User login
CLL boost in PFS came at high cost in side effects
Add-on idelalisib boosted the progression-free survival (PFS) of patients with relapsed and refractory chronic lymphocytic leukemia by over 9 months in a double-blind, placebo-controlled trial of 416 participants. But that improvement came at the price of a 4% absolute increase in treatment-emergent adverse events leading to death.
At a median follow-up of 14 months, the median PFS was 20.8 months in the 207 patients in the idelalisib group and 11.1 months in the 209 patients in the placebo group. However, 11% of patients in the idelalisib group and 7% of those in the placebo group had treatment-related adverse events that resulted in death.
Overall, 68% of those in the idelalisib group, and 44% in the placebo group, had serious adverse events that included febrile neutropenia, pneumonia, and pyrexia. Six deaths resulted from infections in the idelalisib group, and three deaths resulted from infections in the placebo group.
All 416 participants in the international, multicenter study had measurable lymphadenopathy by CT or MRI and progression of chronic lymphocytic leukemia within 36 months of their last therapy. Patients were stratified by high-risk genetic mutations (IGHV, del[17p], or TP53) and refractory versus relapsed disease. Participants were randomly assigned to receive daily idelalisib or placebo, plus a maximum of six cycles of bendamustine and rituximab.
The primary endpoint of PFS was assessed by an independent review committee in the intention-to-treat population.
A 150 mg dose of idelalisib or placebo was given twice daily until disease progressed or drug-related toxicity was intolerable. Bendamustine was given intravenously at 70 mg/m2 on days 1 and 2 for six 28-day cycles. Rituximab was given at 375 mg/m2 on day 1 of cycle 1 and at 500 mg/m2 on day 1 of cycles 2–6.
The trial is ongoing, the researchers reported.
The trial is funded by Gilead Sciences, the maker of idelalisib (Zydelig). Dr. Zelenetz disclosed consulting with multiple drug companies, including Gilead.
[email protected]
On Twitter @maryjodales
Add-on idelalisib boosted the progression-free survival (PFS) of patients with relapsed and refractory chronic lymphocytic leukemia by over 9 months in a double-blind, placebo-controlled trial of 416 participants. But that improvement came at the price of a 4% absolute increase in treatment-emergent adverse events leading to death.
At a median follow-up of 14 months, the median PFS was 20.8 months in the 207 patients in the idelalisib group and 11.1 months in the 209 patients in the placebo group. However, 11% of patients in the idelalisib group and 7% of those in the placebo group had treatment-related adverse events that resulted in death.
Overall, 68% of those in the idelalisib group, and 44% in the placebo group, had serious adverse events that included febrile neutropenia, pneumonia, and pyrexia. Six deaths resulted from infections in the idelalisib group, and three deaths resulted from infections in the placebo group.
All 416 participants in the international, multicenter study had measurable lymphadenopathy by CT or MRI and progression of chronic lymphocytic leukemia within 36 months of their last therapy. Patients were stratified by high-risk genetic mutations (IGHV, del[17p], or TP53) and refractory versus relapsed disease. Participants were randomly assigned to receive daily idelalisib or placebo, plus a maximum of six cycles of bendamustine and rituximab.
The primary endpoint of PFS was assessed by an independent review committee in the intention-to-treat population.
A 150 mg dose of idelalisib or placebo was given twice daily until disease progressed or drug-related toxicity was intolerable. Bendamustine was given intravenously at 70 mg/m2 on days 1 and 2 for six 28-day cycles. Rituximab was given at 375 mg/m2 on day 1 of cycle 1 and at 500 mg/m2 on day 1 of cycles 2–6.
The trial is ongoing, the researchers reported.
The trial is funded by Gilead Sciences, the maker of idelalisib (Zydelig). Dr. Zelenetz disclosed consulting with multiple drug companies, including Gilead.
[email protected]
On Twitter @maryjodales
Add-on idelalisib boosted the progression-free survival (PFS) of patients with relapsed and refractory chronic lymphocytic leukemia by over 9 months in a double-blind, placebo-controlled trial of 416 participants. But that improvement came at the price of a 4% absolute increase in treatment-emergent adverse events leading to death.
At a median follow-up of 14 months, the median PFS was 20.8 months in the 207 patients in the idelalisib group and 11.1 months in the 209 patients in the placebo group. However, 11% of patients in the idelalisib group and 7% of those in the placebo group had treatment-related adverse events that resulted in death.
Overall, 68% of those in the idelalisib group, and 44% in the placebo group, had serious adverse events that included febrile neutropenia, pneumonia, and pyrexia. Six deaths resulted from infections in the idelalisib group, and three deaths resulted from infections in the placebo group.
All 416 participants in the international, multicenter study had measurable lymphadenopathy by CT or MRI and progression of chronic lymphocytic leukemia within 36 months of their last therapy. Patients were stratified by high-risk genetic mutations (IGHV, del[17p], or TP53) and refractory versus relapsed disease. Participants were randomly assigned to receive daily idelalisib or placebo, plus a maximum of six cycles of bendamustine and rituximab.
The primary endpoint of PFS was assessed by an independent review committee in the intention-to-treat population.
A 150 mg dose of idelalisib or placebo was given twice daily until disease progressed or drug-related toxicity was intolerable. Bendamustine was given intravenously at 70 mg/m2 on days 1 and 2 for six 28-day cycles. Rituximab was given at 375 mg/m2 on day 1 of cycle 1 and at 500 mg/m2 on day 1 of cycles 2–6.
The trial is ongoing, the researchers reported.
The trial is funded by Gilead Sciences, the maker of idelalisib (Zydelig). Dr. Zelenetz disclosed consulting with multiple drug companies, including Gilead.
[email protected]
On Twitter @maryjodales
Key clinical point:
Major finding: Add-on idelalisib boosted the progression-free survival of patients with relapsed and refractory chronic lymphocytic leukemia by over 9 months.
Data source: A double-blind, placebo-controlled trial of 416 participants.
Disclosures: Gilead Sciences, the maker of idelalisib (Zydelig), sponsored the study. Dr. Zelenetz disclosed consulting with multiple drug companies, including Gilead.
Thrombotic microangiopathy may signal IV drug abuse
Extended-release oxymorphone hydrochloride (Opana ER) tablets contain an inert ingredient that can trigger acute microangiopathic hemolytic anemia and thrombocytopenia in those who abuse the drug via intravenous injection, Ryan Hunt, of the Food and Drug Administration, and his colleagues reported.
High-molecular-weight polyethylene oxide appeared to be the cause of acute cases of thrombotic microangiopathy in three patients who were treated in the emergency department of a single hospital in Tennessee. All had chest pain, dyspnea, visual impairment, microangiopathic hemolytic anemia and thrombocytopenia, increased lactate dehydrogenase, and undetectable haptoglobin serum levels. Two patients also had acute renal failure. Plasma exchange therapy was initiated in all three; one required additional hemodialysis.
ADAMTS13 activity was normal in blood samples obtained in two patients before they started plasma exchange. Histologic evidence of thrombotic microangiopathy with endothelial swelling of arterioles and acute tubular injury without deposition of immune complexes was seen in kidney biopsies performed in two patients. In addition, gelatinous material occluded the dialysis catheter and apheresis tubings during the initial plasma exchange sessions, the researchers reported (Blood. 2017;129[7]:896-905).
To test the association of polyethylene oxide with thrombotic microangiopathy, the researchers heated a 40-mg Opana ER tablet cut in several pieces in a spoon with 2 mL water until boiling. They injected guinea pigs with 0.1 or 0.3 mg/kg of the extracted polyethylene oxide, either as a single dose or as five doses given at 1.5-hour intervals. A dose-dependent increase of polyethylene oxide in plasma peaked at 8 hours after the first dose was measured and paralleled with intravascular hemolysis with increased plasma concentration of free hemoglobin, schistocytes in the peripheral blood smear, and thrombocytopenia. Spiking control blood samples in vitro with polyethylene oxide did not result in hemolysis.
“Although injection abuse of prescription opioids is highly concentrated in certain regions of the United States, particularly in rural Appalachia, all physicians should be highly inquisitive of IV drug abuse when presented with cases of [thrombotic microangiopathy],” the researchers concluded.
The researchers had no relevant conflicts of interest. One of the researchers is employed by Quest Diagnostics.
[email protected]
On Twitter @maryjodales
Extended-release oxymorphone hydrochloride (Opana ER) tablets contain an inert ingredient that can trigger acute microangiopathic hemolytic anemia and thrombocytopenia in those who abuse the drug via intravenous injection, Ryan Hunt, of the Food and Drug Administration, and his colleagues reported.
High-molecular-weight polyethylene oxide appeared to be the cause of acute cases of thrombotic microangiopathy in three patients who were treated in the emergency department of a single hospital in Tennessee. All had chest pain, dyspnea, visual impairment, microangiopathic hemolytic anemia and thrombocytopenia, increased lactate dehydrogenase, and undetectable haptoglobin serum levels. Two patients also had acute renal failure. Plasma exchange therapy was initiated in all three; one required additional hemodialysis.
ADAMTS13 activity was normal in blood samples obtained in two patients before they started plasma exchange. Histologic evidence of thrombotic microangiopathy with endothelial swelling of arterioles and acute tubular injury without deposition of immune complexes was seen in kidney biopsies performed in two patients. In addition, gelatinous material occluded the dialysis catheter and apheresis tubings during the initial plasma exchange sessions, the researchers reported (Blood. 2017;129[7]:896-905).
To test the association of polyethylene oxide with thrombotic microangiopathy, the researchers heated a 40-mg Opana ER tablet cut in several pieces in a spoon with 2 mL water until boiling. They injected guinea pigs with 0.1 or 0.3 mg/kg of the extracted polyethylene oxide, either as a single dose or as five doses given at 1.5-hour intervals. A dose-dependent increase of polyethylene oxide in plasma peaked at 8 hours after the first dose was measured and paralleled with intravascular hemolysis with increased plasma concentration of free hemoglobin, schistocytes in the peripheral blood smear, and thrombocytopenia. Spiking control blood samples in vitro with polyethylene oxide did not result in hemolysis.
“Although injection abuse of prescription opioids is highly concentrated in certain regions of the United States, particularly in rural Appalachia, all physicians should be highly inquisitive of IV drug abuse when presented with cases of [thrombotic microangiopathy],” the researchers concluded.
The researchers had no relevant conflicts of interest. One of the researchers is employed by Quest Diagnostics.
[email protected]
On Twitter @maryjodales
Extended-release oxymorphone hydrochloride (Opana ER) tablets contain an inert ingredient that can trigger acute microangiopathic hemolytic anemia and thrombocytopenia in those who abuse the drug via intravenous injection, Ryan Hunt, of the Food and Drug Administration, and his colleagues reported.
High-molecular-weight polyethylene oxide appeared to be the cause of acute cases of thrombotic microangiopathy in three patients who were treated in the emergency department of a single hospital in Tennessee. All had chest pain, dyspnea, visual impairment, microangiopathic hemolytic anemia and thrombocytopenia, increased lactate dehydrogenase, and undetectable haptoglobin serum levels. Two patients also had acute renal failure. Plasma exchange therapy was initiated in all three; one required additional hemodialysis.
ADAMTS13 activity was normal in blood samples obtained in two patients before they started plasma exchange. Histologic evidence of thrombotic microangiopathy with endothelial swelling of arterioles and acute tubular injury without deposition of immune complexes was seen in kidney biopsies performed in two patients. In addition, gelatinous material occluded the dialysis catheter and apheresis tubings during the initial plasma exchange sessions, the researchers reported (Blood. 2017;129[7]:896-905).
To test the association of polyethylene oxide with thrombotic microangiopathy, the researchers heated a 40-mg Opana ER tablet cut in several pieces in a spoon with 2 mL water until boiling. They injected guinea pigs with 0.1 or 0.3 mg/kg of the extracted polyethylene oxide, either as a single dose or as five doses given at 1.5-hour intervals. A dose-dependent increase of polyethylene oxide in plasma peaked at 8 hours after the first dose was measured and paralleled with intravascular hemolysis with increased plasma concentration of free hemoglobin, schistocytes in the peripheral blood smear, and thrombocytopenia. Spiking control blood samples in vitro with polyethylene oxide did not result in hemolysis.
“Although injection abuse of prescription opioids is highly concentrated in certain regions of the United States, particularly in rural Appalachia, all physicians should be highly inquisitive of IV drug abuse when presented with cases of [thrombotic microangiopathy],” the researchers concluded.
The researchers had no relevant conflicts of interest. One of the researchers is employed by Quest Diagnostics.
[email protected]
On Twitter @maryjodales
Key clinical point:
Major finding: Extended-release oxymorphone hydrochloride (Opana ER) tablets contain an inert ingredient that can trigger acute microangiopathic hemolytic anemia and thrombocytopenia in those who abuse the drug via intravenous injection.
Data source: Observational study of three patients and translational research study of inert drug components in guinea pigs.
Disclosures: The researchers had no conflicts of interest. One of the researchers is employed by Quest Diagnostics.
VIDEO: First multicenter trial of CAR T cells shows response in DLBCL
SAN DIEGO – Aggressive, refractory non-Hodgkin lymphomas responded to anti-CD19 chimeric antigen receptor T cells in ZUMA-1, the first multicenter trial of the cellular immunotherapy, based on early data reported at the annual meeting of the American Society of Hematology.
In an interim analysis of 51 patients with diffuse large B-cell lymphomas, 47% had complete remissions and 29% had partial remissions. But the remission rate declined to 33% complete remissions and 6% partial remissions after 3 months.
There have really been no new treatments in the last 20 years for patients with non-Hodgkin lymphoma that does not respond to chemotherapy or recurs after autologous stem cell transplant. With median overall survival of 6 months, and about 8% complete remissions with existing therapies, CAR T cells might be a solution for these patients, said ZUMA-1 investigator Sattva S. Neelapu, MD, of the University of Texas MD Anderson Cancer Center in Houston.
In our video interview at the meeting, Dr. Neelapu discussed initial results in the real-world setting of 22 participating centers, most of which had no previous experience with CAR T-cell therapy. With an efficient production and logistics plan, 91% of 110 patients were able to receive the investigational product, known as KTE-C19.
ZUMA-1 is funded by Kite, which makes KTE-C19, and the Leukemia & Lymphoma Society Therapy Acceleration Program. Dr. Neelapu receives research support from and is an advisor to Kite.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @maryjodales
SAN DIEGO – Aggressive, refractory non-Hodgkin lymphomas responded to anti-CD19 chimeric antigen receptor T cells in ZUMA-1, the first multicenter trial of the cellular immunotherapy, based on early data reported at the annual meeting of the American Society of Hematology.
In an interim analysis of 51 patients with diffuse large B-cell lymphomas, 47% had complete remissions and 29% had partial remissions. But the remission rate declined to 33% complete remissions and 6% partial remissions after 3 months.
There have really been no new treatments in the last 20 years for patients with non-Hodgkin lymphoma that does not respond to chemotherapy or recurs after autologous stem cell transplant. With median overall survival of 6 months, and about 8% complete remissions with existing therapies, CAR T cells might be a solution for these patients, said ZUMA-1 investigator Sattva S. Neelapu, MD, of the University of Texas MD Anderson Cancer Center in Houston.
In our video interview at the meeting, Dr. Neelapu discussed initial results in the real-world setting of 22 participating centers, most of which had no previous experience with CAR T-cell therapy. With an efficient production and logistics plan, 91% of 110 patients were able to receive the investigational product, known as KTE-C19.
ZUMA-1 is funded by Kite, which makes KTE-C19, and the Leukemia & Lymphoma Society Therapy Acceleration Program. Dr. Neelapu receives research support from and is an advisor to Kite.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @maryjodales
SAN DIEGO – Aggressive, refractory non-Hodgkin lymphomas responded to anti-CD19 chimeric antigen receptor T cells in ZUMA-1, the first multicenter trial of the cellular immunotherapy, based on early data reported at the annual meeting of the American Society of Hematology.
In an interim analysis of 51 patients with diffuse large B-cell lymphomas, 47% had complete remissions and 29% had partial remissions. But the remission rate declined to 33% complete remissions and 6% partial remissions after 3 months.
There have really been no new treatments in the last 20 years for patients with non-Hodgkin lymphoma that does not respond to chemotherapy or recurs after autologous stem cell transplant. With median overall survival of 6 months, and about 8% complete remissions with existing therapies, CAR T cells might be a solution for these patients, said ZUMA-1 investigator Sattva S. Neelapu, MD, of the University of Texas MD Anderson Cancer Center in Houston.
In our video interview at the meeting, Dr. Neelapu discussed initial results in the real-world setting of 22 participating centers, most of which had no previous experience with CAR T-cell therapy. With an efficient production and logistics plan, 91% of 110 patients were able to receive the investigational product, known as KTE-C19.
ZUMA-1 is funded by Kite, which makes KTE-C19, and the Leukemia & Lymphoma Society Therapy Acceleration Program. Dr. Neelapu receives research support from and is an advisor to Kite.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @maryjodales
VIDEO: SPIN tests hydroxyurea to prevent pediatric stroke in sickle cell disease
SAN DIEGO – Whether hydroxyurea can avert a first stroke in children with sickle cell disease is a question that may be answered by a soon-to-be-started trial in Nigeria.
An estimated 15,000 children with sickle cell anemia have strokes each year in Nigeria, which is considered to have the largest burden of sickle cell disease in the world. Without treatment, about half of those children will have a second stroke within 2 years.
With completion of its feasibility trial results, SPIN (Primary Stroke Prevention in Children With Sickle Cell Anemia in Nigeria) will begin to examine two doses of hydroxyurea therapy, 20 mg/kg per day and 10 mg/kg per day, to determine whether the drug can prevent a first stroke in high-risk children.
In a video interview, Najibah A. Galadanci, MBBS, MPH, of Aminu Kano (Nigeria) Teaching Hospital, discusses how SPIN is addressing the unresolved clinical issue of hydroxyurea’s risks and benefits in children, and how research in Nigeria may provide adequate patient numbers to address a wide range of clinical questions about sickle cell disease.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @maryjodales
SAN DIEGO – Whether hydroxyurea can avert a first stroke in children with sickle cell disease is a question that may be answered by a soon-to-be-started trial in Nigeria.
An estimated 15,000 children with sickle cell anemia have strokes each year in Nigeria, which is considered to have the largest burden of sickle cell disease in the world. Without treatment, about half of those children will have a second stroke within 2 years.
With completion of its feasibility trial results, SPIN (Primary Stroke Prevention in Children With Sickle Cell Anemia in Nigeria) will begin to examine two doses of hydroxyurea therapy, 20 mg/kg per day and 10 mg/kg per day, to determine whether the drug can prevent a first stroke in high-risk children.
In a video interview, Najibah A. Galadanci, MBBS, MPH, of Aminu Kano (Nigeria) Teaching Hospital, discusses how SPIN is addressing the unresolved clinical issue of hydroxyurea’s risks and benefits in children, and how research in Nigeria may provide adequate patient numbers to address a wide range of clinical questions about sickle cell disease.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @maryjodales
SAN DIEGO – Whether hydroxyurea can avert a first stroke in children with sickle cell disease is a question that may be answered by a soon-to-be-started trial in Nigeria.
An estimated 15,000 children with sickle cell anemia have strokes each year in Nigeria, which is considered to have the largest burden of sickle cell disease in the world. Without treatment, about half of those children will have a second stroke within 2 years.
With completion of its feasibility trial results, SPIN (Primary Stroke Prevention in Children With Sickle Cell Anemia in Nigeria) will begin to examine two doses of hydroxyurea therapy, 20 mg/kg per day and 10 mg/kg per day, to determine whether the drug can prevent a first stroke in high-risk children.
In a video interview, Najibah A. Galadanci, MBBS, MPH, of Aminu Kano (Nigeria) Teaching Hospital, discusses how SPIN is addressing the unresolved clinical issue of hydroxyurea’s risks and benefits in children, and how research in Nigeria may provide adequate patient numbers to address a wide range of clinical questions about sickle cell disease.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @maryjodales
AT ASH 2016
VIDEO: Venetoclax shows early good results in multiple myeloma
SAN DIEGO – Venetoclax has shown preliminary good results as an investigational monotherapy in patients with relapsed/refractory multiple myeloma, based on phase I data reported at the annual meeting of the American Society of Hematology.
Shaji Kumar, MD, of the Mayo Clinic, Rochester, Minn., reported that venetoclax monotherapy had anti-myeloma activity in a dose-finding study among patients treated with a median of five previous therapies. As would be expected, the best responses to the small-molecule BCL-2 inhibitor were seen primarily in patients with t(11;14) chromosomal aberrations and high BCL-2, low BCL-XL and low MCL-1 expression levels.
In a video interview, Dr. Kumar discussed the results of this early-stage research as well as ongoing studies that are beginning to examine venetoclax in combination regimens for multiple myeloma.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Venetoclax is approved for the treatment of patients with chronic lymphocytic leukemia with 17p deletion who have received at least one prior treatment. Dr. Kumar receives research funding from Abbvie, the maker of venetoclax (Venclexta), and is a consultant to and receives research funding from several other drug companies.
[email protected]
On Twitter @maryjodales
SAN DIEGO – Venetoclax has shown preliminary good results as an investigational monotherapy in patients with relapsed/refractory multiple myeloma, based on phase I data reported at the annual meeting of the American Society of Hematology.
Shaji Kumar, MD, of the Mayo Clinic, Rochester, Minn., reported that venetoclax monotherapy had anti-myeloma activity in a dose-finding study among patients treated with a median of five previous therapies. As would be expected, the best responses to the small-molecule BCL-2 inhibitor were seen primarily in patients with t(11;14) chromosomal aberrations and high BCL-2, low BCL-XL and low MCL-1 expression levels.
In a video interview, Dr. Kumar discussed the results of this early-stage research as well as ongoing studies that are beginning to examine venetoclax in combination regimens for multiple myeloma.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Venetoclax is approved for the treatment of patients with chronic lymphocytic leukemia with 17p deletion who have received at least one prior treatment. Dr. Kumar receives research funding from Abbvie, the maker of venetoclax (Venclexta), and is a consultant to and receives research funding from several other drug companies.
[email protected]
On Twitter @maryjodales
SAN DIEGO – Venetoclax has shown preliminary good results as an investigational monotherapy in patients with relapsed/refractory multiple myeloma, based on phase I data reported at the annual meeting of the American Society of Hematology.
Shaji Kumar, MD, of the Mayo Clinic, Rochester, Minn., reported that venetoclax monotherapy had anti-myeloma activity in a dose-finding study among patients treated with a median of five previous therapies. As would be expected, the best responses to the small-molecule BCL-2 inhibitor were seen primarily in patients with t(11;14) chromosomal aberrations and high BCL-2, low BCL-XL and low MCL-1 expression levels.
In a video interview, Dr. Kumar discussed the results of this early-stage research as well as ongoing studies that are beginning to examine venetoclax in combination regimens for multiple myeloma.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Venetoclax is approved for the treatment of patients with chronic lymphocytic leukemia with 17p deletion who have received at least one prior treatment. Dr. Kumar receives research funding from Abbvie, the maker of venetoclax (Venclexta), and is a consultant to and receives research funding from several other drug companies.
[email protected]
On Twitter @maryjodales
AT ASH 2016
VIDEO: Artificial blood cells clear first phase of animal testing
SAN DIEGO – An artificial red blood cell has come close to emulating the key functions of natural cells and does not appear to be associated with the side effects such as vasospasm and poor response to changes in blood pH that hampered the development of previous artificial blood products, Allan Doctor, MD, reported at the annual meeting of the American Society of Hematology.
The bio-synthetic cells, called ErythroMer, are about 1/50th the size of natural red blood cells. They can be stored at room temperature and reconstituted with water when needed for use.
In a mouse model, the ErythroMer cells were shown to capture oxygen in the lungs and release it to tissue in a pattern that was nearly identical to blood transfusion. In a rat model of shock, ErythroMer was effective for resuscitation.
In a video interview, Dr. Doctor of Washington University in St. Louis discussed the pharmacokinetics of ErythroMer, the need for a readily available blood substitute for treating trauma patients, other potential uses for artificial blood cells, and next steps for testing the product.
Dr. Doctor has equity ownership in KaloCyte, the company developing ErythroMer. He receives research funding from Children’s Discovery Institute and the National Institutes of Health.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – An artificial red blood cell has come close to emulating the key functions of natural cells and does not appear to be associated with the side effects such as vasospasm and poor response to changes in blood pH that hampered the development of previous artificial blood products, Allan Doctor, MD, reported at the annual meeting of the American Society of Hematology.
The bio-synthetic cells, called ErythroMer, are about 1/50th the size of natural red blood cells. They can be stored at room temperature and reconstituted with water when needed for use.
In a mouse model, the ErythroMer cells were shown to capture oxygen in the lungs and release it to tissue in a pattern that was nearly identical to blood transfusion. In a rat model of shock, ErythroMer was effective for resuscitation.
In a video interview, Dr. Doctor of Washington University in St. Louis discussed the pharmacokinetics of ErythroMer, the need for a readily available blood substitute for treating trauma patients, other potential uses for artificial blood cells, and next steps for testing the product.
Dr. Doctor has equity ownership in KaloCyte, the company developing ErythroMer. He receives research funding from Children’s Discovery Institute and the National Institutes of Health.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – An artificial red blood cell has come close to emulating the key functions of natural cells and does not appear to be associated with the side effects such as vasospasm and poor response to changes in blood pH that hampered the development of previous artificial blood products, Allan Doctor, MD, reported at the annual meeting of the American Society of Hematology.
The bio-synthetic cells, called ErythroMer, are about 1/50th the size of natural red blood cells. They can be stored at room temperature and reconstituted with water when needed for use.
In a mouse model, the ErythroMer cells were shown to capture oxygen in the lungs and release it to tissue in a pattern that was nearly identical to blood transfusion. In a rat model of shock, ErythroMer was effective for resuscitation.
In a video interview, Dr. Doctor of Washington University in St. Louis discussed the pharmacokinetics of ErythroMer, the need for a readily available blood substitute for treating trauma patients, other potential uses for artificial blood cells, and next steps for testing the product.
Dr. Doctor has equity ownership in KaloCyte, the company developing ErythroMer. He receives research funding from Children’s Discovery Institute and the National Institutes of Health.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASH 2016
VIDEO: Cranial ultrasound better than temporal artery biopsy for GCA
PARIS – Cranial ultrasound was more sensitive and just as specific as was temporal artery biopsy for the diagnosis of giant cell arteritis, based on the results of a retrospective cohort study reported by Dr. Adam Croft at a press conference held during the annual European Congress of Rheumatology.
Given that ultrasound is noninvasive, associated with fewer risks, and more sensitive than biopsy, "temporal artery biopsy may now be unnecessary (when) clinical suspicion of GCA [giant cell arteritis] is high or quite low," said Dr. Croft of the University of Birmingham, England. "Cranial ultrasound may soon replace temporal artery biopsy in the assessment of patients with a suspected diagnosis of GCA in routine clinical practice."
Giant cell arteritis typically is associated with severe headaches and scalp tenderness on the sides of the forehead that must be distinguished from more benign causes of headache. In GCA, these symptoms result from ocular arterial inflammation and narrowing that respond to high-dose steroid therapy.
The findings were seen in a study of 87 patients who underwent cranial duplex ultrasound for suspected GCA. At 3-month follow-up, 36 patients (41%) had a confirmed clinical diagnosis of giant cell arteritis. Of the 30 patients with a positive cranial ultrasound result, 29 went on to have a confirmed diagnosis. Of the 36 patients with more than three American College of Rheumatology criteria, 21 (58%) had a diagnosis of GCA.
When compared with clinical diagnosis, ultrasound had 81% sensitivity and 98% specificity with a positive likelihood ratio of 41 and a negative likelihood ratio 0.2. The positive predictive value was 97% and the negative predictive value was 88%, he said. In other words, with a positive ultrasound finding, the probability of giant cell arteritis was 41 times higher.
In contrast, when compared with clinical diagnosis, temporal artery biopsy had a sensitivity of 53% and a specificity of 100%. The positive likelihood ratio was 2.3 and the negative likelihood ratio 0.2. The positive predictive value was 100% and the negative predictive value was 47%.
Relying on temporal arterial biopsy results alone leaves "patients at risk of missing out on potentially sight-saving steroid treatment, or of being treated with high-dose steroids unnecessarily," he said. Further, temporal artery biopsy is not without risks. The biopsy can miss the artery and can result is permanent facial nerve damage. A negative biopsy rarely informs practice.
The availability of cranial ultrasound depends on whether one’s practice is located near facilities with the infrastructure and the availability of well-trained rheumatologists and radiologists who can do the scan rapidly, Dr. Croft said in a video interview.
Dr. Croft had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @maryjodales
PARIS – Cranial ultrasound was more sensitive and just as specific as was temporal artery biopsy for the diagnosis of giant cell arteritis, based on the results of a retrospective cohort study reported by Dr. Adam Croft at a press conference held during the annual European Congress of Rheumatology.
Given that ultrasound is noninvasive, associated with fewer risks, and more sensitive than biopsy, "temporal artery biopsy may now be unnecessary (when) clinical suspicion of GCA [giant cell arteritis] is high or quite low," said Dr. Croft of the University of Birmingham, England. "Cranial ultrasound may soon replace temporal artery biopsy in the assessment of patients with a suspected diagnosis of GCA in routine clinical practice."
Giant cell arteritis typically is associated with severe headaches and scalp tenderness on the sides of the forehead that must be distinguished from more benign causes of headache. In GCA, these symptoms result from ocular arterial inflammation and narrowing that respond to high-dose steroid therapy.
The findings were seen in a study of 87 patients who underwent cranial duplex ultrasound for suspected GCA. At 3-month follow-up, 36 patients (41%) had a confirmed clinical diagnosis of giant cell arteritis. Of the 30 patients with a positive cranial ultrasound result, 29 went on to have a confirmed diagnosis. Of the 36 patients with more than three American College of Rheumatology criteria, 21 (58%) had a diagnosis of GCA.
When compared with clinical diagnosis, ultrasound had 81% sensitivity and 98% specificity with a positive likelihood ratio of 41 and a negative likelihood ratio 0.2. The positive predictive value was 97% and the negative predictive value was 88%, he said. In other words, with a positive ultrasound finding, the probability of giant cell arteritis was 41 times higher.
In contrast, when compared with clinical diagnosis, temporal artery biopsy had a sensitivity of 53% and a specificity of 100%. The positive likelihood ratio was 2.3 and the negative likelihood ratio 0.2. The positive predictive value was 100% and the negative predictive value was 47%.
Relying on temporal arterial biopsy results alone leaves "patients at risk of missing out on potentially sight-saving steroid treatment, or of being treated with high-dose steroids unnecessarily," he said. Further, temporal artery biopsy is not without risks. The biopsy can miss the artery and can result is permanent facial nerve damage. A negative biopsy rarely informs practice.
The availability of cranial ultrasound depends on whether one’s practice is located near facilities with the infrastructure and the availability of well-trained rheumatologists and radiologists who can do the scan rapidly, Dr. Croft said in a video interview.
Dr. Croft had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @maryjodales
PARIS – Cranial ultrasound was more sensitive and just as specific as was temporal artery biopsy for the diagnosis of giant cell arteritis, based on the results of a retrospective cohort study reported by Dr. Adam Croft at a press conference held during the annual European Congress of Rheumatology.
Given that ultrasound is noninvasive, associated with fewer risks, and more sensitive than biopsy, "temporal artery biopsy may now be unnecessary (when) clinical suspicion of GCA [giant cell arteritis] is high or quite low," said Dr. Croft of the University of Birmingham, England. "Cranial ultrasound may soon replace temporal artery biopsy in the assessment of patients with a suspected diagnosis of GCA in routine clinical practice."
Giant cell arteritis typically is associated with severe headaches and scalp tenderness on the sides of the forehead that must be distinguished from more benign causes of headache. In GCA, these symptoms result from ocular arterial inflammation and narrowing that respond to high-dose steroid therapy.
The findings were seen in a study of 87 patients who underwent cranial duplex ultrasound for suspected GCA. At 3-month follow-up, 36 patients (41%) had a confirmed clinical diagnosis of giant cell arteritis. Of the 30 patients with a positive cranial ultrasound result, 29 went on to have a confirmed diagnosis. Of the 36 patients with more than three American College of Rheumatology criteria, 21 (58%) had a diagnosis of GCA.
When compared with clinical diagnosis, ultrasound had 81% sensitivity and 98% specificity with a positive likelihood ratio of 41 and a negative likelihood ratio 0.2. The positive predictive value was 97% and the negative predictive value was 88%, he said. In other words, with a positive ultrasound finding, the probability of giant cell arteritis was 41 times higher.
In contrast, when compared with clinical diagnosis, temporal artery biopsy had a sensitivity of 53% and a specificity of 100%. The positive likelihood ratio was 2.3 and the negative likelihood ratio 0.2. The positive predictive value was 100% and the negative predictive value was 47%.
Relying on temporal arterial biopsy results alone leaves "patients at risk of missing out on potentially sight-saving steroid treatment, or of being treated with high-dose steroids unnecessarily," he said. Further, temporal artery biopsy is not without risks. The biopsy can miss the artery and can result is permanent facial nerve damage. A negative biopsy rarely informs practice.
The availability of cranial ultrasound depends on whether one’s practice is located near facilities with the infrastructure and the availability of well-trained rheumatologists and radiologists who can do the scan rapidly, Dr. Croft said in a video interview.
Dr. Croft had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @maryjodales
AT THE EULAR CONGRESS 2014
Topiramate approved for migraine prevention in adolescents
Topiramate has become the first drug to be approved for migraine prevention in adolescents, according to a release from the Food and Drug Administration.
First approved in 1996 to prevent seizures, topiramate (Topamax) was approved in 2004 for migraine prevention in adults. The new approval extends daily use of topiramate at 100 mg for migraine prophylaxis to adolescents as young as 12 years old.
"Adding dosing and safety information for the adolescent age group to the drug’s prescribing information will help to inform health care professionals and patients in making treatment choices," said Dr. Eric Bastings, deputy director of the division of neurology products in the FDA’s Center for Drug Evaluation and Research.
The approval was based on the results of a clinical trial of 103 adolescents aged 12-17 years. Those on topiramate had a 72% reduction in the frequency of migraines; those treated with placebo had a 44% reduction.
The most common adverse reactions with the approved dose were paresthesia, upper respiratory infection, anorexia, and abdominal pain.
Topiramate and all antiepileptic drugs may increase the risk of suicidal thoughts and behavior. Patients should be advised to be alert for the emergence or worsening of symptoms of depression and unusual changes in mood or behavior.
In women who take the drug during pregnancy, topiramate has been shown to increase the risk in offspring of cleft lip and palate. If the decision is made to use the medication by a woman of childbearing age, effective birth control should be used, the agency said in a release.
Topamax is manufactured by Janssen Pharmaceuticals.
On Twitter @maryjodales
Topiramate has become the first drug to be approved for migraine prevention in adolescents, according to a release from the Food and Drug Administration.
First approved in 1996 to prevent seizures, topiramate (Topamax) was approved in 2004 for migraine prevention in adults. The new approval extends daily use of topiramate at 100 mg for migraine prophylaxis to adolescents as young as 12 years old.
"Adding dosing and safety information for the adolescent age group to the drug’s prescribing information will help to inform health care professionals and patients in making treatment choices," said Dr. Eric Bastings, deputy director of the division of neurology products in the FDA’s Center for Drug Evaluation and Research.
The approval was based on the results of a clinical trial of 103 adolescents aged 12-17 years. Those on topiramate had a 72% reduction in the frequency of migraines; those treated with placebo had a 44% reduction.
The most common adverse reactions with the approved dose were paresthesia, upper respiratory infection, anorexia, and abdominal pain.
Topiramate and all antiepileptic drugs may increase the risk of suicidal thoughts and behavior. Patients should be advised to be alert for the emergence or worsening of symptoms of depression and unusual changes in mood or behavior.
In women who take the drug during pregnancy, topiramate has been shown to increase the risk in offspring of cleft lip and palate. If the decision is made to use the medication by a woman of childbearing age, effective birth control should be used, the agency said in a release.
Topamax is manufactured by Janssen Pharmaceuticals.
On Twitter @maryjodales
Topiramate has become the first drug to be approved for migraine prevention in adolescents, according to a release from the Food and Drug Administration.
First approved in 1996 to prevent seizures, topiramate (Topamax) was approved in 2004 for migraine prevention in adults. The new approval extends daily use of topiramate at 100 mg for migraine prophylaxis to adolescents as young as 12 years old.
"Adding dosing and safety information for the adolescent age group to the drug’s prescribing information will help to inform health care professionals and patients in making treatment choices," said Dr. Eric Bastings, deputy director of the division of neurology products in the FDA’s Center for Drug Evaluation and Research.
The approval was based on the results of a clinical trial of 103 adolescents aged 12-17 years. Those on topiramate had a 72% reduction in the frequency of migraines; those treated with placebo had a 44% reduction.
The most common adverse reactions with the approved dose were paresthesia, upper respiratory infection, anorexia, and abdominal pain.
Topiramate and all antiepileptic drugs may increase the risk of suicidal thoughts and behavior. Patients should be advised to be alert for the emergence or worsening of symptoms of depression and unusual changes in mood or behavior.
In women who take the drug during pregnancy, topiramate has been shown to increase the risk in offspring of cleft lip and palate. If the decision is made to use the medication by a woman of childbearing age, effective birth control should be used, the agency said in a release.
Topamax is manufactured by Janssen Pharmaceuticals.
On Twitter @maryjodales
Autism starts in utero, brain tissue study indicates
Autism may begin in utero, based on a study of postmortem brain tissue from children with and without autism.
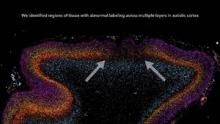
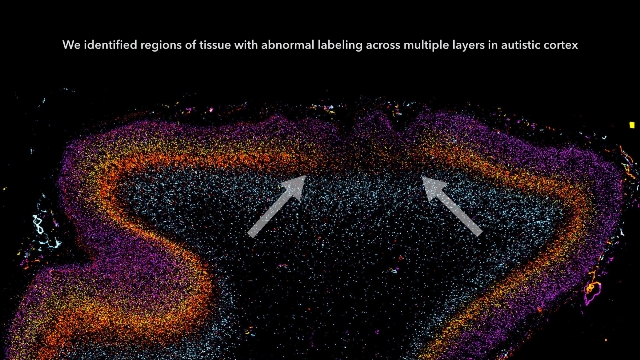
Patchy defects were noted in the six cellular layers of the cortex from 10 of 11 children with autism and from 1 of 11 unaffected children, based on gene expression studies performed on postmortem brain tissue from children aged 2-15 years at death. Abnormal laminar cytoarchitecture and cortical disorganization of neurons, but not of glia, were noted, according to Rich Stoner, Ph.D., of the Autism Center of Excellence at the University of California, San Diego, and his colleagues.
The findings, published in the New England Journal of Medicine, imply that layer formation and layer-specific neuronal differentiation are dysregulated during prenatal development.
The study results suggest that early recognition and treatment of autism may allow the developing brains of autistic children to construct alternative brain pathways around the patchy defects in the cortex. The result could be improved social functioning and communication, the researchers suggested (N. Engl. J. Med. 2014;370:1209-19 [doi:10.1056/NEJMoa1307491]).
In their study, Dr. Stoner and his colleagues used gene expression to examine cellular markers in each of the cortical layers as well as genes that are associated with autism. Markers for several layers of the cortex were absent in the brain tissue of 10 of 11 (91%) children with autism and in 1 of 11 (9%) control children. The areas of disorganization were seen in multiple cortical layers, with most abnormal expression noted in layers 4 and 5 and focal disruption of cortical laminar architecture as patches that were 5-7 mm long.
The research was supported by grants from a variety of nonprofit organizations. Tissue for the study was provided by the National Institute of Child Health and Human Development’s Brain and Tissue Bank for Developmental Disorders, the Brain and Tissue Bank for Developmental Disorders, the Autism Tissue Program, and the Harvard Brain Tissue Resource Center.
On Twitter @maryjodales
Autism may begin in utero, based on a study of postmortem brain tissue from children with and without autism.
Patchy defects were noted in the six cellular layers of the cortex from 10 of 11 children with autism and from 1 of 11 unaffected children, based on gene expression studies performed on postmortem brain tissue from children aged 2-15 years at death. Abnormal laminar cytoarchitecture and cortical disorganization of neurons, but not of glia, were noted, according to Rich Stoner, Ph.D., of the Autism Center of Excellence at the University of California, San Diego, and his colleagues.
The findings, published in the New England Journal of Medicine, imply that layer formation and layer-specific neuronal differentiation are dysregulated during prenatal development.
The study results suggest that early recognition and treatment of autism may allow the developing brains of autistic children to construct alternative brain pathways around the patchy defects in the cortex. The result could be improved social functioning and communication, the researchers suggested (N. Engl. J. Med. 2014;370:1209-19 [doi:10.1056/NEJMoa1307491]).
In their study, Dr. Stoner and his colleagues used gene expression to examine cellular markers in each of the cortical layers as well as genes that are associated with autism. Markers for several layers of the cortex were absent in the brain tissue of 10 of 11 (91%) children with autism and in 1 of 11 (9%) control children. The areas of disorganization were seen in multiple cortical layers, with most abnormal expression noted in layers 4 and 5 and focal disruption of cortical laminar architecture as patches that were 5-7 mm long.
The research was supported by grants from a variety of nonprofit organizations. Tissue for the study was provided by the National Institute of Child Health and Human Development’s Brain and Tissue Bank for Developmental Disorders, the Brain and Tissue Bank for Developmental Disorders, the Autism Tissue Program, and the Harvard Brain Tissue Resource Center.
On Twitter @maryjodales
Autism may begin in utero, based on a study of postmortem brain tissue from children with and without autism.
Patchy defects were noted in the six cellular layers of the cortex from 10 of 11 children with autism and from 1 of 11 unaffected children, based on gene expression studies performed on postmortem brain tissue from children aged 2-15 years at death. Abnormal laminar cytoarchitecture and cortical disorganization of neurons, but not of glia, were noted, according to Rich Stoner, Ph.D., of the Autism Center of Excellence at the University of California, San Diego, and his colleagues.
The findings, published in the New England Journal of Medicine, imply that layer formation and layer-specific neuronal differentiation are dysregulated during prenatal development.
The study results suggest that early recognition and treatment of autism may allow the developing brains of autistic children to construct alternative brain pathways around the patchy defects in the cortex. The result could be improved social functioning and communication, the researchers suggested (N. Engl. J. Med. 2014;370:1209-19 [doi:10.1056/NEJMoa1307491]).
In their study, Dr. Stoner and his colleagues used gene expression to examine cellular markers in each of the cortical layers as well as genes that are associated with autism. Markers for several layers of the cortex were absent in the brain tissue of 10 of 11 (91%) children with autism and in 1 of 11 (9%) control children. The areas of disorganization were seen in multiple cortical layers, with most abnormal expression noted in layers 4 and 5 and focal disruption of cortical laminar architecture as patches that were 5-7 mm long.
The research was supported by grants from a variety of nonprofit organizations. Tissue for the study was provided by the National Institute of Child Health and Human Development’s Brain and Tissue Bank for Developmental Disorders, the Brain and Tissue Bank for Developmental Disorders, the Autism Tissue Program, and the Harvard Brain Tissue Resource Center.
On Twitter @maryjodales
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Major finding: Cellular markers for several layers of the cortex were absent from brain tissue in 91% of the children with autism and in 9% of controls.
Data source: Postmortem brain tissue from 11 children with autism and 11 children without autism.
Disclosures: The research was supported by grants from a variety of nonprofit organizations. Tissue for the study was provided by the National Institute of Child Health and Human Development’s Brain and Tissue Bank for Developmental Disorders, the Brain and Tissue Bank for Developmental Disorders, the Autism Tissue Program, and the Harvard Brain Tissue Resource Center.
Callous-unemotional traits may reflect risk in child with conduct disorder
Evaluating callous-unemotional traits may be a consideration for youths with conduct problems.
Amygdala hypoactivation in response to fearful expressions predicted test results indicative of proactive aggression in adolescents with conduct disorders and elevated levels of callous-unemotional traits, according to Leah M. Lozier and her colleagues at Georgetown University in Washington.
The findings, noted in a study of youth with and without conduct disorders, suggest that proactive aggression may result from dysfunctional empathic responses to victim distress.
"To our knowledge, no previous study has simultaneously modeled CU [callous-unemotional] traits and externalizing behaviors in response to fearful expressions and compared the efficacy of this technique to that of group-based analysis. In addition, no prior study has linked the resulting patterns of neural activation to aggressive behavior," the researchers reported in an article published online March 26 in JAMA Psychiatry. (doi:10.1001/jamapsychiatry.2013.4540).
The researchers performed functional MRI scans on 16 healthy controls and on 30 youths with conduct problems, 16 with low CU traits and 14 with high CU traits. Aggressive behavior was assessed via the youth-completed Reactive-Proactive Aggression Questionnaire. Traits were measured based on scores on the Inventory of Callous-Unemotional Traits. Conduct problems were diagnosed based on the Strengths and Difficulties Questionnaire and the Child Behavior Checklist. The tests were performed in all study participants.
During scanning, participants completed an implicit face emotion processing task that involved the use of stimulus images of 10 men and women from the Pictures of Facial Affect series, which display positive-neutral, fearful, and angry expressions.
Based on a multiple-regression analysis across the full sample, responses in the right amygdala were positively associated with externalizing behaviors (x = 24, y = 0, z = -14; k = 8) and negatively associated with CU traits (x = 26, y = 0, z = -12; k = 1).
A second analysis restricted to youths with conduct problems found similar results: Right amygdala activity was positively associated with externalizing behavior (x = 26, y = -4, z = -12; k = 47) and negatively associated with CU traits (x = 26, y = 0, z = -12; k = 1).
The patterns of results were limited to responses to fearful expressions, and not to full-intensity angry expressions and neutral expressions, the researchers said.
They reported no financial disclosures.
On Twitter @maryjodales
Evaluating callous-unemotional traits may be a consideration for youths with conduct problems.
Amygdala hypoactivation in response to fearful expressions predicted test results indicative of proactive aggression in adolescents with conduct disorders and elevated levels of callous-unemotional traits, according to Leah M. Lozier and her colleagues at Georgetown University in Washington.
The findings, noted in a study of youth with and without conduct disorders, suggest that proactive aggression may result from dysfunctional empathic responses to victim distress.
"To our knowledge, no previous study has simultaneously modeled CU [callous-unemotional] traits and externalizing behaviors in response to fearful expressions and compared the efficacy of this technique to that of group-based analysis. In addition, no prior study has linked the resulting patterns of neural activation to aggressive behavior," the researchers reported in an article published online March 26 in JAMA Psychiatry. (doi:10.1001/jamapsychiatry.2013.4540).
The researchers performed functional MRI scans on 16 healthy controls and on 30 youths with conduct problems, 16 with low CU traits and 14 with high CU traits. Aggressive behavior was assessed via the youth-completed Reactive-Proactive Aggression Questionnaire. Traits were measured based on scores on the Inventory of Callous-Unemotional Traits. Conduct problems were diagnosed based on the Strengths and Difficulties Questionnaire and the Child Behavior Checklist. The tests were performed in all study participants.
During scanning, participants completed an implicit face emotion processing task that involved the use of stimulus images of 10 men and women from the Pictures of Facial Affect series, which display positive-neutral, fearful, and angry expressions.
Based on a multiple-regression analysis across the full sample, responses in the right amygdala were positively associated with externalizing behaviors (x = 24, y = 0, z = -14; k = 8) and negatively associated with CU traits (x = 26, y = 0, z = -12; k = 1).
A second analysis restricted to youths with conduct problems found similar results: Right amygdala activity was positively associated with externalizing behavior (x = 26, y = -4, z = -12; k = 47) and negatively associated with CU traits (x = 26, y = 0, z = -12; k = 1).
The patterns of results were limited to responses to fearful expressions, and not to full-intensity angry expressions and neutral expressions, the researchers said.
They reported no financial disclosures.
On Twitter @maryjodales
Evaluating callous-unemotional traits may be a consideration for youths with conduct problems.
Amygdala hypoactivation in response to fearful expressions predicted test results indicative of proactive aggression in adolescents with conduct disorders and elevated levels of callous-unemotional traits, according to Leah M. Lozier and her colleagues at Georgetown University in Washington.
The findings, noted in a study of youth with and without conduct disorders, suggest that proactive aggression may result from dysfunctional empathic responses to victim distress.
"To our knowledge, no previous study has simultaneously modeled CU [callous-unemotional] traits and externalizing behaviors in response to fearful expressions and compared the efficacy of this technique to that of group-based analysis. In addition, no prior study has linked the resulting patterns of neural activation to aggressive behavior," the researchers reported in an article published online March 26 in JAMA Psychiatry. (doi:10.1001/jamapsychiatry.2013.4540).
The researchers performed functional MRI scans on 16 healthy controls and on 30 youths with conduct problems, 16 with low CU traits and 14 with high CU traits. Aggressive behavior was assessed via the youth-completed Reactive-Proactive Aggression Questionnaire. Traits were measured based on scores on the Inventory of Callous-Unemotional Traits. Conduct problems were diagnosed based on the Strengths and Difficulties Questionnaire and the Child Behavior Checklist. The tests were performed in all study participants.
During scanning, participants completed an implicit face emotion processing task that involved the use of stimulus images of 10 men and women from the Pictures of Facial Affect series, which display positive-neutral, fearful, and angry expressions.
Based on a multiple-regression analysis across the full sample, responses in the right amygdala were positively associated with externalizing behaviors (x = 24, y = 0, z = -14; k = 8) and negatively associated with CU traits (x = 26, y = 0, z = -12; k = 1).
A second analysis restricted to youths with conduct problems found similar results: Right amygdala activity was positively associated with externalizing behavior (x = 26, y = -4, z = -12; k = 47) and negatively associated with CU traits (x = 26, y = 0, z = -12; k = 1).
The patterns of results were limited to responses to fearful expressions, and not to full-intensity angry expressions and neutral expressions, the researchers said.
They reported no financial disclosures.
On Twitter @maryjodales
FROM JAMA PSYCHIATRY
Major finding: In youth with conduct disorders, right amygdala activity was positively associated with externalizing behavior (x = 26, y = -4, z = -12; k = 47) and negatively associated with callous-unemotional traits (x = 26, y = 0, z = -12; k = 11).
Data source: Functional MRI scans and test results in 16 healthy control participants and in 30 youths with conduct problems, 16 with low and 14 with high callous-emotional traits.
Disclosures: The researchers reported no financial disclosures.