User login
Conservative Approach to Treatment of Cyclosporine-Induced Gingival Hyperplasia With Azithromycin and Chlorhexidine
Conservative Approach to Treatment of Cyclosporine-Induced Gingival Hyperplasia With Azithromycin and Chlorhexidine
Cyclosporine is a calcineurin inhibitor and immunosuppressive medication with several indications, including prevention of parenchymal organ and bone marrow transplant rejection as well as treatment of numerous dermatologic conditions (eg, psoriasis, atopic dermatitis). Although it is an effective medication, there are many known adverse effects including nephrotoxicity, hypertension, and gingival hyperplasia.1 Addressing symptomatic cyclosporine-induced gingival hyperplasia can be challenging, especially if continued use of cyclosporine is necessary for adequate control of the underlying disease. We present a simplified approach for conservative management of cyclosporine-induced gingival hyperplasia that allows for continued use of cyclosporine.
Practice Gap
Cyclosporine-induced gingival hyperplasia is a fibrous overgrowth of the interdental papilla and labial gingiva that may lead to gum pain, difficulty eating, gingivitis, and/ or tooth decay or loss.2 The condition usually occurs 3 to 6 months after starting cyclosporine but may occur as soon as 1 month later.1,3 The pathophysiology of this adverse effect is incompletely understood, but several mechanisms have been implicated, including upregulation of the salivary proinflammatory cytokines IL-1α, IL-8, and IL-6.1 Additionally, patients with cyclosporine-induced gingival hyperplasia have increased bacterial colonization with species such as Porphyromonas gingivalis.4 Risk factors for cyclosporine- induced gingival hyperplasia include higher serum concentrations (>400 ng/mL) of cyclosporine, history of gingival hyperplasia, concomitant use of calcium channel blockers, and insufficient oral hygiene.2,3 A study by Seymour and Smith5 found that proper oral hygiene leads to less severe cases of cyclosporine-induced gingival hyperplasia but does not prevent gingival overgrowth. Treatment of cyclosporine-induced gingival hyperplasia traditionally involves targeting oral bacteria and reducing inflammation. Decreasing dental plaque through regular tooth-brushing and interdental cleaning may reduce symptoms such as bleeding and discomfort of the gums.
The intensity of cyclosporine-induced gingival hyperplasia can be reduced with chlorhexidine or azithromycin. Individually, each therapy has been shown to clinically improve cyclosporine-induced gingival hyperplasia; however, to our knowledge the combination of these treatments has not been reported.1 We present a simplified approach to treating cyclosporine-induced gingival hyperplasia using both azithromycin and chlorhexidine. This conservative approach results in effective and sustained improvement of gingival hyperplasia while allowing patients to continue cyclosporine therapy to control underlying disease with minimal adverse effects.
Technique
Before initiating treatment, it is important to confirm that the etiology of gingival hyperplasia is due to cyclosporine use and rule out nutritional deficiencies and autoimmune conditions as potential causes. Be sure to inquire about nutritional intake, systemic symptoms, and family history of autoimmune conditions. Our approach includes the use of azithromycin 500 mg once daily for 7 days followed by chlorhexidine 0.12% oral solution 15 mL twice daily (swish undiluted for 30 seconds, then spit) for at least 3 months for optimal management of gingival hyperplasia. Chlorhexidine should be continued for at least 6 months to maintain symptom resolution. While cyclosporine therapy may be continued throughout the duration of this regimen, consider switching to other immunosuppressive medications that are not associated with gingival hyperplasia (eg, tacrolimus) if symptoms are severe and/or resistant to therapy.1,6
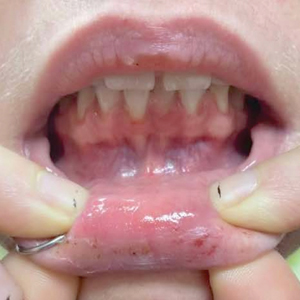
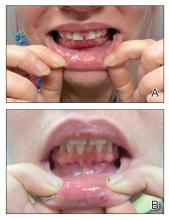
We applied this technique to treat cyclosporine-induced gingival hyperplasia in a 28-year-old woman with a 3-year history of primary aplastic anemia. The patient initially presented with pain and bleeding of the gums of several months’ duration and reported experiencing gum pain when eating solid foods. Her medications included cyclosporine 225 mg daily for aplastic anemia and dapsone 100 mg daily for pneumocystis pneumonia prophylaxis, both of which were taken for the past 6 months. Oral examination revealed pink to bright red hyperplastic gingivae (Figure). She had no other symptoms associated with aplastic anemia and no signs of vitamin or nutritional deficiencies. She denied pre-existing periodontitis prior to starting cyclosporine and reported that the symptoms started several months after initiating cyclosporine therapy. Thus, the clinical diagnosis of cyclosporine-induced gingival hyperplasia was made, and treatment with azithromycin and chlorhexidine was initiated with marked reduction in symptoms.
Conservative management of gingival hyperplasia with oral hygiene including regular tooth-brushing and flossing and antimicrobial therapies was preferred in this patient to reduce gum pain and minimize the risk for tooth loss while also limiting the use of surgically invasive interventions. Due to limited therapeutic options for aplastic anemia, continued administration of cyclosporine was necessary in our patient to prevent further complications.
Practice Implications
The precise mechanism by which azithromycin treats gingival hyperplasia is unclear but may involve its antimicrobial and anti-inflammatory properties. Small concentrations of azithromycin have been shown to persist in macrophages and fibroblasts of the gingiva even with short-term administration of 3 to 5 days.7 Chlorhexidine is another antimicrobial agent often used in oral rinse solutions to decrease plaque formation and prevent gingivitis. Chlorhexidine can reduce cyclosporine-induced gingival overgrowth when used twice daily.8 After rinsing with chlorhexidine, saliva exhibits antibacterial activity for up to 5 hours; however, tooth and gum discoloration may occur.8
Recurrence of gingival hyperplasia is likely if cyclosporine is not discontinued or maintained with treatment.3 Conventional gingivectomy should be considered for cases in which conservative treatment is ineffective, aesthetic concerns arise, or gingival hyperplasia persists for more than 6 to 12 months after discontinuing cyclosporine.1
We theorize that the microbial properties of azithromycin and chlorhexidine help reduce periodontal inflammation and bacterial overgrowth in patients with cyclosporine-induced gingival hyperplasia, which allows for restoration of gingival health. Our case highlights the efficacy of our treatment approach using a 7-day course of azithromycin followed by twice-daily use of chlorhexidine oral rinse in the treatment of cyclosporine-induced gingival hyperplasia with continued use of cyclosporine.
- Chojnacka-Purpurowicz J, Wygonowska E, Placek W, et al. Cyclosporine-induced gingival overgrowth—review. Dermatol Ther. 2022;35:E15912.
- Greenburg KV, Armitage GC, Shiboski CH. Gingival enlargement among renal transplant recipients in the era of new-generation immunosuppressants. J Periodontol. 2008;79:453-460.
- Cyclosporine (ciclosporin)(systemic): drug information. UpToDate. Accessed December 19, 2023. https://www.uptodate.com/contents/table-of-contents/drug-information/general-drug-information
- Gong Y, Bi W, Cao L, et al. Association of CD14-260 polymorphisms, red-complex periodontopathogens and gingival crevicular fluid cytokine levels with cyclosporine A-induced gingival overgrowth in renal transplant patients. J Periodontal Res. 2013;48:203-212.
- Seymour RA, Smith DG. The effect of a plaque control programme on the incidence and severity of cyclosporin-induced gingival changes. J Clin Periodontol. 1991;18:107-110.
- Nash MM, Zaltzman JS. Efficacy of azithromycin in the treatment of cyclosporine-induced gingival hyperplasia in renal transplant recipients. Transplantation. 1998;65:1611-1615.
- Martín JM, Mateo E, Jordá E. Utilidad de la azitromicina en la hyperplasia gingival inducida por ciclosporina [azithromycin for the treatment of ciclosporin-induced gingival hyperplasia]. Actas Dermosifiliogr. 2016;107:780.
- Gau CH, Tu HS, Chin YT, et al. Can chlorhexidine mouthwash twice daily ameliorate cyclosporine-induced gingival overgrowth? J Formos Med Assoc. 2013;112:131-137.
Cyclosporine is a calcineurin inhibitor and immunosuppressive medication with several indications, including prevention of parenchymal organ and bone marrow transplant rejection as well as treatment of numerous dermatologic conditions (eg, psoriasis, atopic dermatitis). Although it is an effective medication, there are many known adverse effects including nephrotoxicity, hypertension, and gingival hyperplasia.1 Addressing symptomatic cyclosporine-induced gingival hyperplasia can be challenging, especially if continued use of cyclosporine is necessary for adequate control of the underlying disease. We present a simplified approach for conservative management of cyclosporine-induced gingival hyperplasia that allows for continued use of cyclosporine.
Practice Gap
Cyclosporine-induced gingival hyperplasia is a fibrous overgrowth of the interdental papilla and labial gingiva that may lead to gum pain, difficulty eating, gingivitis, and/ or tooth decay or loss.2 The condition usually occurs 3 to 6 months after starting cyclosporine but may occur as soon as 1 month later.1,3 The pathophysiology of this adverse effect is incompletely understood, but several mechanisms have been implicated, including upregulation of the salivary proinflammatory cytokines IL-1α, IL-8, and IL-6.1 Additionally, patients with cyclosporine-induced gingival hyperplasia have increased bacterial colonization with species such as Porphyromonas gingivalis.4 Risk factors for cyclosporine- induced gingival hyperplasia include higher serum concentrations (>400 ng/mL) of cyclosporine, history of gingival hyperplasia, concomitant use of calcium channel blockers, and insufficient oral hygiene.2,3 A study by Seymour and Smith5 found that proper oral hygiene leads to less severe cases of cyclosporine-induced gingival hyperplasia but does not prevent gingival overgrowth. Treatment of cyclosporine-induced gingival hyperplasia traditionally involves targeting oral bacteria and reducing inflammation. Decreasing dental plaque through regular tooth-brushing and interdental cleaning may reduce symptoms such as bleeding and discomfort of the gums.
The intensity of cyclosporine-induced gingival hyperplasia can be reduced with chlorhexidine or azithromycin. Individually, each therapy has been shown to clinically improve cyclosporine-induced gingival hyperplasia; however, to our knowledge the combination of these treatments has not been reported.1 We present a simplified approach to treating cyclosporine-induced gingival hyperplasia using both azithromycin and chlorhexidine. This conservative approach results in effective and sustained improvement of gingival hyperplasia while allowing patients to continue cyclosporine therapy to control underlying disease with minimal adverse effects.
Technique
Before initiating treatment, it is important to confirm that the etiology of gingival hyperplasia is due to cyclosporine use and rule out nutritional deficiencies and autoimmune conditions as potential causes. Be sure to inquire about nutritional intake, systemic symptoms, and family history of autoimmune conditions. Our approach includes the use of azithromycin 500 mg once daily for 7 days followed by chlorhexidine 0.12% oral solution 15 mL twice daily (swish undiluted for 30 seconds, then spit) for at least 3 months for optimal management of gingival hyperplasia. Chlorhexidine should be continued for at least 6 months to maintain symptom resolution. While cyclosporine therapy may be continued throughout the duration of this regimen, consider switching to other immunosuppressive medications that are not associated with gingival hyperplasia (eg, tacrolimus) if symptoms are severe and/or resistant to therapy.1,6
We applied this technique to treat cyclosporine-induced gingival hyperplasia in a 28-year-old woman with a 3-year history of primary aplastic anemia. The patient initially presented with pain and bleeding of the gums of several months’ duration and reported experiencing gum pain when eating solid foods. Her medications included cyclosporine 225 mg daily for aplastic anemia and dapsone 100 mg daily for pneumocystis pneumonia prophylaxis, both of which were taken for the past 6 months. Oral examination revealed pink to bright red hyperplastic gingivae (Figure). She had no other symptoms associated with aplastic anemia and no signs of vitamin or nutritional deficiencies. She denied pre-existing periodontitis prior to starting cyclosporine and reported that the symptoms started several months after initiating cyclosporine therapy. Thus, the clinical diagnosis of cyclosporine-induced gingival hyperplasia was made, and treatment with azithromycin and chlorhexidine was initiated with marked reduction in symptoms.
Conservative management of gingival hyperplasia with oral hygiene including regular tooth-brushing and flossing and antimicrobial therapies was preferred in this patient to reduce gum pain and minimize the risk for tooth loss while also limiting the use of surgically invasive interventions. Due to limited therapeutic options for aplastic anemia, continued administration of cyclosporine was necessary in our patient to prevent further complications.
Practice Implications
The precise mechanism by which azithromycin treats gingival hyperplasia is unclear but may involve its antimicrobial and anti-inflammatory properties. Small concentrations of azithromycin have been shown to persist in macrophages and fibroblasts of the gingiva even with short-term administration of 3 to 5 days.7 Chlorhexidine is another antimicrobial agent often used in oral rinse solutions to decrease plaque formation and prevent gingivitis. Chlorhexidine can reduce cyclosporine-induced gingival overgrowth when used twice daily.8 After rinsing with chlorhexidine, saliva exhibits antibacterial activity for up to 5 hours; however, tooth and gum discoloration may occur.8
Recurrence of gingival hyperplasia is likely if cyclosporine is not discontinued or maintained with treatment.3 Conventional gingivectomy should be considered for cases in which conservative treatment is ineffective, aesthetic concerns arise, or gingival hyperplasia persists for more than 6 to 12 months after discontinuing cyclosporine.1
We theorize that the microbial properties of azithromycin and chlorhexidine help reduce periodontal inflammation and bacterial overgrowth in patients with cyclosporine-induced gingival hyperplasia, which allows for restoration of gingival health. Our case highlights the efficacy of our treatment approach using a 7-day course of azithromycin followed by twice-daily use of chlorhexidine oral rinse in the treatment of cyclosporine-induced gingival hyperplasia with continued use of cyclosporine.
Cyclosporine is a calcineurin inhibitor and immunosuppressive medication with several indications, including prevention of parenchymal organ and bone marrow transplant rejection as well as treatment of numerous dermatologic conditions (eg, psoriasis, atopic dermatitis). Although it is an effective medication, there are many known adverse effects including nephrotoxicity, hypertension, and gingival hyperplasia.1 Addressing symptomatic cyclosporine-induced gingival hyperplasia can be challenging, especially if continued use of cyclosporine is necessary for adequate control of the underlying disease. We present a simplified approach for conservative management of cyclosporine-induced gingival hyperplasia that allows for continued use of cyclosporine.
Practice Gap
Cyclosporine-induced gingival hyperplasia is a fibrous overgrowth of the interdental papilla and labial gingiva that may lead to gum pain, difficulty eating, gingivitis, and/ or tooth decay or loss.2 The condition usually occurs 3 to 6 months after starting cyclosporine but may occur as soon as 1 month later.1,3 The pathophysiology of this adverse effect is incompletely understood, but several mechanisms have been implicated, including upregulation of the salivary proinflammatory cytokines IL-1α, IL-8, and IL-6.1 Additionally, patients with cyclosporine-induced gingival hyperplasia have increased bacterial colonization with species such as Porphyromonas gingivalis.4 Risk factors for cyclosporine- induced gingival hyperplasia include higher serum concentrations (>400 ng/mL) of cyclosporine, history of gingival hyperplasia, concomitant use of calcium channel blockers, and insufficient oral hygiene.2,3 A study by Seymour and Smith5 found that proper oral hygiene leads to less severe cases of cyclosporine-induced gingival hyperplasia but does not prevent gingival overgrowth. Treatment of cyclosporine-induced gingival hyperplasia traditionally involves targeting oral bacteria and reducing inflammation. Decreasing dental plaque through regular tooth-brushing and interdental cleaning may reduce symptoms such as bleeding and discomfort of the gums.
The intensity of cyclosporine-induced gingival hyperplasia can be reduced with chlorhexidine or azithromycin. Individually, each therapy has been shown to clinically improve cyclosporine-induced gingival hyperplasia; however, to our knowledge the combination of these treatments has not been reported.1 We present a simplified approach to treating cyclosporine-induced gingival hyperplasia using both azithromycin and chlorhexidine. This conservative approach results in effective and sustained improvement of gingival hyperplasia while allowing patients to continue cyclosporine therapy to control underlying disease with minimal adverse effects.
Technique
Before initiating treatment, it is important to confirm that the etiology of gingival hyperplasia is due to cyclosporine use and rule out nutritional deficiencies and autoimmune conditions as potential causes. Be sure to inquire about nutritional intake, systemic symptoms, and family history of autoimmune conditions. Our approach includes the use of azithromycin 500 mg once daily for 7 days followed by chlorhexidine 0.12% oral solution 15 mL twice daily (swish undiluted for 30 seconds, then spit) for at least 3 months for optimal management of gingival hyperplasia. Chlorhexidine should be continued for at least 6 months to maintain symptom resolution. While cyclosporine therapy may be continued throughout the duration of this regimen, consider switching to other immunosuppressive medications that are not associated with gingival hyperplasia (eg, tacrolimus) if symptoms are severe and/or resistant to therapy.1,6
We applied this technique to treat cyclosporine-induced gingival hyperplasia in a 28-year-old woman with a 3-year history of primary aplastic anemia. The patient initially presented with pain and bleeding of the gums of several months’ duration and reported experiencing gum pain when eating solid foods. Her medications included cyclosporine 225 mg daily for aplastic anemia and dapsone 100 mg daily for pneumocystis pneumonia prophylaxis, both of which were taken for the past 6 months. Oral examination revealed pink to bright red hyperplastic gingivae (Figure). She had no other symptoms associated with aplastic anemia and no signs of vitamin or nutritional deficiencies. She denied pre-existing periodontitis prior to starting cyclosporine and reported that the symptoms started several months after initiating cyclosporine therapy. Thus, the clinical diagnosis of cyclosporine-induced gingival hyperplasia was made, and treatment with azithromycin and chlorhexidine was initiated with marked reduction in symptoms.
Conservative management of gingival hyperplasia with oral hygiene including regular tooth-brushing and flossing and antimicrobial therapies was preferred in this patient to reduce gum pain and minimize the risk for tooth loss while also limiting the use of surgically invasive interventions. Due to limited therapeutic options for aplastic anemia, continued administration of cyclosporine was necessary in our patient to prevent further complications.
Practice Implications
The precise mechanism by which azithromycin treats gingival hyperplasia is unclear but may involve its antimicrobial and anti-inflammatory properties. Small concentrations of azithromycin have been shown to persist in macrophages and fibroblasts of the gingiva even with short-term administration of 3 to 5 days.7 Chlorhexidine is another antimicrobial agent often used in oral rinse solutions to decrease plaque formation and prevent gingivitis. Chlorhexidine can reduce cyclosporine-induced gingival overgrowth when used twice daily.8 After rinsing with chlorhexidine, saliva exhibits antibacterial activity for up to 5 hours; however, tooth and gum discoloration may occur.8
Recurrence of gingival hyperplasia is likely if cyclosporine is not discontinued or maintained with treatment.3 Conventional gingivectomy should be considered for cases in which conservative treatment is ineffective, aesthetic concerns arise, or gingival hyperplasia persists for more than 6 to 12 months after discontinuing cyclosporine.1
We theorize that the microbial properties of azithromycin and chlorhexidine help reduce periodontal inflammation and bacterial overgrowth in patients with cyclosporine-induced gingival hyperplasia, which allows for restoration of gingival health. Our case highlights the efficacy of our treatment approach using a 7-day course of azithromycin followed by twice-daily use of chlorhexidine oral rinse in the treatment of cyclosporine-induced gingival hyperplasia with continued use of cyclosporine.
- Chojnacka-Purpurowicz J, Wygonowska E, Placek W, et al. Cyclosporine-induced gingival overgrowth—review. Dermatol Ther. 2022;35:E15912.
- Greenburg KV, Armitage GC, Shiboski CH. Gingival enlargement among renal transplant recipients in the era of new-generation immunosuppressants. J Periodontol. 2008;79:453-460.
- Cyclosporine (ciclosporin)(systemic): drug information. UpToDate. Accessed December 19, 2023. https://www.uptodate.com/contents/table-of-contents/drug-information/general-drug-information
- Gong Y, Bi W, Cao L, et al. Association of CD14-260 polymorphisms, red-complex periodontopathogens and gingival crevicular fluid cytokine levels with cyclosporine A-induced gingival overgrowth in renal transplant patients. J Periodontal Res. 2013;48:203-212.
- Seymour RA, Smith DG. The effect of a plaque control programme on the incidence and severity of cyclosporin-induced gingival changes. J Clin Periodontol. 1991;18:107-110.
- Nash MM, Zaltzman JS. Efficacy of azithromycin in the treatment of cyclosporine-induced gingival hyperplasia in renal transplant recipients. Transplantation. 1998;65:1611-1615.
- Martín JM, Mateo E, Jordá E. Utilidad de la azitromicina en la hyperplasia gingival inducida por ciclosporina [azithromycin for the treatment of ciclosporin-induced gingival hyperplasia]. Actas Dermosifiliogr. 2016;107:780.
- Gau CH, Tu HS, Chin YT, et al. Can chlorhexidine mouthwash twice daily ameliorate cyclosporine-induced gingival overgrowth? J Formos Med Assoc. 2013;112:131-137.
- Chojnacka-Purpurowicz J, Wygonowska E, Placek W, et al. Cyclosporine-induced gingival overgrowth—review. Dermatol Ther. 2022;35:E15912.
- Greenburg KV, Armitage GC, Shiboski CH. Gingival enlargement among renal transplant recipients in the era of new-generation immunosuppressants. J Periodontol. 2008;79:453-460.
- Cyclosporine (ciclosporin)(systemic): drug information. UpToDate. Accessed December 19, 2023. https://www.uptodate.com/contents/table-of-contents/drug-information/general-drug-information
- Gong Y, Bi W, Cao L, et al. Association of CD14-260 polymorphisms, red-complex periodontopathogens and gingival crevicular fluid cytokine levels with cyclosporine A-induced gingival overgrowth in renal transplant patients. J Periodontal Res. 2013;48:203-212.
- Seymour RA, Smith DG. The effect of a plaque control programme on the incidence and severity of cyclosporin-induced gingival changes. J Clin Periodontol. 1991;18:107-110.
- Nash MM, Zaltzman JS. Efficacy of azithromycin in the treatment of cyclosporine-induced gingival hyperplasia in renal transplant recipients. Transplantation. 1998;65:1611-1615.
- Martín JM, Mateo E, Jordá E. Utilidad de la azitromicina en la hyperplasia gingival inducida por ciclosporina [azithromycin for the treatment of ciclosporin-induced gingival hyperplasia]. Actas Dermosifiliogr. 2016;107:780.
- Gau CH, Tu HS, Chin YT, et al. Can chlorhexidine mouthwash twice daily ameliorate cyclosporine-induced gingival overgrowth? J Formos Med Assoc. 2013;112:131-137.
Conservative Approach to Treatment of Cyclosporine-Induced Gingival Hyperplasia With Azithromycin and Chlorhexidine
Conservative Approach to Treatment of Cyclosporine-Induced Gingival Hyperplasia With Azithromycin and Chlorhexidine