User login
Severely frail elderly patients do not need lipid-lowering drugs
Frail elderly patients are at high risk of adverse clinical outcomes, including those due to polypharmacy. Several groups tackle “deprescribing” by developing lists of medications that are potentially inappropriate for the elderly, such as the Beers or STOPP/START criteria.1–4
See related editorialIn contrast, our group (the Palliative and Therapeutic Harmonization [PATH] program and the Dalhousie Academic Detailing Service) has developed evidence-based, frailty-specific guidelines for treating hypertension5 and diabetes,6 in which we advocate less-stringent treatment targets and tapering or discontinuing medications, as needed.
The PATH program7 is a clinical approach that prioritizes the consideration of frailty when making treatment decisions. The Dalhousie Academic Detailing Service collaborates with the Nova Scotia Health Authority to research and develop evidence-informed educational messages about the treatment of common medical conditions.
Here, we address lipid-lowering therapy in this population.
CONSIDERING FRAILTY
Frailty is defined in several ways. The Fried model8,9 identifies frailty when 3 of the following characteristics are present: unintentional weight loss, exhaustion, muscle weakness, slow walking speed, or low levels of activity. The Clinical Frailty Scale10,11 and the Frailty Assessment for Care-planning Tool (FACT)5 use deficits in cognition, function, and mobility to define frailty. According to these scales, people are considered severely frail when they require assistance with basic activities of daily living (such as bathing or dressing), owing to cognitive or physical deficits from any cause.
In reviewing the evidence, we consider five questions:
- What is the quality of the evidence? (Up to 48% of clinical practice guideline recommendations may be based on low-level evidence or expert opinion.12)
- How did the study population compare with the frail?
- Are study outcomes and potential benefits clinically relevant to those who are frail?
- How long did it take for the clinical benefit of a treatment to become apparent, and are the frail elderly likely to live that long?
- Have the harms of treatment been sufficiently considered?
WHAT IS THE QUALITY OF THE EVIDENCE?
We found no studies that specifically evaluated the benefit of lipid-lowering for severely frail older adults. Therefore, we examined randomized controlled trials that enrolled non-frail older adults,13–28 subgroup analyses of randomized controlled trials,29,30 meta-analyses that analyzed subgroups of elderly populations,31,32 and publications describing the study designs of randomized controlled trials.33–37
Most of the evidence comes from post hoc subgroup analyses of elderly populations. Although meta-analysis is commonly used to compare subgroups, the Cochrane handbook and others consider subgroup comparisons observational by nature.38,39 (See Table 1 for lipid-lowering studies discussed in this article.)
Studies of statins for primary prevention of cardiovascular disease
For evidence of benefit from lipid-lowering for primary prevention (ie, to reduce the risk of cardiovascular events in patients with no known cardiovascular disease at baseline but at increased risk), we reviewed the meta-analysis conducted by the Cholesterol Treatment Trialists’ (CTT) Collaborators.32 Since this meta-analysis included the major trials that enrolled elderly patients, individual publications of post hoc, elderly subgroups were, for the most part, not examined individually. The exception to this approach was a decision to report on the PROSPER13 and JUPITER28 trials separately, because PROSPER is the most representative of the elderly population and JUPITER reached the lowest LDL-C of primary prevention trials published to date and included a large elderly subgroup (n = 5,695).
Savarese et al40 evaluated the benefits of statins for older adults who did not have established cardiovascular disease. We did not report on this meta-analysis, as not all of the subjects that populated the meta-analysis were representative of a typical prevention population. For instance, in the Anglo-Scandinavian Cardiac Outcomes Trial lipid-lowering arm,41 14% of the subjects had had a previous stroke or transient ischemic attack. In the Antihypertensive and Lipid-Lowering Treatment Trial,42 16% of the population had a family history of premature coronary heart disease.
In addition, all the trials in the Savarese meta-analysis were also included in the CTT meta-analysis.32 The CTT reports on baseline risk using patient-level data stratified by age and risk, which may be more relevant to the question of primary prevention for older adults, as highlighted in our review.
PROSPER (Prospective Study of Pravastatin in the Elderly at Risk),13 a well-conducted, double-blind, randomized controlled trial with low probability of bias, compared pravastatin 40 mg and placebo. It was the only study that specifically enrolled older adults, with prespecified analysis of primary and secondary prevention subgroups. The primary prevention subgroup accounted for 56% of the 5,084 participants.
JUPITER (Justification for the Use of Statins in Prevention)28 compared rosuvastatin 20 mg and placebo in 17,802 participants. All had low-density lipoprotein cholesterol (LDL-C) levels below 3.4 mmol/L (130 mg/dL) and elevated levels of the inflammatory biomarker high-sensitivity C-reactive protein (hsCRP), ie, 2 mg/L or higher. Subsequently, Glynn et al performed a post hoc, exploratory subgroup analysis of elderly participants (N = 5,695).29
The JUPITER trial had several limitations.43,44 The planned follow-up period was 5 years, but the trial was stopped early at 1.9 years, after a statistically significant difference was detected in the primary composite outcome of reduction in all vascular events. Studies that are stopped early may exaggerate positive findings.45
Further, JUPITER’s patients were a select group, with normal LDL-C levels, elevated hsCRP values, and without diabetes. Of 90,000 patients screened, 72,000 (80%) did not meet the inclusion criteria and were not enrolled. This high rate of exclusion limits the generalizability of study findings beyond the shortcomings of post hoc subgroup analysis.
The meta-analysis performed by the CTT Collaborators32 used individual participant data from large-scale randomized trials of lipid-modifying treatment. This analysis was specific to people at low risk of vascular disease. In a supplementary appendix, the authors described the reduction in major vascular events for each 1.0 mmol/L decrease in LDL-C in three age categories: under age 60, ages 61 to 70, and over age 70.
The authors also stratified the results by risk category and provided information about those with a risk of major vascular events of less than 20%, which would be more representative of a purer primary prevention population.
For the elderly subgroup at low risk, the CTT Collaborators32 only reported a composite of major vascular events (coronary death, nonfatal myocardial infarction [MI], ischemic stroke, or revascularization) and did not describe individual outcomes, such as prevention of coronary heart disease.
Study results are based on postrandomization findings and therefore may be observational, not experimental.46
Studies of statins for secondary prevention of cardiovascular disease
The aim of secondary prevention is to reduce the risk of recurrent cardiovascular events in patients who already have cardiovascular disease.
To address the question of whether statins reduce cardiovascular risk, we reviewed:
PROSPER,13 which included a preplanned analysis of the secondary prevention population.
Afilalo et al,31,47 who performed a meta-analysis of the elderly subgroups of nine major secondary prevention studies (19,569 patients) using published and unpublished data.
To address the question of whether statins benefit individuals with heart failure, we found two relevant studies:
GISSI-HF (Gruppo Italiano per lo Studio della Sopravvivenza nell’Insufficienza Cardiaca Heart Failure)25 and CORONA (Controlled Rosuvastatin Multinational Trial in Heart Failure),26 which were large, international, well-conducted randomized controlled trials that examined statin use in heart failure.
To answer the question of whether statins benefit individuals after a stroke or transient ischemic attack, we found one relevant study:
SPARCL (Stroke Prevention by Aggressive Reduction in Cholesterol Levels),27 which evaluated the benefit of statins in older adults with a history of stroke or transient ischemic attack. It was a prospective, double-blind, placebo-controlled, international trial conducted at 205 centers. One to 6 months after their cerebrovascular event, patients were randomized to receive either atorvastatin 80 mg or placebo. Given the young age of patients in this trial (mean age 63), we also reviewed a post hoc subgroup analysis of the elderly patients in SPARCL (age > 65).30
HOW DID THE STUDY POPULATION COMPARE WITH THOSE WHO ARE FRAIL?
Frail older adults are almost always excluded from large-scale clinical trials,48 leading to uncertainty about whether the conclusions can be applied to those with advanced frailty.
Although age is an imperfect proxy measure of frailty,49 we consider the age of the study population as well as their comorbidities.
Participants in the studies we reviewed were generally younger and healthier than those who are frail, with mean ages of about 75 or less (Table 1).
PROSPER was the most representative study, as it specifically enrolled older adults, albeit without frailty,13 and excluded people with poor cognitive function as defined by a Mini Mental State Examination score less than 24.
JUPITER enrolled a select population, as described above. The median age in the elderly subgroup was 74 (interquartile range 72–78).29
The Afilalo et al31 meta-analysis primarily included studies of young-elderly patients, with a mean age of less than 70. PROSPER13 was an exception.
The GISSI-HF study,25 which examined the benefit of statins in heart failure, described their study population as frail, although the mean age was only 68. Compared with those in GISSI-HF, the CORONA patients26 with heart failure were older (mean age 73) and had more severe heart failure. Accordingly, it is possible that many of the CORONA participants were frail.
ARE STUDY OUTCOMES CLINICALLY RELEVANT TO THOSE WHO ARE FRAIL?
Because baseline cardiovascular risk increases with age, the elderly should, in theory, experience greater absolute benefit from lipid-lowering. However, there is uncertainty about whether this is true in practice.
Some, but not all, epidemiologic studies show a weaker relationship between cholesterol levels and cardiovascular morbidity and mortality rates in older compared to younger adults.50,51 This may be because those with high cholesterol levels die before they get old (time-related bias), or because those with life-threatening illness may have lower cholesterol levels.50 In addition, classic risk factors such as age, sex, systolic blood pressure, cholesterol values, diabetes, smoking, and left ventricular hypertrophy on electrocardiography may have less power to predict cardiovascular risk among older patients.52
The goal of treatment in frailty is to prevent further disability or improve quality of life. Therefore, meaningful outcomes for lipid-lowering therapy should include symptomatic nonfatal MI and its associated morbidity (eg, heart failure and persistent angina) or symptomatic nonfatal stroke leading to disability. Outcomes without sustained clinical impact, such as transient ischemic attack, nondisabling stroke, or silent MI, while potentially important in other populations, are less relevant in severe frailty. Notably, in many statin studies, outcomes include asymptomatic heart disease (eg, silent MI and “suspected events”) and nondisabling stroke (eg, mild stroke, transient ischemic attack). When symptomatic outcomes are not reported separately, the impact of the reported benefit on quality of life and function is uncertain.
The outcome of all-cause mortality is generally recognized as a gold standard for determining treatment benefit. However, since advanced frailty is characterized by multiple competing causes for mortality, a reduction in all-cause mortality that is achieved by addressing a single issue in nonfrail populations may not extend to the frail.
To more fully understand the impact of lipid-lowering therapy on quality of life and function, we examined the following questions:
Do statins as primary prevention reduce symptomatic heart disease?
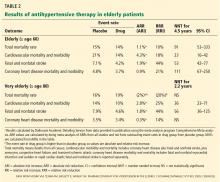
Outcomes for coronary heart disease from PROSPER and JUPITER are summarized in Table 2.
PROSPER. In the PROSPER primary prevention group,13 statin therapy did not reduce the combined outcome of coronary heart disease death and nonfatal MI.
The JUPITER trial demonstrated a statistically significant benefit for preventing MI in the elderly subpopulation (ages 70–97),29 but the number needed to treat was high (211 for 2 years), with a wide confidence interval (CI) (95% CI 106–32,924). The trial did not adequately differentiate between symptomatic and asymptomatic events, making it difficult to determine outcome relevance. Also, due to the methodologic limitations of JUPITER as described above, its results should be interpreted with caution.43,44
The CTT Collaborators32 did not report individual outcomes (eg, coronary heart disease) for the elderly low-risk subgroup and, therefore, this meta-analysis does not answer the question of whether statins reduce symptomatic heart disease in primary prevention populations.
Taken together, these findings do not provide convincing evidence that statin therapy as primary prevention reduces the incidence of symptomatic heart disease for severely frail older adults.
Do statins as secondary prevention reduce symptomatic heart disease?
Most studies defined secondary prevention narrowly as treatment for patients with established coronary artery disease. For instance, in the Afilalo et al meta-analysis,31 the small number of studies that included individuals with other forms of vascular disease (such as peripheral vascular disease) enrolled few participants with noncardiac conditions (eg, 29% in PROSPER13 and 13% in the Heart Protection Study20).
Therefore, any evidence of benefit for secondary prevention demonstrated in these studies is most applicable to patients with coronary heart disease, with less certainty for those with other forms of cardiovascular disease.
In PROSPER,13 the secondary prevention group experienced benefit in the combined outcome of coronary heart disease death or nonfatal MI. In the treatment group, 12.7% experienced this outcome compared with 16.8% with placebo, an absolute risk reduction of 4.1% in 3 years (P = .004, number needed to treat 25, 95% CI 15–77). This measure includes coronary heart disease death, an outcome that may not be generalizable to those who are frail. In addition, the outcome of nonfatal MI includes both symptomatic and suspected events. As such, there is uncertainty whether the realized benefit is clinically relevant to frail older adults.
The Afilalo et al meta-analysis31 showed that the number needed to treat to prevent one nonfatal MI was 38 (95% CI 16–118) over 5 years (Table 2). However, this outcome included both symptomatic and asymptomatic (silent) events.
Based on the available data, we conclude that it is not possible to determine whether statins reduce symptomatic heart disease as secondary prevention for older adults who are frail.
Do statins reduce heart disease in combined populations?
In the combined primary and secondary population from PROSPER,13 pravastatin decreased the risk of nonfatal symptomatic MI from 4.3% in the placebo group to 3.4%, a relatively small reduction in absolute risk (0.9%) and not statistically significant by our chi-square calculation (P = .099).
Do statins prevent a first symptomatic stroke in people with or without preexisting cardiovascular disease?
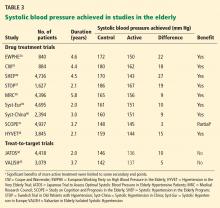
Preventing strokes that cause functional decline is an important outcome for the frail elderly. Stroke outcomes from PROSPER,13 JUPITER,29 and the Afilalo et al meta-analysis31 are summarized in Table 3.
For primary prevention:
In PROSPER (primary prevention),13 there was no statistically significant benefit in the combined outcome of fatal and nonfatal stroke or the single outcome of transient ischemic attack after 3.2 years.
JUPITER,29 in contrast, found that rosuvastatin 20 mg reduced strokes in primary prevention, but the absolute benefit was small. In 2 years, 0.8% of the treatment group had strokes, compared with 1.4% with placebo, an absolute risk reduction of 0.6% (P = .023, number needed to treat 161, 95% CI 86–1,192).
Neither PROSPER nor JUPITER differentiated between disabling and nondisabling strokes.
For secondary prevention:
In PROSPER (secondary prevention),13 there was no statistically significant benefit in the combined outcome of fatal and nonfatal stroke or the single outcome of transient ischemic attack after 3.2 years.
The Afilalo et al secondary prevention meta-analysis demonstrated a 25% relative reduction in stroke (relative risk 0.75, 95% CI 0.56–0.94, number needed to treat 58, 95% CI 27–177).31
Notably, the stroke outcome in Afilalo included both disabling and nondisabling strokes. For example, in the Heart Protection Study,20 the largest study in the Afilalo et al meta-analysis, approximately 50% of nonfatal, classifiable strokes in the overall study population (ie, both younger and older patients) were not disabling. Including disabling and nondisabling strokes in a composite outcome confounds the clinical meaningfulness of these findings in frailty, as the number needed to treat to prevent one disabling stroke cannot be calculated from the data provided.
Do statins prevent a second (symptomatic) stroke in people with a previous stroke?
SPARCL27 (Table 3) examined the question of whether statins decrease the risk of recurrent ischemic stroke for patients with a prior history of stroke or transient ischemic attack. There was a statistically significant reduction in the primary composite outcome of fatal and nonfatal stroke, with 11.2% of the treatment group and 13.1% of the placebo group experiencing this outcome, an absolute risk reduction of 1.9% at 5 years (P = .03; number needed to treat 52, 95% CI 26–1,303). However, the difference in nonfatal stroke, which is the outcome of interest for frailty (since mortality has uncertain relevance), was not statistically significant (10.4% with treatment vs 11.8% with placebo, P =.11).
An exploratory subgroup analysis of SPARCL patients based on age30 showed a smaller, nonsignificant reduction in the primary end point of fatal and nonfatal stroke in the group over age 65 (relative risk 0.90, 95% confidence interval 0.73–1.11, P = .33) compared with the younger group (age < 65) (relative risk 0.74, 95% CI 0.57–0.96, P = .02).
The applicability of these results to the frail elderly is uncertain, since the subgroup analysis was not powered to determine outcomes based on age stratification and there were differences between groups in characteristics such as blood pressure and smoking status. In addition, the outcome of interest, nonfatal stroke, is not provided for the elderly subgroup.
In conclusion, in both primary and secondary prevention populations, the evidence that statins reduce nonfatal, symptomatic stroke rates for older adults is uncertain.
Do statins decrease all-cause mortality for primary or secondary prevention?
Due to competing risks for death, the outcome of mortality may not be relevant to those who are frail; however, studies showed the following:
For primary prevention, there was no decrease in mortality in PROSPER13 or in the elderly subgroup of JUPITER.29
For secondary prevention, an analysis of PROSPER trial data by Afilalo et al31 showed a significant 18% decrease in all-cause mortality (relative risk 0.82, 95% CI 0.69–0.98) using pravastatin 40 mg.
A decrease in all-cause mortality with statins was also reported in the pooled result of the Afilalo et al meta-analysis.31
What are the reported composite outcomes for primary and secondary prevention?
While we were most interested in the symptomatic outcomes described above, we recognize that the small numbers of events make it difficult to draw firm conclusions. Therefore, we also considered composite primary outcomes, even though most included multiple measures that have varying associations with disability and relevancy to frail older adults.
For primary prevention, in the PROSPER preplanned subgroup analysis,13 there was no statistical benefit for any outcome, including the primary composite measure. In contrast, the elderly subpopulation in the JUPITER trial28 showed a treatment benefit with rosuvastatin 20 mg compared with placebo for the primary composite outcome of MI, stroke, cardiovascular death, hospitalization for unstable angina, or revascularization. The number needed to treat for 2 years was 62 (95% CI 39–148).
In the CTT meta-analysis,32 patients at all levels of baseline risk showed benefit up to age 70. However, there was no statistically significant benefit in the composite primary outcome of coronary deaths, nonfatal myocardial infarction, ischemic stroke, or revascularization in the population most representative of elderly primary prevention—those who were more than 70 years old with a 5-year baseline risk of less than 20%.
For secondary prevention, in PROSPER,13 the subpopulation of patients treated for secondary prevention experienced benefit in the primary composite outcome of coronary heart disease death, nonfatal MI, or fatal or nonfatal stroke, achieving a 4% absolute risk reduction with a number needed to treat of 23 (95% CI 14–81) over 3 years.
Do statins decrease disability?
PROSPER was the only study that reported on disability. Compared with placebo, pravastatin did not decrease disability in the total population as measured by basic and instrumental activities of daily living scales.
Do statins help patients with heart failure?
Neither GISSI-HF25 nor CORONA26 found significant benefit from rosuvastatin 10 mg, despite LDL-C lowering of 27% in GISSI-HF and 45% in CORONA.
Do ezetimibe or other nonstatin lipid-lowering agents improve outcomes?
There is no definitive evidence that ezetimibe provides clinically meaningful benefit as a single agent.
For combination therapy, the IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial)53 showed that adding ezetimibe 10 mg to simvastatin 40 mg after an acute coronary syndrome reduced the risk of nonfatal myocardial infarction compared with simvastatin monotherapy (event rate 12.8% vs 14.4%; hazard ratio 0.87, 95% CI 0.80–0.95; P = .002) for a population with a mean age of 64. The risk of any stroke was also reduced; strokes occurred in 4.2% of those receiving combination therapy vs 4.8% with monotherapy (hazard ratio 0.86, 95% CI 0.73–1.00, P = .05). After a median of 6 years, 42% of patients in each group had discontinued treatment. Given the very specific clinical scenario of acute coronary syndrome and the young age of the patients in this trial, we do not think that this study justifies the use of ezetimibe for severely frail older adults.
There is no evidence that other combinations (ie, a statin plus another lipid-lowering drug) improve clinical outcomes for either primary or secondary prevention in any population.54
WILL FRAIL PATIENTS LIVE LONG ENOUGH TO BENEFIT?
It is often difficult to determine the number of years that are needed to achieve benefit, as most trials do not provide a statistical analysis of varying time frames.
The PROSPER trial13 lasted 3.2 years. From the Kaplan-Meier curves in PROSPER, we estimate that it took about 1.5 years to achieve a 1% absolute risk reduction and 2.5 years for a 2% absolute risk reduction in coronary heart disease death and nonfatal MI in the combined primary and secondary groups.
JUPITER28 was stopped early at 1.9 years. The Afilalo et al meta-analysis31 was based on follow-up over 4.9 years.
IMPROVE-IT53 reported event rates at 7 years. The authors note that benefit in the primary composite outcome appeared to emerge at 1 year, although no statistical support is given for this statement and divergence in the Kaplan-Meier curves is not visually apparent.
The duration of other studies ranged between 2.7 and 4.9 years (Table 1).26–28
It has been suggested that statins should be considered for elderly patients who have a life expectancy of at least 5 years.3 However, many older adults have already been taking statins for many years, which makes it difficult to interpret the available timeframe evidence.
In a multicenter, unblinded, randomized trial,55 statins were either stopped or continued in older adults who had a short life expectancy and a median survival of approximately 7 months. Causes of death were evenly divided between cancer and noncancer diagnoses, and 22% of the patients were cognitively impaired. Discontinuing statin therapy did not increase mortality or cardiovascular events within 60 days. Nevertheless, stopping statin therapy did not achieve noninferiority for the primary end point, the proportion of participants who died within 60 days. Statin discontinuation was associated with improved quality of life, although the study was not blinded, which could have influenced results.
HAVE THE HARMS BEEN SUFFICIENTLY CONSIDERED?
Frail older adults commonly take multiple medications and are more vulnerable to adverse events.56
Many statins require dose reduction with severe renal impairment (creatinine clearance < 30 mL/min/1.73 m2), which would be a common consideration in severely frail older adults.
Myopathy
Myopathy, which includes myalgias and muscle weakness, is a statin-related adverse event that can impair quality of life. Myopathy typically develops within the first 6 months but can occur at any time during statin treatment.57 When muscle-related adverse effects occur, they may affect the elderly more significantly, particularly their ability to perform activities of daily living, rise from a chair, or mobilize independently. Another concern is that older adults with dementia may not be able to accurately report muscle-related symptoms.
It is difficult to ascertain the true prevalence of myopathy, especially in advanced age and frailty. Randomized controlled trials report incidence rates of 1.5% to 5%, which is comparable to placebo.57,58 However, inconsistent definitions of myopathy and exclusion of subjects with previous statin intolerance or adverse effects during run-in periods limit interpretability.57 Clinical experience suggests that muscle complaints may be relatively common.59–61
Advanced age, female sex, low body mass index, and multisystem disease are all associated with frailty and have also been described as risk factors for statin-associated muscle syndromes.61 Physiologic changes associated with frailty, such as reduced muscle strength, decreased lean body mass, impaired functional mobility, decreased reserve capacity, and altered drug metabolism may increase the risk and severity of myopathy.62
Adverse cognitive events
Meta-analyses of randomized clinical trials and narrative reviews find no definitive relationship between statin therapy and adverse cognitive events.63–67 Nevertheless, there have been case reports of memory loss associated with the use of statins, and the US Food and Drug Administration has issued a warning that statins have been associated with memory loss and confusion.68
It may be difficult to determine whether a statin is causing or aggravating cognitive symptoms among individuals with dementia without a trial withdrawal of the drug.
OUR RECOMMENDATIONS
The recommendations below are intended for adults with severe or very severe frailty (ie, a score of 7 or 8 on the Clinical Frailty Scale11 or FACT5 and therefore apply to most older adults living in long-term care facilities.
Primary prevention
There is no reason to prescribe or continue statins for primary prevention, as it is unlikely that they would provide benefit for outcomes that are relevant in this population.
Secondary prevention
Statin treatment is probably not necessary for secondary prevention in those with severe frailty, although there may be extenuating circumstances that justify statin use.
Heart failure
There is no reason to start or continue statins for heart failure, as there is insufficient evidence that they are effective for this indication in any population.
Ezetimibe
There is no evidence that ezetimibe reduces cardiovascular events in any population when used as monotherapy. For a select population with acute coronary syndromes, ezetimibe has a modest effect. Given the very specific clinical scenario of acute coronary syndrome, we do not think that the available evidence justifies the use of ezetimibe for severely frail older adults.
Agents other than ezetimibe combined with statins
There is no reason to start or continue other lipid-lowering drugs in conjunction with statins.
Statin dosing
As statin adverse effects have the potential to increase with advancing age and frailty, lower doses may be appropriate.68
Adverse events
Consider stopping statins on a trial basis if there is concern regarding myopathy, drug interactions, or other adverse effects.
BOTTOM LINE: DO STATINS IMPROVE QUALITY OF LIFE OR FUNCTION?
In primary prevention for older adults, there is doubt that statins prevent cardiovascular disease and stroke-related events because the main study involving the elderly did not show a benefit in the primary prevention subgroup.13 Additionally, there is no conclusive evidence that statin treatment decreases mortality in primary prevention.13,29
There is insufficient information to determine whether the frail elderly should receive statins for secondary prevention. Although there is evidence that treatment decreases measures of coronary heart disease and stroke, it is unclear whether it improves quality of life or function for those who are frail. To answer this question, we need more information about whether reported outcomes (such as stroke and MI) are associated with disability, which is not provided in many of the studies we reviewed. When disability was specifically considered in the PROSPER trial for the combined population of primary and secondary prevention, treatment with statins had no impact on basic and instrumental activities of daily living.
Some experts may not agree with our interpretation of the complex evidence presented in this article. Others may ask, “What is the harm in using statins, even if there is no definitive benefit?” However, the harms associated with statin therapy for the frail are poorly defined. In the face of these uncertainties and in the absence of definitive improvement in quality of life, we believe that “less is more” in the context of severe frailty.69
The cost of medications should also be considered, especially in long-term care facilities, where there is an added expense of drug administration that diverts human resources away from interactions that are more congruent with respecting the lifestage of frailty.
Careful review of evidence before applying clinical practice guidelines to those who are frail should become the norm. When considering treatment of frail patients, the five questions described in this review shed light on the applicability of clinical trial evidence. Therapies that are highly effective in healthier populations may be less effective when individuals are severely frail. Accordingly, we propose that medications should only be used if they improve quality of life or function.
- Ontario Pharmacy Research Collaboration. Deprescribing guidelines for the elderly. www.open-pharmacy-research.ca/research-projects/emerging-services/deprescribing-guidelines. Accessed December 28, 2016.
- Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med 2015; 175:827–834.
- O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 2015; 44:213–218.
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2012; 60:616–631.
- Mallery LH, Allen M, Fleming I, et al. Promoting higher blood pressure targets for frail older adults: a consensus guideline from Canada. Cleve Clin J Med 2014; 81:427–437.
- Mallery LH, Ransom T, Steeves B, Cook B, Dunbar P, Moorhouse P. Evidence-informed guidelines for treating frail older adults with type 2 diabetes: from the Diabetes Care Program of Nova Scotia (DCPNS) and the Palliative and Therapeutic Harmonization (PATH) program. J Am Med Dir Assoc 2013; 14:801–808.
- Moorhouse P, Mallery L. Palliative and therapeutic harmonization: a model for appropriate decision-making in frail older adults. J Am Geriatr Soc 2012; 60:2326–2332.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging 2012; 16:601–608.
- Rockwood K, Song Z, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173:489–495.
- Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013; 14:392–397.
- Tricoci P, Allen JM, Kramer JM, Califf RM, Smith SC Jr. Scientific evidence underlying the ACC/AHA clinical practice guidelines. JAMA 2009; 301:831–841.
- Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002; 360:1623–1630.
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- Miettien TA, Pyorala K, Olsson AG, et al. Cholesterol-lowering therapy in women and elderly patients with myocardial infarction or angina pectoris: findings from the Scandinavian Simvastatin Study Group (4S). Circulation 1997; 96:4211–4218.
- Lewis SJ, Moye LA, Sacks FM, et al. Effect of pravastatin on cardiovascular events in older patients with myocardial infarction and cholesterol levels in the average range. Results of the Cholesterol and Recurrent Events (CARE) trial. Ann Intern Med 1998; 129:681–689.
- Hunt D, Young P, Simes J, et al. Benefits of pravastatin on cardiovascular events and mortality in older patients with coronary heart disease are equal to or exceed those seen in younger patients: results from the LIPID trial. Ann Intern Med 2001; 134:931–940.
- Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med 1998; 339:1349–1357.
- Heart Protection Study Collaborative Group. The effects of cholesterol lowering with simvastatin on cause-specific mortality and on cancer incidence in 20,536 high-risk people: a randomized placebo-controlled trial. BMC Med 2005; 3:6.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomized placebo-controlled trial. Lancet 2002; 360:7–22.
- Pitt B, Mancini GB, Ellis SG, Rosman HS, Park JS, McGovern ME. Pravastatin limitation of atherosclerosis in the coronary arteries (PLAC 1): reduction in atherosclerosis progression and clinical events. PLAC 1 investigation. J Am Coll Cardiol 1995; 26:1133–1139.
- Jukema JW, Bruschke AV, van Boven AJ, et al. Effects of lipid lowering by pravastatin on progression and regression of coronary artery disease in symptomatic men with normal to moderately elevated serum cholesterol levels. The Regression Growth Evaluation Statin Study (REGRESS). Circulation 1995; 91:2528–2540.
- Serruys PW, Foley DP, Jackson G, et al. A randomized placebo-controlled trial of fluvastatin for prevention of restenosis after successful coronary balloon angioplasty; final results of the fluvastatin angiographic restenosis (FLARE) trial. Eur Heart J 1999; 20:58–69.
- Serruys PW, de Feyter P, Macaya C, et al; Lescol Intervention Prevention Study (LIPS) Investigators. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 287:3215–3222.
- Tavazzi L, Maggioni AP, Marchioli R, et al; Gissi-HF Investigators. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomized, double-blind, placebo-controlled trial. Lancet 2008; 372:1231–1239.
- Kjekshus J, Apatrei E, Barrios V, et al; CORONA Group. Rosuvastatin in older patients with systolic heart failure. N Engl J Med 2007; 357:2248–2261.
- Amarenco P, Bogousslavsky J, Callahan A, et al; Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006; 355:549–559.
- Ridker PM, Danielson E, Fonseca FA, et al; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Glynn RJ, Koenig W, Nordestgaard BG, Shepherd J, Ridker PM. Rosuvastatin for primary prevention in older persons with elevated C-reactive protein and low to average low-density lipoprotein cholesterol levels: exploratory analysis of a randomized trial. Ann Intern Med 2010; 152:488–496, W174.
- Chaturvedi S, Zivin J, Breazna A, et al; SPARCL Investigators. Effect of atorvastatin in elderly patients with a recent stroke or transient ischemic attack. Neurology 2009; 72:688–694.
- Afilalo J, Duque G, Steele R, Jukema JW, de Craen AJ, Eisenberg MJ. Statins for secondary prevention in elderly patients: a hierarchical bayesian meta-analysis. J Am Coll Cardiol 2008; 51:37–45.
- Cholesterol Treatment Trialists’ (CTT) Collaborators; Mihaylova B, Emberson J, Blackwell L, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 2012; 380:581– 590.
- Sacks FM, Pfeffer MA, Moye L, et al. Rationale and design of a secondary prevention trial of lowering normal plasma cholesterol levels after acute myocardial infarction: the Cholesterol and Recurrent Events (CARE). Am J Cardiol 1991; 68:1436–1446.
- Armitage J, Collins R. Need for large scale randomised evidence about lowering LDL cholesterol in people with diabetes mellitus: MRC/BHF Heart Protection Study and other major trials. Heart 2000; 84:357–360.
- Design features and baseline characteristics of the LIPID (Long-Term Intervention with Pravastatin in Ischemic Disease) study: a randomized trial in patients with previous acute myocardial infarction and/or unstable angina pectoris. Am J Cardiol 1995; 76:474–479.
- Shepherd J, Blauw GJ, Murphy MB, et al. The design of a prospective study of Pravastatin in the Elderly at Risk (PROSPER). Am J Cardiol 1999; 84:1192–1197.
- Amarenco P, Bogousslavsky J, Callahan AS, et al; SPARCL Investigators. Design and baseline characteristics of the stroke prevention by aggressive reduction in cholesterol levels (SPARCL) study. Cerebrovasc Dis 2003; 16:389–395.
- Interpretation of subgroup analyses and meta-regressions. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2011. http://handbook.cochrane.org/chapter_9/9_6_6_interpretation_of_subgroup_analyses_and_meta_regressions.htm. Accessed December 5, 2016.
- Borenstein M, Higgins JP. Meta-analysis and subgroups. Prev Sci 2013; 14:134–143.
- Savarese G, Gotto AM Jr, Paolillo S, et al. Benefits of statins in elderly subjects without established cardiovascular disease: a meta-analysis. J Am Coll Cardiol 2013; 62:2090–2099.
- Sever PS, Dahlof B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003; 361:1149–1158.
- The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). JAMA 2002; 288:2998–3007.
- de Longeril M, Salen P, Abramson J, et al. Cholesterol lowering, cardiovascular diseases, and the rosuvastatin-JUPITER controversy: a critical reappraisal. Arch Intern Med 2010; 170:1032–1036.
- Yusuf S, Lonn E, Bosch J. Lipid lowering for primary prevention. Lancet 2009: 373:1152–1155.
- Briel M, Bassler D, Wang AT, Guyatt GH, Montori VM. The dangers of stopping a trial too early. J Bone Joint Surg Am 2012; 94(suppl 1):56–60.
- Hayward RA, Krumholz HM. Three reasons to abandon low-density lipoprotein targets: an open letter to the Adult Treatment Panel IV of the National Institutes of Health. Circ Cardiovasc Qual Outcomes 2012; 5:2–5.
- Afilalo J, Duque G, Steele R, Jukema JW, de Craen AJ, Eisenberg MJ. Statins for secondary prevention in elderly patients: a hierarchical Bayesian meta-analysis. www.ncbi.nlm.nih.gov/pubmedhealth/PMH0026417. Accessed December 5, 2016.
- Holmes HM, Hayley DC, Alexander GC, Sachs GA. Reconsidering medication appropriateness for patients late in life. Arch Intern Med 2006; 166:605–609.
- Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med 2011; 27:17–26.
- Petersen LK, Christensen K, Kragstrup J. Lipid-lowering treatment to the end? A review of observational studies and RCTs on cholesterol and mortality in 80+-year olds. Age Ageing 2010; 39:674–680.
- Psaty BM, Anderson M, Kronmal RA, et al. The association between lipid levels and the risks of incident myocardial infarction, stroke, and total mortality: the Cardiovascular Health Study. J Am Geriatr Soc 2004; 52:1639–1647.
- de Ruijter W, Westendorp RG, Assendelft WJ, et al. Use of Framingham risk score and new biomarkers to predict cardiovascular mortality in older people: population based observational cohort study. BMJ 2009; 338:a3083.
- Canon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015; 372:2387–2397.
- Anderson TJ, Gregoire J, Hegele RA, et al. 2012 update of the Canadian Cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol 2013; 29:151–167.
- Kutner JS, Blatchford PJ, Taylor DH, et al. Safety and benefit of discontinuing statin therapy in the setting of advanced, life-limiting illness: a randomized clinical trial. JAMA Intern Med 2015; 175:691–700.
- Tinetti ME, Bogardus ST Jr, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med 2004; 351:2870–2874.
- Rosenson RS. Current overview of statin-induced myopathy. Am J Med 2004; 116:408–416.
- Mancini GB, Baker S, Bergeron J, et al. Diagnosis, prevention, and management of statin adverse effects and intolerance: proceedings of a Canadian Working Group Consensus Conference. Can J Cardiol 2011; 27:635–662.
- Cohen JD, Brinton EA, Ito MK, Jacobson TA. Understanding Statin Use in America and Gaps in Patient Education (USAGE): an internet-based survey of 10,138 current and former statin users. J Clin Lipidol 2012; 6:208–215.
- Joy TR, Hegele RA. Narrative review: statin-related myopathy. Ann Intern Med 2009; 150:858–868.
- Talbert RL. Safety issues with statin therapy. J Am Pharm Assoc (2003) 2006; 46:479–490.
- Sewright KA, Clarkson PM, Thompson PD. Statin myopathy: incidence, risk factors, and pathophysiology. Curr Atheroscler Rep 2007; 9:389–396.
- Ott BR, Daiello LA, Dahabreh IJ, et al. Do statins impair cognition? A systematic review and meta-analysis of randomized controlled trials. J Gen Intern Med 2015; 30:348–358.
- Mancini GB, Tashakkor AY, Baker S, et al. Diagnosis, prevention and management of statin adverse effects and intolerance: Canadian Working Group Consensus update. Can J Cardiol 2013: 29:1553–1568.
- Rojas-Fernandez CH, Cameron JC. Is statin-associated cognitive impairment clinically relevant? A narrative review and clinical recommendations. Ann Pharmacother 2012; 46:549–557.
- McGuinness B, O’Hare J, Craig D, Bullock R, Malouf R, Passmore P. Cochrane review on ‘Statins for the treatment of dementia’. Int J Geriatr Psychiatry 2013; 28:119–126.
- Pandey RD, Gupta PP, Jha D, Kumar S. Role of statins in Alzheimer’s disease: a retrospective meta-analysis for commonly investigated clinical parameters in RCTs. Int J Neurosci 2013; 123:521–525.
- Food and Drug Administration (FDA). FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. www.fda.gov/drugs/ drugsafety/ucm293101.htm. Accessed December 5, 2016.
- Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med 2010; 170:1648–1654.
Frail elderly patients are at high risk of adverse clinical outcomes, including those due to polypharmacy. Several groups tackle “deprescribing” by developing lists of medications that are potentially inappropriate for the elderly, such as the Beers or STOPP/START criteria.1–4
See related editorialIn contrast, our group (the Palliative and Therapeutic Harmonization [PATH] program and the Dalhousie Academic Detailing Service) has developed evidence-based, frailty-specific guidelines for treating hypertension5 and diabetes,6 in which we advocate less-stringent treatment targets and tapering or discontinuing medications, as needed.
The PATH program7 is a clinical approach that prioritizes the consideration of frailty when making treatment decisions. The Dalhousie Academic Detailing Service collaborates with the Nova Scotia Health Authority to research and develop evidence-informed educational messages about the treatment of common medical conditions.
Here, we address lipid-lowering therapy in this population.
CONSIDERING FRAILTY
Frailty is defined in several ways. The Fried model8,9 identifies frailty when 3 of the following characteristics are present: unintentional weight loss, exhaustion, muscle weakness, slow walking speed, or low levels of activity. The Clinical Frailty Scale10,11 and the Frailty Assessment for Care-planning Tool (FACT)5 use deficits in cognition, function, and mobility to define frailty. According to these scales, people are considered severely frail when they require assistance with basic activities of daily living (such as bathing or dressing), owing to cognitive or physical deficits from any cause.
In reviewing the evidence, we consider five questions:
- What is the quality of the evidence? (Up to 48% of clinical practice guideline recommendations may be based on low-level evidence or expert opinion.12)
- How did the study population compare with the frail?
- Are study outcomes and potential benefits clinically relevant to those who are frail?
- How long did it take for the clinical benefit of a treatment to become apparent, and are the frail elderly likely to live that long?
- Have the harms of treatment been sufficiently considered?
WHAT IS THE QUALITY OF THE EVIDENCE?
We found no studies that specifically evaluated the benefit of lipid-lowering for severely frail older adults. Therefore, we examined randomized controlled trials that enrolled non-frail older adults,13–28 subgroup analyses of randomized controlled trials,29,30 meta-analyses that analyzed subgroups of elderly populations,31,32 and publications describing the study designs of randomized controlled trials.33–37
Most of the evidence comes from post hoc subgroup analyses of elderly populations. Although meta-analysis is commonly used to compare subgroups, the Cochrane handbook and others consider subgroup comparisons observational by nature.38,39 (See Table 1 for lipid-lowering studies discussed in this article.)
Studies of statins for primary prevention of cardiovascular disease
For evidence of benefit from lipid-lowering for primary prevention (ie, to reduce the risk of cardiovascular events in patients with no known cardiovascular disease at baseline but at increased risk), we reviewed the meta-analysis conducted by the Cholesterol Treatment Trialists’ (CTT) Collaborators.32 Since this meta-analysis included the major trials that enrolled elderly patients, individual publications of post hoc, elderly subgroups were, for the most part, not examined individually. The exception to this approach was a decision to report on the PROSPER13 and JUPITER28 trials separately, because PROSPER is the most representative of the elderly population and JUPITER reached the lowest LDL-C of primary prevention trials published to date and included a large elderly subgroup (n = 5,695).
Savarese et al40 evaluated the benefits of statins for older adults who did not have established cardiovascular disease. We did not report on this meta-analysis, as not all of the subjects that populated the meta-analysis were representative of a typical prevention population. For instance, in the Anglo-Scandinavian Cardiac Outcomes Trial lipid-lowering arm,41 14% of the subjects had had a previous stroke or transient ischemic attack. In the Antihypertensive and Lipid-Lowering Treatment Trial,42 16% of the population had a family history of premature coronary heart disease.
In addition, all the trials in the Savarese meta-analysis were also included in the CTT meta-analysis.32 The CTT reports on baseline risk using patient-level data stratified by age and risk, which may be more relevant to the question of primary prevention for older adults, as highlighted in our review.
PROSPER (Prospective Study of Pravastatin in the Elderly at Risk),13 a well-conducted, double-blind, randomized controlled trial with low probability of bias, compared pravastatin 40 mg and placebo. It was the only study that specifically enrolled older adults, with prespecified analysis of primary and secondary prevention subgroups. The primary prevention subgroup accounted for 56% of the 5,084 participants.
JUPITER (Justification for the Use of Statins in Prevention)28 compared rosuvastatin 20 mg and placebo in 17,802 participants. All had low-density lipoprotein cholesterol (LDL-C) levels below 3.4 mmol/L (130 mg/dL) and elevated levels of the inflammatory biomarker high-sensitivity C-reactive protein (hsCRP), ie, 2 mg/L or higher. Subsequently, Glynn et al performed a post hoc, exploratory subgroup analysis of elderly participants (N = 5,695).29
The JUPITER trial had several limitations.43,44 The planned follow-up period was 5 years, but the trial was stopped early at 1.9 years, after a statistically significant difference was detected in the primary composite outcome of reduction in all vascular events. Studies that are stopped early may exaggerate positive findings.45
Further, JUPITER’s patients were a select group, with normal LDL-C levels, elevated hsCRP values, and without diabetes. Of 90,000 patients screened, 72,000 (80%) did not meet the inclusion criteria and were not enrolled. This high rate of exclusion limits the generalizability of study findings beyond the shortcomings of post hoc subgroup analysis.
The meta-analysis performed by the CTT Collaborators32 used individual participant data from large-scale randomized trials of lipid-modifying treatment. This analysis was specific to people at low risk of vascular disease. In a supplementary appendix, the authors described the reduction in major vascular events for each 1.0 mmol/L decrease in LDL-C in three age categories: under age 60, ages 61 to 70, and over age 70.
The authors also stratified the results by risk category and provided information about those with a risk of major vascular events of less than 20%, which would be more representative of a purer primary prevention population.
For the elderly subgroup at low risk, the CTT Collaborators32 only reported a composite of major vascular events (coronary death, nonfatal myocardial infarction [MI], ischemic stroke, or revascularization) and did not describe individual outcomes, such as prevention of coronary heart disease.
Study results are based on postrandomization findings and therefore may be observational, not experimental.46
Studies of statins for secondary prevention of cardiovascular disease
The aim of secondary prevention is to reduce the risk of recurrent cardiovascular events in patients who already have cardiovascular disease.
To address the question of whether statins reduce cardiovascular risk, we reviewed:
PROSPER,13 which included a preplanned analysis of the secondary prevention population.
Afilalo et al,31,47 who performed a meta-analysis of the elderly subgroups of nine major secondary prevention studies (19,569 patients) using published and unpublished data.
To address the question of whether statins benefit individuals with heart failure, we found two relevant studies:
GISSI-HF (Gruppo Italiano per lo Studio della Sopravvivenza nell’Insufficienza Cardiaca Heart Failure)25 and CORONA (Controlled Rosuvastatin Multinational Trial in Heart Failure),26 which were large, international, well-conducted randomized controlled trials that examined statin use in heart failure.
To answer the question of whether statins benefit individuals after a stroke or transient ischemic attack, we found one relevant study:
SPARCL (Stroke Prevention by Aggressive Reduction in Cholesterol Levels),27 which evaluated the benefit of statins in older adults with a history of stroke or transient ischemic attack. It was a prospective, double-blind, placebo-controlled, international trial conducted at 205 centers. One to 6 months after their cerebrovascular event, patients were randomized to receive either atorvastatin 80 mg or placebo. Given the young age of patients in this trial (mean age 63), we also reviewed a post hoc subgroup analysis of the elderly patients in SPARCL (age > 65).30
HOW DID THE STUDY POPULATION COMPARE WITH THOSE WHO ARE FRAIL?
Frail older adults are almost always excluded from large-scale clinical trials,48 leading to uncertainty about whether the conclusions can be applied to those with advanced frailty.
Although age is an imperfect proxy measure of frailty,49 we consider the age of the study population as well as their comorbidities.
Participants in the studies we reviewed were generally younger and healthier than those who are frail, with mean ages of about 75 or less (Table 1).
PROSPER was the most representative study, as it specifically enrolled older adults, albeit without frailty,13 and excluded people with poor cognitive function as defined by a Mini Mental State Examination score less than 24.
JUPITER enrolled a select population, as described above. The median age in the elderly subgroup was 74 (interquartile range 72–78).29
The Afilalo et al31 meta-analysis primarily included studies of young-elderly patients, with a mean age of less than 70. PROSPER13 was an exception.
The GISSI-HF study,25 which examined the benefit of statins in heart failure, described their study population as frail, although the mean age was only 68. Compared with those in GISSI-HF, the CORONA patients26 with heart failure were older (mean age 73) and had more severe heart failure. Accordingly, it is possible that many of the CORONA participants were frail.
ARE STUDY OUTCOMES CLINICALLY RELEVANT TO THOSE WHO ARE FRAIL?
Because baseline cardiovascular risk increases with age, the elderly should, in theory, experience greater absolute benefit from lipid-lowering. However, there is uncertainty about whether this is true in practice.
Some, but not all, epidemiologic studies show a weaker relationship between cholesterol levels and cardiovascular morbidity and mortality rates in older compared to younger adults.50,51 This may be because those with high cholesterol levels die before they get old (time-related bias), or because those with life-threatening illness may have lower cholesterol levels.50 In addition, classic risk factors such as age, sex, systolic blood pressure, cholesterol values, diabetes, smoking, and left ventricular hypertrophy on electrocardiography may have less power to predict cardiovascular risk among older patients.52
The goal of treatment in frailty is to prevent further disability or improve quality of life. Therefore, meaningful outcomes for lipid-lowering therapy should include symptomatic nonfatal MI and its associated morbidity (eg, heart failure and persistent angina) or symptomatic nonfatal stroke leading to disability. Outcomes without sustained clinical impact, such as transient ischemic attack, nondisabling stroke, or silent MI, while potentially important in other populations, are less relevant in severe frailty. Notably, in many statin studies, outcomes include asymptomatic heart disease (eg, silent MI and “suspected events”) and nondisabling stroke (eg, mild stroke, transient ischemic attack). When symptomatic outcomes are not reported separately, the impact of the reported benefit on quality of life and function is uncertain.
The outcome of all-cause mortality is generally recognized as a gold standard for determining treatment benefit. However, since advanced frailty is characterized by multiple competing causes for mortality, a reduction in all-cause mortality that is achieved by addressing a single issue in nonfrail populations may not extend to the frail.
To more fully understand the impact of lipid-lowering therapy on quality of life and function, we examined the following questions:
Do statins as primary prevention reduce symptomatic heart disease?
Outcomes for coronary heart disease from PROSPER and JUPITER are summarized in Table 2.
PROSPER. In the PROSPER primary prevention group,13 statin therapy did not reduce the combined outcome of coronary heart disease death and nonfatal MI.
The JUPITER trial demonstrated a statistically significant benefit for preventing MI in the elderly subpopulation (ages 70–97),29 but the number needed to treat was high (211 for 2 years), with a wide confidence interval (CI) (95% CI 106–32,924). The trial did not adequately differentiate between symptomatic and asymptomatic events, making it difficult to determine outcome relevance. Also, due to the methodologic limitations of JUPITER as described above, its results should be interpreted with caution.43,44
The CTT Collaborators32 did not report individual outcomes (eg, coronary heart disease) for the elderly low-risk subgroup and, therefore, this meta-analysis does not answer the question of whether statins reduce symptomatic heart disease in primary prevention populations.
Taken together, these findings do not provide convincing evidence that statin therapy as primary prevention reduces the incidence of symptomatic heart disease for severely frail older adults.
Do statins as secondary prevention reduce symptomatic heart disease?
Most studies defined secondary prevention narrowly as treatment for patients with established coronary artery disease. For instance, in the Afilalo et al meta-analysis,31 the small number of studies that included individuals with other forms of vascular disease (such as peripheral vascular disease) enrolled few participants with noncardiac conditions (eg, 29% in PROSPER13 and 13% in the Heart Protection Study20).
Therefore, any evidence of benefit for secondary prevention demonstrated in these studies is most applicable to patients with coronary heart disease, with less certainty for those with other forms of cardiovascular disease.
In PROSPER,13 the secondary prevention group experienced benefit in the combined outcome of coronary heart disease death or nonfatal MI. In the treatment group, 12.7% experienced this outcome compared with 16.8% with placebo, an absolute risk reduction of 4.1% in 3 years (P = .004, number needed to treat 25, 95% CI 15–77). This measure includes coronary heart disease death, an outcome that may not be generalizable to those who are frail. In addition, the outcome of nonfatal MI includes both symptomatic and suspected events. As such, there is uncertainty whether the realized benefit is clinically relevant to frail older adults.
The Afilalo et al meta-analysis31 showed that the number needed to treat to prevent one nonfatal MI was 38 (95% CI 16–118) over 5 years (Table 2). However, this outcome included both symptomatic and asymptomatic (silent) events.
Based on the available data, we conclude that it is not possible to determine whether statins reduce symptomatic heart disease as secondary prevention for older adults who are frail.
Do statins reduce heart disease in combined populations?
In the combined primary and secondary population from PROSPER,13 pravastatin decreased the risk of nonfatal symptomatic MI from 4.3% in the placebo group to 3.4%, a relatively small reduction in absolute risk (0.9%) and not statistically significant by our chi-square calculation (P = .099).
Do statins prevent a first symptomatic stroke in people with or without preexisting cardiovascular disease?
Preventing strokes that cause functional decline is an important outcome for the frail elderly. Stroke outcomes from PROSPER,13 JUPITER,29 and the Afilalo et al meta-analysis31 are summarized in Table 3.
For primary prevention:
In PROSPER (primary prevention),13 there was no statistically significant benefit in the combined outcome of fatal and nonfatal stroke or the single outcome of transient ischemic attack after 3.2 years.
JUPITER,29 in contrast, found that rosuvastatin 20 mg reduced strokes in primary prevention, but the absolute benefit was small. In 2 years, 0.8% of the treatment group had strokes, compared with 1.4% with placebo, an absolute risk reduction of 0.6% (P = .023, number needed to treat 161, 95% CI 86–1,192).
Neither PROSPER nor JUPITER differentiated between disabling and nondisabling strokes.
For secondary prevention:
In PROSPER (secondary prevention),13 there was no statistically significant benefit in the combined outcome of fatal and nonfatal stroke or the single outcome of transient ischemic attack after 3.2 years.
The Afilalo et al secondary prevention meta-analysis demonstrated a 25% relative reduction in stroke (relative risk 0.75, 95% CI 0.56–0.94, number needed to treat 58, 95% CI 27–177).31
Notably, the stroke outcome in Afilalo included both disabling and nondisabling strokes. For example, in the Heart Protection Study,20 the largest study in the Afilalo et al meta-analysis, approximately 50% of nonfatal, classifiable strokes in the overall study population (ie, both younger and older patients) were not disabling. Including disabling and nondisabling strokes in a composite outcome confounds the clinical meaningfulness of these findings in frailty, as the number needed to treat to prevent one disabling stroke cannot be calculated from the data provided.
Do statins prevent a second (symptomatic) stroke in people with a previous stroke?
SPARCL27 (Table 3) examined the question of whether statins decrease the risk of recurrent ischemic stroke for patients with a prior history of stroke or transient ischemic attack. There was a statistically significant reduction in the primary composite outcome of fatal and nonfatal stroke, with 11.2% of the treatment group and 13.1% of the placebo group experiencing this outcome, an absolute risk reduction of 1.9% at 5 years (P = .03; number needed to treat 52, 95% CI 26–1,303). However, the difference in nonfatal stroke, which is the outcome of interest for frailty (since mortality has uncertain relevance), was not statistically significant (10.4% with treatment vs 11.8% with placebo, P =.11).
An exploratory subgroup analysis of SPARCL patients based on age30 showed a smaller, nonsignificant reduction in the primary end point of fatal and nonfatal stroke in the group over age 65 (relative risk 0.90, 95% confidence interval 0.73–1.11, P = .33) compared with the younger group (age < 65) (relative risk 0.74, 95% CI 0.57–0.96, P = .02).
The applicability of these results to the frail elderly is uncertain, since the subgroup analysis was not powered to determine outcomes based on age stratification and there were differences between groups in characteristics such as blood pressure and smoking status. In addition, the outcome of interest, nonfatal stroke, is not provided for the elderly subgroup.
In conclusion, in both primary and secondary prevention populations, the evidence that statins reduce nonfatal, symptomatic stroke rates for older adults is uncertain.
Do statins decrease all-cause mortality for primary or secondary prevention?
Due to competing risks for death, the outcome of mortality may not be relevant to those who are frail; however, studies showed the following:
For primary prevention, there was no decrease in mortality in PROSPER13 or in the elderly subgroup of JUPITER.29
For secondary prevention, an analysis of PROSPER trial data by Afilalo et al31 showed a significant 18% decrease in all-cause mortality (relative risk 0.82, 95% CI 0.69–0.98) using pravastatin 40 mg.
A decrease in all-cause mortality with statins was also reported in the pooled result of the Afilalo et al meta-analysis.31
What are the reported composite outcomes for primary and secondary prevention?
While we were most interested in the symptomatic outcomes described above, we recognize that the small numbers of events make it difficult to draw firm conclusions. Therefore, we also considered composite primary outcomes, even though most included multiple measures that have varying associations with disability and relevancy to frail older adults.
For primary prevention, in the PROSPER preplanned subgroup analysis,13 there was no statistical benefit for any outcome, including the primary composite measure. In contrast, the elderly subpopulation in the JUPITER trial28 showed a treatment benefit with rosuvastatin 20 mg compared with placebo for the primary composite outcome of MI, stroke, cardiovascular death, hospitalization for unstable angina, or revascularization. The number needed to treat for 2 years was 62 (95% CI 39–148).
In the CTT meta-analysis,32 patients at all levels of baseline risk showed benefit up to age 70. However, there was no statistically significant benefit in the composite primary outcome of coronary deaths, nonfatal myocardial infarction, ischemic stroke, or revascularization in the population most representative of elderly primary prevention—those who were more than 70 years old with a 5-year baseline risk of less than 20%.
For secondary prevention, in PROSPER,13 the subpopulation of patients treated for secondary prevention experienced benefit in the primary composite outcome of coronary heart disease death, nonfatal MI, or fatal or nonfatal stroke, achieving a 4% absolute risk reduction with a number needed to treat of 23 (95% CI 14–81) over 3 years.
Do statins decrease disability?
PROSPER was the only study that reported on disability. Compared with placebo, pravastatin did not decrease disability in the total population as measured by basic and instrumental activities of daily living scales.
Do statins help patients with heart failure?
Neither GISSI-HF25 nor CORONA26 found significant benefit from rosuvastatin 10 mg, despite LDL-C lowering of 27% in GISSI-HF and 45% in CORONA.
Do ezetimibe or other nonstatin lipid-lowering agents improve outcomes?
There is no definitive evidence that ezetimibe provides clinically meaningful benefit as a single agent.
For combination therapy, the IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial)53 showed that adding ezetimibe 10 mg to simvastatin 40 mg after an acute coronary syndrome reduced the risk of nonfatal myocardial infarction compared with simvastatin monotherapy (event rate 12.8% vs 14.4%; hazard ratio 0.87, 95% CI 0.80–0.95; P = .002) for a population with a mean age of 64. The risk of any stroke was also reduced; strokes occurred in 4.2% of those receiving combination therapy vs 4.8% with monotherapy (hazard ratio 0.86, 95% CI 0.73–1.00, P = .05). After a median of 6 years, 42% of patients in each group had discontinued treatment. Given the very specific clinical scenario of acute coronary syndrome and the young age of the patients in this trial, we do not think that this study justifies the use of ezetimibe for severely frail older adults.
There is no evidence that other combinations (ie, a statin plus another lipid-lowering drug) improve clinical outcomes for either primary or secondary prevention in any population.54
WILL FRAIL PATIENTS LIVE LONG ENOUGH TO BENEFIT?
It is often difficult to determine the number of years that are needed to achieve benefit, as most trials do not provide a statistical analysis of varying time frames.
The PROSPER trial13 lasted 3.2 years. From the Kaplan-Meier curves in PROSPER, we estimate that it took about 1.5 years to achieve a 1% absolute risk reduction and 2.5 years for a 2% absolute risk reduction in coronary heart disease death and nonfatal MI in the combined primary and secondary groups.
JUPITER28 was stopped early at 1.9 years. The Afilalo et al meta-analysis31 was based on follow-up over 4.9 years.
IMPROVE-IT53 reported event rates at 7 years. The authors note that benefit in the primary composite outcome appeared to emerge at 1 year, although no statistical support is given for this statement and divergence in the Kaplan-Meier curves is not visually apparent.
The duration of other studies ranged between 2.7 and 4.9 years (Table 1).26–28
It has been suggested that statins should be considered for elderly patients who have a life expectancy of at least 5 years.3 However, many older adults have already been taking statins for many years, which makes it difficult to interpret the available timeframe evidence.
In a multicenter, unblinded, randomized trial,55 statins were either stopped or continued in older adults who had a short life expectancy and a median survival of approximately 7 months. Causes of death were evenly divided between cancer and noncancer diagnoses, and 22% of the patients were cognitively impaired. Discontinuing statin therapy did not increase mortality or cardiovascular events within 60 days. Nevertheless, stopping statin therapy did not achieve noninferiority for the primary end point, the proportion of participants who died within 60 days. Statin discontinuation was associated with improved quality of life, although the study was not blinded, which could have influenced results.
HAVE THE HARMS BEEN SUFFICIENTLY CONSIDERED?
Frail older adults commonly take multiple medications and are more vulnerable to adverse events.56
Many statins require dose reduction with severe renal impairment (creatinine clearance < 30 mL/min/1.73 m2), which would be a common consideration in severely frail older adults.
Myopathy
Myopathy, which includes myalgias and muscle weakness, is a statin-related adverse event that can impair quality of life. Myopathy typically develops within the first 6 months but can occur at any time during statin treatment.57 When muscle-related adverse effects occur, they may affect the elderly more significantly, particularly their ability to perform activities of daily living, rise from a chair, or mobilize independently. Another concern is that older adults with dementia may not be able to accurately report muscle-related symptoms.
It is difficult to ascertain the true prevalence of myopathy, especially in advanced age and frailty. Randomized controlled trials report incidence rates of 1.5% to 5%, which is comparable to placebo.57,58 However, inconsistent definitions of myopathy and exclusion of subjects with previous statin intolerance or adverse effects during run-in periods limit interpretability.57 Clinical experience suggests that muscle complaints may be relatively common.59–61
Advanced age, female sex, low body mass index, and multisystem disease are all associated with frailty and have also been described as risk factors for statin-associated muscle syndromes.61 Physiologic changes associated with frailty, such as reduced muscle strength, decreased lean body mass, impaired functional mobility, decreased reserve capacity, and altered drug metabolism may increase the risk and severity of myopathy.62
Adverse cognitive events
Meta-analyses of randomized clinical trials and narrative reviews find no definitive relationship between statin therapy and adverse cognitive events.63–67 Nevertheless, there have been case reports of memory loss associated with the use of statins, and the US Food and Drug Administration has issued a warning that statins have been associated with memory loss and confusion.68
It may be difficult to determine whether a statin is causing or aggravating cognitive symptoms among individuals with dementia without a trial withdrawal of the drug.
OUR RECOMMENDATIONS
The recommendations below are intended for adults with severe or very severe frailty (ie, a score of 7 or 8 on the Clinical Frailty Scale11 or FACT5 and therefore apply to most older adults living in long-term care facilities.
Primary prevention
There is no reason to prescribe or continue statins for primary prevention, as it is unlikely that they would provide benefit for outcomes that are relevant in this population.
Secondary prevention
Statin treatment is probably not necessary for secondary prevention in those with severe frailty, although there may be extenuating circumstances that justify statin use.
Heart failure
There is no reason to start or continue statins for heart failure, as there is insufficient evidence that they are effective for this indication in any population.
Ezetimibe
There is no evidence that ezetimibe reduces cardiovascular events in any population when used as monotherapy. For a select population with acute coronary syndromes, ezetimibe has a modest effect. Given the very specific clinical scenario of acute coronary syndrome, we do not think that the available evidence justifies the use of ezetimibe for severely frail older adults.
Agents other than ezetimibe combined with statins
There is no reason to start or continue other lipid-lowering drugs in conjunction with statins.
Statin dosing
As statin adverse effects have the potential to increase with advancing age and frailty, lower doses may be appropriate.68
Adverse events
Consider stopping statins on a trial basis if there is concern regarding myopathy, drug interactions, or other adverse effects.
BOTTOM LINE: DO STATINS IMPROVE QUALITY OF LIFE OR FUNCTION?
In primary prevention for older adults, there is doubt that statins prevent cardiovascular disease and stroke-related events because the main study involving the elderly did not show a benefit in the primary prevention subgroup.13 Additionally, there is no conclusive evidence that statin treatment decreases mortality in primary prevention.13,29
There is insufficient information to determine whether the frail elderly should receive statins for secondary prevention. Although there is evidence that treatment decreases measures of coronary heart disease and stroke, it is unclear whether it improves quality of life or function for those who are frail. To answer this question, we need more information about whether reported outcomes (such as stroke and MI) are associated with disability, which is not provided in many of the studies we reviewed. When disability was specifically considered in the PROSPER trial for the combined population of primary and secondary prevention, treatment with statins had no impact on basic and instrumental activities of daily living.
Some experts may not agree with our interpretation of the complex evidence presented in this article. Others may ask, “What is the harm in using statins, even if there is no definitive benefit?” However, the harms associated with statin therapy for the frail are poorly defined. In the face of these uncertainties and in the absence of definitive improvement in quality of life, we believe that “less is more” in the context of severe frailty.69
The cost of medications should also be considered, especially in long-term care facilities, where there is an added expense of drug administration that diverts human resources away from interactions that are more congruent with respecting the lifestage of frailty.
Careful review of evidence before applying clinical practice guidelines to those who are frail should become the norm. When considering treatment of frail patients, the five questions described in this review shed light on the applicability of clinical trial evidence. Therapies that are highly effective in healthier populations may be less effective when individuals are severely frail. Accordingly, we propose that medications should only be used if they improve quality of life or function.
Frail elderly patients are at high risk of adverse clinical outcomes, including those due to polypharmacy. Several groups tackle “deprescribing” by developing lists of medications that are potentially inappropriate for the elderly, such as the Beers or STOPP/START criteria.1–4
See related editorialIn contrast, our group (the Palliative and Therapeutic Harmonization [PATH] program and the Dalhousie Academic Detailing Service) has developed evidence-based, frailty-specific guidelines for treating hypertension5 and diabetes,6 in which we advocate less-stringent treatment targets and tapering or discontinuing medications, as needed.
The PATH program7 is a clinical approach that prioritizes the consideration of frailty when making treatment decisions. The Dalhousie Academic Detailing Service collaborates with the Nova Scotia Health Authority to research and develop evidence-informed educational messages about the treatment of common medical conditions.
Here, we address lipid-lowering therapy in this population.
CONSIDERING FRAILTY
Frailty is defined in several ways. The Fried model8,9 identifies frailty when 3 of the following characteristics are present: unintentional weight loss, exhaustion, muscle weakness, slow walking speed, or low levels of activity. The Clinical Frailty Scale10,11 and the Frailty Assessment for Care-planning Tool (FACT)5 use deficits in cognition, function, and mobility to define frailty. According to these scales, people are considered severely frail when they require assistance with basic activities of daily living (such as bathing or dressing), owing to cognitive or physical deficits from any cause.
In reviewing the evidence, we consider five questions:
- What is the quality of the evidence? (Up to 48% of clinical practice guideline recommendations may be based on low-level evidence or expert opinion.12)
- How did the study population compare with the frail?
- Are study outcomes and potential benefits clinically relevant to those who are frail?
- How long did it take for the clinical benefit of a treatment to become apparent, and are the frail elderly likely to live that long?
- Have the harms of treatment been sufficiently considered?
WHAT IS THE QUALITY OF THE EVIDENCE?
We found no studies that specifically evaluated the benefit of lipid-lowering for severely frail older adults. Therefore, we examined randomized controlled trials that enrolled non-frail older adults,13–28 subgroup analyses of randomized controlled trials,29,30 meta-analyses that analyzed subgroups of elderly populations,31,32 and publications describing the study designs of randomized controlled trials.33–37
Most of the evidence comes from post hoc subgroup analyses of elderly populations. Although meta-analysis is commonly used to compare subgroups, the Cochrane handbook and others consider subgroup comparisons observational by nature.38,39 (See Table 1 for lipid-lowering studies discussed in this article.)
Studies of statins for primary prevention of cardiovascular disease
For evidence of benefit from lipid-lowering for primary prevention (ie, to reduce the risk of cardiovascular events in patients with no known cardiovascular disease at baseline but at increased risk), we reviewed the meta-analysis conducted by the Cholesterol Treatment Trialists’ (CTT) Collaborators.32 Since this meta-analysis included the major trials that enrolled elderly patients, individual publications of post hoc, elderly subgroups were, for the most part, not examined individually. The exception to this approach was a decision to report on the PROSPER13 and JUPITER28 trials separately, because PROSPER is the most representative of the elderly population and JUPITER reached the lowest LDL-C of primary prevention trials published to date and included a large elderly subgroup (n = 5,695).
Savarese et al40 evaluated the benefits of statins for older adults who did not have established cardiovascular disease. We did not report on this meta-analysis, as not all of the subjects that populated the meta-analysis were representative of a typical prevention population. For instance, in the Anglo-Scandinavian Cardiac Outcomes Trial lipid-lowering arm,41 14% of the subjects had had a previous stroke or transient ischemic attack. In the Antihypertensive and Lipid-Lowering Treatment Trial,42 16% of the population had a family history of premature coronary heart disease.
In addition, all the trials in the Savarese meta-analysis were also included in the CTT meta-analysis.32 The CTT reports on baseline risk using patient-level data stratified by age and risk, which may be more relevant to the question of primary prevention for older adults, as highlighted in our review.
PROSPER (Prospective Study of Pravastatin in the Elderly at Risk),13 a well-conducted, double-blind, randomized controlled trial with low probability of bias, compared pravastatin 40 mg and placebo. It was the only study that specifically enrolled older adults, with prespecified analysis of primary and secondary prevention subgroups. The primary prevention subgroup accounted for 56% of the 5,084 participants.
JUPITER (Justification for the Use of Statins in Prevention)28 compared rosuvastatin 20 mg and placebo in 17,802 participants. All had low-density lipoprotein cholesterol (LDL-C) levels below 3.4 mmol/L (130 mg/dL) and elevated levels of the inflammatory biomarker high-sensitivity C-reactive protein (hsCRP), ie, 2 mg/L or higher. Subsequently, Glynn et al performed a post hoc, exploratory subgroup analysis of elderly participants (N = 5,695).29
The JUPITER trial had several limitations.43,44 The planned follow-up period was 5 years, but the trial was stopped early at 1.9 years, after a statistically significant difference was detected in the primary composite outcome of reduction in all vascular events. Studies that are stopped early may exaggerate positive findings.45
Further, JUPITER’s patients were a select group, with normal LDL-C levels, elevated hsCRP values, and without diabetes. Of 90,000 patients screened, 72,000 (80%) did not meet the inclusion criteria and were not enrolled. This high rate of exclusion limits the generalizability of study findings beyond the shortcomings of post hoc subgroup analysis.
The meta-analysis performed by the CTT Collaborators32 used individual participant data from large-scale randomized trials of lipid-modifying treatment. This analysis was specific to people at low risk of vascular disease. In a supplementary appendix, the authors described the reduction in major vascular events for each 1.0 mmol/L decrease in LDL-C in three age categories: under age 60, ages 61 to 70, and over age 70.
The authors also stratified the results by risk category and provided information about those with a risk of major vascular events of less than 20%, which would be more representative of a purer primary prevention population.
For the elderly subgroup at low risk, the CTT Collaborators32 only reported a composite of major vascular events (coronary death, nonfatal myocardial infarction [MI], ischemic stroke, or revascularization) and did not describe individual outcomes, such as prevention of coronary heart disease.
Study results are based on postrandomization findings and therefore may be observational, not experimental.46
Studies of statins for secondary prevention of cardiovascular disease
The aim of secondary prevention is to reduce the risk of recurrent cardiovascular events in patients who already have cardiovascular disease.
To address the question of whether statins reduce cardiovascular risk, we reviewed:
PROSPER,13 which included a preplanned analysis of the secondary prevention population.
Afilalo et al,31,47 who performed a meta-analysis of the elderly subgroups of nine major secondary prevention studies (19,569 patients) using published and unpublished data.
To address the question of whether statins benefit individuals with heart failure, we found two relevant studies:
GISSI-HF (Gruppo Italiano per lo Studio della Sopravvivenza nell’Insufficienza Cardiaca Heart Failure)25 and CORONA (Controlled Rosuvastatin Multinational Trial in Heart Failure),26 which were large, international, well-conducted randomized controlled trials that examined statin use in heart failure.
To answer the question of whether statins benefit individuals after a stroke or transient ischemic attack, we found one relevant study:
SPARCL (Stroke Prevention by Aggressive Reduction in Cholesterol Levels),27 which evaluated the benefit of statins in older adults with a history of stroke or transient ischemic attack. It was a prospective, double-blind, placebo-controlled, international trial conducted at 205 centers. One to 6 months after their cerebrovascular event, patients were randomized to receive either atorvastatin 80 mg or placebo. Given the young age of patients in this trial (mean age 63), we also reviewed a post hoc subgroup analysis of the elderly patients in SPARCL (age > 65).30
HOW DID THE STUDY POPULATION COMPARE WITH THOSE WHO ARE FRAIL?
Frail older adults are almost always excluded from large-scale clinical trials,48 leading to uncertainty about whether the conclusions can be applied to those with advanced frailty.
Although age is an imperfect proxy measure of frailty,49 we consider the age of the study population as well as their comorbidities.
Participants in the studies we reviewed were generally younger and healthier than those who are frail, with mean ages of about 75 or less (Table 1).
PROSPER was the most representative study, as it specifically enrolled older adults, albeit without frailty,13 and excluded people with poor cognitive function as defined by a Mini Mental State Examination score less than 24.
JUPITER enrolled a select population, as described above. The median age in the elderly subgroup was 74 (interquartile range 72–78).29
The Afilalo et al31 meta-analysis primarily included studies of young-elderly patients, with a mean age of less than 70. PROSPER13 was an exception.
The GISSI-HF study,25 which examined the benefit of statins in heart failure, described their study population as frail, although the mean age was only 68. Compared with those in GISSI-HF, the CORONA patients26 with heart failure were older (mean age 73) and had more severe heart failure. Accordingly, it is possible that many of the CORONA participants were frail.
ARE STUDY OUTCOMES CLINICALLY RELEVANT TO THOSE WHO ARE FRAIL?
Because baseline cardiovascular risk increases with age, the elderly should, in theory, experience greater absolute benefit from lipid-lowering. However, there is uncertainty about whether this is true in practice.
Some, but not all, epidemiologic studies show a weaker relationship between cholesterol levels and cardiovascular morbidity and mortality rates in older compared to younger adults.50,51 This may be because those with high cholesterol levels die before they get old (time-related bias), or because those with life-threatening illness may have lower cholesterol levels.50 In addition, classic risk factors such as age, sex, systolic blood pressure, cholesterol values, diabetes, smoking, and left ventricular hypertrophy on electrocardiography may have less power to predict cardiovascular risk among older patients.52
The goal of treatment in frailty is to prevent further disability or improve quality of life. Therefore, meaningful outcomes for lipid-lowering therapy should include symptomatic nonfatal MI and its associated morbidity (eg, heart failure and persistent angina) or symptomatic nonfatal stroke leading to disability. Outcomes without sustained clinical impact, such as transient ischemic attack, nondisabling stroke, or silent MI, while potentially important in other populations, are less relevant in severe frailty. Notably, in many statin studies, outcomes include asymptomatic heart disease (eg, silent MI and “suspected events”) and nondisabling stroke (eg, mild stroke, transient ischemic attack). When symptomatic outcomes are not reported separately, the impact of the reported benefit on quality of life and function is uncertain.
The outcome of all-cause mortality is generally recognized as a gold standard for determining treatment benefit. However, since advanced frailty is characterized by multiple competing causes for mortality, a reduction in all-cause mortality that is achieved by addressing a single issue in nonfrail populations may not extend to the frail.
To more fully understand the impact of lipid-lowering therapy on quality of life and function, we examined the following questions:
Do statins as primary prevention reduce symptomatic heart disease?
Outcomes for coronary heart disease from PROSPER and JUPITER are summarized in Table 2.
PROSPER. In the PROSPER primary prevention group,13 statin therapy did not reduce the combined outcome of coronary heart disease death and nonfatal MI.
The JUPITER trial demonstrated a statistically significant benefit for preventing MI in the elderly subpopulation (ages 70–97),29 but the number needed to treat was high (211 for 2 years), with a wide confidence interval (CI) (95% CI 106–32,924). The trial did not adequately differentiate between symptomatic and asymptomatic events, making it difficult to determine outcome relevance. Also, due to the methodologic limitations of JUPITER as described above, its results should be interpreted with caution.43,44
The CTT Collaborators32 did not report individual outcomes (eg, coronary heart disease) for the elderly low-risk subgroup and, therefore, this meta-analysis does not answer the question of whether statins reduce symptomatic heart disease in primary prevention populations.
Taken together, these findings do not provide convincing evidence that statin therapy as primary prevention reduces the incidence of symptomatic heart disease for severely frail older adults.
Do statins as secondary prevention reduce symptomatic heart disease?
Most studies defined secondary prevention narrowly as treatment for patients with established coronary artery disease. For instance, in the Afilalo et al meta-analysis,31 the small number of studies that included individuals with other forms of vascular disease (such as peripheral vascular disease) enrolled few participants with noncardiac conditions (eg, 29% in PROSPER13 and 13% in the Heart Protection Study20).
Therefore, any evidence of benefit for secondary prevention demonstrated in these studies is most applicable to patients with coronary heart disease, with less certainty for those with other forms of cardiovascular disease.
In PROSPER,13 the secondary prevention group experienced benefit in the combined outcome of coronary heart disease death or nonfatal MI. In the treatment group, 12.7% experienced this outcome compared with 16.8% with placebo, an absolute risk reduction of 4.1% in 3 years (P = .004, number needed to treat 25, 95% CI 15–77). This measure includes coronary heart disease death, an outcome that may not be generalizable to those who are frail. In addition, the outcome of nonfatal MI includes both symptomatic and suspected events. As such, there is uncertainty whether the realized benefit is clinically relevant to frail older adults.
The Afilalo et al meta-analysis31 showed that the number needed to treat to prevent one nonfatal MI was 38 (95% CI 16–118) over 5 years (Table 2). However, this outcome included both symptomatic and asymptomatic (silent) events.
Based on the available data, we conclude that it is not possible to determine whether statins reduce symptomatic heart disease as secondary prevention for older adults who are frail.
Do statins reduce heart disease in combined populations?
In the combined primary and secondary population from PROSPER,13 pravastatin decreased the risk of nonfatal symptomatic MI from 4.3% in the placebo group to 3.4%, a relatively small reduction in absolute risk (0.9%) and not statistically significant by our chi-square calculation (P = .099).
Do statins prevent a first symptomatic stroke in people with or without preexisting cardiovascular disease?
Preventing strokes that cause functional decline is an important outcome for the frail elderly. Stroke outcomes from PROSPER,13 JUPITER,29 and the Afilalo et al meta-analysis31 are summarized in Table 3.
For primary prevention:
In PROSPER (primary prevention),13 there was no statistically significant benefit in the combined outcome of fatal and nonfatal stroke or the single outcome of transient ischemic attack after 3.2 years.
JUPITER,29 in contrast, found that rosuvastatin 20 mg reduced strokes in primary prevention, but the absolute benefit was small. In 2 years, 0.8% of the treatment group had strokes, compared with 1.4% with placebo, an absolute risk reduction of 0.6% (P = .023, number needed to treat 161, 95% CI 86–1,192).
Neither PROSPER nor JUPITER differentiated between disabling and nondisabling strokes.
For secondary prevention:
In PROSPER (secondary prevention),13 there was no statistically significant benefit in the combined outcome of fatal and nonfatal stroke or the single outcome of transient ischemic attack after 3.2 years.
The Afilalo et al secondary prevention meta-analysis demonstrated a 25% relative reduction in stroke (relative risk 0.75, 95% CI 0.56–0.94, number needed to treat 58, 95% CI 27–177).31
Notably, the stroke outcome in Afilalo included both disabling and nondisabling strokes. For example, in the Heart Protection Study,20 the largest study in the Afilalo et al meta-analysis, approximately 50% of nonfatal, classifiable strokes in the overall study population (ie, both younger and older patients) were not disabling. Including disabling and nondisabling strokes in a composite outcome confounds the clinical meaningfulness of these findings in frailty, as the number needed to treat to prevent one disabling stroke cannot be calculated from the data provided.
Do statins prevent a second (symptomatic) stroke in people with a previous stroke?
SPARCL27 (Table 3) examined the question of whether statins decrease the risk of recurrent ischemic stroke for patients with a prior history of stroke or transient ischemic attack. There was a statistically significant reduction in the primary composite outcome of fatal and nonfatal stroke, with 11.2% of the treatment group and 13.1% of the placebo group experiencing this outcome, an absolute risk reduction of 1.9% at 5 years (P = .03; number needed to treat 52, 95% CI 26–1,303). However, the difference in nonfatal stroke, which is the outcome of interest for frailty (since mortality has uncertain relevance), was not statistically significant (10.4% with treatment vs 11.8% with placebo, P =.11).
An exploratory subgroup analysis of SPARCL patients based on age30 showed a smaller, nonsignificant reduction in the primary end point of fatal and nonfatal stroke in the group over age 65 (relative risk 0.90, 95% confidence interval 0.73–1.11, P = .33) compared with the younger group (age < 65) (relative risk 0.74, 95% CI 0.57–0.96, P = .02).
The applicability of these results to the frail elderly is uncertain, since the subgroup analysis was not powered to determine outcomes based on age stratification and there were differences between groups in characteristics such as blood pressure and smoking status. In addition, the outcome of interest, nonfatal stroke, is not provided for the elderly subgroup.
In conclusion, in both primary and secondary prevention populations, the evidence that statins reduce nonfatal, symptomatic stroke rates for older adults is uncertain.
Do statins decrease all-cause mortality for primary or secondary prevention?
Due to competing risks for death, the outcome of mortality may not be relevant to those who are frail; however, studies showed the following:
For primary prevention, there was no decrease in mortality in PROSPER13 or in the elderly subgroup of JUPITER.29
For secondary prevention, an analysis of PROSPER trial data by Afilalo et al31 showed a significant 18% decrease in all-cause mortality (relative risk 0.82, 95% CI 0.69–0.98) using pravastatin 40 mg.
A decrease in all-cause mortality with statins was also reported in the pooled result of the Afilalo et al meta-analysis.31
What are the reported composite outcomes for primary and secondary prevention?
While we were most interested in the symptomatic outcomes described above, we recognize that the small numbers of events make it difficult to draw firm conclusions. Therefore, we also considered composite primary outcomes, even though most included multiple measures that have varying associations with disability and relevancy to frail older adults.
For primary prevention, in the PROSPER preplanned subgroup analysis,13 there was no statistical benefit for any outcome, including the primary composite measure. In contrast, the elderly subpopulation in the JUPITER trial28 showed a treatment benefit with rosuvastatin 20 mg compared with placebo for the primary composite outcome of MI, stroke, cardiovascular death, hospitalization for unstable angina, or revascularization. The number needed to treat for 2 years was 62 (95% CI 39–148).
In the CTT meta-analysis,32 patients at all levels of baseline risk showed benefit up to age 70. However, there was no statistically significant benefit in the composite primary outcome of coronary deaths, nonfatal myocardial infarction, ischemic stroke, or revascularization in the population most representative of elderly primary prevention—those who were more than 70 years old with a 5-year baseline risk of less than 20%.
For secondary prevention, in PROSPER,13 the subpopulation of patients treated for secondary prevention experienced benefit in the primary composite outcome of coronary heart disease death, nonfatal MI, or fatal or nonfatal stroke, achieving a 4% absolute risk reduction with a number needed to treat of 23 (95% CI 14–81) over 3 years.
Do statins decrease disability?
PROSPER was the only study that reported on disability. Compared with placebo, pravastatin did not decrease disability in the total population as measured by basic and instrumental activities of daily living scales.
Do statins help patients with heart failure?
Neither GISSI-HF25 nor CORONA26 found significant benefit from rosuvastatin 10 mg, despite LDL-C lowering of 27% in GISSI-HF and 45% in CORONA.
Do ezetimibe or other nonstatin lipid-lowering agents improve outcomes?
There is no definitive evidence that ezetimibe provides clinically meaningful benefit as a single agent.
For combination therapy, the IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial)53 showed that adding ezetimibe 10 mg to simvastatin 40 mg after an acute coronary syndrome reduced the risk of nonfatal myocardial infarction compared with simvastatin monotherapy (event rate 12.8% vs 14.4%; hazard ratio 0.87, 95% CI 0.80–0.95; P = .002) for a population with a mean age of 64. The risk of any stroke was also reduced; strokes occurred in 4.2% of those receiving combination therapy vs 4.8% with monotherapy (hazard ratio 0.86, 95% CI 0.73–1.00, P = .05). After a median of 6 years, 42% of patients in each group had discontinued treatment. Given the very specific clinical scenario of acute coronary syndrome and the young age of the patients in this trial, we do not think that this study justifies the use of ezetimibe for severely frail older adults.
There is no evidence that other combinations (ie, a statin plus another lipid-lowering drug) improve clinical outcomes for either primary or secondary prevention in any population.54
WILL FRAIL PATIENTS LIVE LONG ENOUGH TO BENEFIT?
It is often difficult to determine the number of years that are needed to achieve benefit, as most trials do not provide a statistical analysis of varying time frames.
The PROSPER trial13 lasted 3.2 years. From the Kaplan-Meier curves in PROSPER, we estimate that it took about 1.5 years to achieve a 1% absolute risk reduction and 2.5 years for a 2% absolute risk reduction in coronary heart disease death and nonfatal MI in the combined primary and secondary groups.
JUPITER28 was stopped early at 1.9 years. The Afilalo et al meta-analysis31 was based on follow-up over 4.9 years.
IMPROVE-IT53 reported event rates at 7 years. The authors note that benefit in the primary composite outcome appeared to emerge at 1 year, although no statistical support is given for this statement and divergence in the Kaplan-Meier curves is not visually apparent.
The duration of other studies ranged between 2.7 and 4.9 years (Table 1).26–28
It has been suggested that statins should be considered for elderly patients who have a life expectancy of at least 5 years.3 However, many older adults have already been taking statins for many years, which makes it difficult to interpret the available timeframe evidence.
In a multicenter, unblinded, randomized trial,55 statins were either stopped or continued in older adults who had a short life expectancy and a median survival of approximately 7 months. Causes of death were evenly divided between cancer and noncancer diagnoses, and 22% of the patients were cognitively impaired. Discontinuing statin therapy did not increase mortality or cardiovascular events within 60 days. Nevertheless, stopping statin therapy did not achieve noninferiority for the primary end point, the proportion of participants who died within 60 days. Statin discontinuation was associated with improved quality of life, although the study was not blinded, which could have influenced results.
HAVE THE HARMS BEEN SUFFICIENTLY CONSIDERED?
Frail older adults commonly take multiple medications and are more vulnerable to adverse events.56
Many statins require dose reduction with severe renal impairment (creatinine clearance < 30 mL/min/1.73 m2), which would be a common consideration in severely frail older adults.
Myopathy
Myopathy, which includes myalgias and muscle weakness, is a statin-related adverse event that can impair quality of life. Myopathy typically develops within the first 6 months but can occur at any time during statin treatment.57 When muscle-related adverse effects occur, they may affect the elderly more significantly, particularly their ability to perform activities of daily living, rise from a chair, or mobilize independently. Another concern is that older adults with dementia may not be able to accurately report muscle-related symptoms.
It is difficult to ascertain the true prevalence of myopathy, especially in advanced age and frailty. Randomized controlled trials report incidence rates of 1.5% to 5%, which is comparable to placebo.57,58 However, inconsistent definitions of myopathy and exclusion of subjects with previous statin intolerance or adverse effects during run-in periods limit interpretability.57 Clinical experience suggests that muscle complaints may be relatively common.59–61
Advanced age, female sex, low body mass index, and multisystem disease are all associated with frailty and have also been described as risk factors for statin-associated muscle syndromes.61 Physiologic changes associated with frailty, such as reduced muscle strength, decreased lean body mass, impaired functional mobility, decreased reserve capacity, and altered drug metabolism may increase the risk and severity of myopathy.62
Adverse cognitive events
Meta-analyses of randomized clinical trials and narrative reviews find no definitive relationship between statin therapy and adverse cognitive events.63–67 Nevertheless, there have been case reports of memory loss associated with the use of statins, and the US Food and Drug Administration has issued a warning that statins have been associated with memory loss and confusion.68
It may be difficult to determine whether a statin is causing or aggravating cognitive symptoms among individuals with dementia without a trial withdrawal of the drug.
OUR RECOMMENDATIONS
The recommendations below are intended for adults with severe or very severe frailty (ie, a score of 7 or 8 on the Clinical Frailty Scale11 or FACT5 and therefore apply to most older adults living in long-term care facilities.
Primary prevention
There is no reason to prescribe or continue statins for primary prevention, as it is unlikely that they would provide benefit for outcomes that are relevant in this population.
Secondary prevention
Statin treatment is probably not necessary for secondary prevention in those with severe frailty, although there may be extenuating circumstances that justify statin use.
Heart failure
There is no reason to start or continue statins for heart failure, as there is insufficient evidence that they are effective for this indication in any population.
Ezetimibe
There is no evidence that ezetimibe reduces cardiovascular events in any population when used as monotherapy. For a select population with acute coronary syndromes, ezetimibe has a modest effect. Given the very specific clinical scenario of acute coronary syndrome, we do not think that the available evidence justifies the use of ezetimibe for severely frail older adults.
Agents other than ezetimibe combined with statins
There is no reason to start or continue other lipid-lowering drugs in conjunction with statins.
Statin dosing
As statin adverse effects have the potential to increase with advancing age and frailty, lower doses may be appropriate.68
Adverse events
Consider stopping statins on a trial basis if there is concern regarding myopathy, drug interactions, or other adverse effects.
BOTTOM LINE: DO STATINS IMPROVE QUALITY OF LIFE OR FUNCTION?
In primary prevention for older adults, there is doubt that statins prevent cardiovascular disease and stroke-related events because the main study involving the elderly did not show a benefit in the primary prevention subgroup.13 Additionally, there is no conclusive evidence that statin treatment decreases mortality in primary prevention.13,29
There is insufficient information to determine whether the frail elderly should receive statins for secondary prevention. Although there is evidence that treatment decreases measures of coronary heart disease and stroke, it is unclear whether it improves quality of life or function for those who are frail. To answer this question, we need more information about whether reported outcomes (such as stroke and MI) are associated with disability, which is not provided in many of the studies we reviewed. When disability was specifically considered in the PROSPER trial for the combined population of primary and secondary prevention, treatment with statins had no impact on basic and instrumental activities of daily living.
Some experts may not agree with our interpretation of the complex evidence presented in this article. Others may ask, “What is the harm in using statins, even if there is no definitive benefit?” However, the harms associated with statin therapy for the frail are poorly defined. In the face of these uncertainties and in the absence of definitive improvement in quality of life, we believe that “less is more” in the context of severe frailty.69
The cost of medications should also be considered, especially in long-term care facilities, where there is an added expense of drug administration that diverts human resources away from interactions that are more congruent with respecting the lifestage of frailty.
Careful review of evidence before applying clinical practice guidelines to those who are frail should become the norm. When considering treatment of frail patients, the five questions described in this review shed light on the applicability of clinical trial evidence. Therapies that are highly effective in healthier populations may be less effective when individuals are severely frail. Accordingly, we propose that medications should only be used if they improve quality of life or function.
- Ontario Pharmacy Research Collaboration. Deprescribing guidelines for the elderly. www.open-pharmacy-research.ca/research-projects/emerging-services/deprescribing-guidelines. Accessed December 28, 2016.
- Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med 2015; 175:827–834.
- O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 2015; 44:213–218.
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2012; 60:616–631.
- Mallery LH, Allen M, Fleming I, et al. Promoting higher blood pressure targets for frail older adults: a consensus guideline from Canada. Cleve Clin J Med 2014; 81:427–437.
- Mallery LH, Ransom T, Steeves B, Cook B, Dunbar P, Moorhouse P. Evidence-informed guidelines for treating frail older adults with type 2 diabetes: from the Diabetes Care Program of Nova Scotia (DCPNS) and the Palliative and Therapeutic Harmonization (PATH) program. J Am Med Dir Assoc 2013; 14:801–808.
- Moorhouse P, Mallery L. Palliative and therapeutic harmonization: a model for appropriate decision-making in frail older adults. J Am Geriatr Soc 2012; 60:2326–2332.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging 2012; 16:601–608.
- Rockwood K, Song Z, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173:489–495.
- Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013; 14:392–397.
- Tricoci P, Allen JM, Kramer JM, Califf RM, Smith SC Jr. Scientific evidence underlying the ACC/AHA clinical practice guidelines. JAMA 2009; 301:831–841.
- Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002; 360:1623–1630.
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- Miettien TA, Pyorala K, Olsson AG, et al. Cholesterol-lowering therapy in women and elderly patients with myocardial infarction or angina pectoris: findings from the Scandinavian Simvastatin Study Group (4S). Circulation 1997; 96:4211–4218.
- Lewis SJ, Moye LA, Sacks FM, et al. Effect of pravastatin on cardiovascular events in older patients with myocardial infarction and cholesterol levels in the average range. Results of the Cholesterol and Recurrent Events (CARE) trial. Ann Intern Med 1998; 129:681–689.
- Hunt D, Young P, Simes J, et al. Benefits of pravastatin on cardiovascular events and mortality in older patients with coronary heart disease are equal to or exceed those seen in younger patients: results from the LIPID trial. Ann Intern Med 2001; 134:931–940.
- Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med 1998; 339:1349–1357.
- Heart Protection Study Collaborative Group. The effects of cholesterol lowering with simvastatin on cause-specific mortality and on cancer incidence in 20,536 high-risk people: a randomized placebo-controlled trial. BMC Med 2005; 3:6.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomized placebo-controlled trial. Lancet 2002; 360:7–22.
- Pitt B, Mancini GB, Ellis SG, Rosman HS, Park JS, McGovern ME. Pravastatin limitation of atherosclerosis in the coronary arteries (PLAC 1): reduction in atherosclerosis progression and clinical events. PLAC 1 investigation. J Am Coll Cardiol 1995; 26:1133–1139.
- Jukema JW, Bruschke AV, van Boven AJ, et al. Effects of lipid lowering by pravastatin on progression and regression of coronary artery disease in symptomatic men with normal to moderately elevated serum cholesterol levels. The Regression Growth Evaluation Statin Study (REGRESS). Circulation 1995; 91:2528–2540.
- Serruys PW, Foley DP, Jackson G, et al. A randomized placebo-controlled trial of fluvastatin for prevention of restenosis after successful coronary balloon angioplasty; final results of the fluvastatin angiographic restenosis (FLARE) trial. Eur Heart J 1999; 20:58–69.
- Serruys PW, de Feyter P, Macaya C, et al; Lescol Intervention Prevention Study (LIPS) Investigators. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 287:3215–3222.
- Tavazzi L, Maggioni AP, Marchioli R, et al; Gissi-HF Investigators. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomized, double-blind, placebo-controlled trial. Lancet 2008; 372:1231–1239.
- Kjekshus J, Apatrei E, Barrios V, et al; CORONA Group. Rosuvastatin in older patients with systolic heart failure. N Engl J Med 2007; 357:2248–2261.
- Amarenco P, Bogousslavsky J, Callahan A, et al; Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006; 355:549–559.
- Ridker PM, Danielson E, Fonseca FA, et al; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Glynn RJ, Koenig W, Nordestgaard BG, Shepherd J, Ridker PM. Rosuvastatin for primary prevention in older persons with elevated C-reactive protein and low to average low-density lipoprotein cholesterol levels: exploratory analysis of a randomized trial. Ann Intern Med 2010; 152:488–496, W174.
- Chaturvedi S, Zivin J, Breazna A, et al; SPARCL Investigators. Effect of atorvastatin in elderly patients with a recent stroke or transient ischemic attack. Neurology 2009; 72:688–694.
- Afilalo J, Duque G, Steele R, Jukema JW, de Craen AJ, Eisenberg MJ. Statins for secondary prevention in elderly patients: a hierarchical bayesian meta-analysis. J Am Coll Cardiol 2008; 51:37–45.
- Cholesterol Treatment Trialists’ (CTT) Collaborators; Mihaylova B, Emberson J, Blackwell L, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 2012; 380:581– 590.
- Sacks FM, Pfeffer MA, Moye L, et al. Rationale and design of a secondary prevention trial of lowering normal plasma cholesterol levels after acute myocardial infarction: the Cholesterol and Recurrent Events (CARE). Am J Cardiol 1991; 68:1436–1446.
- Armitage J, Collins R. Need for large scale randomised evidence about lowering LDL cholesterol in people with diabetes mellitus: MRC/BHF Heart Protection Study and other major trials. Heart 2000; 84:357–360.
- Design features and baseline characteristics of the LIPID (Long-Term Intervention with Pravastatin in Ischemic Disease) study: a randomized trial in patients with previous acute myocardial infarction and/or unstable angina pectoris. Am J Cardiol 1995; 76:474–479.
- Shepherd J, Blauw GJ, Murphy MB, et al. The design of a prospective study of Pravastatin in the Elderly at Risk (PROSPER). Am J Cardiol 1999; 84:1192–1197.
- Amarenco P, Bogousslavsky J, Callahan AS, et al; SPARCL Investigators. Design and baseline characteristics of the stroke prevention by aggressive reduction in cholesterol levels (SPARCL) study. Cerebrovasc Dis 2003; 16:389–395.
- Interpretation of subgroup analyses and meta-regressions. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2011. http://handbook.cochrane.org/chapter_9/9_6_6_interpretation_of_subgroup_analyses_and_meta_regressions.htm. Accessed December 5, 2016.
- Borenstein M, Higgins JP. Meta-analysis and subgroups. Prev Sci 2013; 14:134–143.
- Savarese G, Gotto AM Jr, Paolillo S, et al. Benefits of statins in elderly subjects without established cardiovascular disease: a meta-analysis. J Am Coll Cardiol 2013; 62:2090–2099.
- Sever PS, Dahlof B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003; 361:1149–1158.
- The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). JAMA 2002; 288:2998–3007.
- de Longeril M, Salen P, Abramson J, et al. Cholesterol lowering, cardiovascular diseases, and the rosuvastatin-JUPITER controversy: a critical reappraisal. Arch Intern Med 2010; 170:1032–1036.
- Yusuf S, Lonn E, Bosch J. Lipid lowering for primary prevention. Lancet 2009: 373:1152–1155.
- Briel M, Bassler D, Wang AT, Guyatt GH, Montori VM. The dangers of stopping a trial too early. J Bone Joint Surg Am 2012; 94(suppl 1):56–60.
- Hayward RA, Krumholz HM. Three reasons to abandon low-density lipoprotein targets: an open letter to the Adult Treatment Panel IV of the National Institutes of Health. Circ Cardiovasc Qual Outcomes 2012; 5:2–5.
- Afilalo J, Duque G, Steele R, Jukema JW, de Craen AJ, Eisenberg MJ. Statins for secondary prevention in elderly patients: a hierarchical Bayesian meta-analysis. www.ncbi.nlm.nih.gov/pubmedhealth/PMH0026417. Accessed December 5, 2016.
- Holmes HM, Hayley DC, Alexander GC, Sachs GA. Reconsidering medication appropriateness for patients late in life. Arch Intern Med 2006; 166:605–609.
- Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med 2011; 27:17–26.
- Petersen LK, Christensen K, Kragstrup J. Lipid-lowering treatment to the end? A review of observational studies and RCTs on cholesterol and mortality in 80+-year olds. Age Ageing 2010; 39:674–680.
- Psaty BM, Anderson M, Kronmal RA, et al. The association between lipid levels and the risks of incident myocardial infarction, stroke, and total mortality: the Cardiovascular Health Study. J Am Geriatr Soc 2004; 52:1639–1647.
- de Ruijter W, Westendorp RG, Assendelft WJ, et al. Use of Framingham risk score and new biomarkers to predict cardiovascular mortality in older people: population based observational cohort study. BMJ 2009; 338:a3083.
- Canon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015; 372:2387–2397.
- Anderson TJ, Gregoire J, Hegele RA, et al. 2012 update of the Canadian Cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol 2013; 29:151–167.
- Kutner JS, Blatchford PJ, Taylor DH, et al. Safety and benefit of discontinuing statin therapy in the setting of advanced, life-limiting illness: a randomized clinical trial. JAMA Intern Med 2015; 175:691–700.
- Tinetti ME, Bogardus ST Jr, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med 2004; 351:2870–2874.
- Rosenson RS. Current overview of statin-induced myopathy. Am J Med 2004; 116:408–416.
- Mancini GB, Baker S, Bergeron J, et al. Diagnosis, prevention, and management of statin adverse effects and intolerance: proceedings of a Canadian Working Group Consensus Conference. Can J Cardiol 2011; 27:635–662.
- Cohen JD, Brinton EA, Ito MK, Jacobson TA. Understanding Statin Use in America and Gaps in Patient Education (USAGE): an internet-based survey of 10,138 current and former statin users. J Clin Lipidol 2012; 6:208–215.
- Joy TR, Hegele RA. Narrative review: statin-related myopathy. Ann Intern Med 2009; 150:858–868.
- Talbert RL. Safety issues with statin therapy. J Am Pharm Assoc (2003) 2006; 46:479–490.
- Sewright KA, Clarkson PM, Thompson PD. Statin myopathy: incidence, risk factors, and pathophysiology. Curr Atheroscler Rep 2007; 9:389–396.
- Ott BR, Daiello LA, Dahabreh IJ, et al. Do statins impair cognition? A systematic review and meta-analysis of randomized controlled trials. J Gen Intern Med 2015; 30:348–358.
- Mancini GB, Tashakkor AY, Baker S, et al. Diagnosis, prevention and management of statin adverse effects and intolerance: Canadian Working Group Consensus update. Can J Cardiol 2013: 29:1553–1568.
- Rojas-Fernandez CH, Cameron JC. Is statin-associated cognitive impairment clinically relevant? A narrative review and clinical recommendations. Ann Pharmacother 2012; 46:549–557.
- McGuinness B, O’Hare J, Craig D, Bullock R, Malouf R, Passmore P. Cochrane review on ‘Statins for the treatment of dementia’. Int J Geriatr Psychiatry 2013; 28:119–126.
- Pandey RD, Gupta PP, Jha D, Kumar S. Role of statins in Alzheimer’s disease: a retrospective meta-analysis for commonly investigated clinical parameters in RCTs. Int J Neurosci 2013; 123:521–525.
- Food and Drug Administration (FDA). FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. www.fda.gov/drugs/ drugsafety/ucm293101.htm. Accessed December 5, 2016.
- Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med 2010; 170:1648–1654.
- Ontario Pharmacy Research Collaboration. Deprescribing guidelines for the elderly. www.open-pharmacy-research.ca/research-projects/emerging-services/deprescribing-guidelines. Accessed December 28, 2016.
- Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med 2015; 175:827–834.
- O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 2015; 44:213–218.
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2012; 60:616–631.
- Mallery LH, Allen M, Fleming I, et al. Promoting higher blood pressure targets for frail older adults: a consensus guideline from Canada. Cleve Clin J Med 2014; 81:427–437.
- Mallery LH, Ransom T, Steeves B, Cook B, Dunbar P, Moorhouse P. Evidence-informed guidelines for treating frail older adults with type 2 diabetes: from the Diabetes Care Program of Nova Scotia (DCPNS) and the Palliative and Therapeutic Harmonization (PATH) program. J Am Med Dir Assoc 2013; 14:801–808.
- Moorhouse P, Mallery L. Palliative and therapeutic harmonization: a model for appropriate decision-making in frail older adults. J Am Geriatr Soc 2012; 60:2326–2332.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging 2012; 16:601–608.
- Rockwood K, Song Z, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173:489–495.
- Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013; 14:392–397.
- Tricoci P, Allen JM, Kramer JM, Califf RM, Smith SC Jr. Scientific evidence underlying the ACC/AHA clinical practice guidelines. JAMA 2009; 301:831–841.
- Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002; 360:1623–1630.
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- Miettien TA, Pyorala K, Olsson AG, et al. Cholesterol-lowering therapy in women and elderly patients with myocardial infarction or angina pectoris: findings from the Scandinavian Simvastatin Study Group (4S). Circulation 1997; 96:4211–4218.
- Lewis SJ, Moye LA, Sacks FM, et al. Effect of pravastatin on cardiovascular events in older patients with myocardial infarction and cholesterol levels in the average range. Results of the Cholesterol and Recurrent Events (CARE) trial. Ann Intern Med 1998; 129:681–689.
- Hunt D, Young P, Simes J, et al. Benefits of pravastatin on cardiovascular events and mortality in older patients with coronary heart disease are equal to or exceed those seen in younger patients: results from the LIPID trial. Ann Intern Med 2001; 134:931–940.
- Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med 1998; 339:1349–1357.
- Heart Protection Study Collaborative Group. The effects of cholesterol lowering with simvastatin on cause-specific mortality and on cancer incidence in 20,536 high-risk people: a randomized placebo-controlled trial. BMC Med 2005; 3:6.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomized placebo-controlled trial. Lancet 2002; 360:7–22.
- Pitt B, Mancini GB, Ellis SG, Rosman HS, Park JS, McGovern ME. Pravastatin limitation of atherosclerosis in the coronary arteries (PLAC 1): reduction in atherosclerosis progression and clinical events. PLAC 1 investigation. J Am Coll Cardiol 1995; 26:1133–1139.
- Jukema JW, Bruschke AV, van Boven AJ, et al. Effects of lipid lowering by pravastatin on progression and regression of coronary artery disease in symptomatic men with normal to moderately elevated serum cholesterol levels. The Regression Growth Evaluation Statin Study (REGRESS). Circulation 1995; 91:2528–2540.
- Serruys PW, Foley DP, Jackson G, et al. A randomized placebo-controlled trial of fluvastatin for prevention of restenosis after successful coronary balloon angioplasty; final results of the fluvastatin angiographic restenosis (FLARE) trial. Eur Heart J 1999; 20:58–69.
- Serruys PW, de Feyter P, Macaya C, et al; Lescol Intervention Prevention Study (LIPS) Investigators. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 287:3215–3222.
- Tavazzi L, Maggioni AP, Marchioli R, et al; Gissi-HF Investigators. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomized, double-blind, placebo-controlled trial. Lancet 2008; 372:1231–1239.
- Kjekshus J, Apatrei E, Barrios V, et al; CORONA Group. Rosuvastatin in older patients with systolic heart failure. N Engl J Med 2007; 357:2248–2261.
- Amarenco P, Bogousslavsky J, Callahan A, et al; Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006; 355:549–559.
- Ridker PM, Danielson E, Fonseca FA, et al; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Glynn RJ, Koenig W, Nordestgaard BG, Shepherd J, Ridker PM. Rosuvastatin for primary prevention in older persons with elevated C-reactive protein and low to average low-density lipoprotein cholesterol levels: exploratory analysis of a randomized trial. Ann Intern Med 2010; 152:488–496, W174.
- Chaturvedi S, Zivin J, Breazna A, et al; SPARCL Investigators. Effect of atorvastatin in elderly patients with a recent stroke or transient ischemic attack. Neurology 2009; 72:688–694.
- Afilalo J, Duque G, Steele R, Jukema JW, de Craen AJ, Eisenberg MJ. Statins for secondary prevention in elderly patients: a hierarchical bayesian meta-analysis. J Am Coll Cardiol 2008; 51:37–45.
- Cholesterol Treatment Trialists’ (CTT) Collaborators; Mihaylova B, Emberson J, Blackwell L, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 2012; 380:581– 590.
- Sacks FM, Pfeffer MA, Moye L, et al. Rationale and design of a secondary prevention trial of lowering normal plasma cholesterol levels after acute myocardial infarction: the Cholesterol and Recurrent Events (CARE). Am J Cardiol 1991; 68:1436–1446.
- Armitage J, Collins R. Need for large scale randomised evidence about lowering LDL cholesterol in people with diabetes mellitus: MRC/BHF Heart Protection Study and other major trials. Heart 2000; 84:357–360.
- Design features and baseline characteristics of the LIPID (Long-Term Intervention with Pravastatin in Ischemic Disease) study: a randomized trial in patients with previous acute myocardial infarction and/or unstable angina pectoris. Am J Cardiol 1995; 76:474–479.
- Shepherd J, Blauw GJ, Murphy MB, et al. The design of a prospective study of Pravastatin in the Elderly at Risk (PROSPER). Am J Cardiol 1999; 84:1192–1197.
- Amarenco P, Bogousslavsky J, Callahan AS, et al; SPARCL Investigators. Design and baseline characteristics of the stroke prevention by aggressive reduction in cholesterol levels (SPARCL) study. Cerebrovasc Dis 2003; 16:389–395.
- Interpretation of subgroup analyses and meta-regressions. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2011. http://handbook.cochrane.org/chapter_9/9_6_6_interpretation_of_subgroup_analyses_and_meta_regressions.htm. Accessed December 5, 2016.
- Borenstein M, Higgins JP. Meta-analysis and subgroups. Prev Sci 2013; 14:134–143.
- Savarese G, Gotto AM Jr, Paolillo S, et al. Benefits of statins in elderly subjects without established cardiovascular disease: a meta-analysis. J Am Coll Cardiol 2013; 62:2090–2099.
- Sever PS, Dahlof B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003; 361:1149–1158.
- The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). JAMA 2002; 288:2998–3007.
- de Longeril M, Salen P, Abramson J, et al. Cholesterol lowering, cardiovascular diseases, and the rosuvastatin-JUPITER controversy: a critical reappraisal. Arch Intern Med 2010; 170:1032–1036.
- Yusuf S, Lonn E, Bosch J. Lipid lowering for primary prevention. Lancet 2009: 373:1152–1155.
- Briel M, Bassler D, Wang AT, Guyatt GH, Montori VM. The dangers of stopping a trial too early. J Bone Joint Surg Am 2012; 94(suppl 1):56–60.
- Hayward RA, Krumholz HM. Three reasons to abandon low-density lipoprotein targets: an open letter to the Adult Treatment Panel IV of the National Institutes of Health. Circ Cardiovasc Qual Outcomes 2012; 5:2–5.
- Afilalo J, Duque G, Steele R, Jukema JW, de Craen AJ, Eisenberg MJ. Statins for secondary prevention in elderly patients: a hierarchical Bayesian meta-analysis. www.ncbi.nlm.nih.gov/pubmedhealth/PMH0026417. Accessed December 5, 2016.
- Holmes HM, Hayley DC, Alexander GC, Sachs GA. Reconsidering medication appropriateness for patients late in life. Arch Intern Med 2006; 166:605–609.
- Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med 2011; 27:17–26.
- Petersen LK, Christensen K, Kragstrup J. Lipid-lowering treatment to the end? A review of observational studies and RCTs on cholesterol and mortality in 80+-year olds. Age Ageing 2010; 39:674–680.
- Psaty BM, Anderson M, Kronmal RA, et al. The association between lipid levels and the risks of incident myocardial infarction, stroke, and total mortality: the Cardiovascular Health Study. J Am Geriatr Soc 2004; 52:1639–1647.
- de Ruijter W, Westendorp RG, Assendelft WJ, et al. Use of Framingham risk score and new biomarkers to predict cardiovascular mortality in older people: population based observational cohort study. BMJ 2009; 338:a3083.
- Canon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015; 372:2387–2397.
- Anderson TJ, Gregoire J, Hegele RA, et al. 2012 update of the Canadian Cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol 2013; 29:151–167.
- Kutner JS, Blatchford PJ, Taylor DH, et al. Safety and benefit of discontinuing statin therapy in the setting of advanced, life-limiting illness: a randomized clinical trial. JAMA Intern Med 2015; 175:691–700.
- Tinetti ME, Bogardus ST Jr, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med 2004; 351:2870–2874.
- Rosenson RS. Current overview of statin-induced myopathy. Am J Med 2004; 116:408–416.
- Mancini GB, Baker S, Bergeron J, et al. Diagnosis, prevention, and management of statin adverse effects and intolerance: proceedings of a Canadian Working Group Consensus Conference. Can J Cardiol 2011; 27:635–662.
- Cohen JD, Brinton EA, Ito MK, Jacobson TA. Understanding Statin Use in America and Gaps in Patient Education (USAGE): an internet-based survey of 10,138 current and former statin users. J Clin Lipidol 2012; 6:208–215.
- Joy TR, Hegele RA. Narrative review: statin-related myopathy. Ann Intern Med 2009; 150:858–868.
- Talbert RL. Safety issues with statin therapy. J Am Pharm Assoc (2003) 2006; 46:479–490.
- Sewright KA, Clarkson PM, Thompson PD. Statin myopathy: incidence, risk factors, and pathophysiology. Curr Atheroscler Rep 2007; 9:389–396.
- Ott BR, Daiello LA, Dahabreh IJ, et al. Do statins impair cognition? A systematic review and meta-analysis of randomized controlled trials. J Gen Intern Med 2015; 30:348–358.
- Mancini GB, Tashakkor AY, Baker S, et al. Diagnosis, prevention and management of statin adverse effects and intolerance: Canadian Working Group Consensus update. Can J Cardiol 2013: 29:1553–1568.
- Rojas-Fernandez CH, Cameron JC. Is statin-associated cognitive impairment clinically relevant? A narrative review and clinical recommendations. Ann Pharmacother 2012; 46:549–557.
- McGuinness B, O’Hare J, Craig D, Bullock R, Malouf R, Passmore P. Cochrane review on ‘Statins for the treatment of dementia’. Int J Geriatr Psychiatry 2013; 28:119–126.
- Pandey RD, Gupta PP, Jha D, Kumar S. Role of statins in Alzheimer’s disease: a retrospective meta-analysis for commonly investigated clinical parameters in RCTs. Int J Neurosci 2013; 123:521–525.
- Food and Drug Administration (FDA). FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. www.fda.gov/drugs/ drugsafety/ucm293101.htm. Accessed December 5, 2016.
- Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med 2010; 170:1648–1654.
KEY POINTS
- There is no reason to prescribe or continue statins for primary prevention in severely frail elderly patients, as these drugs are unlikely to provide benefit in terms of outcomes relevant to this population.
- Statins are probably not necessary for secondary prevention in patients who are severely frail, although there may be extenuating circumstances for their use.
- There is no reason to start or continue statins for heart failure, as there is insufficient evidence that they are effective for this indication in any population.
- There is no reason to start or continue other lipid-lowering drugs in conjunction with statins.
- As the frail elderly may be more vulnerable to the side effects of statins, lower doses may be more appropriate if these drugs are prescribed.
- If there is concern regarding myopathy, a drug interaction, or other adverse effects, consider a trial of statin discontinuation.
Promoting higher blood pressure targets for frail older adults: A consensus guideline from Canada
Frail older adults deserve guidelines that take frailty into account while assessing the potential benefit and risks of treatment.
Specifically, our group—the Dalhousie Academic Detailing Service (ADS) and the Palliative and Therapeutic Harmonization (PATH) program—recommends that physicians strive to achieve more liberal treatment targets for elderly frail patients who have high blood pressure,1 as evidence does not support an aggressive approach in the frail elderly and the potential exists for harm.
This article reviews the evidence and reasoning that were used to develop and promote a guideline for drug treatment of hypertension in frail older adults. Our recommendations differ from other guidelines in that they focus as much on stopping or decreasing therapy as on starting or increasing it.
FRAILTY INCREASES THE RISK OF ADVERSE EFFECTS
The word frail, applied to older adults, describes those who have complex medical illnesses severe enough to compromise their ability to live independently.2 Many have multiple coexisting medical problems for which they take numerous drugs, in addition to dementia, impaired mobility, compromised functional ability, or a history of falling.
Frailty denotes vulnerability; it increases the risk of adverse effects from medical and surgical procedures,3 complicates drug therapy,4 prolongs hospital length of stay,5 leads to functional and cognitive decline,6 increases the risk of institutionalization,7 and reduces life expectancy8—all of which affect the benefit and harm of medical treatments.
Guidelines for treating hypertension9–11 now acknowledge that little evidence exists to support starting treatment for systolic blood pressure between 140 and 160 mm Hg or aiming for a target of less than 140 mm Hg for “very old” adults, commonly defined as over the age of 80. New guidelines loosen the treatment targets for the very old, but they do not specify targets for the frail and do not describe how to recognize or measure frailty.
RECOGNIZING AND MEASURING FRAILTY
A number of tools are available to recognize and measure frailty.12
The Fried frailty assessment13 has five items:
- Unintentional weight loss
- Self-reported exhaustion
- Weakness in grip
- Slow walking speed
- Low physical activity and energy expenditure.
People are deemed frail if they have three or more of these five. However, experts disagree about whether this system is too sensitive14 or not sensitive enough.15,16
The FRAIL questionnaire17 also has five items:
- Fatigue
- Resistance (inability to climb stairs)
- Ambulation (inability to walk 1 city block)
- Illness (more than 5 major illnesses)
- Weight loss.
People are deemed frail if they have at least three of these five items, and “prefrail” if they have two.
These and other tools are limited by being dichotomous: they classify people as being either frail or not frail18–20 but do not define the spectrum of frailty.
Other frailty assessments such as the Frailty Index21 identify frailty based on the number of accumulated health deficits but take a long time to complete, making them difficult to use in busy clinical settings.22–24
The Clinical Frailty Scale7 is a validated scale that categorizes frailty based on physical and functional indicators of health, such as cognition, function, and mobility, with scores that range from 1 (very fit) to 9 (terminally ill).7,12
The Frailty Assessment for Care-planning Tool (FACT) uses scaling compatible with the Clinical Frailty Scale but has been developed for use as a practical and interpretable frailty screening tool for nonexperts (Table 1). The FACT assesses cognition, mobility, function, and the social situation, using a combination of caregiver report and objective measures. To assess cognition, a health care professional uses items from the Mini-Cog25 (ie, the ability to draw an analog clock face and then recall three unrelated items following the clock-drawing test) and the memory axis of the Brief Cognitive Rating Scale26 (ie, the ability to recall current events, the current US president, and the names of children or spouse). Mobility, function, and social circumstance scores are assigned according to the caregiver report of the patient’s baseline status.
The FACT can be completed in busy clinical settings. Once a caregiver is identified, it takes about 5 minutes to complete.
Our guideline27–31 is intended for those with a score of 7 or more on the Clinical Frailty Scale or FACT,7,12 a score we chose because it describes people who are severely frail with shortened life expectancy.8 At this level, people need help with all instrumental activities of daily living (eg, handling finances, medication management, household chores, and shopping) as well as with basic activities of daily living such as bathing or dressing.
REVIEWING THE LIMITED EVIDENCE
We found no studies that addressed the risks and benefits of treating hypertension in frail older adults; therefore, we concentrated on studies that enrolled individuals who were chronologically old but not frail. We reviewed prominent guidelines,9–11,32,33 the evidence base for these guidelines,34–44 and Cochrane reviews.45,46 A detailed description of the evidence used to build our recommendation can be found online.31
When we deliberated on treatment targets, we reviewed evidence from two types of randomized controlled trials47:
Drug treatment trials randomize patients to different treatments, such as placebo versus a drug or one drug compared with another drug. Patients in different treatment groups may achieve different blood pressures and clinical outcomes, and this information is then used to define optimal targets. However, it may be difficult to determine if the benefit came from lowering blood pressure or from some other effect of the drug, which can be independent of blood pressure lowering.
Treat-to-target trials randomize patients to different blood pressure goals, but the groups are treated with the same or similar drugs. Therefore, any identified benefit can be attributed to the differences in blood pressure rather than the medications used. Compared with a drug treatment trial, this type of trial provides stronger evidence about optimal targets.
We also considered the characteristics of frailty, the dilemma of polypharmacy, and the relevance of the available scientific evidence to those who are frail.
Drug treatment trials
A Cochrane review45 of 15 studies with approximately 24,000 elderly participants found that treating hypertension decreased the rates of cardiovascular morbidity and mortality as well as fatal and nonfatal stroke in the “elderly” (defined as age ≥ 60) and “very elderly” (age ≥ 80). However, in the very elderly, all-cause mortality rates were not statistically significantly different with treatment compared with placebo. The mean duration of treatment was 4.5 years in the elderly and 2.2 years in the very elderly (Table 2). Of importance, all the trials enrolled only those individuals whose systolic blood pressure was at least 160 mm Hg at baseline.
None of the studies were treat-to-target trials—patients were assigned either active medication or placebo. Thus, these trials provide evidence of benefit for treating hypertension in the elderly and very elderly but do not identify the optimal target. All of the drug treatment trials showed benefit, but none achieved a systolic pressure lower than 140 mm Hg with active treatment (Table 3). Therefore, these studies do not support a systolic target of less than 140 mm Hg in the elderly.
Treat-to-target trials: JATOS and VALISH
The Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (JATOS)42 and the Valsartan in Elderly Isolated Systolic Hypertension (VALISH) study43 each enrolled more than 3,000 people age 65 or older (mean age approximately 75). Patients were randomized to either a strict systolic target of less than 140 mm Hg or a higher (more permissive) target of 140 to 160 mm Hg in JATOS and 140 to 149 mm Hg in VALISH.
In both trials, the group with strict targets achieved a systolic pressure of approximately 136 mm Hg, while the group with higher blood pressure targets achieved a systolic pressure of 146 mm Hg in JATOS and 142 mm Hg in VALISH. Despite these differences, there was no statistically significant difference in the primary outcome.
Thus, treat-to-target studies also fail to support a systolic target of less than 140 mm Hg in the elderly, although it is important to recognize the limitations of the studies. Approximately 15% of the participants had cardiovascular disease, so the applicability of the findings to patients with target-organ damage is uncertain. In addition, there were fewer efficacy outcome events than expected, which suggests that the studies were underpowered.
When to start drug treatment
In each of the drug treatment and treat-to-target trials, the inclusion criterion for study entry was a systolic blood pressure above 160 mm Hg, with a mean blood pressure at entry into the drug treatment trials of 182/95 mm Hg.46 Thus, data support starting treatment if the systolic blood pressure is above 160 mm Hg, but not lower.
Notably, in all but one study,46 at least two-thirds of the participants took no more than two antihypertensive medications. Since adverse events become more common as the number of medications increases, the benefit of adding a third drug to lower blood pressure is uncertain.
Evidence in the ‘very elderly’: HYVET
With the exception of the Hypertension in the Very Elderly Trial (HYVET),44 the mean age of elderly patients in the reported studies was between 67 and 76.
HYVET patients were age 80 and older (mean age 84) and were randomized to receive either indapamide (with or without perindopril) or placebo. The trial was stopped early at 2 years because the mortality rate was lower in the treatment group (10.1%) than in the placebo group (12.3%) (number needed to treat 46, 95% confidence interval 24–637, P = .02). There was no significant difference in the primary outcome of fatal and nonfatal stroke.
Notably, trials that are stopped early may overestimate treatment benefit.48
Evidence in frail older adults
While the above studies provide some information about managing hypertension in the elderly, the participants were generally healthy. HYVET44 specifically excluded those with a standing systolic blood pressure of less than 140 mm Hg and enrolled few patients with orthostasis (7.9% in the placebo group and 8.8% in the treatment group), a condition commonly associated with frailty. As such, these studies may be less relevant to the frail elderly, who are at higher risk of adverse drug events and have competing risks for morbidity and mortality.
Observational studies, in fact, raise questions about whether tight blood pressure control improves clinical outcomes for the very elderly. In the Leiden 85-plus study, lower systolic blood pressure was associated with lower cognitive scores, worse functional ability,49,50 and a higher mortality rate51 compared with higher systolic pressure, although it is uncertain whether these outcomes were indicative of underlying disease that could result in lower blood pressure or an effect of blood pressure-lowering.
The National Health and Nutrition Examination Survey52 found an association between blood pressure and mortality rate that varied by walking speed. For slower walkers (based on the 6-minute walk test), higher systolic pressures were not associated with a higher risk of death, suggesting that when older adults are frail (as indicated by their slow walking speed) they are less likely to benefit from aggressive treatment of hypertension.
People at high risk because of stroke
Because the evidence is limited, it is even more difficult to judge whether lowering blood pressure below 140 mm Hg is beneficial for frail patients who have a history of stroke, compared with the possibility that medications will cause adverse effects such as weakness, orthostasis, and falls. When reviewing the evidence to answer this question, we especially looked at outcomes that affect quality of life, such as nonfatal stroke leading to disability. In contrast, because the frail elderly have competing causes of mortality, we could not assume that a mortality benefit shown in nonfrail populations could be applied to frail populations.
The PROGRESS trial (Perindopril Protection Against Recurrent Stroke Study)53 was in patients with a history of stroke or transient ischemic attack and a mean age of 64, who were treated with either perindopril (with or without indapamide) or placebo.
At almost 4 years, the rate of disabling stroke was 2.7% in the treatment group and 4.3% in the placebo group, a relative risk reduction of 38% and an absolute risk reduction of 1.64% (number needed to treat 61, 95% confidence interval 39–139). The relative risk reduction for all strokes (fatal and nonfatal) was similar across a range of baseline systolic pressures, but the absolute risk reduction was greater in the prespecified subgroup that had hypertension at baseline (mean blood pressure 159/94 mm Hg) than in the normotensive subgroup (mean blood pressure 136/79 mm Hg), suggesting that treatment is most beneficial for those with higher systolic blood pressures. Also, the benefit was only demonstrated in the subgroup that received two antihypertensive medications; those who received perindopril alone showed no benefit.
This study involved relatively young patients in relatively good health except for their strokes. The extent to which the results can be extrapolated to older, frail adults is uncertain because of the time needed to achieve benefit and because of the added vulnerability of frailty, which could make treatment with two antihypertensive medications riskier.
PRoFESS (Prevention Regimen for Effectively Avoiding Second Strokes),54 another study in patients with previous stroke (mean age 66) showed no benefit over 2.5 years in the primary outcome of stroke using telmesartan 80 mg daily compared with placebo. This result is concordant with that of PROGRESS,53 in which patients who took only one medication did not show a significant decrease in the rate of stroke.
A possible reason for the lack of benefit from monotherapy was that the differences in blood pressure between the placebo group and the treatment group on monotherapy were small in both studies (3.8/2.0 mm Hg in PRoFESS, 5/3 mm Hg in PROGRESS). In contrast, patients on dual therapy in PROGRESS decreased their blood pressure by 12/5 mm Hg compared with placebo.
CURRENT HYPERTENSION GUIDELINES
Current guidelines make reference to the elderly, but we found none that made specific recommendations for the frail elderly.
JNC 8
In December 2013, members of the Eighth Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 8) released new recommendations.32 One significant revision was to support higher blood pressure targets for older adults (age 60 and older). Whereas JNC 7 stated that lowering blood pressure below 140/90 mm Hg reduced cardiovascular complications,33 JNC 8 now acknowledges that there is no strong evidence to support blood pressure targets below 150/90 mm Hg for hypertensive persons without kidney disease or diabetes age 60 and older. Thus, in the general population age 60 and older, JNC 8 recommends starting antihypertensive treatment when blood pressure is 150/90 mm Hg or higher, and treating to a goal blood pressure of less than 150/90 mm Hg. JNC 8 makes no recommendation about how to adjust blood pressure targets for frailty or how to measure blood pressure.
American College of Cardiology and American Heart Association
In 2011, the American College of Cardiology and American Heart Association published a consensus document on the management of hypertension in the elderly.9
They acknowledged that the generally recommended blood pressure goal of lower than 140/90 mm Hg in uncomplicated elderly patients is based on expert opinion rather than on data from randomized controlled trials, but nevertheless recommended a target systolic pressure lower than 140 mm Hg for older adults, except for octogenarians.
For those over age 80, systolic levels of 140 to 145 mm Hg can be acceptable if tolerated and if the patient does not experience orthostasis when standing. Systolic pressure lower than 130 mm Hg and diastolic pressures lower than 65 mm Hg should be avoided in this age group.
The document acknowledges that systolic pressure may have to remain above 150 mm Hg if there is no response to four “well-selected drugs” or if there are unacceptable side effects. In these cases, the lowest “safely achieved” systolic blood pressure should be the goal.
Canadian Hypertension Education Program
The 2014 Canadian Hypertension Education Program (CHEP) report makes several recommendations for the “very elderly,” a group they define as over the age of 80. The CHEP website and resources include the following recommendations10:
- For the very elderly without diabetes or target-organ damage, drug therapy should be initiated when systolic blood pressure is higher than 160 mm Hg to reach a systolic blood pressure target lower than 150 mm Hg. This is a grade C level recommendation, indicating that it is based on low-quality trials, unvalidated surrogate outcomes, or results from nonrandomized observational studies.
- For the very elderly with macrovascular target-organ damage, antihypertensive therapy should be considered if systolic blood pressure readings average 140 mm Hg or higher (grade D for 140 to 160 mm Hg; grade A for higher than 160 mm Hg), although caution should be exercised in elderly patients who are frail. (Grade D recommendations are the weakest, as they are based on low-powered, imprecise studies or expert opinion, whereas grade A recommendations are based on the strongest evidence from high-quality randomized clinical trials.)
- Decisions regarding initiating and intensifying pharmacotherapy in the very elderly should be based on an individualized risk-benefit analysis.
The European Society of Hypertension and European Society of Cardiology
The 2013 guidelines from the European Society of Hypertension and the European Society of Cardiology11 recommend that for elderly patients under age 80, antihypertensive treatment may be considered at systolic values higher than 140 mm Hg and aimed at values lower than 140 mm Hg if the patient is fit and treatment is well tolerated.
For those over age 80 with an initial systolic pressure of 160 mm Hg or higher, the guidelines recommend lowering systolic pressure to between 150 and 140 mm Hg, provided the patient is in good physical and mental condition. In frail elderly patients, they recommend leaving decisions on antihypertensive therapy to the treating physician, based on monitoring of the clinical effects of treatment.11
The ADS/PATH guidelines
When finalizing our recommendations,1 we considered the characteristics of frailty and the following key points from the evidence:
- Although evidence from drug treatment trials indicates that there is benefit in treating healthy older adults who have hypertension, the benefit of treating frail older adults is unknown.
- Major trials enrolled elderly patients only if they had systolic blood pressures of at least 160 mm Hg. Therefore, evidence supports initiating pharmacotherapy at a systolic pressure of 160 mm Hg or higher.
- No evidence from randomized controlled trials supports a systolic target lower than 140 mm Hg in the elderly, and there is some evidence that such a target does not benefit.
- The benefit of adding a third medication to lower blood pressure has not been studied.
- Frailty makes the potential benefits of strict blood pressure targets even less certain and increases the possibility of harm from adverse drug events.
- The only study of very old adults, HYVET,44 enrolled relatively healthy older adults and few with orthostasis, while excluding those with a standing systolic blood pressure lower than 140 mm Hg.
OUR RECOMMENDATIONS
Based on the above, we advise against unnecessarily strict targets and recommend stopping antihypertensive medications that are used for the sole purpose of keeping the systolic blood pressure below 140 mm Hg. Our guidelines are unique in that they focus equally on when to stop and when to start medications. We concluded that without evidence of definitive benefit, “less is more” with frailty.55 We believe that if physicians and health professionals understand the limitations of the evidence, they can be more confident in stopping medications that lower blood pressure to an unnecessarily low level.
We recommend the following (Table 4):
Before treating
- Carefully review the risks and the potential but unproven benefits of treatment.
- To avoid overtreatment, treatment decisions should be based on blood pressure measurements in the seated (not supine) position, while also considering the presence of orthostasis.
- To evaluate orthostasis, measure blood pressure in the supine position, then immediately on standing, and again after 2 minutes. Ask the patient if he or she feels light-headed or dizzy when standing.
Stop treatment
- If the seated systolic blood pressure is less than 140 mm Hg, medications can be tapered and discontinued to achieve the targets described below.
- Before discontinuation, consider whether the medications are treating additional conditions such as rate control for atrial fibrillation or symptomatic management of heart failure.
- It is uncertain whether to discontinue treatment when there is a history of stroke. Consider that treatment with two medications resulted in an absolute risk reduction for disabling stroke of 1.64% over approximately 4 years for adults with previous stroke and a mean age of 64,57 an effect that may be more prominent at higher systolic pressures.
Start treatment
- Consider starting treatment when systolic pressure is 160 mm Hg or higher.
- Aim for a seated systolic pressure between 140 and 160 mm Hg if there are no adverse effects from treatment that affect quality of life.
- If there is symptomatic orthostasis or if standing systolic pressure is lower than 140 mm Hg, the target seated systolic pressure can be adjusted upwards.
- In the severely frail nearing the end of life, a target systolic pressure of 160 to 190 mm Hg is reasonable.
- The blood pressure target is the same in people with diabetes.
- In general, use no more than two medications.
Dissemination and implementation
The ADS/PATH guideline is intended for use by physicians and other health professionals (eg, pharmacists and nurses) who care for frail older adults or who work in long-term care facilities. Since creating our guideline, we have disseminated it to physicians, pharmacists, and other health professionals through academic detailing, large conferences, and interactive webinars.
While we do not have objective evidence of practice change, our evaluation data found that 34% of 403 family physicians who received academic detailing indicated that the guideline would change their practice, while 36% stated that the guideline confirmed their practice, an indication that family physicians are sensitive to the needs of the frail elderly.
Because health professionals may be wary of stopping medications and not meeting recommended targets, there may be barriers to adopting this guideline. However, our experience with the PATH program indicates that these barriers can be overcome using effective communication strategies between health professionals and consumers.
AN APPROACH APPROPRIATE TO FRAILTY
There is no direct evidence for systolic blood pressure targets in the frail elderly, so we applied evidence from the nonfrail elderly. Our recommendations differ somewhat from those of other groups, which recommend targets below 140 to 150 mm Hg for older adults, although some do advise caution in the elderly for whom a substantial fall in blood pressure might be poorly tolerated. Despite these messages, we believe that clearer guidance is needed to direct health practitioners toward models that acknowledge that frail patients are in a precarious balance of health and may be harmed by treatments that strive to lower blood pressure to unproven targets. For this reason, our guideline clearly indicates when to decrease or stop drug treatment.
After physicians and health professionals examine the evidence and more fully understand the benefits and harms of treating frail older adults, we are confident that they will be more comfortable stopping medications that lower blood pressure to an unnecessarily low level and instead use an approach that is more appropriate to frailty. We hope clinicians can use this guideline with the same enthusiasm applied to other guidelines, and we welcome discussion.
Acknowledgments: We would like to thank and acknowledge Tanya MacLeod and Kathryn Yuill for their review of and advice about the manuscript.
- Palliative and Therapeutic Harmonization program. Hypertension guidelines. Treating hypertension in frailty. http://pathclinic.ca/resources/hypertension/. Accessed May 2, 2014.
- Theou O, Rockwood MR, Mitnitski A, Rockwood K. Disability and co-morbidity in relation to frailty: how much do they overlap? Arch Gerontol Geriatr 2012; 55:e1–e8.
- Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg 2010; 210:901–908.
- Tinetti ME, Bogardus ST, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med 2004; 351:2870–2874.
- Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation 2011; 124:2397–2404.
- Theou O, Rockwood K. Should frailty status always be considered when treating the elderly patient? Aging Health 2012; 8:261–271.
- Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173:489–495.
- Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008; 8:24.
- Aronow WS, Fleg JL, Pepine CJ, et al; ACCF Task Force. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation 2011; 123:2434–2506.
- The Canadian Hypertension Education Program (CHEP). 2014 CHEP recommendations. www.hypertension.ca/en/. Accessed May 2, 2014.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34:2159–2219.
- Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013; 14:392–397.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Ensrud KE, Ewing SK, Cawthon PM, et al; Osteoporotic Fractures in Men Research Group. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc 2009; 57:492–498.
- Avila-Funes JA, Amieva H, Barberger-Gateau P, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J Am Geriatr Soc 2009; 57:453–461.
- Bergman H, Ferrucci L, Guralnik J, et al. Frailty: an emerging research and clinical paradigm—issues and controversies. J Gerontol A Biol Sci Med Sci 2007; 62:731–737.
- Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging 2012; 16:601–608.
- Strawbridge WJ, Shema SJ, Balfour JL, Higby HR, Kaplan GA. Antecedents of frailty over three decades in an older cohort. J Gerontol B Psychol Sci Soc Sci 1998; 53:S9–S16.
- Matthews M, Lucas A, Boland R, et al. Use of a questionnaire to screen for frailty in the elderly: an exploratory study. Aging Clin Exp Res 2004; 16:34–40.
- Salvi F, Morichi V, Grilli A, et al. Screening for frailty in elderly emergency department patients by using the Identification of Seniors At Risk (ISAR). J Nutr Health Aging 2012; 16:313–318.
- Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal 2001; 1:323–336.
- Kellen E, Bulens P, Deckx L, et al. Identifying an accurate pre-screening tool in geriatric oncology. Crit Rev Oncol Hematol 2010; 75:243–248.
- Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing 2006; 35:526–529.
- Martin FC, Brighton P. Frailty: different tools for different purposes? Age Ageing 2008; 37:129–131.
- Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini-cog: a cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry 2000; 15:1021–1027.
- Reisberg B, Ferris SH. Brief Cognitive Rating Scale (BCRS). Psychopharmacol Bull 1988; 24:629–636.
- Moorhouse P, Mallery LH. Palliative and therapeutic harmonization: a model for appropriate decision-making in frail older adults. J Am Geriatr Soc 2012; 60:2326–2332.
- Palliative and Therapeutic Harmonization Clinic (PATH). www.pathclinic.ca. Accessed May 2, 2014.
- Dalhousie University Faculty of Medicine: Continuing Medical Education. http://cme.medicine.dal.ca/ADS.htm. Accessed January 8, 2014.
- Mallery LH, Moorhouse P. Respecting frailty. J Med Ethics 2011; 37:126–128.
- Dalhousie University Faculty of Medicine: Continuing Medical Education. Issues in hypertension 2011. http://cme.medicine.dal.ca/files/Hypertension%20book.pdf. Accessed May 2, 2014.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Amery A, Birkenhäger W, Brixko P, et al. Mortality and morbidity results from the European Working Party on High Blood Pressure in the Elderly trial. Lancet 1985; 1:1349–1354.
- Coope J, Warrender TS. Randomised trial of treatment of hypertension in elderly patients in primary care. Br Med J (Clin Res Ed) 1986; 293:1145–1151.
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991; 265:3255–3264.
- Dahlöf B, Lindholm LH, Hansson L, Scherstén B, Ekbom T, Wester PO. Morbidity and mortality in the Swedish Trial in Old Patients with Hypertension (STOP-Hypertension). Lancet 1991; 338:1281–1285.
- Medical Research Council trial of treatment of hypertension in older adults: principal results. MRC Working Party. BMJ 1992; 304:405–412.
- Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet 1997; 350:757–764.
- Liu L, Wang JG, Gong L, Liu G, Staessen JA. Comparison of active treatment and placebo in older Chinese patients with isolated systolic hypertension. Systolic Hypertension in China (Syst-China) Collaborative Group. J Hypertens 1998; 16:1823–1829.
- Lithell H, Hansson L, Skoog I, et al; SCOPE Study Group. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens 2003; 21:875–886.
- JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008; 31:2115–2127.
- Oparil S, Yarows SA, Patel S, Fang H, Zhang J, Satlin A. Efficacy and safety of combined use of aliskiren and valsartan in patients with hypertension: a randomised, double-blind trial. Lancet 2007; 370:221–229.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Musini VM, Tejani AM, Bassett K, Wright JM. Pharmacotherapy for hypertension in the elderly. Cochrane Database Syst Rev 2009;CD000028.
- He FJ, MacGregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev 2004;CD004937.
- Allen M, Kelly K, Fleming I. Hypertension in elderly patients: recommended systolic targets are not evidence based [in French]. Can Fam Physician 2013; 59:19–24.
- Guyatt GH, Briel M, Glasziou P, Bassler D, Montori VM. Problems of stopping trials early. BMJ 2012; 344:e3863.
- Sabayan B, Oleksik AM, Maier AB, et al. High blood pressure and resilience to physical and cognitive decline in the oldest old: the Leiden 85-plus Study. J Am Geriatr Soc 2012; 60:2014–2019.
- Sabayan B, van Vliet P, de Ruijter W, Gussekloo J, de Craen AJ, Westendorp RG. High blood pressure, physical and cognitive function, and risk of stroke in the oldest old: the Leiden 85-plus Study. Stroke 2013; 44:15–20.
- Poortvliet RK, Blom JW, de Craen AJ, et al. Low blood pressure predicts increased mortality in very old age even without heart failure: the Leiden 85-plus Study. Eur J Heart Fail 2013; 15:528–533.
- Odden MC, Peralta CA, Haan MN, Covinsky KE. Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch Intern Med 2012; 172:1162–1168.
- PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001; 358:1033–1041.
- Yusuf S, Diener HC, Sacco RL, et al; PRoFESS Study Group. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med 2008; 359:1225–1237.
- Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med 2010; 170:1648–1654.
Frail older adults deserve guidelines that take frailty into account while assessing the potential benefit and risks of treatment.
Specifically, our group—the Dalhousie Academic Detailing Service (ADS) and the Palliative and Therapeutic Harmonization (PATH) program—recommends that physicians strive to achieve more liberal treatment targets for elderly frail patients who have high blood pressure,1 as evidence does not support an aggressive approach in the frail elderly and the potential exists for harm.
This article reviews the evidence and reasoning that were used to develop and promote a guideline for drug treatment of hypertension in frail older adults. Our recommendations differ from other guidelines in that they focus as much on stopping or decreasing therapy as on starting or increasing it.
FRAILTY INCREASES THE RISK OF ADVERSE EFFECTS
The word frail, applied to older adults, describes those who have complex medical illnesses severe enough to compromise their ability to live independently.2 Many have multiple coexisting medical problems for which they take numerous drugs, in addition to dementia, impaired mobility, compromised functional ability, or a history of falling.
Frailty denotes vulnerability; it increases the risk of adverse effects from medical and surgical procedures,3 complicates drug therapy,4 prolongs hospital length of stay,5 leads to functional and cognitive decline,6 increases the risk of institutionalization,7 and reduces life expectancy8—all of which affect the benefit and harm of medical treatments.
Guidelines for treating hypertension9–11 now acknowledge that little evidence exists to support starting treatment for systolic blood pressure between 140 and 160 mm Hg or aiming for a target of less than 140 mm Hg for “very old” adults, commonly defined as over the age of 80. New guidelines loosen the treatment targets for the very old, but they do not specify targets for the frail and do not describe how to recognize or measure frailty.
RECOGNIZING AND MEASURING FRAILTY
A number of tools are available to recognize and measure frailty.12
The Fried frailty assessment13 has five items:
- Unintentional weight loss
- Self-reported exhaustion
- Weakness in grip
- Slow walking speed
- Low physical activity and energy expenditure.
People are deemed frail if they have three or more of these five. However, experts disagree about whether this system is too sensitive14 or not sensitive enough.15,16
The FRAIL questionnaire17 also has five items:
- Fatigue
- Resistance (inability to climb stairs)
- Ambulation (inability to walk 1 city block)
- Illness (more than 5 major illnesses)
- Weight loss.
People are deemed frail if they have at least three of these five items, and “prefrail” if they have two.
These and other tools are limited by being dichotomous: they classify people as being either frail or not frail18–20 but do not define the spectrum of frailty.
Other frailty assessments such as the Frailty Index21 identify frailty based on the number of accumulated health deficits but take a long time to complete, making them difficult to use in busy clinical settings.22–24
The Clinical Frailty Scale7 is a validated scale that categorizes frailty based on physical and functional indicators of health, such as cognition, function, and mobility, with scores that range from 1 (very fit) to 9 (terminally ill).7,12
The Frailty Assessment for Care-planning Tool (FACT) uses scaling compatible with the Clinical Frailty Scale but has been developed for use as a practical and interpretable frailty screening tool for nonexperts (Table 1). The FACT assesses cognition, mobility, function, and the social situation, using a combination of caregiver report and objective measures. To assess cognition, a health care professional uses items from the Mini-Cog25 (ie, the ability to draw an analog clock face and then recall three unrelated items following the clock-drawing test) and the memory axis of the Brief Cognitive Rating Scale26 (ie, the ability to recall current events, the current US president, and the names of children or spouse). Mobility, function, and social circumstance scores are assigned according to the caregiver report of the patient’s baseline status.
The FACT can be completed in busy clinical settings. Once a caregiver is identified, it takes about 5 minutes to complete.
Our guideline27–31 is intended for those with a score of 7 or more on the Clinical Frailty Scale or FACT,7,12 a score we chose because it describes people who are severely frail with shortened life expectancy.8 At this level, people need help with all instrumental activities of daily living (eg, handling finances, medication management, household chores, and shopping) as well as with basic activities of daily living such as bathing or dressing.
REVIEWING THE LIMITED EVIDENCE
We found no studies that addressed the risks and benefits of treating hypertension in frail older adults; therefore, we concentrated on studies that enrolled individuals who were chronologically old but not frail. We reviewed prominent guidelines,9–11,32,33 the evidence base for these guidelines,34–44 and Cochrane reviews.45,46 A detailed description of the evidence used to build our recommendation can be found online.31
When we deliberated on treatment targets, we reviewed evidence from two types of randomized controlled trials47:
Drug treatment trials randomize patients to different treatments, such as placebo versus a drug or one drug compared with another drug. Patients in different treatment groups may achieve different blood pressures and clinical outcomes, and this information is then used to define optimal targets. However, it may be difficult to determine if the benefit came from lowering blood pressure or from some other effect of the drug, which can be independent of blood pressure lowering.
Treat-to-target trials randomize patients to different blood pressure goals, but the groups are treated with the same or similar drugs. Therefore, any identified benefit can be attributed to the differences in blood pressure rather than the medications used. Compared with a drug treatment trial, this type of trial provides stronger evidence about optimal targets.
We also considered the characteristics of frailty, the dilemma of polypharmacy, and the relevance of the available scientific evidence to those who are frail.
Drug treatment trials
A Cochrane review45 of 15 studies with approximately 24,000 elderly participants found that treating hypertension decreased the rates of cardiovascular morbidity and mortality as well as fatal and nonfatal stroke in the “elderly” (defined as age ≥ 60) and “very elderly” (age ≥ 80). However, in the very elderly, all-cause mortality rates were not statistically significantly different with treatment compared with placebo. The mean duration of treatment was 4.5 years in the elderly and 2.2 years in the very elderly (Table 2). Of importance, all the trials enrolled only those individuals whose systolic blood pressure was at least 160 mm Hg at baseline.
None of the studies were treat-to-target trials—patients were assigned either active medication or placebo. Thus, these trials provide evidence of benefit for treating hypertension in the elderly and very elderly but do not identify the optimal target. All of the drug treatment trials showed benefit, but none achieved a systolic pressure lower than 140 mm Hg with active treatment (Table 3). Therefore, these studies do not support a systolic target of less than 140 mm Hg in the elderly.
Treat-to-target trials: JATOS and VALISH
The Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (JATOS)42 and the Valsartan in Elderly Isolated Systolic Hypertension (VALISH) study43 each enrolled more than 3,000 people age 65 or older (mean age approximately 75). Patients were randomized to either a strict systolic target of less than 140 mm Hg or a higher (more permissive) target of 140 to 160 mm Hg in JATOS and 140 to 149 mm Hg in VALISH.
In both trials, the group with strict targets achieved a systolic pressure of approximately 136 mm Hg, while the group with higher blood pressure targets achieved a systolic pressure of 146 mm Hg in JATOS and 142 mm Hg in VALISH. Despite these differences, there was no statistically significant difference in the primary outcome.
Thus, treat-to-target studies also fail to support a systolic target of less than 140 mm Hg in the elderly, although it is important to recognize the limitations of the studies. Approximately 15% of the participants had cardiovascular disease, so the applicability of the findings to patients with target-organ damage is uncertain. In addition, there were fewer efficacy outcome events than expected, which suggests that the studies were underpowered.
When to start drug treatment
In each of the drug treatment and treat-to-target trials, the inclusion criterion for study entry was a systolic blood pressure above 160 mm Hg, with a mean blood pressure at entry into the drug treatment trials of 182/95 mm Hg.46 Thus, data support starting treatment if the systolic blood pressure is above 160 mm Hg, but not lower.
Notably, in all but one study,46 at least two-thirds of the participants took no more than two antihypertensive medications. Since adverse events become more common as the number of medications increases, the benefit of adding a third drug to lower blood pressure is uncertain.
Evidence in the ‘very elderly’: HYVET
With the exception of the Hypertension in the Very Elderly Trial (HYVET),44 the mean age of elderly patients in the reported studies was between 67 and 76.
HYVET patients were age 80 and older (mean age 84) and were randomized to receive either indapamide (with or without perindopril) or placebo. The trial was stopped early at 2 years because the mortality rate was lower in the treatment group (10.1%) than in the placebo group (12.3%) (number needed to treat 46, 95% confidence interval 24–637, P = .02). There was no significant difference in the primary outcome of fatal and nonfatal stroke.
Notably, trials that are stopped early may overestimate treatment benefit.48
Evidence in frail older adults
While the above studies provide some information about managing hypertension in the elderly, the participants were generally healthy. HYVET44 specifically excluded those with a standing systolic blood pressure of less than 140 mm Hg and enrolled few patients with orthostasis (7.9% in the placebo group and 8.8% in the treatment group), a condition commonly associated with frailty. As such, these studies may be less relevant to the frail elderly, who are at higher risk of adverse drug events and have competing risks for morbidity and mortality.
Observational studies, in fact, raise questions about whether tight blood pressure control improves clinical outcomes for the very elderly. In the Leiden 85-plus study, lower systolic blood pressure was associated with lower cognitive scores, worse functional ability,49,50 and a higher mortality rate51 compared with higher systolic pressure, although it is uncertain whether these outcomes were indicative of underlying disease that could result in lower blood pressure or an effect of blood pressure-lowering.
The National Health and Nutrition Examination Survey52 found an association between blood pressure and mortality rate that varied by walking speed. For slower walkers (based on the 6-minute walk test), higher systolic pressures were not associated with a higher risk of death, suggesting that when older adults are frail (as indicated by their slow walking speed) they are less likely to benefit from aggressive treatment of hypertension.
People at high risk because of stroke
Because the evidence is limited, it is even more difficult to judge whether lowering blood pressure below 140 mm Hg is beneficial for frail patients who have a history of stroke, compared with the possibility that medications will cause adverse effects such as weakness, orthostasis, and falls. When reviewing the evidence to answer this question, we especially looked at outcomes that affect quality of life, such as nonfatal stroke leading to disability. In contrast, because the frail elderly have competing causes of mortality, we could not assume that a mortality benefit shown in nonfrail populations could be applied to frail populations.
The PROGRESS trial (Perindopril Protection Against Recurrent Stroke Study)53 was in patients with a history of stroke or transient ischemic attack and a mean age of 64, who were treated with either perindopril (with or without indapamide) or placebo.
At almost 4 years, the rate of disabling stroke was 2.7% in the treatment group and 4.3% in the placebo group, a relative risk reduction of 38% and an absolute risk reduction of 1.64% (number needed to treat 61, 95% confidence interval 39–139). The relative risk reduction for all strokes (fatal and nonfatal) was similar across a range of baseline systolic pressures, but the absolute risk reduction was greater in the prespecified subgroup that had hypertension at baseline (mean blood pressure 159/94 mm Hg) than in the normotensive subgroup (mean blood pressure 136/79 mm Hg), suggesting that treatment is most beneficial for those with higher systolic blood pressures. Also, the benefit was only demonstrated in the subgroup that received two antihypertensive medications; those who received perindopril alone showed no benefit.
This study involved relatively young patients in relatively good health except for their strokes. The extent to which the results can be extrapolated to older, frail adults is uncertain because of the time needed to achieve benefit and because of the added vulnerability of frailty, which could make treatment with two antihypertensive medications riskier.
PRoFESS (Prevention Regimen for Effectively Avoiding Second Strokes),54 another study in patients with previous stroke (mean age 66) showed no benefit over 2.5 years in the primary outcome of stroke using telmesartan 80 mg daily compared with placebo. This result is concordant with that of PROGRESS,53 in which patients who took only one medication did not show a significant decrease in the rate of stroke.
A possible reason for the lack of benefit from monotherapy was that the differences in blood pressure between the placebo group and the treatment group on monotherapy were small in both studies (3.8/2.0 mm Hg in PRoFESS, 5/3 mm Hg in PROGRESS). In contrast, patients on dual therapy in PROGRESS decreased their blood pressure by 12/5 mm Hg compared with placebo.
CURRENT HYPERTENSION GUIDELINES
Current guidelines make reference to the elderly, but we found none that made specific recommendations for the frail elderly.
JNC 8
In December 2013, members of the Eighth Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 8) released new recommendations.32 One significant revision was to support higher blood pressure targets for older adults (age 60 and older). Whereas JNC 7 stated that lowering blood pressure below 140/90 mm Hg reduced cardiovascular complications,33 JNC 8 now acknowledges that there is no strong evidence to support blood pressure targets below 150/90 mm Hg for hypertensive persons without kidney disease or diabetes age 60 and older. Thus, in the general population age 60 and older, JNC 8 recommends starting antihypertensive treatment when blood pressure is 150/90 mm Hg or higher, and treating to a goal blood pressure of less than 150/90 mm Hg. JNC 8 makes no recommendation about how to adjust blood pressure targets for frailty or how to measure blood pressure.
American College of Cardiology and American Heart Association
In 2011, the American College of Cardiology and American Heart Association published a consensus document on the management of hypertension in the elderly.9
They acknowledged that the generally recommended blood pressure goal of lower than 140/90 mm Hg in uncomplicated elderly patients is based on expert opinion rather than on data from randomized controlled trials, but nevertheless recommended a target systolic pressure lower than 140 mm Hg for older adults, except for octogenarians.
For those over age 80, systolic levels of 140 to 145 mm Hg can be acceptable if tolerated and if the patient does not experience orthostasis when standing. Systolic pressure lower than 130 mm Hg and diastolic pressures lower than 65 mm Hg should be avoided in this age group.
The document acknowledges that systolic pressure may have to remain above 150 mm Hg if there is no response to four “well-selected drugs” or if there are unacceptable side effects. In these cases, the lowest “safely achieved” systolic blood pressure should be the goal.
Canadian Hypertension Education Program
The 2014 Canadian Hypertension Education Program (CHEP) report makes several recommendations for the “very elderly,” a group they define as over the age of 80. The CHEP website and resources include the following recommendations10:
- For the very elderly without diabetes or target-organ damage, drug therapy should be initiated when systolic blood pressure is higher than 160 mm Hg to reach a systolic blood pressure target lower than 150 mm Hg. This is a grade C level recommendation, indicating that it is based on low-quality trials, unvalidated surrogate outcomes, or results from nonrandomized observational studies.
- For the very elderly with macrovascular target-organ damage, antihypertensive therapy should be considered if systolic blood pressure readings average 140 mm Hg or higher (grade D for 140 to 160 mm Hg; grade A for higher than 160 mm Hg), although caution should be exercised in elderly patients who are frail. (Grade D recommendations are the weakest, as they are based on low-powered, imprecise studies or expert opinion, whereas grade A recommendations are based on the strongest evidence from high-quality randomized clinical trials.)
- Decisions regarding initiating and intensifying pharmacotherapy in the very elderly should be based on an individualized risk-benefit analysis.
The European Society of Hypertension and European Society of Cardiology
The 2013 guidelines from the European Society of Hypertension and the European Society of Cardiology11 recommend that for elderly patients under age 80, antihypertensive treatment may be considered at systolic values higher than 140 mm Hg and aimed at values lower than 140 mm Hg if the patient is fit and treatment is well tolerated.
For those over age 80 with an initial systolic pressure of 160 mm Hg or higher, the guidelines recommend lowering systolic pressure to between 150 and 140 mm Hg, provided the patient is in good physical and mental condition. In frail elderly patients, they recommend leaving decisions on antihypertensive therapy to the treating physician, based on monitoring of the clinical effects of treatment.11
The ADS/PATH guidelines
When finalizing our recommendations,1 we considered the characteristics of frailty and the following key points from the evidence:
- Although evidence from drug treatment trials indicates that there is benefit in treating healthy older adults who have hypertension, the benefit of treating frail older adults is unknown.
- Major trials enrolled elderly patients only if they had systolic blood pressures of at least 160 mm Hg. Therefore, evidence supports initiating pharmacotherapy at a systolic pressure of 160 mm Hg or higher.
- No evidence from randomized controlled trials supports a systolic target lower than 140 mm Hg in the elderly, and there is some evidence that such a target does not benefit.
- The benefit of adding a third medication to lower blood pressure has not been studied.
- Frailty makes the potential benefits of strict blood pressure targets even less certain and increases the possibility of harm from adverse drug events.
- The only study of very old adults, HYVET,44 enrolled relatively healthy older adults and few with orthostasis, while excluding those with a standing systolic blood pressure lower than 140 mm Hg.
OUR RECOMMENDATIONS
Based on the above, we advise against unnecessarily strict targets and recommend stopping antihypertensive medications that are used for the sole purpose of keeping the systolic blood pressure below 140 mm Hg. Our guidelines are unique in that they focus equally on when to stop and when to start medications. We concluded that without evidence of definitive benefit, “less is more” with frailty.55 We believe that if physicians and health professionals understand the limitations of the evidence, they can be more confident in stopping medications that lower blood pressure to an unnecessarily low level.
We recommend the following (Table 4):
Before treating
- Carefully review the risks and the potential but unproven benefits of treatment.
- To avoid overtreatment, treatment decisions should be based on blood pressure measurements in the seated (not supine) position, while also considering the presence of orthostasis.
- To evaluate orthostasis, measure blood pressure in the supine position, then immediately on standing, and again after 2 minutes. Ask the patient if he or she feels light-headed or dizzy when standing.
Stop treatment
- If the seated systolic blood pressure is less than 140 mm Hg, medications can be tapered and discontinued to achieve the targets described below.
- Before discontinuation, consider whether the medications are treating additional conditions such as rate control for atrial fibrillation or symptomatic management of heart failure.
- It is uncertain whether to discontinue treatment when there is a history of stroke. Consider that treatment with two medications resulted in an absolute risk reduction for disabling stroke of 1.64% over approximately 4 years for adults with previous stroke and a mean age of 64,57 an effect that may be more prominent at higher systolic pressures.
Start treatment
- Consider starting treatment when systolic pressure is 160 mm Hg or higher.
- Aim for a seated systolic pressure between 140 and 160 mm Hg if there are no adverse effects from treatment that affect quality of life.
- If there is symptomatic orthostasis or if standing systolic pressure is lower than 140 mm Hg, the target seated systolic pressure can be adjusted upwards.
- In the severely frail nearing the end of life, a target systolic pressure of 160 to 190 mm Hg is reasonable.
- The blood pressure target is the same in people with diabetes.
- In general, use no more than two medications.
Dissemination and implementation
The ADS/PATH guideline is intended for use by physicians and other health professionals (eg, pharmacists and nurses) who care for frail older adults or who work in long-term care facilities. Since creating our guideline, we have disseminated it to physicians, pharmacists, and other health professionals through academic detailing, large conferences, and interactive webinars.
While we do not have objective evidence of practice change, our evaluation data found that 34% of 403 family physicians who received academic detailing indicated that the guideline would change their practice, while 36% stated that the guideline confirmed their practice, an indication that family physicians are sensitive to the needs of the frail elderly.
Because health professionals may be wary of stopping medications and not meeting recommended targets, there may be barriers to adopting this guideline. However, our experience with the PATH program indicates that these barriers can be overcome using effective communication strategies between health professionals and consumers.
AN APPROACH APPROPRIATE TO FRAILTY
There is no direct evidence for systolic blood pressure targets in the frail elderly, so we applied evidence from the nonfrail elderly. Our recommendations differ somewhat from those of other groups, which recommend targets below 140 to 150 mm Hg for older adults, although some do advise caution in the elderly for whom a substantial fall in blood pressure might be poorly tolerated. Despite these messages, we believe that clearer guidance is needed to direct health practitioners toward models that acknowledge that frail patients are in a precarious balance of health and may be harmed by treatments that strive to lower blood pressure to unproven targets. For this reason, our guideline clearly indicates when to decrease or stop drug treatment.
After physicians and health professionals examine the evidence and more fully understand the benefits and harms of treating frail older adults, we are confident that they will be more comfortable stopping medications that lower blood pressure to an unnecessarily low level and instead use an approach that is more appropriate to frailty. We hope clinicians can use this guideline with the same enthusiasm applied to other guidelines, and we welcome discussion.
Acknowledgments: We would like to thank and acknowledge Tanya MacLeod and Kathryn Yuill for their review of and advice about the manuscript.
Frail older adults deserve guidelines that take frailty into account while assessing the potential benefit and risks of treatment.
Specifically, our group—the Dalhousie Academic Detailing Service (ADS) and the Palliative and Therapeutic Harmonization (PATH) program—recommends that physicians strive to achieve more liberal treatment targets for elderly frail patients who have high blood pressure,1 as evidence does not support an aggressive approach in the frail elderly and the potential exists for harm.
This article reviews the evidence and reasoning that were used to develop and promote a guideline for drug treatment of hypertension in frail older adults. Our recommendations differ from other guidelines in that they focus as much on stopping or decreasing therapy as on starting or increasing it.
FRAILTY INCREASES THE RISK OF ADVERSE EFFECTS
The word frail, applied to older adults, describes those who have complex medical illnesses severe enough to compromise their ability to live independently.2 Many have multiple coexisting medical problems for which they take numerous drugs, in addition to dementia, impaired mobility, compromised functional ability, or a history of falling.
Frailty denotes vulnerability; it increases the risk of adverse effects from medical and surgical procedures,3 complicates drug therapy,4 prolongs hospital length of stay,5 leads to functional and cognitive decline,6 increases the risk of institutionalization,7 and reduces life expectancy8—all of which affect the benefit and harm of medical treatments.
Guidelines for treating hypertension9–11 now acknowledge that little evidence exists to support starting treatment for systolic blood pressure between 140 and 160 mm Hg or aiming for a target of less than 140 mm Hg for “very old” adults, commonly defined as over the age of 80. New guidelines loosen the treatment targets for the very old, but they do not specify targets for the frail and do not describe how to recognize or measure frailty.
RECOGNIZING AND MEASURING FRAILTY
A number of tools are available to recognize and measure frailty.12
The Fried frailty assessment13 has five items:
- Unintentional weight loss
- Self-reported exhaustion
- Weakness in grip
- Slow walking speed
- Low physical activity and energy expenditure.
People are deemed frail if they have three or more of these five. However, experts disagree about whether this system is too sensitive14 or not sensitive enough.15,16
The FRAIL questionnaire17 also has five items:
- Fatigue
- Resistance (inability to climb stairs)
- Ambulation (inability to walk 1 city block)
- Illness (more than 5 major illnesses)
- Weight loss.
People are deemed frail if they have at least three of these five items, and “prefrail” if they have two.
These and other tools are limited by being dichotomous: they classify people as being either frail or not frail18–20 but do not define the spectrum of frailty.
Other frailty assessments such as the Frailty Index21 identify frailty based on the number of accumulated health deficits but take a long time to complete, making them difficult to use in busy clinical settings.22–24
The Clinical Frailty Scale7 is a validated scale that categorizes frailty based on physical and functional indicators of health, such as cognition, function, and mobility, with scores that range from 1 (very fit) to 9 (terminally ill).7,12
The Frailty Assessment for Care-planning Tool (FACT) uses scaling compatible with the Clinical Frailty Scale but has been developed for use as a practical and interpretable frailty screening tool for nonexperts (Table 1). The FACT assesses cognition, mobility, function, and the social situation, using a combination of caregiver report and objective measures. To assess cognition, a health care professional uses items from the Mini-Cog25 (ie, the ability to draw an analog clock face and then recall three unrelated items following the clock-drawing test) and the memory axis of the Brief Cognitive Rating Scale26 (ie, the ability to recall current events, the current US president, and the names of children or spouse). Mobility, function, and social circumstance scores are assigned according to the caregiver report of the patient’s baseline status.
The FACT can be completed in busy clinical settings. Once a caregiver is identified, it takes about 5 minutes to complete.
Our guideline27–31 is intended for those with a score of 7 or more on the Clinical Frailty Scale or FACT,7,12 a score we chose because it describes people who are severely frail with shortened life expectancy.8 At this level, people need help with all instrumental activities of daily living (eg, handling finances, medication management, household chores, and shopping) as well as with basic activities of daily living such as bathing or dressing.
REVIEWING THE LIMITED EVIDENCE
We found no studies that addressed the risks and benefits of treating hypertension in frail older adults; therefore, we concentrated on studies that enrolled individuals who were chronologically old but not frail. We reviewed prominent guidelines,9–11,32,33 the evidence base for these guidelines,34–44 and Cochrane reviews.45,46 A detailed description of the evidence used to build our recommendation can be found online.31
When we deliberated on treatment targets, we reviewed evidence from two types of randomized controlled trials47:
Drug treatment trials randomize patients to different treatments, such as placebo versus a drug or one drug compared with another drug. Patients in different treatment groups may achieve different blood pressures and clinical outcomes, and this information is then used to define optimal targets. However, it may be difficult to determine if the benefit came from lowering blood pressure or from some other effect of the drug, which can be independent of blood pressure lowering.
Treat-to-target trials randomize patients to different blood pressure goals, but the groups are treated with the same or similar drugs. Therefore, any identified benefit can be attributed to the differences in blood pressure rather than the medications used. Compared with a drug treatment trial, this type of trial provides stronger evidence about optimal targets.
We also considered the characteristics of frailty, the dilemma of polypharmacy, and the relevance of the available scientific evidence to those who are frail.
Drug treatment trials
A Cochrane review45 of 15 studies with approximately 24,000 elderly participants found that treating hypertension decreased the rates of cardiovascular morbidity and mortality as well as fatal and nonfatal stroke in the “elderly” (defined as age ≥ 60) and “very elderly” (age ≥ 80). However, in the very elderly, all-cause mortality rates were not statistically significantly different with treatment compared with placebo. The mean duration of treatment was 4.5 years in the elderly and 2.2 years in the very elderly (Table 2). Of importance, all the trials enrolled only those individuals whose systolic blood pressure was at least 160 mm Hg at baseline.
None of the studies were treat-to-target trials—patients were assigned either active medication or placebo. Thus, these trials provide evidence of benefit for treating hypertension in the elderly and very elderly but do not identify the optimal target. All of the drug treatment trials showed benefit, but none achieved a systolic pressure lower than 140 mm Hg with active treatment (Table 3). Therefore, these studies do not support a systolic target of less than 140 mm Hg in the elderly.
Treat-to-target trials: JATOS and VALISH
The Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (JATOS)42 and the Valsartan in Elderly Isolated Systolic Hypertension (VALISH) study43 each enrolled more than 3,000 people age 65 or older (mean age approximately 75). Patients were randomized to either a strict systolic target of less than 140 mm Hg or a higher (more permissive) target of 140 to 160 mm Hg in JATOS and 140 to 149 mm Hg in VALISH.
In both trials, the group with strict targets achieved a systolic pressure of approximately 136 mm Hg, while the group with higher blood pressure targets achieved a systolic pressure of 146 mm Hg in JATOS and 142 mm Hg in VALISH. Despite these differences, there was no statistically significant difference in the primary outcome.
Thus, treat-to-target studies also fail to support a systolic target of less than 140 mm Hg in the elderly, although it is important to recognize the limitations of the studies. Approximately 15% of the participants had cardiovascular disease, so the applicability of the findings to patients with target-organ damage is uncertain. In addition, there were fewer efficacy outcome events than expected, which suggests that the studies were underpowered.
When to start drug treatment
In each of the drug treatment and treat-to-target trials, the inclusion criterion for study entry was a systolic blood pressure above 160 mm Hg, with a mean blood pressure at entry into the drug treatment trials of 182/95 mm Hg.46 Thus, data support starting treatment if the systolic blood pressure is above 160 mm Hg, but not lower.
Notably, in all but one study,46 at least two-thirds of the participants took no more than two antihypertensive medications. Since adverse events become more common as the number of medications increases, the benefit of adding a third drug to lower blood pressure is uncertain.
Evidence in the ‘very elderly’: HYVET
With the exception of the Hypertension in the Very Elderly Trial (HYVET),44 the mean age of elderly patients in the reported studies was between 67 and 76.
HYVET patients were age 80 and older (mean age 84) and were randomized to receive either indapamide (with or without perindopril) or placebo. The trial was stopped early at 2 years because the mortality rate was lower in the treatment group (10.1%) than in the placebo group (12.3%) (number needed to treat 46, 95% confidence interval 24–637, P = .02). There was no significant difference in the primary outcome of fatal and nonfatal stroke.
Notably, trials that are stopped early may overestimate treatment benefit.48
Evidence in frail older adults
While the above studies provide some information about managing hypertension in the elderly, the participants were generally healthy. HYVET44 specifically excluded those with a standing systolic blood pressure of less than 140 mm Hg and enrolled few patients with orthostasis (7.9% in the placebo group and 8.8% in the treatment group), a condition commonly associated with frailty. As such, these studies may be less relevant to the frail elderly, who are at higher risk of adverse drug events and have competing risks for morbidity and mortality.
Observational studies, in fact, raise questions about whether tight blood pressure control improves clinical outcomes for the very elderly. In the Leiden 85-plus study, lower systolic blood pressure was associated with lower cognitive scores, worse functional ability,49,50 and a higher mortality rate51 compared with higher systolic pressure, although it is uncertain whether these outcomes were indicative of underlying disease that could result in lower blood pressure or an effect of blood pressure-lowering.
The National Health and Nutrition Examination Survey52 found an association between blood pressure and mortality rate that varied by walking speed. For slower walkers (based on the 6-minute walk test), higher systolic pressures were not associated with a higher risk of death, suggesting that when older adults are frail (as indicated by their slow walking speed) they are less likely to benefit from aggressive treatment of hypertension.
People at high risk because of stroke
Because the evidence is limited, it is even more difficult to judge whether lowering blood pressure below 140 mm Hg is beneficial for frail patients who have a history of stroke, compared with the possibility that medications will cause adverse effects such as weakness, orthostasis, and falls. When reviewing the evidence to answer this question, we especially looked at outcomes that affect quality of life, such as nonfatal stroke leading to disability. In contrast, because the frail elderly have competing causes of mortality, we could not assume that a mortality benefit shown in nonfrail populations could be applied to frail populations.
The PROGRESS trial (Perindopril Protection Against Recurrent Stroke Study)53 was in patients with a history of stroke or transient ischemic attack and a mean age of 64, who were treated with either perindopril (with or without indapamide) or placebo.
At almost 4 years, the rate of disabling stroke was 2.7% in the treatment group and 4.3% in the placebo group, a relative risk reduction of 38% and an absolute risk reduction of 1.64% (number needed to treat 61, 95% confidence interval 39–139). The relative risk reduction for all strokes (fatal and nonfatal) was similar across a range of baseline systolic pressures, but the absolute risk reduction was greater in the prespecified subgroup that had hypertension at baseline (mean blood pressure 159/94 mm Hg) than in the normotensive subgroup (mean blood pressure 136/79 mm Hg), suggesting that treatment is most beneficial for those with higher systolic blood pressures. Also, the benefit was only demonstrated in the subgroup that received two antihypertensive medications; those who received perindopril alone showed no benefit.
This study involved relatively young patients in relatively good health except for their strokes. The extent to which the results can be extrapolated to older, frail adults is uncertain because of the time needed to achieve benefit and because of the added vulnerability of frailty, which could make treatment with two antihypertensive medications riskier.
PRoFESS (Prevention Regimen for Effectively Avoiding Second Strokes),54 another study in patients with previous stroke (mean age 66) showed no benefit over 2.5 years in the primary outcome of stroke using telmesartan 80 mg daily compared with placebo. This result is concordant with that of PROGRESS,53 in which patients who took only one medication did not show a significant decrease in the rate of stroke.
A possible reason for the lack of benefit from monotherapy was that the differences in blood pressure between the placebo group and the treatment group on monotherapy were small in both studies (3.8/2.0 mm Hg in PRoFESS, 5/3 mm Hg in PROGRESS). In contrast, patients on dual therapy in PROGRESS decreased their blood pressure by 12/5 mm Hg compared with placebo.
CURRENT HYPERTENSION GUIDELINES
Current guidelines make reference to the elderly, but we found none that made specific recommendations for the frail elderly.
JNC 8
In December 2013, members of the Eighth Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 8) released new recommendations.32 One significant revision was to support higher blood pressure targets for older adults (age 60 and older). Whereas JNC 7 stated that lowering blood pressure below 140/90 mm Hg reduced cardiovascular complications,33 JNC 8 now acknowledges that there is no strong evidence to support blood pressure targets below 150/90 mm Hg for hypertensive persons without kidney disease or diabetes age 60 and older. Thus, in the general population age 60 and older, JNC 8 recommends starting antihypertensive treatment when blood pressure is 150/90 mm Hg or higher, and treating to a goal blood pressure of less than 150/90 mm Hg. JNC 8 makes no recommendation about how to adjust blood pressure targets for frailty or how to measure blood pressure.
American College of Cardiology and American Heart Association
In 2011, the American College of Cardiology and American Heart Association published a consensus document on the management of hypertension in the elderly.9
They acknowledged that the generally recommended blood pressure goal of lower than 140/90 mm Hg in uncomplicated elderly patients is based on expert opinion rather than on data from randomized controlled trials, but nevertheless recommended a target systolic pressure lower than 140 mm Hg for older adults, except for octogenarians.
For those over age 80, systolic levels of 140 to 145 mm Hg can be acceptable if tolerated and if the patient does not experience orthostasis when standing. Systolic pressure lower than 130 mm Hg and diastolic pressures lower than 65 mm Hg should be avoided in this age group.
The document acknowledges that systolic pressure may have to remain above 150 mm Hg if there is no response to four “well-selected drugs” or if there are unacceptable side effects. In these cases, the lowest “safely achieved” systolic blood pressure should be the goal.
Canadian Hypertension Education Program
The 2014 Canadian Hypertension Education Program (CHEP) report makes several recommendations for the “very elderly,” a group they define as over the age of 80. The CHEP website and resources include the following recommendations10:
- For the very elderly without diabetes or target-organ damage, drug therapy should be initiated when systolic blood pressure is higher than 160 mm Hg to reach a systolic blood pressure target lower than 150 mm Hg. This is a grade C level recommendation, indicating that it is based on low-quality trials, unvalidated surrogate outcomes, or results from nonrandomized observational studies.
- For the very elderly with macrovascular target-organ damage, antihypertensive therapy should be considered if systolic blood pressure readings average 140 mm Hg or higher (grade D for 140 to 160 mm Hg; grade A for higher than 160 mm Hg), although caution should be exercised in elderly patients who are frail. (Grade D recommendations are the weakest, as they are based on low-powered, imprecise studies or expert opinion, whereas grade A recommendations are based on the strongest evidence from high-quality randomized clinical trials.)
- Decisions regarding initiating and intensifying pharmacotherapy in the very elderly should be based on an individualized risk-benefit analysis.
The European Society of Hypertension and European Society of Cardiology
The 2013 guidelines from the European Society of Hypertension and the European Society of Cardiology11 recommend that for elderly patients under age 80, antihypertensive treatment may be considered at systolic values higher than 140 mm Hg and aimed at values lower than 140 mm Hg if the patient is fit and treatment is well tolerated.
For those over age 80 with an initial systolic pressure of 160 mm Hg or higher, the guidelines recommend lowering systolic pressure to between 150 and 140 mm Hg, provided the patient is in good physical and mental condition. In frail elderly patients, they recommend leaving decisions on antihypertensive therapy to the treating physician, based on monitoring of the clinical effects of treatment.11
The ADS/PATH guidelines
When finalizing our recommendations,1 we considered the characteristics of frailty and the following key points from the evidence:
- Although evidence from drug treatment trials indicates that there is benefit in treating healthy older adults who have hypertension, the benefit of treating frail older adults is unknown.
- Major trials enrolled elderly patients only if they had systolic blood pressures of at least 160 mm Hg. Therefore, evidence supports initiating pharmacotherapy at a systolic pressure of 160 mm Hg or higher.
- No evidence from randomized controlled trials supports a systolic target lower than 140 mm Hg in the elderly, and there is some evidence that such a target does not benefit.
- The benefit of adding a third medication to lower blood pressure has not been studied.
- Frailty makes the potential benefits of strict blood pressure targets even less certain and increases the possibility of harm from adverse drug events.
- The only study of very old adults, HYVET,44 enrolled relatively healthy older adults and few with orthostasis, while excluding those with a standing systolic blood pressure lower than 140 mm Hg.
OUR RECOMMENDATIONS
Based on the above, we advise against unnecessarily strict targets and recommend stopping antihypertensive medications that are used for the sole purpose of keeping the systolic blood pressure below 140 mm Hg. Our guidelines are unique in that they focus equally on when to stop and when to start medications. We concluded that without evidence of definitive benefit, “less is more” with frailty.55 We believe that if physicians and health professionals understand the limitations of the evidence, they can be more confident in stopping medications that lower blood pressure to an unnecessarily low level.
We recommend the following (Table 4):
Before treating
- Carefully review the risks and the potential but unproven benefits of treatment.
- To avoid overtreatment, treatment decisions should be based on blood pressure measurements in the seated (not supine) position, while also considering the presence of orthostasis.
- To evaluate orthostasis, measure blood pressure in the supine position, then immediately on standing, and again after 2 minutes. Ask the patient if he or she feels light-headed or dizzy when standing.
Stop treatment
- If the seated systolic blood pressure is less than 140 mm Hg, medications can be tapered and discontinued to achieve the targets described below.
- Before discontinuation, consider whether the medications are treating additional conditions such as rate control for atrial fibrillation or symptomatic management of heart failure.
- It is uncertain whether to discontinue treatment when there is a history of stroke. Consider that treatment with two medications resulted in an absolute risk reduction for disabling stroke of 1.64% over approximately 4 years for adults with previous stroke and a mean age of 64,57 an effect that may be more prominent at higher systolic pressures.
Start treatment
- Consider starting treatment when systolic pressure is 160 mm Hg or higher.
- Aim for a seated systolic pressure between 140 and 160 mm Hg if there are no adverse effects from treatment that affect quality of life.
- If there is symptomatic orthostasis or if standing systolic pressure is lower than 140 mm Hg, the target seated systolic pressure can be adjusted upwards.
- In the severely frail nearing the end of life, a target systolic pressure of 160 to 190 mm Hg is reasonable.
- The blood pressure target is the same in people with diabetes.
- In general, use no more than two medications.
Dissemination and implementation
The ADS/PATH guideline is intended for use by physicians and other health professionals (eg, pharmacists and nurses) who care for frail older adults or who work in long-term care facilities. Since creating our guideline, we have disseminated it to physicians, pharmacists, and other health professionals through academic detailing, large conferences, and interactive webinars.
While we do not have objective evidence of practice change, our evaluation data found that 34% of 403 family physicians who received academic detailing indicated that the guideline would change their practice, while 36% stated that the guideline confirmed their practice, an indication that family physicians are sensitive to the needs of the frail elderly.
Because health professionals may be wary of stopping medications and not meeting recommended targets, there may be barriers to adopting this guideline. However, our experience with the PATH program indicates that these barriers can be overcome using effective communication strategies between health professionals and consumers.
AN APPROACH APPROPRIATE TO FRAILTY
There is no direct evidence for systolic blood pressure targets in the frail elderly, so we applied evidence from the nonfrail elderly. Our recommendations differ somewhat from those of other groups, which recommend targets below 140 to 150 mm Hg for older adults, although some do advise caution in the elderly for whom a substantial fall in blood pressure might be poorly tolerated. Despite these messages, we believe that clearer guidance is needed to direct health practitioners toward models that acknowledge that frail patients are in a precarious balance of health and may be harmed by treatments that strive to lower blood pressure to unproven targets. For this reason, our guideline clearly indicates when to decrease or stop drug treatment.
After physicians and health professionals examine the evidence and more fully understand the benefits and harms of treating frail older adults, we are confident that they will be more comfortable stopping medications that lower blood pressure to an unnecessarily low level and instead use an approach that is more appropriate to frailty. We hope clinicians can use this guideline with the same enthusiasm applied to other guidelines, and we welcome discussion.
Acknowledgments: We would like to thank and acknowledge Tanya MacLeod and Kathryn Yuill for their review of and advice about the manuscript.
- Palliative and Therapeutic Harmonization program. Hypertension guidelines. Treating hypertension in frailty. http://pathclinic.ca/resources/hypertension/. Accessed May 2, 2014.
- Theou O, Rockwood MR, Mitnitski A, Rockwood K. Disability and co-morbidity in relation to frailty: how much do they overlap? Arch Gerontol Geriatr 2012; 55:e1–e8.
- Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg 2010; 210:901–908.
- Tinetti ME, Bogardus ST, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med 2004; 351:2870–2874.
- Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation 2011; 124:2397–2404.
- Theou O, Rockwood K. Should frailty status always be considered when treating the elderly patient? Aging Health 2012; 8:261–271.
- Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173:489–495.
- Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008; 8:24.
- Aronow WS, Fleg JL, Pepine CJ, et al; ACCF Task Force. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation 2011; 123:2434–2506.
- The Canadian Hypertension Education Program (CHEP). 2014 CHEP recommendations. www.hypertension.ca/en/. Accessed May 2, 2014.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34:2159–2219.
- Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013; 14:392–397.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Ensrud KE, Ewing SK, Cawthon PM, et al; Osteoporotic Fractures in Men Research Group. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc 2009; 57:492–498.
- Avila-Funes JA, Amieva H, Barberger-Gateau P, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J Am Geriatr Soc 2009; 57:453–461.
- Bergman H, Ferrucci L, Guralnik J, et al. Frailty: an emerging research and clinical paradigm—issues and controversies. J Gerontol A Biol Sci Med Sci 2007; 62:731–737.
- Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging 2012; 16:601–608.
- Strawbridge WJ, Shema SJ, Balfour JL, Higby HR, Kaplan GA. Antecedents of frailty over three decades in an older cohort. J Gerontol B Psychol Sci Soc Sci 1998; 53:S9–S16.
- Matthews M, Lucas A, Boland R, et al. Use of a questionnaire to screen for frailty in the elderly: an exploratory study. Aging Clin Exp Res 2004; 16:34–40.
- Salvi F, Morichi V, Grilli A, et al. Screening for frailty in elderly emergency department patients by using the Identification of Seniors At Risk (ISAR). J Nutr Health Aging 2012; 16:313–318.
- Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal 2001; 1:323–336.
- Kellen E, Bulens P, Deckx L, et al. Identifying an accurate pre-screening tool in geriatric oncology. Crit Rev Oncol Hematol 2010; 75:243–248.
- Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing 2006; 35:526–529.
- Martin FC, Brighton P. Frailty: different tools for different purposes? Age Ageing 2008; 37:129–131.
- Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini-cog: a cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry 2000; 15:1021–1027.
- Reisberg B, Ferris SH. Brief Cognitive Rating Scale (BCRS). Psychopharmacol Bull 1988; 24:629–636.
- Moorhouse P, Mallery LH. Palliative and therapeutic harmonization: a model for appropriate decision-making in frail older adults. J Am Geriatr Soc 2012; 60:2326–2332.
- Palliative and Therapeutic Harmonization Clinic (PATH). www.pathclinic.ca. Accessed May 2, 2014.
- Dalhousie University Faculty of Medicine: Continuing Medical Education. http://cme.medicine.dal.ca/ADS.htm. Accessed January 8, 2014.
- Mallery LH, Moorhouse P. Respecting frailty. J Med Ethics 2011; 37:126–128.
- Dalhousie University Faculty of Medicine: Continuing Medical Education. Issues in hypertension 2011. http://cme.medicine.dal.ca/files/Hypertension%20book.pdf. Accessed May 2, 2014.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Amery A, Birkenhäger W, Brixko P, et al. Mortality and morbidity results from the European Working Party on High Blood Pressure in the Elderly trial. Lancet 1985; 1:1349–1354.
- Coope J, Warrender TS. Randomised trial of treatment of hypertension in elderly patients in primary care. Br Med J (Clin Res Ed) 1986; 293:1145–1151.
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991; 265:3255–3264.
- Dahlöf B, Lindholm LH, Hansson L, Scherstén B, Ekbom T, Wester PO. Morbidity and mortality in the Swedish Trial in Old Patients with Hypertension (STOP-Hypertension). Lancet 1991; 338:1281–1285.
- Medical Research Council trial of treatment of hypertension in older adults: principal results. MRC Working Party. BMJ 1992; 304:405–412.
- Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet 1997; 350:757–764.
- Liu L, Wang JG, Gong L, Liu G, Staessen JA. Comparison of active treatment and placebo in older Chinese patients with isolated systolic hypertension. Systolic Hypertension in China (Syst-China) Collaborative Group. J Hypertens 1998; 16:1823–1829.
- Lithell H, Hansson L, Skoog I, et al; SCOPE Study Group. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens 2003; 21:875–886.
- JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008; 31:2115–2127.
- Oparil S, Yarows SA, Patel S, Fang H, Zhang J, Satlin A. Efficacy and safety of combined use of aliskiren and valsartan in patients with hypertension: a randomised, double-blind trial. Lancet 2007; 370:221–229.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Musini VM, Tejani AM, Bassett K, Wright JM. Pharmacotherapy for hypertension in the elderly. Cochrane Database Syst Rev 2009;CD000028.
- He FJ, MacGregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev 2004;CD004937.
- Allen M, Kelly K, Fleming I. Hypertension in elderly patients: recommended systolic targets are not evidence based [in French]. Can Fam Physician 2013; 59:19–24.
- Guyatt GH, Briel M, Glasziou P, Bassler D, Montori VM. Problems of stopping trials early. BMJ 2012; 344:e3863.
- Sabayan B, Oleksik AM, Maier AB, et al. High blood pressure and resilience to physical and cognitive decline in the oldest old: the Leiden 85-plus Study. J Am Geriatr Soc 2012; 60:2014–2019.
- Sabayan B, van Vliet P, de Ruijter W, Gussekloo J, de Craen AJ, Westendorp RG. High blood pressure, physical and cognitive function, and risk of stroke in the oldest old: the Leiden 85-plus Study. Stroke 2013; 44:15–20.
- Poortvliet RK, Blom JW, de Craen AJ, et al. Low blood pressure predicts increased mortality in very old age even without heart failure: the Leiden 85-plus Study. Eur J Heart Fail 2013; 15:528–533.
- Odden MC, Peralta CA, Haan MN, Covinsky KE. Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch Intern Med 2012; 172:1162–1168.
- PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001; 358:1033–1041.
- Yusuf S, Diener HC, Sacco RL, et al; PRoFESS Study Group. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med 2008; 359:1225–1237.
- Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med 2010; 170:1648–1654.
- Palliative and Therapeutic Harmonization program. Hypertension guidelines. Treating hypertension in frailty. http://pathclinic.ca/resources/hypertension/. Accessed May 2, 2014.
- Theou O, Rockwood MR, Mitnitski A, Rockwood K. Disability and co-morbidity in relation to frailty: how much do they overlap? Arch Gerontol Geriatr 2012; 55:e1–e8.
- Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg 2010; 210:901–908.
- Tinetti ME, Bogardus ST, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med 2004; 351:2870–2874.
- Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation 2011; 124:2397–2404.
- Theou O, Rockwood K. Should frailty status always be considered when treating the elderly patient? Aging Health 2012; 8:261–271.
- Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173:489–495.
- Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008; 8:24.
- Aronow WS, Fleg JL, Pepine CJ, et al; ACCF Task Force. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation 2011; 123:2434–2506.
- The Canadian Hypertension Education Program (CHEP). 2014 CHEP recommendations. www.hypertension.ca/en/. Accessed May 2, 2014.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34:2159–2219.
- Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013; 14:392–397.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Ensrud KE, Ewing SK, Cawthon PM, et al; Osteoporotic Fractures in Men Research Group. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc 2009; 57:492–498.
- Avila-Funes JA, Amieva H, Barberger-Gateau P, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J Am Geriatr Soc 2009; 57:453–461.
- Bergman H, Ferrucci L, Guralnik J, et al. Frailty: an emerging research and clinical paradigm—issues and controversies. J Gerontol A Biol Sci Med Sci 2007; 62:731–737.
- Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging 2012; 16:601–608.
- Strawbridge WJ, Shema SJ, Balfour JL, Higby HR, Kaplan GA. Antecedents of frailty over three decades in an older cohort. J Gerontol B Psychol Sci Soc Sci 1998; 53:S9–S16.
- Matthews M, Lucas A, Boland R, et al. Use of a questionnaire to screen for frailty in the elderly: an exploratory study. Aging Clin Exp Res 2004; 16:34–40.
- Salvi F, Morichi V, Grilli A, et al. Screening for frailty in elderly emergency department patients by using the Identification of Seniors At Risk (ISAR). J Nutr Health Aging 2012; 16:313–318.
- Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal 2001; 1:323–336.
- Kellen E, Bulens P, Deckx L, et al. Identifying an accurate pre-screening tool in geriatric oncology. Crit Rev Oncol Hematol 2010; 75:243–248.
- Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing 2006; 35:526–529.
- Martin FC, Brighton P. Frailty: different tools for different purposes? Age Ageing 2008; 37:129–131.
- Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini-cog: a cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry 2000; 15:1021–1027.
- Reisberg B, Ferris SH. Brief Cognitive Rating Scale (BCRS). Psychopharmacol Bull 1988; 24:629–636.
- Moorhouse P, Mallery LH. Palliative and therapeutic harmonization: a model for appropriate decision-making in frail older adults. J Am Geriatr Soc 2012; 60:2326–2332.
- Palliative and Therapeutic Harmonization Clinic (PATH). www.pathclinic.ca. Accessed May 2, 2014.
- Dalhousie University Faculty of Medicine: Continuing Medical Education. http://cme.medicine.dal.ca/ADS.htm. Accessed January 8, 2014.
- Mallery LH, Moorhouse P. Respecting frailty. J Med Ethics 2011; 37:126–128.
- Dalhousie University Faculty of Medicine: Continuing Medical Education. Issues in hypertension 2011. http://cme.medicine.dal.ca/files/Hypertension%20book.pdf. Accessed May 2, 2014.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Amery A, Birkenhäger W, Brixko P, et al. Mortality and morbidity results from the European Working Party on High Blood Pressure in the Elderly trial. Lancet 1985; 1:1349–1354.
- Coope J, Warrender TS. Randomised trial of treatment of hypertension in elderly patients in primary care. Br Med J (Clin Res Ed) 1986; 293:1145–1151.
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991; 265:3255–3264.
- Dahlöf B, Lindholm LH, Hansson L, Scherstén B, Ekbom T, Wester PO. Morbidity and mortality in the Swedish Trial in Old Patients with Hypertension (STOP-Hypertension). Lancet 1991; 338:1281–1285.
- Medical Research Council trial of treatment of hypertension in older adults: principal results. MRC Working Party. BMJ 1992; 304:405–412.
- Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet 1997; 350:757–764.
- Liu L, Wang JG, Gong L, Liu G, Staessen JA. Comparison of active treatment and placebo in older Chinese patients with isolated systolic hypertension. Systolic Hypertension in China (Syst-China) Collaborative Group. J Hypertens 1998; 16:1823–1829.
- Lithell H, Hansson L, Skoog I, et al; SCOPE Study Group. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens 2003; 21:875–886.
- JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008; 31:2115–2127.
- Oparil S, Yarows SA, Patel S, Fang H, Zhang J, Satlin A. Efficacy and safety of combined use of aliskiren and valsartan in patients with hypertension: a randomised, double-blind trial. Lancet 2007; 370:221–229.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Musini VM, Tejani AM, Bassett K, Wright JM. Pharmacotherapy for hypertension in the elderly. Cochrane Database Syst Rev 2009;CD000028.
- He FJ, MacGregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev 2004;CD004937.
- Allen M, Kelly K, Fleming I. Hypertension in elderly patients: recommended systolic targets are not evidence based [in French]. Can Fam Physician 2013; 59:19–24.
- Guyatt GH, Briel M, Glasziou P, Bassler D, Montori VM. Problems of stopping trials early. BMJ 2012; 344:e3863.
- Sabayan B, Oleksik AM, Maier AB, et al. High blood pressure and resilience to physical and cognitive decline in the oldest old: the Leiden 85-plus Study. J Am Geriatr Soc 2012; 60:2014–2019.
- Sabayan B, van Vliet P, de Ruijter W, Gussekloo J, de Craen AJ, Westendorp RG. High blood pressure, physical and cognitive function, and risk of stroke in the oldest old: the Leiden 85-plus Study. Stroke 2013; 44:15–20.
- Poortvliet RK, Blom JW, de Craen AJ, et al. Low blood pressure predicts increased mortality in very old age even without heart failure: the Leiden 85-plus Study. Eur J Heart Fail 2013; 15:528–533.
- Odden MC, Peralta CA, Haan MN, Covinsky KE. Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch Intern Med 2012; 172:1162–1168.
- PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001; 358:1033–1041.
- Yusuf S, Diener HC, Sacco RL, et al; PRoFESS Study Group. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med 2008; 359:1225–1237.
- Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med 2010; 170:1648–1654.
KEY POINTS
- For frail elderly patients, consider starting treatment if the systolic blood pressure is 160 mm Hg or higher.
- An appropriate target in this population is a seated systolic pressure between 140 and 160 mm Hg, as long as there is no orthostatic drop to less than 140 mm Hg upon standing from a lying position and treatment does not adversely affect quality of life.
- The blood pressure target does not need to be lower if the patient has diabetes. If the patient is severely frail and has a short life expectancy, a systolic target of 160 to 190 mm Hg may be reasonable.
- If the systolic pressure is below 140 mm Hg, antihypertensive medications can be reduced as long as they are not indicated for other conditions.
- In general, one should prescribe no more than two antihypertensive medications.