User login
Does lowering diastolic BP to less than 90 mm Hg decrease cardiovascular risk?
Although lowering diastolic blood pressure (DBP) is associated with reduced cardiovascular events, systolic blood pressure (SBP) is a more robust predictor of cardiovascular risk than DBP and should now be used to diagnose, stage, and treat hypertension.
Lowering diastolic blood pressure (DBP) to <90 mm Hg in hypertensive individuals of all ages decreases the risk of cardiovascular events including myocardial infarction (MI), heart failure, and sudden death (strength of recommendation [SOR]: A, based on systematic review of randomized controlled trials). However, there is no consensus regarding how far to lower DBP. A “J-shaped” increase in cardiovascular risks with DBP <85 mm Hg may apply under certain conditions.
Evidence summary
The concept of a continuous graded relationship between DBP and cardiovascular risk is supported by a meta-analysis of 14 randomized clinical trials showing that lowering DBP by 6 mm Hg reduced the risk of coronary heart disease by 14% (95% confidence interval [CI], 4%–22%; P<.01; NNT=200).1 Throughout the range of DBP in study subjects, 70–115 mm Hg, a lower DBP was associated with a lower risk of coronary heart disease.
However, there is concern that lowering DBP too much may actually increase cardiovascular risk. A 10-year observational study showed that in patients with a history of ischemic heart disease, the incidence of fatal MI was lowest when DBP was between 85 to 90 mm Hg and increased with DBP <85 mm Hg, thus demonstrating a J-shaped curve.2
Farnett et al3 derived a summary curve from 13 studies that stratified cardiovascular outcomes by level of achieved blood pressure; the nadir of the curve for ischemic heart disease events occurred at 86 to 89 mm Hg DBP. The risk was independent of type of drug therapy, and more pronounced in study subjects with known cardiovascular disease.
A meta-analysis of 7 randomized controlled trials involving 40,233 hypertensive patients used statistical modeling to determine the shape of the “mortality curve” over a range of DBP categories, defined in 10-mm Hg increments from 65 to 106. The subjects received mainly beta-blockers or thiazide diuretics; controls received placebo or no treatment.4 Both groups demonstrated increased risk for cardiovascular and all-cause death at the lowest DBP levels. Among treated patients, overall death rate was lowest with a DBP in the range of 76 to 85 mm Hg; among controls the nadir was 86 to 95 mm Hg.
The Hypertension Optimal Treatment (HOT) trial5 was specifically designed to determine the optimal target blood pressure for hypertensive patients: 18,790 men and women with DBP 100 to 115 mm Hg were randomly assigned to target DBP groups of <90, <85, or <80 mm Hg. All were treated with felodipine and other agents in a stepped-care protocol; average follow-up was 3.8 years. The lowest incidence of cardiovascular events occurred at a mean DBP of 82.6 mm Hg and fewest cardiovascular deaths at 86.5 mm Hg. Further reductions in DBP neither lowered nor increased cardiovascular risk.
A French cohort study6 followed over 4700 hyper-tensive men for an average of 14 years. These men had their hypertension treated in usual fashion by their own physicians. In this group, SBP was much more accurate than DBP in classifying severity of hypertension and in predicting cardiovascular risk.
Recommendations from others
The Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC VII)7 and the World Health Organization–International Society of Hyper-tension Guidelines8 state that the relationship between cardiovascular risk and blood pressure is continuous, without a lower threshold. Target blood pressure goals are <140/90 mm Hg in uncomplicated hypertension and <130/80 mm Hg for individuals with diabetes or kidney disease. The National High Blood Pressure Education Program stressed that SBP, not DBP, should become the major criterion for diagnosis and treatment of hypertension.9
Emphasize education and focus on systolic blood pressure
Randy Ward, MD
Director, Family Medicine/Psychiatry Residency, Medical College of Wisconsin, Milwaukee
In light of JNC VII, there may be some confusion on the part of patients as to “normal” blood pressure and indications for treatment. In fact, on the first page of the NHLBI web site, “Your Guide to Lowering Blood Pressure,” the statement is made that “normal blood pressure is less than 120 mm Hg systolic and less than 80 mm Hg diastolic.” They later go on to describe the category of prehypertension. It is important to understand the concept and implications of prehypertension, and the “J-shaped” curve in counseling our patients on achieving optimal blood pressure control.
1. Collins R, Peto R, MacMahon S, et al. Blood pressure, stroke, and coronary heart disease. Part 2, Short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet 1990;335:827-838.
2. Cruickshank JM, Thorp JM, Zacharias FJ. Benefits and potential harm of lowering high blood pressure. Lancet 1987;1:581-584.
3. Farnett L, Mulrow CD, Linn WD, Lucey CR, Tuley MR. The J-curve phenomenon and the treatment of hypertension. Is there a point beyond which pressure reduction is dangerous? JAMA 1991;265:489-495.
4. Boutitie F, Gueyffier F, Pocock S, Fagard R, Boissel JP. Jshaped relationship between blood pressure and mortality in hypertensive patients: new insights from a meta-analysis of individual-patient data. Ann Intern Med 2002;136:438-448.
5. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood pressure lowering and low-dose aspirin in patients treated with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998;351:1755-1762.
6. Benetos A, Thomas F, Bean K, Gautier S, Smulyan H, Guize L. Prognostic value of systolic and diastolic blood pressure in treated hypertensive men. Arch Intern Med 2002;162:577-581.
7. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 Report. JAMA 2003;289:2560-2572.
8. 1999 World Health Organization–International Society of Hypertension Guidelines for the Management of Hypertension. Guidelines Subcommittee. J Hypertens 1999;17:151-183.
9. Izzo JL, Jr, Levy D, Black HR. Clinical Advisory Statement. Importance of systolic blood pressure in older Americans. Hypertension 2000;35:1021-1024.
Although lowering diastolic blood pressure (DBP) is associated with reduced cardiovascular events, systolic blood pressure (SBP) is a more robust predictor of cardiovascular risk than DBP and should now be used to diagnose, stage, and treat hypertension.
Lowering diastolic blood pressure (DBP) to <90 mm Hg in hypertensive individuals of all ages decreases the risk of cardiovascular events including myocardial infarction (MI), heart failure, and sudden death (strength of recommendation [SOR]: A, based on systematic review of randomized controlled trials). However, there is no consensus regarding how far to lower DBP. A “J-shaped” increase in cardiovascular risks with DBP <85 mm Hg may apply under certain conditions.
Evidence summary
The concept of a continuous graded relationship between DBP and cardiovascular risk is supported by a meta-analysis of 14 randomized clinical trials showing that lowering DBP by 6 mm Hg reduced the risk of coronary heart disease by 14% (95% confidence interval [CI], 4%–22%; P<.01; NNT=200).1 Throughout the range of DBP in study subjects, 70–115 mm Hg, a lower DBP was associated with a lower risk of coronary heart disease.
However, there is concern that lowering DBP too much may actually increase cardiovascular risk. A 10-year observational study showed that in patients with a history of ischemic heart disease, the incidence of fatal MI was lowest when DBP was between 85 to 90 mm Hg and increased with DBP <85 mm Hg, thus demonstrating a J-shaped curve.2
Farnett et al3 derived a summary curve from 13 studies that stratified cardiovascular outcomes by level of achieved blood pressure; the nadir of the curve for ischemic heart disease events occurred at 86 to 89 mm Hg DBP. The risk was independent of type of drug therapy, and more pronounced in study subjects with known cardiovascular disease.
A meta-analysis of 7 randomized controlled trials involving 40,233 hypertensive patients used statistical modeling to determine the shape of the “mortality curve” over a range of DBP categories, defined in 10-mm Hg increments from 65 to 106. The subjects received mainly beta-blockers or thiazide diuretics; controls received placebo or no treatment.4 Both groups demonstrated increased risk for cardiovascular and all-cause death at the lowest DBP levels. Among treated patients, overall death rate was lowest with a DBP in the range of 76 to 85 mm Hg; among controls the nadir was 86 to 95 mm Hg.
The Hypertension Optimal Treatment (HOT) trial5 was specifically designed to determine the optimal target blood pressure for hypertensive patients: 18,790 men and women with DBP 100 to 115 mm Hg were randomly assigned to target DBP groups of <90, <85, or <80 mm Hg. All were treated with felodipine and other agents in a stepped-care protocol; average follow-up was 3.8 years. The lowest incidence of cardiovascular events occurred at a mean DBP of 82.6 mm Hg and fewest cardiovascular deaths at 86.5 mm Hg. Further reductions in DBP neither lowered nor increased cardiovascular risk.
A French cohort study6 followed over 4700 hyper-tensive men for an average of 14 years. These men had their hypertension treated in usual fashion by their own physicians. In this group, SBP was much more accurate than DBP in classifying severity of hypertension and in predicting cardiovascular risk.
Recommendations from others
The Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC VII)7 and the World Health Organization–International Society of Hyper-tension Guidelines8 state that the relationship between cardiovascular risk and blood pressure is continuous, without a lower threshold. Target blood pressure goals are <140/90 mm Hg in uncomplicated hypertension and <130/80 mm Hg for individuals with diabetes or kidney disease. The National High Blood Pressure Education Program stressed that SBP, not DBP, should become the major criterion for diagnosis and treatment of hypertension.9
Emphasize education and focus on systolic blood pressure
Randy Ward, MD
Director, Family Medicine/Psychiatry Residency, Medical College of Wisconsin, Milwaukee
In light of JNC VII, there may be some confusion on the part of patients as to “normal” blood pressure and indications for treatment. In fact, on the first page of the NHLBI web site, “Your Guide to Lowering Blood Pressure,” the statement is made that “normal blood pressure is less than 120 mm Hg systolic and less than 80 mm Hg diastolic.” They later go on to describe the category of prehypertension. It is important to understand the concept and implications of prehypertension, and the “J-shaped” curve in counseling our patients on achieving optimal blood pressure control.
Although lowering diastolic blood pressure (DBP) is associated with reduced cardiovascular events, systolic blood pressure (SBP) is a more robust predictor of cardiovascular risk than DBP and should now be used to diagnose, stage, and treat hypertension.
Lowering diastolic blood pressure (DBP) to <90 mm Hg in hypertensive individuals of all ages decreases the risk of cardiovascular events including myocardial infarction (MI), heart failure, and sudden death (strength of recommendation [SOR]: A, based on systematic review of randomized controlled trials). However, there is no consensus regarding how far to lower DBP. A “J-shaped” increase in cardiovascular risks with DBP <85 mm Hg may apply under certain conditions.
Evidence summary
The concept of a continuous graded relationship between DBP and cardiovascular risk is supported by a meta-analysis of 14 randomized clinical trials showing that lowering DBP by 6 mm Hg reduced the risk of coronary heart disease by 14% (95% confidence interval [CI], 4%–22%; P<.01; NNT=200).1 Throughout the range of DBP in study subjects, 70–115 mm Hg, a lower DBP was associated with a lower risk of coronary heart disease.
However, there is concern that lowering DBP too much may actually increase cardiovascular risk. A 10-year observational study showed that in patients with a history of ischemic heart disease, the incidence of fatal MI was lowest when DBP was between 85 to 90 mm Hg and increased with DBP <85 mm Hg, thus demonstrating a J-shaped curve.2
Farnett et al3 derived a summary curve from 13 studies that stratified cardiovascular outcomes by level of achieved blood pressure; the nadir of the curve for ischemic heart disease events occurred at 86 to 89 mm Hg DBP. The risk was independent of type of drug therapy, and more pronounced in study subjects with known cardiovascular disease.
A meta-analysis of 7 randomized controlled trials involving 40,233 hypertensive patients used statistical modeling to determine the shape of the “mortality curve” over a range of DBP categories, defined in 10-mm Hg increments from 65 to 106. The subjects received mainly beta-blockers or thiazide diuretics; controls received placebo or no treatment.4 Both groups demonstrated increased risk for cardiovascular and all-cause death at the lowest DBP levels. Among treated patients, overall death rate was lowest with a DBP in the range of 76 to 85 mm Hg; among controls the nadir was 86 to 95 mm Hg.
The Hypertension Optimal Treatment (HOT) trial5 was specifically designed to determine the optimal target blood pressure for hypertensive patients: 18,790 men and women with DBP 100 to 115 mm Hg were randomly assigned to target DBP groups of <90, <85, or <80 mm Hg. All were treated with felodipine and other agents in a stepped-care protocol; average follow-up was 3.8 years. The lowest incidence of cardiovascular events occurred at a mean DBP of 82.6 mm Hg and fewest cardiovascular deaths at 86.5 mm Hg. Further reductions in DBP neither lowered nor increased cardiovascular risk.
A French cohort study6 followed over 4700 hyper-tensive men for an average of 14 years. These men had their hypertension treated in usual fashion by their own physicians. In this group, SBP was much more accurate than DBP in classifying severity of hypertension and in predicting cardiovascular risk.
Recommendations from others
The Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC VII)7 and the World Health Organization–International Society of Hyper-tension Guidelines8 state that the relationship between cardiovascular risk and blood pressure is continuous, without a lower threshold. Target blood pressure goals are <140/90 mm Hg in uncomplicated hypertension and <130/80 mm Hg for individuals with diabetes or kidney disease. The National High Blood Pressure Education Program stressed that SBP, not DBP, should become the major criterion for diagnosis and treatment of hypertension.9
Emphasize education and focus on systolic blood pressure
Randy Ward, MD
Director, Family Medicine/Psychiatry Residency, Medical College of Wisconsin, Milwaukee
In light of JNC VII, there may be some confusion on the part of patients as to “normal” blood pressure and indications for treatment. In fact, on the first page of the NHLBI web site, “Your Guide to Lowering Blood Pressure,” the statement is made that “normal blood pressure is less than 120 mm Hg systolic and less than 80 mm Hg diastolic.” They later go on to describe the category of prehypertension. It is important to understand the concept and implications of prehypertension, and the “J-shaped” curve in counseling our patients on achieving optimal blood pressure control.
1. Collins R, Peto R, MacMahon S, et al. Blood pressure, stroke, and coronary heart disease. Part 2, Short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet 1990;335:827-838.
2. Cruickshank JM, Thorp JM, Zacharias FJ. Benefits and potential harm of lowering high blood pressure. Lancet 1987;1:581-584.
3. Farnett L, Mulrow CD, Linn WD, Lucey CR, Tuley MR. The J-curve phenomenon and the treatment of hypertension. Is there a point beyond which pressure reduction is dangerous? JAMA 1991;265:489-495.
4. Boutitie F, Gueyffier F, Pocock S, Fagard R, Boissel JP. Jshaped relationship between blood pressure and mortality in hypertensive patients: new insights from a meta-analysis of individual-patient data. Ann Intern Med 2002;136:438-448.
5. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood pressure lowering and low-dose aspirin in patients treated with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998;351:1755-1762.
6. Benetos A, Thomas F, Bean K, Gautier S, Smulyan H, Guize L. Prognostic value of systolic and diastolic blood pressure in treated hypertensive men. Arch Intern Med 2002;162:577-581.
7. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 Report. JAMA 2003;289:2560-2572.
8. 1999 World Health Organization–International Society of Hypertension Guidelines for the Management of Hypertension. Guidelines Subcommittee. J Hypertens 1999;17:151-183.
9. Izzo JL, Jr, Levy D, Black HR. Clinical Advisory Statement. Importance of systolic blood pressure in older Americans. Hypertension 2000;35:1021-1024.
1. Collins R, Peto R, MacMahon S, et al. Blood pressure, stroke, and coronary heart disease. Part 2, Short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet 1990;335:827-838.
2. Cruickshank JM, Thorp JM, Zacharias FJ. Benefits and potential harm of lowering high blood pressure. Lancet 1987;1:581-584.
3. Farnett L, Mulrow CD, Linn WD, Lucey CR, Tuley MR. The J-curve phenomenon and the treatment of hypertension. Is there a point beyond which pressure reduction is dangerous? JAMA 1991;265:489-495.
4. Boutitie F, Gueyffier F, Pocock S, Fagard R, Boissel JP. Jshaped relationship between blood pressure and mortality in hypertensive patients: new insights from a meta-analysis of individual-patient data. Ann Intern Med 2002;136:438-448.
5. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood pressure lowering and low-dose aspirin in patients treated with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998;351:1755-1762.
6. Benetos A, Thomas F, Bean K, Gautier S, Smulyan H, Guize L. Prognostic value of systolic and diastolic blood pressure in treated hypertensive men. Arch Intern Med 2002;162:577-581.
7. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 Report. JAMA 2003;289:2560-2572.
8. 1999 World Health Organization–International Society of Hypertension Guidelines for the Management of Hypertension. Guidelines Subcommittee. J Hypertens 1999;17:151-183.
9. Izzo JL, Jr, Levy D, Black HR. Clinical Advisory Statement. Importance of systolic blood pressure in older Americans. Hypertension 2000;35:1021-1024.
Evidence-based answers from the Family Physicians Inquiries Network
Should intrathecal narcotics be used as a sole labor analgesic? A prospective comparison of spinal opioids and epidural bupivacaine
OBJECTIVE: Intrathecal narcotics (ITNs) are being used in some settings as a sole labor analgesic. However, they have not been directly compared to epidural analgesia.
STUDY DESIGN: We used a prospective observational design.
POPULATION: Eighty-two women with uncomplicated full-term pregnancies were enrolled upon analgesia request during spontaneous labor with cervical dilation 3 to 7 cm. Sixty-three chose ITNs (morphine and fentanyl), and 19 chose epidural analgesia (continuous infusion of bupivacaine and fentanyl).
OUTCOMES MEASURED: Pain scores were documented using a visual analog scale. Satisfaction and side effects were rated with Likert scales during a structured interview on the first postpartum day. Outcomes were analyzed with multivariate regression techniques.
RESULTS: Intrathecal narcotics were associated with significantly higher pain scores than was epidural analgesia during the first and second stages of labor and on an overall postpartum rating. The median effective duration of action for ITNs was between 60 and 120 minutes; however, ITNs provided excellent analgesia for a subgroup of women who delivered within 2 to 3 hours of receiving them. Although women in both groups were satisfied with their pain management, women receiving ITNs had statistically lower overall satisfaction scores.
CONCLUSIONS: Within the limitations of a nonrandomized study, a single intrathecal injection of morphine and fentanyl has a shorter duration of action and provides less effective pain control than a continuous epidural infusion of bupivacaine and fentanyl. However, ITNs may have a role in settings with limited support from anesthesiologists or for women whose labors are progressing rapidly.
- Stand-alone intrathecal morphine and fentanyl (intrathecal narcotics [ITNs]) are associated with significantly higher pain levels than continuous epidural analgesia with bupivacaine and fentanyl.
- Intrathecal narcotics provide excellent pain relief for women who deliver within 2 to 3 hours of receiving them.
- Lower pain levels are significantly correlated with greater satisfaction with labor pain management.
- Women receiving ITNs were subjectively more satisfied with their ability to walk during labor.
- There was no difference in overall side effect severity between groups.
The subarachnoid injection of opioids, a technique termed “intrathecal narcotics” (ITNs), was first adapted to obstetric practice in the early 1980s1 and has since been achieving increasing acceptance as a safe and effective method for managing labor pain. Compared to epidural local anesthetics, ITNs are easy to administer, provide rapid-onset pain relief, and do not cause motor blockade.2-4 Compared to parenteral opioids, ITNs provide better pain control and are less likely to result in neonatal respiratory depression.5 Despite these advantages, there is uncertainty as to whether ITNs are an analgesic option that deserves wider acceptance, or whether they have a role distinct from the combined spinal epidural technique.
Studies of ITNs given as part of a combined spinal epidural have documented a rapid onset of profound pain relief during the first stage of labor.2-4,6 However, in these studies, when the initial dose of subarachnoid opioid wore off, epidural drugs were administered either immediately or within 1 to 3 hours. Therefore, these studies fail to provide information about the effectiveness of stand-alone ITNs during advanced first- and second-stage labor.
Existing studies of stand-alone ITNs have in fact been favorable to the technique.7-9 However, these studies used patients’ retrospective assessments or nurses’ comments in the medical record rather than pain scores obtained during labor. No prospective studies have documented pain scores during the second stage of labor in women receiving ITNs as a sole labor analgesic. Nor have there been direct comparisons of second-stage pain scores involving women receiving ITNs and women receiving continuous infusion epidural drugs. This prospective study was therefore undertaken to compare the effectiveness of stand-alone ITNs to that of epidural analgesia in the first and second stages of labor, as well as to compare women’s satisfaction with their pain management and their subjective experiences with side effects.
Methods
Setting and subjects
Fairview University Medical Center is a merged community-university teaching hospital with more than 4000 births per year. In 1999, 50% of women undergoing spontaneous vaginal delivery received ITNs and only 6% had epidural analgesia. Although both methods are available to patients, institutional culture has historically favored ITNs, perhaps because a managed care environment favors a simple, cost-effective method.2,10 The Labor Pain Management Study was approved by the University of Minnesota’s Committee on Human Subjects.
Study design
We distributed brochures describing the study during routine prenatal visits and childbirth education classes; women were also informed about the study when they presented to the hospital in spontaneous labor. Parturients with uncomplicated term singleton pregnancies were enrolled when they attained cervical dilation between 3 and 7 cm and requested pain medication. The primary obstetric care providers—including obstetricians, family physicians, and certified nurse midwives—were responsible for managing the participants’ labors.
We originally designed a randomized, 2-arm clinical trial. However, it became clear during the recruiting process that most women in early labor, and even those in prenatal classes during the third trimester, had already made their decisions about the type of pain medication they wanted. Despite receiving an unbiased presentation of the 2 treatment options, women were reluctant to accept random assignment. After 6 weeks, a change in protocol allowed each subject who had refused randomization to choose either ITNs or epidural analgesia, taking into account the recommendations of her care providers. The response rate for eligible women asked to participate under the revised protocol was 66%.
Analgesia
Experienced anesthesiologists from a large private practice provided the analgesia. For ITNs, 0.25 mg morphine sulfate and 25 to 35 μg fentanyl were injected into the subarachnoid space via the L2-L3 or L3-L4 interspace. To decrease postpartum nausea and itching, naltrexone (6.25 mg sublingually) was given to all subjects in the ITNs group within 30 minutes of vaginal delivery. Epidural analgesia consisted of an 8- to 10-mL bolus of 0.25% bupivacaine with 50 μg fentanyl, followed immediately by a continuous infusion of 0.125% bupivacaine with 2 μg/mL fentanyl. The anesthesiologist selected an initial infusion rate between 8 and 12 mL/h. The infusion was discontinued or significantly decreased during the second stage of labor.
Outcomes
Study participants rated their pain using the visual analog scale (VAS). Research assistants instructed participants to place a mark on the 0- to 100-mm VAS scale at the time of request for analgesia, at placement, at 5, 30, and 60 minutes following placement, and every 60 minutes throughout the first stage of labor. One score was collected during the early portion of the second stage, and a retrospective overall rating was obtained within 24 hours of delivery. An analyst uninvolved with the data collection subsequently measured VAS scores to the nearest millimeter.
On the first postpartum day, each subject participated in a structured interview, using 5-point Likert scales to rate her overall satisfaction with pain control and her ability to walk and push during labor. Women used a standardized 3-point scale to rate the severity of symptoms, including nausea, vomiting, pruritus, urinary retention, headache, and the inability to walk or to push.
Primary outcomes were the mean pain scores for the first and second stages of labor, the duration of adequate pain relief (defined as a VAS score ≤ 30 mm), and the retrospective mean score for overall labor pain. Secondary outcomes included women’s satisfaction ratings and subjective experiences with side effects.
Statistical analysis
Initial sample size calculations indicated that 36 women would be needed, equally divided between 2 groups, for the study to have 85% power to detect a 10-mm difference in the group mean VAS scores in the second stage of labor. A 10-mm change in the VAS score represents 1 standard deviation11 and is considered clinically significant.12 As a result of more rapid enrollment in the ITNs group, the group sizes became unequal. We continued to recruit until there were 19 women in the epidural group.
We calculated a time-weighted mean VAS score for each subject for the first stage of labor. A VAS score of 30 mm or less was considered the “zone of analgesic success.”13 Mean VAS scores, the percentage of women whose scores remained in the analgesic success zone, and satisfaction ratings in the ITNs and epidural groups were compared using multivariate linear and logistic regression methods. The outcomes were adjusted for maternal age, parity, previous spinal analgesic use, cervical dilation at time of placement, oxytocin use prior to placement, and baby’s weight. Side effects were compared using 2 tests for categorical outcomes and Student t tests for the mean severity indices.
The duration of successful analgesia was compared using time-to-event methods—Kaplan-Meier life tables and the log-rank test.14 The event of interest was analgesic failure. The time to the event was the number of minutes after placement of spinal analgesic until the VAS score rose above 30.
Results
Eighty-two women enrolled and completed the study between May 1, 1999, and March 1, 2000; 63 received ITNs and 19 received epidurals. Only 9 women underwent random assignment. Demographic and baseline characteristics for the 2 study groups are shown in Table 1. There were no statistically significant differences between groups, although women in the epidural group were older and had slightly less cervical dilation at the time of analgesic placement. The mean VAS scores prior to analgesic placement were similar (65.3 for ITNs, 67.8 for epidural; P = .73).
TABLE 1
Demographic and baseline characteristics of the study participants
| Intrathecal narcotics (n = 63) | Epidural (n = 19) | P | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years) | 28.4 ± 5.7 | 30.7 ± 3.6 | .10 |
| Nulliparous (%) | 63.5 | 47.4 | .21 |
| Caucasian (%) | 74.6 | 84.2 | .39 |
| Marital status single (%) | 20.6 | 10.5 | .32 |
| Employer-paid insurance (%) | 81.0 | 94.7 | .15 |
| Gestational age (weeks) | 39.5 ± 1.1 | 39.4 ± 1.4 | .69 |
| Baseline characteristics | |||
| Previous ITNs or epidural (%)* | 65.2 | 80.0 | .40 |
| Cervical dilation (cm) | |||
| On admission | |||
| Parity 0 | 2.6 ± 1.3 | 2.8 ± 1.3 | .75 |
| Parity > 0 | 2.6 ± 1.3 | 1.7 ± 0.9 | .07 |
| At analgesic placement | |||
| Parity 0 | 4.4 ± 1.4 | 4.1 ± 0.9 | .46 |
| Parity > 0 | 4.6 ± 1.4 | 3.8 ± 1.1 | .09 |
| Negative station at analgesic placement (%) | 51.7 | 63.2 | .38 |
| ROM prior to placement (%) | 55.6 | 68.4 | .32 |
| Oxytocin use prior to placement (%) | 30.2 | 36.8 | .59 |
| VAS at time of placement | 65.3 ± 29.0 | 67.8 ± 18.5 | .73 |
| Data are presented as mean ± SD or as percent. | |||
| *Previous ITNs or epidural analgesia includes parous women only. | |||
| ITNs, intrathecal narcotics; ROM, rupture of membranes; VAS, visual analog scale. | |||
Analgesia
Two women who initially received ITNs were subsequently given an epidural, but they remained in the ITN group for analysis. Eight women (13%) in the ITN group required a second intrathecal injection, and only 5 (8%) received a pudendal nerve block in the second stage of labor.
Pain
Women receiving epidural analgesia had significantly lower mean pain scores for the first and the second stages of labor, as well as for the overall postpartum assessment Table 2. Adjusting with multivariate regression did not significantly alter the results. For women in both groups, the mean postpartum overall VAS scores exceeded both the first-and second-stage scores measured during labor and appeared to reflect the higher of the 2.
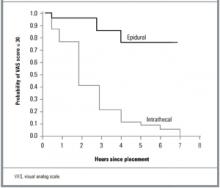
From the standpoint of “analgesic success,” the odds of having a VAS score ≤ 30 throughout the first stage of labor were 24 times greater for women receiving epidural analgesia than for those receiving ITNs (Table 2, adjusted odds ratio [OR] = 24.4, 95% CI = 5.1–116.3, number needed to treat [NNT] = 2). Of note, however, VAS scores remained in the “zone of analgesic success” for 12 women (20% of the ITN group) who delivered within 3 hours of receiving ITNs. In the second stage, the odds of having successful analgesia were 7 times higher for women receiving epidural analgesia (adjusted OR = 7.1, 95% CI = 1.7–29.1, NNT = 3).
The difference in the duration of effective pain relief was also highly significant (Figure 1, log-rank test, P < .001). For women receiving ITNs, the median duration of effective pain relief in the first stage of labor was between 60 and 120 minutes. Continuous epidural infusion, by contrast, maintained successful analgesia for most women for the entire duration of the first stage.
TABLE 2
Pain ratings and overall satisfaction with pain management
| Outcome | Intrathecal narcotics (n = 63) | Epidural (n = 19) | Adjusted differences |
|---|---|---|---|
| Stage 1 VAS | 35.1 ± 20.6 | 9.7 ± 9.6 | 24.7* |
| Stage 2 VAS | 58.5 ± 33.7 | 23.1 ± 23.5 | 42.0* |
| Overall VAS | 61.8 ± 24.9 | 24.6 ± 30.0 | 37.6* |
| Overall satisfaction with pain management (5 = very satisfied) | 3.9 ± 0.9 | 4.7 ± 0.5 | –0.8† |
| Data are presented as mean ± SD. Differences are adjusted for maternal age, parity, previous spinal analgesic use, cervical dilation at time of placement, oxytocin use prior to placement, and weight of baby. | |||
| Differences are *P < .001 or † P = .001. | |||
| VAS, visual analog scale. | |||
FIGURE 1
Kaplan-Meier analysis of the time to analgesic failure (VAS score > 30)
Satisfaction
Although women in both groups expressed overall satisfaction with the way their pain was controlled during labor, there were significant differences between groups. On a Likert scale where 5 = very satisfied, 4 = satisfied, and 3 = neutral, the mean rating for women in the epidural group was 4.7, compared to 3.9 for women receiving ITNs (P = .001). High levels of satisfaction correlated significantly with lower postpartum overall VAS scores (r = 20.50, P < .001). There were similar relationships between overall satisfaction and mean VAS scores in the first stage (r = 2.35, P = .002) and the second stage (r = 20.25, P = .040) of labor.
Side effects
Women receiving ITNs were significantly more likely to experience itching than were those receiving epidural analgesia (P < .001; Figure 2). In fact, 95% of the ITN group experienced itching, although nearly two thirds indicated that the symptom was mild or brief and did not interfere with the overall birthing experience. Nausea and vomiting were reported slightly more often in the ITN group, but the difference was not statistically significant. Women receiving epidural analgesia were significantly more likely to experience headaches (P < .001) and an inability to walk (P < .001). They also reported more difficulty with urination (P = .15) and with pushing (P = .23), neither of which reached statistical significance. None of the women reporting headaches were diagnosed as having postdural puncture headaches, and none required a blood patch for treatment. On the side effect severity index, 29% of women in the ITN group reported at least 1 “very bad” symptom, compared to 17% in the epidural group (P = .30).
FIGURE 2
Side effects of labor analgesia: percentage of women reporting the symptom in each treatment group
Discussion
Evidence-based practice guidelines developed for obstetric analgesia by the American Society of Anesthesiologists are equivocal regarding the analgesic efficacy of spinal opioids compared to epidural local anesthetics.5 Within the limitations of a non-randomized study, our data indicate that intrathecal morphine and fentanyl (ITNs) provide less satisfactory pain control than a continuous epidural infusion of bupivacaine and fentanyl. This was true during the first and second stages of labor and on an overall pain rating from the first postpartum day. The limited duration of action of ITNs is likely to account for their lesser effectiveness. We found that the effective median duration of intrathecal morphine and fentanyl was between 60 and 120 minutes as determined by life table analysis of VAS scores.
Prior studies have evaluated intrathecal opioids in the context of combined spinal epidural analgesia and found the time to request for additional pain medication to be 90 to 150 minutes, with variation depending on which opioid was used and whether a local anesthetic was added.2,3,15 Although our clinical experience is that most women are grateful for 2 to 3 hours of relief from pain during active labor, our participants’ satisfaction with pain management was clearly related to the worst pain they experienced during labor, which in turn influenced their view of the overall effectiveness of the analgesic they received. This finding is similar to that of a survey of 1000 Australian women, where inadequate pain relief was the most frequent cause of dissatisfaction with the childbirth experience as a whole.16
Given our results, it seems reasonable to ask why stand-alone ITNs have become as popular as they have in certain hospital settings. One possibility is that they have offsetting benefits in terms of greater ease of administration and lower cost than epidural analgesia. Community and military hospitals that use ITNs as a sole method cite just such logistical advantages.7-9 Compared to epidural analgesia, ITNs are technically easier to administer and place fewer demands on nurses, obstetricians, and anesthesia personnel.7 In smaller and more rural hospitals that do not have anesthesiologists on staff, anesthetists, obstetricians, or family physicians may perform the spinal injection and safely monitor patients following ITNs.7,17
A second possibility is that ITNs offer the advantage of fewer side effects than epidural analgesia. Our data illustrate the differing side effect profiles of the 2 methods and support the conclusion that there is a trade-off among the expected side effects of opioids and local anesthetics rather than a clear advantage for ITNs. Women receiving intrathecal opioids were subjectively more satisfied with their ability to walk, but they were no more satisfied with their ability to push in the second stage than were women receiving epidural analgesia. It is possible that factors other than motor blockade—such as the constraints of monitors, catheters, and intravenous lines—prevented women in the epidural group from being satisfied with their ability to ambulate. Although the link between reduced motor blockade and fewer operative deliveries is controversial,18 ambulation in labor has been shown to foster a sense of control and improve maternal satisfaction.19,20
Finally, our data indicate that ITNs are an excellent analgesic for a subset of women who deliver rapidly. Studies of combined spinal epidural analgesia have also documented a significant proportion of women who are able to deliver with ITNs alone. In an early case series, 9 (60%) of 15 women receiving intrathecal morphine and fentanyl delivered without any epidural drugs, with pudendal nerve block for perineal anesthesia.2 In 2 recent combined spinal epidural trials, intrathecal sufentanil provided adequate analgesia as a sole agent in 16% to 20% of nulliparous women and in 45% of parous women.4,15
Our observational study is limited by the nonrandomized, nonblinded allocation of the 2 treatments. Observational studies are often viewed as subject to bias due to unrecognized confounders. As noted earlier, women in our study were reluctant to accept randomization and tended to be influenced toward a choice of ITNs due to an institutional history of preference for that method. It is possible that women choosing epidural analgesia in an environment favoring ITNs would be more likely to justify their choices with positive responses during a postpartum interview. However, it seems less likely that the large differences in VAS scores obtained during labor were so influenced. In addition, we used regression analysis to control for several factors shown in others studies to be associated with labor pain.10,21,22
Conclusion
Should ITNs be used as a sole labor analgesic? For hospitals with limited analgesic options, stand-alone ITNs can be a simple and effective method that improves pain management compared to parenteral opioids. Selecting appropriate candidates for ITNs and refraining from offering them too early in labor can improve their success. For hospitals with a full range of analgesic options, the appropriate use of stand-alone ITNs will spare some women added restrictions and side effects compared to continuous epidural analgesia and may improve their satisfaction with mobility. Ultimately, the choice of analgesia for labor rests with the woman herself. True informed consent means that all available options, including stand-alone ITNs, have been presented.
Acknowledgments
The authors wish to acknowledge James Neaton, PhD, Division of Biostatistics, School of Public Health, University of Minnesota; Richard Palahnuik, MD, Department of Anesthesiology; and Preston Williams, MD, FACOG, Department of Obstetrics and Gynecology, for assistance in study design and manuscript review, and the Labor Pain Management Study research assistants, anesthesiologists, and nursing staff of the Fairview University Medical Center BirthPlace who helped develop and implement the clinical protocols.
1. Scott PV, Bowen FE, Cartwright P, et al. Intrathecal morphine as sole analgesic during labour. BMJ 1980;281:351-5.
2. Leighton BL, DeSimone CA, Norris MC, Ben David B. Intrathecal narcotics for labor revisited: the combination of fentanyl and morphine intrathecally provides rapid onset of profound, prolonged analgesia. Anesth Analg 1989;69:122-5.
3. D’Angelo R, Anderson MT, Philip J, Eisenach JC. Intrathecal sufentanil compared to epidural bupivacaine for labor analgesia. Anesthesiology 1994;80:1209-15.
4. Tsen LC, Thue B, Datta S, Segal S. Is combined spinal-epidural analgesia associated with more rapid cervical dilation in nulliparous patients when compared with conventional epidural analgesia? Anesthesiology 1999;91:920-5.
5. Practice guidelines for obstetrical anesthesia: a report by the American Society of Anesthesiologists Task Force on Obstetrical Anesthesia Anesthesiology 1999;90:600-11.
6. Abouleish A, Abouleish E, Camann W. Combined spinal-epidural analgesia in advanced labour. Can J Anaesth 1994;41:575-8.
7. Herpolsheimer A, Schretenthaler J. The use of intrapartum intrathecal narcotic analgesia in a community-based hospital. Obstet Gynecol 1994;84:931-6.
8. Rust LA, Waring RW, Hall GL, Nelson EI. Intrathecal narcotics for obstetric analgesia in a community hospital. Am J Obstet Gynecol 1994;170:1643-6.
9. Zapp J, Thorne T. Comfortable labor with intrathecal narcotics. Mil Med 1995;160:217-9.
10. Fontaine P, Adam P. Intrathecal narcotics are associated with prolonged second-stage labor and increased oxytocin use. J Fam Pract 2000;49:515-20.
11. Ludington E, Dexter F. Statistical analysis of total labor pain using the visual analog scale and application to studies of analgesic effectiveness during childbirth. Anesth Analg 1998;87:723-7.
12. Todd KH, Funk JP. The minimum clinically important difference in physician-assigned visual analog scales. Acad Emerg Med 1996;3:142-6.
13. Mantha S, Thisted R, Foss J, Ellis JE, Roizen MF. A proposal to use confidence intervals for visual analog scale data for pain measurement to determine clinical significance. Anesth Analg 1993;77:1041-7.
14. Lee ET. Statistical Methods for Survival Data Analysis. 2nd ed. New York: John Wiley & Sons; 1992.
15. Gambling DR, Sharma SK, Ramin SM, et al. A randomized study of combined spinal-epidural analgesia versus intravenous meperidine during labor: impact on cesarean delivery rate. Anesthesiology 1998;89:1336-44.
16. Paech MJ. The King Edward Memorial Hospital 1000 mother survey of methods of pain relief in labor. Anaesth Int Care 1991;19:393-9.
17. Stephens MB, Ford RE. Intrathecal narcotics for labor analgesia. Am Fam Physician 1997;56:463-70.
18. Bloom SL, McIntire DD, Kelly MA, et al. Lack of effect of walking on labor and delivery. N Engl J Med 1998;339:76-9.
19. Collis RE, Davies DW, Aveling W. Randomised comparison of combined spinal-epidural and standard epidural analgesia in labour. Lancet 1995;345:1413-6.
20. Collis RE, Harding SA, Morgan BM. Effect of maternal ambulation on labour with low-dose combined spinal-epidural analgesia. Anaesthesia 1999;54:535-9.
21. Melzack R. Severity of labour pain: influence of physical as well as psychologic variables. CMAJ 1984;130:579-84.
22. Green JM. Expectations and experiences of pain in labor: findings from a large prospective study. Birth 1993;20:65-72.
OBJECTIVE: Intrathecal narcotics (ITNs) are being used in some settings as a sole labor analgesic. However, they have not been directly compared to epidural analgesia.
STUDY DESIGN: We used a prospective observational design.
POPULATION: Eighty-two women with uncomplicated full-term pregnancies were enrolled upon analgesia request during spontaneous labor with cervical dilation 3 to 7 cm. Sixty-three chose ITNs (morphine and fentanyl), and 19 chose epidural analgesia (continuous infusion of bupivacaine and fentanyl).
OUTCOMES MEASURED: Pain scores were documented using a visual analog scale. Satisfaction and side effects were rated with Likert scales during a structured interview on the first postpartum day. Outcomes were analyzed with multivariate regression techniques.
RESULTS: Intrathecal narcotics were associated with significantly higher pain scores than was epidural analgesia during the first and second stages of labor and on an overall postpartum rating. The median effective duration of action for ITNs was between 60 and 120 minutes; however, ITNs provided excellent analgesia for a subgroup of women who delivered within 2 to 3 hours of receiving them. Although women in both groups were satisfied with their pain management, women receiving ITNs had statistically lower overall satisfaction scores.
CONCLUSIONS: Within the limitations of a nonrandomized study, a single intrathecal injection of morphine and fentanyl has a shorter duration of action and provides less effective pain control than a continuous epidural infusion of bupivacaine and fentanyl. However, ITNs may have a role in settings with limited support from anesthesiologists or for women whose labors are progressing rapidly.
- Stand-alone intrathecal morphine and fentanyl (intrathecal narcotics [ITNs]) are associated with significantly higher pain levels than continuous epidural analgesia with bupivacaine and fentanyl.
- Intrathecal narcotics provide excellent pain relief for women who deliver within 2 to 3 hours of receiving them.
- Lower pain levels are significantly correlated with greater satisfaction with labor pain management.
- Women receiving ITNs were subjectively more satisfied with their ability to walk during labor.
- There was no difference in overall side effect severity between groups.
The subarachnoid injection of opioids, a technique termed “intrathecal narcotics” (ITNs), was first adapted to obstetric practice in the early 1980s1 and has since been achieving increasing acceptance as a safe and effective method for managing labor pain. Compared to epidural local anesthetics, ITNs are easy to administer, provide rapid-onset pain relief, and do not cause motor blockade.2-4 Compared to parenteral opioids, ITNs provide better pain control and are less likely to result in neonatal respiratory depression.5 Despite these advantages, there is uncertainty as to whether ITNs are an analgesic option that deserves wider acceptance, or whether they have a role distinct from the combined spinal epidural technique.
Studies of ITNs given as part of a combined spinal epidural have documented a rapid onset of profound pain relief during the first stage of labor.2-4,6 However, in these studies, when the initial dose of subarachnoid opioid wore off, epidural drugs were administered either immediately or within 1 to 3 hours. Therefore, these studies fail to provide information about the effectiveness of stand-alone ITNs during advanced first- and second-stage labor.
Existing studies of stand-alone ITNs have in fact been favorable to the technique.7-9 However, these studies used patients’ retrospective assessments or nurses’ comments in the medical record rather than pain scores obtained during labor. No prospective studies have documented pain scores during the second stage of labor in women receiving ITNs as a sole labor analgesic. Nor have there been direct comparisons of second-stage pain scores involving women receiving ITNs and women receiving continuous infusion epidural drugs. This prospective study was therefore undertaken to compare the effectiveness of stand-alone ITNs to that of epidural analgesia in the first and second stages of labor, as well as to compare women’s satisfaction with their pain management and their subjective experiences with side effects.
Methods
Setting and subjects
Fairview University Medical Center is a merged community-university teaching hospital with more than 4000 births per year. In 1999, 50% of women undergoing spontaneous vaginal delivery received ITNs and only 6% had epidural analgesia. Although both methods are available to patients, institutional culture has historically favored ITNs, perhaps because a managed care environment favors a simple, cost-effective method.2,10 The Labor Pain Management Study was approved by the University of Minnesota’s Committee on Human Subjects.
Study design
We distributed brochures describing the study during routine prenatal visits and childbirth education classes; women were also informed about the study when they presented to the hospital in spontaneous labor. Parturients with uncomplicated term singleton pregnancies were enrolled when they attained cervical dilation between 3 and 7 cm and requested pain medication. The primary obstetric care providers—including obstetricians, family physicians, and certified nurse midwives—were responsible for managing the participants’ labors.
We originally designed a randomized, 2-arm clinical trial. However, it became clear during the recruiting process that most women in early labor, and even those in prenatal classes during the third trimester, had already made their decisions about the type of pain medication they wanted. Despite receiving an unbiased presentation of the 2 treatment options, women were reluctant to accept random assignment. After 6 weeks, a change in protocol allowed each subject who had refused randomization to choose either ITNs or epidural analgesia, taking into account the recommendations of her care providers. The response rate for eligible women asked to participate under the revised protocol was 66%.
Analgesia
Experienced anesthesiologists from a large private practice provided the analgesia. For ITNs, 0.25 mg morphine sulfate and 25 to 35 μg fentanyl were injected into the subarachnoid space via the L2-L3 or L3-L4 interspace. To decrease postpartum nausea and itching, naltrexone (6.25 mg sublingually) was given to all subjects in the ITNs group within 30 minutes of vaginal delivery. Epidural analgesia consisted of an 8- to 10-mL bolus of 0.25% bupivacaine with 50 μg fentanyl, followed immediately by a continuous infusion of 0.125% bupivacaine with 2 μg/mL fentanyl. The anesthesiologist selected an initial infusion rate between 8 and 12 mL/h. The infusion was discontinued or significantly decreased during the second stage of labor.
Outcomes
Study participants rated their pain using the visual analog scale (VAS). Research assistants instructed participants to place a mark on the 0- to 100-mm VAS scale at the time of request for analgesia, at placement, at 5, 30, and 60 minutes following placement, and every 60 minutes throughout the first stage of labor. One score was collected during the early portion of the second stage, and a retrospective overall rating was obtained within 24 hours of delivery. An analyst uninvolved with the data collection subsequently measured VAS scores to the nearest millimeter.
On the first postpartum day, each subject participated in a structured interview, using 5-point Likert scales to rate her overall satisfaction with pain control and her ability to walk and push during labor. Women used a standardized 3-point scale to rate the severity of symptoms, including nausea, vomiting, pruritus, urinary retention, headache, and the inability to walk or to push.
Primary outcomes were the mean pain scores for the first and second stages of labor, the duration of adequate pain relief (defined as a VAS score ≤ 30 mm), and the retrospective mean score for overall labor pain. Secondary outcomes included women’s satisfaction ratings and subjective experiences with side effects.
Statistical analysis
Initial sample size calculations indicated that 36 women would be needed, equally divided between 2 groups, for the study to have 85% power to detect a 10-mm difference in the group mean VAS scores in the second stage of labor. A 10-mm change in the VAS score represents 1 standard deviation11 and is considered clinically significant.12 As a result of more rapid enrollment in the ITNs group, the group sizes became unequal. We continued to recruit until there were 19 women in the epidural group.
We calculated a time-weighted mean VAS score for each subject for the first stage of labor. A VAS score of 30 mm or less was considered the “zone of analgesic success.”13 Mean VAS scores, the percentage of women whose scores remained in the analgesic success zone, and satisfaction ratings in the ITNs and epidural groups were compared using multivariate linear and logistic regression methods. The outcomes were adjusted for maternal age, parity, previous spinal analgesic use, cervical dilation at time of placement, oxytocin use prior to placement, and baby’s weight. Side effects were compared using 2 tests for categorical outcomes and Student t tests for the mean severity indices.
The duration of successful analgesia was compared using time-to-event methods—Kaplan-Meier life tables and the log-rank test.14 The event of interest was analgesic failure. The time to the event was the number of minutes after placement of spinal analgesic until the VAS score rose above 30.
Results
Eighty-two women enrolled and completed the study between May 1, 1999, and March 1, 2000; 63 received ITNs and 19 received epidurals. Only 9 women underwent random assignment. Demographic and baseline characteristics for the 2 study groups are shown in Table 1. There were no statistically significant differences between groups, although women in the epidural group were older and had slightly less cervical dilation at the time of analgesic placement. The mean VAS scores prior to analgesic placement were similar (65.3 for ITNs, 67.8 for epidural; P = .73).
TABLE 1
Demographic and baseline characteristics of the study participants
| Intrathecal narcotics (n = 63) | Epidural (n = 19) | P | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years) | 28.4 ± 5.7 | 30.7 ± 3.6 | .10 |
| Nulliparous (%) | 63.5 | 47.4 | .21 |
| Caucasian (%) | 74.6 | 84.2 | .39 |
| Marital status single (%) | 20.6 | 10.5 | .32 |
| Employer-paid insurance (%) | 81.0 | 94.7 | .15 |
| Gestational age (weeks) | 39.5 ± 1.1 | 39.4 ± 1.4 | .69 |
| Baseline characteristics | |||
| Previous ITNs or epidural (%)* | 65.2 | 80.0 | .40 |
| Cervical dilation (cm) | |||
| On admission | |||
| Parity 0 | 2.6 ± 1.3 | 2.8 ± 1.3 | .75 |
| Parity > 0 | 2.6 ± 1.3 | 1.7 ± 0.9 | .07 |
| At analgesic placement | |||
| Parity 0 | 4.4 ± 1.4 | 4.1 ± 0.9 | .46 |
| Parity > 0 | 4.6 ± 1.4 | 3.8 ± 1.1 | .09 |
| Negative station at analgesic placement (%) | 51.7 | 63.2 | .38 |
| ROM prior to placement (%) | 55.6 | 68.4 | .32 |
| Oxytocin use prior to placement (%) | 30.2 | 36.8 | .59 |
| VAS at time of placement | 65.3 ± 29.0 | 67.8 ± 18.5 | .73 |
| Data are presented as mean ± SD or as percent. | |||
| *Previous ITNs or epidural analgesia includes parous women only. | |||
| ITNs, intrathecal narcotics; ROM, rupture of membranes; VAS, visual analog scale. | |||
Analgesia
Two women who initially received ITNs were subsequently given an epidural, but they remained in the ITN group for analysis. Eight women (13%) in the ITN group required a second intrathecal injection, and only 5 (8%) received a pudendal nerve block in the second stage of labor.
Pain
Women receiving epidural analgesia had significantly lower mean pain scores for the first and the second stages of labor, as well as for the overall postpartum assessment Table 2. Adjusting with multivariate regression did not significantly alter the results. For women in both groups, the mean postpartum overall VAS scores exceeded both the first-and second-stage scores measured during labor and appeared to reflect the higher of the 2.
From the standpoint of “analgesic success,” the odds of having a VAS score ≤ 30 throughout the first stage of labor were 24 times greater for women receiving epidural analgesia than for those receiving ITNs (Table 2, adjusted odds ratio [OR] = 24.4, 95% CI = 5.1–116.3, number needed to treat [NNT] = 2). Of note, however, VAS scores remained in the “zone of analgesic success” for 12 women (20% of the ITN group) who delivered within 3 hours of receiving ITNs. In the second stage, the odds of having successful analgesia were 7 times higher for women receiving epidural analgesia (adjusted OR = 7.1, 95% CI = 1.7–29.1, NNT = 3).
The difference in the duration of effective pain relief was also highly significant (Figure 1, log-rank test, P < .001). For women receiving ITNs, the median duration of effective pain relief in the first stage of labor was between 60 and 120 minutes. Continuous epidural infusion, by contrast, maintained successful analgesia for most women for the entire duration of the first stage.
TABLE 2
Pain ratings and overall satisfaction with pain management
| Outcome | Intrathecal narcotics (n = 63) | Epidural (n = 19) | Adjusted differences |
|---|---|---|---|
| Stage 1 VAS | 35.1 ± 20.6 | 9.7 ± 9.6 | 24.7* |
| Stage 2 VAS | 58.5 ± 33.7 | 23.1 ± 23.5 | 42.0* |
| Overall VAS | 61.8 ± 24.9 | 24.6 ± 30.0 | 37.6* |
| Overall satisfaction with pain management (5 = very satisfied) | 3.9 ± 0.9 | 4.7 ± 0.5 | –0.8† |
| Data are presented as mean ± SD. Differences are adjusted for maternal age, parity, previous spinal analgesic use, cervical dilation at time of placement, oxytocin use prior to placement, and weight of baby. | |||
| Differences are *P < .001 or † P = .001. | |||
| VAS, visual analog scale. | |||
FIGURE 1
Kaplan-Meier analysis of the time to analgesic failure (VAS score > 30)
Satisfaction
Although women in both groups expressed overall satisfaction with the way their pain was controlled during labor, there were significant differences between groups. On a Likert scale where 5 = very satisfied, 4 = satisfied, and 3 = neutral, the mean rating for women in the epidural group was 4.7, compared to 3.9 for women receiving ITNs (P = .001). High levels of satisfaction correlated significantly with lower postpartum overall VAS scores (r = 20.50, P < .001). There were similar relationships between overall satisfaction and mean VAS scores in the first stage (r = 2.35, P = .002) and the second stage (r = 20.25, P = .040) of labor.
Side effects
Women receiving ITNs were significantly more likely to experience itching than were those receiving epidural analgesia (P < .001; Figure 2). In fact, 95% of the ITN group experienced itching, although nearly two thirds indicated that the symptom was mild or brief and did not interfere with the overall birthing experience. Nausea and vomiting were reported slightly more often in the ITN group, but the difference was not statistically significant. Women receiving epidural analgesia were significantly more likely to experience headaches (P < .001) and an inability to walk (P < .001). They also reported more difficulty with urination (P = .15) and with pushing (P = .23), neither of which reached statistical significance. None of the women reporting headaches were diagnosed as having postdural puncture headaches, and none required a blood patch for treatment. On the side effect severity index, 29% of women in the ITN group reported at least 1 “very bad” symptom, compared to 17% in the epidural group (P = .30).
FIGURE 2
Side effects of labor analgesia: percentage of women reporting the symptom in each treatment group
Discussion
Evidence-based practice guidelines developed for obstetric analgesia by the American Society of Anesthesiologists are equivocal regarding the analgesic efficacy of spinal opioids compared to epidural local anesthetics.5 Within the limitations of a non-randomized study, our data indicate that intrathecal morphine and fentanyl (ITNs) provide less satisfactory pain control than a continuous epidural infusion of bupivacaine and fentanyl. This was true during the first and second stages of labor and on an overall pain rating from the first postpartum day. The limited duration of action of ITNs is likely to account for their lesser effectiveness. We found that the effective median duration of intrathecal morphine and fentanyl was between 60 and 120 minutes as determined by life table analysis of VAS scores.
Prior studies have evaluated intrathecal opioids in the context of combined spinal epidural analgesia and found the time to request for additional pain medication to be 90 to 150 minutes, with variation depending on which opioid was used and whether a local anesthetic was added.2,3,15 Although our clinical experience is that most women are grateful for 2 to 3 hours of relief from pain during active labor, our participants’ satisfaction with pain management was clearly related to the worst pain they experienced during labor, which in turn influenced their view of the overall effectiveness of the analgesic they received. This finding is similar to that of a survey of 1000 Australian women, where inadequate pain relief was the most frequent cause of dissatisfaction with the childbirth experience as a whole.16
Given our results, it seems reasonable to ask why stand-alone ITNs have become as popular as they have in certain hospital settings. One possibility is that they have offsetting benefits in terms of greater ease of administration and lower cost than epidural analgesia. Community and military hospitals that use ITNs as a sole method cite just such logistical advantages.7-9 Compared to epidural analgesia, ITNs are technically easier to administer and place fewer demands on nurses, obstetricians, and anesthesia personnel.7 In smaller and more rural hospitals that do not have anesthesiologists on staff, anesthetists, obstetricians, or family physicians may perform the spinal injection and safely monitor patients following ITNs.7,17
A second possibility is that ITNs offer the advantage of fewer side effects than epidural analgesia. Our data illustrate the differing side effect profiles of the 2 methods and support the conclusion that there is a trade-off among the expected side effects of opioids and local anesthetics rather than a clear advantage for ITNs. Women receiving intrathecal opioids were subjectively more satisfied with their ability to walk, but they were no more satisfied with their ability to push in the second stage than were women receiving epidural analgesia. It is possible that factors other than motor blockade—such as the constraints of monitors, catheters, and intravenous lines—prevented women in the epidural group from being satisfied with their ability to ambulate. Although the link between reduced motor blockade and fewer operative deliveries is controversial,18 ambulation in labor has been shown to foster a sense of control and improve maternal satisfaction.19,20
Finally, our data indicate that ITNs are an excellent analgesic for a subset of women who deliver rapidly. Studies of combined spinal epidural analgesia have also documented a significant proportion of women who are able to deliver with ITNs alone. In an early case series, 9 (60%) of 15 women receiving intrathecal morphine and fentanyl delivered without any epidural drugs, with pudendal nerve block for perineal anesthesia.2 In 2 recent combined spinal epidural trials, intrathecal sufentanil provided adequate analgesia as a sole agent in 16% to 20% of nulliparous women and in 45% of parous women.4,15
Our observational study is limited by the nonrandomized, nonblinded allocation of the 2 treatments. Observational studies are often viewed as subject to bias due to unrecognized confounders. As noted earlier, women in our study were reluctant to accept randomization and tended to be influenced toward a choice of ITNs due to an institutional history of preference for that method. It is possible that women choosing epidural analgesia in an environment favoring ITNs would be more likely to justify their choices with positive responses during a postpartum interview. However, it seems less likely that the large differences in VAS scores obtained during labor were so influenced. In addition, we used regression analysis to control for several factors shown in others studies to be associated with labor pain.10,21,22
Conclusion
Should ITNs be used as a sole labor analgesic? For hospitals with limited analgesic options, stand-alone ITNs can be a simple and effective method that improves pain management compared to parenteral opioids. Selecting appropriate candidates for ITNs and refraining from offering them too early in labor can improve their success. For hospitals with a full range of analgesic options, the appropriate use of stand-alone ITNs will spare some women added restrictions and side effects compared to continuous epidural analgesia and may improve their satisfaction with mobility. Ultimately, the choice of analgesia for labor rests with the woman herself. True informed consent means that all available options, including stand-alone ITNs, have been presented.
Acknowledgments
The authors wish to acknowledge James Neaton, PhD, Division of Biostatistics, School of Public Health, University of Minnesota; Richard Palahnuik, MD, Department of Anesthesiology; and Preston Williams, MD, FACOG, Department of Obstetrics and Gynecology, for assistance in study design and manuscript review, and the Labor Pain Management Study research assistants, anesthesiologists, and nursing staff of the Fairview University Medical Center BirthPlace who helped develop and implement the clinical protocols.
OBJECTIVE: Intrathecal narcotics (ITNs) are being used in some settings as a sole labor analgesic. However, they have not been directly compared to epidural analgesia.
STUDY DESIGN: We used a prospective observational design.
POPULATION: Eighty-two women with uncomplicated full-term pregnancies were enrolled upon analgesia request during spontaneous labor with cervical dilation 3 to 7 cm. Sixty-three chose ITNs (morphine and fentanyl), and 19 chose epidural analgesia (continuous infusion of bupivacaine and fentanyl).
OUTCOMES MEASURED: Pain scores were documented using a visual analog scale. Satisfaction and side effects were rated with Likert scales during a structured interview on the first postpartum day. Outcomes were analyzed with multivariate regression techniques.
RESULTS: Intrathecal narcotics were associated with significantly higher pain scores than was epidural analgesia during the first and second stages of labor and on an overall postpartum rating. The median effective duration of action for ITNs was between 60 and 120 minutes; however, ITNs provided excellent analgesia for a subgroup of women who delivered within 2 to 3 hours of receiving them. Although women in both groups were satisfied with their pain management, women receiving ITNs had statistically lower overall satisfaction scores.
CONCLUSIONS: Within the limitations of a nonrandomized study, a single intrathecal injection of morphine and fentanyl has a shorter duration of action and provides less effective pain control than a continuous epidural infusion of bupivacaine and fentanyl. However, ITNs may have a role in settings with limited support from anesthesiologists or for women whose labors are progressing rapidly.
- Stand-alone intrathecal morphine and fentanyl (intrathecal narcotics [ITNs]) are associated with significantly higher pain levels than continuous epidural analgesia with bupivacaine and fentanyl.
- Intrathecal narcotics provide excellent pain relief for women who deliver within 2 to 3 hours of receiving them.
- Lower pain levels are significantly correlated with greater satisfaction with labor pain management.
- Women receiving ITNs were subjectively more satisfied with their ability to walk during labor.
- There was no difference in overall side effect severity between groups.
The subarachnoid injection of opioids, a technique termed “intrathecal narcotics” (ITNs), was first adapted to obstetric practice in the early 1980s1 and has since been achieving increasing acceptance as a safe and effective method for managing labor pain. Compared to epidural local anesthetics, ITNs are easy to administer, provide rapid-onset pain relief, and do not cause motor blockade.2-4 Compared to parenteral opioids, ITNs provide better pain control and are less likely to result in neonatal respiratory depression.5 Despite these advantages, there is uncertainty as to whether ITNs are an analgesic option that deserves wider acceptance, or whether they have a role distinct from the combined spinal epidural technique.
Studies of ITNs given as part of a combined spinal epidural have documented a rapid onset of profound pain relief during the first stage of labor.2-4,6 However, in these studies, when the initial dose of subarachnoid opioid wore off, epidural drugs were administered either immediately or within 1 to 3 hours. Therefore, these studies fail to provide information about the effectiveness of stand-alone ITNs during advanced first- and second-stage labor.
Existing studies of stand-alone ITNs have in fact been favorable to the technique.7-9 However, these studies used patients’ retrospective assessments or nurses’ comments in the medical record rather than pain scores obtained during labor. No prospective studies have documented pain scores during the second stage of labor in women receiving ITNs as a sole labor analgesic. Nor have there been direct comparisons of second-stage pain scores involving women receiving ITNs and women receiving continuous infusion epidural drugs. This prospective study was therefore undertaken to compare the effectiveness of stand-alone ITNs to that of epidural analgesia in the first and second stages of labor, as well as to compare women’s satisfaction with their pain management and their subjective experiences with side effects.
Methods
Setting and subjects
Fairview University Medical Center is a merged community-university teaching hospital with more than 4000 births per year. In 1999, 50% of women undergoing spontaneous vaginal delivery received ITNs and only 6% had epidural analgesia. Although both methods are available to patients, institutional culture has historically favored ITNs, perhaps because a managed care environment favors a simple, cost-effective method.2,10 The Labor Pain Management Study was approved by the University of Minnesota’s Committee on Human Subjects.
Study design
We distributed brochures describing the study during routine prenatal visits and childbirth education classes; women were also informed about the study when they presented to the hospital in spontaneous labor. Parturients with uncomplicated term singleton pregnancies were enrolled when they attained cervical dilation between 3 and 7 cm and requested pain medication. The primary obstetric care providers—including obstetricians, family physicians, and certified nurse midwives—were responsible for managing the participants’ labors.
We originally designed a randomized, 2-arm clinical trial. However, it became clear during the recruiting process that most women in early labor, and even those in prenatal classes during the third trimester, had already made their decisions about the type of pain medication they wanted. Despite receiving an unbiased presentation of the 2 treatment options, women were reluctant to accept random assignment. After 6 weeks, a change in protocol allowed each subject who had refused randomization to choose either ITNs or epidural analgesia, taking into account the recommendations of her care providers. The response rate for eligible women asked to participate under the revised protocol was 66%.
Analgesia
Experienced anesthesiologists from a large private practice provided the analgesia. For ITNs, 0.25 mg morphine sulfate and 25 to 35 μg fentanyl were injected into the subarachnoid space via the L2-L3 or L3-L4 interspace. To decrease postpartum nausea and itching, naltrexone (6.25 mg sublingually) was given to all subjects in the ITNs group within 30 minutes of vaginal delivery. Epidural analgesia consisted of an 8- to 10-mL bolus of 0.25% bupivacaine with 50 μg fentanyl, followed immediately by a continuous infusion of 0.125% bupivacaine with 2 μg/mL fentanyl. The anesthesiologist selected an initial infusion rate between 8 and 12 mL/h. The infusion was discontinued or significantly decreased during the second stage of labor.
Outcomes
Study participants rated their pain using the visual analog scale (VAS). Research assistants instructed participants to place a mark on the 0- to 100-mm VAS scale at the time of request for analgesia, at placement, at 5, 30, and 60 minutes following placement, and every 60 minutes throughout the first stage of labor. One score was collected during the early portion of the second stage, and a retrospective overall rating was obtained within 24 hours of delivery. An analyst uninvolved with the data collection subsequently measured VAS scores to the nearest millimeter.
On the first postpartum day, each subject participated in a structured interview, using 5-point Likert scales to rate her overall satisfaction with pain control and her ability to walk and push during labor. Women used a standardized 3-point scale to rate the severity of symptoms, including nausea, vomiting, pruritus, urinary retention, headache, and the inability to walk or to push.
Primary outcomes were the mean pain scores for the first and second stages of labor, the duration of adequate pain relief (defined as a VAS score ≤ 30 mm), and the retrospective mean score for overall labor pain. Secondary outcomes included women’s satisfaction ratings and subjective experiences with side effects.
Statistical analysis
Initial sample size calculations indicated that 36 women would be needed, equally divided between 2 groups, for the study to have 85% power to detect a 10-mm difference in the group mean VAS scores in the second stage of labor. A 10-mm change in the VAS score represents 1 standard deviation11 and is considered clinically significant.12 As a result of more rapid enrollment in the ITNs group, the group sizes became unequal. We continued to recruit until there were 19 women in the epidural group.
We calculated a time-weighted mean VAS score for each subject for the first stage of labor. A VAS score of 30 mm or less was considered the “zone of analgesic success.”13 Mean VAS scores, the percentage of women whose scores remained in the analgesic success zone, and satisfaction ratings in the ITNs and epidural groups were compared using multivariate linear and logistic regression methods. The outcomes were adjusted for maternal age, parity, previous spinal analgesic use, cervical dilation at time of placement, oxytocin use prior to placement, and baby’s weight. Side effects were compared using 2 tests for categorical outcomes and Student t tests for the mean severity indices.
The duration of successful analgesia was compared using time-to-event methods—Kaplan-Meier life tables and the log-rank test.14 The event of interest was analgesic failure. The time to the event was the number of minutes after placement of spinal analgesic until the VAS score rose above 30.
Results
Eighty-two women enrolled and completed the study between May 1, 1999, and March 1, 2000; 63 received ITNs and 19 received epidurals. Only 9 women underwent random assignment. Demographic and baseline characteristics for the 2 study groups are shown in Table 1. There were no statistically significant differences between groups, although women in the epidural group were older and had slightly less cervical dilation at the time of analgesic placement. The mean VAS scores prior to analgesic placement were similar (65.3 for ITNs, 67.8 for epidural; P = .73).
TABLE 1
Demographic and baseline characteristics of the study participants
| Intrathecal narcotics (n = 63) | Epidural (n = 19) | P | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years) | 28.4 ± 5.7 | 30.7 ± 3.6 | .10 |
| Nulliparous (%) | 63.5 | 47.4 | .21 |
| Caucasian (%) | 74.6 | 84.2 | .39 |
| Marital status single (%) | 20.6 | 10.5 | .32 |
| Employer-paid insurance (%) | 81.0 | 94.7 | .15 |
| Gestational age (weeks) | 39.5 ± 1.1 | 39.4 ± 1.4 | .69 |
| Baseline characteristics | |||
| Previous ITNs or epidural (%)* | 65.2 | 80.0 | .40 |
| Cervical dilation (cm) | |||
| On admission | |||
| Parity 0 | 2.6 ± 1.3 | 2.8 ± 1.3 | .75 |
| Parity > 0 | 2.6 ± 1.3 | 1.7 ± 0.9 | .07 |
| At analgesic placement | |||
| Parity 0 | 4.4 ± 1.4 | 4.1 ± 0.9 | .46 |
| Parity > 0 | 4.6 ± 1.4 | 3.8 ± 1.1 | .09 |
| Negative station at analgesic placement (%) | 51.7 | 63.2 | .38 |
| ROM prior to placement (%) | 55.6 | 68.4 | .32 |
| Oxytocin use prior to placement (%) | 30.2 | 36.8 | .59 |
| VAS at time of placement | 65.3 ± 29.0 | 67.8 ± 18.5 | .73 |
| Data are presented as mean ± SD or as percent. | |||
| *Previous ITNs or epidural analgesia includes parous women only. | |||
| ITNs, intrathecal narcotics; ROM, rupture of membranes; VAS, visual analog scale. | |||
Analgesia
Two women who initially received ITNs were subsequently given an epidural, but they remained in the ITN group for analysis. Eight women (13%) in the ITN group required a second intrathecal injection, and only 5 (8%) received a pudendal nerve block in the second stage of labor.
Pain
Women receiving epidural analgesia had significantly lower mean pain scores for the first and the second stages of labor, as well as for the overall postpartum assessment Table 2. Adjusting with multivariate regression did not significantly alter the results. For women in both groups, the mean postpartum overall VAS scores exceeded both the first-and second-stage scores measured during labor and appeared to reflect the higher of the 2.
From the standpoint of “analgesic success,” the odds of having a VAS score ≤ 30 throughout the first stage of labor were 24 times greater for women receiving epidural analgesia than for those receiving ITNs (Table 2, adjusted odds ratio [OR] = 24.4, 95% CI = 5.1–116.3, number needed to treat [NNT] = 2). Of note, however, VAS scores remained in the “zone of analgesic success” for 12 women (20% of the ITN group) who delivered within 3 hours of receiving ITNs. In the second stage, the odds of having successful analgesia were 7 times higher for women receiving epidural analgesia (adjusted OR = 7.1, 95% CI = 1.7–29.1, NNT = 3).
The difference in the duration of effective pain relief was also highly significant (Figure 1, log-rank test, P < .001). For women receiving ITNs, the median duration of effective pain relief in the first stage of labor was between 60 and 120 minutes. Continuous epidural infusion, by contrast, maintained successful analgesia for most women for the entire duration of the first stage.
TABLE 2
Pain ratings and overall satisfaction with pain management
| Outcome | Intrathecal narcotics (n = 63) | Epidural (n = 19) | Adjusted differences |
|---|---|---|---|
| Stage 1 VAS | 35.1 ± 20.6 | 9.7 ± 9.6 | 24.7* |
| Stage 2 VAS | 58.5 ± 33.7 | 23.1 ± 23.5 | 42.0* |
| Overall VAS | 61.8 ± 24.9 | 24.6 ± 30.0 | 37.6* |
| Overall satisfaction with pain management (5 = very satisfied) | 3.9 ± 0.9 | 4.7 ± 0.5 | –0.8† |
| Data are presented as mean ± SD. Differences are adjusted for maternal age, parity, previous spinal analgesic use, cervical dilation at time of placement, oxytocin use prior to placement, and weight of baby. | |||
| Differences are *P < .001 or † P = .001. | |||
| VAS, visual analog scale. | |||
FIGURE 1
Kaplan-Meier analysis of the time to analgesic failure (VAS score > 30)
Satisfaction
Although women in both groups expressed overall satisfaction with the way their pain was controlled during labor, there were significant differences between groups. On a Likert scale where 5 = very satisfied, 4 = satisfied, and 3 = neutral, the mean rating for women in the epidural group was 4.7, compared to 3.9 for women receiving ITNs (P = .001). High levels of satisfaction correlated significantly with lower postpartum overall VAS scores (r = 20.50, P < .001). There were similar relationships between overall satisfaction and mean VAS scores in the first stage (r = 2.35, P = .002) and the second stage (r = 20.25, P = .040) of labor.
Side effects
Women receiving ITNs were significantly more likely to experience itching than were those receiving epidural analgesia (P < .001; Figure 2). In fact, 95% of the ITN group experienced itching, although nearly two thirds indicated that the symptom was mild or brief and did not interfere with the overall birthing experience. Nausea and vomiting were reported slightly more often in the ITN group, but the difference was not statistically significant. Women receiving epidural analgesia were significantly more likely to experience headaches (P < .001) and an inability to walk (P < .001). They also reported more difficulty with urination (P = .15) and with pushing (P = .23), neither of which reached statistical significance. None of the women reporting headaches were diagnosed as having postdural puncture headaches, and none required a blood patch for treatment. On the side effect severity index, 29% of women in the ITN group reported at least 1 “very bad” symptom, compared to 17% in the epidural group (P = .30).
FIGURE 2
Side effects of labor analgesia: percentage of women reporting the symptom in each treatment group
Discussion
Evidence-based practice guidelines developed for obstetric analgesia by the American Society of Anesthesiologists are equivocal regarding the analgesic efficacy of spinal opioids compared to epidural local anesthetics.5 Within the limitations of a non-randomized study, our data indicate that intrathecal morphine and fentanyl (ITNs) provide less satisfactory pain control than a continuous epidural infusion of bupivacaine and fentanyl. This was true during the first and second stages of labor and on an overall pain rating from the first postpartum day. The limited duration of action of ITNs is likely to account for their lesser effectiveness. We found that the effective median duration of intrathecal morphine and fentanyl was between 60 and 120 minutes as determined by life table analysis of VAS scores.
Prior studies have evaluated intrathecal opioids in the context of combined spinal epidural analgesia and found the time to request for additional pain medication to be 90 to 150 minutes, with variation depending on which opioid was used and whether a local anesthetic was added.2,3,15 Although our clinical experience is that most women are grateful for 2 to 3 hours of relief from pain during active labor, our participants’ satisfaction with pain management was clearly related to the worst pain they experienced during labor, which in turn influenced their view of the overall effectiveness of the analgesic they received. This finding is similar to that of a survey of 1000 Australian women, where inadequate pain relief was the most frequent cause of dissatisfaction with the childbirth experience as a whole.16
Given our results, it seems reasonable to ask why stand-alone ITNs have become as popular as they have in certain hospital settings. One possibility is that they have offsetting benefits in terms of greater ease of administration and lower cost than epidural analgesia. Community and military hospitals that use ITNs as a sole method cite just such logistical advantages.7-9 Compared to epidural analgesia, ITNs are technically easier to administer and place fewer demands on nurses, obstetricians, and anesthesia personnel.7 In smaller and more rural hospitals that do not have anesthesiologists on staff, anesthetists, obstetricians, or family physicians may perform the spinal injection and safely monitor patients following ITNs.7,17
A second possibility is that ITNs offer the advantage of fewer side effects than epidural analgesia. Our data illustrate the differing side effect profiles of the 2 methods and support the conclusion that there is a trade-off among the expected side effects of opioids and local anesthetics rather than a clear advantage for ITNs. Women receiving intrathecal opioids were subjectively more satisfied with their ability to walk, but they were no more satisfied with their ability to push in the second stage than were women receiving epidural analgesia. It is possible that factors other than motor blockade—such as the constraints of monitors, catheters, and intravenous lines—prevented women in the epidural group from being satisfied with their ability to ambulate. Although the link between reduced motor blockade and fewer operative deliveries is controversial,18 ambulation in labor has been shown to foster a sense of control and improve maternal satisfaction.19,20
Finally, our data indicate that ITNs are an excellent analgesic for a subset of women who deliver rapidly. Studies of combined spinal epidural analgesia have also documented a significant proportion of women who are able to deliver with ITNs alone. In an early case series, 9 (60%) of 15 women receiving intrathecal morphine and fentanyl delivered without any epidural drugs, with pudendal nerve block for perineal anesthesia.2 In 2 recent combined spinal epidural trials, intrathecal sufentanil provided adequate analgesia as a sole agent in 16% to 20% of nulliparous women and in 45% of parous women.4,15
Our observational study is limited by the nonrandomized, nonblinded allocation of the 2 treatments. Observational studies are often viewed as subject to bias due to unrecognized confounders. As noted earlier, women in our study were reluctant to accept randomization and tended to be influenced toward a choice of ITNs due to an institutional history of preference for that method. It is possible that women choosing epidural analgesia in an environment favoring ITNs would be more likely to justify their choices with positive responses during a postpartum interview. However, it seems less likely that the large differences in VAS scores obtained during labor were so influenced. In addition, we used regression analysis to control for several factors shown in others studies to be associated with labor pain.10,21,22
Conclusion
Should ITNs be used as a sole labor analgesic? For hospitals with limited analgesic options, stand-alone ITNs can be a simple and effective method that improves pain management compared to parenteral opioids. Selecting appropriate candidates for ITNs and refraining from offering them too early in labor can improve their success. For hospitals with a full range of analgesic options, the appropriate use of stand-alone ITNs will spare some women added restrictions and side effects compared to continuous epidural analgesia and may improve their satisfaction with mobility. Ultimately, the choice of analgesia for labor rests with the woman herself. True informed consent means that all available options, including stand-alone ITNs, have been presented.
Acknowledgments
The authors wish to acknowledge James Neaton, PhD, Division of Biostatistics, School of Public Health, University of Minnesota; Richard Palahnuik, MD, Department of Anesthesiology; and Preston Williams, MD, FACOG, Department of Obstetrics and Gynecology, for assistance in study design and manuscript review, and the Labor Pain Management Study research assistants, anesthesiologists, and nursing staff of the Fairview University Medical Center BirthPlace who helped develop and implement the clinical protocols.
1. Scott PV, Bowen FE, Cartwright P, et al. Intrathecal morphine as sole analgesic during labour. BMJ 1980;281:351-5.
2. Leighton BL, DeSimone CA, Norris MC, Ben David B. Intrathecal narcotics for labor revisited: the combination of fentanyl and morphine intrathecally provides rapid onset of profound, prolonged analgesia. Anesth Analg 1989;69:122-5.
3. D’Angelo R, Anderson MT, Philip J, Eisenach JC. Intrathecal sufentanil compared to epidural bupivacaine for labor analgesia. Anesthesiology 1994;80:1209-15.
4. Tsen LC, Thue B, Datta S, Segal S. Is combined spinal-epidural analgesia associated with more rapid cervical dilation in nulliparous patients when compared with conventional epidural analgesia? Anesthesiology 1999;91:920-5.
5. Practice guidelines for obstetrical anesthesia: a report by the American Society of Anesthesiologists Task Force on Obstetrical Anesthesia Anesthesiology 1999;90:600-11.
6. Abouleish A, Abouleish E, Camann W. Combined spinal-epidural analgesia in advanced labour. Can J Anaesth 1994;41:575-8.
7. Herpolsheimer A, Schretenthaler J. The use of intrapartum intrathecal narcotic analgesia in a community-based hospital. Obstet Gynecol 1994;84:931-6.
8. Rust LA, Waring RW, Hall GL, Nelson EI. Intrathecal narcotics for obstetric analgesia in a community hospital. Am J Obstet Gynecol 1994;170:1643-6.
9. Zapp J, Thorne T. Comfortable labor with intrathecal narcotics. Mil Med 1995;160:217-9.
10. Fontaine P, Adam P. Intrathecal narcotics are associated with prolonged second-stage labor and increased oxytocin use. J Fam Pract 2000;49:515-20.
11. Ludington E, Dexter F. Statistical analysis of total labor pain using the visual analog scale and application to studies of analgesic effectiveness during childbirth. Anesth Analg 1998;87:723-7.
12. Todd KH, Funk JP. The minimum clinically important difference in physician-assigned visual analog scales. Acad Emerg Med 1996;3:142-6.
13. Mantha S, Thisted R, Foss J, Ellis JE, Roizen MF. A proposal to use confidence intervals for visual analog scale data for pain measurement to determine clinical significance. Anesth Analg 1993;77:1041-7.
14. Lee ET. Statistical Methods for Survival Data Analysis. 2nd ed. New York: John Wiley & Sons; 1992.
15. Gambling DR, Sharma SK, Ramin SM, et al. A randomized study of combined spinal-epidural analgesia versus intravenous meperidine during labor: impact on cesarean delivery rate. Anesthesiology 1998;89:1336-44.
16. Paech MJ. The King Edward Memorial Hospital 1000 mother survey of methods of pain relief in labor. Anaesth Int Care 1991;19:393-9.
17. Stephens MB, Ford RE. Intrathecal narcotics for labor analgesia. Am Fam Physician 1997;56:463-70.
18. Bloom SL, McIntire DD, Kelly MA, et al. Lack of effect of walking on labor and delivery. N Engl J Med 1998;339:76-9.
19. Collis RE, Davies DW, Aveling W. Randomised comparison of combined spinal-epidural and standard epidural analgesia in labour. Lancet 1995;345:1413-6.
20. Collis RE, Harding SA, Morgan BM. Effect of maternal ambulation on labour with low-dose combined spinal-epidural analgesia. Anaesthesia 1999;54:535-9.
21. Melzack R. Severity of labour pain: influence of physical as well as psychologic variables. CMAJ 1984;130:579-84.
22. Green JM. Expectations and experiences of pain in labor: findings from a large prospective study. Birth 1993;20:65-72.
1. Scott PV, Bowen FE, Cartwright P, et al. Intrathecal morphine as sole analgesic during labour. BMJ 1980;281:351-5.
2. Leighton BL, DeSimone CA, Norris MC, Ben David B. Intrathecal narcotics for labor revisited: the combination of fentanyl and morphine intrathecally provides rapid onset of profound, prolonged analgesia. Anesth Analg 1989;69:122-5.
3. D’Angelo R, Anderson MT, Philip J, Eisenach JC. Intrathecal sufentanil compared to epidural bupivacaine for labor analgesia. Anesthesiology 1994;80:1209-15.
4. Tsen LC, Thue B, Datta S, Segal S. Is combined spinal-epidural analgesia associated with more rapid cervical dilation in nulliparous patients when compared with conventional epidural analgesia? Anesthesiology 1999;91:920-5.
5. Practice guidelines for obstetrical anesthesia: a report by the American Society of Anesthesiologists Task Force on Obstetrical Anesthesia Anesthesiology 1999;90:600-11.
6. Abouleish A, Abouleish E, Camann W. Combined spinal-epidural analgesia in advanced labour. Can J Anaesth 1994;41:575-8.
7. Herpolsheimer A, Schretenthaler J. The use of intrapartum intrathecal narcotic analgesia in a community-based hospital. Obstet Gynecol 1994;84:931-6.
8. Rust LA, Waring RW, Hall GL, Nelson EI. Intrathecal narcotics for obstetric analgesia in a community hospital. Am J Obstet Gynecol 1994;170:1643-6.
9. Zapp J, Thorne T. Comfortable labor with intrathecal narcotics. Mil Med 1995;160:217-9.
10. Fontaine P, Adam P. Intrathecal narcotics are associated with prolonged second-stage labor and increased oxytocin use. J Fam Pract 2000;49:515-20.
11. Ludington E, Dexter F. Statistical analysis of total labor pain using the visual analog scale and application to studies of analgesic effectiveness during childbirth. Anesth Analg 1998;87:723-7.
12. Todd KH, Funk JP. The minimum clinically important difference in physician-assigned visual analog scales. Acad Emerg Med 1996;3:142-6.
13. Mantha S, Thisted R, Foss J, Ellis JE, Roizen MF. A proposal to use confidence intervals for visual analog scale data for pain measurement to determine clinical significance. Anesth Analg 1993;77:1041-7.
14. Lee ET. Statistical Methods for Survival Data Analysis. 2nd ed. New York: John Wiley & Sons; 1992.
15. Gambling DR, Sharma SK, Ramin SM, et al. A randomized study of combined spinal-epidural analgesia versus intravenous meperidine during labor: impact on cesarean delivery rate. Anesthesiology 1998;89:1336-44.
16. Paech MJ. The King Edward Memorial Hospital 1000 mother survey of methods of pain relief in labor. Anaesth Int Care 1991;19:393-9.
17. Stephens MB, Ford RE. Intrathecal narcotics for labor analgesia. Am Fam Physician 1997;56:463-70.
18. Bloom SL, McIntire DD, Kelly MA, et al. Lack of effect of walking on labor and delivery. N Engl J Med 1998;339:76-9.
19. Collis RE, Davies DW, Aveling W. Randomised comparison of combined spinal-epidural and standard epidural analgesia in labour. Lancet 1995;345:1413-6.
20. Collis RE, Harding SA, Morgan BM. Effect of maternal ambulation on labour with low-dose combined spinal-epidural analgesia. Anaesthesia 1999;54:535-9.
21. Melzack R. Severity of labour pain: influence of physical as well as psychologic variables. CMAJ 1984;130:579-84.
22. Green JM. Expectations and experiences of pain in labor: findings from a large prospective study. Birth 1993;20:65-72.