User login
Bedbugs: Helping your patient through an infestation
Bedbugs have been unwelcome bedfellows for humans for thousands of years. An increase in pyrethroid resistance, a ban on the insecticide dichloro-diphenyl-trichloroethane (DDT), increased international travel, and increased population density in large cities have led to an exponential rise in the incidence of bedbug infestations. Physicians are often at the forefront of bedbug infestation diagnosis.
Once the diagnosis is suggested, symptomatic treatment of the patient and extermination of the pests are essential, though time-consuming, costly, and often problematic. Measures to eliminate infestation and to prevent spread include identification of the pest, early detection, patient education, and professional eradication.
BEDBUGS: A BRIEF HISTORY
The term bedbug refers to the obligate parasitic arthropod Cimex lectularius (the common bedbug) and, less commonly, its tropical cousin C hemipterus. Bedbugs have coexisted with humans for centuries, dating back to the ancient Egyptians 3,500 years ago.1 Through the mid-20th century, about 30% of US households were infested with bedbugs.2 The introduction of pesticides during World War II markedly decreased the incidence, but with increased international travel, pesticide resistance, and the banning of certain pesticides in the last decade, bedbugs have reemerged worldwide.3
BIOLOGY
Bedbugs are red-brown, wingless, oval-shaped insects measuring 4 to 5 mm in length (Figure 1). They are hematophagous ectoparasites that preferentially feed on human blood, although they feed on some animals as well.2
Cimex lectularius dwells in temperate climates and C hemipterus in more tropical climates, but overlap and interbreeding are common. The usual life cycle is about 6 months, but some bugs live 12 months or longer. The female bedbug lays 5 to 8 eggs per week, or approximately 500 eggs in her lifetime, and each egg hatches in 5 to 10 days.4
These photophobic parasites do not live on their human hosts but rather simply visit for a meal. They cohabitate in dark locations, attacking human hosts when they are inactive or sleeping for long periods of time. Common living areas include mattress seams, box springs, bed linens and clothes, wallpaper seams, electrical outlets, and furniture seams (Table 1).5 The female bedbug lays her eggs in these secluded crevices, ensuring their safety until hatching. The dense nests of adult bedbugs, their eggs, and accumulated fecal matter allow for easy visual identification of infestation.5
Bedbugs typically feed between 1:00 am and 5:00 am. Though wingless, they successfully navigate towards their human host, attracted by emitted heat and carbon dioxide.2 Once attached to human skin, the bedbug bite releases enzymes and chemicals including nitrophorin and nitric oxide that facilitate bleeding; these substances are responsible for the resultant dermatitis. (Of note, bedbugs with experimentally excised salivary glands do not cause skin disease in humans.6) After feeding for 3 to 20 minutes, the length and weight of the arthropod can increase by 50% to 200%. A fully sated bedbug can survive for a year until its next meal.2,7 Even if an establishment, home, room, or article of clothing infested with bedbugs has been abandoned for several months, without proper eradication the item still represents a possible nidus for recurrent disease if used, inhabited, or worn again.
EPIDEMIOLOGY
From the earliest documented cases of Cimex in ancient Egyptian tombs to the mid-1900s, the cohabitation of humans and bedbugs was seen as inevitable. With the introduction of DDT 60 years ago, the bedbug population significantly decreased.8 Since DDT’s prohibition, coupled with increased travel and heightened resistance to over-the-counter insecticides, the bedbug population has reemerged exponentially.9,10
Infestations have been reported worldwide, on every continent, and in all 50 of the United States. In Australia, infestations have risen 4,500% in the last 10 to 15 years.11 In the United States, infestation occurs exclusively with C lectularius and the incidence is rising. Philadelphia and New York City are among the most bedbug-infested US cities. New York City experienced a 2,000% increase in bedbug complaints between 2004 and 2009.8
Bedbugs can be transmitted either through active migration of colonies from one area to another adjacent living area through wall spaces or ventilation, or through passive transportation in luggage, clothing, furniture, used mattresses, bookbags, and other personal items.1 Although infestation affects people of all socioeconomic classes and backgrounds, the likelihood increases in people who frequently travel and people who live in lower income neighborhoods with tightly packed apartments. Bedbug infestations are also common in refugee camps: 98% of the rooms in a refugee camp in Sierra Leone had bedbugs, and almost 90% of the residents had signs of bites.12 Unlike scabies, direct person-to-person, skin-to-skin transfer is rare.
CLINICAL FINDINGS
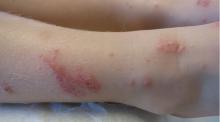
Bedbug bites are analogous, almost identical, to other arthropod bites: bites begin as pink macules that progress to papules (Figure 2), large plaques, or wheals (hives).13 Bites can arise minutes or even days after the initial assault. Some papules and plaques may have a central crust or erosion suggesting a bite.
Bites are typically intensely pruritic, and occasionally, hypersensitive victims can develop bullae, necrotic plaques, or even vasculitis. New papules and plaques form as older ones heal. Some patients may have fever and malaise.13 About 30% of patients may not have skin disease from bedbugs, making diagnosis in those individuals impossible.
The nonspecific nature of this presentation and the subsequent difficulty in prompt diagnosis can lead to a prolonged period of morbidity for the patient, as well as increasing the window of opportunity for the bedbugs to affect other surrounding individuals.
THE DIFFERENTIAL DIAGNOSIS IS BROAD
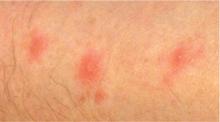
Commonly, bedbug bites have been misdiagnosed as drug eruptions, food allergies, dermatitis herpetiformis, staphylococcal or varicella infection, and scabies, as well as other arthropod bites.11 This broad differential diagnosis can often be narrowed by careful observation of the bite distribution. The clustering of bites in groups of 3, often in a linear pattern, sometimes overlying blood vessels, is known as the “breakfast, lunch, and dinner” sign (Figure 3), and this can help to guide the clinician toward the diagnosis of a bite as opposed to a diffuse urticarial response.2
If the characteristic clusters of bites are not present, distinguishing clinically between the various causes of pruritic urticarial lesions is difficult. Subtle clues that point towards bedbug bites can be that the rash appears to be most edematous in the morning and flattens throughout the day, as the bites occur typically during sleep.14 Likewise, the rash associated with bedbug bites has also been reported to last longer, to blanch less, and to be less responsive to steroid and antihistamine treatment than other urticarial rashes.14 If a skin biopsy specimen is available, histologic assessment can help to rule out similarly presenting conditions such as prodromal bullous pemphigoid, dermatitis herpetiformis, and urticarial dermatosis, even if it cannot provide a definitive answer as to the etiology.15
Bedbug bites vs other arthropod bites
Once a bite is suspected, differentiating between bedbug and other arthropod bites is the next challenge.
Once again, a detailed assessment of the location of the bites can yield valuable information. The waist, axillae, and uncovered parts of the body are the usual sites for bedbug bites.2 Likewise, inflammatory papules along the eyelid (the “eyelid sign”) are highly suggestive of a bedbug bite.16
The scant involvement of covered body areas, the lack of shallow burrows in the skin, and the lack of scabetic elements on skin scrapings exclude scabies as a diagnosis.
Skin biopsy is not helpful in differentiating arthropod bites, as the histologic findings are nonspecific. The key to a definitive diagnosis in these cases is identification of the suspected bug in characteristic locations. Patients should be encouraged to carefully inspect mattresses, floorboards, and other crevices for the small ovaloid bugs or the reddish-brown specks of heme and feces they typically leave behind on bed linens.15 A positive reported sighting of the bugs can lend credence to the diagnosis, whereas capture and laboratory assessment of a specimen is ideal.
BEDBUGS AS DISEASE VECTORS
Extracutaneous manifestations of bedbug assault are rare. Anaphylaxis to proteins in Cimex saliva may occur, as well as significant blood loss, even anemia, from extensive feeding.17 Bedbug infestations can exacerbate asthma, preexisting mental illness, anxiety, and insomnia.18 Since bedbugs extract blood from hosts, they have a putative ability to act as vectors of disease. Some 45 known pathogens have been isolated from the Cimex species including hepatitis B, human immunodeficiency virus (HIV), Trypanosoma cruzi, and methicillin-resistant Staphylococcus aureus. To date, however, there is no evidence to demonstrate transmission of pathogens to humans.5
TREATMENT AND ERADICATION
Treatment is mainly symptomatic—systemic antihistamines and topical corticosteroids to reduce pruritus and alleviate the dermatitis.2 Patients should be instructed to avoid scratching to prevent infection. Secondary bacterial infection can be treated with topical or systemic antibiotics. Rare cases of bite-induced asthma or anaphylaxis necessitate appropriate emergency treatment. Extermination of infestation is critical to therapy.
If bedbug infestation is suggested, mattresses, bedding, sleeping areas, and bed clothing should be inspected for insects, eggs, and fecal spotting. Adhesives or traps that emit heat or carbon dioxide can be used to capture the bedbugs. During widespread infestation, the arthropods release a pungent odor, which allows trained dogs to detect them with 95% to 98% accuracy.19
Eradication techniques
Once infestation is confirmed, patients should contact an exterminator who can confirm the presence of bedbugs. Typical eradication measures often require nonchemical control and chemical pesticides.
Professional exterminators have special equipment that can heat a room to 48 to 50°C (118–122°F). Heat sustained at this temperature for 90 minutes is sufficient to kill bedbugs.20
The infested area should be vacuumed daily, and vacuum bags and unwanted items should be sealed in plastic before discarding. Clothing, linens, and infested fabrics should be washed and dried in heat at 60°C (140°F) or greater.
Mattresses and furniture should be sealed in a special plastic that allows treatment with heat, steaming, or pesticides. Most professional pesticides contain pyrethroids, but resistance to these products is common, necessitating the use of multiple formulations to overcome resistance.8
Over-the-counter pesticides, almost exclusively pyrethroids, are variably effective and potentially hazardous to consumers.8 Patients must be advised to follow label directions to avoid adverse effects and toxicity.
Alternative chemical eradication methods to circumvent the problem of resistance include piperonyl butoxide, S-methoprene, boric acid, silicates (diatomaceous earth dust), and sulfuryl fluoride. Recent research has also posited the use of antiparasitic agents such as ivermectin and moxidectin in cases of resistant bedbug infestation, with promising results.21
All extermination products and techniques have variable risks, efficacies, and costs,8 and repeat inspections and retreatment are often required.
Prevention strategies include visual inspection of possibly infested rooms, with particular attention to mattress seams and crevices, placing luggage on a luggage rack away from the floor and bed, and careful examination of acquired second-hand items.7
Educating patients is the key to success
While all of the above eradication techniques are important curative strategies, the success of any treatment is contingent on appropriate patient education about the nature of the problem.
Resolving a bedbug infestation is notoriously difficult and requires meticulous adherence to hygiene and cleansing instructions throughout the household or institution for a sustained period of time. Information from sources such as the US Environmental Protection Agency (www.epa.gov) can empower patients to perform the necessary eradication protocols, and clinicians should routinely recommended them as part of a holistic treatment strategy.
- Krause-Parello CA, Sciscione P. Bedbugs: an equal opportunist and cosmopolitan creature. J Sch Nurs 2009; 25:126–132.
- Sfeir M, Munoz-Price LS. Scabies and bedbugs in hospital outbreaks. Curr Infect Dis Rep 2014; 16:412.
- Romero A, Potter MF, Potter DA, Haynes KF. Insecticide resistance in the bed bug: a factor in the pest's sudden resurgence? J Med Entomol 2007; 44:175–178.
- Delaunay P, Blanc V, Del Giudice P, et al. Bedbugs and infectious diseases. Clin Infect Dis 2011; 52:200–210.
- Doggett SL, Dwyer DE, Penas PF, Russell RC. Bed bugs: clinical relevance and control options. Clin Microbiol Rev 2012; 25:164–192.
- Goddard J, Edwards KT. Effects of bed bug saliva on human skin. JAMA Dermatol 2013; 149:372–373.
- Goddard J, deShazo R. Bed bugs (Cimex lectularius) and clinical consequences of their bites. JAMA 2009; 301:1358–1366.
- Davies TG, Field LM, Williamson MS. The re-emergence of the bed bug as a nuisance pest: implications of resistance to the pyrethroid insecticides. Med Vet Entomol 2012; 26:241–254.
- Saenz VL, Booth W, Schal C, Vargo EL. Genetic analysis of bed bug populations reveals small propagule size within individual infestations but high genetic diversity across infestations from the eastern United States. J Med Entomol 2012; 49:865–875.
- Jones SC, Bryant JL. Ineffectiveness of over-the-counter total-release foggers against the bed bug (Heteroptera: cimicidae). J Econ Entomol 2012; 105:957–963.
- Doggett SL, Russell R. Bed bugs—what the GP needs to know. Aust Fam Physician 2009; 38:880–884.
- Gbakima AA, Terry BC, Kanja F, Kortequee S, Dukuley I, Sahr F. High prevalence of bedbugs Cimex hemipterus and Cimex lectularis in camps for internally displaced persons in Freetown, Sierra Leone: a pilot humanitarian investigation. West Afr J Med 2002; 21:268–271.
- deShazo RD, Feldlaufer MF, Mihm MC Jr, Goddard J. Bullous reactions to bedbug bites reflect cutaneous vasculitis. Am J Med 2012; 125:688–694.
- Scarupa MD, Economides A. Bedbug bites masquerading as urticaria. J Allergy Clin Immunol 2006; 117:1508–1509.
- Thomas I, Kihiczak GG, Schwartz RA. Bedbug bites: a review. Int J Dermatol 2004; 43:430–433.
- Quach KA, Zaenglein AL. The eyelid sign: a clue to bed bug bites. Pediatr Dermatol 2014; 31:353–355.
- Paulke-Korinek M, Szell M, Laferl H, Auer H, Wenisch C. Bed bugs can cause severe anaemia in adults. Parasitol Res 2012; 110:2577–2579.
- Goddard J, de Shazo R. Psychological effects of bed bug attacks (Cimex lectularius L). Am J Med 2012; 125:101–103.
- Pfiester M, Koehler PG, Pereira RM. Ability of bed bug-detecting canines to locate live bed bugs and viable bed bug eggs. J Econ Entomol 2008; 101:1389–1396.
- Kells SA, Goblirsch MJ. Temperature and time requirements for controlling bed bugs (Cimex lectularius) under commercial heat treatment conditions. Insects 2011; 2:412–422.
- Sheele JM, Ridge GE. Toxicity and potential utility of ivermectin and moxidectin as xenointoxicants against the common bed bug Cimex lectularius L. Parasitol Res 2016; 115:3071–3081.
Bedbugs have been unwelcome bedfellows for humans for thousands of years. An increase in pyrethroid resistance, a ban on the insecticide dichloro-diphenyl-trichloroethane (DDT), increased international travel, and increased population density in large cities have led to an exponential rise in the incidence of bedbug infestations. Physicians are often at the forefront of bedbug infestation diagnosis.
Once the diagnosis is suggested, symptomatic treatment of the patient and extermination of the pests are essential, though time-consuming, costly, and often problematic. Measures to eliminate infestation and to prevent spread include identification of the pest, early detection, patient education, and professional eradication.
BEDBUGS: A BRIEF HISTORY
The term bedbug refers to the obligate parasitic arthropod Cimex lectularius (the common bedbug) and, less commonly, its tropical cousin C hemipterus. Bedbugs have coexisted with humans for centuries, dating back to the ancient Egyptians 3,500 years ago.1 Through the mid-20th century, about 30% of US households were infested with bedbugs.2 The introduction of pesticides during World War II markedly decreased the incidence, but with increased international travel, pesticide resistance, and the banning of certain pesticides in the last decade, bedbugs have reemerged worldwide.3
BIOLOGY
Bedbugs are red-brown, wingless, oval-shaped insects measuring 4 to 5 mm in length (Figure 1). They are hematophagous ectoparasites that preferentially feed on human blood, although they feed on some animals as well.2
Cimex lectularius dwells in temperate climates and C hemipterus in more tropical climates, but overlap and interbreeding are common. The usual life cycle is about 6 months, but some bugs live 12 months or longer. The female bedbug lays 5 to 8 eggs per week, or approximately 500 eggs in her lifetime, and each egg hatches in 5 to 10 days.4
These photophobic parasites do not live on their human hosts but rather simply visit for a meal. They cohabitate in dark locations, attacking human hosts when they are inactive or sleeping for long periods of time. Common living areas include mattress seams, box springs, bed linens and clothes, wallpaper seams, electrical outlets, and furniture seams (Table 1).5 The female bedbug lays her eggs in these secluded crevices, ensuring their safety until hatching. The dense nests of adult bedbugs, their eggs, and accumulated fecal matter allow for easy visual identification of infestation.5
Bedbugs typically feed between 1:00 am and 5:00 am. Though wingless, they successfully navigate towards their human host, attracted by emitted heat and carbon dioxide.2 Once attached to human skin, the bedbug bite releases enzymes and chemicals including nitrophorin and nitric oxide that facilitate bleeding; these substances are responsible for the resultant dermatitis. (Of note, bedbugs with experimentally excised salivary glands do not cause skin disease in humans.6) After feeding for 3 to 20 minutes, the length and weight of the arthropod can increase by 50% to 200%. A fully sated bedbug can survive for a year until its next meal.2,7 Even if an establishment, home, room, or article of clothing infested with bedbugs has been abandoned for several months, without proper eradication the item still represents a possible nidus for recurrent disease if used, inhabited, or worn again.
EPIDEMIOLOGY
From the earliest documented cases of Cimex in ancient Egyptian tombs to the mid-1900s, the cohabitation of humans and bedbugs was seen as inevitable. With the introduction of DDT 60 years ago, the bedbug population significantly decreased.8 Since DDT’s prohibition, coupled with increased travel and heightened resistance to over-the-counter insecticides, the bedbug population has reemerged exponentially.9,10
Infestations have been reported worldwide, on every continent, and in all 50 of the United States. In Australia, infestations have risen 4,500% in the last 10 to 15 years.11 In the United States, infestation occurs exclusively with C lectularius and the incidence is rising. Philadelphia and New York City are among the most bedbug-infested US cities. New York City experienced a 2,000% increase in bedbug complaints between 2004 and 2009.8
Bedbugs can be transmitted either through active migration of colonies from one area to another adjacent living area through wall spaces or ventilation, or through passive transportation in luggage, clothing, furniture, used mattresses, bookbags, and other personal items.1 Although infestation affects people of all socioeconomic classes and backgrounds, the likelihood increases in people who frequently travel and people who live in lower income neighborhoods with tightly packed apartments. Bedbug infestations are also common in refugee camps: 98% of the rooms in a refugee camp in Sierra Leone had bedbugs, and almost 90% of the residents had signs of bites.12 Unlike scabies, direct person-to-person, skin-to-skin transfer is rare.
CLINICAL FINDINGS
Bedbug bites are analogous, almost identical, to other arthropod bites: bites begin as pink macules that progress to papules (Figure 2), large plaques, or wheals (hives).13 Bites can arise minutes or even days after the initial assault. Some papules and plaques may have a central crust or erosion suggesting a bite.
Bites are typically intensely pruritic, and occasionally, hypersensitive victims can develop bullae, necrotic plaques, or even vasculitis. New papules and plaques form as older ones heal. Some patients may have fever and malaise.13 About 30% of patients may not have skin disease from bedbugs, making diagnosis in those individuals impossible.
The nonspecific nature of this presentation and the subsequent difficulty in prompt diagnosis can lead to a prolonged period of morbidity for the patient, as well as increasing the window of opportunity for the bedbugs to affect other surrounding individuals.
THE DIFFERENTIAL DIAGNOSIS IS BROAD
Commonly, bedbug bites have been misdiagnosed as drug eruptions, food allergies, dermatitis herpetiformis, staphylococcal or varicella infection, and scabies, as well as other arthropod bites.11 This broad differential diagnosis can often be narrowed by careful observation of the bite distribution. The clustering of bites in groups of 3, often in a linear pattern, sometimes overlying blood vessels, is known as the “breakfast, lunch, and dinner” sign (Figure 3), and this can help to guide the clinician toward the diagnosis of a bite as opposed to a diffuse urticarial response.2
If the characteristic clusters of bites are not present, distinguishing clinically between the various causes of pruritic urticarial lesions is difficult. Subtle clues that point towards bedbug bites can be that the rash appears to be most edematous in the morning and flattens throughout the day, as the bites occur typically during sleep.14 Likewise, the rash associated with bedbug bites has also been reported to last longer, to blanch less, and to be less responsive to steroid and antihistamine treatment than other urticarial rashes.14 If a skin biopsy specimen is available, histologic assessment can help to rule out similarly presenting conditions such as prodromal bullous pemphigoid, dermatitis herpetiformis, and urticarial dermatosis, even if it cannot provide a definitive answer as to the etiology.15
Bedbug bites vs other arthropod bites
Once a bite is suspected, differentiating between bedbug and other arthropod bites is the next challenge.
Once again, a detailed assessment of the location of the bites can yield valuable information. The waist, axillae, and uncovered parts of the body are the usual sites for bedbug bites.2 Likewise, inflammatory papules along the eyelid (the “eyelid sign”) are highly suggestive of a bedbug bite.16
The scant involvement of covered body areas, the lack of shallow burrows in the skin, and the lack of scabetic elements on skin scrapings exclude scabies as a diagnosis.
Skin biopsy is not helpful in differentiating arthropod bites, as the histologic findings are nonspecific. The key to a definitive diagnosis in these cases is identification of the suspected bug in characteristic locations. Patients should be encouraged to carefully inspect mattresses, floorboards, and other crevices for the small ovaloid bugs or the reddish-brown specks of heme and feces they typically leave behind on bed linens.15 A positive reported sighting of the bugs can lend credence to the diagnosis, whereas capture and laboratory assessment of a specimen is ideal.
BEDBUGS AS DISEASE VECTORS
Extracutaneous manifestations of bedbug assault are rare. Anaphylaxis to proteins in Cimex saliva may occur, as well as significant blood loss, even anemia, from extensive feeding.17 Bedbug infestations can exacerbate asthma, preexisting mental illness, anxiety, and insomnia.18 Since bedbugs extract blood from hosts, they have a putative ability to act as vectors of disease. Some 45 known pathogens have been isolated from the Cimex species including hepatitis B, human immunodeficiency virus (HIV), Trypanosoma cruzi, and methicillin-resistant Staphylococcus aureus. To date, however, there is no evidence to demonstrate transmission of pathogens to humans.5
TREATMENT AND ERADICATION
Treatment is mainly symptomatic—systemic antihistamines and topical corticosteroids to reduce pruritus and alleviate the dermatitis.2 Patients should be instructed to avoid scratching to prevent infection. Secondary bacterial infection can be treated with topical or systemic antibiotics. Rare cases of bite-induced asthma or anaphylaxis necessitate appropriate emergency treatment. Extermination of infestation is critical to therapy.
If bedbug infestation is suggested, mattresses, bedding, sleeping areas, and bed clothing should be inspected for insects, eggs, and fecal spotting. Adhesives or traps that emit heat or carbon dioxide can be used to capture the bedbugs. During widespread infestation, the arthropods release a pungent odor, which allows trained dogs to detect them with 95% to 98% accuracy.19
Eradication techniques
Once infestation is confirmed, patients should contact an exterminator who can confirm the presence of bedbugs. Typical eradication measures often require nonchemical control and chemical pesticides.
Professional exterminators have special equipment that can heat a room to 48 to 50°C (118–122°F). Heat sustained at this temperature for 90 minutes is sufficient to kill bedbugs.20
The infested area should be vacuumed daily, and vacuum bags and unwanted items should be sealed in plastic before discarding. Clothing, linens, and infested fabrics should be washed and dried in heat at 60°C (140°F) or greater.
Mattresses and furniture should be sealed in a special plastic that allows treatment with heat, steaming, or pesticides. Most professional pesticides contain pyrethroids, but resistance to these products is common, necessitating the use of multiple formulations to overcome resistance.8
Over-the-counter pesticides, almost exclusively pyrethroids, are variably effective and potentially hazardous to consumers.8 Patients must be advised to follow label directions to avoid adverse effects and toxicity.
Alternative chemical eradication methods to circumvent the problem of resistance include piperonyl butoxide, S-methoprene, boric acid, silicates (diatomaceous earth dust), and sulfuryl fluoride. Recent research has also posited the use of antiparasitic agents such as ivermectin and moxidectin in cases of resistant bedbug infestation, with promising results.21
All extermination products and techniques have variable risks, efficacies, and costs,8 and repeat inspections and retreatment are often required.
Prevention strategies include visual inspection of possibly infested rooms, with particular attention to mattress seams and crevices, placing luggage on a luggage rack away from the floor and bed, and careful examination of acquired second-hand items.7
Educating patients is the key to success
While all of the above eradication techniques are important curative strategies, the success of any treatment is contingent on appropriate patient education about the nature of the problem.
Resolving a bedbug infestation is notoriously difficult and requires meticulous adherence to hygiene and cleansing instructions throughout the household or institution for a sustained period of time. Information from sources such as the US Environmental Protection Agency (www.epa.gov) can empower patients to perform the necessary eradication protocols, and clinicians should routinely recommended them as part of a holistic treatment strategy.
Bedbugs have been unwelcome bedfellows for humans for thousands of years. An increase in pyrethroid resistance, a ban on the insecticide dichloro-diphenyl-trichloroethane (DDT), increased international travel, and increased population density in large cities have led to an exponential rise in the incidence of bedbug infestations. Physicians are often at the forefront of bedbug infestation diagnosis.
Once the diagnosis is suggested, symptomatic treatment of the patient and extermination of the pests are essential, though time-consuming, costly, and often problematic. Measures to eliminate infestation and to prevent spread include identification of the pest, early detection, patient education, and professional eradication.
BEDBUGS: A BRIEF HISTORY
The term bedbug refers to the obligate parasitic arthropod Cimex lectularius (the common bedbug) and, less commonly, its tropical cousin C hemipterus. Bedbugs have coexisted with humans for centuries, dating back to the ancient Egyptians 3,500 years ago.1 Through the mid-20th century, about 30% of US households were infested with bedbugs.2 The introduction of pesticides during World War II markedly decreased the incidence, but with increased international travel, pesticide resistance, and the banning of certain pesticides in the last decade, bedbugs have reemerged worldwide.3
BIOLOGY
Bedbugs are red-brown, wingless, oval-shaped insects measuring 4 to 5 mm in length (Figure 1). They are hematophagous ectoparasites that preferentially feed on human blood, although they feed on some animals as well.2
Cimex lectularius dwells in temperate climates and C hemipterus in more tropical climates, but overlap and interbreeding are common. The usual life cycle is about 6 months, but some bugs live 12 months or longer. The female bedbug lays 5 to 8 eggs per week, or approximately 500 eggs in her lifetime, and each egg hatches in 5 to 10 days.4
These photophobic parasites do not live on their human hosts but rather simply visit for a meal. They cohabitate in dark locations, attacking human hosts when they are inactive or sleeping for long periods of time. Common living areas include mattress seams, box springs, bed linens and clothes, wallpaper seams, electrical outlets, and furniture seams (Table 1).5 The female bedbug lays her eggs in these secluded crevices, ensuring their safety until hatching. The dense nests of adult bedbugs, their eggs, and accumulated fecal matter allow for easy visual identification of infestation.5
Bedbugs typically feed between 1:00 am and 5:00 am. Though wingless, they successfully navigate towards their human host, attracted by emitted heat and carbon dioxide.2 Once attached to human skin, the bedbug bite releases enzymes and chemicals including nitrophorin and nitric oxide that facilitate bleeding; these substances are responsible for the resultant dermatitis. (Of note, bedbugs with experimentally excised salivary glands do not cause skin disease in humans.6) After feeding for 3 to 20 minutes, the length and weight of the arthropod can increase by 50% to 200%. A fully sated bedbug can survive for a year until its next meal.2,7 Even if an establishment, home, room, or article of clothing infested with bedbugs has been abandoned for several months, without proper eradication the item still represents a possible nidus for recurrent disease if used, inhabited, or worn again.
EPIDEMIOLOGY
From the earliest documented cases of Cimex in ancient Egyptian tombs to the mid-1900s, the cohabitation of humans and bedbugs was seen as inevitable. With the introduction of DDT 60 years ago, the bedbug population significantly decreased.8 Since DDT’s prohibition, coupled with increased travel and heightened resistance to over-the-counter insecticides, the bedbug population has reemerged exponentially.9,10
Infestations have been reported worldwide, on every continent, and in all 50 of the United States. In Australia, infestations have risen 4,500% in the last 10 to 15 years.11 In the United States, infestation occurs exclusively with C lectularius and the incidence is rising. Philadelphia and New York City are among the most bedbug-infested US cities. New York City experienced a 2,000% increase in bedbug complaints between 2004 and 2009.8
Bedbugs can be transmitted either through active migration of colonies from one area to another adjacent living area through wall spaces or ventilation, or through passive transportation in luggage, clothing, furniture, used mattresses, bookbags, and other personal items.1 Although infestation affects people of all socioeconomic classes and backgrounds, the likelihood increases in people who frequently travel and people who live in lower income neighborhoods with tightly packed apartments. Bedbug infestations are also common in refugee camps: 98% of the rooms in a refugee camp in Sierra Leone had bedbugs, and almost 90% of the residents had signs of bites.12 Unlike scabies, direct person-to-person, skin-to-skin transfer is rare.
CLINICAL FINDINGS
Bedbug bites are analogous, almost identical, to other arthropod bites: bites begin as pink macules that progress to papules (Figure 2), large plaques, or wheals (hives).13 Bites can arise minutes or even days after the initial assault. Some papules and plaques may have a central crust or erosion suggesting a bite.
Bites are typically intensely pruritic, and occasionally, hypersensitive victims can develop bullae, necrotic plaques, or even vasculitis. New papules and plaques form as older ones heal. Some patients may have fever and malaise.13 About 30% of patients may not have skin disease from bedbugs, making diagnosis in those individuals impossible.
The nonspecific nature of this presentation and the subsequent difficulty in prompt diagnosis can lead to a prolonged period of morbidity for the patient, as well as increasing the window of opportunity for the bedbugs to affect other surrounding individuals.
THE DIFFERENTIAL DIAGNOSIS IS BROAD
Commonly, bedbug bites have been misdiagnosed as drug eruptions, food allergies, dermatitis herpetiformis, staphylococcal or varicella infection, and scabies, as well as other arthropod bites.11 This broad differential diagnosis can often be narrowed by careful observation of the bite distribution. The clustering of bites in groups of 3, often in a linear pattern, sometimes overlying blood vessels, is known as the “breakfast, lunch, and dinner” sign (Figure 3), and this can help to guide the clinician toward the diagnosis of a bite as opposed to a diffuse urticarial response.2
If the characteristic clusters of bites are not present, distinguishing clinically between the various causes of pruritic urticarial lesions is difficult. Subtle clues that point towards bedbug bites can be that the rash appears to be most edematous in the morning and flattens throughout the day, as the bites occur typically during sleep.14 Likewise, the rash associated with bedbug bites has also been reported to last longer, to blanch less, and to be less responsive to steroid and antihistamine treatment than other urticarial rashes.14 If a skin biopsy specimen is available, histologic assessment can help to rule out similarly presenting conditions such as prodromal bullous pemphigoid, dermatitis herpetiformis, and urticarial dermatosis, even if it cannot provide a definitive answer as to the etiology.15
Bedbug bites vs other arthropod bites
Once a bite is suspected, differentiating between bedbug and other arthropod bites is the next challenge.
Once again, a detailed assessment of the location of the bites can yield valuable information. The waist, axillae, and uncovered parts of the body are the usual sites for bedbug bites.2 Likewise, inflammatory papules along the eyelid (the “eyelid sign”) are highly suggestive of a bedbug bite.16
The scant involvement of covered body areas, the lack of shallow burrows in the skin, and the lack of scabetic elements on skin scrapings exclude scabies as a diagnosis.
Skin biopsy is not helpful in differentiating arthropod bites, as the histologic findings are nonspecific. The key to a definitive diagnosis in these cases is identification of the suspected bug in characteristic locations. Patients should be encouraged to carefully inspect mattresses, floorboards, and other crevices for the small ovaloid bugs or the reddish-brown specks of heme and feces they typically leave behind on bed linens.15 A positive reported sighting of the bugs can lend credence to the diagnosis, whereas capture and laboratory assessment of a specimen is ideal.
BEDBUGS AS DISEASE VECTORS
Extracutaneous manifestations of bedbug assault are rare. Anaphylaxis to proteins in Cimex saliva may occur, as well as significant blood loss, even anemia, from extensive feeding.17 Bedbug infestations can exacerbate asthma, preexisting mental illness, anxiety, and insomnia.18 Since bedbugs extract blood from hosts, they have a putative ability to act as vectors of disease. Some 45 known pathogens have been isolated from the Cimex species including hepatitis B, human immunodeficiency virus (HIV), Trypanosoma cruzi, and methicillin-resistant Staphylococcus aureus. To date, however, there is no evidence to demonstrate transmission of pathogens to humans.5
TREATMENT AND ERADICATION
Treatment is mainly symptomatic—systemic antihistamines and topical corticosteroids to reduce pruritus and alleviate the dermatitis.2 Patients should be instructed to avoid scratching to prevent infection. Secondary bacterial infection can be treated with topical or systemic antibiotics. Rare cases of bite-induced asthma or anaphylaxis necessitate appropriate emergency treatment. Extermination of infestation is critical to therapy.
If bedbug infestation is suggested, mattresses, bedding, sleeping areas, and bed clothing should be inspected for insects, eggs, and fecal spotting. Adhesives or traps that emit heat or carbon dioxide can be used to capture the bedbugs. During widespread infestation, the arthropods release a pungent odor, which allows trained dogs to detect them with 95% to 98% accuracy.19
Eradication techniques
Once infestation is confirmed, patients should contact an exterminator who can confirm the presence of bedbugs. Typical eradication measures often require nonchemical control and chemical pesticides.
Professional exterminators have special equipment that can heat a room to 48 to 50°C (118–122°F). Heat sustained at this temperature for 90 minutes is sufficient to kill bedbugs.20
The infested area should be vacuumed daily, and vacuum bags and unwanted items should be sealed in plastic before discarding. Clothing, linens, and infested fabrics should be washed and dried in heat at 60°C (140°F) or greater.
Mattresses and furniture should be sealed in a special plastic that allows treatment with heat, steaming, or pesticides. Most professional pesticides contain pyrethroids, but resistance to these products is common, necessitating the use of multiple formulations to overcome resistance.8
Over-the-counter pesticides, almost exclusively pyrethroids, are variably effective and potentially hazardous to consumers.8 Patients must be advised to follow label directions to avoid adverse effects and toxicity.
Alternative chemical eradication methods to circumvent the problem of resistance include piperonyl butoxide, S-methoprene, boric acid, silicates (diatomaceous earth dust), and sulfuryl fluoride. Recent research has also posited the use of antiparasitic agents such as ivermectin and moxidectin in cases of resistant bedbug infestation, with promising results.21
All extermination products and techniques have variable risks, efficacies, and costs,8 and repeat inspections and retreatment are often required.
Prevention strategies include visual inspection of possibly infested rooms, with particular attention to mattress seams and crevices, placing luggage on a luggage rack away from the floor and bed, and careful examination of acquired second-hand items.7
Educating patients is the key to success
While all of the above eradication techniques are important curative strategies, the success of any treatment is contingent on appropriate patient education about the nature of the problem.
Resolving a bedbug infestation is notoriously difficult and requires meticulous adherence to hygiene and cleansing instructions throughout the household or institution for a sustained period of time. Information from sources such as the US Environmental Protection Agency (www.epa.gov) can empower patients to perform the necessary eradication protocols, and clinicians should routinely recommended them as part of a holistic treatment strategy.
- Krause-Parello CA, Sciscione P. Bedbugs: an equal opportunist and cosmopolitan creature. J Sch Nurs 2009; 25:126–132.
- Sfeir M, Munoz-Price LS. Scabies and bedbugs in hospital outbreaks. Curr Infect Dis Rep 2014; 16:412.
- Romero A, Potter MF, Potter DA, Haynes KF. Insecticide resistance in the bed bug: a factor in the pest's sudden resurgence? J Med Entomol 2007; 44:175–178.
- Delaunay P, Blanc V, Del Giudice P, et al. Bedbugs and infectious diseases. Clin Infect Dis 2011; 52:200–210.
- Doggett SL, Dwyer DE, Penas PF, Russell RC. Bed bugs: clinical relevance and control options. Clin Microbiol Rev 2012; 25:164–192.
- Goddard J, Edwards KT. Effects of bed bug saliva on human skin. JAMA Dermatol 2013; 149:372–373.
- Goddard J, deShazo R. Bed bugs (Cimex lectularius) and clinical consequences of their bites. JAMA 2009; 301:1358–1366.
- Davies TG, Field LM, Williamson MS. The re-emergence of the bed bug as a nuisance pest: implications of resistance to the pyrethroid insecticides. Med Vet Entomol 2012; 26:241–254.
- Saenz VL, Booth W, Schal C, Vargo EL. Genetic analysis of bed bug populations reveals small propagule size within individual infestations but high genetic diversity across infestations from the eastern United States. J Med Entomol 2012; 49:865–875.
- Jones SC, Bryant JL. Ineffectiveness of over-the-counter total-release foggers against the bed bug (Heteroptera: cimicidae). J Econ Entomol 2012; 105:957–963.
- Doggett SL, Russell R. Bed bugs—what the GP needs to know. Aust Fam Physician 2009; 38:880–884.
- Gbakima AA, Terry BC, Kanja F, Kortequee S, Dukuley I, Sahr F. High prevalence of bedbugs Cimex hemipterus and Cimex lectularis in camps for internally displaced persons in Freetown, Sierra Leone: a pilot humanitarian investigation. West Afr J Med 2002; 21:268–271.
- deShazo RD, Feldlaufer MF, Mihm MC Jr, Goddard J. Bullous reactions to bedbug bites reflect cutaneous vasculitis. Am J Med 2012; 125:688–694.
- Scarupa MD, Economides A. Bedbug bites masquerading as urticaria. J Allergy Clin Immunol 2006; 117:1508–1509.
- Thomas I, Kihiczak GG, Schwartz RA. Bedbug bites: a review. Int J Dermatol 2004; 43:430–433.
- Quach KA, Zaenglein AL. The eyelid sign: a clue to bed bug bites. Pediatr Dermatol 2014; 31:353–355.
- Paulke-Korinek M, Szell M, Laferl H, Auer H, Wenisch C. Bed bugs can cause severe anaemia in adults. Parasitol Res 2012; 110:2577–2579.
- Goddard J, de Shazo R. Psychological effects of bed bug attacks (Cimex lectularius L). Am J Med 2012; 125:101–103.
- Pfiester M, Koehler PG, Pereira RM. Ability of bed bug-detecting canines to locate live bed bugs and viable bed bug eggs. J Econ Entomol 2008; 101:1389–1396.
- Kells SA, Goblirsch MJ. Temperature and time requirements for controlling bed bugs (Cimex lectularius) under commercial heat treatment conditions. Insects 2011; 2:412–422.
- Sheele JM, Ridge GE. Toxicity and potential utility of ivermectin and moxidectin as xenointoxicants against the common bed bug Cimex lectularius L. Parasitol Res 2016; 115:3071–3081.
- Krause-Parello CA, Sciscione P. Bedbugs: an equal opportunist and cosmopolitan creature. J Sch Nurs 2009; 25:126–132.
- Sfeir M, Munoz-Price LS. Scabies and bedbugs in hospital outbreaks. Curr Infect Dis Rep 2014; 16:412.
- Romero A, Potter MF, Potter DA, Haynes KF. Insecticide resistance in the bed bug: a factor in the pest's sudden resurgence? J Med Entomol 2007; 44:175–178.
- Delaunay P, Blanc V, Del Giudice P, et al. Bedbugs and infectious diseases. Clin Infect Dis 2011; 52:200–210.
- Doggett SL, Dwyer DE, Penas PF, Russell RC. Bed bugs: clinical relevance and control options. Clin Microbiol Rev 2012; 25:164–192.
- Goddard J, Edwards KT. Effects of bed bug saliva on human skin. JAMA Dermatol 2013; 149:372–373.
- Goddard J, deShazo R. Bed bugs (Cimex lectularius) and clinical consequences of their bites. JAMA 2009; 301:1358–1366.
- Davies TG, Field LM, Williamson MS. The re-emergence of the bed bug as a nuisance pest: implications of resistance to the pyrethroid insecticides. Med Vet Entomol 2012; 26:241–254.
- Saenz VL, Booth W, Schal C, Vargo EL. Genetic analysis of bed bug populations reveals small propagule size within individual infestations but high genetic diversity across infestations from the eastern United States. J Med Entomol 2012; 49:865–875.
- Jones SC, Bryant JL. Ineffectiveness of over-the-counter total-release foggers against the bed bug (Heteroptera: cimicidae). J Econ Entomol 2012; 105:957–963.
- Doggett SL, Russell R. Bed bugs—what the GP needs to know. Aust Fam Physician 2009; 38:880–884.
- Gbakima AA, Terry BC, Kanja F, Kortequee S, Dukuley I, Sahr F. High prevalence of bedbugs Cimex hemipterus and Cimex lectularis in camps for internally displaced persons in Freetown, Sierra Leone: a pilot humanitarian investigation. West Afr J Med 2002; 21:268–271.
- deShazo RD, Feldlaufer MF, Mihm MC Jr, Goddard J. Bullous reactions to bedbug bites reflect cutaneous vasculitis. Am J Med 2012; 125:688–694.
- Scarupa MD, Economides A. Bedbug bites masquerading as urticaria. J Allergy Clin Immunol 2006; 117:1508–1509.
- Thomas I, Kihiczak GG, Schwartz RA. Bedbug bites: a review. Int J Dermatol 2004; 43:430–433.
- Quach KA, Zaenglein AL. The eyelid sign: a clue to bed bug bites. Pediatr Dermatol 2014; 31:353–355.
- Paulke-Korinek M, Szell M, Laferl H, Auer H, Wenisch C. Bed bugs can cause severe anaemia in adults. Parasitol Res 2012; 110:2577–2579.
- Goddard J, de Shazo R. Psychological effects of bed bug attacks (Cimex lectularius L). Am J Med 2012; 125:101–103.
- Pfiester M, Koehler PG, Pereira RM. Ability of bed bug-detecting canines to locate live bed bugs and viable bed bug eggs. J Econ Entomol 2008; 101:1389–1396.
- Kells SA, Goblirsch MJ. Temperature and time requirements for controlling bed bugs (Cimex lectularius) under commercial heat treatment conditions. Insects 2011; 2:412–422.
- Sheele JM, Ridge GE. Toxicity and potential utility of ivermectin and moxidectin as xenointoxicants against the common bed bug Cimex lectularius L. Parasitol Res 2016; 115:3071–3081.
KEY POINTS
- The increase in pyrethroid resistance, the ban of DDT, the ease and frequency of travel, and the increased population density in large cities have led to an exponential rise in the incidence of bedbug infection.
- Once the diagnosis is suggested, patients deserve symptomatic treatment, and extermination of the pests becomes essential, though time-consuming, costly, and often problematic.
- Measures to eliminate infestation and prevent spread include early detection, identification of the pest, patient education, and professional eradication.
Corneal opacities in a man with chronic kidney disease
A 40-year-old man with end-stage renal disease on intermittent hemodialysis presented to the emergency department with a 1-week history of pain affecting his left lower back, left flank, and left lower abdomen, diagnosed as zoster prodrome.
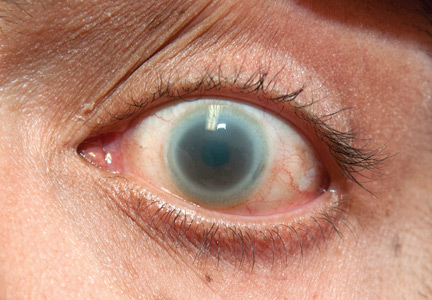
Of note, both corneas were cloudy, most severely in the limbus (Figure 1). His visual acuity and findings on funduscopic examination were normal.
CORNEAL OPACITY
The finding of corneal opacity should prompt an immediate ophthalmologic evaluation by the internist as well as an ophthalmologist. The initial examination should include visual acuity testing; gross examination with the naked eye; penlight examination of the pupil, conjunctiva, and anterior chamber; funduscopic examination to at least confirm a red reflex; and fluorescein examination of the cornea. Fluorescein testing is done last, as the dye may interfere with the other initial tests.1
A number of causes of opacity
A number of conditions can cause corneal opacity. Several genetic conditions can cause developmental anomalies of the cornea, leading to corneal defects present at birth.2 Causes of secondary corneal opacity in early infancy include infections such as herpes, iatrogenic injury during amniocentesis or forceps delivery, and infantile congenital glaucoma.2
Later in life, causes of corneal opacity include cataract, glaucoma, chemical exposure, foreign body injury, irradiation, infection (eg, syphilis, herpes, chlamydia), endophthalmitis, and metabolic genetic disorders such as Fabry disease, trisomy 18 syndrome, and lecithin-cholesterol acyltransferase (LCAT) deficiency.3
LCAT DEFICIENCY
LCAT is a key protein in reverse transport of cholesterol from the systemic circulation to the liver for excretion into the bile. Its deficiency results in low serum concentrations of high-density lipoprotein cholesterol (HDL-C).4 About 80 different mutations in the LCAT gene have been linked to LCAT deficiency.5
LCAT deficiency varies in severity. Patients with complete deficiency can have nearly undetectable levels of HDL-C, eruptive xanthoma, hepatosplenomegaly, and premature coronary artery disease (ie, by age 40).5,6 Features of coronary atherosclerosis can be lacking in patients with partial deficiency.
Regardless of the degree of LCAT deficiency, most patients have corneal opacification that is most severe near the limbus (thus, the term “fish eye syndrome”) and anemia.7 Although corneal opacification presents early in life and persists, it does not seem to affect vision.5 The anemia is associated with enhanced fractional clearance of red blood cells secondary to hypersplenism.8
LCAT deficiency and the kidneys
LCAT deficiency has its most devastating effect on the kidney. Renal disease begins early in life with mild proteinuria and microscopic hematuria. With increasing age, renal function deteriorates and proteinuria and hematuria worsen.9
Renal biopsy study may reveal foam cells in the glomerular tufts, arterioles with thickened intima and narrowed lumens, and subendothelial deposits of lipids in the renal arteries and arterioles.10 Some studies have suggested that kidney disease is most likely initiated by lipid deposition or cellular uptake of lipoproteins in the glomerular basement membrane, mesangium, and capillary subendothelium.
Treatment
There are few treatment options for patients with LCAT deficiency. Control of hypertension, if present, may halt or slow renal deterioration.5 Many patients eventually require dialysis, and some undergo renal transplantation, but the renal disease can recur.
OUR PATIENT
Our patient had a known diagnosis of LCAT deficiency. Five years before this presentation at our emergency department, he developed malignant hypertension, followed shortly by renal disease. Over the next 4 years, his kidney function deteriorated, culminating in the need for dialysis; his corneal opacities manifested and gradually worsened; and after extensive studies including kidney biopsies, he was finally diagnosed with LCAT deficiency.
He also exhibited a chronically low level of HDL-C (2 to 5 mg/dL) and significant coronary artery disease. Although unrelated, his zoster pain was treated with renally dosed acyclovir and gabapentin. He never demonstrated the characteristic rash, and his pain improved significantly within 5 days of treatment.
- Knox KA, McIntee J. Nurse management of corneal abrasion. Br J Nurs 1995; 4:440–460.
- Nischal KK. Congenital corneal opacities—a surgical approach to nomenclature and classification. Eye (Lond) 2007; 21:1326–1337.
- Chiapella AP, Rosenthal AR. One year in an eye casualty clinic. Br J Ophthalmol 1985; 69:865–870.
- Rosenson RS, Brewer HB Jr, Davidson WS, et al. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation 2012; 125:1905–1919.
- Roshan B, Ganda OP, Desilva R, et al. Homozygous lecithin:cholesterol acyltransferase (LCAT) deficiency due to a new loss of function mutation and review of the literature. J Clin Lipidol 2011; 5:493–499.
- Kuivenhoven JA, van Voorst tot Voorst EJ, Wiebusch H, et al. A unique genetic and biochemical presentation of fish-eye disease. J Clin Invest 1995; 96:2783–2791.
- Palmiero PM, Sbeity Z, Liebmann J, Ritch R. In vivo imaging of the cornea in a patient with lecithin-cholesterol acyltransferase deficiency. Cornea 2009; 28:1061–1064.
- Norum KR, Gjone E. Familial serum-cholesterol esterification failure. A new inborn error of metabolism. Biochim Biophys Acta 1967; 144:698–700.
- Gjone E, Norum KR. Familial serum cholesterol ester deficiency. Clinical study of a patient with a new syndrome. Acta Med Scand 1968; 183:107–112.
- Lager DJ, Rosenberg BF, Shapiro H, Bernstein J. Lecithin cholesterol acyltransferase deficiency: ultrastructural examination of sequential renal biopsies. Mod Pathol 1991; 4:331–335.
A 40-year-old man with end-stage renal disease on intermittent hemodialysis presented to the emergency department with a 1-week history of pain affecting his left lower back, left flank, and left lower abdomen, diagnosed as zoster prodrome.
Of note, both corneas were cloudy, most severely in the limbus (Figure 1). His visual acuity and findings on funduscopic examination were normal.
CORNEAL OPACITY
The finding of corneal opacity should prompt an immediate ophthalmologic evaluation by the internist as well as an ophthalmologist. The initial examination should include visual acuity testing; gross examination with the naked eye; penlight examination of the pupil, conjunctiva, and anterior chamber; funduscopic examination to at least confirm a red reflex; and fluorescein examination of the cornea. Fluorescein testing is done last, as the dye may interfere with the other initial tests.1
A number of causes of opacity
A number of conditions can cause corneal opacity. Several genetic conditions can cause developmental anomalies of the cornea, leading to corneal defects present at birth.2 Causes of secondary corneal opacity in early infancy include infections such as herpes, iatrogenic injury during amniocentesis or forceps delivery, and infantile congenital glaucoma.2
Later in life, causes of corneal opacity include cataract, glaucoma, chemical exposure, foreign body injury, irradiation, infection (eg, syphilis, herpes, chlamydia), endophthalmitis, and metabolic genetic disorders such as Fabry disease, trisomy 18 syndrome, and lecithin-cholesterol acyltransferase (LCAT) deficiency.3
LCAT DEFICIENCY
LCAT is a key protein in reverse transport of cholesterol from the systemic circulation to the liver for excretion into the bile. Its deficiency results in low serum concentrations of high-density lipoprotein cholesterol (HDL-C).4 About 80 different mutations in the LCAT gene have been linked to LCAT deficiency.5
LCAT deficiency varies in severity. Patients with complete deficiency can have nearly undetectable levels of HDL-C, eruptive xanthoma, hepatosplenomegaly, and premature coronary artery disease (ie, by age 40).5,6 Features of coronary atherosclerosis can be lacking in patients with partial deficiency.
Regardless of the degree of LCAT deficiency, most patients have corneal opacification that is most severe near the limbus (thus, the term “fish eye syndrome”) and anemia.7 Although corneal opacification presents early in life and persists, it does not seem to affect vision.5 The anemia is associated with enhanced fractional clearance of red blood cells secondary to hypersplenism.8
LCAT deficiency and the kidneys
LCAT deficiency has its most devastating effect on the kidney. Renal disease begins early in life with mild proteinuria and microscopic hematuria. With increasing age, renal function deteriorates and proteinuria and hematuria worsen.9
Renal biopsy study may reveal foam cells in the glomerular tufts, arterioles with thickened intima and narrowed lumens, and subendothelial deposits of lipids in the renal arteries and arterioles.10 Some studies have suggested that kidney disease is most likely initiated by lipid deposition or cellular uptake of lipoproteins in the glomerular basement membrane, mesangium, and capillary subendothelium.
Treatment
There are few treatment options for patients with LCAT deficiency. Control of hypertension, if present, may halt or slow renal deterioration.5 Many patients eventually require dialysis, and some undergo renal transplantation, but the renal disease can recur.
OUR PATIENT
Our patient had a known diagnosis of LCAT deficiency. Five years before this presentation at our emergency department, he developed malignant hypertension, followed shortly by renal disease. Over the next 4 years, his kidney function deteriorated, culminating in the need for dialysis; his corneal opacities manifested and gradually worsened; and after extensive studies including kidney biopsies, he was finally diagnosed with LCAT deficiency.
He also exhibited a chronically low level of HDL-C (2 to 5 mg/dL) and significant coronary artery disease. Although unrelated, his zoster pain was treated with renally dosed acyclovir and gabapentin. He never demonstrated the characteristic rash, and his pain improved significantly within 5 days of treatment.
A 40-year-old man with end-stage renal disease on intermittent hemodialysis presented to the emergency department with a 1-week history of pain affecting his left lower back, left flank, and left lower abdomen, diagnosed as zoster prodrome.
Of note, both corneas were cloudy, most severely in the limbus (Figure 1). His visual acuity and findings on funduscopic examination were normal.
CORNEAL OPACITY
The finding of corneal opacity should prompt an immediate ophthalmologic evaluation by the internist as well as an ophthalmologist. The initial examination should include visual acuity testing; gross examination with the naked eye; penlight examination of the pupil, conjunctiva, and anterior chamber; funduscopic examination to at least confirm a red reflex; and fluorescein examination of the cornea. Fluorescein testing is done last, as the dye may interfere with the other initial tests.1
A number of causes of opacity
A number of conditions can cause corneal opacity. Several genetic conditions can cause developmental anomalies of the cornea, leading to corneal defects present at birth.2 Causes of secondary corneal opacity in early infancy include infections such as herpes, iatrogenic injury during amniocentesis or forceps delivery, and infantile congenital glaucoma.2
Later in life, causes of corneal opacity include cataract, glaucoma, chemical exposure, foreign body injury, irradiation, infection (eg, syphilis, herpes, chlamydia), endophthalmitis, and metabolic genetic disorders such as Fabry disease, trisomy 18 syndrome, and lecithin-cholesterol acyltransferase (LCAT) deficiency.3
LCAT DEFICIENCY
LCAT is a key protein in reverse transport of cholesterol from the systemic circulation to the liver for excretion into the bile. Its deficiency results in low serum concentrations of high-density lipoprotein cholesterol (HDL-C).4 About 80 different mutations in the LCAT gene have been linked to LCAT deficiency.5
LCAT deficiency varies in severity. Patients with complete deficiency can have nearly undetectable levels of HDL-C, eruptive xanthoma, hepatosplenomegaly, and premature coronary artery disease (ie, by age 40).5,6 Features of coronary atherosclerosis can be lacking in patients with partial deficiency.
Regardless of the degree of LCAT deficiency, most patients have corneal opacification that is most severe near the limbus (thus, the term “fish eye syndrome”) and anemia.7 Although corneal opacification presents early in life and persists, it does not seem to affect vision.5 The anemia is associated with enhanced fractional clearance of red blood cells secondary to hypersplenism.8
LCAT deficiency and the kidneys
LCAT deficiency has its most devastating effect on the kidney. Renal disease begins early in life with mild proteinuria and microscopic hematuria. With increasing age, renal function deteriorates and proteinuria and hematuria worsen.9
Renal biopsy study may reveal foam cells in the glomerular tufts, arterioles with thickened intima and narrowed lumens, and subendothelial deposits of lipids in the renal arteries and arterioles.10 Some studies have suggested that kidney disease is most likely initiated by lipid deposition or cellular uptake of lipoproteins in the glomerular basement membrane, mesangium, and capillary subendothelium.
Treatment
There are few treatment options for patients with LCAT deficiency. Control of hypertension, if present, may halt or slow renal deterioration.5 Many patients eventually require dialysis, and some undergo renal transplantation, but the renal disease can recur.
OUR PATIENT
Our patient had a known diagnosis of LCAT deficiency. Five years before this presentation at our emergency department, he developed malignant hypertension, followed shortly by renal disease. Over the next 4 years, his kidney function deteriorated, culminating in the need for dialysis; his corneal opacities manifested and gradually worsened; and after extensive studies including kidney biopsies, he was finally diagnosed with LCAT deficiency.
He also exhibited a chronically low level of HDL-C (2 to 5 mg/dL) and significant coronary artery disease. Although unrelated, his zoster pain was treated with renally dosed acyclovir and gabapentin. He never demonstrated the characteristic rash, and his pain improved significantly within 5 days of treatment.
- Knox KA, McIntee J. Nurse management of corneal abrasion. Br J Nurs 1995; 4:440–460.
- Nischal KK. Congenital corneal opacities—a surgical approach to nomenclature and classification. Eye (Lond) 2007; 21:1326–1337.
- Chiapella AP, Rosenthal AR. One year in an eye casualty clinic. Br J Ophthalmol 1985; 69:865–870.
- Rosenson RS, Brewer HB Jr, Davidson WS, et al. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation 2012; 125:1905–1919.
- Roshan B, Ganda OP, Desilva R, et al. Homozygous lecithin:cholesterol acyltransferase (LCAT) deficiency due to a new loss of function mutation and review of the literature. J Clin Lipidol 2011; 5:493–499.
- Kuivenhoven JA, van Voorst tot Voorst EJ, Wiebusch H, et al. A unique genetic and biochemical presentation of fish-eye disease. J Clin Invest 1995; 96:2783–2791.
- Palmiero PM, Sbeity Z, Liebmann J, Ritch R. In vivo imaging of the cornea in a patient with lecithin-cholesterol acyltransferase deficiency. Cornea 2009; 28:1061–1064.
- Norum KR, Gjone E. Familial serum-cholesterol esterification failure. A new inborn error of metabolism. Biochim Biophys Acta 1967; 144:698–700.
- Gjone E, Norum KR. Familial serum cholesterol ester deficiency. Clinical study of a patient with a new syndrome. Acta Med Scand 1968; 183:107–112.
- Lager DJ, Rosenberg BF, Shapiro H, Bernstein J. Lecithin cholesterol acyltransferase deficiency: ultrastructural examination of sequential renal biopsies. Mod Pathol 1991; 4:331–335.
- Knox KA, McIntee J. Nurse management of corneal abrasion. Br J Nurs 1995; 4:440–460.
- Nischal KK. Congenital corneal opacities—a surgical approach to nomenclature and classification. Eye (Lond) 2007; 21:1326–1337.
- Chiapella AP, Rosenthal AR. One year in an eye casualty clinic. Br J Ophthalmol 1985; 69:865–870.
- Rosenson RS, Brewer HB Jr, Davidson WS, et al. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation 2012; 125:1905–1919.
- Roshan B, Ganda OP, Desilva R, et al. Homozygous lecithin:cholesterol acyltransferase (LCAT) deficiency due to a new loss of function mutation and review of the literature. J Clin Lipidol 2011; 5:493–499.
- Kuivenhoven JA, van Voorst tot Voorst EJ, Wiebusch H, et al. A unique genetic and biochemical presentation of fish-eye disease. J Clin Invest 1995; 96:2783–2791.
- Palmiero PM, Sbeity Z, Liebmann J, Ritch R. In vivo imaging of the cornea in a patient with lecithin-cholesterol acyltransferase deficiency. Cornea 2009; 28:1061–1064.
- Norum KR, Gjone E. Familial serum-cholesterol esterification failure. A new inborn error of metabolism. Biochim Biophys Acta 1967; 144:698–700.
- Gjone E, Norum KR. Familial serum cholesterol ester deficiency. Clinical study of a patient with a new syndrome. Acta Med Scand 1968; 183:107–112.
- Lager DJ, Rosenberg BF, Shapiro H, Bernstein J. Lecithin cholesterol acyltransferase deficiency: ultrastructural examination of sequential renal biopsies. Mod Pathol 1991; 4:331–335.