User login
The Role of Toluidine Blue in Mohs Micrographic Surgery: A Systematic Review
Toluidine blue (TB), a dye with metachromatic staining properties, was developed in 1856 by William Henry Perkin.1 Metachromasia is a perceptible change in the color of staining of living tissue due to the electrochemical properties of the tissue. Tissues that contain high concentrations of ionized sulfate and phosphate groups (high concentrations of free electronegative groups) form polymeric aggregates of the basic dye solution that alter the absorbed wavelengths of light.2 The function of this characteristic is to use a single dye to highlight different structures in tissue based on their relative chemical differences.3
Toluidine blue primarily was used within the dye industry until the 1960s, when it was first used in vital staining of the oral mucosa.2 Because of the tissue absorption potential, this technique was used to detect the location of oral malignancies.4 Since then, TB has progressively been used for staining fresh frozen sections in Mohs micrographic surgery (MMS). In a 2003 survey study (N=310), 16.8% of surgeons performing MMS reported using TB in their laboratory.5 We sought to systematically review the published literature describing the uses of TB in the setting of fresh frozen sections and MMS.
Methods
We conducted a systematic search of the PubMed and Cochrane databases for articles published before December 1, 2019, to identify any relevant studies in English. Electronic searches were performed using the terms toluidine blue and Mohs or Mohs micrographic surgery. We manually checked the bibliographies of the identified articles to further identify eligible studies.
Eligibility Criteria—The inclusion criteria were articles that (1) considered TB in the context of MMS, (2) were published in peer-reviewed journals, (3) were published in English, and (4) were available as full text. Systematic reviews were excluded.
Data Extraction and Outcomes—All relevant information regarding the study characteristics, including design, level of evidence, methodologic quality of evidence, pathology examined, and outcome measures, were collected by 2 independent reviewers (T.L. and A.D.) using a predetermined data sheet. The same 2 reviewers were used for all steps of the review process, data were independently obtained, and any discrepancy was introduced for a third opinion (D.H.) and agreed upon by the majority.
Quality Assessment—The level of evidence was evaluated based on the criteria of the Oxford Centre for Evidence-Based Medicine. Two reviewers (T.L. and A.D.) graded each article included in the review.
Results
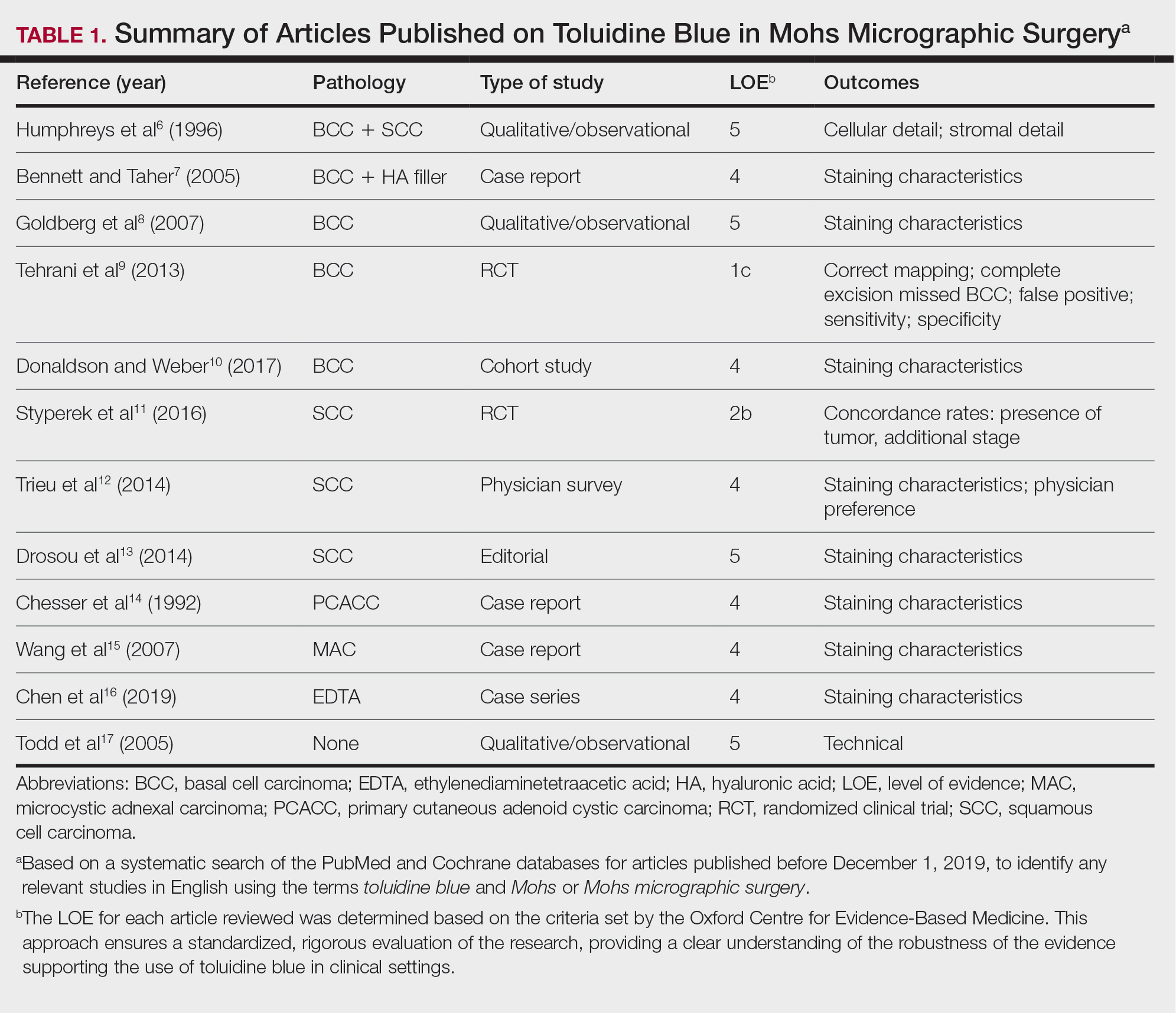
A total of 25 articles were reviewed. After the titles and abstracts were screened for relevance, 12 articles remained (Figure 1). Of these, 1 compared basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), 4 were related to BCC, 3 were related to SCC, 1 was related to microcystic adnexal carcinoma (MAC), 1 was related to primary cutaneous adenoid cystic carcinoma (PCACC), and 2 were related to technical aspects of the staining process (Table 1).
A majority of the articles included in this review were qualitative and observational in nature, describing the staining characteristics of TB. Study characteristics are summarized in Table 1.
Comment
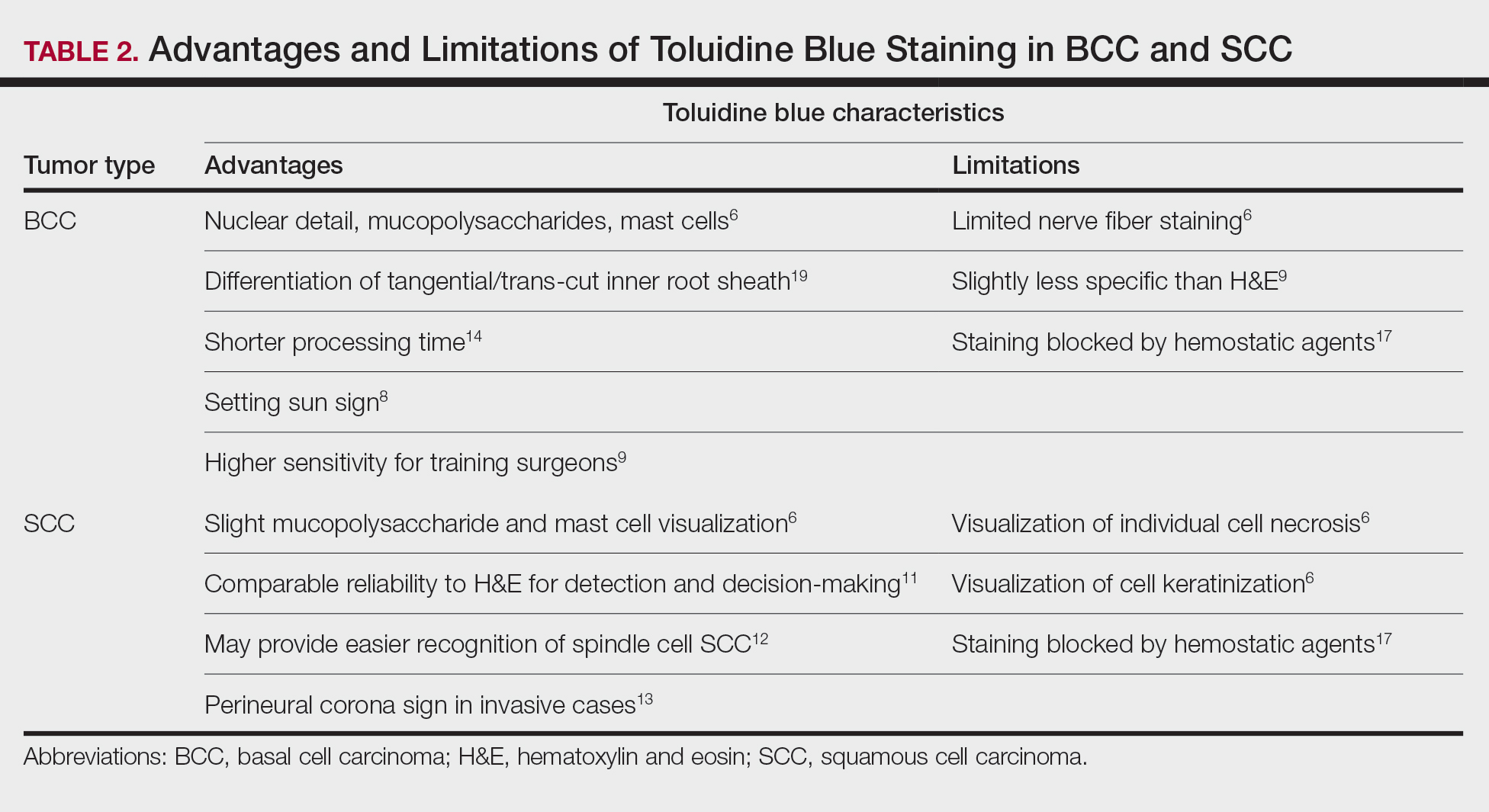
Basal Cell Carcinoma—Toluidine blue staining characteristics help to identify BCC nests by differentiating them from hair follicles in frozen sections. The metachromatic characteristic of TB stains the inner root sheath deep blue and highlights the surrounding stromal mucin of BCC a magenta color.18,19 In hematoxylin and eosin (H&E) stains, these 2 distinct structures can be differentiated by cleft formation around tumor nests, mitotic figures, and the lack of a fibrous sheath present in BCC tumors.20 The advantages and limitations of TB staining of BCC are presented in Table 2.
Humphreys et al6 suggested a noticeable difference between H&E and TB in the staining of cellular and stromal components. The nuclear detail of tumor cells was subjectively sharper and clearer with TB staining. The staining of stromal components may provide the most assistance in locating BCC islands. Mucopolysaccharide staining may be absent in H&E but stain a deep magenta with TB. Although the presence of mucopolysaccharides does not specifically indicate a tumor, it may prompt further attention and provide an indicator for sparse and infiltrative tumor cells.6 The metachromatic stromal change may indicate a narrow tumor-free margin where additional deeper sections often reveal tumor that may warrant additional resection margin in more aggressive malignancies. In particular, sclerosing/morpheaform BCCs have been shown to induce glycosaminoglycan synthesis and are highlighted more readily with TB than with H&E when compared to surrounding tissue.21 This differentiation in staining has remained a popular reason to routinely incorporate TB into the staining of infiltrative and morpheaform variants of BCC. Additionally, stromal mast cells are believed to be more abundant in the stroma of BCC and are more readily visualized in tissue specimens stained with TB, appearing as bright purple metachromatic granules. These granules are larger than normal and are increased in number.6
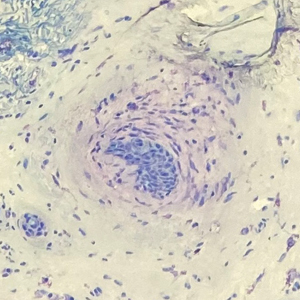
The margin behavior of BCC stained with TB was further characterized by Goldberg et al,8 who coined the term setting sun sign, which may be present in sequential sections of a disappearing nodule of a BCC tumor. Stroma, inflammatory infiltrate, and mast cells produce a magenta glow surrounding BCC tumors that is reminiscent of a setting sun (Figure 2). Invasive BCC is considered variable in this presentation, primarily because of zones of cell-free fluid and edema or the second area of inflammatory cells. This unique sign may benefit the inspecting Mohs surgeon by providing a clue to an underlying process that may have residual BCC tumors. The setting sun sign also may assist in identifying exact surgical margins.8

The nasal surface has a predilection for BCC.22 The skin of the nose has numerous look-alike structures to consider for complete tumor removal and avoidance of unnecessary removal. One challenge is distinguishing follicular basaloid proliferations (FBP) from BCC, a scenario that is more common on the nose.22 When TB staining was used, the sensitivity for detecting FBP reached 100% in 34 cases reviewed by Donaldson and Weber.10 None of the cases examined showed TB metachromasia surrounding FBP, thus indicating that TB can dependably identify this benign entity. Conversely, 5% (N=279) of BCCs confirmed on H&E did not exhibit surrounding TB metachromasia. This finding is concerning regarding the specificity of TB staining for BCC, but the authors of this study suggested the possibility that these exceptions were benign “simulants” (ie, trichoepithelioma) of BCC.10
The use of TB also has been shown to be statistically beneficial in Mohs training. In a single-center, single-fellow experiment, the sensitivity and specificity of using TB for BCC were extrapolated.9 Using TB as an adjunct in deep sections showed superior sensitivity to H&E alone in identifying BCC, increasing sensitivity from 96.3% to 99.7%. In a cohort of 352 BCC excisions and frozen sections, only 1 BCC was not completely excised. If H&E only had been performed, the fellow would have missed 13 residual BCC tumors.9
Bennett and Taher7 described a case in which hyaluronic acid (HA) from a filler injection was confused with the HA surrounding BCC tumor nests. They found that when TB is used as an adjunct, the HA filler is easier to differentiate from the HA surrounding the BCC tumor nests. In frozen sections stained with TB, the HA filler appeared as an amorphous, metachromatic, reddish-purple, whereas the HA surrounding the BCC tumor nests appeared as a well-defined red. These findings were less obvious in the same sections stained with H&E alone.7
Squamous Cell Carcinoma—In early investigations, the utility of TB in identifying SCC in frozen sections was thought to be limited. The description by Humphreys and colleagues6 of staining characteristics in SCC suggested that the nuclear detail that H&E provides is more easily recognized. The deep aqua nuclear staining produced with TB was considered more difficult to observe than the cytoplasmic eosinophilia of pyknotic and keratinizing cells in H&E.6
Toluidine blue may be beneficial in providing unique staining characteristics to further detail tumors that are difficult to interpret, such as spindle cell SCC and perineural invasion of aggressive SCC. In H&E, squamous cells of spindle cell SCC (scSCC) blend into the background of inflammatory cells and can be perceptibly difficult to locate. A small cohort of 3 Mohs surgeons who routinely use H&E were surveyed on their ability to detect a proven scSCC in H&E or TB by photograph.12 All 3 were able to detect the scSCC in the TB photographs, but only 2 of 3 were able to detect it in H&E photographs. All 3 surgeons agreed that TB was preferable to H&E for this tumor type. These findings suggested that TB may be superior and preferred over H&E for visualizing tumor cells of scSCC.12 The TB staining characteristics of perineural invasion of aggressive SCC have been referred to as the perineural corona sign because of the bright magenta stain that forms around affected nerves.13 Drosou et al13 suggested that TB may enhance the diagnostic accuracy for perineural SCC.
Rare Tumors—The adjunctive use of TB with H&E has been examined in rare tumors. Published reports have highlighted its use in MMS for treating MAC and PCACC. Toluidine blue exhibits staining advantages for these tumors. It may render isolated nests and perineural invasion of MAC more easily visible on frozen section.15
Although PCACC is rare, the recurrence rate is high.23 Toluidine blue has been used with MMS to ensure complete removal and higher cure rates. The metachromatic nature of TB is advantageous in staining the HA present in these tumors. Those who have reported the use of TB for PCACC prefer it to H&E for frozen sections.14
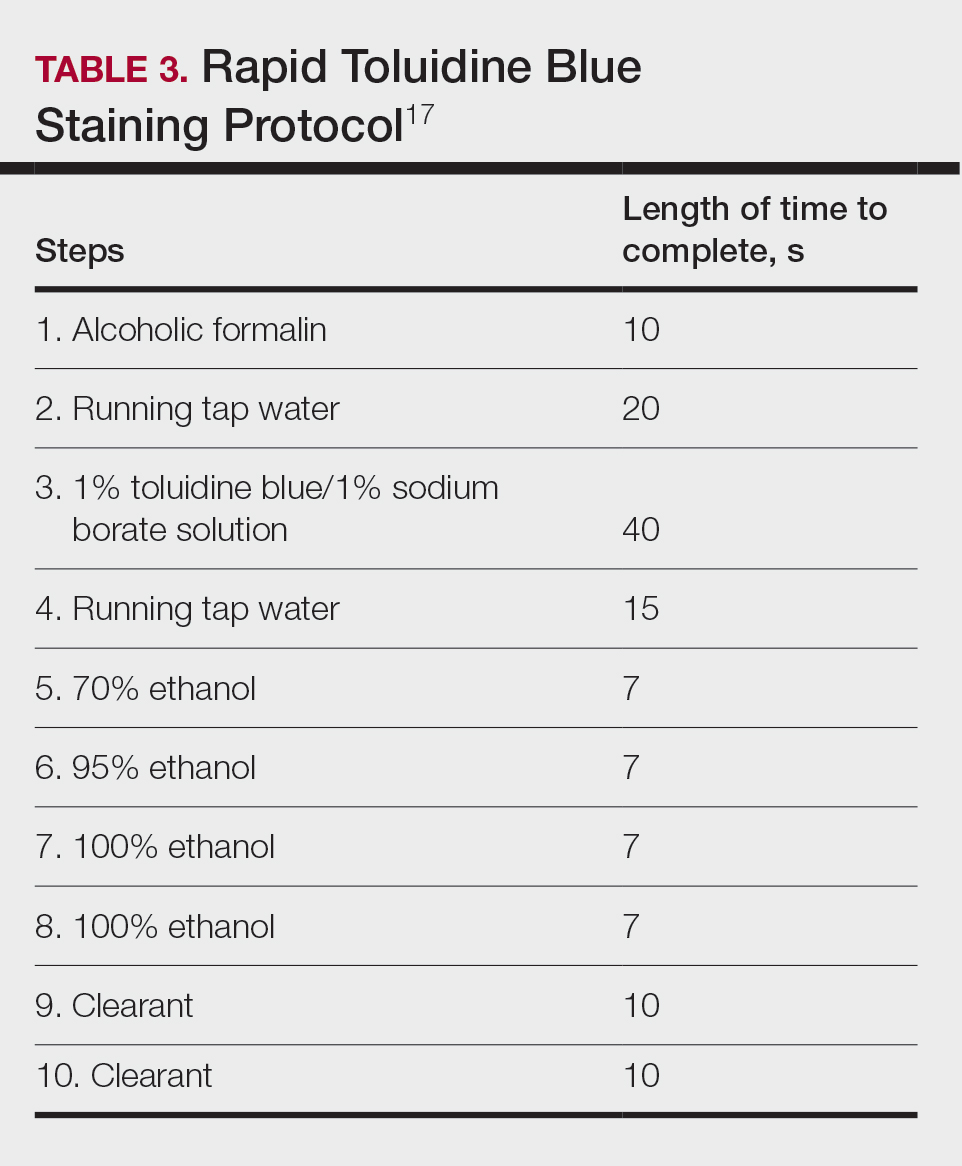
Technical Aspects—The staining time for TB-treated slides is reduced compared to H&E staining; staining can be efficiently done in frozen sections in less than 2.5 minutes using the method shown in Table 3.17 In comparison, typical H&E staining takes 9 minutes, and older TB techniques take 7 minutes.6
Conclusion
Toluidine blue may play an important and helpful role in the successful diagnosis and treatment of particular cutaneous tumors by providing additional diagnostic information. Although surgeons performing MMS will continue using the staining protocols with which they are most comfortable, adjunctive use of TB over time may provide an additional benefit at low risk for disrupting practice efficiency or workflow. Many Mohs surgeons are accustomed to using this stain, even preferring to interpret only TB-stained slides for cutaneous malignancy. Most published studies on this topic have been observational in nature, and additional controlled trials may be warranted to determine the effects on outcomes in real-world practice.
- Culling CF, Allison TR. Cellular Pathology Technique. 4th ed. Butterworths; 1985.
- Bergeron JA, Singer M. Metachromasy: an experimental and theoretical reevaluation. J Biophys Biochem Cytol. 1958;4:433-457. doi:10.1083/jcb.4.4.433
- Epstein JB, Scully C, Spinelli J. Toluidine blue and Lugol’s iodine application in the assessment of oral malignant disease and lesions at risk of malignancy. J Oral Pathol Med. 1992;21:160-163. doi:10.1111/j.1600-0714.1992.tb00094.x
- Warnakulasuriya KA, Johnson NW. Sensitivity and specificity of OraScan (R) toluidine blue mouthrinse in the detection of oral cancer and precancer. J Oral Pathol Med. 1996;25:97-103. doi:10.1111/j.1600-0714.1996.tb00201.x
- Silapunt S, Peterson SR, Alcalay J, et al. Mohs tissue mapping and processing: a survey study. Dermatol Surg. 2003;29:1109-1112; discussion 1112.
- Humphreys TR, Nemeth A, McCrevey S, et al. A pilot study comparing toluidine blue and hematoxylin and eosin staining of basal cell and squamous cell carcinoma during Mohs surgery. Dermatol Surg. 1996;22:693-697. doi:10.1111/j.1524-4725.1996.tb00619.x
- Bennett R, Taher M. Restylane persistent for 23 months found during Mohs micrographic surgery: a source of confusion with hyaluronic acid surrounding basal cell carcinoma. Dermatol Surg. 2005;31:1366-1369. doi:10.1111/j.1524-4725.2005.31223
- Goldberg LH, Wang SQ, Kimyai-Asadi A. The setting sun sign: visualizing the margins of a basal cell carcinoma on serial frozen sections stained with toluidine blue. Dermatol Surg. 2007;33:761-763. doi:10.1111/j.1524-4725.2007.33158.x
- Tehrani H, May K, Morris A, et al. Does the dual use of toluidine blue and hematoxylin and eosin staining improve basal cell carcinoma detection by Mohs surgery trainees? Dermatol Surg. 2013;39:995-1000. doi:10.1111/dsu.12180
- Donaldson MR, Weber LA. Toluidine blue supports differentiation of folliculocentric basaloid proliferation from basal cell carcinoma on frozen sections in a small single-practice cohort. Dermatol Surg. 2017;43:1303-1306. doi:10.1097/DSS.0000000000001107
- Styperek AR, Goldberg LH, Goldschmidt LE, et al. Toluidine blue and hematoxylin and eosin stains are comparable in evaluating squamous cell carcinoma during Mohs. Dermatol Surg. 2016;42:1279-1284. doi:10.1097/DSS.0000000000000872
- Trieu D, Drosou A, Goldberg LH, et al. Detecting spindle cell squamous cell carcinomas with toluidine blue on frozen sections. Dermatol Surg. 2014;40:1259-1260. doi:10.1097/DSS.0000000000000147
- Drosou A, Trieu D, Goldberg LH, et al. The perineural corona sign: enhancing detection of perineural squamous cell carcinoma during Mohs micrographic surgery with toluidine blue stain. J Am Acad Dermatol. 2014;71:826-827. doi:10.1016/j.jaad.2014.04.076
- Chesser RS, Bertler DE, Fitzpatrick JE, et al. Primary cutaneous adenoid cystic carcinoma treated with Mohs micrographic surgery toluidine blue technique. J Dermatol Surg Oncol. 1992;18:175-176. doi:10.1111/j.1524-4725.1992.tb02794.x
- Wang SQ, Goldberg LH, Nemeth A. The merits of adding toluidine blue-stained slides in Mohs surgery in the treatment of a microcystic adnexal carcinoma. J Am Acad Dermatol. 2007;56:1067-1069. doi:10.1016/j.jaad.2007.01.008
- Chen CL, Wilson S, Afzalneia R, et al. Topical aluminum chloride and Monsel’s solution block toluidine blue staining in Mohs frozen sections: mechanism and solution. Dermatol Surg. 2019;45:1019-1025. doi:10.1097/DSS.0000000000001761
- Todd MM, Lee JW, Marks VJ. Rapid toluidine blue stain for Mohs’ micrographic surgery. Dermatol Surg. 2005;31:244-245. doi:10.1111/j.1524-4725.2005.31053
- Picoto AM, Picoto A. Technical procedures for Mohs fresh tissue surgery. J Derm Surg Oncol. 1986;12:134-138. doi:10.1111/j.1524-4725.1986.tb01442.x
- Sperling LC, Winton GB. The transverse anatomy of androgenic alopecia. J Derm Surg Oncol. 1990;16:1127-1133. doi:10.1111/j.1524 -4725.1990.tb00024.x
- Smith-Zagone MJ, Schwartz MR. Frozen section of skin specimens. Arch Pathol Lab Med. 2005;129:1536-1543. doi:10.5858/2005-129-1536-FSOSS
- Moy RL, Potter TS, Uitto J. Increased glycosaminoglycans production in sclerosing basal cell carcinoma–derived fibroblasts and stimulation of normal skin fibroblast glycosaminoglycans production by a cytokine-derived from sclerosing basal cell carcinoma. Dermatol Surg. 2000;26:1029-1036. doi:10.1046/j.1524-4725.2000.0260111029.x
- Leshin B, White WL. Folliculocentric basaloid proliferation. The bulge (der Wulst) revisited. Arch Dermatol. 1990;126:900-906. doi:10.1001/archderm.126.7.900
- Seab JA, Graham JH. Primary cutaneous adenoid cystic carcinoma.J Am Acad Dermatol. 1987;17:113-118. doi:10.1016/s0190 -9622(87)70182-0
Toluidine blue (TB), a dye with metachromatic staining properties, was developed in 1856 by William Henry Perkin.1 Metachromasia is a perceptible change in the color of staining of living tissue due to the electrochemical properties of the tissue. Tissues that contain high concentrations of ionized sulfate and phosphate groups (high concentrations of free electronegative groups) form polymeric aggregates of the basic dye solution that alter the absorbed wavelengths of light.2 The function of this characteristic is to use a single dye to highlight different structures in tissue based on their relative chemical differences.3
Toluidine blue primarily was used within the dye industry until the 1960s, when it was first used in vital staining of the oral mucosa.2 Because of the tissue absorption potential, this technique was used to detect the location of oral malignancies.4 Since then, TB has progressively been used for staining fresh frozen sections in Mohs micrographic surgery (MMS). In a 2003 survey study (N=310), 16.8% of surgeons performing MMS reported using TB in their laboratory.5 We sought to systematically review the published literature describing the uses of TB in the setting of fresh frozen sections and MMS.
Methods
We conducted a systematic search of the PubMed and Cochrane databases for articles published before December 1, 2019, to identify any relevant studies in English. Electronic searches were performed using the terms toluidine blue and Mohs or Mohs micrographic surgery. We manually checked the bibliographies of the identified articles to further identify eligible studies.
Eligibility Criteria—The inclusion criteria were articles that (1) considered TB in the context of MMS, (2) were published in peer-reviewed journals, (3) were published in English, and (4) were available as full text. Systematic reviews were excluded.
Data Extraction and Outcomes—All relevant information regarding the study characteristics, including design, level of evidence, methodologic quality of evidence, pathology examined, and outcome measures, were collected by 2 independent reviewers (T.L. and A.D.) using a predetermined data sheet. The same 2 reviewers were used for all steps of the review process, data were independently obtained, and any discrepancy was introduced for a third opinion (D.H.) and agreed upon by the majority.
Quality Assessment—The level of evidence was evaluated based on the criteria of the Oxford Centre for Evidence-Based Medicine. Two reviewers (T.L. and A.D.) graded each article included in the review.

Results
A total of 25 articles were reviewed. After the titles and abstracts were screened for relevance, 12 articles remained (Figure 1). Of these, 1 compared basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), 4 were related to BCC, 3 were related to SCC, 1 was related to microcystic adnexal carcinoma (MAC), 1 was related to primary cutaneous adenoid cystic carcinoma (PCACC), and 2 were related to technical aspects of the staining process (Table 1).

A majority of the articles included in this review were qualitative and observational in nature, describing the staining characteristics of TB. Study characteristics are summarized in Table 1.
Comment
Basal Cell Carcinoma—Toluidine blue staining characteristics help to identify BCC nests by differentiating them from hair follicles in frozen sections. The metachromatic characteristic of TB stains the inner root sheath deep blue and highlights the surrounding stromal mucin of BCC a magenta color.18,19 In hematoxylin and eosin (H&E) stains, these 2 distinct structures can be differentiated by cleft formation around tumor nests, mitotic figures, and the lack of a fibrous sheath present in BCC tumors.20 The advantages and limitations of TB staining of BCC are presented in Table 2.

Humphreys et al6 suggested a noticeable difference between H&E and TB in the staining of cellular and stromal components. The nuclear detail of tumor cells was subjectively sharper and clearer with TB staining. The staining of stromal components may provide the most assistance in locating BCC islands. Mucopolysaccharide staining may be absent in H&E but stain a deep magenta with TB. Although the presence of mucopolysaccharides does not specifically indicate a tumor, it may prompt further attention and provide an indicator for sparse and infiltrative tumor cells.6 The metachromatic stromal change may indicate a narrow tumor-free margin where additional deeper sections often reveal tumor that may warrant additional resection margin in more aggressive malignancies. In particular, sclerosing/morpheaform BCCs have been shown to induce glycosaminoglycan synthesis and are highlighted more readily with TB than with H&E when compared to surrounding tissue.21 This differentiation in staining has remained a popular reason to routinely incorporate TB into the staining of infiltrative and morpheaform variants of BCC. Additionally, stromal mast cells are believed to be more abundant in the stroma of BCC and are more readily visualized in tissue specimens stained with TB, appearing as bright purple metachromatic granules. These granules are larger than normal and are increased in number.6
The margin behavior of BCC stained with TB was further characterized by Goldberg et al,8 who coined the term setting sun sign, which may be present in sequential sections of a disappearing nodule of a BCC tumor. Stroma, inflammatory infiltrate, and mast cells produce a magenta glow surrounding BCC tumors that is reminiscent of a setting sun (Figure 2). Invasive BCC is considered variable in this presentation, primarily because of zones of cell-free fluid and edema or the second area of inflammatory cells. This unique sign may benefit the inspecting Mohs surgeon by providing a clue to an underlying process that may have residual BCC tumors. The setting sun sign also may assist in identifying exact surgical margins.8

The nasal surface has a predilection for BCC.22 The skin of the nose has numerous look-alike structures to consider for complete tumor removal and avoidance of unnecessary removal. One challenge is distinguishing follicular basaloid proliferations (FBP) from BCC, a scenario that is more common on the nose.22 When TB staining was used, the sensitivity for detecting FBP reached 100% in 34 cases reviewed by Donaldson and Weber.10 None of the cases examined showed TB metachromasia surrounding FBP, thus indicating that TB can dependably identify this benign entity. Conversely, 5% (N=279) of BCCs confirmed on H&E did not exhibit surrounding TB metachromasia. This finding is concerning regarding the specificity of TB staining for BCC, but the authors of this study suggested the possibility that these exceptions were benign “simulants” (ie, trichoepithelioma) of BCC.10
The use of TB also has been shown to be statistically beneficial in Mohs training. In a single-center, single-fellow experiment, the sensitivity and specificity of using TB for BCC were extrapolated.9 Using TB as an adjunct in deep sections showed superior sensitivity to H&E alone in identifying BCC, increasing sensitivity from 96.3% to 99.7%. In a cohort of 352 BCC excisions and frozen sections, only 1 BCC was not completely excised. If H&E only had been performed, the fellow would have missed 13 residual BCC tumors.9
Bennett and Taher7 described a case in which hyaluronic acid (HA) from a filler injection was confused with the HA surrounding BCC tumor nests. They found that when TB is used as an adjunct, the HA filler is easier to differentiate from the HA surrounding the BCC tumor nests. In frozen sections stained with TB, the HA filler appeared as an amorphous, metachromatic, reddish-purple, whereas the HA surrounding the BCC tumor nests appeared as a well-defined red. These findings were less obvious in the same sections stained with H&E alone.7
Squamous Cell Carcinoma—In early investigations, the utility of TB in identifying SCC in frozen sections was thought to be limited. The description by Humphreys and colleagues6 of staining characteristics in SCC suggested that the nuclear detail that H&E provides is more easily recognized. The deep aqua nuclear staining produced with TB was considered more difficult to observe than the cytoplasmic eosinophilia of pyknotic and keratinizing cells in H&E.6
Toluidine blue may be beneficial in providing unique staining characteristics to further detail tumors that are difficult to interpret, such as spindle cell SCC and perineural invasion of aggressive SCC. In H&E, squamous cells of spindle cell SCC (scSCC) blend into the background of inflammatory cells and can be perceptibly difficult to locate. A small cohort of 3 Mohs surgeons who routinely use H&E were surveyed on their ability to detect a proven scSCC in H&E or TB by photograph.12 All 3 were able to detect the scSCC in the TB photographs, but only 2 of 3 were able to detect it in H&E photographs. All 3 surgeons agreed that TB was preferable to H&E for this tumor type. These findings suggested that TB may be superior and preferred over H&E for visualizing tumor cells of scSCC.12 The TB staining characteristics of perineural invasion of aggressive SCC have been referred to as the perineural corona sign because of the bright magenta stain that forms around affected nerves.13 Drosou et al13 suggested that TB may enhance the diagnostic accuracy for perineural SCC.
Rare Tumors—The adjunctive use of TB with H&E has been examined in rare tumors. Published reports have highlighted its use in MMS for treating MAC and PCACC. Toluidine blue exhibits staining advantages for these tumors. It may render isolated nests and perineural invasion of MAC more easily visible on frozen section.15
Although PCACC is rare, the recurrence rate is high.23 Toluidine blue has been used with MMS to ensure complete removal and higher cure rates. The metachromatic nature of TB is advantageous in staining the HA present in these tumors. Those who have reported the use of TB for PCACC prefer it to H&E for frozen sections.14
Technical Aspects—The staining time for TB-treated slides is reduced compared to H&E staining; staining can be efficiently done in frozen sections in less than 2.5 minutes using the method shown in Table 3.17 In comparison, typical H&E staining takes 9 minutes, and older TB techniques take 7 minutes.6

Conclusion
Toluidine blue may play an important and helpful role in the successful diagnosis and treatment of particular cutaneous tumors by providing additional diagnostic information. Although surgeons performing MMS will continue using the staining protocols with which they are most comfortable, adjunctive use of TB over time may provide an additional benefit at low risk for disrupting practice efficiency or workflow. Many Mohs surgeons are accustomed to using this stain, even preferring to interpret only TB-stained slides for cutaneous malignancy. Most published studies on this topic have been observational in nature, and additional controlled trials may be warranted to determine the effects on outcomes in real-world practice.
Toluidine blue (TB), a dye with metachromatic staining properties, was developed in 1856 by William Henry Perkin.1 Metachromasia is a perceptible change in the color of staining of living tissue due to the electrochemical properties of the tissue. Tissues that contain high concentrations of ionized sulfate and phosphate groups (high concentrations of free electronegative groups) form polymeric aggregates of the basic dye solution that alter the absorbed wavelengths of light.2 The function of this characteristic is to use a single dye to highlight different structures in tissue based on their relative chemical differences.3
Toluidine blue primarily was used within the dye industry until the 1960s, when it was first used in vital staining of the oral mucosa.2 Because of the tissue absorption potential, this technique was used to detect the location of oral malignancies.4 Since then, TB has progressively been used for staining fresh frozen sections in Mohs micrographic surgery (MMS). In a 2003 survey study (N=310), 16.8% of surgeons performing MMS reported using TB in their laboratory.5 We sought to systematically review the published literature describing the uses of TB in the setting of fresh frozen sections and MMS.
Methods
We conducted a systematic search of the PubMed and Cochrane databases for articles published before December 1, 2019, to identify any relevant studies in English. Electronic searches were performed using the terms toluidine blue and Mohs or Mohs micrographic surgery. We manually checked the bibliographies of the identified articles to further identify eligible studies.
Eligibility Criteria—The inclusion criteria were articles that (1) considered TB in the context of MMS, (2) were published in peer-reviewed journals, (3) were published in English, and (4) were available as full text. Systematic reviews were excluded.
Data Extraction and Outcomes—All relevant information regarding the study characteristics, including design, level of evidence, methodologic quality of evidence, pathology examined, and outcome measures, were collected by 2 independent reviewers (T.L. and A.D.) using a predetermined data sheet. The same 2 reviewers were used for all steps of the review process, data were independently obtained, and any discrepancy was introduced for a third opinion (D.H.) and agreed upon by the majority.
Quality Assessment—The level of evidence was evaluated based on the criteria of the Oxford Centre for Evidence-Based Medicine. Two reviewers (T.L. and A.D.) graded each article included in the review.

Results
A total of 25 articles were reviewed. After the titles and abstracts were screened for relevance, 12 articles remained (Figure 1). Of these, 1 compared basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), 4 were related to BCC, 3 were related to SCC, 1 was related to microcystic adnexal carcinoma (MAC), 1 was related to primary cutaneous adenoid cystic carcinoma (PCACC), and 2 were related to technical aspects of the staining process (Table 1).

A majority of the articles included in this review were qualitative and observational in nature, describing the staining characteristics of TB. Study characteristics are summarized in Table 1.
Comment
Basal Cell Carcinoma—Toluidine blue staining characteristics help to identify BCC nests by differentiating them from hair follicles in frozen sections. The metachromatic characteristic of TB stains the inner root sheath deep blue and highlights the surrounding stromal mucin of BCC a magenta color.18,19 In hematoxylin and eosin (H&E) stains, these 2 distinct structures can be differentiated by cleft formation around tumor nests, mitotic figures, and the lack of a fibrous sheath present in BCC tumors.20 The advantages and limitations of TB staining of BCC are presented in Table 2.

Humphreys et al6 suggested a noticeable difference between H&E and TB in the staining of cellular and stromal components. The nuclear detail of tumor cells was subjectively sharper and clearer with TB staining. The staining of stromal components may provide the most assistance in locating BCC islands. Mucopolysaccharide staining may be absent in H&E but stain a deep magenta with TB. Although the presence of mucopolysaccharides does not specifically indicate a tumor, it may prompt further attention and provide an indicator for sparse and infiltrative tumor cells.6 The metachromatic stromal change may indicate a narrow tumor-free margin where additional deeper sections often reveal tumor that may warrant additional resection margin in more aggressive malignancies. In particular, sclerosing/morpheaform BCCs have been shown to induce glycosaminoglycan synthesis and are highlighted more readily with TB than with H&E when compared to surrounding tissue.21 This differentiation in staining has remained a popular reason to routinely incorporate TB into the staining of infiltrative and morpheaform variants of BCC. Additionally, stromal mast cells are believed to be more abundant in the stroma of BCC and are more readily visualized in tissue specimens stained with TB, appearing as bright purple metachromatic granules. These granules are larger than normal and are increased in number.6
The margin behavior of BCC stained with TB was further characterized by Goldberg et al,8 who coined the term setting sun sign, which may be present in sequential sections of a disappearing nodule of a BCC tumor. Stroma, inflammatory infiltrate, and mast cells produce a magenta glow surrounding BCC tumors that is reminiscent of a setting sun (Figure 2). Invasive BCC is considered variable in this presentation, primarily because of zones of cell-free fluid and edema or the second area of inflammatory cells. This unique sign may benefit the inspecting Mohs surgeon by providing a clue to an underlying process that may have residual BCC tumors. The setting sun sign also may assist in identifying exact surgical margins.8

The nasal surface has a predilection for BCC.22 The skin of the nose has numerous look-alike structures to consider for complete tumor removal and avoidance of unnecessary removal. One challenge is distinguishing follicular basaloid proliferations (FBP) from BCC, a scenario that is more common on the nose.22 When TB staining was used, the sensitivity for detecting FBP reached 100% in 34 cases reviewed by Donaldson and Weber.10 None of the cases examined showed TB metachromasia surrounding FBP, thus indicating that TB can dependably identify this benign entity. Conversely, 5% (N=279) of BCCs confirmed on H&E did not exhibit surrounding TB metachromasia. This finding is concerning regarding the specificity of TB staining for BCC, but the authors of this study suggested the possibility that these exceptions were benign “simulants” (ie, trichoepithelioma) of BCC.10
The use of TB also has been shown to be statistically beneficial in Mohs training. In a single-center, single-fellow experiment, the sensitivity and specificity of using TB for BCC were extrapolated.9 Using TB as an adjunct in deep sections showed superior sensitivity to H&E alone in identifying BCC, increasing sensitivity from 96.3% to 99.7%. In a cohort of 352 BCC excisions and frozen sections, only 1 BCC was not completely excised. If H&E only had been performed, the fellow would have missed 13 residual BCC tumors.9
Bennett and Taher7 described a case in which hyaluronic acid (HA) from a filler injection was confused with the HA surrounding BCC tumor nests. They found that when TB is used as an adjunct, the HA filler is easier to differentiate from the HA surrounding the BCC tumor nests. In frozen sections stained with TB, the HA filler appeared as an amorphous, metachromatic, reddish-purple, whereas the HA surrounding the BCC tumor nests appeared as a well-defined red. These findings were less obvious in the same sections stained with H&E alone.7
Squamous Cell Carcinoma—In early investigations, the utility of TB in identifying SCC in frozen sections was thought to be limited. The description by Humphreys and colleagues6 of staining characteristics in SCC suggested that the nuclear detail that H&E provides is more easily recognized. The deep aqua nuclear staining produced with TB was considered more difficult to observe than the cytoplasmic eosinophilia of pyknotic and keratinizing cells in H&E.6
Toluidine blue may be beneficial in providing unique staining characteristics to further detail tumors that are difficult to interpret, such as spindle cell SCC and perineural invasion of aggressive SCC. In H&E, squamous cells of spindle cell SCC (scSCC) blend into the background of inflammatory cells and can be perceptibly difficult to locate. A small cohort of 3 Mohs surgeons who routinely use H&E were surveyed on their ability to detect a proven scSCC in H&E or TB by photograph.12 All 3 were able to detect the scSCC in the TB photographs, but only 2 of 3 were able to detect it in H&E photographs. All 3 surgeons agreed that TB was preferable to H&E for this tumor type. These findings suggested that TB may be superior and preferred over H&E for visualizing tumor cells of scSCC.12 The TB staining characteristics of perineural invasion of aggressive SCC have been referred to as the perineural corona sign because of the bright magenta stain that forms around affected nerves.13 Drosou et al13 suggested that TB may enhance the diagnostic accuracy for perineural SCC.
Rare Tumors—The adjunctive use of TB with H&E has been examined in rare tumors. Published reports have highlighted its use in MMS for treating MAC and PCACC. Toluidine blue exhibits staining advantages for these tumors. It may render isolated nests and perineural invasion of MAC more easily visible on frozen section.15
Although PCACC is rare, the recurrence rate is high.23 Toluidine blue has been used with MMS to ensure complete removal and higher cure rates. The metachromatic nature of TB is advantageous in staining the HA present in these tumors. Those who have reported the use of TB for PCACC prefer it to H&E for frozen sections.14
Technical Aspects—The staining time for TB-treated slides is reduced compared to H&E staining; staining can be efficiently done in frozen sections in less than 2.5 minutes using the method shown in Table 3.17 In comparison, typical H&E staining takes 9 minutes, and older TB techniques take 7 minutes.6

Conclusion
Toluidine blue may play an important and helpful role in the successful diagnosis and treatment of particular cutaneous tumors by providing additional diagnostic information. Although surgeons performing MMS will continue using the staining protocols with which they are most comfortable, adjunctive use of TB over time may provide an additional benefit at low risk for disrupting practice efficiency or workflow. Many Mohs surgeons are accustomed to using this stain, even preferring to interpret only TB-stained slides for cutaneous malignancy. Most published studies on this topic have been observational in nature, and additional controlled trials may be warranted to determine the effects on outcomes in real-world practice.
- Culling CF, Allison TR. Cellular Pathology Technique. 4th ed. Butterworths; 1985.
- Bergeron JA, Singer M. Metachromasy: an experimental and theoretical reevaluation. J Biophys Biochem Cytol. 1958;4:433-457. doi:10.1083/jcb.4.4.433
- Epstein JB, Scully C, Spinelli J. Toluidine blue and Lugol’s iodine application in the assessment of oral malignant disease and lesions at risk of malignancy. J Oral Pathol Med. 1992;21:160-163. doi:10.1111/j.1600-0714.1992.tb00094.x
- Warnakulasuriya KA, Johnson NW. Sensitivity and specificity of OraScan (R) toluidine blue mouthrinse in the detection of oral cancer and precancer. J Oral Pathol Med. 1996;25:97-103. doi:10.1111/j.1600-0714.1996.tb00201.x
- Silapunt S, Peterson SR, Alcalay J, et al. Mohs tissue mapping and processing: a survey study. Dermatol Surg. 2003;29:1109-1112; discussion 1112.
- Humphreys TR, Nemeth A, McCrevey S, et al. A pilot study comparing toluidine blue and hematoxylin and eosin staining of basal cell and squamous cell carcinoma during Mohs surgery. Dermatol Surg. 1996;22:693-697. doi:10.1111/j.1524-4725.1996.tb00619.x
- Bennett R, Taher M. Restylane persistent for 23 months found during Mohs micrographic surgery: a source of confusion with hyaluronic acid surrounding basal cell carcinoma. Dermatol Surg. 2005;31:1366-1369. doi:10.1111/j.1524-4725.2005.31223
- Goldberg LH, Wang SQ, Kimyai-Asadi A. The setting sun sign: visualizing the margins of a basal cell carcinoma on serial frozen sections stained with toluidine blue. Dermatol Surg. 2007;33:761-763. doi:10.1111/j.1524-4725.2007.33158.x
- Tehrani H, May K, Morris A, et al. Does the dual use of toluidine blue and hematoxylin and eosin staining improve basal cell carcinoma detection by Mohs surgery trainees? Dermatol Surg. 2013;39:995-1000. doi:10.1111/dsu.12180
- Donaldson MR, Weber LA. Toluidine blue supports differentiation of folliculocentric basaloid proliferation from basal cell carcinoma on frozen sections in a small single-practice cohort. Dermatol Surg. 2017;43:1303-1306. doi:10.1097/DSS.0000000000001107
- Styperek AR, Goldberg LH, Goldschmidt LE, et al. Toluidine blue and hematoxylin and eosin stains are comparable in evaluating squamous cell carcinoma during Mohs. Dermatol Surg. 2016;42:1279-1284. doi:10.1097/DSS.0000000000000872
- Trieu D, Drosou A, Goldberg LH, et al. Detecting spindle cell squamous cell carcinomas with toluidine blue on frozen sections. Dermatol Surg. 2014;40:1259-1260. doi:10.1097/DSS.0000000000000147
- Drosou A, Trieu D, Goldberg LH, et al. The perineural corona sign: enhancing detection of perineural squamous cell carcinoma during Mohs micrographic surgery with toluidine blue stain. J Am Acad Dermatol. 2014;71:826-827. doi:10.1016/j.jaad.2014.04.076
- Chesser RS, Bertler DE, Fitzpatrick JE, et al. Primary cutaneous adenoid cystic carcinoma treated with Mohs micrographic surgery toluidine blue technique. J Dermatol Surg Oncol. 1992;18:175-176. doi:10.1111/j.1524-4725.1992.tb02794.x
- Wang SQ, Goldberg LH, Nemeth A. The merits of adding toluidine blue-stained slides in Mohs surgery in the treatment of a microcystic adnexal carcinoma. J Am Acad Dermatol. 2007;56:1067-1069. doi:10.1016/j.jaad.2007.01.008
- Chen CL, Wilson S, Afzalneia R, et al. Topical aluminum chloride and Monsel’s solution block toluidine blue staining in Mohs frozen sections: mechanism and solution. Dermatol Surg. 2019;45:1019-1025. doi:10.1097/DSS.0000000000001761
- Todd MM, Lee JW, Marks VJ. Rapid toluidine blue stain for Mohs’ micrographic surgery. Dermatol Surg. 2005;31:244-245. doi:10.1111/j.1524-4725.2005.31053
- Picoto AM, Picoto A. Technical procedures for Mohs fresh tissue surgery. J Derm Surg Oncol. 1986;12:134-138. doi:10.1111/j.1524-4725.1986.tb01442.x
- Sperling LC, Winton GB. The transverse anatomy of androgenic alopecia. J Derm Surg Oncol. 1990;16:1127-1133. doi:10.1111/j.1524 -4725.1990.tb00024.x
- Smith-Zagone MJ, Schwartz MR. Frozen section of skin specimens. Arch Pathol Lab Med. 2005;129:1536-1543. doi:10.5858/2005-129-1536-FSOSS
- Moy RL, Potter TS, Uitto J. Increased glycosaminoglycans production in sclerosing basal cell carcinoma–derived fibroblasts and stimulation of normal skin fibroblast glycosaminoglycans production by a cytokine-derived from sclerosing basal cell carcinoma. Dermatol Surg. 2000;26:1029-1036. doi:10.1046/j.1524-4725.2000.0260111029.x
- Leshin B, White WL. Folliculocentric basaloid proliferation. The bulge (der Wulst) revisited. Arch Dermatol. 1990;126:900-906. doi:10.1001/archderm.126.7.900
- Seab JA, Graham JH. Primary cutaneous adenoid cystic carcinoma.J Am Acad Dermatol. 1987;17:113-118. doi:10.1016/s0190 -9622(87)70182-0
- Culling CF, Allison TR. Cellular Pathology Technique. 4th ed. Butterworths; 1985.
- Bergeron JA, Singer M. Metachromasy: an experimental and theoretical reevaluation. J Biophys Biochem Cytol. 1958;4:433-457. doi:10.1083/jcb.4.4.433
- Epstein JB, Scully C, Spinelli J. Toluidine blue and Lugol’s iodine application in the assessment of oral malignant disease and lesions at risk of malignancy. J Oral Pathol Med. 1992;21:160-163. doi:10.1111/j.1600-0714.1992.tb00094.x
- Warnakulasuriya KA, Johnson NW. Sensitivity and specificity of OraScan (R) toluidine blue mouthrinse in the detection of oral cancer and precancer. J Oral Pathol Med. 1996;25:97-103. doi:10.1111/j.1600-0714.1996.tb00201.x
- Silapunt S, Peterson SR, Alcalay J, et al. Mohs tissue mapping and processing: a survey study. Dermatol Surg. 2003;29:1109-1112; discussion 1112.
- Humphreys TR, Nemeth A, McCrevey S, et al. A pilot study comparing toluidine blue and hematoxylin and eosin staining of basal cell and squamous cell carcinoma during Mohs surgery. Dermatol Surg. 1996;22:693-697. doi:10.1111/j.1524-4725.1996.tb00619.x
- Bennett R, Taher M. Restylane persistent for 23 months found during Mohs micrographic surgery: a source of confusion with hyaluronic acid surrounding basal cell carcinoma. Dermatol Surg. 2005;31:1366-1369. doi:10.1111/j.1524-4725.2005.31223
- Goldberg LH, Wang SQ, Kimyai-Asadi A. The setting sun sign: visualizing the margins of a basal cell carcinoma on serial frozen sections stained with toluidine blue. Dermatol Surg. 2007;33:761-763. doi:10.1111/j.1524-4725.2007.33158.x
- Tehrani H, May K, Morris A, et al. Does the dual use of toluidine blue and hematoxylin and eosin staining improve basal cell carcinoma detection by Mohs surgery trainees? Dermatol Surg. 2013;39:995-1000. doi:10.1111/dsu.12180
- Donaldson MR, Weber LA. Toluidine blue supports differentiation of folliculocentric basaloid proliferation from basal cell carcinoma on frozen sections in a small single-practice cohort. Dermatol Surg. 2017;43:1303-1306. doi:10.1097/DSS.0000000000001107
- Styperek AR, Goldberg LH, Goldschmidt LE, et al. Toluidine blue and hematoxylin and eosin stains are comparable in evaluating squamous cell carcinoma during Mohs. Dermatol Surg. 2016;42:1279-1284. doi:10.1097/DSS.0000000000000872
- Trieu D, Drosou A, Goldberg LH, et al. Detecting spindle cell squamous cell carcinomas with toluidine blue on frozen sections. Dermatol Surg. 2014;40:1259-1260. doi:10.1097/DSS.0000000000000147
- Drosou A, Trieu D, Goldberg LH, et al. The perineural corona sign: enhancing detection of perineural squamous cell carcinoma during Mohs micrographic surgery with toluidine blue stain. J Am Acad Dermatol. 2014;71:826-827. doi:10.1016/j.jaad.2014.04.076
- Chesser RS, Bertler DE, Fitzpatrick JE, et al. Primary cutaneous adenoid cystic carcinoma treated with Mohs micrographic surgery toluidine blue technique. J Dermatol Surg Oncol. 1992;18:175-176. doi:10.1111/j.1524-4725.1992.tb02794.x
- Wang SQ, Goldberg LH, Nemeth A. The merits of adding toluidine blue-stained slides in Mohs surgery in the treatment of a microcystic adnexal carcinoma. J Am Acad Dermatol. 2007;56:1067-1069. doi:10.1016/j.jaad.2007.01.008
- Chen CL, Wilson S, Afzalneia R, et al. Topical aluminum chloride and Monsel’s solution block toluidine blue staining in Mohs frozen sections: mechanism and solution. Dermatol Surg. 2019;45:1019-1025. doi:10.1097/DSS.0000000000001761
- Todd MM, Lee JW, Marks VJ. Rapid toluidine blue stain for Mohs’ micrographic surgery. Dermatol Surg. 2005;31:244-245. doi:10.1111/j.1524-4725.2005.31053
- Picoto AM, Picoto A. Technical procedures for Mohs fresh tissue surgery. J Derm Surg Oncol. 1986;12:134-138. doi:10.1111/j.1524-4725.1986.tb01442.x
- Sperling LC, Winton GB. The transverse anatomy of androgenic alopecia. J Derm Surg Oncol. 1990;16:1127-1133. doi:10.1111/j.1524 -4725.1990.tb00024.x
- Smith-Zagone MJ, Schwartz MR. Frozen section of skin specimens. Arch Pathol Lab Med. 2005;129:1536-1543. doi:10.5858/2005-129-1536-FSOSS
- Moy RL, Potter TS, Uitto J. Increased glycosaminoglycans production in sclerosing basal cell carcinoma–derived fibroblasts and stimulation of normal skin fibroblast glycosaminoglycans production by a cytokine-derived from sclerosing basal cell carcinoma. Dermatol Surg. 2000;26:1029-1036. doi:10.1046/j.1524-4725.2000.0260111029.x
- Leshin B, White WL. Folliculocentric basaloid proliferation. The bulge (der Wulst) revisited. Arch Dermatol. 1990;126:900-906. doi:10.1001/archderm.126.7.900
- Seab JA, Graham JH. Primary cutaneous adenoid cystic carcinoma.J Am Acad Dermatol. 1987;17:113-118. doi:10.1016/s0190 -9622(87)70182-0
Practice Points
- Toluidine blue (TB) staining can be integrated into Mohs micrographic surgery (MMS) for enhanced diagnosis of cutaneous tumors. Its metachromatic properties can aid in differentiating tumor cells from surrounding tissues, especially in basal cell carcinomas and squamous cell carcinomas.
- It is important to develop expertise in interpreting TB-stained sections, as it may offer clearer visualization of nuclear details and stromal components, potentially leading to more accurate diagnosis and effective tumor margin identification.
- Toluidine blue staining can be incorporated into routine MMS practice considering its quick staining process and low disruption to workflow. This can potentially improve diagnostic efficiency without significantly lengthening surgery time.