User login
Zoledronate maintains bone loss after denosumab discontinuation
Women with postmenopausal osteoporosis who discontinued denosumab treatment after achieving osteopenia maintained bone mineral density at the spine and hip with a single infusion of zoledronate given 6 months after the last infusion of denosumab, according to results from a small, multicenter, randomized trial published in the Journal of Bone and Mineral Research.
The cessation of the monoclonal antibody denosumab is typically followed by a “rebound phenomenon” often attributed to an increase in bone turnover above pretreatment values caused by the up-regulation of osteoclastogenesis, according to Athanasios D. Anastasilakis, MD, of 424 General Military Hospital, Thessaloníki, Greece, and colleagues. Guidelines recommend that patients take a bisphosphonate to prevent this effect, but the optimal bisphosphonate regimen is unknown and evidence is inconsistent.
To address this question, the investigators randomized 57 postmenopausal women with osteoporosis who had received six monthly injections of denosumab (for an average of 2.2 years) and had achieved nonosteoporotic bone mineral density (BMD) T scores greater than –2.5 but no greater than –1 at the hip or the spine. A total of 27 received a single IV infusion of zoledronate 5 mg given 6 months after the last denosumab injection with a 3-week window, and 30 continued denosumab and received two additional monthly 60-mg injections. Following either the zoledronate infusion or the last denosumab injection, all women received no treatment and were followed until 2 years from randomization. All women were given vitamin D supplements and were seen in clinic appointments at baseline, 6, 12, 15, 18, and 24 months.
Areal BMD of the lumbar spine and femoral neck of the nondominant hip were measured at baseline, 12, and 24 months by dual-energy x-ray absorptiometry, and least significant changes were 5% or less at the spine and 4% or less at the femoral neck, based on proposals from the International Foundation for Osteoporosis and the National Osteoporosis Foundation USA.
At 24 months, lumbar spine BMD (LS‐BMD) returned to baseline in the zoledronate group, but decreased in the denosumab group by 4.82% from the 12‐month value (P less than .001).
The difference in LS-BMD changes between the two groups from month 12 to 24, the primary endpoint of the study, was statistically significant (–0.018 with zoledronate vs. –0.045 with denosumab; P = .025). Differences in changes of femoral neck BMD were also statistically significant (–0.004 with zoledronate vs. –0.038 with denosumab; P = .005), the researchers reported.
The differences in BMD changes between the two groups 24 and 12 months after discontinuation of denosumab (6 months after the last injection) for the zoledronate and denosumab group respectively were also statistically significant both at the lumbar spine (–0.002 with zoledronate vs. –0.045 with denosumab; P = .03) and at the femoral neck (–0.004 with zoledronate vs. –0.038 with denosumab; P = .007).
The authors observed no relationship between the number of denosumab injections and LS-BMD changes in either group of women; however, they noted that responses of individual patients to zoledronate were variable. For example, three women who took zoledronate experienced decreases of LS-BMD greater than the least significant change observed at 24 months, a finding which could not be explained by the timing of the infusion, baseline rate of bone turnover, or baseline BMD.
“It appears that intrinsic factors that still need to be defined may affect the response of a few individuals,” they wrote.
This was further illustrated by one patient in the zoledronate group who sustained clinical vertebral fractures associated with significant, unexplained decreases of BMD that could not be prevented with the zoledronate infusion.
“In clinical practice, it is, therefore, advisable to measure BMD at 12 months after the zoledronate infusion and decide whether additional treatment may be required,” the authors wrote.
Another significant finding reported by the authors was that neither baseline nor 12‐month bone turnover marker (BTM) values were associated with BMD changes in either group of women during the entire study period.
“Particularly important for clinical practice was the lack of a relationship in zoledronate-treated women; even when women were divided according to baseline median BTM values (below or above) there were no significant difference in BMD changes at 12 or 24 months,” they wrote.
“In a substantial number of women in the denosumab group BTMs were still above the upper limit of normal of the postmenopausal age 18 months after the last Dmab [denosumab] injection but also in 7.4% of patients treated with zoledronate at 2 years,” they added.
“Whether in the latter patients BTMs were also increased before the start of Dmab treatment, as it is known to occur in some patients with osteoporosis, or are due to a prolonged effect of Dmab withdrawal on bone metabolism could not be prevented by zoledronate, is not known because pretreatment data were not available,” the study authors noted.
For adverse events, in addition to the one patient in the zoledronate group with clinical vertebral fractures, three patients in the denosumab group sustained vertebral fractures.
“Prevalent vertebral fractures have been previously reported as the most important risk factor for clinical vertebral fractures following cessation of Dmab therapy [which] strongly suggest that spine x-rays should be performed in all patients in whom discontinuation of Dmab treatment is considered,” the authors wrote.
“In most women with postmenopausal osteoporosis treated with [denosumab] in whom discontinuation of treatment is considered when a nonosteoporotic BMD is achieved, a single intravenous infusion of zoledronate 5 mg given 6 months after the last Dmab injection prevents bone loss for at least 2 years independently of the rate of bone turnover. Follow-up is recommended, as in a few patients treatment might not have the expected effect at 2 years for currently unknown reasons,” they concluded.
The study was funded by institutional funds and the Hellenic Endocrine Society. Several authors reported receiving consulting or lecture fees from Amgen, which markets denosumab, as well as other pharmaceutical companies.
SOURCE: Anastasilakis A et al. J Bone Miner Res. 2019 Aug 21. doi: 10.1002/jbmr.3853.
Women with postmenopausal osteoporosis who discontinued denosumab treatment after achieving osteopenia maintained bone mineral density at the spine and hip with a single infusion of zoledronate given 6 months after the last infusion of denosumab, according to results from a small, multicenter, randomized trial published in the Journal of Bone and Mineral Research.
The cessation of the monoclonal antibody denosumab is typically followed by a “rebound phenomenon” often attributed to an increase in bone turnover above pretreatment values caused by the up-regulation of osteoclastogenesis, according to Athanasios D. Anastasilakis, MD, of 424 General Military Hospital, Thessaloníki, Greece, and colleagues. Guidelines recommend that patients take a bisphosphonate to prevent this effect, but the optimal bisphosphonate regimen is unknown and evidence is inconsistent.
To address this question, the investigators randomized 57 postmenopausal women with osteoporosis who had received six monthly injections of denosumab (for an average of 2.2 years) and had achieved nonosteoporotic bone mineral density (BMD) T scores greater than –2.5 but no greater than –1 at the hip or the spine. A total of 27 received a single IV infusion of zoledronate 5 mg given 6 months after the last denosumab injection with a 3-week window, and 30 continued denosumab and received two additional monthly 60-mg injections. Following either the zoledronate infusion or the last denosumab injection, all women received no treatment and were followed until 2 years from randomization. All women were given vitamin D supplements and were seen in clinic appointments at baseline, 6, 12, 15, 18, and 24 months.
Areal BMD of the lumbar spine and femoral neck of the nondominant hip were measured at baseline, 12, and 24 months by dual-energy x-ray absorptiometry, and least significant changes were 5% or less at the spine and 4% or less at the femoral neck, based on proposals from the International Foundation for Osteoporosis and the National Osteoporosis Foundation USA.
At 24 months, lumbar spine BMD (LS‐BMD) returned to baseline in the zoledronate group, but decreased in the denosumab group by 4.82% from the 12‐month value (P less than .001).
The difference in LS-BMD changes between the two groups from month 12 to 24, the primary endpoint of the study, was statistically significant (–0.018 with zoledronate vs. –0.045 with denosumab; P = .025). Differences in changes of femoral neck BMD were also statistically significant (–0.004 with zoledronate vs. –0.038 with denosumab; P = .005), the researchers reported.
The differences in BMD changes between the two groups 24 and 12 months after discontinuation of denosumab (6 months after the last injection) for the zoledronate and denosumab group respectively were also statistically significant both at the lumbar spine (–0.002 with zoledronate vs. –0.045 with denosumab; P = .03) and at the femoral neck (–0.004 with zoledronate vs. –0.038 with denosumab; P = .007).
The authors observed no relationship between the number of denosumab injections and LS-BMD changes in either group of women; however, they noted that responses of individual patients to zoledronate were variable. For example, three women who took zoledronate experienced decreases of LS-BMD greater than the least significant change observed at 24 months, a finding which could not be explained by the timing of the infusion, baseline rate of bone turnover, or baseline BMD.
“It appears that intrinsic factors that still need to be defined may affect the response of a few individuals,” they wrote.
This was further illustrated by one patient in the zoledronate group who sustained clinical vertebral fractures associated with significant, unexplained decreases of BMD that could not be prevented with the zoledronate infusion.
“In clinical practice, it is, therefore, advisable to measure BMD at 12 months after the zoledronate infusion and decide whether additional treatment may be required,” the authors wrote.
Another significant finding reported by the authors was that neither baseline nor 12‐month bone turnover marker (BTM) values were associated with BMD changes in either group of women during the entire study period.
“Particularly important for clinical practice was the lack of a relationship in zoledronate-treated women; even when women were divided according to baseline median BTM values (below or above) there were no significant difference in BMD changes at 12 or 24 months,” they wrote.
“In a substantial number of women in the denosumab group BTMs were still above the upper limit of normal of the postmenopausal age 18 months after the last Dmab [denosumab] injection but also in 7.4% of patients treated with zoledronate at 2 years,” they added.
“Whether in the latter patients BTMs were also increased before the start of Dmab treatment, as it is known to occur in some patients with osteoporosis, or are due to a prolonged effect of Dmab withdrawal on bone metabolism could not be prevented by zoledronate, is not known because pretreatment data were not available,” the study authors noted.
For adverse events, in addition to the one patient in the zoledronate group with clinical vertebral fractures, three patients in the denosumab group sustained vertebral fractures.
“Prevalent vertebral fractures have been previously reported as the most important risk factor for clinical vertebral fractures following cessation of Dmab therapy [which] strongly suggest that spine x-rays should be performed in all patients in whom discontinuation of Dmab treatment is considered,” the authors wrote.
“In most women with postmenopausal osteoporosis treated with [denosumab] in whom discontinuation of treatment is considered when a nonosteoporotic BMD is achieved, a single intravenous infusion of zoledronate 5 mg given 6 months after the last Dmab injection prevents bone loss for at least 2 years independently of the rate of bone turnover. Follow-up is recommended, as in a few patients treatment might not have the expected effect at 2 years for currently unknown reasons,” they concluded.
The study was funded by institutional funds and the Hellenic Endocrine Society. Several authors reported receiving consulting or lecture fees from Amgen, which markets denosumab, as well as other pharmaceutical companies.
SOURCE: Anastasilakis A et al. J Bone Miner Res. 2019 Aug 21. doi: 10.1002/jbmr.3853.
Women with postmenopausal osteoporosis who discontinued denosumab treatment after achieving osteopenia maintained bone mineral density at the spine and hip with a single infusion of zoledronate given 6 months after the last infusion of denosumab, according to results from a small, multicenter, randomized trial published in the Journal of Bone and Mineral Research.
The cessation of the monoclonal antibody denosumab is typically followed by a “rebound phenomenon” often attributed to an increase in bone turnover above pretreatment values caused by the up-regulation of osteoclastogenesis, according to Athanasios D. Anastasilakis, MD, of 424 General Military Hospital, Thessaloníki, Greece, and colleagues. Guidelines recommend that patients take a bisphosphonate to prevent this effect, but the optimal bisphosphonate regimen is unknown and evidence is inconsistent.
To address this question, the investigators randomized 57 postmenopausal women with osteoporosis who had received six monthly injections of denosumab (for an average of 2.2 years) and had achieved nonosteoporotic bone mineral density (BMD) T scores greater than –2.5 but no greater than –1 at the hip or the spine. A total of 27 received a single IV infusion of zoledronate 5 mg given 6 months after the last denosumab injection with a 3-week window, and 30 continued denosumab and received two additional monthly 60-mg injections. Following either the zoledronate infusion or the last denosumab injection, all women received no treatment and were followed until 2 years from randomization. All women were given vitamin D supplements and were seen in clinic appointments at baseline, 6, 12, 15, 18, and 24 months.
Areal BMD of the lumbar spine and femoral neck of the nondominant hip were measured at baseline, 12, and 24 months by dual-energy x-ray absorptiometry, and least significant changes were 5% or less at the spine and 4% or less at the femoral neck, based on proposals from the International Foundation for Osteoporosis and the National Osteoporosis Foundation USA.
At 24 months, lumbar spine BMD (LS‐BMD) returned to baseline in the zoledronate group, but decreased in the denosumab group by 4.82% from the 12‐month value (P less than .001).
The difference in LS-BMD changes between the two groups from month 12 to 24, the primary endpoint of the study, was statistically significant (–0.018 with zoledronate vs. –0.045 with denosumab; P = .025). Differences in changes of femoral neck BMD were also statistically significant (–0.004 with zoledronate vs. –0.038 with denosumab; P = .005), the researchers reported.
The differences in BMD changes between the two groups 24 and 12 months after discontinuation of denosumab (6 months after the last injection) for the zoledronate and denosumab group respectively were also statistically significant both at the lumbar spine (–0.002 with zoledronate vs. –0.045 with denosumab; P = .03) and at the femoral neck (–0.004 with zoledronate vs. –0.038 with denosumab; P = .007).
The authors observed no relationship between the number of denosumab injections and LS-BMD changes in either group of women; however, they noted that responses of individual patients to zoledronate were variable. For example, three women who took zoledronate experienced decreases of LS-BMD greater than the least significant change observed at 24 months, a finding which could not be explained by the timing of the infusion, baseline rate of bone turnover, or baseline BMD.
“It appears that intrinsic factors that still need to be defined may affect the response of a few individuals,” they wrote.
This was further illustrated by one patient in the zoledronate group who sustained clinical vertebral fractures associated with significant, unexplained decreases of BMD that could not be prevented with the zoledronate infusion.
“In clinical practice, it is, therefore, advisable to measure BMD at 12 months after the zoledronate infusion and decide whether additional treatment may be required,” the authors wrote.
Another significant finding reported by the authors was that neither baseline nor 12‐month bone turnover marker (BTM) values were associated with BMD changes in either group of women during the entire study period.
“Particularly important for clinical practice was the lack of a relationship in zoledronate-treated women; even when women were divided according to baseline median BTM values (below or above) there were no significant difference in BMD changes at 12 or 24 months,” they wrote.
“In a substantial number of women in the denosumab group BTMs were still above the upper limit of normal of the postmenopausal age 18 months after the last Dmab [denosumab] injection but also in 7.4% of patients treated with zoledronate at 2 years,” they added.
“Whether in the latter patients BTMs were also increased before the start of Dmab treatment, as it is known to occur in some patients with osteoporosis, or are due to a prolonged effect of Dmab withdrawal on bone metabolism could not be prevented by zoledronate, is not known because pretreatment data were not available,” the study authors noted.
For adverse events, in addition to the one patient in the zoledronate group with clinical vertebral fractures, three patients in the denosumab group sustained vertebral fractures.
“Prevalent vertebral fractures have been previously reported as the most important risk factor for clinical vertebral fractures following cessation of Dmab therapy [which] strongly suggest that spine x-rays should be performed in all patients in whom discontinuation of Dmab treatment is considered,” the authors wrote.
“In most women with postmenopausal osteoporosis treated with [denosumab] in whom discontinuation of treatment is considered when a nonosteoporotic BMD is achieved, a single intravenous infusion of zoledronate 5 mg given 6 months after the last Dmab injection prevents bone loss for at least 2 years independently of the rate of bone turnover. Follow-up is recommended, as in a few patients treatment might not have the expected effect at 2 years for currently unknown reasons,” they concluded.
The study was funded by institutional funds and the Hellenic Endocrine Society. Several authors reported receiving consulting or lecture fees from Amgen, which markets denosumab, as well as other pharmaceutical companies.
SOURCE: Anastasilakis A et al. J Bone Miner Res. 2019 Aug 21. doi: 10.1002/jbmr.3853.
FROM THE JOURNAL OF BONE AND MINERAL RESEARCH
International lupus community sets out top barriers to improving lupus outcomes
The heterogeneity of lupus and the subsequent lack of a clear disease definition have been identified by an international group of experts as the primary barriers hindering timely diagnosis, improved treatment options, and appropriate access to care.
A report published in Lupus Science & Medicine titled “Global Consensus Building and Prioritization of Fundamental Lupus Challenges: The ALPHA Project” describes the results of a first-ever global consensus on key barriers to advances in lupus care, including a lack of validated biomarkers and flawed clinical trial design.
A lack of access to medical professionals familiar with lupus, challenges in managing lupus because of social determinants, and lack of treatment adherence were also considered to be barriers to improving the outcomes of people living with lupus.
First author Susan Manzi, MD, codirector of the Lupus Center of Excellence at Allegheny Health Network, Pittsburgh, and her colleagues said that, in contrast to other autoimmune diseases such as rheumatoid arthritis and psoriasis, the field of lupus has struggled with establishing a clear pathway for lupus drug development because of “persistent challenges in understanding the biology of the disease, defining clinical trial entry criteria and end points, developing instruments to measure changes in clinical activity, and controlling background medications.”
The authors noted that the intention of the Addressing Lupus Pillars for Health Advancement (ALPHA) Project was to build on the work of other initiatives, including some that were international in scope or were still ongoing.
“The ALPHA project was founded as the first step in an ongoing commitment to identify, prioritize, and implement strategies to address the most pressing challenges that limit progress in lupus across the continuum,” they wrote. In a joint initiative, the Lupus Foundation of America (LFA) and the Tufts Center for the Study of Drug Development (Tufts CSDD) set up a Global Advisory Committee (GAC) that included 13 lupus experts from the United States, Australia, United Kingdom, Germany, and South Korea to guide and oversee the study. Members had extensive knowledge of the disease, with specific expertise in rheumatology, dermatology, immunology, nephrology, and pediatrics.
Next, in-depth interviews were conducted with 17 experts who were well respected in the lupus scientific and care communities and represented all stakeholders. Using information garnered from these interviews, the LFA, Tufts CSDD, and GAC collaborated to develop a survey that included 23 questions addressing attitudes and perceptions about lupus as well as the prioritization of the most pressing challenges to improving diagnosis, care, treatment, and research.
The online survey was sent to 366 candidates, from whom the researchers received 127 completed responses. Of these, 82 (65%) were clinician-researcher-scientists and 14 (11%) worked in industry/biotechnology, 13 (10%) were researcher-scientists, and 12 (9%) were clinicians; 5% marked “other.”
The research team used a weighting system to prioritize barriers ranked by respondents, whereby higher ratings represented the challenges of highest impact (a score of 9 was highest rating, with 1 the lowest).
Survey respondents ranked the following as the top barriers to improving outcomes in lupus:
- A lack of diagnostic, predictive, and prognostic biomarkers for lupus (weighted prioritization score of 7.294) and lack of biomarkers to predict drug response in clinical trials (weighted prioritization score of 6.614).
- Flawed clinical trial design (weighted prioritization score of 6.370).
- Lack of access to clinicians familiar with lupus (weighted prioritization score of 6.873), and limited awareness of lupus among nonexpert medical professionals (weighted prioritization score of 5.800).
- Barriers to effective management of lupus because of social determinants of care in predominantly lower socioeconomic status areas (weighted prioritization score of 6.937).
- A lack of treatment adherence (weighted prioritization score of 6.717).
“A strong consensus built throughout the study, as themes and insights gathered from the in-depth interviews were highly consistent with those collected in the survey,” the researchers noted.
They said it was not surprising that the development of biomarkers had received a high ranking, as advances in this area would help accelerate drug development and precision medicine as well as more practical aspects of clinical care.
The research team acknowledged that substantial funds would be needed to address the top priorities identified in the study, and some of the issues may be more easily addressed than others.
“In the past decade, the overall funding landscape for lupus has been on a decline, particularly through the National Institutes of Health – the largest public funder of lupus research in the world – during a time in which arguably, lupus research has been prolific,” they wrote.
They concluded that comprehensive measures were needed to transform the lupus research and health care landscape.
“Lupus experts must convene to determine feasible and coordinated approaches for addressing long-standing barriers across the global lupus community,” they stressed.
The next part of the project will involve an international stakeholder meeting to develop a global road map of specific recommendations to address identified barriers, which “may include multipronged strategies using regulatory and advocacy approaches, scientific consensus building, communication efforts, among other possible tactics,” they added.
The ALPHA Project was launched in partnership with founding partner EMD Serono Research & Development (a business of Merck KGaA) and through additional support by GlaxoSmithKline. Many authors of the report had financial connections to the pharmaceutical industry.
SOURCE: Manzi S et al. Lupus Sci Med. 2019;6:e000342. doi: 10.1136/lupus-2019-000342.
The heterogeneity of lupus and the subsequent lack of a clear disease definition have been identified by an international group of experts as the primary barriers hindering timely diagnosis, improved treatment options, and appropriate access to care.
A report published in Lupus Science & Medicine titled “Global Consensus Building and Prioritization of Fundamental Lupus Challenges: The ALPHA Project” describes the results of a first-ever global consensus on key barriers to advances in lupus care, including a lack of validated biomarkers and flawed clinical trial design.
A lack of access to medical professionals familiar with lupus, challenges in managing lupus because of social determinants, and lack of treatment adherence were also considered to be barriers to improving the outcomes of people living with lupus.
First author Susan Manzi, MD, codirector of the Lupus Center of Excellence at Allegheny Health Network, Pittsburgh, and her colleagues said that, in contrast to other autoimmune diseases such as rheumatoid arthritis and psoriasis, the field of lupus has struggled with establishing a clear pathway for lupus drug development because of “persistent challenges in understanding the biology of the disease, defining clinical trial entry criteria and end points, developing instruments to measure changes in clinical activity, and controlling background medications.”
The authors noted that the intention of the Addressing Lupus Pillars for Health Advancement (ALPHA) Project was to build on the work of other initiatives, including some that were international in scope or were still ongoing.
“The ALPHA project was founded as the first step in an ongoing commitment to identify, prioritize, and implement strategies to address the most pressing challenges that limit progress in lupus across the continuum,” they wrote. In a joint initiative, the Lupus Foundation of America (LFA) and the Tufts Center for the Study of Drug Development (Tufts CSDD) set up a Global Advisory Committee (GAC) that included 13 lupus experts from the United States, Australia, United Kingdom, Germany, and South Korea to guide and oversee the study. Members had extensive knowledge of the disease, with specific expertise in rheumatology, dermatology, immunology, nephrology, and pediatrics.
Next, in-depth interviews were conducted with 17 experts who were well respected in the lupus scientific and care communities and represented all stakeholders. Using information garnered from these interviews, the LFA, Tufts CSDD, and GAC collaborated to develop a survey that included 23 questions addressing attitudes and perceptions about lupus as well as the prioritization of the most pressing challenges to improving diagnosis, care, treatment, and research.
The online survey was sent to 366 candidates, from whom the researchers received 127 completed responses. Of these, 82 (65%) were clinician-researcher-scientists and 14 (11%) worked in industry/biotechnology, 13 (10%) were researcher-scientists, and 12 (9%) were clinicians; 5% marked “other.”
The research team used a weighting system to prioritize barriers ranked by respondents, whereby higher ratings represented the challenges of highest impact (a score of 9 was highest rating, with 1 the lowest).
Survey respondents ranked the following as the top barriers to improving outcomes in lupus:
- A lack of diagnostic, predictive, and prognostic biomarkers for lupus (weighted prioritization score of 7.294) and lack of biomarkers to predict drug response in clinical trials (weighted prioritization score of 6.614).
- Flawed clinical trial design (weighted prioritization score of 6.370).
- Lack of access to clinicians familiar with lupus (weighted prioritization score of 6.873), and limited awareness of lupus among nonexpert medical professionals (weighted prioritization score of 5.800).
- Barriers to effective management of lupus because of social determinants of care in predominantly lower socioeconomic status areas (weighted prioritization score of 6.937).
- A lack of treatment adherence (weighted prioritization score of 6.717).
“A strong consensus built throughout the study, as themes and insights gathered from the in-depth interviews were highly consistent with those collected in the survey,” the researchers noted.
They said it was not surprising that the development of biomarkers had received a high ranking, as advances in this area would help accelerate drug development and precision medicine as well as more practical aspects of clinical care.
The research team acknowledged that substantial funds would be needed to address the top priorities identified in the study, and some of the issues may be more easily addressed than others.
“In the past decade, the overall funding landscape for lupus has been on a decline, particularly through the National Institutes of Health – the largest public funder of lupus research in the world – during a time in which arguably, lupus research has been prolific,” they wrote.
They concluded that comprehensive measures were needed to transform the lupus research and health care landscape.
“Lupus experts must convene to determine feasible and coordinated approaches for addressing long-standing barriers across the global lupus community,” they stressed.
The next part of the project will involve an international stakeholder meeting to develop a global road map of specific recommendations to address identified barriers, which “may include multipronged strategies using regulatory and advocacy approaches, scientific consensus building, communication efforts, among other possible tactics,” they added.
The ALPHA Project was launched in partnership with founding partner EMD Serono Research & Development (a business of Merck KGaA) and through additional support by GlaxoSmithKline. Many authors of the report had financial connections to the pharmaceutical industry.
SOURCE: Manzi S et al. Lupus Sci Med. 2019;6:e000342. doi: 10.1136/lupus-2019-000342.
The heterogeneity of lupus and the subsequent lack of a clear disease definition have been identified by an international group of experts as the primary barriers hindering timely diagnosis, improved treatment options, and appropriate access to care.
A report published in Lupus Science & Medicine titled “Global Consensus Building and Prioritization of Fundamental Lupus Challenges: The ALPHA Project” describes the results of a first-ever global consensus on key barriers to advances in lupus care, including a lack of validated biomarkers and flawed clinical trial design.
A lack of access to medical professionals familiar with lupus, challenges in managing lupus because of social determinants, and lack of treatment adherence were also considered to be barriers to improving the outcomes of people living with lupus.
First author Susan Manzi, MD, codirector of the Lupus Center of Excellence at Allegheny Health Network, Pittsburgh, and her colleagues said that, in contrast to other autoimmune diseases such as rheumatoid arthritis and psoriasis, the field of lupus has struggled with establishing a clear pathway for lupus drug development because of “persistent challenges in understanding the biology of the disease, defining clinical trial entry criteria and end points, developing instruments to measure changes in clinical activity, and controlling background medications.”
The authors noted that the intention of the Addressing Lupus Pillars for Health Advancement (ALPHA) Project was to build on the work of other initiatives, including some that were international in scope or were still ongoing.
“The ALPHA project was founded as the first step in an ongoing commitment to identify, prioritize, and implement strategies to address the most pressing challenges that limit progress in lupus across the continuum,” they wrote. In a joint initiative, the Lupus Foundation of America (LFA) and the Tufts Center for the Study of Drug Development (Tufts CSDD) set up a Global Advisory Committee (GAC) that included 13 lupus experts from the United States, Australia, United Kingdom, Germany, and South Korea to guide and oversee the study. Members had extensive knowledge of the disease, with specific expertise in rheumatology, dermatology, immunology, nephrology, and pediatrics.
Next, in-depth interviews were conducted with 17 experts who were well respected in the lupus scientific and care communities and represented all stakeholders. Using information garnered from these interviews, the LFA, Tufts CSDD, and GAC collaborated to develop a survey that included 23 questions addressing attitudes and perceptions about lupus as well as the prioritization of the most pressing challenges to improving diagnosis, care, treatment, and research.
The online survey was sent to 366 candidates, from whom the researchers received 127 completed responses. Of these, 82 (65%) were clinician-researcher-scientists and 14 (11%) worked in industry/biotechnology, 13 (10%) were researcher-scientists, and 12 (9%) were clinicians; 5% marked “other.”
The research team used a weighting system to prioritize barriers ranked by respondents, whereby higher ratings represented the challenges of highest impact (a score of 9 was highest rating, with 1 the lowest).
Survey respondents ranked the following as the top barriers to improving outcomes in lupus:
- A lack of diagnostic, predictive, and prognostic biomarkers for lupus (weighted prioritization score of 7.294) and lack of biomarkers to predict drug response in clinical trials (weighted prioritization score of 6.614).
- Flawed clinical trial design (weighted prioritization score of 6.370).
- Lack of access to clinicians familiar with lupus (weighted prioritization score of 6.873), and limited awareness of lupus among nonexpert medical professionals (weighted prioritization score of 5.800).
- Barriers to effective management of lupus because of social determinants of care in predominantly lower socioeconomic status areas (weighted prioritization score of 6.937).
- A lack of treatment adherence (weighted prioritization score of 6.717).
“A strong consensus built throughout the study, as themes and insights gathered from the in-depth interviews were highly consistent with those collected in the survey,” the researchers noted.
They said it was not surprising that the development of biomarkers had received a high ranking, as advances in this area would help accelerate drug development and precision medicine as well as more practical aspects of clinical care.
The research team acknowledged that substantial funds would be needed to address the top priorities identified in the study, and some of the issues may be more easily addressed than others.
“In the past decade, the overall funding landscape for lupus has been on a decline, particularly through the National Institutes of Health – the largest public funder of lupus research in the world – during a time in which arguably, lupus research has been prolific,” they wrote.
They concluded that comprehensive measures were needed to transform the lupus research and health care landscape.
“Lupus experts must convene to determine feasible and coordinated approaches for addressing long-standing barriers across the global lupus community,” they stressed.
The next part of the project will involve an international stakeholder meeting to develop a global road map of specific recommendations to address identified barriers, which “may include multipronged strategies using regulatory and advocacy approaches, scientific consensus building, communication efforts, among other possible tactics,” they added.
The ALPHA Project was launched in partnership with founding partner EMD Serono Research & Development (a business of Merck KGaA) and through additional support by GlaxoSmithKline. Many authors of the report had financial connections to the pharmaceutical industry.
SOURCE: Manzi S et al. Lupus Sci Med. 2019;6:e000342. doi: 10.1136/lupus-2019-000342.
REPORTING FROM LUPUS SCIENCE & MEDICINE
Machine-learning model predicts anti-TNF nonresponse in RA patients
A machine-learning model that uses clinical profiles and genetic information has shown promise in predicting which rheumatoid arthritis patients respond to anti–tumor necrosis factor drugs in a patient population of European descent.
The model can “help up to 40% of European-descent anti–tumor necrosis factor [TNF] nonresponders avoid ineffective treatments” when compared with the usual “trial-and-error practice,” according to the authors led by Yuanfang Guan, PhD, of the department of computational medicine and bioinformatics at the University of Michigan, Ann Arbor.
The ability to accurately predict rheumatoid arthritis patients’ response to treatments would provide valuable information for optimal drug selection and would help potential nonresponders avoid drug expenses and side effects, such as an increased risk of infections, Dr. Guan and coauthors noted in Arthritis & Rheumatology.
The investigators used a modeling technique called Gaussian process regression (GPR) to predict anti-TNF drug responses. “GPR is designed to predict the unknown dependent variable for any given independent variables based on known but noisy observations of the dependent and independent variables,” they explained.
The model they used won first place in the Dialogue on Reverse Engineering Assessment and Methods: Rheumatoid Arthritis Responder Challenge, which used a crowd-based competition framework to develop a validated molecular predictor of anti-TNF response in RA.
The model was developed and cross-validated using 1,892 patients randomly selected from a training data set of 2,706 individuals of European ancestry compiled from 13 patient cohorts. All patients met 1987 American College of Rheumatology criteria for RA or were diagnosed by a board-certified rheumatologist. In addition, patients were required to have at least moderate disease activity at baseline, based on a 28-joint Disease Activity Score (DAS28) greater than 3.2.
The research team also evaluated the model using an independent dataset of 680 patients from the CERTAIN (Comparative Effectiveness Registry to study Therapies for Arthritis and Inflammatory Conditions) study.
The model combined demographic, clinical, and genetic markers to predict patients’ changes in DAS28 24 months after their baseline assessment, and identify nonresponders to anti-TNF treatments, the authors explained.
“Specifically, the [model] predicts the changes in [DAS28] of patients who have taken 12 months of anti-TNF treatments, and also classifies the patients’ responses based on the EULAR response metric,” they wrote.
Results showed that, in cross-validation tests, the model predicted changes in DAS28 with a correlation coefficient of 0.406, correctly classifying responses of 78% of subjects, with an area under the receiver operating characteristic curve (AUROC) of about 0.66.
In the independent test, the method achieved a Pearson correlation coefficient of 0.393 in predicting the change in DAS28.
Genetic SNP biomarkers provided a small additional contribution to the prediction on top of the clinical models, the authors noted.
“Compared to traditional trial-and-error practice, our model can help up to 40% of European-descent anti-TNF nonresponders avoid ineffective treatments. The model performance is even comparable to some published models utilizing additional biomarker data, whose AUROC ranges from 55% to 74% over various testing sets,” they wrote.
The GPR model has practical advantages in clinical application, unlike many sophisticated machine-learning algorithms, according to the authors. For example, GPR is a well-studied statistical model, its similarity-modeling approach is intuitive, and its results are easy to interpret.
“Our GPR model can predict subpopulations that do not respond to the treatment. This can help physicians tailor treatments for individual patients based on their conditions. ... The model can also estimate confidence intervals for its predictions, allowing physicians to judge how confident the predictions are,” the study authors wrote.
However, they cautioned that because the model was built using patients of European descent they did not expect it to achieve a similar performance in other populations. “Extension of the model over other populations requires new patient data and separate feature selection.”
The research was supported by the National Science Foundation and the National Natural Science Foundation of China. Several of the researchers reported financial relationships with pharmaceutical or technology companies.
SOURCE: Guan Y et al. Arthritis Rheumatol. 2019 Jul 24. doi: 10.1002/art.41056.
A machine-learning model that uses clinical profiles and genetic information has shown promise in predicting which rheumatoid arthritis patients respond to anti–tumor necrosis factor drugs in a patient population of European descent.
The model can “help up to 40% of European-descent anti–tumor necrosis factor [TNF] nonresponders avoid ineffective treatments” when compared with the usual “trial-and-error practice,” according to the authors led by Yuanfang Guan, PhD, of the department of computational medicine and bioinformatics at the University of Michigan, Ann Arbor.
The ability to accurately predict rheumatoid arthritis patients’ response to treatments would provide valuable information for optimal drug selection and would help potential nonresponders avoid drug expenses and side effects, such as an increased risk of infections, Dr. Guan and coauthors noted in Arthritis & Rheumatology.
The investigators used a modeling technique called Gaussian process regression (GPR) to predict anti-TNF drug responses. “GPR is designed to predict the unknown dependent variable for any given independent variables based on known but noisy observations of the dependent and independent variables,” they explained.
The model they used won first place in the Dialogue on Reverse Engineering Assessment and Methods: Rheumatoid Arthritis Responder Challenge, which used a crowd-based competition framework to develop a validated molecular predictor of anti-TNF response in RA.
The model was developed and cross-validated using 1,892 patients randomly selected from a training data set of 2,706 individuals of European ancestry compiled from 13 patient cohorts. All patients met 1987 American College of Rheumatology criteria for RA or were diagnosed by a board-certified rheumatologist. In addition, patients were required to have at least moderate disease activity at baseline, based on a 28-joint Disease Activity Score (DAS28) greater than 3.2.
The research team also evaluated the model using an independent dataset of 680 patients from the CERTAIN (Comparative Effectiveness Registry to study Therapies for Arthritis and Inflammatory Conditions) study.
The model combined demographic, clinical, and genetic markers to predict patients’ changes in DAS28 24 months after their baseline assessment, and identify nonresponders to anti-TNF treatments, the authors explained.
“Specifically, the [model] predicts the changes in [DAS28] of patients who have taken 12 months of anti-TNF treatments, and also classifies the patients’ responses based on the EULAR response metric,” they wrote.
Results showed that, in cross-validation tests, the model predicted changes in DAS28 with a correlation coefficient of 0.406, correctly classifying responses of 78% of subjects, with an area under the receiver operating characteristic curve (AUROC) of about 0.66.
In the independent test, the method achieved a Pearson correlation coefficient of 0.393 in predicting the change in DAS28.
Genetic SNP biomarkers provided a small additional contribution to the prediction on top of the clinical models, the authors noted.
“Compared to traditional trial-and-error practice, our model can help up to 40% of European-descent anti-TNF nonresponders avoid ineffective treatments. The model performance is even comparable to some published models utilizing additional biomarker data, whose AUROC ranges from 55% to 74% over various testing sets,” they wrote.
The GPR model has practical advantages in clinical application, unlike many sophisticated machine-learning algorithms, according to the authors. For example, GPR is a well-studied statistical model, its similarity-modeling approach is intuitive, and its results are easy to interpret.
“Our GPR model can predict subpopulations that do not respond to the treatment. This can help physicians tailor treatments for individual patients based on their conditions. ... The model can also estimate confidence intervals for its predictions, allowing physicians to judge how confident the predictions are,” the study authors wrote.
However, they cautioned that because the model was built using patients of European descent they did not expect it to achieve a similar performance in other populations. “Extension of the model over other populations requires new patient data and separate feature selection.”
The research was supported by the National Science Foundation and the National Natural Science Foundation of China. Several of the researchers reported financial relationships with pharmaceutical or technology companies.
SOURCE: Guan Y et al. Arthritis Rheumatol. 2019 Jul 24. doi: 10.1002/art.41056.
A machine-learning model that uses clinical profiles and genetic information has shown promise in predicting which rheumatoid arthritis patients respond to anti–tumor necrosis factor drugs in a patient population of European descent.
The model can “help up to 40% of European-descent anti–tumor necrosis factor [TNF] nonresponders avoid ineffective treatments” when compared with the usual “trial-and-error practice,” according to the authors led by Yuanfang Guan, PhD, of the department of computational medicine and bioinformatics at the University of Michigan, Ann Arbor.
The ability to accurately predict rheumatoid arthritis patients’ response to treatments would provide valuable information for optimal drug selection and would help potential nonresponders avoid drug expenses and side effects, such as an increased risk of infections, Dr. Guan and coauthors noted in Arthritis & Rheumatology.
The investigators used a modeling technique called Gaussian process regression (GPR) to predict anti-TNF drug responses. “GPR is designed to predict the unknown dependent variable for any given independent variables based on known but noisy observations of the dependent and independent variables,” they explained.
The model they used won first place in the Dialogue on Reverse Engineering Assessment and Methods: Rheumatoid Arthritis Responder Challenge, which used a crowd-based competition framework to develop a validated molecular predictor of anti-TNF response in RA.
The model was developed and cross-validated using 1,892 patients randomly selected from a training data set of 2,706 individuals of European ancestry compiled from 13 patient cohorts. All patients met 1987 American College of Rheumatology criteria for RA or were diagnosed by a board-certified rheumatologist. In addition, patients were required to have at least moderate disease activity at baseline, based on a 28-joint Disease Activity Score (DAS28) greater than 3.2.
The research team also evaluated the model using an independent dataset of 680 patients from the CERTAIN (Comparative Effectiveness Registry to study Therapies for Arthritis and Inflammatory Conditions) study.
The model combined demographic, clinical, and genetic markers to predict patients’ changes in DAS28 24 months after their baseline assessment, and identify nonresponders to anti-TNF treatments, the authors explained.
“Specifically, the [model] predicts the changes in [DAS28] of patients who have taken 12 months of anti-TNF treatments, and also classifies the patients’ responses based on the EULAR response metric,” they wrote.
Results showed that, in cross-validation tests, the model predicted changes in DAS28 with a correlation coefficient of 0.406, correctly classifying responses of 78% of subjects, with an area under the receiver operating characteristic curve (AUROC) of about 0.66.
In the independent test, the method achieved a Pearson correlation coefficient of 0.393 in predicting the change in DAS28.
Genetic SNP biomarkers provided a small additional contribution to the prediction on top of the clinical models, the authors noted.
“Compared to traditional trial-and-error practice, our model can help up to 40% of European-descent anti-TNF nonresponders avoid ineffective treatments. The model performance is even comparable to some published models utilizing additional biomarker data, whose AUROC ranges from 55% to 74% over various testing sets,” they wrote.
The GPR model has practical advantages in clinical application, unlike many sophisticated machine-learning algorithms, according to the authors. For example, GPR is a well-studied statistical model, its similarity-modeling approach is intuitive, and its results are easy to interpret.
“Our GPR model can predict subpopulations that do not respond to the treatment. This can help physicians tailor treatments for individual patients based on their conditions. ... The model can also estimate confidence intervals for its predictions, allowing physicians to judge how confident the predictions are,” the study authors wrote.
However, they cautioned that because the model was built using patients of European descent they did not expect it to achieve a similar performance in other populations. “Extension of the model over other populations requires new patient data and separate feature selection.”
The research was supported by the National Science Foundation and the National Natural Science Foundation of China. Several of the researchers reported financial relationships with pharmaceutical or technology companies.
SOURCE: Guan Y et al. Arthritis Rheumatol. 2019 Jul 24. doi: 10.1002/art.41056.
FROM ARTHRITIS & RHEUMATOLOGY
Almost half of sudden cardiac deaths linked to prior silent MI
Almost half of individuals who died of sudden cardiac death (SCD) had a myocardial scar at autopsy, indicating a prior silent myocardial infarction (SMI), in a case-controlled study.
The research team led by Juha H. Vähätalo, MD, from the University of Oulu (Finland), compared autopsy findings, clinical characteristics, and ECG markers associated with SMI in 5,869 people in Northern Finland who had sudden cardiac deaths during 1998-2017.
Overall, 75% of the deaths were caused by coronary artery disease (CAD), and of them, 71% had no previous diagnosis of CAD. Of these latter individuals, 42% had a myocardial scar at autopsy (detected by macroscopic and microscopic evaluation of myocardium), a finding that the authors said indicated a previous, unrecognized MI.
The analysis showed that individuals with SMI were slightly older, at 69.9 years, than were those with no SMI, at 65.5 years, and were more likely to be male (83.4% vs. 75.5%).
The group with prior SMI also died during physical activity at a greater rate than did those without (18.2% vs. 12.4%), the study authors reported in their paper published in JAMA Cardiology.
The research team obtained 438 ECGs prior to SCD; 187 in individuals with SMI and 251 in the group previously diagnosed with CAD.
Of the premortem ECGs in the individuals who had had an SCD after an SMI, 67% were abnormal, the researchers reported.
The SMI group had more frequently inverted T waves (16.6% vs. 8.4%) and pathologic Q waves (12.8% vs. 6.8%), compared with the non-SMI group. Both differences were statistically significant.
Fragmented QRS was the most common marker of a scar in the SMI group, however the authors noted that the fQRS complex was “probably a sensitive marker of myocardial scarring, but its specificity is not very high”.
Overall, having at least one of the following ECG abnormalities – fQRS, Q wave, T-wave inversion, or QRS of at least 110 msec – was more common in the SMI group (66.8%) compared with the non-SMI group (55.4%).
“Among patients in whom SCD without a prior MI is the first sign of cardiac disease, a previous ECG result is likely to be normal. ... ECGs were available only in 187 individuals with SMI, so the data are not sufficient to draw definite conclusions. Rather, they support motivation for further studies on this question,” the study authors noted.
“In the future, other, more efficient methods might be useful for diagnosing SMI, in addition to standard ECGs,” such as cardiac magnetic resonance imaging, but the cost-effectiveness “is likely to be unreasonable. Therefore, screening high-risk populations with ECG to identify individuals for further examinations would probably be reasonable,” they wrote.
The research team noted some limitations of the study such as the autopsy data not revealing the size of the scar detected in the myocardium and not all individuals had an ECG recorded prior to death.
SOURCE: JAMA Cardiol. 2019 Jul 10; doi: 10.1001/jamacardio.2019.2210
Almost half of individuals who died of sudden cardiac death (SCD) had a myocardial scar at autopsy, indicating a prior silent myocardial infarction (SMI), in a case-controlled study.
The research team led by Juha H. Vähätalo, MD, from the University of Oulu (Finland), compared autopsy findings, clinical characteristics, and ECG markers associated with SMI in 5,869 people in Northern Finland who had sudden cardiac deaths during 1998-2017.
Overall, 75% of the deaths were caused by coronary artery disease (CAD), and of them, 71% had no previous diagnosis of CAD. Of these latter individuals, 42% had a myocardial scar at autopsy (detected by macroscopic and microscopic evaluation of myocardium), a finding that the authors said indicated a previous, unrecognized MI.
The analysis showed that individuals with SMI were slightly older, at 69.9 years, than were those with no SMI, at 65.5 years, and were more likely to be male (83.4% vs. 75.5%).
The group with prior SMI also died during physical activity at a greater rate than did those without (18.2% vs. 12.4%), the study authors reported in their paper published in JAMA Cardiology.
The research team obtained 438 ECGs prior to SCD; 187 in individuals with SMI and 251 in the group previously diagnosed with CAD.
Of the premortem ECGs in the individuals who had had an SCD after an SMI, 67% were abnormal, the researchers reported.
The SMI group had more frequently inverted T waves (16.6% vs. 8.4%) and pathologic Q waves (12.8% vs. 6.8%), compared with the non-SMI group. Both differences were statistically significant.
Fragmented QRS was the most common marker of a scar in the SMI group, however the authors noted that the fQRS complex was “probably a sensitive marker of myocardial scarring, but its specificity is not very high”.
Overall, having at least one of the following ECG abnormalities – fQRS, Q wave, T-wave inversion, or QRS of at least 110 msec – was more common in the SMI group (66.8%) compared with the non-SMI group (55.4%).
“Among patients in whom SCD without a prior MI is the first sign of cardiac disease, a previous ECG result is likely to be normal. ... ECGs were available only in 187 individuals with SMI, so the data are not sufficient to draw definite conclusions. Rather, they support motivation for further studies on this question,” the study authors noted.
“In the future, other, more efficient methods might be useful for diagnosing SMI, in addition to standard ECGs,” such as cardiac magnetic resonance imaging, but the cost-effectiveness “is likely to be unreasonable. Therefore, screening high-risk populations with ECG to identify individuals for further examinations would probably be reasonable,” they wrote.
The research team noted some limitations of the study such as the autopsy data not revealing the size of the scar detected in the myocardium and not all individuals had an ECG recorded prior to death.
SOURCE: JAMA Cardiol. 2019 Jul 10; doi: 10.1001/jamacardio.2019.2210
Almost half of individuals who died of sudden cardiac death (SCD) had a myocardial scar at autopsy, indicating a prior silent myocardial infarction (SMI), in a case-controlled study.
The research team led by Juha H. Vähätalo, MD, from the University of Oulu (Finland), compared autopsy findings, clinical characteristics, and ECG markers associated with SMI in 5,869 people in Northern Finland who had sudden cardiac deaths during 1998-2017.
Overall, 75% of the deaths were caused by coronary artery disease (CAD), and of them, 71% had no previous diagnosis of CAD. Of these latter individuals, 42% had a myocardial scar at autopsy (detected by macroscopic and microscopic evaluation of myocardium), a finding that the authors said indicated a previous, unrecognized MI.
The analysis showed that individuals with SMI were slightly older, at 69.9 years, than were those with no SMI, at 65.5 years, and were more likely to be male (83.4% vs. 75.5%).
The group with prior SMI also died during physical activity at a greater rate than did those without (18.2% vs. 12.4%), the study authors reported in their paper published in JAMA Cardiology.
The research team obtained 438 ECGs prior to SCD; 187 in individuals with SMI and 251 in the group previously diagnosed with CAD.
Of the premortem ECGs in the individuals who had had an SCD after an SMI, 67% were abnormal, the researchers reported.
The SMI group had more frequently inverted T waves (16.6% vs. 8.4%) and pathologic Q waves (12.8% vs. 6.8%), compared with the non-SMI group. Both differences were statistically significant.
Fragmented QRS was the most common marker of a scar in the SMI group, however the authors noted that the fQRS complex was “probably a sensitive marker of myocardial scarring, but its specificity is not very high”.
Overall, having at least one of the following ECG abnormalities – fQRS, Q wave, T-wave inversion, or QRS of at least 110 msec – was more common in the SMI group (66.8%) compared with the non-SMI group (55.4%).
“Among patients in whom SCD without a prior MI is the first sign of cardiac disease, a previous ECG result is likely to be normal. ... ECGs were available only in 187 individuals with SMI, so the data are not sufficient to draw definite conclusions. Rather, they support motivation for further studies on this question,” the study authors noted.
“In the future, other, more efficient methods might be useful for diagnosing SMI, in addition to standard ECGs,” such as cardiac magnetic resonance imaging, but the cost-effectiveness “is likely to be unreasonable. Therefore, screening high-risk populations with ECG to identify individuals for further examinations would probably be reasonable,” they wrote.
The research team noted some limitations of the study such as the autopsy data not revealing the size of the scar detected in the myocardium and not all individuals had an ECG recorded prior to death.
SOURCE: JAMA Cardiol. 2019 Jul 10; doi: 10.1001/jamacardio.2019.2210
FROM JAMA CARDIOLOGY
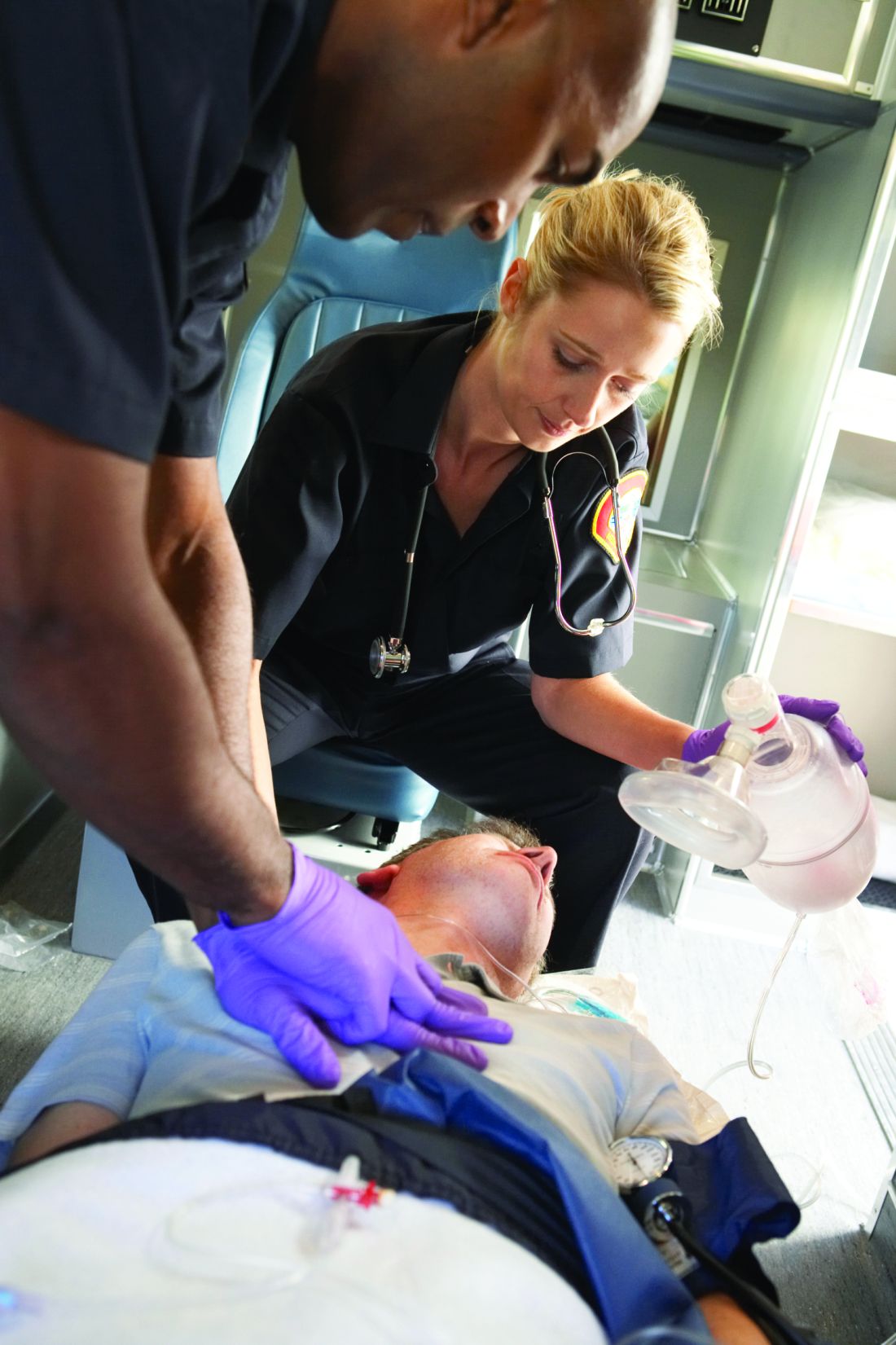
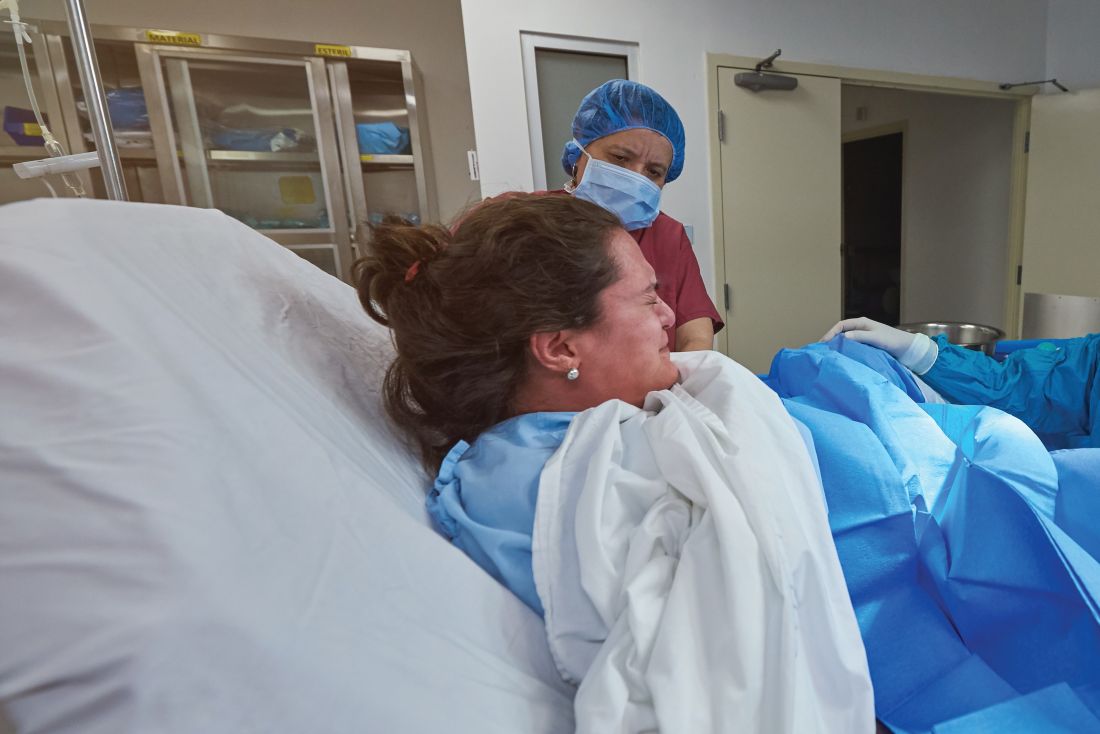
Hyponatremia is a potential and serious complication of prolonged labor
Sarah C. Lassey, MD, of the Brigham and Women’s Hospital, Boston, and colleagues noted that a rise in the number of women choosing a home labor means health care professionals may be more likely to encounter patients with hyponatremia secondary to prolonged labor and excessive hypotonic fluid consumption.
They presented the cases of two patients who were transferred to their labor and delivery unit with hyponatremia after a prolonged labor, published in Obstetrics & Gynecology. One woman presented with somnolence and confusion. The other woman was alert on arrival, but post partum had slurred speech and blurry vision.
“A likely mechanism for hyponatremia in these cases includes endogenous oxytocin and excessive free water consumption in the setting of hypovolemia,” they suggested.
One of the patients was managed with an intravenous infusion of normal saline and went on to make a full recovery after being monitored in the intensive care unit.
The other patient also was managed with normal saline, but her serum sodium level rose by 6 mmol/L within 3 hours of delivery, raising concern that the serum sodium correction was dangerously rapid. After consultation with a nephrologist, the patient’s saline IV was discontinued, and she was given desmopressin acetate to “reduce rapid diuresis of free water excretion,” they said.
“The goal of treatment should be to raise the serum sodium concentration by 1-2 mmol/L per hour, with a maximum increase ranging from 8 mmol/L in 24 hours to 25 mmol/L in 48 hours,” Dr. Lassey and colleagues noted. “The risk of raising the serum sodium concentration too quickly is osmotic demyelination syndrome, which has been reported with a serum sodium level increase of more than 12 mmol/L in 24 hours.”
Hyponatremia should be considered in women presenting as home birth transfers and in women undergoing prolonged labor at the hospital, they concluded. It also makes sense to perform electrolyte testing on admission for women with a long labor prior to coming to the hospital and for those whose labor becomes prolonged who drink a lot of fluids.
Considering the risks associated with hyponatremia, if suspicion is high, it may be prudent to start isotonic fluids before lab results are back, they said. Symptoms of mild hyponatremia include headache, lethargy, nausea, vomiting, and anorexia. Moderate hyponatremia can present as agitation, disorientation, and psychosis; if severe, hyponatremia may present as seizure, coma, or death.
Also alert your neonatal colleagues in cases of maternal intrapartum hyponatremia, because “neonatal serum sodium level will be reflective of the mother’s serum sodium level,” Dr. Lassey and associates added.
Dr. Daniela Carusi received payment from UptoDate. The other authors did not report any potential conflicts of interest.
SOURCE: Lassey SC et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003306.
Sarah C. Lassey, MD, of the Brigham and Women’s Hospital, Boston, and colleagues noted that a rise in the number of women choosing a home labor means health care professionals may be more likely to encounter patients with hyponatremia secondary to prolonged labor and excessive hypotonic fluid consumption.
They presented the cases of two patients who were transferred to their labor and delivery unit with hyponatremia after a prolonged labor, published in Obstetrics & Gynecology. One woman presented with somnolence and confusion. The other woman was alert on arrival, but post partum had slurred speech and blurry vision.
“A likely mechanism for hyponatremia in these cases includes endogenous oxytocin and excessive free water consumption in the setting of hypovolemia,” they suggested.
One of the patients was managed with an intravenous infusion of normal saline and went on to make a full recovery after being monitored in the intensive care unit.
The other patient also was managed with normal saline, but her serum sodium level rose by 6 mmol/L within 3 hours of delivery, raising concern that the serum sodium correction was dangerously rapid. After consultation with a nephrologist, the patient’s saline IV was discontinued, and she was given desmopressin acetate to “reduce rapid diuresis of free water excretion,” they said.
“The goal of treatment should be to raise the serum sodium concentration by 1-2 mmol/L per hour, with a maximum increase ranging from 8 mmol/L in 24 hours to 25 mmol/L in 48 hours,” Dr. Lassey and colleagues noted. “The risk of raising the serum sodium concentration too quickly is osmotic demyelination syndrome, which has been reported with a serum sodium level increase of more than 12 mmol/L in 24 hours.”
Hyponatremia should be considered in women presenting as home birth transfers and in women undergoing prolonged labor at the hospital, they concluded. It also makes sense to perform electrolyte testing on admission for women with a long labor prior to coming to the hospital and for those whose labor becomes prolonged who drink a lot of fluids.
Considering the risks associated with hyponatremia, if suspicion is high, it may be prudent to start isotonic fluids before lab results are back, they said. Symptoms of mild hyponatremia include headache, lethargy, nausea, vomiting, and anorexia. Moderate hyponatremia can present as agitation, disorientation, and psychosis; if severe, hyponatremia may present as seizure, coma, or death.
Also alert your neonatal colleagues in cases of maternal intrapartum hyponatremia, because “neonatal serum sodium level will be reflective of the mother’s serum sodium level,” Dr. Lassey and associates added.
Dr. Daniela Carusi received payment from UptoDate. The other authors did not report any potential conflicts of interest.
SOURCE: Lassey SC et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003306.
Sarah C. Lassey, MD, of the Brigham and Women’s Hospital, Boston, and colleagues noted that a rise in the number of women choosing a home labor means health care professionals may be more likely to encounter patients with hyponatremia secondary to prolonged labor and excessive hypotonic fluid consumption.
They presented the cases of two patients who were transferred to their labor and delivery unit with hyponatremia after a prolonged labor, published in Obstetrics & Gynecology. One woman presented with somnolence and confusion. The other woman was alert on arrival, but post partum had slurred speech and blurry vision.
“A likely mechanism for hyponatremia in these cases includes endogenous oxytocin and excessive free water consumption in the setting of hypovolemia,” they suggested.
One of the patients was managed with an intravenous infusion of normal saline and went on to make a full recovery after being monitored in the intensive care unit.
The other patient also was managed with normal saline, but her serum sodium level rose by 6 mmol/L within 3 hours of delivery, raising concern that the serum sodium correction was dangerously rapid. After consultation with a nephrologist, the patient’s saline IV was discontinued, and she was given desmopressin acetate to “reduce rapid diuresis of free water excretion,” they said.
“The goal of treatment should be to raise the serum sodium concentration by 1-2 mmol/L per hour, with a maximum increase ranging from 8 mmol/L in 24 hours to 25 mmol/L in 48 hours,” Dr. Lassey and colleagues noted. “The risk of raising the serum sodium concentration too quickly is osmotic demyelination syndrome, which has been reported with a serum sodium level increase of more than 12 mmol/L in 24 hours.”
Hyponatremia should be considered in women presenting as home birth transfers and in women undergoing prolonged labor at the hospital, they concluded. It also makes sense to perform electrolyte testing on admission for women with a long labor prior to coming to the hospital and for those whose labor becomes prolonged who drink a lot of fluids.
Considering the risks associated with hyponatremia, if suspicion is high, it may be prudent to start isotonic fluids before lab results are back, they said. Symptoms of mild hyponatremia include headache, lethargy, nausea, vomiting, and anorexia. Moderate hyponatremia can present as agitation, disorientation, and psychosis; if severe, hyponatremia may present as seizure, coma, or death.
Also alert your neonatal colleagues in cases of maternal intrapartum hyponatremia, because “neonatal serum sodium level will be reflective of the mother’s serum sodium level,” Dr. Lassey and associates added.
Dr. Daniela Carusi received payment from UptoDate. The other authors did not report any potential conflicts of interest.
SOURCE: Lassey SC et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003306.
FROM OBSTETRICS & GYNECOLOGY
Lupus pregnancy outcomes show marked improvement in past 20 years
research shows.
A retrospective cohort study led by rheumatologist Bella Mehta, MBBS, of the Hospital for Special Surgery in New York, and colleagues looked at data from the National Inpatient Sample database involving 93,820 pregnant women with SLE and 78,045,054 without SLE who were hospitalized in the United States between 1998 and 2015.
The results showed that over the 18-year study period, in-hospital maternal deaths per 100,000 admissions declined among patients with as well as those without SLE (442 vs. 13 for 1998-2000 and less than 50 vs. 10 for 2013-2015), although the decrease was greater in women with SLE (difference in trends, P less than .002).
Fetal mortality declined both for patients with SLE (268 deaths per 10,000 deliveries in 1998-2000 vs. 153 in 2013-2015) and those without SLE (72 deaths per 10,000 deliveries in 1998-2000 vs. 66 in 2013-2015).
“Although the decrease in fetal mortality seems somewhat greater in patients with SLE than those without it, the difference in trends is not statistically significant (P = .064),” the study authors noted in their paper, published in Annals of Internal Medicine.
Although patients with SLE in their study showed greater progress in rates of preeclampsia or eclampsia and length of stay, they still had worse outcomes in all measures when compared against women without SLE, the authors noted.
“Our study provides nationwide evidence that SLE pregnancy outcomes have become markedly better in the past 2 decades and continue to improve. However, SLE pregnancy risks remain high, and more work is needed to ensure good pregnancy outcomes among women with SLE,” they concluded.
Writing in an accompanying editorial, Megan E.B. Clowse, MD, of Duke University, Durham, N.C., noted that although the study findings of a progressive reduction in maternal mortality seemed very likely, the absolute decrease seen – from 140 maternal deaths per 100,000 births to fewer than 50 in 2013-2015 – warranted some reflection (Ann Intern Med. 2019;171:212-3. doi: 10.7326/M19-1667).
“SLE pregnancy management has not advanced within the past 5 years to an extent great enough to explain such a large drop in mortality,” she wrote.
There were also question marks around whether the NIS data should be used to analyze very rare events, such as maternal deaths in women with SLE. The SLE diagnosis in the database may also have included pregnancies in women who did not have SLE but instead had an elevated level of antinuclear antibody or lupus anticoagulant, which “may have diluted the frequency of poor outcomes,” Dr. Clowse wrote.
“For these reasons, I am concerned that the observed decline in maternal mortality may be an artifact, underestimating the ongoing risk of pregnancy for women with SLE,” Dr. Clowse wrote.
She added that recent analyses demonstrated that the use of hydroxychloroquine and aspirin in SLE pregnancy was not widespread.
“The inaugural reproductive health guidelines soon to be published by the American College of Rheumatology will have the potential to help expand state-of-the-art approaches to the management of pregnant women with SLE seen in everyday practice,” she concluded.
The study had no primary funding source and no conflicts of interest were declared. Dr. Mehta is supported by the C. Ronald MacKenzie Young Scientist Endowment Award. Dr. Clowse reported grants from GlaxoSmithKline and personal fees from UCB, both outside the submitted work.
SOURCE: Mehta B et al. Ann Intern Med. 2019;171:164-71. doi: 10.7326/M19-0120.
research shows.
A retrospective cohort study led by rheumatologist Bella Mehta, MBBS, of the Hospital for Special Surgery in New York, and colleagues looked at data from the National Inpatient Sample database involving 93,820 pregnant women with SLE and 78,045,054 without SLE who were hospitalized in the United States between 1998 and 2015.
The results showed that over the 18-year study period, in-hospital maternal deaths per 100,000 admissions declined among patients with as well as those without SLE (442 vs. 13 for 1998-2000 and less than 50 vs. 10 for 2013-2015), although the decrease was greater in women with SLE (difference in trends, P less than .002).
Fetal mortality declined both for patients with SLE (268 deaths per 10,000 deliveries in 1998-2000 vs. 153 in 2013-2015) and those without SLE (72 deaths per 10,000 deliveries in 1998-2000 vs. 66 in 2013-2015).
“Although the decrease in fetal mortality seems somewhat greater in patients with SLE than those without it, the difference in trends is not statistically significant (P = .064),” the study authors noted in their paper, published in Annals of Internal Medicine.
Although patients with SLE in their study showed greater progress in rates of preeclampsia or eclampsia and length of stay, they still had worse outcomes in all measures when compared against women without SLE, the authors noted.
“Our study provides nationwide evidence that SLE pregnancy outcomes have become markedly better in the past 2 decades and continue to improve. However, SLE pregnancy risks remain high, and more work is needed to ensure good pregnancy outcomes among women with SLE,” they concluded.
Writing in an accompanying editorial, Megan E.B. Clowse, MD, of Duke University, Durham, N.C., noted that although the study findings of a progressive reduction in maternal mortality seemed very likely, the absolute decrease seen – from 140 maternal deaths per 100,000 births to fewer than 50 in 2013-2015 – warranted some reflection (Ann Intern Med. 2019;171:212-3. doi: 10.7326/M19-1667).
“SLE pregnancy management has not advanced within the past 5 years to an extent great enough to explain such a large drop in mortality,” she wrote.
There were also question marks around whether the NIS data should be used to analyze very rare events, such as maternal deaths in women with SLE. The SLE diagnosis in the database may also have included pregnancies in women who did not have SLE but instead had an elevated level of antinuclear antibody or lupus anticoagulant, which “may have diluted the frequency of poor outcomes,” Dr. Clowse wrote.
“For these reasons, I am concerned that the observed decline in maternal mortality may be an artifact, underestimating the ongoing risk of pregnancy for women with SLE,” Dr. Clowse wrote.
She added that recent analyses demonstrated that the use of hydroxychloroquine and aspirin in SLE pregnancy was not widespread.
“The inaugural reproductive health guidelines soon to be published by the American College of Rheumatology will have the potential to help expand state-of-the-art approaches to the management of pregnant women with SLE seen in everyday practice,” she concluded.
The study had no primary funding source and no conflicts of interest were declared. Dr. Mehta is supported by the C. Ronald MacKenzie Young Scientist Endowment Award. Dr. Clowse reported grants from GlaxoSmithKline and personal fees from UCB, both outside the submitted work.
SOURCE: Mehta B et al. Ann Intern Med. 2019;171:164-71. doi: 10.7326/M19-0120.
research shows.
A retrospective cohort study led by rheumatologist Bella Mehta, MBBS, of the Hospital for Special Surgery in New York, and colleagues looked at data from the National Inpatient Sample database involving 93,820 pregnant women with SLE and 78,045,054 without SLE who were hospitalized in the United States between 1998 and 2015.
The results showed that over the 18-year study period, in-hospital maternal deaths per 100,000 admissions declined among patients with as well as those without SLE (442 vs. 13 for 1998-2000 and less than 50 vs. 10 for 2013-2015), although the decrease was greater in women with SLE (difference in trends, P less than .002).
Fetal mortality declined both for patients with SLE (268 deaths per 10,000 deliveries in 1998-2000 vs. 153 in 2013-2015) and those without SLE (72 deaths per 10,000 deliveries in 1998-2000 vs. 66 in 2013-2015).
“Although the decrease in fetal mortality seems somewhat greater in patients with SLE than those without it, the difference in trends is not statistically significant (P = .064),” the study authors noted in their paper, published in Annals of Internal Medicine.
Although patients with SLE in their study showed greater progress in rates of preeclampsia or eclampsia and length of stay, they still had worse outcomes in all measures when compared against women without SLE, the authors noted.
“Our study provides nationwide evidence that SLE pregnancy outcomes have become markedly better in the past 2 decades and continue to improve. However, SLE pregnancy risks remain high, and more work is needed to ensure good pregnancy outcomes among women with SLE,” they concluded.
Writing in an accompanying editorial, Megan E.B. Clowse, MD, of Duke University, Durham, N.C., noted that although the study findings of a progressive reduction in maternal mortality seemed very likely, the absolute decrease seen – from 140 maternal deaths per 100,000 births to fewer than 50 in 2013-2015 – warranted some reflection (Ann Intern Med. 2019;171:212-3. doi: 10.7326/M19-1667).
“SLE pregnancy management has not advanced within the past 5 years to an extent great enough to explain such a large drop in mortality,” she wrote.
There were also question marks around whether the NIS data should be used to analyze very rare events, such as maternal deaths in women with SLE. The SLE diagnosis in the database may also have included pregnancies in women who did not have SLE but instead had an elevated level of antinuclear antibody or lupus anticoagulant, which “may have diluted the frequency of poor outcomes,” Dr. Clowse wrote.
“For these reasons, I am concerned that the observed decline in maternal mortality may be an artifact, underestimating the ongoing risk of pregnancy for women with SLE,” Dr. Clowse wrote.
She added that recent analyses demonstrated that the use of hydroxychloroquine and aspirin in SLE pregnancy was not widespread.
“The inaugural reproductive health guidelines soon to be published by the American College of Rheumatology will have the potential to help expand state-of-the-art approaches to the management of pregnant women with SLE seen in everyday practice,” she concluded.
The study had no primary funding source and no conflicts of interest were declared. Dr. Mehta is supported by the C. Ronald MacKenzie Young Scientist Endowment Award. Dr. Clowse reported grants from GlaxoSmithKline and personal fees from UCB, both outside the submitted work.
SOURCE: Mehta B et al. Ann Intern Med. 2019;171:164-71. doi: 10.7326/M19-0120.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: In-hospital maternal mortality and outcomes have improved markedly among women with SLE but improvements are still needed.
Major finding: In-hospital maternal deaths per 100,000 admissions declined among patients with as well as those without SLE (442 vs. 13 for 1998-2000 and less than 50 vs. 10 for 2013-2015), although the decrease was greater in women with SLE (difference in trends, P less than .002).
Study details: A retrospective cohort study using the National Inpatient Sample database involving 93,820 pregnant women with SLE and 78,045,054 without SLE who were hospitalized in the United States between 1998 and 2015.
Disclosure: The study had no primary funding source and no conflicts of interest were declared. Dr. Mehta is supported by the C. Ronald MacKenzie Young Scientist Endowment award.
Source: Mehta B et al. Ann Intern Med. 2019;171:164-71. doi: 10.7326/M19-0120.
More cognitive rigidity found in patients with depression plus fibromyalgia
Increasing cognitive complexity cited as a possible therapeutic target
More attention might need to be paid to the role of chronic pain in the treatment of patients with comorbid depression, researchers suggest.
“Maybe models of depression should differentiate between depressed patients with a chronic pain condition, such as [fibromyalgia], and those without pain, wrote Mari Aguilera of the department of cognition, development, and educational psychology at the University of Barcelona and associates.
The research involved 62 patients who had participated in a previous randomized controlled trial that had assessed the efficacy of a dilemma-focused intervention for depression. All patients in the trial had met the criteria for major depressive disorder and/or dysthymia and had a score of more than 19 on the Beck Depression Inventory-II (BDI-II) scale, the investigators reported in the International Journal of Clinical and Health Psychology.
For the current trial, the researchers studied 31 patients from the trial who had an average age of 50, a concurrent diagnosis of fibromyalgia for an average of 8.14 years, an average of 2.06 depressive episodes, and a mean pain intensity of 76.21 on the visual analog scale.
The matched group of 31 patients who were used as a comparator group did not have a diagnosis of fibromyalgia and did not report high levels of pain intensity. Results showed that, in line with the researchers’ expectations, depressed patients with fibromyalgia had significantly higher BDI-II scores than patients with depression alone.
The researchers noted that patients with comorbid fibromyalgia had higher scores in pessimism, irritability, concentration/difficulty, tiredness or fatigue, and loss of interest in sex, compared with the control group.
“The nature of the relationship between pain and depression needs further studies to develop a better understanding in the future,” they wrote. The study was published in the International Journal of Clinical and Health Psychology.
Patients with comorbid fibromyalgia had higher levels of depressive symptoms and greater cognitive rigidity than did controls, the researchers found. Those with comorbid depression and pain also displayed higher levels of polarization, compared with the matched patients, with a medium-sized effect.
The small study size was cited as a limitation for generalizability. However, if confirmed by other larger studies, the researchers said, the findings might have implications for the treatment of depressed patients with comorbid fibromyalgia. “For patients with chronic pain, increasing their cognitive complexity might lead to better therapeutic results,” they wrote. “Overall, our study points to the need for more attention to the role of chronic pain in the study and treatment of depressed patients.
The research was funded by Spain’s Ministry of Science and Innovation.
SOURCE: Aguilera M et al. Int J Clin Health Psychol. 2019 May;19(2):160-4.
Increasing cognitive complexity cited as a possible therapeutic target
Increasing cognitive complexity cited as a possible therapeutic target
More attention might need to be paid to the role of chronic pain in the treatment of patients with comorbid depression, researchers suggest.
“Maybe models of depression should differentiate between depressed patients with a chronic pain condition, such as [fibromyalgia], and those without pain, wrote Mari Aguilera of the department of cognition, development, and educational psychology at the University of Barcelona and associates.
The research involved 62 patients who had participated in a previous randomized controlled trial that had assessed the efficacy of a dilemma-focused intervention for depression. All patients in the trial had met the criteria for major depressive disorder and/or dysthymia and had a score of more than 19 on the Beck Depression Inventory-II (BDI-II) scale, the investigators reported in the International Journal of Clinical and Health Psychology.
For the current trial, the researchers studied 31 patients from the trial who had an average age of 50, a concurrent diagnosis of fibromyalgia for an average of 8.14 years, an average of 2.06 depressive episodes, and a mean pain intensity of 76.21 on the visual analog scale.
The matched group of 31 patients who were used as a comparator group did not have a diagnosis of fibromyalgia and did not report high levels of pain intensity. Results showed that, in line with the researchers’ expectations, depressed patients with fibromyalgia had significantly higher BDI-II scores than patients with depression alone.
The researchers noted that patients with comorbid fibromyalgia had higher scores in pessimism, irritability, concentration/difficulty, tiredness or fatigue, and loss of interest in sex, compared with the control group.
“The nature of the relationship between pain and depression needs further studies to develop a better understanding in the future,” they wrote. The study was published in the International Journal of Clinical and Health Psychology.
Patients with comorbid fibromyalgia had higher levels of depressive symptoms and greater cognitive rigidity than did controls, the researchers found. Those with comorbid depression and pain also displayed higher levels of polarization, compared with the matched patients, with a medium-sized effect.
The small study size was cited as a limitation for generalizability. However, if confirmed by other larger studies, the researchers said, the findings might have implications for the treatment of depressed patients with comorbid fibromyalgia. “For patients with chronic pain, increasing their cognitive complexity might lead to better therapeutic results,” they wrote. “Overall, our study points to the need for more attention to the role of chronic pain in the study and treatment of depressed patients.
The research was funded by Spain’s Ministry of Science and Innovation.
SOURCE: Aguilera M et al. Int J Clin Health Psychol. 2019 May;19(2):160-4.
More attention might need to be paid to the role of chronic pain in the treatment of patients with comorbid depression, researchers suggest.
“Maybe models of depression should differentiate between depressed patients with a chronic pain condition, such as [fibromyalgia], and those without pain, wrote Mari Aguilera of the department of cognition, development, and educational psychology at the University of Barcelona and associates.
The research involved 62 patients who had participated in a previous randomized controlled trial that had assessed the efficacy of a dilemma-focused intervention for depression. All patients in the trial had met the criteria for major depressive disorder and/or dysthymia and had a score of more than 19 on the Beck Depression Inventory-II (BDI-II) scale, the investigators reported in the International Journal of Clinical and Health Psychology.
For the current trial, the researchers studied 31 patients from the trial who had an average age of 50, a concurrent diagnosis of fibromyalgia for an average of 8.14 years, an average of 2.06 depressive episodes, and a mean pain intensity of 76.21 on the visual analog scale.
The matched group of 31 patients who were used as a comparator group did not have a diagnosis of fibromyalgia and did not report high levels of pain intensity. Results showed that, in line with the researchers’ expectations, depressed patients with fibromyalgia had significantly higher BDI-II scores than patients with depression alone.
The researchers noted that patients with comorbid fibromyalgia had higher scores in pessimism, irritability, concentration/difficulty, tiredness or fatigue, and loss of interest in sex, compared with the control group.
“The nature of the relationship between pain and depression needs further studies to develop a better understanding in the future,” they wrote. The study was published in the International Journal of Clinical and Health Psychology.
Patients with comorbid fibromyalgia had higher levels of depressive symptoms and greater cognitive rigidity than did controls, the researchers found. Those with comorbid depression and pain also displayed higher levels of polarization, compared with the matched patients, with a medium-sized effect.
The small study size was cited as a limitation for generalizability. However, if confirmed by other larger studies, the researchers said, the findings might have implications for the treatment of depressed patients with comorbid fibromyalgia. “For patients with chronic pain, increasing their cognitive complexity might lead to better therapeutic results,” they wrote. “Overall, our study points to the need for more attention to the role of chronic pain in the study and treatment of depressed patients.
The research was funded by Spain’s Ministry of Science and Innovation.
SOURCE: Aguilera M et al. Int J Clin Health Psychol. 2019 May;19(2):160-4.
FROM THE INTERNATIONAL JOURNAL OF CLINICAL AND HEALTH PSYCHOLOGY
Upadacitinib monotherapy appears promising in RA patients with inadequate methotrexate response
The investigational oral Janus kinase (JAK) inhibitor upadacitinib has shown promise as a monotherapy treatment option for patients with active rheumatoid arthritis despite treatment with methotrexate, based on results from the phase 3 SELECT-MONOTHERAPY trial.
“The SELECT-MONOTHERAPY trial showed that patients who were having an inadequate response to methotrexate could be switched to oral upadacitinib monotherapy (15 mg or 30 mg) once a day, with improvements in clinical signs and symptoms, physical function, and quality of life measures, compared with patients who continued on their previous methotrexate dose,” Josef S. Smolen, MD, of the Medical University of Vienna, and his colleagues wrote in the Lancet.
The phase 3, double-blind, placebo-controlled trial randomized 648 RA patients with active disease despite methotrexate therapy from 138 sites in 24 countries.
Higher proportions of patients receiving upadacitinib 15 mg and 30 mg versus continued methotrexate achieved the primary endpoints of the proportion of patients achieving a 20% improvement in American College of Rheumatology (ACR 20) criteria at 14 weeks and the proportion achieving low disease activity defined as a 28-joint Disease Activity Score using C-reactive protein (DAS28-CRP) of 3.2 or lower.
An ACR 20 response was achieved by 89 (41%) of 216 patients (95% confidence interval, 35%-48%) in the continued methotrexate group, 147 (68%) of 217 patients (95% CI, 62%-74%) receiving upadacitinib 15 mg, and 153 (71%) of 215 patients (95% CI, 65%-77%) receiving upadacitinib 30 mg (P less than .0001 for both doses vs. continued methotrexate).
A DAS28-CRP score of 3.2 or lower was met by 42 (19%) of 216 (95% CI, 14%-25%) receiving methotrexate, 97 (45%) of 217 (95% CI, 38%-51%) receiving upadacitinib 15 mg, and 114 (53%) of 215 (95% CI, 46%-60%) receiving upadacitinib 30 mg (P less than .0001 for both doses vs. continued methotrexate).
The investigators noted that responses were observed with both 15-mg and 30-mg doses of upadacitinib, although numerically higher responses were seen with the 30-mg dose.
“Whether the 15-mg or the 30-mg dose is the more appropriate one for patients who switch from methotrexate to upadacitinib will have to be established in conjunction with data from the other phase 3 upadacitinib trials,” they wrote.
Treatment-emergent adverse events were reported at similar frequencies across the arms (n = 102 with methotrexate; n = 103 with upadacitinib 15 mg; n = 105 with upadacitinib 30 mg).
Serious adverse events were reported in 11 (5%) of 217 patients in the upadacitinib 15 mg arm, 6 (3%) of 216 patients in the continued methotrexate arm, and 6 (3%) of 215 patients in the upadacitinib 30 mg arm.
“This favorable benefit-risk profile of upadacitinib monotherapy has the potential to provide a treatment option for patients who are intolerant to methotrexate or who prefer a treatment without the need for concomitant [conventional synthetic] DMARDs,” the authors wrote.
The study was funded by AbbVie. Dr. Smolen and most authors reported financial ties to AbbVie and other companies marketing rheumatoid arthritis treatments. Five authors are employees of AbbVie.
SOURCE: Smolen JS et al. Lancet. 2019;393[10188]:2303-11. doi: 10.1016/S0140-6736(19)30419-2
Treatment recommendations for the management of RA do not currently include the use of novel disease-modifying antirheumatic drugs (DMARDs) as monotherapy. However, there is growing interest in this concept, most likely because of poor tolerability and contraindications to conventional synthetic DMARDs such as methotrexate.
The observed treatment effects of SELECT-NEXT and the current trial by Prof. Smolen and associates are similar for both control groups (responders: 36% vs. 41% on methotrexate plus placebo) and for the upadacitinib treatment groups, regardless of concomitant methotrexate status (64% on methotrexate plus upadacitinib 15 mg or 66% on methotrexate plus upadacitinib 30 mg vs. 68% on upadacitinib 15 mg or 71% on upadacitinib 30 mg).
Prof. Smolen and associates chose a trial design that assessed the continuation of methotrexate plus placebo versus switching from methotrexate to upadacitinib monotherapy, but does this research question adequately reflect the question often raised in clinical practice: Should a novel DMARD be added to methotrexate or should patients switch from methotrexate to a novel DMARD?
But we now need a trial that compares a switch versus an add-on strategy to adequately answer the question on the treatment of resistant RA.
These comments are adapted from an accompanying editorial by Anna Moltó, MD, and Maxime Dougados, MD, both with the rheumatology department at Cochin Hospital, Paris (Lancet. 2019;393[10188]:2277-8. doi: 10.1016/S0140-6736(19)30768-8). The rheumatology department at Cochin Hospital has received grants to do clinical studies and trials from AbbVie and other manufacturers of drugs for RA. Both Dr. Dougados and Dr. Moltó reported receiving personal fees for advisory boards and symposia from AbbVie and other manufacturers of drugs for RA outside the area of work commented on here.
Treatment recommendations for the management of RA do not currently include the use of novel disease-modifying antirheumatic drugs (DMARDs) as monotherapy. However, there is growing interest in this concept, most likely because of poor tolerability and contraindications to conventional synthetic DMARDs such as methotrexate.
The observed treatment effects of SELECT-NEXT and the current trial by Prof. Smolen and associates are similar for both control groups (responders: 36% vs. 41% on methotrexate plus placebo) and for the upadacitinib treatment groups, regardless of concomitant methotrexate status (64% on methotrexate plus upadacitinib 15 mg or 66% on methotrexate plus upadacitinib 30 mg vs. 68% on upadacitinib 15 mg or 71% on upadacitinib 30 mg).
Prof. Smolen and associates chose a trial design that assessed the continuation of methotrexate plus placebo versus switching from methotrexate to upadacitinib monotherapy, but does this research question adequately reflect the question often raised in clinical practice: Should a novel DMARD be added to methotrexate or should patients switch from methotrexate to a novel DMARD?
But we now need a trial that compares a switch versus an add-on strategy to adequately answer the question on the treatment of resistant RA.
These comments are adapted from an accompanying editorial by Anna Moltó, MD, and Maxime Dougados, MD, both with the rheumatology department at Cochin Hospital, Paris (Lancet. 2019;393[10188]:2277-8. doi: 10.1016/S0140-6736(19)30768-8). The rheumatology department at Cochin Hospital has received grants to do clinical studies and trials from AbbVie and other manufacturers of drugs for RA. Both Dr. Dougados and Dr. Moltó reported receiving personal fees for advisory boards and symposia from AbbVie and other manufacturers of drugs for RA outside the area of work commented on here.
Treatment recommendations for the management of RA do not currently include the use of novel disease-modifying antirheumatic drugs (DMARDs) as monotherapy. However, there is growing interest in this concept, most likely because of poor tolerability and contraindications to conventional synthetic DMARDs such as methotrexate.
The observed treatment effects of SELECT-NEXT and the current trial by Prof. Smolen and associates are similar for both control groups (responders: 36% vs. 41% on methotrexate plus placebo) and for the upadacitinib treatment groups, regardless of concomitant methotrexate status (64% on methotrexate plus upadacitinib 15 mg or 66% on methotrexate plus upadacitinib 30 mg vs. 68% on upadacitinib 15 mg or 71% on upadacitinib 30 mg).
Prof. Smolen and associates chose a trial design that assessed the continuation of methotrexate plus placebo versus switching from methotrexate to upadacitinib monotherapy, but does this research question adequately reflect the question often raised in clinical practice: Should a novel DMARD be added to methotrexate or should patients switch from methotrexate to a novel DMARD?
But we now need a trial that compares a switch versus an add-on strategy to adequately answer the question on the treatment of resistant RA.
These comments are adapted from an accompanying editorial by Anna Moltó, MD, and Maxime Dougados, MD, both with the rheumatology department at Cochin Hospital, Paris (Lancet. 2019;393[10188]:2277-8. doi: 10.1016/S0140-6736(19)30768-8). The rheumatology department at Cochin Hospital has received grants to do clinical studies and trials from AbbVie and other manufacturers of drugs for RA. Both Dr. Dougados and Dr. Moltó reported receiving personal fees for advisory boards and symposia from AbbVie and other manufacturers of drugs for RA outside the area of work commented on here.
The investigational oral Janus kinase (JAK) inhibitor upadacitinib has shown promise as a monotherapy treatment option for patients with active rheumatoid arthritis despite treatment with methotrexate, based on results from the phase 3 SELECT-MONOTHERAPY trial.
“The SELECT-MONOTHERAPY trial showed that patients who were having an inadequate response to methotrexate could be switched to oral upadacitinib monotherapy (15 mg or 30 mg) once a day, with improvements in clinical signs and symptoms, physical function, and quality of life measures, compared with patients who continued on their previous methotrexate dose,” Josef S. Smolen, MD, of the Medical University of Vienna, and his colleagues wrote in the Lancet.
The phase 3, double-blind, placebo-controlled trial randomized 648 RA patients with active disease despite methotrexate therapy from 138 sites in 24 countries.
Higher proportions of patients receiving upadacitinib 15 mg and 30 mg versus continued methotrexate achieved the primary endpoints of the proportion of patients achieving a 20% improvement in American College of Rheumatology (ACR 20) criteria at 14 weeks and the proportion achieving low disease activity defined as a 28-joint Disease Activity Score using C-reactive protein (DAS28-CRP) of 3.2 or lower.
An ACR 20 response was achieved by 89 (41%) of 216 patients (95% confidence interval, 35%-48%) in the continued methotrexate group, 147 (68%) of 217 patients (95% CI, 62%-74%) receiving upadacitinib 15 mg, and 153 (71%) of 215 patients (95% CI, 65%-77%) receiving upadacitinib 30 mg (P less than .0001 for both doses vs. continued methotrexate).
A DAS28-CRP score of 3.2 or lower was met by 42 (19%) of 216 (95% CI, 14%-25%) receiving methotrexate, 97 (45%) of 217 (95% CI, 38%-51%) receiving upadacitinib 15 mg, and 114 (53%) of 215 (95% CI, 46%-60%) receiving upadacitinib 30 mg (P less than .0001 for both doses vs. continued methotrexate).
The investigators noted that responses were observed with both 15-mg and 30-mg doses of upadacitinib, although numerically higher responses were seen with the 30-mg dose.
“Whether the 15-mg or the 30-mg dose is the more appropriate one for patients who switch from methotrexate to upadacitinib will have to be established in conjunction with data from the other phase 3 upadacitinib trials,” they wrote.
Treatment-emergent adverse events were reported at similar frequencies across the arms (n = 102 with methotrexate; n = 103 with upadacitinib 15 mg; n = 105 with upadacitinib 30 mg).
Serious adverse events were reported in 11 (5%) of 217 patients in the upadacitinib 15 mg arm, 6 (3%) of 216 patients in the continued methotrexate arm, and 6 (3%) of 215 patients in the upadacitinib 30 mg arm.
“This favorable benefit-risk profile of upadacitinib monotherapy has the potential to provide a treatment option for patients who are intolerant to methotrexate or who prefer a treatment without the need for concomitant [conventional synthetic] DMARDs,” the authors wrote.
The study was funded by AbbVie. Dr. Smolen and most authors reported financial ties to AbbVie and other companies marketing rheumatoid arthritis treatments. Five authors are employees of AbbVie.
SOURCE: Smolen JS et al. Lancet. 2019;393[10188]:2303-11. doi: 10.1016/S0140-6736(19)30419-2
The investigational oral Janus kinase (JAK) inhibitor upadacitinib has shown promise as a monotherapy treatment option for patients with active rheumatoid arthritis despite treatment with methotrexate, based on results from the phase 3 SELECT-MONOTHERAPY trial.
“The SELECT-MONOTHERAPY trial showed that patients who were having an inadequate response to methotrexate could be switched to oral upadacitinib monotherapy (15 mg or 30 mg) once a day, with improvements in clinical signs and symptoms, physical function, and quality of life measures, compared with patients who continued on their previous methotrexate dose,” Josef S. Smolen, MD, of the Medical University of Vienna, and his colleagues wrote in the Lancet.
The phase 3, double-blind, placebo-controlled trial randomized 648 RA patients with active disease despite methotrexate therapy from 138 sites in 24 countries.
Higher proportions of patients receiving upadacitinib 15 mg and 30 mg versus continued methotrexate achieved the primary endpoints of the proportion of patients achieving a 20% improvement in American College of Rheumatology (ACR 20) criteria at 14 weeks and the proportion achieving low disease activity defined as a 28-joint Disease Activity Score using C-reactive protein (DAS28-CRP) of 3.2 or lower.
An ACR 20 response was achieved by 89 (41%) of 216 patients (95% confidence interval, 35%-48%) in the continued methotrexate group, 147 (68%) of 217 patients (95% CI, 62%-74%) receiving upadacitinib 15 mg, and 153 (71%) of 215 patients (95% CI, 65%-77%) receiving upadacitinib 30 mg (P less than .0001 for both doses vs. continued methotrexate).
A DAS28-CRP score of 3.2 or lower was met by 42 (19%) of 216 (95% CI, 14%-25%) receiving methotrexate, 97 (45%) of 217 (95% CI, 38%-51%) receiving upadacitinib 15 mg, and 114 (53%) of 215 (95% CI, 46%-60%) receiving upadacitinib 30 mg (P less than .0001 for both doses vs. continued methotrexate).
The investigators noted that responses were observed with both 15-mg and 30-mg doses of upadacitinib, although numerically higher responses were seen with the 30-mg dose.
“Whether the 15-mg or the 30-mg dose is the more appropriate one for patients who switch from methotrexate to upadacitinib will have to be established in conjunction with data from the other phase 3 upadacitinib trials,” they wrote.
Treatment-emergent adverse events were reported at similar frequencies across the arms (n = 102 with methotrexate; n = 103 with upadacitinib 15 mg; n = 105 with upadacitinib 30 mg).
Serious adverse events were reported in 11 (5%) of 217 patients in the upadacitinib 15 mg arm, 6 (3%) of 216 patients in the continued methotrexate arm, and 6 (3%) of 215 patients in the upadacitinib 30 mg arm.
“This favorable benefit-risk profile of upadacitinib monotherapy has the potential to provide a treatment option for patients who are intolerant to methotrexate or who prefer a treatment without the need for concomitant [conventional synthetic] DMARDs,” the authors wrote.
The study was funded by AbbVie. Dr. Smolen and most authors reported financial ties to AbbVie and other companies marketing rheumatoid arthritis treatments. Five authors are employees of AbbVie.
SOURCE: Smolen JS et al. Lancet. 2019;393[10188]:2303-11. doi: 10.1016/S0140-6736(19)30419-2
FROM THE LANCET
Flu shot can be given irrespective of the time of last methotrexate dose
Immune response to influenza vaccination in rheumatoid arthritis patients taking methotrexate appears to depend most on stopping the next two weekly doses of the drug rather than any effect from the timing of the last dose, new research concludes.
The new finding, reported in Annals of the Rheumatic Diseases, stems from a post hoc analysis of a randomized, controlled trial that Jin Kyun Park, MD, of Seoul (Korea) National University, and his colleagues had conducted earlier on immune response when patients stopped methotrexate for either 2 or 4 weeks after vaccination. While the main endpoint of that study showed no difference in the improvement in vaccine response with either stopping methotrexate for 2 or 4 weeks and no increase in disease activity with stopping for 2 weeks, it was unclear whether the timing of the last dose mattered when stopping for 2 weeks.
In a bid to identify the optimal time between the last dose of methotrexate and administration of a flu vaccine, Dr. Park and his colleagues conducted a post hoc analysis of the trial, which involved 316 patients with RA receiving methotrexate for 6 weeks or longer to continue (n = 156) or to hold methotrexate (n = 160) for 2 weeks after receiving a quadrivalent influenza vaccine containing H1N1, H3N2, B-Yamagata, and B-Victoria.
The study authors defined a positive vaccine response as a fourfold or greater increase in hemagglutination inhibition (HI) antibody titer. A satisfactory vaccine response was a positive response to two or more of four vaccine antigens.
Patients who stopped taking methotrexate were divided into eight subgroups according to the number of days between their last dose and their vaccination.
The research team reported that response to vaccine, fold increase in HI antibody titers, and postvaccination seroprotection rates were not associated with the time between the last methotrexate dose and the time of vaccination.
However, they conceded that “the absence of impact of the number of days between the last methotrexate dose and vaccination could be due to the small patient numbers in eight subgroups.”
Vaccine response also did not differ between patients who received the influenza vaccination within 3 days of the last methotrexate dose (n = 65) and those who received it between 4-7 days of the last methotrexate dose (n = 95).
Furthermore, RA disease activity, seropositivity, or use of conventional or biologic disease-modifying antirheumatic drugs did not have an impact on methotrexate discontinuation.
The authors concluded that vaccinations could be given irrespective of the time of the last methotrexate dose, and patients should be advised to skip two weekly doses following vaccination.
“This supports the notion that the effects of methotrexate on humeral immunity occur rapidly, despite the delayed effects on arthritis; therefore, the absence of methotrexate during the first 2 weeks postvaccination is critical for humoral immunity,” they wrote.
The study was sponsored by GC Pharma. One author disclosed serving as a consultant to Pfizer and receiving research grants from GC Pharma and Hanmi Pharma.
SOURCE: Park JK et al. Ann Rheum Dis. 2019 Mar 23. doi: 10.1136/annrheumdis-2019-215187.
Immune response to influenza vaccination in rheumatoid arthritis patients taking methotrexate appears to depend most on stopping the next two weekly doses of the drug rather than any effect from the timing of the last dose, new research concludes.
The new finding, reported in Annals of the Rheumatic Diseases, stems from a post hoc analysis of a randomized, controlled trial that Jin Kyun Park, MD, of Seoul (Korea) National University, and his colleagues had conducted earlier on immune response when patients stopped methotrexate for either 2 or 4 weeks after vaccination. While the main endpoint of that study showed no difference in the improvement in vaccine response with either stopping methotrexate for 2 or 4 weeks and no increase in disease activity with stopping for 2 weeks, it was unclear whether the timing of the last dose mattered when stopping for 2 weeks.
In a bid to identify the optimal time between the last dose of methotrexate and administration of a flu vaccine, Dr. Park and his colleagues conducted a post hoc analysis of the trial, which involved 316 patients with RA receiving methotrexate for 6 weeks or longer to continue (n = 156) or to hold methotrexate (n = 160) for 2 weeks after receiving a quadrivalent influenza vaccine containing H1N1, H3N2, B-Yamagata, and B-Victoria.
The study authors defined a positive vaccine response as a fourfold or greater increase in hemagglutination inhibition (HI) antibody titer. A satisfactory vaccine response was a positive response to two or more of four vaccine antigens.
Patients who stopped taking methotrexate were divided into eight subgroups according to the number of days between their last dose and their vaccination.
The research team reported that response to vaccine, fold increase in HI antibody titers, and postvaccination seroprotection rates were not associated with the time between the last methotrexate dose and the time of vaccination.
However, they conceded that “the absence of impact of the number of days between the last methotrexate dose and vaccination could be due to the small patient numbers in eight subgroups.”
Vaccine response also did not differ between patients who received the influenza vaccination within 3 days of the last methotrexate dose (n = 65) and those who received it between 4-7 days of the last methotrexate dose (n = 95).
Furthermore, RA disease activity, seropositivity, or use of conventional or biologic disease-modifying antirheumatic drugs did not have an impact on methotrexate discontinuation.
The authors concluded that vaccinations could be given irrespective of the time of the last methotrexate dose, and patients should be advised to skip two weekly doses following vaccination.
“This supports the notion that the effects of methotrexate on humeral immunity occur rapidly, despite the delayed effects on arthritis; therefore, the absence of methotrexate during the first 2 weeks postvaccination is critical for humoral immunity,” they wrote.
The study was sponsored by GC Pharma. One author disclosed serving as a consultant to Pfizer and receiving research grants from GC Pharma and Hanmi Pharma.
SOURCE: Park JK et al. Ann Rheum Dis. 2019 Mar 23. doi: 10.1136/annrheumdis-2019-215187.
Immune response to influenza vaccination in rheumatoid arthritis patients taking methotrexate appears to depend most on stopping the next two weekly doses of the drug rather than any effect from the timing of the last dose, new research concludes.
The new finding, reported in Annals of the Rheumatic Diseases, stems from a post hoc analysis of a randomized, controlled trial that Jin Kyun Park, MD, of Seoul (Korea) National University, and his colleagues had conducted earlier on immune response when patients stopped methotrexate for either 2 or 4 weeks after vaccination. While the main endpoint of that study showed no difference in the improvement in vaccine response with either stopping methotrexate for 2 or 4 weeks and no increase in disease activity with stopping for 2 weeks, it was unclear whether the timing of the last dose mattered when stopping for 2 weeks.
In a bid to identify the optimal time between the last dose of methotrexate and administration of a flu vaccine, Dr. Park and his colleagues conducted a post hoc analysis of the trial, which involved 316 patients with RA receiving methotrexate for 6 weeks or longer to continue (n = 156) or to hold methotrexate (n = 160) for 2 weeks after receiving a quadrivalent influenza vaccine containing H1N1, H3N2, B-Yamagata, and B-Victoria.
The study authors defined a positive vaccine response as a fourfold or greater increase in hemagglutination inhibition (HI) antibody titer. A satisfactory vaccine response was a positive response to two or more of four vaccine antigens.
Patients who stopped taking methotrexate were divided into eight subgroups according to the number of days between their last dose and their vaccination.
The research team reported that response to vaccine, fold increase in HI antibody titers, and postvaccination seroprotection rates were not associated with the time between the last methotrexate dose and the time of vaccination.
However, they conceded that “the absence of impact of the number of days between the last methotrexate dose and vaccination could be due to the small patient numbers in eight subgroups.”
Vaccine response also did not differ between patients who received the influenza vaccination within 3 days of the last methotrexate dose (n = 65) and those who received it between 4-7 days of the last methotrexate dose (n = 95).
Furthermore, RA disease activity, seropositivity, or use of conventional or biologic disease-modifying antirheumatic drugs did not have an impact on methotrexate discontinuation.
The authors concluded that vaccinations could be given irrespective of the time of the last methotrexate dose, and patients should be advised to skip two weekly doses following vaccination.
“This supports the notion that the effects of methotrexate on humeral immunity occur rapidly, despite the delayed effects on arthritis; therefore, the absence of methotrexate during the first 2 weeks postvaccination is critical for humoral immunity,” they wrote.
The study was sponsored by GC Pharma. One author disclosed serving as a consultant to Pfizer and receiving research grants from GC Pharma and Hanmi Pharma.
SOURCE: Park JK et al. Ann Rheum Dis. 2019 Mar 23. doi: 10.1136/annrheumdis-2019-215187.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point:
Major finding: Response to vaccine, fold increase in HI antibody titers, and postvaccination seroprotection rates were not associated with the time between the last methotrexate dose and the time of vaccination.
Study details: A post hoc analysis of a randomized, controlled trial involving 316 patients with rheumatoid arthritis who continued or stopped methotrexate for 2 weeks following influenza vaccination.
Disclosures: The study was sponsored by GC Pharma. One author disclosed serving as a consultant to Pfizer and receiving research grants from GC Pharma and Hanmi Pharma.
Source: Park JK et al. Ann Rheum Dis. 2019 Mar 23. doi: 10.1136/annrheumdis-2019-215187
TNF inhibitor prices rose despite increased drug class competition
according to a new analysis of Medicare claims data and wholesale acquisition costs published online in JAMA Internal Medicine.
A research team led by Alvaro San-Juan-Rodriguez, PharmD, of the University of Pittsburgh said their analysis illustrates “a market failure contributing to the rising costs of prescription drugs.”
Before 2009, etanercept (Enbrel), infliximab (Remicade), and adalimumab (Humira) were the only tumor necrosis factor (TNF) inhibitors approved by the Food and Drug Administration for treating rheumatoid arthritis; infliximab and adalimumab are also approved to treat inflammatory bowel disease. In 2009, subcutaneous golimumab (Simponi) and certolizumab pegol (Cimzia) entered the market, followed by intravenous golimumab (Simponi ARIA) in 2013.
The researchers used an interrupted time series analysis with a linear model that “regressed the annual cost of existing TNF inhibitors against a continuous variable for month, two indicator variables for each period after market entry of new drugs, and the interactions between them.”
Using estimates from this model, the researchers calculated the trends in costs that would have been expected if new anti-TNFs had not entered the market. They examined costs for TNF inhibitors typically reimbursed under Medicare Part D (Enbrel, Humira, Simponi, and Cimzia) and adjusted the data for increases in manufacturer rebates, but “owing to lack of data,” they could not “assess how purchasing prices for drugs typically reimbursed under Medicare Part B [Remicade and Simponi ARIA] changed over time.” All estimates for annual costs of treatment were based on dosing recommendations for a standard 80-kg patient with rheumatoid arthritis.
The annual treatment costs with existing TNF inhibitors increased after the three new agents entered the market. For example, when wholesale acquisition cost data was applied, annual treatment costs with existing TNF inhibitors increased by 144% from April 2009 to December 2016 after new drug entry (from $15.809 to $38,574). However, in the absence of new drugs’ entry, the researchers estimated that annual treatment costs would have increased by 34% (from $15,809 to $21,184).
Medicare annual treatment costs increased by 139% (from $14,901 to $35,613), compared with a 43% increase expected in the absence of new drugs’ entry (from $14,901 to $21,308). Medicare spending increased in parallel with increases in annual treatment costs, but out-of-pocket costs and manufacturer coverage gap discounts remained relatively constant over time.
The research team noted that if cost trends had not changed after the entry of new products, the costs of Enbrel, Remicade, and Humira in December 2016 would have been 40%-45% lower.
“These increases were born solely by Medicare, while patient out-of-pocket spending remained flat. In addition, these increases were not offset by manufacturer discounts in the Medicare Part D coverage gap. The rising costs of existing products may reflect manufacturers’ opportunism in response to payers’ increased willingness to pay for TNF inhibitors after market entry of new, more expensive agents,” the research team noted.
The study was funded in part by the Myers Family Foundation and one author reported funding from the National Heart, Lung, and Blood Institute.
SOURCE: San-Juan-Rodriguez A et al. JAMA Intern Med. 2019 Feb 18. doi: 10.1001/jamainternmed.2018.7656
according to a new analysis of Medicare claims data and wholesale acquisition costs published online in JAMA Internal Medicine.
A research team led by Alvaro San-Juan-Rodriguez, PharmD, of the University of Pittsburgh said their analysis illustrates “a market failure contributing to the rising costs of prescription drugs.”
Before 2009, etanercept (Enbrel), infliximab (Remicade), and adalimumab (Humira) were the only tumor necrosis factor (TNF) inhibitors approved by the Food and Drug Administration for treating rheumatoid arthritis; infliximab and adalimumab are also approved to treat inflammatory bowel disease. In 2009, subcutaneous golimumab (Simponi) and certolizumab pegol (Cimzia) entered the market, followed by intravenous golimumab (Simponi ARIA) in 2013.
The researchers used an interrupted time series analysis with a linear model that “regressed the annual cost of existing TNF inhibitors against a continuous variable for month, two indicator variables for each period after market entry of new drugs, and the interactions between them.”
Using estimates from this model, the researchers calculated the trends in costs that would have been expected if new anti-TNFs had not entered the market. They examined costs for TNF inhibitors typically reimbursed under Medicare Part D (Enbrel, Humira, Simponi, and Cimzia) and adjusted the data for increases in manufacturer rebates, but “owing to lack of data,” they could not “assess how purchasing prices for drugs typically reimbursed under Medicare Part B [Remicade and Simponi ARIA] changed over time.” All estimates for annual costs of treatment were based on dosing recommendations for a standard 80-kg patient with rheumatoid arthritis.
The annual treatment costs with existing TNF inhibitors increased after the three new agents entered the market. For example, when wholesale acquisition cost data was applied, annual treatment costs with existing TNF inhibitors increased by 144% from April 2009 to December 2016 after new drug entry (from $15.809 to $38,574). However, in the absence of new drugs’ entry, the researchers estimated that annual treatment costs would have increased by 34% (from $15,809 to $21,184).
Medicare annual treatment costs increased by 139% (from $14,901 to $35,613), compared with a 43% increase expected in the absence of new drugs’ entry (from $14,901 to $21,308). Medicare spending increased in parallel with increases in annual treatment costs, but out-of-pocket costs and manufacturer coverage gap discounts remained relatively constant over time.
The research team noted that if cost trends had not changed after the entry of new products, the costs of Enbrel, Remicade, and Humira in December 2016 would have been 40%-45% lower.
“These increases were born solely by Medicare, while patient out-of-pocket spending remained flat. In addition, these increases were not offset by manufacturer discounts in the Medicare Part D coverage gap. The rising costs of existing products may reflect manufacturers’ opportunism in response to payers’ increased willingness to pay for TNF inhibitors after market entry of new, more expensive agents,” the research team noted.
The study was funded in part by the Myers Family Foundation and one author reported funding from the National Heart, Lung, and Blood Institute.
SOURCE: San-Juan-Rodriguez A et al. JAMA Intern Med. 2019 Feb 18. doi: 10.1001/jamainternmed.2018.7656
according to a new analysis of Medicare claims data and wholesale acquisition costs published online in JAMA Internal Medicine.
A research team led by Alvaro San-Juan-Rodriguez, PharmD, of the University of Pittsburgh said their analysis illustrates “a market failure contributing to the rising costs of prescription drugs.”
Before 2009, etanercept (Enbrel), infliximab (Remicade), and adalimumab (Humira) were the only tumor necrosis factor (TNF) inhibitors approved by the Food and Drug Administration for treating rheumatoid arthritis; infliximab and adalimumab are also approved to treat inflammatory bowel disease. In 2009, subcutaneous golimumab (Simponi) and certolizumab pegol (Cimzia) entered the market, followed by intravenous golimumab (Simponi ARIA) in 2013.
The researchers used an interrupted time series analysis with a linear model that “regressed the annual cost of existing TNF inhibitors against a continuous variable for month, two indicator variables for each period after market entry of new drugs, and the interactions between them.”
Using estimates from this model, the researchers calculated the trends in costs that would have been expected if new anti-TNFs had not entered the market. They examined costs for TNF inhibitors typically reimbursed under Medicare Part D (Enbrel, Humira, Simponi, and Cimzia) and adjusted the data for increases in manufacturer rebates, but “owing to lack of data,” they could not “assess how purchasing prices for drugs typically reimbursed under Medicare Part B [Remicade and Simponi ARIA] changed over time.” All estimates for annual costs of treatment were based on dosing recommendations for a standard 80-kg patient with rheumatoid arthritis.
The annual treatment costs with existing TNF inhibitors increased after the three new agents entered the market. For example, when wholesale acquisition cost data was applied, annual treatment costs with existing TNF inhibitors increased by 144% from April 2009 to December 2016 after new drug entry (from $15.809 to $38,574). However, in the absence of new drugs’ entry, the researchers estimated that annual treatment costs would have increased by 34% (from $15,809 to $21,184).
Medicare annual treatment costs increased by 139% (from $14,901 to $35,613), compared with a 43% increase expected in the absence of new drugs’ entry (from $14,901 to $21,308). Medicare spending increased in parallel with increases in annual treatment costs, but out-of-pocket costs and manufacturer coverage gap discounts remained relatively constant over time.
The research team noted that if cost trends had not changed after the entry of new products, the costs of Enbrel, Remicade, and Humira in December 2016 would have been 40%-45% lower.
“These increases were born solely by Medicare, while patient out-of-pocket spending remained flat. In addition, these increases were not offset by manufacturer discounts in the Medicare Part D coverage gap. The rising costs of existing products may reflect manufacturers’ opportunism in response to payers’ increased willingness to pay for TNF inhibitors after market entry of new, more expensive agents,” the research team noted.
The study was funded in part by the Myers Family Foundation and one author reported funding from the National Heart, Lung, and Blood Institute.
SOURCE: San-Juan-Rodriguez A et al. JAMA Intern Med. 2019 Feb 18. doi: 10.1001/jamainternmed.2018.7656
FROM JAMA INTERNAL MEDICINE