User login
Anticipating the care adolescents will need
Adolescents are an increasingly diverse population reflecting changes in the racial, ethnic, and geopolitical milieus of the United States. The World Health Organization classifies adolescence as ages 10 to 19 years.1 However, given the complexity of adolescent development physically, behaviorally, emotionally, and socially, others propose that adolescence may extend to age 24.2
Recognizing the specific challenges adolescents face is key to providing comprehensive longitudinal health care. Moreover, creating an environment of trust helps to ensure open 2-way communication that can facilitate anticipatory guidance.
Our review focuses on common adolescent issues, including injury from vehicles and firearms, tobacco and substance misuse, obesity, behavioral health, sexual health, and social media use. We discuss current trends and recommend strategies to maximize health and wellness.
Start by framing the visit
Confidentiality
Laws governing confidentiality in adolescent health care vary by state. Be aware of the laws pertaining to your practice setting. In addition, health care facilities may have their own policies regarding consent and confidentiality in adolescent care. Discuss confidentiality with both an adolescent and the parent/guardian at the initial visit. And, to help avoid potential misunderstandings, let them know in advance what will (and will not) be divulged.
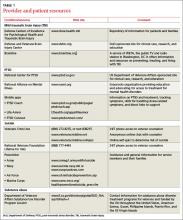
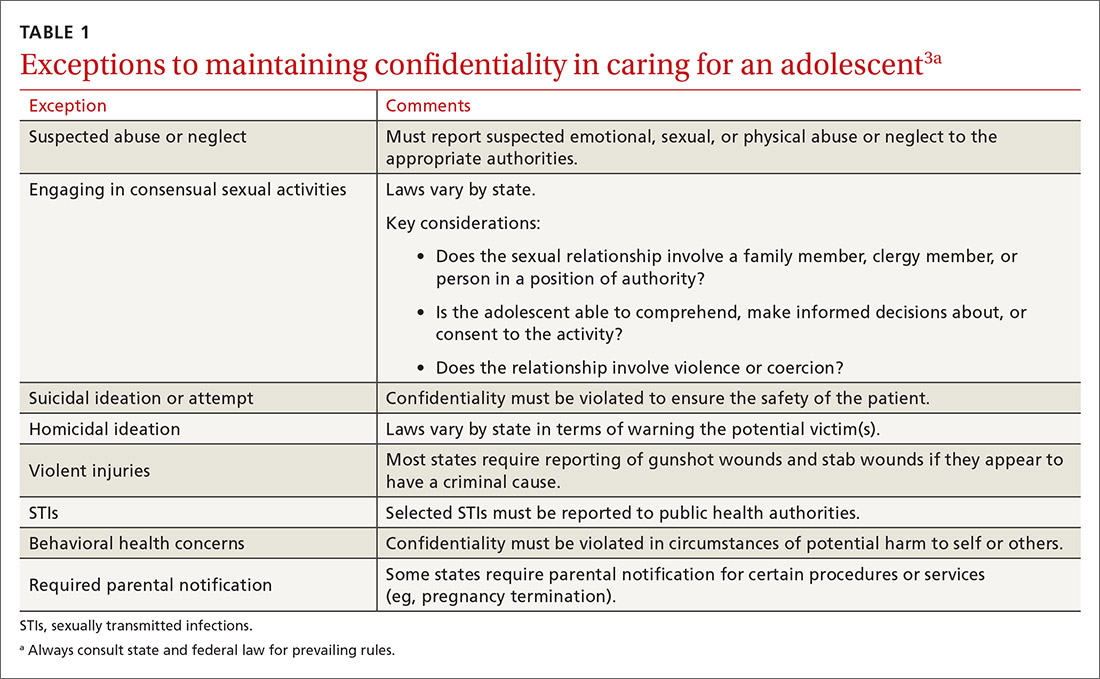
The American Academy of Pediatrics has developed a useful tip sheet regarding confidentiality laws (www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/healthy-foster-care-america/Documents/Confidentiality_Laws.pdf). Examples of required (conditional) disclosure include abuse and suicidal or homicidal ideations. Patients should understand that sexually transmitted infections (STIs) are reportable to public health authorities and that potentially injurious behaviors to self or others (eg, excessive drinking prior to driving) may also warrant disclosure(TABLE 13).
Privacy and general visit structure
Create a safe atmosphere where adolescents can discuss personal issues without fear of repercussion or judgment. While parents may prefer to be present during the visit, allowing for time to visit independently with an adolescent offers the opportunity to reinforce issues of privacy and confidentiality. Also discuss your office policies regarding electronic communication, phone communication, and relaying test results.

A useful paradigm for organizing a visit for routine adolescent care is to use an expanded version of the HEADSS mnemonic (TABLE 24,5), which includes questions about an adolescent’s Home, Education, Activities, Drug and alcohol use, Sexual behavior, Suicidality and depression, and other topics. Other validated screening tools include RAAPS (Rapid Adolescent Prevention Screening)6 (www.possibilitiesforchange.com/raaps/); the Guidelines for Adolescent Preventive Services7; and the Bright Futures recommendations for preventive care from the American Academy of Pediatrics.8 Below, we consider important topics addressed with the HEADSS approach.

Continue to: Injury from vehicles and firearms
Injury from vehicles and firearms
Motor vehicle accidents and firearm wounds are the 2 leading causes of adolescent injury. In 2016, of the more than 20,000 deaths in children and adolescents (ages 1-19 years), 20% were due to motor vehicle accidents (4074) and 15% were a result of firearm-related injuries (3143). Among firearm-related deaths, 60% were homicides, 35% were suicides, and 4% were due to accidental discharge.9 The rate of firearm-related deaths among American teens is 36 times greater than that of any other developed nation.9 Currently, 1 of every 3 US households with children younger than 18 has a firearm. Data suggest that in 43% of these households, the firearm is loaded and kept in an unlocked location.10
To aid anticipatory guidance, ask adolescents about firearm and seat belt use, drinking and driving, and suicidal thoughts (TABLE 24,5). Advise them to always wear seat belts whether driving or riding as a passenger. They should never drink and drive (or get in a car with someone who has been drinking). Advise parents that if firearms are present in the household, they should be kept in a secure, locked location. Weapons should be separated from ammunition and safety mechanisms should be engaged on all devices.
Tobacco and substance misuse
Tobacco use, the leading preventable cause of death in the United States,11 is responsible for more deaths than alcohol, motor vehicle accidents, suicides, homicides, and HIV disease combined.12 Most tobacco-associated mortality occurs in individuals who began smoking before the age of 18.12 Individuals who start smoking early are also more likely to continue smoking through adulthood.
Encouragingly, tobacco use has declined significantly among adolescents over the past several decades. Roughly 1 in 25 high school seniors reports daily tobacco use.13 Adolescent smoking behaviors are also changing dramatically with the increasing popularity of electronic cigarettes (“vaping”). Currently, more adolescents vape than smoke cigarettes.13 Vaping has additional health risks including toxic lung injury.
Multiple resources can help combat tobacco and nicotine use in adolescents. The US Preventive Services Task Force recommends that primary care clinicians intervene through education or brief counselling to prevent initiation of tobacco use in school-aged children and adolescents.14 Ask teens about tobacco and electronic cigarette use and encourage them to quit when use is acknowledged. Other helpful office-based tools are the “Quit Line” 800-QUIT-NOW and texting “Quit” to 47848. Smokefree teen (https://teen.smokefree.gov/) is a website that reviews the risks of tobacco and nicotine use and provides age-appropriate cessation tools and tips (including a smartphone app and a live-chat feature). Other useful information is available in a report from the Surgeon General on preventing tobacco use among young adults.15
Continue to: Alcohol use
Alcohol use. Three in 5 high school students report ever having used alcohol.13 As with tobacco, adolescent alcohol use has declined over the past decade. However, binge drinking (≥ 5 drinks on 1 occasion for males; ≥ 4 drinks on 1 occasion for females) remains a common high-risk behavior among adolescents (particularly college students). Based on the Monitoring the Future Survey, 1 in 6 high school seniors reported binge drinking in the past 2 weeks.13 While historically more common among males, rates of binge drinking are now basically similar between male and female adolescents.13
The National Institute on Alcohol Abuse and Alcoholism has a screening and intervention guide specifically for adolescents.16
Illicit drug use. Half of adolescents report using an illicit drug by their senior year in high school.13 Marijuana is the most commonly used substance, and laws governing its use are rapidly changing across the United States. Marijuana is illegal in 10 states and legal in 10 states (and the District of Columbia). The remaining states have varying policies on the medical use of marijuana and the decriminalization of marijuana. In addition, cannabinoid (CBD) products are increasingly available. Frequent cannabis use in adolescence has an adverse impact on general executive function (compared with adult users) and learning.17 Marijuana may serve as a gateway drug in the abuse of other substances,18 and its use should be strongly discouraged in adolescents.
Of note, there has been a sharp rise in the illicit use of prescription drugs, particularly opioids, creating a public health emergency across the United States.19 In 2015, more than 4000 young people, ages 15 to 24, died from a drug-related overdose (> 50% of these attributable to opioids).20 Adolescents with a history of substance abuse and behavioral illness are at particular risk. Many adolescents who misuse opioids and other prescription drugs obtain them from friends and relatives.21
The Substance Abuse and Mental Health Services Administration (SAMHSA) recommends universal screening of adolescents for substance abuse. This screening should be accompanied by a brief intervention to prevent, mitigate, or eliminate substance use, or a referral to appropriate treatment sources. This process of screening, brief intervention, and referral to treatment (SBIRT) is recommended as part of routine health care.22
Continue to: Obesity and physical activity
Obesity and physical activity
The percentage of overweight and obese adolescents in the United States has more than tripled over the past 40 years,23 and 1 in 5 US adolescents is obese.23 Obese teens are at higher risk for multiple chronic diseases, including type 2 diabetes, sleep apnea, and heart disease.24 They are also more likely to be bullied and to have poor self-esteem.25 Only 1 in 5 American high school students engages in 60 or more minutes of moderate-to-vigorous physical activity on 5 or more days per week.26
Regular physical activity is, of course, beneficial for cardiorespiratory fitness, bone health, weight control, and improved indices of behavioral health.26 Adolescents who are physically active consistently demonstrate better school attendance and grades.17 Higher levels of physical fitness are also associated with improved overall cognitive performance.24
General recommendations. The Department of Health and Human Services recommends that adolescents get at least 60 minutes of mostly moderate physical activity every day.26 Encourage adolescents to engage in vigorous physical activity (heavy breathing, sweating) at least 3 days a week. As part of their physical activity patterns, adolescents should also engage in muscle-strengthening and bone-strengthening activities on at least 3 days per week.
Behavioral health
As young people develop their sense of personal identity, they also strive for independence. It can be difficult, at times, to differentiate normal adolescent rebellion from true mental illness. An estimated 17% to 19% of adolescents meet criteria for mental illness, and about 7% have a severe psychiatric disorder.27 Only one-third of adolescents with mental illness receive any mental health services.28
Depression. The 1-year incidence of major depression in adolescents is 3% to 4%, and the lifetime prevalence of depressive symptoms is 25% in all high school students.27 Risk factors include ethnic minority status, poor self-esteem, poor health, recent personal crisis, insomnia, and alcohol/substance abuse. Depression in adolescent girls is correlated with becoming sexually active at a younger age, failure to use contraception, having an STI, and suicide attempts. Depressed boys are more likely to have unprotected intercourse and participate in physical fights.29 Depressed teens have a 2- to 3-fold greater risk for behavioral disorders, anxiety, and attention-deficit/hyperactivity disorder (ADHD).30
Continue to: Suicide
Suicide. Among individuals 15 to 29 years of age, suicide is the second leading cause of death globally, with an annual incidence of 11 to 15 per 100,000.31 Suicide attempts are 10 to 20 times more common than completed suicide.31 Males are more likely than females to die by suicide,32 and boys with a history of attempted suicide have a 30-fold increased risk of subsequent successful suicide.31 Hanging, drug poisoning, and firearms (particularly for males) are the most common means of suicide in adolescents. More than half of adolescents dying by suicide have coexisting depression.31
Characteristics associated with suicidal behaviors in adolescents include impulsivity, poor problem-solving skills, and dichotomous thinking.31 There may be a genetic component as well. In 1 of 5 teenage suicides, a precipitating life event such as the break-up of a relationship, cyber-bullying, or peer rejection is felt to contribute.31
ADHD. The prevalence of ADHD is 7% to 9% in US school-aged children.33 Boys more commonly exhibit hyperactive behaviors, while girls have more inattention. Hyperactivity often diminishes in teens, but inattention and impulsivity persist. Sequelae of ADHD include high-risk sexual behaviors, motor vehicle accidents, incarceration, and substance abuse.34 Poor self-esteem, suicidal ideation, smoking, and obesity are also increased.34 ADHD often persists into adulthood, with implications for social relationships and job performance.34
Eating disorders. The distribution of eating disorders is now known to increasingly include more minorities and males, the latter representing 5% to 10% of cases.35 Eating disorders show a strong genetic tendency and appear to be accelerated by puberty. The most common eating disorder (diagnosed in 0.8%-14% of teens) is eating disorder not otherwise specified (NOS).35 Anorexia nervosa is diagnosed in 0.5% of adolescent girls, and bulimia nervosa in 1% to 2%—particularly among athletes and performers.35 Unanticipated loss of weight, amenorrhea, excessive concern about weight, and deceleration in height/weight curves are potential indicators of an eating disorder. When identified, eating disorders are best managed by a trusted family physician, acting as a coordinator of a multidisciplinary team.
Sexual health
Girls begin to menstruate at an average age of 12, and it takes about 4 years for them to reach reproductive maturity.36 Puberty has been documented to start at younger ages over the past 30 years, likely due to an increase in average body mass index and a decrease in levels of physical activity.37 Girls with early maturation are often insecure and self-conscious, with higher levels of psychological distress.38 In boys, the average age for spermarche (first ejaculation) is 13.39 Boys who mature early tend to be taller, be more confident, and express a good body image.40 Those who have early puberty are more likely to be sexually active or participate in high-risk behaviors.41
Continue to: Pregnancy and contraception
Pregnancy and contraception
Over the past several decades, more US teens have been abstaining from sexual intercourse or have been using effective forms of birth control, particularly condoms and long-acting reversible contraceptives (LARCs).42 Teenage birth rates in girls ages 15 to 19 have declined significantly since the 1980s.42 Despite this, the teenage birth rate in the United States remains higher than in other industrialized nations, and most teen pregnancies are unintended.
There are numerous interventions to reduce teen pregnancy, including sex education, contraceptive counseling, the use of mobile apps that track a user’s monthly fertility cycle or issue reminders to take oral contraceptives,45 and the liberal distribution of contraceptives and condoms. The Contraceptive CHOICE Project shows that providing free (or low-cost) LARCs influences young women to choose these as their preferred contraceptive method.46 Other programs specifically empower girls to convince partners to use condoms and to resist unwanted sexual advances or intimate partner violence.
Adolescents prefer to have their health care providers address the topic of sexual health. Teens are more likely to share information with providers if asked directly about sexual behaviors.47TABLE 24,5 offers tips for anticipatory guidance and potential ways to frame questions with adolescents in this context. State laws vary with regard to the ability of minors to seek contraception, pregnancy testing, or care/screening for STIs without parental consent. Contraceptive counseling combined with effective screening decrease the incidence of STIs and pelvic inflammatory disease for sexually active teens.48

Sexually transmitted infections
Young adolescents often have a limited ability to imagine consequences related to specific actions. In general, there is also an increased desire to engage in experimental behaviors as an expression of developing autonomy, which may expose them to STIs. About half of all STIs contracted in the United States occur in individuals 15 to 24 years of age.49 Girls are at particular risk for the sequelae of these infections, including cervical dysplasia and infertility. Many teens erroneously believe that sexual activities other than intercourse decrease their risk of contracting an STI.50
Human papillomavirus (HPV) infection is the most common STI in adolescence.51 In most cases, HPV is transient and asymptomatic. Oncogenic strains may cause cervical cancer or cancers of the anogenital or oropharyngeal systems. Due to viral latency, it is not recommended to perform HPV typing in men or in women younger than 30 years of age; however, Pap tests are recommended every 3 years for women ages 21 to 29. Primary care providers are pivotal in the public health struggle to prevent HPV infection.
Continue to: Universal immunization of all children...
Universal immunization of all children older than 11 years of age against HPV is strongly advised as part of routine well-child care. Emphasize the proven role of HPV vaccination in preventing cervical52 and oropharyngeal53 cancers. And be prepared to address concerns raised by parents in the context of vaccine safety and the initiation of sexual behaviors (www.cdc.gov/hpv/hcp/answering-questions.html).
Chlamydia is the second most common STI in the United States, usually occurring in individuals younger than 24.54 The CDC estimates that more than 3 million new chlamydial infections occur yearly. These infections are often asymptomatic, particularly in females, but may cause urethritis, cervicitis, epididymitis, proctitis, or pelvic inflammatory disease. Indolent chlamydial infection is the leading cause of tubal infertility in women.54 Routine annual screening for chlamydia is recommended for all sexually active females ≤ 25 years (and for older women with specific risks).55 Annual screening is also recommended for men who have sex with men (MSM).55
Chlamydial infection may be diagnosed with first-catch urine sampling (men or women), urethral swab (men), endocervical swab (women), or self-collected vaginal swab. Nucleic acid amplification testing is the most sensitive test that is widely available.56 First-line treatment includes either azithromycin (1 g orally, single dose) or doxycycline (100 mg orally, twice daily for 7 days).56
Gonorrhea. In 2018, there were more than 500,000 annual cases of gonorrhea, with the majority occurring in those between 15 and 24 years of age.57 Gonorrhea may increase rates of HIV infection transmission up to 5-fold.57 As more adolescents practice oral sex, cases of pharyngeal gonorrhea (and oropharyngeal HPV) have increased. Symptoms of urethritis occur more frequently in men. Screening is recommended for all sexually active women younger than 25.56 Importantly, the organism Neisseria gonorrhoeae has developed significant antibiotic resistance over the past decade. The CDC currently recommends dual therapy for the treatment of gonorrhea using 250 mg of intramuscular ceftriaxone and 1 g of oral azithromycin.56
Syphilis. Rates of syphilis are increasing among individuals ages 15 to 24.51 Screening is particularly recommended for MSM and individuals infected with HIV. Benzathine penicillin G, 50,000 U/kg IM, remains the treatment of choice.56
Continue to: HIV
HIV. Globally, HIV impacts young people disproportionately. HIV infection also facilitates infection with other STIs. In the United States, the highest burden of HIV infection is borne by young MSM, with prevalence among those 18 to 24 years old varying between 26% to 30% (black) and 3% to 5.5% (non-Hispanic white).51 The use of emtricitabine/tenofovir disoproxil fumarate for pre-exposure prophylaxis (PrEP) has recently been approved for the prevention of HIV. PrEP reduces risk by up to 92% for MSM and transgender women.58
Sexual identity
One in 10 high school students self-identifies as “nonheterosexual,” and 1 in 15 reports same-sex sexual contact.59 The term LGBTQ+ includes the communities of lesbian, gay, bisexual, transgender, transsexual, queer, questioning, intersex, and asexual individuals. Developing a safe sense of sexual identity is fundamental to adolescent psychological development, and many adolescents struggle to develop a positive sexual identity. Suicide rates and self-harm behaviors among LGBTQ+ adolescents can be 4 times higher than among their heterosexual peers.60 Rates of mood disorders, substance abuse, and high-risk sexual behaviors are also increased in the LGBTQ+ population.61
The LGBTQ+ community often seeks health care advice and affirmation from primary care providers. Resources to enhance this care are available at www.lgbthealtheducation.org.
Social media
Adolescents today have more media exposure than any prior generation, with smartphone and computer use increasing exponentially. Most (95%) teens have access to a smartphone,62 45% describe themselves as constantly connected to the Internet, and 14% feel that social media is “addictive.”62 Most manage their social media portfolio on multiple sites. Patterns of adolescents' online activities show that boys prefer online gaming, while girls tend to spend more time on social networking.62
Whether extensive media use is psychologically beneficial or deleterious has been widely debated. Increased time online correlates with decreased levels of physical activity.63 And sleep disturbances have been associated with excessive screen time and the presence of mobile devices in the bedroom.64 The use of social media prior to bedtime also has an adverse impact on academic performance—particularly for girls. This adverse impact on academics persists after correcting for daytime sleepiness, body mass index, and number of hours spent on homework.64
Continue to: Due to growing concerns...
Due to growing concerns about the risks of social media in children and adolescents, the American Academy of Pediatrics has developed the Family Media Plan (www.healthychildren.org/English/media/Pages/default.aspx). Some specific questions that providers may ask are outlined in TABLE 3.64 The Family Media Plan can provide age-specific guidelines to assist parents or caregivers in answering these questions.
Cyber-bullying. One in 3 adolescents (primarily female) has been a victim of cyber-bullying.65 Sadly, 1 in 5 teens has received some form of electronic sexual solicitation.66 The likelihood of unsolicited stranger contact correlates with teens’ online habits and the amount of information disclosed. Predictors include female sex, visiting chat rooms, posting photos, and disclosing personal information. Restricting computer use to an area with parental supervision or installing monitoring programs does not seem to exert any protective influence on cyber-bullying or unsolicited stranger contact.65 While 63% of cyber-bullying victims feel upset, embarrassed, or stressed by these contacts,66 few events are actually reported. To address this, some states have adopted laws adding cyber-bullying to school disciplinary codes.
Negative health impacts associated with cyber-bullying include anxiety, sadness, and greater difficulty in concentrating on school work.65 Victims of bullying are more likely to have school disciplinary actions and depression and to be truant or to carry weapons to school.66 Cyber-bullying is uniquely destructive due to its ubiquitous presence. A sense of relative anonymity online may encourage perpetrators to act more cruelly, with less concern for punishment.
Young people are also more likely to share passwords as a sign of friendship. This may result in others assuming their identity online. Adolescents rarely disclose bullying to parents or other adults, fearing restriction of Internet access, and many of them think that adults may downplay the seriousness of the events.66
CORRESPONDENCE
Mark B. Stephens, MD, Penn State Health Medical Group, 1850 East Park Avenue, State College, PA 16803; [email protected].
1. World Health Organization. Adolescent health. Accessed February 23, 2021. www.who.int/maternal_child_adolescent/adolescence/en/
2. Sawyer SM, Azzopardi PS, Wickremarathne D, et al. The age of adolescence. Lancet Child Adolesc Health. 2018;2:223-228.
3. Pathak PR, Chou A. Confidential care for adoloscents in the U.S. healthcare system. J Patient Cent Res Rev. 2019;6:46-50.
4. AMA Journal of Ethics. HEADSS: the “review of systems” for adolescents. Accessed February 23, 2021. https://journalofethics.ama-assn.org/article/headss-review-systems-adolescents/2005-03
5. Cohen E, MacKenzie RG, Yates GL. HEADSS, a psychosocial risk assessment instrument: implications for designing effective intervention programs for runaway youth. J Adolesc Health. 1991;12:539-544.
6. Possibilities for Change. Rapid Adolescent Prevention Screening (RAAPS). Accessed February 23, 2021. www.possibilitiesforchange.com/raaps/
7. Elster AB, Kuznets NJ. AMA Guidelines for Adolescent Preventive Services (GAPS): Recommendations and Rationale. Williams & Wilkins; 1994.
8. AAP. Engaging patients and families - periodicity schedule. Accessed February 23, 2021. www.aap.org/en-us/professional-resources/practice-support/Pages/PeriodicitySchedule.aspx
9. Cunningham RM, Walton MA, Carter PM. The major causes of death in children and adolescents in the United States. N Eng J Med. 2018;379:2468-2475.
10. Schuster MA, Franke TM, Bastian AM, et al. Firearm storage patterns in US homes with children. Am J Public Health. 2000;90:588-594.
11. Mokdad AH, Marks JS, Stroup DF, et al. Actual causes of death in the United States. JAMA. 2004;291:1238-1245.
12. HHS. Health consequences of smoking, surgeon general fact sheet. Accessed February 23, 2021. www.hhs.gov/surgeongeneral/reports-and-publications/tobacco/consequences-smoking-factsheet/index.html
13. Johnston LD, Miech RA, O’Malley PM, et al. Monitoring the future: national survey results on drug use, 1975-2017. The University of Michigan. 2018. Accessed February 23, 2021. https://eric.ed.gov/?id=ED589762
14. US Preventive Services Task Force. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. Accessed February 23, 2021. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
15. HHS. Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta, GA: HHS, CDC, NCCDPHP, OSH; 2012. Accessed February 23, 2021. www.ncbi.nlm.nih.gov/books/NBK99237/
16. NIH. Alcohol screening and brief intervention for youth: a pocket guide. Accessed February 23, 2021. https://pubs.niaaa.nih.gov/publications/Practitioner/YouthGuide/YouthGuidePocket.pdf
17. Gorey C, Kuhns L, Smaragdi E, et al. Age-related differences in the impact of cannabis use on the brain and cognition: a systematic review. Eur Arch Psychiatry Clin Neurosci. 2019;269:37-58.
18. Secades-Villa R, Garcia-Rodriguez O, Jin CJ, et al. Probability and predictors of the cannabis gateway effect: a national study. Int J Drug Policy. 2015;26:135-142.
19. Kann L, McManus T, Harris WA, et al. Youth risk behavior surveillance—United States, 2017. MMWR Surveill Summ. 2018;67:1-114.
20. NIH. Drug overdoses in youth. How do drug overdoses happen?. Accessed February 23, 2021. https://teens.drugabuse.gov/drug-facts/drug-overdoses-youth
21. Branstetter SA, Low S, Furman W. The influence of parents and friends on adolescent substance use: a multidimensional approach. J Subst Use. 2011;162:150-160.
22. AAP. Committee on Substance Use and Prevention. Substance use screening, brief intervention, and referral to treatment. Pediatrics. 2016;138:e20161210.
23. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief. 2017;288:1-8.
24. Halfon N, Larson K, Slusser W. Associations between obesity and comorbid mental health, developmental and physical health conditions in a nationally representative sample of US children aged 10 to 17. Acad Pediatr. 2013;13:6-13.
25. Griffiths LJ, Parsons TJ, Hill AJ. Self-esteem and quality of life in obese children and adolescents: a systematic review. Int J Pediatr Obes. 2010;5:282-304.
26. National Physical Activity Plan Alliance. The 2018 United States report card on physical activity for children and youth. Accessed February 23, 2021. http://physicalactivityplan.org/projects/PA/2018/2018%20US%20Report%20Card%20Full%20Version_WEB.PDF?pdf=page-link
27. HHS. NIMH. Child and adolescent mental health. Accessed February 23, 2021. www.nimh.nih.gov/health/topics/child-and-adolescent-mental-health/index.shtml
28. Yonek JC, Jordan N, Dunlop D, et al. Patient-centered medical home care for adolescents in need of mental health treatment. J Adolesc Health. 2018;63:172-180.
29. Brooks TL, Harris SK, Thrall JS, et al. Association of adolescent risk behaviors with mental health symptoms in high school students. |J Adolesc Health. 2002;31:240-246.
30. Weller BE, Blanford KL, Butler AM. Estimated prevalence of psychiatric comorbidities in US adolescents with depression by race/ethnicity, 2011-2012. J Adolesc Health. 2018;62:716-721.
31. Bilsen J. Suicide and youth: risk factors. Front Psychiatry. 2018;9:540.
32. Shain B, AAP Committee on Adolescence. Suicide and suicide attempts in adolescents. Pediatrics. 2016;138:e20161420.
33. Brahmbhatt K, Hilty DM, Hah M, et al. Diagnosis and treatment of attention deficit hyperactivity disorder during adolescence in the primary care setting: review and future directions. J Adolesc Health. 2016;59:135-143.
34. Bravender T. Attention-deficit/hyperactivity disorder and disordered eating. [editorial] J Adolesc Health. 2017;61:125-126.
35. Rosen DS, AAP Committee on Adolescence. Identification and management of eating disorders in children and adolescents. Pediatrics. 2010;126:1240-1253.
36. Susman EJ, Houts RM, Steinberg L, et al. Longitudinal development of secondary sexual characteristics in girls and boys between ages 9 ½ and 15 ½ years. Arch Pediatr Adolesc Med. 2010;164:166-173.
37. Kaplowitz PB. Link between body fat and the timing of puberty. Pediatrics. 2008;121(suppl 3):S208-S217.
38. Ge X, Conger RD, Elder GH. Coming of age too early: pubertal influences on girl’s vulnerability to psychologic distress. Child Dev. 1996;67:3386-3400.
39. Jørgensen M, Keiding N, Skakkebaek NE. Estimation of spermarche from longitudinal spermaturia data. Biometrics. 1991;47:177-193.
40. Kar SK, Choudhury A, Singh AP. Understanding normal development of adolescent sexuality: a bumpy ride. J Hum Reprod Sci. 2015;8:70-74.
41. Susman EJ, Dorn LD, Schiefelbein VL. Puberty, sexuality and health. In: Lerner MA, Easterbrooks MA, Mistry J (eds). Comprehensive Handbook of Psychology. Wiley; 2003.
42. Lindberg LD, Santelli JS, Desai S. Changing patterns of contraceptive use and the decline in rates of pregnancy and birth among U.S. adolescents, 2007-2014. J Adolesc Health. 2018;63:253-256.
43. Guttmacher Institute. Teen pregnancy. www.guttmacher.org/united-states/teens/teen-pregnancy. Accessed February 23, 2021.
44. CDC. Social determinants and eliminating disparities in teen pregnancy. Accessed February 23, 2021. www.cdc.gov/teenpregnancy/about/social-determinants-disparities-teen-pregnancy.htm
45. Widman L, Nesi J, Kamke K, et al. Technology-based interventions to reduce sexually transmitted infection and unintended pregnancy among youth. J Adolesc Health. 2018;62:651-660.
46. Secura GM, Allsworth JE, Madden T, et al. The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol. 2010;203:115.e1-115.e7.
47. Ham P, Allen C. Adolescent health screening and counseling. Am Fam Physician. 2012;86:1109-1116.
48. ACOG. Committee on Adolescent Health Care. Adolescent pregnancy, contraception and sexual activity. 2017. Accessed February 23, 2021. www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2017/05/adolescent-pregnancy-contraception-and-sexual-activity
49. Wangu Z, Burstein GR. Adolescent sexuality: updates to the sexually transmitted infection guidelines. Pediatr Clin N Am. 2017;64:389-411.
50. Holway GV, Hernandez SM. Oral sex and condom use in a U.S. national sample of adolescents and young adults. J Adolesc Health. 2018;62:402-410.
51. CDC. STDs in adults and adolescents. Accessed February 23, 2021. www.cdc.gov/std/stats17/adolescents.htm
52. McClung N, Gargano J, Bennett N, et al. Trends in human papillomavirus vaccine types 16 and 18 in cervical precancers, 2008-2014. Accessed February 23, 2021. https://cebp.aacrjournals.org/content/28/3/602
53. Timbang MR, Sim MW, Bewley AF, et al. HPV-related oropharyngeal cancer: a review on burden of the disease and opportunities for prevention and early detection. Hum Vaccin Immunother. 2019;15:1920-1928.
54. Carey AJ, Beagley KW. Chlamydia trachomatis, a hidden epidemic: effects on female reproduction and options for treatment. Am J Reprod Immunol. 2010;63:576-586.
55. USPSTF. Chlamydia and gonorrhea screening. Accessed February 23, 2021. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/chlamydia-and-gonorrhea-screening
56. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:1-135.
57. CDC. Sexually transmitted disease surveillance 2018. Accessed February 23, 2021. www.cdc.gov/std/stats18/gonorrhea.htm
5
59. Kann L, McManus T, Harris WA, et al. Youth risk behavior surveillance–United States, 2015. MMWR Surveill Summ. 2016;65:1-174.
60. CDC. LGBT youth. Accessed February 23, 2021. www.cdc.gov/lgbthealth/youth.htm
61. Johns MM, Lowry R, Rasberry CN, et al. Violence victimization, substance use, and suicide risk among sexual minority high school students – United States, 2015-2017. MMWR Morb Mortal Wkly Rep. 2018;67:1211-1215.
62. Pew Research Center. Teens, social media & technology 2018. . Accessed February 23, 2021. www.pewinternet.org/2018/05/31/teens-social-media-technology-2018/
63. Chassiakos YLR, Radesky J, Christakis D, et al. Children and adolescents and digital media. Pediatrics. 2016;138:e20162593.
64. Arora T, Albahri A, Omar OM, et al. The prospective association between electronic device use before bedtime and academic attainment in adolescents. J Adolesc Health. 2018;63:451-458.
65. Mishna F, Saini M, Solomon S. Ongoing and online: children and youth’s perceptions of cyber bullying. Child Youth Serv Rev. 2009;31:1222-1228.
66. Sengupta A, Chaudhuri A. Are social networking sites a source of online harassment for teens? Evidence from survey data. Child Youth Serv Rev. 2011;33:284-290.
Adolescents are an increasingly diverse population reflecting changes in the racial, ethnic, and geopolitical milieus of the United States. The World Health Organization classifies adolescence as ages 10 to 19 years.1 However, given the complexity of adolescent development physically, behaviorally, emotionally, and socially, others propose that adolescence may extend to age 24.2
Recognizing the specific challenges adolescents face is key to providing comprehensive longitudinal health care. Moreover, creating an environment of trust helps to ensure open 2-way communication that can facilitate anticipatory guidance.
Our review focuses on common adolescent issues, including injury from vehicles and firearms, tobacco and substance misuse, obesity, behavioral health, sexual health, and social media use. We discuss current trends and recommend strategies to maximize health and wellness.
Start by framing the visit
Confidentiality
Laws governing confidentiality in adolescent health care vary by state. Be aware of the laws pertaining to your practice setting. In addition, health care facilities may have their own policies regarding consent and confidentiality in adolescent care. Discuss confidentiality with both an adolescent and the parent/guardian at the initial visit. And, to help avoid potential misunderstandings, let them know in advance what will (and will not) be divulged.
The American Academy of Pediatrics has developed a useful tip sheet regarding confidentiality laws (www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/healthy-foster-care-america/Documents/Confidentiality_Laws.pdf). Examples of required (conditional) disclosure include abuse and suicidal or homicidal ideations. Patients should understand that sexually transmitted infections (STIs) are reportable to public health authorities and that potentially injurious behaviors to self or others (eg, excessive drinking prior to driving) may also warrant disclosure(TABLE 13).

Privacy and general visit structure
Create a safe atmosphere where adolescents can discuss personal issues without fear of repercussion or judgment. While parents may prefer to be present during the visit, allowing for time to visit independently with an adolescent offers the opportunity to reinforce issues of privacy and confidentiality. Also discuss your office policies regarding electronic communication, phone communication, and relaying test results.

A useful paradigm for organizing a visit for routine adolescent care is to use an expanded version of the HEADSS mnemonic (TABLE 24,5), which includes questions about an adolescent’s Home, Education, Activities, Drug and alcohol use, Sexual behavior, Suicidality and depression, and other topics. Other validated screening tools include RAAPS (Rapid Adolescent Prevention Screening)6 (www.possibilitiesforchange.com/raaps/); the Guidelines for Adolescent Preventive Services7; and the Bright Futures recommendations for preventive care from the American Academy of Pediatrics.8 Below, we consider important topics addressed with the HEADSS approach.

Continue to: Injury from vehicles and firearms
Injury from vehicles and firearms
Motor vehicle accidents and firearm wounds are the 2 leading causes of adolescent injury. In 2016, of the more than 20,000 deaths in children and adolescents (ages 1-19 years), 20% were due to motor vehicle accidents (4074) and 15% were a result of firearm-related injuries (3143). Among firearm-related deaths, 60% were homicides, 35% were suicides, and 4% were due to accidental discharge.9 The rate of firearm-related deaths among American teens is 36 times greater than that of any other developed nation.9 Currently, 1 of every 3 US households with children younger than 18 has a firearm. Data suggest that in 43% of these households, the firearm is loaded and kept in an unlocked location.10
To aid anticipatory guidance, ask adolescents about firearm and seat belt use, drinking and driving, and suicidal thoughts (TABLE 24,5). Advise them to always wear seat belts whether driving or riding as a passenger. They should never drink and drive (or get in a car with someone who has been drinking). Advise parents that if firearms are present in the household, they should be kept in a secure, locked location. Weapons should be separated from ammunition and safety mechanisms should be engaged on all devices.
Tobacco and substance misuse
Tobacco use, the leading preventable cause of death in the United States,11 is responsible for more deaths than alcohol, motor vehicle accidents, suicides, homicides, and HIV disease combined.12 Most tobacco-associated mortality occurs in individuals who began smoking before the age of 18.12 Individuals who start smoking early are also more likely to continue smoking through adulthood.
Encouragingly, tobacco use has declined significantly among adolescents over the past several decades. Roughly 1 in 25 high school seniors reports daily tobacco use.13 Adolescent smoking behaviors are also changing dramatically with the increasing popularity of electronic cigarettes (“vaping”). Currently, more adolescents vape than smoke cigarettes.13 Vaping has additional health risks including toxic lung injury.
Multiple resources can help combat tobacco and nicotine use in adolescents. The US Preventive Services Task Force recommends that primary care clinicians intervene through education or brief counselling to prevent initiation of tobacco use in school-aged children and adolescents.14 Ask teens about tobacco and electronic cigarette use and encourage them to quit when use is acknowledged. Other helpful office-based tools are the “Quit Line” 800-QUIT-NOW and texting “Quit” to 47848. Smokefree teen (https://teen.smokefree.gov/) is a website that reviews the risks of tobacco and nicotine use and provides age-appropriate cessation tools and tips (including a smartphone app and a live-chat feature). Other useful information is available in a report from the Surgeon General on preventing tobacco use among young adults.15
Continue to: Alcohol use
Alcohol use. Three in 5 high school students report ever having used alcohol.13 As with tobacco, adolescent alcohol use has declined over the past decade. However, binge drinking (≥ 5 drinks on 1 occasion for males; ≥ 4 drinks on 1 occasion for females) remains a common high-risk behavior among adolescents (particularly college students). Based on the Monitoring the Future Survey, 1 in 6 high school seniors reported binge drinking in the past 2 weeks.13 While historically more common among males, rates of binge drinking are now basically similar between male and female adolescents.13
The National Institute on Alcohol Abuse and Alcoholism has a screening and intervention guide specifically for adolescents.16
Illicit drug use. Half of adolescents report using an illicit drug by their senior year in high school.13 Marijuana is the most commonly used substance, and laws governing its use are rapidly changing across the United States. Marijuana is illegal in 10 states and legal in 10 states (and the District of Columbia). The remaining states have varying policies on the medical use of marijuana and the decriminalization of marijuana. In addition, cannabinoid (CBD) products are increasingly available. Frequent cannabis use in adolescence has an adverse impact on general executive function (compared with adult users) and learning.17 Marijuana may serve as a gateway drug in the abuse of other substances,18 and its use should be strongly discouraged in adolescents.
Of note, there has been a sharp rise in the illicit use of prescription drugs, particularly opioids, creating a public health emergency across the United States.19 In 2015, more than 4000 young people, ages 15 to 24, died from a drug-related overdose (> 50% of these attributable to opioids).20 Adolescents with a history of substance abuse and behavioral illness are at particular risk. Many adolescents who misuse opioids and other prescription drugs obtain them from friends and relatives.21
The Substance Abuse and Mental Health Services Administration (SAMHSA) recommends universal screening of adolescents for substance abuse. This screening should be accompanied by a brief intervention to prevent, mitigate, or eliminate substance use, or a referral to appropriate treatment sources. This process of screening, brief intervention, and referral to treatment (SBIRT) is recommended as part of routine health care.22
Continue to: Obesity and physical activity
Obesity and physical activity
The percentage of overweight and obese adolescents in the United States has more than tripled over the past 40 years,23 and 1 in 5 US adolescents is obese.23 Obese teens are at higher risk for multiple chronic diseases, including type 2 diabetes, sleep apnea, and heart disease.24 They are also more likely to be bullied and to have poor self-esteem.25 Only 1 in 5 American high school students engages in 60 or more minutes of moderate-to-vigorous physical activity on 5 or more days per week.26
Regular physical activity is, of course, beneficial for cardiorespiratory fitness, bone health, weight control, and improved indices of behavioral health.26 Adolescents who are physically active consistently demonstrate better school attendance and grades.17 Higher levels of physical fitness are also associated with improved overall cognitive performance.24
General recommendations. The Department of Health and Human Services recommends that adolescents get at least 60 minutes of mostly moderate physical activity every day.26 Encourage adolescents to engage in vigorous physical activity (heavy breathing, sweating) at least 3 days a week. As part of their physical activity patterns, adolescents should also engage in muscle-strengthening and bone-strengthening activities on at least 3 days per week.
Behavioral health
As young people develop their sense of personal identity, they also strive for independence. It can be difficult, at times, to differentiate normal adolescent rebellion from true mental illness. An estimated 17% to 19% of adolescents meet criteria for mental illness, and about 7% have a severe psychiatric disorder.27 Only one-third of adolescents with mental illness receive any mental health services.28
Depression. The 1-year incidence of major depression in adolescents is 3% to 4%, and the lifetime prevalence of depressive symptoms is 25% in all high school students.27 Risk factors include ethnic minority status, poor self-esteem, poor health, recent personal crisis, insomnia, and alcohol/substance abuse. Depression in adolescent girls is correlated with becoming sexually active at a younger age, failure to use contraception, having an STI, and suicide attempts. Depressed boys are more likely to have unprotected intercourse and participate in physical fights.29 Depressed teens have a 2- to 3-fold greater risk for behavioral disorders, anxiety, and attention-deficit/hyperactivity disorder (ADHD).30
Continue to: Suicide
Suicide. Among individuals 15 to 29 years of age, suicide is the second leading cause of death globally, with an annual incidence of 11 to 15 per 100,000.31 Suicide attempts are 10 to 20 times more common than completed suicide.31 Males are more likely than females to die by suicide,32 and boys with a history of attempted suicide have a 30-fold increased risk of subsequent successful suicide.31 Hanging, drug poisoning, and firearms (particularly for males) are the most common means of suicide in adolescents. More than half of adolescents dying by suicide have coexisting depression.31
Characteristics associated with suicidal behaviors in adolescents include impulsivity, poor problem-solving skills, and dichotomous thinking.31 There may be a genetic component as well. In 1 of 5 teenage suicides, a precipitating life event such as the break-up of a relationship, cyber-bullying, or peer rejection is felt to contribute.31
ADHD. The prevalence of ADHD is 7% to 9% in US school-aged children.33 Boys more commonly exhibit hyperactive behaviors, while girls have more inattention. Hyperactivity often diminishes in teens, but inattention and impulsivity persist. Sequelae of ADHD include high-risk sexual behaviors, motor vehicle accidents, incarceration, and substance abuse.34 Poor self-esteem, suicidal ideation, smoking, and obesity are also increased.34 ADHD often persists into adulthood, with implications for social relationships and job performance.34
Eating disorders. The distribution of eating disorders is now known to increasingly include more minorities and males, the latter representing 5% to 10% of cases.35 Eating disorders show a strong genetic tendency and appear to be accelerated by puberty. The most common eating disorder (diagnosed in 0.8%-14% of teens) is eating disorder not otherwise specified (NOS).35 Anorexia nervosa is diagnosed in 0.5% of adolescent girls, and bulimia nervosa in 1% to 2%—particularly among athletes and performers.35 Unanticipated loss of weight, amenorrhea, excessive concern about weight, and deceleration in height/weight curves are potential indicators of an eating disorder. When identified, eating disorders are best managed by a trusted family physician, acting as a coordinator of a multidisciplinary team.
Sexual health
Girls begin to menstruate at an average age of 12, and it takes about 4 years for them to reach reproductive maturity.36 Puberty has been documented to start at younger ages over the past 30 years, likely due to an increase in average body mass index and a decrease in levels of physical activity.37 Girls with early maturation are often insecure and self-conscious, with higher levels of psychological distress.38 In boys, the average age for spermarche (first ejaculation) is 13.39 Boys who mature early tend to be taller, be more confident, and express a good body image.40 Those who have early puberty are more likely to be sexually active or participate in high-risk behaviors.41
Continue to: Pregnancy and contraception
Pregnancy and contraception
Over the past several decades, more US teens have been abstaining from sexual intercourse or have been using effective forms of birth control, particularly condoms and long-acting reversible contraceptives (LARCs).42 Teenage birth rates in girls ages 15 to 19 have declined significantly since the 1980s.42 Despite this, the teenage birth rate in the United States remains higher than in other industrialized nations, and most teen pregnancies are unintended.
There are numerous interventions to reduce teen pregnancy, including sex education, contraceptive counseling, the use of mobile apps that track a user’s monthly fertility cycle or issue reminders to take oral contraceptives,45 and the liberal distribution of contraceptives and condoms. The Contraceptive CHOICE Project shows that providing free (or low-cost) LARCs influences young women to choose these as their preferred contraceptive method.46 Other programs specifically empower girls to convince partners to use condoms and to resist unwanted sexual advances or intimate partner violence.
Adolescents prefer to have their health care providers address the topic of sexual health. Teens are more likely to share information with providers if asked directly about sexual behaviors.47TABLE 24,5 offers tips for anticipatory guidance and potential ways to frame questions with adolescents in this context. State laws vary with regard to the ability of minors to seek contraception, pregnancy testing, or care/screening for STIs without parental consent. Contraceptive counseling combined with effective screening decrease the incidence of STIs and pelvic inflammatory disease for sexually active teens.48

Sexually transmitted infections
Young adolescents often have a limited ability to imagine consequences related to specific actions. In general, there is also an increased desire to engage in experimental behaviors as an expression of developing autonomy, which may expose them to STIs. About half of all STIs contracted in the United States occur in individuals 15 to 24 years of age.49 Girls are at particular risk for the sequelae of these infections, including cervical dysplasia and infertility. Many teens erroneously believe that sexual activities other than intercourse decrease their risk of contracting an STI.50
Human papillomavirus (HPV) infection is the most common STI in adolescence.51 In most cases, HPV is transient and asymptomatic. Oncogenic strains may cause cervical cancer or cancers of the anogenital or oropharyngeal systems. Due to viral latency, it is not recommended to perform HPV typing in men or in women younger than 30 years of age; however, Pap tests are recommended every 3 years for women ages 21 to 29. Primary care providers are pivotal in the public health struggle to prevent HPV infection.
Continue to: Universal immunization of all children...
Universal immunization of all children older than 11 years of age against HPV is strongly advised as part of routine well-child care. Emphasize the proven role of HPV vaccination in preventing cervical52 and oropharyngeal53 cancers. And be prepared to address concerns raised by parents in the context of vaccine safety and the initiation of sexual behaviors (www.cdc.gov/hpv/hcp/answering-questions.html).
Chlamydia is the second most common STI in the United States, usually occurring in individuals younger than 24.54 The CDC estimates that more than 3 million new chlamydial infections occur yearly. These infections are often asymptomatic, particularly in females, but may cause urethritis, cervicitis, epididymitis, proctitis, or pelvic inflammatory disease. Indolent chlamydial infection is the leading cause of tubal infertility in women.54 Routine annual screening for chlamydia is recommended for all sexually active females ≤ 25 years (and for older women with specific risks).55 Annual screening is also recommended for men who have sex with men (MSM).55
Chlamydial infection may be diagnosed with first-catch urine sampling (men or women), urethral swab (men), endocervical swab (women), or self-collected vaginal swab. Nucleic acid amplification testing is the most sensitive test that is widely available.56 First-line treatment includes either azithromycin (1 g orally, single dose) or doxycycline (100 mg orally, twice daily for 7 days).56
Gonorrhea. In 2018, there were more than 500,000 annual cases of gonorrhea, with the majority occurring in those between 15 and 24 years of age.57 Gonorrhea may increase rates of HIV infection transmission up to 5-fold.57 As more adolescents practice oral sex, cases of pharyngeal gonorrhea (and oropharyngeal HPV) have increased. Symptoms of urethritis occur more frequently in men. Screening is recommended for all sexually active women younger than 25.56 Importantly, the organism Neisseria gonorrhoeae has developed significant antibiotic resistance over the past decade. The CDC currently recommends dual therapy for the treatment of gonorrhea using 250 mg of intramuscular ceftriaxone and 1 g of oral azithromycin.56
Syphilis. Rates of syphilis are increasing among individuals ages 15 to 24.51 Screening is particularly recommended for MSM and individuals infected with HIV. Benzathine penicillin G, 50,000 U/kg IM, remains the treatment of choice.56
Continue to: HIV
HIV. Globally, HIV impacts young people disproportionately. HIV infection also facilitates infection with other STIs. In the United States, the highest burden of HIV infection is borne by young MSM, with prevalence among those 18 to 24 years old varying between 26% to 30% (black) and 3% to 5.5% (non-Hispanic white).51 The use of emtricitabine/tenofovir disoproxil fumarate for pre-exposure prophylaxis (PrEP) has recently been approved for the prevention of HIV. PrEP reduces risk by up to 92% for MSM and transgender women.58
Sexual identity
One in 10 high school students self-identifies as “nonheterosexual,” and 1 in 15 reports same-sex sexual contact.59 The term LGBTQ+ includes the communities of lesbian, gay, bisexual, transgender, transsexual, queer, questioning, intersex, and asexual individuals. Developing a safe sense of sexual identity is fundamental to adolescent psychological development, and many adolescents struggle to develop a positive sexual identity. Suicide rates and self-harm behaviors among LGBTQ+ adolescents can be 4 times higher than among their heterosexual peers.60 Rates of mood disorders, substance abuse, and high-risk sexual behaviors are also increased in the LGBTQ+ population.61
The LGBTQ+ community often seeks health care advice and affirmation from primary care providers. Resources to enhance this care are available at www.lgbthealtheducation.org.
Social media
Adolescents today have more media exposure than any prior generation, with smartphone and computer use increasing exponentially. Most (95%) teens have access to a smartphone,62 45% describe themselves as constantly connected to the Internet, and 14% feel that social media is “addictive.”62 Most manage their social media portfolio on multiple sites. Patterns of adolescents' online activities show that boys prefer online gaming, while girls tend to spend more time on social networking.62
Whether extensive media use is psychologically beneficial or deleterious has been widely debated. Increased time online correlates with decreased levels of physical activity.63 And sleep disturbances have been associated with excessive screen time and the presence of mobile devices in the bedroom.64 The use of social media prior to bedtime also has an adverse impact on academic performance—particularly for girls. This adverse impact on academics persists after correcting for daytime sleepiness, body mass index, and number of hours spent on homework.64
Continue to: Due to growing concerns...
Due to growing concerns about the risks of social media in children and adolescents, the American Academy of Pediatrics has developed the Family Media Plan (www.healthychildren.org/English/media/Pages/default.aspx). Some specific questions that providers may ask are outlined in TABLE 3.64 The Family Media Plan can provide age-specific guidelines to assist parents or caregivers in answering these questions.
Cyber-bullying. One in 3 adolescents (primarily female) has been a victim of cyber-bullying.65 Sadly, 1 in 5 teens has received some form of electronic sexual solicitation.66 The likelihood of unsolicited stranger contact correlates with teens’ online habits and the amount of information disclosed. Predictors include female sex, visiting chat rooms, posting photos, and disclosing personal information. Restricting computer use to an area with parental supervision or installing monitoring programs does not seem to exert any protective influence on cyber-bullying or unsolicited stranger contact.65 While 63% of cyber-bullying victims feel upset, embarrassed, or stressed by these contacts,66 few events are actually reported. To address this, some states have adopted laws adding cyber-bullying to school disciplinary codes.
Negative health impacts associated with cyber-bullying include anxiety, sadness, and greater difficulty in concentrating on school work.65 Victims of bullying are more likely to have school disciplinary actions and depression and to be truant or to carry weapons to school.66 Cyber-bullying is uniquely destructive due to its ubiquitous presence. A sense of relative anonymity online may encourage perpetrators to act more cruelly, with less concern for punishment.
Young people are also more likely to share passwords as a sign of friendship. This may result in others assuming their identity online. Adolescents rarely disclose bullying to parents or other adults, fearing restriction of Internet access, and many of them think that adults may downplay the seriousness of the events.66
CORRESPONDENCE
Mark B. Stephens, MD, Penn State Health Medical Group, 1850 East Park Avenue, State College, PA 16803; [email protected].
Adolescents are an increasingly diverse population reflecting changes in the racial, ethnic, and geopolitical milieus of the United States. The World Health Organization classifies adolescence as ages 10 to 19 years.1 However, given the complexity of adolescent development physically, behaviorally, emotionally, and socially, others propose that adolescence may extend to age 24.2
Recognizing the specific challenges adolescents face is key to providing comprehensive longitudinal health care. Moreover, creating an environment of trust helps to ensure open 2-way communication that can facilitate anticipatory guidance.
Our review focuses on common adolescent issues, including injury from vehicles and firearms, tobacco and substance misuse, obesity, behavioral health, sexual health, and social media use. We discuss current trends and recommend strategies to maximize health and wellness.
Start by framing the visit
Confidentiality
Laws governing confidentiality in adolescent health care vary by state. Be aware of the laws pertaining to your practice setting. In addition, health care facilities may have their own policies regarding consent and confidentiality in adolescent care. Discuss confidentiality with both an adolescent and the parent/guardian at the initial visit. And, to help avoid potential misunderstandings, let them know in advance what will (and will not) be divulged.
The American Academy of Pediatrics has developed a useful tip sheet regarding confidentiality laws (www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/healthy-foster-care-america/Documents/Confidentiality_Laws.pdf). Examples of required (conditional) disclosure include abuse and suicidal or homicidal ideations. Patients should understand that sexually transmitted infections (STIs) are reportable to public health authorities and that potentially injurious behaviors to self or others (eg, excessive drinking prior to driving) may also warrant disclosure(TABLE 13).

Privacy and general visit structure
Create a safe atmosphere where adolescents can discuss personal issues without fear of repercussion or judgment. While parents may prefer to be present during the visit, allowing for time to visit independently with an adolescent offers the opportunity to reinforce issues of privacy and confidentiality. Also discuss your office policies regarding electronic communication, phone communication, and relaying test results.

A useful paradigm for organizing a visit for routine adolescent care is to use an expanded version of the HEADSS mnemonic (TABLE 24,5), which includes questions about an adolescent’s Home, Education, Activities, Drug and alcohol use, Sexual behavior, Suicidality and depression, and other topics. Other validated screening tools include RAAPS (Rapid Adolescent Prevention Screening)6 (www.possibilitiesforchange.com/raaps/); the Guidelines for Adolescent Preventive Services7; and the Bright Futures recommendations for preventive care from the American Academy of Pediatrics.8 Below, we consider important topics addressed with the HEADSS approach.

Continue to: Injury from vehicles and firearms
Injury from vehicles and firearms
Motor vehicle accidents and firearm wounds are the 2 leading causes of adolescent injury. In 2016, of the more than 20,000 deaths in children and adolescents (ages 1-19 years), 20% were due to motor vehicle accidents (4074) and 15% were a result of firearm-related injuries (3143). Among firearm-related deaths, 60% were homicides, 35% were suicides, and 4% were due to accidental discharge.9 The rate of firearm-related deaths among American teens is 36 times greater than that of any other developed nation.9 Currently, 1 of every 3 US households with children younger than 18 has a firearm. Data suggest that in 43% of these households, the firearm is loaded and kept in an unlocked location.10
To aid anticipatory guidance, ask adolescents about firearm and seat belt use, drinking and driving, and suicidal thoughts (TABLE 24,5). Advise them to always wear seat belts whether driving or riding as a passenger. They should never drink and drive (or get in a car with someone who has been drinking). Advise parents that if firearms are present in the household, they should be kept in a secure, locked location. Weapons should be separated from ammunition and safety mechanisms should be engaged on all devices.
Tobacco and substance misuse
Tobacco use, the leading preventable cause of death in the United States,11 is responsible for more deaths than alcohol, motor vehicle accidents, suicides, homicides, and HIV disease combined.12 Most tobacco-associated mortality occurs in individuals who began smoking before the age of 18.12 Individuals who start smoking early are also more likely to continue smoking through adulthood.
Encouragingly, tobacco use has declined significantly among adolescents over the past several decades. Roughly 1 in 25 high school seniors reports daily tobacco use.13 Adolescent smoking behaviors are also changing dramatically with the increasing popularity of electronic cigarettes (“vaping”). Currently, more adolescents vape than smoke cigarettes.13 Vaping has additional health risks including toxic lung injury.
Multiple resources can help combat tobacco and nicotine use in adolescents. The US Preventive Services Task Force recommends that primary care clinicians intervene through education or brief counselling to prevent initiation of tobacco use in school-aged children and adolescents.14 Ask teens about tobacco and electronic cigarette use and encourage them to quit when use is acknowledged. Other helpful office-based tools are the “Quit Line” 800-QUIT-NOW and texting “Quit” to 47848. Smokefree teen (https://teen.smokefree.gov/) is a website that reviews the risks of tobacco and nicotine use and provides age-appropriate cessation tools and tips (including a smartphone app and a live-chat feature). Other useful information is available in a report from the Surgeon General on preventing tobacco use among young adults.15
Continue to: Alcohol use
Alcohol use. Three in 5 high school students report ever having used alcohol.13 As with tobacco, adolescent alcohol use has declined over the past decade. However, binge drinking (≥ 5 drinks on 1 occasion for males; ≥ 4 drinks on 1 occasion for females) remains a common high-risk behavior among adolescents (particularly college students). Based on the Monitoring the Future Survey, 1 in 6 high school seniors reported binge drinking in the past 2 weeks.13 While historically more common among males, rates of binge drinking are now basically similar between male and female adolescents.13
The National Institute on Alcohol Abuse and Alcoholism has a screening and intervention guide specifically for adolescents.16
Illicit drug use. Half of adolescents report using an illicit drug by their senior year in high school.13 Marijuana is the most commonly used substance, and laws governing its use are rapidly changing across the United States. Marijuana is illegal in 10 states and legal in 10 states (and the District of Columbia). The remaining states have varying policies on the medical use of marijuana and the decriminalization of marijuana. In addition, cannabinoid (CBD) products are increasingly available. Frequent cannabis use in adolescence has an adverse impact on general executive function (compared with adult users) and learning.17 Marijuana may serve as a gateway drug in the abuse of other substances,18 and its use should be strongly discouraged in adolescents.
Of note, there has been a sharp rise in the illicit use of prescription drugs, particularly opioids, creating a public health emergency across the United States.19 In 2015, more than 4000 young people, ages 15 to 24, died from a drug-related overdose (> 50% of these attributable to opioids).20 Adolescents with a history of substance abuse and behavioral illness are at particular risk. Many adolescents who misuse opioids and other prescription drugs obtain them from friends and relatives.21
The Substance Abuse and Mental Health Services Administration (SAMHSA) recommends universal screening of adolescents for substance abuse. This screening should be accompanied by a brief intervention to prevent, mitigate, or eliminate substance use, or a referral to appropriate treatment sources. This process of screening, brief intervention, and referral to treatment (SBIRT) is recommended as part of routine health care.22
Continue to: Obesity and physical activity
Obesity and physical activity
The percentage of overweight and obese adolescents in the United States has more than tripled over the past 40 years,23 and 1 in 5 US adolescents is obese.23 Obese teens are at higher risk for multiple chronic diseases, including type 2 diabetes, sleep apnea, and heart disease.24 They are also more likely to be bullied and to have poor self-esteem.25 Only 1 in 5 American high school students engages in 60 or more minutes of moderate-to-vigorous physical activity on 5 or more days per week.26
Regular physical activity is, of course, beneficial for cardiorespiratory fitness, bone health, weight control, and improved indices of behavioral health.26 Adolescents who are physically active consistently demonstrate better school attendance and grades.17 Higher levels of physical fitness are also associated with improved overall cognitive performance.24
General recommendations. The Department of Health and Human Services recommends that adolescents get at least 60 minutes of mostly moderate physical activity every day.26 Encourage adolescents to engage in vigorous physical activity (heavy breathing, sweating) at least 3 days a week. As part of their physical activity patterns, adolescents should also engage in muscle-strengthening and bone-strengthening activities on at least 3 days per week.
Behavioral health
As young people develop their sense of personal identity, they also strive for independence. It can be difficult, at times, to differentiate normal adolescent rebellion from true mental illness. An estimated 17% to 19% of adolescents meet criteria for mental illness, and about 7% have a severe psychiatric disorder.27 Only one-third of adolescents with mental illness receive any mental health services.28
Depression. The 1-year incidence of major depression in adolescents is 3% to 4%, and the lifetime prevalence of depressive symptoms is 25% in all high school students.27 Risk factors include ethnic minority status, poor self-esteem, poor health, recent personal crisis, insomnia, and alcohol/substance abuse. Depression in adolescent girls is correlated with becoming sexually active at a younger age, failure to use contraception, having an STI, and suicide attempts. Depressed boys are more likely to have unprotected intercourse and participate in physical fights.29 Depressed teens have a 2- to 3-fold greater risk for behavioral disorders, anxiety, and attention-deficit/hyperactivity disorder (ADHD).30
Continue to: Suicide
Suicide. Among individuals 15 to 29 years of age, suicide is the second leading cause of death globally, with an annual incidence of 11 to 15 per 100,000.31 Suicide attempts are 10 to 20 times more common than completed suicide.31 Males are more likely than females to die by suicide,32 and boys with a history of attempted suicide have a 30-fold increased risk of subsequent successful suicide.31 Hanging, drug poisoning, and firearms (particularly for males) are the most common means of suicide in adolescents. More than half of adolescents dying by suicide have coexisting depression.31
Characteristics associated with suicidal behaviors in adolescents include impulsivity, poor problem-solving skills, and dichotomous thinking.31 There may be a genetic component as well. In 1 of 5 teenage suicides, a precipitating life event such as the break-up of a relationship, cyber-bullying, or peer rejection is felt to contribute.31
ADHD. The prevalence of ADHD is 7% to 9% in US school-aged children.33 Boys more commonly exhibit hyperactive behaviors, while girls have more inattention. Hyperactivity often diminishes in teens, but inattention and impulsivity persist. Sequelae of ADHD include high-risk sexual behaviors, motor vehicle accidents, incarceration, and substance abuse.34 Poor self-esteem, suicidal ideation, smoking, and obesity are also increased.34 ADHD often persists into adulthood, with implications for social relationships and job performance.34
Eating disorders. The distribution of eating disorders is now known to increasingly include more minorities and males, the latter representing 5% to 10% of cases.35 Eating disorders show a strong genetic tendency and appear to be accelerated by puberty. The most common eating disorder (diagnosed in 0.8%-14% of teens) is eating disorder not otherwise specified (NOS).35 Anorexia nervosa is diagnosed in 0.5% of adolescent girls, and bulimia nervosa in 1% to 2%—particularly among athletes and performers.35 Unanticipated loss of weight, amenorrhea, excessive concern about weight, and deceleration in height/weight curves are potential indicators of an eating disorder. When identified, eating disorders are best managed by a trusted family physician, acting as a coordinator of a multidisciplinary team.
Sexual health
Girls begin to menstruate at an average age of 12, and it takes about 4 years for them to reach reproductive maturity.36 Puberty has been documented to start at younger ages over the past 30 years, likely due to an increase in average body mass index and a decrease in levels of physical activity.37 Girls with early maturation are often insecure and self-conscious, with higher levels of psychological distress.38 In boys, the average age for spermarche (first ejaculation) is 13.39 Boys who mature early tend to be taller, be more confident, and express a good body image.40 Those who have early puberty are more likely to be sexually active or participate in high-risk behaviors.41
Continue to: Pregnancy and contraception
Pregnancy and contraception
Over the past several decades, more US teens have been abstaining from sexual intercourse or have been using effective forms of birth control, particularly condoms and long-acting reversible contraceptives (LARCs).42 Teenage birth rates in girls ages 15 to 19 have declined significantly since the 1980s.42 Despite this, the teenage birth rate in the United States remains higher than in other industrialized nations, and most teen pregnancies are unintended.
There are numerous interventions to reduce teen pregnancy, including sex education, contraceptive counseling, the use of mobile apps that track a user’s monthly fertility cycle or issue reminders to take oral contraceptives,45 and the liberal distribution of contraceptives and condoms. The Contraceptive CHOICE Project shows that providing free (or low-cost) LARCs influences young women to choose these as their preferred contraceptive method.46 Other programs specifically empower girls to convince partners to use condoms and to resist unwanted sexual advances or intimate partner violence.
Adolescents prefer to have their health care providers address the topic of sexual health. Teens are more likely to share information with providers if asked directly about sexual behaviors.47TABLE 24,5 offers tips for anticipatory guidance and potential ways to frame questions with adolescents in this context. State laws vary with regard to the ability of minors to seek contraception, pregnancy testing, or care/screening for STIs without parental consent. Contraceptive counseling combined with effective screening decrease the incidence of STIs and pelvic inflammatory disease for sexually active teens.48

Sexually transmitted infections
Young adolescents often have a limited ability to imagine consequences related to specific actions. In general, there is also an increased desire to engage in experimental behaviors as an expression of developing autonomy, which may expose them to STIs. About half of all STIs contracted in the United States occur in individuals 15 to 24 years of age.49 Girls are at particular risk for the sequelae of these infections, including cervical dysplasia and infertility. Many teens erroneously believe that sexual activities other than intercourse decrease their risk of contracting an STI.50
Human papillomavirus (HPV) infection is the most common STI in adolescence.51 In most cases, HPV is transient and asymptomatic. Oncogenic strains may cause cervical cancer or cancers of the anogenital or oropharyngeal systems. Due to viral latency, it is not recommended to perform HPV typing in men or in women younger than 30 years of age; however, Pap tests are recommended every 3 years for women ages 21 to 29. Primary care providers are pivotal in the public health struggle to prevent HPV infection.
Continue to: Universal immunization of all children...
Universal immunization of all children older than 11 years of age against HPV is strongly advised as part of routine well-child care. Emphasize the proven role of HPV vaccination in preventing cervical52 and oropharyngeal53 cancers. And be prepared to address concerns raised by parents in the context of vaccine safety and the initiation of sexual behaviors (www.cdc.gov/hpv/hcp/answering-questions.html).
Chlamydia is the second most common STI in the United States, usually occurring in individuals younger than 24.54 The CDC estimates that more than 3 million new chlamydial infections occur yearly. These infections are often asymptomatic, particularly in females, but may cause urethritis, cervicitis, epididymitis, proctitis, or pelvic inflammatory disease. Indolent chlamydial infection is the leading cause of tubal infertility in women.54 Routine annual screening for chlamydia is recommended for all sexually active females ≤ 25 years (and for older women with specific risks).55 Annual screening is also recommended for men who have sex with men (MSM).55
Chlamydial infection may be diagnosed with first-catch urine sampling (men or women), urethral swab (men), endocervical swab (women), or self-collected vaginal swab. Nucleic acid amplification testing is the most sensitive test that is widely available.56 First-line treatment includes either azithromycin (1 g orally, single dose) or doxycycline (100 mg orally, twice daily for 7 days).56
Gonorrhea. In 2018, there were more than 500,000 annual cases of gonorrhea, with the majority occurring in those between 15 and 24 years of age.57 Gonorrhea may increase rates of HIV infection transmission up to 5-fold.57 As more adolescents practice oral sex, cases of pharyngeal gonorrhea (and oropharyngeal HPV) have increased. Symptoms of urethritis occur more frequently in men. Screening is recommended for all sexually active women younger than 25.56 Importantly, the organism Neisseria gonorrhoeae has developed significant antibiotic resistance over the past decade. The CDC currently recommends dual therapy for the treatment of gonorrhea using 250 mg of intramuscular ceftriaxone and 1 g of oral azithromycin.56
Syphilis. Rates of syphilis are increasing among individuals ages 15 to 24.51 Screening is particularly recommended for MSM and individuals infected with HIV. Benzathine penicillin G, 50,000 U/kg IM, remains the treatment of choice.56
Continue to: HIV
HIV. Globally, HIV impacts young people disproportionately. HIV infection also facilitates infection with other STIs. In the United States, the highest burden of HIV infection is borne by young MSM, with prevalence among those 18 to 24 years old varying between 26% to 30% (black) and 3% to 5.5% (non-Hispanic white).51 The use of emtricitabine/tenofovir disoproxil fumarate for pre-exposure prophylaxis (PrEP) has recently been approved for the prevention of HIV. PrEP reduces risk by up to 92% for MSM and transgender women.58
Sexual identity
One in 10 high school students self-identifies as “nonheterosexual,” and 1 in 15 reports same-sex sexual contact.59 The term LGBTQ+ includes the communities of lesbian, gay, bisexual, transgender, transsexual, queer, questioning, intersex, and asexual individuals. Developing a safe sense of sexual identity is fundamental to adolescent psychological development, and many adolescents struggle to develop a positive sexual identity. Suicide rates and self-harm behaviors among LGBTQ+ adolescents can be 4 times higher than among their heterosexual peers.60 Rates of mood disorders, substance abuse, and high-risk sexual behaviors are also increased in the LGBTQ+ population.61
The LGBTQ+ community often seeks health care advice and affirmation from primary care providers. Resources to enhance this care are available at www.lgbthealtheducation.org.
Social media
Adolescents today have more media exposure than any prior generation, with smartphone and computer use increasing exponentially. Most (95%) teens have access to a smartphone,62 45% describe themselves as constantly connected to the Internet, and 14% feel that social media is “addictive.”62 Most manage their social media portfolio on multiple sites. Patterns of adolescents' online activities show that boys prefer online gaming, while girls tend to spend more time on social networking.62
Whether extensive media use is psychologically beneficial or deleterious has been widely debated. Increased time online correlates with decreased levels of physical activity.63 And sleep disturbances have been associated with excessive screen time and the presence of mobile devices in the bedroom.64 The use of social media prior to bedtime also has an adverse impact on academic performance—particularly for girls. This adverse impact on academics persists after correcting for daytime sleepiness, body mass index, and number of hours spent on homework.64
Continue to: Due to growing concerns...
Due to growing concerns about the risks of social media in children and adolescents, the American Academy of Pediatrics has developed the Family Media Plan (www.healthychildren.org/English/media/Pages/default.aspx). Some specific questions that providers may ask are outlined in TABLE 3.64 The Family Media Plan can provide age-specific guidelines to assist parents or caregivers in answering these questions.
Cyber-bullying. One in 3 adolescents (primarily female) has been a victim of cyber-bullying.65 Sadly, 1 in 5 teens has received some form of electronic sexual solicitation.66 The likelihood of unsolicited stranger contact correlates with teens’ online habits and the amount of information disclosed. Predictors include female sex, visiting chat rooms, posting photos, and disclosing personal information. Restricting computer use to an area with parental supervision or installing monitoring programs does not seem to exert any protective influence on cyber-bullying or unsolicited stranger contact.65 While 63% of cyber-bullying victims feel upset, embarrassed, or stressed by these contacts,66 few events are actually reported. To address this, some states have adopted laws adding cyber-bullying to school disciplinary codes.
Negative health impacts associated with cyber-bullying include anxiety, sadness, and greater difficulty in concentrating on school work.65 Victims of bullying are more likely to have school disciplinary actions and depression and to be truant or to carry weapons to school.66 Cyber-bullying is uniquely destructive due to its ubiquitous presence. A sense of relative anonymity online may encourage perpetrators to act more cruelly, with less concern for punishment.
Young people are also more likely to share passwords as a sign of friendship. This may result in others assuming their identity online. Adolescents rarely disclose bullying to parents or other adults, fearing restriction of Internet access, and many of them think that adults may downplay the seriousness of the events.66
CORRESPONDENCE
Mark B. Stephens, MD, Penn State Health Medical Group, 1850 East Park Avenue, State College, PA 16803; [email protected].
1. World Health Organization. Adolescent health. Accessed February 23, 2021. www.who.int/maternal_child_adolescent/adolescence/en/
2. Sawyer SM, Azzopardi PS, Wickremarathne D, et al. The age of adolescence. Lancet Child Adolesc Health. 2018;2:223-228.
3. Pathak PR, Chou A. Confidential care for adoloscents in the U.S. healthcare system. J Patient Cent Res Rev. 2019;6:46-50.
4. AMA Journal of Ethics. HEADSS: the “review of systems” for adolescents. Accessed February 23, 2021. https://journalofethics.ama-assn.org/article/headss-review-systems-adolescents/2005-03
5. Cohen E, MacKenzie RG, Yates GL. HEADSS, a psychosocial risk assessment instrument: implications for designing effective intervention programs for runaway youth. J Adolesc Health. 1991;12:539-544.
6. Possibilities for Change. Rapid Adolescent Prevention Screening (RAAPS). Accessed February 23, 2021. www.possibilitiesforchange.com/raaps/
7. Elster AB, Kuznets NJ. AMA Guidelines for Adolescent Preventive Services (GAPS): Recommendations and Rationale. Williams & Wilkins; 1994.
8. AAP. Engaging patients and families - periodicity schedule. Accessed February 23, 2021. www.aap.org/en-us/professional-resources/practice-support/Pages/PeriodicitySchedule.aspx
9. Cunningham RM, Walton MA, Carter PM. The major causes of death in children and adolescents in the United States. N Eng J Med. 2018;379:2468-2475.
10. Schuster MA, Franke TM, Bastian AM, et al. Firearm storage patterns in US homes with children. Am J Public Health. 2000;90:588-594.
11. Mokdad AH, Marks JS, Stroup DF, et al. Actual causes of death in the United States. JAMA. 2004;291:1238-1245.
12. HHS. Health consequences of smoking, surgeon general fact sheet. Accessed February 23, 2021. www.hhs.gov/surgeongeneral/reports-and-publications/tobacco/consequences-smoking-factsheet/index.html
13. Johnston LD, Miech RA, O’Malley PM, et al. Monitoring the future: national survey results on drug use, 1975-2017. The University of Michigan. 2018. Accessed February 23, 2021. https://eric.ed.gov/?id=ED589762
14. US Preventive Services Task Force. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. Accessed February 23, 2021. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
15. HHS. Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta, GA: HHS, CDC, NCCDPHP, OSH; 2012. Accessed February 23, 2021. www.ncbi.nlm.nih.gov/books/NBK99237/
16. NIH. Alcohol screening and brief intervention for youth: a pocket guide. Accessed February 23, 2021. https://pubs.niaaa.nih.gov/publications/Practitioner/YouthGuide/YouthGuidePocket.pdf
17. Gorey C, Kuhns L, Smaragdi E, et al. Age-related differences in the impact of cannabis use on the brain and cognition: a systematic review. Eur Arch Psychiatry Clin Neurosci. 2019;269:37-58.
18. Secades-Villa R, Garcia-Rodriguez O, Jin CJ, et al. Probability and predictors of the cannabis gateway effect: a national study. Int J Drug Policy. 2015;26:135-142.
19. Kann L, McManus T, Harris WA, et al. Youth risk behavior surveillance—United States, 2017. MMWR Surveill Summ. 2018;67:1-114.
20. NIH. Drug overdoses in youth. How do drug overdoses happen?. Accessed February 23, 2021. https://teens.drugabuse.gov/drug-facts/drug-overdoses-youth
21. Branstetter SA, Low S, Furman W. The influence of parents and friends on adolescent substance use: a multidimensional approach. J Subst Use. 2011;162:150-160.
22. AAP. Committee on Substance Use and Prevention. Substance use screening, brief intervention, and referral to treatment. Pediatrics. 2016;138:e20161210.
23. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief. 2017;288:1-8.
24. Halfon N, Larson K, Slusser W. Associations between obesity and comorbid mental health, developmental and physical health conditions in a nationally representative sample of US children aged 10 to 17. Acad Pediatr. 2013;13:6-13.
25. Griffiths LJ, Parsons TJ, Hill AJ. Self-esteem and quality of life in obese children and adolescents: a systematic review. Int J Pediatr Obes. 2010;5:282-304.
26. National Physical Activity Plan Alliance. The 2018 United States report card on physical activity for children and youth. Accessed February 23, 2021. http://physicalactivityplan.org/projects/PA/2018/2018%20US%20Report%20Card%20Full%20Version_WEB.PDF?pdf=page-link
27. HHS. NIMH. Child and adolescent mental health. Accessed February 23, 2021. www.nimh.nih.gov/health/topics/child-and-adolescent-mental-health/index.shtml
28. Yonek JC, Jordan N, Dunlop D, et al. Patient-centered medical home care for adolescents in need of mental health treatment. J Adolesc Health. 2018;63:172-180.
29. Brooks TL, Harris SK, Thrall JS, et al. Association of adolescent risk behaviors with mental health symptoms in high school students. |J Adolesc Health. 2002;31:240-246.
30. Weller BE, Blanford KL, Butler AM. Estimated prevalence of psychiatric comorbidities in US adolescents with depression by race/ethnicity, 2011-2012. J Adolesc Health. 2018;62:716-721.
31. Bilsen J. Suicide and youth: risk factors. Front Psychiatry. 2018;9:540.
32. Shain B, AAP Committee on Adolescence. Suicide and suicide attempts in adolescents. Pediatrics. 2016;138:e20161420.
33. Brahmbhatt K, Hilty DM, Hah M, et al. Diagnosis and treatment of attention deficit hyperactivity disorder during adolescence in the primary care setting: review and future directions. J Adolesc Health. 2016;59:135-143.
34. Bravender T. Attention-deficit/hyperactivity disorder and disordered eating. [editorial] J Adolesc Health. 2017;61:125-126.
35. Rosen DS, AAP Committee on Adolescence. Identification and management of eating disorders in children and adolescents. Pediatrics. 2010;126:1240-1253.
36. Susman EJ, Houts RM, Steinberg L, et al. Longitudinal development of secondary sexual characteristics in girls and boys between ages 9 ½ and 15 ½ years. Arch Pediatr Adolesc Med. 2010;164:166-173.
37. Kaplowitz PB. Link between body fat and the timing of puberty. Pediatrics. 2008;121(suppl 3):S208-S217.
38. Ge X, Conger RD, Elder GH. Coming of age too early: pubertal influences on girl’s vulnerability to psychologic distress. Child Dev. 1996;67:3386-3400.
39. Jørgensen M, Keiding N, Skakkebaek NE. Estimation of spermarche from longitudinal spermaturia data. Biometrics. 1991;47:177-193.
40. Kar SK, Choudhury A, Singh AP. Understanding normal development of adolescent sexuality: a bumpy ride. J Hum Reprod Sci. 2015;8:70-74.
41. Susman EJ, Dorn LD, Schiefelbein VL. Puberty, sexuality and health. In: Lerner MA, Easterbrooks MA, Mistry J (eds). Comprehensive Handbook of Psychology. Wiley; 2003.
42. Lindberg LD, Santelli JS, Desai S. Changing patterns of contraceptive use and the decline in rates of pregnancy and birth among U.S. adolescents, 2007-2014. J Adolesc Health. 2018;63:253-256.
43. Guttmacher Institute. Teen pregnancy. www.guttmacher.org/united-states/teens/teen-pregnancy. Accessed February 23, 2021.
44. CDC. Social determinants and eliminating disparities in teen pregnancy. Accessed February 23, 2021. www.cdc.gov/teenpregnancy/about/social-determinants-disparities-teen-pregnancy.htm
45. Widman L, Nesi J, Kamke K, et al. Technology-based interventions to reduce sexually transmitted infection and unintended pregnancy among youth. J Adolesc Health. 2018;62:651-660.
46. Secura GM, Allsworth JE, Madden T, et al. The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol. 2010;203:115.e1-115.e7.
47. Ham P, Allen C. Adolescent health screening and counseling. Am Fam Physician. 2012;86:1109-1116.
48. ACOG. Committee on Adolescent Health Care. Adolescent pregnancy, contraception and sexual activity. 2017. Accessed February 23, 2021. www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2017/05/adolescent-pregnancy-contraception-and-sexual-activity
49. Wangu Z, Burstein GR. Adolescent sexuality: updates to the sexually transmitted infection guidelines. Pediatr Clin N Am. 2017;64:389-411.
50. Holway GV, Hernandez SM. Oral sex and condom use in a U.S. national sample of adolescents and young adults. J Adolesc Health. 2018;62:402-410.
51. CDC. STDs in adults and adolescents. Accessed February 23, 2021. www.cdc.gov/std/stats17/adolescents.htm
52. McClung N, Gargano J, Bennett N, et al. Trends in human papillomavirus vaccine types 16 and 18 in cervical precancers, 2008-2014. Accessed February 23, 2021. https://cebp.aacrjournals.org/content/28/3/602
53. Timbang MR, Sim MW, Bewley AF, et al. HPV-related oropharyngeal cancer: a review on burden of the disease and opportunities for prevention and early detection. Hum Vaccin Immunother. 2019;15:1920-1928.
54. Carey AJ, Beagley KW. Chlamydia trachomatis, a hidden epidemic: effects on female reproduction and options for treatment. Am J Reprod Immunol. 2010;63:576-586.
55. USPSTF. Chlamydia and gonorrhea screening. Accessed February 23, 2021. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/chlamydia-and-gonorrhea-screening
56. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:1-135.
57. CDC. Sexually transmitted disease surveillance 2018. Accessed February 23, 2021. www.cdc.gov/std/stats18/gonorrhea.htm
5
59. Kann L, McManus T, Harris WA, et al. Youth risk behavior surveillance–United States, 2015. MMWR Surveill Summ. 2016;65:1-174.
60. CDC. LGBT youth. Accessed February 23, 2021. www.cdc.gov/lgbthealth/youth.htm
61. Johns MM, Lowry R, Rasberry CN, et al. Violence victimization, substance use, and suicide risk among sexual minority high school students – United States, 2015-2017. MMWR Morb Mortal Wkly Rep. 2018;67:1211-1215.
62. Pew Research Center. Teens, social media & technology 2018. . Accessed February 23, 2021. www.pewinternet.org/2018/05/31/teens-social-media-technology-2018/
63. Chassiakos YLR, Radesky J, Christakis D, et al. Children and adolescents and digital media. Pediatrics. 2016;138:e20162593.
64. Arora T, Albahri A, Omar OM, et al. The prospective association between electronic device use before bedtime and academic attainment in adolescents. J Adolesc Health. 2018;63:451-458.
65. Mishna F, Saini M, Solomon S. Ongoing and online: children and youth’s perceptions of cyber bullying. Child Youth Serv Rev. 2009;31:1222-1228.
66. Sengupta A, Chaudhuri A. Are social networking sites a source of online harassment for teens? Evidence from survey data. Child Youth Serv Rev. 2011;33:284-290.
1. World Health Organization. Adolescent health. Accessed February 23, 2021. www.who.int/maternal_child_adolescent/adolescence/en/
2. Sawyer SM, Azzopardi PS, Wickremarathne D, et al. The age of adolescence. Lancet Child Adolesc Health. 2018;2:223-228.
3. Pathak PR, Chou A. Confidential care for adoloscents in the U.S. healthcare system. J Patient Cent Res Rev. 2019;6:46-50.
4. AMA Journal of Ethics. HEADSS: the “review of systems” for adolescents. Accessed February 23, 2021. https://journalofethics.ama-assn.org/article/headss-review-systems-adolescents/2005-03
5. Cohen E, MacKenzie RG, Yates GL. HEADSS, a psychosocial risk assessment instrument: implications for designing effective intervention programs for runaway youth. J Adolesc Health. 1991;12:539-544.
6. Possibilities for Change. Rapid Adolescent Prevention Screening (RAAPS). Accessed February 23, 2021. www.possibilitiesforchange.com/raaps/
7. Elster AB, Kuznets NJ. AMA Guidelines for Adolescent Preventive Services (GAPS): Recommendations and Rationale. Williams & Wilkins; 1994.
8. AAP. Engaging patients and families - periodicity schedule. Accessed February 23, 2021. www.aap.org/en-us/professional-resources/practice-support/Pages/PeriodicitySchedule.aspx
9. Cunningham RM, Walton MA, Carter PM. The major causes of death in children and adolescents in the United States. N Eng J Med. 2018;379:2468-2475.
10. Schuster MA, Franke TM, Bastian AM, et al. Firearm storage patterns in US homes with children. Am J Public Health. 2000;90:588-594.
11. Mokdad AH, Marks JS, Stroup DF, et al. Actual causes of death in the United States. JAMA. 2004;291:1238-1245.
12. HHS. Health consequences of smoking, surgeon general fact sheet. Accessed February 23, 2021. www.hhs.gov/surgeongeneral/reports-and-publications/tobacco/consequences-smoking-factsheet/index.html
13. Johnston LD, Miech RA, O’Malley PM, et al. Monitoring the future: national survey results on drug use, 1975-2017. The University of Michigan. 2018. Accessed February 23, 2021. https://eric.ed.gov/?id=ED589762
14. US Preventive Services Task Force. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. Accessed February 23, 2021. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
15. HHS. Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta, GA: HHS, CDC, NCCDPHP, OSH; 2012. Accessed February 23, 2021. www.ncbi.nlm.nih.gov/books/NBK99237/
16. NIH. Alcohol screening and brief intervention for youth: a pocket guide. Accessed February 23, 2021. https://pubs.niaaa.nih.gov/publications/Practitioner/YouthGuide/YouthGuidePocket.pdf
17. Gorey C, Kuhns L, Smaragdi E, et al. Age-related differences in the impact of cannabis use on the brain and cognition: a systematic review. Eur Arch Psychiatry Clin Neurosci. 2019;269:37-58.
18. Secades-Villa R, Garcia-Rodriguez O, Jin CJ, et al. Probability and predictors of the cannabis gateway effect: a national study. Int J Drug Policy. 2015;26:135-142.
19. Kann L, McManus T, Harris WA, et al. Youth risk behavior surveillance—United States, 2017. MMWR Surveill Summ. 2018;67:1-114.
20. NIH. Drug overdoses in youth. How do drug overdoses happen?. Accessed February 23, 2021. https://teens.drugabuse.gov/drug-facts/drug-overdoses-youth
21. Branstetter SA, Low S, Furman W. The influence of parents and friends on adolescent substance use: a multidimensional approach. J Subst Use. 2011;162:150-160.
22. AAP. Committee on Substance Use and Prevention. Substance use screening, brief intervention, and referral to treatment. Pediatrics. 2016;138:e20161210.
23. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief. 2017;288:1-8.
24. Halfon N, Larson K, Slusser W. Associations between obesity and comorbid mental health, developmental and physical health conditions in a nationally representative sample of US children aged 10 to 17. Acad Pediatr. 2013;13:6-13.
25. Griffiths LJ, Parsons TJ, Hill AJ. Self-esteem and quality of life in obese children and adolescents: a systematic review. Int J Pediatr Obes. 2010;5:282-304.
26. National Physical Activity Plan Alliance. The 2018 United States report card on physical activity for children and youth. Accessed February 23, 2021. http://physicalactivityplan.org/projects/PA/2018/2018%20US%20Report%20Card%20Full%20Version_WEB.PDF?pdf=page-link
27. HHS. NIMH. Child and adolescent mental health. Accessed February 23, 2021. www.nimh.nih.gov/health/topics/child-and-adolescent-mental-health/index.shtml
28. Yonek JC, Jordan N, Dunlop D, et al. Patient-centered medical home care for adolescents in need of mental health treatment. J Adolesc Health. 2018;63:172-180.
29. Brooks TL, Harris SK, Thrall JS, et al. Association of adolescent risk behaviors with mental health symptoms in high school students. |J Adolesc Health. 2002;31:240-246.
30. Weller BE, Blanford KL, Butler AM. Estimated prevalence of psychiatric comorbidities in US adolescents with depression by race/ethnicity, 2011-2012. J Adolesc Health. 2018;62:716-721.
31. Bilsen J. Suicide and youth: risk factors. Front Psychiatry. 2018;9:540.
32. Shain B, AAP Committee on Adolescence. Suicide and suicide attempts in adolescents. Pediatrics. 2016;138:e20161420.
33. Brahmbhatt K, Hilty DM, Hah M, et al. Diagnosis and treatment of attention deficit hyperactivity disorder during adolescence in the primary care setting: review and future directions. J Adolesc Health. 2016;59:135-143.
34. Bravender T. Attention-deficit/hyperactivity disorder and disordered eating. [editorial] J Adolesc Health. 2017;61:125-126.
35. Rosen DS, AAP Committee on Adolescence. Identification and management of eating disorders in children and adolescents. Pediatrics. 2010;126:1240-1253.
36. Susman EJ, Houts RM, Steinberg L, et al. Longitudinal development of secondary sexual characteristics in girls and boys between ages 9 ½ and 15 ½ years. Arch Pediatr Adolesc Med. 2010;164:166-173.
37. Kaplowitz PB. Link between body fat and the timing of puberty. Pediatrics. 2008;121(suppl 3):S208-S217.
38. Ge X, Conger RD, Elder GH. Coming of age too early: pubertal influences on girl’s vulnerability to psychologic distress. Child Dev. 1996;67:3386-3400.
39. Jørgensen M, Keiding N, Skakkebaek NE. Estimation of spermarche from longitudinal spermaturia data. Biometrics. 1991;47:177-193.
40. Kar SK, Choudhury A, Singh AP. Understanding normal development of adolescent sexuality: a bumpy ride. J Hum Reprod Sci. 2015;8:70-74.
41. Susman EJ, Dorn LD, Schiefelbein VL. Puberty, sexuality and health. In: Lerner MA, Easterbrooks MA, Mistry J (eds). Comprehensive Handbook of Psychology. Wiley; 2003.
42. Lindberg LD, Santelli JS, Desai S. Changing patterns of contraceptive use and the decline in rates of pregnancy and birth among U.S. adolescents, 2007-2014. J Adolesc Health. 2018;63:253-256.
43. Guttmacher Institute. Teen pregnancy. www.guttmacher.org/united-states/teens/teen-pregnancy. Accessed February 23, 2021.
44. CDC. Social determinants and eliminating disparities in teen pregnancy. Accessed February 23, 2021. www.cdc.gov/teenpregnancy/about/social-determinants-disparities-teen-pregnancy.htm
45. Widman L, Nesi J, Kamke K, et al. Technology-based interventions to reduce sexually transmitted infection and unintended pregnancy among youth. J Adolesc Health. 2018;62:651-660.
46. Secura GM, Allsworth JE, Madden T, et al. The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol. 2010;203:115.e1-115.e7.
47. Ham P, Allen C. Adolescent health screening and counseling. Am Fam Physician. 2012;86:1109-1116.
48. ACOG. Committee on Adolescent Health Care. Adolescent pregnancy, contraception and sexual activity. 2017. Accessed February 23, 2021. www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2017/05/adolescent-pregnancy-contraception-and-sexual-activity
49. Wangu Z, Burstein GR. Adolescent sexuality: updates to the sexually transmitted infection guidelines. Pediatr Clin N Am. 2017;64:389-411.
50. Holway GV, Hernandez SM. Oral sex and condom use in a U.S. national sample of adolescents and young adults. J Adolesc Health. 2018;62:402-410.
51. CDC. STDs in adults and adolescents. Accessed February 23, 2021. www.cdc.gov/std/stats17/adolescents.htm
52. McClung N, Gargano J, Bennett N, et al. Trends in human papillomavirus vaccine types 16 and 18 in cervical precancers, 2008-2014. Accessed February 23, 2021. https://cebp.aacrjournals.org/content/28/3/602
53. Timbang MR, Sim MW, Bewley AF, et al. HPV-related oropharyngeal cancer: a review on burden of the disease and opportunities for prevention and early detection. Hum Vaccin Immunother. 2019;15:1920-1928.
54. Carey AJ, Beagley KW. Chlamydia trachomatis, a hidden epidemic: effects on female reproduction and options for treatment. Am J Reprod Immunol. 2010;63:576-586.
55. USPSTF. Chlamydia and gonorrhea screening. Accessed February 23, 2021. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/chlamydia-and-gonorrhea-screening
56. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:1-135.
57. CDC. Sexually transmitted disease surveillance 2018. Accessed February 23, 2021. www.cdc.gov/std/stats18/gonorrhea.htm
5
59. Kann L, McManus T, Harris WA, et al. Youth risk behavior surveillance–United States, 2015. MMWR Surveill Summ. 2016;65:1-174.
60. CDC. LGBT youth. Accessed February 23, 2021. www.cdc.gov/lgbthealth/youth.htm
61. Johns MM, Lowry R, Rasberry CN, et al. Violence victimization, substance use, and suicide risk among sexual minority high school students – United States, 2015-2017. MMWR Morb Mortal Wkly Rep. 2018;67:1211-1215.
62. Pew Research Center. Teens, social media & technology 2018. . Accessed February 23, 2021. www.pewinternet.org/2018/05/31/teens-social-media-technology-2018/
63. Chassiakos YLR, Radesky J, Christakis D, et al. Children and adolescents and digital media. Pediatrics. 2016;138:e20162593.
64. Arora T, Albahri A, Omar OM, et al. The prospective association between electronic device use before bedtime and academic attainment in adolescents. J Adolesc Health. 2018;63:451-458.
65. Mishna F, Saini M, Solomon S. Ongoing and online: children and youth’s perceptions of cyber bullying. Child Youth Serv Rev. 2009;31:1222-1228.
66. Sengupta A, Chaudhuri A. Are social networking sites a source of online harassment for teens? Evidence from survey data. Child Youth Serv Rev. 2011;33:284-290.
PRACTICE RECOMMENDATIONS
› Consider using a 2-question screening tool for adolescents that asks about personal use of alcohol and use of alcohol by friends; this resource offers a risk assessment with recommendations. C
› Consider using the American Academy of Pediatrics Family Media Plan to provide age-specific guidelines to help parents or caregivers establish rules for online activities. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
When war follows combat veterans home
› Ask, “Have you or a loved one ever served in the military?” as a way to uncover service-related concerns. C
› Conduct a thorough neurological evaluation with suspected mild traumatic brain injury, including vestibular, vision, postural, and neuro-cognitive assessments. C
› Use the Post-Traumatic Checklist–Military to assess individuals with possible post-traumatic stress disorder. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE A 37-year-old white woman presents for an employment physical. Your nurse reports that she also has a complaint of headaches, that she scored an 8 on the Alcohol Use Disorders identification Test-consumption (AUDiT-c), and that the result on her patient health Questionnaire (phQ-2) suggests a depressive disorder. You ask the patient whether she has served in the military and discover that, in the last 4 years, she served 2 year-long tours in Afghanistan with her Army reserve unit, returning home 6 months ago. Since her return, she has lost her job due to chronic tardiness (sleeping through her alarm, she says) and admits she has “started drinking again.” Her visit with you this day is only to undergo the physical exam required by her new employer. What are your next steps with this patient? What resources can you use to help her?
As long as human beings have engaged in combat, there have often been extraordinarily damaging psychiatric1 injuries among those who survive. Combat survivability today is 84% to 90%, the highest in the history of armed conflict,2,3 thanks to improvements in personal protective gear, vehicle armor, rapid casualty evacuation, and surgical resuscitation and stabilization that is “far forward” on the battlefield. These survivors are subsequently at high risk for a host of other medical conditions, which commonly include traumatic brain injury (TBI), post-traumatic stress disorder (PTSD), depression, suicide, and substance abuse.4-8
Family physicians—both civilian and uniformed—may be the first to encounter these individuals. Of the more than 2.4 million US service members who have been deployed to Afghanistan or Iraq in support of Operation Enduring Freedom (OEF) or Operation Iraqi Freedom (OIF), nearly 60% are no longer on active duty.
Among this group, only half receive care from the US Department of Veterans Affairs (VA).9 Despite a concerted effort on the part of the Department of Defense (DoD) and the VA to develop and distribute effective, evidenced-based treatment protocols for veterans with combat-related conditions, major gaps remain in the care provided to combat veterans.10
This article seeks to help fill that gap by providing the information you need to recognize and treat common combat-related illness, as well as resources to help improve the quality of life for veterans and their families (TABLE 1).
Initial roadblocks to care
One of the biggest challenges in treating veterans with behavioral health issues is the fact that only 23% to 40% of those with mental illness seek care.11 Among the reasons veterans have offered for avoiding behavioral health care are a fear of the stigma associated with mental illness, concern that treatment will negatively affect their career, lack of comfort with mental health professionals, and the perception that mental health treatment is a “last resort.”12 Unfortunately, efforts by the DoD leadership to overcome these inherent biases have been largely unsuccessful13 and much work is still required to see that service members get the care they need.
Due to low rates of self-reporting, effective screening is essential. With this in mind, the DoD has implemented the deployment health assessment program (DHAP), which requires service members to be screened for common conditions within 60 days of deployment, within 30 days of returning, and again at 90 to 180 days after their return.
While the long-term effects of this program are yet to be determined, results to date are promising. Since the DHAP was implemented, there has been a significant decrease in occupationally impairing mental health problems and suicidal ideation requiring medical evacuation from a combat theater.14
FPs should begin with a simple question. Many of the 20+ million veterans living in the United States will not be wearing a uniform when they enter your office. Simply asking all of your patients, “Have you or a loved one ever served in the military?” may help you discover service-related questions or concerns.15,16 Underscoring the importance of such screening is the recent decision by the American Academy of Family Physicians to partner with First Lady Michelle Obama and Dr. Jill Biden in a new campaign called “Joining Forces,” which aims to support veterans and their families.16
Mild traumatic brain injury: Common—though overlooked
A TBI is any temporary or permanent neurologic dysfunction after a blow to the head.10,17 TBI is classified based on severity and mechanism (direct blow to the head or exposure to blast waves). Mild TBI (mTBI) is commonly referred to as a concussion and usually is not associated with loss of consciousness or altered mental status. Brain imaging results are also normal with mTBI. Severe TBI, on the other hand, is associated with prolonged loss of consciousness, altered mental status, and abnormal brain imaging results (TABLE 2).17
A unique obstacle to accurate evaluation in the field. It is important to emphasize that mTBI is a clinical diagnosis, and its detection requires honest patient communication. This can be problematic with motivated soldiers who are anxious to continue the mission and fear that any admission of symptoms might delay a return to their unit. As with a concussed athlete eager to return to the field of play, the clinical diagnosis of mTBI requires a high index of clinical suspicion and constant vigilance by the health care provider. Despite being the most common combat- related injury, mTBI is often overlooked due to the absence of obvious physical injuries.4 Recent data suggest that 28% to 60% of ser- vice members evacuated from combat have a TBI. Most of these injuries (77%) are mTBI.18-20 Improved personal protective equipment (including Kevlar helmets and body armor) and the high number of blast-related injuries are likely responsible for the high incidence of mTBI among OEF/OIF veterans.8,21,22 The prevalence of mTBI among service members not evacuated is estimated to be 20% to 30%.20
Symptoms can persist. Most patients with mTBI completely recover within 30 days of the injury. Unfortunately, 10% to 15% of mTBI patients develop chronic problems lasting months to years.4 Residual symptoms most commonly include headache, irritability, depression, sleep disturbance, impaired reasoning, memory problems, and difficulty concentrating. These symptoms are not unique to mTBI and overlap with comorbid combat diagnoses like PTSD, depression, and sleep deprivation.10 The following tools can help physicians determine whether mTBI is present.
Checking for possible mtBi. In the field, patients with possible mTBI can be screened rapidly using the Military Acute Concussion Evaluation (MACE, found at www.dvbic.org), a modification of the validated and widely used Sideline Assessment of Concussion (SAC) tool. More challenging is evaluating potential mTBI patients who present weeks or months after a traumatic event, for which there are no simple confirmatory tests. In this event, conduct a thorough neurological evaluation that includes vestibular, vision, postural, and neurocognitive assessments. For patients with persistent symptoms or possible anatomic brain abnormalities, magnetic resonance imaging (MRI) is the imaging modality of choice. Patients with complications or a questionable diagnosis are best managed in consultation with a neurologist.
Initial treatment of mtBi is symptom-based. When practical, try nonpharmacologic interventions first (TABLE 3).10 In particular, have the patient avoid further high-risk exposures that could lead to second impact syndrome (an issue increasingly recognized in contact sports). Also critical are physical and cognitive rest and the restoration of sleep until the patient is completely asymptomatic.
If the patient exhibits irritability and depression, selective serotonin reuptake inhibitors (SSRIs) are first-line treatment. Avoid narcotics and sedative-hypnotic sleep medications if treating comorbidities such as pain and sleep deprivation. The VA/DoD guideline on managing concussion and mTBI provides additional detailed, evidence-based treatment recommendations.17
Reliving the horror again and again: PTSD
PTSD is a persistent and, at times, debilitating clinical syndrome that develops after exposure to a psychologically traumatic event. It’s the second most common illness among OEF/OIF combat veterans, with an estimated prevalence of 3% to 20%, a finding consistent with prior wars.6,23-25 In the case of combat veterans, the inciting event usually involves an actual or perceived risk of death or serious injury. The individual’s response to the event involves intense fear, helplessness, or horror. The traumatic event is persistently re-experienced through intrusive and disturbing recollections or dreams that cause intense psychological distress. This, in turn, leads to a state of persistent sympathetic arousal. As symptoms are often triggered by specific cues, individuals with PTSD actively seek to avoid thoughts, situations, or stimuli associated with the event.23,26
Symptoms commonly associated with PTSD include difficulty falling or staying asleep, recurrent nightmares, hypervigilance, and an exaggerated startle response. Individuals with PTSD also have a poorer sense of well-being, a higher rate of work absenteeism, and significantly more somatic complaints than age-matched peers.27 For symptoms to be attributable to PTSD, their onset must follow a recent inciting event and must also cause clinically significant distress or impairment in social, occupational, or other areas of daily living. Common comorbid illnesses include mTBI, depression, and substance abuse. As with mTBI, the presence of multiple comorbidities in patients with PTSD can complicate evaluation, diagnosis, and treatment.
Diagnosis. PTSD is subdivided into acute (symptoms lasting more than one month but less than 3 months after the traumatic event) and chronic (symptoms lasting longer than 3 months after the traumatic event).28 The distinction of acute or chronic does not affect treatment, but it is useful information for the patient to have regarding prognosis and eventual outcome. Like mTBI, PTSD is a clinical diagnosis made only after a thorough, structured diagnostic interview. The use of a validated, self-administered checklist, such as the Post-Traumatic Checklist-Military (PCL-M), allows for an efficient review of a patient’s symptoms and a reliable way to track treatment progress (http://www.ptsd.va.gov/professional/ pages/assessments/ptsd-checklist.asp).
Treatment Options. Effective evidence-based treatments for PTSD are cognitive behavioral therapy, eye movement desensitization and reprocessing (EMDR), and pharmacotherapy. SSRIs and serotonin- norepinephrine reuptake inhibitors (SNRIs) have the strongest evidence for pharmacologic benefit in the treatment of PTSD.28,29 Other helpful medications are prazosin for nightmares and trazodone for sleep. Family physicians can use these medications as part of a patient-centered collaboration with the rest of the integrated care team, to offer the best chance for treatment success.10,28,30
Depression: Vets are reluctant to self-report
Combat experience is a significant risk factor for major depression. Estimates of the lifetime prevalence of depression in the general US population vary from 9% to 25% in women and 5% to 12% in men. By contrast, the prevalence of depression in OIF/OEF veterans ranges from 2% to 37%.24,31,32
Screening can yield false negatives. Many combat veterans are reluctant to self-report behavioral conditions, including depression. Screening, therefore, is important to identify potential depression and allow for intervention. Validated screening tools for depression include the PHQ-2 and PHQ-9, which are easy to use in the office setting. (See http://www.cqaimh.org/pdf/ tool_phq2.pdf [PHQ-2] and http://www. integration.samhsa.gov/images/res/ PHQ%20-%20Questions.pdf [PHQ-9]). Importantly, some veterans will have a negative depression screen on return from deployment, and then test positive 6 to 12 months later.24 Explanations for the early false-negative results include the excitement of being home and patients intentionally answering questions inaccurately to avoid excessive screening at their home base.11
Treatment is most effective with a combination approach. As with most cases of depression, combining psychotherapy and psychopharmacology appears to be most effective for treating depression related to combat experience.33,34 While SSRIs and SNRIs are typical first-line pharmacologic agents, combat veterans often have comorbid mTBI, PTSD, or substance abuse issues that may influence the initial choice of therapy35 (TABLE 3).10
Suicide is on the rise in the military
Historically, the incidence of suicide has been 25% lower in military personnel than in civilian peers.36 However, between 2005 and 2009, the incidence of suicide in the Marine Corps and Army almost doubled.37 While the exact reasons remain unknown, it is likely due to prolonged and repeated deployments to a combat environment.12 While the incidence of suicide has been particularly high in the Army (22 per 100,000 active-duty and reserve personnel per year), all services have been affected. In fact, since 2009, the number of suicides among active duty service members exceeds those killed in action.37
Consider all veterans to be at risk for suicide, and screen accordingly. An effective screening tool is the Columbia-Suicide Severity Rating Scale (C-SSRS), which is able to predict those most at risk for an impending suicide attempt.34 Service members identified as high risk for suicide require unhindered access to care. The VA has worked to improve access to care and provide evidence-based point-of-care treatment strategies.38 Available resources can be found in TABLE 1.
Unfortunately, even with effective screening and treatment, not all suicides can be prevented. Studies have demonstrated that approximately 65% of service members who commit suicide had no known history of communicating their suicidal intent. Sadly, 25% of service members who committed suicide had seen a mental health provider within the previous 30 days.39
Alcohol abuse is common; opioids present a unique risk
Excessive use of alcohol and recreational and prescription drugs is common among OEF/ OIF veterans, especially those with comorbid mental health disorders. Retrospective cross-sectional studies show that 11% to 20% of OEF/OIF veterans met DSM-IV-TR diagnostic criteria for substance use disorders.40-42 At highest risk are single enlisted men under the age of 24 in the Army or Marine Corps who serve in a combat-specific capacity. Interestingly, the prevalence of substance use disorders among OEF/OIF veterans closely mirrors that reported in epidemiologic studies of Vietnam veterans (11%-14%).41 This similarity, combined with the 39% lifetime prevalence of substance use disorders among Vietnam veterans, may foreshadow a similar lifetime prevalence of substance use disorders among OEF/OIF veterans.41
Most-abused substances. Alcohol is the most commonly abused substance among OEF/OIF veterans (10%-20%).40,41,43-45 Other abused substances include opioids (prescribed or illicitly obtained), synthetic marijuana (“Spice” and “K2”), and “bath salts” (synthetic stimulants) (W.M. Sauve, MD, personal communication, August 27, 2012).
OEF/OIF veterans seem to be at particular risk for developing problems related to opioid use. A 2012 retrospective cohort study showed that veterans with non–cancer- related pain diagnoses treated with opioid analgesics had an increased risk for adverse clinical outcomes compared with those not treated with opioid analgesics (9.5% vs 4.1%; relative risk [RR]=2.33; 95% confidence interval [CI], 2.20-2.46). These outcomes included traumatic accidents, overdoses, self-inflicted injuries, and injuries related to violence. This study also demonstrated that, compared with veterans without mental illness, veterans with mental illness (particularly PTSD) and non–cancer-related pain were significantly more likely to receive opioids to treat their pain and had a higher risk of adverse clinical outcomes, including overdose.46,47
Recreational use of synthetic marijuana and “bath salts” has increased in recent years. These substances are commonly labeled “not for human consumption,” which allows them to remain outside US Food and Drug Administration (FDA) regulation and be sold legally in the United States. Efforts to prohibit the sale or possession of these drugs, including the Federal Synthetic Bath Salt Ban in 2012, have fallen short, often due to creative product ”re-engineering.”33 Synthetic marijuana and stimulants are inexpensive, readily available, and perceived by users to be safe. Health care providers are often unaware that their patients are using these products. Adverse health outcomes associated with the use of these synthetic drugs include memory loss, depression, and psychosis.
These alcohol and drug screens can help
One efficient screening tool to identify veterans at risk for alcohol abuse is the AUDIT-C, developed by the World Health Organization. This brief 3-question test identifies past-year hazardous drinking and alcohol abuse or dependence with >79% sensitivity and >56% specificity in male veterans, and >66% sensitivity and >87% specificity in female veterans. These numbers are similar to those provided by the full 10-question AUDIT.48,49 The Drug Abuse Screen Test-10 (DAST-10) provides a similar screening instrument for other substances. Condensed from the original DAST-28 instrument, the DAST-10 identifies high-risk substance abuse with 74% to 94% sensitivity and 68% to 88% specificity.3
Screen for comorbidities. When you see veterans with a diagnosis of substance abuse, also evaluate for comorbid disease. Most veterans with substance use disorders (82%-93%) have at least one other mental health diagnosis (a 45% greater risk than that of civilians with substance abuse disorders),50 most commonly PTSD, depression, anxiety, and adjustment disorders.41,44,45 A number of hypotheses exist to explain the association between substance use disorders and other mental health diagnoses (“dual diagnoses”). The prevailing theory, in both veteran and civilian populations, is that substance abuse is an attempt to self-treat mental illness. Other evidence suggests that substance abuse promotes the development of mental illness, either by leading to a higher risk for traumatic experiences (increasing the chance of developing PTSD) or through a direct biochemical mechanism. Finally, con- current substance use disorder and mental illness may be due to an undefined genetic or biological vulnerability.38,44 This complicated relationship between substance abuse and behavioral health reinforces the need for screening, early diagnosis, and a comprehensive, multidisciplinary approach to treatment.
Treatment options. Office-based treatment options for narcotic and alcohol abuse and dependency are available to family physicians. Methadone has been used since the 1950s to treat opioid addiction and remains one of the mainstays of outpatient treatment.47,51 Originally, methadone was restricted to detoxification and maintenance treatment in narcotic addiction treatment programs approved by the FDA. In 1976, this restriction was lifted, and all physicians registered with the Drug Enforcement Agency (DEA) were permitted to prescribe methadone for analgesia.
In 2002, the FDA approved buprenorphine monotherapy and the combination product buprenorphine/naloxone for the treatment of opioid addiction. The prescribing of buprenorphine products requires physicians to undergo extra training, declare to the DEA their intent to prescribe buprenorphine, and obtain a special DEA identification number.52,53 Physicians interested in finding out more about buprenorphine treatment and prescribing requirements can go to the Substance Abuse and Mental Health Services Administration (SAMHSA) Web page at http://samhsa.gov.
Naltrexone is an opioid receptor agonist that is used primarily to treat alcohol dependency, and is thought to work by reducing the craving for alcohol. Multiple studies have proven the efficacy of naltrexone in an outpatient setting when used alone or in combination with psychotherapy.54,55 If you are uncomfortable or unfamiliar with the use or prescribing of these medications, referral to a substance abuse clinic specializing in dual-diagnosis treatment (TABLE 1) may offer optimal outcomes for patients with substance abuse disorders and other mental illness.
Cognitive behavioral therapy—including coping skills training, relapse prevention, contingency management, and behavioral couples’ therapy—and 12-step treatment programs are evidence-based options for the treatment of substance abuse disorders. Behavioral counseling interventions in the primary care setting (typically lasting 5-15 minutes) result in decreases in alcohol consumption, heavy drinking episodes, drinking above recommended amounts, and the number of days spent in the hospital, but have not been demonstrated to affect mortality, alcohol-related liver problems, outpatient visits, legal problems, or quality of life.56 Resources can be found at www.niaaa.nih.gov. For patients with dual diagnoses, it is not yet known whether sequential therapy (in which substance abuse is treated first, followed by treatment of the comorbid mental illness) or concurrent therapy results in better outcomes.57
CASE Your patient’s history of recent combat service, acknowledgement of employment and behavioral difficulties, and initial screening results lead you to diagnose alcoholism and depression. Additionally, she denies any suicidal ideation, but admits to experimenting with synthetic marijuana. After some discussion, she agrees to see your clinic’s social worker, and you start her on an SSri with scheduled follow-up.
CORRESPONDENCE
Shawn Kane, MD, USASoc, Attn: Surgeon (AomD), 2929 Desert Storm Drive, Ft. Bragg, NC 28310, or PO Box 3639 Pinehurst, NC 28374; [email protected]
1. Wessely S. Risk, psychiatry and the military. Br J Psychiatry. 2005;186:459-466.
2. Gawande A. Casualties of war—military care for the wounded from Iraq and Afghanistan. N Engl J Med. 2004;351:2471-2475.
3. Kotwal RS, Montgomery HR, Kotwal BM, et al. Eliminating pre- ventable death on the battlefield. Arch Surg. 2011;146:1350-1358.
4. Belanger HG, Uomoto JM, Vanderploeg RD. The Veterans Health Administration’s (VHA’s) Polytrauma System of Care for mild traumatic brain injury: costs, benefits, and controversies. J Head Trauma Rehabil. 2009;24:4-13.
5. Galarneau MR, Woodruff SI, Dye JL, et al. Traumatic brain in- jury during Operation Iraqi Freedom: findings from the United States Navy-Marine Corps Combat Trauma Registry. J Neurosurg. 2008;108:950-957.
6. Hermann BA, Shiner B, Friedman MJ. Epidemiology and preven- tion of combat-related post-traumatic stress in OEF/OIF/OND service members. Mil Med. 2012;177:1-6.
7. Uomoto JM. Best practices in veteran traumatic brain injury care. J Head Trauma Rehabil. 2012;27:241-243.
8. Warden D. Military TBI during the Iraq and Afghanistan wars. J Head Trauma Rehabil. 2006;21:398-402.
9. Taylor BC, Hagel EM, Carlson KF, et al. Prevalence and costs of co-occurring traumatic brain injury with and without psychiatric disturbance and pain among Afghanistan and Iraq War Veteran V.A. users. Med Care. 2012;50:342-346.
10. Quinlan JD, Guaron MR, Deschere BR, et al. Care of the returning veteran. Am Fam Physician. 2010;82:43-49.
11. Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004;351:13-22.
12. Hoge CW, Castro CA. Preventing suicides in US service mem- bers and veterans: concerns after a decade of war. JAMA. 2012;308:671-672.
13. Jaffe G. New name for PTSD could mean less stigma. The Washington Post. May 5, 2012. Available at: http://articles. washingtonpost.com/2012-05-05/world/35454931_1_ptsd-post- traumatic-stress-psychiatrists. Accessed June 19, 2013.
14. Warner CH, Appenzeller GN, Parker JR, et al. Effectiveness of mental health screening and coordination of in-theater care prior to deployment to Iraq: a cohort study. Am J Psychiatry. 2011;168:378-385.
15. United States Census Bureau. Sex by age by veteran sta- tus for civilian population 18 years and over. 2010 American community survey 1-year estimates. Available at: https:// d3gqux9sl0z33u.cloudfront.net/AA/AT/gambillingonjustice- com/downloads/206273/ACS_10_1YR_B21001A.pdf. Accessed June 19, 2013.
16. American Academy of Family Physicians. Joining forces. Avail- able at: http://www.aafp.org/online/en/home/membership/ initiatives/joiningforces.html. Accessed June 19, 2013.
17. Department of Veterans Affairs and Department of Defense. Clinical Practice Guideline for Management of Concussion/Mild Traumatic Brain Injury. April 2009. Available at: http://www. healthquality.va.gov/mtbi/concussion_mtbi_full_1_0.pdf. Accessed June 19, 2013.
18. Lew HL, Poole JH, Alvarez S, et al. Soldiers with occult traumatic brain injury. Am J Phys Med Rehabil. 2005;84:393-398.
19. Marshall KR, Holland SL, Meyer KS, et al. Mild traumatic brain injury screening, diagnosis, and treatment. Mil Med. 2012;177:67- 75.
20. Terrio H, Brenner LA, Ivins BJ, et al. Traumatic brain injury screening: preliminary findings in a US Army Brigade Combat Team. J Head Trauma Rehabil. 2009;24:14-23.
21. Mossadegh S, Tai N, Midwinter M, et al. Improvised explosive de- vice related pelvi-perineal trauma: anatomic injuries and surgical management. J Trauma Acute Care Surg. 2012;73:S24-S31.
22. Okie S. Traumatic brain injury in the war zone. N Engl J Med. 2005;352:2043-2047.
23. Espinoza JM. Posttraumatic stress disorder and the perceived consequences of seeking therapy among US Army special forces operators exposed to combat. J Psychol Issues Organ Culture. 2010;1:6-28.
24. Grieger TA, Cozza SJ, Ursano RJ, et al. Posttraumatic stress dis- order and depression in battle-injured soldiers. Am J Psychiatry. 2006;163:1777-1783.
25. Hoge CW, Auchterlonie JL, Milliken CS. Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. JAMA. 2006;295:1023-1032.
26. Adler AB, Wright KM, Bliese PD, et al. A2 diagnostic criterion for combat-related posttraumatic stress disorder. J Trauma Stress. 2008;21:301-308.
27. Hoge CW, Terhakopian A, Castro CA, et al. Association of post- traumatic stress disorder with somatic symptoms, health care vis- its, and absenteeism among Iraq war veterans. Am J Psychiatry. 2007;164:150-153.
28. Department of Veterans Affairs and Department of Defense. Clin- ical Practice Guideline for Management of Post-Traumatic Stress. October 2010. Available at: http://www.healthquality.va.gov/ ptsd/cpg_PTSD-FULL-201011612.pdf. Accessed June 19, 2013.
29. Alexander W. Pharmacotherapy for post-traumatic stress disor- der in combat veterans: focus on antidepressants and atypical antipsychotic agents. P T. 2012;37:32-38.
30. Wisco BE, Marx BP, Keane TM. Screening, diagnosis, and treat- ment of post-traumatic stress disorder. Mil Med. 2012;177:7-13.
31. Gadermann AM, Engel CC, Naifeh JA, et al. Prevalence of DSM-IV major depression among U.S. military personnel: meta-analysis and simulation. Mil Med. 2012;177:47-59.
32. Seal KH, Shi Y, Cohen G, et al. Association of mental health dis- orders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307:940-947.
33. Perrone M. Many drugs remain legal after ‘bath salts’ ban. Boston. com. July 25, 2012. Available at: http://articles.boston.com/2012- 07-25/lifestyle/32850962_1_bath-salts-mdpv-synthetic-drugs. Accessed June 19, 2013.
34. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Se- verity Rating Scale: initial validity and internal consistency find- ings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266-1277.
35. Greenberg J, Tesfazion AA, Robinson CS. Screening, diagnosis, and treatment of depression. Mil Med. 2012;177:60-66.
36. Eaton KM, Messer SC, Garvey Wilson AL, et al. Strengthening the validity of population-based suicide rate comparisons: an il- lustration using U.S. military and civilian data. Suicide Life Threat Behav. 2006;36:182-191.
37. Miller M, Azrael D, Barber C, et al. A call to link data to answer pressing questions about suicide risk among veterans. Am J Pub Health. 2012;102(suppl 1):S20-S22.
38. Department of Veterans Affairs. Report of the Blue Ribbon Work Group on suicide prevention in the veteran population. June 2008. Available at: http://www.mentalhealth.va.gov/suicide_ prevention/Blue_Ribbon_Report-FINAL_June-30-08.pdf. Accessed July 18, 2013.
39. Kinn JT, Luxton DD, Reger MA, et al. Department of Defense sui- cide event report: calendar year 2010 annual report. September 2011. Available at: http://t2health.org/sites/default/files/dodser/ DoDSER_2010_Annual_Report.pdf. Accessed June 19, 2013.
40. Fontana A, Rosenheck R. Treatment-seeking veterans of Iraq and Afghanistan: comparison with veterans of previous wars. J Nerv Ment Dis. 2008;196:513-521.
41. Seal KH, Cohen G, Waldrop A, et al. Substance use disorders in Iraq and Afghanistan veterans in VA healthcare, 2001-2010: implications for screening, diagnosis and treatment. Drug Alcohol Depend. 2011;116:93-101.
42. Mirza RA, Eick-Cost A, Otto JL. The risk of mental health disor- ders among U.S. military personnel infected with human immu- nodeficiency virus, active component, U.S. Armed Forces, 2000- 2011. MSMR. 2012;19:10-13.
43. Bohnert AS, Ilgen MA, Bossarte RM, et al. Veteran status and alco- hol use in men in the United States. Mil Med. 2012;177:198-203.
44. Erbes CR, Kaler ME, Schult T, et al. Mental health diagnosis and occupational functioning in National Guard/Reserve veterans re- turning from Iraq. J Rehabil Res Dev. 2011;48:1159-1170.
45. Stecker T, Fortney J, Owen R, et al. Co-occurring medical, psychi- atric, and alcohol-related disorders among veterans returning from Iraq and Afghanistan. Psychosomatics. 2010;51:503-507.
46. Seal KH, Shi Y, Cohen G, et al. Association of mental health dis- orders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307:940-947.
47. Praveen KT, Law F, O’Shea J, et al. Opioid dependence. Am Fam Physician. 2012;86:565-566.
48. Bradley KA, Bush KR, Epler AJ, et al. Two brief alcohol-screening tests from the Alcohol Use Disorders Identification Test (AUDIT): validation in a female Veterans Affairs patient population. Arch Intern Med. 2003;163:821-829.
49. Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789-1795.
50. Farrell M, Howes S, Taylor C, et al. Substance misuse and psychi- atric comorbidity: an overview of the OPCS National Psychiatric Morbidity Survey. Addict Behav. 1998;23:909-918.
51. Toombs JD, Kral LA. Methadone treatment for pain states. Am Fam Physician. 2005;71:1353-1358.
52. Center for Substance Abuse Treatment. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. Treatment Improvement Protocol (TIP) series 40. DHHS pub- lication (SMA) 04-3939. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004. Available at: http:// buprenorphine.samhsa.gov/Bup_Guidelines.pdf. Accessed June 19, 2013.
53. U.S.DepartmentofHealthandHumanServices,SubstanceAbuse and Mental Health Services Administration Web site. About buprenorphine therapy. Available at: http://buprenorphine. samhsa.gov/about.html. Accessed June 19, 2013.
54. Volpicelli JR, Alterman AI, Hayashida M, et al. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876-880.
55. O’Brien CP, Volpicelli LA, Volpicelli JR. Naltrexone in the treat- ment of alcoholism: a clinical review. Alcohol. 1996;13:35-39.
56. Jonas DE, Garbutt JC, Amick HR, et al. Behavioral counseling after screening for alcohol misuse in primary care: a systematic review and meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2012;157:645-654.
57. van Dam D, Vedel E, Ehring T, et al. Psychological treatments for concurrent posttraumatic stress disorder and substance use dis- order: a systematic review. Clin Psychol Rev. 2012;32:202-214.
› Ask, “Have you or a loved one ever served in the military?” as a way to uncover service-related concerns. C
› Conduct a thorough neurological evaluation with suspected mild traumatic brain injury, including vestibular, vision, postural, and neuro-cognitive assessments. C
› Use the Post-Traumatic Checklist–Military to assess individuals with possible post-traumatic stress disorder. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE A 37-year-old white woman presents for an employment physical. Your nurse reports that she also has a complaint of headaches, that she scored an 8 on the Alcohol Use Disorders identification Test-consumption (AUDiT-c), and that the result on her patient health Questionnaire (phQ-2) suggests a depressive disorder. You ask the patient whether she has served in the military and discover that, in the last 4 years, she served 2 year-long tours in Afghanistan with her Army reserve unit, returning home 6 months ago. Since her return, she has lost her job due to chronic tardiness (sleeping through her alarm, she says) and admits she has “started drinking again.” Her visit with you this day is only to undergo the physical exam required by her new employer. What are your next steps with this patient? What resources can you use to help her?
As long as human beings have engaged in combat, there have often been extraordinarily damaging psychiatric1 injuries among those who survive. Combat survivability today is 84% to 90%, the highest in the history of armed conflict,2,3 thanks to improvements in personal protective gear, vehicle armor, rapid casualty evacuation, and surgical resuscitation and stabilization that is “far forward” on the battlefield. These survivors are subsequently at high risk for a host of other medical conditions, which commonly include traumatic brain injury (TBI), post-traumatic stress disorder (PTSD), depression, suicide, and substance abuse.4-8
Family physicians—both civilian and uniformed—may be the first to encounter these individuals. Of the more than 2.4 million US service members who have been deployed to Afghanistan or Iraq in support of Operation Enduring Freedom (OEF) or Operation Iraqi Freedom (OIF), nearly 60% are no longer on active duty.
Among this group, only half receive care from the US Department of Veterans Affairs (VA).9 Despite a concerted effort on the part of the Department of Defense (DoD) and the VA to develop and distribute effective, evidenced-based treatment protocols for veterans with combat-related conditions, major gaps remain in the care provided to combat veterans.10
This article seeks to help fill that gap by providing the information you need to recognize and treat common combat-related illness, as well as resources to help improve the quality of life for veterans and their families (TABLE 1).
Initial roadblocks to care
One of the biggest challenges in treating veterans with behavioral health issues is the fact that only 23% to 40% of those with mental illness seek care.11 Among the reasons veterans have offered for avoiding behavioral health care are a fear of the stigma associated with mental illness, concern that treatment will negatively affect their career, lack of comfort with mental health professionals, and the perception that mental health treatment is a “last resort.”12 Unfortunately, efforts by the DoD leadership to overcome these inherent biases have been largely unsuccessful13 and much work is still required to see that service members get the care they need.
Due to low rates of self-reporting, effective screening is essential. With this in mind, the DoD has implemented the deployment health assessment program (DHAP), which requires service members to be screened for common conditions within 60 days of deployment, within 30 days of returning, and again at 90 to 180 days after their return.
While the long-term effects of this program are yet to be determined, results to date are promising. Since the DHAP was implemented, there has been a significant decrease in occupationally impairing mental health problems and suicidal ideation requiring medical evacuation from a combat theater.14
FPs should begin with a simple question. Many of the 20+ million veterans living in the United States will not be wearing a uniform when they enter your office. Simply asking all of your patients, “Have you or a loved one ever served in the military?” may help you discover service-related questions or concerns.15,16 Underscoring the importance of such screening is the recent decision by the American Academy of Family Physicians to partner with First Lady Michelle Obama and Dr. Jill Biden in a new campaign called “Joining Forces,” which aims to support veterans and their families.16
Mild traumatic brain injury: Common—though overlooked
A TBI is any temporary or permanent neurologic dysfunction after a blow to the head.10,17 TBI is classified based on severity and mechanism (direct blow to the head or exposure to blast waves). Mild TBI (mTBI) is commonly referred to as a concussion and usually is not associated with loss of consciousness or altered mental status. Brain imaging results are also normal with mTBI. Severe TBI, on the other hand, is associated with prolonged loss of consciousness, altered mental status, and abnormal brain imaging results (TABLE 2).17
A unique obstacle to accurate evaluation in the field. It is important to emphasize that mTBI is a clinical diagnosis, and its detection requires honest patient communication. This can be problematic with motivated soldiers who are anxious to continue the mission and fear that any admission of symptoms might delay a return to their unit. As with a concussed athlete eager to return to the field of play, the clinical diagnosis of mTBI requires a high index of clinical suspicion and constant vigilance by the health care provider. Despite being the most common combat- related injury, mTBI is often overlooked due to the absence of obvious physical injuries.4 Recent data suggest that 28% to 60% of ser- vice members evacuated from combat have a TBI. Most of these injuries (77%) are mTBI.18-20 Improved personal protective equipment (including Kevlar helmets and body armor) and the high number of blast-related injuries are likely responsible for the high incidence of mTBI among OEF/OIF veterans.8,21,22 The prevalence of mTBI among service members not evacuated is estimated to be 20% to 30%.20
Symptoms can persist. Most patients with mTBI completely recover within 30 days of the injury. Unfortunately, 10% to 15% of mTBI patients develop chronic problems lasting months to years.4 Residual symptoms most commonly include headache, irritability, depression, sleep disturbance, impaired reasoning, memory problems, and difficulty concentrating. These symptoms are not unique to mTBI and overlap with comorbid combat diagnoses like PTSD, depression, and sleep deprivation.10 The following tools can help physicians determine whether mTBI is present.
Checking for possible mtBi. In the field, patients with possible mTBI can be screened rapidly using the Military Acute Concussion Evaluation (MACE, found at www.dvbic.org), a modification of the validated and widely used Sideline Assessment of Concussion (SAC) tool. More challenging is evaluating potential mTBI patients who present weeks or months after a traumatic event, for which there are no simple confirmatory tests. In this event, conduct a thorough neurological evaluation that includes vestibular, vision, postural, and neurocognitive assessments. For patients with persistent symptoms or possible anatomic brain abnormalities, magnetic resonance imaging (MRI) is the imaging modality of choice. Patients with complications or a questionable diagnosis are best managed in consultation with a neurologist.
Initial treatment of mtBi is symptom-based. When practical, try nonpharmacologic interventions first (TABLE 3).10 In particular, have the patient avoid further high-risk exposures that could lead to second impact syndrome (an issue increasingly recognized in contact sports). Also critical are physical and cognitive rest and the restoration of sleep until the patient is completely asymptomatic.
If the patient exhibits irritability and depression, selective serotonin reuptake inhibitors (SSRIs) are first-line treatment. Avoid narcotics and sedative-hypnotic sleep medications if treating comorbidities such as pain and sleep deprivation. The VA/DoD guideline on managing concussion and mTBI provides additional detailed, evidence-based treatment recommendations.17
Reliving the horror again and again: PTSD
PTSD is a persistent and, at times, debilitating clinical syndrome that develops after exposure to a psychologically traumatic event. It’s the second most common illness among OEF/OIF combat veterans, with an estimated prevalence of 3% to 20%, a finding consistent with prior wars.6,23-25 In the case of combat veterans, the inciting event usually involves an actual or perceived risk of death or serious injury. The individual’s response to the event involves intense fear, helplessness, or horror. The traumatic event is persistently re-experienced through intrusive and disturbing recollections or dreams that cause intense psychological distress. This, in turn, leads to a state of persistent sympathetic arousal. As symptoms are often triggered by specific cues, individuals with PTSD actively seek to avoid thoughts, situations, or stimuli associated with the event.23,26
Symptoms commonly associated with PTSD include difficulty falling or staying asleep, recurrent nightmares, hypervigilance, and an exaggerated startle response. Individuals with PTSD also have a poorer sense of well-being, a higher rate of work absenteeism, and significantly more somatic complaints than age-matched peers.27 For symptoms to be attributable to PTSD, their onset must follow a recent inciting event and must also cause clinically significant distress or impairment in social, occupational, or other areas of daily living. Common comorbid illnesses include mTBI, depression, and substance abuse. As with mTBI, the presence of multiple comorbidities in patients with PTSD can complicate evaluation, diagnosis, and treatment.
Diagnosis. PTSD is subdivided into acute (symptoms lasting more than one month but less than 3 months after the traumatic event) and chronic (symptoms lasting longer than 3 months after the traumatic event).28 The distinction of acute or chronic does not affect treatment, but it is useful information for the patient to have regarding prognosis and eventual outcome. Like mTBI, PTSD is a clinical diagnosis made only after a thorough, structured diagnostic interview. The use of a validated, self-administered checklist, such as the Post-Traumatic Checklist-Military (PCL-M), allows for an efficient review of a patient’s symptoms and a reliable way to track treatment progress (http://www.ptsd.va.gov/professional/ pages/assessments/ptsd-checklist.asp).
Treatment Options. Effective evidence-based treatments for PTSD are cognitive behavioral therapy, eye movement desensitization and reprocessing (EMDR), and pharmacotherapy. SSRIs and serotonin- norepinephrine reuptake inhibitors (SNRIs) have the strongest evidence for pharmacologic benefit in the treatment of PTSD.28,29 Other helpful medications are prazosin for nightmares and trazodone for sleep. Family physicians can use these medications as part of a patient-centered collaboration with the rest of the integrated care team, to offer the best chance for treatment success.10,28,30
Depression: Vets are reluctant to self-report
Combat experience is a significant risk factor for major depression. Estimates of the lifetime prevalence of depression in the general US population vary from 9% to 25% in women and 5% to 12% in men. By contrast, the prevalence of depression in OIF/OEF veterans ranges from 2% to 37%.24,31,32
Screening can yield false negatives. Many combat veterans are reluctant to self-report behavioral conditions, including depression. Screening, therefore, is important to identify potential depression and allow for intervention. Validated screening tools for depression include the PHQ-2 and PHQ-9, which are easy to use in the office setting. (See http://www.cqaimh.org/pdf/ tool_phq2.pdf [PHQ-2] and http://www. integration.samhsa.gov/images/res/ PHQ%20-%20Questions.pdf [PHQ-9]). Importantly, some veterans will have a negative depression screen on return from deployment, and then test positive 6 to 12 months later.24 Explanations for the early false-negative results include the excitement of being home and patients intentionally answering questions inaccurately to avoid excessive screening at their home base.11
Treatment is most effective with a combination approach. As with most cases of depression, combining psychotherapy and psychopharmacology appears to be most effective for treating depression related to combat experience.33,34 While SSRIs and SNRIs are typical first-line pharmacologic agents, combat veterans often have comorbid mTBI, PTSD, or substance abuse issues that may influence the initial choice of therapy35 (TABLE 3).10
Suicide is on the rise in the military
Historically, the incidence of suicide has been 25% lower in military personnel than in civilian peers.36 However, between 2005 and 2009, the incidence of suicide in the Marine Corps and Army almost doubled.37 While the exact reasons remain unknown, it is likely due to prolonged and repeated deployments to a combat environment.12 While the incidence of suicide has been particularly high in the Army (22 per 100,000 active-duty and reserve personnel per year), all services have been affected. In fact, since 2009, the number of suicides among active duty service members exceeds those killed in action.37
Consider all veterans to be at risk for suicide, and screen accordingly. An effective screening tool is the Columbia-Suicide Severity Rating Scale (C-SSRS), which is able to predict those most at risk for an impending suicide attempt.34 Service members identified as high risk for suicide require unhindered access to care. The VA has worked to improve access to care and provide evidence-based point-of-care treatment strategies.38 Available resources can be found in TABLE 1.
Unfortunately, even with effective screening and treatment, not all suicides can be prevented. Studies have demonstrated that approximately 65% of service members who commit suicide had no known history of communicating their suicidal intent. Sadly, 25% of service members who committed suicide had seen a mental health provider within the previous 30 days.39
Alcohol abuse is common; opioids present a unique risk
Excessive use of alcohol and recreational and prescription drugs is common among OEF/ OIF veterans, especially those with comorbid mental health disorders. Retrospective cross-sectional studies show that 11% to 20% of OEF/OIF veterans met DSM-IV-TR diagnostic criteria for substance use disorders.40-42 At highest risk are single enlisted men under the age of 24 in the Army or Marine Corps who serve in a combat-specific capacity. Interestingly, the prevalence of substance use disorders among OEF/OIF veterans closely mirrors that reported in epidemiologic studies of Vietnam veterans (11%-14%).41 This similarity, combined with the 39% lifetime prevalence of substance use disorders among Vietnam veterans, may foreshadow a similar lifetime prevalence of substance use disorders among OEF/OIF veterans.41
Most-abused substances. Alcohol is the most commonly abused substance among OEF/OIF veterans (10%-20%).40,41,43-45 Other abused substances include opioids (prescribed or illicitly obtained), synthetic marijuana (“Spice” and “K2”), and “bath salts” (synthetic stimulants) (W.M. Sauve, MD, personal communication, August 27, 2012).
OEF/OIF veterans seem to be at particular risk for developing problems related to opioid use. A 2012 retrospective cohort study showed that veterans with non–cancer- related pain diagnoses treated with opioid analgesics had an increased risk for adverse clinical outcomes compared with those not treated with opioid analgesics (9.5% vs 4.1%; relative risk [RR]=2.33; 95% confidence interval [CI], 2.20-2.46). These outcomes included traumatic accidents, overdoses, self-inflicted injuries, and injuries related to violence. This study also demonstrated that, compared with veterans without mental illness, veterans with mental illness (particularly PTSD) and non–cancer-related pain were significantly more likely to receive opioids to treat their pain and had a higher risk of adverse clinical outcomes, including overdose.46,47
Recreational use of synthetic marijuana and “bath salts” has increased in recent years. These substances are commonly labeled “not for human consumption,” which allows them to remain outside US Food and Drug Administration (FDA) regulation and be sold legally in the United States. Efforts to prohibit the sale or possession of these drugs, including the Federal Synthetic Bath Salt Ban in 2012, have fallen short, often due to creative product ”re-engineering.”33 Synthetic marijuana and stimulants are inexpensive, readily available, and perceived by users to be safe. Health care providers are often unaware that their patients are using these products. Adverse health outcomes associated with the use of these synthetic drugs include memory loss, depression, and psychosis.
These alcohol and drug screens can help
One efficient screening tool to identify veterans at risk for alcohol abuse is the AUDIT-C, developed by the World Health Organization. This brief 3-question test identifies past-year hazardous drinking and alcohol abuse or dependence with >79% sensitivity and >56% specificity in male veterans, and >66% sensitivity and >87% specificity in female veterans. These numbers are similar to those provided by the full 10-question AUDIT.48,49 The Drug Abuse Screen Test-10 (DAST-10) provides a similar screening instrument for other substances. Condensed from the original DAST-28 instrument, the DAST-10 identifies high-risk substance abuse with 74% to 94% sensitivity and 68% to 88% specificity.3
Screen for comorbidities. When you see veterans with a diagnosis of substance abuse, also evaluate for comorbid disease. Most veterans with substance use disorders (82%-93%) have at least one other mental health diagnosis (a 45% greater risk than that of civilians with substance abuse disorders),50 most commonly PTSD, depression, anxiety, and adjustment disorders.41,44,45 A number of hypotheses exist to explain the association between substance use disorders and other mental health diagnoses (“dual diagnoses”). The prevailing theory, in both veteran and civilian populations, is that substance abuse is an attempt to self-treat mental illness. Other evidence suggests that substance abuse promotes the development of mental illness, either by leading to a higher risk for traumatic experiences (increasing the chance of developing PTSD) or through a direct biochemical mechanism. Finally, con- current substance use disorder and mental illness may be due to an undefined genetic or biological vulnerability.38,44 This complicated relationship between substance abuse and behavioral health reinforces the need for screening, early diagnosis, and a comprehensive, multidisciplinary approach to treatment.
Treatment options. Office-based treatment options for narcotic and alcohol abuse and dependency are available to family physicians. Methadone has been used since the 1950s to treat opioid addiction and remains one of the mainstays of outpatient treatment.47,51 Originally, methadone was restricted to detoxification and maintenance treatment in narcotic addiction treatment programs approved by the FDA. In 1976, this restriction was lifted, and all physicians registered with the Drug Enforcement Agency (DEA) were permitted to prescribe methadone for analgesia.
In 2002, the FDA approved buprenorphine monotherapy and the combination product buprenorphine/naloxone for the treatment of opioid addiction. The prescribing of buprenorphine products requires physicians to undergo extra training, declare to the DEA their intent to prescribe buprenorphine, and obtain a special DEA identification number.52,53 Physicians interested in finding out more about buprenorphine treatment and prescribing requirements can go to the Substance Abuse and Mental Health Services Administration (SAMHSA) Web page at http://samhsa.gov.
Naltrexone is an opioid receptor agonist that is used primarily to treat alcohol dependency, and is thought to work by reducing the craving for alcohol. Multiple studies have proven the efficacy of naltrexone in an outpatient setting when used alone or in combination with psychotherapy.54,55 If you are uncomfortable or unfamiliar with the use or prescribing of these medications, referral to a substance abuse clinic specializing in dual-diagnosis treatment (TABLE 1) may offer optimal outcomes for patients with substance abuse disorders and other mental illness.
Cognitive behavioral therapy—including coping skills training, relapse prevention, contingency management, and behavioral couples’ therapy—and 12-step treatment programs are evidence-based options for the treatment of substance abuse disorders. Behavioral counseling interventions in the primary care setting (typically lasting 5-15 minutes) result in decreases in alcohol consumption, heavy drinking episodes, drinking above recommended amounts, and the number of days spent in the hospital, but have not been demonstrated to affect mortality, alcohol-related liver problems, outpatient visits, legal problems, or quality of life.56 Resources can be found at www.niaaa.nih.gov. For patients with dual diagnoses, it is not yet known whether sequential therapy (in which substance abuse is treated first, followed by treatment of the comorbid mental illness) or concurrent therapy results in better outcomes.57
CASE Your patient’s history of recent combat service, acknowledgement of employment and behavioral difficulties, and initial screening results lead you to diagnose alcoholism and depression. Additionally, she denies any suicidal ideation, but admits to experimenting with synthetic marijuana. After some discussion, she agrees to see your clinic’s social worker, and you start her on an SSri with scheduled follow-up.
CORRESPONDENCE
Shawn Kane, MD, USASoc, Attn: Surgeon (AomD), 2929 Desert Storm Drive, Ft. Bragg, NC 28310, or PO Box 3639 Pinehurst, NC 28374; [email protected]
› Ask, “Have you or a loved one ever served in the military?” as a way to uncover service-related concerns. C
› Conduct a thorough neurological evaluation with suspected mild traumatic brain injury, including vestibular, vision, postural, and neuro-cognitive assessments. C
› Use the Post-Traumatic Checklist–Military to assess individuals with possible post-traumatic stress disorder. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE A 37-year-old white woman presents for an employment physical. Your nurse reports that she also has a complaint of headaches, that she scored an 8 on the Alcohol Use Disorders identification Test-consumption (AUDiT-c), and that the result on her patient health Questionnaire (phQ-2) suggests a depressive disorder. You ask the patient whether she has served in the military and discover that, in the last 4 years, she served 2 year-long tours in Afghanistan with her Army reserve unit, returning home 6 months ago. Since her return, she has lost her job due to chronic tardiness (sleeping through her alarm, she says) and admits she has “started drinking again.” Her visit with you this day is only to undergo the physical exam required by her new employer. What are your next steps with this patient? What resources can you use to help her?
As long as human beings have engaged in combat, there have often been extraordinarily damaging psychiatric1 injuries among those who survive. Combat survivability today is 84% to 90%, the highest in the history of armed conflict,2,3 thanks to improvements in personal protective gear, vehicle armor, rapid casualty evacuation, and surgical resuscitation and stabilization that is “far forward” on the battlefield. These survivors are subsequently at high risk for a host of other medical conditions, which commonly include traumatic brain injury (TBI), post-traumatic stress disorder (PTSD), depression, suicide, and substance abuse.4-8
Family physicians—both civilian and uniformed—may be the first to encounter these individuals. Of the more than 2.4 million US service members who have been deployed to Afghanistan or Iraq in support of Operation Enduring Freedom (OEF) or Operation Iraqi Freedom (OIF), nearly 60% are no longer on active duty.
Among this group, only half receive care from the US Department of Veterans Affairs (VA).9 Despite a concerted effort on the part of the Department of Defense (DoD) and the VA to develop and distribute effective, evidenced-based treatment protocols for veterans with combat-related conditions, major gaps remain in the care provided to combat veterans.10
This article seeks to help fill that gap by providing the information you need to recognize and treat common combat-related illness, as well as resources to help improve the quality of life for veterans and their families (TABLE 1).
Initial roadblocks to care
One of the biggest challenges in treating veterans with behavioral health issues is the fact that only 23% to 40% of those with mental illness seek care.11 Among the reasons veterans have offered for avoiding behavioral health care are a fear of the stigma associated with mental illness, concern that treatment will negatively affect their career, lack of comfort with mental health professionals, and the perception that mental health treatment is a “last resort.”12 Unfortunately, efforts by the DoD leadership to overcome these inherent biases have been largely unsuccessful13 and much work is still required to see that service members get the care they need.
Due to low rates of self-reporting, effective screening is essential. With this in mind, the DoD has implemented the deployment health assessment program (DHAP), which requires service members to be screened for common conditions within 60 days of deployment, within 30 days of returning, and again at 90 to 180 days after their return.
While the long-term effects of this program are yet to be determined, results to date are promising. Since the DHAP was implemented, there has been a significant decrease in occupationally impairing mental health problems and suicidal ideation requiring medical evacuation from a combat theater.14
FPs should begin with a simple question. Many of the 20+ million veterans living in the United States will not be wearing a uniform when they enter your office. Simply asking all of your patients, “Have you or a loved one ever served in the military?” may help you discover service-related questions or concerns.15,16 Underscoring the importance of such screening is the recent decision by the American Academy of Family Physicians to partner with First Lady Michelle Obama and Dr. Jill Biden in a new campaign called “Joining Forces,” which aims to support veterans and their families.16
Mild traumatic brain injury: Common—though overlooked
A TBI is any temporary or permanent neurologic dysfunction after a blow to the head.10,17 TBI is classified based on severity and mechanism (direct blow to the head or exposure to blast waves). Mild TBI (mTBI) is commonly referred to as a concussion and usually is not associated with loss of consciousness or altered mental status. Brain imaging results are also normal with mTBI. Severe TBI, on the other hand, is associated with prolonged loss of consciousness, altered mental status, and abnormal brain imaging results (TABLE 2).17
A unique obstacle to accurate evaluation in the field. It is important to emphasize that mTBI is a clinical diagnosis, and its detection requires honest patient communication. This can be problematic with motivated soldiers who are anxious to continue the mission and fear that any admission of symptoms might delay a return to their unit. As with a concussed athlete eager to return to the field of play, the clinical diagnosis of mTBI requires a high index of clinical suspicion and constant vigilance by the health care provider. Despite being the most common combat- related injury, mTBI is often overlooked due to the absence of obvious physical injuries.4 Recent data suggest that 28% to 60% of ser- vice members evacuated from combat have a TBI. Most of these injuries (77%) are mTBI.18-20 Improved personal protective equipment (including Kevlar helmets and body armor) and the high number of blast-related injuries are likely responsible for the high incidence of mTBI among OEF/OIF veterans.8,21,22 The prevalence of mTBI among service members not evacuated is estimated to be 20% to 30%.20
Symptoms can persist. Most patients with mTBI completely recover within 30 days of the injury. Unfortunately, 10% to 15% of mTBI patients develop chronic problems lasting months to years.4 Residual symptoms most commonly include headache, irritability, depression, sleep disturbance, impaired reasoning, memory problems, and difficulty concentrating. These symptoms are not unique to mTBI and overlap with comorbid combat diagnoses like PTSD, depression, and sleep deprivation.10 The following tools can help physicians determine whether mTBI is present.
Checking for possible mtBi. In the field, patients with possible mTBI can be screened rapidly using the Military Acute Concussion Evaluation (MACE, found at www.dvbic.org), a modification of the validated and widely used Sideline Assessment of Concussion (SAC) tool. More challenging is evaluating potential mTBI patients who present weeks or months after a traumatic event, for which there are no simple confirmatory tests. In this event, conduct a thorough neurological evaluation that includes vestibular, vision, postural, and neurocognitive assessments. For patients with persistent symptoms or possible anatomic brain abnormalities, magnetic resonance imaging (MRI) is the imaging modality of choice. Patients with complications or a questionable diagnosis are best managed in consultation with a neurologist.
Initial treatment of mtBi is symptom-based. When practical, try nonpharmacologic interventions first (TABLE 3).10 In particular, have the patient avoid further high-risk exposures that could lead to second impact syndrome (an issue increasingly recognized in contact sports). Also critical are physical and cognitive rest and the restoration of sleep until the patient is completely asymptomatic.
If the patient exhibits irritability and depression, selective serotonin reuptake inhibitors (SSRIs) are first-line treatment. Avoid narcotics and sedative-hypnotic sleep medications if treating comorbidities such as pain and sleep deprivation. The VA/DoD guideline on managing concussion and mTBI provides additional detailed, evidence-based treatment recommendations.17
Reliving the horror again and again: PTSD
PTSD is a persistent and, at times, debilitating clinical syndrome that develops after exposure to a psychologically traumatic event. It’s the second most common illness among OEF/OIF combat veterans, with an estimated prevalence of 3% to 20%, a finding consistent with prior wars.6,23-25 In the case of combat veterans, the inciting event usually involves an actual or perceived risk of death or serious injury. The individual’s response to the event involves intense fear, helplessness, or horror. The traumatic event is persistently re-experienced through intrusive and disturbing recollections or dreams that cause intense psychological distress. This, in turn, leads to a state of persistent sympathetic arousal. As symptoms are often triggered by specific cues, individuals with PTSD actively seek to avoid thoughts, situations, or stimuli associated with the event.23,26
Symptoms commonly associated with PTSD include difficulty falling or staying asleep, recurrent nightmares, hypervigilance, and an exaggerated startle response. Individuals with PTSD also have a poorer sense of well-being, a higher rate of work absenteeism, and significantly more somatic complaints than age-matched peers.27 For symptoms to be attributable to PTSD, their onset must follow a recent inciting event and must also cause clinically significant distress or impairment in social, occupational, or other areas of daily living. Common comorbid illnesses include mTBI, depression, and substance abuse. As with mTBI, the presence of multiple comorbidities in patients with PTSD can complicate evaluation, diagnosis, and treatment.
Diagnosis. PTSD is subdivided into acute (symptoms lasting more than one month but less than 3 months after the traumatic event) and chronic (symptoms lasting longer than 3 months after the traumatic event).28 The distinction of acute or chronic does not affect treatment, but it is useful information for the patient to have regarding prognosis and eventual outcome. Like mTBI, PTSD is a clinical diagnosis made only after a thorough, structured diagnostic interview. The use of a validated, self-administered checklist, such as the Post-Traumatic Checklist-Military (PCL-M), allows for an efficient review of a patient’s symptoms and a reliable way to track treatment progress (http://www.ptsd.va.gov/professional/ pages/assessments/ptsd-checklist.asp).
Treatment Options. Effective evidence-based treatments for PTSD are cognitive behavioral therapy, eye movement desensitization and reprocessing (EMDR), and pharmacotherapy. SSRIs and serotonin- norepinephrine reuptake inhibitors (SNRIs) have the strongest evidence for pharmacologic benefit in the treatment of PTSD.28,29 Other helpful medications are prazosin for nightmares and trazodone for sleep. Family physicians can use these medications as part of a patient-centered collaboration with the rest of the integrated care team, to offer the best chance for treatment success.10,28,30
Depression: Vets are reluctant to self-report
Combat experience is a significant risk factor for major depression. Estimates of the lifetime prevalence of depression in the general US population vary from 9% to 25% in women and 5% to 12% in men. By contrast, the prevalence of depression in OIF/OEF veterans ranges from 2% to 37%.24,31,32
Screening can yield false negatives. Many combat veterans are reluctant to self-report behavioral conditions, including depression. Screening, therefore, is important to identify potential depression and allow for intervention. Validated screening tools for depression include the PHQ-2 and PHQ-9, which are easy to use in the office setting. (See http://www.cqaimh.org/pdf/ tool_phq2.pdf [PHQ-2] and http://www. integration.samhsa.gov/images/res/ PHQ%20-%20Questions.pdf [PHQ-9]). Importantly, some veterans will have a negative depression screen on return from deployment, and then test positive 6 to 12 months later.24 Explanations for the early false-negative results include the excitement of being home and patients intentionally answering questions inaccurately to avoid excessive screening at their home base.11
Treatment is most effective with a combination approach. As with most cases of depression, combining psychotherapy and psychopharmacology appears to be most effective for treating depression related to combat experience.33,34 While SSRIs and SNRIs are typical first-line pharmacologic agents, combat veterans often have comorbid mTBI, PTSD, or substance abuse issues that may influence the initial choice of therapy35 (TABLE 3).10
Suicide is on the rise in the military
Historically, the incidence of suicide has been 25% lower in military personnel than in civilian peers.36 However, between 2005 and 2009, the incidence of suicide in the Marine Corps and Army almost doubled.37 While the exact reasons remain unknown, it is likely due to prolonged and repeated deployments to a combat environment.12 While the incidence of suicide has been particularly high in the Army (22 per 100,000 active-duty and reserve personnel per year), all services have been affected. In fact, since 2009, the number of suicides among active duty service members exceeds those killed in action.37
Consider all veterans to be at risk for suicide, and screen accordingly. An effective screening tool is the Columbia-Suicide Severity Rating Scale (C-SSRS), which is able to predict those most at risk for an impending suicide attempt.34 Service members identified as high risk for suicide require unhindered access to care. The VA has worked to improve access to care and provide evidence-based point-of-care treatment strategies.38 Available resources can be found in TABLE 1.
Unfortunately, even with effective screening and treatment, not all suicides can be prevented. Studies have demonstrated that approximately 65% of service members who commit suicide had no known history of communicating their suicidal intent. Sadly, 25% of service members who committed suicide had seen a mental health provider within the previous 30 days.39
Alcohol abuse is common; opioids present a unique risk
Excessive use of alcohol and recreational and prescription drugs is common among OEF/ OIF veterans, especially those with comorbid mental health disorders. Retrospective cross-sectional studies show that 11% to 20% of OEF/OIF veterans met DSM-IV-TR diagnostic criteria for substance use disorders.40-42 At highest risk are single enlisted men under the age of 24 in the Army or Marine Corps who serve in a combat-specific capacity. Interestingly, the prevalence of substance use disorders among OEF/OIF veterans closely mirrors that reported in epidemiologic studies of Vietnam veterans (11%-14%).41 This similarity, combined with the 39% lifetime prevalence of substance use disorders among Vietnam veterans, may foreshadow a similar lifetime prevalence of substance use disorders among OEF/OIF veterans.41
Most-abused substances. Alcohol is the most commonly abused substance among OEF/OIF veterans (10%-20%).40,41,43-45 Other abused substances include opioids (prescribed or illicitly obtained), synthetic marijuana (“Spice” and “K2”), and “bath salts” (synthetic stimulants) (W.M. Sauve, MD, personal communication, August 27, 2012).
OEF/OIF veterans seem to be at particular risk for developing problems related to opioid use. A 2012 retrospective cohort study showed that veterans with non–cancer- related pain diagnoses treated with opioid analgesics had an increased risk for adverse clinical outcomes compared with those not treated with opioid analgesics (9.5% vs 4.1%; relative risk [RR]=2.33; 95% confidence interval [CI], 2.20-2.46). These outcomes included traumatic accidents, overdoses, self-inflicted injuries, and injuries related to violence. This study also demonstrated that, compared with veterans without mental illness, veterans with mental illness (particularly PTSD) and non–cancer-related pain were significantly more likely to receive opioids to treat their pain and had a higher risk of adverse clinical outcomes, including overdose.46,47
Recreational use of synthetic marijuana and “bath salts” has increased in recent years. These substances are commonly labeled “not for human consumption,” which allows them to remain outside US Food and Drug Administration (FDA) regulation and be sold legally in the United States. Efforts to prohibit the sale or possession of these drugs, including the Federal Synthetic Bath Salt Ban in 2012, have fallen short, often due to creative product ”re-engineering.”33 Synthetic marijuana and stimulants are inexpensive, readily available, and perceived by users to be safe. Health care providers are often unaware that their patients are using these products. Adverse health outcomes associated with the use of these synthetic drugs include memory loss, depression, and psychosis.
These alcohol and drug screens can help
One efficient screening tool to identify veterans at risk for alcohol abuse is the AUDIT-C, developed by the World Health Organization. This brief 3-question test identifies past-year hazardous drinking and alcohol abuse or dependence with >79% sensitivity and >56% specificity in male veterans, and >66% sensitivity and >87% specificity in female veterans. These numbers are similar to those provided by the full 10-question AUDIT.48,49 The Drug Abuse Screen Test-10 (DAST-10) provides a similar screening instrument for other substances. Condensed from the original DAST-28 instrument, the DAST-10 identifies high-risk substance abuse with 74% to 94% sensitivity and 68% to 88% specificity.3
Screen for comorbidities. When you see veterans with a diagnosis of substance abuse, also evaluate for comorbid disease. Most veterans with substance use disorders (82%-93%) have at least one other mental health diagnosis (a 45% greater risk than that of civilians with substance abuse disorders),50 most commonly PTSD, depression, anxiety, and adjustment disorders.41,44,45 A number of hypotheses exist to explain the association between substance use disorders and other mental health diagnoses (“dual diagnoses”). The prevailing theory, in both veteran and civilian populations, is that substance abuse is an attempt to self-treat mental illness. Other evidence suggests that substance abuse promotes the development of mental illness, either by leading to a higher risk for traumatic experiences (increasing the chance of developing PTSD) or through a direct biochemical mechanism. Finally, con- current substance use disorder and mental illness may be due to an undefined genetic or biological vulnerability.38,44 This complicated relationship between substance abuse and behavioral health reinforces the need for screening, early diagnosis, and a comprehensive, multidisciplinary approach to treatment.
Treatment options. Office-based treatment options for narcotic and alcohol abuse and dependency are available to family physicians. Methadone has been used since the 1950s to treat opioid addiction and remains one of the mainstays of outpatient treatment.47,51 Originally, methadone was restricted to detoxification and maintenance treatment in narcotic addiction treatment programs approved by the FDA. In 1976, this restriction was lifted, and all physicians registered with the Drug Enforcement Agency (DEA) were permitted to prescribe methadone for analgesia.
In 2002, the FDA approved buprenorphine monotherapy and the combination product buprenorphine/naloxone for the treatment of opioid addiction. The prescribing of buprenorphine products requires physicians to undergo extra training, declare to the DEA their intent to prescribe buprenorphine, and obtain a special DEA identification number.52,53 Physicians interested in finding out more about buprenorphine treatment and prescribing requirements can go to the Substance Abuse and Mental Health Services Administration (SAMHSA) Web page at http://samhsa.gov.
Naltrexone is an opioid receptor agonist that is used primarily to treat alcohol dependency, and is thought to work by reducing the craving for alcohol. Multiple studies have proven the efficacy of naltrexone in an outpatient setting when used alone or in combination with psychotherapy.54,55 If you are uncomfortable or unfamiliar with the use or prescribing of these medications, referral to a substance abuse clinic specializing in dual-diagnosis treatment (TABLE 1) may offer optimal outcomes for patients with substance abuse disorders and other mental illness.
Cognitive behavioral therapy—including coping skills training, relapse prevention, contingency management, and behavioral couples’ therapy—and 12-step treatment programs are evidence-based options for the treatment of substance abuse disorders. Behavioral counseling interventions in the primary care setting (typically lasting 5-15 minutes) result in decreases in alcohol consumption, heavy drinking episodes, drinking above recommended amounts, and the number of days spent in the hospital, but have not been demonstrated to affect mortality, alcohol-related liver problems, outpatient visits, legal problems, or quality of life.56 Resources can be found at www.niaaa.nih.gov. For patients with dual diagnoses, it is not yet known whether sequential therapy (in which substance abuse is treated first, followed by treatment of the comorbid mental illness) or concurrent therapy results in better outcomes.57
CASE Your patient’s history of recent combat service, acknowledgement of employment and behavioral difficulties, and initial screening results lead you to diagnose alcoholism and depression. Additionally, she denies any suicidal ideation, but admits to experimenting with synthetic marijuana. After some discussion, she agrees to see your clinic’s social worker, and you start her on an SSri with scheduled follow-up.
CORRESPONDENCE
Shawn Kane, MD, USASoc, Attn: Surgeon (AomD), 2929 Desert Storm Drive, Ft. Bragg, NC 28310, or PO Box 3639 Pinehurst, NC 28374; [email protected]
1. Wessely S. Risk, psychiatry and the military. Br J Psychiatry. 2005;186:459-466.
2. Gawande A. Casualties of war—military care for the wounded from Iraq and Afghanistan. N Engl J Med. 2004;351:2471-2475.
3. Kotwal RS, Montgomery HR, Kotwal BM, et al. Eliminating pre- ventable death on the battlefield. Arch Surg. 2011;146:1350-1358.
4. Belanger HG, Uomoto JM, Vanderploeg RD. The Veterans Health Administration’s (VHA’s) Polytrauma System of Care for mild traumatic brain injury: costs, benefits, and controversies. J Head Trauma Rehabil. 2009;24:4-13.
5. Galarneau MR, Woodruff SI, Dye JL, et al. Traumatic brain in- jury during Operation Iraqi Freedom: findings from the United States Navy-Marine Corps Combat Trauma Registry. J Neurosurg. 2008;108:950-957.
6. Hermann BA, Shiner B, Friedman MJ. Epidemiology and preven- tion of combat-related post-traumatic stress in OEF/OIF/OND service members. Mil Med. 2012;177:1-6.
7. Uomoto JM. Best practices in veteran traumatic brain injury care. J Head Trauma Rehabil. 2012;27:241-243.
8. Warden D. Military TBI during the Iraq and Afghanistan wars. J Head Trauma Rehabil. 2006;21:398-402.
9. Taylor BC, Hagel EM, Carlson KF, et al. Prevalence and costs of co-occurring traumatic brain injury with and without psychiatric disturbance and pain among Afghanistan and Iraq War Veteran V.A. users. Med Care. 2012;50:342-346.
10. Quinlan JD, Guaron MR, Deschere BR, et al. Care of the returning veteran. Am Fam Physician. 2010;82:43-49.
11. Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004;351:13-22.
12. Hoge CW, Castro CA. Preventing suicides in US service mem- bers and veterans: concerns after a decade of war. JAMA. 2012;308:671-672.
13. Jaffe G. New name for PTSD could mean less stigma. The Washington Post. May 5, 2012. Available at: http://articles. washingtonpost.com/2012-05-05/world/35454931_1_ptsd-post- traumatic-stress-psychiatrists. Accessed June 19, 2013.
14. Warner CH, Appenzeller GN, Parker JR, et al. Effectiveness of mental health screening and coordination of in-theater care prior to deployment to Iraq: a cohort study. Am J Psychiatry. 2011;168:378-385.
15. United States Census Bureau. Sex by age by veteran sta- tus for civilian population 18 years and over. 2010 American community survey 1-year estimates. Available at: https:// d3gqux9sl0z33u.cloudfront.net/AA/AT/gambillingonjustice- com/downloads/206273/ACS_10_1YR_B21001A.pdf. Accessed June 19, 2013.
16. American Academy of Family Physicians. Joining forces. Avail- able at: http://www.aafp.org/online/en/home/membership/ initiatives/joiningforces.html. Accessed June 19, 2013.
17. Department of Veterans Affairs and Department of Defense. Clinical Practice Guideline for Management of Concussion/Mild Traumatic Brain Injury. April 2009. Available at: http://www. healthquality.va.gov/mtbi/concussion_mtbi_full_1_0.pdf. Accessed June 19, 2013.
18. Lew HL, Poole JH, Alvarez S, et al. Soldiers with occult traumatic brain injury. Am J Phys Med Rehabil. 2005;84:393-398.
19. Marshall KR, Holland SL, Meyer KS, et al. Mild traumatic brain injury screening, diagnosis, and treatment. Mil Med. 2012;177:67- 75.
20. Terrio H, Brenner LA, Ivins BJ, et al. Traumatic brain injury screening: preliminary findings in a US Army Brigade Combat Team. J Head Trauma Rehabil. 2009;24:14-23.
21. Mossadegh S, Tai N, Midwinter M, et al. Improvised explosive de- vice related pelvi-perineal trauma: anatomic injuries and surgical management. J Trauma Acute Care Surg. 2012;73:S24-S31.
22. Okie S. Traumatic brain injury in the war zone. N Engl J Med. 2005;352:2043-2047.
23. Espinoza JM. Posttraumatic stress disorder and the perceived consequences of seeking therapy among US Army special forces operators exposed to combat. J Psychol Issues Organ Culture. 2010;1:6-28.
24. Grieger TA, Cozza SJ, Ursano RJ, et al. Posttraumatic stress dis- order and depression in battle-injured soldiers. Am J Psychiatry. 2006;163:1777-1783.
25. Hoge CW, Auchterlonie JL, Milliken CS. Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. JAMA. 2006;295:1023-1032.
26. Adler AB, Wright KM, Bliese PD, et al. A2 diagnostic criterion for combat-related posttraumatic stress disorder. J Trauma Stress. 2008;21:301-308.
27. Hoge CW, Terhakopian A, Castro CA, et al. Association of post- traumatic stress disorder with somatic symptoms, health care vis- its, and absenteeism among Iraq war veterans. Am J Psychiatry. 2007;164:150-153.
28. Department of Veterans Affairs and Department of Defense. Clin- ical Practice Guideline for Management of Post-Traumatic Stress. October 2010. Available at: http://www.healthquality.va.gov/ ptsd/cpg_PTSD-FULL-201011612.pdf. Accessed June 19, 2013.
29. Alexander W. Pharmacotherapy for post-traumatic stress disor- der in combat veterans: focus on antidepressants and atypical antipsychotic agents. P T. 2012;37:32-38.
30. Wisco BE, Marx BP, Keane TM. Screening, diagnosis, and treat- ment of post-traumatic stress disorder. Mil Med. 2012;177:7-13.
31. Gadermann AM, Engel CC, Naifeh JA, et al. Prevalence of DSM-IV major depression among U.S. military personnel: meta-analysis and simulation. Mil Med. 2012;177:47-59.
32. Seal KH, Shi Y, Cohen G, et al. Association of mental health dis- orders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307:940-947.
33. Perrone M. Many drugs remain legal after ‘bath salts’ ban. Boston. com. July 25, 2012. Available at: http://articles.boston.com/2012- 07-25/lifestyle/32850962_1_bath-salts-mdpv-synthetic-drugs. Accessed June 19, 2013.
34. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Se- verity Rating Scale: initial validity and internal consistency find- ings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266-1277.
35. Greenberg J, Tesfazion AA, Robinson CS. Screening, diagnosis, and treatment of depression. Mil Med. 2012;177:60-66.
36. Eaton KM, Messer SC, Garvey Wilson AL, et al. Strengthening the validity of population-based suicide rate comparisons: an il- lustration using U.S. military and civilian data. Suicide Life Threat Behav. 2006;36:182-191.
37. Miller M, Azrael D, Barber C, et al. A call to link data to answer pressing questions about suicide risk among veterans. Am J Pub Health. 2012;102(suppl 1):S20-S22.
38. Department of Veterans Affairs. Report of the Blue Ribbon Work Group on suicide prevention in the veteran population. June 2008. Available at: http://www.mentalhealth.va.gov/suicide_ prevention/Blue_Ribbon_Report-FINAL_June-30-08.pdf. Accessed July 18, 2013.
39. Kinn JT, Luxton DD, Reger MA, et al. Department of Defense sui- cide event report: calendar year 2010 annual report. September 2011. Available at: http://t2health.org/sites/default/files/dodser/ DoDSER_2010_Annual_Report.pdf. Accessed June 19, 2013.
40. Fontana A, Rosenheck R. Treatment-seeking veterans of Iraq and Afghanistan: comparison with veterans of previous wars. J Nerv Ment Dis. 2008;196:513-521.
41. Seal KH, Cohen G, Waldrop A, et al. Substance use disorders in Iraq and Afghanistan veterans in VA healthcare, 2001-2010: implications for screening, diagnosis and treatment. Drug Alcohol Depend. 2011;116:93-101.
42. Mirza RA, Eick-Cost A, Otto JL. The risk of mental health disor- ders among U.S. military personnel infected with human immu- nodeficiency virus, active component, U.S. Armed Forces, 2000- 2011. MSMR. 2012;19:10-13.
43. Bohnert AS, Ilgen MA, Bossarte RM, et al. Veteran status and alco- hol use in men in the United States. Mil Med. 2012;177:198-203.
44. Erbes CR, Kaler ME, Schult T, et al. Mental health diagnosis and occupational functioning in National Guard/Reserve veterans re- turning from Iraq. J Rehabil Res Dev. 2011;48:1159-1170.
45. Stecker T, Fortney J, Owen R, et al. Co-occurring medical, psychi- atric, and alcohol-related disorders among veterans returning from Iraq and Afghanistan. Psychosomatics. 2010;51:503-507.
46. Seal KH, Shi Y, Cohen G, et al. Association of mental health dis- orders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307:940-947.
47. Praveen KT, Law F, O’Shea J, et al. Opioid dependence. Am Fam Physician. 2012;86:565-566.
48. Bradley KA, Bush KR, Epler AJ, et al. Two brief alcohol-screening tests from the Alcohol Use Disorders Identification Test (AUDIT): validation in a female Veterans Affairs patient population. Arch Intern Med. 2003;163:821-829.
49. Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789-1795.
50. Farrell M, Howes S, Taylor C, et al. Substance misuse and psychi- atric comorbidity: an overview of the OPCS National Psychiatric Morbidity Survey. Addict Behav. 1998;23:909-918.
51. Toombs JD, Kral LA. Methadone treatment for pain states. Am Fam Physician. 2005;71:1353-1358.
52. Center for Substance Abuse Treatment. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. Treatment Improvement Protocol (TIP) series 40. DHHS pub- lication (SMA) 04-3939. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004. Available at: http:// buprenorphine.samhsa.gov/Bup_Guidelines.pdf. Accessed June 19, 2013.
53. U.S.DepartmentofHealthandHumanServices,SubstanceAbuse and Mental Health Services Administration Web site. About buprenorphine therapy. Available at: http://buprenorphine. samhsa.gov/about.html. Accessed June 19, 2013.
54. Volpicelli JR, Alterman AI, Hayashida M, et al. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876-880.
55. O’Brien CP, Volpicelli LA, Volpicelli JR. Naltrexone in the treat- ment of alcoholism: a clinical review. Alcohol. 1996;13:35-39.
56. Jonas DE, Garbutt JC, Amick HR, et al. Behavioral counseling after screening for alcohol misuse in primary care: a systematic review and meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2012;157:645-654.
57. van Dam D, Vedel E, Ehring T, et al. Psychological treatments for concurrent posttraumatic stress disorder and substance use dis- order: a systematic review. Clin Psychol Rev. 2012;32:202-214.
1. Wessely S. Risk, psychiatry and the military. Br J Psychiatry. 2005;186:459-466.
2. Gawande A. Casualties of war—military care for the wounded from Iraq and Afghanistan. N Engl J Med. 2004;351:2471-2475.
3. Kotwal RS, Montgomery HR, Kotwal BM, et al. Eliminating pre- ventable death on the battlefield. Arch Surg. 2011;146:1350-1358.
4. Belanger HG, Uomoto JM, Vanderploeg RD. The Veterans Health Administration’s (VHA’s) Polytrauma System of Care for mild traumatic brain injury: costs, benefits, and controversies. J Head Trauma Rehabil. 2009;24:4-13.
5. Galarneau MR, Woodruff SI, Dye JL, et al. Traumatic brain in- jury during Operation Iraqi Freedom: findings from the United States Navy-Marine Corps Combat Trauma Registry. J Neurosurg. 2008;108:950-957.
6. Hermann BA, Shiner B, Friedman MJ. Epidemiology and preven- tion of combat-related post-traumatic stress in OEF/OIF/OND service members. Mil Med. 2012;177:1-6.
7. Uomoto JM. Best practices in veteran traumatic brain injury care. J Head Trauma Rehabil. 2012;27:241-243.
8. Warden D. Military TBI during the Iraq and Afghanistan wars. J Head Trauma Rehabil. 2006;21:398-402.
9. Taylor BC, Hagel EM, Carlson KF, et al. Prevalence and costs of co-occurring traumatic brain injury with and without psychiatric disturbance and pain among Afghanistan and Iraq War Veteran V.A. users. Med Care. 2012;50:342-346.
10. Quinlan JD, Guaron MR, Deschere BR, et al. Care of the returning veteran. Am Fam Physician. 2010;82:43-49.
11. Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004;351:13-22.
12. Hoge CW, Castro CA. Preventing suicides in US service mem- bers and veterans: concerns after a decade of war. JAMA. 2012;308:671-672.
13. Jaffe G. New name for PTSD could mean less stigma. The Washington Post. May 5, 2012. Available at: http://articles. washingtonpost.com/2012-05-05/world/35454931_1_ptsd-post- traumatic-stress-psychiatrists. Accessed June 19, 2013.
14. Warner CH, Appenzeller GN, Parker JR, et al. Effectiveness of mental health screening and coordination of in-theater care prior to deployment to Iraq: a cohort study. Am J Psychiatry. 2011;168:378-385.
15. United States Census Bureau. Sex by age by veteran sta- tus for civilian population 18 years and over. 2010 American community survey 1-year estimates. Available at: https:// d3gqux9sl0z33u.cloudfront.net/AA/AT/gambillingonjustice- com/downloads/206273/ACS_10_1YR_B21001A.pdf. Accessed June 19, 2013.
16. American Academy of Family Physicians. Joining forces. Avail- able at: http://www.aafp.org/online/en/home/membership/ initiatives/joiningforces.html. Accessed June 19, 2013.
17. Department of Veterans Affairs and Department of Defense. Clinical Practice Guideline for Management of Concussion/Mild Traumatic Brain Injury. April 2009. Available at: http://www. healthquality.va.gov/mtbi/concussion_mtbi_full_1_0.pdf. Accessed June 19, 2013.
18. Lew HL, Poole JH, Alvarez S, et al. Soldiers with occult traumatic brain injury. Am J Phys Med Rehabil. 2005;84:393-398.
19. Marshall KR, Holland SL, Meyer KS, et al. Mild traumatic brain injury screening, diagnosis, and treatment. Mil Med. 2012;177:67- 75.
20. Terrio H, Brenner LA, Ivins BJ, et al. Traumatic brain injury screening: preliminary findings in a US Army Brigade Combat Team. J Head Trauma Rehabil. 2009;24:14-23.
21. Mossadegh S, Tai N, Midwinter M, et al. Improvised explosive de- vice related pelvi-perineal trauma: anatomic injuries and surgical management. J Trauma Acute Care Surg. 2012;73:S24-S31.
22. Okie S. Traumatic brain injury in the war zone. N Engl J Med. 2005;352:2043-2047.
23. Espinoza JM. Posttraumatic stress disorder and the perceived consequences of seeking therapy among US Army special forces operators exposed to combat. J Psychol Issues Organ Culture. 2010;1:6-28.
24. Grieger TA, Cozza SJ, Ursano RJ, et al. Posttraumatic stress dis- order and depression in battle-injured soldiers. Am J Psychiatry. 2006;163:1777-1783.
25. Hoge CW, Auchterlonie JL, Milliken CS. Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. JAMA. 2006;295:1023-1032.
26. Adler AB, Wright KM, Bliese PD, et al. A2 diagnostic criterion for combat-related posttraumatic stress disorder. J Trauma Stress. 2008;21:301-308.
27. Hoge CW, Terhakopian A, Castro CA, et al. Association of post- traumatic stress disorder with somatic symptoms, health care vis- its, and absenteeism among Iraq war veterans. Am J Psychiatry. 2007;164:150-153.
28. Department of Veterans Affairs and Department of Defense. Clin- ical Practice Guideline for Management of Post-Traumatic Stress. October 2010. Available at: http://www.healthquality.va.gov/ ptsd/cpg_PTSD-FULL-201011612.pdf. Accessed June 19, 2013.
29. Alexander W. Pharmacotherapy for post-traumatic stress disor- der in combat veterans: focus on antidepressants and atypical antipsychotic agents. P T. 2012;37:32-38.
30. Wisco BE, Marx BP, Keane TM. Screening, diagnosis, and treat- ment of post-traumatic stress disorder. Mil Med. 2012;177:7-13.
31. Gadermann AM, Engel CC, Naifeh JA, et al. Prevalence of DSM-IV major depression among U.S. military personnel: meta-analysis and simulation. Mil Med. 2012;177:47-59.
32. Seal KH, Shi Y, Cohen G, et al. Association of mental health dis- orders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307:940-947.
33. Perrone M. Many drugs remain legal after ‘bath salts’ ban. Boston. com. July 25, 2012. Available at: http://articles.boston.com/2012- 07-25/lifestyle/32850962_1_bath-salts-mdpv-synthetic-drugs. Accessed June 19, 2013.
34. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Se- verity Rating Scale: initial validity and internal consistency find- ings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266-1277.
35. Greenberg J, Tesfazion AA, Robinson CS. Screening, diagnosis, and treatment of depression. Mil Med. 2012;177:60-66.
36. Eaton KM, Messer SC, Garvey Wilson AL, et al. Strengthening the validity of population-based suicide rate comparisons: an il- lustration using U.S. military and civilian data. Suicide Life Threat Behav. 2006;36:182-191.
37. Miller M, Azrael D, Barber C, et al. A call to link data to answer pressing questions about suicide risk among veterans. Am J Pub Health. 2012;102(suppl 1):S20-S22.
38. Department of Veterans Affairs. Report of the Blue Ribbon Work Group on suicide prevention in the veteran population. June 2008. Available at: http://www.mentalhealth.va.gov/suicide_ prevention/Blue_Ribbon_Report-FINAL_June-30-08.pdf. Accessed July 18, 2013.
39. Kinn JT, Luxton DD, Reger MA, et al. Department of Defense sui- cide event report: calendar year 2010 annual report. September 2011. Available at: http://t2health.org/sites/default/files/dodser/ DoDSER_2010_Annual_Report.pdf. Accessed June 19, 2013.
40. Fontana A, Rosenheck R. Treatment-seeking veterans of Iraq and Afghanistan: comparison with veterans of previous wars. J Nerv Ment Dis. 2008;196:513-521.
41. Seal KH, Cohen G, Waldrop A, et al. Substance use disorders in Iraq and Afghanistan veterans in VA healthcare, 2001-2010: implications for screening, diagnosis and treatment. Drug Alcohol Depend. 2011;116:93-101.
42. Mirza RA, Eick-Cost A, Otto JL. The risk of mental health disor- ders among U.S. military personnel infected with human immu- nodeficiency virus, active component, U.S. Armed Forces, 2000- 2011. MSMR. 2012;19:10-13.
43. Bohnert AS, Ilgen MA, Bossarte RM, et al. Veteran status and alco- hol use in men in the United States. Mil Med. 2012;177:198-203.
44. Erbes CR, Kaler ME, Schult T, et al. Mental health diagnosis and occupational functioning in National Guard/Reserve veterans re- turning from Iraq. J Rehabil Res Dev. 2011;48:1159-1170.
45. Stecker T, Fortney J, Owen R, et al. Co-occurring medical, psychi- atric, and alcohol-related disorders among veterans returning from Iraq and Afghanistan. Psychosomatics. 2010;51:503-507.
46. Seal KH, Shi Y, Cohen G, et al. Association of mental health dis- orders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307:940-947.
47. Praveen KT, Law F, O’Shea J, et al. Opioid dependence. Am Fam Physician. 2012;86:565-566.
48. Bradley KA, Bush KR, Epler AJ, et al. Two brief alcohol-screening tests from the Alcohol Use Disorders Identification Test (AUDIT): validation in a female Veterans Affairs patient population. Arch Intern Med. 2003;163:821-829.
49. Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789-1795.
50. Farrell M, Howes S, Taylor C, et al. Substance misuse and psychi- atric comorbidity: an overview of the OPCS National Psychiatric Morbidity Survey. Addict Behav. 1998;23:909-918.
51. Toombs JD, Kral LA. Methadone treatment for pain states. Am Fam Physician. 2005;71:1353-1358.
52. Center for Substance Abuse Treatment. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. Treatment Improvement Protocol (TIP) series 40. DHHS pub- lication (SMA) 04-3939. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004. Available at: http:// buprenorphine.samhsa.gov/Bup_Guidelines.pdf. Accessed June 19, 2013.
53. U.S.DepartmentofHealthandHumanServices,SubstanceAbuse and Mental Health Services Administration Web site. About buprenorphine therapy. Available at: http://buprenorphine. samhsa.gov/about.html. Accessed June 19, 2013.
54. Volpicelli JR, Alterman AI, Hayashida M, et al. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876-880.
55. O’Brien CP, Volpicelli LA, Volpicelli JR. Naltrexone in the treat- ment of alcoholism: a clinical review. Alcohol. 1996;13:35-39.
56. Jonas DE, Garbutt JC, Amick HR, et al. Behavioral counseling after screening for alcohol misuse in primary care: a systematic review and meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2012;157:645-654.
57. van Dam D, Vedel E, Ehring T, et al. Psychological treatments for concurrent posttraumatic stress disorder and substance use dis- order: a systematic review. Clin Psychol Rev. 2012;32:202-214.
What is the clinical workup for failure to thrive?
The clinical evaluation of failure to thrive (FTT) includes a thorough history and physical examination; observation of parent–child interactions; observation and documentation of the child’s feeding patterns; and a home visit by an appropriately trained health care professional (Strength of Recommendation [SOR]: C). Further diagnostic testing should be performed as indicated by positive findings from the history and physical exam or if the child’s weight has not improved at follow-up (SOR: C).
A complex problem that requires a team approach
Robert Gauer, MD
Family Medicine Residency Clinic Faculty, Womack Army Medical Center, Fort Bragg, NC
We admit several infants with FTT to the hospital each month from a large population of young families at Fort Bragg, NC, and manage many more in our outpatient practice. Our experience confirms that FTT is a complex problem with many potential causes.
Laboratory and other evaluation beyond history, physical examination, and observation rarely help establish the diagnosis or prognosis. Incidental abnormalities occasionally change management, but more often result in false positives.
Close follow-up and a multidisciplinary team approach generally uncover the cause and lead to successful treatment. Children who don’t respond to treatment or have a suspected “organic” cause of FTT always warrant further laboratory investigation to identify the 1% of cases that result from a diagnosable disease. FTT can also be the sole indication of neglect or nonaccidental trauma, with devastating consequences.
Early identification of an infant or child approaching the diagnostic criteria for FTT is critical. Diagnosis and intervention may be delayed by inaccurate growth curve points, loss of a growth chart in a busy practice, or lack of well-child visits. Our experience with early detection and a multidisciplinary team treatment approach has been highly successful.
Evidence summary
FTT is a generic term used to describe a child whose current weight (or trajectory of weight gain) does not equal that of other children of similar age, gender, and ethnicity. No single accepted anthropometric measure can be used to diagnose the condition.1
FTT has been variously defined in children who:
- drop more than 2 standard percentile lines on standardized growth charts,2
- are below the third percentile for weight,
- have weight-for-length <80% of ideal weight,3
- have height-for-weight less than the third percentile,4
- have weight-for-height less than the 10th percentile, or have weight-for-age less than 2 standard deviations below the mean for age.
Recent updates of standardized growth charts for children are available from the Centers for Disease Control and Prevention at www.cdc.gov/nchs/about/major/nhanes/growthcharts/clinical_charts.htm.
A complex diagnosis
FTT occurs when nutritive intake is insufficient to meet demands for growth (TABLE). It is usually manifested by failure to gain weight. In more severe cases, height and head circumference are affected. FTT is also associated with lower developmental testing scores,5 persistent poor growth, increased susceptibility to infections, and an increased prevalence of behavioral disorders and neurologic disability.4 As many as 10% of children seen in primary-care settings show signs of FTT.2
Children with FTT are most often identified when parents raise concerns about the child’s feeding or growth patterns or when a physician notes a decrease in the child’s growth on physical examination. The terms “organic” and “inorganic” or “nonorganic” FTT, often used to guide diagnostic thinking, are outdated because most cases of FTT are influenced by many variables.6 FTT represents the final common pathway of disruptions in the complex system of biological, psychosocial, and environmental factors contributing to a child’s growth and development.
TABLE
Failure to thrive: Causes and physical findings
| GENERIC CAUSE | ASSOCIATED CONDITIONS | PHYSICAL FINDINGS* | DIAGNOSTIC EVALUATION |
|---|---|---|---|
| Inadequate caloric intake | Poor food intake Chronic illness Inappropriate type/volume of feeding Food not available Parental withholding Neglect Poverty | Signs of neglect or abuse Minimal subcutaneous fat Protuberant abdomen | Complete dietary history and psychosocial evaluation Complete blood count (CBC) Basic metabolic profile Lead screening |
| Inadequate caloric absorption | Gastrointestinal causes Malabsorption Chronic vomiting Pancreatic insufficiency Celiac disease Chronic reflux Inflammatory bowel disease Chronic renal disease Cystic fibrosis | Dysmorphism suggestive of chronic disease Organomegaly Skin/mucosal changes | Stool pathogens Stool fat Cystic fibrosis screening CBC/erythrocyte sedimentation rate (ESR) Basic metabolic profile Urinalysis (U/A) |
| Excessive caloric expenditure | Hyperthyroidism Chronic disease (cardiac, renal, endocrine, hepatic) Malignancy | Dysmporphism Skin dysmorphology Cardiac findings | TSH CBC/ESR Basic metabolic profile Liver function tests |
| *Abnormal weight is observed in all cases. | |||
| Modified from Skuse DH et al., .8 Bergman P et al., .13 Krugman SD et al.14 | |||
FTT has 3 basic causes
1. Inadequate caloric intake. More than 80% of children with poor growth do not have an underlying medical disorder.7 The initial workup, therefore, should include a thorough dietary and psychosocial history. Find out exactly what the child eats, how often he eats, and what behaviors he exhibits at mealtimes.
A detailed prenatal history (including birth weight and pregnancy complications) and medical history for both the child and parents can identify underlying metabolic, endocrine, or familial disorders. It is always important to look for signs of child abuse, because children with FTT are more likely to be victims of abuse than normal-weight peers.3 That said, other factors are responsible for poor nutritional intake in as many as 80% of cases.8
2. Inadequate caloric absorption (mal-absorption). This usually results from persistent emesis or malabsorption. Emesis can be caused by reflux, obstruction, medication, food sensitivities, or underlying metabolic disease. Malabsorption most often arises from chronic diarrhea, celiac disease, protein-losing enteropathy, food sensitivities, or excessive juice intake.
3. Excessive caloric expenditure. Such expenditure is associated with underlying medical conditions such as congenital heart disease, chronic hypoxia (pulmonary disease), hyperthyroidism, metabolic disease (diabetes, renal tubular acidosis), chronic immunodeficiency, recurrent infection, or malignancy.
FTT accounts for between 1% and 5% of all pediatric hospitalizations.9 Children who continue to exhibit poor growth despite adequate outpatient evaluation should be admitted to the hospital. Admission is also indicated if abuse is suspected.
Recommendations
The American Academy of Pediatrics recommends that physicians consider child neglect as a cause of FTT, particularly in cases that do not resolve with appropriate medical intervention.3
The American Gastroenterological Association10 and World Gastroenterology Organization11 recommend that physicians consider celiac sprue in children presenting with FTT. Interestingly, the Cochrane Database of Systematic Reviews suggests that there is little systematic evidence to support routine growth monitoring in children.12
1. Olsen EM. Failure to thrive: still a problem of definition. Clin Pediatr. 2006;45(1):1-6.
2. Wright CM. Identification and management of failure to thrive: a community perspective. Arch Dis Child. 2000;82(1):5-9.
3. Block RW, Krebs NF. Failure to thrive as a manifestation of child neglect. Pediatrics. 2005;116:1234-1237.
4. Perrin EC, Cole CH, Frank DA, et al. Criteria for determining disability in infants and children: failure to thrive. Evid Rep Technol Assess (Summ). 2003;(72):1-5.
5. Rudolf MC, Logan S. What is the long-term outcome for children who fail to thrive? A systematic review. Arch Dis Child. 2005;90:925-931.
6. Gahagan S, Holmes R. A stepwise approach to evaluation of undernutrition and failure to thrive. Pediatr Clin North Am. 1998;45:169-187.
7. Gahagan S. Failure to thrive: a consequence of undernutrition. Pediatr Rev. 2006;27:e1-11.
8. Skuse DH, Gill D, Reilly S, Wolke D, et al. Failure to thrive and the risk of child abuse: a prospective population survey. J Med Screen. 1995;2:145-149.
9. Shah MD. Failure to thrive in children. J Clin Gastroenterol. 2002;35:371-374.
10. American Gastroenterological Association. American Gastroenterological Association medical position statement: celiac sprue. Gastroenterology. 2001;120:1522-1525.
11. WGO-OMGE practice guideline: celiac disease 2005. Available at: www.worldgastroenterology.org/globalguidelines/guide13/guideline13.htm. Accessed September 30, 2007.
12. Panpanich R, Garner P. Growth monitoring in children. Cochrane Database Syst Rev. 1999;(4):CD001443.-
13. Bergman P, Graham J. An approach to “failure to thrive.” Aust Fam Physician. 2005;34:725-729.
14. Krugman SD, Dubowitz H. Failure to thrive. Am Fam Physician. 2003;68:879-884.
The clinical evaluation of failure to thrive (FTT) includes a thorough history and physical examination; observation of parent–child interactions; observation and documentation of the child’s feeding patterns; and a home visit by an appropriately trained health care professional (Strength of Recommendation [SOR]: C). Further diagnostic testing should be performed as indicated by positive findings from the history and physical exam or if the child’s weight has not improved at follow-up (SOR: C).
A complex problem that requires a team approach
Robert Gauer, MD
Family Medicine Residency Clinic Faculty, Womack Army Medical Center, Fort Bragg, NC
We admit several infants with FTT to the hospital each month from a large population of young families at Fort Bragg, NC, and manage many more in our outpatient practice. Our experience confirms that FTT is a complex problem with many potential causes.
Laboratory and other evaluation beyond history, physical examination, and observation rarely help establish the diagnosis or prognosis. Incidental abnormalities occasionally change management, but more often result in false positives.
Close follow-up and a multidisciplinary team approach generally uncover the cause and lead to successful treatment. Children who don’t respond to treatment or have a suspected “organic” cause of FTT always warrant further laboratory investigation to identify the 1% of cases that result from a diagnosable disease. FTT can also be the sole indication of neglect or nonaccidental trauma, with devastating consequences.
Early identification of an infant or child approaching the diagnostic criteria for FTT is critical. Diagnosis and intervention may be delayed by inaccurate growth curve points, loss of a growth chart in a busy practice, or lack of well-child visits. Our experience with early detection and a multidisciplinary team treatment approach has been highly successful.
Evidence summary
FTT is a generic term used to describe a child whose current weight (or trajectory of weight gain) does not equal that of other children of similar age, gender, and ethnicity. No single accepted anthropometric measure can be used to diagnose the condition.1
FTT has been variously defined in children who:
- drop more than 2 standard percentile lines on standardized growth charts,2
- are below the third percentile for weight,
- have weight-for-length <80% of ideal weight,3
- have height-for-weight less than the third percentile,4
- have weight-for-height less than the 10th percentile, or have weight-for-age less than 2 standard deviations below the mean for age.
Recent updates of standardized growth charts for children are available from the Centers for Disease Control and Prevention at www.cdc.gov/nchs/about/major/nhanes/growthcharts/clinical_charts.htm.
A complex diagnosis
FTT occurs when nutritive intake is insufficient to meet demands for growth (TABLE). It is usually manifested by failure to gain weight. In more severe cases, height and head circumference are affected. FTT is also associated with lower developmental testing scores,5 persistent poor growth, increased susceptibility to infections, and an increased prevalence of behavioral disorders and neurologic disability.4 As many as 10% of children seen in primary-care settings show signs of FTT.2
Children with FTT are most often identified when parents raise concerns about the child’s feeding or growth patterns or when a physician notes a decrease in the child’s growth on physical examination. The terms “organic” and “inorganic” or “nonorganic” FTT, often used to guide diagnostic thinking, are outdated because most cases of FTT are influenced by many variables.6 FTT represents the final common pathway of disruptions in the complex system of biological, psychosocial, and environmental factors contributing to a child’s growth and development.
TABLE
Failure to thrive: Causes and physical findings
| GENERIC CAUSE | ASSOCIATED CONDITIONS | PHYSICAL FINDINGS* | DIAGNOSTIC EVALUATION |
|---|---|---|---|
| Inadequate caloric intake | Poor food intake Chronic illness Inappropriate type/volume of feeding Food not available Parental withholding Neglect Poverty | Signs of neglect or abuse Minimal subcutaneous fat Protuberant abdomen | Complete dietary history and psychosocial evaluation Complete blood count (CBC) Basic metabolic profile Lead screening |
| Inadequate caloric absorption | Gastrointestinal causes Malabsorption Chronic vomiting Pancreatic insufficiency Celiac disease Chronic reflux Inflammatory bowel disease Chronic renal disease Cystic fibrosis | Dysmorphism suggestive of chronic disease Organomegaly Skin/mucosal changes | Stool pathogens Stool fat Cystic fibrosis screening CBC/erythrocyte sedimentation rate (ESR) Basic metabolic profile Urinalysis (U/A) |
| Excessive caloric expenditure | Hyperthyroidism Chronic disease (cardiac, renal, endocrine, hepatic) Malignancy | Dysmporphism Skin dysmorphology Cardiac findings | TSH CBC/ESR Basic metabolic profile Liver function tests |
| *Abnormal weight is observed in all cases. | |||
| Modified from Skuse DH et al., .8 Bergman P et al., .13 Krugman SD et al.14 | |||
FTT has 3 basic causes
1. Inadequate caloric intake. More than 80% of children with poor growth do not have an underlying medical disorder.7 The initial workup, therefore, should include a thorough dietary and psychosocial history. Find out exactly what the child eats, how often he eats, and what behaviors he exhibits at mealtimes.
A detailed prenatal history (including birth weight and pregnancy complications) and medical history for both the child and parents can identify underlying metabolic, endocrine, or familial disorders. It is always important to look for signs of child abuse, because children with FTT are more likely to be victims of abuse than normal-weight peers.3 That said, other factors are responsible for poor nutritional intake in as many as 80% of cases.8
2. Inadequate caloric absorption (mal-absorption). This usually results from persistent emesis or malabsorption. Emesis can be caused by reflux, obstruction, medication, food sensitivities, or underlying metabolic disease. Malabsorption most often arises from chronic diarrhea, celiac disease, protein-losing enteropathy, food sensitivities, or excessive juice intake.
3. Excessive caloric expenditure. Such expenditure is associated with underlying medical conditions such as congenital heart disease, chronic hypoxia (pulmonary disease), hyperthyroidism, metabolic disease (diabetes, renal tubular acidosis), chronic immunodeficiency, recurrent infection, or malignancy.
FTT accounts for between 1% and 5% of all pediatric hospitalizations.9 Children who continue to exhibit poor growth despite adequate outpatient evaluation should be admitted to the hospital. Admission is also indicated if abuse is suspected.
Recommendations
The American Academy of Pediatrics recommends that physicians consider child neglect as a cause of FTT, particularly in cases that do not resolve with appropriate medical intervention.3
The American Gastroenterological Association10 and World Gastroenterology Organization11 recommend that physicians consider celiac sprue in children presenting with FTT. Interestingly, the Cochrane Database of Systematic Reviews suggests that there is little systematic evidence to support routine growth monitoring in children.12
The clinical evaluation of failure to thrive (FTT) includes a thorough history and physical examination; observation of parent–child interactions; observation and documentation of the child’s feeding patterns; and a home visit by an appropriately trained health care professional (Strength of Recommendation [SOR]: C). Further diagnostic testing should be performed as indicated by positive findings from the history and physical exam or if the child’s weight has not improved at follow-up (SOR: C).
A complex problem that requires a team approach
Robert Gauer, MD
Family Medicine Residency Clinic Faculty, Womack Army Medical Center, Fort Bragg, NC
We admit several infants with FTT to the hospital each month from a large population of young families at Fort Bragg, NC, and manage many more in our outpatient practice. Our experience confirms that FTT is a complex problem with many potential causes.
Laboratory and other evaluation beyond history, physical examination, and observation rarely help establish the diagnosis or prognosis. Incidental abnormalities occasionally change management, but more often result in false positives.
Close follow-up and a multidisciplinary team approach generally uncover the cause and lead to successful treatment. Children who don’t respond to treatment or have a suspected “organic” cause of FTT always warrant further laboratory investigation to identify the 1% of cases that result from a diagnosable disease. FTT can also be the sole indication of neglect or nonaccidental trauma, with devastating consequences.
Early identification of an infant or child approaching the diagnostic criteria for FTT is critical. Diagnosis and intervention may be delayed by inaccurate growth curve points, loss of a growth chart in a busy practice, or lack of well-child visits. Our experience with early detection and a multidisciplinary team treatment approach has been highly successful.
Evidence summary
FTT is a generic term used to describe a child whose current weight (or trajectory of weight gain) does not equal that of other children of similar age, gender, and ethnicity. No single accepted anthropometric measure can be used to diagnose the condition.1
FTT has been variously defined in children who:
- drop more than 2 standard percentile lines on standardized growth charts,2
- are below the third percentile for weight,
- have weight-for-length <80% of ideal weight,3
- have height-for-weight less than the third percentile,4
- have weight-for-height less than the 10th percentile, or have weight-for-age less than 2 standard deviations below the mean for age.
Recent updates of standardized growth charts for children are available from the Centers for Disease Control and Prevention at www.cdc.gov/nchs/about/major/nhanes/growthcharts/clinical_charts.htm.
A complex diagnosis
FTT occurs when nutritive intake is insufficient to meet demands for growth (TABLE). It is usually manifested by failure to gain weight. In more severe cases, height and head circumference are affected. FTT is also associated with lower developmental testing scores,5 persistent poor growth, increased susceptibility to infections, and an increased prevalence of behavioral disorders and neurologic disability.4 As many as 10% of children seen in primary-care settings show signs of FTT.2
Children with FTT are most often identified when parents raise concerns about the child’s feeding or growth patterns or when a physician notes a decrease in the child’s growth on physical examination. The terms “organic” and “inorganic” or “nonorganic” FTT, often used to guide diagnostic thinking, are outdated because most cases of FTT are influenced by many variables.6 FTT represents the final common pathway of disruptions in the complex system of biological, psychosocial, and environmental factors contributing to a child’s growth and development.
TABLE
Failure to thrive: Causes and physical findings
| GENERIC CAUSE | ASSOCIATED CONDITIONS | PHYSICAL FINDINGS* | DIAGNOSTIC EVALUATION |
|---|---|---|---|
| Inadequate caloric intake | Poor food intake Chronic illness Inappropriate type/volume of feeding Food not available Parental withholding Neglect Poverty | Signs of neglect or abuse Minimal subcutaneous fat Protuberant abdomen | Complete dietary history and psychosocial evaluation Complete blood count (CBC) Basic metabolic profile Lead screening |
| Inadequate caloric absorption | Gastrointestinal causes Malabsorption Chronic vomiting Pancreatic insufficiency Celiac disease Chronic reflux Inflammatory bowel disease Chronic renal disease Cystic fibrosis | Dysmorphism suggestive of chronic disease Organomegaly Skin/mucosal changes | Stool pathogens Stool fat Cystic fibrosis screening CBC/erythrocyte sedimentation rate (ESR) Basic metabolic profile Urinalysis (U/A) |
| Excessive caloric expenditure | Hyperthyroidism Chronic disease (cardiac, renal, endocrine, hepatic) Malignancy | Dysmporphism Skin dysmorphology Cardiac findings | TSH CBC/ESR Basic metabolic profile Liver function tests |
| *Abnormal weight is observed in all cases. | |||
| Modified from Skuse DH et al., .8 Bergman P et al., .13 Krugman SD et al.14 | |||
FTT has 3 basic causes
1. Inadequate caloric intake. More than 80% of children with poor growth do not have an underlying medical disorder.7 The initial workup, therefore, should include a thorough dietary and psychosocial history. Find out exactly what the child eats, how often he eats, and what behaviors he exhibits at mealtimes.
A detailed prenatal history (including birth weight and pregnancy complications) and medical history for both the child and parents can identify underlying metabolic, endocrine, or familial disorders. It is always important to look for signs of child abuse, because children with FTT are more likely to be victims of abuse than normal-weight peers.3 That said, other factors are responsible for poor nutritional intake in as many as 80% of cases.8
2. Inadequate caloric absorption (mal-absorption). This usually results from persistent emesis or malabsorption. Emesis can be caused by reflux, obstruction, medication, food sensitivities, or underlying metabolic disease. Malabsorption most often arises from chronic diarrhea, celiac disease, protein-losing enteropathy, food sensitivities, or excessive juice intake.
3. Excessive caloric expenditure. Such expenditure is associated with underlying medical conditions such as congenital heart disease, chronic hypoxia (pulmonary disease), hyperthyroidism, metabolic disease (diabetes, renal tubular acidosis), chronic immunodeficiency, recurrent infection, or malignancy.
FTT accounts for between 1% and 5% of all pediatric hospitalizations.9 Children who continue to exhibit poor growth despite adequate outpatient evaluation should be admitted to the hospital. Admission is also indicated if abuse is suspected.
Recommendations
The American Academy of Pediatrics recommends that physicians consider child neglect as a cause of FTT, particularly in cases that do not resolve with appropriate medical intervention.3
The American Gastroenterological Association10 and World Gastroenterology Organization11 recommend that physicians consider celiac sprue in children presenting with FTT. Interestingly, the Cochrane Database of Systematic Reviews suggests that there is little systematic evidence to support routine growth monitoring in children.12
1. Olsen EM. Failure to thrive: still a problem of definition. Clin Pediatr. 2006;45(1):1-6.
2. Wright CM. Identification and management of failure to thrive: a community perspective. Arch Dis Child. 2000;82(1):5-9.
3. Block RW, Krebs NF. Failure to thrive as a manifestation of child neglect. Pediatrics. 2005;116:1234-1237.
4. Perrin EC, Cole CH, Frank DA, et al. Criteria for determining disability in infants and children: failure to thrive. Evid Rep Technol Assess (Summ). 2003;(72):1-5.
5. Rudolf MC, Logan S. What is the long-term outcome for children who fail to thrive? A systematic review. Arch Dis Child. 2005;90:925-931.
6. Gahagan S, Holmes R. A stepwise approach to evaluation of undernutrition and failure to thrive. Pediatr Clin North Am. 1998;45:169-187.
7. Gahagan S. Failure to thrive: a consequence of undernutrition. Pediatr Rev. 2006;27:e1-11.
8. Skuse DH, Gill D, Reilly S, Wolke D, et al. Failure to thrive and the risk of child abuse: a prospective population survey. J Med Screen. 1995;2:145-149.
9. Shah MD. Failure to thrive in children. J Clin Gastroenterol. 2002;35:371-374.
10. American Gastroenterological Association. American Gastroenterological Association medical position statement: celiac sprue. Gastroenterology. 2001;120:1522-1525.
11. WGO-OMGE practice guideline: celiac disease 2005. Available at: www.worldgastroenterology.org/globalguidelines/guide13/guideline13.htm. Accessed September 30, 2007.
12. Panpanich R, Garner P. Growth monitoring in children. Cochrane Database Syst Rev. 1999;(4):CD001443.-
13. Bergman P, Graham J. An approach to “failure to thrive.” Aust Fam Physician. 2005;34:725-729.
14. Krugman SD, Dubowitz H. Failure to thrive. Am Fam Physician. 2003;68:879-884.
1. Olsen EM. Failure to thrive: still a problem of definition. Clin Pediatr. 2006;45(1):1-6.
2. Wright CM. Identification and management of failure to thrive: a community perspective. Arch Dis Child. 2000;82(1):5-9.
3. Block RW, Krebs NF. Failure to thrive as a manifestation of child neglect. Pediatrics. 2005;116:1234-1237.
4. Perrin EC, Cole CH, Frank DA, et al. Criteria for determining disability in infants and children: failure to thrive. Evid Rep Technol Assess (Summ). 2003;(72):1-5.
5. Rudolf MC, Logan S. What is the long-term outcome for children who fail to thrive? A systematic review. Arch Dis Child. 2005;90:925-931.
6. Gahagan S, Holmes R. A stepwise approach to evaluation of undernutrition and failure to thrive. Pediatr Clin North Am. 1998;45:169-187.
7. Gahagan S. Failure to thrive: a consequence of undernutrition. Pediatr Rev. 2006;27:e1-11.
8. Skuse DH, Gill D, Reilly S, Wolke D, et al. Failure to thrive and the risk of child abuse: a prospective population survey. J Med Screen. 1995;2:145-149.
9. Shah MD. Failure to thrive in children. J Clin Gastroenterol. 2002;35:371-374.
10. American Gastroenterological Association. American Gastroenterological Association medical position statement: celiac sprue. Gastroenterology. 2001;120:1522-1525.
11. WGO-OMGE practice guideline: celiac disease 2005. Available at: www.worldgastroenterology.org/globalguidelines/guide13/guideline13.htm. Accessed September 30, 2007.
12. Panpanich R, Garner P. Growth monitoring in children. Cochrane Database Syst Rev. 1999;(4):CD001443.-
13. Bergman P, Graham J. An approach to “failure to thrive.” Aust Fam Physician. 2005;34:725-729.
14. Krugman SD, Dubowitz H. Failure to thrive. Am Fam Physician. 2003;68:879-884.
Evidence-based answers from the Family Physicians Inquiries Network
Estrogen-Progestin Increases Breast Cancer Risk
CLINICAL QUESTION: For postmenopausal women taking hormone replacement therapy (HRT), does the addition of progestin to estrogen increase the risk of breast cancer above the risk associated with estrogen replacement therapy (ERT) alone?
BACKGROUND: It is clear that postmenopausal HRT is associated with an increase in the risk of a diagnosis of breast cancer. This risk is related to the duration and type of HRT used. ERT and combination estrogen-progestin hormone therapy (CHRT) are the most commonly prescribed regimens. This study examines the impact of CHRT on breast cancer risk.
POPULATION STUDIED: This study is a follow-up to the Breast Cancer Detection Demonstration Project (BCDDP) originally conducted from 1973 to 1980. The original sample included 59,907 patients. Subsequent phone interviews and mailed questionnaires conducted between 1980 and 1995 tracked participants and their behaviors related to breast health. Specifically, individual risk factors for breast cancer, the use of breast cancer screening practices (particularly mammography), the use of hormone replacement therapy (type and duration), and the rate of breast-related procedures were assessed. Participants were predominantly white (86%). For this study, subjects were excluded if they had a prophylactic mastectomy or if they had used hormone replacement in shots, patches, or creams.
STUDY DESIGN AND VALIDITY: This cohort study examined follow-up BCDDP data collected between 1980 and 1995. A total of 46,355 subjects were available for analysis. Cases of breast cancer were identified in study participants, and regression analyses were used to calculate the relative risk (RR) of breast cancer associated with different patterns of HRT use. The weaknesses of this study included the ethnic homogeneity of the sample, the use of 10-year-old data to calculate body mass index (BMI), and the lack of differentiation between continuous and sequential CHRT.
OUTCOMES MEASURED: The primary outcome measured was the incidence of breast cancer relative to the type and duration of HRT.
RESULTS: A total of 2082 cases of breast cancer were identified during 473,687 person-years of accumulated follow-up (4.4% of the women). Increases in risk of breast cancer with estrogen only (RR=1.2; 95% confidence interval [CI], 1.0-1.4; number needed to harm [NNH]=1100) and estrogen-progestin (RR=1.4; 95% CI, 1.1-1.8; NNH=641) were found only with use within the previous 4 years. Current use of CHRT was also associated with an increase in breast cancer risk. Lean women (BMI <24.4 kg/m2) who had been using CHRT for at least 4 years had the highest risk of breast cancer, and there was no statistically significant increased risk of cancer in heavier women.
The combination of estrogen and progestin slightly increases the risk of breast cancer beyond that associated with estrogen alone in lean women only. The risk of breast cancer with postmenopausal HRT is most apparent in current or recent users of HRT and is related to duration of use (>4 years). An increase in the diagnosis of breast cancer in women taking postmenopausal HRT does not necessarily translate to an associated increase in breast cancer mortality.1,2 For many women the benefits of HRT may outweigh the risks. The following should be considered when counseling postmenopausal women about HRT: (1) postmenopausal HRT use leads to an annual increase in the risk of breast cancer equivalent to an extra year of remaining premenopausal; (2) the increase in breast cancer risk associated with postmenopausal HRT is particularly apparent for lean white women with current or recent HRT use; (3) postmenopausal women without a uterus who are considering HRT should take estrogen alone; and (4) preventive counseling to promote favorable diet, exercise, and lifestyle behaviors is the cornerstone of healthy aging. The use of medication to manage menopause should not be viewed as the de facto clinical standard of care.
CLINICAL QUESTION: For postmenopausal women taking hormone replacement therapy (HRT), does the addition of progestin to estrogen increase the risk of breast cancer above the risk associated with estrogen replacement therapy (ERT) alone?
BACKGROUND: It is clear that postmenopausal HRT is associated with an increase in the risk of a diagnosis of breast cancer. This risk is related to the duration and type of HRT used. ERT and combination estrogen-progestin hormone therapy (CHRT) are the most commonly prescribed regimens. This study examines the impact of CHRT on breast cancer risk.
POPULATION STUDIED: This study is a follow-up to the Breast Cancer Detection Demonstration Project (BCDDP) originally conducted from 1973 to 1980. The original sample included 59,907 patients. Subsequent phone interviews and mailed questionnaires conducted between 1980 and 1995 tracked participants and their behaviors related to breast health. Specifically, individual risk factors for breast cancer, the use of breast cancer screening practices (particularly mammography), the use of hormone replacement therapy (type and duration), and the rate of breast-related procedures were assessed. Participants were predominantly white (86%). For this study, subjects were excluded if they had a prophylactic mastectomy or if they had used hormone replacement in shots, patches, or creams.
STUDY DESIGN AND VALIDITY: This cohort study examined follow-up BCDDP data collected between 1980 and 1995. A total of 46,355 subjects were available for analysis. Cases of breast cancer were identified in study participants, and regression analyses were used to calculate the relative risk (RR) of breast cancer associated with different patterns of HRT use. The weaknesses of this study included the ethnic homogeneity of the sample, the use of 10-year-old data to calculate body mass index (BMI), and the lack of differentiation between continuous and sequential CHRT.
OUTCOMES MEASURED: The primary outcome measured was the incidence of breast cancer relative to the type and duration of HRT.
RESULTS: A total of 2082 cases of breast cancer were identified during 473,687 person-years of accumulated follow-up (4.4% of the women). Increases in risk of breast cancer with estrogen only (RR=1.2; 95% confidence interval [CI], 1.0-1.4; number needed to harm [NNH]=1100) and estrogen-progestin (RR=1.4; 95% CI, 1.1-1.8; NNH=641) were found only with use within the previous 4 years. Current use of CHRT was also associated with an increase in breast cancer risk. Lean women (BMI <24.4 kg/m2) who had been using CHRT for at least 4 years had the highest risk of breast cancer, and there was no statistically significant increased risk of cancer in heavier women.
The combination of estrogen and progestin slightly increases the risk of breast cancer beyond that associated with estrogen alone in lean women only. The risk of breast cancer with postmenopausal HRT is most apparent in current or recent users of HRT and is related to duration of use (>4 years). An increase in the diagnosis of breast cancer in women taking postmenopausal HRT does not necessarily translate to an associated increase in breast cancer mortality.1,2 For many women the benefits of HRT may outweigh the risks. The following should be considered when counseling postmenopausal women about HRT: (1) postmenopausal HRT use leads to an annual increase in the risk of breast cancer equivalent to an extra year of remaining premenopausal; (2) the increase in breast cancer risk associated with postmenopausal HRT is particularly apparent for lean white women with current or recent HRT use; (3) postmenopausal women without a uterus who are considering HRT should take estrogen alone; and (4) preventive counseling to promote favorable diet, exercise, and lifestyle behaviors is the cornerstone of healthy aging. The use of medication to manage menopause should not be viewed as the de facto clinical standard of care.
CLINICAL QUESTION: For postmenopausal women taking hormone replacement therapy (HRT), does the addition of progestin to estrogen increase the risk of breast cancer above the risk associated with estrogen replacement therapy (ERT) alone?
BACKGROUND: It is clear that postmenopausal HRT is associated with an increase in the risk of a diagnosis of breast cancer. This risk is related to the duration and type of HRT used. ERT and combination estrogen-progestin hormone therapy (CHRT) are the most commonly prescribed regimens. This study examines the impact of CHRT on breast cancer risk.
POPULATION STUDIED: This study is a follow-up to the Breast Cancer Detection Demonstration Project (BCDDP) originally conducted from 1973 to 1980. The original sample included 59,907 patients. Subsequent phone interviews and mailed questionnaires conducted between 1980 and 1995 tracked participants and their behaviors related to breast health. Specifically, individual risk factors for breast cancer, the use of breast cancer screening practices (particularly mammography), the use of hormone replacement therapy (type and duration), and the rate of breast-related procedures were assessed. Participants were predominantly white (86%). For this study, subjects were excluded if they had a prophylactic mastectomy or if they had used hormone replacement in shots, patches, or creams.
STUDY DESIGN AND VALIDITY: This cohort study examined follow-up BCDDP data collected between 1980 and 1995. A total of 46,355 subjects were available for analysis. Cases of breast cancer were identified in study participants, and regression analyses were used to calculate the relative risk (RR) of breast cancer associated with different patterns of HRT use. The weaknesses of this study included the ethnic homogeneity of the sample, the use of 10-year-old data to calculate body mass index (BMI), and the lack of differentiation between continuous and sequential CHRT.
OUTCOMES MEASURED: The primary outcome measured was the incidence of breast cancer relative to the type and duration of HRT.
RESULTS: A total of 2082 cases of breast cancer were identified during 473,687 person-years of accumulated follow-up (4.4% of the women). Increases in risk of breast cancer with estrogen only (RR=1.2; 95% confidence interval [CI], 1.0-1.4; number needed to harm [NNH]=1100) and estrogen-progestin (RR=1.4; 95% CI, 1.1-1.8; NNH=641) were found only with use within the previous 4 years. Current use of CHRT was also associated with an increase in breast cancer risk. Lean women (BMI <24.4 kg/m2) who had been using CHRT for at least 4 years had the highest risk of breast cancer, and there was no statistically significant increased risk of cancer in heavier women.
The combination of estrogen and progestin slightly increases the risk of breast cancer beyond that associated with estrogen alone in lean women only. The risk of breast cancer with postmenopausal HRT is most apparent in current or recent users of HRT and is related to duration of use (>4 years). An increase in the diagnosis of breast cancer in women taking postmenopausal HRT does not necessarily translate to an associated increase in breast cancer mortality.1,2 For many women the benefits of HRT may outweigh the risks. The following should be considered when counseling postmenopausal women about HRT: (1) postmenopausal HRT use leads to an annual increase in the risk of breast cancer equivalent to an extra year of remaining premenopausal; (2) the increase in breast cancer risk associated with postmenopausal HRT is particularly apparent for lean white women with current or recent HRT use; (3) postmenopausal women without a uterus who are considering HRT should take estrogen alone; and (4) preventive counseling to promote favorable diet, exercise, and lifestyle behaviors is the cornerstone of healthy aging. The use of medication to manage menopause should not be viewed as the de facto clinical standard of care.
Routine Preoperative Testing Before Cataract Surgery
CLINICAL QUESTION: Does routine medical testing before cataract surgery reduce the rate of perioperative complications?
BACKGROUND: Although routine medical tests (serum chemistries, complete blood counts, and electrocardiograms) are commonly ordered before elective surgery, their value is questionable. This study prospectively analyzes the usefulness of routine medical testing before cataract surgery.
POPULATION STUDIED: Patients aged older than 50 years presenting to one of 9 clinical centers for elective cataract surgery were enrolled (18,189 patients and 19,557 cataract surgeries). Study centers included private, academic, and community-based hospitals. Exclusion criteria were minimal; only patients who did not speak English or Spanish, who were scheduled for general anesthesia, who had a history of a myocardial infarction in the preceding 3 months, or who had routine preoperative medical testing 28 days before enrollment were excluded.
STUDY DESIGN AND VALIDITY: This is a randomized prospective trial. Patients were given a letter to take to their primary care physician that explained the study and whether the physician was to order routine tests. One group had a battery of routine preoperative tests, and the other did not. All patients had a complete history taken and a physical examination performed before surgery. Adverse medical events were recorded on the day of surgery (intraoperative) and for 1 week following surgery (postoperative). Each adverse event was independently documented by 2 investigators and confirmed in a blinded fashion. This study is elegant in design and execution. Patient representativeness and generalizability were addressed in the study design, and crossovers were appropriately analyzed by intention to treat. The authors were specific in their aim to maximize the generalizability of their results.
OUTCOMES MEASURED: The primary outcomes measured were recorded as adverse events. These included myocardial infarction/ischemia, congestive heart failure, arrhythmia, hypertension, hypotension, cerebral infarct/ischemia, bronchospasm, respiratory failure, hypoglycemia, diabetic ketoacidosis, and oxygen desaturation.
RESULTS: Only 5.9% of the patients crossed over from one group to another, and most of those were from routine testing to no testing. There was no difference between the rates of intraoperative events in the routine testing (19.2 events per 1000 operations) and the no-testing (19.7 events per 1000 operations) groups. There was also no significant difference between the rates of postoperative events in the routine testing (12.6 events per 1000 operations) and no-testing (12.1 events per 1000 operations) groups. Subgroup analyses revealed no benefit of routine testing among groups stratified according to age, ethnicity, sex, or health status.
Routine preoperative medical testing before elective cataract surgery does not improve patient outcomes but does increase the cost of care. It contributes nothing that cannot be elicited by a careful medical history and physical examination. This confirms the age-old dictum: Do not order a test unless you know what you are going to do with the result. In this situation, physicians should rely less on technology and more on clinical skills.
CLINICAL QUESTION: Does routine medical testing before cataract surgery reduce the rate of perioperative complications?
BACKGROUND: Although routine medical tests (serum chemistries, complete blood counts, and electrocardiograms) are commonly ordered before elective surgery, their value is questionable. This study prospectively analyzes the usefulness of routine medical testing before cataract surgery.
POPULATION STUDIED: Patients aged older than 50 years presenting to one of 9 clinical centers for elective cataract surgery were enrolled (18,189 patients and 19,557 cataract surgeries). Study centers included private, academic, and community-based hospitals. Exclusion criteria were minimal; only patients who did not speak English or Spanish, who were scheduled for general anesthesia, who had a history of a myocardial infarction in the preceding 3 months, or who had routine preoperative medical testing 28 days before enrollment were excluded.
STUDY DESIGN AND VALIDITY: This is a randomized prospective trial. Patients were given a letter to take to their primary care physician that explained the study and whether the physician was to order routine tests. One group had a battery of routine preoperative tests, and the other did not. All patients had a complete history taken and a physical examination performed before surgery. Adverse medical events were recorded on the day of surgery (intraoperative) and for 1 week following surgery (postoperative). Each adverse event was independently documented by 2 investigators and confirmed in a blinded fashion. This study is elegant in design and execution. Patient representativeness and generalizability were addressed in the study design, and crossovers were appropriately analyzed by intention to treat. The authors were specific in their aim to maximize the generalizability of their results.
OUTCOMES MEASURED: The primary outcomes measured were recorded as adverse events. These included myocardial infarction/ischemia, congestive heart failure, arrhythmia, hypertension, hypotension, cerebral infarct/ischemia, bronchospasm, respiratory failure, hypoglycemia, diabetic ketoacidosis, and oxygen desaturation.
RESULTS: Only 5.9% of the patients crossed over from one group to another, and most of those were from routine testing to no testing. There was no difference between the rates of intraoperative events in the routine testing (19.2 events per 1000 operations) and the no-testing (19.7 events per 1000 operations) groups. There was also no significant difference between the rates of postoperative events in the routine testing (12.6 events per 1000 operations) and no-testing (12.1 events per 1000 operations) groups. Subgroup analyses revealed no benefit of routine testing among groups stratified according to age, ethnicity, sex, or health status.
Routine preoperative medical testing before elective cataract surgery does not improve patient outcomes but does increase the cost of care. It contributes nothing that cannot be elicited by a careful medical history and physical examination. This confirms the age-old dictum: Do not order a test unless you know what you are going to do with the result. In this situation, physicians should rely less on technology and more on clinical skills.
CLINICAL QUESTION: Does routine medical testing before cataract surgery reduce the rate of perioperative complications?
BACKGROUND: Although routine medical tests (serum chemistries, complete blood counts, and electrocardiograms) are commonly ordered before elective surgery, their value is questionable. This study prospectively analyzes the usefulness of routine medical testing before cataract surgery.
POPULATION STUDIED: Patients aged older than 50 years presenting to one of 9 clinical centers for elective cataract surgery were enrolled (18,189 patients and 19,557 cataract surgeries). Study centers included private, academic, and community-based hospitals. Exclusion criteria were minimal; only patients who did not speak English or Spanish, who were scheduled for general anesthesia, who had a history of a myocardial infarction in the preceding 3 months, or who had routine preoperative medical testing 28 days before enrollment were excluded.
STUDY DESIGN AND VALIDITY: This is a randomized prospective trial. Patients were given a letter to take to their primary care physician that explained the study and whether the physician was to order routine tests. One group had a battery of routine preoperative tests, and the other did not. All patients had a complete history taken and a physical examination performed before surgery. Adverse medical events were recorded on the day of surgery (intraoperative) and for 1 week following surgery (postoperative). Each adverse event was independently documented by 2 investigators and confirmed in a blinded fashion. This study is elegant in design and execution. Patient representativeness and generalizability were addressed in the study design, and crossovers were appropriately analyzed by intention to treat. The authors were specific in their aim to maximize the generalizability of their results.
OUTCOMES MEASURED: The primary outcomes measured were recorded as adverse events. These included myocardial infarction/ischemia, congestive heart failure, arrhythmia, hypertension, hypotension, cerebral infarct/ischemia, bronchospasm, respiratory failure, hypoglycemia, diabetic ketoacidosis, and oxygen desaturation.
RESULTS: Only 5.9% of the patients crossed over from one group to another, and most of those were from routine testing to no testing. There was no difference between the rates of intraoperative events in the routine testing (19.2 events per 1000 operations) and the no-testing (19.7 events per 1000 operations) groups. There was also no significant difference between the rates of postoperative events in the routine testing (12.6 events per 1000 operations) and no-testing (12.1 events per 1000 operations) groups. Subgroup analyses revealed no benefit of routine testing among groups stratified according to age, ethnicity, sex, or health status.
Routine preoperative medical testing before elective cataract surgery does not improve patient outcomes but does increase the cost of care. It contributes nothing that cannot be elicited by a careful medical history and physical examination. This confirms the age-old dictum: Do not order a test unless you know what you are going to do with the result. In this situation, physicians should rely less on technology and more on clinical skills.