User login
Neuromodulation for Treatment-Refractory PTSD (FULL)
Failure of fear extinction is a core feature of posttraumatic stress disorder (PTSD).1 Recently, it was confirmed that the amygdala and the orbitofrontal cortex are crucial for both fear acquisition and fear extinction.2 The amygdala was found to have neurons active only during fear acquisition, and other neurons active only during fear extinction.3 In essence, the balance of activity between these 2 neuronal populations determines whether if an incoming stimulus is feared or not feared. This balance is under the influence of several cognitive domains, including memory, reward, and executive function.
In PTSD, the equilibrium is shifted heavily toward fear acquisition. The majority of patients spontaneously regain the capacity for fear extinction over time4 or with the help of treatment.5,6 Nonetheless, some patients with severe PTSD seem unable to recover the ability of fear extinction and remain refractory to both standard and novel psychotherapeutic or psychopharmacologic treatments.7 For these patients, direct modulation of the neural activity in the amygdala may permit fear extinction. This article describes the rationale for using deep brain stimulation (DBS) and initial results from the first-ever clinical trial.
Deep Brain Stimulation
Deep brain stimulation involves inserting electrodes in precise cerebral targets and then connecting the leads to a pulse generator (similar to a pacemaker) inserted in a subclavicular pocket. The generator controls the electrical signal (amplitude, pulse width, pulse frequency) delivered to the brain target and can be adjusted with use of a noninvasive programmer. In 1997, the FDA approved DBS for patients with Parkinson disease or essential tremor. Since then, its efficacy in these movement disorders has been confirmed in several studies.8,9
The mechanism by which the small electrical pulses of DBS influence activity is not clear. Clinically, DBS functionally inhibits the activity of local neurons.10 One theory describes “frequency jamming,” a concept similar to cardiac overdrive pacing in which the resultant high-frequency neuronal signal is meaningless and discounted by the rest of the brain.11
Over the years, DBS has demonstrated a strong safety profile.12 The risks of electrode insertion are mitigated with targeting based on high-quality magnetic resonance imaging (MRI) and computed tomography (Figure). Unlike a destructive lesion, DBS is reversible, and the implanted system can be removed in its entirety. Histologic analyses have shown only a small amount of scarring around the electrode tip.13 In movement disorder treatment, clinical experience has shown that stimulation-related adverse effects (AEs) are reversible with readjustment of stimulation parameters by external programmer.14
Novel Applications of DBS
The advantageous safety profile of DBS has permitted its evaluation in the treatment of other conditions thought to have malfunctioning networks at their core—such as intractable epilepsy (in resective surgery noncandidates).15,16 Although several trials have shown promising results of using DBS for treatment-resistant depression,17 the results of pivotal sham-controlled trials have been mixed.18,19 Obsessive-compulsive disorder, on the other hand, received the FDA humanitarian device exemption designation on the basis of positive long-term results.20 In treatment-resistant depression and obsessive-compulsive disorder, functional neuroimaging has identified DBS targets.21,22 Functional MRI or positron emission tomography (PET) images can be compared at resting state, at symptomatic state, and after treatment response. Nodes hyperactive during a symptomatic state and less active after successful treatment can be targeted with high-frequency DBS to directly reduce the hyperactivity and indirectly modulate or normalize the overall function of the circuit.23
Given the functional MRI and O15 (oxygen-15) PET evidence of amygdala hyperactivity in patients with PTSD having core symptoms,24-26 the authors hypothesized that high-frequency DBS targeting of the amygdala would improve PTSD-associated hyperarousal and reexperiencing symptoms in treatment-refractory patients. Indirect data supporting this hypothesis include a correlation between amygdala hyperactivity of increased intensity and symptom severity measured with the Clinician-Administered PTSD Scale (CAPS),27 and a correlation between reduced pretreatment amygdala hyperactivity and successful cognitive-behavioral treatment.28,29
Preclinical Work
Using a rodent model in which a novel object serves as a cue reminder of foot shocks (traumatic event), the authors tested the hypothesis that amygdala DBS would reduce PTSD-like symptoms.30 When untreated rats were presented with the object in their cage a week after the initial exposure, they immediately buried the object under bedding to avoid being reminded of the shocks. In contrast, rats treated with DBS did not bury the object. In most cases, in fact, they played with it.
The authors also replicated their results but with the addition of rats treated with paroxetine.31 Using the same rodent model, they found DBS superior to paroxetine in treating PTSD-like symptoms. This study had a crossover design: DBS and sham DBS. Briefly, 20 rats received an electrode in the amygdala and were exposed to inescapable shocks in the presence of the cue object. The rats were then randomly assigned to a DBS group (10 rats) or a sham-DBS group (10 rats). After 1 week, behavioral testing showed fear extinction in the DBS group and no improvement in the sham-DBS group. Then the groups were switched: The rats originally treated with DBS received no treatment, and the rats that were originally sham-treated underwent DBS. One week later, behavioral testing showed acquisition of fear extinction in all the rats. These results suggested DBS can be effective even when delayed after establishment of fear persistence and PTSD symptoms. These results also showed that DBS effects persist even after therapy discontinuation.
Similarly, other investigators have reported that the role of the amygdala is not limited to fear acquisition; it extends to fear expression. A lesion in the amygdala can prevent fear expression even if the disruption is performed subsequent to fear-conditioning training.32 This finding is important for humans, as DBS would be initiated during the chronic phase of the disorder, after failure of less invasive treatment options, such as pharmacotherapy and psychotherapy.
Early Clinical Experience
The authors have initiated the first ever clinical trial (NCT02091843) evaluating use of DBS for PTSD and are now recruiting patients. Enrollment is limited to 6 combat veterans with disabling PTSD that has not responded to pharmacotherapy and psychotherapy. This VA-funded single-site study, being conducted at the VA Greater Los Angeles Healthcare System (VAGLAHS), was approved by the VAGLAHS Institutional Review Board and the FDA. The authors have published the 2-year trial’s protocol, which includes an active-versus-sham stimulation phase; continuous electroencephalogram monitoring; baseline and posttreatment 18FDG (fluorodeoxyglucose) PET performed during a resting state vs during investigator-guided exposure to trauma reminders; and extensive psychological and neuropsychological assessments.33 The literature includes only 1 case report on amygdala DBS.34 The authors of that report used DBS of the basolateral nucleus of the amygdala to treat a teenaged boy with severe autism and found that the therapy was safe.
As of this writing, the authors have recruited and implanted 1 patient and reported on his clinical results (including baseline PET) over the first 8 months of stimulation35 and on the electrophysiologic findings over the first year.36 After experiencing extremely severe combat PTSD refractory to pharmacotherapy and psychotherapy treatments for more than 20 years, the patient treated with DBS is now experiencing substantial symptom relief, and his CAPS score (primary outcome measure) has improved by about 40%. He has tolerated continuous stimulation without any serious DBS-related AEs for up to 16 months. Notably, he has not had a single severe combat nightmare in a year—in stark contrast to nightly combat nightmares during the 20-year period leading to the trial. Furthermore, he has not been having any episodes of severe dissociation, which had been a common disabling problem before the trial. He has taken a second trip out of the country, improved his relationships with family, and made strides (albeit limited) in pursuing additional social interactions.
Avoidance remains a major problem. He recently left his job after 7 years, because he prefers a more nature-oriented rather than people-oriented environment. In addition, his interest in intensive psychotherapy has increased, and he has been considering options for spending more time working on his therapy.
Over 15 months of treatment, the patient’s CAPS total and subscale scores have decreased—his symptoms have improved (Table).21 He has had rapid and substantial reductions in recurrence and hyperarousal symptoms but slower improvement in avoidance. Improvements in emotional reactivity would be expected to occur before any change in behavior (eg, avoidance). Patients likely must first recognize changes in emotional reactivity to events before they can engage in a cognitive process to modify learned behavioral responses to those events.
After about 9 months of treatment, all of the study patient’s symptoms were somewhat stabilized, and the authors began making gradual stimulation adjustments to the latest parameters—3.5 V, 60 µs, and 160 Hz for the right electrode and 1.5 V, 60 µs, and 160 Hz for the left electrode—using the contacts in the basolateral nucleus of the amygdala, per postoperative neuroimaging.3
After 15 to 18 months, when improvement peaked at 48% symptom reduction from baseline, the patient experienced psychiatric decompensation (depression, suicide gesture) not attributable to changes in stimulation settings and not associated with exacerbation of PTSD symptoms. Treatment team members and independent psychiatric consultants attributed the decompensation to the patient’s difficulty in changing a long-standing avoidant behavior routine, owing to severe recurrence and hyperarousal symptoms in the past. His persistent inability to overcome avoidance and isolation, despite core PTSD symptom improvement, had left him feeling worthless.
The patient remains in the study but also is participating in other medication and psychotherapy trials and is making a career change. Periodic decompensations may be part of the treatment course as patients reach a more complex and volatile phase of improvement that requires more intensive cognitive reprocessing. If this proves to be the case with other patients enrolling in the study, intensive psychotherapy that addresses cognitive and emotional PTSD symptoms may be needed once there is improvement in intrusive and hyperarousal symptoms.
Conclusion
Deep brain stimulation has been successful in treating Parkinson disease and essential tremor. Physiologically, DBS seems to inhibit specific brain regions’ dysfunctional activity stemming from a disease process. Deep brain stimulation-induced inhibition of a dysfunctional node improves clinical outcomes in movement disorders.
Given the reversibility and positive safety profile of DBS, new applications are being studied. The authors propose that DBS may benefit patients with severe treatment-refractory PTSD. Their first patient’s core PTSD symptoms have improved significantly, as expected, but as in other psychiatric DBS cases, the seriousness and chronicity of his illness may be complicating the course of recovery. The authors plan to recruit 6 patients for this early-phase safety trial.
Click here to read the digital edition.
1. Milad MR, Pitman RK, Ellis CB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66(12):1075-1082.
2. Marin MF, Song H, VanElzakker MB, et al. Association of resting metabolism in the fear neural network with extinction recall activations and clinical measures in trauma-exposed individuals. Am J Psychiatry. 2016;173(9):930-938.
3. Herry C, Ciocchi S, Senn V, Demmou L, Müller C, Lüthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454(7204):600-606.
4. Morina N, Wicherts JM, Lobbrecht J, Priebe S. Remission from post-traumatic stress disorder in adults: a systematic review and meta-analysis of long term outcome studies. Clin Psychol Rev. 2014;34(3):249-255.
5. Steenkamp MM, Litz BT, Hoge CW, Marmar CR. Psychotherapy for military-related PTSD: a review of randomized clinical trials. JAMA. 2015;314(5):489-500.
6. Hoskins M, Pearce J, Bethell A, et al. Pharmacotherapy for post-traumatic stress disorder: systematic review and meta-analysis. Br J Psychiatry. 2015;206(2):93-100.
7. Koek RJ, Schwartz HN, Scully S, et al. Treatment-refractory posttraumatic stress disorder (TRPTSD): a review and framework for the future. Prog Neuropsychopharmacol Biol Psychiatry. 2016;70:170-218.
8. Wagle Shukla A, Okun MS. State of the art for deep brain stimulation therapy in movement disorders: a clinical and technological perspective. IEEE Rev Biomed Eng. 2016;9:219-233.
9. Weaver FM, Follett K, Stern M, et al; CSP 468 Study Group. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009;301(1):63-73.
10. Benabid AL, Benazzouz A, Hoffmann D, Limousin P, Krack P, Pollack P. Long-term electrical inhibition of deep brain targets in movement disorders. Mov Disord. 1998;13(suppl 3):119-125.
11. Benabid AL, Wallace B, Mitrofanis J, et al. A putative generalized model of the effects and mechanism of action of high frequency electrical stimulation of the central nervous system. Acta Neurol Belg. 2005;105(3):149-157.
12. Fenoy AJ, Simpson RK Jr. Risks of common complications in deep brain stimulation surgery: management and avoidance. J Neurosurg. 2014;120(1):132-139.
13. DiLorenzo DJ, Jankovic J, Simpson RK, Takei H, Powell SZ. Neurohistopathological findings at the electrode–tissue interface in long-term deep brain stimulation: systematic literature review, case report, and assessment of stimulation threshold safety. Neuromodulation. 2014;17(5):405-418.
14. Revell MA. Deep brain stimulation for movement disorders. Nurs Clin North Am. 2015;50(4):691-701.
15. Fisher R, Salanova V, Witt T, et al; SANTE Study Group. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51(5):899-908.
16. Salanova V, Witt T, Worth R, et al; SANTE Study Group. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology. 2015;84(10):1017-1025.
17. Berlim MT, McGirr A, Van den Eynde F, Fleck MP, Giacobbe P. Effectiveness and acceptability of deep brain stimulation (DBS) of the subgenual cingulate cortex for treatment-resistant depression: a systematic review and exploratory meta-analysis. J Affect Disord. 2014;159:31-38.
18. Dougherty DD, Rezai AR, Carpenter LL, et al. A randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. Biol Psychiatry. 2015;78(4):240-248.
19. Bergfeld IO, Mantione M, Hoogendoorn ML, et al. Deep brain stimulation of the ventral anterior limb of the internal capsule for treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2016;73(5):456-464.
20. Greenberg BD, Malone DA, Friehs GM, et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology. 2006;31(11):2384-2393.
21. Mayber HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156(5):675-682.
22. Rauch SL, Jenike MA, Alpert NM, et al. Regional cerebral blood flow measured during symptom provocation in obsessive-compulsive disorder using oxygen 15-labeled carbon dioxide and positron emission tomography. Arch Gen Psychiatry. 1994;51(1):62-70.
23. Williams NR, Taylor JJ, Lamb K, Hanlon CA, Short EB, George MS. Role of functional imaging in the development and refinement of invasive neuromodulation for psychiatric disorders. World J Radiol. 2014;6(10):756-778.
24. Francati V, Vermetten E, Bremner JD. Functional neuroimaging studies in posttraumatic stress disorder: review of current methods and findings. Depress Anxiety. 2007;24(3):202-218.
25. Shin LM, Orr SP, Carson MA, et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61(2):168-176.
26. Armony JL, Corbo V, Clément MH, Brunet A. Amygdala response in patients with acute PTSD to masked and unmasked emotional facial expressions. Am J Psychiatry. 2005;162(10):1961-1963.
27. Blake DD, Weathers FW, Nagy LM, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8(1):75-90.
28. Felmingham K, Kemp A, Williams L, et al. Changes in anterior cingulate and amygdala after cognitive behavior therapy of posttraumatic stress disorder. Psychol Sci. 2007;18(2):127-129.
29. Peres JF, Newberg AB, Mercante JP, et al. Cerebral blood flow changes during retrieval of traumatic memories before and after psychotherapy: a SPECT study. Psychol Med. 2007;37(10):1481-1491.
30. Langevin JP, De Salles AA, Kosoyan HP, Krahl SE. Deep brain stimulation of the amygdala alleviates post-traumatic stress disorder symptoms in a rat model. J Psychiatr Res. 2010;44(16):1241-1245.
31. Stidd DA, Vogelsang K, Krahl SE, Langevin JP, Fellous JM. Amygdala deep brain stimulation is superior to paroxetine treatment in a rat model of posttraumatic stress disorder. Brain Stimul. 2013;6(6):837-844.
32. Anglada-Figueroa D, Quirk GJ. Lesions of the basal amygdala block expression of conditioned fear but not extinction. J Neurosci. 2005;25(42):9680-9685.
33. Koek RJ, Langevin JP, Krahl SE, et al. Deep brain stimulation of the basolateral amygdala for treatment-refractory combat post-traumatic stress disorder (PTSD): study protocol for a pilot randomized controlled trial with blinded, staggered onset of stimulation. Trials. 2014;15:356.
34. Sturm V, Fricke O, Bührle CP, et al. DBS in the basolateral amygdala improves symptoms of autism and related self-injurious behavior: a case report and hypothesis on the pathogenesis of the disorder. Front Hum Neurosci. 2013;6:341.
35. Langevin JP, Koek RJ, Schwartz HN, et al. Deep brain stimulation of the basolateral amygdala for treatment-refractory posttraumatic stress disorder. Biol Psychiatry. 2016;79(10):e82-e84.
36. Langevin JP, Chen JW, Koek RJ, et al. Deep brain stimulation of the basolateral amygdala: targeting technique and electrodiagnostic findings. Brain Sci. 2016;6(3):E28.
Failure of fear extinction is a core feature of posttraumatic stress disorder (PTSD).1 Recently, it was confirmed that the amygdala and the orbitofrontal cortex are crucial for both fear acquisition and fear extinction.2 The amygdala was found to have neurons active only during fear acquisition, and other neurons active only during fear extinction.3 In essence, the balance of activity between these 2 neuronal populations determines whether if an incoming stimulus is feared or not feared. This balance is under the influence of several cognitive domains, including memory, reward, and executive function.
In PTSD, the equilibrium is shifted heavily toward fear acquisition. The majority of patients spontaneously regain the capacity for fear extinction over time4 or with the help of treatment.5,6 Nonetheless, some patients with severe PTSD seem unable to recover the ability of fear extinction and remain refractory to both standard and novel psychotherapeutic or psychopharmacologic treatments.7 For these patients, direct modulation of the neural activity in the amygdala may permit fear extinction. This article describes the rationale for using deep brain stimulation (DBS) and initial results from the first-ever clinical trial.
Deep Brain Stimulation
Deep brain stimulation involves inserting electrodes in precise cerebral targets and then connecting the leads to a pulse generator (similar to a pacemaker) inserted in a subclavicular pocket. The generator controls the electrical signal (amplitude, pulse width, pulse frequency) delivered to the brain target and can be adjusted with use of a noninvasive programmer. In 1997, the FDA approved DBS for patients with Parkinson disease or essential tremor. Since then, its efficacy in these movement disorders has been confirmed in several studies.8,9
The mechanism by which the small electrical pulses of DBS influence activity is not clear. Clinically, DBS functionally inhibits the activity of local neurons.10 One theory describes “frequency jamming,” a concept similar to cardiac overdrive pacing in which the resultant high-frequency neuronal signal is meaningless and discounted by the rest of the brain.11
Over the years, DBS has demonstrated a strong safety profile.12 The risks of electrode insertion are mitigated with targeting based on high-quality magnetic resonance imaging (MRI) and computed tomography (Figure). Unlike a destructive lesion, DBS is reversible, and the implanted system can be removed in its entirety. Histologic analyses have shown only a small amount of scarring around the electrode tip.13 In movement disorder treatment, clinical experience has shown that stimulation-related adverse effects (AEs) are reversible with readjustment of stimulation parameters by external programmer.14
Novel Applications of DBS
The advantageous safety profile of DBS has permitted its evaluation in the treatment of other conditions thought to have malfunctioning networks at their core—such as intractable epilepsy (in resective surgery noncandidates).15,16 Although several trials have shown promising results of using DBS for treatment-resistant depression,17 the results of pivotal sham-controlled trials have been mixed.18,19 Obsessive-compulsive disorder, on the other hand, received the FDA humanitarian device exemption designation on the basis of positive long-term results.20 In treatment-resistant depression and obsessive-compulsive disorder, functional neuroimaging has identified DBS targets.21,22 Functional MRI or positron emission tomography (PET) images can be compared at resting state, at symptomatic state, and after treatment response. Nodes hyperactive during a symptomatic state and less active after successful treatment can be targeted with high-frequency DBS to directly reduce the hyperactivity and indirectly modulate or normalize the overall function of the circuit.23
Given the functional MRI and O15 (oxygen-15) PET evidence of amygdala hyperactivity in patients with PTSD having core symptoms,24-26 the authors hypothesized that high-frequency DBS targeting of the amygdala would improve PTSD-associated hyperarousal and reexperiencing symptoms in treatment-refractory patients. Indirect data supporting this hypothesis include a correlation between amygdala hyperactivity of increased intensity and symptom severity measured with the Clinician-Administered PTSD Scale (CAPS),27 and a correlation between reduced pretreatment amygdala hyperactivity and successful cognitive-behavioral treatment.28,29
Preclinical Work
Using a rodent model in which a novel object serves as a cue reminder of foot shocks (traumatic event), the authors tested the hypothesis that amygdala DBS would reduce PTSD-like symptoms.30 When untreated rats were presented with the object in their cage a week after the initial exposure, they immediately buried the object under bedding to avoid being reminded of the shocks. In contrast, rats treated with DBS did not bury the object. In most cases, in fact, they played with it.
The authors also replicated their results but with the addition of rats treated with paroxetine.31 Using the same rodent model, they found DBS superior to paroxetine in treating PTSD-like symptoms. This study had a crossover design: DBS and sham DBS. Briefly, 20 rats received an electrode in the amygdala and were exposed to inescapable shocks in the presence of the cue object. The rats were then randomly assigned to a DBS group (10 rats) or a sham-DBS group (10 rats). After 1 week, behavioral testing showed fear extinction in the DBS group and no improvement in the sham-DBS group. Then the groups were switched: The rats originally treated with DBS received no treatment, and the rats that were originally sham-treated underwent DBS. One week later, behavioral testing showed acquisition of fear extinction in all the rats. These results suggested DBS can be effective even when delayed after establishment of fear persistence and PTSD symptoms. These results also showed that DBS effects persist even after therapy discontinuation.
Similarly, other investigators have reported that the role of the amygdala is not limited to fear acquisition; it extends to fear expression. A lesion in the amygdala can prevent fear expression even if the disruption is performed subsequent to fear-conditioning training.32 This finding is important for humans, as DBS would be initiated during the chronic phase of the disorder, after failure of less invasive treatment options, such as pharmacotherapy and psychotherapy.
Early Clinical Experience
The authors have initiated the first ever clinical trial (NCT02091843) evaluating use of DBS for PTSD and are now recruiting patients. Enrollment is limited to 6 combat veterans with disabling PTSD that has not responded to pharmacotherapy and psychotherapy. This VA-funded single-site study, being conducted at the VA Greater Los Angeles Healthcare System (VAGLAHS), was approved by the VAGLAHS Institutional Review Board and the FDA. The authors have published the 2-year trial’s protocol, which includes an active-versus-sham stimulation phase; continuous electroencephalogram monitoring; baseline and posttreatment 18FDG (fluorodeoxyglucose) PET performed during a resting state vs during investigator-guided exposure to trauma reminders; and extensive psychological and neuropsychological assessments.33 The literature includes only 1 case report on amygdala DBS.34 The authors of that report used DBS of the basolateral nucleus of the amygdala to treat a teenaged boy with severe autism and found that the therapy was safe.
As of this writing, the authors have recruited and implanted 1 patient and reported on his clinical results (including baseline PET) over the first 8 months of stimulation35 and on the electrophysiologic findings over the first year.36 After experiencing extremely severe combat PTSD refractory to pharmacotherapy and psychotherapy treatments for more than 20 years, the patient treated with DBS is now experiencing substantial symptom relief, and his CAPS score (primary outcome measure) has improved by about 40%. He has tolerated continuous stimulation without any serious DBS-related AEs for up to 16 months. Notably, he has not had a single severe combat nightmare in a year—in stark contrast to nightly combat nightmares during the 20-year period leading to the trial. Furthermore, he has not been having any episodes of severe dissociation, which had been a common disabling problem before the trial. He has taken a second trip out of the country, improved his relationships with family, and made strides (albeit limited) in pursuing additional social interactions.
Avoidance remains a major problem. He recently left his job after 7 years, because he prefers a more nature-oriented rather than people-oriented environment. In addition, his interest in intensive psychotherapy has increased, and he has been considering options for spending more time working on his therapy.
Over 15 months of treatment, the patient’s CAPS total and subscale scores have decreased—his symptoms have improved (Table).21 He has had rapid and substantial reductions in recurrence and hyperarousal symptoms but slower improvement in avoidance. Improvements in emotional reactivity would be expected to occur before any change in behavior (eg, avoidance). Patients likely must first recognize changes in emotional reactivity to events before they can engage in a cognitive process to modify learned behavioral responses to those events.
After about 9 months of treatment, all of the study patient’s symptoms were somewhat stabilized, and the authors began making gradual stimulation adjustments to the latest parameters—3.5 V, 60 µs, and 160 Hz for the right electrode and 1.5 V, 60 µs, and 160 Hz for the left electrode—using the contacts in the basolateral nucleus of the amygdala, per postoperative neuroimaging.3
After 15 to 18 months, when improvement peaked at 48% symptom reduction from baseline, the patient experienced psychiatric decompensation (depression, suicide gesture) not attributable to changes in stimulation settings and not associated with exacerbation of PTSD symptoms. Treatment team members and independent psychiatric consultants attributed the decompensation to the patient’s difficulty in changing a long-standing avoidant behavior routine, owing to severe recurrence and hyperarousal symptoms in the past. His persistent inability to overcome avoidance and isolation, despite core PTSD symptom improvement, had left him feeling worthless.
The patient remains in the study but also is participating in other medication and psychotherapy trials and is making a career change. Periodic decompensations may be part of the treatment course as patients reach a more complex and volatile phase of improvement that requires more intensive cognitive reprocessing. If this proves to be the case with other patients enrolling in the study, intensive psychotherapy that addresses cognitive and emotional PTSD symptoms may be needed once there is improvement in intrusive and hyperarousal symptoms.
Conclusion
Deep brain stimulation has been successful in treating Parkinson disease and essential tremor. Physiologically, DBS seems to inhibit specific brain regions’ dysfunctional activity stemming from a disease process. Deep brain stimulation-induced inhibition of a dysfunctional node improves clinical outcomes in movement disorders.
Given the reversibility and positive safety profile of DBS, new applications are being studied. The authors propose that DBS may benefit patients with severe treatment-refractory PTSD. Their first patient’s core PTSD symptoms have improved significantly, as expected, but as in other psychiatric DBS cases, the seriousness and chronicity of his illness may be complicating the course of recovery. The authors plan to recruit 6 patients for this early-phase safety trial.
Click here to read the digital edition.
Failure of fear extinction is a core feature of posttraumatic stress disorder (PTSD).1 Recently, it was confirmed that the amygdala and the orbitofrontal cortex are crucial for both fear acquisition and fear extinction.2 The amygdala was found to have neurons active only during fear acquisition, and other neurons active only during fear extinction.3 In essence, the balance of activity between these 2 neuronal populations determines whether if an incoming stimulus is feared or not feared. This balance is under the influence of several cognitive domains, including memory, reward, and executive function.
In PTSD, the equilibrium is shifted heavily toward fear acquisition. The majority of patients spontaneously regain the capacity for fear extinction over time4 or with the help of treatment.5,6 Nonetheless, some patients with severe PTSD seem unable to recover the ability of fear extinction and remain refractory to both standard and novel psychotherapeutic or psychopharmacologic treatments.7 For these patients, direct modulation of the neural activity in the amygdala may permit fear extinction. This article describes the rationale for using deep brain stimulation (DBS) and initial results from the first-ever clinical trial.
Deep Brain Stimulation
Deep brain stimulation involves inserting electrodes in precise cerebral targets and then connecting the leads to a pulse generator (similar to a pacemaker) inserted in a subclavicular pocket. The generator controls the electrical signal (amplitude, pulse width, pulse frequency) delivered to the brain target and can be adjusted with use of a noninvasive programmer. In 1997, the FDA approved DBS for patients with Parkinson disease or essential tremor. Since then, its efficacy in these movement disorders has been confirmed in several studies.8,9
The mechanism by which the small electrical pulses of DBS influence activity is not clear. Clinically, DBS functionally inhibits the activity of local neurons.10 One theory describes “frequency jamming,” a concept similar to cardiac overdrive pacing in which the resultant high-frequency neuronal signal is meaningless and discounted by the rest of the brain.11
Over the years, DBS has demonstrated a strong safety profile.12 The risks of electrode insertion are mitigated with targeting based on high-quality magnetic resonance imaging (MRI) and computed tomography (Figure). Unlike a destructive lesion, DBS is reversible, and the implanted system can be removed in its entirety. Histologic analyses have shown only a small amount of scarring around the electrode tip.13 In movement disorder treatment, clinical experience has shown that stimulation-related adverse effects (AEs) are reversible with readjustment of stimulation parameters by external programmer.14
Novel Applications of DBS
The advantageous safety profile of DBS has permitted its evaluation in the treatment of other conditions thought to have malfunctioning networks at their core—such as intractable epilepsy (in resective surgery noncandidates).15,16 Although several trials have shown promising results of using DBS for treatment-resistant depression,17 the results of pivotal sham-controlled trials have been mixed.18,19 Obsessive-compulsive disorder, on the other hand, received the FDA humanitarian device exemption designation on the basis of positive long-term results.20 In treatment-resistant depression and obsessive-compulsive disorder, functional neuroimaging has identified DBS targets.21,22 Functional MRI or positron emission tomography (PET) images can be compared at resting state, at symptomatic state, and after treatment response. Nodes hyperactive during a symptomatic state and less active after successful treatment can be targeted with high-frequency DBS to directly reduce the hyperactivity and indirectly modulate or normalize the overall function of the circuit.23
Given the functional MRI and O15 (oxygen-15) PET evidence of amygdala hyperactivity in patients with PTSD having core symptoms,24-26 the authors hypothesized that high-frequency DBS targeting of the amygdala would improve PTSD-associated hyperarousal and reexperiencing symptoms in treatment-refractory patients. Indirect data supporting this hypothesis include a correlation between amygdala hyperactivity of increased intensity and symptom severity measured with the Clinician-Administered PTSD Scale (CAPS),27 and a correlation between reduced pretreatment amygdala hyperactivity and successful cognitive-behavioral treatment.28,29
Preclinical Work
Using a rodent model in which a novel object serves as a cue reminder of foot shocks (traumatic event), the authors tested the hypothesis that amygdala DBS would reduce PTSD-like symptoms.30 When untreated rats were presented with the object in their cage a week after the initial exposure, they immediately buried the object under bedding to avoid being reminded of the shocks. In contrast, rats treated with DBS did not bury the object. In most cases, in fact, they played with it.
The authors also replicated their results but with the addition of rats treated with paroxetine.31 Using the same rodent model, they found DBS superior to paroxetine in treating PTSD-like symptoms. This study had a crossover design: DBS and sham DBS. Briefly, 20 rats received an electrode in the amygdala and were exposed to inescapable shocks in the presence of the cue object. The rats were then randomly assigned to a DBS group (10 rats) or a sham-DBS group (10 rats). After 1 week, behavioral testing showed fear extinction in the DBS group and no improvement in the sham-DBS group. Then the groups were switched: The rats originally treated with DBS received no treatment, and the rats that were originally sham-treated underwent DBS. One week later, behavioral testing showed acquisition of fear extinction in all the rats. These results suggested DBS can be effective even when delayed after establishment of fear persistence and PTSD symptoms. These results also showed that DBS effects persist even after therapy discontinuation.
Similarly, other investigators have reported that the role of the amygdala is not limited to fear acquisition; it extends to fear expression. A lesion in the amygdala can prevent fear expression even if the disruption is performed subsequent to fear-conditioning training.32 This finding is important for humans, as DBS would be initiated during the chronic phase of the disorder, after failure of less invasive treatment options, such as pharmacotherapy and psychotherapy.
Early Clinical Experience
The authors have initiated the first ever clinical trial (NCT02091843) evaluating use of DBS for PTSD and are now recruiting patients. Enrollment is limited to 6 combat veterans with disabling PTSD that has not responded to pharmacotherapy and psychotherapy. This VA-funded single-site study, being conducted at the VA Greater Los Angeles Healthcare System (VAGLAHS), was approved by the VAGLAHS Institutional Review Board and the FDA. The authors have published the 2-year trial’s protocol, which includes an active-versus-sham stimulation phase; continuous electroencephalogram monitoring; baseline and posttreatment 18FDG (fluorodeoxyglucose) PET performed during a resting state vs during investigator-guided exposure to trauma reminders; and extensive psychological and neuropsychological assessments.33 The literature includes only 1 case report on amygdala DBS.34 The authors of that report used DBS of the basolateral nucleus of the amygdala to treat a teenaged boy with severe autism and found that the therapy was safe.
As of this writing, the authors have recruited and implanted 1 patient and reported on his clinical results (including baseline PET) over the first 8 months of stimulation35 and on the electrophysiologic findings over the first year.36 After experiencing extremely severe combat PTSD refractory to pharmacotherapy and psychotherapy treatments for more than 20 years, the patient treated with DBS is now experiencing substantial symptom relief, and his CAPS score (primary outcome measure) has improved by about 40%. He has tolerated continuous stimulation without any serious DBS-related AEs for up to 16 months. Notably, he has not had a single severe combat nightmare in a year—in stark contrast to nightly combat nightmares during the 20-year period leading to the trial. Furthermore, he has not been having any episodes of severe dissociation, which had been a common disabling problem before the trial. He has taken a second trip out of the country, improved his relationships with family, and made strides (albeit limited) in pursuing additional social interactions.
Avoidance remains a major problem. He recently left his job after 7 years, because he prefers a more nature-oriented rather than people-oriented environment. In addition, his interest in intensive psychotherapy has increased, and he has been considering options for spending more time working on his therapy.
Over 15 months of treatment, the patient’s CAPS total and subscale scores have decreased—his symptoms have improved (Table).21 He has had rapid and substantial reductions in recurrence and hyperarousal symptoms but slower improvement in avoidance. Improvements in emotional reactivity would be expected to occur before any change in behavior (eg, avoidance). Patients likely must first recognize changes in emotional reactivity to events before they can engage in a cognitive process to modify learned behavioral responses to those events.
After about 9 months of treatment, all of the study patient’s symptoms were somewhat stabilized, and the authors began making gradual stimulation adjustments to the latest parameters—3.5 V, 60 µs, and 160 Hz for the right electrode and 1.5 V, 60 µs, and 160 Hz for the left electrode—using the contacts in the basolateral nucleus of the amygdala, per postoperative neuroimaging.3
After 15 to 18 months, when improvement peaked at 48% symptom reduction from baseline, the patient experienced psychiatric decompensation (depression, suicide gesture) not attributable to changes in stimulation settings and not associated with exacerbation of PTSD symptoms. Treatment team members and independent psychiatric consultants attributed the decompensation to the patient’s difficulty in changing a long-standing avoidant behavior routine, owing to severe recurrence and hyperarousal symptoms in the past. His persistent inability to overcome avoidance and isolation, despite core PTSD symptom improvement, had left him feeling worthless.
The patient remains in the study but also is participating in other medication and psychotherapy trials and is making a career change. Periodic decompensations may be part of the treatment course as patients reach a more complex and volatile phase of improvement that requires more intensive cognitive reprocessing. If this proves to be the case with other patients enrolling in the study, intensive psychotherapy that addresses cognitive and emotional PTSD symptoms may be needed once there is improvement in intrusive and hyperarousal symptoms.
Conclusion
Deep brain stimulation has been successful in treating Parkinson disease and essential tremor. Physiologically, DBS seems to inhibit specific brain regions’ dysfunctional activity stemming from a disease process. Deep brain stimulation-induced inhibition of a dysfunctional node improves clinical outcomes in movement disorders.
Given the reversibility and positive safety profile of DBS, new applications are being studied. The authors propose that DBS may benefit patients with severe treatment-refractory PTSD. Their first patient’s core PTSD symptoms have improved significantly, as expected, but as in other psychiatric DBS cases, the seriousness and chronicity of his illness may be complicating the course of recovery. The authors plan to recruit 6 patients for this early-phase safety trial.
Click here to read the digital edition.
1. Milad MR, Pitman RK, Ellis CB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66(12):1075-1082.
2. Marin MF, Song H, VanElzakker MB, et al. Association of resting metabolism in the fear neural network with extinction recall activations and clinical measures in trauma-exposed individuals. Am J Psychiatry. 2016;173(9):930-938.
3. Herry C, Ciocchi S, Senn V, Demmou L, Müller C, Lüthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454(7204):600-606.
4. Morina N, Wicherts JM, Lobbrecht J, Priebe S. Remission from post-traumatic stress disorder in adults: a systematic review and meta-analysis of long term outcome studies. Clin Psychol Rev. 2014;34(3):249-255.
5. Steenkamp MM, Litz BT, Hoge CW, Marmar CR. Psychotherapy for military-related PTSD: a review of randomized clinical trials. JAMA. 2015;314(5):489-500.
6. Hoskins M, Pearce J, Bethell A, et al. Pharmacotherapy for post-traumatic stress disorder: systematic review and meta-analysis. Br J Psychiatry. 2015;206(2):93-100.
7. Koek RJ, Schwartz HN, Scully S, et al. Treatment-refractory posttraumatic stress disorder (TRPTSD): a review and framework for the future. Prog Neuropsychopharmacol Biol Psychiatry. 2016;70:170-218.
8. Wagle Shukla A, Okun MS. State of the art for deep brain stimulation therapy in movement disorders: a clinical and technological perspective. IEEE Rev Biomed Eng. 2016;9:219-233.
9. Weaver FM, Follett K, Stern M, et al; CSP 468 Study Group. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009;301(1):63-73.
10. Benabid AL, Benazzouz A, Hoffmann D, Limousin P, Krack P, Pollack P. Long-term electrical inhibition of deep brain targets in movement disorders. Mov Disord. 1998;13(suppl 3):119-125.
11. Benabid AL, Wallace B, Mitrofanis J, et al. A putative generalized model of the effects and mechanism of action of high frequency electrical stimulation of the central nervous system. Acta Neurol Belg. 2005;105(3):149-157.
12. Fenoy AJ, Simpson RK Jr. Risks of common complications in deep brain stimulation surgery: management and avoidance. J Neurosurg. 2014;120(1):132-139.
13. DiLorenzo DJ, Jankovic J, Simpson RK, Takei H, Powell SZ. Neurohistopathological findings at the electrode–tissue interface in long-term deep brain stimulation: systematic literature review, case report, and assessment of stimulation threshold safety. Neuromodulation. 2014;17(5):405-418.
14. Revell MA. Deep brain stimulation for movement disorders. Nurs Clin North Am. 2015;50(4):691-701.
15. Fisher R, Salanova V, Witt T, et al; SANTE Study Group. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51(5):899-908.
16. Salanova V, Witt T, Worth R, et al; SANTE Study Group. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology. 2015;84(10):1017-1025.
17. Berlim MT, McGirr A, Van den Eynde F, Fleck MP, Giacobbe P. Effectiveness and acceptability of deep brain stimulation (DBS) of the subgenual cingulate cortex for treatment-resistant depression: a systematic review and exploratory meta-analysis. J Affect Disord. 2014;159:31-38.
18. Dougherty DD, Rezai AR, Carpenter LL, et al. A randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. Biol Psychiatry. 2015;78(4):240-248.
19. Bergfeld IO, Mantione M, Hoogendoorn ML, et al. Deep brain stimulation of the ventral anterior limb of the internal capsule for treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2016;73(5):456-464.
20. Greenberg BD, Malone DA, Friehs GM, et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology. 2006;31(11):2384-2393.
21. Mayber HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156(5):675-682.
22. Rauch SL, Jenike MA, Alpert NM, et al. Regional cerebral blood flow measured during symptom provocation in obsessive-compulsive disorder using oxygen 15-labeled carbon dioxide and positron emission tomography. Arch Gen Psychiatry. 1994;51(1):62-70.
23. Williams NR, Taylor JJ, Lamb K, Hanlon CA, Short EB, George MS. Role of functional imaging in the development and refinement of invasive neuromodulation for psychiatric disorders. World J Radiol. 2014;6(10):756-778.
24. Francati V, Vermetten E, Bremner JD. Functional neuroimaging studies in posttraumatic stress disorder: review of current methods and findings. Depress Anxiety. 2007;24(3):202-218.
25. Shin LM, Orr SP, Carson MA, et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61(2):168-176.
26. Armony JL, Corbo V, Clément MH, Brunet A. Amygdala response in patients with acute PTSD to masked and unmasked emotional facial expressions. Am J Psychiatry. 2005;162(10):1961-1963.
27. Blake DD, Weathers FW, Nagy LM, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8(1):75-90.
28. Felmingham K, Kemp A, Williams L, et al. Changes in anterior cingulate and amygdala after cognitive behavior therapy of posttraumatic stress disorder. Psychol Sci. 2007;18(2):127-129.
29. Peres JF, Newberg AB, Mercante JP, et al. Cerebral blood flow changes during retrieval of traumatic memories before and after psychotherapy: a SPECT study. Psychol Med. 2007;37(10):1481-1491.
30. Langevin JP, De Salles AA, Kosoyan HP, Krahl SE. Deep brain stimulation of the amygdala alleviates post-traumatic stress disorder symptoms in a rat model. J Psychiatr Res. 2010;44(16):1241-1245.
31. Stidd DA, Vogelsang K, Krahl SE, Langevin JP, Fellous JM. Amygdala deep brain stimulation is superior to paroxetine treatment in a rat model of posttraumatic stress disorder. Brain Stimul. 2013;6(6):837-844.
32. Anglada-Figueroa D, Quirk GJ. Lesions of the basal amygdala block expression of conditioned fear but not extinction. J Neurosci. 2005;25(42):9680-9685.
33. Koek RJ, Langevin JP, Krahl SE, et al. Deep brain stimulation of the basolateral amygdala for treatment-refractory combat post-traumatic stress disorder (PTSD): study protocol for a pilot randomized controlled trial with blinded, staggered onset of stimulation. Trials. 2014;15:356.
34. Sturm V, Fricke O, Bührle CP, et al. DBS in the basolateral amygdala improves symptoms of autism and related self-injurious behavior: a case report and hypothesis on the pathogenesis of the disorder. Front Hum Neurosci. 2013;6:341.
35. Langevin JP, Koek RJ, Schwartz HN, et al. Deep brain stimulation of the basolateral amygdala for treatment-refractory posttraumatic stress disorder. Biol Psychiatry. 2016;79(10):e82-e84.
36. Langevin JP, Chen JW, Koek RJ, et al. Deep brain stimulation of the basolateral amygdala: targeting technique and electrodiagnostic findings. Brain Sci. 2016;6(3):E28.
1. Milad MR, Pitman RK, Ellis CB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66(12):1075-1082.
2. Marin MF, Song H, VanElzakker MB, et al. Association of resting metabolism in the fear neural network with extinction recall activations and clinical measures in trauma-exposed individuals. Am J Psychiatry. 2016;173(9):930-938.
3. Herry C, Ciocchi S, Senn V, Demmou L, Müller C, Lüthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454(7204):600-606.
4. Morina N, Wicherts JM, Lobbrecht J, Priebe S. Remission from post-traumatic stress disorder in adults: a systematic review and meta-analysis of long term outcome studies. Clin Psychol Rev. 2014;34(3):249-255.
5. Steenkamp MM, Litz BT, Hoge CW, Marmar CR. Psychotherapy for military-related PTSD: a review of randomized clinical trials. JAMA. 2015;314(5):489-500.
6. Hoskins M, Pearce J, Bethell A, et al. Pharmacotherapy for post-traumatic stress disorder: systematic review and meta-analysis. Br J Psychiatry. 2015;206(2):93-100.
7. Koek RJ, Schwartz HN, Scully S, et al. Treatment-refractory posttraumatic stress disorder (TRPTSD): a review and framework for the future. Prog Neuropsychopharmacol Biol Psychiatry. 2016;70:170-218.
8. Wagle Shukla A, Okun MS. State of the art for deep brain stimulation therapy in movement disorders: a clinical and technological perspective. IEEE Rev Biomed Eng. 2016;9:219-233.
9. Weaver FM, Follett K, Stern M, et al; CSP 468 Study Group. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009;301(1):63-73.
10. Benabid AL, Benazzouz A, Hoffmann D, Limousin P, Krack P, Pollack P. Long-term electrical inhibition of deep brain targets in movement disorders. Mov Disord. 1998;13(suppl 3):119-125.
11. Benabid AL, Wallace B, Mitrofanis J, et al. A putative generalized model of the effects and mechanism of action of high frequency electrical stimulation of the central nervous system. Acta Neurol Belg. 2005;105(3):149-157.
12. Fenoy AJ, Simpson RK Jr. Risks of common complications in deep brain stimulation surgery: management and avoidance. J Neurosurg. 2014;120(1):132-139.
13. DiLorenzo DJ, Jankovic J, Simpson RK, Takei H, Powell SZ. Neurohistopathological findings at the electrode–tissue interface in long-term deep brain stimulation: systematic literature review, case report, and assessment of stimulation threshold safety. Neuromodulation. 2014;17(5):405-418.
14. Revell MA. Deep brain stimulation for movement disorders. Nurs Clin North Am. 2015;50(4):691-701.
15. Fisher R, Salanova V, Witt T, et al; SANTE Study Group. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51(5):899-908.
16. Salanova V, Witt T, Worth R, et al; SANTE Study Group. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology. 2015;84(10):1017-1025.
17. Berlim MT, McGirr A, Van den Eynde F, Fleck MP, Giacobbe P. Effectiveness and acceptability of deep brain stimulation (DBS) of the subgenual cingulate cortex for treatment-resistant depression: a systematic review and exploratory meta-analysis. J Affect Disord. 2014;159:31-38.
18. Dougherty DD, Rezai AR, Carpenter LL, et al. A randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. Biol Psychiatry. 2015;78(4):240-248.
19. Bergfeld IO, Mantione M, Hoogendoorn ML, et al. Deep brain stimulation of the ventral anterior limb of the internal capsule for treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2016;73(5):456-464.
20. Greenberg BD, Malone DA, Friehs GM, et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology. 2006;31(11):2384-2393.
21. Mayber HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156(5):675-682.
22. Rauch SL, Jenike MA, Alpert NM, et al. Regional cerebral blood flow measured during symptom provocation in obsessive-compulsive disorder using oxygen 15-labeled carbon dioxide and positron emission tomography. Arch Gen Psychiatry. 1994;51(1):62-70.
23. Williams NR, Taylor JJ, Lamb K, Hanlon CA, Short EB, George MS. Role of functional imaging in the development and refinement of invasive neuromodulation for psychiatric disorders. World J Radiol. 2014;6(10):756-778.
24. Francati V, Vermetten E, Bremner JD. Functional neuroimaging studies in posttraumatic stress disorder: review of current methods and findings. Depress Anxiety. 2007;24(3):202-218.
25. Shin LM, Orr SP, Carson MA, et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61(2):168-176.
26. Armony JL, Corbo V, Clément MH, Brunet A. Amygdala response in patients with acute PTSD to masked and unmasked emotional facial expressions. Am J Psychiatry. 2005;162(10):1961-1963.
27. Blake DD, Weathers FW, Nagy LM, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8(1):75-90.
28. Felmingham K, Kemp A, Williams L, et al. Changes in anterior cingulate and amygdala after cognitive behavior therapy of posttraumatic stress disorder. Psychol Sci. 2007;18(2):127-129.
29. Peres JF, Newberg AB, Mercante JP, et al. Cerebral blood flow changes during retrieval of traumatic memories before and after psychotherapy: a SPECT study. Psychol Med. 2007;37(10):1481-1491.
30. Langevin JP, De Salles AA, Kosoyan HP, Krahl SE. Deep brain stimulation of the amygdala alleviates post-traumatic stress disorder symptoms in a rat model. J Psychiatr Res. 2010;44(16):1241-1245.
31. Stidd DA, Vogelsang K, Krahl SE, Langevin JP, Fellous JM. Amygdala deep brain stimulation is superior to paroxetine treatment in a rat model of posttraumatic stress disorder. Brain Stimul. 2013;6(6):837-844.
32. Anglada-Figueroa D, Quirk GJ. Lesions of the basal amygdala block expression of conditioned fear but not extinction. J Neurosci. 2005;25(42):9680-9685.
33. Koek RJ, Langevin JP, Krahl SE, et al. Deep brain stimulation of the basolateral amygdala for treatment-refractory combat post-traumatic stress disorder (PTSD): study protocol for a pilot randomized controlled trial with blinded, staggered onset of stimulation. Trials. 2014;15:356.
34. Sturm V, Fricke O, Bührle CP, et al. DBS in the basolateral amygdala improves symptoms of autism and related self-injurious behavior: a case report and hypothesis on the pathogenesis of the disorder. Front Hum Neurosci. 2013;6:341.
35. Langevin JP, Koek RJ, Schwartz HN, et al. Deep brain stimulation of the basolateral amygdala for treatment-refractory posttraumatic stress disorder. Biol Psychiatry. 2016;79(10):e82-e84.
36. Langevin JP, Chen JW, Koek RJ, et al. Deep brain stimulation of the basolateral amygdala: targeting technique and electrodiagnostic findings. Brain Sci. 2016;6(3):E28.
Defensive medicine’s stranglehold on the realities of practice
In the September 2017 issue of JAMA Neurology, Louis R. Caplan, MD, wrote an excellent editorial, “Patient care is all about stories.” He notes that we all hear from patients about a recurrence of their previous stroke deficits, typically caused by infections, medications, or metabolic changes.
His point is that, telling the difference between true vascular events and recrudescence of old deficits can be difficult, but generally can be gleaned by taking a thorough history. He also notes, quite correctly, that the generic, automated features of modern charting systems often make it harder to get the details you need from previous visits.
Obviously, being able to accurately tell the difference between them can save health care costs, too. In a study in the same issue, Mehmet Topcuoglo, MD, and his colleagues discuss methodologies to differentiate between the causes of recrudescence of stroke-related deficits. Currently, the main approach is to admit patients to the hospital, do a knee-jerk repeat work-up with MRI, magnetic resonance angiogram, and echocardiogram (typically ordered before the neurologist has even been told of the consult) and then conclude that nothing has changed neurologically and that it was all caused by a bladder infection.
Surely, if we had an accurate way of telling the difference between them with a careful history, we’d save a lot of time and money on unnecessary hospital admissions. Right?
It sounds good in principle, but, sadly, the answer is “probably not.”
This is where the idealism of medicine meets the reality of its practice.
In the world of the emergency department, time and resources are limited. Emergency medicine physicians don’t have the luxury of taking a detailed neurologic history, nor are they trained (or expected) to be able to do so. Their job is to decide what is (and isn’t) life-threatening and who does (or doesn’t) need to be admitted.
But probably the main reason why Dr. Topcuoglo and his colleagues’ methodologies will never be implemented is defensive medicine. It’s a heck of lot easier and safer for any doctor – emergency medicine, hospitalist, and neurologist – to admit the patient and order more studies than it is to get served for malpractice and have to defend why you didn’t do that.
People can bemoan defensive medicine and its costs all they want. But, if you’ve been sued, you won’t care. You’ll order any test to protect yourself. Claiming that you followed a guideline from a journal, no matter how well researched it was, will likely be worthless the one time a stroke was missed. It’s easy for a plaintiff’s attorney to find someone to say you fell below the standard of care for doing so.
For an example of where this stands, here’s something from personal experience: One of my patients went to the emergency department for recrudescence of an old left hemiparesis, likely caused by a urinary tract infection. This wasn’t the first time it had happened. A head CT was stable while a urine analysis was abnormal. Because of my schedule, I wasn’t in a position to go see him in the ED in an expedient fashion. The ED physician was planning on admitting him and called to notify me. Knowing the history, I suggested sending him home with treatment for the UTI and to follow up with me the next day.
I thought that seemed reasonable, but the ED doctor didn’t. He said, “If you want to do that, then I am going to document that it’s on your instructions, that you are assuming all responsibility for care and outcome if a stroke is missed, and that I entirely disagree with your decision.”
I’m sure another neurologist might have said, “Okay, tell him to come in here tomorrow,” and hung up, but I really don’t have that kind of fortitude or desire for conflict with another physician. So I backed down and let the person on the scene make the decision. I saw the patient later that day as a consult, all his tests (except the urine analysis in the ED) were fine, and he went home the next day. I’m sure the bill was at least $50,000 (what really got paid is another matter), and defensive medicine had, for better or worse, won out over probability and reason.
Dr. Caplan, quite correctly, emphasizes the importance of taking a careful history, and I absolutely agree with him. Unfortunately, the lack of time in the ED setting, and fears driven by legal consequences, often make a good history irrelevant. Even when it’s done, there are other forces that push it to the background in making medical decisions.
I’m not saying that’s a good thing – it isn’t. But that’s the way it is right now in American medicine, and this aspect of the system shows no sign of changing.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In the September 2017 issue of JAMA Neurology, Louis R. Caplan, MD, wrote an excellent editorial, “Patient care is all about stories.” He notes that we all hear from patients about a recurrence of their previous stroke deficits, typically caused by infections, medications, or metabolic changes.
His point is that, telling the difference between true vascular events and recrudescence of old deficits can be difficult, but generally can be gleaned by taking a thorough history. He also notes, quite correctly, that the generic, automated features of modern charting systems often make it harder to get the details you need from previous visits.
Obviously, being able to accurately tell the difference between them can save health care costs, too. In a study in the same issue, Mehmet Topcuoglo, MD, and his colleagues discuss methodologies to differentiate between the causes of recrudescence of stroke-related deficits. Currently, the main approach is to admit patients to the hospital, do a knee-jerk repeat work-up with MRI, magnetic resonance angiogram, and echocardiogram (typically ordered before the neurologist has even been told of the consult) and then conclude that nothing has changed neurologically and that it was all caused by a bladder infection.
Surely, if we had an accurate way of telling the difference between them with a careful history, we’d save a lot of time and money on unnecessary hospital admissions. Right?
It sounds good in principle, but, sadly, the answer is “probably not.”
This is where the idealism of medicine meets the reality of its practice.
In the world of the emergency department, time and resources are limited. Emergency medicine physicians don’t have the luxury of taking a detailed neurologic history, nor are they trained (or expected) to be able to do so. Their job is to decide what is (and isn’t) life-threatening and who does (or doesn’t) need to be admitted.
But probably the main reason why Dr. Topcuoglo and his colleagues’ methodologies will never be implemented is defensive medicine. It’s a heck of lot easier and safer for any doctor – emergency medicine, hospitalist, and neurologist – to admit the patient and order more studies than it is to get served for malpractice and have to defend why you didn’t do that.
People can bemoan defensive medicine and its costs all they want. But, if you’ve been sued, you won’t care. You’ll order any test to protect yourself. Claiming that you followed a guideline from a journal, no matter how well researched it was, will likely be worthless the one time a stroke was missed. It’s easy for a plaintiff’s attorney to find someone to say you fell below the standard of care for doing so.
For an example of where this stands, here’s something from personal experience: One of my patients went to the emergency department for recrudescence of an old left hemiparesis, likely caused by a urinary tract infection. This wasn’t the first time it had happened. A head CT was stable while a urine analysis was abnormal. Because of my schedule, I wasn’t in a position to go see him in the ED in an expedient fashion. The ED physician was planning on admitting him and called to notify me. Knowing the history, I suggested sending him home with treatment for the UTI and to follow up with me the next day.
I thought that seemed reasonable, but the ED doctor didn’t. He said, “If you want to do that, then I am going to document that it’s on your instructions, that you are assuming all responsibility for care and outcome if a stroke is missed, and that I entirely disagree with your decision.”
I’m sure another neurologist might have said, “Okay, tell him to come in here tomorrow,” and hung up, but I really don’t have that kind of fortitude or desire for conflict with another physician. So I backed down and let the person on the scene make the decision. I saw the patient later that day as a consult, all his tests (except the urine analysis in the ED) were fine, and he went home the next day. I’m sure the bill was at least $50,000 (what really got paid is another matter), and defensive medicine had, for better or worse, won out over probability and reason.
Dr. Caplan, quite correctly, emphasizes the importance of taking a careful history, and I absolutely agree with him. Unfortunately, the lack of time in the ED setting, and fears driven by legal consequences, often make a good history irrelevant. Even when it’s done, there are other forces that push it to the background in making medical decisions.
I’m not saying that’s a good thing – it isn’t. But that’s the way it is right now in American medicine, and this aspect of the system shows no sign of changing.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In the September 2017 issue of JAMA Neurology, Louis R. Caplan, MD, wrote an excellent editorial, “Patient care is all about stories.” He notes that we all hear from patients about a recurrence of their previous stroke deficits, typically caused by infections, medications, or metabolic changes.
His point is that, telling the difference between true vascular events and recrudescence of old deficits can be difficult, but generally can be gleaned by taking a thorough history. He also notes, quite correctly, that the generic, automated features of modern charting systems often make it harder to get the details you need from previous visits.
Obviously, being able to accurately tell the difference between them can save health care costs, too. In a study in the same issue, Mehmet Topcuoglo, MD, and his colleagues discuss methodologies to differentiate between the causes of recrudescence of stroke-related deficits. Currently, the main approach is to admit patients to the hospital, do a knee-jerk repeat work-up with MRI, magnetic resonance angiogram, and echocardiogram (typically ordered before the neurologist has even been told of the consult) and then conclude that nothing has changed neurologically and that it was all caused by a bladder infection.
Surely, if we had an accurate way of telling the difference between them with a careful history, we’d save a lot of time and money on unnecessary hospital admissions. Right?
It sounds good in principle, but, sadly, the answer is “probably not.”
This is where the idealism of medicine meets the reality of its practice.
In the world of the emergency department, time and resources are limited. Emergency medicine physicians don’t have the luxury of taking a detailed neurologic history, nor are they trained (or expected) to be able to do so. Their job is to decide what is (and isn’t) life-threatening and who does (or doesn’t) need to be admitted.
But probably the main reason why Dr. Topcuoglo and his colleagues’ methodologies will never be implemented is defensive medicine. It’s a heck of lot easier and safer for any doctor – emergency medicine, hospitalist, and neurologist – to admit the patient and order more studies than it is to get served for malpractice and have to defend why you didn’t do that.
People can bemoan defensive medicine and its costs all they want. But, if you’ve been sued, you won’t care. You’ll order any test to protect yourself. Claiming that you followed a guideline from a journal, no matter how well researched it was, will likely be worthless the one time a stroke was missed. It’s easy for a plaintiff’s attorney to find someone to say you fell below the standard of care for doing so.
For an example of where this stands, here’s something from personal experience: One of my patients went to the emergency department for recrudescence of an old left hemiparesis, likely caused by a urinary tract infection. This wasn’t the first time it had happened. A head CT was stable while a urine analysis was abnormal. Because of my schedule, I wasn’t in a position to go see him in the ED in an expedient fashion. The ED physician was planning on admitting him and called to notify me. Knowing the history, I suggested sending him home with treatment for the UTI and to follow up with me the next day.
I thought that seemed reasonable, but the ED doctor didn’t. He said, “If you want to do that, then I am going to document that it’s on your instructions, that you are assuming all responsibility for care and outcome if a stroke is missed, and that I entirely disagree with your decision.”
I’m sure another neurologist might have said, “Okay, tell him to come in here tomorrow,” and hung up, but I really don’t have that kind of fortitude or desire for conflict with another physician. So I backed down and let the person on the scene make the decision. I saw the patient later that day as a consult, all his tests (except the urine analysis in the ED) were fine, and he went home the next day. I’m sure the bill was at least $50,000 (what really got paid is another matter), and defensive medicine had, for better or worse, won out over probability and reason.
Dr. Caplan, quite correctly, emphasizes the importance of taking a careful history, and I absolutely agree with him. Unfortunately, the lack of time in the ED setting, and fears driven by legal consequences, often make a good history irrelevant. Even when it’s done, there are other forces that push it to the background in making medical decisions.
I’m not saying that’s a good thing – it isn’t. But that’s the way it is right now in American medicine, and this aspect of the system shows no sign of changing.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Clinical Challenges - March 2018 What's your diagnosis?
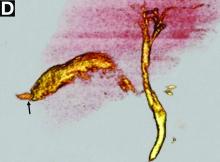
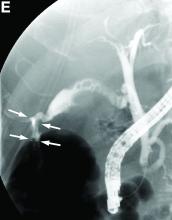
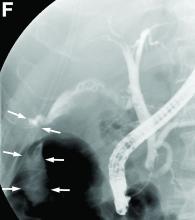
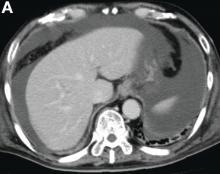
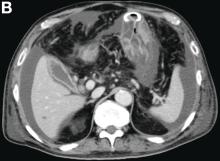
The diagnosis: Spontaneous gallbladder perforation
References
1. Ausania, F., Guzman Suarez, S., Alvarez Garcia, H. et al. Gallbladder perforation: morbidity, mortality and preoperative risk prediction. Surg Endosc. 2015;29:955-60.
2. Niemeier, O.W. Acute free perforation of the gall-bladder. Ann Surg. 1934;99:922-4.
3. Hyodo, T., Kumano, S., Kushihata, F. et al. CT and MR cholangiography: advantages and pitfalls in perioperative evaluation of biliary tree. Br J Radiol. 2012;85:887-96.
The diagnosis: Spontaneous gallbladder perforation
References
1. Ausania, F., Guzman Suarez, S., Alvarez Garcia, H. et al. Gallbladder perforation: morbidity, mortality and preoperative risk prediction. Surg Endosc. 2015;29:955-60.
2. Niemeier, O.W. Acute free perforation of the gall-bladder. Ann Surg. 1934;99:922-4.
3. Hyodo, T., Kumano, S., Kushihata, F. et al. CT and MR cholangiography: advantages and pitfalls in perioperative evaluation of biliary tree. Br J Radiol. 2012;85:887-96.
The diagnosis: Spontaneous gallbladder perforation
References
1. Ausania, F., Guzman Suarez, S., Alvarez Garcia, H. et al. Gallbladder perforation: morbidity, mortality and preoperative risk prediction. Surg Endosc. 2015;29:955-60.
2. Niemeier, O.W. Acute free perforation of the gall-bladder. Ann Surg. 1934;99:922-4.
3. Hyodo, T., Kumano, S., Kushihata, F. et al. CT and MR cholangiography: advantages and pitfalls in perioperative evaluation of biliary tree. Br J Radiol. 2012;85:887-96.
Published previously in Gastroenterology (2016;151[1]:40-2).
What is your diagnosis and treatment?
Triple therapy in question
Clinical question: In patients with nonvalvular atrial fibrillation undergoing percutaneous coronary intervention (PCI), is dabigatran plus a P2Y12 inhibitor safer than, and as efficacious as, triple therapy with warfarin?
Background: Recent studies have shown that patients on long-term anticoagulation who undergo PCI can be managed on oral anticoagulants and P2Y12 inhibitors with lower bleeding rates than do those who receive triple therapy.
Study design: Randomized, controlled trial.
Setting: 414 sites in 41 countries.
Synopsis: In 2,725 patients with nonvalvular atrial fibrillation undergoing PCI, low-dose (110 mg, twice daily) and high-dose (150 mg, twice daily) dabigatran plus a P2Y12 inhibitor lowered absolute bleeding risk by 11.5% and 5.5%, respectively, compared with triple therapy. Rates of thrombosis, death, and unexpected revascularization as a composite endpoint were noninferior to triple therapy for both dabigatran doses studied. In patients on dabigatran for atrial fibrillation, it is reasonable to continue dabigatran and add a single P2Y12 inhibitor (clopidogrel or ticagrelor) but not aspirin after PCI. In patients at high risk for bleeding complications, it may be reasonable to dose reduce the dabigatran from 150 mg twice daily to 110 mg twice daily before starting antiplatelet therapy, although the study was underpowered to examine this.
Bottom line: In patients with atrial fibrillation undergoing PCI, dabigatran plus clopidogrel or ticagrelor had lower bleeding rates and was noninferior with respect to the risk of thromboembolic events when compared with triple therapy with warfarin.
Citation: Cannon CP et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017 Oct 19. doi: 10.1056/NEJMoa1708454.
Dr. Theobald is a hospitalist at the University of Colorado School of Medicine.
Clinical question: In patients with nonvalvular atrial fibrillation undergoing percutaneous coronary intervention (PCI), is dabigatran plus a P2Y12 inhibitor safer than, and as efficacious as, triple therapy with warfarin?
Background: Recent studies have shown that patients on long-term anticoagulation who undergo PCI can be managed on oral anticoagulants and P2Y12 inhibitors with lower bleeding rates than do those who receive triple therapy.
Study design: Randomized, controlled trial.
Setting: 414 sites in 41 countries.
Synopsis: In 2,725 patients with nonvalvular atrial fibrillation undergoing PCI, low-dose (110 mg, twice daily) and high-dose (150 mg, twice daily) dabigatran plus a P2Y12 inhibitor lowered absolute bleeding risk by 11.5% and 5.5%, respectively, compared with triple therapy. Rates of thrombosis, death, and unexpected revascularization as a composite endpoint were noninferior to triple therapy for both dabigatran doses studied. In patients on dabigatran for atrial fibrillation, it is reasonable to continue dabigatran and add a single P2Y12 inhibitor (clopidogrel or ticagrelor) but not aspirin after PCI. In patients at high risk for bleeding complications, it may be reasonable to dose reduce the dabigatran from 150 mg twice daily to 110 mg twice daily before starting antiplatelet therapy, although the study was underpowered to examine this.
Bottom line: In patients with atrial fibrillation undergoing PCI, dabigatran plus clopidogrel or ticagrelor had lower bleeding rates and was noninferior with respect to the risk of thromboembolic events when compared with triple therapy with warfarin.
Citation: Cannon CP et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017 Oct 19. doi: 10.1056/NEJMoa1708454.
Dr. Theobald is a hospitalist at the University of Colorado School of Medicine.
Clinical question: In patients with nonvalvular atrial fibrillation undergoing percutaneous coronary intervention (PCI), is dabigatran plus a P2Y12 inhibitor safer than, and as efficacious as, triple therapy with warfarin?
Background: Recent studies have shown that patients on long-term anticoagulation who undergo PCI can be managed on oral anticoagulants and P2Y12 inhibitors with lower bleeding rates than do those who receive triple therapy.
Study design: Randomized, controlled trial.
Setting: 414 sites in 41 countries.
Synopsis: In 2,725 patients with nonvalvular atrial fibrillation undergoing PCI, low-dose (110 mg, twice daily) and high-dose (150 mg, twice daily) dabigatran plus a P2Y12 inhibitor lowered absolute bleeding risk by 11.5% and 5.5%, respectively, compared with triple therapy. Rates of thrombosis, death, and unexpected revascularization as a composite endpoint were noninferior to triple therapy for both dabigatran doses studied. In patients on dabigatran for atrial fibrillation, it is reasonable to continue dabigatran and add a single P2Y12 inhibitor (clopidogrel or ticagrelor) but not aspirin after PCI. In patients at high risk for bleeding complications, it may be reasonable to dose reduce the dabigatran from 150 mg twice daily to 110 mg twice daily before starting antiplatelet therapy, although the study was underpowered to examine this.
Bottom line: In patients with atrial fibrillation undergoing PCI, dabigatran plus clopidogrel or ticagrelor had lower bleeding rates and was noninferior with respect to the risk of thromboembolic events when compared with triple therapy with warfarin.
Citation: Cannon CP et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017 Oct 19. doi: 10.1056/NEJMoa1708454.
Dr. Theobald is a hospitalist at the University of Colorado School of Medicine.
The Frontier of Transition Medicine: A Unique Inpatient Model for Transitions of Care
The transition of care from pediatric to adult providers has drawn increased national attention to the survival of patients with chronic childhood conditions into adulthood.ttps://www.ncbi.nlm.nih.gov/books/NBK11432/ While survival outcomes have improved due to advances in care, many of these patients experience gaps in medical care when they move from pediatric to adult healthcare systems, resulting in age-inappropriate and fragmented care in adulthood.4 Many youth with chronic childhood conditions are not prepared to move into adult healthcare, and this lack of transition preparation is associated with poorer health outcomes, including elevated glycosylated hemoglobin and loss of transplanted organs.5-7 National transition efforts have largely focused on the outpatient setting and there remains a paucity of literature on inpatient transitions of care.8,9 Although transition-age patients represent a small percentage of patients at children’s hospitals, they accumulate more hospital days and have higher resource utilization compared to their pediatric cohorts.10 In this issue, Coller et al.11 characterize the current state of pediatric to adult inpatient transitions of care among general pediatric services at US children’s hospitals. Over 50% of children’s hospitals did not have a specific adult-oriented hospital identified to receive transitioning patients. Fewer than half of hospitals (38%) had an explicit inpatient transition policy. Notably only 2% of hospitals could track patient outcomes through transitions; however, 41% had systems in place to address insurance issues. Institutions with combined internal medicine-pediatric (Med-Peds) providers more frequently had inpatient transition initiatives (P = .04). It is clear from Coller et al.11 that the adoption of transition initiatives has been delayed since its introduction at the US Surgeon’s conference in 1989, and much work is needed to bridge this gap.12
Coller et al.11 spearhead establishing standardized transition programs using the multidisciplinary Six Core Elements framework and highlight effective techniques from existing inpatient transition processes.13 While we encourage providers to utilize existing partnerships in the outpatient community to bridge the gap for this at-risk population, shifting to adult care continues to be disorganized in the face of some key barriers including challenges in addressing psychosocial needs, gaps in insurance, and poor care coordination between pediatric and adult healthcare systems.4
We propose several inpatient activities to improve transitions. First, we suggest the development of an inpatient transition or Med-Peds consult service across all hospitals. The Med-Peds consult service would implement the Six Core Elements, including transition readiness, transition planning, and providing insurance and referral resources. A Med-Peds consult service has been well received at our institution as it identifies clear leaders with expertise in transition. Coller et al.11 report only 11% of children’s hospitals surveyed had transition policies that referenced inpatient transitions of care. For those institutions without Med-Peds providers, we recommend establishing a hospital-wide transition policy, and identifying hospitalists trained in transitions, with multidisciplinary approaches to staff their transition consult service.
Tracking and monitoring youth in the inpatient transition process occurred in only 2% of hospitals surveyed. We urge for automatic consults to the transition service for adult aged patients admitted to children’s hospitals. With current electronic health records (EHRs), admission order sets with built-in transition consults for adolescents and young adults would improve the identification and tracking of youths. Assuming care of a pediatric patient with multiple comorbidities can be overwhelming for providers.14 The transition consult service could alleviate some of this anxiety with clear and concise documentation using standardized, readily available transition templates. These templates would summarize the patient’s past medical history and outline current medical problems, necessary subspecialty referrals, insurance status, limitations in activities of daily living, ancillary services (including physical therapy, occupational therapy, speech therapy, transportation services), and current level of readiness and independence.
In summary, the transition of care from pediatric to adult providers is a particularly vulnerable time for young adults with chronic medical conditions, and efforts focused on inpatient transitions of medical care have overall been limited. Crucial barriers include addressing psychosocial needs, gaps in insurance, and poor communication between pediatric and adult providers.4 Coller et al.11 have identified several gaps in inpatient transitions of care as well as multiple areas of focus to improve the patient experience. Based on the findings of this study, we urge children’s hospitals caring for adult patients to identify transition leaders, partner with an adult hospital to foster effective transitions, and to protocolize inpatient and outpatient models of transition. Perhaps the most concerning finding of this study was the widespread inability to track transition outcomes. Our group’s experience has led us to believe that coupling an inpatient transition consult team with EHR-based interventions to identify patients and follow outcomes has the most potential to improve inpatient transitions of care from pediatric to adult providers.
Disclosure
The authors have no conflicts of interests or financial disclosures.
1. Elborn JS, Shale DJ, Britton JR. Cystic fibrosis: current survival and population estimates to the year 2000. Thorax. 1991;46(12):881-885.
2. Reid GJ, Webb GD, Barzel M, McCrindle BW, Irvine MJ, Siu SC. Estimates of life expectancy by adolescents and young adults with congenital heart disease. J Am Coll Cardiol. 2006;48(2):349-355. doi:10.1016/j.jacc.2006.03.041.
3. Ferris ME, Gipson DS, Kimmel PL, Eggers PW. Trends in treatment and outcomes of survival of adolescents initiating end-stage renal disease care in the United States of America. Pediatr Nephrol. 2006;21(7):1020-1026. doi:10.1007/s00467-006-0059-9.
4. Sharma N, O’Hare K, Antonelli RC, Sawicki GS. Transition care: future directions in education, health policy, and outcomes research. Acad Pediatr. 2014;14(2):120-127. doi:10.1016/j.acap.2013.11.007.
5. Harden PN, Walsh G, Bandler N, et al. Bridging the gap: an integrated paediatric to adult clinical service for young adults with kidney failure. BMJ. 2012;344:e3718. doi:10.1136/bmj.e3718.
6. Watson AR. Non-compliance and transfer from paediatric to adult transplant unit. Pediatr Nephrol. 2000;14(6):469-472.
7. Lotstein DS, Seid M, Klingensmith G, et al. Transition from pediatric to adult care for youth diagnosed with type 1 diabetes in adolescence. Pediatrics. 2013;131(4):e1062-1070. doi:10.1542/peds.2012-1450.
8. Scal P. Transition for youth with chronic conditions: primary care physicians’ approaches. Pediatrics. 2002;110(6 Pt 2):1315-1321.
9. Kelly AM, Kratz B, Bielski M, Rinehart PM. Implementing transitions for youth with complex chronic conditions using the medical home model. Pediatrics. 2002;110(6 Pt 2):1322-1327.
10. Goodman DM, Hall M, Levin A, et al. Adults with chronic health conditions originating in childhood: inpatient experience in children’s hospitals. Pediatrics. 2011;128(1):5-13. doi:10.1542/peds.2010-2037.
11. Coller RJ, Ahrens S, Ehlenbach M, et al. Transitioning from General Pediatric to Adult-Oriented Inpatient Care: National Survey of US Children’s Hospitals. J Hosp Med. 2018;13(1):13-20.
12. Olson D. Health Care Transitions for Young People. In Field MJ, Jette AM, Institute of Medicine (US) Committee on Disability in America, editors. The Future of Disability in America. Washington, DC: National Academy Press; 2007. https://www.ncbi.nlm.nih.gov/books/NBK11432/.
13. GotTransition.org. http://www.gottransition.org/. Accessed September 15, 2017.
14. Okumura MJ, Kerr EA, Cabana MD, Davis MM, Demonner S, Heisler M. Physician views on barriers to primary care for young adults with childhood-onset chronic disease. Pediatrics. 2010;125(4):e748-754. doi:10.1542/peds.2008-3451.
The transition of care from pediatric to adult providers has drawn increased national attention to the survival of patients with chronic childhood conditions into adulthood.ttps://www.ncbi.nlm.nih.gov/books/NBK11432/ While survival outcomes have improved due to advances in care, many of these patients experience gaps in medical care when they move from pediatric to adult healthcare systems, resulting in age-inappropriate and fragmented care in adulthood.4 Many youth with chronic childhood conditions are not prepared to move into adult healthcare, and this lack of transition preparation is associated with poorer health outcomes, including elevated glycosylated hemoglobin and loss of transplanted organs.5-7 National transition efforts have largely focused on the outpatient setting and there remains a paucity of literature on inpatient transitions of care.8,9 Although transition-age patients represent a small percentage of patients at children’s hospitals, they accumulate more hospital days and have higher resource utilization compared to their pediatric cohorts.10 In this issue, Coller et al.11 characterize the current state of pediatric to adult inpatient transitions of care among general pediatric services at US children’s hospitals. Over 50% of children’s hospitals did not have a specific adult-oriented hospital identified to receive transitioning patients. Fewer than half of hospitals (38%) had an explicit inpatient transition policy. Notably only 2% of hospitals could track patient outcomes through transitions; however, 41% had systems in place to address insurance issues. Institutions with combined internal medicine-pediatric (Med-Peds) providers more frequently had inpatient transition initiatives (P = .04). It is clear from Coller et al.11 that the adoption of transition initiatives has been delayed since its introduction at the US Surgeon’s conference in 1989, and much work is needed to bridge this gap.12
Coller et al.11 spearhead establishing standardized transition programs using the multidisciplinary Six Core Elements framework and highlight effective techniques from existing inpatient transition processes.13 While we encourage providers to utilize existing partnerships in the outpatient community to bridge the gap for this at-risk population, shifting to adult care continues to be disorganized in the face of some key barriers including challenges in addressing psychosocial needs, gaps in insurance, and poor care coordination between pediatric and adult healthcare systems.4
We propose several inpatient activities to improve transitions. First, we suggest the development of an inpatient transition or Med-Peds consult service across all hospitals. The Med-Peds consult service would implement the Six Core Elements, including transition readiness, transition planning, and providing insurance and referral resources. A Med-Peds consult service has been well received at our institution as it identifies clear leaders with expertise in transition. Coller et al.11 report only 11% of children’s hospitals surveyed had transition policies that referenced inpatient transitions of care. For those institutions without Med-Peds providers, we recommend establishing a hospital-wide transition policy, and identifying hospitalists trained in transitions, with multidisciplinary approaches to staff their transition consult service.
Tracking and monitoring youth in the inpatient transition process occurred in only 2% of hospitals surveyed. We urge for automatic consults to the transition service for adult aged patients admitted to children’s hospitals. With current electronic health records (EHRs), admission order sets with built-in transition consults for adolescents and young adults would improve the identification and tracking of youths. Assuming care of a pediatric patient with multiple comorbidities can be overwhelming for providers.14 The transition consult service could alleviate some of this anxiety with clear and concise documentation using standardized, readily available transition templates. These templates would summarize the patient’s past medical history and outline current medical problems, necessary subspecialty referrals, insurance status, limitations in activities of daily living, ancillary services (including physical therapy, occupational therapy, speech therapy, transportation services), and current level of readiness and independence.
In summary, the transition of care from pediatric to adult providers is a particularly vulnerable time for young adults with chronic medical conditions, and efforts focused on inpatient transitions of medical care have overall been limited. Crucial barriers include addressing psychosocial needs, gaps in insurance, and poor communication between pediatric and adult providers.4 Coller et al.11 have identified several gaps in inpatient transitions of care as well as multiple areas of focus to improve the patient experience. Based on the findings of this study, we urge children’s hospitals caring for adult patients to identify transition leaders, partner with an adult hospital to foster effective transitions, and to protocolize inpatient and outpatient models of transition. Perhaps the most concerning finding of this study was the widespread inability to track transition outcomes. Our group’s experience has led us to believe that coupling an inpatient transition consult team with EHR-based interventions to identify patients and follow outcomes has the most potential to improve inpatient transitions of care from pediatric to adult providers.
Disclosure
The authors have no conflicts of interests or financial disclosures.
The transition of care from pediatric to adult providers has drawn increased national attention to the survival of patients with chronic childhood conditions into adulthood.ttps://www.ncbi.nlm.nih.gov/books/NBK11432/ While survival outcomes have improved due to advances in care, many of these patients experience gaps in medical care when they move from pediatric to adult healthcare systems, resulting in age-inappropriate and fragmented care in adulthood.4 Many youth with chronic childhood conditions are not prepared to move into adult healthcare, and this lack of transition preparation is associated with poorer health outcomes, including elevated glycosylated hemoglobin and loss of transplanted organs.5-7 National transition efforts have largely focused on the outpatient setting and there remains a paucity of literature on inpatient transitions of care.8,9 Although transition-age patients represent a small percentage of patients at children’s hospitals, they accumulate more hospital days and have higher resource utilization compared to their pediatric cohorts.10 In this issue, Coller et al.11 characterize the current state of pediatric to adult inpatient transitions of care among general pediatric services at US children’s hospitals. Over 50% of children’s hospitals did not have a specific adult-oriented hospital identified to receive transitioning patients. Fewer than half of hospitals (38%) had an explicit inpatient transition policy. Notably only 2% of hospitals could track patient outcomes through transitions; however, 41% had systems in place to address insurance issues. Institutions with combined internal medicine-pediatric (Med-Peds) providers more frequently had inpatient transition initiatives (P = .04). It is clear from Coller et al.11 that the adoption of transition initiatives has been delayed since its introduction at the US Surgeon’s conference in 1989, and much work is needed to bridge this gap.12
Coller et al.11 spearhead establishing standardized transition programs using the multidisciplinary Six Core Elements framework and highlight effective techniques from existing inpatient transition processes.13 While we encourage providers to utilize existing partnerships in the outpatient community to bridge the gap for this at-risk population, shifting to adult care continues to be disorganized in the face of some key barriers including challenges in addressing psychosocial needs, gaps in insurance, and poor care coordination between pediatric and adult healthcare systems.4
We propose several inpatient activities to improve transitions. First, we suggest the development of an inpatient transition or Med-Peds consult service across all hospitals. The Med-Peds consult service would implement the Six Core Elements, including transition readiness, transition planning, and providing insurance and referral resources. A Med-Peds consult service has been well received at our institution as it identifies clear leaders with expertise in transition. Coller et al.11 report only 11% of children’s hospitals surveyed had transition policies that referenced inpatient transitions of care. For those institutions without Med-Peds providers, we recommend establishing a hospital-wide transition policy, and identifying hospitalists trained in transitions, with multidisciplinary approaches to staff their transition consult service.
Tracking and monitoring youth in the inpatient transition process occurred in only 2% of hospitals surveyed. We urge for automatic consults to the transition service for adult aged patients admitted to children’s hospitals. With current electronic health records (EHRs), admission order sets with built-in transition consults for adolescents and young adults would improve the identification and tracking of youths. Assuming care of a pediatric patient with multiple comorbidities can be overwhelming for providers.14 The transition consult service could alleviate some of this anxiety with clear and concise documentation using standardized, readily available transition templates. These templates would summarize the patient’s past medical history and outline current medical problems, necessary subspecialty referrals, insurance status, limitations in activities of daily living, ancillary services (including physical therapy, occupational therapy, speech therapy, transportation services), and current level of readiness and independence.
In summary, the transition of care from pediatric to adult providers is a particularly vulnerable time for young adults with chronic medical conditions, and efforts focused on inpatient transitions of medical care have overall been limited. Crucial barriers include addressing psychosocial needs, gaps in insurance, and poor communication between pediatric and adult providers.4 Coller et al.11 have identified several gaps in inpatient transitions of care as well as multiple areas of focus to improve the patient experience. Based on the findings of this study, we urge children’s hospitals caring for adult patients to identify transition leaders, partner with an adult hospital to foster effective transitions, and to protocolize inpatient and outpatient models of transition. Perhaps the most concerning finding of this study was the widespread inability to track transition outcomes. Our group’s experience has led us to believe that coupling an inpatient transition consult team with EHR-based interventions to identify patients and follow outcomes has the most potential to improve inpatient transitions of care from pediatric to adult providers.
Disclosure
The authors have no conflicts of interests or financial disclosures.
1. Elborn JS, Shale DJ, Britton JR. Cystic fibrosis: current survival and population estimates to the year 2000. Thorax. 1991;46(12):881-885.
2. Reid GJ, Webb GD, Barzel M, McCrindle BW, Irvine MJ, Siu SC. Estimates of life expectancy by adolescents and young adults with congenital heart disease. J Am Coll Cardiol. 2006;48(2):349-355. doi:10.1016/j.jacc.2006.03.041.
3. Ferris ME, Gipson DS, Kimmel PL, Eggers PW. Trends in treatment and outcomes of survival of adolescents initiating end-stage renal disease care in the United States of America. Pediatr Nephrol. 2006;21(7):1020-1026. doi:10.1007/s00467-006-0059-9.
4. Sharma N, O’Hare K, Antonelli RC, Sawicki GS. Transition care: future directions in education, health policy, and outcomes research. Acad Pediatr. 2014;14(2):120-127. doi:10.1016/j.acap.2013.11.007.
5. Harden PN, Walsh G, Bandler N, et al. Bridging the gap: an integrated paediatric to adult clinical service for young adults with kidney failure. BMJ. 2012;344:e3718. doi:10.1136/bmj.e3718.
6. Watson AR. Non-compliance and transfer from paediatric to adult transplant unit. Pediatr Nephrol. 2000;14(6):469-472.
7. Lotstein DS, Seid M, Klingensmith G, et al. Transition from pediatric to adult care for youth diagnosed with type 1 diabetes in adolescence. Pediatrics. 2013;131(4):e1062-1070. doi:10.1542/peds.2012-1450.
8. Scal P. Transition for youth with chronic conditions: primary care physicians’ approaches. Pediatrics. 2002;110(6 Pt 2):1315-1321.
9. Kelly AM, Kratz B, Bielski M, Rinehart PM. Implementing transitions for youth with complex chronic conditions using the medical home model. Pediatrics. 2002;110(6 Pt 2):1322-1327.
10. Goodman DM, Hall M, Levin A, et al. Adults with chronic health conditions originating in childhood: inpatient experience in children’s hospitals. Pediatrics. 2011;128(1):5-13. doi:10.1542/peds.2010-2037.
11. Coller RJ, Ahrens S, Ehlenbach M, et al. Transitioning from General Pediatric to Adult-Oriented Inpatient Care: National Survey of US Children’s Hospitals. J Hosp Med. 2018;13(1):13-20.
12. Olson D. Health Care Transitions for Young People. In Field MJ, Jette AM, Institute of Medicine (US) Committee on Disability in America, editors. The Future of Disability in America. Washington, DC: National Academy Press; 2007. https://www.ncbi.nlm.nih.gov/books/NBK11432/.
13. GotTransition.org. http://www.gottransition.org/. Accessed September 15, 2017.
14. Okumura MJ, Kerr EA, Cabana MD, Davis MM, Demonner S, Heisler M. Physician views on barriers to primary care for young adults with childhood-onset chronic disease. Pediatrics. 2010;125(4):e748-754. doi:10.1542/peds.2008-3451.
1. Elborn JS, Shale DJ, Britton JR. Cystic fibrosis: current survival and population estimates to the year 2000. Thorax. 1991;46(12):881-885.
2. Reid GJ, Webb GD, Barzel M, McCrindle BW, Irvine MJ, Siu SC. Estimates of life expectancy by adolescents and young adults with congenital heart disease. J Am Coll Cardiol. 2006;48(2):349-355. doi:10.1016/j.jacc.2006.03.041.
3. Ferris ME, Gipson DS, Kimmel PL, Eggers PW. Trends in treatment and outcomes of survival of adolescents initiating end-stage renal disease care in the United States of America. Pediatr Nephrol. 2006;21(7):1020-1026. doi:10.1007/s00467-006-0059-9.
4. Sharma N, O’Hare K, Antonelli RC, Sawicki GS. Transition care: future directions in education, health policy, and outcomes research. Acad Pediatr. 2014;14(2):120-127. doi:10.1016/j.acap.2013.11.007.
5. Harden PN, Walsh G, Bandler N, et al. Bridging the gap: an integrated paediatric to adult clinical service for young adults with kidney failure. BMJ. 2012;344:e3718. doi:10.1136/bmj.e3718.
6. Watson AR. Non-compliance and transfer from paediatric to adult transplant unit. Pediatr Nephrol. 2000;14(6):469-472.
7. Lotstein DS, Seid M, Klingensmith G, et al. Transition from pediatric to adult care for youth diagnosed with type 1 diabetes in adolescence. Pediatrics. 2013;131(4):e1062-1070. doi:10.1542/peds.2012-1450.
8. Scal P. Transition for youth with chronic conditions: primary care physicians’ approaches. Pediatrics. 2002;110(6 Pt 2):1315-1321.
9. Kelly AM, Kratz B, Bielski M, Rinehart PM. Implementing transitions for youth with complex chronic conditions using the medical home model. Pediatrics. 2002;110(6 Pt 2):1322-1327.
10. Goodman DM, Hall M, Levin A, et al. Adults with chronic health conditions originating in childhood: inpatient experience in children’s hospitals. Pediatrics. 2011;128(1):5-13. doi:10.1542/peds.2010-2037.
11. Coller RJ, Ahrens S, Ehlenbach M, et al. Transitioning from General Pediatric to Adult-Oriented Inpatient Care: National Survey of US Children’s Hospitals. J Hosp Med. 2018;13(1):13-20.
12. Olson D. Health Care Transitions for Young People. In Field MJ, Jette AM, Institute of Medicine (US) Committee on Disability in America, editors. The Future of Disability in America. Washington, DC: National Academy Press; 2007. https://www.ncbi.nlm.nih.gov/books/NBK11432/.
13. GotTransition.org. http://www.gottransition.org/. Accessed September 15, 2017.
14. Okumura MJ, Kerr EA, Cabana MD, Davis MM, Demonner S, Heisler M. Physician views on barriers to primary care for young adults with childhood-onset chronic disease. Pediatrics. 2010;125(4):e748-754. doi:10.1542/peds.2008-3451.
© 2018 Society of Hospital Medicine
Penalizing Physicians for Low-Value Care in Hospital Medicine: A Randomized Survey
Reducing low-value care—services for which there is little to no benefit, little benefit relative to cost, or outsized potential harm compared with benefit—is an essential step toward maintaining or improving quality while lowering cost. Unfortunately, low-value services persist widelydespite professional consensus, guidelines, and national campaigns aimed to reduce them.1-3 In turn, policy makers are beginning to consider financially penalizing physicians in order to deter low-value services.4,5 Physician support for such penalties remains unknown. In this study, we used a randomized survey experiment to evaluate how the framing of harms from low-value care—in terms of those to patients, healthcare institutions, or society—influenced physician support of financial penalties for low-value care services.
METHODS
Study Sample
By using a stratified random sample maintained by the American College of Physicians, we conducted a web-based survey among 484 physicians who were either internal medicine residents or internists practicing hospital medicine.
Instrument Design and Administration
Our study focused on 3 low-value services relevant to inpatient medicine: (1) placing, and leaving in, urinary catheters for urine output monitoring in noncritically ill patients; (2) ordering continuous telemetry monitoring for nonintensive care unit (non-ICU) patients without a protocol governing continuation; and (3) prescribing stress ulcer prophylaxis for medical patients not at a high risk for gastrointestinal (GI) complications. Although the nature and trade-offs between costs, harms, and benefits vary by individual service, all 3 are promulgated through the Choosing Wisely® guidelines as low value based on existing data and professional consensus from the Society of Hospital Medicine.6
To evaluate intended behavior related to these 3 low-value services, respondents were first presented with 3 clinical vignettes focused on the care of patients hospitalized for pneumonia, congestive heart failure, and alcohol withdrawal, which were selected to reflect common inpatient medicine scenarios. Respondents were asked to use a 4-point scale (very likely to very unlikely) to estimate how likely they were to recommend various tests or treatments, including the low-value services noted above. Respondents who were “somewhat unlikely” and “very unlikely” to recommend low-value services were considered concordant with low-value care guidelines.
Following the vignettes, respondents then used a 5-point scale (strongly agree to strongly disagree) to indicate their agreement with a policy that financially penalizes physicians for prescribing each service. Support was defined as “somewhat or strongly” agreeing with the policy. Respondents were randomized to receive 1 of 3 versions of this question (supplementary Appendix).
All versions stated that, “According to research and expert opinion, certain aspects of inpatient care provide little benefit to patients” and listed the 3 low-value services noted above. The “patient harm” version also described the harm of low-value care as costs to patients and risk for clinical harms and complications. The “societal harm” version described the harms as costs to society and utilization of limited healthcare resources. The “institutional harm” version described harms as costs to hospitals and insurers.
Other survey items were adapted from existing literature7-9 and evaluated respondent beliefs about the effectiveness of physician incentives in improving the value of care, as well as the appropriateness of including cost considerations in clinical decision-making.
The instrument was pilot tested among study team members and several independent internists affiliated with the University of Pennsylvania. After incorporating feedback into the final instrument, the web-based survey was distributed to eligible physicians via e-mail. Responses were anonymous and respondents received a $15 gift card for participation. The protocol was reviewed and deemed exempt by the University of Pennsylvania Institutional Review Board.
Statistical Analysis
Respondent characteristics (sociodemographic, intended clinical behavior, and cost control attitudes) were described by using percentages for categorical variables and medians and interquartile ranges for continuous variables. Balance in respondent characteristics across survey versions was evaluated using χ2 and Kruskal-Wallis tests. Multivariable logistic regression, adjusted for characteristics in the Table, was used to evaluate the association between survey version and policy support. All tests of significance were 2-tailed with significance level alpha = 0.05. Analyses were performed using STATA version 14.1 (StataCorp LLC, College Station, TX, http://www.stata.com).
RESULTS
Of 484 eligible respondents, 187 (39%) completed the survey. Compared with nonrespondents, respondents were more likely to be female (30% vs 26%, P = 0.001), older (mean age 41 vs 36 years, P < 0.001), and practicing clinicians rather than internal medicine residents (87% vs 69%, P < 0.001). Physician characteristics were similar across the 3 survey versions (Table). Most respondents agreed that financial incentives for individual physicians is an effective way to improve the value of healthcare (73.3%) and that physicians should consider the costs of a test or treatment to society when making clinical decisions for patients (79.1%). The majority also felt that clinicians have a duty to offer a test or treatment to a patient if it has any chance of helping them (70.1%) and that it is inappropriate for anyone beyond the clinician and patient to decide if a test or treatment is “worth the cost” (63.6%).
Overall, policy support rate was 39.6% and was the highest for the “societal harm” version (48.4%), followed by the “institutional harm” (36.9%) and “patient harm” (33.3%) versions. Compared with respondents receiving the “patient harm” version, those receiving the “societal harm” version (adjusted odds ratio [OR] 2.83; 95% confidence interval [CI], 1.20-6.69), but not the “institutional harm” framing (adjusted OR 1.53; 95% CI, 0.66-3.53), were more likely to report policy support. Policy support was also higher among those who agreed that providing financial incentives to individual physicians is an effective way to improve the value of healthcare (adjusted OR 4.61; 95% CI, 1.80-11.80).
DISCUSSION
To our knowledge, this study is the first to prospectively evaluate physician support of financial penalties for low-value services relevant to hospital medicine. It has 2 main findings.
First, although overall policy support was relatively low (39.6%), it varied significantly on the basis of how the harms of low-value care were framed. Support was highest in the “societal harm” version, suggesting that emphasizing these harms may increase acceptability of financial penalties among physicians and contribute to the larger effort to decrease low-value care in hospital settings. The comparatively low support for the “patient harm” version is somewhat surprising but may reflect variation in the nature of harm, benefit, and cost trade-offs for individual low-value services, as noted above, and physician belief that some low-value services do not in fact produce significant clinical harms.
For example, whereas evidence demonstrates that stress ulcer prophylaxis in non-ICU patients can harm patients through nosocomial infections and adverse drug effects,10,11 the clinical harms of telemetry are less obvious. Telemetry’s low value derives more from its high cost relative to benefit, rather than its potential for clinical harm.6 The many paths to “low value” underscore the need to examine attitudes and uptake toward these services separately and may explain the wide range in concordance between intended clinical behavior and low-value care guidelines (11.8% to 78.6%).
Reinforcing policies could more effectively deter low-value care. For example, multiple forces, including Medicare payment reform and national accreditation policies,12,13 have converged to discourage low-value use of urinary catheters in hospitalized patients. In contrast, there has been little reinforcement beyond consensus guidelines to reduce low-value use of telemetric monitoring. Given questions about whether consensus methods alone can deter low-value care beyond obvious “low hanging fruit,”14 policy makers could coordinate policies to accelerate progress within other priority areas.
Broad policies should also be paired with local initiatives to influence physician behavior. For example, health systems have begun successfully leveraging the electronic medical record and utilizing behavioral economics principles to design interventions to reduce inappropriate overuse of antibiotics for upper respiratory infections in primary care clinics.15 Organizations are also redesigning care processes in response to resource utilization imperatives under ongoing value-based care payment reform. Care redesign and behavioral interventions embedded at the point of care can both help deter low-value services in inpatient settings.
Study limitations include a relatively low response rate, which limits generalizability. However, all 3 randomized groups were similar on measured characteristics, and experimental randomization reduces the nonresponse bias concerns accompanying descriptive surveys. Additionally, although we evaluated intended clinical behavior in a national sample, our results may not reflect actual behavior among all physicians practicing hospital medicine. Future work could include assessments of actual or self-reported practices or examine additional factors, including site, years of practice, knowledge about guidelines, and other possible determinants of guideline-concordant behaviors.
Despite these limitations, our study provides important early evidence about physician support of financial penalties for low-value care relevant to hospital medicine. As policy makers design and organizational leaders implement financial incentive policies, this information can help increase their acceptability among physicians and more effectively reduce low-value care within hospitals.
Disclosure
Drs. Liao, Schapira, Mitra, and Weissman have no conflicts to disclose. Dr. Navathe serves as advisor to Navvis and Company, Navigant Inc, Lynx Medical, Indegene Inc, and Sutherland Global Services and receives an honorarium from Elsevier Press, none of which have relationship to this manuscript. Dr. Asch is a partner and part owner of VAL Health, which has no relationship to this manuscript.
Funding
This work was supported by The Leonard Davis Institute of Health Economics at the University of Pennsylvania, which had no role in the study design, data collection, analysis, or interpretation of results.
1. The MedPAC blog. Use of low-value care in Medicare is substantial. http://www.medpac.gov/-blog-/medpacblog/2015/05/21/use-of-low-value-care-in-medicare-is-substantial. Accessed on September 18, 2017.
2. American Board of Internal Medicine Foundation. Choosing Wisely. http://www.choosingwisely.org/. Accessed on September 18, 2017.
3. Rosenberg A, Agiro A, Gottlieb M, et al. Early Trends Among Seven Recommendations From the Choosing Wisely Campaign. JAMA Intern Med. 2015;175(12):1913-1920. PubMed
4. Centers for Medicare & Medicaid Services. CMS Response to Public Comments on Non-Recommended PSA-Based Screening Measure. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/MMS/Downloads/eCQM-Development-and-Maintenance-for-Eligible-Professionals_CMS_PSA_Response_Public-Comment.pdf. Accessed September 18, 2017.
5. Berwick DM. Avoiding overuse-the next quality frontier. Lancet. 2017;390(10090):102-104. doi: 10.1016/S0140-6736(16)32570-3. PubMed
6. Society of Hospital Medicine. Choosing Wisely. https://www.hospitalmedicine.org/choosingwisely. Accessed on September 18, 2017.
7. Tilburt JC, Wynia MK, Sheeler RD, et al. Views of US Physicians About Controlling Health Care Costs. JAMA. 2013;310(4):380-388. PubMed
8. Ginsburg ME, Kravitz RL, Sandberg WA. A survey of physician attitudes and practices concerning cost-effectiveness in patient care. West J Med. 2000;173(6):309-394. PubMed
9. Colla CH, Kinsella EA, Morden NE, Meyers DJ, Rosenthal MB, Sequist TD. Physician perceptions of Choosing Wisely and drivers of overuse. Am J Manag Care. 2016;22(5):337-343. PubMed
10. Herzig SJ, Vaughn BP, Howell MD, Ngo LH, Marcantonio ER. Acid-suppressive medication use and the risk for nosocomial gastrointestinal tract bleeding. Arch Intern Med. 2011;171(11):991-997. PubMed
11. Pappas M, Jolly S, Vijan S. Defining Appropriate Use of Proton-Pump Inhibitors Among Medical Inpatients. J Gen Intern Med. 2016;31(4):364-371. PubMed
12. Centers for Medicare & Medicaid Services. CMS’ Value-Based Programs. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/Value-Based-Programs.html. Accessed September 18, 2017.
13. The Joint Commission. Requirements for the Catheter-Associated Urinary Tract Infections (CAUTI) National Patient Safety Goal for Hospitals. https://www.jointcommission.org/assets/1/6/R3_Cauti_HAP.pdf. Accessed September 18, 2017 .
14. Beaudin-Seiler B, Ciarametaro M, Dubois R, Lee J, Fendrick AM. Reducing Low-Value Care. Health Affairs Blog. http://healthaffairs.org/blog/2016/09/20/reducing-low-value-care/. Accessed on September 18, 2017.
15. Meeker D, Linder JA, Fox CR, et al. Effect of Behavioral Interventions on Inappropriate Antibiotic Prescribing Among Primary Care Practices: A Randomized Clinical Trial. JAMA. 2016;315(6):562-570. PubMed
Reducing low-value care—services for which there is little to no benefit, little benefit relative to cost, or outsized potential harm compared with benefit—is an essential step toward maintaining or improving quality while lowering cost. Unfortunately, low-value services persist widelydespite professional consensus, guidelines, and national campaigns aimed to reduce them.1-3 In turn, policy makers are beginning to consider financially penalizing physicians in order to deter low-value services.4,5 Physician support for such penalties remains unknown. In this study, we used a randomized survey experiment to evaluate how the framing of harms from low-value care—in terms of those to patients, healthcare institutions, or society—influenced physician support of financial penalties for low-value care services.
METHODS
Study Sample
By using a stratified random sample maintained by the American College of Physicians, we conducted a web-based survey among 484 physicians who were either internal medicine residents or internists practicing hospital medicine.
Instrument Design and Administration
Our study focused on 3 low-value services relevant to inpatient medicine: (1) placing, and leaving in, urinary catheters for urine output monitoring in noncritically ill patients; (2) ordering continuous telemetry monitoring for nonintensive care unit (non-ICU) patients without a protocol governing continuation; and (3) prescribing stress ulcer prophylaxis for medical patients not at a high risk for gastrointestinal (GI) complications. Although the nature and trade-offs between costs, harms, and benefits vary by individual service, all 3 are promulgated through the Choosing Wisely® guidelines as low value based on existing data and professional consensus from the Society of Hospital Medicine.6
To evaluate intended behavior related to these 3 low-value services, respondents were first presented with 3 clinical vignettes focused on the care of patients hospitalized for pneumonia, congestive heart failure, and alcohol withdrawal, which were selected to reflect common inpatient medicine scenarios. Respondents were asked to use a 4-point scale (very likely to very unlikely) to estimate how likely they were to recommend various tests or treatments, including the low-value services noted above. Respondents who were “somewhat unlikely” and “very unlikely” to recommend low-value services were considered concordant with low-value care guidelines.
Following the vignettes, respondents then used a 5-point scale (strongly agree to strongly disagree) to indicate their agreement with a policy that financially penalizes physicians for prescribing each service. Support was defined as “somewhat or strongly” agreeing with the policy. Respondents were randomized to receive 1 of 3 versions of this question (supplementary Appendix).
All versions stated that, “According to research and expert opinion, certain aspects of inpatient care provide little benefit to patients” and listed the 3 low-value services noted above. The “patient harm” version also described the harm of low-value care as costs to patients and risk for clinical harms and complications. The “societal harm” version described the harms as costs to society and utilization of limited healthcare resources. The “institutional harm” version described harms as costs to hospitals and insurers.
Other survey items were adapted from existing literature7-9 and evaluated respondent beliefs about the effectiveness of physician incentives in improving the value of care, as well as the appropriateness of including cost considerations in clinical decision-making.
The instrument was pilot tested among study team members and several independent internists affiliated with the University of Pennsylvania. After incorporating feedback into the final instrument, the web-based survey was distributed to eligible physicians via e-mail. Responses were anonymous and respondents received a $15 gift card for participation. The protocol was reviewed and deemed exempt by the University of Pennsylvania Institutional Review Board.
Statistical Analysis
Respondent characteristics (sociodemographic, intended clinical behavior, and cost control attitudes) were described by using percentages for categorical variables and medians and interquartile ranges for continuous variables. Balance in respondent characteristics across survey versions was evaluated using χ2 and Kruskal-Wallis tests. Multivariable logistic regression, adjusted for characteristics in the Table, was used to evaluate the association between survey version and policy support. All tests of significance were 2-tailed with significance level alpha = 0.05. Analyses were performed using STATA version 14.1 (StataCorp LLC, College Station, TX, http://www.stata.com).
RESULTS
Of 484 eligible respondents, 187 (39%) completed the survey. Compared with nonrespondents, respondents were more likely to be female (30% vs 26%, P = 0.001), older (mean age 41 vs 36 years, P < 0.001), and practicing clinicians rather than internal medicine residents (87% vs 69%, P < 0.001). Physician characteristics were similar across the 3 survey versions (Table). Most respondents agreed that financial incentives for individual physicians is an effective way to improve the value of healthcare (73.3%) and that physicians should consider the costs of a test or treatment to society when making clinical decisions for patients (79.1%). The majority also felt that clinicians have a duty to offer a test or treatment to a patient if it has any chance of helping them (70.1%) and that it is inappropriate for anyone beyond the clinician and patient to decide if a test or treatment is “worth the cost” (63.6%).
Overall, policy support rate was 39.6% and was the highest for the “societal harm” version (48.4%), followed by the “institutional harm” (36.9%) and “patient harm” (33.3%) versions. Compared with respondents receiving the “patient harm” version, those receiving the “societal harm” version (adjusted odds ratio [OR] 2.83; 95% confidence interval [CI], 1.20-6.69), but not the “institutional harm” framing (adjusted OR 1.53; 95% CI, 0.66-3.53), were more likely to report policy support. Policy support was also higher among those who agreed that providing financial incentives to individual physicians is an effective way to improve the value of healthcare (adjusted OR 4.61; 95% CI, 1.80-11.80).
DISCUSSION
To our knowledge, this study is the first to prospectively evaluate physician support of financial penalties for low-value services relevant to hospital medicine. It has 2 main findings.
First, although overall policy support was relatively low (39.6%), it varied significantly on the basis of how the harms of low-value care were framed. Support was highest in the “societal harm” version, suggesting that emphasizing these harms may increase acceptability of financial penalties among physicians and contribute to the larger effort to decrease low-value care in hospital settings. The comparatively low support for the “patient harm” version is somewhat surprising but may reflect variation in the nature of harm, benefit, and cost trade-offs for individual low-value services, as noted above, and physician belief that some low-value services do not in fact produce significant clinical harms.
For example, whereas evidence demonstrates that stress ulcer prophylaxis in non-ICU patients can harm patients through nosocomial infections and adverse drug effects,10,11 the clinical harms of telemetry are less obvious. Telemetry’s low value derives more from its high cost relative to benefit, rather than its potential for clinical harm.6 The many paths to “low value” underscore the need to examine attitudes and uptake toward these services separately and may explain the wide range in concordance between intended clinical behavior and low-value care guidelines (11.8% to 78.6%).
Reinforcing policies could more effectively deter low-value care. For example, multiple forces, including Medicare payment reform and national accreditation policies,12,13 have converged to discourage low-value use of urinary catheters in hospitalized patients. In contrast, there has been little reinforcement beyond consensus guidelines to reduce low-value use of telemetric monitoring. Given questions about whether consensus methods alone can deter low-value care beyond obvious “low hanging fruit,”14 policy makers could coordinate policies to accelerate progress within other priority areas.
Broad policies should also be paired with local initiatives to influence physician behavior. For example, health systems have begun successfully leveraging the electronic medical record and utilizing behavioral economics principles to design interventions to reduce inappropriate overuse of antibiotics for upper respiratory infections in primary care clinics.15 Organizations are also redesigning care processes in response to resource utilization imperatives under ongoing value-based care payment reform. Care redesign and behavioral interventions embedded at the point of care can both help deter low-value services in inpatient settings.
Study limitations include a relatively low response rate, which limits generalizability. However, all 3 randomized groups were similar on measured characteristics, and experimental randomization reduces the nonresponse bias concerns accompanying descriptive surveys. Additionally, although we evaluated intended clinical behavior in a national sample, our results may not reflect actual behavior among all physicians practicing hospital medicine. Future work could include assessments of actual or self-reported practices or examine additional factors, including site, years of practice, knowledge about guidelines, and other possible determinants of guideline-concordant behaviors.
Despite these limitations, our study provides important early evidence about physician support of financial penalties for low-value care relevant to hospital medicine. As policy makers design and organizational leaders implement financial incentive policies, this information can help increase their acceptability among physicians and more effectively reduce low-value care within hospitals.
Disclosure
Drs. Liao, Schapira, Mitra, and Weissman have no conflicts to disclose. Dr. Navathe serves as advisor to Navvis and Company, Navigant Inc, Lynx Medical, Indegene Inc, and Sutherland Global Services and receives an honorarium from Elsevier Press, none of which have relationship to this manuscript. Dr. Asch is a partner and part owner of VAL Health, which has no relationship to this manuscript.
Funding
This work was supported by The Leonard Davis Institute of Health Economics at the University of Pennsylvania, which had no role in the study design, data collection, analysis, or interpretation of results.
Reducing low-value care—services for which there is little to no benefit, little benefit relative to cost, or outsized potential harm compared with benefit—is an essential step toward maintaining or improving quality while lowering cost. Unfortunately, low-value services persist widelydespite professional consensus, guidelines, and national campaigns aimed to reduce them.1-3 In turn, policy makers are beginning to consider financially penalizing physicians in order to deter low-value services.4,5 Physician support for such penalties remains unknown. In this study, we used a randomized survey experiment to evaluate how the framing of harms from low-value care—in terms of those to patients, healthcare institutions, or society—influenced physician support of financial penalties for low-value care services.
METHODS
Study Sample
By using a stratified random sample maintained by the American College of Physicians, we conducted a web-based survey among 484 physicians who were either internal medicine residents or internists practicing hospital medicine.
Instrument Design and Administration
Our study focused on 3 low-value services relevant to inpatient medicine: (1) placing, and leaving in, urinary catheters for urine output monitoring in noncritically ill patients; (2) ordering continuous telemetry monitoring for nonintensive care unit (non-ICU) patients without a protocol governing continuation; and (3) prescribing stress ulcer prophylaxis for medical patients not at a high risk for gastrointestinal (GI) complications. Although the nature and trade-offs between costs, harms, and benefits vary by individual service, all 3 are promulgated through the Choosing Wisely® guidelines as low value based on existing data and professional consensus from the Society of Hospital Medicine.6
To evaluate intended behavior related to these 3 low-value services, respondents were first presented with 3 clinical vignettes focused on the care of patients hospitalized for pneumonia, congestive heart failure, and alcohol withdrawal, which were selected to reflect common inpatient medicine scenarios. Respondents were asked to use a 4-point scale (very likely to very unlikely) to estimate how likely they were to recommend various tests or treatments, including the low-value services noted above. Respondents who were “somewhat unlikely” and “very unlikely” to recommend low-value services were considered concordant with low-value care guidelines.
Following the vignettes, respondents then used a 5-point scale (strongly agree to strongly disagree) to indicate their agreement with a policy that financially penalizes physicians for prescribing each service. Support was defined as “somewhat or strongly” agreeing with the policy. Respondents were randomized to receive 1 of 3 versions of this question (supplementary Appendix).
All versions stated that, “According to research and expert opinion, certain aspects of inpatient care provide little benefit to patients” and listed the 3 low-value services noted above. The “patient harm” version also described the harm of low-value care as costs to patients and risk for clinical harms and complications. The “societal harm” version described the harms as costs to society and utilization of limited healthcare resources. The “institutional harm” version described harms as costs to hospitals and insurers.
Other survey items were adapted from existing literature7-9 and evaluated respondent beliefs about the effectiveness of physician incentives in improving the value of care, as well as the appropriateness of including cost considerations in clinical decision-making.
The instrument was pilot tested among study team members and several independent internists affiliated with the University of Pennsylvania. After incorporating feedback into the final instrument, the web-based survey was distributed to eligible physicians via e-mail. Responses were anonymous and respondents received a $15 gift card for participation. The protocol was reviewed and deemed exempt by the University of Pennsylvania Institutional Review Board.
Statistical Analysis
Respondent characteristics (sociodemographic, intended clinical behavior, and cost control attitudes) were described by using percentages for categorical variables and medians and interquartile ranges for continuous variables. Balance in respondent characteristics across survey versions was evaluated using χ2 and Kruskal-Wallis tests. Multivariable logistic regression, adjusted for characteristics in the Table, was used to evaluate the association between survey version and policy support. All tests of significance were 2-tailed with significance level alpha = 0.05. Analyses were performed using STATA version 14.1 (StataCorp LLC, College Station, TX, http://www.stata.com).
RESULTS
Of 484 eligible respondents, 187 (39%) completed the survey. Compared with nonrespondents, respondents were more likely to be female (30% vs 26%, P = 0.001), older (mean age 41 vs 36 years, P < 0.001), and practicing clinicians rather than internal medicine residents (87% vs 69%, P < 0.001). Physician characteristics were similar across the 3 survey versions (Table). Most respondents agreed that financial incentives for individual physicians is an effective way to improve the value of healthcare (73.3%) and that physicians should consider the costs of a test or treatment to society when making clinical decisions for patients (79.1%). The majority also felt that clinicians have a duty to offer a test or treatment to a patient if it has any chance of helping them (70.1%) and that it is inappropriate for anyone beyond the clinician and patient to decide if a test or treatment is “worth the cost” (63.6%).
Overall, policy support rate was 39.6% and was the highest for the “societal harm” version (48.4%), followed by the “institutional harm” (36.9%) and “patient harm” (33.3%) versions. Compared with respondents receiving the “patient harm” version, those receiving the “societal harm” version (adjusted odds ratio [OR] 2.83; 95% confidence interval [CI], 1.20-6.69), but not the “institutional harm” framing (adjusted OR 1.53; 95% CI, 0.66-3.53), were more likely to report policy support. Policy support was also higher among those who agreed that providing financial incentives to individual physicians is an effective way to improve the value of healthcare (adjusted OR 4.61; 95% CI, 1.80-11.80).
DISCUSSION
To our knowledge, this study is the first to prospectively evaluate physician support of financial penalties for low-value services relevant to hospital medicine. It has 2 main findings.
First, although overall policy support was relatively low (39.6%), it varied significantly on the basis of how the harms of low-value care were framed. Support was highest in the “societal harm” version, suggesting that emphasizing these harms may increase acceptability of financial penalties among physicians and contribute to the larger effort to decrease low-value care in hospital settings. The comparatively low support for the “patient harm” version is somewhat surprising but may reflect variation in the nature of harm, benefit, and cost trade-offs for individual low-value services, as noted above, and physician belief that some low-value services do not in fact produce significant clinical harms.
For example, whereas evidence demonstrates that stress ulcer prophylaxis in non-ICU patients can harm patients through nosocomial infections and adverse drug effects,10,11 the clinical harms of telemetry are less obvious. Telemetry’s low value derives more from its high cost relative to benefit, rather than its potential for clinical harm.6 The many paths to “low value” underscore the need to examine attitudes and uptake toward these services separately and may explain the wide range in concordance between intended clinical behavior and low-value care guidelines (11.8% to 78.6%).
Reinforcing policies could more effectively deter low-value care. For example, multiple forces, including Medicare payment reform and national accreditation policies,12,13 have converged to discourage low-value use of urinary catheters in hospitalized patients. In contrast, there has been little reinforcement beyond consensus guidelines to reduce low-value use of telemetric monitoring. Given questions about whether consensus methods alone can deter low-value care beyond obvious “low hanging fruit,”14 policy makers could coordinate policies to accelerate progress within other priority areas.
Broad policies should also be paired with local initiatives to influence physician behavior. For example, health systems have begun successfully leveraging the electronic medical record and utilizing behavioral economics principles to design interventions to reduce inappropriate overuse of antibiotics for upper respiratory infections in primary care clinics.15 Organizations are also redesigning care processes in response to resource utilization imperatives under ongoing value-based care payment reform. Care redesign and behavioral interventions embedded at the point of care can both help deter low-value services in inpatient settings.
Study limitations include a relatively low response rate, which limits generalizability. However, all 3 randomized groups were similar on measured characteristics, and experimental randomization reduces the nonresponse bias concerns accompanying descriptive surveys. Additionally, although we evaluated intended clinical behavior in a national sample, our results may not reflect actual behavior among all physicians practicing hospital medicine. Future work could include assessments of actual or self-reported practices or examine additional factors, including site, years of practice, knowledge about guidelines, and other possible determinants of guideline-concordant behaviors.
Despite these limitations, our study provides important early evidence about physician support of financial penalties for low-value care relevant to hospital medicine. As policy makers design and organizational leaders implement financial incentive policies, this information can help increase their acceptability among physicians and more effectively reduce low-value care within hospitals.
Disclosure
Drs. Liao, Schapira, Mitra, and Weissman have no conflicts to disclose. Dr. Navathe serves as advisor to Navvis and Company, Navigant Inc, Lynx Medical, Indegene Inc, and Sutherland Global Services and receives an honorarium from Elsevier Press, none of which have relationship to this manuscript. Dr. Asch is a partner and part owner of VAL Health, which has no relationship to this manuscript.
Funding
This work was supported by The Leonard Davis Institute of Health Economics at the University of Pennsylvania, which had no role in the study design, data collection, analysis, or interpretation of results.
1. The MedPAC blog. Use of low-value care in Medicare is substantial. http://www.medpac.gov/-blog-/medpacblog/2015/05/21/use-of-low-value-care-in-medicare-is-substantial. Accessed on September 18, 2017.
2. American Board of Internal Medicine Foundation. Choosing Wisely. http://www.choosingwisely.org/. Accessed on September 18, 2017.
3. Rosenberg A, Agiro A, Gottlieb M, et al. Early Trends Among Seven Recommendations From the Choosing Wisely Campaign. JAMA Intern Med. 2015;175(12):1913-1920. PubMed
4. Centers for Medicare & Medicaid Services. CMS Response to Public Comments on Non-Recommended PSA-Based Screening Measure. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/MMS/Downloads/eCQM-Development-and-Maintenance-for-Eligible-Professionals_CMS_PSA_Response_Public-Comment.pdf. Accessed September 18, 2017.
5. Berwick DM. Avoiding overuse-the next quality frontier. Lancet. 2017;390(10090):102-104. doi: 10.1016/S0140-6736(16)32570-3. PubMed
6. Society of Hospital Medicine. Choosing Wisely. https://www.hospitalmedicine.org/choosingwisely. Accessed on September 18, 2017.
7. Tilburt JC, Wynia MK, Sheeler RD, et al. Views of US Physicians About Controlling Health Care Costs. JAMA. 2013;310(4):380-388. PubMed
8. Ginsburg ME, Kravitz RL, Sandberg WA. A survey of physician attitudes and practices concerning cost-effectiveness in patient care. West J Med. 2000;173(6):309-394. PubMed
9. Colla CH, Kinsella EA, Morden NE, Meyers DJ, Rosenthal MB, Sequist TD. Physician perceptions of Choosing Wisely and drivers of overuse. Am J Manag Care. 2016;22(5):337-343. PubMed
10. Herzig SJ, Vaughn BP, Howell MD, Ngo LH, Marcantonio ER. Acid-suppressive medication use and the risk for nosocomial gastrointestinal tract bleeding. Arch Intern Med. 2011;171(11):991-997. PubMed
11. Pappas M, Jolly S, Vijan S. Defining Appropriate Use of Proton-Pump Inhibitors Among Medical Inpatients. J Gen Intern Med. 2016;31(4):364-371. PubMed
12. Centers for Medicare & Medicaid Services. CMS’ Value-Based Programs. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/Value-Based-Programs.html. Accessed September 18, 2017.
13. The Joint Commission. Requirements for the Catheter-Associated Urinary Tract Infections (CAUTI) National Patient Safety Goal for Hospitals. https://www.jointcommission.org/assets/1/6/R3_Cauti_HAP.pdf. Accessed September 18, 2017 .
14. Beaudin-Seiler B, Ciarametaro M, Dubois R, Lee J, Fendrick AM. Reducing Low-Value Care. Health Affairs Blog. http://healthaffairs.org/blog/2016/09/20/reducing-low-value-care/. Accessed on September 18, 2017.
15. Meeker D, Linder JA, Fox CR, et al. Effect of Behavioral Interventions on Inappropriate Antibiotic Prescribing Among Primary Care Practices: A Randomized Clinical Trial. JAMA. 2016;315(6):562-570. PubMed
1. The MedPAC blog. Use of low-value care in Medicare is substantial. http://www.medpac.gov/-blog-/medpacblog/2015/05/21/use-of-low-value-care-in-medicare-is-substantial. Accessed on September 18, 2017.
2. American Board of Internal Medicine Foundation. Choosing Wisely. http://www.choosingwisely.org/. Accessed on September 18, 2017.
3. Rosenberg A, Agiro A, Gottlieb M, et al. Early Trends Among Seven Recommendations From the Choosing Wisely Campaign. JAMA Intern Med. 2015;175(12):1913-1920. PubMed
4. Centers for Medicare & Medicaid Services. CMS Response to Public Comments on Non-Recommended PSA-Based Screening Measure. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/MMS/Downloads/eCQM-Development-and-Maintenance-for-Eligible-Professionals_CMS_PSA_Response_Public-Comment.pdf. Accessed September 18, 2017.
5. Berwick DM. Avoiding overuse-the next quality frontier. Lancet. 2017;390(10090):102-104. doi: 10.1016/S0140-6736(16)32570-3. PubMed
6. Society of Hospital Medicine. Choosing Wisely. https://www.hospitalmedicine.org/choosingwisely. Accessed on September 18, 2017.
7. Tilburt JC, Wynia MK, Sheeler RD, et al. Views of US Physicians About Controlling Health Care Costs. JAMA. 2013;310(4):380-388. PubMed
8. Ginsburg ME, Kravitz RL, Sandberg WA. A survey of physician attitudes and practices concerning cost-effectiveness in patient care. West J Med. 2000;173(6):309-394. PubMed
9. Colla CH, Kinsella EA, Morden NE, Meyers DJ, Rosenthal MB, Sequist TD. Physician perceptions of Choosing Wisely and drivers of overuse. Am J Manag Care. 2016;22(5):337-343. PubMed
10. Herzig SJ, Vaughn BP, Howell MD, Ngo LH, Marcantonio ER. Acid-suppressive medication use and the risk for nosocomial gastrointestinal tract bleeding. Arch Intern Med. 2011;171(11):991-997. PubMed
11. Pappas M, Jolly S, Vijan S. Defining Appropriate Use of Proton-Pump Inhibitors Among Medical Inpatients. J Gen Intern Med. 2016;31(4):364-371. PubMed
12. Centers for Medicare & Medicaid Services. CMS’ Value-Based Programs. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/Value-Based-Programs.html. Accessed September 18, 2017.
13. The Joint Commission. Requirements for the Catheter-Associated Urinary Tract Infections (CAUTI) National Patient Safety Goal for Hospitals. https://www.jointcommission.org/assets/1/6/R3_Cauti_HAP.pdf. Accessed September 18, 2017 .
14. Beaudin-Seiler B, Ciarametaro M, Dubois R, Lee J, Fendrick AM. Reducing Low-Value Care. Health Affairs Blog. http://healthaffairs.org/blog/2016/09/20/reducing-low-value-care/. Accessed on September 18, 2017.
15. Meeker D, Linder JA, Fox CR, et al. Effect of Behavioral Interventions on Inappropriate Antibiotic Prescribing Among Primary Care Practices: A Randomized Clinical Trial. JAMA. 2016;315(6):562-570. PubMed
© 2018 Society of Hospital Medicine
Isolation precautions are associated with higher costs, longer LOS
Clinical question: What are the effects of isolation precautions on hospital outcomes and cost of care?
Background: Previous studies have found that isolation precautions negatively affect various aspects of patient care, including frequency of contact with clinicians, adverse events in the hospital, measures of patient well-being, and patient experience scores. It is not known how isolation precautions affect other hospital-based metrics, such as 30-day readmissions, length of stay (LOS), in-hospital mortality, and cost of care.
Study design: Multisite, retrospective, propensity score–matched cohort study.
Setting: Three academic tertiary care hospitals in Toronto.
Synopsis: The authors used administrative databases and propensity-score modeling to match isolated patients and nonisolated controls. Researchers included 17,649 control patients, 737 patients isolated for methicillin-resistant Staphylococcus aureus (contact isolation), and 1,502 patients isolated for respiratory illnesses (contact and droplet isolation) in the study. Patients isolated for MRSA had a higher 30-day readmission rate than did controls (19% vs. 14.7%), a longer average length of stay (11.9 days vs. 9.1 days), and higher direct costs ($11,009 vs. $7,670). Patients isolated for respiratory illnesses had a longer average length of stay (8.5 days vs. 7.6 days) and higher direct costs ($7,194 vs. $6,294). No differences in adverse events rates or in-hospital mortality were observed between control patients and patients in either isolation group.
Some of the differences observed may be from illness severity rather than from the effects of isolation, especially in the MRSA group. There was no difference observed in rates of adverse outcomes, such as falls or medication errors, or in rates of formal patient complaints to the hospital. It is possible that propensity score modeling corrected for unidentified biases in prior studies that found differences in these types of outcomes.
Bottom line: Isolation precautions are associated with higher costs and longer LOS in hospitalized general medicine patients.
Citation: Tran K et al. The effect of hospital isolation precautions on patient outcomes and cost of care: A multisite, retrospective, propensity score-matched cohort study. J Gen Intern Med. 2017;32(3):262-8.
Dr. Wachter is an assistant professor of medicine at Duke University.
Clinical question: What are the effects of isolation precautions on hospital outcomes and cost of care?
Background: Previous studies have found that isolation precautions negatively affect various aspects of patient care, including frequency of contact with clinicians, adverse events in the hospital, measures of patient well-being, and patient experience scores. It is not known how isolation precautions affect other hospital-based metrics, such as 30-day readmissions, length of stay (LOS), in-hospital mortality, and cost of care.
Study design: Multisite, retrospective, propensity score–matched cohort study.
Setting: Three academic tertiary care hospitals in Toronto.
Synopsis: The authors used administrative databases and propensity-score modeling to match isolated patients and nonisolated controls. Researchers included 17,649 control patients, 737 patients isolated for methicillin-resistant Staphylococcus aureus (contact isolation), and 1,502 patients isolated for respiratory illnesses (contact and droplet isolation) in the study. Patients isolated for MRSA had a higher 30-day readmission rate than did controls (19% vs. 14.7%), a longer average length of stay (11.9 days vs. 9.1 days), and higher direct costs ($11,009 vs. $7,670). Patients isolated for respiratory illnesses had a longer average length of stay (8.5 days vs. 7.6 days) and higher direct costs ($7,194 vs. $6,294). No differences in adverse events rates or in-hospital mortality were observed between control patients and patients in either isolation group.
Some of the differences observed may be from illness severity rather than from the effects of isolation, especially in the MRSA group. There was no difference observed in rates of adverse outcomes, such as falls or medication errors, or in rates of formal patient complaints to the hospital. It is possible that propensity score modeling corrected for unidentified biases in prior studies that found differences in these types of outcomes.
Bottom line: Isolation precautions are associated with higher costs and longer LOS in hospitalized general medicine patients.
Citation: Tran K et al. The effect of hospital isolation precautions on patient outcomes and cost of care: A multisite, retrospective, propensity score-matched cohort study. J Gen Intern Med. 2017;32(3):262-8.
Dr. Wachter is an assistant professor of medicine at Duke University.
Clinical question: What are the effects of isolation precautions on hospital outcomes and cost of care?
Background: Previous studies have found that isolation precautions negatively affect various aspects of patient care, including frequency of contact with clinicians, adverse events in the hospital, measures of patient well-being, and patient experience scores. It is not known how isolation precautions affect other hospital-based metrics, such as 30-day readmissions, length of stay (LOS), in-hospital mortality, and cost of care.
Study design: Multisite, retrospective, propensity score–matched cohort study.
Setting: Three academic tertiary care hospitals in Toronto.
Synopsis: The authors used administrative databases and propensity-score modeling to match isolated patients and nonisolated controls. Researchers included 17,649 control patients, 737 patients isolated for methicillin-resistant Staphylococcus aureus (contact isolation), and 1,502 patients isolated for respiratory illnesses (contact and droplet isolation) in the study. Patients isolated for MRSA had a higher 30-day readmission rate than did controls (19% vs. 14.7%), a longer average length of stay (11.9 days vs. 9.1 days), and higher direct costs ($11,009 vs. $7,670). Patients isolated for respiratory illnesses had a longer average length of stay (8.5 days vs. 7.6 days) and higher direct costs ($7,194 vs. $6,294). No differences in adverse events rates or in-hospital mortality were observed between control patients and patients in either isolation group.
Some of the differences observed may be from illness severity rather than from the effects of isolation, especially in the MRSA group. There was no difference observed in rates of adverse outcomes, such as falls or medication errors, or in rates of formal patient complaints to the hospital. It is possible that propensity score modeling corrected for unidentified biases in prior studies that found differences in these types of outcomes.
Bottom line: Isolation precautions are associated with higher costs and longer LOS in hospitalized general medicine patients.
Citation: Tran K et al. The effect of hospital isolation precautions on patient outcomes and cost of care: A multisite, retrospective, propensity score-matched cohort study. J Gen Intern Med. 2017;32(3):262-8.
Dr. Wachter is an assistant professor of medicine at Duke University.
Urgent endoscopy is associated with lower mortality in high-risk patients with acute nonvariceal GI bleeding
Clinical question: Is urgent endoscopy (less than 6 hours after ED presentation) better than elective endoscopy (6-48 hours after presentation) to decrease mortality and rebleeding in high-risk patients with acute nonvariceal upper GI bleeding (ANVGIB)?
Background: High-risk ANVGIB patients (Glasgow-Blatchford score greater than 7) are recommended to undergo early endoscopy, within 24 hours of presentation. The impact of urgent endoscopy (less than 6 hours) on patient outcomes is not clear.
Study design: Retrospective observation study.
Setting: Single tertiary referral center in South Korea.
Synopsis: Investigators retrospectively reviewed 961 high-risk ANVGIB patients, 571 patients underwent urgent endoscopy and 390 patients had elective endoscopy (6-48 hours), to compare clinical features and outcomes. The urgent group was slightly older, had a higher Rockall score, lower blood pressure, and higher incidence of shock on admission.
Urgent endoscopy was associated with significantly lower 28-day mortality (1.6% vs 3.8%). Urgent endoscopy also was associated with higher packed red blood cell transfusion volume (2.6 U vs. 2.3 U) and greater need for endoscopic intervention (69.5% vs. 53.5%) and embolization (2.8% vs. 0.5%). There was no significant difference in rebleeding rates, need for ICU admission, vasopressor use, and length of hospital stay between the urgent and elective endoscopy groups. The authors conclude that urgent endoscopy was associated with lower mortality rate but not rebleeding in high-risk patients with ANVGIB.
Despite differences between these two groups, based on this retrospective data, it is reasonable to suggest that urgent endoscopy may be beneficial for reducing mortality in high-risk patients with ANVGIB.
Bottom line: Urgent endoscopy may be beneficial in reducing mortality in high-risk patients with acute nonvariceal gastrointestinal bleeding.
Citation: Cho SH et al. Outcomes and role of urgent endoscopy in high-risk patients with acute nonvariceal gastrointestinal bleeding. Clin Gastroenterol Hepatol. 2017 Jun 19. pii: S1542-3565(17)30736-X.
Dr. Patel is a hospitalist and an assistant professor of medicine, Duke University Health System.
Clinical question: Is urgent endoscopy (less than 6 hours after ED presentation) better than elective endoscopy (6-48 hours after presentation) to decrease mortality and rebleeding in high-risk patients with acute nonvariceal upper GI bleeding (ANVGIB)?
Background: High-risk ANVGIB patients (Glasgow-Blatchford score greater than 7) are recommended to undergo early endoscopy, within 24 hours of presentation. The impact of urgent endoscopy (less than 6 hours) on patient outcomes is not clear.
Study design: Retrospective observation study.
Setting: Single tertiary referral center in South Korea.
Synopsis: Investigators retrospectively reviewed 961 high-risk ANVGIB patients, 571 patients underwent urgent endoscopy and 390 patients had elective endoscopy (6-48 hours), to compare clinical features and outcomes. The urgent group was slightly older, had a higher Rockall score, lower blood pressure, and higher incidence of shock on admission.
Urgent endoscopy was associated with significantly lower 28-day mortality (1.6% vs 3.8%). Urgent endoscopy also was associated with higher packed red blood cell transfusion volume (2.6 U vs. 2.3 U) and greater need for endoscopic intervention (69.5% vs. 53.5%) and embolization (2.8% vs. 0.5%). There was no significant difference in rebleeding rates, need for ICU admission, vasopressor use, and length of hospital stay between the urgent and elective endoscopy groups. The authors conclude that urgent endoscopy was associated with lower mortality rate but not rebleeding in high-risk patients with ANVGIB.
Despite differences between these two groups, based on this retrospective data, it is reasonable to suggest that urgent endoscopy may be beneficial for reducing mortality in high-risk patients with ANVGIB.
Bottom line: Urgent endoscopy may be beneficial in reducing mortality in high-risk patients with acute nonvariceal gastrointestinal bleeding.
Citation: Cho SH et al. Outcomes and role of urgent endoscopy in high-risk patients with acute nonvariceal gastrointestinal bleeding. Clin Gastroenterol Hepatol. 2017 Jun 19. pii: S1542-3565(17)30736-X.
Dr. Patel is a hospitalist and an assistant professor of medicine, Duke University Health System.
Clinical question: Is urgent endoscopy (less than 6 hours after ED presentation) better than elective endoscopy (6-48 hours after presentation) to decrease mortality and rebleeding in high-risk patients with acute nonvariceal upper GI bleeding (ANVGIB)?
Background: High-risk ANVGIB patients (Glasgow-Blatchford score greater than 7) are recommended to undergo early endoscopy, within 24 hours of presentation. The impact of urgent endoscopy (less than 6 hours) on patient outcomes is not clear.
Study design: Retrospective observation study.
Setting: Single tertiary referral center in South Korea.
Synopsis: Investigators retrospectively reviewed 961 high-risk ANVGIB patients, 571 patients underwent urgent endoscopy and 390 patients had elective endoscopy (6-48 hours), to compare clinical features and outcomes. The urgent group was slightly older, had a higher Rockall score, lower blood pressure, and higher incidence of shock on admission.
Urgent endoscopy was associated with significantly lower 28-day mortality (1.6% vs 3.8%). Urgent endoscopy also was associated with higher packed red blood cell transfusion volume (2.6 U vs. 2.3 U) and greater need for endoscopic intervention (69.5% vs. 53.5%) and embolization (2.8% vs. 0.5%). There was no significant difference in rebleeding rates, need for ICU admission, vasopressor use, and length of hospital stay between the urgent and elective endoscopy groups. The authors conclude that urgent endoscopy was associated with lower mortality rate but not rebleeding in high-risk patients with ANVGIB.
Despite differences between these two groups, based on this retrospective data, it is reasonable to suggest that urgent endoscopy may be beneficial for reducing mortality in high-risk patients with ANVGIB.
Bottom line: Urgent endoscopy may be beneficial in reducing mortality in high-risk patients with acute nonvariceal gastrointestinal bleeding.
Citation: Cho SH et al. Outcomes and role of urgent endoscopy in high-risk patients with acute nonvariceal gastrointestinal bleeding. Clin Gastroenterol Hepatol. 2017 Jun 19. pii: S1542-3565(17)30736-X.
Dr. Patel is a hospitalist and an assistant professor of medicine, Duke University Health System.
Journal of Hospital Medicine – Dec. 2017
BACKGROUND: Identifying hospitals that are both early and consistent adopters of high-value care can help shed light on the culture and practices at those institutions that are necessary to promote high-value care nationwide. The use of troponin testing to diagnose acute myocardial infarction (AMI), and not testing for myoglobin or creatine kinase-MB (CK-MB), is a high-value recommendation of the Choosing Wisely® campaign.
OBJECTIVE: To examine the variation in cardiac biomarker testing and the effect of the Choosing Wisely® troponin-only testing recommendation for the diagnosis of AMI.
DESIGN: A retrospective, observational study using administrative ordering data from Vizient’s Clinical Database/Resource Manager.
PATIENTS: Hospitalized patients with a principal discharge diagnosis of AMI.
INTERVENTION: The Choosing Wisely® recommendation to order troponin-only testing to diagnose AMI was released during the first quarter of 2015.
RESULTS: In 19 hospitals, troponin-only testing was consistently ordered to diagnose AMI before the Choosing Wisely® recommendation and throughout the study period. In 34 hospitals, both troponin testing and myoglobin/CK-MB testing were ordered to diagnose AMI even after the Choosing Wisely® recommendation. In 26 hospitals with low rates of troponin-only testing before the Choosing Wisely® recommendation, the release of the recommendation was associated with a statistically significant increase in the rate of troponin-only testing to diagnose AMI.
CONCLUSION: In institutions with low rates of troponin-only testing prior to the Choosing Wisely® recommendation, the recommendation was associated with a significant increase in the rate of troponin-only testing.
Read the entire article in the Dec. 2017 issue of the Journal of Hospital Medicine.
Also in JHM this month
Hospital perceptions of Medicare’s Sepsis Quality Reporting Initiative
AUTHORS: Ian J. Barbash, MD, MS; Kimberly J. Rak, PhD; Courtney C. Kuza, MPH; and Jeremy M. Kahn, MD, MS
Health literacy and hospital length of stay: An inpatient cohort study
AUTHORS: Ethan G. Jaffee, MD; Vineet M. Arora, MD, MAPP; Madeleine I. Matthiesen, MD; David O. Meltzer, MD, PhD, MHM; and Valerie G. Press, MD, FAAP, FACP, MPH
How exemplary teaching physicians interact with hospitalized patients
AUTHORS: Sanjay Saint, MD, MPH, FHM; Molly Harrod, PhD; Karen E. Fowler, MPH; and Nathan Houchens, MD, FACP, FHM
A randomized cohort controlled trial to compare intern sign-out training interventions
AUTHORS: Soo-Hoon Lee, PhD; Christopher Terndrup, MD; Phillip H. Phan, PhD; Sandra E. Zaeh, MD; Kwame Atsina, MD; Nicole Minkove, MD; Alexander Billioux, MD; DPhil, Souvik Chatterjee, MD; Idoreyin Montague, MD; Bennett Clark, MD; Andrew Hughes, MD; and Sanjay V. Desai, MD
BACKGROUND: Identifying hospitals that are both early and consistent adopters of high-value care can help shed light on the culture and practices at those institutions that are necessary to promote high-value care nationwide. The use of troponin testing to diagnose acute myocardial infarction (AMI), and not testing for myoglobin or creatine kinase-MB (CK-MB), is a high-value recommendation of the Choosing Wisely® campaign.
OBJECTIVE: To examine the variation in cardiac biomarker testing and the effect of the Choosing Wisely® troponin-only testing recommendation for the diagnosis of AMI.
DESIGN: A retrospective, observational study using administrative ordering data from Vizient’s Clinical Database/Resource Manager.
PATIENTS: Hospitalized patients with a principal discharge diagnosis of AMI.
INTERVENTION: The Choosing Wisely® recommendation to order troponin-only testing to diagnose AMI was released during the first quarter of 2015.
RESULTS: In 19 hospitals, troponin-only testing was consistently ordered to diagnose AMI before the Choosing Wisely® recommendation and throughout the study period. In 34 hospitals, both troponin testing and myoglobin/CK-MB testing were ordered to diagnose AMI even after the Choosing Wisely® recommendation. In 26 hospitals with low rates of troponin-only testing before the Choosing Wisely® recommendation, the release of the recommendation was associated with a statistically significant increase in the rate of troponin-only testing to diagnose AMI.
CONCLUSION: In institutions with low rates of troponin-only testing prior to the Choosing Wisely® recommendation, the recommendation was associated with a significant increase in the rate of troponin-only testing.
Read the entire article in the Dec. 2017 issue of the Journal of Hospital Medicine.
Also in JHM this month
Hospital perceptions of Medicare’s Sepsis Quality Reporting Initiative
AUTHORS: Ian J. Barbash, MD, MS; Kimberly J. Rak, PhD; Courtney C. Kuza, MPH; and Jeremy M. Kahn, MD, MS
Health literacy and hospital length of stay: An inpatient cohort study
AUTHORS: Ethan G. Jaffee, MD; Vineet M. Arora, MD, MAPP; Madeleine I. Matthiesen, MD; David O. Meltzer, MD, PhD, MHM; and Valerie G. Press, MD, FAAP, FACP, MPH
How exemplary teaching physicians interact with hospitalized patients
AUTHORS: Sanjay Saint, MD, MPH, FHM; Molly Harrod, PhD; Karen E. Fowler, MPH; and Nathan Houchens, MD, FACP, FHM
A randomized cohort controlled trial to compare intern sign-out training interventions
AUTHORS: Soo-Hoon Lee, PhD; Christopher Terndrup, MD; Phillip H. Phan, PhD; Sandra E. Zaeh, MD; Kwame Atsina, MD; Nicole Minkove, MD; Alexander Billioux, MD; DPhil, Souvik Chatterjee, MD; Idoreyin Montague, MD; Bennett Clark, MD; Andrew Hughes, MD; and Sanjay V. Desai, MD
BACKGROUND: Identifying hospitals that are both early and consistent adopters of high-value care can help shed light on the culture and practices at those institutions that are necessary to promote high-value care nationwide. The use of troponin testing to diagnose acute myocardial infarction (AMI), and not testing for myoglobin or creatine kinase-MB (CK-MB), is a high-value recommendation of the Choosing Wisely® campaign.
OBJECTIVE: To examine the variation in cardiac biomarker testing and the effect of the Choosing Wisely® troponin-only testing recommendation for the diagnosis of AMI.
DESIGN: A retrospective, observational study using administrative ordering data from Vizient’s Clinical Database/Resource Manager.
PATIENTS: Hospitalized patients with a principal discharge diagnosis of AMI.
INTERVENTION: The Choosing Wisely® recommendation to order troponin-only testing to diagnose AMI was released during the first quarter of 2015.
RESULTS: In 19 hospitals, troponin-only testing was consistently ordered to diagnose AMI before the Choosing Wisely® recommendation and throughout the study period. In 34 hospitals, both troponin testing and myoglobin/CK-MB testing were ordered to diagnose AMI even after the Choosing Wisely® recommendation. In 26 hospitals with low rates of troponin-only testing before the Choosing Wisely® recommendation, the release of the recommendation was associated with a statistically significant increase in the rate of troponin-only testing to diagnose AMI.
CONCLUSION: In institutions with low rates of troponin-only testing prior to the Choosing Wisely® recommendation, the recommendation was associated with a significant increase in the rate of troponin-only testing.
Read the entire article in the Dec. 2017 issue of the Journal of Hospital Medicine.
Also in JHM this month
Hospital perceptions of Medicare’s Sepsis Quality Reporting Initiative
AUTHORS: Ian J. Barbash, MD, MS; Kimberly J. Rak, PhD; Courtney C. Kuza, MPH; and Jeremy M. Kahn, MD, MS
Health literacy and hospital length of stay: An inpatient cohort study
AUTHORS: Ethan G. Jaffee, MD; Vineet M. Arora, MD, MAPP; Madeleine I. Matthiesen, MD; David O. Meltzer, MD, PhD, MHM; and Valerie G. Press, MD, FAAP, FACP, MPH
How exemplary teaching physicians interact with hospitalized patients
AUTHORS: Sanjay Saint, MD, MPH, FHM; Molly Harrod, PhD; Karen E. Fowler, MPH; and Nathan Houchens, MD, FACP, FHM
A randomized cohort controlled trial to compare intern sign-out training interventions
AUTHORS: Soo-Hoon Lee, PhD; Christopher Terndrup, MD; Phillip H. Phan, PhD; Sandra E. Zaeh, MD; Kwame Atsina, MD; Nicole Minkove, MD; Alexander Billioux, MD; DPhil, Souvik Chatterjee, MD; Idoreyin Montague, MD; Bennett Clark, MD; Andrew Hughes, MD; and Sanjay V. Desai, MD
Adopting the patient’s perspective
Editor’s note: “Everything We Say and Do” provides readers with thoughtful and actionable communication tactics that can positively impact patients’ experience of care. In the current series of columns, physicians share how their experiences as patients have shaped their professional approach.
I have been fortunate to have had very few major health issues throughout my life. I have, however, had three major surgical procedures in the last 10 years – two total hip arthroplasties and a cataract removal with lens implant in between. The most recent THA was October 2017. Going through each procedure helped me see things from a patient’s perspective, and that showed me how important little things are to a patient, things which we may not think are all that big a deal as a provider.
Almost all of the medical personnel who came to care for me during my stays identified themselves and why they were there, and that made me feel comfortable, knowing who they were and their role. However, there were a few who did not do this, and that made me uncomfortable, not knowing who they were and why they were in my room. Not knowing is an uncomfortable feeling for a patient.
Almost every registered nurse who came to me with medication explained what the medicine was and why they were administering it, with the exception of one preop RN I met before to my cataract procedure. She walked up to me, told me to open my eye wide, held the affected eye open, and started dripping cold drops into my eye without explanation. She then said she would be back every 10 minutes to repeat the process. I had to inquire as to what the medication was and why there was a need for this process. It was a jolting experience, and she showed no compassion toward me as a patient or a person, even after I inquired.
This was not a good experience. Although cataract surgery was a totally new experience for me, she had obviously done this many times before and had to do it many times that day. However, she acted as if I should have known what she was going to do and as if she need not explain herself to anyone – which she did not, even after being queried.
Everyone during the admission process for all three procedures was solicitous and warm except for one person. Unfortunately, this individual was the first person to greet my wife and me when we arrived for my last total hip arthroplasty. She was seated at the welcome desk with her head down. After we arrived, she kept her head down and asked “How can I help you?” without ever looking up. I did not realize how unwelcome I would feel when the first person I encountered in the surgical preop admissions area failed to make eye contact with me. Her demeanor was nice enough, but she did not even attempt to make a personal connection with me – and she was at the welcome desk!
Overall, I had tremendously good experiences at three facilities in three different parts of the United States, but as we all know, it is the things that do not go well that stand out. I choose to use those things, along with some of the good things, as “reinforcers” for many of the patient-experience behaviors we identify as best practices.
What I say and do
During each patient encounter, I make eye contact with the patient and each person in the room and identify who I am and why I am there. I sit down during each visit unless there is simply no place for me to do so. I explain the procedures that are to take place, set expectations for those procedures, and then use “teachback” to ensure that my discussion with the patient has been effective. Setting expectations is very important to me: If you do not ensure that patients have appropriate expectations, their expectations will never be met and they will never have a good experience. I explain any new medication I am ordering, what it is for, and any possible significant side effects and again use teachback. The last thing I do is ask “What questions do you have for me today?” giving the patient permission to have questions, and then I respond to those questions with plain talk and teachback.
Why I do it
Not knowing what was going on and feeling marginalized were the most uncomfortable things I experienced as a patient. Using best practices for patient experience shows courtesy and respect. These practices show a willingness to take time with the patient and demonstrate my concern that I am effectively communicating my message for that visit. All of these behaviors decrease uncertainty and/or raise the patient’s feelings of importance, thereby decreasing marginalization.
How I do it
I remind myself each day I am on a clinical shift that my goal is to treat each patient like I would want my family (or myself) to be treated, and then I go out and do it. After “forcing” myself to put these behaviors into my rounding routine, they have become second nature, and I feel better for providing this level of care because it made me feel so good when I was cared for in this manner.
Dr. Sharp is chief hospitalist with Sound Physicians at University of Florida Health in Jacksonville, Fla.
Editor’s note: “Everything We Say and Do” provides readers with thoughtful and actionable communication tactics that can positively impact patients’ experience of care. In the current series of columns, physicians share how their experiences as patients have shaped their professional approach.
I have been fortunate to have had very few major health issues throughout my life. I have, however, had three major surgical procedures in the last 10 years – two total hip arthroplasties and a cataract removal with lens implant in between. The most recent THA was October 2017. Going through each procedure helped me see things from a patient’s perspective, and that showed me how important little things are to a patient, things which we may not think are all that big a deal as a provider.
Almost all of the medical personnel who came to care for me during my stays identified themselves and why they were there, and that made me feel comfortable, knowing who they were and their role. However, there were a few who did not do this, and that made me uncomfortable, not knowing who they were and why they were in my room. Not knowing is an uncomfortable feeling for a patient.
Almost every registered nurse who came to me with medication explained what the medicine was and why they were administering it, with the exception of one preop RN I met before to my cataract procedure. She walked up to me, told me to open my eye wide, held the affected eye open, and started dripping cold drops into my eye without explanation. She then said she would be back every 10 minutes to repeat the process. I had to inquire as to what the medication was and why there was a need for this process. It was a jolting experience, and she showed no compassion toward me as a patient or a person, even after I inquired.
This was not a good experience. Although cataract surgery was a totally new experience for me, she had obviously done this many times before and had to do it many times that day. However, she acted as if I should have known what she was going to do and as if she need not explain herself to anyone – which she did not, even after being queried.
Everyone during the admission process for all three procedures was solicitous and warm except for one person. Unfortunately, this individual was the first person to greet my wife and me when we arrived for my last total hip arthroplasty. She was seated at the welcome desk with her head down. After we arrived, she kept her head down and asked “How can I help you?” without ever looking up. I did not realize how unwelcome I would feel when the first person I encountered in the surgical preop admissions area failed to make eye contact with me. Her demeanor was nice enough, but she did not even attempt to make a personal connection with me – and she was at the welcome desk!
Overall, I had tremendously good experiences at three facilities in three different parts of the United States, but as we all know, it is the things that do not go well that stand out. I choose to use those things, along with some of the good things, as “reinforcers” for many of the patient-experience behaviors we identify as best practices.
What I say and do
During each patient encounter, I make eye contact with the patient and each person in the room and identify who I am and why I am there. I sit down during each visit unless there is simply no place for me to do so. I explain the procedures that are to take place, set expectations for those procedures, and then use “teachback” to ensure that my discussion with the patient has been effective. Setting expectations is very important to me: If you do not ensure that patients have appropriate expectations, their expectations will never be met and they will never have a good experience. I explain any new medication I am ordering, what it is for, and any possible significant side effects and again use teachback. The last thing I do is ask “What questions do you have for me today?” giving the patient permission to have questions, and then I respond to those questions with plain talk and teachback.
Why I do it
Not knowing what was going on and feeling marginalized were the most uncomfortable things I experienced as a patient. Using best practices for patient experience shows courtesy and respect. These practices show a willingness to take time with the patient and demonstrate my concern that I am effectively communicating my message for that visit. All of these behaviors decrease uncertainty and/or raise the patient’s feelings of importance, thereby decreasing marginalization.
How I do it
I remind myself each day I am on a clinical shift that my goal is to treat each patient like I would want my family (or myself) to be treated, and then I go out and do it. After “forcing” myself to put these behaviors into my rounding routine, they have become second nature, and I feel better for providing this level of care because it made me feel so good when I was cared for in this manner.
Dr. Sharp is chief hospitalist with Sound Physicians at University of Florida Health in Jacksonville, Fla.
Editor’s note: “Everything We Say and Do” provides readers with thoughtful and actionable communication tactics that can positively impact patients’ experience of care. In the current series of columns, physicians share how their experiences as patients have shaped their professional approach.
I have been fortunate to have had very few major health issues throughout my life. I have, however, had three major surgical procedures in the last 10 years – two total hip arthroplasties and a cataract removal with lens implant in between. The most recent THA was October 2017. Going through each procedure helped me see things from a patient’s perspective, and that showed me how important little things are to a patient, things which we may not think are all that big a deal as a provider.
Almost all of the medical personnel who came to care for me during my stays identified themselves and why they were there, and that made me feel comfortable, knowing who they were and their role. However, there were a few who did not do this, and that made me uncomfortable, not knowing who they were and why they were in my room. Not knowing is an uncomfortable feeling for a patient.
Almost every registered nurse who came to me with medication explained what the medicine was and why they were administering it, with the exception of one preop RN I met before to my cataract procedure. She walked up to me, told me to open my eye wide, held the affected eye open, and started dripping cold drops into my eye without explanation. She then said she would be back every 10 minutes to repeat the process. I had to inquire as to what the medication was and why there was a need for this process. It was a jolting experience, and she showed no compassion toward me as a patient or a person, even after I inquired.
This was not a good experience. Although cataract surgery was a totally new experience for me, she had obviously done this many times before and had to do it many times that day. However, she acted as if I should have known what she was going to do and as if she need not explain herself to anyone – which she did not, even after being queried.
Everyone during the admission process for all three procedures was solicitous and warm except for one person. Unfortunately, this individual was the first person to greet my wife and me when we arrived for my last total hip arthroplasty. She was seated at the welcome desk with her head down. After we arrived, she kept her head down and asked “How can I help you?” without ever looking up. I did not realize how unwelcome I would feel when the first person I encountered in the surgical preop admissions area failed to make eye contact with me. Her demeanor was nice enough, but she did not even attempt to make a personal connection with me – and she was at the welcome desk!
Overall, I had tremendously good experiences at three facilities in three different parts of the United States, but as we all know, it is the things that do not go well that stand out. I choose to use those things, along with some of the good things, as “reinforcers” for many of the patient-experience behaviors we identify as best practices.
What I say and do
During each patient encounter, I make eye contact with the patient and each person in the room and identify who I am and why I am there. I sit down during each visit unless there is simply no place for me to do so. I explain the procedures that are to take place, set expectations for those procedures, and then use “teachback” to ensure that my discussion with the patient has been effective. Setting expectations is very important to me: If you do not ensure that patients have appropriate expectations, their expectations will never be met and they will never have a good experience. I explain any new medication I am ordering, what it is for, and any possible significant side effects and again use teachback. The last thing I do is ask “What questions do you have for me today?” giving the patient permission to have questions, and then I respond to those questions with plain talk and teachback.
Why I do it
Not knowing what was going on and feeling marginalized were the most uncomfortable things I experienced as a patient. Using best practices for patient experience shows courtesy and respect. These practices show a willingness to take time with the patient and demonstrate my concern that I am effectively communicating my message for that visit. All of these behaviors decrease uncertainty and/or raise the patient’s feelings of importance, thereby decreasing marginalization.
How I do it
I remind myself each day I am on a clinical shift that my goal is to treat each patient like I would want my family (or myself) to be treated, and then I go out and do it. After “forcing” myself to put these behaviors into my rounding routine, they have become second nature, and I feel better for providing this level of care because it made me feel so good when I was cared for in this manner.
Dr. Sharp is chief hospitalist with Sound Physicians at University of Florida Health in Jacksonville, Fla.