User login
2017 ACC/AHA hypertension guidelines: Toward tighter control
In 2017, the American College of Cardiology (ACC), American Heart Association (AHA), and 9 other professional associations published a new guideline on high blood pressure in adults.1 Their document addresses a range of topics relevant to preventing, diagnosing, and managing hypertension. It incorporates evidence from randomized controlled trials, including the Systolic Blood Pressure Intervention Trial (SPRINT),2 systematic reviews, and expert opinion.
The new guidelines contain many noteworthy changes, some of which are generating intense debate and discussion. Here, we provide our opinions to help practicing clinicians broaden their perspective and make informed decisions about management.
ACC AND AHA ARE NOW RESPONSIBLE FOR HYPERTENSION GUIDELINES
The Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC), organized by the National Heart, Lung, and Blood Institute, began issuing hypertension guidelines in 1977. Based on observational and clinical trial data, succeeding JNC reports recommended ever-lower blood pressure goals, with emphasis shifting to treatment of systolic hypertension.
The last official JNC report—JNC 7—was published in 2003.3 In 2013, the Institute transferred the responsibility for cardiovascular prevention guidelines to the ACC and AHA.4
A report from the panel members appointed to JNC 8 was published independently in 2014.5 It focused on a few key questions and used evidence limited to randomized controlled trials. In this report, the panel relaxed the goals for many subgroups, leading to criticism from many professional societies and from some members of the panel writing group.6
WHAT'S NEW IN THE 2017 GUIDELINES?
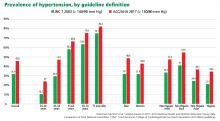
The new ACC/AHA guidelines contain a number of changes from previous documents that have been the topic of debate.
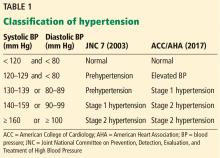
New definition and classification of hypertension
Strong recommendation, based on moderate-quality evidence.
Our opinion. While this new classification is intended to promote closer monitoring and earlier intervention to lower cardiovascular event rates, creating a new level of disease may lead to more pharmacologic treatment for those with lower risk, without emphasis on lifestyle modifications.
Emphasis on measurement technique and out-of-office measurements
Strong recommendation, based on expert opinion, for accurate measurement of blood pressure in the office, high-quality evidence from systematic review for out-of-office measurement.
Appropriate management of hypertension entails accurate blood pressure measurement. While office-based measurement remains the most commonly used method, this “snapshot” may not reflect a patient’s true baseline blood pressure.
Out-of-office measurements. Based on the results of a systematic review commissioned by the guideline committee, out-of-office measurements are now recommended to confirm the diagnosis of hypertension and to assess response to therapy.
Ambulatory blood pressure monitoring should be strongly considered as the preferred method for out-of-office monitoring; home blood pressure monitoring can be done if ambulatory monitoring is not feasible. Ambulatory monitoring provides additional information on nighttime blood pressure, including the dipping status (normal defined as a nighttime blood pressure decrease of 10% to 20%). Ambulatory monitoring predicts long-term cardiovascular outcomes independent of office blood pressure, and elevated nighttime pressure and non-dipping have been shown to be independently associated with increased cardiovascular mortality rates.8,9 Unfortunately, despite evidence supporting its use, ambulatory blood pressure monitoring is not widely available for a variety of reasons, including high cost (roughly $2,000–$4,000) and minimal reimbursement.
Out-of-office measurements can also detect white coat hypertension and masked hypertension. White coat hypertension is defined as blood pressure that is elevated in the office but normal in an out-of-office setting, and masked hypertension is blood pressure that is normal in the office and elevated in an out-of-office setting. Currently, pharmacologic therapy is not recommended to treat white coat hypertension, and treatment for masked hypertension should be the same as for sustained hypertension.
While the guidelines do not comment specifically on manual office measurement vs automated office measurements using devices that take multiple measurements with the patient alone in the room to reduce the white coat effect, they acknowledge “increasing evidence” favoring the use of automated office measurement.
Proper technique for measuring blood pressure is appropriately emphasized; correct patient positioning, allowing a period of rest, and using the appropriate cuff size are all important. Unfortunately, many busy clinical practices may not follow correct technique when measuring blood pressure in the office, leading to misdiagnosis and unnecessary pharmacologic therapy that may result in adverse events.
Of note, the SPRINT trial, which informed many of the new guideline recommendations, followed a strict protocol of blood pressure measurement with an automated device, checking sitting blood pressure 3 times at 1-minute intervals, with the patient alone in the room and without an observer present at many of the sites.10
Most guidelines11,12 agree on an average of at least 135/85 mm Hg as the threshold for diagnosing hypertension by home monitoring, or an average daytime pressure of at least 135/85 mm Hg by ambulatory monitoring, corresponding with office-based blood pressure of 140/90 mm Hg. However, the new guidelines recommend a lower threshold of 130/80 mm Hg for both home monitoring and average daytime ambulatory monitoring, corresponding with an office blood pressure of 130/80 mm Hg. They do not specify whether the office-based measurement is manual or automated.
Our opinion. Since office-based measurement will likely remain the principal method for managing hypertension due to constraints with ambulatory or home monitoring, the use of automated devices for office measurement should be strongly considered. Studies have shown that, compared with routine office measurements, automated measurements more closely approximate those obtained by ambulatory and home blood pressure monitoring.13
Risk-based approach to hypertension management
The algorithm for hypertension management now incorporates objective assessment of cardiovascular risk. Specifically, it calls for estimation of the 10-year risk of atherosclerotic cardiovascular disease, defined as coronary heart disease death, nonfatal myocardial infarction, or fatal or nonfatal stroke.
The information required to estimate risk includes age, sex, race, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, use of blood pressure-lowering medication, diabetes status, and smoking status. The guideline recommends an easy-to-use online risk calculator (http://tools.acc.org/ASCVD-Risk-Estimator).
A 10-year risk of 10% or more is designated as the cutoff between high risk and low risk. However, this is not based on trial evidence, and the risk calculator has not been verified in prospective trials to show that its use reduces cardiovascular events. The SPRINT trial,2 which was a study of blood pressure-lowering in high-risk patients, used a 10-year risk of 15% or more based on the Framingham risk score to delineate high risk.
Additionally, the 10-year risk calculator is valid only in patients ages 40 through 79, and some studies indicate that it may overestimate risk in older adults.14,15 This overestimation may lead to patients being started on pharmacologic therapy when it may not truly be indicated. The risk calculator controversy has been discussed in a previous issue of this journal.16
Blood pressure goals
Strong recommendation for known cardiovascular disease or atherosclerotic cardiovascular disease risk 10% or greater, weak recommendation for risk less than 10%, based on moderate-quality evidence for systolic blood pressure, expert opinion for diastolic.
The guidelines recommend a blood pressure goal of less than 130/80 mm Hg for all patients, including the elderly and patients with chronic kidney disease or diabetes.
The SPRINT trial,2 which showed better cardiovascular outcomes in the intensive treatment group (aiming for systolic pressure < 120 mm Hg) compared with a standard treatment group (aiming for systolic pressure < 140 mm Hg), excluded participants with diabetes and severe chronic kidney disease (estimated glomerular filtration rate < 20 mL/min/m2 and proteinuria > 1 g/day), and those who were in nursing homes or had dementia.
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) blood pressure trial showed that intensive blood pressure control did not have cardiovascular benefits compared with standard therapy.17 However, many now believe that the study may have been underpowered due to its design, and a meta-analysis of the results from SPRINT and ACCORD suggested that findings from both trials were consistent, favoring intensive blood pressure control in a high-risk population.18
While the totality of evidence favors a lower achieved blood pressure for many patients, this lower goal may be difficult to achieve in many, particularly those with vascular stiffness, which is common in the elderly. These patients also tend to have low diastolic pressure, and lowering diastolic pressure below 60 mm Hg in those with documented coronary artery disease could increase the risk of adverse cardiovascular outcomes.19,20 The guidelines do not address the potential issues with lowering diastolic blood pressure.
Our opinion. While a “universal” blood pressure goal may simplify decision-making, we believe it is important to individualize goals, taking into account patient characteristics, lifestyle factors, medication side effects, patient preferences, cost issues, and adherence to therapy.
The goal blood pressure should also consider the method of measurement. Systolic blood pressure readings have been reported to be 5 to 10 mm Hg lower with automated office measurement than with routine office measurement.21
It is also not clear that the magnitude of absolute benefit from pursuing more intensive blood pressure control with antihypertensive therapy in patients with high cardiovascular risk (as in SPRINT) would translate to similar benefits in a lower-risk population. Thus, we believe that in patients with lower cardiovascular risk, a goal blood pressure of less than 140/90 mm Hg (if routine office measurement is done) and less than 135/85 mm Hg (if automated office measurement is done) would be reasonable.
We also believe that it is reasonable to relax these goals in the very elderly (age ≥ 80), especially those who are frail and at risk of falls, with low diastolic pressures. In these patients, we recommend individualizing blood pressure goals that can be achieved without significant side effects from antihypertensive therapy.
Nonpharmacologic therapy
Strong recommendation, based on high-quality evidence from randomized controlled trials
Nonpharmacologic therapy and lifestyle modification are appropriately emphasized in the new guidelines. Most of the lifestyle changes that are recommended are in concordance with prior JNC 7 recommendations.3
Recognizing the roles of sodium and potassium in the pathogenesis of hypertension, the guidelines emphasize a diet that is higher in potassium, the DASH (Dietary Approaches to Stop Hypertension) diet, and a low-sodium diet. The recommended optimal goal of sodium intake of less than 1,500 mg/day may be difficult to achieve with a Western diet, and there is debate about the potential adverse effects of a very-low sodium diet.22 The general recommendation for sodium intake of less than 2,300 mg/day is supported in the literature, and it is unclear if further reduction has additional beneficial effects on blood pressure.23
The guidelines recommend a 3- to 6-month reassessment of patients who are prescribed risk-factor modification, but are unclear about initiation of pharmacologic therapy or other steps if these low-risk patients have not responded to lifestyle modifications alone at the time of reassessment.
Pharmacologic therapy
Strong recommendation, based on high-quality evidence from randomized controlled trials for systolic blood pressure, expert opinion for diastolic blood pressure for those with atherosclerotic cardiovascular disease risk 10% or greater, and limited data for those with risk less than 10%.
Pharmacologic therapy is recommended in patients with stage 1 hypertension and pre-existing cardiovascular disease or 10-year risk of atherosclerotic cardiovascular disease of 10% or more, and in those with stage 2 hypertension even if their 10-year risk is less than 10%.
In the absence of compelling indications, the primary drugs recommended for initial therapy are:
- Thiazide or thiazide-type diuretics (preferably chlorthalidone)
- Angiotensin-converting enzyme (ACE) inhibitors
- Angiotensin II receptor blockers (ARBs)
- Calcium channel blockers (CCBs).
In black adults, thiazide diuretics or CCBs are recommended for initial therapy. Beta-blockers are not recommended as first-line agents in the absence of a compelling indication, although meta-analyses that suggested beta-blockers are less effective than other classes of agents included trials that used beta-blockers in doses now considered suboptimal. ACE inhibitors or ARBs are recommended as initial therapy in proteinuric patients with chronic kidney disease or diabetes. Combining an ACE inhibitor and an ARB or renin inhibitor is potentially harmful and is not recommended. The guidelines provide a helpful table describing important characteristics and available dosage forms of the commonly used antihypertensive agents.
These recommendations are concordant with the JNC 8 panel recommendations,5 and differ from JNC 7, which recommended thiazide-type diuretics as first-line therapy.3 The European guidelines recommend that all major classes of antihypertensive agents, including beta-blockers, are suitable for initiation of therapy.24 The UK National Institute for Clinical Excellence guidelines adopt an age-based approach to deciding initial therapy—with ACE inhibitors or ARBs favored in those below the age of 55 and CCBs in those who are 55 and older.25
Starting with a single antihypertensive agent is recommended for stage 1 hypertension with increased cardiovascular risk, and starting with 2 agents (either separately or in fixed-dose combination) is recommended for stage 2 hypertension. The guidelines emphasize a team-based approach to improve hypertension care, using adjunctive interventions such as telehealth strategies and leveraging electronic medical records to guide quality improvement initiatives.
Our opinion. We agree with Bakris and Sorrentino26 that general patient profiles should be considered to decide on efficient pharmacologic management in clinical practice—thiazide diuretics would be best in those who are volume-expanded; ACE inhibitors, ARBs, or CCBs in those who are obese or have metabolic syndrome; and beta-blockers or nondihydropyridine CCBs in those who are hyperadrenergic. More patients will likely be classified as having resistant hypertension based on the blood pressure goal of less than 130/80 mm Hg, which may require greater use of mineralocorticoid receptor antagonists such as spironolactone.
COMPARISONS WITH OTHER GUIDELINES
STRENGTHS AND LIMITATIONS
The new guidelines stress correct technique of blood pressure measurement, out-of-office and self-monitoring of blood pressure, and lifestyle modifications. In addition, they comprehensively review topics relevant to hypertension management of practical use for healthcare providers, including resistant hypertension, secondary hypertension, hypertensive crises, and special populations. The guidelines also incorporate multiple lines of evidence rather than just randomized controlled trials (which may not be available for every scenario).
There will be ongoing debate and discussion about the new definition and classification of hypertension, and the “conversion” of previously healthy adults to a new disease category. The blood pressure goals will also be debated: Should the goal for a young patient be applied to an elderly patient? The pathophysiology of the disease process should be considered rather than a one-size-fits-all approach. For example, older patients with stiff arteries and low diastolic blood pressure will have more difficulty achieving a lower systolic pressure, are more likely to experience medication side effects, and may have adherence issues due to polypharmacy.
A clinical trial, with strict adherence to protocols and rigorous follow-up procedures, is different from real-world clinical practice. Busy clinical practices with time and space constraints may forgo the steps needed for accurate blood pressure measurement in the office and may not reinforce lifestyle modifications, instead opting for more pharmacologic therapy to achieve a blood pressure goal that may become mandated by healthcare payment models without consideration for clinical judgment and individual patient characteristics.
The ACC/AHA guidelines have not been universally endorsed. The American College of Physicians and the American Academy of Family Physicians released their own guidelines for older adults earlier in 2017, echoing the recommendations from the panel appointed to JNC 8.27 Contrasting recommendations can unfortunately lead to confusion among healthcare providers and patients and can undermine confidence and trust in the healthcare system.
In the background of ongoing debate, where battle lines have been drawn by key stakeholders with regard to their contrasting positions, it is even more important for the practicing clinician who is in the front lines of hypertension management to be knowledgeable about the pros and cons of different recommendations as they apply to individual patients, and to be able to clearly communicate this with patients when deciding on a treatment plan.
FINAL THOUGHTS
- Accurate measurement of blood pressure in the office is imperative—position the patient properly, use an appropriately sized cuff, and allow for a period of rest. Consider using automated office measurement to minimize potential white coat effect.
- Out-of-office blood pressure monitoring is recommended to confirm the diagnosis of hypertension and for monitoring response to therapy. Ambulatory monitoring is preferred, but home blood pressure monitoring can be done if ambulatory monitoring is unavailable or unfeasible.
- Nonpharmacologic therapy should be emphasized for everyone, regardless of blood pressure level.
- Guidelines should be used as a framework for management. Individualize decisions about blood pressure goals and pharmacologic therapy based on patient characteristics and clinical judgment.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017. doi:10.1016/j.jacc.2017.11.006
- SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373(22):2103-2116. doi:10.1056/NEJMoa1511939
- Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289(19):2560–2571. doi:10.1001/jama.289.19.2560
- Gibbons GH, Shurin SB, Mensah GA, Lauer MS. Refocusing the agenda on cardiovascular guidelines: an announcement from the National Heart, Lung, and Blood Institute. Circulation 2013; 128(15)1713–1715. doi:10.1161/CIRCULATIONAHA.113.004587
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311(5):507–520. doi:10.1001/jama.2013.284427
- Wright JT, Fine LJ, Lackland DT, Ogedegbe G, Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med 2014; 160(7):499–503. doi:10.7326/M13-2981
- Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. Circulation 2018; 137(2):109–118. doi:10.1161/CIRCULATIONAHA.117.032582
- Piper MA, Evans CV, Burda BU, Margolis KL, O’Connor E, Whitlock EP. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the US Preventive Services Task Force. Ann Intern Med 2015; 162(3):192–204. doi:10.7326/M14-1539
- Boggia J, Li Y, Thijs L, et al. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet 2007; 370(9594): 1219–1229. doi:10.1016/S0140-6736(07)61538-4
- Drawz PE, Ix JH. BP measurement in clinical practice: time to SPRINT to guideline-recommended protocols. J Am Soc Nephrol 2017: 29(2):383–388. doi:10.1681/ASN.2017070753
- O’Brien E, Parati G, Stergiou G, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens 2013; 31(9):1731–1768. doi:10.1097/HJH.0b013e328363e964
- Nerenberg KA, Zarnke KB, Leung AA, et al. Hypertension Canada’s 2018 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults and children. Can J Cardiol 2018; 34(5):506–525. doi:10.1016/j.cjca.2018.02.022
- Myers MG, Godwin M, Dawes M, et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomised parallel design controlled trial. BMJ 2011; 342:d286. doi:10.1136/bmj.d286
- Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet 2013; 382(9907):1762–1765. doi:10.1016/S0140-6736(13)62388-0
- DeFilippis AP, Young R, McEvoy JW, et al. Risk score overestimation: the impact of individual cardiovascular risk factors and preventive therapies on the performance of the American Heart Association-American College of Cardiology-Atherosclerotic Cardiovascular Disease risk score in a modern multi-ethnic cohort. Eur Heart J 2017; 38(8):598–608. doi:10.1093/eurheartj/ehw301
- Raymond C, Cho L, Rocco M, Hazen SL. New cholesterol guidelines: worth the wait? Cleve Clin J Med 2014; 81(1):11–19. doi:10.3949/ccjm.81a.13161
- ACCORD Study Group, Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362(17):1575–1585. doi:10.1056/NEJMoa1001286
- Perkovic V, Rodgers A. Redefining blood-pressure targets – SPRINT starts the marathon. N Engl J Med 2015; 373(22):2175–2178. doi:10.1056/NEJMe1513301
- Vidal-Petiot E, Ford I, Greenlaw N, et al. Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet 2016; 388(10056):2142–2152. doi:10.1016/S0140-6736(16)31326-5
- McEvoy JW, Chen Y, Rawlings A, et al. Diastolic blood pressure, subclinical myocardial damage, and cardiac events: implications for blood pressure control. J Am Coll Cardiol 2016; 68(16):1713–1722. doi:10.1016/j.jacc.2016.07.754
- Bakris GL. The implications of blood pressure measurement methods on treatment targets for blood pressure. Circulation 2016; 134(13):904–905. doi:10.1161/CIRCULATIONAHA.116.022536
- O’Donnell M, Mente A, Rangarajan S, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med 2014; 371(7):612–623. doi:10.1056/NEJMoa1311889
- Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 2001; 344(1):3–10. doi:10.1056/NEJM200101043440101
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34(28):2159–2219. doi:10.1093/eurheartj/eht151
- National Institute for Health and Care Excellence (NICE). Hypertension in adults: diagnosis and management. Clinical guideline CG127. http://www.nice.org.uk/guidance/CG127. Accessed August 6, 2018.
- Bakris G, Sorrentino M. Redefining hypertension—assessing the new blood-pressure guidelines. N Engl Med 2018; 378(6):497–499. doi:10.1056/NEJMp1716193
- Qaseem A, Wilt TJ, Rich R, Humphrey LL, Frost J, Forciea MA. Pharmacologic treatment of hypertension in adults aged 60 years or older to higher versus lower blood pressure targets: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 2017; 166(6): 430-437. doi:10.7326/M16-1785
- Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hyperten 2014; 16(1):14–26. doi:10.1111/jch.12237
- KDIGO Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl 2012; 2(5):337–414.
- De Boer IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care 2017; 40(9):1273–1284. doi:10.2337/dci17-0026
In 2017, the American College of Cardiology (ACC), American Heart Association (AHA), and 9 other professional associations published a new guideline on high blood pressure in adults.1 Their document addresses a range of topics relevant to preventing, diagnosing, and managing hypertension. It incorporates evidence from randomized controlled trials, including the Systolic Blood Pressure Intervention Trial (SPRINT),2 systematic reviews, and expert opinion.
The new guidelines contain many noteworthy changes, some of which are generating intense debate and discussion. Here, we provide our opinions to help practicing clinicians broaden their perspective and make informed decisions about management.
ACC AND AHA ARE NOW RESPONSIBLE FOR HYPERTENSION GUIDELINES
The Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC), organized by the National Heart, Lung, and Blood Institute, began issuing hypertension guidelines in 1977. Based on observational and clinical trial data, succeeding JNC reports recommended ever-lower blood pressure goals, with emphasis shifting to treatment of systolic hypertension.
The last official JNC report—JNC 7—was published in 2003.3 In 2013, the Institute transferred the responsibility for cardiovascular prevention guidelines to the ACC and AHA.4
A report from the panel members appointed to JNC 8 was published independently in 2014.5 It focused on a few key questions and used evidence limited to randomized controlled trials. In this report, the panel relaxed the goals for many subgroups, leading to criticism from many professional societies and from some members of the panel writing group.6
WHAT'S NEW IN THE 2017 GUIDELINES?
The new ACC/AHA guidelines contain a number of changes from previous documents that have been the topic of debate.
New definition and classification of hypertension
Strong recommendation, based on moderate-quality evidence.
Our opinion. While this new classification is intended to promote closer monitoring and earlier intervention to lower cardiovascular event rates, creating a new level of disease may lead to more pharmacologic treatment for those with lower risk, without emphasis on lifestyle modifications.
Emphasis on measurement technique and out-of-office measurements
Strong recommendation, based on expert opinion, for accurate measurement of blood pressure in the office, high-quality evidence from systematic review for out-of-office measurement.
Appropriate management of hypertension entails accurate blood pressure measurement. While office-based measurement remains the most commonly used method, this “snapshot” may not reflect a patient’s true baseline blood pressure.
Out-of-office measurements. Based on the results of a systematic review commissioned by the guideline committee, out-of-office measurements are now recommended to confirm the diagnosis of hypertension and to assess response to therapy.
Ambulatory blood pressure monitoring should be strongly considered as the preferred method for out-of-office monitoring; home blood pressure monitoring can be done if ambulatory monitoring is not feasible. Ambulatory monitoring provides additional information on nighttime blood pressure, including the dipping status (normal defined as a nighttime blood pressure decrease of 10% to 20%). Ambulatory monitoring predicts long-term cardiovascular outcomes independent of office blood pressure, and elevated nighttime pressure and non-dipping have been shown to be independently associated with increased cardiovascular mortality rates.8,9 Unfortunately, despite evidence supporting its use, ambulatory blood pressure monitoring is not widely available for a variety of reasons, including high cost (roughly $2,000–$4,000) and minimal reimbursement.
Out-of-office measurements can also detect white coat hypertension and masked hypertension. White coat hypertension is defined as blood pressure that is elevated in the office but normal in an out-of-office setting, and masked hypertension is blood pressure that is normal in the office and elevated in an out-of-office setting. Currently, pharmacologic therapy is not recommended to treat white coat hypertension, and treatment for masked hypertension should be the same as for sustained hypertension.
While the guidelines do not comment specifically on manual office measurement vs automated office measurements using devices that take multiple measurements with the patient alone in the room to reduce the white coat effect, they acknowledge “increasing evidence” favoring the use of automated office measurement.
Proper technique for measuring blood pressure is appropriately emphasized; correct patient positioning, allowing a period of rest, and using the appropriate cuff size are all important. Unfortunately, many busy clinical practices may not follow correct technique when measuring blood pressure in the office, leading to misdiagnosis and unnecessary pharmacologic therapy that may result in adverse events.
Of note, the SPRINT trial, which informed many of the new guideline recommendations, followed a strict protocol of blood pressure measurement with an automated device, checking sitting blood pressure 3 times at 1-minute intervals, with the patient alone in the room and without an observer present at many of the sites.10
Most guidelines11,12 agree on an average of at least 135/85 mm Hg as the threshold for diagnosing hypertension by home monitoring, or an average daytime pressure of at least 135/85 mm Hg by ambulatory monitoring, corresponding with office-based blood pressure of 140/90 mm Hg. However, the new guidelines recommend a lower threshold of 130/80 mm Hg for both home monitoring and average daytime ambulatory monitoring, corresponding with an office blood pressure of 130/80 mm Hg. They do not specify whether the office-based measurement is manual or automated.
Our opinion. Since office-based measurement will likely remain the principal method for managing hypertension due to constraints with ambulatory or home monitoring, the use of automated devices for office measurement should be strongly considered. Studies have shown that, compared with routine office measurements, automated measurements more closely approximate those obtained by ambulatory and home blood pressure monitoring.13
Risk-based approach to hypertension management
The algorithm for hypertension management now incorporates objective assessment of cardiovascular risk. Specifically, it calls for estimation of the 10-year risk of atherosclerotic cardiovascular disease, defined as coronary heart disease death, nonfatal myocardial infarction, or fatal or nonfatal stroke.
The information required to estimate risk includes age, sex, race, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, use of blood pressure-lowering medication, diabetes status, and smoking status. The guideline recommends an easy-to-use online risk calculator (http://tools.acc.org/ASCVD-Risk-Estimator).
A 10-year risk of 10% or more is designated as the cutoff between high risk and low risk. However, this is not based on trial evidence, and the risk calculator has not been verified in prospective trials to show that its use reduces cardiovascular events. The SPRINT trial,2 which was a study of blood pressure-lowering in high-risk patients, used a 10-year risk of 15% or more based on the Framingham risk score to delineate high risk.
Additionally, the 10-year risk calculator is valid only in patients ages 40 through 79, and some studies indicate that it may overestimate risk in older adults.14,15 This overestimation may lead to patients being started on pharmacologic therapy when it may not truly be indicated. The risk calculator controversy has been discussed in a previous issue of this journal.16
Blood pressure goals
Strong recommendation for known cardiovascular disease or atherosclerotic cardiovascular disease risk 10% or greater, weak recommendation for risk less than 10%, based on moderate-quality evidence for systolic blood pressure, expert opinion for diastolic.
The guidelines recommend a blood pressure goal of less than 130/80 mm Hg for all patients, including the elderly and patients with chronic kidney disease or diabetes.
The SPRINT trial,2 which showed better cardiovascular outcomes in the intensive treatment group (aiming for systolic pressure < 120 mm Hg) compared with a standard treatment group (aiming for systolic pressure < 140 mm Hg), excluded participants with diabetes and severe chronic kidney disease (estimated glomerular filtration rate < 20 mL/min/m2 and proteinuria > 1 g/day), and those who were in nursing homes or had dementia.
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) blood pressure trial showed that intensive blood pressure control did not have cardiovascular benefits compared with standard therapy.17 However, many now believe that the study may have been underpowered due to its design, and a meta-analysis of the results from SPRINT and ACCORD suggested that findings from both trials were consistent, favoring intensive blood pressure control in a high-risk population.18
While the totality of evidence favors a lower achieved blood pressure for many patients, this lower goal may be difficult to achieve in many, particularly those with vascular stiffness, which is common in the elderly. These patients also tend to have low diastolic pressure, and lowering diastolic pressure below 60 mm Hg in those with documented coronary artery disease could increase the risk of adverse cardiovascular outcomes.19,20 The guidelines do not address the potential issues with lowering diastolic blood pressure.
Our opinion. While a “universal” blood pressure goal may simplify decision-making, we believe it is important to individualize goals, taking into account patient characteristics, lifestyle factors, medication side effects, patient preferences, cost issues, and adherence to therapy.
The goal blood pressure should also consider the method of measurement. Systolic blood pressure readings have been reported to be 5 to 10 mm Hg lower with automated office measurement than with routine office measurement.21
It is also not clear that the magnitude of absolute benefit from pursuing more intensive blood pressure control with antihypertensive therapy in patients with high cardiovascular risk (as in SPRINT) would translate to similar benefits in a lower-risk population. Thus, we believe that in patients with lower cardiovascular risk, a goal blood pressure of less than 140/90 mm Hg (if routine office measurement is done) and less than 135/85 mm Hg (if automated office measurement is done) would be reasonable.
We also believe that it is reasonable to relax these goals in the very elderly (age ≥ 80), especially those who are frail and at risk of falls, with low diastolic pressures. In these patients, we recommend individualizing blood pressure goals that can be achieved without significant side effects from antihypertensive therapy.
Nonpharmacologic therapy
Strong recommendation, based on high-quality evidence from randomized controlled trials
Nonpharmacologic therapy and lifestyle modification are appropriately emphasized in the new guidelines. Most of the lifestyle changes that are recommended are in concordance with prior JNC 7 recommendations.3
Recognizing the roles of sodium and potassium in the pathogenesis of hypertension, the guidelines emphasize a diet that is higher in potassium, the DASH (Dietary Approaches to Stop Hypertension) diet, and a low-sodium diet. The recommended optimal goal of sodium intake of less than 1,500 mg/day may be difficult to achieve with a Western diet, and there is debate about the potential adverse effects of a very-low sodium diet.22 The general recommendation for sodium intake of less than 2,300 mg/day is supported in the literature, and it is unclear if further reduction has additional beneficial effects on blood pressure.23
The guidelines recommend a 3- to 6-month reassessment of patients who are prescribed risk-factor modification, but are unclear about initiation of pharmacologic therapy or other steps if these low-risk patients have not responded to lifestyle modifications alone at the time of reassessment.
Pharmacologic therapy
Strong recommendation, based on high-quality evidence from randomized controlled trials for systolic blood pressure, expert opinion for diastolic blood pressure for those with atherosclerotic cardiovascular disease risk 10% or greater, and limited data for those with risk less than 10%.
Pharmacologic therapy is recommended in patients with stage 1 hypertension and pre-existing cardiovascular disease or 10-year risk of atherosclerotic cardiovascular disease of 10% or more, and in those with stage 2 hypertension even if their 10-year risk is less than 10%.
In the absence of compelling indications, the primary drugs recommended for initial therapy are:
- Thiazide or thiazide-type diuretics (preferably chlorthalidone)
- Angiotensin-converting enzyme (ACE) inhibitors
- Angiotensin II receptor blockers (ARBs)
- Calcium channel blockers (CCBs).
In black adults, thiazide diuretics or CCBs are recommended for initial therapy. Beta-blockers are not recommended as first-line agents in the absence of a compelling indication, although meta-analyses that suggested beta-blockers are less effective than other classes of agents included trials that used beta-blockers in doses now considered suboptimal. ACE inhibitors or ARBs are recommended as initial therapy in proteinuric patients with chronic kidney disease or diabetes. Combining an ACE inhibitor and an ARB or renin inhibitor is potentially harmful and is not recommended. The guidelines provide a helpful table describing important characteristics and available dosage forms of the commonly used antihypertensive agents.
These recommendations are concordant with the JNC 8 panel recommendations,5 and differ from JNC 7, which recommended thiazide-type diuretics as first-line therapy.3 The European guidelines recommend that all major classes of antihypertensive agents, including beta-blockers, are suitable for initiation of therapy.24 The UK National Institute for Clinical Excellence guidelines adopt an age-based approach to deciding initial therapy—with ACE inhibitors or ARBs favored in those below the age of 55 and CCBs in those who are 55 and older.25
Starting with a single antihypertensive agent is recommended for stage 1 hypertension with increased cardiovascular risk, and starting with 2 agents (either separately or in fixed-dose combination) is recommended for stage 2 hypertension. The guidelines emphasize a team-based approach to improve hypertension care, using adjunctive interventions such as telehealth strategies and leveraging electronic medical records to guide quality improvement initiatives.
Our opinion. We agree with Bakris and Sorrentino26 that general patient profiles should be considered to decide on efficient pharmacologic management in clinical practice—thiazide diuretics would be best in those who are volume-expanded; ACE inhibitors, ARBs, or CCBs in those who are obese or have metabolic syndrome; and beta-blockers or nondihydropyridine CCBs in those who are hyperadrenergic. More patients will likely be classified as having resistant hypertension based on the blood pressure goal of less than 130/80 mm Hg, which may require greater use of mineralocorticoid receptor antagonists such as spironolactone.
COMPARISONS WITH OTHER GUIDELINES
STRENGTHS AND LIMITATIONS
The new guidelines stress correct technique of blood pressure measurement, out-of-office and self-monitoring of blood pressure, and lifestyle modifications. In addition, they comprehensively review topics relevant to hypertension management of practical use for healthcare providers, including resistant hypertension, secondary hypertension, hypertensive crises, and special populations. The guidelines also incorporate multiple lines of evidence rather than just randomized controlled trials (which may not be available for every scenario).
There will be ongoing debate and discussion about the new definition and classification of hypertension, and the “conversion” of previously healthy adults to a new disease category. The blood pressure goals will also be debated: Should the goal for a young patient be applied to an elderly patient? The pathophysiology of the disease process should be considered rather than a one-size-fits-all approach. For example, older patients with stiff arteries and low diastolic blood pressure will have more difficulty achieving a lower systolic pressure, are more likely to experience medication side effects, and may have adherence issues due to polypharmacy.
A clinical trial, with strict adherence to protocols and rigorous follow-up procedures, is different from real-world clinical practice. Busy clinical practices with time and space constraints may forgo the steps needed for accurate blood pressure measurement in the office and may not reinforce lifestyle modifications, instead opting for more pharmacologic therapy to achieve a blood pressure goal that may become mandated by healthcare payment models without consideration for clinical judgment and individual patient characteristics.
The ACC/AHA guidelines have not been universally endorsed. The American College of Physicians and the American Academy of Family Physicians released their own guidelines for older adults earlier in 2017, echoing the recommendations from the panel appointed to JNC 8.27 Contrasting recommendations can unfortunately lead to confusion among healthcare providers and patients and can undermine confidence and trust in the healthcare system.
In the background of ongoing debate, where battle lines have been drawn by key stakeholders with regard to their contrasting positions, it is even more important for the practicing clinician who is in the front lines of hypertension management to be knowledgeable about the pros and cons of different recommendations as they apply to individual patients, and to be able to clearly communicate this with patients when deciding on a treatment plan.
FINAL THOUGHTS
- Accurate measurement of blood pressure in the office is imperative—position the patient properly, use an appropriately sized cuff, and allow for a period of rest. Consider using automated office measurement to minimize potential white coat effect.
- Out-of-office blood pressure monitoring is recommended to confirm the diagnosis of hypertension and for monitoring response to therapy. Ambulatory monitoring is preferred, but home blood pressure monitoring can be done if ambulatory monitoring is unavailable or unfeasible.
- Nonpharmacologic therapy should be emphasized for everyone, regardless of blood pressure level.
- Guidelines should be used as a framework for management. Individualize decisions about blood pressure goals and pharmacologic therapy based on patient characteristics and clinical judgment.
In 2017, the American College of Cardiology (ACC), American Heart Association (AHA), and 9 other professional associations published a new guideline on high blood pressure in adults.1 Their document addresses a range of topics relevant to preventing, diagnosing, and managing hypertension. It incorporates evidence from randomized controlled trials, including the Systolic Blood Pressure Intervention Trial (SPRINT),2 systematic reviews, and expert opinion.
The new guidelines contain many noteworthy changes, some of which are generating intense debate and discussion. Here, we provide our opinions to help practicing clinicians broaden their perspective and make informed decisions about management.
ACC AND AHA ARE NOW RESPONSIBLE FOR HYPERTENSION GUIDELINES
The Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC), organized by the National Heart, Lung, and Blood Institute, began issuing hypertension guidelines in 1977. Based on observational and clinical trial data, succeeding JNC reports recommended ever-lower blood pressure goals, with emphasis shifting to treatment of systolic hypertension.
The last official JNC report—JNC 7—was published in 2003.3 In 2013, the Institute transferred the responsibility for cardiovascular prevention guidelines to the ACC and AHA.4
A report from the panel members appointed to JNC 8 was published independently in 2014.5 It focused on a few key questions and used evidence limited to randomized controlled trials. In this report, the panel relaxed the goals for many subgroups, leading to criticism from many professional societies and from some members of the panel writing group.6
WHAT'S NEW IN THE 2017 GUIDELINES?
The new ACC/AHA guidelines contain a number of changes from previous documents that have been the topic of debate.
New definition and classification of hypertension
Strong recommendation, based on moderate-quality evidence.
Our opinion. While this new classification is intended to promote closer monitoring and earlier intervention to lower cardiovascular event rates, creating a new level of disease may lead to more pharmacologic treatment for those with lower risk, without emphasis on lifestyle modifications.
Emphasis on measurement technique and out-of-office measurements
Strong recommendation, based on expert opinion, for accurate measurement of blood pressure in the office, high-quality evidence from systematic review for out-of-office measurement.
Appropriate management of hypertension entails accurate blood pressure measurement. While office-based measurement remains the most commonly used method, this “snapshot” may not reflect a patient’s true baseline blood pressure.
Out-of-office measurements. Based on the results of a systematic review commissioned by the guideline committee, out-of-office measurements are now recommended to confirm the diagnosis of hypertension and to assess response to therapy.
Ambulatory blood pressure monitoring should be strongly considered as the preferred method for out-of-office monitoring; home blood pressure monitoring can be done if ambulatory monitoring is not feasible. Ambulatory monitoring provides additional information on nighttime blood pressure, including the dipping status (normal defined as a nighttime blood pressure decrease of 10% to 20%). Ambulatory monitoring predicts long-term cardiovascular outcomes independent of office blood pressure, and elevated nighttime pressure and non-dipping have been shown to be independently associated with increased cardiovascular mortality rates.8,9 Unfortunately, despite evidence supporting its use, ambulatory blood pressure monitoring is not widely available for a variety of reasons, including high cost (roughly $2,000–$4,000) and minimal reimbursement.
Out-of-office measurements can also detect white coat hypertension and masked hypertension. White coat hypertension is defined as blood pressure that is elevated in the office but normal in an out-of-office setting, and masked hypertension is blood pressure that is normal in the office and elevated in an out-of-office setting. Currently, pharmacologic therapy is not recommended to treat white coat hypertension, and treatment for masked hypertension should be the same as for sustained hypertension.
While the guidelines do not comment specifically on manual office measurement vs automated office measurements using devices that take multiple measurements with the patient alone in the room to reduce the white coat effect, they acknowledge “increasing evidence” favoring the use of automated office measurement.
Proper technique for measuring blood pressure is appropriately emphasized; correct patient positioning, allowing a period of rest, and using the appropriate cuff size are all important. Unfortunately, many busy clinical practices may not follow correct technique when measuring blood pressure in the office, leading to misdiagnosis and unnecessary pharmacologic therapy that may result in adverse events.
Of note, the SPRINT trial, which informed many of the new guideline recommendations, followed a strict protocol of blood pressure measurement with an automated device, checking sitting blood pressure 3 times at 1-minute intervals, with the patient alone in the room and without an observer present at many of the sites.10
Most guidelines11,12 agree on an average of at least 135/85 mm Hg as the threshold for diagnosing hypertension by home monitoring, or an average daytime pressure of at least 135/85 mm Hg by ambulatory monitoring, corresponding with office-based blood pressure of 140/90 mm Hg. However, the new guidelines recommend a lower threshold of 130/80 mm Hg for both home monitoring and average daytime ambulatory monitoring, corresponding with an office blood pressure of 130/80 mm Hg. They do not specify whether the office-based measurement is manual or automated.
Our opinion. Since office-based measurement will likely remain the principal method for managing hypertension due to constraints with ambulatory or home monitoring, the use of automated devices for office measurement should be strongly considered. Studies have shown that, compared with routine office measurements, automated measurements more closely approximate those obtained by ambulatory and home blood pressure monitoring.13
Risk-based approach to hypertension management
The algorithm for hypertension management now incorporates objective assessment of cardiovascular risk. Specifically, it calls for estimation of the 10-year risk of atherosclerotic cardiovascular disease, defined as coronary heart disease death, nonfatal myocardial infarction, or fatal or nonfatal stroke.
The information required to estimate risk includes age, sex, race, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, use of blood pressure-lowering medication, diabetes status, and smoking status. The guideline recommends an easy-to-use online risk calculator (http://tools.acc.org/ASCVD-Risk-Estimator).
A 10-year risk of 10% or more is designated as the cutoff between high risk and low risk. However, this is not based on trial evidence, and the risk calculator has not been verified in prospective trials to show that its use reduces cardiovascular events. The SPRINT trial,2 which was a study of blood pressure-lowering in high-risk patients, used a 10-year risk of 15% or more based on the Framingham risk score to delineate high risk.
Additionally, the 10-year risk calculator is valid only in patients ages 40 through 79, and some studies indicate that it may overestimate risk in older adults.14,15 This overestimation may lead to patients being started on pharmacologic therapy when it may not truly be indicated. The risk calculator controversy has been discussed in a previous issue of this journal.16
Blood pressure goals
Strong recommendation for known cardiovascular disease or atherosclerotic cardiovascular disease risk 10% or greater, weak recommendation for risk less than 10%, based on moderate-quality evidence for systolic blood pressure, expert opinion for diastolic.
The guidelines recommend a blood pressure goal of less than 130/80 mm Hg for all patients, including the elderly and patients with chronic kidney disease or diabetes.
The SPRINT trial,2 which showed better cardiovascular outcomes in the intensive treatment group (aiming for systolic pressure < 120 mm Hg) compared with a standard treatment group (aiming for systolic pressure < 140 mm Hg), excluded participants with diabetes and severe chronic kidney disease (estimated glomerular filtration rate < 20 mL/min/m2 and proteinuria > 1 g/day), and those who were in nursing homes or had dementia.
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) blood pressure trial showed that intensive blood pressure control did not have cardiovascular benefits compared with standard therapy.17 However, many now believe that the study may have been underpowered due to its design, and a meta-analysis of the results from SPRINT and ACCORD suggested that findings from both trials were consistent, favoring intensive blood pressure control in a high-risk population.18
While the totality of evidence favors a lower achieved blood pressure for many patients, this lower goal may be difficult to achieve in many, particularly those with vascular stiffness, which is common in the elderly. These patients also tend to have low diastolic pressure, and lowering diastolic pressure below 60 mm Hg in those with documented coronary artery disease could increase the risk of adverse cardiovascular outcomes.19,20 The guidelines do not address the potential issues with lowering diastolic blood pressure.
Our opinion. While a “universal” blood pressure goal may simplify decision-making, we believe it is important to individualize goals, taking into account patient characteristics, lifestyle factors, medication side effects, patient preferences, cost issues, and adherence to therapy.
The goal blood pressure should also consider the method of measurement. Systolic blood pressure readings have been reported to be 5 to 10 mm Hg lower with automated office measurement than with routine office measurement.21
It is also not clear that the magnitude of absolute benefit from pursuing more intensive blood pressure control with antihypertensive therapy in patients with high cardiovascular risk (as in SPRINT) would translate to similar benefits in a lower-risk population. Thus, we believe that in patients with lower cardiovascular risk, a goal blood pressure of less than 140/90 mm Hg (if routine office measurement is done) and less than 135/85 mm Hg (if automated office measurement is done) would be reasonable.
We also believe that it is reasonable to relax these goals in the very elderly (age ≥ 80), especially those who are frail and at risk of falls, with low diastolic pressures. In these patients, we recommend individualizing blood pressure goals that can be achieved without significant side effects from antihypertensive therapy.
Nonpharmacologic therapy
Strong recommendation, based on high-quality evidence from randomized controlled trials
Nonpharmacologic therapy and lifestyle modification are appropriately emphasized in the new guidelines. Most of the lifestyle changes that are recommended are in concordance with prior JNC 7 recommendations.3
Recognizing the roles of sodium and potassium in the pathogenesis of hypertension, the guidelines emphasize a diet that is higher in potassium, the DASH (Dietary Approaches to Stop Hypertension) diet, and a low-sodium diet. The recommended optimal goal of sodium intake of less than 1,500 mg/day may be difficult to achieve with a Western diet, and there is debate about the potential adverse effects of a very-low sodium diet.22 The general recommendation for sodium intake of less than 2,300 mg/day is supported in the literature, and it is unclear if further reduction has additional beneficial effects on blood pressure.23
The guidelines recommend a 3- to 6-month reassessment of patients who are prescribed risk-factor modification, but are unclear about initiation of pharmacologic therapy or other steps if these low-risk patients have not responded to lifestyle modifications alone at the time of reassessment.
Pharmacologic therapy
Strong recommendation, based on high-quality evidence from randomized controlled trials for systolic blood pressure, expert opinion for diastolic blood pressure for those with atherosclerotic cardiovascular disease risk 10% or greater, and limited data for those with risk less than 10%.
Pharmacologic therapy is recommended in patients with stage 1 hypertension and pre-existing cardiovascular disease or 10-year risk of atherosclerotic cardiovascular disease of 10% or more, and in those with stage 2 hypertension even if their 10-year risk is less than 10%.
In the absence of compelling indications, the primary drugs recommended for initial therapy are:
- Thiazide or thiazide-type diuretics (preferably chlorthalidone)
- Angiotensin-converting enzyme (ACE) inhibitors
- Angiotensin II receptor blockers (ARBs)
- Calcium channel blockers (CCBs).
In black adults, thiazide diuretics or CCBs are recommended for initial therapy. Beta-blockers are not recommended as first-line agents in the absence of a compelling indication, although meta-analyses that suggested beta-blockers are less effective than other classes of agents included trials that used beta-blockers in doses now considered suboptimal. ACE inhibitors or ARBs are recommended as initial therapy in proteinuric patients with chronic kidney disease or diabetes. Combining an ACE inhibitor and an ARB or renin inhibitor is potentially harmful and is not recommended. The guidelines provide a helpful table describing important characteristics and available dosage forms of the commonly used antihypertensive agents.
These recommendations are concordant with the JNC 8 panel recommendations,5 and differ from JNC 7, which recommended thiazide-type diuretics as first-line therapy.3 The European guidelines recommend that all major classes of antihypertensive agents, including beta-blockers, are suitable for initiation of therapy.24 The UK National Institute for Clinical Excellence guidelines adopt an age-based approach to deciding initial therapy—with ACE inhibitors or ARBs favored in those below the age of 55 and CCBs in those who are 55 and older.25
Starting with a single antihypertensive agent is recommended for stage 1 hypertension with increased cardiovascular risk, and starting with 2 agents (either separately or in fixed-dose combination) is recommended for stage 2 hypertension. The guidelines emphasize a team-based approach to improve hypertension care, using adjunctive interventions such as telehealth strategies and leveraging electronic medical records to guide quality improvement initiatives.
Our opinion. We agree with Bakris and Sorrentino26 that general patient profiles should be considered to decide on efficient pharmacologic management in clinical practice—thiazide diuretics would be best in those who are volume-expanded; ACE inhibitors, ARBs, or CCBs in those who are obese or have metabolic syndrome; and beta-blockers or nondihydropyridine CCBs in those who are hyperadrenergic. More patients will likely be classified as having resistant hypertension based on the blood pressure goal of less than 130/80 mm Hg, which may require greater use of mineralocorticoid receptor antagonists such as spironolactone.
COMPARISONS WITH OTHER GUIDELINES
STRENGTHS AND LIMITATIONS
The new guidelines stress correct technique of blood pressure measurement, out-of-office and self-monitoring of blood pressure, and lifestyle modifications. In addition, they comprehensively review topics relevant to hypertension management of practical use for healthcare providers, including resistant hypertension, secondary hypertension, hypertensive crises, and special populations. The guidelines also incorporate multiple lines of evidence rather than just randomized controlled trials (which may not be available for every scenario).
There will be ongoing debate and discussion about the new definition and classification of hypertension, and the “conversion” of previously healthy adults to a new disease category. The blood pressure goals will also be debated: Should the goal for a young patient be applied to an elderly patient? The pathophysiology of the disease process should be considered rather than a one-size-fits-all approach. For example, older patients with stiff arteries and low diastolic blood pressure will have more difficulty achieving a lower systolic pressure, are more likely to experience medication side effects, and may have adherence issues due to polypharmacy.
A clinical trial, with strict adherence to protocols and rigorous follow-up procedures, is different from real-world clinical practice. Busy clinical practices with time and space constraints may forgo the steps needed for accurate blood pressure measurement in the office and may not reinforce lifestyle modifications, instead opting for more pharmacologic therapy to achieve a blood pressure goal that may become mandated by healthcare payment models without consideration for clinical judgment and individual patient characteristics.
The ACC/AHA guidelines have not been universally endorsed. The American College of Physicians and the American Academy of Family Physicians released their own guidelines for older adults earlier in 2017, echoing the recommendations from the panel appointed to JNC 8.27 Contrasting recommendations can unfortunately lead to confusion among healthcare providers and patients and can undermine confidence and trust in the healthcare system.
In the background of ongoing debate, where battle lines have been drawn by key stakeholders with regard to their contrasting positions, it is even more important for the practicing clinician who is in the front lines of hypertension management to be knowledgeable about the pros and cons of different recommendations as they apply to individual patients, and to be able to clearly communicate this with patients when deciding on a treatment plan.
FINAL THOUGHTS
- Accurate measurement of blood pressure in the office is imperative—position the patient properly, use an appropriately sized cuff, and allow for a period of rest. Consider using automated office measurement to minimize potential white coat effect.
- Out-of-office blood pressure monitoring is recommended to confirm the diagnosis of hypertension and for monitoring response to therapy. Ambulatory monitoring is preferred, but home blood pressure monitoring can be done if ambulatory monitoring is unavailable or unfeasible.
- Nonpharmacologic therapy should be emphasized for everyone, regardless of blood pressure level.
- Guidelines should be used as a framework for management. Individualize decisions about blood pressure goals and pharmacologic therapy based on patient characteristics and clinical judgment.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017. doi:10.1016/j.jacc.2017.11.006
- SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373(22):2103-2116. doi:10.1056/NEJMoa1511939
- Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289(19):2560–2571. doi:10.1001/jama.289.19.2560
- Gibbons GH, Shurin SB, Mensah GA, Lauer MS. Refocusing the agenda on cardiovascular guidelines: an announcement from the National Heart, Lung, and Blood Institute. Circulation 2013; 128(15)1713–1715. doi:10.1161/CIRCULATIONAHA.113.004587
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311(5):507–520. doi:10.1001/jama.2013.284427
- Wright JT, Fine LJ, Lackland DT, Ogedegbe G, Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med 2014; 160(7):499–503. doi:10.7326/M13-2981
- Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. Circulation 2018; 137(2):109–118. doi:10.1161/CIRCULATIONAHA.117.032582
- Piper MA, Evans CV, Burda BU, Margolis KL, O’Connor E, Whitlock EP. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the US Preventive Services Task Force. Ann Intern Med 2015; 162(3):192–204. doi:10.7326/M14-1539
- Boggia J, Li Y, Thijs L, et al. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet 2007; 370(9594): 1219–1229. doi:10.1016/S0140-6736(07)61538-4
- Drawz PE, Ix JH. BP measurement in clinical practice: time to SPRINT to guideline-recommended protocols. J Am Soc Nephrol 2017: 29(2):383–388. doi:10.1681/ASN.2017070753
- O’Brien E, Parati G, Stergiou G, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens 2013; 31(9):1731–1768. doi:10.1097/HJH.0b013e328363e964
- Nerenberg KA, Zarnke KB, Leung AA, et al. Hypertension Canada’s 2018 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults and children. Can J Cardiol 2018; 34(5):506–525. doi:10.1016/j.cjca.2018.02.022
- Myers MG, Godwin M, Dawes M, et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomised parallel design controlled trial. BMJ 2011; 342:d286. doi:10.1136/bmj.d286
- Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet 2013; 382(9907):1762–1765. doi:10.1016/S0140-6736(13)62388-0
- DeFilippis AP, Young R, McEvoy JW, et al. Risk score overestimation: the impact of individual cardiovascular risk factors and preventive therapies on the performance of the American Heart Association-American College of Cardiology-Atherosclerotic Cardiovascular Disease risk score in a modern multi-ethnic cohort. Eur Heart J 2017; 38(8):598–608. doi:10.1093/eurheartj/ehw301
- Raymond C, Cho L, Rocco M, Hazen SL. New cholesterol guidelines: worth the wait? Cleve Clin J Med 2014; 81(1):11–19. doi:10.3949/ccjm.81a.13161
- ACCORD Study Group, Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362(17):1575–1585. doi:10.1056/NEJMoa1001286
- Perkovic V, Rodgers A. Redefining blood-pressure targets – SPRINT starts the marathon. N Engl J Med 2015; 373(22):2175–2178. doi:10.1056/NEJMe1513301
- Vidal-Petiot E, Ford I, Greenlaw N, et al. Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet 2016; 388(10056):2142–2152. doi:10.1016/S0140-6736(16)31326-5
- McEvoy JW, Chen Y, Rawlings A, et al. Diastolic blood pressure, subclinical myocardial damage, and cardiac events: implications for blood pressure control. J Am Coll Cardiol 2016; 68(16):1713–1722. doi:10.1016/j.jacc.2016.07.754
- Bakris GL. The implications of blood pressure measurement methods on treatment targets for blood pressure. Circulation 2016; 134(13):904–905. doi:10.1161/CIRCULATIONAHA.116.022536
- O’Donnell M, Mente A, Rangarajan S, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med 2014; 371(7):612–623. doi:10.1056/NEJMoa1311889
- Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 2001; 344(1):3–10. doi:10.1056/NEJM200101043440101
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34(28):2159–2219. doi:10.1093/eurheartj/eht151
- National Institute for Health and Care Excellence (NICE). Hypertension in adults: diagnosis and management. Clinical guideline CG127. http://www.nice.org.uk/guidance/CG127. Accessed August 6, 2018.
- Bakris G, Sorrentino M. Redefining hypertension—assessing the new blood-pressure guidelines. N Engl Med 2018; 378(6):497–499. doi:10.1056/NEJMp1716193
- Qaseem A, Wilt TJ, Rich R, Humphrey LL, Frost J, Forciea MA. Pharmacologic treatment of hypertension in adults aged 60 years or older to higher versus lower blood pressure targets: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 2017; 166(6): 430-437. doi:10.7326/M16-1785
- Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hyperten 2014; 16(1):14–26. doi:10.1111/jch.12237
- KDIGO Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl 2012; 2(5):337–414.
- De Boer IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care 2017; 40(9):1273–1284. doi:10.2337/dci17-0026
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017. doi:10.1016/j.jacc.2017.11.006
- SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373(22):2103-2116. doi:10.1056/NEJMoa1511939
- Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289(19):2560–2571. doi:10.1001/jama.289.19.2560
- Gibbons GH, Shurin SB, Mensah GA, Lauer MS. Refocusing the agenda on cardiovascular guidelines: an announcement from the National Heart, Lung, and Blood Institute. Circulation 2013; 128(15)1713–1715. doi:10.1161/CIRCULATIONAHA.113.004587
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311(5):507–520. doi:10.1001/jama.2013.284427
- Wright JT, Fine LJ, Lackland DT, Ogedegbe G, Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med 2014; 160(7):499–503. doi:10.7326/M13-2981
- Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. Circulation 2018; 137(2):109–118. doi:10.1161/CIRCULATIONAHA.117.032582
- Piper MA, Evans CV, Burda BU, Margolis KL, O’Connor E, Whitlock EP. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the US Preventive Services Task Force. Ann Intern Med 2015; 162(3):192–204. doi:10.7326/M14-1539
- Boggia J, Li Y, Thijs L, et al. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet 2007; 370(9594): 1219–1229. doi:10.1016/S0140-6736(07)61538-4
- Drawz PE, Ix JH. BP measurement in clinical practice: time to SPRINT to guideline-recommended protocols. J Am Soc Nephrol 2017: 29(2):383–388. doi:10.1681/ASN.2017070753
- O’Brien E, Parati G, Stergiou G, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens 2013; 31(9):1731–1768. doi:10.1097/HJH.0b013e328363e964
- Nerenberg KA, Zarnke KB, Leung AA, et al. Hypertension Canada’s 2018 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults and children. Can J Cardiol 2018; 34(5):506–525. doi:10.1016/j.cjca.2018.02.022
- Myers MG, Godwin M, Dawes M, et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomised parallel design controlled trial. BMJ 2011; 342:d286. doi:10.1136/bmj.d286
- Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet 2013; 382(9907):1762–1765. doi:10.1016/S0140-6736(13)62388-0
- DeFilippis AP, Young R, McEvoy JW, et al. Risk score overestimation: the impact of individual cardiovascular risk factors and preventive therapies on the performance of the American Heart Association-American College of Cardiology-Atherosclerotic Cardiovascular Disease risk score in a modern multi-ethnic cohort. Eur Heart J 2017; 38(8):598–608. doi:10.1093/eurheartj/ehw301
- Raymond C, Cho L, Rocco M, Hazen SL. New cholesterol guidelines: worth the wait? Cleve Clin J Med 2014; 81(1):11–19. doi:10.3949/ccjm.81a.13161
- ACCORD Study Group, Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362(17):1575–1585. doi:10.1056/NEJMoa1001286
- Perkovic V, Rodgers A. Redefining blood-pressure targets – SPRINT starts the marathon. N Engl J Med 2015; 373(22):2175–2178. doi:10.1056/NEJMe1513301
- Vidal-Petiot E, Ford I, Greenlaw N, et al. Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet 2016; 388(10056):2142–2152. doi:10.1016/S0140-6736(16)31326-5
- McEvoy JW, Chen Y, Rawlings A, et al. Diastolic blood pressure, subclinical myocardial damage, and cardiac events: implications for blood pressure control. J Am Coll Cardiol 2016; 68(16):1713–1722. doi:10.1016/j.jacc.2016.07.754
- Bakris GL. The implications of blood pressure measurement methods on treatment targets for blood pressure. Circulation 2016; 134(13):904–905. doi:10.1161/CIRCULATIONAHA.116.022536
- O’Donnell M, Mente A, Rangarajan S, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med 2014; 371(7):612–623. doi:10.1056/NEJMoa1311889
- Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 2001; 344(1):3–10. doi:10.1056/NEJM200101043440101
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34(28):2159–2219. doi:10.1093/eurheartj/eht151
- National Institute for Health and Care Excellence (NICE). Hypertension in adults: diagnosis and management. Clinical guideline CG127. http://www.nice.org.uk/guidance/CG127. Accessed August 6, 2018.
- Bakris G, Sorrentino M. Redefining hypertension—assessing the new blood-pressure guidelines. N Engl Med 2018; 378(6):497–499. doi:10.1056/NEJMp1716193
- Qaseem A, Wilt TJ, Rich R, Humphrey LL, Frost J, Forciea MA. Pharmacologic treatment of hypertension in adults aged 60 years or older to higher versus lower blood pressure targets: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 2017; 166(6): 430-437. doi:10.7326/M16-1785
- Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hyperten 2014; 16(1):14–26. doi:10.1111/jch.12237
- KDIGO Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl 2012; 2(5):337–414.
- De Boer IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care 2017; 40(9):1273–1284. doi:10.2337/dci17-0026