User login
Vitamin D supplements during pregnancy may protect infants from atopic eczema
according to results of a clinical trial.
“Our data provide the first randomized controlled trial evidence of a protective effect of antenatal cholecalciferol supplementation on risk of infantile atopic eczema, with the effect only seen in infants that were breastfed for more than 1 month,” lead study author Sarah El-Heis, MRCP, DM, and colleagues wrote.
“The findings support a developmental influence on infantile atopic eczema and point to gestational cholecalciferol supplementation as a preventive strategy to reduce the burden of atopic eczema during infancy,” Dr. El-Heis, an academic clinical lecturer in dermatology at the Medical Research Council Lifecourse Epidemiology Center of the University of Southampton (England), said in a presentation at the annual meeting of the Society for Investigative Dermatology.
The study also was published in the British Journal of Dermatology.
Dr. El-Heis and colleagues analyzed data from one of the three U.K. study sites involved in the double-blind Maternal Vitamin D Osteoporosis Study (MAVIDOS), which enrolled participants between 2008 and 2014.
The women enrolled at the University of Southampton site were of age 18 or older, and had a singleton pregnancy. Serum 25-hydroxy vitamin D (25[OH]D) levels were 25-100 nmol/L, and calcium levels were less than 2.75 mmol/L.
Those who had metabolic bone disease, kidney stones, hyperparathyroidism, or hypercalciuria or who were taking more than 400 IU/day of vitamin D supplements or medication known to interfere with fetal growth or whose fetus had a major anomaly were excluded.
The study included 1,134 women. Half of the participants were randomly assigned to receive cholecalciferol 1,000 IU/day from around 14 weeks’ gestation until delivery, and half were assigned to receive placebo. Their babies were assessed for atopic eczema at 12, 24, and 48 months of age.
The maternal and infant characteristics were similar in both groups, but the treatment group tended to breastfeed longer.
Infants appear to be protected up to 1 year of age
Using logistic regression, the researchers analyzed links between maternal cholecalciferol 1,000 IU/day supplements or placebo and atopic eczema risk in their offspring.
After adjustments for breastfeeding duration, among the 636 infants assessed at 12 months, those whose mothers received cholecalciferol had lower odds ratios of atopic eczema than those whose mothers received placebo (OR, 0.55; 95% confidence interval, 0.32-0.97).
The risk of atopic eczema at 12 months was reduced only for children in the treatment group who were breastfed longer than 1 month (OR, 0.48; 95% CI, 0.24-0.94), further analysis showed. Those who were breastfed for less than 1 month showed no reduced risk.
The combined effect of vitamin D and breastfeeding for longer than 1 month weakened after 1 year and was not statistically significant among the 611 children assessed at 24 months and the 450 children assessed at 48 months. The ORs of atopic eczema in the treatment group and in the control group increased to 0.76 (95% CI, 0.47-1.23) and 0.75 (95% CI, 0.37-1.52), respectively.
At baseline, the mean maternal serum 25(OH)D levels in the treatment group (46.0 nmol/L) and in the control group (44.7 nmol/L) were similar. But by late pregnancy, maternal serum 25(OH)D levels in the treatment group were higher (67.4 nmol/L) than in the control group (42.4 nmol/L).
The authors note that strengths of the study include its design, the uniformity of criteria used to diagnose atopic eczema, and the similarity of both pregnant groups in their intake of vitamin D during the study.
Limitations included the lack of ultraviolet B light exposure data, the lack of non-White women in the study, the lack of measurement of cord blood and offspring 25(OH)D levels, and the exclusion of women with baseline 25(OH)D concentrations less than 25 nmol/L.
“This is an interesting study that brings up the possibility that maternal factors during pregnancy may impact atopic dermatitis,” Kalyani S. Marathe, MD, MPH, the director of the division of dermatology at Cincinnati Children’s Hospital Medical Center, told this news organization.
The results are mixed, though, she noted.
“While some impact on the risk of eczema is seen at 1 year of age, that protective effect is gone by 2 years and 4 years,” Dr. Marathe, who was not involved in the study, said in an email. “So if maternal supplementation does improve eczema, the effect is not long-lasting.
“The other complicating factor is that the babies who showed reduction in eczema were also the ones who were breastfed longer than 1 month,” she added. “We know that breastfeeding is associated with several factors, including socioeconomic status, so it is difficult to tease out the relationships here.
“Vitamin D has become a very hot topic lately and seems to have protective effects in many areas of health care,” Dr. Marathe said. “These results may motivate pregnant women to be compliant with their prenatal vitamins that contain the amount of vitamin D studied here.”
The study received grant support. Several authors disclosed financial relationships with pharmaceutical and nutritional products industries. Dr. El-Heis and Dr. Marathe reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to results of a clinical trial.
“Our data provide the first randomized controlled trial evidence of a protective effect of antenatal cholecalciferol supplementation on risk of infantile atopic eczema, with the effect only seen in infants that were breastfed for more than 1 month,” lead study author Sarah El-Heis, MRCP, DM, and colleagues wrote.
“The findings support a developmental influence on infantile atopic eczema and point to gestational cholecalciferol supplementation as a preventive strategy to reduce the burden of atopic eczema during infancy,” Dr. El-Heis, an academic clinical lecturer in dermatology at the Medical Research Council Lifecourse Epidemiology Center of the University of Southampton (England), said in a presentation at the annual meeting of the Society for Investigative Dermatology.
The study also was published in the British Journal of Dermatology.
Dr. El-Heis and colleagues analyzed data from one of the three U.K. study sites involved in the double-blind Maternal Vitamin D Osteoporosis Study (MAVIDOS), which enrolled participants between 2008 and 2014.
The women enrolled at the University of Southampton site were of age 18 or older, and had a singleton pregnancy. Serum 25-hydroxy vitamin D (25[OH]D) levels were 25-100 nmol/L, and calcium levels were less than 2.75 mmol/L.
Those who had metabolic bone disease, kidney stones, hyperparathyroidism, or hypercalciuria or who were taking more than 400 IU/day of vitamin D supplements or medication known to interfere with fetal growth or whose fetus had a major anomaly were excluded.
The study included 1,134 women. Half of the participants were randomly assigned to receive cholecalciferol 1,000 IU/day from around 14 weeks’ gestation until delivery, and half were assigned to receive placebo. Their babies were assessed for atopic eczema at 12, 24, and 48 months of age.
The maternal and infant characteristics were similar in both groups, but the treatment group tended to breastfeed longer.
Infants appear to be protected up to 1 year of age
Using logistic regression, the researchers analyzed links between maternal cholecalciferol 1,000 IU/day supplements or placebo and atopic eczema risk in their offspring.
After adjustments for breastfeeding duration, among the 636 infants assessed at 12 months, those whose mothers received cholecalciferol had lower odds ratios of atopic eczema than those whose mothers received placebo (OR, 0.55; 95% confidence interval, 0.32-0.97).
The risk of atopic eczema at 12 months was reduced only for children in the treatment group who were breastfed longer than 1 month (OR, 0.48; 95% CI, 0.24-0.94), further analysis showed. Those who were breastfed for less than 1 month showed no reduced risk.
The combined effect of vitamin D and breastfeeding for longer than 1 month weakened after 1 year and was not statistically significant among the 611 children assessed at 24 months and the 450 children assessed at 48 months. The ORs of atopic eczema in the treatment group and in the control group increased to 0.76 (95% CI, 0.47-1.23) and 0.75 (95% CI, 0.37-1.52), respectively.
At baseline, the mean maternal serum 25(OH)D levels in the treatment group (46.0 nmol/L) and in the control group (44.7 nmol/L) were similar. But by late pregnancy, maternal serum 25(OH)D levels in the treatment group were higher (67.4 nmol/L) than in the control group (42.4 nmol/L).
The authors note that strengths of the study include its design, the uniformity of criteria used to diagnose atopic eczema, and the similarity of both pregnant groups in their intake of vitamin D during the study.
Limitations included the lack of ultraviolet B light exposure data, the lack of non-White women in the study, the lack of measurement of cord blood and offspring 25(OH)D levels, and the exclusion of women with baseline 25(OH)D concentrations less than 25 nmol/L.
“This is an interesting study that brings up the possibility that maternal factors during pregnancy may impact atopic dermatitis,” Kalyani S. Marathe, MD, MPH, the director of the division of dermatology at Cincinnati Children’s Hospital Medical Center, told this news organization.
The results are mixed, though, she noted.
“While some impact on the risk of eczema is seen at 1 year of age, that protective effect is gone by 2 years and 4 years,” Dr. Marathe, who was not involved in the study, said in an email. “So if maternal supplementation does improve eczema, the effect is not long-lasting.
“The other complicating factor is that the babies who showed reduction in eczema were also the ones who were breastfed longer than 1 month,” she added. “We know that breastfeeding is associated with several factors, including socioeconomic status, so it is difficult to tease out the relationships here.
“Vitamin D has become a very hot topic lately and seems to have protective effects in many areas of health care,” Dr. Marathe said. “These results may motivate pregnant women to be compliant with their prenatal vitamins that contain the amount of vitamin D studied here.”
The study received grant support. Several authors disclosed financial relationships with pharmaceutical and nutritional products industries. Dr. El-Heis and Dr. Marathe reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to results of a clinical trial.
“Our data provide the first randomized controlled trial evidence of a protective effect of antenatal cholecalciferol supplementation on risk of infantile atopic eczema, with the effect only seen in infants that were breastfed for more than 1 month,” lead study author Sarah El-Heis, MRCP, DM, and colleagues wrote.
“The findings support a developmental influence on infantile atopic eczema and point to gestational cholecalciferol supplementation as a preventive strategy to reduce the burden of atopic eczema during infancy,” Dr. El-Heis, an academic clinical lecturer in dermatology at the Medical Research Council Lifecourse Epidemiology Center of the University of Southampton (England), said in a presentation at the annual meeting of the Society for Investigative Dermatology.
The study also was published in the British Journal of Dermatology.
Dr. El-Heis and colleagues analyzed data from one of the three U.K. study sites involved in the double-blind Maternal Vitamin D Osteoporosis Study (MAVIDOS), which enrolled participants between 2008 and 2014.
The women enrolled at the University of Southampton site were of age 18 or older, and had a singleton pregnancy. Serum 25-hydroxy vitamin D (25[OH]D) levels were 25-100 nmol/L, and calcium levels were less than 2.75 mmol/L.
Those who had metabolic bone disease, kidney stones, hyperparathyroidism, or hypercalciuria or who were taking more than 400 IU/day of vitamin D supplements or medication known to interfere with fetal growth or whose fetus had a major anomaly were excluded.
The study included 1,134 women. Half of the participants were randomly assigned to receive cholecalciferol 1,000 IU/day from around 14 weeks’ gestation until delivery, and half were assigned to receive placebo. Their babies were assessed for atopic eczema at 12, 24, and 48 months of age.
The maternal and infant characteristics were similar in both groups, but the treatment group tended to breastfeed longer.
Infants appear to be protected up to 1 year of age
Using logistic regression, the researchers analyzed links between maternal cholecalciferol 1,000 IU/day supplements or placebo and atopic eczema risk in their offspring.
After adjustments for breastfeeding duration, among the 636 infants assessed at 12 months, those whose mothers received cholecalciferol had lower odds ratios of atopic eczema than those whose mothers received placebo (OR, 0.55; 95% confidence interval, 0.32-0.97).
The risk of atopic eczema at 12 months was reduced only for children in the treatment group who were breastfed longer than 1 month (OR, 0.48; 95% CI, 0.24-0.94), further analysis showed. Those who were breastfed for less than 1 month showed no reduced risk.
The combined effect of vitamin D and breastfeeding for longer than 1 month weakened after 1 year and was not statistically significant among the 611 children assessed at 24 months and the 450 children assessed at 48 months. The ORs of atopic eczema in the treatment group and in the control group increased to 0.76 (95% CI, 0.47-1.23) and 0.75 (95% CI, 0.37-1.52), respectively.
At baseline, the mean maternal serum 25(OH)D levels in the treatment group (46.0 nmol/L) and in the control group (44.7 nmol/L) were similar. But by late pregnancy, maternal serum 25(OH)D levels in the treatment group were higher (67.4 nmol/L) than in the control group (42.4 nmol/L).
The authors note that strengths of the study include its design, the uniformity of criteria used to diagnose atopic eczema, and the similarity of both pregnant groups in their intake of vitamin D during the study.
Limitations included the lack of ultraviolet B light exposure data, the lack of non-White women in the study, the lack of measurement of cord blood and offspring 25(OH)D levels, and the exclusion of women with baseline 25(OH)D concentrations less than 25 nmol/L.
“This is an interesting study that brings up the possibility that maternal factors during pregnancy may impact atopic dermatitis,” Kalyani S. Marathe, MD, MPH, the director of the division of dermatology at Cincinnati Children’s Hospital Medical Center, told this news organization.
The results are mixed, though, she noted.
“While some impact on the risk of eczema is seen at 1 year of age, that protective effect is gone by 2 years and 4 years,” Dr. Marathe, who was not involved in the study, said in an email. “So if maternal supplementation does improve eczema, the effect is not long-lasting.
“The other complicating factor is that the babies who showed reduction in eczema were also the ones who were breastfed longer than 1 month,” she added. “We know that breastfeeding is associated with several factors, including socioeconomic status, so it is difficult to tease out the relationships here.
“Vitamin D has become a very hot topic lately and seems to have protective effects in many areas of health care,” Dr. Marathe said. “These results may motivate pregnant women to be compliant with their prenatal vitamins that contain the amount of vitamin D studied here.”
The study received grant support. Several authors disclosed financial relationships with pharmaceutical and nutritional products industries. Dr. El-Heis and Dr. Marathe reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM SID 2022
Diabetes devices may give children contact dermatitis
Devices that help children control their diabetes and lead fuller lives may also give them contact dermatitis, report the authors of a new study that calls for mandatory labeling of ingredients for allergy patch testing.
“A high share of patients showed positive reactions to isobornyl acrylate adhesive (IBOA) and/or their medical devices (insulin pumps or glucose devices),” the study authors write in Contact Dermatitis. “A third of patients showed positive reactions to benzoyl peroxide (BP),” used in adhesives.
“The presence of additional unidentified allergens cannot be excluded,” they add. “Overall, our experience once more highlights the importance of having access to a full description of the chemical composition of diabetes devices and related medical devices to efficiently manage patients (including children) who experience adverse skin reactions from such devices.”
Lead study author Catarina Alves da Silva, MD, of the department of dermatology and venereology of Aarhus (Denmark) University Hospital, and her colleagues conducted a retrospective study of 15 referred patients younger than 18 years who had type 1 diabetes. The children were patch tested in the university’s dermatology clinic between 2018 and 2020 in a study of skin reactions linked with diabetes devices.
Contact dermatitis from device-related allergens may be common
Many children in the study reacted to chemical compounds related to their devices.
- Of the 15 patients, seven showed positive patch test reactions to IBOA, and five showed positive reactions to BP.
- Ten children had positive patch test reactions to materials from glucose sensors and insulin pumps.
- Three showed positive reactions to adhesive remover wipes.
- Five reacted to .
Marcia Hogeling, MD, a pediatric dermatologist at UCLA Health in Santa Monica, Calif., told this news organization that she expected acrylates to cause problems but was surprised that BP caused positive patch test reactions.
BP is known to be a strong irritant but a weak allergen, the authors wrote.
“It was important to identify the allergens in these devices. Hopefully, this information will be used by manufacturers to create safer products for patients,” Dr. Hogeling, who was not involved in the study, said in an email.
Dr. Hogeling acknowledged that the small sample size is a weakness of the study, although she added that the findings may help providers select devices that do not contain their patients’ contact allergens.
Ryan J. McDonough, DO, a pediatric endocrinologist and the codirector of the Diabetes Center at Children’s Mercy Kansas City (Mo.), said in an email that, despite the small sample size, the study “highlights important device-related experiences of those living with type 1 diabetes that clinicians often encounter.
“We often spend considerable time aiding patients and their families in finding ways to mitigate the reactions,” he explained. “Having a broader understanding of these chemical compositions would help clinicians choose the right devices for their patients and prevent and treat these types of reactions.”
Dr. McDonough, who was not involved in the study, noted that the patients were in Denmark, and they were able to easily transition between insulin pumps and glucose monitoring devices.
“In the U.S., it is often more challenging to switch between devices, due to insurance-related concerns.
“The true rates of reaction in the broad type 1 diabetes population are difficult to assess,” Dr. McDonough said. “The study participants were drawn from patients referred to a dermatology clinic for evaluation of reaction. Many patients either don’t develop reactions or are treated for mild symptoms locally by their endocrinologists.
“This study should serve as a call to action for continued improvements in the transparency of the components that make up the devices and adhesives, and it can provide an opportunity to develop additional interventions to prevent these reactions,” he advised.
No information regarding funding for the study was provided. The authors, Dr. Hogeling, and Dr. McDonough reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Devices that help children control their diabetes and lead fuller lives may also give them contact dermatitis, report the authors of a new study that calls for mandatory labeling of ingredients for allergy patch testing.
“A high share of patients showed positive reactions to isobornyl acrylate adhesive (IBOA) and/or their medical devices (insulin pumps or glucose devices),” the study authors write in Contact Dermatitis. “A third of patients showed positive reactions to benzoyl peroxide (BP),” used in adhesives.
“The presence of additional unidentified allergens cannot be excluded,” they add. “Overall, our experience once more highlights the importance of having access to a full description of the chemical composition of diabetes devices and related medical devices to efficiently manage patients (including children) who experience adverse skin reactions from such devices.”
Lead study author Catarina Alves da Silva, MD, of the department of dermatology and venereology of Aarhus (Denmark) University Hospital, and her colleagues conducted a retrospective study of 15 referred patients younger than 18 years who had type 1 diabetes. The children were patch tested in the university’s dermatology clinic between 2018 and 2020 in a study of skin reactions linked with diabetes devices.
Contact dermatitis from device-related allergens may be common
Many children in the study reacted to chemical compounds related to their devices.
- Of the 15 patients, seven showed positive patch test reactions to IBOA, and five showed positive reactions to BP.
- Ten children had positive patch test reactions to materials from glucose sensors and insulin pumps.
- Three showed positive reactions to adhesive remover wipes.
- Five reacted to .
Marcia Hogeling, MD, a pediatric dermatologist at UCLA Health in Santa Monica, Calif., told this news organization that she expected acrylates to cause problems but was surprised that BP caused positive patch test reactions.
BP is known to be a strong irritant but a weak allergen, the authors wrote.
“It was important to identify the allergens in these devices. Hopefully, this information will be used by manufacturers to create safer products for patients,” Dr. Hogeling, who was not involved in the study, said in an email.
Dr. Hogeling acknowledged that the small sample size is a weakness of the study, although she added that the findings may help providers select devices that do not contain their patients’ contact allergens.
Ryan J. McDonough, DO, a pediatric endocrinologist and the codirector of the Diabetes Center at Children’s Mercy Kansas City (Mo.), said in an email that, despite the small sample size, the study “highlights important device-related experiences of those living with type 1 diabetes that clinicians often encounter.
“We often spend considerable time aiding patients and their families in finding ways to mitigate the reactions,” he explained. “Having a broader understanding of these chemical compositions would help clinicians choose the right devices for their patients and prevent and treat these types of reactions.”
Dr. McDonough, who was not involved in the study, noted that the patients were in Denmark, and they were able to easily transition between insulin pumps and glucose monitoring devices.
“In the U.S., it is often more challenging to switch between devices, due to insurance-related concerns.
“The true rates of reaction in the broad type 1 diabetes population are difficult to assess,” Dr. McDonough said. “The study participants were drawn from patients referred to a dermatology clinic for evaluation of reaction. Many patients either don’t develop reactions or are treated for mild symptoms locally by their endocrinologists.
“This study should serve as a call to action for continued improvements in the transparency of the components that make up the devices and adhesives, and it can provide an opportunity to develop additional interventions to prevent these reactions,” he advised.
No information regarding funding for the study was provided. The authors, Dr. Hogeling, and Dr. McDonough reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Devices that help children control their diabetes and lead fuller lives may also give them contact dermatitis, report the authors of a new study that calls for mandatory labeling of ingredients for allergy patch testing.
“A high share of patients showed positive reactions to isobornyl acrylate adhesive (IBOA) and/or their medical devices (insulin pumps or glucose devices),” the study authors write in Contact Dermatitis. “A third of patients showed positive reactions to benzoyl peroxide (BP),” used in adhesives.
“The presence of additional unidentified allergens cannot be excluded,” they add. “Overall, our experience once more highlights the importance of having access to a full description of the chemical composition of diabetes devices and related medical devices to efficiently manage patients (including children) who experience adverse skin reactions from such devices.”
Lead study author Catarina Alves da Silva, MD, of the department of dermatology and venereology of Aarhus (Denmark) University Hospital, and her colleagues conducted a retrospective study of 15 referred patients younger than 18 years who had type 1 diabetes. The children were patch tested in the university’s dermatology clinic between 2018 and 2020 in a study of skin reactions linked with diabetes devices.
Contact dermatitis from device-related allergens may be common
Many children in the study reacted to chemical compounds related to their devices.
- Of the 15 patients, seven showed positive patch test reactions to IBOA, and five showed positive reactions to BP.
- Ten children had positive patch test reactions to materials from glucose sensors and insulin pumps.
- Three showed positive reactions to adhesive remover wipes.
- Five reacted to .
Marcia Hogeling, MD, a pediatric dermatologist at UCLA Health in Santa Monica, Calif., told this news organization that she expected acrylates to cause problems but was surprised that BP caused positive patch test reactions.
BP is known to be a strong irritant but a weak allergen, the authors wrote.
“It was important to identify the allergens in these devices. Hopefully, this information will be used by manufacturers to create safer products for patients,” Dr. Hogeling, who was not involved in the study, said in an email.
Dr. Hogeling acknowledged that the small sample size is a weakness of the study, although she added that the findings may help providers select devices that do not contain their patients’ contact allergens.
Ryan J. McDonough, DO, a pediatric endocrinologist and the codirector of the Diabetes Center at Children’s Mercy Kansas City (Mo.), said in an email that, despite the small sample size, the study “highlights important device-related experiences of those living with type 1 diabetes that clinicians often encounter.
“We often spend considerable time aiding patients and their families in finding ways to mitigate the reactions,” he explained. “Having a broader understanding of these chemical compositions would help clinicians choose the right devices for their patients and prevent and treat these types of reactions.”
Dr. McDonough, who was not involved in the study, noted that the patients were in Denmark, and they were able to easily transition between insulin pumps and glucose monitoring devices.
“In the U.S., it is often more challenging to switch between devices, due to insurance-related concerns.
“The true rates of reaction in the broad type 1 diabetes population are difficult to assess,” Dr. McDonough said. “The study participants were drawn from patients referred to a dermatology clinic for evaluation of reaction. Many patients either don’t develop reactions or are treated for mild symptoms locally by their endocrinologists.
“This study should serve as a call to action for continued improvements in the transparency of the components that make up the devices and adhesives, and it can provide an opportunity to develop additional interventions to prevent these reactions,” he advised.
No information regarding funding for the study was provided. The authors, Dr. Hogeling, and Dr. McDonough reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TDF use in HBV-HIV coinfection linked with kidney, bone issues
Patients coinfected with hepatitis B virus (HBV) and human immunodeficiency virus who take tenofovir disoproxil fumarate (TDF) may have worsening renal function and bone turnover, according to a small, prospective cohort study in HIV Medicine.
“In this HBV-HIV cohort of adults with high prevalence of tenofovir use, several biomarkers of renal function and bone turnover indicated worsening status over approximately 4 years, highlighting the importance of clinicians’ awareness,” lead author Richard K. Sterling, MD, MSc, assistant chair of research in the department of internal medicine of Virginia Commonwealth University, Richmond, told this news organization in an email.
TDF is a common component of antiretroviral therapy (ART) in adults coinfected with HBV and HIV. The drug is known to adversely affect kidney function and bone turnover, but few studies have evaluated these issues, the authors write.
Dr. Sterling and colleagues enrolled adults coinfected with HBV and HIV who were taking any type of ART in their study at eight sites in North America.
The authors assessed demographics, medical history, current health status reports, physical exams, and blood and urine tests. They extracted clinical, laboratory, and radiologic data from medical records, and they processed whole blood, stored serum at -70 °C (-94 °F) at each site, and tested specimens in central laboratories.
The researchers assessed the participants at baseline and every 24 weeks for up to 192 weeks (3.7 years). They tested bone markers from stored serum at baseline, week 96, and week 192. And they recorded changes in renal function markers and bone turnover over time.
At baseline, the median age of the 115 patients was 49 years; 91% were male, and 52% were non-Hispanic Black. Their median body mass index was 26 kg/m2, with 6.3% of participants underweight and 59% overweight or obese. The participants had been living with HIV for a median of about 20 years.
Overall, 84% of participants reported tenofovir use, 3% reported no HBV therapy, and 80% had HBV/HIV suppression. In addition, 13% had stage 2 liver fibrosis and 23% had stage 3 to 4 liver fibrosis. No participants reported using immunosuppressants, 4% reported using an anticoagulant, 3% reported taking calcium plus vitamin D, and 33% reported taking multivitamins.
Throughout the follow-up period, TDF use ranged from 80% to 92%. Estimated glomerular filtration rate (eGFR) dropped from 87.1 to 79.9 ml/min/1.73m2 over 192 weeks (P < .001); but eGFR prevalence < 60 ml/min/1.73m2 did not appear to change over time (always < 16%; P = .43).
From baseline to week 192, procollagen type 1 N-terminal propeptide (P1NP) dropped from 146.7 to 130.5 ng/ml (P = .001), osteocalcin dropped from 14.4 to 10.2 ng/ml (P < .001), and C-terminal telopeptides of type I collagen (CTX-1) dropped from 373 to 273 pg/ml (P < .001).
Predictors of decrease in eGFR included younger age, male sex, and overweight or obesity. Predictors of worsening bone turnover included Black race, healthy weight, advanced fibrosis, undetectable HBV DNA, and lower parathyroid hormone level.
Monitor patients with HBV and HIV closely
“The long-term effects of TDF on renal and bone health are important to monitor,” Dr. Sterling advised. “For renal health, physicians should monitor GFR as well as creatinine. For bone health, monitoring serum calcium, vitamin D, parathyroid hormone, and phosphate may not catch increased bone turnover.”
“We knew that TDF can cause renal dysfunction; however, we were surprised that we did not observe significant rise in serum creatinine but did observe decline in glomerular filtration rate and several markers of increased bone turnover,” he added.
Dr. Sterling acknowledged that limitations of the study include its small cohort, short follow-up, and lack of control participants who were taking TDF while mono-infected with either HBV or HIV. He added that strengths include close follow-up, use of bone turnover markers, and control for severity of liver disease.
Joseph Alvarnas, MD, a hematologist and oncologist in the department of hematology & hematopoietic cell transplant at City of Hope Comprehensive Cancer Center, Duarte, California, told this news organization that he welcomes the rigor of the study. “This study provides an important reminder of the complexities of taking a comprehensive management approach to the care of patients with long-term HIV infection,” Dr. Alvarnas wrote in an email. He was not involved in the study.
“More than 6 million people worldwide live with coinfection,” he added. “Patients coinfected with HBV and HIV have additional care needs over those living with only chronic HIV infection. With more HIV-infected patients becoming long-term survivors who are managed through the use of effective ART, fully understanding the differentiated long-term care needs of this population is important.”
Debika Bhattacharya, MD, a specialist in HIV and viral hepatitis coinfection in the Division of Infectious Diseases at UCLA Health, Los Angeles, joined Dr. Sterling and Dr. Alvarnas in advising clinicians to regularly evaluate the kidney and bone health of their coinfected patients.
“While this study focuses the very common antiretroviral agent TDF, it will be important to see the impact of a similar drug, tenofovir alafenamide (TAF) – which has been associated with less impact on bone and kidney health – on clinical outcomes in HBV-HIV coinfection,” Dr. Bhattacharya, who also was not involved in the study, wrote in an email.
The National Institute of Diabetes and Digestive and Kidney Diseases funded the study. Dr. Sterling has served on boards for Pfizer and AskBio, and he reports research grants from Gilead, Abbott, AbbVie, and Roche to his institution. Most other authors report financial relationships with pharmaceutical companies. Dr. Alvarnas reports no relevant financial relationships. Dr. Bhattacharya has received a research grant from Gilead Sciences, paid to her institution.
A version of this article first appeared on Medscape.com.
Patients coinfected with hepatitis B virus (HBV) and human immunodeficiency virus who take tenofovir disoproxil fumarate (TDF) may have worsening renal function and bone turnover, according to a small, prospective cohort study in HIV Medicine.
“In this HBV-HIV cohort of adults with high prevalence of tenofovir use, several biomarkers of renal function and bone turnover indicated worsening status over approximately 4 years, highlighting the importance of clinicians’ awareness,” lead author Richard K. Sterling, MD, MSc, assistant chair of research in the department of internal medicine of Virginia Commonwealth University, Richmond, told this news organization in an email.
TDF is a common component of antiretroviral therapy (ART) in adults coinfected with HBV and HIV. The drug is known to adversely affect kidney function and bone turnover, but few studies have evaluated these issues, the authors write.
Dr. Sterling and colleagues enrolled adults coinfected with HBV and HIV who were taking any type of ART in their study at eight sites in North America.
The authors assessed demographics, medical history, current health status reports, physical exams, and blood and urine tests. They extracted clinical, laboratory, and radiologic data from medical records, and they processed whole blood, stored serum at -70 °C (-94 °F) at each site, and tested specimens in central laboratories.
The researchers assessed the participants at baseline and every 24 weeks for up to 192 weeks (3.7 years). They tested bone markers from stored serum at baseline, week 96, and week 192. And they recorded changes in renal function markers and bone turnover over time.
At baseline, the median age of the 115 patients was 49 years; 91% were male, and 52% were non-Hispanic Black. Their median body mass index was 26 kg/m2, with 6.3% of participants underweight and 59% overweight or obese. The participants had been living with HIV for a median of about 20 years.
Overall, 84% of participants reported tenofovir use, 3% reported no HBV therapy, and 80% had HBV/HIV suppression. In addition, 13% had stage 2 liver fibrosis and 23% had stage 3 to 4 liver fibrosis. No participants reported using immunosuppressants, 4% reported using an anticoagulant, 3% reported taking calcium plus vitamin D, and 33% reported taking multivitamins.
Throughout the follow-up period, TDF use ranged from 80% to 92%. Estimated glomerular filtration rate (eGFR) dropped from 87.1 to 79.9 ml/min/1.73m2 over 192 weeks (P < .001); but eGFR prevalence < 60 ml/min/1.73m2 did not appear to change over time (always < 16%; P = .43).
From baseline to week 192, procollagen type 1 N-terminal propeptide (P1NP) dropped from 146.7 to 130.5 ng/ml (P = .001), osteocalcin dropped from 14.4 to 10.2 ng/ml (P < .001), and C-terminal telopeptides of type I collagen (CTX-1) dropped from 373 to 273 pg/ml (P < .001).
Predictors of decrease in eGFR included younger age, male sex, and overweight or obesity. Predictors of worsening bone turnover included Black race, healthy weight, advanced fibrosis, undetectable HBV DNA, and lower parathyroid hormone level.
Monitor patients with HBV and HIV closely
“The long-term effects of TDF on renal and bone health are important to monitor,” Dr. Sterling advised. “For renal health, physicians should monitor GFR as well as creatinine. For bone health, monitoring serum calcium, vitamin D, parathyroid hormone, and phosphate may not catch increased bone turnover.”
“We knew that TDF can cause renal dysfunction; however, we were surprised that we did not observe significant rise in serum creatinine but did observe decline in glomerular filtration rate and several markers of increased bone turnover,” he added.
Dr. Sterling acknowledged that limitations of the study include its small cohort, short follow-up, and lack of control participants who were taking TDF while mono-infected with either HBV or HIV. He added that strengths include close follow-up, use of bone turnover markers, and control for severity of liver disease.
Joseph Alvarnas, MD, a hematologist and oncologist in the department of hematology & hematopoietic cell transplant at City of Hope Comprehensive Cancer Center, Duarte, California, told this news organization that he welcomes the rigor of the study. “This study provides an important reminder of the complexities of taking a comprehensive management approach to the care of patients with long-term HIV infection,” Dr. Alvarnas wrote in an email. He was not involved in the study.
“More than 6 million people worldwide live with coinfection,” he added. “Patients coinfected with HBV and HIV have additional care needs over those living with only chronic HIV infection. With more HIV-infected patients becoming long-term survivors who are managed through the use of effective ART, fully understanding the differentiated long-term care needs of this population is important.”
Debika Bhattacharya, MD, a specialist in HIV and viral hepatitis coinfection in the Division of Infectious Diseases at UCLA Health, Los Angeles, joined Dr. Sterling and Dr. Alvarnas in advising clinicians to regularly evaluate the kidney and bone health of their coinfected patients.
“While this study focuses the very common antiretroviral agent TDF, it will be important to see the impact of a similar drug, tenofovir alafenamide (TAF) – which has been associated with less impact on bone and kidney health – on clinical outcomes in HBV-HIV coinfection,” Dr. Bhattacharya, who also was not involved in the study, wrote in an email.
The National Institute of Diabetes and Digestive and Kidney Diseases funded the study. Dr. Sterling has served on boards for Pfizer and AskBio, and he reports research grants from Gilead, Abbott, AbbVie, and Roche to his institution. Most other authors report financial relationships with pharmaceutical companies. Dr. Alvarnas reports no relevant financial relationships. Dr. Bhattacharya has received a research grant from Gilead Sciences, paid to her institution.
A version of this article first appeared on Medscape.com.
Patients coinfected with hepatitis B virus (HBV) and human immunodeficiency virus who take tenofovir disoproxil fumarate (TDF) may have worsening renal function and bone turnover, according to a small, prospective cohort study in HIV Medicine.
“In this HBV-HIV cohort of adults with high prevalence of tenofovir use, several biomarkers of renal function and bone turnover indicated worsening status over approximately 4 years, highlighting the importance of clinicians’ awareness,” lead author Richard K. Sterling, MD, MSc, assistant chair of research in the department of internal medicine of Virginia Commonwealth University, Richmond, told this news organization in an email.
TDF is a common component of antiretroviral therapy (ART) in adults coinfected with HBV and HIV. The drug is known to adversely affect kidney function and bone turnover, but few studies have evaluated these issues, the authors write.
Dr. Sterling and colleagues enrolled adults coinfected with HBV and HIV who were taking any type of ART in their study at eight sites in North America.
The authors assessed demographics, medical history, current health status reports, physical exams, and blood and urine tests. They extracted clinical, laboratory, and radiologic data from medical records, and they processed whole blood, stored serum at -70 °C (-94 °F) at each site, and tested specimens in central laboratories.
The researchers assessed the participants at baseline and every 24 weeks for up to 192 weeks (3.7 years). They tested bone markers from stored serum at baseline, week 96, and week 192. And they recorded changes in renal function markers and bone turnover over time.
At baseline, the median age of the 115 patients was 49 years; 91% were male, and 52% were non-Hispanic Black. Their median body mass index was 26 kg/m2, with 6.3% of participants underweight and 59% overweight or obese. The participants had been living with HIV for a median of about 20 years.
Overall, 84% of participants reported tenofovir use, 3% reported no HBV therapy, and 80% had HBV/HIV suppression. In addition, 13% had stage 2 liver fibrosis and 23% had stage 3 to 4 liver fibrosis. No participants reported using immunosuppressants, 4% reported using an anticoagulant, 3% reported taking calcium plus vitamin D, and 33% reported taking multivitamins.
Throughout the follow-up period, TDF use ranged from 80% to 92%. Estimated glomerular filtration rate (eGFR) dropped from 87.1 to 79.9 ml/min/1.73m2 over 192 weeks (P < .001); but eGFR prevalence < 60 ml/min/1.73m2 did not appear to change over time (always < 16%; P = .43).
From baseline to week 192, procollagen type 1 N-terminal propeptide (P1NP) dropped from 146.7 to 130.5 ng/ml (P = .001), osteocalcin dropped from 14.4 to 10.2 ng/ml (P < .001), and C-terminal telopeptides of type I collagen (CTX-1) dropped from 373 to 273 pg/ml (P < .001).
Predictors of decrease in eGFR included younger age, male sex, and overweight or obesity. Predictors of worsening bone turnover included Black race, healthy weight, advanced fibrosis, undetectable HBV DNA, and lower parathyroid hormone level.
Monitor patients with HBV and HIV closely
“The long-term effects of TDF on renal and bone health are important to monitor,” Dr. Sterling advised. “For renal health, physicians should monitor GFR as well as creatinine. For bone health, monitoring serum calcium, vitamin D, parathyroid hormone, and phosphate may not catch increased bone turnover.”
“We knew that TDF can cause renal dysfunction; however, we were surprised that we did not observe significant rise in serum creatinine but did observe decline in glomerular filtration rate and several markers of increased bone turnover,” he added.
Dr. Sterling acknowledged that limitations of the study include its small cohort, short follow-up, and lack of control participants who were taking TDF while mono-infected with either HBV or HIV. He added that strengths include close follow-up, use of bone turnover markers, and control for severity of liver disease.
Joseph Alvarnas, MD, a hematologist and oncologist in the department of hematology & hematopoietic cell transplant at City of Hope Comprehensive Cancer Center, Duarte, California, told this news organization that he welcomes the rigor of the study. “This study provides an important reminder of the complexities of taking a comprehensive management approach to the care of patients with long-term HIV infection,” Dr. Alvarnas wrote in an email. He was not involved in the study.
“More than 6 million people worldwide live with coinfection,” he added. “Patients coinfected with HBV and HIV have additional care needs over those living with only chronic HIV infection. With more HIV-infected patients becoming long-term survivors who are managed through the use of effective ART, fully understanding the differentiated long-term care needs of this population is important.”
Debika Bhattacharya, MD, a specialist in HIV and viral hepatitis coinfection in the Division of Infectious Diseases at UCLA Health, Los Angeles, joined Dr. Sterling and Dr. Alvarnas in advising clinicians to regularly evaluate the kidney and bone health of their coinfected patients.
“While this study focuses the very common antiretroviral agent TDF, it will be important to see the impact of a similar drug, tenofovir alafenamide (TAF) – which has been associated with less impact on bone and kidney health – on clinical outcomes in HBV-HIV coinfection,” Dr. Bhattacharya, who also was not involved in the study, wrote in an email.
The National Institute of Diabetes and Digestive and Kidney Diseases funded the study. Dr. Sterling has served on boards for Pfizer and AskBio, and he reports research grants from Gilead, Abbott, AbbVie, and Roche to his institution. Most other authors report financial relationships with pharmaceutical companies. Dr. Alvarnas reports no relevant financial relationships. Dr. Bhattacharya has received a research grant from Gilead Sciences, paid to her institution.
A version of this article first appeared on Medscape.com.
Emerging tick-borne pathogen has spread to state of Georgia
Heartland virus (HRTV), an emerging infection first detected in lone star ticks in Missouri in 2009, has spread to lone star ticks in Georgia, a study published in Emerging Infectious Diseases reports.
HRTV disease is transmitted by the bite of an infected Amblyomma americanum tick, named “lone star” because of the silver-white spot on the female scutum (back).
“By … sampling … in an area with reported exposure to HRTV in wildlife and humans and testing for infection in thousands of ticks from multiple sites and physiologic stages, we confirmed the presence of HRTV in Georgia,” the authors write.
“This information about the expanding geographic range of lone star ticks, combined with increased human presence in tick-infested habitats, can be used to improve strategies for preventing tick bites and to alert physicians about this emerging tickborne virus infection,” a press release by the Centers for Disease Control and Prevention notes.
Persistent field and lab work led to HRTV discovery in Georgia
The search for infected lone star ticks began after a retroactive analysis confirmed that a person who died in Georgia in 2005 from an unidentified illness was infected with HRTV. A subsequent analysis of serum samples collected earlier from local white-tailed deer showed that the deer had been exposed to HRTV since at least 2001, according to a press release by Emory University.
These discoveries prompted local researchers to investigate whether lone star ticks in rural, woodsy central Georgia were carrying HRTV.
Lead study author Yamila Romer, MD, an infectious disease clinician and microbiologist in the department of environmental sciences at Emory University in Atlanta, and her colleagues collected samples of ticks in 2018 at 26 sites near the location of the patient who died and the seropositive deer. In 2019, they focused their collections on the two sites that had provided the most ticks in 2018.
From April to October in both years, the research team visited sites weekly to swish white flannel flags through underbrush. They picked off adult and nymph Amblyomma americanum ticks, placed them into vials, and transported them to their lab. They sorted 9,294 ticks by sex, life stage, and collection site. Then they crushed the ticks and extracted their RNA.
To confirm viral infection, the team tested RNA extracted from cell culture supernatants using a real-time polymerase chain reaction test specific for HRTV.
In the three pools of ticks that tested positive for HRTV, the researchers found a minimum infection rate of 0.46/1,000 ticks, suggesting that about 1 of every 2,000 ticks carried HRTV. They sequenced the genome of the three isolates and found that the genomes were similar to one another but were very different from the genomes from HRTV samples taken outside Georgia.
Catherine A. Hill, PhD, a professor of entomology and vector biology and the interim head of the department of entomology at Purdue University in West Lafayette, Ind., was impressed with the researchers’ discovery.
“Heartland virus is difficult to detect,” she said in an email. “The prevalence of human cases is low, and the virus appears to be present at very low levels in populations of lone star tick. The investigators went to some lengths to survey for the virus, collect, and process thousands of ticks – and they found the needle in the haystack.” Dr. Hill was not involved in the study.
Georgia data help researchers monitor HRTV spread
HRTV was first identified in 2009 in Missouri in two people hospitalized with fever, muscle pain, diarrhea, and low white blood cell and platelet counts. Researchers traced the infections to lone star ticks, and they found antibodies to the virus in blood samples from deer and other wild mammals.
According to the CDC, U.S. cases of tick-borne diseases more than doubled between 2004 and 2016. As of January 2021, more than 50 human cases of HRTV disease had been reported in 11 Midwestern and Southeastern states: Arkansas, Georgia, Illinois, Indiana, Iowa, Kansas, Kentucky, Missouri, North Carolina, Oklahoma, and Tennessee.
Precautions, signs, symptoms, testing, and treatment
“The lone star tick is aggressive and will actively seek out a human host to bite,” Dr. Hill noted.
She recommends that health care providers advise patients to avoid tick habitat, wear protective clothing, apply repellants, know the signs and symptoms of tick-borne disease, and seek immediate medical care if they become ill.
Common symptoms of HRTV disease include fatigue, fever, nausea, diarrhea, and anorexia. Treatment is supportive. Many patients have been hospitalized, and some with comorbidities have died.
HRTV infection is rarely tested for, and the disease burden is unknown. With no commercial tests available in the United States, the CDC performs molecular and serologic testing for HRTV infection. The agency advises doctors to contact their state health department if they suspect a patient may have HRTV disease.
Further research is needed
Samantha M. Wisely, PhD, a professor of wildlife ecology and the director of the Cervidae Health Research Initiative at the University of Florida in Gainesville, was not surprised by the study finding.
“The more we look for heartland virus, the more places we find it,” Dr. Wisely told this news organization in an email.
“Little is known about which wildlife play a role in maintaining the virus on the landscape,” said Dr. Wisely, who was not involved in the study. “White-tailed deer have been shown to produce antibodies, meaning they have been exposed to the virus, but no one has actually found the virus in a wildlife species.”
The whole-genome sequencing of the virus was particularly important, Dr. Wisely explained. “Whole-genome data allow researchers to better understand viral evolution, pathogenicity, and viral dynamics across space and time – how it is evolving.”
The study was supported by a grant from the Emory University Research Council. The authors, Dr. Wisely, and Dr. Hill have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Heartland virus (HRTV), an emerging infection first detected in lone star ticks in Missouri in 2009, has spread to lone star ticks in Georgia, a study published in Emerging Infectious Diseases reports.
HRTV disease is transmitted by the bite of an infected Amblyomma americanum tick, named “lone star” because of the silver-white spot on the female scutum (back).
“By … sampling … in an area with reported exposure to HRTV in wildlife and humans and testing for infection in thousands of ticks from multiple sites and physiologic stages, we confirmed the presence of HRTV in Georgia,” the authors write.
“This information about the expanding geographic range of lone star ticks, combined with increased human presence in tick-infested habitats, can be used to improve strategies for preventing tick bites and to alert physicians about this emerging tickborne virus infection,” a press release by the Centers for Disease Control and Prevention notes.
Persistent field and lab work led to HRTV discovery in Georgia
The search for infected lone star ticks began after a retroactive analysis confirmed that a person who died in Georgia in 2005 from an unidentified illness was infected with HRTV. A subsequent analysis of serum samples collected earlier from local white-tailed deer showed that the deer had been exposed to HRTV since at least 2001, according to a press release by Emory University.
These discoveries prompted local researchers to investigate whether lone star ticks in rural, woodsy central Georgia were carrying HRTV.
Lead study author Yamila Romer, MD, an infectious disease clinician and microbiologist in the department of environmental sciences at Emory University in Atlanta, and her colleagues collected samples of ticks in 2018 at 26 sites near the location of the patient who died and the seropositive deer. In 2019, they focused their collections on the two sites that had provided the most ticks in 2018.
From April to October in both years, the research team visited sites weekly to swish white flannel flags through underbrush. They picked off adult and nymph Amblyomma americanum ticks, placed them into vials, and transported them to their lab. They sorted 9,294 ticks by sex, life stage, and collection site. Then they crushed the ticks and extracted their RNA.
To confirm viral infection, the team tested RNA extracted from cell culture supernatants using a real-time polymerase chain reaction test specific for HRTV.
In the three pools of ticks that tested positive for HRTV, the researchers found a minimum infection rate of 0.46/1,000 ticks, suggesting that about 1 of every 2,000 ticks carried HRTV. They sequenced the genome of the three isolates and found that the genomes were similar to one another but were very different from the genomes from HRTV samples taken outside Georgia.
Catherine A. Hill, PhD, a professor of entomology and vector biology and the interim head of the department of entomology at Purdue University in West Lafayette, Ind., was impressed with the researchers’ discovery.
“Heartland virus is difficult to detect,” she said in an email. “The prevalence of human cases is low, and the virus appears to be present at very low levels in populations of lone star tick. The investigators went to some lengths to survey for the virus, collect, and process thousands of ticks – and they found the needle in the haystack.” Dr. Hill was not involved in the study.
Georgia data help researchers monitor HRTV spread
HRTV was first identified in 2009 in Missouri in two people hospitalized with fever, muscle pain, diarrhea, and low white blood cell and platelet counts. Researchers traced the infections to lone star ticks, and they found antibodies to the virus in blood samples from deer and other wild mammals.
According to the CDC, U.S. cases of tick-borne diseases more than doubled between 2004 and 2016. As of January 2021, more than 50 human cases of HRTV disease had been reported in 11 Midwestern and Southeastern states: Arkansas, Georgia, Illinois, Indiana, Iowa, Kansas, Kentucky, Missouri, North Carolina, Oklahoma, and Tennessee.
Precautions, signs, symptoms, testing, and treatment
“The lone star tick is aggressive and will actively seek out a human host to bite,” Dr. Hill noted.
She recommends that health care providers advise patients to avoid tick habitat, wear protective clothing, apply repellants, know the signs and symptoms of tick-borne disease, and seek immediate medical care if they become ill.
Common symptoms of HRTV disease include fatigue, fever, nausea, diarrhea, and anorexia. Treatment is supportive. Many patients have been hospitalized, and some with comorbidities have died.
HRTV infection is rarely tested for, and the disease burden is unknown. With no commercial tests available in the United States, the CDC performs molecular and serologic testing for HRTV infection. The agency advises doctors to contact their state health department if they suspect a patient may have HRTV disease.
Further research is needed
Samantha M. Wisely, PhD, a professor of wildlife ecology and the director of the Cervidae Health Research Initiative at the University of Florida in Gainesville, was not surprised by the study finding.
“The more we look for heartland virus, the more places we find it,” Dr. Wisely told this news organization in an email.
“Little is known about which wildlife play a role in maintaining the virus on the landscape,” said Dr. Wisely, who was not involved in the study. “White-tailed deer have been shown to produce antibodies, meaning they have been exposed to the virus, but no one has actually found the virus in a wildlife species.”
The whole-genome sequencing of the virus was particularly important, Dr. Wisely explained. “Whole-genome data allow researchers to better understand viral evolution, pathogenicity, and viral dynamics across space and time – how it is evolving.”
The study was supported by a grant from the Emory University Research Council. The authors, Dr. Wisely, and Dr. Hill have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Heartland virus (HRTV), an emerging infection first detected in lone star ticks in Missouri in 2009, has spread to lone star ticks in Georgia, a study published in Emerging Infectious Diseases reports.
HRTV disease is transmitted by the bite of an infected Amblyomma americanum tick, named “lone star” because of the silver-white spot on the female scutum (back).
“By … sampling … in an area with reported exposure to HRTV in wildlife and humans and testing for infection in thousands of ticks from multiple sites and physiologic stages, we confirmed the presence of HRTV in Georgia,” the authors write.
“This information about the expanding geographic range of lone star ticks, combined with increased human presence in tick-infested habitats, can be used to improve strategies for preventing tick bites and to alert physicians about this emerging tickborne virus infection,” a press release by the Centers for Disease Control and Prevention notes.
Persistent field and lab work led to HRTV discovery in Georgia
The search for infected lone star ticks began after a retroactive analysis confirmed that a person who died in Georgia in 2005 from an unidentified illness was infected with HRTV. A subsequent analysis of serum samples collected earlier from local white-tailed deer showed that the deer had been exposed to HRTV since at least 2001, according to a press release by Emory University.
These discoveries prompted local researchers to investigate whether lone star ticks in rural, woodsy central Georgia were carrying HRTV.
Lead study author Yamila Romer, MD, an infectious disease clinician and microbiologist in the department of environmental sciences at Emory University in Atlanta, and her colleagues collected samples of ticks in 2018 at 26 sites near the location of the patient who died and the seropositive deer. In 2019, they focused their collections on the two sites that had provided the most ticks in 2018.
From April to October in both years, the research team visited sites weekly to swish white flannel flags through underbrush. They picked off adult and nymph Amblyomma americanum ticks, placed them into vials, and transported them to their lab. They sorted 9,294 ticks by sex, life stage, and collection site. Then they crushed the ticks and extracted their RNA.
To confirm viral infection, the team tested RNA extracted from cell culture supernatants using a real-time polymerase chain reaction test specific for HRTV.
In the three pools of ticks that tested positive for HRTV, the researchers found a minimum infection rate of 0.46/1,000 ticks, suggesting that about 1 of every 2,000 ticks carried HRTV. They sequenced the genome of the three isolates and found that the genomes were similar to one another but were very different from the genomes from HRTV samples taken outside Georgia.
Catherine A. Hill, PhD, a professor of entomology and vector biology and the interim head of the department of entomology at Purdue University in West Lafayette, Ind., was impressed with the researchers’ discovery.
“Heartland virus is difficult to detect,” she said in an email. “The prevalence of human cases is low, and the virus appears to be present at very low levels in populations of lone star tick. The investigators went to some lengths to survey for the virus, collect, and process thousands of ticks – and they found the needle in the haystack.” Dr. Hill was not involved in the study.
Georgia data help researchers monitor HRTV spread
HRTV was first identified in 2009 in Missouri in two people hospitalized with fever, muscle pain, diarrhea, and low white blood cell and platelet counts. Researchers traced the infections to lone star ticks, and they found antibodies to the virus in blood samples from deer and other wild mammals.
According to the CDC, U.S. cases of tick-borne diseases more than doubled between 2004 and 2016. As of January 2021, more than 50 human cases of HRTV disease had been reported in 11 Midwestern and Southeastern states: Arkansas, Georgia, Illinois, Indiana, Iowa, Kansas, Kentucky, Missouri, North Carolina, Oklahoma, and Tennessee.
Precautions, signs, symptoms, testing, and treatment
“The lone star tick is aggressive and will actively seek out a human host to bite,” Dr. Hill noted.
She recommends that health care providers advise patients to avoid tick habitat, wear protective clothing, apply repellants, know the signs and symptoms of tick-borne disease, and seek immediate medical care if they become ill.
Common symptoms of HRTV disease include fatigue, fever, nausea, diarrhea, and anorexia. Treatment is supportive. Many patients have been hospitalized, and some with comorbidities have died.
HRTV infection is rarely tested for, and the disease burden is unknown. With no commercial tests available in the United States, the CDC performs molecular and serologic testing for HRTV infection. The agency advises doctors to contact their state health department if they suspect a patient may have HRTV disease.
Further research is needed
Samantha M. Wisely, PhD, a professor of wildlife ecology and the director of the Cervidae Health Research Initiative at the University of Florida in Gainesville, was not surprised by the study finding.
“The more we look for heartland virus, the more places we find it,” Dr. Wisely told this news organization in an email.
“Little is known about which wildlife play a role in maintaining the virus on the landscape,” said Dr. Wisely, who was not involved in the study. “White-tailed deer have been shown to produce antibodies, meaning they have been exposed to the virus, but no one has actually found the virus in a wildlife species.”
The whole-genome sequencing of the virus was particularly important, Dr. Wisely explained. “Whole-genome data allow researchers to better understand viral evolution, pathogenicity, and viral dynamics across space and time – how it is evolving.”
The study was supported by a grant from the Emory University Research Council. The authors, Dr. Wisely, and Dr. Hill have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EMERGING INFECTIOUS DISEASES
Treat or refer? New primary care flow diagrams for allergy patients
Most patients with allergy problems first see PCPs, not allergists, the authors write in Allergy. The new flow diagrams help PCPs treat anaphylaxis, asthma, drug allergy, food allergy, and urticaria.
“The European Academy of Allergy and Clinical Immunology established the Logogram Task Force to create a set of simple flow diagrams to assist allergy nonspecialist, generalist, and primary care teams in the diagnosis of five common allergic diseases encountered in primary care,” lead author Dermot Ryan, MB BCh, BAO, FRGCP, of the University of Edinburgh told this news organization.
“The source documents were mainstream guidelines coupled with ancillary literature,” he added in an email. “A multi-disciplinary taskforce ... distilled these guidelines into accessible, comprehensible, usable, and context-specific flow diagrams.”
The flow diagrams developed in Europe can be used by providers in the United States and elsewhere
“These diagrams are consistent with practices in the U.S.,” Christina E. Ciaccio, MD, an associate professor of pediatrics and the section chief of pediatric allergy and immunology at the University of Chicago Medicine, said in an email. “They will prove helpful to PCPs in the U.S. and elsewhere, particularly to young physicians new to practice.
“Treating allergies is part of the ‘bread-and-butter’ practice of primary care physicians in the U.S.,” Dr. Ciaccio, who was not involved in developing the flow diagrams, explained. “Up to 30% of Americans are atopic, and the vast majority seek treatment advice from their PCP first.”
The flow diagrams can help providers in developing countries, where allergic diseases are common, provide the best patient care possible, she said.
At some point, a PCP may need to think beyond flow diagrams and refer the patient to an allergist
“If the treatment plan for a patient falls outside first- or second-line medications, or if a diagnosis is unclear with preliminary testing, a PCP may reach out to an allergy/immunology specialist to assist in providing care,” Dr. Ciaccio advised. “Allergists may provide treatment options, such as immunotherapy, that the PCP does not offer. PCPs also often reach out to allergy team members for help with patients whose allergies are not ‘run-of-the-mill.’
“The flow diagrams are complex and may not be practical in the middle of a busy clinic,” she cautioned. “However, when a patient comes into a primary care clinic with an atypical presentation of an allergic disease, the diagrams are likely to help a physician feel confident that an allergist is the right physician for consultation.”
Patricia Lynne Lugar, MD, an associate professor of medicine in pulmonary, allergy, and critical care medicine at Duke University in Durham, N.C., noted that providers in the U.S. can use the flow diagrams because the definitions, differential diagnosis, and treatments for the conditions they cover are similar.
“The flow diagrams are comprehensive, and they attempt to condense a great deal of information into summary points. They are very useful in the U.S., and not just for generalists,” Dr. Lugar, who also was not involved in the project, said. “Even emergency rooms would benefit from these flow diagrams, especially regarding the recognition of symptoms and differential diagnosis.”
Asthma and seasonal and environmental allergies are often managed by PCPs, and the flow diagrams would help them decide when to refer their patients to an allergist, she added in an email.
Dr. Lugar advises PCPs to “recognize the symptoms of an allergic condition, offer treatment based on confidence the diagnosis is correct, and offer a referral for testing to confirm the allergy.
“Because 50% or more of asthmatics are allergic, all asthmatics should be offered an allergy evaluation to determine their allergies and avoid exacerbating the asthma,” she added. “I do not see the flow diagrams as comprehensive enough to manage chronic urticaria, asthma, venom allergy, and drug allergy.”
With food allergy, environmental allergy, venom allergy, or anaphylaxis, “allergists are experts at considering the differential diagnosis and providing the next steps in the diagnostic workup,” Dr. Lugar said. “Allergists can also provide special treatments, such as allergen-specific immunotherapy or desensitization.”
The flow diagrams guide nonspecialists in diagnosis and treatment of their patients with allergy, with supplementary information as needed. The diagrams recommend referral to a specialist when appropriate, as in cases of anaphylaxis, or chronic urticaria.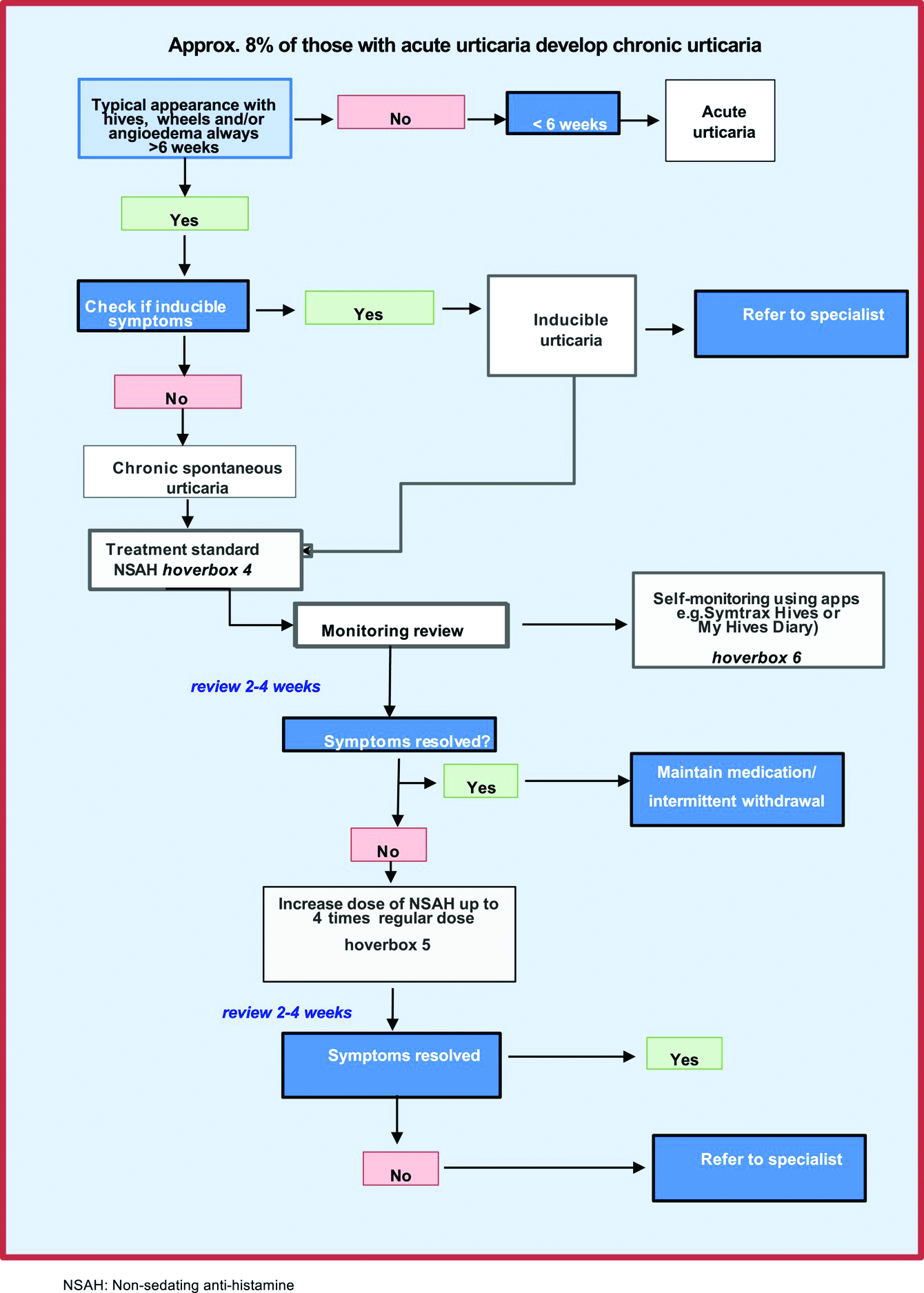
The task force was funded by EAACI. Dr. Ryan and several other authors report financial relationships with pharmaceutical companies. Dr. Ciaccio and Dr. Lugar report no such relationships.
A version of this article first appeared on Medscape.com.
Most patients with allergy problems first see PCPs, not allergists, the authors write in Allergy. The new flow diagrams help PCPs treat anaphylaxis, asthma, drug allergy, food allergy, and urticaria.
“The European Academy of Allergy and Clinical Immunology established the Logogram Task Force to create a set of simple flow diagrams to assist allergy nonspecialist, generalist, and primary care teams in the diagnosis of five common allergic diseases encountered in primary care,” lead author Dermot Ryan, MB BCh, BAO, FRGCP, of the University of Edinburgh told this news organization.
“The source documents were mainstream guidelines coupled with ancillary literature,” he added in an email. “A multi-disciplinary taskforce ... distilled these guidelines into accessible, comprehensible, usable, and context-specific flow diagrams.”
The flow diagrams developed in Europe can be used by providers in the United States and elsewhere
“These diagrams are consistent with practices in the U.S.,” Christina E. Ciaccio, MD, an associate professor of pediatrics and the section chief of pediatric allergy and immunology at the University of Chicago Medicine, said in an email. “They will prove helpful to PCPs in the U.S. and elsewhere, particularly to young physicians new to practice.
“Treating allergies is part of the ‘bread-and-butter’ practice of primary care physicians in the U.S.,” Dr. Ciaccio, who was not involved in developing the flow diagrams, explained. “Up to 30% of Americans are atopic, and the vast majority seek treatment advice from their PCP first.”
The flow diagrams can help providers in developing countries, where allergic diseases are common, provide the best patient care possible, she said.
At some point, a PCP may need to think beyond flow diagrams and refer the patient to an allergist
“If the treatment plan for a patient falls outside first- or second-line medications, or if a diagnosis is unclear with preliminary testing, a PCP may reach out to an allergy/immunology specialist to assist in providing care,” Dr. Ciaccio advised. “Allergists may provide treatment options, such as immunotherapy, that the PCP does not offer. PCPs also often reach out to allergy team members for help with patients whose allergies are not ‘run-of-the-mill.’
“The flow diagrams are complex and may not be practical in the middle of a busy clinic,” she cautioned. “However, when a patient comes into a primary care clinic with an atypical presentation of an allergic disease, the diagrams are likely to help a physician feel confident that an allergist is the right physician for consultation.”
Patricia Lynne Lugar, MD, an associate professor of medicine in pulmonary, allergy, and critical care medicine at Duke University in Durham, N.C., noted that providers in the U.S. can use the flow diagrams because the definitions, differential diagnosis, and treatments for the conditions they cover are similar.
“The flow diagrams are comprehensive, and they attempt to condense a great deal of information into summary points. They are very useful in the U.S., and not just for generalists,” Dr. Lugar, who also was not involved in the project, said. “Even emergency rooms would benefit from these flow diagrams, especially regarding the recognition of symptoms and differential diagnosis.”
Asthma and seasonal and environmental allergies are often managed by PCPs, and the flow diagrams would help them decide when to refer their patients to an allergist, she added in an email.
Dr. Lugar advises PCPs to “recognize the symptoms of an allergic condition, offer treatment based on confidence the diagnosis is correct, and offer a referral for testing to confirm the allergy.
“Because 50% or more of asthmatics are allergic, all asthmatics should be offered an allergy evaluation to determine their allergies and avoid exacerbating the asthma,” she added. “I do not see the flow diagrams as comprehensive enough to manage chronic urticaria, asthma, venom allergy, and drug allergy.”
With food allergy, environmental allergy, venom allergy, or anaphylaxis, “allergists are experts at considering the differential diagnosis and providing the next steps in the diagnostic workup,” Dr. Lugar said. “Allergists can also provide special treatments, such as allergen-specific immunotherapy or desensitization.”
The flow diagrams guide nonspecialists in diagnosis and treatment of their patients with allergy, with supplementary information as needed. The diagrams recommend referral to a specialist when appropriate, as in cases of anaphylaxis, or chronic urticaria.
The task force was funded by EAACI. Dr. Ryan and several other authors report financial relationships with pharmaceutical companies. Dr. Ciaccio and Dr. Lugar report no such relationships.
A version of this article first appeared on Medscape.com.
Most patients with allergy problems first see PCPs, not allergists, the authors write in Allergy. The new flow diagrams help PCPs treat anaphylaxis, asthma, drug allergy, food allergy, and urticaria.
“The European Academy of Allergy and Clinical Immunology established the Logogram Task Force to create a set of simple flow diagrams to assist allergy nonspecialist, generalist, and primary care teams in the diagnosis of five common allergic diseases encountered in primary care,” lead author Dermot Ryan, MB BCh, BAO, FRGCP, of the University of Edinburgh told this news organization.
“The source documents were mainstream guidelines coupled with ancillary literature,” he added in an email. “A multi-disciplinary taskforce ... distilled these guidelines into accessible, comprehensible, usable, and context-specific flow diagrams.”
The flow diagrams developed in Europe can be used by providers in the United States and elsewhere
“These diagrams are consistent with practices in the U.S.,” Christina E. Ciaccio, MD, an associate professor of pediatrics and the section chief of pediatric allergy and immunology at the University of Chicago Medicine, said in an email. “They will prove helpful to PCPs in the U.S. and elsewhere, particularly to young physicians new to practice.
“Treating allergies is part of the ‘bread-and-butter’ practice of primary care physicians in the U.S.,” Dr. Ciaccio, who was not involved in developing the flow diagrams, explained. “Up to 30% of Americans are atopic, and the vast majority seek treatment advice from their PCP first.”
The flow diagrams can help providers in developing countries, where allergic diseases are common, provide the best patient care possible, she said.
At some point, a PCP may need to think beyond flow diagrams and refer the patient to an allergist
“If the treatment plan for a patient falls outside first- or second-line medications, or if a diagnosis is unclear with preliminary testing, a PCP may reach out to an allergy/immunology specialist to assist in providing care,” Dr. Ciaccio advised. “Allergists may provide treatment options, such as immunotherapy, that the PCP does not offer. PCPs also often reach out to allergy team members for help with patients whose allergies are not ‘run-of-the-mill.’
“The flow diagrams are complex and may not be practical in the middle of a busy clinic,” she cautioned. “However, when a patient comes into a primary care clinic with an atypical presentation of an allergic disease, the diagrams are likely to help a physician feel confident that an allergist is the right physician for consultation.”
Patricia Lynne Lugar, MD, an associate professor of medicine in pulmonary, allergy, and critical care medicine at Duke University in Durham, N.C., noted that providers in the U.S. can use the flow diagrams because the definitions, differential diagnosis, and treatments for the conditions they cover are similar.
“The flow diagrams are comprehensive, and they attempt to condense a great deal of information into summary points. They are very useful in the U.S., and not just for generalists,” Dr. Lugar, who also was not involved in the project, said. “Even emergency rooms would benefit from these flow diagrams, especially regarding the recognition of symptoms and differential diagnosis.”
Asthma and seasonal and environmental allergies are often managed by PCPs, and the flow diagrams would help them decide when to refer their patients to an allergist, she added in an email.
Dr. Lugar advises PCPs to “recognize the symptoms of an allergic condition, offer treatment based on confidence the diagnosis is correct, and offer a referral for testing to confirm the allergy.
“Because 50% or more of asthmatics are allergic, all asthmatics should be offered an allergy evaluation to determine their allergies and avoid exacerbating the asthma,” she added. “I do not see the flow diagrams as comprehensive enough to manage chronic urticaria, asthma, venom allergy, and drug allergy.”
With food allergy, environmental allergy, venom allergy, or anaphylaxis, “allergists are experts at considering the differential diagnosis and providing the next steps in the diagnostic workup,” Dr. Lugar said. “Allergists can also provide special treatments, such as allergen-specific immunotherapy or desensitization.”
The flow diagrams guide nonspecialists in diagnosis and treatment of their patients with allergy, with supplementary information as needed. The diagrams recommend referral to a specialist when appropriate, as in cases of anaphylaxis, or chronic urticaria.
The task force was funded by EAACI. Dr. Ryan and several other authors report financial relationships with pharmaceutical companies. Dr. Ciaccio and Dr. Lugar report no such relationships.
A version of this article first appeared on Medscape.com.
FROM ALLERGY
Children and teens with food allergies face quality-of-life issues
Children and adolescents with food allergies appear to fare worse physically, socially, and emotionally, and have poorer overall health-related quality of life (HRQL) than their food allergy–free peers, a new systematic review suggests.
“Findings from the current review suggest that food allergy has a negative impact on the HRQL of children and teens, particularly older children and those with severe food allergy,” the authors wrote. “By comparison, the link between food allergy and psychosocial functioning is less clear.
“Evidence from the qualitative literature suggests that the burden of childhood food allergy largely stems from worries surrounding exposures outside of the home and the social consequences of the condition,” they added.
Lead study author Michael A. Golding, a research coordinator at Children’s Hospital Research Institute of Manitoba in Winnipeg, Canada, and colleagues searched PubMed, Scopus, PsycInfo, and CINAHL (Cumulative Index to Nursing and Allied Health Literature) databases on several days between November 2019 and March 2021 for peer-reviewed articles published in English in any year.
They reviewed articles focused on HRQL, psychological health, or social well-being in children and teens with food allergy from birth through 19 years of age. Food allergy comprised both immunoglobulin E (IgE)-mediated food allergies and non-IgE-mediated allergies, including food protein–induced enterocolitis, enteropathy, and proctocolitis.
From the 3,789 publications the researchers screened, they included 8,202 patients in 45 studies in their quantitative synthesis and 186 patients in 9 studies in their qualitative synthesis. Using a segregated, mixed research synthesis design, they analyzed and synthesized the quantitative and qualitative articles separately, then integrated those findings.
Navigating through many challenges
The authors found that food allergy lowered the young people’s HRQL. In 11 of the 14 studies (78%) that included a comparison group, young patients with food allergy showed significantly lower HRQL in at least one domain. Most significant differences occurred in domains related to total HRQL (66%), social functioning (58%), emotional functioning (54%), and physical functioning (54%).
Parents were often more likely than their children to perceive that the child’s food allergy was causing problems.
Between 20% and 32% of children reported bullying related to their food allergy. Many children reported that their allergy sometimes isolated them from their classmates.
Many children described feeling comfortable at home but worried in places where they had less control, such as school, restaurants, or when traveling.
Children and teens tended to downplay their limitations and the negative impacts of their condition.
Older children who had been diagnosed early in life tended to accept managing their food allergy as a way of life, whereas those diagnosed when they were older reported the need to adapt, accept, and grieve the loss of foods and experiences.
“This study highlights the importance of addressing the underlying impact that food allergy can have on patients’ mental health and social functioning,” Kelly Marie O’Shea, MD, assistant professor of allergy and immunology at University of Michigan Health in Ann Arbor, said in an interview.
“While it has been shown previously that food-allergic patients have lower HRQL, this systematic review aptly reveals that for children and teens with food allergy, overall quality of life, including psychosocial functioning, can also be negatively affected,” said Dr. O’Shea, who was not involved in the study.
“Symptoms of anxiety and depression are reported at higher rates in the food-allergic population, and social limitations have been shown to play a role,” she explained. “However, as revealed in this study, longitudinal and appropriately controlled studies to investigate the impact of food allergy on psychosocial outcomes in children and teens are scarce.”
Robert Alan Wood, MD, professor of pediatrics at Johns Hopkins University and director of pediatric allergy and immunology at Johns Hopkins Children’s Center, Baltimore, told this news organization that the effects of food allergy on mental health are not fully appreciated by the public or by many clinicians.
“These findings emphasize the need to recognize the emotional consequences of food allergy and to take steps to be proactive in managing these issues among our patients,” said Dr. Wood, who was not associated with the study.
More research is needed
The authors noted that more research is needed to examine links between food allergy, HRQL, and psychosocial outcome; links between food allergy and bullying; and how challenges change over time. They recommend exploring the relative impacts of specific types of food allergy and whether specific traits in young people with food allergy make them more susceptible to its psychological effects. They also call for efforts to identify and help young people with food allergy overcome their many challenges.
The study was funded by the Canadian Institutes for Health Research, the Children’s Hospital Research Institute of Manitoba, and the University of Manitoba.
Study senior author Jennifer L. P. Protudjer, PhD, reported involvement with Canada’s National Food Allergy Action Plan and Allied Health at the Canadian Society of Allergy and Clinical Immunology, and receipt of fees from Novartis. The remaining authors, as well as Dr. O’Shea and Dr. Wood, reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children and adolescents with food allergies appear to fare worse physically, socially, and emotionally, and have poorer overall health-related quality of life (HRQL) than their food allergy–free peers, a new systematic review suggests.
“Findings from the current review suggest that food allergy has a negative impact on the HRQL of children and teens, particularly older children and those with severe food allergy,” the authors wrote. “By comparison, the link between food allergy and psychosocial functioning is less clear.
“Evidence from the qualitative literature suggests that the burden of childhood food allergy largely stems from worries surrounding exposures outside of the home and the social consequences of the condition,” they added.
Lead study author Michael A. Golding, a research coordinator at Children’s Hospital Research Institute of Manitoba in Winnipeg, Canada, and colleagues searched PubMed, Scopus, PsycInfo, and CINAHL (Cumulative Index to Nursing and Allied Health Literature) databases on several days between November 2019 and March 2021 for peer-reviewed articles published in English in any year.
They reviewed articles focused on HRQL, psychological health, or social well-being in children and teens with food allergy from birth through 19 years of age. Food allergy comprised both immunoglobulin E (IgE)-mediated food allergies and non-IgE-mediated allergies, including food protein–induced enterocolitis, enteropathy, and proctocolitis.
From the 3,789 publications the researchers screened, they included 8,202 patients in 45 studies in their quantitative synthesis and 186 patients in 9 studies in their qualitative synthesis. Using a segregated, mixed research synthesis design, they analyzed and synthesized the quantitative and qualitative articles separately, then integrated those findings.
Navigating through many challenges
The authors found that food allergy lowered the young people’s HRQL. In 11 of the 14 studies (78%) that included a comparison group, young patients with food allergy showed significantly lower HRQL in at least one domain. Most significant differences occurred in domains related to total HRQL (66%), social functioning (58%), emotional functioning (54%), and physical functioning (54%).
Parents were often more likely than their children to perceive that the child’s food allergy was causing problems.
Between 20% and 32% of children reported bullying related to their food allergy. Many children reported that their allergy sometimes isolated them from their classmates.
Many children described feeling comfortable at home but worried in places where they had less control, such as school, restaurants, or when traveling.
Children and teens tended to downplay their limitations and the negative impacts of their condition.
Older children who had been diagnosed early in life tended to accept managing their food allergy as a way of life, whereas those diagnosed when they were older reported the need to adapt, accept, and grieve the loss of foods and experiences.
“This study highlights the importance of addressing the underlying impact that food allergy can have on patients’ mental health and social functioning,” Kelly Marie O’Shea, MD, assistant professor of allergy and immunology at University of Michigan Health in Ann Arbor, said in an interview.
“While it has been shown previously that food-allergic patients have lower HRQL, this systematic review aptly reveals that for children and teens with food allergy, overall quality of life, including psychosocial functioning, can also be negatively affected,” said Dr. O’Shea, who was not involved in the study.
“Symptoms of anxiety and depression are reported at higher rates in the food-allergic population, and social limitations have been shown to play a role,” she explained. “However, as revealed in this study, longitudinal and appropriately controlled studies to investigate the impact of food allergy on psychosocial outcomes in children and teens are scarce.”
Robert Alan Wood, MD, professor of pediatrics at Johns Hopkins University and director of pediatric allergy and immunology at Johns Hopkins Children’s Center, Baltimore, told this news organization that the effects of food allergy on mental health are not fully appreciated by the public or by many clinicians.
“These findings emphasize the need to recognize the emotional consequences of food allergy and to take steps to be proactive in managing these issues among our patients,” said Dr. Wood, who was not associated with the study.
More research is needed
The authors noted that more research is needed to examine links between food allergy, HRQL, and psychosocial outcome; links between food allergy and bullying; and how challenges change over time. They recommend exploring the relative impacts of specific types of food allergy and whether specific traits in young people with food allergy make them more susceptible to its psychological effects. They also call for efforts to identify and help young people with food allergy overcome their many challenges.
The study was funded by the Canadian Institutes for Health Research, the Children’s Hospital Research Institute of Manitoba, and the University of Manitoba.
Study senior author Jennifer L. P. Protudjer, PhD, reported involvement with Canada’s National Food Allergy Action Plan and Allied Health at the Canadian Society of Allergy and Clinical Immunology, and receipt of fees from Novartis. The remaining authors, as well as Dr. O’Shea and Dr. Wood, reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children and adolescents with food allergies appear to fare worse physically, socially, and emotionally, and have poorer overall health-related quality of life (HRQL) than their food allergy–free peers, a new systematic review suggests.
“Findings from the current review suggest that food allergy has a negative impact on the HRQL of children and teens, particularly older children and those with severe food allergy,” the authors wrote. “By comparison, the link between food allergy and psychosocial functioning is less clear.
“Evidence from the qualitative literature suggests that the burden of childhood food allergy largely stems from worries surrounding exposures outside of the home and the social consequences of the condition,” they added.
Lead study author Michael A. Golding, a research coordinator at Children’s Hospital Research Institute of Manitoba in Winnipeg, Canada, and colleagues searched PubMed, Scopus, PsycInfo, and CINAHL (Cumulative Index to Nursing and Allied Health Literature) databases on several days between November 2019 and March 2021 for peer-reviewed articles published in English in any year.
They reviewed articles focused on HRQL, psychological health, or social well-being in children and teens with food allergy from birth through 19 years of age. Food allergy comprised both immunoglobulin E (IgE)-mediated food allergies and non-IgE-mediated allergies, including food protein–induced enterocolitis, enteropathy, and proctocolitis.
From the 3,789 publications the researchers screened, they included 8,202 patients in 45 studies in their quantitative synthesis and 186 patients in 9 studies in their qualitative synthesis. Using a segregated, mixed research synthesis design, they analyzed and synthesized the quantitative and qualitative articles separately, then integrated those findings.
Navigating through many challenges
The authors found that food allergy lowered the young people’s HRQL. In 11 of the 14 studies (78%) that included a comparison group, young patients with food allergy showed significantly lower HRQL in at least one domain. Most significant differences occurred in domains related to total HRQL (66%), social functioning (58%), emotional functioning (54%), and physical functioning (54%).
Parents were often more likely than their children to perceive that the child’s food allergy was causing problems.
Between 20% and 32% of children reported bullying related to their food allergy. Many children reported that their allergy sometimes isolated them from their classmates.
Many children described feeling comfortable at home but worried in places where they had less control, such as school, restaurants, or when traveling.
Children and teens tended to downplay their limitations and the negative impacts of their condition.
Older children who had been diagnosed early in life tended to accept managing their food allergy as a way of life, whereas those diagnosed when they were older reported the need to adapt, accept, and grieve the loss of foods and experiences.
“This study highlights the importance of addressing the underlying impact that food allergy can have on patients’ mental health and social functioning,” Kelly Marie O’Shea, MD, assistant professor of allergy and immunology at University of Michigan Health in Ann Arbor, said in an interview.
“While it has been shown previously that food-allergic patients have lower HRQL, this systematic review aptly reveals that for children and teens with food allergy, overall quality of life, including psychosocial functioning, can also be negatively affected,” said Dr. O’Shea, who was not involved in the study.
“Symptoms of anxiety and depression are reported at higher rates in the food-allergic population, and social limitations have been shown to play a role,” she explained. “However, as revealed in this study, longitudinal and appropriately controlled studies to investigate the impact of food allergy on psychosocial outcomes in children and teens are scarce.”
Robert Alan Wood, MD, professor of pediatrics at Johns Hopkins University and director of pediatric allergy and immunology at Johns Hopkins Children’s Center, Baltimore, told this news organization that the effects of food allergy on mental health are not fully appreciated by the public or by many clinicians.
“These findings emphasize the need to recognize the emotional consequences of food allergy and to take steps to be proactive in managing these issues among our patients,” said Dr. Wood, who was not associated with the study.
More research is needed
The authors noted that more research is needed to examine links between food allergy, HRQL, and psychosocial outcome; links between food allergy and bullying; and how challenges change over time. They recommend exploring the relative impacts of specific types of food allergy and whether specific traits in young people with food allergy make them more susceptible to its psychological effects. They also call for efforts to identify and help young people with food allergy overcome their many challenges.
The study was funded by the Canadian Institutes for Health Research, the Children’s Hospital Research Institute of Manitoba, and the University of Manitoba.
Study senior author Jennifer L. P. Protudjer, PhD, reported involvement with Canada’s National Food Allergy Action Plan and Allied Health at the Canadian Society of Allergy and Clinical Immunology, and receipt of fees from Novartis. The remaining authors, as well as Dr. O’Shea and Dr. Wood, reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cancer of the appendix on the rise in younger patients with appendicitis
Younger patients with appendicitis appear to be increasingly likely to have cancer of the appendix, a new study suggests.
While acute appendicitis can often be managed with antibiotics instead of surgery, patients who do not have surgery need to be closely followed to avoid missed diagnoses, the authors write.
“The most important finding is that between 2004 and 2017, relative to right-sided colon cancer, the number of appendiceal cancer cases increased,” said Mustafa Raoof, MD, who was not involved in the study. He is a surgical oncologist and an assistant professor of surgical oncology at City of Hope Comprehensive Cancer Center, Duarte, Calif. “Approximately one-fourth of these were carcinoid tumors, and this category saw the greatest increase, particularly in individuals under 50 years of age.”
“There is a push to treat acute appendicitis with only antibiotics to avoid surgery,” Dr. Raoof told this news organization by email. “One underappreciated downside of this strategy is that an appendiceal cancer can be missed if the appendix is not removed.”
As reported in Journal of the American College of Surgeons, lead study author Michelle C. Salazar, MD, MHS, a general surgery resident at Yale School of Medicine in New Haven, Conn., and her colleagues conducted a retrospective analysis of the National Cancer Database (NCDB). The NCDB contains hospital registry data of around 70% of new cancer diagnoses in the United States.
The researchers extracted data on patients aged 18 years and older who were diagnosed with right-sided colon cancer between 2004 and 2017 and who underwent appendectomy. The authors also identified all patients with appendiceal cancer. They divided that group into patients with carcinoid tumors and those with other types of appendiceal cancer. They also investigated trends by age group.
Of 387,867 patients with right-sided colon cancer, 19,570 (5%) had appendiceal cancer, and 5,628 of those patients (29%) had carcinoid tumor. The odds of appendiceal cancer, compared with other right-sided colon cancers, rose over the years studied (odds ratio, 2.56; 95% confidence interval, 2.35-2.79).
This increase in odds occurred in all age groups but was steepest among patients between 40 and 49 years of age, among whom it increased from 10% in 2004 (95% CI, 9-12) to 18% in 2017 (95% CI, 16-20; P < .001).
Odds of appendiceal carcinoid, compared with other appendiceal cancers, also rose during this period (OR, 1.70; 95% CI, 1.40-2.07). The sharpest increase occurred in the likelihood of carcinoid in patients younger than 40, from 24% in 2004 (95% CI, 15-34) to 45% in 2017 (95% CI, 37-53; P < .001).
“The findings in this study are yet another reason to temper excitement about nonoperative management of acute appendicitis in adults,” Richard S. Hoehn, MD, a surgical oncologist at University Hospitals Seidman Cancer Center, Cleveland, said. “The study is important because it reveals a potential risk of deferring appendectomy in adult patients with appendiceal pathology.”
“As our pathologic examinations of appendiceal cancers improve, we are gaining better understanding of these tumors and how to best manage these patients,” Dr. Hoehn, who also was not involved in the study, added in an email. “The findings should make surgeons more suspicious of malignant causes of acute appendicitis in adults, especially those under 40. Colonoscopy and appendectomy are necessary in adult patients with appendicitis.”
Gregory Botta, MD, PhD, a medical oncologist and associate professor of medicine at the University of California San Diego Health, mentioned limitations of the study in an email. They include the NCDB’s omission of around 30% of cases nationally and the study’s omission of patients diagnosed with primary appendiceal tumor, as well as patients who underwent total colectomies and those who did not have surgery.
“Appendiceal tumors are not normally seen during colonoscopies; screening colonoscopies are not recommended to younger patients under 40, and there is less surgical management for appendicitis,” explained Dr. Botta, who also was not involved in the study. “Thus, the cause of the increasing incidence is not due to increased detection on surveillance.
“Gastrointestinal oncologists are seeing an increase in colon cancer in younger patients and a parallel increase in appendix tumors. While carcinoid tumors are usually found localized and [are] therefore curable postsurgical resection, adenocarcinoma or mucinous subtypes tend to be more diffuse, metastatic, and noncurable,” he added. “Although the increase in colon cancer is being found in our younger population, the authors hint at causes not captured by the NCDB, including environmental exposures or each patient’s diet.”
“There is no good answer as to why there is an increase in carcinoids,” Dr. Salazar said in a press release. “It could be due to environmental reasons, or it could be due to better diagnostics technology.”
The researchers and independent experts share concerns that, because surgery provides the only definitive diagnosis of appendiceal cancer, among patients managed nonsurgically, there may be a delay in cancer diagnosis.
“I would counsel patients based on age,” Dr. Salazar added. “If you’re older, you are at less risk for appendiceal cancer and greater risk for complications from surgery. Younger, healthy patients are more likely to be able to tolerate an operation and may want to rule out cancer by undergoing the operation. The characteristics of appendicitis should be considered in the decision.”
Funding information was not provided. The authors, Dr. Raoof, Dr. Hoehn, and Dr. Botta have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Younger patients with appendicitis appear to be increasingly likely to have cancer of the appendix, a new study suggests.
While acute appendicitis can often be managed with antibiotics instead of surgery, patients who do not have surgery need to be closely followed to avoid missed diagnoses, the authors write.
“The most important finding is that between 2004 and 2017, relative to right-sided colon cancer, the number of appendiceal cancer cases increased,” said Mustafa Raoof, MD, who was not involved in the study. He is a surgical oncologist and an assistant professor of surgical oncology at City of Hope Comprehensive Cancer Center, Duarte, Calif. “Approximately one-fourth of these were carcinoid tumors, and this category saw the greatest increase, particularly in individuals under 50 years of age.”
“There is a push to treat acute appendicitis with only antibiotics to avoid surgery,” Dr. Raoof told this news organization by email. “One underappreciated downside of this strategy is that an appendiceal cancer can be missed if the appendix is not removed.”
As reported in Journal of the American College of Surgeons, lead study author Michelle C. Salazar, MD, MHS, a general surgery resident at Yale School of Medicine in New Haven, Conn., and her colleagues conducted a retrospective analysis of the National Cancer Database (NCDB). The NCDB contains hospital registry data of around 70% of new cancer diagnoses in the United States.
The researchers extracted data on patients aged 18 years and older who were diagnosed with right-sided colon cancer between 2004 and 2017 and who underwent appendectomy. The authors also identified all patients with appendiceal cancer. They divided that group into patients with carcinoid tumors and those with other types of appendiceal cancer. They also investigated trends by age group.
Of 387,867 patients with right-sided colon cancer, 19,570 (5%) had appendiceal cancer, and 5,628 of those patients (29%) had carcinoid tumor. The odds of appendiceal cancer, compared with other right-sided colon cancers, rose over the years studied (odds ratio, 2.56; 95% confidence interval, 2.35-2.79).
This increase in odds occurred in all age groups but was steepest among patients between 40 and 49 years of age, among whom it increased from 10% in 2004 (95% CI, 9-12) to 18% in 2017 (95% CI, 16-20; P < .001).
Odds of appendiceal carcinoid, compared with other appendiceal cancers, also rose during this period (OR, 1.70; 95% CI, 1.40-2.07). The sharpest increase occurred in the likelihood of carcinoid in patients younger than 40, from 24% in 2004 (95% CI, 15-34) to 45% in 2017 (95% CI, 37-53; P < .001).
“The findings in this study are yet another reason to temper excitement about nonoperative management of acute appendicitis in adults,” Richard S. Hoehn, MD, a surgical oncologist at University Hospitals Seidman Cancer Center, Cleveland, said. “The study is important because it reveals a potential risk of deferring appendectomy in adult patients with appendiceal pathology.”
“As our pathologic examinations of appendiceal cancers improve, we are gaining better understanding of these tumors and how to best manage these patients,” Dr. Hoehn, who also was not involved in the study, added in an email. “The findings should make surgeons more suspicious of malignant causes of acute appendicitis in adults, especially those under 40. Colonoscopy and appendectomy are necessary in adult patients with appendicitis.”
Gregory Botta, MD, PhD, a medical oncologist and associate professor of medicine at the University of California San Diego Health, mentioned limitations of the study in an email. They include the NCDB’s omission of around 30% of cases nationally and the study’s omission of patients diagnosed with primary appendiceal tumor, as well as patients who underwent total colectomies and those who did not have surgery.
“Appendiceal tumors are not normally seen during colonoscopies; screening colonoscopies are not recommended to younger patients under 40, and there is less surgical management for appendicitis,” explained Dr. Botta, who also was not involved in the study. “Thus, the cause of the increasing incidence is not due to increased detection on surveillance.
“Gastrointestinal oncologists are seeing an increase in colon cancer in younger patients and a parallel increase in appendix tumors. While carcinoid tumors are usually found localized and [are] therefore curable postsurgical resection, adenocarcinoma or mucinous subtypes tend to be more diffuse, metastatic, and noncurable,” he added. “Although the increase in colon cancer is being found in our younger population, the authors hint at causes not captured by the NCDB, including environmental exposures or each patient’s diet.”
“There is no good answer as to why there is an increase in carcinoids,” Dr. Salazar said in a press release. “It could be due to environmental reasons, or it could be due to better diagnostics technology.”
The researchers and independent experts share concerns that, because surgery provides the only definitive diagnosis of appendiceal cancer, among patients managed nonsurgically, there may be a delay in cancer diagnosis.
“I would counsel patients based on age,” Dr. Salazar added. “If you’re older, you are at less risk for appendiceal cancer and greater risk for complications from surgery. Younger, healthy patients are more likely to be able to tolerate an operation and may want to rule out cancer by undergoing the operation. The characteristics of appendicitis should be considered in the decision.”
Funding information was not provided. The authors, Dr. Raoof, Dr. Hoehn, and Dr. Botta have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Younger patients with appendicitis appear to be increasingly likely to have cancer of the appendix, a new study suggests.
While acute appendicitis can often be managed with antibiotics instead of surgery, patients who do not have surgery need to be closely followed to avoid missed diagnoses, the authors write.
“The most important finding is that between 2004 and 2017, relative to right-sided colon cancer, the number of appendiceal cancer cases increased,” said Mustafa Raoof, MD, who was not involved in the study. He is a surgical oncologist and an assistant professor of surgical oncology at City of Hope Comprehensive Cancer Center, Duarte, Calif. “Approximately one-fourth of these were carcinoid tumors, and this category saw the greatest increase, particularly in individuals under 50 years of age.”
“There is a push to treat acute appendicitis with only antibiotics to avoid surgery,” Dr. Raoof told this news organization by email. “One underappreciated downside of this strategy is that an appendiceal cancer can be missed if the appendix is not removed.”
As reported in Journal of the American College of Surgeons, lead study author Michelle C. Salazar, MD, MHS, a general surgery resident at Yale School of Medicine in New Haven, Conn., and her colleagues conducted a retrospective analysis of the National Cancer Database (NCDB). The NCDB contains hospital registry data of around 70% of new cancer diagnoses in the United States.
The researchers extracted data on patients aged 18 years and older who were diagnosed with right-sided colon cancer between 2004 and 2017 and who underwent appendectomy. The authors also identified all patients with appendiceal cancer. They divided that group into patients with carcinoid tumors and those with other types of appendiceal cancer. They also investigated trends by age group.
Of 387,867 patients with right-sided colon cancer, 19,570 (5%) had appendiceal cancer, and 5,628 of those patients (29%) had carcinoid tumor. The odds of appendiceal cancer, compared with other right-sided colon cancers, rose over the years studied (odds ratio, 2.56; 95% confidence interval, 2.35-2.79).
This increase in odds occurred in all age groups but was steepest among patients between 40 and 49 years of age, among whom it increased from 10% in 2004 (95% CI, 9-12) to 18% in 2017 (95% CI, 16-20; P < .001).
Odds of appendiceal carcinoid, compared with other appendiceal cancers, also rose during this period (OR, 1.70; 95% CI, 1.40-2.07). The sharpest increase occurred in the likelihood of carcinoid in patients younger than 40, from 24% in 2004 (95% CI, 15-34) to 45% in 2017 (95% CI, 37-53; P < .001).
“The findings in this study are yet another reason to temper excitement about nonoperative management of acute appendicitis in adults,” Richard S. Hoehn, MD, a surgical oncologist at University Hospitals Seidman Cancer Center, Cleveland, said. “The study is important because it reveals a potential risk of deferring appendectomy in adult patients with appendiceal pathology.”
“As our pathologic examinations of appendiceal cancers improve, we are gaining better understanding of these tumors and how to best manage these patients,” Dr. Hoehn, who also was not involved in the study, added in an email. “The findings should make surgeons more suspicious of malignant causes of acute appendicitis in adults, especially those under 40. Colonoscopy and appendectomy are necessary in adult patients with appendicitis.”
Gregory Botta, MD, PhD, a medical oncologist and associate professor of medicine at the University of California San Diego Health, mentioned limitations of the study in an email. They include the NCDB’s omission of around 30% of cases nationally and the study’s omission of patients diagnosed with primary appendiceal tumor, as well as patients who underwent total colectomies and those who did not have surgery.
“Appendiceal tumors are not normally seen during colonoscopies; screening colonoscopies are not recommended to younger patients under 40, and there is less surgical management for appendicitis,” explained Dr. Botta, who also was not involved in the study. “Thus, the cause of the increasing incidence is not due to increased detection on surveillance.
“Gastrointestinal oncologists are seeing an increase in colon cancer in younger patients and a parallel increase in appendix tumors. While carcinoid tumors are usually found localized and [are] therefore curable postsurgical resection, adenocarcinoma or mucinous subtypes tend to be more diffuse, metastatic, and noncurable,” he added. “Although the increase in colon cancer is being found in our younger population, the authors hint at causes not captured by the NCDB, including environmental exposures or each patient’s diet.”
“There is no good answer as to why there is an increase in carcinoids,” Dr. Salazar said in a press release. “It could be due to environmental reasons, or it could be due to better diagnostics technology.”
The researchers and independent experts share concerns that, because surgery provides the only definitive diagnosis of appendiceal cancer, among patients managed nonsurgically, there may be a delay in cancer diagnosis.
“I would counsel patients based on age,” Dr. Salazar added. “If you’re older, you are at less risk for appendiceal cancer and greater risk for complications from surgery. Younger, healthy patients are more likely to be able to tolerate an operation and may want to rule out cancer by undergoing the operation. The characteristics of appendicitis should be considered in the decision.”
Funding information was not provided. The authors, Dr. Raoof, Dr. Hoehn, and Dr. Botta have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF THE AMERICAN COLLEGE OF SURGEONS
Breakfast Based on Whey Protein May Help Manage Type 2 Diabetes
NEW YORK (Reuters Health) - A breakfast rich in whey protein may help people with type 2 diabetes manage their illness better, new research from Israel suggests.
"Whey protein, a byproduct of cheese manufacturing, lowers postprandial glycemia more than other protein sources," said lead author Dr. Daniela Jakubowicz from Wolfson Medical Center at Tel Aviv University."
We found that in type 2 diabetes, increasing protein content at breakfast has a greater impact on weight loss, glycated hemoglobin (HbA1C), satiety and postprandial glycemia when the protein source is whey protein, compared with other protein sources, such as eggs, tuna and soy," she told Reuters Health by email.
Dr. Jakubowicz and her group presented their findings April 1 at ENDO 2016, the annual meeting of the Endocrine Society, in Boston.
They randomly assigned 48 overweight and obese patients with type 2 diabetes to one of three isocaloric diets. Over 12 weeks, everyone ate a large breakfast, a medium-sized lunch and a small dinner, but the amount and source of each group's breakfast proteins differed.
At breakfast, the 17 participants in the whey group ate 36 g of protein as part of a whey protein shake consisting of 40% carbohydrate, 40% protein and 20% fat. The 16 participants in the high-protein group ate 36 g of protein in the form of eggs, tuna and cheese (40% carbs; 40% protein; 20% fat). The 15 in the high-carbohydrate group ate 13 g of protein in ready-to-eat cereals (65% carbs; 15% protein; 20% fat).
All three diets included a 660 kcal breakfast, a 567 cal lunch and a 276 cal dinner, with the same composition at lunch and dinner.
After 12 weeks, the participants in the whey protein group lost the most weight (7.6 kg vs. 6.1 kg for participants in the high-protein group and 3.5 kg for those in the high-carbohydrate group (p<0.0001).
Participants on the whey protein diet were less hungry during the day and had lower glucose spikes after meals compared with those on the other two diets.
The drop in HbA1C was 11.5% in the whey group, 7.7% in the protein group and 4.6% in the carbohydrate group (p<0.0001). Compared with the carbohydrate group, the percentage drop in HbA1c was greater by 41% in the protein group and by 64% in the whey group (p<0.0001).
"Whey protein was consumed only at breakfast; however, the improvement of glucose, insulin and glucagon-like peptide 1 (GLP-1) was also observed after lunch and dinner. The mechanism of this persistent beneficial effect of whey protein needs further research," Dr. Jakubowicz said.
Co-author Dr. Julio Wainstein, also at Wolfson Medical Center, added by email, "Usually, patients with type 2 diabetes are treated with a combination of several antidiabetic drugs to achieve adequate glucose regulation and decrease HbA1c. Whey protein should be considered an important adjuvant in the management of type 2 diabetes."
"Furthermore," Dr. Wainstein added, "it is possible that by adding whey protein to the diet, glucose regulation might be achieved with less medication, which is a valuable advantage in type 2 diabetes treatment."
The study had no commercial funding, and the authors declared no conflicts of interest.
NEW YORK (Reuters Health) - A breakfast rich in whey protein may help people with type 2 diabetes manage their illness better, new research from Israel suggests.
"Whey protein, a byproduct of cheese manufacturing, lowers postprandial glycemia more than other protein sources," said lead author Dr. Daniela Jakubowicz from Wolfson Medical Center at Tel Aviv University."
We found that in type 2 diabetes, increasing protein content at breakfast has a greater impact on weight loss, glycated hemoglobin (HbA1C), satiety and postprandial glycemia when the protein source is whey protein, compared with other protein sources, such as eggs, tuna and soy," she told Reuters Health by email.
Dr. Jakubowicz and her group presented their findings April 1 at ENDO 2016, the annual meeting of the Endocrine Society, in Boston.
They randomly assigned 48 overweight and obese patients with type 2 diabetes to one of three isocaloric diets. Over 12 weeks, everyone ate a large breakfast, a medium-sized lunch and a small dinner, but the amount and source of each group's breakfast proteins differed.
At breakfast, the 17 participants in the whey group ate 36 g of protein as part of a whey protein shake consisting of 40% carbohydrate, 40% protein and 20% fat. The 16 participants in the high-protein group ate 36 g of protein in the form of eggs, tuna and cheese (40% carbs; 40% protein; 20% fat). The 15 in the high-carbohydrate group ate 13 g of protein in ready-to-eat cereals (65% carbs; 15% protein; 20% fat).
All three diets included a 660 kcal breakfast, a 567 cal lunch and a 276 cal dinner, with the same composition at lunch and dinner.
After 12 weeks, the participants in the whey protein group lost the most weight (7.6 kg vs. 6.1 kg for participants in the high-protein group and 3.5 kg for those in the high-carbohydrate group (p<0.0001).
Participants on the whey protein diet were less hungry during the day and had lower glucose spikes after meals compared with those on the other two diets.
The drop in HbA1C was 11.5% in the whey group, 7.7% in the protein group and 4.6% in the carbohydrate group (p<0.0001). Compared with the carbohydrate group, the percentage drop in HbA1c was greater by 41% in the protein group and by 64% in the whey group (p<0.0001).
"Whey protein was consumed only at breakfast; however, the improvement of glucose, insulin and glucagon-like peptide 1 (GLP-1) was also observed after lunch and dinner. The mechanism of this persistent beneficial effect of whey protein needs further research," Dr. Jakubowicz said.
Co-author Dr. Julio Wainstein, also at Wolfson Medical Center, added by email, "Usually, patients with type 2 diabetes are treated with a combination of several antidiabetic drugs to achieve adequate glucose regulation and decrease HbA1c. Whey protein should be considered an important adjuvant in the management of type 2 diabetes."
"Furthermore," Dr. Wainstein added, "it is possible that by adding whey protein to the diet, glucose regulation might be achieved with less medication, which is a valuable advantage in type 2 diabetes treatment."
The study had no commercial funding, and the authors declared no conflicts of interest.
NEW YORK (Reuters Health) - A breakfast rich in whey protein may help people with type 2 diabetes manage their illness better, new research from Israel suggests.
"Whey protein, a byproduct of cheese manufacturing, lowers postprandial glycemia more than other protein sources," said lead author Dr. Daniela Jakubowicz from Wolfson Medical Center at Tel Aviv University."
We found that in type 2 diabetes, increasing protein content at breakfast has a greater impact on weight loss, glycated hemoglobin (HbA1C), satiety and postprandial glycemia when the protein source is whey protein, compared with other protein sources, such as eggs, tuna and soy," she told Reuters Health by email.
Dr. Jakubowicz and her group presented their findings April 1 at ENDO 2016, the annual meeting of the Endocrine Society, in Boston.
They randomly assigned 48 overweight and obese patients with type 2 diabetes to one of three isocaloric diets. Over 12 weeks, everyone ate a large breakfast, a medium-sized lunch and a small dinner, but the amount and source of each group's breakfast proteins differed.
At breakfast, the 17 participants in the whey group ate 36 g of protein as part of a whey protein shake consisting of 40% carbohydrate, 40% protein and 20% fat. The 16 participants in the high-protein group ate 36 g of protein in the form of eggs, tuna and cheese (40% carbs; 40% protein; 20% fat). The 15 in the high-carbohydrate group ate 13 g of protein in ready-to-eat cereals (65% carbs; 15% protein; 20% fat).
All three diets included a 660 kcal breakfast, a 567 cal lunch and a 276 cal dinner, with the same composition at lunch and dinner.
After 12 weeks, the participants in the whey protein group lost the most weight (7.6 kg vs. 6.1 kg for participants in the high-protein group and 3.5 kg for those in the high-carbohydrate group (p<0.0001).
Participants on the whey protein diet were less hungry during the day and had lower glucose spikes after meals compared with those on the other two diets.
The drop in HbA1C was 11.5% in the whey group, 7.7% in the protein group and 4.6% in the carbohydrate group (p<0.0001). Compared with the carbohydrate group, the percentage drop in HbA1c was greater by 41% in the protein group and by 64% in the whey group (p<0.0001).
"Whey protein was consumed only at breakfast; however, the improvement of glucose, insulin and glucagon-like peptide 1 (GLP-1) was also observed after lunch and dinner. The mechanism of this persistent beneficial effect of whey protein needs further research," Dr. Jakubowicz said.
Co-author Dr. Julio Wainstein, also at Wolfson Medical Center, added by email, "Usually, patients with type 2 diabetes are treated with a combination of several antidiabetic drugs to achieve adequate glucose regulation and decrease HbA1c. Whey protein should be considered an important adjuvant in the management of type 2 diabetes."
"Furthermore," Dr. Wainstein added, "it is possible that by adding whey protein to the diet, glucose regulation might be achieved with less medication, which is a valuable advantage in type 2 diabetes treatment."
The study had no commercial funding, and the authors declared no conflicts of interest.