User login
Neurology shortfall to worsen by 2025
The shortage of adult and child neurologists in the United States is set to go from bad to worse, according to a simulation study sponsored by the American Academy of Neurology.
The demand for neurologists already exceeds the supply by about 11% overall. But by 2025 the difference between supply and demand is expected to hit 19% as Americans age and as more people have access to insurance through the Affordable Care Act.
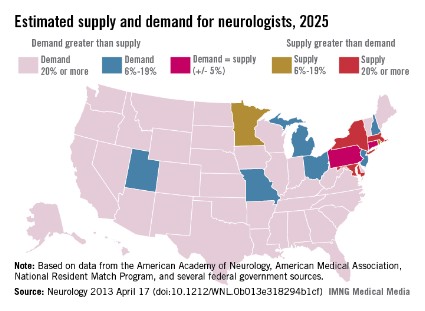
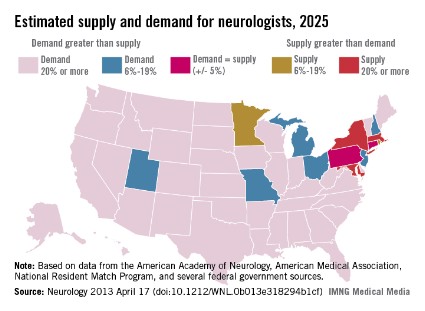
The result of the shortfall is likely to be more of what physicians and patients are already experiencing: long wait times to get an appointment, difficulty hiring new neurologists, and large numbers of neurologists who will not accept Medicaid. Already the average wait time for a new patient to see a neurologist is 34.8 business days. In child neurology, the average wait time is 45 business days.
Officials at the American Academy of Neurology (AAN) are taking the findings to Capitol Hill in the hope of getting some legislative relief that could help improve access to neurology services.
One solution they are proposing is to increase the payment for evaluation and management codes under the Medicare payment system. Undervalued cognitive care services are part of the reason that medical students and residents, who are burdened with significant educational debt, sometimes shy away from careers in neurology, Dr. Timothy A. Pedley, AAN President, said in an interview.
"This is a problem that will only increase in magnitude unless something is done to reverse this trend," Dr. Pedley said.
In 2012, the supply of adult and child neurologists was 16,366, but the demand was for 18,180 neurologists, a shortfall of about 11% nationally. By 2025, the supply is expected to grow to about 18,060, but the demand will hit 21,440, a shortfall of 19% nationally. The figures include both adult and child neurologists, but shortages for child neurologists alone are even more severe, according to the study.
Researchers conducted a microsimulation supply and demand model study to look at how the shortfall of neurologists was likely to change between 2012 and 2025. The model simulates the career choices of neurologists throughout their careers, taking into account deaths, retirements, new graduates, and the number of patient care hours worked. The investigators relied on several sources, including databases from the AAN and the American Medical Association. They also used data and surveys from the National Residency Match Program (Neurology 2013 April 17 [doi: 10.1212/WNL.0b013e318294b1cf]).
The researchers modeled the demand for services by taking demographic, socioeconomic, and health risk factors for a representative sample in each state. They then used that data to forecast the use of neurology services, and applied estimates of neurologist productivity. They used data from the U.S. Census Bureau and the Centers for Disease Control and Prevention to make the demand projections.
The shortage of neurologists varies by region. However, the researchers estimate that by 2025 most of the country will be facing a neurologist shortage of 20% or more, while only a few states will have enough neurologists to meet the demand.
There are several reasons for the increased demand, the researchers noted. One factor is the aging of the population and, with it, the rising prevalence of neurologic conditions. Another factor is the increased use of nonphysician providers in the primary care setting. Since these providers have limited training in neurology, there are likely to be more referrals made to neurologists.
Increased access to health insurance coverage under the Affordable Care Act also will drive demand for neurologists. The researchers estimate that it will push the projected shortfall up from 16% in 2025 to 19% overall by 2025.
The study was supported by the AAN and an educational grant from Lilly USA.
The shortage of adult and child neurologists in the United States is set to go from bad to worse, according to a simulation study sponsored by the American Academy of Neurology.
The demand for neurologists already exceeds the supply by about 11% overall. But by 2025 the difference between supply and demand is expected to hit 19% as Americans age and as more people have access to insurance through the Affordable Care Act.

The result of the shortfall is likely to be more of what physicians and patients are already experiencing: long wait times to get an appointment, difficulty hiring new neurologists, and large numbers of neurologists who will not accept Medicaid. Already the average wait time for a new patient to see a neurologist is 34.8 business days. In child neurology, the average wait time is 45 business days.
Officials at the American Academy of Neurology (AAN) are taking the findings to Capitol Hill in the hope of getting some legislative relief that could help improve access to neurology services.
One solution they are proposing is to increase the payment for evaluation and management codes under the Medicare payment system. Undervalued cognitive care services are part of the reason that medical students and residents, who are burdened with significant educational debt, sometimes shy away from careers in neurology, Dr. Timothy A. Pedley, AAN President, said in an interview.
"This is a problem that will only increase in magnitude unless something is done to reverse this trend," Dr. Pedley said.
In 2012, the supply of adult and child neurologists was 16,366, but the demand was for 18,180 neurologists, a shortfall of about 11% nationally. By 2025, the supply is expected to grow to about 18,060, but the demand will hit 21,440, a shortfall of 19% nationally. The figures include both adult and child neurologists, but shortages for child neurologists alone are even more severe, according to the study.
Researchers conducted a microsimulation supply and demand model study to look at how the shortfall of neurologists was likely to change between 2012 and 2025. The model simulates the career choices of neurologists throughout their careers, taking into account deaths, retirements, new graduates, and the number of patient care hours worked. The investigators relied on several sources, including databases from the AAN and the American Medical Association. They also used data and surveys from the National Residency Match Program (Neurology 2013 April 17 [doi: 10.1212/WNL.0b013e318294b1cf]).
The researchers modeled the demand for services by taking demographic, socioeconomic, and health risk factors for a representative sample in each state. They then used that data to forecast the use of neurology services, and applied estimates of neurologist productivity. They used data from the U.S. Census Bureau and the Centers for Disease Control and Prevention to make the demand projections.
The shortage of neurologists varies by region. However, the researchers estimate that by 2025 most of the country will be facing a neurologist shortage of 20% or more, while only a few states will have enough neurologists to meet the demand.
There are several reasons for the increased demand, the researchers noted. One factor is the aging of the population and, with it, the rising prevalence of neurologic conditions. Another factor is the increased use of nonphysician providers in the primary care setting. Since these providers have limited training in neurology, there are likely to be more referrals made to neurologists.
Increased access to health insurance coverage under the Affordable Care Act also will drive demand for neurologists. The researchers estimate that it will push the projected shortfall up from 16% in 2025 to 19% overall by 2025.
The study was supported by the AAN and an educational grant from Lilly USA.
The shortage of adult and child neurologists in the United States is set to go from bad to worse, according to a simulation study sponsored by the American Academy of Neurology.
The demand for neurologists already exceeds the supply by about 11% overall. But by 2025 the difference between supply and demand is expected to hit 19% as Americans age and as more people have access to insurance through the Affordable Care Act.

The result of the shortfall is likely to be more of what physicians and patients are already experiencing: long wait times to get an appointment, difficulty hiring new neurologists, and large numbers of neurologists who will not accept Medicaid. Already the average wait time for a new patient to see a neurologist is 34.8 business days. In child neurology, the average wait time is 45 business days.
Officials at the American Academy of Neurology (AAN) are taking the findings to Capitol Hill in the hope of getting some legislative relief that could help improve access to neurology services.
One solution they are proposing is to increase the payment for evaluation and management codes under the Medicare payment system. Undervalued cognitive care services are part of the reason that medical students and residents, who are burdened with significant educational debt, sometimes shy away from careers in neurology, Dr. Timothy A. Pedley, AAN President, said in an interview.
"This is a problem that will only increase in magnitude unless something is done to reverse this trend," Dr. Pedley said.
In 2012, the supply of adult and child neurologists was 16,366, but the demand was for 18,180 neurologists, a shortfall of about 11% nationally. By 2025, the supply is expected to grow to about 18,060, but the demand will hit 21,440, a shortfall of 19% nationally. The figures include both adult and child neurologists, but shortages for child neurologists alone are even more severe, according to the study.
Researchers conducted a microsimulation supply and demand model study to look at how the shortfall of neurologists was likely to change between 2012 and 2025. The model simulates the career choices of neurologists throughout their careers, taking into account deaths, retirements, new graduates, and the number of patient care hours worked. The investigators relied on several sources, including databases from the AAN and the American Medical Association. They also used data and surveys from the National Residency Match Program (Neurology 2013 April 17 [doi: 10.1212/WNL.0b013e318294b1cf]).
The researchers modeled the demand for services by taking demographic, socioeconomic, and health risk factors for a representative sample in each state. They then used that data to forecast the use of neurology services, and applied estimates of neurologist productivity. They used data from the U.S. Census Bureau and the Centers for Disease Control and Prevention to make the demand projections.
The shortage of neurologists varies by region. However, the researchers estimate that by 2025 most of the country will be facing a neurologist shortage of 20% or more, while only a few states will have enough neurologists to meet the demand.
There are several reasons for the increased demand, the researchers noted. One factor is the aging of the population and, with it, the rising prevalence of neurologic conditions. Another factor is the increased use of nonphysician providers in the primary care setting. Since these providers have limited training in neurology, there are likely to be more referrals made to neurologists.
Increased access to health insurance coverage under the Affordable Care Act also will drive demand for neurologists. The researchers estimate that it will push the projected shortfall up from 16% in 2025 to 19% overall by 2025.
The study was supported by the AAN and an educational grant from Lilly USA.
FROM NEUROLOGY
Major finding: By 2025, the demand for neurologists will exceed the supply by 19%.
Data source: A microsimulation supply and demand model study to estimate the supply and demand of neurologists in the United States from 2012 to 2025.
Disclosures: The study was funded by the AAN and an educational grant from Lilly USA.
Supreme Court ponders patenting of human genes
Can human genes be patented? The Supreme Court is set to decide just that later this summer in a decision that could have a deep and lasting effect on medical research.
In Association for Molecular Pathology, et al. v. Myriad Genetics, the high court is considering the validity of patents held by Utah-based Myriad Genetics for the BRCA1 and BRCA2 genes, which are associated with an increased risk of breast and ovarian cancer.
Scientists at Myriad Genetics uncovered the link between the BRCA1 and BRCA2 genes and the increased risk for cancer and patented the discovery in the mid-1990s. Since then, they have held the exclusive rights to the diagnostic testing for the mutations.
The company, however, has been criticized for setting the price of its BRACAnalysis, which tests for mutations on both genes, too high. Also, critics say that, since no one else can run the test, it’s impossible to get a second opinion.
So in 2009, the Association for Molecular Pathology, the American Civil Liberties Union, and several women’s health groups filed suit against Myriad in an effort to invalidate the company’s patents. In the most recent court ruling, a federal appeals court sided with Myriad and upheld the patents.
The Supreme Court recently heard oral arguments in the case and is expected to release its decision at the end of June.
Opponents of gene patenting, including the American Medical Association, contend that Myriad doesn’t have the right to patent human genes because these are products of nature and have not been altered. The AMA, which filed an amicus brief in the case, is also opposed to the patents on ethical grounds.
"Medical innovations that provide insight into natural human biology must remain freely accessible and widely disseminated, not hidden behind a vast thicket of exclusive rights," Dr. Jeremy Lazarus, AMA president, said in a statement. "Blocking this information interferes with diagnosis and treatment of patients and inhibits new medical discoveries."
Myriad argues that its patents aren’t for the genes themselves but for the synthetic molecules based on the genes, which are created in the lab. These molecules are different from what is found in nature or the human body, according to Myriad.
On a practical level, invalidating the patents would "chill" future biomedical research, according to the Biotechnology Industry Organization (BIO). In an amicus brief in support of Myriad, the organization warned that the chilling effect would apply to nondiagnostic uses for isolated human DNA, the untapped potential of isolated nonhuman DNA, the use of RNA molecules such as microRNA, and other isolated molecules such as therapeutic proteins and antibiotics. BIO said that future advances in vaccination also could be threatened if the Myriad patent case failed. For example, without the hope of patent protection, companies might not continue to work toward making vaccines that use small pieces of DNA to trigger antibody production. This area of research, which is in the early stages, holds the potential to produce vaccines for diseases such as HIV and cancer, according to BIO.
But bioethicist Arthur Caplan, Ph.D., said that the idea that research would be stifled if patents on genes were overturned is "sheer nonsense."
"Most of the money to understand human genes came from the taxpayer, through the form of the genome mapping project," said Dr. Caplan, who is the director of the division of medical ethics at the New York University Langone Medical Center. "It wasn’t funded by private companies."
No matter what the court decides, there isn’t likely to be a major practical impact, Dr. Caplan said. Since Myriad’s 20-year patent exclusivity is about to expire, diagnostic testing on BRCA1 and BRCA2 will soon open up. So in terms of a fight about cost and access, the case is a little late, he said.
"I don’t think the case is momentous if you’re someone thinking about getting a breast cancer test because the situation is going to likely change pretty soon no matter what the court says," Dr. Caplan said.
Dr. James P. Evans, professor of genetics and medicine at the University of North Carolina at Chapel Hill, agreed.
"This is a classic example of the science having outstripped the legislative and judicial arenas," Dr. Evans said.
For starters, genome sequencing has become so routine that there is patent infringement going on "right and left," he said, which is likely to continue regardless of what the Supreme Court rules in the case. "I think the horse is out of the barn," he said.
As for research, Dr. Evans said that the removal of patents is likely to open up this area. Most of the progress in genetics so far has come from researchers who have not been seeking patents. And patents would still be available for other advances, such as the creation of new gene-sequencing platforms, he said.
On Twitter @MaryEllenNY
Opponents of gene patenting, American Medical Association,
Can human genes be patented? The Supreme Court is set to decide just that later this summer in a decision that could have a deep and lasting effect on medical research.
In Association for Molecular Pathology, et al. v. Myriad Genetics, the high court is considering the validity of patents held by Utah-based Myriad Genetics for the BRCA1 and BRCA2 genes, which are associated with an increased risk of breast and ovarian cancer.
Scientists at Myriad Genetics uncovered the link between the BRCA1 and BRCA2 genes and the increased risk for cancer and patented the discovery in the mid-1990s. Since then, they have held the exclusive rights to the diagnostic testing for the mutations.
The company, however, has been criticized for setting the price of its BRACAnalysis, which tests for mutations on both genes, too high. Also, critics say that, since no one else can run the test, it’s impossible to get a second opinion.
So in 2009, the Association for Molecular Pathology, the American Civil Liberties Union, and several women’s health groups filed suit against Myriad in an effort to invalidate the company’s patents. In the most recent court ruling, a federal appeals court sided with Myriad and upheld the patents.
The Supreme Court recently heard oral arguments in the case and is expected to release its decision at the end of June.
Opponents of gene patenting, including the American Medical Association, contend that Myriad doesn’t have the right to patent human genes because these are products of nature and have not been altered. The AMA, which filed an amicus brief in the case, is also opposed to the patents on ethical grounds.
"Medical innovations that provide insight into natural human biology must remain freely accessible and widely disseminated, not hidden behind a vast thicket of exclusive rights," Dr. Jeremy Lazarus, AMA president, said in a statement. "Blocking this information interferes with diagnosis and treatment of patients and inhibits new medical discoveries."
Myriad argues that its patents aren’t for the genes themselves but for the synthetic molecules based on the genes, which are created in the lab. These molecules are different from what is found in nature or the human body, according to Myriad.
On a practical level, invalidating the patents would "chill" future biomedical research, according to the Biotechnology Industry Organization (BIO). In an amicus brief in support of Myriad, the organization warned that the chilling effect would apply to nondiagnostic uses for isolated human DNA, the untapped potential of isolated nonhuman DNA, the use of RNA molecules such as microRNA, and other isolated molecules such as therapeutic proteins and antibiotics. BIO said that future advances in vaccination also could be threatened if the Myriad patent case failed. For example, without the hope of patent protection, companies might not continue to work toward making vaccines that use small pieces of DNA to trigger antibody production. This area of research, which is in the early stages, holds the potential to produce vaccines for diseases such as HIV and cancer, according to BIO.
But bioethicist Arthur Caplan, Ph.D., said that the idea that research would be stifled if patents on genes were overturned is "sheer nonsense."
"Most of the money to understand human genes came from the taxpayer, through the form of the genome mapping project," said Dr. Caplan, who is the director of the division of medical ethics at the New York University Langone Medical Center. "It wasn’t funded by private companies."
No matter what the court decides, there isn’t likely to be a major practical impact, Dr. Caplan said. Since Myriad’s 20-year patent exclusivity is about to expire, diagnostic testing on BRCA1 and BRCA2 will soon open up. So in terms of a fight about cost and access, the case is a little late, he said.
"I don’t think the case is momentous if you’re someone thinking about getting a breast cancer test because the situation is going to likely change pretty soon no matter what the court says," Dr. Caplan said.
Dr. James P. Evans, professor of genetics and medicine at the University of North Carolina at Chapel Hill, agreed.
"This is a classic example of the science having outstripped the legislative and judicial arenas," Dr. Evans said.
For starters, genome sequencing has become so routine that there is patent infringement going on "right and left," he said, which is likely to continue regardless of what the Supreme Court rules in the case. "I think the horse is out of the barn," he said.
As for research, Dr. Evans said that the removal of patents is likely to open up this area. Most of the progress in genetics so far has come from researchers who have not been seeking patents. And patents would still be available for other advances, such as the creation of new gene-sequencing platforms, he said.
On Twitter @MaryEllenNY
Can human genes be patented? The Supreme Court is set to decide just that later this summer in a decision that could have a deep and lasting effect on medical research.
In Association for Molecular Pathology, et al. v. Myriad Genetics, the high court is considering the validity of patents held by Utah-based Myriad Genetics for the BRCA1 and BRCA2 genes, which are associated with an increased risk of breast and ovarian cancer.
Scientists at Myriad Genetics uncovered the link between the BRCA1 and BRCA2 genes and the increased risk for cancer and patented the discovery in the mid-1990s. Since then, they have held the exclusive rights to the diagnostic testing for the mutations.
The company, however, has been criticized for setting the price of its BRACAnalysis, which tests for mutations on both genes, too high. Also, critics say that, since no one else can run the test, it’s impossible to get a second opinion.
So in 2009, the Association for Molecular Pathology, the American Civil Liberties Union, and several women’s health groups filed suit against Myriad in an effort to invalidate the company’s patents. In the most recent court ruling, a federal appeals court sided with Myriad and upheld the patents.
The Supreme Court recently heard oral arguments in the case and is expected to release its decision at the end of June.
Opponents of gene patenting, including the American Medical Association, contend that Myriad doesn’t have the right to patent human genes because these are products of nature and have not been altered. The AMA, which filed an amicus brief in the case, is also opposed to the patents on ethical grounds.
"Medical innovations that provide insight into natural human biology must remain freely accessible and widely disseminated, not hidden behind a vast thicket of exclusive rights," Dr. Jeremy Lazarus, AMA president, said in a statement. "Blocking this information interferes with diagnosis and treatment of patients and inhibits new medical discoveries."
Myriad argues that its patents aren’t for the genes themselves but for the synthetic molecules based on the genes, which are created in the lab. These molecules are different from what is found in nature or the human body, according to Myriad.
On a practical level, invalidating the patents would "chill" future biomedical research, according to the Biotechnology Industry Organization (BIO). In an amicus brief in support of Myriad, the organization warned that the chilling effect would apply to nondiagnostic uses for isolated human DNA, the untapped potential of isolated nonhuman DNA, the use of RNA molecules such as microRNA, and other isolated molecules such as therapeutic proteins and antibiotics. BIO said that future advances in vaccination also could be threatened if the Myriad patent case failed. For example, without the hope of patent protection, companies might not continue to work toward making vaccines that use small pieces of DNA to trigger antibody production. This area of research, which is in the early stages, holds the potential to produce vaccines for diseases such as HIV and cancer, according to BIO.
But bioethicist Arthur Caplan, Ph.D., said that the idea that research would be stifled if patents on genes were overturned is "sheer nonsense."
"Most of the money to understand human genes came from the taxpayer, through the form of the genome mapping project," said Dr. Caplan, who is the director of the division of medical ethics at the New York University Langone Medical Center. "It wasn’t funded by private companies."
No matter what the court decides, there isn’t likely to be a major practical impact, Dr. Caplan said. Since Myriad’s 20-year patent exclusivity is about to expire, diagnostic testing on BRCA1 and BRCA2 will soon open up. So in terms of a fight about cost and access, the case is a little late, he said.
"I don’t think the case is momentous if you’re someone thinking about getting a breast cancer test because the situation is going to likely change pretty soon no matter what the court says," Dr. Caplan said.
Dr. James P. Evans, professor of genetics and medicine at the University of North Carolina at Chapel Hill, agreed.
"This is a classic example of the science having outstripped the legislative and judicial arenas," Dr. Evans said.
For starters, genome sequencing has become so routine that there is patent infringement going on "right and left," he said, which is likely to continue regardless of what the Supreme Court rules in the case. "I think the horse is out of the barn," he said.
As for research, Dr. Evans said that the removal of patents is likely to open up this area. Most of the progress in genetics so far has come from researchers who have not been seeking patents. And patents would still be available for other advances, such as the creation of new gene-sequencing platforms, he said.
On Twitter @MaryEllenNY
Opponents of gene patenting, American Medical Association,
Opponents of gene patenting, American Medical Association,
Joint Commission issues alert on 'alarm fatigue'
A single hospitalized patient can generate up to several hundred alarm signals each day, causing physicians to quickly become desensitized to the noise. But ignoring these alarms can have fatal consequences for patients, the Joint Commission warns.
Between January 2009 and June 2012, the Joint Commission’s Sentinel Event Alert database recorded 98 alarm-related adverse events, 80 of which resulted in death. Another 13 resulted in permanent loss of function and 5 led to unexpected additional care or an extended stay in the hospital.
The Joint Commission found that inadequate alarms, improper settings, and signals that were not loud enough all contributed to the reported adverse events. Alarms that were improperly turned off also were a problem, according to the Joint Commission.
In the Sentinel Event Alert issued on April 8, the Joint Commission recommended several steps hospital leaders can take to curb the "alarm fatigue" common in hospitals.
– Set up a process for alarm management and response, especially in high-risk areas.
– Perform an inventory of all devices with alarms in high-risk areas and their default settings.
– Establish guidelines for alarm settings in high-risk areas and for high-risk conditions, including situations when alarms are not clinically necessary.
– Establish guidelines for tailoring alarm settings and limits for individual patients.
– Inspect and maintain alarm-equipped devices.
"Alarm fatigue and management of alarms are important safety issues that we must confront," Dr. Ana McKee, executive vice president and chief medical officer at the Joint Commission, said in a statement. "The recommendations in this alert offer hospitals a framework on which to assess their individual circumstances and develop a systematic, coordinated approach to alarms. By making alarm safety a priority, lives can be saved."
The Sentinel Event Alert also calls on hospitals to provide training and education on safe alarm management and response in high-risk areas to all members of the clinical care team.
In addition to the guidance to hospitals, the Joint Commission is considering the creation of a National Patient Safety Goal on the issue of alarm fatigue.
Twitter: @MaryEllenNY
A single hospitalized patient can generate up to several hundred alarm signals each day, causing physicians to quickly become desensitized to the noise. But ignoring these alarms can have fatal consequences for patients, the Joint Commission warns.
Between January 2009 and June 2012, the Joint Commission’s Sentinel Event Alert database recorded 98 alarm-related adverse events, 80 of which resulted in death. Another 13 resulted in permanent loss of function and 5 led to unexpected additional care or an extended stay in the hospital.
The Joint Commission found that inadequate alarms, improper settings, and signals that were not loud enough all contributed to the reported adverse events. Alarms that were improperly turned off also were a problem, according to the Joint Commission.
In the Sentinel Event Alert issued on April 8, the Joint Commission recommended several steps hospital leaders can take to curb the "alarm fatigue" common in hospitals.
– Set up a process for alarm management and response, especially in high-risk areas.
– Perform an inventory of all devices with alarms in high-risk areas and their default settings.
– Establish guidelines for alarm settings in high-risk areas and for high-risk conditions, including situations when alarms are not clinically necessary.
– Establish guidelines for tailoring alarm settings and limits for individual patients.
– Inspect and maintain alarm-equipped devices.
"Alarm fatigue and management of alarms are important safety issues that we must confront," Dr. Ana McKee, executive vice president and chief medical officer at the Joint Commission, said in a statement. "The recommendations in this alert offer hospitals a framework on which to assess their individual circumstances and develop a systematic, coordinated approach to alarms. By making alarm safety a priority, lives can be saved."
The Sentinel Event Alert also calls on hospitals to provide training and education on safe alarm management and response in high-risk areas to all members of the clinical care team.
In addition to the guidance to hospitals, the Joint Commission is considering the creation of a National Patient Safety Goal on the issue of alarm fatigue.
Twitter: @MaryEllenNY
A single hospitalized patient can generate up to several hundred alarm signals each day, causing physicians to quickly become desensitized to the noise. But ignoring these alarms can have fatal consequences for patients, the Joint Commission warns.
Between January 2009 and June 2012, the Joint Commission’s Sentinel Event Alert database recorded 98 alarm-related adverse events, 80 of which resulted in death. Another 13 resulted in permanent loss of function and 5 led to unexpected additional care or an extended stay in the hospital.
The Joint Commission found that inadequate alarms, improper settings, and signals that were not loud enough all contributed to the reported adverse events. Alarms that were improperly turned off also were a problem, according to the Joint Commission.
In the Sentinel Event Alert issued on April 8, the Joint Commission recommended several steps hospital leaders can take to curb the "alarm fatigue" common in hospitals.
– Set up a process for alarm management and response, especially in high-risk areas.
– Perform an inventory of all devices with alarms in high-risk areas and their default settings.
– Establish guidelines for alarm settings in high-risk areas and for high-risk conditions, including situations when alarms are not clinically necessary.
– Establish guidelines for tailoring alarm settings and limits for individual patients.
– Inspect and maintain alarm-equipped devices.
"Alarm fatigue and management of alarms are important safety issues that we must confront," Dr. Ana McKee, executive vice president and chief medical officer at the Joint Commission, said in a statement. "The recommendations in this alert offer hospitals a framework on which to assess their individual circumstances and develop a systematic, coordinated approach to alarms. By making alarm safety a priority, lives can be saved."
The Sentinel Event Alert also calls on hospitals to provide training and education on safe alarm management and response in high-risk areas to all members of the clinical care team.
In addition to the guidance to hospitals, the Joint Commission is considering the creation of a National Patient Safety Goal on the issue of alarm fatigue.
Twitter: @MaryEllenNY
Taking the guesswork out of patient handoffs
Dr. Jennifer O’Toole is a pediatric and adult hospitalist who splits her time between two different Cincinnati hospitals where she teaches residents, conducts educational research, and sees patients. She understands that hospitalists and residents are facing work-hour compression while caring for more complicated patients. But no matter how busy they are, physicians need to take the time to perform a concise, standardized handoff of their patients, she said.
Dr. O’Toole is the site principal investigator for Cincinnati Children’s Hospital in the I-PASS Study Group. I-PASS is an acronym of acronyms: the IIPE (Initiative for Innovation in Pediatric Education) and PRIS (Pediatric Research in Inpatient Settings) Network Accelerating Safe Sign-outs Study. The project, which is currently underway at 10 sites across North America, tests the use of a standardized, evidence-based handoff bundle among residents. The bundle includes team training, a verbal mnemonic, and a structured printed tool. Each of the sites is implementing the bundle and measuring how it impacts medical errors, verbal and written miscommunications, and satisfaction and work flow among residents.
Dr. O’Toole is also the lead investigator for an I-PASS ancillary study, which is developing and evaluating a handoff bundle for medical students. In an interview with Hospitalist News, she shared her thoughts on how hospitalists can improve their handoff techniques.
Question: The data collection for the I-PASS study ends in May. What have you learned so far at your site?
Dr. O’Toole: We have learned that handoffs need to be a standardized process that is practiced consistently. Practicing a standardized handoff is not something that you can turn on or off. One must use the same consistent structure for every patient handoff.
We have also learned that effective handoff education requires more than just a one-time workshop training session. In order to develop and refine one’s skill in handoffs, there need to be intermittent refreshers and reinforcement via observation and feedback from peers and faculty. This feedback needs to be targeted at the various components of an effective handoff. For instance, did they speak too quickly, did they include superfluous information, or did they include an assessment of a patient’s illness severity?
Last, we learned that robust faculty development is critical to successfully implementing a handoff training program during medical school or residency. Many of our faculty were never formally trained in handoff communication and therefore were not well equipped to teach and evaluate these skills. We had to implement an intensive faculty development program for the I-PASS study, and in response, we noticed a change in how our faculty executed their own handoff communication.
Question: Can you have successful handoffs without a standardized process?
Dr. O’Toole: No. I believe that standardization during handoffs is critical.
We developed a standardized mnemonic for the I-PASS study and tailored our handoff documents to reflect the structure of the mnemonic. There are a lot of handoff mnemonics out there, and I don’t think we have the evidence to say one is better than another. Regardless of what handoff process or mnemonic one uses, I think it all comes down to being very structured and methodical about how you perform a handoff. For example, one needs to consistently provide a patient summary in a familiar format and consistently articulate contingency planning for every patient. Reliable use of a standardized structure is imperative to ensure that critical elements are not omitted during handoff communication.
When residents or hospitalists are tired, stressed, or busy, they run the risk of leaving out important elements during a handoff. A structured mnemonic and printed handoff document provide a stable framework to prevent lapses in effective handoff communication.
Question: Does the handoff protocol need to be site specific?
Dr. O’Toole: While we implemented a consistent handoff bundle during the I-PASS project at all of our sites, we found that there were minor ways each site tweaked the elements of the handoff bundle to fit the needs of its program and its individual institutional culture. These site-specific adaptations were critical for the successful implementation of the program at each site. For example, at our site we were able to easily incorporate our I-PASS printed handoff document into our electronic health record. However, this wasn’t the case for all of the study sites. Each site had to develop a printed handoff document according to the resources available at its institution. I have encountered similar experiences during the dissemination of the I-PASS handoff bundle to my internal medicine residency program at the University of Cincinnati Medical Center.
Question: Everyone is talking about handoffs right now. What are the biggest mistakes that hospitalists make in this area?
Dr. O’Toole: I think the biggest mistakes hospitalists make during handoffs are not using a standard structure and not embracing communication best practices of high-performing teams.
Within a hospitalist group each individual may handoff their patients, in both written and verbal fashion, slightly differently. Having consistency within a group is critical. Embracing good communication techniques, such as the TeamSTEPPS techniques we used during I-PASS, is also essential.
I’m also a strong believer that you need to have a verbal interaction and a written component to the handoff. It’s not enough to send a colleague a well-composed written handoff document via a secure e-mail. You need to have a verbal communication so that you can emphasize important patient information with verbal cues and so that the receiver can have an opportunity to ask questions. This verbal communication does not have to be lengthy. I am well aware that time is a scarce commodity for all practicing hospitalists. However, a concise, well-composed verbal interaction is an essential element to the safe handoff of patients.
Question: Fast forward 5 years. Do you think that most hospitalist programs will be using some type of standardized handoff tool?
Dr. O’Toole: Yes, I hope this will be the case. However, for this to occur we need to have solid research and outcomes surrounding standardized handoff programs and their impact on medical errors. It’s an area that has been lacking. As a result, a lot of institutions and hospitalist groups have been apprehensive about saying, ‘This is the best way to execute a handoff,’ since they don’t have solid data to show that a handoff process or program improves patient care and safety outcomes. That’s one of the benefits of the work we’ve been doing with the I-PASS project. We are evaluating the effects of a standardized handoff bundle on medical errors, verbal and written miscommunications, and satisfaction and work flow among residents.
A study with the scale and scope of the I-PASS study has never been attempted before and will hopefully provide the evidence hospitalist programs, residency programs, and institutions need to get full support to implement a comprehensive, evidence-based handoff program.
Take us to your leader. Nominate a hospitalist whose work inspires you. E-mail suggestions to [email protected].
Dr. Jennifer O’Toole is a pediatric and adult hospitalist who splits her time between two different Cincinnati hospitals where she teaches residents, conducts educational research, and sees patients. She understands that hospitalists and residents are facing work-hour compression while caring for more complicated patients. But no matter how busy they are, physicians need to take the time to perform a concise, standardized handoff of their patients, she said.
Dr. O’Toole is the site principal investigator for Cincinnati Children’s Hospital in the I-PASS Study Group. I-PASS is an acronym of acronyms: the IIPE (Initiative for Innovation in Pediatric Education) and PRIS (Pediatric Research in Inpatient Settings) Network Accelerating Safe Sign-outs Study. The project, which is currently underway at 10 sites across North America, tests the use of a standardized, evidence-based handoff bundle among residents. The bundle includes team training, a verbal mnemonic, and a structured printed tool. Each of the sites is implementing the bundle and measuring how it impacts medical errors, verbal and written miscommunications, and satisfaction and work flow among residents.
Dr. O’Toole is also the lead investigator for an I-PASS ancillary study, which is developing and evaluating a handoff bundle for medical students. In an interview with Hospitalist News, she shared her thoughts on how hospitalists can improve their handoff techniques.
Question: The data collection for the I-PASS study ends in May. What have you learned so far at your site?
Dr. O’Toole: We have learned that handoffs need to be a standardized process that is practiced consistently. Practicing a standardized handoff is not something that you can turn on or off. One must use the same consistent structure for every patient handoff.
We have also learned that effective handoff education requires more than just a one-time workshop training session. In order to develop and refine one’s skill in handoffs, there need to be intermittent refreshers and reinforcement via observation and feedback from peers and faculty. This feedback needs to be targeted at the various components of an effective handoff. For instance, did they speak too quickly, did they include superfluous information, or did they include an assessment of a patient’s illness severity?
Last, we learned that robust faculty development is critical to successfully implementing a handoff training program during medical school or residency. Many of our faculty were never formally trained in handoff communication and therefore were not well equipped to teach and evaluate these skills. We had to implement an intensive faculty development program for the I-PASS study, and in response, we noticed a change in how our faculty executed their own handoff communication.
Question: Can you have successful handoffs without a standardized process?
Dr. O’Toole: No. I believe that standardization during handoffs is critical.
We developed a standardized mnemonic for the I-PASS study and tailored our handoff documents to reflect the structure of the mnemonic. There are a lot of handoff mnemonics out there, and I don’t think we have the evidence to say one is better than another. Regardless of what handoff process or mnemonic one uses, I think it all comes down to being very structured and methodical about how you perform a handoff. For example, one needs to consistently provide a patient summary in a familiar format and consistently articulate contingency planning for every patient. Reliable use of a standardized structure is imperative to ensure that critical elements are not omitted during handoff communication.
When residents or hospitalists are tired, stressed, or busy, they run the risk of leaving out important elements during a handoff. A structured mnemonic and printed handoff document provide a stable framework to prevent lapses in effective handoff communication.
Question: Does the handoff protocol need to be site specific?
Dr. O’Toole: While we implemented a consistent handoff bundle during the I-PASS project at all of our sites, we found that there were minor ways each site tweaked the elements of the handoff bundle to fit the needs of its program and its individual institutional culture. These site-specific adaptations were critical for the successful implementation of the program at each site. For example, at our site we were able to easily incorporate our I-PASS printed handoff document into our electronic health record. However, this wasn’t the case for all of the study sites. Each site had to develop a printed handoff document according to the resources available at its institution. I have encountered similar experiences during the dissemination of the I-PASS handoff bundle to my internal medicine residency program at the University of Cincinnati Medical Center.
Question: Everyone is talking about handoffs right now. What are the biggest mistakes that hospitalists make in this area?
Dr. O’Toole: I think the biggest mistakes hospitalists make during handoffs are not using a standard structure and not embracing communication best practices of high-performing teams.
Within a hospitalist group each individual may handoff their patients, in both written and verbal fashion, slightly differently. Having consistency within a group is critical. Embracing good communication techniques, such as the TeamSTEPPS techniques we used during I-PASS, is also essential.
I’m also a strong believer that you need to have a verbal interaction and a written component to the handoff. It’s not enough to send a colleague a well-composed written handoff document via a secure e-mail. You need to have a verbal communication so that you can emphasize important patient information with verbal cues and so that the receiver can have an opportunity to ask questions. This verbal communication does not have to be lengthy. I am well aware that time is a scarce commodity for all practicing hospitalists. However, a concise, well-composed verbal interaction is an essential element to the safe handoff of patients.
Question: Fast forward 5 years. Do you think that most hospitalist programs will be using some type of standardized handoff tool?
Dr. O’Toole: Yes, I hope this will be the case. However, for this to occur we need to have solid research and outcomes surrounding standardized handoff programs and their impact on medical errors. It’s an area that has been lacking. As a result, a lot of institutions and hospitalist groups have been apprehensive about saying, ‘This is the best way to execute a handoff,’ since they don’t have solid data to show that a handoff process or program improves patient care and safety outcomes. That’s one of the benefits of the work we’ve been doing with the I-PASS project. We are evaluating the effects of a standardized handoff bundle on medical errors, verbal and written miscommunications, and satisfaction and work flow among residents.
A study with the scale and scope of the I-PASS study has never been attempted before and will hopefully provide the evidence hospitalist programs, residency programs, and institutions need to get full support to implement a comprehensive, evidence-based handoff program.
Take us to your leader. Nominate a hospitalist whose work inspires you. E-mail suggestions to [email protected].
Dr. Jennifer O’Toole is a pediatric and adult hospitalist who splits her time between two different Cincinnati hospitals where she teaches residents, conducts educational research, and sees patients. She understands that hospitalists and residents are facing work-hour compression while caring for more complicated patients. But no matter how busy they are, physicians need to take the time to perform a concise, standardized handoff of their patients, she said.
Dr. O’Toole is the site principal investigator for Cincinnati Children’s Hospital in the I-PASS Study Group. I-PASS is an acronym of acronyms: the IIPE (Initiative for Innovation in Pediatric Education) and PRIS (Pediatric Research in Inpatient Settings) Network Accelerating Safe Sign-outs Study. The project, which is currently underway at 10 sites across North America, tests the use of a standardized, evidence-based handoff bundle among residents. The bundle includes team training, a verbal mnemonic, and a structured printed tool. Each of the sites is implementing the bundle and measuring how it impacts medical errors, verbal and written miscommunications, and satisfaction and work flow among residents.
Dr. O’Toole is also the lead investigator for an I-PASS ancillary study, which is developing and evaluating a handoff bundle for medical students. In an interview with Hospitalist News, she shared her thoughts on how hospitalists can improve their handoff techniques.
Question: The data collection for the I-PASS study ends in May. What have you learned so far at your site?
Dr. O’Toole: We have learned that handoffs need to be a standardized process that is practiced consistently. Practicing a standardized handoff is not something that you can turn on or off. One must use the same consistent structure for every patient handoff.
We have also learned that effective handoff education requires more than just a one-time workshop training session. In order to develop and refine one’s skill in handoffs, there need to be intermittent refreshers and reinforcement via observation and feedback from peers and faculty. This feedback needs to be targeted at the various components of an effective handoff. For instance, did they speak too quickly, did they include superfluous information, or did they include an assessment of a patient’s illness severity?
Last, we learned that robust faculty development is critical to successfully implementing a handoff training program during medical school or residency. Many of our faculty were never formally trained in handoff communication and therefore were not well equipped to teach and evaluate these skills. We had to implement an intensive faculty development program for the I-PASS study, and in response, we noticed a change in how our faculty executed their own handoff communication.
Question: Can you have successful handoffs without a standardized process?
Dr. O’Toole: No. I believe that standardization during handoffs is critical.
We developed a standardized mnemonic for the I-PASS study and tailored our handoff documents to reflect the structure of the mnemonic. There are a lot of handoff mnemonics out there, and I don’t think we have the evidence to say one is better than another. Regardless of what handoff process or mnemonic one uses, I think it all comes down to being very structured and methodical about how you perform a handoff. For example, one needs to consistently provide a patient summary in a familiar format and consistently articulate contingency planning for every patient. Reliable use of a standardized structure is imperative to ensure that critical elements are not omitted during handoff communication.
When residents or hospitalists are tired, stressed, or busy, they run the risk of leaving out important elements during a handoff. A structured mnemonic and printed handoff document provide a stable framework to prevent lapses in effective handoff communication.
Question: Does the handoff protocol need to be site specific?
Dr. O’Toole: While we implemented a consistent handoff bundle during the I-PASS project at all of our sites, we found that there were minor ways each site tweaked the elements of the handoff bundle to fit the needs of its program and its individual institutional culture. These site-specific adaptations were critical for the successful implementation of the program at each site. For example, at our site we were able to easily incorporate our I-PASS printed handoff document into our electronic health record. However, this wasn’t the case for all of the study sites. Each site had to develop a printed handoff document according to the resources available at its institution. I have encountered similar experiences during the dissemination of the I-PASS handoff bundle to my internal medicine residency program at the University of Cincinnati Medical Center.
Question: Everyone is talking about handoffs right now. What are the biggest mistakes that hospitalists make in this area?
Dr. O’Toole: I think the biggest mistakes hospitalists make during handoffs are not using a standard structure and not embracing communication best practices of high-performing teams.
Within a hospitalist group each individual may handoff their patients, in both written and verbal fashion, slightly differently. Having consistency within a group is critical. Embracing good communication techniques, such as the TeamSTEPPS techniques we used during I-PASS, is also essential.
I’m also a strong believer that you need to have a verbal interaction and a written component to the handoff. It’s not enough to send a colleague a well-composed written handoff document via a secure e-mail. You need to have a verbal communication so that you can emphasize important patient information with verbal cues and so that the receiver can have an opportunity to ask questions. This verbal communication does not have to be lengthy. I am well aware that time is a scarce commodity for all practicing hospitalists. However, a concise, well-composed verbal interaction is an essential element to the safe handoff of patients.
Question: Fast forward 5 years. Do you think that most hospitalist programs will be using some type of standardized handoff tool?
Dr. O’Toole: Yes, I hope this will be the case. However, for this to occur we need to have solid research and outcomes surrounding standardized handoff programs and their impact on medical errors. It’s an area that has been lacking. As a result, a lot of institutions and hospitalist groups have been apprehensive about saying, ‘This is the best way to execute a handoff,’ since they don’t have solid data to show that a handoff process or program improves patient care and safety outcomes. That’s one of the benefits of the work we’ve been doing with the I-PASS project. We are evaluating the effects of a standardized handoff bundle on medical errors, verbal and written miscommunications, and satisfaction and work flow among residents.
A study with the scale and scope of the I-PASS study has never been attempted before and will hopefully provide the evidence hospitalist programs, residency programs, and institutions need to get full support to implement a comprehensive, evidence-based handoff program.
Take us to your leader. Nominate a hospitalist whose work inspires you. E-mail suggestions to [email protected].
Bill would waive Medicare copays for polyp removal
Legislation in Congress aims to eliminate cost sharing for Medicare beneficiaries who have polyps removed during a colonoscopy.
Under the Affordable Care Act, preventive care services – including screening colonoscopy – are covered with no copayment or coinsurance to the patients. Under Medicare, however, colonoscopies are reclassified as therapeutic if polyps are removed during the procedure. In that case, patients must pay coinsurance of 20% of the Medicare-approved charge. Changing the Medicare policy requires action by Congress.
Rep. Charlie Dent (R-Pa.) introduced H.R. 1070, the Removing Barriers to Colorectal Cancer Screening Act, which would waive the cost sharing for Medicare beneficiaries if polyps were removed during a screening colonoscopy. This legislation is strongly supported by the American Gastroenterological Association (AGA), the American Society of Gastrointestinal Endoscopy, the American Cancer Society Cancer Action Network, Fight Colorectal Cancer, Prevent Cancer Foundation, and other patient advocacy groups. At press time, it was cosponsored by 17 Democrats and 3 Republicans.
"For years, the AGA has been working to increase access to colorectal cancer screenings for patients, including removing financial barriers that many patients face when seeking lifesaving colorectal cancer screenings," said Dr. Carla Ginsburg, AGAF, chair of the Public Affairs and Advocacy Committee of the AGA. "The removal of polyps during a screening is an integral part of the screening and we believe that patients should not be responsible for the cost sharing. H.R. 1070 ensures that a screening colonoscopy is fully covered, regardless of the outcome, and that patients will not be responsible for the coinsurance."
Also in March, Sen. Ben Cardin (D-Md.) introduced S.608, the Supporting Colorectal Examination and Education Now (SCREEN) Act. The bill would waive cost sharing for Medicare beneficiaries if polyps were removed during a colonoscopy. The proposed legislation also calls for notifying all Medicare beneficiaries about the screening benefit and would cover a prescreening visit under Medicare.
Rep. Richard Neal (D-Mass.) introduced companion legislation in the House (H.R. 1320).
The SCREEN Act also would give physicians a chance to earn higher payments for providing colonoscopies. The legislation would create a preventive services payment modifier for colorectal cancer screening. Under the bill, physicians could earn incentive payments by meeting national colorectal cancer screening goals and minimum standards for knowledge, training, continuing education, and documentation.
At press time, all three bills had been referred to committee; no other action had been taken.
On Twitter @MaryEllenNY
Legislation in Congress aims to eliminate cost sharing for Medicare beneficiaries who have polyps removed during a colonoscopy.
Under the Affordable Care Act, preventive care services – including screening colonoscopy – are covered with no copayment or coinsurance to the patients. Under Medicare, however, colonoscopies are reclassified as therapeutic if polyps are removed during the procedure. In that case, patients must pay coinsurance of 20% of the Medicare-approved charge. Changing the Medicare policy requires action by Congress.
Rep. Charlie Dent (R-Pa.) introduced H.R. 1070, the Removing Barriers to Colorectal Cancer Screening Act, which would waive the cost sharing for Medicare beneficiaries if polyps were removed during a screening colonoscopy. This legislation is strongly supported by the American Gastroenterological Association (AGA), the American Society of Gastrointestinal Endoscopy, the American Cancer Society Cancer Action Network, Fight Colorectal Cancer, Prevent Cancer Foundation, and other patient advocacy groups. At press time, it was cosponsored by 17 Democrats and 3 Republicans.
"For years, the AGA has been working to increase access to colorectal cancer screenings for patients, including removing financial barriers that many patients face when seeking lifesaving colorectal cancer screenings," said Dr. Carla Ginsburg, AGAF, chair of the Public Affairs and Advocacy Committee of the AGA. "The removal of polyps during a screening is an integral part of the screening and we believe that patients should not be responsible for the cost sharing. H.R. 1070 ensures that a screening colonoscopy is fully covered, regardless of the outcome, and that patients will not be responsible for the coinsurance."
Also in March, Sen. Ben Cardin (D-Md.) introduced S.608, the Supporting Colorectal Examination and Education Now (SCREEN) Act. The bill would waive cost sharing for Medicare beneficiaries if polyps were removed during a colonoscopy. The proposed legislation also calls for notifying all Medicare beneficiaries about the screening benefit and would cover a prescreening visit under Medicare.
Rep. Richard Neal (D-Mass.) introduced companion legislation in the House (H.R. 1320).
The SCREEN Act also would give physicians a chance to earn higher payments for providing colonoscopies. The legislation would create a preventive services payment modifier for colorectal cancer screening. Under the bill, physicians could earn incentive payments by meeting national colorectal cancer screening goals and minimum standards for knowledge, training, continuing education, and documentation.
At press time, all three bills had been referred to committee; no other action had been taken.
On Twitter @MaryEllenNY
Legislation in Congress aims to eliminate cost sharing for Medicare beneficiaries who have polyps removed during a colonoscopy.
Under the Affordable Care Act, preventive care services – including screening colonoscopy – are covered with no copayment or coinsurance to the patients. Under Medicare, however, colonoscopies are reclassified as therapeutic if polyps are removed during the procedure. In that case, patients must pay coinsurance of 20% of the Medicare-approved charge. Changing the Medicare policy requires action by Congress.
Rep. Charlie Dent (R-Pa.) introduced H.R. 1070, the Removing Barriers to Colorectal Cancer Screening Act, which would waive the cost sharing for Medicare beneficiaries if polyps were removed during a screening colonoscopy. This legislation is strongly supported by the American Gastroenterological Association (AGA), the American Society of Gastrointestinal Endoscopy, the American Cancer Society Cancer Action Network, Fight Colorectal Cancer, Prevent Cancer Foundation, and other patient advocacy groups. At press time, it was cosponsored by 17 Democrats and 3 Republicans.
"For years, the AGA has been working to increase access to colorectal cancer screenings for patients, including removing financial barriers that many patients face when seeking lifesaving colorectal cancer screenings," said Dr. Carla Ginsburg, AGAF, chair of the Public Affairs and Advocacy Committee of the AGA. "The removal of polyps during a screening is an integral part of the screening and we believe that patients should not be responsible for the cost sharing. H.R. 1070 ensures that a screening colonoscopy is fully covered, regardless of the outcome, and that patients will not be responsible for the coinsurance."
Also in March, Sen. Ben Cardin (D-Md.) introduced S.608, the Supporting Colorectal Examination and Education Now (SCREEN) Act. The bill would waive cost sharing for Medicare beneficiaries if polyps were removed during a colonoscopy. The proposed legislation also calls for notifying all Medicare beneficiaries about the screening benefit and would cover a prescreening visit under Medicare.
Rep. Richard Neal (D-Mass.) introduced companion legislation in the House (H.R. 1320).
The SCREEN Act also would give physicians a chance to earn higher payments for providing colonoscopies. The legislation would create a preventive services payment modifier for colorectal cancer screening. Under the bill, physicians could earn incentive payments by meeting national colorectal cancer screening goals and minimum standards for knowledge, training, continuing education, and documentation.
At press time, all three bills had been referred to committee; no other action had been taken.
On Twitter @MaryEllenNY
Obama's budget proposal gives more power to IPAB
President Obama is proposing to cut more than $370 billion from the Medicare program over the next decade, a move aimed at reducing the federal deficit and putting the program on firmer financial footing.
The president’s fiscal year 2014 budget proposal, sent to Congress April 10, includes cuts for physicians, drug companies, hospitals, and long-term facilities, as well as increased cost-sharing for some Medicare beneficiaries.
The part of the budget with the potential to have the biggest impact on doctors is the increased authority for the Independent Payment Advisory Board (IPAB). The 15-member board was created under the Affordable Care Act (ACA) and is charged with recommending to Congress how to reduce spending growth in Medicare. Under current law, IPAB would make recommendations only if the projected Medicare per capita growth rate exceeded the gross domestic product (GDP) plus 1%. In the president’s budget proposal, that target would be triggered early, when Medicare spending was projected to exceed GDP plus 0.5%.
| Source: U.S. Dept. of Health and Human Services |
This change is projected to save the federal government $4.1 billion over the next decade.
The bulk of the Medicare savings will come from proposals to cut payments to drug companies, long-term care facilities, and increases in cost sharing by beneficiaries.
For instance, the administration estimates it will save about $123 billion over 10 years by allowing the Medicare Part D program to pay the lower Medicaid rate for prescription drugs for its low-income beneficiaries.
A change involving post–acute care providers would save $79 billion. The administration wants to reduce the market basket updates for inpatient rehabilitation facilities, long-term care hospitals, skilled nursing facilities, and home health agencies by 1.1% starting in 2014 and running through 2023.
Beneficiaries also would contribute more for their Medicare premiums under the Obama budget proposal. Starting in 2017, certain Medicare beneficiaries will pay more for their Part B and D premiums, which is expected to generate $50 billion in savings over 10 years.
The budget also includes about $22.1 billion in cuts to Medicaid over 10 years.
The budget also includes a little good news for physicians: It assumes that the Congress will eliminate the Sustainable Growth Rate (SGR) formula used in setting Medicare physician payments.
The American Medical Association praised the administration for its commitment to move toward new ways to pay for health care. "The President’s proposals align with many of the principles developed by the AMA and 110 other physician organizations on transitioning Medicare to include an array of accountable payment models," Dr. Jeremy A. Lazarus, AMA president, said in a statement. "It is critical for physicians to have a period of stability and the flexibility to choose options that will help them lower costs and improve the quality of care for their patients. We are encouraged that the president and members of Congress are focused this year on eliminating this failed formula and strengthening Medicare for patients now and in the future."
The cost-cutting proposals aren’t new; President Obama included some of them in last year’s budget proposal. He also called for cuts to Medicare when negotiating for a deficit reduction deal with House Speaker John Boehner (R-Ohio) late last year.
President Obama said his proposed budget would lower the federal deficit in a "balanced way," allowing more targeted cuts to replace the across-the-board budget cuts set out in the sequester, which took effect in March. Overall, the Obama administration estimates that the new budget would achieve $1.8 trillion in deficit reduction over the next 10 years.
But the budget is already getting a cool reception from Republicans on Capitol Hill. House Budget Committee Chairman Paul Ryan (R-Wisc.) said that after eliminating the sequester, there is only about $119 billion in deficit reduction over the next decade in the president’s proposed budget. "I’m disappointed by the president’s proposal because it merely ratifies the status quo," Rep. Ryan said in a statement. "It doesn’t break new ground; it goes over old ground."
Both the House and the Senate have already passed their own budget proposals for fiscal year 2014.
In addition to the Medicare and Medicaid cuts, the President’s budget offers some targeted increases.
For instance, the Centers for Medicare and Medicaid Services is seeking about $1.5 billion in new funding to help support operations and outreach related to the ACA’s health insurance exchanges. The funds would support the federally operated exchanges and offer assistance to states that are running their own exchanges. The exchanges will open for enrollment on Oct. 1, 2013, the first day of fiscal year 2014. Coverage under the exchanges is set to begin on Jan. 1, 2014.
Health and Human Services Secretary Kathleen Sebelius said she is hopeful that Congress will come through with the money to help launch the new program. "We intend to implement the law," she said at a press conference April 10.
The budget also includes funding for mental health. The proposal invests $130 million to add 5,000 mental health professionals to the behavioral health workforce. The money will also fund Project AWARE (Advancing Wellness and Resilience in Education), which trains teachers to detect and respond to mental illness in their students.
The Centers for Disease Control and Prevention would get an additional $30 million to track gun violence and research ways to prevent it under the budget proposal sent to Congress.
The American Psychiatric Association supported the administration’s effort to identify at-risk individuals early through Project AWARE. But the APA said in a statement that it was concerned that the effort to expand the supply of mental health professionals seems to stop at nonphysician providers.
"We recognize the growing need for mental health providers; however, providing a small amount of training to lesser-qualified health professionals at the expense of utilizing veteran medical psychiatrists will only serve to exacerbate the problem we are trying to solve," the APA wrote. "As a nation, we should ensure that patients have access to the full range of services, from physician care to hospital care to outpatient clinics and long-term follow-up."
The National Institutes of Health would receive a $471 million funding increase over its fiscal year 2012 funding, bringing its total budget to $31.1 billion. That includes about $40 billion toward an effort to map the human brain. The agency will also invest $80 million to speed up drug development and the testing of new therapies for Alzheimer’s disease.
President Obama is proposing to cut more than $370 billion from the Medicare program over the next decade, a move aimed at reducing the federal deficit and putting the program on firmer financial footing.
The president’s fiscal year 2014 budget proposal, sent to Congress April 10, includes cuts for physicians, drug companies, hospitals, and long-term facilities, as well as increased cost-sharing for some Medicare beneficiaries.
The part of the budget with the potential to have the biggest impact on doctors is the increased authority for the Independent Payment Advisory Board (IPAB). The 15-member board was created under the Affordable Care Act (ACA) and is charged with recommending to Congress how to reduce spending growth in Medicare. Under current law, IPAB would make recommendations only if the projected Medicare per capita growth rate exceeded the gross domestic product (GDP) plus 1%. In the president’s budget proposal, that target would be triggered early, when Medicare spending was projected to exceed GDP plus 0.5%.
| Source: U.S. Dept. of Health and Human Services |
This change is projected to save the federal government $4.1 billion over the next decade.
The bulk of the Medicare savings will come from proposals to cut payments to drug companies, long-term care facilities, and increases in cost sharing by beneficiaries.
For instance, the administration estimates it will save about $123 billion over 10 years by allowing the Medicare Part D program to pay the lower Medicaid rate for prescription drugs for its low-income beneficiaries.
A change involving post–acute care providers would save $79 billion. The administration wants to reduce the market basket updates for inpatient rehabilitation facilities, long-term care hospitals, skilled nursing facilities, and home health agencies by 1.1% starting in 2014 and running through 2023.
Beneficiaries also would contribute more for their Medicare premiums under the Obama budget proposal. Starting in 2017, certain Medicare beneficiaries will pay more for their Part B and D premiums, which is expected to generate $50 billion in savings over 10 years.
The budget also includes about $22.1 billion in cuts to Medicaid over 10 years.
The budget also includes a little good news for physicians: It assumes that the Congress will eliminate the Sustainable Growth Rate (SGR) formula used in setting Medicare physician payments.
The American Medical Association praised the administration for its commitment to move toward new ways to pay for health care. "The President’s proposals align with many of the principles developed by the AMA and 110 other physician organizations on transitioning Medicare to include an array of accountable payment models," Dr. Jeremy A. Lazarus, AMA president, said in a statement. "It is critical for physicians to have a period of stability and the flexibility to choose options that will help them lower costs and improve the quality of care for their patients. We are encouraged that the president and members of Congress are focused this year on eliminating this failed formula and strengthening Medicare for patients now and in the future."
The cost-cutting proposals aren’t new; President Obama included some of them in last year’s budget proposal. He also called for cuts to Medicare when negotiating for a deficit reduction deal with House Speaker John Boehner (R-Ohio) late last year.
President Obama said his proposed budget would lower the federal deficit in a "balanced way," allowing more targeted cuts to replace the across-the-board budget cuts set out in the sequester, which took effect in March. Overall, the Obama administration estimates that the new budget would achieve $1.8 trillion in deficit reduction over the next 10 years.
But the budget is already getting a cool reception from Republicans on Capitol Hill. House Budget Committee Chairman Paul Ryan (R-Wisc.) said that after eliminating the sequester, there is only about $119 billion in deficit reduction over the next decade in the president’s proposed budget. "I’m disappointed by the president’s proposal because it merely ratifies the status quo," Rep. Ryan said in a statement. "It doesn’t break new ground; it goes over old ground."
Both the House and the Senate have already passed their own budget proposals for fiscal year 2014.
In addition to the Medicare and Medicaid cuts, the President’s budget offers some targeted increases.
For instance, the Centers for Medicare and Medicaid Services is seeking about $1.5 billion in new funding to help support operations and outreach related to the ACA’s health insurance exchanges. The funds would support the federally operated exchanges and offer assistance to states that are running their own exchanges. The exchanges will open for enrollment on Oct. 1, 2013, the first day of fiscal year 2014. Coverage under the exchanges is set to begin on Jan. 1, 2014.
Health and Human Services Secretary Kathleen Sebelius said she is hopeful that Congress will come through with the money to help launch the new program. "We intend to implement the law," she said at a press conference April 10.
The budget also includes funding for mental health. The proposal invests $130 million to add 5,000 mental health professionals to the behavioral health workforce. The money will also fund Project AWARE (Advancing Wellness and Resilience in Education), which trains teachers to detect and respond to mental illness in their students.
The Centers for Disease Control and Prevention would get an additional $30 million to track gun violence and research ways to prevent it under the budget proposal sent to Congress.
The American Psychiatric Association supported the administration’s effort to identify at-risk individuals early through Project AWARE. But the APA said in a statement that it was concerned that the effort to expand the supply of mental health professionals seems to stop at nonphysician providers.
"We recognize the growing need for mental health providers; however, providing a small amount of training to lesser-qualified health professionals at the expense of utilizing veteran medical psychiatrists will only serve to exacerbate the problem we are trying to solve," the APA wrote. "As a nation, we should ensure that patients have access to the full range of services, from physician care to hospital care to outpatient clinics and long-term follow-up."
The National Institutes of Health would receive a $471 million funding increase over its fiscal year 2012 funding, bringing its total budget to $31.1 billion. That includes about $40 billion toward an effort to map the human brain. The agency will also invest $80 million to speed up drug development and the testing of new therapies for Alzheimer’s disease.
President Obama is proposing to cut more than $370 billion from the Medicare program over the next decade, a move aimed at reducing the federal deficit and putting the program on firmer financial footing.
The president’s fiscal year 2014 budget proposal, sent to Congress April 10, includes cuts for physicians, drug companies, hospitals, and long-term facilities, as well as increased cost-sharing for some Medicare beneficiaries.
The part of the budget with the potential to have the biggest impact on doctors is the increased authority for the Independent Payment Advisory Board (IPAB). The 15-member board was created under the Affordable Care Act (ACA) and is charged with recommending to Congress how to reduce spending growth in Medicare. Under current law, IPAB would make recommendations only if the projected Medicare per capita growth rate exceeded the gross domestic product (GDP) plus 1%. In the president’s budget proposal, that target would be triggered early, when Medicare spending was projected to exceed GDP plus 0.5%.
| Source: U.S. Dept. of Health and Human Services |
This change is projected to save the federal government $4.1 billion over the next decade.
The bulk of the Medicare savings will come from proposals to cut payments to drug companies, long-term care facilities, and increases in cost sharing by beneficiaries.
For instance, the administration estimates it will save about $123 billion over 10 years by allowing the Medicare Part D program to pay the lower Medicaid rate for prescription drugs for its low-income beneficiaries.
A change involving post–acute care providers would save $79 billion. The administration wants to reduce the market basket updates for inpatient rehabilitation facilities, long-term care hospitals, skilled nursing facilities, and home health agencies by 1.1% starting in 2014 and running through 2023.
Beneficiaries also would contribute more for their Medicare premiums under the Obama budget proposal. Starting in 2017, certain Medicare beneficiaries will pay more for their Part B and D premiums, which is expected to generate $50 billion in savings over 10 years.
The budget also includes about $22.1 billion in cuts to Medicaid over 10 years.
The budget also includes a little good news for physicians: It assumes that the Congress will eliminate the Sustainable Growth Rate (SGR) formula used in setting Medicare physician payments.
The American Medical Association praised the administration for its commitment to move toward new ways to pay for health care. "The President’s proposals align with many of the principles developed by the AMA and 110 other physician organizations on transitioning Medicare to include an array of accountable payment models," Dr. Jeremy A. Lazarus, AMA president, said in a statement. "It is critical for physicians to have a period of stability and the flexibility to choose options that will help them lower costs and improve the quality of care for their patients. We are encouraged that the president and members of Congress are focused this year on eliminating this failed formula and strengthening Medicare for patients now and in the future."
The cost-cutting proposals aren’t new; President Obama included some of them in last year’s budget proposal. He also called for cuts to Medicare when negotiating for a deficit reduction deal with House Speaker John Boehner (R-Ohio) late last year.
President Obama said his proposed budget would lower the federal deficit in a "balanced way," allowing more targeted cuts to replace the across-the-board budget cuts set out in the sequester, which took effect in March. Overall, the Obama administration estimates that the new budget would achieve $1.8 trillion in deficit reduction over the next 10 years.
But the budget is already getting a cool reception from Republicans on Capitol Hill. House Budget Committee Chairman Paul Ryan (R-Wisc.) said that after eliminating the sequester, there is only about $119 billion in deficit reduction over the next decade in the president’s proposed budget. "I’m disappointed by the president’s proposal because it merely ratifies the status quo," Rep. Ryan said in a statement. "It doesn’t break new ground; it goes over old ground."
Both the House and the Senate have already passed their own budget proposals for fiscal year 2014.
In addition to the Medicare and Medicaid cuts, the President’s budget offers some targeted increases.
For instance, the Centers for Medicare and Medicaid Services is seeking about $1.5 billion in new funding to help support operations and outreach related to the ACA’s health insurance exchanges. The funds would support the federally operated exchanges and offer assistance to states that are running their own exchanges. The exchanges will open for enrollment on Oct. 1, 2013, the first day of fiscal year 2014. Coverage under the exchanges is set to begin on Jan. 1, 2014.
Health and Human Services Secretary Kathleen Sebelius said she is hopeful that Congress will come through with the money to help launch the new program. "We intend to implement the law," she said at a press conference April 10.
The budget also includes funding for mental health. The proposal invests $130 million to add 5,000 mental health professionals to the behavioral health workforce. The money will also fund Project AWARE (Advancing Wellness and Resilience in Education), which trains teachers to detect and respond to mental illness in their students.
The Centers for Disease Control and Prevention would get an additional $30 million to track gun violence and research ways to prevent it under the budget proposal sent to Congress.
The American Psychiatric Association supported the administration’s effort to identify at-risk individuals early through Project AWARE. But the APA said in a statement that it was concerned that the effort to expand the supply of mental health professionals seems to stop at nonphysician providers.
"We recognize the growing need for mental health providers; however, providing a small amount of training to lesser-qualified health professionals at the expense of utilizing veteran medical psychiatrists will only serve to exacerbate the problem we are trying to solve," the APA wrote. "As a nation, we should ensure that patients have access to the full range of services, from physician care to hospital care to outpatient clinics and long-term follow-up."
The National Institutes of Health would receive a $471 million funding increase over its fiscal year 2012 funding, bringing its total budget to $31.1 billion. That includes about $40 billion toward an effort to map the human brain. The agency will also invest $80 million to speed up drug development and the testing of new therapies for Alzheimer’s disease.
States act early to restrict abortion
In the first few months of the year, a handful of state legislatures have moved to curb abortions by imposing restrictions on admitting privileges and beefing up regulatory requirements on abortion clinics.
In North Dakota, Gov. Jack Dalrymple (R) recently signed into law (S.B. 2305) a measure requiring any physician who performs abortions to have admitting and staff privileges at a nearby hospital. The new law also requires that the hospital allow abortions to take place in its facility.
When signing the law back in March, Gov. Dalrymple acknowledged that the additional requirement that hospitals must allow abortions to be performed on site would likely result in a legal showdown. "Nevertheless, it is a legitimate and new question for the courts regarding a precise restriction on doctors who perform abortions," he said in a statement.
The law goes into effect Aug. 1.
The Center for Reproductive Rights is planning to challenge the law in court. The group, which is seeking to overturn a similar law passed last year in Mississippi, said the requirement would shut down the state’s only abortion clinic – Red River Women’s Clinic in Fargo – which provides reproductive health services to nearly 1,500 women each year.
Gov. Dalrymple also signed two other antiabortion measures into law, which are likely to be challenged in court. One would ban abortions performed solely for the purpose of gender selection or due to genetic abnormalities. The second would ban any abortions performed after a fetal heartbeat could be detected.
The American Congress of Obstetricians and Gynecologists opposed all three of the new North Dakota laws.
In a statement, ACOG officials said the three laws were the most recent examples of state legislatures regulating medical care without the benefit of scientific evidence.
"Legislators should not needlessly interfere in the patient-physician relationship or the practice of medicine," ACOG officials wrote. "ACOG, as an organization, joins other women’s health advocates in supporting the legal right of women to obtain an abortion and opposing laws that are dangerous, unscientific, and criminalize medical care. Physicians, not legislators, should be making medical decisions with North Dakota women about their care."
North Dakota lawmakers aren’t the only ones seeking to put tighter restrictions on abortions. North Carolina legislators are considering a bill (SB 308) with similar hospital admitting requirements. And Texas lawmakers are considering a bill (SB 537) that would require abortion clinics to conform to the same building requirements as those of ambulatory surgical centers. The Planned Parenthood Federation of America estimates that if enacted, that requirement would force many health centers to stop offering abortion services, dropping the number of health centers that perform abortions from 42 to 5 in Texas.
In Alabama, the state legislature passed a bill that includes both admitting privilege requirements and rules that abortion clinics meet the same building specifications as those of ambulatory surgical centers. Gov. Robert J. Bentley (R) is expected to sign the bill.
In Indiana, lawmakers have taken the ambulatory surgical center requirements a step further. The state legislature passed a bill imposing the surgical requirements on health centers even if they only perform nonsurgical abortions.
In the first few months of the year, a handful of state legislatures have moved to curb abortions by imposing restrictions on admitting privileges and beefing up regulatory requirements on abortion clinics.
In North Dakota, Gov. Jack Dalrymple (R) recently signed into law (S.B. 2305) a measure requiring any physician who performs abortions to have admitting and staff privileges at a nearby hospital. The new law also requires that the hospital allow abortions to take place in its facility.
When signing the law back in March, Gov. Dalrymple acknowledged that the additional requirement that hospitals must allow abortions to be performed on site would likely result in a legal showdown. "Nevertheless, it is a legitimate and new question for the courts regarding a precise restriction on doctors who perform abortions," he said in a statement.
The law goes into effect Aug. 1.
The Center for Reproductive Rights is planning to challenge the law in court. The group, which is seeking to overturn a similar law passed last year in Mississippi, said the requirement would shut down the state’s only abortion clinic – Red River Women’s Clinic in Fargo – which provides reproductive health services to nearly 1,500 women each year.
Gov. Dalrymple also signed two other antiabortion measures into law, which are likely to be challenged in court. One would ban abortions performed solely for the purpose of gender selection or due to genetic abnormalities. The second would ban any abortions performed after a fetal heartbeat could be detected.
The American Congress of Obstetricians and Gynecologists opposed all three of the new North Dakota laws.
In a statement, ACOG officials said the three laws were the most recent examples of state legislatures regulating medical care without the benefit of scientific evidence.
"Legislators should not needlessly interfere in the patient-physician relationship or the practice of medicine," ACOG officials wrote. "ACOG, as an organization, joins other women’s health advocates in supporting the legal right of women to obtain an abortion and opposing laws that are dangerous, unscientific, and criminalize medical care. Physicians, not legislators, should be making medical decisions with North Dakota women about their care."
North Dakota lawmakers aren’t the only ones seeking to put tighter restrictions on abortions. North Carolina legislators are considering a bill (SB 308) with similar hospital admitting requirements. And Texas lawmakers are considering a bill (SB 537) that would require abortion clinics to conform to the same building requirements as those of ambulatory surgical centers. The Planned Parenthood Federation of America estimates that if enacted, that requirement would force many health centers to stop offering abortion services, dropping the number of health centers that perform abortions from 42 to 5 in Texas.
In Alabama, the state legislature passed a bill that includes both admitting privilege requirements and rules that abortion clinics meet the same building specifications as those of ambulatory surgical centers. Gov. Robert J. Bentley (R) is expected to sign the bill.
In Indiana, lawmakers have taken the ambulatory surgical center requirements a step further. The state legislature passed a bill imposing the surgical requirements on health centers even if they only perform nonsurgical abortions.
In the first few months of the year, a handful of state legislatures have moved to curb abortions by imposing restrictions on admitting privileges and beefing up regulatory requirements on abortion clinics.
In North Dakota, Gov. Jack Dalrymple (R) recently signed into law (S.B. 2305) a measure requiring any physician who performs abortions to have admitting and staff privileges at a nearby hospital. The new law also requires that the hospital allow abortions to take place in its facility.
When signing the law back in March, Gov. Dalrymple acknowledged that the additional requirement that hospitals must allow abortions to be performed on site would likely result in a legal showdown. "Nevertheless, it is a legitimate and new question for the courts regarding a precise restriction on doctors who perform abortions," he said in a statement.
The law goes into effect Aug. 1.
The Center for Reproductive Rights is planning to challenge the law in court. The group, which is seeking to overturn a similar law passed last year in Mississippi, said the requirement would shut down the state’s only abortion clinic – Red River Women’s Clinic in Fargo – which provides reproductive health services to nearly 1,500 women each year.
Gov. Dalrymple also signed two other antiabortion measures into law, which are likely to be challenged in court. One would ban abortions performed solely for the purpose of gender selection or due to genetic abnormalities. The second would ban any abortions performed after a fetal heartbeat could be detected.
The American Congress of Obstetricians and Gynecologists opposed all three of the new North Dakota laws.
In a statement, ACOG officials said the three laws were the most recent examples of state legislatures regulating medical care without the benefit of scientific evidence.
"Legislators should not needlessly interfere in the patient-physician relationship or the practice of medicine," ACOG officials wrote. "ACOG, as an organization, joins other women’s health advocates in supporting the legal right of women to obtain an abortion and opposing laws that are dangerous, unscientific, and criminalize medical care. Physicians, not legislators, should be making medical decisions with North Dakota women about their care."
North Dakota lawmakers aren’t the only ones seeking to put tighter restrictions on abortions. North Carolina legislators are considering a bill (SB 308) with similar hospital admitting requirements. And Texas lawmakers are considering a bill (SB 537) that would require abortion clinics to conform to the same building requirements as those of ambulatory surgical centers. The Planned Parenthood Federation of America estimates that if enacted, that requirement would force many health centers to stop offering abortion services, dropping the number of health centers that perform abortions from 42 to 5 in Texas.
In Alabama, the state legislature passed a bill that includes both admitting privilege requirements and rules that abortion clinics meet the same building specifications as those of ambulatory surgical centers. Gov. Robert J. Bentley (R) is expected to sign the bill.
In Indiana, lawmakers have taken the ambulatory surgical center requirements a step further. The state legislature passed a bill imposing the surgical requirements on health centers even if they only perform nonsurgical abortions.
AHRQ releases updated discharge toolkit
The Agency for Healthcare Research and Quality has published an expanded version of the Re-Engineered Discharge (RED) Toolkit, including tools for dealing with patients with limited English proficiency.
The RED Toolkit, which was developed by researchers at Boston University Medical Center, outlines a series of steps that hospitals can take both during and after a hospital stay to ensure a smooth transition at the time of discharge.
In one randomized controlled trial, patients who were discharged using the RED process had a 30% lower rate of hospital utilization within 30 days of discharge, compared with patients receiving usual care. And use of the RED toolkit prevented one readmission or emergency department visit for every seven patients, according to the Agency for Healthcare Research and Quality (AHRQ).
In the updated version of the toolkit, the RED developers added several new tools, including health literacy strategies to help patients learn how to care for themselves once they return home. The expanded toolkit also includes more details on how to conduct a post-discharge follow-up telephone call within 72 hours of discharge and how to monitor a hospital’s progress on reducing readmissions.
On Twitter @MaryEllenNY
The Agency for Healthcare Research and Quality has published an expanded version of the Re-Engineered Discharge (RED) Toolkit, including tools for dealing with patients with limited English proficiency.
The RED Toolkit, which was developed by researchers at Boston University Medical Center, outlines a series of steps that hospitals can take both during and after a hospital stay to ensure a smooth transition at the time of discharge.
In one randomized controlled trial, patients who were discharged using the RED process had a 30% lower rate of hospital utilization within 30 days of discharge, compared with patients receiving usual care. And use of the RED toolkit prevented one readmission or emergency department visit for every seven patients, according to the Agency for Healthcare Research and Quality (AHRQ).
In the updated version of the toolkit, the RED developers added several new tools, including health literacy strategies to help patients learn how to care for themselves once they return home. The expanded toolkit also includes more details on how to conduct a post-discharge follow-up telephone call within 72 hours of discharge and how to monitor a hospital’s progress on reducing readmissions.
On Twitter @MaryEllenNY
The Agency for Healthcare Research and Quality has published an expanded version of the Re-Engineered Discharge (RED) Toolkit, including tools for dealing with patients with limited English proficiency.
The RED Toolkit, which was developed by researchers at Boston University Medical Center, outlines a series of steps that hospitals can take both during and after a hospital stay to ensure a smooth transition at the time of discharge.
In one randomized controlled trial, patients who were discharged using the RED process had a 30% lower rate of hospital utilization within 30 days of discharge, compared with patients receiving usual care. And use of the RED toolkit prevented one readmission or emergency department visit for every seven patients, according to the Agency for Healthcare Research and Quality (AHRQ).
In the updated version of the toolkit, the RED developers added several new tools, including health literacy strategies to help patients learn how to care for themselves once they return home. The expanded toolkit also includes more details on how to conduct a post-discharge follow-up telephone call within 72 hours of discharge and how to monitor a hospital’s progress on reducing readmissions.
On Twitter @MaryEllenNY
Tavenner CMS confirmation chances looking brighter
It’s looking more and more like the U.S. Senate will confirm Marilyn Tavenner as administrator of the Centers for Medicare and Medicaid Services.
Ms. Tavenner, a nurse and former hospital executive, has been serving as acting administrator at the agency since Dr. Donald Berwick stepped down in November 2011. But until recently, she couldn’t even get a hearing on her nomination.
That all changed on April 9, when Ms. Tavenner appeared before the Senate Finance Committee. Not only was she praised by senators of both parties, she was strongly endorsed by House Majority Leader Eric Cantor (R.-Va.), a chief opponent of the Affordable Care Act (ACA) and most of the administration’s health care policies. He attended the Senate hearing to introduce Ms. Tavenner, with whom he worked during his time in the Virginia House of Delegates.
Ms. Tavenner’s supporters praised her as a "practical" and "nonideological" leader who works hard to solve problems. The senators on the committee also gushed over her diverse work experience.
Ms. Tavenner started her career in the trenches, as a nurse. Later she became a hospital CEO and in 2001 was promoted to president of the Hospital Corporation of America’s Central Atlantic division, overseeing 20 hospitals. She also has government experience. She served for 4 years as Virginia’s Secretary of Health and Human Resources, working for then-Gov. Tim Kaine. Mr. Kaine (D-Va.) now serves in the U.S. Senate.
While the senators were complimentary about Ms. Tavenner, they also complained about the lack of information coming out of her agency on the implementation of the ACA, in particular the health insurance exchanges. Sen. Orrin Hatch (R-Utah) said they are still waiting for detailed answers from the CMS about the cost of running the federally facilitated exchanges.
She was also asked about the Sustainable Growth Rate formula and the looming cuts to Medicare physician fees. Ms. Tavenner said she agrees that the SGR should be replaced.
On Twitter @MaryEllenNY
It’s looking more and more like the U.S. Senate will confirm Marilyn Tavenner as administrator of the Centers for Medicare and Medicaid Services.
Ms. Tavenner, a nurse and former hospital executive, has been serving as acting administrator at the agency since Dr. Donald Berwick stepped down in November 2011. But until recently, she couldn’t even get a hearing on her nomination.
That all changed on April 9, when Ms. Tavenner appeared before the Senate Finance Committee. Not only was she praised by senators of both parties, she was strongly endorsed by House Majority Leader Eric Cantor (R.-Va.), a chief opponent of the Affordable Care Act (ACA) and most of the administration’s health care policies. He attended the Senate hearing to introduce Ms. Tavenner, with whom he worked during his time in the Virginia House of Delegates.
Ms. Tavenner’s supporters praised her as a "practical" and "nonideological" leader who works hard to solve problems. The senators on the committee also gushed over her diverse work experience.
Ms. Tavenner started her career in the trenches, as a nurse. Later she became a hospital CEO and in 2001 was promoted to president of the Hospital Corporation of America’s Central Atlantic division, overseeing 20 hospitals. She also has government experience. She served for 4 years as Virginia’s Secretary of Health and Human Resources, working for then-Gov. Tim Kaine. Mr. Kaine (D-Va.) now serves in the U.S. Senate.
While the senators were complimentary about Ms. Tavenner, they also complained about the lack of information coming out of her agency on the implementation of the ACA, in particular the health insurance exchanges. Sen. Orrin Hatch (R-Utah) said they are still waiting for detailed answers from the CMS about the cost of running the federally facilitated exchanges.
She was also asked about the Sustainable Growth Rate formula and the looming cuts to Medicare physician fees. Ms. Tavenner said she agrees that the SGR should be replaced.
On Twitter @MaryEllenNY
It’s looking more and more like the U.S. Senate will confirm Marilyn Tavenner as administrator of the Centers for Medicare and Medicaid Services.
Ms. Tavenner, a nurse and former hospital executive, has been serving as acting administrator at the agency since Dr. Donald Berwick stepped down in November 2011. But until recently, she couldn’t even get a hearing on her nomination.
That all changed on April 9, when Ms. Tavenner appeared before the Senate Finance Committee. Not only was she praised by senators of both parties, she was strongly endorsed by House Majority Leader Eric Cantor (R.-Va.), a chief opponent of the Affordable Care Act (ACA) and most of the administration’s health care policies. He attended the Senate hearing to introduce Ms. Tavenner, with whom he worked during his time in the Virginia House of Delegates.
Ms. Tavenner’s supporters praised her as a "practical" and "nonideological" leader who works hard to solve problems. The senators on the committee also gushed over her diverse work experience.
Ms. Tavenner started her career in the trenches, as a nurse. Later she became a hospital CEO and in 2001 was promoted to president of the Hospital Corporation of America’s Central Atlantic division, overseeing 20 hospitals. She also has government experience. She served for 4 years as Virginia’s Secretary of Health and Human Resources, working for then-Gov. Tim Kaine. Mr. Kaine (D-Va.) now serves in the U.S. Senate.
While the senators were complimentary about Ms. Tavenner, they also complained about the lack of information coming out of her agency on the implementation of the ACA, in particular the health insurance exchanges. Sen. Orrin Hatch (R-Utah) said they are still waiting for detailed answers from the CMS about the cost of running the federally facilitated exchanges.
She was also asked about the Sustainable Growth Rate formula and the looming cuts to Medicare physician fees. Ms. Tavenner said she agrees that the SGR should be replaced.
On Twitter @MaryEllenNY
Feds bolster efforts to understand the human brain
President Obama is calling on neuroscientists inside and outside of the federal government to join an ambitious effort to understand and map the activity of the human brain.
If successful, this basic science work could lay the foundation for effective treatment and prevention strategies for many neurologic and psychiatric diseases.
Right now, scientists are able to study individual neurons and can identify the main function of certain areas of the brain, but the underlying causes of most neurologic and psychiatric diseases are still largely unknown.
"There’s this enormous mystery waiting to be unlocked," President Obama said during a speech at the White House on April 2. "The BRAIN Initiative will change that by giving scientists the tools they need to get a dynamic picture of the brain in action and better understand how we think, how we learn, and how we remember. That knowledge will be transformative."
The Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative is part of President Obama’s fiscal year 2014 budget proposal and seeks to invest $100 million to accelerate the development of technologies to better understand human brain function.
The National Institutes of Health (NIH) plans to spend about $40 million on the project in 2014 and will build upon the work already underway as part of the agency’s Blueprint for Neuroscience Research, which coordinates activities across its 15 institutes and centers. The Defense Advanced Research Projects Agency (DARPA) will contribute $50 million. The DARPA activities will focus on developing new tools to capture and process dynamic neural and synaptic activities. The rest of the federal funding will come from the National Science Foundation. The agency plans to support research advances across biology, the physical sciences, engineering, computer science, and social and behavioral sciences.
Exactly how the money will be spent is unclear. But the NIH is creating a working group that will be cochaired by Dr. Cornelia Bargmann of Rockefeller University, New York, and Dr. William Newsome of Stanford (Calif.) University to spell out the goals of the BRAIN Initiative. The working group will present a preliminary plan later this year and deliver a final report in June 2014.
"This initiative is an idea whose time has come," said NIH Director Francis Collins.
While the $100 million investment is less than 1% of the NIH’s total annual funding, Dr. Collins said the initiative is a "strong start out of the gate."
The federal agencies won’t be the only players in the new BRAIN Initiative. At least four private foundations are making significant financial investments in brain mapping, including the Howard Hughes Medical Institute, the Allen Institute for Brain Science, the Kavli Foundation, and the Salk Institute for Biological Studies.
It was some of these same private organizations that originally brought forward the idea to systematically map the human brain. In September 2011, scientists from the Kavli Foundation, the Allen Institute, and the Gatsby Charitable Foundation met in the United Kingdom and formed the idea. After that, they held a series of meetings and produced a white paper before catching the attention of the White House.
The attention of the federal government and the president in particular, is "energizing," said Dr. R. Clay Reid, the senior investigator for neural coding at the Allen Institute. The BRAIN Initiative will help accelerate the activity already underway and kick start the next series of technological advances in nanotechnology and advanced optics that will give neuroscientists the tools they need to make new discoveries, he said.
"It’s building enabling technologies that will push the entire field forward," Dr. Reid said in an interview.
The basic science initiative won’t lead to immediate clinical advances, said Christof Koch, Ph.D., the chief scientific officer at the Allen Institute. But the next clinical application is likely to be in the area of prosthetics. The discoveries that come out of the BRAIN Initiative will help enable better communication between patients’ brain and their prosthetics, he said.
Further off, however, are advances in such diseases as Parkinson’s and autism. "We’re not going to see in the next couple of years significant in-roads into those pathologies," Dr. Koch said in an interview. "We will understand them ultimately, but it will take time."
Dr. Daniel G. Amen, a psychiatrist and an expert in using brain scans as part of psychiatric treatment, said the launch of the BRAIN Initiative is also good news for the field of psychiatry.
"It’s great to have the president of the United States saying the brain is important," he said in an interview.
Dr. Amen said he’s hopeful the initiative will help get more people to equate mental health with brain health and offer new imaging advances that psychiatrists can use in their everyday practice. Dr. Amen, who has a database of about 80,000 brain scans, said showing patients images of their brain helps to decrease stigma about their condition and to increase their compliance with medication and behavioral changes. "It radically changes the conversation," he said.
On Twitter @MaryEllenNY
President Obama is calling on neuroscientists inside and outside of the federal government to join an ambitious effort to understand and map the activity of the human brain.
If successful, this basic science work could lay the foundation for effective treatment and prevention strategies for many neurologic and psychiatric diseases.
Right now, scientists are able to study individual neurons and can identify the main function of certain areas of the brain, but the underlying causes of most neurologic and psychiatric diseases are still largely unknown.
"There’s this enormous mystery waiting to be unlocked," President Obama said during a speech at the White House on April 2. "The BRAIN Initiative will change that by giving scientists the tools they need to get a dynamic picture of the brain in action and better understand how we think, how we learn, and how we remember. That knowledge will be transformative."
The Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative is part of President Obama’s fiscal year 2014 budget proposal and seeks to invest $100 million to accelerate the development of technologies to better understand human brain function.
The National Institutes of Health (NIH) plans to spend about $40 million on the project in 2014 and will build upon the work already underway as part of the agency’s Blueprint for Neuroscience Research, which coordinates activities across its 15 institutes and centers. The Defense Advanced Research Projects Agency (DARPA) will contribute $50 million. The DARPA activities will focus on developing new tools to capture and process dynamic neural and synaptic activities. The rest of the federal funding will come from the National Science Foundation. The agency plans to support research advances across biology, the physical sciences, engineering, computer science, and social and behavioral sciences.
Exactly how the money will be spent is unclear. But the NIH is creating a working group that will be cochaired by Dr. Cornelia Bargmann of Rockefeller University, New York, and Dr. William Newsome of Stanford (Calif.) University to spell out the goals of the BRAIN Initiative. The working group will present a preliminary plan later this year and deliver a final report in June 2014.
"This initiative is an idea whose time has come," said NIH Director Francis Collins.
While the $100 million investment is less than 1% of the NIH’s total annual funding, Dr. Collins said the initiative is a "strong start out of the gate."
The federal agencies won’t be the only players in the new BRAIN Initiative. At least four private foundations are making significant financial investments in brain mapping, including the Howard Hughes Medical Institute, the Allen Institute for Brain Science, the Kavli Foundation, and the Salk Institute for Biological Studies.
It was some of these same private organizations that originally brought forward the idea to systematically map the human brain. In September 2011, scientists from the Kavli Foundation, the Allen Institute, and the Gatsby Charitable Foundation met in the United Kingdom and formed the idea. After that, they held a series of meetings and produced a white paper before catching the attention of the White House.
The attention of the federal government and the president in particular, is "energizing," said Dr. R. Clay Reid, the senior investigator for neural coding at the Allen Institute. The BRAIN Initiative will help accelerate the activity already underway and kick start the next series of technological advances in nanotechnology and advanced optics that will give neuroscientists the tools they need to make new discoveries, he said.
"It’s building enabling technologies that will push the entire field forward," Dr. Reid said in an interview.
The basic science initiative won’t lead to immediate clinical advances, said Christof Koch, Ph.D., the chief scientific officer at the Allen Institute. But the next clinical application is likely to be in the area of prosthetics. The discoveries that come out of the BRAIN Initiative will help enable better communication between patients’ brain and their prosthetics, he said.
Further off, however, are advances in such diseases as Parkinson’s and autism. "We’re not going to see in the next couple of years significant in-roads into those pathologies," Dr. Koch said in an interview. "We will understand them ultimately, but it will take time."
Dr. Daniel G. Amen, a psychiatrist and an expert in using brain scans as part of psychiatric treatment, said the launch of the BRAIN Initiative is also good news for the field of psychiatry.
"It’s great to have the president of the United States saying the brain is important," he said in an interview.
Dr. Amen said he’s hopeful the initiative will help get more people to equate mental health with brain health and offer new imaging advances that psychiatrists can use in their everyday practice. Dr. Amen, who has a database of about 80,000 brain scans, said showing patients images of their brain helps to decrease stigma about their condition and to increase their compliance with medication and behavioral changes. "It radically changes the conversation," he said.
On Twitter @MaryEllenNY
President Obama is calling on neuroscientists inside and outside of the federal government to join an ambitious effort to understand and map the activity of the human brain.
If successful, this basic science work could lay the foundation for effective treatment and prevention strategies for many neurologic and psychiatric diseases.
Right now, scientists are able to study individual neurons and can identify the main function of certain areas of the brain, but the underlying causes of most neurologic and psychiatric diseases are still largely unknown.
"There’s this enormous mystery waiting to be unlocked," President Obama said during a speech at the White House on April 2. "The BRAIN Initiative will change that by giving scientists the tools they need to get a dynamic picture of the brain in action and better understand how we think, how we learn, and how we remember. That knowledge will be transformative."
The Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative is part of President Obama’s fiscal year 2014 budget proposal and seeks to invest $100 million to accelerate the development of technologies to better understand human brain function.
The National Institutes of Health (NIH) plans to spend about $40 million on the project in 2014 and will build upon the work already underway as part of the agency’s Blueprint for Neuroscience Research, which coordinates activities across its 15 institutes and centers. The Defense Advanced Research Projects Agency (DARPA) will contribute $50 million. The DARPA activities will focus on developing new tools to capture and process dynamic neural and synaptic activities. The rest of the federal funding will come from the National Science Foundation. The agency plans to support research advances across biology, the physical sciences, engineering, computer science, and social and behavioral sciences.
Exactly how the money will be spent is unclear. But the NIH is creating a working group that will be cochaired by Dr. Cornelia Bargmann of Rockefeller University, New York, and Dr. William Newsome of Stanford (Calif.) University to spell out the goals of the BRAIN Initiative. The working group will present a preliminary plan later this year and deliver a final report in June 2014.
"This initiative is an idea whose time has come," said NIH Director Francis Collins.
While the $100 million investment is less than 1% of the NIH’s total annual funding, Dr. Collins said the initiative is a "strong start out of the gate."
The federal agencies won’t be the only players in the new BRAIN Initiative. At least four private foundations are making significant financial investments in brain mapping, including the Howard Hughes Medical Institute, the Allen Institute for Brain Science, the Kavli Foundation, and the Salk Institute for Biological Studies.
It was some of these same private organizations that originally brought forward the idea to systematically map the human brain. In September 2011, scientists from the Kavli Foundation, the Allen Institute, and the Gatsby Charitable Foundation met in the United Kingdom and formed the idea. After that, they held a series of meetings and produced a white paper before catching the attention of the White House.
The attention of the federal government and the president in particular, is "energizing," said Dr. R. Clay Reid, the senior investigator for neural coding at the Allen Institute. The BRAIN Initiative will help accelerate the activity already underway and kick start the next series of technological advances in nanotechnology and advanced optics that will give neuroscientists the tools they need to make new discoveries, he said.
"It’s building enabling technologies that will push the entire field forward," Dr. Reid said in an interview.
The basic science initiative won’t lead to immediate clinical advances, said Christof Koch, Ph.D., the chief scientific officer at the Allen Institute. But the next clinical application is likely to be in the area of prosthetics. The discoveries that come out of the BRAIN Initiative will help enable better communication between patients’ brain and their prosthetics, he said.
Further off, however, are advances in such diseases as Parkinson’s and autism. "We’re not going to see in the next couple of years significant in-roads into those pathologies," Dr. Koch said in an interview. "We will understand them ultimately, but it will take time."
Dr. Daniel G. Amen, a psychiatrist and an expert in using brain scans as part of psychiatric treatment, said the launch of the BRAIN Initiative is also good news for the field of psychiatry.
"It’s great to have the president of the United States saying the brain is important," he said in an interview.
Dr. Amen said he’s hopeful the initiative will help get more people to equate mental health with brain health and offer new imaging advances that psychiatrists can use in their everyday practice. Dr. Amen, who has a database of about 80,000 brain scans, said showing patients images of their brain helps to decrease stigma about their condition and to increase their compliance with medication and behavioral changes. "It radically changes the conversation," he said.
On Twitter @MaryEllenNY