retired military service members and their families (51% male; 49% female).13,14 Data were fully anonymized and complied in accordance with federal and state laws, including the Health Insurance Portability and Accountability Act of 1996. The Naval Medical Center Portsmouth Institutional Review Board approved this study.
Study Design
This retrospective, observational cohort study identified MHS patients with WPW syndrome from January 1, 2014, to December 31, 2019. Patients were included if they had ≥ 2 International Classification of Diseases, Ninth Revision (ICD-9) or International Classification of Diseases, Tenth Revision (ICD-10) diagnosis codes for WPW syndrome (ICD-9, 426.7; ICD-10, I45.6) on separate dates; were aged ≥ 18 years at index date; and had ≥ 1 year of continuous eligibility prior to the index date (enrollment gaps ≤ 30 days were considered continuous). Patients were then divided into 2 subgroups by the presence or absence of AF/AFL using diagnostic codes. Patients were excluded if they had evidence of an implantable cardioverter-defibrillator, permanent pacemaker or were missing age or sex data. Patients were followed from index date until the first occurrence of the outcome of interest, MHS disenrollment, or the end of the study period.
Cardiac composite outcomes comprised of sudden cardiac arrest (SCA), ventricular fibrillation (VF), ventricular tachycardia and death, as well as death specifically, were the outcomes of interest and assessed after index date using ICD-9 and ICD-10 codes. Death was defined as all-cause mortality. Time to event was calculated based on the date of the initial component from the composite outcome and date of death specifically for mortality. Those not experiencing an outcome were followed until MHS disenrollment or the end of the study period.
Various patient characteristics were assessed at index including age, sex, military sponsor (the patient’s active or retired duty member through which their dependent receives TRICARE benefits) rank and branch, geographic region, type of US Department of Defense beneficiary, and index year. Clinical characteristics were assessed over a 1-year baseline period prior to index date and included the number of cardiologist and clinical visits for WPW syndrome, Charlson Comorbidity Index (CCI) scores calculated from diagnostic codes outlined in the Quan coding method, and preindex time.15 Comorbidities were assessed at baseline and defined as having ≥ 1 ICD-9 or ICD-10 code for a corresponding condition within 1 year prior to index.
Statistical Analysis
Baseline characteristics were assessed and descriptive statistics for categorical and continuous variables were presented accordingly. To assess bivariate association with exposure, χ2 tests were used to compare categorical variables, while t tests were used to compare continuous variables by exposure status. Incidence proportions and rates were reported for each outcome of interest. Kaplan-Meier curves were constructed to assess the bivariate association between exposure and study outcomes. Cox proportional hazard modeling was performed to estimate the association between AF/AFL and time to each of the outcomes. Multiple models were designed to assess cardiac and metabolic covariates, in addition to baseline characteristics. This included a base model adjusted for age, sex, military sponsor rank and branch, geographic region, and duty status.
Additional models adjusted for cardiac and metabolic confounders and CCI score. A comprehensive model included the base, cardiac, and metabolic covariates. Multicollinearity between covariates was assessed. Variables with a variance inflation factor > 4 or a tolerance level < 0.1 were added to the models. Cox proportional hazard models were used to estimate the unadjusted and adjusted hazard ratios (HRs) and 95% CIs of the association between AF/AFL and the study outcomes. Data were analyzed using SAS, version 9.4 for Windows.
RESULTS
From 2014 through 2019, 35,539 patients with WPW syndrome were identified in the MHS, 5291 had AF/AFL (14.9%); 19,961 were female (56.2%), the mean (SD) age was 62.9 (18.0) years, and 11,742 were aged ≥ 75 years (33.0%) (Table 1).
There were 4121 (11.6%), 322 (0.9%), and 848 (2.4%) patients with AF, AFL, and both arrhythmias, respectively. The mean (SD) number of cardiology visits was 3.9 (3.0). The mean (SD) baseline CCI score for the AF/AFL subgroup was 5.9 (3.5) vs 3.7 (2.2) for the non-AF/AFL subgroup (P < .001). The most prevalent comorbid conditions were hypertension, hyperlipidemia, chronic obstructive pulmonary disease, and diabetes (P < .001) (Figure 1).
Composite Outcomes
In the overall cohort, during a mean (SD) follow-up time of 3.4 (2.0) years comprising 119,682 total person-years, the components of the composite outcome occurred 6506 times with an incidence rate of 5.44 per 100 person-years. Ventricular tachycardia was the most common event, occurring 3281 times with an incidence rate of 2.74 per 100 person-years. SCA and VF occurred 841 and 135 times with incidence rates of 0.70 and 0.11 per 100 person-years, respectively. Death was the initial event 2249 times with an incidence rate of 1.88 per 100 person-years. Figure 2 shows the Kaplan-Meier curve of cardiac composite outcome by AF/AFL status.
The subgroup with AF/AFL comprised 17,412 total person-years and 1424 cardiac composite incidences compared with 102,270 person years and 5082 incidences in the no AF/AFL group (Table 2). Comparing AF/AFL vs no AF/AFL incidence rates were 8.18 vs 4.97 per 100 person-years, respectively (P < .001). SCA and VF occurred 233 and 38 times and respectively had incidence rates of 1.34 and 0.22 per 100 person-years in the AF/AFL group vs 0.59 and 0.09 per 100 person-years in the no AF/AFL group (P < .001). There were 549 deaths and a 3.15 per 100 person-years incidence rate in the AF/AFL group vs 1700 deaths and a 1.66 incidence rate in the no AF/AFL group (P < .001).
The HR for the composite outcome in the base model was 1.33 (95% CI, 1.26-1.42, P < .001) (Table 3). The association between AF/AFL and the composite outcome remained significant after adjusting for additional metabolic and cardiac covariates. The HRs for the metabolic and cardiac models were 1.30 (95% CI, 1.23-1.38, P < .001) and 1.11 (95% CI, 1.05-1.18, P < .001), respectively. After adjusting for the full model, the HR was 1.12 (95% CI, 1.05-1.19, P < .001).
Mortality
Over the 6-year study period, there was a lower survival probability for patients with AF/AFL. In the overall cohort, during a mean (SD) follow-up time of 3.7 (1.9) years comprising 129,391 total person-years, there were 3130 (8.8%) deaths and an incidence rate of 2.42 per 100 person-years. Death occurred 786 times with a 4.09 incidence rate per 100 person-years in the AF/AFL vs 2344 deaths and a 2.13 incidence rate per 100 person-years in the no AF/AFL group (P < .001). In the non-AF/AFL subgroup, death occurred 2344 times during a mean (SD) follow-up of 3.7 (1.9) years comprising 110,151 total person-years. Figure 3 shows the Kaplan-Meier curve of mortality by AF/AFL status.
After adjusting for the base, metabolic and cardiac covariates, the HRs for mortality were 1.45 (95% CI, 1.33-1.57, P < .001), 1.40 (95% CI, 1.29-1.51, P < .001) and 1.15 (95% CI, 1.06-1.25, P = .001), respectively (Table 4). The HR after adjusting for the full model was 1.16 (95% CI, 1.07-1.26, P < .001).
DISCUSSION
In this large retrospective cohort study, patients with WPW syndrome and comorbid AF/AFL had a significantly higher association with the cardiac composite outcome and death during a 3-year follow-up period when compared with patients without AF/AFL. After adjusting for confounding variables, the AF/AFL subgroup maintained a 12% and 16% higher association with the composite outcome and mortality, respectively. There was minimal difference in confounding effects between demographic data and metabolic profiles, suggesting one may serve as a proxy for the other.
To our knowledge, this is the largest WPW syndrome cohort study evaluating cardiac outcomes and mortality to date. Although previous research has shown the relatively low and mostly anecdotal SCD incidence within this population, our results demonstrate a higher association of adverse cardiac outcomes and death in an AF/AFL subgroup.16-18 Notably, in this study the AF/AFL cohort was older and had higher CCI scores than their counterparts (P < .001), thus inferring an inherently greater degree of morbidity and 10-year mortality risk. Our study is also unique in that the mean patient age was significantly older than previously reported (63 vs 27 years), which may suggest a longer living history of both ventricular pre-excitation and the comorbidities outlined in Figure 1.19 Given these age discrepancies, it is possible that our overall study population was still relatively low risk and that not all reported deaths were necessarily related to WPW syndrome. Despite these assumptions, when comparing the WPW syndrome subgroups, we still found the AF/AFL cohort maintained a statistically significant higher association with the 2 study outcomes, even after adjusting for the greater presence of comorbidities. This suggests that the presence of AF/AFL may still portend a worse prognosis in patients with WPW syndrome.
Although the association of AF and development of VF in patients with WPW syndrome—due to rapid conduction over the accessory pathway(s)—was first reported > 40 years ago, there has still been few large, long-term data studies exploring mortality in this cohort.19-25 Furthermore, even though the current literature attributes the development of AF with the electrophysiologic properties of the accessory pathway, as well as intrinsic atrial architecture and muscle vulnerability, there is still equivocal consensus regarding EPT screening and ablation indications for asymptomatic patients with WPW syndrome.26-28 Notably, Pappone and colleagues demonstrated the potential benefit of liberal ablation indications for asymptomatic patients, arguing that the intrinsic electrophysiologic properties of the accessory pathway—ie, short accessory-pathway antegrade effective refractory period, inducibility of atrioventricular reentrant tachycardia triggering AF, and multiple accessory pathway—rather than symptoms, are independent predictors of developing malignant arrhythmia.1-5
These findings contradict those reported by Obeyesekere and colleagues, who concluded that the low SCD incidence rates in patients with WPW syndrome precluded routine invasive screening.19,28 They argued that Pappone and colleagues used malignant arrhythmia as a surrogate marker for death, and that the positive predictive value of a short accessory-pathway antegrade effective refractory period for developing malignant arrhythmia was lower than reported (15% vs 82%, respectively) and that its negative predictive value was 100%.1,19,28 Given these conflicting recommendations, we hope our data elucidates the higher association of adverse outcomes and support considerations for more intensive EPT indications in patients with WPW syndrome.
While our study does not report SCD incidence, it does provide robust and reliable mortality data that suggests a greater association of death within an AF/AFL subgroup. Our findings would support more liberal EPT recommendations in patients with WPW syndrome.1-5,8,9 In this study, the SCA incidence rate was more than double the rate in the AF/AFL cohort (P < .001) and is commonly the initial presenting event in WPW syndrome.9 Even though the reported SCD incidence rate is low in WPW syndrome, our data demonstrated an increased association of death within the AF/AFL cohort. Physicians should consider early risk stratification and ablation to prevent potential recurrent malignant arrhythmia leading to death.1-5,8,9,12,19,20
Limitations
As a retrospective study and without access to the National Death Index, we were unable to determine the exact cause or events leading to death and instead utilized all-cause mortality data. Subsequently, our observations may only demonstrate association, rather than causality, between AF/AFL and death in patients with WPW syndrome. Additionally, we could not distinguish between AF and AFL as the arrhythmia leading to death. However, since overall survivability was the outcome of interest, our adjusted HR models were still able to demonstrate the increased association of the composite outcome and death within an AF/AFL cohort.
Although a large cohort was analyzed, due to the constraints of utilizing diagnostic codes to determine study outcomes, we could not distinguish between symptomatic and asymptomatic patients, nor how they were managed prior to the outcome event. However, as recent literature demonstrates, updated predictors of malignant arrhythmia and decisions for early EPT are similar for both symptomatic and asymptomatic patients and should be driven by the intrinsic electrophysiologic properties of the accessory pathway, rather than symptomatology; thus, our inability to discern this should have negligible consequence in determining when to perform risk stratification and ablation.1
MHS eligible patients have direct access to care; the generalizability of our data may not necessarily correspond to a community population with lower socioeconomic status (we did adjust for military sponsor rank which has been used as a proxy), reduced access to care, or uninsured individuals. However, the prevalence of WPW syndrome within our cohort was comparable to the general population, 0.4% vs 0.1%-0.3%, respectively.13,14,19 Similarly, the incidence of AF within our population was comparable to the general population, 15% vs 16%-26%, respectively.23 These similar data points suggest our results may apply beyond MHS patients.
CONCLUSIONS
Patients with WPW syndrome and AF/AFL have a higher association with adverse cardiac outcomes and death. Despite previously reported low SCD incidence rates in this population, our study demonstrates the increased association of mortality in an AF/AFL cohort. The limitations of utilizing all-cause mortality data necessitate further investigation into the etiology behind the deaths in our study population. Since ventricular pre-excitation can predispose patients to AF and potentially lead to malignant arrhythmia and SCD, understanding the cause of mortality will allow physicians to determine the appropriate monitoring and intervention strategies to improve outcomes in this population. Our results suggest consideration for more aggressive EPT screening and ablation recommendations in patients with WPW syndrome may be warranted.
Wolff-Parkinson-White (WPW) syndrome is characterized by the presence of ≥ 1 accessory pathways and the development of both recurrent paroxysmal atrial fibrillation (AF) and supraventricular tachycardia that can lead to further malignant arrhythmias resulting in sudden cardiac death (SCD).1-7 Historically, incidental, ventricular pre-excitation on electrocardiogram has conferred a relatively low SCD risk in adults; however, newer WPW syndrome data suggest the endpoint may not be as benign as previously thought.7 The current literature has defined atrioventricular reentrant tachycardia triggering AF, rather than symptoms, as an independent risk factor for malignant arrhythmias. Still, long-term data detailing the association of AF with serious cardiac events and death in patients with WPW syndrome are still limited.1-7
While previous guidelines for the treatment of WPW syndrome only recommended routine electrophysiology testing (EPT) with liberal catheter ablation for symptomatic individuals, the 2015 American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines now suggest its potential benefit for risk stratification in the asymptomatic population.8-12 Given the limited existing data, more long-term studies are needed to corroborate the latest EPT recommendations before routinely applying them in practice. Furthermore, since concomitant AF can lead to adverse cardiac outcomes in patients with WPW syndrome, additional data evaluating this association are also necessary. In this study, we aimed to determine the impact of atrial fibrillation and/or flutter (AF/AFL) on adverse cardiac outcomes and mortality in patients with WPW syndrome.
METHODS
This study used data from the Military Health System (MHS) Database Repository. The MHS is one of the largest health care systems in the country and includes information on about 10 million active duty and retired military service members and their families (51% male; 49% female).13,14 Data were fully anonymized and complied in accordance with federal and state laws, including the Health Insurance Portability and Accountability Act of 1996. The Naval Medical Center Portsmouth Institutional Review Board approved this study.
Study Design
This retrospective, observational cohort study identified MHS patients with WPW syndrome from January 1, 2014, to December 31, 2019. Patients were included if they had ≥ 2 International Classification of Diseases, Ninth Revision (ICD-9) or International Classification of Diseases, Tenth Revision (ICD-10) diagnosis codes for WPW syndrome (ICD-9, 426.7; ICD-10, I45.6) on separate dates; were aged ≥ 18 years at index date; and had ≥ 1 year of continuous eligibility prior to the index date (enrollment gaps ≤ 30 days were considered continuous). Patients were then divided into 2 subgroups by the presence or absence of AF/AFL using diagnostic codes. Patients were excluded if they had evidence of an implantable cardioverter-defibrillator, permanent pacemaker or were missing age or sex data. Patients were followed from index date until the first occurrence of the outcome of interest, MHS disenrollment, or the end of the study period.
Cardiac composite outcomes comprised of sudden cardiac arrest (SCA), ventricular fibrillation (VF), ventricular tachycardia and death, as well as death specifically, were the outcomes of interest and assessed after index date using ICD-9 and ICD-10 codes. Death was defined as all-cause mortality. Time to event was calculated based on the date of the initial component from the composite outcome and date of death specifically for mortality. Those not experiencing an outcome were followed until MHS disenrollment or the end of the study period.
Various patient characteristics were assessed at index including age, sex, military sponsor (the patient’s active or retired duty member through which their dependent receives TRICARE benefits) rank and branch, geographic region, type of US Department of Defense beneficiary, and index year. Clinical characteristics were assessed over a 1-year baseline period prior to index date and included the number of cardiologist and clinical visits for WPW syndrome, Charlson Comorbidity Index (CCI) scores calculated from diagnostic codes outlined in the Quan coding method, and preindex time.15 Comorbidities were assessed at baseline and defined as having ≥ 1 ICD-9 or ICD-10 code for a corresponding condition within 1 year prior to index.
Statistical Analysis
Baseline characteristics were assessed and descriptive statistics for categorical and continuous variables were presented accordingly. To assess bivariate association with exposure, χ2 tests were used to compare categorical variables, while t tests were used to compare continuous variables by exposure status. Incidence proportions and rates were reported for each outcome of interest. Kaplan-Meier curves were constructed to assess the bivariate association between exposure and study outcomes. Cox proportional hazard modeling was performed to estimate the association between AF/AFL and time to each of the outcomes. Multiple models were designed to assess cardiac and metabolic covariates, in addition to baseline characteristics. This included a base model adjusted for age, sex, military sponsor rank and branch, geographic region, and duty status.
Additional models adjusted for cardiac and metabolic confounders and CCI score. A comprehensive model included the base, cardiac, and metabolic covariates. Multicollinearity between covariates was assessed. Variables with a variance inflation factor > 4 or a tolerance level < 0.1 were added to the models. Cox proportional hazard models were used to estimate the unadjusted and adjusted hazard ratios (HRs) and 95% CIs of the association between AF/AFL and the study outcomes. Data were analyzed using SAS, version 9.4 for Windows.
RESULTS
From 2014 through 2019, 35,539 patients with WPW syndrome were identified in the MHS, 5291 had AF/AFL (14.9%); 19,961 were female (56.2%), the mean (SD) age was 62.9 (18.0) years, and 11,742 were aged ≥ 75 years (33.0%) (Table 1).
There were 4121 (11.6%), 322 (0.9%), and 848 (2.4%) patients with AF, AFL, and both arrhythmias, respectively. The mean (SD) number of cardiology visits was 3.9 (3.0). The mean (SD) baseline CCI score for the AF/AFL subgroup was 5.9 (3.5) vs 3.7 (2.2) for the non-AF/AFL subgroup (P < .001). The most prevalent comorbid conditions were hypertension, hyperlipidemia, chronic obstructive pulmonary disease, and diabetes (P < .001) (Figure 1).
Composite Outcomes
In the overall cohort, during a mean (SD) follow-up time of 3.4 (2.0) years comprising 119,682 total person-years, the components of the composite outcome occurred 6506 times with an incidence rate of 5.44 per 100 person-years. Ventricular tachycardia was the most common event, occurring 3281 times with an incidence rate of 2.74 per 100 person-years. SCA and VF occurred 841 and 135 times with incidence rates of 0.70 and 0.11 per 100 person-years, respectively. Death was the initial event 2249 times with an incidence rate of 1.88 per 100 person-years. Figure 2 shows the Kaplan-Meier curve of cardiac composite outcome by AF/AFL status.
The subgroup with AF/AFL comprised 17,412 total person-years and 1424 cardiac composite incidences compared with 102,270 person years and 5082 incidences in the no AF/AFL group (Table 2). Comparing AF/AFL vs no AF/AFL incidence rates were 8.18 vs 4.97 per 100 person-years, respectively (P < .001). SCA and VF occurred 233 and 38 times and respectively had incidence rates of 1.34 and 0.22 per 100 person-years in the AF/AFL group vs 0.59 and 0.09 per 100 person-years in the no AF/AFL group (P < .001). There were 549 deaths and a 3.15 per 100 person-years incidence rate in the AF/AFL group vs 1700 deaths and a 1.66 incidence rate in the no AF/AFL group (P < .001).
The HR for the composite outcome in the base model was 1.33 (95% CI, 1.26-1.42, P < .001) (Table 3). The association between AF/AFL and the composite outcome remained significant after adjusting for additional metabolic and cardiac covariates. The HRs for the metabolic and cardiac models were 1.30 (95% CI, 1.23-1.38, P < .001) and 1.11 (95% CI, 1.05-1.18, P < .001), respectively. After adjusting for the full model, the HR was 1.12 (95% CI, 1.05-1.19, P < .001).
Mortality
Over the 6-year study period, there was a lower survival probability for patients with AF/AFL. In the overall cohort, during a mean (SD) follow-up time of 3.7 (1.9) years comprising 129,391 total person-years, there were 3130 (8.8%) deaths and an incidence rate of 2.42 per 100 person-years. Death occurred 786 times with a 4.09 incidence rate per 100 person-years in the AF/AFL vs 2344 deaths and a 2.13 incidence rate per 100 person-years in the no AF/AFL group (P < .001). In the non-AF/AFL subgroup, death occurred 2344 times during a mean (SD) follow-up of 3.7 (1.9) years comprising 110,151 total person-years. Figure 3 shows the Kaplan-Meier curve of mortality by AF/AFL status.
After adjusting for the base, metabolic and cardiac covariates, the HRs for mortality were 1.45 (95% CI, 1.33-1.57, P < .001), 1.40 (95% CI, 1.29-1.51, P < .001) and 1.15 (95% CI, 1.06-1.25, P = .001), respectively (Table 4). The HR after adjusting for the full model was 1.16 (95% CI, 1.07-1.26, P < .001).
DISCUSSION
In this large retrospective cohort study, patients with WPW syndrome and comorbid AF/AFL had a significantly higher association with the cardiac composite outcome and death during a 3-year follow-up period when compared with patients without AF/AFL. After adjusting for confounding variables, the AF/AFL subgroup maintained a 12% and 16% higher association with the composite outcome and mortality, respectively. There was minimal difference in confounding effects between demographic data and metabolic profiles, suggesting one may serve as a proxy for the other.
To our knowledge, this is the largest WPW syndrome cohort study evaluating cardiac outcomes and mortality to date. Although previous research has shown the relatively low and mostly anecdotal SCD incidence within this population, our results demonstrate a higher association of adverse cardiac outcomes and death in an AF/AFL subgroup.16-18 Notably, in this study the AF/AFL cohort was older and had higher CCI scores than their counterparts (P < .001), thus inferring an inherently greater degree of morbidity and 10-year mortality risk. Our study is also unique in that the mean patient age was significantly older than previously reported (63 vs 27 years), which may suggest a longer living history of both ventricular pre-excitation and the comorbidities outlined in Figure 1.19 Given these age discrepancies, it is possible that our overall study population was still relatively low risk and that not all reported deaths were necessarily related to WPW syndrome. Despite these assumptions, when comparing the WPW syndrome subgroups, we still found the AF/AFL cohort maintained a statistically significant higher association with the 2 study outcomes, even after adjusting for the greater presence of comorbidities. This suggests that the presence of AF/AFL may still portend a worse prognosis in patients with WPW syndrome.
Although the association of AF and development of VF in patients with WPW syndrome—due to rapid conduction over the accessory pathway(s)—was first reported > 40 years ago, there has still been few large, long-term data studies exploring mortality in this cohort.19-25 Furthermore, even though the current literature attributes the development of AF with the electrophysiologic properties of the accessory pathway, as well as intrinsic atrial architecture and muscle vulnerability, there is still equivocal consensus regarding EPT screening and ablation indications for asymptomatic patients with WPW syndrome.26-28 Notably, Pappone and colleagues demonstrated the potential benefit of liberal ablation indications for asymptomatic patients, arguing that the intrinsic electrophysiologic properties of the accessory pathway—ie, short accessory-pathway antegrade effective refractory period, inducibility of atrioventricular reentrant tachycardia triggering AF, and multiple accessory pathway—rather than symptoms, are independent predictors of developing malignant arrhythmia.1-5
These findings contradict those reported by Obeyesekere and colleagues, who concluded that the low SCD incidence rates in patients with WPW syndrome precluded routine invasive screening.19,28 They argued that Pappone and colleagues used malignant arrhythmia as a surrogate marker for death, and that the positive predictive value of a short accessory-pathway antegrade effective refractory period for developing malignant arrhythmia was lower than reported (15% vs 82%, respectively) and that its negative predictive value was 100%.1,19,28 Given these conflicting recommendations, we hope our data elucidates the higher association of adverse outcomes and support considerations for more intensive EPT indications in patients with WPW syndrome.
While our study does not report SCD incidence, it does provide robust and reliable mortality data that suggests a greater association of death within an AF/AFL subgroup. Our findings would support more liberal EPT recommendations in patients with WPW syndrome.1-5,8,9 In this study, the SCA incidence rate was more than double the rate in the AF/AFL cohort (P < .001) and is commonly the initial presenting event in WPW syndrome.9 Even though the reported SCD incidence rate is low in WPW syndrome, our data demonstrated an increased association of death within the AF/AFL cohort. Physicians should consider early risk stratification and ablation to prevent potential recurrent malignant arrhythmia leading to death.1-5,8,9,12,19,20
Limitations
As a retrospective study and without access to the National Death Index, we were unable to determine the exact cause or events leading to death and instead utilized all-cause mortality data. Subsequently, our observations may only demonstrate association, rather than causality, between AF/AFL and death in patients with WPW syndrome. Additionally, we could not distinguish between AF and AFL as the arrhythmia leading to death. However, since overall survivability was the outcome of interest, our adjusted HR models were still able to demonstrate the increased association of the composite outcome and death within an AF/AFL cohort.
Although a large cohort was analyzed, due to the constraints of utilizing diagnostic codes to determine study outcomes, we could not distinguish between symptomatic and asymptomatic patients, nor how they were managed prior to the outcome event. However, as recent literature demonstrates, updated predictors of malignant arrhythmia and decisions for early EPT are similar for both symptomatic and asymptomatic patients and should be driven by the intrinsic electrophysiologic properties of the accessory pathway, rather than symptomatology; thus, our inability to discern this should have negligible consequence in determining when to perform risk stratification and ablation.1
MHS eligible patients have direct access to care; the generalizability of our data may not necessarily correspond to a community population with lower socioeconomic status (we did adjust for military sponsor rank which has been used as a proxy), reduced access to care, or uninsured individuals. However, the prevalence of WPW syndrome within our cohort was comparable to the general population, 0.4% vs 0.1%-0.3%, respectively.13,14,19 Similarly, the incidence of AF within our population was comparable to the general population, 15% vs 16%-26%, respectively.23 These similar data points suggest our results may apply beyond MHS patients.
CONCLUSIONS
Patients with WPW syndrome and AF/AFL have a higher association with adverse cardiac outcomes and death. Despite previously reported low SCD incidence rates in this population, our study demonstrates the increased association of mortality in an AF/AFL cohort. The limitations of utilizing all-cause mortality data necessitate further investigation into the etiology behind the deaths in our study population. Since ventricular pre-excitation can predispose patients to AF and potentially lead to malignant arrhythmia and SCD, understanding the cause of mortality will allow physicians to determine the appropriate monitoring and intervention strategies to improve outcomes in this population. Our results suggest consideration for more aggressive EPT screening and ablation recommendations in patients with WPW syndrome may be warranted.
1. Pappone C, Vicedomini G, Manguso F, et al. The natural history of WPW syndrome. Eur Heart J Suppl. 2015; 17 (Supplement A):A8-A11.doi:10.1093/eurheartj/suv004
2. Pappone C, Vicedomini G, Manguso F, et al. Risk of malignant arrhythmias in initially symptomatic patients with Wolff-Parkinson-White syndrome: results of a prospective long-term electrophysiological follow-up study. Circulation. 2012;125(5):661-668. doi:10.1161/CIRCULATIONAHA.111.065722
3. Pappone C, Santinelli V, Rosanio S, et al. Usefulness of invasive electrophysiologic testing to stratify the risk of arrhythmic events in asymptomatic patients with Wolff-Parkinson-White pattern: results from a large prospective long-term follow-up study. J Am Coll Cardiol. 2003;41(2):239-244. doi:10.1016/s0735-1097(02)02706-7
4. Pappone C, Vicedomini G, Manguso F, et al. Wolff-Parkinson-White syndrome in the era of catheter ablation: insights from a registry study of 2169 patients. Circulation. 2014;130(10):811-819. doi:10.1161/CIRCULATIONAHA.114.011154
5. Pappone C, Santinelli V, Manguso F, et al. A randomized study of prophylactic catheter ablation in asymptomatic patients with the Wolff-Parkinson-White syndrome. N Engl J Med. 2003;349(19):1803-1811. doi:10.1056/NEJMoa035345
6. Santinelli V, Radinovic A, Manguso F, et al. Asymptomatic ventricular preexcitation: a long-term prospective follow-up study of 293 adult patients. Circ Arrhythm Electrophysiol. 2009;2(2):102-107. doi:10.1161/CIRCEP.108.827550
7. Santinelli V, Radinovic A, Manguso F, et al. The natural history of asymptomatic ventricular pre-excitation a long-term prospective follow-up study of 184 asymptomatic children. J Am Coll Cardiol. 2009;53(3):275-280. doi:10.1016/j.jacc.2008.09.037
8. Al-Khatib SM, Arshad A, Balk EM, et al. Risk Stratification for Arrhythmic Events in Patients With Asymptomatic Pre-Excitation: A Systematic Review for the 2015 ACC/AHA/HRS Guideline for the Management of Adult Patients With Supraventricular Tachycardia: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2016;67(13):1624-1638. doi:10.1016/j.jacc.2015.09.018
9. Blomström-Lundqvist C, Scheinman MM, Aliot EM, et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Supraventricular Arrhythmias). Circulation. 2003;108(15):1871-1909.doi:10.1161/01.CIR.0000091380.04100.84
10. Pediatric and Congenital Electrophysiology Society (PACES); Heart Rhythm Society (HRS); American College of Cardiology Foundation (ACCF); PACES/HRS expert consensus statement on the management of the asymptomatic young patient with a Wolff-Parkinson-White (WPW, ventricular preexcitation) electrocardiographic pattern: developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology Foundation (ACCF), the American Heart Association (AHA), the American Academy of Pediatrics (AAP), and the Canadian Heart Rhythm Society (CHRS). Heart Rhythm. 2012;9(6):1006-1024. doi:10.1016/j.hrthm.2012.03.050
11. Cohen M, Triedman J. Guidelines for management of asymptomatic ventricular pre-excitation: brave new world or Pandora’s box?. Circ Arrhythm Electrophysiol. 2014;7(2):187-189. doi:10.1161/CIRCEP.114.001528
12. Svendsen JH, Dagres N, Dobreanu D, et al. Current strategy for treatment of patients with Wolff-Parkinson-White syndrome and asymptomatic preexcitation in Europe: European Heart Rhythm Association survey. Europace. 2013;15(5):750-753. doi:10.1093/europace/eut094
13. Gimbel RW, Pangaro L, Barbour G. America’s “undiscovered” laboratory for health services research. Med Care. 2010;48(8):751-756. doi:10.1097/MLR.0b013e3181e35be8
14. Dorrance KA, Ramchandani S, Neil N, Fisher H. Leveraging the military health system as a laboratory for health care reform. Mil Med. 2013;178(2):142-145. doi:10.7205/milmed-d-12-00168
15. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. doi:10.1097/01.mlr.0000182534.19832.83
16. Finocchiaro G, Papadakis M, Behr ER, Sharma S, Sheppard M. Sudden Cardiac Death in Pre-Excitation and Wolff-Parkinson-White: Demographic and Clinical Features. J Am Coll Cardiol. 2017;69(12):1644-1645. doi:10.1016/j.jacc.2017.01.023
17. Munger TM, Packer DL, Hammill SC, et al. A population study of the natural history of Wolff-Parkinson-White syndrome in Olmsted County, Minnesota, 1953-1989. Circulation. 1993;87(3):866-873. doi:10.1161/01.cir.87.3.866
18. Fitzsimmons PJ, McWhirter PD, Peterson DW, Kruyer WB. The natural history of Wolff-Parkinson-White syndrome in 228 military aviators: a long-term follow-up of 22 years. Am Heart J. 2001;142(3):530-536. doi:10.1067/mhj.2001.117779
19. Obeyesekere MN, Leong-Sit P, Massel D, et al. Risk of arrhythmia and sudden death in patients with asymptomatic preexcitation: a meta-analysis. Circulation. 2012;125(19):2308-2315. doi:10.1161/CIRCULATIONAHA.111.055350
20. Waspe LE, Brodman R, Kim SG, Fisher JD. Susceptibility to atrial fibrillation and ventricular tachyarrhythmia in the Wolff-Parkinson-White syndrome: role of the accessory pathway. Am Heart J. 1986;112(6):1141-1152. doi:10.1016/0002-8703(86)90342-x
21. Pietersen AH, Andersen ED, Sandøe E. Atrial fibrillation in the Wolff-Parkinson-White syndrome. Am J Cardiol. 1992;70(5):38A-43A. doi:10.1016/0002-9149(92)91076-g
22. Della Bella P, Brugada P, Talajic M, et al. Atrial fibrillation in patients with an accessory pathway: importance of the conduction properties of the accessory pathway. J Am Coll Cardiol. 1991;17(6):1352-1356. doi:10.1016/s0735-1097(10)80146-9
23. Fujimura O, Klein GJ, Yee R, Sharma AD. Mode of onset of atrial fibrillation in the Wolff-Parkinson-White syndrome: how important is the accessory pathway?. J Am Coll Cardiol. 1990;15(5):1082-1086. doi:10.1016/0735-1097(90)90244-j
24. Montoya PT, Brugada P, Smeets J, et al. Ventricular fibrillation in the Wolff-Parkinson-White syndrome. Eur Heart J. 1991;12(2):144-150. doi:10.1093/oxfordjournals.eurheartj.a059860
25. Klein GJ, Bashore TM, Sellers TD, Pritchett EL, Smith WM, Gallagher JJ. Ventricular fibrillation in the Wolff-Parkinson-White syndrome. N Engl J Med. 1979;301(20):1080-1085. doi:10.1056/NEJM197911153012003
26. Centurion OA. Atrial Fibrillation in the Wolff-Parkinson-White Syndrome. J Atr Fibrillation. 2011;4(1):287. Published 2011 May 4. doi:10.4022/jafib.287
27. Song C, Guo Y, Zheng X, et al. Prognostic Significance and Risk of Atrial Fibrillation of Wolff-Parkinson-White Syndrome in Patients With Hypertrophic Cardiomyopathy. Am J Cardiol. 2018;122(9):1546-1550. doi:10.1016/j.amjcard.2018.07.021
28. Obeyesekere M, Gula LJ, Skanes AC, Leong-Sit P, Klein GJ. Risk of sudden death in Wolff-Parkinson-White syndrome: how high is the risk?. Circulation. 2012;125(5):659-660. doi:10.1161/CIRCULATIONAHA.111.085159
1. Pappone C, Vicedomini G, Manguso F, et al. The natural history of WPW syndrome. Eur Heart J Suppl. 2015; 17 (Supplement A):A8-A11.doi:10.1093/eurheartj/suv004
2. Pappone C, Vicedomini G, Manguso F, et al. Risk of malignant arrhythmias in initially symptomatic patients with Wolff-Parkinson-White syndrome: results of a prospective long-term electrophysiological follow-up study. Circulation. 2012;125(5):661-668. doi:10.1161/CIRCULATIONAHA.111.065722
3. Pappone C, Santinelli V, Rosanio S, et al. Usefulness of invasive electrophysiologic testing to stratify the risk of arrhythmic events in asymptomatic patients with Wolff-Parkinson-White pattern: results from a large prospective long-term follow-up study. J Am Coll Cardiol. 2003;41(2):239-244. doi:10.1016/s0735-1097(02)02706-7
4. Pappone C, Vicedomini G, Manguso F, et al. Wolff-Parkinson-White syndrome in the era of catheter ablation: insights from a registry study of 2169 patients. Circulation. 2014;130(10):811-819. doi:10.1161/CIRCULATIONAHA.114.011154
5. Pappone C, Santinelli V, Manguso F, et al. A randomized study of prophylactic catheter ablation in asymptomatic patients with the Wolff-Parkinson-White syndrome. N Engl J Med. 2003;349(19):1803-1811. doi:10.1056/NEJMoa035345
6. Santinelli V, Radinovic A, Manguso F, et al. Asymptomatic ventricular preexcitation: a long-term prospective follow-up study of 293 adult patients. Circ Arrhythm Electrophysiol. 2009;2(2):102-107. doi:10.1161/CIRCEP.108.827550
7. Santinelli V, Radinovic A, Manguso F, et al. The natural history of asymptomatic ventricular pre-excitation a long-term prospective follow-up study of 184 asymptomatic children. J Am Coll Cardiol. 2009;53(3):275-280. doi:10.1016/j.jacc.2008.09.037
8. Al-Khatib SM, Arshad A, Balk EM, et al. Risk Stratification for Arrhythmic Events in Patients With Asymptomatic Pre-Excitation: A Systematic Review for the 2015 ACC/AHA/HRS Guideline for the Management of Adult Patients With Supraventricular Tachycardia: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2016;67(13):1624-1638. doi:10.1016/j.jacc.2015.09.018
9. Blomström-Lundqvist C, Scheinman MM, Aliot EM, et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Supraventricular Arrhythmias). Circulation. 2003;108(15):1871-1909.doi:10.1161/01.CIR.0000091380.04100.84
10. Pediatric and Congenital Electrophysiology Society (PACES); Heart Rhythm Society (HRS); American College of Cardiology Foundation (ACCF); PACES/HRS expert consensus statement on the management of the asymptomatic young patient with a Wolff-Parkinson-White (WPW, ventricular preexcitation) electrocardiographic pattern: developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology Foundation (ACCF), the American Heart Association (AHA), the American Academy of Pediatrics (AAP), and the Canadian Heart Rhythm Society (CHRS). Heart Rhythm. 2012;9(6):1006-1024. doi:10.1016/j.hrthm.2012.03.050
11. Cohen M, Triedman J. Guidelines for management of asymptomatic ventricular pre-excitation: brave new world or Pandora’s box?. Circ Arrhythm Electrophysiol. 2014;7(2):187-189. doi:10.1161/CIRCEP.114.001528
12. Svendsen JH, Dagres N, Dobreanu D, et al. Current strategy for treatment of patients with Wolff-Parkinson-White syndrome and asymptomatic preexcitation in Europe: European Heart Rhythm Association survey. Europace. 2013;15(5):750-753. doi:10.1093/europace/eut094
13. Gimbel RW, Pangaro L, Barbour G. America’s “undiscovered” laboratory for health services research. Med Care. 2010;48(8):751-756. doi:10.1097/MLR.0b013e3181e35be8
14. Dorrance KA, Ramchandani S, Neil N, Fisher H. Leveraging the military health system as a laboratory for health care reform. Mil Med. 2013;178(2):142-145. doi:10.7205/milmed-d-12-00168
15. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. doi:10.1097/01.mlr.0000182534.19832.83
16. Finocchiaro G, Papadakis M, Behr ER, Sharma S, Sheppard M. Sudden Cardiac Death in Pre-Excitation and Wolff-Parkinson-White: Demographic and Clinical Features. J Am Coll Cardiol. 2017;69(12):1644-1645. doi:10.1016/j.jacc.2017.01.023
17. Munger TM, Packer DL, Hammill SC, et al. A population study of the natural history of Wolff-Parkinson-White syndrome in Olmsted County, Minnesota, 1953-1989. Circulation. 1993;87(3):866-873. doi:10.1161/01.cir.87.3.866
18. Fitzsimmons PJ, McWhirter PD, Peterson DW, Kruyer WB. The natural history of Wolff-Parkinson-White syndrome in 228 military aviators: a long-term follow-up of 22 years. Am Heart J. 2001;142(3):530-536. doi:10.1067/mhj.2001.117779
19. Obeyesekere MN, Leong-Sit P, Massel D, et al. Risk of arrhythmia and sudden death in patients with asymptomatic preexcitation: a meta-analysis. Circulation. 2012;125(19):2308-2315. doi:10.1161/CIRCULATIONAHA.111.055350
20. Waspe LE, Brodman R, Kim SG, Fisher JD. Susceptibility to atrial fibrillation and ventricular tachyarrhythmia in the Wolff-Parkinson-White syndrome: role of the accessory pathway. Am Heart J. 1986;112(6):1141-1152. doi:10.1016/0002-8703(86)90342-x
21. Pietersen AH, Andersen ED, Sandøe E. Atrial fibrillation in the Wolff-Parkinson-White syndrome. Am J Cardiol. 1992;70(5):38A-43A. doi:10.1016/0002-9149(92)91076-g
22. Della Bella P, Brugada P, Talajic M, et al. Atrial fibrillation in patients with an accessory pathway: importance of the conduction properties of the accessory pathway. J Am Coll Cardiol. 1991;17(6):1352-1356. doi:10.1016/s0735-1097(10)80146-9
23. Fujimura O, Klein GJ, Yee R, Sharma AD. Mode of onset of atrial fibrillation in the Wolff-Parkinson-White syndrome: how important is the accessory pathway?. J Am Coll Cardiol. 1990;15(5):1082-1086. doi:10.1016/0735-1097(90)90244-j
24. Montoya PT, Brugada P, Smeets J, et al. Ventricular fibrillation in the Wolff-Parkinson-White syndrome. Eur Heart J. 1991;12(2):144-150. doi:10.1093/oxfordjournals.eurheartj.a059860
25. Klein GJ, Bashore TM, Sellers TD, Pritchett EL, Smith WM, Gallagher JJ. Ventricular fibrillation in the Wolff-Parkinson-White syndrome. N Engl J Med. 1979;301(20):1080-1085. doi:10.1056/NEJM197911153012003
26. Centurion OA. Atrial Fibrillation in the Wolff-Parkinson-White Syndrome. J Atr Fibrillation. 2011;4(1):287. Published 2011 May 4. doi:10.4022/jafib.287
27. Song C, Guo Y, Zheng X, et al. Prognostic Significance and Risk of Atrial Fibrillation of Wolff-Parkinson-White Syndrome in Patients With Hypertrophic Cardiomyopathy. Am J Cardiol. 2018;122(9):1546-1550. doi:10.1016/j.amjcard.2018.07.021
28. Obeyesekere M, Gula LJ, Skanes AC, Leong-Sit P, Klein GJ. Risk of sudden death in Wolff-Parkinson-White syndrome: how high is the risk?. Circulation. 2012;125(5):659-660. doi:10.1161/CIRCULATIONAHA.111.085159
Reporting Coronary Artery Calcium on Low-Dose Computed Tomography Impacts Statin Management in a Lung Cancer Screening Population
Cigarette smoking is an independent risk factor for lung cancer and atherosclerotic cardiovascular disease (ASCVD).1-3 The National Lung Screening Trial (NLST) demonstrated both lung cancer mortality reduction with the use of surveillance low-dose computed tomography (LDCT) and ASCVD as the most common cause of death among smokers.4,5 ASCVD remains the leading cause of death in the lung cancer screening (LCS) population.2,3 After publication of the NLST results, the US Preventive Services Task Force (USPSTF) established LCS eligibility among smokers and the Center for Medicare and Medicaid Services approved payment for annual LDCT in this group.1,6,7
Recently LDCT has been proposed as an adjunct diagnostic tool for detecting coronary artery calcium (CAC), which is independently associated with ASCVD and mortality.8-13 CAC scores have been recommended by the 2019 American College of Cardiology/American Heart Association cholesterol treatment guidelines and shown to be cost-effective in guiding statin therapy for patients with borderline to intermediate ASCVD risk.14-16 While CAC is conventionally quantified using electrocardiogram (ECG)-gated CT, these scans are not routinely performed in clinical practice because preventive CAC screening is neither recommended by the USPSTF nor covered by most insurance providers.17,18 LDCT, conversely, is reimbursable and a well-validated ASCVD risk predictor.18,19
In this study, we aimed to determine the validity of LDCT in identifying CAC among the military LCS population and whether it would impact statin recommendations based on 10-year ASCVD risk.
Methods
Participants were recruited from a retrospective cohort of 563 Military Health System (MHS) beneficiaries who received LCS with LDCT at Naval Medical Center Portsmouth (NMCP) in Virginia between January 1, 2019, and December 31, 2020. The 2013 USPSTF LCS guidelines were followed as the 2021 guidelines had not been published before the start of the study; thus, eligible participants included adults aged 55 to 80 years with at least a 30-pack-year smoking history and currently smoked or had quit within 15 years from the date of study consent.6,7
Between November 2020 and May 2021, study investigators screened 287 patient records and recruited 190 participants by telephone, starting with individuals who had the most recent LDCT and working backward until reaching the predetermined 170 subjects who had undergone in-office consents before ECG-gated CT scans. Since LDCT was not obtained simultaneously with the ECG-gated CT, participants were required to complete their gated CT within 24 months of their last LDCT. Of the 190 subjects initially recruited, those who were ineligible for LCS (n = 4), had a history of angioplasty, stent, or bypass revascularization procedure (n = 4), did not complete their ECG-gated CT within the specified time frame (n = 8), or withdrew from the study (n = 4) were excluded. While gated CT scans were scored for CAC in the present time, LDCT (previously only read for general lung pathology) was not scored until after participant consent. Patients were peripherally followed, via health record reviews, for 3 months after their gated CT to document any additional imaging ordered by their primary care practitioners. The study was approved by the NMCP Institutional Review Board.
Coronary Artery Calcification Scoring
We performed CT scans using Siemens SOMATOM Flash, a second-generation dual-source scanner; and GE LightSpeed VCT, a single-source, 64-slice scanner. A step-and-shoot prospective trigger technique was used, and contiguous axial images were reconstructed at 2.5-mm or 3-mm intervals for CAC quantification using the Agatston method.20 ECG-gated CT scans were electrocardiographically triggered at mid-diastole (70% of the R-R interval). Radiation dose reduction techniques involved adjustments of the mA according to body mass index and iterative reconstruction. LDCT scans were performed without ECG gating. We reconstructed contiguous axial images at 1-mm intervals for evaluation of the lung parenchyma. Similar dose-reduction techniques were used, to limit radiation exposure for each LDCT scan to < 1.5 mSv, per established guidelines.21 CAC on LDCT was also scored using the Agatston method. CAC was scored on the 2 scan types by different blinded reviewers.
Covariates
We reviewed outpatient health records to obtain participants’ age, sex, medical history, statin use, smoking status (current or former), and pack-years. International Classification of Diseases, Tenth Revision codes within medical encounters were used to document prevalent hypertension, hyperlipidemia, and diabetes mellitus. Participants’ most recent low-density lipoprotein value (within 24 months of ECG-gated CT) was recorded and 10-year ASCVD risk scores were calculated using the pooled cohorts equation.
Statistical Analysis
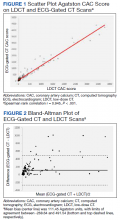
A power analysis performed before study initiation determined that a prospective sample size of 170 would be sufficient to provide strength of correlation between CAC scores calculated from ECG-gated CT and LDCT and achieve a statistical power of at least 80%. The Wilcoxon rank sum and Fisher exact tests were used to evaluate differences in continuous and categorical CAC scores, respectively. Given skewed distributions, Spearman rank correlations and Kendall W coefficient of concordance were respectively used to evaluate correlation and concordance of CAC scores between the 2 scan types. κ statistics were used to rate agreement between categorical CAC scores. Bland-Altman analysis was performed to determine the bias and limits of agreement between ECG-gated CT and LDCT.22 For categorical CAC score analysis, participants were categorized into 5 groups according to standard Agatston score cut-off points. We defined the 5 categories of CAC for both scan types based on previous analysis from Rumberger and colleagues: CAC = 0 (absent), CAC = 1-10 (minimal), CAC = 11-100 (mild), CAC = 101-400 (moderate), CAC > 400 (severe).23 Of note, LDCT reports at NMCP include a visual CAC score using these qualitative descriptors that were available to LDCT reviewers. Analyses were conducted using SAS version 9.4 and Microsoft Excel; P values < .05 were considered statistically significant.
Results
The 170 participants had a mean (SD) age of 62.1 (4.6) years and were 70.6% male (Table 1). Hyperlipidemia was the most prevalent cardiac risk factor with almost 70% of participants on a statin. There was no incidence of ischemic ASCVD during follow-up, although 1 participant was later diagnosed with lung cancer after evaluation of suspicious pulmonary findings on ECG-gated CT. CAC was identified on both scan types in 126 participants; however, LDCT was discordant with gated CT in identifying CAC in 24 subjects (P < .001).
The correlation between CAC scores on ECG-gated CT and LDCT was 0.945 (P < .001) and the concordance was 0.643, indicating moderate agreement between CAC scores on the 2 different scans (Figure 1). Median CAC scores were significantly higher on ECG-gated CT when compared with LDCT (107.5 vs 48.1 Agatston units, respectively; P < .05). Table 2 shows the CAC score characteristics for both scan types. The κ statistic for agreement between categorical CAC scores on ECG-gated CT compared with LDCT was 0.49 (SEκ= 0.05; 95% CI, -0.73-1.71), and the weighted κ statistic was 0.71, indicating moderate to substantial agreement between the 2 scans using the specified cutoff points. The Bland-Altman analysis presented a mean bias of 111.45 Agatston units, with limits of agreement between -268.64 and 491.54, as shown in Figure 2, suggesting that CAC scores on ECG-gated CT were, on average, about 111 units higher than those on LDCT. Finally, there were 24 participants with CAC seen on ECG-gated CT but none identified on LDCT (P < .001); of this cohort 20 were already on a statin, and of the remaining 4 individuals, 1 met statin criteria based on a > 20% ASCVD risk score alone (regardless of CAC score), 1 with an intermediate risk score met statin criteria based on CAC score reporting, 1 did not meet criteria due to a low-risk score, and the last had no reportable ASCVD risk score.
In the study, there were 80 participants with reportable borderline to intermediate 10-year ASCVD risk scores (5% ≤ 10-year ASCVD risk < 20%), 49 of which were taking a statin. Of the remaining 31 participants not on a statin, 19 met statin criteria after CAC was identified on ECG-gated CT (of these 18 also had CAC identified on LDCT). Subsequently, the number of participants who met statin criteria after additional CAC reporting (on ECG-gated CT and LDCT) was statistically significant (P < .001 and P < .05, respectively). Of the 49 participants on a statin, only 1 individual no longer met statin criteria due to a CAC score < 1 on gated CT.
Discussion
In this study population of recruited MHS beneficiaries, there was a strong correlation and moderate to substantial agreement between CAC scores calculated from LDCT and conventional ECG-gated CT. The number of nonstatin participants who met statin criteria and would have benefited from additional CAC score reporting was statistically significant as compared to their statin counterparts who no longer met the criteria.
CAC screening using nongated CT has become an increasingly available and consistently reproducible means for stratifying ASCVD risk and guiding statin therapy in individuals with equivocal ASCVD risk scores.24-26 As has been demonstrated in previous studies, our study additionally highlights the effective use of LDCT in not only identifying CAC, but also in beneficially impacting statin decisions in the high-risk smoking population.24-26 Our results also showed LDCT missed CAC in participants, the majority of which were already on a statin, and only 1 nonstatin individual benefited from additional CAC reporting. CAC scoring on LDCT should be an adjunct, not a substitute, for ASCVD risk stratification to help guide statin management.25,27
Our results may provide cost considerate implications for preventive CAC screening. While TRICARE covers the cost of ECG-gated CT for MHS beneficiaries, the same is not true of most nonmilitary insurance providers. Concerns about cancer risk from radiation exposure may also lead to hesitation about receiving additional CTs in the smoking population. Since the LCS population already receives annual LDCT, these scans can also be used for CAC scoring to help primary care professionals risk stratify their patients, as has been previously shown.28-31 Clinicians should consider implementing CAC scoring with annual LDCT scans, which would curtail further risks and expenses from CAC-specified scans.
Although CAC is scored visually and routinely reported in the body of LDCT reports at our facility, this is not a universal practice and was performed in only 44% of subjects with known CAC by a previous study.32 In 2007, there were 600,000 CAC scoring scans and > 9 million routine chest CTs performed in the United States.33 Based on our results and the growing consensus in the existing literature, CAC scoring on nongated CT is not only valid and reliable, but also can estimate ASCVD risk and subsequent mortality.34-36 Routine chest CTs remain an available resource for providing additional ASCVD risk stratification.
As we demonstrated, median CAC scores on LDCT were on average significantly lower than those from gated CT. This could be due to slice thickness variability between the GE and Siemens scanners or CAC progression between the time of the retrospective LDCT and prospective ECG-gated CT. Aside from this potential limitation, LDCT has been shown to have a high level of agreement with gated CT in predicting CAC, both visually and by the Agatston technique.37-39 Our results further support previous recommendations of utilizing CAC score categories when determining ASCVD risk from LDCT and that establishing scoring cutoff points warrants further development for potential standardization.37-39 Readers should be mindful that LDCT may still be less sensitive and underestimate low CAC levels and that ECG-gated CT may occasionally be more optimal in determining ASCVD risk when considering the negative predictive value of CAC.40
Limitations
Our study cohort was composed of MHS beneficiaries. Compared with the general population, these individuals may have greater access to care and be more likely to receive statins after preventive screenings. Additional studies may be required to assess CAC-associated statin eligibility among the general population. As discussed previously LDCT was not performed concomitantly with the ECG-gated CT. Although there was moderate to substantial CAC agreement between the 2 scan types, the timing difference could have led to absolute differences in CAC scores across both scan types and impacted the ability to detect low-level CAC on LDCT. CAC values should be interpreted based on the respective scan type.
Conclusions
LDCT is a reliable diagnostic alternative to ECG-gated CT in predicting CAC. CAC scores from LDCT are highly correlated and concordant with those from gated CT and can help guide statin management in individuals with intermediate ASCVD risk. The proposed duality of LDCT to assess ASCVD risk in addition to lung cancer can reduce the need for unnecessary scans while optimizing preventive clinical care. While coronary calcium and elevated CAC scores can facilitate clinical decision making to initiate statin therapy for intermediate-risk patients, physicians must still determine whether additional cardiac testing is warranted to avoid unnecessary procedures and health care costs. Smokers undergoing annual LDCT may benefit from standardized CAC scoring to help further stratify ASCVD risk while limiting the expense and radiation of additional scans.
Acknowledgments
The authors thank Ms. Lorie Gower for her contributions to the study.
1. Leigh A, McEvoy JW, Garg P, et al. Coronary artery calcium scores and atherosclerotic cardiovascular disease risk stratification in smokers. JACC Cardiovasc Imaging. 2019;12(5):852-861. doi:10.1016/j.jcmg.2017.12.017
2. Lu MT, Onuma OK, Massaro JM, D’Agostino RB Sr, O’Donnell CJ, Hoffmann U. Lung cancer screening eligibility in the community: cardiovascular risk factors, coronary artery calcification, and cardiovascular events. Circulation. 2016;134(12):897-899. doi:10.1161/CIRCULATIONAHA.116.023957
3. Tailor TD, Chiles C, Yeboah J, et al. Cardiovascular risk in the lung cancer screening population: a multicenter study evaluating the association between coronary artery calcification and preventive statin prescription. J Am Coll Radiol. 2021;18(9):1258-1266. doi:10.1016/j.jacr.2021.01.015
4. National Lung Screening Trial Research Team, Church TR, Black WC, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368(21):1980-1991. doi:10.1056/NEJMoa1209120
5. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29-e322. doi:10.1161/CIR.0000000000000152
6. Moyer VA; U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330-338. doi:10.7326/M13-2771
7. US Preventive Services Task Force, Krist AH, Davidson KW, et al. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325(10):962-970. doi:10.1001/jama.2021.1117
8. Arcadi T, Maffei E, Sverzellati N, et al. Coronary artery calcium score on low-dose computed tomography for lung cancer screening. World J Radiol. 2014;6(6):381-387. doi:10.4329/wjr.v6.i6.381
9. Kim SM, Chung MJ, Lee KS, Choe YH, Yi CA, Choe BK. Coronary calcium screening using low-dose lung cancer screening: effectiveness of MDCT with retrospective reconstruction. AJR Am J Roentgenol. 2008;190(4):917-922. doi:10.2214/AJR.07.2979
10. Ruparel M, Quaife SL, Dickson JL, et al. Evaluation of cardiovascular risk in a lung cancer screening cohort. Thorax. 2019;74(12):1140-1146. doi:10.1136/thoraxjnl-2018-212812
11. Jacobs PC, Gondrie MJ, van der Graaf Y, et al. Coronary artery calcium can predict all-cause mortality and cardiovascular events on low-dose CT screening for lung cancer. AJR Am J Roentgenol. 2012;198(3):505-511. doi:10.2214/AJR.10.5577
12. Fan L, Fan K. Lung cancer screening CT-based coronary artery calcification in predicting cardiovascular events: A systematic review and meta-analysis. Medicine (Baltimore). 2018;97(20):e10461. doi:10.1097/MD.0000000000010461
13. Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol. 2018;72(4):434-447. doi:10.1016/j.jacc.2018.05.027
14. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e563-e595. doi:10.1161/CIR.0000000000000677
15. Pletcher MJ, Pignone M, Earnshaw S, et al. Using the coronary artery calcium score to guide statin therapy: a cost-effectiveness analysis. Circ Cardiovasc Qual Outcomes. 2014;7(2):276-284. doi:10.1161/CIRCOUTCOMES.113.000799
16. Hong JC, Blankstein R, Shaw LJ, et al. Implications of coronary artery calcium testing for treatment decisions among statin candidates according to the ACC/AHA Cholesterol Management Guidelines: a cost-effectiveness analysis. JACC Cardiovasc Imaging. 2017;10(8):938-952. doi:10.1016/j.jcmg.2017.04.014
17. US Preventive Services Task Force, Curry SJ, Krist AH, et al. Risk assessment for cardiovascular disease with nontraditional risk factors: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320(3):272-280. doi:10.1001/jama.2018.8359
18. Hughes-Austin JM, Dominguez A 3rd, Allison MA, et al. Relationship of coronary calcium on standard chest CT scans with mortality. JACC Cardiovasc Imaging. 2016;9(2):152-159. doi:10.1016/j.jcmg.2015.06.030
19. Haller C, Vandehei A, Fisher R, et al. Incidence and implication of coronary artery calcium on non-gated chest computed tomography scans: a large observational cohort. Cureus. 2019;11(11):e6218. Published 2019 Nov 22. doi:10.7759/cureus.6218
20. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827-832. doi:10.1016/0735-1097(90)90282-t
21. Aberle D, Berg C, Black W, et al. The National Lung Screening Trial: overview and study design. Radiology. 2011;258(1):243-53. doi:10.1148/radiol.10091808
22. Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135-160. doi:10.1177/096228029900800204
23. Rumberger JA, Brundage BH, Rader DJ, Kondos G. Electron beam computed tomographic coronary calcium scanning: a review and guidelines for use in asymptomatic persons. Mayo Clin Proc. 1999;74(3):243-252. doi:10.4065/74.3.243
24. Douthit NT, Wyatt N, Schwartz B. Clinical impact of reporting coronary artery calcium scores of non-gated chest computed tomography on statin management. Cureus. 2021;13(5):e14856. Published 2021 May 5. doi:10.7759/cureus.14856
25. Miedema MD, Dardari ZA, Kianoush S, et al. Statin eligibility, coronary artery calcium, and subsequent cardiovascular events according to the 2016 United States Preventive Services Task Force (USPSTF) Statin Guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Heart Assoc. 2018;7(12):e008920. Published 2018 Jun 13. doi:10.1161/JAHA.118.008920
26. Fisher R, Vandehei A, Haller C, et al. Reporting the presence of coronary artery calcium in the final impression of non-gated CT chest scans increases the appropriate utilization of statins. Cureus. 2020;12(9):e10579. Published 2020 Sep 21. doi:10.7759/cureus.10579
27. Blaha MJ, Budoff MJ, DeFilippis AP, et al. Associations between C-reactive protein, coronary artery calcium, and cardiovascular events: implications for the JUPITER population from MESA, a population-based cohort study. Lancet. 2011;378(9792):684-692. doi:10.1016/S0140-6736(11)60784-8
28. Waheed S, Pollack S, Roth M, Reichek N, Guerci A, Cao JJ. Collective impact of conventional cardiovascular risk factors and coronary calcium score on clinical outcomes with or without statin therapy: the St Francis Heart Study. Atherosclerosis. 2016;255:193-199. doi:10.1016/j.atherosclerosis.2016.09.060
29. Mahabadi AA, Möhlenkamp S, Lehmann N, et al. CAC score improves coronary and CV risk assessment above statin indication by ESC and AHA/ACC Primary Prevention Guidelines. JACC Cardiovasc Imaging. 2017;10(2):143-153. doi:10.1016/j.jcmg.2016.03.022
30. Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2016;133(9):849-858. doi:10.1161/CIRCULATIONAHA.115.018524
31. Hoffmann U, Massaro JM, D’Agostino RB Sr, Kathiresan S, Fox CS, O’Donnell CJ. Cardiovascular event prediction and risk reclassification by coronary, aortic, and valvular calcification in the Framingham Heart Study. J Am Heart Assoc. 2016;5(2):e003144. Published 2016 Feb 22. doi:10.1161/JAHA.115.003144
32. Williams KA Sr, Kim JT, Holohan KM. Frequency of unrecognized, unreported, or underreported coronary artery and cardiovascular calcification on noncardiac chest CT. J Cardiovasc Comput Tomogr. 2013;7(3):167-172. doi:10.1016/j.jcct.2013.05.003

33. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077. doi:10.1001/archinternmed.2009.440
34. Azour L, Kadoch MA, Ward TJ, Eber CD, Jacobi AH. Estimation of cardiovascular risk on routine chest CT: Ordinal coronary artery calcium scoring as an accurate predictor of Agatston score ranges. J Cardiovasc Comput Tomogr. 2017;11(1):8-15. doi:10.1016/j.jcct.2016.10.001
35. Waltz J, Kocher M, Kahn J, Dirr M, Burt JR. The future of concurrent automated coronary artery calcium scoring on screening low-dose computed tomography. Cureus. 2020;12(6):e8574. Published 2020 Jun 12. doi:10.7759/cureus.8574
36. Huang YL, Wu FZ, Wang YC, et al. Reliable categorisation of visual scoring of coronary artery calcification on low-dose CT for lung cancer screening: validation with the standard Agatston score. Eur Radiol. 2013;23(5):1226-1233. doi:10.1007/s00330-012-2726-5
37. Kim YK, Sung YM, Cho SH, Park YN, Choi HY. Reliability analysis of visual ranking of coronary artery calcification on low-dose CT of the thorax for lung cancer screening: comparison with ECG-gated calcium scoring CT. Int J Cardiovasc Imaging. 2014;30 Suppl 2:81-87. doi:10.1007/s10554-014-0507-8
38. Xia C, Vonder M, Pelgrim GJ, et al. High-pitch dual-source CT for coronary artery calcium scoring: A head-to-head comparison of non-triggered chest versus triggered cardiac acquisition. J Cardiovasc Comput Tomogr. 2021;15(1):65-72. doi:10.1016/j.jcct.2020.04.013
39. Hutt A, Duhamel A, Deken V, et al. Coronary calcium screening with dual-source CT: reliability of ungated, high-pitch chest CT in comparison with dedicated calcium-scoring CT. Eur Radiol. 2016;26(6):1521-1528. doi:10.1007/s00330-015-3978-7
40. Blaha MJ, Budoff MJ, Tota-Maharaj R, et al. Improving the CAC score by addition of regional measures of calcium distribution: Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc Imaging. 2016;9(12):1407-1416. doi:10.1016/j.jcmg.2016.03.001
Cigarette smoking is an independent risk factor for lung cancer and atherosclerotic cardiovascular disease (ASCVD).1-3 The National Lung Screening Trial (NLST) demonstrated both lung cancer mortality reduction with the use of surveillance low-dose computed tomography (LDCT) and ASCVD as the most common cause of death among smokers.4,5 ASCVD remains the leading cause of death in the lung cancer screening (LCS) population.2,3 After publication of the NLST results, the US Preventive Services Task Force (USPSTF) established LCS eligibility among smokers and the Center for Medicare and Medicaid Services approved payment for annual LDCT in this group.1,6,7
Recently LDCT has been proposed as an adjunct diagnostic tool for detecting coronary artery calcium (CAC), which is independently associated with ASCVD and mortality.8-13 CAC scores have been recommended by the 2019 American College of Cardiology/American Heart Association cholesterol treatment guidelines and shown to be cost-effective in guiding statin therapy for patients with borderline to intermediate ASCVD risk.14-16 While CAC is conventionally quantified using electrocardiogram (ECG)-gated CT, these scans are not routinely performed in clinical practice because preventive CAC screening is neither recommended by the USPSTF nor covered by most insurance providers.17,18 LDCT, conversely, is reimbursable and a well-validated ASCVD risk predictor.18,19
In this study, we aimed to determine the validity of LDCT in identifying CAC among the military LCS population and whether it would impact statin recommendations based on 10-year ASCVD risk.
Methods
Participants were recruited from a retrospective cohort of 563 Military Health System (MHS) beneficiaries who received LCS with LDCT at Naval Medical Center Portsmouth (NMCP) in Virginia between January 1, 2019, and December 31, 2020. The 2013 USPSTF LCS guidelines were followed as the 2021 guidelines had not been published before the start of the study; thus, eligible participants included adults aged 55 to 80 years with at least a 30-pack-year smoking history and currently smoked or had quit within 15 years from the date of study consent.6,7
Between November 2020 and May 2021, study investigators screened 287 patient records and recruited 190 participants by telephone, starting with individuals who had the most recent LDCT and working backward until reaching the predetermined 170 subjects who had undergone in-office consents before ECG-gated CT scans. Since LDCT was not obtained simultaneously with the ECG-gated CT, participants were required to complete their gated CT within 24 months of their last LDCT. Of the 190 subjects initially recruited, those who were ineligible for LCS (n = 4), had a history of angioplasty, stent, or bypass revascularization procedure (n = 4), did not complete their ECG-gated CT within the specified time frame (n = 8), or withdrew from the study (n = 4) were excluded. While gated CT scans were scored for CAC in the present time, LDCT (previously only read for general lung pathology) was not scored until after participant consent. Patients were peripherally followed, via health record reviews, for 3 months after their gated CT to document any additional imaging ordered by their primary care practitioners. The study was approved by the NMCP Institutional Review Board.
Coronary Artery Calcification Scoring
We performed CT scans using Siemens SOMATOM Flash, a second-generation dual-source scanner; and GE LightSpeed VCT, a single-source, 64-slice scanner. A step-and-shoot prospective trigger technique was used, and contiguous axial images were reconstructed at 2.5-mm or 3-mm intervals for CAC quantification using the Agatston method.20 ECG-gated CT scans were electrocardiographically triggered at mid-diastole (70% of the R-R interval). Radiation dose reduction techniques involved adjustments of the mA according to body mass index and iterative reconstruction. LDCT scans were performed without ECG gating. We reconstructed contiguous axial images at 1-mm intervals for evaluation of the lung parenchyma. Similar dose-reduction techniques were used, to limit radiation exposure for each LDCT scan to < 1.5 mSv, per established guidelines.21 CAC on LDCT was also scored using the Agatston method. CAC was scored on the 2 scan types by different blinded reviewers.
Covariates
We reviewed outpatient health records to obtain participants’ age, sex, medical history, statin use, smoking status (current or former), and pack-years. International Classification of Diseases, Tenth Revision codes within medical encounters were used to document prevalent hypertension, hyperlipidemia, and diabetes mellitus. Participants’ most recent low-density lipoprotein value (within 24 months of ECG-gated CT) was recorded and 10-year ASCVD risk scores were calculated using the pooled cohorts equation.
Statistical Analysis
A power analysis performed before study initiation determined that a prospective sample size of 170 would be sufficient to provide strength of correlation between CAC scores calculated from ECG-gated CT and LDCT and achieve a statistical power of at least 80%. The Wilcoxon rank sum and Fisher exact tests were used to evaluate differences in continuous and categorical CAC scores, respectively. Given skewed distributions, Spearman rank correlations and Kendall W coefficient of concordance were respectively used to evaluate correlation and concordance of CAC scores between the 2 scan types. κ statistics were used to rate agreement between categorical CAC scores. Bland-Altman analysis was performed to determine the bias and limits of agreement between ECG-gated CT and LDCT.22 For categorical CAC score analysis, participants were categorized into 5 groups according to standard Agatston score cut-off points. We defined the 5 categories of CAC for both scan types based on previous analysis from Rumberger and colleagues: CAC = 0 (absent), CAC = 1-10 (minimal), CAC = 11-100 (mild), CAC = 101-400 (moderate), CAC > 400 (severe).23 Of note, LDCT reports at NMCP include a visual CAC score using these qualitative descriptors that were available to LDCT reviewers. Analyses were conducted using SAS version 9.4 and Microsoft Excel; P values < .05 were considered statistically significant.
Results
The 170 participants had a mean (SD) age of 62.1 (4.6) years and were 70.6% male (Table 1). Hyperlipidemia was the most prevalent cardiac risk factor with almost 70% of participants on a statin. There was no incidence of ischemic ASCVD during follow-up, although 1 participant was later diagnosed with lung cancer after evaluation of suspicious pulmonary findings on ECG-gated CT. CAC was identified on both scan types in 126 participants; however, LDCT was discordant with gated CT in identifying CAC in 24 subjects (P < .001).
The correlation between CAC scores on ECG-gated CT and LDCT was 0.945 (P < .001) and the concordance was 0.643, indicating moderate agreement between CAC scores on the 2 different scans (Figure 1). Median CAC scores were significantly higher on ECG-gated CT when compared with LDCT (107.5 vs 48.1 Agatston units, respectively; P < .05). Table 2 shows the CAC score characteristics for both scan types. The κ statistic for agreement between categorical CAC scores on ECG-gated CT compared with LDCT was 0.49 (SEκ= 0.05; 95% CI, -0.73-1.71), and the weighted κ statistic was 0.71, indicating moderate to substantial agreement between the 2 scans using the specified cutoff points. The Bland-Altman analysis presented a mean bias of 111.45 Agatston units, with limits of agreement between -268.64 and 491.54, as shown in Figure 2, suggesting that CAC scores on ECG-gated CT were, on average, about 111 units higher than those on LDCT. Finally, there were 24 participants with CAC seen on ECG-gated CT but none identified on LDCT (P < .001); of this cohort 20 were already on a statin, and of the remaining 4 individuals, 1 met statin criteria based on a > 20% ASCVD risk score alone (regardless of CAC score), 1 with an intermediate risk score met statin criteria based on CAC score reporting, 1 did not meet criteria due to a low-risk score, and the last had no reportable ASCVD risk score.
In the study, there were 80 participants with reportable borderline to intermediate 10-year ASCVD risk scores (5% ≤ 10-year ASCVD risk < 20%), 49 of which were taking a statin. Of the remaining 31 participants not on a statin, 19 met statin criteria after CAC was identified on ECG-gated CT (of these 18 also had CAC identified on LDCT). Subsequently, the number of participants who met statin criteria after additional CAC reporting (on ECG-gated CT and LDCT) was statistically significant (P < .001 and P < .05, respectively). Of the 49 participants on a statin, only 1 individual no longer met statin criteria due to a CAC score < 1 on gated CT.
Discussion
In this study population of recruited MHS beneficiaries, there was a strong correlation and moderate to substantial agreement between CAC scores calculated from LDCT and conventional ECG-gated CT. The number of nonstatin participants who met statin criteria and would have benefited from additional CAC score reporting was statistically significant as compared to their statin counterparts who no longer met the criteria.
CAC screening using nongated CT has become an increasingly available and consistently reproducible means for stratifying ASCVD risk and guiding statin therapy in individuals with equivocal ASCVD risk scores.24-26 As has been demonstrated in previous studies, our study additionally highlights the effective use of LDCT in not only identifying CAC, but also in beneficially impacting statin decisions in the high-risk smoking population.24-26 Our results also showed LDCT missed CAC in participants, the majority of which were already on a statin, and only 1 nonstatin individual benefited from additional CAC reporting. CAC scoring on LDCT should be an adjunct, not a substitute, for ASCVD risk stratification to help guide statin management.25,27
Our results may provide cost considerate implications for preventive CAC screening. While TRICARE covers the cost of ECG-gated CT for MHS beneficiaries, the same is not true of most nonmilitary insurance providers. Concerns about cancer risk from radiation exposure may also lead to hesitation about receiving additional CTs in the smoking population. Since the LCS population already receives annual LDCT, these scans can also be used for CAC scoring to help primary care professionals risk stratify their patients, as has been previously shown.28-31 Clinicians should consider implementing CAC scoring with annual LDCT scans, which would curtail further risks and expenses from CAC-specified scans.
Although CAC is scored visually and routinely reported in the body of LDCT reports at our facility, this is not a universal practice and was performed in only 44% of subjects with known CAC by a previous study.32 In 2007, there were 600,000 CAC scoring scans and > 9 million routine chest CTs performed in the United States.33 Based on our results and the growing consensus in the existing literature, CAC scoring on nongated CT is not only valid and reliable, but also can estimate ASCVD risk and subsequent mortality.34-36 Routine chest CTs remain an available resource for providing additional ASCVD risk stratification.
As we demonstrated, median CAC scores on LDCT were on average significantly lower than those from gated CT. This could be due to slice thickness variability between the GE and Siemens scanners or CAC progression between the time of the retrospective LDCT and prospective ECG-gated CT. Aside from this potential limitation, LDCT has been shown to have a high level of agreement with gated CT in predicting CAC, both visually and by the Agatston technique.37-39 Our results further support previous recommendations of utilizing CAC score categories when determining ASCVD risk from LDCT and that establishing scoring cutoff points warrants further development for potential standardization.37-39 Readers should be mindful that LDCT may still be less sensitive and underestimate low CAC levels and that ECG-gated CT may occasionally be more optimal in determining ASCVD risk when considering the negative predictive value of CAC.40
Limitations
Our study cohort was composed of MHS beneficiaries. Compared with the general population, these individuals may have greater access to care and be more likely to receive statins after preventive screenings. Additional studies may be required to assess CAC-associated statin eligibility among the general population. As discussed previously LDCT was not performed concomitantly with the ECG-gated CT. Although there was moderate to substantial CAC agreement between the 2 scan types, the timing difference could have led to absolute differences in CAC scores across both scan types and impacted the ability to detect low-level CAC on LDCT. CAC values should be interpreted based on the respective scan type.
Conclusions
LDCT is a reliable diagnostic alternative to ECG-gated CT in predicting CAC. CAC scores from LDCT are highly correlated and concordant with those from gated CT and can help guide statin management in individuals with intermediate ASCVD risk. The proposed duality of LDCT to assess ASCVD risk in addition to lung cancer can reduce the need for unnecessary scans while optimizing preventive clinical care. While coronary calcium and elevated CAC scores can facilitate clinical decision making to initiate statin therapy for intermediate-risk patients, physicians must still determine whether additional cardiac testing is warranted to avoid unnecessary procedures and health care costs. Smokers undergoing annual LDCT may benefit from standardized CAC scoring to help further stratify ASCVD risk while limiting the expense and radiation of additional scans.
Acknowledgments
The authors thank Ms. Lorie Gower for her contributions to the study.
Cigarette smoking is an independent risk factor for lung cancer and atherosclerotic cardiovascular disease (ASCVD).1-3 The National Lung Screening Trial (NLST) demonstrated both lung cancer mortality reduction with the use of surveillance low-dose computed tomography (LDCT) and ASCVD as the most common cause of death among smokers.4,5 ASCVD remains the leading cause of death in the lung cancer screening (LCS) population.2,3 After publication of the NLST results, the US Preventive Services Task Force (USPSTF) established LCS eligibility among smokers and the Center for Medicare and Medicaid Services approved payment for annual LDCT in this group.1,6,7
Recently LDCT has been proposed as an adjunct diagnostic tool for detecting coronary artery calcium (CAC), which is independently associated with ASCVD and mortality.8-13 CAC scores have been recommended by the 2019 American College of Cardiology/American Heart Association cholesterol treatment guidelines and shown to be cost-effective in guiding statin therapy for patients with borderline to intermediate ASCVD risk.14-16 While CAC is conventionally quantified using electrocardiogram (ECG)-gated CT, these scans are not routinely performed in clinical practice because preventive CAC screening is neither recommended by the USPSTF nor covered by most insurance providers.17,18 LDCT, conversely, is reimbursable and a well-validated ASCVD risk predictor.18,19
In this study, we aimed to determine the validity of LDCT in identifying CAC among the military LCS population and whether it would impact statin recommendations based on 10-year ASCVD risk.
Methods
Participants were recruited from a retrospective cohort of 563 Military Health System (MHS) beneficiaries who received LCS with LDCT at Naval Medical Center Portsmouth (NMCP) in Virginia between January 1, 2019, and December 31, 2020. The 2013 USPSTF LCS guidelines were followed as the 2021 guidelines had not been published before the start of the study; thus, eligible participants included adults aged 55 to 80 years with at least a 30-pack-year smoking history and currently smoked or had quit within 15 years from the date of study consent.6,7
Between November 2020 and May 2021, study investigators screened 287 patient records and recruited 190 participants by telephone, starting with individuals who had the most recent LDCT and working backward until reaching the predetermined 170 subjects who had undergone in-office consents before ECG-gated CT scans. Since LDCT was not obtained simultaneously with the ECG-gated CT, participants were required to complete their gated CT within 24 months of their last LDCT. Of the 190 subjects initially recruited, those who were ineligible for LCS (n = 4), had a history of angioplasty, stent, or bypass revascularization procedure (n = 4), did not complete their ECG-gated CT within the specified time frame (n = 8), or withdrew from the study (n = 4) were excluded. While gated CT scans were scored for CAC in the present time, LDCT (previously only read for general lung pathology) was not scored until after participant consent. Patients were peripherally followed, via health record reviews, for 3 months after their gated CT to document any additional imaging ordered by their primary care practitioners. The study was approved by the NMCP Institutional Review Board.
Coronary Artery Calcification Scoring
We performed CT scans using Siemens SOMATOM Flash, a second-generation dual-source scanner; and GE LightSpeed VCT, a single-source, 64-slice scanner. A step-and-shoot prospective trigger technique was used, and contiguous axial images were reconstructed at 2.5-mm or 3-mm intervals for CAC quantification using the Agatston method.20 ECG-gated CT scans were electrocardiographically triggered at mid-diastole (70% of the R-R interval). Radiation dose reduction techniques involved adjustments of the mA according to body mass index and iterative reconstruction. LDCT scans were performed without ECG gating. We reconstructed contiguous axial images at 1-mm intervals for evaluation of the lung parenchyma. Similar dose-reduction techniques were used, to limit radiation exposure for each LDCT scan to < 1.5 mSv, per established guidelines.21 CAC on LDCT was also scored using the Agatston method. CAC was scored on the 2 scan types by different blinded reviewers.
Covariates
We reviewed outpatient health records to obtain participants’ age, sex, medical history, statin use, smoking status (current or former), and pack-years. International Classification of Diseases, Tenth Revision codes within medical encounters were used to document prevalent hypertension, hyperlipidemia, and diabetes mellitus. Participants’ most recent low-density lipoprotein value (within 24 months of ECG-gated CT) was recorded and 10-year ASCVD risk scores were calculated using the pooled cohorts equation.
Statistical Analysis
A power analysis performed before study initiation determined that a prospective sample size of 170 would be sufficient to provide strength of correlation between CAC scores calculated from ECG-gated CT and LDCT and achieve a statistical power of at least 80%. The Wilcoxon rank sum and Fisher exact tests were used to evaluate differences in continuous and categorical CAC scores, respectively. Given skewed distributions, Spearman rank correlations and Kendall W coefficient of concordance were respectively used to evaluate correlation and concordance of CAC scores between the 2 scan types. κ statistics were used to rate agreement between categorical CAC scores. Bland-Altman analysis was performed to determine the bias and limits of agreement between ECG-gated CT and LDCT.22 For categorical CAC score analysis, participants were categorized into 5 groups according to standard Agatston score cut-off points. We defined the 5 categories of CAC for both scan types based on previous analysis from Rumberger and colleagues: CAC = 0 (absent), CAC = 1-10 (minimal), CAC = 11-100 (mild), CAC = 101-400 (moderate), CAC > 400 (severe).23 Of note, LDCT reports at NMCP include a visual CAC score using these qualitative descriptors that were available to LDCT reviewers. Analyses were conducted using SAS version 9.4 and Microsoft Excel; P values < .05 were considered statistically significant.
Results
The 170 participants had a mean (SD) age of 62.1 (4.6) years and were 70.6% male (Table 1). Hyperlipidemia was the most prevalent cardiac risk factor with almost 70% of participants on a statin. There was no incidence of ischemic ASCVD during follow-up, although 1 participant was later diagnosed with lung cancer after evaluation of suspicious pulmonary findings on ECG-gated CT. CAC was identified on both scan types in 126 participants; however, LDCT was discordant with gated CT in identifying CAC in 24 subjects (P < .001).
The correlation between CAC scores on ECG-gated CT and LDCT was 0.945 (P < .001) and the concordance was 0.643, indicating moderate agreement between CAC scores on the 2 different scans (Figure 1). Median CAC scores were significantly higher on ECG-gated CT when compared with LDCT (107.5 vs 48.1 Agatston units, respectively; P < .05). Table 2 shows the CAC score characteristics for both scan types. The κ statistic for agreement between categorical CAC scores on ECG-gated CT compared with LDCT was 0.49 (SEκ= 0.05; 95% CI, -0.73-1.71), and the weighted κ statistic was 0.71, indicating moderate to substantial agreement between the 2 scans using the specified cutoff points. The Bland-Altman analysis presented a mean bias of 111.45 Agatston units, with limits of agreement between -268.64 and 491.54, as shown in Figure 2, suggesting that CAC scores on ECG-gated CT were, on average, about 111 units higher than those on LDCT. Finally, there were 24 participants with CAC seen on ECG-gated CT but none identified on LDCT (P < .001); of this cohort 20 were already on a statin, and of the remaining 4 individuals, 1 met statin criteria based on a > 20% ASCVD risk score alone (regardless of CAC score), 1 with an intermediate risk score met statin criteria based on CAC score reporting, 1 did not meet criteria due to a low-risk score, and the last had no reportable ASCVD risk score.
In the study, there were 80 participants with reportable borderline to intermediate 10-year ASCVD risk scores (5% ≤ 10-year ASCVD risk < 20%), 49 of which were taking a statin. Of the remaining 31 participants not on a statin, 19 met statin criteria after CAC was identified on ECG-gated CT (of these 18 also had CAC identified on LDCT). Subsequently, the number of participants who met statin criteria after additional CAC reporting (on ECG-gated CT and LDCT) was statistically significant (P < .001 and P < .05, respectively). Of the 49 participants on a statin, only 1 individual no longer met statin criteria due to a CAC score < 1 on gated CT.
Discussion
In this study population of recruited MHS beneficiaries, there was a strong correlation and moderate to substantial agreement between CAC scores calculated from LDCT and conventional ECG-gated CT. The number of nonstatin participants who met statin criteria and would have benefited from additional CAC score reporting was statistically significant as compared to their statin counterparts who no longer met the criteria.
CAC screening using nongated CT has become an increasingly available and consistently reproducible means for stratifying ASCVD risk and guiding statin therapy in individuals with equivocal ASCVD risk scores.24-26 As has been demonstrated in previous studies, our study additionally highlights the effective use of LDCT in not only identifying CAC, but also in beneficially impacting statin decisions in the high-risk smoking population.24-26 Our results also showed LDCT missed CAC in participants, the majority of which were already on a statin, and only 1 nonstatin individual benefited from additional CAC reporting. CAC scoring on LDCT should be an adjunct, not a substitute, for ASCVD risk stratification to help guide statin management.25,27
Our results may provide cost considerate implications for preventive CAC screening. While TRICARE covers the cost of ECG-gated CT for MHS beneficiaries, the same is not true of most nonmilitary insurance providers. Concerns about cancer risk from radiation exposure may also lead to hesitation about receiving additional CTs in the smoking population. Since the LCS population already receives annual LDCT, these scans can also be used for CAC scoring to help primary care professionals risk stratify their patients, as has been previously shown.28-31 Clinicians should consider implementing CAC scoring with annual LDCT scans, which would curtail further risks and expenses from CAC-specified scans.
Although CAC is scored visually and routinely reported in the body of LDCT reports at our facility, this is not a universal practice and was performed in only 44% of subjects with known CAC by a previous study.32 In 2007, there were 600,000 CAC scoring scans and > 9 million routine chest CTs performed in the United States.33 Based on our results and the growing consensus in the existing literature, CAC scoring on nongated CT is not only valid and reliable, but also can estimate ASCVD risk and subsequent mortality.34-36 Routine chest CTs remain an available resource for providing additional ASCVD risk stratification.
As we demonstrated, median CAC scores on LDCT were on average significantly lower than those from gated CT. This could be due to slice thickness variability between the GE and Siemens scanners or CAC progression between the time of the retrospective LDCT and prospective ECG-gated CT. Aside from this potential limitation, LDCT has been shown to have a high level of agreement with gated CT in predicting CAC, both visually and by the Agatston technique.37-39 Our results further support previous recommendations of utilizing CAC score categories when determining ASCVD risk from LDCT and that establishing scoring cutoff points warrants further development for potential standardization.37-39 Readers should be mindful that LDCT may still be less sensitive and underestimate low CAC levels and that ECG-gated CT may occasionally be more optimal in determining ASCVD risk when considering the negative predictive value of CAC.40
Limitations
Our study cohort was composed of MHS beneficiaries. Compared with the general population, these individuals may have greater access to care and be more likely to receive statins after preventive screenings. Additional studies may be required to assess CAC-associated statin eligibility among the general population. As discussed previously LDCT was not performed concomitantly with the ECG-gated CT. Although there was moderate to substantial CAC agreement between the 2 scan types, the timing difference could have led to absolute differences in CAC scores across both scan types and impacted the ability to detect low-level CAC on LDCT. CAC values should be interpreted based on the respective scan type.
Conclusions
LDCT is a reliable diagnostic alternative to ECG-gated CT in predicting CAC. CAC scores from LDCT are highly correlated and concordant with those from gated CT and can help guide statin management in individuals with intermediate ASCVD risk. The proposed duality of LDCT to assess ASCVD risk in addition to lung cancer can reduce the need for unnecessary scans while optimizing preventive clinical care. While coronary calcium and elevated CAC scores can facilitate clinical decision making to initiate statin therapy for intermediate-risk patients, physicians must still determine whether additional cardiac testing is warranted to avoid unnecessary procedures and health care costs. Smokers undergoing annual LDCT may benefit from standardized CAC scoring to help further stratify ASCVD risk while limiting the expense and radiation of additional scans.
Acknowledgments
The authors thank Ms. Lorie Gower for her contributions to the study.
1. Leigh A, McEvoy JW, Garg P, et al. Coronary artery calcium scores and atherosclerotic cardiovascular disease risk stratification in smokers. JACC Cardiovasc Imaging. 2019;12(5):852-861. doi:10.1016/j.jcmg.2017.12.017
2. Lu MT, Onuma OK, Massaro JM, D’Agostino RB Sr, O’Donnell CJ, Hoffmann U. Lung cancer screening eligibility in the community: cardiovascular risk factors, coronary artery calcification, and cardiovascular events. Circulation. 2016;134(12):897-899. doi:10.1161/CIRCULATIONAHA.116.023957
3. Tailor TD, Chiles C, Yeboah J, et al. Cardiovascular risk in the lung cancer screening population: a multicenter study evaluating the association between coronary artery calcification and preventive statin prescription. J Am Coll Radiol. 2021;18(9):1258-1266. doi:10.1016/j.jacr.2021.01.015
4. National Lung Screening Trial Research Team, Church TR, Black WC, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368(21):1980-1991. doi:10.1056/NEJMoa1209120
5. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29-e322. doi:10.1161/CIR.0000000000000152
6. Moyer VA; U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330-338. doi:10.7326/M13-2771
7. US Preventive Services Task Force, Krist AH, Davidson KW, et al. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325(10):962-970. doi:10.1001/jama.2021.1117
8. Arcadi T, Maffei E, Sverzellati N, et al. Coronary artery calcium score on low-dose computed tomography for lung cancer screening. World J Radiol. 2014;6(6):381-387. doi:10.4329/wjr.v6.i6.381
9. Kim SM, Chung MJ, Lee KS, Choe YH, Yi CA, Choe BK. Coronary calcium screening using low-dose lung cancer screening: effectiveness of MDCT with retrospective reconstruction. AJR Am J Roentgenol. 2008;190(4):917-922. doi:10.2214/AJR.07.2979
10. Ruparel M, Quaife SL, Dickson JL, et al. Evaluation of cardiovascular risk in a lung cancer screening cohort. Thorax. 2019;74(12):1140-1146. doi:10.1136/thoraxjnl-2018-212812
11. Jacobs PC, Gondrie MJ, van der Graaf Y, et al. Coronary artery calcium can predict all-cause mortality and cardiovascular events on low-dose CT screening for lung cancer. AJR Am J Roentgenol. 2012;198(3):505-511. doi:10.2214/AJR.10.5577
12. Fan L, Fan K. Lung cancer screening CT-based coronary artery calcification in predicting cardiovascular events: A systematic review and meta-analysis. Medicine (Baltimore). 2018;97(20):e10461. doi:10.1097/MD.0000000000010461
13. Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol. 2018;72(4):434-447. doi:10.1016/j.jacc.2018.05.027
14. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e563-e595. doi:10.1161/CIR.0000000000000677
15. Pletcher MJ, Pignone M, Earnshaw S, et al. Using the coronary artery calcium score to guide statin therapy: a cost-effectiveness analysis. Circ Cardiovasc Qual Outcomes. 2014;7(2):276-284. doi:10.1161/CIRCOUTCOMES.113.000799
16. Hong JC, Blankstein R, Shaw LJ, et al. Implications of coronary artery calcium testing for treatment decisions among statin candidates according to the ACC/AHA Cholesterol Management Guidelines: a cost-effectiveness analysis. JACC Cardiovasc Imaging. 2017;10(8):938-952. doi:10.1016/j.jcmg.2017.04.014
17. US Preventive Services Task Force, Curry SJ, Krist AH, et al. Risk assessment for cardiovascular disease with nontraditional risk factors: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320(3):272-280. doi:10.1001/jama.2018.8359
18. Hughes-Austin JM, Dominguez A 3rd, Allison MA, et al. Relationship of coronary calcium on standard chest CT scans with mortality. JACC Cardiovasc Imaging. 2016;9(2):152-159. doi:10.1016/j.jcmg.2015.06.030
19. Haller C, Vandehei A, Fisher R, et al. Incidence and implication of coronary artery calcium on non-gated chest computed tomography scans: a large observational cohort. Cureus. 2019;11(11):e6218. Published 2019 Nov 22. doi:10.7759/cureus.6218
20. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827-832. doi:10.1016/0735-1097(90)90282-t
21. Aberle D, Berg C, Black W, et al. The National Lung Screening Trial: overview and study design. Radiology. 2011;258(1):243-53. doi:10.1148/radiol.10091808
22. Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135-160. doi:10.1177/096228029900800204
23. Rumberger JA, Brundage BH, Rader DJ, Kondos G. Electron beam computed tomographic coronary calcium scanning: a review and guidelines for use in asymptomatic persons. Mayo Clin Proc. 1999;74(3):243-252. doi:10.4065/74.3.243
24. Douthit NT, Wyatt N, Schwartz B. Clinical impact of reporting coronary artery calcium scores of non-gated chest computed tomography on statin management. Cureus. 2021;13(5):e14856. Published 2021 May 5. doi:10.7759/cureus.14856
25. Miedema MD, Dardari ZA, Kianoush S, et al. Statin eligibility, coronary artery calcium, and subsequent cardiovascular events according to the 2016 United States Preventive Services Task Force (USPSTF) Statin Guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Heart Assoc. 2018;7(12):e008920. Published 2018 Jun 13. doi:10.1161/JAHA.118.008920
26. Fisher R, Vandehei A, Haller C, et al. Reporting the presence of coronary artery calcium in the final impression of non-gated CT chest scans increases the appropriate utilization of statins. Cureus. 2020;12(9):e10579. Published 2020 Sep 21. doi:10.7759/cureus.10579
27. Blaha MJ, Budoff MJ, DeFilippis AP, et al. Associations between C-reactive protein, coronary artery calcium, and cardiovascular events: implications for the JUPITER population from MESA, a population-based cohort study. Lancet. 2011;378(9792):684-692. doi:10.1016/S0140-6736(11)60784-8
28. Waheed S, Pollack S, Roth M, Reichek N, Guerci A, Cao JJ. Collective impact of conventional cardiovascular risk factors and coronary calcium score on clinical outcomes with or without statin therapy: the St Francis Heart Study. Atherosclerosis. 2016;255:193-199. doi:10.1016/j.atherosclerosis.2016.09.060
29. Mahabadi AA, Möhlenkamp S, Lehmann N, et al. CAC score improves coronary and CV risk assessment above statin indication by ESC and AHA/ACC Primary Prevention Guidelines. JACC Cardiovasc Imaging. 2017;10(2):143-153. doi:10.1016/j.jcmg.2016.03.022
30. Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2016;133(9):849-858. doi:10.1161/CIRCULATIONAHA.115.018524
31. Hoffmann U, Massaro JM, D’Agostino RB Sr, Kathiresan S, Fox CS, O’Donnell CJ. Cardiovascular event prediction and risk reclassification by coronary, aortic, and valvular calcification in the Framingham Heart Study. J Am Heart Assoc. 2016;5(2):e003144. Published 2016 Feb 22. doi:10.1161/JAHA.115.003144
32. Williams KA Sr, Kim JT, Holohan KM. Frequency of unrecognized, unreported, or underreported coronary artery and cardiovascular calcification on noncardiac chest CT. J Cardiovasc Comput Tomogr. 2013;7(3):167-172. doi:10.1016/j.jcct.2013.05.003

33. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077. doi:10.1001/archinternmed.2009.440
34. Azour L, Kadoch MA, Ward TJ, Eber CD, Jacobi AH. Estimation of cardiovascular risk on routine chest CT: Ordinal coronary artery calcium scoring as an accurate predictor of Agatston score ranges. J Cardiovasc Comput Tomogr. 2017;11(1):8-15. doi:10.1016/j.jcct.2016.10.001
35. Waltz J, Kocher M, Kahn J, Dirr M, Burt JR. The future of concurrent automated coronary artery calcium scoring on screening low-dose computed tomography. Cureus. 2020;12(6):e8574. Published 2020 Jun 12. doi:10.7759/cureus.8574
36. Huang YL, Wu FZ, Wang YC, et al. Reliable categorisation of visual scoring of coronary artery calcification on low-dose CT for lung cancer screening: validation with the standard Agatston score. Eur Radiol. 2013;23(5):1226-1233. doi:10.1007/s00330-012-2726-5
37. Kim YK, Sung YM, Cho SH, Park YN, Choi HY. Reliability analysis of visual ranking of coronary artery calcification on low-dose CT of the thorax for lung cancer screening: comparison with ECG-gated calcium scoring CT. Int J Cardiovasc Imaging. 2014;30 Suppl 2:81-87. doi:10.1007/s10554-014-0507-8
38. Xia C, Vonder M, Pelgrim GJ, et al. High-pitch dual-source CT for coronary artery calcium scoring: A head-to-head comparison of non-triggered chest versus triggered cardiac acquisition. J Cardiovasc Comput Tomogr. 2021;15(1):65-72. doi:10.1016/j.jcct.2020.04.013
39. Hutt A, Duhamel A, Deken V, et al. Coronary calcium screening with dual-source CT: reliability of ungated, high-pitch chest CT in comparison with dedicated calcium-scoring CT. Eur Radiol. 2016;26(6):1521-1528. doi:10.1007/s00330-015-3978-7
40. Blaha MJ, Budoff MJ, Tota-Maharaj R, et al. Improving the CAC score by addition of regional measures of calcium distribution: Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc Imaging. 2016;9(12):1407-1416. doi:10.1016/j.jcmg.2016.03.001
1. Leigh A, McEvoy JW, Garg P, et al. Coronary artery calcium scores and atherosclerotic cardiovascular disease risk stratification in smokers. JACC Cardiovasc Imaging. 2019;12(5):852-861. doi:10.1016/j.jcmg.2017.12.017
2. Lu MT, Onuma OK, Massaro JM, D’Agostino RB Sr, O’Donnell CJ, Hoffmann U. Lung cancer screening eligibility in the community: cardiovascular risk factors, coronary artery calcification, and cardiovascular events. Circulation. 2016;134(12):897-899. doi:10.1161/CIRCULATIONAHA.116.023957
3. Tailor TD, Chiles C, Yeboah J, et al. Cardiovascular risk in the lung cancer screening population: a multicenter study evaluating the association between coronary artery calcification and preventive statin prescription. J Am Coll Radiol. 2021;18(9):1258-1266. doi:10.1016/j.jacr.2021.01.015
4. National Lung Screening Trial Research Team, Church TR, Black WC, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368(21):1980-1991. doi:10.1056/NEJMoa1209120
5. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29-e322. doi:10.1161/CIR.0000000000000152
6. Moyer VA; U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330-338. doi:10.7326/M13-2771
7. US Preventive Services Task Force, Krist AH, Davidson KW, et al. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325(10):962-970. doi:10.1001/jama.2021.1117
8. Arcadi T, Maffei E, Sverzellati N, et al. Coronary artery calcium score on low-dose computed tomography for lung cancer screening. World J Radiol. 2014;6(6):381-387. doi:10.4329/wjr.v6.i6.381
9. Kim SM, Chung MJ, Lee KS, Choe YH, Yi CA, Choe BK. Coronary calcium screening using low-dose lung cancer screening: effectiveness of MDCT with retrospective reconstruction. AJR Am J Roentgenol. 2008;190(4):917-922. doi:10.2214/AJR.07.2979
10. Ruparel M, Quaife SL, Dickson JL, et al. Evaluation of cardiovascular risk in a lung cancer screening cohort. Thorax. 2019;74(12):1140-1146. doi:10.1136/thoraxjnl-2018-212812
11. Jacobs PC, Gondrie MJ, van der Graaf Y, et al. Coronary artery calcium can predict all-cause mortality and cardiovascular events on low-dose CT screening for lung cancer. AJR Am J Roentgenol. 2012;198(3):505-511. doi:10.2214/AJR.10.5577
12. Fan L, Fan K. Lung cancer screening CT-based coronary artery calcification in predicting cardiovascular events: A systematic review and meta-analysis. Medicine (Baltimore). 2018;97(20):e10461. doi:10.1097/MD.0000000000010461
13. Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol. 2018;72(4):434-447. doi:10.1016/j.jacc.2018.05.027
14. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e563-e595. doi:10.1161/CIR.0000000000000677
15. Pletcher MJ, Pignone M, Earnshaw S, et al. Using the coronary artery calcium score to guide statin therapy: a cost-effectiveness analysis. Circ Cardiovasc Qual Outcomes. 2014;7(2):276-284. doi:10.1161/CIRCOUTCOMES.113.000799
16. Hong JC, Blankstein R, Shaw LJ, et al. Implications of coronary artery calcium testing for treatment decisions among statin candidates according to the ACC/AHA Cholesterol Management Guidelines: a cost-effectiveness analysis. JACC Cardiovasc Imaging. 2017;10(8):938-952. doi:10.1016/j.jcmg.2017.04.014
17. US Preventive Services Task Force, Curry SJ, Krist AH, et al. Risk assessment for cardiovascular disease with nontraditional risk factors: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320(3):272-280. doi:10.1001/jama.2018.8359
18. Hughes-Austin JM, Dominguez A 3rd, Allison MA, et al. Relationship of coronary calcium on standard chest CT scans with mortality. JACC Cardiovasc Imaging. 2016;9(2):152-159. doi:10.1016/j.jcmg.2015.06.030
19. Haller C, Vandehei A, Fisher R, et al. Incidence and implication of coronary artery calcium on non-gated chest computed tomography scans: a large observational cohort. Cureus. 2019;11(11):e6218. Published 2019 Nov 22. doi:10.7759/cureus.6218
20. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827-832. doi:10.1016/0735-1097(90)90282-t
21. Aberle D, Berg C, Black W, et al. The National Lung Screening Trial: overview and study design. Radiology. 2011;258(1):243-53. doi:10.1148/radiol.10091808
22. Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135-160. doi:10.1177/096228029900800204
23. Rumberger JA, Brundage BH, Rader DJ, Kondos G. Electron beam computed tomographic coronary calcium scanning: a review and guidelines for use in asymptomatic persons. Mayo Clin Proc. 1999;74(3):243-252. doi:10.4065/74.3.243
24. Douthit NT, Wyatt N, Schwartz B. Clinical impact of reporting coronary artery calcium scores of non-gated chest computed tomography on statin management. Cureus. 2021;13(5):e14856. Published 2021 May 5. doi:10.7759/cureus.14856
25. Miedema MD, Dardari ZA, Kianoush S, et al. Statin eligibility, coronary artery calcium, and subsequent cardiovascular events according to the 2016 United States Preventive Services Task Force (USPSTF) Statin Guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Heart Assoc. 2018;7(12):e008920. Published 2018 Jun 13. doi:10.1161/JAHA.118.008920
26. Fisher R, Vandehei A, Haller C, et al. Reporting the presence of coronary artery calcium in the final impression of non-gated CT chest scans increases the appropriate utilization of statins. Cureus. 2020;12(9):e10579. Published 2020 Sep 21. doi:10.7759/cureus.10579
27. Blaha MJ, Budoff MJ, DeFilippis AP, et al. Associations between C-reactive protein, coronary artery calcium, and cardiovascular events: implications for the JUPITER population from MESA, a population-based cohort study. Lancet. 2011;378(9792):684-692. doi:10.1016/S0140-6736(11)60784-8
28. Waheed S, Pollack S, Roth M, Reichek N, Guerci A, Cao JJ. Collective impact of conventional cardiovascular risk factors and coronary calcium score on clinical outcomes with or without statin therapy: the St Francis Heart Study. Atherosclerosis. 2016;255:193-199. doi:10.1016/j.atherosclerosis.2016.09.060
29. Mahabadi AA, Möhlenkamp S, Lehmann N, et al. CAC score improves coronary and CV risk assessment above statin indication by ESC and AHA/ACC Primary Prevention Guidelines. JACC Cardiovasc Imaging. 2017;10(2):143-153. doi:10.1016/j.jcmg.2016.03.022
30. Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2016;133(9):849-858. doi:10.1161/CIRCULATIONAHA.115.018524
31. Hoffmann U, Massaro JM, D’Agostino RB Sr, Kathiresan S, Fox CS, O’Donnell CJ. Cardiovascular event prediction and risk reclassification by coronary, aortic, and valvular calcification in the Framingham Heart Study. J Am Heart Assoc. 2016;5(2):e003144. Published 2016 Feb 22. doi:10.1161/JAHA.115.003144
32. Williams KA Sr, Kim JT, Holohan KM. Frequency of unrecognized, unreported, or underreported coronary artery and cardiovascular calcification on noncardiac chest CT. J Cardiovasc Comput Tomogr. 2013;7(3):167-172. doi:10.1016/j.jcct.2013.05.003

33. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077. doi:10.1001/archinternmed.2009.440
34. Azour L, Kadoch MA, Ward TJ, Eber CD, Jacobi AH. Estimation of cardiovascular risk on routine chest CT: Ordinal coronary artery calcium scoring as an accurate predictor of Agatston score ranges. J Cardiovasc Comput Tomogr. 2017;11(1):8-15. doi:10.1016/j.jcct.2016.10.001
35. Waltz J, Kocher M, Kahn J, Dirr M, Burt JR. The future of concurrent automated coronary artery calcium scoring on screening low-dose computed tomography. Cureus. 2020;12(6):e8574. Published 2020 Jun 12. doi:10.7759/cureus.8574
36. Huang YL, Wu FZ, Wang YC, et al. Reliable categorisation of visual scoring of coronary artery calcification on low-dose CT for lung cancer screening: validation with the standard Agatston score. Eur Radiol. 2013;23(5):1226-1233. doi:10.1007/s00330-012-2726-5
37. Kim YK, Sung YM, Cho SH, Park YN, Choi HY. Reliability analysis of visual ranking of coronary artery calcification on low-dose CT of the thorax for lung cancer screening: comparison with ECG-gated calcium scoring CT. Int J Cardiovasc Imaging. 2014;30 Suppl 2:81-87. doi:10.1007/s10554-014-0507-8
38. Xia C, Vonder M, Pelgrim GJ, et al. High-pitch dual-source CT for coronary artery calcium scoring: A head-to-head comparison of non-triggered chest versus triggered cardiac acquisition. J Cardiovasc Comput Tomogr. 2021;15(1):65-72. doi:10.1016/j.jcct.2020.04.013
39. Hutt A, Duhamel A, Deken V, et al. Coronary calcium screening with dual-source CT: reliability of ungated, high-pitch chest CT in comparison with dedicated calcium-scoring CT. Eur Radiol. 2016;26(6):1521-1528. doi:10.1007/s00330-015-3978-7
40. Blaha MJ, Budoff MJ, Tota-Maharaj R, et al. Improving the CAC score by addition of regional measures of calcium distribution: Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc Imaging. 2016;9(12):1407-1416. doi:10.1016/j.jcmg.2016.03.001
Bacteroides Fragilis Vertebral Osteomyelitis and Discitis: “Back” to Susceptibility Testing
Acute pyogenic vertebral osteomyelitis is often due to hematogenous spread of aerobic bacteria.1-4 Conversely, only 0.5% of anaerobic bacteremias lead to osteomyelitis.5 Anaerobic osteomyelitis typically results from the contiguous spread of polymicrobial infections through breaks in the gut mucosal barrier and involves the vertebral bodies in only 2% to 5% of cases.5,6 Although Bacteroides fragilis (B fragilis) is the most common anaerobic pathogen cultivated from blood, accounting for about half of all anaerobic blood isolates, it seldom leads to osteomyelitis.1,2,7-11 We report an uncommon case of B fragilis bacteremia and vertebral osteomyelitis confounded by uncertainties in anaerobic identification and susceptibilities.
Case Presentation
A healthy-appearing male aged 55 years presented to the Naval Medical Center Portsmouth (NMCP) with subacute low back pain and fevers of 103 °F for > 3 weeks. While traveling 4 weeks prior, he completed a course of oseltamivir for influenza B infection; afterward, he was diagnosed with community-acquired pneumonia and treated with a dose of ceftriaxone and a 7-day course of doxycycline. The patient presented to the same facility a week later for low back pain and nonresolving respiratory symptoms, and his therapy was changed to azithromycin, cefuroxime, prednisone, and inhalers. Additionally, after being treated for influenza, he developed constipation and hematochezia for which he did not seek care. The hematochezia was similar to a previous episode from an anal fissure 1 year prior that resolved with stool softeners. When he was finally seen at NMCP after 3 weeks of worsening back pain and fevers, lumbosacral magnetic resonance imaging (MRI) demonstrated vertebral osteomyelitis and discitis at L4-L5 and admitted to the hospital (Figure 1).
After a fluoroscopy-guided biopsy of the L4 vertebral body on hospital day 1, the patient was started on cefepime and vancomycin. The biopsy sample was inoculated onto solid media (blood agar, chocolate agar, and MacConkey agar) and incubated at 36 °C for 24 hours in a 5% CO2 atmosphere, as well as onto Shaedler agar with vitamin K and chopped meat glucose broth and incubated at 36 °C for 48 hours under anaerobic conditions. Metronidazole was added and vancomycin discontinued after 2 anaerobic blood culture vials obtained on hospital day 1, incubated in a Becton Dickinson BACTEC FX automated system, which demonstrated Gram-negative bacilli after 48 hours. The blood culture isolates demonstrated a > 99% probability of being identified as ß-lactamase positive Prevotella loescheii using Thermo Fischer Scientific RapID ANA II biochemical testing. Nitrocefinase discs were used to detect ß-lactamase activity.
The biopsy demonstrated nongranulomatous focal areas of necrotic bone and neutrophilia in a hematopoietic background consistent with acute osteomyelitis (Figure 2); on hospital day 4, ß-lactamase positive B fragilis grew from the bone culture. Additionally, 1 anaerobic vial from a surveillance blood culture set that was obtained on hospital day 3 grew ß-lactamasepositive B fragilis using the same identification methods. With these results he was thought to have a polymicrobial infection (B fragilis and Prevotella loescheii [P loescheii]) from a suspected bowel source based on his hematochezia and history of anal fissure. No aerobic, Gram-negative enterobacteriaceae were isolated, but he had previously been on cefuroxime, which has potential activity against these organisms, for ≥ 2 weeks prior to hospitalization and cultures. He was discharged on moxifloxacin and metronidazole pending final culture results, including requested anaerobic susceptibility testing.
At 1-week follow-up, both aerobic and anaerobic vials from surveillance blood cultures remained negative for any microbes, so antibiotics were deescalated to moxifloxacin monotherapy. However, after 3 days the patient was readmitted for increasing C-reactive protein (CRP) levels and intractable back pain with worsening bilateral radiculopathy. A repeat MRI demonstrated interval disease progression with near obliteration of the L4-L5 disc space and hyperenhancement of the prevertebral soft tissues and adjacent psoas musculature without focal rim-enhancing fluid collection (Figure 3). After repeat L4 biopsy, metronidazole was restarted and ertapenem added for enterobacteriaceae coverage, given the known B fragilis and potential suppression from previous cephalosporin therapy; moxifloxacin was discontinued. L4 biopsy cultures showed no growth, and CRP levels trended down from 154.2 mg/L (start of first admission) to 42.4 mg/L (start of second admission) to 14.9 mg/L (day of discharge) (reference range, 5-9.9 mg/L). He was discharged on ertapenem and metronidazole. He completed a 6-week course without further complication.
During antibiotic therapy he had an unremarkable colonoscopy, CRP normalized to 2.6 mg/L (reference range, 0-4.9 mg/L), and he underwent successful L4-L5 transforaminal lumbar interbody fusion 2 weeks after finishing antibiotics.
We retroactively sent both P loescheii isolates and the 1 B fragilis isolate that grew from the surveillance blood culture to the Multidrug-resistant Organism Repository and Surveillance Network (MRSN) at the Walter Reed Army Institute of Research for identification confirmation and susceptibility analysis. Whole genome sequencing with single nucleotide polymorphism (SNP)-based analysis revealed all isolates were 100% identical and consistent with B fragilis and not P loescheii, based on clustering around other B fragilis sequences found in the National Center for Biotechnology Information (NCBI) Genbank database (Figure 4). All isolates carried the antibiotic resistance genes— cepA, sul(2), tetQ— encoding for possible resistance to cephalosporins, sulphonamides, and tetracyclines, respectively; as well as a point mutation in the gyrA gene (Ser82Phe). None of the isolates carried the nim gene, and screening for the 3 subtypes of B fragilis enterotoxin gene (bft-1, bft-2, bft-3) was negative. Eventual susceptibility testing at the Mayo Clinic several months after the conclusion of the case indicated that the B fragilis isolate was sensitive to piperacillin-tazobactam, ertapenem, clindamycin, and metronidazole; however, testing was not performed against moxifloxacin.
Discussion
In the era of growing antibiotic resistance patterns, antimicrobial stewardship programs recommend interventions to improve antimicrobial use through targeted narrow- spectrum antibiotics.12 The Clinical and Laboratory Standards Institute (CLSI) maintains guidelines on the major indications for anaerobic antimicrobial susceptibility testing (AST) to help direct narrow-targeted antimicrobial therapy. However, in a 2008 practice survey Goldstein and colleagues reported that less than half of US hospitals performed anaerobic AST, and only 21% of these facilities did it in-house, while the remainder sent out their isolates for testing.11-14 The CLSI major indications for AST include situations in which the selection of agents is important because of the (1) known resistance of a particular species; (2) confirmation of appropriate therapy for severe infections or for those that may require long-term therapy; (3) persistence of infection despite adequate treatment with an appropriate therapeutic regimen; and (4) difficulty in making empirical decisions based on precedent.14 Additionally, isolates from brain abscess, endocarditis, osteomyelitis, joint infection, infection of prosthetic devices or vascular grafts, bacteremia, and normally sterile body sites (unless contamination suspected) should be tested.14
Because of the lack of anaerobic AST, health care providers must base empiric treatment on reported sensitivities from the medical literature. Empiric selection of antimicrobials for anaerobic infections is made even more challenging by the increased rates of resistance reported in the literature, leading to recommendations to increase susceptibility testing to guide therapy.13,15,16 Empiric therapy of deep-seated anaerobic infections may lead to use of inactive agents or overly broad-spectrum antibiotics. Current antimicrobial stewardship initiatives recognize the importance of narrow-spectrum antibiotics to minimize risk of adverse events and selective pressure for antimicrobial resistance.
Although we attempted to confirm the identification of the anaerobic isolates via commercially available methods, it was not until we performed genetic testing that we were able verify the isolates as B fragilis. Furthermore, earlier susceptibility testing would have allowed for more narrow-targeted antimicrobial therapy and could have potentially prevented our patient’s readmission and use of ertapenem, despite its > 98% susceptibility rates against B fragilis.13,17
All of the B fragilis isolates carried the cepA gene, which is a cephalosporinase that encodes for resistance to cephalosporins and aminopenicillins but not to ß-lactam ß-lactamase inhibitor combinations.13 Although not a substitution for susceptibility analysis, genetic testing showed that all of the isolates carried a nonsynonymous mutation from serine to a phenylalanine at amino acid position 82 (S82F) in the gyrA gene. The S82F mutation has been implicated in fluoroquinolone resistance, via inhibition of substrate–target recognition and binding between fluoroquinolones and the target topoisomerase protein,18 and may potentially explain why our patient clinically worsened while on moxifloxacin monotherapy. Although moxifloxacin susceptibility was not performed, susceptibility rates remain highly variable, ranging from 50% to 70% for B fragilis.13,15,16
It is important to note that the metronidazole the patient received during his first hospital admission could have sterilized the vertebral body without completely eradicating the microbe; thus could explain his clinical worsening while on moxifloxacin monotherapy despite no growth from the repeat biopsy culture. Our rationale for initially continuing moxifloxacin was based on its excellent bioavailability and bone penetration properties. Additionally, of the fluoroquinolones it has the most reliable anaerobic activity and is the only one recommended as monotherapy for complicated intraabdominal infections.19 However, guidelines recommend avoiding its use in patients who have received a fluoroquinolone in the past 90 days or at institutions with high rates of resistance. At our institution Escherichia coli has a > 90% susceptibility rate to fluoroquinolones. Given this rate and our concern that the patient had a polymicrobial infection, we felt that moxifloxacin would provide appropriate anaerobic and aerobic coverage, especially since he had no previous fluoroquinolone exposure.
Additionally, none of the isolates carried the nim or bft toxin genes. Although the nim gene is associated with metronidazole resistance,its presence does not invariably result in resistant strains of B fragilis; in fact, metronidazole resistance is relatively uncommon, with the majority of B fragilis showing < 1% resistance, based on CLSI breakpoints (≥ 32 mg/L).13,20,21 However, one recent epidemiologic study on anaerobic wound isolates from Iraq and Afghanistan casualties found that 12% (2/17) of B fragilis isolates were resistant to metronidazole.15 Given the improvement of the patient’s symptoms while on metronidazole, it is likely that the B fragilis was susceptible. Nevertheless, susceptibility testing with minimum inhibitory concentrations is necessary to verify this result. Also, although enterotoxigenic strains of B fragilis have been associated with bloodstream infections, our patient’s isolates lacked the 3 subtypes of B fragilis enterotoxin gene.22
Conclusions
We report a case of B fragilis bacteremia and vertebral osteomyelitis complicated by challenges in anaerobic identification and sensitivities that led to brief use of a possibly inactive antimicrobial and the subsequent use of carbapenem therapy, which may have been avoided if susceptibility testing were more readily available. This case led to changes in our hospital’s processing of anaerobic isolates to include susceptibility testing on request.
Acknowledgments
We thank Keith Thompson, MD (staff pathologist, Naval Medical Center Portsmouth Virginia), for providing the pathology images from the initial vertebral biopsy, and Dr. Kate Hinkle (director, Multidrug-Resistant Organism Repository and Surveillance Network, Silver Spring, Maryland ) for providing the whole genome sequencing results from the B fragilis isolates.
1. Zimmerli W. Vertebral osteomyelitis. N Eng J Med. 2010;362(11):1022-1029.
2. Chazan B, Strahilevitz J, Millgram MA, Kaufmann S, Raz R. Bacteroides fragilis vertebral osteomyelitis secondary to anal dilatation. Spine (Phila PA 1976). 2001;26(16):E377-E378.
3. Kierzkowska M, Pedzisz P, Babiak I, et al. Orthopedic infections caused by obligatory anaerobic Gram-negative rods: report of two cases. Med Microbiol Immunol. 2017;206(5):363-366.
4. McHenry M, Easley K, Locker G. Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin Infect Dis. 2002;34(10):1342-1350.
5. Raff MJ, Melo JC. Anaerobic osteomyelitis. Medicine (Baltimore).1978;57(1):83-103.
6. Lewis R, Sutter V, Finegold S. Bone infections involving anaerobic bacteria. Medicine (Baltimore). 1978;57(1):279-305.
7. Brook I. The role of anaerobic bacteria in bacteremia. Anaerobe. 2010;16(3):183-189.
8. Lassmann B, Gustafson DR, Wood CM, Rosenblatt JE. Reemergence of anaerobic bacteremia. Clin Infect Dis. 2007;44(7):895-900.
9. Lazarovitch T, Freimann S, Shapira G, Blank H. Decrease in anaerobe-related bacteraemias and increase in Bacteroides species isolation rate from 1998 to 2007: a retrospective study. Anaerobe. 2010;16(3):201-205.
10. Keukeleire S, Wybo I, Naessens A, et al. Anaerobic bacteraemia: a 10-year retrospective epidemiological survey. Anaerobe. 2016;39:54-59.
11. Goldstein EJC, Citron DM, Goldman PJ, Goldman RJ. National hospital survey of anaerobic culture and susceptibility methods: III. Anaerobe. 2008;14(2):68-72.
12. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51-e77.
13. Schuetz AN. Antimicrobial resistance and susceptibility testing of anaerobic bacteria. Antimicr Resist. 2014;59(5):698-705.
14. Clinical and Laboratory Standards Institute. M11-A8: Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria; Approved Standard. 8th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2012.
15. White B, Mende K, Weintrob A, et al; Infectious Disease Clinical Research Program Trauma Infectious Disease Outcome Study Group. Epidemiology and antimicrobial susceptibilities of wound isolates of obligate anaerobes from combat casualties. Diagn Mircrobiol Infect Dis. 2016;84(2):144-150.
16. Hastey CJ, Boyd H, Schuetz AN, et al; Ad Hoc Working Group on Antimicrobial Susceptibility Testing of Anaerobic Bacteria of CLSI. Changes in the antibiotic susceptibility of anaerobic bacteria from 2007-2009 to 2010-2012 based on the CLSI methodology. Anaerobe. 2016;42:27-30.
17. Brook I, Wexler HM, Goldstein EJC. Antianaerobic antimicrobials: spectrum and susceptibility testing. Clin Microbiol Rev. 2013;26(3):526-546.
18. Pumbwe L, Wareham D, Aduse-Opoku J, Brazier JS, Wexler HM. Genetic analysis of mechanisms of multidrug resistance in a clinical isolate of Bacteroides fragilis. Clin Microbiol Infect. 2007;13(2):183-189.
19. Solomkin J, Mazuski J, Bradley J, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133-164.
20. Breuil J, Dublanchet A, Truffaut N, Sebald M. Transferable 5-nitroimidazole resistance Bacteroides fragilis group. Plasmid. 1989;21(2):151-154.
21. Nagy E, Urbán E, Nord CE; ESCMID Study Group on Antimicrobial Resistance in Anaerobic Bacteria. Antimicrobial susceptibility of Bacteroides fragilis group isolates in Europe: 20 years of experience. Clin Microbiol Infect. 2011;17(3):371-379.
22. Avila-Campos M, Liu C, Song Y, Rowlinson M-C, Finegold SM. Determination of bft gene subtypes in Bacteroides fragilis clinical isolates. J Clin Microbiol. 2007;45(4):1336-1338.
Acute pyogenic vertebral osteomyelitis is often due to hematogenous spread of aerobic bacteria.1-4 Conversely, only 0.5% of anaerobic bacteremias lead to osteomyelitis.5 Anaerobic osteomyelitis typically results from the contiguous spread of polymicrobial infections through breaks in the gut mucosal barrier and involves the vertebral bodies in only 2% to 5% of cases.5,6 Although Bacteroides fragilis (B fragilis) is the most common anaerobic pathogen cultivated from blood, accounting for about half of all anaerobic blood isolates, it seldom leads to osteomyelitis.1,2,7-11 We report an uncommon case of B fragilis bacteremia and vertebral osteomyelitis confounded by uncertainties in anaerobic identification and susceptibilities.
Case Presentation
A healthy-appearing male aged 55 years presented to the Naval Medical Center Portsmouth (NMCP) with subacute low back pain and fevers of 103 °F for > 3 weeks. While traveling 4 weeks prior, he completed a course of oseltamivir for influenza B infection; afterward, he was diagnosed with community-acquired pneumonia and treated with a dose of ceftriaxone and a 7-day course of doxycycline. The patient presented to the same facility a week later for low back pain and nonresolving respiratory symptoms, and his therapy was changed to azithromycin, cefuroxime, prednisone, and inhalers. Additionally, after being treated for influenza, he developed constipation and hematochezia for which he did not seek care. The hematochezia was similar to a previous episode from an anal fissure 1 year prior that resolved with stool softeners. When he was finally seen at NMCP after 3 weeks of worsening back pain and fevers, lumbosacral magnetic resonance imaging (MRI) demonstrated vertebral osteomyelitis and discitis at L4-L5 and admitted to the hospital (Figure 1).
After a fluoroscopy-guided biopsy of the L4 vertebral body on hospital day 1, the patient was started on cefepime and vancomycin. The biopsy sample was inoculated onto solid media (blood agar, chocolate agar, and MacConkey agar) and incubated at 36 °C for 24 hours in a 5% CO2 atmosphere, as well as onto Shaedler agar with vitamin K and chopped meat glucose broth and incubated at 36 °C for 48 hours under anaerobic conditions. Metronidazole was added and vancomycin discontinued after 2 anaerobic blood culture vials obtained on hospital day 1, incubated in a Becton Dickinson BACTEC FX automated system, which demonstrated Gram-negative bacilli after 48 hours. The blood culture isolates demonstrated a > 99% probability of being identified as ß-lactamase positive Prevotella loescheii using Thermo Fischer Scientific RapID ANA II biochemical testing. Nitrocefinase discs were used to detect ß-lactamase activity.
The biopsy demonstrated nongranulomatous focal areas of necrotic bone and neutrophilia in a hematopoietic background consistent with acute osteomyelitis (Figure 2); on hospital day 4, ß-lactamase positive B fragilis grew from the bone culture. Additionally, 1 anaerobic vial from a surveillance blood culture set that was obtained on hospital day 3 grew ß-lactamasepositive B fragilis using the same identification methods. With these results he was thought to have a polymicrobial infection (B fragilis and Prevotella loescheii [P loescheii]) from a suspected bowel source based on his hematochezia and history of anal fissure. No aerobic, Gram-negative enterobacteriaceae were isolated, but he had previously been on cefuroxime, which has potential activity against these organisms, for ≥ 2 weeks prior to hospitalization and cultures. He was discharged on moxifloxacin and metronidazole pending final culture results, including requested anaerobic susceptibility testing.
At 1-week follow-up, both aerobic and anaerobic vials from surveillance blood cultures remained negative for any microbes, so antibiotics were deescalated to moxifloxacin monotherapy. However, after 3 days the patient was readmitted for increasing C-reactive protein (CRP) levels and intractable back pain with worsening bilateral radiculopathy. A repeat MRI demonstrated interval disease progression with near obliteration of the L4-L5 disc space and hyperenhancement of the prevertebral soft tissues and adjacent psoas musculature without focal rim-enhancing fluid collection (Figure 3). After repeat L4 biopsy, metronidazole was restarted and ertapenem added for enterobacteriaceae coverage, given the known B fragilis and potential suppression from previous cephalosporin therapy; moxifloxacin was discontinued. L4 biopsy cultures showed no growth, and CRP levels trended down from 154.2 mg/L (start of first admission) to 42.4 mg/L (start of second admission) to 14.9 mg/L (day of discharge) (reference range, 5-9.9 mg/L). He was discharged on ertapenem and metronidazole. He completed a 6-week course without further complication.
During antibiotic therapy he had an unremarkable colonoscopy, CRP normalized to 2.6 mg/L (reference range, 0-4.9 mg/L), and he underwent successful L4-L5 transforaminal lumbar interbody fusion 2 weeks after finishing antibiotics.
We retroactively sent both P loescheii isolates and the 1 B fragilis isolate that grew from the surveillance blood culture to the Multidrug-resistant Organism Repository and Surveillance Network (MRSN) at the Walter Reed Army Institute of Research for identification confirmation and susceptibility analysis. Whole genome sequencing with single nucleotide polymorphism (SNP)-based analysis revealed all isolates were 100% identical and consistent with B fragilis and not P loescheii, based on clustering around other B fragilis sequences found in the National Center for Biotechnology Information (NCBI) Genbank database (Figure 4). All isolates carried the antibiotic resistance genes— cepA, sul(2), tetQ— encoding for possible resistance to cephalosporins, sulphonamides, and tetracyclines, respectively; as well as a point mutation in the gyrA gene (Ser82Phe). None of the isolates carried the nim gene, and screening for the 3 subtypes of B fragilis enterotoxin gene (bft-1, bft-2, bft-3) was negative. Eventual susceptibility testing at the Mayo Clinic several months after the conclusion of the case indicated that the B fragilis isolate was sensitive to piperacillin-tazobactam, ertapenem, clindamycin, and metronidazole; however, testing was not performed against moxifloxacin.
Discussion
In the era of growing antibiotic resistance patterns, antimicrobial stewardship programs recommend interventions to improve antimicrobial use through targeted narrow- spectrum antibiotics.12 The Clinical and Laboratory Standards Institute (CLSI) maintains guidelines on the major indications for anaerobic antimicrobial susceptibility testing (AST) to help direct narrow-targeted antimicrobial therapy. However, in a 2008 practice survey Goldstein and colleagues reported that less than half of US hospitals performed anaerobic AST, and only 21% of these facilities did it in-house, while the remainder sent out their isolates for testing.11-14 The CLSI major indications for AST include situations in which the selection of agents is important because of the (1) known resistance of a particular species; (2) confirmation of appropriate therapy for severe infections or for those that may require long-term therapy; (3) persistence of infection despite adequate treatment with an appropriate therapeutic regimen; and (4) difficulty in making empirical decisions based on precedent.14 Additionally, isolates from brain abscess, endocarditis, osteomyelitis, joint infection, infection of prosthetic devices or vascular grafts, bacteremia, and normally sterile body sites (unless contamination suspected) should be tested.14
Because of the lack of anaerobic AST, health care providers must base empiric treatment on reported sensitivities from the medical literature. Empiric selection of antimicrobials for anaerobic infections is made even more challenging by the increased rates of resistance reported in the literature, leading to recommendations to increase susceptibility testing to guide therapy.13,15,16 Empiric therapy of deep-seated anaerobic infections may lead to use of inactive agents or overly broad-spectrum antibiotics. Current antimicrobial stewardship initiatives recognize the importance of narrow-spectrum antibiotics to minimize risk of adverse events and selective pressure for antimicrobial resistance.
Although we attempted to confirm the identification of the anaerobic isolates via commercially available methods, it was not until we performed genetic testing that we were able verify the isolates as B fragilis. Furthermore, earlier susceptibility testing would have allowed for more narrow-targeted antimicrobial therapy and could have potentially prevented our patient’s readmission and use of ertapenem, despite its > 98% susceptibility rates against B fragilis.13,17
All of the B fragilis isolates carried the cepA gene, which is a cephalosporinase that encodes for resistance to cephalosporins and aminopenicillins but not to ß-lactam ß-lactamase inhibitor combinations.13 Although not a substitution for susceptibility analysis, genetic testing showed that all of the isolates carried a nonsynonymous mutation from serine to a phenylalanine at amino acid position 82 (S82F) in the gyrA gene. The S82F mutation has been implicated in fluoroquinolone resistance, via inhibition of substrate–target recognition and binding between fluoroquinolones and the target topoisomerase protein,18 and may potentially explain why our patient clinically worsened while on moxifloxacin monotherapy. Although moxifloxacin susceptibility was not performed, susceptibility rates remain highly variable, ranging from 50% to 70% for B fragilis.13,15,16
It is important to note that the metronidazole the patient received during his first hospital admission could have sterilized the vertebral body without completely eradicating the microbe; thus could explain his clinical worsening while on moxifloxacin monotherapy despite no growth from the repeat biopsy culture. Our rationale for initially continuing moxifloxacin was based on its excellent bioavailability and bone penetration properties. Additionally, of the fluoroquinolones it has the most reliable anaerobic activity and is the only one recommended as monotherapy for complicated intraabdominal infections.19 However, guidelines recommend avoiding its use in patients who have received a fluoroquinolone in the past 90 days or at institutions with high rates of resistance. At our institution Escherichia coli has a > 90% susceptibility rate to fluoroquinolones. Given this rate and our concern that the patient had a polymicrobial infection, we felt that moxifloxacin would provide appropriate anaerobic and aerobic coverage, especially since he had no previous fluoroquinolone exposure.
Additionally, none of the isolates carried the nim or bft toxin genes. Although the nim gene is associated with metronidazole resistance,its presence does not invariably result in resistant strains of B fragilis; in fact, metronidazole resistance is relatively uncommon, with the majority of B fragilis showing < 1% resistance, based on CLSI breakpoints (≥ 32 mg/L).13,20,21 However, one recent epidemiologic study on anaerobic wound isolates from Iraq and Afghanistan casualties found that 12% (2/17) of B fragilis isolates were resistant to metronidazole.15 Given the improvement of the patient’s symptoms while on metronidazole, it is likely that the B fragilis was susceptible. Nevertheless, susceptibility testing with minimum inhibitory concentrations is necessary to verify this result. Also, although enterotoxigenic strains of B fragilis have been associated with bloodstream infections, our patient’s isolates lacked the 3 subtypes of B fragilis enterotoxin gene.22
Conclusions
We report a case of B fragilis bacteremia and vertebral osteomyelitis complicated by challenges in anaerobic identification and sensitivities that led to brief use of a possibly inactive antimicrobial and the subsequent use of carbapenem therapy, which may have been avoided if susceptibility testing were more readily available. This case led to changes in our hospital’s processing of anaerobic isolates to include susceptibility testing on request.
Acknowledgments
We thank Keith Thompson, MD (staff pathologist, Naval Medical Center Portsmouth Virginia), for providing the pathology images from the initial vertebral biopsy, and Dr. Kate Hinkle (director, Multidrug-Resistant Organism Repository and Surveillance Network, Silver Spring, Maryland ) for providing the whole genome sequencing results from the B fragilis isolates.
Acute pyogenic vertebral osteomyelitis is often due to hematogenous spread of aerobic bacteria.1-4 Conversely, only 0.5% of anaerobic bacteremias lead to osteomyelitis.5 Anaerobic osteomyelitis typically results from the contiguous spread of polymicrobial infections through breaks in the gut mucosal barrier and involves the vertebral bodies in only 2% to 5% of cases.5,6 Although Bacteroides fragilis (B fragilis) is the most common anaerobic pathogen cultivated from blood, accounting for about half of all anaerobic blood isolates, it seldom leads to osteomyelitis.1,2,7-11 We report an uncommon case of B fragilis bacteremia and vertebral osteomyelitis confounded by uncertainties in anaerobic identification and susceptibilities.
Case Presentation
A healthy-appearing male aged 55 years presented to the Naval Medical Center Portsmouth (NMCP) with subacute low back pain and fevers of 103 °F for > 3 weeks. While traveling 4 weeks prior, he completed a course of oseltamivir for influenza B infection; afterward, he was diagnosed with community-acquired pneumonia and treated with a dose of ceftriaxone and a 7-day course of doxycycline. The patient presented to the same facility a week later for low back pain and nonresolving respiratory symptoms, and his therapy was changed to azithromycin, cefuroxime, prednisone, and inhalers. Additionally, after being treated for influenza, he developed constipation and hematochezia for which he did not seek care. The hematochezia was similar to a previous episode from an anal fissure 1 year prior that resolved with stool softeners. When he was finally seen at NMCP after 3 weeks of worsening back pain and fevers, lumbosacral magnetic resonance imaging (MRI) demonstrated vertebral osteomyelitis and discitis at L4-L5 and admitted to the hospital (Figure 1).
After a fluoroscopy-guided biopsy of the L4 vertebral body on hospital day 1, the patient was started on cefepime and vancomycin. The biopsy sample was inoculated onto solid media (blood agar, chocolate agar, and MacConkey agar) and incubated at 36 °C for 24 hours in a 5% CO2 atmosphere, as well as onto Shaedler agar with vitamin K and chopped meat glucose broth and incubated at 36 °C for 48 hours under anaerobic conditions. Metronidazole was added and vancomycin discontinued after 2 anaerobic blood culture vials obtained on hospital day 1, incubated in a Becton Dickinson BACTEC FX automated system, which demonstrated Gram-negative bacilli after 48 hours. The blood culture isolates demonstrated a > 99% probability of being identified as ß-lactamase positive Prevotella loescheii using Thermo Fischer Scientific RapID ANA II biochemical testing. Nitrocefinase discs were used to detect ß-lactamase activity.
The biopsy demonstrated nongranulomatous focal areas of necrotic bone and neutrophilia in a hematopoietic background consistent with acute osteomyelitis (Figure 2); on hospital day 4, ß-lactamase positive B fragilis grew from the bone culture. Additionally, 1 anaerobic vial from a surveillance blood culture set that was obtained on hospital day 3 grew ß-lactamasepositive B fragilis using the same identification methods. With these results he was thought to have a polymicrobial infection (B fragilis and Prevotella loescheii [P loescheii]) from a suspected bowel source based on his hematochezia and history of anal fissure. No aerobic, Gram-negative enterobacteriaceae were isolated, but he had previously been on cefuroxime, which has potential activity against these organisms, for ≥ 2 weeks prior to hospitalization and cultures. He was discharged on moxifloxacin and metronidazole pending final culture results, including requested anaerobic susceptibility testing.
At 1-week follow-up, both aerobic and anaerobic vials from surveillance blood cultures remained negative for any microbes, so antibiotics were deescalated to moxifloxacin monotherapy. However, after 3 days the patient was readmitted for increasing C-reactive protein (CRP) levels and intractable back pain with worsening bilateral radiculopathy. A repeat MRI demonstrated interval disease progression with near obliteration of the L4-L5 disc space and hyperenhancement of the prevertebral soft tissues and adjacent psoas musculature without focal rim-enhancing fluid collection (Figure 3). After repeat L4 biopsy, metronidazole was restarted and ertapenem added for enterobacteriaceae coverage, given the known B fragilis and potential suppression from previous cephalosporin therapy; moxifloxacin was discontinued. L4 biopsy cultures showed no growth, and CRP levels trended down from 154.2 mg/L (start of first admission) to 42.4 mg/L (start of second admission) to 14.9 mg/L (day of discharge) (reference range, 5-9.9 mg/L). He was discharged on ertapenem and metronidazole. He completed a 6-week course without further complication.
During antibiotic therapy he had an unremarkable colonoscopy, CRP normalized to 2.6 mg/L (reference range, 0-4.9 mg/L), and he underwent successful L4-L5 transforaminal lumbar interbody fusion 2 weeks after finishing antibiotics.
We retroactively sent both P loescheii isolates and the 1 B fragilis isolate that grew from the surveillance blood culture to the Multidrug-resistant Organism Repository and Surveillance Network (MRSN) at the Walter Reed Army Institute of Research for identification confirmation and susceptibility analysis. Whole genome sequencing with single nucleotide polymorphism (SNP)-based analysis revealed all isolates were 100% identical and consistent with B fragilis and not P loescheii, based on clustering around other B fragilis sequences found in the National Center for Biotechnology Information (NCBI) Genbank database (Figure 4). All isolates carried the antibiotic resistance genes— cepA, sul(2), tetQ— encoding for possible resistance to cephalosporins, sulphonamides, and tetracyclines, respectively; as well as a point mutation in the gyrA gene (Ser82Phe). None of the isolates carried the nim gene, and screening for the 3 subtypes of B fragilis enterotoxin gene (bft-1, bft-2, bft-3) was negative. Eventual susceptibility testing at the Mayo Clinic several months after the conclusion of the case indicated that the B fragilis isolate was sensitive to piperacillin-tazobactam, ertapenem, clindamycin, and metronidazole; however, testing was not performed against moxifloxacin.
Discussion
In the era of growing antibiotic resistance patterns, antimicrobial stewardship programs recommend interventions to improve antimicrobial use through targeted narrow- spectrum antibiotics.12 The Clinical and Laboratory Standards Institute (CLSI) maintains guidelines on the major indications for anaerobic antimicrobial susceptibility testing (AST) to help direct narrow-targeted antimicrobial therapy. However, in a 2008 practice survey Goldstein and colleagues reported that less than half of US hospitals performed anaerobic AST, and only 21% of these facilities did it in-house, while the remainder sent out their isolates for testing.11-14 The CLSI major indications for AST include situations in which the selection of agents is important because of the (1) known resistance of a particular species; (2) confirmation of appropriate therapy for severe infections or for those that may require long-term therapy; (3) persistence of infection despite adequate treatment with an appropriate therapeutic regimen; and (4) difficulty in making empirical decisions based on precedent.14 Additionally, isolates from brain abscess, endocarditis, osteomyelitis, joint infection, infection of prosthetic devices or vascular grafts, bacteremia, and normally sterile body sites (unless contamination suspected) should be tested.14
Because of the lack of anaerobic AST, health care providers must base empiric treatment on reported sensitivities from the medical literature. Empiric selection of antimicrobials for anaerobic infections is made even more challenging by the increased rates of resistance reported in the literature, leading to recommendations to increase susceptibility testing to guide therapy.13,15,16 Empiric therapy of deep-seated anaerobic infections may lead to use of inactive agents or overly broad-spectrum antibiotics. Current antimicrobial stewardship initiatives recognize the importance of narrow-spectrum antibiotics to minimize risk of adverse events and selective pressure for antimicrobial resistance.
Although we attempted to confirm the identification of the anaerobic isolates via commercially available methods, it was not until we performed genetic testing that we were able verify the isolates as B fragilis. Furthermore, earlier susceptibility testing would have allowed for more narrow-targeted antimicrobial therapy and could have potentially prevented our patient’s readmission and use of ertapenem, despite its > 98% susceptibility rates against B fragilis.13,17
All of the B fragilis isolates carried the cepA gene, which is a cephalosporinase that encodes for resistance to cephalosporins and aminopenicillins but not to ß-lactam ß-lactamase inhibitor combinations.13 Although not a substitution for susceptibility analysis, genetic testing showed that all of the isolates carried a nonsynonymous mutation from serine to a phenylalanine at amino acid position 82 (S82F) in the gyrA gene. The S82F mutation has been implicated in fluoroquinolone resistance, via inhibition of substrate–target recognition and binding between fluoroquinolones and the target topoisomerase protein,18 and may potentially explain why our patient clinically worsened while on moxifloxacin monotherapy. Although moxifloxacin susceptibility was not performed, susceptibility rates remain highly variable, ranging from 50% to 70% for B fragilis.13,15,16
It is important to note that the metronidazole the patient received during his first hospital admission could have sterilized the vertebral body without completely eradicating the microbe; thus could explain his clinical worsening while on moxifloxacin monotherapy despite no growth from the repeat biopsy culture. Our rationale for initially continuing moxifloxacin was based on its excellent bioavailability and bone penetration properties. Additionally, of the fluoroquinolones it has the most reliable anaerobic activity and is the only one recommended as monotherapy for complicated intraabdominal infections.19 However, guidelines recommend avoiding its use in patients who have received a fluoroquinolone in the past 90 days or at institutions with high rates of resistance. At our institution Escherichia coli has a > 90% susceptibility rate to fluoroquinolones. Given this rate and our concern that the patient had a polymicrobial infection, we felt that moxifloxacin would provide appropriate anaerobic and aerobic coverage, especially since he had no previous fluoroquinolone exposure.
Additionally, none of the isolates carried the nim or bft toxin genes. Although the nim gene is associated with metronidazole resistance,its presence does not invariably result in resistant strains of B fragilis; in fact, metronidazole resistance is relatively uncommon, with the majority of B fragilis showing < 1% resistance, based on CLSI breakpoints (≥ 32 mg/L).13,20,21 However, one recent epidemiologic study on anaerobic wound isolates from Iraq and Afghanistan casualties found that 12% (2/17) of B fragilis isolates were resistant to metronidazole.15 Given the improvement of the patient’s symptoms while on metronidazole, it is likely that the B fragilis was susceptible. Nevertheless, susceptibility testing with minimum inhibitory concentrations is necessary to verify this result. Also, although enterotoxigenic strains of B fragilis have been associated with bloodstream infections, our patient’s isolates lacked the 3 subtypes of B fragilis enterotoxin gene.22
Conclusions
We report a case of B fragilis bacteremia and vertebral osteomyelitis complicated by challenges in anaerobic identification and sensitivities that led to brief use of a possibly inactive antimicrobial and the subsequent use of carbapenem therapy, which may have been avoided if susceptibility testing were more readily available. This case led to changes in our hospital’s processing of anaerobic isolates to include susceptibility testing on request.
Acknowledgments
We thank Keith Thompson, MD (staff pathologist, Naval Medical Center Portsmouth Virginia), for providing the pathology images from the initial vertebral biopsy, and Dr. Kate Hinkle (director, Multidrug-Resistant Organism Repository and Surveillance Network, Silver Spring, Maryland ) for providing the whole genome sequencing results from the B fragilis isolates.
1. Zimmerli W. Vertebral osteomyelitis. N Eng J Med. 2010;362(11):1022-1029.
2. Chazan B, Strahilevitz J, Millgram MA, Kaufmann S, Raz R. Bacteroides fragilis vertebral osteomyelitis secondary to anal dilatation. Spine (Phila PA 1976). 2001;26(16):E377-E378.
3. Kierzkowska M, Pedzisz P, Babiak I, et al. Orthopedic infections caused by obligatory anaerobic Gram-negative rods: report of two cases. Med Microbiol Immunol. 2017;206(5):363-366.
4. McHenry M, Easley K, Locker G. Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin Infect Dis. 2002;34(10):1342-1350.
5. Raff MJ, Melo JC. Anaerobic osteomyelitis. Medicine (Baltimore).1978;57(1):83-103.
6. Lewis R, Sutter V, Finegold S. Bone infections involving anaerobic bacteria. Medicine (Baltimore). 1978;57(1):279-305.
7. Brook I. The role of anaerobic bacteria in bacteremia. Anaerobe. 2010;16(3):183-189.
8. Lassmann B, Gustafson DR, Wood CM, Rosenblatt JE. Reemergence of anaerobic bacteremia. Clin Infect Dis. 2007;44(7):895-900.
9. Lazarovitch T, Freimann S, Shapira G, Blank H. Decrease in anaerobe-related bacteraemias and increase in Bacteroides species isolation rate from 1998 to 2007: a retrospective study. Anaerobe. 2010;16(3):201-205.
10. Keukeleire S, Wybo I, Naessens A, et al. Anaerobic bacteraemia: a 10-year retrospective epidemiological survey. Anaerobe. 2016;39:54-59.
11. Goldstein EJC, Citron DM, Goldman PJ, Goldman RJ. National hospital survey of anaerobic culture and susceptibility methods: III. Anaerobe. 2008;14(2):68-72.
12. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51-e77.
13. Schuetz AN. Antimicrobial resistance and susceptibility testing of anaerobic bacteria. Antimicr Resist. 2014;59(5):698-705.
14. Clinical and Laboratory Standards Institute. M11-A8: Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria; Approved Standard. 8th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2012.
15. White B, Mende K, Weintrob A, et al; Infectious Disease Clinical Research Program Trauma Infectious Disease Outcome Study Group. Epidemiology and antimicrobial susceptibilities of wound isolates of obligate anaerobes from combat casualties. Diagn Mircrobiol Infect Dis. 2016;84(2):144-150.
16. Hastey CJ, Boyd H, Schuetz AN, et al; Ad Hoc Working Group on Antimicrobial Susceptibility Testing of Anaerobic Bacteria of CLSI. Changes in the antibiotic susceptibility of anaerobic bacteria from 2007-2009 to 2010-2012 based on the CLSI methodology. Anaerobe. 2016;42:27-30.
17. Brook I, Wexler HM, Goldstein EJC. Antianaerobic antimicrobials: spectrum and susceptibility testing. Clin Microbiol Rev. 2013;26(3):526-546.
18. Pumbwe L, Wareham D, Aduse-Opoku J, Brazier JS, Wexler HM. Genetic analysis of mechanisms of multidrug resistance in a clinical isolate of Bacteroides fragilis. Clin Microbiol Infect. 2007;13(2):183-189.
19. Solomkin J, Mazuski J, Bradley J, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133-164.
20. Breuil J, Dublanchet A, Truffaut N, Sebald M. Transferable 5-nitroimidazole resistance Bacteroides fragilis group. Plasmid. 1989;21(2):151-154.
21. Nagy E, Urbán E, Nord CE; ESCMID Study Group on Antimicrobial Resistance in Anaerobic Bacteria. Antimicrobial susceptibility of Bacteroides fragilis group isolates in Europe: 20 years of experience. Clin Microbiol Infect. 2011;17(3):371-379.
22. Avila-Campos M, Liu C, Song Y, Rowlinson M-C, Finegold SM. Determination of bft gene subtypes in Bacteroides fragilis clinical isolates. J Clin Microbiol. 2007;45(4):1336-1338.
1. Zimmerli W. Vertebral osteomyelitis. N Eng J Med. 2010;362(11):1022-1029.
2. Chazan B, Strahilevitz J, Millgram MA, Kaufmann S, Raz R. Bacteroides fragilis vertebral osteomyelitis secondary to anal dilatation. Spine (Phila PA 1976). 2001;26(16):E377-E378.
3. Kierzkowska M, Pedzisz P, Babiak I, et al. Orthopedic infections caused by obligatory anaerobic Gram-negative rods: report of two cases. Med Microbiol Immunol. 2017;206(5):363-366.
4. McHenry M, Easley K, Locker G. Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin Infect Dis. 2002;34(10):1342-1350.
5. Raff MJ, Melo JC. Anaerobic osteomyelitis. Medicine (Baltimore).1978;57(1):83-103.
6. Lewis R, Sutter V, Finegold S. Bone infections involving anaerobic bacteria. Medicine (Baltimore). 1978;57(1):279-305.
7. Brook I. The role of anaerobic bacteria in bacteremia. Anaerobe. 2010;16(3):183-189.
8. Lassmann B, Gustafson DR, Wood CM, Rosenblatt JE. Reemergence of anaerobic bacteremia. Clin Infect Dis. 2007;44(7):895-900.
9. Lazarovitch T, Freimann S, Shapira G, Blank H. Decrease in anaerobe-related bacteraemias and increase in Bacteroides species isolation rate from 1998 to 2007: a retrospective study. Anaerobe. 2010;16(3):201-205.
10. Keukeleire S, Wybo I, Naessens A, et al. Anaerobic bacteraemia: a 10-year retrospective epidemiological survey. Anaerobe. 2016;39:54-59.
11. Goldstein EJC, Citron DM, Goldman PJ, Goldman RJ. National hospital survey of anaerobic culture and susceptibility methods: III. Anaerobe. 2008;14(2):68-72.
12. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51-e77.
13. Schuetz AN. Antimicrobial resistance and susceptibility testing of anaerobic bacteria. Antimicr Resist. 2014;59(5):698-705.
14. Clinical and Laboratory Standards Institute. M11-A8: Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria; Approved Standard. 8th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2012.
15. White B, Mende K, Weintrob A, et al; Infectious Disease Clinical Research Program Trauma Infectious Disease Outcome Study Group. Epidemiology and antimicrobial susceptibilities of wound isolates of obligate anaerobes from combat casualties. Diagn Mircrobiol Infect Dis. 2016;84(2):144-150.
16. Hastey CJ, Boyd H, Schuetz AN, et al; Ad Hoc Working Group on Antimicrobial Susceptibility Testing of Anaerobic Bacteria of CLSI. Changes in the antibiotic susceptibility of anaerobic bacteria from 2007-2009 to 2010-2012 based on the CLSI methodology. Anaerobe. 2016;42:27-30.
17. Brook I, Wexler HM, Goldstein EJC. Antianaerobic antimicrobials: spectrum and susceptibility testing. Clin Microbiol Rev. 2013;26(3):526-546.
18. Pumbwe L, Wareham D, Aduse-Opoku J, Brazier JS, Wexler HM. Genetic analysis of mechanisms of multidrug resistance in a clinical isolate of Bacteroides fragilis. Clin Microbiol Infect. 2007;13(2):183-189.
19. Solomkin J, Mazuski J, Bradley J, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133-164.
20. Breuil J, Dublanchet A, Truffaut N, Sebald M. Transferable 5-nitroimidazole resistance Bacteroides fragilis group. Plasmid. 1989;21(2):151-154.
21. Nagy E, Urbán E, Nord CE; ESCMID Study Group on Antimicrobial Resistance in Anaerobic Bacteria. Antimicrobial susceptibility of Bacteroides fragilis group isolates in Europe: 20 years of experience. Clin Microbiol Infect. 2011;17(3):371-379.
22. Avila-Campos M, Liu C, Song Y, Rowlinson M-C, Finegold SM. Determination of bft gene subtypes in Bacteroides fragilis clinical isolates. J Clin Microbiol. 2007;45(4):1336-1338.