User login
M. Alexander Otto began his reporting career early in 1999 covering the pharmaceutical industry for a national pharmacists' magazine and freelancing for the Washington Post and other newspapers. He then joined BNA, now part of Bloomberg News, covering health law and the protection of people and animals in medical research. Alex next worked for the McClatchy Company. Based on his work, Alex won a year-long Knight Science Journalism Fellowship to MIT in 2008-2009. He joined the company shortly thereafter. Alex has a newspaper journalism degree from Syracuse (N.Y.) University and a master's degree in medical science -- a physician assistant degree -- from George Washington University. Alex is based in Seattle.
ACR: Don’t be fooled by contaminated synovial fluid
SAN FRANCISCO – Hold off on surgery in patients with presumed septic arthritis if they’re not otherwise too sick and their cultures don’t grow out a pathogenic organism within 48 hours.
The reason is because those patients are likely to have synovial fluid that was contaminated during collection, not a true joint infection.
The advice comes from investigators at Beth Israel Deaconess Medical Center, Boston, who compared 425 monoarticular septic arthritis cases with 25 cases that turned out to be false positives due to synovial fluid contamination; most of the false positives got antibiotics, and three (12%) had joint operations that they did not need.
“Rushing off to the operating room isn’t” always warranted. “You can suspect contamination if patients have milder disease manifestations and cultures grow late,” said investigator Dr. Robert H. Shmerling, clinical chief of Beth Israel’s division of rheumatology.
The findings help determine when – and when not – to be aggressive with patients who present with what looks to be septic arthritis. “No one’s ever really looked at this before,” he said at the annual meeting of the American College of Rheumatology.
“These are very different sorts of patients. Look at the full range of clinical characteristics and lab values, not just the synovial fluid tap. If contamination is suspected, you can wait until the cultures come back or possibly do serial taps before going to the operating room,” said coinvestigator Clara Zhu, a medical student at Boston University.
Patients with true joint infections had higher mean peripheral polymorphonuclear neutrophil percentages (78% vs. 68% in false positives) and synovial fluid polymorphonuclear cell percentages (88% vs. 74% in false positives). True cases also had substantially higher mean synovial fluid white blood cell counts (88,000 vs. 29,000).
Unlike true cases, contaminated synovial fluid took about 4 days to grow out a positive culture, and the most common organisms by far were coagulase-negative staphylococci, typically normal skin bacteria.
Patients with contaminated fluid also tended to be older (71 vs. 59 years), with fewer prior admissions. They were far less likely to have had recent joint procedures and histories of septic arthritis but were more likely to have synovial fluid crystals, as in gout. False positives also left the hospital sooner (7 vs. 11 days) and were less likely to be readmitted within 2 months. They were also less likely to present with fever (19% vs. 37%) but not significantly so.
This “study suggests that contaminated synovial fluid is found in up to 6% of patients with suspected septic arthritis and positive synovial fluid or synovial biopsy cultures. We recommend a conservative approach for patients with ... mild disease manifestations and no growth of pathogenic organisms within the first 48 hours,” the investigators concluded.
The authors have no disclosures, and there was no outside funding for the work.
SAN FRANCISCO – Hold off on surgery in patients with presumed septic arthritis if they’re not otherwise too sick and their cultures don’t grow out a pathogenic organism within 48 hours.
The reason is because those patients are likely to have synovial fluid that was contaminated during collection, not a true joint infection.
The advice comes from investigators at Beth Israel Deaconess Medical Center, Boston, who compared 425 monoarticular septic arthritis cases with 25 cases that turned out to be false positives due to synovial fluid contamination; most of the false positives got antibiotics, and three (12%) had joint operations that they did not need.
“Rushing off to the operating room isn’t” always warranted. “You can suspect contamination if patients have milder disease manifestations and cultures grow late,” said investigator Dr. Robert H. Shmerling, clinical chief of Beth Israel’s division of rheumatology.
The findings help determine when – and when not – to be aggressive with patients who present with what looks to be septic arthritis. “No one’s ever really looked at this before,” he said at the annual meeting of the American College of Rheumatology.
“These are very different sorts of patients. Look at the full range of clinical characteristics and lab values, not just the synovial fluid tap. If contamination is suspected, you can wait until the cultures come back or possibly do serial taps before going to the operating room,” said coinvestigator Clara Zhu, a medical student at Boston University.
Patients with true joint infections had higher mean peripheral polymorphonuclear neutrophil percentages (78% vs. 68% in false positives) and synovial fluid polymorphonuclear cell percentages (88% vs. 74% in false positives). True cases also had substantially higher mean synovial fluid white blood cell counts (88,000 vs. 29,000).
Unlike true cases, contaminated synovial fluid took about 4 days to grow out a positive culture, and the most common organisms by far were coagulase-negative staphylococci, typically normal skin bacteria.
Patients with contaminated fluid also tended to be older (71 vs. 59 years), with fewer prior admissions. They were far less likely to have had recent joint procedures and histories of septic arthritis but were more likely to have synovial fluid crystals, as in gout. False positives also left the hospital sooner (7 vs. 11 days) and were less likely to be readmitted within 2 months. They were also less likely to present with fever (19% vs. 37%) but not significantly so.
This “study suggests that contaminated synovial fluid is found in up to 6% of patients with suspected septic arthritis and positive synovial fluid or synovial biopsy cultures. We recommend a conservative approach for patients with ... mild disease manifestations and no growth of pathogenic organisms within the first 48 hours,” the investigators concluded.
The authors have no disclosures, and there was no outside funding for the work.
SAN FRANCISCO – Hold off on surgery in patients with presumed septic arthritis if they’re not otherwise too sick and their cultures don’t grow out a pathogenic organism within 48 hours.
The reason is because those patients are likely to have synovial fluid that was contaminated during collection, not a true joint infection.
The advice comes from investigators at Beth Israel Deaconess Medical Center, Boston, who compared 425 monoarticular septic arthritis cases with 25 cases that turned out to be false positives due to synovial fluid contamination; most of the false positives got antibiotics, and three (12%) had joint operations that they did not need.
“Rushing off to the operating room isn’t” always warranted. “You can suspect contamination if patients have milder disease manifestations and cultures grow late,” said investigator Dr. Robert H. Shmerling, clinical chief of Beth Israel’s division of rheumatology.
The findings help determine when – and when not – to be aggressive with patients who present with what looks to be septic arthritis. “No one’s ever really looked at this before,” he said at the annual meeting of the American College of Rheumatology.
“These are very different sorts of patients. Look at the full range of clinical characteristics and lab values, not just the synovial fluid tap. If contamination is suspected, you can wait until the cultures come back or possibly do serial taps before going to the operating room,” said coinvestigator Clara Zhu, a medical student at Boston University.
Patients with true joint infections had higher mean peripheral polymorphonuclear neutrophil percentages (78% vs. 68% in false positives) and synovial fluid polymorphonuclear cell percentages (88% vs. 74% in false positives). True cases also had substantially higher mean synovial fluid white blood cell counts (88,000 vs. 29,000).
Unlike true cases, contaminated synovial fluid took about 4 days to grow out a positive culture, and the most common organisms by far were coagulase-negative staphylococci, typically normal skin bacteria.
Patients with contaminated fluid also tended to be older (71 vs. 59 years), with fewer prior admissions. They were far less likely to have had recent joint procedures and histories of septic arthritis but were more likely to have synovial fluid crystals, as in gout. False positives also left the hospital sooner (7 vs. 11 days) and were less likely to be readmitted within 2 months. They were also less likely to present with fever (19% vs. 37%) but not significantly so.
This “study suggests that contaminated synovial fluid is found in up to 6% of patients with suspected septic arthritis and positive synovial fluid or synovial biopsy cultures. We recommend a conservative approach for patients with ... mild disease manifestations and no growth of pathogenic organisms within the first 48 hours,” the investigators concluded.
The authors have no disclosures, and there was no outside funding for the work.
AT THE ACR ANNUAL MEETING
Key clinical point: It’s probably not really septic arthritis if patients have mild disease manifestations and slow-growing synovial fluid cultures.
Major finding: True cases of septic arthritis had substantially higher mean synovial fluid white blood cell counts than did false-positive cases (88,000 vs. 29,000).
Data source: Review of 450 patients with presumed septic arthritis.
Disclosures: The authors have no disclosures, and there was no outside funding for the work.
ACR: Don’t stop TNFis during rheumatoid arthritis pregnancy
SAN FRANCISCO – It might be best to keep women with rheumatoid arthritis on their tumor necrosis factor blockers during pregnancy, according to German investigators.
They found that women are likely to flare without them and need more prednisolone, which is associated with preterm birth and other problems, while an increasing body of evidence suggests that tumor necrosis factor inhibitors (TNFis) are relatively safe during pregnancy.
“We should” rethink discontinuing TNFis during pregnancy, as recommended in some quarters. “We do not want women to flare during pregnancy,” said investigator Dr. Rebecca Fischer-Betz of the department of rheumatology at Heinrich Heine University in Düsseldorf.
She and her colleagues compared birth outcomes in 18 rheumatoid arthritis (RA) patients who discontinued TNFi treatment shortly after they got pregnant against those of 24 women with RA who were never exposed to a TNFi because, in general, they had less severe disease.
Twelve of the women (75%) in the TNFi group flared, versus four women (17%) in the control group. Although patients in both groups started with a mean 28-joint Disease Activity Score using C-reactive protein (DAS28-CRP) below 3.0, women in the TNFi group had a rise in activity to a mean of about 3.5 in the second trimester, while disease activity in control patients remained stable.
Compared with controls, women who stopped TNFis were also far more likely to flare (odds ratio, 10.0; 95% confidence interval, 2.3-42.8; P = .002), even after adjusting for age, DAS28-CRP at conception, rheumatoid factor and cyclic citrullinated peptide status, and other potential confounders. They also relied more heavily on prednisolone, taking, for example, a mean dose of 13 mg in the second trimester versus 8 mg in the control group. Perhaps not surprisingly, the mean duration of pregnancy was 37 weeks in the TNFi group, with six women (33%) delivering at or before 37 weeks; women in the control group delivered, on average, at 39 weeks, with four (17%) delivering at or before week 37.
The investigators found that the risk of preterm birth increased with every cumulative milligram of prednisolone (OR, 1.08; 95% CI, 1.02-1.15; P less than .01).
“Women with RA who discontinue TNFis at conception face a high risk for flares during pregnancy, independently of known risk factors like seropositivity. Flares are usually treated with prednisolone. We found a dose-dependent, significant increased risk for preterm birth associated with prednisolone. In this era of treat-to-target management of RA, our paradigm for RA pregnancy management may need adjusting. By controlling RA activity with medications considered relatively safe in pregnancy, we may be able to improve both the pregnancy experience and pregnancy outcomes,” the investigators concluded.
The women were 33 years old on average, and all had live births; four early miscarriages were excluded from analysis. All the pregnancies were planned, with methotrexate discontinued at least 3 months before conception. There was no statistical difference in the rate of seropositivity between the groups, “which is interesting because we know seropositivity is a risk factor for staying active during pregnancy,” Dr. Fischer-Betz said.
Two boys born to women who took TNFis had minor malformations, one with nasal bone aplasia, retrognathia, and hydronephrosis, and the other with hypospadias. Both of their mothers had taken etanercept (Enbrel) in the first trimester. There was one malformation in the control group, a girl born with hydronephrosis.
There was no outside funding for the work, and the investigators have no disclosures.
SAN FRANCISCO – It might be best to keep women with rheumatoid arthritis on their tumor necrosis factor blockers during pregnancy, according to German investigators.
They found that women are likely to flare without them and need more prednisolone, which is associated with preterm birth and other problems, while an increasing body of evidence suggests that tumor necrosis factor inhibitors (TNFis) are relatively safe during pregnancy.
“We should” rethink discontinuing TNFis during pregnancy, as recommended in some quarters. “We do not want women to flare during pregnancy,” said investigator Dr. Rebecca Fischer-Betz of the department of rheumatology at Heinrich Heine University in Düsseldorf.
She and her colleagues compared birth outcomes in 18 rheumatoid arthritis (RA) patients who discontinued TNFi treatment shortly after they got pregnant against those of 24 women with RA who were never exposed to a TNFi because, in general, they had less severe disease.
Twelve of the women (75%) in the TNFi group flared, versus four women (17%) in the control group. Although patients in both groups started with a mean 28-joint Disease Activity Score using C-reactive protein (DAS28-CRP) below 3.0, women in the TNFi group had a rise in activity to a mean of about 3.5 in the second trimester, while disease activity in control patients remained stable.
Compared with controls, women who stopped TNFis were also far more likely to flare (odds ratio, 10.0; 95% confidence interval, 2.3-42.8; P = .002), even after adjusting for age, DAS28-CRP at conception, rheumatoid factor and cyclic citrullinated peptide status, and other potential confounders. They also relied more heavily on prednisolone, taking, for example, a mean dose of 13 mg in the second trimester versus 8 mg in the control group. Perhaps not surprisingly, the mean duration of pregnancy was 37 weeks in the TNFi group, with six women (33%) delivering at or before 37 weeks; women in the control group delivered, on average, at 39 weeks, with four (17%) delivering at or before week 37.
The investigators found that the risk of preterm birth increased with every cumulative milligram of prednisolone (OR, 1.08; 95% CI, 1.02-1.15; P less than .01).
“Women with RA who discontinue TNFis at conception face a high risk for flares during pregnancy, independently of known risk factors like seropositivity. Flares are usually treated with prednisolone. We found a dose-dependent, significant increased risk for preterm birth associated with prednisolone. In this era of treat-to-target management of RA, our paradigm for RA pregnancy management may need adjusting. By controlling RA activity with medications considered relatively safe in pregnancy, we may be able to improve both the pregnancy experience and pregnancy outcomes,” the investigators concluded.
The women were 33 years old on average, and all had live births; four early miscarriages were excluded from analysis. All the pregnancies were planned, with methotrexate discontinued at least 3 months before conception. There was no statistical difference in the rate of seropositivity between the groups, “which is interesting because we know seropositivity is a risk factor for staying active during pregnancy,” Dr. Fischer-Betz said.
Two boys born to women who took TNFis had minor malformations, one with nasal bone aplasia, retrognathia, and hydronephrosis, and the other with hypospadias. Both of their mothers had taken etanercept (Enbrel) in the first trimester. There was one malformation in the control group, a girl born with hydronephrosis.
There was no outside funding for the work, and the investigators have no disclosures.
SAN FRANCISCO – It might be best to keep women with rheumatoid arthritis on their tumor necrosis factor blockers during pregnancy, according to German investigators.
They found that women are likely to flare without them and need more prednisolone, which is associated with preterm birth and other problems, while an increasing body of evidence suggests that tumor necrosis factor inhibitors (TNFis) are relatively safe during pregnancy.
“We should” rethink discontinuing TNFis during pregnancy, as recommended in some quarters. “We do not want women to flare during pregnancy,” said investigator Dr. Rebecca Fischer-Betz of the department of rheumatology at Heinrich Heine University in Düsseldorf.
She and her colleagues compared birth outcomes in 18 rheumatoid arthritis (RA) patients who discontinued TNFi treatment shortly after they got pregnant against those of 24 women with RA who were never exposed to a TNFi because, in general, they had less severe disease.
Twelve of the women (75%) in the TNFi group flared, versus four women (17%) in the control group. Although patients in both groups started with a mean 28-joint Disease Activity Score using C-reactive protein (DAS28-CRP) below 3.0, women in the TNFi group had a rise in activity to a mean of about 3.5 in the second trimester, while disease activity in control patients remained stable.
Compared with controls, women who stopped TNFis were also far more likely to flare (odds ratio, 10.0; 95% confidence interval, 2.3-42.8; P = .002), even after adjusting for age, DAS28-CRP at conception, rheumatoid factor and cyclic citrullinated peptide status, and other potential confounders. They also relied more heavily on prednisolone, taking, for example, a mean dose of 13 mg in the second trimester versus 8 mg in the control group. Perhaps not surprisingly, the mean duration of pregnancy was 37 weeks in the TNFi group, with six women (33%) delivering at or before 37 weeks; women in the control group delivered, on average, at 39 weeks, with four (17%) delivering at or before week 37.
The investigators found that the risk of preterm birth increased with every cumulative milligram of prednisolone (OR, 1.08; 95% CI, 1.02-1.15; P less than .01).
“Women with RA who discontinue TNFis at conception face a high risk for flares during pregnancy, independently of known risk factors like seropositivity. Flares are usually treated with prednisolone. We found a dose-dependent, significant increased risk for preterm birth associated with prednisolone. In this era of treat-to-target management of RA, our paradigm for RA pregnancy management may need adjusting. By controlling RA activity with medications considered relatively safe in pregnancy, we may be able to improve both the pregnancy experience and pregnancy outcomes,” the investigators concluded.
The women were 33 years old on average, and all had live births; four early miscarriages were excluded from analysis. All the pregnancies were planned, with methotrexate discontinued at least 3 months before conception. There was no statistical difference in the rate of seropositivity between the groups, “which is interesting because we know seropositivity is a risk factor for staying active during pregnancy,” Dr. Fischer-Betz said.
Two boys born to women who took TNFis had minor malformations, one with nasal bone aplasia, retrognathia, and hydronephrosis, and the other with hypospadias. Both of their mothers had taken etanercept (Enbrel) in the first trimester. There was one malformation in the control group, a girl born with hydronephrosis.
There was no outside funding for the work, and the investigators have no disclosures.
AT THE ACR ANNUAL MEETING
Key clinical point: Stopping a TNFi during pregnancy comes at the cost of increased reliance on prednisolone.
Major finding: Compared with controls, women who stopped a TNFi during pregnancy were far more likely to flare (OR, 10.0; 95% CI, 2.3-42.8; P = .002).
Data source: Birth outcomes in 42 women with rheumatoid arthritis.
Disclosures: There was no outside funding for the work, and the investigators have no disclosures.
ACR: Rheumatologists aren’t giving methotrexate a fair shot
SAN FRANCISCO – Oral methotrexate is frequently underdosed, given for an inadequate length of time, and rarely switched to subcutaneous formulations before rheumatologists move on to biologics, according to an analysis of claims data from 35,640 rheumatoid arthritis patients.
“There’re some major concerns here. Methotrexate is the anchor drug for rheumatoid arthritis, the best drug we have. More appropriate [use] could lead to better control” and “produce significant cost savings,” said investigator Dr. James O’Dell, chief of the division of rheumatology at the University of Nebraska Medical Center, Omaha.
When patients don’t fully respond to lower doses, the ground rules for oral methotrexate include escalation up to 25 mg or a switch to subcutaneous formulations, which have better bioavailability. Those moves should be considered before turning to biologics, Dr. O’Dell explained at the American College of Rheumatology annual meeting.
Rheumatologists, by and large, aren’t playing by those rules, according to the analysis. “We need to own these data because the majority of patients, over three-quarters, were treated by rheumatologists. We are not doing a great job,” Dr. ODell said.
The claims data came from Symphony Health Solutions, which captures about 92% of prescriptions written in the United States. The 35,640 rheumatoid arthritis patients in the study were started on methotrexate in 2009 and followed through 2014; 15,599 (43.8%) didn’t need anything else and stayed on oral methotrexate alone throughout the study period.
Prescribers, however, gave up on oral methotrexate at a mean dose of 15.3 mg and moved 17,528 patients (49%) straight to a biologic without giving subcutaneous methotrexate a shot. They did that after a median of less than 6 months, and within 3 months in more than 40% of patients.
Just 2,513 patients (7%) moved on to subcutaneous methotrexate when their oral formulation wasn’t enough. That’s all most of them needed; 1,802 (72%) remained on subcutaneous methotrexate alone for the remainder of the study period. The rest moved on to a biologic, but after a median of almost a year, not a few months. When their time on oral and subcutaneous methotrexate was included, their median time to a biologic was more than 2 years.
The investigators checked to see if things improved for patients who started on oral methotrexate in 2012. “The answer was no. We didn’t do better,” Dr. O’Dell said.
He didn’t speculate on why methotrexate is underused in the United States.
Claims data can’t address why patients were switched from methotrexate and other issues, but such nuances “don’t even begin to explain the doses and the timing of switch that we saw here,” he said.
Dr. O’Dell is an adviser for AbbVie, Lilly, Coherus, Bristol-Myers Squibb, Antares, and Medac. Other investigators disclosed relationships with those or other companies.
SAN FRANCISCO – Oral methotrexate is frequently underdosed, given for an inadequate length of time, and rarely switched to subcutaneous formulations before rheumatologists move on to biologics, according to an analysis of claims data from 35,640 rheumatoid arthritis patients.
“There’re some major concerns here. Methotrexate is the anchor drug for rheumatoid arthritis, the best drug we have. More appropriate [use] could lead to better control” and “produce significant cost savings,” said investigator Dr. James O’Dell, chief of the division of rheumatology at the University of Nebraska Medical Center, Omaha.
When patients don’t fully respond to lower doses, the ground rules for oral methotrexate include escalation up to 25 mg or a switch to subcutaneous formulations, which have better bioavailability. Those moves should be considered before turning to biologics, Dr. O’Dell explained at the American College of Rheumatology annual meeting.
Rheumatologists, by and large, aren’t playing by those rules, according to the analysis. “We need to own these data because the majority of patients, over three-quarters, were treated by rheumatologists. We are not doing a great job,” Dr. ODell said.
The claims data came from Symphony Health Solutions, which captures about 92% of prescriptions written in the United States. The 35,640 rheumatoid arthritis patients in the study were started on methotrexate in 2009 and followed through 2014; 15,599 (43.8%) didn’t need anything else and stayed on oral methotrexate alone throughout the study period.
Prescribers, however, gave up on oral methotrexate at a mean dose of 15.3 mg and moved 17,528 patients (49%) straight to a biologic without giving subcutaneous methotrexate a shot. They did that after a median of less than 6 months, and within 3 months in more than 40% of patients.
Just 2,513 patients (7%) moved on to subcutaneous methotrexate when their oral formulation wasn’t enough. That’s all most of them needed; 1,802 (72%) remained on subcutaneous methotrexate alone for the remainder of the study period. The rest moved on to a biologic, but after a median of almost a year, not a few months. When their time on oral and subcutaneous methotrexate was included, their median time to a biologic was more than 2 years.
The investigators checked to see if things improved for patients who started on oral methotrexate in 2012. “The answer was no. We didn’t do better,” Dr. O’Dell said.
He didn’t speculate on why methotrexate is underused in the United States.
Claims data can’t address why patients were switched from methotrexate and other issues, but such nuances “don’t even begin to explain the doses and the timing of switch that we saw here,” he said.
Dr. O’Dell is an adviser for AbbVie, Lilly, Coherus, Bristol-Myers Squibb, Antares, and Medac. Other investigators disclosed relationships with those or other companies.
SAN FRANCISCO – Oral methotrexate is frequently underdosed, given for an inadequate length of time, and rarely switched to subcutaneous formulations before rheumatologists move on to biologics, according to an analysis of claims data from 35,640 rheumatoid arthritis patients.
“There’re some major concerns here. Methotrexate is the anchor drug for rheumatoid arthritis, the best drug we have. More appropriate [use] could lead to better control” and “produce significant cost savings,” said investigator Dr. James O’Dell, chief of the division of rheumatology at the University of Nebraska Medical Center, Omaha.
When patients don’t fully respond to lower doses, the ground rules for oral methotrexate include escalation up to 25 mg or a switch to subcutaneous formulations, which have better bioavailability. Those moves should be considered before turning to biologics, Dr. O’Dell explained at the American College of Rheumatology annual meeting.
Rheumatologists, by and large, aren’t playing by those rules, according to the analysis. “We need to own these data because the majority of patients, over three-quarters, were treated by rheumatologists. We are not doing a great job,” Dr. ODell said.
The claims data came from Symphony Health Solutions, which captures about 92% of prescriptions written in the United States. The 35,640 rheumatoid arthritis patients in the study were started on methotrexate in 2009 and followed through 2014; 15,599 (43.8%) didn’t need anything else and stayed on oral methotrexate alone throughout the study period.
Prescribers, however, gave up on oral methotrexate at a mean dose of 15.3 mg and moved 17,528 patients (49%) straight to a biologic without giving subcutaneous methotrexate a shot. They did that after a median of less than 6 months, and within 3 months in more than 40% of patients.
Just 2,513 patients (7%) moved on to subcutaneous methotrexate when their oral formulation wasn’t enough. That’s all most of them needed; 1,802 (72%) remained on subcutaneous methotrexate alone for the remainder of the study period. The rest moved on to a biologic, but after a median of almost a year, not a few months. When their time on oral and subcutaneous methotrexate was included, their median time to a biologic was more than 2 years.
The investigators checked to see if things improved for patients who started on oral methotrexate in 2012. “The answer was no. We didn’t do better,” Dr. O’Dell said.
He didn’t speculate on why methotrexate is underused in the United States.
Claims data can’t address why patients were switched from methotrexate and other issues, but such nuances “don’t even begin to explain the doses and the timing of switch that we saw here,” he said.
Dr. O’Dell is an adviser for AbbVie, Lilly, Coherus, Bristol-Myers Squibb, Antares, and Medac. Other investigators disclosed relationships with those or other companies.
AT THE ACR ANNUAL MEETING
Key clinical point: Give methotrexate a chance in rheumatoid arthritis; don’t be too quick with the biologics.
Major finding: Prescribers gave up on oral methotrexate at a mean dose of 15.3 mg, and moved 49% of patients straight to a biologic without first trying subcutaneous methotrexate.
Data source: Claims data from 35,640 rheumatoid arthritis patients.
Disclosures: The presenter is an adviser for AbbVie, Lilly, Coherus, Bristol-Myers Squibb, Antares, and Medac. Other investigators disclosed relationships with those or other companies.
ACR: Don’t give pneumococcal vaccine to CAPS, Behçet’s patients
SAN FRANCISCO – Pneumococcal vaccines can trigger severe local and systemic inflammatory reactions in patients with cryopyrin-associated periodic syndromes (CAPS), Behçet’s disease, and possibly other autoinflammatory disorders, according to a report from the annual meeting of the American College of Rheumatology.
The European League Against Rheumatism currently recommends pneumococcal vaccine in patients with inflammatory rheumatic diseases, and the Centers for Disease Control and Prevention recommends the vaccine in patients treated with immunosuppressive drugs.
“Careful consideration is warranted when implicating” these “guidelines in these patient populations. The risk of coming down with pneumococcal disease in these patients has to be balanced against the risk of severe reactions. We have here a 100% risk of unusually severe reactions in CAPS and Behçet’s patients with one shot, so we and others find it unethical to continue giving this vaccine to these patients. We no longer do this,” said investigator Dr. Ulrich Walker, a rheumatologist at Basel University in Switzerland and a member of the steering committee for the Ilaris registry, a Novartis database of CAPS patients treated with the company’s biologic canakinumab (Ilaris).
He and his colleagues reported on seven consecutive CAPS patients vaccinated with pneumococcal polysaccharide or conjugate vaccines according to current guidelines. Dr. Walker noted, however, that the Ilaris registry now has at least 14 CAPS patients who ran into serious trouble after receiving the vaccine.
“This is definitely real, and it’s not an adjuvant problem because not all pneumococcal vaccines contain adjuvants.” Instead, vaccine antigens trigger a flare. “The same thing happens with Behçet’s, where we’ve seen clear evidence of the triggering of underlying disease because patients had folliculitis around the vaccination site,” said Dr. Walker, who’s also published on that problem (Rheumatology [Oxford]. 2012 Apr;51[4]:761-2).
Within a few hours of vaccination, all seven patients developed severe, local injection site reactions and fever. Most received antibiotics, and two had to be hospitalized for systemic reactions. Patients ranged in age from 7 to 52 years old.
One patient, a 43-year-old woman, developed a severe headache associated with neck stiffness and photophobia. A florid red rash covered much of the arm where she got the shot. She was admitted to the hospital that evening with a presumed diagnosis of viral meningitis. Lumbar puncture revealed elevated white cells, normal glucose, and elevated protein. She was hospitalized for 18 days.
The reactions occurred with pneumococcal vaccines from three different companies and in patients with different CAPS phenotypes. Four vaccine reactions occurred in temporal association with concomitant coinjections of canakinumab; two reactions were separated by 15 days from the last canakinumab dose; and one reaction occurred in a patient who had never been exposed to canakinumab. Some patients had been vaccinated with a variety of other vaccines without any problem, so “this is something specific to pneumococcal vaccines,” Dr. Walker said.
Symptoms resolved in all the patients in 3-18 days.
The rapid onset, severity, and systemic nature of the reactions suggest that the vaccine triggers tau-like receptor activity, with subsequent inflammasome hyperactivation. “Patients with CAPS already have inflammasome constitutively turned on, so when you activate it even further you come down with a fever. The same thing happens with Behçet’s, which is also thought to be a multigenic disease associated with aberrant inflammasome activation,” Dr. Walker said.
The findings were so strong that the investigators are now testing low-dose pneumococcal vaccine for diagnostic screening before genetic testing for CAPs, Behçet’s, and some other autoimmune diseases. “A similar problem has been described in patients with mevalonate kinase deficiency,” Dr. Walker noted.
Novartis is worried the problem will be associated with canakinumab, but it doesn’t seem to be. “We had a lot of discussion about this and decided that we need to bring this out. Patients need to be aware of this,” said Dr. Jasmin Kümmerle-Deschner, a pediatrician at the University Hospital of Tübingen, Germany, and also a member of the Ilaris registry steering committee.
Dr. Walker disclosed financial relationships with Novartis, as did Dr. Kümmerle-Deschner, who also reported involvement with Sobi.
SAN FRANCISCO – Pneumococcal vaccines can trigger severe local and systemic inflammatory reactions in patients with cryopyrin-associated periodic syndromes (CAPS), Behçet’s disease, and possibly other autoinflammatory disorders, according to a report from the annual meeting of the American College of Rheumatology.
The European League Against Rheumatism currently recommends pneumococcal vaccine in patients with inflammatory rheumatic diseases, and the Centers for Disease Control and Prevention recommends the vaccine in patients treated with immunosuppressive drugs.
“Careful consideration is warranted when implicating” these “guidelines in these patient populations. The risk of coming down with pneumococcal disease in these patients has to be balanced against the risk of severe reactions. We have here a 100% risk of unusually severe reactions in CAPS and Behçet’s patients with one shot, so we and others find it unethical to continue giving this vaccine to these patients. We no longer do this,” said investigator Dr. Ulrich Walker, a rheumatologist at Basel University in Switzerland and a member of the steering committee for the Ilaris registry, a Novartis database of CAPS patients treated with the company’s biologic canakinumab (Ilaris).
He and his colleagues reported on seven consecutive CAPS patients vaccinated with pneumococcal polysaccharide or conjugate vaccines according to current guidelines. Dr. Walker noted, however, that the Ilaris registry now has at least 14 CAPS patients who ran into serious trouble after receiving the vaccine.
“This is definitely real, and it’s not an adjuvant problem because not all pneumococcal vaccines contain adjuvants.” Instead, vaccine antigens trigger a flare. “The same thing happens with Behçet’s, where we’ve seen clear evidence of the triggering of underlying disease because patients had folliculitis around the vaccination site,” said Dr. Walker, who’s also published on that problem (Rheumatology [Oxford]. 2012 Apr;51[4]:761-2).
Within a few hours of vaccination, all seven patients developed severe, local injection site reactions and fever. Most received antibiotics, and two had to be hospitalized for systemic reactions. Patients ranged in age from 7 to 52 years old.
One patient, a 43-year-old woman, developed a severe headache associated with neck stiffness and photophobia. A florid red rash covered much of the arm where she got the shot. She was admitted to the hospital that evening with a presumed diagnosis of viral meningitis. Lumbar puncture revealed elevated white cells, normal glucose, and elevated protein. She was hospitalized for 18 days.
The reactions occurred with pneumococcal vaccines from three different companies and in patients with different CAPS phenotypes. Four vaccine reactions occurred in temporal association with concomitant coinjections of canakinumab; two reactions were separated by 15 days from the last canakinumab dose; and one reaction occurred in a patient who had never been exposed to canakinumab. Some patients had been vaccinated with a variety of other vaccines without any problem, so “this is something specific to pneumococcal vaccines,” Dr. Walker said.
Symptoms resolved in all the patients in 3-18 days.
The rapid onset, severity, and systemic nature of the reactions suggest that the vaccine triggers tau-like receptor activity, with subsequent inflammasome hyperactivation. “Patients with CAPS already have inflammasome constitutively turned on, so when you activate it even further you come down with a fever. The same thing happens with Behçet’s, which is also thought to be a multigenic disease associated with aberrant inflammasome activation,” Dr. Walker said.
The findings were so strong that the investigators are now testing low-dose pneumococcal vaccine for diagnostic screening before genetic testing for CAPs, Behçet’s, and some other autoimmune diseases. “A similar problem has been described in patients with mevalonate kinase deficiency,” Dr. Walker noted.
Novartis is worried the problem will be associated with canakinumab, but it doesn’t seem to be. “We had a lot of discussion about this and decided that we need to bring this out. Patients need to be aware of this,” said Dr. Jasmin Kümmerle-Deschner, a pediatrician at the University Hospital of Tübingen, Germany, and also a member of the Ilaris registry steering committee.
Dr. Walker disclosed financial relationships with Novartis, as did Dr. Kümmerle-Deschner, who also reported involvement with Sobi.
SAN FRANCISCO – Pneumococcal vaccines can trigger severe local and systemic inflammatory reactions in patients with cryopyrin-associated periodic syndromes (CAPS), Behçet’s disease, and possibly other autoinflammatory disorders, according to a report from the annual meeting of the American College of Rheumatology.
The European League Against Rheumatism currently recommends pneumococcal vaccine in patients with inflammatory rheumatic diseases, and the Centers for Disease Control and Prevention recommends the vaccine in patients treated with immunosuppressive drugs.
“Careful consideration is warranted when implicating” these “guidelines in these patient populations. The risk of coming down with pneumococcal disease in these patients has to be balanced against the risk of severe reactions. We have here a 100% risk of unusually severe reactions in CAPS and Behçet’s patients with one shot, so we and others find it unethical to continue giving this vaccine to these patients. We no longer do this,” said investigator Dr. Ulrich Walker, a rheumatologist at Basel University in Switzerland and a member of the steering committee for the Ilaris registry, a Novartis database of CAPS patients treated with the company’s biologic canakinumab (Ilaris).
He and his colleagues reported on seven consecutive CAPS patients vaccinated with pneumococcal polysaccharide or conjugate vaccines according to current guidelines. Dr. Walker noted, however, that the Ilaris registry now has at least 14 CAPS patients who ran into serious trouble after receiving the vaccine.
“This is definitely real, and it’s not an adjuvant problem because not all pneumococcal vaccines contain adjuvants.” Instead, vaccine antigens trigger a flare. “The same thing happens with Behçet’s, where we’ve seen clear evidence of the triggering of underlying disease because patients had folliculitis around the vaccination site,” said Dr. Walker, who’s also published on that problem (Rheumatology [Oxford]. 2012 Apr;51[4]:761-2).
Within a few hours of vaccination, all seven patients developed severe, local injection site reactions and fever. Most received antibiotics, and two had to be hospitalized for systemic reactions. Patients ranged in age from 7 to 52 years old.
One patient, a 43-year-old woman, developed a severe headache associated with neck stiffness and photophobia. A florid red rash covered much of the arm where she got the shot. She was admitted to the hospital that evening with a presumed diagnosis of viral meningitis. Lumbar puncture revealed elevated white cells, normal glucose, and elevated protein. She was hospitalized for 18 days.
The reactions occurred with pneumococcal vaccines from three different companies and in patients with different CAPS phenotypes. Four vaccine reactions occurred in temporal association with concomitant coinjections of canakinumab; two reactions were separated by 15 days from the last canakinumab dose; and one reaction occurred in a patient who had never been exposed to canakinumab. Some patients had been vaccinated with a variety of other vaccines without any problem, so “this is something specific to pneumococcal vaccines,” Dr. Walker said.
Symptoms resolved in all the patients in 3-18 days.
The rapid onset, severity, and systemic nature of the reactions suggest that the vaccine triggers tau-like receptor activity, with subsequent inflammasome hyperactivation. “Patients with CAPS already have inflammasome constitutively turned on, so when you activate it even further you come down with a fever. The same thing happens with Behçet’s, which is also thought to be a multigenic disease associated with aberrant inflammasome activation,” Dr. Walker said.
The findings were so strong that the investigators are now testing low-dose pneumococcal vaccine for diagnostic screening before genetic testing for CAPs, Behçet’s, and some other autoimmune diseases. “A similar problem has been described in patients with mevalonate kinase deficiency,” Dr. Walker noted.
Novartis is worried the problem will be associated with canakinumab, but it doesn’t seem to be. “We had a lot of discussion about this and decided that we need to bring this out. Patients need to be aware of this,” said Dr. Jasmin Kümmerle-Deschner, a pediatrician at the University Hospital of Tübingen, Germany, and also a member of the Ilaris registry steering committee.
Dr. Walker disclosed financial relationships with Novartis, as did Dr. Kümmerle-Deschner, who also reported involvement with Sobi.
AT THE ACR ANNUAL MEETING
Key clinical point: Pneumococcal vaccines trigger severe local and systemic inflammatory reactions in patients with CAPS, Behçet’s disease, and perhaps other autoinflammatory disorders.
Major finding: Pneumococcal vaccine triggered disease flares in 100% of the CAPS and Behçet’s disease patients who received it.
Data source: Small case series of patients vaccinated against pneumococcal disease.
Disclosures: The investigators disclosed relationships with Novartis and Sobi.
ACR: Adalimumab best TNFi for anterior uveitis in ankylosing spondylitis
SAN FRANCISCO – Adalimumab was the clear winner over infliximab and etanercept for anterior uveitis in a Scandinavian database study of 1,365 patients with ankylosing spondylitis.
Office visits for uveitis dropped from 42.6 to 13.4 per 100 patient-years for the 406 patients who started on adalimumab (Humira). Flares per 100 patient-years dropped by almost 70%.
There was less improvement with infliximab (Remicade), which tended to be used in low doses in the study, less than 5 mg/kg. Office visits dropped from 50.4 to 27.3 per 100 patient-years for the 605 patients who started on it. Flares decreased by about 40%.
Etanercept (Enbrel), meanwhile, seemed to make uveitis worse, a concern that’s been raised previously. Office visits for uveitis increased from 38.1 to 56.5 per 100 patient-years for the 354 patients started on etanercept. Flares increased by about 20%.
The results were presented at the annual meeting of the American College of Rheumatology.
Among patients who were uveitis free in the 2 years prior to starting a tumor necrosis factor inhibitor (TNFi), the risk of flare was far greater with etanercept than with adalimumab (adjusted hazard ratio, 3.69; 95% confidence interval, 1.61-8.46). The risk also was higher for infliximab, though not significantly so (adjusted HR, 1.67; 95% CI, 0.69-4.04).
About 2% of adalimumab patients had their first bout of uveitis after starting it, versus 5.3% of infliximab and 13.7% of etanercept patients.
“A clear reduction in uveitis rates was observed for adalimumab, a slight reduction for infliximab, and a marked increase for etanercept, irrespective of the method for counting flares,” reported Dr. Elisabeth Lie, a rheumatology fellow at Gothenburg (Sweden) University.
In the adjusted analysis, “adalimumab and infliximab were associated with significantly lower hazard for first uveitis flare than etanercept,” noted Dr. Lie.
“Right now, we are still favoring infliximab because it comes as a biosimilar, so it’s inexpensive; I think we are giving it the benefit of the doubt. Still, these results are so favorable for adalimumab” that they make “a strong case to at least have a lower threshold to switch to it if you are not successful with your first choice,” Dr. Lie said in an interview.
The etanercept findings are “at least a reminder that if you have a patient with any history of uveitis, choose another option. I’ve seen a few” cases apparently triggered by etanercept. When that happens, “switch to something else,” she said.
The problem with etanercept may be related to its structure or immunologic effects, but no one really knows for sure, she added.
The study linked the first-time use of a TNFi by ankylosing spondylitis patients in the Swedish Biologics Register to their office visits for anterior uveitis as captured in the country’s National Patient Register. Dr. Lie and her coinvestigators analyzed the 2 years before and after the start of a TNFi. Adalimumab was more frequently used toward the end of the study period, which ran from 2003 through 2010.
Patients were in their early 40s, on average, and almost three-quarters were men. Infliximab patients most often used concomitant disease-modifying antirheumatic drugs and had the highest rate of comorbid inflammatory bowel disease, at 10.4%. Adalimumab patients had the lowest median baseline C-reactive protein at 10 mg/L, but highest pretreatment rate of uveitis, at 28%.
Dr. Lie is a speaker and consultant for AbbVie, the maker of adalimumab, and Pfizer, the maker of etanercept.
SAN FRANCISCO – Adalimumab was the clear winner over infliximab and etanercept for anterior uveitis in a Scandinavian database study of 1,365 patients with ankylosing spondylitis.
Office visits for uveitis dropped from 42.6 to 13.4 per 100 patient-years for the 406 patients who started on adalimumab (Humira). Flares per 100 patient-years dropped by almost 70%.
There was less improvement with infliximab (Remicade), which tended to be used in low doses in the study, less than 5 mg/kg. Office visits dropped from 50.4 to 27.3 per 100 patient-years for the 605 patients who started on it. Flares decreased by about 40%.
Etanercept (Enbrel), meanwhile, seemed to make uveitis worse, a concern that’s been raised previously. Office visits for uveitis increased from 38.1 to 56.5 per 100 patient-years for the 354 patients started on etanercept. Flares increased by about 20%.
The results were presented at the annual meeting of the American College of Rheumatology.
Among patients who were uveitis free in the 2 years prior to starting a tumor necrosis factor inhibitor (TNFi), the risk of flare was far greater with etanercept than with adalimumab (adjusted hazard ratio, 3.69; 95% confidence interval, 1.61-8.46). The risk also was higher for infliximab, though not significantly so (adjusted HR, 1.67; 95% CI, 0.69-4.04).
About 2% of adalimumab patients had their first bout of uveitis after starting it, versus 5.3% of infliximab and 13.7% of etanercept patients.
“A clear reduction in uveitis rates was observed for adalimumab, a slight reduction for infliximab, and a marked increase for etanercept, irrespective of the method for counting flares,” reported Dr. Elisabeth Lie, a rheumatology fellow at Gothenburg (Sweden) University.
In the adjusted analysis, “adalimumab and infliximab were associated with significantly lower hazard for first uveitis flare than etanercept,” noted Dr. Lie.
“Right now, we are still favoring infliximab because it comes as a biosimilar, so it’s inexpensive; I think we are giving it the benefit of the doubt. Still, these results are so favorable for adalimumab” that they make “a strong case to at least have a lower threshold to switch to it if you are not successful with your first choice,” Dr. Lie said in an interview.
The etanercept findings are “at least a reminder that if you have a patient with any history of uveitis, choose another option. I’ve seen a few” cases apparently triggered by etanercept. When that happens, “switch to something else,” she said.
The problem with etanercept may be related to its structure or immunologic effects, but no one really knows for sure, she added.
The study linked the first-time use of a TNFi by ankylosing spondylitis patients in the Swedish Biologics Register to their office visits for anterior uveitis as captured in the country’s National Patient Register. Dr. Lie and her coinvestigators analyzed the 2 years before and after the start of a TNFi. Adalimumab was more frequently used toward the end of the study period, which ran from 2003 through 2010.
Patients were in their early 40s, on average, and almost three-quarters were men. Infliximab patients most often used concomitant disease-modifying antirheumatic drugs and had the highest rate of comorbid inflammatory bowel disease, at 10.4%. Adalimumab patients had the lowest median baseline C-reactive protein at 10 mg/L, but highest pretreatment rate of uveitis, at 28%.
Dr. Lie is a speaker and consultant for AbbVie, the maker of adalimumab, and Pfizer, the maker of etanercept.
SAN FRANCISCO – Adalimumab was the clear winner over infliximab and etanercept for anterior uveitis in a Scandinavian database study of 1,365 patients with ankylosing spondylitis.
Office visits for uveitis dropped from 42.6 to 13.4 per 100 patient-years for the 406 patients who started on adalimumab (Humira). Flares per 100 patient-years dropped by almost 70%.
There was less improvement with infliximab (Remicade), which tended to be used in low doses in the study, less than 5 mg/kg. Office visits dropped from 50.4 to 27.3 per 100 patient-years for the 605 patients who started on it. Flares decreased by about 40%.
Etanercept (Enbrel), meanwhile, seemed to make uveitis worse, a concern that’s been raised previously. Office visits for uveitis increased from 38.1 to 56.5 per 100 patient-years for the 354 patients started on etanercept. Flares increased by about 20%.
The results were presented at the annual meeting of the American College of Rheumatology.
Among patients who were uveitis free in the 2 years prior to starting a tumor necrosis factor inhibitor (TNFi), the risk of flare was far greater with etanercept than with adalimumab (adjusted hazard ratio, 3.69; 95% confidence interval, 1.61-8.46). The risk also was higher for infliximab, though not significantly so (adjusted HR, 1.67; 95% CI, 0.69-4.04).
About 2% of adalimumab patients had their first bout of uveitis after starting it, versus 5.3% of infliximab and 13.7% of etanercept patients.
“A clear reduction in uveitis rates was observed for adalimumab, a slight reduction for infliximab, and a marked increase for etanercept, irrespective of the method for counting flares,” reported Dr. Elisabeth Lie, a rheumatology fellow at Gothenburg (Sweden) University.
In the adjusted analysis, “adalimumab and infliximab were associated with significantly lower hazard for first uveitis flare than etanercept,” noted Dr. Lie.
“Right now, we are still favoring infliximab because it comes as a biosimilar, so it’s inexpensive; I think we are giving it the benefit of the doubt. Still, these results are so favorable for adalimumab” that they make “a strong case to at least have a lower threshold to switch to it if you are not successful with your first choice,” Dr. Lie said in an interview.
The etanercept findings are “at least a reminder that if you have a patient with any history of uveitis, choose another option. I’ve seen a few” cases apparently triggered by etanercept. When that happens, “switch to something else,” she said.
The problem with etanercept may be related to its structure or immunologic effects, but no one really knows for sure, she added.
The study linked the first-time use of a TNFi by ankylosing spondylitis patients in the Swedish Biologics Register to their office visits for anterior uveitis as captured in the country’s National Patient Register. Dr. Lie and her coinvestigators analyzed the 2 years before and after the start of a TNFi. Adalimumab was more frequently used toward the end of the study period, which ran from 2003 through 2010.
Patients were in their early 40s, on average, and almost three-quarters were men. Infliximab patients most often used concomitant disease-modifying antirheumatic drugs and had the highest rate of comorbid inflammatory bowel disease, at 10.4%. Adalimumab patients had the lowest median baseline C-reactive protein at 10 mg/L, but highest pretreatment rate of uveitis, at 28%.
Dr. Lie is a speaker and consultant for AbbVie, the maker of adalimumab, and Pfizer, the maker of etanercept.
AT The ACR ANNUAL MEETING
Key clinical point: Pick adalimumab or perhaps infliximab for anterior uveitis in AS, not etanercept.
Major finding: Office visits for uveitis dropped from 42.6 to 13.4 per 100 patient-years for the 406 patients who started on adalimumab; office visits increased from 38.1 to 56.5 per 100 patient-years for the 354 patients who started on etanercept.
Data source: Database study of 1,365 ankylosing spondylitis patients with anterior uveitis.
Disclosures: The presenting investigator is a speaker and consultant for AbbVie, maker of adalimumab, and Pfizer, maker of etanercept.
ACR: A reminder about ADHD-stimulant vasculopathy
SAN FRANCISCO – Forty-seven children referred to Children’s Hospital of Georgia’s rheumatology clinic in Augusta for work-up of autoimmune vasculopathy had vasculopathy symptoms triggered by their ADHD stimulants.
They had been referred by primary care providers who told families that their children probably had an autoimmune disease; the families were relieved to find out that they did not, according to Dr. Rita Jerath, a pediatric rheumatologist in the department of rheumatology, Medical College of Georgia, Augusta.
The findings show that psychostimulant-induced peripheral vasculopathy, a known side effect included in the labeling of drugs such as methylphenidate (Ritalin or Concerta), dexmethylphenidate (Focalin), and amphetamine/dextroamphetamine (Adderall), is underrecognized in the medical community, leading to needless anxiety and unnecessary medical expenses, she noted.
Although uncommon, “there is a strong association between the use of psychostimulants and peripheral vasculopathy symptoms. Our study demonstrates the need of making prescribing physicians aware of this significant side effect so they may educate the families and also elucidate occurrence of new vascular symptoms [in] this patient population,” noted Dr. Jerath.
“A lot of rheumatologists will recognize this, but we want to get this message across to primary care physicians and families,” she added.
She had several tips for recognizing the problem. Otherwise well children seem to have Raynaud’s disease, “but their signs and symptoms are different,” she said. The color change is more extensive, on the tops of hands and feet, and more dusky and cyanotic. Hands and feet can be cold, numb, and painful, but the condition doesn’t change with cold or stress, as in Raynaud’s. Children can be positive for antinuclear antibodies, as several were in the study, Dr. Jerath said.
Children are usually on their psychostimulants from a few months or few years before an attack, and attacks seem to be triggered by a change in dose or drug.
The symptoms often improve with a drug holiday, for instance during vacation.
Although families are glad to learn their children don’t have an autoimmune disease, they are often reluctant to discontinue the stimulants. “A lot of times, the question is, ‘What else is available?’ There are a few medications that are not psychostimulants – Straterra [atomoxetine] is one – but usually physicians find they are not as helpful,” Dr. Jerath said.
The investigators had no disclosures.
SAN FRANCISCO – Forty-seven children referred to Children’s Hospital of Georgia’s rheumatology clinic in Augusta for work-up of autoimmune vasculopathy had vasculopathy symptoms triggered by their ADHD stimulants.
They had been referred by primary care providers who told families that their children probably had an autoimmune disease; the families were relieved to find out that they did not, according to Dr. Rita Jerath, a pediatric rheumatologist in the department of rheumatology, Medical College of Georgia, Augusta.
The findings show that psychostimulant-induced peripheral vasculopathy, a known side effect included in the labeling of drugs such as methylphenidate (Ritalin or Concerta), dexmethylphenidate (Focalin), and amphetamine/dextroamphetamine (Adderall), is underrecognized in the medical community, leading to needless anxiety and unnecessary medical expenses, she noted.
Although uncommon, “there is a strong association between the use of psychostimulants and peripheral vasculopathy symptoms. Our study demonstrates the need of making prescribing physicians aware of this significant side effect so they may educate the families and also elucidate occurrence of new vascular symptoms [in] this patient population,” noted Dr. Jerath.
“A lot of rheumatologists will recognize this, but we want to get this message across to primary care physicians and families,” she added.
She had several tips for recognizing the problem. Otherwise well children seem to have Raynaud’s disease, “but their signs and symptoms are different,” she said. The color change is more extensive, on the tops of hands and feet, and more dusky and cyanotic. Hands and feet can be cold, numb, and painful, but the condition doesn’t change with cold or stress, as in Raynaud’s. Children can be positive for antinuclear antibodies, as several were in the study, Dr. Jerath said.
Children are usually on their psychostimulants from a few months or few years before an attack, and attacks seem to be triggered by a change in dose or drug.
The symptoms often improve with a drug holiday, for instance during vacation.
Although families are glad to learn their children don’t have an autoimmune disease, they are often reluctant to discontinue the stimulants. “A lot of times, the question is, ‘What else is available?’ There are a few medications that are not psychostimulants – Straterra [atomoxetine] is one – but usually physicians find they are not as helpful,” Dr. Jerath said.
The investigators had no disclosures.
SAN FRANCISCO – Forty-seven children referred to Children’s Hospital of Georgia’s rheumatology clinic in Augusta for work-up of autoimmune vasculopathy had vasculopathy symptoms triggered by their ADHD stimulants.
They had been referred by primary care providers who told families that their children probably had an autoimmune disease; the families were relieved to find out that they did not, according to Dr. Rita Jerath, a pediatric rheumatologist in the department of rheumatology, Medical College of Georgia, Augusta.
The findings show that psychostimulant-induced peripheral vasculopathy, a known side effect included in the labeling of drugs such as methylphenidate (Ritalin or Concerta), dexmethylphenidate (Focalin), and amphetamine/dextroamphetamine (Adderall), is underrecognized in the medical community, leading to needless anxiety and unnecessary medical expenses, she noted.
Although uncommon, “there is a strong association between the use of psychostimulants and peripheral vasculopathy symptoms. Our study demonstrates the need of making prescribing physicians aware of this significant side effect so they may educate the families and also elucidate occurrence of new vascular symptoms [in] this patient population,” noted Dr. Jerath.
“A lot of rheumatologists will recognize this, but we want to get this message across to primary care physicians and families,” she added.
She had several tips for recognizing the problem. Otherwise well children seem to have Raynaud’s disease, “but their signs and symptoms are different,” she said. The color change is more extensive, on the tops of hands and feet, and more dusky and cyanotic. Hands and feet can be cold, numb, and painful, but the condition doesn’t change with cold or stress, as in Raynaud’s. Children can be positive for antinuclear antibodies, as several were in the study, Dr. Jerath said.
Children are usually on their psychostimulants from a few months or few years before an attack, and attacks seem to be triggered by a change in dose or drug.
The symptoms often improve with a drug holiday, for instance during vacation.
Although families are glad to learn their children don’t have an autoimmune disease, they are often reluctant to discontinue the stimulants. “A lot of times, the question is, ‘What else is available?’ There are a few medications that are not psychostimulants – Straterra [atomoxetine] is one – but usually physicians find they are not as helpful,” Dr. Jerath said.
The investigators had no disclosures.
AT THE ACR ANNUAL MEETING
Key clinical point: ADHD stimulants can cause peripheral vasculopathy.
Major finding: Forty-seven children with stimulant-induced peripheral vasoconstriction were misdiagnosed with autoimmune disease.
Data source: A review of 47 pediatric ADHD cases.
Disclosures: The investigators had no disclosures.
ACR: Mycophenolate reduces need for GAVE endoscopy
SAN FRANCISCO – Mycophenolate significantly reduces the need for endoscopic interventions for gastric antral vascular ectasia in patients with systemic sclerosis, according to investigators from the Cleveland Clinic.
In a retrospective chart review of 48 systemic sclerosis patients with gastric antral vascular ectasia (GAVE), the 19 patients not on immunosuppressive agents needed 1.37 endoscopies per year. The 14 patients on mycophenolate needed 0.25 per year. Mycophenolate patients were far less likely to need endoscopy (relative risk, 0.19; 95% confidence interval, 0.05-0.70; P = .013).
Other immunosuppressives such as glucocorticoids, hydroxychloroquine, cyclophosphamide, and methotrexate also reduced the frequency of endoscopies, but not as much. The investigators found that patients on any immunosuppressive agent needed 0.51 endoscopies per year.
“Mycophenolate should be considered a potential treatment to alter the course of disease particularly in patients that need frequent therapeutic endoscopies. We are considering it more and more in our GAVE patients,” said investigator Dr. Tiffany Lin, a rheumatology fellow at the Cleveland Clinic Foundation.
“We had a suspicion that” immunosuppression might help “based on prior reports that looked at cyclophosphamide. We sought to” find other “immunosuppressive therapies that were less toxic and were surprised and excited about the results with mycophenolate,” she said at the annual meeting of the American College of Rheumatology.
The mean dose of mycophenolate in the study was 1 g twice a day. The duration of treatment varied from months to years.
In GAVE, also known as “watermelon stomach,” antral blood vessels are dilated and likely to bleed. It is an increasingly recognized cause of upper gastrointestinal bleeding in patients with systemic sclerosis.
There’s currently no defined role for immunosuppressive therapy in GAVE, which is usually treated with endoscopic argon plasma coagulation of the aberrant vessels.
Patients in the study were in their mid-60s on average, and the majority were women. There were no significant differences in hospitalization or blood transfusion frequencies between immunosuppressed and nonimmunosuppressed patients.
The investigators have no disclosures, and there was no outside funding for the project.
SAN FRANCISCO – Mycophenolate significantly reduces the need for endoscopic interventions for gastric antral vascular ectasia in patients with systemic sclerosis, according to investigators from the Cleveland Clinic.
In a retrospective chart review of 48 systemic sclerosis patients with gastric antral vascular ectasia (GAVE), the 19 patients not on immunosuppressive agents needed 1.37 endoscopies per year. The 14 patients on mycophenolate needed 0.25 per year. Mycophenolate patients were far less likely to need endoscopy (relative risk, 0.19; 95% confidence interval, 0.05-0.70; P = .013).
Other immunosuppressives such as glucocorticoids, hydroxychloroquine, cyclophosphamide, and methotrexate also reduced the frequency of endoscopies, but not as much. The investigators found that patients on any immunosuppressive agent needed 0.51 endoscopies per year.
“Mycophenolate should be considered a potential treatment to alter the course of disease particularly in patients that need frequent therapeutic endoscopies. We are considering it more and more in our GAVE patients,” said investigator Dr. Tiffany Lin, a rheumatology fellow at the Cleveland Clinic Foundation.
“We had a suspicion that” immunosuppression might help “based on prior reports that looked at cyclophosphamide. We sought to” find other “immunosuppressive therapies that were less toxic and were surprised and excited about the results with mycophenolate,” she said at the annual meeting of the American College of Rheumatology.
The mean dose of mycophenolate in the study was 1 g twice a day. The duration of treatment varied from months to years.
In GAVE, also known as “watermelon stomach,” antral blood vessels are dilated and likely to bleed. It is an increasingly recognized cause of upper gastrointestinal bleeding in patients with systemic sclerosis.
There’s currently no defined role for immunosuppressive therapy in GAVE, which is usually treated with endoscopic argon plasma coagulation of the aberrant vessels.
Patients in the study were in their mid-60s on average, and the majority were women. There were no significant differences in hospitalization or blood transfusion frequencies between immunosuppressed and nonimmunosuppressed patients.
The investigators have no disclosures, and there was no outside funding for the project.
SAN FRANCISCO – Mycophenolate significantly reduces the need for endoscopic interventions for gastric antral vascular ectasia in patients with systemic sclerosis, according to investigators from the Cleveland Clinic.
In a retrospective chart review of 48 systemic sclerosis patients with gastric antral vascular ectasia (GAVE), the 19 patients not on immunosuppressive agents needed 1.37 endoscopies per year. The 14 patients on mycophenolate needed 0.25 per year. Mycophenolate patients were far less likely to need endoscopy (relative risk, 0.19; 95% confidence interval, 0.05-0.70; P = .013).
Other immunosuppressives such as glucocorticoids, hydroxychloroquine, cyclophosphamide, and methotrexate also reduced the frequency of endoscopies, but not as much. The investigators found that patients on any immunosuppressive agent needed 0.51 endoscopies per year.
“Mycophenolate should be considered a potential treatment to alter the course of disease particularly in patients that need frequent therapeutic endoscopies. We are considering it more and more in our GAVE patients,” said investigator Dr. Tiffany Lin, a rheumatology fellow at the Cleveland Clinic Foundation.
“We had a suspicion that” immunosuppression might help “based on prior reports that looked at cyclophosphamide. We sought to” find other “immunosuppressive therapies that were less toxic and were surprised and excited about the results with mycophenolate,” she said at the annual meeting of the American College of Rheumatology.
The mean dose of mycophenolate in the study was 1 g twice a day. The duration of treatment varied from months to years.
In GAVE, also known as “watermelon stomach,” antral blood vessels are dilated and likely to bleed. It is an increasingly recognized cause of upper gastrointestinal bleeding in patients with systemic sclerosis.
There’s currently no defined role for immunosuppressive therapy in GAVE, which is usually treated with endoscopic argon plasma coagulation of the aberrant vessels.
Patients in the study were in their mid-60s on average, and the majority were women. There were no significant differences in hospitalization or blood transfusion frequencies between immunosuppressed and nonimmunosuppressed patients.
The investigators have no disclosures, and there was no outside funding for the project.
AT THE ACR ANNUAL MEETING
Key clinical point: Mycophenolate reduces the frequency of recurrent gastric antral vascular ectasia in systemic sclerosis.
Major finding: Nineteen patients not on immunosuppressive agents needed 1.37 endoscopies per year. The 14 patients on mycophenolate needed 0.25 per year.
Data source: The study was a retrospective review of 48 systemic sclerosis patients with GAVE.
Disclosures: The investigators have no disclosures, and there was no outside funding for the project.
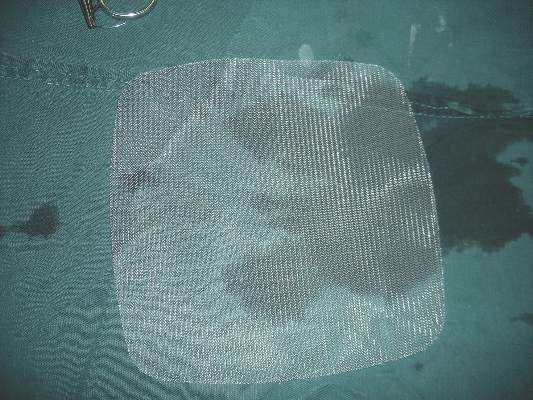
ACS: Infected hernia salvage success most likely with lightweight polypropylene
CHICAGO – Infected ventral hernia mesh is most likely to be salvaged if it’s made from lightweight polypropylene, according to data from a series of 161 mesh infection cases at Carolinas Medical Center in Charlotte, N.C.
Investigators there were able to salvage 33% of lightweight polypropylene and 8% of polytetrafluoroethylene mesh patients, but salvage failed in patients with composite, polyester, or heavyweight polypropylene meshes. The overall salvage rate was less than 10%.
“We sometimes think that we’ve salvaged the mesh, but if we follow these patients long enough” – follow-up was an average of 37 months in the series – “the majority of them will need to be excised,” said investigator Dr. Vedra Augenstein of the department of surgery at the medical center.
Wound complications are common in ventral hernia repairs, and mesh infections, said Dr. Augenstein, are among “the most dreaded.” The North Carolina findings help define the small pool of patients in whom salvage might work.
The average body mass index in the series was 36 kg/m2, and patients had an average of 2.6 previous ventral hernia repairs. The majority had a polypropylene mesh. Most of the cases were referred to the medical center from elsewhere, so mesh excision came an average of 10 months after the diagnosis of infection.
Almost a third of patients had their infections diagnosed a year or more after mesh implant, which goes against the common notion that mesh patients are out of the woods after a year. “It took a very long time for some of these infections to present. This was a big surprise for us,” Dr. Augenstein said.
The team tried to salvage all of their patients, using antibiotics in 90%, vacuum-assisted closure and/or debridement in 57%, and percutaneous drainage in 17%.
No patient presented with an obvious fistula, but the investigators found fistulas during surgery in about 16% of patients. Meanwhile, salvage failed in every patient who continued to smoke despite being diagnosed with a mesh infection.
The team assembled their findings into an informal algorithm for considering mesh salvage.
“Think about what you need to ask yourself. First, does the patient have a fistula or do you suspect one” from, for instance, gut bacteria in the wound or oral contrast above the mesh on CT? If so, “they are going to need an operation,” Dr. Augenstein said.
Smoking is the next stop point; salvage is likely to fail in smokers.
Mesh type is the third consideration; lightweight polypropylene is a good sign. “If they have heavyweight polypropylene, composite, or polyester mesh, they were not salvageable in our series,” she said.
For patients still in the running, Methicillin-resistant Staphylococcus aureus (MRSA) is the next concern. If it’s in the wound, mesh is going to be harder to salvage, said Dr. Augenstein. Most of the infections in the series were caused by Staphylococcus, with MRSA present in 45% of patients.
Infection recurrence is common even when salvage seems to work, so “we need to follow these patients for a very, very long time,” Dr. Augenstein said.
Along with clinical exams, the North Carolina team follows C-reactive protein and erythrocyte sedimentation rate. “If the abdomen gets red or if, for example, the C-reactive protein goes up, we’ll do a CT scan or ultrasound to make sure they are not brewing an infection,” she said.
Dr. Augenstein has received research and educational grants from Gore, Ethicon, Novadaq, Bard, and LifeCell.
CHICAGO – Infected ventral hernia mesh is most likely to be salvaged if it’s made from lightweight polypropylene, according to data from a series of 161 mesh infection cases at Carolinas Medical Center in Charlotte, N.C.
Investigators there were able to salvage 33% of lightweight polypropylene and 8% of polytetrafluoroethylene mesh patients, but salvage failed in patients with composite, polyester, or heavyweight polypropylene meshes. The overall salvage rate was less than 10%.
“We sometimes think that we’ve salvaged the mesh, but if we follow these patients long enough” – follow-up was an average of 37 months in the series – “the majority of them will need to be excised,” said investigator Dr. Vedra Augenstein of the department of surgery at the medical center.
Wound complications are common in ventral hernia repairs, and mesh infections, said Dr. Augenstein, are among “the most dreaded.” The North Carolina findings help define the small pool of patients in whom salvage might work.
The average body mass index in the series was 36 kg/m2, and patients had an average of 2.6 previous ventral hernia repairs. The majority had a polypropylene mesh. Most of the cases were referred to the medical center from elsewhere, so mesh excision came an average of 10 months after the diagnosis of infection.
Almost a third of patients had their infections diagnosed a year or more after mesh implant, which goes against the common notion that mesh patients are out of the woods after a year. “It took a very long time for some of these infections to present. This was a big surprise for us,” Dr. Augenstein said.
The team tried to salvage all of their patients, using antibiotics in 90%, vacuum-assisted closure and/or debridement in 57%, and percutaneous drainage in 17%.
No patient presented with an obvious fistula, but the investigators found fistulas during surgery in about 16% of patients. Meanwhile, salvage failed in every patient who continued to smoke despite being diagnosed with a mesh infection.
The team assembled their findings into an informal algorithm for considering mesh salvage.
“Think about what you need to ask yourself. First, does the patient have a fistula or do you suspect one” from, for instance, gut bacteria in the wound or oral contrast above the mesh on CT? If so, “they are going to need an operation,” Dr. Augenstein said.
Smoking is the next stop point; salvage is likely to fail in smokers.
Mesh type is the third consideration; lightweight polypropylene is a good sign. “If they have heavyweight polypropylene, composite, or polyester mesh, they were not salvageable in our series,” she said.
For patients still in the running, Methicillin-resistant Staphylococcus aureus (MRSA) is the next concern. If it’s in the wound, mesh is going to be harder to salvage, said Dr. Augenstein. Most of the infections in the series were caused by Staphylococcus, with MRSA present in 45% of patients.
Infection recurrence is common even when salvage seems to work, so “we need to follow these patients for a very, very long time,” Dr. Augenstein said.
Along with clinical exams, the North Carolina team follows C-reactive protein and erythrocyte sedimentation rate. “If the abdomen gets red or if, for example, the C-reactive protein goes up, we’ll do a CT scan or ultrasound to make sure they are not brewing an infection,” she said.
Dr. Augenstein has received research and educational grants from Gore, Ethicon, Novadaq, Bard, and LifeCell.
CHICAGO – Infected ventral hernia mesh is most likely to be salvaged if it’s made from lightweight polypropylene, according to data from a series of 161 mesh infection cases at Carolinas Medical Center in Charlotte, N.C.
Investigators there were able to salvage 33% of lightweight polypropylene and 8% of polytetrafluoroethylene mesh patients, but salvage failed in patients with composite, polyester, or heavyweight polypropylene meshes. The overall salvage rate was less than 10%.
“We sometimes think that we’ve salvaged the mesh, but if we follow these patients long enough” – follow-up was an average of 37 months in the series – “the majority of them will need to be excised,” said investigator Dr. Vedra Augenstein of the department of surgery at the medical center.
Wound complications are common in ventral hernia repairs, and mesh infections, said Dr. Augenstein, are among “the most dreaded.” The North Carolina findings help define the small pool of patients in whom salvage might work.
The average body mass index in the series was 36 kg/m2, and patients had an average of 2.6 previous ventral hernia repairs. The majority had a polypropylene mesh. Most of the cases were referred to the medical center from elsewhere, so mesh excision came an average of 10 months after the diagnosis of infection.
Almost a third of patients had their infections diagnosed a year or more after mesh implant, which goes against the common notion that mesh patients are out of the woods after a year. “It took a very long time for some of these infections to present. This was a big surprise for us,” Dr. Augenstein said.
The team tried to salvage all of their patients, using antibiotics in 90%, vacuum-assisted closure and/or debridement in 57%, and percutaneous drainage in 17%.
No patient presented with an obvious fistula, but the investigators found fistulas during surgery in about 16% of patients. Meanwhile, salvage failed in every patient who continued to smoke despite being diagnosed with a mesh infection.
The team assembled their findings into an informal algorithm for considering mesh salvage.
“Think about what you need to ask yourself. First, does the patient have a fistula or do you suspect one” from, for instance, gut bacteria in the wound or oral contrast above the mesh on CT? If so, “they are going to need an operation,” Dr. Augenstein said.
Smoking is the next stop point; salvage is likely to fail in smokers.
Mesh type is the third consideration; lightweight polypropylene is a good sign. “If they have heavyweight polypropylene, composite, or polyester mesh, they were not salvageable in our series,” she said.
For patients still in the running, Methicillin-resistant Staphylococcus aureus (MRSA) is the next concern. If it’s in the wound, mesh is going to be harder to salvage, said Dr. Augenstein. Most of the infections in the series were caused by Staphylococcus, with MRSA present in 45% of patients.
Infection recurrence is common even when salvage seems to work, so “we need to follow these patients for a very, very long time,” Dr. Augenstein said.
Along with clinical exams, the North Carolina team follows C-reactive protein and erythrocyte sedimentation rate. “If the abdomen gets red or if, for example, the C-reactive protein goes up, we’ll do a CT scan or ultrasound to make sure they are not brewing an infection,” she said.
Dr. Augenstein has received research and educational grants from Gore, Ethicon, Novadaq, Bard, and LifeCell.
AT THE ACS CLINICAL CONGRESS
Key clinical point: The ideal infected mesh salvage candidate is a MRSA-free nonsmoker with a lightweight polypropylene implant and no fistulas.
Major finding: Mesh salvage worked in about a third of lightweight polypropylene patients and 8% of polytetrafluoroethylene mesh patients but failed in patients with composite, polyester, or heavyweight polypropylene meshes.
Data source: Series of 161 infected ventral hernia mesh patients.
Disclosures: The presenting investigator has received research and educational grants from Gore, Ethicon, Novadaq, Bard, and LifeCell.
ACS: Watchful waiting for some rectal cancers almost ready for ‘prime time’
CHICAGO – Watchful waiting with careful surveillance may become an option for the majority of locally advanced rectal cancer patients who have a complete clinical response to neoadjuvant therapy, according to a review of 442 rectal cancer patients at Memorial Sloan Kettering Cancer Center in New York.
Seventy-three of those patients had a complete clinical response to neoadjuvant therapy and opted for watchful waiting instead of surgery after weighing the risks and benefits – including about a 25% chance of local recurrence – with their doctors.
At 4 years’ follow-up, 54 (74%) remained cancer free. Nineteen patients had local tumor recurrence, generally within 13 months. Two of those patients had successful local excisions, and the remaining 17 had salvage total mesorectal excisions (TME).
There were no statistically significant differences in 4-year disease-specific and overall survival among the 73 patients and 72 other patients who opted for TME after neoadjuvant chemotherapy and were found to have had pathologic complete responses.
“In our cohort, watch and wait was safe. It’s an effective treatment strategy achieving a high rate of rectal preservation in tumors that respond to neoadjuvant therapy. I don’t think the rectum needs to come out in everybody,” said investigator Dr. J. Joshua Smith, a surgical oncologist at Sloan Kettering.
Several studies have reported similar results similar to the Sloan Kettering study, but other investigations have been retrospective, so optimal patient selection, assessment of response, surveillance protocols, and other matters remain uncertain. Sloan Kettering and about 20 other cancer centers in United States – all members of the Rectal Cancer Consortium – recently launched a randomized clinical trial to get a better handle on those issues.
Locally advanced rectal cancer patients will be randomized to either chemoradiation for 5.5 weeks followed by folinic acid, fluorouracil, and oxaliplatin (FOLFOX) or capecitabine and oxaliplatin (CapeOX) over about 16 weeks, or FOLFOX/CapeOX first and chemoradiation second. Those who have a significant clinical response will then undergo watchful waiting; those who do not will have TME.
About 50 patients have enrolled in the phase II trial so far; the investigators are looking for more than 200.
“I think ‘prime time for watchful waiting’ is around the corner, but not yet here. It must be preceded by a prospective trial.” Meanwhile, “how we define complete clinical response is important” when considering watchful waiting, Dr. Smith said at the annual clinical congress of the American College of Surgeons..
At Sloan Kettering, where watchful waiting has become more popular in recent years, complete clinical response means no tumor or lymph nodes on imaging, and, on digital rectal exam (DRE) and proctoscopy, normal flat mucosa, smooth induration, no mass, no nodules, no ulcerations, and no luminal narrowing; a pale scar and telangiectasias are okay.
In the first year, surveillance includes DRE and endoscopy every 3 months and imaging every 6 months. In the second year, DRE and endoscopy come every 4 months, and imaging again every 6 months. From years 3 to 5, DRE and endoscopy are done every 6 months, and imaging every 6-12 months. After 5 years, surveillance is by yearly DRE and endoscopy.
When discussing the option with patients, they need to know – besides the risk of recurrence – that watchful waiting is currently not standard medical management; surveillance must be frequent; they are at risk for a more extensive salvage TME than they might have had otherwise; and the approach might compromise the chance of a cure, Dr. Smith said.
Dr. Smith said he has no relevant disclosures.
CHICAGO – Watchful waiting with careful surveillance may become an option for the majority of locally advanced rectal cancer patients who have a complete clinical response to neoadjuvant therapy, according to a review of 442 rectal cancer patients at Memorial Sloan Kettering Cancer Center in New York.
Seventy-three of those patients had a complete clinical response to neoadjuvant therapy and opted for watchful waiting instead of surgery after weighing the risks and benefits – including about a 25% chance of local recurrence – with their doctors.
At 4 years’ follow-up, 54 (74%) remained cancer free. Nineteen patients had local tumor recurrence, generally within 13 months. Two of those patients had successful local excisions, and the remaining 17 had salvage total mesorectal excisions (TME).
There were no statistically significant differences in 4-year disease-specific and overall survival among the 73 patients and 72 other patients who opted for TME after neoadjuvant chemotherapy and were found to have had pathologic complete responses.
“In our cohort, watch and wait was safe. It’s an effective treatment strategy achieving a high rate of rectal preservation in tumors that respond to neoadjuvant therapy. I don’t think the rectum needs to come out in everybody,” said investigator Dr. J. Joshua Smith, a surgical oncologist at Sloan Kettering.
Several studies have reported similar results similar to the Sloan Kettering study, but other investigations have been retrospective, so optimal patient selection, assessment of response, surveillance protocols, and other matters remain uncertain. Sloan Kettering and about 20 other cancer centers in United States – all members of the Rectal Cancer Consortium – recently launched a randomized clinical trial to get a better handle on those issues.
Locally advanced rectal cancer patients will be randomized to either chemoradiation for 5.5 weeks followed by folinic acid, fluorouracil, and oxaliplatin (FOLFOX) or capecitabine and oxaliplatin (CapeOX) over about 16 weeks, or FOLFOX/CapeOX first and chemoradiation second. Those who have a significant clinical response will then undergo watchful waiting; those who do not will have TME.
About 50 patients have enrolled in the phase II trial so far; the investigators are looking for more than 200.
“I think ‘prime time for watchful waiting’ is around the corner, but not yet here. It must be preceded by a prospective trial.” Meanwhile, “how we define complete clinical response is important” when considering watchful waiting, Dr. Smith said at the annual clinical congress of the American College of Surgeons..
At Sloan Kettering, where watchful waiting has become more popular in recent years, complete clinical response means no tumor or lymph nodes on imaging, and, on digital rectal exam (DRE) and proctoscopy, normal flat mucosa, smooth induration, no mass, no nodules, no ulcerations, and no luminal narrowing; a pale scar and telangiectasias are okay.
In the first year, surveillance includes DRE and endoscopy every 3 months and imaging every 6 months. In the second year, DRE and endoscopy come every 4 months, and imaging again every 6 months. From years 3 to 5, DRE and endoscopy are done every 6 months, and imaging every 6-12 months. After 5 years, surveillance is by yearly DRE and endoscopy.
When discussing the option with patients, they need to know – besides the risk of recurrence – that watchful waiting is currently not standard medical management; surveillance must be frequent; they are at risk for a more extensive salvage TME than they might have had otherwise; and the approach might compromise the chance of a cure, Dr. Smith said.
Dr. Smith said he has no relevant disclosures.
CHICAGO – Watchful waiting with careful surveillance may become an option for the majority of locally advanced rectal cancer patients who have a complete clinical response to neoadjuvant therapy, according to a review of 442 rectal cancer patients at Memorial Sloan Kettering Cancer Center in New York.
Seventy-three of those patients had a complete clinical response to neoadjuvant therapy and opted for watchful waiting instead of surgery after weighing the risks and benefits – including about a 25% chance of local recurrence – with their doctors.
At 4 years’ follow-up, 54 (74%) remained cancer free. Nineteen patients had local tumor recurrence, generally within 13 months. Two of those patients had successful local excisions, and the remaining 17 had salvage total mesorectal excisions (TME).
There were no statistically significant differences in 4-year disease-specific and overall survival among the 73 patients and 72 other patients who opted for TME after neoadjuvant chemotherapy and were found to have had pathologic complete responses.
“In our cohort, watch and wait was safe. It’s an effective treatment strategy achieving a high rate of rectal preservation in tumors that respond to neoadjuvant therapy. I don’t think the rectum needs to come out in everybody,” said investigator Dr. J. Joshua Smith, a surgical oncologist at Sloan Kettering.
Several studies have reported similar results similar to the Sloan Kettering study, but other investigations have been retrospective, so optimal patient selection, assessment of response, surveillance protocols, and other matters remain uncertain. Sloan Kettering and about 20 other cancer centers in United States – all members of the Rectal Cancer Consortium – recently launched a randomized clinical trial to get a better handle on those issues.
Locally advanced rectal cancer patients will be randomized to either chemoradiation for 5.5 weeks followed by folinic acid, fluorouracil, and oxaliplatin (FOLFOX) or capecitabine and oxaliplatin (CapeOX) over about 16 weeks, or FOLFOX/CapeOX first and chemoradiation second. Those who have a significant clinical response will then undergo watchful waiting; those who do not will have TME.
About 50 patients have enrolled in the phase II trial so far; the investigators are looking for more than 200.
“I think ‘prime time for watchful waiting’ is around the corner, but not yet here. It must be preceded by a prospective trial.” Meanwhile, “how we define complete clinical response is important” when considering watchful waiting, Dr. Smith said at the annual clinical congress of the American College of Surgeons..
At Sloan Kettering, where watchful waiting has become more popular in recent years, complete clinical response means no tumor or lymph nodes on imaging, and, on digital rectal exam (DRE) and proctoscopy, normal flat mucosa, smooth induration, no mass, no nodules, no ulcerations, and no luminal narrowing; a pale scar and telangiectasias are okay.
In the first year, surveillance includes DRE and endoscopy every 3 months and imaging every 6 months. In the second year, DRE and endoscopy come every 4 months, and imaging again every 6 months. From years 3 to 5, DRE and endoscopy are done every 6 months, and imaging every 6-12 months. After 5 years, surveillance is by yearly DRE and endoscopy.
When discussing the option with patients, they need to know – besides the risk of recurrence – that watchful waiting is currently not standard medical management; surveillance must be frequent; they are at risk for a more extensive salvage TME than they might have had otherwise; and the approach might compromise the chance of a cure, Dr. Smith said.
Dr. Smith said he has no relevant disclosures.
AT THE ACS CLINICAL CONGRESS
Key clinical point: Organ preservation seems to be a valid option when locally advanced rectal cancers respond completely to neoadjuvant therapy.
Major finding: Almost three-quarters of 73 patients who opted for watchful waiting after complete clinical responses to neodjuvant therapy remained cancer free at 4 years.
Data source: Review of 442 patients at Memorial Sloan Kettering Cancer Center.
Disclosures: The presenting investigator has no relevant financial disclosures.
VIDEO: How to handle pregnancy and breast feeding in rheumatoid arthritis
San Francisco – Should you give rheumatoid arthritis patients tumor necrosis factor blockers during pregnancy? What can you substitute for methotrexate?
And what in the world do you do about breast feeding?
“The management of rheumatoid arthritis in pregnancy seems to be evolving,” explained Dr. Megan Clowse, a specialist in rheumatology and pregnancy at Duke University, Durham, N.C. “The old strategy of just stopping all the medications and letting the woman flare and using some prednisone really doesn’t seem to actually be achieving the outcomes that we need to get.”
Dr. Clowse answered a host of pregnancy-related questions and more in a pearl-filled interview at the annual meeting of the American College of Rheumatology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
San Francisco – Should you give rheumatoid arthritis patients tumor necrosis factor blockers during pregnancy? What can you substitute for methotrexate?
And what in the world do you do about breast feeding?
“The management of rheumatoid arthritis in pregnancy seems to be evolving,” explained Dr. Megan Clowse, a specialist in rheumatology and pregnancy at Duke University, Durham, N.C. “The old strategy of just stopping all the medications and letting the woman flare and using some prednisone really doesn’t seem to actually be achieving the outcomes that we need to get.”
Dr. Clowse answered a host of pregnancy-related questions and more in a pearl-filled interview at the annual meeting of the American College of Rheumatology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
San Francisco – Should you give rheumatoid arthritis patients tumor necrosis factor blockers during pregnancy? What can you substitute for methotrexate?
And what in the world do you do about breast feeding?
“The management of rheumatoid arthritis in pregnancy seems to be evolving,” explained Dr. Megan Clowse, a specialist in rheumatology and pregnancy at Duke University, Durham, N.C. “The old strategy of just stopping all the medications and letting the woman flare and using some prednisone really doesn’t seem to actually be achieving the outcomes that we need to get.”
Dr. Clowse answered a host of pregnancy-related questions and more in a pearl-filled interview at the annual meeting of the American College of Rheumatology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE ACR ANNUAL MEETING