User login
Lilly cuts insulin price by 70%, caps out-of-pocket cost
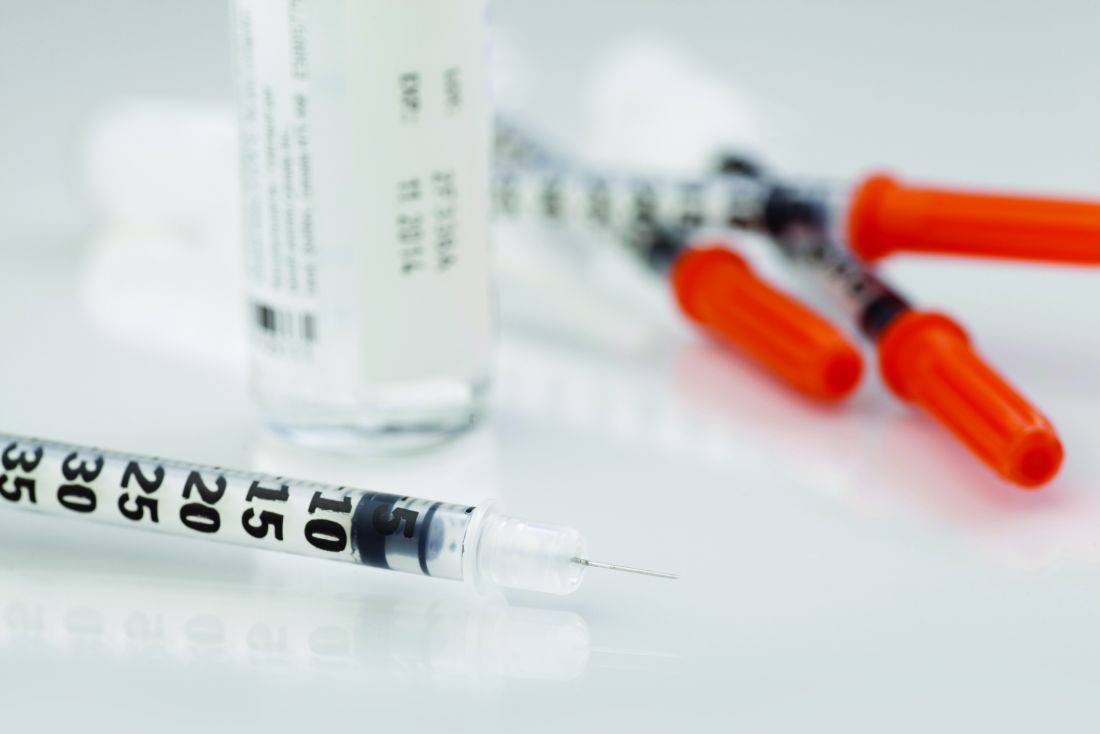
Eli Lilly will cut prices for most of its insulins in the United States by 70% and cap out-of-pocket costs for insulin at $35 per month, the company announced on March 1.
“Lilly is taking these actions to make it easier to access Lilly insulin and help Americans who may have difficulty navigating a complex healthcare system that may keep them from getting affordable insulin,” the company said in a statement.
The $35 price cap is effective immediately at participating retail pharmacies for people with commercial insurance. Those without insurance can go to InsulinAffordability.com and download the Lilly Insulin Value Program savings card to receive Lilly insulins for $35 per month.
The company says it will cut the list price of its nonbranded Insulin Lispro Injection 100 units/mL to $25 a vial, effective May 1, 2023. The list price of the branded Humalog (insulin lispro injection) 100 units/mL will be cut by 70%, effective in the fourth quarter of 2023.
Lilly is among the three main companies that manufacture insulin, along with Novo Nordisk and Sanofi, that have come under fire over the cost of insulin in the US. Studies have shown that up to 25% of people with type 1 diabetes ration insulin because of costs, putting their health and often their lives in jeopardy.
Prices in the United States are around 10 times higher than in other countries. California is the latest state to say it plans to sue these big three companies over the high price of insulin and has announced plans to make its own cheaper versions.
Asked at a telephone press briefing if the lawsuit prompted the company’s move, Lilly chair and CEO David A. Ricks said: “Of course there are complaints against the industry and the company. We see those as completely unfounded. However, we can probably all agree that patients should have a consistent and lower-cost experience at the pharmacy counter, and that’s what today’s announcement is about. We’re doing this completely voluntarily because it’s time and it’s the right thing to do.”
On hearing the company announcement, Laura Nally, MD, a pediatric endocrinologist living with type 1 diabetes, @drnallypants, tweeted: “YES. After years of advocacy, the list price of Lispro/Humalog is now similar to what it was in the late 1990s. Cheers to all the #pwd [people with diabetes] who have advocated through #insulin4all! But we still have work to do to improve access to other diabetes medications & supplies.”
#insulin4all is a worldwide campaign to ensure that people with type 1 diabetes have access to affordable insulin and other supplies needed to manage the condition, such as glucose strips. It is supported, among others, by the advocacy group T1International.
Also giving his reaction to the Lilly announcement, Chuck Henderson, CEO of the American Diabetes Association, said: “We applaud Eli Lilly for taking the important step to limit cost-sharing for its insulin, and we encourage other insulin manufacturers to do the same.
“While we have been able to help achieve significant progress on the issue of insulin affordability, including Medicare’s new out-of-pocket cost cap on insulin, state copay caps, and patient assistance developments from insulin manufacturers, we know that our work is not done,” he added.
“ADA will work to ensure that Eli Lilly’s patient assistance program is benefiting patients as intended and continue the fight so that everyone who needs insulin has access.”
And Endocrine Society chief medical officer Robert Lash, MD, said: “Lilly’s move to apply a $35/month cap for people with private insurance will be a significant improvement for adults and children with diabetes who use Lilly’s products.
“We encourage all insulin manufacturers to join in the effort to reduce out-of-pocket costs for people who need insulin.”
Lilly will also launch a new insulin biosimilar, Rezvoglar (insulin glargine-aglr) injection, which is similar to and interchangeable with insulin glargine (Lantus). The cost will by $92 for a five pack of KwikPens, a 78% discount, compared with the cost of Lantus, beginning April 1, 2023.
A version of this article first appeared on Medscape.com.
Eli Lilly will cut prices for most of its insulins in the United States by 70% and cap out-of-pocket costs for insulin at $35 per month, the company announced on March 1.
“Lilly is taking these actions to make it easier to access Lilly insulin and help Americans who may have difficulty navigating a complex healthcare system that may keep them from getting affordable insulin,” the company said in a statement.
The $35 price cap is effective immediately at participating retail pharmacies for people with commercial insurance. Those without insurance can go to InsulinAffordability.com and download the Lilly Insulin Value Program savings card to receive Lilly insulins for $35 per month.
The company says it will cut the list price of its nonbranded Insulin Lispro Injection 100 units/mL to $25 a vial, effective May 1, 2023. The list price of the branded Humalog (insulin lispro injection) 100 units/mL will be cut by 70%, effective in the fourth quarter of 2023.
Lilly is among the three main companies that manufacture insulin, along with Novo Nordisk and Sanofi, that have come under fire over the cost of insulin in the US. Studies have shown that up to 25% of people with type 1 diabetes ration insulin because of costs, putting their health and often their lives in jeopardy.
Prices in the United States are around 10 times higher than in other countries. California is the latest state to say it plans to sue these big three companies over the high price of insulin and has announced plans to make its own cheaper versions.
Asked at a telephone press briefing if the lawsuit prompted the company’s move, Lilly chair and CEO David A. Ricks said: “Of course there are complaints against the industry and the company. We see those as completely unfounded. However, we can probably all agree that patients should have a consistent and lower-cost experience at the pharmacy counter, and that’s what today’s announcement is about. We’re doing this completely voluntarily because it’s time and it’s the right thing to do.”
On hearing the company announcement, Laura Nally, MD, a pediatric endocrinologist living with type 1 diabetes, @drnallypants, tweeted: “YES. After years of advocacy, the list price of Lispro/Humalog is now similar to what it was in the late 1990s. Cheers to all the #pwd [people with diabetes] who have advocated through #insulin4all! But we still have work to do to improve access to other diabetes medications & supplies.”
#insulin4all is a worldwide campaign to ensure that people with type 1 diabetes have access to affordable insulin and other supplies needed to manage the condition, such as glucose strips. It is supported, among others, by the advocacy group T1International.
Also giving his reaction to the Lilly announcement, Chuck Henderson, CEO of the American Diabetes Association, said: “We applaud Eli Lilly for taking the important step to limit cost-sharing for its insulin, and we encourage other insulin manufacturers to do the same.
“While we have been able to help achieve significant progress on the issue of insulin affordability, including Medicare’s new out-of-pocket cost cap on insulin, state copay caps, and patient assistance developments from insulin manufacturers, we know that our work is not done,” he added.
“ADA will work to ensure that Eli Lilly’s patient assistance program is benefiting patients as intended and continue the fight so that everyone who needs insulin has access.”
And Endocrine Society chief medical officer Robert Lash, MD, said: “Lilly’s move to apply a $35/month cap for people with private insurance will be a significant improvement for adults and children with diabetes who use Lilly’s products.
“We encourage all insulin manufacturers to join in the effort to reduce out-of-pocket costs for people who need insulin.”
Lilly will also launch a new insulin biosimilar, Rezvoglar (insulin glargine-aglr) injection, which is similar to and interchangeable with insulin glargine (Lantus). The cost will by $92 for a five pack of KwikPens, a 78% discount, compared with the cost of Lantus, beginning April 1, 2023.
A version of this article first appeared on Medscape.com.
Eli Lilly will cut prices for most of its insulins in the United States by 70% and cap out-of-pocket costs for insulin at $35 per month, the company announced on March 1.
“Lilly is taking these actions to make it easier to access Lilly insulin and help Americans who may have difficulty navigating a complex healthcare system that may keep them from getting affordable insulin,” the company said in a statement.
The $35 price cap is effective immediately at participating retail pharmacies for people with commercial insurance. Those without insurance can go to InsulinAffordability.com and download the Lilly Insulin Value Program savings card to receive Lilly insulins for $35 per month.
The company says it will cut the list price of its nonbranded Insulin Lispro Injection 100 units/mL to $25 a vial, effective May 1, 2023. The list price of the branded Humalog (insulin lispro injection) 100 units/mL will be cut by 70%, effective in the fourth quarter of 2023.
Lilly is among the three main companies that manufacture insulin, along with Novo Nordisk and Sanofi, that have come under fire over the cost of insulin in the US. Studies have shown that up to 25% of people with type 1 diabetes ration insulin because of costs, putting their health and often their lives in jeopardy.
Prices in the United States are around 10 times higher than in other countries. California is the latest state to say it plans to sue these big three companies over the high price of insulin and has announced plans to make its own cheaper versions.
Asked at a telephone press briefing if the lawsuit prompted the company’s move, Lilly chair and CEO David A. Ricks said: “Of course there are complaints against the industry and the company. We see those as completely unfounded. However, we can probably all agree that patients should have a consistent and lower-cost experience at the pharmacy counter, and that’s what today’s announcement is about. We’re doing this completely voluntarily because it’s time and it’s the right thing to do.”
On hearing the company announcement, Laura Nally, MD, a pediatric endocrinologist living with type 1 diabetes, @drnallypants, tweeted: “YES. After years of advocacy, the list price of Lispro/Humalog is now similar to what it was in the late 1990s. Cheers to all the #pwd [people with diabetes] who have advocated through #insulin4all! But we still have work to do to improve access to other diabetes medications & supplies.”
#insulin4all is a worldwide campaign to ensure that people with type 1 diabetes have access to affordable insulin and other supplies needed to manage the condition, such as glucose strips. It is supported, among others, by the advocacy group T1International.
Also giving his reaction to the Lilly announcement, Chuck Henderson, CEO of the American Diabetes Association, said: “We applaud Eli Lilly for taking the important step to limit cost-sharing for its insulin, and we encourage other insulin manufacturers to do the same.
“While we have been able to help achieve significant progress on the issue of insulin affordability, including Medicare’s new out-of-pocket cost cap on insulin, state copay caps, and patient assistance developments from insulin manufacturers, we know that our work is not done,” he added.
“ADA will work to ensure that Eli Lilly’s patient assistance program is benefiting patients as intended and continue the fight so that everyone who needs insulin has access.”
And Endocrine Society chief medical officer Robert Lash, MD, said: “Lilly’s move to apply a $35/month cap for people with private insurance will be a significant improvement for adults and children with diabetes who use Lilly’s products.
“We encourage all insulin manufacturers to join in the effort to reduce out-of-pocket costs for people who need insulin.”
Lilly will also launch a new insulin biosimilar, Rezvoglar (insulin glargine-aglr) injection, which is similar to and interchangeable with insulin glargine (Lantus). The cost will by $92 for a five pack of KwikPens, a 78% discount, compared with the cost of Lantus, beginning April 1, 2023.
A version of this article first appeared on Medscape.com.
Old drug verapamil may have new use in type 1 diabetes
In children and adolescents with new-onset type 1 diabetes, the calcium channel blocker verapamil slowed the destruction of insulin-producing pancreatic beta cells for up to a year, new data show.
Use of daily verapamil within a month of diagnosis resulted in a 30% increase in C-peptide secretion (a measure of preserved beta-cell function), compared with placebo at 52 weeks, without serious adverse events.
To put it another way, verapamil delayed the expected decline in C-peptide production from 3 months after diagnosis of type 1 diabetes to 6 months after diagnosis.
“We think this is a really, really exciting finding that’s hopefully going to impact the care for children with type 1 diabetes in the new-onset period,” lead author Gregory P. Forlenza, MD, said during his presentation of the data on Feb. 24 at the annual Advanced Technologies & Treatments for Diabetes (ATTD) meeting in Berlin.
“In view of the favorable safety profile, particularly compared with immune-suppressive agents, once-a-day oral administration, and low cost, initiation of verapamil should be a consideration for newly diagnosed patients with type 1 diabetes,” added Dr. Forlenza, a pediatric endocrinologist at the Barbara Davis Center for Diabetes, Anschutz Medical Campus, University of Colorado, Aurora.
The data were also simultaneously published in JAMA, as part of the CLVer (Hybrid Closed Loop Therapy and Verapamil for Beta Cell Preservation in New Onset Type 1 Diabetes) trial.
The randomized, double-blind, six-center trial involved 113 participants, aged 7-17 years, with newly diagnosed type 1 diabetes. They were randomized to the most advanced commercially available automated insulin delivery systems available or standard care to test the effects of intensive glucose control on C-peptide levels for 52 weeks during the COVID-19 pandemic (July 2020 to September 2022). Eighty-eight patients who weighed 30 kg (66 lb) or more were further randomized (1:1) to daily extended-release verapamil or placebo for the same duration.
The positive findings for verapamil, published in one paper, contrasted with the negative ones for the automated insulin delivery (AID) system. The latter did not prevent the expected decline in C-peptide, putting to rest a long-held hypothesis that reducing glucotoxicity might preserve beta-cell function in newly diagnosed individuals with type 1 diabetes, noted Dr. Forlenza.
Could combination therapy work?
In recent years, immune-modulating agents have increasingly been shown to preserve beta-cell function in both new-onset and preclinical type 1 diabetes. One such agent, teplizumab (Tzield, Provention Bio), was approved by the U.S. Food and Drug Administration in November 2022 to delay type 1 diabetes onset in those at high risk.
Calcium channel blockers such as verapamil – used for years to treat hypertension and cardiac arrhythmias – may accomplish the same goal as teplizumab but in a different way, by reducing the protein overexpression that induces beta-cell apoptosis and death.
Dr. Forlenza showed a slide comparing the preservation of C-peptide, which was much lower with verapamil, at 30%, than with teplizumab, at 75%.
Asked to comment, session moderator Torben Biester, MD, a pediatric diabetologist at Auf der Bult-Zentrum Diabetes-Center for Children and Adolescents, Hanover, Germany, said: “[Verapamil] is a very cheap [daily] pill. [Teplizumab] is a very high-priced ... immune therapy in the United States ... an infusion twice for 10 days, so it’s a lot more burden for the patients and a lot more risk of side effects.”
“The future might be combination therapy,” added Dr. Biester.
And in an editorial published in JAMA and accompanying the two CLVer papers, Jennifer Couper, MD, of the University of Adelaide, agrees: “A well-tolerated, inexpensive, oral treatment such as verapamil with modest benefits on C-peptide production is relevant to practice.”
The new work “supports investigation of verapamil in combination with other effective agents during the earlier stages of type 1 diabetes before insulin dependence develops,” she noted.
Verapamil results ‘brilliant’ but more work needed
In the verapamil part of the CLVer trial, by 52 weeks, verapamil doses in the youth who received it ranged from 120-360 mg/day based on weight and tolerance.
The primary outcome, C-peptide area under the curve, stayed stable, from 0.66 pmol/mL at baseline to 0.65 pmol/mL at 52 weeks in the verapamil group, compared with a drop from 0.60 pmol/mL down to 0.44 pmol/mL with placebo, a significant difference of 0.14 pmol/mL (P = .04), representing a 30% higher C-peptide level in the verapamil group.
“For us, this is a phenomenally exciting result,” Dr. Forlenza commented during his presentation.
At 52 weeks, A1c was 6.6% in the verapamil group versus 6.9% with placebo, which was not significantly different. Daily insulin dose was 0.65 versus 0.74 units/kg per day, respectively, also not significantly different.
One severe hypoglycemic event occurred in each group, and one diabetic ketoacidosis event occurred in the placebo group. In the verapamil group, three participants experienced “nonserious” electrocardiogram abnormalities and one had hypertension.
Dr. Biester said he isn’t “that concerned” about the small number of mild ECG abnormalities seen in the study with verapamil, as this is a known side effect. But overall, he said, “I would think that for a recommendation for routine use it’s too early after one study, even though the results are brilliant.”
He noted that he is involved in a similar ongoing study of verapamil in adults with new-onset type 1 diabetes, called Ver-A-T1D.
No C-peptide effect of tight glycemic control: ‘A tough pill’
In the AID part of the study, the 113 participants were randomized 2:1 to one of two commercially available AID systems (Tandem t:slim X2 with Control-IQ or Medtronic 670G or 780G) plus frequent contact (a median of 35 times) by study staff, or standard management using a continuous glucose monitor (CGM) with an insulin pump or multiple daily injections.
At 52 weeks, A1c was 6.5% for the intensive group versus 7.1% with standard care, a significant difference. Time in blood glucose range of 70-180 mg/dL was significantly longer with intensive management, at 78%, compared with standard care, at 64%.
Nonetheless, the change in C-peptide area under the curve did not differ between the two groups, decreasing from 0.57 pmol/mL at baseline to 0.45 pmol/mL at 52 weeks with the AID system, compared with a decrease from 0.60 pmol/L down to 0.50 pmol/L with standard care (P = .89).
Dr. Forlenza commented that the hypothesis that tight glycemic control would delay the decline in C-peptide secretion “is something I think a lot of endocrinologists assumed to be true and something I’ve heard lots of colleagues over the years talk about.”
Consequently, he said these findings are “a tough pill for us to swallow ... but it’s important for us in the field to understand.”
“Even with frequent contacts that are well above the level we’d be able to do in standard clinical care, and even with use of the most advanced AID systems we have ... we saw absolutely no difference in stimulated C-peptide levels at any of the timepoints throughout the first year or at 52 weeks.”
“So, in our opinion, this,” combined with a prior study from 2022, “should put this hypothesis to rest,” he said.
“Excellent glycemic control has a benefit in and of itself, but it was not a successful intervention for beta-cell preservation.”
Dr. Forlenza has reported serving as a consultant, speaker, or advisory board member for Medtronic, Dexcom, Abbott, Tandem Diabetes Care, Insulet, Lilly, and Beta Bionics, and his institution has also received funding on his behalf for research grants from these companies. Dr. Biester has reported receiving speaker’s fees from DexCom, Medtronic, Novo Nordisk, F. Hoffmann–La Roche, Sanofi, and Ypsomed Holding; serving on advisory boards for Ascensia Diabetes Care Holdings, AstraZeneca, DexCom, and Medtronic; and receiving personal fees from SYNLAB; and is a member of the European Commission Expert Panel for Medical Devices for Endocrinology and Diabetes. Dr. Couper has reported no relevant financial relationships.
The rationale for the companion CLVer analysis of the effect of reducing glucose toxicity via tight glycemic control on C-peptide progression dates back to an inpatient study published in 1989 involving 26 adolescents using an early artificial pancreas prototype called a Biostator, in which beta-cell preservation was achieved. However, two more recent studies of this approach, including one published in late 2022, did not show a difference. The CLVer analysis involved 113 participants randomized 2:1 to one of two commercially available AID systems (Tandem t:slim X2 with Control-IQ or Medtronic 670G or 780G) plus frequent contact by study staff, or standard management using a CGM with a pump or multiple daily injections.
A version of this article originally appeared on Medscape.com.
In children and adolescents with new-onset type 1 diabetes, the calcium channel blocker verapamil slowed the destruction of insulin-producing pancreatic beta cells for up to a year, new data show.
Use of daily verapamil within a month of diagnosis resulted in a 30% increase in C-peptide secretion (a measure of preserved beta-cell function), compared with placebo at 52 weeks, without serious adverse events.
To put it another way, verapamil delayed the expected decline in C-peptide production from 3 months after diagnosis of type 1 diabetes to 6 months after diagnosis.
“We think this is a really, really exciting finding that’s hopefully going to impact the care for children with type 1 diabetes in the new-onset period,” lead author Gregory P. Forlenza, MD, said during his presentation of the data on Feb. 24 at the annual Advanced Technologies & Treatments for Diabetes (ATTD) meeting in Berlin.
“In view of the favorable safety profile, particularly compared with immune-suppressive agents, once-a-day oral administration, and low cost, initiation of verapamil should be a consideration for newly diagnosed patients with type 1 diabetes,” added Dr. Forlenza, a pediatric endocrinologist at the Barbara Davis Center for Diabetes, Anschutz Medical Campus, University of Colorado, Aurora.
The data were also simultaneously published in JAMA, as part of the CLVer (Hybrid Closed Loop Therapy and Verapamil for Beta Cell Preservation in New Onset Type 1 Diabetes) trial.
The randomized, double-blind, six-center trial involved 113 participants, aged 7-17 years, with newly diagnosed type 1 diabetes. They were randomized to the most advanced commercially available automated insulin delivery systems available or standard care to test the effects of intensive glucose control on C-peptide levels for 52 weeks during the COVID-19 pandemic (July 2020 to September 2022). Eighty-eight patients who weighed 30 kg (66 lb) or more were further randomized (1:1) to daily extended-release verapamil or placebo for the same duration.
The positive findings for verapamil, published in one paper, contrasted with the negative ones for the automated insulin delivery (AID) system. The latter did not prevent the expected decline in C-peptide, putting to rest a long-held hypothesis that reducing glucotoxicity might preserve beta-cell function in newly diagnosed individuals with type 1 diabetes, noted Dr. Forlenza.
Could combination therapy work?
In recent years, immune-modulating agents have increasingly been shown to preserve beta-cell function in both new-onset and preclinical type 1 diabetes. One such agent, teplizumab (Tzield, Provention Bio), was approved by the U.S. Food and Drug Administration in November 2022 to delay type 1 diabetes onset in those at high risk.
Calcium channel blockers such as verapamil – used for years to treat hypertension and cardiac arrhythmias – may accomplish the same goal as teplizumab but in a different way, by reducing the protein overexpression that induces beta-cell apoptosis and death.
Dr. Forlenza showed a slide comparing the preservation of C-peptide, which was much lower with verapamil, at 30%, than with teplizumab, at 75%.
Asked to comment, session moderator Torben Biester, MD, a pediatric diabetologist at Auf der Bult-Zentrum Diabetes-Center for Children and Adolescents, Hanover, Germany, said: “[Verapamil] is a very cheap [daily] pill. [Teplizumab] is a very high-priced ... immune therapy in the United States ... an infusion twice for 10 days, so it’s a lot more burden for the patients and a lot more risk of side effects.”
“The future might be combination therapy,” added Dr. Biester.
And in an editorial published in JAMA and accompanying the two CLVer papers, Jennifer Couper, MD, of the University of Adelaide, agrees: “A well-tolerated, inexpensive, oral treatment such as verapamil with modest benefits on C-peptide production is relevant to practice.”
The new work “supports investigation of verapamil in combination with other effective agents during the earlier stages of type 1 diabetes before insulin dependence develops,” she noted.
Verapamil results ‘brilliant’ but more work needed
In the verapamil part of the CLVer trial, by 52 weeks, verapamil doses in the youth who received it ranged from 120-360 mg/day based on weight and tolerance.
The primary outcome, C-peptide area under the curve, stayed stable, from 0.66 pmol/mL at baseline to 0.65 pmol/mL at 52 weeks in the verapamil group, compared with a drop from 0.60 pmol/mL down to 0.44 pmol/mL with placebo, a significant difference of 0.14 pmol/mL (P = .04), representing a 30% higher C-peptide level in the verapamil group.
“For us, this is a phenomenally exciting result,” Dr. Forlenza commented during his presentation.
At 52 weeks, A1c was 6.6% in the verapamil group versus 6.9% with placebo, which was not significantly different. Daily insulin dose was 0.65 versus 0.74 units/kg per day, respectively, also not significantly different.
One severe hypoglycemic event occurred in each group, and one diabetic ketoacidosis event occurred in the placebo group. In the verapamil group, three participants experienced “nonserious” electrocardiogram abnormalities and one had hypertension.
Dr. Biester said he isn’t “that concerned” about the small number of mild ECG abnormalities seen in the study with verapamil, as this is a known side effect. But overall, he said, “I would think that for a recommendation for routine use it’s too early after one study, even though the results are brilliant.”
He noted that he is involved in a similar ongoing study of verapamil in adults with new-onset type 1 diabetes, called Ver-A-T1D.
No C-peptide effect of tight glycemic control: ‘A tough pill’
In the AID part of the study, the 113 participants were randomized 2:1 to one of two commercially available AID systems (Tandem t:slim X2 with Control-IQ or Medtronic 670G or 780G) plus frequent contact (a median of 35 times) by study staff, or standard management using a continuous glucose monitor (CGM) with an insulin pump or multiple daily injections.
At 52 weeks, A1c was 6.5% for the intensive group versus 7.1% with standard care, a significant difference. Time in blood glucose range of 70-180 mg/dL was significantly longer with intensive management, at 78%, compared with standard care, at 64%.
Nonetheless, the change in C-peptide area under the curve did not differ between the two groups, decreasing from 0.57 pmol/mL at baseline to 0.45 pmol/mL at 52 weeks with the AID system, compared with a decrease from 0.60 pmol/L down to 0.50 pmol/L with standard care (P = .89).
Dr. Forlenza commented that the hypothesis that tight glycemic control would delay the decline in C-peptide secretion “is something I think a lot of endocrinologists assumed to be true and something I’ve heard lots of colleagues over the years talk about.”
Consequently, he said these findings are “a tough pill for us to swallow ... but it’s important for us in the field to understand.”
“Even with frequent contacts that are well above the level we’d be able to do in standard clinical care, and even with use of the most advanced AID systems we have ... we saw absolutely no difference in stimulated C-peptide levels at any of the timepoints throughout the first year or at 52 weeks.”
“So, in our opinion, this,” combined with a prior study from 2022, “should put this hypothesis to rest,” he said.
“Excellent glycemic control has a benefit in and of itself, but it was not a successful intervention for beta-cell preservation.”
Dr. Forlenza has reported serving as a consultant, speaker, or advisory board member for Medtronic, Dexcom, Abbott, Tandem Diabetes Care, Insulet, Lilly, and Beta Bionics, and his institution has also received funding on his behalf for research grants from these companies. Dr. Biester has reported receiving speaker’s fees from DexCom, Medtronic, Novo Nordisk, F. Hoffmann–La Roche, Sanofi, and Ypsomed Holding; serving on advisory boards for Ascensia Diabetes Care Holdings, AstraZeneca, DexCom, and Medtronic; and receiving personal fees from SYNLAB; and is a member of the European Commission Expert Panel for Medical Devices for Endocrinology and Diabetes. Dr. Couper has reported no relevant financial relationships.
The rationale for the companion CLVer analysis of the effect of reducing glucose toxicity via tight glycemic control on C-peptide progression dates back to an inpatient study published in 1989 involving 26 adolescents using an early artificial pancreas prototype called a Biostator, in which beta-cell preservation was achieved. However, two more recent studies of this approach, including one published in late 2022, did not show a difference. The CLVer analysis involved 113 participants randomized 2:1 to one of two commercially available AID systems (Tandem t:slim X2 with Control-IQ or Medtronic 670G or 780G) plus frequent contact by study staff, or standard management using a CGM with a pump or multiple daily injections.
A version of this article originally appeared on Medscape.com.
In children and adolescents with new-onset type 1 diabetes, the calcium channel blocker verapamil slowed the destruction of insulin-producing pancreatic beta cells for up to a year, new data show.
Use of daily verapamil within a month of diagnosis resulted in a 30% increase in C-peptide secretion (a measure of preserved beta-cell function), compared with placebo at 52 weeks, without serious adverse events.
To put it another way, verapamil delayed the expected decline in C-peptide production from 3 months after diagnosis of type 1 diabetes to 6 months after diagnosis.
“We think this is a really, really exciting finding that’s hopefully going to impact the care for children with type 1 diabetes in the new-onset period,” lead author Gregory P. Forlenza, MD, said during his presentation of the data on Feb. 24 at the annual Advanced Technologies & Treatments for Diabetes (ATTD) meeting in Berlin.
“In view of the favorable safety profile, particularly compared with immune-suppressive agents, once-a-day oral administration, and low cost, initiation of verapamil should be a consideration for newly diagnosed patients with type 1 diabetes,” added Dr. Forlenza, a pediatric endocrinologist at the Barbara Davis Center for Diabetes, Anschutz Medical Campus, University of Colorado, Aurora.
The data were also simultaneously published in JAMA, as part of the CLVer (Hybrid Closed Loop Therapy and Verapamil for Beta Cell Preservation in New Onset Type 1 Diabetes) trial.
The randomized, double-blind, six-center trial involved 113 participants, aged 7-17 years, with newly diagnosed type 1 diabetes. They were randomized to the most advanced commercially available automated insulin delivery systems available or standard care to test the effects of intensive glucose control on C-peptide levels for 52 weeks during the COVID-19 pandemic (July 2020 to September 2022). Eighty-eight patients who weighed 30 kg (66 lb) or more were further randomized (1:1) to daily extended-release verapamil or placebo for the same duration.
The positive findings for verapamil, published in one paper, contrasted with the negative ones for the automated insulin delivery (AID) system. The latter did not prevent the expected decline in C-peptide, putting to rest a long-held hypothesis that reducing glucotoxicity might preserve beta-cell function in newly diagnosed individuals with type 1 diabetes, noted Dr. Forlenza.
Could combination therapy work?
In recent years, immune-modulating agents have increasingly been shown to preserve beta-cell function in both new-onset and preclinical type 1 diabetes. One such agent, teplizumab (Tzield, Provention Bio), was approved by the U.S. Food and Drug Administration in November 2022 to delay type 1 diabetes onset in those at high risk.
Calcium channel blockers such as verapamil – used for years to treat hypertension and cardiac arrhythmias – may accomplish the same goal as teplizumab but in a different way, by reducing the protein overexpression that induces beta-cell apoptosis and death.
Dr. Forlenza showed a slide comparing the preservation of C-peptide, which was much lower with verapamil, at 30%, than with teplizumab, at 75%.
Asked to comment, session moderator Torben Biester, MD, a pediatric diabetologist at Auf der Bult-Zentrum Diabetes-Center for Children and Adolescents, Hanover, Germany, said: “[Verapamil] is a very cheap [daily] pill. [Teplizumab] is a very high-priced ... immune therapy in the United States ... an infusion twice for 10 days, so it’s a lot more burden for the patients and a lot more risk of side effects.”
“The future might be combination therapy,” added Dr. Biester.
And in an editorial published in JAMA and accompanying the two CLVer papers, Jennifer Couper, MD, of the University of Adelaide, agrees: “A well-tolerated, inexpensive, oral treatment such as verapamil with modest benefits on C-peptide production is relevant to practice.”
The new work “supports investigation of verapamil in combination with other effective agents during the earlier stages of type 1 diabetes before insulin dependence develops,” she noted.
Verapamil results ‘brilliant’ but more work needed
In the verapamil part of the CLVer trial, by 52 weeks, verapamil doses in the youth who received it ranged from 120-360 mg/day based on weight and tolerance.
The primary outcome, C-peptide area under the curve, stayed stable, from 0.66 pmol/mL at baseline to 0.65 pmol/mL at 52 weeks in the verapamil group, compared with a drop from 0.60 pmol/mL down to 0.44 pmol/mL with placebo, a significant difference of 0.14 pmol/mL (P = .04), representing a 30% higher C-peptide level in the verapamil group.
“For us, this is a phenomenally exciting result,” Dr. Forlenza commented during his presentation.
At 52 weeks, A1c was 6.6% in the verapamil group versus 6.9% with placebo, which was not significantly different. Daily insulin dose was 0.65 versus 0.74 units/kg per day, respectively, also not significantly different.
One severe hypoglycemic event occurred in each group, and one diabetic ketoacidosis event occurred in the placebo group. In the verapamil group, three participants experienced “nonserious” electrocardiogram abnormalities and one had hypertension.
Dr. Biester said he isn’t “that concerned” about the small number of mild ECG abnormalities seen in the study with verapamil, as this is a known side effect. But overall, he said, “I would think that for a recommendation for routine use it’s too early after one study, even though the results are brilliant.”
He noted that he is involved in a similar ongoing study of verapamil in adults with new-onset type 1 diabetes, called Ver-A-T1D.
No C-peptide effect of tight glycemic control: ‘A tough pill’
In the AID part of the study, the 113 participants were randomized 2:1 to one of two commercially available AID systems (Tandem t:slim X2 with Control-IQ or Medtronic 670G or 780G) plus frequent contact (a median of 35 times) by study staff, or standard management using a continuous glucose monitor (CGM) with an insulin pump or multiple daily injections.
At 52 weeks, A1c was 6.5% for the intensive group versus 7.1% with standard care, a significant difference. Time in blood glucose range of 70-180 mg/dL was significantly longer with intensive management, at 78%, compared with standard care, at 64%.
Nonetheless, the change in C-peptide area under the curve did not differ between the two groups, decreasing from 0.57 pmol/mL at baseline to 0.45 pmol/mL at 52 weeks with the AID system, compared with a decrease from 0.60 pmol/L down to 0.50 pmol/L with standard care (P = .89).
Dr. Forlenza commented that the hypothesis that tight glycemic control would delay the decline in C-peptide secretion “is something I think a lot of endocrinologists assumed to be true and something I’ve heard lots of colleagues over the years talk about.”
Consequently, he said these findings are “a tough pill for us to swallow ... but it’s important for us in the field to understand.”
“Even with frequent contacts that are well above the level we’d be able to do in standard clinical care, and even with use of the most advanced AID systems we have ... we saw absolutely no difference in stimulated C-peptide levels at any of the timepoints throughout the first year or at 52 weeks.”
“So, in our opinion, this,” combined with a prior study from 2022, “should put this hypothesis to rest,” he said.
“Excellent glycemic control has a benefit in and of itself, but it was not a successful intervention for beta-cell preservation.”
Dr. Forlenza has reported serving as a consultant, speaker, or advisory board member for Medtronic, Dexcom, Abbott, Tandem Diabetes Care, Insulet, Lilly, and Beta Bionics, and his institution has also received funding on his behalf for research grants from these companies. Dr. Biester has reported receiving speaker’s fees from DexCom, Medtronic, Novo Nordisk, F. Hoffmann–La Roche, Sanofi, and Ypsomed Holding; serving on advisory boards for Ascensia Diabetes Care Holdings, AstraZeneca, DexCom, and Medtronic; and receiving personal fees from SYNLAB; and is a member of the European Commission Expert Panel for Medical Devices for Endocrinology and Diabetes. Dr. Couper has reported no relevant financial relationships.
The rationale for the companion CLVer analysis of the effect of reducing glucose toxicity via tight glycemic control on C-peptide progression dates back to an inpatient study published in 1989 involving 26 adolescents using an early artificial pancreas prototype called a Biostator, in which beta-cell preservation was achieved. However, two more recent studies of this approach, including one published in late 2022, did not show a difference. The CLVer analysis involved 113 participants randomized 2:1 to one of two commercially available AID systems (Tandem t:slim X2 with Control-IQ or Medtronic 670G or 780G) plus frequent contact by study staff, or standard management using a CGM with a pump or multiple daily injections.
A version of this article originally appeared on Medscape.com.
Real-time CGM plus insulin pump best for type 1 diabetes
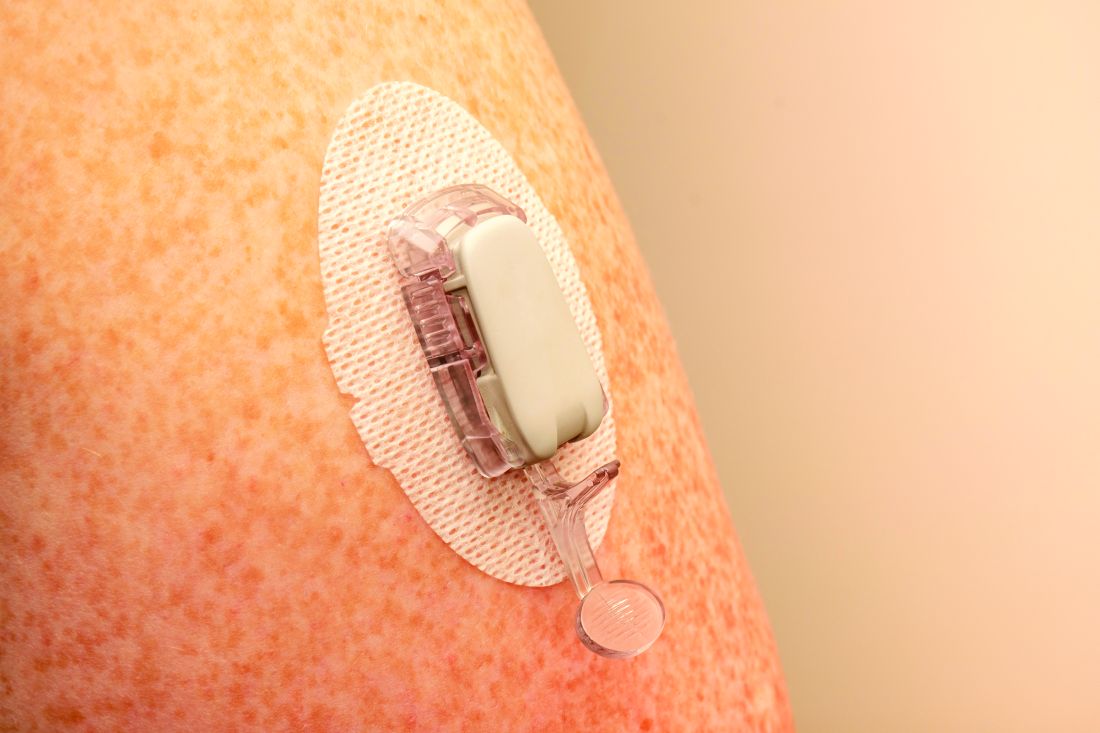
Youth with type 1 diabetes who use real-time continuous glucose monitoring (rtCGM) and an insulin pump spend more time in target glucose range than do those using intermittently scanned CGM (isCGM) and/or multiple daily insulin injections, new data show.
In the multinational cohort study of more than 4,500 people younger than age 21 with type 1 diabetes, those using rtCGM and pumps also spent less time above and below glucose targets and had fewer severe adverse events – either severe hypoglycemia or diabetic ketoacidosis (DKA) – compared with injections and isCGM.
The findings were published online in JAMA Network Open by Klemen Dovc, MD, PhD, assistant professor in the department of pediatric endocrinology, diabetes, and metabolic diseases, University Children’s Hospital, Ljubljana, Slovenia, and colleagues.
“These results underscore the synergistic effect of advanced diabetes technologies that should be more readily available to youths with type 1 diabetes for further improvement of diabetes-related clinical outcomes,” the authors wrote.
Moreover, Dr. Dovc told this news organization: “Clinicians should be aware that there may be differences in effectiveness between different types of devices, and that choosing the right device for each individual may be important for achieving optimal outcomes.”
Real-time CGM + insulin pump = highest time in range
The researchers explained that two modalities of CGM are broadly available: rtCGM, which continuously displays glucose concentration in the interstitial fluid (usually at intervals of 1-5 minutes) on a dedicated receiver or other portable device, such as a smartphone, and provides various adjustable alarms, and isCGM, which displays data on demand when the transmitter is scanned using either a dedicated reader or smartphone-based application.
rtCGMs include devices from Dexcom and Medtronic. The isCGM, or “flash,” generally refers to the Abbott FreeStyle Libre.
The study included individuals younger than 21 years from 34 centers in 21 countries in the SWEET registry, a worldwide network of diabetes care centers for youth, between Jan. 1, 2016, and Dec. 31, 2021.
The researchers didn’t report which particular devices were used in the trial, rather they just divided patients into four groups: 850 used isCGM with a pump, 1,231 used isCGM with multiple daily injections, 2,252 used rtCGM with a pump, and 886 used rtCGM with insulin injections.
After adjustments for sex, age, diabetes duration, and body mass index standard deviation score, rtCGM plus insulin pump was the most likely group to achieve the recommended greater than 70% time in target glycemic range (70-180 mg/dL), with 36.2% achieving it, followed by rtCGM plus injections, at 20.9%, and isCGM plus injections, at 12.5%. Those using isCGM with an insulin pump were the least likely to achieve time in range, at just 11.3%.
Similar trends were seen for the recommended goal of less than 4% of time spent below range (< 70 mg/dL) and less than 25% of time spent above range (> 180 mg/dL). Those using rtCGM with a pump had the highest proportions achieving both of those goals, 73.1% and 32.5%, respectively.
The use of rtCGM, with or without a pump, was associated with lower rates of severe hypoglycemia (2.5% and 2.0%, respectively) than isCGM with or without a pump (5.5% and 5.2%, respectively).
Similarly, the proportion experiencing at least one DKA episode varied from 1.4% for rtCGM plus insulin pump and 0.7% for rtCGM plus injections to 3.0% for isCGM plus pump and 1.5% isCGM plus injections.
Study looked at older technology but results still reflect benefit
Among the rtCGM plus insulin pump group were 264 participants (5% of the total study population) recorded in the database as using automated insulin delivery (AID) systems, also known as the artificial pancreas, although this is likely an undercount as the presence of communication between the two devices was not automatically recorded, Dr. Dovc explained.
Those individuals recorded as using AIDs had a higher unadjusted time in range compared with non-AID users (66.3% vs. 59.0%) and lower time above range (30.1% vs. 37.0%) but didn’t differ in time below range (2.9% vs. 3.0%).
Dr. Dovc told this news organization: “While automated systems are becoming more common, there are still many individuals who do not have access to glucose-responsive devices.” Reasons include lack of reimbursement, or decisions not to use them, he said.
But, he added, “Despite the low reported numbers of AID users, results achieved in the pump with real-time CGM [group] are admirable and approaching recommended consensus targets with a clinically meaningful difference towards all other treatment modalities. As our findings may not be directly applicable to all participants using automated systems, they may still provide useful insights into the factors that influence glycemic control.”
Similarly, the intermittently scanned CGMs used by most in the study, and particularly in the earlier period, didn’t have low- or high-glucose alarms as do later versions. And an even more recent version also doesn’t require scanning either, so is essentially also “real-time.”
Dr. Dovc noted, “in the first half of our observational period only first generation of intermittently-scanned CGM was generally available, and we can speculate that only a small proportion started to use second generation towards the end of our observational period. The exact number of second-generation users was not available in this analysis.”
He acknowledged that because the study was observational and not randomized, patient choice of device could have influenced the outcomes.
“For example, participants who choose to use a more expensive device may have more resources or support available to them, which could influence their ability to manage their diabetes effectively. Additionally, individuals who choose to use a particular device may be more motivated or engaged in their diabetes care, which could also impact their outcomes. It would be important for future studies to explore the impact of device selection on device effectiveness and to control for this potential confounding factor in the analysis.”
This study was supported by the international Better Control in Pediatric and Adolescent Diabetes: Working to Create Centers of Reference (SWEET) corporate members, including Abbott Laboratories, Boehringer Ingelheim, Dexcom, Insulet, Eli Lilly, Medtronic, Sanofi, and the Slovenian National Research Agency. Dr. Dovc disclosed ties with Abbott Laboratories, Medtronic, Novo Nordisk, Eli Lilly, and Pfizer. He served as a member of the European Commission Expert Panel for Medical Devices for Endocrinology and Diabetes.
A version of this article originally appeared on Medscape.com.
Youth with type 1 diabetes who use real-time continuous glucose monitoring (rtCGM) and an insulin pump spend more time in target glucose range than do those using intermittently scanned CGM (isCGM) and/or multiple daily insulin injections, new data show.
In the multinational cohort study of more than 4,500 people younger than age 21 with type 1 diabetes, those using rtCGM and pumps also spent less time above and below glucose targets and had fewer severe adverse events – either severe hypoglycemia or diabetic ketoacidosis (DKA) – compared with injections and isCGM.
The findings were published online in JAMA Network Open by Klemen Dovc, MD, PhD, assistant professor in the department of pediatric endocrinology, diabetes, and metabolic diseases, University Children’s Hospital, Ljubljana, Slovenia, and colleagues.
“These results underscore the synergistic effect of advanced diabetes technologies that should be more readily available to youths with type 1 diabetes for further improvement of diabetes-related clinical outcomes,” the authors wrote.
Moreover, Dr. Dovc told this news organization: “Clinicians should be aware that there may be differences in effectiveness between different types of devices, and that choosing the right device for each individual may be important for achieving optimal outcomes.”
Real-time CGM + insulin pump = highest time in range
The researchers explained that two modalities of CGM are broadly available: rtCGM, which continuously displays glucose concentration in the interstitial fluid (usually at intervals of 1-5 minutes) on a dedicated receiver or other portable device, such as a smartphone, and provides various adjustable alarms, and isCGM, which displays data on demand when the transmitter is scanned using either a dedicated reader or smartphone-based application.
rtCGMs include devices from Dexcom and Medtronic. The isCGM, or “flash,” generally refers to the Abbott FreeStyle Libre.
The study included individuals younger than 21 years from 34 centers in 21 countries in the SWEET registry, a worldwide network of diabetes care centers for youth, between Jan. 1, 2016, and Dec. 31, 2021.
The researchers didn’t report which particular devices were used in the trial, rather they just divided patients into four groups: 850 used isCGM with a pump, 1,231 used isCGM with multiple daily injections, 2,252 used rtCGM with a pump, and 886 used rtCGM with insulin injections.
After adjustments for sex, age, diabetes duration, and body mass index standard deviation score, rtCGM plus insulin pump was the most likely group to achieve the recommended greater than 70% time in target glycemic range (70-180 mg/dL), with 36.2% achieving it, followed by rtCGM plus injections, at 20.9%, and isCGM plus injections, at 12.5%. Those using isCGM with an insulin pump were the least likely to achieve time in range, at just 11.3%.
Similar trends were seen for the recommended goal of less than 4% of time spent below range (< 70 mg/dL) and less than 25% of time spent above range (> 180 mg/dL). Those using rtCGM with a pump had the highest proportions achieving both of those goals, 73.1% and 32.5%, respectively.
The use of rtCGM, with or without a pump, was associated with lower rates of severe hypoglycemia (2.5% and 2.0%, respectively) than isCGM with or without a pump (5.5% and 5.2%, respectively).
Similarly, the proportion experiencing at least one DKA episode varied from 1.4% for rtCGM plus insulin pump and 0.7% for rtCGM plus injections to 3.0% for isCGM plus pump and 1.5% isCGM plus injections.
Study looked at older technology but results still reflect benefit
Among the rtCGM plus insulin pump group were 264 participants (5% of the total study population) recorded in the database as using automated insulin delivery (AID) systems, also known as the artificial pancreas, although this is likely an undercount as the presence of communication between the two devices was not automatically recorded, Dr. Dovc explained.
Those individuals recorded as using AIDs had a higher unadjusted time in range compared with non-AID users (66.3% vs. 59.0%) and lower time above range (30.1% vs. 37.0%) but didn’t differ in time below range (2.9% vs. 3.0%).
Dr. Dovc told this news organization: “While automated systems are becoming more common, there are still many individuals who do not have access to glucose-responsive devices.” Reasons include lack of reimbursement, or decisions not to use them, he said.
But, he added, “Despite the low reported numbers of AID users, results achieved in the pump with real-time CGM [group] are admirable and approaching recommended consensus targets with a clinically meaningful difference towards all other treatment modalities. As our findings may not be directly applicable to all participants using automated systems, they may still provide useful insights into the factors that influence glycemic control.”
Similarly, the intermittently scanned CGMs used by most in the study, and particularly in the earlier period, didn’t have low- or high-glucose alarms as do later versions. And an even more recent version also doesn’t require scanning either, so is essentially also “real-time.”
Dr. Dovc noted, “in the first half of our observational period only first generation of intermittently-scanned CGM was generally available, and we can speculate that only a small proportion started to use second generation towards the end of our observational period. The exact number of second-generation users was not available in this analysis.”
He acknowledged that because the study was observational and not randomized, patient choice of device could have influenced the outcomes.
“For example, participants who choose to use a more expensive device may have more resources or support available to them, which could influence their ability to manage their diabetes effectively. Additionally, individuals who choose to use a particular device may be more motivated or engaged in their diabetes care, which could also impact their outcomes. It would be important for future studies to explore the impact of device selection on device effectiveness and to control for this potential confounding factor in the analysis.”
This study was supported by the international Better Control in Pediatric and Adolescent Diabetes: Working to Create Centers of Reference (SWEET) corporate members, including Abbott Laboratories, Boehringer Ingelheim, Dexcom, Insulet, Eli Lilly, Medtronic, Sanofi, and the Slovenian National Research Agency. Dr. Dovc disclosed ties with Abbott Laboratories, Medtronic, Novo Nordisk, Eli Lilly, and Pfizer. He served as a member of the European Commission Expert Panel for Medical Devices for Endocrinology and Diabetes.
A version of this article originally appeared on Medscape.com.
Youth with type 1 diabetes who use real-time continuous glucose monitoring (rtCGM) and an insulin pump spend more time in target glucose range than do those using intermittently scanned CGM (isCGM) and/or multiple daily insulin injections, new data show.
In the multinational cohort study of more than 4,500 people younger than age 21 with type 1 diabetes, those using rtCGM and pumps also spent less time above and below glucose targets and had fewer severe adverse events – either severe hypoglycemia or diabetic ketoacidosis (DKA) – compared with injections and isCGM.
The findings were published online in JAMA Network Open by Klemen Dovc, MD, PhD, assistant professor in the department of pediatric endocrinology, diabetes, and metabolic diseases, University Children’s Hospital, Ljubljana, Slovenia, and colleagues.
“These results underscore the synergistic effect of advanced diabetes technologies that should be more readily available to youths with type 1 diabetes for further improvement of diabetes-related clinical outcomes,” the authors wrote.
Moreover, Dr. Dovc told this news organization: “Clinicians should be aware that there may be differences in effectiveness between different types of devices, and that choosing the right device for each individual may be important for achieving optimal outcomes.”
Real-time CGM + insulin pump = highest time in range
The researchers explained that two modalities of CGM are broadly available: rtCGM, which continuously displays glucose concentration in the interstitial fluid (usually at intervals of 1-5 minutes) on a dedicated receiver or other portable device, such as a smartphone, and provides various adjustable alarms, and isCGM, which displays data on demand when the transmitter is scanned using either a dedicated reader or smartphone-based application.
rtCGMs include devices from Dexcom and Medtronic. The isCGM, or “flash,” generally refers to the Abbott FreeStyle Libre.
The study included individuals younger than 21 years from 34 centers in 21 countries in the SWEET registry, a worldwide network of diabetes care centers for youth, between Jan. 1, 2016, and Dec. 31, 2021.
The researchers didn’t report which particular devices were used in the trial, rather they just divided patients into four groups: 850 used isCGM with a pump, 1,231 used isCGM with multiple daily injections, 2,252 used rtCGM with a pump, and 886 used rtCGM with insulin injections.
After adjustments for sex, age, diabetes duration, and body mass index standard deviation score, rtCGM plus insulin pump was the most likely group to achieve the recommended greater than 70% time in target glycemic range (70-180 mg/dL), with 36.2% achieving it, followed by rtCGM plus injections, at 20.9%, and isCGM plus injections, at 12.5%. Those using isCGM with an insulin pump were the least likely to achieve time in range, at just 11.3%.
Similar trends were seen for the recommended goal of less than 4% of time spent below range (< 70 mg/dL) and less than 25% of time spent above range (> 180 mg/dL). Those using rtCGM with a pump had the highest proportions achieving both of those goals, 73.1% and 32.5%, respectively.
The use of rtCGM, with or without a pump, was associated with lower rates of severe hypoglycemia (2.5% and 2.0%, respectively) than isCGM with or without a pump (5.5% and 5.2%, respectively).
Similarly, the proportion experiencing at least one DKA episode varied from 1.4% for rtCGM plus insulin pump and 0.7% for rtCGM plus injections to 3.0% for isCGM plus pump and 1.5% isCGM plus injections.
Study looked at older technology but results still reflect benefit
Among the rtCGM plus insulin pump group were 264 participants (5% of the total study population) recorded in the database as using automated insulin delivery (AID) systems, also known as the artificial pancreas, although this is likely an undercount as the presence of communication between the two devices was not automatically recorded, Dr. Dovc explained.
Those individuals recorded as using AIDs had a higher unadjusted time in range compared with non-AID users (66.3% vs. 59.0%) and lower time above range (30.1% vs. 37.0%) but didn’t differ in time below range (2.9% vs. 3.0%).
Dr. Dovc told this news organization: “While automated systems are becoming more common, there are still many individuals who do not have access to glucose-responsive devices.” Reasons include lack of reimbursement, or decisions not to use them, he said.
But, he added, “Despite the low reported numbers of AID users, results achieved in the pump with real-time CGM [group] are admirable and approaching recommended consensus targets with a clinically meaningful difference towards all other treatment modalities. As our findings may not be directly applicable to all participants using automated systems, they may still provide useful insights into the factors that influence glycemic control.”
Similarly, the intermittently scanned CGMs used by most in the study, and particularly in the earlier period, didn’t have low- or high-glucose alarms as do later versions. And an even more recent version also doesn’t require scanning either, so is essentially also “real-time.”
Dr. Dovc noted, “in the first half of our observational period only first generation of intermittently-scanned CGM was generally available, and we can speculate that only a small proportion started to use second generation towards the end of our observational period. The exact number of second-generation users was not available in this analysis.”
He acknowledged that because the study was observational and not randomized, patient choice of device could have influenced the outcomes.
“For example, participants who choose to use a more expensive device may have more resources or support available to them, which could influence their ability to manage their diabetes effectively. Additionally, individuals who choose to use a particular device may be more motivated or engaged in their diabetes care, which could also impact their outcomes. It would be important for future studies to explore the impact of device selection on device effectiveness and to control for this potential confounding factor in the analysis.”
This study was supported by the international Better Control in Pediatric and Adolescent Diabetes: Working to Create Centers of Reference (SWEET) corporate members, including Abbott Laboratories, Boehringer Ingelheim, Dexcom, Insulet, Eli Lilly, Medtronic, Sanofi, and the Slovenian National Research Agency. Dr. Dovc disclosed ties with Abbott Laboratories, Medtronic, Novo Nordisk, Eli Lilly, and Pfizer. He served as a member of the European Commission Expert Panel for Medical Devices for Endocrinology and Diabetes.
A version of this article originally appeared on Medscape.com.
COVID-19 shot appears to reduce diabetes risk, even after Omicron
new data suggest.
The findings, from more than 20,000 patients in the Cedars-Sinai Health System in Los Angeles, suggest that “continued efforts to prevent COVID-19 infection may be beneficial to patient health until we develop better understanding of the effects of potential long-term effects of COVID-19,” lead author Alan C. Kwan, MD, of the department of cardiology at Cedars Sinai’s Smidt Heart Institute, said in an interview.
Several studies conducted early in the pandemic suggested increased risks for both new-onset diabetes and cardiometabolic diseases following COVID-19 infection, possibly because of persistent inflammation contributing to insulin resistance.
However, it hasn’t been clear if those risks have persisted with the more recent predominance of the less-virulent Omicron variant or whether the COVID-19 vaccine influences the risk. This new study suggests that both are the case.
“Our results verify that the risk of developing type 2 diabetes after a COVID-19 infection was not just an early observation but, in fact, a real risk that has, unfortunately, persisted through the Omicron era,” Dr. Kwan noted.
“While the level of evidence by our study and others may not reach the degree needed to affect formal guidelines at this time, we believe it is reasonable to have increased clinical suspicion for diabetes after COVID-19 infection and a lower threshold for testing,” he added.
Moreover, “we believe that our study and others suggest the potential role of COVID-19 to affect cardiovascular risk, and so both prevention of COVID-19 infection, through reasonable personal practices and vaccination, and an increased attention to cardiovascular health after COVID-19 infection is warranted.”
The findings were published online in JAMA Network Open.
Dr. Kwan and colleagues analyzed data for a total of 23,709 patients treated (inpatient and outpatient) for at least one COVID-19 infection between March 2020 and June 2022.
Rates of new-onset diabetes (using ICD-10 codes, primarily type 2 diabetes), hypertension, and hyperlipidemia were all elevated in the 90 days following COVID-19 infection compared with the 90 days prior. The same was true of two diagnoses unrelated to COVID-19, urinary tract infection and gastroesophageal reflux, used as benchmarks of health care engagement.
The highest odds for post versus preinfection were for diabetes (odds ratio, 2.35; P < .001), followed by hypertension (OR, 1.54; P < .001), the benchmark diagnoses (OR, 1.42; P < .001), and hyperlipidemia (OR, 1.22; P = .03).
Following adjustments, the risk versus the benchmark conditions for new-onset diabetes before versus after COVID-19 was significantly elevated (OR, 1.58; P < .001), while the risks for hypertension and hyperlipidemia versus benchmark diagnoses were not (OR, 1.06; P = .52 and 0.91, P = .43, respectively).
The diabetes risk after versus before COVID-19 infection was higher among those who had not been vaccinated (OR, 1.78; P < .001), compared with those who had received the vaccine (OR, 1.07; P = .80).
However, there was no significant interaction between vaccination and diabetes diagnosis (P = .08). “For this reason, we believe our data are suggestive of a protective effect in the population who received vaccination prior to infection, but [this is] not definitive,” Dr. Kwan said.
There were no apparent interactions by age, sex, or pre-existing cardiovascular risk factors, including hypertension or hyperlipidemia. Age, sex, and timing of index infection regarding the Omicron variant were not associated with an increased risk of a new cardiometabolic diagnosis before or after COVID-19 infection in any of the models.
Dr. Kwan said in an interview: “We have continued to be surprised by the evolving understanding of the SARS-CoV-2 virus and the effects on human health. In the beginning of the pandemic it was framed as a purely respiratory virus, which we now know to be a severely limited description of all of its potential effects on the human body. We believe that our research and others raise a concern for increased cardiometabolic risk after COVID infection.”
He added that, “while knowledge is incomplete on this topic, we believe that clinical providers may wish to have a higher degree of suspicion for both diabetes and risk of future cardiac events in patients after COVID infection, and that continued efforts to prevent COVID infection may be beneficial to patient health until we develop better understanding of the potential long-term effects of COVID.”
This study was funded by the Erika J. Glazer Family Foundation, the Doris Duke Charitable Foundation, and grants from the National Institutes of Health. Dr. Kwan reported receiving grants from the Doris Duke Charitable Foundation during the conduct of the study.
A version of this article originally appeared on Medscape.com.
new data suggest.
The findings, from more than 20,000 patients in the Cedars-Sinai Health System in Los Angeles, suggest that “continued efforts to prevent COVID-19 infection may be beneficial to patient health until we develop better understanding of the effects of potential long-term effects of COVID-19,” lead author Alan C. Kwan, MD, of the department of cardiology at Cedars Sinai’s Smidt Heart Institute, said in an interview.
Several studies conducted early in the pandemic suggested increased risks for both new-onset diabetes and cardiometabolic diseases following COVID-19 infection, possibly because of persistent inflammation contributing to insulin resistance.
However, it hasn’t been clear if those risks have persisted with the more recent predominance of the less-virulent Omicron variant or whether the COVID-19 vaccine influences the risk. This new study suggests that both are the case.
“Our results verify that the risk of developing type 2 diabetes after a COVID-19 infection was not just an early observation but, in fact, a real risk that has, unfortunately, persisted through the Omicron era,” Dr. Kwan noted.
“While the level of evidence by our study and others may not reach the degree needed to affect formal guidelines at this time, we believe it is reasonable to have increased clinical suspicion for diabetes after COVID-19 infection and a lower threshold for testing,” he added.
Moreover, “we believe that our study and others suggest the potential role of COVID-19 to affect cardiovascular risk, and so both prevention of COVID-19 infection, through reasonable personal practices and vaccination, and an increased attention to cardiovascular health after COVID-19 infection is warranted.”
The findings were published online in JAMA Network Open.
Dr. Kwan and colleagues analyzed data for a total of 23,709 patients treated (inpatient and outpatient) for at least one COVID-19 infection between March 2020 and June 2022.
Rates of new-onset diabetes (using ICD-10 codes, primarily type 2 diabetes), hypertension, and hyperlipidemia were all elevated in the 90 days following COVID-19 infection compared with the 90 days prior. The same was true of two diagnoses unrelated to COVID-19, urinary tract infection and gastroesophageal reflux, used as benchmarks of health care engagement.
The highest odds for post versus preinfection were for diabetes (odds ratio, 2.35; P < .001), followed by hypertension (OR, 1.54; P < .001), the benchmark diagnoses (OR, 1.42; P < .001), and hyperlipidemia (OR, 1.22; P = .03).
Following adjustments, the risk versus the benchmark conditions for new-onset diabetes before versus after COVID-19 was significantly elevated (OR, 1.58; P < .001), while the risks for hypertension and hyperlipidemia versus benchmark diagnoses were not (OR, 1.06; P = .52 and 0.91, P = .43, respectively).
The diabetes risk after versus before COVID-19 infection was higher among those who had not been vaccinated (OR, 1.78; P < .001), compared with those who had received the vaccine (OR, 1.07; P = .80).
However, there was no significant interaction between vaccination and diabetes diagnosis (P = .08). “For this reason, we believe our data are suggestive of a protective effect in the population who received vaccination prior to infection, but [this is] not definitive,” Dr. Kwan said.
There were no apparent interactions by age, sex, or pre-existing cardiovascular risk factors, including hypertension or hyperlipidemia. Age, sex, and timing of index infection regarding the Omicron variant were not associated with an increased risk of a new cardiometabolic diagnosis before or after COVID-19 infection in any of the models.
Dr. Kwan said in an interview: “We have continued to be surprised by the evolving understanding of the SARS-CoV-2 virus and the effects on human health. In the beginning of the pandemic it was framed as a purely respiratory virus, which we now know to be a severely limited description of all of its potential effects on the human body. We believe that our research and others raise a concern for increased cardiometabolic risk after COVID infection.”
He added that, “while knowledge is incomplete on this topic, we believe that clinical providers may wish to have a higher degree of suspicion for both diabetes and risk of future cardiac events in patients after COVID infection, and that continued efforts to prevent COVID infection may be beneficial to patient health until we develop better understanding of the potential long-term effects of COVID.”
This study was funded by the Erika J. Glazer Family Foundation, the Doris Duke Charitable Foundation, and grants from the National Institutes of Health. Dr. Kwan reported receiving grants from the Doris Duke Charitable Foundation during the conduct of the study.
A version of this article originally appeared on Medscape.com.
new data suggest.
The findings, from more than 20,000 patients in the Cedars-Sinai Health System in Los Angeles, suggest that “continued efforts to prevent COVID-19 infection may be beneficial to patient health until we develop better understanding of the effects of potential long-term effects of COVID-19,” lead author Alan C. Kwan, MD, of the department of cardiology at Cedars Sinai’s Smidt Heart Institute, said in an interview.
Several studies conducted early in the pandemic suggested increased risks for both new-onset diabetes and cardiometabolic diseases following COVID-19 infection, possibly because of persistent inflammation contributing to insulin resistance.
However, it hasn’t been clear if those risks have persisted with the more recent predominance of the less-virulent Omicron variant or whether the COVID-19 vaccine influences the risk. This new study suggests that both are the case.
“Our results verify that the risk of developing type 2 diabetes after a COVID-19 infection was not just an early observation but, in fact, a real risk that has, unfortunately, persisted through the Omicron era,” Dr. Kwan noted.
“While the level of evidence by our study and others may not reach the degree needed to affect formal guidelines at this time, we believe it is reasonable to have increased clinical suspicion for diabetes after COVID-19 infection and a lower threshold for testing,” he added.
Moreover, “we believe that our study and others suggest the potential role of COVID-19 to affect cardiovascular risk, and so both prevention of COVID-19 infection, through reasonable personal practices and vaccination, and an increased attention to cardiovascular health after COVID-19 infection is warranted.”
The findings were published online in JAMA Network Open.
Dr. Kwan and colleagues analyzed data for a total of 23,709 patients treated (inpatient and outpatient) for at least one COVID-19 infection between March 2020 and June 2022.
Rates of new-onset diabetes (using ICD-10 codes, primarily type 2 diabetes), hypertension, and hyperlipidemia were all elevated in the 90 days following COVID-19 infection compared with the 90 days prior. The same was true of two diagnoses unrelated to COVID-19, urinary tract infection and gastroesophageal reflux, used as benchmarks of health care engagement.
The highest odds for post versus preinfection were for diabetes (odds ratio, 2.35; P < .001), followed by hypertension (OR, 1.54; P < .001), the benchmark diagnoses (OR, 1.42; P < .001), and hyperlipidemia (OR, 1.22; P = .03).
Following adjustments, the risk versus the benchmark conditions for new-onset diabetes before versus after COVID-19 was significantly elevated (OR, 1.58; P < .001), while the risks for hypertension and hyperlipidemia versus benchmark diagnoses were not (OR, 1.06; P = .52 and 0.91, P = .43, respectively).
The diabetes risk after versus before COVID-19 infection was higher among those who had not been vaccinated (OR, 1.78; P < .001), compared with those who had received the vaccine (OR, 1.07; P = .80).
However, there was no significant interaction between vaccination and diabetes diagnosis (P = .08). “For this reason, we believe our data are suggestive of a protective effect in the population who received vaccination prior to infection, but [this is] not definitive,” Dr. Kwan said.
There were no apparent interactions by age, sex, or pre-existing cardiovascular risk factors, including hypertension or hyperlipidemia. Age, sex, and timing of index infection regarding the Omicron variant were not associated with an increased risk of a new cardiometabolic diagnosis before or after COVID-19 infection in any of the models.
Dr. Kwan said in an interview: “We have continued to be surprised by the evolving understanding of the SARS-CoV-2 virus and the effects on human health. In the beginning of the pandemic it was framed as a purely respiratory virus, which we now know to be a severely limited description of all of its potential effects on the human body. We believe that our research and others raise a concern for increased cardiometabolic risk after COVID infection.”
He added that, “while knowledge is incomplete on this topic, we believe that clinical providers may wish to have a higher degree of suspicion for both diabetes and risk of future cardiac events in patients after COVID infection, and that continued efforts to prevent COVID infection may be beneficial to patient health until we develop better understanding of the potential long-term effects of COVID.”
This study was funded by the Erika J. Glazer Family Foundation, the Doris Duke Charitable Foundation, and grants from the National Institutes of Health. Dr. Kwan reported receiving grants from the Doris Duke Charitable Foundation during the conduct of the study.
A version of this article originally appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Type 1 diabetes no longer a disease of the thin: Lifestyle advice needed
About two-thirds of people with type 1 diabetes in the United States have overweight or obesity, nearly the same proportion as Americans without diabetes, new nationwide survey data suggest.
What’s more, among people with overweight or obesity, those with type 1 diabetes are less likely to receive lifestyle recommendations from health care professionals than those with type 2 diabetes, and are less likely to actually engage in lifestyle weight management activities than others with overweight or obesity, with or without type 2 diabetes.
“Among U.S. adults with type 1 diabetes, the burden of overweight and obesity is substantial and remains poorly managed,” write Michael Fang, PhD, of the Johns Hopkins Bloomberg School of Public Health, Baltimore, and colleagues.
Their data, from the National Health Interview Survey (NHIS), were published online in Annals of Internal Medicine.
The need for insulin complicates weight management in people with type 1 diabetes because changes in diet and physical activity typically require adjustments to insulin timing and dosage to prevent hypoglycemia. There is little evidence to guide this for weight management, Dr. Fang and colleagues explain.
Consequently, “the lack of evidence for safe, effective methods of diet- and exercise-based weight control in people with type 1 diabetes may be keeping doctors from recommending such methods,” Dr. Fang said in a statement.
“Large clinical trials have been done in type 2 diabetes patients to establish guidelines for diet- and exercise-based weight management, and we now need something similar for type 1 diabetes patients.”
Asked to comment, M. Sue Kirkman, MD, told this news organization: “The days when we could teach simple concepts about diabetes type like ‘those with type 1 are lean and those with type 2 are overweight’ are long gone. … Of concern, fewer adults with type 1 diabetes and overweight/obesity report that they are engaging in physical activity or caloric restriction than those without diabetes or those with type 2 diabetes.”
There are several likely reasons for the low rates of obesity/overweight lifestyle modification advice and implementation for those with type 1 diabetes, noted Dr. Kirkman, of the University of North Carolina at Chapel Hill, who coauthored joint American/European guidance on type 1 diabetes management.
“Medical visits are often primarily focused on glycemic management and complications screening, and we know that physicians in general are not very knowledgeable about how to counsel people – even those without diabetes – on weight loss. When you add in potential worries, real or not, about hypoglycemia, ketosis with carbohydrate restriction … it’s no wonder that this may not be addressed in busy visits.”
She also observed, “In years of going to diabetes meetings, I’ve noticed occasional sessions on managing ‘elite athletes’ with type 1 diabetes, but rarely are there sessions on how to counsel people about everyday healthy living.”
Many with type 1 diabetes have overweight/obesity
Dr. Fang and colleagues analyzed NHIS data for the years 2016, 2017, 2019, 2020, and 2021, when diabetes subtype data were available, for 128,571 adults. Diabetes type and height/weight data were self-reported. In the 2016, 2017, and 2020 surveys, participants were asked whether their physicians had recommended increasing physical activity and/or reducing calorie or fat consumption, and whether they were currently engaging in those activities.
The study population comprised 733 people with type 1 diabetes, 12,397 with type 2 diabetes, and 115,441 without diabetes. The proportions with overweight (body mass index, 25 to < 30 kg/m2) or obesity (≥ 30 kg/m2) were 62% among those with type 1 diabetes and 64% among those without diabetes, compared with 86% among those with type 2 diabetes.
Among those with overweight or obesity, the proportions who reported having received lifestyle recommendations were greatest among those with type 2 diabetes and least among those without diabetes, with the type 1 diabetes group in the middle.
After adjustment for age, sex, and race/ethnicity, the adjusted prevalence of receiving a provider recommendation to increase physical activity was 60% for those with type 2 diabetes, 54% for type 1 diabetes, and 44% for those without diabetes. Proportions for receiving recommendations for reducing fat/caloric intake were similar, at 60%, 51%, and 41%, respectively.
The proportions who reported actually engaging in lifestyle activities for weight management were lowest among those with type 1 diabetes, with 52% and 56% of them reporting having increased their physical activity and reducing fat/calories, respectively, compared with proportions ranging from 56% to 63% among the other two groups.
Regarding those findings, Dr. Kirkman commented, “In addition to the factors regarding physician interactions, people with type 1 diabetes may see this as a lower-priority health issue after years of being told that glucose control is the main priority.”
“I also wonder if the many, many tasks people with type 1 diabetes must do every day to manage their diabetes – along with other life issues all adults face – mean that there is just too much on the plate to add more lifestyle changes,” she added.
Asked about the potential for off-label use of glucagonlike peptide–1 agonists for weight management for people with type 1 diabetes, Dr. Kirkman said they could probably help some patients. However, she also pointed to two clinical trials in which liraglutide added to insulin therapy helped with glycemic control and weight reduction, but also increased the risk for hypoglycemia and diabetic ketoacidosis.
“It’s really important that researchers engage with adults with type 1 diabetes to better understand the unique priorities and barriers they face in addressing body weight,” Dr. Kirkman said.
Senior study author Elizabeth Selvin, PhD, professor of epidemiology at the Bloomberg School, said in the statement: “Our study busts the myth that people with type 1 diabetes are not being affected by the global obesity epidemic. … These findings should be a wake-up call that we need to be aggressive in addressing the obesity epidemic in persons with type 1 diabetes.”
The study was funded by the U.S. National Institutes of Health. Dr. Fang and Dr. Kirkman have reported no relevant financial relationships. Dr. Selvin has reported receiving royalty payments from Wolters Kluwer for chapters and laboratory monographs in UpToDate. She also reports receiving honoraria for editorial work on journals published by the American Diabetes Association and European Association for the Study of Diabetes.
A version of this article originally appeared on Medscape.com.
About two-thirds of people with type 1 diabetes in the United States have overweight or obesity, nearly the same proportion as Americans without diabetes, new nationwide survey data suggest.
What’s more, among people with overweight or obesity, those with type 1 diabetes are less likely to receive lifestyle recommendations from health care professionals than those with type 2 diabetes, and are less likely to actually engage in lifestyle weight management activities than others with overweight or obesity, with or without type 2 diabetes.
“Among U.S. adults with type 1 diabetes, the burden of overweight and obesity is substantial and remains poorly managed,” write Michael Fang, PhD, of the Johns Hopkins Bloomberg School of Public Health, Baltimore, and colleagues.
Their data, from the National Health Interview Survey (NHIS), were published online in Annals of Internal Medicine.
The need for insulin complicates weight management in people with type 1 diabetes because changes in diet and physical activity typically require adjustments to insulin timing and dosage to prevent hypoglycemia. There is little evidence to guide this for weight management, Dr. Fang and colleagues explain.
Consequently, “the lack of evidence for safe, effective methods of diet- and exercise-based weight control in people with type 1 diabetes may be keeping doctors from recommending such methods,” Dr. Fang said in a statement.
“Large clinical trials have been done in type 2 diabetes patients to establish guidelines for diet- and exercise-based weight management, and we now need something similar for type 1 diabetes patients.”
Asked to comment, M. Sue Kirkman, MD, told this news organization: “The days when we could teach simple concepts about diabetes type like ‘those with type 1 are lean and those with type 2 are overweight’ are long gone. … Of concern, fewer adults with type 1 diabetes and overweight/obesity report that they are engaging in physical activity or caloric restriction than those without diabetes or those with type 2 diabetes.”
There are several likely reasons for the low rates of obesity/overweight lifestyle modification advice and implementation for those with type 1 diabetes, noted Dr. Kirkman, of the University of North Carolina at Chapel Hill, who coauthored joint American/European guidance on type 1 diabetes management.
“Medical visits are often primarily focused on glycemic management and complications screening, and we know that physicians in general are not very knowledgeable about how to counsel people – even those without diabetes – on weight loss. When you add in potential worries, real or not, about hypoglycemia, ketosis with carbohydrate restriction … it’s no wonder that this may not be addressed in busy visits.”
She also observed, “In years of going to diabetes meetings, I’ve noticed occasional sessions on managing ‘elite athletes’ with type 1 diabetes, but rarely are there sessions on how to counsel people about everyday healthy living.”
Many with type 1 diabetes have overweight/obesity
Dr. Fang and colleagues analyzed NHIS data for the years 2016, 2017, 2019, 2020, and 2021, when diabetes subtype data were available, for 128,571 adults. Diabetes type and height/weight data were self-reported. In the 2016, 2017, and 2020 surveys, participants were asked whether their physicians had recommended increasing physical activity and/or reducing calorie or fat consumption, and whether they were currently engaging in those activities.
The study population comprised 733 people with type 1 diabetes, 12,397 with type 2 diabetes, and 115,441 without diabetes. The proportions with overweight (body mass index, 25 to < 30 kg/m2) or obesity (≥ 30 kg/m2) were 62% among those with type 1 diabetes and 64% among those without diabetes, compared with 86% among those with type 2 diabetes.
Among those with overweight or obesity, the proportions who reported having received lifestyle recommendations were greatest among those with type 2 diabetes and least among those without diabetes, with the type 1 diabetes group in the middle.
After adjustment for age, sex, and race/ethnicity, the adjusted prevalence of receiving a provider recommendation to increase physical activity was 60% for those with type 2 diabetes, 54% for type 1 diabetes, and 44% for those without diabetes. Proportions for receiving recommendations for reducing fat/caloric intake were similar, at 60%, 51%, and 41%, respectively.
The proportions who reported actually engaging in lifestyle activities for weight management were lowest among those with type 1 diabetes, with 52% and 56% of them reporting having increased their physical activity and reducing fat/calories, respectively, compared with proportions ranging from 56% to 63% among the other two groups.
Regarding those findings, Dr. Kirkman commented, “In addition to the factors regarding physician interactions, people with type 1 diabetes may see this as a lower-priority health issue after years of being told that glucose control is the main priority.”
“I also wonder if the many, many tasks people with type 1 diabetes must do every day to manage their diabetes – along with other life issues all adults face – mean that there is just too much on the plate to add more lifestyle changes,” she added.
Asked about the potential for off-label use of glucagonlike peptide–1 agonists for weight management for people with type 1 diabetes, Dr. Kirkman said they could probably help some patients. However, she also pointed to two clinical trials in which liraglutide added to insulin therapy helped with glycemic control and weight reduction, but also increased the risk for hypoglycemia and diabetic ketoacidosis.
“It’s really important that researchers engage with adults with type 1 diabetes to better understand the unique priorities and barriers they face in addressing body weight,” Dr. Kirkman said.
Senior study author Elizabeth Selvin, PhD, professor of epidemiology at the Bloomberg School, said in the statement: “Our study busts the myth that people with type 1 diabetes are not being affected by the global obesity epidemic. … These findings should be a wake-up call that we need to be aggressive in addressing the obesity epidemic in persons with type 1 diabetes.”
The study was funded by the U.S. National Institutes of Health. Dr. Fang and Dr. Kirkman have reported no relevant financial relationships. Dr. Selvin has reported receiving royalty payments from Wolters Kluwer for chapters and laboratory monographs in UpToDate. She also reports receiving honoraria for editorial work on journals published by the American Diabetes Association and European Association for the Study of Diabetes.
A version of this article originally appeared on Medscape.com.
About two-thirds of people with type 1 diabetes in the United States have overweight or obesity, nearly the same proportion as Americans without diabetes, new nationwide survey data suggest.
What’s more, among people with overweight or obesity, those with type 1 diabetes are less likely to receive lifestyle recommendations from health care professionals than those with type 2 diabetes, and are less likely to actually engage in lifestyle weight management activities than others with overweight or obesity, with or without type 2 diabetes.
“Among U.S. adults with type 1 diabetes, the burden of overweight and obesity is substantial and remains poorly managed,” write Michael Fang, PhD, of the Johns Hopkins Bloomberg School of Public Health, Baltimore, and colleagues.
Their data, from the National Health Interview Survey (NHIS), were published online in Annals of Internal Medicine.
The need for insulin complicates weight management in people with type 1 diabetes because changes in diet and physical activity typically require adjustments to insulin timing and dosage to prevent hypoglycemia. There is little evidence to guide this for weight management, Dr. Fang and colleagues explain.
Consequently, “the lack of evidence for safe, effective methods of diet- and exercise-based weight control in people with type 1 diabetes may be keeping doctors from recommending such methods,” Dr. Fang said in a statement.
“Large clinical trials have been done in type 2 diabetes patients to establish guidelines for diet- and exercise-based weight management, and we now need something similar for type 1 diabetes patients.”
Asked to comment, M. Sue Kirkman, MD, told this news organization: “The days when we could teach simple concepts about diabetes type like ‘those with type 1 are lean and those with type 2 are overweight’ are long gone. … Of concern, fewer adults with type 1 diabetes and overweight/obesity report that they are engaging in physical activity or caloric restriction than those without diabetes or those with type 2 diabetes.”
There are several likely reasons for the low rates of obesity/overweight lifestyle modification advice and implementation for those with type 1 diabetes, noted Dr. Kirkman, of the University of North Carolina at Chapel Hill, who coauthored joint American/European guidance on type 1 diabetes management.
“Medical visits are often primarily focused on glycemic management and complications screening, and we know that physicians in general are not very knowledgeable about how to counsel people – even those without diabetes – on weight loss. When you add in potential worries, real or not, about hypoglycemia, ketosis with carbohydrate restriction … it’s no wonder that this may not be addressed in busy visits.”
She also observed, “In years of going to diabetes meetings, I’ve noticed occasional sessions on managing ‘elite athletes’ with type 1 diabetes, but rarely are there sessions on how to counsel people about everyday healthy living.”
Many with type 1 diabetes have overweight/obesity
Dr. Fang and colleagues analyzed NHIS data for the years 2016, 2017, 2019, 2020, and 2021, when diabetes subtype data were available, for 128,571 adults. Diabetes type and height/weight data were self-reported. In the 2016, 2017, and 2020 surveys, participants were asked whether their physicians had recommended increasing physical activity and/or reducing calorie or fat consumption, and whether they were currently engaging in those activities.
The study population comprised 733 people with type 1 diabetes, 12,397 with type 2 diabetes, and 115,441 without diabetes. The proportions with overweight (body mass index, 25 to < 30 kg/m2) or obesity (≥ 30 kg/m2) were 62% among those with type 1 diabetes and 64% among those without diabetes, compared with 86% among those with type 2 diabetes.
Among those with overweight or obesity, the proportions who reported having received lifestyle recommendations were greatest among those with type 2 diabetes and least among those without diabetes, with the type 1 diabetes group in the middle.
After adjustment for age, sex, and race/ethnicity, the adjusted prevalence of receiving a provider recommendation to increase physical activity was 60% for those with type 2 diabetes, 54% for type 1 diabetes, and 44% for those without diabetes. Proportions for receiving recommendations for reducing fat/caloric intake were similar, at 60%, 51%, and 41%, respectively.
The proportions who reported actually engaging in lifestyle activities for weight management were lowest among those with type 1 diabetes, with 52% and 56% of them reporting having increased their physical activity and reducing fat/calories, respectively, compared with proportions ranging from 56% to 63% among the other two groups.
Regarding those findings, Dr. Kirkman commented, “In addition to the factors regarding physician interactions, people with type 1 diabetes may see this as a lower-priority health issue after years of being told that glucose control is the main priority.”
“I also wonder if the many, many tasks people with type 1 diabetes must do every day to manage their diabetes – along with other life issues all adults face – mean that there is just too much on the plate to add more lifestyle changes,” she added.
Asked about the potential for off-label use of glucagonlike peptide–1 agonists for weight management for people with type 1 diabetes, Dr. Kirkman said they could probably help some patients. However, she also pointed to two clinical trials in which liraglutide added to insulin therapy helped with glycemic control and weight reduction, but also increased the risk for hypoglycemia and diabetic ketoacidosis.
“It’s really important that researchers engage with adults with type 1 diabetes to better understand the unique priorities and barriers they face in addressing body weight,” Dr. Kirkman said.
Senior study author Elizabeth Selvin, PhD, professor of epidemiology at the Bloomberg School, said in the statement: “Our study busts the myth that people with type 1 diabetes are not being affected by the global obesity epidemic. … These findings should be a wake-up call that we need to be aggressive in addressing the obesity epidemic in persons with type 1 diabetes.”
The study was funded by the U.S. National Institutes of Health. Dr. Fang and Dr. Kirkman have reported no relevant financial relationships. Dr. Selvin has reported receiving royalty payments from Wolters Kluwer for chapters and laboratory monographs in UpToDate. She also reports receiving honoraria for editorial work on journals published by the American Diabetes Association and European Association for the Study of Diabetes.
A version of this article originally appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
Eye check important before starting semaglutide for diabetes
A small potential increased risk of retinopathy worsening at 1 year with injected semaglutide (Ozempic, Novo Nordisk), a glucagon-like peptide 1 (GLP-1) agonist approved for type 2 diabetes, doesn’t outweigh the drug’s cardiovascular benefits but does highlight the need for baseline ophthalmologic evaluation before initiating treatment and ongoing retinal monitoring, researchers say.
That conclusion was based on data from a meta-analysis of the seven major cardiovascular outcomes trials of GLP-1 agonists currently on the market.
The findings were recently published in Diabetes & Metabolic Syndrome: Clinical Research & Reviews, by Stewart G. Albert, MD, and colleagues.
Concerns about retinopathy worsening with the GLP-1 agonist drug class first arose from the SUSTAIN-6 cardiovascular outcomes trial for injectable semaglutide, although a subsequent analysis of data from that trial appeared to suggest the problem is likely due to rapid glucose-lowering in already vulnerable patients rather than a drug-specific effect. This effect had been previously reported, most notably in the landmark Diabetes Control and Complications Trial.
In this new meta-analysis, “we showed that with improvements in A1c there were correlations with decreases in the rate of cardiovascular events but increases in the rate of retinopathy,” Dr. Albert, of St. Louis University, told this news organization.
“As a class of drugs, we did not find an increased rate of retinopathy. The effect of GLP-1 agonists on retinopathy did not appear to be due to an immediate direct toxic effect of the drug. The worsening of the rate of retinopathy was seen with semaglutide after 1 year of therapy and when there was a decrease in A1c of 1%,” he explained.
He noted that because the increased risk was seen primarily among those who already had retinopathy at baseline, “it would seem prudent to know the level of retinopathy either before or plan for close ophthalmologic monitoring around the time of drug initiation ... We routinely evaluate patients with known type 2 diabetes mellitus at yearly intervals for retinopathy. From our data, we saw worsening at 1 year of drug exposure, but we do not know the exact time when the changes occurred during that year.”
The Ozempic label advises that “patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy” but doesn’t specifically mention baseline assessment at the time of drug initiation.
No increase in retinopathy risk for GLP-1 agonist class overall
The seven trials in the meta-analysis comprised 56,004 participants, with baseline retinopathy prevalence ranging from 9% to 31%.
For the GLP-1 agonist class overall, there was no significant increase in the relative rate (RR) of retinopathy (RR, 1.09; P = .36), while there were significant reductions in relative rates of major adverse cardiac events, overall deaths, and cardiovascular deaths (all P < .001 or P = .001).
The increased retinopathy risk was seen only in the subcutaneous semaglutide group (RR, 1.73; P = .02).
The overall number needed to harm was 1,000 and the number to treat was 77. For semaglutide, those values were 77 and 43, respectively.
There was a significant correlation between a decrease in major adverse cardiac events and a decrease in A1c (P = .014), while for retinopathy, the risk increased with improved A1c (P = .076).
Semaglutide subanalysis finds increased retinopathy worsening
Dr. Albert and colleagues conducted a separate subanalysis of 11 studies of semaglutide that enrolled 11,894 patients, of which 6 studies (n = 5,610) were of oral semaglutide (Rybelsus) and 5 studies were of subcutaneous semaglutide (Ozempic; n = 6,284).
In the subanalysis, there was an overall increase in relative rates of new or worsening retinopathy (RR, 1.218; P = .049).
The change in relative rate of retinopathy was predominantly found for subcutaneous semaglutide given for longer than 1 year (RR, 1.559; P = .022) and decreases in A1c of more than 1.0% (RR, 1.590; P = .016). No such differences were seen with oral semaglutide.
A further evaluation of the data without the SUSTAIN 6 trial showed no effect on retinopathy but the analysis lacked power.
Dr. Albert told this news organization: “We did not find an immediate toxic effect of any drug. However, we cannot rule out that there was a cumulative effect of the dose over longer times.”
No disclosures were given.
A version of this article first appeared on Medscape.com.
A small potential increased risk of retinopathy worsening at 1 year with injected semaglutide (Ozempic, Novo Nordisk), a glucagon-like peptide 1 (GLP-1) agonist approved for type 2 diabetes, doesn’t outweigh the drug’s cardiovascular benefits but does highlight the need for baseline ophthalmologic evaluation before initiating treatment and ongoing retinal monitoring, researchers say.
That conclusion was based on data from a meta-analysis of the seven major cardiovascular outcomes trials of GLP-1 agonists currently on the market.
The findings were recently published in Diabetes & Metabolic Syndrome: Clinical Research & Reviews, by Stewart G. Albert, MD, and colleagues.
Concerns about retinopathy worsening with the GLP-1 agonist drug class first arose from the SUSTAIN-6 cardiovascular outcomes trial for injectable semaglutide, although a subsequent analysis of data from that trial appeared to suggest the problem is likely due to rapid glucose-lowering in already vulnerable patients rather than a drug-specific effect. This effect had been previously reported, most notably in the landmark Diabetes Control and Complications Trial.
In this new meta-analysis, “we showed that with improvements in A1c there were correlations with decreases in the rate of cardiovascular events but increases in the rate of retinopathy,” Dr. Albert, of St. Louis University, told this news organization.
“As a class of drugs, we did not find an increased rate of retinopathy. The effect of GLP-1 agonists on retinopathy did not appear to be due to an immediate direct toxic effect of the drug. The worsening of the rate of retinopathy was seen with semaglutide after 1 year of therapy and when there was a decrease in A1c of 1%,” he explained.
He noted that because the increased risk was seen primarily among those who already had retinopathy at baseline, “it would seem prudent to know the level of retinopathy either before or plan for close ophthalmologic monitoring around the time of drug initiation ... We routinely evaluate patients with known type 2 diabetes mellitus at yearly intervals for retinopathy. From our data, we saw worsening at 1 year of drug exposure, but we do not know the exact time when the changes occurred during that year.”
The Ozempic label advises that “patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy” but doesn’t specifically mention baseline assessment at the time of drug initiation.
No increase in retinopathy risk for GLP-1 agonist class overall
The seven trials in the meta-analysis comprised 56,004 participants, with baseline retinopathy prevalence ranging from 9% to 31%.
For the GLP-1 agonist class overall, there was no significant increase in the relative rate (RR) of retinopathy (RR, 1.09; P = .36), while there were significant reductions in relative rates of major adverse cardiac events, overall deaths, and cardiovascular deaths (all P < .001 or P = .001).
The increased retinopathy risk was seen only in the subcutaneous semaglutide group (RR, 1.73; P = .02).
The overall number needed to harm was 1,000 and the number to treat was 77. For semaglutide, those values were 77 and 43, respectively.
There was a significant correlation between a decrease in major adverse cardiac events and a decrease in A1c (P = .014), while for retinopathy, the risk increased with improved A1c (P = .076).
Semaglutide subanalysis finds increased retinopathy worsening
Dr. Albert and colleagues conducted a separate subanalysis of 11 studies of semaglutide that enrolled 11,894 patients, of which 6 studies (n = 5,610) were of oral semaglutide (Rybelsus) and 5 studies were of subcutaneous semaglutide (Ozempic; n = 6,284).
In the subanalysis, there was an overall increase in relative rates of new or worsening retinopathy (RR, 1.218; P = .049).
The change in relative rate of retinopathy was predominantly found for subcutaneous semaglutide given for longer than 1 year (RR, 1.559; P = .022) and decreases in A1c of more than 1.0% (RR, 1.590; P = .016). No such differences were seen with oral semaglutide.
A further evaluation of the data without the SUSTAIN 6 trial showed no effect on retinopathy but the analysis lacked power.
Dr. Albert told this news organization: “We did not find an immediate toxic effect of any drug. However, we cannot rule out that there was a cumulative effect of the dose over longer times.”
No disclosures were given.
A version of this article first appeared on Medscape.com.
A small potential increased risk of retinopathy worsening at 1 year with injected semaglutide (Ozempic, Novo Nordisk), a glucagon-like peptide 1 (GLP-1) agonist approved for type 2 diabetes, doesn’t outweigh the drug’s cardiovascular benefits but does highlight the need for baseline ophthalmologic evaluation before initiating treatment and ongoing retinal monitoring, researchers say.
That conclusion was based on data from a meta-analysis of the seven major cardiovascular outcomes trials of GLP-1 agonists currently on the market.
The findings were recently published in Diabetes & Metabolic Syndrome: Clinical Research & Reviews, by Stewart G. Albert, MD, and colleagues.
Concerns about retinopathy worsening with the GLP-1 agonist drug class first arose from the SUSTAIN-6 cardiovascular outcomes trial for injectable semaglutide, although a subsequent analysis of data from that trial appeared to suggest the problem is likely due to rapid glucose-lowering in already vulnerable patients rather than a drug-specific effect. This effect had been previously reported, most notably in the landmark Diabetes Control and Complications Trial.
In this new meta-analysis, “we showed that with improvements in A1c there were correlations with decreases in the rate of cardiovascular events but increases in the rate of retinopathy,” Dr. Albert, of St. Louis University, told this news organization.
“As a class of drugs, we did not find an increased rate of retinopathy. The effect of GLP-1 agonists on retinopathy did not appear to be due to an immediate direct toxic effect of the drug. The worsening of the rate of retinopathy was seen with semaglutide after 1 year of therapy and when there was a decrease in A1c of 1%,” he explained.
He noted that because the increased risk was seen primarily among those who already had retinopathy at baseline, “it would seem prudent to know the level of retinopathy either before or plan for close ophthalmologic monitoring around the time of drug initiation ... We routinely evaluate patients with known type 2 diabetes mellitus at yearly intervals for retinopathy. From our data, we saw worsening at 1 year of drug exposure, but we do not know the exact time when the changes occurred during that year.”
The Ozempic label advises that “patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy” but doesn’t specifically mention baseline assessment at the time of drug initiation.
No increase in retinopathy risk for GLP-1 agonist class overall
The seven trials in the meta-analysis comprised 56,004 participants, with baseline retinopathy prevalence ranging from 9% to 31%.
For the GLP-1 agonist class overall, there was no significant increase in the relative rate (RR) of retinopathy (RR, 1.09; P = .36), while there were significant reductions in relative rates of major adverse cardiac events, overall deaths, and cardiovascular deaths (all P < .001 or P = .001).
The increased retinopathy risk was seen only in the subcutaneous semaglutide group (RR, 1.73; P = .02).
The overall number needed to harm was 1,000 and the number to treat was 77. For semaglutide, those values were 77 and 43, respectively.
There was a significant correlation between a decrease in major adverse cardiac events and a decrease in A1c (P = .014), while for retinopathy, the risk increased with improved A1c (P = .076).
Semaglutide subanalysis finds increased retinopathy worsening
Dr. Albert and colleagues conducted a separate subanalysis of 11 studies of semaglutide that enrolled 11,894 patients, of which 6 studies (n = 5,610) were of oral semaglutide (Rybelsus) and 5 studies were of subcutaneous semaglutide (Ozempic; n = 6,284).
In the subanalysis, there was an overall increase in relative rates of new or worsening retinopathy (RR, 1.218; P = .049).
The change in relative rate of retinopathy was predominantly found for subcutaneous semaglutide given for longer than 1 year (RR, 1.559; P = .022) and decreases in A1c of more than 1.0% (RR, 1.590; P = .016). No such differences were seen with oral semaglutide.
A further evaluation of the data without the SUSTAIN 6 trial showed no effect on retinopathy but the analysis lacked power.
Dr. Albert told this news organization: “We did not find an immediate toxic effect of any drug. However, we cannot rule out that there was a cumulative effect of the dose over longer times.”
No disclosures were given.
A version of this article first appeared on Medscape.com.
FROM DIABETES & METABOLIC SYNDROME: CLINICAL RESEARCH & REVIEWS
FDA okays Tidepool Loop app to help guide insulin delivery
The Food and Drug Administration has cleared the Tidepool Loop, a mobile application for use with compatible continuous glucose monitors (CGMs) and insulin pumps to enable automated insulin delivery.
Indicated for people with type 1 diabetes ages 6 years and up, the app algorithm was developed by the diabetes startup Tidepool, which already hosts a cloud-based platform for users to download and review data from different glucose meters, insulin pumps, and CGM systems. The Tidepool Loop project arose from patient-led, open-source initiatives to enable interoperability between the devices.
“The [FDA] authorization of the Tidepool Loop is a huge win for the type 1 diabetes (T1D) community and is a vital step towards a world where people with T1D can choose the pump, CGM, and algorithm that are best for them – and have all three work together seamlessly,” Aaron Kowalski, PhD, CEO of the advocacy organization JDRF, said in a statement.
JDRF helped support preclinical and clinical research in the development of the Loop algorithm, along with The Leona M. and Harry B. Helmsley Charitable Trust, the Tullman Foundation, and partnerships with device makers and donations from the T1D community.
Available by prescription only, the Tidepool app is for single patient use. It works with designated “integrated CGMs” and “alternate controller enabled pumps” to automatically increase, decrease, or suspend insulin delivery, based on the glucose readings and predicted values. The app can also recommend correction doses, which the user can confirm.
According to an FDA statement:“Tidepool Loop’s algorithm technology is designed to be compatible with other individual interoperable devices that meet prespecified acceptance criteria set forth in a validation and integration plan provided by the sponsor and cleared by the FDA as part of the premarket submission.”
Tidepool is finalizing agreements with the various device manufacturers “to create a seamless experience for both physicians prescribing Tidepool Loop and the patients using it,” according to a company statement.
Tidepool’s initial launch device partners have not yet been announced, but the company “has a development partnership with Dexcom and other yet-to-be-named medical device companies for future inclusion of their components with the Tidepool Loop platform,” the statement says.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has cleared the Tidepool Loop, a mobile application for use with compatible continuous glucose monitors (CGMs) and insulin pumps to enable automated insulin delivery.
Indicated for people with type 1 diabetes ages 6 years and up, the app algorithm was developed by the diabetes startup Tidepool, which already hosts a cloud-based platform for users to download and review data from different glucose meters, insulin pumps, and CGM systems. The Tidepool Loop project arose from patient-led, open-source initiatives to enable interoperability between the devices.
“The [FDA] authorization of the Tidepool Loop is a huge win for the type 1 diabetes (T1D) community and is a vital step towards a world where people with T1D can choose the pump, CGM, and algorithm that are best for them – and have all three work together seamlessly,” Aaron Kowalski, PhD, CEO of the advocacy organization JDRF, said in a statement.
JDRF helped support preclinical and clinical research in the development of the Loop algorithm, along with The Leona M. and Harry B. Helmsley Charitable Trust, the Tullman Foundation, and partnerships with device makers and donations from the T1D community.
Available by prescription only, the Tidepool app is for single patient use. It works with designated “integrated CGMs” and “alternate controller enabled pumps” to automatically increase, decrease, or suspend insulin delivery, based on the glucose readings and predicted values. The app can also recommend correction doses, which the user can confirm.
According to an FDA statement:“Tidepool Loop’s algorithm technology is designed to be compatible with other individual interoperable devices that meet prespecified acceptance criteria set forth in a validation and integration plan provided by the sponsor and cleared by the FDA as part of the premarket submission.”
Tidepool is finalizing agreements with the various device manufacturers “to create a seamless experience for both physicians prescribing Tidepool Loop and the patients using it,” according to a company statement.
Tidepool’s initial launch device partners have not yet been announced, but the company “has a development partnership with Dexcom and other yet-to-be-named medical device companies for future inclusion of their components with the Tidepool Loop platform,” the statement says.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has cleared the Tidepool Loop, a mobile application for use with compatible continuous glucose monitors (CGMs) and insulin pumps to enable automated insulin delivery.
Indicated for people with type 1 diabetes ages 6 years and up, the app algorithm was developed by the diabetes startup Tidepool, which already hosts a cloud-based platform for users to download and review data from different glucose meters, insulin pumps, and CGM systems. The Tidepool Loop project arose from patient-led, open-source initiatives to enable interoperability between the devices.
“The [FDA] authorization of the Tidepool Loop is a huge win for the type 1 diabetes (T1D) community and is a vital step towards a world where people with T1D can choose the pump, CGM, and algorithm that are best for them – and have all three work together seamlessly,” Aaron Kowalski, PhD, CEO of the advocacy organization JDRF, said in a statement.
JDRF helped support preclinical and clinical research in the development of the Loop algorithm, along with The Leona M. and Harry B. Helmsley Charitable Trust, the Tullman Foundation, and partnerships with device makers and donations from the T1D community.
Available by prescription only, the Tidepool app is for single patient use. It works with designated “integrated CGMs” and “alternate controller enabled pumps” to automatically increase, decrease, or suspend insulin delivery, based on the glucose readings and predicted values. The app can also recommend correction doses, which the user can confirm.
According to an FDA statement:“Tidepool Loop’s algorithm technology is designed to be compatible with other individual interoperable devices that meet prespecified acceptance criteria set forth in a validation and integration plan provided by the sponsor and cleared by the FDA as part of the premarket submission.”
Tidepool is finalizing agreements with the various device manufacturers “to create a seamless experience for both physicians prescribing Tidepool Loop and the patients using it,” according to a company statement.
Tidepool’s initial launch device partners have not yet been announced, but the company “has a development partnership with Dexcom and other yet-to-be-named medical device companies for future inclusion of their components with the Tidepool Loop platform,” the statement says.
A version of this article first appeared on Medscape.com.
FDA approves new type 2 diabetes drug bexagliflozin
The U.S. Food and Drug Administration has approved bexagliflozin (Brenzavvy, TheracosBio) for the treatment of adults with type 2 diabetes.
The once-daily 20-mg oral sodium-glucose cotransporter 2 (SGLT2) inhibitor is indicated as an adjunct to diet and exercise to improve glycemic control for those with type 2 diabetes, but not type 1 diabetes. It can be used in adults with an estimated glomerular filtration rate (eGFR) > 30 mL/min per 1.73 m2.
Approval was based on results from 23 clinical trials with more than 5,000 participants, including more than 300 patients with stage 3 kidney disease (eGFR < 60 and > 30 mL/min per 1.73 m2).
In the phase 3 studies, bexagliflozin significantly reduced hemoglobin A1c and fasting blood glucose at 24 weeks as monotherapy or as add-on to metformin and other glucose-lowering drugs and combinations. It also produced modest reductions in body weight and systolic blood pressure.
In the phase 3 Bexagliflozin Efficacy and Safety Trial (BEST) cardiovascular outcomes trial, the drug met its efficacy and safety objectives in patients at high cardiovascular risk. Noninferiority was demonstrated for the composite outcome of cardiovascular death, myocardial infarction, stroke, or unstable angina.
“As a class of drugs, SGLT2 inhibitors have shown tremendous benefit in treating adults with type 2 diabetes,” said Mason Freeman, MD, director of the Translational Research Center at Massachusetts General Hospital, Boston, in a press release from TheracosBio.
“Being involved in all of the clinical trials for Brenzavvy, I am greatly impressed with the efficacy of the drug in reducing blood glucose levels and I believe it is an important addition to the SGLT2 inhibitor class of drugs.”
As with other SGLT2 inhibitors, adverse events seen in the trials include ketoacidosis, lower limb amputation, volume depletion, urosepsis, pyelonephritis, Fournier’s gangrene, genital mycotic infections, and hypoglycemia when used with insulin or insulin secretagogues.
Bexagliflozin joins an already crowded field of SGLT2 inhibitors, some of which have been approved for additional cardiovascular and kidney indications.
Of interest, bexagliflozin was approved by the FDA for diabetes in cats in December 2022, as the first oral new animal drug to improve glycemic control in otherwise healthy cats with diabetes not previously treated with insulin.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved bexagliflozin (Brenzavvy, TheracosBio) for the treatment of adults with type 2 diabetes.
The once-daily 20-mg oral sodium-glucose cotransporter 2 (SGLT2) inhibitor is indicated as an adjunct to diet and exercise to improve glycemic control for those with type 2 diabetes, but not type 1 diabetes. It can be used in adults with an estimated glomerular filtration rate (eGFR) > 30 mL/min per 1.73 m2.
Approval was based on results from 23 clinical trials with more than 5,000 participants, including more than 300 patients with stage 3 kidney disease (eGFR < 60 and > 30 mL/min per 1.73 m2).
In the phase 3 studies, bexagliflozin significantly reduced hemoglobin A1c and fasting blood glucose at 24 weeks as monotherapy or as add-on to metformin and other glucose-lowering drugs and combinations. It also produced modest reductions in body weight and systolic blood pressure.
In the phase 3 Bexagliflozin Efficacy and Safety Trial (BEST) cardiovascular outcomes trial, the drug met its efficacy and safety objectives in patients at high cardiovascular risk. Noninferiority was demonstrated for the composite outcome of cardiovascular death, myocardial infarction, stroke, or unstable angina.
“As a class of drugs, SGLT2 inhibitors have shown tremendous benefit in treating adults with type 2 diabetes,” said Mason Freeman, MD, director of the Translational Research Center at Massachusetts General Hospital, Boston, in a press release from TheracosBio.
“Being involved in all of the clinical trials for Brenzavvy, I am greatly impressed with the efficacy of the drug in reducing blood glucose levels and I believe it is an important addition to the SGLT2 inhibitor class of drugs.”
As with other SGLT2 inhibitors, adverse events seen in the trials include ketoacidosis, lower limb amputation, volume depletion, urosepsis, pyelonephritis, Fournier’s gangrene, genital mycotic infections, and hypoglycemia when used with insulin or insulin secretagogues.
Bexagliflozin joins an already crowded field of SGLT2 inhibitors, some of which have been approved for additional cardiovascular and kidney indications.
Of interest, bexagliflozin was approved by the FDA for diabetes in cats in December 2022, as the first oral new animal drug to improve glycemic control in otherwise healthy cats with diabetes not previously treated with insulin.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved bexagliflozin (Brenzavvy, TheracosBio) for the treatment of adults with type 2 diabetes.
The once-daily 20-mg oral sodium-glucose cotransporter 2 (SGLT2) inhibitor is indicated as an adjunct to diet and exercise to improve glycemic control for those with type 2 diabetes, but not type 1 diabetes. It can be used in adults with an estimated glomerular filtration rate (eGFR) > 30 mL/min per 1.73 m2.
Approval was based on results from 23 clinical trials with more than 5,000 participants, including more than 300 patients with stage 3 kidney disease (eGFR < 60 and > 30 mL/min per 1.73 m2).
In the phase 3 studies, bexagliflozin significantly reduced hemoglobin A1c and fasting blood glucose at 24 weeks as monotherapy or as add-on to metformin and other glucose-lowering drugs and combinations. It also produced modest reductions in body weight and systolic blood pressure.
In the phase 3 Bexagliflozin Efficacy and Safety Trial (BEST) cardiovascular outcomes trial, the drug met its efficacy and safety objectives in patients at high cardiovascular risk. Noninferiority was demonstrated for the composite outcome of cardiovascular death, myocardial infarction, stroke, or unstable angina.
“As a class of drugs, SGLT2 inhibitors have shown tremendous benefit in treating adults with type 2 diabetes,” said Mason Freeman, MD, director of the Translational Research Center at Massachusetts General Hospital, Boston, in a press release from TheracosBio.
“Being involved in all of the clinical trials for Brenzavvy, I am greatly impressed with the efficacy of the drug in reducing blood glucose levels and I believe it is an important addition to the SGLT2 inhibitor class of drugs.”
As with other SGLT2 inhibitors, adverse events seen in the trials include ketoacidosis, lower limb amputation, volume depletion, urosepsis, pyelonephritis, Fournier’s gangrene, genital mycotic infections, and hypoglycemia when used with insulin or insulin secretagogues.
Bexagliflozin joins an already crowded field of SGLT2 inhibitors, some of which have been approved for additional cardiovascular and kidney indications.
Of interest, bexagliflozin was approved by the FDA for diabetes in cats in December 2022, as the first oral new animal drug to improve glycemic control in otherwise healthy cats with diabetes not previously treated with insulin.
A version of this article first appeared on Medscape.com.
Metformin monotherapy not always best start in type 2 diabetes
Metformin failure in people with type 2 diabetes is very common, particularly among those with high hemoglobin A1c levels at the time of diagnosis, new findings suggest.
An analysis of electronic health record data for more than 22,000 patients starting metformin at three U.S. clinical sites found that over 40% experienced metformin failure.
This was defined as either failure to achieve or maintain A1c less than 7% within 18 months or the use of additional glucose-lowering medications.
Other predictors that metformin use wouldn’t be successful included increasing age, male sex, and race/ethnicity. However, the latter ceased to be linked after adjustment for other clinical risk factors.
“Our study results suggest increased monitoring with potentially earlier treatment intensification to achieve glycemic control may be appropriate in patients with clinical parameters described in this paper,” Suzette J. Bielinski, PhD, and colleagues wrote.
“Further, these results call into question the ubiquitous use of metformin as the first-line therapy and suggest a more individualized approach may be needed to optimize therapy,” they added in their article, published online in the Journal of Clinical Endocrinology and Metabolism.
The study is also noteworthy in that it demonstrated the feasibility of using EHR data with a machine-learning approach to discover risk biomarkers, Dr. Bielinski, professor of epidemiology at the Mayo Clinic, Rochester, Minn., said in an interview.
“We wanted to repurpose clinical data to answer questions ... I think more studies using these types of techniques repurposing data meant for one thing could potentially impact care in other domains. ... If we can get the bang for the buck from all these data that we generate on people I just think it will improve health care and maybe save health care dollars.”
Baseline A1c strongest predictor of metformin failure
The investigators identified a total of 22,047 metformin initiators from three clinical primary care sites: the University of Mississippi’s Jackson centers, which serves a mostly African American population, the Mountain Park Health Center in Arizona, a seven-clinic federally qualified community health center in Phoenix that serves a mostly Latino population, and the Rochester Epidemiology Project, which includes the Mayo Clinic and serves a primarily White population.
Overall, a total of 43% (9,407) of patients met one of two criteria for metformin failure by 18 months. Among those, median time to failure on metformin was 3.9 months.
Unadjusted failure rates were higher among African Americans, Hispanics, and other racial groups, compared with non-Hispanic White patients.
However, the racial groups also differed by baseline characteristics. Mean A1c was 7.7% overall, 8.1% for the African American group, 7.9% for Asians, and 8.2% for Hispanics, compared with 7.6% for non-Hispanic Whites.
Of 150 clinical factors examined, higher A1c was the strongest predictor of metformin failure, with a rapid increase in risk appearing between 7.5% and 8.0%.
“The slope is steep. It gives us some clinical guidance,” Dr. Bielinski said.
Other variables positively correlated with metformin failure included “diabetes with complications,” increased age, and higher levels of potassium, triglycerides, heart rate, and mean cell hemoglobin.
Factors inversely correlated with metformin failure were having received screening for other suspected conditions and medical examination/evaluation, and lower levels of sodium, albumin, and HDL cholesterol.
Three variables – body mass index, LDL cholesterol, and creatinine – had a U-shaped relationship with metformin failure, so that both high and low values were associated with increased risk.
“The racial/ethnic differences disappeared once other clinical factors were considered suggesting that the biological response to metformin is similar regardless of race/ethnicity,” Dr. Bielinski and colleagues wrote.
They also noted that the abnormal lab results which correlated with metformin failure “likely represent biomarkers for chronic illnesses. However, the effect size for lab abnormalities was small compared with that of baseline A1c.”
Dr. Bielinski urged caution in interpreting the findings. “Electronic health records data have limitations. We have evidence that these people were prescribed metformin. We have no idea if they took it. ... I would really be hesitant to be too strong in making clinical recommendations.”
However, she said that the data are “suggestive to say maybe we need to have some kind of threshold where if someone comes in with an A1c of X that they go on dual therapy right away. I think this is opening the door to that.”
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Metformin failure in people with type 2 diabetes is very common, particularly among those with high hemoglobin A1c levels at the time of diagnosis, new findings suggest.
An analysis of electronic health record data for more than 22,000 patients starting metformin at three U.S. clinical sites found that over 40% experienced metformin failure.
This was defined as either failure to achieve or maintain A1c less than 7% within 18 months or the use of additional glucose-lowering medications.
Other predictors that metformin use wouldn’t be successful included increasing age, male sex, and race/ethnicity. However, the latter ceased to be linked after adjustment for other clinical risk factors.
“Our study results suggest increased monitoring with potentially earlier treatment intensification to achieve glycemic control may be appropriate in patients with clinical parameters described in this paper,” Suzette J. Bielinski, PhD, and colleagues wrote.
“Further, these results call into question the ubiquitous use of metformin as the first-line therapy and suggest a more individualized approach may be needed to optimize therapy,” they added in their article, published online in the Journal of Clinical Endocrinology and Metabolism.
The study is also noteworthy in that it demonstrated the feasibility of using EHR data with a machine-learning approach to discover risk biomarkers, Dr. Bielinski, professor of epidemiology at the Mayo Clinic, Rochester, Minn., said in an interview.
“We wanted to repurpose clinical data to answer questions ... I think more studies using these types of techniques repurposing data meant for one thing could potentially impact care in other domains. ... If we can get the bang for the buck from all these data that we generate on people I just think it will improve health care and maybe save health care dollars.”
Baseline A1c strongest predictor of metformin failure
The investigators identified a total of 22,047 metformin initiators from three clinical primary care sites: the University of Mississippi’s Jackson centers, which serves a mostly African American population, the Mountain Park Health Center in Arizona, a seven-clinic federally qualified community health center in Phoenix that serves a mostly Latino population, and the Rochester Epidemiology Project, which includes the Mayo Clinic and serves a primarily White population.
Overall, a total of 43% (9,407) of patients met one of two criteria for metformin failure by 18 months. Among those, median time to failure on metformin was 3.9 months.
Unadjusted failure rates were higher among African Americans, Hispanics, and other racial groups, compared with non-Hispanic White patients.
However, the racial groups also differed by baseline characteristics. Mean A1c was 7.7% overall, 8.1% for the African American group, 7.9% for Asians, and 8.2% for Hispanics, compared with 7.6% for non-Hispanic Whites.
Of 150 clinical factors examined, higher A1c was the strongest predictor of metformin failure, with a rapid increase in risk appearing between 7.5% and 8.0%.
“The slope is steep. It gives us some clinical guidance,” Dr. Bielinski said.
Other variables positively correlated with metformin failure included “diabetes with complications,” increased age, and higher levels of potassium, triglycerides, heart rate, and mean cell hemoglobin.
Factors inversely correlated with metformin failure were having received screening for other suspected conditions and medical examination/evaluation, and lower levels of sodium, albumin, and HDL cholesterol.
Three variables – body mass index, LDL cholesterol, and creatinine – had a U-shaped relationship with metformin failure, so that both high and low values were associated with increased risk.
“The racial/ethnic differences disappeared once other clinical factors were considered suggesting that the biological response to metformin is similar regardless of race/ethnicity,” Dr. Bielinski and colleagues wrote.
They also noted that the abnormal lab results which correlated with metformin failure “likely represent biomarkers for chronic illnesses. However, the effect size for lab abnormalities was small compared with that of baseline A1c.”
Dr. Bielinski urged caution in interpreting the findings. “Electronic health records data have limitations. We have evidence that these people were prescribed metformin. We have no idea if they took it. ... I would really be hesitant to be too strong in making clinical recommendations.”
However, she said that the data are “suggestive to say maybe we need to have some kind of threshold where if someone comes in with an A1c of X that they go on dual therapy right away. I think this is opening the door to that.”
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Metformin failure in people with type 2 diabetes is very common, particularly among those with high hemoglobin A1c levels at the time of diagnosis, new findings suggest.
An analysis of electronic health record data for more than 22,000 patients starting metformin at three U.S. clinical sites found that over 40% experienced metformin failure.
This was defined as either failure to achieve or maintain A1c less than 7% within 18 months or the use of additional glucose-lowering medications.
Other predictors that metformin use wouldn’t be successful included increasing age, male sex, and race/ethnicity. However, the latter ceased to be linked after adjustment for other clinical risk factors.
“Our study results suggest increased monitoring with potentially earlier treatment intensification to achieve glycemic control may be appropriate in patients with clinical parameters described in this paper,” Suzette J. Bielinski, PhD, and colleagues wrote.
“Further, these results call into question the ubiquitous use of metformin as the first-line therapy and suggest a more individualized approach may be needed to optimize therapy,” they added in their article, published online in the Journal of Clinical Endocrinology and Metabolism.
The study is also noteworthy in that it demonstrated the feasibility of using EHR data with a machine-learning approach to discover risk biomarkers, Dr. Bielinski, professor of epidemiology at the Mayo Clinic, Rochester, Minn., said in an interview.
“We wanted to repurpose clinical data to answer questions ... I think more studies using these types of techniques repurposing data meant for one thing could potentially impact care in other domains. ... If we can get the bang for the buck from all these data that we generate on people I just think it will improve health care and maybe save health care dollars.”
Baseline A1c strongest predictor of metformin failure
The investigators identified a total of 22,047 metformin initiators from three clinical primary care sites: the University of Mississippi’s Jackson centers, which serves a mostly African American population, the Mountain Park Health Center in Arizona, a seven-clinic federally qualified community health center in Phoenix that serves a mostly Latino population, and the Rochester Epidemiology Project, which includes the Mayo Clinic and serves a primarily White population.
Overall, a total of 43% (9,407) of patients met one of two criteria for metformin failure by 18 months. Among those, median time to failure on metformin was 3.9 months.
Unadjusted failure rates were higher among African Americans, Hispanics, and other racial groups, compared with non-Hispanic White patients.
However, the racial groups also differed by baseline characteristics. Mean A1c was 7.7% overall, 8.1% for the African American group, 7.9% for Asians, and 8.2% for Hispanics, compared with 7.6% for non-Hispanic Whites.
Of 150 clinical factors examined, higher A1c was the strongest predictor of metformin failure, with a rapid increase in risk appearing between 7.5% and 8.0%.
“The slope is steep. It gives us some clinical guidance,” Dr. Bielinski said.
Other variables positively correlated with metformin failure included “diabetes with complications,” increased age, and higher levels of potassium, triglycerides, heart rate, and mean cell hemoglobin.
Factors inversely correlated with metformin failure were having received screening for other suspected conditions and medical examination/evaluation, and lower levels of sodium, albumin, and HDL cholesterol.
Three variables – body mass index, LDL cholesterol, and creatinine – had a U-shaped relationship with metformin failure, so that both high and low values were associated with increased risk.
“The racial/ethnic differences disappeared once other clinical factors were considered suggesting that the biological response to metformin is similar regardless of race/ethnicity,” Dr. Bielinski and colleagues wrote.
They also noted that the abnormal lab results which correlated with metformin failure “likely represent biomarkers for chronic illnesses. However, the effect size for lab abnormalities was small compared with that of baseline A1c.”
Dr. Bielinski urged caution in interpreting the findings. “Electronic health records data have limitations. We have evidence that these people were prescribed metformin. We have no idea if they took it. ... I would really be hesitant to be too strong in making clinical recommendations.”
However, she said that the data are “suggestive to say maybe we need to have some kind of threshold where if someone comes in with an A1c of X that they go on dual therapy right away. I think this is opening the door to that.”
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Oramed oral insulin fails to meet goal in type 2 diabetes
Oramed Pharmaceuticals’ investigational oral insulin failed to achieve its primary endpoint in a phase 3 trial, according to top-line results announced by the company.
“Therefore, Oramed expects to discontinue its oral insulin clinical activities for [type 2 diabetes],” according to a company statement.
, ORA-D-013-1, comparing the efficacy of the insulin product ORMD-0801 to placebo in 710 people with type 2 diabetes with inadequate glycemic control on two or three oral glucose-lowering agents.
The participants were randomized 2:2:1:1 into ORMD-0801 dosed at 8 mg once or twice daily, or placebo dosed once or twice daily. They completed a 21-day screening period, followed by a 26-week double-blind treatment period.
The product didn’t achieve the primary endpoint comparing reduction in hemoglobin A1c from baseline to 26 weeks, or the secondary endpoint of mean change in fasting plasma glucose at 26 weeks. There were no serious adverse events.
Oramed Pharmaceuticals specializes in developing oral delivery formulations of drugs currently delivered via injection. The company has offices in the United States and Israel.
Oramed CEO Nadav Kidron commented in the statement, “Today’s outcome is very disappointing, given the positive results from prior trials. Once full data from the studies are available, we expect to share relevant learnings and future plans. We thank all the patients, families, and health care professionals who participated in the trial.”
Insulin manufacturer Novo Nordisk had also been developing an oral insulin product. Successful phase 2a results were presented at the American Diabetes Association’s 2017 Scientific Sessions and full phase 2 feasibility results were published in Lancet Diabetes & Endocrinology in 2019.
However, Novo Nordisk, which manufactures the oral glucagon-like peptide-1 receptor agonist semaglutide (Rybelsus), subsequently discontinued development of their oral insulin product. According to a statement, “Initial results raised questions about truly addressing patients’ unmet needs with insulin therapy. Therefore, we discontinued this work to focus on projects that could in fact improve cardiometabolic outcomes for people living with diabetes.”
A version of this article first appeared on Medscape.com.
Oramed Pharmaceuticals’ investigational oral insulin failed to achieve its primary endpoint in a phase 3 trial, according to top-line results announced by the company.
“Therefore, Oramed expects to discontinue its oral insulin clinical activities for [type 2 diabetes],” according to a company statement.
, ORA-D-013-1, comparing the efficacy of the insulin product ORMD-0801 to placebo in 710 people with type 2 diabetes with inadequate glycemic control on two or three oral glucose-lowering agents.
The participants were randomized 2:2:1:1 into ORMD-0801 dosed at 8 mg once or twice daily, or placebo dosed once or twice daily. They completed a 21-day screening period, followed by a 26-week double-blind treatment period.
The product didn’t achieve the primary endpoint comparing reduction in hemoglobin A1c from baseline to 26 weeks, or the secondary endpoint of mean change in fasting plasma glucose at 26 weeks. There were no serious adverse events.
Oramed Pharmaceuticals specializes in developing oral delivery formulations of drugs currently delivered via injection. The company has offices in the United States and Israel.
Oramed CEO Nadav Kidron commented in the statement, “Today’s outcome is very disappointing, given the positive results from prior trials. Once full data from the studies are available, we expect to share relevant learnings and future plans. We thank all the patients, families, and health care professionals who participated in the trial.”
Insulin manufacturer Novo Nordisk had also been developing an oral insulin product. Successful phase 2a results were presented at the American Diabetes Association’s 2017 Scientific Sessions and full phase 2 feasibility results were published in Lancet Diabetes & Endocrinology in 2019.
However, Novo Nordisk, which manufactures the oral glucagon-like peptide-1 receptor agonist semaglutide (Rybelsus), subsequently discontinued development of their oral insulin product. According to a statement, “Initial results raised questions about truly addressing patients’ unmet needs with insulin therapy. Therefore, we discontinued this work to focus on projects that could in fact improve cardiometabolic outcomes for people living with diabetes.”
A version of this article first appeared on Medscape.com.
Oramed Pharmaceuticals’ investigational oral insulin failed to achieve its primary endpoint in a phase 3 trial, according to top-line results announced by the company.
“Therefore, Oramed expects to discontinue its oral insulin clinical activities for [type 2 diabetes],” according to a company statement.
, ORA-D-013-1, comparing the efficacy of the insulin product ORMD-0801 to placebo in 710 people with type 2 diabetes with inadequate glycemic control on two or three oral glucose-lowering agents.
The participants were randomized 2:2:1:1 into ORMD-0801 dosed at 8 mg once or twice daily, or placebo dosed once or twice daily. They completed a 21-day screening period, followed by a 26-week double-blind treatment period.
The product didn’t achieve the primary endpoint comparing reduction in hemoglobin A1c from baseline to 26 weeks, or the secondary endpoint of mean change in fasting plasma glucose at 26 weeks. There were no serious adverse events.
Oramed Pharmaceuticals specializes in developing oral delivery formulations of drugs currently delivered via injection. The company has offices in the United States and Israel.
Oramed CEO Nadav Kidron commented in the statement, “Today’s outcome is very disappointing, given the positive results from prior trials. Once full data from the studies are available, we expect to share relevant learnings and future plans. We thank all the patients, families, and health care professionals who participated in the trial.”
Insulin manufacturer Novo Nordisk had also been developing an oral insulin product. Successful phase 2a results were presented at the American Diabetes Association’s 2017 Scientific Sessions and full phase 2 feasibility results were published in Lancet Diabetes & Endocrinology in 2019.
However, Novo Nordisk, which manufactures the oral glucagon-like peptide-1 receptor agonist semaglutide (Rybelsus), subsequently discontinued development of their oral insulin product. According to a statement, “Initial results raised questions about truly addressing patients’ unmet needs with insulin therapy. Therefore, we discontinued this work to focus on projects that could in fact improve cardiometabolic outcomes for people living with diabetes.”
A version of this article first appeared on Medscape.com.