User login
The Role of Toluidine Blue in Mohs Micrographic Surgery: A Systematic Review
Toluidine blue (TB), a dye with metachromatic staining properties, was developed in 1856 by William Henry Perkin.1 Metachromasia is a perceptible change in the color of staining of living tissue due to the electrochemical properties of the tissue. Tissues that contain high concentrations of ionized sulfate and phosphate groups (high concentrations of free electronegative groups) form polymeric aggregates of the basic dye solution that alter the absorbed wavelengths of light.2 The function of this characteristic is to use a single dye to highlight different structures in tissue based on their relative chemical differences.3
Toluidine blue primarily was used within the dye industry until the 1960s, when it was first used in vital staining of the oral mucosa.2 Because of the tissue absorption potential, this technique was used to detect the location of oral malignancies.4 Since then, TB has progressively been used for staining fresh frozen sections in Mohs micrographic surgery (MMS). In a 2003 survey study (N=310), 16.8% of surgeons performing MMS reported using TB in their laboratory.5 We sought to systematically review the published literature describing the uses of TB in the setting of fresh frozen sections and MMS.
Methods
We conducted a systematic search of the PubMed and Cochrane databases for articles published before December 1, 2019, to identify any relevant studies in English. Electronic searches were performed using the terms toluidine blue and Mohs or Mohs micrographic surgery. We manually checked the bibliographies of the identified articles to further identify eligible studies.
Eligibility Criteria—The inclusion criteria were articles that (1) considered TB in the context of MMS, (2) were published in peer-reviewed journals, (3) were published in English, and (4) were available as full text. Systematic reviews were excluded.
Data Extraction and Outcomes—All relevant information regarding the study characteristics, including design, level of evidence, methodologic quality of evidence, pathology examined, and outcome measures, were collected by 2 independent reviewers (T.L. and A.D.) using a predetermined data sheet. The same 2 reviewers were used for all steps of the review process, data were independently obtained, and any discrepancy was introduced for a third opinion (D.H.) and agreed upon by the majority.
Quality Assessment—The level of evidence was evaluated based on the criteria of the Oxford Centre for Evidence-Based Medicine. Two reviewers (T.L. and A.D.) graded each article included in the review.
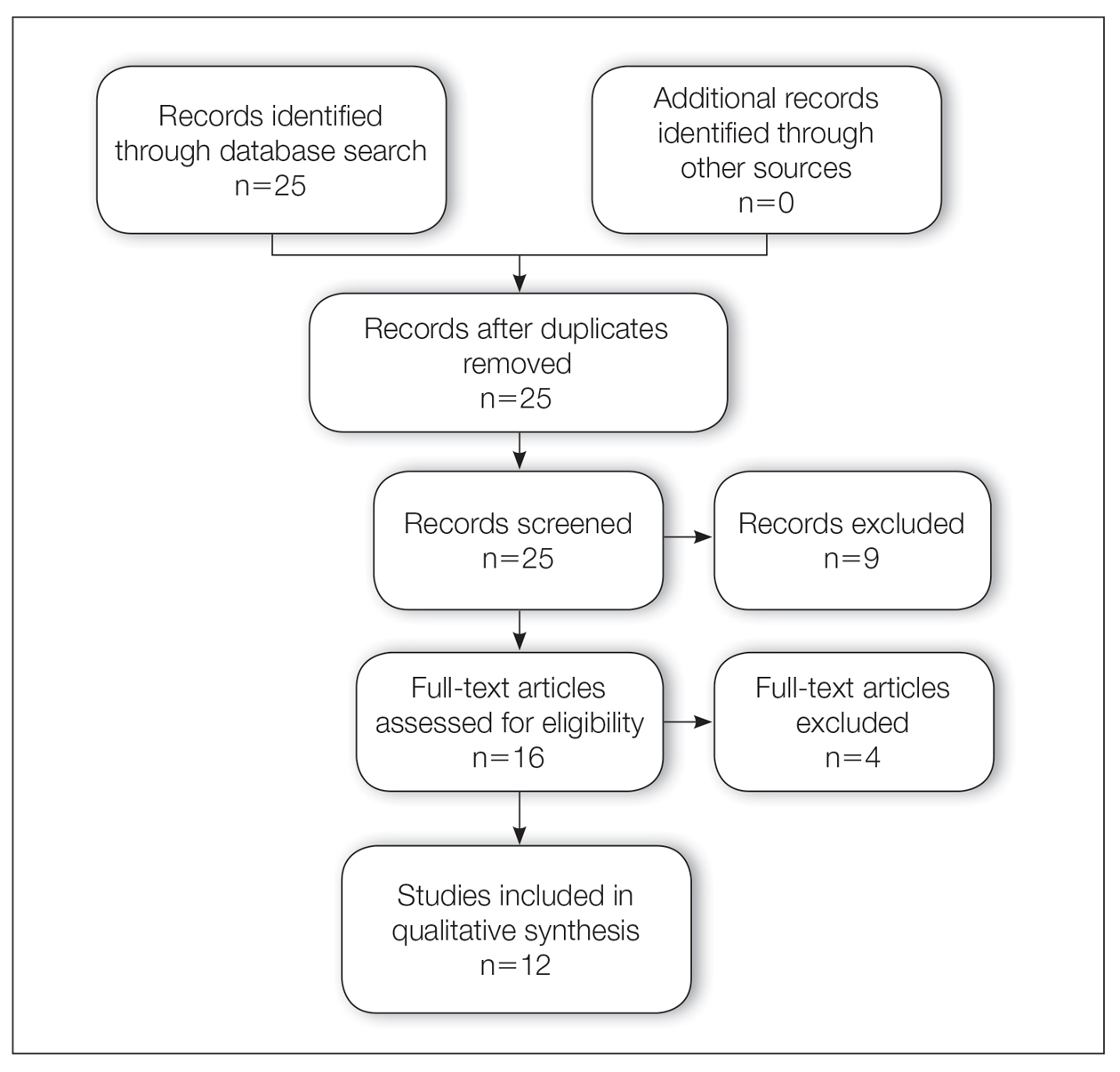
Results
A total of 25 articles were reviewed. After the titles and abstracts were screened for relevance, 12 articles remained (Figure 1). Of these, 1 compared basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), 4 were related to BCC, 3 were related to SCC, 1 was related to microcystic adnexal carcinoma (MAC), 1 was related to primary cutaneous adenoid cystic carcinoma (PCACC), and 2 were related to technical aspects of the staining process (Table 1).
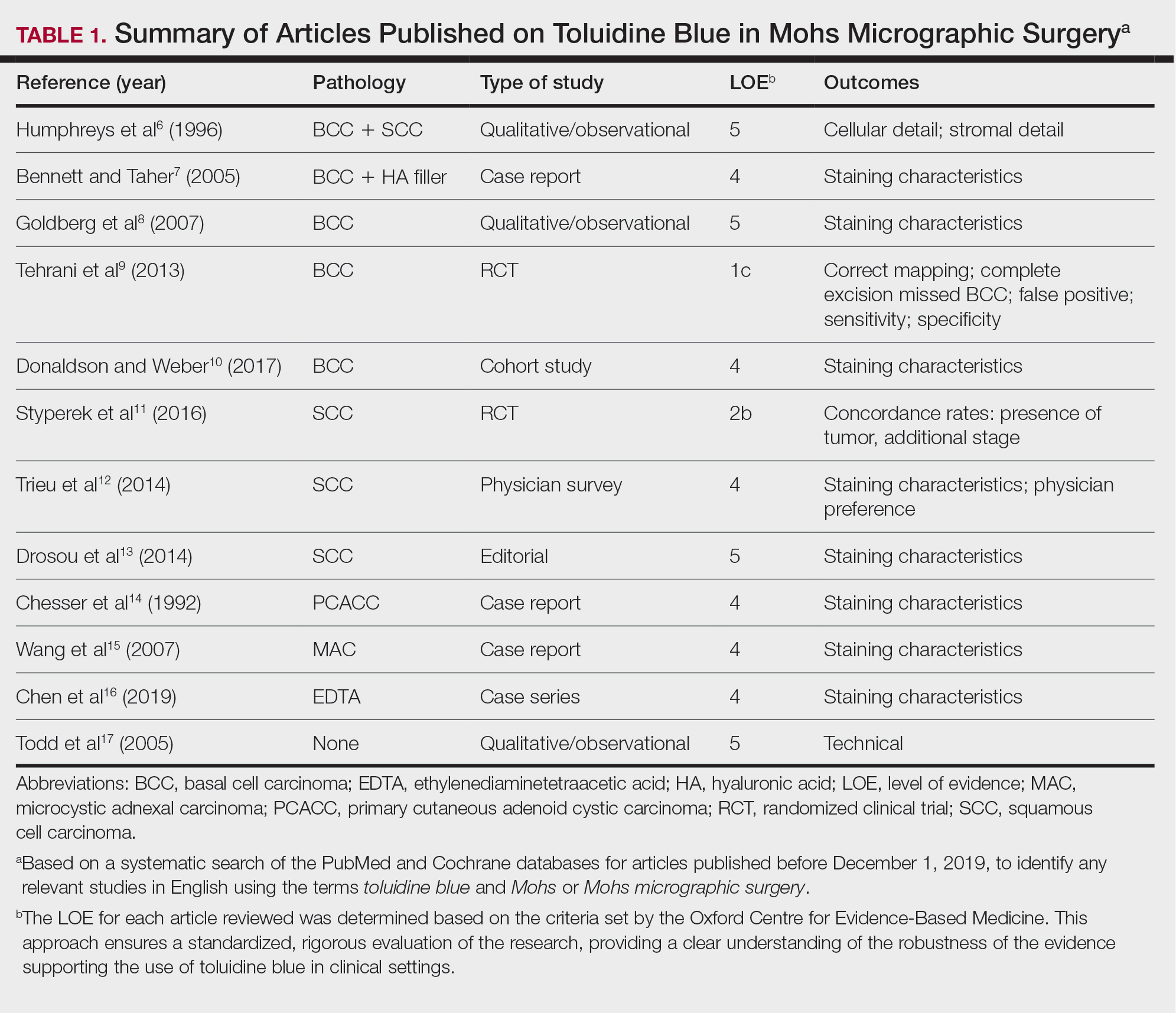
A majority of the articles included in this review were qualitative and observational in nature, describing the staining characteristics of TB. Study characteristics are summarized in Table 1.
Comment
Basal Cell Carcinoma—Toluidine blue staining characteristics help to identify BCC nests by differentiating them from hair follicles in frozen sections. The metachromatic characteristic of TB stains the inner root sheath deep blue and highlights the surrounding stromal mucin of BCC a magenta color.18,19 In hematoxylin and eosin (H&E) stains, these 2 distinct structures can be differentiated by cleft formation around tumor nests, mitotic figures, and the lack of a fibrous sheath present in BCC tumors.20 The advantages and limitations of TB staining of BCC are presented in Table 2.
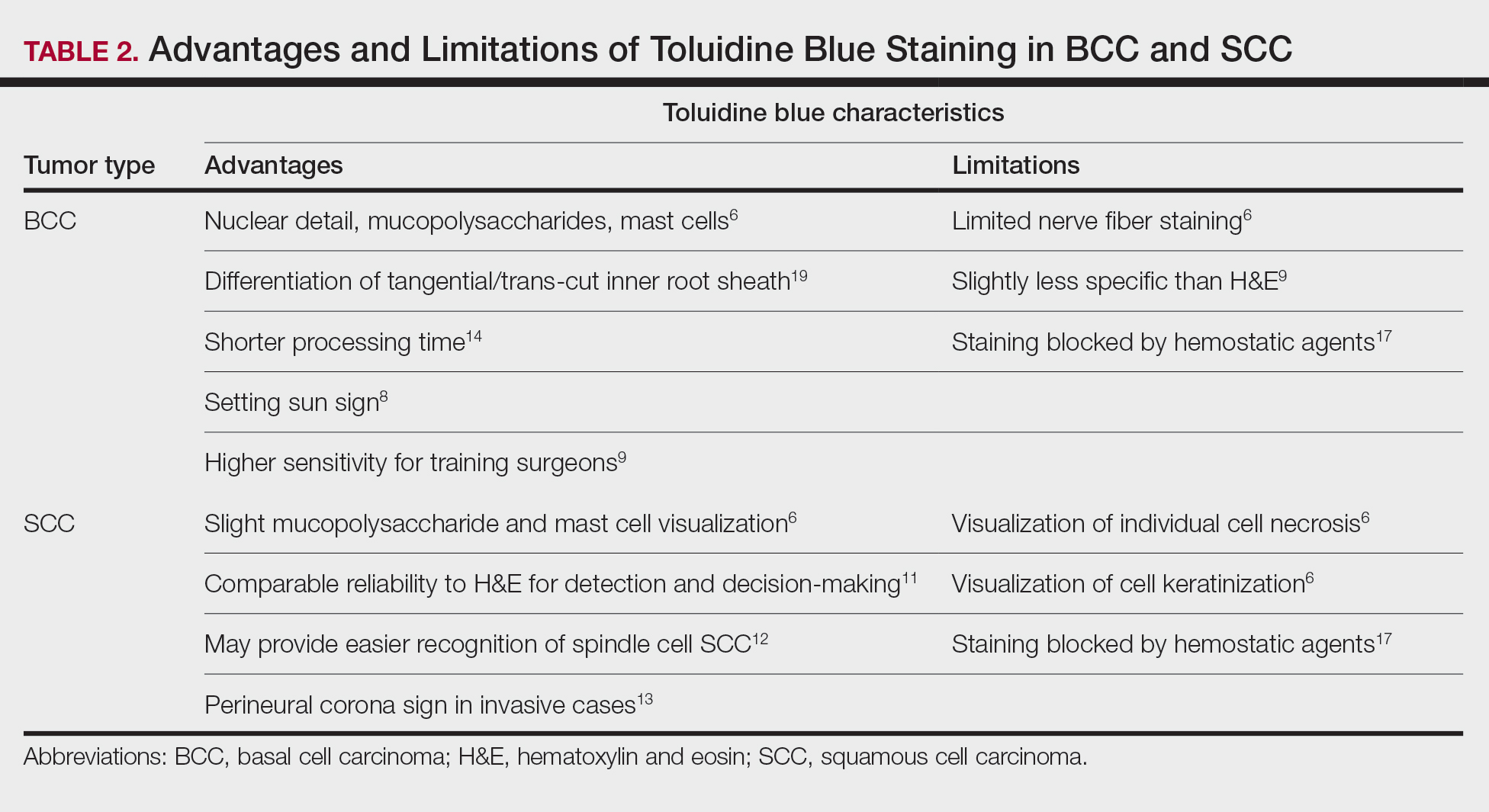
Humphreys et al6 suggested a noticeable difference between H&E and TB in the staining of cellular and stromal components. The nuclear detail of tumor cells was subjectively sharper and clearer with TB staining. The staining of stromal components may provide the most assistance in locating BCC islands. Mucopolysaccharide staining may be absent in H&E but stain a deep magenta with TB. Although the presence of mucopolysaccharides does not specifically indicate a tumor, it may prompt further attention and provide an indicator for sparse and infiltrative tumor cells.6 The metachromatic stromal change may indicate a narrow tumor-free margin where additional deeper sections often reveal tumor that may warrant additional resection margin in more aggressive malignancies. In particular, sclerosing/morpheaform BCCs have been shown to induce glycosaminoglycan synthesis and are highlighted more readily with TB than with H&E when compared to surrounding tissue.21 This differentiation in staining has remained a popular reason to routinely incorporate TB into the staining of infiltrative and morpheaform variants of BCC. Additionally, stromal mast cells are believed to be more abundant in the stroma of BCC and are more readily visualized in tissue specimens stained with TB, appearing as bright purple metachromatic granules. These granules are larger than normal and are increased in number.6
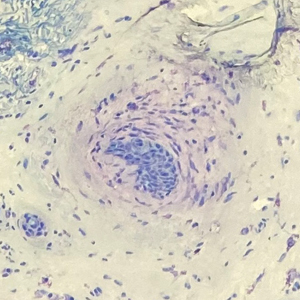
The margin behavior of BCC stained with TB was further characterized by Goldberg et al,8 who coined the term setting sun sign, which may be present in sequential sections of a disappearing nodule of a BCC tumor. Stroma, inflammatory infiltrate, and mast cells produce a magenta glow surrounding BCC tumors that is reminiscent of a setting sun (Figure 2). Invasive BCC is considered variable in this presentation, primarily because of zones of cell-free fluid and edema or the second area of inflammatory cells. This unique sign may benefit the inspecting Mohs surgeon by providing a clue to an underlying process that may have residual BCC tumors. The setting sun sign also may assist in identifying exact surgical margins.8

The nasal surface has a predilection for BCC.22 The skin of the nose has numerous look-alike structures to consider for complete tumor removal and avoidance of unnecessary removal. One challenge is distinguishing follicular basaloid proliferations (FBP) from BCC, a scenario that is more common on the nose.22 When TB staining was used, the sensitivity for detecting FBP reached 100% in 34 cases reviewed by Donaldson and Weber.10 None of the cases examined showed TB metachromasia surrounding FBP, thus indicating that TB can dependably identify this benign entity. Conversely, 5% (N=279) of BCCs confirmed on H&E did not exhibit surrounding TB metachromasia. This finding is concerning regarding the specificity of TB staining for BCC, but the authors of this study suggested the possibility that these exceptions were benign “simulants” (ie, trichoepithelioma) of BCC.10
The use of TB also has been shown to be statistically beneficial in Mohs training. In a single-center, single-fellow experiment, the sensitivity and specificity of using TB for BCC were extrapolated.9 Using TB as an adjunct in deep sections showed superior sensitivity to H&E alone in identifying BCC, increasing sensitivity from 96.3% to 99.7%. In a cohort of 352 BCC excisions and frozen sections, only 1 BCC was not completely excised. If H&E only had been performed, the fellow would have missed 13 residual BCC tumors.9
Bennett and Taher7 described a case in which hyaluronic acid (HA) from a filler injection was confused with the HA surrounding BCC tumor nests. They found that when TB is used as an adjunct, the HA filler is easier to differentiate from the HA surrounding the BCC tumor nests. In frozen sections stained with TB, the HA filler appeared as an amorphous, metachromatic, reddish-purple, whereas the HA surrounding the BCC tumor nests appeared as a well-defined red. These findings were less obvious in the same sections stained with H&E alone.7
Squamous Cell Carcinoma—In early investigations, the utility of TB in identifying SCC in frozen sections was thought to be limited. The description by Humphreys and colleagues6 of staining characteristics in SCC suggested that the nuclear detail that H&E provides is more easily recognized. The deep aqua nuclear staining produced with TB was considered more difficult to observe than the cytoplasmic eosinophilia of pyknotic and keratinizing cells in H&E.6
Toluidine blue may be beneficial in providing unique staining characteristics to further detail tumors that are difficult to interpret, such as spindle cell SCC and perineural invasion of aggressive SCC. In H&E, squamous cells of spindle cell SCC (scSCC) blend into the background of inflammatory cells and can be perceptibly difficult to locate. A small cohort of 3 Mohs surgeons who routinely use H&E were surveyed on their ability to detect a proven scSCC in H&E or TB by photograph.12 All 3 were able to detect the scSCC in the TB photographs, but only 2 of 3 were able to detect it in H&E photographs. All 3 surgeons agreed that TB was preferable to H&E for this tumor type. These findings suggested that TB may be superior and preferred over H&E for visualizing tumor cells of scSCC.12 The TB staining characteristics of perineural invasion of aggressive SCC have been referred to as the perineural corona sign because of the bright magenta stain that forms around affected nerves.13 Drosou et al13 suggested that TB may enhance the diagnostic accuracy for perineural SCC.
Rare Tumors—The adjunctive use of TB with H&E has been examined in rare tumors. Published reports have highlighted its use in MMS for treating MAC and PCACC. Toluidine blue exhibits staining advantages for these tumors. It may render isolated nests and perineural invasion of MAC more easily visible on frozen section.15
Although PCACC is rare, the recurrence rate is high.23 Toluidine blue has been used with MMS to ensure complete removal and higher cure rates. The metachromatic nature of TB is advantageous in staining the HA present in these tumors. Those who have reported the use of TB for PCACC prefer it to H&E for frozen sections.14
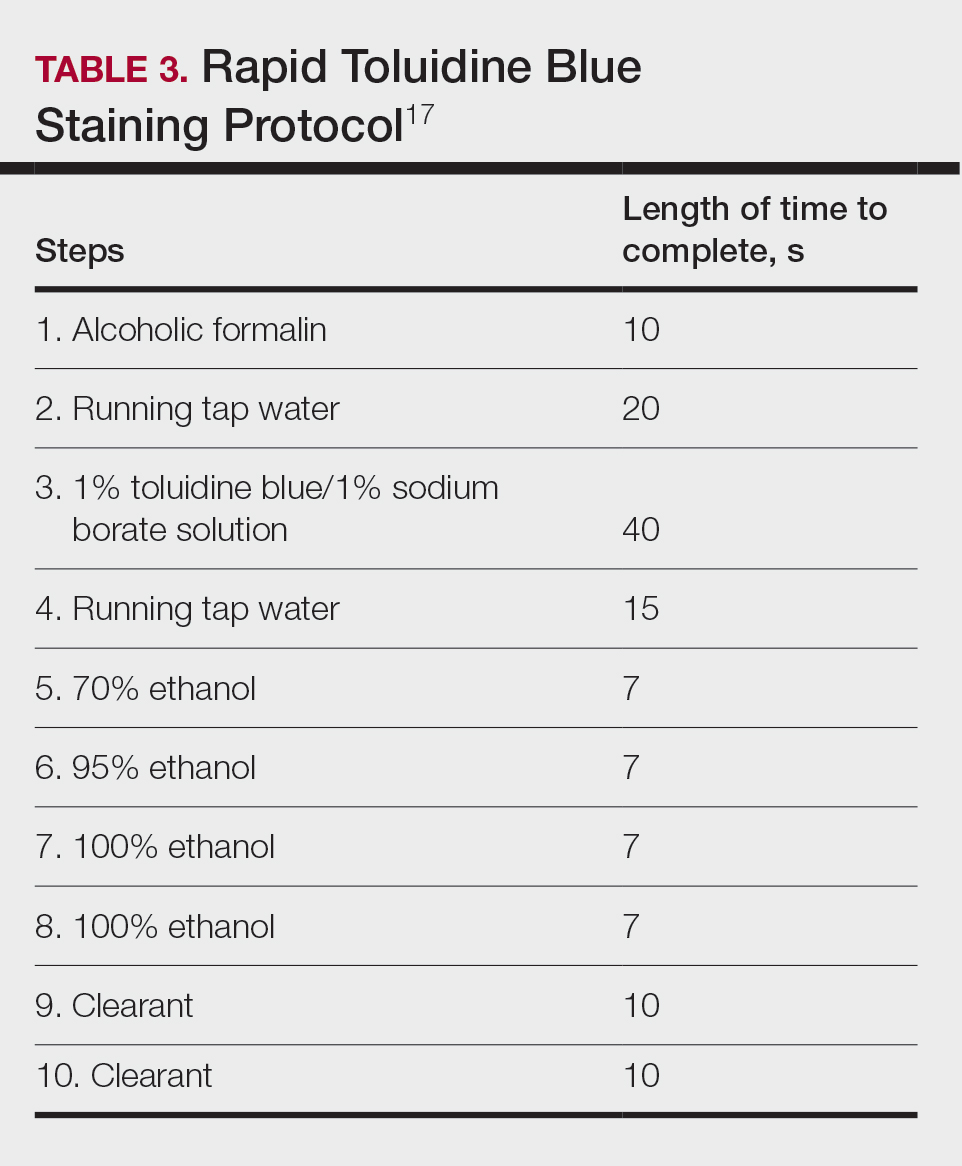
Technical Aspects—The staining time for TB-treated slides is reduced compared to H&E staining; staining can be efficiently done in frozen sections in less than 2.5 minutes using the method shown in Table 3.17 In comparison, typical H&E staining takes 9 minutes, and older TB techniques take 7 minutes.6
Conclusion
Toluidine blue may play an important and helpful role in the successful diagnosis and treatment of particular cutaneous tumors by providing additional diagnostic information. Although surgeons performing MMS will continue using the staining protocols with which they are most comfortable, adjunctive use of TB over time may provide an additional benefit at low risk for disrupting practice efficiency or workflow. Many Mohs surgeons are accustomed to using this stain, even preferring to interpret only TB-stained slides for cutaneous malignancy. Most published studies on this topic have been observational in nature, and additional controlled trials may be warranted to determine the effects on outcomes in real-world practice.
- Culling CF, Allison TR. Cellular Pathology Technique. 4th ed. Butterworths; 1985.
- Bergeron JA, Singer M. Metachromasy: an experimental and theoretical reevaluation. J Biophys Biochem Cytol. 1958;4:433-457. doi:10.1083/jcb.4.4.433
- Epstein JB, Scully C, Spinelli J. Toluidine blue and Lugol’s iodine application in the assessment of oral malignant disease and lesions at risk of malignancy. J Oral Pathol Med. 1992;21:160-163. doi:10.1111/j.1600-0714.1992.tb00094.x
- Warnakulasuriya KA, Johnson NW. Sensitivity and specificity of OraScan (R) toluidine blue mouthrinse in the detection of oral cancer and precancer. J Oral Pathol Med. 1996;25:97-103. doi:10.1111/j.1600-0714.1996.tb00201.x
- Silapunt S, Peterson SR, Alcalay J, et al. Mohs tissue mapping and processing: a survey study. Dermatol Surg. 2003;29:1109-1112; discussion 1112.
- Humphreys TR, Nemeth A, McCrevey S, et al. A pilot study comparing toluidine blue and hematoxylin and eosin staining of basal cell and squamous cell carcinoma during Mohs surgery. Dermatol Surg. 1996;22:693-697. doi:10.1111/j.1524-4725.1996.tb00619.x
- Bennett R, Taher M. Restylane persistent for 23 months found during Mohs micrographic surgery: a source of confusion with hyaluronic acid surrounding basal cell carcinoma. Dermatol Surg. 2005;31:1366-1369. doi:10.1111/j.1524-4725.2005.31223
- Goldberg LH, Wang SQ, Kimyai-Asadi A. The setting sun sign: visualizing the margins of a basal cell carcinoma on serial frozen sections stained with toluidine blue. Dermatol Surg. 2007;33:761-763. doi:10.1111/j.1524-4725.2007.33158.x
- Tehrani H, May K, Morris A, et al. Does the dual use of toluidine blue and hematoxylin and eosin staining improve basal cell carcinoma detection by Mohs surgery trainees? Dermatol Surg. 2013;39:995-1000. doi:10.1111/dsu.12180
- Donaldson MR, Weber LA. Toluidine blue supports differentiation of folliculocentric basaloid proliferation from basal cell carcinoma on frozen sections in a small single-practice cohort. Dermatol Surg. 2017;43:1303-1306. doi:10.1097/DSS.0000000000001107
- Styperek AR, Goldberg LH, Goldschmidt LE, et al. Toluidine blue and hematoxylin and eosin stains are comparable in evaluating squamous cell carcinoma during Mohs. Dermatol Surg. 2016;42:1279-1284. doi:10.1097/DSS.0000000000000872
- Trieu D, Drosou A, Goldberg LH, et al. Detecting spindle cell squamous cell carcinomas with toluidine blue on frozen sections. Dermatol Surg. 2014;40:1259-1260. doi:10.1097/DSS.0000000000000147
- Drosou A, Trieu D, Goldberg LH, et al. The perineural corona sign: enhancing detection of perineural squamous cell carcinoma during Mohs micrographic surgery with toluidine blue stain. J Am Acad Dermatol. 2014;71:826-827. doi:10.1016/j.jaad.2014.04.076
- Chesser RS, Bertler DE, Fitzpatrick JE, et al. Primary cutaneous adenoid cystic carcinoma treated with Mohs micrographic surgery toluidine blue technique. J Dermatol Surg Oncol. 1992;18:175-176. doi:10.1111/j.1524-4725.1992.tb02794.x
- Wang SQ, Goldberg LH, Nemeth A. The merits of adding toluidine blue-stained slides in Mohs surgery in the treatment of a microcystic adnexal carcinoma. J Am Acad Dermatol. 2007;56:1067-1069. doi:10.1016/j.jaad.2007.01.008
- Chen CL, Wilson S, Afzalneia R, et al. Topical aluminum chloride and Monsel’s solution block toluidine blue staining in Mohs frozen sections: mechanism and solution. Dermatol Surg. 2019;45:1019-1025. doi:10.1097/DSS.0000000000001761
- Todd MM, Lee JW, Marks VJ. Rapid toluidine blue stain for Mohs’ micrographic surgery. Dermatol Surg. 2005;31:244-245. doi:10.1111/j.1524-4725.2005.31053
- Picoto AM, Picoto A. Technical procedures for Mohs fresh tissue surgery. J Derm Surg Oncol. 1986;12:134-138. doi:10.1111/j.1524-4725.1986.tb01442.x
- Sperling LC, Winton GB. The transverse anatomy of androgenic alopecia. J Derm Surg Oncol. 1990;16:1127-1133. doi:10.1111/j.1524 -4725.1990.tb00024.x
- Smith-Zagone MJ, Schwartz MR. Frozen section of skin specimens. Arch Pathol Lab Med. 2005;129:1536-1543. doi:10.5858/2005-129-1536-FSOSS
- Moy RL, Potter TS, Uitto J. Increased glycosaminoglycans production in sclerosing basal cell carcinoma–derived fibroblasts and stimulation of normal skin fibroblast glycosaminoglycans production by a cytokine-derived from sclerosing basal cell carcinoma. Dermatol Surg. 2000;26:1029-1036. doi:10.1046/j.1524-4725.2000.0260111029.x
- Leshin B, White WL. Folliculocentric basaloid proliferation. The bulge (der Wulst) revisited. Arch Dermatol. 1990;126:900-906. doi:10.1001/archderm.126.7.900
- Seab JA, Graham JH. Primary cutaneous adenoid cystic carcinoma.J Am Acad Dermatol. 1987;17:113-118. doi:10.1016/s0190 -9622(87)70182-0
Toluidine blue (TB), a dye with metachromatic staining properties, was developed in 1856 by William Henry Perkin.1 Metachromasia is a perceptible change in the color of staining of living tissue due to the electrochemical properties of the tissue. Tissues that contain high concentrations of ionized sulfate and phosphate groups (high concentrations of free electronegative groups) form polymeric aggregates of the basic dye solution that alter the absorbed wavelengths of light.2 The function of this characteristic is to use a single dye to highlight different structures in tissue based on their relative chemical differences.3
Toluidine blue primarily was used within the dye industry until the 1960s, when it was first used in vital staining of the oral mucosa.2 Because of the tissue absorption potential, this technique was used to detect the location of oral malignancies.4 Since then, TB has progressively been used for staining fresh frozen sections in Mohs micrographic surgery (MMS). In a 2003 survey study (N=310), 16.8% of surgeons performing MMS reported using TB in their laboratory.5 We sought to systematically review the published literature describing the uses of TB in the setting of fresh frozen sections and MMS.
Methods
We conducted a systematic search of the PubMed and Cochrane databases for articles published before December 1, 2019, to identify any relevant studies in English. Electronic searches were performed using the terms toluidine blue and Mohs or Mohs micrographic surgery. We manually checked the bibliographies of the identified articles to further identify eligible studies.
Eligibility Criteria—The inclusion criteria were articles that (1) considered TB in the context of MMS, (2) were published in peer-reviewed journals, (3) were published in English, and (4) were available as full text. Systematic reviews were excluded.
Data Extraction and Outcomes—All relevant information regarding the study characteristics, including design, level of evidence, methodologic quality of evidence, pathology examined, and outcome measures, were collected by 2 independent reviewers (T.L. and A.D.) using a predetermined data sheet. The same 2 reviewers were used for all steps of the review process, data were independently obtained, and any discrepancy was introduced for a third opinion (D.H.) and agreed upon by the majority.
Quality Assessment—The level of evidence was evaluated based on the criteria of the Oxford Centre for Evidence-Based Medicine. Two reviewers (T.L. and A.D.) graded each article included in the review.

Results
A total of 25 articles were reviewed. After the titles and abstracts were screened for relevance, 12 articles remained (Figure 1). Of these, 1 compared basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), 4 were related to BCC, 3 were related to SCC, 1 was related to microcystic adnexal carcinoma (MAC), 1 was related to primary cutaneous adenoid cystic carcinoma (PCACC), and 2 were related to technical aspects of the staining process (Table 1).

A majority of the articles included in this review were qualitative and observational in nature, describing the staining characteristics of TB. Study characteristics are summarized in Table 1.
Comment
Basal Cell Carcinoma—Toluidine blue staining characteristics help to identify BCC nests by differentiating them from hair follicles in frozen sections. The metachromatic characteristic of TB stains the inner root sheath deep blue and highlights the surrounding stromal mucin of BCC a magenta color.18,19 In hematoxylin and eosin (H&E) stains, these 2 distinct structures can be differentiated by cleft formation around tumor nests, mitotic figures, and the lack of a fibrous sheath present in BCC tumors.20 The advantages and limitations of TB staining of BCC are presented in Table 2.

Humphreys et al6 suggested a noticeable difference between H&E and TB in the staining of cellular and stromal components. The nuclear detail of tumor cells was subjectively sharper and clearer with TB staining. The staining of stromal components may provide the most assistance in locating BCC islands. Mucopolysaccharide staining may be absent in H&E but stain a deep magenta with TB. Although the presence of mucopolysaccharides does not specifically indicate a tumor, it may prompt further attention and provide an indicator for sparse and infiltrative tumor cells.6 The metachromatic stromal change may indicate a narrow tumor-free margin where additional deeper sections often reveal tumor that may warrant additional resection margin in more aggressive malignancies. In particular, sclerosing/morpheaform BCCs have been shown to induce glycosaminoglycan synthesis and are highlighted more readily with TB than with H&E when compared to surrounding tissue.21 This differentiation in staining has remained a popular reason to routinely incorporate TB into the staining of infiltrative and morpheaform variants of BCC. Additionally, stromal mast cells are believed to be more abundant in the stroma of BCC and are more readily visualized in tissue specimens stained with TB, appearing as bright purple metachromatic granules. These granules are larger than normal and are increased in number.6
The margin behavior of BCC stained with TB was further characterized by Goldberg et al,8 who coined the term setting sun sign, which may be present in sequential sections of a disappearing nodule of a BCC tumor. Stroma, inflammatory infiltrate, and mast cells produce a magenta glow surrounding BCC tumors that is reminiscent of a setting sun (Figure 2). Invasive BCC is considered variable in this presentation, primarily because of zones of cell-free fluid and edema or the second area of inflammatory cells. This unique sign may benefit the inspecting Mohs surgeon by providing a clue to an underlying process that may have residual BCC tumors. The setting sun sign also may assist in identifying exact surgical margins.8

The nasal surface has a predilection for BCC.22 The skin of the nose has numerous look-alike structures to consider for complete tumor removal and avoidance of unnecessary removal. One challenge is distinguishing follicular basaloid proliferations (FBP) from BCC, a scenario that is more common on the nose.22 When TB staining was used, the sensitivity for detecting FBP reached 100% in 34 cases reviewed by Donaldson and Weber.10 None of the cases examined showed TB metachromasia surrounding FBP, thus indicating that TB can dependably identify this benign entity. Conversely, 5% (N=279) of BCCs confirmed on H&E did not exhibit surrounding TB metachromasia. This finding is concerning regarding the specificity of TB staining for BCC, but the authors of this study suggested the possibility that these exceptions were benign “simulants” (ie, trichoepithelioma) of BCC.10
The use of TB also has been shown to be statistically beneficial in Mohs training. In a single-center, single-fellow experiment, the sensitivity and specificity of using TB for BCC were extrapolated.9 Using TB as an adjunct in deep sections showed superior sensitivity to H&E alone in identifying BCC, increasing sensitivity from 96.3% to 99.7%. In a cohort of 352 BCC excisions and frozen sections, only 1 BCC was not completely excised. If H&E only had been performed, the fellow would have missed 13 residual BCC tumors.9
Bennett and Taher7 described a case in which hyaluronic acid (HA) from a filler injection was confused with the HA surrounding BCC tumor nests. They found that when TB is used as an adjunct, the HA filler is easier to differentiate from the HA surrounding the BCC tumor nests. In frozen sections stained with TB, the HA filler appeared as an amorphous, metachromatic, reddish-purple, whereas the HA surrounding the BCC tumor nests appeared as a well-defined red. These findings were less obvious in the same sections stained with H&E alone.7
Squamous Cell Carcinoma—In early investigations, the utility of TB in identifying SCC in frozen sections was thought to be limited. The description by Humphreys and colleagues6 of staining characteristics in SCC suggested that the nuclear detail that H&E provides is more easily recognized. The deep aqua nuclear staining produced with TB was considered more difficult to observe than the cytoplasmic eosinophilia of pyknotic and keratinizing cells in H&E.6
Toluidine blue may be beneficial in providing unique staining characteristics to further detail tumors that are difficult to interpret, such as spindle cell SCC and perineural invasion of aggressive SCC. In H&E, squamous cells of spindle cell SCC (scSCC) blend into the background of inflammatory cells and can be perceptibly difficult to locate. A small cohort of 3 Mohs surgeons who routinely use H&E were surveyed on their ability to detect a proven scSCC in H&E or TB by photograph.12 All 3 were able to detect the scSCC in the TB photographs, but only 2 of 3 were able to detect it in H&E photographs. All 3 surgeons agreed that TB was preferable to H&E for this tumor type. These findings suggested that TB may be superior and preferred over H&E for visualizing tumor cells of scSCC.12 The TB staining characteristics of perineural invasion of aggressive SCC have been referred to as the perineural corona sign because of the bright magenta stain that forms around affected nerves.13 Drosou et al13 suggested that TB may enhance the diagnostic accuracy for perineural SCC.
Rare Tumors—The adjunctive use of TB with H&E has been examined in rare tumors. Published reports have highlighted its use in MMS for treating MAC and PCACC. Toluidine blue exhibits staining advantages for these tumors. It may render isolated nests and perineural invasion of MAC more easily visible on frozen section.15
Although PCACC is rare, the recurrence rate is high.23 Toluidine blue has been used with MMS to ensure complete removal and higher cure rates. The metachromatic nature of TB is advantageous in staining the HA present in these tumors. Those who have reported the use of TB for PCACC prefer it to H&E for frozen sections.14
Technical Aspects—The staining time for TB-treated slides is reduced compared to H&E staining; staining can be efficiently done in frozen sections in less than 2.5 minutes using the method shown in Table 3.17 In comparison, typical H&E staining takes 9 minutes, and older TB techniques take 7 minutes.6

Conclusion
Toluidine blue may play an important and helpful role in the successful diagnosis and treatment of particular cutaneous tumors by providing additional diagnostic information. Although surgeons performing MMS will continue using the staining protocols with which they are most comfortable, adjunctive use of TB over time may provide an additional benefit at low risk for disrupting practice efficiency or workflow. Many Mohs surgeons are accustomed to using this stain, even preferring to interpret only TB-stained slides for cutaneous malignancy. Most published studies on this topic have been observational in nature, and additional controlled trials may be warranted to determine the effects on outcomes in real-world practice.
Toluidine blue (TB), a dye with metachromatic staining properties, was developed in 1856 by William Henry Perkin.1 Metachromasia is a perceptible change in the color of staining of living tissue due to the electrochemical properties of the tissue. Tissues that contain high concentrations of ionized sulfate and phosphate groups (high concentrations of free electronegative groups) form polymeric aggregates of the basic dye solution that alter the absorbed wavelengths of light.2 The function of this characteristic is to use a single dye to highlight different structures in tissue based on their relative chemical differences.3
Toluidine blue primarily was used within the dye industry until the 1960s, when it was first used in vital staining of the oral mucosa.2 Because of the tissue absorption potential, this technique was used to detect the location of oral malignancies.4 Since then, TB has progressively been used for staining fresh frozen sections in Mohs micrographic surgery (MMS). In a 2003 survey study (N=310), 16.8% of surgeons performing MMS reported using TB in their laboratory.5 We sought to systematically review the published literature describing the uses of TB in the setting of fresh frozen sections and MMS.
Methods
We conducted a systematic search of the PubMed and Cochrane databases for articles published before December 1, 2019, to identify any relevant studies in English. Electronic searches were performed using the terms toluidine blue and Mohs or Mohs micrographic surgery. We manually checked the bibliographies of the identified articles to further identify eligible studies.
Eligibility Criteria—The inclusion criteria were articles that (1) considered TB in the context of MMS, (2) were published in peer-reviewed journals, (3) were published in English, and (4) were available as full text. Systematic reviews were excluded.
Data Extraction and Outcomes—All relevant information regarding the study characteristics, including design, level of evidence, methodologic quality of evidence, pathology examined, and outcome measures, were collected by 2 independent reviewers (T.L. and A.D.) using a predetermined data sheet. The same 2 reviewers were used for all steps of the review process, data were independently obtained, and any discrepancy was introduced for a third opinion (D.H.) and agreed upon by the majority.
Quality Assessment—The level of evidence was evaluated based on the criteria of the Oxford Centre for Evidence-Based Medicine. Two reviewers (T.L. and A.D.) graded each article included in the review.

Results
A total of 25 articles were reviewed. After the titles and abstracts were screened for relevance, 12 articles remained (Figure 1). Of these, 1 compared basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), 4 were related to BCC, 3 were related to SCC, 1 was related to microcystic adnexal carcinoma (MAC), 1 was related to primary cutaneous adenoid cystic carcinoma (PCACC), and 2 were related to technical aspects of the staining process (Table 1).

A majority of the articles included in this review were qualitative and observational in nature, describing the staining characteristics of TB. Study characteristics are summarized in Table 1.
Comment
Basal Cell Carcinoma—Toluidine blue staining characteristics help to identify BCC nests by differentiating them from hair follicles in frozen sections. The metachromatic characteristic of TB stains the inner root sheath deep blue and highlights the surrounding stromal mucin of BCC a magenta color.18,19 In hematoxylin and eosin (H&E) stains, these 2 distinct structures can be differentiated by cleft formation around tumor nests, mitotic figures, and the lack of a fibrous sheath present in BCC tumors.20 The advantages and limitations of TB staining of BCC are presented in Table 2.

Humphreys et al6 suggested a noticeable difference between H&E and TB in the staining of cellular and stromal components. The nuclear detail of tumor cells was subjectively sharper and clearer with TB staining. The staining of stromal components may provide the most assistance in locating BCC islands. Mucopolysaccharide staining may be absent in H&E but stain a deep magenta with TB. Although the presence of mucopolysaccharides does not specifically indicate a tumor, it may prompt further attention and provide an indicator for sparse and infiltrative tumor cells.6 The metachromatic stromal change may indicate a narrow tumor-free margin where additional deeper sections often reveal tumor that may warrant additional resection margin in more aggressive malignancies. In particular, sclerosing/morpheaform BCCs have been shown to induce glycosaminoglycan synthesis and are highlighted more readily with TB than with H&E when compared to surrounding tissue.21 This differentiation in staining has remained a popular reason to routinely incorporate TB into the staining of infiltrative and morpheaform variants of BCC. Additionally, stromal mast cells are believed to be more abundant in the stroma of BCC and are more readily visualized in tissue specimens stained with TB, appearing as bright purple metachromatic granules. These granules are larger than normal and are increased in number.6
The margin behavior of BCC stained with TB was further characterized by Goldberg et al,8 who coined the term setting sun sign, which may be present in sequential sections of a disappearing nodule of a BCC tumor. Stroma, inflammatory infiltrate, and mast cells produce a magenta glow surrounding BCC tumors that is reminiscent of a setting sun (Figure 2). Invasive BCC is considered variable in this presentation, primarily because of zones of cell-free fluid and edema or the second area of inflammatory cells. This unique sign may benefit the inspecting Mohs surgeon by providing a clue to an underlying process that may have residual BCC tumors. The setting sun sign also may assist in identifying exact surgical margins.8

The nasal surface has a predilection for BCC.22 The skin of the nose has numerous look-alike structures to consider for complete tumor removal and avoidance of unnecessary removal. One challenge is distinguishing follicular basaloid proliferations (FBP) from BCC, a scenario that is more common on the nose.22 When TB staining was used, the sensitivity for detecting FBP reached 100% in 34 cases reviewed by Donaldson and Weber.10 None of the cases examined showed TB metachromasia surrounding FBP, thus indicating that TB can dependably identify this benign entity. Conversely, 5% (N=279) of BCCs confirmed on H&E did not exhibit surrounding TB metachromasia. This finding is concerning regarding the specificity of TB staining for BCC, but the authors of this study suggested the possibility that these exceptions were benign “simulants” (ie, trichoepithelioma) of BCC.10
The use of TB also has been shown to be statistically beneficial in Mohs training. In a single-center, single-fellow experiment, the sensitivity and specificity of using TB for BCC were extrapolated.9 Using TB as an adjunct in deep sections showed superior sensitivity to H&E alone in identifying BCC, increasing sensitivity from 96.3% to 99.7%. In a cohort of 352 BCC excisions and frozen sections, only 1 BCC was not completely excised. If H&E only had been performed, the fellow would have missed 13 residual BCC tumors.9
Bennett and Taher7 described a case in which hyaluronic acid (HA) from a filler injection was confused with the HA surrounding BCC tumor nests. They found that when TB is used as an adjunct, the HA filler is easier to differentiate from the HA surrounding the BCC tumor nests. In frozen sections stained with TB, the HA filler appeared as an amorphous, metachromatic, reddish-purple, whereas the HA surrounding the BCC tumor nests appeared as a well-defined red. These findings were less obvious in the same sections stained with H&E alone.7
Squamous Cell Carcinoma—In early investigations, the utility of TB in identifying SCC in frozen sections was thought to be limited. The description by Humphreys and colleagues6 of staining characteristics in SCC suggested that the nuclear detail that H&E provides is more easily recognized. The deep aqua nuclear staining produced with TB was considered more difficult to observe than the cytoplasmic eosinophilia of pyknotic and keratinizing cells in H&E.6
Toluidine blue may be beneficial in providing unique staining characteristics to further detail tumors that are difficult to interpret, such as spindle cell SCC and perineural invasion of aggressive SCC. In H&E, squamous cells of spindle cell SCC (scSCC) blend into the background of inflammatory cells and can be perceptibly difficult to locate. A small cohort of 3 Mohs surgeons who routinely use H&E were surveyed on their ability to detect a proven scSCC in H&E or TB by photograph.12 All 3 were able to detect the scSCC in the TB photographs, but only 2 of 3 were able to detect it in H&E photographs. All 3 surgeons agreed that TB was preferable to H&E for this tumor type. These findings suggested that TB may be superior and preferred over H&E for visualizing tumor cells of scSCC.12 The TB staining characteristics of perineural invasion of aggressive SCC have been referred to as the perineural corona sign because of the bright magenta stain that forms around affected nerves.13 Drosou et al13 suggested that TB may enhance the diagnostic accuracy for perineural SCC.
Rare Tumors—The adjunctive use of TB with H&E has been examined in rare tumors. Published reports have highlighted its use in MMS for treating MAC and PCACC. Toluidine blue exhibits staining advantages for these tumors. It may render isolated nests and perineural invasion of MAC more easily visible on frozen section.15
Although PCACC is rare, the recurrence rate is high.23 Toluidine blue has been used with MMS to ensure complete removal and higher cure rates. The metachromatic nature of TB is advantageous in staining the HA present in these tumors. Those who have reported the use of TB for PCACC prefer it to H&E for frozen sections.14
Technical Aspects—The staining time for TB-treated slides is reduced compared to H&E staining; staining can be efficiently done in frozen sections in less than 2.5 minutes using the method shown in Table 3.17 In comparison, typical H&E staining takes 9 minutes, and older TB techniques take 7 minutes.6

Conclusion
Toluidine blue may play an important and helpful role in the successful diagnosis and treatment of particular cutaneous tumors by providing additional diagnostic information. Although surgeons performing MMS will continue using the staining protocols with which they are most comfortable, adjunctive use of TB over time may provide an additional benefit at low risk for disrupting practice efficiency or workflow. Many Mohs surgeons are accustomed to using this stain, even preferring to interpret only TB-stained slides for cutaneous malignancy. Most published studies on this topic have been observational in nature, and additional controlled trials may be warranted to determine the effects on outcomes in real-world practice.
- Culling CF, Allison TR. Cellular Pathology Technique. 4th ed. Butterworths; 1985.
- Bergeron JA, Singer M. Metachromasy: an experimental and theoretical reevaluation. J Biophys Biochem Cytol. 1958;4:433-457. doi:10.1083/jcb.4.4.433
- Epstein JB, Scully C, Spinelli J. Toluidine blue and Lugol’s iodine application in the assessment of oral malignant disease and lesions at risk of malignancy. J Oral Pathol Med. 1992;21:160-163. doi:10.1111/j.1600-0714.1992.tb00094.x
- Warnakulasuriya KA, Johnson NW. Sensitivity and specificity of OraScan (R) toluidine blue mouthrinse in the detection of oral cancer and precancer. J Oral Pathol Med. 1996;25:97-103. doi:10.1111/j.1600-0714.1996.tb00201.x
- Silapunt S, Peterson SR, Alcalay J, et al. Mohs tissue mapping and processing: a survey study. Dermatol Surg. 2003;29:1109-1112; discussion 1112.
- Humphreys TR, Nemeth A, McCrevey S, et al. A pilot study comparing toluidine blue and hematoxylin and eosin staining of basal cell and squamous cell carcinoma during Mohs surgery. Dermatol Surg. 1996;22:693-697. doi:10.1111/j.1524-4725.1996.tb00619.x
- Bennett R, Taher M. Restylane persistent for 23 months found during Mohs micrographic surgery: a source of confusion with hyaluronic acid surrounding basal cell carcinoma. Dermatol Surg. 2005;31:1366-1369. doi:10.1111/j.1524-4725.2005.31223
- Goldberg LH, Wang SQ, Kimyai-Asadi A. The setting sun sign: visualizing the margins of a basal cell carcinoma on serial frozen sections stained with toluidine blue. Dermatol Surg. 2007;33:761-763. doi:10.1111/j.1524-4725.2007.33158.x
- Tehrani H, May K, Morris A, et al. Does the dual use of toluidine blue and hematoxylin and eosin staining improve basal cell carcinoma detection by Mohs surgery trainees? Dermatol Surg. 2013;39:995-1000. doi:10.1111/dsu.12180
- Donaldson MR, Weber LA. Toluidine blue supports differentiation of folliculocentric basaloid proliferation from basal cell carcinoma on frozen sections in a small single-practice cohort. Dermatol Surg. 2017;43:1303-1306. doi:10.1097/DSS.0000000000001107
- Styperek AR, Goldberg LH, Goldschmidt LE, et al. Toluidine blue and hematoxylin and eosin stains are comparable in evaluating squamous cell carcinoma during Mohs. Dermatol Surg. 2016;42:1279-1284. doi:10.1097/DSS.0000000000000872
- Trieu D, Drosou A, Goldberg LH, et al. Detecting spindle cell squamous cell carcinomas with toluidine blue on frozen sections. Dermatol Surg. 2014;40:1259-1260. doi:10.1097/DSS.0000000000000147
- Drosou A, Trieu D, Goldberg LH, et al. The perineural corona sign: enhancing detection of perineural squamous cell carcinoma during Mohs micrographic surgery with toluidine blue stain. J Am Acad Dermatol. 2014;71:826-827. doi:10.1016/j.jaad.2014.04.076
- Chesser RS, Bertler DE, Fitzpatrick JE, et al. Primary cutaneous adenoid cystic carcinoma treated with Mohs micrographic surgery toluidine blue technique. J Dermatol Surg Oncol. 1992;18:175-176. doi:10.1111/j.1524-4725.1992.tb02794.x
- Wang SQ, Goldberg LH, Nemeth A. The merits of adding toluidine blue-stained slides in Mohs surgery in the treatment of a microcystic adnexal carcinoma. J Am Acad Dermatol. 2007;56:1067-1069. doi:10.1016/j.jaad.2007.01.008
- Chen CL, Wilson S, Afzalneia R, et al. Topical aluminum chloride and Monsel’s solution block toluidine blue staining in Mohs frozen sections: mechanism and solution. Dermatol Surg. 2019;45:1019-1025. doi:10.1097/DSS.0000000000001761
- Todd MM, Lee JW, Marks VJ. Rapid toluidine blue stain for Mohs’ micrographic surgery. Dermatol Surg. 2005;31:244-245. doi:10.1111/j.1524-4725.2005.31053
- Picoto AM, Picoto A. Technical procedures for Mohs fresh tissue surgery. J Derm Surg Oncol. 1986;12:134-138. doi:10.1111/j.1524-4725.1986.tb01442.x
- Sperling LC, Winton GB. The transverse anatomy of androgenic alopecia. J Derm Surg Oncol. 1990;16:1127-1133. doi:10.1111/j.1524 -4725.1990.tb00024.x
- Smith-Zagone MJ, Schwartz MR. Frozen section of skin specimens. Arch Pathol Lab Med. 2005;129:1536-1543. doi:10.5858/2005-129-1536-FSOSS
- Moy RL, Potter TS, Uitto J. Increased glycosaminoglycans production in sclerosing basal cell carcinoma–derived fibroblasts and stimulation of normal skin fibroblast glycosaminoglycans production by a cytokine-derived from sclerosing basal cell carcinoma. Dermatol Surg. 2000;26:1029-1036. doi:10.1046/j.1524-4725.2000.0260111029.x
- Leshin B, White WL. Folliculocentric basaloid proliferation. The bulge (der Wulst) revisited. Arch Dermatol. 1990;126:900-906. doi:10.1001/archderm.126.7.900
- Seab JA, Graham JH. Primary cutaneous adenoid cystic carcinoma.J Am Acad Dermatol. 1987;17:113-118. doi:10.1016/s0190 -9622(87)70182-0
- Culling CF, Allison TR. Cellular Pathology Technique. 4th ed. Butterworths; 1985.
- Bergeron JA, Singer M. Metachromasy: an experimental and theoretical reevaluation. J Biophys Biochem Cytol. 1958;4:433-457. doi:10.1083/jcb.4.4.433
- Epstein JB, Scully C, Spinelli J. Toluidine blue and Lugol’s iodine application in the assessment of oral malignant disease and lesions at risk of malignancy. J Oral Pathol Med. 1992;21:160-163. doi:10.1111/j.1600-0714.1992.tb00094.x
- Warnakulasuriya KA, Johnson NW. Sensitivity and specificity of OraScan (R) toluidine blue mouthrinse in the detection of oral cancer and precancer. J Oral Pathol Med. 1996;25:97-103. doi:10.1111/j.1600-0714.1996.tb00201.x
- Silapunt S, Peterson SR, Alcalay J, et al. Mohs tissue mapping and processing: a survey study. Dermatol Surg. 2003;29:1109-1112; discussion 1112.
- Humphreys TR, Nemeth A, McCrevey S, et al. A pilot study comparing toluidine blue and hematoxylin and eosin staining of basal cell and squamous cell carcinoma during Mohs surgery. Dermatol Surg. 1996;22:693-697. doi:10.1111/j.1524-4725.1996.tb00619.x
- Bennett R, Taher M. Restylane persistent for 23 months found during Mohs micrographic surgery: a source of confusion with hyaluronic acid surrounding basal cell carcinoma. Dermatol Surg. 2005;31:1366-1369. doi:10.1111/j.1524-4725.2005.31223
- Goldberg LH, Wang SQ, Kimyai-Asadi A. The setting sun sign: visualizing the margins of a basal cell carcinoma on serial frozen sections stained with toluidine blue. Dermatol Surg. 2007;33:761-763. doi:10.1111/j.1524-4725.2007.33158.x
- Tehrani H, May K, Morris A, et al. Does the dual use of toluidine blue and hematoxylin and eosin staining improve basal cell carcinoma detection by Mohs surgery trainees? Dermatol Surg. 2013;39:995-1000. doi:10.1111/dsu.12180
- Donaldson MR, Weber LA. Toluidine blue supports differentiation of folliculocentric basaloid proliferation from basal cell carcinoma on frozen sections in a small single-practice cohort. Dermatol Surg. 2017;43:1303-1306. doi:10.1097/DSS.0000000000001107
- Styperek AR, Goldberg LH, Goldschmidt LE, et al. Toluidine blue and hematoxylin and eosin stains are comparable in evaluating squamous cell carcinoma during Mohs. Dermatol Surg. 2016;42:1279-1284. doi:10.1097/DSS.0000000000000872
- Trieu D, Drosou A, Goldberg LH, et al. Detecting spindle cell squamous cell carcinomas with toluidine blue on frozen sections. Dermatol Surg. 2014;40:1259-1260. doi:10.1097/DSS.0000000000000147
- Drosou A, Trieu D, Goldberg LH, et al. The perineural corona sign: enhancing detection of perineural squamous cell carcinoma during Mohs micrographic surgery with toluidine blue stain. J Am Acad Dermatol. 2014;71:826-827. doi:10.1016/j.jaad.2014.04.076
- Chesser RS, Bertler DE, Fitzpatrick JE, et al. Primary cutaneous adenoid cystic carcinoma treated with Mohs micrographic surgery toluidine blue technique. J Dermatol Surg Oncol. 1992;18:175-176. doi:10.1111/j.1524-4725.1992.tb02794.x
- Wang SQ, Goldberg LH, Nemeth A. The merits of adding toluidine blue-stained slides in Mohs surgery in the treatment of a microcystic adnexal carcinoma. J Am Acad Dermatol. 2007;56:1067-1069. doi:10.1016/j.jaad.2007.01.008
- Chen CL, Wilson S, Afzalneia R, et al. Topical aluminum chloride and Monsel’s solution block toluidine blue staining in Mohs frozen sections: mechanism and solution. Dermatol Surg. 2019;45:1019-1025. doi:10.1097/DSS.0000000000001761
- Todd MM, Lee JW, Marks VJ. Rapid toluidine blue stain for Mohs’ micrographic surgery. Dermatol Surg. 2005;31:244-245. doi:10.1111/j.1524-4725.2005.31053
- Picoto AM, Picoto A. Technical procedures for Mohs fresh tissue surgery. J Derm Surg Oncol. 1986;12:134-138. doi:10.1111/j.1524-4725.1986.tb01442.x
- Sperling LC, Winton GB. The transverse anatomy of androgenic alopecia. J Derm Surg Oncol. 1990;16:1127-1133. doi:10.1111/j.1524 -4725.1990.tb00024.x
- Smith-Zagone MJ, Schwartz MR. Frozen section of skin specimens. Arch Pathol Lab Med. 2005;129:1536-1543. doi:10.5858/2005-129-1536-FSOSS
- Moy RL, Potter TS, Uitto J. Increased glycosaminoglycans production in sclerosing basal cell carcinoma–derived fibroblasts and stimulation of normal skin fibroblast glycosaminoglycans production by a cytokine-derived from sclerosing basal cell carcinoma. Dermatol Surg. 2000;26:1029-1036. doi:10.1046/j.1524-4725.2000.0260111029.x
- Leshin B, White WL. Folliculocentric basaloid proliferation. The bulge (der Wulst) revisited. Arch Dermatol. 1990;126:900-906. doi:10.1001/archderm.126.7.900
- Seab JA, Graham JH. Primary cutaneous adenoid cystic carcinoma.J Am Acad Dermatol. 1987;17:113-118. doi:10.1016/s0190 -9622(87)70182-0
Practice Points
- Toluidine blue (TB) staining can be integrated into Mohs micrographic surgery (MMS) for enhanced diagnosis of cutaneous tumors. Its metachromatic properties can aid in differentiating tumor cells from surrounding tissues, especially in basal cell carcinomas and squamous cell carcinomas.
- It is important to develop expertise in interpreting TB-stained sections, as it may offer clearer visualization of nuclear details and stromal components, potentially leading to more accurate diagnosis and effective tumor margin identification.
- Toluidine blue staining can be incorporated into routine MMS practice considering its quick staining process and low disruption to workflow. This can potentially improve diagnostic efficiency without significantly lengthening surgery time.
Is your patient a candidate for Mohs micrographic surgery?
Mohs micrographic surgery (MMS) is a unique dermatologic surgery technique that allows the dermatologist to fill the concomitant roles of surgeon and pathologist. It is utilized for the extirpation of skin malignancy, with an emphasis on tissue preservation and immediate surgical margin evaluation. In MMS, the Mohs surgeon acts as the surgeon for physical removal of the lesion and the pathologist during evaluation of frozen section margins.1
Primary care providers (PCPs) are on the frontlines of management of cutaneous malignancy. Whether referring to Dermatology for biopsy or performing a biopsy themselves, PCPs can assure optimal treatment outcomes by guiding patients to evidence-based treatments, while still respecting the patient’s wishes. In this evidence-based review of the advantages, improved outcomes, and safety of Mohs surgery for the treatment of common and rare skin neoplasms, we provide our primary care colleagues with information on the indications, process (the order in which steps of the procedure are performed), and techniques used for treating cutaneous malignancies with Mohs surgery.
When is Mohs surgery appropriate?
MMS has typically been reserved for treatment of cutaneous malignancy in cosmetically sensitive areas where tissue preservation is key. In 2012, Connolly et al released appropriate use criteria (AUC) for MMS.2 (See “An app that helps clinicians apply the criteria for Mohs surgery.”) Within the AUC, there are 4 major qualitative and quantitative categories when considering referral for MMS:
- area of the body in which the lesion manifests
- the patient’s medical characteristics
- tumor characteristics
- the size of the lesion to be treated.2
Areas of the body are divided into 3 categories by the AUC according to how challenging tumor extirpation is expected to be and how critical tissue preservation is. Areas termed “H” receive the highest score for appropriate Mohs usage, followed by areas “M” and “L.”
SIDEBAR
An app that helps clinicians apply the criteria for Mohs surgery
“Mohs Surgery Appropriate Use Criteria” is a free and easy-to-use smartphone application to help determine whether Mohs micrographic surgery (MMS) is appropriate for a particular patient. Clinicians can enter the details of a recent skin cancer biopsy along with patient information into the app and it will calculate a score automatically categorized into 1 of 3 categories: “appropriate,” “uncertain,” and “not appropriate” for MMS. The clinician can then talk to the patient about a possible referral to a Mohs surgeon, depending on the appropriateness of the procedure for the patient and their tumor.
Patient medical characteristics that should be taken into account when referring for Mohs surgery are the patient’s immune status, genetic syndromes that may predispose the patient to cutaneous malignancies (eg, xeroderma pigmentosa), history of radiation to the area of involvement, and the patient’s history of aggressive cutaneous malignancies.
Tumor characteristics. The most common malignancies treated with MMS include basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). These malignancies are further delineated through histologic evaluation by a pathologist or dermatopathologist. Aggressive features of a BCC on any area of the body that warrant referral to a Mohs surgeon include morpheaform/fibrosing/sclerosing histologic findings, as well as micronodular architecture and perineural invasion. Concerning histologic SCC findings that warrant Mohs surgery through the AUC include sclerosing, basosquamous, and small cell histology, as well as poorly differentiated and/or undifferentiated SCC.
Melanoma in situ and lentigo maligna, which are variants of melanoma limited to the epidermis without invasion into the underlying dermis, are included within the AUC for MMS. For invasive melanoma (melanoma that has invaded into the dermis or subcutaneous tissue), MMS has been shown to have marginal benefit but currently is not included within the AUC.3
Continue to: Due to excellent margin control...
Due to excellent margin control via immediate microscopic evaluation of surgical margins, MMS is an appropriate treatment choice and indicated for many more uncommon cutaneous malignancies, including sebaceous and mucinous carcinoma, microcystic adnexal carcinoma, Merkel cell carcinoma, leiomyosarcoma, dermatofibrosarcoma protuberans, atypical fibroxanthoma, angiosarcoma, and other more rarely encountered clinical malignancies.2
Tumor size. When considering a referral to MMS for cancer extirpation, the size of the tumor does play a role; however, size depends on the type of tumor as well as the location on the body. In general, most skin cancers of any size on the face, perianal area, genitalia, nipples, hands, feet and ankles, or pretibial surface are appropriate for Mohs surgery. Skin cancers on the trunk and extremities are also appropriate if they are above a certain size specified by the AUC. Tumor type and whether they are recurrences also factor into the equation.
Who will do the procedure?
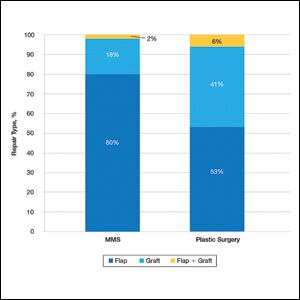
A recent review showed that PCPs were more likely to refer patients to plastic surgery rather than Mohs surgery for skin cancer removal, especially among younger female patients.4 This is likely because of the perception that plastic surgeons do more complex closures and have more experience removing difficult cancers. Interestingly, this same study showed that Mohs surgeons may actually be doing several-fold more complex closures (flaps and grafts) on the nose and ears than plastic surgeons at similar practice settings.4
Aside from Mohs surgeons doing more closures, perhaps the biggest difference between Mohs surgeons and plastic surgeons is the pathology training of the Mohs surgeon. Mohs surgeons evaluate 100% of the tissue margins at the time of the procedure to both ensure complete tumor removal and to preserve as much tumor-free skin as possible, ultimately resulting in decreased recurrences and smaller scars. In contrast, the plastic surgeon’s rigorous training typically does not include extensive dermatopathology training, particularly the pathology of cutaneous neoplasms. Plastic surgeons will often send pathologic specimens for evaluation, meaning patients have to wait for outside histologic confirmation before their wounds can be closed. Additionally, the histologic evaluation is often not a full-margin assessment, as not all labs are equipped for this technique.
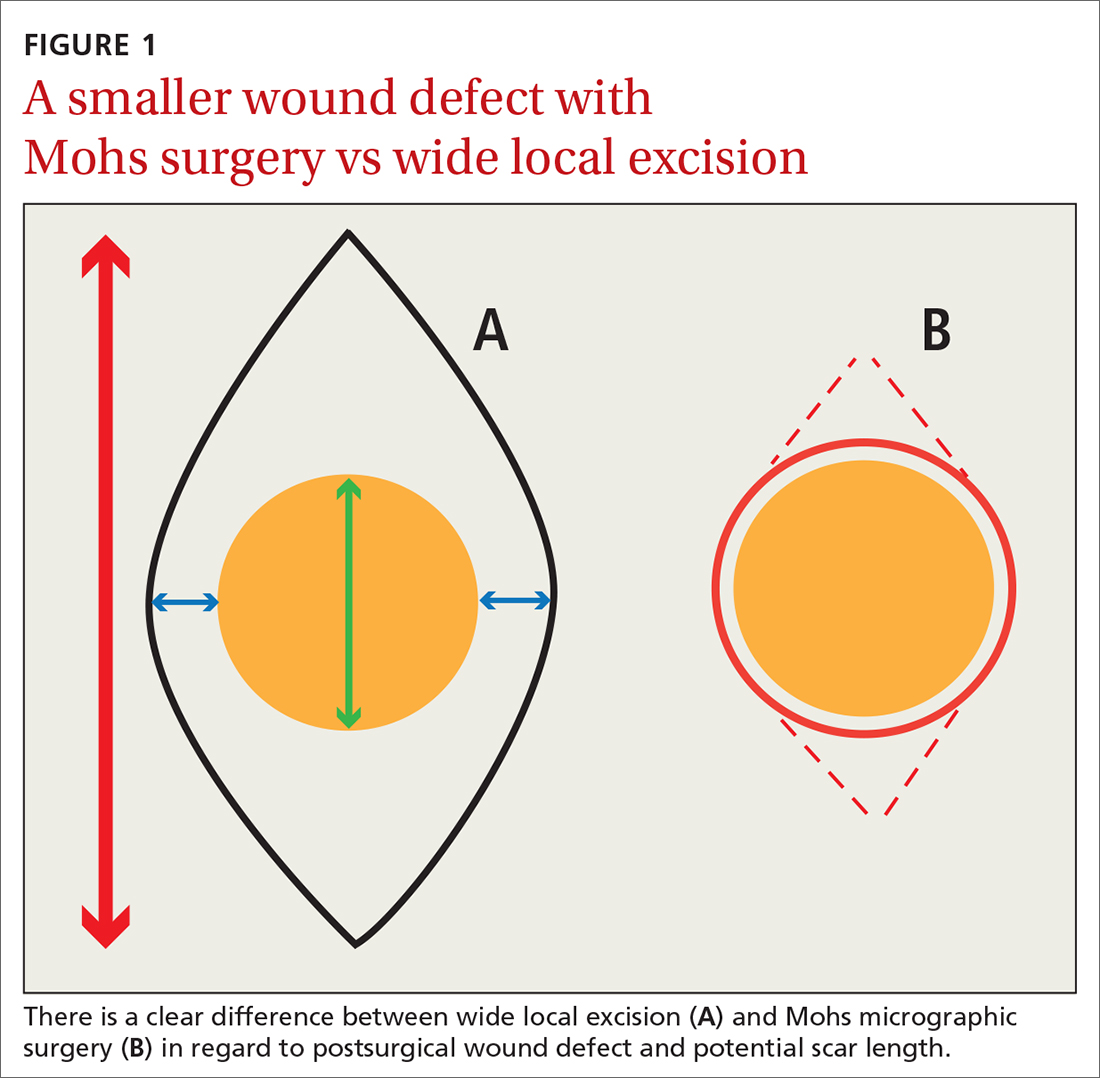
Consider early consultation with a Mohs surgeon for tumor extirpation to keep the defect size as small as possible, as MMS does not require taking margins of healthy surrounding tissue, in contrast to wide local excisions (WLEs; FIGURE 1). A smaller initial incision will result in a smaller scar, which is likely to have better cosmetic outcomes and decreased risk for wound infection.
Continue to: Before consultation...
Before consultation, include a picture of the surgical site with the patient’s referral documentation or have the patient present a photo from his or her phone to the Mohs surgeon. (If a camera or cell phone is not available, triangulation of the site’s location using cosmetic landmarks can be documented in the patient’s chart.)
What the patient can expect during preop visits
During an initial consultation, patients can expect an evaluation by the surgeon that will include more photo taking, a discussion of the surgery, and possibly, performance of an in-clinic biopsy of suspicious lesions. Many practices, including the authors’, use a photo capturing add-on for the EMR in the office.5-7
During the consent process, MMS is described to the patient using lay language and, often, pictorial depictions of the procedure. While explaining that the procedure helps preserve healthy tissue and limit the size of the resulting scar, the surgeon will typically manage the expectations of the patient prior to the first incision. Many clinically small lesions can have significant subclinical extension adjacent to, or on top of, cosmetic landmarks, requiring a flap or graft to close the surgical defect with acceptable cosmetic outcomes.8
One more time. Immediately before surgery, the surgeon will again review the procedure with the patient, using photos of the biopsy site taken during the initial consult, in conjunction with patient verification of the biopsy site, to verify the surgical site and confirm that the patient understands and agrees to the surgery.
A look at how Mohs surgery is performed
MMS typically is performed in the outpatient setting but can also be performed in an operating room or outpatient surgical center. MMS can be performed in a nonsterile procedure room with surgeons and assistants typically utilizing clean, nonsterile gloves, although many Mohs surgeons prefer to perform part, or all, of the technique using sterile gloves.9 A recent systematic review and large meta-analysis showed no significant difference in postsurgical site infections when comparing the use of sterile vs nonsterile gloves.10
Continue to: Prior to initial incision...
Prior to initial incision, the site is marked with a surgical pen and given 1-mm margins around the clinically visualized lesion. The site is then cleansed with an antiseptic, typically a chlorhexidine solution. Local anesthesia is employed, most commonly with a 1:100,000 lidocaine and epinephrine injection. Marking of the tumor prior to numbing is imperative, as the boundaries of the tumor are typically obscured when the local cutaneous vasculature constricts and causes visualized blanching of adjacent skin. Many Mohs surgeons perform a brief curettage of the lesion with a nondisposable, dull curette to better define the tumor edges and to debulk any obvious exophytic tumor noted by the naked eye.
Prior to the first incision, the surgical site is scored in a variety of ways in order to properly orient the tissue after it has been removed from the patient. Mohs surgeons have differing opinions on how to score and/or mark the tissue, but a common practice is to make a nick at the 12 o’clock position. Following removal of the first stage, the nick will be visible on both the extirpated tissue and the tissue just above the surgical defect. This prevents potential confusion regarding orientation during tissue processing.
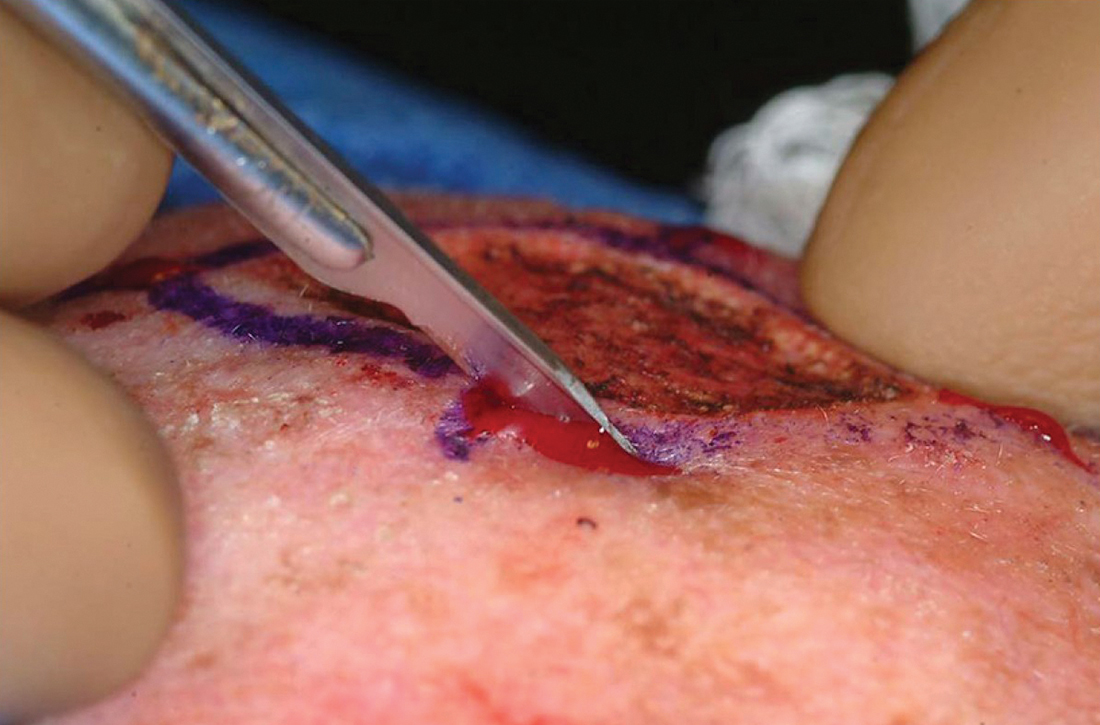
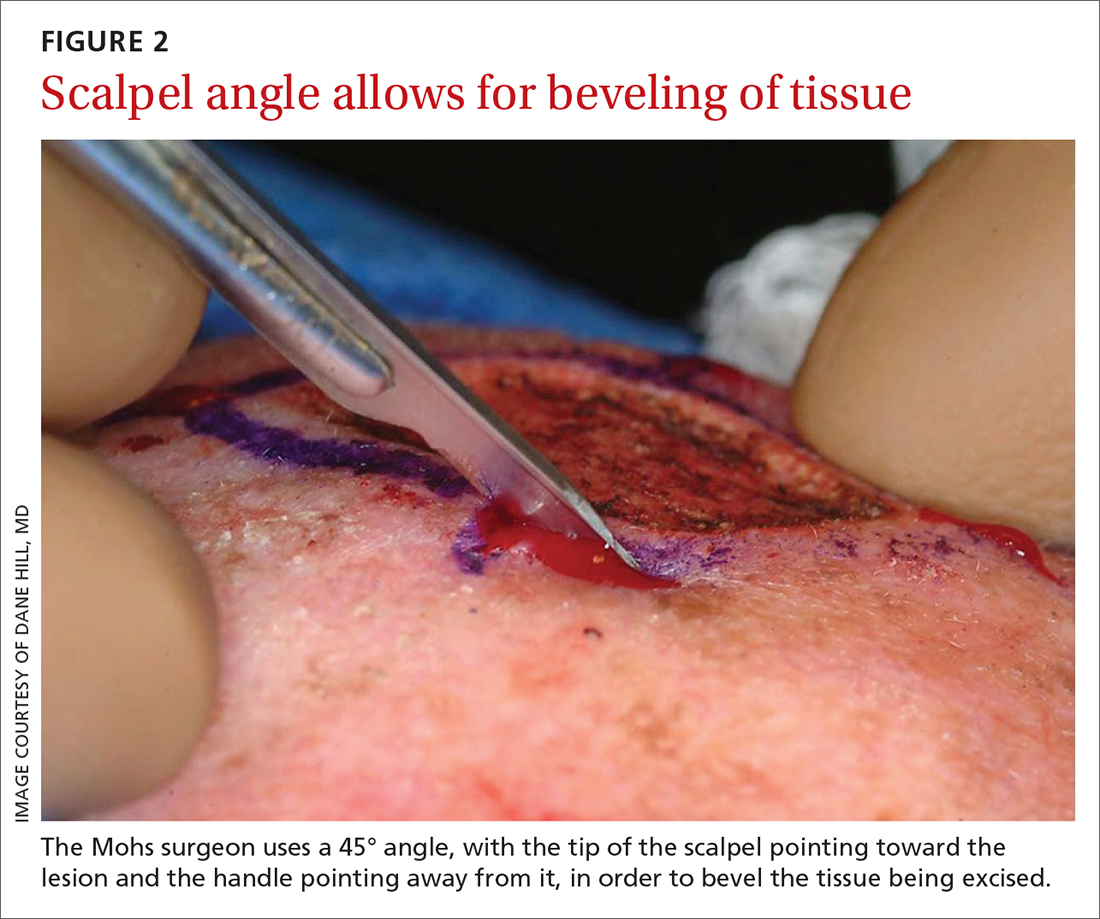
The majority of all WLEs are performed utilizing the scalpel blade at an angle 90° perpendicular to the plane of the skin. In MMS, a signature 45° angle with the tip of the scalpel pointing toward, and the handle pointing away from, the lesion is commonly used in order to bevel the tissue being excised (FIGURE 2). Once the tissue is excised, hemostasis is obtained using electrodessication/electrofulguration or electrocoagulation.
Tissue processing and microscopic evaluation
The technique of beveling allows the epidermis, dermis, and subcutaneous tissue to lie flat on the tissue block, so the Mohs surgeon can evaluate 100% of the excised tissue’s margins. The tissue is transported to a nearby lab for staining and processing. Even if near-perfect beveling is achieved, many stages will require bisecting, quadrissecting, or relaxing cuts in order to allow the margins to lie flat on the tissue block.
Using the scoring system made prior to incision, the tissue is oriented and stained with colored ink. Subsequently, a map is made with sections highlighting the colors used to stain designated areas of the tissue. This step is imperative for orientation during microscopic evaluation. Additionally, the map serves as a guide and log, should a section of the specimen have an involved margin and require another stage.
Continue to: Once fixed to the block...
Once fixed to the block, the tissue is engulfed in appropriate embedding medium and placed within the cryostat. The block is slowly cut to produce several micron-thin wafers of tissue that are then mounted on glass slides and processed with hematoxylin and eosin (H&E) or various stains. The first wafers of tissue that come from the tissue block are those that are closest to the margin that was excised. Thus, 100% of the epidermis and deep margin can be visualized. “Deeper sections” are those that come from deeper cuts within the tissue and are more likely to show the malignant neoplasm.
The evaluation of immediate margins at the very edge of the tissue is in contrast to the technique of “bread-loafing,” which is the standard of evaluating margins after a WLE.11 With this process, the pathologist examines sections that are cut 2- to 4-mm apart. This process only allows the pathologist to examine roughly 1% of the total tissue that was excised, and large variability in cutaneous representation can occur depending on the individual who cuts and processes the tissue.11
Closing the defect
Once the site is deemed clear of residual tumor, the Mohs surgeon approaches the defect and determines the most appropriate way to close the surgical wound. Mohs surgeons are trained to close wounds using a variety of methods, including complex linear closures, flaps, and full-thickness skin grafts. Thoughtful consideration of local anatomy, cosmetic landmarks that may be affected by the closure method, and local tissue laxity are evaluated.
Depending on the location, a secondary intention closure may prove to be just as effective and cosmetically satisfying as a primary intention closure. In light of the many methods of closure, a complex or large surface area defect may better be suited for evaluation and closure by another specialist such as an ENT physician, ophthalmologist, or plastic surgeon.12
Lower recurrence rates for patients who undergo Mohs surgery
As noted earlier, the cutaneous malignancies most commonly treated with MMS are BCCs, followed by SCCs.13 Comparison studies between WLE and MMS show clinically significant differences in terms of recurrence rates between the 2 procedures.
Continue to: For BCCs
For BCCs, recurrence rates for excisions vs MMS are 10% and 1%, respectively.14-16 A randomized trial reviewing 10-year recurrence of primary BCCs on the face showed recurrence rates for MMS of 4.4% compared to 12.2% for WLE.17 This study also showed recurrence rates for recurrent facial BCCs treated with MMS to be 3.9% vs 13.5% for standard WLE.17
SCC. The evidence similarly supports the efficacy of MMS for SCCs. A recent study showed primary T2a tumors had a 1.2% local recurrence rate with Mohs vs a 4% recurrence rate with WLE at an average follow-up of 2.8 years.18 Another study showed that primary tumors that were < 2 cm in diameter had a 5-year cure rate of 99% with Mohs surgery.11
Melanoma in situ. A few studies have shown no clinically significant benefit of MMS compared to WLE when it comes to melanoma in situ.19,20 However, a more recent article by Etzkom et al noted the ability to potentially upstage melanoma in situ and invasive melanoma after reviewing peripheral and deep margins during MMS.21 In this study, the authors uniquely delayed wound closure if upstaging was established and the need for a sentinel lymph node biopsy was warranted. This approach to MMS with delayed closure ultimately paved the way for very low recurrence rates.
CORRESPONDENCE
Andres Garcia, MD, 2612 112th Street, Lubbock, TX 79423; [email protected]
1. Dim-Jamora KC, Perone JB. Management of cutaneous tumors with Mohs micrographic surgery. Semin Plast Surg. 2008;22:247-256.
2. Ad Hoc Task Force, Connolly SM, Baker DR, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550. Published correction appears in J Am Acad Dermatol. 2015;72:748.
3. Cheraghlou S, Christensen S, Agogo G, et al. Comparison of survival after Mohs micrographic surgery vs wide margin excision for early-stage invasive melanoma. JAMA Dermatol. 2019;155:1252-1259.
4. Hill D, Kim K, Mansouri B, et al. Quantity and characteristics of flap or graft repairs for skin cancer on the nose or ears: a comparison between Mohs micrographic surgery and plastic surgery. Cutis. 2019;103:284-287.
5. McGinness JL, Goldstein G. The value of preoperative biopsy-site photography for identifying cutaneous lesions. Dermatol Surg. 2010;36:194-197.
6. Ke M, Moul D, Camouse M, et al. Where is it? The utility of biopsy-site photography. Dermatol Surg. 2010;36:198-202.
7. Nijhawan RI, Lee EH, Nehal KS. Biopsy site selfies—a quality improvement pilot study to assist with correct surgical site identification. Dermatol Surg. 2015;41:499-504
8. Breuninger H, Dietz K. Prediction of subclinical tumor infiltration in basal cell carcinoma. J Dermatol Surg Oncol. 1991;17:574-578.
9. Rhinehart BM, Murphy Me, Farley MF, et al. Sterile versus nonsterile gloves during Mohs micrographic surgery: infection rate is not affected. Dermatol Surg. 2006;32:170-176.
10. Brewer JD, Gonzalez AB, Baum CL, et al. Comparison of sterile vs nonsterile gloves in cutaneous surgery and common outpatient dental procedures: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:1008-1014.
11. Shriner DL, McCoy DK, Goldberg DJ, et al. Mohs micrographic surgery. J Am Acad Dermatol. 1998;39:79-97.
12. Gladstone HB, Stewart D. An algorithm for the reconstruction of complex facial defects. Skin Therapy Lett. 2007;12:6-9.
13. Robinson JK. Mohs micrographic surgery. Clin Plast Surg. 1993;20:149-156.
14. Swanson NA. Mohs surgery. Technique, indications, applications, and the future. Arch Dermatol. 1983;119:761-773.
15. Robins P. Chemosurgery: my 15 years of experience. J Dermatol Surg Oncol. 1981;7:779-789.
16. Rowe DE, Carroll RJ, Day CL Jr. Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow-up. J Dermatol Surg Oncol. 1989;15:315-328.
17. van Loo E, Mosterd K, Krekels GA, et al. Surgical excision versus Mohs’ micrographic surgery for basal cell carcinoma of the face: a randomised clinical trial with 10 year follow-up. Eur J Cancer. 2014;50:3011-3020.
18. Xiong DD, Beal BT, Varra V, et al. Outcomes in intermediate-risk squamous cell carcinomas treated with Mohs micrographic surgery compared with wide local excision. J Am Acad Dermatol. 2020;82: 1195-1204.
19. Trofymenko O, Bordeaux JS, Zeitouni NC. Melanoma of the face and Mohs micrographic surgery: nationwide mortality data analysis. Dermatol Surg. 2018;44:481-492.
20. Nosrati A, Berliner JG, Goel S, et al. Outcomes of melanoma in situ treated with Mohs micrographic surgery compared with wide local excision. JAMA Dermatol. 2017;153:436-441.
21. Etzkom JR, Sobanko JF, Elenitsas R, et al. Low recurrences for in situ and invasive melanomas using Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining: tissue processing methodology to optimize pathologic and margin assessment. J Am Acad Dermatol. 2015;72:840-850.
Mohs micrographic surgery (MMS) is a unique dermatologic surgery technique that allows the dermatologist to fill the concomitant roles of surgeon and pathologist. It is utilized for the extirpation of skin malignancy, with an emphasis on tissue preservation and immediate surgical margin evaluation. In MMS, the Mohs surgeon acts as the surgeon for physical removal of the lesion and the pathologist during evaluation of frozen section margins.1
Primary care providers (PCPs) are on the frontlines of management of cutaneous malignancy. Whether referring to Dermatology for biopsy or performing a biopsy themselves, PCPs can assure optimal treatment outcomes by guiding patients to evidence-based treatments, while still respecting the patient’s wishes. In this evidence-based review of the advantages, improved outcomes, and safety of Mohs surgery for the treatment of common and rare skin neoplasms, we provide our primary care colleagues with information on the indications, process (the order in which steps of the procedure are performed), and techniques used for treating cutaneous malignancies with Mohs surgery.
When is Mohs surgery appropriate?
MMS has typically been reserved for treatment of cutaneous malignancy in cosmetically sensitive areas where tissue preservation is key. In 2012, Connolly et al released appropriate use criteria (AUC) for MMS.2 (See “An app that helps clinicians apply the criteria for Mohs surgery.”) Within the AUC, there are 4 major qualitative and quantitative categories when considering referral for MMS:
- area of the body in which the lesion manifests
- the patient’s medical characteristics
- tumor characteristics
- the size of the lesion to be treated.2
Areas of the body are divided into 3 categories by the AUC according to how challenging tumor extirpation is expected to be and how critical tissue preservation is. Areas termed “H” receive the highest score for appropriate Mohs usage, followed by areas “M” and “L.”
SIDEBAR
An app that helps clinicians apply the criteria for Mohs surgery
“Mohs Surgery Appropriate Use Criteria” is a free and easy-to-use smartphone application to help determine whether Mohs micrographic surgery (MMS) is appropriate for a particular patient. Clinicians can enter the details of a recent skin cancer biopsy along with patient information into the app and it will calculate a score automatically categorized into 1 of 3 categories: “appropriate,” “uncertain,” and “not appropriate” for MMS. The clinician can then talk to the patient about a possible referral to a Mohs surgeon, depending on the appropriateness of the procedure for the patient and their tumor.
Patient medical characteristics that should be taken into account when referring for Mohs surgery are the patient’s immune status, genetic syndromes that may predispose the patient to cutaneous malignancies (eg, xeroderma pigmentosa), history of radiation to the area of involvement, and the patient’s history of aggressive cutaneous malignancies.
Tumor characteristics. The most common malignancies treated with MMS include basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). These malignancies are further delineated through histologic evaluation by a pathologist or dermatopathologist. Aggressive features of a BCC on any area of the body that warrant referral to a Mohs surgeon include morpheaform/fibrosing/sclerosing histologic findings, as well as micronodular architecture and perineural invasion. Concerning histologic SCC findings that warrant Mohs surgery through the AUC include sclerosing, basosquamous, and small cell histology, as well as poorly differentiated and/or undifferentiated SCC.
Melanoma in situ and lentigo maligna, which are variants of melanoma limited to the epidermis without invasion into the underlying dermis, are included within the AUC for MMS. For invasive melanoma (melanoma that has invaded into the dermis or subcutaneous tissue), MMS has been shown to have marginal benefit but currently is not included within the AUC.3
Continue to: Due to excellent margin control...
Due to excellent margin control via immediate microscopic evaluation of surgical margins, MMS is an appropriate treatment choice and indicated for many more uncommon cutaneous malignancies, including sebaceous and mucinous carcinoma, microcystic adnexal carcinoma, Merkel cell carcinoma, leiomyosarcoma, dermatofibrosarcoma protuberans, atypical fibroxanthoma, angiosarcoma, and other more rarely encountered clinical malignancies.2
Tumor size. When considering a referral to MMS for cancer extirpation, the size of the tumor does play a role; however, size depends on the type of tumor as well as the location on the body. In general, most skin cancers of any size on the face, perianal area, genitalia, nipples, hands, feet and ankles, or pretibial surface are appropriate for Mohs surgery. Skin cancers on the trunk and extremities are also appropriate if they are above a certain size specified by the AUC. Tumor type and whether they are recurrences also factor into the equation.
Who will do the procedure?
A recent review showed that PCPs were more likely to refer patients to plastic surgery rather than Mohs surgery for skin cancer removal, especially among younger female patients.4 This is likely because of the perception that plastic surgeons do more complex closures and have more experience removing difficult cancers. Interestingly, this same study showed that Mohs surgeons may actually be doing several-fold more complex closures (flaps and grafts) on the nose and ears than plastic surgeons at similar practice settings.4
Aside from Mohs surgeons doing more closures, perhaps the biggest difference between Mohs surgeons and plastic surgeons is the pathology training of the Mohs surgeon. Mohs surgeons evaluate 100% of the tissue margins at the time of the procedure to both ensure complete tumor removal and to preserve as much tumor-free skin as possible, ultimately resulting in decreased recurrences and smaller scars. In contrast, the plastic surgeon’s rigorous training typically does not include extensive dermatopathology training, particularly the pathology of cutaneous neoplasms. Plastic surgeons will often send pathologic specimens for evaluation, meaning patients have to wait for outside histologic confirmation before their wounds can be closed. Additionally, the histologic evaluation is often not a full-margin assessment, as not all labs are equipped for this technique.
Consider early consultation with a Mohs surgeon for tumor extirpation to keep the defect size as small as possible, as MMS does not require taking margins of healthy surrounding tissue, in contrast to wide local excisions (WLEs; FIGURE 1). A smaller initial incision will result in a smaller scar, which is likely to have better cosmetic outcomes and decreased risk for wound infection.

Continue to: Before consultation...
Before consultation, include a picture of the surgical site with the patient’s referral documentation or have the patient present a photo from his or her phone to the Mohs surgeon. (If a camera or cell phone is not available, triangulation of the site’s location using cosmetic landmarks can be documented in the patient’s chart.)
What the patient can expect during preop visits
During an initial consultation, patients can expect an evaluation by the surgeon that will include more photo taking, a discussion of the surgery, and possibly, performance of an in-clinic biopsy of suspicious lesions. Many practices, including the authors’, use a photo capturing add-on for the EMR in the office.5-7
During the consent process, MMS is described to the patient using lay language and, often, pictorial depictions of the procedure. While explaining that the procedure helps preserve healthy tissue and limit the size of the resulting scar, the surgeon will typically manage the expectations of the patient prior to the first incision. Many clinically small lesions can have significant subclinical extension adjacent to, or on top of, cosmetic landmarks, requiring a flap or graft to close the surgical defect with acceptable cosmetic outcomes.8
One more time. Immediately before surgery, the surgeon will again review the procedure with the patient, using photos of the biopsy site taken during the initial consult, in conjunction with patient verification of the biopsy site, to verify the surgical site and confirm that the patient understands and agrees to the surgery.
A look at how Mohs surgery is performed
MMS typically is performed in the outpatient setting but can also be performed in an operating room or outpatient surgical center. MMS can be performed in a nonsterile procedure room with surgeons and assistants typically utilizing clean, nonsterile gloves, although many Mohs surgeons prefer to perform part, or all, of the technique using sterile gloves.9 A recent systematic review and large meta-analysis showed no significant difference in postsurgical site infections when comparing the use of sterile vs nonsterile gloves.10
Continue to: Prior to initial incision...
Prior to initial incision, the site is marked with a surgical pen and given 1-mm margins around the clinically visualized lesion. The site is then cleansed with an antiseptic, typically a chlorhexidine solution. Local anesthesia is employed, most commonly with a 1:100,000 lidocaine and epinephrine injection. Marking of the tumor prior to numbing is imperative, as the boundaries of the tumor are typically obscured when the local cutaneous vasculature constricts and causes visualized blanching of adjacent skin. Many Mohs surgeons perform a brief curettage of the lesion with a nondisposable, dull curette to better define the tumor edges and to debulk any obvious exophytic tumor noted by the naked eye.
Prior to the first incision, the surgical site is scored in a variety of ways in order to properly orient the tissue after it has been removed from the patient. Mohs surgeons have differing opinions on how to score and/or mark the tissue, but a common practice is to make a nick at the 12 o’clock position. Following removal of the first stage, the nick will be visible on both the extirpated tissue and the tissue just above the surgical defect. This prevents potential confusion regarding orientation during tissue processing.
The majority of all WLEs are performed utilizing the scalpel blade at an angle 90° perpendicular to the plane of the skin. In MMS, a signature 45° angle with the tip of the scalpel pointing toward, and the handle pointing away from, the lesion is commonly used in order to bevel the tissue being excised (FIGURE 2). Once the tissue is excised, hemostasis is obtained using electrodessication/electrofulguration or electrocoagulation.

Tissue processing and microscopic evaluation
The technique of beveling allows the epidermis, dermis, and subcutaneous tissue to lie flat on the tissue block, so the Mohs surgeon can evaluate 100% of the excised tissue’s margins. The tissue is transported to a nearby lab for staining and processing. Even if near-perfect beveling is achieved, many stages will require bisecting, quadrissecting, or relaxing cuts in order to allow the margins to lie flat on the tissue block.
Using the scoring system made prior to incision, the tissue is oriented and stained with colored ink. Subsequently, a map is made with sections highlighting the colors used to stain designated areas of the tissue. This step is imperative for orientation during microscopic evaluation. Additionally, the map serves as a guide and log, should a section of the specimen have an involved margin and require another stage.
Continue to: Once fixed to the block...
Once fixed to the block, the tissue is engulfed in appropriate embedding medium and placed within the cryostat. The block is slowly cut to produce several micron-thin wafers of tissue that are then mounted on glass slides and processed with hematoxylin and eosin (H&E) or various stains. The first wafers of tissue that come from the tissue block are those that are closest to the margin that was excised. Thus, 100% of the epidermis and deep margin can be visualized. “Deeper sections” are those that come from deeper cuts within the tissue and are more likely to show the malignant neoplasm.
The evaluation of immediate margins at the very edge of the tissue is in contrast to the technique of “bread-loafing,” which is the standard of evaluating margins after a WLE.11 With this process, the pathologist examines sections that are cut 2- to 4-mm apart. This process only allows the pathologist to examine roughly 1% of the total tissue that was excised, and large variability in cutaneous representation can occur depending on the individual who cuts and processes the tissue.11
Closing the defect
Once the site is deemed clear of residual tumor, the Mohs surgeon approaches the defect and determines the most appropriate way to close the surgical wound. Mohs surgeons are trained to close wounds using a variety of methods, including complex linear closures, flaps, and full-thickness skin grafts. Thoughtful consideration of local anatomy, cosmetic landmarks that may be affected by the closure method, and local tissue laxity are evaluated.
Depending on the location, a secondary intention closure may prove to be just as effective and cosmetically satisfying as a primary intention closure. In light of the many methods of closure, a complex or large surface area defect may better be suited for evaluation and closure by another specialist such as an ENT physician, ophthalmologist, or plastic surgeon.12
Lower recurrence rates for patients who undergo Mohs surgery
As noted earlier, the cutaneous malignancies most commonly treated with MMS are BCCs, followed by SCCs.13 Comparison studies between WLE and MMS show clinically significant differences in terms of recurrence rates between the 2 procedures.
Continue to: For BCCs
For BCCs, recurrence rates for excisions vs MMS are 10% and 1%, respectively.14-16 A randomized trial reviewing 10-year recurrence of primary BCCs on the face showed recurrence rates for MMS of 4.4% compared to 12.2% for WLE.17 This study also showed recurrence rates for recurrent facial BCCs treated with MMS to be 3.9% vs 13.5% for standard WLE.17
SCC. The evidence similarly supports the efficacy of MMS for SCCs. A recent study showed primary T2a tumors had a 1.2% local recurrence rate with Mohs vs a 4% recurrence rate with WLE at an average follow-up of 2.8 years.18 Another study showed that primary tumors that were < 2 cm in diameter had a 5-year cure rate of 99% with Mohs surgery.11
Melanoma in situ. A few studies have shown no clinically significant benefit of MMS compared to WLE when it comes to melanoma in situ.19,20 However, a more recent article by Etzkom et al noted the ability to potentially upstage melanoma in situ and invasive melanoma after reviewing peripheral and deep margins during MMS.21 In this study, the authors uniquely delayed wound closure if upstaging was established and the need for a sentinel lymph node biopsy was warranted. This approach to MMS with delayed closure ultimately paved the way for very low recurrence rates.
CORRESPONDENCE
Andres Garcia, MD, 2612 112th Street, Lubbock, TX 79423; [email protected]
Mohs micrographic surgery (MMS) is a unique dermatologic surgery technique that allows the dermatologist to fill the concomitant roles of surgeon and pathologist. It is utilized for the extirpation of skin malignancy, with an emphasis on tissue preservation and immediate surgical margin evaluation. In MMS, the Mohs surgeon acts as the surgeon for physical removal of the lesion and the pathologist during evaluation of frozen section margins.1
Primary care providers (PCPs) are on the frontlines of management of cutaneous malignancy. Whether referring to Dermatology for biopsy or performing a biopsy themselves, PCPs can assure optimal treatment outcomes by guiding patients to evidence-based treatments, while still respecting the patient’s wishes. In this evidence-based review of the advantages, improved outcomes, and safety of Mohs surgery for the treatment of common and rare skin neoplasms, we provide our primary care colleagues with information on the indications, process (the order in which steps of the procedure are performed), and techniques used for treating cutaneous malignancies with Mohs surgery.
When is Mohs surgery appropriate?
MMS has typically been reserved for treatment of cutaneous malignancy in cosmetically sensitive areas where tissue preservation is key. In 2012, Connolly et al released appropriate use criteria (AUC) for MMS.2 (See “An app that helps clinicians apply the criteria for Mohs surgery.”) Within the AUC, there are 4 major qualitative and quantitative categories when considering referral for MMS:
- area of the body in which the lesion manifests
- the patient’s medical characteristics
- tumor characteristics
- the size of the lesion to be treated.2
Areas of the body are divided into 3 categories by the AUC according to how challenging tumor extirpation is expected to be and how critical tissue preservation is. Areas termed “H” receive the highest score for appropriate Mohs usage, followed by areas “M” and “L.”
SIDEBAR
An app that helps clinicians apply the criteria for Mohs surgery
“Mohs Surgery Appropriate Use Criteria” is a free and easy-to-use smartphone application to help determine whether Mohs micrographic surgery (MMS) is appropriate for a particular patient. Clinicians can enter the details of a recent skin cancer biopsy along with patient information into the app and it will calculate a score automatically categorized into 1 of 3 categories: “appropriate,” “uncertain,” and “not appropriate” for MMS. The clinician can then talk to the patient about a possible referral to a Mohs surgeon, depending on the appropriateness of the procedure for the patient and their tumor.
Patient medical characteristics that should be taken into account when referring for Mohs surgery are the patient’s immune status, genetic syndromes that may predispose the patient to cutaneous malignancies (eg, xeroderma pigmentosa), history of radiation to the area of involvement, and the patient’s history of aggressive cutaneous malignancies.
Tumor characteristics. The most common malignancies treated with MMS include basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). These malignancies are further delineated through histologic evaluation by a pathologist or dermatopathologist. Aggressive features of a BCC on any area of the body that warrant referral to a Mohs surgeon include morpheaform/fibrosing/sclerosing histologic findings, as well as micronodular architecture and perineural invasion. Concerning histologic SCC findings that warrant Mohs surgery through the AUC include sclerosing, basosquamous, and small cell histology, as well as poorly differentiated and/or undifferentiated SCC.
Melanoma in situ and lentigo maligna, which are variants of melanoma limited to the epidermis without invasion into the underlying dermis, are included within the AUC for MMS. For invasive melanoma (melanoma that has invaded into the dermis or subcutaneous tissue), MMS has been shown to have marginal benefit but currently is not included within the AUC.3
Continue to: Due to excellent margin control...
Due to excellent margin control via immediate microscopic evaluation of surgical margins, MMS is an appropriate treatment choice and indicated for many more uncommon cutaneous malignancies, including sebaceous and mucinous carcinoma, microcystic adnexal carcinoma, Merkel cell carcinoma, leiomyosarcoma, dermatofibrosarcoma protuberans, atypical fibroxanthoma, angiosarcoma, and other more rarely encountered clinical malignancies.2
Tumor size. When considering a referral to MMS for cancer extirpation, the size of the tumor does play a role; however, size depends on the type of tumor as well as the location on the body. In general, most skin cancers of any size on the face, perianal area, genitalia, nipples, hands, feet and ankles, or pretibial surface are appropriate for Mohs surgery. Skin cancers on the trunk and extremities are also appropriate if they are above a certain size specified by the AUC. Tumor type and whether they are recurrences also factor into the equation.
Who will do the procedure?
A recent review showed that PCPs were more likely to refer patients to plastic surgery rather than Mohs surgery for skin cancer removal, especially among younger female patients.4 This is likely because of the perception that plastic surgeons do more complex closures and have more experience removing difficult cancers. Interestingly, this same study showed that Mohs surgeons may actually be doing several-fold more complex closures (flaps and grafts) on the nose and ears than plastic surgeons at similar practice settings.4
Aside from Mohs surgeons doing more closures, perhaps the biggest difference between Mohs surgeons and plastic surgeons is the pathology training of the Mohs surgeon. Mohs surgeons evaluate 100% of the tissue margins at the time of the procedure to both ensure complete tumor removal and to preserve as much tumor-free skin as possible, ultimately resulting in decreased recurrences and smaller scars. In contrast, the plastic surgeon’s rigorous training typically does not include extensive dermatopathology training, particularly the pathology of cutaneous neoplasms. Plastic surgeons will often send pathologic specimens for evaluation, meaning patients have to wait for outside histologic confirmation before their wounds can be closed. Additionally, the histologic evaluation is often not a full-margin assessment, as not all labs are equipped for this technique.
Consider early consultation with a Mohs surgeon for tumor extirpation to keep the defect size as small as possible, as MMS does not require taking margins of healthy surrounding tissue, in contrast to wide local excisions (WLEs; FIGURE 1). A smaller initial incision will result in a smaller scar, which is likely to have better cosmetic outcomes and decreased risk for wound infection.

Continue to: Before consultation...
Before consultation, include a picture of the surgical site with the patient’s referral documentation or have the patient present a photo from his or her phone to the Mohs surgeon. (If a camera or cell phone is not available, triangulation of the site’s location using cosmetic landmarks can be documented in the patient’s chart.)
What the patient can expect during preop visits
During an initial consultation, patients can expect an evaluation by the surgeon that will include more photo taking, a discussion of the surgery, and possibly, performance of an in-clinic biopsy of suspicious lesions. Many practices, including the authors’, use a photo capturing add-on for the EMR in the office.5-7
During the consent process, MMS is described to the patient using lay language and, often, pictorial depictions of the procedure. While explaining that the procedure helps preserve healthy tissue and limit the size of the resulting scar, the surgeon will typically manage the expectations of the patient prior to the first incision. Many clinically small lesions can have significant subclinical extension adjacent to, or on top of, cosmetic landmarks, requiring a flap or graft to close the surgical defect with acceptable cosmetic outcomes.8
One more time. Immediately before surgery, the surgeon will again review the procedure with the patient, using photos of the biopsy site taken during the initial consult, in conjunction with patient verification of the biopsy site, to verify the surgical site and confirm that the patient understands and agrees to the surgery.
A look at how Mohs surgery is performed
MMS typically is performed in the outpatient setting but can also be performed in an operating room or outpatient surgical center. MMS can be performed in a nonsterile procedure room with surgeons and assistants typically utilizing clean, nonsterile gloves, although many Mohs surgeons prefer to perform part, or all, of the technique using sterile gloves.9 A recent systematic review and large meta-analysis showed no significant difference in postsurgical site infections when comparing the use of sterile vs nonsterile gloves.10
Continue to: Prior to initial incision...
Prior to initial incision, the site is marked with a surgical pen and given 1-mm margins around the clinically visualized lesion. The site is then cleansed with an antiseptic, typically a chlorhexidine solution. Local anesthesia is employed, most commonly with a 1:100,000 lidocaine and epinephrine injection. Marking of the tumor prior to numbing is imperative, as the boundaries of the tumor are typically obscured when the local cutaneous vasculature constricts and causes visualized blanching of adjacent skin. Many Mohs surgeons perform a brief curettage of the lesion with a nondisposable, dull curette to better define the tumor edges and to debulk any obvious exophytic tumor noted by the naked eye.
Prior to the first incision, the surgical site is scored in a variety of ways in order to properly orient the tissue after it has been removed from the patient. Mohs surgeons have differing opinions on how to score and/or mark the tissue, but a common practice is to make a nick at the 12 o’clock position. Following removal of the first stage, the nick will be visible on both the extirpated tissue and the tissue just above the surgical defect. This prevents potential confusion regarding orientation during tissue processing.
The majority of all WLEs are performed utilizing the scalpel blade at an angle 90° perpendicular to the plane of the skin. In MMS, a signature 45° angle with the tip of the scalpel pointing toward, and the handle pointing away from, the lesion is commonly used in order to bevel the tissue being excised (FIGURE 2). Once the tissue is excised, hemostasis is obtained using electrodessication/electrofulguration or electrocoagulation.

Tissue processing and microscopic evaluation
The technique of beveling allows the epidermis, dermis, and subcutaneous tissue to lie flat on the tissue block, so the Mohs surgeon can evaluate 100% of the excised tissue’s margins. The tissue is transported to a nearby lab for staining and processing. Even if near-perfect beveling is achieved, many stages will require bisecting, quadrissecting, or relaxing cuts in order to allow the margins to lie flat on the tissue block.
Using the scoring system made prior to incision, the tissue is oriented and stained with colored ink. Subsequently, a map is made with sections highlighting the colors used to stain designated areas of the tissue. This step is imperative for orientation during microscopic evaluation. Additionally, the map serves as a guide and log, should a section of the specimen have an involved margin and require another stage.
Continue to: Once fixed to the block...
Once fixed to the block, the tissue is engulfed in appropriate embedding medium and placed within the cryostat. The block is slowly cut to produce several micron-thin wafers of tissue that are then mounted on glass slides and processed with hematoxylin and eosin (H&E) or various stains. The first wafers of tissue that come from the tissue block are those that are closest to the margin that was excised. Thus, 100% of the epidermis and deep margin can be visualized. “Deeper sections” are those that come from deeper cuts within the tissue and are more likely to show the malignant neoplasm.
The evaluation of immediate margins at the very edge of the tissue is in contrast to the technique of “bread-loafing,” which is the standard of evaluating margins after a WLE.11 With this process, the pathologist examines sections that are cut 2- to 4-mm apart. This process only allows the pathologist to examine roughly 1% of the total tissue that was excised, and large variability in cutaneous representation can occur depending on the individual who cuts and processes the tissue.11
Closing the defect
Once the site is deemed clear of residual tumor, the Mohs surgeon approaches the defect and determines the most appropriate way to close the surgical wound. Mohs surgeons are trained to close wounds using a variety of methods, including complex linear closures, flaps, and full-thickness skin grafts. Thoughtful consideration of local anatomy, cosmetic landmarks that may be affected by the closure method, and local tissue laxity are evaluated.
Depending on the location, a secondary intention closure may prove to be just as effective and cosmetically satisfying as a primary intention closure. In light of the many methods of closure, a complex or large surface area defect may better be suited for evaluation and closure by another specialist such as an ENT physician, ophthalmologist, or plastic surgeon.12
Lower recurrence rates for patients who undergo Mohs surgery
As noted earlier, the cutaneous malignancies most commonly treated with MMS are BCCs, followed by SCCs.13 Comparison studies between WLE and MMS show clinically significant differences in terms of recurrence rates between the 2 procedures.
Continue to: For BCCs
For BCCs, recurrence rates for excisions vs MMS are 10% and 1%, respectively.14-16 A randomized trial reviewing 10-year recurrence of primary BCCs on the face showed recurrence rates for MMS of 4.4% compared to 12.2% for WLE.17 This study also showed recurrence rates for recurrent facial BCCs treated with MMS to be 3.9% vs 13.5% for standard WLE.17
SCC. The evidence similarly supports the efficacy of MMS for SCCs. A recent study showed primary T2a tumors had a 1.2% local recurrence rate with Mohs vs a 4% recurrence rate with WLE at an average follow-up of 2.8 years.18 Another study showed that primary tumors that were < 2 cm in diameter had a 5-year cure rate of 99% with Mohs surgery.11
Melanoma in situ. A few studies have shown no clinically significant benefit of MMS compared to WLE when it comes to melanoma in situ.19,20 However, a more recent article by Etzkom et al noted the ability to potentially upstage melanoma in situ and invasive melanoma after reviewing peripheral and deep margins during MMS.21 In this study, the authors uniquely delayed wound closure if upstaging was established and the need for a sentinel lymph node biopsy was warranted. This approach to MMS with delayed closure ultimately paved the way for very low recurrence rates.
CORRESPONDENCE
Andres Garcia, MD, 2612 112th Street, Lubbock, TX 79423; [email protected]
1. Dim-Jamora KC, Perone JB. Management of cutaneous tumors with Mohs micrographic surgery. Semin Plast Surg. 2008;22:247-256.
2. Ad Hoc Task Force, Connolly SM, Baker DR, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550. Published correction appears in J Am Acad Dermatol. 2015;72:748.
3. Cheraghlou S, Christensen S, Agogo G, et al. Comparison of survival after Mohs micrographic surgery vs wide margin excision for early-stage invasive melanoma. JAMA Dermatol. 2019;155:1252-1259.
4. Hill D, Kim K, Mansouri B, et al. Quantity and characteristics of flap or graft repairs for skin cancer on the nose or ears: a comparison between Mohs micrographic surgery and plastic surgery. Cutis. 2019;103:284-287.
5. McGinness JL, Goldstein G. The value of preoperative biopsy-site photography for identifying cutaneous lesions. Dermatol Surg. 2010;36:194-197.
6. Ke M, Moul D, Camouse M, et al. Where is it? The utility of biopsy-site photography. Dermatol Surg. 2010;36:198-202.
7. Nijhawan RI, Lee EH, Nehal KS. Biopsy site selfies—a quality improvement pilot study to assist with correct surgical site identification. Dermatol Surg. 2015;41:499-504
8. Breuninger H, Dietz K. Prediction of subclinical tumor infiltration in basal cell carcinoma. J Dermatol Surg Oncol. 1991;17:574-578.
9. Rhinehart BM, Murphy Me, Farley MF, et al. Sterile versus nonsterile gloves during Mohs micrographic surgery: infection rate is not affected. Dermatol Surg. 2006;32:170-176.
10. Brewer JD, Gonzalez AB, Baum CL, et al. Comparison of sterile vs nonsterile gloves in cutaneous surgery and common outpatient dental procedures: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:1008-1014.
11. Shriner DL, McCoy DK, Goldberg DJ, et al. Mohs micrographic surgery. J Am Acad Dermatol. 1998;39:79-97.
12. Gladstone HB, Stewart D. An algorithm for the reconstruction of complex facial defects. Skin Therapy Lett. 2007;12:6-9.
13. Robinson JK. Mohs micrographic surgery. Clin Plast Surg. 1993;20:149-156.
14. Swanson NA. Mohs surgery. Technique, indications, applications, and the future. Arch Dermatol. 1983;119:761-773.
15. Robins P. Chemosurgery: my 15 years of experience. J Dermatol Surg Oncol. 1981;7:779-789.
16. Rowe DE, Carroll RJ, Day CL Jr. Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow-up. J Dermatol Surg Oncol. 1989;15:315-328.
17. van Loo E, Mosterd K, Krekels GA, et al. Surgical excision versus Mohs’ micrographic surgery for basal cell carcinoma of the face: a randomised clinical trial with 10 year follow-up. Eur J Cancer. 2014;50:3011-3020.
18. Xiong DD, Beal BT, Varra V, et al. Outcomes in intermediate-risk squamous cell carcinomas treated with Mohs micrographic surgery compared with wide local excision. J Am Acad Dermatol. 2020;82: 1195-1204.
19. Trofymenko O, Bordeaux JS, Zeitouni NC. Melanoma of the face and Mohs micrographic surgery: nationwide mortality data analysis. Dermatol Surg. 2018;44:481-492.
20. Nosrati A, Berliner JG, Goel S, et al. Outcomes of melanoma in situ treated with Mohs micrographic surgery compared with wide local excision. JAMA Dermatol. 2017;153:436-441.
21. Etzkom JR, Sobanko JF, Elenitsas R, et al. Low recurrences for in situ and invasive melanomas using Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining: tissue processing methodology to optimize pathologic and margin assessment. J Am Acad Dermatol. 2015;72:840-850.
1. Dim-Jamora KC, Perone JB. Management of cutaneous tumors with Mohs micrographic surgery. Semin Plast Surg. 2008;22:247-256.
2. Ad Hoc Task Force, Connolly SM, Baker DR, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550. Published correction appears in J Am Acad Dermatol. 2015;72:748.
3. Cheraghlou S, Christensen S, Agogo G, et al. Comparison of survival after Mohs micrographic surgery vs wide margin excision for early-stage invasive melanoma. JAMA Dermatol. 2019;155:1252-1259.
4. Hill D, Kim K, Mansouri B, et al. Quantity and characteristics of flap or graft repairs for skin cancer on the nose or ears: a comparison between Mohs micrographic surgery and plastic surgery. Cutis. 2019;103:284-287.
5. McGinness JL, Goldstein G. The value of preoperative biopsy-site photography for identifying cutaneous lesions. Dermatol Surg. 2010;36:194-197.
6. Ke M, Moul D, Camouse M, et al. Where is it? The utility of biopsy-site photography. Dermatol Surg. 2010;36:198-202.
7. Nijhawan RI, Lee EH, Nehal KS. Biopsy site selfies—a quality improvement pilot study to assist with correct surgical site identification. Dermatol Surg. 2015;41:499-504
8. Breuninger H, Dietz K. Prediction of subclinical tumor infiltration in basal cell carcinoma. J Dermatol Surg Oncol. 1991;17:574-578.
9. Rhinehart BM, Murphy Me, Farley MF, et al. Sterile versus nonsterile gloves during Mohs micrographic surgery: infection rate is not affected. Dermatol Surg. 2006;32:170-176.
10. Brewer JD, Gonzalez AB, Baum CL, et al. Comparison of sterile vs nonsterile gloves in cutaneous surgery and common outpatient dental procedures: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:1008-1014.
11. Shriner DL, McCoy DK, Goldberg DJ, et al. Mohs micrographic surgery. J Am Acad Dermatol. 1998;39:79-97.
12. Gladstone HB, Stewart D. An algorithm for the reconstruction of complex facial defects. Skin Therapy Lett. 2007;12:6-9.
13. Robinson JK. Mohs micrographic surgery. Clin Plast Surg. 1993;20:149-156.
14. Swanson NA. Mohs surgery. Technique, indications, applications, and the future. Arch Dermatol. 1983;119:761-773.
15. Robins P. Chemosurgery: my 15 years of experience. J Dermatol Surg Oncol. 1981;7:779-789.
16. Rowe DE, Carroll RJ, Day CL Jr. Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow-up. J Dermatol Surg Oncol. 1989;15:315-328.
17. van Loo E, Mosterd K, Krekels GA, et al. Surgical excision versus Mohs’ micrographic surgery for basal cell carcinoma of the face: a randomised clinical trial with 10 year follow-up. Eur J Cancer. 2014;50:3011-3020.
18. Xiong DD, Beal BT, Varra V, et al. Outcomes in intermediate-risk squamous cell carcinomas treated with Mohs micrographic surgery compared with wide local excision. J Am Acad Dermatol. 2020;82: 1195-1204.
19. Trofymenko O, Bordeaux JS, Zeitouni NC. Melanoma of the face and Mohs micrographic surgery: nationwide mortality data analysis. Dermatol Surg. 2018;44:481-492.
20. Nosrati A, Berliner JG, Goel S, et al. Outcomes of melanoma in situ treated with Mohs micrographic surgery compared with wide local excision. JAMA Dermatol. 2017;153:436-441.
21. Etzkom JR, Sobanko JF, Elenitsas R, et al. Low recurrences for in situ and invasive melanomas using Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining: tissue processing methodology to optimize pathologic and margin assessment. J Am Acad Dermatol. 2015;72:840-850.
PRACTICE RECOMMENDATIONS
› Consider Mohs surgery for patients who have lesions located mainly in regions of the face that make excision difficult without significant scarring. A
› Consider Mohs surgery for basal cell carcinoma and squamous cell carcinoma that typically involve (but are not necessarily limited to) the face, as the procedure significantly reduces recurrence rates and leads to cure rates of up to 99%. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Quantity and Characteristics of Flap or Graft Repairs for Skin Cancer on the Nose or Ears: A Comparison Between Mohs Micrographic Surgery and Plastic Surgery
The incidence of nonmelanoma skin cancer (NMSC) is steadily increasing, and it accounts for more annual cancer diagnoses than all other malignancies combined.1,2 For NMSCs of the head and neck, Mohs micrographic surgery (MMS) has become a preferred technique because of its high cure rates, intraprocedural margin control, and improved tissue preservation in cosmetically sensitive areas.3 The nose and ears are especially sensitive anatomic locations given their prominent positions and relative lack of skin reservoir and laxity compared to other areas of the head and neck. For the nose and ears, both patients and referring providers may question who is best suited to surgically remove a malignancy and repair the defect with positive functional and cosmetic results, as a large portion of the defects following tumor extirpation will require a flap or graft for repair.
The notion of plastic surgery is strongly associated with supreme cosmesis for many patients and providers, as the specialty trains in several surgical and nonsurgical elective techniques to preserve and improve appearance. Consequently, patients commonly ask dermatologists if they should be referred to a plastic surgeon for skin cancer removal in cosmetically sensitive areas, especially areas that may require more complex surgical repairs. However, recent Medicare data indicate that dermatologists perform the vast majority of reconstructive skin surgeries, with more than 15 times the number of intermediate and complex closures and more than 4 times the number of flaps and grafts as the next closest specialty.4 Earlier studies using Medicare data revealed similar findings, with dermatologic surgeons performing more reconstructions of head and neck skin than both plastic surgeons and otorhinolaryngologists.5 However, these studies did not address the characteristics of the tumor, defects, or repairs performed by the specialties for comparison.
We sought to compare the quantity and characteristics of flaps or grafts performed for skin cancer on the nose or ears by fellowship-trained Mohs surgeons and plastic surgeons at 1 academic institution.
Methods
We performed a retrospective chart review of all skin cancer surgeries requiring a flap or graft on the nose or ears at Baylor Scott & White Health (Temple, Texas) from October 1, 2016, to October 1, 2017. This study was approved by the Baylor Scott & White Health institutional review board.
Data Collection
The analysis included full-time, fellowship-trained Mohs surgeons and all full-time plastic surgeons who accepted skin cancer surgery patient referrals as part of their practice and performed all procedures within our hospital system. We reviewed individual provider schedules for both outpatient consultation and operating room notes to capture each procedure performed. To ensure we captured all procedures for both Mohs and plastic surgeons, we used billing codes for any flap or graft repair done on the nose or ears to cross-reference and confirm the cases found by chart review. The total number of flaps or grafts on the nose or ears were collected. Data also were collected regarding the anatomic location of the skin cancer, final defect size prior to the repair, skin tumor type, repair type (flap or graft), and flap (transposition vs advancement) or graft (full thickness vs partial thickness) type. All surgical data were collected from operative notes. Demographic data, including age, race, and sex, also were collected. We also collected data on the specialty of the physicians who referred patients for surgical management of biopsy-proven skin malignancy.
Statistical Analysis
Sample characteristics were described using descriptive statistics. Frequencies and percentages were used to describe categorical variables. Medians and ranges were used to describe continuous variables due to nonsymmetrically distributed data. χ2 tests (or Fisher exact tests when low cell counts were present) for categorical variables and Wilcoxon signed rank tests for continuous variables were used to test for associations in bivariate comparisons between MMS and plastic surgery.
Results
A total of 7 physicians (1 fellowship-trained Mohs surgeon and 6 plastic surgeons) at our institution met the inclusion criteria. The Mohs surgeon performed a significantly higher number of flaps and grafts (n=276) than the plastic surgeons (n=17 combined; average per plastic surgeon, 2.83) on the nose or ears in a 12-month period (P<.05)(Table). The median final defect size was not significantly different between MMS (1.5 cm) and plastic surgery (1.8 cm)(P=.306). Flap repairs were more common in patients undergoing MMS (80%) vs plastic surgery (53%)(P=.022)(Figure). For flap repair, advancement flaps were used more commonly (MMS, 53%; plastic surgery, 35%) than transposition flaps (MMS, 27%; plastic surgery, 12%) by both specialties.
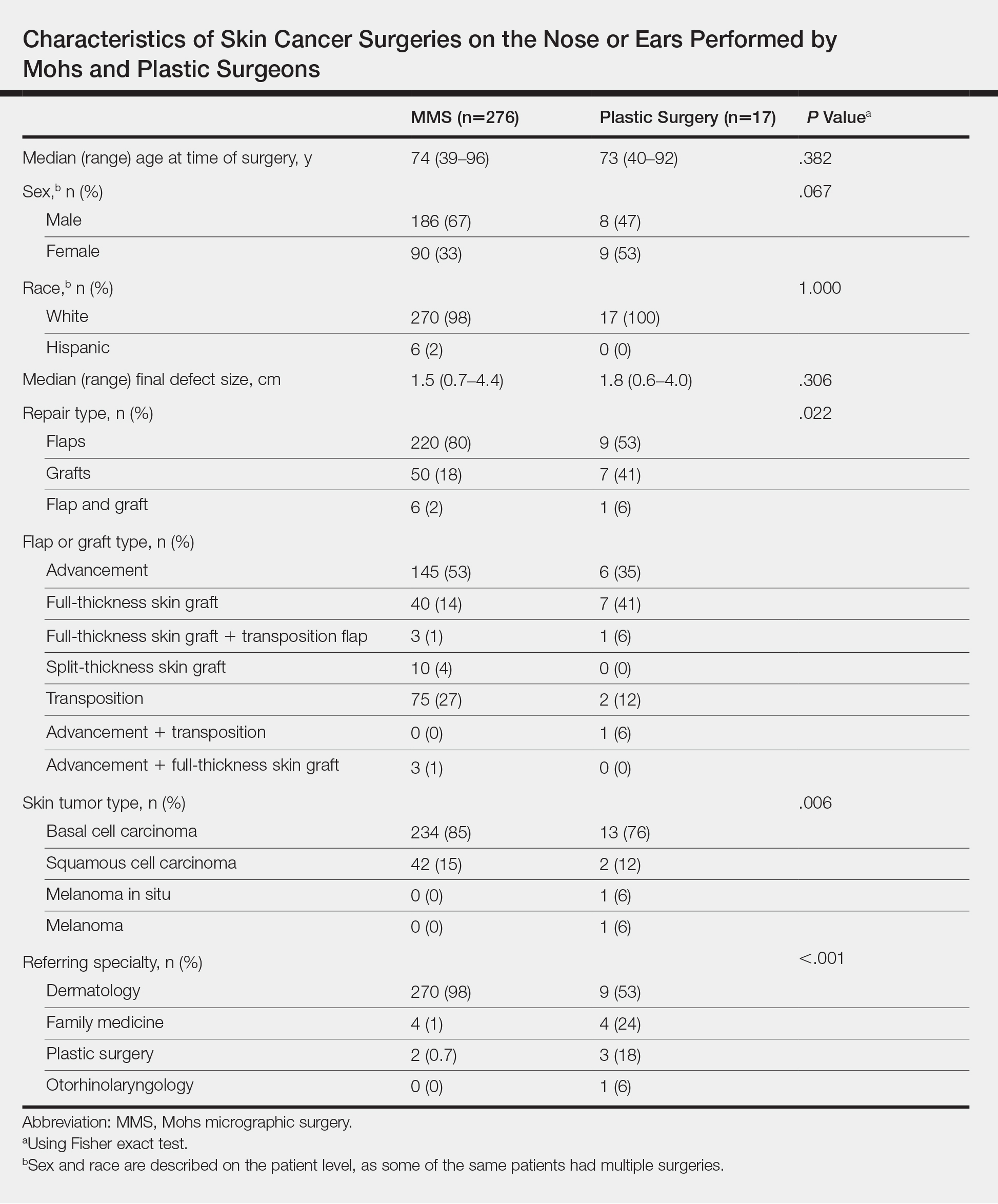
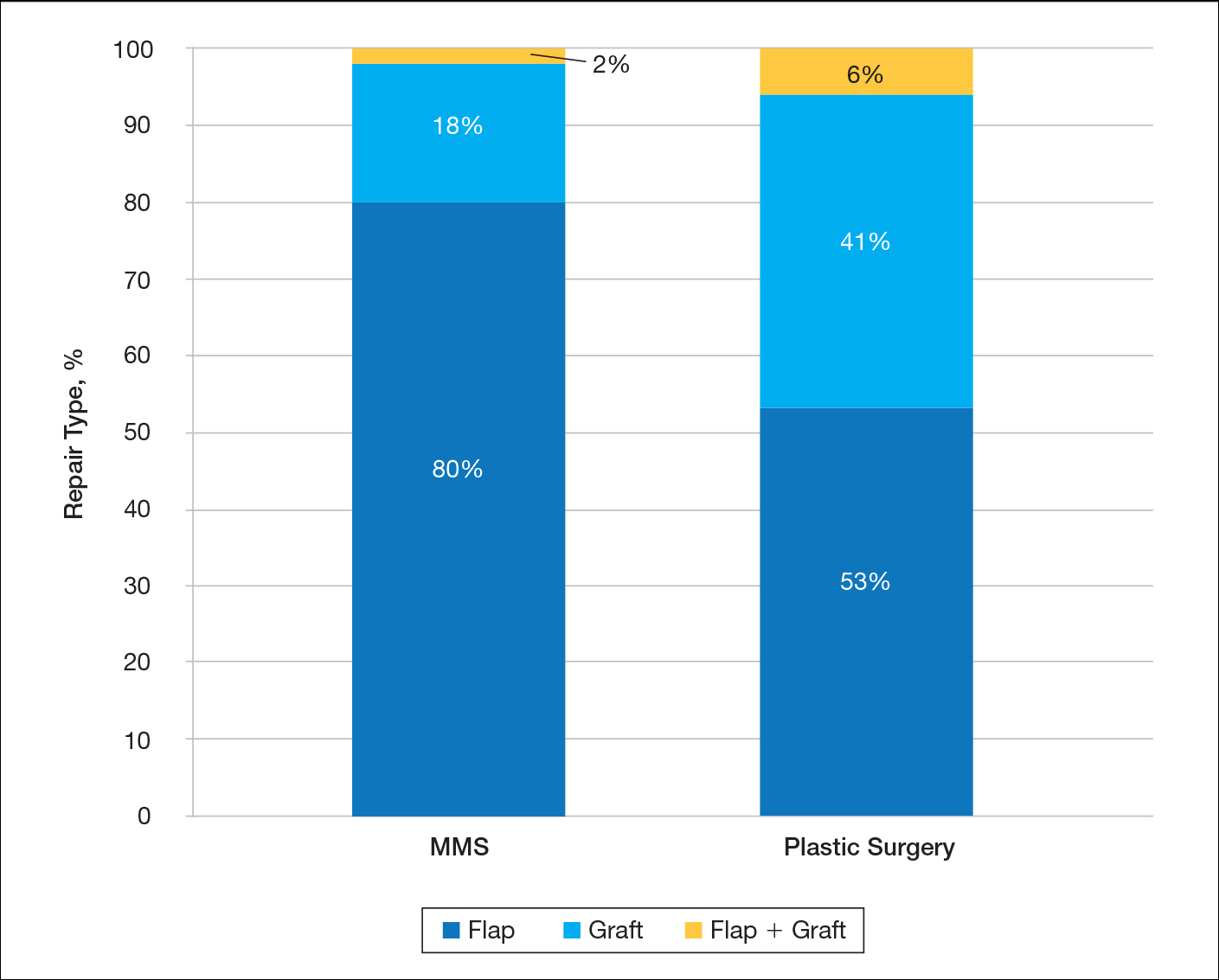
Patient age was similar between MMS (median, 74 years) and plastic surgery (median, 73 years) patients (P=.382), but a greater percentage of women were treated by plastic surgeons (53%) compared with Mohs surgeons (33%). The predominant skin tumor type for both specialties was basal cell carcinoma (MMS, 85%; plastic surgery, 76%). Dermatology was the largest referring specialty to both MMS (98%) and plastic surgery (53%). Family medicine referrals comprised a much larger percentage of cases for plastic surgery (24%) compared to MMS (1%).
Comment
This study supports and adds to recent studies and data regarding the utilization of MMS for the treatment of NMSCs. Although the percentage of all skin cancer surgery is increasing for dermatology, little has been reported on more complex repairs. This study highlights the volume and complexity of skin surgery performed by Mohs surgeons compared to our colleagues in plastic surgery.
Defect Size
The defect sizes prior to repair were not statistically different between the 2 types of surgeries, though the median size was slightly larger for plastic surgery (1.8 cm) compared to MMS (1.5 cm). These non–statistically significant differences may be explained by potentially larger tumors requiring repair by plastic surgeons in an operating room. Plastic surgeons, however, may be more likely to take a larger margin of clinically unaffected tissue as part of the initial layer. Plastic surgeons also may be less likely to curette the lesion prior to excision to obtain more clear tumor margins, possibly leading to more stages and a subsequently larger defect. Knowing the clinical sizes of these NMSCs prior to biopsy would have been beneficial to our study, but these data often were not available from the referring providers.
Repair Type
Most patients who underwent MMS had surgical defects repaired with a flap vs a graft, and a much higher percentage of patients who had undergone MMS vs surgical excision with plastic surgery had their defects repaired with flaps. Using a visual analog scale score and Hollander Wound Evaluation Scale, Jacobs et al6 found flaps to be cosmetically superior to grafts following tumor extirpation on the nose. The more frequent use of grafts by plastic surgeons could be at least partially explained by larger defect size or by a few outlier larger lesions among an otherwise small sample size. Larger studies may be needed to see if a true discrepancy in repair preferences exists between the specialties.
Referring Specialty
Primary care physician referral comprised a much larger percentage of cases sent for treatment with plastic surgery (24%) compared to MMS (1%). This statistic may represent a practice gap in the perception of MMS and its benefits among our primary care colleagues, particularly among female patients, as a much higher percentage of women were treated with plastic surgery. Important potential benefits of MMS, particularly tissue conservation, cure rates for skin cancer, and the volume of repairs performed by Mohs surgeons, may need to be emphasized.
Scope of Practice
Our colleagues in plastic surgery are extremely gifted and perform numerous repairs outside the scope of most Mohs surgeons. They are vital to multidisciplinary approaches to patients with skin cancer. Although Mohs surgeons focus on treating skin cancers that arise in a narrower range of anatomic locations, the breadth and variety of surgical procedures performed by plastic surgeons is more diverse. Skin cancer surgery may account for a smaller portion of procedures in a plastic surgery practice.
Limitations
There are several limitations to this study. We did not compare cosmesis or wound healing in patients treated by MMS or plastic surgery. The sample size, particularly with plastic surgery, was small and did not allow for a larger, more powerful comparison of data between the 2 specialties. Finally, our study only represents 1 institution over the course of 1 year.
Conclusion
To provide the best care possible, it is imperative for referring physicians to possess an accurate understanding of the volume of cases and the types of repairs that treating specialties perform on a regular basis for NMSCs. This knowledge is particularly important when there is a treatment overlap among specialties. Our data show Mohs surgeons are performing more complex repairs and reconstructions on even the most cosmetically sensitive areas; therefore, primary care physicians and other specialists may be more likely to involve dermatology in the care of skin cancer.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the united states, 2006. Arch Dermatol. 2010;146:283-287.
- Mansouri B, Bicknell LM, Hill D, et al. Mohs micrographic surgery for the management of cutaneous malignancies. Facial Plast Surg Clin North Am. 2017;25:291-301.
- Kantor J. Dermatologists perform more reconstructive surgery in the Medicare population than any other specialist group: a cross-sectional individual-level analysis of Medicare volume and specialist type in cutaneous and reconstructive surgery. J Am Acad Dermatol. 2018;78:171-173.e1.
- Donaldson MR, Coldiron BM. Dermatologists perform the majority of cutaneous reconstructions in the Medicare population: numbers and trends from 2004 to 2009. J Am Acad Dermatol. 2013;68:803-808.
- Jacobs MA, Christenson LJ, Weaver AL, et al. Clinical outcome of cutaneous flaps versus full-thickness skin grafts after Mohs surgery on the nose. Dermatol Surg. 2010;36:23-30.
The incidence of nonmelanoma skin cancer (NMSC) is steadily increasing, and it accounts for more annual cancer diagnoses than all other malignancies combined.1,2 For NMSCs of the head and neck, Mohs micrographic surgery (MMS) has become a preferred technique because of its high cure rates, intraprocedural margin control, and improved tissue preservation in cosmetically sensitive areas.3 The nose and ears are especially sensitive anatomic locations given their prominent positions and relative lack of skin reservoir and laxity compared to other areas of the head and neck. For the nose and ears, both patients and referring providers may question who is best suited to surgically remove a malignancy and repair the defect with positive functional and cosmetic results, as a large portion of the defects following tumor extirpation will require a flap or graft for repair.
The notion of plastic surgery is strongly associated with supreme cosmesis for many patients and providers, as the specialty trains in several surgical and nonsurgical elective techniques to preserve and improve appearance. Consequently, patients commonly ask dermatologists if they should be referred to a plastic surgeon for skin cancer removal in cosmetically sensitive areas, especially areas that may require more complex surgical repairs. However, recent Medicare data indicate that dermatologists perform the vast majority of reconstructive skin surgeries, with more than 15 times the number of intermediate and complex closures and more than 4 times the number of flaps and grafts as the next closest specialty.4 Earlier studies using Medicare data revealed similar findings, with dermatologic surgeons performing more reconstructions of head and neck skin than both plastic surgeons and otorhinolaryngologists.5 However, these studies did not address the characteristics of the tumor, defects, or repairs performed by the specialties for comparison.
We sought to compare the quantity and characteristics of flaps or grafts performed for skin cancer on the nose or ears by fellowship-trained Mohs surgeons and plastic surgeons at 1 academic institution.
Methods
We performed a retrospective chart review of all skin cancer surgeries requiring a flap or graft on the nose or ears at Baylor Scott & White Health (Temple, Texas) from October 1, 2016, to October 1, 2017. This study was approved by the Baylor Scott & White Health institutional review board.
Data Collection
The analysis included full-time, fellowship-trained Mohs surgeons and all full-time plastic surgeons who accepted skin cancer surgery patient referrals as part of their practice and performed all procedures within our hospital system. We reviewed individual provider schedules for both outpatient consultation and operating room notes to capture each procedure performed. To ensure we captured all procedures for both Mohs and plastic surgeons, we used billing codes for any flap or graft repair done on the nose or ears to cross-reference and confirm the cases found by chart review. The total number of flaps or grafts on the nose or ears were collected. Data also were collected regarding the anatomic location of the skin cancer, final defect size prior to the repair, skin tumor type, repair type (flap or graft), and flap (transposition vs advancement) or graft (full thickness vs partial thickness) type. All surgical data were collected from operative notes. Demographic data, including age, race, and sex, also were collected. We also collected data on the specialty of the physicians who referred patients for surgical management of biopsy-proven skin malignancy.
Statistical Analysis
Sample characteristics were described using descriptive statistics. Frequencies and percentages were used to describe categorical variables. Medians and ranges were used to describe continuous variables due to nonsymmetrically distributed data. χ2 tests (or Fisher exact tests when low cell counts were present) for categorical variables and Wilcoxon signed rank tests for continuous variables were used to test for associations in bivariate comparisons between MMS and plastic surgery.
Results
A total of 7 physicians (1 fellowship-trained Mohs surgeon and 6 plastic surgeons) at our institution met the inclusion criteria. The Mohs surgeon performed a significantly higher number of flaps and grafts (n=276) than the plastic surgeons (n=17 combined; average per plastic surgeon, 2.83) on the nose or ears in a 12-month period (P<.05)(Table). The median final defect size was not significantly different between MMS (1.5 cm) and plastic surgery (1.8 cm)(P=.306). Flap repairs were more common in patients undergoing MMS (80%) vs plastic surgery (53%)(P=.022)(Figure). For flap repair, advancement flaps were used more commonly (MMS, 53%; plastic surgery, 35%) than transposition flaps (MMS, 27%; plastic surgery, 12%) by both specialties.


Patient age was similar between MMS (median, 74 years) and plastic surgery (median, 73 years) patients (P=.382), but a greater percentage of women were treated by plastic surgeons (53%) compared with Mohs surgeons (33%). The predominant skin tumor type for both specialties was basal cell carcinoma (MMS, 85%; plastic surgery, 76%). Dermatology was the largest referring specialty to both MMS (98%) and plastic surgery (53%). Family medicine referrals comprised a much larger percentage of cases for plastic surgery (24%) compared to MMS (1%).
Comment
This study supports and adds to recent studies and data regarding the utilization of MMS for the treatment of NMSCs. Although the percentage of all skin cancer surgery is increasing for dermatology, little has been reported on more complex repairs. This study highlights the volume and complexity of skin surgery performed by Mohs surgeons compared to our colleagues in plastic surgery.
Defect Size
The defect sizes prior to repair were not statistically different between the 2 types of surgeries, though the median size was slightly larger for plastic surgery (1.8 cm) compared to MMS (1.5 cm). These non–statistically significant differences may be explained by potentially larger tumors requiring repair by plastic surgeons in an operating room. Plastic surgeons, however, may be more likely to take a larger margin of clinically unaffected tissue as part of the initial layer. Plastic surgeons also may be less likely to curette the lesion prior to excision to obtain more clear tumor margins, possibly leading to more stages and a subsequently larger defect. Knowing the clinical sizes of these NMSCs prior to biopsy would have been beneficial to our study, but these data often were not available from the referring providers.
Repair Type
Most patients who underwent MMS had surgical defects repaired with a flap vs a graft, and a much higher percentage of patients who had undergone MMS vs surgical excision with plastic surgery had their defects repaired with flaps. Using a visual analog scale score and Hollander Wound Evaluation Scale, Jacobs et al6 found flaps to be cosmetically superior to grafts following tumor extirpation on the nose. The more frequent use of grafts by plastic surgeons could be at least partially explained by larger defect size or by a few outlier larger lesions among an otherwise small sample size. Larger studies may be needed to see if a true discrepancy in repair preferences exists between the specialties.
Referring Specialty
Primary care physician referral comprised a much larger percentage of cases sent for treatment with plastic surgery (24%) compared to MMS (1%). This statistic may represent a practice gap in the perception of MMS and its benefits among our primary care colleagues, particularly among female patients, as a much higher percentage of women were treated with plastic surgery. Important potential benefits of MMS, particularly tissue conservation, cure rates for skin cancer, and the volume of repairs performed by Mohs surgeons, may need to be emphasized.
Scope of Practice
Our colleagues in plastic surgery are extremely gifted and perform numerous repairs outside the scope of most Mohs surgeons. They are vital to multidisciplinary approaches to patients with skin cancer. Although Mohs surgeons focus on treating skin cancers that arise in a narrower range of anatomic locations, the breadth and variety of surgical procedures performed by plastic surgeons is more diverse. Skin cancer surgery may account for a smaller portion of procedures in a plastic surgery practice.
Limitations
There are several limitations to this study. We did not compare cosmesis or wound healing in patients treated by MMS or plastic surgery. The sample size, particularly with plastic surgery, was small and did not allow for a larger, more powerful comparison of data between the 2 specialties. Finally, our study only represents 1 institution over the course of 1 year.
Conclusion
To provide the best care possible, it is imperative for referring physicians to possess an accurate understanding of the volume of cases and the types of repairs that treating specialties perform on a regular basis for NMSCs. This knowledge is particularly important when there is a treatment overlap among specialties. Our data show Mohs surgeons are performing more complex repairs and reconstructions on even the most cosmetically sensitive areas; therefore, primary care physicians and other specialists may be more likely to involve dermatology in the care of skin cancer.
The incidence of nonmelanoma skin cancer (NMSC) is steadily increasing, and it accounts for more annual cancer diagnoses than all other malignancies combined.1,2 For NMSCs of the head and neck, Mohs micrographic surgery (MMS) has become a preferred technique because of its high cure rates, intraprocedural margin control, and improved tissue preservation in cosmetically sensitive areas.3 The nose and ears are especially sensitive anatomic locations given their prominent positions and relative lack of skin reservoir and laxity compared to other areas of the head and neck. For the nose and ears, both patients and referring providers may question who is best suited to surgically remove a malignancy and repair the defect with positive functional and cosmetic results, as a large portion of the defects following tumor extirpation will require a flap or graft for repair.
The notion of plastic surgery is strongly associated with supreme cosmesis for many patients and providers, as the specialty trains in several surgical and nonsurgical elective techniques to preserve and improve appearance. Consequently, patients commonly ask dermatologists if they should be referred to a plastic surgeon for skin cancer removal in cosmetically sensitive areas, especially areas that may require more complex surgical repairs. However, recent Medicare data indicate that dermatologists perform the vast majority of reconstructive skin surgeries, with more than 15 times the number of intermediate and complex closures and more than 4 times the number of flaps and grafts as the next closest specialty.4 Earlier studies using Medicare data revealed similar findings, with dermatologic surgeons performing more reconstructions of head and neck skin than both plastic surgeons and otorhinolaryngologists.5 However, these studies did not address the characteristics of the tumor, defects, or repairs performed by the specialties for comparison.
We sought to compare the quantity and characteristics of flaps or grafts performed for skin cancer on the nose or ears by fellowship-trained Mohs surgeons and plastic surgeons at 1 academic institution.
Methods
We performed a retrospective chart review of all skin cancer surgeries requiring a flap or graft on the nose or ears at Baylor Scott & White Health (Temple, Texas) from October 1, 2016, to October 1, 2017. This study was approved by the Baylor Scott & White Health institutional review board.
Data Collection
The analysis included full-time, fellowship-trained Mohs surgeons and all full-time plastic surgeons who accepted skin cancer surgery patient referrals as part of their practice and performed all procedures within our hospital system. We reviewed individual provider schedules for both outpatient consultation and operating room notes to capture each procedure performed. To ensure we captured all procedures for both Mohs and plastic surgeons, we used billing codes for any flap or graft repair done on the nose or ears to cross-reference and confirm the cases found by chart review. The total number of flaps or grafts on the nose or ears were collected. Data also were collected regarding the anatomic location of the skin cancer, final defect size prior to the repair, skin tumor type, repair type (flap or graft), and flap (transposition vs advancement) or graft (full thickness vs partial thickness) type. All surgical data were collected from operative notes. Demographic data, including age, race, and sex, also were collected. We also collected data on the specialty of the physicians who referred patients for surgical management of biopsy-proven skin malignancy.
Statistical Analysis
Sample characteristics were described using descriptive statistics. Frequencies and percentages were used to describe categorical variables. Medians and ranges were used to describe continuous variables due to nonsymmetrically distributed data. χ2 tests (or Fisher exact tests when low cell counts were present) for categorical variables and Wilcoxon signed rank tests for continuous variables were used to test for associations in bivariate comparisons between MMS and plastic surgery.
Results
A total of 7 physicians (1 fellowship-trained Mohs surgeon and 6 plastic surgeons) at our institution met the inclusion criteria. The Mohs surgeon performed a significantly higher number of flaps and grafts (n=276) than the plastic surgeons (n=17 combined; average per plastic surgeon, 2.83) on the nose or ears in a 12-month period (P<.05)(Table). The median final defect size was not significantly different between MMS (1.5 cm) and plastic surgery (1.8 cm)(P=.306). Flap repairs were more common in patients undergoing MMS (80%) vs plastic surgery (53%)(P=.022)(Figure). For flap repair, advancement flaps were used more commonly (MMS, 53%; plastic surgery, 35%) than transposition flaps (MMS, 27%; plastic surgery, 12%) by both specialties.


Patient age was similar between MMS (median, 74 years) and plastic surgery (median, 73 years) patients (P=.382), but a greater percentage of women were treated by plastic surgeons (53%) compared with Mohs surgeons (33%). The predominant skin tumor type for both specialties was basal cell carcinoma (MMS, 85%; plastic surgery, 76%). Dermatology was the largest referring specialty to both MMS (98%) and plastic surgery (53%). Family medicine referrals comprised a much larger percentage of cases for plastic surgery (24%) compared to MMS (1%).
Comment
This study supports and adds to recent studies and data regarding the utilization of MMS for the treatment of NMSCs. Although the percentage of all skin cancer surgery is increasing for dermatology, little has been reported on more complex repairs. This study highlights the volume and complexity of skin surgery performed by Mohs surgeons compared to our colleagues in plastic surgery.
Defect Size
The defect sizes prior to repair were not statistically different between the 2 types of surgeries, though the median size was slightly larger for plastic surgery (1.8 cm) compared to MMS (1.5 cm). These non–statistically significant differences may be explained by potentially larger tumors requiring repair by plastic surgeons in an operating room. Plastic surgeons, however, may be more likely to take a larger margin of clinically unaffected tissue as part of the initial layer. Plastic surgeons also may be less likely to curette the lesion prior to excision to obtain more clear tumor margins, possibly leading to more stages and a subsequently larger defect. Knowing the clinical sizes of these NMSCs prior to biopsy would have been beneficial to our study, but these data often were not available from the referring providers.
Repair Type
Most patients who underwent MMS had surgical defects repaired with a flap vs a graft, and a much higher percentage of patients who had undergone MMS vs surgical excision with plastic surgery had their defects repaired with flaps. Using a visual analog scale score and Hollander Wound Evaluation Scale, Jacobs et al6 found flaps to be cosmetically superior to grafts following tumor extirpation on the nose. The more frequent use of grafts by plastic surgeons could be at least partially explained by larger defect size or by a few outlier larger lesions among an otherwise small sample size. Larger studies may be needed to see if a true discrepancy in repair preferences exists between the specialties.
Referring Specialty
Primary care physician referral comprised a much larger percentage of cases sent for treatment with plastic surgery (24%) compared to MMS (1%). This statistic may represent a practice gap in the perception of MMS and its benefits among our primary care colleagues, particularly among female patients, as a much higher percentage of women were treated with plastic surgery. Important potential benefits of MMS, particularly tissue conservation, cure rates for skin cancer, and the volume of repairs performed by Mohs surgeons, may need to be emphasized.
Scope of Practice
Our colleagues in plastic surgery are extremely gifted and perform numerous repairs outside the scope of most Mohs surgeons. They are vital to multidisciplinary approaches to patients with skin cancer. Although Mohs surgeons focus on treating skin cancers that arise in a narrower range of anatomic locations, the breadth and variety of surgical procedures performed by plastic surgeons is more diverse. Skin cancer surgery may account for a smaller portion of procedures in a plastic surgery practice.
Limitations
There are several limitations to this study. We did not compare cosmesis or wound healing in patients treated by MMS or plastic surgery. The sample size, particularly with plastic surgery, was small and did not allow for a larger, more powerful comparison of data between the 2 specialties. Finally, our study only represents 1 institution over the course of 1 year.
Conclusion
To provide the best care possible, it is imperative for referring physicians to possess an accurate understanding of the volume of cases and the types of repairs that treating specialties perform on a regular basis for NMSCs. This knowledge is particularly important when there is a treatment overlap among specialties. Our data show Mohs surgeons are performing more complex repairs and reconstructions on even the most cosmetically sensitive areas; therefore, primary care physicians and other specialists may be more likely to involve dermatology in the care of skin cancer.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the united states, 2006. Arch Dermatol. 2010;146:283-287.
- Mansouri B, Bicknell LM, Hill D, et al. Mohs micrographic surgery for the management of cutaneous malignancies. Facial Plast Surg Clin North Am. 2017;25:291-301.
- Kantor J. Dermatologists perform more reconstructive surgery in the Medicare population than any other specialist group: a cross-sectional individual-level analysis of Medicare volume and specialist type in cutaneous and reconstructive surgery. J Am Acad Dermatol. 2018;78:171-173.e1.
- Donaldson MR, Coldiron BM. Dermatologists perform the majority of cutaneous reconstructions in the Medicare population: numbers and trends from 2004 to 2009. J Am Acad Dermatol. 2013;68:803-808.
- Jacobs MA, Christenson LJ, Weaver AL, et al. Clinical outcome of cutaneous flaps versus full-thickness skin grafts after Mohs surgery on the nose. Dermatol Surg. 2010;36:23-30.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the united states, 2006. Arch Dermatol. 2010;146:283-287.
- Mansouri B, Bicknell LM, Hill D, et al. Mohs micrographic surgery for the management of cutaneous malignancies. Facial Plast Surg Clin North Am. 2017;25:291-301.
- Kantor J. Dermatologists perform more reconstructive surgery in the Medicare population than any other specialist group: a cross-sectional individual-level analysis of Medicare volume and specialist type in cutaneous and reconstructive surgery. J Am Acad Dermatol. 2018;78:171-173.e1.
- Donaldson MR, Coldiron BM. Dermatologists perform the majority of cutaneous reconstructions in the Medicare population: numbers and trends from 2004 to 2009. J Am Acad Dermatol. 2013;68:803-808.
- Jacobs MA, Christenson LJ, Weaver AL, et al. Clinical outcome of cutaneous flaps versus full-thickness skin grafts after Mohs surgery on the nose. Dermatol Surg. 2010;36:23-30.
Practice Points
- Patients and nondermatologist physicians may be unaware of how frequently Mohs surgeons perform complex surgical repairs compared to other specialists.
- Compared to plastic surgeons, Mohs surgeons performed a larger number of complex skin cancer repairs on the nose or ears with similar-sized defects.
- Primary care physicians and other specialists may be more likely to involve dermatology in the care of skin cancer through awareness of this type of data.
Cost of Diagnosing Psoriasis and Rosacea for Dermatologists Versus Primary Care Physicians
Growing incentives to control health care costs may cause accountable care organizations (ACOs) to reconsider how diseases are best managed. Few studies have examined the cost difference between primary care providers (PCPs) and specialists in managing the same disease. Limited data have suggested that management of some diseases by a PCP may be less costly compared to a specialist1,2; however, it is not clear if this finding extends to skin disease. This study sought to assess the cost of seeing a dermatologist versus a PCP for diagnosis of the common skin diseases psoriasis and rosacea.
Methods
Patient data were obtained from the Humana database, a large commercial data set for claims and reimbursed costs encompassing 18,162,539 patients covered between January 2007 and December 2014. Our study population consisted of 3,944,465 patients with claims that included International Classification of Diseases, Ninth Revision (ICD-9), codes for dermatological diagnoses (680.0–709.9). We searched by ICD-9 code for US patients with primary diagnoses of psoriasis (696.1) and rosacea (695.3). We narrowed the search to include patients aged 30 to 64 years, as the diagnoses for these diseases are most common in patients older than 30 years. Patients who were older than 64 years were not included in the study, as most are covered by Medicare and therefore costs covered by Humana in this age group would not be as representative as in younger age groups. Total and average diagnosis-related costs per patient were compared between dermatologists and PCPs. Diagnosis-related costs encompassed physician reimbursement; laboratory and imaging costs, including skin biopsies; inpatient hospitalization cost; and any other charge that could be coded or billed by providers and reimbursed by the insurance company. To be eligible for reimbursement from Humana, dermatologists and PCPs must be registered with the insurer according to specialty board certification and practice credentialing, and they are reimbursed differently based on specialty. Drug costs, which would possibly skew the data toward providers using more expensive systemic medications (ie, dermatologists), were not included in this study, as the discussion is better reserved for long-term management of disease rather than diagnosis-related costs. All diagnoses of psoriasis were included in the study, which likely includes all severities of psoriasis, though we did not have the ability to further break down these diagnoses by severity.
Results
We identified 30,217 psoriasis patients and 37,561 rosacea patients. Of those patients with a primary diagnosis of psoriasis, 26,112 (86%) were seen by a dermatologist and 4105 (14%) were seen by a PCP (Table). Of those patients with a primary diagnosis of rosacea, 34,694 (92%) were seen by a dermatologist and 2867 (8%) were seen by a PCP (Table). There was little difference in the average diagnosis-related cost per patient for psoriasis in males (dermatologists, $638; PCPs, $657) versus females (dermatologists, $592; PCPs, $586) or between specialties (Figure). Findings were similar for rosacea in males (dermatologists, $179; PCPs, $168) versus females (dermatologists, $157; PCPs, $161). For these skin diseases, i

Comment
For the management of common skin disorders such as psoriasis and rosacea, there is little cost difference in seeing a dermatologist versus a PCP. Through extensive training and repeated exposure to many skin diseases, dermatologists are expected to be more comfortable in diagnosing and managing psoriasis and rosacea. Compared to PCPs, dermatologists have demonstrated increased diagnostic accuracy and efficiency when examining pigmented lesions and other dermatologic diseases in several studies.3-6 Although the current study shows that diagnosis-related costs for psoriasis and rosacea are essentially equal between dermatologists and PCPs, it actually may be less expensive for patients to see a dermatologist, as unnecessary tests, biopsies, or medications are more likely to be ordered/prescribed when there is less clinical diagnostic certainty.7,8 Additionally, seeing a PCP for diagnosis of a skin disease may be inefficient if subsequent referral to a dermatologist is needed, a common scenario that occurs when patients see a PCP for skin conditions.9
Our study had limitations, which is typical of a study using a claims database. We used ICD-9 codes recorded in patients’ medical claims to determine diagnosis of psoriasis and rosacea; therefore, our study and data are subject to coding errors. We could not assess the severity of disease, only the presence of disease. Further confirmation of diagnosis could have been made through searching for a second ICD-9 code in the patient’s history. Our data also are from a limited time period and may not represent costs from other time periods.
Conclusion
Given the lack of cost difference between both specialties, we conclude that ACOs should consider encouraging patients to seek care for dermatologic diseases by dermatologists who generally are more accurate and efficient skin diagnosticians, particularly if there is a shortage of PCPs within the ACO network.
- Wimo A, Religa D, Spångberg K, et al. Costs of diagnosing dementia: results from SveDem, the Swedish Dementia Registry. Int J Geriatr Psychiatry. 2013;28:1039-1044.
- Grunfeld E, Fitzpatrick R, Mant D, et al. Comparison of breast cancer patient satisfaction with follow-up in primary care versus specialist care: results from a randomized controlled trial. Br J Gen Pract. 1999;49:705-710.
- Chen SC, Pennie ML, Kolm P, et al. Diagnosing and managing cutaneous pigmented lesions: primary care physicians versus dermatologists. J Gen Intern Med. 2006;21:678-682.
- Federman D, Hogan D, Taylor JR, et al. A comparison of diagnosis, evaluation, and treatment of patients with dermatologic disorders. J Am Acad Dermatol. 1995;32:726-729.
- Feldman SR, Fleischer AB, Young AC, et al. Time-efficiency of nondermatologists compared with dermatologists in the care of skin disease. J Am Acad Dermatol. 1999;40:194-199.
- Feldman SR, Peterson SR, Fleischer AB Jr. Dermatologists meet the primary care standard for first contact management of skin disease. J Am Acad Dermatol. 1998;39(2, pt 1):182-186.
- Smith ES, Fleischer AB, Feldman SR. Nondermatologists are more likely than dermatologists to prescribe antifungal/corticosteroid products: an analysis of office visits for cutaneous fungal infections, 1990-1994. J Am Acad Dermatol. 1998;39:43-47.
- Shaffer MP, Feldman SR, Fleischer AB. Use of clotrimazole/betamethasone diproprionate by family physicians. Fam Med. 2000;32:561-565.
- Feldman SR, Fleischer AB, Chen JG. The gatekeeper model is inefficient for the delivery of dermatologic services. J Am Acad Dermatol. 1999;40:426-432.
Growing incentives to control health care costs may cause accountable care organizations (ACOs) to reconsider how diseases are best managed. Few studies have examined the cost difference between primary care providers (PCPs) and specialists in managing the same disease. Limited data have suggested that management of some diseases by a PCP may be less costly compared to a specialist1,2; however, it is not clear if this finding extends to skin disease. This study sought to assess the cost of seeing a dermatologist versus a PCP for diagnosis of the common skin diseases psoriasis and rosacea.
Methods
Patient data were obtained from the Humana database, a large commercial data set for claims and reimbursed costs encompassing 18,162,539 patients covered between January 2007 and December 2014. Our study population consisted of 3,944,465 patients with claims that included International Classification of Diseases, Ninth Revision (ICD-9), codes for dermatological diagnoses (680.0–709.9). We searched by ICD-9 code for US patients with primary diagnoses of psoriasis (696.1) and rosacea (695.3). We narrowed the search to include patients aged 30 to 64 years, as the diagnoses for these diseases are most common in patients older than 30 years. Patients who were older than 64 years were not included in the study, as most are covered by Medicare and therefore costs covered by Humana in this age group would not be as representative as in younger age groups. Total and average diagnosis-related costs per patient were compared between dermatologists and PCPs. Diagnosis-related costs encompassed physician reimbursement; laboratory and imaging costs, including skin biopsies; inpatient hospitalization cost; and any other charge that could be coded or billed by providers and reimbursed by the insurance company. To be eligible for reimbursement from Humana, dermatologists and PCPs must be registered with the insurer according to specialty board certification and practice credentialing, and they are reimbursed differently based on specialty. Drug costs, which would possibly skew the data toward providers using more expensive systemic medications (ie, dermatologists), were not included in this study, as the discussion is better reserved for long-term management of disease rather than diagnosis-related costs. All diagnoses of psoriasis were included in the study, which likely includes all severities of psoriasis, though we did not have the ability to further break down these diagnoses by severity.
Results
We identified 30,217 psoriasis patients and 37,561 rosacea patients. Of those patients with a primary diagnosis of psoriasis, 26,112 (86%) were seen by a dermatologist and 4105 (14%) were seen by a PCP (Table). Of those patients with a primary diagnosis of rosacea, 34,694 (92%) were seen by a dermatologist and 2867 (8%) were seen by a PCP (Table). There was little difference in the average diagnosis-related cost per patient for psoriasis in males (dermatologists, $638; PCPs, $657) versus females (dermatologists, $592; PCPs, $586) or between specialties (Figure). Findings were similar for rosacea in males (dermatologists, $179; PCPs, $168) versus females (dermatologists, $157; PCPs, $161). For these skin diseases, i


Comment
For the management of common skin disorders such as psoriasis and rosacea, there is little cost difference in seeing a dermatologist versus a PCP. Through extensive training and repeated exposure to many skin diseases, dermatologists are expected to be more comfortable in diagnosing and managing psoriasis and rosacea. Compared to PCPs, dermatologists have demonstrated increased diagnostic accuracy and efficiency when examining pigmented lesions and other dermatologic diseases in several studies.3-6 Although the current study shows that diagnosis-related costs for psoriasis and rosacea are essentially equal between dermatologists and PCPs, it actually may be less expensive for patients to see a dermatologist, as unnecessary tests, biopsies, or medications are more likely to be ordered/prescribed when there is less clinical diagnostic certainty.7,8 Additionally, seeing a PCP for diagnosis of a skin disease may be inefficient if subsequent referral to a dermatologist is needed, a common scenario that occurs when patients see a PCP for skin conditions.9
Our study had limitations, which is typical of a study using a claims database. We used ICD-9 codes recorded in patients’ medical claims to determine diagnosis of psoriasis and rosacea; therefore, our study and data are subject to coding errors. We could not assess the severity of disease, only the presence of disease. Further confirmation of diagnosis could have been made through searching for a second ICD-9 code in the patient’s history. Our data also are from a limited time period and may not represent costs from other time periods.
Conclusion
Given the lack of cost difference between both specialties, we conclude that ACOs should consider encouraging patients to seek care for dermatologic diseases by dermatologists who generally are more accurate and efficient skin diagnosticians, particularly if there is a shortage of PCPs within the ACO network.
Growing incentives to control health care costs may cause accountable care organizations (ACOs) to reconsider how diseases are best managed. Few studies have examined the cost difference between primary care providers (PCPs) and specialists in managing the same disease. Limited data have suggested that management of some diseases by a PCP may be less costly compared to a specialist1,2; however, it is not clear if this finding extends to skin disease. This study sought to assess the cost of seeing a dermatologist versus a PCP for diagnosis of the common skin diseases psoriasis and rosacea.
Methods
Patient data were obtained from the Humana database, a large commercial data set for claims and reimbursed costs encompassing 18,162,539 patients covered between January 2007 and December 2014. Our study population consisted of 3,944,465 patients with claims that included International Classification of Diseases, Ninth Revision (ICD-9), codes for dermatological diagnoses (680.0–709.9). We searched by ICD-9 code for US patients with primary diagnoses of psoriasis (696.1) and rosacea (695.3). We narrowed the search to include patients aged 30 to 64 years, as the diagnoses for these diseases are most common in patients older than 30 years. Patients who were older than 64 years were not included in the study, as most are covered by Medicare and therefore costs covered by Humana in this age group would not be as representative as in younger age groups. Total and average diagnosis-related costs per patient were compared between dermatologists and PCPs. Diagnosis-related costs encompassed physician reimbursement; laboratory and imaging costs, including skin biopsies; inpatient hospitalization cost; and any other charge that could be coded or billed by providers and reimbursed by the insurance company. To be eligible for reimbursement from Humana, dermatologists and PCPs must be registered with the insurer according to specialty board certification and practice credentialing, and they are reimbursed differently based on specialty. Drug costs, which would possibly skew the data toward providers using more expensive systemic medications (ie, dermatologists), were not included in this study, as the discussion is better reserved for long-term management of disease rather than diagnosis-related costs. All diagnoses of psoriasis were included in the study, which likely includes all severities of psoriasis, though we did not have the ability to further break down these diagnoses by severity.
Results
We identified 30,217 psoriasis patients and 37,561 rosacea patients. Of those patients with a primary diagnosis of psoriasis, 26,112 (86%) were seen by a dermatologist and 4105 (14%) were seen by a PCP (Table). Of those patients with a primary diagnosis of rosacea, 34,694 (92%) were seen by a dermatologist and 2867 (8%) were seen by a PCP (Table). There was little difference in the average diagnosis-related cost per patient for psoriasis in males (dermatologists, $638; PCPs, $657) versus females (dermatologists, $592; PCPs, $586) or between specialties (Figure). Findings were similar for rosacea in males (dermatologists, $179; PCPs, $168) versus females (dermatologists, $157; PCPs, $161). For these skin diseases, i


Comment
For the management of common skin disorders such as psoriasis and rosacea, there is little cost difference in seeing a dermatologist versus a PCP. Through extensive training and repeated exposure to many skin diseases, dermatologists are expected to be more comfortable in diagnosing and managing psoriasis and rosacea. Compared to PCPs, dermatologists have demonstrated increased diagnostic accuracy and efficiency when examining pigmented lesions and other dermatologic diseases in several studies.3-6 Although the current study shows that diagnosis-related costs for psoriasis and rosacea are essentially equal between dermatologists and PCPs, it actually may be less expensive for patients to see a dermatologist, as unnecessary tests, biopsies, or medications are more likely to be ordered/prescribed when there is less clinical diagnostic certainty.7,8 Additionally, seeing a PCP for diagnosis of a skin disease may be inefficient if subsequent referral to a dermatologist is needed, a common scenario that occurs when patients see a PCP for skin conditions.9
Our study had limitations, which is typical of a study using a claims database. We used ICD-9 codes recorded in patients’ medical claims to determine diagnosis of psoriasis and rosacea; therefore, our study and data are subject to coding errors. We could not assess the severity of disease, only the presence of disease. Further confirmation of diagnosis could have been made through searching for a second ICD-9 code in the patient’s history. Our data also are from a limited time period and may not represent costs from other time periods.
Conclusion
Given the lack of cost difference between both specialties, we conclude that ACOs should consider encouraging patients to seek care for dermatologic diseases by dermatologists who generally are more accurate and efficient skin diagnosticians, particularly if there is a shortage of PCPs within the ACO network.
- Wimo A, Religa D, Spångberg K, et al. Costs of diagnosing dementia: results from SveDem, the Swedish Dementia Registry. Int J Geriatr Psychiatry. 2013;28:1039-1044.
- Grunfeld E, Fitzpatrick R, Mant D, et al. Comparison of breast cancer patient satisfaction with follow-up in primary care versus specialist care: results from a randomized controlled trial. Br J Gen Pract. 1999;49:705-710.
- Chen SC, Pennie ML, Kolm P, et al. Diagnosing and managing cutaneous pigmented lesions: primary care physicians versus dermatologists. J Gen Intern Med. 2006;21:678-682.
- Federman D, Hogan D, Taylor JR, et al. A comparison of diagnosis, evaluation, and treatment of patients with dermatologic disorders. J Am Acad Dermatol. 1995;32:726-729.
- Feldman SR, Fleischer AB, Young AC, et al. Time-efficiency of nondermatologists compared with dermatologists in the care of skin disease. J Am Acad Dermatol. 1999;40:194-199.
- Feldman SR, Peterson SR, Fleischer AB Jr. Dermatologists meet the primary care standard for first contact management of skin disease. J Am Acad Dermatol. 1998;39(2, pt 1):182-186.
- Smith ES, Fleischer AB, Feldman SR. Nondermatologists are more likely than dermatologists to prescribe antifungal/corticosteroid products: an analysis of office visits for cutaneous fungal infections, 1990-1994. J Am Acad Dermatol. 1998;39:43-47.
- Shaffer MP, Feldman SR, Fleischer AB. Use of clotrimazole/betamethasone diproprionate by family physicians. Fam Med. 2000;32:561-565.
- Feldman SR, Fleischer AB, Chen JG. The gatekeeper model is inefficient for the delivery of dermatologic services. J Am Acad Dermatol. 1999;40:426-432.
- Wimo A, Religa D, Spångberg K, et al. Costs of diagnosing dementia: results from SveDem, the Swedish Dementia Registry. Int J Geriatr Psychiatry. 2013;28:1039-1044.
- Grunfeld E, Fitzpatrick R, Mant D, et al. Comparison of breast cancer patient satisfaction with follow-up in primary care versus specialist care: results from a randomized controlled trial. Br J Gen Pract. 1999;49:705-710.
- Chen SC, Pennie ML, Kolm P, et al. Diagnosing and managing cutaneous pigmented lesions: primary care physicians versus dermatologists. J Gen Intern Med. 2006;21:678-682.
- Federman D, Hogan D, Taylor JR, et al. A comparison of diagnosis, evaluation, and treatment of patients with dermatologic disorders. J Am Acad Dermatol. 1995;32:726-729.
- Feldman SR, Fleischer AB, Young AC, et al. Time-efficiency of nondermatologists compared with dermatologists in the care of skin disease. J Am Acad Dermatol. 1999;40:194-199.
- Feldman SR, Peterson SR, Fleischer AB Jr. Dermatologists meet the primary care standard for first contact management of skin disease. J Am Acad Dermatol. 1998;39(2, pt 1):182-186.
- Smith ES, Fleischer AB, Feldman SR. Nondermatologists are more likely than dermatologists to prescribe antifungal/corticosteroid products: an analysis of office visits for cutaneous fungal infections, 1990-1994. J Am Acad Dermatol. 1998;39:43-47.
- Shaffer MP, Feldman SR, Fleischer AB. Use of clotrimazole/betamethasone diproprionate by family physicians. Fam Med. 2000;32:561-565.
- Feldman SR, Fleischer AB, Chen JG. The gatekeeper model is inefficient for the delivery of dermatologic services. J Am Acad Dermatol. 1999;40:426-432.
Practice Points
- Growing health care costs are causing accountable care organizations (ACOs) to reconsider how to best manage skin disease.
- There is little difference in average diagnosis-related cost between primary care physicians and dermatologists in diagnosing psoriasis or rosacea.
- With diagnosis costs essentially equal and increased dermatologist diagnostic accuracy, ACOs may encourage skin disease to be managed by dermatologists.