User login
MV disease in children requires modified strategies
NEW YORK – Repairing mitral valves in pediatric patients must overcome two issues: the wide variability in their anatomy and their growth. Using strategies and techniques common in adult mitral surgery can accomplish good mitral valve function in children, but some techniques in children differ, like using combined resorbable material with autologous tissue or transferring native chords instead of placing artificial chords to a malfunctioning leaflet.
Pedro del Nido, MD, of Boston Children’s Hospital, said the spectrum of mitral valve pathology in children goes from congenital mitral stenosis with a thick annulus with leaflet immobility to leaflet hypermobility that involves anterior leaflet prolapse and can involve a cleft that causes regurgitation. Dr. del Nido explained his surgical approaches for mitral valve disease in children at the 2017 Mitral Conclave, sponsored by the American Association of Thoracic Surgery.
Accessing the mitral valve in children requires a different approach than in adults, Dr. del Nido said. “Going through the left atrium is generally difficult, so we often enter through a trans-septal incision,” he said. “The main reason for that is because the tricuspid valve is often associated with the mitral valve problem and this gives us the most direct exposure.”
Once the surgeon gains exposure, the surgical analysis for a diseased adult or child valve is almost identical, with the exception that adult disease is acquired whereas childhood disease tends to be congenital, Dr. del Nido said. “In the congenital patient, we often find fibroelastic tissue that the child is born with,” he said. “We see this in neonates and young infants. It thickens over time; it doesn’t often calcify, but it does often restrict the leaflets and it tends to fuse the chords, so in essence you have direct attachments of the leaflets to the papillaries.”
He explained that this pathology requires an approach similar to that for rheumatic mitral disease in adults. “Start splitting the commissures and start resecting the tissue off the chords creating fenestrations in order to improve the inflow.” Dr. del Nido added, “If you don’t do this, the child will always have a gradient, and if you think about an adult having problems and symptoms with a gradient, think about a 10-year-old running around trying to do athletics; it’s impossible.”
Dysfunctional chords also require a somewhat different approach in children than they require in adults. “We find elongation of the chords and the anterior support structure is abnormal; the secondary chords are totally intact,” Dr. del Nido said. When confronting a torn-edge chord, resection is often an option in adults, but is uncommon in children. “We don’t usually have very much leaflet tissue,” he said. Artificial chords do not accommodate growth.
“We tend to use native tissue,” said Dr. del Nido. “You can transfer the strut chord; you can transfer the secondary chord in order to achieve support for the edge of that prolapsed leaflet.”
Leaflet problems are probably the biggest single source of recurrence in children, Dr. del Nido said. A cleft on the anterior leaflet can be particularly vexing. For example, cleft edges attached to the septum can prevent the valve leaflet from coaptation with the posterior leaflet. “If you don’t recognize that on 3-D echocardiography, you’re going to have a problem; that leaflet will never create the coaptation surface that you want,” he said.
The solution may lie underneath the leaflet. Said Dr. del Nido, “We tend to want to close a cleft, and, yes, that will get you relief of regurgitation in the central portion, but if you end up with immobility of that leaflet, then look underneath. Most often there are very abnormal attachments to the edges of that cleft to the septum. You have to get rid of that; if you don’t resect all that, you’ll never have a leaflet that truly floats up to coapt against the posterior leaflet.”
Annular dilation in children can also challenge a cardiothoracic surgeon’s skill.
In rare cases, a suture commissuroplasty may correct the problem. Sometimes Dr. del Nido will use the DeVega suture annuloplasty – “even though it is very much user dependent; it’s very easy in pediatrics to create stenosis with the DeVega.” As an alternative, synthetic ring annuloplasties can confine valve growth and are rarely used.
Dr. del Nido’s preference is to use a hybrid approach of tissue and resorbable material. “The advantage of the resorbable material is that it will go away, but that’s also the problem with the resorbable material,” he said. “Once it does go away, there’s nothing there to support the annulus, so a combination of tissue and resorbable suture is probably the best answer.”
In posterior leaflet deficiency, a patch of pericardium posteriorly can augment the dysfunctional leaflet. You can also use pericardium as an annuloplasty ring. “You can use it circumferentially,” Dr. del Nido said. “It’s a soft ring; you can certainly use this material which is autologous; it does provide strength to the fibrous annulus; it does support that valve; and you do see growth.” He added that bovine pericardium is not ideal for this use.
Dr. del Nido reported no relevant financial relationships.
NEW YORK – Repairing mitral valves in pediatric patients must overcome two issues: the wide variability in their anatomy and their growth. Using strategies and techniques common in adult mitral surgery can accomplish good mitral valve function in children, but some techniques in children differ, like using combined resorbable material with autologous tissue or transferring native chords instead of placing artificial chords to a malfunctioning leaflet.
Pedro del Nido, MD, of Boston Children’s Hospital, said the spectrum of mitral valve pathology in children goes from congenital mitral stenosis with a thick annulus with leaflet immobility to leaflet hypermobility that involves anterior leaflet prolapse and can involve a cleft that causes regurgitation. Dr. del Nido explained his surgical approaches for mitral valve disease in children at the 2017 Mitral Conclave, sponsored by the American Association of Thoracic Surgery.
Accessing the mitral valve in children requires a different approach than in adults, Dr. del Nido said. “Going through the left atrium is generally difficult, so we often enter through a trans-septal incision,” he said. “The main reason for that is because the tricuspid valve is often associated with the mitral valve problem and this gives us the most direct exposure.”
Once the surgeon gains exposure, the surgical analysis for a diseased adult or child valve is almost identical, with the exception that adult disease is acquired whereas childhood disease tends to be congenital, Dr. del Nido said. “In the congenital patient, we often find fibroelastic tissue that the child is born with,” he said. “We see this in neonates and young infants. It thickens over time; it doesn’t often calcify, but it does often restrict the leaflets and it tends to fuse the chords, so in essence you have direct attachments of the leaflets to the papillaries.”
He explained that this pathology requires an approach similar to that for rheumatic mitral disease in adults. “Start splitting the commissures and start resecting the tissue off the chords creating fenestrations in order to improve the inflow.” Dr. del Nido added, “If you don’t do this, the child will always have a gradient, and if you think about an adult having problems and symptoms with a gradient, think about a 10-year-old running around trying to do athletics; it’s impossible.”
Dysfunctional chords also require a somewhat different approach in children than they require in adults. “We find elongation of the chords and the anterior support structure is abnormal; the secondary chords are totally intact,” Dr. del Nido said. When confronting a torn-edge chord, resection is often an option in adults, but is uncommon in children. “We don’t usually have very much leaflet tissue,” he said. Artificial chords do not accommodate growth.
“We tend to use native tissue,” said Dr. del Nido. “You can transfer the strut chord; you can transfer the secondary chord in order to achieve support for the edge of that prolapsed leaflet.”
Leaflet problems are probably the biggest single source of recurrence in children, Dr. del Nido said. A cleft on the anterior leaflet can be particularly vexing. For example, cleft edges attached to the septum can prevent the valve leaflet from coaptation with the posterior leaflet. “If you don’t recognize that on 3-D echocardiography, you’re going to have a problem; that leaflet will never create the coaptation surface that you want,” he said.
The solution may lie underneath the leaflet. Said Dr. del Nido, “We tend to want to close a cleft, and, yes, that will get you relief of regurgitation in the central portion, but if you end up with immobility of that leaflet, then look underneath. Most often there are very abnormal attachments to the edges of that cleft to the septum. You have to get rid of that; if you don’t resect all that, you’ll never have a leaflet that truly floats up to coapt against the posterior leaflet.”
Annular dilation in children can also challenge a cardiothoracic surgeon’s skill.
In rare cases, a suture commissuroplasty may correct the problem. Sometimes Dr. del Nido will use the DeVega suture annuloplasty – “even though it is very much user dependent; it’s very easy in pediatrics to create stenosis with the DeVega.” As an alternative, synthetic ring annuloplasties can confine valve growth and are rarely used.
Dr. del Nido’s preference is to use a hybrid approach of tissue and resorbable material. “The advantage of the resorbable material is that it will go away, but that’s also the problem with the resorbable material,” he said. “Once it does go away, there’s nothing there to support the annulus, so a combination of tissue and resorbable suture is probably the best answer.”
In posterior leaflet deficiency, a patch of pericardium posteriorly can augment the dysfunctional leaflet. You can also use pericardium as an annuloplasty ring. “You can use it circumferentially,” Dr. del Nido said. “It’s a soft ring; you can certainly use this material which is autologous; it does provide strength to the fibrous annulus; it does support that valve; and you do see growth.” He added that bovine pericardium is not ideal for this use.
Dr. del Nido reported no relevant financial relationships.
NEW YORK – Repairing mitral valves in pediatric patients must overcome two issues: the wide variability in their anatomy and their growth. Using strategies and techniques common in adult mitral surgery can accomplish good mitral valve function in children, but some techniques in children differ, like using combined resorbable material with autologous tissue or transferring native chords instead of placing artificial chords to a malfunctioning leaflet.
Pedro del Nido, MD, of Boston Children’s Hospital, said the spectrum of mitral valve pathology in children goes from congenital mitral stenosis with a thick annulus with leaflet immobility to leaflet hypermobility that involves anterior leaflet prolapse and can involve a cleft that causes regurgitation. Dr. del Nido explained his surgical approaches for mitral valve disease in children at the 2017 Mitral Conclave, sponsored by the American Association of Thoracic Surgery.
Accessing the mitral valve in children requires a different approach than in adults, Dr. del Nido said. “Going through the left atrium is generally difficult, so we often enter through a trans-septal incision,” he said. “The main reason for that is because the tricuspid valve is often associated with the mitral valve problem and this gives us the most direct exposure.”
Once the surgeon gains exposure, the surgical analysis for a diseased adult or child valve is almost identical, with the exception that adult disease is acquired whereas childhood disease tends to be congenital, Dr. del Nido said. “In the congenital patient, we often find fibroelastic tissue that the child is born with,” he said. “We see this in neonates and young infants. It thickens over time; it doesn’t often calcify, but it does often restrict the leaflets and it tends to fuse the chords, so in essence you have direct attachments of the leaflets to the papillaries.”
He explained that this pathology requires an approach similar to that for rheumatic mitral disease in adults. “Start splitting the commissures and start resecting the tissue off the chords creating fenestrations in order to improve the inflow.” Dr. del Nido added, “If you don’t do this, the child will always have a gradient, and if you think about an adult having problems and symptoms with a gradient, think about a 10-year-old running around trying to do athletics; it’s impossible.”
Dysfunctional chords also require a somewhat different approach in children than they require in adults. “We find elongation of the chords and the anterior support structure is abnormal; the secondary chords are totally intact,” Dr. del Nido said. When confronting a torn-edge chord, resection is often an option in adults, but is uncommon in children. “We don’t usually have very much leaflet tissue,” he said. Artificial chords do not accommodate growth.
“We tend to use native tissue,” said Dr. del Nido. “You can transfer the strut chord; you can transfer the secondary chord in order to achieve support for the edge of that prolapsed leaflet.”
Leaflet problems are probably the biggest single source of recurrence in children, Dr. del Nido said. A cleft on the anterior leaflet can be particularly vexing. For example, cleft edges attached to the septum can prevent the valve leaflet from coaptation with the posterior leaflet. “If you don’t recognize that on 3-D echocardiography, you’re going to have a problem; that leaflet will never create the coaptation surface that you want,” he said.
The solution may lie underneath the leaflet. Said Dr. del Nido, “We tend to want to close a cleft, and, yes, that will get you relief of regurgitation in the central portion, but if you end up with immobility of that leaflet, then look underneath. Most often there are very abnormal attachments to the edges of that cleft to the septum. You have to get rid of that; if you don’t resect all that, you’ll never have a leaflet that truly floats up to coapt against the posterior leaflet.”
Annular dilation in children can also challenge a cardiothoracic surgeon’s skill.
In rare cases, a suture commissuroplasty may correct the problem. Sometimes Dr. del Nido will use the DeVega suture annuloplasty – “even though it is very much user dependent; it’s very easy in pediatrics to create stenosis with the DeVega.” As an alternative, synthetic ring annuloplasties can confine valve growth and are rarely used.
Dr. del Nido’s preference is to use a hybrid approach of tissue and resorbable material. “The advantage of the resorbable material is that it will go away, but that’s also the problem with the resorbable material,” he said. “Once it does go away, there’s nothing there to support the annulus, so a combination of tissue and resorbable suture is probably the best answer.”
In posterior leaflet deficiency, a patch of pericardium posteriorly can augment the dysfunctional leaflet. You can also use pericardium as an annuloplasty ring. “You can use it circumferentially,” Dr. del Nido said. “It’s a soft ring; you can certainly use this material which is autologous; it does provide strength to the fibrous annulus; it does support that valve; and you do see growth.” He added that bovine pericardium is not ideal for this use.
Dr. del Nido reported no relevant financial relationships.
AT THE 2017 MITRAL VALVE CONCLAVE
Key clinical point: Operating on mitral valves in pediatric patients must overcome the wide variability in anatomy among young patients and accommodate growth.
Major finding: Strategies that involve a combination of resorbable material with autologous tissue can accomplish repair in most of these patients.
Data source: Review based on Dr. del Nido’s experience.
Disclosures: Dr. del Nido reported having no relevant financial disclosures.
Age, anticoagulation prime issues in choice of mechanical valves
NEW YORK – While bioprosthetic valves have become the predominant choice for cardiothoracic surgeons performing heart valve replacement, situations exist in which a mechanical valve may be a better choice. Young and middle-aged adults are the ideal candidates for mechanical valves, but achieving long-term success with mechanical valves also depends on a patient’s circumstances, according to an expert panel at the American Association for Thoracic Surgery Mitral Conclave 2017 here.
“I think we should put in more mechanical valves,” said panel chair Thoralf M. Sundt III, MD, of Massachusetts General Hospital, Boston and former AATS president. “I think mechanical valves have gotten a bad rap. If patients have a supportive, stable social structure and they can manage their anticoagulation, I don’t think there’s anything wrong with a mechanical valve. I think the pendulum has swung too far.”
Anelechi C. Anyanwu, MD, of Mount Sinai Health System, New York, acknowledged another patient factor that would enter his calculus for recommending a mechanical mitral valve. “We would consider a mechanical valve in the patient who is compliant [and] well informed and understands well the requirements and implications of long-term anticoagulation,” he said. In another scenario – “the patient who’s had multiple reoperations – we may consider a mechanical valve.” Particularly, the patient who has had a reoperation for a bioprosthetic valve resulting from early degeneration will merit consideration of a mechanical prosthesis, Dr. Anyanwu said.
Michael A. Borger, MD, of Columbia University Medical Center, New York, agreed that the younger patient who has had a series of operations is a good candidate for a mechanical mitral valve. “In addition, the mechanical valve does have some hemodynamic advantages over bioprosthetic valves,” Dr. Borger said. “If a surgeon implants a 25-mm tissue valve with a plan for the patient to undergo a series of valve-in-valve operations in the future, mitral stenosis will definitely become a factor at some point.”
Pedro J. del Nido, MD, a pediatric cardiac surgeon at Children’s Hospital, Boston, expanded on that point. “We very, very rarely use mechanical valves in young patients,” Dr. del Nido said. “In very young patients, the reoperation rates for mechanical or biological valves are not much different. We still have to reoperate on both sets of patients. But, the reoperation itself for a mechanical valve is more difficult, and there is the need for full anticoagulation.”
Instead, Dr. del Nido has used the bovine Melody valve (Medtronic) in the mitral position for these patients because it accommodates some growth. Typically, the only time Dr. del Nido considers a mechanical valve in these young patients is for an aortic valve replacement.
Another younger patient that may be a good candidate for a mechanical valve is the 25-year-old male with rheumatic mitral stenosis. “I would err on the side of the mechanical valve in this patient if I were to make a choice, but I would present the patient with informed data on the outcomes of both mechanical valve and bioprosthesis,” according to Dr. Anyanwu
For even younger patients, Dr. del Nido bases his valve choice on their activity level. “I have a lot of young teenagers, and my decision is entirely based on what their background is like – what their regular life is like,” he said. “If they have a support structure than can help them manage anticoagulation, absolutely the mechanical valve is probably the best thing.”
However, there are exceptions because the couching of the device can change over time. “Eventually that 12-year-old [or] that 15-year-old is going to decide he can still snowboard or ride a motorcycle, and that’s when he’s going to get into trouble,” Dr. del Nido said. “If a reoperation is not problematic, I would still say it’s a tossup between the mechanical and biological valve. I would still offer the possibility of a bioprosthesis knowing that they’ll be back in 6, 8, or 10 years.”
Bleeding risk is another factor that can influence valve choice, as mechanical valve recipients must stay on anticoagulation. “The bleeding complication rates are very low when patients are younger, in their 40s or 50s, but the bleeding rates increase exponentially with warfarin for patients in their 80s and 90s,” Dr. Borger said. “The older you are, the more difficult it is to manage anticoagulation. In addition, the ability to stop anticoagulants because of bleeding is different for the two types of prostheses. If a patient develops bleeding during anticoagulation for leaflet immobility after a tissue valve-in-valve procedure, the physician can simply stop the warfarin. But, if you have a mechanical mitral valve in place that’s not an option.”
That’s not necessarily a bad problem to have, Dr. Sundt said, quoting Steven Bolling, MD, of the University of Michigan. “If you’re 25 and you’ve had your valve replaced and you’re worried about bleeding at the age of 80, I call that a success.”
Dr. Borger disclosed he is a speaker for and consultant to Edwards Lifesciences, Medtronic, and CryoLife; a speaker for St. Jude; and a recipient of research support from NeoChord. Dr. Lange disclosed he is a consultant and speaker for Medtronic, St. Jude/Abbott, LivaNova and NeoChord and cofounder of HighLife. Dr. Anyanwu, Dr. del Nido, and Dr. Sundt reported no relevant financial relationships.
NEW YORK – While bioprosthetic valves have become the predominant choice for cardiothoracic surgeons performing heart valve replacement, situations exist in which a mechanical valve may be a better choice. Young and middle-aged adults are the ideal candidates for mechanical valves, but achieving long-term success with mechanical valves also depends on a patient’s circumstances, according to an expert panel at the American Association for Thoracic Surgery Mitral Conclave 2017 here.
“I think we should put in more mechanical valves,” said panel chair Thoralf M. Sundt III, MD, of Massachusetts General Hospital, Boston and former AATS president. “I think mechanical valves have gotten a bad rap. If patients have a supportive, stable social structure and they can manage their anticoagulation, I don’t think there’s anything wrong with a mechanical valve. I think the pendulum has swung too far.”
Anelechi C. Anyanwu, MD, of Mount Sinai Health System, New York, acknowledged another patient factor that would enter his calculus for recommending a mechanical mitral valve. “We would consider a mechanical valve in the patient who is compliant [and] well informed and understands well the requirements and implications of long-term anticoagulation,” he said. In another scenario – “the patient who’s had multiple reoperations – we may consider a mechanical valve.” Particularly, the patient who has had a reoperation for a bioprosthetic valve resulting from early degeneration will merit consideration of a mechanical prosthesis, Dr. Anyanwu said.
Michael A. Borger, MD, of Columbia University Medical Center, New York, agreed that the younger patient who has had a series of operations is a good candidate for a mechanical mitral valve. “In addition, the mechanical valve does have some hemodynamic advantages over bioprosthetic valves,” Dr. Borger said. “If a surgeon implants a 25-mm tissue valve with a plan for the patient to undergo a series of valve-in-valve operations in the future, mitral stenosis will definitely become a factor at some point.”
Pedro J. del Nido, MD, a pediatric cardiac surgeon at Children’s Hospital, Boston, expanded on that point. “We very, very rarely use mechanical valves in young patients,” Dr. del Nido said. “In very young patients, the reoperation rates for mechanical or biological valves are not much different. We still have to reoperate on both sets of patients. But, the reoperation itself for a mechanical valve is more difficult, and there is the need for full anticoagulation.”
Instead, Dr. del Nido has used the bovine Melody valve (Medtronic) in the mitral position for these patients because it accommodates some growth. Typically, the only time Dr. del Nido considers a mechanical valve in these young patients is for an aortic valve replacement.
Another younger patient that may be a good candidate for a mechanical valve is the 25-year-old male with rheumatic mitral stenosis. “I would err on the side of the mechanical valve in this patient if I were to make a choice, but I would present the patient with informed data on the outcomes of both mechanical valve and bioprosthesis,” according to Dr. Anyanwu
For even younger patients, Dr. del Nido bases his valve choice on their activity level. “I have a lot of young teenagers, and my decision is entirely based on what their background is like – what their regular life is like,” he said. “If they have a support structure than can help them manage anticoagulation, absolutely the mechanical valve is probably the best thing.”
However, there are exceptions because the couching of the device can change over time. “Eventually that 12-year-old [or] that 15-year-old is going to decide he can still snowboard or ride a motorcycle, and that’s when he’s going to get into trouble,” Dr. del Nido said. “If a reoperation is not problematic, I would still say it’s a tossup between the mechanical and biological valve. I would still offer the possibility of a bioprosthesis knowing that they’ll be back in 6, 8, or 10 years.”
Bleeding risk is another factor that can influence valve choice, as mechanical valve recipients must stay on anticoagulation. “The bleeding complication rates are very low when patients are younger, in their 40s or 50s, but the bleeding rates increase exponentially with warfarin for patients in their 80s and 90s,” Dr. Borger said. “The older you are, the more difficult it is to manage anticoagulation. In addition, the ability to stop anticoagulants because of bleeding is different for the two types of prostheses. If a patient develops bleeding during anticoagulation for leaflet immobility after a tissue valve-in-valve procedure, the physician can simply stop the warfarin. But, if you have a mechanical mitral valve in place that’s not an option.”
That’s not necessarily a bad problem to have, Dr. Sundt said, quoting Steven Bolling, MD, of the University of Michigan. “If you’re 25 and you’ve had your valve replaced and you’re worried about bleeding at the age of 80, I call that a success.”
Dr. Borger disclosed he is a speaker for and consultant to Edwards Lifesciences, Medtronic, and CryoLife; a speaker for St. Jude; and a recipient of research support from NeoChord. Dr. Lange disclosed he is a consultant and speaker for Medtronic, St. Jude/Abbott, LivaNova and NeoChord and cofounder of HighLife. Dr. Anyanwu, Dr. del Nido, and Dr. Sundt reported no relevant financial relationships.
NEW YORK – While bioprosthetic valves have become the predominant choice for cardiothoracic surgeons performing heart valve replacement, situations exist in which a mechanical valve may be a better choice. Young and middle-aged adults are the ideal candidates for mechanical valves, but achieving long-term success with mechanical valves also depends on a patient’s circumstances, according to an expert panel at the American Association for Thoracic Surgery Mitral Conclave 2017 here.
“I think we should put in more mechanical valves,” said panel chair Thoralf M. Sundt III, MD, of Massachusetts General Hospital, Boston and former AATS president. “I think mechanical valves have gotten a bad rap. If patients have a supportive, stable social structure and they can manage their anticoagulation, I don’t think there’s anything wrong with a mechanical valve. I think the pendulum has swung too far.”
Anelechi C. Anyanwu, MD, of Mount Sinai Health System, New York, acknowledged another patient factor that would enter his calculus for recommending a mechanical mitral valve. “We would consider a mechanical valve in the patient who is compliant [and] well informed and understands well the requirements and implications of long-term anticoagulation,” he said. In another scenario – “the patient who’s had multiple reoperations – we may consider a mechanical valve.” Particularly, the patient who has had a reoperation for a bioprosthetic valve resulting from early degeneration will merit consideration of a mechanical prosthesis, Dr. Anyanwu said.
Michael A. Borger, MD, of Columbia University Medical Center, New York, agreed that the younger patient who has had a series of operations is a good candidate for a mechanical mitral valve. “In addition, the mechanical valve does have some hemodynamic advantages over bioprosthetic valves,” Dr. Borger said. “If a surgeon implants a 25-mm tissue valve with a plan for the patient to undergo a series of valve-in-valve operations in the future, mitral stenosis will definitely become a factor at some point.”
Pedro J. del Nido, MD, a pediatric cardiac surgeon at Children’s Hospital, Boston, expanded on that point. “We very, very rarely use mechanical valves in young patients,” Dr. del Nido said. “In very young patients, the reoperation rates for mechanical or biological valves are not much different. We still have to reoperate on both sets of patients. But, the reoperation itself for a mechanical valve is more difficult, and there is the need for full anticoagulation.”
Instead, Dr. del Nido has used the bovine Melody valve (Medtronic) in the mitral position for these patients because it accommodates some growth. Typically, the only time Dr. del Nido considers a mechanical valve in these young patients is for an aortic valve replacement.
Another younger patient that may be a good candidate for a mechanical valve is the 25-year-old male with rheumatic mitral stenosis. “I would err on the side of the mechanical valve in this patient if I were to make a choice, but I would present the patient with informed data on the outcomes of both mechanical valve and bioprosthesis,” according to Dr. Anyanwu
For even younger patients, Dr. del Nido bases his valve choice on their activity level. “I have a lot of young teenagers, and my decision is entirely based on what their background is like – what their regular life is like,” he said. “If they have a support structure than can help them manage anticoagulation, absolutely the mechanical valve is probably the best thing.”
However, there are exceptions because the couching of the device can change over time. “Eventually that 12-year-old [or] that 15-year-old is going to decide he can still snowboard or ride a motorcycle, and that’s when he’s going to get into trouble,” Dr. del Nido said. “If a reoperation is not problematic, I would still say it’s a tossup between the mechanical and biological valve. I would still offer the possibility of a bioprosthesis knowing that they’ll be back in 6, 8, or 10 years.”
Bleeding risk is another factor that can influence valve choice, as mechanical valve recipients must stay on anticoagulation. “The bleeding complication rates are very low when patients are younger, in their 40s or 50s, but the bleeding rates increase exponentially with warfarin for patients in their 80s and 90s,” Dr. Borger said. “The older you are, the more difficult it is to manage anticoagulation. In addition, the ability to stop anticoagulants because of bleeding is different for the two types of prostheses. If a patient develops bleeding during anticoagulation for leaflet immobility after a tissue valve-in-valve procedure, the physician can simply stop the warfarin. But, if you have a mechanical mitral valve in place that’s not an option.”
That’s not necessarily a bad problem to have, Dr. Sundt said, quoting Steven Bolling, MD, of the University of Michigan. “If you’re 25 and you’ve had your valve replaced and you’re worried about bleeding at the age of 80, I call that a success.”
Dr. Borger disclosed he is a speaker for and consultant to Edwards Lifesciences, Medtronic, and CryoLife; a speaker for St. Jude; and a recipient of research support from NeoChord. Dr. Lange disclosed he is a consultant and speaker for Medtronic, St. Jude/Abbott, LivaNova and NeoChord and cofounder of HighLife. Dr. Anyanwu, Dr. del Nido, and Dr. Sundt reported no relevant financial relationships.
EXPERT ANALYSIS FROM THE 2017 MITRAL VALVE CONCLAVE
Shift to minimally invasive MV surgery picks up
NEW YORK – An analysis of a Society of Thoracic Surgeons database has identified a significant increase in volumes for isolated mitral valve surgery and leaflet prolapse this decade, with a shift toward minimally invasive approaches, according to a study of trends in mitral valve surgery in the United States presented here at the American Association for Thoracic Surgery Mitral Conclave 2017.
James Gammie, MD, of the University of Maryland School of Medicine, Baltimore, reported on the analysis of the STS Adult Cardiac Surgery Database in which more than 90% of the adult cardiac surgery centers in North America participate.
“Degenerative disease remains the most common reason patients are referred for surgery,” Dr. Gammie said, noting that 60.7% of patients with an identified etiology had degenerative leaflet prolapse (etiology was unknown in 31% of the patients in the dataset).
“The operative approach has changed and continues to shift toward a less invasive approach,” Dr. Gammie said. Overall, 74.1% of operations involved sternotomy, but only 67.5% of those in the leaflet prolapse subgroup, with less invasive operations comprising 23% of all operations and 29.1% of those in the leaflet prolapse subgroup. From 2011 to 2016, total mitral surgical volume grew at a rate of 1.1% annually, but the volume for isolated MV operations grew 4.4% annually while leaflet prolapse procedures increased 7.6% annually, Dr. Gammie said.
Dr. Gammie described surgeons’ decision to perform ablation for preoperative AF during MV surgery a “coin toss.” One-third (34%) had AF, but only 51.2% of patients with AF in the total cohort and 54.4% of those in the leaflet prolapse subgroup got ablation. The overall MV repair rate was 65% for the total cohort but 83% for those with leaflet prolapse. For those who had MV replacement, the share of bioprosthetic devices increased steadily through the study period, from around 65% to 75.8%, Dr. Gammie said.
In the leaflet prolapse subgroup, 96.1% had annuloplasty and 29.2% had artificial chords implanted, with an average of two chords per operation. “There’s an increasing use of artificial ePTFE chords,” Dr. Gammie said. He noted the leaflet prolapse subgroup was composed of low-risk healthy patients. The mean ejection fraction (EF) for the cohort was 57%, and 47% of patients had EF of less than 60%. The overwhelming majority of patients (88%) had Class I indications for surgery with the remainder having Class IIa indications.
Dr. Gammie noted a few other emerging trends of MV surgery during the study period. “Patients with functional mitral regurgitation are rarely referred for operation: these patients made up fewer than 5% of patients undergoing mitral valve operations during the study period,” he said.;
With regard to outcomes, Dr. Gammie said, “There remain substantial differences between repair and replacement.” Replacement had almost twice the rate of reoperation for bleeding than repair (4.1% vs. 2.1%) and renal failure (3.4% vs. 1.4%).
“We observed that a substantial number of patients have an unsuccessful attempt at mitral valve repair before undergoing replacement – 16 % of the overall replacement group and 27% of patients having replacement for degenerative leaflet prolapse,” he said. “This does not appear to penalize patients in terms of outcome.”
The rate of permanent pacemaker was also significantly higher in the replacement cohort, 9.8% vs. 3.8% for repair operations, as was operative mortality, 3.7% vs. 1.1%. Said Dr. Gammie.
“This is something to think about as we move to less invasive approaches and overall operative mortality remains over threefold higher for replacement than repair.”
The leaflet prolapse subgroup showed similar disparities between replacement and repair groups. “The increased application of repair when feasible will be of value to improve outcomes, as will referral of patients earlier in the disease process ,” Dr. Gammie said.
“In our own STS database, about one-third of patients who were coded as having AF had no evidence of it, and that’s why in our institution they don’t get treated.” He added, “I’m not that surprised at the low repair rate when the average surgeon in the United States does five mitral repairs a year.”
Dr. Gammie disclosed that he is a consultant to Edwards Lifesciences and has an ownership interest in Harpoon Medical. Dr. Damiano disclosed that he is a speaker for LivaNova and a consultant to and a research grant recipient of Atricure.
NEW YORK – An analysis of a Society of Thoracic Surgeons database has identified a significant increase in volumes for isolated mitral valve surgery and leaflet prolapse this decade, with a shift toward minimally invasive approaches, according to a study of trends in mitral valve surgery in the United States presented here at the American Association for Thoracic Surgery Mitral Conclave 2017.
James Gammie, MD, of the University of Maryland School of Medicine, Baltimore, reported on the analysis of the STS Adult Cardiac Surgery Database in which more than 90% of the adult cardiac surgery centers in North America participate.
“Degenerative disease remains the most common reason patients are referred for surgery,” Dr. Gammie said, noting that 60.7% of patients with an identified etiology had degenerative leaflet prolapse (etiology was unknown in 31% of the patients in the dataset).
“The operative approach has changed and continues to shift toward a less invasive approach,” Dr. Gammie said. Overall, 74.1% of operations involved sternotomy, but only 67.5% of those in the leaflet prolapse subgroup, with less invasive operations comprising 23% of all operations and 29.1% of those in the leaflet prolapse subgroup. From 2011 to 2016, total mitral surgical volume grew at a rate of 1.1% annually, but the volume for isolated MV operations grew 4.4% annually while leaflet prolapse procedures increased 7.6% annually, Dr. Gammie said.
Dr. Gammie described surgeons’ decision to perform ablation for preoperative AF during MV surgery a “coin toss.” One-third (34%) had AF, but only 51.2% of patients with AF in the total cohort and 54.4% of those in the leaflet prolapse subgroup got ablation. The overall MV repair rate was 65% for the total cohort but 83% for those with leaflet prolapse. For those who had MV replacement, the share of bioprosthetic devices increased steadily through the study period, from around 65% to 75.8%, Dr. Gammie said.
In the leaflet prolapse subgroup, 96.1% had annuloplasty and 29.2% had artificial chords implanted, with an average of two chords per operation. “There’s an increasing use of artificial ePTFE chords,” Dr. Gammie said. He noted the leaflet prolapse subgroup was composed of low-risk healthy patients. The mean ejection fraction (EF) for the cohort was 57%, and 47% of patients had EF of less than 60%. The overwhelming majority of patients (88%) had Class I indications for surgery with the remainder having Class IIa indications.
Dr. Gammie noted a few other emerging trends of MV surgery during the study period. “Patients with functional mitral regurgitation are rarely referred for operation: these patients made up fewer than 5% of patients undergoing mitral valve operations during the study period,” he said.;
With regard to outcomes, Dr. Gammie said, “There remain substantial differences between repair and replacement.” Replacement had almost twice the rate of reoperation for bleeding than repair (4.1% vs. 2.1%) and renal failure (3.4% vs. 1.4%).
“We observed that a substantial number of patients have an unsuccessful attempt at mitral valve repair before undergoing replacement – 16 % of the overall replacement group and 27% of patients having replacement for degenerative leaflet prolapse,” he said. “This does not appear to penalize patients in terms of outcome.”
The rate of permanent pacemaker was also significantly higher in the replacement cohort, 9.8% vs. 3.8% for repair operations, as was operative mortality, 3.7% vs. 1.1%. Said Dr. Gammie.
“This is something to think about as we move to less invasive approaches and overall operative mortality remains over threefold higher for replacement than repair.”
The leaflet prolapse subgroup showed similar disparities between replacement and repair groups. “The increased application of repair when feasible will be of value to improve outcomes, as will referral of patients earlier in the disease process ,” Dr. Gammie said.
“In our own STS database, about one-third of patients who were coded as having AF had no evidence of it, and that’s why in our institution they don’t get treated.” He added, “I’m not that surprised at the low repair rate when the average surgeon in the United States does five mitral repairs a year.”
Dr. Gammie disclosed that he is a consultant to Edwards Lifesciences and has an ownership interest in Harpoon Medical. Dr. Damiano disclosed that he is a speaker for LivaNova and a consultant to and a research grant recipient of Atricure.
NEW YORK – An analysis of a Society of Thoracic Surgeons database has identified a significant increase in volumes for isolated mitral valve surgery and leaflet prolapse this decade, with a shift toward minimally invasive approaches, according to a study of trends in mitral valve surgery in the United States presented here at the American Association for Thoracic Surgery Mitral Conclave 2017.
James Gammie, MD, of the University of Maryland School of Medicine, Baltimore, reported on the analysis of the STS Adult Cardiac Surgery Database in which more than 90% of the adult cardiac surgery centers in North America participate.
“Degenerative disease remains the most common reason patients are referred for surgery,” Dr. Gammie said, noting that 60.7% of patients with an identified etiology had degenerative leaflet prolapse (etiology was unknown in 31% of the patients in the dataset).
“The operative approach has changed and continues to shift toward a less invasive approach,” Dr. Gammie said. Overall, 74.1% of operations involved sternotomy, but only 67.5% of those in the leaflet prolapse subgroup, with less invasive operations comprising 23% of all operations and 29.1% of those in the leaflet prolapse subgroup. From 2011 to 2016, total mitral surgical volume grew at a rate of 1.1% annually, but the volume for isolated MV operations grew 4.4% annually while leaflet prolapse procedures increased 7.6% annually, Dr. Gammie said.
Dr. Gammie described surgeons’ decision to perform ablation for preoperative AF during MV surgery a “coin toss.” One-third (34%) had AF, but only 51.2% of patients with AF in the total cohort and 54.4% of those in the leaflet prolapse subgroup got ablation. The overall MV repair rate was 65% for the total cohort but 83% for those with leaflet prolapse. For those who had MV replacement, the share of bioprosthetic devices increased steadily through the study period, from around 65% to 75.8%, Dr. Gammie said.
In the leaflet prolapse subgroup, 96.1% had annuloplasty and 29.2% had artificial chords implanted, with an average of two chords per operation. “There’s an increasing use of artificial ePTFE chords,” Dr. Gammie said. He noted the leaflet prolapse subgroup was composed of low-risk healthy patients. The mean ejection fraction (EF) for the cohort was 57%, and 47% of patients had EF of less than 60%. The overwhelming majority of patients (88%) had Class I indications for surgery with the remainder having Class IIa indications.
Dr. Gammie noted a few other emerging trends of MV surgery during the study period. “Patients with functional mitral regurgitation are rarely referred for operation: these patients made up fewer than 5% of patients undergoing mitral valve operations during the study period,” he said.;
With regard to outcomes, Dr. Gammie said, “There remain substantial differences between repair and replacement.” Replacement had almost twice the rate of reoperation for bleeding than repair (4.1% vs. 2.1%) and renal failure (3.4% vs. 1.4%).
“We observed that a substantial number of patients have an unsuccessful attempt at mitral valve repair before undergoing replacement – 16 % of the overall replacement group and 27% of patients having replacement for degenerative leaflet prolapse,” he said. “This does not appear to penalize patients in terms of outcome.”
The rate of permanent pacemaker was also significantly higher in the replacement cohort, 9.8% vs. 3.8% for repair operations, as was operative mortality, 3.7% vs. 1.1%. Said Dr. Gammie.
“This is something to think about as we move to less invasive approaches and overall operative mortality remains over threefold higher for replacement than repair.”
The leaflet prolapse subgroup showed similar disparities between replacement and repair groups. “The increased application of repair when feasible will be of value to improve outcomes, as will referral of patients earlier in the disease process ,” Dr. Gammie said.
“In our own STS database, about one-third of patients who were coded as having AF had no evidence of it, and that’s why in our institution they don’t get treated.” He added, “I’m not that surprised at the low repair rate when the average surgeon in the United States does five mitral repairs a year.”
Dr. Gammie disclosed that he is a consultant to Edwards Lifesciences and has an ownership interest in Harpoon Medical. Dr. Damiano disclosed that he is a speaker for LivaNova and a consultant to and a research grant recipient of Atricure.
AT THE AATS MITRAL CONCLAVE 2017
Key clinical point: The volume of mitral valve surgery has increased substantially, according to an analysis of the Society for Thoracic Surgery database, and an increasing percentage of procedures are minimally invasive in nature.
Major finding: Sternotomy continues to be the most widely used approach for mitral valve surgery, but less invasive options most recently comprised 23% of the overall group and 29.1% of those with isolated leaflet prolapse.
Data source: Retrospective study of 15,360 isolated mitral valve operations performed from July 2011 to September 2016 in the Society of Thoracic Surgeons database.
Disclosures: Dr. Gammie reported being a consultant to Edwards Lifesciences and having an ownership interest in Harpoon Medical.
In 8 years, TMVR means more procedures
NEW YORK – Since the commercialization of transcatheter mitral valve repair, the share of these procedures among all mitral operations has grown exponentially and has also contributed to an increase in the number of overall mitral procedures, including surgical repair, according to an analysis of a national German database reported at the American Association for Thoracic Surgery Mitral Conclave here.
Transcatheter mitral valve repair (TMVR) using the MitraClip device (Abbott Vascular) was first commercialized in Germany in 2008, and in the years since the share of TMVR procedures among all mitral operations increased from 0.3% to 18.1% in 2015 throughout Germany, said Lenard Conradi, MD, surgical director of minimally-invasive and transcatheter heart valve procedures at the University Heart Center Hamburg.
The goal of the study was to gain insights into how cardiothoracic surgeons in Germany and at Dr. Conradi’s center in particular were approaching TMVR and what types of patients were having the procedure, Dr. Conradi said.
“While the EuroSCORE I of patients we operated on didn’t change at all between these two time frames, there were subtle changes in the patients that we operated on,” he said. “Before the commercialization of TMVR, we tended to have a ratio of organic vs. functional MR [mitral regurgitation] of 50-50; after commercialization of TMVR, many of the functional MR patients were allocated to TMVR.”
Dr. Conradi noted the profile of patients who had mitral valve repair also changed once the transcatheter approach became available. “Ischemic disease with coronary artery disease, previous infarction, or previous cardiac surgery – mostly coronary artery bypass grafting – were much less prevalent in this surgery population after TMVR became available,” he said.
The study also found that 30-day mortality declined from 7% to 4% during the study period. “That was probably due to more adequate patient selection because we had a more appropriate treatment that we could offer these high-risk patients as an alternative to high-risk surgical approaches,” Dr. Conradi said.
The analysis stratified procedure volumes and growth by four age groups: younger than 65 years; 65-74; 75-84; and greater than ore equal to 85. Older patients were more likely to have TMVR. Surgical procedure volumes increased most in the less than 65 group and least in the greater than or equal to 85 group.
In Germany, transcatheter mitral valve procedures are reimbursed at a higher rate than surgical procedures, but that doesn’t fully explain the uptake in TMVR, Dr. Conradi said. “The patients receiving the transcatheter approach vs. surgery still differ fundamentally, but the addition of an interventional program decreases the surgical patient’s mean risk profile and thus optimizes surgical results,” he said. “I think this can only happen if surgeons are closely involved. The indications will broaden for these therapies. There’s no doubt about that.”
Dr. Conradi disclosed receiving travel support and lecture fees from Abbott Vascular.
NEW YORK – Since the commercialization of transcatheter mitral valve repair, the share of these procedures among all mitral operations has grown exponentially and has also contributed to an increase in the number of overall mitral procedures, including surgical repair, according to an analysis of a national German database reported at the American Association for Thoracic Surgery Mitral Conclave here.
Transcatheter mitral valve repair (TMVR) using the MitraClip device (Abbott Vascular) was first commercialized in Germany in 2008, and in the years since the share of TMVR procedures among all mitral operations increased from 0.3% to 18.1% in 2015 throughout Germany, said Lenard Conradi, MD, surgical director of minimally-invasive and transcatheter heart valve procedures at the University Heart Center Hamburg.
The goal of the study was to gain insights into how cardiothoracic surgeons in Germany and at Dr. Conradi’s center in particular were approaching TMVR and what types of patients were having the procedure, Dr. Conradi said.
“While the EuroSCORE I of patients we operated on didn’t change at all between these two time frames, there were subtle changes in the patients that we operated on,” he said. “Before the commercialization of TMVR, we tended to have a ratio of organic vs. functional MR [mitral regurgitation] of 50-50; after commercialization of TMVR, many of the functional MR patients were allocated to TMVR.”
Dr. Conradi noted the profile of patients who had mitral valve repair also changed once the transcatheter approach became available. “Ischemic disease with coronary artery disease, previous infarction, or previous cardiac surgery – mostly coronary artery bypass grafting – were much less prevalent in this surgery population after TMVR became available,” he said.
The study also found that 30-day mortality declined from 7% to 4% during the study period. “That was probably due to more adequate patient selection because we had a more appropriate treatment that we could offer these high-risk patients as an alternative to high-risk surgical approaches,” Dr. Conradi said.
The analysis stratified procedure volumes and growth by four age groups: younger than 65 years; 65-74; 75-84; and greater than ore equal to 85. Older patients were more likely to have TMVR. Surgical procedure volumes increased most in the less than 65 group and least in the greater than or equal to 85 group.
In Germany, transcatheter mitral valve procedures are reimbursed at a higher rate than surgical procedures, but that doesn’t fully explain the uptake in TMVR, Dr. Conradi said. “The patients receiving the transcatheter approach vs. surgery still differ fundamentally, but the addition of an interventional program decreases the surgical patient’s mean risk profile and thus optimizes surgical results,” he said. “I think this can only happen if surgeons are closely involved. The indications will broaden for these therapies. There’s no doubt about that.”
Dr. Conradi disclosed receiving travel support and lecture fees from Abbott Vascular.
NEW YORK – Since the commercialization of transcatheter mitral valve repair, the share of these procedures among all mitral operations has grown exponentially and has also contributed to an increase in the number of overall mitral procedures, including surgical repair, according to an analysis of a national German database reported at the American Association for Thoracic Surgery Mitral Conclave here.
Transcatheter mitral valve repair (TMVR) using the MitraClip device (Abbott Vascular) was first commercialized in Germany in 2008, and in the years since the share of TMVR procedures among all mitral operations increased from 0.3% to 18.1% in 2015 throughout Germany, said Lenard Conradi, MD, surgical director of minimally-invasive and transcatheter heart valve procedures at the University Heart Center Hamburg.
The goal of the study was to gain insights into how cardiothoracic surgeons in Germany and at Dr. Conradi’s center in particular were approaching TMVR and what types of patients were having the procedure, Dr. Conradi said.
“While the EuroSCORE I of patients we operated on didn’t change at all between these two time frames, there were subtle changes in the patients that we operated on,” he said. “Before the commercialization of TMVR, we tended to have a ratio of organic vs. functional MR [mitral regurgitation] of 50-50; after commercialization of TMVR, many of the functional MR patients were allocated to TMVR.”
Dr. Conradi noted the profile of patients who had mitral valve repair also changed once the transcatheter approach became available. “Ischemic disease with coronary artery disease, previous infarction, or previous cardiac surgery – mostly coronary artery bypass grafting – were much less prevalent in this surgery population after TMVR became available,” he said.
The study also found that 30-day mortality declined from 7% to 4% during the study period. “That was probably due to more adequate patient selection because we had a more appropriate treatment that we could offer these high-risk patients as an alternative to high-risk surgical approaches,” Dr. Conradi said.
The analysis stratified procedure volumes and growth by four age groups: younger than 65 years; 65-74; 75-84; and greater than ore equal to 85. Older patients were more likely to have TMVR. Surgical procedure volumes increased most in the less than 65 group and least in the greater than or equal to 85 group.
In Germany, transcatheter mitral valve procedures are reimbursed at a higher rate than surgical procedures, but that doesn’t fully explain the uptake in TMVR, Dr. Conradi said. “The patients receiving the transcatheter approach vs. surgery still differ fundamentally, but the addition of an interventional program decreases the surgical patient’s mean risk profile and thus optimizes surgical results,” he said. “I think this can only happen if surgeons are closely involved. The indications will broaden for these therapies. There’s no doubt about that.”
Dr. Conradi disclosed receiving travel support and lecture fees from Abbott Vascular.
AT THE AATS MITRAL CONCLAVE 2017
Key clinical point: Since its commercialization, transcatheter mitral valve repair (TMVR) has accounted for an increasing share of all mitral surgeries in Germany and driven greater mitral surgery volume overall.
Major finding: Volume of surgical mitral valve procedures increased 70% overall and TMVR procedures 57% in Germany since 2008.
Data source: Analysis of data from German Federal statistics office.
Disclosures: Dr. Conradi reported receiving travel support and lecture fees from Abbott Vascular.
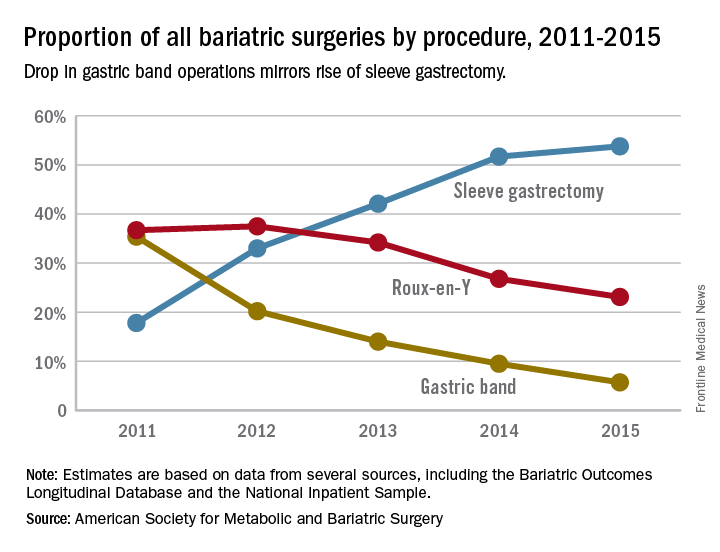
Gastric bands hit with high reoperation rates, rising costs
About one in five laparoscopic gastric band surgeries result in device-related reoperations and reoperations account for almost half of all Medicare expenditures for gastric band surgery, a large retrospective study has found.
The laparoscopic adjustable gastric band for treatment of morbid obesity, approved in 2001 by the Food and Drug Administration, was once a common choice for bariatric patients. Although its use has declined from in recent years, the American Society for Metabolic and Bariatric Surgery estimated that 11,000 bands were placed in 2015 and many others remain in place (ASMBS, Estimate of bariatric surgery numbers, 2011-2015, https://goo.gl/f8iByl). Many of these gastric bands will need to be removed, replaced, or revised in a series of procedures over the coming years.
Of the 24,042 gastric band patients in this study group, 4,636 (18.5%) underwent reoperation, defined as band removal, band replacement, or revision to a different bariatric procedure, but not including band size adjustment. Patients who had reoperations were more likely to be women, to be white, and to have slightly lower rates of hypertension and diabetes. But they were also more likely to have received a psychiatric, anemia, or electrolyte disorder diagnosis at the time of their index operations.
Among the 4,636 patients who had reoperations, 17,539 such procedures were performed, an average of 3.8 procedures per patient, in addition to the index operation, over an average follow-up of 4.5 years. The most common reoperation was for band removal (41.8%). Other reasons included conversion to laparoscopic Roux-en-Y gastric bypass (13.1%) or laparoscopic sleeve gastrectomy (5.3%).
The study also looked at the regional differences, reflecting the comparative success of some programs in managing laparoscopic gastric band placement. Reoperation rates across the referral hospitals ranged from 5% to 95.5%, The study found a nearly a threefold variation in reoperation rates across geographic regions. The bottom quartile of hospital referral regions had an average reoperation rate of 13.3% (0.3 standard deviation) and the top quartile had an average reoperation rate of 39.1% (0.21 SD). Top-quartile regions were concentrated in the West, but were otherwise distributed throughout the country.
Most reoperations were elective admissions (79.9%), while 10% were classified as urgent and another 10.1% as emergency. So although previous studies have documented complications such as band slippage and gastric erosion, the preponderance of elective admissions suggests patient and clinician preferences, or weight loss failure, rather than emergency situations, may be the driving force in the reoperation trend.
The investigators concluded that patients should be fully informed about the likelihood of reoperation with the gastric band. In addition, the wide range of reoperation rates across regions and institutions suggests that more training or better patient selection may be needed to improve outcomes. However, they suggested that “taken together, these findings indicate that the gastric band is associated with high reoperation rates and considerable costs to the payers, which raises concerns about its safety, effectiveness, and value.” They added that “payers should reconsider their coverage of the gastric band device.”
Coauthor Justin B. Dimick, MD, disclosed a financial interest in ArborMetrix. The other coauthors reported having no financial disclosures. The Robert Wood Johnson Foundation, U.S. Department of Veterans Affairs, National Institute on Aging, and National Institute of Diabetes and Digestive and Kidney Diseases provided funding.
Dr. Ibrahim and his colleagues have suggested that payers reconsider covering the adjustable laparoscopic gastric band. I disagree and feel that this device still has a role, albeit limited in the modern bariatric surgical program. Many patients do well for a long period. A committed surgeon and program, and the ideal patient with a similar level of commitment, are needed to achieve these best outcomes. Now that patients and surgeons are better informed of the drawbacks to the device, use has decreased without external regulations or policies to drive this change. No single bariatric procedure is appropriate for all patients. Patients need options, and we need better data to help guide their decisions. Do not throw the baby out with the bathwater.
Jon C. Gould, MD, FACS, is with the Medical College of Wisconsin, Milwaukee. Dr. Gould made these comments in an editorial (JAMA Surg. 2017 May 17; doi: 10.1001/jamasurg.2017.1082) that accompanied the study. He has no disclosures.
Dr. Ibrahim and his colleagues have suggested that payers reconsider covering the adjustable laparoscopic gastric band. I disagree and feel that this device still has a role, albeit limited in the modern bariatric surgical program. Many patients do well for a long period. A committed surgeon and program, and the ideal patient with a similar level of commitment, are needed to achieve these best outcomes. Now that patients and surgeons are better informed of the drawbacks to the device, use has decreased without external regulations or policies to drive this change. No single bariatric procedure is appropriate for all patients. Patients need options, and we need better data to help guide their decisions. Do not throw the baby out with the bathwater.
Jon C. Gould, MD, FACS, is with the Medical College of Wisconsin, Milwaukee. Dr. Gould made these comments in an editorial (JAMA Surg. 2017 May 17; doi: 10.1001/jamasurg.2017.1082) that accompanied the study. He has no disclosures.
Dr. Ibrahim and his colleagues have suggested that payers reconsider covering the adjustable laparoscopic gastric band. I disagree and feel that this device still has a role, albeit limited in the modern bariatric surgical program. Many patients do well for a long period. A committed surgeon and program, and the ideal patient with a similar level of commitment, are needed to achieve these best outcomes. Now that patients and surgeons are better informed of the drawbacks to the device, use has decreased without external regulations or policies to drive this change. No single bariatric procedure is appropriate for all patients. Patients need options, and we need better data to help guide their decisions. Do not throw the baby out with the bathwater.
Jon C. Gould, MD, FACS, is with the Medical College of Wisconsin, Milwaukee. Dr. Gould made these comments in an editorial (JAMA Surg. 2017 May 17; doi: 10.1001/jamasurg.2017.1082) that accompanied the study. He has no disclosures.
About one in five laparoscopic gastric band surgeries result in device-related reoperations and reoperations account for almost half of all Medicare expenditures for gastric band surgery, a large retrospective study has found.
The laparoscopic adjustable gastric band for treatment of morbid obesity, approved in 2001 by the Food and Drug Administration, was once a common choice for bariatric patients. Although its use has declined from in recent years, the American Society for Metabolic and Bariatric Surgery estimated that 11,000 bands were placed in 2015 and many others remain in place (ASMBS, Estimate of bariatric surgery numbers, 2011-2015, https://goo.gl/f8iByl). Many of these gastric bands will need to be removed, replaced, or revised in a series of procedures over the coming years.
Of the 24,042 gastric band patients in this study group, 4,636 (18.5%) underwent reoperation, defined as band removal, band replacement, or revision to a different bariatric procedure, but not including band size adjustment. Patients who had reoperations were more likely to be women, to be white, and to have slightly lower rates of hypertension and diabetes. But they were also more likely to have received a psychiatric, anemia, or electrolyte disorder diagnosis at the time of their index operations.
Among the 4,636 patients who had reoperations, 17,539 such procedures were performed, an average of 3.8 procedures per patient, in addition to the index operation, over an average follow-up of 4.5 years. The most common reoperation was for band removal (41.8%). Other reasons included conversion to laparoscopic Roux-en-Y gastric bypass (13.1%) or laparoscopic sleeve gastrectomy (5.3%).

The study also looked at the regional differences, reflecting the comparative success of some programs in managing laparoscopic gastric band placement. Reoperation rates across the referral hospitals ranged from 5% to 95.5%, The study found a nearly a threefold variation in reoperation rates across geographic regions. The bottom quartile of hospital referral regions had an average reoperation rate of 13.3% (0.3 standard deviation) and the top quartile had an average reoperation rate of 39.1% (0.21 SD). Top-quartile regions were concentrated in the West, but were otherwise distributed throughout the country.
Most reoperations were elective admissions (79.9%), while 10% were classified as urgent and another 10.1% as emergency. So although previous studies have documented complications such as band slippage and gastric erosion, the preponderance of elective admissions suggests patient and clinician preferences, or weight loss failure, rather than emergency situations, may be the driving force in the reoperation trend.
The investigators concluded that patients should be fully informed about the likelihood of reoperation with the gastric band. In addition, the wide range of reoperation rates across regions and institutions suggests that more training or better patient selection may be needed to improve outcomes. However, they suggested that “taken together, these findings indicate that the gastric band is associated with high reoperation rates and considerable costs to the payers, which raises concerns about its safety, effectiveness, and value.” They added that “payers should reconsider their coverage of the gastric band device.”
Coauthor Justin B. Dimick, MD, disclosed a financial interest in ArborMetrix. The other coauthors reported having no financial disclosures. The Robert Wood Johnson Foundation, U.S. Department of Veterans Affairs, National Institute on Aging, and National Institute of Diabetes and Digestive and Kidney Diseases provided funding.
About one in five laparoscopic gastric band surgeries result in device-related reoperations and reoperations account for almost half of all Medicare expenditures for gastric band surgery, a large retrospective study has found.
The laparoscopic adjustable gastric band for treatment of morbid obesity, approved in 2001 by the Food and Drug Administration, was once a common choice for bariatric patients. Although its use has declined from in recent years, the American Society for Metabolic and Bariatric Surgery estimated that 11,000 bands were placed in 2015 and many others remain in place (ASMBS, Estimate of bariatric surgery numbers, 2011-2015, https://goo.gl/f8iByl). Many of these gastric bands will need to be removed, replaced, or revised in a series of procedures over the coming years.
Of the 24,042 gastric band patients in this study group, 4,636 (18.5%) underwent reoperation, defined as band removal, band replacement, or revision to a different bariatric procedure, but not including band size adjustment. Patients who had reoperations were more likely to be women, to be white, and to have slightly lower rates of hypertension and diabetes. But they were also more likely to have received a psychiatric, anemia, or electrolyte disorder diagnosis at the time of their index operations.
Among the 4,636 patients who had reoperations, 17,539 such procedures were performed, an average of 3.8 procedures per patient, in addition to the index operation, over an average follow-up of 4.5 years. The most common reoperation was for band removal (41.8%). Other reasons included conversion to laparoscopic Roux-en-Y gastric bypass (13.1%) or laparoscopic sleeve gastrectomy (5.3%).

The study also looked at the regional differences, reflecting the comparative success of some programs in managing laparoscopic gastric band placement. Reoperation rates across the referral hospitals ranged from 5% to 95.5%, The study found a nearly a threefold variation in reoperation rates across geographic regions. The bottom quartile of hospital referral regions had an average reoperation rate of 13.3% (0.3 standard deviation) and the top quartile had an average reoperation rate of 39.1% (0.21 SD). Top-quartile regions were concentrated in the West, but were otherwise distributed throughout the country.
Most reoperations were elective admissions (79.9%), while 10% were classified as urgent and another 10.1% as emergency. So although previous studies have documented complications such as band slippage and gastric erosion, the preponderance of elective admissions suggests patient and clinician preferences, or weight loss failure, rather than emergency situations, may be the driving force in the reoperation trend.
The investigators concluded that patients should be fully informed about the likelihood of reoperation with the gastric band. In addition, the wide range of reoperation rates across regions and institutions suggests that more training or better patient selection may be needed to improve outcomes. However, they suggested that “taken together, these findings indicate that the gastric band is associated with high reoperation rates and considerable costs to the payers, which raises concerns about its safety, effectiveness, and value.” They added that “payers should reconsider their coverage of the gastric band device.”
Coauthor Justin B. Dimick, MD, disclosed a financial interest in ArborMetrix. The other coauthors reported having no financial disclosures. The Robert Wood Johnson Foundation, U.S. Department of Veterans Affairs, National Institute on Aging, and National Institute of Diabetes and Digestive and Kidney Diseases provided funding.
FROM JAMA SURGERY
Key clinical point: Reoperations after gastric band placement are common and raise concerns about the safety, effectiveness, and value of the device.
Major finding: During the study period, reoperations accounted for 47.6% of Medicare payments for laparoscopic gastric band procedures.
Data source: Medicare Provider Analysis and Review file of 25,042 beneficiaries who had gastric band procedures between 2006 and 2013.
Disclosures: Coauthor Justin B. Dimick, MD, disclosed a financial interest in ArborMetrix. The other coauthors reported having no financial disclosures. The Robert Wood Johnson Foundation, U.S. Department of Veterans Affairs, National Institute on Aging, and National Institute of Diabetes and Digestive and Kidney Diseases provided funding.
Omitting ALND in some breast cancer patients may be the right choice
PHILADELPHIA – The safety of sentinel lymph node biopsy (SLNB) alone without axillary lymph node dissection (ALND) has been established for patients with cT1-2N0 cancer that are found to have one or two metastatic sentinel lymph nodes who undergo breast conservation therapy, but questions regarding the role of regional radiation have persisted.
This issue is addressed by the results of a large, prospective, 5+ year study at Memorial Sloan Kettering Cancer Center which confirmed the safety of omitting axillary lymph node dissection and suggested that regional radiation provides minimal benefit.
Dr. Morrow explained that, in August 2010, the breast surgery service at MSKCC adopted the guidelines that arose from the American College of Surgeons Oncology Group’s multicenter Z0011 trial and abandoned routine use of ALND in eligible patients. The goal of the study, she reported, was to determine how frequently axillary dissection was avoided in a consecutive, otherwise unselected, series of patients and to determine the incidence of local regional recurrence after SLNB alone in a population treated with known radiotherapy fields.
Eligible subjects had T1 or T2 node-negative breast cancer, were undergoing breast-conserving surgery with planned whole-breast irradiation, and were found to have hematoxylin-eosin-detected sentinel node metastases. Patients receiving neoadjuvant chemotherapy or requiring conversion to mastectomy, or those in whom partial breast irradiation or no radiotherapy was planned, were ineligible. Axillary imaging was not used in select patients. Criteria for axillary dissection were metastases in three or more sentinel nodes or the presence of matted nodes identified intraoperatively. The researchers did not use the MSKCC nomogram to predict the likelihood of non–sentinel node metastases.
Median patient age was 58 years and median tumor size 1.7 cm. With regard to tumor pathology, 87% had infiltrating ductal tumors, 94% had grade 2 or 3 disease, and the most common subtype was HR+, HER2– disease in 84%. “In this node-positive cohort of patients, 98% received adjuvant systemic therapy, most commonly both chemotherapy and endocrine therapy (received by 65%), and 93% completed radiotherapy,” Dr. Morrow said.
In the entire patient cohort, 84% (663) were treated with SLNB alone, Dr. Morrow said. Among the 130 patients requiring ALND, 68% (88) had metastases in three or more nodes, 26% (34) were found to have had matted nodes intraoperatively, and 6% (8) were eligible for SLNB alone but opted for ALND or had it recommended by their surgeon. “All of these occurred early in our experience, and this has not been repeated since,” Dr. Morrow said.
Among the SLNB-only patients, the 5-year event-free survival was 93%. “There were no isolated axillary recurrences,” Dr. Morrow said. The study reported four combined breast and axillary recurrences, three in nonradiated patients, and four combined nodal and distant recurrences, only one of which involved the axillary nodes. “The median time to any nodal recurrence was 25 months,” Dr. Morrow added. Among 484 patients who had 1 year or more of follow-up, 58% (280) received conventional supine breast tangents, 21% were treated prone – “meaning their axilla received essentially no radiotherapy,” Dr. Morrow said – and 21% had node field irradiation.
“If we compare patient characteristics based on radiotherapy fields treated, it’s clear that the patients who received nodal irradiation were a higher-risk group,” Dr. Morrow said. While all three groups had a median of one positive sentinel node, that “skewed towards two” in the nodal irradiation group, she said. This group also had higher rates of lymphovascular invasion (72% vs. 56% and 49% in the supine and prone groups, respectively) and extracapsular extension (41% vs. 31% and 25%).
The rates of nodal relapse were not statistically significant among the three groups: 1% in the prone group, 1.4% in the supine group, and 0% in the node irradiation group.
“Factors associated with a higher risk of distant metastases, such as young patient age, estrogen receptor negativity, or HER2 over-expression, were not associated with the need for axillary dissection and should not be used as priority selection criteria,” Dr. Morrow said. “Nodal recurrence was uncommon in the absence of routine nodal radiation therapy, and no isolated nodal failures were observed.
In his comments, Armando Giuliano, MD, of Cedars Sinai Medical Center in Los Angeles, principal investigator of the Z0011 trial, said the MSKCC study “extends and informs” the Z0011 findings. He noted that the prone treatment group in the MSKCC trial had a low rate of axillary recurrence. “Can you speculate how such excellent results are achieved without resection or irradiation?” he asked Dr. Morrow. “To me it appears that nodal irradiation provides very little benefit to this selected group of patients.”
The patients in the prone group were in the lowest-risk category of the study, Dr. Morrow said, but the fact that not all nodal disease becomes clinically evident, even in patients who do not receive radiotherapy or systemic therapy, along with the high use of systemic therapy in this group, may explain the low rates of axillary recurrence. “What I think we still need to find out, though, is whether or not failure to irradiate the nodes at all is in any way associated with decreased survival, as would be suggested in the MA.20 trial,” she said. “I think we will find that out from ongoing trials looking at no axillary dissection in mastectomy patients.”
Dr. Morrow and Dr. Giuliano reported no financial disclosures.
The complete manuscript of this study and its presentation at the American Surgical Association’s 137th Annual Meeting, April 2017, in Philadelphia, Pennsylvania, is to be published in Annals of Surgery pending editorial review.
PHILADELPHIA – The safety of sentinel lymph node biopsy (SLNB) alone without axillary lymph node dissection (ALND) has been established for patients with cT1-2N0 cancer that are found to have one or two metastatic sentinel lymph nodes who undergo breast conservation therapy, but questions regarding the role of regional radiation have persisted.
This issue is addressed by the results of a large, prospective, 5+ year study at Memorial Sloan Kettering Cancer Center which confirmed the safety of omitting axillary lymph node dissection and suggested that regional radiation provides minimal benefit.
Dr. Morrow explained that, in August 2010, the breast surgery service at MSKCC adopted the guidelines that arose from the American College of Surgeons Oncology Group’s multicenter Z0011 trial and abandoned routine use of ALND in eligible patients. The goal of the study, she reported, was to determine how frequently axillary dissection was avoided in a consecutive, otherwise unselected, series of patients and to determine the incidence of local regional recurrence after SLNB alone in a population treated with known radiotherapy fields.
Eligible subjects had T1 or T2 node-negative breast cancer, were undergoing breast-conserving surgery with planned whole-breast irradiation, and were found to have hematoxylin-eosin-detected sentinel node metastases. Patients receiving neoadjuvant chemotherapy or requiring conversion to mastectomy, or those in whom partial breast irradiation or no radiotherapy was planned, were ineligible. Axillary imaging was not used in select patients. Criteria for axillary dissection were metastases in three or more sentinel nodes or the presence of matted nodes identified intraoperatively. The researchers did not use the MSKCC nomogram to predict the likelihood of non–sentinel node metastases.
Median patient age was 58 years and median tumor size 1.7 cm. With regard to tumor pathology, 87% had infiltrating ductal tumors, 94% had grade 2 or 3 disease, and the most common subtype was HR+, HER2– disease in 84%. “In this node-positive cohort of patients, 98% received adjuvant systemic therapy, most commonly both chemotherapy and endocrine therapy (received by 65%), and 93% completed radiotherapy,” Dr. Morrow said.
In the entire patient cohort, 84% (663) were treated with SLNB alone, Dr. Morrow said. Among the 130 patients requiring ALND, 68% (88) had metastases in three or more nodes, 26% (34) were found to have had matted nodes intraoperatively, and 6% (8) were eligible for SLNB alone but opted for ALND or had it recommended by their surgeon. “All of these occurred early in our experience, and this has not been repeated since,” Dr. Morrow said.
Among the SLNB-only patients, the 5-year event-free survival was 93%. “There were no isolated axillary recurrences,” Dr. Morrow said. The study reported four combined breast and axillary recurrences, three in nonradiated patients, and four combined nodal and distant recurrences, only one of which involved the axillary nodes. “The median time to any nodal recurrence was 25 months,” Dr. Morrow added. Among 484 patients who had 1 year or more of follow-up, 58% (280) received conventional supine breast tangents, 21% were treated prone – “meaning their axilla received essentially no radiotherapy,” Dr. Morrow said – and 21% had node field irradiation.
“If we compare patient characteristics based on radiotherapy fields treated, it’s clear that the patients who received nodal irradiation were a higher-risk group,” Dr. Morrow said. While all three groups had a median of one positive sentinel node, that “skewed towards two” in the nodal irradiation group, she said. This group also had higher rates of lymphovascular invasion (72% vs. 56% and 49% in the supine and prone groups, respectively) and extracapsular extension (41% vs. 31% and 25%).
The rates of nodal relapse were not statistically significant among the three groups: 1% in the prone group, 1.4% in the supine group, and 0% in the node irradiation group.
“Factors associated with a higher risk of distant metastases, such as young patient age, estrogen receptor negativity, or HER2 over-expression, were not associated with the need for axillary dissection and should not be used as priority selection criteria,” Dr. Morrow said. “Nodal recurrence was uncommon in the absence of routine nodal radiation therapy, and no isolated nodal failures were observed.
In his comments, Armando Giuliano, MD, of Cedars Sinai Medical Center in Los Angeles, principal investigator of the Z0011 trial, said the MSKCC study “extends and informs” the Z0011 findings. He noted that the prone treatment group in the MSKCC trial had a low rate of axillary recurrence. “Can you speculate how such excellent results are achieved without resection or irradiation?” he asked Dr. Morrow. “To me it appears that nodal irradiation provides very little benefit to this selected group of patients.”
The patients in the prone group were in the lowest-risk category of the study, Dr. Morrow said, but the fact that not all nodal disease becomes clinically evident, even in patients who do not receive radiotherapy or systemic therapy, along with the high use of systemic therapy in this group, may explain the low rates of axillary recurrence. “What I think we still need to find out, though, is whether or not failure to irradiate the nodes at all is in any way associated with decreased survival, as would be suggested in the MA.20 trial,” she said. “I think we will find that out from ongoing trials looking at no axillary dissection in mastectomy patients.”
Dr. Morrow and Dr. Giuliano reported no financial disclosures.
The complete manuscript of this study and its presentation at the American Surgical Association’s 137th Annual Meeting, April 2017, in Philadelphia, Pennsylvania, is to be published in Annals of Surgery pending editorial review.
PHILADELPHIA – The safety of sentinel lymph node biopsy (SLNB) alone without axillary lymph node dissection (ALND) has been established for patients with cT1-2N0 cancer that are found to have one or two metastatic sentinel lymph nodes who undergo breast conservation therapy, but questions regarding the role of regional radiation have persisted.
This issue is addressed by the results of a large, prospective, 5+ year study at Memorial Sloan Kettering Cancer Center which confirmed the safety of omitting axillary lymph node dissection and suggested that regional radiation provides minimal benefit.
Dr. Morrow explained that, in August 2010, the breast surgery service at MSKCC adopted the guidelines that arose from the American College of Surgeons Oncology Group’s multicenter Z0011 trial and abandoned routine use of ALND in eligible patients. The goal of the study, she reported, was to determine how frequently axillary dissection was avoided in a consecutive, otherwise unselected, series of patients and to determine the incidence of local regional recurrence after SLNB alone in a population treated with known radiotherapy fields.
Eligible subjects had T1 or T2 node-negative breast cancer, were undergoing breast-conserving surgery with planned whole-breast irradiation, and were found to have hematoxylin-eosin-detected sentinel node metastases. Patients receiving neoadjuvant chemotherapy or requiring conversion to mastectomy, or those in whom partial breast irradiation or no radiotherapy was planned, were ineligible. Axillary imaging was not used in select patients. Criteria for axillary dissection were metastases in three or more sentinel nodes or the presence of matted nodes identified intraoperatively. The researchers did not use the MSKCC nomogram to predict the likelihood of non–sentinel node metastases.
Median patient age was 58 years and median tumor size 1.7 cm. With regard to tumor pathology, 87% had infiltrating ductal tumors, 94% had grade 2 or 3 disease, and the most common subtype was HR+, HER2– disease in 84%. “In this node-positive cohort of patients, 98% received adjuvant systemic therapy, most commonly both chemotherapy and endocrine therapy (received by 65%), and 93% completed radiotherapy,” Dr. Morrow said.
In the entire patient cohort, 84% (663) were treated with SLNB alone, Dr. Morrow said. Among the 130 patients requiring ALND, 68% (88) had metastases in three or more nodes, 26% (34) were found to have had matted nodes intraoperatively, and 6% (8) were eligible for SLNB alone but opted for ALND or had it recommended by their surgeon. “All of these occurred early in our experience, and this has not been repeated since,” Dr. Morrow said.
Among the SLNB-only patients, the 5-year event-free survival was 93%. “There were no isolated axillary recurrences,” Dr. Morrow said. The study reported four combined breast and axillary recurrences, three in nonradiated patients, and four combined nodal and distant recurrences, only one of which involved the axillary nodes. “The median time to any nodal recurrence was 25 months,” Dr. Morrow added. Among 484 patients who had 1 year or more of follow-up, 58% (280) received conventional supine breast tangents, 21% were treated prone – “meaning their axilla received essentially no radiotherapy,” Dr. Morrow said – and 21% had node field irradiation.
“If we compare patient characteristics based on radiotherapy fields treated, it’s clear that the patients who received nodal irradiation were a higher-risk group,” Dr. Morrow said. While all three groups had a median of one positive sentinel node, that “skewed towards two” in the nodal irradiation group, she said. This group also had higher rates of lymphovascular invasion (72% vs. 56% and 49% in the supine and prone groups, respectively) and extracapsular extension (41% vs. 31% and 25%).
The rates of nodal relapse were not statistically significant among the three groups: 1% in the prone group, 1.4% in the supine group, and 0% in the node irradiation group.
“Factors associated with a higher risk of distant metastases, such as young patient age, estrogen receptor negativity, or HER2 over-expression, were not associated with the need for axillary dissection and should not be used as priority selection criteria,” Dr. Morrow said. “Nodal recurrence was uncommon in the absence of routine nodal radiation therapy, and no isolated nodal failures were observed.
In his comments, Armando Giuliano, MD, of Cedars Sinai Medical Center in Los Angeles, principal investigator of the Z0011 trial, said the MSKCC study “extends and informs” the Z0011 findings. He noted that the prone treatment group in the MSKCC trial had a low rate of axillary recurrence. “Can you speculate how such excellent results are achieved without resection or irradiation?” he asked Dr. Morrow. “To me it appears that nodal irradiation provides very little benefit to this selected group of patients.”
The patients in the prone group were in the lowest-risk category of the study, Dr. Morrow said, but the fact that not all nodal disease becomes clinically evident, even in patients who do not receive radiotherapy or systemic therapy, along with the high use of systemic therapy in this group, may explain the low rates of axillary recurrence. “What I think we still need to find out, though, is whether or not failure to irradiate the nodes at all is in any way associated with decreased survival, as would be suggested in the MA.20 trial,” she said. “I think we will find that out from ongoing trials looking at no axillary dissection in mastectomy patients.”
Dr. Morrow and Dr. Giuliano reported no financial disclosures.
The complete manuscript of this study and its presentation at the American Surgical Association’s 137th Annual Meeting, April 2017, in Philadelphia, Pennsylvania, is to be published in Annals of Surgery pending editorial review.
AT THE ANNUAL ASA MEETING
Nomogram may direct diabetes patients to best operation
PHILADELPHIA – A nomogram that assigns a disease severity score to individuals with type 2 diabetes may provide a tool that helps surgeons, endocrinologists, and primary care physicians determine which weight-loss surgical procedure would be most effective, according to an analysis of 900 patients from Cleveland Clinic and University Hospital Clinic, Barcelona, reported at the annual meeting of the American Surgical Association.
“This is the largest reported cohort with long-term glycemic follow-up data that categorizes diabetes into three validated stages of severity to guide procedure selection,” said Ali Aminian, MD, of Cleveland Clinic. The study also highlighted the importance of surgery in early diabetes. The study involved a modeling cohort of 659 patients who had bariatric procedures at Cleveland Clinic from 2005 to 2011 and a separate data set of 241 patients from Barcelona to validate the findings. Roux-en-Y gastric bypass (RYGB) was performed in 78% of the Cleveland Clinic group and 49% of the Barcelona group, with the remainder having sleeve gastrectomy (SG).
RYGB and SG account for more than 95% of all bariatric procedures in people with type 2 diabetes, Dr. Aminian said, but outcomes of clinical trials have been variable, some reporting up to half of patients having long-term relapses. The Cleveland Clinic study involved all patients with type 2 diabetes who had RYGB or SG from 2005 to 2011 with 5 years or more of glycemic data, with a median follow-up of 7 years. The study used American Diabetes Association targets to define remission and glycemic control.
“Long-term response after bariatric surgery in patients with diabetes significantly differs according to diabetes severity,” Dr. Aminian said. “For example, the outcome of surgery in a patient who has diabetes for 2 years is significantly different than a patient who has diabetes for 15 years taking three medications, including insulin.”
The researchers generated the nomogram based on these four independent preoperative factors:
- Number of preoperative diabetes medications (P less than .0001).
- Insulin use (P = .002).
- Duration of diabetes (P less than .0001).
- Glycemic control (P = .002).
“These factors are readily available in clinical practice and are considered a proxy of functional pancreatic beta-cell reserve,” Dr. Aminian said. The nomogram scores the severity of each factor on a scale of 0 to 100. For example, one preoperative medication scores 12, but five scores 63; duration of diabetes of 1 year scores five points, but 16 years scores 60. The patient’s total points represent the Individualized Metabolic Surgery score.
The researchers assigned three categories: A score of 25 or less represents mild disease, a score of 25-95 represents moderate, and 95 to the maximum 180 is severe disease.
Based on the Cleveland Clinic and Barcelona cohorts, the researchers next developed recommendations for average risk patients in each category.
RYGB is “suggested” in mild disease based on remission rates of 92% in the Cleveland subgroup and 91% in the Barcelona subgroup vs. SG remission rates of 74% and 91%, respectively. On the other end of the spectrum, in patients with severe diabetes, both procedures were less effective in achieving long-term diabetes remission. The disparities were more pronounced for the moderate group: 60% and 70% for RYGB and 25% and 56% for SG in the Cleveland Clinic and Barcelona subgroups, respectively. Hence the nomogram highly “recommends” RYGB. An online calculator to determine Individualized Metabolic Surgery score is available at http://riskcalc.org/Metabolic_Surgery_Score/.
“Obviously, this is the first attempt toward individualized procedure selection and more work needs to be done,” Dr. Aminian said. “Our findings also highlight the importance of surgical intervention in early stages of diabetes in order to achieve sustainable remission.”
In his discussion, Matthew M. Hutter, MD, FACS, of Harvard Medical School, Boston, offered to “quibble” with Dr. Aminian’s conclusions. “I challenge you on your conclusion for the mild and severe categories,” Dr. Hutter said. “My rate-of-cure data makes me want to recommend bypass for any patient with diabetes – mild, moderate or severe.”
Dr. Aminian acknowledged that the number of SG cases in the severe subgroup – 51 – was not great, and long-term diabetes remission was comparable between the two procedures. The key distinguishing measure in the severe category was a net 8% difference between two procedures in glycemic control at last follow-up. “This is the difference that we must decide whether it’s clinically important or not – whether we’re willing to recommend a riskier procedure for an extra 8% achieving glycemic control,” Dr. Aminian said. “If someone thinks it’s worth the risk, then they may suggest gastric bypass. If someone thinks it’s not worth the risk, then they may suggest a sleeve gastrectomy. But, we should remember that patients in the severe group are very high-risk patients.”
Dr. Aminian reported no financial disclosures. Dr. Hutter disclosed receiving conference reimbursement from Olympus.
The complete manuscript of this study and its presentation at the American Surgical Association’s 137th Annual Meeting, April 2017, in Philadelphia, Pennsylvania, is to be published in Annals of Surgery pending editorial review.
PHILADELPHIA – A nomogram that assigns a disease severity score to individuals with type 2 diabetes may provide a tool that helps surgeons, endocrinologists, and primary care physicians determine which weight-loss surgical procedure would be most effective, according to an analysis of 900 patients from Cleveland Clinic and University Hospital Clinic, Barcelona, reported at the annual meeting of the American Surgical Association.
“This is the largest reported cohort with long-term glycemic follow-up data that categorizes diabetes into three validated stages of severity to guide procedure selection,” said Ali Aminian, MD, of Cleveland Clinic. The study also highlighted the importance of surgery in early diabetes. The study involved a modeling cohort of 659 patients who had bariatric procedures at Cleveland Clinic from 2005 to 2011 and a separate data set of 241 patients from Barcelona to validate the findings. Roux-en-Y gastric bypass (RYGB) was performed in 78% of the Cleveland Clinic group and 49% of the Barcelona group, with the remainder having sleeve gastrectomy (SG).
RYGB and SG account for more than 95% of all bariatric procedures in people with type 2 diabetes, Dr. Aminian said, but outcomes of clinical trials have been variable, some reporting up to half of patients having long-term relapses. The Cleveland Clinic study involved all patients with type 2 diabetes who had RYGB or SG from 2005 to 2011 with 5 years or more of glycemic data, with a median follow-up of 7 years. The study used American Diabetes Association targets to define remission and glycemic control.
“Long-term response after bariatric surgery in patients with diabetes significantly differs according to diabetes severity,” Dr. Aminian said. “For example, the outcome of surgery in a patient who has diabetes for 2 years is significantly different than a patient who has diabetes for 15 years taking three medications, including insulin.”
The researchers generated the nomogram based on these four independent preoperative factors:
- Number of preoperative diabetes medications (P less than .0001).
- Insulin use (P = .002).
- Duration of diabetes (P less than .0001).
- Glycemic control (P = .002).
“These factors are readily available in clinical practice and are considered a proxy of functional pancreatic beta-cell reserve,” Dr. Aminian said. The nomogram scores the severity of each factor on a scale of 0 to 100. For example, one preoperative medication scores 12, but five scores 63; duration of diabetes of 1 year scores five points, but 16 years scores 60. The patient’s total points represent the Individualized Metabolic Surgery score.
The researchers assigned three categories: A score of 25 or less represents mild disease, a score of 25-95 represents moderate, and 95 to the maximum 180 is severe disease.
Based on the Cleveland Clinic and Barcelona cohorts, the researchers next developed recommendations for average risk patients in each category.
RYGB is “suggested” in mild disease based on remission rates of 92% in the Cleveland subgroup and 91% in the Barcelona subgroup vs. SG remission rates of 74% and 91%, respectively. On the other end of the spectrum, in patients with severe diabetes, both procedures were less effective in achieving long-term diabetes remission. The disparities were more pronounced for the moderate group: 60% and 70% for RYGB and 25% and 56% for SG in the Cleveland Clinic and Barcelona subgroups, respectively. Hence the nomogram highly “recommends” RYGB. An online calculator to determine Individualized Metabolic Surgery score is available at http://riskcalc.org/Metabolic_Surgery_Score/.
“Obviously, this is the first attempt toward individualized procedure selection and more work needs to be done,” Dr. Aminian said. “Our findings also highlight the importance of surgical intervention in early stages of diabetes in order to achieve sustainable remission.”
In his discussion, Matthew M. Hutter, MD, FACS, of Harvard Medical School, Boston, offered to “quibble” with Dr. Aminian’s conclusions. “I challenge you on your conclusion for the mild and severe categories,” Dr. Hutter said. “My rate-of-cure data makes me want to recommend bypass for any patient with diabetes – mild, moderate or severe.”
Dr. Aminian acknowledged that the number of SG cases in the severe subgroup – 51 – was not great, and long-term diabetes remission was comparable between the two procedures. The key distinguishing measure in the severe category was a net 8% difference between two procedures in glycemic control at last follow-up. “This is the difference that we must decide whether it’s clinically important or not – whether we’re willing to recommend a riskier procedure for an extra 8% achieving glycemic control,” Dr. Aminian said. “If someone thinks it’s worth the risk, then they may suggest gastric bypass. If someone thinks it’s not worth the risk, then they may suggest a sleeve gastrectomy. But, we should remember that patients in the severe group are very high-risk patients.”
Dr. Aminian reported no financial disclosures. Dr. Hutter disclosed receiving conference reimbursement from Olympus.
The complete manuscript of this study and its presentation at the American Surgical Association’s 137th Annual Meeting, April 2017, in Philadelphia, Pennsylvania, is to be published in Annals of Surgery pending editorial review.
PHILADELPHIA – A nomogram that assigns a disease severity score to individuals with type 2 diabetes may provide a tool that helps surgeons, endocrinologists, and primary care physicians determine which weight-loss surgical procedure would be most effective, according to an analysis of 900 patients from Cleveland Clinic and University Hospital Clinic, Barcelona, reported at the annual meeting of the American Surgical Association.
“This is the largest reported cohort with long-term glycemic follow-up data that categorizes diabetes into three validated stages of severity to guide procedure selection,” said Ali Aminian, MD, of Cleveland Clinic. The study also highlighted the importance of surgery in early diabetes. The study involved a modeling cohort of 659 patients who had bariatric procedures at Cleveland Clinic from 2005 to 2011 and a separate data set of 241 patients from Barcelona to validate the findings. Roux-en-Y gastric bypass (RYGB) was performed in 78% of the Cleveland Clinic group and 49% of the Barcelona group, with the remainder having sleeve gastrectomy (SG).
RYGB and SG account for more than 95% of all bariatric procedures in people with type 2 diabetes, Dr. Aminian said, but outcomes of clinical trials have been variable, some reporting up to half of patients having long-term relapses. The Cleveland Clinic study involved all patients with type 2 diabetes who had RYGB or SG from 2005 to 2011 with 5 years or more of glycemic data, with a median follow-up of 7 years. The study used American Diabetes Association targets to define remission and glycemic control.
“Long-term response after bariatric surgery in patients with diabetes significantly differs according to diabetes severity,” Dr. Aminian said. “For example, the outcome of surgery in a patient who has diabetes for 2 years is significantly different than a patient who has diabetes for 15 years taking three medications, including insulin.”
The researchers generated the nomogram based on these four independent preoperative factors:
- Number of preoperative diabetes medications (P less than .0001).
- Insulin use (P = .002).
- Duration of diabetes (P less than .0001).
- Glycemic control (P = .002).
“These factors are readily available in clinical practice and are considered a proxy of functional pancreatic beta-cell reserve,” Dr. Aminian said. The nomogram scores the severity of each factor on a scale of 0 to 100. For example, one preoperative medication scores 12, but five scores 63; duration of diabetes of 1 year scores five points, but 16 years scores 60. The patient’s total points represent the Individualized Metabolic Surgery score.
The researchers assigned three categories: A score of 25 or less represents mild disease, a score of 25-95 represents moderate, and 95 to the maximum 180 is severe disease.
Based on the Cleveland Clinic and Barcelona cohorts, the researchers next developed recommendations for average risk patients in each category.
RYGB is “suggested” in mild disease based on remission rates of 92% in the Cleveland subgroup and 91% in the Barcelona subgroup vs. SG remission rates of 74% and 91%, respectively. On the other end of the spectrum, in patients with severe diabetes, both procedures were less effective in achieving long-term diabetes remission. The disparities were more pronounced for the moderate group: 60% and 70% for RYGB and 25% and 56% for SG in the Cleveland Clinic and Barcelona subgroups, respectively. Hence the nomogram highly “recommends” RYGB. An online calculator to determine Individualized Metabolic Surgery score is available at http://riskcalc.org/Metabolic_Surgery_Score/.
“Obviously, this is the first attempt toward individualized procedure selection and more work needs to be done,” Dr. Aminian said. “Our findings also highlight the importance of surgical intervention in early stages of diabetes in order to achieve sustainable remission.”
In his discussion, Matthew M. Hutter, MD, FACS, of Harvard Medical School, Boston, offered to “quibble” with Dr. Aminian’s conclusions. “I challenge you on your conclusion for the mild and severe categories,” Dr. Hutter said. “My rate-of-cure data makes me want to recommend bypass for any patient with diabetes – mild, moderate or severe.”
Dr. Aminian acknowledged that the number of SG cases in the severe subgroup – 51 – was not great, and long-term diabetes remission was comparable between the two procedures. The key distinguishing measure in the severe category was a net 8% difference between two procedures in glycemic control at last follow-up. “This is the difference that we must decide whether it’s clinically important or not – whether we’re willing to recommend a riskier procedure for an extra 8% achieving glycemic control,” Dr. Aminian said. “If someone thinks it’s worth the risk, then they may suggest gastric bypass. If someone thinks it’s not worth the risk, then they may suggest a sleeve gastrectomy. But, we should remember that patients in the severe group are very high-risk patients.”
Dr. Aminian reported no financial disclosures. Dr. Hutter disclosed receiving conference reimbursement from Olympus.
The complete manuscript of this study and its presentation at the American Surgical Association’s 137th Annual Meeting, April 2017, in Philadelphia, Pennsylvania, is to be published in Annals of Surgery pending editorial review.
AT THE ASA ANNUAL MEETING
Key clinical point: A nomogram has been developed that assigns an Individualized Metabolic Surgery score to individuals with type 2 diabetes to help determine which type of bariatric procedure would provide best outcomes.
Major finding: In mild diabetes (Individualized Metabolic Surgery score less than or equal to 25), Roux-en-Y gastric bypass and sleeve gastrectomy significantly improve diabetes. For patients with severe diabetes (IMS Score greater than 95), both procedures have similarly low efficacy for diabetes remission.
Data source: Analysis of 900 patients with type 2 diabetes who had either Roux-en-Y gastric bypass or sleeve gastrectomy with a minimum 5-year follow-up.
Disclosure: Dr. Aminian reported no financial disclosures. Dr. Hutter disclosed receiving conference reimbursement from Olympus.
Big data study looks at safety of concurrent surgical procedures
PHILADELPHIA – Overlapping and concurrent surgical procedures operations are not uncommon, but data on their safety and quality are scarce.
An analysis of the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) data suggests that concurrent operations do not pose any greater risk to patients than do nonconcurrent operations.
“We found that concurrent operations were more often elective inpatient operations at large academic hospitals,” Jason Liu, MD, a clinical scholar-in-residence with the American College of Surgeons, Chicago, said at the annual meeting of the American Surgical Association.
The study evaluated 12,010 concurrent and 521,656 nonconcurrent operations in the ASC NSQIP registry between 2014 and 2015, and propensity-score matched 11,044 operations of each type to evaluate safety. “We then analyzed the three primary outcomes – death or serious morbidity, unplanned reoperation, and unplanned readmission,” Dr. Liu said. “After propensity matching and risk adjustment, we detected no association of concurrent operations with these adverse outcomes.”
Death or serious morbidity had an odds ratio of 1.08, while the odds ratio for reoperation and readmission were 1.16 and 1.14, respectively.
Of the five surgery subspecialty groups presented, ear, nose and throat surgeons performed the highest proportion of concurrent operations, comprising 11.2% of all operations within the subspecialty, followed by neurosurgeons (8.4%) and urological surgeons (5.2%). General surgeons performed more total concurrent cases than did any of the other surgical subspecialties, but these comprised only 1.5% of all general surgery cases.
Among the individual operations presented, the highest percentage of concurrent cases was for spinal operations, comprising 7.1% of all spinal surgeries, followed by total knee and hip replacements, at 2.1% each. Among general surgery procedures, concurrent operations comprised 1.9% of colon surgeries, 1.5% of ventral hernia repairs, and 0.9% of cholecystectomies.
“We found that patients who had concurrent operations tended to have fewer comorbidities,” Dr. Liu said.
Despite the findings suggesting concurrent operations are safe, Dr. Liu added that “failure to detect an effect does not prove its absence. Additional studies and continued vigilance are certainly needed moving forward.”
In her discussion, Valerie W. Rusch, MD, FACS, of Memorial Sloan-Kettering Cancer Center, New York, pointed out that the American College of Surgeons has not revised its statement that considers concurrent surgery “not appropriate,” and that the study itself “reflects both the strengths and weaknesses of ‘big data.’ ” While ACS NSQIP provides a large number of patients, the study authors were still constrained in using time as a proxy for concurrence, she said. “Also, as noted, this analysis reports only a sample of operations rather than all the procedures performed, and the authors were constrained to using simulated statistical methods to avoid misclassification of cases,” Dr. Rusch said.
The death or serious morbidity composite measure the study used may not identify procedure-specific or specialty-specific adverse events that could reflect differences in outcomes between concurrent and nonconcurrent operations, she said. “Nonetheless, this study represents an important step toward bringing science to an extremely important and rightfully contentious aspect of surgical practice,” Dr. Rusch said.
Senior coauthor David Hoyt, MD, FACS, of the ACS, acknowledged the many study limitations Dr. Rusch pointed out. “What this analysis shows is that probably there’s not a huge problem with morbidity and mortality,” Dr. Hoyt said. “It doesn’t really say that we should pursue this without some care of how we do this.”
A key element in planning concurrent surgery is disclosing that to the patient. “Much of what came out of the discussion that led to this was because patients felt that they were not informed,” Dr. Hoyt said. “It really has to do with an ethical issue of patient autonomy.”
Dr. Liu, Dr. Hoyt, and Dr. Rusch had no financial relationships to disclose.
The complete manuscript of this study and its presentation at the American Surgical Association’s 137th Annual Meeting, April 2017, in Philadelphia, Pennsylvania, is anticipated to be published in the Annals of Surgery pending editorial review.
PHILADELPHIA – Overlapping and concurrent surgical procedures operations are not uncommon, but data on their safety and quality are scarce.
An analysis of the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) data suggests that concurrent operations do not pose any greater risk to patients than do nonconcurrent operations.
“We found that concurrent operations were more often elective inpatient operations at large academic hospitals,” Jason Liu, MD, a clinical scholar-in-residence with the American College of Surgeons, Chicago, said at the annual meeting of the American Surgical Association.
The study evaluated 12,010 concurrent and 521,656 nonconcurrent operations in the ASC NSQIP registry between 2014 and 2015, and propensity-score matched 11,044 operations of each type to evaluate safety. “We then analyzed the three primary outcomes – death or serious morbidity, unplanned reoperation, and unplanned readmission,” Dr. Liu said. “After propensity matching and risk adjustment, we detected no association of concurrent operations with these adverse outcomes.”
Death or serious morbidity had an odds ratio of 1.08, while the odds ratio for reoperation and readmission were 1.16 and 1.14, respectively.
Of the five surgery subspecialty groups presented, ear, nose and throat surgeons performed the highest proportion of concurrent operations, comprising 11.2% of all operations within the subspecialty, followed by neurosurgeons (8.4%) and urological surgeons (5.2%). General surgeons performed more total concurrent cases than did any of the other surgical subspecialties, but these comprised only 1.5% of all general surgery cases.
Among the individual operations presented, the highest percentage of concurrent cases was for spinal operations, comprising 7.1% of all spinal surgeries, followed by total knee and hip replacements, at 2.1% each. Among general surgery procedures, concurrent operations comprised 1.9% of colon surgeries, 1.5% of ventral hernia repairs, and 0.9% of cholecystectomies.
“We found that patients who had concurrent operations tended to have fewer comorbidities,” Dr. Liu said.
Despite the findings suggesting concurrent operations are safe, Dr. Liu added that “failure to detect an effect does not prove its absence. Additional studies and continued vigilance are certainly needed moving forward.”
In her discussion, Valerie W. Rusch, MD, FACS, of Memorial Sloan-Kettering Cancer Center, New York, pointed out that the American College of Surgeons has not revised its statement that considers concurrent surgery “not appropriate,” and that the study itself “reflects both the strengths and weaknesses of ‘big data.’ ” While ACS NSQIP provides a large number of patients, the study authors were still constrained in using time as a proxy for concurrence, she said. “Also, as noted, this analysis reports only a sample of operations rather than all the procedures performed, and the authors were constrained to using simulated statistical methods to avoid misclassification of cases,” Dr. Rusch said.
The death or serious morbidity composite measure the study used may not identify procedure-specific or specialty-specific adverse events that could reflect differences in outcomes between concurrent and nonconcurrent operations, she said. “Nonetheless, this study represents an important step toward bringing science to an extremely important and rightfully contentious aspect of surgical practice,” Dr. Rusch said.
Senior coauthor David Hoyt, MD, FACS, of the ACS, acknowledged the many study limitations Dr. Rusch pointed out. “What this analysis shows is that probably there’s not a huge problem with morbidity and mortality,” Dr. Hoyt said. “It doesn’t really say that we should pursue this without some care of how we do this.”
A key element in planning concurrent surgery is disclosing that to the patient. “Much of what came out of the discussion that led to this was because patients felt that they were not informed,” Dr. Hoyt said. “It really has to do with an ethical issue of patient autonomy.”
Dr. Liu, Dr. Hoyt, and Dr. Rusch had no financial relationships to disclose.
The complete manuscript of this study and its presentation at the American Surgical Association’s 137th Annual Meeting, April 2017, in Philadelphia, Pennsylvania, is anticipated to be published in the Annals of Surgery pending editorial review.
PHILADELPHIA – Overlapping and concurrent surgical procedures operations are not uncommon, but data on their safety and quality are scarce.
An analysis of the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) data suggests that concurrent operations do not pose any greater risk to patients than do nonconcurrent operations.
“We found that concurrent operations were more often elective inpatient operations at large academic hospitals,” Jason Liu, MD, a clinical scholar-in-residence with the American College of Surgeons, Chicago, said at the annual meeting of the American Surgical Association.
The study evaluated 12,010 concurrent and 521,656 nonconcurrent operations in the ASC NSQIP registry between 2014 and 2015, and propensity-score matched 11,044 operations of each type to evaluate safety. “We then analyzed the three primary outcomes – death or serious morbidity, unplanned reoperation, and unplanned readmission,” Dr. Liu said. “After propensity matching and risk adjustment, we detected no association of concurrent operations with these adverse outcomes.”
Death or serious morbidity had an odds ratio of 1.08, while the odds ratio for reoperation and readmission were 1.16 and 1.14, respectively.
Of the five surgery subspecialty groups presented, ear, nose and throat surgeons performed the highest proportion of concurrent operations, comprising 11.2% of all operations within the subspecialty, followed by neurosurgeons (8.4%) and urological surgeons (5.2%). General surgeons performed more total concurrent cases than did any of the other surgical subspecialties, but these comprised only 1.5% of all general surgery cases.
Among the individual operations presented, the highest percentage of concurrent cases was for spinal operations, comprising 7.1% of all spinal surgeries, followed by total knee and hip replacements, at 2.1% each. Among general surgery procedures, concurrent operations comprised 1.9% of colon surgeries, 1.5% of ventral hernia repairs, and 0.9% of cholecystectomies.
“We found that patients who had concurrent operations tended to have fewer comorbidities,” Dr. Liu said.
Despite the findings suggesting concurrent operations are safe, Dr. Liu added that “failure to detect an effect does not prove its absence. Additional studies and continued vigilance are certainly needed moving forward.”
In her discussion, Valerie W. Rusch, MD, FACS, of Memorial Sloan-Kettering Cancer Center, New York, pointed out that the American College of Surgeons has not revised its statement that considers concurrent surgery “not appropriate,” and that the study itself “reflects both the strengths and weaknesses of ‘big data.’ ” While ACS NSQIP provides a large number of patients, the study authors were still constrained in using time as a proxy for concurrence, she said. “Also, as noted, this analysis reports only a sample of operations rather than all the procedures performed, and the authors were constrained to using simulated statistical methods to avoid misclassification of cases,” Dr. Rusch said.
The death or serious morbidity composite measure the study used may not identify procedure-specific or specialty-specific adverse events that could reflect differences in outcomes between concurrent and nonconcurrent operations, she said. “Nonetheless, this study represents an important step toward bringing science to an extremely important and rightfully contentious aspect of surgical practice,” Dr. Rusch said.
Senior coauthor David Hoyt, MD, FACS, of the ACS, acknowledged the many study limitations Dr. Rusch pointed out. “What this analysis shows is that probably there’s not a huge problem with morbidity and mortality,” Dr. Hoyt said. “It doesn’t really say that we should pursue this without some care of how we do this.”
A key element in planning concurrent surgery is disclosing that to the patient. “Much of what came out of the discussion that led to this was because patients felt that they were not informed,” Dr. Hoyt said. “It really has to do with an ethical issue of patient autonomy.”
Dr. Liu, Dr. Hoyt, and Dr. Rusch had no financial relationships to disclose.
The complete manuscript of this study and its presentation at the American Surgical Association’s 137th Annual Meeting, April 2017, in Philadelphia, Pennsylvania, is anticipated to be published in the Annals of Surgery pending editorial review.
AT THE ASA ANNUAL MEETING
Key clinical point: Concurrent operations were not associated with an increased risk for worse outcomes, compared with nonconcurrent operations.
Major finding: After propensity-score matching and risk adjustment, the adjusted odds ratio for death or serious mortality was 1.08, and for reoperation, 1.16, for concurrent operations vs. nonconcurrent operations.
Data source: Propensity-score-matched concurrent and nonconcurrent operations (n = 11,044 for each) done in 2014 and 2015 in the American College of Surgeons National Surgical Quality Improvement Program registry.
Disclosures: Dr. Liu and coauthors had no financial relationships to disclose.
OR staff perception of safety linked to better surgical outcomes
PHILADELPHIA – No large-scale study has evaluated the culture of operating room safety and 30-day postoperative death, but a statewide evaluation of South Carolina hospitals found that those in which operating-room personnel perceived a high degree of safety reported lower all-cause 30-day postoperative mortality rates.
“Reducing postoperative death rates and improving patient safety require that, in addition to technical competence, surgeons lead surgical teams in ways that foster the creation of a culture in operating rooms where personnel feel respected and invited to speak up on behalf of patient safety,” George Molina, MD, MPH, said at the annual meeting of the American Surgical Association. Dr. Molina is with Brigham and Women’s Hospital, Harvard T.H. Chan School of Public Health, and Massachusetts General Hospital, Boston.
The study drew on a statewide quality and safety program of the South Carolina Hospital Association known as Safe Surgery 2015, one component of which was a survey sent to operating-room (OR) personnel to measure baseline culture of surgical safety. The survey evaluated five teamwork factors: mutual respect, clinical leadership, assertiveness, coordination, and effective communication. The overall response rate of OR staff at 31 surveyed hospitals was 38.1% with 1,793 completed surveys, with a physicians response rate of 29%.
The researchers analyzed statewide claims data of nearly all surgery in the state (except for operations at Veterans Affairs, military and Shriners’ centers) and a state-level death registry to identify patients who died within 30 days after an inpatient operation. The unadjusted median 30-day postoperative death rate at the 31 participating hospitals was 3.2%, Dr. Molina said.
The study evaluated the association between hospital-level mean scores for each survey statement and postoperative death, and used machine learning to adjust for potential confounders. The analysis also took into account hospital-level variables such as patient gender, Charlson Comorbidity Index, primary payer status, and procedure type.
“Among the factors that make up the teamwork dimension, respect, leadership and assertiveness on behalf of patient safety were significantly associated with lower 30-day postoperative death rates,” Dr. Molina said. “For every one-point increase on a seven-point Likert scale and the hospital level mean score for respect, clinical leadership, and assertiveness among all survey respondents, there were associated decreases in postoperative mortality following surgery, ranging from 14% to 29%.”
Of the five teamwork variables, assertiveness had the lowest relative risk, 0.71 (P = .01), whereas communication had the highest, 0.98 (P = .77).
Dr. Molina noted a number of limitations to the study, such as the inability to generalize findings from a single state and the use of cross-sectional data, which precludes a causal link between culture and mortality. “Postoperative mortality is most likely due to multiple factors that take place not only in the operating room, but also during the course of preoperative and postoperative course,” he said.
In his discussion, Justin Dimick, MD, of the University of Michigan, Ann Arbor, asked Dr. Molina what interventions beyond OR checklists are available to improve safety culture.
“It’s not a matter of changing perceptions but actually changing behaviors and then actually improving the culture,” Dr. Molina said. OR team training that includes simulated emergency response is one such intervention. Other strategies he offered: “Working on communication, in particular closed-loop communication among health care providers working in the operating room, and then also quality and safety programs such as Safe Surgery 2015.”
Dr. Molina reported no financial disclosures. Dr. Dimick is cofounder of Arbor Metrix and receives royalties.
The complete manuscript of this study and its presentation at the American Surgical Association’s 137th Annual Meeting, April 2017, in Philadelphia, Pennsylvania, is anticipated to be published in the Annals of Surgery pending editorial review.
PHILADELPHIA – No large-scale study has evaluated the culture of operating room safety and 30-day postoperative death, but a statewide evaluation of South Carolina hospitals found that those in which operating-room personnel perceived a high degree of safety reported lower all-cause 30-day postoperative mortality rates.
“Reducing postoperative death rates and improving patient safety require that, in addition to technical competence, surgeons lead surgical teams in ways that foster the creation of a culture in operating rooms where personnel feel respected and invited to speak up on behalf of patient safety,” George Molina, MD, MPH, said at the annual meeting of the American Surgical Association. Dr. Molina is with Brigham and Women’s Hospital, Harvard T.H. Chan School of Public Health, and Massachusetts General Hospital, Boston.
The study drew on a statewide quality and safety program of the South Carolina Hospital Association known as Safe Surgery 2015, one component of which was a survey sent to operating-room (OR) personnel to measure baseline culture of surgical safety. The survey evaluated five teamwork factors: mutual respect, clinical leadership, assertiveness, coordination, and effective communication. The overall response rate of OR staff at 31 surveyed hospitals was 38.1% with 1,793 completed surveys, with a physicians response rate of 29%.
The researchers analyzed statewide claims data of nearly all surgery in the state (except for operations at Veterans Affairs, military and Shriners’ centers) and a state-level death registry to identify patients who died within 30 days after an inpatient operation. The unadjusted median 30-day postoperative death rate at the 31 participating hospitals was 3.2%, Dr. Molina said.
The study evaluated the association between hospital-level mean scores for each survey statement and postoperative death, and used machine learning to adjust for potential confounders. The analysis also took into account hospital-level variables such as patient gender, Charlson Comorbidity Index, primary payer status, and procedure type.
“Among the factors that make up the teamwork dimension, respect, leadership and assertiveness on behalf of patient safety were significantly associated with lower 30-day postoperative death rates,” Dr. Molina said. “For every one-point increase on a seven-point Likert scale and the hospital level mean score for respect, clinical leadership, and assertiveness among all survey respondents, there were associated decreases in postoperative mortality following surgery, ranging from 14% to 29%.”
Of the five teamwork variables, assertiveness had the lowest relative risk, 0.71 (P = .01), whereas communication had the highest, 0.98 (P = .77).
Dr. Molina noted a number of limitations to the study, such as the inability to generalize findings from a single state and the use of cross-sectional data, which precludes a causal link between culture and mortality. “Postoperative mortality is most likely due to multiple factors that take place not only in the operating room, but also during the course of preoperative and postoperative course,” he said.
In his discussion, Justin Dimick, MD, of the University of Michigan, Ann Arbor, asked Dr. Molina what interventions beyond OR checklists are available to improve safety culture.
“It’s not a matter of changing perceptions but actually changing behaviors and then actually improving the culture,” Dr. Molina said. OR team training that includes simulated emergency response is one such intervention. Other strategies he offered: “Working on communication, in particular closed-loop communication among health care providers working in the operating room, and then also quality and safety programs such as Safe Surgery 2015.”
Dr. Molina reported no financial disclosures. Dr. Dimick is cofounder of Arbor Metrix and receives royalties.
The complete manuscript of this study and its presentation at the American Surgical Association’s 137th Annual Meeting, April 2017, in Philadelphia, Pennsylvania, is anticipated to be published in the Annals of Surgery pending editorial review.
PHILADELPHIA – No large-scale study has evaluated the culture of operating room safety and 30-day postoperative death, but a statewide evaluation of South Carolina hospitals found that those in which operating-room personnel perceived a high degree of safety reported lower all-cause 30-day postoperative mortality rates.
“Reducing postoperative death rates and improving patient safety require that, in addition to technical competence, surgeons lead surgical teams in ways that foster the creation of a culture in operating rooms where personnel feel respected and invited to speak up on behalf of patient safety,” George Molina, MD, MPH, said at the annual meeting of the American Surgical Association. Dr. Molina is with Brigham and Women’s Hospital, Harvard T.H. Chan School of Public Health, and Massachusetts General Hospital, Boston.
The study drew on a statewide quality and safety program of the South Carolina Hospital Association known as Safe Surgery 2015, one component of which was a survey sent to operating-room (OR) personnel to measure baseline culture of surgical safety. The survey evaluated five teamwork factors: mutual respect, clinical leadership, assertiveness, coordination, and effective communication. The overall response rate of OR staff at 31 surveyed hospitals was 38.1% with 1,793 completed surveys, with a physicians response rate of 29%.
The researchers analyzed statewide claims data of nearly all surgery in the state (except for operations at Veterans Affairs, military and Shriners’ centers) and a state-level death registry to identify patients who died within 30 days after an inpatient operation. The unadjusted median 30-day postoperative death rate at the 31 participating hospitals was 3.2%, Dr. Molina said.
The study evaluated the association between hospital-level mean scores for each survey statement and postoperative death, and used machine learning to adjust for potential confounders. The analysis also took into account hospital-level variables such as patient gender, Charlson Comorbidity Index, primary payer status, and procedure type.
“Among the factors that make up the teamwork dimension, respect, leadership and assertiveness on behalf of patient safety were significantly associated with lower 30-day postoperative death rates,” Dr. Molina said. “For every one-point increase on a seven-point Likert scale and the hospital level mean score for respect, clinical leadership, and assertiveness among all survey respondents, there were associated decreases in postoperative mortality following surgery, ranging from 14% to 29%.”
Of the five teamwork variables, assertiveness had the lowest relative risk, 0.71 (P = .01), whereas communication had the highest, 0.98 (P = .77).
Dr. Molina noted a number of limitations to the study, such as the inability to generalize findings from a single state and the use of cross-sectional data, which precludes a causal link between culture and mortality. “Postoperative mortality is most likely due to multiple factors that take place not only in the operating room, but also during the course of preoperative and postoperative course,” he said.
In his discussion, Justin Dimick, MD, of the University of Michigan, Ann Arbor, asked Dr. Molina what interventions beyond OR checklists are available to improve safety culture.
“It’s not a matter of changing perceptions but actually changing behaviors and then actually improving the culture,” Dr. Molina said. OR team training that includes simulated emergency response is one such intervention. Other strategies he offered: “Working on communication, in particular closed-loop communication among health care providers working in the operating room, and then also quality and safety programs such as Safe Surgery 2015.”
Dr. Molina reported no financial disclosures. Dr. Dimick is cofounder of Arbor Metrix and receives royalties.
The complete manuscript of this study and its presentation at the American Surgical Association’s 137th Annual Meeting, April 2017, in Philadelphia, Pennsylvania, is anticipated to be published in the Annals of Surgery pending editorial review.
Key clinical point: Perceptions of safety among operating room personnel may influence postoperative death rates.
Major finding: Higher scores for key teamwork variables were associated with decreases in postoperative hospital death rates ranging from 14% to 29%.
Data source: Survey responses of 1,793 operating room personnel at 31 member hospitals of the South Carolina Hospital Association.
Disclosures: Dr. Molina reported no financial disclosures. Dr. Dimick is cofounder of Arbor Metrix and receives royalties.
Choice of lap vs. open SBO surgery still hinges on patient selection
PHILADELPHIA – In surgery for small-bowel obstruction, laparoscopy is more likely to result in bowel injury than is open surgery but with ultimately better outcomes overall. This means that surgeons should proceed with “considerable caution” when performing minimally invasive surgery in the small bowel, researchers at University of Toronto reported during the annual meeting of the American Surgical Association.
In explaining the rationale for the study, Dr. Behman said, “Several studies have shown that over the last several years [that] the utilization of laparoscopic techniques for adhesive small-bowel obstruction has become increasingly common. However, these procedures have some inherent challenges.” Those technical challenges include introducing the trocar into an abdomen filled with distended bowel, trying to manipulate the distended bowel with laparoscopic bowel graspers, and the potentially ischemic bowel wall, he said.
The University of Toronto researchers performed a population-based retrospective cohort study of 8,584 patients in the Ministry of Health Ontario database from 2005 to 2014. The primary outcome was a composite, consisting of bowel repair, including operative billing codes for either via intraoperative enterotomy or suture repair of the intestine, or bowel resection. Secondary outcomes were serious complications and 30-day mortality.
During the study period, the share of SBO procedures performed laparoscopically increased from 4% in 2005 to 14.3% in 2014, Dr. Behman said.
“Patients in the laparoscopic group were slightly younger, had a lower overall comorbidity burden, and tended to be treated at larger hospitals and hospitals without teaching designations,” Dr. Behman said. Specifically, the average age of the 673 patients who had laparoscopy was 63 years vs. 67 years for the 7,911 open-procedure patients. As for comorbidities, 9% and 50% in the laparoscopic group were healthy and low users and high and very high users, respectively, vs. 7% and 57% of the open-procedure group.
The incidence of any bowel intervention was 53.5% in the laparoscopic group vs. 42% in the open group, Dr. Behman said. After a multivariable regression analysis, the researchers determined the following factors raised a patient’s odds of having a bowel intervention: older age; female sex; having had an after-hours procedure; and having had the procedure earlier in the study period. “However, even after [the researchers adjusted] for all of these covariates, the variable in our model that was most significantly associated with a bowel intervention was having had a laparoscopic procedure, with an odds ratio of 1.6,” Dr. Behman said.
When analyzing secondary outcomes, the researchers found that laparoscopy was associated with a lower risk of 30-day mortality (OR, 0.6) and serious complication (OR, 0.8). “So, there does appear to be a direct trade-off with laparoscopy probably having better clinical outcomes, but also being a risk factor for bowel intervention,” Dr. Behman said. So the researchers preformed a secondary analysis that divided the cohort into four subgroups: laparoscopy with and without bowel intervention; and open surgery with and without bowel intervention.
“The next question we wanted to ask was, in patients that are at high risk of a laparoscopic bowel injury, is an open approach or early conversion to open beneficial?” Dr. Behman said. “To put this another way, is it more important to avoid a bowel intervention or to avoid the morbidity associated with an open procedure?”
The secondary analysis confirmed that laparoscopic patients had lower rates of serious complications: in those without bowel intervention, 5% for laparoscopy vs. 10% for open; and in those who had a bowel intervention, 15% for laparoscopy vs. 22% for open. However, those who had an open procedure without a bowel intervention had lower complication rates than did those who had a laparoscopic operation with a bowel intervention, Dr. Behman said, “suggesting that perhaps the bowel intervention is a greater driver of serious complications than having an open procedure.”
These findings can inform patient selection, Dr. Behman added. “In light of the fact that laparoscopic approaches are likely associated with a greater risk of bowel intervention, appropriate caution should accompany these procedures,” he said. “In patients who are at high risk for a bowel intervention, an open approach or early conversion to a laparotomy should be considered.”
In his discussion of the study, Lawrence Diebel, MD, FACS, of Wayne State University, Detroit, asked if the study accounted for timing of surgery, the etiology of laparoscopic-related bowel interventions, and if serosal tears were included as bowel injuries. Dr. Behman said that the study did include timing of surgery in the initial analysis and found it to be a nonfactor. However, the database did not specify the causes for bowel resections or reasons for conversions.
Dr. Behman and Dr. Diebel reported having no financial disclosures
The complete manuscript of this study and its presentation at the American Surgical Association’s 137th Annual Meeting, April 2017, in Philadelphia, is to be published in the Annals of Surgery pending editorial review.
PHILADELPHIA – In surgery for small-bowel obstruction, laparoscopy is more likely to result in bowel injury than is open surgery but with ultimately better outcomes overall. This means that surgeons should proceed with “considerable caution” when performing minimally invasive surgery in the small bowel, researchers at University of Toronto reported during the annual meeting of the American Surgical Association.
In explaining the rationale for the study, Dr. Behman said, “Several studies have shown that over the last several years [that] the utilization of laparoscopic techniques for adhesive small-bowel obstruction has become increasingly common. However, these procedures have some inherent challenges.” Those technical challenges include introducing the trocar into an abdomen filled with distended bowel, trying to manipulate the distended bowel with laparoscopic bowel graspers, and the potentially ischemic bowel wall, he said.
The University of Toronto researchers performed a population-based retrospective cohort study of 8,584 patients in the Ministry of Health Ontario database from 2005 to 2014. The primary outcome was a composite, consisting of bowel repair, including operative billing codes for either via intraoperative enterotomy or suture repair of the intestine, or bowel resection. Secondary outcomes were serious complications and 30-day mortality.
During the study period, the share of SBO procedures performed laparoscopically increased from 4% in 2005 to 14.3% in 2014, Dr. Behman said.
“Patients in the laparoscopic group were slightly younger, had a lower overall comorbidity burden, and tended to be treated at larger hospitals and hospitals without teaching designations,” Dr. Behman said. Specifically, the average age of the 673 patients who had laparoscopy was 63 years vs. 67 years for the 7,911 open-procedure patients. As for comorbidities, 9% and 50% in the laparoscopic group were healthy and low users and high and very high users, respectively, vs. 7% and 57% of the open-procedure group.
The incidence of any bowel intervention was 53.5% in the laparoscopic group vs. 42% in the open group, Dr. Behman said. After a multivariable regression analysis, the researchers determined the following factors raised a patient’s odds of having a bowel intervention: older age; female sex; having had an after-hours procedure; and having had the procedure earlier in the study period. “However, even after [the researchers adjusted] for all of these covariates, the variable in our model that was most significantly associated with a bowel intervention was having had a laparoscopic procedure, with an odds ratio of 1.6,” Dr. Behman said.
When analyzing secondary outcomes, the researchers found that laparoscopy was associated with a lower risk of 30-day mortality (OR, 0.6) and serious complication (OR, 0.8). “So, there does appear to be a direct trade-off with laparoscopy probably having better clinical outcomes, but also being a risk factor for bowel intervention,” Dr. Behman said. So the researchers preformed a secondary analysis that divided the cohort into four subgroups: laparoscopy with and without bowel intervention; and open surgery with and without bowel intervention.
“The next question we wanted to ask was, in patients that are at high risk of a laparoscopic bowel injury, is an open approach or early conversion to open beneficial?” Dr. Behman said. “To put this another way, is it more important to avoid a bowel intervention or to avoid the morbidity associated with an open procedure?”
The secondary analysis confirmed that laparoscopic patients had lower rates of serious complications: in those without bowel intervention, 5% for laparoscopy vs. 10% for open; and in those who had a bowel intervention, 15% for laparoscopy vs. 22% for open. However, those who had an open procedure without a bowel intervention had lower complication rates than did those who had a laparoscopic operation with a bowel intervention, Dr. Behman said, “suggesting that perhaps the bowel intervention is a greater driver of serious complications than having an open procedure.”
These findings can inform patient selection, Dr. Behman added. “In light of the fact that laparoscopic approaches are likely associated with a greater risk of bowel intervention, appropriate caution should accompany these procedures,” he said. “In patients who are at high risk for a bowel intervention, an open approach or early conversion to a laparotomy should be considered.”
In his discussion of the study, Lawrence Diebel, MD, FACS, of Wayne State University, Detroit, asked if the study accounted for timing of surgery, the etiology of laparoscopic-related bowel interventions, and if serosal tears were included as bowel injuries. Dr. Behman said that the study did include timing of surgery in the initial analysis and found it to be a nonfactor. However, the database did not specify the causes for bowel resections or reasons for conversions.
Dr. Behman and Dr. Diebel reported having no financial disclosures
The complete manuscript of this study and its presentation at the American Surgical Association’s 137th Annual Meeting, April 2017, in Philadelphia, is to be published in the Annals of Surgery pending editorial review.
PHILADELPHIA – In surgery for small-bowel obstruction, laparoscopy is more likely to result in bowel injury than is open surgery but with ultimately better outcomes overall. This means that surgeons should proceed with “considerable caution” when performing minimally invasive surgery in the small bowel, researchers at University of Toronto reported during the annual meeting of the American Surgical Association.
In explaining the rationale for the study, Dr. Behman said, “Several studies have shown that over the last several years [that] the utilization of laparoscopic techniques for adhesive small-bowel obstruction has become increasingly common. However, these procedures have some inherent challenges.” Those technical challenges include introducing the trocar into an abdomen filled with distended bowel, trying to manipulate the distended bowel with laparoscopic bowel graspers, and the potentially ischemic bowel wall, he said.
The University of Toronto researchers performed a population-based retrospective cohort study of 8,584 patients in the Ministry of Health Ontario database from 2005 to 2014. The primary outcome was a composite, consisting of bowel repair, including operative billing codes for either via intraoperative enterotomy or suture repair of the intestine, or bowel resection. Secondary outcomes were serious complications and 30-day mortality.
During the study period, the share of SBO procedures performed laparoscopically increased from 4% in 2005 to 14.3% in 2014, Dr. Behman said.
“Patients in the laparoscopic group were slightly younger, had a lower overall comorbidity burden, and tended to be treated at larger hospitals and hospitals without teaching designations,” Dr. Behman said. Specifically, the average age of the 673 patients who had laparoscopy was 63 years vs. 67 years for the 7,911 open-procedure patients. As for comorbidities, 9% and 50% in the laparoscopic group were healthy and low users and high and very high users, respectively, vs. 7% and 57% of the open-procedure group.
The incidence of any bowel intervention was 53.5% in the laparoscopic group vs. 42% in the open group, Dr. Behman said. After a multivariable regression analysis, the researchers determined the following factors raised a patient’s odds of having a bowel intervention: older age; female sex; having had an after-hours procedure; and having had the procedure earlier in the study period. “However, even after [the researchers adjusted] for all of these covariates, the variable in our model that was most significantly associated with a bowel intervention was having had a laparoscopic procedure, with an odds ratio of 1.6,” Dr. Behman said.
When analyzing secondary outcomes, the researchers found that laparoscopy was associated with a lower risk of 30-day mortality (OR, 0.6) and serious complication (OR, 0.8). “So, there does appear to be a direct trade-off with laparoscopy probably having better clinical outcomes, but also being a risk factor for bowel intervention,” Dr. Behman said. So the researchers preformed a secondary analysis that divided the cohort into four subgroups: laparoscopy with and without bowel intervention; and open surgery with and without bowel intervention.
“The next question we wanted to ask was, in patients that are at high risk of a laparoscopic bowel injury, is an open approach or early conversion to open beneficial?” Dr. Behman said. “To put this another way, is it more important to avoid a bowel intervention or to avoid the morbidity associated with an open procedure?”
The secondary analysis confirmed that laparoscopic patients had lower rates of serious complications: in those without bowel intervention, 5% for laparoscopy vs. 10% for open; and in those who had a bowel intervention, 15% for laparoscopy vs. 22% for open. However, those who had an open procedure without a bowel intervention had lower complication rates than did those who had a laparoscopic operation with a bowel intervention, Dr. Behman said, “suggesting that perhaps the bowel intervention is a greater driver of serious complications than having an open procedure.”
These findings can inform patient selection, Dr. Behman added. “In light of the fact that laparoscopic approaches are likely associated with a greater risk of bowel intervention, appropriate caution should accompany these procedures,” he said. “In patients who are at high risk for a bowel intervention, an open approach or early conversion to a laparotomy should be considered.”
In his discussion of the study, Lawrence Diebel, MD, FACS, of Wayne State University, Detroit, asked if the study accounted for timing of surgery, the etiology of laparoscopic-related bowel interventions, and if serosal tears were included as bowel injuries. Dr. Behman said that the study did include timing of surgery in the initial analysis and found it to be a nonfactor. However, the database did not specify the causes for bowel resections or reasons for conversions.
Dr. Behman and Dr. Diebel reported having no financial disclosures
The complete manuscript of this study and its presentation at the American Surgical Association’s 137th Annual Meeting, April 2017, in Philadelphia, is to be published in the Annals of Surgery pending editorial review.
AT THE ASA ANNUAL MEETING
Key clinical point: Laparoscopic surgery for SBO is associated with a greater risk of bowel intervention.
Major finding: The incidence of bowel intervention was 53.5% vs. 43.4% in laparoscopic and open procedures, respectively.
Data source: Review of 8,584 procedures for SBO in the Ministry of Health Ontario database performed from 2005 to 2014.
Disclosures: Dr. Behman and Dr. Diebel reported having no financial disclosures.