User login
Spontaneously Regressing Primary Nodular Melanoma of the Glans Penis
To the Editor:
Primary malignant melanoma (PMM) of the penis is rare, comprising 1% of melanomas overall and less than 4% of malignancies in the male genitourinary tract.1 However, regression of PMM is not rare. Melanoma is 6 times more likely to undergo regression compared to other malignancies.2 Approximately 10% to 35% of cutaneous PMMs undergo partial regression, but only 42 cases of completely regressed cutaneous PMMs have been reported,3,4 which may be due to underreporting of completely regressed cutaneous PMMs, as they often are clinically inconspicuous. Additionally, completely regressed cutaneous PMMs may be incorrectly reported as metastatic melanoma of unknown primary.5 Clinical characteristics of regression include pink coloration and a lightening or whitening of baseline lesional color. Dermatoscopic features of regression include white areas, blue areas, or vascular structures that translate microscopically to dermal fibrosis, melanophages, and telangiectases.5 We report a case of complete clinical regression of a nodular, mucosal, penile PMM with no evidence of metastatic disease.
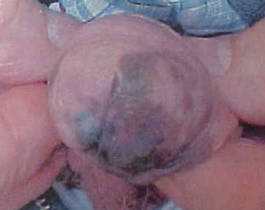
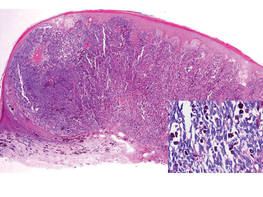
An 86-year-old man presented with a progressively enlarging, pigmented lesion on the glans penis of 2 years’ duration. His medical history was notable for retinal detachment, macular degeneration, lumbar stenosis, and seizures postneurosurgery for a subdural hematoma. Physical examination revealed a healthy man with a mottled, black-brown, macular, and nodular lesion with irregular margins and irregular shape on the glans penis (Figure 1). No other similar skin lesions or lymphadenopathy were detectable. A lesional deep shave biopsy obtained at presentation demonstrated a nodular-type malignant melanoma with a Breslow thickness of approximately 3.5 mm (Figure 2).
|
| Figure 1. Mottled, black-brown, macular, and nodular lesion with irregular margins and irregular shape on the glans penis. |
|
| Figure 2. Histologic section showed a nodular melanocytic proliferation with a dense sheetlike collection of melanocytes, predominantly in the dermis (H&E, original magnification ×20). Prominent cytologic atypia and multiple mitotic figures were consistent with melanoma (inset)(H&E, original magnification ×400). |
|
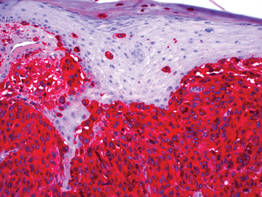
| Figure 3. High-power view of HMB-45 stain showed strong and diffuse staining, including several pagetoid intraepidermal melanocytes (original magnification ×200). |
|
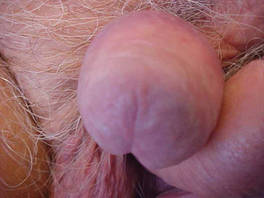
| Figure 4. Skin examination 8 years following the initial primary malignant melanoma diagnosis showed no clinical evidence of recurrent or metastatic melanoma and almost complete loss of pigmentation at the prior melanoma site. |
Histologic examination showed nodular nests of malignant melanocytes that were dispersed along the dermal-epidermal junction and coalesced into sheets within the dermis. Numerous dermal mitoses were present. The tumor was strongly and diffusely positive with Melan-A and HMB-45, which also highlighted scattered pagetoid intraepidermal cells (Figure 3). These findings were diagnostic of PMM of the mucocutaneous glans penis. The tumor was nonulcerated and invaded to a Breslow thickness of approximately 3.5 mm, corresponding to American Joint Committee on Cancer stage IIA (T3aN0M0) with an expected 5-year survival rate of 79%.6
He was referred to the urology department and was offered cystoscopy, urethrography, and phallectomy, which he refused. He also refused a trial of imiquimod. Computed tomography (CT) scans of his brain, chest, abdomen, and pelvis were negative for metastatic disease. Following the initial melanoma diagnosis, he had yearly dermatologic evaluations consisting of total-body skin and lymph node (LN) examinations. At 87 years of age (1 year following the initial diagnosis), the melanoma became dramatically smaller. At 88 years of age (2 years after diagnosis), the melanoma had near-complete clinical resolution. At 89 years of age, the patient reported asymmetric hearing loss. A cranial magnetic resonance imaging study showed no evidence of metastases.
At 92 years of age (6 years after the initial diagnosis), the patient reported bilateral leg pain. A CT scan of the lumbar spine showed no evidence of metastasis. He also reported abdominal pain. A CT scan of the abdomen and pelvis revealed an ileocecal mass. Biopsy of the ileocecal mass showed moderately differentiated invasive adenocarcinoma and no evidence of metastatic melanoma. The adenocarcinoma was resected and he continues to do well. Skin and LN examination 8 years after the initial diagnosis showed no clinical evidence of recurrent penile mucosal melanoma or metastatic melanoma (Figure 4). The PMM appeared to have clinically regressed spontaneously. He refused repeat skin biopsy and additional imaging studies.
The criteria for complete melanoma regression were initially described in 19657 and revised in 2005.2 Although our patient demonstrated complete clinical regression of his PMM, he did not meet the revised criteria for complete regression because there was no histopathologic confirmation of regression or of the absence of melanoma as well as no lymphatic involvement. It is extremely difficult to quantify the percentage of PMMs that completely regress. A case of a completely regressed untreated PMM with no metastatic disease 4 years after diagnosis has been reported. This case involved a nonulcerated melanoma with a Breslow thickness of 0.7 mm (American Joint Committee on Cancer stage IA).4 The prognosis of penile mucosal PMM is comparable to that of cutaneous PMM with a similar Breslow thickness.1
The prognostic significance of melanoma regression is controversial. Regression may be mediated by host immunity, apoptosis, and/or antiangiogenesis. The lymphocytic infiltrate in regressive melanomas consists of cytotoxic T cells with selective antitumor activity, which induces HLA class I–restricted melanoma lysis.8 Lymph node migration may result in T-lymphocyte priming and induction of antitumor immunity.9 Therefore, regression may indicate risk for sentinel LN metastasis.
It is possible that complete regression of melanoma does not truly exist, and late recurrence due to cancer dormancy is inevitable. Late recurrence is defined as first metastasis 10 years after complete removal of the PMM.10 Our patient has only been followed for 8 years, so this possibility cannot be entirely excluded.
1. van Geel AN, den Bakker MA, Kirkels W, et al. Prognosis of primary mucosal penile melanoma: a series of 19 Dutch patients and 47 patients from the literature. Urology. 2007;70:143-147.
2. High WA, Stewart D, Wilbers CR, et al. Completely regressed primary cutaneous malignant melanoma with nodal and/or visceral metastases: a report of 5 cases and assessment of the literature and diagnostic criteria. J Am Acad Dermatol. 2005;53:89-100.
3. Emanuel PO, Mannion M, Phelps RG. Complete regression of primary malignant melanoma. Am J Dermatopathol. 2008;30:178-181.
4. Muniesa C, Ferreres JR, Moreno A, et al. Completely regressed primary cutaneous malignant melanoma with metastases [published online ahead of print June 23, 2008]. J Eur Acad Dermatol Venereol. 2009;23:327-328.
5. Bories N, Dalle S, Debarbieux S, et al. Dermoscopy of fully regressive cutaneous melanoma [published online ahead of print March 13, 2008]. Br J Dermatol. 2008;158:1224-1229.
6. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification [published online ahead of print November 16, 2009]. J Clin Oncol. 2009;27:6199-6206.
7. Smith JL Jr, Stehlin JS Jr. Spontaneous regression of primary malignant melanomas with regional metastasis. Cancer. 1965;18:1399-1415.
8. Bottger D, Dowden RV, Kay PP. Complete spontaneous regression of cutaneous primary malignant melanoma. Plast Reconstr Surg. 1992;89:548-553.
9. Shaw HM, McCarthy SW, McCarthy WH, et al. Thin regressing malignant melanoma: significance of concurrent regional lymph node metastases. Histopathology. 1989;15:257-265.
10. Hansel G, Schönlebe J, Haroske G, et al. Late recurrence (10 years or more) of malignant melanoma in south-east Germany (Saxony). a single-centre analysis of 1881 patients with a follow-up of 10 years or more [published online ahead of print January 11, 2010]. J Eur Acad Dermatol Venereol. 2010;24:833-836.
To the Editor:
Primary malignant melanoma (PMM) of the penis is rare, comprising 1% of melanomas overall and less than 4% of malignancies in the male genitourinary tract.1 However, regression of PMM is not rare. Melanoma is 6 times more likely to undergo regression compared to other malignancies.2 Approximately 10% to 35% of cutaneous PMMs undergo partial regression, but only 42 cases of completely regressed cutaneous PMMs have been reported,3,4 which may be due to underreporting of completely regressed cutaneous PMMs, as they often are clinically inconspicuous. Additionally, completely regressed cutaneous PMMs may be incorrectly reported as metastatic melanoma of unknown primary.5 Clinical characteristics of regression include pink coloration and a lightening or whitening of baseline lesional color. Dermatoscopic features of regression include white areas, blue areas, or vascular structures that translate microscopically to dermal fibrosis, melanophages, and telangiectases.5 We report a case of complete clinical regression of a nodular, mucosal, penile PMM with no evidence of metastatic disease.
An 86-year-old man presented with a progressively enlarging, pigmented lesion on the glans penis of 2 years’ duration. His medical history was notable for retinal detachment, macular degeneration, lumbar stenosis, and seizures postneurosurgery for a subdural hematoma. Physical examination revealed a healthy man with a mottled, black-brown, macular, and nodular lesion with irregular margins and irregular shape on the glans penis (Figure 1). No other similar skin lesions or lymphadenopathy were detectable. A lesional deep shave biopsy obtained at presentation demonstrated a nodular-type malignant melanoma with a Breslow thickness of approximately 3.5 mm (Figure 2).

|
| Figure 1. Mottled, black-brown, macular, and nodular lesion with irregular margins and irregular shape on the glans penis. |

|
| Figure 2. Histologic section showed a nodular melanocytic proliferation with a dense sheetlike collection of melanocytes, predominantly in the dermis (H&E, original magnification ×20). Prominent cytologic atypia and multiple mitotic figures were consistent with melanoma (inset)(H&E, original magnification ×400). |

|
| Figure 3. High-power view of HMB-45 stain showed strong and diffuse staining, including several pagetoid intraepidermal melanocytes (original magnification ×200). |

|
| Figure 4. Skin examination 8 years following the initial primary malignant melanoma diagnosis showed no clinical evidence of recurrent or metastatic melanoma and almost complete loss of pigmentation at the prior melanoma site. |
Histologic examination showed nodular nests of malignant melanocytes that were dispersed along the dermal-epidermal junction and coalesced into sheets within the dermis. Numerous dermal mitoses were present. The tumor was strongly and diffusely positive with Melan-A and HMB-45, which also highlighted scattered pagetoid intraepidermal cells (Figure 3). These findings were diagnostic of PMM of the mucocutaneous glans penis. The tumor was nonulcerated and invaded to a Breslow thickness of approximately 3.5 mm, corresponding to American Joint Committee on Cancer stage IIA (T3aN0M0) with an expected 5-year survival rate of 79%.6
He was referred to the urology department and was offered cystoscopy, urethrography, and phallectomy, which he refused. He also refused a trial of imiquimod. Computed tomography (CT) scans of his brain, chest, abdomen, and pelvis were negative for metastatic disease. Following the initial melanoma diagnosis, he had yearly dermatologic evaluations consisting of total-body skin and lymph node (LN) examinations. At 87 years of age (1 year following the initial diagnosis), the melanoma became dramatically smaller. At 88 years of age (2 years after diagnosis), the melanoma had near-complete clinical resolution. At 89 years of age, the patient reported asymmetric hearing loss. A cranial magnetic resonance imaging study showed no evidence of metastases.
At 92 years of age (6 years after the initial diagnosis), the patient reported bilateral leg pain. A CT scan of the lumbar spine showed no evidence of metastasis. He also reported abdominal pain. A CT scan of the abdomen and pelvis revealed an ileocecal mass. Biopsy of the ileocecal mass showed moderately differentiated invasive adenocarcinoma and no evidence of metastatic melanoma. The adenocarcinoma was resected and he continues to do well. Skin and LN examination 8 years after the initial diagnosis showed no clinical evidence of recurrent penile mucosal melanoma or metastatic melanoma (Figure 4). The PMM appeared to have clinically regressed spontaneously. He refused repeat skin biopsy and additional imaging studies.
The criteria for complete melanoma regression were initially described in 19657 and revised in 2005.2 Although our patient demonstrated complete clinical regression of his PMM, he did not meet the revised criteria for complete regression because there was no histopathologic confirmation of regression or of the absence of melanoma as well as no lymphatic involvement. It is extremely difficult to quantify the percentage of PMMs that completely regress. A case of a completely regressed untreated PMM with no metastatic disease 4 years after diagnosis has been reported. This case involved a nonulcerated melanoma with a Breslow thickness of 0.7 mm (American Joint Committee on Cancer stage IA).4 The prognosis of penile mucosal PMM is comparable to that of cutaneous PMM with a similar Breslow thickness.1
The prognostic significance of melanoma regression is controversial. Regression may be mediated by host immunity, apoptosis, and/or antiangiogenesis. The lymphocytic infiltrate in regressive melanomas consists of cytotoxic T cells with selective antitumor activity, which induces HLA class I–restricted melanoma lysis.8 Lymph node migration may result in T-lymphocyte priming and induction of antitumor immunity.9 Therefore, regression may indicate risk for sentinel LN metastasis.
It is possible that complete regression of melanoma does not truly exist, and late recurrence due to cancer dormancy is inevitable. Late recurrence is defined as first metastasis 10 years after complete removal of the PMM.10 Our patient has only been followed for 8 years, so this possibility cannot be entirely excluded.
To the Editor:
Primary malignant melanoma (PMM) of the penis is rare, comprising 1% of melanomas overall and less than 4% of malignancies in the male genitourinary tract.1 However, regression of PMM is not rare. Melanoma is 6 times more likely to undergo regression compared to other malignancies.2 Approximately 10% to 35% of cutaneous PMMs undergo partial regression, but only 42 cases of completely regressed cutaneous PMMs have been reported,3,4 which may be due to underreporting of completely regressed cutaneous PMMs, as they often are clinically inconspicuous. Additionally, completely regressed cutaneous PMMs may be incorrectly reported as metastatic melanoma of unknown primary.5 Clinical characteristics of regression include pink coloration and a lightening or whitening of baseline lesional color. Dermatoscopic features of regression include white areas, blue areas, or vascular structures that translate microscopically to dermal fibrosis, melanophages, and telangiectases.5 We report a case of complete clinical regression of a nodular, mucosal, penile PMM with no evidence of metastatic disease.
An 86-year-old man presented with a progressively enlarging, pigmented lesion on the glans penis of 2 years’ duration. His medical history was notable for retinal detachment, macular degeneration, lumbar stenosis, and seizures postneurosurgery for a subdural hematoma. Physical examination revealed a healthy man with a mottled, black-brown, macular, and nodular lesion with irregular margins and irregular shape on the glans penis (Figure 1). No other similar skin lesions or lymphadenopathy were detectable. A lesional deep shave biopsy obtained at presentation demonstrated a nodular-type malignant melanoma with a Breslow thickness of approximately 3.5 mm (Figure 2).

|
| Figure 1. Mottled, black-brown, macular, and nodular lesion with irregular margins and irregular shape on the glans penis. |

|
| Figure 2. Histologic section showed a nodular melanocytic proliferation with a dense sheetlike collection of melanocytes, predominantly in the dermis (H&E, original magnification ×20). Prominent cytologic atypia and multiple mitotic figures were consistent with melanoma (inset)(H&E, original magnification ×400). |

|
| Figure 3. High-power view of HMB-45 stain showed strong and diffuse staining, including several pagetoid intraepidermal melanocytes (original magnification ×200). |

|
| Figure 4. Skin examination 8 years following the initial primary malignant melanoma diagnosis showed no clinical evidence of recurrent or metastatic melanoma and almost complete loss of pigmentation at the prior melanoma site. |
Histologic examination showed nodular nests of malignant melanocytes that were dispersed along the dermal-epidermal junction and coalesced into sheets within the dermis. Numerous dermal mitoses were present. The tumor was strongly and diffusely positive with Melan-A and HMB-45, which also highlighted scattered pagetoid intraepidermal cells (Figure 3). These findings were diagnostic of PMM of the mucocutaneous glans penis. The tumor was nonulcerated and invaded to a Breslow thickness of approximately 3.5 mm, corresponding to American Joint Committee on Cancer stage IIA (T3aN0M0) with an expected 5-year survival rate of 79%.6
He was referred to the urology department and was offered cystoscopy, urethrography, and phallectomy, which he refused. He also refused a trial of imiquimod. Computed tomography (CT) scans of his brain, chest, abdomen, and pelvis were negative for metastatic disease. Following the initial melanoma diagnosis, he had yearly dermatologic evaluations consisting of total-body skin and lymph node (LN) examinations. At 87 years of age (1 year following the initial diagnosis), the melanoma became dramatically smaller. At 88 years of age (2 years after diagnosis), the melanoma had near-complete clinical resolution. At 89 years of age, the patient reported asymmetric hearing loss. A cranial magnetic resonance imaging study showed no evidence of metastases.
At 92 years of age (6 years after the initial diagnosis), the patient reported bilateral leg pain. A CT scan of the lumbar spine showed no evidence of metastasis. He also reported abdominal pain. A CT scan of the abdomen and pelvis revealed an ileocecal mass. Biopsy of the ileocecal mass showed moderately differentiated invasive adenocarcinoma and no evidence of metastatic melanoma. The adenocarcinoma was resected and he continues to do well. Skin and LN examination 8 years after the initial diagnosis showed no clinical evidence of recurrent penile mucosal melanoma or metastatic melanoma (Figure 4). The PMM appeared to have clinically regressed spontaneously. He refused repeat skin biopsy and additional imaging studies.
The criteria for complete melanoma regression were initially described in 19657 and revised in 2005.2 Although our patient demonstrated complete clinical regression of his PMM, he did not meet the revised criteria for complete regression because there was no histopathologic confirmation of regression or of the absence of melanoma as well as no lymphatic involvement. It is extremely difficult to quantify the percentage of PMMs that completely regress. A case of a completely regressed untreated PMM with no metastatic disease 4 years after diagnosis has been reported. This case involved a nonulcerated melanoma with a Breslow thickness of 0.7 mm (American Joint Committee on Cancer stage IA).4 The prognosis of penile mucosal PMM is comparable to that of cutaneous PMM with a similar Breslow thickness.1
The prognostic significance of melanoma regression is controversial. Regression may be mediated by host immunity, apoptosis, and/or antiangiogenesis. The lymphocytic infiltrate in regressive melanomas consists of cytotoxic T cells with selective antitumor activity, which induces HLA class I–restricted melanoma lysis.8 Lymph node migration may result in T-lymphocyte priming and induction of antitumor immunity.9 Therefore, regression may indicate risk for sentinel LN metastasis.
It is possible that complete regression of melanoma does not truly exist, and late recurrence due to cancer dormancy is inevitable. Late recurrence is defined as first metastasis 10 years after complete removal of the PMM.10 Our patient has only been followed for 8 years, so this possibility cannot be entirely excluded.
1. van Geel AN, den Bakker MA, Kirkels W, et al. Prognosis of primary mucosal penile melanoma: a series of 19 Dutch patients and 47 patients from the literature. Urology. 2007;70:143-147.
2. High WA, Stewart D, Wilbers CR, et al. Completely regressed primary cutaneous malignant melanoma with nodal and/or visceral metastases: a report of 5 cases and assessment of the literature and diagnostic criteria. J Am Acad Dermatol. 2005;53:89-100.
3. Emanuel PO, Mannion M, Phelps RG. Complete regression of primary malignant melanoma. Am J Dermatopathol. 2008;30:178-181.
4. Muniesa C, Ferreres JR, Moreno A, et al. Completely regressed primary cutaneous malignant melanoma with metastases [published online ahead of print June 23, 2008]. J Eur Acad Dermatol Venereol. 2009;23:327-328.
5. Bories N, Dalle S, Debarbieux S, et al. Dermoscopy of fully regressive cutaneous melanoma [published online ahead of print March 13, 2008]. Br J Dermatol. 2008;158:1224-1229.
6. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification [published online ahead of print November 16, 2009]. J Clin Oncol. 2009;27:6199-6206.
7. Smith JL Jr, Stehlin JS Jr. Spontaneous regression of primary malignant melanomas with regional metastasis. Cancer. 1965;18:1399-1415.
8. Bottger D, Dowden RV, Kay PP. Complete spontaneous regression of cutaneous primary malignant melanoma. Plast Reconstr Surg. 1992;89:548-553.
9. Shaw HM, McCarthy SW, McCarthy WH, et al. Thin regressing malignant melanoma: significance of concurrent regional lymph node metastases. Histopathology. 1989;15:257-265.
10. Hansel G, Schönlebe J, Haroske G, et al. Late recurrence (10 years or more) of malignant melanoma in south-east Germany (Saxony). a single-centre analysis of 1881 patients with a follow-up of 10 years or more [published online ahead of print January 11, 2010]. J Eur Acad Dermatol Venereol. 2010;24:833-836.
1. van Geel AN, den Bakker MA, Kirkels W, et al. Prognosis of primary mucosal penile melanoma: a series of 19 Dutch patients and 47 patients from the literature. Urology. 2007;70:143-147.
2. High WA, Stewart D, Wilbers CR, et al. Completely regressed primary cutaneous malignant melanoma with nodal and/or visceral metastases: a report of 5 cases and assessment of the literature and diagnostic criteria. J Am Acad Dermatol. 2005;53:89-100.
3. Emanuel PO, Mannion M, Phelps RG. Complete regression of primary malignant melanoma. Am J Dermatopathol. 2008;30:178-181.
4. Muniesa C, Ferreres JR, Moreno A, et al. Completely regressed primary cutaneous malignant melanoma with metastases [published online ahead of print June 23, 2008]. J Eur Acad Dermatol Venereol. 2009;23:327-328.
5. Bories N, Dalle S, Debarbieux S, et al. Dermoscopy of fully regressive cutaneous melanoma [published online ahead of print March 13, 2008]. Br J Dermatol. 2008;158:1224-1229.
6. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification [published online ahead of print November 16, 2009]. J Clin Oncol. 2009;27:6199-6206.
7. Smith JL Jr, Stehlin JS Jr. Spontaneous regression of primary malignant melanomas with regional metastasis. Cancer. 1965;18:1399-1415.
8. Bottger D, Dowden RV, Kay PP. Complete spontaneous regression of cutaneous primary malignant melanoma. Plast Reconstr Surg. 1992;89:548-553.
9. Shaw HM, McCarthy SW, McCarthy WH, et al. Thin regressing malignant melanoma: significance of concurrent regional lymph node metastases. Histopathology. 1989;15:257-265.
10. Hansel G, Schönlebe J, Haroske G, et al. Late recurrence (10 years or more) of malignant melanoma in south-east Germany (Saxony). a single-centre analysis of 1881 patients with a follow-up of 10 years or more [published online ahead of print January 11, 2010]. J Eur Acad Dermatol Venereol. 2010;24:833-836.