User login
Multiple Keratoacanthomas Occurring in Surgical Margins and De Novo Treated With Intralesional Methotrexate
Keratoacanthomas (KAs) are rapidly growing tumors most prominently found on sun-exposed areas of the skin. The normal progression of a KA is to show rapid growth followed by spontaneous resolution.1 Most KAs are solitary; however, there are several variants of multiple KAs including the familial Ferguson-Smith type, Gryzbowski syndrome (generalized eruptive KAs), KA centrifugum marginatum, Muir-Torre syndrome, and xeroderma pigmentosum.2-4 Keratoacanthomas also may develop in areas of trauma, including burns, laser treatment, radiation, and surgical margins from excisional biopsies or skin grafting.5 Treatment of multiple KAs can be difficult due to a potentially large field size and number of lesions.6 We present a case of multiple KAs developing both in the surgical margins and de novo that responded dramatically to treatment with intralesional methotrexate (MTX).
Case Report
A 55-year-old man with a history of a surgically treated squamous cell carcinoma (SCC) on the anterior aspect of the right leg developed multiple nodules involving the surgical scar. He previously underwent Mohs micrographic surgery (MMS); within a month after the second surgery the patient noticed increased pruritus along with scaly pink changes at the site of the surgical scar.
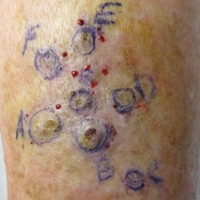
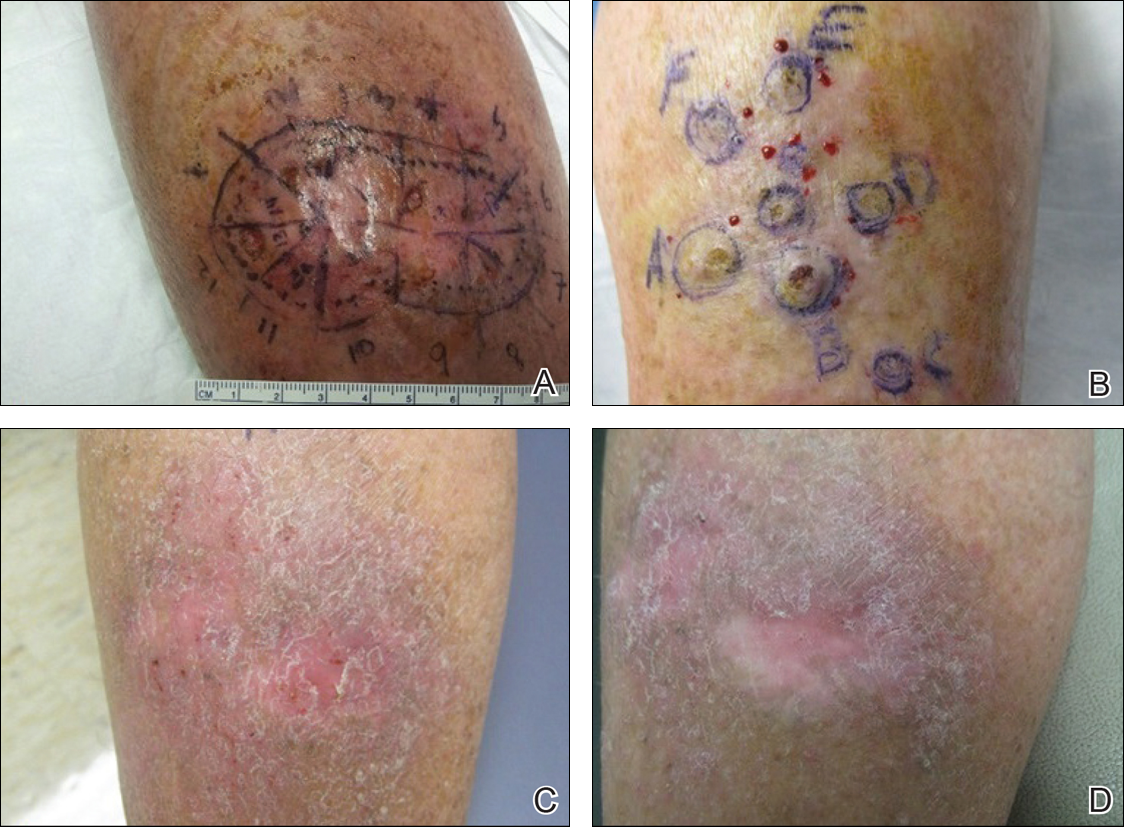
One month prior to presentation, biopsies from the anterior aspect of the right leg demonstrated well-differentiated SCC and he was subsequently treated with MMS; however, examination 1 month after MMS revealed an 11×7-cm indurated plaque with multiple nodules ranging from 1 to 2 cm near the periphery of the plaque with central atrophy and scarring, reminiscent of KA centrifugum marginatum (Figure, A). In a similar fashion, an 8×5-cm plaque composed of 7 nodular areas was noted on the posterior aspect of the right leg (Figure, B). The patient denied any history of trauma to this area. There was no palpable regional lymphadenopathy and the remainder of the skin examination was normal, except for signs of venous stasis in both legs.
Based on the location and morphology of the lesions, the clinical presentation was consistent with multiple KAs. Histologic examination from punch biopsies taken from the plaque's periphery demonstrated well-differentiated SCC (KA type), as well as a lichenoid inflammatory process, epidermal hyperplasia, and cystic and endophytic squamous proliferation suggestive of hypertrophic lichen planus (HLP).
In consideration of the size and number of the lesions as well as the prolonged wound healing with prior surgery, the patient consented to treatment with intralesional MTX (1 mL of 12.5 mg/mL every 2 weeks) rather than undergoing further surgery. The MTX injection was distributed between the lesions on the anterior and posterior aspects of the lower right leg. At each injection session, the size, thickness, and nodularity of the tumor decreased with markedly less pruritus and symptomatic relief was achieved. After 3 injection sessions, resulting in a total of 3 mL of 12.5 mg/mL of MTX, biopsies were taken from the residual atrophic scar on the anterior aspect of the right leg and the remaining 3 papules on the posterior aspect of the right leg to rule out HLP and invasive SCC. The pathology report commented on the presence of prurigo nodules without any evidence of SCC.
At 3-month follow-up, the patient demonstrated no new lesions or recurrence (Figure, C and D). The right leg continued to heal with scarring and postinflammatory pigmentary changes. The patient was monitored for recurrence and to determine the diagnosis of HLP.
Comment
We report the development of multiple KAs arising both from within surgical margins and de novo, and resolution with intralesional MTX. Keratoacanthomas, especially various KA types, have been observed to develop due to various types of trauma, including sites of surgical scars, lichen planus, tattoos, thermal burns, radiation, and discoid lupus erythematosus, and within skin grafts and donor sites.5-19
Hypertrophic lichen planus is a chronic variant of lichen planus that often is found on the pretibial areas of the lower legs.13 Both SCC and reactive KAs have been observed to develop within lesions of HLP.14 Our pathologist commented on the presence of a lichenoid infiltrate with necrotic keratinocytes and epidermal hyperplasia suspicious for HLP, with a small focus of cystic and endophytic squamous proliferation. The latter lacked notable atypia or an invasive component and could represent an irritated infundibular cyst versus an early evolving KA.
The lichenoid inflammation is suspicious for HLP, which has been associated with eruptive KAs13-16 and may have contributed to the development of persistent KAs in our patient, both in sites of surgical scars (the anterior aspect of the leg) and in uninvolved skin (the posterior aspect of the leg). Trauma from the prior surgery may have stimulated a local inflammatory response and, if coupled with a preexisting underlying chronic inflammatory condition such as HLP, may have triggered the development of new lesions on the posterior leg. Skin pathergy reactions also are caused by an upregulated inflammatory response, which is reduced with immunosuppressive agents such as MTX.12
In our patient, there was both an isotopic and isomorphic response. The term isotopic response refers to the occurrence of a new skin disorder at the site of another unrelated and already healed skin disease. It was first defined by Wolf and Wolf20 in 1985 and hence is also known as Wolf isotopic response. The isotopic response in our patient occurred in the setting of lichen planus. The isomorphic response indicates the appearance of typical skin lesions of an existing dermatosis at sites of other skin injuries.
Initially, we thought the patient had recurrence of SCC, but with the rapid development of multiple lesions, the diagnosis of multiple KAs was more likely. Kimyai-Asadi et al8 demonstrated that surgical trauma can precede the development of KAs, as they reported a patient who developed a KA at an excision site. Tamir et al7 reported the simultaneous appearance of KAs in burn scars and skin graft donor sites 4 months after a 40% total body surface area burn. Hamilton et al11 described surgical trauma from a split-skin graft donor site as a trigger for the onset of a KA.
Multiple treatment alternatives exist for KAs, with the standard of care for large or high-risk KAs being excisional surgery21,22; however, other approaches may need to be considered in certain cases, such as with multiple KAs in which lesions may be large and extensive, thereby yielding poor cosmetic outcomes, or with increased surgical risk.23 Furthermore, multiple KAs that develop in the setting of surgical scars require special consideration. Topical 5-fluorouracil, various systemic and intralesional agents (eg, retinoids, interferon, bleomycin, MTX), laser therapy, electrodesiccation and curettage, radiotherapy, and photodynamic therapy all have been reported as methods employed for the treatment of KA.23-27 Goldberg et al5 reported cases of resolution of eruptive KAs arising in both surgical and nonsurgical sites with a combination of deep shave excision, MMS, curettage and desiccation, and oral isotretinoin.
For our patient, we opted for treatment with intralesional MTX, both due to its effectiveness for solitary KAs and reasonably decreased risk of morbidity compared to surgical excision of regions of the pretibial calves. Treatment with MTX would not have been attempted if there was any clinical doubt that the lesions were not the well-differentiated KA type. Also, we had a low threshold for discontinuing therapy and reverting to MMS treatment if any of the lesions displayed a paradoxical growth post-MTX treatment or failed to respond after 3 treatments. Intralesional MTX is less invasive, relatively inexpensive, and a treatment modality with decreased morbidity for KAs, especially for multiple KAs. It should be considered as a potential alternative to surgery in such cases.23-27
- Schwartz RA. Keratoacanthoma. J Am Acad Dermatol. 1994;30:1-19.
- Feldman RJ, Maize JC. Multiple keratoacanthomas in a young woman: report of a case emphasizing medical management and a review of the spectrum of multiple keratoacanthomas. Int J Dermatol. 2007;46:77-79.
- Ereaux LP, Schopflocher P, Fornier CJ. Keratoacanthoma. Arch Dermatol. 1955;71:73-83.
- Lloyd KM, Madsen DK, Lin PY. Grzybowski's eruptive keratoacanthoma. J Am Acad Dermatol. 1989;21(5, pt 1):1023-1024.
- Goldberg LH, Silapunt S, Beyrau KK, et al. Keratoacanthoma as a postoperative complication of skin cancer excision. J Am Acad Dermatol. 2004;50:753-758.
- Pillsbury DM, Beerman H. Multiple keratoacanthoma. Am J Med Sci. 1958;236:614-623.
- Tamir G, Morgenstern S, Ben-Amitay D, et al. Synchronous appearance of keratoacanthomas in burn scar and skin graft donor site shortly after injury. J Am Acad Dermatol. 1999;400(5, pt 2):870-871.
- Kimyai-Asadi A, Shaffer C, Levine VJ, et al. Keratoacanthomas arising from an excisional surgery scar. J Drugs Dermatol. 2004;3:193-194.
- Pattee SF, Silvis NG. Keratoacanthoma developing in sites of previous trauma: a report of two cases and review of the literature. J Am Acad Dermatol. 2003;48(suppl 2):S35-S38.
- Hendricks WM. Sudden appearance of multiple keratoacanthomas three weeks after thermal burns. Cutis. 1991;47:410-412.
- Hamilton SA, Dickson WA, O'Brien CJ. Keratoacanthoma developing in a split skin graft donor site. Br J Plast Surg. 1997;50:560-561.
- Bangash SJ, Green WH, Dolson DJ, et al. Eruptive postoperative squamous cell carcinomas exhibiting a pathergy-like reaction around surgical wound sites. J Am Acad Dermatol. 2009;61:892-897.
- Badell A, Marcoval J, Gallego I, et al. Keratoacanthomas arising in hypertrophic lichen planus. Br J Dermatol. 2000;142:370-393.
- Chave TA, Graham-Brown RAC. Keratoacanthoma developing in hypertrophic lichen planus. Br J Dermatol. 2003;148:592.
- Epstein R. Treatment of keratoacanthoma arising from hypertrophic lichen planus. J Am Acad Dermatol. 2010;62(3, suppl 1):AB28.
- Giesecke LM, Reid CM, James CL, et al. Giant keratoacanthoma arising in hypertrophic lichen planus. Australas J Dermatol. 2003;44:267-269.
- Toll A, Salgado R, Espinet B, et al. "Eruptive postoperative squamous cell carcinomas" or "Hypertrophic lichen planus-like reactions combined with infundibulocystic hyperplasia"? J Am Acad Dermatol. 2010;63:910-911.
- Fanti PA, Tosti A, Peluso AM, et al. Multiple keratoacanthoma in discoid lupus erythematosus. J Am Acad Dermatol. 1989;21(4, pt 1):809-810.
- Kossard S, Thompson C, Duncan GM. Hypertrophic lichen planus-like reactions combined with infundibulocystic hyperplasia: pathway to neoplasia. Arch Dermatol. 2004;140:1262-1267.
- Wolf R, Wolf D. Tinea in a site of healed herpes zoster (Isoloci response). Int J Dermatol. 1985;24:539.
- Larson PO. Keratoacanthomas treated with Mohs' micrographic surgery (chemosurgery): a review of forty-three cases. J Am Acad Dermatol. 1987;16:1040-1044.
- Benest L, Kaplan RP, Salit R, et al. Keratoacanthoma centrifugum marginatum of the lower extremity treated with Mohs micrographic surgery. J Am Acad Dermatol. 1994;31:501-502.
- Remling R, Mempel M, Schnopp N, et al. Intralesional methotrexate injection: an effective time and cost saving therapy alternative in keratoacanthomas that are difficult to treat surgically. Hautarzt. 2000;51:612-614.
- Annest NM, VanBeek MJ, Arpey CJ, et al. Intralesional methotrexate treatment for keratoacanthoma tumors: a retrospective study and review of the literature. J Am Acad Dermatol. 2007;56:989-993.
- Melton JL, Nelson BR, Stough DB, et al. Treatment of keratoacanthoma with intralesional methotrexate. J Am Acad Dermatol. 1991;25:1017-1023.
- Cuesta-Romero C, de Grado-Pena J. Intralesional methotrexate in solitary keratoacanthoma. Arch Dermatol. 1998;134:513-514.
- Richard MA, Gachon J, Choux R, et al. Treatment of keratoacanthoma with intralesional methotrexate injections. An Dermatol Venereol. 2000;127:1097.
Keratoacanthomas (KAs) are rapidly growing tumors most prominently found on sun-exposed areas of the skin. The normal progression of a KA is to show rapid growth followed by spontaneous resolution.1 Most KAs are solitary; however, there are several variants of multiple KAs including the familial Ferguson-Smith type, Gryzbowski syndrome (generalized eruptive KAs), KA centrifugum marginatum, Muir-Torre syndrome, and xeroderma pigmentosum.2-4 Keratoacanthomas also may develop in areas of trauma, including burns, laser treatment, radiation, and surgical margins from excisional biopsies or skin grafting.5 Treatment of multiple KAs can be difficult due to a potentially large field size and number of lesions.6 We present a case of multiple KAs developing both in the surgical margins and de novo that responded dramatically to treatment with intralesional methotrexate (MTX).
Case Report
A 55-year-old man with a history of a surgically treated squamous cell carcinoma (SCC) on the anterior aspect of the right leg developed multiple nodules involving the surgical scar. He previously underwent Mohs micrographic surgery (MMS); within a month after the second surgery the patient noticed increased pruritus along with scaly pink changes at the site of the surgical scar.
One month prior to presentation, biopsies from the anterior aspect of the right leg demonstrated well-differentiated SCC and he was subsequently treated with MMS; however, examination 1 month after MMS revealed an 11×7-cm indurated plaque with multiple nodules ranging from 1 to 2 cm near the periphery of the plaque with central atrophy and scarring, reminiscent of KA centrifugum marginatum (Figure, A). In a similar fashion, an 8×5-cm plaque composed of 7 nodular areas was noted on the posterior aspect of the right leg (Figure, B). The patient denied any history of trauma to this area. There was no palpable regional lymphadenopathy and the remainder of the skin examination was normal, except for signs of venous stasis in both legs.
Based on the location and morphology of the lesions, the clinical presentation was consistent with multiple KAs. Histologic examination from punch biopsies taken from the plaque's periphery demonstrated well-differentiated SCC (KA type), as well as a lichenoid inflammatory process, epidermal hyperplasia, and cystic and endophytic squamous proliferation suggestive of hypertrophic lichen planus (HLP).
In consideration of the size and number of the lesions as well as the prolonged wound healing with prior surgery, the patient consented to treatment with intralesional MTX (1 mL of 12.5 mg/mL every 2 weeks) rather than undergoing further surgery. The MTX injection was distributed between the lesions on the anterior and posterior aspects of the lower right leg. At each injection session, the size, thickness, and nodularity of the tumor decreased with markedly less pruritus and symptomatic relief was achieved. After 3 injection sessions, resulting in a total of 3 mL of 12.5 mg/mL of MTX, biopsies were taken from the residual atrophic scar on the anterior aspect of the right leg and the remaining 3 papules on the posterior aspect of the right leg to rule out HLP and invasive SCC. The pathology report commented on the presence of prurigo nodules without any evidence of SCC.
At 3-month follow-up, the patient demonstrated no new lesions or recurrence (Figure, C and D). The right leg continued to heal with scarring and postinflammatory pigmentary changes. The patient was monitored for recurrence and to determine the diagnosis of HLP.

Comment
We report the development of multiple KAs arising both from within surgical margins and de novo, and resolution with intralesional MTX. Keratoacanthomas, especially various KA types, have been observed to develop due to various types of trauma, including sites of surgical scars, lichen planus, tattoos, thermal burns, radiation, and discoid lupus erythematosus, and within skin grafts and donor sites.5-19
Hypertrophic lichen planus is a chronic variant of lichen planus that often is found on the pretibial areas of the lower legs.13 Both SCC and reactive KAs have been observed to develop within lesions of HLP.14 Our pathologist commented on the presence of a lichenoid infiltrate with necrotic keratinocytes and epidermal hyperplasia suspicious for HLP, with a small focus of cystic and endophytic squamous proliferation. The latter lacked notable atypia or an invasive component and could represent an irritated infundibular cyst versus an early evolving KA.
The lichenoid inflammation is suspicious for HLP, which has been associated with eruptive KAs13-16 and may have contributed to the development of persistent KAs in our patient, both in sites of surgical scars (the anterior aspect of the leg) and in uninvolved skin (the posterior aspect of the leg). Trauma from the prior surgery may have stimulated a local inflammatory response and, if coupled with a preexisting underlying chronic inflammatory condition such as HLP, may have triggered the development of new lesions on the posterior leg. Skin pathergy reactions also are caused by an upregulated inflammatory response, which is reduced with immunosuppressive agents such as MTX.12
In our patient, there was both an isotopic and isomorphic response. The term isotopic response refers to the occurrence of a new skin disorder at the site of another unrelated and already healed skin disease. It was first defined by Wolf and Wolf20 in 1985 and hence is also known as Wolf isotopic response. The isotopic response in our patient occurred in the setting of lichen planus. The isomorphic response indicates the appearance of typical skin lesions of an existing dermatosis at sites of other skin injuries.
Initially, we thought the patient had recurrence of SCC, but with the rapid development of multiple lesions, the diagnosis of multiple KAs was more likely. Kimyai-Asadi et al8 demonstrated that surgical trauma can precede the development of KAs, as they reported a patient who developed a KA at an excision site. Tamir et al7 reported the simultaneous appearance of KAs in burn scars and skin graft donor sites 4 months after a 40% total body surface area burn. Hamilton et al11 described surgical trauma from a split-skin graft donor site as a trigger for the onset of a KA.
Multiple treatment alternatives exist for KAs, with the standard of care for large or high-risk KAs being excisional surgery21,22; however, other approaches may need to be considered in certain cases, such as with multiple KAs in which lesions may be large and extensive, thereby yielding poor cosmetic outcomes, or with increased surgical risk.23 Furthermore, multiple KAs that develop in the setting of surgical scars require special consideration. Topical 5-fluorouracil, various systemic and intralesional agents (eg, retinoids, interferon, bleomycin, MTX), laser therapy, electrodesiccation and curettage, radiotherapy, and photodynamic therapy all have been reported as methods employed for the treatment of KA.23-27 Goldberg et al5 reported cases of resolution of eruptive KAs arising in both surgical and nonsurgical sites with a combination of deep shave excision, MMS, curettage and desiccation, and oral isotretinoin.
For our patient, we opted for treatment with intralesional MTX, both due to its effectiveness for solitary KAs and reasonably decreased risk of morbidity compared to surgical excision of regions of the pretibial calves. Treatment with MTX would not have been attempted if there was any clinical doubt that the lesions were not the well-differentiated KA type. Also, we had a low threshold for discontinuing therapy and reverting to MMS treatment if any of the lesions displayed a paradoxical growth post-MTX treatment or failed to respond after 3 treatments. Intralesional MTX is less invasive, relatively inexpensive, and a treatment modality with decreased morbidity for KAs, especially for multiple KAs. It should be considered as a potential alternative to surgery in such cases.23-27
Keratoacanthomas (KAs) are rapidly growing tumors most prominently found on sun-exposed areas of the skin. The normal progression of a KA is to show rapid growth followed by spontaneous resolution.1 Most KAs are solitary; however, there are several variants of multiple KAs including the familial Ferguson-Smith type, Gryzbowski syndrome (generalized eruptive KAs), KA centrifugum marginatum, Muir-Torre syndrome, and xeroderma pigmentosum.2-4 Keratoacanthomas also may develop in areas of trauma, including burns, laser treatment, radiation, and surgical margins from excisional biopsies or skin grafting.5 Treatment of multiple KAs can be difficult due to a potentially large field size and number of lesions.6 We present a case of multiple KAs developing both in the surgical margins and de novo that responded dramatically to treatment with intralesional methotrexate (MTX).
Case Report
A 55-year-old man with a history of a surgically treated squamous cell carcinoma (SCC) on the anterior aspect of the right leg developed multiple nodules involving the surgical scar. He previously underwent Mohs micrographic surgery (MMS); within a month after the second surgery the patient noticed increased pruritus along with scaly pink changes at the site of the surgical scar.
One month prior to presentation, biopsies from the anterior aspect of the right leg demonstrated well-differentiated SCC and he was subsequently treated with MMS; however, examination 1 month after MMS revealed an 11×7-cm indurated plaque with multiple nodules ranging from 1 to 2 cm near the periphery of the plaque with central atrophy and scarring, reminiscent of KA centrifugum marginatum (Figure, A). In a similar fashion, an 8×5-cm plaque composed of 7 nodular areas was noted on the posterior aspect of the right leg (Figure, B). The patient denied any history of trauma to this area. There was no palpable regional lymphadenopathy and the remainder of the skin examination was normal, except for signs of venous stasis in both legs.
Based on the location and morphology of the lesions, the clinical presentation was consistent with multiple KAs. Histologic examination from punch biopsies taken from the plaque's periphery demonstrated well-differentiated SCC (KA type), as well as a lichenoid inflammatory process, epidermal hyperplasia, and cystic and endophytic squamous proliferation suggestive of hypertrophic lichen planus (HLP).
In consideration of the size and number of the lesions as well as the prolonged wound healing with prior surgery, the patient consented to treatment with intralesional MTX (1 mL of 12.5 mg/mL every 2 weeks) rather than undergoing further surgery. The MTX injection was distributed between the lesions on the anterior and posterior aspects of the lower right leg. At each injection session, the size, thickness, and nodularity of the tumor decreased with markedly less pruritus and symptomatic relief was achieved. After 3 injection sessions, resulting in a total of 3 mL of 12.5 mg/mL of MTX, biopsies were taken from the residual atrophic scar on the anterior aspect of the right leg and the remaining 3 papules on the posterior aspect of the right leg to rule out HLP and invasive SCC. The pathology report commented on the presence of prurigo nodules without any evidence of SCC.
At 3-month follow-up, the patient demonstrated no new lesions or recurrence (Figure, C and D). The right leg continued to heal with scarring and postinflammatory pigmentary changes. The patient was monitored for recurrence and to determine the diagnosis of HLP.

Comment
We report the development of multiple KAs arising both from within surgical margins and de novo, and resolution with intralesional MTX. Keratoacanthomas, especially various KA types, have been observed to develop due to various types of trauma, including sites of surgical scars, lichen planus, tattoos, thermal burns, radiation, and discoid lupus erythematosus, and within skin grafts and donor sites.5-19
Hypertrophic lichen planus is a chronic variant of lichen planus that often is found on the pretibial areas of the lower legs.13 Both SCC and reactive KAs have been observed to develop within lesions of HLP.14 Our pathologist commented on the presence of a lichenoid infiltrate with necrotic keratinocytes and epidermal hyperplasia suspicious for HLP, with a small focus of cystic and endophytic squamous proliferation. The latter lacked notable atypia or an invasive component and could represent an irritated infundibular cyst versus an early evolving KA.
The lichenoid inflammation is suspicious for HLP, which has been associated with eruptive KAs13-16 and may have contributed to the development of persistent KAs in our patient, both in sites of surgical scars (the anterior aspect of the leg) and in uninvolved skin (the posterior aspect of the leg). Trauma from the prior surgery may have stimulated a local inflammatory response and, if coupled with a preexisting underlying chronic inflammatory condition such as HLP, may have triggered the development of new lesions on the posterior leg. Skin pathergy reactions also are caused by an upregulated inflammatory response, which is reduced with immunosuppressive agents such as MTX.12
In our patient, there was both an isotopic and isomorphic response. The term isotopic response refers to the occurrence of a new skin disorder at the site of another unrelated and already healed skin disease. It was first defined by Wolf and Wolf20 in 1985 and hence is also known as Wolf isotopic response. The isotopic response in our patient occurred in the setting of lichen planus. The isomorphic response indicates the appearance of typical skin lesions of an existing dermatosis at sites of other skin injuries.
Initially, we thought the patient had recurrence of SCC, but with the rapid development of multiple lesions, the diagnosis of multiple KAs was more likely. Kimyai-Asadi et al8 demonstrated that surgical trauma can precede the development of KAs, as they reported a patient who developed a KA at an excision site. Tamir et al7 reported the simultaneous appearance of KAs in burn scars and skin graft donor sites 4 months after a 40% total body surface area burn. Hamilton et al11 described surgical trauma from a split-skin graft donor site as a trigger for the onset of a KA.
Multiple treatment alternatives exist for KAs, with the standard of care for large or high-risk KAs being excisional surgery21,22; however, other approaches may need to be considered in certain cases, such as with multiple KAs in which lesions may be large and extensive, thereby yielding poor cosmetic outcomes, or with increased surgical risk.23 Furthermore, multiple KAs that develop in the setting of surgical scars require special consideration. Topical 5-fluorouracil, various systemic and intralesional agents (eg, retinoids, interferon, bleomycin, MTX), laser therapy, electrodesiccation and curettage, radiotherapy, and photodynamic therapy all have been reported as methods employed for the treatment of KA.23-27 Goldberg et al5 reported cases of resolution of eruptive KAs arising in both surgical and nonsurgical sites with a combination of deep shave excision, MMS, curettage and desiccation, and oral isotretinoin.
For our patient, we opted for treatment with intralesional MTX, both due to its effectiveness for solitary KAs and reasonably decreased risk of morbidity compared to surgical excision of regions of the pretibial calves. Treatment with MTX would not have been attempted if there was any clinical doubt that the lesions were not the well-differentiated KA type. Also, we had a low threshold for discontinuing therapy and reverting to MMS treatment if any of the lesions displayed a paradoxical growth post-MTX treatment or failed to respond after 3 treatments. Intralesional MTX is less invasive, relatively inexpensive, and a treatment modality with decreased morbidity for KAs, especially for multiple KAs. It should be considered as a potential alternative to surgery in such cases.23-27
- Schwartz RA. Keratoacanthoma. J Am Acad Dermatol. 1994;30:1-19.
- Feldman RJ, Maize JC. Multiple keratoacanthomas in a young woman: report of a case emphasizing medical management and a review of the spectrum of multiple keratoacanthomas. Int J Dermatol. 2007;46:77-79.
- Ereaux LP, Schopflocher P, Fornier CJ. Keratoacanthoma. Arch Dermatol. 1955;71:73-83.
- Lloyd KM, Madsen DK, Lin PY. Grzybowski's eruptive keratoacanthoma. J Am Acad Dermatol. 1989;21(5, pt 1):1023-1024.
- Goldberg LH, Silapunt S, Beyrau KK, et al. Keratoacanthoma as a postoperative complication of skin cancer excision. J Am Acad Dermatol. 2004;50:753-758.
- Pillsbury DM, Beerman H. Multiple keratoacanthoma. Am J Med Sci. 1958;236:614-623.
- Tamir G, Morgenstern S, Ben-Amitay D, et al. Synchronous appearance of keratoacanthomas in burn scar and skin graft donor site shortly after injury. J Am Acad Dermatol. 1999;400(5, pt 2):870-871.
- Kimyai-Asadi A, Shaffer C, Levine VJ, et al. Keratoacanthomas arising from an excisional surgery scar. J Drugs Dermatol. 2004;3:193-194.
- Pattee SF, Silvis NG. Keratoacanthoma developing in sites of previous trauma: a report of two cases and review of the literature. J Am Acad Dermatol. 2003;48(suppl 2):S35-S38.
- Hendricks WM. Sudden appearance of multiple keratoacanthomas three weeks after thermal burns. Cutis. 1991;47:410-412.
- Hamilton SA, Dickson WA, O'Brien CJ. Keratoacanthoma developing in a split skin graft donor site. Br J Plast Surg. 1997;50:560-561.
- Bangash SJ, Green WH, Dolson DJ, et al. Eruptive postoperative squamous cell carcinomas exhibiting a pathergy-like reaction around surgical wound sites. J Am Acad Dermatol. 2009;61:892-897.
- Badell A, Marcoval J, Gallego I, et al. Keratoacanthomas arising in hypertrophic lichen planus. Br J Dermatol. 2000;142:370-393.
- Chave TA, Graham-Brown RAC. Keratoacanthoma developing in hypertrophic lichen planus. Br J Dermatol. 2003;148:592.
- Epstein R. Treatment of keratoacanthoma arising from hypertrophic lichen planus. J Am Acad Dermatol. 2010;62(3, suppl 1):AB28.
- Giesecke LM, Reid CM, James CL, et al. Giant keratoacanthoma arising in hypertrophic lichen planus. Australas J Dermatol. 2003;44:267-269.
- Toll A, Salgado R, Espinet B, et al. "Eruptive postoperative squamous cell carcinomas" or "Hypertrophic lichen planus-like reactions combined with infundibulocystic hyperplasia"? J Am Acad Dermatol. 2010;63:910-911.
- Fanti PA, Tosti A, Peluso AM, et al. Multiple keratoacanthoma in discoid lupus erythematosus. J Am Acad Dermatol. 1989;21(4, pt 1):809-810.
- Kossard S, Thompson C, Duncan GM. Hypertrophic lichen planus-like reactions combined with infundibulocystic hyperplasia: pathway to neoplasia. Arch Dermatol. 2004;140:1262-1267.
- Wolf R, Wolf D. Tinea in a site of healed herpes zoster (Isoloci response). Int J Dermatol. 1985;24:539.
- Larson PO. Keratoacanthomas treated with Mohs' micrographic surgery (chemosurgery): a review of forty-three cases. J Am Acad Dermatol. 1987;16:1040-1044.
- Benest L, Kaplan RP, Salit R, et al. Keratoacanthoma centrifugum marginatum of the lower extremity treated with Mohs micrographic surgery. J Am Acad Dermatol. 1994;31:501-502.
- Remling R, Mempel M, Schnopp N, et al. Intralesional methotrexate injection: an effective time and cost saving therapy alternative in keratoacanthomas that are difficult to treat surgically. Hautarzt. 2000;51:612-614.
- Annest NM, VanBeek MJ, Arpey CJ, et al. Intralesional methotrexate treatment for keratoacanthoma tumors: a retrospective study and review of the literature. J Am Acad Dermatol. 2007;56:989-993.
- Melton JL, Nelson BR, Stough DB, et al. Treatment of keratoacanthoma with intralesional methotrexate. J Am Acad Dermatol. 1991;25:1017-1023.
- Cuesta-Romero C, de Grado-Pena J. Intralesional methotrexate in solitary keratoacanthoma. Arch Dermatol. 1998;134:513-514.
- Richard MA, Gachon J, Choux R, et al. Treatment of keratoacanthoma with intralesional methotrexate injections. An Dermatol Venereol. 2000;127:1097.
- Schwartz RA. Keratoacanthoma. J Am Acad Dermatol. 1994;30:1-19.
- Feldman RJ, Maize JC. Multiple keratoacanthomas in a young woman: report of a case emphasizing medical management and a review of the spectrum of multiple keratoacanthomas. Int J Dermatol. 2007;46:77-79.
- Ereaux LP, Schopflocher P, Fornier CJ. Keratoacanthoma. Arch Dermatol. 1955;71:73-83.
- Lloyd KM, Madsen DK, Lin PY. Grzybowski's eruptive keratoacanthoma. J Am Acad Dermatol. 1989;21(5, pt 1):1023-1024.
- Goldberg LH, Silapunt S, Beyrau KK, et al. Keratoacanthoma as a postoperative complication of skin cancer excision. J Am Acad Dermatol. 2004;50:753-758.
- Pillsbury DM, Beerman H. Multiple keratoacanthoma. Am J Med Sci. 1958;236:614-623.
- Tamir G, Morgenstern S, Ben-Amitay D, et al. Synchronous appearance of keratoacanthomas in burn scar and skin graft donor site shortly after injury. J Am Acad Dermatol. 1999;400(5, pt 2):870-871.
- Kimyai-Asadi A, Shaffer C, Levine VJ, et al. Keratoacanthomas arising from an excisional surgery scar. J Drugs Dermatol. 2004;3:193-194.
- Pattee SF, Silvis NG. Keratoacanthoma developing in sites of previous trauma: a report of two cases and review of the literature. J Am Acad Dermatol. 2003;48(suppl 2):S35-S38.
- Hendricks WM. Sudden appearance of multiple keratoacanthomas three weeks after thermal burns. Cutis. 1991;47:410-412.
- Hamilton SA, Dickson WA, O'Brien CJ. Keratoacanthoma developing in a split skin graft donor site. Br J Plast Surg. 1997;50:560-561.
- Bangash SJ, Green WH, Dolson DJ, et al. Eruptive postoperative squamous cell carcinomas exhibiting a pathergy-like reaction around surgical wound sites. J Am Acad Dermatol. 2009;61:892-897.
- Badell A, Marcoval J, Gallego I, et al. Keratoacanthomas arising in hypertrophic lichen planus. Br J Dermatol. 2000;142:370-393.
- Chave TA, Graham-Brown RAC. Keratoacanthoma developing in hypertrophic lichen planus. Br J Dermatol. 2003;148:592.
- Epstein R. Treatment of keratoacanthoma arising from hypertrophic lichen planus. J Am Acad Dermatol. 2010;62(3, suppl 1):AB28.
- Giesecke LM, Reid CM, James CL, et al. Giant keratoacanthoma arising in hypertrophic lichen planus. Australas J Dermatol. 2003;44:267-269.
- Toll A, Salgado R, Espinet B, et al. "Eruptive postoperative squamous cell carcinomas" or "Hypertrophic lichen planus-like reactions combined with infundibulocystic hyperplasia"? J Am Acad Dermatol. 2010;63:910-911.
- Fanti PA, Tosti A, Peluso AM, et al. Multiple keratoacanthoma in discoid lupus erythematosus. J Am Acad Dermatol. 1989;21(4, pt 1):809-810.
- Kossard S, Thompson C, Duncan GM. Hypertrophic lichen planus-like reactions combined with infundibulocystic hyperplasia: pathway to neoplasia. Arch Dermatol. 2004;140:1262-1267.
- Wolf R, Wolf D. Tinea in a site of healed herpes zoster (Isoloci response). Int J Dermatol. 1985;24:539.
- Larson PO. Keratoacanthomas treated with Mohs' micrographic surgery (chemosurgery): a review of forty-three cases. J Am Acad Dermatol. 1987;16:1040-1044.
- Benest L, Kaplan RP, Salit R, et al. Keratoacanthoma centrifugum marginatum of the lower extremity treated with Mohs micrographic surgery. J Am Acad Dermatol. 1994;31:501-502.
- Remling R, Mempel M, Schnopp N, et al. Intralesional methotrexate injection: an effective time and cost saving therapy alternative in keratoacanthomas that are difficult to treat surgically. Hautarzt. 2000;51:612-614.
- Annest NM, VanBeek MJ, Arpey CJ, et al. Intralesional methotrexate treatment for keratoacanthoma tumors: a retrospective study and review of the literature. J Am Acad Dermatol. 2007;56:989-993.
- Melton JL, Nelson BR, Stough DB, et al. Treatment of keratoacanthoma with intralesional methotrexate. J Am Acad Dermatol. 1991;25:1017-1023.
- Cuesta-Romero C, de Grado-Pena J. Intralesional methotrexate in solitary keratoacanthoma. Arch Dermatol. 1998;134:513-514.
- Richard MA, Gachon J, Choux R, et al. Treatment of keratoacanthoma with intralesional methotrexate injections. An Dermatol Venereol. 2000;127:1097.
Practice Points
- Keratoacanthomas (KAs) are rapidly growing tumors most prominently found on sun-exposed areas but also may develop in areas of trauma including burns, laser treatment, radiation, and surgical margins from excisional biopsies or skin grafting.
- Intralesional methotrexate is a potential alternative to surgical treatment of KAs as a less invasive and less costly treatment modality with decreased morbidity for multiple KAs.
- Isotopic response refers to the occurrence of a new skin disorder arising at the site of another unrelated and already healed skin disease. Isomorphic response indicates the appearance of typical skin lesions of an existing dermatosis at sites of injuries.
Best Practices in Pulsed Dye Laser Treatment
For more information, access Dr. Ezra's article from the August 2014 issue, "Linear Scarring Following Treatment With a 595-nm Pulsed Dye Laser."
For more information, access Dr. Ezra's article from the August 2014 issue, "Linear Scarring Following Treatment With a 595-nm Pulsed Dye Laser."
For more information, access Dr. Ezra's article from the August 2014 issue, "Linear Scarring Following Treatment With a 595-nm Pulsed Dye Laser."
Linear Scarring Following Treatment With a 595-nm Pulsed Dye Laser
The pulsed dye laser (PDL) has been widely used in the treatment of port-wine stains, telangiectases, and other cutaneous vascular lesions since the late 1980s.1 This treatment modality generally is considered to have few serious adverse effects. There have been few reports of PDL treatment with subsequent complications,1-3 which may include ulceration developing immediately after treatment as well as scarring with a spotlike pattern caused by laser therapy. Numerous studies within the last 2 decades have documented improvement in the appearance of scars and telangiectases following treatment with PDL.4-6 We report the case of a 42-year-old woman who developed atrophic linear scarring of the nasal ala following cosmetic treatment with a 595-nm PDL.
Case Report
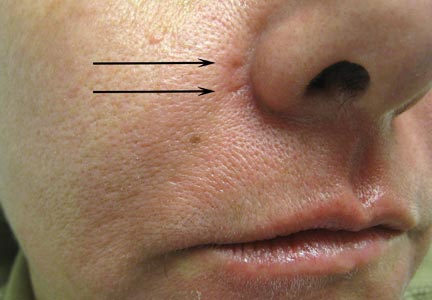
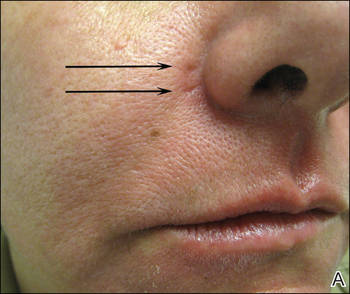
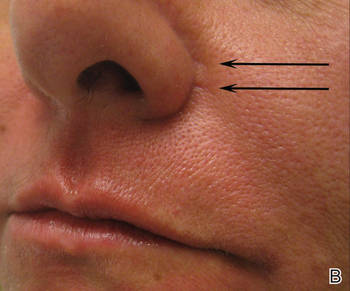
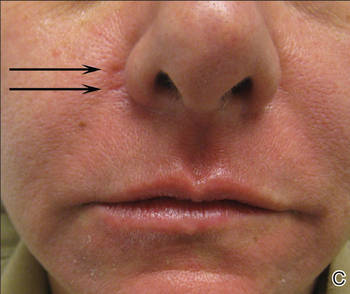
A healthy 42-year-old woman presented with atrophic linear scarring of the bilateral nasal alae following treatment with a 595-nm PDL. The patient had initially presented to an outside clinic 13 months prior for treatment of multiple telangiectases in this area. She received a single, 1-pass treatment with the 595-nm PDL (spot size, 3×10 mm; fluence, 11 J/cm2; pulse duration, 1.5 milliseconds) and returned to the clinic approximately 2 months later for a second treatment with the same settings. Seven months later she returned for a third treatment of the recalcitrant alar telangiectases with the same settings to maximize clinical outcome. Dynamic cooling was used during all treatment sessions with 30/20 setting. After the third treatment, immediate blanching followed by purpura was noted in the treated area. The patient initially was lost to follow-up but returned to the outside clinic 6 months later. On physical examination white atrophic skin with linear scarred depressions were noted on the nasofacial angle of the nasal alae (Figure). The patient denied any postoperative complications such as scabbing, blistering, or pain. At that time she was referred to our office for evaluation, and treatment with a hyaluronic acid filler was initiated. Examination and medical history were otherwise unremarkable at the time of presentation to our office. Resolution of skin atrophy and excellent correction of the depressions was maintained at a follow-up 2 months later. She declined photographs at that time.
|
|
|
| Right (A), left (B), and frontal (C) views of atrophic linear scars (arrows) caused by high-energy purpuric doses of a 595-nm pulsed dye laser. |
Comment
The PDL often is employed in the treatment of vascular lesions such as telangiectases.7 The most common adverse effect is postinflammatory hyperpigmentation; atrophic and hypertrophic scarring rarely are seen.1,8,9 In a study of adverse reactions following pulsed tunable dye laser treatment of port-wine stains in 701 patients, atrophic scarring occurred in 5% of patients and 0.83% of treatments; clinical resolution was noted over the following 6 to 12 months in 30% of patients.8
Following treatment with the PDL, thermal damage occurs primarily to vessel walls with little or no damage to surrounding nonvascular structures. The depth of vascular injury after PDL treatment has been shown to be approximately 1.2 mm.10
Although scarring in our patient was a result of PDL treatment, PDL therapy is commonly used as a treatment option for scars. In conjunction with intralesional steroids directed at flattening hypertrophic scars and keloids, the PDL is used to reduce scar redness and enhance pliability.11 Although redness and telangiectases that develop in surgical scars usually spontaneously remit, they often can show prolonged and incomplete healing. Surgical scars have been shown to benefit from PDL treatment as it advances the end point closer to the complete absence of redness.11-14
The off-label use of hyaluronic acid filler in our patient is notable, as the injection of the nasal ala is not an ordinary injection site for this filler material. It can be associated with risk for necrosis and thus must be performed by an experienced injector combined with informed consent from the patient. The nasal ala is particularly sebaceous and consists of fibrofatty tissue, which is not easily amenable to infiltration. Despite this usual characteristic of the nasal area, the scarring in our patient was fortuitously lateral to the nasal ala and easily filled with hyaluronic acid, as a linear tract was created by the high energies and linear spot size used to treat the patient.
We report a 595-nm PDL treatment that resulted in atrophic linear scarring in a distribution mimicking the linear spot size used by the laser operator. No adverse effects were noted following the first 2 treatments, thereby suggesting either a cumulative insult or more likely cutaneous necrosis from excessive fluence and short pulse durations due to operator inexperience. Other possibilities include rapid and overlapping passes with the laser leading to bulk heating and thermal injury to the skin.
Alternative laser treatment protocols have been proposed in the literature. Rohrer et al15 recommended multiple passes at subpurpuric doses for treatment of facial telangiectases with the PDL. It has been suggested that multiple stacked pulses at lower fluences may have similar effects on targets as a single pulse at a higher fluence, thereby minimizing thermal injury and leading to decreased risk for adverse events such as scarring. When treating vascular lesions such as telangiectases, increasing the fluence will increase the risk for purpura due to the constant pulse duration. Stacking pulses of lower fluence may have the advantage of heating vessels to a critical temperature without creating purpura, leading to similar clearance rates with decreased adverse risk profiles.15
It may be better to err on the side of safety by performing a greater number of treatment sessions with increased pulse width and decreased fluence (subpurpuric treatment settings) to minimize the risk for atrophic scarring from treatment with the PDL. Treating superficial facial telangiectases with a pulse-stacking technique may improve clinical results without a remarkable increase in adverse effects. It may be wrongfully intuitive to try to maximize results by using high fluences and purpuric narrow pulse durations; this case report reiterates the danger of using these settings in an attempt to rapidly achieve clearance of telangiectases. Lastly, this case underscores the value of verbal and written postoperative instructions that should be given to every patient prior to undergoing laser therapy. Specifically, with regard to our case, the laser operator must be aware at all times of potential adverse events, which may be foreseen during treatment if persistent or prolonged blanching and/or blistering occurs. The physician operator and patient must be prepared to rapidly respond to adverse reactions such as skin necrosis or blistering. Meticulous wound care is necessary if skin breakdown occurs. We recommend using a hydrating petrolatum ointment or a topical emulsion to minimize the risks for scarring, if possible.
1. Levine VJ, Geronemus RG. Adverse effects associated with the 577- and 585-nanometer pulsed dye laser in the treatment of cutaneous vascular lesions: a study of 500 patients. J Am Acad Dermatol. 1995;32:613-617.
2. Witman PM, Wagner AM, Scherer K, et al. Complications following pulsed dye laser treatment of superficial hemangiomas. Lasers Surg Med. 2006;38:116-123.
3. Sommer S, Sheehan-Dare RA. Atrophie blanche-like scarring after pulsed dye laser treatment. J Am Acad Dermatol. 1999;41:100-102.
4. Dover JS, Geronemus R, Stern RS, et al. Dye laser treatment of port-wine stains: comparison of the continuous-wave dye laser with a robotized scanning device and the pulsed dye laser. J Am Acad Dermatol. 1995;32(2, pt 1):237-240.
5. Dierickx C, Goldman MP, Fitzpatrick RE. Laser treatment of erythematous/hypertrophic and pigmented scars in 26 patients. Plast Reconstr Surg. 1995;95:84-90.
6. Wittenberg GP, Fabian BG, Bogomilsky JL, et al. Prospective, single-blind, randomized, controlled study to assess the efficacy of the 585-nm flashlamp-pumped pulsed-dye laser and silicone gel sheeting in hypertrophic scar treatment. Arch Dermatol. 1999;135:1049-1055.
7. Ross EV, Uebelhoer NS, Domankevitz Y. Use of a novel pulse dye laser for rapid single-pass purpura-free treatment of telangiectases. Dermatol Surg. 2007;33:1466-1469.
8. Seukeran DC, Collins P, Sheehan-Dare RA. Adverse reactions following pulsed tunable dye laser treatment of port wine stains in 701 patients. Br J Dermatol. 1997;136:725-729.
9. Wlotzke U, Hohenleutner U, Abd-El-Raheem TA, et al. Side-effects and complications of flashlamp-pumped pulsed dye laser therapy of port-wine stains. a prospective study. Br J Dermatol. 1996;134:475-480.
10. Tan OT, Morrison P, Kurban AK. 585 nm for the treatment of port-wine stains. Plast Reconstr Surg. 1990;86:1112-1117.
11. Alam M, Pon K, Van Laborde S, et al. Clinical effect of a single pulsed dye laser treatment of fresh surgical scars: randomized controlled trial. Dermatol Surg. 2006;32:21-25.
12. Bowes LE, Nouri K, Berman B, et al. Treatment of pigmented hypertrophic scars with the 585 nm pulsed dye laser and the 532 nm frequency-doubled Nd:YAG laser in the Q-switched and variable pulse modes: a comparative study. Dermatol Surg. 2002;28:714-719.
13. Alster TS, Williams CM. Treatment of keloid sternotomy scars with 585 nm flashlamp-pumped pulsed-dye laser. Lancet. 1995;345:1198-1200.
14. Manuskiatti W, Fitzpatrick RE. Treatment response of keloidal and hypertrophic sternotomy scars: comparison among intralesional corticosteroid, 5-fluorouracil, and 585-nm flashlamp-pumped pulsed-dye laser treatments. Arch Dermatol. 2002;138:1149-1155.
15. Rohrer TE, Chatrath V, Iyengar V. Does pulse stacking improve the results of treatment with variable-pulse pulsed-dye lasers? Dermatol Surg. 2004;30(2, pt 1):163-167.
The pulsed dye laser (PDL) has been widely used in the treatment of port-wine stains, telangiectases, and other cutaneous vascular lesions since the late 1980s.1 This treatment modality generally is considered to have few serious adverse effects. There have been few reports of PDL treatment with subsequent complications,1-3 which may include ulceration developing immediately after treatment as well as scarring with a spotlike pattern caused by laser therapy. Numerous studies within the last 2 decades have documented improvement in the appearance of scars and telangiectases following treatment with PDL.4-6 We report the case of a 42-year-old woman who developed atrophic linear scarring of the nasal ala following cosmetic treatment with a 595-nm PDL.
Case Report
A healthy 42-year-old woman presented with atrophic linear scarring of the bilateral nasal alae following treatment with a 595-nm PDL. The patient had initially presented to an outside clinic 13 months prior for treatment of multiple telangiectases in this area. She received a single, 1-pass treatment with the 595-nm PDL (spot size, 3×10 mm; fluence, 11 J/cm2; pulse duration, 1.5 milliseconds) and returned to the clinic approximately 2 months later for a second treatment with the same settings. Seven months later she returned for a third treatment of the recalcitrant alar telangiectases with the same settings to maximize clinical outcome. Dynamic cooling was used during all treatment sessions with 30/20 setting. After the third treatment, immediate blanching followed by purpura was noted in the treated area. The patient initially was lost to follow-up but returned to the outside clinic 6 months later. On physical examination white atrophic skin with linear scarred depressions were noted on the nasofacial angle of the nasal alae (Figure). The patient denied any postoperative complications such as scabbing, blistering, or pain. At that time she was referred to our office for evaluation, and treatment with a hyaluronic acid filler was initiated. Examination and medical history were otherwise unremarkable at the time of presentation to our office. Resolution of skin atrophy and excellent correction of the depressions was maintained at a follow-up 2 months later. She declined photographs at that time.

|

|

|
| Right (A), left (B), and frontal (C) views of atrophic linear scars (arrows) caused by high-energy purpuric doses of a 595-nm pulsed dye laser. |
Comment
The PDL often is employed in the treatment of vascular lesions such as telangiectases.7 The most common adverse effect is postinflammatory hyperpigmentation; atrophic and hypertrophic scarring rarely are seen.1,8,9 In a study of adverse reactions following pulsed tunable dye laser treatment of port-wine stains in 701 patients, atrophic scarring occurred in 5% of patients and 0.83% of treatments; clinical resolution was noted over the following 6 to 12 months in 30% of patients.8
Following treatment with the PDL, thermal damage occurs primarily to vessel walls with little or no damage to surrounding nonvascular structures. The depth of vascular injury after PDL treatment has been shown to be approximately 1.2 mm.10
Although scarring in our patient was a result of PDL treatment, PDL therapy is commonly used as a treatment option for scars. In conjunction with intralesional steroids directed at flattening hypertrophic scars and keloids, the PDL is used to reduce scar redness and enhance pliability.11 Although redness and telangiectases that develop in surgical scars usually spontaneously remit, they often can show prolonged and incomplete healing. Surgical scars have been shown to benefit from PDL treatment as it advances the end point closer to the complete absence of redness.11-14
The off-label use of hyaluronic acid filler in our patient is notable, as the injection of the nasal ala is not an ordinary injection site for this filler material. It can be associated with risk for necrosis and thus must be performed by an experienced injector combined with informed consent from the patient. The nasal ala is particularly sebaceous and consists of fibrofatty tissue, which is not easily amenable to infiltration. Despite this usual characteristic of the nasal area, the scarring in our patient was fortuitously lateral to the nasal ala and easily filled with hyaluronic acid, as a linear tract was created by the high energies and linear spot size used to treat the patient.
We report a 595-nm PDL treatment that resulted in atrophic linear scarring in a distribution mimicking the linear spot size used by the laser operator. No adverse effects were noted following the first 2 treatments, thereby suggesting either a cumulative insult or more likely cutaneous necrosis from excessive fluence and short pulse durations due to operator inexperience. Other possibilities include rapid and overlapping passes with the laser leading to bulk heating and thermal injury to the skin.
Alternative laser treatment protocols have been proposed in the literature. Rohrer et al15 recommended multiple passes at subpurpuric doses for treatment of facial telangiectases with the PDL. It has been suggested that multiple stacked pulses at lower fluences may have similar effects on targets as a single pulse at a higher fluence, thereby minimizing thermal injury and leading to decreased risk for adverse events such as scarring. When treating vascular lesions such as telangiectases, increasing the fluence will increase the risk for purpura due to the constant pulse duration. Stacking pulses of lower fluence may have the advantage of heating vessels to a critical temperature without creating purpura, leading to similar clearance rates with decreased adverse risk profiles.15
It may be better to err on the side of safety by performing a greater number of treatment sessions with increased pulse width and decreased fluence (subpurpuric treatment settings) to minimize the risk for atrophic scarring from treatment with the PDL. Treating superficial facial telangiectases with a pulse-stacking technique may improve clinical results without a remarkable increase in adverse effects. It may be wrongfully intuitive to try to maximize results by using high fluences and purpuric narrow pulse durations; this case report reiterates the danger of using these settings in an attempt to rapidly achieve clearance of telangiectases. Lastly, this case underscores the value of verbal and written postoperative instructions that should be given to every patient prior to undergoing laser therapy. Specifically, with regard to our case, the laser operator must be aware at all times of potential adverse events, which may be foreseen during treatment if persistent or prolonged blanching and/or blistering occurs. The physician operator and patient must be prepared to rapidly respond to adverse reactions such as skin necrosis or blistering. Meticulous wound care is necessary if skin breakdown occurs. We recommend using a hydrating petrolatum ointment or a topical emulsion to minimize the risks for scarring, if possible.
The pulsed dye laser (PDL) has been widely used in the treatment of port-wine stains, telangiectases, and other cutaneous vascular lesions since the late 1980s.1 This treatment modality generally is considered to have few serious adverse effects. There have been few reports of PDL treatment with subsequent complications,1-3 which may include ulceration developing immediately after treatment as well as scarring with a spotlike pattern caused by laser therapy. Numerous studies within the last 2 decades have documented improvement in the appearance of scars and telangiectases following treatment with PDL.4-6 We report the case of a 42-year-old woman who developed atrophic linear scarring of the nasal ala following cosmetic treatment with a 595-nm PDL.
Case Report
A healthy 42-year-old woman presented with atrophic linear scarring of the bilateral nasal alae following treatment with a 595-nm PDL. The patient had initially presented to an outside clinic 13 months prior for treatment of multiple telangiectases in this area. She received a single, 1-pass treatment with the 595-nm PDL (spot size, 3×10 mm; fluence, 11 J/cm2; pulse duration, 1.5 milliseconds) and returned to the clinic approximately 2 months later for a second treatment with the same settings. Seven months later she returned for a third treatment of the recalcitrant alar telangiectases with the same settings to maximize clinical outcome. Dynamic cooling was used during all treatment sessions with 30/20 setting. After the third treatment, immediate blanching followed by purpura was noted in the treated area. The patient initially was lost to follow-up but returned to the outside clinic 6 months later. On physical examination white atrophic skin with linear scarred depressions were noted on the nasofacial angle of the nasal alae (Figure). The patient denied any postoperative complications such as scabbing, blistering, or pain. At that time she was referred to our office for evaluation, and treatment with a hyaluronic acid filler was initiated. Examination and medical history were otherwise unremarkable at the time of presentation to our office. Resolution of skin atrophy and excellent correction of the depressions was maintained at a follow-up 2 months later. She declined photographs at that time.

|

|

|
| Right (A), left (B), and frontal (C) views of atrophic linear scars (arrows) caused by high-energy purpuric doses of a 595-nm pulsed dye laser. |
Comment
The PDL often is employed in the treatment of vascular lesions such as telangiectases.7 The most common adverse effect is postinflammatory hyperpigmentation; atrophic and hypertrophic scarring rarely are seen.1,8,9 In a study of adverse reactions following pulsed tunable dye laser treatment of port-wine stains in 701 patients, atrophic scarring occurred in 5% of patients and 0.83% of treatments; clinical resolution was noted over the following 6 to 12 months in 30% of patients.8
Following treatment with the PDL, thermal damage occurs primarily to vessel walls with little or no damage to surrounding nonvascular structures. The depth of vascular injury after PDL treatment has been shown to be approximately 1.2 mm.10
Although scarring in our patient was a result of PDL treatment, PDL therapy is commonly used as a treatment option for scars. In conjunction with intralesional steroids directed at flattening hypertrophic scars and keloids, the PDL is used to reduce scar redness and enhance pliability.11 Although redness and telangiectases that develop in surgical scars usually spontaneously remit, they often can show prolonged and incomplete healing. Surgical scars have been shown to benefit from PDL treatment as it advances the end point closer to the complete absence of redness.11-14
The off-label use of hyaluronic acid filler in our patient is notable, as the injection of the nasal ala is not an ordinary injection site for this filler material. It can be associated with risk for necrosis and thus must be performed by an experienced injector combined with informed consent from the patient. The nasal ala is particularly sebaceous and consists of fibrofatty tissue, which is not easily amenable to infiltration. Despite this usual characteristic of the nasal area, the scarring in our patient was fortuitously lateral to the nasal ala and easily filled with hyaluronic acid, as a linear tract was created by the high energies and linear spot size used to treat the patient.
We report a 595-nm PDL treatment that resulted in atrophic linear scarring in a distribution mimicking the linear spot size used by the laser operator. No adverse effects were noted following the first 2 treatments, thereby suggesting either a cumulative insult or more likely cutaneous necrosis from excessive fluence and short pulse durations due to operator inexperience. Other possibilities include rapid and overlapping passes with the laser leading to bulk heating and thermal injury to the skin.
Alternative laser treatment protocols have been proposed in the literature. Rohrer et al15 recommended multiple passes at subpurpuric doses for treatment of facial telangiectases with the PDL. It has been suggested that multiple stacked pulses at lower fluences may have similar effects on targets as a single pulse at a higher fluence, thereby minimizing thermal injury and leading to decreased risk for adverse events such as scarring. When treating vascular lesions such as telangiectases, increasing the fluence will increase the risk for purpura due to the constant pulse duration. Stacking pulses of lower fluence may have the advantage of heating vessels to a critical temperature without creating purpura, leading to similar clearance rates with decreased adverse risk profiles.15
It may be better to err on the side of safety by performing a greater number of treatment sessions with increased pulse width and decreased fluence (subpurpuric treatment settings) to minimize the risk for atrophic scarring from treatment with the PDL. Treating superficial facial telangiectases with a pulse-stacking technique may improve clinical results without a remarkable increase in adverse effects. It may be wrongfully intuitive to try to maximize results by using high fluences and purpuric narrow pulse durations; this case report reiterates the danger of using these settings in an attempt to rapidly achieve clearance of telangiectases. Lastly, this case underscores the value of verbal and written postoperative instructions that should be given to every patient prior to undergoing laser therapy. Specifically, with regard to our case, the laser operator must be aware at all times of potential adverse events, which may be foreseen during treatment if persistent or prolonged blanching and/or blistering occurs. The physician operator and patient must be prepared to rapidly respond to adverse reactions such as skin necrosis or blistering. Meticulous wound care is necessary if skin breakdown occurs. We recommend using a hydrating petrolatum ointment or a topical emulsion to minimize the risks for scarring, if possible.
1. Levine VJ, Geronemus RG. Adverse effects associated with the 577- and 585-nanometer pulsed dye laser in the treatment of cutaneous vascular lesions: a study of 500 patients. J Am Acad Dermatol. 1995;32:613-617.
2. Witman PM, Wagner AM, Scherer K, et al. Complications following pulsed dye laser treatment of superficial hemangiomas. Lasers Surg Med. 2006;38:116-123.
3. Sommer S, Sheehan-Dare RA. Atrophie blanche-like scarring after pulsed dye laser treatment. J Am Acad Dermatol. 1999;41:100-102.
4. Dover JS, Geronemus R, Stern RS, et al. Dye laser treatment of port-wine stains: comparison of the continuous-wave dye laser with a robotized scanning device and the pulsed dye laser. J Am Acad Dermatol. 1995;32(2, pt 1):237-240.
5. Dierickx C, Goldman MP, Fitzpatrick RE. Laser treatment of erythematous/hypertrophic and pigmented scars in 26 patients. Plast Reconstr Surg. 1995;95:84-90.
6. Wittenberg GP, Fabian BG, Bogomilsky JL, et al. Prospective, single-blind, randomized, controlled study to assess the efficacy of the 585-nm flashlamp-pumped pulsed-dye laser and silicone gel sheeting in hypertrophic scar treatment. Arch Dermatol. 1999;135:1049-1055.
7. Ross EV, Uebelhoer NS, Domankevitz Y. Use of a novel pulse dye laser for rapid single-pass purpura-free treatment of telangiectases. Dermatol Surg. 2007;33:1466-1469.
8. Seukeran DC, Collins P, Sheehan-Dare RA. Adverse reactions following pulsed tunable dye laser treatment of port wine stains in 701 patients. Br J Dermatol. 1997;136:725-729.
9. Wlotzke U, Hohenleutner U, Abd-El-Raheem TA, et al. Side-effects and complications of flashlamp-pumped pulsed dye laser therapy of port-wine stains. a prospective study. Br J Dermatol. 1996;134:475-480.
10. Tan OT, Morrison P, Kurban AK. 585 nm for the treatment of port-wine stains. Plast Reconstr Surg. 1990;86:1112-1117.
11. Alam M, Pon K, Van Laborde S, et al. Clinical effect of a single pulsed dye laser treatment of fresh surgical scars: randomized controlled trial. Dermatol Surg. 2006;32:21-25.
12. Bowes LE, Nouri K, Berman B, et al. Treatment of pigmented hypertrophic scars with the 585 nm pulsed dye laser and the 532 nm frequency-doubled Nd:YAG laser in the Q-switched and variable pulse modes: a comparative study. Dermatol Surg. 2002;28:714-719.
13. Alster TS, Williams CM. Treatment of keloid sternotomy scars with 585 nm flashlamp-pumped pulsed-dye laser. Lancet. 1995;345:1198-1200.
14. Manuskiatti W, Fitzpatrick RE. Treatment response of keloidal and hypertrophic sternotomy scars: comparison among intralesional corticosteroid, 5-fluorouracil, and 585-nm flashlamp-pumped pulsed-dye laser treatments. Arch Dermatol. 2002;138:1149-1155.
15. Rohrer TE, Chatrath V, Iyengar V. Does pulse stacking improve the results of treatment with variable-pulse pulsed-dye lasers? Dermatol Surg. 2004;30(2, pt 1):163-167.
1. Levine VJ, Geronemus RG. Adverse effects associated with the 577- and 585-nanometer pulsed dye laser in the treatment of cutaneous vascular lesions: a study of 500 patients. J Am Acad Dermatol. 1995;32:613-617.
2. Witman PM, Wagner AM, Scherer K, et al. Complications following pulsed dye laser treatment of superficial hemangiomas. Lasers Surg Med. 2006;38:116-123.
3. Sommer S, Sheehan-Dare RA. Atrophie blanche-like scarring after pulsed dye laser treatment. J Am Acad Dermatol. 1999;41:100-102.
4. Dover JS, Geronemus R, Stern RS, et al. Dye laser treatment of port-wine stains: comparison of the continuous-wave dye laser with a robotized scanning device and the pulsed dye laser. J Am Acad Dermatol. 1995;32(2, pt 1):237-240.
5. Dierickx C, Goldman MP, Fitzpatrick RE. Laser treatment of erythematous/hypertrophic and pigmented scars in 26 patients. Plast Reconstr Surg. 1995;95:84-90.
6. Wittenberg GP, Fabian BG, Bogomilsky JL, et al. Prospective, single-blind, randomized, controlled study to assess the efficacy of the 585-nm flashlamp-pumped pulsed-dye laser and silicone gel sheeting in hypertrophic scar treatment. Arch Dermatol. 1999;135:1049-1055.
7. Ross EV, Uebelhoer NS, Domankevitz Y. Use of a novel pulse dye laser for rapid single-pass purpura-free treatment of telangiectases. Dermatol Surg. 2007;33:1466-1469.
8. Seukeran DC, Collins P, Sheehan-Dare RA. Adverse reactions following pulsed tunable dye laser treatment of port wine stains in 701 patients. Br J Dermatol. 1997;136:725-729.
9. Wlotzke U, Hohenleutner U, Abd-El-Raheem TA, et al. Side-effects and complications of flashlamp-pumped pulsed dye laser therapy of port-wine stains. a prospective study. Br J Dermatol. 1996;134:475-480.
10. Tan OT, Morrison P, Kurban AK. 585 nm for the treatment of port-wine stains. Plast Reconstr Surg. 1990;86:1112-1117.
11. Alam M, Pon K, Van Laborde S, et al. Clinical effect of a single pulsed dye laser treatment of fresh surgical scars: randomized controlled trial. Dermatol Surg. 2006;32:21-25.
12. Bowes LE, Nouri K, Berman B, et al. Treatment of pigmented hypertrophic scars with the 585 nm pulsed dye laser and the 532 nm frequency-doubled Nd:YAG laser in the Q-switched and variable pulse modes: a comparative study. Dermatol Surg. 2002;28:714-719.
13. Alster TS, Williams CM. Treatment of keloid sternotomy scars with 585 nm flashlamp-pumped pulsed-dye laser. Lancet. 1995;345:1198-1200.
14. Manuskiatti W, Fitzpatrick RE. Treatment response of keloidal and hypertrophic sternotomy scars: comparison among intralesional corticosteroid, 5-fluorouracil, and 585-nm flashlamp-pumped pulsed-dye laser treatments. Arch Dermatol. 2002;138:1149-1155.
15. Rohrer TE, Chatrath V, Iyengar V. Does pulse stacking improve the results of treatment with variable-pulse pulsed-dye lasers? Dermatol Surg. 2004;30(2, pt 1):163-167.
Practice Points
- Lasers should be used by experienced operators and treatments should be tailored to individual patient needs.
- Multiple passes at subpurpuric settings with the pulsed dye laser may lead to safer results with fewer adverse events and at the same time more tolerable treatments for the patient by minimizing downtime associated with purpura.
- Although scarring is rare, it can occur and should be part of the patient’s informed consent prior to treatment.