User login
Direct Measurement of T3 Is Likely Vital, Say Researchers
To assess thyroid function, clinicians typically determine blood levels of thyroid-stimulating hormone (TSH) and thyroxine (T4) and do not directly measure triiodothyronine (T3).
However, linking socioeconomic forces, human biology, and aging,” researchers reported.
The study, with lead author Ralph I. Lawton, an MD-PhD student at Harvard Medical School, Boston, was published online on January 4, 2024, in Proceedings of the National Academy of Sciences. In this national sample of healthy adults older than 20, free T3 was negatively related to household income. At age 40 and older, free T3 was significantly linked to being employed. Among adults from age 51 to 80, free T3 levels were inversely related to mortality.
“It is important to note that the more typically measured biomarkers for thyroid function (T4 and TSH) are poorly linked to free T3 levels,” Dr. Lawton and colleagues wrote.
The ratio of TSH:T4 explained only 1.7% of variation in T3 levels, they noted, which suggested that TSH and T4 may not be accurate surrogates of free T3. Thus, “direct measurement of free T3 is likely vital to properly stratify the effects of HPT-axis variation,” they maintained. “Improved methods for measurement [of T3] and further investigation of the role of free T3 in clinical conditions may be high yield,” according to the researchers.
Further studies of the relationship between the action of deiodinase in converting T4 to T3 and social forces and aging could elucidate critical biology. “New therapeutics targeting T3/T4 ratios,” they suggested, “may be more beneficial for a significant cohort of older individuals [with hypothyroidism] currently treated with levothyroxine [(LT4)].”
‘Cherry on Top’
“I think this is a really important paper,” thyroidology expert Antonio Bianco, MD, PhD, professor of medicine, The University of Chicago, who was not involved with this research, told this news organization.
His research group and others “have slowly been providing evidence of the relevance of measuring T3 levels,” he said. “This paper is sort of a cherry on top. It is really the most recent strong evidence that looking at T3 levels in plasma is really important. We need to do a better job at developing a much more precise assay for T3,” he said, “because now we know T3 is a very important parameter that endocrinologists should be looking for. We know that about 20 million Americans have hypothyroidism, and are being treated with T4 (levothyroxine),” Dr. Bianco continued.
“As endocrinologists, we believe that if you treat someone with levothyroxine, you normalize T3 levels. However, many times, with standard of care with levothyroxine, T3 levels are not normalized.”
Larger Variations in T3
Thyroid hormones (TSH, free T4, and free T3) influence many aspects of human physiology and behavior, but it is not known how variations in these levels in a population free of thyroid disease are associated with mortality and socioeconomic factors.
The researchers analyzed data from 7626 adults over age 20 up to age 80 who participated in the National Health and Nutrition Examination Survey during 2007-2012 and had blood tests to determine TSH, free T4, and free T3 and were not being actively treated for thyroid conditions. Among the 3603 older adults (over age 50), there were 981 deaths up until the end of 2019. TSH levels were not linked to mortality, but increasing levels of free T3 and decreasing levels of free T4 were protective for mortality (hazard ratios, 0.88 and 1.26, respectively; P < .01).
There was a robust negative relationship between household income and employment and free T3 but no relationship between these factors and T4. There was a much larger variation across age in levels of free T3 than in levels of TSH or free T4. Free T4 and free T3 diverged at older ages, when free T4 increased and free T3 continually decreased.
Older adults with symptoms of hypothyroidism may have high free T4 levels despite low free T3, the researchers noted.
“In a nonclinical sample representative of the US population,” they summarized, “we find that free T3 is much more strongly related to all domains studied — age, sex, seasonality, household income, employment, and longevity — than the other molecules of the HPT axis.”
This work was supported by the Burroughs Wellcome Fund Career at Scientific Interface award, a Brain and Behavior Research Foundation young investigator award, the Howard Hughes Medical Institute, the National Institute of Aging, and the National Institute of General Medical Sciences. Dr. Bianco served as a consultant for AbbVie, Avion Pharmaceuticals, Synthonics, Synthion, and Thyron and recently published the book Rethinking Hypothyroidism: Why Treatment Must Change and What Patients Can Do.
A version of this article appeared on Medscape.com.
To assess thyroid function, clinicians typically determine blood levels of thyroid-stimulating hormone (TSH) and thyroxine (T4) and do not directly measure triiodothyronine (T3).
However, linking socioeconomic forces, human biology, and aging,” researchers reported.
The study, with lead author Ralph I. Lawton, an MD-PhD student at Harvard Medical School, Boston, was published online on January 4, 2024, in Proceedings of the National Academy of Sciences. In this national sample of healthy adults older than 20, free T3 was negatively related to household income. At age 40 and older, free T3 was significantly linked to being employed. Among adults from age 51 to 80, free T3 levels were inversely related to mortality.
“It is important to note that the more typically measured biomarkers for thyroid function (T4 and TSH) are poorly linked to free T3 levels,” Dr. Lawton and colleagues wrote.
The ratio of TSH:T4 explained only 1.7% of variation in T3 levels, they noted, which suggested that TSH and T4 may not be accurate surrogates of free T3. Thus, “direct measurement of free T3 is likely vital to properly stratify the effects of HPT-axis variation,” they maintained. “Improved methods for measurement [of T3] and further investigation of the role of free T3 in clinical conditions may be high yield,” according to the researchers.
Further studies of the relationship between the action of deiodinase in converting T4 to T3 and social forces and aging could elucidate critical biology. “New therapeutics targeting T3/T4 ratios,” they suggested, “may be more beneficial for a significant cohort of older individuals [with hypothyroidism] currently treated with levothyroxine [(LT4)].”
‘Cherry on Top’
“I think this is a really important paper,” thyroidology expert Antonio Bianco, MD, PhD, professor of medicine, The University of Chicago, who was not involved with this research, told this news organization.
His research group and others “have slowly been providing evidence of the relevance of measuring T3 levels,” he said. “This paper is sort of a cherry on top. It is really the most recent strong evidence that looking at T3 levels in plasma is really important. We need to do a better job at developing a much more precise assay for T3,” he said, “because now we know T3 is a very important parameter that endocrinologists should be looking for. We know that about 20 million Americans have hypothyroidism, and are being treated with T4 (levothyroxine),” Dr. Bianco continued.
“As endocrinologists, we believe that if you treat someone with levothyroxine, you normalize T3 levels. However, many times, with standard of care with levothyroxine, T3 levels are not normalized.”
Larger Variations in T3
Thyroid hormones (TSH, free T4, and free T3) influence many aspects of human physiology and behavior, but it is not known how variations in these levels in a population free of thyroid disease are associated with mortality and socioeconomic factors.
The researchers analyzed data from 7626 adults over age 20 up to age 80 who participated in the National Health and Nutrition Examination Survey during 2007-2012 and had blood tests to determine TSH, free T4, and free T3 and were not being actively treated for thyroid conditions. Among the 3603 older adults (over age 50), there were 981 deaths up until the end of 2019. TSH levels were not linked to mortality, but increasing levels of free T3 and decreasing levels of free T4 were protective for mortality (hazard ratios, 0.88 and 1.26, respectively; P < .01).
There was a robust negative relationship between household income and employment and free T3 but no relationship between these factors and T4. There was a much larger variation across age in levels of free T3 than in levels of TSH or free T4. Free T4 and free T3 diverged at older ages, when free T4 increased and free T3 continually decreased.
Older adults with symptoms of hypothyroidism may have high free T4 levels despite low free T3, the researchers noted.
“In a nonclinical sample representative of the US population,” they summarized, “we find that free T3 is much more strongly related to all domains studied — age, sex, seasonality, household income, employment, and longevity — than the other molecules of the HPT axis.”
This work was supported by the Burroughs Wellcome Fund Career at Scientific Interface award, a Brain and Behavior Research Foundation young investigator award, the Howard Hughes Medical Institute, the National Institute of Aging, and the National Institute of General Medical Sciences. Dr. Bianco served as a consultant for AbbVie, Avion Pharmaceuticals, Synthonics, Synthion, and Thyron and recently published the book Rethinking Hypothyroidism: Why Treatment Must Change and What Patients Can Do.
A version of this article appeared on Medscape.com.
To assess thyroid function, clinicians typically determine blood levels of thyroid-stimulating hormone (TSH) and thyroxine (T4) and do not directly measure triiodothyronine (T3).
However, linking socioeconomic forces, human biology, and aging,” researchers reported.
The study, with lead author Ralph I. Lawton, an MD-PhD student at Harvard Medical School, Boston, was published online on January 4, 2024, in Proceedings of the National Academy of Sciences. In this national sample of healthy adults older than 20, free T3 was negatively related to household income. At age 40 and older, free T3 was significantly linked to being employed. Among adults from age 51 to 80, free T3 levels were inversely related to mortality.
“It is important to note that the more typically measured biomarkers for thyroid function (T4 and TSH) are poorly linked to free T3 levels,” Dr. Lawton and colleagues wrote.
The ratio of TSH:T4 explained only 1.7% of variation in T3 levels, they noted, which suggested that TSH and T4 may not be accurate surrogates of free T3. Thus, “direct measurement of free T3 is likely vital to properly stratify the effects of HPT-axis variation,” they maintained. “Improved methods for measurement [of T3] and further investigation of the role of free T3 in clinical conditions may be high yield,” according to the researchers.
Further studies of the relationship between the action of deiodinase in converting T4 to T3 and social forces and aging could elucidate critical biology. “New therapeutics targeting T3/T4 ratios,” they suggested, “may be more beneficial for a significant cohort of older individuals [with hypothyroidism] currently treated with levothyroxine [(LT4)].”
‘Cherry on Top’
“I think this is a really important paper,” thyroidology expert Antonio Bianco, MD, PhD, professor of medicine, The University of Chicago, who was not involved with this research, told this news organization.
His research group and others “have slowly been providing evidence of the relevance of measuring T3 levels,” he said. “This paper is sort of a cherry on top. It is really the most recent strong evidence that looking at T3 levels in plasma is really important. We need to do a better job at developing a much more precise assay for T3,” he said, “because now we know T3 is a very important parameter that endocrinologists should be looking for. We know that about 20 million Americans have hypothyroidism, and are being treated with T4 (levothyroxine),” Dr. Bianco continued.
“As endocrinologists, we believe that if you treat someone with levothyroxine, you normalize T3 levels. However, many times, with standard of care with levothyroxine, T3 levels are not normalized.”
Larger Variations in T3
Thyroid hormones (TSH, free T4, and free T3) influence many aspects of human physiology and behavior, but it is not known how variations in these levels in a population free of thyroid disease are associated with mortality and socioeconomic factors.
The researchers analyzed data from 7626 adults over age 20 up to age 80 who participated in the National Health and Nutrition Examination Survey during 2007-2012 and had blood tests to determine TSH, free T4, and free T3 and were not being actively treated for thyroid conditions. Among the 3603 older adults (over age 50), there were 981 deaths up until the end of 2019. TSH levels were not linked to mortality, but increasing levels of free T3 and decreasing levels of free T4 were protective for mortality (hazard ratios, 0.88 and 1.26, respectively; P < .01).
There was a robust negative relationship between household income and employment and free T3 but no relationship between these factors and T4. There was a much larger variation across age in levels of free T3 than in levels of TSH or free T4. Free T4 and free T3 diverged at older ages, when free T4 increased and free T3 continually decreased.
Older adults with symptoms of hypothyroidism may have high free T4 levels despite low free T3, the researchers noted.
“In a nonclinical sample representative of the US population,” they summarized, “we find that free T3 is much more strongly related to all domains studied — age, sex, seasonality, household income, employment, and longevity — than the other molecules of the HPT axis.”
This work was supported by the Burroughs Wellcome Fund Career at Scientific Interface award, a Brain and Behavior Research Foundation young investigator award, the Howard Hughes Medical Institute, the National Institute of Aging, and the National Institute of General Medical Sciences. Dr. Bianco served as a consultant for AbbVie, Avion Pharmaceuticals, Synthonics, Synthion, and Thyron and recently published the book Rethinking Hypothyroidism: Why Treatment Must Change and What Patients Can Do.
A version of this article appeared on Medscape.com.
Early age at first period raises type 2 diabetes risk
TOPLINE:
, a retrospective study of US women under age 65 found.
METHODOLOGY:
- Researchers analyzed data from 17,377 women who were aged 20-65 years when they participated in a National Health and Nutrition Examination Survey (NHANES) from 1999 to 2018 and reported their age at first menstruation, which was classified as ≤ 10, 11, 12, 13, 14, or ≥ 15 years of age.
- In total, 0.2% of the women (1773) had type 2 diabetes; of these, 11.5% (205) had cardiovascular disease (CVD), defined as coronary heart disease (CHD), myocardial infarction, or stroke.
- Compared with women who had their first menstrual period at age 13 (the mean age in this population), those who had their period at age ≤ 10 had a significantly greater risk of having type 2 diabetes, after adjustment for age, race/ethnicity, education, parity, menopause status, family history of diabetes, smoking status, physical activity, alcohol consumption, and body mass index (odds ratio, 1.32; 95% CI, 1.03-1.69; P trend = .03).
- Among the women with diabetes, compared with those who had their first menstrual period at age 13, those who had it at age ≤ 10 had a significantly greater risk of having stroke (OR, 2.66; 95% CI, 1.07-6.64; P trend = .02), but not CVD or CHD, after adjustment for these multiple variables.
TAKEAWAY:
- In a racially and ethnically diverse national sample of US women younger than 65, “extremely early” age at first menstrual period was associated with significantly increased risk for type 2 diabetes; among the women with type 2 diabetes, it was associated with significantly increased risk for stroke but not CVD or CHD, after adjustment for multiple variables.
- Early age at menarche may be an early indicator of the cardiometabolic disease trajectory in women.
IN PRACTICE:
“Women with early-life exposures such as early age at menarche need to be further examined for diabetes and prevention research and strategies for progression of diabetes complications,” the study authors write.
SOURCE:
The authors, mainly from Tulane University School of Public Health and Tropical Medicine, New Orleans, Louisiana, and also from Harvard Medical School, Boston, Massachusetts, published their findings in BMJ Nutrition, Prevention & Health.
LIMITATIONS:
- The women who participated in NHANES may not be representative of all women in the United States (selection bias).
- The study only included women who reported the age when they had their first menstrual period (selection bias).
- This was a cross-sectional, observational study, so it cannot show causality.
- The women may have reported the wrong age at which they had their first period (recall bias and social desirability bias).
- The women may have inaccurately reported CVD and type 2 diabetes (recall bias and social desirability bias).
DISCLOSURES:
The researchers were supported by grants from the National Heart, Lung, and Blood Institute and from the National Institute of General Medical Sciences of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
TOPLINE:
, a retrospective study of US women under age 65 found.
METHODOLOGY:
- Researchers analyzed data from 17,377 women who were aged 20-65 years when they participated in a National Health and Nutrition Examination Survey (NHANES) from 1999 to 2018 and reported their age at first menstruation, which was classified as ≤ 10, 11, 12, 13, 14, or ≥ 15 years of age.
- In total, 0.2% of the women (1773) had type 2 diabetes; of these, 11.5% (205) had cardiovascular disease (CVD), defined as coronary heart disease (CHD), myocardial infarction, or stroke.
- Compared with women who had their first menstrual period at age 13 (the mean age in this population), those who had their period at age ≤ 10 had a significantly greater risk of having type 2 diabetes, after adjustment for age, race/ethnicity, education, parity, menopause status, family history of diabetes, smoking status, physical activity, alcohol consumption, and body mass index (odds ratio, 1.32; 95% CI, 1.03-1.69; P trend = .03).
- Among the women with diabetes, compared with those who had their first menstrual period at age 13, those who had it at age ≤ 10 had a significantly greater risk of having stroke (OR, 2.66; 95% CI, 1.07-6.64; P trend = .02), but not CVD or CHD, after adjustment for these multiple variables.
TAKEAWAY:
- In a racially and ethnically diverse national sample of US women younger than 65, “extremely early” age at first menstrual period was associated with significantly increased risk for type 2 diabetes; among the women with type 2 diabetes, it was associated with significantly increased risk for stroke but not CVD or CHD, after adjustment for multiple variables.
- Early age at menarche may be an early indicator of the cardiometabolic disease trajectory in women.
IN PRACTICE:
“Women with early-life exposures such as early age at menarche need to be further examined for diabetes and prevention research and strategies for progression of diabetes complications,” the study authors write.
SOURCE:
The authors, mainly from Tulane University School of Public Health and Tropical Medicine, New Orleans, Louisiana, and also from Harvard Medical School, Boston, Massachusetts, published their findings in BMJ Nutrition, Prevention & Health.
LIMITATIONS:
- The women who participated in NHANES may not be representative of all women in the United States (selection bias).
- The study only included women who reported the age when they had their first menstrual period (selection bias).
- This was a cross-sectional, observational study, so it cannot show causality.
- The women may have reported the wrong age at which they had their first period (recall bias and social desirability bias).
- The women may have inaccurately reported CVD and type 2 diabetes (recall bias and social desirability bias).
DISCLOSURES:
The researchers were supported by grants from the National Heart, Lung, and Blood Institute and from the National Institute of General Medical Sciences of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
TOPLINE:
, a retrospective study of US women under age 65 found.
METHODOLOGY:
- Researchers analyzed data from 17,377 women who were aged 20-65 years when they participated in a National Health and Nutrition Examination Survey (NHANES) from 1999 to 2018 and reported their age at first menstruation, which was classified as ≤ 10, 11, 12, 13, 14, or ≥ 15 years of age.
- In total, 0.2% of the women (1773) had type 2 diabetes; of these, 11.5% (205) had cardiovascular disease (CVD), defined as coronary heart disease (CHD), myocardial infarction, or stroke.
- Compared with women who had their first menstrual period at age 13 (the mean age in this population), those who had their period at age ≤ 10 had a significantly greater risk of having type 2 diabetes, after adjustment for age, race/ethnicity, education, parity, menopause status, family history of diabetes, smoking status, physical activity, alcohol consumption, and body mass index (odds ratio, 1.32; 95% CI, 1.03-1.69; P trend = .03).
- Among the women with diabetes, compared with those who had their first menstrual period at age 13, those who had it at age ≤ 10 had a significantly greater risk of having stroke (OR, 2.66; 95% CI, 1.07-6.64; P trend = .02), but not CVD or CHD, after adjustment for these multiple variables.
TAKEAWAY:
- In a racially and ethnically diverse national sample of US women younger than 65, “extremely early” age at first menstrual period was associated with significantly increased risk for type 2 diabetes; among the women with type 2 diabetes, it was associated with significantly increased risk for stroke but not CVD or CHD, after adjustment for multiple variables.
- Early age at menarche may be an early indicator of the cardiometabolic disease trajectory in women.
IN PRACTICE:
“Women with early-life exposures such as early age at menarche need to be further examined for diabetes and prevention research and strategies for progression of diabetes complications,” the study authors write.
SOURCE:
The authors, mainly from Tulane University School of Public Health and Tropical Medicine, New Orleans, Louisiana, and also from Harvard Medical School, Boston, Massachusetts, published their findings in BMJ Nutrition, Prevention & Health.
LIMITATIONS:
- The women who participated in NHANES may not be representative of all women in the United States (selection bias).
- The study only included women who reported the age when they had their first menstrual period (selection bias).
- This was a cross-sectional, observational study, so it cannot show causality.
- The women may have reported the wrong age at which they had their first period (recall bias and social desirability bias).
- The women may have inaccurately reported CVD and type 2 diabetes (recall bias and social desirability bias).
DISCLOSURES:
The researchers were supported by grants from the National Heart, Lung, and Blood Institute and from the National Institute of General Medical Sciences of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
‘Smart’ stethoscope spots peripartum cardiomyopathy
in a large study of obstetric patients in Nigeria.
Demilade A. Adedinsewo, MD, MPH, from Mayo Clinic, Jacksonville, Fla., reported these findings from the Screening for Pregnancy Related Heart Failure in Nigeria (SPEC-AI Nigeria) trial in a press briefing and in a late-breaking trial session at the annual scientific sessions of the American Heart Association.
“The key takeaway,” Dr. Adedinsewo said in an interview, “is recognizing that a simple, low-impact tool like a digital stethoscope can dramatically improve the diagnosis of a life-threatening condition, and we can treat it. A large proportion of the women will recover; if we identify them early and treat them appropriately, we can reduce the risk of dying.”
If the device predicted low ejection fraction, the patient went on to have an echocardiogram to confirm cardiomyopathy, defined as a left ventricular ejection fraction (LVEF) <50%.
Peripartum cardiomyopathy was detected in 4% of the women who were screened with this tool, compared with 1.8% of those who received usual care, which included a traditional ECG.
“I believe that the control arm also has about 4% of cardiomyopathy cases, but because they didn’t have the same screening and echo, we’re missing them,” Dr. Adedinsewo said.
Diagnosis of peripartum cardiomyopathy is challenging, she noted, owing to overlap of common symptoms in pregnancy, such as lower-extremity swelling, fatigue, and shortness of breath with mild activity, which are also cardinal symptoms of heart failure.
“We were really impressed by the effectiveness of the tool, looking at how accurate it was when it comes to the sensitivity,” she added. She noted that the digital stethoscope correctly identified 92% of women with LVEF < 50% and 100% of those with LVEF < 40%.
This was the first large, clinical trial to evaluate an AI intervention in pregnancy. The investigators used a portable, battery-operated device that yielded AI results in real time.
Nigeria has the highest rate of pericardium cardiomyopathy of any country. However, one study showed a 16-fold higher rate of cardiomyopathy among African American women, compared with White women in the United States, Dr. Adedinsewo noted. “It will be important to identify who we should be screening to identify more cases,” she said.
A digital stethoscope that provides an ECG is currently available, but the algorithm that powers detection of cardiomyopathy is not yet commercially available.
Findings ‘absolutely startling’
The study discussant in the press briefing, Alexander Tarlochan Singh Sandhu, MD, from Stanford (Calif.) University congratulated the authors on this “valuable study that uses AI tools to solve a real health problem.”
Finding that 4% of the women in the intervention arm had reduced ejection fraction is “absolutely startling,” he said, “and speaks to how important improving our diagnosis in this space is.
“Where the burden of disease is high, a tool like this can be so incredibly valuable,” he said. He noted that the investigators identified 2% more patients with peripartum cardiomyopathy.
“This is an example of the potential of AI tools that can actually improve access to care and improve quality of care in resource-limited settings,” he said. “We need to move to understanding how to implement this into subsequent care [and] figure out what the next steps are to improve their outcomes.”
“The main takeaway is that, in areas where there is a very high prevalence of a morbid condition, a prescreening tool like this may be helpful” for diagnosis, the assigned discussant in the session, Marco Perez, MD, also from Stanford University, told this news organization.
The number of women needed to screen to detect peripartum cardiomyopathy by echocardiography alone is 1 in 23 in Nigeria and 1 in 970 in the United States, he said.
With an AI tool such as this one (sensitivity, 92%; specificity, 80%), the number needed to screen would be 1 in 5.7 in Nigeria and 1 in 194 in the United States, he estimates on the basis of incidence data.
“Because it is so common in Nigeria, a screening method makes a lot of sense,” Dr. Perez said. “The big question that remains is, what is the best screening modality?
“Certainly, this tool helped in bringing down the number of echoes needed to find a case, from the mid 20s down to about 5 or 6, so it certainly does seem to be helpful.”
However, the investigators did not say whether this tool is better than a clinical review of ECG or an AI analysis of ECG alone. It’s not clear whether the phonocardiogram component is significant in conjunction with the ECG component.
Nevertheless, “In a place where there’s a very high prevalence of peripartum cardiomyopathy, like Haiti, like Nigeria, doing something like this makes a lot of sense.
“For the U.S. and the rest of the world, where the prevalence is much lower, even with a tool like this you still would need to do a lot of echoes to find one case, and that may end up not being cost-effective. You would need to screen 200 women with echo to find one case.”
AI-guided screening study
Nigeria has the highest reported incidence of peripartum cardiomyopathy mortality (1 in 100 live births) and the highest number of maternal deaths.
In the United States, where rates of peripartum cardiomyopathy are much lower, maternal deaths are nevertheless higher than in other developed countries and have trended up over the past 3 decades; cardiomyopathy is a key contributor.
The investigators enrolled 1,195 women who were pregnant or had given birth in the past 12 months. The patients were from six teaching hospitals in Nigeria (two in the north and four in the south). They were randomly assigned in a 1:1 ratio to the intervention group (587) or the control group (608).
In the intervention group, clinicians used a smart stethoscope to record a phonocardiogram and a single-lead ECG reading in the V2 position and in an angled position on the patient’s chest wall and to record an ECG from the patient’s fingers. The recordings were sent to a Bluetooth-enabled mobile device (tablet or smartphone), which displayed the phonocardiogram and ECG images and that indicated whether the ejection fraction was normal or low. All patients in the intervention group received an echocardiogram.
In the control group, patients received usual care plus a traditional ECG. They were not required to have an echocardiogram because undergoing an echocardiogram is not part of usual care; however, they could receive an echocardiogram if the ECG suggested that they might need further testing.
The mean age of all the patients was 31 years, and all were Black. At study entry, 73% were pregnant, and 26% were post partum. They had similar comorbidities.
The primary outcome, cardiomyopathy (LVEF <50%) was detected in 24 of 587 patients (4.1%) in the intervention group and in 11 of 608 patients (1.8%) in the control group (odds ratio, 2.3; 95% confidence interval, 1.1-4.8; P = .02).
For the detection of LVEF <50%, the sensitivity was 92% and the specificity was 80%. For the detection of LVEF <40% (a secondary outcome), the sensitivity was 100% and the specificity was 79%.
Dr. Adedinsewo is supported by the Mayo Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) Program, which is funded by the National Institutes of Health. The trial was funded by Mayo Clinic (Centers for Digital Health and Community Health and Engagement Research) and in part by the Mayo Clinic BIRCWH Program. Portable ECG, phonocardiogram recordings, and AI predictions using the digital stethoscope were extracted by the Eko Health team and were sent to the coordinating center for analysis. Eko Health had no role in study design, data collection, data analysis, or data interpretation.
A version of this article appeared on Medscape.com.
in a large study of obstetric patients in Nigeria.
Demilade A. Adedinsewo, MD, MPH, from Mayo Clinic, Jacksonville, Fla., reported these findings from the Screening for Pregnancy Related Heart Failure in Nigeria (SPEC-AI Nigeria) trial in a press briefing and in a late-breaking trial session at the annual scientific sessions of the American Heart Association.
“The key takeaway,” Dr. Adedinsewo said in an interview, “is recognizing that a simple, low-impact tool like a digital stethoscope can dramatically improve the diagnosis of a life-threatening condition, and we can treat it. A large proportion of the women will recover; if we identify them early and treat them appropriately, we can reduce the risk of dying.”
If the device predicted low ejection fraction, the patient went on to have an echocardiogram to confirm cardiomyopathy, defined as a left ventricular ejection fraction (LVEF) <50%.
Peripartum cardiomyopathy was detected in 4% of the women who were screened with this tool, compared with 1.8% of those who received usual care, which included a traditional ECG.
“I believe that the control arm also has about 4% of cardiomyopathy cases, but because they didn’t have the same screening and echo, we’re missing them,” Dr. Adedinsewo said.
Diagnosis of peripartum cardiomyopathy is challenging, she noted, owing to overlap of common symptoms in pregnancy, such as lower-extremity swelling, fatigue, and shortness of breath with mild activity, which are also cardinal symptoms of heart failure.
“We were really impressed by the effectiveness of the tool, looking at how accurate it was when it comes to the sensitivity,” she added. She noted that the digital stethoscope correctly identified 92% of women with LVEF < 50% and 100% of those with LVEF < 40%.
This was the first large, clinical trial to evaluate an AI intervention in pregnancy. The investigators used a portable, battery-operated device that yielded AI results in real time.
Nigeria has the highest rate of pericardium cardiomyopathy of any country. However, one study showed a 16-fold higher rate of cardiomyopathy among African American women, compared with White women in the United States, Dr. Adedinsewo noted. “It will be important to identify who we should be screening to identify more cases,” she said.
A digital stethoscope that provides an ECG is currently available, but the algorithm that powers detection of cardiomyopathy is not yet commercially available.
Findings ‘absolutely startling’
The study discussant in the press briefing, Alexander Tarlochan Singh Sandhu, MD, from Stanford (Calif.) University congratulated the authors on this “valuable study that uses AI tools to solve a real health problem.”
Finding that 4% of the women in the intervention arm had reduced ejection fraction is “absolutely startling,” he said, “and speaks to how important improving our diagnosis in this space is.
“Where the burden of disease is high, a tool like this can be so incredibly valuable,” he said. He noted that the investigators identified 2% more patients with peripartum cardiomyopathy.
“This is an example of the potential of AI tools that can actually improve access to care and improve quality of care in resource-limited settings,” he said. “We need to move to understanding how to implement this into subsequent care [and] figure out what the next steps are to improve their outcomes.”
“The main takeaway is that, in areas where there is a very high prevalence of a morbid condition, a prescreening tool like this may be helpful” for diagnosis, the assigned discussant in the session, Marco Perez, MD, also from Stanford University, told this news organization.
The number of women needed to screen to detect peripartum cardiomyopathy by echocardiography alone is 1 in 23 in Nigeria and 1 in 970 in the United States, he said.
With an AI tool such as this one (sensitivity, 92%; specificity, 80%), the number needed to screen would be 1 in 5.7 in Nigeria and 1 in 194 in the United States, he estimates on the basis of incidence data.
“Because it is so common in Nigeria, a screening method makes a lot of sense,” Dr. Perez said. “The big question that remains is, what is the best screening modality?
“Certainly, this tool helped in bringing down the number of echoes needed to find a case, from the mid 20s down to about 5 or 6, so it certainly does seem to be helpful.”
However, the investigators did not say whether this tool is better than a clinical review of ECG or an AI analysis of ECG alone. It’s not clear whether the phonocardiogram component is significant in conjunction with the ECG component.
Nevertheless, “In a place where there’s a very high prevalence of peripartum cardiomyopathy, like Haiti, like Nigeria, doing something like this makes a lot of sense.
“For the U.S. and the rest of the world, where the prevalence is much lower, even with a tool like this you still would need to do a lot of echoes to find one case, and that may end up not being cost-effective. You would need to screen 200 women with echo to find one case.”
AI-guided screening study
Nigeria has the highest reported incidence of peripartum cardiomyopathy mortality (1 in 100 live births) and the highest number of maternal deaths.
In the United States, where rates of peripartum cardiomyopathy are much lower, maternal deaths are nevertheless higher than in other developed countries and have trended up over the past 3 decades; cardiomyopathy is a key contributor.
The investigators enrolled 1,195 women who were pregnant or had given birth in the past 12 months. The patients were from six teaching hospitals in Nigeria (two in the north and four in the south). They were randomly assigned in a 1:1 ratio to the intervention group (587) or the control group (608).
In the intervention group, clinicians used a smart stethoscope to record a phonocardiogram and a single-lead ECG reading in the V2 position and in an angled position on the patient’s chest wall and to record an ECG from the patient’s fingers. The recordings were sent to a Bluetooth-enabled mobile device (tablet or smartphone), which displayed the phonocardiogram and ECG images and that indicated whether the ejection fraction was normal or low. All patients in the intervention group received an echocardiogram.
In the control group, patients received usual care plus a traditional ECG. They were not required to have an echocardiogram because undergoing an echocardiogram is not part of usual care; however, they could receive an echocardiogram if the ECG suggested that they might need further testing.
The mean age of all the patients was 31 years, and all were Black. At study entry, 73% were pregnant, and 26% were post partum. They had similar comorbidities.
The primary outcome, cardiomyopathy (LVEF <50%) was detected in 24 of 587 patients (4.1%) in the intervention group and in 11 of 608 patients (1.8%) in the control group (odds ratio, 2.3; 95% confidence interval, 1.1-4.8; P = .02).
For the detection of LVEF <50%, the sensitivity was 92% and the specificity was 80%. For the detection of LVEF <40% (a secondary outcome), the sensitivity was 100% and the specificity was 79%.
Dr. Adedinsewo is supported by the Mayo Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) Program, which is funded by the National Institutes of Health. The trial was funded by Mayo Clinic (Centers for Digital Health and Community Health and Engagement Research) and in part by the Mayo Clinic BIRCWH Program. Portable ECG, phonocardiogram recordings, and AI predictions using the digital stethoscope were extracted by the Eko Health team and were sent to the coordinating center for analysis. Eko Health had no role in study design, data collection, data analysis, or data interpretation.
A version of this article appeared on Medscape.com.
in a large study of obstetric patients in Nigeria.
Demilade A. Adedinsewo, MD, MPH, from Mayo Clinic, Jacksonville, Fla., reported these findings from the Screening for Pregnancy Related Heart Failure in Nigeria (SPEC-AI Nigeria) trial in a press briefing and in a late-breaking trial session at the annual scientific sessions of the American Heart Association.
“The key takeaway,” Dr. Adedinsewo said in an interview, “is recognizing that a simple, low-impact tool like a digital stethoscope can dramatically improve the diagnosis of a life-threatening condition, and we can treat it. A large proportion of the women will recover; if we identify them early and treat them appropriately, we can reduce the risk of dying.”
If the device predicted low ejection fraction, the patient went on to have an echocardiogram to confirm cardiomyopathy, defined as a left ventricular ejection fraction (LVEF) <50%.
Peripartum cardiomyopathy was detected in 4% of the women who were screened with this tool, compared with 1.8% of those who received usual care, which included a traditional ECG.
“I believe that the control arm also has about 4% of cardiomyopathy cases, but because they didn’t have the same screening and echo, we’re missing them,” Dr. Adedinsewo said.
Diagnosis of peripartum cardiomyopathy is challenging, she noted, owing to overlap of common symptoms in pregnancy, such as lower-extremity swelling, fatigue, and shortness of breath with mild activity, which are also cardinal symptoms of heart failure.
“We were really impressed by the effectiveness of the tool, looking at how accurate it was when it comes to the sensitivity,” she added. She noted that the digital stethoscope correctly identified 92% of women with LVEF < 50% and 100% of those with LVEF < 40%.
This was the first large, clinical trial to evaluate an AI intervention in pregnancy. The investigators used a portable, battery-operated device that yielded AI results in real time.
Nigeria has the highest rate of pericardium cardiomyopathy of any country. However, one study showed a 16-fold higher rate of cardiomyopathy among African American women, compared with White women in the United States, Dr. Adedinsewo noted. “It will be important to identify who we should be screening to identify more cases,” she said.
A digital stethoscope that provides an ECG is currently available, but the algorithm that powers detection of cardiomyopathy is not yet commercially available.
Findings ‘absolutely startling’
The study discussant in the press briefing, Alexander Tarlochan Singh Sandhu, MD, from Stanford (Calif.) University congratulated the authors on this “valuable study that uses AI tools to solve a real health problem.”
Finding that 4% of the women in the intervention arm had reduced ejection fraction is “absolutely startling,” he said, “and speaks to how important improving our diagnosis in this space is.
“Where the burden of disease is high, a tool like this can be so incredibly valuable,” he said. He noted that the investigators identified 2% more patients with peripartum cardiomyopathy.
“This is an example of the potential of AI tools that can actually improve access to care and improve quality of care in resource-limited settings,” he said. “We need to move to understanding how to implement this into subsequent care [and] figure out what the next steps are to improve their outcomes.”
“The main takeaway is that, in areas where there is a very high prevalence of a morbid condition, a prescreening tool like this may be helpful” for diagnosis, the assigned discussant in the session, Marco Perez, MD, also from Stanford University, told this news organization.
The number of women needed to screen to detect peripartum cardiomyopathy by echocardiography alone is 1 in 23 in Nigeria and 1 in 970 in the United States, he said.
With an AI tool such as this one (sensitivity, 92%; specificity, 80%), the number needed to screen would be 1 in 5.7 in Nigeria and 1 in 194 in the United States, he estimates on the basis of incidence data.
“Because it is so common in Nigeria, a screening method makes a lot of sense,” Dr. Perez said. “The big question that remains is, what is the best screening modality?
“Certainly, this tool helped in bringing down the number of echoes needed to find a case, from the mid 20s down to about 5 or 6, so it certainly does seem to be helpful.”
However, the investigators did not say whether this tool is better than a clinical review of ECG or an AI analysis of ECG alone. It’s not clear whether the phonocardiogram component is significant in conjunction with the ECG component.
Nevertheless, “In a place where there’s a very high prevalence of peripartum cardiomyopathy, like Haiti, like Nigeria, doing something like this makes a lot of sense.
“For the U.S. and the rest of the world, where the prevalence is much lower, even with a tool like this you still would need to do a lot of echoes to find one case, and that may end up not being cost-effective. You would need to screen 200 women with echo to find one case.”
AI-guided screening study
Nigeria has the highest reported incidence of peripartum cardiomyopathy mortality (1 in 100 live births) and the highest number of maternal deaths.
In the United States, where rates of peripartum cardiomyopathy are much lower, maternal deaths are nevertheless higher than in other developed countries and have trended up over the past 3 decades; cardiomyopathy is a key contributor.
The investigators enrolled 1,195 women who were pregnant or had given birth in the past 12 months. The patients were from six teaching hospitals in Nigeria (two in the north and four in the south). They were randomly assigned in a 1:1 ratio to the intervention group (587) or the control group (608).
In the intervention group, clinicians used a smart stethoscope to record a phonocardiogram and a single-lead ECG reading in the V2 position and in an angled position on the patient’s chest wall and to record an ECG from the patient’s fingers. The recordings were sent to a Bluetooth-enabled mobile device (tablet or smartphone), which displayed the phonocardiogram and ECG images and that indicated whether the ejection fraction was normal or low. All patients in the intervention group received an echocardiogram.
In the control group, patients received usual care plus a traditional ECG. They were not required to have an echocardiogram because undergoing an echocardiogram is not part of usual care; however, they could receive an echocardiogram if the ECG suggested that they might need further testing.
The mean age of all the patients was 31 years, and all were Black. At study entry, 73% were pregnant, and 26% were post partum. They had similar comorbidities.
The primary outcome, cardiomyopathy (LVEF <50%) was detected in 24 of 587 patients (4.1%) in the intervention group and in 11 of 608 patients (1.8%) in the control group (odds ratio, 2.3; 95% confidence interval, 1.1-4.8; P = .02).
For the detection of LVEF <50%, the sensitivity was 92% and the specificity was 80%. For the detection of LVEF <40% (a secondary outcome), the sensitivity was 100% and the specificity was 79%.
Dr. Adedinsewo is supported by the Mayo Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) Program, which is funded by the National Institutes of Health. The trial was funded by Mayo Clinic (Centers for Digital Health and Community Health and Engagement Research) and in part by the Mayo Clinic BIRCWH Program. Portable ECG, phonocardiogram recordings, and AI predictions using the digital stethoscope were extracted by the Eko Health team and were sent to the coordinating center for analysis. Eko Health had no role in study design, data collection, data analysis, or data interpretation.
A version of this article appeared on Medscape.com.
FROM AHA 2023
Better postpartum BP control with self-monitoring: POP-HT
, new research suggests.
In a randomized trial of 220 women with preeclampsia or gestational hypertension, those who took daily postpartum BP readings and received clinician-guided advice for titrating antihypertensives had a 5 mm Hg–lower average diastolic BP at 9 months, compared with those receiving usual care.
Jamie Kitt, DPhil, from the University of Oxford (England) presented these findings from the Physicians Optimized Postpartum Hypertension Treatment (POP-HT, NCT04273854) clinical trial at the American Heart Association scientific sessions. The study was simultaneously published online in JAMA, and a cardiac imaging substudy was published online in Circulation.
“This trial identifies a potential need for a paradigm shift in the way women affected by hypertensive pregnancy are managed postnatally,” Dr. Kitt said. “If a 5–mm Hg improvement in BP is maintained longer term, it can result in about a 20% reduction in lifetime cardiovascular risk.”
The imaging substudy suggests that short-term postnatal optimization of BP control following hypertensive pregnancy through self-monitoring and physician-guided antihypertensive titration is linked with better cardiac remodeling changes seen by cardiovascular magnetic resonance and echocardiography.
POP-HT “proves for the first time that the first few weeks after delivery are a critical time that can determine the long-term cardiovascular health of the mother,” senior author Paul Leeson, PhD, also from the University of Oxford, who presented the findings in a press briefing, said in an interview.
“Interventions during this period can have long-term beneficial impacts on cardiovascular health,” he said. “These findings rewrite the textbook on our understanding of how and why hypertensive pregnancies associate with later cardiovascular disease in the mother.”
Next, Dr. Leeson said, “We need to work out the best ways to implement these interventions “at scale. Then we can ensure all women who have hypertensive pregnancies can get access to the long-term cardiovascular benefits we have demonstrated are possible through improving postpartum cardiac care,” he said, adding that “this is entirely achievable using current available technologies.”
Hypertension in pregnancy
About 1 in 10 pregnant women develop hypertension in pregnancy (preeclampsia or gestational hypertension), and 1 in 3 such women go on to develop chronic hypertension within 10 years, “when they are usually still in their 30s or 40s,” Dr. Leeson said.
During pregnancy, the heart remodels to cope with pregnancy, and it undergoes more severe changes if BP is high. Then during the 6 weeks after giving birth, this remodeling rapidly reverses.
Higher blood pressure in young adulthood is associated with a twofold higher risk of subsequent myocardial infarction and stroke. And abnormal cardiac remodeling postpartum is also linked with higher cardiovascular risk.
Self-monitoring blood pressure during the postpartum period may be a “critical window” for intervention.
Previously, the research group performed a pilot study, the Self-Management of Postnatal Antihypertensive Treatment (SNAP-HT) trial and the SNAP-extension trial, which compared a BP self-monitoring intervention with usual care in 91 women with gestational hypertension or preeclampsia requiring postnatal antihypertensive treatment.
Diastolic BP, which drives cardiovascular risk in younger populations, was 4.5–mm Hg lower at 6 months postpartum and 7–mm Hg lower at 4 years post partum in patients randomly assigned to BP self-management vs. usual care – even after they were no longer taking antihypertensives.
Building on these findings, the POP-HT trial enrolled 220 pregnant women seen at Oxford University Hospitals in the United Kingdom who were age 18 years or older, had either gestational hypertension or preeclampsia, and still required antihypertensives when they were being discharged from hospital after giving birth.
Following a baseline visit at day 1-6 after delivery, while in the postnatal ward, the patients were randomly assigned 1:1 to the intervention group (112 women) or usual-care group (108 women).
They had an average age of 32.6 years; 40% had gestational hypertension, and 60% had preeclampsia.
Women in the usual-care group typically received a BP review at 7-10 days after hospital discharge with a community midwife, and another at 6-8 weeks with their general practitioner.
The women in the intervention group were given and taught to use a Bluetooth-enabled OMRON Evolv BP monitor (Omron Healthcare Europe) while on the postnatal ward, and they installed a smartphone app on their mobile phones that transmitted self-monitored BP readings to a National Health Service-hosted, web-based platform.
They were instructed to take daily BP measurements (twice daily if out of target range). Dose titration of antihypertensives after hospital discharge was guided remotely by research clinicians, according to a guideline-based algorithm.
Patients in both groups had four study visits when their BP was measured: visit 1 (baseline) between days 1 and 6 post partum; visit 2 at week 1; visit 3 at week 6; and visit 4 between months 6 and 9 post partum.
Similar antihypertensive classes were prescribed in each group (enalapril 57%, nifedipine 27%, and labetalol 30% for intervention vs. enalapril 43%, nifedipine 30%, and labetalol 27% for control).
At 6 weeks, approximately 30% of participants in each group were still taking medication; this dropped to approximately 12% by visit 4.
The primary outcome – the mean 24-hour diastolic BP at visit 4 (roughly 9 months post partum), adjusted for baseline postnatal diastolic blood pressure – was 5.8–mm Hg lower in the intervention group than in the control group (71.2 mm Hg vs. 76.6 mm Hg; P < .001).
Secondary outcomes – between-group differences in systolic BP at 9 months, BP-related postnatal admission, and cardiac remodeling assessed by cardiac magnetic resonance – were all better in the intervention group.
The mean 24-hour average systolic BP at 9 months post partum, adjusted for baseline postnatal systolic BP was 6.5–mm Hg lower in the intervention group than in the control group (114.0 mm Hg vs. 120.3 mm Hg; P < .001).
There was an absolute risk reduction of 20% and a relative risk reduction of 73.5% in postnatal readmission. The number needed to treat to avoid one postnatal readmission was five, which “has potential for big cost savings,” said Dr. Leeson.
Blood pressure post partum can be improved with self-monitoring and physician-guided medication adjustment, Dr. Leeson summarized. The blood pressure remains low for at least 9 months, even when medication is stopped, and the intervention leads to beneficial cardiac remodeling.
U.S. pilot study
Non-Hispanic Black adults have a high hypertension and cardiovascular disease burden, and a related small U.S. study showed benefits of BP self-monitoring in a population comprising mainly Black women, Keith Ferdinand, MD, discussant of the POP-HT trial in the press briefing, said in an interview.
Dr. Ferdinand, from Tulane University, New Orleans, Louisiana, was lead author of the Text My Hypertension BP Meds NOLA pilot study that was published in February in the American Heart Journal Plus: Cardiology Research and Practice.
The study showed that text-messaging and social support increased hypertension medication adherence.
They enrolled 36 individuals, of whom 32 (89%) were non-Hispanic Black, and 23 (64%) were women. The participants received validated Bluetooth-enabled BP-monitoring devices that were synced to smartphones via a secured cloud-based application. The participants could send and receive messages to health care practitioners.
This intervention significantly improved medication adherence and systolic BP without modifying pharmacotherapy.
‘Need to be passionate about monitoring BP’
“The take-home messages from these exciting findings is that physicians and women who have had high BP during pregnancy need to be passionate about monitoring and controlling their blood pressure and not ignore it,” Anastasia Mihailidou, PhD, Royal North Shore Hospital, Sydney, the assigned discussant in the late-breaking trial session, said in an interview.
“It also resulted in fewer postpartum hospital readmissions for high blood pressure and benefit at 9 months in the structure and function of the heart and blood vessels of the women,” she said.
“While we need to see further studies in ethnically diverse women to see that they are reproducible, there are simple measures that clinicians can implement, and women can ask to have their BP monitored more frequently than the current practice. In the U.K. it is 5-10 days after delivery and then at 6-8 weeks after giving birth when changes in heart structure have already started,” Dr. Mihailidou noted.
“The procedure will need to be modified if there are no telemedicine facilities, but that should not stop having close monitoring of BP and treating it adequately. Monitoring requires an accurate BP monitor. There also has to be monitoring BP for the children.”
The trial was funded by a BHF Clinical Research Training Fellowship to Dr. Kitt, with additional support from the NIHR Oxford Biomedical Research Centre and Oxford BHF Centre for Research Excellence.
A version of this article first appeared on Medscape.com.
, new research suggests.
In a randomized trial of 220 women with preeclampsia or gestational hypertension, those who took daily postpartum BP readings and received clinician-guided advice for titrating antihypertensives had a 5 mm Hg–lower average diastolic BP at 9 months, compared with those receiving usual care.
Jamie Kitt, DPhil, from the University of Oxford (England) presented these findings from the Physicians Optimized Postpartum Hypertension Treatment (POP-HT, NCT04273854) clinical trial at the American Heart Association scientific sessions. The study was simultaneously published online in JAMA, and a cardiac imaging substudy was published online in Circulation.
“This trial identifies a potential need for a paradigm shift in the way women affected by hypertensive pregnancy are managed postnatally,” Dr. Kitt said. “If a 5–mm Hg improvement in BP is maintained longer term, it can result in about a 20% reduction in lifetime cardiovascular risk.”
The imaging substudy suggests that short-term postnatal optimization of BP control following hypertensive pregnancy through self-monitoring and physician-guided antihypertensive titration is linked with better cardiac remodeling changes seen by cardiovascular magnetic resonance and echocardiography.
POP-HT “proves for the first time that the first few weeks after delivery are a critical time that can determine the long-term cardiovascular health of the mother,” senior author Paul Leeson, PhD, also from the University of Oxford, who presented the findings in a press briefing, said in an interview.
“Interventions during this period can have long-term beneficial impacts on cardiovascular health,” he said. “These findings rewrite the textbook on our understanding of how and why hypertensive pregnancies associate with later cardiovascular disease in the mother.”
Next, Dr. Leeson said, “We need to work out the best ways to implement these interventions “at scale. Then we can ensure all women who have hypertensive pregnancies can get access to the long-term cardiovascular benefits we have demonstrated are possible through improving postpartum cardiac care,” he said, adding that “this is entirely achievable using current available technologies.”
Hypertension in pregnancy
About 1 in 10 pregnant women develop hypertension in pregnancy (preeclampsia or gestational hypertension), and 1 in 3 such women go on to develop chronic hypertension within 10 years, “when they are usually still in their 30s or 40s,” Dr. Leeson said.
During pregnancy, the heart remodels to cope with pregnancy, and it undergoes more severe changes if BP is high. Then during the 6 weeks after giving birth, this remodeling rapidly reverses.
Higher blood pressure in young adulthood is associated with a twofold higher risk of subsequent myocardial infarction and stroke. And abnormal cardiac remodeling postpartum is also linked with higher cardiovascular risk.
Self-monitoring blood pressure during the postpartum period may be a “critical window” for intervention.
Previously, the research group performed a pilot study, the Self-Management of Postnatal Antihypertensive Treatment (SNAP-HT) trial and the SNAP-extension trial, which compared a BP self-monitoring intervention with usual care in 91 women with gestational hypertension or preeclampsia requiring postnatal antihypertensive treatment.
Diastolic BP, which drives cardiovascular risk in younger populations, was 4.5–mm Hg lower at 6 months postpartum and 7–mm Hg lower at 4 years post partum in patients randomly assigned to BP self-management vs. usual care – even after they were no longer taking antihypertensives.
Building on these findings, the POP-HT trial enrolled 220 pregnant women seen at Oxford University Hospitals in the United Kingdom who were age 18 years or older, had either gestational hypertension or preeclampsia, and still required antihypertensives when they were being discharged from hospital after giving birth.
Following a baseline visit at day 1-6 after delivery, while in the postnatal ward, the patients were randomly assigned 1:1 to the intervention group (112 women) or usual-care group (108 women).
They had an average age of 32.6 years; 40% had gestational hypertension, and 60% had preeclampsia.
Women in the usual-care group typically received a BP review at 7-10 days after hospital discharge with a community midwife, and another at 6-8 weeks with their general practitioner.
The women in the intervention group were given and taught to use a Bluetooth-enabled OMRON Evolv BP monitor (Omron Healthcare Europe) while on the postnatal ward, and they installed a smartphone app on their mobile phones that transmitted self-monitored BP readings to a National Health Service-hosted, web-based platform.
They were instructed to take daily BP measurements (twice daily if out of target range). Dose titration of antihypertensives after hospital discharge was guided remotely by research clinicians, according to a guideline-based algorithm.
Patients in both groups had four study visits when their BP was measured: visit 1 (baseline) between days 1 and 6 post partum; visit 2 at week 1; visit 3 at week 6; and visit 4 between months 6 and 9 post partum.
Similar antihypertensive classes were prescribed in each group (enalapril 57%, nifedipine 27%, and labetalol 30% for intervention vs. enalapril 43%, nifedipine 30%, and labetalol 27% for control).
At 6 weeks, approximately 30% of participants in each group were still taking medication; this dropped to approximately 12% by visit 4.
The primary outcome – the mean 24-hour diastolic BP at visit 4 (roughly 9 months post partum), adjusted for baseline postnatal diastolic blood pressure – was 5.8–mm Hg lower in the intervention group than in the control group (71.2 mm Hg vs. 76.6 mm Hg; P < .001).
Secondary outcomes – between-group differences in systolic BP at 9 months, BP-related postnatal admission, and cardiac remodeling assessed by cardiac magnetic resonance – were all better in the intervention group.
The mean 24-hour average systolic BP at 9 months post partum, adjusted for baseline postnatal systolic BP was 6.5–mm Hg lower in the intervention group than in the control group (114.0 mm Hg vs. 120.3 mm Hg; P < .001).
There was an absolute risk reduction of 20% and a relative risk reduction of 73.5% in postnatal readmission. The number needed to treat to avoid one postnatal readmission was five, which “has potential for big cost savings,” said Dr. Leeson.
Blood pressure post partum can be improved with self-monitoring and physician-guided medication adjustment, Dr. Leeson summarized. The blood pressure remains low for at least 9 months, even when medication is stopped, and the intervention leads to beneficial cardiac remodeling.
U.S. pilot study
Non-Hispanic Black adults have a high hypertension and cardiovascular disease burden, and a related small U.S. study showed benefits of BP self-monitoring in a population comprising mainly Black women, Keith Ferdinand, MD, discussant of the POP-HT trial in the press briefing, said in an interview.
Dr. Ferdinand, from Tulane University, New Orleans, Louisiana, was lead author of the Text My Hypertension BP Meds NOLA pilot study that was published in February in the American Heart Journal Plus: Cardiology Research and Practice.
The study showed that text-messaging and social support increased hypertension medication adherence.
They enrolled 36 individuals, of whom 32 (89%) were non-Hispanic Black, and 23 (64%) were women. The participants received validated Bluetooth-enabled BP-monitoring devices that were synced to smartphones via a secured cloud-based application. The participants could send and receive messages to health care practitioners.
This intervention significantly improved medication adherence and systolic BP without modifying pharmacotherapy.
‘Need to be passionate about monitoring BP’
“The take-home messages from these exciting findings is that physicians and women who have had high BP during pregnancy need to be passionate about monitoring and controlling their blood pressure and not ignore it,” Anastasia Mihailidou, PhD, Royal North Shore Hospital, Sydney, the assigned discussant in the late-breaking trial session, said in an interview.
“It also resulted in fewer postpartum hospital readmissions for high blood pressure and benefit at 9 months in the structure and function of the heart and blood vessels of the women,” she said.
“While we need to see further studies in ethnically diverse women to see that they are reproducible, there are simple measures that clinicians can implement, and women can ask to have their BP monitored more frequently than the current practice. In the U.K. it is 5-10 days after delivery and then at 6-8 weeks after giving birth when changes in heart structure have already started,” Dr. Mihailidou noted.
“The procedure will need to be modified if there are no telemedicine facilities, but that should not stop having close monitoring of BP and treating it adequately. Monitoring requires an accurate BP monitor. There also has to be monitoring BP for the children.”
The trial was funded by a BHF Clinical Research Training Fellowship to Dr. Kitt, with additional support from the NIHR Oxford Biomedical Research Centre and Oxford BHF Centre for Research Excellence.
A version of this article first appeared on Medscape.com.
, new research suggests.
In a randomized trial of 220 women with preeclampsia or gestational hypertension, those who took daily postpartum BP readings and received clinician-guided advice for titrating antihypertensives had a 5 mm Hg–lower average diastolic BP at 9 months, compared with those receiving usual care.
Jamie Kitt, DPhil, from the University of Oxford (England) presented these findings from the Physicians Optimized Postpartum Hypertension Treatment (POP-HT, NCT04273854) clinical trial at the American Heart Association scientific sessions. The study was simultaneously published online in JAMA, and a cardiac imaging substudy was published online in Circulation.
“This trial identifies a potential need for a paradigm shift in the way women affected by hypertensive pregnancy are managed postnatally,” Dr. Kitt said. “If a 5–mm Hg improvement in BP is maintained longer term, it can result in about a 20% reduction in lifetime cardiovascular risk.”
The imaging substudy suggests that short-term postnatal optimization of BP control following hypertensive pregnancy through self-monitoring and physician-guided antihypertensive titration is linked with better cardiac remodeling changes seen by cardiovascular magnetic resonance and echocardiography.
POP-HT “proves for the first time that the first few weeks after delivery are a critical time that can determine the long-term cardiovascular health of the mother,” senior author Paul Leeson, PhD, also from the University of Oxford, who presented the findings in a press briefing, said in an interview.
“Interventions during this period can have long-term beneficial impacts on cardiovascular health,” he said. “These findings rewrite the textbook on our understanding of how and why hypertensive pregnancies associate with later cardiovascular disease in the mother.”
Next, Dr. Leeson said, “We need to work out the best ways to implement these interventions “at scale. Then we can ensure all women who have hypertensive pregnancies can get access to the long-term cardiovascular benefits we have demonstrated are possible through improving postpartum cardiac care,” he said, adding that “this is entirely achievable using current available technologies.”
Hypertension in pregnancy
About 1 in 10 pregnant women develop hypertension in pregnancy (preeclampsia or gestational hypertension), and 1 in 3 such women go on to develop chronic hypertension within 10 years, “when they are usually still in their 30s or 40s,” Dr. Leeson said.
During pregnancy, the heart remodels to cope with pregnancy, and it undergoes more severe changes if BP is high. Then during the 6 weeks after giving birth, this remodeling rapidly reverses.
Higher blood pressure in young adulthood is associated with a twofold higher risk of subsequent myocardial infarction and stroke. And abnormal cardiac remodeling postpartum is also linked with higher cardiovascular risk.
Self-monitoring blood pressure during the postpartum period may be a “critical window” for intervention.
Previously, the research group performed a pilot study, the Self-Management of Postnatal Antihypertensive Treatment (SNAP-HT) trial and the SNAP-extension trial, which compared a BP self-monitoring intervention with usual care in 91 women with gestational hypertension or preeclampsia requiring postnatal antihypertensive treatment.
Diastolic BP, which drives cardiovascular risk in younger populations, was 4.5–mm Hg lower at 6 months postpartum and 7–mm Hg lower at 4 years post partum in patients randomly assigned to BP self-management vs. usual care – even after they were no longer taking antihypertensives.
Building on these findings, the POP-HT trial enrolled 220 pregnant women seen at Oxford University Hospitals in the United Kingdom who were age 18 years or older, had either gestational hypertension or preeclampsia, and still required antihypertensives when they were being discharged from hospital after giving birth.
Following a baseline visit at day 1-6 after delivery, while in the postnatal ward, the patients were randomly assigned 1:1 to the intervention group (112 women) or usual-care group (108 women).
They had an average age of 32.6 years; 40% had gestational hypertension, and 60% had preeclampsia.
Women in the usual-care group typically received a BP review at 7-10 days after hospital discharge with a community midwife, and another at 6-8 weeks with their general practitioner.
The women in the intervention group were given and taught to use a Bluetooth-enabled OMRON Evolv BP monitor (Omron Healthcare Europe) while on the postnatal ward, and they installed a smartphone app on their mobile phones that transmitted self-monitored BP readings to a National Health Service-hosted, web-based platform.
They were instructed to take daily BP measurements (twice daily if out of target range). Dose titration of antihypertensives after hospital discharge was guided remotely by research clinicians, according to a guideline-based algorithm.
Patients in both groups had four study visits when their BP was measured: visit 1 (baseline) between days 1 and 6 post partum; visit 2 at week 1; visit 3 at week 6; and visit 4 between months 6 and 9 post partum.
Similar antihypertensive classes were prescribed in each group (enalapril 57%, nifedipine 27%, and labetalol 30% for intervention vs. enalapril 43%, nifedipine 30%, and labetalol 27% for control).
At 6 weeks, approximately 30% of participants in each group were still taking medication; this dropped to approximately 12% by visit 4.
The primary outcome – the mean 24-hour diastolic BP at visit 4 (roughly 9 months post partum), adjusted for baseline postnatal diastolic blood pressure – was 5.8–mm Hg lower in the intervention group than in the control group (71.2 mm Hg vs. 76.6 mm Hg; P < .001).
Secondary outcomes – between-group differences in systolic BP at 9 months, BP-related postnatal admission, and cardiac remodeling assessed by cardiac magnetic resonance – were all better in the intervention group.
The mean 24-hour average systolic BP at 9 months post partum, adjusted for baseline postnatal systolic BP was 6.5–mm Hg lower in the intervention group than in the control group (114.0 mm Hg vs. 120.3 mm Hg; P < .001).
There was an absolute risk reduction of 20% and a relative risk reduction of 73.5% in postnatal readmission. The number needed to treat to avoid one postnatal readmission was five, which “has potential for big cost savings,” said Dr. Leeson.
Blood pressure post partum can be improved with self-monitoring and physician-guided medication adjustment, Dr. Leeson summarized. The blood pressure remains low for at least 9 months, even when medication is stopped, and the intervention leads to beneficial cardiac remodeling.
U.S. pilot study
Non-Hispanic Black adults have a high hypertension and cardiovascular disease burden, and a related small U.S. study showed benefits of BP self-monitoring in a population comprising mainly Black women, Keith Ferdinand, MD, discussant of the POP-HT trial in the press briefing, said in an interview.
Dr. Ferdinand, from Tulane University, New Orleans, Louisiana, was lead author of the Text My Hypertension BP Meds NOLA pilot study that was published in February in the American Heart Journal Plus: Cardiology Research and Practice.
The study showed that text-messaging and social support increased hypertension medication adherence.
They enrolled 36 individuals, of whom 32 (89%) were non-Hispanic Black, and 23 (64%) were women. The participants received validated Bluetooth-enabled BP-monitoring devices that were synced to smartphones via a secured cloud-based application. The participants could send and receive messages to health care practitioners.
This intervention significantly improved medication adherence and systolic BP without modifying pharmacotherapy.
‘Need to be passionate about monitoring BP’
“The take-home messages from these exciting findings is that physicians and women who have had high BP during pregnancy need to be passionate about monitoring and controlling their blood pressure and not ignore it,” Anastasia Mihailidou, PhD, Royal North Shore Hospital, Sydney, the assigned discussant in the late-breaking trial session, said in an interview.
“It also resulted in fewer postpartum hospital readmissions for high blood pressure and benefit at 9 months in the structure and function of the heart and blood vessels of the women,” she said.
“While we need to see further studies in ethnically diverse women to see that they are reproducible, there are simple measures that clinicians can implement, and women can ask to have their BP monitored more frequently than the current practice. In the U.K. it is 5-10 days after delivery and then at 6-8 weeks after giving birth when changes in heart structure have already started,” Dr. Mihailidou noted.
“The procedure will need to be modified if there are no telemedicine facilities, but that should not stop having close monitoring of BP and treating it adequately. Monitoring requires an accurate BP monitor. There also has to be monitoring BP for the children.”
The trial was funded by a BHF Clinical Research Training Fellowship to Dr. Kitt, with additional support from the NIHR Oxford Biomedical Research Centre and Oxford BHF Centre for Research Excellence.
A version of this article first appeared on Medscape.com.
FROM AHA 2023
In MI with anemia, results may favor liberal transfusion: MINT
In patients with myocardial infarction and anemia, a “liberal” red blood cell transfusion strategy did not significantly reduce the risk of recurrent MI or death within 30 days, compared with a “restrictive” transfusion strategy, in the 3,500-patient MINT trial.
Jeffrey L. Carson, MD, from Robert Wood Johnson Medical School, New Brunswick, N.J., said in a press briefing.
He presented the study in a late-breaking trial session at the annual scientific sessions of the American Heart Association, and it was simultaneously published online in the New England Journal of Medicine.
“Whether to transfuse is an everyday decision faced by clinicians caring for patients with acute MI,” Dr. Carson said.
“We cannot claim that a liberal transfusion strategy is definitively superior based on our primary outcome,” he said, but “the 95% confidence interval is consistent with treatment effects corresponding to no difference between the two transfusion strategies and to a clinically relevant benefit with the liberal strategy.”
“In contrast to other trials in other settings,” such as anemia and cardiac surgery, Dr. Carson said, “the results suggest that a liberal transfusion strategy has the potential for clinical benefit with an acceptable risk of harm.”
“A liberal transfusion strategy may be the most prudent approach to transfusion in anemic patients with MI,” he added.
Not a home run
Others agreed with this interpretation. Martin B. Leon, MD, from Columbia University, New York, the study discussant in the press briefing, said the study “addresses a question that is common” in clinical practice. It was well conducted, and international (although most patients were in the United States and Canada), in a very broad group of patients, designed to make the results more generalizable. The 98% follow-up was extremely good, Dr. Leon added, and the trialists achieved their goal in that they did show a difference between the two transfusion strategies.
The number needed to treat was 40 to see a benefit in the combined outcome of death or recurrent MI at 30 days, Dr. Leon said. The P value for this was .07, “right on the edge” of statistical significance.
This study is “not a home run,” for the primary outcome, he noted; however, many of the outcomes tended to be in favor of a liberal transfusion strategy. Notably, cardiovascular death, which was not a specified outcome, was significantly lower in the group who received a liberal transfusion strategy.
Although a liberal transfusion strategy was “not definitely superior” in these patients with MI and anemia, Dr. Carson said, he thinks the trial will be interpreted as favoring a liberal transfusion strategy.
C. Michael Gibson, MD, professor of medicine at Harvard Medical School, Boston, and CEO of Harvard’s Baim and PERFUSE institutes for clinical research, voiced similar views.
“Given the lack of acute harm associated with liberal transfusion and the preponderance of evidence favoring liberal transfusion in the largest trial to date,” concluded Dr. Gibson, the assigned discussant at the session, “liberal transfusion appears to be a viable management strategy, particularly among patients with non-STEMI type 1 MI and as clinical judgment dictates.”
Only three small randomized controlled trials have compared transfusion thresholds in a total of 820 patients with MI and anemia, Dr. Gibson said, a point that the trial investigators also made. The results were inconsistent between trials: the CRIT trial (n = 45) favored a restrictive strategy, the MINT pilot study (n = 110) favored a liberal one, and the REALITY trial (n = 668) showed noninferiority of a restrictive strategy, compared with a liberal strategy in 30-day MACE.
The MINT trial was four times larger than all prior studies combined. However, most outcomes were negative or of borderline significance for benefit.
Cardiac death was more common in the restrictive group at 5.5% than the liberal group at 3.2% (risk ratio, 1.74, 95% CI, 1.26-2.40), but this was nonadjudicated, and not designated as a primary, secondary, or tertiary outcome – which the researchers also noted. Fewer than half of the deaths were classified as cardiac, which was “odd,” Dr. Gibson observed.
A restrictive transfusion strategy was associated with increased events among participants with type 1 MI (RR, 1.32, 95% CI, 1.04-1.67), he noted.
Study strengths included that 45.5% of participants were women, Dr. Gibson said. Limitations included that the trial was “somewhat underpowered.” Also, even in the restrictive group, participants received a mean of 0.7 units of packed red blood cells.
Adherence to the 10 g/dL threshold in the liberal transfusion group was moderate (86.3% at hospital discharge), which the researchers acknowledged. They noted that this was frequently caused by clinical discretion, such as concern about fluid overload, and to the timing of hospital discharge. In addition, long-term potential for harm (microchimerism) is not known.
“There was a consistent nonsignificant acute benefit for liberal transfusion and a nominal reduction in CV mortality and improved outcomes in patients with type 1 MI in exploratory analyses, in a trial that ended up underpowered,” Dr. Gibson summarized. “Long-term follow up would be helpful to evaluate chronic outcomes.”
This is a very well-conducted, high-quality, important study that will be considered a landmark trial, C. David Mazer, MD, University of Toronto and St. Michael’s Hospital, also in Toronto, said in an interview.
Unfortunately, “it was not as definitive as hoped for,” Dr. Mazer lamented. Nevertheless, “I think people may interpret it as providing support for a liberal transfusion strategy” in patients with anemia and MI, he said.
Dr. Mazer, who was not involved with this research, was a principal investigator on the TRICS-3 trial, which disputed a liberal RBC transfusion strategy in patients with anemia undergoing cardiac surgery, as previously reported.
The “Red Blood Cell Transfusion: 2023 AABB International Guidelines,” led by Dr. Carson and published in JAMA, recommend a restrictive strategy in stable patients, although these guidelines did not include the current study, Dr. Mazer observed.
In the REALITY trial, there were fewer major adverse cardiac events (MACE) events in the restrictive strategy, he noted.
MINT can be viewed as comparing a high versus low hemoglobin threshold. “It is possible that the best is in between,” he said.
Dr. Mazer also noted that MINT may have achieved significance if it was designed with a larger enrollment and a higher power (for example, 90% instead of 80%) to detect between-group difference for the primary outcome.
Study rationale, design, and findings
Anemia, or low RBC count, is common in patients with MI, Dr. Carson noted. A normal hemoglobin is 13 g/dL in men and 12 g/dL in women. Administering a packed RBC transfusion only when a patient’s hemoglobin falls below 7 or 8 g/dL has been widely adopted, but it is unclear if patients with acute MI may benefit from a higher hemoglobin level.
“Blood transfusion may decrease ischemic injury by improving oxygen delivery to myocardial tissues and reduce the risk of reinfarction or death,” the researchers wrote. “Alternatively, administering more blood could result in more frequent heart failure from fluid overload, infection from immunosuppression, thrombosis from higher viscosity, and inflammation.”
From 2017 to 2023, investigators enrolled 3,504 adults aged 18 and older at 144 sites in the United States (2,157 patients), Canada (885), France (323), Brazil (105), New Zealand (25), and Australia (9).
The participants had ST-elevation or non–ST-elevation MI and hemoglobin less than 10 g/dL within 24 hours. Patients with type 1 (atherosclerotic plaque disruption), type 2 (supply-demand mismatch without atherothrombotic plaque disruption), type 4b, or type 4c MI were eligible.
They were randomly assigned to receive:
- A ‘restrictive’ transfusion strategy (1,749 patients): Transfusion was permitted but not required when a patient’s hemoglobin was less than 8 g/dL and was strongly recommended when it was less than 7 g/dL or when anginal symptoms were not controlled with medications.
- A ‘liberal’ transfusion strategy (1,755 patients): One unit of RBCs was administered after randomization, and RBCs were transfused to maintain hemoglobin 10 g/dL or higher until hospital discharge or 30 days.
The patients had a mean age of 72 years and 46% were women. More than three-quarters (78%) were White and 14% were Black. They had frequent coexisting illnesses, about a third had a history of MI, percutaneous coronary intervention, or heart failure; 14% were on a ventilator and 12% had renal dialysis. The median duration of hospitalization was 5 days in the two groups.
At baseline, the mean hemoglobin was 8.6 g/dL in both groups. At days 1, 2, and 3, the mean hemoglobin was 8.8, 8.9, and 8.9 g/dL, respectively, in the restrictive transfusion group, and 10.1, 10.4, and 10.5 g/dL, respectively, in the liberal transfusion group.
The mean number of transfused blood units was 0.7 units in the restrictive strategy group and 2.5 units in the liberal strategy group, roughly a 3.5-fold difference.
After adjustment for site and incomplete follow-up in 57 patients (20 with the restrictive strategy and 37 with the liberal strategy), the estimated RR for the primary outcome in the restrictive group versus the liberal group was 1.15 (P = .07).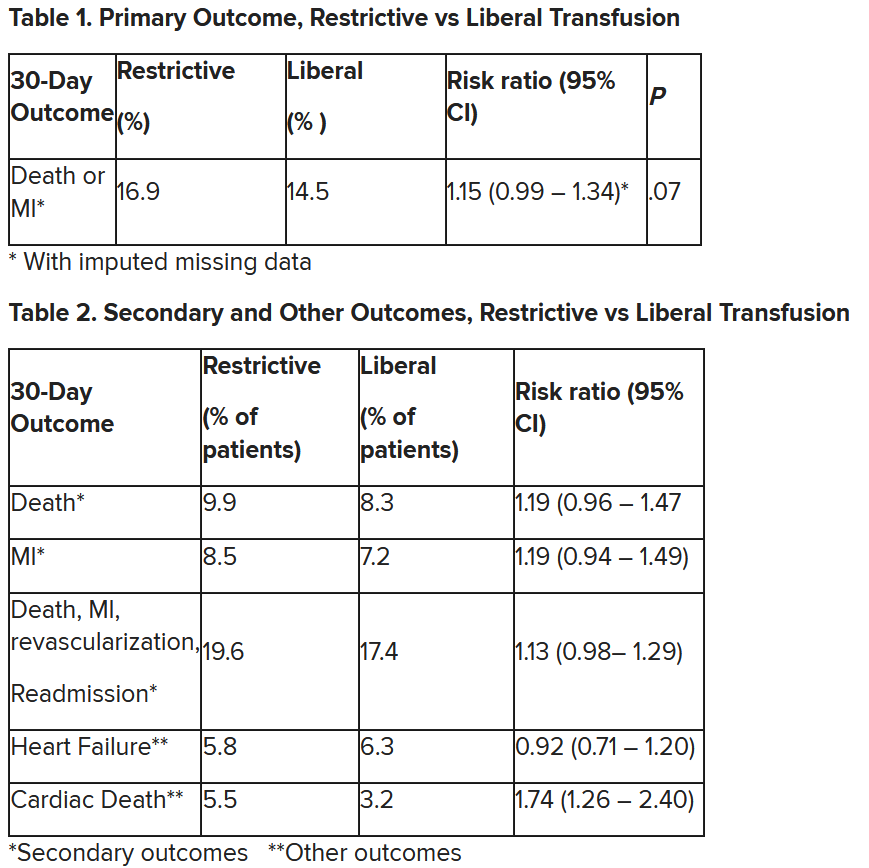
“We observed that the 95% confidence interval contains values that suggest a clinical benefit for the liberal transfusion strategy and does not include values that suggest a benefit for the more restrictive transfusion strategy,” the researchers wrote. Heart failure and other safety outcomes were comparable in the two groups.
The trial was supported by grants from the National Heart, Lung, and Blood Institute and by the Canadian Blood Services and Canadian Institutes of Health Research Institute of Circulatory and Respiratory Health. Dr. Carson, Dr. Leon, Dr. Gibson, and Dr. Mazer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In patients with myocardial infarction and anemia, a “liberal” red blood cell transfusion strategy did not significantly reduce the risk of recurrent MI or death within 30 days, compared with a “restrictive” transfusion strategy, in the 3,500-patient MINT trial.
Jeffrey L. Carson, MD, from Robert Wood Johnson Medical School, New Brunswick, N.J., said in a press briefing.
He presented the study in a late-breaking trial session at the annual scientific sessions of the American Heart Association, and it was simultaneously published online in the New England Journal of Medicine.
“Whether to transfuse is an everyday decision faced by clinicians caring for patients with acute MI,” Dr. Carson said.
“We cannot claim that a liberal transfusion strategy is definitively superior based on our primary outcome,” he said, but “the 95% confidence interval is consistent with treatment effects corresponding to no difference between the two transfusion strategies and to a clinically relevant benefit with the liberal strategy.”
“In contrast to other trials in other settings,” such as anemia and cardiac surgery, Dr. Carson said, “the results suggest that a liberal transfusion strategy has the potential for clinical benefit with an acceptable risk of harm.”
“A liberal transfusion strategy may be the most prudent approach to transfusion in anemic patients with MI,” he added.
Not a home run
Others agreed with this interpretation. Martin B. Leon, MD, from Columbia University, New York, the study discussant in the press briefing, said the study “addresses a question that is common” in clinical practice. It was well conducted, and international (although most patients were in the United States and Canada), in a very broad group of patients, designed to make the results more generalizable. The 98% follow-up was extremely good, Dr. Leon added, and the trialists achieved their goal in that they did show a difference between the two transfusion strategies.
The number needed to treat was 40 to see a benefit in the combined outcome of death or recurrent MI at 30 days, Dr. Leon said. The P value for this was .07, “right on the edge” of statistical significance.
This study is “not a home run,” for the primary outcome, he noted; however, many of the outcomes tended to be in favor of a liberal transfusion strategy. Notably, cardiovascular death, which was not a specified outcome, was significantly lower in the group who received a liberal transfusion strategy.
Although a liberal transfusion strategy was “not definitely superior” in these patients with MI and anemia, Dr. Carson said, he thinks the trial will be interpreted as favoring a liberal transfusion strategy.
C. Michael Gibson, MD, professor of medicine at Harvard Medical School, Boston, and CEO of Harvard’s Baim and PERFUSE institutes for clinical research, voiced similar views.
“Given the lack of acute harm associated with liberal transfusion and the preponderance of evidence favoring liberal transfusion in the largest trial to date,” concluded Dr. Gibson, the assigned discussant at the session, “liberal transfusion appears to be a viable management strategy, particularly among patients with non-STEMI type 1 MI and as clinical judgment dictates.”
Only three small randomized controlled trials have compared transfusion thresholds in a total of 820 patients with MI and anemia, Dr. Gibson said, a point that the trial investigators also made. The results were inconsistent between trials: the CRIT trial (n = 45) favored a restrictive strategy, the MINT pilot study (n = 110) favored a liberal one, and the REALITY trial (n = 668) showed noninferiority of a restrictive strategy, compared with a liberal strategy in 30-day MACE.
The MINT trial was four times larger than all prior studies combined. However, most outcomes were negative or of borderline significance for benefit.
Cardiac death was more common in the restrictive group at 5.5% than the liberal group at 3.2% (risk ratio, 1.74, 95% CI, 1.26-2.40), but this was nonadjudicated, and not designated as a primary, secondary, or tertiary outcome – which the researchers also noted. Fewer than half of the deaths were classified as cardiac, which was “odd,” Dr. Gibson observed.
A restrictive transfusion strategy was associated with increased events among participants with type 1 MI (RR, 1.32, 95% CI, 1.04-1.67), he noted.
Study strengths included that 45.5% of participants were women, Dr. Gibson said. Limitations included that the trial was “somewhat underpowered.” Also, even in the restrictive group, participants received a mean of 0.7 units of packed red blood cells.
Adherence to the 10 g/dL threshold in the liberal transfusion group was moderate (86.3% at hospital discharge), which the researchers acknowledged. They noted that this was frequently caused by clinical discretion, such as concern about fluid overload, and to the timing of hospital discharge. In addition, long-term potential for harm (microchimerism) is not known.
“There was a consistent nonsignificant acute benefit for liberal transfusion and a nominal reduction in CV mortality and improved outcomes in patients with type 1 MI in exploratory analyses, in a trial that ended up underpowered,” Dr. Gibson summarized. “Long-term follow up would be helpful to evaluate chronic outcomes.”
This is a very well-conducted, high-quality, important study that will be considered a landmark trial, C. David Mazer, MD, University of Toronto and St. Michael’s Hospital, also in Toronto, said in an interview.
Unfortunately, “it was not as definitive as hoped for,” Dr. Mazer lamented. Nevertheless, “I think people may interpret it as providing support for a liberal transfusion strategy” in patients with anemia and MI, he said.
Dr. Mazer, who was not involved with this research, was a principal investigator on the TRICS-3 trial, which disputed a liberal RBC transfusion strategy in patients with anemia undergoing cardiac surgery, as previously reported.
The “Red Blood Cell Transfusion: 2023 AABB International Guidelines,” led by Dr. Carson and published in JAMA, recommend a restrictive strategy in stable patients, although these guidelines did not include the current study, Dr. Mazer observed.
In the REALITY trial, there were fewer major adverse cardiac events (MACE) events in the restrictive strategy, he noted.
MINT can be viewed as comparing a high versus low hemoglobin threshold. “It is possible that the best is in between,” he said.
Dr. Mazer also noted that MINT may have achieved significance if it was designed with a larger enrollment and a higher power (for example, 90% instead of 80%) to detect between-group difference for the primary outcome.
Study rationale, design, and findings
Anemia, or low RBC count, is common in patients with MI, Dr. Carson noted. A normal hemoglobin is 13 g/dL in men and 12 g/dL in women. Administering a packed RBC transfusion only when a patient’s hemoglobin falls below 7 or 8 g/dL has been widely adopted, but it is unclear if patients with acute MI may benefit from a higher hemoglobin level.
“Blood transfusion may decrease ischemic injury by improving oxygen delivery to myocardial tissues and reduce the risk of reinfarction or death,” the researchers wrote. “Alternatively, administering more blood could result in more frequent heart failure from fluid overload, infection from immunosuppression, thrombosis from higher viscosity, and inflammation.”
From 2017 to 2023, investigators enrolled 3,504 adults aged 18 and older at 144 sites in the United States (2,157 patients), Canada (885), France (323), Brazil (105), New Zealand (25), and Australia (9).
The participants had ST-elevation or non–ST-elevation MI and hemoglobin less than 10 g/dL within 24 hours. Patients with type 1 (atherosclerotic plaque disruption), type 2 (supply-demand mismatch without atherothrombotic plaque disruption), type 4b, or type 4c MI were eligible.
They were randomly assigned to receive:
- A ‘restrictive’ transfusion strategy (1,749 patients): Transfusion was permitted but not required when a patient’s hemoglobin was less than 8 g/dL and was strongly recommended when it was less than 7 g/dL or when anginal symptoms were not controlled with medications.
- A ‘liberal’ transfusion strategy (1,755 patients): One unit of RBCs was administered after randomization, and RBCs were transfused to maintain hemoglobin 10 g/dL or higher until hospital discharge or 30 days.
The patients had a mean age of 72 years and 46% were women. More than three-quarters (78%) were White and 14% were Black. They had frequent coexisting illnesses, about a third had a history of MI, percutaneous coronary intervention, or heart failure; 14% were on a ventilator and 12% had renal dialysis. The median duration of hospitalization was 5 days in the two groups.
At baseline, the mean hemoglobin was 8.6 g/dL in both groups. At days 1, 2, and 3, the mean hemoglobin was 8.8, 8.9, and 8.9 g/dL, respectively, in the restrictive transfusion group, and 10.1, 10.4, and 10.5 g/dL, respectively, in the liberal transfusion group.
The mean number of transfused blood units was 0.7 units in the restrictive strategy group and 2.5 units in the liberal strategy group, roughly a 3.5-fold difference.
After adjustment for site and incomplete follow-up in 57 patients (20 with the restrictive strategy and 37 with the liberal strategy), the estimated RR for the primary outcome in the restrictive group versus the liberal group was 1.15 (P = .07).
“We observed that the 95% confidence interval contains values that suggest a clinical benefit for the liberal transfusion strategy and does not include values that suggest a benefit for the more restrictive transfusion strategy,” the researchers wrote. Heart failure and other safety outcomes were comparable in the two groups.
The trial was supported by grants from the National Heart, Lung, and Blood Institute and by the Canadian Blood Services and Canadian Institutes of Health Research Institute of Circulatory and Respiratory Health. Dr. Carson, Dr. Leon, Dr. Gibson, and Dr. Mazer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In patients with myocardial infarction and anemia, a “liberal” red blood cell transfusion strategy did not significantly reduce the risk of recurrent MI or death within 30 days, compared with a “restrictive” transfusion strategy, in the 3,500-patient MINT trial.
Jeffrey L. Carson, MD, from Robert Wood Johnson Medical School, New Brunswick, N.J., said in a press briefing.
He presented the study in a late-breaking trial session at the annual scientific sessions of the American Heart Association, and it was simultaneously published online in the New England Journal of Medicine.
“Whether to transfuse is an everyday decision faced by clinicians caring for patients with acute MI,” Dr. Carson said.
“We cannot claim that a liberal transfusion strategy is definitively superior based on our primary outcome,” he said, but “the 95% confidence interval is consistent with treatment effects corresponding to no difference between the two transfusion strategies and to a clinically relevant benefit with the liberal strategy.”
“In contrast to other trials in other settings,” such as anemia and cardiac surgery, Dr. Carson said, “the results suggest that a liberal transfusion strategy has the potential for clinical benefit with an acceptable risk of harm.”
“A liberal transfusion strategy may be the most prudent approach to transfusion in anemic patients with MI,” he added.
Not a home run
Others agreed with this interpretation. Martin B. Leon, MD, from Columbia University, New York, the study discussant in the press briefing, said the study “addresses a question that is common” in clinical practice. It was well conducted, and international (although most patients were in the United States and Canada), in a very broad group of patients, designed to make the results more generalizable. The 98% follow-up was extremely good, Dr. Leon added, and the trialists achieved their goal in that they did show a difference between the two transfusion strategies.
The number needed to treat was 40 to see a benefit in the combined outcome of death or recurrent MI at 30 days, Dr. Leon said. The P value for this was .07, “right on the edge” of statistical significance.
This study is “not a home run,” for the primary outcome, he noted; however, many of the outcomes tended to be in favor of a liberal transfusion strategy. Notably, cardiovascular death, which was not a specified outcome, was significantly lower in the group who received a liberal transfusion strategy.
Although a liberal transfusion strategy was “not definitely superior” in these patients with MI and anemia, Dr. Carson said, he thinks the trial will be interpreted as favoring a liberal transfusion strategy.
C. Michael Gibson, MD, professor of medicine at Harvard Medical School, Boston, and CEO of Harvard’s Baim and PERFUSE institutes for clinical research, voiced similar views.
“Given the lack of acute harm associated with liberal transfusion and the preponderance of evidence favoring liberal transfusion in the largest trial to date,” concluded Dr. Gibson, the assigned discussant at the session, “liberal transfusion appears to be a viable management strategy, particularly among patients with non-STEMI type 1 MI and as clinical judgment dictates.”
Only three small randomized controlled trials have compared transfusion thresholds in a total of 820 patients with MI and anemia, Dr. Gibson said, a point that the trial investigators also made. The results were inconsistent between trials: the CRIT trial (n = 45) favored a restrictive strategy, the MINT pilot study (n = 110) favored a liberal one, and the REALITY trial (n = 668) showed noninferiority of a restrictive strategy, compared with a liberal strategy in 30-day MACE.
The MINT trial was four times larger than all prior studies combined. However, most outcomes were negative or of borderline significance for benefit.
Cardiac death was more common in the restrictive group at 5.5% than the liberal group at 3.2% (risk ratio, 1.74, 95% CI, 1.26-2.40), but this was nonadjudicated, and not designated as a primary, secondary, or tertiary outcome – which the researchers also noted. Fewer than half of the deaths were classified as cardiac, which was “odd,” Dr. Gibson observed.
A restrictive transfusion strategy was associated with increased events among participants with type 1 MI (RR, 1.32, 95% CI, 1.04-1.67), he noted.
Study strengths included that 45.5% of participants were women, Dr. Gibson said. Limitations included that the trial was “somewhat underpowered.” Also, even in the restrictive group, participants received a mean of 0.7 units of packed red blood cells.
Adherence to the 10 g/dL threshold in the liberal transfusion group was moderate (86.3% at hospital discharge), which the researchers acknowledged. They noted that this was frequently caused by clinical discretion, such as concern about fluid overload, and to the timing of hospital discharge. In addition, long-term potential for harm (microchimerism) is not known.
“There was a consistent nonsignificant acute benefit for liberal transfusion and a nominal reduction in CV mortality and improved outcomes in patients with type 1 MI in exploratory analyses, in a trial that ended up underpowered,” Dr. Gibson summarized. “Long-term follow up would be helpful to evaluate chronic outcomes.”
This is a very well-conducted, high-quality, important study that will be considered a landmark trial, C. David Mazer, MD, University of Toronto and St. Michael’s Hospital, also in Toronto, said in an interview.
Unfortunately, “it was not as definitive as hoped for,” Dr. Mazer lamented. Nevertheless, “I think people may interpret it as providing support for a liberal transfusion strategy” in patients with anemia and MI, he said.
Dr. Mazer, who was not involved with this research, was a principal investigator on the TRICS-3 trial, which disputed a liberal RBC transfusion strategy in patients with anemia undergoing cardiac surgery, as previously reported.
The “Red Blood Cell Transfusion: 2023 AABB International Guidelines,” led by Dr. Carson and published in JAMA, recommend a restrictive strategy in stable patients, although these guidelines did not include the current study, Dr. Mazer observed.
In the REALITY trial, there were fewer major adverse cardiac events (MACE) events in the restrictive strategy, he noted.
MINT can be viewed as comparing a high versus low hemoglobin threshold. “It is possible that the best is in between,” he said.
Dr. Mazer also noted that MINT may have achieved significance if it was designed with a larger enrollment and a higher power (for example, 90% instead of 80%) to detect between-group difference for the primary outcome.
Study rationale, design, and findings
Anemia, or low RBC count, is common in patients with MI, Dr. Carson noted. A normal hemoglobin is 13 g/dL in men and 12 g/dL in women. Administering a packed RBC transfusion only when a patient’s hemoglobin falls below 7 or 8 g/dL has been widely adopted, but it is unclear if patients with acute MI may benefit from a higher hemoglobin level.
“Blood transfusion may decrease ischemic injury by improving oxygen delivery to myocardial tissues and reduce the risk of reinfarction or death,” the researchers wrote. “Alternatively, administering more blood could result in more frequent heart failure from fluid overload, infection from immunosuppression, thrombosis from higher viscosity, and inflammation.”
From 2017 to 2023, investigators enrolled 3,504 adults aged 18 and older at 144 sites in the United States (2,157 patients), Canada (885), France (323), Brazil (105), New Zealand (25), and Australia (9).
The participants had ST-elevation or non–ST-elevation MI and hemoglobin less than 10 g/dL within 24 hours. Patients with type 1 (atherosclerotic plaque disruption), type 2 (supply-demand mismatch without atherothrombotic plaque disruption), type 4b, or type 4c MI were eligible.
They were randomly assigned to receive:
- A ‘restrictive’ transfusion strategy (1,749 patients): Transfusion was permitted but not required when a patient’s hemoglobin was less than 8 g/dL and was strongly recommended when it was less than 7 g/dL or when anginal symptoms were not controlled with medications.
- A ‘liberal’ transfusion strategy (1,755 patients): One unit of RBCs was administered after randomization, and RBCs were transfused to maintain hemoglobin 10 g/dL or higher until hospital discharge or 30 days.
The patients had a mean age of 72 years and 46% were women. More than three-quarters (78%) were White and 14% were Black. They had frequent coexisting illnesses, about a third had a history of MI, percutaneous coronary intervention, or heart failure; 14% were on a ventilator and 12% had renal dialysis. The median duration of hospitalization was 5 days in the two groups.
At baseline, the mean hemoglobin was 8.6 g/dL in both groups. At days 1, 2, and 3, the mean hemoglobin was 8.8, 8.9, and 8.9 g/dL, respectively, in the restrictive transfusion group, and 10.1, 10.4, and 10.5 g/dL, respectively, in the liberal transfusion group.
The mean number of transfused blood units was 0.7 units in the restrictive strategy group and 2.5 units in the liberal strategy group, roughly a 3.5-fold difference.
After adjustment for site and incomplete follow-up in 57 patients (20 with the restrictive strategy and 37 with the liberal strategy), the estimated RR for the primary outcome in the restrictive group versus the liberal group was 1.15 (P = .07).
“We observed that the 95% confidence interval contains values that suggest a clinical benefit for the liberal transfusion strategy and does not include values that suggest a benefit for the more restrictive transfusion strategy,” the researchers wrote. Heart failure and other safety outcomes were comparable in the two groups.
The trial was supported by grants from the National Heart, Lung, and Blood Institute and by the Canadian Blood Services and Canadian Institutes of Health Research Institute of Circulatory and Respiratory Health. Dr. Carson, Dr. Leon, Dr. Gibson, and Dr. Mazer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AHA 2023
Newer antiobesity meds lower the body’s defended fat mass
The current highly effective antiobesity medications approved for treating obesity (semaglutide), under review (tirzepatide), or in late-stage clinical trials “appear to lower the body’s target and defended fat mass [set point]” but do not permanently fix it at a lower point, Lee M. Kaplan, MD, PhD, explained in a lecture during the annual meeting of the Obesity Society.
It is very likely that patients with obesity will have to take these antiobesity medications “forever,” he said, “until we identify and can repair the cellular and molecular mechanisms that the body uses to regulate body fat mass throughout the life cycle and that are dysfunctional in obesity.”
“The body is able to regulate fat mass at multiple stages during development,” Dr. Kaplan, from Massachusetts General Hospital and Harvard Medical School, Boston, explained, “and when it doesn’t do it appropriately, that becomes the physiological basis of obesity.”
The loss of baby fat, as well as fat changes during puberty, menopause, aging, and, in particular, during and after pregnancy, “all occur without conscious or purposeful input,” he noted.
The body uses food intake and energy expenditure to reach and defend its intended fat mass, and there is an evolutionary benefit to doing this.
For example, people recovering from an acute illness can regain the lost fat and weight. A woman can support a pregnancy and lactation by increasing fat mass.
However, “the idea that [with antiobesity medications] we should be aiming for a fixed lower amount of fat is probably not a good idea.” Dr. Kaplan cautioned.
People need the flexibility to recover lost fat and weight after an acute illness or injury, and pregnant women need to gain an appropriate amount of body fat to support pregnancy and lactation.
Intermittent therapy: A practical strategy?
The long-term benefit of antiobesity medications requires continuous use, Dr. Kaplan noted. For example, in the STEP 1 trial of semaglutide in patients with obesity and without diabetes, when treatment was stopped at 68 weeks, average weight increased through 120 weeks, although it did not return to baseline levels.
Intermittent antiobesity therapy may be an effective, “very practical strategy” to maintain weight loss, which would also “address current challenges of high cost, limited drug availability, and inadequate access to care.”
“Until we have strategies for decreasing the cost of effective obesity treatment, and ensuring more equitable access to obesity care,” Dr. Kaplan said, “optimizing algorithms for the use of intermittent therapy may be an effective stopgap measure.”
Dr. Kaplan is or has recently been a paid consultant for Eli Lilly, Novo Nordisk, and multiple pharmaceutical companies developing antiobesity medications.
A version of this article appeared on Medscape.com.
The current highly effective antiobesity medications approved for treating obesity (semaglutide), under review (tirzepatide), or in late-stage clinical trials “appear to lower the body’s target and defended fat mass [set point]” but do not permanently fix it at a lower point, Lee M. Kaplan, MD, PhD, explained in a lecture during the annual meeting of the Obesity Society.
It is very likely that patients with obesity will have to take these antiobesity medications “forever,” he said, “until we identify and can repair the cellular and molecular mechanisms that the body uses to regulate body fat mass throughout the life cycle and that are dysfunctional in obesity.”
“The body is able to regulate fat mass at multiple stages during development,” Dr. Kaplan, from Massachusetts General Hospital and Harvard Medical School, Boston, explained, “and when it doesn’t do it appropriately, that becomes the physiological basis of obesity.”
The loss of baby fat, as well as fat changes during puberty, menopause, aging, and, in particular, during and after pregnancy, “all occur without conscious or purposeful input,” he noted.
The body uses food intake and energy expenditure to reach and defend its intended fat mass, and there is an evolutionary benefit to doing this.
For example, people recovering from an acute illness can regain the lost fat and weight. A woman can support a pregnancy and lactation by increasing fat mass.
However, “the idea that [with antiobesity medications] we should be aiming for a fixed lower amount of fat is probably not a good idea.” Dr. Kaplan cautioned.
People need the flexibility to recover lost fat and weight after an acute illness or injury, and pregnant women need to gain an appropriate amount of body fat to support pregnancy and lactation.
Intermittent therapy: A practical strategy?
The long-term benefit of antiobesity medications requires continuous use, Dr. Kaplan noted. For example, in the STEP 1 trial of semaglutide in patients with obesity and without diabetes, when treatment was stopped at 68 weeks, average weight increased through 120 weeks, although it did not return to baseline levels.
Intermittent antiobesity therapy may be an effective, “very practical strategy” to maintain weight loss, which would also “address current challenges of high cost, limited drug availability, and inadequate access to care.”
“Until we have strategies for decreasing the cost of effective obesity treatment, and ensuring more equitable access to obesity care,” Dr. Kaplan said, “optimizing algorithms for the use of intermittent therapy may be an effective stopgap measure.”
Dr. Kaplan is or has recently been a paid consultant for Eli Lilly, Novo Nordisk, and multiple pharmaceutical companies developing antiobesity medications.
A version of this article appeared on Medscape.com.
The current highly effective antiobesity medications approved for treating obesity (semaglutide), under review (tirzepatide), or in late-stage clinical trials “appear to lower the body’s target and defended fat mass [set point]” but do not permanently fix it at a lower point, Lee M. Kaplan, MD, PhD, explained in a lecture during the annual meeting of the Obesity Society.
It is very likely that patients with obesity will have to take these antiobesity medications “forever,” he said, “until we identify and can repair the cellular and molecular mechanisms that the body uses to regulate body fat mass throughout the life cycle and that are dysfunctional in obesity.”
“The body is able to regulate fat mass at multiple stages during development,” Dr. Kaplan, from Massachusetts General Hospital and Harvard Medical School, Boston, explained, “and when it doesn’t do it appropriately, that becomes the physiological basis of obesity.”
The loss of baby fat, as well as fat changes during puberty, menopause, aging, and, in particular, during and after pregnancy, “all occur without conscious or purposeful input,” he noted.
The body uses food intake and energy expenditure to reach and defend its intended fat mass, and there is an evolutionary benefit to doing this.
For example, people recovering from an acute illness can regain the lost fat and weight. A woman can support a pregnancy and lactation by increasing fat mass.
However, “the idea that [with antiobesity medications] we should be aiming for a fixed lower amount of fat is probably not a good idea.” Dr. Kaplan cautioned.
People need the flexibility to recover lost fat and weight after an acute illness or injury, and pregnant women need to gain an appropriate amount of body fat to support pregnancy and lactation.
Intermittent therapy: A practical strategy?
The long-term benefit of antiobesity medications requires continuous use, Dr. Kaplan noted. For example, in the STEP 1 trial of semaglutide in patients with obesity and without diabetes, when treatment was stopped at 68 weeks, average weight increased through 120 weeks, although it did not return to baseline levels.
Intermittent antiobesity therapy may be an effective, “very practical strategy” to maintain weight loss, which would also “address current challenges of high cost, limited drug availability, and inadequate access to care.”
“Until we have strategies for decreasing the cost of effective obesity treatment, and ensuring more equitable access to obesity care,” Dr. Kaplan said, “optimizing algorithms for the use of intermittent therapy may be an effective stopgap measure.”
Dr. Kaplan is or has recently been a paid consultant for Eli Lilly, Novo Nordisk, and multiple pharmaceutical companies developing antiobesity medications.
A version of this article appeared on Medscape.com.
FROM OBESITYWEEK® 2023
Beyond semaglutide, a coming pipeline of new antiobesity meds
“We are at a watershed [moment] brought on by the recent introduction of highly effective antiobesity medications,” Ania M. Jastreboff, MD, PhD, said in a lecture at the annual meeting of the Obesity Society.
Dr. Jastreboff, of Yale University and the Yale Center for Weight Management, New Haven, Conn., provided an overview of the many nutrient-stimulated hormone-based antiobesity therapies in late phases of development – including dual and triple therapies with glucagon-like peptide 1 receptor agonists (GLP-1 RAs), glucose-dependent insulinotropic polypeptide (GIP) agonists, glucagon, and amylin.
“I’ve shown you all of these agents that clearly produce substantial weight reduction,” she said. “The fact that these nutrient-stimulated, hormone-based therapies are not all the same is a good thing,” she stressed, because “it’s not likely that everyone will respond to each of these, and they are likely to respond differently.”
She then briefly touched on activin receptor inhibitors –”the next [medication] class that I think will be up and coming,” she speculated.
“Beyond (just) weight reduction,” Dr. Jastreboff concluded, clinicians “need to focus on optimizing health as we are treating obesity.” Clinicians need to consider the patient’s severity of obesity, overall health, and metabolic profile, and match the obesity treatment to the patient. They also need to consider the rate of weight reduction, potential bone loss, vitamin deficiencies, muscle loss and function, and side effects, and be mindful of affordability, bias, and stigma.
Looking forward to multiple options
W. Timothy Garvey, MD, of the University of Alabama at Birmingham, told this news organization that clinicians treating patients with obesity are looking forward to the decision from the Food and Drug Administration about tirzepatide (Mounjaro), expected by year’s end. Tirzepatide “is really the best medicine that we have for diabetes in terms of A1c control without much hypoglycemia,” he said, “and also the best medicine for treating obesity in patients with diabetes.”
A recent study found that people with type 2 diabetes who adhered to their tirzepatide regimen achieved a 15% weight loss from their baseline after 40-42 weeks.
Dr. Garvey added that he is looking forward to drugs in development such as survodutide (a GLP-1/glucagon agonist) and orforglipron (a small oral daily nonpeptide GLP-1 RA). “Orforglipron wouldn’t have to be refrigerated,” he noted, and it “could be cheaper to manufacture, might be preferred over subcutaneous medication by some people, and it showed pretty good efficacy in early studies.”
Retatrutide, a triple agonist (GLP-1/GIP/glucagon) and CagriSema (cagrilintide plus semaglutide) showed “pretty impressive weight loss in early studies,” Dr. Garvey said. “We’re optimistic.”
Also invited to comment, Sean Wharton, MD, PharmD, Wharton Medical Clinic and York University, Toronto, said that the recent developments in antiobesity medications are “so exciting that it’s difficult to make direct comments,” since “maybe there will be something bigger, or maybe something will go wrong with these molecules and we’ll have to back-step.”
Further studies are needed, he added, to determine outcomes in patients who reduce their intake to half or three-quarters of a dose, or who transition to intermittent therapy.
Nutrient-stimulated, hormone-based antiobesity medications
Here’s a status overview of the nutrient-stimulated hormone-based medications already approved and on the horizon:
Semaglutide. The GLP-1 RA semaglutide (Ozempic), was approved by the FDA for type 2 diabetes in 2017. In June 2021, the FDA approved the use of semaglutide (Wegovy) for obesity.
Topline results from the Semaglutide Effects on Heart Disease and Stroke in Patients with Overweight or Obesity (SELECT) cardiovascular outcome trial showed that in individuals with obesity without type 2 diabetes, semaglutide led to a 20% reduction in major cardiovascular events, Dr. Jastreboff noted, adding that full results will be presented at the American Heart Association meeting on Nov. 11.
Tirzepatide. In May 2022, the FDA approved tirzepatide (Mounjaro), a GIP/GLP-1RA, for type 2 diabetes, and a decision about the use of tirzepatide for obesity is expected by year’s end.
The full results of the phase 3 SURMOUNT-3 trial were presented at ObesityWeek (just after this session), as reported by this news organization.
And the full results of the phase 3 SURMOUNT-4 trial of tirzepatide for obesity were presented at the European Association for the Study of Diabetes meeting, Dr. Jastreboff noted. At 88 weeks, in the continued tirzepatide group, average weight reduction was 26%, absolute weight reduction was 62 pounds (28.1 kg), and > 50% of individuals achieved ≥ 25% weight loss.
The phase 3 SURMOUNT MMO trial of morbidity and mortality with tirzepatide in obesity is estimated to be completed in 2027.
Cagrilintide. In a phase 2 trial of the amylin analog cagrilintide in patients with obesity, more than half of participants lost at least 10% of their weight at 26 weeks.
CagriSema. In a phase 1b trial of the amylin analog/GLP-1 RA combination of cagrilintide/semaglutide (CagriSema), average weight reduction at 20 weeks was 17.1%. The estimated primary completion dates of phase 3 trials of CagriSema, REDEFINE 1 (obesity), REDEFINE 2 (obesity and type 2 diabetes), and REDEFINE 3 (obesity and established cardiovascular disease), are 2025, 2024, and 2027, respectively.
Survodutide. Findings from a phase 2 trial of the glucagon/GLP-1 RA survodutide were presented at the American Diabetes Association (ADA) meeting in June. With 46 weeks of treatment, the average weight reduction was 18.7%, and up to 40% of participants lost at least 20% of their body weight.
Survodutide is being studied in the phase 3 SYNCHRONIZE trials.
Retatrutide. Phase 2 findings of 12-mg weekly of the GIP/GLP-1/glucagon triple hormone receptor agonist retatrutide were also presented at ADA. On average, at 48 weeks, the placebo group lost 2.1% of their weight and the retatrutide group lost 24.2% of their weight, with an average absolute reduction of 58 pounds (26.3 kg). At the highest dose (12 mg), 9 out of 10 individuals lost ≥ 10%, nearly two-thirds lost ≥ 20%, and a quarter lost ≥ 30% of their weight, at 48 weeks.
With the two highest doses of retatrutide, 100% of participants lost ≥ 5% of weight, Dr. Jastreboff reported, adding, “I’m not sure how many other times I will ever be able to say ‘100%’ in any scientific presentation.”
TRIUMPH phase 3 studies of retatrutide are ongoing.
“All the agents I’ve spoken about thus far are once-weekly injectable,” Dr. Jastreboff said, turning her attention to oral drugs.
Oral semaglutide (Rybelsus) is already FDA-approved for type 2 diabetes. The phase 2 OASIS trial results presented at ADA showed that participants with obesity who received 50 mg daily of the oral medication had an average weight reduction of 17.4% at 68 weeks, which is comparable to the 16.9% weight reduction with subcutaneous semaglutide 2.4 once weekly. More than a third of patients receiving the treatment lost ≥ 20% weight at 68 weeks.
The phase 3 OASIS study of oral semaglutide in obesity is ongoing.
Orforglipron. Phase 2 data of the small molecule oral GLP-1 RA orforglipron presented at ADA showed that participants with obesity had up to a 14.7% body weight reduction at 36 weeks. Nearly half of participants lost ≥ 15% of their body weight at 36 weeks.
The phase 3 ATTAIN study of orforglipron in obesity is ongoing.
AMG133. In a phase 2 trial, participants with obesity who received the monthly GIP receptor antagonist/ GLP-1 receptor agonist AMG133 (Amgen) had an average weight reduction of 14.5% at just 12 weeks.
Activin receptor inhibitors
Bimagrumab. This drug is a monoclonal antibody activin receptor inhibitor that binds to activin type II receptors. In a phase 2 study of 58 individuals with type 2 diabetes and obesity who received monthly medication or placebo, participants receiving bimagrumab lost 20.5% of fat mass and gained 3.6% of lean mass at 48 weeks, and the most common adverse events were mild diarrhea and muscle spasm.
Bimagrumab and semaglutide for obesity are being studied in BELIEVE, an ongoing phase 2b study. Topline results are anticipated by the end of 2024.
Taldefgrobep. The fusion protein taldefgrobep binds active myostatin. A phase 2 study of taldefgrobep for obesity is planned to start in 2024.
Dr. Jastreboff is on the scientific advisory board for Amgen, Boehringer Ingelheim, Eli Lilly, and Novo Nordisk, and has received research support form Novo Nordisk, Eli Lilly, Rhythm, and NIH/NIDDK.
A version of this article first appeared on Medscape.com.
“We are at a watershed [moment] brought on by the recent introduction of highly effective antiobesity medications,” Ania M. Jastreboff, MD, PhD, said in a lecture at the annual meeting of the Obesity Society.
Dr. Jastreboff, of Yale University and the Yale Center for Weight Management, New Haven, Conn., provided an overview of the many nutrient-stimulated hormone-based antiobesity therapies in late phases of development – including dual and triple therapies with glucagon-like peptide 1 receptor agonists (GLP-1 RAs), glucose-dependent insulinotropic polypeptide (GIP) agonists, glucagon, and amylin.
“I’ve shown you all of these agents that clearly produce substantial weight reduction,” she said. “The fact that these nutrient-stimulated, hormone-based therapies are not all the same is a good thing,” she stressed, because “it’s not likely that everyone will respond to each of these, and they are likely to respond differently.”
She then briefly touched on activin receptor inhibitors –”the next [medication] class that I think will be up and coming,” she speculated.
“Beyond (just) weight reduction,” Dr. Jastreboff concluded, clinicians “need to focus on optimizing health as we are treating obesity.” Clinicians need to consider the patient’s severity of obesity, overall health, and metabolic profile, and match the obesity treatment to the patient. They also need to consider the rate of weight reduction, potential bone loss, vitamin deficiencies, muscle loss and function, and side effects, and be mindful of affordability, bias, and stigma.
Looking forward to multiple options
W. Timothy Garvey, MD, of the University of Alabama at Birmingham, told this news organization that clinicians treating patients with obesity are looking forward to the decision from the Food and Drug Administration about tirzepatide (Mounjaro), expected by year’s end. Tirzepatide “is really the best medicine that we have for diabetes in terms of A1c control without much hypoglycemia,” he said, “and also the best medicine for treating obesity in patients with diabetes.”
A recent study found that people with type 2 diabetes who adhered to their tirzepatide regimen achieved a 15% weight loss from their baseline after 40-42 weeks.
Dr. Garvey added that he is looking forward to drugs in development such as survodutide (a GLP-1/glucagon agonist) and orforglipron (a small oral daily nonpeptide GLP-1 RA). “Orforglipron wouldn’t have to be refrigerated,” he noted, and it “could be cheaper to manufacture, might be preferred over subcutaneous medication by some people, and it showed pretty good efficacy in early studies.”
Retatrutide, a triple agonist (GLP-1/GIP/glucagon) and CagriSema (cagrilintide plus semaglutide) showed “pretty impressive weight loss in early studies,” Dr. Garvey said. “We’re optimistic.”
Also invited to comment, Sean Wharton, MD, PharmD, Wharton Medical Clinic and York University, Toronto, said that the recent developments in antiobesity medications are “so exciting that it’s difficult to make direct comments,” since “maybe there will be something bigger, or maybe something will go wrong with these molecules and we’ll have to back-step.”
Further studies are needed, he added, to determine outcomes in patients who reduce their intake to half or three-quarters of a dose, or who transition to intermittent therapy.
Nutrient-stimulated, hormone-based antiobesity medications
Here’s a status overview of the nutrient-stimulated hormone-based medications already approved and on the horizon:
Semaglutide. The GLP-1 RA semaglutide (Ozempic), was approved by the FDA for type 2 diabetes in 2017. In June 2021, the FDA approved the use of semaglutide (Wegovy) for obesity.
Topline results from the Semaglutide Effects on Heart Disease and Stroke in Patients with Overweight or Obesity (SELECT) cardiovascular outcome trial showed that in individuals with obesity without type 2 diabetes, semaglutide led to a 20% reduction in major cardiovascular events, Dr. Jastreboff noted, adding that full results will be presented at the American Heart Association meeting on Nov. 11.
Tirzepatide. In May 2022, the FDA approved tirzepatide (Mounjaro), a GIP/GLP-1RA, for type 2 diabetes, and a decision about the use of tirzepatide for obesity is expected by year’s end.
The full results of the phase 3 SURMOUNT-3 trial were presented at ObesityWeek (just after this session), as reported by this news organization.
And the full results of the phase 3 SURMOUNT-4 trial of tirzepatide for obesity were presented at the European Association for the Study of Diabetes meeting, Dr. Jastreboff noted. At 88 weeks, in the continued tirzepatide group, average weight reduction was 26%, absolute weight reduction was 62 pounds (28.1 kg), and > 50% of individuals achieved ≥ 25% weight loss.
The phase 3 SURMOUNT MMO trial of morbidity and mortality with tirzepatide in obesity is estimated to be completed in 2027.
Cagrilintide. In a phase 2 trial of the amylin analog cagrilintide in patients with obesity, more than half of participants lost at least 10% of their weight at 26 weeks.
CagriSema. In a phase 1b trial of the amylin analog/GLP-1 RA combination of cagrilintide/semaglutide (CagriSema), average weight reduction at 20 weeks was 17.1%. The estimated primary completion dates of phase 3 trials of CagriSema, REDEFINE 1 (obesity), REDEFINE 2 (obesity and type 2 diabetes), and REDEFINE 3 (obesity and established cardiovascular disease), are 2025, 2024, and 2027, respectively.
Survodutide. Findings from a phase 2 trial of the glucagon/GLP-1 RA survodutide were presented at the American Diabetes Association (ADA) meeting in June. With 46 weeks of treatment, the average weight reduction was 18.7%, and up to 40% of participants lost at least 20% of their body weight.
Survodutide is being studied in the phase 3 SYNCHRONIZE trials.
Retatrutide. Phase 2 findings of 12-mg weekly of the GIP/GLP-1/glucagon triple hormone receptor agonist retatrutide were also presented at ADA. On average, at 48 weeks, the placebo group lost 2.1% of their weight and the retatrutide group lost 24.2% of their weight, with an average absolute reduction of 58 pounds (26.3 kg). At the highest dose (12 mg), 9 out of 10 individuals lost ≥ 10%, nearly two-thirds lost ≥ 20%, and a quarter lost ≥ 30% of their weight, at 48 weeks.
With the two highest doses of retatrutide, 100% of participants lost ≥ 5% of weight, Dr. Jastreboff reported, adding, “I’m not sure how many other times I will ever be able to say ‘100%’ in any scientific presentation.”
TRIUMPH phase 3 studies of retatrutide are ongoing.
“All the agents I’ve spoken about thus far are once-weekly injectable,” Dr. Jastreboff said, turning her attention to oral drugs.
Oral semaglutide (Rybelsus) is already FDA-approved for type 2 diabetes. The phase 2 OASIS trial results presented at ADA showed that participants with obesity who received 50 mg daily of the oral medication had an average weight reduction of 17.4% at 68 weeks, which is comparable to the 16.9% weight reduction with subcutaneous semaglutide 2.4 once weekly. More than a third of patients receiving the treatment lost ≥ 20% weight at 68 weeks.
The phase 3 OASIS study of oral semaglutide in obesity is ongoing.
Orforglipron. Phase 2 data of the small molecule oral GLP-1 RA orforglipron presented at ADA showed that participants with obesity had up to a 14.7% body weight reduction at 36 weeks. Nearly half of participants lost ≥ 15% of their body weight at 36 weeks.
The phase 3 ATTAIN study of orforglipron in obesity is ongoing.
AMG133. In a phase 2 trial, participants with obesity who received the monthly GIP receptor antagonist/ GLP-1 receptor agonist AMG133 (Amgen) had an average weight reduction of 14.5% at just 12 weeks.
Activin receptor inhibitors
Bimagrumab. This drug is a monoclonal antibody activin receptor inhibitor that binds to activin type II receptors. In a phase 2 study of 58 individuals with type 2 diabetes and obesity who received monthly medication or placebo, participants receiving bimagrumab lost 20.5% of fat mass and gained 3.6% of lean mass at 48 weeks, and the most common adverse events were mild diarrhea and muscle spasm.
Bimagrumab and semaglutide for obesity are being studied in BELIEVE, an ongoing phase 2b study. Topline results are anticipated by the end of 2024.
Taldefgrobep. The fusion protein taldefgrobep binds active myostatin. A phase 2 study of taldefgrobep for obesity is planned to start in 2024.
Dr. Jastreboff is on the scientific advisory board for Amgen, Boehringer Ingelheim, Eli Lilly, and Novo Nordisk, and has received research support form Novo Nordisk, Eli Lilly, Rhythm, and NIH/NIDDK.
A version of this article first appeared on Medscape.com.
“We are at a watershed [moment] brought on by the recent introduction of highly effective antiobesity medications,” Ania M. Jastreboff, MD, PhD, said in a lecture at the annual meeting of the Obesity Society.
Dr. Jastreboff, of Yale University and the Yale Center for Weight Management, New Haven, Conn., provided an overview of the many nutrient-stimulated hormone-based antiobesity therapies in late phases of development – including dual and triple therapies with glucagon-like peptide 1 receptor agonists (GLP-1 RAs), glucose-dependent insulinotropic polypeptide (GIP) agonists, glucagon, and amylin.
“I’ve shown you all of these agents that clearly produce substantial weight reduction,” she said. “The fact that these nutrient-stimulated, hormone-based therapies are not all the same is a good thing,” she stressed, because “it’s not likely that everyone will respond to each of these, and they are likely to respond differently.”
She then briefly touched on activin receptor inhibitors –”the next [medication] class that I think will be up and coming,” she speculated.
“Beyond (just) weight reduction,” Dr. Jastreboff concluded, clinicians “need to focus on optimizing health as we are treating obesity.” Clinicians need to consider the patient’s severity of obesity, overall health, and metabolic profile, and match the obesity treatment to the patient. They also need to consider the rate of weight reduction, potential bone loss, vitamin deficiencies, muscle loss and function, and side effects, and be mindful of affordability, bias, and stigma.
Looking forward to multiple options
W. Timothy Garvey, MD, of the University of Alabama at Birmingham, told this news organization that clinicians treating patients with obesity are looking forward to the decision from the Food and Drug Administration about tirzepatide (Mounjaro), expected by year’s end. Tirzepatide “is really the best medicine that we have for diabetes in terms of A1c control without much hypoglycemia,” he said, “and also the best medicine for treating obesity in patients with diabetes.”
A recent study found that people with type 2 diabetes who adhered to their tirzepatide regimen achieved a 15% weight loss from their baseline after 40-42 weeks.
Dr. Garvey added that he is looking forward to drugs in development such as survodutide (a GLP-1/glucagon agonist) and orforglipron (a small oral daily nonpeptide GLP-1 RA). “Orforglipron wouldn’t have to be refrigerated,” he noted, and it “could be cheaper to manufacture, might be preferred over subcutaneous medication by some people, and it showed pretty good efficacy in early studies.”
Retatrutide, a triple agonist (GLP-1/GIP/glucagon) and CagriSema (cagrilintide plus semaglutide) showed “pretty impressive weight loss in early studies,” Dr. Garvey said. “We’re optimistic.”
Also invited to comment, Sean Wharton, MD, PharmD, Wharton Medical Clinic and York University, Toronto, said that the recent developments in antiobesity medications are “so exciting that it’s difficult to make direct comments,” since “maybe there will be something bigger, or maybe something will go wrong with these molecules and we’ll have to back-step.”
Further studies are needed, he added, to determine outcomes in patients who reduce their intake to half or three-quarters of a dose, or who transition to intermittent therapy.
Nutrient-stimulated, hormone-based antiobesity medications
Here’s a status overview of the nutrient-stimulated hormone-based medications already approved and on the horizon:
Semaglutide. The GLP-1 RA semaglutide (Ozempic), was approved by the FDA for type 2 diabetes in 2017. In June 2021, the FDA approved the use of semaglutide (Wegovy) for obesity.
Topline results from the Semaglutide Effects on Heart Disease and Stroke in Patients with Overweight or Obesity (SELECT) cardiovascular outcome trial showed that in individuals with obesity without type 2 diabetes, semaglutide led to a 20% reduction in major cardiovascular events, Dr. Jastreboff noted, adding that full results will be presented at the American Heart Association meeting on Nov. 11.
Tirzepatide. In May 2022, the FDA approved tirzepatide (Mounjaro), a GIP/GLP-1RA, for type 2 diabetes, and a decision about the use of tirzepatide for obesity is expected by year’s end.
The full results of the phase 3 SURMOUNT-3 trial were presented at ObesityWeek (just after this session), as reported by this news organization.
And the full results of the phase 3 SURMOUNT-4 trial of tirzepatide for obesity were presented at the European Association for the Study of Diabetes meeting, Dr. Jastreboff noted. At 88 weeks, in the continued tirzepatide group, average weight reduction was 26%, absolute weight reduction was 62 pounds (28.1 kg), and > 50% of individuals achieved ≥ 25% weight loss.
The phase 3 SURMOUNT MMO trial of morbidity and mortality with tirzepatide in obesity is estimated to be completed in 2027.
Cagrilintide. In a phase 2 trial of the amylin analog cagrilintide in patients with obesity, more than half of participants lost at least 10% of their weight at 26 weeks.
CagriSema. In a phase 1b trial of the amylin analog/GLP-1 RA combination of cagrilintide/semaglutide (CagriSema), average weight reduction at 20 weeks was 17.1%. The estimated primary completion dates of phase 3 trials of CagriSema, REDEFINE 1 (obesity), REDEFINE 2 (obesity and type 2 diabetes), and REDEFINE 3 (obesity and established cardiovascular disease), are 2025, 2024, and 2027, respectively.
Survodutide. Findings from a phase 2 trial of the glucagon/GLP-1 RA survodutide were presented at the American Diabetes Association (ADA) meeting in June. With 46 weeks of treatment, the average weight reduction was 18.7%, and up to 40% of participants lost at least 20% of their body weight.
Survodutide is being studied in the phase 3 SYNCHRONIZE trials.
Retatrutide. Phase 2 findings of 12-mg weekly of the GIP/GLP-1/glucagon triple hormone receptor agonist retatrutide were also presented at ADA. On average, at 48 weeks, the placebo group lost 2.1% of their weight and the retatrutide group lost 24.2% of their weight, with an average absolute reduction of 58 pounds (26.3 kg). At the highest dose (12 mg), 9 out of 10 individuals lost ≥ 10%, nearly two-thirds lost ≥ 20%, and a quarter lost ≥ 30% of their weight, at 48 weeks.
With the two highest doses of retatrutide, 100% of participants lost ≥ 5% of weight, Dr. Jastreboff reported, adding, “I’m not sure how many other times I will ever be able to say ‘100%’ in any scientific presentation.”
TRIUMPH phase 3 studies of retatrutide are ongoing.
“All the agents I’ve spoken about thus far are once-weekly injectable,” Dr. Jastreboff said, turning her attention to oral drugs.
Oral semaglutide (Rybelsus) is already FDA-approved for type 2 diabetes. The phase 2 OASIS trial results presented at ADA showed that participants with obesity who received 50 mg daily of the oral medication had an average weight reduction of 17.4% at 68 weeks, which is comparable to the 16.9% weight reduction with subcutaneous semaglutide 2.4 once weekly. More than a third of patients receiving the treatment lost ≥ 20% weight at 68 weeks.
The phase 3 OASIS study of oral semaglutide in obesity is ongoing.
Orforglipron. Phase 2 data of the small molecule oral GLP-1 RA orforglipron presented at ADA showed that participants with obesity had up to a 14.7% body weight reduction at 36 weeks. Nearly half of participants lost ≥ 15% of their body weight at 36 weeks.
The phase 3 ATTAIN study of orforglipron in obesity is ongoing.
AMG133. In a phase 2 trial, participants with obesity who received the monthly GIP receptor antagonist/ GLP-1 receptor agonist AMG133 (Amgen) had an average weight reduction of 14.5% at just 12 weeks.
Activin receptor inhibitors
Bimagrumab. This drug is a monoclonal antibody activin receptor inhibitor that binds to activin type II receptors. In a phase 2 study of 58 individuals with type 2 diabetes and obesity who received monthly medication or placebo, participants receiving bimagrumab lost 20.5% of fat mass and gained 3.6% of lean mass at 48 weeks, and the most common adverse events were mild diarrhea and muscle spasm.
Bimagrumab and semaglutide for obesity are being studied in BELIEVE, an ongoing phase 2b study. Topline results are anticipated by the end of 2024.
Taldefgrobep. The fusion protein taldefgrobep binds active myostatin. A phase 2 study of taldefgrobep for obesity is planned to start in 2024.
Dr. Jastreboff is on the scientific advisory board for Amgen, Boehringer Ingelheim, Eli Lilly, and Novo Nordisk, and has received research support form Novo Nordisk, Eli Lilly, Rhythm, and NIH/NIDDK.
A version of this article first appeared on Medscape.com.
FROM OBESITYWEEK® 2023
What predicts successful weight loss maintenance in WeightWatchers?
DALLAS – Researchers identified behavioral, psychological, and environmental predictors of continued weight loss maintenance vs. weight regain in a large cohort of members of WeightWatchers who had successful long-term weight loss.
On average, the participants had lost 25.5 kg (56 lb) and kept it off for 3.5 years, when they entered the 1-year study.
At study entry and 1 year later, the participants replied to several questionnaires that asked about predictors of weight loss maintenance.
Compared with people who gained weight over the 1-year study,
They also had reduced disinhibition (tendency to overeat) when faced with food cues, as well as less pain and a more positive body image “at any weight, shape, or size,” Suzanne Phelan, MD, PhD, reported.
Dr. Phelan, from the department of kinesiology and public health, California Polytechnic State University, San Luis Obispo, presented the study in an Obesity symposium at the annual meeting of the Obesity Society, and it was simultaneously published in the journal. The study was selected as one of five top papers submitted to the journal to coincide with the meeting.
Future interventions to prevent weight regain should target overeating in response to internal and external food cues and declines in self-monitoring and body image, Dr. Phelan said.
The study aimed to identify behaviors that might predict who might “beat the odds” and sustain long-term weight loss, she said in an interview.
The findings suggest that the people who maintained their weight loss had developed skills to help them cope with cravings and not respond by eating, she said. Continued self-monitoring and body acceptance and appreciation (all body sizes are beautiful) were key elements of successful weight-loss maintenance.
No antiobesity drugs or surgery; don’t forget behavioral stuff
Importantly, although 43% of the study participants regained more than five pounds during this 1-year study, they still remained at 18% below their starting weight, “indicating that they were largely successful at weight loss,” Dr. Phelan said.
Michael D. Jensen, MD, editor-in-chief of Obesity, echoed this.
The researchers “did find some weak predictors of success,” said Dr. Jensen, from Mayo Clinic, Rochester, Minn. “But perhaps as important,” he said, “was that at the end of the trial, even those who had regained some slight weight still had 18% weight loss – which is not trivial – after, on average, 4.5 years with a standard commercial weight management program.
“At every talk I go to here,” the message is, “Let’s stampede towards use of the drugs and skip diet and exercise and behavioral stuff,” he observed. “I would argue,” he said, “that when it works, it works really well, and it’s free. So this idea that we shouldn’t even try it, because we know it’s going to fail, is wrong.
“If you have the right group, they have a decent chance of having a sufficiently good response that you don’t have to give the medications and you don’t have to send them for bariatric surgery.
“Once you learn from these programs what to do, you’re not paying $1,000 a month for a drug and you haven’t had bariatric surgery,” Dr. Jensen noted. “Their 3 years of follow-up of WeightWatchers cost less than 1 month worth of one of these [antiobesity medications].”
The predictive findings were like ‘”icing on the cake,” he said. Anybody can find five people who’ve done well with therapy, but this study was in more than 2,800 people who did well with a commercial program that is not expensive.
Study design and findings
Between 2019 and 2020, WeightWatchers invited adult members who had maintained weight loss of at least 9.1 kg (20 lb) for at least 1 year to participate in this study.
Of 7,025 participants who entered the study, 4,004 individuals (57%) who did not complete the 1-year questionnaires and others with implausible weight were excluded, leaving a final sample of 2,843 participants.
Most participants were women (92%), non-Hispanic White (95%), married (92%), and college educated. They had a mean age of 56 years.
On average, the participants had a body mass index (BMI) of 35.9 kg/m2 (grade 2 obesity) at the start of the WeightWatchers program and a BMI of 26.7 when they entered the current study.
At the 1-year follow-up, 57% of the participants had maintained the same weight (within 2.3 kg) as when they enrolled in the study, and 43% had gained ≥ 2.3 kg.
On average, the weight-loss maintainers had gained 0.4 kg (0.88 lb). The weight gainers had gained 7.2 kg (15.9 lb) but were still 19.1 kg (42.1 lb) below the weight they had when they started the WeightWatchers program.
At baseline, compared with the weight gainers, the weight-loss maintainers were on average older (58 vs. 52 years), had a lower initial BMI (26 vs. 28), and had longer duration of weight loss maintenance (4 vs. 3 years).
At 1 year, those who had maintained their weight loss had greater self-monitoring, psychological coping, physical activity strategies, dietary choice strategies, and eating and physical activity habits, and they had less eating initiation in the absence of hunger.
They also had less disinhibition, more willingness to ignore cravings and accept food urges, more future orientation, more mindfulness, more positive body image and body satisfaction, better general health and mental health, and less bodily pain.
This research was supported by a grant to Dr. Phelan from WeightWatchers (WW) International, and three study authors are employees and shareholders of the company. Dr. Jensen discloses consulting for Biohaven Pharmaceuticals and for Seattle Gummy Co.
A version of this article first appeared on Medscape.com.
DALLAS – Researchers identified behavioral, psychological, and environmental predictors of continued weight loss maintenance vs. weight regain in a large cohort of members of WeightWatchers who had successful long-term weight loss.
On average, the participants had lost 25.5 kg (56 lb) and kept it off for 3.5 years, when they entered the 1-year study.
At study entry and 1 year later, the participants replied to several questionnaires that asked about predictors of weight loss maintenance.
Compared with people who gained weight over the 1-year study,
They also had reduced disinhibition (tendency to overeat) when faced with food cues, as well as less pain and a more positive body image “at any weight, shape, or size,” Suzanne Phelan, MD, PhD, reported.
Dr. Phelan, from the department of kinesiology and public health, California Polytechnic State University, San Luis Obispo, presented the study in an Obesity symposium at the annual meeting of the Obesity Society, and it was simultaneously published in the journal. The study was selected as one of five top papers submitted to the journal to coincide with the meeting.
Future interventions to prevent weight regain should target overeating in response to internal and external food cues and declines in self-monitoring and body image, Dr. Phelan said.
The study aimed to identify behaviors that might predict who might “beat the odds” and sustain long-term weight loss, she said in an interview.
The findings suggest that the people who maintained their weight loss had developed skills to help them cope with cravings and not respond by eating, she said. Continued self-monitoring and body acceptance and appreciation (all body sizes are beautiful) were key elements of successful weight-loss maintenance.
No antiobesity drugs or surgery; don’t forget behavioral stuff
Importantly, although 43% of the study participants regained more than five pounds during this 1-year study, they still remained at 18% below their starting weight, “indicating that they were largely successful at weight loss,” Dr. Phelan said.
Michael D. Jensen, MD, editor-in-chief of Obesity, echoed this.
The researchers “did find some weak predictors of success,” said Dr. Jensen, from Mayo Clinic, Rochester, Minn. “But perhaps as important,” he said, “was that at the end of the trial, even those who had regained some slight weight still had 18% weight loss – which is not trivial – after, on average, 4.5 years with a standard commercial weight management program.
“At every talk I go to here,” the message is, “Let’s stampede towards use of the drugs and skip diet and exercise and behavioral stuff,” he observed. “I would argue,” he said, “that when it works, it works really well, and it’s free. So this idea that we shouldn’t even try it, because we know it’s going to fail, is wrong.
“If you have the right group, they have a decent chance of having a sufficiently good response that you don’t have to give the medications and you don’t have to send them for bariatric surgery.
“Once you learn from these programs what to do, you’re not paying $1,000 a month for a drug and you haven’t had bariatric surgery,” Dr. Jensen noted. “Their 3 years of follow-up of WeightWatchers cost less than 1 month worth of one of these [antiobesity medications].”
The predictive findings were like ‘”icing on the cake,” he said. Anybody can find five people who’ve done well with therapy, but this study was in more than 2,800 people who did well with a commercial program that is not expensive.
Study design and findings
Between 2019 and 2020, WeightWatchers invited adult members who had maintained weight loss of at least 9.1 kg (20 lb) for at least 1 year to participate in this study.
Of 7,025 participants who entered the study, 4,004 individuals (57%) who did not complete the 1-year questionnaires and others with implausible weight were excluded, leaving a final sample of 2,843 participants.
Most participants were women (92%), non-Hispanic White (95%), married (92%), and college educated. They had a mean age of 56 years.
On average, the participants had a body mass index (BMI) of 35.9 kg/m2 (grade 2 obesity) at the start of the WeightWatchers program and a BMI of 26.7 when they entered the current study.
At the 1-year follow-up, 57% of the participants had maintained the same weight (within 2.3 kg) as when they enrolled in the study, and 43% had gained ≥ 2.3 kg.
On average, the weight-loss maintainers had gained 0.4 kg (0.88 lb). The weight gainers had gained 7.2 kg (15.9 lb) but were still 19.1 kg (42.1 lb) below the weight they had when they started the WeightWatchers program.
At baseline, compared with the weight gainers, the weight-loss maintainers were on average older (58 vs. 52 years), had a lower initial BMI (26 vs. 28), and had longer duration of weight loss maintenance (4 vs. 3 years).
At 1 year, those who had maintained their weight loss had greater self-monitoring, psychological coping, physical activity strategies, dietary choice strategies, and eating and physical activity habits, and they had less eating initiation in the absence of hunger.
They also had less disinhibition, more willingness to ignore cravings and accept food urges, more future orientation, more mindfulness, more positive body image and body satisfaction, better general health and mental health, and less bodily pain.
This research was supported by a grant to Dr. Phelan from WeightWatchers (WW) International, and three study authors are employees and shareholders of the company. Dr. Jensen discloses consulting for Biohaven Pharmaceuticals and for Seattle Gummy Co.
A version of this article first appeared on Medscape.com.
DALLAS – Researchers identified behavioral, psychological, and environmental predictors of continued weight loss maintenance vs. weight regain in a large cohort of members of WeightWatchers who had successful long-term weight loss.
On average, the participants had lost 25.5 kg (56 lb) and kept it off for 3.5 years, when they entered the 1-year study.
At study entry and 1 year later, the participants replied to several questionnaires that asked about predictors of weight loss maintenance.
Compared with people who gained weight over the 1-year study,
They also had reduced disinhibition (tendency to overeat) when faced with food cues, as well as less pain and a more positive body image “at any weight, shape, or size,” Suzanne Phelan, MD, PhD, reported.
Dr. Phelan, from the department of kinesiology and public health, California Polytechnic State University, San Luis Obispo, presented the study in an Obesity symposium at the annual meeting of the Obesity Society, and it was simultaneously published in the journal. The study was selected as one of five top papers submitted to the journal to coincide with the meeting.
Future interventions to prevent weight regain should target overeating in response to internal and external food cues and declines in self-monitoring and body image, Dr. Phelan said.
The study aimed to identify behaviors that might predict who might “beat the odds” and sustain long-term weight loss, she said in an interview.
The findings suggest that the people who maintained their weight loss had developed skills to help them cope with cravings and not respond by eating, she said. Continued self-monitoring and body acceptance and appreciation (all body sizes are beautiful) were key elements of successful weight-loss maintenance.
No antiobesity drugs or surgery; don’t forget behavioral stuff
Importantly, although 43% of the study participants regained more than five pounds during this 1-year study, they still remained at 18% below their starting weight, “indicating that they were largely successful at weight loss,” Dr. Phelan said.
Michael D. Jensen, MD, editor-in-chief of Obesity, echoed this.
The researchers “did find some weak predictors of success,” said Dr. Jensen, from Mayo Clinic, Rochester, Minn. “But perhaps as important,” he said, “was that at the end of the trial, even those who had regained some slight weight still had 18% weight loss – which is not trivial – after, on average, 4.5 years with a standard commercial weight management program.
“At every talk I go to here,” the message is, “Let’s stampede towards use of the drugs and skip diet and exercise and behavioral stuff,” he observed. “I would argue,” he said, “that when it works, it works really well, and it’s free. So this idea that we shouldn’t even try it, because we know it’s going to fail, is wrong.
“If you have the right group, they have a decent chance of having a sufficiently good response that you don’t have to give the medications and you don’t have to send them for bariatric surgery.
“Once you learn from these programs what to do, you’re not paying $1,000 a month for a drug and you haven’t had bariatric surgery,” Dr. Jensen noted. “Their 3 years of follow-up of WeightWatchers cost less than 1 month worth of one of these [antiobesity medications].”
The predictive findings were like ‘”icing on the cake,” he said. Anybody can find five people who’ve done well with therapy, but this study was in more than 2,800 people who did well with a commercial program that is not expensive.
Study design and findings
Between 2019 and 2020, WeightWatchers invited adult members who had maintained weight loss of at least 9.1 kg (20 lb) for at least 1 year to participate in this study.
Of 7,025 participants who entered the study, 4,004 individuals (57%) who did not complete the 1-year questionnaires and others with implausible weight were excluded, leaving a final sample of 2,843 participants.
Most participants were women (92%), non-Hispanic White (95%), married (92%), and college educated. They had a mean age of 56 years.
On average, the participants had a body mass index (BMI) of 35.9 kg/m2 (grade 2 obesity) at the start of the WeightWatchers program and a BMI of 26.7 when they entered the current study.
At the 1-year follow-up, 57% of the participants had maintained the same weight (within 2.3 kg) as when they enrolled in the study, and 43% had gained ≥ 2.3 kg.
On average, the weight-loss maintainers had gained 0.4 kg (0.88 lb). The weight gainers had gained 7.2 kg (15.9 lb) but were still 19.1 kg (42.1 lb) below the weight they had when they started the WeightWatchers program.
At baseline, compared with the weight gainers, the weight-loss maintainers were on average older (58 vs. 52 years), had a lower initial BMI (26 vs. 28), and had longer duration of weight loss maintenance (4 vs. 3 years).
At 1 year, those who had maintained their weight loss had greater self-monitoring, psychological coping, physical activity strategies, dietary choice strategies, and eating and physical activity habits, and they had less eating initiation in the absence of hunger.
They also had less disinhibition, more willingness to ignore cravings and accept food urges, more future orientation, more mindfulness, more positive body image and body satisfaction, better general health and mental health, and less bodily pain.
This research was supported by a grant to Dr. Phelan from WeightWatchers (WW) International, and three study authors are employees and shareholders of the company. Dr. Jensen discloses consulting for Biohaven Pharmaceuticals and for Seattle Gummy Co.
A version of this article first appeared on Medscape.com.
FROM OBESITY WEEK® 2023
Antidepressants ‘don’t blunt’ semaglutide and weight loss
in a post hoc analysis of the Semaglutide Treatment Effect in People with Obesity (STEP) program.
Adverse events, including psychiatric events, were slightly more usual in the patients on antidepressants, Robert Kushner, MD, noted, in an oral session at the annual meeting of the Obesity Society.
“It is very common that patients who present for weight management are taking antidepressants for various reasons, including depression, anxiety, insomnia, or chronic pain,”Dr. Kushner, from Northwestern University in Chicago, said in an email. “We wanted to see if these participants responded differently to semaglutide, compared to those not on antidepressants.”
“We found that antidepressants do not blunt the effect of semaglutide for weight loss,” he said. “However, there is a slight increase in reported adverse effects.”
“Semaglutide 2.4 mg provides an effective treatment option for weight management, regardless of antidepressant use at baseline,” Dr. Kushner summarized. “Clinicians should be assured that we can use semaglutide in this population of patients.”
Jack Yanovski, MD, PhD, said this was a “great presentation,” noting that “it’s really important that we understand what goes on in patients with depression.”
“Of course, all these trials still had rules that prevent the folks with the most severe depressive symptoms or past suicidality to participate,” added Dr. Yanovski, chief of the Growth and Obesity Section, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Rockville, Md. “We need specific trials to know exactly how well we do.”
Dr. Kushner agreed, but also noted that, ever since some earlier antidepressants were associated with risk for suicidal ideation and death, strict guidelines were put in place that exclude certain patients from participating in clinical trials.
Dr. Yanovski suggested that now that the drugs are approved, it would be possible to study this, and the information would be important for clinicians.
Dr. Kushner said he hopes that such studies are forthcoming. In the meantime, “data like this will add some support and understanding,” he suggested.
36,000 Patients with obesity, 500 on antidepressants
Many people living with obesity report taking antidepressants for depression, anxiety, chronic pain, obsessive-compulsive disorder, sleep disturbance, neuropathy, panic disorder, or posttraumatic stress disorder, Dr. Kushner noted.
However, some of these medications can cause weight gain, and little is known about treatment outcomes for people with obesity who are on antidepressants, since most weight-loss studies exclude people with active major depressive disorder.
The researchers analyzed data from 1,961 patients in STEP 1 and 807 patients in STEP 2 as well as 611 patients in STEP 3 and 304 patients in STEP 5 – 3,683 participants in total, of which 539 were on antidepressants at baseline.
The patients were randomly assigned to 2.4 mg semaglutide vs. placebo plus a lifestyle intervention (STEP 1, 2, and 5) or intensive behavioral therapy (STEP 3 only), for 68 weeks, except STEP 5, which was 104 weeks.
Patients were included if they were aged 18 or older with a body mass index ≥30 kg/m2, or ≥27 kg/m2 with more than one weight-related complication (STEP 1, 3, and 5) or BMI ≥27 kg/m2 with type 2 diabetes (STEP 2 only), and at least one self-reported unsuccessful effort to lose weight by diet.
They were excluded if they had active major depressive disorder within 2 years prior to screening (or other severe psychiatric disorders such as schizophrenia or bipolar disorder) or a Patient Health Questionnaire-9 score of 15 or higher (indicating moderately severe or severe depression), or suicide ideation (type 4 or 5 on the Columbia Suicide Severity Rating Scale) or suicide behavior, within 30 days of screening.
From baseline to week 68, patients on semaglutide (with/without baseline antidepressant use) had a significantly greater change in weight vs. patients on placebo (with/without baseline antidepressant use), respectively:
- STEP 1: –15.7% / –14.7% vs. –0.2% / –2.8%
- STEP 2: –10.7% / –9.5% vs. –3.3% / –3.4%
- STEP 3: –16.2% / –15.9% vs. –5.0% / –5.9%
- STEP 5: –19.0% / –14.1% vs. +1.6% / – 4.0%.
The proportion of reported adverse events was generally slightly greater in patients receiving semaglutide (with/without baseline antidepressant use) than those on placebo (with/without baseline antidepressant use), respectively:
- STEP 1: 97.7% vs 88.6% and 92.9% vs. 86%
- STEP 2: 97.6% vs 86.5% and 88.6% vs. 77.2%
- STEP 3: 97.6% vs 95.3% and 100% vs. 95.8%
- STEP 5: 100% vs 94.8% and 95.5% vs. 89.2%.
Gastrointestinal adverse events were more frequently reported in the semaglutide group and in patients on antidepressants at baseline. The proportion of patients with psychiatric adverse events was greater in participants on antidepressants at baseline. There were no differences in suicidal ideation/behavior in patients with/without antidepressant use at baseline.
The STEP trials were funded by Novo Nordisk. Dr. Kushner discloses that he served as a consultant for Novo Nordisk, WeightWatchers, Eli Lilly, and Pfizer, and received a research grant from Epitomee.
A version of this article appeared on Medscape.com.
in a post hoc analysis of the Semaglutide Treatment Effect in People with Obesity (STEP) program.
Adverse events, including psychiatric events, were slightly more usual in the patients on antidepressants, Robert Kushner, MD, noted, in an oral session at the annual meeting of the Obesity Society.
“It is very common that patients who present for weight management are taking antidepressants for various reasons, including depression, anxiety, insomnia, or chronic pain,”Dr. Kushner, from Northwestern University in Chicago, said in an email. “We wanted to see if these participants responded differently to semaglutide, compared to those not on antidepressants.”
“We found that antidepressants do not blunt the effect of semaglutide for weight loss,” he said. “However, there is a slight increase in reported adverse effects.”
“Semaglutide 2.4 mg provides an effective treatment option for weight management, regardless of antidepressant use at baseline,” Dr. Kushner summarized. “Clinicians should be assured that we can use semaglutide in this population of patients.”
Jack Yanovski, MD, PhD, said this was a “great presentation,” noting that “it’s really important that we understand what goes on in patients with depression.”
“Of course, all these trials still had rules that prevent the folks with the most severe depressive symptoms or past suicidality to participate,” added Dr. Yanovski, chief of the Growth and Obesity Section, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Rockville, Md. “We need specific trials to know exactly how well we do.”
Dr. Kushner agreed, but also noted that, ever since some earlier antidepressants were associated with risk for suicidal ideation and death, strict guidelines were put in place that exclude certain patients from participating in clinical trials.
Dr. Yanovski suggested that now that the drugs are approved, it would be possible to study this, and the information would be important for clinicians.
Dr. Kushner said he hopes that such studies are forthcoming. In the meantime, “data like this will add some support and understanding,” he suggested.
36,000 Patients with obesity, 500 on antidepressants
Many people living with obesity report taking antidepressants for depression, anxiety, chronic pain, obsessive-compulsive disorder, sleep disturbance, neuropathy, panic disorder, or posttraumatic stress disorder, Dr. Kushner noted.
However, some of these medications can cause weight gain, and little is known about treatment outcomes for people with obesity who are on antidepressants, since most weight-loss studies exclude people with active major depressive disorder.
The researchers analyzed data from 1,961 patients in STEP 1 and 807 patients in STEP 2 as well as 611 patients in STEP 3 and 304 patients in STEP 5 – 3,683 participants in total, of which 539 were on antidepressants at baseline.
The patients were randomly assigned to 2.4 mg semaglutide vs. placebo plus a lifestyle intervention (STEP 1, 2, and 5) or intensive behavioral therapy (STEP 3 only), for 68 weeks, except STEP 5, which was 104 weeks.
Patients were included if they were aged 18 or older with a body mass index ≥30 kg/m2, or ≥27 kg/m2 with more than one weight-related complication (STEP 1, 3, and 5) or BMI ≥27 kg/m2 with type 2 diabetes (STEP 2 only), and at least one self-reported unsuccessful effort to lose weight by diet.
They were excluded if they had active major depressive disorder within 2 years prior to screening (or other severe psychiatric disorders such as schizophrenia or bipolar disorder) or a Patient Health Questionnaire-9 score of 15 or higher (indicating moderately severe or severe depression), or suicide ideation (type 4 or 5 on the Columbia Suicide Severity Rating Scale) or suicide behavior, within 30 days of screening.
From baseline to week 68, patients on semaglutide (with/without baseline antidepressant use) had a significantly greater change in weight vs. patients on placebo (with/without baseline antidepressant use), respectively:
- STEP 1: –15.7% / –14.7% vs. –0.2% / –2.8%
- STEP 2: –10.7% / –9.5% vs. –3.3% / –3.4%
- STEP 3: –16.2% / –15.9% vs. –5.0% / –5.9%
- STEP 5: –19.0% / –14.1% vs. +1.6% / – 4.0%.
The proportion of reported adverse events was generally slightly greater in patients receiving semaglutide (with/without baseline antidepressant use) than those on placebo (with/without baseline antidepressant use), respectively:
- STEP 1: 97.7% vs 88.6% and 92.9% vs. 86%
- STEP 2: 97.6% vs 86.5% and 88.6% vs. 77.2%
- STEP 3: 97.6% vs 95.3% and 100% vs. 95.8%
- STEP 5: 100% vs 94.8% and 95.5% vs. 89.2%.
Gastrointestinal adverse events were more frequently reported in the semaglutide group and in patients on antidepressants at baseline. The proportion of patients with psychiatric adverse events was greater in participants on antidepressants at baseline. There were no differences in suicidal ideation/behavior in patients with/without antidepressant use at baseline.
The STEP trials were funded by Novo Nordisk. Dr. Kushner discloses that he served as a consultant for Novo Nordisk, WeightWatchers, Eli Lilly, and Pfizer, and received a research grant from Epitomee.
A version of this article appeared on Medscape.com.
in a post hoc analysis of the Semaglutide Treatment Effect in People with Obesity (STEP) program.
Adverse events, including psychiatric events, were slightly more usual in the patients on antidepressants, Robert Kushner, MD, noted, in an oral session at the annual meeting of the Obesity Society.
“It is very common that patients who present for weight management are taking antidepressants for various reasons, including depression, anxiety, insomnia, or chronic pain,”Dr. Kushner, from Northwestern University in Chicago, said in an email. “We wanted to see if these participants responded differently to semaglutide, compared to those not on antidepressants.”
“We found that antidepressants do not blunt the effect of semaglutide for weight loss,” he said. “However, there is a slight increase in reported adverse effects.”
“Semaglutide 2.4 mg provides an effective treatment option for weight management, regardless of antidepressant use at baseline,” Dr. Kushner summarized. “Clinicians should be assured that we can use semaglutide in this population of patients.”
Jack Yanovski, MD, PhD, said this was a “great presentation,” noting that “it’s really important that we understand what goes on in patients with depression.”
“Of course, all these trials still had rules that prevent the folks with the most severe depressive symptoms or past suicidality to participate,” added Dr. Yanovski, chief of the Growth and Obesity Section, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Rockville, Md. “We need specific trials to know exactly how well we do.”
Dr. Kushner agreed, but also noted that, ever since some earlier antidepressants were associated with risk for suicidal ideation and death, strict guidelines were put in place that exclude certain patients from participating in clinical trials.
Dr. Yanovski suggested that now that the drugs are approved, it would be possible to study this, and the information would be important for clinicians.
Dr. Kushner said he hopes that such studies are forthcoming. In the meantime, “data like this will add some support and understanding,” he suggested.
36,000 Patients with obesity, 500 on antidepressants
Many people living with obesity report taking antidepressants for depression, anxiety, chronic pain, obsessive-compulsive disorder, sleep disturbance, neuropathy, panic disorder, or posttraumatic stress disorder, Dr. Kushner noted.
However, some of these medications can cause weight gain, and little is known about treatment outcomes for people with obesity who are on antidepressants, since most weight-loss studies exclude people with active major depressive disorder.
The researchers analyzed data from 1,961 patients in STEP 1 and 807 patients in STEP 2 as well as 611 patients in STEP 3 and 304 patients in STEP 5 – 3,683 participants in total, of which 539 were on antidepressants at baseline.
The patients were randomly assigned to 2.4 mg semaglutide vs. placebo plus a lifestyle intervention (STEP 1, 2, and 5) or intensive behavioral therapy (STEP 3 only), for 68 weeks, except STEP 5, which was 104 weeks.
Patients were included if they were aged 18 or older with a body mass index ≥30 kg/m2, or ≥27 kg/m2 with more than one weight-related complication (STEP 1, 3, and 5) or BMI ≥27 kg/m2 with type 2 diabetes (STEP 2 only), and at least one self-reported unsuccessful effort to lose weight by diet.
They were excluded if they had active major depressive disorder within 2 years prior to screening (or other severe psychiatric disorders such as schizophrenia or bipolar disorder) or a Patient Health Questionnaire-9 score of 15 or higher (indicating moderately severe or severe depression), or suicide ideation (type 4 or 5 on the Columbia Suicide Severity Rating Scale) or suicide behavior, within 30 days of screening.
From baseline to week 68, patients on semaglutide (with/without baseline antidepressant use) had a significantly greater change in weight vs. patients on placebo (with/without baseline antidepressant use), respectively:
- STEP 1: –15.7% / –14.7% vs. –0.2% / –2.8%
- STEP 2: –10.7% / –9.5% vs. –3.3% / –3.4%
- STEP 3: –16.2% / –15.9% vs. –5.0% / –5.9%
- STEP 5: –19.0% / –14.1% vs. +1.6% / – 4.0%.
The proportion of reported adverse events was generally slightly greater in patients receiving semaglutide (with/without baseline antidepressant use) than those on placebo (with/without baseline antidepressant use), respectively:
- STEP 1: 97.7% vs 88.6% and 92.9% vs. 86%
- STEP 2: 97.6% vs 86.5% and 88.6% vs. 77.2%
- STEP 3: 97.6% vs 95.3% and 100% vs. 95.8%
- STEP 5: 100% vs 94.8% and 95.5% vs. 89.2%.
Gastrointestinal adverse events were more frequently reported in the semaglutide group and in patients on antidepressants at baseline. The proportion of patients with psychiatric adverse events was greater in participants on antidepressants at baseline. There were no differences in suicidal ideation/behavior in patients with/without antidepressant use at baseline.
The STEP trials were funded by Novo Nordisk. Dr. Kushner discloses that he served as a consultant for Novo Nordisk, WeightWatchers, Eli Lilly, and Pfizer, and received a research grant from Epitomee.
A version of this article appeared on Medscape.com.
FROM OBESITYWEEK® 2023
Alert! A decade of type 2 diabetes shortens life by 3.5 years
researchers estimate on the basis of data from studies conducted in 19 high-income countries.
They estimated that, among 50-year-olds, life expectancy of those diagnosed with type 2 diabetes at age 30 is 14 years shorter than that of their peers without diabetes. Among those diagnosed at age 50, life expectancy is 6 years shorter.
The study was recently published in The Lancet – Diabetes and Endocrinology.
The team analyzed data from the Emerging Risk Factors Collaboration and the UK Biobank. The data were from 97 long-term, prospective cohorts and involved 1.5 million participants who were followed for 23.1 million person-years.
“The strongest associations with earlier age at diagnosis of diabetes were for vascular (for example, myocardial infarction and stroke) and other causes of death – mainly respiratory, neurological, and infectious diseases and external causes,” they reported.
Their findings are consistent with previous studies that suggested that younger individuals who develop type 2 diabetes might have higher body mass index (BMI), blood pressure, and lipid levels and that they might experience faster deterioration in glycemic control than individuals who develop diabetes later, potentially leading to premature mortality.
Asked to comment, Anne Peters, MD, director of clinical diabetes programs at the University of Southern California, Los Angeles, who was not involved with this study, said: “We’ve long known that diabetes reduces life expectancy, and the younger you get it the more years you lose. However, this study was from a broader and larger population base than prior studies.
“In this study, the major reason for death was vascular disease, and undertreatment of cardiovascular risk factors may have occurred in the younger individuals. We also don’t know about glucose control.
“I personally think the findings show that we should treat cardiovascular risk factors more aggressively in people diagnosed with [type 2] diabetes in their 30s and 40s,” urged Dr. Peters.
High priority should be given to prevention globally
“Type 2 diabetes used to be seen as a disease that affected older adults, but we’re increasingly seeing people diagnosed earlier in life,” senior author Emanuele Di Angelantonio, MD, PhD, from the University of Cambridge (England), explained in a press release. “As we’ve shown, this means they are at risk of a much shorter life expectancy than they would otherwise have.”
The findings suggest that “high priority should be given to developing and implementing interventions that prevent or delay the onset of [type 2 diabetes], especially as its prevalence among younger age groups is increasing globally,” the study authors wrote.
The results “support the idea that the younger an individual is when they develop type 2 diabetes, the more damage their body accumulates from its impaired metabolism,” added co–senior author Naveed Sattar, MD, PhD, of the University of Glasgow,
Dr. Peters agreed: “People who develop type 2 diabetes at a younger age might have a different, potentially more aggressive type of type 2 diabetes and perhaps need treatment targets that are lower than people who develop type 2 diabetes when they are older.”
“The findings ... suggest that early detection of diabetes by screening followed by intensive glucose management could help prevent long-term complications from the condition,” Dr. Sattar said.
Dr. Peters added: “An issue for some is pregnancy. ... Many of the medications taken for management of CVD [cardiovascular disease] risk factors are contraindicated in pregnancy (as are many of the medications [for treating type 2 diabetes]).
“We need to be careful to risk reduce but take care of the ‘whole person,’ and if of childbearing age, consider the safest approaches to healthy management,” she emphasized.
Study results: Type 2 diabetes diagnosed at age 30, 40, and 50
Previous studies estimated that adults with type 2 diabetes die 6 years earlier on average in comparison with their counterparts who do not have diabetes, but it was not known how diabetes duration affects life span.
To investigate this, the team analyzed individual records from the Emerging Risk Factors Collaboration and the UK Biobank. The primary outcome was all-cause mortality. Other outcomes were deaths from CVD, cancer, and other causes.
Over a median follow-up of 12.5 years, there were 246,670 deaths: 84,443 from cardiovascular causes, 150, 972 from noncardiovascular causes, and 11,255 from unknown/ill-defined causes.
Compared with participants who did not have a history of type 2 diabetes, the hazard ratios for all-cause mortality, adjusted for age and sex, were 2.69 for participants diagnosed at age 30-39, 2.26 for those diagnosed aged 40-49, 1.84 aged 50-59, 1.57 for those aged 60-69, and 1.39 for those diagnosed 70 and older.
These hazard ratios were similar after adjusting for BMI, systolic blood pressure, and total cholesterol, but they were substantially attenuated after further adjusting for fasting glucose or hemoglobin A1c level.
Similar patterns were observed for cause-specific mortality.
“Every decade of earlier diagnosis of diabetes was associated with about 3-4 years of lower life expectancy, highlighting the need to develop and implement interventions that prevent or delay the onset of diabetes and to intensify the treatment of risk factors among young adults diagnosed with diabetes,” the researchers wrote.
The study was funded the British Heart Foundation, the Medical Research Council, the National Institute for Health and Care Research, and Health Data Research UK. Dr. Peters is on advisory boards for Vertex, Eli Lilly, and Medscape, receives research funding from Abbott Diabetes Care and Insulet, and has stock options for Omada Health.
A version of this article first appeared on Medscape.com.
researchers estimate on the basis of data from studies conducted in 19 high-income countries.
They estimated that, among 50-year-olds, life expectancy of those diagnosed with type 2 diabetes at age 30 is 14 years shorter than that of their peers without diabetes. Among those diagnosed at age 50, life expectancy is 6 years shorter.
The study was recently published in The Lancet – Diabetes and Endocrinology.
The team analyzed data from the Emerging Risk Factors Collaboration and the UK Biobank. The data were from 97 long-term, prospective cohorts and involved 1.5 million participants who were followed for 23.1 million person-years.
“The strongest associations with earlier age at diagnosis of diabetes were for vascular (for example, myocardial infarction and stroke) and other causes of death – mainly respiratory, neurological, and infectious diseases and external causes,” they reported.
Their findings are consistent with previous studies that suggested that younger individuals who develop type 2 diabetes might have higher body mass index (BMI), blood pressure, and lipid levels and that they might experience faster deterioration in glycemic control than individuals who develop diabetes later, potentially leading to premature mortality.
Asked to comment, Anne Peters, MD, director of clinical diabetes programs at the University of Southern California, Los Angeles, who was not involved with this study, said: “We’ve long known that diabetes reduces life expectancy, and the younger you get it the more years you lose. However, this study was from a broader and larger population base than prior studies.
“In this study, the major reason for death was vascular disease, and undertreatment of cardiovascular risk factors may have occurred in the younger individuals. We also don’t know about glucose control.
“I personally think the findings show that we should treat cardiovascular risk factors more aggressively in people diagnosed with [type 2] diabetes in their 30s and 40s,” urged Dr. Peters.
High priority should be given to prevention globally
“Type 2 diabetes used to be seen as a disease that affected older adults, but we’re increasingly seeing people diagnosed earlier in life,” senior author Emanuele Di Angelantonio, MD, PhD, from the University of Cambridge (England), explained in a press release. “As we’ve shown, this means they are at risk of a much shorter life expectancy than they would otherwise have.”
The findings suggest that “high priority should be given to developing and implementing interventions that prevent or delay the onset of [type 2 diabetes], especially as its prevalence among younger age groups is increasing globally,” the study authors wrote.
The results “support the idea that the younger an individual is when they develop type 2 diabetes, the more damage their body accumulates from its impaired metabolism,” added co–senior author Naveed Sattar, MD, PhD, of the University of Glasgow,
Dr. Peters agreed: “People who develop type 2 diabetes at a younger age might have a different, potentially more aggressive type of type 2 diabetes and perhaps need treatment targets that are lower than people who develop type 2 diabetes when they are older.”
“The findings ... suggest that early detection of diabetes by screening followed by intensive glucose management could help prevent long-term complications from the condition,” Dr. Sattar said.
Dr. Peters added: “An issue for some is pregnancy. ... Many of the medications taken for management of CVD [cardiovascular disease] risk factors are contraindicated in pregnancy (as are many of the medications [for treating type 2 diabetes]).
“We need to be careful to risk reduce but take care of the ‘whole person,’ and if of childbearing age, consider the safest approaches to healthy management,” she emphasized.
Study results: Type 2 diabetes diagnosed at age 30, 40, and 50
Previous studies estimated that adults with type 2 diabetes die 6 years earlier on average in comparison with their counterparts who do not have diabetes, but it was not known how diabetes duration affects life span.
To investigate this, the team analyzed individual records from the Emerging Risk Factors Collaboration and the UK Biobank. The primary outcome was all-cause mortality. Other outcomes were deaths from CVD, cancer, and other causes.
Over a median follow-up of 12.5 years, there were 246,670 deaths: 84,443 from cardiovascular causes, 150, 972 from noncardiovascular causes, and 11,255 from unknown/ill-defined causes.
Compared with participants who did not have a history of type 2 diabetes, the hazard ratios for all-cause mortality, adjusted for age and sex, were 2.69 for participants diagnosed at age 30-39, 2.26 for those diagnosed aged 40-49, 1.84 aged 50-59, 1.57 for those aged 60-69, and 1.39 for those diagnosed 70 and older.
These hazard ratios were similar after adjusting for BMI, systolic blood pressure, and total cholesterol, but they were substantially attenuated after further adjusting for fasting glucose or hemoglobin A1c level.
Similar patterns were observed for cause-specific mortality.
“Every decade of earlier diagnosis of diabetes was associated with about 3-4 years of lower life expectancy, highlighting the need to develop and implement interventions that prevent or delay the onset of diabetes and to intensify the treatment of risk factors among young adults diagnosed with diabetes,” the researchers wrote.
The study was funded the British Heart Foundation, the Medical Research Council, the National Institute for Health and Care Research, and Health Data Research UK. Dr. Peters is on advisory boards for Vertex, Eli Lilly, and Medscape, receives research funding from Abbott Diabetes Care and Insulet, and has stock options for Omada Health.
A version of this article first appeared on Medscape.com.
researchers estimate on the basis of data from studies conducted in 19 high-income countries.
They estimated that, among 50-year-olds, life expectancy of those diagnosed with type 2 diabetes at age 30 is 14 years shorter than that of their peers without diabetes. Among those diagnosed at age 50, life expectancy is 6 years shorter.
The study was recently published in The Lancet – Diabetes and Endocrinology.
The team analyzed data from the Emerging Risk Factors Collaboration and the UK Biobank. The data were from 97 long-term, prospective cohorts and involved 1.5 million participants who were followed for 23.1 million person-years.
“The strongest associations with earlier age at diagnosis of diabetes were for vascular (for example, myocardial infarction and stroke) and other causes of death – mainly respiratory, neurological, and infectious diseases and external causes,” they reported.
Their findings are consistent with previous studies that suggested that younger individuals who develop type 2 diabetes might have higher body mass index (BMI), blood pressure, and lipid levels and that they might experience faster deterioration in glycemic control than individuals who develop diabetes later, potentially leading to premature mortality.
Asked to comment, Anne Peters, MD, director of clinical diabetes programs at the University of Southern California, Los Angeles, who was not involved with this study, said: “We’ve long known that diabetes reduces life expectancy, and the younger you get it the more years you lose. However, this study was from a broader and larger population base than prior studies.
“In this study, the major reason for death was vascular disease, and undertreatment of cardiovascular risk factors may have occurred in the younger individuals. We also don’t know about glucose control.
“I personally think the findings show that we should treat cardiovascular risk factors more aggressively in people diagnosed with [type 2] diabetes in their 30s and 40s,” urged Dr. Peters.
High priority should be given to prevention globally
“Type 2 diabetes used to be seen as a disease that affected older adults, but we’re increasingly seeing people diagnosed earlier in life,” senior author Emanuele Di Angelantonio, MD, PhD, from the University of Cambridge (England), explained in a press release. “As we’ve shown, this means they are at risk of a much shorter life expectancy than they would otherwise have.”
The findings suggest that “high priority should be given to developing and implementing interventions that prevent or delay the onset of [type 2 diabetes], especially as its prevalence among younger age groups is increasing globally,” the study authors wrote.
The results “support the idea that the younger an individual is when they develop type 2 diabetes, the more damage their body accumulates from its impaired metabolism,” added co–senior author Naveed Sattar, MD, PhD, of the University of Glasgow,
Dr. Peters agreed: “People who develop type 2 diabetes at a younger age might have a different, potentially more aggressive type of type 2 diabetes and perhaps need treatment targets that are lower than people who develop type 2 diabetes when they are older.”
“The findings ... suggest that early detection of diabetes by screening followed by intensive glucose management could help prevent long-term complications from the condition,” Dr. Sattar said.
Dr. Peters added: “An issue for some is pregnancy. ... Many of the medications taken for management of CVD [cardiovascular disease] risk factors are contraindicated in pregnancy (as are many of the medications [for treating type 2 diabetes]).
“We need to be careful to risk reduce but take care of the ‘whole person,’ and if of childbearing age, consider the safest approaches to healthy management,” she emphasized.
Study results: Type 2 diabetes diagnosed at age 30, 40, and 50
Previous studies estimated that adults with type 2 diabetes die 6 years earlier on average in comparison with their counterparts who do not have diabetes, but it was not known how diabetes duration affects life span.
To investigate this, the team analyzed individual records from the Emerging Risk Factors Collaboration and the UK Biobank. The primary outcome was all-cause mortality. Other outcomes were deaths from CVD, cancer, and other causes.
Over a median follow-up of 12.5 years, there were 246,670 deaths: 84,443 from cardiovascular causes, 150, 972 from noncardiovascular causes, and 11,255 from unknown/ill-defined causes.
Compared with participants who did not have a history of type 2 diabetes, the hazard ratios for all-cause mortality, adjusted for age and sex, were 2.69 for participants diagnosed at age 30-39, 2.26 for those diagnosed aged 40-49, 1.84 aged 50-59, 1.57 for those aged 60-69, and 1.39 for those diagnosed 70 and older.
These hazard ratios were similar after adjusting for BMI, systolic blood pressure, and total cholesterol, but they were substantially attenuated after further adjusting for fasting glucose or hemoglobin A1c level.
Similar patterns were observed for cause-specific mortality.
“Every decade of earlier diagnosis of diabetes was associated with about 3-4 years of lower life expectancy, highlighting the need to develop and implement interventions that prevent or delay the onset of diabetes and to intensify the treatment of risk factors among young adults diagnosed with diabetes,” the researchers wrote.
The study was funded the British Heart Foundation, the Medical Research Council, the National Institute for Health and Care Research, and Health Data Research UK. Dr. Peters is on advisory boards for Vertex, Eli Lilly, and Medscape, receives research funding from Abbott Diabetes Care and Insulet, and has stock options for Omada Health.
A version of this article first appeared on Medscape.com.
FROM THE LANCET – DIABETES AND ENDOCRINOLOGY

