User login
Glenoid Damage From Articular Protrusion of Metal Suture Anchor After Arthroscopic Rotator Cuff Repair
Complications with the use of anchor screws in shoulder surgery have been well-documented1,2 and can be divided into 3 categories: insertion (eg, incomplete seating, inadequate insertion, and migration), biologic (eg, large tacks producing synovitis and bone reaction), and, less commonly, mechanical (eg, intra- and extra-articular bone pull-out with migration) complications.
Prominent hardware, including suture anchors, as a cause of arthritis and joint damage has been well-documented in shoulder surgery.3,4 For example, anchors placed on the glenoid rim have been implicated in severe cartilage loss if they protrude above the level of the glenoid rim.3 However, to the authors’ knowledge, prominent anchor placement after rotator cuff repair has not been reported as a cause of arthritis unless the anchor dislodges into the glenohumeral joint. The authors present a case in which a suture anchor used for rotator cuff repair protruded through the humeral head, resulting in glenohumeral arthritis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 61-year-old woman presented with complaints of persistent right shoulder pain for 5 months after a fall from a bicycle. She had taken nonsteroidal anti-inflammatory medication without pain relief. On presentation, she had no atrophy or deformity, was neurologically intact for sensation and reflexes, and had full range of motion (ROM) but a painful arc. She had tenderness over the greater tuberosity and positive Neer and Hawkins-Kennedy impingement signs. She had pain but no weakness to resisted abduction or to resisted external rotation with the arms at the sides.
Preoperative conventional radiographs of the shoulder were normal. A gadolinium-enhanced magnetic resonance arthrogram showed a high-grade articular partial tear of the supraspinatus, which was judged to be at least two-thirds of the tendon width. Because nonoperative methods had failed, the patient elected operative intervention for this tear.
Diagnostic arthroscopy (with the patient in a lateral decubitus position) showed a normal joint except for a high-grade, 8×8-mm, greater than 6 mm deep, partial tear of the articular side of the supraspinatus tendon. The subacromial space had moderate to severe bursal tissue inflammation but no full-thickness component to the rotator cuff tear. A bursectomy, coracoacromial ligament release, and partial anterolateral acromioplasty were performed.
A transtendinous technique was used to repair this high-grade tear. For an anatomically rigid repair, we used 3 suture anchors with a straight configuration because each metal anchor has only 1 suture. According to the standard arthroscopic transtendinous repair technique, the suture anchors were placed through the rotator cuff tendon (at the lateral articular margin at the medial extent of the footprint) after localization of the angle with a spinal needle. A shuttle relay was used to pass the sutures, and the knot was pulled into the subacromial space, cinching the rotator cuff on top of the suture anchors and reestablishing the contact of the tendon to the footprint. We used two 2.4-mm FASTak suture anchors (Arthrex, Naples, Florida) and one 3.5-mm Corkscrew suture anchor (Arthrex). This process was repeated for the remaining suture limbs. The placement of the suture anchors adequately reduced the articular part of the cuff to the footprint.
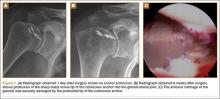
After surgery, the patient had no complications, and radiographs taken the next day suggested no abnormalities (Figure 1A). The shoulder was immobilized for 4 weeks after surgery, and passive, gentle ROM exercise was supervised by a physical therapist twice a week during this period. After the first 4 weeks, an active ROM program was begun. However, shortly after initiating motion in the shoulder, the patient complained of a recurrence of pain that she described as a sharp and grinding sensation.
The patient was reevaluated 8 weeks after surgery. Her pain was worsening, and she was having difficulty regaining ROM. Conventional radiographs showed the tip of the metal anchor protruding through the articular cartilage of the humeral head (Figure 1B). The patient was informed of the findings, and immediate surgery was performed to remove the anchor.
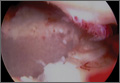
Arthroscopic examination showed extensive damage to the glenoid cartilage (Figure 1C) and an intra-articularly intact rotator cuff repair. The cartilage damage was located in the posterior and inferior half of the glenoid, which is related to the forward flexion of the arm; the depth of the cartilage defect was approximately 2 mm. Under the image intensifier, an empty suture anchor driver was inserted into the previous screw insertion hole, and the anchor was screwed back out and removed.
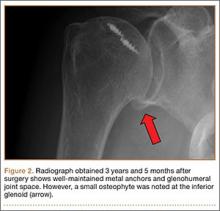
After surgery, the patient’s arm was placed in a sling, and an ROM program began 4 weeks later. The sensation of grinding was eliminated, and her pain gradually improved. Three years after surgery, she had no pain, no weakness, and full ROM without limitations (Figure 2).
Discussion
Protrusion and migration of suture anchors in shoulder surgery has been documented extensively.3,4 Zuckerman and Matsen4 divided these complications into 4 groups: (1) incorrect placement, (2) migration after placement, (3) loosening, and (4) device breakage. These complications may be frequently related to surgical technique, and all these studies describe backward migration of the anchor out of the drill hole. In the current case, the anchor tip penetrated the articular surface of the humeral head, not because of anchor migration but because the anchor was inserted too far. To the authors’ knowledge, there is only 1 reported case of anchor protrusion through the humeral head; it involved a different type of anchor insertion system.5 In that case, there was only mild cartilage damage to the glenoid, and the patient recovered after removal of the anchors.
Several factors contributed to the improper insertion of the anchor in the current patient. First, repairing a high-grade articular side defect or partial articular supraspinatus tendon avulsion lesion can be technically challenging because rotator cuff tissue obscures the view when inserting the anchor. Second, the anchor was inserted too medially on the greater tuberosity, which made the distance from the tuberosity to the joint shorter. Wong and colleagues5 performed an analysis of the angle of insertion that would be safe using a PEEK PushLock SP system (Arthrex), but they emphasized that the angle depends on the configuration of the particular insertion system. The current case also shows that the surgeon should be cognizant of the fact that penetration of the humeral head by the anchor can occur if the surgeon is unaware of the distance from the anchor to the laser line on the insertion device or of the distance from the tuberosity to the articular surface of the humeral head.
The current case also shows that the type of anchor and delivery system may contribute to this complication. Double-loaded suture anchors can decrease the number of anchors needed for secure fixation. Bioabsorbable anchors can be used for this purpose, but they may be technically more difficult to use for repairing partial tears of the rotator cuff. Better visualization of the laser line on the anchor may be facilitated by using a probe from an anterior portal to hold the cuff up while the anchor is inserted.
This case has shown the importance of obtaining postoperative radiographic studies in patients who have metal anchors placed during shoulder surgery, especially if they complain of continued pain, new pain, crepitus, or grinding. When conventional radiography is insufficient for locating the anchor or its proximity to the joint line, computed tomography can be helpful.1
Conclusion
Removing failed suture anchors can be challenging, especially when they protrude into the joint on the humeral side.1,6 The best way to prevent this complication is through careful technique. The anchors should not be inserted beyond the depth of the laser line on the anchors, and every attempt should be made to make sure the laser line is visible at the time of anchor insertion. Postoperative radiographs should be considered for patients with metal anchors in the shoulder, especially if the patient continues to have symptoms or develops new symptoms in the shoulder after surgery.
1. Park HB, Keyurapan E, Gill HS, Selhi HS, McFarland EG. Suture anchors and tacks for shoulder surgery. Part II: The prevention and treatment of complications. Am J Sports Med. 2006;34(1):136-144.
2. McFarland EG, Park HB, Keyurapan E, Gill HS, Selhi HS. Suture anchors and tacks for shoulder surgery. Part I: Biology and biomechanics. Am J Sports Med. 2005;33(12):1918-1923.
3. Rhee YG, Lee DH, Chun IH, Bae SC. Glenohumeral arthropathy after arthroscopic anterior shoulder stabilization. Arthroscopy. 2004;20(4):402-406.
4. Zuckerman JD, Matsen FA III. Complications about the glenohumeral joint related to the use of screws and staples. J Bone Joint Surg Am. 1984;66(2):175-180.
5. Wong AS, Kokkalis ZT, Schmidt CC. Proper insertion angle is essential to prevent intra-articular protrusion of a knotless suture anchor in shoulder rotator cuff repair. Arthroscopy. 2010;26(2):286-290.
6. Grutter PW, McFarland EG, Zikria BA, Dai Z, Petersen SA. Techniques for suture anchor removal in shoulder surgery. Am J Sports Med. 2010;38(8):1706-1710.
Complications with the use of anchor screws in shoulder surgery have been well-documented1,2 and can be divided into 3 categories: insertion (eg, incomplete seating, inadequate insertion, and migration), biologic (eg, large tacks producing synovitis and bone reaction), and, less commonly, mechanical (eg, intra- and extra-articular bone pull-out with migration) complications.
Prominent hardware, including suture anchors, as a cause of arthritis and joint damage has been well-documented in shoulder surgery.3,4 For example, anchors placed on the glenoid rim have been implicated in severe cartilage loss if they protrude above the level of the glenoid rim.3 However, to the authors’ knowledge, prominent anchor placement after rotator cuff repair has not been reported as a cause of arthritis unless the anchor dislodges into the glenohumeral joint. The authors present a case in which a suture anchor used for rotator cuff repair protruded through the humeral head, resulting in glenohumeral arthritis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 61-year-old woman presented with complaints of persistent right shoulder pain for 5 months after a fall from a bicycle. She had taken nonsteroidal anti-inflammatory medication without pain relief. On presentation, she had no atrophy or deformity, was neurologically intact for sensation and reflexes, and had full range of motion (ROM) but a painful arc. She had tenderness over the greater tuberosity and positive Neer and Hawkins-Kennedy impingement signs. She had pain but no weakness to resisted abduction or to resisted external rotation with the arms at the sides.
Preoperative conventional radiographs of the shoulder were normal. A gadolinium-enhanced magnetic resonance arthrogram showed a high-grade articular partial tear of the supraspinatus, which was judged to be at least two-thirds of the tendon width. Because nonoperative methods had failed, the patient elected operative intervention for this tear.
Diagnostic arthroscopy (with the patient in a lateral decubitus position) showed a normal joint except for a high-grade, 8×8-mm, greater than 6 mm deep, partial tear of the articular side of the supraspinatus tendon. The subacromial space had moderate to severe bursal tissue inflammation but no full-thickness component to the rotator cuff tear. A bursectomy, coracoacromial ligament release, and partial anterolateral acromioplasty were performed.
A transtendinous technique was used to repair this high-grade tear. For an anatomically rigid repair, we used 3 suture anchors with a straight configuration because each metal anchor has only 1 suture. According to the standard arthroscopic transtendinous repair technique, the suture anchors were placed through the rotator cuff tendon (at the lateral articular margin at the medial extent of the footprint) after localization of the angle with a spinal needle. A shuttle relay was used to pass the sutures, and the knot was pulled into the subacromial space, cinching the rotator cuff on top of the suture anchors and reestablishing the contact of the tendon to the footprint. We used two 2.4-mm FASTak suture anchors (Arthrex, Naples, Florida) and one 3.5-mm Corkscrew suture anchor (Arthrex). This process was repeated for the remaining suture limbs. The placement of the suture anchors adequately reduced the articular part of the cuff to the footprint.
After surgery, the patient had no complications, and radiographs taken the next day suggested no abnormalities (Figure 1A). The shoulder was immobilized for 4 weeks after surgery, and passive, gentle ROM exercise was supervised by a physical therapist twice a week during this period. After the first 4 weeks, an active ROM program was begun. However, shortly after initiating motion in the shoulder, the patient complained of a recurrence of pain that she described as a sharp and grinding sensation.
The patient was reevaluated 8 weeks after surgery. Her pain was worsening, and she was having difficulty regaining ROM. Conventional radiographs showed the tip of the metal anchor protruding through the articular cartilage of the humeral head (Figure 1B). The patient was informed of the findings, and immediate surgery was performed to remove the anchor.
Arthroscopic examination showed extensive damage to the glenoid cartilage (Figure 1C) and an intra-articularly intact rotator cuff repair. The cartilage damage was located in the posterior and inferior half of the glenoid, which is related to the forward flexion of the arm; the depth of the cartilage defect was approximately 2 mm. Under the image intensifier, an empty suture anchor driver was inserted into the previous screw insertion hole, and the anchor was screwed back out and removed.
After surgery, the patient’s arm was placed in a sling, and an ROM program began 4 weeks later. The sensation of grinding was eliminated, and her pain gradually improved. Three years after surgery, she had no pain, no weakness, and full ROM without limitations (Figure 2).
Discussion
Protrusion and migration of suture anchors in shoulder surgery has been documented extensively.3,4 Zuckerman and Matsen4 divided these complications into 4 groups: (1) incorrect placement, (2) migration after placement, (3) loosening, and (4) device breakage. These complications may be frequently related to surgical technique, and all these studies describe backward migration of the anchor out of the drill hole. In the current case, the anchor tip penetrated the articular surface of the humeral head, not because of anchor migration but because the anchor was inserted too far. To the authors’ knowledge, there is only 1 reported case of anchor protrusion through the humeral head; it involved a different type of anchor insertion system.5 In that case, there was only mild cartilage damage to the glenoid, and the patient recovered after removal of the anchors.
Several factors contributed to the improper insertion of the anchor in the current patient. First, repairing a high-grade articular side defect or partial articular supraspinatus tendon avulsion lesion can be technically challenging because rotator cuff tissue obscures the view when inserting the anchor. Second, the anchor was inserted too medially on the greater tuberosity, which made the distance from the tuberosity to the joint shorter. Wong and colleagues5 performed an analysis of the angle of insertion that would be safe using a PEEK PushLock SP system (Arthrex), but they emphasized that the angle depends on the configuration of the particular insertion system. The current case also shows that the surgeon should be cognizant of the fact that penetration of the humeral head by the anchor can occur if the surgeon is unaware of the distance from the anchor to the laser line on the insertion device or of the distance from the tuberosity to the articular surface of the humeral head.
The current case also shows that the type of anchor and delivery system may contribute to this complication. Double-loaded suture anchors can decrease the number of anchors needed for secure fixation. Bioabsorbable anchors can be used for this purpose, but they may be technically more difficult to use for repairing partial tears of the rotator cuff. Better visualization of the laser line on the anchor may be facilitated by using a probe from an anterior portal to hold the cuff up while the anchor is inserted.
This case has shown the importance of obtaining postoperative radiographic studies in patients who have metal anchors placed during shoulder surgery, especially if they complain of continued pain, new pain, crepitus, or grinding. When conventional radiography is insufficient for locating the anchor or its proximity to the joint line, computed tomography can be helpful.1
Conclusion
Removing failed suture anchors can be challenging, especially when they protrude into the joint on the humeral side.1,6 The best way to prevent this complication is through careful technique. The anchors should not be inserted beyond the depth of the laser line on the anchors, and every attempt should be made to make sure the laser line is visible at the time of anchor insertion. Postoperative radiographs should be considered for patients with metal anchors in the shoulder, especially if the patient continues to have symptoms or develops new symptoms in the shoulder after surgery.
Complications with the use of anchor screws in shoulder surgery have been well-documented1,2 and can be divided into 3 categories: insertion (eg, incomplete seating, inadequate insertion, and migration), biologic (eg, large tacks producing synovitis and bone reaction), and, less commonly, mechanical (eg, intra- and extra-articular bone pull-out with migration) complications.
Prominent hardware, including suture anchors, as a cause of arthritis and joint damage has been well-documented in shoulder surgery.3,4 For example, anchors placed on the glenoid rim have been implicated in severe cartilage loss if they protrude above the level of the glenoid rim.3 However, to the authors’ knowledge, prominent anchor placement after rotator cuff repair has not been reported as a cause of arthritis unless the anchor dislodges into the glenohumeral joint. The authors present a case in which a suture anchor used for rotator cuff repair protruded through the humeral head, resulting in glenohumeral arthritis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 61-year-old woman presented with complaints of persistent right shoulder pain for 5 months after a fall from a bicycle. She had taken nonsteroidal anti-inflammatory medication without pain relief. On presentation, she had no atrophy or deformity, was neurologically intact for sensation and reflexes, and had full range of motion (ROM) but a painful arc. She had tenderness over the greater tuberosity and positive Neer and Hawkins-Kennedy impingement signs. She had pain but no weakness to resisted abduction or to resisted external rotation with the arms at the sides.
Preoperative conventional radiographs of the shoulder were normal. A gadolinium-enhanced magnetic resonance arthrogram showed a high-grade articular partial tear of the supraspinatus, which was judged to be at least two-thirds of the tendon width. Because nonoperative methods had failed, the patient elected operative intervention for this tear.
Diagnostic arthroscopy (with the patient in a lateral decubitus position) showed a normal joint except for a high-grade, 8×8-mm, greater than 6 mm deep, partial tear of the articular side of the supraspinatus tendon. The subacromial space had moderate to severe bursal tissue inflammation but no full-thickness component to the rotator cuff tear. A bursectomy, coracoacromial ligament release, and partial anterolateral acromioplasty were performed.
A transtendinous technique was used to repair this high-grade tear. For an anatomically rigid repair, we used 3 suture anchors with a straight configuration because each metal anchor has only 1 suture. According to the standard arthroscopic transtendinous repair technique, the suture anchors were placed through the rotator cuff tendon (at the lateral articular margin at the medial extent of the footprint) after localization of the angle with a spinal needle. A shuttle relay was used to pass the sutures, and the knot was pulled into the subacromial space, cinching the rotator cuff on top of the suture anchors and reestablishing the contact of the tendon to the footprint. We used two 2.4-mm FASTak suture anchors (Arthrex, Naples, Florida) and one 3.5-mm Corkscrew suture anchor (Arthrex). This process was repeated for the remaining suture limbs. The placement of the suture anchors adequately reduced the articular part of the cuff to the footprint.
After surgery, the patient had no complications, and radiographs taken the next day suggested no abnormalities (Figure 1A). The shoulder was immobilized for 4 weeks after surgery, and passive, gentle ROM exercise was supervised by a physical therapist twice a week during this period. After the first 4 weeks, an active ROM program was begun. However, shortly after initiating motion in the shoulder, the patient complained of a recurrence of pain that she described as a sharp and grinding sensation.
The patient was reevaluated 8 weeks after surgery. Her pain was worsening, and she was having difficulty regaining ROM. Conventional radiographs showed the tip of the metal anchor protruding through the articular cartilage of the humeral head (Figure 1B). The patient was informed of the findings, and immediate surgery was performed to remove the anchor.
Arthroscopic examination showed extensive damage to the glenoid cartilage (Figure 1C) and an intra-articularly intact rotator cuff repair. The cartilage damage was located in the posterior and inferior half of the glenoid, which is related to the forward flexion of the arm; the depth of the cartilage defect was approximately 2 mm. Under the image intensifier, an empty suture anchor driver was inserted into the previous screw insertion hole, and the anchor was screwed back out and removed.
After surgery, the patient’s arm was placed in a sling, and an ROM program began 4 weeks later. The sensation of grinding was eliminated, and her pain gradually improved. Three years after surgery, she had no pain, no weakness, and full ROM without limitations (Figure 2).
Discussion
Protrusion and migration of suture anchors in shoulder surgery has been documented extensively.3,4 Zuckerman and Matsen4 divided these complications into 4 groups: (1) incorrect placement, (2) migration after placement, (3) loosening, and (4) device breakage. These complications may be frequently related to surgical technique, and all these studies describe backward migration of the anchor out of the drill hole. In the current case, the anchor tip penetrated the articular surface of the humeral head, not because of anchor migration but because the anchor was inserted too far. To the authors’ knowledge, there is only 1 reported case of anchor protrusion through the humeral head; it involved a different type of anchor insertion system.5 In that case, there was only mild cartilage damage to the glenoid, and the patient recovered after removal of the anchors.
Several factors contributed to the improper insertion of the anchor in the current patient. First, repairing a high-grade articular side defect or partial articular supraspinatus tendon avulsion lesion can be technically challenging because rotator cuff tissue obscures the view when inserting the anchor. Second, the anchor was inserted too medially on the greater tuberosity, which made the distance from the tuberosity to the joint shorter. Wong and colleagues5 performed an analysis of the angle of insertion that would be safe using a PEEK PushLock SP system (Arthrex), but they emphasized that the angle depends on the configuration of the particular insertion system. The current case also shows that the surgeon should be cognizant of the fact that penetration of the humeral head by the anchor can occur if the surgeon is unaware of the distance from the anchor to the laser line on the insertion device or of the distance from the tuberosity to the articular surface of the humeral head.
The current case also shows that the type of anchor and delivery system may contribute to this complication. Double-loaded suture anchors can decrease the number of anchors needed for secure fixation. Bioabsorbable anchors can be used for this purpose, but they may be technically more difficult to use for repairing partial tears of the rotator cuff. Better visualization of the laser line on the anchor may be facilitated by using a probe from an anterior portal to hold the cuff up while the anchor is inserted.
This case has shown the importance of obtaining postoperative radiographic studies in patients who have metal anchors placed during shoulder surgery, especially if they complain of continued pain, new pain, crepitus, or grinding. When conventional radiography is insufficient for locating the anchor or its proximity to the joint line, computed tomography can be helpful.1
Conclusion
Removing failed suture anchors can be challenging, especially when they protrude into the joint on the humeral side.1,6 The best way to prevent this complication is through careful technique. The anchors should not be inserted beyond the depth of the laser line on the anchors, and every attempt should be made to make sure the laser line is visible at the time of anchor insertion. Postoperative radiographs should be considered for patients with metal anchors in the shoulder, especially if the patient continues to have symptoms or develops new symptoms in the shoulder after surgery.
1. Park HB, Keyurapan E, Gill HS, Selhi HS, McFarland EG. Suture anchors and tacks for shoulder surgery. Part II: The prevention and treatment of complications. Am J Sports Med. 2006;34(1):136-144.
2. McFarland EG, Park HB, Keyurapan E, Gill HS, Selhi HS. Suture anchors and tacks for shoulder surgery. Part I: Biology and biomechanics. Am J Sports Med. 2005;33(12):1918-1923.
3. Rhee YG, Lee DH, Chun IH, Bae SC. Glenohumeral arthropathy after arthroscopic anterior shoulder stabilization. Arthroscopy. 2004;20(4):402-406.
4. Zuckerman JD, Matsen FA III. Complications about the glenohumeral joint related to the use of screws and staples. J Bone Joint Surg Am. 1984;66(2):175-180.
5. Wong AS, Kokkalis ZT, Schmidt CC. Proper insertion angle is essential to prevent intra-articular protrusion of a knotless suture anchor in shoulder rotator cuff repair. Arthroscopy. 2010;26(2):286-290.
6. Grutter PW, McFarland EG, Zikria BA, Dai Z, Petersen SA. Techniques for suture anchor removal in shoulder surgery. Am J Sports Med. 2010;38(8):1706-1710.
1. Park HB, Keyurapan E, Gill HS, Selhi HS, McFarland EG. Suture anchors and tacks for shoulder surgery. Part II: The prevention and treatment of complications. Am J Sports Med. 2006;34(1):136-144.
2. McFarland EG, Park HB, Keyurapan E, Gill HS, Selhi HS. Suture anchors and tacks for shoulder surgery. Part I: Biology and biomechanics. Am J Sports Med. 2005;33(12):1918-1923.
3. Rhee YG, Lee DH, Chun IH, Bae SC. Glenohumeral arthropathy after arthroscopic anterior shoulder stabilization. Arthroscopy. 2004;20(4):402-406.
4. Zuckerman JD, Matsen FA III. Complications about the glenohumeral joint related to the use of screws and staples. J Bone Joint Surg Am. 1984;66(2):175-180.
5. Wong AS, Kokkalis ZT, Schmidt CC. Proper insertion angle is essential to prevent intra-articular protrusion of a knotless suture anchor in shoulder rotator cuff repair. Arthroscopy. 2010;26(2):286-290.
6. Grutter PW, McFarland EG, Zikria BA, Dai Z, Petersen SA. Techniques for suture anchor removal in shoulder surgery. Am J Sports Med. 2010;38(8):1706-1710.
Cutaneous Burn Caused by Radiofrequency Ablation Probe During Shoulder Arthroscopy
Cautery and radiofrequency ablation (RFA) devices are commonly used in shoulder arthroscopic surgery for hemostasis and ablation of soft tissue. Although these devices are easily used and applied, complications (eg, extensive release of deltoid muscle,1 nerve damage,2 tendon damage,3 cartilage damage from heat transfer4) can occur during arthroscopic surgery. Radiofrequency devices can elevate fluid temperatures to unsafe levels and directly or indirectly injure surrounding tissue.5,6 Skin complications from using these devices include direct burns to the subcutaneous tissues from the joint to the skin surface7 and skin burns related to overheated arthroscopic fluid.8
In our English-language literature review, however, we found no report of a skin burn secondary to contact between a RFA device and a spinal needle used in identifying structures during an arthroscopic acromioplasty. We report such a case here. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old woman injured her left, nondominant shoulder when a descending garage door hit her directly on the superior aspect of the shoulder. She had immediate onset of pain on the top and lateral side of the shoulder and was evaluated by a primary care physician. Radiographs and magnetic resonance imaging (MRI) were normal. The patient was referred to an orthopedic surgeon for further evaluation.
The orthopedic surgeon found her to be in good health, with no history of diabetes, vascular conditions, or skin disorders. The initial diagnosis after history taking and physical examination was impingement syndrome with subacromial bursitis. The surgeon recommended nonoperative treatment: ice, nonsteroidal anti-inflammatory drugs, and physical therapy. After 3 months, the patient’s examination was unchanged, and there was no improvement in pain. Cortisone injected into the subacromial space helped for a few weeks, but the pain returned. After 2 more cortisone injections over 9 months failed, repeat MRI showed no tears of the rotator cuff or any other salient abnormalities. The treatment options were discussed with the patient, and, because the physical examination findings were consistent with impingement syndrome and nonoperative measures had failed, she consented to arthroscopic evaluation of the shoulder and arthroscopic partial anterior-lateral acromioplasty.
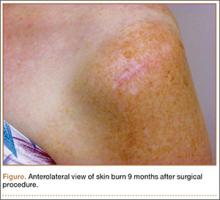
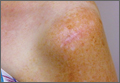
The procedure was performed 8 months after initial injury. With the patient under general anesthesia and in a lateral decubitus position, her arm was placed in an arm holder. Before the partial acromioplasty, two 18-gauge spinal needles were inserted from the skin surface into the subacromial space to help localize the anterolateral acromion and the acromioclavicular joint. The procedure was performed with a pump using saline bags kept at room temperature. A bipolar radiofrequency device (Stryker Energy Radiofrequency Ablation System; Stryker, Mahwah, New Jersey) was used to débride the subacromial bursa and the periosteum of the undersurface of the acromion. While the bursa was being débrided, the radiofrequency device inadvertently touched the anterior lateral needle probe, and a small skin burn formed around the needle on the surface of the shoulder (Figure). The radiofrequency device did not directly contact the skin, and the deltoid fascia was intact. The spinal needle was removed, and the skin around the burn was excised; the muscle beneath the skin was intact and showed no signs of thermal damage. The skin was mobilized and closed with interrupted simple sutures using a 4-0 nylon suture. The procedure was then completed with no other complications.
After surgery, the patient recovered without complications, and the skin lesion healed with no signs of infection and no skin or muscle defects. Some stiffness was treated with medication and physical therapy. Nine months after surgery, the patient reported mild shoulder stiffness and remained dissatisfied with the appearance of the skin in the area of the burn.
Discussion
Our patient’s case is a reminder that contact between a radiofrequency device and metal needles can transfer heat to tissues and cause skin burns. When using a radiofrequency device around metal needles or cannulas, surgeons should be sure to avoid prolonged contact with the metal. Our patient’s case is the first reported case of a thermal skin injury occurring when a spinal needle was heated by an arthroscopic ablater.
Other authors have reported indirect thermal skin injuries caused by radiofrequency devices during arthroscopic surgery, but the causes were postulated to be direct contact between device and skin7 and overheating of the arthroscopy fluid.5,6,8 Huang and colleagues8 reported that full-thickness skin burns occurred when normal saline used during routine knee arthroscopy overheated from use of a radiofrequency device. Burn lesions, noted on their patient’s leg within 1 day after surgery, required subsequent débridement, a muscle flap, and split-skin grafting. Skin burns caused by overheated fluid have occurred irrespective of type of fluid used (eg, 1.5% glycine or lactated Ringer solution).6 There was no evidence that our patient’s burn resulted from extravasated overheated fluid, as the lesion was localized to the area immediately around the needle and was not geographic, as was described by Huang and colleagues.8
Other possible causes of skin burns during arthroscopic surgery have been described, but none applies in our patient’s case. Segami and colleagues7 described a burn resulting from direct transfer of heat from the radiofrequency device to the skin because of their proximity. This mechanism was not the cause in our patient’s case; there was no evidence of a defect or burned deltoid muscle at time of surgery. Lau and Dao9 reported 2 small full-thickness skin burns caused by a fiberoptic-light cable tip placed on a patient’s leg; in addition, the hot (>170°C) cables caused the paper drapes to combust.9 Skin burns secondary to use of skin antiseptics have been reported,10 but such lesions typically are located beneath tourniquets or in areas of friction from surgical drapes. In some cases, lesions described as skin burns may actually have been pressure lesions secondary to moist skin and friction.11
Whether type of radiofrequency device contributes to the occurrence of heat-related lesions during arthroscopic surgery is unknown. Some investigators have suggested there is more potential for harm with bipolar RFA devices than with monopolar devices.12,13 Monopolar devices pass energy between a probe and a grounding plate, whereas bipolar devices pass energy through 2 points on the probe.14 Because the heat for the monopolar probe derives from the frictional resistance of tissues to each other rather than from the probe itself, the bipolar probe theoretically allows for better temperature control. In addition, bipolar probes require less current to achieve the same heating effect. However, recent studies have suggested that, compared with monopolar radiofrequency devices, bipolar radiofrequency devices are associated with larger increases in temperature at equal depths after an equal number of applications.12,13
To our knowledge, no one has specifically investigated the type of bipolar device used in the present case. This case report, the first to describe a thermal skin injury caused by direct contact between a radiofrequency device and a metal needle inserted in the skin, is a reminder that contact between radiofrequency devices and spinal needles or other metal cannulas used in arthroscopic surgery should be avoided.
1. Bonsell S. Detached deltoid during arthroscopic subacromial decompression. Arthroscopy. 2000;16(7):745-748.
2. Mohammed KD, Hayes MG, Saies AD. Unusual complications of shoulder arthroscopy. J Shoulder Elbow Surg. 2000;9(4):350-353.
3. Pell RF 4th, Uhl RL. Complications of thermal ablation in wrist arthroscopy. Arthroscopy. 2004;20(suppl 2):84-86.
4. Lu Y, Hayashi K, Hecht P, et al. The effect of monopolar radiofrequency energy on partial-thickness defects of articular cartilage. Arthroscopy. 2000;16(5):527-536.
5. Kouk SN, Zoric B, Stetson WB. Complication of the use of a radiofrequency device in arthroscopic shoulder surgery: second-degree burn of the shoulder girdle. Arthroscopy. 2011;27(1):136-141.
6. Lord MJ, Maltry JA, Shall LM. Thermal injury resulting from arthroscopic lateral retinacular release by electrocautery: report of three cases and a review of the literature. Arthroscopy. 1991;7(1):33-37.
7. Segami N, Yamada T, Nishimura M. Thermal injury during temporomandibular joint arthroscopy: a case report. J Oral Maxillofac Surg. 2004;62(4):508-510.
8. Huang S, Gateley D, Moss ALH. Accidental burn injury during knee arthroscopy. Arthroscopy. 2007;23(12):1363.e1-e3.
9. Lau YJ, Dao Q. Cutaneous burns from a fiberoptic cable tip during arthroscopy of the knee. Knee. 2008;15(4):333-335.
10. Sanders TH, Hawken SM. Chlorhexidine burns after shoulder arthroscopy. Am J Orthop. 2012;41(4):172-174.
11. Keyurapan E, Hu SJ, Redett R, McCarthy EF, McFarland EG. Pressure ulcers of the thorax after shoulder surgery. Knee Surg Sports Traumatol Arthrosc. 2007;15(12):1489-1493.
12. Edwards RB 3rd, Lu Y, Rodriguez E, Markel MD. Thermometric determination of cartilage matrix temperatures during thermal chondroplasty: comparison of bipolar and monopolar radiofrequency devices. Arthroscopy. 2002;18(4):339-346.
13. Figueroa D, Calvo R, Vaisman A, et al. Bipolar radiofrequency in the human meniscus. Comparative study between patients younger and older than 40 years of age. Knee. 2007;14(5):357-360.
14. Sahasrabudhe A, McMahon PJ. Thermal probes: what’s available in 2004. Oper Tech Sports Med. 2004;12:206-209.
Cautery and radiofrequency ablation (RFA) devices are commonly used in shoulder arthroscopic surgery for hemostasis and ablation of soft tissue. Although these devices are easily used and applied, complications (eg, extensive release of deltoid muscle,1 nerve damage,2 tendon damage,3 cartilage damage from heat transfer4) can occur during arthroscopic surgery. Radiofrequency devices can elevate fluid temperatures to unsafe levels and directly or indirectly injure surrounding tissue.5,6 Skin complications from using these devices include direct burns to the subcutaneous tissues from the joint to the skin surface7 and skin burns related to overheated arthroscopic fluid.8
In our English-language literature review, however, we found no report of a skin burn secondary to contact between a RFA device and a spinal needle used in identifying structures during an arthroscopic acromioplasty. We report such a case here. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old woman injured her left, nondominant shoulder when a descending garage door hit her directly on the superior aspect of the shoulder. She had immediate onset of pain on the top and lateral side of the shoulder and was evaluated by a primary care physician. Radiographs and magnetic resonance imaging (MRI) were normal. The patient was referred to an orthopedic surgeon for further evaluation.
The orthopedic surgeon found her to be in good health, with no history of diabetes, vascular conditions, or skin disorders. The initial diagnosis after history taking and physical examination was impingement syndrome with subacromial bursitis. The surgeon recommended nonoperative treatment: ice, nonsteroidal anti-inflammatory drugs, and physical therapy. After 3 months, the patient’s examination was unchanged, and there was no improvement in pain. Cortisone injected into the subacromial space helped for a few weeks, but the pain returned. After 2 more cortisone injections over 9 months failed, repeat MRI showed no tears of the rotator cuff or any other salient abnormalities. The treatment options were discussed with the patient, and, because the physical examination findings were consistent with impingement syndrome and nonoperative measures had failed, she consented to arthroscopic evaluation of the shoulder and arthroscopic partial anterior-lateral acromioplasty.
The procedure was performed 8 months after initial injury. With the patient under general anesthesia and in a lateral decubitus position, her arm was placed in an arm holder. Before the partial acromioplasty, two 18-gauge spinal needles were inserted from the skin surface into the subacromial space to help localize the anterolateral acromion and the acromioclavicular joint. The procedure was performed with a pump using saline bags kept at room temperature. A bipolar radiofrequency device (Stryker Energy Radiofrequency Ablation System; Stryker, Mahwah, New Jersey) was used to débride the subacromial bursa and the periosteum of the undersurface of the acromion. While the bursa was being débrided, the radiofrequency device inadvertently touched the anterior lateral needle probe, and a small skin burn formed around the needle on the surface of the shoulder (Figure). The radiofrequency device did not directly contact the skin, and the deltoid fascia was intact. The spinal needle was removed, and the skin around the burn was excised; the muscle beneath the skin was intact and showed no signs of thermal damage. The skin was mobilized and closed with interrupted simple sutures using a 4-0 nylon suture. The procedure was then completed with no other complications.
After surgery, the patient recovered without complications, and the skin lesion healed with no signs of infection and no skin or muscle defects. Some stiffness was treated with medication and physical therapy. Nine months after surgery, the patient reported mild shoulder stiffness and remained dissatisfied with the appearance of the skin in the area of the burn.
Discussion
Our patient’s case is a reminder that contact between a radiofrequency device and metal needles can transfer heat to tissues and cause skin burns. When using a radiofrequency device around metal needles or cannulas, surgeons should be sure to avoid prolonged contact with the metal. Our patient’s case is the first reported case of a thermal skin injury occurring when a spinal needle was heated by an arthroscopic ablater.
Other authors have reported indirect thermal skin injuries caused by radiofrequency devices during arthroscopic surgery, but the causes were postulated to be direct contact between device and skin7 and overheating of the arthroscopy fluid.5,6,8 Huang and colleagues8 reported that full-thickness skin burns occurred when normal saline used during routine knee arthroscopy overheated from use of a radiofrequency device. Burn lesions, noted on their patient’s leg within 1 day after surgery, required subsequent débridement, a muscle flap, and split-skin grafting. Skin burns caused by overheated fluid have occurred irrespective of type of fluid used (eg, 1.5% glycine or lactated Ringer solution).6 There was no evidence that our patient’s burn resulted from extravasated overheated fluid, as the lesion was localized to the area immediately around the needle and was not geographic, as was described by Huang and colleagues.8
Other possible causes of skin burns during arthroscopic surgery have been described, but none applies in our patient’s case. Segami and colleagues7 described a burn resulting from direct transfer of heat from the radiofrequency device to the skin because of their proximity. This mechanism was not the cause in our patient’s case; there was no evidence of a defect or burned deltoid muscle at time of surgery. Lau and Dao9 reported 2 small full-thickness skin burns caused by a fiberoptic-light cable tip placed on a patient’s leg; in addition, the hot (>170°C) cables caused the paper drapes to combust.9 Skin burns secondary to use of skin antiseptics have been reported,10 but such lesions typically are located beneath tourniquets or in areas of friction from surgical drapes. In some cases, lesions described as skin burns may actually have been pressure lesions secondary to moist skin and friction.11
Whether type of radiofrequency device contributes to the occurrence of heat-related lesions during arthroscopic surgery is unknown. Some investigators have suggested there is more potential for harm with bipolar RFA devices than with monopolar devices.12,13 Monopolar devices pass energy between a probe and a grounding plate, whereas bipolar devices pass energy through 2 points on the probe.14 Because the heat for the monopolar probe derives from the frictional resistance of tissues to each other rather than from the probe itself, the bipolar probe theoretically allows for better temperature control. In addition, bipolar probes require less current to achieve the same heating effect. However, recent studies have suggested that, compared with monopolar radiofrequency devices, bipolar radiofrequency devices are associated with larger increases in temperature at equal depths after an equal number of applications.12,13
To our knowledge, no one has specifically investigated the type of bipolar device used in the present case. This case report, the first to describe a thermal skin injury caused by direct contact between a radiofrequency device and a metal needle inserted in the skin, is a reminder that contact between radiofrequency devices and spinal needles or other metal cannulas used in arthroscopic surgery should be avoided.
Cautery and radiofrequency ablation (RFA) devices are commonly used in shoulder arthroscopic surgery for hemostasis and ablation of soft tissue. Although these devices are easily used and applied, complications (eg, extensive release of deltoid muscle,1 nerve damage,2 tendon damage,3 cartilage damage from heat transfer4) can occur during arthroscopic surgery. Radiofrequency devices can elevate fluid temperatures to unsafe levels and directly or indirectly injure surrounding tissue.5,6 Skin complications from using these devices include direct burns to the subcutaneous tissues from the joint to the skin surface7 and skin burns related to overheated arthroscopic fluid.8
In our English-language literature review, however, we found no report of a skin burn secondary to contact between a RFA device and a spinal needle used in identifying structures during an arthroscopic acromioplasty. We report such a case here. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old woman injured her left, nondominant shoulder when a descending garage door hit her directly on the superior aspect of the shoulder. She had immediate onset of pain on the top and lateral side of the shoulder and was evaluated by a primary care physician. Radiographs and magnetic resonance imaging (MRI) were normal. The patient was referred to an orthopedic surgeon for further evaluation.
The orthopedic surgeon found her to be in good health, with no history of diabetes, vascular conditions, or skin disorders. The initial diagnosis after history taking and physical examination was impingement syndrome with subacromial bursitis. The surgeon recommended nonoperative treatment: ice, nonsteroidal anti-inflammatory drugs, and physical therapy. After 3 months, the patient’s examination was unchanged, and there was no improvement in pain. Cortisone injected into the subacromial space helped for a few weeks, but the pain returned. After 2 more cortisone injections over 9 months failed, repeat MRI showed no tears of the rotator cuff or any other salient abnormalities. The treatment options were discussed with the patient, and, because the physical examination findings were consistent with impingement syndrome and nonoperative measures had failed, she consented to arthroscopic evaluation of the shoulder and arthroscopic partial anterior-lateral acromioplasty.
The procedure was performed 8 months after initial injury. With the patient under general anesthesia and in a lateral decubitus position, her arm was placed in an arm holder. Before the partial acromioplasty, two 18-gauge spinal needles were inserted from the skin surface into the subacromial space to help localize the anterolateral acromion and the acromioclavicular joint. The procedure was performed with a pump using saline bags kept at room temperature. A bipolar radiofrequency device (Stryker Energy Radiofrequency Ablation System; Stryker, Mahwah, New Jersey) was used to débride the subacromial bursa and the periosteum of the undersurface of the acromion. While the bursa was being débrided, the radiofrequency device inadvertently touched the anterior lateral needle probe, and a small skin burn formed around the needle on the surface of the shoulder (Figure). The radiofrequency device did not directly contact the skin, and the deltoid fascia was intact. The spinal needle was removed, and the skin around the burn was excised; the muscle beneath the skin was intact and showed no signs of thermal damage. The skin was mobilized and closed with interrupted simple sutures using a 4-0 nylon suture. The procedure was then completed with no other complications.
After surgery, the patient recovered without complications, and the skin lesion healed with no signs of infection and no skin or muscle defects. Some stiffness was treated with medication and physical therapy. Nine months after surgery, the patient reported mild shoulder stiffness and remained dissatisfied with the appearance of the skin in the area of the burn.
Discussion
Our patient’s case is a reminder that contact between a radiofrequency device and metal needles can transfer heat to tissues and cause skin burns. When using a radiofrequency device around metal needles or cannulas, surgeons should be sure to avoid prolonged contact with the metal. Our patient’s case is the first reported case of a thermal skin injury occurring when a spinal needle was heated by an arthroscopic ablater.
Other authors have reported indirect thermal skin injuries caused by radiofrequency devices during arthroscopic surgery, but the causes were postulated to be direct contact between device and skin7 and overheating of the arthroscopy fluid.5,6,8 Huang and colleagues8 reported that full-thickness skin burns occurred when normal saline used during routine knee arthroscopy overheated from use of a radiofrequency device. Burn lesions, noted on their patient’s leg within 1 day after surgery, required subsequent débridement, a muscle flap, and split-skin grafting. Skin burns caused by overheated fluid have occurred irrespective of type of fluid used (eg, 1.5% glycine or lactated Ringer solution).6 There was no evidence that our patient’s burn resulted from extravasated overheated fluid, as the lesion was localized to the area immediately around the needle and was not geographic, as was described by Huang and colleagues.8
Other possible causes of skin burns during arthroscopic surgery have been described, but none applies in our patient’s case. Segami and colleagues7 described a burn resulting from direct transfer of heat from the radiofrequency device to the skin because of their proximity. This mechanism was not the cause in our patient’s case; there was no evidence of a defect or burned deltoid muscle at time of surgery. Lau and Dao9 reported 2 small full-thickness skin burns caused by a fiberoptic-light cable tip placed on a patient’s leg; in addition, the hot (>170°C) cables caused the paper drapes to combust.9 Skin burns secondary to use of skin antiseptics have been reported,10 but such lesions typically are located beneath tourniquets or in areas of friction from surgical drapes. In some cases, lesions described as skin burns may actually have been pressure lesions secondary to moist skin and friction.11
Whether type of radiofrequency device contributes to the occurrence of heat-related lesions during arthroscopic surgery is unknown. Some investigators have suggested there is more potential for harm with bipolar RFA devices than with monopolar devices.12,13 Monopolar devices pass energy between a probe and a grounding plate, whereas bipolar devices pass energy through 2 points on the probe.14 Because the heat for the monopolar probe derives from the frictional resistance of tissues to each other rather than from the probe itself, the bipolar probe theoretically allows for better temperature control. In addition, bipolar probes require less current to achieve the same heating effect. However, recent studies have suggested that, compared with monopolar radiofrequency devices, bipolar radiofrequency devices are associated with larger increases in temperature at equal depths after an equal number of applications.12,13
To our knowledge, no one has specifically investigated the type of bipolar device used in the present case. This case report, the first to describe a thermal skin injury caused by direct contact between a radiofrequency device and a metal needle inserted in the skin, is a reminder that contact between radiofrequency devices and spinal needles or other metal cannulas used in arthroscopic surgery should be avoided.
1. Bonsell S. Detached deltoid during arthroscopic subacromial decompression. Arthroscopy. 2000;16(7):745-748.
2. Mohammed KD, Hayes MG, Saies AD. Unusual complications of shoulder arthroscopy. J Shoulder Elbow Surg. 2000;9(4):350-353.
3. Pell RF 4th, Uhl RL. Complications of thermal ablation in wrist arthroscopy. Arthroscopy. 2004;20(suppl 2):84-86.
4. Lu Y, Hayashi K, Hecht P, et al. The effect of monopolar radiofrequency energy on partial-thickness defects of articular cartilage. Arthroscopy. 2000;16(5):527-536.
5. Kouk SN, Zoric B, Stetson WB. Complication of the use of a radiofrequency device in arthroscopic shoulder surgery: second-degree burn of the shoulder girdle. Arthroscopy. 2011;27(1):136-141.
6. Lord MJ, Maltry JA, Shall LM. Thermal injury resulting from arthroscopic lateral retinacular release by electrocautery: report of three cases and a review of the literature. Arthroscopy. 1991;7(1):33-37.
7. Segami N, Yamada T, Nishimura M. Thermal injury during temporomandibular joint arthroscopy: a case report. J Oral Maxillofac Surg. 2004;62(4):508-510.
8. Huang S, Gateley D, Moss ALH. Accidental burn injury during knee arthroscopy. Arthroscopy. 2007;23(12):1363.e1-e3.
9. Lau YJ, Dao Q. Cutaneous burns from a fiberoptic cable tip during arthroscopy of the knee. Knee. 2008;15(4):333-335.
10. Sanders TH, Hawken SM. Chlorhexidine burns after shoulder arthroscopy. Am J Orthop. 2012;41(4):172-174.
11. Keyurapan E, Hu SJ, Redett R, McCarthy EF, McFarland EG. Pressure ulcers of the thorax after shoulder surgery. Knee Surg Sports Traumatol Arthrosc. 2007;15(12):1489-1493.
12. Edwards RB 3rd, Lu Y, Rodriguez E, Markel MD. Thermometric determination of cartilage matrix temperatures during thermal chondroplasty: comparison of bipolar and monopolar radiofrequency devices. Arthroscopy. 2002;18(4):339-346.
13. Figueroa D, Calvo R, Vaisman A, et al. Bipolar radiofrequency in the human meniscus. Comparative study between patients younger and older than 40 years of age. Knee. 2007;14(5):357-360.
14. Sahasrabudhe A, McMahon PJ. Thermal probes: what’s available in 2004. Oper Tech Sports Med. 2004;12:206-209.
1. Bonsell S. Detached deltoid during arthroscopic subacromial decompression. Arthroscopy. 2000;16(7):745-748.
2. Mohammed KD, Hayes MG, Saies AD. Unusual complications of shoulder arthroscopy. J Shoulder Elbow Surg. 2000;9(4):350-353.
3. Pell RF 4th, Uhl RL. Complications of thermal ablation in wrist arthroscopy. Arthroscopy. 2004;20(suppl 2):84-86.
4. Lu Y, Hayashi K, Hecht P, et al. The effect of monopolar radiofrequency energy on partial-thickness defects of articular cartilage. Arthroscopy. 2000;16(5):527-536.
5. Kouk SN, Zoric B, Stetson WB. Complication of the use of a radiofrequency device in arthroscopic shoulder surgery: second-degree burn of the shoulder girdle. Arthroscopy. 2011;27(1):136-141.
6. Lord MJ, Maltry JA, Shall LM. Thermal injury resulting from arthroscopic lateral retinacular release by electrocautery: report of three cases and a review of the literature. Arthroscopy. 1991;7(1):33-37.
7. Segami N, Yamada T, Nishimura M. Thermal injury during temporomandibular joint arthroscopy: a case report. J Oral Maxillofac Surg. 2004;62(4):508-510.
8. Huang S, Gateley D, Moss ALH. Accidental burn injury during knee arthroscopy. Arthroscopy. 2007;23(12):1363.e1-e3.
9. Lau YJ, Dao Q. Cutaneous burns from a fiberoptic cable tip during arthroscopy of the knee. Knee. 2008;15(4):333-335.
10. Sanders TH, Hawken SM. Chlorhexidine burns after shoulder arthroscopy. Am J Orthop. 2012;41(4):172-174.
11. Keyurapan E, Hu SJ, Redett R, McCarthy EF, McFarland EG. Pressure ulcers of the thorax after shoulder surgery. Knee Surg Sports Traumatol Arthrosc. 2007;15(12):1489-1493.
12. Edwards RB 3rd, Lu Y, Rodriguez E, Markel MD. Thermometric determination of cartilage matrix temperatures during thermal chondroplasty: comparison of bipolar and monopolar radiofrequency devices. Arthroscopy. 2002;18(4):339-346.
13. Figueroa D, Calvo R, Vaisman A, et al. Bipolar radiofrequency in the human meniscus. Comparative study between patients younger and older than 40 years of age. Knee. 2007;14(5):357-360.
14. Sahasrabudhe A, McMahon PJ. Thermal probes: what’s available in 2004. Oper Tech Sports Med. 2004;12:206-209.