User login
Geriatrics update 2018: Challenges in mental health, mobility, and postdischarge care
Unfortunately, recent research has not unveiled a breakthrough for preventing or treating cognitive impairment or Alzheimer disease. But several studies from the last 2 years are helping to drive the field of geriatrics forward, providing evidence of what does and does not help a variety of issues specific to the elderly.
Based on a search of the 2017 and 2018 literature, this article presents new evidence on preventing and treating cognitive impairment, managing dementia-associated behavioral disturbances and delirium, preventing falls, and improving inpatient mobility and posthospital care transitions.
COGNITIVE IMPAIRMENT, DEMENTIA: STILL NO SILVER BULLET
With the exception of oral anticoagulation treatment for atrial fibrillation, there is little evidence that pharmacologic or nonpharmacologic interventions slow the onset or progression of Alzheimer disease.
Nonpharmacologic interventions
Home occupational therapy. A 2-year home-based occupational therapy intervention1 showed no evidence of slowing functional decline in patients with Alzheimer disease. The randomized controlled trial involving 180 participants consisted of monthly sessions of an intensive, well-established collaborative-care management model that included fall prevention and other safety strategies, personalized training in activities of daily living, exercise, and education. Outcome measures for activities of daily living did not differ significantly between the treatment and control groups.1
Physical activity. Whether physical activity interventions slow cognitive decline and prevent dementia in cognitively intact adults was examined in a systematic review of 32 trials.2 Most of the trials followed patients for 6 months; a few stretched for 1 or 2 years.
Evidence was insufficient to prove cognitive benefit for short-term, single-component or multicomponent physical activity interventions. However, a multidomain physical activity intervention that also included dietary modifications and cognitive training did show a delay in cognitive decline, but only “low-strength” evidence.2
Nutritional supplements. The antioxidants vitamin E and selenium were studied for their possible cognitive benefit in the double-blind randomized Prevention of Alzheimer Disease by Vitamin E and Selenium trial3 in 3,786 asymptomatic men ages 60 and older. Neither supplement was found to prevent dementia over a 7-year follow-up period.
A review of 38 trials4 evaluated the effects on cognition of omega-3 fatty acids, soy, ginkgo biloba, B vitamins, vitamin D plus calcium, vitamin C, beta-carotene, and multi-ingredient supplements. It found insufficient evidence to recommend any over-the-counter supplement for cognitive protection in adults with normal cognition or mild cognitive impairment.
Pharmacologic treatments
Testosterone supplementation. The Testosterone Trials tested the effects of testosterone gel vs placebo for 1 year on 493 men over age 65 with low testosterone (< 275 ng/mL) and with subjective memory complaints and objective memory performance deficits. Treatment was not associated with improved memory or other cognitive functions compared with placebo.5
Antiamyloid drugs. A randomized, double-blind, placebo-controlled trial in nearly 2,000 patients evaluated verubecestat, an oral beta-site amyloid precursor protein-cleaving enzyme-1 inhibitor that reduces the amyloid-beta level in cerebrospinal fluid.6
Verubecestat did not reduce cognitive or functional decline in patients with mild-to-moderate Alzheimer disease, while adverse events including rashes, falls, injuries, sleep disturbances, suicidal ideation, weight loss, and hair color change were more common in the treatment groups. The trial was terminated early because of futility at 50 months.
And in a placebo-controlled trial of solanezumab, a monoclonal antibody directed against the amyloid beta peptide, no benefit was demonstrated at 80 weeks in more than 2,000 patients with Alzheimer disease.7
Multiple common agents. A well-conducted systematic review8 of 51 trials of at least a 6-month duration did not support the use of antihypertensive agents, diabetes medications, nonsteroidal anti-inflammatory drugs, aspirin, hormones, or lipid-lowering drugs for cognitive protection for people with normal cognition or mild cognitive impairment.
However, some studies found reassuring evidence that standard therapies for other conditions do not worsen cognitive decline and are protective for atrial fibrillation.8
Proton-pump inhibitors. Concern exists for a potential link between dementia risk and proton-pump inhibitors, which are widely used to treat acid-related gastrointestinal disorders.9
A prospective population-based cohort study10 of nearly 3,500 people ages 65 and older without baseline dementia screened participants for dementia every 2 years over a mean period of 7.5 years and provided further evaluation for those who screened positive. Use of proton-pump inhibitors was not found to be associated with dementia risk, even with high cumulative exposure.
Results from this study do not support avoiding proton-pump inhibitors out of concern for dementia risk, although long-term use is associated with other safety concerns.
Oral anticoagulation. The increased risk of dementia with atrial fibrillation is well documented.11
A retrospective study12 based on a Swedish health registry and using more than 444,000 patients covering more than 1.5 million years at risk found that oral anticoagulant treatment at baseline conferred a 29% lower risk of dementia in an intention-to-treat analysis and a 48% lower risk in on-treatment analysis compared with no oral anticoagulation therapy. No difference was found between new oral anticoagulants and warfarin.
Transcatheter aortic valve implantation is not associated with cognitive decline
For patients with severe aortic stenosis who are not surgical candidates, transcatheter aortic valve implantation is superior to standard medical therapy,13 but there are concerns of neurologic and cognitive changes after the procedure.14 A meta-analysis of 18 studies assessing cognitive performance in more than 1,000 patients (average age ≥ 80) after undergoing the procedure for severe aortic stenosis found no significant cognitive performance changes from baseline perioperatively or 3 or 6 months later.15
TREATING DEMENTIA-ASSOCIATED BEHAVIORAL DISTURBANCES
Behavioral and psychiatric symptoms often accompany dementia, but no drugs have yet been approved by the US Food and Drug Administration (FDA) to address them in this population. Nonpharmacologic interventions are recommended as first-line therapy.
Antipsychotics are not recommended
Antipsychotics are often prescribed,16 although they are associated with metabolic syndrome17 and increased risks of stroke and death.18 The FDA has issued black box warnings against using antipsychotics for behavioral management in patients with dementia. Further, the American Geriatrics Society and the American Psychiatric Association do not endorse using them as initial therapy for behavioral and psychological symptoms of dementia.16,19
The Centers for Medicare and Medicaid Services partnered with nursing homes to improve the quality of care for patients with dementia, with results measured as the rate of prescribing antipsychotic medications. Although the use of psychotropic medications declined after initiating the partnership, the use of mood stabilizers increased, possibly as a substitute for antipsychotics.20
Dextromethorphan-quinidine use is up, despite modest evidence of benefit
A consumer news report in 2017 stated that the use of dextromethorphan-quinidine in long-term care facilities increased by nearly 400% between 2012 and 2016.21
Evidence for its benefits comes from a 10-week, phase 2, randomized controlled trial conducted at 42 US study sites with 194 patients with probable Alzheimer disease. Compared with the placebo group, the active treatment group had mildly reduced agitation but an increased risk of falls, dizziness, and diarrhea. However, rates of adverse effects were low, and the authors concluded that treatment was generally well tolerated.22
Pimavanserin: No long-term benefit for psychosis
In a phase 2, randomized, double-blind, placebo-controlled trial in 181 patients with possible or probable Alzheimer disease and psychotic symptoms, pimavanserin was associated with improved symptoms as measured by the Neuropsychiatric Inventory–Nursing Home Version psychosis score at 6 weeks, but no difference was found compared with placebo at 12 weeks. The treatment group had more adverse events, including agitation, aggression, peripheral edema, anxiety, and symptoms of dementia, although the differences were not statistically significant.23
DELIRIUM: AVOID ANTIPSYCHOTICS
Delirium is common in hospitalized older adults, especially those who have baseline cognitive or functional impairment and are exposed to precipitating factors such as treatment with anticholinergic or narcotic medications, infection, surgery, or admission to an intensive care unit.24
Delirium at discharge predicts poor outcomes
In a prospective study of 152 hospitalized patients with delirium, those who either did not recover from delirium or had only partially recovered at discharge were more likely to visit the emergency department, be rehospitalized, or die during the subsequent 3 months than those who had fully recovered from delirium at discharge.25
Multicomponent, patient-centered approach can help
A randomized trial in 377 patients in Taiwan evaluated the use of a modified Hospital Elder Life Program, consisting of 3 protocols focused on orienting communication, oral and nutritional assistance, and early mobilization. Patients were at least 65 years old and undergoing elective abdominal surgery with expected length of hospital stay longer than 6 days. The program, administered daily during hospitalization, significantly lowered postoperative delirium by 56% and hospital stay by 2 days compared with usual care.26
Prophylactic haloperidol does not improve outcomes
In a multicenter randomized, double-blind, placebo-controlled trial, van den Boogaard et al studied prophylactic intravenous haloperidol in nearly 1,800 critically ill patients at high risk of delirium.27 Haloperidol did not improve survival at 28 days compared with placebo. For secondary outcomes, including delirium incidence, delirium-free and coma-free days, duration of mechanical ventilation, and hospital and intensive care department length of stay, treatment was not found to differ statistically from placebo.
Antipsychotics may worsen delirium
A double-blind, parallel-arm, dose-titrated randomized trial, conducted at 11 Australian hospices or hospitals with palliative care services, administered oral risperidone, haloperidol, or placebo to 247 patients with life-limiting illness and delirium. Both treatment groups had higher delirium symptom scores than the placebo group.28
In addition, a systematic review and meta-analysis of 19 studies found no benefit of antipsychotic medications for preventing or treating delirium in hospitalized adults.29
Antipsychotics are often continued indefinitely
A retrospective chart review at a US academic health system found30 that among 487 patients with a new antipsychotic medication prescribed during hospitalization, 147 (30.2%) were discharged on an antipsychotic. Of these, 121 (82.3%) had a diagnosis of delirium. Only 15 (12.4%) had discharge summaries that included instructions for discontinuing the drug.
Another US health system retrospectively reviewed antipsychotic use and found31 that out of 260 patients who were newly exposed to an antipsychotic drug during hospitalization, 146 (56.2%) were discharged on an antipsychotic drug, and 65% of these patients were still on the drug at the time of the next hospital admission.
EXERCISE, EXERCISE, EXERCISE
Exercise recommended, but not vitamin D, to prevent falls
In 2018, the US Preventive Services Task Force updated its recommendations for preventing falls in community-dwelling older adults.32 Based on the findings of several trials, the task force recommends exercise interventions for adults age 65 and older who are at increased risk for falls. Gait, balance, and functional training were studied in 17 trials, resistance training in 13, flexibility in 8, endurance training in 5, and tai chi in 3, with 5 studies including general physical activity. Exercise interventions most commonly took place for 3 sessions per week for 12 months (range 2–42 months).
The task force also recommends against vitamin D supplementation for fall prevention in community-dwelling adults age 65 or older who are not known to have osteoporosis or vitamin D deficiency.
Early mobilization helps inpatients
Hospitalized older adults usually spend most of their time in bed. Forty-five previously ambulatory patients (age ≥ 65 without dementia or delirium) in a Veterans Affairs hospital were monitored with wireless accelerometers and were found to spend, on average, 83% of the measured hospital stay in bed. Standing or walking time ranged from 0.2% to 21%, with a median of only 3% (43 minutes a day).33
Since falls with injury became a Centers for Medicare and Medicaid Services nonreimbursable hospital-acquired condition, tension has arisen between promoting mobility and preventing falls.34 Two studies evaluating the adoption of mobility-restricting approaches such as bed-alarms, “fall-alert” signs, supervision of patients in the bathroom, and ensuring patients’ walking aids are within reach, did not find a significant reduction in falls or fall-related injuries.35,36
A clinically significant loss of community mobility is common after hospitalization in older adults.37 Older adults who developed mobility impairment during hospitalization had a higher risk of death in a large, retrospective study.38 A large Canadian multisite intervention trial39 that promoted early mobilization in older patients who were admitted to general medical wards resulted in increased mobilization and significantly shorter hospital stays.
POSTHOSPITAL CARE NEEDS IMPROVEMENT
After hospitalization, older adults who have difficulty with activities of daily living or complex medical needs often require continued care.
About 20% of hospitalized Medicare beneficiaries in the United States are discharged to skilled nursing facilities.40 This is often a stressful transition, and most people have little guidance on selecting a facility and simply choose one based on its proximity to home.41
A program of frequent visits by hospital-employed physicians and advanced practice professionals at skilled nursing facilities resulted in a significantly lower 30-day readmission rate compared with nonparticipating skilled nursing facilities in the same geographic area.42
Home healthcare is recommended after hospital discharge at a rapidly increasing rate. Overall referral rates increased from 8.6% to 14.1% between 2001 and 2012, and from 14.3% to 24.0% for patients with heart failure.43 A qualitative study of home healthcare nurses found a need for improved care coordination between home healthcare agencies and discharging hospitals, including defining accountability for orders and enhancing communication.44
- Callahan CM, Boustani MA, Schmid AA, et al. Targeting functional decline in Alzheimer disease: a randomized trial. Ann Intern Med 2017; 166(3):164–171. doi:10.7326/M16-0830
- Brasure M, Desai P, Davila H, et al. Physical activity interventions in preventing cognitive decline and Alzheimer-type dementia: a systematic review. Ann Intern Med 2018; 168(1):30–38. doi:10.7326/M17-1528
- Kryscio RJ, Abner EL, Caban-Holt A, et al. Association of antioxidant supplement use and dementia in the Prevention of Alzheimer’s Disease by Vitamin E and Selenium Trial (PREADViSE). JAMA Neurol 2017; 74(5):567–573. doi:10.1001/jamaneurol.2016.5778
- Butler M, Nelson VA, Davila H, et al. Over-the-counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med 2018; 168(1):52–62. doi:10.7326/M17-1530
- Resnick SM, Matsumoto AM, Stephens-Shields AJ, et al. Testosterone treatment and cognitive function in older men with low testosterone and age-associated memory impairment. JAMA 2017; 317(7):717–727. doi:10.1001/jama.2016.21044
- Egan MF, Kost J, Tariot PN, et al. Randomized trial of verubecestat for mild-to-moderate Alzheimer’s disease. N Engl J Med 2018; 378(18):1691–1703. doi:10.1056/NEJMoa1706441
- Honig LS, Vellas B, Woodward M, et al. Trial of solanezumab for mild dementia due to Alzheimer’s disease. N Engl J Med 2018; 378(4):321–330. doi:10.1056/NEJMoa1705971
- Fink HA, Jutkowitz E, McCarten JR, et al. Pharmacologic interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med 2018; 168(1):39–51. doi:10.7326/M17-1529
- Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol 2016; 73(4):410–416. doi:10.1001/jamaneurol.2015.4791
- Gray SL, Walker RL, Dublin S, et al. Proton pump inhibitor use and dementia risk: prospective population-based study. J Am Geriatr Soc 2018; 66(2):247–253. doi:10.1111/jgs.15073
- de Bruijn RF, Heeringa J, Wolters FJ, et al. Association between atrial fibrillation and dementia in the general population. JAMA Neurol 2015; 72(11):1288–1294. doi:10.1001/jamaneurol.2015.2161
- Friberg L, Rosenqvist M. Less dementia with oral anticoagulation in atrial fibrillation. Eur Heart J 2018; 39(6):453–460. doi:10.1093/eurheartj/ehx579
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363(17):1597–1607. doi:10.1056/NEJMoa1008232
- Haussig S, Mangner N, Dwyer MG, et al. Effect of a cerebral protection device on brain lesions following transcatheter aortic valve implantation in patients with severe aortic stenosis: the CLEAN-TAVI randomized clinical trial. JAMA 2016; 316(6):592–601. doi:10.1001/jama.2016.10302
- Khan MM, Herrmann N, Gallagher D, et al. Cognitive outcomes after transcatheter aortic valve implantation: a metaanalysis. J Am Geriatr Soc 2018; 66(2):254–262. doi:10.1111/jgs.15123
- Choosing Wisely; ABIM Foundation. American Geriatrics Society: ten things physicians and patients should question. www.choosingwisely.org/societies/american-geriatrics-society. Accessed November 6, 2018.
- Lieberman JA 3rd. Metabolic changes associated with antipsychotic use. Prim Care Companion J Clin Psychiatry 2004; 6(suppl 2):8–13. pmid:16001095
- Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA 2005; 294(15):1934–1943. doi:10.1001/jama.294.15.1934
- Choosing Wisely; ABIM Foundation. American Psychiatric Association: five things physicians and patients should question. www.choosingwisely.org/societies/american-psychiatric-association. Accessed November 6, 2018.
- Maust DT, Kim HM, Chiang C, Kales HC. Association of the Centers for Medicare & Medicaid Services’ National Partnership to improve dementia care with the use of antipsychotics and other psychotropics in long-term care in the United States from 2009 to 2014. JAMA Intern Med 2018; 178(5):640–647. doi:10.1001/jamainternmed.2018.0379
- CNN. The little red pill being pushed on the elderly. www.cnn.com/2017/10/12/health/nuedexta-nursing-homes-invs/index.html. Accessed November 6, 2018.
- Cummings JL, Lyketsos CG, Peskind ER, et al. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA 2015; 314(12):1242–1254. doi:10.1001/jama.2015.10214
- Ballard C, Banister C, Khan Z, et al; ADP Investigators. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer’s disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol 2018; 17(3):213–222. doi:10.1016/S1474-4422(18)30039-5
- Inouye SK. Delirium in older persons. N Engl J Med 2006; 354(11):1157–1165. doi:10.1056/NEJMra052321
- Cole MG, McCusker J, Bailey R, et al. Partial and no recovery from delirium after hospital discharge predict increased adverse events. Age Ageing 2017; 46(1):90–95. doi:10.1093/ageing/afw153
- Chen CC, Li HC, Liang JT, et al. Effect of a modified hospital elder life program on delirium and length of hospital stay in patients undergoing abdominal surgery: a cluster randomized clinical trial. JAMA Surg 2017; 152(9):827–834. doi:10.1001/jamasurg.2017.1083
- van den Boogaard M, Slooter AJC, Brüggemann RJM, et al. Effect of haloperidol on survival among critically ill adults with a high risk of delirium: the REDUCE randomized clinical trial. JAMA 2018; 319(7):680–690. doi:10.1001/jama.2018.0160
- Agar MR, Lawlor PG, Quinn S, et al. Efficacy of oral risperidone, haloperidol, or placebo for symptoms of delirium among patients in palliative care: a randomized clinical trial. JAMA Intern Med 2017; 177(1):34–42. doi:10.1001/jamainternmed.2016.7491
- Neufeld KJ, Yue J, Robinson TN, Inouye SK, Needham DM. Antipsychotic medication for prevention and treatment of delirium in hospitalized adults: a systematic review and meta-analysis. J Am Geriatr Soc 2016; 64(4):705–714. doi:10.1111/jgs.14076
- Johnson KG, Fashoyin A, Madden-Fuentes R, Muzyk AJ, Gagliardi JP, Yanamadala M. Discharge plans for geriatric inpatients with delirium: a plan to stop antipsychotics? J Am Geriatr Soc 2017; 65(10):2278–2281. doi:10.1111/jgs.15026
- Loh KP, Ramdass S, Garb JL, et al. Long-term outcomes of elders discharged on antipsychotics. J Hosp Med 2016; 11(8):550–555. doi:10.1002/jhm.2585
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Interventions to prevent falls in community-dwelling older adults: US Preventive Services Task Force Recommendation statement. JAMA 2018; 319(16):1696–1704. doi:10.1001/jama.2018.3097
- Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc 2009; 57(9):1660–1665. doi:10.1111/j.1532-5415.2009.02393.x
- Growdon ME, Shorr RI, Inouye SK. The tension between promoting mobility and preventing falls in the hospital. JAMA Intern Med 2017; 177(6):759–760. doi:10.1001/jamainternmed.2017.0840
- Barker AL, Morello RT, Wolfe R, et al. 6-PACK programme to decrease fall injuries in acute hospitals: cluster randomised controlled trial. BMJ 2016; 352:h6781. doi:10.1136/bmj.h6781
- Shorr RI, Chandler AM, Mion LC, et al. Effects of an intervention to increase bed alarm use to prevent falls in hospitalized patients: a cluster randomized trial. Ann Intern Med 2012; 157(10):692–699. doi:10.7326/0003-4819-157-10-201211200-00005
- Loyd C, Beasley TM, Miltner RS, Clark D, King B, Brown CJ. Trajectories of community mobility recovery after hospitalization in older adults. J Am Geriatr Soc 2018; 66(7):1399–1403. doi:10.1111/jgs.15397
- Valiani V, Chen Z, Lipori G, Pahor M, Sabbá C, Manini TM. Prognostic value of Braden Activity subscale for mobility status in hospitalized older adults. J Hosp Med 2017; 12(6):396–401. doi:10.12788/jhm.2748
- Liu B, Moore JE, Almaawiy U, et al; MOVE ON Collaboration. Outcomes of mobilisation of vulnerable elders in Ontario (MOVE ON): a multisite interrupted time series evaluation of an implementation intervention to increase patient mobilisation. Age Ageing 2018; 47(1):112–119. doi:10.1093/ageing/afx128
- Report to Congress: Medicare Payment Policy. Medicare Payment Advisory Commission 2016. www.medpac.gov/docs/default-source/reports/march-2016-report-to-the-congress-medicare-payment-policy.pdf?sfvrsn=0. Accessed November 6, 2018.
- Gadbois EA, Tyler DA, Mor V. Selecting a skilled nursing facility for postacute care: individual and family perspectives. J Am Geriatr Soc 2017; 65(11):2459–2465. doi:10.1111/jgs.14988
- Kim LD, Kou L, Hu B, Gorodeski EZ, Rothberg MB. Impact of a connected care model on 30-day readmission rates from skilled nursing facilities. J Hosp Med 2017; 12(4):238–244. doi:10.12788/jhm.2710
- Jones CD, Ginde AA, Burke RE, Wald HL, Masoudi FA, Boxer RS. Increasing home healthcare referrals upon discharge from U.S. hospitals: 2001-2012. J Am Geriatr Soc 2015; 63(6):1265–1266. doi:10.1111/jgs.13467
- Jones CD, Jones J, Richard A, et al. “Connecting the dots”: a qualitative study of home health nurse perspectives on coordinating care for recently discharged patients. J Gen Intern Med 2017; 32(10):1114–1121. doi:10.1007/s11606-017-4104-0
Unfortunately, recent research has not unveiled a breakthrough for preventing or treating cognitive impairment or Alzheimer disease. But several studies from the last 2 years are helping to drive the field of geriatrics forward, providing evidence of what does and does not help a variety of issues specific to the elderly.
Based on a search of the 2017 and 2018 literature, this article presents new evidence on preventing and treating cognitive impairment, managing dementia-associated behavioral disturbances and delirium, preventing falls, and improving inpatient mobility and posthospital care transitions.
COGNITIVE IMPAIRMENT, DEMENTIA: STILL NO SILVER BULLET
With the exception of oral anticoagulation treatment for atrial fibrillation, there is little evidence that pharmacologic or nonpharmacologic interventions slow the onset or progression of Alzheimer disease.
Nonpharmacologic interventions
Home occupational therapy. A 2-year home-based occupational therapy intervention1 showed no evidence of slowing functional decline in patients with Alzheimer disease. The randomized controlled trial involving 180 participants consisted of monthly sessions of an intensive, well-established collaborative-care management model that included fall prevention and other safety strategies, personalized training in activities of daily living, exercise, and education. Outcome measures for activities of daily living did not differ significantly between the treatment and control groups.1
Physical activity. Whether physical activity interventions slow cognitive decline and prevent dementia in cognitively intact adults was examined in a systematic review of 32 trials.2 Most of the trials followed patients for 6 months; a few stretched for 1 or 2 years.
Evidence was insufficient to prove cognitive benefit for short-term, single-component or multicomponent physical activity interventions. However, a multidomain physical activity intervention that also included dietary modifications and cognitive training did show a delay in cognitive decline, but only “low-strength” evidence.2
Nutritional supplements. The antioxidants vitamin E and selenium were studied for their possible cognitive benefit in the double-blind randomized Prevention of Alzheimer Disease by Vitamin E and Selenium trial3 in 3,786 asymptomatic men ages 60 and older. Neither supplement was found to prevent dementia over a 7-year follow-up period.
A review of 38 trials4 evaluated the effects on cognition of omega-3 fatty acids, soy, ginkgo biloba, B vitamins, vitamin D plus calcium, vitamin C, beta-carotene, and multi-ingredient supplements. It found insufficient evidence to recommend any over-the-counter supplement for cognitive protection in adults with normal cognition or mild cognitive impairment.
Pharmacologic treatments
Testosterone supplementation. The Testosterone Trials tested the effects of testosterone gel vs placebo for 1 year on 493 men over age 65 with low testosterone (< 275 ng/mL) and with subjective memory complaints and objective memory performance deficits. Treatment was not associated with improved memory or other cognitive functions compared with placebo.5
Antiamyloid drugs. A randomized, double-blind, placebo-controlled trial in nearly 2,000 patients evaluated verubecestat, an oral beta-site amyloid precursor protein-cleaving enzyme-1 inhibitor that reduces the amyloid-beta level in cerebrospinal fluid.6
Verubecestat did not reduce cognitive or functional decline in patients with mild-to-moderate Alzheimer disease, while adverse events including rashes, falls, injuries, sleep disturbances, suicidal ideation, weight loss, and hair color change were more common in the treatment groups. The trial was terminated early because of futility at 50 months.
And in a placebo-controlled trial of solanezumab, a monoclonal antibody directed against the amyloid beta peptide, no benefit was demonstrated at 80 weeks in more than 2,000 patients with Alzheimer disease.7
Multiple common agents. A well-conducted systematic review8 of 51 trials of at least a 6-month duration did not support the use of antihypertensive agents, diabetes medications, nonsteroidal anti-inflammatory drugs, aspirin, hormones, or lipid-lowering drugs for cognitive protection for people with normal cognition or mild cognitive impairment.
However, some studies found reassuring evidence that standard therapies for other conditions do not worsen cognitive decline and are protective for atrial fibrillation.8
Proton-pump inhibitors. Concern exists for a potential link between dementia risk and proton-pump inhibitors, which are widely used to treat acid-related gastrointestinal disorders.9
A prospective population-based cohort study10 of nearly 3,500 people ages 65 and older without baseline dementia screened participants for dementia every 2 years over a mean period of 7.5 years and provided further evaluation for those who screened positive. Use of proton-pump inhibitors was not found to be associated with dementia risk, even with high cumulative exposure.
Results from this study do not support avoiding proton-pump inhibitors out of concern for dementia risk, although long-term use is associated with other safety concerns.
Oral anticoagulation. The increased risk of dementia with atrial fibrillation is well documented.11
A retrospective study12 based on a Swedish health registry and using more than 444,000 patients covering more than 1.5 million years at risk found that oral anticoagulant treatment at baseline conferred a 29% lower risk of dementia in an intention-to-treat analysis and a 48% lower risk in on-treatment analysis compared with no oral anticoagulation therapy. No difference was found between new oral anticoagulants and warfarin.
Transcatheter aortic valve implantation is not associated with cognitive decline
For patients with severe aortic stenosis who are not surgical candidates, transcatheter aortic valve implantation is superior to standard medical therapy,13 but there are concerns of neurologic and cognitive changes after the procedure.14 A meta-analysis of 18 studies assessing cognitive performance in more than 1,000 patients (average age ≥ 80) after undergoing the procedure for severe aortic stenosis found no significant cognitive performance changes from baseline perioperatively or 3 or 6 months later.15
TREATING DEMENTIA-ASSOCIATED BEHAVIORAL DISTURBANCES
Behavioral and psychiatric symptoms often accompany dementia, but no drugs have yet been approved by the US Food and Drug Administration (FDA) to address them in this population. Nonpharmacologic interventions are recommended as first-line therapy.
Antipsychotics are not recommended
Antipsychotics are often prescribed,16 although they are associated with metabolic syndrome17 and increased risks of stroke and death.18 The FDA has issued black box warnings against using antipsychotics for behavioral management in patients with dementia. Further, the American Geriatrics Society and the American Psychiatric Association do not endorse using them as initial therapy for behavioral and psychological symptoms of dementia.16,19
The Centers for Medicare and Medicaid Services partnered with nursing homes to improve the quality of care for patients with dementia, with results measured as the rate of prescribing antipsychotic medications. Although the use of psychotropic medications declined after initiating the partnership, the use of mood stabilizers increased, possibly as a substitute for antipsychotics.20
Dextromethorphan-quinidine use is up, despite modest evidence of benefit
A consumer news report in 2017 stated that the use of dextromethorphan-quinidine in long-term care facilities increased by nearly 400% between 2012 and 2016.21
Evidence for its benefits comes from a 10-week, phase 2, randomized controlled trial conducted at 42 US study sites with 194 patients with probable Alzheimer disease. Compared with the placebo group, the active treatment group had mildly reduced agitation but an increased risk of falls, dizziness, and diarrhea. However, rates of adverse effects were low, and the authors concluded that treatment was generally well tolerated.22
Pimavanserin: No long-term benefit for psychosis
In a phase 2, randomized, double-blind, placebo-controlled trial in 181 patients with possible or probable Alzheimer disease and psychotic symptoms, pimavanserin was associated with improved symptoms as measured by the Neuropsychiatric Inventory–Nursing Home Version psychosis score at 6 weeks, but no difference was found compared with placebo at 12 weeks. The treatment group had more adverse events, including agitation, aggression, peripheral edema, anxiety, and symptoms of dementia, although the differences were not statistically significant.23
DELIRIUM: AVOID ANTIPSYCHOTICS
Delirium is common in hospitalized older adults, especially those who have baseline cognitive or functional impairment and are exposed to precipitating factors such as treatment with anticholinergic or narcotic medications, infection, surgery, or admission to an intensive care unit.24
Delirium at discharge predicts poor outcomes
In a prospective study of 152 hospitalized patients with delirium, those who either did not recover from delirium or had only partially recovered at discharge were more likely to visit the emergency department, be rehospitalized, or die during the subsequent 3 months than those who had fully recovered from delirium at discharge.25
Multicomponent, patient-centered approach can help
A randomized trial in 377 patients in Taiwan evaluated the use of a modified Hospital Elder Life Program, consisting of 3 protocols focused on orienting communication, oral and nutritional assistance, and early mobilization. Patients were at least 65 years old and undergoing elective abdominal surgery with expected length of hospital stay longer than 6 days. The program, administered daily during hospitalization, significantly lowered postoperative delirium by 56% and hospital stay by 2 days compared with usual care.26
Prophylactic haloperidol does not improve outcomes
In a multicenter randomized, double-blind, placebo-controlled trial, van den Boogaard et al studied prophylactic intravenous haloperidol in nearly 1,800 critically ill patients at high risk of delirium.27 Haloperidol did not improve survival at 28 days compared with placebo. For secondary outcomes, including delirium incidence, delirium-free and coma-free days, duration of mechanical ventilation, and hospital and intensive care department length of stay, treatment was not found to differ statistically from placebo.
Antipsychotics may worsen delirium
A double-blind, parallel-arm, dose-titrated randomized trial, conducted at 11 Australian hospices or hospitals with palliative care services, administered oral risperidone, haloperidol, or placebo to 247 patients with life-limiting illness and delirium. Both treatment groups had higher delirium symptom scores than the placebo group.28
In addition, a systematic review and meta-analysis of 19 studies found no benefit of antipsychotic medications for preventing or treating delirium in hospitalized adults.29
Antipsychotics are often continued indefinitely
A retrospective chart review at a US academic health system found30 that among 487 patients with a new antipsychotic medication prescribed during hospitalization, 147 (30.2%) were discharged on an antipsychotic. Of these, 121 (82.3%) had a diagnosis of delirium. Only 15 (12.4%) had discharge summaries that included instructions for discontinuing the drug.
Another US health system retrospectively reviewed antipsychotic use and found31 that out of 260 patients who were newly exposed to an antipsychotic drug during hospitalization, 146 (56.2%) were discharged on an antipsychotic drug, and 65% of these patients were still on the drug at the time of the next hospital admission.
EXERCISE, EXERCISE, EXERCISE
Exercise recommended, but not vitamin D, to prevent falls
In 2018, the US Preventive Services Task Force updated its recommendations for preventing falls in community-dwelling older adults.32 Based on the findings of several trials, the task force recommends exercise interventions for adults age 65 and older who are at increased risk for falls. Gait, balance, and functional training were studied in 17 trials, resistance training in 13, flexibility in 8, endurance training in 5, and tai chi in 3, with 5 studies including general physical activity. Exercise interventions most commonly took place for 3 sessions per week for 12 months (range 2–42 months).
The task force also recommends against vitamin D supplementation for fall prevention in community-dwelling adults age 65 or older who are not known to have osteoporosis or vitamin D deficiency.
Early mobilization helps inpatients
Hospitalized older adults usually spend most of their time in bed. Forty-five previously ambulatory patients (age ≥ 65 without dementia or delirium) in a Veterans Affairs hospital were monitored with wireless accelerometers and were found to spend, on average, 83% of the measured hospital stay in bed. Standing or walking time ranged from 0.2% to 21%, with a median of only 3% (43 minutes a day).33
Since falls with injury became a Centers for Medicare and Medicaid Services nonreimbursable hospital-acquired condition, tension has arisen between promoting mobility and preventing falls.34 Two studies evaluating the adoption of mobility-restricting approaches such as bed-alarms, “fall-alert” signs, supervision of patients in the bathroom, and ensuring patients’ walking aids are within reach, did not find a significant reduction in falls or fall-related injuries.35,36
A clinically significant loss of community mobility is common after hospitalization in older adults.37 Older adults who developed mobility impairment during hospitalization had a higher risk of death in a large, retrospective study.38 A large Canadian multisite intervention trial39 that promoted early mobilization in older patients who were admitted to general medical wards resulted in increased mobilization and significantly shorter hospital stays.
POSTHOSPITAL CARE NEEDS IMPROVEMENT
After hospitalization, older adults who have difficulty with activities of daily living or complex medical needs often require continued care.
About 20% of hospitalized Medicare beneficiaries in the United States are discharged to skilled nursing facilities.40 This is often a stressful transition, and most people have little guidance on selecting a facility and simply choose one based on its proximity to home.41
A program of frequent visits by hospital-employed physicians and advanced practice professionals at skilled nursing facilities resulted in a significantly lower 30-day readmission rate compared with nonparticipating skilled nursing facilities in the same geographic area.42
Home healthcare is recommended after hospital discharge at a rapidly increasing rate. Overall referral rates increased from 8.6% to 14.1% between 2001 and 2012, and from 14.3% to 24.0% for patients with heart failure.43 A qualitative study of home healthcare nurses found a need for improved care coordination between home healthcare agencies and discharging hospitals, including defining accountability for orders and enhancing communication.44
Unfortunately, recent research has not unveiled a breakthrough for preventing or treating cognitive impairment or Alzheimer disease. But several studies from the last 2 years are helping to drive the field of geriatrics forward, providing evidence of what does and does not help a variety of issues specific to the elderly.
Based on a search of the 2017 and 2018 literature, this article presents new evidence on preventing and treating cognitive impairment, managing dementia-associated behavioral disturbances and delirium, preventing falls, and improving inpatient mobility and posthospital care transitions.
COGNITIVE IMPAIRMENT, DEMENTIA: STILL NO SILVER BULLET
With the exception of oral anticoagulation treatment for atrial fibrillation, there is little evidence that pharmacologic or nonpharmacologic interventions slow the onset or progression of Alzheimer disease.
Nonpharmacologic interventions
Home occupational therapy. A 2-year home-based occupational therapy intervention1 showed no evidence of slowing functional decline in patients with Alzheimer disease. The randomized controlled trial involving 180 participants consisted of monthly sessions of an intensive, well-established collaborative-care management model that included fall prevention and other safety strategies, personalized training in activities of daily living, exercise, and education. Outcome measures for activities of daily living did not differ significantly between the treatment and control groups.1
Physical activity. Whether physical activity interventions slow cognitive decline and prevent dementia in cognitively intact adults was examined in a systematic review of 32 trials.2 Most of the trials followed patients for 6 months; a few stretched for 1 or 2 years.
Evidence was insufficient to prove cognitive benefit for short-term, single-component or multicomponent physical activity interventions. However, a multidomain physical activity intervention that also included dietary modifications and cognitive training did show a delay in cognitive decline, but only “low-strength” evidence.2
Nutritional supplements. The antioxidants vitamin E and selenium were studied for their possible cognitive benefit in the double-blind randomized Prevention of Alzheimer Disease by Vitamin E and Selenium trial3 in 3,786 asymptomatic men ages 60 and older. Neither supplement was found to prevent dementia over a 7-year follow-up period.
A review of 38 trials4 evaluated the effects on cognition of omega-3 fatty acids, soy, ginkgo biloba, B vitamins, vitamin D plus calcium, vitamin C, beta-carotene, and multi-ingredient supplements. It found insufficient evidence to recommend any over-the-counter supplement for cognitive protection in adults with normal cognition or mild cognitive impairment.
Pharmacologic treatments
Testosterone supplementation. The Testosterone Trials tested the effects of testosterone gel vs placebo for 1 year on 493 men over age 65 with low testosterone (< 275 ng/mL) and with subjective memory complaints and objective memory performance deficits. Treatment was not associated with improved memory or other cognitive functions compared with placebo.5
Antiamyloid drugs. A randomized, double-blind, placebo-controlled trial in nearly 2,000 patients evaluated verubecestat, an oral beta-site amyloid precursor protein-cleaving enzyme-1 inhibitor that reduces the amyloid-beta level in cerebrospinal fluid.6
Verubecestat did not reduce cognitive or functional decline in patients with mild-to-moderate Alzheimer disease, while adverse events including rashes, falls, injuries, sleep disturbances, suicidal ideation, weight loss, and hair color change were more common in the treatment groups. The trial was terminated early because of futility at 50 months.
And in a placebo-controlled trial of solanezumab, a monoclonal antibody directed against the amyloid beta peptide, no benefit was demonstrated at 80 weeks in more than 2,000 patients with Alzheimer disease.7
Multiple common agents. A well-conducted systematic review8 of 51 trials of at least a 6-month duration did not support the use of antihypertensive agents, diabetes medications, nonsteroidal anti-inflammatory drugs, aspirin, hormones, or lipid-lowering drugs for cognitive protection for people with normal cognition or mild cognitive impairment.
However, some studies found reassuring evidence that standard therapies for other conditions do not worsen cognitive decline and are protective for atrial fibrillation.8
Proton-pump inhibitors. Concern exists for a potential link between dementia risk and proton-pump inhibitors, which are widely used to treat acid-related gastrointestinal disorders.9
A prospective population-based cohort study10 of nearly 3,500 people ages 65 and older without baseline dementia screened participants for dementia every 2 years over a mean period of 7.5 years and provided further evaluation for those who screened positive. Use of proton-pump inhibitors was not found to be associated with dementia risk, even with high cumulative exposure.
Results from this study do not support avoiding proton-pump inhibitors out of concern for dementia risk, although long-term use is associated with other safety concerns.
Oral anticoagulation. The increased risk of dementia with atrial fibrillation is well documented.11
A retrospective study12 based on a Swedish health registry and using more than 444,000 patients covering more than 1.5 million years at risk found that oral anticoagulant treatment at baseline conferred a 29% lower risk of dementia in an intention-to-treat analysis and a 48% lower risk in on-treatment analysis compared with no oral anticoagulation therapy. No difference was found between new oral anticoagulants and warfarin.
Transcatheter aortic valve implantation is not associated with cognitive decline
For patients with severe aortic stenosis who are not surgical candidates, transcatheter aortic valve implantation is superior to standard medical therapy,13 but there are concerns of neurologic and cognitive changes after the procedure.14 A meta-analysis of 18 studies assessing cognitive performance in more than 1,000 patients (average age ≥ 80) after undergoing the procedure for severe aortic stenosis found no significant cognitive performance changes from baseline perioperatively or 3 or 6 months later.15
TREATING DEMENTIA-ASSOCIATED BEHAVIORAL DISTURBANCES
Behavioral and psychiatric symptoms often accompany dementia, but no drugs have yet been approved by the US Food and Drug Administration (FDA) to address them in this population. Nonpharmacologic interventions are recommended as first-line therapy.
Antipsychotics are not recommended
Antipsychotics are often prescribed,16 although they are associated with metabolic syndrome17 and increased risks of stroke and death.18 The FDA has issued black box warnings against using antipsychotics for behavioral management in patients with dementia. Further, the American Geriatrics Society and the American Psychiatric Association do not endorse using them as initial therapy for behavioral and psychological symptoms of dementia.16,19
The Centers for Medicare and Medicaid Services partnered with nursing homes to improve the quality of care for patients with dementia, with results measured as the rate of prescribing antipsychotic medications. Although the use of psychotropic medications declined after initiating the partnership, the use of mood stabilizers increased, possibly as a substitute for antipsychotics.20
Dextromethorphan-quinidine use is up, despite modest evidence of benefit
A consumer news report in 2017 stated that the use of dextromethorphan-quinidine in long-term care facilities increased by nearly 400% between 2012 and 2016.21
Evidence for its benefits comes from a 10-week, phase 2, randomized controlled trial conducted at 42 US study sites with 194 patients with probable Alzheimer disease. Compared with the placebo group, the active treatment group had mildly reduced agitation but an increased risk of falls, dizziness, and diarrhea. However, rates of adverse effects were low, and the authors concluded that treatment was generally well tolerated.22
Pimavanserin: No long-term benefit for psychosis
In a phase 2, randomized, double-blind, placebo-controlled trial in 181 patients with possible or probable Alzheimer disease and psychotic symptoms, pimavanserin was associated with improved symptoms as measured by the Neuropsychiatric Inventory–Nursing Home Version psychosis score at 6 weeks, but no difference was found compared with placebo at 12 weeks. The treatment group had more adverse events, including agitation, aggression, peripheral edema, anxiety, and symptoms of dementia, although the differences were not statistically significant.23
DELIRIUM: AVOID ANTIPSYCHOTICS
Delirium is common in hospitalized older adults, especially those who have baseline cognitive or functional impairment and are exposed to precipitating factors such as treatment with anticholinergic or narcotic medications, infection, surgery, or admission to an intensive care unit.24
Delirium at discharge predicts poor outcomes
In a prospective study of 152 hospitalized patients with delirium, those who either did not recover from delirium or had only partially recovered at discharge were more likely to visit the emergency department, be rehospitalized, or die during the subsequent 3 months than those who had fully recovered from delirium at discharge.25
Multicomponent, patient-centered approach can help
A randomized trial in 377 patients in Taiwan evaluated the use of a modified Hospital Elder Life Program, consisting of 3 protocols focused on orienting communication, oral and nutritional assistance, and early mobilization. Patients were at least 65 years old and undergoing elective abdominal surgery with expected length of hospital stay longer than 6 days. The program, administered daily during hospitalization, significantly lowered postoperative delirium by 56% and hospital stay by 2 days compared with usual care.26
Prophylactic haloperidol does not improve outcomes
In a multicenter randomized, double-blind, placebo-controlled trial, van den Boogaard et al studied prophylactic intravenous haloperidol in nearly 1,800 critically ill patients at high risk of delirium.27 Haloperidol did not improve survival at 28 days compared with placebo. For secondary outcomes, including delirium incidence, delirium-free and coma-free days, duration of mechanical ventilation, and hospital and intensive care department length of stay, treatment was not found to differ statistically from placebo.
Antipsychotics may worsen delirium
A double-blind, parallel-arm, dose-titrated randomized trial, conducted at 11 Australian hospices or hospitals with palliative care services, administered oral risperidone, haloperidol, or placebo to 247 patients with life-limiting illness and delirium. Both treatment groups had higher delirium symptom scores than the placebo group.28
In addition, a systematic review and meta-analysis of 19 studies found no benefit of antipsychotic medications for preventing or treating delirium in hospitalized adults.29
Antipsychotics are often continued indefinitely
A retrospective chart review at a US academic health system found30 that among 487 patients with a new antipsychotic medication prescribed during hospitalization, 147 (30.2%) were discharged on an antipsychotic. Of these, 121 (82.3%) had a diagnosis of delirium. Only 15 (12.4%) had discharge summaries that included instructions for discontinuing the drug.
Another US health system retrospectively reviewed antipsychotic use and found31 that out of 260 patients who were newly exposed to an antipsychotic drug during hospitalization, 146 (56.2%) were discharged on an antipsychotic drug, and 65% of these patients were still on the drug at the time of the next hospital admission.
EXERCISE, EXERCISE, EXERCISE
Exercise recommended, but not vitamin D, to prevent falls
In 2018, the US Preventive Services Task Force updated its recommendations for preventing falls in community-dwelling older adults.32 Based on the findings of several trials, the task force recommends exercise interventions for adults age 65 and older who are at increased risk for falls. Gait, balance, and functional training were studied in 17 trials, resistance training in 13, flexibility in 8, endurance training in 5, and tai chi in 3, with 5 studies including general physical activity. Exercise interventions most commonly took place for 3 sessions per week for 12 months (range 2–42 months).
The task force also recommends against vitamin D supplementation for fall prevention in community-dwelling adults age 65 or older who are not known to have osteoporosis or vitamin D deficiency.
Early mobilization helps inpatients
Hospitalized older adults usually spend most of their time in bed. Forty-five previously ambulatory patients (age ≥ 65 without dementia or delirium) in a Veterans Affairs hospital were monitored with wireless accelerometers and were found to spend, on average, 83% of the measured hospital stay in bed. Standing or walking time ranged from 0.2% to 21%, with a median of only 3% (43 minutes a day).33
Since falls with injury became a Centers for Medicare and Medicaid Services nonreimbursable hospital-acquired condition, tension has arisen between promoting mobility and preventing falls.34 Two studies evaluating the adoption of mobility-restricting approaches such as bed-alarms, “fall-alert” signs, supervision of patients in the bathroom, and ensuring patients’ walking aids are within reach, did not find a significant reduction in falls or fall-related injuries.35,36
A clinically significant loss of community mobility is common after hospitalization in older adults.37 Older adults who developed mobility impairment during hospitalization had a higher risk of death in a large, retrospective study.38 A large Canadian multisite intervention trial39 that promoted early mobilization in older patients who were admitted to general medical wards resulted in increased mobilization and significantly shorter hospital stays.
POSTHOSPITAL CARE NEEDS IMPROVEMENT
After hospitalization, older adults who have difficulty with activities of daily living or complex medical needs often require continued care.
About 20% of hospitalized Medicare beneficiaries in the United States are discharged to skilled nursing facilities.40 This is often a stressful transition, and most people have little guidance on selecting a facility and simply choose one based on its proximity to home.41
A program of frequent visits by hospital-employed physicians and advanced practice professionals at skilled nursing facilities resulted in a significantly lower 30-day readmission rate compared with nonparticipating skilled nursing facilities in the same geographic area.42
Home healthcare is recommended after hospital discharge at a rapidly increasing rate. Overall referral rates increased from 8.6% to 14.1% between 2001 and 2012, and from 14.3% to 24.0% for patients with heart failure.43 A qualitative study of home healthcare nurses found a need for improved care coordination between home healthcare agencies and discharging hospitals, including defining accountability for orders and enhancing communication.44
- Callahan CM, Boustani MA, Schmid AA, et al. Targeting functional decline in Alzheimer disease: a randomized trial. Ann Intern Med 2017; 166(3):164–171. doi:10.7326/M16-0830
- Brasure M, Desai P, Davila H, et al. Physical activity interventions in preventing cognitive decline and Alzheimer-type dementia: a systematic review. Ann Intern Med 2018; 168(1):30–38. doi:10.7326/M17-1528
- Kryscio RJ, Abner EL, Caban-Holt A, et al. Association of antioxidant supplement use and dementia in the Prevention of Alzheimer’s Disease by Vitamin E and Selenium Trial (PREADViSE). JAMA Neurol 2017; 74(5):567–573. doi:10.1001/jamaneurol.2016.5778
- Butler M, Nelson VA, Davila H, et al. Over-the-counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med 2018; 168(1):52–62. doi:10.7326/M17-1530
- Resnick SM, Matsumoto AM, Stephens-Shields AJ, et al. Testosterone treatment and cognitive function in older men with low testosterone and age-associated memory impairment. JAMA 2017; 317(7):717–727. doi:10.1001/jama.2016.21044
- Egan MF, Kost J, Tariot PN, et al. Randomized trial of verubecestat for mild-to-moderate Alzheimer’s disease. N Engl J Med 2018; 378(18):1691–1703. doi:10.1056/NEJMoa1706441
- Honig LS, Vellas B, Woodward M, et al. Trial of solanezumab for mild dementia due to Alzheimer’s disease. N Engl J Med 2018; 378(4):321–330. doi:10.1056/NEJMoa1705971
- Fink HA, Jutkowitz E, McCarten JR, et al. Pharmacologic interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med 2018; 168(1):39–51. doi:10.7326/M17-1529
- Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol 2016; 73(4):410–416. doi:10.1001/jamaneurol.2015.4791
- Gray SL, Walker RL, Dublin S, et al. Proton pump inhibitor use and dementia risk: prospective population-based study. J Am Geriatr Soc 2018; 66(2):247–253. doi:10.1111/jgs.15073
- de Bruijn RF, Heeringa J, Wolters FJ, et al. Association between atrial fibrillation and dementia in the general population. JAMA Neurol 2015; 72(11):1288–1294. doi:10.1001/jamaneurol.2015.2161
- Friberg L, Rosenqvist M. Less dementia with oral anticoagulation in atrial fibrillation. Eur Heart J 2018; 39(6):453–460. doi:10.1093/eurheartj/ehx579
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363(17):1597–1607. doi:10.1056/NEJMoa1008232
- Haussig S, Mangner N, Dwyer MG, et al. Effect of a cerebral protection device on brain lesions following transcatheter aortic valve implantation in patients with severe aortic stenosis: the CLEAN-TAVI randomized clinical trial. JAMA 2016; 316(6):592–601. doi:10.1001/jama.2016.10302
- Khan MM, Herrmann N, Gallagher D, et al. Cognitive outcomes after transcatheter aortic valve implantation: a metaanalysis. J Am Geriatr Soc 2018; 66(2):254–262. doi:10.1111/jgs.15123
- Choosing Wisely; ABIM Foundation. American Geriatrics Society: ten things physicians and patients should question. www.choosingwisely.org/societies/american-geriatrics-society. Accessed November 6, 2018.
- Lieberman JA 3rd. Metabolic changes associated with antipsychotic use. Prim Care Companion J Clin Psychiatry 2004; 6(suppl 2):8–13. pmid:16001095
- Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA 2005; 294(15):1934–1943. doi:10.1001/jama.294.15.1934
- Choosing Wisely; ABIM Foundation. American Psychiatric Association: five things physicians and patients should question. www.choosingwisely.org/societies/american-psychiatric-association. Accessed November 6, 2018.
- Maust DT, Kim HM, Chiang C, Kales HC. Association of the Centers for Medicare & Medicaid Services’ National Partnership to improve dementia care with the use of antipsychotics and other psychotropics in long-term care in the United States from 2009 to 2014. JAMA Intern Med 2018; 178(5):640–647. doi:10.1001/jamainternmed.2018.0379
- CNN. The little red pill being pushed on the elderly. www.cnn.com/2017/10/12/health/nuedexta-nursing-homes-invs/index.html. Accessed November 6, 2018.
- Cummings JL, Lyketsos CG, Peskind ER, et al. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA 2015; 314(12):1242–1254. doi:10.1001/jama.2015.10214
- Ballard C, Banister C, Khan Z, et al; ADP Investigators. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer’s disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol 2018; 17(3):213–222. doi:10.1016/S1474-4422(18)30039-5
- Inouye SK. Delirium in older persons. N Engl J Med 2006; 354(11):1157–1165. doi:10.1056/NEJMra052321
- Cole MG, McCusker J, Bailey R, et al. Partial and no recovery from delirium after hospital discharge predict increased adverse events. Age Ageing 2017; 46(1):90–95. doi:10.1093/ageing/afw153
- Chen CC, Li HC, Liang JT, et al. Effect of a modified hospital elder life program on delirium and length of hospital stay in patients undergoing abdominal surgery: a cluster randomized clinical trial. JAMA Surg 2017; 152(9):827–834. doi:10.1001/jamasurg.2017.1083
- van den Boogaard M, Slooter AJC, Brüggemann RJM, et al. Effect of haloperidol on survival among critically ill adults with a high risk of delirium: the REDUCE randomized clinical trial. JAMA 2018; 319(7):680–690. doi:10.1001/jama.2018.0160
- Agar MR, Lawlor PG, Quinn S, et al. Efficacy of oral risperidone, haloperidol, or placebo for symptoms of delirium among patients in palliative care: a randomized clinical trial. JAMA Intern Med 2017; 177(1):34–42. doi:10.1001/jamainternmed.2016.7491
- Neufeld KJ, Yue J, Robinson TN, Inouye SK, Needham DM. Antipsychotic medication for prevention and treatment of delirium in hospitalized adults: a systematic review and meta-analysis. J Am Geriatr Soc 2016; 64(4):705–714. doi:10.1111/jgs.14076
- Johnson KG, Fashoyin A, Madden-Fuentes R, Muzyk AJ, Gagliardi JP, Yanamadala M. Discharge plans for geriatric inpatients with delirium: a plan to stop antipsychotics? J Am Geriatr Soc 2017; 65(10):2278–2281. doi:10.1111/jgs.15026
- Loh KP, Ramdass S, Garb JL, et al. Long-term outcomes of elders discharged on antipsychotics. J Hosp Med 2016; 11(8):550–555. doi:10.1002/jhm.2585
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Interventions to prevent falls in community-dwelling older adults: US Preventive Services Task Force Recommendation statement. JAMA 2018; 319(16):1696–1704. doi:10.1001/jama.2018.3097
- Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc 2009; 57(9):1660–1665. doi:10.1111/j.1532-5415.2009.02393.x
- Growdon ME, Shorr RI, Inouye SK. The tension between promoting mobility and preventing falls in the hospital. JAMA Intern Med 2017; 177(6):759–760. doi:10.1001/jamainternmed.2017.0840
- Barker AL, Morello RT, Wolfe R, et al. 6-PACK programme to decrease fall injuries in acute hospitals: cluster randomised controlled trial. BMJ 2016; 352:h6781. doi:10.1136/bmj.h6781
- Shorr RI, Chandler AM, Mion LC, et al. Effects of an intervention to increase bed alarm use to prevent falls in hospitalized patients: a cluster randomized trial. Ann Intern Med 2012; 157(10):692–699. doi:10.7326/0003-4819-157-10-201211200-00005
- Loyd C, Beasley TM, Miltner RS, Clark D, King B, Brown CJ. Trajectories of community mobility recovery after hospitalization in older adults. J Am Geriatr Soc 2018; 66(7):1399–1403. doi:10.1111/jgs.15397
- Valiani V, Chen Z, Lipori G, Pahor M, Sabbá C, Manini TM. Prognostic value of Braden Activity subscale for mobility status in hospitalized older adults. J Hosp Med 2017; 12(6):396–401. doi:10.12788/jhm.2748
- Liu B, Moore JE, Almaawiy U, et al; MOVE ON Collaboration. Outcomes of mobilisation of vulnerable elders in Ontario (MOVE ON): a multisite interrupted time series evaluation of an implementation intervention to increase patient mobilisation. Age Ageing 2018; 47(1):112–119. doi:10.1093/ageing/afx128
- Report to Congress: Medicare Payment Policy. Medicare Payment Advisory Commission 2016. www.medpac.gov/docs/default-source/reports/march-2016-report-to-the-congress-medicare-payment-policy.pdf?sfvrsn=0. Accessed November 6, 2018.
- Gadbois EA, Tyler DA, Mor V. Selecting a skilled nursing facility for postacute care: individual and family perspectives. J Am Geriatr Soc 2017; 65(11):2459–2465. doi:10.1111/jgs.14988
- Kim LD, Kou L, Hu B, Gorodeski EZ, Rothberg MB. Impact of a connected care model on 30-day readmission rates from skilled nursing facilities. J Hosp Med 2017; 12(4):238–244. doi:10.12788/jhm.2710
- Jones CD, Ginde AA, Burke RE, Wald HL, Masoudi FA, Boxer RS. Increasing home healthcare referrals upon discharge from U.S. hospitals: 2001-2012. J Am Geriatr Soc 2015; 63(6):1265–1266. doi:10.1111/jgs.13467
- Jones CD, Jones J, Richard A, et al. “Connecting the dots”: a qualitative study of home health nurse perspectives on coordinating care for recently discharged patients. J Gen Intern Med 2017; 32(10):1114–1121. doi:10.1007/s11606-017-4104-0
- Callahan CM, Boustani MA, Schmid AA, et al. Targeting functional decline in Alzheimer disease: a randomized trial. Ann Intern Med 2017; 166(3):164–171. doi:10.7326/M16-0830
- Brasure M, Desai P, Davila H, et al. Physical activity interventions in preventing cognitive decline and Alzheimer-type dementia: a systematic review. Ann Intern Med 2018; 168(1):30–38. doi:10.7326/M17-1528
- Kryscio RJ, Abner EL, Caban-Holt A, et al. Association of antioxidant supplement use and dementia in the Prevention of Alzheimer’s Disease by Vitamin E and Selenium Trial (PREADViSE). JAMA Neurol 2017; 74(5):567–573. doi:10.1001/jamaneurol.2016.5778
- Butler M, Nelson VA, Davila H, et al. Over-the-counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med 2018; 168(1):52–62. doi:10.7326/M17-1530
- Resnick SM, Matsumoto AM, Stephens-Shields AJ, et al. Testosterone treatment and cognitive function in older men with low testosterone and age-associated memory impairment. JAMA 2017; 317(7):717–727. doi:10.1001/jama.2016.21044
- Egan MF, Kost J, Tariot PN, et al. Randomized trial of verubecestat for mild-to-moderate Alzheimer’s disease. N Engl J Med 2018; 378(18):1691–1703. doi:10.1056/NEJMoa1706441
- Honig LS, Vellas B, Woodward M, et al. Trial of solanezumab for mild dementia due to Alzheimer’s disease. N Engl J Med 2018; 378(4):321–330. doi:10.1056/NEJMoa1705971
- Fink HA, Jutkowitz E, McCarten JR, et al. Pharmacologic interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med 2018; 168(1):39–51. doi:10.7326/M17-1529
- Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol 2016; 73(4):410–416. doi:10.1001/jamaneurol.2015.4791
- Gray SL, Walker RL, Dublin S, et al. Proton pump inhibitor use and dementia risk: prospective population-based study. J Am Geriatr Soc 2018; 66(2):247–253. doi:10.1111/jgs.15073
- de Bruijn RF, Heeringa J, Wolters FJ, et al. Association between atrial fibrillation and dementia in the general population. JAMA Neurol 2015; 72(11):1288–1294. doi:10.1001/jamaneurol.2015.2161
- Friberg L, Rosenqvist M. Less dementia with oral anticoagulation in atrial fibrillation. Eur Heart J 2018; 39(6):453–460. doi:10.1093/eurheartj/ehx579
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363(17):1597–1607. doi:10.1056/NEJMoa1008232
- Haussig S, Mangner N, Dwyer MG, et al. Effect of a cerebral protection device on brain lesions following transcatheter aortic valve implantation in patients with severe aortic stenosis: the CLEAN-TAVI randomized clinical trial. JAMA 2016; 316(6):592–601. doi:10.1001/jama.2016.10302
- Khan MM, Herrmann N, Gallagher D, et al. Cognitive outcomes after transcatheter aortic valve implantation: a metaanalysis. J Am Geriatr Soc 2018; 66(2):254–262. doi:10.1111/jgs.15123
- Choosing Wisely; ABIM Foundation. American Geriatrics Society: ten things physicians and patients should question. www.choosingwisely.org/societies/american-geriatrics-society. Accessed November 6, 2018.
- Lieberman JA 3rd. Metabolic changes associated with antipsychotic use. Prim Care Companion J Clin Psychiatry 2004; 6(suppl 2):8–13. pmid:16001095
- Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA 2005; 294(15):1934–1943. doi:10.1001/jama.294.15.1934
- Choosing Wisely; ABIM Foundation. American Psychiatric Association: five things physicians and patients should question. www.choosingwisely.org/societies/american-psychiatric-association. Accessed November 6, 2018.
- Maust DT, Kim HM, Chiang C, Kales HC. Association of the Centers for Medicare & Medicaid Services’ National Partnership to improve dementia care with the use of antipsychotics and other psychotropics in long-term care in the United States from 2009 to 2014. JAMA Intern Med 2018; 178(5):640–647. doi:10.1001/jamainternmed.2018.0379
- CNN. The little red pill being pushed on the elderly. www.cnn.com/2017/10/12/health/nuedexta-nursing-homes-invs/index.html. Accessed November 6, 2018.
- Cummings JL, Lyketsos CG, Peskind ER, et al. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA 2015; 314(12):1242–1254. doi:10.1001/jama.2015.10214
- Ballard C, Banister C, Khan Z, et al; ADP Investigators. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer’s disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol 2018; 17(3):213–222. doi:10.1016/S1474-4422(18)30039-5
- Inouye SK. Delirium in older persons. N Engl J Med 2006; 354(11):1157–1165. doi:10.1056/NEJMra052321
- Cole MG, McCusker J, Bailey R, et al. Partial and no recovery from delirium after hospital discharge predict increased adverse events. Age Ageing 2017; 46(1):90–95. doi:10.1093/ageing/afw153
- Chen CC, Li HC, Liang JT, et al. Effect of a modified hospital elder life program on delirium and length of hospital stay in patients undergoing abdominal surgery: a cluster randomized clinical trial. JAMA Surg 2017; 152(9):827–834. doi:10.1001/jamasurg.2017.1083
- van den Boogaard M, Slooter AJC, Brüggemann RJM, et al. Effect of haloperidol on survival among critically ill adults with a high risk of delirium: the REDUCE randomized clinical trial. JAMA 2018; 319(7):680–690. doi:10.1001/jama.2018.0160
- Agar MR, Lawlor PG, Quinn S, et al. Efficacy of oral risperidone, haloperidol, or placebo for symptoms of delirium among patients in palliative care: a randomized clinical trial. JAMA Intern Med 2017; 177(1):34–42. doi:10.1001/jamainternmed.2016.7491
- Neufeld KJ, Yue J, Robinson TN, Inouye SK, Needham DM. Antipsychotic medication for prevention and treatment of delirium in hospitalized adults: a systematic review and meta-analysis. J Am Geriatr Soc 2016; 64(4):705–714. doi:10.1111/jgs.14076
- Johnson KG, Fashoyin A, Madden-Fuentes R, Muzyk AJ, Gagliardi JP, Yanamadala M. Discharge plans for geriatric inpatients with delirium: a plan to stop antipsychotics? J Am Geriatr Soc 2017; 65(10):2278–2281. doi:10.1111/jgs.15026
- Loh KP, Ramdass S, Garb JL, et al. Long-term outcomes of elders discharged on antipsychotics. J Hosp Med 2016; 11(8):550–555. doi:10.1002/jhm.2585
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Interventions to prevent falls in community-dwelling older adults: US Preventive Services Task Force Recommendation statement. JAMA 2018; 319(16):1696–1704. doi:10.1001/jama.2018.3097
- Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc 2009; 57(9):1660–1665. doi:10.1111/j.1532-5415.2009.02393.x
- Growdon ME, Shorr RI, Inouye SK. The tension between promoting mobility and preventing falls in the hospital. JAMA Intern Med 2017; 177(6):759–760. doi:10.1001/jamainternmed.2017.0840
- Barker AL, Morello RT, Wolfe R, et al. 6-PACK programme to decrease fall injuries in acute hospitals: cluster randomised controlled trial. BMJ 2016; 352:h6781. doi:10.1136/bmj.h6781
- Shorr RI, Chandler AM, Mion LC, et al. Effects of an intervention to increase bed alarm use to prevent falls in hospitalized patients: a cluster randomized trial. Ann Intern Med 2012; 157(10):692–699. doi:10.7326/0003-4819-157-10-201211200-00005
- Loyd C, Beasley TM, Miltner RS, Clark D, King B, Brown CJ. Trajectories of community mobility recovery after hospitalization in older adults. J Am Geriatr Soc 2018; 66(7):1399–1403. doi:10.1111/jgs.15397
- Valiani V, Chen Z, Lipori G, Pahor M, Sabbá C, Manini TM. Prognostic value of Braden Activity subscale for mobility status in hospitalized older adults. J Hosp Med 2017; 12(6):396–401. doi:10.12788/jhm.2748
- Liu B, Moore JE, Almaawiy U, et al; MOVE ON Collaboration. Outcomes of mobilisation of vulnerable elders in Ontario (MOVE ON): a multisite interrupted time series evaluation of an implementation intervention to increase patient mobilisation. Age Ageing 2018; 47(1):112–119. doi:10.1093/ageing/afx128
- Report to Congress: Medicare Payment Policy. Medicare Payment Advisory Commission 2016. www.medpac.gov/docs/default-source/reports/march-2016-report-to-the-congress-medicare-payment-policy.pdf?sfvrsn=0. Accessed November 6, 2018.
- Gadbois EA, Tyler DA, Mor V. Selecting a skilled nursing facility for postacute care: individual and family perspectives. J Am Geriatr Soc 2017; 65(11):2459–2465. doi:10.1111/jgs.14988
- Kim LD, Kou L, Hu B, Gorodeski EZ, Rothberg MB. Impact of a connected care model on 30-day readmission rates from skilled nursing facilities. J Hosp Med 2017; 12(4):238–244. doi:10.12788/jhm.2710
- Jones CD, Ginde AA, Burke RE, Wald HL, Masoudi FA, Boxer RS. Increasing home healthcare referrals upon discharge from U.S. hospitals: 2001-2012. J Am Geriatr Soc 2015; 63(6):1265–1266. doi:10.1111/jgs.13467
- Jones CD, Jones J, Richard A, et al. “Connecting the dots”: a qualitative study of home health nurse perspectives on coordinating care for recently discharged patients. J Gen Intern Med 2017; 32(10):1114–1121. doi:10.1007/s11606-017-4104-0
KEY POINTS
- Oral anticoagulant treatment for atrial fibrillation helps preserve cognitive function.
- Antipsychotics are not recommended as initial therapy for dementia-associated behavioral disturbances or for hospitalization-induced delirium.
- A multicomponent inpatient program can help prevent postoperative delirium in hospitalized patients.
- The US Preventive Services Task Force recommends exercise to prevent falls.
- Early mobility should be encouraged for hospitalized patients.
- Better continuity of care between hospitals and skilled nursing facilities can reduce hospital readmission rates.
Alzheimer dementia: Starting, stopping drug therapy
Alzheimer disease is the most common form of dementia. In 2016, an estimated 5.2 million Americans age 65 and older had Alzheimer disease. The prevalence is projected to increase to 13.8 million by 2050, including 7 million people age 85 and older.1
Although no cure for dementia exists, several cognition-enhancing drugs have been approved by the US Food and Drug Administration (FDA) to treat the symptoms of Alzheimer dementia. The purpose of these drugs is to stabilize cognitive and functional status, with a secondary benefit of potentially reducing behavioral problems associated with dementia.
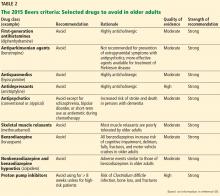
CURRENTLY APPROVED DRUGS
Two classes of drugs are approved to treat Alzheimer disease: cholinesterase inhibitors and an N-methyl-d-aspartate (NMDA) receptor antagonist (Table 1).
Cholinesterase inhibitors
The cholinesterase inhibitors act by reversibly binding and inactivating acetylcholinesterase, consequently increasing the time the neurotransmitter acetylcholine remains in the synaptic cleft. The 3 FDA-approved cholinesterase inhibitors are donepezil, galantamine, and rivastigmine. Tacrine, the first approved cholinesterase inhibitor, was removed from the US market after reports of severe hepatic toxicity.2
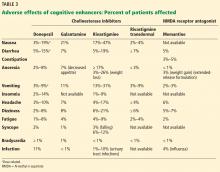
The clinical efficacy of cholinesterase inhibitors in improving cognitive function has been shown in several randomized controlled trials.3–10 However, benefits were generally modest, and some trials used questionable methodology, leading experts to challenge the overall efficacy of these agents.
All 3 drugs are approved for mild to moderate Alzheimer disease (stages 4–6 on the Global Deterioration Scale; Table 2)11,12; only donepezil is approved for severe Alzheimer disease. Rivastigmine has an added indication for treating mild to moderate dementia associated with Parkinson disease. Cholinesterase inhibitors are often used off-label to treat other forms of dementia such as vascular dementia, mixed dementia, and dementia with Lewy bodies.13
NMDA receptor antagonist
Memantine, currently the only FDA-approved NMDA receptor antagonist, acts by reducing neuronal calcium ion influx and its associated excitation and toxicity. Memantine is approved for moderate to severe Alzheimer disease.
Combination therapy
Often, these 2 classes of medications are prescribed in combination. In a randomized controlled trial that added memantine to stable doses of donepezil, patients had significantly better clinical response on combination therapy than on cholinesterase inhibitor monotherapy.14
In December 2014, the FDA approved a capsule formulation combining donepezil and memantine to treat symptoms of Alzheimer dementia. However, no novel pharmacologic treatment for Alzheimer disease has been approved since 2003. Furthermore, recently Pfizer announced a plan to eliminate 300 research positions aimed at finding new drugs to treat Alzheimer disease and Parkinson disease.15
CONSIDERATIONS WHEN STARTING COGNITIVE ENHANCERS
Cholinesterase inhibitors
Adverse effects of cholinesterase inhibitors are generally mild and well tolerated and subside within 1 to 2 weeks. Gastrointestinal effects are common, primarily diarrhea, nausea, and vomiting. They are transient but can occur in about 20% of patients (Table 3).
Other potential adverse effects include bradycardia, syncope, rhabdomyolysis, neuroleptic malignant syndrome, and esophageal rupture. Often, the side-effect profile helps determine which patients are appropriate candidates for these medications.
As expected, higher doses of donepezil (23 mg vs 5–10 mg) are associated with higher rates of nausea, diarrhea, and vomiting.
Dosing. The cholinesterase inhibitors should be slowly titrated to minimize side effects. Starting at the lowest dose and maintaining it for 4 weeks allows sufficient time for transient side effects to abate. Some patients may require a longer titration period.
As the dose is escalated, the probability of side effects may increase. If they do not subside, dose reduction with maintenance at the next lower dose is appropriate.
Gastrointestinal effects. Given the adverse gastrointestinal effects associated with this class of medications, patients experiencing significant anorexia and weight loss should generally avoid cholinesterase inhibitors. However, the rivastigmine patch, a transdermal formulation, is an alternative for patients who experience gastrointestinal side effects.
Bradycardia risk. Patients with significant bradycardia or who are taking medications that lower the heart rate may experience a worsening of their bradycardia or associated symptoms if they take a cholinesterase inhibitor. Syncope from bradycardia is a significant concern, especially in patients already at risk of falls or fracture due to osteoporosis.
NMDA receptor antagonist
The side-effect profile of memantine is generally more favorable than that of cholinesterase inhibitors. In clinical trials, it has been better tolerated with fewer adverse effects than placebo, with the exception of an increased incidence of dizziness, confusion, and delusions.16,17
Caution is required when treating patients with renal impairment. In patients with a creatinine clearance of 5 to 29 mL/min, the recommended maximum total daily dose is 10 mg (twice-daily formulation) or 14 mg (once-daily formulation).
Off-label use to treat behavioral problems
These medications have been used off-label to treat behavioral problems associated with dementia. A systematic review and meta-analysis showed cholinesterase inhibitor therapy had a statistically significant effect in reducing the severity of behavioral problems.18 Unfortunately, the number of dropouts increased in the active-treatment groups.
Patients with behavioral problems associated with dementia with Lewy bodies may experience a greater response to cholinesterase inhibitors than those with Alzheimer disease.19 Published post hoc analyses suggest that patients with moderate to severe Alzheimer disease receiving memantine therapy have less severe agitation, aggression, irritability, and other behavioral disturbances compared with those on placebo.20,21 However, systematic reviews have not found that memantine has a clinically significant effect on neuropsychiatric symptoms of dementia.18,22,23
Combination therapy
In early randomized controlled trials, adding memantine to a cholinesterase inhibitor provided additional cognitive benefit in patients with Alzheimer disease.15,24 However, a more recent randomized controlled trial did not show significant benefits for combined memantine and donepezil vs donepezil alone in moderate to severe dementia.25
In patients who had mild to moderate Alzheimer disease at 14 Veterans Affairs medical centers who were already on cholinesterase inhibitor treatment, adding memantine did not show benefit. However, the group receiving alpha-tocopherol (vitamin E) showed slower functional decline than those on placebo.26 Cognition and function are not expected to improve with memantine.
CONSIDERATIONS WHEN STOPPING COGNITIVE ENHANCERS
The cholinesterase inhibitors are usually prescribed early in the course of dementia, and some patients take these drugs for years, although no studies have investigated benefit or risk beyond 1 year. It is generally recommended that cholinesterase inhibitor therapy be assessed periodically, eg, every 3 to 6 months, for perceived cognitive benefits and adverse gastrointestinal effects.
These medications should be stopped if the desired effects—stabilizing cognitive and functional status—are not perceived within a reasonable time, such as 12 weeks. In some cases, stopping cholinesterase inhibitor therapy may cause negative effects on cognition and neuropsychiatric symptoms.27
Deciding whether benefit has occurred during a trial of cholinesterase inhibitors often requires input and observations from the family and caregivers. Soliciting this information is key for practitioners to determine the correct treatment approach for each patient.
Although some patients with moderately severe disease experience clinical benefits from cholinesterase inhibitor therapy, it is reasonable to consider discontinuing therapy when a patient has progressed to advanced dementia with loss of functional independence, thus making the use of the therapy—ie, to preserve functional status—less relevant. Results from a randomized discontinuation trial of cholinesterase inhibitors in institutionalized patients with moderate to severe dementia suggest that discontinuation is safe and well tolerated in most of these patients.28
Abruptly stopping high-dose cholinesterase inhibitors is not recommended. Most clinical trials tapered these medications over 2 to 4 weeks. Patients taking the maximum dose of a cholinesterase inhibitor should have the dose reduced to the next lowest dose for 2 weeks before the dose is reduced further or stopped completely.
CONSIDERATIONS FOR OTHER DEMENTIA THERAPY
Behavioral and psychiatric problems often accompany dementia; however, no drugs are approved to treat these symptoms in patients with Alzheimer disease. Nonpharmacologic interventions are recommended as the initial treatment.29 Some practitioners prescribe psychotropic drugs off-label for Alzheimer disease, but most clinical trials have not found these therapies to be very effective for psychiatric symptoms associated with Alzheimer disease.30,31
Recently, a randomized controlled trial of dextromethorphan-quinidine showed mild reduction in agitation in patients with Alzheimer disease, but there were significant increases in falls, dizziness, and diarrhea.32
Patients prescribed medications for behavioral and psychological symptoms of dementia should be assessed every 3 to 6 months to determine if the medications have been effective in reducing the symptoms they were meant to reduce. If there has been no clear reduction in the target behaviors, a trial off the drug should be initiated, with careful monitoring to see if the target behavior changes. Dementia-related behaviors may worsen off the medication, but a lower dose may be found to be as effective as a higher dose. As dementia advances, behaviors initially encountered during one stage may diminish or abate.
In a long-term care setting, a gradual dose-reduction trial of psychotropic medications should be conducted every year to determine if the medications are still necessary.33 This should be considered during routine management and follow-up of patients with dementia-associated behavioral problems.
REASONABLE TO TRY
Cognitive enhancers have been around for more than 10 years and are reasonable to try in patients with Alzheimer disease. All the available drugs are FDA-approved for reducing dementia symptoms associated with mild to moderate Alzheimer disease; donepezil and memantine are also approved for severe Alzheimer disease, either in combination or as monotherapy.
When selecting a cognitive enhancer, practitioners need to consider the potential for adverse effects. And if a cholinesterase inhibitor is prescribed, it is important to periodically assess for perceived cognitive benefits and adverse gastrointestinal effects. The NMDA receptor antagonist has a more favorable side effect profile. Combining the drugs is also an option.
Similarly, patients prescribed psychotropic medications for behavioral problems related to dementia should be reassessed to determine if the dose could be reduced or eliminated, particularly if targeted behaviors have not responded to the treatment or the dementia has advanced.
For patients on cognitive enhancers, discontinuation should be considered when the dementia advances to the point where the patient is totally dependent for all basic activities of daily living, and the initial intended purpose of these medications—preservation of cognitive and functional status—is no longer achievable.
- Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013; 80:1778–1783.
- Watkins PB, Zimmerman HJ, Knapp MJ, et al. Hepatotoxic effects of tacrine administration in patients with Alzheimer’s disease. JAMA 1994; 271:992–998.
- Courtney C, Farrell D, Gray R, et al. Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): randomised double-blind trial. Lancet 2004; 363:2105–2115.
- Wang J, Yu JT, Wang HF, et al. Pharmacological treatment of neuropsychiatric symptoms in Alzheimer’s disease: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2015; 86:101–109.
- Raina P, Santaguida P, Ismaila A, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med 2008; 148:379–397.
- Lanctot KL, Hermann N, Yau KK, et al. Efficacy and safety of cholinesterase inhibitors in Alzheimer’s disease: a meta-analysis. CMAJ 2003; 169:557–564.
- Qaseem A, Snow V, Cross JT Jr, et al. Current pharmacologic treatment of dementia: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 2008; 148:370–378.
- Trinh NH, Hoblyn J, Mohanty S, Yaffe K. Efficacy of cholinesterase inhibitors in the treatment of neuropsychiatric symptoms and functional impairment in Alzheimer disease: a meta-analysis. JAMA 2003; 289:210–216.
- Kaduszkiewicz H, Zimmermann T, Beck-Bornholdt HP, van den Bussche H. Cholinesterase inhibitors for patients with Alzheimer’s disease: systematic review of randomised clinical trials. BMJ 2005; 331:321–327.
- Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev 2006; 1:CD005593.
- Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry 1982; 139:1136–1139.
- Mitchell SL. Advanced dementia. N Engl J Med 2015; 372:2533–2540.
- Rolinski M, Fox C, Maidment I, McShane R. Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson’s disease dementia and cognitive impairment in Parkinson’s disease. Cochrane Database Syst Rev 2012; 3:CD006504.
- Tariot PN, Farlow MR, Grossberg GT, et al. Memantine treatment in patients with moderate to severe Alzheimer’s disease already receiving donepezil: a randomized controlled trial. JAMA 2004; 291:317–324.
- Reuters Staff. Pfizer ends research for new Alzheimer’s, Parkinson’s drugs. January 7, 2018. https://www.reuters.com/article/us-pfizer-alzheimers/pfizer-ends-research-for-new-alzheimers-parkinsons-drugs-idUSKBN1EW0TN. Accessed February 2, 2018.
- Aerosa SA, Sherriff F, McShane R. Memantine for dementia. Cochrane Database Syst Rev 2005 Jul 20;(3):CD003154.
- Rossom R, Adityanjee, Dysken M. Efficacy and tolerability of memantine in the treatment of dementia. Am J Geriatr Pharmacother 2004; 2:303–312.
- Wang J, Yu JT, Wang HF, et al. Pharmacological treatment of neuropsychiatric symptoms in Alzheimer’s disease: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2015; 86:101–109.
- McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomized, double-blind, placebo-controlled international study. Lancet 2000; 356:2031–2036.
- Cummings JL, Schneider E, Tariot PN, Graham SM, Memantine MEM-MD-02 Study Group. Behavioral effects of memantine in Alzheimer disease patients receiving donepezil treatment. Neurology 2006; 67:57–63.
- Wilcock GK, Ballard CG, Cooper JA, Loft H. Memantine for agitation/aggression and psychosis in moderately severe to severe Alzheimer’s disease: a pooled analysis of 3 studies. J Clin Psychiatry 2008; 69:341–348.
- Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA 2005; 293:596–608.
- McShane R, Areosa Sastre A, Minakaran N. Memantine for dementia. Cochrane Database Syst Rev 2006; 2:CD003154.
- Reisberg B, Doody R, Stoffler A, et al. Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med 2003; 348:1333–1341.
- Howard R, McShane R, Lindesay J, et al. Donepezil and memantine for moderate-to-severe Alzheimer’s disease. N Engl J Med 2012; 366:893–903.
- Dysken MW, Sano M, Asthana S, et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomized trial. JAMA 2014; 311:33–44.
- O’Regan J, Lanctot KL, Mazereeuw G, Herrmann N. Cholinesterase inhibitor discontinuation in patients with Alzheimer’s disease: a meta-analysis of randomized controlled trials. J Clin Psychiatry 2015; 76:e1424–e1431.
- Herrmann N, O’Reagan J, Ruthirahukhan M, et al. A randomized placebo-controlled discontinuation study of cholinesterase inhibitors in institutionalized patients with moderate to severe Alzheimer disease. J Am Med Dir Assoc 2016; 17:142–174.
- Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacological management of behavioral symptoms in dementia. JAMA 2012; 308:2020–2029.
- Schwab W, Messinger-Rapport B, Franco K. Psychiatric symptoms of dementia: treatable, but no silver bullet. Cleve Clin J Med 2009; 76:167–174.
- Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA 2005; 293:596–608.
- Cummings JL, Lyketsos CG, Peskind ER, et al. Effects of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA 2015; 314:1242–1254.
- Centers for Medicare and Medicaid Services. Dementia care in nursing homes: clarification to Appendix P State Operations Manual (SOM) and Appendix PP in the SOM for F309—quality of care and F329—unnecessary drugs. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/Downloads/Survey-and-Cert-Letter-13-35.pdf. Accessed February 1, 2018.
Alzheimer disease is the most common form of dementia. In 2016, an estimated 5.2 million Americans age 65 and older had Alzheimer disease. The prevalence is projected to increase to 13.8 million by 2050, including 7 million people age 85 and older.1
Although no cure for dementia exists, several cognition-enhancing drugs have been approved by the US Food and Drug Administration (FDA) to treat the symptoms of Alzheimer dementia. The purpose of these drugs is to stabilize cognitive and functional status, with a secondary benefit of potentially reducing behavioral problems associated with dementia.
CURRENTLY APPROVED DRUGS
Two classes of drugs are approved to treat Alzheimer disease: cholinesterase inhibitors and an N-methyl-d-aspartate (NMDA) receptor antagonist (Table 1).
Cholinesterase inhibitors
The cholinesterase inhibitors act by reversibly binding and inactivating acetylcholinesterase, consequently increasing the time the neurotransmitter acetylcholine remains in the synaptic cleft. The 3 FDA-approved cholinesterase inhibitors are donepezil, galantamine, and rivastigmine. Tacrine, the first approved cholinesterase inhibitor, was removed from the US market after reports of severe hepatic toxicity.2
The clinical efficacy of cholinesterase inhibitors in improving cognitive function has been shown in several randomized controlled trials.3–10 However, benefits were generally modest, and some trials used questionable methodology, leading experts to challenge the overall efficacy of these agents.
All 3 drugs are approved for mild to moderate Alzheimer disease (stages 4–6 on the Global Deterioration Scale; Table 2)11,12; only donepezil is approved for severe Alzheimer disease. Rivastigmine has an added indication for treating mild to moderate dementia associated with Parkinson disease. Cholinesterase inhibitors are often used off-label to treat other forms of dementia such as vascular dementia, mixed dementia, and dementia with Lewy bodies.13
NMDA receptor antagonist
Memantine, currently the only FDA-approved NMDA receptor antagonist, acts by reducing neuronal calcium ion influx and its associated excitation and toxicity. Memantine is approved for moderate to severe Alzheimer disease.
Combination therapy
Often, these 2 classes of medications are prescribed in combination. In a randomized controlled trial that added memantine to stable doses of donepezil, patients had significantly better clinical response on combination therapy than on cholinesterase inhibitor monotherapy.14
In December 2014, the FDA approved a capsule formulation combining donepezil and memantine to treat symptoms of Alzheimer dementia. However, no novel pharmacologic treatment for Alzheimer disease has been approved since 2003. Furthermore, recently Pfizer announced a plan to eliminate 300 research positions aimed at finding new drugs to treat Alzheimer disease and Parkinson disease.15
CONSIDERATIONS WHEN STARTING COGNITIVE ENHANCERS
Cholinesterase inhibitors
Adverse effects of cholinesterase inhibitors are generally mild and well tolerated and subside within 1 to 2 weeks. Gastrointestinal effects are common, primarily diarrhea, nausea, and vomiting. They are transient but can occur in about 20% of patients (Table 3).
Other potential adverse effects include bradycardia, syncope, rhabdomyolysis, neuroleptic malignant syndrome, and esophageal rupture. Often, the side-effect profile helps determine which patients are appropriate candidates for these medications.
As expected, higher doses of donepezil (23 mg vs 5–10 mg) are associated with higher rates of nausea, diarrhea, and vomiting.
Dosing. The cholinesterase inhibitors should be slowly titrated to minimize side effects. Starting at the lowest dose and maintaining it for 4 weeks allows sufficient time for transient side effects to abate. Some patients may require a longer titration period.
As the dose is escalated, the probability of side effects may increase. If they do not subside, dose reduction with maintenance at the next lower dose is appropriate.
Gastrointestinal effects. Given the adverse gastrointestinal effects associated with this class of medications, patients experiencing significant anorexia and weight loss should generally avoid cholinesterase inhibitors. However, the rivastigmine patch, a transdermal formulation, is an alternative for patients who experience gastrointestinal side effects.
Bradycardia risk. Patients with significant bradycardia or who are taking medications that lower the heart rate may experience a worsening of their bradycardia or associated symptoms if they take a cholinesterase inhibitor. Syncope from bradycardia is a significant concern, especially in patients already at risk of falls or fracture due to osteoporosis.
NMDA receptor antagonist
The side-effect profile of memantine is generally more favorable than that of cholinesterase inhibitors. In clinical trials, it has been better tolerated with fewer adverse effects than placebo, with the exception of an increased incidence of dizziness, confusion, and delusions.16,17
Caution is required when treating patients with renal impairment. In patients with a creatinine clearance of 5 to 29 mL/min, the recommended maximum total daily dose is 10 mg (twice-daily formulation) or 14 mg (once-daily formulation).
Off-label use to treat behavioral problems
These medications have been used off-label to treat behavioral problems associated with dementia. A systematic review and meta-analysis showed cholinesterase inhibitor therapy had a statistically significant effect in reducing the severity of behavioral problems.18 Unfortunately, the number of dropouts increased in the active-treatment groups.
Patients with behavioral problems associated with dementia with Lewy bodies may experience a greater response to cholinesterase inhibitors than those with Alzheimer disease.19 Published post hoc analyses suggest that patients with moderate to severe Alzheimer disease receiving memantine therapy have less severe agitation, aggression, irritability, and other behavioral disturbances compared with those on placebo.20,21 However, systematic reviews have not found that memantine has a clinically significant effect on neuropsychiatric symptoms of dementia.18,22,23
Combination therapy
In early randomized controlled trials, adding memantine to a cholinesterase inhibitor provided additional cognitive benefit in patients with Alzheimer disease.15,24 However, a more recent randomized controlled trial did not show significant benefits for combined memantine and donepezil vs donepezil alone in moderate to severe dementia.25
In patients who had mild to moderate Alzheimer disease at 14 Veterans Affairs medical centers who were already on cholinesterase inhibitor treatment, adding memantine did not show benefit. However, the group receiving alpha-tocopherol (vitamin E) showed slower functional decline than those on placebo.26 Cognition and function are not expected to improve with memantine.
CONSIDERATIONS WHEN STOPPING COGNITIVE ENHANCERS
The cholinesterase inhibitors are usually prescribed early in the course of dementia, and some patients take these drugs for years, although no studies have investigated benefit or risk beyond 1 year. It is generally recommended that cholinesterase inhibitor therapy be assessed periodically, eg, every 3 to 6 months, for perceived cognitive benefits and adverse gastrointestinal effects.
These medications should be stopped if the desired effects—stabilizing cognitive and functional status—are not perceived within a reasonable time, such as 12 weeks. In some cases, stopping cholinesterase inhibitor therapy may cause negative effects on cognition and neuropsychiatric symptoms.27
Deciding whether benefit has occurred during a trial of cholinesterase inhibitors often requires input and observations from the family and caregivers. Soliciting this information is key for practitioners to determine the correct treatment approach for each patient.
Although some patients with moderately severe disease experience clinical benefits from cholinesterase inhibitor therapy, it is reasonable to consider discontinuing therapy when a patient has progressed to advanced dementia with loss of functional independence, thus making the use of the therapy—ie, to preserve functional status—less relevant. Results from a randomized discontinuation trial of cholinesterase inhibitors in institutionalized patients with moderate to severe dementia suggest that discontinuation is safe and well tolerated in most of these patients.28
Abruptly stopping high-dose cholinesterase inhibitors is not recommended. Most clinical trials tapered these medications over 2 to 4 weeks. Patients taking the maximum dose of a cholinesterase inhibitor should have the dose reduced to the next lowest dose for 2 weeks before the dose is reduced further or stopped completely.
CONSIDERATIONS FOR OTHER DEMENTIA THERAPY
Behavioral and psychiatric problems often accompany dementia; however, no drugs are approved to treat these symptoms in patients with Alzheimer disease. Nonpharmacologic interventions are recommended as the initial treatment.29 Some practitioners prescribe psychotropic drugs off-label for Alzheimer disease, but most clinical trials have not found these therapies to be very effective for psychiatric symptoms associated with Alzheimer disease.30,31
Recently, a randomized controlled trial of dextromethorphan-quinidine showed mild reduction in agitation in patients with Alzheimer disease, but there were significant increases in falls, dizziness, and diarrhea.32
Patients prescribed medications for behavioral and psychological symptoms of dementia should be assessed every 3 to 6 months to determine if the medications have been effective in reducing the symptoms they were meant to reduce. If there has been no clear reduction in the target behaviors, a trial off the drug should be initiated, with careful monitoring to see if the target behavior changes. Dementia-related behaviors may worsen off the medication, but a lower dose may be found to be as effective as a higher dose. As dementia advances, behaviors initially encountered during one stage may diminish or abate.
In a long-term care setting, a gradual dose-reduction trial of psychotropic medications should be conducted every year to determine if the medications are still necessary.33 This should be considered during routine management and follow-up of patients with dementia-associated behavioral problems.
REASONABLE TO TRY
Cognitive enhancers have been around for more than 10 years and are reasonable to try in patients with Alzheimer disease. All the available drugs are FDA-approved for reducing dementia symptoms associated with mild to moderate Alzheimer disease; donepezil and memantine are also approved for severe Alzheimer disease, either in combination or as monotherapy.
When selecting a cognitive enhancer, practitioners need to consider the potential for adverse effects. And if a cholinesterase inhibitor is prescribed, it is important to periodically assess for perceived cognitive benefits and adverse gastrointestinal effects. The NMDA receptor antagonist has a more favorable side effect profile. Combining the drugs is also an option.
Similarly, patients prescribed psychotropic medications for behavioral problems related to dementia should be reassessed to determine if the dose could be reduced or eliminated, particularly if targeted behaviors have not responded to the treatment or the dementia has advanced.
For patients on cognitive enhancers, discontinuation should be considered when the dementia advances to the point where the patient is totally dependent for all basic activities of daily living, and the initial intended purpose of these medications—preservation of cognitive and functional status—is no longer achievable.
Alzheimer disease is the most common form of dementia. In 2016, an estimated 5.2 million Americans age 65 and older had Alzheimer disease. The prevalence is projected to increase to 13.8 million by 2050, including 7 million people age 85 and older.1
Although no cure for dementia exists, several cognition-enhancing drugs have been approved by the US Food and Drug Administration (FDA) to treat the symptoms of Alzheimer dementia. The purpose of these drugs is to stabilize cognitive and functional status, with a secondary benefit of potentially reducing behavioral problems associated with dementia.
CURRENTLY APPROVED DRUGS
Two classes of drugs are approved to treat Alzheimer disease: cholinesterase inhibitors and an N-methyl-d-aspartate (NMDA) receptor antagonist (Table 1).
Cholinesterase inhibitors
The cholinesterase inhibitors act by reversibly binding and inactivating acetylcholinesterase, consequently increasing the time the neurotransmitter acetylcholine remains in the synaptic cleft. The 3 FDA-approved cholinesterase inhibitors are donepezil, galantamine, and rivastigmine. Tacrine, the first approved cholinesterase inhibitor, was removed from the US market after reports of severe hepatic toxicity.2
The clinical efficacy of cholinesterase inhibitors in improving cognitive function has been shown in several randomized controlled trials.3–10 However, benefits were generally modest, and some trials used questionable methodology, leading experts to challenge the overall efficacy of these agents.
All 3 drugs are approved for mild to moderate Alzheimer disease (stages 4–6 on the Global Deterioration Scale; Table 2)11,12; only donepezil is approved for severe Alzheimer disease. Rivastigmine has an added indication for treating mild to moderate dementia associated with Parkinson disease. Cholinesterase inhibitors are often used off-label to treat other forms of dementia such as vascular dementia, mixed dementia, and dementia with Lewy bodies.13
NMDA receptor antagonist
Memantine, currently the only FDA-approved NMDA receptor antagonist, acts by reducing neuronal calcium ion influx and its associated excitation and toxicity. Memantine is approved for moderate to severe Alzheimer disease.
Combination therapy
Often, these 2 classes of medications are prescribed in combination. In a randomized controlled trial that added memantine to stable doses of donepezil, patients had significantly better clinical response on combination therapy than on cholinesterase inhibitor monotherapy.14
In December 2014, the FDA approved a capsule formulation combining donepezil and memantine to treat symptoms of Alzheimer dementia. However, no novel pharmacologic treatment for Alzheimer disease has been approved since 2003. Furthermore, recently Pfizer announced a plan to eliminate 300 research positions aimed at finding new drugs to treat Alzheimer disease and Parkinson disease.15
CONSIDERATIONS WHEN STARTING COGNITIVE ENHANCERS
Cholinesterase inhibitors
Adverse effects of cholinesterase inhibitors are generally mild and well tolerated and subside within 1 to 2 weeks. Gastrointestinal effects are common, primarily diarrhea, nausea, and vomiting. They are transient but can occur in about 20% of patients (Table 3).
Other potential adverse effects include bradycardia, syncope, rhabdomyolysis, neuroleptic malignant syndrome, and esophageal rupture. Often, the side-effect profile helps determine which patients are appropriate candidates for these medications.
As expected, higher doses of donepezil (23 mg vs 5–10 mg) are associated with higher rates of nausea, diarrhea, and vomiting.
Dosing. The cholinesterase inhibitors should be slowly titrated to minimize side effects. Starting at the lowest dose and maintaining it for 4 weeks allows sufficient time for transient side effects to abate. Some patients may require a longer titration period.
As the dose is escalated, the probability of side effects may increase. If they do not subside, dose reduction with maintenance at the next lower dose is appropriate.
Gastrointestinal effects. Given the adverse gastrointestinal effects associated with this class of medications, patients experiencing significant anorexia and weight loss should generally avoid cholinesterase inhibitors. However, the rivastigmine patch, a transdermal formulation, is an alternative for patients who experience gastrointestinal side effects.
Bradycardia risk. Patients with significant bradycardia or who are taking medications that lower the heart rate may experience a worsening of their bradycardia or associated symptoms if they take a cholinesterase inhibitor. Syncope from bradycardia is a significant concern, especially in patients already at risk of falls or fracture due to osteoporosis.
NMDA receptor antagonist
The side-effect profile of memantine is generally more favorable than that of cholinesterase inhibitors. In clinical trials, it has been better tolerated with fewer adverse effects than placebo, with the exception of an increased incidence of dizziness, confusion, and delusions.16,17
Caution is required when treating patients with renal impairment. In patients with a creatinine clearance of 5 to 29 mL/min, the recommended maximum total daily dose is 10 mg (twice-daily formulation) or 14 mg (once-daily formulation).
Off-label use to treat behavioral problems
These medications have been used off-label to treat behavioral problems associated with dementia. A systematic review and meta-analysis showed cholinesterase inhibitor therapy had a statistically significant effect in reducing the severity of behavioral problems.18 Unfortunately, the number of dropouts increased in the active-treatment groups.
Patients with behavioral problems associated with dementia with Lewy bodies may experience a greater response to cholinesterase inhibitors than those with Alzheimer disease.19 Published post hoc analyses suggest that patients with moderate to severe Alzheimer disease receiving memantine therapy have less severe agitation, aggression, irritability, and other behavioral disturbances compared with those on placebo.20,21 However, systematic reviews have not found that memantine has a clinically significant effect on neuropsychiatric symptoms of dementia.18,22,23
Combination therapy
In early randomized controlled trials, adding memantine to a cholinesterase inhibitor provided additional cognitive benefit in patients with Alzheimer disease.15,24 However, a more recent randomized controlled trial did not show significant benefits for combined memantine and donepezil vs donepezil alone in moderate to severe dementia.25
In patients who had mild to moderate Alzheimer disease at 14 Veterans Affairs medical centers who were already on cholinesterase inhibitor treatment, adding memantine did not show benefit. However, the group receiving alpha-tocopherol (vitamin E) showed slower functional decline than those on placebo.26 Cognition and function are not expected to improve with memantine.
CONSIDERATIONS WHEN STOPPING COGNITIVE ENHANCERS
The cholinesterase inhibitors are usually prescribed early in the course of dementia, and some patients take these drugs for years, although no studies have investigated benefit or risk beyond 1 year. It is generally recommended that cholinesterase inhibitor therapy be assessed periodically, eg, every 3 to 6 months, for perceived cognitive benefits and adverse gastrointestinal effects.
These medications should be stopped if the desired effects—stabilizing cognitive and functional status—are not perceived within a reasonable time, such as 12 weeks. In some cases, stopping cholinesterase inhibitor therapy may cause negative effects on cognition and neuropsychiatric symptoms.27
Deciding whether benefit has occurred during a trial of cholinesterase inhibitors often requires input and observations from the family and caregivers. Soliciting this information is key for practitioners to determine the correct treatment approach for each patient.
Although some patients with moderately severe disease experience clinical benefits from cholinesterase inhibitor therapy, it is reasonable to consider discontinuing therapy when a patient has progressed to advanced dementia with loss of functional independence, thus making the use of the therapy—ie, to preserve functional status—less relevant. Results from a randomized discontinuation trial of cholinesterase inhibitors in institutionalized patients with moderate to severe dementia suggest that discontinuation is safe and well tolerated in most of these patients.28
Abruptly stopping high-dose cholinesterase inhibitors is not recommended. Most clinical trials tapered these medications over 2 to 4 weeks. Patients taking the maximum dose of a cholinesterase inhibitor should have the dose reduced to the next lowest dose for 2 weeks before the dose is reduced further or stopped completely.
CONSIDERATIONS FOR OTHER DEMENTIA THERAPY
Behavioral and psychiatric problems often accompany dementia; however, no drugs are approved to treat these symptoms in patients with Alzheimer disease. Nonpharmacologic interventions are recommended as the initial treatment.29 Some practitioners prescribe psychotropic drugs off-label for Alzheimer disease, but most clinical trials have not found these therapies to be very effective for psychiatric symptoms associated with Alzheimer disease.30,31
Recently, a randomized controlled trial of dextromethorphan-quinidine showed mild reduction in agitation in patients with Alzheimer disease, but there were significant increases in falls, dizziness, and diarrhea.32
Patients prescribed medications for behavioral and psychological symptoms of dementia should be assessed every 3 to 6 months to determine if the medications have been effective in reducing the symptoms they were meant to reduce. If there has been no clear reduction in the target behaviors, a trial off the drug should be initiated, with careful monitoring to see if the target behavior changes. Dementia-related behaviors may worsen off the medication, but a lower dose may be found to be as effective as a higher dose. As dementia advances, behaviors initially encountered during one stage may diminish or abate.
In a long-term care setting, a gradual dose-reduction trial of psychotropic medications should be conducted every year to determine if the medications are still necessary.33 This should be considered during routine management and follow-up of patients with dementia-associated behavioral problems.
REASONABLE TO TRY
Cognitive enhancers have been around for more than 10 years and are reasonable to try in patients with Alzheimer disease. All the available drugs are FDA-approved for reducing dementia symptoms associated with mild to moderate Alzheimer disease; donepezil and memantine are also approved for severe Alzheimer disease, either in combination or as monotherapy.
When selecting a cognitive enhancer, practitioners need to consider the potential for adverse effects. And if a cholinesterase inhibitor is prescribed, it is important to periodically assess for perceived cognitive benefits and adverse gastrointestinal effects. The NMDA receptor antagonist has a more favorable side effect profile. Combining the drugs is also an option.
Similarly, patients prescribed psychotropic medications for behavioral problems related to dementia should be reassessed to determine if the dose could be reduced or eliminated, particularly if targeted behaviors have not responded to the treatment or the dementia has advanced.
For patients on cognitive enhancers, discontinuation should be considered when the dementia advances to the point where the patient is totally dependent for all basic activities of daily living, and the initial intended purpose of these medications—preservation of cognitive and functional status—is no longer achievable.
- Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013; 80:1778–1783.
- Watkins PB, Zimmerman HJ, Knapp MJ, et al. Hepatotoxic effects of tacrine administration in patients with Alzheimer’s disease. JAMA 1994; 271:992–998.
- Courtney C, Farrell D, Gray R, et al. Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): randomised double-blind trial. Lancet 2004; 363:2105–2115.
- Wang J, Yu JT, Wang HF, et al. Pharmacological treatment of neuropsychiatric symptoms in Alzheimer’s disease: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2015; 86:101–109.
- Raina P, Santaguida P, Ismaila A, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med 2008; 148:379–397.
- Lanctot KL, Hermann N, Yau KK, et al. Efficacy and safety of cholinesterase inhibitors in Alzheimer’s disease: a meta-analysis. CMAJ 2003; 169:557–564.
- Qaseem A, Snow V, Cross JT Jr, et al. Current pharmacologic treatment of dementia: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 2008; 148:370–378.
- Trinh NH, Hoblyn J, Mohanty S, Yaffe K. Efficacy of cholinesterase inhibitors in the treatment of neuropsychiatric symptoms and functional impairment in Alzheimer disease: a meta-analysis. JAMA 2003; 289:210–216.
- Kaduszkiewicz H, Zimmermann T, Beck-Bornholdt HP, van den Bussche H. Cholinesterase inhibitors for patients with Alzheimer’s disease: systematic review of randomised clinical trials. BMJ 2005; 331:321–327.
- Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev 2006; 1:CD005593.
- Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry 1982; 139:1136–1139.
- Mitchell SL. Advanced dementia. N Engl J Med 2015; 372:2533–2540.
- Rolinski M, Fox C, Maidment I, McShane R. Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson’s disease dementia and cognitive impairment in Parkinson’s disease. Cochrane Database Syst Rev 2012; 3:CD006504.
- Tariot PN, Farlow MR, Grossberg GT, et al. Memantine treatment in patients with moderate to severe Alzheimer’s disease already receiving donepezil: a randomized controlled trial. JAMA 2004; 291:317–324.
- Reuters Staff. Pfizer ends research for new Alzheimer’s, Parkinson’s drugs. January 7, 2018. https://www.reuters.com/article/us-pfizer-alzheimers/pfizer-ends-research-for-new-alzheimers-parkinsons-drugs-idUSKBN1EW0TN. Accessed February 2, 2018.
- Aerosa SA, Sherriff F, McShane R. Memantine for dementia. Cochrane Database Syst Rev 2005 Jul 20;(3):CD003154.
- Rossom R, Adityanjee, Dysken M. Efficacy and tolerability of memantine in the treatment of dementia. Am J Geriatr Pharmacother 2004; 2:303–312.
- Wang J, Yu JT, Wang HF, et al. Pharmacological treatment of neuropsychiatric symptoms in Alzheimer’s disease: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2015; 86:101–109.
- McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomized, double-blind, placebo-controlled international study. Lancet 2000; 356:2031–2036.
- Cummings JL, Schneider E, Tariot PN, Graham SM, Memantine MEM-MD-02 Study Group. Behavioral effects of memantine in Alzheimer disease patients receiving donepezil treatment. Neurology 2006; 67:57–63.
- Wilcock GK, Ballard CG, Cooper JA, Loft H. Memantine for agitation/aggression and psychosis in moderately severe to severe Alzheimer’s disease: a pooled analysis of 3 studies. J Clin Psychiatry 2008; 69:341–348.
- Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA 2005; 293:596–608.
- McShane R, Areosa Sastre A, Minakaran N. Memantine for dementia. Cochrane Database Syst Rev 2006; 2:CD003154.
- Reisberg B, Doody R, Stoffler A, et al. Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med 2003; 348:1333–1341.
- Howard R, McShane R, Lindesay J, et al. Donepezil and memantine for moderate-to-severe Alzheimer’s disease. N Engl J Med 2012; 366:893–903.
- Dysken MW, Sano M, Asthana S, et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomized trial. JAMA 2014; 311:33–44.
- O’Regan J, Lanctot KL, Mazereeuw G, Herrmann N. Cholinesterase inhibitor discontinuation in patients with Alzheimer’s disease: a meta-analysis of randomized controlled trials. J Clin Psychiatry 2015; 76:e1424–e1431.
- Herrmann N, O’Reagan J, Ruthirahukhan M, et al. A randomized placebo-controlled discontinuation study of cholinesterase inhibitors in institutionalized patients with moderate to severe Alzheimer disease. J Am Med Dir Assoc 2016; 17:142–174.
- Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacological management of behavioral symptoms in dementia. JAMA 2012; 308:2020–2029.
- Schwab W, Messinger-Rapport B, Franco K. Psychiatric symptoms of dementia: treatable, but no silver bullet. Cleve Clin J Med 2009; 76:167–174.
- Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA 2005; 293:596–608.
- Cummings JL, Lyketsos CG, Peskind ER, et al. Effects of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA 2015; 314:1242–1254.
- Centers for Medicare and Medicaid Services. Dementia care in nursing homes: clarification to Appendix P State Operations Manual (SOM) and Appendix PP in the SOM for F309—quality of care and F329—unnecessary drugs. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/Downloads/Survey-and-Cert-Letter-13-35.pdf. Accessed February 1, 2018.
- Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013; 80:1778–1783.
- Watkins PB, Zimmerman HJ, Knapp MJ, et al. Hepatotoxic effects of tacrine administration in patients with Alzheimer’s disease. JAMA 1994; 271:992–998.
- Courtney C, Farrell D, Gray R, et al. Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): randomised double-blind trial. Lancet 2004; 363:2105–2115.
- Wang J, Yu JT, Wang HF, et al. Pharmacological treatment of neuropsychiatric symptoms in Alzheimer’s disease: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2015; 86:101–109.
- Raina P, Santaguida P, Ismaila A, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med 2008; 148:379–397.
- Lanctot KL, Hermann N, Yau KK, et al. Efficacy and safety of cholinesterase inhibitors in Alzheimer’s disease: a meta-analysis. CMAJ 2003; 169:557–564.
- Qaseem A, Snow V, Cross JT Jr, et al. Current pharmacologic treatment of dementia: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 2008; 148:370–378.
- Trinh NH, Hoblyn J, Mohanty S, Yaffe K. Efficacy of cholinesterase inhibitors in the treatment of neuropsychiatric symptoms and functional impairment in Alzheimer disease: a meta-analysis. JAMA 2003; 289:210–216.
- Kaduszkiewicz H, Zimmermann T, Beck-Bornholdt HP, van den Bussche H. Cholinesterase inhibitors for patients with Alzheimer’s disease: systematic review of randomised clinical trials. BMJ 2005; 331:321–327.
- Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev 2006; 1:CD005593.
- Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry 1982; 139:1136–1139.
- Mitchell SL. Advanced dementia. N Engl J Med 2015; 372:2533–2540.
- Rolinski M, Fox C, Maidment I, McShane R. Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson’s disease dementia and cognitive impairment in Parkinson’s disease. Cochrane Database Syst Rev 2012; 3:CD006504.
- Tariot PN, Farlow MR, Grossberg GT, et al. Memantine treatment in patients with moderate to severe Alzheimer’s disease already receiving donepezil: a randomized controlled trial. JAMA 2004; 291:317–324.
- Reuters Staff. Pfizer ends research for new Alzheimer’s, Parkinson’s drugs. January 7, 2018. https://www.reuters.com/article/us-pfizer-alzheimers/pfizer-ends-research-for-new-alzheimers-parkinsons-drugs-idUSKBN1EW0TN. Accessed February 2, 2018.
- Aerosa SA, Sherriff F, McShane R. Memantine for dementia. Cochrane Database Syst Rev 2005 Jul 20;(3):CD003154.
- Rossom R, Adityanjee, Dysken M. Efficacy and tolerability of memantine in the treatment of dementia. Am J Geriatr Pharmacother 2004; 2:303–312.
- Wang J, Yu JT, Wang HF, et al. Pharmacological treatment of neuropsychiatric symptoms in Alzheimer’s disease: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2015; 86:101–109.
- McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomized, double-blind, placebo-controlled international study. Lancet 2000; 356:2031–2036.
- Cummings JL, Schneider E, Tariot PN, Graham SM, Memantine MEM-MD-02 Study Group. Behavioral effects of memantine in Alzheimer disease patients receiving donepezil treatment. Neurology 2006; 67:57–63.
- Wilcock GK, Ballard CG, Cooper JA, Loft H. Memantine for agitation/aggression and psychosis in moderately severe to severe Alzheimer’s disease: a pooled analysis of 3 studies. J Clin Psychiatry 2008; 69:341–348.
- Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA 2005; 293:596–608.
- McShane R, Areosa Sastre A, Minakaran N. Memantine for dementia. Cochrane Database Syst Rev 2006; 2:CD003154.
- Reisberg B, Doody R, Stoffler A, et al. Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med 2003; 348:1333–1341.
- Howard R, McShane R, Lindesay J, et al. Donepezil and memantine for moderate-to-severe Alzheimer’s disease. N Engl J Med 2012; 366:893–903.
- Dysken MW, Sano M, Asthana S, et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomized trial. JAMA 2014; 311:33–44.
- O’Regan J, Lanctot KL, Mazereeuw G, Herrmann N. Cholinesterase inhibitor discontinuation in patients with Alzheimer’s disease: a meta-analysis of randomized controlled trials. J Clin Psychiatry 2015; 76:e1424–e1431.
- Herrmann N, O’Reagan J, Ruthirahukhan M, et al. A randomized placebo-controlled discontinuation study of cholinesterase inhibitors in institutionalized patients with moderate to severe Alzheimer disease. J Am Med Dir Assoc 2016; 17:142–174.
- Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacological management of behavioral symptoms in dementia. JAMA 2012; 308:2020–2029.
- Schwab W, Messinger-Rapport B, Franco K. Psychiatric symptoms of dementia: treatable, but no silver bullet. Cleve Clin J Med 2009; 76:167–174.
- Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA 2005; 293:596–608.
- Cummings JL, Lyketsos CG, Peskind ER, et al. Effects of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA 2015; 314:1242–1254.
- Centers for Medicare and Medicaid Services. Dementia care in nursing homes: clarification to Appendix P State Operations Manual (SOM) and Appendix PP in the SOM for F309—quality of care and F329—unnecessary drugs. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/Downloads/Survey-and-Cert-Letter-13-35.pdf. Accessed February 1, 2018.
KEY POINTS
- In 2016, an estimated 5.2 million Americans age 65 and older had Alzheimer disease; by 2050, the prevalence is expected to be 13.8 million.
- Cognitive enhancers (cholinesterase inhibitors and an N-methyl-d-aspartate receptor antagonist) have shown modest efficacy in preserving cognitive function.
- When evaluating therapy with a cognitive enhancer, practitioners need to consider the potential adverse effects, especially gastrointestinal effects with cholinesterase inhibitors.
- Discontinuation should be considered when the dementia reaches the advanced stage and the initial intended purpose of these drugs is no longer achievable.
Medication management in older adults
Medications started for appropriate indications in middle age may need to be monitored more closely as the patient ages. Some drugs may become unnecessary or even dangerous as the patient ages, functional status and renal function decline, and goals of care change.
Older adults tend to have multiple illnesses and therefore take more drugs, and polypharmacy increases the risk of poor outcomes. The number of medications a person uses is a risk factor for adverse drug reactions, nonadherence, financial burden, drug-drug interactions, and worse outcomes.1
The prevalence of polypharmacy increased from an estimated 8.2% to 15% from 1999 to 2011 based on the National Health and Nutrition Examination Survey.2 Guideline-based therapy for specific diseases may lead to the addition of more medications to reach disease targets.3 Most older adults in the United States compound the risk of prescribed medications by also taking over-the-counter medications and dietary supplements.4
In addition, medications are often used in older adults based on studies of younger persons without significant comorbidities. Applying clinical guidelines based on these studies to older adults with comorbidity and functional impairment is challenging.5 Age-related pharmacokinetic and pharmacodynamic changes increase the risk of adverse drug reactions.6
In this article, we review commonly used medications that are potentially inappropriate based on clinical practice. We also review tools to evaluate appropriate drug therapy in older adults.
DRUGS THAT ARE COMMONLY USED, BUT POTENTIALLY INAPPROPRIATE
Statins
Statins are effective when used as secondary prevention in older adults,7 but their efficacy when used as primary prevention of atherosclerotic cardiovascular disease in people age 75 and older is questionable.8 Nevertheless, they are widely used for this purpose. For example, before the 2013 joint guidelines of the American College of Cardiology and the American Heart Association (ACC/AHA) were released, 22% of patients age 80 and older in the Geisinger health system were taking a statin for primary prevention.9
The 2013 ACC/AHA guidelines included a limited recommendation for statins for primary prevention of atherosclerotic cardiovascular disease in adults age 75 and older.10 The guideline noted, however, that few data were available to support this recommendation.10
In a systematic review of 18 randomized clinical trials of statins for primary prevention of atherosclerotic cardiovascular disease, the mean age was 57, yet conclusions were extrapolated to an older patient population.11 The estimated 10-year risk of atherosclerotic cardiovascular disease based on pooled cohort risk equations of adults age 75 and older always exceeds the 7.5% treatment threshold recommended by the guidelines.8
Myopathy is a common adverse effect of statins. In addition, statins interact with other drugs that inhibit the cytochrome P450 3A4 isoenzyme, such as amlodipine, amiodarone, and diltiazem.8,12 If statin therapy caused no functional limitation due to muscle pain or weakness, statins for primary prevention would be cost-effective, but even a small increase in adverse effects in an elderly patient can offset the cardiovascular benefit.13 A recent post hoc secondary analysis found no benefit of pravastatin for primary prevention in adults age 75 and older.14
Thus, statin treatment for primary prevention in older patients should be individualized, based on life expectancy, function, and cardiovascular risk. Statin therapy does not replace modification of other risk factors.
Anticholinergics
Drugs with anticholinergic properties are commonly prescribed in the elderly for conditions such as muscle spasm, overactive bladder, psychiatric disorders, insomnia, extrapyramidal symptoms, vertigo, pruritus, peptic ulcer disease, seasonal allergies, and even the common cold,15 and many of the drugs often prescribed have strong anticholinergic properties (Table 1). Taking multiple medications with anticholinergic properties results in a high “anticholinergic burden,” which is associated with falls, impulsive behavior, poor physical performance, loss of independence, dementia, delirium, and brain atrophy.15–18
The 2014 American College of Physicians guideline on nonsurgical management of urinary incontinence in women recommends pharmacologic treatment for urgency and stress urinary incontinence after failure of nonpharmacologic therapy,19 and many drugs for these urinary symptoms have anticholinergic properties. If an anticholinergic is necessary, an agent that results in a lower anticholinergic burden should be considered in older patients.
A pharmacist-initiated medication review and intervention may be another way to adjust medications to reduce the patient’s anticholinergic burden.20,21 The common use of anticholinergic drugs in older adults reminds us to monitor their use closely.22
Benzodiazepines and nonbenzodiazepines
Benzodiazepines are among the most commonly prescribed psychotropics in developed countries and are prescribed mainly by primary care physicians rather than psychiatrists.23
In 2008, 5.2% of US adults ages 18 to 80 used a benzodiazepine, and long-term use was more prevalent in older patients (ages 65–80).23
Benzodiazepines are prescribed for anxiety,24 insomnia,25 and agitation. They can cause withdrawal26 and have potential for abuse.27 Benzodiazepines are associated with cognitive decline,28 impaired driving,29 falls,30 and hip fractures31 in older adults.
In addition, use of nonbenzodiazepine hypnotics (eg, zolpidem) is on the rise,32 and these drugs are known to increase the risk of hip fracture in nursing home residents.33
The American Geriatrics Society, through the American Board of Internal Medicine’s Choosing Wisely campaign, recommends avoiding benzodiazepines as a first-line treatment for insomnia, agitation, or delirium in older adults.34 Yet prescribing practices with these drugs in primary care settings conflict with guidelines, partly due to lack of training in constructive strategies regarding appropriate use of benzodiazepines.35 Educating patients on the risks and benefits of benzodiazepine treatment, especially long-term use, has been shown to reduce the rate of benzodiazepine-associated secondary events.36
Antipsychotics
Off-label use of antipsychotics is common and is increasing in the United States. In 2008, off-label use of antipsychotic drugs accounted for an estimated $6 billion.37 A common off-label use is to manage behavioral symptoms of dementia, despite a black-box warning about an increased risk of death in patients with dementia who are treated with antipsychotics.38,39 The Choosing Wisely campaign recommends against prescribing antipsychotics as a first-line treatment of behavioral and psychological symptoms of dementia.34
Antipsychotic drugs are associated with risk of acute kidney injury,40 as well as increased risk of falls and fractures (eg, a 52% higher risk of a serious fall, and a 50% higher risk of a nonvertebral osteoporotic fracture).41
Patients with dementia often exhibit aggression, resistance to care, and other challenging or disruptive behaviors. In such instances, antipsychotic drugs are often prescribed, but they provide limited and inconsistent benefits, while causing oversedation and worsening of cognitive function and increasing the likelihood of falling, stroke, and death.38,39,41
Because pharmacologic treatments for dementia are only modestly effective, have notable risks, and do not treat some of the behaviors that family members and caregivers find most distressing, nonpharmacologic measures are recommended as first-line treatment.42 These include caregiver education and support, training in problem-solving, and targeted therapy directed at the underlying causes of specific behaviors (eg, implementing nighttime routines to address sleep disturbances).42 Nonpharmacologic management of behavioral symptoms in dementia can significantly improve quality of life for patients and caregivers.42 Use of antipsychotic drugs in patients with dementia should be limited to cases in which nonpharmacologic measures have failed and patients pose an imminent threat to themselves or others.43
Proton pump inhibitors
Proton pump inhibitors are among the most commonly prescribed medications in the United States, and their use has increased significantly over the decade. It has been estimated that between 25% and 70% of these prescriptions have no appropriate indication.44
There is considerable excess use of acid suppressants in both inpatient and outpatient settings.45,46 In one study, at discharge from an internal medicine service, almost half of patients were taking a proton pump inhibitor.47
Evidence-based guidelines recommend these drugs to treat gastroesophageal reflux disease, nonerosive reflux disease, erosive esophagitis, dyspepsia, and peptic ulcer disease. However, long-term use (ie, beyond 8 weeks) is recommended only for patients with erosive esophagitis, Barrett esophagus, a pathologic hypersecretory condition, or a demonstrated need for maintenance treatment for reflux disease.48
Although proton pump inhibitors are highly effective and have low toxicity, there are reports of an association with Clostridium difficile infection,49 community-acquired pneumonia,50 hip fracture,51 vitamin B12 deficiency,52 atrophic gastritis,53 kidney disease,54 and dementia.55
Nondrug therapies such as weight loss and elevation of the head of the bed may improve esophageal pH levels and reflux symptoms.56
Deprescribing.org has practical advice for healthcare providers, patients, and caregivers on how to discontinue proton pump inhibitors, including videos, algorithms, and guidelines.
TOOLS TO EVALUATE APPROPRIATE DRUG THERAPY
Beers criteria
The Beers criteria (Table 2), developed in 1991 by a geriatrician as an approach to safer, more effective drug therapy in frail elderly nursing home patients,57 were updated by the American Geriatrics Society in 2015 for use in any clinical setting.58 (The criteria are also available as a smartphone application through the American Geriatrics Society at www.americangeriatrics.org.)
The Beers criteria offer evidence-based recommendations on drugs to avoid in the elderly, along with the rationale for use, the quality of evidence behind the recommendation, and the graded strength of the recommendation. The Beers criteria should be viewed through the lens of clinical judgment to offer safer nonpharmacologic and pharmacologic treatments.
The Joint Commission recommends medication reconciliation at every transition of care.59 The Beers criteria are a good starting point for a comprehensive medication review.
STOPP/START criteria
Another tool to aid safe prescribing in older adults is the Screening Tool of Older Persons’ Potentially Inappropriate Prescriptions (STOPP), used in conjuction with the Screening Tool to Alert Doctors to Right Treatment (START). The STOPP/START criteria60,61 are based on an up-to-date literature review and consensus (Table 3).
THE BOTTOM LINE
Physicians caring for older adults need to diligently weigh the benefits of drug therapy and consider the patient’s care goals, current level of functioning, life expectancy, values, and preferences. Statin therapy for primary prevention, anticholinergics, benzodiazepines, antipsychotics, and proton pump inhibitors are widely used without proper indications, pointing to the need for a periodic comprehensive review of medications to reevaluate the risks vs the benefits of the patient’s medications. The Beers criteria and the STOPP/ START criteria can be useful tools for this purpose.
- Steinman MA. Polypharmacy—time to get beyond numbers. JAMA Intern Med 2016; 176:482–483.
- Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA 2015; 314:1818–1831.
- Tinetti ME, Bogardus ST Jr, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med 2004; 351:2870–2874.
- Qato DM, Wilder J, Schumm LP, Gillet V, Alexander GC. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med 2016; 176:473–482.
- Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA 2005; 294:716–724.
- Atkin PA, Veitch PC, Veitch EM, Ogle SJ. The epidemiology of serious adverse drug reactions among the elderly. Drugs Aging 1999; 14:141–152.
- Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016; 338:2532–2561.
- Gurwitz JH, Go AS, Fortman SP. Statins for primary prevention in older adults: uncertainty and the need for more evidence. JAMA 2016; 316:1971–1972.
- Chokshi NP, Messerli FH, Sutin D, Supariwala AA, Shah NR. Appropriateness of statins in patients aged ≥ 80 years and comparison to other age groups. Am J Cardiol 2012; 110:1477–1481.
- Stone NJ, Robinson J, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(suppl 2):S1–S45.
- Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013; 1:CD004816.
- Chatzizisis YS, Koskinas KC, Misirli G, Vaklavas C, Hatzitolios A, Giannoglou GD. Risk factors and drug interactions predisposing to statin-induced myopathy: implications for risk assessment, prevention and treatment. Drug Saf 2010; 33:171–187.
- Odden MC, Pletcher MJ, Coxson PG, et al. Cost-effectiveness and population impact of statins for primary prevention in adults aged 75 years or older in the United States. Ann Intern Med 2015; 162:533–541.
- Han BH, Sutin D, Williamson JD, et al. Effect of statin treatment vs usual care on primary cardiovascular prevention among older adults. The ALLHAT-LLT randomized clinical trial. JAMA Intern Med 2017; 177:955–965.
- Gray SL, Anderson ML, Dublin S, et al. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med 2015; 175:401–407.
- Rudolph JL, Salow MJ, Angelini MC, McGlinchey RE. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med 2008; 168:508–513.
- Hilmer SN, Mager DE, Simonsick EM, et al. A drug burden index to define the functional burden of medications in older people. Arch Intern Med 2007; 167:781–787.
- Risacher SL, McDonald BC, Tallman EF, et al; Alzheimer’s Disease Neuroimaging Initiative. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol 2016; 73:721–732.
- Qaseem A, Dallas P, Forciea MA, Starkey M, Denberg TD, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Nonsurgical management of urinary incontinence in women: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2014; 161:429–440.
- Efjestad AS, Molden E, Oksengard AR. Pharmacist-initiated management of antagonistic interactions between anticholinergic drugs and acetyl cholinesterase inhibitors in individuals with dementia. J Am Geriatr Soc 2013; 61:1624–1625.
- Kersten H, Molden E, Tolo IK, Skovlund E, Engedal K, Wyller TB. Cognitive effects of reducing anticholinergic drug burden in a frail elderly population: a randomized controlled trial. J Gerontol A Biol Sci Med Sci 2013; 68:271–278.
- Curtis LH, Østbye T, Sendersky V, et al. Inappropriate prescribing for elderly Americans in a large outpatient population. Arch Intern Med 2004; 164:1621–1625.
- Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry 2015; 72:136–142.
- Martin JL, Sainz-Pardo M, Furukawa TA, Martín-Sánchez E, Seoane T, Galán C. Benzodiazepines in generalized anxiety disorder: heterogeneity of outcomes based on a systematic review and meta-analysis of clinical trials. J Psychopharmacol 2007; 21:774–782.
- Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med 2007; 22:1335–1350.
- Rickels K, Schweizer E, Case WG, Greenblatt DJ. Long-term therapeutic use of benzodiazepines, I. Effects of abrupt discontinuation. Arch Gen Psychiatry 1990; 47:899–907.
- Fenton MC, Keyes KM, Martins SS, Hasin DS. The role of a prescription in anxiety medication use, abuse, and dependence. Am J Psychiatry 2010; 167:1247–1253.
- Billoti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ 2014; 349:g5205.
- Smink BE, Egberts AC, Lusthof KJ, Uges DR, de Gier JJ. The relationship between benzodiazepine use and traffic accidents: a systemic literature review. CNS Drugs 2010; 24:639–653.
- Tinett, ME, Speechley M, Ginter S. Risk factors for falls among elderly persons living in the community. N Engl J Med 1988; 319:1701–1707.
- Zint K, Haefeli WE, Glynn RJ, Mogun H, Avorn J, Stürmer T. Impact of drug interactions, dosage, and duration of therapy on the risk of hip fracture associated with benzodiazepine use in older adults. Pharmacoepidemiol Drug Saf 2010; 19:1248–1255.
- Briesacher BA, Soumerai SB, Field TS, Fouayzi H, Gurwitz JH. Medicare Part D’s exclusion of benzodiazepines and fracture risk in nursing homes. Arch Intern Med 2010; 170:693–698.
- Berry SD, Lee Y, Cai S, Dore DD. Nonbenzodiazepine sleep medication use and hip fractures in nursing home residents. JAMA Intern Med 2013; 173:754–761.
- American Geriatrics Society. Choosing Wisely. Ten things clinicians and patients should question. www.choosingwisely.org/societies/american-geriatrics-society/. Accessed December 3, 2017.
- Cook JM, Marshall R, Masci C, Coyne JC. Physicians’ perspectives on prescribing benzodiazepines for older adults: a qualitative study. J Gen Intern Med 2007; 22:303–307.
- Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med 2014; 174:890–898.
- Alexander GC, Gallagher SA, Mascola A, Moloney RM, Stafford RS. Increasing off-label use of antipsychotic medications in the United States, 1995–2008. Phamacoepidemiol Drug Saf 2011; 20:177–184.
- Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med 2007; 146:775–786.
- US Food and Drug Administration (FDA). Public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm053171.htm. Accessed December 4, 2017.
- Hwang YJ, Dixon SN, Reiss JP, et al. Atypical antipsychotic drugs and the risk for acute kidney injury and other adverse outcomes in older adults. Ann Intern Med 2014; 161:242–248.
- Fraser L, Liu K, Naylor KL, et al. Falls and fractures with atypical antipsychotic medication use: a population-based cohort study. JAMA Intern Med 2015; 175:450–452.
- Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA 2012; 308:2020–2029.
- Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 2006; 355:1525–1538.
- Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. BMJ 2008; 336:2–3.
- Mazer-Amirshahi M, Mullins PM, van den Anker J, Meltzer A, Pines JM. Rising rates of proton pump inhibitor prescribing in US emergency departments. Am J Emerg Med 2014; 32:618–622.
- Heidelbaugh JJ, Goldberg KL, Inadomi JM. Magnitude and economic effect of overuse of antisecretory therapy in the ambulatory care setting. Am J Manag Care 2010; 16:e228–e324.
- Pham CQ, Regal RE, Bostwich TR, Knauf KS. Acid suppressive therapy used on an inpatient internal medicine service. Ann Pharmacother 2006; 40:1261–1266.
- Kahrilas PJ, Shaheen NJ, Vaezi MF, et al; American Gastroenterological Association. American Gastroenterological Association medical position statement on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135:1383–1391.e1–e5.
- Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med 2010; 170:784–790.
- Gulmez SE, Holm A, Frederiksen H, Jensen TG, Pedersen C, Hallas J. Use of proton pump inhibitors and the risk of community-acquired pneumonia: a population-based case-control study. Arch Intern Med 2007; 167:950–955.
- Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006; 296:2947–2953.
- Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA 2013; 310:2435–2442.
- Kuipers EJ, Lundell L, Klinkenberg-Knol EC, et al. Atrophic gastritis and Helicobacter pylori infection in patients with reflux esophagitis treated with omeprazole or fundoplication. N Engl J Med 1996; 334:1018–1022.
- Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med 2016; 176:238–246.
- Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol 2016; 73:410–416.
- Kaltenbach T, Crockett S, Gerson LB. Are lifestyle measures effective in patients with gastroesophageal reflux disease? An evidence-based approach. Arch Intern Med 2006; 166:965–971.
- Beers MH, Ouslander JG, Rollingher I, Reuben DB, Brooks J, Beck JC. Explicit criteria for determining inappropriate medication use in nursing home residents. Arch Intern Med 1991; 151:1825–1832.
- American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63:2227–2246.
- Joint Commission. Sentinel event alert, Issue 35: using medication reconciliation to prevent errors. www.jointcommission.org/sentinel_event_alert_issue_35_using_medication_reconciliation_to_prevent_errors/. Accessed August 18, 2017.
- Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther 2008; 46:72–83.
- O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 2015; 44:213–218.
Medications started for appropriate indications in middle age may need to be monitored more closely as the patient ages. Some drugs may become unnecessary or even dangerous as the patient ages, functional status and renal function decline, and goals of care change.
Older adults tend to have multiple illnesses and therefore take more drugs, and polypharmacy increases the risk of poor outcomes. The number of medications a person uses is a risk factor for adverse drug reactions, nonadherence, financial burden, drug-drug interactions, and worse outcomes.1
The prevalence of polypharmacy increased from an estimated 8.2% to 15% from 1999 to 2011 based on the National Health and Nutrition Examination Survey.2 Guideline-based therapy for specific diseases may lead to the addition of more medications to reach disease targets.3 Most older adults in the United States compound the risk of prescribed medications by also taking over-the-counter medications and dietary supplements.4
In addition, medications are often used in older adults based on studies of younger persons without significant comorbidities. Applying clinical guidelines based on these studies to older adults with comorbidity and functional impairment is challenging.5 Age-related pharmacokinetic and pharmacodynamic changes increase the risk of adverse drug reactions.6
In this article, we review commonly used medications that are potentially inappropriate based on clinical practice. We also review tools to evaluate appropriate drug therapy in older adults.
DRUGS THAT ARE COMMONLY USED, BUT POTENTIALLY INAPPROPRIATE
Statins
Statins are effective when used as secondary prevention in older adults,7 but their efficacy when used as primary prevention of atherosclerotic cardiovascular disease in people age 75 and older is questionable.8 Nevertheless, they are widely used for this purpose. For example, before the 2013 joint guidelines of the American College of Cardiology and the American Heart Association (ACC/AHA) were released, 22% of patients age 80 and older in the Geisinger health system were taking a statin for primary prevention.9
The 2013 ACC/AHA guidelines included a limited recommendation for statins for primary prevention of atherosclerotic cardiovascular disease in adults age 75 and older.10 The guideline noted, however, that few data were available to support this recommendation.10
In a systematic review of 18 randomized clinical trials of statins for primary prevention of atherosclerotic cardiovascular disease, the mean age was 57, yet conclusions were extrapolated to an older patient population.11 The estimated 10-year risk of atherosclerotic cardiovascular disease based on pooled cohort risk equations of adults age 75 and older always exceeds the 7.5% treatment threshold recommended by the guidelines.8
Myopathy is a common adverse effect of statins. In addition, statins interact with other drugs that inhibit the cytochrome P450 3A4 isoenzyme, such as amlodipine, amiodarone, and diltiazem.8,12 If statin therapy caused no functional limitation due to muscle pain or weakness, statins for primary prevention would be cost-effective, but even a small increase in adverse effects in an elderly patient can offset the cardiovascular benefit.13 A recent post hoc secondary analysis found no benefit of pravastatin for primary prevention in adults age 75 and older.14
Thus, statin treatment for primary prevention in older patients should be individualized, based on life expectancy, function, and cardiovascular risk. Statin therapy does not replace modification of other risk factors.
Anticholinergics
Drugs with anticholinergic properties are commonly prescribed in the elderly for conditions such as muscle spasm, overactive bladder, psychiatric disorders, insomnia, extrapyramidal symptoms, vertigo, pruritus, peptic ulcer disease, seasonal allergies, and even the common cold,15 and many of the drugs often prescribed have strong anticholinergic properties (Table 1). Taking multiple medications with anticholinergic properties results in a high “anticholinergic burden,” which is associated with falls, impulsive behavior, poor physical performance, loss of independence, dementia, delirium, and brain atrophy.15–18
The 2014 American College of Physicians guideline on nonsurgical management of urinary incontinence in women recommends pharmacologic treatment for urgency and stress urinary incontinence after failure of nonpharmacologic therapy,19 and many drugs for these urinary symptoms have anticholinergic properties. If an anticholinergic is necessary, an agent that results in a lower anticholinergic burden should be considered in older patients.
A pharmacist-initiated medication review and intervention may be another way to adjust medications to reduce the patient’s anticholinergic burden.20,21 The common use of anticholinergic drugs in older adults reminds us to monitor their use closely.22
Benzodiazepines and nonbenzodiazepines
Benzodiazepines are among the most commonly prescribed psychotropics in developed countries and are prescribed mainly by primary care physicians rather than psychiatrists.23
In 2008, 5.2% of US adults ages 18 to 80 used a benzodiazepine, and long-term use was more prevalent in older patients (ages 65–80).23
Benzodiazepines are prescribed for anxiety,24 insomnia,25 and agitation. They can cause withdrawal26 and have potential for abuse.27 Benzodiazepines are associated with cognitive decline,28 impaired driving,29 falls,30 and hip fractures31 in older adults.
In addition, use of nonbenzodiazepine hypnotics (eg, zolpidem) is on the rise,32 and these drugs are known to increase the risk of hip fracture in nursing home residents.33
The American Geriatrics Society, through the American Board of Internal Medicine’s Choosing Wisely campaign, recommends avoiding benzodiazepines as a first-line treatment for insomnia, agitation, or delirium in older adults.34 Yet prescribing practices with these drugs in primary care settings conflict with guidelines, partly due to lack of training in constructive strategies regarding appropriate use of benzodiazepines.35 Educating patients on the risks and benefits of benzodiazepine treatment, especially long-term use, has been shown to reduce the rate of benzodiazepine-associated secondary events.36
Antipsychotics
Off-label use of antipsychotics is common and is increasing in the United States. In 2008, off-label use of antipsychotic drugs accounted for an estimated $6 billion.37 A common off-label use is to manage behavioral symptoms of dementia, despite a black-box warning about an increased risk of death in patients with dementia who are treated with antipsychotics.38,39 The Choosing Wisely campaign recommends against prescribing antipsychotics as a first-line treatment of behavioral and psychological symptoms of dementia.34
Antipsychotic drugs are associated with risk of acute kidney injury,40 as well as increased risk of falls and fractures (eg, a 52% higher risk of a serious fall, and a 50% higher risk of a nonvertebral osteoporotic fracture).41
Patients with dementia often exhibit aggression, resistance to care, and other challenging or disruptive behaviors. In such instances, antipsychotic drugs are often prescribed, but they provide limited and inconsistent benefits, while causing oversedation and worsening of cognitive function and increasing the likelihood of falling, stroke, and death.38,39,41
Because pharmacologic treatments for dementia are only modestly effective, have notable risks, and do not treat some of the behaviors that family members and caregivers find most distressing, nonpharmacologic measures are recommended as first-line treatment.42 These include caregiver education and support, training in problem-solving, and targeted therapy directed at the underlying causes of specific behaviors (eg, implementing nighttime routines to address sleep disturbances).42 Nonpharmacologic management of behavioral symptoms in dementia can significantly improve quality of life for patients and caregivers.42 Use of antipsychotic drugs in patients with dementia should be limited to cases in which nonpharmacologic measures have failed and patients pose an imminent threat to themselves or others.43
Proton pump inhibitors
Proton pump inhibitors are among the most commonly prescribed medications in the United States, and their use has increased significantly over the decade. It has been estimated that between 25% and 70% of these prescriptions have no appropriate indication.44
There is considerable excess use of acid suppressants in both inpatient and outpatient settings.45,46 In one study, at discharge from an internal medicine service, almost half of patients were taking a proton pump inhibitor.47
Evidence-based guidelines recommend these drugs to treat gastroesophageal reflux disease, nonerosive reflux disease, erosive esophagitis, dyspepsia, and peptic ulcer disease. However, long-term use (ie, beyond 8 weeks) is recommended only for patients with erosive esophagitis, Barrett esophagus, a pathologic hypersecretory condition, or a demonstrated need for maintenance treatment for reflux disease.48
Although proton pump inhibitors are highly effective and have low toxicity, there are reports of an association with Clostridium difficile infection,49 community-acquired pneumonia,50 hip fracture,51 vitamin B12 deficiency,52 atrophic gastritis,53 kidney disease,54 and dementia.55
Nondrug therapies such as weight loss and elevation of the head of the bed may improve esophageal pH levels and reflux symptoms.56
Deprescribing.org has practical advice for healthcare providers, patients, and caregivers on how to discontinue proton pump inhibitors, including videos, algorithms, and guidelines.
TOOLS TO EVALUATE APPROPRIATE DRUG THERAPY
Beers criteria
The Beers criteria (Table 2), developed in 1991 by a geriatrician as an approach to safer, more effective drug therapy in frail elderly nursing home patients,57 were updated by the American Geriatrics Society in 2015 for use in any clinical setting.58 (The criteria are also available as a smartphone application through the American Geriatrics Society at www.americangeriatrics.org.)
The Beers criteria offer evidence-based recommendations on drugs to avoid in the elderly, along with the rationale for use, the quality of evidence behind the recommendation, and the graded strength of the recommendation. The Beers criteria should be viewed through the lens of clinical judgment to offer safer nonpharmacologic and pharmacologic treatments.
The Joint Commission recommends medication reconciliation at every transition of care.59 The Beers criteria are a good starting point for a comprehensive medication review.
STOPP/START criteria
Another tool to aid safe prescribing in older adults is the Screening Tool of Older Persons’ Potentially Inappropriate Prescriptions (STOPP), used in conjuction with the Screening Tool to Alert Doctors to Right Treatment (START). The STOPP/START criteria60,61 are based on an up-to-date literature review and consensus (Table 3).
THE BOTTOM LINE
Physicians caring for older adults need to diligently weigh the benefits of drug therapy and consider the patient’s care goals, current level of functioning, life expectancy, values, and preferences. Statin therapy for primary prevention, anticholinergics, benzodiazepines, antipsychotics, and proton pump inhibitors are widely used without proper indications, pointing to the need for a periodic comprehensive review of medications to reevaluate the risks vs the benefits of the patient’s medications. The Beers criteria and the STOPP/ START criteria can be useful tools for this purpose.
Medications started for appropriate indications in middle age may need to be monitored more closely as the patient ages. Some drugs may become unnecessary or even dangerous as the patient ages, functional status and renal function decline, and goals of care change.
Older adults tend to have multiple illnesses and therefore take more drugs, and polypharmacy increases the risk of poor outcomes. The number of medications a person uses is a risk factor for adverse drug reactions, nonadherence, financial burden, drug-drug interactions, and worse outcomes.1
The prevalence of polypharmacy increased from an estimated 8.2% to 15% from 1999 to 2011 based on the National Health and Nutrition Examination Survey.2 Guideline-based therapy for specific diseases may lead to the addition of more medications to reach disease targets.3 Most older adults in the United States compound the risk of prescribed medications by also taking over-the-counter medications and dietary supplements.4
In addition, medications are often used in older adults based on studies of younger persons without significant comorbidities. Applying clinical guidelines based on these studies to older adults with comorbidity and functional impairment is challenging.5 Age-related pharmacokinetic and pharmacodynamic changes increase the risk of adverse drug reactions.6
In this article, we review commonly used medications that are potentially inappropriate based on clinical practice. We also review tools to evaluate appropriate drug therapy in older adults.
DRUGS THAT ARE COMMONLY USED, BUT POTENTIALLY INAPPROPRIATE
Statins
Statins are effective when used as secondary prevention in older adults,7 but their efficacy when used as primary prevention of atherosclerotic cardiovascular disease in people age 75 and older is questionable.8 Nevertheless, they are widely used for this purpose. For example, before the 2013 joint guidelines of the American College of Cardiology and the American Heart Association (ACC/AHA) were released, 22% of patients age 80 and older in the Geisinger health system were taking a statin for primary prevention.9
The 2013 ACC/AHA guidelines included a limited recommendation for statins for primary prevention of atherosclerotic cardiovascular disease in adults age 75 and older.10 The guideline noted, however, that few data were available to support this recommendation.10
In a systematic review of 18 randomized clinical trials of statins for primary prevention of atherosclerotic cardiovascular disease, the mean age was 57, yet conclusions were extrapolated to an older patient population.11 The estimated 10-year risk of atherosclerotic cardiovascular disease based on pooled cohort risk equations of adults age 75 and older always exceeds the 7.5% treatment threshold recommended by the guidelines.8
Myopathy is a common adverse effect of statins. In addition, statins interact with other drugs that inhibit the cytochrome P450 3A4 isoenzyme, such as amlodipine, amiodarone, and diltiazem.8,12 If statin therapy caused no functional limitation due to muscle pain or weakness, statins for primary prevention would be cost-effective, but even a small increase in adverse effects in an elderly patient can offset the cardiovascular benefit.13 A recent post hoc secondary analysis found no benefit of pravastatin for primary prevention in adults age 75 and older.14
Thus, statin treatment for primary prevention in older patients should be individualized, based on life expectancy, function, and cardiovascular risk. Statin therapy does not replace modification of other risk factors.
Anticholinergics
Drugs with anticholinergic properties are commonly prescribed in the elderly for conditions such as muscle spasm, overactive bladder, psychiatric disorders, insomnia, extrapyramidal symptoms, vertigo, pruritus, peptic ulcer disease, seasonal allergies, and even the common cold,15 and many of the drugs often prescribed have strong anticholinergic properties (Table 1). Taking multiple medications with anticholinergic properties results in a high “anticholinergic burden,” which is associated with falls, impulsive behavior, poor physical performance, loss of independence, dementia, delirium, and brain atrophy.15–18
The 2014 American College of Physicians guideline on nonsurgical management of urinary incontinence in women recommends pharmacologic treatment for urgency and stress urinary incontinence after failure of nonpharmacologic therapy,19 and many drugs for these urinary symptoms have anticholinergic properties. If an anticholinergic is necessary, an agent that results in a lower anticholinergic burden should be considered in older patients.
A pharmacist-initiated medication review and intervention may be another way to adjust medications to reduce the patient’s anticholinergic burden.20,21 The common use of anticholinergic drugs in older adults reminds us to monitor their use closely.22
Benzodiazepines and nonbenzodiazepines
Benzodiazepines are among the most commonly prescribed psychotropics in developed countries and are prescribed mainly by primary care physicians rather than psychiatrists.23
In 2008, 5.2% of US adults ages 18 to 80 used a benzodiazepine, and long-term use was more prevalent in older patients (ages 65–80).23
Benzodiazepines are prescribed for anxiety,24 insomnia,25 and agitation. They can cause withdrawal26 and have potential for abuse.27 Benzodiazepines are associated with cognitive decline,28 impaired driving,29 falls,30 and hip fractures31 in older adults.
In addition, use of nonbenzodiazepine hypnotics (eg, zolpidem) is on the rise,32 and these drugs are known to increase the risk of hip fracture in nursing home residents.33
The American Geriatrics Society, through the American Board of Internal Medicine’s Choosing Wisely campaign, recommends avoiding benzodiazepines as a first-line treatment for insomnia, agitation, or delirium in older adults.34 Yet prescribing practices with these drugs in primary care settings conflict with guidelines, partly due to lack of training in constructive strategies regarding appropriate use of benzodiazepines.35 Educating patients on the risks and benefits of benzodiazepine treatment, especially long-term use, has been shown to reduce the rate of benzodiazepine-associated secondary events.36
Antipsychotics
Off-label use of antipsychotics is common and is increasing in the United States. In 2008, off-label use of antipsychotic drugs accounted for an estimated $6 billion.37 A common off-label use is to manage behavioral symptoms of dementia, despite a black-box warning about an increased risk of death in patients with dementia who are treated with antipsychotics.38,39 The Choosing Wisely campaign recommends against prescribing antipsychotics as a first-line treatment of behavioral and psychological symptoms of dementia.34
Antipsychotic drugs are associated with risk of acute kidney injury,40 as well as increased risk of falls and fractures (eg, a 52% higher risk of a serious fall, and a 50% higher risk of a nonvertebral osteoporotic fracture).41
Patients with dementia often exhibit aggression, resistance to care, and other challenging or disruptive behaviors. In such instances, antipsychotic drugs are often prescribed, but they provide limited and inconsistent benefits, while causing oversedation and worsening of cognitive function and increasing the likelihood of falling, stroke, and death.38,39,41
Because pharmacologic treatments for dementia are only modestly effective, have notable risks, and do not treat some of the behaviors that family members and caregivers find most distressing, nonpharmacologic measures are recommended as first-line treatment.42 These include caregiver education and support, training in problem-solving, and targeted therapy directed at the underlying causes of specific behaviors (eg, implementing nighttime routines to address sleep disturbances).42 Nonpharmacologic management of behavioral symptoms in dementia can significantly improve quality of life for patients and caregivers.42 Use of antipsychotic drugs in patients with dementia should be limited to cases in which nonpharmacologic measures have failed and patients pose an imminent threat to themselves or others.43
Proton pump inhibitors
Proton pump inhibitors are among the most commonly prescribed medications in the United States, and their use has increased significantly over the decade. It has been estimated that between 25% and 70% of these prescriptions have no appropriate indication.44
There is considerable excess use of acid suppressants in both inpatient and outpatient settings.45,46 In one study, at discharge from an internal medicine service, almost half of patients were taking a proton pump inhibitor.47
Evidence-based guidelines recommend these drugs to treat gastroesophageal reflux disease, nonerosive reflux disease, erosive esophagitis, dyspepsia, and peptic ulcer disease. However, long-term use (ie, beyond 8 weeks) is recommended only for patients with erosive esophagitis, Barrett esophagus, a pathologic hypersecretory condition, or a demonstrated need for maintenance treatment for reflux disease.48
Although proton pump inhibitors are highly effective and have low toxicity, there are reports of an association with Clostridium difficile infection,49 community-acquired pneumonia,50 hip fracture,51 vitamin B12 deficiency,52 atrophic gastritis,53 kidney disease,54 and dementia.55
Nondrug therapies such as weight loss and elevation of the head of the bed may improve esophageal pH levels and reflux symptoms.56
Deprescribing.org has practical advice for healthcare providers, patients, and caregivers on how to discontinue proton pump inhibitors, including videos, algorithms, and guidelines.
TOOLS TO EVALUATE APPROPRIATE DRUG THERAPY
Beers criteria
The Beers criteria (Table 2), developed in 1991 by a geriatrician as an approach to safer, more effective drug therapy in frail elderly nursing home patients,57 were updated by the American Geriatrics Society in 2015 for use in any clinical setting.58 (The criteria are also available as a smartphone application through the American Geriatrics Society at www.americangeriatrics.org.)
The Beers criteria offer evidence-based recommendations on drugs to avoid in the elderly, along with the rationale for use, the quality of evidence behind the recommendation, and the graded strength of the recommendation. The Beers criteria should be viewed through the lens of clinical judgment to offer safer nonpharmacologic and pharmacologic treatments.
The Joint Commission recommends medication reconciliation at every transition of care.59 The Beers criteria are a good starting point for a comprehensive medication review.
STOPP/START criteria
Another tool to aid safe prescribing in older adults is the Screening Tool of Older Persons’ Potentially Inappropriate Prescriptions (STOPP), used in conjuction with the Screening Tool to Alert Doctors to Right Treatment (START). The STOPP/START criteria60,61 are based on an up-to-date literature review and consensus (Table 3).
THE BOTTOM LINE
Physicians caring for older adults need to diligently weigh the benefits of drug therapy and consider the patient’s care goals, current level of functioning, life expectancy, values, and preferences. Statin therapy for primary prevention, anticholinergics, benzodiazepines, antipsychotics, and proton pump inhibitors are widely used without proper indications, pointing to the need for a periodic comprehensive review of medications to reevaluate the risks vs the benefits of the patient’s medications. The Beers criteria and the STOPP/ START criteria can be useful tools for this purpose.
- Steinman MA. Polypharmacy—time to get beyond numbers. JAMA Intern Med 2016; 176:482–483.
- Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA 2015; 314:1818–1831.
- Tinetti ME, Bogardus ST Jr, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med 2004; 351:2870–2874.
- Qato DM, Wilder J, Schumm LP, Gillet V, Alexander GC. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med 2016; 176:473–482.
- Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA 2005; 294:716–724.
- Atkin PA, Veitch PC, Veitch EM, Ogle SJ. The epidemiology of serious adverse drug reactions among the elderly. Drugs Aging 1999; 14:141–152.
- Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016; 338:2532–2561.
- Gurwitz JH, Go AS, Fortman SP. Statins for primary prevention in older adults: uncertainty and the need for more evidence. JAMA 2016; 316:1971–1972.
- Chokshi NP, Messerli FH, Sutin D, Supariwala AA, Shah NR. Appropriateness of statins in patients aged ≥ 80 years and comparison to other age groups. Am J Cardiol 2012; 110:1477–1481.
- Stone NJ, Robinson J, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(suppl 2):S1–S45.
- Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013; 1:CD004816.
- Chatzizisis YS, Koskinas KC, Misirli G, Vaklavas C, Hatzitolios A, Giannoglou GD. Risk factors and drug interactions predisposing to statin-induced myopathy: implications for risk assessment, prevention and treatment. Drug Saf 2010; 33:171–187.
- Odden MC, Pletcher MJ, Coxson PG, et al. Cost-effectiveness and population impact of statins for primary prevention in adults aged 75 years or older in the United States. Ann Intern Med 2015; 162:533–541.
- Han BH, Sutin D, Williamson JD, et al. Effect of statin treatment vs usual care on primary cardiovascular prevention among older adults. The ALLHAT-LLT randomized clinical trial. JAMA Intern Med 2017; 177:955–965.
- Gray SL, Anderson ML, Dublin S, et al. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med 2015; 175:401–407.
- Rudolph JL, Salow MJ, Angelini MC, McGlinchey RE. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med 2008; 168:508–513.
- Hilmer SN, Mager DE, Simonsick EM, et al. A drug burden index to define the functional burden of medications in older people. Arch Intern Med 2007; 167:781–787.
- Risacher SL, McDonald BC, Tallman EF, et al; Alzheimer’s Disease Neuroimaging Initiative. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol 2016; 73:721–732.
- Qaseem A, Dallas P, Forciea MA, Starkey M, Denberg TD, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Nonsurgical management of urinary incontinence in women: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2014; 161:429–440.
- Efjestad AS, Molden E, Oksengard AR. Pharmacist-initiated management of antagonistic interactions between anticholinergic drugs and acetyl cholinesterase inhibitors in individuals with dementia. J Am Geriatr Soc 2013; 61:1624–1625.
- Kersten H, Molden E, Tolo IK, Skovlund E, Engedal K, Wyller TB. Cognitive effects of reducing anticholinergic drug burden in a frail elderly population: a randomized controlled trial. J Gerontol A Biol Sci Med Sci 2013; 68:271–278.
- Curtis LH, Østbye T, Sendersky V, et al. Inappropriate prescribing for elderly Americans in a large outpatient population. Arch Intern Med 2004; 164:1621–1625.
- Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry 2015; 72:136–142.
- Martin JL, Sainz-Pardo M, Furukawa TA, Martín-Sánchez E, Seoane T, Galán C. Benzodiazepines in generalized anxiety disorder: heterogeneity of outcomes based on a systematic review and meta-analysis of clinical trials. J Psychopharmacol 2007; 21:774–782.
- Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med 2007; 22:1335–1350.
- Rickels K, Schweizer E, Case WG, Greenblatt DJ. Long-term therapeutic use of benzodiazepines, I. Effects of abrupt discontinuation. Arch Gen Psychiatry 1990; 47:899–907.
- Fenton MC, Keyes KM, Martins SS, Hasin DS. The role of a prescription in anxiety medication use, abuse, and dependence. Am J Psychiatry 2010; 167:1247–1253.
- Billoti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ 2014; 349:g5205.
- Smink BE, Egberts AC, Lusthof KJ, Uges DR, de Gier JJ. The relationship between benzodiazepine use and traffic accidents: a systemic literature review. CNS Drugs 2010; 24:639–653.
- Tinett, ME, Speechley M, Ginter S. Risk factors for falls among elderly persons living in the community. N Engl J Med 1988; 319:1701–1707.
- Zint K, Haefeli WE, Glynn RJ, Mogun H, Avorn J, Stürmer T. Impact of drug interactions, dosage, and duration of therapy on the risk of hip fracture associated with benzodiazepine use in older adults. Pharmacoepidemiol Drug Saf 2010; 19:1248–1255.
- Briesacher BA, Soumerai SB, Field TS, Fouayzi H, Gurwitz JH. Medicare Part D’s exclusion of benzodiazepines and fracture risk in nursing homes. Arch Intern Med 2010; 170:693–698.
- Berry SD, Lee Y, Cai S, Dore DD. Nonbenzodiazepine sleep medication use and hip fractures in nursing home residents. JAMA Intern Med 2013; 173:754–761.
- American Geriatrics Society. Choosing Wisely. Ten things clinicians and patients should question. www.choosingwisely.org/societies/american-geriatrics-society/. Accessed December 3, 2017.
- Cook JM, Marshall R, Masci C, Coyne JC. Physicians’ perspectives on prescribing benzodiazepines for older adults: a qualitative study. J Gen Intern Med 2007; 22:303–307.
- Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med 2014; 174:890–898.
- Alexander GC, Gallagher SA, Mascola A, Moloney RM, Stafford RS. Increasing off-label use of antipsychotic medications in the United States, 1995–2008. Phamacoepidemiol Drug Saf 2011; 20:177–184.
- Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med 2007; 146:775–786.
- US Food and Drug Administration (FDA). Public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm053171.htm. Accessed December 4, 2017.
- Hwang YJ, Dixon SN, Reiss JP, et al. Atypical antipsychotic drugs and the risk for acute kidney injury and other adverse outcomes in older adults. Ann Intern Med 2014; 161:242–248.
- Fraser L, Liu K, Naylor KL, et al. Falls and fractures with atypical antipsychotic medication use: a population-based cohort study. JAMA Intern Med 2015; 175:450–452.
- Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA 2012; 308:2020–2029.
- Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 2006; 355:1525–1538.
- Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. BMJ 2008; 336:2–3.
- Mazer-Amirshahi M, Mullins PM, van den Anker J, Meltzer A, Pines JM. Rising rates of proton pump inhibitor prescribing in US emergency departments. Am J Emerg Med 2014; 32:618–622.
- Heidelbaugh JJ, Goldberg KL, Inadomi JM. Magnitude and economic effect of overuse of antisecretory therapy in the ambulatory care setting. Am J Manag Care 2010; 16:e228–e324.
- Pham CQ, Regal RE, Bostwich TR, Knauf KS. Acid suppressive therapy used on an inpatient internal medicine service. Ann Pharmacother 2006; 40:1261–1266.
- Kahrilas PJ, Shaheen NJ, Vaezi MF, et al; American Gastroenterological Association. American Gastroenterological Association medical position statement on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135:1383–1391.e1–e5.
- Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med 2010; 170:784–790.
- Gulmez SE, Holm A, Frederiksen H, Jensen TG, Pedersen C, Hallas J. Use of proton pump inhibitors and the risk of community-acquired pneumonia: a population-based case-control study. Arch Intern Med 2007; 167:950–955.
- Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006; 296:2947–2953.
- Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA 2013; 310:2435–2442.
- Kuipers EJ, Lundell L, Klinkenberg-Knol EC, et al. Atrophic gastritis and Helicobacter pylori infection in patients with reflux esophagitis treated with omeprazole or fundoplication. N Engl J Med 1996; 334:1018–1022.
- Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med 2016; 176:238–246.
- Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol 2016; 73:410–416.
- Kaltenbach T, Crockett S, Gerson LB. Are lifestyle measures effective in patients with gastroesophageal reflux disease? An evidence-based approach. Arch Intern Med 2006; 166:965–971.
- Beers MH, Ouslander JG, Rollingher I, Reuben DB, Brooks J, Beck JC. Explicit criteria for determining inappropriate medication use in nursing home residents. Arch Intern Med 1991; 151:1825–1832.
- American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63:2227–2246.
- Joint Commission. Sentinel event alert, Issue 35: using medication reconciliation to prevent errors. www.jointcommission.org/sentinel_event_alert_issue_35_using_medication_reconciliation_to_prevent_errors/. Accessed August 18, 2017.
- Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther 2008; 46:72–83.
- O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 2015; 44:213–218.
- Steinman MA. Polypharmacy—time to get beyond numbers. JAMA Intern Med 2016; 176:482–483.
- Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA 2015; 314:1818–1831.
- Tinetti ME, Bogardus ST Jr, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med 2004; 351:2870–2874.
- Qato DM, Wilder J, Schumm LP, Gillet V, Alexander GC. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med 2016; 176:473–482.
- Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA 2005; 294:716–724.
- Atkin PA, Veitch PC, Veitch EM, Ogle SJ. The epidemiology of serious adverse drug reactions among the elderly. Drugs Aging 1999; 14:141–152.
- Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016; 338:2532–2561.
- Gurwitz JH, Go AS, Fortman SP. Statins for primary prevention in older adults: uncertainty and the need for more evidence. JAMA 2016; 316:1971–1972.
- Chokshi NP, Messerli FH, Sutin D, Supariwala AA, Shah NR. Appropriateness of statins in patients aged ≥ 80 years and comparison to other age groups. Am J Cardiol 2012; 110:1477–1481.
- Stone NJ, Robinson J, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(suppl 2):S1–S45.
- Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013; 1:CD004816.
- Chatzizisis YS, Koskinas KC, Misirli G, Vaklavas C, Hatzitolios A, Giannoglou GD. Risk factors and drug interactions predisposing to statin-induced myopathy: implications for risk assessment, prevention and treatment. Drug Saf 2010; 33:171–187.
- Odden MC, Pletcher MJ, Coxson PG, et al. Cost-effectiveness and population impact of statins for primary prevention in adults aged 75 years or older in the United States. Ann Intern Med 2015; 162:533–541.
- Han BH, Sutin D, Williamson JD, et al. Effect of statin treatment vs usual care on primary cardiovascular prevention among older adults. The ALLHAT-LLT randomized clinical trial. JAMA Intern Med 2017; 177:955–965.
- Gray SL, Anderson ML, Dublin S, et al. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med 2015; 175:401–407.
- Rudolph JL, Salow MJ, Angelini MC, McGlinchey RE. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med 2008; 168:508–513.
- Hilmer SN, Mager DE, Simonsick EM, et al. A drug burden index to define the functional burden of medications in older people. Arch Intern Med 2007; 167:781–787.
- Risacher SL, McDonald BC, Tallman EF, et al; Alzheimer’s Disease Neuroimaging Initiative. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol 2016; 73:721–732.
- Qaseem A, Dallas P, Forciea MA, Starkey M, Denberg TD, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Nonsurgical management of urinary incontinence in women: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2014; 161:429–440.
- Efjestad AS, Molden E, Oksengard AR. Pharmacist-initiated management of antagonistic interactions between anticholinergic drugs and acetyl cholinesterase inhibitors in individuals with dementia. J Am Geriatr Soc 2013; 61:1624–1625.
- Kersten H, Molden E, Tolo IK, Skovlund E, Engedal K, Wyller TB. Cognitive effects of reducing anticholinergic drug burden in a frail elderly population: a randomized controlled trial. J Gerontol A Biol Sci Med Sci 2013; 68:271–278.
- Curtis LH, Østbye T, Sendersky V, et al. Inappropriate prescribing for elderly Americans in a large outpatient population. Arch Intern Med 2004; 164:1621–1625.
- Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry 2015; 72:136–142.
- Martin JL, Sainz-Pardo M, Furukawa TA, Martín-Sánchez E, Seoane T, Galán C. Benzodiazepines in generalized anxiety disorder: heterogeneity of outcomes based on a systematic review and meta-analysis of clinical trials. J Psychopharmacol 2007; 21:774–782.
- Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med 2007; 22:1335–1350.
- Rickels K, Schweizer E, Case WG, Greenblatt DJ. Long-term therapeutic use of benzodiazepines, I. Effects of abrupt discontinuation. Arch Gen Psychiatry 1990; 47:899–907.
- Fenton MC, Keyes KM, Martins SS, Hasin DS. The role of a prescription in anxiety medication use, abuse, and dependence. Am J Psychiatry 2010; 167:1247–1253.
- Billoti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ 2014; 349:g5205.
- Smink BE, Egberts AC, Lusthof KJ, Uges DR, de Gier JJ. The relationship between benzodiazepine use and traffic accidents: a systemic literature review. CNS Drugs 2010; 24:639–653.
- Tinett, ME, Speechley M, Ginter S. Risk factors for falls among elderly persons living in the community. N Engl J Med 1988; 319:1701–1707.
- Zint K, Haefeli WE, Glynn RJ, Mogun H, Avorn J, Stürmer T. Impact of drug interactions, dosage, and duration of therapy on the risk of hip fracture associated with benzodiazepine use in older adults. Pharmacoepidemiol Drug Saf 2010; 19:1248–1255.
- Briesacher BA, Soumerai SB, Field TS, Fouayzi H, Gurwitz JH. Medicare Part D’s exclusion of benzodiazepines and fracture risk in nursing homes. Arch Intern Med 2010; 170:693–698.
- Berry SD, Lee Y, Cai S, Dore DD. Nonbenzodiazepine sleep medication use and hip fractures in nursing home residents. JAMA Intern Med 2013; 173:754–761.
- American Geriatrics Society. Choosing Wisely. Ten things clinicians and patients should question. www.choosingwisely.org/societies/american-geriatrics-society/. Accessed December 3, 2017.
- Cook JM, Marshall R, Masci C, Coyne JC. Physicians’ perspectives on prescribing benzodiazepines for older adults: a qualitative study. J Gen Intern Med 2007; 22:303–307.
- Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med 2014; 174:890–898.
- Alexander GC, Gallagher SA, Mascola A, Moloney RM, Stafford RS. Increasing off-label use of antipsychotic medications in the United States, 1995–2008. Phamacoepidemiol Drug Saf 2011; 20:177–184.
- Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med 2007; 146:775–786.
- US Food and Drug Administration (FDA). Public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm053171.htm. Accessed December 4, 2017.
- Hwang YJ, Dixon SN, Reiss JP, et al. Atypical antipsychotic drugs and the risk for acute kidney injury and other adverse outcomes in older adults. Ann Intern Med 2014; 161:242–248.
- Fraser L, Liu K, Naylor KL, et al. Falls and fractures with atypical antipsychotic medication use: a population-based cohort study. JAMA Intern Med 2015; 175:450–452.
- Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA 2012; 308:2020–2029.
- Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 2006; 355:1525–1538.
- Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. BMJ 2008; 336:2–3.
- Mazer-Amirshahi M, Mullins PM, van den Anker J, Meltzer A, Pines JM. Rising rates of proton pump inhibitor prescribing in US emergency departments. Am J Emerg Med 2014; 32:618–622.
- Heidelbaugh JJ, Goldberg KL, Inadomi JM. Magnitude and economic effect of overuse of antisecretory therapy in the ambulatory care setting. Am J Manag Care 2010; 16:e228–e324.
- Pham CQ, Regal RE, Bostwich TR, Knauf KS. Acid suppressive therapy used on an inpatient internal medicine service. Ann Pharmacother 2006; 40:1261–1266.
- Kahrilas PJ, Shaheen NJ, Vaezi MF, et al; American Gastroenterological Association. American Gastroenterological Association medical position statement on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135:1383–1391.e1–e5.
- Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med 2010; 170:784–790.
- Gulmez SE, Holm A, Frederiksen H, Jensen TG, Pedersen C, Hallas J. Use of proton pump inhibitors and the risk of community-acquired pneumonia: a population-based case-control study. Arch Intern Med 2007; 167:950–955.
- Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006; 296:2947–2953.
- Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA 2013; 310:2435–2442.
- Kuipers EJ, Lundell L, Klinkenberg-Knol EC, et al. Atrophic gastritis and Helicobacter pylori infection in patients with reflux esophagitis treated with omeprazole or fundoplication. N Engl J Med 1996; 334:1018–1022.
- Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med 2016; 176:238–246.
- Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol 2016; 73:410–416.
- Kaltenbach T, Crockett S, Gerson LB. Are lifestyle measures effective in patients with gastroesophageal reflux disease? An evidence-based approach. Arch Intern Med 2006; 166:965–971.
- Beers MH, Ouslander JG, Rollingher I, Reuben DB, Brooks J, Beck JC. Explicit criteria for determining inappropriate medication use in nursing home residents. Arch Intern Med 1991; 151:1825–1832.
- American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63:2227–2246.
- Joint Commission. Sentinel event alert, Issue 35: using medication reconciliation to prevent errors. www.jointcommission.org/sentinel_event_alert_issue_35_using_medication_reconciliation_to_prevent_errors/. Accessed August 18, 2017.
- Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther 2008; 46:72–83.
- O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 2015; 44:213–218.
KEY POINTS
- Statins, anticholinergics, benzodiazepines, antipsychotics, and proton pump inhibitors are widely prescribed.
- In older patients, a periodic comprehensive medication review is needed to reevaluate the risks and the benefits of current medications in light of goals of care, life expectancy, and the patient’s preferences.
- The Beers criteria and the Screening Tool of Older Persons’ Potentially Inappropriate Prescriptions provide valuable guidance for safe prescribing in older adults.