User login
Spectral gradient acoustic reflectometry aids diagnosis of acute otitis media and otitis media with effusion
Spectral gradient acoustic reflectometer (SGAR) is a technology to assist in the detection of middle ear fluid occurring in the context of diagnosing acute otitis media (AOM) and otitis media with effusion (OME). The technology involves sending a harmless, inaudible sonar-like sound wave from the emitter that goes through the tympanic membrane, hits the posterior wall of the middle ear space, and bounces back to the sound detector in the device. If there is only air in the middle ear space, the sound wave bounces back quickly, and you get a high reading. If the sound wave bounces back more slowly, there is middle ear effusion. The thicker the effusion, the more likely it is pus and an AOM or a chronic OME (depending on the clinical situation), causing the sound wave to bounce back more slowly and giving a low reading.
The specificity of a high reading is remarkable at around 95%, so a high reading is a big reassurance that middle ear effusion is absent. A lower reading suggests effusion and the lower it is, the greater the sensitivity. When I get an unexpected higher or lower reading, I go back and reexamine the patient.
I asked our nurses to compare the handheld tympanometer to the SGAR. They actually perform the testing, and I interpret it. The nurses said:
• The SGAR is easier to use because of how quickly a readout is obtained.
• If a child is crying or moving, they can still get a readout.
• You don’t have to change the tip of the SGAR for the size of the external ear canal.
• The SGAR is easier to read than the tympanometer.
• The SGAR is easier to interpret for the parents.
• You don’t have to get a seal with the ear canal with SGAR, as you do with a tympanometer.
• The SGAR uses a disposable tip.
I asked our office manager to look up our use of the SGAR and tympanometer during our everyday practice. We found that SGAR or tympanometry was used in 12% of patient encounters in which the diagnosis of AOM or OME was part of the chief complaint. The ratio of use was 3:1, favoring SGAR. The most frequent use was in 30% of patient encounters tied to the diagnosis of "otalgia" (388.70) because with that diagnosis, we are stating to parents and patients that there is no middle ear pathology seen on exam, and it is confirmed by a test using sonar waves with the SGAR device. Our nurse practitioners and physician assistants particularly find the use of the SGAR beneficial in helping to reassure the parents and patients that they have not missed an AOM or OME.
The billing code is the same for SGAR and tympanometry (92567), so the fee payment is the same for both tests. Our second most common use is in association with possible AOM (382.9) at 12% of visits. Third is OME (381.02) used in a follow-up visit to determine the presence and thickness of persisting effusion.
About one-quarter of children seen in our practice with a chief complaint of "earache" receive the diagnosis of otalgia, often confirmed by SGAR, and do not receive an antibiotic. Thus, they are offsetting the charge for the procedure by saving on the costs of antibiotics and the accumulation of excessive diagnoses of AOM and OME leading to ear tube surgeries and tonsillectomy/adenoidectomy. The diagnosis of AOM and OME requires a middle ear effusion to be accurate, and only SGAR measures detection of middle ear effusion. SGAR is a must own device for clinicians who exam ears. SGAR can help in conjunction with otoscopy for a difficult diagnosis of AOM. If I am having troubleremoving wax, or if the external ear canal is particularly curved, or if I’m on the fence or the parent seems to need further evidence of my diagnosis, I turn to the SGAR. If I can get a reading, then it can really help, and my nurses are successful in getting a reading about 90% of the time. The main issue is ear canal wax, because occlusion by wax of more than 50% of the external ear canal opening causes invalid readings.
We should prescribe antibiotics for AOM in my opinion, but not for otalgia and not if the diagnosis is uncertain. The SGAR device when properly used can help to reduce unnecessary use of antibiotics and their complications. In prior "ID Consult" columns, I have discussed improving the diagnostic accuracy of AOM and OME. Performing a good otoscopic exam with the best tools available and combining that exam with SGAR or tympanometry, in selected cases, is the best practice in my opinion, and what I do in my own practice.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester General Hospital, N.Y. He is also a pediatrician at Legacy Pediatrics in Rochester. E-mail him at [email protected]. Innovia Medical, the company that is bringing the SGAR EarCheck Pro back to market in 2014 after improvement and the addition of a USB port to allow the import of the data readout into the electronic medical record, asked Dr. Pichichero to assess the SGAR device.
Spectral gradient acoustic reflectometer (SGAR) is a technology to assist in the detection of middle ear fluid occurring in the context of diagnosing acute otitis media (AOM) and otitis media with effusion (OME). The technology involves sending a harmless, inaudible sonar-like sound wave from the emitter that goes through the tympanic membrane, hits the posterior wall of the middle ear space, and bounces back to the sound detector in the device. If there is only air in the middle ear space, the sound wave bounces back quickly, and you get a high reading. If the sound wave bounces back more slowly, there is middle ear effusion. The thicker the effusion, the more likely it is pus and an AOM or a chronic OME (depending on the clinical situation), causing the sound wave to bounce back more slowly and giving a low reading.
The specificity of a high reading is remarkable at around 95%, so a high reading is a big reassurance that middle ear effusion is absent. A lower reading suggests effusion and the lower it is, the greater the sensitivity. When I get an unexpected higher or lower reading, I go back and reexamine the patient.
I asked our nurses to compare the handheld tympanometer to the SGAR. They actually perform the testing, and I interpret it. The nurses said:
• The SGAR is easier to use because of how quickly a readout is obtained.
• If a child is crying or moving, they can still get a readout.
• You don’t have to change the tip of the SGAR for the size of the external ear canal.
• The SGAR is easier to read than the tympanometer.
• The SGAR is easier to interpret for the parents.
• You don’t have to get a seal with the ear canal with SGAR, as you do with a tympanometer.
• The SGAR uses a disposable tip.
I asked our office manager to look up our use of the SGAR and tympanometer during our everyday practice. We found that SGAR or tympanometry was used in 12% of patient encounters in which the diagnosis of AOM or OME was part of the chief complaint. The ratio of use was 3:1, favoring SGAR. The most frequent use was in 30% of patient encounters tied to the diagnosis of "otalgia" (388.70) because with that diagnosis, we are stating to parents and patients that there is no middle ear pathology seen on exam, and it is confirmed by a test using sonar waves with the SGAR device. Our nurse practitioners and physician assistants particularly find the use of the SGAR beneficial in helping to reassure the parents and patients that they have not missed an AOM or OME.
The billing code is the same for SGAR and tympanometry (92567), so the fee payment is the same for both tests. Our second most common use is in association with possible AOM (382.9) at 12% of visits. Third is OME (381.02) used in a follow-up visit to determine the presence and thickness of persisting effusion.
About one-quarter of children seen in our practice with a chief complaint of "earache" receive the diagnosis of otalgia, often confirmed by SGAR, and do not receive an antibiotic. Thus, they are offsetting the charge for the procedure by saving on the costs of antibiotics and the accumulation of excessive diagnoses of AOM and OME leading to ear tube surgeries and tonsillectomy/adenoidectomy. The diagnosis of AOM and OME requires a middle ear effusion to be accurate, and only SGAR measures detection of middle ear effusion. SGAR is a must own device for clinicians who exam ears. SGAR can help in conjunction with otoscopy for a difficult diagnosis of AOM. If I am having troubleremoving wax, or if the external ear canal is particularly curved, or if I’m on the fence or the parent seems to need further evidence of my diagnosis, I turn to the SGAR. If I can get a reading, then it can really help, and my nurses are successful in getting a reading about 90% of the time. The main issue is ear canal wax, because occlusion by wax of more than 50% of the external ear canal opening causes invalid readings.
We should prescribe antibiotics for AOM in my opinion, but not for otalgia and not if the diagnosis is uncertain. The SGAR device when properly used can help to reduce unnecessary use of antibiotics and their complications. In prior "ID Consult" columns, I have discussed improving the diagnostic accuracy of AOM and OME. Performing a good otoscopic exam with the best tools available and combining that exam with SGAR or tympanometry, in selected cases, is the best practice in my opinion, and what I do in my own practice.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester General Hospital, N.Y. He is also a pediatrician at Legacy Pediatrics in Rochester. E-mail him at [email protected]. Innovia Medical, the company that is bringing the SGAR EarCheck Pro back to market in 2014 after improvement and the addition of a USB port to allow the import of the data readout into the electronic medical record, asked Dr. Pichichero to assess the SGAR device.
Spectral gradient acoustic reflectometer (SGAR) is a technology to assist in the detection of middle ear fluid occurring in the context of diagnosing acute otitis media (AOM) and otitis media with effusion (OME). The technology involves sending a harmless, inaudible sonar-like sound wave from the emitter that goes through the tympanic membrane, hits the posterior wall of the middle ear space, and bounces back to the sound detector in the device. If there is only air in the middle ear space, the sound wave bounces back quickly, and you get a high reading. If the sound wave bounces back more slowly, there is middle ear effusion. The thicker the effusion, the more likely it is pus and an AOM or a chronic OME (depending on the clinical situation), causing the sound wave to bounce back more slowly and giving a low reading.
The specificity of a high reading is remarkable at around 95%, so a high reading is a big reassurance that middle ear effusion is absent. A lower reading suggests effusion and the lower it is, the greater the sensitivity. When I get an unexpected higher or lower reading, I go back and reexamine the patient.
I asked our nurses to compare the handheld tympanometer to the SGAR. They actually perform the testing, and I interpret it. The nurses said:
• The SGAR is easier to use because of how quickly a readout is obtained.
• If a child is crying or moving, they can still get a readout.
• You don’t have to change the tip of the SGAR for the size of the external ear canal.
• The SGAR is easier to read than the tympanometer.
• The SGAR is easier to interpret for the parents.
• You don’t have to get a seal with the ear canal with SGAR, as you do with a tympanometer.
• The SGAR uses a disposable tip.
I asked our office manager to look up our use of the SGAR and tympanometer during our everyday practice. We found that SGAR or tympanometry was used in 12% of patient encounters in which the diagnosis of AOM or OME was part of the chief complaint. The ratio of use was 3:1, favoring SGAR. The most frequent use was in 30% of patient encounters tied to the diagnosis of "otalgia" (388.70) because with that diagnosis, we are stating to parents and patients that there is no middle ear pathology seen on exam, and it is confirmed by a test using sonar waves with the SGAR device. Our nurse practitioners and physician assistants particularly find the use of the SGAR beneficial in helping to reassure the parents and patients that they have not missed an AOM or OME.
The billing code is the same for SGAR and tympanometry (92567), so the fee payment is the same for both tests. Our second most common use is in association with possible AOM (382.9) at 12% of visits. Third is OME (381.02) used in a follow-up visit to determine the presence and thickness of persisting effusion.
About one-quarter of children seen in our practice with a chief complaint of "earache" receive the diagnosis of otalgia, often confirmed by SGAR, and do not receive an antibiotic. Thus, they are offsetting the charge for the procedure by saving on the costs of antibiotics and the accumulation of excessive diagnoses of AOM and OME leading to ear tube surgeries and tonsillectomy/adenoidectomy. The diagnosis of AOM and OME requires a middle ear effusion to be accurate, and only SGAR measures detection of middle ear effusion. SGAR is a must own device for clinicians who exam ears. SGAR can help in conjunction with otoscopy for a difficult diagnosis of AOM. If I am having troubleremoving wax, or if the external ear canal is particularly curved, or if I’m on the fence or the parent seems to need further evidence of my diagnosis, I turn to the SGAR. If I can get a reading, then it can really help, and my nurses are successful in getting a reading about 90% of the time. The main issue is ear canal wax, because occlusion by wax of more than 50% of the external ear canal opening causes invalid readings.
We should prescribe antibiotics for AOM in my opinion, but not for otalgia and not if the diagnosis is uncertain. The SGAR device when properly used can help to reduce unnecessary use of antibiotics and their complications. In prior "ID Consult" columns, I have discussed improving the diagnostic accuracy of AOM and OME. Performing a good otoscopic exam with the best tools available and combining that exam with SGAR or tympanometry, in selected cases, is the best practice in my opinion, and what I do in my own practice.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester General Hospital, N.Y. He is also a pediatrician at Legacy Pediatrics in Rochester. E-mail him at [email protected]. Innovia Medical, the company that is bringing the SGAR EarCheck Pro back to market in 2014 after improvement and the addition of a USB port to allow the import of the data readout into the electronic medical record, asked Dr. Pichichero to assess the SGAR device.
Improving diagnosis of otitis media
The diagnosis of otitis media absolutely requires visualization of the tympanic membrane. So it may be time to upgrade your tools to do a better job in diagnosing. Think about how often you use your otoscope. Are you using the best available technology, or are you using the otoscope you got in medical school, perhaps quite a few years ago? It may be time for an upgrade. Considering how often you might use an otoscope, you can afford it. You deserve it.
The improved features of new otoscopes include remarkably better illumination. The quality of the light not only has to do with the lumens, but also the color of the light. Also there is a version of an otoscope called a Macro View (Welch Allyn, Skaneateles Falls, N.Y.). It allows you to increase the magnification on the tympanic membrane (TM) as needed. There is an option to purchase a lighter and smaller handle for the scope, and that can improve ease of use for persons with small hands.
For all otoscopes, the bulb should be replaced when illumination begins to fade and you cannot get back the intensity of light with a battery recharge. For most primary care practitioners, bulbs usually require replacement annually.
Speculum size is key to getting the most light onto the TM; the bigger the speculum, the better. Advancing the speculum as far into the external ear canal as you can without causing discomfort helps improve the intensity of the light shone on the TM. While it is convenient to use disposable specula, they are not as good as reusable ones because the finish on the inside of disposable specula is duller than on reusable specula, thus decreasing the amount of light shone on the TM. Also, disposable specula often are too short, and that too reduces the light shone on the TM.
Many clinicians have not been trained on using pneumatic otoscopy, or even if trained, they find it inconvenient and/or problematic to use because it requires a seal of the speculum against the external auditory canal; this makes children cry. The problem is that you really need to use pneumatic otoscopy in some cases to determine if the TM is retracted (no acute infection) or bulging (acute infection, or AOM). I use pneumatic otoscopy in about one-third of cases, and to this day I am surprised sometimes when the negative pressure pulls a retracted TM forward when I was pretty sure the TM more likely was bulging. There are specula with a semisoft sleeve midway down the shaft, but I have not found they are any less likely to cause the child to cry, because as anyone knows who has stuck a Q-tip swab into their ear canal, it is sensitive skin.
Then there is the wax! Clinical studies show that about half of children have wax in their external auditory canal blocking 25% of the view, and one-quarter have wax blocking 50% of the view. The best tool I have found to clear the wax is a plastic cerumen spoon (called a safe ear curette) made by Bionix Medical Technologies (Toledo, Ohio). I use the white ones as they are the most flexible. Ninety percent of the time I can scoop the wax out of the way and get a good view. For the remaining difficult cases, the ear canal needs to be irrigated with warm water (code 69210), and then the remaining wax can be scooped out.
Tympanometry (code 92567) is another tool to aid in accurate diagnosis and follow-up of otitis media. A key aspect of the diagnostic algorithm advocated by the American Academy of Pediatrics is a determination of whether the TM is bulging (AOM) or not (no AOM). A retracted TM is inconsistent with the diagnosis of AOM. Tympanometry requires a seal with the external auditory canal because a pressure is applied to the TM to determine TM movement. After positive and negative pressure are applied by the instrument, the readout will be a positive peaked curve (bulging), a negative peaked curve (retracted), a normal peaked curve (normal), or flat, no curve (stiff TM).
The first three readouts are very helpful in distinguishing AOM from no AOM. The flat curve indicates three possibilities: The TM is stiff, perhaps due to thickening; the TM is not moving because the middle ear space is filled with pus behind it, meaning it is AOM; or the TM is not moving because the middle ear space is filled with effusion fluid behind it, meaning the patient has otitis media with effusion. In the case of a flat readout, the tie breaker should come from the visual exam and/or the use of spectral gradient acoustic reflectometry (code 92567).
These better tools and techniques should improve your diagnosis of otitis media.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester General Hospital, N.Y. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said he had no financial disclosures relevant to this article. To comment, e-mail him at [email protected].
The diagnosis of otitis media absolutely requires visualization of the tympanic membrane. So it may be time to upgrade your tools to do a better job in diagnosing. Think about how often you use your otoscope. Are you using the best available technology, or are you using the otoscope you got in medical school, perhaps quite a few years ago? It may be time for an upgrade. Considering how often you might use an otoscope, you can afford it. You deserve it.
The improved features of new otoscopes include remarkably better illumination. The quality of the light not only has to do with the lumens, but also the color of the light. Also there is a version of an otoscope called a Macro View (Welch Allyn, Skaneateles Falls, N.Y.). It allows you to increase the magnification on the tympanic membrane (TM) as needed. There is an option to purchase a lighter and smaller handle for the scope, and that can improve ease of use for persons with small hands.
For all otoscopes, the bulb should be replaced when illumination begins to fade and you cannot get back the intensity of light with a battery recharge. For most primary care practitioners, bulbs usually require replacement annually.
Speculum size is key to getting the most light onto the TM; the bigger the speculum, the better. Advancing the speculum as far into the external ear canal as you can without causing discomfort helps improve the intensity of the light shone on the TM. While it is convenient to use disposable specula, they are not as good as reusable ones because the finish on the inside of disposable specula is duller than on reusable specula, thus decreasing the amount of light shone on the TM. Also, disposable specula often are too short, and that too reduces the light shone on the TM.
Many clinicians have not been trained on using pneumatic otoscopy, or even if trained, they find it inconvenient and/or problematic to use because it requires a seal of the speculum against the external auditory canal; this makes children cry. The problem is that you really need to use pneumatic otoscopy in some cases to determine if the TM is retracted (no acute infection) or bulging (acute infection, or AOM). I use pneumatic otoscopy in about one-third of cases, and to this day I am surprised sometimes when the negative pressure pulls a retracted TM forward when I was pretty sure the TM more likely was bulging. There are specula with a semisoft sleeve midway down the shaft, but I have not found they are any less likely to cause the child to cry, because as anyone knows who has stuck a Q-tip swab into their ear canal, it is sensitive skin.
Then there is the wax! Clinical studies show that about half of children have wax in their external auditory canal blocking 25% of the view, and one-quarter have wax blocking 50% of the view. The best tool I have found to clear the wax is a plastic cerumen spoon (called a safe ear curette) made by Bionix Medical Technologies (Toledo, Ohio). I use the white ones as they are the most flexible. Ninety percent of the time I can scoop the wax out of the way and get a good view. For the remaining difficult cases, the ear canal needs to be irrigated with warm water (code 69210), and then the remaining wax can be scooped out.
Tympanometry (code 92567) is another tool to aid in accurate diagnosis and follow-up of otitis media. A key aspect of the diagnostic algorithm advocated by the American Academy of Pediatrics is a determination of whether the TM is bulging (AOM) or not (no AOM). A retracted TM is inconsistent with the diagnosis of AOM. Tympanometry requires a seal with the external auditory canal because a pressure is applied to the TM to determine TM movement. After positive and negative pressure are applied by the instrument, the readout will be a positive peaked curve (bulging), a negative peaked curve (retracted), a normal peaked curve (normal), or flat, no curve (stiff TM).
The first three readouts are very helpful in distinguishing AOM from no AOM. The flat curve indicates three possibilities: The TM is stiff, perhaps due to thickening; the TM is not moving because the middle ear space is filled with pus behind it, meaning it is AOM; or the TM is not moving because the middle ear space is filled with effusion fluid behind it, meaning the patient has otitis media with effusion. In the case of a flat readout, the tie breaker should come from the visual exam and/or the use of spectral gradient acoustic reflectometry (code 92567).
These better tools and techniques should improve your diagnosis of otitis media.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester General Hospital, N.Y. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said he had no financial disclosures relevant to this article. To comment, e-mail him at [email protected].
The diagnosis of otitis media absolutely requires visualization of the tympanic membrane. So it may be time to upgrade your tools to do a better job in diagnosing. Think about how often you use your otoscope. Are you using the best available technology, or are you using the otoscope you got in medical school, perhaps quite a few years ago? It may be time for an upgrade. Considering how often you might use an otoscope, you can afford it. You deserve it.
The improved features of new otoscopes include remarkably better illumination. The quality of the light not only has to do with the lumens, but also the color of the light. Also there is a version of an otoscope called a Macro View (Welch Allyn, Skaneateles Falls, N.Y.). It allows you to increase the magnification on the tympanic membrane (TM) as needed. There is an option to purchase a lighter and smaller handle for the scope, and that can improve ease of use for persons with small hands.
For all otoscopes, the bulb should be replaced when illumination begins to fade and you cannot get back the intensity of light with a battery recharge. For most primary care practitioners, bulbs usually require replacement annually.
Speculum size is key to getting the most light onto the TM; the bigger the speculum, the better. Advancing the speculum as far into the external ear canal as you can without causing discomfort helps improve the intensity of the light shone on the TM. While it is convenient to use disposable specula, they are not as good as reusable ones because the finish on the inside of disposable specula is duller than on reusable specula, thus decreasing the amount of light shone on the TM. Also, disposable specula often are too short, and that too reduces the light shone on the TM.
Many clinicians have not been trained on using pneumatic otoscopy, or even if trained, they find it inconvenient and/or problematic to use because it requires a seal of the speculum against the external auditory canal; this makes children cry. The problem is that you really need to use pneumatic otoscopy in some cases to determine if the TM is retracted (no acute infection) or bulging (acute infection, or AOM). I use pneumatic otoscopy in about one-third of cases, and to this day I am surprised sometimes when the negative pressure pulls a retracted TM forward when I was pretty sure the TM more likely was bulging. There are specula with a semisoft sleeve midway down the shaft, but I have not found they are any less likely to cause the child to cry, because as anyone knows who has stuck a Q-tip swab into their ear canal, it is sensitive skin.
Then there is the wax! Clinical studies show that about half of children have wax in their external auditory canal blocking 25% of the view, and one-quarter have wax blocking 50% of the view. The best tool I have found to clear the wax is a plastic cerumen spoon (called a safe ear curette) made by Bionix Medical Technologies (Toledo, Ohio). I use the white ones as they are the most flexible. Ninety percent of the time I can scoop the wax out of the way and get a good view. For the remaining difficult cases, the ear canal needs to be irrigated with warm water (code 69210), and then the remaining wax can be scooped out.
Tympanometry (code 92567) is another tool to aid in accurate diagnosis and follow-up of otitis media. A key aspect of the diagnostic algorithm advocated by the American Academy of Pediatrics is a determination of whether the TM is bulging (AOM) or not (no AOM). A retracted TM is inconsistent with the diagnosis of AOM. Tympanometry requires a seal with the external auditory canal because a pressure is applied to the TM to determine TM movement. After positive and negative pressure are applied by the instrument, the readout will be a positive peaked curve (bulging), a negative peaked curve (retracted), a normal peaked curve (normal), or flat, no curve (stiff TM).
The first three readouts are very helpful in distinguishing AOM from no AOM. The flat curve indicates three possibilities: The TM is stiff, perhaps due to thickening; the TM is not moving because the middle ear space is filled with pus behind it, meaning it is AOM; or the TM is not moving because the middle ear space is filled with effusion fluid behind it, meaning the patient has otitis media with effusion. In the case of a flat readout, the tie breaker should come from the visual exam and/or the use of spectral gradient acoustic reflectometry (code 92567).
These better tools and techniques should improve your diagnosis of otitis media.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester General Hospital, N.Y. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said he had no financial disclosures relevant to this article. To comment, e-mail him at [email protected].
The human microbiome
Microbiome refers to all the microbial life that exists in a specific niche. In the case of humans that means a lot of bacteria, viruses, fungi, parasites, and a very old class of single-celled organisms called archaea. The organisms include commensals and pathogenic microorganisms. Many articles distinguish "microbiome" and "microbiota" to differentiate the collective genomes of the microorganisms or the microorganisms themselves, respectively. However, these terms are largely synonymous.
A number of advances have allowed scientists to make major advances in understanding the microbiome. Specifically, we now have the molecular tools to perform gene expression analysis for an entire microbial community in the new discipline of metagenomics and analyze the massive results with new methods of mathematical analysis.
The human body contains over 10 times more microorganisms than human cells. The existence of a remarkably diverse and enormously large microbial world on us and in us first began to come to light in the late 1990s. We are learning more and more about the individual locations of the human host that have different populations of microbes and about differences among humans that contribute to or account for susceptibility to infectious diseases as well as autoimmune diseases and even obesity and cancer.
The nasopharyngeal microbiome has become an area of research by our group led by Qingfu Xu, Ph.D., at the Rochester (N.Y.) General Hospital Research Institute in collaboration with Melinda M. Pettigrew, Ph.D., at the Yale School of Public Health, New Haven, Conn., and Dr. Janet R. Casey at Legacy Pediatrics, also in Rochester. The traditional view of the immune system is undergoing reassessment as we learn that our microbiota has coevolved with our immune system, and each exerts influence over the other. Our group has a special interest in the impact of the nasopharyngeal microbiome on the innate immune response in that physiologic niche, and the way the innate immune system modifies the microbiome. With a special interest in the bacteria that cause respiratory infections such as acute otitis media, acute sinusitis, bronchopneumonia, and pneumonia, we have identified how microbes like Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis compete and synergize in the nasopharynx to cause infections.
Also, we seek to better understand how respiratory viruses like respiratory syncytial virus (RSV), influenzae, parainfluenzae, rhinovirus, and others facilitate the overgrowth of S. pneumoniae, H. flu, and M. catarrhalis in the nose such that they convert from commensals to pathogens. But the synergy goes both ways, as we have recently found that S. pneumoniae facilitates upper respiratory viral infections.
Up to now most of the work on the human microbiome has focused on the gut, and nearly all studies have occurred in adults. Perhaps readers are aware of the use of "fecal microbiota transplantation" as a treatment/cure for Clostridium difficile infection. Unhealthy gut microbiota in premature neonates are a major contributing factor in necrotizing enterocolitis.
For decades, physicians have been taught that obesity is a problem derived from excessive caloric intake and inadequate caloric consumption through activity, plus vaguely defined differences in "metabolism." As a consequence, we checked for hypothyroidism � I never found a case. New research has shown that there is a difference in the "metabolism" of obese patients, but the difference is how the individual gut microbiota metabolizes our food. It turns out the thinner individuals have a microbiota that is less efficient in breaking down the food we ingest to allow efficient absorption into the bloodstream, whereas obese individuals have a more efficient microbiota that facilitates absorption of a greater percentage of the proteins, carbohydrates, and fats that are ingested. So the pathway to treatment of obesity may lie in the study of the microbiome!
It turns out that the microbiota of the skin is highly diverse. The microbiota colonizing the antecubital fossa is different from that of the forearm or biceps or axillae. When atopic dermatitis flares, it is often in the antecubital fossa, and it is caused by overgrowth of Staphylococcus aureus. The microbiome of a patient with atopic dermatitis is different from that of a person without atopic dermatitis, and the former microbiota is more permissive to S. aureus becoming a pathogen rather than a commensal of the skin.
Prevention of urogenital infections in girls depends on a healthy vaginal microbiota. Bacterial vaginosis requires the establishment of overgrowth by Gardnerella vaginalis and Peptostreptococcus anaerobius that can only occur if the resident microbiota is unable to control the proliferation of these bacteria. Only if the microbiota of the perineum, urethra, and bladder will allow potential urinary tract infection pathogens access to epithelial attachment sites can infection become established.
A last topic for this column is the role of the microbiota in autoimmune diseases. In particular, I find it fascinating to learn that aberrant, unstable intestinal microbiota can lead to a leaky intestinal mucosal barrier. Combined with inadequate innate immune responses in the gut, progression may occur that allows antigens from microbes that cross-react with antigens of self in the pancreas to stimulate autoimmune antibodies. Similar pathogenic mechanisms may contribute to inflammatory bowel disease, rheumatoid arthritis, multiple sclerosis, and other autoimmune diseases.
I anticipate future research will establish the makeup of a healthy microbiota associated with protection from the diseases mentioned here. With that knowledge, the next efforts in research will focus on how to convert an unhealthy microbiota to a healthy one. If the efforts succeed, I see new promising treatments in the future.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Rochester (N.Y.) General Research Institute. He is also a pediatrician at Legacy Pediatrics in Rochester. The microbiome research at the Rochester General Hospital Research Institute is supported by the National Institutes of Health and the National Institute for Deafness and Communication Disorders. To comment, e-mail him at pdnews@ frontlinemedcom.com.
Microbiome refers to all the microbial life that exists in a specific niche. In the case of humans that means a lot of bacteria, viruses, fungi, parasites, and a very old class of single-celled organisms called archaea. The organisms include commensals and pathogenic microorganisms. Many articles distinguish "microbiome" and "microbiota" to differentiate the collective genomes of the microorganisms or the microorganisms themselves, respectively. However, these terms are largely synonymous.
A number of advances have allowed scientists to make major advances in understanding the microbiome. Specifically, we now have the molecular tools to perform gene expression analysis for an entire microbial community in the new discipline of metagenomics and analyze the massive results with new methods of mathematical analysis.
The human body contains over 10 times more microorganisms than human cells. The existence of a remarkably diverse and enormously large microbial world on us and in us first began to come to light in the late 1990s. We are learning more and more about the individual locations of the human host that have different populations of microbes and about differences among humans that contribute to or account for susceptibility to infectious diseases as well as autoimmune diseases and even obesity and cancer.
The nasopharyngeal microbiome has become an area of research by our group led by Qingfu Xu, Ph.D., at the Rochester (N.Y.) General Hospital Research Institute in collaboration with Melinda M. Pettigrew, Ph.D., at the Yale School of Public Health, New Haven, Conn., and Dr. Janet R. Casey at Legacy Pediatrics, also in Rochester. The traditional view of the immune system is undergoing reassessment as we learn that our microbiota has coevolved with our immune system, and each exerts influence over the other. Our group has a special interest in the impact of the nasopharyngeal microbiome on the innate immune response in that physiologic niche, and the way the innate immune system modifies the microbiome. With a special interest in the bacteria that cause respiratory infections such as acute otitis media, acute sinusitis, bronchopneumonia, and pneumonia, we have identified how microbes like Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis compete and synergize in the nasopharynx to cause infections.
Also, we seek to better understand how respiratory viruses like respiratory syncytial virus (RSV), influenzae, parainfluenzae, rhinovirus, and others facilitate the overgrowth of S. pneumoniae, H. flu, and M. catarrhalis in the nose such that they convert from commensals to pathogens. But the synergy goes both ways, as we have recently found that S. pneumoniae facilitates upper respiratory viral infections.
Up to now most of the work on the human microbiome has focused on the gut, and nearly all studies have occurred in adults. Perhaps readers are aware of the use of "fecal microbiota transplantation" as a treatment/cure for Clostridium difficile infection. Unhealthy gut microbiota in premature neonates are a major contributing factor in necrotizing enterocolitis.
For decades, physicians have been taught that obesity is a problem derived from excessive caloric intake and inadequate caloric consumption through activity, plus vaguely defined differences in "metabolism." As a consequence, we checked for hypothyroidism � I never found a case. New research has shown that there is a difference in the "metabolism" of obese patients, but the difference is how the individual gut microbiota metabolizes our food. It turns out the thinner individuals have a microbiota that is less efficient in breaking down the food we ingest to allow efficient absorption into the bloodstream, whereas obese individuals have a more efficient microbiota that facilitates absorption of a greater percentage of the proteins, carbohydrates, and fats that are ingested. So the pathway to treatment of obesity may lie in the study of the microbiome!
It turns out that the microbiota of the skin is highly diverse. The microbiota colonizing the antecubital fossa is different from that of the forearm or biceps or axillae. When atopic dermatitis flares, it is often in the antecubital fossa, and it is caused by overgrowth of Staphylococcus aureus. The microbiome of a patient with atopic dermatitis is different from that of a person without atopic dermatitis, and the former microbiota is more permissive to S. aureus becoming a pathogen rather than a commensal of the skin.
Prevention of urogenital infections in girls depends on a healthy vaginal microbiota. Bacterial vaginosis requires the establishment of overgrowth by Gardnerella vaginalis and Peptostreptococcus anaerobius that can only occur if the resident microbiota is unable to control the proliferation of these bacteria. Only if the microbiota of the perineum, urethra, and bladder will allow potential urinary tract infection pathogens access to epithelial attachment sites can infection become established.
A last topic for this column is the role of the microbiota in autoimmune diseases. In particular, I find it fascinating to learn that aberrant, unstable intestinal microbiota can lead to a leaky intestinal mucosal barrier. Combined with inadequate innate immune responses in the gut, progression may occur that allows antigens from microbes that cross-react with antigens of self in the pancreas to stimulate autoimmune antibodies. Similar pathogenic mechanisms may contribute to inflammatory bowel disease, rheumatoid arthritis, multiple sclerosis, and other autoimmune diseases.
I anticipate future research will establish the makeup of a healthy microbiota associated with protection from the diseases mentioned here. With that knowledge, the next efforts in research will focus on how to convert an unhealthy microbiota to a healthy one. If the efforts succeed, I see new promising treatments in the future.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Rochester (N.Y.) General Research Institute. He is also a pediatrician at Legacy Pediatrics in Rochester. The microbiome research at the Rochester General Hospital Research Institute is supported by the National Institutes of Health and the National Institute for Deafness and Communication Disorders. To comment, e-mail him at pdnews@ frontlinemedcom.com.
Microbiome refers to all the microbial life that exists in a specific niche. In the case of humans that means a lot of bacteria, viruses, fungi, parasites, and a very old class of single-celled organisms called archaea. The organisms include commensals and pathogenic microorganisms. Many articles distinguish "microbiome" and "microbiota" to differentiate the collective genomes of the microorganisms or the microorganisms themselves, respectively. However, these terms are largely synonymous.
A number of advances have allowed scientists to make major advances in understanding the microbiome. Specifically, we now have the molecular tools to perform gene expression analysis for an entire microbial community in the new discipline of metagenomics and analyze the massive results with new methods of mathematical analysis.
The human body contains over 10 times more microorganisms than human cells. The existence of a remarkably diverse and enormously large microbial world on us and in us first began to come to light in the late 1990s. We are learning more and more about the individual locations of the human host that have different populations of microbes and about differences among humans that contribute to or account for susceptibility to infectious diseases as well as autoimmune diseases and even obesity and cancer.
The nasopharyngeal microbiome has become an area of research by our group led by Qingfu Xu, Ph.D., at the Rochester (N.Y.) General Hospital Research Institute in collaboration with Melinda M. Pettigrew, Ph.D., at the Yale School of Public Health, New Haven, Conn., and Dr. Janet R. Casey at Legacy Pediatrics, also in Rochester. The traditional view of the immune system is undergoing reassessment as we learn that our microbiota has coevolved with our immune system, and each exerts influence over the other. Our group has a special interest in the impact of the nasopharyngeal microbiome on the innate immune response in that physiologic niche, and the way the innate immune system modifies the microbiome. With a special interest in the bacteria that cause respiratory infections such as acute otitis media, acute sinusitis, bronchopneumonia, and pneumonia, we have identified how microbes like Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis compete and synergize in the nasopharynx to cause infections.
Also, we seek to better understand how respiratory viruses like respiratory syncytial virus (RSV), influenzae, parainfluenzae, rhinovirus, and others facilitate the overgrowth of S. pneumoniae, H. flu, and M. catarrhalis in the nose such that they convert from commensals to pathogens. But the synergy goes both ways, as we have recently found that S. pneumoniae facilitates upper respiratory viral infections.
Up to now most of the work on the human microbiome has focused on the gut, and nearly all studies have occurred in adults. Perhaps readers are aware of the use of "fecal microbiota transplantation" as a treatment/cure for Clostridium difficile infection. Unhealthy gut microbiota in premature neonates are a major contributing factor in necrotizing enterocolitis.
For decades, physicians have been taught that obesity is a problem derived from excessive caloric intake and inadequate caloric consumption through activity, plus vaguely defined differences in "metabolism." As a consequence, we checked for hypothyroidism � I never found a case. New research has shown that there is a difference in the "metabolism" of obese patients, but the difference is how the individual gut microbiota metabolizes our food. It turns out the thinner individuals have a microbiota that is less efficient in breaking down the food we ingest to allow efficient absorption into the bloodstream, whereas obese individuals have a more efficient microbiota that facilitates absorption of a greater percentage of the proteins, carbohydrates, and fats that are ingested. So the pathway to treatment of obesity may lie in the study of the microbiome!
It turns out that the microbiota of the skin is highly diverse. The microbiota colonizing the antecubital fossa is different from that of the forearm or biceps or axillae. When atopic dermatitis flares, it is often in the antecubital fossa, and it is caused by overgrowth of Staphylococcus aureus. The microbiome of a patient with atopic dermatitis is different from that of a person without atopic dermatitis, and the former microbiota is more permissive to S. aureus becoming a pathogen rather than a commensal of the skin.
Prevention of urogenital infections in girls depends on a healthy vaginal microbiota. Bacterial vaginosis requires the establishment of overgrowth by Gardnerella vaginalis and Peptostreptococcus anaerobius that can only occur if the resident microbiota is unable to control the proliferation of these bacteria. Only if the microbiota of the perineum, urethra, and bladder will allow potential urinary tract infection pathogens access to epithelial attachment sites can infection become established.
A last topic for this column is the role of the microbiota in autoimmune diseases. In particular, I find it fascinating to learn that aberrant, unstable intestinal microbiota can lead to a leaky intestinal mucosal barrier. Combined with inadequate innate immune responses in the gut, progression may occur that allows antigens from microbes that cross-react with antigens of self in the pancreas to stimulate autoimmune antibodies. Similar pathogenic mechanisms may contribute to inflammatory bowel disease, rheumatoid arthritis, multiple sclerosis, and other autoimmune diseases.
I anticipate future research will establish the makeup of a healthy microbiota associated with protection from the diseases mentioned here. With that knowledge, the next efforts in research will focus on how to convert an unhealthy microbiota to a healthy one. If the efforts succeed, I see new promising treatments in the future.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Rochester (N.Y.) General Research Institute. He is also a pediatrician at Legacy Pediatrics in Rochester. The microbiome research at the Rochester General Hospital Research Institute is supported by the National Institutes of Health and the National Institute for Deafness and Communication Disorders. To comment, e-mail him at pdnews@ frontlinemedcom.com.
A new way to treat ear infections
A new way to treat ear infections in children called "individualized care" is described in the May 2013 issue of Pediatric Infectious Diseases Journal. It explains how to reduce the frequency of repeated ear infections nearly 500% and how to reduce the need for ear tube surgery by 600% in your practice.
Dr. Janet Casey at Legacy Pediatrics in Rochester, N.Y.; Anthony Almudevar, Ph.D., of the University of Rochester; and I conducted the prospective, longitudinal multiyear study with the support of the National Institutes of Health’s National Institute for Deafness and Communication Disorders and the Thrasher Research Fund (Pediatr. Infect. Dis. J. 2013 Jan. 21 [Epub ahead of print]).
The study compared three groups: children who were in the Legacy Pediatrics practice and received individualized care; control children in the Legacy practice who did not participate because their parents declined participation (they did not want venipunctures or ear taps); and community controls drawn from a different pediatric practice in the suburbs of Rochester that used the diagnostic criteria of the American Academy of Pediatrics and treated all children empirically with high-dose amoxicillin as endorsed by the former and new AAP treatment guidelines (Pediatrics 2013;131:e964-99).
The new treatment paradigm of individualized care included a tympanocentesis procedure, also called an ear tap, to determine precisely the bacteria causing the ear infection. Treatment was started with high-dose amoxicillin/clavulanate. The sample of fluid then was taken to my laboratory at the Rochester General Hospital Research Institute, where the bacteria isolated were tested against a panel of antibiotics to determine whether to continue with amoxicillin/clavulanate or switch to a more effective antibiotic for the child based on culture susceptibility. By doing the ear tap and antibiotic testing, the frequency of repeated ear infections was reduced by 250%, compared with the Legacy practice controls who did not participate, and by 460%, compared with the community controls.
The most common reason for children to receive ear tubes is repeated ear infections, so when the frequency of ear infections was reduced so too was the frequency of ear tube surgery. The new treatment approach resulted in 260% fewer ear tube surgeries in the individualized care group, compared with the Legacy Pediatrics controls, and 620% fewer surgeries than the community controls.
Allowing the child to receive an ear tap was a requirement for the study. Dr. Casey and I found a way to do the procedure painlessly by instilling 8% Novocain in the ear canal as drops to anesthetize the tympanic membrane. After 15 minutes there was no pain when the tap was done. We used a papoose to hold the child still.
The ear-tap procedure not only allowed individualized care with the astonishing results reported, it also allowed more rapid healing of the ear since removal of the pus and bacteria from behind the ear allowed the antibiotics to work better and the immune system to clear the infection more effectively.
The article discusses reasons for the remarkable difference in results with the individualized care approach. First, Dr. Casey and I have undergone special training from ear, nose, and throat (ENT) doctors in the diagnosis of ear infections.
In earlier studies, a group of experts in otitis media diagnosis joined together in a continuing medical education course sponsored by Outcomes Management Education Workshops to use video exams to test whether pediatricians, family physicians, and urgent care physicians knew how to correctly distinguish true acute otitis media (AOM) from otitis media with effusion (OME) and variations of normal in the tympanic membrane exam. We found that all three specialty groups and residents in training in all three specialties and nurse practitioners and physician assistants overdiagnosed AOM about half the time.
Second, the selection of antibiotic proved to be key. Dr. Casey and I have the only otitis media research center in the United States providing tympanocentesis data at the current time. We have found that amoxicillin kills the bacteria causing AOM infections in children in the Rochester area only about 30% of the time. By knowing the bacteria, an evidence-based antibiotic can be chosen.
I expect that readers of this column will believe they diagnose AOM correctly nearly all the time and that it is the other physician who overdiagnoses. I expect that readers will be reluctant to not adhere to the AAP guideline recommendation of using amoxicillin as the treatment of first choice. Most of all, I expect readers to be reluctant to undertake training on how to do the ear tap procedure. Change is always resisted by the majority, and only with time does it occur if the evidence is strong and there is growing adoption.
Nevertheless, I encourage all to find an opportunity to attend a CME course on AOM diagnosis and I hope that resident training programs will incorporate more effective teaching on AOM diagnosis. I recommend high-dose amoxicillin/clavulanate as the treatment of choice for AOM; if it is not tolerated, then one of the preferred cephalosporins endorsed by the AAP guideline should be chosen.
I recommend that resident training programs include tympanocentesis as part of the curriculum. Why are residents taught how to do a spinal tap, arterial artery puncture, and lung tap but not an ear tap? I also recommend that practicing pediatricians gain the skill to perform tympanocentesis as well. I recognize that some just won’t have the hand/eye coordination or steady hand needed, so it’s not for everyone. However, especially in group practices, a few trained providers could become an internal referral resource for getting the procedure done.
Arguments about malpractice are a smokescreen. The risks of tympanocentesis are no greater than venipuncture in trained and skilled hands. It is included as a standard procedure for pediatricians in our state without any additional malpractice insurance costs. And Dr. Casey and I have effectively managed to get the procedure done when a patient needs it without blowing our schedules off the map and raising the ire of patients and staff. It just takes a commitment.
It would be convenient to refer to an ENT doctor for a tympanocentesis, but most ENT doctors have not been trained to do the procedure while the child is awake and prefer to have the child asleep. Also, try to get a child in for an appointment with an ENT with no notice on the same day! Moreover, ENT doctors have been trained that if an ear tap is needed then it is advisable to go ahead and put in an ear tube.
Because of the success of this research, our center received a renewal of support from NIH in 2012 to continue the study through 2017. Several pediatric practices in Rochester are part of the research – Long Pond Pediatrics, Westfall Pediatrics, Sunrise Pediatrics, Lewis Pediatrics, and Pathway Pediatrics – as well as Dr. Margo Benoit of the department of otolaryngology at the University of Rochester and Dr. Frank Salamone and Dr. Kevin Kozara of the Rochester Otolaryngology Group, which is affiliated with Rochester General Hospital.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Rochester (N.Y.) General Hospital Research Institute. He is also a pediatrician at Legacy Pediatrics in Rochester. He said he had no relevant financial conflicts of interest to disclose.
A new way to treat ear infections in children called "individualized care" is described in the May 2013 issue of Pediatric Infectious Diseases Journal. It explains how to reduce the frequency of repeated ear infections nearly 500% and how to reduce the need for ear tube surgery by 600% in your practice.
Dr. Janet Casey at Legacy Pediatrics in Rochester, N.Y.; Anthony Almudevar, Ph.D., of the University of Rochester; and I conducted the prospective, longitudinal multiyear study with the support of the National Institutes of Health’s National Institute for Deafness and Communication Disorders and the Thrasher Research Fund (Pediatr. Infect. Dis. J. 2013 Jan. 21 [Epub ahead of print]).
The study compared three groups: children who were in the Legacy Pediatrics practice and received individualized care; control children in the Legacy practice who did not participate because their parents declined participation (they did not want venipunctures or ear taps); and community controls drawn from a different pediatric practice in the suburbs of Rochester that used the diagnostic criteria of the American Academy of Pediatrics and treated all children empirically with high-dose amoxicillin as endorsed by the former and new AAP treatment guidelines (Pediatrics 2013;131:e964-99).
The new treatment paradigm of individualized care included a tympanocentesis procedure, also called an ear tap, to determine precisely the bacteria causing the ear infection. Treatment was started with high-dose amoxicillin/clavulanate. The sample of fluid then was taken to my laboratory at the Rochester General Hospital Research Institute, where the bacteria isolated were tested against a panel of antibiotics to determine whether to continue with amoxicillin/clavulanate or switch to a more effective antibiotic for the child based on culture susceptibility. By doing the ear tap and antibiotic testing, the frequency of repeated ear infections was reduced by 250%, compared with the Legacy practice controls who did not participate, and by 460%, compared with the community controls.
The most common reason for children to receive ear tubes is repeated ear infections, so when the frequency of ear infections was reduced so too was the frequency of ear tube surgery. The new treatment approach resulted in 260% fewer ear tube surgeries in the individualized care group, compared with the Legacy Pediatrics controls, and 620% fewer surgeries than the community controls.
Allowing the child to receive an ear tap was a requirement for the study. Dr. Casey and I found a way to do the procedure painlessly by instilling 8% Novocain in the ear canal as drops to anesthetize the tympanic membrane. After 15 minutes there was no pain when the tap was done. We used a papoose to hold the child still.
The ear-tap procedure not only allowed individualized care with the astonishing results reported, it also allowed more rapid healing of the ear since removal of the pus and bacteria from behind the ear allowed the antibiotics to work better and the immune system to clear the infection more effectively.
The article discusses reasons for the remarkable difference in results with the individualized care approach. First, Dr. Casey and I have undergone special training from ear, nose, and throat (ENT) doctors in the diagnosis of ear infections.
In earlier studies, a group of experts in otitis media diagnosis joined together in a continuing medical education course sponsored by Outcomes Management Education Workshops to use video exams to test whether pediatricians, family physicians, and urgent care physicians knew how to correctly distinguish true acute otitis media (AOM) from otitis media with effusion (OME) and variations of normal in the tympanic membrane exam. We found that all three specialty groups and residents in training in all three specialties and nurse practitioners and physician assistants overdiagnosed AOM about half the time.
Second, the selection of antibiotic proved to be key. Dr. Casey and I have the only otitis media research center in the United States providing tympanocentesis data at the current time. We have found that amoxicillin kills the bacteria causing AOM infections in children in the Rochester area only about 30% of the time. By knowing the bacteria, an evidence-based antibiotic can be chosen.
I expect that readers of this column will believe they diagnose AOM correctly nearly all the time and that it is the other physician who overdiagnoses. I expect that readers will be reluctant to not adhere to the AAP guideline recommendation of using amoxicillin as the treatment of first choice. Most of all, I expect readers to be reluctant to undertake training on how to do the ear tap procedure. Change is always resisted by the majority, and only with time does it occur if the evidence is strong and there is growing adoption.
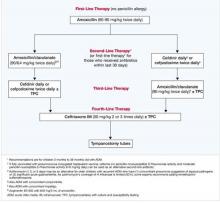
Nevertheless, I encourage all to find an opportunity to attend a CME course on AOM diagnosis and I hope that resident training programs will incorporate more effective teaching on AOM diagnosis. I recommend high-dose amoxicillin/clavulanate as the treatment of choice for AOM; if it is not tolerated, then one of the preferred cephalosporins endorsed by the AAP guideline should be chosen.
I recommend that resident training programs include tympanocentesis as part of the curriculum. Why are residents taught how to do a spinal tap, arterial artery puncture, and lung tap but not an ear tap? I also recommend that practicing pediatricians gain the skill to perform tympanocentesis as well. I recognize that some just won’t have the hand/eye coordination or steady hand needed, so it’s not for everyone. However, especially in group practices, a few trained providers could become an internal referral resource for getting the procedure done.
Arguments about malpractice are a smokescreen. The risks of tympanocentesis are no greater than venipuncture in trained and skilled hands. It is included as a standard procedure for pediatricians in our state without any additional malpractice insurance costs. And Dr. Casey and I have effectively managed to get the procedure done when a patient needs it without blowing our schedules off the map and raising the ire of patients and staff. It just takes a commitment.
It would be convenient to refer to an ENT doctor for a tympanocentesis, but most ENT doctors have not been trained to do the procedure while the child is awake and prefer to have the child asleep. Also, try to get a child in for an appointment with an ENT with no notice on the same day! Moreover, ENT doctors have been trained that if an ear tap is needed then it is advisable to go ahead and put in an ear tube.
Because of the success of this research, our center received a renewal of support from NIH in 2012 to continue the study through 2017. Several pediatric practices in Rochester are part of the research – Long Pond Pediatrics, Westfall Pediatrics, Sunrise Pediatrics, Lewis Pediatrics, and Pathway Pediatrics – as well as Dr. Margo Benoit of the department of otolaryngology at the University of Rochester and Dr. Frank Salamone and Dr. Kevin Kozara of the Rochester Otolaryngology Group, which is affiliated with Rochester General Hospital.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Rochester (N.Y.) General Hospital Research Institute. He is also a pediatrician at Legacy Pediatrics in Rochester. He said he had no relevant financial conflicts of interest to disclose.
A new way to treat ear infections in children called "individualized care" is described in the May 2013 issue of Pediatric Infectious Diseases Journal. It explains how to reduce the frequency of repeated ear infections nearly 500% and how to reduce the need for ear tube surgery by 600% in your practice.
Dr. Janet Casey at Legacy Pediatrics in Rochester, N.Y.; Anthony Almudevar, Ph.D., of the University of Rochester; and I conducted the prospective, longitudinal multiyear study with the support of the National Institutes of Health’s National Institute for Deafness and Communication Disorders and the Thrasher Research Fund (Pediatr. Infect. Dis. J. 2013 Jan. 21 [Epub ahead of print]).
The study compared three groups: children who were in the Legacy Pediatrics practice and received individualized care; control children in the Legacy practice who did not participate because their parents declined participation (they did not want venipunctures or ear taps); and community controls drawn from a different pediatric practice in the suburbs of Rochester that used the diagnostic criteria of the American Academy of Pediatrics and treated all children empirically with high-dose amoxicillin as endorsed by the former and new AAP treatment guidelines (Pediatrics 2013;131:e964-99).
The new treatment paradigm of individualized care included a tympanocentesis procedure, also called an ear tap, to determine precisely the bacteria causing the ear infection. Treatment was started with high-dose amoxicillin/clavulanate. The sample of fluid then was taken to my laboratory at the Rochester General Hospital Research Institute, where the bacteria isolated were tested against a panel of antibiotics to determine whether to continue with amoxicillin/clavulanate or switch to a more effective antibiotic for the child based on culture susceptibility. By doing the ear tap and antibiotic testing, the frequency of repeated ear infections was reduced by 250%, compared with the Legacy practice controls who did not participate, and by 460%, compared with the community controls.
The most common reason for children to receive ear tubes is repeated ear infections, so when the frequency of ear infections was reduced so too was the frequency of ear tube surgery. The new treatment approach resulted in 260% fewer ear tube surgeries in the individualized care group, compared with the Legacy Pediatrics controls, and 620% fewer surgeries than the community controls.
Allowing the child to receive an ear tap was a requirement for the study. Dr. Casey and I found a way to do the procedure painlessly by instilling 8% Novocain in the ear canal as drops to anesthetize the tympanic membrane. After 15 minutes there was no pain when the tap was done. We used a papoose to hold the child still.
The ear-tap procedure not only allowed individualized care with the astonishing results reported, it also allowed more rapid healing of the ear since removal of the pus and bacteria from behind the ear allowed the antibiotics to work better and the immune system to clear the infection more effectively.
The article discusses reasons for the remarkable difference in results with the individualized care approach. First, Dr. Casey and I have undergone special training from ear, nose, and throat (ENT) doctors in the diagnosis of ear infections.
In earlier studies, a group of experts in otitis media diagnosis joined together in a continuing medical education course sponsored by Outcomes Management Education Workshops to use video exams to test whether pediatricians, family physicians, and urgent care physicians knew how to correctly distinguish true acute otitis media (AOM) from otitis media with effusion (OME) and variations of normal in the tympanic membrane exam. We found that all three specialty groups and residents in training in all three specialties and nurse practitioners and physician assistants overdiagnosed AOM about half the time.
Second, the selection of antibiotic proved to be key. Dr. Casey and I have the only otitis media research center in the United States providing tympanocentesis data at the current time. We have found that amoxicillin kills the bacteria causing AOM infections in children in the Rochester area only about 30% of the time. By knowing the bacteria, an evidence-based antibiotic can be chosen.
I expect that readers of this column will believe they diagnose AOM correctly nearly all the time and that it is the other physician who overdiagnoses. I expect that readers will be reluctant to not adhere to the AAP guideline recommendation of using amoxicillin as the treatment of first choice. Most of all, I expect readers to be reluctant to undertake training on how to do the ear tap procedure. Change is always resisted by the majority, and only with time does it occur if the evidence is strong and there is growing adoption.
Nevertheless, I encourage all to find an opportunity to attend a CME course on AOM diagnosis and I hope that resident training programs will incorporate more effective teaching on AOM diagnosis. I recommend high-dose amoxicillin/clavulanate as the treatment of choice for AOM; if it is not tolerated, then one of the preferred cephalosporins endorsed by the AAP guideline should be chosen.
I recommend that resident training programs include tympanocentesis as part of the curriculum. Why are residents taught how to do a spinal tap, arterial artery puncture, and lung tap but not an ear tap? I also recommend that practicing pediatricians gain the skill to perform tympanocentesis as well. I recognize that some just won’t have the hand/eye coordination or steady hand needed, so it’s not for everyone. However, especially in group practices, a few trained providers could become an internal referral resource for getting the procedure done.
Arguments about malpractice are a smokescreen. The risks of tympanocentesis are no greater than venipuncture in trained and skilled hands. It is included as a standard procedure for pediatricians in our state without any additional malpractice insurance costs. And Dr. Casey and I have effectively managed to get the procedure done when a patient needs it without blowing our schedules off the map and raising the ire of patients and staff. It just takes a commitment.
It would be convenient to refer to an ENT doctor for a tympanocentesis, but most ENT doctors have not been trained to do the procedure while the child is awake and prefer to have the child asleep. Also, try to get a child in for an appointment with an ENT with no notice on the same day! Moreover, ENT doctors have been trained that if an ear tap is needed then it is advisable to go ahead and put in an ear tube.
Because of the success of this research, our center received a renewal of support from NIH in 2012 to continue the study through 2017. Several pediatric practices in Rochester are part of the research – Long Pond Pediatrics, Westfall Pediatrics, Sunrise Pediatrics, Lewis Pediatrics, and Pathway Pediatrics – as well as Dr. Margo Benoit of the department of otolaryngology at the University of Rochester and Dr. Frank Salamone and Dr. Kevin Kozara of the Rochester Otolaryngology Group, which is affiliated with Rochester General Hospital.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Rochester (N.Y.) General Hospital Research Institute. He is also a pediatrician at Legacy Pediatrics in Rochester. He said he had no relevant financial conflicts of interest to disclose.
Infant Meningococcal Vaccine: Why Not?
Haemophilus influenzae type B-Neisseria meningitidis serogroups C (MenC) and Y (MenY)-tetanus toxoid (Hib-MenCY-TT, MenHibrix) vaccine has been approved by the Food and Drug Administration for an infant indication to be administered according to the standard 2-, 4-, 6-, and 12 months vaccine schedule in the United States as endorsed by the American Academy of Pediatrics (AAP), the American Academy of Family Physicians (AAFP), and the Centers for Disease Control and Prevention (CDC).
According to the presentation given at the CDC meeting Oct. 14, 2011, by Dr. Ismael Ortega-Sanchez, giving the vaccine could prevent 130 of the projected 377 (34.5%) cases of meningococcal infection in the 4-million-child birth cohort of the United States cumulatively to age 10 years. Also infant vaccination would prevent one death per 642,000 infants (seven deaths/year). The vaccine could be given along with a DTaP/inactivated polio vaccine/hepatitis b vaccine (DTaP/IPV/HepB, Pediarix) and the 13-valent pneumococcal conjugate vaccine (PCV13, Prevnar13) without increasing the total number of shots in a visit. The vaccine has been proven safe and effective.
Yet at the October meeting of the CDC Advisory Committee on Immunization Practices (ACIP), Hib-MenCY-TT (MenHibrix) vaccine was not recommended for universal use. Instead, it was recommended for high risk children as previously defined as complement deficient and asplenic. Why the restricted recommendation?*
• It isn’t the right time. When the ACIP/AAP/AAFP endorsed meningococcal vaccination for 11- to 12-year-olds 7 years ago, the annual incidence of meningococcal disease was fivefold higher than it is now. The drop in incidence cannot be fully attributed to the initiation of our national vaccination campaign. It was known before the meningococcal conjugate vaccine was recommended that meningococcal disease had a cyclical pattern with high and low years of incidence. But, had the incidence been as low as it is now, then one might speculate that the vote to recommend universal vaccination might have been different. The passionate pleas of concerned parents and the desire by all of us in health care to protect every single adolescent against the devastation of meningococcal infections carried the day, even though the cost for prevention of those cases and deaths was the highest ever seen up to that time. So, if the incidence of meningococcal infections is now at an all time low, the calculations of cost to prevent cases and deaths would be a multiple of what it was 7 years ago.
• The vaccine doesn’t include all the serotypes. Hib-MenCY-TT has meningococcal serotypes C and Y. The vaccine does not include serotype A or serotype W-135 (because these serotypes are virtually absent and uncommon, respectively, in the United States and other developed countries. So, a concern could be that serotype replacement might occur over time as we have seen with Prevnar7, now replaced in the United States with Prevnar13 because of serotype replacement. But more importantly is the absence of serotype B in the vaccine. Serotype B meningococci cause 60%-65% of meningococcal disease in the United States in infants. That is why the number of cases projected to be prevented with Hib-MenCY-TT is about one-third of all cases among infants.
• The total number of shots goes up per visit unless GlaxoSmithKline vaccines are preferentially used. Hib-MenCY-TT was developed and is licensed by GlaxoSmithKline, a world leader in pediatric vaccines, and they are building a portfolio of vaccines that can fit together well. There is nothing wrong with that – it is good marketing. Sanofi Pasteur Vaccines is doing the same thing and, as more products are forthcoming from Pfizer Vaccines, Novartis Vaccines, and others, we can expect the same strategy. However, the CDC, AAP, and AAFP do not want to endorse products that limit choices and/or provide any single company with a competitive advantage. So, to endorse Hib-MenCY-TT that clearly fits best with only GlaxoSmithKline vaccine products may be an unspoken concern.
• The National Immunization Program cannot afford it. Going back to the presentation to ACIP at the CDC in October 2011, a key aspect was the cost of vaccination calculated against cases prevented and lives saved. The calculation for Quality-Adjusted Life Year (QALY) saved for a two-dose schedule among adolescents came out to $157,000/case. For the infant vaccination, the numbers were pretty staggering at $3.6 million/case, based on the current incidence of meningococcal infections in the United States (see sidebar). Even if the incidence of meningococcal infections were currently as high as they were back in 1997-1999, the cost would be $0.5 million/case. For those thinking about the option of toddler vaccination with the quadrivalent meningococcal conjugate vaccine (Menactra), the calculations for QALYs concluded that such a strategy prevented half as many cases at half the cost.
So where do we go from here? The lack of an endorsement by ACIP/AAP/AAFP for universal use* normally means that the vaccine will not be available within the Vaccines for Children free program, and it will not be covered by commercial health insurance plans except for the specific indications endorsed by the recommending bodies.* So these are huge barriers to use. Nevertheless, it is a licensed vaccine and it is safe and effective, just not perfect and not cost-effective for widespread public use at government expense. How much is a child’s life worth? If it is your child, then the life is priceless. But in public health there are limits to what can be afforded. We will see more of these types of issues in the future. Another unspoken concern of the ACIP/AAP/AAFP is that a two-tiered vaccine access situation develops. In other words, for those who can afford to pay, Hib-MenCY-TT is available and, if they can pay for it out-of-pocket, then they can buy it to protect their child.
The following are cost-effectiveness analysis conclusions that were presented to the CDC:
• Vaccinating infants or toddlers with meningococcal vaccine has a high cost per case prevented – even at a low vaccine price.
• Cost estimates are much higher than prior analyses because of declining incidence and shorter duration of protection.
• Infant vaccination prevents twice as many cases as toddler vaccination but at twice the cost – cost per QALY saved is similar for both strategies.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Rochester (N.Y.) General Hospital Research Institute. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero disclosed that in the past 3 years, he has served as a consultant to Sanofi Pasteur, Pfizer, Novartis, and Crucell for their vaccines currently licensed and in development. Dr. Pichichero also disclosed that in the past 3 years, his academic institution has received research grants to support vaccine work from GlaxoSmithKline, Sanofi Pasteur, Pfizer, Novartis, and Crucell, including studies involving Hib-MenCY-TT produced by GlaxoSmithKline and Quadrivalent meningococcal vaccine by Sanofi Pasteur and Novartis.
* This article was updated on 10/26/12.
Haemophilus influenzae type B-Neisseria meningitidis serogroups C (MenC) and Y (MenY)-tetanus toxoid (Hib-MenCY-TT, MenHibrix) vaccine has been approved by the Food and Drug Administration for an infant indication to be administered according to the standard 2-, 4-, 6-, and 12 months vaccine schedule in the United States as endorsed by the American Academy of Pediatrics (AAP), the American Academy of Family Physicians (AAFP), and the Centers for Disease Control and Prevention (CDC).
According to the presentation given at the CDC meeting Oct. 14, 2011, by Dr. Ismael Ortega-Sanchez, giving the vaccine could prevent 130 of the projected 377 (34.5%) cases of meningococcal infection in the 4-million-child birth cohort of the United States cumulatively to age 10 years. Also infant vaccination would prevent one death per 642,000 infants (seven deaths/year). The vaccine could be given along with a DTaP/inactivated polio vaccine/hepatitis b vaccine (DTaP/IPV/HepB, Pediarix) and the 13-valent pneumococcal conjugate vaccine (PCV13, Prevnar13) without increasing the total number of shots in a visit. The vaccine has been proven safe and effective.
Yet at the October meeting of the CDC Advisory Committee on Immunization Practices (ACIP), Hib-MenCY-TT (MenHibrix) vaccine was not recommended for universal use. Instead, it was recommended for high risk children as previously defined as complement deficient and asplenic. Why the restricted recommendation?*
• It isn’t the right time. When the ACIP/AAP/AAFP endorsed meningococcal vaccination for 11- to 12-year-olds 7 years ago, the annual incidence of meningococcal disease was fivefold higher than it is now. The drop in incidence cannot be fully attributed to the initiation of our national vaccination campaign. It was known before the meningococcal conjugate vaccine was recommended that meningococcal disease had a cyclical pattern with high and low years of incidence. But, had the incidence been as low as it is now, then one might speculate that the vote to recommend universal vaccination might have been different. The passionate pleas of concerned parents and the desire by all of us in health care to protect every single adolescent against the devastation of meningococcal infections carried the day, even though the cost for prevention of those cases and deaths was the highest ever seen up to that time. So, if the incidence of meningococcal infections is now at an all time low, the calculations of cost to prevent cases and deaths would be a multiple of what it was 7 years ago.
• The vaccine doesn’t include all the serotypes. Hib-MenCY-TT has meningococcal serotypes C and Y. The vaccine does not include serotype A or serotype W-135 (because these serotypes are virtually absent and uncommon, respectively, in the United States and other developed countries. So, a concern could be that serotype replacement might occur over time as we have seen with Prevnar7, now replaced in the United States with Prevnar13 because of serotype replacement. But more importantly is the absence of serotype B in the vaccine. Serotype B meningococci cause 60%-65% of meningococcal disease in the United States in infants. That is why the number of cases projected to be prevented with Hib-MenCY-TT is about one-third of all cases among infants.
• The total number of shots goes up per visit unless GlaxoSmithKline vaccines are preferentially used. Hib-MenCY-TT was developed and is licensed by GlaxoSmithKline, a world leader in pediatric vaccines, and they are building a portfolio of vaccines that can fit together well. There is nothing wrong with that – it is good marketing. Sanofi Pasteur Vaccines is doing the same thing and, as more products are forthcoming from Pfizer Vaccines, Novartis Vaccines, and others, we can expect the same strategy. However, the CDC, AAP, and AAFP do not want to endorse products that limit choices and/or provide any single company with a competitive advantage. So, to endorse Hib-MenCY-TT that clearly fits best with only GlaxoSmithKline vaccine products may be an unspoken concern.
• The National Immunization Program cannot afford it. Going back to the presentation to ACIP at the CDC in October 2011, a key aspect was the cost of vaccination calculated against cases prevented and lives saved. The calculation for Quality-Adjusted Life Year (QALY) saved for a two-dose schedule among adolescents came out to $157,000/case. For the infant vaccination, the numbers were pretty staggering at $3.6 million/case, based on the current incidence of meningococcal infections in the United States (see sidebar). Even if the incidence of meningococcal infections were currently as high as they were back in 1997-1999, the cost would be $0.5 million/case. For those thinking about the option of toddler vaccination with the quadrivalent meningococcal conjugate vaccine (Menactra), the calculations for QALYs concluded that such a strategy prevented half as many cases at half the cost.
So where do we go from here? The lack of an endorsement by ACIP/AAP/AAFP for universal use* normally means that the vaccine will not be available within the Vaccines for Children free program, and it will not be covered by commercial health insurance plans except for the specific indications endorsed by the recommending bodies.* So these are huge barriers to use. Nevertheless, it is a licensed vaccine and it is safe and effective, just not perfect and not cost-effective for widespread public use at government expense. How much is a child’s life worth? If it is your child, then the life is priceless. But in public health there are limits to what can be afforded. We will see more of these types of issues in the future. Another unspoken concern of the ACIP/AAP/AAFP is that a two-tiered vaccine access situation develops. In other words, for those who can afford to pay, Hib-MenCY-TT is available and, if they can pay for it out-of-pocket, then they can buy it to protect their child.
The following are cost-effectiveness analysis conclusions that were presented to the CDC:
• Vaccinating infants or toddlers with meningococcal vaccine has a high cost per case prevented – even at a low vaccine price.
• Cost estimates are much higher than prior analyses because of declining incidence and shorter duration of protection.
• Infant vaccination prevents twice as many cases as toddler vaccination but at twice the cost – cost per QALY saved is similar for both strategies.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Rochester (N.Y.) General Hospital Research Institute. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero disclosed that in the past 3 years, he has served as a consultant to Sanofi Pasteur, Pfizer, Novartis, and Crucell for their vaccines currently licensed and in development. Dr. Pichichero also disclosed that in the past 3 years, his academic institution has received research grants to support vaccine work from GlaxoSmithKline, Sanofi Pasteur, Pfizer, Novartis, and Crucell, including studies involving Hib-MenCY-TT produced by GlaxoSmithKline and Quadrivalent meningococcal vaccine by Sanofi Pasteur and Novartis.
* This article was updated on 10/26/12.
Haemophilus influenzae type B-Neisseria meningitidis serogroups C (MenC) and Y (MenY)-tetanus toxoid (Hib-MenCY-TT, MenHibrix) vaccine has been approved by the Food and Drug Administration for an infant indication to be administered according to the standard 2-, 4-, 6-, and 12 months vaccine schedule in the United States as endorsed by the American Academy of Pediatrics (AAP), the American Academy of Family Physicians (AAFP), and the Centers for Disease Control and Prevention (CDC).
According to the presentation given at the CDC meeting Oct. 14, 2011, by Dr. Ismael Ortega-Sanchez, giving the vaccine could prevent 130 of the projected 377 (34.5%) cases of meningococcal infection in the 4-million-child birth cohort of the United States cumulatively to age 10 years. Also infant vaccination would prevent one death per 642,000 infants (seven deaths/year). The vaccine could be given along with a DTaP/inactivated polio vaccine/hepatitis b vaccine (DTaP/IPV/HepB, Pediarix) and the 13-valent pneumococcal conjugate vaccine (PCV13, Prevnar13) without increasing the total number of shots in a visit. The vaccine has been proven safe and effective.
Yet at the October meeting of the CDC Advisory Committee on Immunization Practices (ACIP), Hib-MenCY-TT (MenHibrix) vaccine was not recommended for universal use. Instead, it was recommended for high risk children as previously defined as complement deficient and asplenic. Why the restricted recommendation?*
• It isn’t the right time. When the ACIP/AAP/AAFP endorsed meningococcal vaccination for 11- to 12-year-olds 7 years ago, the annual incidence of meningococcal disease was fivefold higher than it is now. The drop in incidence cannot be fully attributed to the initiation of our national vaccination campaign. It was known before the meningococcal conjugate vaccine was recommended that meningococcal disease had a cyclical pattern with high and low years of incidence. But, had the incidence been as low as it is now, then one might speculate that the vote to recommend universal vaccination might have been different. The passionate pleas of concerned parents and the desire by all of us in health care to protect every single adolescent against the devastation of meningococcal infections carried the day, even though the cost for prevention of those cases and deaths was the highest ever seen up to that time. So, if the incidence of meningococcal infections is now at an all time low, the calculations of cost to prevent cases and deaths would be a multiple of what it was 7 years ago.
• The vaccine doesn’t include all the serotypes. Hib-MenCY-TT has meningococcal serotypes C and Y. The vaccine does not include serotype A or serotype W-135 (because these serotypes are virtually absent and uncommon, respectively, in the United States and other developed countries. So, a concern could be that serotype replacement might occur over time as we have seen with Prevnar7, now replaced in the United States with Prevnar13 because of serotype replacement. But more importantly is the absence of serotype B in the vaccine. Serotype B meningococci cause 60%-65% of meningococcal disease in the United States in infants. That is why the number of cases projected to be prevented with Hib-MenCY-TT is about one-third of all cases among infants.
• The total number of shots goes up per visit unless GlaxoSmithKline vaccines are preferentially used. Hib-MenCY-TT was developed and is licensed by GlaxoSmithKline, a world leader in pediatric vaccines, and they are building a portfolio of vaccines that can fit together well. There is nothing wrong with that – it is good marketing. Sanofi Pasteur Vaccines is doing the same thing and, as more products are forthcoming from Pfizer Vaccines, Novartis Vaccines, and others, we can expect the same strategy. However, the CDC, AAP, and AAFP do not want to endorse products that limit choices and/or provide any single company with a competitive advantage. So, to endorse Hib-MenCY-TT that clearly fits best with only GlaxoSmithKline vaccine products may be an unspoken concern.
• The National Immunization Program cannot afford it. Going back to the presentation to ACIP at the CDC in October 2011, a key aspect was the cost of vaccination calculated against cases prevented and lives saved. The calculation for Quality-Adjusted Life Year (QALY) saved for a two-dose schedule among adolescents came out to $157,000/case. For the infant vaccination, the numbers were pretty staggering at $3.6 million/case, based on the current incidence of meningococcal infections in the United States (see sidebar). Even if the incidence of meningococcal infections were currently as high as they were back in 1997-1999, the cost would be $0.5 million/case. For those thinking about the option of toddler vaccination with the quadrivalent meningococcal conjugate vaccine (Menactra), the calculations for QALYs concluded that such a strategy prevented half as many cases at half the cost.
So where do we go from here? The lack of an endorsement by ACIP/AAP/AAFP for universal use* normally means that the vaccine will not be available within the Vaccines for Children free program, and it will not be covered by commercial health insurance plans except for the specific indications endorsed by the recommending bodies.* So these are huge barriers to use. Nevertheless, it is a licensed vaccine and it is safe and effective, just not perfect and not cost-effective for widespread public use at government expense. How much is a child’s life worth? If it is your child, then the life is priceless. But in public health there are limits to what can be afforded. We will see more of these types of issues in the future. Another unspoken concern of the ACIP/AAP/AAFP is that a two-tiered vaccine access situation develops. In other words, for those who can afford to pay, Hib-MenCY-TT is available and, if they can pay for it out-of-pocket, then they can buy it to protect their child.
The following are cost-effectiveness analysis conclusions that were presented to the CDC:
• Vaccinating infants or toddlers with meningococcal vaccine has a high cost per case prevented – even at a low vaccine price.
• Cost estimates are much higher than prior analyses because of declining incidence and shorter duration of protection.
• Infant vaccination prevents twice as many cases as toddler vaccination but at twice the cost – cost per QALY saved is similar for both strategies.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Rochester (N.Y.) General Hospital Research Institute. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero disclosed that in the past 3 years, he has served as a consultant to Sanofi Pasteur, Pfizer, Novartis, and Crucell for their vaccines currently licensed and in development. Dr. Pichichero also disclosed that in the past 3 years, his academic institution has received research grants to support vaccine work from GlaxoSmithKline, Sanofi Pasteur, Pfizer, Novartis, and Crucell, including studies involving Hib-MenCY-TT produced by GlaxoSmithKline and Quadrivalent meningococcal vaccine by Sanofi Pasteur and Novartis.
* This article was updated on 10/26/12.
WBC Count Prevents Unnecessary Antibiotics for Enterovirus
It’s summertime, which means that pediatric and family medicine offices are filled with children who have enteroviral infections, and too many of them will receive inappropriate antibiotic prescriptions.
From June through September, children who present with fever and other flulike symptoms nearly always have a nonpolio enterovirus (NPEV) infection, with coxsackievirus groups A and B, echoviruses, and the newer numbered enteroviruses being the most common in the United States. Epidemiologic surveillance suggests that 10 million to 15 million illnesses attributable to NPEV occur in the United States each year. I tell parents that their child has a "summer flu," which helps to communicate that it is usually self-limited and that antibiotics will not work to treat it.
A sudden high fever is usually what brings the child in, and the classic blistering hand-foot-mouth presentation of enterovirus helps us nail down the diagnosis. But sometimes, especially in younger children, symptoms such as myalgia, malaise, irritability, upper respiratory symptoms, or gastrointestinal symptoms may be nonspecific. In addition, the fever can precede hand-foot-mouth blisters by several days. A 9-month-old with a sudden fever of 103° F and a mildly red-looking throat can cause panic for parents, and a physician will often be led to prescribe an antibiotic out of an abundance of caution. In fact, more than 80% of inappropriate antibiotic use in the summertime is for febrile illness related to early enteroviral infection combined with worried parents and physicians.
Obtaining a complete blood count and differential can solve this problem, but it’s often not done because typically it involves sending the patient out for the blood work and waiting 4-6 hours for the results. Depending on when the test is done, results may not be available until the following day. It’s much simpler to "cover" with an antibiotic than to order the diagnostic test.
But I believe we should be using the white blood cell (WBC) count rather than prescribing antibiotics. The WBC count will almost always be low, and if a differential is added it will show a predominance of lymphocytes, indicating a viral infection. With that, parents and physicians can be reassured it’s enterovirus, and that no antibiotic is required.
In recent years, large pediatric practices and urgent care centers have begun purchasing a point-of-care WBC test made by Hemacue. Currently, it is licensed for use only in level 3 Clinical Laboratory Improvement Amendments (CLIA) facilities. A couple of years ago, I testified before a Food and Drug Administration device panel urging broader availability for the machine in clinical practice, but the panel had concerns that physicians would overrely on the WBC – the machine doesn’t give the differential – and possibly miss a more significant illness, such as leukemia.
Of course, the current widespread practice of empirically prescribing an antibiotic with no lab testing won’t pick up leukemia, either. In my view, the point-of-care test is an aid. It’s a piece of information you add to your clinical history and physical exam that assists in your diagnosis. I believe the benefits of avoiding unnecessary antibiotic use in millions of children every summer far outweigh the theoretical possibility that pediatricians would not rely on the broader context, including the time of year, disease rates in the community, and their clinical judgment. I really believe that WBC testing constitutes better care.
My colleagues and I have published two papers regarding the use of the WBC test to aid in judicious antibiotic use. In one, a prospective, 3-year study of 1,956 patients aged 3 months to 21 years with acute upper respiratory illness and fever, 737 did not have a diagnosis established by history and physical. Of those patients, we had WBC counts done for 351 children who appeared ill, had a temperature greater than 101° F, and parents who were demanding an antibiotic or physicians who were inclined to give an antibiotic. Of those, just 14 had a WBC count of 15,000/mcL or greater, and an antibiotic was prescribed for 13 of them. With the selective use of WBC testing, no child had significant bacterial illness that was missed (Clin. Pediatr. [Phila] 2003;42:113-9).
In another study of 120 acutely ill children and potential antibiotic recipients, we found that the point-of-care Hemacue WBC device produced comparable results to the Cell-Dyn countertop machine for total WBC counts (Clin. Pediatr. [Phila] 2009;48:291-4).
I’d just like to add a few more points about enterovirus to keep in mind when you advise patients and families. There is a long-held notion that summer viruses enteroviruses in particular – are of shorter duration than are winter viruses such as influenza, parainfluenza, and respiratory syncytial virus. That’s actually not true. Several years ago, my colleagues and I prospectively studied 380 children aged 4-18 years with systemic NPEV syndromes who presented to private suburban pediatric practices. Overall, NPEV infections were virologically confirmed in 122 of 372 patients (33%). The median duration of illness was 6 days for those with rash, 7 days for those with hand-foot-mouth and viral meningitis, 8 days for those with pleurodynia, and 9 days for those with myalgia/malaise (Pediatrics 1998;102:1126-34). Many children were ill for 10 days to 2 weeks.
Interestingly, in that study we also found that in more than half of the families, there was more than one individual with NPEV illness that was often similar but sometimes had a different clinical presentation. For example, one child might have classic hand-foot-mouth while another just has upper respiratory symptoms or gastrointestinal manifestations. This creates a confusing picture for the family because it is not intuitive to attribute different clinical presentations to the same viral etiology and, as a consequence, medical care may be sought more often.
We also found that 20% of the mothers caught the illness from the child, compared with fewer than 10% of the fathers. Luckily, the mother’s illness was usually less severe.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Rochester (N.Y.) General Research Institute. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero’s institution received a research grant in 2008 from Hemacue, and he has served as a paid consultant to testify before the FDA in 2009 for Hemacue. He said he has no other conflicts to declare.
It’s summertime, which means that pediatric and family medicine offices are filled with children who have enteroviral infections, and too many of them will receive inappropriate antibiotic prescriptions.
From June through September, children who present with fever and other flulike symptoms nearly always have a nonpolio enterovirus (NPEV) infection, with coxsackievirus groups A and B, echoviruses, and the newer numbered enteroviruses being the most common in the United States. Epidemiologic surveillance suggests that 10 million to 15 million illnesses attributable to NPEV occur in the United States each year. I tell parents that their child has a "summer flu," which helps to communicate that it is usually self-limited and that antibiotics will not work to treat it.
A sudden high fever is usually what brings the child in, and the classic blistering hand-foot-mouth presentation of enterovirus helps us nail down the diagnosis. But sometimes, especially in younger children, symptoms such as myalgia, malaise, irritability, upper respiratory symptoms, or gastrointestinal symptoms may be nonspecific. In addition, the fever can precede hand-foot-mouth blisters by several days. A 9-month-old with a sudden fever of 103° F and a mildly red-looking throat can cause panic for parents, and a physician will often be led to prescribe an antibiotic out of an abundance of caution. In fact, more than 80% of inappropriate antibiotic use in the summertime is for febrile illness related to early enteroviral infection combined with worried parents and physicians.
Obtaining a complete blood count and differential can solve this problem, but it’s often not done because typically it involves sending the patient out for the blood work and waiting 4-6 hours for the results. Depending on when the test is done, results may not be available until the following day. It’s much simpler to "cover" with an antibiotic than to order the diagnostic test.
But I believe we should be using the white blood cell (WBC) count rather than prescribing antibiotics. The WBC count will almost always be low, and if a differential is added it will show a predominance of lymphocytes, indicating a viral infection. With that, parents and physicians can be reassured it’s enterovirus, and that no antibiotic is required.
In recent years, large pediatric practices and urgent care centers have begun purchasing a point-of-care WBC test made by Hemacue. Currently, it is licensed for use only in level 3 Clinical Laboratory Improvement Amendments (CLIA) facilities. A couple of years ago, I testified before a Food and Drug Administration device panel urging broader availability for the machine in clinical practice, but the panel had concerns that physicians would overrely on the WBC – the machine doesn’t give the differential – and possibly miss a more significant illness, such as leukemia.
Of course, the current widespread practice of empirically prescribing an antibiotic with no lab testing won’t pick up leukemia, either. In my view, the point-of-care test is an aid. It’s a piece of information you add to your clinical history and physical exam that assists in your diagnosis. I believe the benefits of avoiding unnecessary antibiotic use in millions of children every summer far outweigh the theoretical possibility that pediatricians would not rely on the broader context, including the time of year, disease rates in the community, and their clinical judgment. I really believe that WBC testing constitutes better care.
My colleagues and I have published two papers regarding the use of the WBC test to aid in judicious antibiotic use. In one, a prospective, 3-year study of 1,956 patients aged 3 months to 21 years with acute upper respiratory illness and fever, 737 did not have a diagnosis established by history and physical. Of those patients, we had WBC counts done for 351 children who appeared ill, had a temperature greater than 101° F, and parents who were demanding an antibiotic or physicians who were inclined to give an antibiotic. Of those, just 14 had a WBC count of 15,000/mcL or greater, and an antibiotic was prescribed for 13 of them. With the selective use of WBC testing, no child had significant bacterial illness that was missed (Clin. Pediatr. [Phila] 2003;42:113-9).
In another study of 120 acutely ill children and potential antibiotic recipients, we found that the point-of-care Hemacue WBC device produced comparable results to the Cell-Dyn countertop machine for total WBC counts (Clin. Pediatr. [Phila] 2009;48:291-4).
I’d just like to add a few more points about enterovirus to keep in mind when you advise patients and families. There is a long-held notion that summer viruses enteroviruses in particular – are of shorter duration than are winter viruses such as influenza, parainfluenza, and respiratory syncytial virus. That’s actually not true. Several years ago, my colleagues and I prospectively studied 380 children aged 4-18 years with systemic NPEV syndromes who presented to private suburban pediatric practices. Overall, NPEV infections were virologically confirmed in 122 of 372 patients (33%). The median duration of illness was 6 days for those with rash, 7 days for those with hand-foot-mouth and viral meningitis, 8 days for those with pleurodynia, and 9 days for those with myalgia/malaise (Pediatrics 1998;102:1126-34). Many children were ill for 10 days to 2 weeks.
Interestingly, in that study we also found that in more than half of the families, there was more than one individual with NPEV illness that was often similar but sometimes had a different clinical presentation. For example, one child might have classic hand-foot-mouth while another just has upper respiratory symptoms or gastrointestinal manifestations. This creates a confusing picture for the family because it is not intuitive to attribute different clinical presentations to the same viral etiology and, as a consequence, medical care may be sought more often.
We also found that 20% of the mothers caught the illness from the child, compared with fewer than 10% of the fathers. Luckily, the mother’s illness was usually less severe.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Rochester (N.Y.) General Research Institute. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero’s institution received a research grant in 2008 from Hemacue, and he has served as a paid consultant to testify before the FDA in 2009 for Hemacue. He said he has no other conflicts to declare.
It’s summertime, which means that pediatric and family medicine offices are filled with children who have enteroviral infections, and too many of them will receive inappropriate antibiotic prescriptions.
From June through September, children who present with fever and other flulike symptoms nearly always have a nonpolio enterovirus (NPEV) infection, with coxsackievirus groups A and B, echoviruses, and the newer numbered enteroviruses being the most common in the United States. Epidemiologic surveillance suggests that 10 million to 15 million illnesses attributable to NPEV occur in the United States each year. I tell parents that their child has a "summer flu," which helps to communicate that it is usually self-limited and that antibiotics will not work to treat it.
A sudden high fever is usually what brings the child in, and the classic blistering hand-foot-mouth presentation of enterovirus helps us nail down the diagnosis. But sometimes, especially in younger children, symptoms such as myalgia, malaise, irritability, upper respiratory symptoms, or gastrointestinal symptoms may be nonspecific. In addition, the fever can precede hand-foot-mouth blisters by several days. A 9-month-old with a sudden fever of 103° F and a mildly red-looking throat can cause panic for parents, and a physician will often be led to prescribe an antibiotic out of an abundance of caution. In fact, more than 80% of inappropriate antibiotic use in the summertime is for febrile illness related to early enteroviral infection combined with worried parents and physicians.
Obtaining a complete blood count and differential can solve this problem, but it’s often not done because typically it involves sending the patient out for the blood work and waiting 4-6 hours for the results. Depending on when the test is done, results may not be available until the following day. It’s much simpler to "cover" with an antibiotic than to order the diagnostic test.
But I believe we should be using the white blood cell (WBC) count rather than prescribing antibiotics. The WBC count will almost always be low, and if a differential is added it will show a predominance of lymphocytes, indicating a viral infection. With that, parents and physicians can be reassured it’s enterovirus, and that no antibiotic is required.
In recent years, large pediatric practices and urgent care centers have begun purchasing a point-of-care WBC test made by Hemacue. Currently, it is licensed for use only in level 3 Clinical Laboratory Improvement Amendments (CLIA) facilities. A couple of years ago, I testified before a Food and Drug Administration device panel urging broader availability for the machine in clinical practice, but the panel had concerns that physicians would overrely on the WBC – the machine doesn’t give the differential – and possibly miss a more significant illness, such as leukemia.
Of course, the current widespread practice of empirically prescribing an antibiotic with no lab testing won’t pick up leukemia, either. In my view, the point-of-care test is an aid. It’s a piece of information you add to your clinical history and physical exam that assists in your diagnosis. I believe the benefits of avoiding unnecessary antibiotic use in millions of children every summer far outweigh the theoretical possibility that pediatricians would not rely on the broader context, including the time of year, disease rates in the community, and their clinical judgment. I really believe that WBC testing constitutes better care.
My colleagues and I have published two papers regarding the use of the WBC test to aid in judicious antibiotic use. In one, a prospective, 3-year study of 1,956 patients aged 3 months to 21 years with acute upper respiratory illness and fever, 737 did not have a diagnosis established by history and physical. Of those patients, we had WBC counts done for 351 children who appeared ill, had a temperature greater than 101° F, and parents who were demanding an antibiotic or physicians who were inclined to give an antibiotic. Of those, just 14 had a WBC count of 15,000/mcL or greater, and an antibiotic was prescribed for 13 of them. With the selective use of WBC testing, no child had significant bacterial illness that was missed (Clin. Pediatr. [Phila] 2003;42:113-9).
In another study of 120 acutely ill children and potential antibiotic recipients, we found that the point-of-care Hemacue WBC device produced comparable results to the Cell-Dyn countertop machine for total WBC counts (Clin. Pediatr. [Phila] 2009;48:291-4).
I’d just like to add a few more points about enterovirus to keep in mind when you advise patients and families. There is a long-held notion that summer viruses enteroviruses in particular – are of shorter duration than are winter viruses such as influenza, parainfluenza, and respiratory syncytial virus. That’s actually not true. Several years ago, my colleagues and I prospectively studied 380 children aged 4-18 years with systemic NPEV syndromes who presented to private suburban pediatric practices. Overall, NPEV infections were virologically confirmed in 122 of 372 patients (33%). The median duration of illness was 6 days for those with rash, 7 days for those with hand-foot-mouth and viral meningitis, 8 days for those with pleurodynia, and 9 days for those with myalgia/malaise (Pediatrics 1998;102:1126-34). Many children were ill for 10 days to 2 weeks.
Interestingly, in that study we also found that in more than half of the families, there was more than one individual with NPEV illness that was often similar but sometimes had a different clinical presentation. For example, one child might have classic hand-foot-mouth while another just has upper respiratory symptoms or gastrointestinal manifestations. This creates a confusing picture for the family because it is not intuitive to attribute different clinical presentations to the same viral etiology and, as a consequence, medical care may be sought more often.
We also found that 20% of the mothers caught the illness from the child, compared with fewer than 10% of the fathers. Luckily, the mother’s illness was usually less severe.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Rochester (N.Y.) General Research Institute. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero’s institution received a research grant in 2008 from Hemacue, and he has served as a paid consultant to testify before the FDA in 2009 for Hemacue. He said he has no other conflicts to declare.
Observation Option for Acute Otitis Media: A Second Look
Two new, well-designed trials published in the New England Journal of Medicine have demonstrated that when acute otitis media is correctly diagnosed, treatment with effective antibiotics is of clear and substantial benefit. To me, this suggests that the confusion about whether antibiotics help children get better faster is about getting the diagnosis right, a challenging task for pediatricians and family physicians with squirming patients and ear canal wax occluding visualization of the eardrum.
All along, I have believed that the American Academy of Pediatrics’ 2004 "watchful waiting" option for treating acute otitis media (AOM) was well intentioned but not based on good evidence. In an effort to address the growing problem of antimicrobial resistance, the AAP recommended the "observation option" for otherwise healthy children aged 6 months to 2 years with nonsevere illness and an uncertain diagnosis, and for all children above the age of 2 years who were not systemically ill (Pediatrics 2004;113:1451-65).
Problem is, the studies cited by the AAP as evidence for this recommendation were nearly all seriously flawed, because they excluded children with the very criteria that signal a true AOM diagnosis: a full or bulging eardrum ... and in some studies, because it was determined that they were too "unwell" and/or they "needed an antibiotic"! And, many of these trials excluded children younger than 2 years old and included many children who likely did not have AOM at all or had otitis media with effusion.
Dr. Janet R. Casey and I reviewed 25 of the studies in a paper published 3 years ago (Pediatr. Infect. Dis. J. 2008;27:958-62). We found so many serious flaws in the inclusion and exclusion criteria, and diagnostic and outcome criteria, that we were obliged to conclude that no evidence-based conclusion could be drawn.
The flaws we found in individual AOM trials call into question the validity of the conclusions of two major meta-analyses cited by the AAP, one involving 5,400 children from 33 randomized trials (J. Pediatr. 1994;124:355-67), the other of 6 studies of children aged 7 months to 15 years (BMJ 1997;314:1526-9), both of which found only modest benefit for the use of antimicrobials.
Now in the New England Journal of Medicine papers, we have two well-designed studies clearly demonstrating that treatment should not be withheld in children with proven AOM.
One of the studies, from the University of Pittsburgh, randomized 291 children aged 6-23 months to receive amoxicillin-clavulanate or placebo for 10 days. To be eligible, patients had to have AOM that was diagnosed on the basis of three criteria:
• Onset of symptoms within 48 hours that parents rated with a score of at least 3 on the Acute Otitis Media Severity of Symptoms scale,
• Presence of middle-ear effusion, and
• Moderate or marked bulging of the tympanic membrane or slight bulging accompanied by either otalgia or marked erythema of the membrane.
Patients also had to have received at least two doses of pneumococcal conjugate vaccine.
Among the children who received amoxicillin-clavulanate, 35% had initial resolution of symptoms by day 2, 61% by day 4, and 80% by day 7, compared with 28%, 54%, and 74% among those who received placebo, respectively. For sustained resolution of symptoms, the corresponding values were 20%, 41%, and 67% with amoxicillin-clavulanate, vs. 14%, 36%, and 53% with placebo (N. Engl. J. Med. 2011;364:105-15).
The other trial, from Finland, used equally strict criteria for 319 children aged 6-35 months who were randomized to receive amoxicillin-clavulanate or placebo for 7 days. Treatment failure occurred in 18.6% of the children who received amoxicillin-clavulanate, compared with 44.9% of the children who received placebo, a highly statistically significant difference that was already apparent at the first scheduled visit on day 3 (13.7% vs. 25.3%). Overall, amoxicillin-clavulanate reduced the progression to treatment failure by 62% (N. Engl. J. Med. 2011;364:116-26).
As I see it, the problem really lies in our inability to adequately diagnose AOM. For one thing, it’s essential to clean the wax out of the child’s ear in order to visualize the eardrum, given that two-thirds of children diagnosed with AOM have partially or fully occluded ear canals blocking visualization of the eardrum. Yet, physicians often don’t do that because it takes time and it’s difficult to get the child to hold still. It’s far simpler to simply take a quick look and say that the diagnosis is "uncertain," or to say that the eardrum is "red" in order to justify a diagnosis and antibiotic prescription.
Pediatricians and family physicians should all have a good, high-grade otoscope with a fresh battery and bulb, along with the training and ability to use the pneumatic attachment in order to distinguish between a bulging and retracted eardrum, which often look alike with just the otoscope.
Frankly, I find it embarrassing that with a condition as common as AOM, pediatricians and family physicians receive so little training in diagnosing it and, therefore, just don’t do a good job. In otitis media workshops that include testing for competency in diagnosis (Outcomes Management Educational Workshops, West Palm Beach, Fla.), I found that physicians got the diagnosis of AOM wrong at least 50% of the time on video presentation testing. And that was without wax, under ideal classroom conditions.
Diagnosing otitis media needs to become a critical part of medical education, and physicians in practice should be retrained via CME courses. Pharmaceutical companies no longer sponsor those, so now the professional societies such as the American Academy of Pediatrics, the American Academy of Family Physicians, and the nursing organizations need to step up.
With the new evidence from the two well-controlled trials, I don’t see how any clinician can withhold antibiotic treatment in good conscience. AOM is a painful condition that infants and toddlers are too young to explain to us. Can you imagine asking an adult to agree to withholding effective treatment when they are in pain and propose they take acetaminophen instead? Or can you imagine telling an adult who seeks care for an earache that the diagnosis is uncertain after examination, so the recommendation is to "observe"?
As advocates for our pediatric patients, how in the world can we allow a child to remain in severe pain for 24-48 hours longer than is necessary and keep parents up all night and away from work for 2-3 extra days?
Once everyone learns how to better diagnose AOM, we will stop overprescribing antibiotics for those children who don’t have the condition. For the rest, I contend that treatment is a moral imperative.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Rochester (N.Y.) General Research Institute. Dr. Pichichero disclosed that he is the medical director of Outcome Management Educational Workshops that train providers to improve diagnosis but has no disclosures related to antibiotic use in AOM.
Two new, well-designed trials published in the New England Journal of Medicine have demonstrated that when acute otitis media is correctly diagnosed, treatment with effective antibiotics is of clear and substantial benefit. To me, this suggests that the confusion about whether antibiotics help children get better faster is about getting the diagnosis right, a challenging task for pediatricians and family physicians with squirming patients and ear canal wax occluding visualization of the eardrum.
All along, I have believed that the American Academy of Pediatrics’ 2004 "watchful waiting" option for treating acute otitis media (AOM) was well intentioned but not based on good evidence. In an effort to address the growing problem of antimicrobial resistance, the AAP recommended the "observation option" for otherwise healthy children aged 6 months to 2 years with nonsevere illness and an uncertain diagnosis, and for all children above the age of 2 years who were not systemically ill (Pediatrics 2004;113:1451-65).
Problem is, the studies cited by the AAP as evidence for this recommendation were nearly all seriously flawed, because they excluded children with the very criteria that signal a true AOM diagnosis: a full or bulging eardrum ... and in some studies, because it was determined that they were too "unwell" and/or they "needed an antibiotic"! And, many of these trials excluded children younger than 2 years old and included many children who likely did not have AOM at all or had otitis media with effusion.
Dr. Janet R. Casey and I reviewed 25 of the studies in a paper published 3 years ago (Pediatr. Infect. Dis. J. 2008;27:958-62). We found so many serious flaws in the inclusion and exclusion criteria, and diagnostic and outcome criteria, that we were obliged to conclude that no evidence-based conclusion could be drawn.
The flaws we found in individual AOM trials call into question the validity of the conclusions of two major meta-analyses cited by the AAP, one involving 5,400 children from 33 randomized trials (J. Pediatr. 1994;124:355-67), the other of 6 studies of children aged 7 months to 15 years (BMJ 1997;314:1526-9), both of which found only modest benefit for the use of antimicrobials.
Now in the New England Journal of Medicine papers, we have two well-designed studies clearly demonstrating that treatment should not be withheld in children with proven AOM.
One of the studies, from the University of Pittsburgh, randomized 291 children aged 6-23 months to receive amoxicillin-clavulanate or placebo for 10 days. To be eligible, patients had to have AOM that was diagnosed on the basis of three criteria:
• Onset of symptoms within 48 hours that parents rated with a score of at least 3 on the Acute Otitis Media Severity of Symptoms scale,
• Presence of middle-ear effusion, and
• Moderate or marked bulging of the tympanic membrane or slight bulging accompanied by either otalgia or marked erythema of the membrane.
Patients also had to have received at least two doses of pneumococcal conjugate vaccine.
Among the children who received amoxicillin-clavulanate, 35% had initial resolution of symptoms by day 2, 61% by day 4, and 80% by day 7, compared with 28%, 54%, and 74% among those who received placebo, respectively. For sustained resolution of symptoms, the corresponding values were 20%, 41%, and 67% with amoxicillin-clavulanate, vs. 14%, 36%, and 53% with placebo (N. Engl. J. Med. 2011;364:105-15).
The other trial, from Finland, used equally strict criteria for 319 children aged 6-35 months who were randomized to receive amoxicillin-clavulanate or placebo for 7 days. Treatment failure occurred in 18.6% of the children who received amoxicillin-clavulanate, compared with 44.9% of the children who received placebo, a highly statistically significant difference that was already apparent at the first scheduled visit on day 3 (13.7% vs. 25.3%). Overall, amoxicillin-clavulanate reduced the progression to treatment failure by 62% (N. Engl. J. Med. 2011;364:116-26).
As I see it, the problem really lies in our inability to adequately diagnose AOM. For one thing, it’s essential to clean the wax out of the child’s ear in order to visualize the eardrum, given that two-thirds of children diagnosed with AOM have partially or fully occluded ear canals blocking visualization of the eardrum. Yet, physicians often don’t do that because it takes time and it’s difficult to get the child to hold still. It’s far simpler to simply take a quick look and say that the diagnosis is "uncertain," or to say that the eardrum is "red" in order to justify a diagnosis and antibiotic prescription.
Pediatricians and family physicians should all have a good, high-grade otoscope with a fresh battery and bulb, along with the training and ability to use the pneumatic attachment in order to distinguish between a bulging and retracted eardrum, which often look alike with just the otoscope.
Frankly, I find it embarrassing that with a condition as common as AOM, pediatricians and family physicians receive so little training in diagnosing it and, therefore, just don’t do a good job. In otitis media workshops that include testing for competency in diagnosis (Outcomes Management Educational Workshops, West Palm Beach, Fla.), I found that physicians got the diagnosis of AOM wrong at least 50% of the time on video presentation testing. And that was without wax, under ideal classroom conditions.
Diagnosing otitis media needs to become a critical part of medical education, and physicians in practice should be retrained via CME courses. Pharmaceutical companies no longer sponsor those, so now the professional societies such as the American Academy of Pediatrics, the American Academy of Family Physicians, and the nursing organizations need to step up.
With the new evidence from the two well-controlled trials, I don’t see how any clinician can withhold antibiotic treatment in good conscience. AOM is a painful condition that infants and toddlers are too young to explain to us. Can you imagine asking an adult to agree to withholding effective treatment when they are in pain and propose they take acetaminophen instead? Or can you imagine telling an adult who seeks care for an earache that the diagnosis is uncertain after examination, so the recommendation is to "observe"?
As advocates for our pediatric patients, how in the world can we allow a child to remain in severe pain for 24-48 hours longer than is necessary and keep parents up all night and away from work for 2-3 extra days?
Once everyone learns how to better diagnose AOM, we will stop overprescribing antibiotics for those children who don’t have the condition. For the rest, I contend that treatment is a moral imperative.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Rochester (N.Y.) General Research Institute. Dr. Pichichero disclosed that he is the medical director of Outcome Management Educational Workshops that train providers to improve diagnosis but has no disclosures related to antibiotic use in AOM.
Two new, well-designed trials published in the New England Journal of Medicine have demonstrated that when acute otitis media is correctly diagnosed, treatment with effective antibiotics is of clear and substantial benefit. To me, this suggests that the confusion about whether antibiotics help children get better faster is about getting the diagnosis right, a challenging task for pediatricians and family physicians with squirming patients and ear canal wax occluding visualization of the eardrum.
All along, I have believed that the American Academy of Pediatrics’ 2004 "watchful waiting" option for treating acute otitis media (AOM) was well intentioned but not based on good evidence. In an effort to address the growing problem of antimicrobial resistance, the AAP recommended the "observation option" for otherwise healthy children aged 6 months to 2 years with nonsevere illness and an uncertain diagnosis, and for all children above the age of 2 years who were not systemically ill (Pediatrics 2004;113:1451-65).
Problem is, the studies cited by the AAP as evidence for this recommendation were nearly all seriously flawed, because they excluded children with the very criteria that signal a true AOM diagnosis: a full or bulging eardrum ... and in some studies, because it was determined that they were too "unwell" and/or they "needed an antibiotic"! And, many of these trials excluded children younger than 2 years old and included many children who likely did not have AOM at all or had otitis media with effusion.
Dr. Janet R. Casey and I reviewed 25 of the studies in a paper published 3 years ago (Pediatr. Infect. Dis. J. 2008;27:958-62). We found so many serious flaws in the inclusion and exclusion criteria, and diagnostic and outcome criteria, that we were obliged to conclude that no evidence-based conclusion could be drawn.
The flaws we found in individual AOM trials call into question the validity of the conclusions of two major meta-analyses cited by the AAP, one involving 5,400 children from 33 randomized trials (J. Pediatr. 1994;124:355-67), the other of 6 studies of children aged 7 months to 15 years (BMJ 1997;314:1526-9), both of which found only modest benefit for the use of antimicrobials.
Now in the New England Journal of Medicine papers, we have two well-designed studies clearly demonstrating that treatment should not be withheld in children with proven AOM.
One of the studies, from the University of Pittsburgh, randomized 291 children aged 6-23 months to receive amoxicillin-clavulanate or placebo for 10 days. To be eligible, patients had to have AOM that was diagnosed on the basis of three criteria:
• Onset of symptoms within 48 hours that parents rated with a score of at least 3 on the Acute Otitis Media Severity of Symptoms scale,
• Presence of middle-ear effusion, and
• Moderate or marked bulging of the tympanic membrane or slight bulging accompanied by either otalgia or marked erythema of the membrane.
Patients also had to have received at least two doses of pneumococcal conjugate vaccine.
Among the children who received amoxicillin-clavulanate, 35% had initial resolution of symptoms by day 2, 61% by day 4, and 80% by day 7, compared with 28%, 54%, and 74% among those who received placebo, respectively. For sustained resolution of symptoms, the corresponding values were 20%, 41%, and 67% with amoxicillin-clavulanate, vs. 14%, 36%, and 53% with placebo (N. Engl. J. Med. 2011;364:105-15).
The other trial, from Finland, used equally strict criteria for 319 children aged 6-35 months who were randomized to receive amoxicillin-clavulanate or placebo for 7 days. Treatment failure occurred in 18.6% of the children who received amoxicillin-clavulanate, compared with 44.9% of the children who received placebo, a highly statistically significant difference that was already apparent at the first scheduled visit on day 3 (13.7% vs. 25.3%). Overall, amoxicillin-clavulanate reduced the progression to treatment failure by 62% (N. Engl. J. Med. 2011;364:116-26).
As I see it, the problem really lies in our inability to adequately diagnose AOM. For one thing, it’s essential to clean the wax out of the child’s ear in order to visualize the eardrum, given that two-thirds of children diagnosed with AOM have partially or fully occluded ear canals blocking visualization of the eardrum. Yet, physicians often don’t do that because it takes time and it’s difficult to get the child to hold still. It’s far simpler to simply take a quick look and say that the diagnosis is "uncertain," or to say that the eardrum is "red" in order to justify a diagnosis and antibiotic prescription.
Pediatricians and family physicians should all have a good, high-grade otoscope with a fresh battery and bulb, along with the training and ability to use the pneumatic attachment in order to distinguish between a bulging and retracted eardrum, which often look alike with just the otoscope.
Frankly, I find it embarrassing that with a condition as common as AOM, pediatricians and family physicians receive so little training in diagnosing it and, therefore, just don’t do a good job. In otitis media workshops that include testing for competency in diagnosis (Outcomes Management Educational Workshops, West Palm Beach, Fla.), I found that physicians got the diagnosis of AOM wrong at least 50% of the time on video presentation testing. And that was without wax, under ideal classroom conditions.
Diagnosing otitis media needs to become a critical part of medical education, and physicians in practice should be retrained via CME courses. Pharmaceutical companies no longer sponsor those, so now the professional societies such as the American Academy of Pediatrics, the American Academy of Family Physicians, and the nursing organizations need to step up.
With the new evidence from the two well-controlled trials, I don’t see how any clinician can withhold antibiotic treatment in good conscience. AOM is a painful condition that infants and toddlers are too young to explain to us. Can you imagine asking an adult to agree to withholding effective treatment when they are in pain and propose they take acetaminophen instead? Or can you imagine telling an adult who seeks care for an earache that the diagnosis is uncertain after examination, so the recommendation is to "observe"?
As advocates for our pediatric patients, how in the world can we allow a child to remain in severe pain for 24-48 hours longer than is necessary and keep parents up all night and away from work for 2-3 extra days?
Once everyone learns how to better diagnose AOM, we will stop overprescribing antibiotics for those children who don’t have the condition. For the rest, I contend that treatment is a moral imperative.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Rochester (N.Y.) General Research Institute. Dr. Pichichero disclosed that he is the medical director of Outcome Management Educational Workshops that train providers to improve diagnosis but has no disclosures related to antibiotic use in AOM.
Who should get the HPV vaccine?
- Consider recommending HPV vaccine for 11- and 12-year-old girls in your practice, before sexual activity puts them at risk of viral infection (A). The FDA has also approved the HPV vaccine for women up to 26 years of age.
- If women older than 26 years ask to be vaccinated, make sure they understand it is an off-label use for them (A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Presexual adolescent girls and sexually active women can now lower their lifetime risk of cervical cancer, thanks to a newly available quadrivalent vaccine (Gardasil) directed at human papillomavirus (HPV). This gives us the opportunity to educate parents and adolescents (the primary target group for the vaccine), many of whom remain uninformed about the direct link between HPV infection and cervical cancer.
Ethical, cultural, social, and religious issues that will require attention1 are beyond the scope of this article.
Who should receive the HPV vaccine?
Pre-adolescent and adolescent girls
Girls ages 11 to 12 years—most of whom have not started sexual activity—are the primary targets of immunization. However, the US Food and Drug Administration also approved the use of Gardasil for girls as young as 9. Girls this age may require other vaccines, such as meningococcal conjugate and tetanus-diphtheria-acellular pertussis, and experience thus far indicates no negative immune effects with co-administration of vaccines.1,2
According to one study, vaccination of the entire US population of 12-year-old girls would prevent more than 200,000 HPV infections, 100,000 abnormal Pap tests, and 3300 cases of cervical cancer.3 Parental as well as health care provider acceptance of HPV vaccines for adolescents will be critical to the success of the vaccination effort (see “What makes FPs recommend the HPV vaccine” ).4
Practical issues. As with any new vaccine added to the childhood/adolescent vaccination schedule, a host of issues will need to be resolved to ensure adequate coverage. Factors likely to influence use of HPV vaccine among adolescents are cost and reimbursement, and adherence to the 3-dose regimen that spans 6 months.
The American Academy of Pediatrics’ Committee on Infectious Diseases and the Advisory Committee on Immunization Practices (ACIP) recommends universal use of the HPV vaccine for girls, with a focus on 11- to 12-year-olds. The vaccine is also recommended for 13- to 26-year-old girls and women who have received or completed the 3-dose vaccine series.
Why not vaccinate boys? HPV infection is highly prevalent in sexually active men.5 The efficacy of vaccinating boys against HPV infection is currently being explored.6 However, one model has suggested that vaccinating adolescent males with a bivalent HPV vaccine would only slightly reduce the incidence of cervical cancer cases beyond that achieved by vaccination of adolescent girls, and with an extremely high cost-effectiveness ratio compared with female-only vaccination.5
Women ≤26 years
Indications under FDA approval also include women up to 26 years. Even adults who have been sexually active for years may not have been exposed to all high-risk HPV covered by the vaccine.
Are women older than 26 years eligible?
Though FDA approval of the vaccine is for females aged 9 to 26 years, a recent working group on HPV prevention concluded that any sexually active person may benefit from vaccination and should have the opportunity to receive the vaccine.1 Importantly, women older than 26 years who request the vaccine should be made fully aware of its off-label application in their case.
The rationale behind the recommendations
HPV transmission occurs easily with skin-to-skin contact.8-11 HPV can infect the external genitalia during non-intercourse sexual activities, including manual and oral genital contact. Sexual intercourse is the most frequent mode of infection of the cervix. Condoms may help protect against transmission of HPV but are not fully effective.8,12
Adolescents are particularly vulnerable to HPV, but respond best to vaccine. The cervix is especially susceptible to HPV infection in adolescence because the squamous columnar cell junction transformation zone is more exposed. The adult cervix is less susceptible to HPV than the adolescent cervix because of the smaller area of cervical ectopy comprised of columnar epithelial cells.13 However, in adolescents, the immune response to HPV exposure is greater than in than adults.
Risk for acquiring HPV infection. Risk factors for acquiring HPV infection are listed in the TABLE .8,14,15 According to the Centers for Disease Control and Prevention, sexually active men and women have a 50% lifetime risk of acquiring HPV infection.16 An estimated 6.2 million people in the US become infected with HPV each year,16 and approximately 20 million currently harbor HPV infections.17 This estimate includes more than 9 million sexually active adolescents and young adults 15 to 24 years of age, the group in which nearly 75% of new HPV infections occur.18 Among women <25 years of age, between 28% and 46% are infected with HPV.19,20
Infection cannot always be cleared. Most HPV infections (whether high-risk or low-risk type) are asymptomatic and are efficiently cleared (ie, no detection of DNA for a specific HPV type) by the immune system.21,22 However, if the infection cannot be cleared or controlled by the immune system, it may become a persistent infection.
Persistent infection with HPV increases the probability of progression to high-grade cervical intraepithelial neoplasia (CIN) and invasive carcinoma ( FIGURE ).18-19 Evidence also increasingly shows that high-risk HPV types likely cause anal, penile, scrotal, vulvar, vaginal, and some head and neck cancers.25
After HPV vaccination, neutralizing antibodies are secreted from memory B cells, and bind to their target HPV type, preventing infection before it occurs, thereby blocking the initial step toward development of cervical cancer.
15 high-risk oncogenic types. Papillomaviruses such as HPV are nonenveloped, double-stranded, DNA viruses. They infect cutaneous and mucosal epithelial tissues. More than 100 HPV types have been identified,3 about 30 to 40 of which are spread by sexual contact.4 Of the many known HPVs, only 15 are high-risk oncogenic types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, and 73) that can cause cervical cancer.5.6 Of these high-risk oncogenic types, HPV 16 and 18 account for about 70% of all cervical cancers.7
The new HPV vaccines, Gardasil and Cervarix, (see Web table) both contain virosomal antigens to vaccinate against HPV types 16 and 18. Persistent infection with these high-risk HPV types is necessary for the development of cervical cancer. Chronic infection with low-risk HPV types (eg, HPV 6 or 11) may lead to the development of anogenital warts and other low-grade genital abnormalities, as well as laryngeal cancer or recurrent respiratory papillomatosis. Gardasil also contains virosome antigens for these 2 HPV types. Warts on the hands are usually attributable to HPV 7.8
Viral integration is a necessary step in the malignant transformation of HPV infection; infection may progress from residential to episomal, and, finally, to an integrated form. Residential infection typically occurs a minimum of 6 weeks from exposure, can persist without detection for decades, and can be low risk or high risk. In the episomal state, virally active HPV is located in the cell nucleus, separate from the human DNA. In the integrated form of infection, the HPV DNA circle has opened and joined the human DNA. Integrated HPV—always high risk—produces an abnormal Papanicolaou (Pap) test. If recognized on colposcopy, it must be treated to prevent progression to cervical cancer.
FIGURE
How HPV infection progresses to cervical cancer
Adapted with permission from Pinto and Crum 200023 and Schlecht et al 2001.24
TABLE
Factors that put women at risk for HPV infection
| Young age (peak age group: 20–24 years) |
| Lifetime number of sexual partners |
| First sexual intercourse at early age |
| Male partner sexual behavior |
| Smoking |
| Oral contraceptive use |
| Uncircumcised male partners |
| Sources: Winer et al 2003;8 Schiffman and castle 2003;14 Insinga et al 2003.15 |
Why screening alone isn’t enough
New technologies for Pap testing, HPV DNA testing, and revisions in the Bethesda system for reporting cervical cytology have led to better treatment recommendations for patients with abnormal cytology results.26 But despite these advances, cervical screening is underused or not used at all for many women at risk.
For example, some women with abnormal cervical cytology—especially those of lower socioeconomic status, who often are medically underserved or lack insurance—may not receive adequate follow-up care.27 Though widespread cervical screening in the future may significantly decrease morbidity and mortality associated with cervical cancer, HPV vaccination can also help achieve this goal.
The case for vaccination plus screening
It will likely take at least a decade to assess the impact of HPV vaccination on invasive cervical cancer, and perhaps 20 to 30 years to achieve the maximum benefit from such a program. A computer-based model of the natural history of HPV and cervical cancer developed by the Harvard School of Public Health considered different cancer prevention policies, including vaccination against HPV types 16 and 18 (initiated at the age of 12 years), cytologic screening (initiated at 18, 21, 25, 30, or 35 years,) and combined vaccination and screening strategies. The model showed the combination strategy to be most effective.28
Dramatic reductions expected. The model predicts that with current screening and vaccination against HPV, low-grade cervical abnormalities associated with HPV-16 and HPV-18 infections would be reduced by 15% and high-grade lesions by 49%. Vaccination would decrease the number of cases of cervical cancer by about 66% in conjunction with screening. The vaccine, however, would not prevent cancers caused by other high-risk HPV types.
According to the model, HPV vaccination would produce health gains that are well worth the cost. Specifically, the cost per additional quality-adjusted life-year gained with vaccinating only females was estimated to be $21,000. This ratio compares favorably with many adult and pediatric vaccines currently used in the US.
A recent survey of attitudes about HPV vaccination among members of the American Academy of Family Physicians (AAFP) found that survey respondents would be more likely to administer an HPV vaccine to girls than to boys and to older rather than younger adolescents.4 Female gender, knowledge about HPV, and attitudes about vaccination were independently associated with family physicians’ intentions to recommend HPV vaccination.
It will take decades to see cervical cancer rates drop, but we will soon see fewer CIN 2/3 lesions once HPV 16/18 vaccination is routine.
HPV types 6 and 11 cause 90% of genital warts
Looking forward
The long-term efficacy of HPV vaccines remains to be determined. Sustained efficacy up to 4.5 years has been documented29 but it could be that boosters will be needed.
Research has shown that adolescents and parents, and even some providers of adolescent health care, may have a significant misunderstanding about HPV infection and its possible sequelae,30 suggesting the need for educational programs about the disease and its prevention. Education and vaccine advocacy from professional organizations such as the AAFP, the American Academy of Pediatrics, and the American College of Obstetricians and Gynecologists will be essential to foster acceptance of HPV vaccination.
CORRESPONDENCE
Michael E. Pichichero, MD, Elmwood Pediatric Group, 601 Elmwood Avenue, Box 672, Rochester, NY 14642. [email protected]
1. Frazer IH, Cox JT, Mayeaux Jr EJ, et al. advances in prevention of cervical cancer and other human papillomavirus-related diseases. Pediatr Infect Dis J 2006;25:S65-S81.
2. Bonnez W. Immunization against genital human papillomaviruses. J Infect Dis 2005;24:1005-1006.
3. Sanders GD, Taira AV. cost-effectiveness of a potential vaccine for human papillomavirus. Emerg Infect Dis 2003;9:37-48.
4. Riedesel JM, Rosenthal SL, Zimet GD, et al. Attitudes about HPV vaccine among family physicians. J Pediatr Adolesc Gynecol 2005;18:391-398.
5. Dunne EF, Nielson CM, Stone KM, Markowitz LE, Giuliano AR. Prevalence of HPV infection among men: A systematic review of the literature. J Infect Dis 2006;194:1044-1057.
6. Block SL, Nolan T, Sattler C, et al. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in mail and female adolescents and young adult women. Pediatrics 2006;118:2135-2145.
7. Taira AV, Neukermans CP, Sanders GD. Evaluating human papillomavirus vaccination programs. Emerg Infect Dis 2004;19:1915-1923.
8. Winer RL, Lee SK, Hughes JP, et al. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol 2003;157:218-226.
8. Kjaer SK, Chackerian B, van den Brule AJC, et al. High-risk human papillomavirus is sexually transmitted: Evidence from a follow-up study of virgins starting sexual activity (intercourse). Cancer Epidemiol Biomarkers Prev 2001;10:101-106.
10. Herrero R, Castellsague X, Pawlita M, et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst 2003;95:1772-1783.
11. Smith EM, Ritchie JM, Yankowitz J, et al. Human papillomavirus prevalence and types in newborns and parents: concordance and modes of transmission. Sex Transm Dis 2004;31:57-62.
12. Winer RL, Hughes JP, Feng Q, et al. Condom use and the risk of genital human papillomavirus infection in young women. N Engl J Med 2006;354:2645-2654.
13. Kahn JA, Hillard PA. Human papillomavirus and cervical cytology in adolescents. Adolesc Med Clin 2004;15:301-321.
14. Schiffman M, Castle PE. Human papillomavirus: epidemiology and public health. Arch Pathol Lab Med 2003;127:930-934.
15. Insinga RP, Dasbach EF, Myers ER. The health and economic burden of genital warts in a set of private health plans in the United States. Clin Infect Dis 2003;36:1397-1403.
16. Centers for Disease Control and Prevention. Genital HPV Infection Fact Sheet. Rockville, Md: CDC National Prevention Information Network; 2004.
17. Cates W, Jr. and the American Social Health Association Panel. Estimates of the incidence and prevalence of sexually transmitted diseases in the United States. Sex Transm Dis 1999;26(suppl):S2-S7.
18. Weinstock H, Berman S, Cates W, Jr. Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health 2004;36:6-10.
19. Tarkowski TA, Koumans EH, Sawyer M, et al. Epidemiology of human papillomavirus infection and abnormal cytologic test results in an urban adolescent population. J Infect Dis 2004;189:46-50.
20. Revzina NV, Diclemente RJ. Prevalence and incidence of human papillomavirus infection in women in the USA: a systematic review. Int J STD AIDS 2005;16:528-537.
21. Brown DR, Shew ML, Qadadri B, et al. A longitudinal study of genital human papillomavirus infection in a cohort of closely followed adolescent women. J Infect Dis 2005;191:182-192.
22. Richardson H, Kelsall G, Tellier P, et al. The natural history of type-specific human papillomavirus infections in female university students. Cancer Epidemiol Biomarkers Prev 2003;12:485-490.
23. Pinto AP, Crum CP. Natural history of cervical neoplasia: defining progression and its consequence. Clin Obstet Gynecol 2000;43:352-362.
24. Schlecht NF, Kulaga S, Robitaille J, et al. Persistent human papillomavirus infection as a predictor of CIN. JAMA 2001;286:3106-3114.
25. Hernandez BY, McDuffie K, Zhu X, et al. Anal human papillomavirus infection in women and its relationship with cervical infection. Cancer Epidemiol Biomarkers Prev 2005;14:2550-2556.
26. Holcomb K, Runowicz CD. Cervical cancer screening. Surg Oncol Clin N Am 2005;14:777-797.
27. Benard VB, Lawson HW, Eheman CR, Anderson C, Helsel W. Adherence to guidelines for follow-up of low-grade cytologic abnormalities among medically underserved women. Obstet Gynecol 2005;105:1323-1328.
28. Goldie SJ, Kohli M, Grimm D, et al. Projected clinical benefits and cost-effectiveness of a human papillomavirus 16/18 vaccine. J Natl Cancer Inst 2004;96:604-615.
29. Harper DM, Franco EL, Wheeler CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomized control trial. Lancet 2006;367:1247-1255.
30. Dell DL, Chen H, Ahmad F, Stewart DE. Knowledge about human papillomavirus among adolescents. Obstet Gynecol 2000;96:653-656.
31. de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology 2004;324:17-27.
32. Howley PM. Papillomavirinae: The viruses and their replication. In: Fields BN, knipe DM, Howley PM, eds. Fields Virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996:2045-2076.
33. Schiffman M, Castle PE. Human papillomavirus: epidemiology and public health. Arch Pathol Lab Med 2003;127:930-934.
34. Wiley DJ, Douglas J, Beutner K, et al. External genital warts: diagnosis, treatment, and prevention. Clin Infect Dis 2002;35:S210-S224.
35. Clifford GM, Smith JS, Aguadp T, Franceschi S. Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: a meta-analysis. Br J Cancer 2003;89:101-105.
36. de Villiers EM, Neumann C, Oltersdorf T, Fierlbeck G, zur Hausen H. Butcher’s wart virus (HPV 7) infections in non-butchers. J Invest Dermatol 1986;87:236-238.
- Consider recommending HPV vaccine for 11- and 12-year-old girls in your practice, before sexual activity puts them at risk of viral infection (A). The FDA has also approved the HPV vaccine for women up to 26 years of age.
- If women older than 26 years ask to be vaccinated, make sure they understand it is an off-label use for them (A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Presexual adolescent girls and sexually active women can now lower their lifetime risk of cervical cancer, thanks to a newly available quadrivalent vaccine (Gardasil) directed at human papillomavirus (HPV). This gives us the opportunity to educate parents and adolescents (the primary target group for the vaccine), many of whom remain uninformed about the direct link between HPV infection and cervical cancer.
Ethical, cultural, social, and religious issues that will require attention1 are beyond the scope of this article.
Who should receive the HPV vaccine?
Pre-adolescent and adolescent girls
Girls ages 11 to 12 years—most of whom have not started sexual activity—are the primary targets of immunization. However, the US Food and Drug Administration also approved the use of Gardasil for girls as young as 9. Girls this age may require other vaccines, such as meningococcal conjugate and tetanus-diphtheria-acellular pertussis, and experience thus far indicates no negative immune effects with co-administration of vaccines.1,2
According to one study, vaccination of the entire US population of 12-year-old girls would prevent more than 200,000 HPV infections, 100,000 abnormal Pap tests, and 3300 cases of cervical cancer.3 Parental as well as health care provider acceptance of HPV vaccines for adolescents will be critical to the success of the vaccination effort (see “What makes FPs recommend the HPV vaccine” ).4
Practical issues. As with any new vaccine added to the childhood/adolescent vaccination schedule, a host of issues will need to be resolved to ensure adequate coverage. Factors likely to influence use of HPV vaccine among adolescents are cost and reimbursement, and adherence to the 3-dose regimen that spans 6 months.
The American Academy of Pediatrics’ Committee on Infectious Diseases and the Advisory Committee on Immunization Practices (ACIP) recommends universal use of the HPV vaccine for girls, with a focus on 11- to 12-year-olds. The vaccine is also recommended for 13- to 26-year-old girls and women who have received or completed the 3-dose vaccine series.
Why not vaccinate boys? HPV infection is highly prevalent in sexually active men.5 The efficacy of vaccinating boys against HPV infection is currently being explored.6 However, one model has suggested that vaccinating adolescent males with a bivalent HPV vaccine would only slightly reduce the incidence of cervical cancer cases beyond that achieved by vaccination of adolescent girls, and with an extremely high cost-effectiveness ratio compared with female-only vaccination.5
Women ≤26 years
Indications under FDA approval also include women up to 26 years. Even adults who have been sexually active for years may not have been exposed to all high-risk HPV covered by the vaccine.
Are women older than 26 years eligible?
Though FDA approval of the vaccine is for females aged 9 to 26 years, a recent working group on HPV prevention concluded that any sexually active person may benefit from vaccination and should have the opportunity to receive the vaccine.1 Importantly, women older than 26 years who request the vaccine should be made fully aware of its off-label application in their case.
The rationale behind the recommendations
HPV transmission occurs easily with skin-to-skin contact.8-11 HPV can infect the external genitalia during non-intercourse sexual activities, including manual and oral genital contact. Sexual intercourse is the most frequent mode of infection of the cervix. Condoms may help protect against transmission of HPV but are not fully effective.8,12
Adolescents are particularly vulnerable to HPV, but respond best to vaccine. The cervix is especially susceptible to HPV infection in adolescence because the squamous columnar cell junction transformation zone is more exposed. The adult cervix is less susceptible to HPV than the adolescent cervix because of the smaller area of cervical ectopy comprised of columnar epithelial cells.13 However, in adolescents, the immune response to HPV exposure is greater than in than adults.
Risk for acquiring HPV infection. Risk factors for acquiring HPV infection are listed in the TABLE .8,14,15 According to the Centers for Disease Control and Prevention, sexually active men and women have a 50% lifetime risk of acquiring HPV infection.16 An estimated 6.2 million people in the US become infected with HPV each year,16 and approximately 20 million currently harbor HPV infections.17 This estimate includes more than 9 million sexually active adolescents and young adults 15 to 24 years of age, the group in which nearly 75% of new HPV infections occur.18 Among women <25 years of age, between 28% and 46% are infected with HPV.19,20
Infection cannot always be cleared. Most HPV infections (whether high-risk or low-risk type) are asymptomatic and are efficiently cleared (ie, no detection of DNA for a specific HPV type) by the immune system.21,22 However, if the infection cannot be cleared or controlled by the immune system, it may become a persistent infection.
Persistent infection with HPV increases the probability of progression to high-grade cervical intraepithelial neoplasia (CIN) and invasive carcinoma ( FIGURE ).18-19 Evidence also increasingly shows that high-risk HPV types likely cause anal, penile, scrotal, vulvar, vaginal, and some head and neck cancers.25
After HPV vaccination, neutralizing antibodies are secreted from memory B cells, and bind to their target HPV type, preventing infection before it occurs, thereby blocking the initial step toward development of cervical cancer.
15 high-risk oncogenic types. Papillomaviruses such as HPV are nonenveloped, double-stranded, DNA viruses. They infect cutaneous and mucosal epithelial tissues. More than 100 HPV types have been identified,3 about 30 to 40 of which are spread by sexual contact.4 Of the many known HPVs, only 15 are high-risk oncogenic types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, and 73) that can cause cervical cancer.5.6 Of these high-risk oncogenic types, HPV 16 and 18 account for about 70% of all cervical cancers.7
The new HPV vaccines, Gardasil and Cervarix, (see Web table) both contain virosomal antigens to vaccinate against HPV types 16 and 18. Persistent infection with these high-risk HPV types is necessary for the development of cervical cancer. Chronic infection with low-risk HPV types (eg, HPV 6 or 11) may lead to the development of anogenital warts and other low-grade genital abnormalities, as well as laryngeal cancer or recurrent respiratory papillomatosis. Gardasil also contains virosome antigens for these 2 HPV types. Warts on the hands are usually attributable to HPV 7.8
Viral integration is a necessary step in the malignant transformation of HPV infection; infection may progress from residential to episomal, and, finally, to an integrated form. Residential infection typically occurs a minimum of 6 weeks from exposure, can persist without detection for decades, and can be low risk or high risk. In the episomal state, virally active HPV is located in the cell nucleus, separate from the human DNA. In the integrated form of infection, the HPV DNA circle has opened and joined the human DNA. Integrated HPV—always high risk—produces an abnormal Papanicolaou (Pap) test. If recognized on colposcopy, it must be treated to prevent progression to cervical cancer.
FIGURE
How HPV infection progresses to cervical cancer
Adapted with permission from Pinto and Crum 200023 and Schlecht et al 2001.24
TABLE
Factors that put women at risk for HPV infection
| Young age (peak age group: 20–24 years) |
| Lifetime number of sexual partners |
| First sexual intercourse at early age |
| Male partner sexual behavior |
| Smoking |
| Oral contraceptive use |
| Uncircumcised male partners |
| Sources: Winer et al 2003;8 Schiffman and castle 2003;14 Insinga et al 2003.15 |
Why screening alone isn’t enough
New technologies for Pap testing, HPV DNA testing, and revisions in the Bethesda system for reporting cervical cytology have led to better treatment recommendations for patients with abnormal cytology results.26 But despite these advances, cervical screening is underused or not used at all for many women at risk.
For example, some women with abnormal cervical cytology—especially those of lower socioeconomic status, who often are medically underserved or lack insurance—may not receive adequate follow-up care.27 Though widespread cervical screening in the future may significantly decrease morbidity and mortality associated with cervical cancer, HPV vaccination can also help achieve this goal.
The case for vaccination plus screening
It will likely take at least a decade to assess the impact of HPV vaccination on invasive cervical cancer, and perhaps 20 to 30 years to achieve the maximum benefit from such a program. A computer-based model of the natural history of HPV and cervical cancer developed by the Harvard School of Public Health considered different cancer prevention policies, including vaccination against HPV types 16 and 18 (initiated at the age of 12 years), cytologic screening (initiated at 18, 21, 25, 30, or 35 years,) and combined vaccination and screening strategies. The model showed the combination strategy to be most effective.28
Dramatic reductions expected. The model predicts that with current screening and vaccination against HPV, low-grade cervical abnormalities associated with HPV-16 and HPV-18 infections would be reduced by 15% and high-grade lesions by 49%. Vaccination would decrease the number of cases of cervical cancer by about 66% in conjunction with screening. The vaccine, however, would not prevent cancers caused by other high-risk HPV types.
According to the model, HPV vaccination would produce health gains that are well worth the cost. Specifically, the cost per additional quality-adjusted life-year gained with vaccinating only females was estimated to be $21,000. This ratio compares favorably with many adult and pediatric vaccines currently used in the US.
A recent survey of attitudes about HPV vaccination among members of the American Academy of Family Physicians (AAFP) found that survey respondents would be more likely to administer an HPV vaccine to girls than to boys and to older rather than younger adolescents.4 Female gender, knowledge about HPV, and attitudes about vaccination were independently associated with family physicians’ intentions to recommend HPV vaccination.
It will take decades to see cervical cancer rates drop, but we will soon see fewer CIN 2/3 lesions once HPV 16/18 vaccination is routine.
HPV types 6 and 11 cause 90% of genital warts
Looking forward
The long-term efficacy of HPV vaccines remains to be determined. Sustained efficacy up to 4.5 years has been documented29 but it could be that boosters will be needed.
Research has shown that adolescents and parents, and even some providers of adolescent health care, may have a significant misunderstanding about HPV infection and its possible sequelae,30 suggesting the need for educational programs about the disease and its prevention. Education and vaccine advocacy from professional organizations such as the AAFP, the American Academy of Pediatrics, and the American College of Obstetricians and Gynecologists will be essential to foster acceptance of HPV vaccination.
CORRESPONDENCE
Michael E. Pichichero, MD, Elmwood Pediatric Group, 601 Elmwood Avenue, Box 672, Rochester, NY 14642. [email protected]
- Consider recommending HPV vaccine for 11- and 12-year-old girls in your practice, before sexual activity puts them at risk of viral infection (A). The FDA has also approved the HPV vaccine for women up to 26 years of age.
- If women older than 26 years ask to be vaccinated, make sure they understand it is an off-label use for them (A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Presexual adolescent girls and sexually active women can now lower their lifetime risk of cervical cancer, thanks to a newly available quadrivalent vaccine (Gardasil) directed at human papillomavirus (HPV). This gives us the opportunity to educate parents and adolescents (the primary target group for the vaccine), many of whom remain uninformed about the direct link between HPV infection and cervical cancer.
Ethical, cultural, social, and religious issues that will require attention1 are beyond the scope of this article.
Who should receive the HPV vaccine?
Pre-adolescent and adolescent girls
Girls ages 11 to 12 years—most of whom have not started sexual activity—are the primary targets of immunization. However, the US Food and Drug Administration also approved the use of Gardasil for girls as young as 9. Girls this age may require other vaccines, such as meningococcal conjugate and tetanus-diphtheria-acellular pertussis, and experience thus far indicates no negative immune effects with co-administration of vaccines.1,2
According to one study, vaccination of the entire US population of 12-year-old girls would prevent more than 200,000 HPV infections, 100,000 abnormal Pap tests, and 3300 cases of cervical cancer.3 Parental as well as health care provider acceptance of HPV vaccines for adolescents will be critical to the success of the vaccination effort (see “What makes FPs recommend the HPV vaccine” ).4
Practical issues. As with any new vaccine added to the childhood/adolescent vaccination schedule, a host of issues will need to be resolved to ensure adequate coverage. Factors likely to influence use of HPV vaccine among adolescents are cost and reimbursement, and adherence to the 3-dose regimen that spans 6 months.
The American Academy of Pediatrics’ Committee on Infectious Diseases and the Advisory Committee on Immunization Practices (ACIP) recommends universal use of the HPV vaccine for girls, with a focus on 11- to 12-year-olds. The vaccine is also recommended for 13- to 26-year-old girls and women who have received or completed the 3-dose vaccine series.
Why not vaccinate boys? HPV infection is highly prevalent in sexually active men.5 The efficacy of vaccinating boys against HPV infection is currently being explored.6 However, one model has suggested that vaccinating adolescent males with a bivalent HPV vaccine would only slightly reduce the incidence of cervical cancer cases beyond that achieved by vaccination of adolescent girls, and with an extremely high cost-effectiveness ratio compared with female-only vaccination.5
Women ≤26 years
Indications under FDA approval also include women up to 26 years. Even adults who have been sexually active for years may not have been exposed to all high-risk HPV covered by the vaccine.
Are women older than 26 years eligible?
Though FDA approval of the vaccine is for females aged 9 to 26 years, a recent working group on HPV prevention concluded that any sexually active person may benefit from vaccination and should have the opportunity to receive the vaccine.1 Importantly, women older than 26 years who request the vaccine should be made fully aware of its off-label application in their case.
The rationale behind the recommendations
HPV transmission occurs easily with skin-to-skin contact.8-11 HPV can infect the external genitalia during non-intercourse sexual activities, including manual and oral genital contact. Sexual intercourse is the most frequent mode of infection of the cervix. Condoms may help protect against transmission of HPV but are not fully effective.8,12
Adolescents are particularly vulnerable to HPV, but respond best to vaccine. The cervix is especially susceptible to HPV infection in adolescence because the squamous columnar cell junction transformation zone is more exposed. The adult cervix is less susceptible to HPV than the adolescent cervix because of the smaller area of cervical ectopy comprised of columnar epithelial cells.13 However, in adolescents, the immune response to HPV exposure is greater than in than adults.
Risk for acquiring HPV infection. Risk factors for acquiring HPV infection are listed in the TABLE .8,14,15 According to the Centers for Disease Control and Prevention, sexually active men and women have a 50% lifetime risk of acquiring HPV infection.16 An estimated 6.2 million people in the US become infected with HPV each year,16 and approximately 20 million currently harbor HPV infections.17 This estimate includes more than 9 million sexually active adolescents and young adults 15 to 24 years of age, the group in which nearly 75% of new HPV infections occur.18 Among women <25 years of age, between 28% and 46% are infected with HPV.19,20
Infection cannot always be cleared. Most HPV infections (whether high-risk or low-risk type) are asymptomatic and are efficiently cleared (ie, no detection of DNA for a specific HPV type) by the immune system.21,22 However, if the infection cannot be cleared or controlled by the immune system, it may become a persistent infection.
Persistent infection with HPV increases the probability of progression to high-grade cervical intraepithelial neoplasia (CIN) and invasive carcinoma ( FIGURE ).18-19 Evidence also increasingly shows that high-risk HPV types likely cause anal, penile, scrotal, vulvar, vaginal, and some head and neck cancers.25
After HPV vaccination, neutralizing antibodies are secreted from memory B cells, and bind to their target HPV type, preventing infection before it occurs, thereby blocking the initial step toward development of cervical cancer.
15 high-risk oncogenic types. Papillomaviruses such as HPV are nonenveloped, double-stranded, DNA viruses. They infect cutaneous and mucosal epithelial tissues. More than 100 HPV types have been identified,3 about 30 to 40 of which are spread by sexual contact.4 Of the many known HPVs, only 15 are high-risk oncogenic types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, and 73) that can cause cervical cancer.5.6 Of these high-risk oncogenic types, HPV 16 and 18 account for about 70% of all cervical cancers.7
The new HPV vaccines, Gardasil and Cervarix, (see Web table) both contain virosomal antigens to vaccinate against HPV types 16 and 18. Persistent infection with these high-risk HPV types is necessary for the development of cervical cancer. Chronic infection with low-risk HPV types (eg, HPV 6 or 11) may lead to the development of anogenital warts and other low-grade genital abnormalities, as well as laryngeal cancer or recurrent respiratory papillomatosis. Gardasil also contains virosome antigens for these 2 HPV types. Warts on the hands are usually attributable to HPV 7.8
Viral integration is a necessary step in the malignant transformation of HPV infection; infection may progress from residential to episomal, and, finally, to an integrated form. Residential infection typically occurs a minimum of 6 weeks from exposure, can persist without detection for decades, and can be low risk or high risk. In the episomal state, virally active HPV is located in the cell nucleus, separate from the human DNA. In the integrated form of infection, the HPV DNA circle has opened and joined the human DNA. Integrated HPV—always high risk—produces an abnormal Papanicolaou (Pap) test. If recognized on colposcopy, it must be treated to prevent progression to cervical cancer.
FIGURE
How HPV infection progresses to cervical cancer
Adapted with permission from Pinto and Crum 200023 and Schlecht et al 2001.24
TABLE
Factors that put women at risk for HPV infection
| Young age (peak age group: 20–24 years) |
| Lifetime number of sexual partners |
| First sexual intercourse at early age |
| Male partner sexual behavior |
| Smoking |
| Oral contraceptive use |
| Uncircumcised male partners |
| Sources: Winer et al 2003;8 Schiffman and castle 2003;14 Insinga et al 2003.15 |
Why screening alone isn’t enough
New technologies for Pap testing, HPV DNA testing, and revisions in the Bethesda system for reporting cervical cytology have led to better treatment recommendations for patients with abnormal cytology results.26 But despite these advances, cervical screening is underused or not used at all for many women at risk.
For example, some women with abnormal cervical cytology—especially those of lower socioeconomic status, who often are medically underserved or lack insurance—may not receive adequate follow-up care.27 Though widespread cervical screening in the future may significantly decrease morbidity and mortality associated with cervical cancer, HPV vaccination can also help achieve this goal.
The case for vaccination plus screening
It will likely take at least a decade to assess the impact of HPV vaccination on invasive cervical cancer, and perhaps 20 to 30 years to achieve the maximum benefit from such a program. A computer-based model of the natural history of HPV and cervical cancer developed by the Harvard School of Public Health considered different cancer prevention policies, including vaccination against HPV types 16 and 18 (initiated at the age of 12 years), cytologic screening (initiated at 18, 21, 25, 30, or 35 years,) and combined vaccination and screening strategies. The model showed the combination strategy to be most effective.28
Dramatic reductions expected. The model predicts that with current screening and vaccination against HPV, low-grade cervical abnormalities associated with HPV-16 and HPV-18 infections would be reduced by 15% and high-grade lesions by 49%. Vaccination would decrease the number of cases of cervical cancer by about 66% in conjunction with screening. The vaccine, however, would not prevent cancers caused by other high-risk HPV types.
According to the model, HPV vaccination would produce health gains that are well worth the cost. Specifically, the cost per additional quality-adjusted life-year gained with vaccinating only females was estimated to be $21,000. This ratio compares favorably with many adult and pediatric vaccines currently used in the US.
A recent survey of attitudes about HPV vaccination among members of the American Academy of Family Physicians (AAFP) found that survey respondents would be more likely to administer an HPV vaccine to girls than to boys and to older rather than younger adolescents.4 Female gender, knowledge about HPV, and attitudes about vaccination were independently associated with family physicians’ intentions to recommend HPV vaccination.
It will take decades to see cervical cancer rates drop, but we will soon see fewer CIN 2/3 lesions once HPV 16/18 vaccination is routine.
HPV types 6 and 11 cause 90% of genital warts
Looking forward
The long-term efficacy of HPV vaccines remains to be determined. Sustained efficacy up to 4.5 years has been documented29 but it could be that boosters will be needed.
Research has shown that adolescents and parents, and even some providers of adolescent health care, may have a significant misunderstanding about HPV infection and its possible sequelae,30 suggesting the need for educational programs about the disease and its prevention. Education and vaccine advocacy from professional organizations such as the AAFP, the American Academy of Pediatrics, and the American College of Obstetricians and Gynecologists will be essential to foster acceptance of HPV vaccination.
CORRESPONDENCE
Michael E. Pichichero, MD, Elmwood Pediatric Group, 601 Elmwood Avenue, Box 672, Rochester, NY 14642. [email protected]
1. Frazer IH, Cox JT, Mayeaux Jr EJ, et al. advances in prevention of cervical cancer and other human papillomavirus-related diseases. Pediatr Infect Dis J 2006;25:S65-S81.
2. Bonnez W. Immunization against genital human papillomaviruses. J Infect Dis 2005;24:1005-1006.
3. Sanders GD, Taira AV. cost-effectiveness of a potential vaccine for human papillomavirus. Emerg Infect Dis 2003;9:37-48.
4. Riedesel JM, Rosenthal SL, Zimet GD, et al. Attitudes about HPV vaccine among family physicians. J Pediatr Adolesc Gynecol 2005;18:391-398.
5. Dunne EF, Nielson CM, Stone KM, Markowitz LE, Giuliano AR. Prevalence of HPV infection among men: A systematic review of the literature. J Infect Dis 2006;194:1044-1057.
6. Block SL, Nolan T, Sattler C, et al. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in mail and female adolescents and young adult women. Pediatrics 2006;118:2135-2145.
7. Taira AV, Neukermans CP, Sanders GD. Evaluating human papillomavirus vaccination programs. Emerg Infect Dis 2004;19:1915-1923.
8. Winer RL, Lee SK, Hughes JP, et al. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol 2003;157:218-226.
8. Kjaer SK, Chackerian B, van den Brule AJC, et al. High-risk human papillomavirus is sexually transmitted: Evidence from a follow-up study of virgins starting sexual activity (intercourse). Cancer Epidemiol Biomarkers Prev 2001;10:101-106.
10. Herrero R, Castellsague X, Pawlita M, et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst 2003;95:1772-1783.
11. Smith EM, Ritchie JM, Yankowitz J, et al. Human papillomavirus prevalence and types in newborns and parents: concordance and modes of transmission. Sex Transm Dis 2004;31:57-62.
12. Winer RL, Hughes JP, Feng Q, et al. Condom use and the risk of genital human papillomavirus infection in young women. N Engl J Med 2006;354:2645-2654.
13. Kahn JA, Hillard PA. Human papillomavirus and cervical cytology in adolescents. Adolesc Med Clin 2004;15:301-321.
14. Schiffman M, Castle PE. Human papillomavirus: epidemiology and public health. Arch Pathol Lab Med 2003;127:930-934.
15. Insinga RP, Dasbach EF, Myers ER. The health and economic burden of genital warts in a set of private health plans in the United States. Clin Infect Dis 2003;36:1397-1403.
16. Centers for Disease Control and Prevention. Genital HPV Infection Fact Sheet. Rockville, Md: CDC National Prevention Information Network; 2004.
17. Cates W, Jr. and the American Social Health Association Panel. Estimates of the incidence and prevalence of sexually transmitted diseases in the United States. Sex Transm Dis 1999;26(suppl):S2-S7.
18. Weinstock H, Berman S, Cates W, Jr. Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health 2004;36:6-10.
19. Tarkowski TA, Koumans EH, Sawyer M, et al. Epidemiology of human papillomavirus infection and abnormal cytologic test results in an urban adolescent population. J Infect Dis 2004;189:46-50.
20. Revzina NV, Diclemente RJ. Prevalence and incidence of human papillomavirus infection in women in the USA: a systematic review. Int J STD AIDS 2005;16:528-537.
21. Brown DR, Shew ML, Qadadri B, et al. A longitudinal study of genital human papillomavirus infection in a cohort of closely followed adolescent women. J Infect Dis 2005;191:182-192.
22. Richardson H, Kelsall G, Tellier P, et al. The natural history of type-specific human papillomavirus infections in female university students. Cancer Epidemiol Biomarkers Prev 2003;12:485-490.
23. Pinto AP, Crum CP. Natural history of cervical neoplasia: defining progression and its consequence. Clin Obstet Gynecol 2000;43:352-362.
24. Schlecht NF, Kulaga S, Robitaille J, et al. Persistent human papillomavirus infection as a predictor of CIN. JAMA 2001;286:3106-3114.
25. Hernandez BY, McDuffie K, Zhu X, et al. Anal human papillomavirus infection in women and its relationship with cervical infection. Cancer Epidemiol Biomarkers Prev 2005;14:2550-2556.
26. Holcomb K, Runowicz CD. Cervical cancer screening. Surg Oncol Clin N Am 2005;14:777-797.
27. Benard VB, Lawson HW, Eheman CR, Anderson C, Helsel W. Adherence to guidelines for follow-up of low-grade cytologic abnormalities among medically underserved women. Obstet Gynecol 2005;105:1323-1328.
28. Goldie SJ, Kohli M, Grimm D, et al. Projected clinical benefits and cost-effectiveness of a human papillomavirus 16/18 vaccine. J Natl Cancer Inst 2004;96:604-615.
29. Harper DM, Franco EL, Wheeler CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomized control trial. Lancet 2006;367:1247-1255.
30. Dell DL, Chen H, Ahmad F, Stewart DE. Knowledge about human papillomavirus among adolescents. Obstet Gynecol 2000;96:653-656.
31. de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology 2004;324:17-27.
32. Howley PM. Papillomavirinae: The viruses and their replication. In: Fields BN, knipe DM, Howley PM, eds. Fields Virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996:2045-2076.
33. Schiffman M, Castle PE. Human papillomavirus: epidemiology and public health. Arch Pathol Lab Med 2003;127:930-934.
34. Wiley DJ, Douglas J, Beutner K, et al. External genital warts: diagnosis, treatment, and prevention. Clin Infect Dis 2002;35:S210-S224.
35. Clifford GM, Smith JS, Aguadp T, Franceschi S. Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: a meta-analysis. Br J Cancer 2003;89:101-105.
36. de Villiers EM, Neumann C, Oltersdorf T, Fierlbeck G, zur Hausen H. Butcher’s wart virus (HPV 7) infections in non-butchers. J Invest Dermatol 1986;87:236-238.
1. Frazer IH, Cox JT, Mayeaux Jr EJ, et al. advances in prevention of cervical cancer and other human papillomavirus-related diseases. Pediatr Infect Dis J 2006;25:S65-S81.
2. Bonnez W. Immunization against genital human papillomaviruses. J Infect Dis 2005;24:1005-1006.
3. Sanders GD, Taira AV. cost-effectiveness of a potential vaccine for human papillomavirus. Emerg Infect Dis 2003;9:37-48.
4. Riedesel JM, Rosenthal SL, Zimet GD, et al. Attitudes about HPV vaccine among family physicians. J Pediatr Adolesc Gynecol 2005;18:391-398.
5. Dunne EF, Nielson CM, Stone KM, Markowitz LE, Giuliano AR. Prevalence of HPV infection among men: A systematic review of the literature. J Infect Dis 2006;194:1044-1057.
6. Block SL, Nolan T, Sattler C, et al. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in mail and female adolescents and young adult women. Pediatrics 2006;118:2135-2145.
7. Taira AV, Neukermans CP, Sanders GD. Evaluating human papillomavirus vaccination programs. Emerg Infect Dis 2004;19:1915-1923.
8. Winer RL, Lee SK, Hughes JP, et al. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol 2003;157:218-226.
8. Kjaer SK, Chackerian B, van den Brule AJC, et al. High-risk human papillomavirus is sexually transmitted: Evidence from a follow-up study of virgins starting sexual activity (intercourse). Cancer Epidemiol Biomarkers Prev 2001;10:101-106.
10. Herrero R, Castellsague X, Pawlita M, et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst 2003;95:1772-1783.
11. Smith EM, Ritchie JM, Yankowitz J, et al. Human papillomavirus prevalence and types in newborns and parents: concordance and modes of transmission. Sex Transm Dis 2004;31:57-62.
12. Winer RL, Hughes JP, Feng Q, et al. Condom use and the risk of genital human papillomavirus infection in young women. N Engl J Med 2006;354:2645-2654.
13. Kahn JA, Hillard PA. Human papillomavirus and cervical cytology in adolescents. Adolesc Med Clin 2004;15:301-321.
14. Schiffman M, Castle PE. Human papillomavirus: epidemiology and public health. Arch Pathol Lab Med 2003;127:930-934.
15. Insinga RP, Dasbach EF, Myers ER. The health and economic burden of genital warts in a set of private health plans in the United States. Clin Infect Dis 2003;36:1397-1403.
16. Centers for Disease Control and Prevention. Genital HPV Infection Fact Sheet. Rockville, Md: CDC National Prevention Information Network; 2004.
17. Cates W, Jr. and the American Social Health Association Panel. Estimates of the incidence and prevalence of sexually transmitted diseases in the United States. Sex Transm Dis 1999;26(suppl):S2-S7.
18. Weinstock H, Berman S, Cates W, Jr. Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health 2004;36:6-10.
19. Tarkowski TA, Koumans EH, Sawyer M, et al. Epidemiology of human papillomavirus infection and abnormal cytologic test results in an urban adolescent population. J Infect Dis 2004;189:46-50.
20. Revzina NV, Diclemente RJ. Prevalence and incidence of human papillomavirus infection in women in the USA: a systematic review. Int J STD AIDS 2005;16:528-537.
21. Brown DR, Shew ML, Qadadri B, et al. A longitudinal study of genital human papillomavirus infection in a cohort of closely followed adolescent women. J Infect Dis 2005;191:182-192.
22. Richardson H, Kelsall G, Tellier P, et al. The natural history of type-specific human papillomavirus infections in female university students. Cancer Epidemiol Biomarkers Prev 2003;12:485-490.
23. Pinto AP, Crum CP. Natural history of cervical neoplasia: defining progression and its consequence. Clin Obstet Gynecol 2000;43:352-362.
24. Schlecht NF, Kulaga S, Robitaille J, et al. Persistent human papillomavirus infection as a predictor of CIN. JAMA 2001;286:3106-3114.
25. Hernandez BY, McDuffie K, Zhu X, et al. Anal human papillomavirus infection in women and its relationship with cervical infection. Cancer Epidemiol Biomarkers Prev 2005;14:2550-2556.
26. Holcomb K, Runowicz CD. Cervical cancer screening. Surg Oncol Clin N Am 2005;14:777-797.
27. Benard VB, Lawson HW, Eheman CR, Anderson C, Helsel W. Adherence to guidelines for follow-up of low-grade cytologic abnormalities among medically underserved women. Obstet Gynecol 2005;105:1323-1328.
28. Goldie SJ, Kohli M, Grimm D, et al. Projected clinical benefits and cost-effectiveness of a human papillomavirus 16/18 vaccine. J Natl Cancer Inst 2004;96:604-615.
29. Harper DM, Franco EL, Wheeler CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomized control trial. Lancet 2006;367:1247-1255.
30. Dell DL, Chen H, Ahmad F, Stewart DE. Knowledge about human papillomavirus among adolescents. Obstet Gynecol 2000;96:653-656.
31. de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology 2004;324:17-27.
32. Howley PM. Papillomavirinae: The viruses and their replication. In: Fields BN, knipe DM, Howley PM, eds. Fields Virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996:2045-2076.
33. Schiffman M, Castle PE. Human papillomavirus: epidemiology and public health. Arch Pathol Lab Med 2003;127:930-934.
34. Wiley DJ, Douglas J, Beutner K, et al. External genital warts: diagnosis, treatment, and prevention. Clin Infect Dis 2002;35:S210-S224.
35. Clifford GM, Smith JS, Aguadp T, Franceschi S. Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: a meta-analysis. Br J Cancer 2003;89:101-105.
36. de Villiers EM, Neumann C, Oltersdorf T, Fierlbeck G, zur Hausen H. Butcher’s wart virus (HPV 7) infections in non-butchers. J Invest Dermatol 1986;87:236-238.
Cephalosporins can be prescribed safely for penicillin-allergic patients
- The widely quoted cross-allergy risk of 10% between penicillin and cephalosporins is a myth (A).
- Cephalothin, cephalexin, cefadroxil, and cefazolin confer an increased risk of allergic reaction among patients with penicillin allergy (B).
- Cefprozil, cefuroxime, cefpodoxime, ceftazidime, and ceftriaxone do not increase risk of an allergic reaction (B).
Undoubtedly you have patients who say they are allergic to penicillin but have difficulty recalling details of the reactions they experienced. To be safe, we often label these patients as penicillin-allergic without further questioning and withhold not only penicillins but cephalosporins due to concerns about potential cross-reactivity and resultant IgE-mediated, type I reactions. But even for patients truly allergic to penicillin, is the concern over cephalosporins justified? It depends on the specific agent. What is certain is that a blanket dismissal of all cephalosporins is unfounded.
The truth about the myth
Despite myriad studies spanning decades and involving varied patient populations, results have not conclusively established that penicillin allergy increases the risk of an allergic reaction to cephalosporins, compared with the incidence of a primary (and unrelated) cephalosporin allergy. Most people produce IgG and IgM antibodies in response to exposure to penicillin1 that may cross-react with cephalosporin antigens.2 The presence of these antibodies does not predict allergic, IgE cross-sensitivity to a cephalosporin. Even penicillin skin testing is generally not predictive of cephalosporin allergy.3
Reliably predicting cross-reactivity
A comprehensive review of the evidence shows that the attributable risk of a cross-reactive allergic reaction varies and is strongest when the chemical side chain of the specific cephalosporin is similar to that of penicillin or amoxicillin.
Administration of cephalothin, cephalexin, cefadroxil, and cefazolin in penicillin-allergic patients is associated with a significant increase in the rate of allergic reactions; whereas administration of cefprozil, cefuroxime, cefpodoxime, ceftazidime, and ceftriaxone is not.
Penicillin skin testing can accurately predict a penicillin-allergic reaction, but is not predictive for cephalosporin allergy unless the side chain of the penicillin or ampicillin testing reagent is similar to the cephalosporin side chain being evaluated. Patients who have a reaction to a penicillin or a cephalosporin that is not IgE mediated and not serious may receive repeated courses of that antibiotic and related antibiotics.
This article provides a comprehensive review of the frequency of allergic cross-reactivity between penicillin/amoxicillin and cephalosporin antibiotics, supporting the recent American Academy of Family Physicians evidence-based clinical practice guideline on treatment of acute otitis media recommending the use of cefuroxime, cefpodoxime, cefdinir, and ceftriaxone cephalosporins for patients allergic to penicillin.
Methods
We searched Medline and EMBASE databases for English-language articles using the keywords cephalosporin, penicillin, allergy, and cross-sensitivity for the years 1960 to 2005. Among 219 articles identified, 101 were included as source material for this review. Articles we excluded were reviews, republication of results, or ones irrelevant to our purpose.
Five articles described the rate of rashes following use of penicillin and cephalosporins,4-8 and 4 articles described rates of anaphylaxis.5,9-11 We included 26 articles for the evidence base evaluating penicillin/amoxicillin cross-allergy.3,12-36 Eleven articles relied on patient history of penicillin/amoxicillin allergy to categorize results and establish reaction rates and relative risks for the penicillin/amoxicillin allergic vs nonallergic when receiving cephalosporins.12-15,17-20,27,28,31 Fourteen articles relied on patient history of penicillin/amoxicillin allergy plus skin testing results to penicillin/amoxicillin to categorize patients.16,21-25,29,30,32-37 One article3 provided data on a subset where penicillin/amoxicillin allergy was established based on history, and a separate subset where penicillin/amoxicillin allergy was established by skin testing. Other articles related to antibiotic chemical structures, animal studies, monoclonal antibody studies, cross-reactive antibody studies, and antibiotic skin testing were also reviewed.
Results
True incidence of reactions to cephalosporins
The most frequent reactions to cephalosporins are non-pruritic, non-urticarial rashes, which occur in 1.0% to 2.8% of patients;4-8 for most, the mechanism is idiopathic and not a contraindication for future use.38 Retrospective studies suggest a 1% to 3% incidence of immune or allergic reactions to cephalosporins independent of any history of penicillin/amoxicillin allergy.31 Anaphylactic reactions from cephalosporins are extremely rare, with the risk estimated at 0.0001% to 0.1%.31,38 A seminal study suggested approximately 0.004% to 0.015% of treatment courses with penicillin results in anaphylaxis.5,9-11 Several studies suggest that cephalosporin-induced anaphylaxis occurs no more frequently among patients with known penicillin allergy than among those without such allergy.23,27,38-41
Determining cross-reactivity
Penicillins and cephalosporins both possess a beta-lactam ring for antimicrobial activity. They differ in that the 5-membered thiazolidine ring of penicillin is replaced in the cephalosporins with a 6-membered dihydrothiazine ring. After degradation, penicillin forms a stable ring, whereas cephalosporins undergo rapid fragmentation of their rings.42 Immunologic cross-reactivity between the penicillin and cephalosporin beta-lactam rings is, therefore, very unlikely—an observation confirmed by monoclonal antibody analysis.43
How the “10% cross-reactivity” myth took hold. When the first-generation cephalosporins cephaloridine and cephalothin were introduced in the 1960s, allergic and anaphylactic reactions were reported in patients with previous allergic reactions to penicillins. Subsequent reports, which attributed up to 10% cross-reactivity between the 2 drug classes, involved these same first-generation cephalosporins plus cephalexin and cefadroxil and a second-generation drug, cefamandole. However, these studies were flawed because the penicillin test compounds had been contaminated with cephalosporins. Until 1982, penicillin was produced commercially using the cephalosporium mold.38
Many recent studies have established that the rate of cross-reactivity between penicillin and cephalosporins has been grossly overestimated. In fact, the rate of cross-reactivity between penicillin/amoxicillin and second- or third-generation cephalosporins is very low and may actually be lower than that between penicillins and other classes of antibiotics.44
The evidence for limited cross-reactivity. A summary of publications evaluating 38,846 children and adults with and without a history of penicillin allergy is presented in TABLE 1. The database included 2435 patients with a history of penicillin allergy and 961 patients with a history of penicillin allergy and positive skin-test results for penicillin or amoxicillin (total penicillin-allergic patients=3396). The allergic reaction rate is compared with 34,047 patients without a history of penicillin allergy and 1403 patients without a history of penicillin allergy and negative skin-test results for penicillin or amoxicillin (total penicillin-nonallergic patients=35,450).
When patients with a positive history of penicillin-allergy received first-generation cephalosporins, which share a chemical side chain similar to penicillin or amoxicillin (cephalothin, cephaloridine, cephalexin, cefadroxil, and cefazolin, plus the early second-generation cephalosporin, cefamandole), they exhibited a significant increased risk of an allergic reaction to the cephalosporin.
Second- and third-generation cephalosporins modified in size and the complexity of their side chains (eg, cefprozil, cefuroxime, ceftazidime, cefpodoxime, and ceftriaxone) were different enough from penicillin and ampicillin that they did not increase risk of allergic cross-reactivity (TABLE 1).
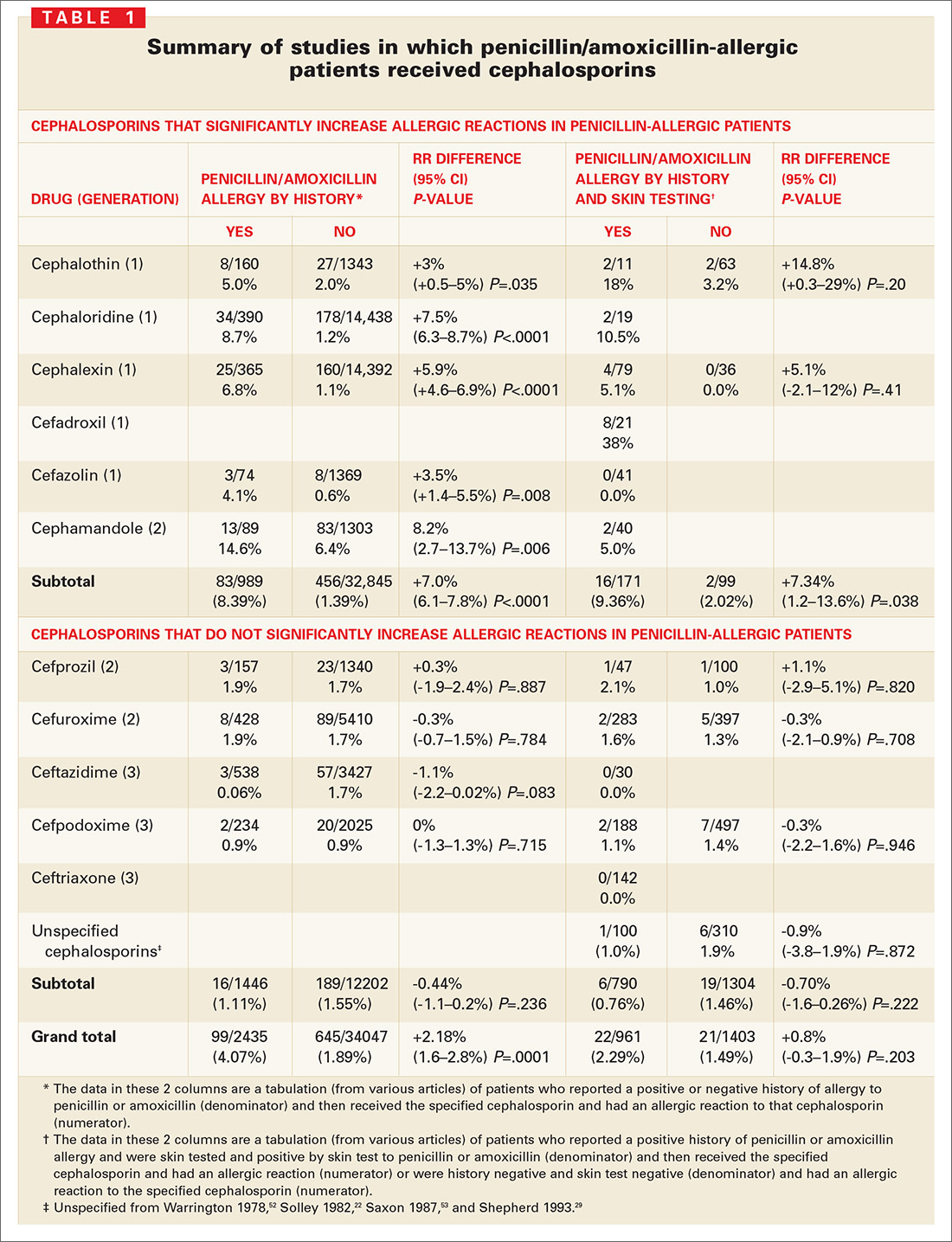
Many other studies have suggested that cross-reactive immune responses to cephalosporins depend on side chain structure;22,23,27,32,37,38,44-49 that is, cephalosporins with a 7-position side chain similar to benzylpenicillin are more likely to cross-react with penicillin (TABLE 2). Cephalosporins that share a similar 7-position or 3-position side chain are more likely to cross-react with each other.
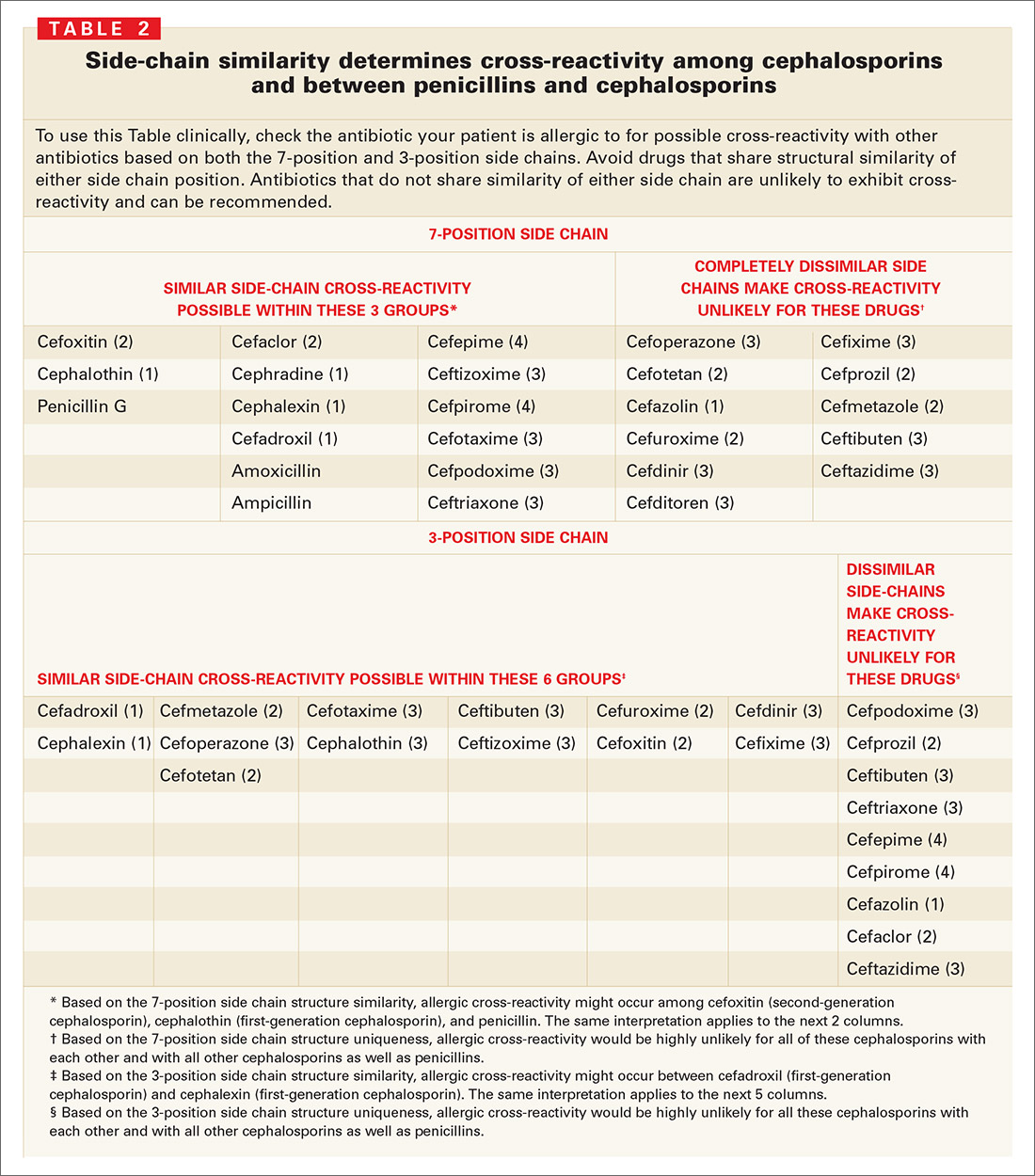
Cephalosporin/penicillin cross-reactivity
Few studies have evaluated whether patients with primary hypersensitivity to cephalosporins will experience cross-reactivity with penicillin. Romano et al49 conducted skin tests and RASTs in patients with immediate allergic reactions to cephalosporins to examine responses to other cephalosporins and to classic penicillin determinants. About 1 in 5 patients allergic to a cephalosporin reacted to penicillin determinants, while most had positive results to other cephalosporins with the same or similar side-chains.
Limitations of skin testing
Penicillin skin testing in patients with a history of penicillin allergy does not reliably predict allergy to a cephalosporin unless the side chain of the penicillin or ampicillin reagent is similar to the cephalosporin side chain being tested.3 The positive and negative predictive values of skin testing results for cephalosporins are not well established; if the haptens that cause cephalosporin allergy were known, cross-reactivity with penicillins could be assessed directly. Cephalosporin skin testing works only for the specific drug and drugs with the same side chains, and can be done only if the drug is available in an IV or IM formulation.
Even a positive result does not guarantee a clinical reaction. When penicillin and cephalosporin skin tests or radioallergosorbent tests (RASTs) are positive, a clinical reaction is observed in only 10% to 60% of patients, depending on the reagent and study.50 For example, among 19 well-characterized patients allergic to penicillin who were studied for their sensitivity to the cephalosporins, cephaloridine and cefamandole (which have identical or very similar side chains to penicillin and were therefore potentially cross-reactive) only 2 (10.5%) reacted to cefamandole, while the other 17 patients tolerated both agents.26 In another study of clinical cross-reactivity between amoxicillin and cefadroxil in patients allergic to amoxicillin with good tolerance of penicillin, only 12% had an immediate allergic reaction to cefadroxil, despite the 2 drugs sharing an identical side chain.33 In a third study, allergenic cross-reactivity with cefadroxil and cefamandole was studied among 21 patients selectively allergic to amoxicillin; 8 (38%) had a positive response to cefadroxil (same side chain) and none to cefamandole (different side chain).32
Discussion
Sensible approach to penicillin-allergic patients
Question patients who report penicillin allergy. In many cases, penicillin may not actually have been taken, or patients may have had non-immunologic adverse events such as vomiting, diarrhea, or nonspecific rash; toxic effects; or contemporaneous side effects inappropriately attributed to the drug. These patients can receive penicillin, amoxicillin, or the cephalosporins.
Without the ability to detect patients with IgE antibody to penicillin prospectively or to distinguish true IgE immunologic reactions from idiopathic reactions in patients receiving cephalosporins, it is impossible to definitely claim that increased immune or IgE-mediated reactions to cephalosporins occur in true penicillinallergic (IgE) patients.
When a cephalosporin is/is not safe for a penicillin-allergic patient. Only IgE-mediated reactions—such as anaphylaxis or hypotension, laryngeal edema, wheezing, angioedema, or urticaria—are likely to become more severe with time. Therefore, with a patient who has had a true IgE-mediated reaction to a penicillin, avoid using cephalosporins with a similar side chain. You may, however, give cephalosporins that have different side chains. Cephalosporins may also be used for patients who have had non-IgE-mediated adverse reactions (“non-type I allergy”)21 to a penicillin, such as a non-pruritic, non-urticarial morbilliform or maculopapular rash.
How prevalent is primary cephalosporin allergy? Even if the patient is not allergic to penicillin, cephalosporins can cause allergic or immune-mediated reactions in approximately 1% to 3% of patients. A patient who had an allergic reaction to a specific cephalosporin probably should not receive that cephalosporin again. The risk of a reaction with a different cephalosporin is very low to nonexistent if the side chains of the 2 drugs are dissimilar.
Bottom line. Penicillin-allergic patients have indeed shown an increased incidence of allergic reactions to cephalothin, cephaloridine, cephalexin, cefadroxil, cefazolin, and cefamandole. However, the risk has been overestimated because most studies reporting this cross-reactivity were flawed (because penicillins were contaminated with cephalosporins) and then failed to account for the fact that penicillin-allergic patients have a 3-fold increased risk of allergic reactions even to nonrelated drugs.51
For patients truly allergic to penicillin, the risk of a reaction from a cephalosporin with side chains that differ from penicillin/amoxicillin (cefuroxime, cefpodoxime, cefdinir, and ceftriaxone, as endorsed by the AAFP) is so low that use is justified and medico-legally defensible by the currently available evidence.
CORRESPONDENCE
Michael E. Pichichero, MD, University of Rochester Medical Center, 601 Elmwood Avenue, Box 672, Rochester, New York 14642. E-mail: [email protected]
1. Levine B. Immunologic mechanisms of penicillin allergy. A haptenic model system for the study of allergic diseases of man. N Engl J Med 1966;275:1115-1125.
2. Torres M, Gonzales F, Mayorga C, et al. IgG and IgE antibodies in subjects allergic to penicillins recognize different parts of the penicillin molecule. Int Arch Allergy Immunol 1997;113:342-344.
3. Pichichero M. A review of evidence supporting the American Academy of Pediatrics recommendation for prescribing cephalosporin antibiotics for penicillin-allergic patients. Pediatrics 2005;115:1048-1057.
4. Arndt J, Jick H. Rates of cutaneous reactions to drugs. A report from the Boston Collaborative Drug Surveillance Program. JAMA 1976;235:918-923.
5. Platt R. Adverse effects of third-generation cephalosporins. J Antimicrob Chemother 1982;10(C):135-140.
6. Sanders CV, Greenberg RN, Marier RL. Cefamandole and cefoxitin. Ann Intern Med 1985;103:70-78.
7. Levine LR. Quantitative comparison of adverse reactions to cefaclor vs. amoxicillin in a surveillance study. Pediatr Infect Dis 1985;4:358-361.
8. Norrby S. Side effects of cephalosporins. Drug 1987;34(Suppl 2):105-120.
9. Idsoe O, Guthe T, Wilcox R. Nature and extent of penicillin side-reactions with particular reference to fatalities from anaphylactic shock. Bull WHO 1968;38:159-188.
10. Petz L. Immunologic reactions of humans to cephalosporins. Post Grad Med J 1971;47(Suppl):64-69.
11. Gadde J, Spence M, Wheeler B, et al. Clinical experience with penicillin skin testing in a large inner-city STD clinic. JAMA 1993;270:2456-2463.
12. Walter E, Moelling K, Pavlovic J, et al. Micro-encapsulation of DNA using poly (DL-lactide-co-glycolide): stability issues and release characteristics. J Controlled Release 1999;61:361-374.
13. Weinstein L, Kaplan K, Chang T. Treatment of infections in man with cephalothin. JAMA 1964;189:829-834.
14. Griffith R, Black H. Cephalothin—a new antibiotic. Preliminary clinical and laboratory studies. JAMA 1964;189:823-828.
15. Apicella M, Perkins R, Salsaw S. Cephaloridine treatment of bacterial infections. Am J Med Sci 1966;251:266-276.
16. Assem E, Vickers M. Tests for penicillin allergy in man: The immunological cross-reaction between penicillins and cephalosporins. Immunology 1974;27:255-269.
17. Marks J, Garrett R. Cephalexin in general practice. Post Grad Med J 1970;46(Suppl):113-117.
18. Stewart G. Cross-allergenicity of penicillin G and related substances. Lancet 1962;1:509-510.
19. Dash C. Penicillin allergy and the cephalosporins. J Antimicrob Chemother 1975;(1 Suppl):107-118.
20. Petz L. Immunologic cross-reactivity between penicillins and cephalosporins. J Infect Dis 1978;137:S74-S79.
21. Thoburn R, Johnson J, Cluff L. Studies on the epidemiology of adverse drug reactions. IV. The relationship of cephalothin and penicillin allergy. JAMA 1966;198:345-348.
22. Solley G, Gleich G, Van Dellen R. Penicillin allergy: Clincal experience with a battery of skin test reagents. J Allergy Clin Immunol 1982;69:238-244.
23. Warrington R, McPhillipps S. Independent anaphylaxis to cefazolin without allergy to other beta-lactam antibiotics. J Allergy Clin Immunol 1996;98:460-462.
24. Sullivan T, Wedner H, Shatz G, et al. Skin testing to detect penicillin allergy. J Allergy Clin Immunol 1981;68:171-180.
25. Saxon A. Immediate hypersensitivity reactions to b-lactam antibiotics. Rev Infect Dis 1983;5(Suppl 2):S368-S378.
26. Blanca M, Fernandez J, Miranda A, et al. Cross reactivity between penicillins and cephalosporins: Clinical and immunological studies. J Allergy Clin Immunol 1989;83:381-385.
27. Lin R. A perspective on penicillin allergy. Arch Intern Med 1992;152:930-937.
28. Martin J, Igea J, Fraj J, et al. Allergy to amoxicillin in patients who tolerated benzylpenicillin, aztreonam, and ceftazidime. Clin Infect Dis 1992;14:592-593.
29. Shepherd G, Burton D. Administration of cephalosporin antibiotics to patients with a history of penicillin allergy [abstract]. J Allergy Clin Immunol 1993;91:262.-
30. Audicana M, Bernaola G, Urrutia I, et al. Allergic reactions to betalactams: Studies in a group of patients allergic to penicillin and evaluation of cross-reactivity with cephalosporins. Allergy 1994;49:108-113.
31. Anne S, Reisman R. Risk of administering cephalosporin antibiotics to patients with histories of penicillin allergy. Annals Allergy Asthma Immunology 1995;74:167-170.
32. Miranda A, Blanca M, Vega J, et al. Cross-reactivity between a penicillin and a cephalosporin with the same side chain. J Allergy Clin Immunol 1996;98:671-677.
33. Sastre J, Quijano L, Novalbos A, et al. Clinical cross-reactivity between amoxicillin and cephadroxil in patients allergic to amoxicillin and with good tolerance of penicillin. Allergy 1996;51:383-386.
34. Pichichero M, Pichichero D. Selecting skin testing reagents to predict amoxicillin and cephalosporin allergy. Pediatr Asthma Allergy Immunol 1997;11:79-93.
35. Novalbos A, Sastre J, Cuesta J, et al. Lack of allergic cross reactivity to cephalosporins among patients allergic to penicillins. Clin Exp Allergy 2001;31:438-443.
36. Romano A, Gueant-Rodriguez RM, Viola M, et al. Cross-reactivity and tolerability of cephalosporins in patients with immediate hypersensitivity to penicillins. Ann Intern Med 2004;141:16-22.
37. Torres M, Blanca M, Garcia J. Evaluation of a large cohort of subjects allergic to penicillins [abstract]. J Allergy Clin Immunol 1995;95:285.-
38. Kelkar P, Li J. Cephalosporin allergy. N Engl J Med 2001;385:804-809.
39. Kabins S, Einstein B, Cohen S. Anaphylactic reaction to an initial dose of sodium cephalothin. JAMA 1965;193:165.-
40. Romano A, Piunti E, De Fronso M, et al. Selective immediate hypersensitivity to ceftriaxone. Allergy 2000;55:418-419.
41. Pumphrey R, Davis S. Under-reporting of antibiotic anaphylaxis may put patients at risk. Lancet 1999;353:1157-1158.
42. Mayorga C, Torres M, Blanca M. Cephalosporin allergy. N Engl J Med 2002;236:380-381.
43. Mayorga C, Ovispo T, Jimeno L. Epitope mapping of betalactam antibiotics with the use of monoclonal antibodies. Toxicology 1995;97:225-34.
44. Weiss M, Adkinson N. Immediate hypersensitivity reactions to penicillin and related antibiotics. Clin Allergy 1998;18:515-540.
45. Blaiss M, DeShazo R. Drug allergy. Pediatr Clin North Am 1998;35:1131-1147.
46. Baumgart K, Baldo B. Cephalosporin allergy. N Engl J Med 2002;346:380.-
47. James J. Diagnosis of penicillin, amoxicillin, and cephalosporin allergy: reliability of examination assessed by skin testing and oral challenge. Pediatrics 1999;104:367.-
48. Pichichero M, Pichichero D. Diagnosis of penicillin, amoxicillin, and cephalosporin allergy: Reliability of examination assessed by skin testing and oral challenge. J Pediatr 1998;132:137-143.
49. Romano A, Mayorga C, Torres M, et al. Immediate allergic reactions to cephalosporins: Cross-reactivity and selective responses. J Allergy Clin Immunol 2000;106:1177-1183.
50. Salkind A, Cuddy P, Foxworth J. Is this patient allergic to penicillin? An evidence-based analysis of the likelihood of penicillin allergy. JAMA 2001;285:2498-2505.
51. Smith J, Johnson J, Cluff L. Studies on the epidemiology of adverse drug reactions: II. An evaluation of penicillin allergy. N Engl J Med 1966;274:998-1002.
52. Warrington R, Simons F, Ho H, et al. Diagnosis of penicillin allergy by skin testing: the Manitoba experience. Can Med Assoc J 1978;118:797-791.
53. Saxon A, Beall G, Rohr A. Immediate hypersensitivity reactions to beta-lactam antibiotics. Ann Intern Med 1987;107:204-215.
- The widely quoted cross-allergy risk of 10% between penicillin and cephalosporins is a myth (A).
- Cephalothin, cephalexin, cefadroxil, and cefazolin confer an increased risk of allergic reaction among patients with penicillin allergy (B).
- Cefprozil, cefuroxime, cefpodoxime, ceftazidime, and ceftriaxone do not increase risk of an allergic reaction (B).
Undoubtedly you have patients who say they are allergic to penicillin but have difficulty recalling details of the reactions they experienced. To be safe, we often label these patients as penicillin-allergic without further questioning and withhold not only penicillins but cephalosporins due to concerns about potential cross-reactivity and resultant IgE-mediated, type I reactions. But even for patients truly allergic to penicillin, is the concern over cephalosporins justified? It depends on the specific agent. What is certain is that a blanket dismissal of all cephalosporins is unfounded.
The truth about the myth
Despite myriad studies spanning decades and involving varied patient populations, results have not conclusively established that penicillin allergy increases the risk of an allergic reaction to cephalosporins, compared with the incidence of a primary (and unrelated) cephalosporin allergy. Most people produce IgG and IgM antibodies in response to exposure to penicillin1 that may cross-react with cephalosporin antigens.2 The presence of these antibodies does not predict allergic, IgE cross-sensitivity to a cephalosporin. Even penicillin skin testing is generally not predictive of cephalosporin allergy.3
Reliably predicting cross-reactivity
A comprehensive review of the evidence shows that the attributable risk of a cross-reactive allergic reaction varies and is strongest when the chemical side chain of the specific cephalosporin is similar to that of penicillin or amoxicillin.
Administration of cephalothin, cephalexin, cefadroxil, and cefazolin in penicillin-allergic patients is associated with a significant increase in the rate of allergic reactions; whereas administration of cefprozil, cefuroxime, cefpodoxime, ceftazidime, and ceftriaxone is not.
Penicillin skin testing can accurately predict a penicillin-allergic reaction, but is not predictive for cephalosporin allergy unless the side chain of the penicillin or ampicillin testing reagent is similar to the cephalosporin side chain being evaluated. Patients who have a reaction to a penicillin or a cephalosporin that is not IgE mediated and not serious may receive repeated courses of that antibiotic and related antibiotics.
This article provides a comprehensive review of the frequency of allergic cross-reactivity between penicillin/amoxicillin and cephalosporin antibiotics, supporting the recent American Academy of Family Physicians evidence-based clinical practice guideline on treatment of acute otitis media recommending the use of cefuroxime, cefpodoxime, cefdinir, and ceftriaxone cephalosporins for patients allergic to penicillin.
Methods
We searched Medline and EMBASE databases for English-language articles using the keywords cephalosporin, penicillin, allergy, and cross-sensitivity for the years 1960 to 2005. Among 219 articles identified, 101 were included as source material for this review. Articles we excluded were reviews, republication of results, or ones irrelevant to our purpose.
Five articles described the rate of rashes following use of penicillin and cephalosporins,4-8 and 4 articles described rates of anaphylaxis.5,9-11 We included 26 articles for the evidence base evaluating penicillin/amoxicillin cross-allergy.3,12-36 Eleven articles relied on patient history of penicillin/amoxicillin allergy to categorize results and establish reaction rates and relative risks for the penicillin/amoxicillin allergic vs nonallergic when receiving cephalosporins.12-15,17-20,27,28,31 Fourteen articles relied on patient history of penicillin/amoxicillin allergy plus skin testing results to penicillin/amoxicillin to categorize patients.16,21-25,29,30,32-37 One article3 provided data on a subset where penicillin/amoxicillin allergy was established based on history, and a separate subset where penicillin/amoxicillin allergy was established by skin testing. Other articles related to antibiotic chemical structures, animal studies, monoclonal antibody studies, cross-reactive antibody studies, and antibiotic skin testing were also reviewed.
Results
True incidence of reactions to cephalosporins
The most frequent reactions to cephalosporins are non-pruritic, non-urticarial rashes, which occur in 1.0% to 2.8% of patients;4-8 for most, the mechanism is idiopathic and not a contraindication for future use.38 Retrospective studies suggest a 1% to 3% incidence of immune or allergic reactions to cephalosporins independent of any history of penicillin/amoxicillin allergy.31 Anaphylactic reactions from cephalosporins are extremely rare, with the risk estimated at 0.0001% to 0.1%.31,38 A seminal study suggested approximately 0.004% to 0.015% of treatment courses with penicillin results in anaphylaxis.5,9-11 Several studies suggest that cephalosporin-induced anaphylaxis occurs no more frequently among patients with known penicillin allergy than among those without such allergy.23,27,38-41
Determining cross-reactivity
Penicillins and cephalosporins both possess a beta-lactam ring for antimicrobial activity. They differ in that the 5-membered thiazolidine ring of penicillin is replaced in the cephalosporins with a 6-membered dihydrothiazine ring. After degradation, penicillin forms a stable ring, whereas cephalosporins undergo rapid fragmentation of their rings.42 Immunologic cross-reactivity between the penicillin and cephalosporin beta-lactam rings is, therefore, very unlikely—an observation confirmed by monoclonal antibody analysis.43
How the “10% cross-reactivity” myth took hold. When the first-generation cephalosporins cephaloridine and cephalothin were introduced in the 1960s, allergic and anaphylactic reactions were reported in patients with previous allergic reactions to penicillins. Subsequent reports, which attributed up to 10% cross-reactivity between the 2 drug classes, involved these same first-generation cephalosporins plus cephalexin and cefadroxil and a second-generation drug, cefamandole. However, these studies were flawed because the penicillin test compounds had been contaminated with cephalosporins. Until 1982, penicillin was produced commercially using the cephalosporium mold.38
Many recent studies have established that the rate of cross-reactivity between penicillin and cephalosporins has been grossly overestimated. In fact, the rate of cross-reactivity between penicillin/amoxicillin and second- or third-generation cephalosporins is very low and may actually be lower than that between penicillins and other classes of antibiotics.44
The evidence for limited cross-reactivity. A summary of publications evaluating 38,846 children and adults with and without a history of penicillin allergy is presented in TABLE 1. The database included 2435 patients with a history of penicillin allergy and 961 patients with a history of penicillin allergy and positive skin-test results for penicillin or amoxicillin (total penicillin-allergic patients=3396). The allergic reaction rate is compared with 34,047 patients without a history of penicillin allergy and 1403 patients without a history of penicillin allergy and negative skin-test results for penicillin or amoxicillin (total penicillin-nonallergic patients=35,450).
When patients with a positive history of penicillin-allergy received first-generation cephalosporins, which share a chemical side chain similar to penicillin or amoxicillin (cephalothin, cephaloridine, cephalexin, cefadroxil, and cefazolin, plus the early second-generation cephalosporin, cefamandole), they exhibited a significant increased risk of an allergic reaction to the cephalosporin.
Second- and third-generation cephalosporins modified in size and the complexity of their side chains (eg, cefprozil, cefuroxime, ceftazidime, cefpodoxime, and ceftriaxone) were different enough from penicillin and ampicillin that they did not increase risk of allergic cross-reactivity (TABLE 1).

Many other studies have suggested that cross-reactive immune responses to cephalosporins depend on side chain structure;22,23,27,32,37,38,44-49 that is, cephalosporins with a 7-position side chain similar to benzylpenicillin are more likely to cross-react with penicillin (TABLE 2). Cephalosporins that share a similar 7-position or 3-position side chain are more likely to cross-react with each other.

Cephalosporin/penicillin cross-reactivity
Few studies have evaluated whether patients with primary hypersensitivity to cephalosporins will experience cross-reactivity with penicillin. Romano et al49 conducted skin tests and RASTs in patients with immediate allergic reactions to cephalosporins to examine responses to other cephalosporins and to classic penicillin determinants. About 1 in 5 patients allergic to a cephalosporin reacted to penicillin determinants, while most had positive results to other cephalosporins with the same or similar side-chains.
Limitations of skin testing
Penicillin skin testing in patients with a history of penicillin allergy does not reliably predict allergy to a cephalosporin unless the side chain of the penicillin or ampicillin reagent is similar to the cephalosporin side chain being tested.3 The positive and negative predictive values of skin testing results for cephalosporins are not well established; if the haptens that cause cephalosporin allergy were known, cross-reactivity with penicillins could be assessed directly. Cephalosporin skin testing works only for the specific drug and drugs with the same side chains, and can be done only if the drug is available in an IV or IM formulation.
Even a positive result does not guarantee a clinical reaction. When penicillin and cephalosporin skin tests or radioallergosorbent tests (RASTs) are positive, a clinical reaction is observed in only 10% to 60% of patients, depending on the reagent and study.50 For example, among 19 well-characterized patients allergic to penicillin who were studied for their sensitivity to the cephalosporins, cephaloridine and cefamandole (which have identical or very similar side chains to penicillin and were therefore potentially cross-reactive) only 2 (10.5%) reacted to cefamandole, while the other 17 patients tolerated both agents.26 In another study of clinical cross-reactivity between amoxicillin and cefadroxil in patients allergic to amoxicillin with good tolerance of penicillin, only 12% had an immediate allergic reaction to cefadroxil, despite the 2 drugs sharing an identical side chain.33 In a third study, allergenic cross-reactivity with cefadroxil and cefamandole was studied among 21 patients selectively allergic to amoxicillin; 8 (38%) had a positive response to cefadroxil (same side chain) and none to cefamandole (different side chain).32
Discussion
Sensible approach to penicillin-allergic patients
Question patients who report penicillin allergy. In many cases, penicillin may not actually have been taken, or patients may have had non-immunologic adverse events such as vomiting, diarrhea, or nonspecific rash; toxic effects; or contemporaneous side effects inappropriately attributed to the drug. These patients can receive penicillin, amoxicillin, or the cephalosporins.
Without the ability to detect patients with IgE antibody to penicillin prospectively or to distinguish true IgE immunologic reactions from idiopathic reactions in patients receiving cephalosporins, it is impossible to definitely claim that increased immune or IgE-mediated reactions to cephalosporins occur in true penicillinallergic (IgE) patients.
When a cephalosporin is/is not safe for a penicillin-allergic patient. Only IgE-mediated reactions—such as anaphylaxis or hypotension, laryngeal edema, wheezing, angioedema, or urticaria—are likely to become more severe with time. Therefore, with a patient who has had a true IgE-mediated reaction to a penicillin, avoid using cephalosporins with a similar side chain. You may, however, give cephalosporins that have different side chains. Cephalosporins may also be used for patients who have had non-IgE-mediated adverse reactions (“non-type I allergy”)21 to a penicillin, such as a non-pruritic, non-urticarial morbilliform or maculopapular rash.
How prevalent is primary cephalosporin allergy? Even if the patient is not allergic to penicillin, cephalosporins can cause allergic or immune-mediated reactions in approximately 1% to 3% of patients. A patient who had an allergic reaction to a specific cephalosporin probably should not receive that cephalosporin again. The risk of a reaction with a different cephalosporin is very low to nonexistent if the side chains of the 2 drugs are dissimilar.
Bottom line. Penicillin-allergic patients have indeed shown an increased incidence of allergic reactions to cephalothin, cephaloridine, cephalexin, cefadroxil, cefazolin, and cefamandole. However, the risk has been overestimated because most studies reporting this cross-reactivity were flawed (because penicillins were contaminated with cephalosporins) and then failed to account for the fact that penicillin-allergic patients have a 3-fold increased risk of allergic reactions even to nonrelated drugs.51
For patients truly allergic to penicillin, the risk of a reaction from a cephalosporin with side chains that differ from penicillin/amoxicillin (cefuroxime, cefpodoxime, cefdinir, and ceftriaxone, as endorsed by the AAFP) is so low that use is justified and medico-legally defensible by the currently available evidence.
CORRESPONDENCE
Michael E. Pichichero, MD, University of Rochester Medical Center, 601 Elmwood Avenue, Box 672, Rochester, New York 14642. E-mail: [email protected]
- The widely quoted cross-allergy risk of 10% between penicillin and cephalosporins is a myth (A).
- Cephalothin, cephalexin, cefadroxil, and cefazolin confer an increased risk of allergic reaction among patients with penicillin allergy (B).
- Cefprozil, cefuroxime, cefpodoxime, ceftazidime, and ceftriaxone do not increase risk of an allergic reaction (B).
Undoubtedly you have patients who say they are allergic to penicillin but have difficulty recalling details of the reactions they experienced. To be safe, we often label these patients as penicillin-allergic without further questioning and withhold not only penicillins but cephalosporins due to concerns about potential cross-reactivity and resultant IgE-mediated, type I reactions. But even for patients truly allergic to penicillin, is the concern over cephalosporins justified? It depends on the specific agent. What is certain is that a blanket dismissal of all cephalosporins is unfounded.
The truth about the myth
Despite myriad studies spanning decades and involving varied patient populations, results have not conclusively established that penicillin allergy increases the risk of an allergic reaction to cephalosporins, compared with the incidence of a primary (and unrelated) cephalosporin allergy. Most people produce IgG and IgM antibodies in response to exposure to penicillin1 that may cross-react with cephalosporin antigens.2 The presence of these antibodies does not predict allergic, IgE cross-sensitivity to a cephalosporin. Even penicillin skin testing is generally not predictive of cephalosporin allergy.3
Reliably predicting cross-reactivity
A comprehensive review of the evidence shows that the attributable risk of a cross-reactive allergic reaction varies and is strongest when the chemical side chain of the specific cephalosporin is similar to that of penicillin or amoxicillin.
Administration of cephalothin, cephalexin, cefadroxil, and cefazolin in penicillin-allergic patients is associated with a significant increase in the rate of allergic reactions; whereas administration of cefprozil, cefuroxime, cefpodoxime, ceftazidime, and ceftriaxone is not.
Penicillin skin testing can accurately predict a penicillin-allergic reaction, but is not predictive for cephalosporin allergy unless the side chain of the penicillin or ampicillin testing reagent is similar to the cephalosporin side chain being evaluated. Patients who have a reaction to a penicillin or a cephalosporin that is not IgE mediated and not serious may receive repeated courses of that antibiotic and related antibiotics.
This article provides a comprehensive review of the frequency of allergic cross-reactivity between penicillin/amoxicillin and cephalosporin antibiotics, supporting the recent American Academy of Family Physicians evidence-based clinical practice guideline on treatment of acute otitis media recommending the use of cefuroxime, cefpodoxime, cefdinir, and ceftriaxone cephalosporins for patients allergic to penicillin.
Methods
We searched Medline and EMBASE databases for English-language articles using the keywords cephalosporin, penicillin, allergy, and cross-sensitivity for the years 1960 to 2005. Among 219 articles identified, 101 were included as source material for this review. Articles we excluded were reviews, republication of results, or ones irrelevant to our purpose.
Five articles described the rate of rashes following use of penicillin and cephalosporins,4-8 and 4 articles described rates of anaphylaxis.5,9-11 We included 26 articles for the evidence base evaluating penicillin/amoxicillin cross-allergy.3,12-36 Eleven articles relied on patient history of penicillin/amoxicillin allergy to categorize results and establish reaction rates and relative risks for the penicillin/amoxicillin allergic vs nonallergic when receiving cephalosporins.12-15,17-20,27,28,31 Fourteen articles relied on patient history of penicillin/amoxicillin allergy plus skin testing results to penicillin/amoxicillin to categorize patients.16,21-25,29,30,32-37 One article3 provided data on a subset where penicillin/amoxicillin allergy was established based on history, and a separate subset where penicillin/amoxicillin allergy was established by skin testing. Other articles related to antibiotic chemical structures, animal studies, monoclonal antibody studies, cross-reactive antibody studies, and antibiotic skin testing were also reviewed.
Results
True incidence of reactions to cephalosporins
The most frequent reactions to cephalosporins are non-pruritic, non-urticarial rashes, which occur in 1.0% to 2.8% of patients;4-8 for most, the mechanism is idiopathic and not a contraindication for future use.38 Retrospective studies suggest a 1% to 3% incidence of immune or allergic reactions to cephalosporins independent of any history of penicillin/amoxicillin allergy.31 Anaphylactic reactions from cephalosporins are extremely rare, with the risk estimated at 0.0001% to 0.1%.31,38 A seminal study suggested approximately 0.004% to 0.015% of treatment courses with penicillin results in anaphylaxis.5,9-11 Several studies suggest that cephalosporin-induced anaphylaxis occurs no more frequently among patients with known penicillin allergy than among those without such allergy.23,27,38-41
Determining cross-reactivity
Penicillins and cephalosporins both possess a beta-lactam ring for antimicrobial activity. They differ in that the 5-membered thiazolidine ring of penicillin is replaced in the cephalosporins with a 6-membered dihydrothiazine ring. After degradation, penicillin forms a stable ring, whereas cephalosporins undergo rapid fragmentation of their rings.42 Immunologic cross-reactivity between the penicillin and cephalosporin beta-lactam rings is, therefore, very unlikely—an observation confirmed by monoclonal antibody analysis.43
How the “10% cross-reactivity” myth took hold. When the first-generation cephalosporins cephaloridine and cephalothin were introduced in the 1960s, allergic and anaphylactic reactions were reported in patients with previous allergic reactions to penicillins. Subsequent reports, which attributed up to 10% cross-reactivity between the 2 drug classes, involved these same first-generation cephalosporins plus cephalexin and cefadroxil and a second-generation drug, cefamandole. However, these studies were flawed because the penicillin test compounds had been contaminated with cephalosporins. Until 1982, penicillin was produced commercially using the cephalosporium mold.38
Many recent studies have established that the rate of cross-reactivity between penicillin and cephalosporins has been grossly overestimated. In fact, the rate of cross-reactivity between penicillin/amoxicillin and second- or third-generation cephalosporins is very low and may actually be lower than that between penicillins and other classes of antibiotics.44
The evidence for limited cross-reactivity. A summary of publications evaluating 38,846 children and adults with and without a history of penicillin allergy is presented in TABLE 1. The database included 2435 patients with a history of penicillin allergy and 961 patients with a history of penicillin allergy and positive skin-test results for penicillin or amoxicillin (total penicillin-allergic patients=3396). The allergic reaction rate is compared with 34,047 patients without a history of penicillin allergy and 1403 patients without a history of penicillin allergy and negative skin-test results for penicillin or amoxicillin (total penicillin-nonallergic patients=35,450).
When patients with a positive history of penicillin-allergy received first-generation cephalosporins, which share a chemical side chain similar to penicillin or amoxicillin (cephalothin, cephaloridine, cephalexin, cefadroxil, and cefazolin, plus the early second-generation cephalosporin, cefamandole), they exhibited a significant increased risk of an allergic reaction to the cephalosporin.
Second- and third-generation cephalosporins modified in size and the complexity of their side chains (eg, cefprozil, cefuroxime, ceftazidime, cefpodoxime, and ceftriaxone) were different enough from penicillin and ampicillin that they did not increase risk of allergic cross-reactivity (TABLE 1).

Many other studies have suggested that cross-reactive immune responses to cephalosporins depend on side chain structure;22,23,27,32,37,38,44-49 that is, cephalosporins with a 7-position side chain similar to benzylpenicillin are more likely to cross-react with penicillin (TABLE 2). Cephalosporins that share a similar 7-position or 3-position side chain are more likely to cross-react with each other.

Cephalosporin/penicillin cross-reactivity
Few studies have evaluated whether patients with primary hypersensitivity to cephalosporins will experience cross-reactivity with penicillin. Romano et al49 conducted skin tests and RASTs in patients with immediate allergic reactions to cephalosporins to examine responses to other cephalosporins and to classic penicillin determinants. About 1 in 5 patients allergic to a cephalosporin reacted to penicillin determinants, while most had positive results to other cephalosporins with the same or similar side-chains.
Limitations of skin testing
Penicillin skin testing in patients with a history of penicillin allergy does not reliably predict allergy to a cephalosporin unless the side chain of the penicillin or ampicillin reagent is similar to the cephalosporin side chain being tested.3 The positive and negative predictive values of skin testing results for cephalosporins are not well established; if the haptens that cause cephalosporin allergy were known, cross-reactivity with penicillins could be assessed directly. Cephalosporin skin testing works only for the specific drug and drugs with the same side chains, and can be done only if the drug is available in an IV or IM formulation.
Even a positive result does not guarantee a clinical reaction. When penicillin and cephalosporin skin tests or radioallergosorbent tests (RASTs) are positive, a clinical reaction is observed in only 10% to 60% of patients, depending on the reagent and study.50 For example, among 19 well-characterized patients allergic to penicillin who were studied for their sensitivity to the cephalosporins, cephaloridine and cefamandole (which have identical or very similar side chains to penicillin and were therefore potentially cross-reactive) only 2 (10.5%) reacted to cefamandole, while the other 17 patients tolerated both agents.26 In another study of clinical cross-reactivity between amoxicillin and cefadroxil in patients allergic to amoxicillin with good tolerance of penicillin, only 12% had an immediate allergic reaction to cefadroxil, despite the 2 drugs sharing an identical side chain.33 In a third study, allergenic cross-reactivity with cefadroxil and cefamandole was studied among 21 patients selectively allergic to amoxicillin; 8 (38%) had a positive response to cefadroxil (same side chain) and none to cefamandole (different side chain).32
Discussion
Sensible approach to penicillin-allergic patients
Question patients who report penicillin allergy. In many cases, penicillin may not actually have been taken, or patients may have had non-immunologic adverse events such as vomiting, diarrhea, or nonspecific rash; toxic effects; or contemporaneous side effects inappropriately attributed to the drug. These patients can receive penicillin, amoxicillin, or the cephalosporins.
Without the ability to detect patients with IgE antibody to penicillin prospectively or to distinguish true IgE immunologic reactions from idiopathic reactions in patients receiving cephalosporins, it is impossible to definitely claim that increased immune or IgE-mediated reactions to cephalosporins occur in true penicillinallergic (IgE) patients.
When a cephalosporin is/is not safe for a penicillin-allergic patient. Only IgE-mediated reactions—such as anaphylaxis or hypotension, laryngeal edema, wheezing, angioedema, or urticaria—are likely to become more severe with time. Therefore, with a patient who has had a true IgE-mediated reaction to a penicillin, avoid using cephalosporins with a similar side chain. You may, however, give cephalosporins that have different side chains. Cephalosporins may also be used for patients who have had non-IgE-mediated adverse reactions (“non-type I allergy”)21 to a penicillin, such as a non-pruritic, non-urticarial morbilliform or maculopapular rash.
How prevalent is primary cephalosporin allergy? Even if the patient is not allergic to penicillin, cephalosporins can cause allergic or immune-mediated reactions in approximately 1% to 3% of patients. A patient who had an allergic reaction to a specific cephalosporin probably should not receive that cephalosporin again. The risk of a reaction with a different cephalosporin is very low to nonexistent if the side chains of the 2 drugs are dissimilar.
Bottom line. Penicillin-allergic patients have indeed shown an increased incidence of allergic reactions to cephalothin, cephaloridine, cephalexin, cefadroxil, cefazolin, and cefamandole. However, the risk has been overestimated because most studies reporting this cross-reactivity were flawed (because penicillins were contaminated with cephalosporins) and then failed to account for the fact that penicillin-allergic patients have a 3-fold increased risk of allergic reactions even to nonrelated drugs.51
For patients truly allergic to penicillin, the risk of a reaction from a cephalosporin with side chains that differ from penicillin/amoxicillin (cefuroxime, cefpodoxime, cefdinir, and ceftriaxone, as endorsed by the AAFP) is so low that use is justified and medico-legally defensible by the currently available evidence.
CORRESPONDENCE
Michael E. Pichichero, MD, University of Rochester Medical Center, 601 Elmwood Avenue, Box 672, Rochester, New York 14642. E-mail: [email protected]
1. Levine B. Immunologic mechanisms of penicillin allergy. A haptenic model system for the study of allergic diseases of man. N Engl J Med 1966;275:1115-1125.
2. Torres M, Gonzales F, Mayorga C, et al. IgG and IgE antibodies in subjects allergic to penicillins recognize different parts of the penicillin molecule. Int Arch Allergy Immunol 1997;113:342-344.
3. Pichichero M. A review of evidence supporting the American Academy of Pediatrics recommendation for prescribing cephalosporin antibiotics for penicillin-allergic patients. Pediatrics 2005;115:1048-1057.
4. Arndt J, Jick H. Rates of cutaneous reactions to drugs. A report from the Boston Collaborative Drug Surveillance Program. JAMA 1976;235:918-923.
5. Platt R. Adverse effects of third-generation cephalosporins. J Antimicrob Chemother 1982;10(C):135-140.
6. Sanders CV, Greenberg RN, Marier RL. Cefamandole and cefoxitin. Ann Intern Med 1985;103:70-78.
7. Levine LR. Quantitative comparison of adverse reactions to cefaclor vs. amoxicillin in a surveillance study. Pediatr Infect Dis 1985;4:358-361.
8. Norrby S. Side effects of cephalosporins. Drug 1987;34(Suppl 2):105-120.
9. Idsoe O, Guthe T, Wilcox R. Nature and extent of penicillin side-reactions with particular reference to fatalities from anaphylactic shock. Bull WHO 1968;38:159-188.
10. Petz L. Immunologic reactions of humans to cephalosporins. Post Grad Med J 1971;47(Suppl):64-69.
11. Gadde J, Spence M, Wheeler B, et al. Clinical experience with penicillin skin testing in a large inner-city STD clinic. JAMA 1993;270:2456-2463.
12. Walter E, Moelling K, Pavlovic J, et al. Micro-encapsulation of DNA using poly (DL-lactide-co-glycolide): stability issues and release characteristics. J Controlled Release 1999;61:361-374.
13. Weinstein L, Kaplan K, Chang T. Treatment of infections in man with cephalothin. JAMA 1964;189:829-834.
14. Griffith R, Black H. Cephalothin—a new antibiotic. Preliminary clinical and laboratory studies. JAMA 1964;189:823-828.
15. Apicella M, Perkins R, Salsaw S. Cephaloridine treatment of bacterial infections. Am J Med Sci 1966;251:266-276.
16. Assem E, Vickers M. Tests for penicillin allergy in man: The immunological cross-reaction between penicillins and cephalosporins. Immunology 1974;27:255-269.
17. Marks J, Garrett R. Cephalexin in general practice. Post Grad Med J 1970;46(Suppl):113-117.
18. Stewart G. Cross-allergenicity of penicillin G and related substances. Lancet 1962;1:509-510.
19. Dash C. Penicillin allergy and the cephalosporins. J Antimicrob Chemother 1975;(1 Suppl):107-118.
20. Petz L. Immunologic cross-reactivity between penicillins and cephalosporins. J Infect Dis 1978;137:S74-S79.
21. Thoburn R, Johnson J, Cluff L. Studies on the epidemiology of adverse drug reactions. IV. The relationship of cephalothin and penicillin allergy. JAMA 1966;198:345-348.
22. Solley G, Gleich G, Van Dellen R. Penicillin allergy: Clincal experience with a battery of skin test reagents. J Allergy Clin Immunol 1982;69:238-244.
23. Warrington R, McPhillipps S. Independent anaphylaxis to cefazolin without allergy to other beta-lactam antibiotics. J Allergy Clin Immunol 1996;98:460-462.
24. Sullivan T, Wedner H, Shatz G, et al. Skin testing to detect penicillin allergy. J Allergy Clin Immunol 1981;68:171-180.
25. Saxon A. Immediate hypersensitivity reactions to b-lactam antibiotics. Rev Infect Dis 1983;5(Suppl 2):S368-S378.
26. Blanca M, Fernandez J, Miranda A, et al. Cross reactivity between penicillins and cephalosporins: Clinical and immunological studies. J Allergy Clin Immunol 1989;83:381-385.
27. Lin R. A perspective on penicillin allergy. Arch Intern Med 1992;152:930-937.
28. Martin J, Igea J, Fraj J, et al. Allergy to amoxicillin in patients who tolerated benzylpenicillin, aztreonam, and ceftazidime. Clin Infect Dis 1992;14:592-593.
29. Shepherd G, Burton D. Administration of cephalosporin antibiotics to patients with a history of penicillin allergy [abstract]. J Allergy Clin Immunol 1993;91:262.-
30. Audicana M, Bernaola G, Urrutia I, et al. Allergic reactions to betalactams: Studies in a group of patients allergic to penicillin and evaluation of cross-reactivity with cephalosporins. Allergy 1994;49:108-113.
31. Anne S, Reisman R. Risk of administering cephalosporin antibiotics to patients with histories of penicillin allergy. Annals Allergy Asthma Immunology 1995;74:167-170.
32. Miranda A, Blanca M, Vega J, et al. Cross-reactivity between a penicillin and a cephalosporin with the same side chain. J Allergy Clin Immunol 1996;98:671-677.
33. Sastre J, Quijano L, Novalbos A, et al. Clinical cross-reactivity between amoxicillin and cephadroxil in patients allergic to amoxicillin and with good tolerance of penicillin. Allergy 1996;51:383-386.
34. Pichichero M, Pichichero D. Selecting skin testing reagents to predict amoxicillin and cephalosporin allergy. Pediatr Asthma Allergy Immunol 1997;11:79-93.
35. Novalbos A, Sastre J, Cuesta J, et al. Lack of allergic cross reactivity to cephalosporins among patients allergic to penicillins. Clin Exp Allergy 2001;31:438-443.
36. Romano A, Gueant-Rodriguez RM, Viola M, et al. Cross-reactivity and tolerability of cephalosporins in patients with immediate hypersensitivity to penicillins. Ann Intern Med 2004;141:16-22.
37. Torres M, Blanca M, Garcia J. Evaluation of a large cohort of subjects allergic to penicillins [abstract]. J Allergy Clin Immunol 1995;95:285.-
38. Kelkar P, Li J. Cephalosporin allergy. N Engl J Med 2001;385:804-809.
39. Kabins S, Einstein B, Cohen S. Anaphylactic reaction to an initial dose of sodium cephalothin. JAMA 1965;193:165.-
40. Romano A, Piunti E, De Fronso M, et al. Selective immediate hypersensitivity to ceftriaxone. Allergy 2000;55:418-419.
41. Pumphrey R, Davis S. Under-reporting of antibiotic anaphylaxis may put patients at risk. Lancet 1999;353:1157-1158.
42. Mayorga C, Torres M, Blanca M. Cephalosporin allergy. N Engl J Med 2002;236:380-381.
43. Mayorga C, Ovispo T, Jimeno L. Epitope mapping of betalactam antibiotics with the use of monoclonal antibodies. Toxicology 1995;97:225-34.
44. Weiss M, Adkinson N. Immediate hypersensitivity reactions to penicillin and related antibiotics. Clin Allergy 1998;18:515-540.
45. Blaiss M, DeShazo R. Drug allergy. Pediatr Clin North Am 1998;35:1131-1147.
46. Baumgart K, Baldo B. Cephalosporin allergy. N Engl J Med 2002;346:380.-
47. James J. Diagnosis of penicillin, amoxicillin, and cephalosporin allergy: reliability of examination assessed by skin testing and oral challenge. Pediatrics 1999;104:367.-
48. Pichichero M, Pichichero D. Diagnosis of penicillin, amoxicillin, and cephalosporin allergy: Reliability of examination assessed by skin testing and oral challenge. J Pediatr 1998;132:137-143.
49. Romano A, Mayorga C, Torres M, et al. Immediate allergic reactions to cephalosporins: Cross-reactivity and selective responses. J Allergy Clin Immunol 2000;106:1177-1183.
50. Salkind A, Cuddy P, Foxworth J. Is this patient allergic to penicillin? An evidence-based analysis of the likelihood of penicillin allergy. JAMA 2001;285:2498-2505.
51. Smith J, Johnson J, Cluff L. Studies on the epidemiology of adverse drug reactions: II. An evaluation of penicillin allergy. N Engl J Med 1966;274:998-1002.
52. Warrington R, Simons F, Ho H, et al. Diagnosis of penicillin allergy by skin testing: the Manitoba experience. Can Med Assoc J 1978;118:797-791.
53. Saxon A, Beall G, Rohr A. Immediate hypersensitivity reactions to beta-lactam antibiotics. Ann Intern Med 1987;107:204-215.
1. Levine B. Immunologic mechanisms of penicillin allergy. A haptenic model system for the study of allergic diseases of man. N Engl J Med 1966;275:1115-1125.
2. Torres M, Gonzales F, Mayorga C, et al. IgG and IgE antibodies in subjects allergic to penicillins recognize different parts of the penicillin molecule. Int Arch Allergy Immunol 1997;113:342-344.
3. Pichichero M. A review of evidence supporting the American Academy of Pediatrics recommendation for prescribing cephalosporin antibiotics for penicillin-allergic patients. Pediatrics 2005;115:1048-1057.
4. Arndt J, Jick H. Rates of cutaneous reactions to drugs. A report from the Boston Collaborative Drug Surveillance Program. JAMA 1976;235:918-923.
5. Platt R. Adverse effects of third-generation cephalosporins. J Antimicrob Chemother 1982;10(C):135-140.
6. Sanders CV, Greenberg RN, Marier RL. Cefamandole and cefoxitin. Ann Intern Med 1985;103:70-78.
7. Levine LR. Quantitative comparison of adverse reactions to cefaclor vs. amoxicillin in a surveillance study. Pediatr Infect Dis 1985;4:358-361.
8. Norrby S. Side effects of cephalosporins. Drug 1987;34(Suppl 2):105-120.
9. Idsoe O, Guthe T, Wilcox R. Nature and extent of penicillin side-reactions with particular reference to fatalities from anaphylactic shock. Bull WHO 1968;38:159-188.
10. Petz L. Immunologic reactions of humans to cephalosporins. Post Grad Med J 1971;47(Suppl):64-69.
11. Gadde J, Spence M, Wheeler B, et al. Clinical experience with penicillin skin testing in a large inner-city STD clinic. JAMA 1993;270:2456-2463.
12. Walter E, Moelling K, Pavlovic J, et al. Micro-encapsulation of DNA using poly (DL-lactide-co-glycolide): stability issues and release characteristics. J Controlled Release 1999;61:361-374.
13. Weinstein L, Kaplan K, Chang T. Treatment of infections in man with cephalothin. JAMA 1964;189:829-834.
14. Griffith R, Black H. Cephalothin—a new antibiotic. Preliminary clinical and laboratory studies. JAMA 1964;189:823-828.
15. Apicella M, Perkins R, Salsaw S. Cephaloridine treatment of bacterial infections. Am J Med Sci 1966;251:266-276.
16. Assem E, Vickers M. Tests for penicillin allergy in man: The immunological cross-reaction between penicillins and cephalosporins. Immunology 1974;27:255-269.
17. Marks J, Garrett R. Cephalexin in general practice. Post Grad Med J 1970;46(Suppl):113-117.
18. Stewart G. Cross-allergenicity of penicillin G and related substances. Lancet 1962;1:509-510.
19. Dash C. Penicillin allergy and the cephalosporins. J Antimicrob Chemother 1975;(1 Suppl):107-118.
20. Petz L. Immunologic cross-reactivity between penicillins and cephalosporins. J Infect Dis 1978;137:S74-S79.
21. Thoburn R, Johnson J, Cluff L. Studies on the epidemiology of adverse drug reactions. IV. The relationship of cephalothin and penicillin allergy. JAMA 1966;198:345-348.
22. Solley G, Gleich G, Van Dellen R. Penicillin allergy: Clincal experience with a battery of skin test reagents. J Allergy Clin Immunol 1982;69:238-244.
23. Warrington R, McPhillipps S. Independent anaphylaxis to cefazolin without allergy to other beta-lactam antibiotics. J Allergy Clin Immunol 1996;98:460-462.
24. Sullivan T, Wedner H, Shatz G, et al. Skin testing to detect penicillin allergy. J Allergy Clin Immunol 1981;68:171-180.
25. Saxon A. Immediate hypersensitivity reactions to b-lactam antibiotics. Rev Infect Dis 1983;5(Suppl 2):S368-S378.
26. Blanca M, Fernandez J, Miranda A, et al. Cross reactivity between penicillins and cephalosporins: Clinical and immunological studies. J Allergy Clin Immunol 1989;83:381-385.
27. Lin R. A perspective on penicillin allergy. Arch Intern Med 1992;152:930-937.
28. Martin J, Igea J, Fraj J, et al. Allergy to amoxicillin in patients who tolerated benzylpenicillin, aztreonam, and ceftazidime. Clin Infect Dis 1992;14:592-593.
29. Shepherd G, Burton D. Administration of cephalosporin antibiotics to patients with a history of penicillin allergy [abstract]. J Allergy Clin Immunol 1993;91:262.-
30. Audicana M, Bernaola G, Urrutia I, et al. Allergic reactions to betalactams: Studies in a group of patients allergic to penicillin and evaluation of cross-reactivity with cephalosporins. Allergy 1994;49:108-113.
31. Anne S, Reisman R. Risk of administering cephalosporin antibiotics to patients with histories of penicillin allergy. Annals Allergy Asthma Immunology 1995;74:167-170.
32. Miranda A, Blanca M, Vega J, et al. Cross-reactivity between a penicillin and a cephalosporin with the same side chain. J Allergy Clin Immunol 1996;98:671-677.
33. Sastre J, Quijano L, Novalbos A, et al. Clinical cross-reactivity between amoxicillin and cephadroxil in patients allergic to amoxicillin and with good tolerance of penicillin. Allergy 1996;51:383-386.
34. Pichichero M, Pichichero D. Selecting skin testing reagents to predict amoxicillin and cephalosporin allergy. Pediatr Asthma Allergy Immunol 1997;11:79-93.
35. Novalbos A, Sastre J, Cuesta J, et al. Lack of allergic cross reactivity to cephalosporins among patients allergic to penicillins. Clin Exp Allergy 2001;31:438-443.
36. Romano A, Gueant-Rodriguez RM, Viola M, et al. Cross-reactivity and tolerability of cephalosporins in patients with immediate hypersensitivity to penicillins. Ann Intern Med 2004;141:16-22.
37. Torres M, Blanca M, Garcia J. Evaluation of a large cohort of subjects allergic to penicillins [abstract]. J Allergy Clin Immunol 1995;95:285.-
38. Kelkar P, Li J. Cephalosporin allergy. N Engl J Med 2001;385:804-809.
39. Kabins S, Einstein B, Cohen S. Anaphylactic reaction to an initial dose of sodium cephalothin. JAMA 1965;193:165.-
40. Romano A, Piunti E, De Fronso M, et al. Selective immediate hypersensitivity to ceftriaxone. Allergy 2000;55:418-419.
41. Pumphrey R, Davis S. Under-reporting of antibiotic anaphylaxis may put patients at risk. Lancet 1999;353:1157-1158.
42. Mayorga C, Torres M, Blanca M. Cephalosporin allergy. N Engl J Med 2002;236:380-381.
43. Mayorga C, Ovispo T, Jimeno L. Epitope mapping of betalactam antibiotics with the use of monoclonal antibodies. Toxicology 1995;97:225-34.
44. Weiss M, Adkinson N. Immediate hypersensitivity reactions to penicillin and related antibiotics. Clin Allergy 1998;18:515-540.
45. Blaiss M, DeShazo R. Drug allergy. Pediatr Clin North Am 1998;35:1131-1147.
46. Baumgart K, Baldo B. Cephalosporin allergy. N Engl J Med 2002;346:380.-
47. James J. Diagnosis of penicillin, amoxicillin, and cephalosporin allergy: reliability of examination assessed by skin testing and oral challenge. Pediatrics 1999;104:367.-
48. Pichichero M, Pichichero D. Diagnosis of penicillin, amoxicillin, and cephalosporin allergy: Reliability of examination assessed by skin testing and oral challenge. J Pediatr 1998;132:137-143.
49. Romano A, Mayorga C, Torres M, et al. Immediate allergic reactions to cephalosporins: Cross-reactivity and selective responses. J Allergy Clin Immunol 2000;106:1177-1183.
50. Salkind A, Cuddy P, Foxworth J. Is this patient allergic to penicillin? An evidence-based analysis of the likelihood of penicillin allergy. JAMA 2001;285:2498-2505.
51. Smith J, Johnson J, Cluff L. Studies on the epidemiology of adverse drug reactions: II. An evaluation of penicillin allergy. N Engl J Med 1966;274:998-1002.
52. Warrington R, Simons F, Ho H, et al. Diagnosis of penicillin allergy by skin testing: the Manitoba experience. Can Med Assoc J 1978;118:797-791.
53. Saxon A, Beall G, Rohr A. Immediate hypersensitivity reactions to beta-lactam antibiotics. Ann Intern Med 1987;107:204-215.
Acute Otitis Media: Influence of the PCV-7 vaccine on changes in the disease and its management
- Widespread use of the 7-valent pneumococcal conjugate vaccine has resulted in a shift in frequency of causative bacterial pathogens responsible for recurrent and persistent acute otitis media (AOM); disease management practice should encompass this change (SOR: B).
- High-dose amoxicillin is the first choice for antibiotic therapy in uncomplicated bacterial AOM, although β-lactamase–producing pathogens are increasingly common primary causative agents, and amoxicillin is susceptible to β-lactamase (SOR: B).
- Adding clavulanate to amoxicillin increases resistance to and improves effectiveness against β-lactamase–producing pathogens. Specific third-generation cephalosporins also should be included as antibiotic choices because of excellent activity against β-lactamase–producing pathogens and because of compliance advantages, such as better taste, less frequent dosing, and even shorter duration of therapy (SOR: B).
Since the approval of the 7-valent pneumococcal conjugate vaccine (PCV-7) for use in children younger than 24 months in February 2000, occurrences of acute otitis media (AOM) and the frequency of recurrent AOM have declined. Based on results from early clinical trials, PCV-7 may reduce total AOM by 6% to 8%, recurrent AOM by 10% to 26%, and tympanostomy tube placements by 24%.1,2
Acute otitis media occurs most frequently in children between the ages of 6 months and 18 months. By the end of their first year, approximately 86% of children will experience at least 1 episode of AOM.3
The condition remains a leading reason for visits to pediatricians and family physicians in the United States.4 It accounted for 16 million visits in 2000.4 This is a decrease from almost 25 million visits in 1995, prior to use of PCV-7. Additionally, AOM is associated with significant costs: In 1995, the direct and indirect costs of AOM were estimated to be about $3 billion.5
Changes in pathogen frequency for AOM in the ERA of PCV-7
The true impact of PCV-7 on management practice is not characterized by the modest reduction in incidence of uncomplicated AOM but in the PCV-7–associated shift in causative pathogens. Pre-PCV-7, 40% to 50% of cases of AOM in young children were caused by Streptococcus pneumoniae, 20% to 30% by Haemophilus influenzae, and 10% to 15% by Moraxella catarrhalis.6 In studies conducted prior to 2000, diagnostic tympanocentesis isolated S pneumoniae from 25% to 55% of all middle ear aspirates from children with AOM.6-8
Conversely, 1 study published in 2001 and 2 studies published in 2004 appear to document a reverse trend with the advent of the conjugate pneumococcal vaccine.9,10 Compared with children studied in an earlier era, those vaccinated with PCV-7 may be more likely to have H influenzae isolates. These studies will be described in detail below.
Bacterial AOM: initial antibiotic therapy and specific pathogens
Current guidelines recommend amoxicillin (45 mg/kg/day) or high-dose amoxicillin (80-90 mg/kg/day) as initial therapy in presumed or documented bacterial AOM.5 Although amoxicillin is effective against pneumococcus and β-lactamase–negative strains of H influenzae, it is ineffective against β-lactamase–positive strains of H influenzae.9 Significant initial failures may point to a changing pathogen per population frequency. A 2004 review assessed children with continued (persistence of infection detected within 30 days after treatment completion) or refractory (clinical failure while receiving antimicrobial therapy) AOM who have received high-dose amoxicillin as initial empiric therapy. The authors noted that the rate of infection due to H influenzae has increased from 43% among those treated prior to the licensure of PCV-7 to 57% among those who received 2 or more doses of PCV-7.10
Evidence from the medical literature
Three studies provide the major evidence concerning the pathogen shift associated with the adoption of the PCV-7 conjugate vaccine:
- The Finnish Otitis Media Vaccine Trial2
- A published collection of clinical trial results from a rural practice in Kentucky in which 94% of children were immunized with PCV-79
- A prospective study conducted in a suburban community-based private practice in Rochester, NY that evaluated children with persistent or nonresponsive AOM.10
The Finnish Otitis Media Vaccine Trial
In this trial, 1662 infants received either the PCV-7 vaccine or a control vaccine at ages 2, 4, 6, and 12 months and were monitored from ages 6.5 months to 24 months.2 An overall 6.9% reduction in episodes of clinical AOM were diagnosed (n=1251) in PCV-7–vaccinated children compared with the control group (n=1345). This suggested that fewer AOM cases were caused by the S pneumoniae vaccine serotypes than nonvaccine serotypes. The bacteriologic findings in the samples of middle ear fluid taken during 93% of the visits for AOM (TABLE 1) show a 34% reduction in culture-confirmed episodes in the PCV-7–vaccinated group, a decrease of more than 50% in pneumococcal AOM episodes caused by vaccine or vaccine cross-reactive serotypes, a 33% increase in infections caused by other pneumococcal serotypes, and an 11% increase in the proportion of AOM cases due to H influenzae.2
TABLE 1
Finnish Otitis Media Vaccine Trial: Causes of AOM Episodes and Impact of PCV-7 Immunization on Incidence
| CAUSE | PCV-7 EPISODES | CONTROL EPISODES | DIFFERENCE (%) |
|---|---|---|---|
| Culture-confirmed pneumococcus | 271 | 414 | 34 |
| Pneumococcal serotype in vaccine | 107 | 250 | 57 |
| Vaccine cross-reactive serotypes* | 41 | 84 | 51 |
| Other pneumococcal serotypes | 125 | 95 | 33 |
| Haemophilus influenzae | 315 | 287 | 11 |
| Moraxella catarrhalis | 379 | 381 | 1 |
| *6A, 9N, 18B, 19A, 23A. | |||
| AOM, acute otitis media; PCV-7, 7-valent pneumococcal conjugate vaccine. | |||
| Adapted with permission from Eskola J, et al. N Engl J Med. 2001;344:403-409. Copyright 2001 Massachusetts Medical Society. All rights reserved. | |||
Study Results From a Rural Kentucky Practice
In this practice, data on isolates from middle ear fluid were collected in children with severe or refractory AOM aged 7 to 24 months.9 Data were obtained from 1992 to 1998, before the introduction of PCV-7, and from 2000 to 2003, following immunization with 3 or 4 doses of PCV-7 during the first 18 months of life.
As shown in TABLE 2, the pre-PCV-7 group of children (N=336; 1992-1998) had a proportion of 48% culture-confirmed pneumococcus vs a proportion of 31% in the PCV-7-vaccinated group (N=83; 2000-2003), a 17% decrease. The decrease in proportion of AOM episodes resulting from vaccine serotypes was 34%.
In this investigation, 28% of the pre-PCV-7 group and 34% of the post-PCV-7 group had received antibiotic therapy within the previous 3 days. Additionally, 59% and 76%, respectively, had received antibiotic therapy within the preceding 30 days. There were increases of 30% in vaccine cross-reactive serotypes and 45% in nonvaccine serotypes. Vaccine cross-reactive serotypes 6A and 19A accounted for most of the penicillin-nonsusceptible S pneumoniae strains in the vaccinated population.
Most impressive, however, in the post-PCV-7 group, was that gram-negative bacteria, mainly H influenzae, accounted for two thirds of AOM isolates, an increase from 41% in the pre-PCV-7 group to 56% in the vaccinated group. A 56% increase was noted in β-lactamase–positive organisms from the pre-PCV-7 group to the post-PCV-7 group. The combined H influenzae and M catarrhalis β-lactamase–producing organisms accounted for nearly half of all isolates.9
TABLE 2
Results From a Rural Kentucky Practice: Change in AOM Microbiology From Pre-PCV-7 (1992–1998) to Post-PCV-7 (2000–2003)
| PATHOGEN | PRE-PCV-7 1992-1998 (N=336) | POST-PCV-7 2000-2003 (N=83) | CHANGE (%) | P VALUE | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Culture-confirmed pneumococcus | 160 | 48 | 26 | 31 | 17 | .007 |
| Pneumococcal serotype in vaccine | 236 | 70 | 30 | 36 | 34 | .003 |
| Vaccine cross-reactive serotypes* | 27 | 8 | 27 | 32 | 24 | .003 |
| Other pneumococcal serotypes† | 74 | 22 | 27 | 32 | 10 | NS |
| Haemophilus influenzae | 137 | 41 | 46 | 32 | 15 | .01 |
| β-lactamase-positive | 108 | 23 | 39 | 36 | 15 | .007 |
| Moraxella catarrhalis, β-lactamase-positive | 31 | 9 | 9 | 11 | 2 | NS |
| AOM, acute otitis media; n, total isolates; NS, nonsignificant; PCV-7, 7-valent pneumococcal conjugate vaccine. | ||||||
| *Includes 6A and 19A. | ||||||
| †Nonvaccine serotypes in post-PCV-7 group: 1, 11A, 15A, 29, and 33F. | ||||||
| Adapted with permission from Block SL, et al. Pediatr Infect Dis J. 2004;23:829-833. | ||||||
The Prospective Rochester, New York Study
Changes in pre- and post-PVC-7 patterns also were seen in a prospective study of 551 children with persistent or nonresponsive AOM (defined as nonresponders after 1 or 2 empiric antibiotic courses or failures after 48 hours of treatment). These children underwent tympanocentesis to identify bacterial isolates during the 9-year period from 1995 to 2003.10
From 1995 to 1997, enrollees received a standard dose of amoxicillin (40-50 mg/kg, divided into 3 doses daily) as initial empiric treatment. From 1998 to 2000 and 2001 to 2003, all children received high-dose amoxicillin (80-100 mg/kg, divided into twice-daily doses).
During the latter period, the children also were vaccinated with PCV-7, with 63% receiving the primary series of 3 doses and 10% receiving the booster dose. In this investigation, shortages in vaccine supply, discussed below, caused vaccination schedules to be compromised.
Study results (TABLE 3) show that in the post-PCV-7 group, there was a 13% decrease in the proportion of S pneumoniae isolates and a 14% increase in the proportion of H influenzae isolates compared with the pre-PCV-7 group (1998-2000 enrollees). An increase of 22% for β-lactamase–positive bacteria was also observed, along with a trend toward an increased proportion of penicillin-susceptible S pneumoniae isolates (58% vs 72%; P=.017) post-PCV-7.10 A 24% reduction (P=.009) in the frequency of the diagnosis of persistent or AOM treatment failure occurred in the period after PCV-7 vaccination. These changes were considered to be the result of the use of the conjugate pneumococcal vaccine rather than of the change in amoxicillin dosing.10
TABLE 3
The Prospective Rochester, New York Study: Pathogens Isolated in Persistent AOM and AOM Treatment Failure Pre- and Post-PCV-7
| PATHOGEN | PRE-PCV-7 1998-2000 (N=204) | POST-PCV-7 2000-2003 (N=152) | CHANGE (%) | P VALUE | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Streptococcus pneumoniae* | 50 | 44 | 28 | 31 | 13 | .017 |
| Penicillin nonsusceptible | 12 | 24 | 4 | 14 | 10 | NS |
| Haemophilus influenzae | 49 | 43 | 51 | 57 | 14 | .012 |
| β-lactamase-positive | 16 | 33 | 28 | 55 | 22 | .044 |
| Moraxella catarrhalis | 6 | 5 | 1 | 1 | 4 | NS |
| AOM, acute otitis media; N, total isolates; NS, nonsignificant; PCV-7, 7-valent pneumococcal conjugate vaccine. | ||||||
| *Pneumococcal serotyping was not done. | ||||||
| Adapted with permission from Casey JR, Pichichero ME. Pediatr Infect Dis J. 2004;23:824-828. | ||||||
Pneumococcal serotype shifts
In addition to the change in causative pathogens, use of the conjugate pneumococcal vaccine appears to have led to a significant shift in the pneumococcal strains causing AOM. Studies at urban medical centers and in the Kentucky practice documented an increase in the proportion of nonvaccine serotypes, accounting for 32% to 38% of pneumococcal AOM.10-12 A 33% increase was seen in the Finnish Trial.2 These nonvaccine pneumococcal serotypes do not carry the same level of resistance seen with those serotypes included in PCV-7.
PCV-7 conjugate vaccine
The PCV-7 conjugate vaccine was approved for use in February 2000. It is a 7-valent pneumococcal conjugate of the capsular antigens of the S pneumoniae serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F, individually conjugated to diphtheria CRM197 protein.13 These serotypes have been responsible for approximately 80% of invasive pneumococcal disease in children younger than 6 years in the United States.13,14 They also accounted for 74% of penicillin-susceptible S pneumoniae and 100% of pneumococci with high-level penicillin resistance isolated from children younger than age 6 years with invasive disease during a 1993-1994 surveillance by the Centers for Disease Control and Prevention (CDC).13
Mechanism of Action and Recommended Immunization Schedule
The conjugate vaccine is converted to a T-cell–dependent antigen, antibody formation is enhanced, and memory B cells are primed.14
The recommended immunization schedule was established as 3 primary doses at ages 2, 4, and 6 months and a booster dose at 12 to 15 months.1 It is the first multivalent pneumococcal vaccine approved for use in children younger than 24 months.
An 89% reduction in invasive pneumococcal disease was observed in children receiving 1 or more doses, and the vaccine appears to reduce nasopharyngeal carriage of vaccine serotypes.15,16
The older 23-valent polysaccharide vaccine does not stimulate good response in children younger than 2 years of age14 and does not reduce mucosal carriage or limit the spread of resistant strains.15
PCV-7 Supply Since 2000
In August 2001, a serious shortage of the vaccine developed in 34 state immunization programs.17 The following month, the CDC advised physicians to administer it only to children younger than 12 months and to those aged 1 to 5 years at increased risk of pneumococcal disease.18 As demand continued despite the change in recommendations, the CDC further changed recommendations to conserve vaccine supply, first suspending the fourth dose temporarily in healthy children19 and then discontinuing both the third and fourth doses.11 In July 2004, production problems seemed to have resolved; the CDC recommended that every child receive 3 doses. In September, supplies were adequate for return to the 4-dose schedule.12 As of June 2004, 67.7% of children aged 24 months had received 3 or more doses of PCV-7.20 Thus, the effects of PCV-7 on the changing microbiology of AOM may only now, at the end of 2005, be fully realized.
Herd Immunity and Reduction in Carriage
Despite the shortages of vaccine during the first years of use, evidence of herd immunity and a decrease in antibiotic resistance in pneumococcal pathogens has been reported throughout the United States.21,22 A 29% decrease in the rate of pneumococcal disease in both young children and adults has also been observed, along with a 35% reduction in the rate of disease caused by nonpenicillin-susceptible pneumococcal strains.21 The reduction in carriage among vaccinated children may be the reason.21,22 Because of the impact of PCV-7, it will be important to record immunization history when collecting AOM data.
AOM treatment choices
The basis of recommendations for treating AOM depends on the presumed responsible pathogens, their susceptibility to antibiotics, and concerns for developing resistance, all influenced by clinical trial data. In practice, however, empiric choices are often made based on knowledge of local resistance patterns and of other patient characteristics; that is cost concerns, adverse event profiles, need to avoid initial treatment failure, adherence issues (eg, taste or palatability), convenience, and duration of dosing regimen.
All current guidelines recommend oral amoxicillin as first-line therapy in documented or presumed bacterial AOM. The 2004 American Academy of Pediatrics/American Academy of Family Physicians’ (AAP/AAFP) guidelines4 recommended increasing the dosage used for empiric treatment from 40 to 45 mg/kg/day to 80 to 90 mg/kg/day for all children. This was a result of concerns about the prevalence of penicillin-resistant S pneumoniae for which standard-dose amoxicillin is inadequate.23
The guidelines were written and published before the data from the Kentucky and New York studies were available; therefore, although the guidelines recommended that empiric treatment of bacterial AOM should target S pneumoniae, H influenzae, and M catarrhalis, the pathogen shift discussed previously might today produce a modified antibiotic selection paradigm. The pathogen mix in persistent or recurrent AOM has already led to a guideline recommendation for high-dose amoxicillin/clavulanate, 90/6.4 mg/kg/day, cefdinir, cefprozil, cefpodoxime, cefuroxime, or ceftriaxone in these patients.23
If an increase in the proportion of β-lactamase–producing pathogens due to PCV-7 occurs, amoxicillin may no longer be the best first choice.
Selecting Among Recommended Antibiotic Choices
As antibiotic preparations for treating bacterial AOM are oral suspensions, taste is a major factor for pediatric patients. TABLE 4 summarizes comparative taste ratings for antibiotic suspensions based on several studies and shows the range, from those that can enhance compliance to those that discourage compliance.23
Adverse events, especially diarrhea, nausea/vomiting, and gastritis, are also of concern. These are shortcomings of amoxicillin/clavulanate, which has a higher incidence of diarrhea and nausea than cephalosporins.24
Dosing frequency is also a factor among recommended agents. Amoxicillin, amoxicillin/clavulanate, cefprozil, and cefpodoxime require twice-daily dosing. Cefdinir can be effective at once-daily dosing.24
Duration of approved therapy is perhaps the most critical selection factor given the reality of patient behaviors. Cefpodoxime and cefdinir are the only 2 FDA-approved agents for 5-day treatment of bacterial AOM that are also guideline-recommended.
TABLE 4
Compliance-Enhancing Ranking of Antibiotic Suspensions
| STRONGLY COMPLIANCE-ENHANCING | |
|
|
| MODERATELY COMPLIANCE-ENHANCING | |
|
|
| EQUIVOCAL COMPLIANCE-ENHANCING | |
| |
| NOT COMPLIANCE-ENHANCING | |
| |
| DISCOURAGES COMPLIANCE | |
|
|
| TMP-SMX, trimethoprim sulfamethoxazole | |
| Sources: Adapted from Steele RW, et al. Pediatr Infect Dis J. 2001;20:1-5. | |
| Demers DM, et al. Pediatr Infect Dis J. 1994;13:87-89. | |
| Ruff ME, et al. Pediatr Infect Dis J. 1991;10:30-33. | |
Choices for Effective Initial Therapy
Considering the changing microbial population in bacterial AOM and the increasing concern of effectiveness of amoxicillin and other antibiotics against β-lactamase–producing H influenzae, the choice of therapy may need modification. Specifically, that may mean changing the choice of effective antibiotic, taking into consideration the compliance-enhancing advantages of available options.
Based on efficacy, the overall prevalence of antibiotic-resistant AOM pathogens for PCV-7-vaccinated children, the potential for adverse effects, and patient compliance issues, Block and Harrison developed an algorithm (FIGURE) for the management of AOM diagnosed by strict criteria in an otherwise healthy child between 4 months and 36 months old.24 As the environment of AOM evolves, the choices for treatment must be not only effective but also the best and most appropriate.
FIGURE Antibiotic Choices for Acute Otitis Media in the 2000s
1. Fireman B, Black SB, Shinefield HR, et al. Impact of the pneumococcal conjugate vaccine on otitis media. Pediatr Infect Dis J. 2003;22:10-16.
2. Eskola J, Kilpi T, Palmu A, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med. 2001;344:403-409.
3. Block SL, Harrison CH, Hedrick J, et al. Restricted use of antibiotic prophylaxis for recurrent acute otitis media in the era of penicillin non-susceptible Streptococcus pneumoniae. Int J Pediatr Otorhinolaryngol. 2001;61:47-60.
4. Advisory Committee on Immunization Practices. Preventing pneumococcal disease among infants and young children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2000;49(RR-9):1-35.
5. American Academy of Family Physicians Subcommittee on Management of Acute Otitis Media. Diagnosis and management of acute otitis media. Pediatrics. 2004;113:1451-1465.
6. Dowell SF, Butler JC, Giebink GS, et al. Acute otitis media: management and surveillance in an era of pneumococcal resistance—a report from the Drug-resistant Streptococcus pneumoniae Therapeutic Working Group. Pediatr Infect Dis J. 1999;18:1-9.
7. Block SL. Causative pathogens, antibiotic resistance and therapeutic considerations in acute otitis media. Pediatr Infect Dis J. 1997;16:449-456.
8. Bluestone CD, Stephenson JS, Martin LM. Ten-year review of otitis media pathogens. Pediatr Infect Dis J. 1992;11(suppl 8):S7-S11.
9. Block SL, Hedrick J, Harrison CJ, et al. Community-wide vaccination with the heptavalent pneumococcal conjugate significantly alters the microbiology of acute otitis media. Pediatr Infect Dis J. 2004;23:829-833.
10. Casey JR, Pichichero ME. Changes in frequency and pathogens causing acute otitis media in 1995-2003. Pediatr Infect Dis J. 2004;23:824-828.
11. Centers for Disease Control and Prevention. Updated recommendations on the use of pneumococcal conjugate vaccine: suspension of recommendation for third and fourth dose. MMWR Morb Mortal Wkly Rep. 2004;53:177-178.
12. Centers for Disease Control and Prevention. Pneumococcal conjugate vaccine shortage resolved. MMWR Morb Mortal Wkly Rep. 2004;53:851-852.
13. Prevnar® (pneumococcal 7-valent vaccine) [prescribing information]. Philadelphia, Pa: Wyeth Pharmaceuticals. Rev. 01/04.
14. Watson W. Pneumococcal conjugate vaccines. Pediatr Infect Dis J. 2000;19:331-332.
15. Giebink GS. The prevention of pneumococcal disease in children. N Engl J Med. 2001;345:1177-1183.
16. Pelton SI, Loughlin AM, Marchand CD. Seven valent pneumococcal conjugate vaccine immunization in two Boston communities: changes in serotypes and antimicrobial susceptibility among Streptococcus pneumoniae isolates. Pediatr Infect Dis J. 2003;23:1015-1022.
17. Freed GL, Davis MM, Clark SJ. Variation in public and private supply of pneumococcal conjugate vaccine during a shortage. JAMA. 2003;289:575-578.
18. Centers for Disease Control and Prevention. Decreased availability of pneumococcal conjugate vaccine. MMWR Morb Mortal Wkly Rep. 2001;50:783-784.
19. Centers for Disease Control and Prevention. Limited supply of pneumococcal conjugate vaccine: suspension of recommendation for fourth dose. MMWR Morb Mortal Wkly Rep. 2004;53:108-109.
20. CDC National Immunization Survey. Estimated vaccination coverage with individual vaccines and selected vaccination series by 24 months of age by state and immunization action plan area US, Q3/2003-Q4/2004. Available at: http://www2a.cdc.gov/nip/coverage/nis/nis_iap.asp?fmt=v&rpt=tab09_24mo_iap_0304&qtr=Q3/2003-Q2/2004. Accessed September 20, 2005.
21. Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737-1746.
22. Black S, Shinefield H, Baxter R, et al. Postlicensure surveillance for pneumococcal invasive disease after use of heptavalent pneumococcal vaccine in Northern California Kaiser Permanente. Pediatr Infect Dis J. 2004;23:485-489.
23. Pichichero ME, Casey JR. Acute otitis media: Making sense of recent guidelines on antimicrobial treatment. J Fam Pract. 2005;54:313-332.
24. Block SL, Harrison CJ. Diagnosis and Management of Acute Otitis Media, 3rd ed. In press.
- Widespread use of the 7-valent pneumococcal conjugate vaccine has resulted in a shift in frequency of causative bacterial pathogens responsible for recurrent and persistent acute otitis media (AOM); disease management practice should encompass this change (SOR: B).
- High-dose amoxicillin is the first choice for antibiotic therapy in uncomplicated bacterial AOM, although β-lactamase–producing pathogens are increasingly common primary causative agents, and amoxicillin is susceptible to β-lactamase (SOR: B).
- Adding clavulanate to amoxicillin increases resistance to and improves effectiveness against β-lactamase–producing pathogens. Specific third-generation cephalosporins also should be included as antibiotic choices because of excellent activity against β-lactamase–producing pathogens and because of compliance advantages, such as better taste, less frequent dosing, and even shorter duration of therapy (SOR: B).
Since the approval of the 7-valent pneumococcal conjugate vaccine (PCV-7) for use in children younger than 24 months in February 2000, occurrences of acute otitis media (AOM) and the frequency of recurrent AOM have declined. Based on results from early clinical trials, PCV-7 may reduce total AOM by 6% to 8%, recurrent AOM by 10% to 26%, and tympanostomy tube placements by 24%.1,2
Acute otitis media occurs most frequently in children between the ages of 6 months and 18 months. By the end of their first year, approximately 86% of children will experience at least 1 episode of AOM.3
The condition remains a leading reason for visits to pediatricians and family physicians in the United States.4 It accounted for 16 million visits in 2000.4 This is a decrease from almost 25 million visits in 1995, prior to use of PCV-7. Additionally, AOM is associated with significant costs: In 1995, the direct and indirect costs of AOM were estimated to be about $3 billion.5
Changes in pathogen frequency for AOM in the ERA of PCV-7
The true impact of PCV-7 on management practice is not characterized by the modest reduction in incidence of uncomplicated AOM but in the PCV-7–associated shift in causative pathogens. Pre-PCV-7, 40% to 50% of cases of AOM in young children were caused by Streptococcus pneumoniae, 20% to 30% by Haemophilus influenzae, and 10% to 15% by Moraxella catarrhalis.6 In studies conducted prior to 2000, diagnostic tympanocentesis isolated S pneumoniae from 25% to 55% of all middle ear aspirates from children with AOM.6-8
Conversely, 1 study published in 2001 and 2 studies published in 2004 appear to document a reverse trend with the advent of the conjugate pneumococcal vaccine.9,10 Compared with children studied in an earlier era, those vaccinated with PCV-7 may be more likely to have H influenzae isolates. These studies will be described in detail below.
Bacterial AOM: initial antibiotic therapy and specific pathogens
Current guidelines recommend amoxicillin (45 mg/kg/day) or high-dose amoxicillin (80-90 mg/kg/day) as initial therapy in presumed or documented bacterial AOM.5 Although amoxicillin is effective against pneumococcus and β-lactamase–negative strains of H influenzae, it is ineffective against β-lactamase–positive strains of H influenzae.9 Significant initial failures may point to a changing pathogen per population frequency. A 2004 review assessed children with continued (persistence of infection detected within 30 days after treatment completion) or refractory (clinical failure while receiving antimicrobial therapy) AOM who have received high-dose amoxicillin as initial empiric therapy. The authors noted that the rate of infection due to H influenzae has increased from 43% among those treated prior to the licensure of PCV-7 to 57% among those who received 2 or more doses of PCV-7.10
Evidence from the medical literature
Three studies provide the major evidence concerning the pathogen shift associated with the adoption of the PCV-7 conjugate vaccine:
- The Finnish Otitis Media Vaccine Trial2
- A published collection of clinical trial results from a rural practice in Kentucky in which 94% of children were immunized with PCV-79
- A prospective study conducted in a suburban community-based private practice in Rochester, NY that evaluated children with persistent or nonresponsive AOM.10
The Finnish Otitis Media Vaccine Trial
In this trial, 1662 infants received either the PCV-7 vaccine or a control vaccine at ages 2, 4, 6, and 12 months and were monitored from ages 6.5 months to 24 months.2 An overall 6.9% reduction in episodes of clinical AOM were diagnosed (n=1251) in PCV-7–vaccinated children compared with the control group (n=1345). This suggested that fewer AOM cases were caused by the S pneumoniae vaccine serotypes than nonvaccine serotypes. The bacteriologic findings in the samples of middle ear fluid taken during 93% of the visits for AOM (TABLE 1) show a 34% reduction in culture-confirmed episodes in the PCV-7–vaccinated group, a decrease of more than 50% in pneumococcal AOM episodes caused by vaccine or vaccine cross-reactive serotypes, a 33% increase in infections caused by other pneumococcal serotypes, and an 11% increase in the proportion of AOM cases due to H influenzae.2
TABLE 1
Finnish Otitis Media Vaccine Trial: Causes of AOM Episodes and Impact of PCV-7 Immunization on Incidence
| CAUSE | PCV-7 EPISODES | CONTROL EPISODES | DIFFERENCE (%) |
|---|---|---|---|
| Culture-confirmed pneumococcus | 271 | 414 | 34 |
| Pneumococcal serotype in vaccine | 107 | 250 | 57 |
| Vaccine cross-reactive serotypes* | 41 | 84 | 51 |
| Other pneumococcal serotypes | 125 | 95 | 33 |
| Haemophilus influenzae | 315 | 287 | 11 |
| Moraxella catarrhalis | 379 | 381 | 1 |
| *6A, 9N, 18B, 19A, 23A. | |||
| AOM, acute otitis media; PCV-7, 7-valent pneumococcal conjugate vaccine. | |||
| Adapted with permission from Eskola J, et al. N Engl J Med. 2001;344:403-409. Copyright 2001 Massachusetts Medical Society. All rights reserved. | |||
Study Results From a Rural Kentucky Practice
In this practice, data on isolates from middle ear fluid were collected in children with severe or refractory AOM aged 7 to 24 months.9 Data were obtained from 1992 to 1998, before the introduction of PCV-7, and from 2000 to 2003, following immunization with 3 or 4 doses of PCV-7 during the first 18 months of life.
As shown in TABLE 2, the pre-PCV-7 group of children (N=336; 1992-1998) had a proportion of 48% culture-confirmed pneumococcus vs a proportion of 31% in the PCV-7-vaccinated group (N=83; 2000-2003), a 17% decrease. The decrease in proportion of AOM episodes resulting from vaccine serotypes was 34%.
In this investigation, 28% of the pre-PCV-7 group and 34% of the post-PCV-7 group had received antibiotic therapy within the previous 3 days. Additionally, 59% and 76%, respectively, had received antibiotic therapy within the preceding 30 days. There were increases of 30% in vaccine cross-reactive serotypes and 45% in nonvaccine serotypes. Vaccine cross-reactive serotypes 6A and 19A accounted for most of the penicillin-nonsusceptible S pneumoniae strains in the vaccinated population.
Most impressive, however, in the post-PCV-7 group, was that gram-negative bacteria, mainly H influenzae, accounted for two thirds of AOM isolates, an increase from 41% in the pre-PCV-7 group to 56% in the vaccinated group. A 56% increase was noted in β-lactamase–positive organisms from the pre-PCV-7 group to the post-PCV-7 group. The combined H influenzae and M catarrhalis β-lactamase–producing organisms accounted for nearly half of all isolates.9
TABLE 2
Results From a Rural Kentucky Practice: Change in AOM Microbiology From Pre-PCV-7 (1992–1998) to Post-PCV-7 (2000–2003)
| PATHOGEN | PRE-PCV-7 1992-1998 (N=336) | POST-PCV-7 2000-2003 (N=83) | CHANGE (%) | P VALUE | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Culture-confirmed pneumococcus | 160 | 48 | 26 | 31 | 17 | .007 |
| Pneumococcal serotype in vaccine | 236 | 70 | 30 | 36 | 34 | .003 |
| Vaccine cross-reactive serotypes* | 27 | 8 | 27 | 32 | 24 | .003 |
| Other pneumococcal serotypes† | 74 | 22 | 27 | 32 | 10 | NS |
| Haemophilus influenzae | 137 | 41 | 46 | 32 | 15 | .01 |
| β-lactamase-positive | 108 | 23 | 39 | 36 | 15 | .007 |
| Moraxella catarrhalis, β-lactamase-positive | 31 | 9 | 9 | 11 | 2 | NS |
| AOM, acute otitis media; n, total isolates; NS, nonsignificant; PCV-7, 7-valent pneumococcal conjugate vaccine. | ||||||
| *Includes 6A and 19A. | ||||||
| †Nonvaccine serotypes in post-PCV-7 group: 1, 11A, 15A, 29, and 33F. | ||||||
| Adapted with permission from Block SL, et al. Pediatr Infect Dis J. 2004;23:829-833. | ||||||
The Prospective Rochester, New York Study
Changes in pre- and post-PVC-7 patterns also were seen in a prospective study of 551 children with persistent or nonresponsive AOM (defined as nonresponders after 1 or 2 empiric antibiotic courses or failures after 48 hours of treatment). These children underwent tympanocentesis to identify bacterial isolates during the 9-year period from 1995 to 2003.10
From 1995 to 1997, enrollees received a standard dose of amoxicillin (40-50 mg/kg, divided into 3 doses daily) as initial empiric treatment. From 1998 to 2000 and 2001 to 2003, all children received high-dose amoxicillin (80-100 mg/kg, divided into twice-daily doses).
During the latter period, the children also were vaccinated with PCV-7, with 63% receiving the primary series of 3 doses and 10% receiving the booster dose. In this investigation, shortages in vaccine supply, discussed below, caused vaccination schedules to be compromised.
Study results (TABLE 3) show that in the post-PCV-7 group, there was a 13% decrease in the proportion of S pneumoniae isolates and a 14% increase in the proportion of H influenzae isolates compared with the pre-PCV-7 group (1998-2000 enrollees). An increase of 22% for β-lactamase–positive bacteria was also observed, along with a trend toward an increased proportion of penicillin-susceptible S pneumoniae isolates (58% vs 72%; P=.017) post-PCV-7.10 A 24% reduction (P=.009) in the frequency of the diagnosis of persistent or AOM treatment failure occurred in the period after PCV-7 vaccination. These changes were considered to be the result of the use of the conjugate pneumococcal vaccine rather than of the change in amoxicillin dosing.10
TABLE 3
The Prospective Rochester, New York Study: Pathogens Isolated in Persistent AOM and AOM Treatment Failure Pre- and Post-PCV-7
| PATHOGEN | PRE-PCV-7 1998-2000 (N=204) | POST-PCV-7 2000-2003 (N=152) | CHANGE (%) | P VALUE | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Streptococcus pneumoniae* | 50 | 44 | 28 | 31 | 13 | .017 |
| Penicillin nonsusceptible | 12 | 24 | 4 | 14 | 10 | NS |
| Haemophilus influenzae | 49 | 43 | 51 | 57 | 14 | .012 |
| β-lactamase-positive | 16 | 33 | 28 | 55 | 22 | .044 |
| Moraxella catarrhalis | 6 | 5 | 1 | 1 | 4 | NS |
| AOM, acute otitis media; N, total isolates; NS, nonsignificant; PCV-7, 7-valent pneumococcal conjugate vaccine. | ||||||
| *Pneumococcal serotyping was not done. | ||||||
| Adapted with permission from Casey JR, Pichichero ME. Pediatr Infect Dis J. 2004;23:824-828. | ||||||
Pneumococcal serotype shifts
In addition to the change in causative pathogens, use of the conjugate pneumococcal vaccine appears to have led to a significant shift in the pneumococcal strains causing AOM. Studies at urban medical centers and in the Kentucky practice documented an increase in the proportion of nonvaccine serotypes, accounting for 32% to 38% of pneumococcal AOM.10-12 A 33% increase was seen in the Finnish Trial.2 These nonvaccine pneumococcal serotypes do not carry the same level of resistance seen with those serotypes included in PCV-7.
PCV-7 conjugate vaccine
The PCV-7 conjugate vaccine was approved for use in February 2000. It is a 7-valent pneumococcal conjugate of the capsular antigens of the S pneumoniae serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F, individually conjugated to diphtheria CRM197 protein.13 These serotypes have been responsible for approximately 80% of invasive pneumococcal disease in children younger than 6 years in the United States.13,14 They also accounted for 74% of penicillin-susceptible S pneumoniae and 100% of pneumococci with high-level penicillin resistance isolated from children younger than age 6 years with invasive disease during a 1993-1994 surveillance by the Centers for Disease Control and Prevention (CDC).13
Mechanism of Action and Recommended Immunization Schedule
The conjugate vaccine is converted to a T-cell–dependent antigen, antibody formation is enhanced, and memory B cells are primed.14
The recommended immunization schedule was established as 3 primary doses at ages 2, 4, and 6 months and a booster dose at 12 to 15 months.1 It is the first multivalent pneumococcal vaccine approved for use in children younger than 24 months.
An 89% reduction in invasive pneumococcal disease was observed in children receiving 1 or more doses, and the vaccine appears to reduce nasopharyngeal carriage of vaccine serotypes.15,16
The older 23-valent polysaccharide vaccine does not stimulate good response in children younger than 2 years of age14 and does not reduce mucosal carriage or limit the spread of resistant strains.15
PCV-7 Supply Since 2000
In August 2001, a serious shortage of the vaccine developed in 34 state immunization programs.17 The following month, the CDC advised physicians to administer it only to children younger than 12 months and to those aged 1 to 5 years at increased risk of pneumococcal disease.18 As demand continued despite the change in recommendations, the CDC further changed recommendations to conserve vaccine supply, first suspending the fourth dose temporarily in healthy children19 and then discontinuing both the third and fourth doses.11 In July 2004, production problems seemed to have resolved; the CDC recommended that every child receive 3 doses. In September, supplies were adequate for return to the 4-dose schedule.12 As of June 2004, 67.7% of children aged 24 months had received 3 or more doses of PCV-7.20 Thus, the effects of PCV-7 on the changing microbiology of AOM may only now, at the end of 2005, be fully realized.
Herd Immunity and Reduction in Carriage
Despite the shortages of vaccine during the first years of use, evidence of herd immunity and a decrease in antibiotic resistance in pneumococcal pathogens has been reported throughout the United States.21,22 A 29% decrease in the rate of pneumococcal disease in both young children and adults has also been observed, along with a 35% reduction in the rate of disease caused by nonpenicillin-susceptible pneumococcal strains.21 The reduction in carriage among vaccinated children may be the reason.21,22 Because of the impact of PCV-7, it will be important to record immunization history when collecting AOM data.
AOM treatment choices
The basis of recommendations for treating AOM depends on the presumed responsible pathogens, their susceptibility to antibiotics, and concerns for developing resistance, all influenced by clinical trial data. In practice, however, empiric choices are often made based on knowledge of local resistance patterns and of other patient characteristics; that is cost concerns, adverse event profiles, need to avoid initial treatment failure, adherence issues (eg, taste or palatability), convenience, and duration of dosing regimen.
All current guidelines recommend oral amoxicillin as first-line therapy in documented or presumed bacterial AOM. The 2004 American Academy of Pediatrics/American Academy of Family Physicians’ (AAP/AAFP) guidelines4 recommended increasing the dosage used for empiric treatment from 40 to 45 mg/kg/day to 80 to 90 mg/kg/day for all children. This was a result of concerns about the prevalence of penicillin-resistant S pneumoniae for which standard-dose amoxicillin is inadequate.23
The guidelines were written and published before the data from the Kentucky and New York studies were available; therefore, although the guidelines recommended that empiric treatment of bacterial AOM should target S pneumoniae, H influenzae, and M catarrhalis, the pathogen shift discussed previously might today produce a modified antibiotic selection paradigm. The pathogen mix in persistent or recurrent AOM has already led to a guideline recommendation for high-dose amoxicillin/clavulanate, 90/6.4 mg/kg/day, cefdinir, cefprozil, cefpodoxime, cefuroxime, or ceftriaxone in these patients.23
If an increase in the proportion of β-lactamase–producing pathogens due to PCV-7 occurs, amoxicillin may no longer be the best first choice.
Selecting Among Recommended Antibiotic Choices
As antibiotic preparations for treating bacterial AOM are oral suspensions, taste is a major factor for pediatric patients. TABLE 4 summarizes comparative taste ratings for antibiotic suspensions based on several studies and shows the range, from those that can enhance compliance to those that discourage compliance.23
Adverse events, especially diarrhea, nausea/vomiting, and gastritis, are also of concern. These are shortcomings of amoxicillin/clavulanate, which has a higher incidence of diarrhea and nausea than cephalosporins.24
Dosing frequency is also a factor among recommended agents. Amoxicillin, amoxicillin/clavulanate, cefprozil, and cefpodoxime require twice-daily dosing. Cefdinir can be effective at once-daily dosing.24
Duration of approved therapy is perhaps the most critical selection factor given the reality of patient behaviors. Cefpodoxime and cefdinir are the only 2 FDA-approved agents for 5-day treatment of bacterial AOM that are also guideline-recommended.
TABLE 4
Compliance-Enhancing Ranking of Antibiotic Suspensions
| STRONGLY COMPLIANCE-ENHANCING | |
|
|
| MODERATELY COMPLIANCE-ENHANCING | |
|
|
| EQUIVOCAL COMPLIANCE-ENHANCING | |
| |
| NOT COMPLIANCE-ENHANCING | |
| |
| DISCOURAGES COMPLIANCE | |
|
|
| TMP-SMX, trimethoprim sulfamethoxazole | |
| Sources: Adapted from Steele RW, et al. Pediatr Infect Dis J. 2001;20:1-5. | |
| Demers DM, et al. Pediatr Infect Dis J. 1994;13:87-89. | |
| Ruff ME, et al. Pediatr Infect Dis J. 1991;10:30-33. | |
Choices for Effective Initial Therapy
Considering the changing microbial population in bacterial AOM and the increasing concern of effectiveness of amoxicillin and other antibiotics against β-lactamase–producing H influenzae, the choice of therapy may need modification. Specifically, that may mean changing the choice of effective antibiotic, taking into consideration the compliance-enhancing advantages of available options.
Based on efficacy, the overall prevalence of antibiotic-resistant AOM pathogens for PCV-7-vaccinated children, the potential for adverse effects, and patient compliance issues, Block and Harrison developed an algorithm (FIGURE) for the management of AOM diagnosed by strict criteria in an otherwise healthy child between 4 months and 36 months old.24 As the environment of AOM evolves, the choices for treatment must be not only effective but also the best and most appropriate.
FIGURE Antibiotic Choices for Acute Otitis Media in the 2000s
- Widespread use of the 7-valent pneumococcal conjugate vaccine has resulted in a shift in frequency of causative bacterial pathogens responsible for recurrent and persistent acute otitis media (AOM); disease management practice should encompass this change (SOR: B).
- High-dose amoxicillin is the first choice for antibiotic therapy in uncomplicated bacterial AOM, although β-lactamase–producing pathogens are increasingly common primary causative agents, and amoxicillin is susceptible to β-lactamase (SOR: B).
- Adding clavulanate to amoxicillin increases resistance to and improves effectiveness against β-lactamase–producing pathogens. Specific third-generation cephalosporins also should be included as antibiotic choices because of excellent activity against β-lactamase–producing pathogens and because of compliance advantages, such as better taste, less frequent dosing, and even shorter duration of therapy (SOR: B).
Since the approval of the 7-valent pneumococcal conjugate vaccine (PCV-7) for use in children younger than 24 months in February 2000, occurrences of acute otitis media (AOM) and the frequency of recurrent AOM have declined. Based on results from early clinical trials, PCV-7 may reduce total AOM by 6% to 8%, recurrent AOM by 10% to 26%, and tympanostomy tube placements by 24%.1,2
Acute otitis media occurs most frequently in children between the ages of 6 months and 18 months. By the end of their first year, approximately 86% of children will experience at least 1 episode of AOM.3
The condition remains a leading reason for visits to pediatricians and family physicians in the United States.4 It accounted for 16 million visits in 2000.4 This is a decrease from almost 25 million visits in 1995, prior to use of PCV-7. Additionally, AOM is associated with significant costs: In 1995, the direct and indirect costs of AOM were estimated to be about $3 billion.5
Changes in pathogen frequency for AOM in the ERA of PCV-7
The true impact of PCV-7 on management practice is not characterized by the modest reduction in incidence of uncomplicated AOM but in the PCV-7–associated shift in causative pathogens. Pre-PCV-7, 40% to 50% of cases of AOM in young children were caused by Streptococcus pneumoniae, 20% to 30% by Haemophilus influenzae, and 10% to 15% by Moraxella catarrhalis.6 In studies conducted prior to 2000, diagnostic tympanocentesis isolated S pneumoniae from 25% to 55% of all middle ear aspirates from children with AOM.6-8
Conversely, 1 study published in 2001 and 2 studies published in 2004 appear to document a reverse trend with the advent of the conjugate pneumococcal vaccine.9,10 Compared with children studied in an earlier era, those vaccinated with PCV-7 may be more likely to have H influenzae isolates. These studies will be described in detail below.
Bacterial AOM: initial antibiotic therapy and specific pathogens
Current guidelines recommend amoxicillin (45 mg/kg/day) or high-dose amoxicillin (80-90 mg/kg/day) as initial therapy in presumed or documented bacterial AOM.5 Although amoxicillin is effective against pneumococcus and β-lactamase–negative strains of H influenzae, it is ineffective against β-lactamase–positive strains of H influenzae.9 Significant initial failures may point to a changing pathogen per population frequency. A 2004 review assessed children with continued (persistence of infection detected within 30 days after treatment completion) or refractory (clinical failure while receiving antimicrobial therapy) AOM who have received high-dose amoxicillin as initial empiric therapy. The authors noted that the rate of infection due to H influenzae has increased from 43% among those treated prior to the licensure of PCV-7 to 57% among those who received 2 or more doses of PCV-7.10
Evidence from the medical literature
Three studies provide the major evidence concerning the pathogen shift associated with the adoption of the PCV-7 conjugate vaccine:
- The Finnish Otitis Media Vaccine Trial2
- A published collection of clinical trial results from a rural practice in Kentucky in which 94% of children were immunized with PCV-79
- A prospective study conducted in a suburban community-based private practice in Rochester, NY that evaluated children with persistent or nonresponsive AOM.10
The Finnish Otitis Media Vaccine Trial
In this trial, 1662 infants received either the PCV-7 vaccine or a control vaccine at ages 2, 4, 6, and 12 months and were monitored from ages 6.5 months to 24 months.2 An overall 6.9% reduction in episodes of clinical AOM were diagnosed (n=1251) in PCV-7–vaccinated children compared with the control group (n=1345). This suggested that fewer AOM cases were caused by the S pneumoniae vaccine serotypes than nonvaccine serotypes. The bacteriologic findings in the samples of middle ear fluid taken during 93% of the visits for AOM (TABLE 1) show a 34% reduction in culture-confirmed episodes in the PCV-7–vaccinated group, a decrease of more than 50% in pneumococcal AOM episodes caused by vaccine or vaccine cross-reactive serotypes, a 33% increase in infections caused by other pneumococcal serotypes, and an 11% increase in the proportion of AOM cases due to H influenzae.2
TABLE 1
Finnish Otitis Media Vaccine Trial: Causes of AOM Episodes and Impact of PCV-7 Immunization on Incidence
| CAUSE | PCV-7 EPISODES | CONTROL EPISODES | DIFFERENCE (%) |
|---|---|---|---|
| Culture-confirmed pneumococcus | 271 | 414 | 34 |
| Pneumococcal serotype in vaccine | 107 | 250 | 57 |
| Vaccine cross-reactive serotypes* | 41 | 84 | 51 |
| Other pneumococcal serotypes | 125 | 95 | 33 |
| Haemophilus influenzae | 315 | 287 | 11 |
| Moraxella catarrhalis | 379 | 381 | 1 |
| *6A, 9N, 18B, 19A, 23A. | |||
| AOM, acute otitis media; PCV-7, 7-valent pneumococcal conjugate vaccine. | |||
| Adapted with permission from Eskola J, et al. N Engl J Med. 2001;344:403-409. Copyright 2001 Massachusetts Medical Society. All rights reserved. | |||
Study Results From a Rural Kentucky Practice
In this practice, data on isolates from middle ear fluid were collected in children with severe or refractory AOM aged 7 to 24 months.9 Data were obtained from 1992 to 1998, before the introduction of PCV-7, and from 2000 to 2003, following immunization with 3 or 4 doses of PCV-7 during the first 18 months of life.
As shown in TABLE 2, the pre-PCV-7 group of children (N=336; 1992-1998) had a proportion of 48% culture-confirmed pneumococcus vs a proportion of 31% in the PCV-7-vaccinated group (N=83; 2000-2003), a 17% decrease. The decrease in proportion of AOM episodes resulting from vaccine serotypes was 34%.
In this investigation, 28% of the pre-PCV-7 group and 34% of the post-PCV-7 group had received antibiotic therapy within the previous 3 days. Additionally, 59% and 76%, respectively, had received antibiotic therapy within the preceding 30 days. There were increases of 30% in vaccine cross-reactive serotypes and 45% in nonvaccine serotypes. Vaccine cross-reactive serotypes 6A and 19A accounted for most of the penicillin-nonsusceptible S pneumoniae strains in the vaccinated population.
Most impressive, however, in the post-PCV-7 group, was that gram-negative bacteria, mainly H influenzae, accounted for two thirds of AOM isolates, an increase from 41% in the pre-PCV-7 group to 56% in the vaccinated group. A 56% increase was noted in β-lactamase–positive organisms from the pre-PCV-7 group to the post-PCV-7 group. The combined H influenzae and M catarrhalis β-lactamase–producing organisms accounted for nearly half of all isolates.9
TABLE 2
Results From a Rural Kentucky Practice: Change in AOM Microbiology From Pre-PCV-7 (1992–1998) to Post-PCV-7 (2000–2003)
| PATHOGEN | PRE-PCV-7 1992-1998 (N=336) | POST-PCV-7 2000-2003 (N=83) | CHANGE (%) | P VALUE | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Culture-confirmed pneumococcus | 160 | 48 | 26 | 31 | 17 | .007 |
| Pneumococcal serotype in vaccine | 236 | 70 | 30 | 36 | 34 | .003 |
| Vaccine cross-reactive serotypes* | 27 | 8 | 27 | 32 | 24 | .003 |
| Other pneumococcal serotypes† | 74 | 22 | 27 | 32 | 10 | NS |
| Haemophilus influenzae | 137 | 41 | 46 | 32 | 15 | .01 |
| β-lactamase-positive | 108 | 23 | 39 | 36 | 15 | .007 |
| Moraxella catarrhalis, β-lactamase-positive | 31 | 9 | 9 | 11 | 2 | NS |
| AOM, acute otitis media; n, total isolates; NS, nonsignificant; PCV-7, 7-valent pneumococcal conjugate vaccine. | ||||||
| *Includes 6A and 19A. | ||||||
| †Nonvaccine serotypes in post-PCV-7 group: 1, 11A, 15A, 29, and 33F. | ||||||
| Adapted with permission from Block SL, et al. Pediatr Infect Dis J. 2004;23:829-833. | ||||||
The Prospective Rochester, New York Study
Changes in pre- and post-PVC-7 patterns also were seen in a prospective study of 551 children with persistent or nonresponsive AOM (defined as nonresponders after 1 or 2 empiric antibiotic courses or failures after 48 hours of treatment). These children underwent tympanocentesis to identify bacterial isolates during the 9-year period from 1995 to 2003.10
From 1995 to 1997, enrollees received a standard dose of amoxicillin (40-50 mg/kg, divided into 3 doses daily) as initial empiric treatment. From 1998 to 2000 and 2001 to 2003, all children received high-dose amoxicillin (80-100 mg/kg, divided into twice-daily doses).
During the latter period, the children also were vaccinated with PCV-7, with 63% receiving the primary series of 3 doses and 10% receiving the booster dose. In this investigation, shortages in vaccine supply, discussed below, caused vaccination schedules to be compromised.
Study results (TABLE 3) show that in the post-PCV-7 group, there was a 13% decrease in the proportion of S pneumoniae isolates and a 14% increase in the proportion of H influenzae isolates compared with the pre-PCV-7 group (1998-2000 enrollees). An increase of 22% for β-lactamase–positive bacteria was also observed, along with a trend toward an increased proportion of penicillin-susceptible S pneumoniae isolates (58% vs 72%; P=.017) post-PCV-7.10 A 24% reduction (P=.009) in the frequency of the diagnosis of persistent or AOM treatment failure occurred in the period after PCV-7 vaccination. These changes were considered to be the result of the use of the conjugate pneumococcal vaccine rather than of the change in amoxicillin dosing.10
TABLE 3
The Prospective Rochester, New York Study: Pathogens Isolated in Persistent AOM and AOM Treatment Failure Pre- and Post-PCV-7
| PATHOGEN | PRE-PCV-7 1998-2000 (N=204) | POST-PCV-7 2000-2003 (N=152) | CHANGE (%) | P VALUE | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Streptococcus pneumoniae* | 50 | 44 | 28 | 31 | 13 | .017 |
| Penicillin nonsusceptible | 12 | 24 | 4 | 14 | 10 | NS |
| Haemophilus influenzae | 49 | 43 | 51 | 57 | 14 | .012 |
| β-lactamase-positive | 16 | 33 | 28 | 55 | 22 | .044 |
| Moraxella catarrhalis | 6 | 5 | 1 | 1 | 4 | NS |
| AOM, acute otitis media; N, total isolates; NS, nonsignificant; PCV-7, 7-valent pneumococcal conjugate vaccine. | ||||||
| *Pneumococcal serotyping was not done. | ||||||
| Adapted with permission from Casey JR, Pichichero ME. Pediatr Infect Dis J. 2004;23:824-828. | ||||||
Pneumococcal serotype shifts
In addition to the change in causative pathogens, use of the conjugate pneumococcal vaccine appears to have led to a significant shift in the pneumococcal strains causing AOM. Studies at urban medical centers and in the Kentucky practice documented an increase in the proportion of nonvaccine serotypes, accounting for 32% to 38% of pneumococcal AOM.10-12 A 33% increase was seen in the Finnish Trial.2 These nonvaccine pneumococcal serotypes do not carry the same level of resistance seen with those serotypes included in PCV-7.
PCV-7 conjugate vaccine
The PCV-7 conjugate vaccine was approved for use in February 2000. It is a 7-valent pneumococcal conjugate of the capsular antigens of the S pneumoniae serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F, individually conjugated to diphtheria CRM197 protein.13 These serotypes have been responsible for approximately 80% of invasive pneumococcal disease in children younger than 6 years in the United States.13,14 They also accounted for 74% of penicillin-susceptible S pneumoniae and 100% of pneumococci with high-level penicillin resistance isolated from children younger than age 6 years with invasive disease during a 1993-1994 surveillance by the Centers for Disease Control and Prevention (CDC).13
Mechanism of Action and Recommended Immunization Schedule
The conjugate vaccine is converted to a T-cell–dependent antigen, antibody formation is enhanced, and memory B cells are primed.14
The recommended immunization schedule was established as 3 primary doses at ages 2, 4, and 6 months and a booster dose at 12 to 15 months.1 It is the first multivalent pneumococcal vaccine approved for use in children younger than 24 months.
An 89% reduction in invasive pneumococcal disease was observed in children receiving 1 or more doses, and the vaccine appears to reduce nasopharyngeal carriage of vaccine serotypes.15,16
The older 23-valent polysaccharide vaccine does not stimulate good response in children younger than 2 years of age14 and does not reduce mucosal carriage or limit the spread of resistant strains.15
PCV-7 Supply Since 2000
In August 2001, a serious shortage of the vaccine developed in 34 state immunization programs.17 The following month, the CDC advised physicians to administer it only to children younger than 12 months and to those aged 1 to 5 years at increased risk of pneumococcal disease.18 As demand continued despite the change in recommendations, the CDC further changed recommendations to conserve vaccine supply, first suspending the fourth dose temporarily in healthy children19 and then discontinuing both the third and fourth doses.11 In July 2004, production problems seemed to have resolved; the CDC recommended that every child receive 3 doses. In September, supplies were adequate for return to the 4-dose schedule.12 As of June 2004, 67.7% of children aged 24 months had received 3 or more doses of PCV-7.20 Thus, the effects of PCV-7 on the changing microbiology of AOM may only now, at the end of 2005, be fully realized.
Herd Immunity and Reduction in Carriage
Despite the shortages of vaccine during the first years of use, evidence of herd immunity and a decrease in antibiotic resistance in pneumococcal pathogens has been reported throughout the United States.21,22 A 29% decrease in the rate of pneumococcal disease in both young children and adults has also been observed, along with a 35% reduction in the rate of disease caused by nonpenicillin-susceptible pneumococcal strains.21 The reduction in carriage among vaccinated children may be the reason.21,22 Because of the impact of PCV-7, it will be important to record immunization history when collecting AOM data.
AOM treatment choices
The basis of recommendations for treating AOM depends on the presumed responsible pathogens, their susceptibility to antibiotics, and concerns for developing resistance, all influenced by clinical trial data. In practice, however, empiric choices are often made based on knowledge of local resistance patterns and of other patient characteristics; that is cost concerns, adverse event profiles, need to avoid initial treatment failure, adherence issues (eg, taste or palatability), convenience, and duration of dosing regimen.
All current guidelines recommend oral amoxicillin as first-line therapy in documented or presumed bacterial AOM. The 2004 American Academy of Pediatrics/American Academy of Family Physicians’ (AAP/AAFP) guidelines4 recommended increasing the dosage used for empiric treatment from 40 to 45 mg/kg/day to 80 to 90 mg/kg/day for all children. This was a result of concerns about the prevalence of penicillin-resistant S pneumoniae for which standard-dose amoxicillin is inadequate.23
The guidelines were written and published before the data from the Kentucky and New York studies were available; therefore, although the guidelines recommended that empiric treatment of bacterial AOM should target S pneumoniae, H influenzae, and M catarrhalis, the pathogen shift discussed previously might today produce a modified antibiotic selection paradigm. The pathogen mix in persistent or recurrent AOM has already led to a guideline recommendation for high-dose amoxicillin/clavulanate, 90/6.4 mg/kg/day, cefdinir, cefprozil, cefpodoxime, cefuroxime, or ceftriaxone in these patients.23
If an increase in the proportion of β-lactamase–producing pathogens due to PCV-7 occurs, amoxicillin may no longer be the best first choice.
Selecting Among Recommended Antibiotic Choices
As antibiotic preparations for treating bacterial AOM are oral suspensions, taste is a major factor for pediatric patients. TABLE 4 summarizes comparative taste ratings for antibiotic suspensions based on several studies and shows the range, from those that can enhance compliance to those that discourage compliance.23
Adverse events, especially diarrhea, nausea/vomiting, and gastritis, are also of concern. These are shortcomings of amoxicillin/clavulanate, which has a higher incidence of diarrhea and nausea than cephalosporins.24
Dosing frequency is also a factor among recommended agents. Amoxicillin, amoxicillin/clavulanate, cefprozil, and cefpodoxime require twice-daily dosing. Cefdinir can be effective at once-daily dosing.24
Duration of approved therapy is perhaps the most critical selection factor given the reality of patient behaviors. Cefpodoxime and cefdinir are the only 2 FDA-approved agents for 5-day treatment of bacterial AOM that are also guideline-recommended.
TABLE 4
Compliance-Enhancing Ranking of Antibiotic Suspensions
| STRONGLY COMPLIANCE-ENHANCING | |
|
|
| MODERATELY COMPLIANCE-ENHANCING | |
|
|
| EQUIVOCAL COMPLIANCE-ENHANCING | |
| |
| NOT COMPLIANCE-ENHANCING | |
| |
| DISCOURAGES COMPLIANCE | |
|
|
| TMP-SMX, trimethoprim sulfamethoxazole | |
| Sources: Adapted from Steele RW, et al. Pediatr Infect Dis J. 2001;20:1-5. | |
| Demers DM, et al. Pediatr Infect Dis J. 1994;13:87-89. | |
| Ruff ME, et al. Pediatr Infect Dis J. 1991;10:30-33. | |
Choices for Effective Initial Therapy
Considering the changing microbial population in bacterial AOM and the increasing concern of effectiveness of amoxicillin and other antibiotics against β-lactamase–producing H influenzae, the choice of therapy may need modification. Specifically, that may mean changing the choice of effective antibiotic, taking into consideration the compliance-enhancing advantages of available options.
Based on efficacy, the overall prevalence of antibiotic-resistant AOM pathogens for PCV-7-vaccinated children, the potential for adverse effects, and patient compliance issues, Block and Harrison developed an algorithm (FIGURE) for the management of AOM diagnosed by strict criteria in an otherwise healthy child between 4 months and 36 months old.24 As the environment of AOM evolves, the choices for treatment must be not only effective but also the best and most appropriate.
FIGURE Antibiotic Choices for Acute Otitis Media in the 2000s
1. Fireman B, Black SB, Shinefield HR, et al. Impact of the pneumococcal conjugate vaccine on otitis media. Pediatr Infect Dis J. 2003;22:10-16.
2. Eskola J, Kilpi T, Palmu A, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med. 2001;344:403-409.
3. Block SL, Harrison CH, Hedrick J, et al. Restricted use of antibiotic prophylaxis for recurrent acute otitis media in the era of penicillin non-susceptible Streptococcus pneumoniae. Int J Pediatr Otorhinolaryngol. 2001;61:47-60.
4. Advisory Committee on Immunization Practices. Preventing pneumococcal disease among infants and young children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2000;49(RR-9):1-35.
5. American Academy of Family Physicians Subcommittee on Management of Acute Otitis Media. Diagnosis and management of acute otitis media. Pediatrics. 2004;113:1451-1465.
6. Dowell SF, Butler JC, Giebink GS, et al. Acute otitis media: management and surveillance in an era of pneumococcal resistance—a report from the Drug-resistant Streptococcus pneumoniae Therapeutic Working Group. Pediatr Infect Dis J. 1999;18:1-9.
7. Block SL. Causative pathogens, antibiotic resistance and therapeutic considerations in acute otitis media. Pediatr Infect Dis J. 1997;16:449-456.
8. Bluestone CD, Stephenson JS, Martin LM. Ten-year review of otitis media pathogens. Pediatr Infect Dis J. 1992;11(suppl 8):S7-S11.
9. Block SL, Hedrick J, Harrison CJ, et al. Community-wide vaccination with the heptavalent pneumococcal conjugate significantly alters the microbiology of acute otitis media. Pediatr Infect Dis J. 2004;23:829-833.
10. Casey JR, Pichichero ME. Changes in frequency and pathogens causing acute otitis media in 1995-2003. Pediatr Infect Dis J. 2004;23:824-828.
11. Centers for Disease Control and Prevention. Updated recommendations on the use of pneumococcal conjugate vaccine: suspension of recommendation for third and fourth dose. MMWR Morb Mortal Wkly Rep. 2004;53:177-178.
12. Centers for Disease Control and Prevention. Pneumococcal conjugate vaccine shortage resolved. MMWR Morb Mortal Wkly Rep. 2004;53:851-852.
13. Prevnar® (pneumococcal 7-valent vaccine) [prescribing information]. Philadelphia, Pa: Wyeth Pharmaceuticals. Rev. 01/04.
14. Watson W. Pneumococcal conjugate vaccines. Pediatr Infect Dis J. 2000;19:331-332.
15. Giebink GS. The prevention of pneumococcal disease in children. N Engl J Med. 2001;345:1177-1183.
16. Pelton SI, Loughlin AM, Marchand CD. Seven valent pneumococcal conjugate vaccine immunization in two Boston communities: changes in serotypes and antimicrobial susceptibility among Streptococcus pneumoniae isolates. Pediatr Infect Dis J. 2003;23:1015-1022.
17. Freed GL, Davis MM, Clark SJ. Variation in public and private supply of pneumococcal conjugate vaccine during a shortage. JAMA. 2003;289:575-578.
18. Centers for Disease Control and Prevention. Decreased availability of pneumococcal conjugate vaccine. MMWR Morb Mortal Wkly Rep. 2001;50:783-784.
19. Centers for Disease Control and Prevention. Limited supply of pneumococcal conjugate vaccine: suspension of recommendation for fourth dose. MMWR Morb Mortal Wkly Rep. 2004;53:108-109.
20. CDC National Immunization Survey. Estimated vaccination coverage with individual vaccines and selected vaccination series by 24 months of age by state and immunization action plan area US, Q3/2003-Q4/2004. Available at: http://www2a.cdc.gov/nip/coverage/nis/nis_iap.asp?fmt=v&rpt=tab09_24mo_iap_0304&qtr=Q3/2003-Q2/2004. Accessed September 20, 2005.
21. Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737-1746.
22. Black S, Shinefield H, Baxter R, et al. Postlicensure surveillance for pneumococcal invasive disease after use of heptavalent pneumococcal vaccine in Northern California Kaiser Permanente. Pediatr Infect Dis J. 2004;23:485-489.
23. Pichichero ME, Casey JR. Acute otitis media: Making sense of recent guidelines on antimicrobial treatment. J Fam Pract. 2005;54:313-332.
24. Block SL, Harrison CJ. Diagnosis and Management of Acute Otitis Media, 3rd ed. In press.
1. Fireman B, Black SB, Shinefield HR, et al. Impact of the pneumococcal conjugate vaccine on otitis media. Pediatr Infect Dis J. 2003;22:10-16.
2. Eskola J, Kilpi T, Palmu A, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med. 2001;344:403-409.
3. Block SL, Harrison CH, Hedrick J, et al. Restricted use of antibiotic prophylaxis for recurrent acute otitis media in the era of penicillin non-susceptible Streptococcus pneumoniae. Int J Pediatr Otorhinolaryngol. 2001;61:47-60.
4. Advisory Committee on Immunization Practices. Preventing pneumococcal disease among infants and young children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2000;49(RR-9):1-35.
5. American Academy of Family Physicians Subcommittee on Management of Acute Otitis Media. Diagnosis and management of acute otitis media. Pediatrics. 2004;113:1451-1465.
6. Dowell SF, Butler JC, Giebink GS, et al. Acute otitis media: management and surveillance in an era of pneumococcal resistance—a report from the Drug-resistant Streptococcus pneumoniae Therapeutic Working Group. Pediatr Infect Dis J. 1999;18:1-9.
7. Block SL. Causative pathogens, antibiotic resistance and therapeutic considerations in acute otitis media. Pediatr Infect Dis J. 1997;16:449-456.
8. Bluestone CD, Stephenson JS, Martin LM. Ten-year review of otitis media pathogens. Pediatr Infect Dis J. 1992;11(suppl 8):S7-S11.
9. Block SL, Hedrick J, Harrison CJ, et al. Community-wide vaccination with the heptavalent pneumococcal conjugate significantly alters the microbiology of acute otitis media. Pediatr Infect Dis J. 2004;23:829-833.
10. Casey JR, Pichichero ME. Changes in frequency and pathogens causing acute otitis media in 1995-2003. Pediatr Infect Dis J. 2004;23:824-828.
11. Centers for Disease Control and Prevention. Updated recommendations on the use of pneumococcal conjugate vaccine: suspension of recommendation for third and fourth dose. MMWR Morb Mortal Wkly Rep. 2004;53:177-178.
12. Centers for Disease Control and Prevention. Pneumococcal conjugate vaccine shortage resolved. MMWR Morb Mortal Wkly Rep. 2004;53:851-852.
13. Prevnar® (pneumococcal 7-valent vaccine) [prescribing information]. Philadelphia, Pa: Wyeth Pharmaceuticals. Rev. 01/04.
14. Watson W. Pneumococcal conjugate vaccines. Pediatr Infect Dis J. 2000;19:331-332.
15. Giebink GS. The prevention of pneumococcal disease in children. N Engl J Med. 2001;345:1177-1183.
16. Pelton SI, Loughlin AM, Marchand CD. Seven valent pneumococcal conjugate vaccine immunization in two Boston communities: changes in serotypes and antimicrobial susceptibility among Streptococcus pneumoniae isolates. Pediatr Infect Dis J. 2003;23:1015-1022.
17. Freed GL, Davis MM, Clark SJ. Variation in public and private supply of pneumococcal conjugate vaccine during a shortage. JAMA. 2003;289:575-578.
18. Centers for Disease Control and Prevention. Decreased availability of pneumococcal conjugate vaccine. MMWR Morb Mortal Wkly Rep. 2001;50:783-784.
19. Centers for Disease Control and Prevention. Limited supply of pneumococcal conjugate vaccine: suspension of recommendation for fourth dose. MMWR Morb Mortal Wkly Rep. 2004;53:108-109.
20. CDC National Immunization Survey. Estimated vaccination coverage with individual vaccines and selected vaccination series by 24 months of age by state and immunization action plan area US, Q3/2003-Q4/2004. Available at: http://www2a.cdc.gov/nip/coverage/nis/nis_iap.asp?fmt=v&rpt=tab09_24mo_iap_0304&qtr=Q3/2003-Q2/2004. Accessed September 20, 2005.
21. Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737-1746.
22. Black S, Shinefield H, Baxter R, et al. Postlicensure surveillance for pneumococcal invasive disease after use of heptavalent pneumococcal vaccine in Northern California Kaiser Permanente. Pediatr Infect Dis J. 2004;23:485-489.
23. Pichichero ME, Casey JR. Acute otitis media: Making sense of recent guidelines on antimicrobial treatment. J Fam Pract. 2005;54:313-332.
24. Block SL, Harrison CJ. Diagnosis and Management of Acute Otitis Media, 3rd ed. In press.