User login
Updates in Multiple Sclerosis Imaging
Updates in Multiple Sclerosis Imaging
Multiple sclerosis (MS) is a complex, chronic immune-mediated disease of the central nervous system characterized by focal inflammation, demyelination, and neurodegeneration. Magnetic resonance imaging (MRI), first incorporated into the McDonald Criteria for the diagnosis of MS in 2001, is an integral tool in the diagnosis, prognosis, and therapeutic monitoring of people with MS (PwMS).1
MRI research in MS is rapidly expanding and offers insights into the pathophysiology of MS with important implications for the routine clinical care of PwMS. At the Consortium of Multiple Sclerosis Centers 2024 Annual Meeting, the US Department of Veterans Affairs (VA) MS Centers of Excellence hosted an educational symposium highlighting MRI biomarkers in MS, including T2-lesions, chronic black holes (cBHs), brain atrophy, paramagnetic rim lesions (PRLs), and the central vein sign (CVS). The symposium also provided a brief overview of quantitative MRI techniques used to characterize MS lesion severity and research applications of these techniques. This clinical review summarizes the main points of that symposium with the goal of introducing key concepts to federal health care practitioners caring for PwMS.
MRI Biomarkers in MS
T2-lesions, Chronic Black Holes, and Brain Atrophy
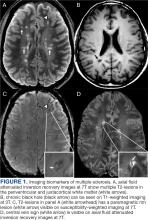
Focal immune-mediated inflammation and demyelination in MS may be detected by MRI as hyperintense foci on T2-weighted (T2-w) imaging (eg, T2-w turbo spin echo or T2-w fluid attenuated inversion recovery sequences). These T2-lesions, critical for diagnosing MS, are typically ovoid and occur in the periventricular, juxtacortical, infratentorial spinal cord white matter (Figure 1A). T2-lesion number and volume show some association with disability and optic nerve.
Wattjes et al highlight 2 cases to demonstrate this point: a man aged 52 years with MS for 23 years and a woman aged 50 years with MS for 11 years. Despite having MS for a much shorter duration, the woman had worse disability due to a higher lesion number and volume.2 T2-lesion volume also impacts disability progression in PwMS. Gauthier et al compared the probability of progression in 3 women, all of whom were aged 39 years and had MS for 6 years. The profile with highest probability of disability progression had the highest quartile of T2-lesion volume.3 T2-lesion volume over 2 years correlates with worse scores on disability metrics such as the MS functional composite, paced auditory serial addition task, and brain volume.4 A 2024 systematic review and meta-analysis demonstrated that T2-lesion volume is significantly correlated with clinical disability in PwMS.5
Select T2-lesions are also hypointense on T1-w spin echo images and are known as cBHs (Figure 1B). Histologically, T2-lesions with cBHs have more severe architectural disruption than those without cBHs.6 cBH number and volume are significantly correlated with disability, regardless of the degree of hypointensity on T1-w imaging.5,7 A 10-year longitudinal study demonstrated that cBHs were associated with disease progression after 5 years while T2-lesion volume was not, indicating that cBHs may be a more accurate predictor of disability.8
Brain atrophy, another imaging biomarker of MS, affects both the cerebral white and gray matter. White matter fraction (the volume of white matter relative to the intracranial compartment volume) and gray matter fraction (the volume of gray matter relative to the intracranial compartment) are significantly lower among PwMS compared with healthy controls. In addition, gray matter fraction is lower among patients with primary and secondary progressive MS compared with those with relapsing-remitting MS, clinically isolated syndrome (CIS), and radiologically isolated syndrome (RIS). Gray matter fraction is also correlated with several motor and cognitive disability indices.9
Paramagnetic Rim Lesions
Neurologic worsening in PwMS occurs by 2 distinct mechanisms: relapse-associated worsening, a stepwise worsening of symptoms due to incomplete recovery following a relapse; and progression independent of relapse activity (PIRA), which is an irreversible neurologic deterioration in the absence of clinical or radiological relapses.10 PIRA is associated with neurodegeneration and predominates in both primary and secondary progressive MS. However, recent data demonstrated that PIRA may contribute to as much as 50% of disability worsening in relapsing MS and occurs early in the RMS disease course.10,11 Current high-efficacy disease modifying therapy, such as ocrelizumab, are extraordinarily successful at preventing focal inflammation and relapses but are less effective for preventing the slow march of disability progression characterizing PIRA.12,13 The prevention of PIRA is therefore an unmet treatment need.
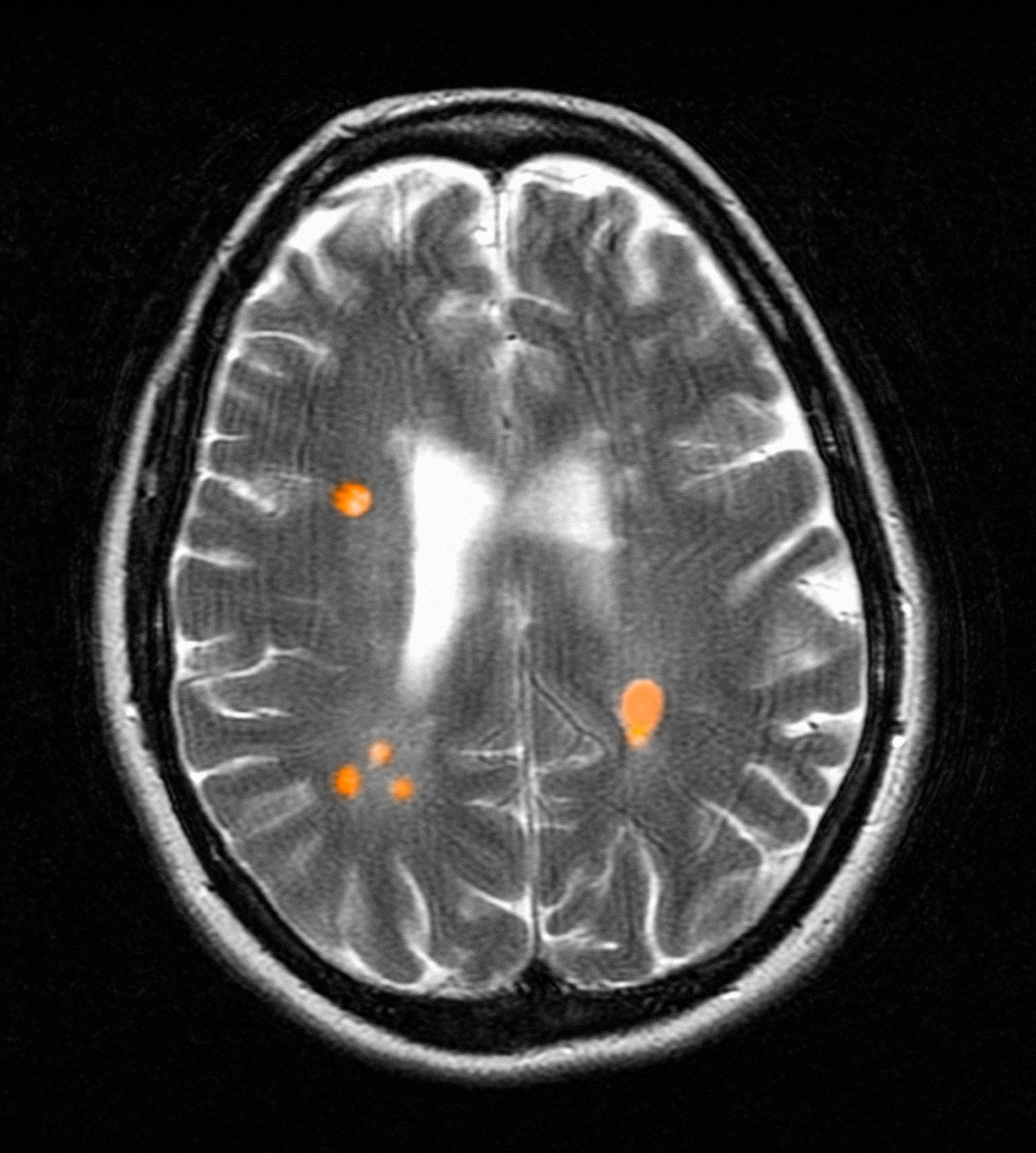
Chronic active lesions (CALs) are an important driver of PIRA. When an acute gadolinium-enhancing lesion develops in PwMS, there are 3 possible fates of this lesion. The lesion may become chronically inactive, remyelinate, or transition to CALs.14 The histopathologic signature of CALs is compartmentalized, low-grade inflammation behind an intact blood-brain barrier with evidence of both active and chronic components.15 CALs may be found not only in cerebral white matter but also in the cerebral cortex and spinal cord.16,17 Combined MRI and histopathological studies have shown that iron-laden microglia/macrophages can be detected by susceptibility-based MRI as a rim of paramagnetic signal surrounding select T2-lesions.19 These PRLs represent an in vivo imaging biomarker of CAL (Figure 1C). According to the North American Imaging in MS Cooperative (NAIMS) consensus criteria, a PRL must surround at least two-thirds of the outer edge of a T2-lesion, be visible in ≥ 2 consecutive MRI slices, and cannot be contrast enhancing.20
PRLs can be visualized on multiple susceptibility-based imaging methods, including multiecho derived R2*/T2*, phase maps, susceptibility-weighted imaging, and quantitative susceptibility mapping.21-23 Retrospective analyses have shown no significant differences in sensitivity across these imaging modalities.24 Although first visualized with 7T MRI, PRLs may also be detected by 1.5T and 3T MRI with comparable sensitivities.25-27 However, there remains a significant knowledge gap regarding the accuracy of each imaging modality. Systematic, prospectively designed studies are needed to ascertain the comparative value of each method.
The presence of PRL is a poor prognostic indicator. PwMS without PRLs have higher levels of disability, are more likely to progress, and demonstrate greater gray matter atrophy and cognitive dysfunction when compared with PwMS with PRLs.27-29 Lesions with PRL tend to slowly expand, exhibit greater demyelination, and have diminished white matter integrity.21,22,30
PRLs may also be used as a diagnostic tool. PRLs are highly specific for MS/CIS with a 99.7% specificity and 98.4% positive predictive value, although the sensitivity is limited to 24%.31 Taken together, these data indicate that the presence of a PRL substantially increases the likelihood of an MS/CIS diagnosis, whereas the absence of a PRL does not exclude these diagnoses.
Several unanswered questions remain: Why do select acute MS lesions transition to CALs? How may investigators utilize PRLs as outcome measures in future clinical trials? How should PRLs be incorporated into the routine care of PwMS? As the role of this imaging biomarker is clarified both in the research and clinical settings, clinicians caring for PwMS can expect to increasingly encounter the topic of PRLs in the near future.
Central Vein Sign
A CVS is defined by the presence of a central vessel within a demyelinating plaque (Figure 1D). As early as the 1820s, MS plaques on gross pathology were noted to follow the course of a vessel. Early histological studies reported that up to 91% of MS plaques had a central vessel present.32 Lesion formation is dependent on the movement of lymphocytes and other inflammatory cells from the systemic circulation across the blood brain barrier into the perivascular space, a privileged site where immune cells interact with antigen presenting cells to launch an inflammatory cascade and eventual demyelinating lesion.33
CVS can be visualized on 1.5T, 3T and 7T MRI. However, 7T MRI is superior to 3T in the detection of CVS, with 85% of MS lesions having CVS visible compared with 45% on 3T.34 With advances in 7T MRI, fluid attenuated inversion recovery and T2* susceptibility, weighted sequences can be overlaid, allowing simultaneous visualization of the vessel and the demyelinating lesion. With higher density of parenchymal veins in the periventricular regions, the CVS is most seen in lesions of this territory but can also be present in juxtacortical, thalamic and infratentorial lesions with decreasing prevalence as these approach the cortex.35
MS lesions are more likely to have CVS than T2 hyperintense white matter lesions of other causes, with a large study reporting 78% of MS lesions were CVS positive. Further, CVS positive lesions can be found across all MS phenotypes including relapsing remitting, primary progressive, and secondary progressive.35 The CVS is also specific to MS lesions and is an effective tool for differentiating MS lesions from other common causes of T2 hyperintense lesions including chronic ischemic white matter disease,36 migraines,37 neuromyelitis optica spectrum disorders,38,39 Susac syndrome,40 and systemic autoimmune diseases (Behcet disease, systemic lupus erythematosus, and antiphospholipid syndrome).41
With CVS emerging as a promising radiographic biomarker for MS, NAIMS issued a consensus statement on necessary properties of a CVS. These criteria included appearance of a thin hypointense line or small dot, visualized in ≥ 2 perpendicular planes, with diameter < 2 mm, and running partially or entirely through the center of the lesion. They also clarified that lesions < 3 mm, confluent lesions, lesions with multiple vessels present or poorly visualized lesions were excluded.42
A shared CVS definition was a necessary step toward routine use of CVS as a radiographic biomarker and its incorporation in the 2024 revised McDonald criteria.43 Remaining limitations including 7T MRI is primarily available in research settings and the lack of consensus on a diagnostic threshold. There have been many proposed methods, including a 40% cut off,44 60% cut off,45 and Select 3* or Select 6* methods.46 The goal of each method is to optimize sensitivity and specificity while not compromising efficiency of MRI review for both neurologists and radiologists.
The CVS has significant potential as a radiographic biomarker for MS and may allow the early stages of MS to be differentiated from other common causes of white matter lesions on MRI. However, it remains unclear whether CVS holds prognostic value for patients, if CVS is suggestive of differing underlying pathology, or if the presence of a CVS is dynamic over time. Progress in these areas is anticipated as CVS is incorporated into routine clinical practice.
Quantitative MRI Techniques
In the research setting, several imaging modalities can be used to quantify the degree of microstructural injury in PwMS. The goal of these methods is to identify and quantify myelin and axonal damage, the major drivers of neurodegeneration. Among these methods, diffusion-based imaging is a measure of the amount of diffusion or fluid mobility across the tissues of the brain.47 Diffusion-weighted imaging (DWI) yields several parametric maps including axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (Figure 2 A, B, and C). These parametric maps provide information on different directions of water molecules’ movements. Myelin surrounds the axons preventing water molecules diffusion perpendicular to axons (RD) while axonal content prevents water diffusion horizontal to the axons (AD).Thus, AD is considered more specific to axonal injury, whereas RD is specific to myelin content.48 A higher value of any of these metrics is associated with a higher degree of tissue injury.
Although sensitive to axonal and myelin injury, AD and RD computed from single b-shell DWI experience several limitations including being affected by nonpathologic factors such as fiber orientation, distribution, and crossing, and by various nonmyelin specific pathologies including fluid accumulation during inflammation, myelin sheath thickness, and axonal intactness.48 Several multi b-shell methods have been developed to overcome diffusion imaging limitations. For example, work at the Nashville VA MS Center of Excellence has focused on the use of the multicompartment diffusion MRI with spherical mean technique (SMT). This method removes the orientation dependency of the diffusion MRI signal, increasing the signal-to-noise ratio and reducing biases from fiber undulation, crossing, and dispersion.49 SMT generates the apparent axonal volume fraction (Vax), which is a direct measure of axonal integrity with lower values indicating lower axonal content and higher tissue destruction (Figure 2D). Vax was previously validated in MS as a measure of axonal integrity.49
In terms of myelin, several other specific measures have been developed. Magnetization transfer ratio (MTR) is another measure of tissue integrity that has been validated as a measure of tissue injury in MS (Figure 2E).50,51 Zheng et al found that the percentage of lesions with low MTR was significantly higher among patients whose disease disability progressed compared with patients who did not.52Selective inversion recovery with quantitative magnetization transfer (SIR-qMT) was developed to account for the limitations of MTR, including its sensitivity to edema and axonal density.52 Germane to myelin measurements, SIR-qMT generates the macromolecular to free size ratio (PSR). PSR represents the ratio of protons bound to macromolecules (myelin) to free protons (Figure 2F). PSR is considered a marker of myelin integrity, with lower values correlating with disability severity and indicating higher tissue damage and lower myelin content. Previous studies from the Nashville VA MS Center of Excellence validated the use of SIR-qMT among patients with MS, CIS, RIS, and healthy controls.53
Quantitative MRI has several research applications in the field of MS. We demonstrated that PRL harbor a higher degree of myelin injury indicated by PSR compared with rimless lesions.54 These MRI techniques are also helpful to investigate tissues surrounding the lesions, called normal appearing white matter (NAWM). Using quantitative MRI techniques such as MTR,52 PSR,53 and Vax,49 investigators have demonstrated that NAWM is injured in PwMS, and proximal NAWM may have higher degree of tissue damage compared with distant NAWM.55
Anticipated Innovations and Challenges
In the field of quantitative MRI, several new techniques are being adopted. Researchers are developing techniques such as myelin water fraction which evaluates the interaction between water and protons to measure myelin content. This is considered an advancement as it takes into account edema resulting from MS injury.56 Another example is multicompartment diffusion imaging, such as standard model imaging,57 and neurite orientation dispersion and density imaging,58 which considers water as an additional compartment compared with the SMT derived Vax. For PRL identification, more advanced methodologic techniques are developing such quantitative susceptibility mapping (QSM), which can detect iron deposits that surround the lesions with relatively high sensitivity and specificity of identifying PRL.59
Despite these innovations, several challenges remain before possible incorporation into the clinical setting. These limitations include longer scan time, familiarity of clinicians in using these maps, higher financial cost, and the necessity of advanced imaging processing skills. Artificial intelligence is a promising tool that may overcome these challenges through creating automated processing pipelines and developing synthetic maps without the need for additional acquisition.60
Conclusions
MRI is the most important tool for diagnosing and treating PwMS. Imaging biomarkers such as T2-lesions, cBHs, brain atrophy, PRLs, and CVS provide insight into the disease’s pathogenesis and are invaluable for the accurate diagnosis and prognostication of MS. Quantitative MRI techniques, while not available in the clinical setting, are important tools for translational research that may help direct the development of future therapeutics. In the near future, clinicians caring for PwMS should expect to encounter these imaging biomarkers more frequently in the clinical setting, especially with the inclusion of PRLs and CVS in the next iteration of the McDonald diagnostic criteria.
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121-127. doi:10.1002/ana.1032
Wattjes MP, Steenwijk MD, Stangel M. MRI in the diagnosis and monitoring of multiple sclerosis: an update. Clin Neuroradiol. 2015;25:157-165. doi:10.1007/s00062-015-0430-y
Gauthier SA, Mandel M, Guttmann CR, et al. Predicting short-term disability in multiple sclerosis. Neurology. 2007;68:2059-2065.doi:10.1212/01.wnl.0000264890.97479.b1
Rudick RA, Lee JC, Simon J, Fisher E. Significance of T2 lesions in multiple sclerosis: a 13-year longitudinal study. Ann Neurol. 2006;60:236-242. doi:10.1002/ana.20883
Nabizadeh F, Zafari R, Mohamadi M, et al. MRI features and disability in multiple sclerosis: a systematic review and meta-analysis. J Neuroradiol. 2024;51:24-37. doi:10.1016/j.neurad.2023.11.007
Bagnato F, Jeffries N, Richert ND, et al. Evolution of T1 black holes in patients with multiple sclerosis imaged monthly for 4 years. Brain. 2003;126:1782-1789. doi:10.1093/brain/awg182
Jacobsen C, Hagemeier J, Myhr KM, et al. Brain atrophy and disability progression in multiple sclerosis patients: a 10-year follow-up study. J Neurol Neurosurg Psychiatry. 2014;85:1109-1115. doi:10.1136/jnnp-2013-306906
Rovaris M, Gass A, Bammer R, et al. Diffusion MRI in multiple sclerosis. Neurology. 2005;65:1526-1532. doi:10.1212/01.wnl.0000184471.83948.e0
Fisniku LK, Chard DT, Jackson JS, et al. Gray matter atrophy is related to long-term disability in multiple sclerosis. Ann Neurol. 2008;64:247-254. doi:10.1002/ana.21423
Lublin FD, Häring DA, Ganjgahi H, et al. How patients with multiple sclerosis acquire disability. Brain. 2022;145:3147-3161. doi:10.1093/brain/awac016
Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77:1132-1140. doi:10.1001/jamaneurol.2020.1568
Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376:221-234. doi:10.1056/NEJMoa1601277
Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376:209-220. doi:10.1056/NEJMoa1606468
Prineas JW, Kwon EE, Cho ES, et al. Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol. 2001;50:646-657. doi:10.1002/ana.1255
Kuhlmann T, Ludwin S, Prat A, Antel J, Brück W, Lassmann H. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 2017;133:13-24. doi:10.1007/s00401-016-1653-y
Pitt D, Boster A, Pei W, et al. Imaging cortical lesions in multiple sclerosis with ultra-high-field magnetic resonance imaging. Arch Neurol. 2010;67:812-818. doi:10.1001/archneurol.2010.148
Gilmore CP, Geurts JJ, Evangelou N, et al. Spinal cord grey matter lesions in multiple sclerosis detected by post-mortem high field MR imaging. Mult Scler. 2009;15:180-188. doi:10.1177/1352458508096876
Lassmann H, Brück W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210-218. doi:10.1111/j.1750-3639.2007.00064.x
Bagnato F, Hametner S, Yao B, et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain. 2011;134:3602-3615. doi:10.1093/brain/awr278
Bagnato F, Sati P, Hemond CC, et al. Imaging chronic active lesions in multiple sclerosis: a consensus statement. Brain. 2024;147:2913-2933. doi:10.1093/brain/awae013
Dal-Bianco A, Grabner G, Kronnerwetter C, et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol. 2017;133:25-42. doi:10.1007/s00401-016-1636-z
Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest. 2016;126:2597-2609. doi:10.1172/JCI86198
Gillen KM, Mubarak M, Park C, et al. QSM is an imaging biomarker for chronic glial activation in multiple sclerosis lesions. Ann Clin Transl Neurol. 2021;8:877-886. doi:10.1002/acn3.51338
Ng Kee Kwong KC, Mollison D, Meijboom R, et al. The prevalence of paramagnetic rim lesions in multiple sclerosis: a systematic review and meta-analysis. PLoS One. 2021;16:e0256845. doi:10.1371/journal.pone.0256845
Absinta M, Sati P, Fechner A, et al. Identification of chronic active multiple sclerosis lesions on 3T MRI. AJNR Am J Neuroradiol. 2018;39:1233-1238. doi:10.3174/ajnr.A5660
Hemond CC, Reich DS, Dundamadappa SK. Paramagnetic rim lesions in multiple sclerosis: comparison of visualization at 1.5-T and 3-T MRI. AJR Am J Roentgenol. 2022;219:120-131. doi:10.2214/AJR.21.26777
Altokhis AI, Hibbert AM, Allen CM, et al. Longitudinal clinical study of patients with iron rim lesions in multiple sclerosis. Mult Scler. 2022;28:2202-2211. doi:10.1177/13524585221114750
Choi S, Lake S, Harrison DM. Evaluation of the blood-brain barrier, demyelination, and neurodegeneration in paramagnetic rim lesions in multiple sclerosis on 7 tesla MRI. J Magn Reson Imaging. 2024;59:941-951. doi:10.1002/jmri.28847
Kazimuddin HF, Wang J, Hernandez B, et al. Paramagnetic rim lesions and their relationship with neurodegeneration and clinical disability at the time of multiple sclerosis diagnosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Rohm Z, Koch C, Kazimuddin H, et al. Longitudinal characterization of paramagnetic rim lesions in early multiple sclerosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Meaton I, Altokhis A, Allen CM, et al. Paramagnetic rims are a promising diagnostic imaging biomarker in multiple sclerosis. Mult Scler. 2022;28:2212-2220. doi:10.1177/13524585221118677
Fog T. On the vessel-plaque relationships in the brain in multiple sclerosis. Acta Neurol Scand Suppl. 1964;40:9-15.
Ineichen BV, Okar SV, Proulx ST, et al. Perivascular spaces and their role in neuroinflammation. Neuron. 2022;110:3566-3581. doi:10.1016/j.neuron.2022.10.024
Tallantyre EC, Morgan PS, Dixon JE, et al. A comparison of 3T and 7T in the detection of small parenchymal veins within MS lesions. Invest Radiol. 2009;44:491-494. doi:10.1097/RLI.0b013e3181b4c144
Kilsdonk ID, Lopez-Soriano A, Kuijer JP, et al. Morphological features of MS lesions on FLAIR* at 7 T and their relation to patient characteristics. J Neurol. 2014;261:1356-1364. doi:10.1007/s00415-014-7351-6
Tallantyre EC, Dixon JE, Donaldson I, et al. Ultra-high-field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology. 2011;76:534-539. doi:10.1212/WNL.0b013e31820b7630
Solomon AJ, Schindler MK, Howard DB, et al. “Central vessel sign” on 3T FLAIR* MRI for the differentiation of multiple sclerosis from migraine. Ann Clin Transl Neurol. 2015;3:82-87. doi:10.1002/acn3.273
Sinnecker T, Dörr J, Pfueller CF, et al. Distinct lesion morphology at 7-T MRI differentiates neuromyelitis optica from multiple sclerosis. Neurology. 2012;79:708-714. doi:10.1212/WNL.0b013e3182648bc8
Kister I, Herbert J, Zhou Y, Ge Y. Ultrahigh-field MR (7 T) imaging of brain lesions in neuromyelitis optica. Mult Scler Int. 2013;2013:398259. doi:10.1155/2013/398259
Wuerfel J, Sinnecker T, Ringelstein EB, et al. Lesion morphology at 7 Tesla MRI differentiates Susac syndrome from multiple sclerosis. Mult Scler. 2012;18:1592-1599. doi:10.1177/1352458512441270
Massacesi L. Perivenular distribution of white matter lesions evaluated by MRI can differentiate MS lesions from inflammatory small vessel diseases. Eur J Neurol. 2016;23:86. doi:10.1212/WNL.86.16_supplement.P6.121
Sati P, Oh J, Constable RT, et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol. 2016;12:714-722. doi:10.1038/nrneurol.2016.166
Montalban X, Lebrun-Frénay C, Oh J, et al. Diagnosis of multiple sclerosis: 2024 revisions of the McDonald criteria. Lancet Neurol. 2025;24:850-865. doi:10.1016/S1474-4422(25)00270-4
Mistry N, Dixon J, Tallantyre E, et al. Central veins in brain lesions visualized with high-field magnetic resonance imaging: a pathologically specific diagnostic biomarker for inflammatory demyelination in the brain. JAMA Neurol. 2013;70:623-628. doi:10.1001/jamaneurol.2013.1405
Campion T, Smith RJP, Altmann DR, et al. FLAIR* to visualize veins in white matter lesions: a new tool for the diagnosis of multiple sclerosis? Eur Radiol. 2017;27:4257-4263. doi:10.1007/s00330-017-4822-z
Solomon AJ, Watts R, Ontaneda D, et al. Diagnostic performance of central vein sign for multiple sclerosis with a simplified three-lesion algorithm. Mult Scler. 2018;24:750-757. doi:10.1177/1352458517726383
Cercignani M, Bozzali M, Iannucci G, Comi G, Filippi M. Intra-voxel and inter-voxel coherence in patients with multiple sclerosis assessed using diffusion tensor MRI. J Neurol. 2002;249:875-883. doi:10.1007/s00415-002-0752-y
Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132-140. doi:10.1016/j.neuroimage.2005.01.028
Bagnato F, Franco G, Li H, et al. Probing axons using multi-compartmental diffusion in multiple sclerosis. Ann Clin Transl Neurol. 2019;6:1595-1605. doi:10.1002/acn3.50836
Filippi M, Cercignani M, Inglese M, et al. Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology. 2001;56:304-311. doi:10.1212/wnl.56.3.304
Bagnato F. Clinical application of magnetization transfer imaging. In: Advanced Neuro MR Techniques and Applications. Elsevier; 2022:403-417. doi:10.1016/B978-0-12-822479-3.00041-5
Zheng Y, Lee JC, Rudick R, Fisher E. Long-term magnetization transfer ratio evolution in multiple sclerosis white matter lesions. J Neuroimaging. 2018;28:191-198. doi:10.1111/jon.12480
Bagnato F, Hametner S, Franco G, et al. Selective inversion recovery quantitative magnetization transfer brain MRI at 7T: clinical and postmortem validation in multiple sclerosis. J Neuroimaging. 2018;28:380-388. doi:10.1111/jon.12511
Clarke MA, Cheek R, Hernandez B, et al. Paramagnetic rim lesions and the central vein sign: characterizing multiple sclerosis imaging markers. J Neuroimaging. 2024;34:86-94. doi:10.1111/jon.13173
Clarke MA, Lakhani DA, Wen S, et al. Perilesional neurodegenerative injury in multiple sclerosis: relation to focal lesions and impact on disability. Mult Scler Relat Disord. 2021;49:102738. doi:10.1016/j.msard.2021.102738
Laule C, Moore GRW. Myelin water imaging to detect demyelination and remyelination and its validation in pathology. Brain Pathol. 2018;28:750-764. doi:10.1111/bpa.12645
Coelho S, Baete SH, Lemberskiy G, et al. Reproducibility of the standard model of diffusion in white matter on clinical MRI systems. Neuroimage. 2022;257:119290. doi:10.1016/j.neuroimage.2022.119290
Novikov DS, Veraart J, Jelescu IO, et al. Rotationally-invariant mapping of scalar and orientational metrics of neuronal microstructure with diffusion MRI. Neuroimage. 2018;174:518-538. doi:10.1016/j.neuroimage.2018.03.006
Langkammer C, Liu T, Khalil M, et al. Quantitative susceptibility mapping in multiple sclerosis. Radiology. 2013;267:551-559. doi:10.1148/radiol.12120707
Collorone S, Coll L, Lorenzi M, et al. Artificial intelligence applied to MRI data to tackle key challenges in multiple sclerosis. Mult Scler. 2024;30:767-784. doi:10.1177/13524585241249422
Multiple sclerosis (MS) is a complex, chronic immune-mediated disease of the central nervous system characterized by focal inflammation, demyelination, and neurodegeneration. Magnetic resonance imaging (MRI), first incorporated into the McDonald Criteria for the diagnosis of MS in 2001, is an integral tool in the diagnosis, prognosis, and therapeutic monitoring of people with MS (PwMS).1
MRI research in MS is rapidly expanding and offers insights into the pathophysiology of MS with important implications for the routine clinical care of PwMS. At the Consortium of Multiple Sclerosis Centers 2024 Annual Meeting, the US Department of Veterans Affairs (VA) MS Centers of Excellence hosted an educational symposium highlighting MRI biomarkers in MS, including T2-lesions, chronic black holes (cBHs), brain atrophy, paramagnetic rim lesions (PRLs), and the central vein sign (CVS). The symposium also provided a brief overview of quantitative MRI techniques used to characterize MS lesion severity and research applications of these techniques. This clinical review summarizes the main points of that symposium with the goal of introducing key concepts to federal health care practitioners caring for PwMS.
MRI Biomarkers in MS
T2-lesions, Chronic Black Holes, and Brain Atrophy
Focal immune-mediated inflammation and demyelination in MS may be detected by MRI as hyperintense foci on T2-weighted (T2-w) imaging (eg, T2-w turbo spin echo or T2-w fluid attenuated inversion recovery sequences). These T2-lesions, critical for diagnosing MS, are typically ovoid and occur in the periventricular, juxtacortical, infratentorial spinal cord white matter (Figure 1A). T2-lesion number and volume show some association with disability and optic nerve.
Wattjes et al highlight 2 cases to demonstrate this point: a man aged 52 years with MS for 23 years and a woman aged 50 years with MS for 11 years. Despite having MS for a much shorter duration, the woman had worse disability due to a higher lesion number and volume.2 T2-lesion volume also impacts disability progression in PwMS. Gauthier et al compared the probability of progression in 3 women, all of whom were aged 39 years and had MS for 6 years. The profile with highest probability of disability progression had the highest quartile of T2-lesion volume.3 T2-lesion volume over 2 years correlates with worse scores on disability metrics such as the MS functional composite, paced auditory serial addition task, and brain volume.4 A 2024 systematic review and meta-analysis demonstrated that T2-lesion volume is significantly correlated with clinical disability in PwMS.5
Select T2-lesions are also hypointense on T1-w spin echo images and are known as cBHs (Figure 1B). Histologically, T2-lesions with cBHs have more severe architectural disruption than those without cBHs.6 cBH number and volume are significantly correlated with disability, regardless of the degree of hypointensity on T1-w imaging.5,7 A 10-year longitudinal study demonstrated that cBHs were associated with disease progression after 5 years while T2-lesion volume was not, indicating that cBHs may be a more accurate predictor of disability.8
Brain atrophy, another imaging biomarker of MS, affects both the cerebral white and gray matter. White matter fraction (the volume of white matter relative to the intracranial compartment volume) and gray matter fraction (the volume of gray matter relative to the intracranial compartment) are significantly lower among PwMS compared with healthy controls. In addition, gray matter fraction is lower among patients with primary and secondary progressive MS compared with those with relapsing-remitting MS, clinically isolated syndrome (CIS), and radiologically isolated syndrome (RIS). Gray matter fraction is also correlated with several motor and cognitive disability indices.9
Paramagnetic Rim Lesions
Neurologic worsening in PwMS occurs by 2 distinct mechanisms: relapse-associated worsening, a stepwise worsening of symptoms due to incomplete recovery following a relapse; and progression independent of relapse activity (PIRA), which is an irreversible neurologic deterioration in the absence of clinical or radiological relapses.10 PIRA is associated with neurodegeneration and predominates in both primary and secondary progressive MS. However, recent data demonstrated that PIRA may contribute to as much as 50% of disability worsening in relapsing MS and occurs early in the RMS disease course.10,11 Current high-efficacy disease modifying therapy, such as ocrelizumab, are extraordinarily successful at preventing focal inflammation and relapses but are less effective for preventing the slow march of disability progression characterizing PIRA.12,13 The prevention of PIRA is therefore an unmet treatment need.
Chronic active lesions (CALs) are an important driver of PIRA. When an acute gadolinium-enhancing lesion develops in PwMS, there are 3 possible fates of this lesion. The lesion may become chronically inactive, remyelinate, or transition to CALs.14 The histopathologic signature of CALs is compartmentalized, low-grade inflammation behind an intact blood-brain barrier with evidence of both active and chronic components.15 CALs may be found not only in cerebral white matter but also in the cerebral cortex and spinal cord.16,17 Combined MRI and histopathological studies have shown that iron-laden microglia/macrophages can be detected by susceptibility-based MRI as a rim of paramagnetic signal surrounding select T2-lesions.19 These PRLs represent an in vivo imaging biomarker of CAL (Figure 1C). According to the North American Imaging in MS Cooperative (NAIMS) consensus criteria, a PRL must surround at least two-thirds of the outer edge of a T2-lesion, be visible in ≥ 2 consecutive MRI slices, and cannot be contrast enhancing.20
PRLs can be visualized on multiple susceptibility-based imaging methods, including multiecho derived R2*/T2*, phase maps, susceptibility-weighted imaging, and quantitative susceptibility mapping.21-23 Retrospective analyses have shown no significant differences in sensitivity across these imaging modalities.24 Although first visualized with 7T MRI, PRLs may also be detected by 1.5T and 3T MRI with comparable sensitivities.25-27 However, there remains a significant knowledge gap regarding the accuracy of each imaging modality. Systematic, prospectively designed studies are needed to ascertain the comparative value of each method.
The presence of PRL is a poor prognostic indicator. PwMS without PRLs have higher levels of disability, are more likely to progress, and demonstrate greater gray matter atrophy and cognitive dysfunction when compared with PwMS with PRLs.27-29 Lesions with PRL tend to slowly expand, exhibit greater demyelination, and have diminished white matter integrity.21,22,30
PRLs may also be used as a diagnostic tool. PRLs are highly specific for MS/CIS with a 99.7% specificity and 98.4% positive predictive value, although the sensitivity is limited to 24%.31 Taken together, these data indicate that the presence of a PRL substantially increases the likelihood of an MS/CIS diagnosis, whereas the absence of a PRL does not exclude these diagnoses.
Several unanswered questions remain: Why do select acute MS lesions transition to CALs? How may investigators utilize PRLs as outcome measures in future clinical trials? How should PRLs be incorporated into the routine care of PwMS? As the role of this imaging biomarker is clarified both in the research and clinical settings, clinicians caring for PwMS can expect to increasingly encounter the topic of PRLs in the near future.
Central Vein Sign
A CVS is defined by the presence of a central vessel within a demyelinating plaque (Figure 1D). As early as the 1820s, MS plaques on gross pathology were noted to follow the course of a vessel. Early histological studies reported that up to 91% of MS plaques had a central vessel present.32 Lesion formation is dependent on the movement of lymphocytes and other inflammatory cells from the systemic circulation across the blood brain barrier into the perivascular space, a privileged site where immune cells interact with antigen presenting cells to launch an inflammatory cascade and eventual demyelinating lesion.33
CVS can be visualized on 1.5T, 3T and 7T MRI. However, 7T MRI is superior to 3T in the detection of CVS, with 85% of MS lesions having CVS visible compared with 45% on 3T.34 With advances in 7T MRI, fluid attenuated inversion recovery and T2* susceptibility, weighted sequences can be overlaid, allowing simultaneous visualization of the vessel and the demyelinating lesion. With higher density of parenchymal veins in the periventricular regions, the CVS is most seen in lesions of this territory but can also be present in juxtacortical, thalamic and infratentorial lesions with decreasing prevalence as these approach the cortex.35
MS lesions are more likely to have CVS than T2 hyperintense white matter lesions of other causes, with a large study reporting 78% of MS lesions were CVS positive. Further, CVS positive lesions can be found across all MS phenotypes including relapsing remitting, primary progressive, and secondary progressive.35 The CVS is also specific to MS lesions and is an effective tool for differentiating MS lesions from other common causes of T2 hyperintense lesions including chronic ischemic white matter disease,36 migraines,37 neuromyelitis optica spectrum disorders,38,39 Susac syndrome,40 and systemic autoimmune diseases (Behcet disease, systemic lupus erythematosus, and antiphospholipid syndrome).41
With CVS emerging as a promising radiographic biomarker for MS, NAIMS issued a consensus statement on necessary properties of a CVS. These criteria included appearance of a thin hypointense line or small dot, visualized in ≥ 2 perpendicular planes, with diameter < 2 mm, and running partially or entirely through the center of the lesion. They also clarified that lesions < 3 mm, confluent lesions, lesions with multiple vessels present or poorly visualized lesions were excluded.42
A shared CVS definition was a necessary step toward routine use of CVS as a radiographic biomarker and its incorporation in the 2024 revised McDonald criteria.43 Remaining limitations including 7T MRI is primarily available in research settings and the lack of consensus on a diagnostic threshold. There have been many proposed methods, including a 40% cut off,44 60% cut off,45 and Select 3* or Select 6* methods.46 The goal of each method is to optimize sensitivity and specificity while not compromising efficiency of MRI review for both neurologists and radiologists.
The CVS has significant potential as a radiographic biomarker for MS and may allow the early stages of MS to be differentiated from other common causes of white matter lesions on MRI. However, it remains unclear whether CVS holds prognostic value for patients, if CVS is suggestive of differing underlying pathology, or if the presence of a CVS is dynamic over time. Progress in these areas is anticipated as CVS is incorporated into routine clinical practice.
Quantitative MRI Techniques
In the research setting, several imaging modalities can be used to quantify the degree of microstructural injury in PwMS. The goal of these methods is to identify and quantify myelin and axonal damage, the major drivers of neurodegeneration. Among these methods, diffusion-based imaging is a measure of the amount of diffusion or fluid mobility across the tissues of the brain.47 Diffusion-weighted imaging (DWI) yields several parametric maps including axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (Figure 2 A, B, and C). These parametric maps provide information on different directions of water molecules’ movements. Myelin surrounds the axons preventing water molecules diffusion perpendicular to axons (RD) while axonal content prevents water diffusion horizontal to the axons (AD).Thus, AD is considered more specific to axonal injury, whereas RD is specific to myelin content.48 A higher value of any of these metrics is associated with a higher degree of tissue injury.
Although sensitive to axonal and myelin injury, AD and RD computed from single b-shell DWI experience several limitations including being affected by nonpathologic factors such as fiber orientation, distribution, and crossing, and by various nonmyelin specific pathologies including fluid accumulation during inflammation, myelin sheath thickness, and axonal intactness.48 Several multi b-shell methods have been developed to overcome diffusion imaging limitations. For example, work at the Nashville VA MS Center of Excellence has focused on the use of the multicompartment diffusion MRI with spherical mean technique (SMT). This method removes the orientation dependency of the diffusion MRI signal, increasing the signal-to-noise ratio and reducing biases from fiber undulation, crossing, and dispersion.49 SMT generates the apparent axonal volume fraction (Vax), which is a direct measure of axonal integrity with lower values indicating lower axonal content and higher tissue destruction (Figure 2D). Vax was previously validated in MS as a measure of axonal integrity.49
In terms of myelin, several other specific measures have been developed. Magnetization transfer ratio (MTR) is another measure of tissue integrity that has been validated as a measure of tissue injury in MS (Figure 2E).50,51 Zheng et al found that the percentage of lesions with low MTR was significantly higher among patients whose disease disability progressed compared with patients who did not.52Selective inversion recovery with quantitative magnetization transfer (SIR-qMT) was developed to account for the limitations of MTR, including its sensitivity to edema and axonal density.52 Germane to myelin measurements, SIR-qMT generates the macromolecular to free size ratio (PSR). PSR represents the ratio of protons bound to macromolecules (myelin) to free protons (Figure 2F). PSR is considered a marker of myelin integrity, with lower values correlating with disability severity and indicating higher tissue damage and lower myelin content. Previous studies from the Nashville VA MS Center of Excellence validated the use of SIR-qMT among patients with MS, CIS, RIS, and healthy controls.53
Quantitative MRI has several research applications in the field of MS. We demonstrated that PRL harbor a higher degree of myelin injury indicated by PSR compared with rimless lesions.54 These MRI techniques are also helpful to investigate tissues surrounding the lesions, called normal appearing white matter (NAWM). Using quantitative MRI techniques such as MTR,52 PSR,53 and Vax,49 investigators have demonstrated that NAWM is injured in PwMS, and proximal NAWM may have higher degree of tissue damage compared with distant NAWM.55
Anticipated Innovations and Challenges
In the field of quantitative MRI, several new techniques are being adopted. Researchers are developing techniques such as myelin water fraction which evaluates the interaction between water and protons to measure myelin content. This is considered an advancement as it takes into account edema resulting from MS injury.56 Another example is multicompartment diffusion imaging, such as standard model imaging,57 and neurite orientation dispersion and density imaging,58 which considers water as an additional compartment compared with the SMT derived Vax. For PRL identification, more advanced methodologic techniques are developing such quantitative susceptibility mapping (QSM), which can detect iron deposits that surround the lesions with relatively high sensitivity and specificity of identifying PRL.59
Despite these innovations, several challenges remain before possible incorporation into the clinical setting. These limitations include longer scan time, familiarity of clinicians in using these maps, higher financial cost, and the necessity of advanced imaging processing skills. Artificial intelligence is a promising tool that may overcome these challenges through creating automated processing pipelines and developing synthetic maps without the need for additional acquisition.60
Conclusions
MRI is the most important tool for diagnosing and treating PwMS. Imaging biomarkers such as T2-lesions, cBHs, brain atrophy, PRLs, and CVS provide insight into the disease’s pathogenesis and are invaluable for the accurate diagnosis and prognostication of MS. Quantitative MRI techniques, while not available in the clinical setting, are important tools for translational research that may help direct the development of future therapeutics. In the near future, clinicians caring for PwMS should expect to encounter these imaging biomarkers more frequently in the clinical setting, especially with the inclusion of PRLs and CVS in the next iteration of the McDonald diagnostic criteria.
Multiple sclerosis (MS) is a complex, chronic immune-mediated disease of the central nervous system characterized by focal inflammation, demyelination, and neurodegeneration. Magnetic resonance imaging (MRI), first incorporated into the McDonald Criteria for the diagnosis of MS in 2001, is an integral tool in the diagnosis, prognosis, and therapeutic monitoring of people with MS (PwMS).1
MRI research in MS is rapidly expanding and offers insights into the pathophysiology of MS with important implications for the routine clinical care of PwMS. At the Consortium of Multiple Sclerosis Centers 2024 Annual Meeting, the US Department of Veterans Affairs (VA) MS Centers of Excellence hosted an educational symposium highlighting MRI biomarkers in MS, including T2-lesions, chronic black holes (cBHs), brain atrophy, paramagnetic rim lesions (PRLs), and the central vein sign (CVS). The symposium also provided a brief overview of quantitative MRI techniques used to characterize MS lesion severity and research applications of these techniques. This clinical review summarizes the main points of that symposium with the goal of introducing key concepts to federal health care practitioners caring for PwMS.
MRI Biomarkers in MS
T2-lesions, Chronic Black Holes, and Brain Atrophy
Focal immune-mediated inflammation and demyelination in MS may be detected by MRI as hyperintense foci on T2-weighted (T2-w) imaging (eg, T2-w turbo spin echo or T2-w fluid attenuated inversion recovery sequences). These T2-lesions, critical for diagnosing MS, are typically ovoid and occur in the periventricular, juxtacortical, infratentorial spinal cord white matter (Figure 1A). T2-lesion number and volume show some association with disability and optic nerve.
Wattjes et al highlight 2 cases to demonstrate this point: a man aged 52 years with MS for 23 years and a woman aged 50 years with MS for 11 years. Despite having MS for a much shorter duration, the woman had worse disability due to a higher lesion number and volume.2 T2-lesion volume also impacts disability progression in PwMS. Gauthier et al compared the probability of progression in 3 women, all of whom were aged 39 years and had MS for 6 years. The profile with highest probability of disability progression had the highest quartile of T2-lesion volume.3 T2-lesion volume over 2 years correlates with worse scores on disability metrics such as the MS functional composite, paced auditory serial addition task, and brain volume.4 A 2024 systematic review and meta-analysis demonstrated that T2-lesion volume is significantly correlated with clinical disability in PwMS.5
Select T2-lesions are also hypointense on T1-w spin echo images and are known as cBHs (Figure 1B). Histologically, T2-lesions with cBHs have more severe architectural disruption than those without cBHs.6 cBH number and volume are significantly correlated with disability, regardless of the degree of hypointensity on T1-w imaging.5,7 A 10-year longitudinal study demonstrated that cBHs were associated with disease progression after 5 years while T2-lesion volume was not, indicating that cBHs may be a more accurate predictor of disability.8
Brain atrophy, another imaging biomarker of MS, affects both the cerebral white and gray matter. White matter fraction (the volume of white matter relative to the intracranial compartment volume) and gray matter fraction (the volume of gray matter relative to the intracranial compartment) are significantly lower among PwMS compared with healthy controls. In addition, gray matter fraction is lower among patients with primary and secondary progressive MS compared with those with relapsing-remitting MS, clinically isolated syndrome (CIS), and radiologically isolated syndrome (RIS). Gray matter fraction is also correlated with several motor and cognitive disability indices.9
Paramagnetic Rim Lesions
Neurologic worsening in PwMS occurs by 2 distinct mechanisms: relapse-associated worsening, a stepwise worsening of symptoms due to incomplete recovery following a relapse; and progression independent of relapse activity (PIRA), which is an irreversible neurologic deterioration in the absence of clinical or radiological relapses.10 PIRA is associated with neurodegeneration and predominates in both primary and secondary progressive MS. However, recent data demonstrated that PIRA may contribute to as much as 50% of disability worsening in relapsing MS and occurs early in the RMS disease course.10,11 Current high-efficacy disease modifying therapy, such as ocrelizumab, are extraordinarily successful at preventing focal inflammation and relapses but are less effective for preventing the slow march of disability progression characterizing PIRA.12,13 The prevention of PIRA is therefore an unmet treatment need.
Chronic active lesions (CALs) are an important driver of PIRA. When an acute gadolinium-enhancing lesion develops in PwMS, there are 3 possible fates of this lesion. The lesion may become chronically inactive, remyelinate, or transition to CALs.14 The histopathologic signature of CALs is compartmentalized, low-grade inflammation behind an intact blood-brain barrier with evidence of both active and chronic components.15 CALs may be found not only in cerebral white matter but also in the cerebral cortex and spinal cord.16,17 Combined MRI and histopathological studies have shown that iron-laden microglia/macrophages can be detected by susceptibility-based MRI as a rim of paramagnetic signal surrounding select T2-lesions.19 These PRLs represent an in vivo imaging biomarker of CAL (Figure 1C). According to the North American Imaging in MS Cooperative (NAIMS) consensus criteria, a PRL must surround at least two-thirds of the outer edge of a T2-lesion, be visible in ≥ 2 consecutive MRI slices, and cannot be contrast enhancing.20
PRLs can be visualized on multiple susceptibility-based imaging methods, including multiecho derived R2*/T2*, phase maps, susceptibility-weighted imaging, and quantitative susceptibility mapping.21-23 Retrospective analyses have shown no significant differences in sensitivity across these imaging modalities.24 Although first visualized with 7T MRI, PRLs may also be detected by 1.5T and 3T MRI with comparable sensitivities.25-27 However, there remains a significant knowledge gap regarding the accuracy of each imaging modality. Systematic, prospectively designed studies are needed to ascertain the comparative value of each method.
The presence of PRL is a poor prognostic indicator. PwMS without PRLs have higher levels of disability, are more likely to progress, and demonstrate greater gray matter atrophy and cognitive dysfunction when compared with PwMS with PRLs.27-29 Lesions with PRL tend to slowly expand, exhibit greater demyelination, and have diminished white matter integrity.21,22,30
PRLs may also be used as a diagnostic tool. PRLs are highly specific for MS/CIS with a 99.7% specificity and 98.4% positive predictive value, although the sensitivity is limited to 24%.31 Taken together, these data indicate that the presence of a PRL substantially increases the likelihood of an MS/CIS diagnosis, whereas the absence of a PRL does not exclude these diagnoses.
Several unanswered questions remain: Why do select acute MS lesions transition to CALs? How may investigators utilize PRLs as outcome measures in future clinical trials? How should PRLs be incorporated into the routine care of PwMS? As the role of this imaging biomarker is clarified both in the research and clinical settings, clinicians caring for PwMS can expect to increasingly encounter the topic of PRLs in the near future.
Central Vein Sign
A CVS is defined by the presence of a central vessel within a demyelinating plaque (Figure 1D). As early as the 1820s, MS plaques on gross pathology were noted to follow the course of a vessel. Early histological studies reported that up to 91% of MS plaques had a central vessel present.32 Lesion formation is dependent on the movement of lymphocytes and other inflammatory cells from the systemic circulation across the blood brain barrier into the perivascular space, a privileged site where immune cells interact with antigen presenting cells to launch an inflammatory cascade and eventual demyelinating lesion.33
CVS can be visualized on 1.5T, 3T and 7T MRI. However, 7T MRI is superior to 3T in the detection of CVS, with 85% of MS lesions having CVS visible compared with 45% on 3T.34 With advances in 7T MRI, fluid attenuated inversion recovery and T2* susceptibility, weighted sequences can be overlaid, allowing simultaneous visualization of the vessel and the demyelinating lesion. With higher density of parenchymal veins in the periventricular regions, the CVS is most seen in lesions of this territory but can also be present in juxtacortical, thalamic and infratentorial lesions with decreasing prevalence as these approach the cortex.35
MS lesions are more likely to have CVS than T2 hyperintense white matter lesions of other causes, with a large study reporting 78% of MS lesions were CVS positive. Further, CVS positive lesions can be found across all MS phenotypes including relapsing remitting, primary progressive, and secondary progressive.35 The CVS is also specific to MS lesions and is an effective tool for differentiating MS lesions from other common causes of T2 hyperintense lesions including chronic ischemic white matter disease,36 migraines,37 neuromyelitis optica spectrum disorders,38,39 Susac syndrome,40 and systemic autoimmune diseases (Behcet disease, systemic lupus erythematosus, and antiphospholipid syndrome).41
With CVS emerging as a promising radiographic biomarker for MS, NAIMS issued a consensus statement on necessary properties of a CVS. These criteria included appearance of a thin hypointense line or small dot, visualized in ≥ 2 perpendicular planes, with diameter < 2 mm, and running partially or entirely through the center of the lesion. They also clarified that lesions < 3 mm, confluent lesions, lesions with multiple vessels present or poorly visualized lesions were excluded.42
A shared CVS definition was a necessary step toward routine use of CVS as a radiographic biomarker and its incorporation in the 2024 revised McDonald criteria.43 Remaining limitations including 7T MRI is primarily available in research settings and the lack of consensus on a diagnostic threshold. There have been many proposed methods, including a 40% cut off,44 60% cut off,45 and Select 3* or Select 6* methods.46 The goal of each method is to optimize sensitivity and specificity while not compromising efficiency of MRI review for both neurologists and radiologists.
The CVS has significant potential as a radiographic biomarker for MS and may allow the early stages of MS to be differentiated from other common causes of white matter lesions on MRI. However, it remains unclear whether CVS holds prognostic value for patients, if CVS is suggestive of differing underlying pathology, or if the presence of a CVS is dynamic over time. Progress in these areas is anticipated as CVS is incorporated into routine clinical practice.
Quantitative MRI Techniques
In the research setting, several imaging modalities can be used to quantify the degree of microstructural injury in PwMS. The goal of these methods is to identify and quantify myelin and axonal damage, the major drivers of neurodegeneration. Among these methods, diffusion-based imaging is a measure of the amount of diffusion or fluid mobility across the tissues of the brain.47 Diffusion-weighted imaging (DWI) yields several parametric maps including axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (Figure 2 A, B, and C). These parametric maps provide information on different directions of water molecules’ movements. Myelin surrounds the axons preventing water molecules diffusion perpendicular to axons (RD) while axonal content prevents water diffusion horizontal to the axons (AD).Thus, AD is considered more specific to axonal injury, whereas RD is specific to myelin content.48 A higher value of any of these metrics is associated with a higher degree of tissue injury.
Although sensitive to axonal and myelin injury, AD and RD computed from single b-shell DWI experience several limitations including being affected by nonpathologic factors such as fiber orientation, distribution, and crossing, and by various nonmyelin specific pathologies including fluid accumulation during inflammation, myelin sheath thickness, and axonal intactness.48 Several multi b-shell methods have been developed to overcome diffusion imaging limitations. For example, work at the Nashville VA MS Center of Excellence has focused on the use of the multicompartment diffusion MRI with spherical mean technique (SMT). This method removes the orientation dependency of the diffusion MRI signal, increasing the signal-to-noise ratio and reducing biases from fiber undulation, crossing, and dispersion.49 SMT generates the apparent axonal volume fraction (Vax), which is a direct measure of axonal integrity with lower values indicating lower axonal content and higher tissue destruction (Figure 2D). Vax was previously validated in MS as a measure of axonal integrity.49
In terms of myelin, several other specific measures have been developed. Magnetization transfer ratio (MTR) is another measure of tissue integrity that has been validated as a measure of tissue injury in MS (Figure 2E).50,51 Zheng et al found that the percentage of lesions with low MTR was significantly higher among patients whose disease disability progressed compared with patients who did not.52Selective inversion recovery with quantitative magnetization transfer (SIR-qMT) was developed to account for the limitations of MTR, including its sensitivity to edema and axonal density.52 Germane to myelin measurements, SIR-qMT generates the macromolecular to free size ratio (PSR). PSR represents the ratio of protons bound to macromolecules (myelin) to free protons (Figure 2F). PSR is considered a marker of myelin integrity, with lower values correlating with disability severity and indicating higher tissue damage and lower myelin content. Previous studies from the Nashville VA MS Center of Excellence validated the use of SIR-qMT among patients with MS, CIS, RIS, and healthy controls.53
Quantitative MRI has several research applications in the field of MS. We demonstrated that PRL harbor a higher degree of myelin injury indicated by PSR compared with rimless lesions.54 These MRI techniques are also helpful to investigate tissues surrounding the lesions, called normal appearing white matter (NAWM). Using quantitative MRI techniques such as MTR,52 PSR,53 and Vax,49 investigators have demonstrated that NAWM is injured in PwMS, and proximal NAWM may have higher degree of tissue damage compared with distant NAWM.55
Anticipated Innovations and Challenges
In the field of quantitative MRI, several new techniques are being adopted. Researchers are developing techniques such as myelin water fraction which evaluates the interaction between water and protons to measure myelin content. This is considered an advancement as it takes into account edema resulting from MS injury.56 Another example is multicompartment diffusion imaging, such as standard model imaging,57 and neurite orientation dispersion and density imaging,58 which considers water as an additional compartment compared with the SMT derived Vax. For PRL identification, more advanced methodologic techniques are developing such quantitative susceptibility mapping (QSM), which can detect iron deposits that surround the lesions with relatively high sensitivity and specificity of identifying PRL.59
Despite these innovations, several challenges remain before possible incorporation into the clinical setting. These limitations include longer scan time, familiarity of clinicians in using these maps, higher financial cost, and the necessity of advanced imaging processing skills. Artificial intelligence is a promising tool that may overcome these challenges through creating automated processing pipelines and developing synthetic maps without the need for additional acquisition.60
Conclusions
MRI is the most important tool for diagnosing and treating PwMS. Imaging biomarkers such as T2-lesions, cBHs, brain atrophy, PRLs, and CVS provide insight into the disease’s pathogenesis and are invaluable for the accurate diagnosis and prognostication of MS. Quantitative MRI techniques, while not available in the clinical setting, are important tools for translational research that may help direct the development of future therapeutics. In the near future, clinicians caring for PwMS should expect to encounter these imaging biomarkers more frequently in the clinical setting, especially with the inclusion of PRLs and CVS in the next iteration of the McDonald diagnostic criteria.
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121-127. doi:10.1002/ana.1032
Wattjes MP, Steenwijk MD, Stangel M. MRI in the diagnosis and monitoring of multiple sclerosis: an update. Clin Neuroradiol. 2015;25:157-165. doi:10.1007/s00062-015-0430-y
Gauthier SA, Mandel M, Guttmann CR, et al. Predicting short-term disability in multiple sclerosis. Neurology. 2007;68:2059-2065.doi:10.1212/01.wnl.0000264890.97479.b1
Rudick RA, Lee JC, Simon J, Fisher E. Significance of T2 lesions in multiple sclerosis: a 13-year longitudinal study. Ann Neurol. 2006;60:236-242. doi:10.1002/ana.20883
Nabizadeh F, Zafari R, Mohamadi M, et al. MRI features and disability in multiple sclerosis: a systematic review and meta-analysis. J Neuroradiol. 2024;51:24-37. doi:10.1016/j.neurad.2023.11.007
Bagnato F, Jeffries N, Richert ND, et al. Evolution of T1 black holes in patients with multiple sclerosis imaged monthly for 4 years. Brain. 2003;126:1782-1789. doi:10.1093/brain/awg182
Jacobsen C, Hagemeier J, Myhr KM, et al. Brain atrophy and disability progression in multiple sclerosis patients: a 10-year follow-up study. J Neurol Neurosurg Psychiatry. 2014;85:1109-1115. doi:10.1136/jnnp-2013-306906
Rovaris M, Gass A, Bammer R, et al. Diffusion MRI in multiple sclerosis. Neurology. 2005;65:1526-1532. doi:10.1212/01.wnl.0000184471.83948.e0
Fisniku LK, Chard DT, Jackson JS, et al. Gray matter atrophy is related to long-term disability in multiple sclerosis. Ann Neurol. 2008;64:247-254. doi:10.1002/ana.21423
Lublin FD, Häring DA, Ganjgahi H, et al. How patients with multiple sclerosis acquire disability. Brain. 2022;145:3147-3161. doi:10.1093/brain/awac016
Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77:1132-1140. doi:10.1001/jamaneurol.2020.1568
Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376:221-234. doi:10.1056/NEJMoa1601277
Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376:209-220. doi:10.1056/NEJMoa1606468
Prineas JW, Kwon EE, Cho ES, et al. Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol. 2001;50:646-657. doi:10.1002/ana.1255
Kuhlmann T, Ludwin S, Prat A, Antel J, Brück W, Lassmann H. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 2017;133:13-24. doi:10.1007/s00401-016-1653-y
Pitt D, Boster A, Pei W, et al. Imaging cortical lesions in multiple sclerosis with ultra-high-field magnetic resonance imaging. Arch Neurol. 2010;67:812-818. doi:10.1001/archneurol.2010.148
Gilmore CP, Geurts JJ, Evangelou N, et al. Spinal cord grey matter lesions in multiple sclerosis detected by post-mortem high field MR imaging. Mult Scler. 2009;15:180-188. doi:10.1177/1352458508096876
Lassmann H, Brück W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210-218. doi:10.1111/j.1750-3639.2007.00064.x
Bagnato F, Hametner S, Yao B, et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain. 2011;134:3602-3615. doi:10.1093/brain/awr278
Bagnato F, Sati P, Hemond CC, et al. Imaging chronic active lesions in multiple sclerosis: a consensus statement. Brain. 2024;147:2913-2933. doi:10.1093/brain/awae013
Dal-Bianco A, Grabner G, Kronnerwetter C, et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol. 2017;133:25-42. doi:10.1007/s00401-016-1636-z
Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest. 2016;126:2597-2609. doi:10.1172/JCI86198
Gillen KM, Mubarak M, Park C, et al. QSM is an imaging biomarker for chronic glial activation in multiple sclerosis lesions. Ann Clin Transl Neurol. 2021;8:877-886. doi:10.1002/acn3.51338
Ng Kee Kwong KC, Mollison D, Meijboom R, et al. The prevalence of paramagnetic rim lesions in multiple sclerosis: a systematic review and meta-analysis. PLoS One. 2021;16:e0256845. doi:10.1371/journal.pone.0256845
Absinta M, Sati P, Fechner A, et al. Identification of chronic active multiple sclerosis lesions on 3T MRI. AJNR Am J Neuroradiol. 2018;39:1233-1238. doi:10.3174/ajnr.A5660
Hemond CC, Reich DS, Dundamadappa SK. Paramagnetic rim lesions in multiple sclerosis: comparison of visualization at 1.5-T and 3-T MRI. AJR Am J Roentgenol. 2022;219:120-131. doi:10.2214/AJR.21.26777
Altokhis AI, Hibbert AM, Allen CM, et al. Longitudinal clinical study of patients with iron rim lesions in multiple sclerosis. Mult Scler. 2022;28:2202-2211. doi:10.1177/13524585221114750
Choi S, Lake S, Harrison DM. Evaluation of the blood-brain barrier, demyelination, and neurodegeneration in paramagnetic rim lesions in multiple sclerosis on 7 tesla MRI. J Magn Reson Imaging. 2024;59:941-951. doi:10.1002/jmri.28847
Kazimuddin HF, Wang J, Hernandez B, et al. Paramagnetic rim lesions and their relationship with neurodegeneration and clinical disability at the time of multiple sclerosis diagnosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Rohm Z, Koch C, Kazimuddin H, et al. Longitudinal characterization of paramagnetic rim lesions in early multiple sclerosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Meaton I, Altokhis A, Allen CM, et al. Paramagnetic rims are a promising diagnostic imaging biomarker in multiple sclerosis. Mult Scler. 2022;28:2212-2220. doi:10.1177/13524585221118677
Fog T. On the vessel-plaque relationships in the brain in multiple sclerosis. Acta Neurol Scand Suppl. 1964;40:9-15.
Ineichen BV, Okar SV, Proulx ST, et al. Perivascular spaces and their role in neuroinflammation. Neuron. 2022;110:3566-3581. doi:10.1016/j.neuron.2022.10.024
Tallantyre EC, Morgan PS, Dixon JE, et al. A comparison of 3T and 7T in the detection of small parenchymal veins within MS lesions. Invest Radiol. 2009;44:491-494. doi:10.1097/RLI.0b013e3181b4c144
Kilsdonk ID, Lopez-Soriano A, Kuijer JP, et al. Morphological features of MS lesions on FLAIR* at 7 T and their relation to patient characteristics. J Neurol. 2014;261:1356-1364. doi:10.1007/s00415-014-7351-6
Tallantyre EC, Dixon JE, Donaldson I, et al. Ultra-high-field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology. 2011;76:534-539. doi:10.1212/WNL.0b013e31820b7630
Solomon AJ, Schindler MK, Howard DB, et al. “Central vessel sign” on 3T FLAIR* MRI for the differentiation of multiple sclerosis from migraine. Ann Clin Transl Neurol. 2015;3:82-87. doi:10.1002/acn3.273
Sinnecker T, Dörr J, Pfueller CF, et al. Distinct lesion morphology at 7-T MRI differentiates neuromyelitis optica from multiple sclerosis. Neurology. 2012;79:708-714. doi:10.1212/WNL.0b013e3182648bc8
Kister I, Herbert J, Zhou Y, Ge Y. Ultrahigh-field MR (7 T) imaging of brain lesions in neuromyelitis optica. Mult Scler Int. 2013;2013:398259. doi:10.1155/2013/398259
Wuerfel J, Sinnecker T, Ringelstein EB, et al. Lesion morphology at 7 Tesla MRI differentiates Susac syndrome from multiple sclerosis. Mult Scler. 2012;18:1592-1599. doi:10.1177/1352458512441270
Massacesi L. Perivenular distribution of white matter lesions evaluated by MRI can differentiate MS lesions from inflammatory small vessel diseases. Eur J Neurol. 2016;23:86. doi:10.1212/WNL.86.16_supplement.P6.121
Sati P, Oh J, Constable RT, et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol. 2016;12:714-722. doi:10.1038/nrneurol.2016.166
Montalban X, Lebrun-Frénay C, Oh J, et al. Diagnosis of multiple sclerosis: 2024 revisions of the McDonald criteria. Lancet Neurol. 2025;24:850-865. doi:10.1016/S1474-4422(25)00270-4
Mistry N, Dixon J, Tallantyre E, et al. Central veins in brain lesions visualized with high-field magnetic resonance imaging: a pathologically specific diagnostic biomarker for inflammatory demyelination in the brain. JAMA Neurol. 2013;70:623-628. doi:10.1001/jamaneurol.2013.1405
Campion T, Smith RJP, Altmann DR, et al. FLAIR* to visualize veins in white matter lesions: a new tool for the diagnosis of multiple sclerosis? Eur Radiol. 2017;27:4257-4263. doi:10.1007/s00330-017-4822-z
Solomon AJ, Watts R, Ontaneda D, et al. Diagnostic performance of central vein sign for multiple sclerosis with a simplified three-lesion algorithm. Mult Scler. 2018;24:750-757. doi:10.1177/1352458517726383
Cercignani M, Bozzali M, Iannucci G, Comi G, Filippi M. Intra-voxel and inter-voxel coherence in patients with multiple sclerosis assessed using diffusion tensor MRI. J Neurol. 2002;249:875-883. doi:10.1007/s00415-002-0752-y
Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132-140. doi:10.1016/j.neuroimage.2005.01.028
Bagnato F, Franco G, Li H, et al. Probing axons using multi-compartmental diffusion in multiple sclerosis. Ann Clin Transl Neurol. 2019;6:1595-1605. doi:10.1002/acn3.50836
Filippi M, Cercignani M, Inglese M, et al. Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology. 2001;56:304-311. doi:10.1212/wnl.56.3.304
Bagnato F. Clinical application of magnetization transfer imaging. In: Advanced Neuro MR Techniques and Applications. Elsevier; 2022:403-417. doi:10.1016/B978-0-12-822479-3.00041-5
Zheng Y, Lee JC, Rudick R, Fisher E. Long-term magnetization transfer ratio evolution in multiple sclerosis white matter lesions. J Neuroimaging. 2018;28:191-198. doi:10.1111/jon.12480
Bagnato F, Hametner S, Franco G, et al. Selective inversion recovery quantitative magnetization transfer brain MRI at 7T: clinical and postmortem validation in multiple sclerosis. J Neuroimaging. 2018;28:380-388. doi:10.1111/jon.12511
Clarke MA, Cheek R, Hernandez B, et al. Paramagnetic rim lesions and the central vein sign: characterizing multiple sclerosis imaging markers. J Neuroimaging. 2024;34:86-94. doi:10.1111/jon.13173
Clarke MA, Lakhani DA, Wen S, et al. Perilesional neurodegenerative injury in multiple sclerosis: relation to focal lesions and impact on disability. Mult Scler Relat Disord. 2021;49:102738. doi:10.1016/j.msard.2021.102738
Laule C, Moore GRW. Myelin water imaging to detect demyelination and remyelination and its validation in pathology. Brain Pathol. 2018;28:750-764. doi:10.1111/bpa.12645
Coelho S, Baete SH, Lemberskiy G, et al. Reproducibility of the standard model of diffusion in white matter on clinical MRI systems. Neuroimage. 2022;257:119290. doi:10.1016/j.neuroimage.2022.119290
Novikov DS, Veraart J, Jelescu IO, et al. Rotationally-invariant mapping of scalar and orientational metrics of neuronal microstructure with diffusion MRI. Neuroimage. 2018;174:518-538. doi:10.1016/j.neuroimage.2018.03.006
Langkammer C, Liu T, Khalil M, et al. Quantitative susceptibility mapping in multiple sclerosis. Radiology. 2013;267:551-559. doi:10.1148/radiol.12120707
Collorone S, Coll L, Lorenzi M, et al. Artificial intelligence applied to MRI data to tackle key challenges in multiple sclerosis. Mult Scler. 2024;30:767-784. doi:10.1177/13524585241249422
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121-127. doi:10.1002/ana.1032
Wattjes MP, Steenwijk MD, Stangel M. MRI in the diagnosis and monitoring of multiple sclerosis: an update. Clin Neuroradiol. 2015;25:157-165. doi:10.1007/s00062-015-0430-y
Gauthier SA, Mandel M, Guttmann CR, et al. Predicting short-term disability in multiple sclerosis. Neurology. 2007;68:2059-2065.doi:10.1212/01.wnl.0000264890.97479.b1
Rudick RA, Lee JC, Simon J, Fisher E. Significance of T2 lesions in multiple sclerosis: a 13-year longitudinal study. Ann Neurol. 2006;60:236-242. doi:10.1002/ana.20883
Nabizadeh F, Zafari R, Mohamadi M, et al. MRI features and disability in multiple sclerosis: a systematic review and meta-analysis. J Neuroradiol. 2024;51:24-37. doi:10.1016/j.neurad.2023.11.007
Bagnato F, Jeffries N, Richert ND, et al. Evolution of T1 black holes in patients with multiple sclerosis imaged monthly for 4 years. Brain. 2003;126:1782-1789. doi:10.1093/brain/awg182
Jacobsen C, Hagemeier J, Myhr KM, et al. Brain atrophy and disability progression in multiple sclerosis patients: a 10-year follow-up study. J Neurol Neurosurg Psychiatry. 2014;85:1109-1115. doi:10.1136/jnnp-2013-306906
Rovaris M, Gass A, Bammer R, et al. Diffusion MRI in multiple sclerosis. Neurology. 2005;65:1526-1532. doi:10.1212/01.wnl.0000184471.83948.e0
Fisniku LK, Chard DT, Jackson JS, et al. Gray matter atrophy is related to long-term disability in multiple sclerosis. Ann Neurol. 2008;64:247-254. doi:10.1002/ana.21423
Lublin FD, Häring DA, Ganjgahi H, et al. How patients with multiple sclerosis acquire disability. Brain. 2022;145:3147-3161. doi:10.1093/brain/awac016
Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77:1132-1140. doi:10.1001/jamaneurol.2020.1568
Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376:221-234. doi:10.1056/NEJMoa1601277
Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376:209-220. doi:10.1056/NEJMoa1606468
Prineas JW, Kwon EE, Cho ES, et al. Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol. 2001;50:646-657. doi:10.1002/ana.1255
Kuhlmann T, Ludwin S, Prat A, Antel J, Brück W, Lassmann H. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 2017;133:13-24. doi:10.1007/s00401-016-1653-y
Pitt D, Boster A, Pei W, et al. Imaging cortical lesions in multiple sclerosis with ultra-high-field magnetic resonance imaging. Arch Neurol. 2010;67:812-818. doi:10.1001/archneurol.2010.148
Gilmore CP, Geurts JJ, Evangelou N, et al. Spinal cord grey matter lesions in multiple sclerosis detected by post-mortem high field MR imaging. Mult Scler. 2009;15:180-188. doi:10.1177/1352458508096876
Lassmann H, Brück W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210-218. doi:10.1111/j.1750-3639.2007.00064.x
Bagnato F, Hametner S, Yao B, et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain. 2011;134:3602-3615. doi:10.1093/brain/awr278
Bagnato F, Sati P, Hemond CC, et al. Imaging chronic active lesions in multiple sclerosis: a consensus statement. Brain. 2024;147:2913-2933. doi:10.1093/brain/awae013
Dal-Bianco A, Grabner G, Kronnerwetter C, et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol. 2017;133:25-42. doi:10.1007/s00401-016-1636-z
Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest. 2016;126:2597-2609. doi:10.1172/JCI86198
Gillen KM, Mubarak M, Park C, et al. QSM is an imaging biomarker for chronic glial activation in multiple sclerosis lesions. Ann Clin Transl Neurol. 2021;8:877-886. doi:10.1002/acn3.51338
Ng Kee Kwong KC, Mollison D, Meijboom R, et al. The prevalence of paramagnetic rim lesions in multiple sclerosis: a systematic review and meta-analysis. PLoS One. 2021;16:e0256845. doi:10.1371/journal.pone.0256845
Absinta M, Sati P, Fechner A, et al. Identification of chronic active multiple sclerosis lesions on 3T MRI. AJNR Am J Neuroradiol. 2018;39:1233-1238. doi:10.3174/ajnr.A5660
Hemond CC, Reich DS, Dundamadappa SK. Paramagnetic rim lesions in multiple sclerosis: comparison of visualization at 1.5-T and 3-T MRI. AJR Am J Roentgenol. 2022;219:120-131. doi:10.2214/AJR.21.26777
Altokhis AI, Hibbert AM, Allen CM, et al. Longitudinal clinical study of patients with iron rim lesions in multiple sclerosis. Mult Scler. 2022;28:2202-2211. doi:10.1177/13524585221114750
Choi S, Lake S, Harrison DM. Evaluation of the blood-brain barrier, demyelination, and neurodegeneration in paramagnetic rim lesions in multiple sclerosis on 7 tesla MRI. J Magn Reson Imaging. 2024;59:941-951. doi:10.1002/jmri.28847
Kazimuddin HF, Wang J, Hernandez B, et al. Paramagnetic rim lesions and their relationship with neurodegeneration and clinical disability at the time of multiple sclerosis diagnosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Rohm Z, Koch C, Kazimuddin H, et al. Longitudinal characterization of paramagnetic rim lesions in early multiple sclerosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Meaton I, Altokhis A, Allen CM, et al. Paramagnetic rims are a promising diagnostic imaging biomarker in multiple sclerosis. Mult Scler. 2022;28:2212-2220. doi:10.1177/13524585221118677
Fog T. On the vessel-plaque relationships in the brain in multiple sclerosis. Acta Neurol Scand Suppl. 1964;40:9-15.
Ineichen BV, Okar SV, Proulx ST, et al. Perivascular spaces and their role in neuroinflammation. Neuron. 2022;110:3566-3581. doi:10.1016/j.neuron.2022.10.024
Tallantyre EC, Morgan PS, Dixon JE, et al. A comparison of 3T and 7T in the detection of small parenchymal veins within MS lesions. Invest Radiol. 2009;44:491-494. doi:10.1097/RLI.0b013e3181b4c144
Kilsdonk ID, Lopez-Soriano A, Kuijer JP, et al. Morphological features of MS lesions on FLAIR* at 7 T and their relation to patient characteristics. J Neurol. 2014;261:1356-1364. doi:10.1007/s00415-014-7351-6
Tallantyre EC, Dixon JE, Donaldson I, et al. Ultra-high-field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology. 2011;76:534-539. doi:10.1212/WNL.0b013e31820b7630
Solomon AJ, Schindler MK, Howard DB, et al. “Central vessel sign” on 3T FLAIR* MRI for the differentiation of multiple sclerosis from migraine. Ann Clin Transl Neurol. 2015;3:82-87. doi:10.1002/acn3.273
Sinnecker T, Dörr J, Pfueller CF, et al. Distinct lesion morphology at 7-T MRI differentiates neuromyelitis optica from multiple sclerosis. Neurology. 2012;79:708-714. doi:10.1212/WNL.0b013e3182648bc8
Kister I, Herbert J, Zhou Y, Ge Y. Ultrahigh-field MR (7 T) imaging of brain lesions in neuromyelitis optica. Mult Scler Int. 2013;2013:398259. doi:10.1155/2013/398259
Wuerfel J, Sinnecker T, Ringelstein EB, et al. Lesion morphology at 7 Tesla MRI differentiates Susac syndrome from multiple sclerosis. Mult Scler. 2012;18:1592-1599. doi:10.1177/1352458512441270
Massacesi L. Perivenular distribution of white matter lesions evaluated by MRI can differentiate MS lesions from inflammatory small vessel diseases. Eur J Neurol. 2016;23:86. doi:10.1212/WNL.86.16_supplement.P6.121
Sati P, Oh J, Constable RT, et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol. 2016;12:714-722. doi:10.1038/nrneurol.2016.166
Montalban X, Lebrun-Frénay C, Oh J, et al. Diagnosis of multiple sclerosis: 2024 revisions of the McDonald criteria. Lancet Neurol. 2025;24:850-865. doi:10.1016/S1474-4422(25)00270-4
Mistry N, Dixon J, Tallantyre E, et al. Central veins in brain lesions visualized with high-field magnetic resonance imaging: a pathologically specific diagnostic biomarker for inflammatory demyelination in the brain. JAMA Neurol. 2013;70:623-628. doi:10.1001/jamaneurol.2013.1405
Campion T, Smith RJP, Altmann DR, et al. FLAIR* to visualize veins in white matter lesions: a new tool for the diagnosis of multiple sclerosis? Eur Radiol. 2017;27:4257-4263. doi:10.1007/s00330-017-4822-z
Solomon AJ, Watts R, Ontaneda D, et al. Diagnostic performance of central vein sign for multiple sclerosis with a simplified three-lesion algorithm. Mult Scler. 2018;24:750-757. doi:10.1177/1352458517726383
Cercignani M, Bozzali M, Iannucci G, Comi G, Filippi M. Intra-voxel and inter-voxel coherence in patients with multiple sclerosis assessed using diffusion tensor MRI. J Neurol. 2002;249:875-883. doi:10.1007/s00415-002-0752-y
Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132-140. doi:10.1016/j.neuroimage.2005.01.028
Bagnato F, Franco G, Li H, et al. Probing axons using multi-compartmental diffusion in multiple sclerosis. Ann Clin Transl Neurol. 2019;6:1595-1605. doi:10.1002/acn3.50836
Filippi M, Cercignani M, Inglese M, et al. Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology. 2001;56:304-311. doi:10.1212/wnl.56.3.304
Bagnato F. Clinical application of magnetization transfer imaging. In: Advanced Neuro MR Techniques and Applications. Elsevier; 2022:403-417. doi:10.1016/B978-0-12-822479-3.00041-5
Zheng Y, Lee JC, Rudick R, Fisher E. Long-term magnetization transfer ratio evolution in multiple sclerosis white matter lesions. J Neuroimaging. 2018;28:191-198. doi:10.1111/jon.12480
Bagnato F, Hametner S, Franco G, et al. Selective inversion recovery quantitative magnetization transfer brain MRI at 7T: clinical and postmortem validation in multiple sclerosis. J Neuroimaging. 2018;28:380-388. doi:10.1111/jon.12511
Clarke MA, Cheek R, Hernandez B, et al. Paramagnetic rim lesions and the central vein sign: characterizing multiple sclerosis imaging markers. J Neuroimaging. 2024;34:86-94. doi:10.1111/jon.13173
Clarke MA, Lakhani DA, Wen S, et al. Perilesional neurodegenerative injury in multiple sclerosis: relation to focal lesions and impact on disability. Mult Scler Relat Disord. 2021;49:102738. doi:10.1016/j.msard.2021.102738
Laule C, Moore GRW. Myelin water imaging to detect demyelination and remyelination and its validation in pathology. Brain Pathol. 2018;28:750-764. doi:10.1111/bpa.12645
Coelho S, Baete SH, Lemberskiy G, et al. Reproducibility of the standard model of diffusion in white matter on clinical MRI systems. Neuroimage. 2022;257:119290. doi:10.1016/j.neuroimage.2022.119290
Novikov DS, Veraart J, Jelescu IO, et al. Rotationally-invariant mapping of scalar and orientational metrics of neuronal microstructure with diffusion MRI. Neuroimage. 2018;174:518-538. doi:10.1016/j.neuroimage.2018.03.006
Langkammer C, Liu T, Khalil M, et al. Quantitative susceptibility mapping in multiple sclerosis. Radiology. 2013;267:551-559. doi:10.1148/radiol.12120707
Collorone S, Coll L, Lorenzi M, et al. Artificial intelligence applied to MRI data to tackle key challenges in multiple sclerosis. Mult Scler. 2024;30:767-784. doi:10.1177/13524585241249422
Updates in Multiple Sclerosis Imaging
Updates in Multiple Sclerosis Imaging
Harmonizing Magnetic Resonance Imaging Protocols for Veterans With Multiple Sclerosis
Multiple sclerosis (MS) is a lifelong disease that affects about a million people in the United States.1,2 Since 1998 more than 45,000 veterans have been diagnosed with MS and about 20,000 are evaluated in the Veterans Health Administration (VHA) annually.3
Magnetic resonance imaging (MRI) is a cornerstone for the assessment of persons with multiple sclerosis (pwMS).4-6 MRI assists with disease diagnosis, allowing for timely therapeutic interventions and withthe evaluation of its progression, treatment effect, and safety. 4,5 MRI-based outcomes also are used as primary endpoints in clinical trials.4,5
MS has its clinical onset in early adulthood in most individuals and is diagnosed at a mean age of 30 years.7 As a result, pwMS may receive care and MRIs in different facilities during their lifetime. Mitigating interscan variabilities that can challenge intra- and interperson comparisons is crucial for accurate care. Radiologists may find it difficult to compare scans acquired in different facilities, as dissimilarities in acquisition protocols may mask or uncover focal disease, creating false negative or false positive findings. Moreover, lack of a standardized method to report MRI changes may compromise neurologists’ ability to correctly interpret scans and disease progression.
Accordingly, in October 2019, an international task force of neurologists, radiologists, MRI technologists, and imaging scientists with expertise in MS, including representatives from the VHA, worked together to update guidelines for imaging the brain, spinal cord, and optic nerve in pwMS.8,9 Recognizing the importance of this effort, the VHA Multiple Sclerosis Centers of Excellence (MSCoE), in collaboration with a team of subject matter expert neuroradiologists promptly committed to this effort, advocating the updated consensus recommendations, and favoring their dissemination within the VHA.10
As part of this commitment and dissemination effort, in this report we summarize the core points of the newly proposed MRI guidelines and ways to adapt them for use within the VHA. We then discuss key elements for their successful implementation and dissemination, specifically regarding the clinical operations of VHA.
Updated Guidelines
MRI Scan at Different Timepoints of MS
There are 3 crucial milestones within a the lifespan of a pwMS that require an MRI to reach appropriate conclusions and avoid clinical errors. These include the initial diagnosis, the follow-up to monitor disease and/or treatment effect, and the assessment of medication safety.
In the interest of efficiency, MRI protocols may vary slightly depending on these clinical indications. The Table lists core sequences of the updated 2021 consensus recommendations at each timepoint along with the proposed alternatives or preferences from the VHA workgroup.
At the time of diagnosis, both brain and spine (cervical and thoracic) MRIs are recommended. Routine MRI of the optic nerve is considered optional at diagnosis. However, imaging the optic nerve may be useful in specific clinical scenarios when the optic nerve is selectively involved, and the diagnosis or etiology of an optic neuritis is not clear. A repeat brain MRI is advised every 6 to 12 months in patients with clinically or radiologically isolated syndrome who do not fulfill the diagnostic criteria of MS but present risk factors for conversion to MS or paraclinical features of it.
Once the diagnosis is established, brain MRI is recommended for follow-up and for surveillance of drug safety. Spinal cord and optic nerve MRIs are desirable but optional in the follow-up of pwMS and are not required for drug surveillance. Spinal cord MRIs are required at follow-up for patients whose progression cannot be explained by brain MRI features, or who manifest with recurrent spinal cord symptoms, or have spinal cord comorbidities. In these cases, spinal cord MRI also may assist with treatment decisions. Similarly, optic nerve MRI is necessary during follow-up only when optic nerve comorbidities are suspected or when there is progression or reoccurrence of optic nerve–related symptoms.
Brain MRIs are recommended for monitoring drug effect yearly (or at longer intervals, after a few years of disease stability). Conversely, a repeat brain MRI is advised after 6 months if nonsymptomatic radiological disease activity is discovered on surveillance scans.
Abbreviated but more frequent serial brain MRI protocols (eg, every 3 to 4 months) are recommended for pwMS treated with natalizumab and at high risk of developing progressive multifocal leukoencephalopathy (eg, pwMS who are John Cunningham virus [JCV]–positive, and have been treated with natalizumabfor ≥ 18 months, have a JCV antibody index > 0.9, or have a history of immunosuppression). A similar approach is recommended for carryover cases, such as those with high JCV antibody index who are switched to other immunosuppressive treatments.
MRI Field, Scan Resolution, and Coverage
Both 1.5-Tesla (1.5-T) and 3-T scans are believed to be equally effective in imaging pwMS, providing that the 1.5-T scans are good quality. Although imaging at < 1.5 T is not recommended due to suboptimal disease detection, the use of scanners > 3 T is equally discouraged outside the supervision of trained investigators. Signal-to-noise ratio and resolution are key factors impacting scan quality, and their optimization is prioritized over the number of sequences in the updated 2021 consensus recommendations. For brain imaging, a resolution of 1 mm3 isotropic is preferred for 3-dimensional (3D) imaging and slice thickness ≤ 3 mm without gap (≤ 5 mm with 10-30% gaps for diffusion-weighted imaging only) is recommended for 2D sequences. Images should cover the entire brain and as much of the cervical spine as possible; images should be prescribed axial for 2D or reformatted axial oblique for 3D using the subcallosal plane as reference. For spine imaging, sites should aim at an in-plane resolution of 1 mm2; using sagittal slices ≤ 3 mm thick and axial slices ≤ 5 mm thick, both with no gap. Scans should cover the entire cervical and thoracolumbar region inclusive of the conus. For the optic nerve images, slices should be ≤ 2 or 3 mm thick with an in-plane resolution of 1 mm2. Images should be aligned to the orientation of the optic nerve and chiasms, both of which should be entirely covered.
Postgadolinium Images Use
The discovery of the higher sensitivity of post-gadolinium (Gd) T1-weighted (T1-w) MRI relative to high iodine (88.1 g I) computed tomography scans in demonstrating contrast-enhancing MS lesions has revolutionized the way clinicians diagnose and monitor this disease.11 However, in recent years the role of postcontrast MRI has been debated, considering the potential safety concerns secondary to Gd tissue deposition. For this reason, an intentionally more judicious use of postcontrast MRI is proposed by the consensus recommendations. At disease diagnosis, the use of Gd is advisable to (1) show disease dissemination in time; (2) differentiate the diagnosis based on the Gd pattern; (3) predict short-term disease activity; and (4) characterize activity in the setting of progression. When monitoring pwMS, the use of Gd may be useful in the first year of follow-up, particularly if in the setting of low potency medications or for patients for whom the detection of one or more active lesions would lead to a change in disease-modifying agents. Gd also should be used to first, confirm a clinical exacerbation (if needed); second, further characterize a lesion suggestive of progressive multifocal encephalopathy or monitor this disease over time; and third, monitor lesion burden change in patients with large confluent lesions, the count of which otherwise may be difficult.
MRI During Pregnancy and Lactation
The consensus recommendations state that Gd contrast–enhanced MRI is not absolutely contraindicated during pregnancy, although its use should be limited to strictly necessary situations, particularly those involving differential diagnosis, such as cerebral venous thrombosis or monitoring of possibly enlarging lesion burden. The use of Gd is not contraindicated during lactation, as only a small proportion (< 0.4%) passes into the breast milk, leading to an exposure to < 1% of the permitted Gd dose for neonates.12,13
Harmonizing MRI Reports
The consensus recommendations propose reporting the exact lesion count on T2-weighted (T2-w) images when lesions are < 20, or specifying if the number of T2 lesions is between 20 and 50, between 50 and 100, or uncountable, eg, confluent large lesions. Similarly, for the spinal cord, the consensus recommendations propose reporting the exact lesion count on T2-w images when lesions are < 10, or otherwise report that > 10 lesions are seen.
The VHA workgroup proposed reporting a mild, moderate, or severe T2-lesion burden for a T2-lesion count < 20, between 20 and 50, and > 50, respectively. For follow-up MRIs, notation should be made if there is any change in lesion number, indicating the number of new lesions whenever possible. At each timepoint, the presence of active lesions on postcontrast images should be accurately defined.
Dissemination and Implementation
To implement and disseminate these proposed recommendations within the VHA, a workgroup of neurologists and radiologists was formed in late 2020. A review and discussion of the importance of each of the proposed MRI protocols for veterans with MS was held along with possible modifications to balance the intent of meeting standards of care with resources of individual US Department of Veterans Affairs (VA) medical centers and veterans’ needs. The final protocol recommendations were agreed on by group consensus.
In general, this VHA workgroup felt that the current adopted MRI protocols in several VA medical centers (based on previously proposed recommendations) were similar to the ones newly proposed and that implementing changes to meet the 2021 criteria would not be a major challenge.14,15 Possible regional and nonregional barriers were discussed. The result of these discussions led to a modified version of what could be considered more stringent guidelines to accommodate medical centers that had fewer imaging resources. This modified protocol offers a viable alternative that allows for minimizing heterogeneities while recognizing the capabilities of the available scanner fleet and meeting the needs of specific centers or veterans. Finally, the workgroup recognized a fundamental obstacle toward this harmonization process in the heterogeneity in vendors and scanner field strength, factors that have previously limited implementation.
The guidelines and proposed changes were then presented to the VA National Radiology Program Office, examined, and discussed for consensus. No changes were felt to be needed, and the recommendation to implement these guidelines in MS regional programs, whenever possible, was deemed appropriate.
At this time, a focused communication plan has been implemented to diffuse the use of this protocol at MS regional programs in the MSCoE network. We will work iteratively with individual sites to practically apply the guidelines, learn about challenges, and work through them to optimize local implementation.
Conclusions
Standardized MRI protocols are fundamental for the care of veterans with MS. Mitigating interscan variabilities should be recognized as a priority by scientific and clinical expert committees. Several guidelines have been developed over the years to standardize MRI acquisition protocols and interpretations, while updating the same to the latest discoveries.4,5,8,14,15 The VHA has been historically committed to these international efforts, with the goal to excel in the care of veterans with MS by providing access to state-of-the-art technologies. To this end, the initial Consortium of MS Centers MRI protocol was implemented in several MSCoE VA Regional Program sites a decade ago.14 Efforts continue to update protocol recommendations as needed and to promote their dissemination across the VHA enterprise.
This commentary is part of the continuous effort of the MSCoE to align with contemporary guidelines, apply the highest scientific standards, and achieve consistent outcomes for veterans with MS. For more important details of the clinical scenarios when additional/optional sequences or scans can be acquired, we advise the reader to refer to the 2021 MAGNIMS-CMSC-NAIMS Consensus Recommendations on the Use of MRI in Patients With Multiple Sclerosis.8
1. Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: A population-based estimate using health claims data. Neurology. 2019;92(10):e1029-e1040. doi:10.1212/WNL.0000000000007035
2. Nelson LM, Wallin MT, Marrie RA, et al. A new way to estimate neurologic disease prevalence in the United States: Illustrated with MS. Neurology. 2019;92(10):469-480. doi:10.1212/WNL.0000000000007044
3. Culpepper WJ, Wallin MT, Magder LS, et al. VHA Multiple Sclerosis Surveillance Registry and its similarities to other contemporary multiple sclerosis cohorts. J Rehabil Res Dev. 2015;52(3):263-272. doi:10.1682/JRRD.2014.07.0172
4. Wattjes MP, Rovira À, Miller D, et al. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis--establishing disease prognosis and monitoring patients. Nat Rev Neurol. 2015;11(10):597-606. doi:10.1038/nrneurol.2015.157
5. Rovira À, Wattjes MP, Tintoré M, et al. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis-clinical implementation in the diagnostic process. Nat Rev Neurol. 2015;11(8):471-482. doi:10.1038/nrneurol.2015.106
6. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173. doi:10.1016/S1474-4422(17)30470-2
7. Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. 2018;378(2):169-180. doi:10.1056/NEJMra1401483
8. Wattjes MP, Ciccarelli O, Reich DS, et al. 2021 MAGNIMS-CMSC-NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol. 2021;20(8):653-670. doi:10.1016/S1474-4422(21)00095-8
9. Saslow L, Li DKB, Halper J, et al. An International Standardized Magnetic Resonance Imaging Protocol for Diagnosis and Follow-up of Patients with Multiple Sclerosis: Advocacy, Dissemination, and Implementation Strategies. Int J MS Care. 2020;22(5):226-232. doi:10.7224/1537-2073.2020-094
10. Cameron MH, Haselkorn JK, Wallin MT. The Multiple Sclerosis Centers of Excellence: a model of excellence in the VA. Fed Pract. 2020;37(suppl 1):S6-S10.
11. Grossman RI, Gonzalez-Scarano F, Atlas SW, Galetta S, Silberberg DH. Multiple sclerosis: gadolinium enhancement in MR imaging. Radiology. 1986;161(3):721-725. doi:10.1148/radiology.161.3.3786722
12. European Society of Urogenital Radiology. ESUR guidelines on contrast agent, 10.0. March 2018. Accessed March 11, 2022. https://www.esur.org/fileadmin/content/2019/ESUR_Guidelines_10.0_Final_Version.pdf
13. Sundgren PC, Leander P. Is administration of gadolinium-based contrast media to pregnant women and small children justified?. J Magn Reson Imaging. 2011;34(4):750-757. doi:10.1002/jmri.22413
14. Simon JH, Li D, Traboulsee A, et al. Standardized MR imaging protocol for multiple sclerosis: Consortium of MS Centers consensus guidelines. AJNR Am J Neuroradiol. 2006;27(2):455-461.
15. Traboulsee A, Simon JH, Stone L, et al. Revised Recommendations of the Consortium of MS Centers Task Force for a Standardized MRI Protocol and Clinical Guidelines for the Diagnosis and Follow-Up of Multiple Sclerosis. AJNR Am J Neuroradiol. 2016;37(3):394-401. doi:10.3174/ajnr.A4539
Multiple sclerosis (MS) is a lifelong disease that affects about a million people in the United States.1,2 Since 1998 more than 45,000 veterans have been diagnosed with MS and about 20,000 are evaluated in the Veterans Health Administration (VHA) annually.3
Magnetic resonance imaging (MRI) is a cornerstone for the assessment of persons with multiple sclerosis (pwMS).4-6 MRI assists with disease diagnosis, allowing for timely therapeutic interventions and withthe evaluation of its progression, treatment effect, and safety. 4,5 MRI-based outcomes also are used as primary endpoints in clinical trials.4,5
MS has its clinical onset in early adulthood in most individuals and is diagnosed at a mean age of 30 years.7 As a result, pwMS may receive care and MRIs in different facilities during their lifetime. Mitigating interscan variabilities that can challenge intra- and interperson comparisons is crucial for accurate care. Radiologists may find it difficult to compare scans acquired in different facilities, as dissimilarities in acquisition protocols may mask or uncover focal disease, creating false negative or false positive findings. Moreover, lack of a standardized method to report MRI changes may compromise neurologists’ ability to correctly interpret scans and disease progression.
Accordingly, in October 2019, an international task force of neurologists, radiologists, MRI technologists, and imaging scientists with expertise in MS, including representatives from the VHA, worked together to update guidelines for imaging the brain, spinal cord, and optic nerve in pwMS.8,9 Recognizing the importance of this effort, the VHA Multiple Sclerosis Centers of Excellence (MSCoE), in collaboration with a team of subject matter expert neuroradiologists promptly committed to this effort, advocating the updated consensus recommendations, and favoring their dissemination within the VHA.10
As part of this commitment and dissemination effort, in this report we summarize the core points of the newly proposed MRI guidelines and ways to adapt them for use within the VHA. We then discuss key elements for their successful implementation and dissemination, specifically regarding the clinical operations of VHA.
Updated Guidelines
MRI Scan at Different Timepoints of MS
There are 3 crucial milestones within a the lifespan of a pwMS that require an MRI to reach appropriate conclusions and avoid clinical errors. These include the initial diagnosis, the follow-up to monitor disease and/or treatment effect, and the assessment of medication safety.
In the interest of efficiency, MRI protocols may vary slightly depending on these clinical indications. The Table lists core sequences of the updated 2021 consensus recommendations at each timepoint along with the proposed alternatives or preferences from the VHA workgroup.
At the time of diagnosis, both brain and spine (cervical and thoracic) MRIs are recommended. Routine MRI of the optic nerve is considered optional at diagnosis. However, imaging the optic nerve may be useful in specific clinical scenarios when the optic nerve is selectively involved, and the diagnosis or etiology of an optic neuritis is not clear. A repeat brain MRI is advised every 6 to 12 months in patients with clinically or radiologically isolated syndrome who do not fulfill the diagnostic criteria of MS but present risk factors for conversion to MS or paraclinical features of it.
Once the diagnosis is established, brain MRI is recommended for follow-up and for surveillance of drug safety. Spinal cord and optic nerve MRIs are desirable but optional in the follow-up of pwMS and are not required for drug surveillance. Spinal cord MRIs are required at follow-up for patients whose progression cannot be explained by brain MRI features, or who manifest with recurrent spinal cord symptoms, or have spinal cord comorbidities. In these cases, spinal cord MRI also may assist with treatment decisions. Similarly, optic nerve MRI is necessary during follow-up only when optic nerve comorbidities are suspected or when there is progression or reoccurrence of optic nerve–related symptoms.
Brain MRIs are recommended for monitoring drug effect yearly (or at longer intervals, after a few years of disease stability). Conversely, a repeat brain MRI is advised after 6 months if nonsymptomatic radiological disease activity is discovered on surveillance scans.
Abbreviated but more frequent serial brain MRI protocols (eg, every 3 to 4 months) are recommended for pwMS treated with natalizumab and at high risk of developing progressive multifocal leukoencephalopathy (eg, pwMS who are John Cunningham virus [JCV]–positive, and have been treated with natalizumabfor ≥ 18 months, have a JCV antibody index > 0.9, or have a history of immunosuppression). A similar approach is recommended for carryover cases, such as those with high JCV antibody index who are switched to other immunosuppressive treatments.
MRI Field, Scan Resolution, and Coverage
Both 1.5-Tesla (1.5-T) and 3-T scans are believed to be equally effective in imaging pwMS, providing that the 1.5-T scans are good quality. Although imaging at < 1.5 T is not recommended due to suboptimal disease detection, the use of scanners > 3 T is equally discouraged outside the supervision of trained investigators. Signal-to-noise ratio and resolution are key factors impacting scan quality, and their optimization is prioritized over the number of sequences in the updated 2021 consensus recommendations. For brain imaging, a resolution of 1 mm3 isotropic is preferred for 3-dimensional (3D) imaging and slice thickness ≤ 3 mm without gap (≤ 5 mm with 10-30% gaps for diffusion-weighted imaging only) is recommended for 2D sequences. Images should cover the entire brain and as much of the cervical spine as possible; images should be prescribed axial for 2D or reformatted axial oblique for 3D using the subcallosal plane as reference. For spine imaging, sites should aim at an in-plane resolution of 1 mm2; using sagittal slices ≤ 3 mm thick and axial slices ≤ 5 mm thick, both with no gap. Scans should cover the entire cervical and thoracolumbar region inclusive of the conus. For the optic nerve images, slices should be ≤ 2 or 3 mm thick with an in-plane resolution of 1 mm2. Images should be aligned to the orientation of the optic nerve and chiasms, both of which should be entirely covered.
Postgadolinium Images Use
The discovery of the higher sensitivity of post-gadolinium (Gd) T1-weighted (T1-w) MRI relative to high iodine (88.1 g I) computed tomography scans in demonstrating contrast-enhancing MS lesions has revolutionized the way clinicians diagnose and monitor this disease.11 However, in recent years the role of postcontrast MRI has been debated, considering the potential safety concerns secondary to Gd tissue deposition. For this reason, an intentionally more judicious use of postcontrast MRI is proposed by the consensus recommendations. At disease diagnosis, the use of Gd is advisable to (1) show disease dissemination in time; (2) differentiate the diagnosis based on the Gd pattern; (3) predict short-term disease activity; and (4) characterize activity in the setting of progression. When monitoring pwMS, the use of Gd may be useful in the first year of follow-up, particularly if in the setting of low potency medications or for patients for whom the detection of one or more active lesions would lead to a change in disease-modifying agents. Gd also should be used to first, confirm a clinical exacerbation (if needed); second, further characterize a lesion suggestive of progressive multifocal encephalopathy or monitor this disease over time; and third, monitor lesion burden change in patients with large confluent lesions, the count of which otherwise may be difficult.
MRI During Pregnancy and Lactation
The consensus recommendations state that Gd contrast–enhanced MRI is not absolutely contraindicated during pregnancy, although its use should be limited to strictly necessary situations, particularly those involving differential diagnosis, such as cerebral venous thrombosis or monitoring of possibly enlarging lesion burden. The use of Gd is not contraindicated during lactation, as only a small proportion (< 0.4%) passes into the breast milk, leading to an exposure to < 1% of the permitted Gd dose for neonates.12,13
Harmonizing MRI Reports
The consensus recommendations propose reporting the exact lesion count on T2-weighted (T2-w) images when lesions are < 20, or specifying if the number of T2 lesions is between 20 and 50, between 50 and 100, or uncountable, eg, confluent large lesions. Similarly, for the spinal cord, the consensus recommendations propose reporting the exact lesion count on T2-w images when lesions are < 10, or otherwise report that > 10 lesions are seen.
The VHA workgroup proposed reporting a mild, moderate, or severe T2-lesion burden for a T2-lesion count < 20, between 20 and 50, and > 50, respectively. For follow-up MRIs, notation should be made if there is any change in lesion number, indicating the number of new lesions whenever possible. At each timepoint, the presence of active lesions on postcontrast images should be accurately defined.
Dissemination and Implementation
To implement and disseminate these proposed recommendations within the VHA, a workgroup of neurologists and radiologists was formed in late 2020. A review and discussion of the importance of each of the proposed MRI protocols for veterans with MS was held along with possible modifications to balance the intent of meeting standards of care with resources of individual US Department of Veterans Affairs (VA) medical centers and veterans’ needs. The final protocol recommendations were agreed on by group consensus.
In general, this VHA workgroup felt that the current adopted MRI protocols in several VA medical centers (based on previously proposed recommendations) were similar to the ones newly proposed and that implementing changes to meet the 2021 criteria would not be a major challenge.14,15 Possible regional and nonregional barriers were discussed. The result of these discussions led to a modified version of what could be considered more stringent guidelines to accommodate medical centers that had fewer imaging resources. This modified protocol offers a viable alternative that allows for minimizing heterogeneities while recognizing the capabilities of the available scanner fleet and meeting the needs of specific centers or veterans. Finally, the workgroup recognized a fundamental obstacle toward this harmonization process in the heterogeneity in vendors and scanner field strength, factors that have previously limited implementation.
The guidelines and proposed changes were then presented to the VA National Radiology Program Office, examined, and discussed for consensus. No changes were felt to be needed, and the recommendation to implement these guidelines in MS regional programs, whenever possible, was deemed appropriate.
At this time, a focused communication plan has been implemented to diffuse the use of this protocol at MS regional programs in the MSCoE network. We will work iteratively with individual sites to practically apply the guidelines, learn about challenges, and work through them to optimize local implementation.
Conclusions
Standardized MRI protocols are fundamental for the care of veterans with MS. Mitigating interscan variabilities should be recognized as a priority by scientific and clinical expert committees. Several guidelines have been developed over the years to standardize MRI acquisition protocols and interpretations, while updating the same to the latest discoveries.4,5,8,14,15 The VHA has been historically committed to these international efforts, with the goal to excel in the care of veterans with MS by providing access to state-of-the-art technologies. To this end, the initial Consortium of MS Centers MRI protocol was implemented in several MSCoE VA Regional Program sites a decade ago.14 Efforts continue to update protocol recommendations as needed and to promote their dissemination across the VHA enterprise.
This commentary is part of the continuous effort of the MSCoE to align with contemporary guidelines, apply the highest scientific standards, and achieve consistent outcomes for veterans with MS. For more important details of the clinical scenarios when additional/optional sequences or scans can be acquired, we advise the reader to refer to the 2021 MAGNIMS-CMSC-NAIMS Consensus Recommendations on the Use of MRI in Patients With Multiple Sclerosis.8
Multiple sclerosis (MS) is a lifelong disease that affects about a million people in the United States.1,2 Since 1998 more than 45,000 veterans have been diagnosed with MS and about 20,000 are evaluated in the Veterans Health Administration (VHA) annually.3
Magnetic resonance imaging (MRI) is a cornerstone for the assessment of persons with multiple sclerosis (pwMS).4-6 MRI assists with disease diagnosis, allowing for timely therapeutic interventions and withthe evaluation of its progression, treatment effect, and safety. 4,5 MRI-based outcomes also are used as primary endpoints in clinical trials.4,5
MS has its clinical onset in early adulthood in most individuals and is diagnosed at a mean age of 30 years.7 As a result, pwMS may receive care and MRIs in different facilities during their lifetime. Mitigating interscan variabilities that can challenge intra- and interperson comparisons is crucial for accurate care. Radiologists may find it difficult to compare scans acquired in different facilities, as dissimilarities in acquisition protocols may mask or uncover focal disease, creating false negative or false positive findings. Moreover, lack of a standardized method to report MRI changes may compromise neurologists’ ability to correctly interpret scans and disease progression.
Accordingly, in October 2019, an international task force of neurologists, radiologists, MRI technologists, and imaging scientists with expertise in MS, including representatives from the VHA, worked together to update guidelines for imaging the brain, spinal cord, and optic nerve in pwMS.8,9 Recognizing the importance of this effort, the VHA Multiple Sclerosis Centers of Excellence (MSCoE), in collaboration with a team of subject matter expert neuroradiologists promptly committed to this effort, advocating the updated consensus recommendations, and favoring their dissemination within the VHA.10
As part of this commitment and dissemination effort, in this report we summarize the core points of the newly proposed MRI guidelines and ways to adapt them for use within the VHA. We then discuss key elements for their successful implementation and dissemination, specifically regarding the clinical operations of VHA.
Updated Guidelines
MRI Scan at Different Timepoints of MS
There are 3 crucial milestones within a the lifespan of a pwMS that require an MRI to reach appropriate conclusions and avoid clinical errors. These include the initial diagnosis, the follow-up to monitor disease and/or treatment effect, and the assessment of medication safety.
In the interest of efficiency, MRI protocols may vary slightly depending on these clinical indications. The Table lists core sequences of the updated 2021 consensus recommendations at each timepoint along with the proposed alternatives or preferences from the VHA workgroup.
At the time of diagnosis, both brain and spine (cervical and thoracic) MRIs are recommended. Routine MRI of the optic nerve is considered optional at diagnosis. However, imaging the optic nerve may be useful in specific clinical scenarios when the optic nerve is selectively involved, and the diagnosis or etiology of an optic neuritis is not clear. A repeat brain MRI is advised every 6 to 12 months in patients with clinically or radiologically isolated syndrome who do not fulfill the diagnostic criteria of MS but present risk factors for conversion to MS or paraclinical features of it.
Once the diagnosis is established, brain MRI is recommended for follow-up and for surveillance of drug safety. Spinal cord and optic nerve MRIs are desirable but optional in the follow-up of pwMS and are not required for drug surveillance. Spinal cord MRIs are required at follow-up for patients whose progression cannot be explained by brain MRI features, or who manifest with recurrent spinal cord symptoms, or have spinal cord comorbidities. In these cases, spinal cord MRI also may assist with treatment decisions. Similarly, optic nerve MRI is necessary during follow-up only when optic nerve comorbidities are suspected or when there is progression or reoccurrence of optic nerve–related symptoms.
Brain MRIs are recommended for monitoring drug effect yearly (or at longer intervals, after a few years of disease stability). Conversely, a repeat brain MRI is advised after 6 months if nonsymptomatic radiological disease activity is discovered on surveillance scans.
Abbreviated but more frequent serial brain MRI protocols (eg, every 3 to 4 months) are recommended for pwMS treated with natalizumab and at high risk of developing progressive multifocal leukoencephalopathy (eg, pwMS who are John Cunningham virus [JCV]–positive, and have been treated with natalizumabfor ≥ 18 months, have a JCV antibody index > 0.9, or have a history of immunosuppression). A similar approach is recommended for carryover cases, such as those with high JCV antibody index who are switched to other immunosuppressive treatments.
MRI Field, Scan Resolution, and Coverage
Both 1.5-Tesla (1.5-T) and 3-T scans are believed to be equally effective in imaging pwMS, providing that the 1.5-T scans are good quality. Although imaging at < 1.5 T is not recommended due to suboptimal disease detection, the use of scanners > 3 T is equally discouraged outside the supervision of trained investigators. Signal-to-noise ratio and resolution are key factors impacting scan quality, and their optimization is prioritized over the number of sequences in the updated 2021 consensus recommendations. For brain imaging, a resolution of 1 mm3 isotropic is preferred for 3-dimensional (3D) imaging and slice thickness ≤ 3 mm without gap (≤ 5 mm with 10-30% gaps for diffusion-weighted imaging only) is recommended for 2D sequences. Images should cover the entire brain and as much of the cervical spine as possible; images should be prescribed axial for 2D or reformatted axial oblique for 3D using the subcallosal plane as reference. For spine imaging, sites should aim at an in-plane resolution of 1 mm2; using sagittal slices ≤ 3 mm thick and axial slices ≤ 5 mm thick, both with no gap. Scans should cover the entire cervical and thoracolumbar region inclusive of the conus. For the optic nerve images, slices should be ≤ 2 or 3 mm thick with an in-plane resolution of 1 mm2. Images should be aligned to the orientation of the optic nerve and chiasms, both of which should be entirely covered.
Postgadolinium Images Use
The discovery of the higher sensitivity of post-gadolinium (Gd) T1-weighted (T1-w) MRI relative to high iodine (88.1 g I) computed tomography scans in demonstrating contrast-enhancing MS lesions has revolutionized the way clinicians diagnose and monitor this disease.11 However, in recent years the role of postcontrast MRI has been debated, considering the potential safety concerns secondary to Gd tissue deposition. For this reason, an intentionally more judicious use of postcontrast MRI is proposed by the consensus recommendations. At disease diagnosis, the use of Gd is advisable to (1) show disease dissemination in time; (2) differentiate the diagnosis based on the Gd pattern; (3) predict short-term disease activity; and (4) characterize activity in the setting of progression. When monitoring pwMS, the use of Gd may be useful in the first year of follow-up, particularly if in the setting of low potency medications or for patients for whom the detection of one or more active lesions would lead to a change in disease-modifying agents. Gd also should be used to first, confirm a clinical exacerbation (if needed); second, further characterize a lesion suggestive of progressive multifocal encephalopathy or monitor this disease over time; and third, monitor lesion burden change in patients with large confluent lesions, the count of which otherwise may be difficult.
MRI During Pregnancy and Lactation
The consensus recommendations state that Gd contrast–enhanced MRI is not absolutely contraindicated during pregnancy, although its use should be limited to strictly necessary situations, particularly those involving differential diagnosis, such as cerebral venous thrombosis or monitoring of possibly enlarging lesion burden. The use of Gd is not contraindicated during lactation, as only a small proportion (< 0.4%) passes into the breast milk, leading to an exposure to < 1% of the permitted Gd dose for neonates.12,13
Harmonizing MRI Reports
The consensus recommendations propose reporting the exact lesion count on T2-weighted (T2-w) images when lesions are < 20, or specifying if the number of T2 lesions is between 20 and 50, between 50 and 100, or uncountable, eg, confluent large lesions. Similarly, for the spinal cord, the consensus recommendations propose reporting the exact lesion count on T2-w images when lesions are < 10, or otherwise report that > 10 lesions are seen.
The VHA workgroup proposed reporting a mild, moderate, or severe T2-lesion burden for a T2-lesion count < 20, between 20 and 50, and > 50, respectively. For follow-up MRIs, notation should be made if there is any change in lesion number, indicating the number of new lesions whenever possible. At each timepoint, the presence of active lesions on postcontrast images should be accurately defined.
Dissemination and Implementation
To implement and disseminate these proposed recommendations within the VHA, a workgroup of neurologists and radiologists was formed in late 2020. A review and discussion of the importance of each of the proposed MRI protocols for veterans with MS was held along with possible modifications to balance the intent of meeting standards of care with resources of individual US Department of Veterans Affairs (VA) medical centers and veterans’ needs. The final protocol recommendations were agreed on by group consensus.
In general, this VHA workgroup felt that the current adopted MRI protocols in several VA medical centers (based on previously proposed recommendations) were similar to the ones newly proposed and that implementing changes to meet the 2021 criteria would not be a major challenge.14,15 Possible regional and nonregional barriers were discussed. The result of these discussions led to a modified version of what could be considered more stringent guidelines to accommodate medical centers that had fewer imaging resources. This modified protocol offers a viable alternative that allows for minimizing heterogeneities while recognizing the capabilities of the available scanner fleet and meeting the needs of specific centers or veterans. Finally, the workgroup recognized a fundamental obstacle toward this harmonization process in the heterogeneity in vendors and scanner field strength, factors that have previously limited implementation.
The guidelines and proposed changes were then presented to the VA National Radiology Program Office, examined, and discussed for consensus. No changes were felt to be needed, and the recommendation to implement these guidelines in MS regional programs, whenever possible, was deemed appropriate.
At this time, a focused communication plan has been implemented to diffuse the use of this protocol at MS regional programs in the MSCoE network. We will work iteratively with individual sites to practically apply the guidelines, learn about challenges, and work through them to optimize local implementation.
Conclusions
Standardized MRI protocols are fundamental for the care of veterans with MS. Mitigating interscan variabilities should be recognized as a priority by scientific and clinical expert committees. Several guidelines have been developed over the years to standardize MRI acquisition protocols and interpretations, while updating the same to the latest discoveries.4,5,8,14,15 The VHA has been historically committed to these international efforts, with the goal to excel in the care of veterans with MS by providing access to state-of-the-art technologies. To this end, the initial Consortium of MS Centers MRI protocol was implemented in several MSCoE VA Regional Program sites a decade ago.14 Efforts continue to update protocol recommendations as needed and to promote their dissemination across the VHA enterprise.
This commentary is part of the continuous effort of the MSCoE to align with contemporary guidelines, apply the highest scientific standards, and achieve consistent outcomes for veterans with MS. For more important details of the clinical scenarios when additional/optional sequences or scans can be acquired, we advise the reader to refer to the 2021 MAGNIMS-CMSC-NAIMS Consensus Recommendations on the Use of MRI in Patients With Multiple Sclerosis.8
1. Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: A population-based estimate using health claims data. Neurology. 2019;92(10):e1029-e1040. doi:10.1212/WNL.0000000000007035
2. Nelson LM, Wallin MT, Marrie RA, et al. A new way to estimate neurologic disease prevalence in the United States: Illustrated with MS. Neurology. 2019;92(10):469-480. doi:10.1212/WNL.0000000000007044
3. Culpepper WJ, Wallin MT, Magder LS, et al. VHA Multiple Sclerosis Surveillance Registry and its similarities to other contemporary multiple sclerosis cohorts. J Rehabil Res Dev. 2015;52(3):263-272. doi:10.1682/JRRD.2014.07.0172
4. Wattjes MP, Rovira À, Miller D, et al. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis--establishing disease prognosis and monitoring patients. Nat Rev Neurol. 2015;11(10):597-606. doi:10.1038/nrneurol.2015.157
5. Rovira À, Wattjes MP, Tintoré M, et al. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis-clinical implementation in the diagnostic process. Nat Rev Neurol. 2015;11(8):471-482. doi:10.1038/nrneurol.2015.106
6. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173. doi:10.1016/S1474-4422(17)30470-2
7. Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. 2018;378(2):169-180. doi:10.1056/NEJMra1401483
8. Wattjes MP, Ciccarelli O, Reich DS, et al. 2021 MAGNIMS-CMSC-NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol. 2021;20(8):653-670. doi:10.1016/S1474-4422(21)00095-8
9. Saslow L, Li DKB, Halper J, et al. An International Standardized Magnetic Resonance Imaging Protocol for Diagnosis and Follow-up of Patients with Multiple Sclerosis: Advocacy, Dissemination, and Implementation Strategies. Int J MS Care. 2020;22(5):226-232. doi:10.7224/1537-2073.2020-094
10. Cameron MH, Haselkorn JK, Wallin MT. The Multiple Sclerosis Centers of Excellence: a model of excellence in the VA. Fed Pract. 2020;37(suppl 1):S6-S10.
11. Grossman RI, Gonzalez-Scarano F, Atlas SW, Galetta S, Silberberg DH. Multiple sclerosis: gadolinium enhancement in MR imaging. Radiology. 1986;161(3):721-725. doi:10.1148/radiology.161.3.3786722
12. European Society of Urogenital Radiology. ESUR guidelines on contrast agent, 10.0. March 2018. Accessed March 11, 2022. https://www.esur.org/fileadmin/content/2019/ESUR_Guidelines_10.0_Final_Version.pdf
13. Sundgren PC, Leander P. Is administration of gadolinium-based contrast media to pregnant women and small children justified?. J Magn Reson Imaging. 2011;34(4):750-757. doi:10.1002/jmri.22413
14. Simon JH, Li D, Traboulsee A, et al. Standardized MR imaging protocol for multiple sclerosis: Consortium of MS Centers consensus guidelines. AJNR Am J Neuroradiol. 2006;27(2):455-461.
15. Traboulsee A, Simon JH, Stone L, et al. Revised Recommendations of the Consortium of MS Centers Task Force for a Standardized MRI Protocol and Clinical Guidelines for the Diagnosis and Follow-Up of Multiple Sclerosis. AJNR Am J Neuroradiol. 2016;37(3):394-401. doi:10.3174/ajnr.A4539
1. Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: A population-based estimate using health claims data. Neurology. 2019;92(10):e1029-e1040. doi:10.1212/WNL.0000000000007035
2. Nelson LM, Wallin MT, Marrie RA, et al. A new way to estimate neurologic disease prevalence in the United States: Illustrated with MS. Neurology. 2019;92(10):469-480. doi:10.1212/WNL.0000000000007044
3. Culpepper WJ, Wallin MT, Magder LS, et al. VHA Multiple Sclerosis Surveillance Registry and its similarities to other contemporary multiple sclerosis cohorts. J Rehabil Res Dev. 2015;52(3):263-272. doi:10.1682/JRRD.2014.07.0172
4. Wattjes MP, Rovira À, Miller D, et al. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis--establishing disease prognosis and monitoring patients. Nat Rev Neurol. 2015;11(10):597-606. doi:10.1038/nrneurol.2015.157
5. Rovira À, Wattjes MP, Tintoré M, et al. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis-clinical implementation in the diagnostic process. Nat Rev Neurol. 2015;11(8):471-482. doi:10.1038/nrneurol.2015.106
6. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173. doi:10.1016/S1474-4422(17)30470-2
7. Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. 2018;378(2):169-180. doi:10.1056/NEJMra1401483
8. Wattjes MP, Ciccarelli O, Reich DS, et al. 2021 MAGNIMS-CMSC-NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol. 2021;20(8):653-670. doi:10.1016/S1474-4422(21)00095-8
9. Saslow L, Li DKB, Halper J, et al. An International Standardized Magnetic Resonance Imaging Protocol for Diagnosis and Follow-up of Patients with Multiple Sclerosis: Advocacy, Dissemination, and Implementation Strategies. Int J MS Care. 2020;22(5):226-232. doi:10.7224/1537-2073.2020-094
10. Cameron MH, Haselkorn JK, Wallin MT. The Multiple Sclerosis Centers of Excellence: a model of excellence in the VA. Fed Pract. 2020;37(suppl 1):S6-S10.
11. Grossman RI, Gonzalez-Scarano F, Atlas SW, Galetta S, Silberberg DH. Multiple sclerosis: gadolinium enhancement in MR imaging. Radiology. 1986;161(3):721-725. doi:10.1148/radiology.161.3.3786722
12. European Society of Urogenital Radiology. ESUR guidelines on contrast agent, 10.0. March 2018. Accessed March 11, 2022. https://www.esur.org/fileadmin/content/2019/ESUR_Guidelines_10.0_Final_Version.pdf
13. Sundgren PC, Leander P. Is administration of gadolinium-based contrast media to pregnant women and small children justified?. J Magn Reson Imaging. 2011;34(4):750-757. doi:10.1002/jmri.22413
14. Simon JH, Li D, Traboulsee A, et al. Standardized MR imaging protocol for multiple sclerosis: Consortium of MS Centers consensus guidelines. AJNR Am J Neuroradiol. 2006;27(2):455-461.
15. Traboulsee A, Simon JH, Stone L, et al. Revised Recommendations of the Consortium of MS Centers Task Force for a Standardized MRI Protocol and Clinical Guidelines for the Diagnosis and Follow-Up of Multiple Sclerosis. AJNR Am J Neuroradiol. 2016;37(3):394-401. doi:10.3174/ajnr.A4539
Impact of the COVID-19 Pandemic on Multiple Sclerosis Care for Veterans
The following is a lightly edited transcript of a teleconference recorded in February 2021.
How has COVID impacted Veterans with multiple sclerosis?
Mitchell Wallin, MD, MPH: There has been a lot of concern in the multiple sclerosis (MS) patient community about getting infected with COVID-19 and what to do about it. Now that there are vaccines, the concern is whether and how to take a vaccine. At least here, in the Washington DC/Baltimore area where I practice, we have seen many veterans being hospitalized with COVID-19, some with multiple sclerosis (MS), and some who have died of COVID-19. So, there has been a lot of fear, especially in veterans that are older with comorbid diseases.
Rebecca Spain, MD, MSPH: There also has been an impact on our ability to provide care to our veterans with MS. There are challenges having them come into the office or providing virtual care. There are additional challenges and concerns this year about making changes in MS medications because we can’t see patients in person to or understand their needs or current status of their MS. So, providing care has been a challenge this year as well.
There has also been an impact on our day to day lives, like there has been for all of us, from the lockdown particularly not being able to exercise and socialize as much. There have been physical and social and emotional tolls that this disease has taken on veterans with MS.
Jodie Haselkorn, MD, MPH: The survivors of COVID-19, that are transferred to an inpatient multidisciplinary rehabilitation program unit to address impairments related to the cardiopulmonary, immobility, psychological impacts and other medical complications are highly motivated to work with the team to achieve a safe discharge. The US Department of Veterans Affairs (VA) Rehabilitation Services has much to offer them.
Heidi Maloni, PhD, NP: Veterans with MS are not at greater risk because they are diagnosed with MS. But, their comorbidities such as hypertension, obesity, or factors such as older age and increased disability can increase the risk of COVID-19 infection and poorer outcomes if infected. might place them at greater risk.
Veterans have asked “Am I at greater risk? Do I need to do something more to protect myself?” I have had innumerable veterans call and ask whether I can write them letters for their employer to ensure that they work at home longer rather than go into the workplace because they’re very nervous and don’t feel confident that masking and distancing is really going to be protective.
Mitchell Wallin: We are analyzing some of our data in the VA health care system related to COVID-19 infections in the MS population. We can’t say for sure what are numbers are, but our rates of infection and hospitalization are higher than the general population and we will soon have a report. We have a majority male population, which is different from the general MS population, which is predominantly female. The proportion of minority patients in VA mirrors those of the US population. These demographic factors along with a high level of comorbid disease put veterans at high risk for acquiring COVID-19. So, in some ways it’s hard to compare when you look at reports from other countries or the US National MS-COVID-19 Registry, which captures a population that is predominantly female. In the VA, our age range spans from the 20s to almost 100 years. We must understand our population to prevent COVID-19 and better care for the most vulnerable.
Rebecca Spain: Heidi, my understanding, although the numbers are small, that for the most part, Veterans with MS who are older are at higher risk of complications and death, which is also true of the general population. But that there is an additional risk for people with MS who have higher disability levels. My understanding from reading the literature, was that people with MS needing or requiring a cane to walk or greater assistance for mobility were at a higher risk for COVID-19 complications, including mortality. I have been particularly encouraged that in many places this special population of people with MS are getting vaccinated sooner.
Heidi Maloni: I completely agree, you said it very clearly, Becca. Their disability level puts them at risk
Rebecca Spain: Disability is a comorbidity.
Heidi Maloni: Yes. Just sitting in a wheelchair and not being able to get a full breath or having problems with respiratory effort really does put you at risk for doing well if you were to have COVID-19.
Are there other ancillary impacts from COVID-19 for patients with MS?
Jodie Haselkorn: Individuals who are hospitalized with COVID-19 miss social touch and social support from family and friends. They miss familiar conversations, a hug and having someone hold their hand. The acute phase of the infection limits professional face-to-face interaction with patients due to time and protective garments. There are reports of negative consequences with isolation and social reintegration of the COVID-19 survivors is necessary and a necessary part of rehabilitation.
Mitchell Wallin: For certain procedures (eg, magnetic resonance imaging [MRI]) or consultations, we need to bring people into the medical center. Many clinical encounters, however, can be done through telemedicine and both the VA and the US Department of Defense systems were set up to execute this type of visit. We had been doing telemedicine for a long time before the pandemic and we were in a better position than a lot of other health systems to shift to a virtual format with COVID-19. We had to ramp up a little bit and get our tools working a little more effectively for all clinics, but I think we were prepared to broadly execute telemedicine clinics for the pandemic.
Jodie Haselkorn: I agree that the he VA infrastructure was ahead of most other health system in terms of readiness for telehealth and maintaining access to care. Not all health care providers (HCPs) were using it, but the system was there, and included a telehealth coordinator in all of the facilities who could gear health care professionals up quickly. Additionally, a system was in place to provide veterans and caregivers with telehealth home equipment and provide training. Another thing that really helped was the MISSION Act. Veterans who have difficulty travelling for an appointment may have the ability to seek care outside of the VA within their own community. They may be able to go into a local facility to get laboratory or radiologic studies done or continue rehabilitation closer to home.
VA MS Registry Data
Rebecca Spain: Mitch, there are many interesting things we can learn about the interplay between COVID-19 and MS using registries such as how it affects people based on rural vs metropolitan living, whether people are living in single family homes or not as a proxy marker for social support, and so on.
Mitchell Wallin: We have both an MS registry to track and follow patients through our clinical network and a specific COVID-19 registry as well in VA. We have identified the MS cases infected with CoVID-19 and are putting them together.
Jodie Haselkorn: There are a number of efforts in mental health that are moving forward to examine depression and in anxiety during COVID-19. Individuals with MS have increased rates of depression and anxiety above that of the general population during usual times. The literature reports an increase in anxiety and depression in general population associated with the pandemic and veterans with MS seem to be reporting these symptoms more frequently as well. We will be able to track use the registry to assess the impacts of COVID-19 on depression and anxiety in Veterans with MS.
Providing MS Care During COVID-19
Jodie Haselkorn: The transition to telehealth in COVID-19 has been surprisingly seamless with some additional training for veterans and HCPs. I initially experienced an inefficiency in my clinic visit productivity. It took me longer to see a veteran because I wasn’t doing telehealth in our clinic with support staff and residents, my examination had to change, my documentation template needed to be restructured, and the coding was different. Sometimes I saw a veteran in clinic the and my next appointment required me to move back to my office in another building for a telehealth appointment. Teaching virtual trainees who also participated in the clinic encounters had its own challenges and rewards. My ‘motor routine’ was disrupted.
Rebecca Spain: There’s a real learning curve for telehealth in terms of how comfortable you feel with the data you get by telephone or video and how reliable that is. There are issues based on technology factors—like the patient’s bandwidth—because determining how smooth their motions are is challenging if you have a jerky, intermittent signal. I learned quickly to always do the physical examination first because I might lose video connection partway through and have to switch to a phone visit!
It’s still an open question, how much are we missing by using a video and not in-person visits. And what are the long-term health outcomes and implications of that? That is something that needs to be studied in neurology where we pride ourselves on the physical examination. When move to a virtual physical examination, is there cost? There are incredible gains using telehealth in terms of convenience and access to care, which may outweigh some of the drawbacks in particular cases.
There are also pandemic challenge in terms of clinic workflow. At VA Portland Health Care System in Oregon, I have 3 clinics for Friday morning: telephone, virtual, and face-to-face clinics. It’s a real struggle for the schedulers. And because of that transition to new system workflows to accommodate this, some patient visits have been dropped, lost, or scheduled incorrectly.
Heidi Maloni: As the nurse in this group, I agree with everything that Becca and Jodie have said about telehealth. But, I have found some benefits, and one of them is a greater intimacy with my patients. What do I mean by that? For instance, if a patient has taken me to their kitchen and opened their cupboard to show me the breakfast cereal, I’m also observing that there’s nothing else in that cupboard other than cereal. I’m also putting some things together about health and wellness. Or, for the first time, I might meet their significant other who can’t come to clinic because they’re working, but they are at home with the patient. And then having that 3-way conversation with the patient and the significant other, that’s kind of opened up my sense of who that person is.
You are right about the neurological examination. It’s challenging to make exacting assessments. When gathering household objects, ice bags and pronged forks to assess sensation, you remember that this exam is subjective and there is meaning in this remote evaluation. But all in all, I have been blessed with telehealth. Patients don’t mind it at all. They’re completely open to the idea. They like the telehealth for the contact they are able to have with their HCP.
Jodie Haselkorn: As you were saying that, Heidi, I thought, I’ve been inside my veterans’ bathrooms virtually and have seen all of their equipment that they have at home. In a face-to-face clinic visit, you don’t have an opportunity to see all their canes and walkers, braces, and other assistive technology. Some of it’s stashed in a closet, some of it under the bed. In a virtual visit, I get to understand why some is not used, what veterans prefer, and see their own innovations for mobility and self-care.
Mitchell Wallin: There’s a typical ritual that patients talk about when they go to a clinic. They check in, sit down, and wait for the nurse to give them their vital signs and set them up in the room. And then they meet with their HCP, and finally they complete the tasks on the checklist. And part of that may mean scheduling an MRI or going to the lab. But some of these handoffs don’t happen as well on telehealth. Maybe we haven’t integrated these segments of a clinical visit into telehealth platforms. But it could be developed, and there could be new neurologic tools to improve the interview and physical examination. Twenty years ago, you couldn’t deposit a check on your phone; but now you can do everything on your phone you could do in a physical bank. With some creativity, we can improve parts of the neurological exam that are currently difficult to assess remotely.
Jodie Haselkorn: I have not used peripherals in video telehealth to home and I would need to become accustomed to their use with current technology and train patients and caregivers. I would like telehealth peripherals such as a stethoscope to listen to the abdomen of a veteran with neurogenic bowel or a user-friendly ultrasound probe to measure postvoid residual urine in an individual with symptoms of neurogenic bladder, in addition to devices that measure walking speed and pulmonary function. I look forward to the development, use, and the incorporation peripherals that will enable a more extensive virtual exam within the home.
What are the MS Centers of Excellence working on now?
Jodie Haselkorn: We are working to understand the healthcare needs of veterans with MS by evaluating not only care for MS within the VA, but also the types and quantity of MS specialty care VA that is being received in the community during the pandemic. Dr. Wallin is also using the registry to lead a telehealth study to capture the variety of different codes that VA health professionals in MS have used to document workload by telehealth, and face-to-face, and telephone encounters.
Rebecca Spain: The MS Center of Excellence (MSCoE) is coming out with note templates to be available for HCPs, which we can refine as we get experience. This is s one way we can promote high standards in MS care by making these ancillary tools more productive.
Jodie Haselkorn: We are looking at different ways to achieve a high-quality virtual examination using standardized examination strategies and patient and caregiver information to prepare for a specialty MS visit.
Rebecca Spain: I would like to, in more of a research setting, study health outcomes using telehealth vs in person and start tracking that long term.
Mitchell Wallin: We can probably do more in terms of standardization, such as the routine patient reported surveys and implementing the new Consortium of Multiple Sclerosis Centers’ International MRI criteria. The COVID pandemic has affected everything in medical care. But we want to have a regular standardized outcome to assess, and if we can start to do some of the standard data collection through telemedicine, it becomes part of our regular clinic data.
Heidi Maloni: We need better technology. You can do electrocardiograms on your watch. Could we do Dinamaps? Could we figure out strength? That’s a wish list.
Jodie Haselkorn: Since the MSCoE is a national program, we were set up to do what we needed to do for education. We were able to continue on with all of our HCP webinars, including the series with the National MS Society (NMSS). We also have a Specialty Care Access Network-Extension for Community Healthcare Outcomes (SCAN-ECHO) series with the Northwest ECHO VA program and collaborated with the Can Do MS program on patient education as well. We’ve sent out 2 printed newsletters for veterans. The training of HCPs for the future has continued as well. All of our postdoctoral fellows who have finished their programs on time and moved on to either clinical practice or received career development grants to continue their VA careers, a new fellow has joined, and our other fellows are continuing as planned.
The loss that we sustained was in-person meetings. We held MSCoE Regional Program meetings in the East and West that combined education and administrative goals. Both of these were well attended and successful. There was a lot of virtual education available from multiple sources. It was challenging this year was to anticipate what education programming people wanted from MSCoE. Interestingly, a lot of our regional HCPs did not want much more COVID-19 education. They wanted other education and we were able to meet those needs.
Did the pandemic impact the VA MS registry?
Mitchell Wallin: Like any electronic product, the VA MS Surveillance Registry must be maintained, and we have tried to encourage people to use it. Our biggest concern was to identify cases of MS that got infected with COVID-19 and to put those people into the registry. In some cases, Veterans with MS were in locations without a MS clinic. So, we’ve spent a lot more time identifying those cases and adjudicating them to make sure their infection and MS were documented correctly.
During the COVID-19 pandemic, the VA healthcare system has been taxed like others and so HCPs have been a lot busier than normal, forcing new workflows. It has been a hard year that way because a lot of health care providers have been doing many other jobs to help maintain patient care during the COVID-19 pandemic.
Heidi Maloni: The impact of COVID-19 has been positive for the registry because we’ve had more opportunities to populate it.
Jodie Haselkorn: Dr. Wallin and the COVID-19 Registry group began building the combined registry at the onset of the pandemic. We have developed the capacity to identify COVID-19 infections in veterans who have MS and receive care in the VA. We entered these cases in the MS Surveillance Registry and have developed a linkage with the COVID-19 national VA registry. We are in the middle of the grunt work part case entry, but it is a rich resource.
How has the pandemic impacted MS research?
Rebecca Spain: COVID-19 has put a big damper on clinical research progress, including some of our MSCoE studies. It has been difficult to have subjects come in for clinical visits. It’s been difficult to get approval for new studies. It’s shifted timelines dramatically, and then that always increases budgets in a time when there’s not a lot of extra money. So, for clinical research, it’s been a real struggle and a strain and an ever-moving target. For laboratory research most, if not all, centers that have laboratory research at some point were closed and have only slowly reopened. Some still haven’t reopened to any kind of research or laboratory. So, it’s been tough, I think, on research in general.
Heidi Maloni: I would say the word is devastating. The pandemic essentially put a stop to in-person research studies. Our hospital was in research phase I, meaning human subjects can only participate in a research study if they are an inpatient or outpatient with an established clinic visit (clinics open to 25% occupancy) or involved in a study requiring safety monitoring, This plan limits risk of COVID-19 exposure.
Rebecca Spain: There is risk for a higher dropout rate of subjects from studies meaning there’s less chance of success for finding answers if enough people don’t stay in. At a certain point, you have to say, “Is this going to be a successful study?”
Jodie Haselkorn: Dr. Spain has done an amazing job leading a multisite, international clinical trial funded by the VA and the NMSS and kept it afloat, despite challenges. The pandemic has had impacts, but the study continues to move towards completion. I’ve appreciated the efforts of the Research Service at VA Puget Sound to ensure that we could safely obtain many of the 12-month outcomes for all the participants enrolled in that study.
Mitchell Wallin: The funding for some of our nonprofit partners, including the Paralyzed Veterans Association (PVA) and the NMSS, has suffered as well and so a lot of their funding programs have closed or been cut back during the pandemic. Despite that, we still have been able to use televideo technology for our clinical and educational programs with our network.
Jodie Haselkorn: MSCoE also does health services and epidemiological studies in addition to clinical trials and that work has continued. Quite a few of the studies that had human subjects in them were completed in terms of data collection, and so those are being analyzed. There will be a drop in funded studies, publications and posters as the pandemic continues and for a recovery period. We have a robust baseline for research productivity and a talented team. We’ll be able to track drop off and recovery over time.
Rebecca Spain: There’s going to be long-term consequences that we don’t see right now, especially for young researchers who have missed getting pilot data which would have led to additional small grants and then later large grants. There’s going to be an education gap that’s going on with all of the kids who are not able to go to school properly. It’s part of that whole swath of lost time and lost opportunity that we will have to deal with.
However, there are going to be some positive changes. We’re now busy designing clinical trials that can be done virtually to minimize any contact with the health facility, and then looking at things like shifting to research ideas that are more focused around health services.
Jodie Haselkorn: Given the current impacts of the pandemic on delivery of health care there is a strong interest in looking at how we can deliver health care in ways that accommodates the consumers and the providers perspectives. In the future we see marked impacts in our abilities to deliver care to Veterans with MS.
As a final thought, I wanted to put in a plug for this talented team. One of our pandemic resolutions was to innovatively find new possibilities and avoid negative focus on small changes. We are fortunate that all our staff have remained healthy and been supportive and compassionate with each other throughout this period. We have met our goals and are still moving forward.
MSCoE has benefited from the supportive leadership of Sharyl Martini, MD, PhD, and Glenn Graham, MD, PhD, in VA Specialty Care Neurology and leadership and space from VA Puget Sound, VA Portland Health Care System, the Washington DC VA Medical Center and VA Maryland Health Care System in Baltimore.
We also have a national advisory system that is actively involved, sets high standards and performs a rigorous annual review. We have rich inputs from the VA National Regional Programs and Veterans. Additionally, we have had the leadership and opportunities to collaborate with outside organizations including, the Consortium of MS Centers, the NMSS, and the PVA. We have been fortunate.
The following is a lightly edited transcript of a teleconference recorded in February 2021.
How has COVID impacted Veterans with multiple sclerosis?
Mitchell Wallin, MD, MPH: There has been a lot of concern in the multiple sclerosis (MS) patient community about getting infected with COVID-19 and what to do about it. Now that there are vaccines, the concern is whether and how to take a vaccine. At least here, in the Washington DC/Baltimore area where I practice, we have seen many veterans being hospitalized with COVID-19, some with multiple sclerosis (MS), and some who have died of COVID-19. So, there has been a lot of fear, especially in veterans that are older with comorbid diseases.
Rebecca Spain, MD, MSPH: There also has been an impact on our ability to provide care to our veterans with MS. There are challenges having them come into the office or providing virtual care. There are additional challenges and concerns this year about making changes in MS medications because we can’t see patients in person to or understand their needs or current status of their MS. So, providing care has been a challenge this year as well.
There has also been an impact on our day to day lives, like there has been for all of us, from the lockdown particularly not being able to exercise and socialize as much. There have been physical and social and emotional tolls that this disease has taken on veterans with MS.
Jodie Haselkorn, MD, MPH: The survivors of COVID-19, that are transferred to an inpatient multidisciplinary rehabilitation program unit to address impairments related to the cardiopulmonary, immobility, psychological impacts and other medical complications are highly motivated to work with the team to achieve a safe discharge. The US Department of Veterans Affairs (VA) Rehabilitation Services has much to offer them.
Heidi Maloni, PhD, NP: Veterans with MS are not at greater risk because they are diagnosed with MS. But, their comorbidities such as hypertension, obesity, or factors such as older age and increased disability can increase the risk of COVID-19 infection and poorer outcomes if infected. might place them at greater risk.
Veterans have asked “Am I at greater risk? Do I need to do something more to protect myself?” I have had innumerable veterans call and ask whether I can write them letters for their employer to ensure that they work at home longer rather than go into the workplace because they’re very nervous and don’t feel confident that masking and distancing is really going to be protective.
Mitchell Wallin: We are analyzing some of our data in the VA health care system related to COVID-19 infections in the MS population. We can’t say for sure what are numbers are, but our rates of infection and hospitalization are higher than the general population and we will soon have a report. We have a majority male population, which is different from the general MS population, which is predominantly female. The proportion of minority patients in VA mirrors those of the US population. These demographic factors along with a high level of comorbid disease put veterans at high risk for acquiring COVID-19. So, in some ways it’s hard to compare when you look at reports from other countries or the US National MS-COVID-19 Registry, which captures a population that is predominantly female. In the VA, our age range spans from the 20s to almost 100 years. We must understand our population to prevent COVID-19 and better care for the most vulnerable.
Rebecca Spain: Heidi, my understanding, although the numbers are small, that for the most part, Veterans with MS who are older are at higher risk of complications and death, which is also true of the general population. But that there is an additional risk for people with MS who have higher disability levels. My understanding from reading the literature, was that people with MS needing or requiring a cane to walk or greater assistance for mobility were at a higher risk for COVID-19 complications, including mortality. I have been particularly encouraged that in many places this special population of people with MS are getting vaccinated sooner.
Heidi Maloni: I completely agree, you said it very clearly, Becca. Their disability level puts them at risk
Rebecca Spain: Disability is a comorbidity.
Heidi Maloni: Yes. Just sitting in a wheelchair and not being able to get a full breath or having problems with respiratory effort really does put you at risk for doing well if you were to have COVID-19.
Are there other ancillary impacts from COVID-19 for patients with MS?
Jodie Haselkorn: Individuals who are hospitalized with COVID-19 miss social touch and social support from family and friends. They miss familiar conversations, a hug and having someone hold their hand. The acute phase of the infection limits professional face-to-face interaction with patients due to time and protective garments. There are reports of negative consequences with isolation and social reintegration of the COVID-19 survivors is necessary and a necessary part of rehabilitation.
Mitchell Wallin: For certain procedures (eg, magnetic resonance imaging [MRI]) or consultations, we need to bring people into the medical center. Many clinical encounters, however, can be done through telemedicine and both the VA and the US Department of Defense systems were set up to execute this type of visit. We had been doing telemedicine for a long time before the pandemic and we were in a better position than a lot of other health systems to shift to a virtual format with COVID-19. We had to ramp up a little bit and get our tools working a little more effectively for all clinics, but I think we were prepared to broadly execute telemedicine clinics for the pandemic.
Jodie Haselkorn: I agree that the he VA infrastructure was ahead of most other health system in terms of readiness for telehealth and maintaining access to care. Not all health care providers (HCPs) were using it, but the system was there, and included a telehealth coordinator in all of the facilities who could gear health care professionals up quickly. Additionally, a system was in place to provide veterans and caregivers with telehealth home equipment and provide training. Another thing that really helped was the MISSION Act. Veterans who have difficulty travelling for an appointment may have the ability to seek care outside of the VA within their own community. They may be able to go into a local facility to get laboratory or radiologic studies done or continue rehabilitation closer to home.
VA MS Registry Data
Rebecca Spain: Mitch, there are many interesting things we can learn about the interplay between COVID-19 and MS using registries such as how it affects people based on rural vs metropolitan living, whether people are living in single family homes or not as a proxy marker for social support, and so on.
Mitchell Wallin: We have both an MS registry to track and follow patients through our clinical network and a specific COVID-19 registry as well in VA. We have identified the MS cases infected with CoVID-19 and are putting them together.
Jodie Haselkorn: There are a number of efforts in mental health that are moving forward to examine depression and in anxiety during COVID-19. Individuals with MS have increased rates of depression and anxiety above that of the general population during usual times. The literature reports an increase in anxiety and depression in general population associated with the pandemic and veterans with MS seem to be reporting these symptoms more frequently as well. We will be able to track use the registry to assess the impacts of COVID-19 on depression and anxiety in Veterans with MS.
Providing MS Care During COVID-19
Jodie Haselkorn: The transition to telehealth in COVID-19 has been surprisingly seamless with some additional training for veterans and HCPs. I initially experienced an inefficiency in my clinic visit productivity. It took me longer to see a veteran because I wasn’t doing telehealth in our clinic with support staff and residents, my examination had to change, my documentation template needed to be restructured, and the coding was different. Sometimes I saw a veteran in clinic the and my next appointment required me to move back to my office in another building for a telehealth appointment. Teaching virtual trainees who also participated in the clinic encounters had its own challenges and rewards. My ‘motor routine’ was disrupted.
Rebecca Spain: There’s a real learning curve for telehealth in terms of how comfortable you feel with the data you get by telephone or video and how reliable that is. There are issues based on technology factors—like the patient’s bandwidth—because determining how smooth their motions are is challenging if you have a jerky, intermittent signal. I learned quickly to always do the physical examination first because I might lose video connection partway through and have to switch to a phone visit!
It’s still an open question, how much are we missing by using a video and not in-person visits. And what are the long-term health outcomes and implications of that? That is something that needs to be studied in neurology where we pride ourselves on the physical examination. When move to a virtual physical examination, is there cost? There are incredible gains using telehealth in terms of convenience and access to care, which may outweigh some of the drawbacks in particular cases.
There are also pandemic challenge in terms of clinic workflow. At VA Portland Health Care System in Oregon, I have 3 clinics for Friday morning: telephone, virtual, and face-to-face clinics. It’s a real struggle for the schedulers. And because of that transition to new system workflows to accommodate this, some patient visits have been dropped, lost, or scheduled incorrectly.
Heidi Maloni: As the nurse in this group, I agree with everything that Becca and Jodie have said about telehealth. But, I have found some benefits, and one of them is a greater intimacy with my patients. What do I mean by that? For instance, if a patient has taken me to their kitchen and opened their cupboard to show me the breakfast cereal, I’m also observing that there’s nothing else in that cupboard other than cereal. I’m also putting some things together about health and wellness. Or, for the first time, I might meet their significant other who can’t come to clinic because they’re working, but they are at home with the patient. And then having that 3-way conversation with the patient and the significant other, that’s kind of opened up my sense of who that person is.
You are right about the neurological examination. It’s challenging to make exacting assessments. When gathering household objects, ice bags and pronged forks to assess sensation, you remember that this exam is subjective and there is meaning in this remote evaluation. But all in all, I have been blessed with telehealth. Patients don’t mind it at all. They’re completely open to the idea. They like the telehealth for the contact they are able to have with their HCP.
Jodie Haselkorn: As you were saying that, Heidi, I thought, I’ve been inside my veterans’ bathrooms virtually and have seen all of their equipment that they have at home. In a face-to-face clinic visit, you don’t have an opportunity to see all their canes and walkers, braces, and other assistive technology. Some of it’s stashed in a closet, some of it under the bed. In a virtual visit, I get to understand why some is not used, what veterans prefer, and see their own innovations for mobility and self-care.
Mitchell Wallin: There’s a typical ritual that patients talk about when they go to a clinic. They check in, sit down, and wait for the nurse to give them their vital signs and set them up in the room. And then they meet with their HCP, and finally they complete the tasks on the checklist. And part of that may mean scheduling an MRI or going to the lab. But some of these handoffs don’t happen as well on telehealth. Maybe we haven’t integrated these segments of a clinical visit into telehealth platforms. But it could be developed, and there could be new neurologic tools to improve the interview and physical examination. Twenty years ago, you couldn’t deposit a check on your phone; but now you can do everything on your phone you could do in a physical bank. With some creativity, we can improve parts of the neurological exam that are currently difficult to assess remotely.
Jodie Haselkorn: I have not used peripherals in video telehealth to home and I would need to become accustomed to their use with current technology and train patients and caregivers. I would like telehealth peripherals such as a stethoscope to listen to the abdomen of a veteran with neurogenic bowel or a user-friendly ultrasound probe to measure postvoid residual urine in an individual with symptoms of neurogenic bladder, in addition to devices that measure walking speed and pulmonary function. I look forward to the development, use, and the incorporation peripherals that will enable a more extensive virtual exam within the home.
What are the MS Centers of Excellence working on now?
Jodie Haselkorn: We are working to understand the healthcare needs of veterans with MS by evaluating not only care for MS within the VA, but also the types and quantity of MS specialty care VA that is being received in the community during the pandemic. Dr. Wallin is also using the registry to lead a telehealth study to capture the variety of different codes that VA health professionals in MS have used to document workload by telehealth, and face-to-face, and telephone encounters.
Rebecca Spain: The MS Center of Excellence (MSCoE) is coming out with note templates to be available for HCPs, which we can refine as we get experience. This is s one way we can promote high standards in MS care by making these ancillary tools more productive.
Jodie Haselkorn: We are looking at different ways to achieve a high-quality virtual examination using standardized examination strategies and patient and caregiver information to prepare for a specialty MS visit.
Rebecca Spain: I would like to, in more of a research setting, study health outcomes using telehealth vs in person and start tracking that long term.
Mitchell Wallin: We can probably do more in terms of standardization, such as the routine patient reported surveys and implementing the new Consortium of Multiple Sclerosis Centers’ International MRI criteria. The COVID pandemic has affected everything in medical care. But we want to have a regular standardized outcome to assess, and if we can start to do some of the standard data collection through telemedicine, it becomes part of our regular clinic data.
Heidi Maloni: We need better technology. You can do electrocardiograms on your watch. Could we do Dinamaps? Could we figure out strength? That’s a wish list.
Jodie Haselkorn: Since the MSCoE is a national program, we were set up to do what we needed to do for education. We were able to continue on with all of our HCP webinars, including the series with the National MS Society (NMSS). We also have a Specialty Care Access Network-Extension for Community Healthcare Outcomes (SCAN-ECHO) series with the Northwest ECHO VA program and collaborated with the Can Do MS program on patient education as well. We’ve sent out 2 printed newsletters for veterans. The training of HCPs for the future has continued as well. All of our postdoctoral fellows who have finished their programs on time and moved on to either clinical practice or received career development grants to continue their VA careers, a new fellow has joined, and our other fellows are continuing as planned.
The loss that we sustained was in-person meetings. We held MSCoE Regional Program meetings in the East and West that combined education and administrative goals. Both of these were well attended and successful. There was a lot of virtual education available from multiple sources. It was challenging this year was to anticipate what education programming people wanted from MSCoE. Interestingly, a lot of our regional HCPs did not want much more COVID-19 education. They wanted other education and we were able to meet those needs.
Did the pandemic impact the VA MS registry?
Mitchell Wallin: Like any electronic product, the VA MS Surveillance Registry must be maintained, and we have tried to encourage people to use it. Our biggest concern was to identify cases of MS that got infected with COVID-19 and to put those people into the registry. In some cases, Veterans with MS were in locations without a MS clinic. So, we’ve spent a lot more time identifying those cases and adjudicating them to make sure their infection and MS were documented correctly.
During the COVID-19 pandemic, the VA healthcare system has been taxed like others and so HCPs have been a lot busier than normal, forcing new workflows. It has been a hard year that way because a lot of health care providers have been doing many other jobs to help maintain patient care during the COVID-19 pandemic.
Heidi Maloni: The impact of COVID-19 has been positive for the registry because we’ve had more opportunities to populate it.
Jodie Haselkorn: Dr. Wallin and the COVID-19 Registry group began building the combined registry at the onset of the pandemic. We have developed the capacity to identify COVID-19 infections in veterans who have MS and receive care in the VA. We entered these cases in the MS Surveillance Registry and have developed a linkage with the COVID-19 national VA registry. We are in the middle of the grunt work part case entry, but it is a rich resource.
How has the pandemic impacted MS research?
Rebecca Spain: COVID-19 has put a big damper on clinical research progress, including some of our MSCoE studies. It has been difficult to have subjects come in for clinical visits. It’s been difficult to get approval for new studies. It’s shifted timelines dramatically, and then that always increases budgets in a time when there’s not a lot of extra money. So, for clinical research, it’s been a real struggle and a strain and an ever-moving target. For laboratory research most, if not all, centers that have laboratory research at some point were closed and have only slowly reopened. Some still haven’t reopened to any kind of research or laboratory. So, it’s been tough, I think, on research in general.
Heidi Maloni: I would say the word is devastating. The pandemic essentially put a stop to in-person research studies. Our hospital was in research phase I, meaning human subjects can only participate in a research study if they are an inpatient or outpatient with an established clinic visit (clinics open to 25% occupancy) or involved in a study requiring safety monitoring, This plan limits risk of COVID-19 exposure.
Rebecca Spain: There is risk for a higher dropout rate of subjects from studies meaning there’s less chance of success for finding answers if enough people don’t stay in. At a certain point, you have to say, “Is this going to be a successful study?”
Jodie Haselkorn: Dr. Spain has done an amazing job leading a multisite, international clinical trial funded by the VA and the NMSS and kept it afloat, despite challenges. The pandemic has had impacts, but the study continues to move towards completion. I’ve appreciated the efforts of the Research Service at VA Puget Sound to ensure that we could safely obtain many of the 12-month outcomes for all the participants enrolled in that study.
Mitchell Wallin: The funding for some of our nonprofit partners, including the Paralyzed Veterans Association (PVA) and the NMSS, has suffered as well and so a lot of their funding programs have closed or been cut back during the pandemic. Despite that, we still have been able to use televideo technology for our clinical and educational programs with our network.
Jodie Haselkorn: MSCoE also does health services and epidemiological studies in addition to clinical trials and that work has continued. Quite a few of the studies that had human subjects in them were completed in terms of data collection, and so those are being analyzed. There will be a drop in funded studies, publications and posters as the pandemic continues and for a recovery period. We have a robust baseline for research productivity and a talented team. We’ll be able to track drop off and recovery over time.
Rebecca Spain: There’s going to be long-term consequences that we don’t see right now, especially for young researchers who have missed getting pilot data which would have led to additional small grants and then later large grants. There’s going to be an education gap that’s going on with all of the kids who are not able to go to school properly. It’s part of that whole swath of lost time and lost opportunity that we will have to deal with.
However, there are going to be some positive changes. We’re now busy designing clinical trials that can be done virtually to minimize any contact with the health facility, and then looking at things like shifting to research ideas that are more focused around health services.
Jodie Haselkorn: Given the current impacts of the pandemic on delivery of health care there is a strong interest in looking at how we can deliver health care in ways that accommodates the consumers and the providers perspectives. In the future we see marked impacts in our abilities to deliver care to Veterans with MS.
As a final thought, I wanted to put in a plug for this talented team. One of our pandemic resolutions was to innovatively find new possibilities and avoid negative focus on small changes. We are fortunate that all our staff have remained healthy and been supportive and compassionate with each other throughout this period. We have met our goals and are still moving forward.
MSCoE has benefited from the supportive leadership of Sharyl Martini, MD, PhD, and Glenn Graham, MD, PhD, in VA Specialty Care Neurology and leadership and space from VA Puget Sound, VA Portland Health Care System, the Washington DC VA Medical Center and VA Maryland Health Care System in Baltimore.
We also have a national advisory system that is actively involved, sets high standards and performs a rigorous annual review. We have rich inputs from the VA National Regional Programs and Veterans. Additionally, we have had the leadership and opportunities to collaborate with outside organizations including, the Consortium of MS Centers, the NMSS, and the PVA. We have been fortunate.
The following is a lightly edited transcript of a teleconference recorded in February 2021.
How has COVID impacted Veterans with multiple sclerosis?
Mitchell Wallin, MD, MPH: There has been a lot of concern in the multiple sclerosis (MS) patient community about getting infected with COVID-19 and what to do about it. Now that there are vaccines, the concern is whether and how to take a vaccine. At least here, in the Washington DC/Baltimore area where I practice, we have seen many veterans being hospitalized with COVID-19, some with multiple sclerosis (MS), and some who have died of COVID-19. So, there has been a lot of fear, especially in veterans that are older with comorbid diseases.
Rebecca Spain, MD, MSPH: There also has been an impact on our ability to provide care to our veterans with MS. There are challenges having them come into the office or providing virtual care. There are additional challenges and concerns this year about making changes in MS medications because we can’t see patients in person to or understand their needs or current status of their MS. So, providing care has been a challenge this year as well.
There has also been an impact on our day to day lives, like there has been for all of us, from the lockdown particularly not being able to exercise and socialize as much. There have been physical and social and emotional tolls that this disease has taken on veterans with MS.
Jodie Haselkorn, MD, MPH: The survivors of COVID-19, that are transferred to an inpatient multidisciplinary rehabilitation program unit to address impairments related to the cardiopulmonary, immobility, psychological impacts and other medical complications are highly motivated to work with the team to achieve a safe discharge. The US Department of Veterans Affairs (VA) Rehabilitation Services has much to offer them.
Heidi Maloni, PhD, NP: Veterans with MS are not at greater risk because they are diagnosed with MS. But, their comorbidities such as hypertension, obesity, or factors such as older age and increased disability can increase the risk of COVID-19 infection and poorer outcomes if infected. might place them at greater risk.
Veterans have asked “Am I at greater risk? Do I need to do something more to protect myself?” I have had innumerable veterans call and ask whether I can write them letters for their employer to ensure that they work at home longer rather than go into the workplace because they’re very nervous and don’t feel confident that masking and distancing is really going to be protective.
Mitchell Wallin: We are analyzing some of our data in the VA health care system related to COVID-19 infections in the MS population. We can’t say for sure what are numbers are, but our rates of infection and hospitalization are higher than the general population and we will soon have a report. We have a majority male population, which is different from the general MS population, which is predominantly female. The proportion of minority patients in VA mirrors those of the US population. These demographic factors along with a high level of comorbid disease put veterans at high risk for acquiring COVID-19. So, in some ways it’s hard to compare when you look at reports from other countries or the US National MS-COVID-19 Registry, which captures a population that is predominantly female. In the VA, our age range spans from the 20s to almost 100 years. We must understand our population to prevent COVID-19 and better care for the most vulnerable.
Rebecca Spain: Heidi, my understanding, although the numbers are small, that for the most part, Veterans with MS who are older are at higher risk of complications and death, which is also true of the general population. But that there is an additional risk for people with MS who have higher disability levels. My understanding from reading the literature, was that people with MS needing or requiring a cane to walk or greater assistance for mobility were at a higher risk for COVID-19 complications, including mortality. I have been particularly encouraged that in many places this special population of people with MS are getting vaccinated sooner.
Heidi Maloni: I completely agree, you said it very clearly, Becca. Their disability level puts them at risk
Rebecca Spain: Disability is a comorbidity.
Heidi Maloni: Yes. Just sitting in a wheelchair and not being able to get a full breath or having problems with respiratory effort really does put you at risk for doing well if you were to have COVID-19.
Are there other ancillary impacts from COVID-19 for patients with MS?
Jodie Haselkorn: Individuals who are hospitalized with COVID-19 miss social touch and social support from family and friends. They miss familiar conversations, a hug and having someone hold their hand. The acute phase of the infection limits professional face-to-face interaction with patients due to time and protective garments. There are reports of negative consequences with isolation and social reintegration of the COVID-19 survivors is necessary and a necessary part of rehabilitation.
Mitchell Wallin: For certain procedures (eg, magnetic resonance imaging [MRI]) or consultations, we need to bring people into the medical center. Many clinical encounters, however, can be done through telemedicine and both the VA and the US Department of Defense systems were set up to execute this type of visit. We had been doing telemedicine for a long time before the pandemic and we were in a better position than a lot of other health systems to shift to a virtual format with COVID-19. We had to ramp up a little bit and get our tools working a little more effectively for all clinics, but I think we were prepared to broadly execute telemedicine clinics for the pandemic.
Jodie Haselkorn: I agree that the he VA infrastructure was ahead of most other health system in terms of readiness for telehealth and maintaining access to care. Not all health care providers (HCPs) were using it, but the system was there, and included a telehealth coordinator in all of the facilities who could gear health care professionals up quickly. Additionally, a system was in place to provide veterans and caregivers with telehealth home equipment and provide training. Another thing that really helped was the MISSION Act. Veterans who have difficulty travelling for an appointment may have the ability to seek care outside of the VA within their own community. They may be able to go into a local facility to get laboratory or radiologic studies done or continue rehabilitation closer to home.
VA MS Registry Data
Rebecca Spain: Mitch, there are many interesting things we can learn about the interplay between COVID-19 and MS using registries such as how it affects people based on rural vs metropolitan living, whether people are living in single family homes or not as a proxy marker for social support, and so on.
Mitchell Wallin: We have both an MS registry to track and follow patients through our clinical network and a specific COVID-19 registry as well in VA. We have identified the MS cases infected with CoVID-19 and are putting them together.
Jodie Haselkorn: There are a number of efforts in mental health that are moving forward to examine depression and in anxiety during COVID-19. Individuals with MS have increased rates of depression and anxiety above that of the general population during usual times. The literature reports an increase in anxiety and depression in general population associated with the pandemic and veterans with MS seem to be reporting these symptoms more frequently as well. We will be able to track use the registry to assess the impacts of COVID-19 on depression and anxiety in Veterans with MS.
Providing MS Care During COVID-19
Jodie Haselkorn: The transition to telehealth in COVID-19 has been surprisingly seamless with some additional training for veterans and HCPs. I initially experienced an inefficiency in my clinic visit productivity. It took me longer to see a veteran because I wasn’t doing telehealth in our clinic with support staff and residents, my examination had to change, my documentation template needed to be restructured, and the coding was different. Sometimes I saw a veteran in clinic the and my next appointment required me to move back to my office in another building for a telehealth appointment. Teaching virtual trainees who also participated in the clinic encounters had its own challenges and rewards. My ‘motor routine’ was disrupted.
Rebecca Spain: There’s a real learning curve for telehealth in terms of how comfortable you feel with the data you get by telephone or video and how reliable that is. There are issues based on technology factors—like the patient’s bandwidth—because determining how smooth their motions are is challenging if you have a jerky, intermittent signal. I learned quickly to always do the physical examination first because I might lose video connection partway through and have to switch to a phone visit!
It’s still an open question, how much are we missing by using a video and not in-person visits. And what are the long-term health outcomes and implications of that? That is something that needs to be studied in neurology where we pride ourselves on the physical examination. When move to a virtual physical examination, is there cost? There are incredible gains using telehealth in terms of convenience and access to care, which may outweigh some of the drawbacks in particular cases.
There are also pandemic challenge in terms of clinic workflow. At VA Portland Health Care System in Oregon, I have 3 clinics for Friday morning: telephone, virtual, and face-to-face clinics. It’s a real struggle for the schedulers. And because of that transition to new system workflows to accommodate this, some patient visits have been dropped, lost, or scheduled incorrectly.
Heidi Maloni: As the nurse in this group, I agree with everything that Becca and Jodie have said about telehealth. But, I have found some benefits, and one of them is a greater intimacy with my patients. What do I mean by that? For instance, if a patient has taken me to their kitchen and opened their cupboard to show me the breakfast cereal, I’m also observing that there’s nothing else in that cupboard other than cereal. I’m also putting some things together about health and wellness. Or, for the first time, I might meet their significant other who can’t come to clinic because they’re working, but they are at home with the patient. And then having that 3-way conversation with the patient and the significant other, that’s kind of opened up my sense of who that person is.
You are right about the neurological examination. It’s challenging to make exacting assessments. When gathering household objects, ice bags and pronged forks to assess sensation, you remember that this exam is subjective and there is meaning in this remote evaluation. But all in all, I have been blessed with telehealth. Patients don’t mind it at all. They’re completely open to the idea. They like the telehealth for the contact they are able to have with their HCP.
Jodie Haselkorn: As you were saying that, Heidi, I thought, I’ve been inside my veterans’ bathrooms virtually and have seen all of their equipment that they have at home. In a face-to-face clinic visit, you don’t have an opportunity to see all their canes and walkers, braces, and other assistive technology. Some of it’s stashed in a closet, some of it under the bed. In a virtual visit, I get to understand why some is not used, what veterans prefer, and see their own innovations for mobility and self-care.
Mitchell Wallin: There’s a typical ritual that patients talk about when they go to a clinic. They check in, sit down, and wait for the nurse to give them their vital signs and set them up in the room. And then they meet with their HCP, and finally they complete the tasks on the checklist. And part of that may mean scheduling an MRI or going to the lab. But some of these handoffs don’t happen as well on telehealth. Maybe we haven’t integrated these segments of a clinical visit into telehealth platforms. But it could be developed, and there could be new neurologic tools to improve the interview and physical examination. Twenty years ago, you couldn’t deposit a check on your phone; but now you can do everything on your phone you could do in a physical bank. With some creativity, we can improve parts of the neurological exam that are currently difficult to assess remotely.
Jodie Haselkorn: I have not used peripherals in video telehealth to home and I would need to become accustomed to their use with current technology and train patients and caregivers. I would like telehealth peripherals such as a stethoscope to listen to the abdomen of a veteran with neurogenic bowel or a user-friendly ultrasound probe to measure postvoid residual urine in an individual with symptoms of neurogenic bladder, in addition to devices that measure walking speed and pulmonary function. I look forward to the development, use, and the incorporation peripherals that will enable a more extensive virtual exam within the home.
What are the MS Centers of Excellence working on now?
Jodie Haselkorn: We are working to understand the healthcare needs of veterans with MS by evaluating not only care for MS within the VA, but also the types and quantity of MS specialty care VA that is being received in the community during the pandemic. Dr. Wallin is also using the registry to lead a telehealth study to capture the variety of different codes that VA health professionals in MS have used to document workload by telehealth, and face-to-face, and telephone encounters.
Rebecca Spain: The MS Center of Excellence (MSCoE) is coming out with note templates to be available for HCPs, which we can refine as we get experience. This is s one way we can promote high standards in MS care by making these ancillary tools more productive.
Jodie Haselkorn: We are looking at different ways to achieve a high-quality virtual examination using standardized examination strategies and patient and caregiver information to prepare for a specialty MS visit.
Rebecca Spain: I would like to, in more of a research setting, study health outcomes using telehealth vs in person and start tracking that long term.
Mitchell Wallin: We can probably do more in terms of standardization, such as the routine patient reported surveys and implementing the new Consortium of Multiple Sclerosis Centers’ International MRI criteria. The COVID pandemic has affected everything in medical care. But we want to have a regular standardized outcome to assess, and if we can start to do some of the standard data collection through telemedicine, it becomes part of our regular clinic data.
Heidi Maloni: We need better technology. You can do electrocardiograms on your watch. Could we do Dinamaps? Could we figure out strength? That’s a wish list.
Jodie Haselkorn: Since the MSCoE is a national program, we were set up to do what we needed to do for education. We were able to continue on with all of our HCP webinars, including the series with the National MS Society (NMSS). We also have a Specialty Care Access Network-Extension for Community Healthcare Outcomes (SCAN-ECHO) series with the Northwest ECHO VA program and collaborated with the Can Do MS program on patient education as well. We’ve sent out 2 printed newsletters for veterans. The training of HCPs for the future has continued as well. All of our postdoctoral fellows who have finished their programs on time and moved on to either clinical practice or received career development grants to continue their VA careers, a new fellow has joined, and our other fellows are continuing as planned.
The loss that we sustained was in-person meetings. We held MSCoE Regional Program meetings in the East and West that combined education and administrative goals. Both of these were well attended and successful. There was a lot of virtual education available from multiple sources. It was challenging this year was to anticipate what education programming people wanted from MSCoE. Interestingly, a lot of our regional HCPs did not want much more COVID-19 education. They wanted other education and we were able to meet those needs.
Did the pandemic impact the VA MS registry?
Mitchell Wallin: Like any electronic product, the VA MS Surveillance Registry must be maintained, and we have tried to encourage people to use it. Our biggest concern was to identify cases of MS that got infected with COVID-19 and to put those people into the registry. In some cases, Veterans with MS were in locations without a MS clinic. So, we’ve spent a lot more time identifying those cases and adjudicating them to make sure their infection and MS were documented correctly.
During the COVID-19 pandemic, the VA healthcare system has been taxed like others and so HCPs have been a lot busier than normal, forcing new workflows. It has been a hard year that way because a lot of health care providers have been doing many other jobs to help maintain patient care during the COVID-19 pandemic.
Heidi Maloni: The impact of COVID-19 has been positive for the registry because we’ve had more opportunities to populate it.
Jodie Haselkorn: Dr. Wallin and the COVID-19 Registry group began building the combined registry at the onset of the pandemic. We have developed the capacity to identify COVID-19 infections in veterans who have MS and receive care in the VA. We entered these cases in the MS Surveillance Registry and have developed a linkage with the COVID-19 national VA registry. We are in the middle of the grunt work part case entry, but it is a rich resource.
How has the pandemic impacted MS research?
Rebecca Spain: COVID-19 has put a big damper on clinical research progress, including some of our MSCoE studies. It has been difficult to have subjects come in for clinical visits. It’s been difficult to get approval for new studies. It’s shifted timelines dramatically, and then that always increases budgets in a time when there’s not a lot of extra money. So, for clinical research, it’s been a real struggle and a strain and an ever-moving target. For laboratory research most, if not all, centers that have laboratory research at some point were closed and have only slowly reopened. Some still haven’t reopened to any kind of research or laboratory. So, it’s been tough, I think, on research in general.
Heidi Maloni: I would say the word is devastating. The pandemic essentially put a stop to in-person research studies. Our hospital was in research phase I, meaning human subjects can only participate in a research study if they are an inpatient or outpatient with an established clinic visit (clinics open to 25% occupancy) or involved in a study requiring safety monitoring, This plan limits risk of COVID-19 exposure.
Rebecca Spain: There is risk for a higher dropout rate of subjects from studies meaning there’s less chance of success for finding answers if enough people don’t stay in. At a certain point, you have to say, “Is this going to be a successful study?”
Jodie Haselkorn: Dr. Spain has done an amazing job leading a multisite, international clinical trial funded by the VA and the NMSS and kept it afloat, despite challenges. The pandemic has had impacts, but the study continues to move towards completion. I’ve appreciated the efforts of the Research Service at VA Puget Sound to ensure that we could safely obtain many of the 12-month outcomes for all the participants enrolled in that study.
Mitchell Wallin: The funding for some of our nonprofit partners, including the Paralyzed Veterans Association (PVA) and the NMSS, has suffered as well and so a lot of their funding programs have closed or been cut back during the pandemic. Despite that, we still have been able to use televideo technology for our clinical and educational programs with our network.
Jodie Haselkorn: MSCoE also does health services and epidemiological studies in addition to clinical trials and that work has continued. Quite a few of the studies that had human subjects in them were completed in terms of data collection, and so those are being analyzed. There will be a drop in funded studies, publications and posters as the pandemic continues and for a recovery period. We have a robust baseline for research productivity and a talented team. We’ll be able to track drop off and recovery over time.
Rebecca Spain: There’s going to be long-term consequences that we don’t see right now, especially for young researchers who have missed getting pilot data which would have led to additional small grants and then later large grants. There’s going to be an education gap that’s going on with all of the kids who are not able to go to school properly. It’s part of that whole swath of lost time and lost opportunity that we will have to deal with.
However, there are going to be some positive changes. We’re now busy designing clinical trials that can be done virtually to minimize any contact with the health facility, and then looking at things like shifting to research ideas that are more focused around health services.
Jodie Haselkorn: Given the current impacts of the pandemic on delivery of health care there is a strong interest in looking at how we can deliver health care in ways that accommodates the consumers and the providers perspectives. In the future we see marked impacts in our abilities to deliver care to Veterans with MS.
As a final thought, I wanted to put in a plug for this talented team. One of our pandemic resolutions was to innovatively find new possibilities and avoid negative focus on small changes. We are fortunate that all our staff have remained healthy and been supportive and compassionate with each other throughout this period. We have met our goals and are still moving forward.
MSCoE has benefited from the supportive leadership of Sharyl Martini, MD, PhD, and Glenn Graham, MD, PhD, in VA Specialty Care Neurology and leadership and space from VA Puget Sound, VA Portland Health Care System, the Washington DC VA Medical Center and VA Maryland Health Care System in Baltimore.
We also have a national advisory system that is actively involved, sets high standards and performs a rigorous annual review. We have rich inputs from the VA National Regional Programs and Veterans. Additionally, we have had the leadership and opportunities to collaborate with outside organizations including, the Consortium of MS Centers, the NMSS, and the PVA. We have been fortunate.
COVID-19 Vaccine in Veterans with Multiple Sclerosis: Protect the Vulnerable
Older veterans with progressive MS and associated comorbidities are at higher risk of death should they be infected by COVID-19 and we urge health care providers to educate every veteran about the benefits of being vaccinated against COVID-19.
This article has been updated to reflect new US Food and Drug Administration and Centers for Disease Control and Prevention recommendations to pause administration of the Johnson and Johnson Jansen (JNJ-78436735) COVID-19 vaccine.1
Since the outbreak of the pandemic caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2),a plethora of studies have been performed to increase our knowledge of its associated illness COVID-19.2 There is no cure for COVID-19, which can be lethal. In the absence of a cure, preventive measures are of vital importance. In order to help prevent the spread of the virus, the Centers for Diseases Control and Prevention (CDC) advocates for: (1) the use of a face mask over the mouth and nose; (2) a minimum of 6-foot distance between individuals; and (3) avoidance of gatherings.As of March 2021, the US Food and Drug Administration (FDA) approved 3 vaccines for the prevention of COVID-19, under an emergency use authorization (EUA).3-5
COVID-19 and Multiple Sclerosis
Since the beginning of the pandemic, neurologists have faced a new challenge—determining whether persons with multiple sclerosis (pwMS) were more at risk than others of becoming ill from COVID-19 or were destined for a worse outcome. The National MS Society has advised a personalized approach in relation to particularly vulnerable persons when needed and has also initiated worldwide registries to collect information regarding incidence and outcome of COVID-19 in pwMS. Accordingly, through the MS Center of Excellence (MSCoE), the Veterans Health Administration (VHA) has established a national registry assembling data regarding COVID-19 in veterans with MS.
A recent descriptive literature review summarized the outcomes of 873 persons with both MS and COVID-19 and reported that about 36% of COVID-19 cases were treated with B-cell depleting therapies (ocrelizumab or rituximab).6 This proportion was relatively higher when compared with other disease modifying agents. Of those who became infected with SARS-CoV-2, death from COVID-19 occurred in about 4%, and an additional 3% required assisted invasive or noninvasive ventilation. Persons reported to have passed away from COVID-19 generally were older; had progressive MS; or had associated comorbidities such as obesity, hypertension, heart or lung conditions, or cancers. Of these, 50% were not on any disease modifying agent, 25% were on B-cell depleting therapies (ocrelizumab or rituximab), and the remaining 25% were on various medications for MS. It is important to highlight that no formal statistical analyses were performed in this review. On the contrary, in the recently published Italian report on 844 pwMS who had suspected or confirmed COVID-19, the authors used univariate and multivariate models to analyze their findings and noted that the use of ocrelizumab was significantly associated with a worse clinical outcome.7 These authors also identified age, sex, disability score, and recent (within 1 month) use of steroids as risk factors for a severe COVID-19 outcome. The incidence of death from COVID-19 in this cohort was 1.54%.
The recently published data from the North American Registry of the National MS Society based on 1,626 patients reported a 3.3% incidence of death from COVID-19.8 The following factors were identified as risks for worse outcome: male sex, nonambulatory status, age, Black race, and cardiovascular disease. The use of rituximab, ocrelizumab, and steroids (the latter medication over the preceding 2 months) increased the risks of hospitalization for COVID-19.
COVID-19 Vaccines
Of the 3 available vaccines, the Pfizer-BioNTech COVID-19 (BNT162b2) vaccine is approved for individuals aged ≥ 16 years, while the Moderna COVID-19 (mRNA-1273) and the Johnson and Johnson/Jannsen COVID-19 (JNJ-78436735) vaccines are approved for individuals aged ≥ 18 years, though the latter vaccine has been temporarily suspended.1,3-5 The EUAs were released following the disclosure of the results of 3 phase 3 clinical trials and several phase 1 and 2 clinical trials.9-16
The BNT162b2 vaccine from Pfizer-BioNTech encodes the SARS-CoV-2 full-length spike protein (S) in prefusion conformation locked by the mutation in 2 prolines.9 Differently from the BNT162b2 vaccine, the BNT162b1 vaccine encodes a secreted trimerized SARS-CoV-2 receptor–binding domain. The S-glycoprotein is required for viral entry, as implicated in host cell attachment, and is the target of the neutralizing antibodies. In a phase 1 clinical study on 195 volunteers treated with BNT162b1 (10 mg, 20 mg, 30 mg, or 100 mg doses) or BNT162b2 (10 mg, 20 mg, or 30 mg doses) vaccines or placebo 21 days apart, both the binding and neutralizing antibody response was found to be age and “somewhat” dose dependent.9
Higher neutralization titers were measured at day 28 and 35 (7 and 14 days after the second dose, respectively) and compared with titers of persons who recovered from a COVID-19 infection.9 Serum neutralization was measured using a fluorescence-based high-throughput neutralization assay, while binding activity was assessed using the receptor-binding domain (RBD)–binding or S1-binding IgG direct Luminex immunoassays.
The overall reactogenicity/immunogenicity profile of BNT162b2 administered twice (30 mg each time) led to its selection for the phase 3 clinical trial.9,10 In a large phase 3 clinical trial on 43,458 participants, the BNT162b2 vaccine given at 30 mg doses 21 days apart conferred 95% clinical efficacy in reducing the likelihood of being affected by symptomatic COVID-19.10 No safety concerns to stop the trial were identified, though related severe and life-threatening events were reported in 0.3% and 0.1% of the volunteers, respectively. We note that these incidence rates were the same for the treated and the placebo group.
The mRNA-1273 vaccine from Moderna also encodes the SARS-CoV-2 S-glycoprotein. In a dose escalation phase 1 trial of 45 participants aged between 18 and 55 years (25 mg, 100 mg or 250 mg, given at days 1 and 29) and 40 participants aged ≥ 57 years (25 mg and 100 mg, given at days 1 and 29), a dose-dependent effect was observed for both binding (receptor-binding domain and S-2p IgG on enzyme-linked immunosorbent assay [ELISA])and neutralizing antibodies (SARS-CoV-2 nanoluciferase high-throughput neutralization assay, focus reduction neutralization test mNeonGreen and SARS-CoV-2 plaque-reduction neutralization testing assay) development.11,12 The geometric mean of both binding and neutralizing antibodies declined over time but persisted high as late as 119 days after the first burst of 100 mg dose.13 The same dose of the vaccine also elicited a strong T helper-1 response with little T helper-2 response across all ages.11 The strength of the memory cellular response remains to be defined and is the subject of ongoing investigations. In a large phase 3 clinical trial with 30,420 participants, the Moderna COVID-19 mRNA-1273 vaccine, given 28 days apart at the dose of 100 mg, met 94.1% clinical efficacy in reducing the likelihood of being affected by symptomatic COVID-19.14
Less than 0.1% of volunteers in both groups withdrew from the trial due to adverse effects (AEs); 0.5% in the placebo group and 0.3% in the treated group had AEs after the first dose, which precluded receiving the second dose.14
The Johnson and Johnson/Jannsen JNJ-78436735 vaccine is based upon a recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector, which encodes the full-length, stabilized S-glycoprotein of SARS-CoV-2. The currently reported results of the phase 1 and 2 clinical study indicated that 805 volunteers (402 participants between ages 18 and 55 years and 403 individuals aged ≥ 65 years) were randomized to receive a single or double dose of either 5 x 1010 viral particles per 0.5 mL (low dose) or 1 x 1011 viral particles per 0.5 mL (high dose), each compared with a placebo group. Incidence of seroconversion to binding antibodies against the full-length stabilized S-glycoprotein, as measured by ELISA, showed ≥ 96% seroconversion by day 29 after the first dose. The incidence of seroconversion to neutralizing antibodies was ≥ 90% as early as early as 29 days after the first of either dose. In this study, neutralization activity was measured using the wild-type virus microneutralization assay based on the Victoria/1/2020/ SARS-CoV-2 strain.15 We note that the data related to this study have been partially reported and additional information will be available when each participant will have received the second dose.
In a large phase 3 clinical trial with 40,000 participants aged between 18 and 100 years, the Johnson and Johnson/Jannsen JNJ-78436735 vaccine, given as single dose of 5 x 1010 viral particles per 0.5 mL, met 65.5% clinical efficacy in the likelihood of being affected by symptomatic COVID-19 ≥ 28 days postimmunization.16 In this study, the vaccine efficacy was found to have a geographic distribution with highest efficacy in the US (74.4%), followed by Latin America (64.7%) where Brazil showed a predominance of the P2 COVID-19 lineage (64.7%), and Africa (52%) where the B.1.351 lineage was most frequent (94.5%). The vaccine also proved to be effective in reducing the likelihood of asymptomatic seroconversion, as measured by the level of a non-S protein, eg, 0.7% of positive cases in the vaccine group vs 2.8% in the placebo group. Immunological data indicated that the vaccine response was mainly driven by T-helper 1 lymphocytes. As of April 13, 2021 the FDA has recommend suspending the administration of the Johnson and Johnson/Janssen vaccine due to the occurrence of severe blood clots reported in a 6 subjects out of ~6.8 millions administered doses.1
It is noteworthy to highlight that all vaccines reduced the likelihood of hospitalizations and deaths due to COVID-19.
As of April 17, 2021, the CDC reports that more than 130 million (40%) Americans, nearly 1/3 of the population, have received at least 1 dose of any of the 3 available vaccines, including 4.6 million at the VHA.17 Using the Vaccine Adverse Event Reporting System and v-safe, the US is conducting what has been defined the most “intense and comprehensive safety monitoring in the US history.”18 Thus far, data affirm the overall safety of the available vaccines against COVID-19. Individuals should not receive the COVID-19 vaccines if they have had a severe allergic reaction to any ingredient in the vaccine or a severe allergic reaction to a prior dose of the vaccine. Additionally, individuals who have received convalescent plasma should wait 90 days before getting the COVID-19 vaccine.
Vaccination for Persons with MS
PwMS or those on immunosuppressive medications were excluded from the clinical trial led by Pfizer-BioNTech. There is no mention of MS as comorbidity in the study from Moderna, although this condition is not listed as an exclusion criterion either. The results of the phase 3 clinical trial for the Johnson and Johnson/Janssen vaccine are not fully public yet, thus this information is not known as well. As a result, the use of this vaccine in pwMS under immunomodulatory agents is based on previous knowledge of other vaccines. Evidence is growing for the safety of the BNT162b2 COVID-19 vaccination in pwMS.19 Data regarding COVID-19 efficacy and safety are still largely based on previous knowledge on other vaccines.20,21
Immunization of pwMS is considered safe and should proceed with confidence in those persons who have no other contraindication to receive a vaccine. A fundamental problem for pwMS treated with immunomodulatory or immunosuppressive medications is whether the vaccine will remain safe or be able to solicit an adequate immune response.20,21 As of the time of publication 2021, there is consensus that mRNA based or inactivated vaccines are also considered safe in pwMS undergoing immunomodulatory or immunosuppressive treatments.20-23 We advise a one-on-one conversation between each veteran with MS and their primary neurologist to understand the importance of the vaccination, the minimal risks associated with it and if any specific treatment modification should be made.
To provide guidance, the National MS Society released a position statement that is regularly updated.22 Given the risks associated with discontinuation of disease modifying agents, pwMS opting to receive a COVID-19 vaccine should continue taking their medications unless recommended otherwise by their primary neurologist. In addition, on the basis of available literature and the American Academy of Neurology recommendations on the use of vaccines in general, the following recommendations are proposed.20-23
Recommendation 1: injections, orals, and natalizumab. Given the risks associated with discontinuation of disease modifying agents, pwMS opting to receive a COVID-19 vaccine should continue taking their medications unless recommended otherwise by their primary neurologist. Neither delay in start nor adjustments in dosing or timing of administration are advised for pwMS taking currently available either generic or brand formulations of β interferons, glatiramer acetate, teriflunomide, dimethyl or monomethyl fumarate, or natalizumab.22
Recommendation 2: anti-CD20 monoclonal infusions. As an attenuated humoral response is predicted in pwMS treated with anti-CD20 monoclonal infusions, coordinating the timing of vaccination with treatment schedule may maximize efficacy of the vaccine. Whenever possible, it is advised to be vaccinated ≥ 12 weeks after the last infusion and to resume infusion 4 weeks after the last dose of the vaccine. PwMS starting anti-CD20 monoclonal infusions are advised to be fully vaccinated first and start these medications ≥ 2 to 4 weeks later.22
Recommendation 3: alemtuzumab infusion. Given its effect on CD52+ cells, it is advised to be vaccinated ≥ 24 weeks after the last infusion and to resume infusion 4 weeks after the last dose of the vaccine. PwMS starting alemtuzumab infusions are advised to get fully vaccinated first and start this medication 4 weeks or more after completing the vaccine.22
Recommendation 4: sphingosine 1 phosphate receptor modulators, oral cladribine, and ofatumumab. PwMS starting any of these medications are advised to be fully vaccinated first and start these medications 2 to 4 weeks after completing the vaccine. PwMS already on those medications are not advised to change the schedule of administration. When possible, though, one should resume the dose of cladribine or ofatumumab 2 to 4 weeks after the last dose of the vaccine. 20
Notably, all these recommendations hold true when there is enough disease stability to allow delaying treatment. We also add that it remains unclear if persons with an overall very low number of lymphocytes will be able to elicit a strong reaction to the vaccine. Blood collection and analysis of white blood cell count and lymphocyte subset estimates should be obtained in those persons with a markedly suppressed immune system. Whenever possible, to maximize outcome, timing the vaccination with treatment should be considered in those persons with a markedly reduced number of T-helper 1 cells.
Vaccination for Veterans
Currently the VHA is offering to veterans the Pfizer and Moderna COVID-19 vaccines with FDA EUAs. In accordance with FDA regulations, the VHA has paused administration of the Johnson and Johnson/Janssen vaccine. The VHA has launched its vaccination program in December 2020 by first providing the vaccine to health care personnel, nursing home patients, spinal cord injury patients, chemotherapy patients, dialysis and transplant patients, as well as homeless veterans. Most VA health care systems have passed this phase and are now able to provide vaccines to veterans with MS.
In December 2020, the MSCoE released a position statement regarding the importance and safety of the COVID-19 vaccine for veterans with MS.24 This statement will be updated on a regular basis as new information becomes available from major organizations like the National MS Society, FDA, CDC, and World Health Organization (WHO) or relevant literature.
Conclusions
Older veterans with progressive MS and associated comorbidities are at higher risk of death should they be infected by COVID-19. Fortunately, we live in a time where vaccines are recognized as a critical tool to prevent this infection and to significantly reduce its morbidity and mortality. Yet, hesitancy to vaccinate has been identified as one of the most important threats to public health by the WHO in 2019.25 Understandably such hesitancy is even more profound for the COVID-19 vaccine, which is being administered under an EUA. In light of this indecision, and given the current state of the pandemic, we urge health care providers to educate every veteran about the benefits of being vaccinated against COVID-19. Within the VHA, a solid campaign of vaccination has been put in place at an unprecedented speed.
Health care providers interacting with veterans with MS are encouraged to use the MSCoE website (www.va.gov/ms) for any questions or concerns, or to reach out to MSCoE staff. It is vitally important that our community of veterans receives appropriate education on the importance of this vaccination for their own safety, for that of their household and society.
1. Centers for Disease Control and Prevention. Recommendation to pause use of Johnson & Johnson’s Janssen COVID-19 vaccine. Updated April 16, 2021. Accessed April 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/JJUpdate.html
2. World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. Accessed March 9, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it
3. US Food and Drug Administration. Pfizer-BioNTech COVID-19 vaccine. Updated February 3, 2021. Accessed March 22, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine
4. US Food and Drug Administration. Moderna COVID-19 vaccine. Updated February 3, 2021. Accessed March 22, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine
5. US Food and Drug Administration. FDA issues emergency use authorization for third COVID-19 vaccine [press release]. Published February 27, 2021. Accessed March 22, 2021. https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-third-covid-19-vaccine
6. Möhn N, Konen FF, Pul R, et al. Experience in multiple sclerosis patients with COVID-19 and disease-modifying therapies: a review of 873 published cases. J Clin Med. 2020;9(12):4067. Published 2020 Dec 16. doi:10.3390/jcm9124067
7. Sormani MP, De Rossi N, Schiavetti I, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. 2021;89(4):780-789. doi:10.1002/ana.26028
8. Salter A, Fox RJ, Newsome SD, et al. Outcomes and risk factors associated with SARS-CoV-2 infection in a North American registry of patients with multiple sclerosis [published online ahead of print, 2021 Mar 19]. JAMA Neurol. 2021;10.1001/jamaneurol.2021.0688. doi:10.1001/jamaneurol.2021.0688
9. Walsh EE, Frenck RW Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439-2450. doi:10.1056/NEJMoa2027906
10. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/NEJMoa2034577
11. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2 - preliminary Report. N Engl J Med. 2020;383(20):1920-1931. doi:10.1056/NEJMoa2022483
12. Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383(25):2427-2438. doi:10.1056/NEJMoa2028436
13. Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2021;384(1):80-82. doi:10.1056/NEJMc2032195
14. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. doi:10.1056/NEJMoa2035389
15. Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1-2a Trial of Ad26.COV2.S Covid-19 Vaccine [published online ahead of print, 2021 Jan 13]. N Engl J Med. 2021;NEJMoa2034201. doi:10.1056/NEJMoa2034201
16. Oliver SE, Gargano JW, Scobie H, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Janssen COVID-19 vaccine - United States, February 2021. MMWR Morb Mortal Wkly Rep. 2021;70(9):329-332. Published 2021 Mar 5. doi:10.15585/mmwr.mm7009e4
17. US Centers for Disease Control and Prevention. COVID-19 vaccinations in the United States. Updated March 21, 2021. Accessed March 22, 2021. https://covid.cdc.gov/covid-data-tracker/#vaccinations
18. Gee J, Marquez P, Su J, et al. First month of COVID-19 vaccine safety monitoring - United States, December 14, 2020-January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(8):283-288. Published 2021 Feb 26. doi:10.15585/mmwr.mm7008e3
19. Achiron A, Dolev M, Menascu S, et al. COVID-19 vaccination in patients with multiple sclerosis: What we have learnt by February 2021 [published online ahead of print, 2021 Apr 15]. Mult Scler. 2021;13524585211003476. doi:10.1177/13524585211003476
20. Righi E, Gallo T, Azzini AM, et al. A review of vaccinations in adult patients with secondary immunodeficiency [published online ahead of print, 2021 Mar 9]. Infect Dis Ther. 2021;1-25. doi:10.1007/s40121-021-00404-y
21. Ciotti JR, Valtcheva MV, Cross AH. Effects of MS disease-modifying therapies on responses to vaccinations: A review. Mult Scler Relat Disord. 2020;45:102439. doi:10.1016/j.msard.2020.102439
22. National Multiple Sclerosis Society. COVID-19 vaccine guidance for people living with MS. Accessed March 22, 2021. https://www.nationalmssociety.org/coronavirus-covid-19-information/multiple-sclerosis-and-coronavirus/covid-19-vaccine-guidance
23. Farez MF, Correale J, Armstrong MJ, et al. Practice guideline update summary: vaccine-preventable infections and immunization in multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2019;93(13):584-594. doi:10.1212/WNL.0000000000008157
24. US Department of Veterans Affairs, Multiple Sclerosis Centers of Excellence. Coronavirus (COVID-19) and vaccine information. Updated February 25. 2021. Accessed March 9, 2021. https://www.va.gov/ms
25. World Health Organization. Ten threats to global health in 2019. Accessed March 18, 2021. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019.
Older veterans with progressive MS and associated comorbidities are at higher risk of death should they be infected by COVID-19 and we urge health care providers to educate every veteran about the benefits of being vaccinated against COVID-19.
Older veterans with progressive MS and associated comorbidities are at higher risk of death should they be infected by COVID-19 and we urge health care providers to educate every veteran about the benefits of being vaccinated against COVID-19.
This article has been updated to reflect new US Food and Drug Administration and Centers for Disease Control and Prevention recommendations to pause administration of the Johnson and Johnson Jansen (JNJ-78436735) COVID-19 vaccine.1
Since the outbreak of the pandemic caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2),a plethora of studies have been performed to increase our knowledge of its associated illness COVID-19.2 There is no cure for COVID-19, which can be lethal. In the absence of a cure, preventive measures are of vital importance. In order to help prevent the spread of the virus, the Centers for Diseases Control and Prevention (CDC) advocates for: (1) the use of a face mask over the mouth and nose; (2) a minimum of 6-foot distance between individuals; and (3) avoidance of gatherings.As of March 2021, the US Food and Drug Administration (FDA) approved 3 vaccines for the prevention of COVID-19, under an emergency use authorization (EUA).3-5
COVID-19 and Multiple Sclerosis
Since the beginning of the pandemic, neurologists have faced a new challenge—determining whether persons with multiple sclerosis (pwMS) were more at risk than others of becoming ill from COVID-19 or were destined for a worse outcome. The National MS Society has advised a personalized approach in relation to particularly vulnerable persons when needed and has also initiated worldwide registries to collect information regarding incidence and outcome of COVID-19 in pwMS. Accordingly, through the MS Center of Excellence (MSCoE), the Veterans Health Administration (VHA) has established a national registry assembling data regarding COVID-19 in veterans with MS.
A recent descriptive literature review summarized the outcomes of 873 persons with both MS and COVID-19 and reported that about 36% of COVID-19 cases were treated with B-cell depleting therapies (ocrelizumab or rituximab).6 This proportion was relatively higher when compared with other disease modifying agents. Of those who became infected with SARS-CoV-2, death from COVID-19 occurred in about 4%, and an additional 3% required assisted invasive or noninvasive ventilation. Persons reported to have passed away from COVID-19 generally were older; had progressive MS; or had associated comorbidities such as obesity, hypertension, heart or lung conditions, or cancers. Of these, 50% were not on any disease modifying agent, 25% were on B-cell depleting therapies (ocrelizumab or rituximab), and the remaining 25% were on various medications for MS. It is important to highlight that no formal statistical analyses were performed in this review. On the contrary, in the recently published Italian report on 844 pwMS who had suspected or confirmed COVID-19, the authors used univariate and multivariate models to analyze their findings and noted that the use of ocrelizumab was significantly associated with a worse clinical outcome.7 These authors also identified age, sex, disability score, and recent (within 1 month) use of steroids as risk factors for a severe COVID-19 outcome. The incidence of death from COVID-19 in this cohort was 1.54%.
The recently published data from the North American Registry of the National MS Society based on 1,626 patients reported a 3.3% incidence of death from COVID-19.8 The following factors were identified as risks for worse outcome: male sex, nonambulatory status, age, Black race, and cardiovascular disease. The use of rituximab, ocrelizumab, and steroids (the latter medication over the preceding 2 months) increased the risks of hospitalization for COVID-19.
COVID-19 Vaccines
Of the 3 available vaccines, the Pfizer-BioNTech COVID-19 (BNT162b2) vaccine is approved for individuals aged ≥ 16 years, while the Moderna COVID-19 (mRNA-1273) and the Johnson and Johnson/Jannsen COVID-19 (JNJ-78436735) vaccines are approved for individuals aged ≥ 18 years, though the latter vaccine has been temporarily suspended.1,3-5 The EUAs were released following the disclosure of the results of 3 phase 3 clinical trials and several phase 1 and 2 clinical trials.9-16
The BNT162b2 vaccine from Pfizer-BioNTech encodes the SARS-CoV-2 full-length spike protein (S) in prefusion conformation locked by the mutation in 2 prolines.9 Differently from the BNT162b2 vaccine, the BNT162b1 vaccine encodes a secreted trimerized SARS-CoV-2 receptor–binding domain. The S-glycoprotein is required for viral entry, as implicated in host cell attachment, and is the target of the neutralizing antibodies. In a phase 1 clinical study on 195 volunteers treated with BNT162b1 (10 mg, 20 mg, 30 mg, or 100 mg doses) or BNT162b2 (10 mg, 20 mg, or 30 mg doses) vaccines or placebo 21 days apart, both the binding and neutralizing antibody response was found to be age and “somewhat” dose dependent.9
Higher neutralization titers were measured at day 28 and 35 (7 and 14 days after the second dose, respectively) and compared with titers of persons who recovered from a COVID-19 infection.9 Serum neutralization was measured using a fluorescence-based high-throughput neutralization assay, while binding activity was assessed using the receptor-binding domain (RBD)–binding or S1-binding IgG direct Luminex immunoassays.
The overall reactogenicity/immunogenicity profile of BNT162b2 administered twice (30 mg each time) led to its selection for the phase 3 clinical trial.9,10 In a large phase 3 clinical trial on 43,458 participants, the BNT162b2 vaccine given at 30 mg doses 21 days apart conferred 95% clinical efficacy in reducing the likelihood of being affected by symptomatic COVID-19.10 No safety concerns to stop the trial were identified, though related severe and life-threatening events were reported in 0.3% and 0.1% of the volunteers, respectively. We note that these incidence rates were the same for the treated and the placebo group.
The mRNA-1273 vaccine from Moderna also encodes the SARS-CoV-2 S-glycoprotein. In a dose escalation phase 1 trial of 45 participants aged between 18 and 55 years (25 mg, 100 mg or 250 mg, given at days 1 and 29) and 40 participants aged ≥ 57 years (25 mg and 100 mg, given at days 1 and 29), a dose-dependent effect was observed for both binding (receptor-binding domain and S-2p IgG on enzyme-linked immunosorbent assay [ELISA])and neutralizing antibodies (SARS-CoV-2 nanoluciferase high-throughput neutralization assay, focus reduction neutralization test mNeonGreen and SARS-CoV-2 plaque-reduction neutralization testing assay) development.11,12 The geometric mean of both binding and neutralizing antibodies declined over time but persisted high as late as 119 days after the first burst of 100 mg dose.13 The same dose of the vaccine also elicited a strong T helper-1 response with little T helper-2 response across all ages.11 The strength of the memory cellular response remains to be defined and is the subject of ongoing investigations. In a large phase 3 clinical trial with 30,420 participants, the Moderna COVID-19 mRNA-1273 vaccine, given 28 days apart at the dose of 100 mg, met 94.1% clinical efficacy in reducing the likelihood of being affected by symptomatic COVID-19.14
Less than 0.1% of volunteers in both groups withdrew from the trial due to adverse effects (AEs); 0.5% in the placebo group and 0.3% in the treated group had AEs after the first dose, which precluded receiving the second dose.14
The Johnson and Johnson/Jannsen JNJ-78436735 vaccine is based upon a recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector, which encodes the full-length, stabilized S-glycoprotein of SARS-CoV-2. The currently reported results of the phase 1 and 2 clinical study indicated that 805 volunteers (402 participants between ages 18 and 55 years and 403 individuals aged ≥ 65 years) were randomized to receive a single or double dose of either 5 x 1010 viral particles per 0.5 mL (low dose) or 1 x 1011 viral particles per 0.5 mL (high dose), each compared with a placebo group. Incidence of seroconversion to binding antibodies against the full-length stabilized S-glycoprotein, as measured by ELISA, showed ≥ 96% seroconversion by day 29 after the first dose. The incidence of seroconversion to neutralizing antibodies was ≥ 90% as early as early as 29 days after the first of either dose. In this study, neutralization activity was measured using the wild-type virus microneutralization assay based on the Victoria/1/2020/ SARS-CoV-2 strain.15 We note that the data related to this study have been partially reported and additional information will be available when each participant will have received the second dose.
In a large phase 3 clinical trial with 40,000 participants aged between 18 and 100 years, the Johnson and Johnson/Jannsen JNJ-78436735 vaccine, given as single dose of 5 x 1010 viral particles per 0.5 mL, met 65.5% clinical efficacy in the likelihood of being affected by symptomatic COVID-19 ≥ 28 days postimmunization.16 In this study, the vaccine efficacy was found to have a geographic distribution with highest efficacy in the US (74.4%), followed by Latin America (64.7%) where Brazil showed a predominance of the P2 COVID-19 lineage (64.7%), and Africa (52%) where the B.1.351 lineage was most frequent (94.5%). The vaccine also proved to be effective in reducing the likelihood of asymptomatic seroconversion, as measured by the level of a non-S protein, eg, 0.7% of positive cases in the vaccine group vs 2.8% in the placebo group. Immunological data indicated that the vaccine response was mainly driven by T-helper 1 lymphocytes. As of April 13, 2021 the FDA has recommend suspending the administration of the Johnson and Johnson/Janssen vaccine due to the occurrence of severe blood clots reported in a 6 subjects out of ~6.8 millions administered doses.1
It is noteworthy to highlight that all vaccines reduced the likelihood of hospitalizations and deaths due to COVID-19.
As of April 17, 2021, the CDC reports that more than 130 million (40%) Americans, nearly 1/3 of the population, have received at least 1 dose of any of the 3 available vaccines, including 4.6 million at the VHA.17 Using the Vaccine Adverse Event Reporting System and v-safe, the US is conducting what has been defined the most “intense and comprehensive safety monitoring in the US history.”18 Thus far, data affirm the overall safety of the available vaccines against COVID-19. Individuals should not receive the COVID-19 vaccines if they have had a severe allergic reaction to any ingredient in the vaccine or a severe allergic reaction to a prior dose of the vaccine. Additionally, individuals who have received convalescent plasma should wait 90 days before getting the COVID-19 vaccine.
Vaccination for Persons with MS
PwMS or those on immunosuppressive medications were excluded from the clinical trial led by Pfizer-BioNTech. There is no mention of MS as comorbidity in the study from Moderna, although this condition is not listed as an exclusion criterion either. The results of the phase 3 clinical trial for the Johnson and Johnson/Janssen vaccine are not fully public yet, thus this information is not known as well. As a result, the use of this vaccine in pwMS under immunomodulatory agents is based on previous knowledge of other vaccines. Evidence is growing for the safety of the BNT162b2 COVID-19 vaccination in pwMS.19 Data regarding COVID-19 efficacy and safety are still largely based on previous knowledge on other vaccines.20,21
Immunization of pwMS is considered safe and should proceed with confidence in those persons who have no other contraindication to receive a vaccine. A fundamental problem for pwMS treated with immunomodulatory or immunosuppressive medications is whether the vaccine will remain safe or be able to solicit an adequate immune response.20,21 As of the time of publication 2021, there is consensus that mRNA based or inactivated vaccines are also considered safe in pwMS undergoing immunomodulatory or immunosuppressive treatments.20-23 We advise a one-on-one conversation between each veteran with MS and their primary neurologist to understand the importance of the vaccination, the minimal risks associated with it and if any specific treatment modification should be made.
To provide guidance, the National MS Society released a position statement that is regularly updated.22 Given the risks associated with discontinuation of disease modifying agents, pwMS opting to receive a COVID-19 vaccine should continue taking their medications unless recommended otherwise by their primary neurologist. In addition, on the basis of available literature and the American Academy of Neurology recommendations on the use of vaccines in general, the following recommendations are proposed.20-23
Recommendation 1: injections, orals, and natalizumab. Given the risks associated with discontinuation of disease modifying agents, pwMS opting to receive a COVID-19 vaccine should continue taking their medications unless recommended otherwise by their primary neurologist. Neither delay in start nor adjustments in dosing or timing of administration are advised for pwMS taking currently available either generic or brand formulations of β interferons, glatiramer acetate, teriflunomide, dimethyl or monomethyl fumarate, or natalizumab.22
Recommendation 2: anti-CD20 monoclonal infusions. As an attenuated humoral response is predicted in pwMS treated with anti-CD20 monoclonal infusions, coordinating the timing of vaccination with treatment schedule may maximize efficacy of the vaccine. Whenever possible, it is advised to be vaccinated ≥ 12 weeks after the last infusion and to resume infusion 4 weeks after the last dose of the vaccine. PwMS starting anti-CD20 monoclonal infusions are advised to be fully vaccinated first and start these medications ≥ 2 to 4 weeks later.22
Recommendation 3: alemtuzumab infusion. Given its effect on CD52+ cells, it is advised to be vaccinated ≥ 24 weeks after the last infusion and to resume infusion 4 weeks after the last dose of the vaccine. PwMS starting alemtuzumab infusions are advised to get fully vaccinated first and start this medication 4 weeks or more after completing the vaccine.22
Recommendation 4: sphingosine 1 phosphate receptor modulators, oral cladribine, and ofatumumab. PwMS starting any of these medications are advised to be fully vaccinated first and start these medications 2 to 4 weeks after completing the vaccine. PwMS already on those medications are not advised to change the schedule of administration. When possible, though, one should resume the dose of cladribine or ofatumumab 2 to 4 weeks after the last dose of the vaccine. 20
Notably, all these recommendations hold true when there is enough disease stability to allow delaying treatment. We also add that it remains unclear if persons with an overall very low number of lymphocytes will be able to elicit a strong reaction to the vaccine. Blood collection and analysis of white blood cell count and lymphocyte subset estimates should be obtained in those persons with a markedly suppressed immune system. Whenever possible, to maximize outcome, timing the vaccination with treatment should be considered in those persons with a markedly reduced number of T-helper 1 cells.
Vaccination for Veterans
Currently the VHA is offering to veterans the Pfizer and Moderna COVID-19 vaccines with FDA EUAs. In accordance with FDA regulations, the VHA has paused administration of the Johnson and Johnson/Janssen vaccine. The VHA has launched its vaccination program in December 2020 by first providing the vaccine to health care personnel, nursing home patients, spinal cord injury patients, chemotherapy patients, dialysis and transplant patients, as well as homeless veterans. Most VA health care systems have passed this phase and are now able to provide vaccines to veterans with MS.
In December 2020, the MSCoE released a position statement regarding the importance and safety of the COVID-19 vaccine for veterans with MS.24 This statement will be updated on a regular basis as new information becomes available from major organizations like the National MS Society, FDA, CDC, and World Health Organization (WHO) or relevant literature.
Conclusions
Older veterans with progressive MS and associated comorbidities are at higher risk of death should they be infected by COVID-19. Fortunately, we live in a time where vaccines are recognized as a critical tool to prevent this infection and to significantly reduce its morbidity and mortality. Yet, hesitancy to vaccinate has been identified as one of the most important threats to public health by the WHO in 2019.25 Understandably such hesitancy is even more profound for the COVID-19 vaccine, which is being administered under an EUA. In light of this indecision, and given the current state of the pandemic, we urge health care providers to educate every veteran about the benefits of being vaccinated against COVID-19. Within the VHA, a solid campaign of vaccination has been put in place at an unprecedented speed.
Health care providers interacting with veterans with MS are encouraged to use the MSCoE website (www.va.gov/ms) for any questions or concerns, or to reach out to MSCoE staff. It is vitally important that our community of veterans receives appropriate education on the importance of this vaccination for their own safety, for that of their household and society.
This article has been updated to reflect new US Food and Drug Administration and Centers for Disease Control and Prevention recommendations to pause administration of the Johnson and Johnson Jansen (JNJ-78436735) COVID-19 vaccine.1
Since the outbreak of the pandemic caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2),a plethora of studies have been performed to increase our knowledge of its associated illness COVID-19.2 There is no cure for COVID-19, which can be lethal. In the absence of a cure, preventive measures are of vital importance. In order to help prevent the spread of the virus, the Centers for Diseases Control and Prevention (CDC) advocates for: (1) the use of a face mask over the mouth and nose; (2) a minimum of 6-foot distance between individuals; and (3) avoidance of gatherings.As of March 2021, the US Food and Drug Administration (FDA) approved 3 vaccines for the prevention of COVID-19, under an emergency use authorization (EUA).3-5
COVID-19 and Multiple Sclerosis
Since the beginning of the pandemic, neurologists have faced a new challenge—determining whether persons with multiple sclerosis (pwMS) were more at risk than others of becoming ill from COVID-19 or were destined for a worse outcome. The National MS Society has advised a personalized approach in relation to particularly vulnerable persons when needed and has also initiated worldwide registries to collect information regarding incidence and outcome of COVID-19 in pwMS. Accordingly, through the MS Center of Excellence (MSCoE), the Veterans Health Administration (VHA) has established a national registry assembling data regarding COVID-19 in veterans with MS.
A recent descriptive literature review summarized the outcomes of 873 persons with both MS and COVID-19 and reported that about 36% of COVID-19 cases were treated with B-cell depleting therapies (ocrelizumab or rituximab).6 This proportion was relatively higher when compared with other disease modifying agents. Of those who became infected with SARS-CoV-2, death from COVID-19 occurred in about 4%, and an additional 3% required assisted invasive or noninvasive ventilation. Persons reported to have passed away from COVID-19 generally were older; had progressive MS; or had associated comorbidities such as obesity, hypertension, heart or lung conditions, or cancers. Of these, 50% were not on any disease modifying agent, 25% were on B-cell depleting therapies (ocrelizumab or rituximab), and the remaining 25% were on various medications for MS. It is important to highlight that no formal statistical analyses were performed in this review. On the contrary, in the recently published Italian report on 844 pwMS who had suspected or confirmed COVID-19, the authors used univariate and multivariate models to analyze their findings and noted that the use of ocrelizumab was significantly associated with a worse clinical outcome.7 These authors also identified age, sex, disability score, and recent (within 1 month) use of steroids as risk factors for a severe COVID-19 outcome. The incidence of death from COVID-19 in this cohort was 1.54%.
The recently published data from the North American Registry of the National MS Society based on 1,626 patients reported a 3.3% incidence of death from COVID-19.8 The following factors were identified as risks for worse outcome: male sex, nonambulatory status, age, Black race, and cardiovascular disease. The use of rituximab, ocrelizumab, and steroids (the latter medication over the preceding 2 months) increased the risks of hospitalization for COVID-19.
COVID-19 Vaccines
Of the 3 available vaccines, the Pfizer-BioNTech COVID-19 (BNT162b2) vaccine is approved for individuals aged ≥ 16 years, while the Moderna COVID-19 (mRNA-1273) and the Johnson and Johnson/Jannsen COVID-19 (JNJ-78436735) vaccines are approved for individuals aged ≥ 18 years, though the latter vaccine has been temporarily suspended.1,3-5 The EUAs were released following the disclosure of the results of 3 phase 3 clinical trials and several phase 1 and 2 clinical trials.9-16
The BNT162b2 vaccine from Pfizer-BioNTech encodes the SARS-CoV-2 full-length spike protein (S) in prefusion conformation locked by the mutation in 2 prolines.9 Differently from the BNT162b2 vaccine, the BNT162b1 vaccine encodes a secreted trimerized SARS-CoV-2 receptor–binding domain. The S-glycoprotein is required for viral entry, as implicated in host cell attachment, and is the target of the neutralizing antibodies. In a phase 1 clinical study on 195 volunteers treated with BNT162b1 (10 mg, 20 mg, 30 mg, or 100 mg doses) or BNT162b2 (10 mg, 20 mg, or 30 mg doses) vaccines or placebo 21 days apart, both the binding and neutralizing antibody response was found to be age and “somewhat” dose dependent.9
Higher neutralization titers were measured at day 28 and 35 (7 and 14 days after the second dose, respectively) and compared with titers of persons who recovered from a COVID-19 infection.9 Serum neutralization was measured using a fluorescence-based high-throughput neutralization assay, while binding activity was assessed using the receptor-binding domain (RBD)–binding or S1-binding IgG direct Luminex immunoassays.
The overall reactogenicity/immunogenicity profile of BNT162b2 administered twice (30 mg each time) led to its selection for the phase 3 clinical trial.9,10 In a large phase 3 clinical trial on 43,458 participants, the BNT162b2 vaccine given at 30 mg doses 21 days apart conferred 95% clinical efficacy in reducing the likelihood of being affected by symptomatic COVID-19.10 No safety concerns to stop the trial were identified, though related severe and life-threatening events were reported in 0.3% and 0.1% of the volunteers, respectively. We note that these incidence rates were the same for the treated and the placebo group.
The mRNA-1273 vaccine from Moderna also encodes the SARS-CoV-2 S-glycoprotein. In a dose escalation phase 1 trial of 45 participants aged between 18 and 55 years (25 mg, 100 mg or 250 mg, given at days 1 and 29) and 40 participants aged ≥ 57 years (25 mg and 100 mg, given at days 1 and 29), a dose-dependent effect was observed for both binding (receptor-binding domain and S-2p IgG on enzyme-linked immunosorbent assay [ELISA])and neutralizing antibodies (SARS-CoV-2 nanoluciferase high-throughput neutralization assay, focus reduction neutralization test mNeonGreen and SARS-CoV-2 plaque-reduction neutralization testing assay) development.11,12 The geometric mean of both binding and neutralizing antibodies declined over time but persisted high as late as 119 days after the first burst of 100 mg dose.13 The same dose of the vaccine also elicited a strong T helper-1 response with little T helper-2 response across all ages.11 The strength of the memory cellular response remains to be defined and is the subject of ongoing investigations. In a large phase 3 clinical trial with 30,420 participants, the Moderna COVID-19 mRNA-1273 vaccine, given 28 days apart at the dose of 100 mg, met 94.1% clinical efficacy in reducing the likelihood of being affected by symptomatic COVID-19.14
Less than 0.1% of volunteers in both groups withdrew from the trial due to adverse effects (AEs); 0.5% in the placebo group and 0.3% in the treated group had AEs after the first dose, which precluded receiving the second dose.14
The Johnson and Johnson/Jannsen JNJ-78436735 vaccine is based upon a recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector, which encodes the full-length, stabilized S-glycoprotein of SARS-CoV-2. The currently reported results of the phase 1 and 2 clinical study indicated that 805 volunteers (402 participants between ages 18 and 55 years and 403 individuals aged ≥ 65 years) were randomized to receive a single or double dose of either 5 x 1010 viral particles per 0.5 mL (low dose) or 1 x 1011 viral particles per 0.5 mL (high dose), each compared with a placebo group. Incidence of seroconversion to binding antibodies against the full-length stabilized S-glycoprotein, as measured by ELISA, showed ≥ 96% seroconversion by day 29 after the first dose. The incidence of seroconversion to neutralizing antibodies was ≥ 90% as early as early as 29 days after the first of either dose. In this study, neutralization activity was measured using the wild-type virus microneutralization assay based on the Victoria/1/2020/ SARS-CoV-2 strain.15 We note that the data related to this study have been partially reported and additional information will be available when each participant will have received the second dose.
In a large phase 3 clinical trial with 40,000 participants aged between 18 and 100 years, the Johnson and Johnson/Jannsen JNJ-78436735 vaccine, given as single dose of 5 x 1010 viral particles per 0.5 mL, met 65.5% clinical efficacy in the likelihood of being affected by symptomatic COVID-19 ≥ 28 days postimmunization.16 In this study, the vaccine efficacy was found to have a geographic distribution with highest efficacy in the US (74.4%), followed by Latin America (64.7%) where Brazil showed a predominance of the P2 COVID-19 lineage (64.7%), and Africa (52%) where the B.1.351 lineage was most frequent (94.5%). The vaccine also proved to be effective in reducing the likelihood of asymptomatic seroconversion, as measured by the level of a non-S protein, eg, 0.7% of positive cases in the vaccine group vs 2.8% in the placebo group. Immunological data indicated that the vaccine response was mainly driven by T-helper 1 lymphocytes. As of April 13, 2021 the FDA has recommend suspending the administration of the Johnson and Johnson/Janssen vaccine due to the occurrence of severe blood clots reported in a 6 subjects out of ~6.8 millions administered doses.1
It is noteworthy to highlight that all vaccines reduced the likelihood of hospitalizations and deaths due to COVID-19.
As of April 17, 2021, the CDC reports that more than 130 million (40%) Americans, nearly 1/3 of the population, have received at least 1 dose of any of the 3 available vaccines, including 4.6 million at the VHA.17 Using the Vaccine Adverse Event Reporting System and v-safe, the US is conducting what has been defined the most “intense and comprehensive safety monitoring in the US history.”18 Thus far, data affirm the overall safety of the available vaccines against COVID-19. Individuals should not receive the COVID-19 vaccines if they have had a severe allergic reaction to any ingredient in the vaccine or a severe allergic reaction to a prior dose of the vaccine. Additionally, individuals who have received convalescent plasma should wait 90 days before getting the COVID-19 vaccine.
Vaccination for Persons with MS
PwMS or those on immunosuppressive medications were excluded from the clinical trial led by Pfizer-BioNTech. There is no mention of MS as comorbidity in the study from Moderna, although this condition is not listed as an exclusion criterion either. The results of the phase 3 clinical trial for the Johnson and Johnson/Janssen vaccine are not fully public yet, thus this information is not known as well. As a result, the use of this vaccine in pwMS under immunomodulatory agents is based on previous knowledge of other vaccines. Evidence is growing for the safety of the BNT162b2 COVID-19 vaccination in pwMS.19 Data regarding COVID-19 efficacy and safety are still largely based on previous knowledge on other vaccines.20,21
Immunization of pwMS is considered safe and should proceed with confidence in those persons who have no other contraindication to receive a vaccine. A fundamental problem for pwMS treated with immunomodulatory or immunosuppressive medications is whether the vaccine will remain safe or be able to solicit an adequate immune response.20,21 As of the time of publication 2021, there is consensus that mRNA based or inactivated vaccines are also considered safe in pwMS undergoing immunomodulatory or immunosuppressive treatments.20-23 We advise a one-on-one conversation between each veteran with MS and their primary neurologist to understand the importance of the vaccination, the minimal risks associated with it and if any specific treatment modification should be made.
To provide guidance, the National MS Society released a position statement that is regularly updated.22 Given the risks associated with discontinuation of disease modifying agents, pwMS opting to receive a COVID-19 vaccine should continue taking their medications unless recommended otherwise by their primary neurologist. In addition, on the basis of available literature and the American Academy of Neurology recommendations on the use of vaccines in general, the following recommendations are proposed.20-23
Recommendation 1: injections, orals, and natalizumab. Given the risks associated with discontinuation of disease modifying agents, pwMS opting to receive a COVID-19 vaccine should continue taking their medications unless recommended otherwise by their primary neurologist. Neither delay in start nor adjustments in dosing or timing of administration are advised for pwMS taking currently available either generic or brand formulations of β interferons, glatiramer acetate, teriflunomide, dimethyl or monomethyl fumarate, or natalizumab.22
Recommendation 2: anti-CD20 monoclonal infusions. As an attenuated humoral response is predicted in pwMS treated with anti-CD20 monoclonal infusions, coordinating the timing of vaccination with treatment schedule may maximize efficacy of the vaccine. Whenever possible, it is advised to be vaccinated ≥ 12 weeks after the last infusion and to resume infusion 4 weeks after the last dose of the vaccine. PwMS starting anti-CD20 monoclonal infusions are advised to be fully vaccinated first and start these medications ≥ 2 to 4 weeks later.22
Recommendation 3: alemtuzumab infusion. Given its effect on CD52+ cells, it is advised to be vaccinated ≥ 24 weeks after the last infusion and to resume infusion 4 weeks after the last dose of the vaccine. PwMS starting alemtuzumab infusions are advised to get fully vaccinated first and start this medication 4 weeks or more after completing the vaccine.22
Recommendation 4: sphingosine 1 phosphate receptor modulators, oral cladribine, and ofatumumab. PwMS starting any of these medications are advised to be fully vaccinated first and start these medications 2 to 4 weeks after completing the vaccine. PwMS already on those medications are not advised to change the schedule of administration. When possible, though, one should resume the dose of cladribine or ofatumumab 2 to 4 weeks after the last dose of the vaccine. 20
Notably, all these recommendations hold true when there is enough disease stability to allow delaying treatment. We also add that it remains unclear if persons with an overall very low number of lymphocytes will be able to elicit a strong reaction to the vaccine. Blood collection and analysis of white blood cell count and lymphocyte subset estimates should be obtained in those persons with a markedly suppressed immune system. Whenever possible, to maximize outcome, timing the vaccination with treatment should be considered in those persons with a markedly reduced number of T-helper 1 cells.
Vaccination for Veterans
Currently the VHA is offering to veterans the Pfizer and Moderna COVID-19 vaccines with FDA EUAs. In accordance with FDA regulations, the VHA has paused administration of the Johnson and Johnson/Janssen vaccine. The VHA has launched its vaccination program in December 2020 by first providing the vaccine to health care personnel, nursing home patients, spinal cord injury patients, chemotherapy patients, dialysis and transplant patients, as well as homeless veterans. Most VA health care systems have passed this phase and are now able to provide vaccines to veterans with MS.
In December 2020, the MSCoE released a position statement regarding the importance and safety of the COVID-19 vaccine for veterans with MS.24 This statement will be updated on a regular basis as new information becomes available from major organizations like the National MS Society, FDA, CDC, and World Health Organization (WHO) or relevant literature.
Conclusions
Older veterans with progressive MS and associated comorbidities are at higher risk of death should they be infected by COVID-19. Fortunately, we live in a time where vaccines are recognized as a critical tool to prevent this infection and to significantly reduce its morbidity and mortality. Yet, hesitancy to vaccinate has been identified as one of the most important threats to public health by the WHO in 2019.25 Understandably such hesitancy is even more profound for the COVID-19 vaccine, which is being administered under an EUA. In light of this indecision, and given the current state of the pandemic, we urge health care providers to educate every veteran about the benefits of being vaccinated against COVID-19. Within the VHA, a solid campaign of vaccination has been put in place at an unprecedented speed.
Health care providers interacting with veterans with MS are encouraged to use the MSCoE website (www.va.gov/ms) for any questions or concerns, or to reach out to MSCoE staff. It is vitally important that our community of veterans receives appropriate education on the importance of this vaccination for their own safety, for that of their household and society.
1. Centers for Disease Control and Prevention. Recommendation to pause use of Johnson & Johnson’s Janssen COVID-19 vaccine. Updated April 16, 2021. Accessed April 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/JJUpdate.html
2. World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. Accessed March 9, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it
3. US Food and Drug Administration. Pfizer-BioNTech COVID-19 vaccine. Updated February 3, 2021. Accessed March 22, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine
4. US Food and Drug Administration. Moderna COVID-19 vaccine. Updated February 3, 2021. Accessed March 22, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine
5. US Food and Drug Administration. FDA issues emergency use authorization for third COVID-19 vaccine [press release]. Published February 27, 2021. Accessed March 22, 2021. https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-third-covid-19-vaccine
6. Möhn N, Konen FF, Pul R, et al. Experience in multiple sclerosis patients with COVID-19 and disease-modifying therapies: a review of 873 published cases. J Clin Med. 2020;9(12):4067. Published 2020 Dec 16. doi:10.3390/jcm9124067
7. Sormani MP, De Rossi N, Schiavetti I, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. 2021;89(4):780-789. doi:10.1002/ana.26028
8. Salter A, Fox RJ, Newsome SD, et al. Outcomes and risk factors associated with SARS-CoV-2 infection in a North American registry of patients with multiple sclerosis [published online ahead of print, 2021 Mar 19]. JAMA Neurol. 2021;10.1001/jamaneurol.2021.0688. doi:10.1001/jamaneurol.2021.0688
9. Walsh EE, Frenck RW Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439-2450. doi:10.1056/NEJMoa2027906
10. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/NEJMoa2034577
11. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2 - preliminary Report. N Engl J Med. 2020;383(20):1920-1931. doi:10.1056/NEJMoa2022483
12. Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383(25):2427-2438. doi:10.1056/NEJMoa2028436
13. Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2021;384(1):80-82. doi:10.1056/NEJMc2032195
14. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. doi:10.1056/NEJMoa2035389
15. Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1-2a Trial of Ad26.COV2.S Covid-19 Vaccine [published online ahead of print, 2021 Jan 13]. N Engl J Med. 2021;NEJMoa2034201. doi:10.1056/NEJMoa2034201
16. Oliver SE, Gargano JW, Scobie H, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Janssen COVID-19 vaccine - United States, February 2021. MMWR Morb Mortal Wkly Rep. 2021;70(9):329-332. Published 2021 Mar 5. doi:10.15585/mmwr.mm7009e4
17. US Centers for Disease Control and Prevention. COVID-19 vaccinations in the United States. Updated March 21, 2021. Accessed March 22, 2021. https://covid.cdc.gov/covid-data-tracker/#vaccinations
18. Gee J, Marquez P, Su J, et al. First month of COVID-19 vaccine safety monitoring - United States, December 14, 2020-January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(8):283-288. Published 2021 Feb 26. doi:10.15585/mmwr.mm7008e3
19. Achiron A, Dolev M, Menascu S, et al. COVID-19 vaccination in patients with multiple sclerosis: What we have learnt by February 2021 [published online ahead of print, 2021 Apr 15]. Mult Scler. 2021;13524585211003476. doi:10.1177/13524585211003476
20. Righi E, Gallo T, Azzini AM, et al. A review of vaccinations in adult patients with secondary immunodeficiency [published online ahead of print, 2021 Mar 9]. Infect Dis Ther. 2021;1-25. doi:10.1007/s40121-021-00404-y
21. Ciotti JR, Valtcheva MV, Cross AH. Effects of MS disease-modifying therapies on responses to vaccinations: A review. Mult Scler Relat Disord. 2020;45:102439. doi:10.1016/j.msard.2020.102439
22. National Multiple Sclerosis Society. COVID-19 vaccine guidance for people living with MS. Accessed March 22, 2021. https://www.nationalmssociety.org/coronavirus-covid-19-information/multiple-sclerosis-and-coronavirus/covid-19-vaccine-guidance
23. Farez MF, Correale J, Armstrong MJ, et al. Practice guideline update summary: vaccine-preventable infections and immunization in multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2019;93(13):584-594. doi:10.1212/WNL.0000000000008157
24. US Department of Veterans Affairs, Multiple Sclerosis Centers of Excellence. Coronavirus (COVID-19) and vaccine information. Updated February 25. 2021. Accessed March 9, 2021. https://www.va.gov/ms
25. World Health Organization. Ten threats to global health in 2019. Accessed March 18, 2021. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019.
1. Centers for Disease Control and Prevention. Recommendation to pause use of Johnson & Johnson’s Janssen COVID-19 vaccine. Updated April 16, 2021. Accessed April 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/JJUpdate.html
2. World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. Accessed March 9, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it
3. US Food and Drug Administration. Pfizer-BioNTech COVID-19 vaccine. Updated February 3, 2021. Accessed March 22, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine
4. US Food and Drug Administration. Moderna COVID-19 vaccine. Updated February 3, 2021. Accessed March 22, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine
5. US Food and Drug Administration. FDA issues emergency use authorization for third COVID-19 vaccine [press release]. Published February 27, 2021. Accessed March 22, 2021. https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-third-covid-19-vaccine
6. Möhn N, Konen FF, Pul R, et al. Experience in multiple sclerosis patients with COVID-19 and disease-modifying therapies: a review of 873 published cases. J Clin Med. 2020;9(12):4067. Published 2020 Dec 16. doi:10.3390/jcm9124067
7. Sormani MP, De Rossi N, Schiavetti I, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. 2021;89(4):780-789. doi:10.1002/ana.26028
8. Salter A, Fox RJ, Newsome SD, et al. Outcomes and risk factors associated with SARS-CoV-2 infection in a North American registry of patients with multiple sclerosis [published online ahead of print, 2021 Mar 19]. JAMA Neurol. 2021;10.1001/jamaneurol.2021.0688. doi:10.1001/jamaneurol.2021.0688
9. Walsh EE, Frenck RW Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439-2450. doi:10.1056/NEJMoa2027906
10. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/NEJMoa2034577
11. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2 - preliminary Report. N Engl J Med. 2020;383(20):1920-1931. doi:10.1056/NEJMoa2022483
12. Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383(25):2427-2438. doi:10.1056/NEJMoa2028436
13. Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2021;384(1):80-82. doi:10.1056/NEJMc2032195
14. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. doi:10.1056/NEJMoa2035389
15. Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1-2a Trial of Ad26.COV2.S Covid-19 Vaccine [published online ahead of print, 2021 Jan 13]. N Engl J Med. 2021;NEJMoa2034201. doi:10.1056/NEJMoa2034201
16. Oliver SE, Gargano JW, Scobie H, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Janssen COVID-19 vaccine - United States, February 2021. MMWR Morb Mortal Wkly Rep. 2021;70(9):329-332. Published 2021 Mar 5. doi:10.15585/mmwr.mm7009e4
17. US Centers for Disease Control and Prevention. COVID-19 vaccinations in the United States. Updated March 21, 2021. Accessed March 22, 2021. https://covid.cdc.gov/covid-data-tracker/#vaccinations
18. Gee J, Marquez P, Su J, et al. First month of COVID-19 vaccine safety monitoring - United States, December 14, 2020-January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(8):283-288. Published 2021 Feb 26. doi:10.15585/mmwr.mm7008e3
19. Achiron A, Dolev M, Menascu S, et al. COVID-19 vaccination in patients with multiple sclerosis: What we have learnt by February 2021 [published online ahead of print, 2021 Apr 15]. Mult Scler. 2021;13524585211003476. doi:10.1177/13524585211003476
20. Righi E, Gallo T, Azzini AM, et al. A review of vaccinations in adult patients with secondary immunodeficiency [published online ahead of print, 2021 Mar 9]. Infect Dis Ther. 2021;1-25. doi:10.1007/s40121-021-00404-y
21. Ciotti JR, Valtcheva MV, Cross AH. Effects of MS disease-modifying therapies on responses to vaccinations: A review. Mult Scler Relat Disord. 2020;45:102439. doi:10.1016/j.msard.2020.102439
22. National Multiple Sclerosis Society. COVID-19 vaccine guidance for people living with MS. Accessed March 22, 2021. https://www.nationalmssociety.org/coronavirus-covid-19-information/multiple-sclerosis-and-coronavirus/covid-19-vaccine-guidance
23. Farez MF, Correale J, Armstrong MJ, et al. Practice guideline update summary: vaccine-preventable infections and immunization in multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2019;93(13):584-594. doi:10.1212/WNL.0000000000008157
24. US Department of Veterans Affairs, Multiple Sclerosis Centers of Excellence. Coronavirus (COVID-19) and vaccine information. Updated February 25. 2021. Accessed March 9, 2021. https://www.va.gov/ms
25. World Health Organization. Ten threats to global health in 2019. Accessed March 18, 2021. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019.