User login
A practical approach to knee OA
CASE A 73-year-old woman presents to your clinic with 1 year of gradual-onset left knee pain. The pain is worse at the medial knee and at the beginning and end of the day, with some mild improvement after activity in the morning. The patient has already tried oral acetaminophen, an over-the-counter menthol cream, and a soft elastic knee brace, but these interventions have helped only minimally.
On physical exam, there is no obvious deformity of the knee. There is a bit of small joint effusion without redness or warmth. There is mild tenderness to palpation of the medial joint line. Radiographic findings include osteophytes of the medial and lateral tibial plateaus and medial and lateral femoral condyles with mild joint-space narrowing of the medial compartment, consistent with mild osteoarthritis.
How would you manage this patient’s care?
The knee is the most common joint to be affected by osteoarthritis (OA) and accounts for the majority of the disease’s total burden.1 More than 19% of American adults ages ≥ 45 years have knee OA,1,2 and more than half of the people with symptomatic knee OA in the United States are younger than 65 years of age.3 Longer lifespan and increasing rates of obesity are thought to be driving the increasing prevalence of knee OA, although this remains debated.1 Risk factors for knee OA are outlined in TABLE.1,4-8

Diagnosis: Radiographs are helpful, not essential
The diagnosis of knee OA is relatively straightforward. Gradual onset of knee joint pain is present most days, with pain worse after activity and better with rest. Patients are usually middle-aged or older and/or have a distant history of knee joint injury. Other signs, symptoms, and physical exam findings associated with knee OA include: morning stiffness < 30 minutes, crepitus, instability, range-of-motion deficit, varus or valgus deformity, bony exostosis, joint-line tenderness, joint swelling/effusion, and the absence of erythema/warmth.1,9,10
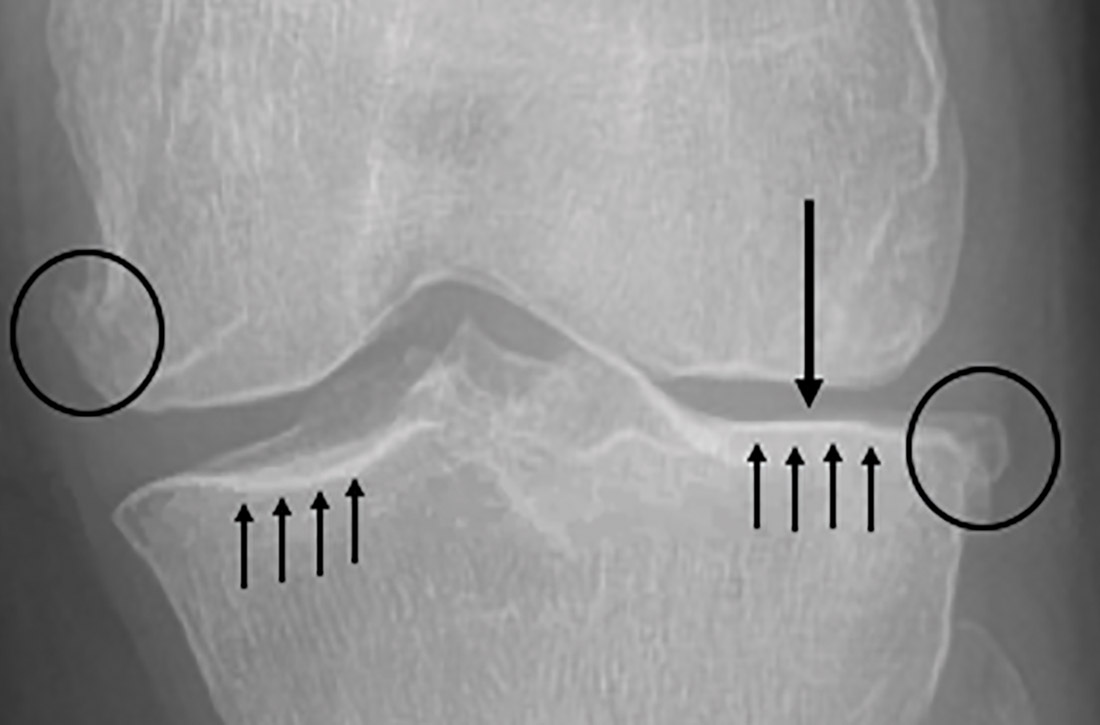
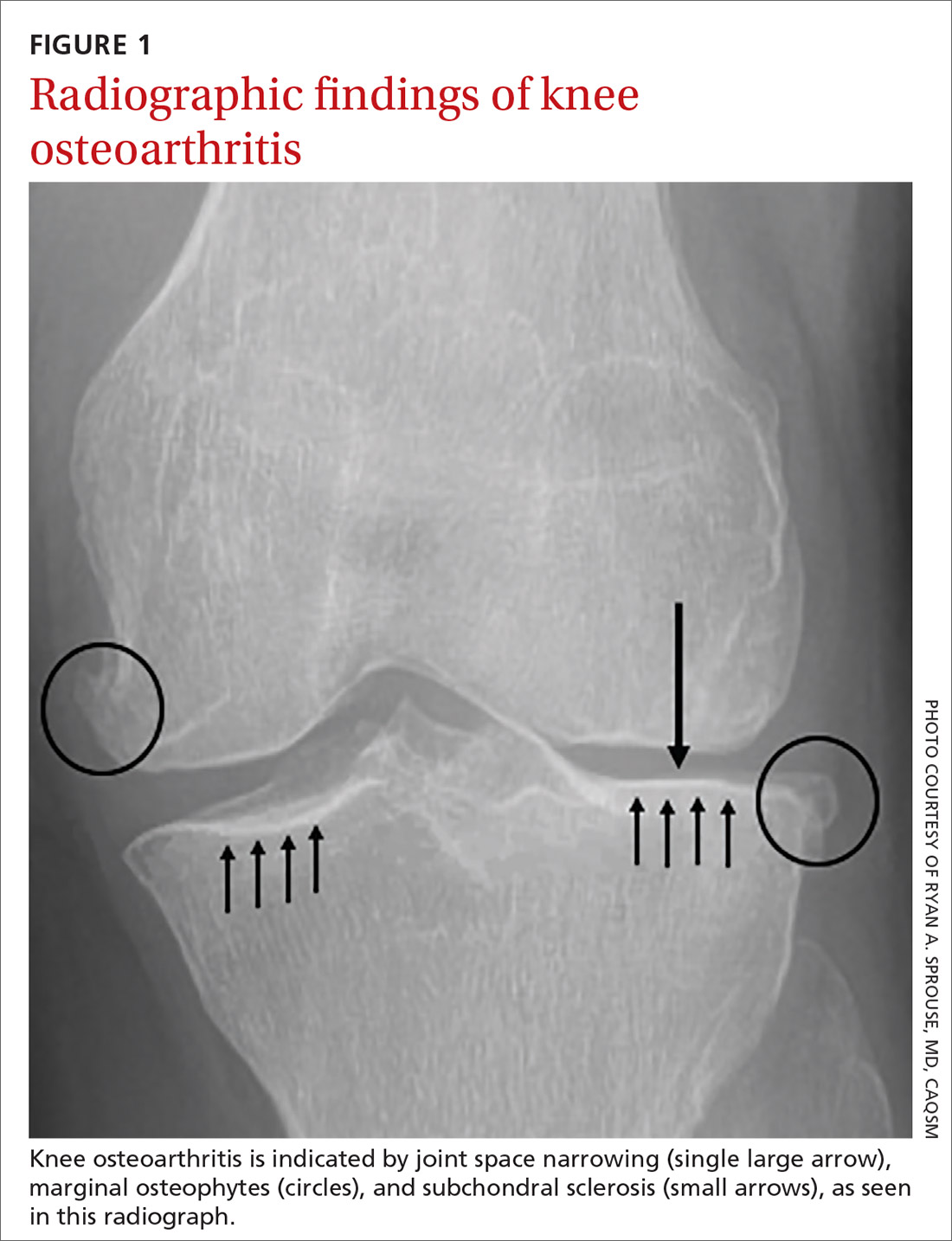
Although radiographs are not necessary to diagnose knee OA, they can be helpful in confirming the diagnosis by assessing the degree and location of OA and ruling out other pathology. Standing, weight-bearing radiographs are particularly helpful for assessing the degree of joint-space narrowing. In addition to joint-space narrowing, radiographic findings indicative of knee OA include marginal osteophytes, subchondral sclerosis, and subchondral cysts. (See FIGURE 1.)
Keep in mind that radiographs are less sensitive for early OA, that the degree of OA seen on radiographs does not correlate well with symptoms, and that radiographic evidence of OA is a common incidental finding—especially in elderly individuals.11 Although not routinely utilized for knee OA diagnosis, magnetic resonance imaging (MRI) can be used to assess for earlier stages of the disease and to rule out pathology associated with the soft tissue and cartilage that is not directly associated with OA.
Continue to: Management
Management: Decrease pain, improve function, slow progression
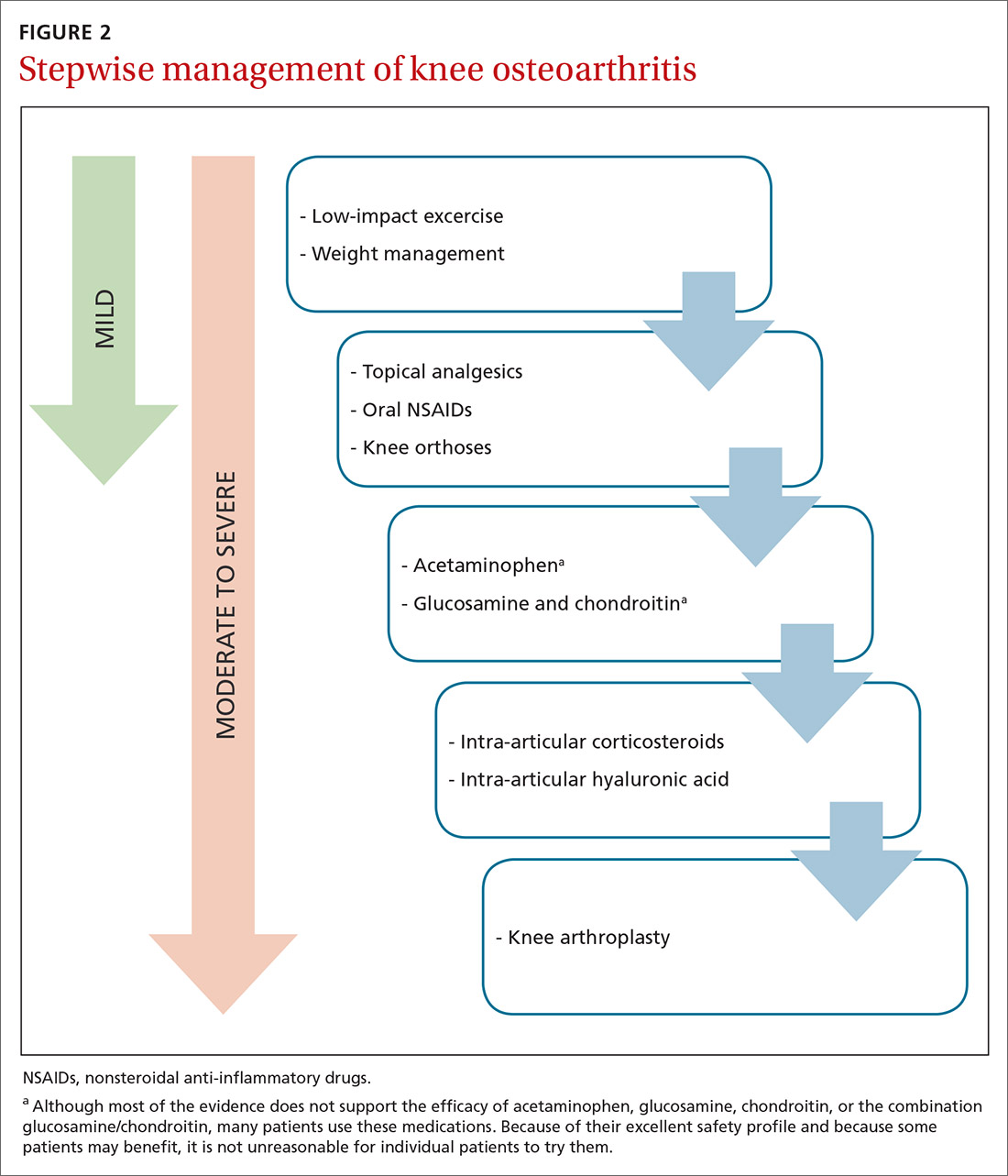
Because there is no cure for OA, the primary goals of treatment are to decrease pain, improve function of the joint, and slow progression of the disease. As a result, a multifaceted treatment approach is usually undertaken that includes weight reduction and exercise therapy and may include pharmacotherapy, depending on the degree of symptoms. FIGURE 2 contains a summary of the stepwise management of knee OA.
Weight management can slow progression of the disease
Obesity is a causative factor in knee OA.12,13 Patients with knee OA who achieve and maintain an appropriate body weight can potentially slow progression of the disease.13,14 One pound of weight loss can lead to a 4-fold reduction in the load exerted on the knee per step.15
Specific methods of weight reduction are beyond the scope of this article; however, one randomized controlled trial (RCT) involving 399 overweight and obese adults with knee OA found that individuals who participated in a dietary intervention or a combined diet and exercise intervention achieved more weight loss than those who undertook exercise alone.16 Additionally, the diet group had greater reductions in knee compression forces compared to the exercise group, and the combined diet and exercise group had less pain and better function than both the diet group and the exercise group.16 This would suggest that both diet and exercise interventions should be employed in the treatment of knee OA, not only for weight management, but also for knee joint health.
What kind of exercise? Evidence exists to support the utilization of various forms of exercise. In general, land-based therapeutic exercise improves knee pain, physical function, and quality of life, but these benefits often last less than 1 year because people often fail to maintain exercise programs for the long term.17
Specific therapies such as yoga, Tai Chi, balance training, and aquatic exercise have shown some minor improvement in symptoms related to knee OA.18-22 Weight-bearing strength training, non–weight-bearing strength training, and aerobic exercise have all been shown to be effective for short-term pain relief in knee OA, with non–weight-bearing strength training being the most effective.23
Continue to: Strengthening of the upper leg muscles...
Strengthening of the upper leg muscles is thought to be one of the factors involved in reducing pain associated with knee OA.24 Strength training, Tai Chi, and aerobic exercise have also been shown to decrease fall risk in the elderly with knee OA.25 In general, lower impact activities (eg, walking, swimming, biking, yoga) are preferred over higher impact activities (eg, running, jumping) in order to lessen pain with exercise.26-28
Knee orthoses: Many forms and mixed findings
Knee braces come in many forms, including soft braces (eg, elastic sleeves, simple hinged braces) and unloading braces. Many of these braces have been purported to help with knee OA although the evidence remains mixed, with a lack of high-quality trials. A systematic review of RCTs comparing various knee braces, foot orthotics, and conservative treatment for the management of medial compartment OA concluded that the optimal choice for orthosis remains unclear, and long-term evidence is lacking.29
The medial unloading (valgus) knee brace is often used to treat medial compartment OA and varus malalignment of the knee by applying a valgus force, thereby reducing the load on the medial compartment. One recent systematic review concluded that medial unloading braces improve pain from medial compartment OA, but whether they improve function and stiffness is unclear.30 Another study showed that compared to conservative treatment alone, valgus knee bracing has some benefit in decreasing pain and improving knee function.31 Additionally, an 8-year prospective study found that the valgus unloading brace can delay the time before patients need to undergo knee arthroplasty.32 However, another prospective study examining the efficacy of valgus bracing at 2.7 years and 11.2 years showed short-term but not long-term benefit.33
Soft knee braces include a variety of elastic sleeves and simple hinged knee braces. These braces are available commercially at most pharmacies and athletic retail stores. Soft braces are thought to improve pain by a thermal and compressive effect, and to provide stability to the knee joint. One systematic review concluded that soft knee braces have a moderate effect on pain and a small-to-moderate effect on self-reported physical function.34 A small trial showed that soft knee braces reduced pain and dynamic instability in individuals with knee OA.35
In summary, many types of soft knee braces exist, but the evidence for recommending them individually or collectively is limited, as high-quality trials are lacking. However, the available evidence does suggest some mild benefit with regard to pain and function with no concern for adverse effects.
Continue to: Pharmacotherapy
Pharmacotherapy: Oral agents
Acetaminophen. Although people commonly use this over-the-counter analgesic for knee OA pain, recent meta-analyses have shown that acetaminophen provides little to no benefit.36,37 Furthermore, although many believe acetaminophen causes fewer adverse effects than oral nonsteroidal anti-inflammatory drugs (NSAIDs), liver, gastrointestinal, and renal complications are not uncommon with long-term acetaminophen use. Nevertheless, a trial of acetaminophen may be beneficial in patients with cardiovascular disease or who are taking oral anticoagulants.
Oral NSAIDs. Many studies have concluded that NSAIDs are more effective at controlling pain from knee OA than acetaminophen.37,38 They are among the most commonly prescribed treatments for knee OA, but patients and their physicians should be cautious about long-term use because of potential cardiac, renal, gastrointestinal, and other adverse effects. Although evidence regarding optimal frequency of use is scarce, oral NSAIDs should be used intermittently and at the minimal effective dose in order to decrease the risk of adverse events.
One recent meta-analysis of RCTs concluded that diclofenac at a dose of 150 mg/d is the most effective NSAID for improving pain and function associated with knee OA.37 Another recent systematic review and meta-analysis analyzing multiple pharmacologic treatments found an association between celecoxib and decreased pain from knee OA.39 However, this study also concluded that uncertainty surrounded all of the estimates of effect size for change in pain compared to placebo for all of the pharmacologic treatments included in the study.39
A meta-analysis of RCTs comparing celecoxib to no treatment, placebo, naproxen, and diclofenac concluded that celecoxib is slightly better than placebo and the aforementioned NSAIDs in reducing pain and improving function in general OA. However, the authors had reservations regarding pharmaceutical industry involvement in the studies and overall limited data.40
With all of that said, the American Academy of Orthopaedic Surgeons (AAOS) recommends strongly for the use of oral NSAIDs in the management of knee OA.41
Continue to: Glucosamine and chondroitin
Glucosamine and chondroitin. Glucosamine and chondroitin are supplements that have gained popularity in the treatment of knee OA. These constituents are found naturally in articular cartilage, which explains the rationale for their use. Glucosamine and chondroitin (or a combination of the 2) are associated with few adverse effects, but the evidence to support their use in knee OA management is mixed.
One large double-blind RCT (the Glucosamine/Chondroitin Arthritis Intervention Trial [GAIT]) concluded that glucosamine, chondroitin, or the combination of the 2 did not have a significant effect on reducing pain from knee OA compared to placebo and did not slow structural joint disease.42 However, this same study found that in a subset of patients with moderate-to-severe knee OA, the combination of glucosamine and chondroitin was mildly effective in reducing pain.42
Multiple studies have shown either no benefit, inconsistent results, or limited benefit of glucosamine and chondroitin in the treatment of knee OA, with the patented crystalline form of glucosamine showing the most efficacy.43-47 The AAOS and the American College of Rheumatology (ACR) do not recommend glucosamine and chondroitin for knee OA management.10,41
In summary, the evidence for glucosamine, chondroitin, or a combination of the 2 for knee OA is mixed with likely limited benefit, but because they are associated with few adverse effects, patients may be offered a 3- to 6-month trial of these supplements if other effective options are exhausted.
Injections
Limited-quality evidence suggests that oral NSAIDs and intra-articular (IA) hyaluronic acid (HA) injections are equally efficacious for knee OA pain.38,48 There is insufficient evidence directly comparing oral NSAIDs with IA corticosteroid (CS) injections.
Continue to: HA is found naturally...
HA is found naturally in articular cartilage, which explains the rationale behind its use. A network meta-analysis performed by the American Medical Society for Sports Medicine concluded that knee OA is more likely to respond to IAHA than to IACS or IA placebo, leading the society to recommend the use of IAHA in knee OA management, especially for patients > 60 years with mild-to-moderate knee OA.9 Conversely, the AAOS does not recommend the use of IAHA, and the ACR does not recommend for or against the use of IAHA.10,41
IACSs are commonly used to provide pain relief in those with moderate-to-severe knee OA. There is evidence that a single IACS injection provides mild pain relief for up to 6 weeks.49 However, there is some concern that repetitive IACS injections may speed cartilage loss. A 2-year randomized double-blind placebo-controlled trial comparing the effectiveness of repetitive IA triamcinolone vs saline in knee OA found no difference in pain severity and concluded that there was greater cartilage volume loss in the triamcinolone group.50
AAOS does not recommend for or against the use of IACSs, whereas the ACR does recommend for the use of IACSs.10,41 Given the available evidence, conservative use of IACS injections remains an option for patients with refractory moderate-to-severe knee OA.
Topicals
Topical analgesics are often utilized for knee OA because of their efficacy, tolerability, low risk of adverse effects, and ease of use. They are generally recommended over oral NSAIDs in the elderly and in individuals at risk for cardiac, renal, and gastrointestinal complications from oral NSAIDs.
One review found that topical diclofenac and topical ketoprofen were comparable to the oral forms of these medications.51 One RCT concluded that topical and oral diclofenac were equally efficacious in treating knee OA symptoms, although topical diclofenac was associated with significantly fewer gastrointestinal adverse effects.52 In multiple randomized trials, topical diclofenac has shown efficacy compared to placebo.53-55 A recent systematic review and meta-analysis of RCTs concluded that topical NSAIDs were safe and effective for treating general OA compared to placebo, with diclofenac patches most effective for pain relief and piroxicam most effective for functional improvement.56
Continue to: Topical capsaicin has shown...
Topical capsaicin has shown some efficacy in treating pain associated with knee OA.57 One meta-analysis of RCTs concluded that topical NSAIDs and capsaicin may be equally efficacious for OA-associated pain relief, although none of the RCTs directly compared the two.58 The major limitation of capsaicin is a patient-reported mild-to-moderate burning sensation with application that may decrease compliance.
Emerging treatments: IA PRP & extended-release IA triamcinolone acetonide
IA platelet-rich plasma (PRP) has been investigated for efficacy in treating knee OA. PRP is thought to decrease inflammation in the joint, although its exact mechanism remains unknown.59 Multiple studies have shown some benefit of PRP in reducing pain and improving function in individuals with knee OA, but nearly all of these studies have failed to show a clear benefit of PRP over HA injections.59-63 Additionally, the authors of most of these studies mention a high risk of bias. PRP therapy is expensive and generally is not covered by insurance companies, which precludes its use for many people.
Extended-release (ER) IA triamcinolone acetonide (Zilretta) has shown some superiority to standard IA triamcinolone acetonide in both degree and duration of pain relief for knee OA.64-66 The ER version tolerability did not differ from placebo and also showed prolonged synovial presence, lower systemic absorption, and lower blood glucose elevations compared with standard triamcinolone.64-66
Surgical intervention: A last resort
Select patients with severe pain and disability from knee OA that is refractory to conservative management options should be referred for consideration of knee arthroplasty. Age, weight, OA location, and degree of OA are all considered with respect to knee arthroplasty timing and technique.
There is good evidence that arthroscopy with debridement, on the other hand, is no more effective than conservative management.67
Continue to: Unicompartmental or "partial"...
Unicompartmental or “partial” knee replacements are reserved for select cases when 1 knee compartment has a significantly higher degree of degenerative change.
CASE After reviewing the therapeutic options with your patient, you agree that she will undergo a course of physical therapy and try using topical diclofenac along with a hinged knee brace. Because of the patient’s age and co-morbidities of cardiovascular disease and mild chronic kidney disease, oral NSAIDs are avoided at this time.
The patient returns to the office in 2 months reporting mild improvement in her pain. To provide additional pain relief, an ultrasound-guided IA steroid injection is attempted. The patient also continues home physical therapy, activity modification, topical diclofenac, and use of a hinged knee brace.
She returns to the office 2 months later, reporting continued improvement in her pain. No further intervention is undertaken at this time.
CORRESPONDENCE
Ryan A. Sprouse, MD, CAQSM, West Virginia University School of Medicine–Eastern Campus, WVU Medicine Orthopaedics and Sports Medicine, 912 Somerset Boulevard, Charles Town, WV 25414; [email protected].
1. Wallace IJ, Worthington S,Felson DT, et al. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc Natl Acad Sci. 2017;114:9332-9336.
2. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35.
3. Vina ER, Kwoh CK. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol. 2018;30:160-167.
4. Warner SC, Valdes AM. Genetic association studies in osteoarthritis: is it fairytale? Curr Opin Rheumatol. 2017;29:103-109.
5. Srikanth VK, Fryer JL, Zhai G, et al. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. 2005;13:769-781.
6. Palazzo C, Nguyen C, Lefevre-Colau MM, et al. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med. 2016;59:134-138.
7. Tanamas S, Hanna FS, Cicuttini FM, et al. Does knee malalignment increase the risk of development and progression of knee osteoarthritis? A systematic review. Arthritis Rheum. 2009;61:459-467.
8. Yucesoy B, Charles LE, Baker B, et al. Occupational and genetic risk factors for osteoarthritis: a review. Work. 2015;50:261-273.
9. Trojian TH, Concoff AL, Joy SM, et al. AMSSM scientific statement concerning viscosupplementation injections for knee osteoarthritis: importance for individual patient outcomes. Br J Sports Med. 2016;50:84-92.
10. Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 Recommendations for the Use of Nonpharmacologic and Pharmacologic Therapies in Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2012;64:465-474.
11. Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord. 2008;9:116.
12. Felson DT, Anderson JJ, Naimark A, et al. Obesity and knee osteoarthritis. The Framingham Study. Ann Intern Med. 1988;109:18-24.
13. Yusuf E, Bijsterbosch J, Slagboom PE, et al. Body mass index and alignment and their interaction as risk factors for progression of knees with radiographic signs of osteoarthritis. Osteoarthritis Cartilage. 2011;19:1117-1122.
14. Niu J, Zhang YQ, Torner J, et al. Is obesity a risk factor for progressive radiographic knee osteoarthritis? Arthritis Rheum. 2009;61:329-335.
15. Messier SP, Gutekunst DJ, Davis C, et al. Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis Rheum. 2005;52:2026-2032.
16. Messier SP, Mihalko SL, Legault C, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310:1263-1273.
17. Fransen M, McConnell S, Harmer AR, et al. Exercise for osteoarthritis of the knee: a Cochrane systematic review. Br J Sports Med.
18. Kan L, Zhang J, Yang Y, et al. The effects of yoga on pain, mobility, and quality of life in patients with knee osteoarthritis: a systematic review. Evid Based Complement Alternat Med. 2016;2016:6016532.
19. Chang WD, Chen S, Lee CL, et al. The effects of tai chi chuan on improving mind-body health for knee osteoarthritis patients: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2016;2016:1813979.
20. Takacs J, Krowchuk NM, Garland SJ, et al. Dynamic balance training improves physical function in individuals with knee osteoarthritis: a pilot randomized controlled trial. Arch Phys Med Rehabil. 2017;98:1586-1593.
21. Bartels EM, Juhl CB, Christensen R, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016;(3):CD005523.
22. Hinman RS, Heywood SE, Day AR. Aquatic physical therapy for hip and knee osteoarthritis: results of a single-blind randomized controlled trial. Phys Ther. 2007;87:32-43.
23. Tanaka R, Ozawa J, Kito N, et al. Efficacy of strengthening or aerobic exercise on pain relief in people with knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Clin Rehabil. 2013;27:1059-1071.
24. Knoop J, Steultjens MP, Roorda LD, et al. Improvement in upper leg muscle strength underlies beneficial effects of exercise therapy in knee osteoarthritis: secondary analysis from a randomised controlled trial. Physiotherapy. 2015;101:171-177.
25. Mat S, Tan MP, Kamaruzzaman SB, et al. Physical therapies for improving balance and reducing falls risk in osteoarthritis of the knee: a systematic review. Age Ageing. 2015;44:16-24.
26. Peeler J, Christian M, Cooper J, et al. Managing knee osteoarthritis: the effects of body weight supported physical activity on joint pain, function, and thigh muscle strength. Clin J Sport Med. 2015;25:518-523.
27. Peeler J, Ripat J. The effect of low-load exercise on joint pain, function, and activities of daily living in patients with knee osteoarthritis. Knee. 2018;25:135-145.
28. Takacs J, Anderson JE, Leiter JR, et al. Lower body positive pressure: an emerging technology in the battle against knee osteoarthritis? Clin Interv Aging. 2013;8:983-991.
29. Duivenvoorden T, Brouwer RW, van Raaij TM, et al. Braces and orthoses for treating osteoarthritis of the knee. Cochrane Database Syst Rev. 2015;(3):CD004020.
30. Gohal C, Shanmugaraj A, Tate P, et al. Effectiveness of valgus offloading knee braces in the treatment of medial compartment knee osteoarthritis: a systematic review. Sports Health. 2018;10:500-514.
31. Brouwer RW, van Raaij TM, Verhaar JA, et al. Brace treatment for osteoarthritis of the knee: a prospective randomized multi-centre trial. Osteoarthritis Cartilage. 2006;14:777-783.
32. Lee PY, Winfield TG, Harris SR, et al. Unloading knee brace is a cost-effective method to bridge and delay surgery in unicompartmental knee arthritis. BMJ Open Sport Exerc Med. 2017;2:e000195.
33. Wilson B, Rankin H, Barnes CL. Long-term results of an unloader brace in patients with unicompartmental knee osteoarthritis. Orthopedics. 2011;34:334-347.
34. Cudejko T, van der Esch M, van der Leeden M, et al. Effect of soft braces on pain and physical function in patients with knee osteoarthritis: systematic review with meta-analyses. Arch Phys Med Rehabil. 2018;99:153-163.
35. Cudejko T, van der Esch M, van den Noort JC. Decreased pain and improved dynamic knee instability mediate the beneficial effect of wearing a soft knee brace on activity limitations in persons with knee osteoarthritis. Arthritis Care Res (Hoboken). 2019;71:1036-1043.
36. Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225.
37. da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2017;390:e21-e33.
38. Bannuru RR, Schmid CH, Kent DM, et al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162:46-54.
39. Gregori D, Giacovelli G, Minto C, et al. Association of pharmacological treatments with long-term pain control in patients with knee osteoarthritis: a systematic review and meta-analysis. JAMA. 2018;320:2564-2579.
40. Puljak L, Marin A, Vrdoljak D, et al. Celecoxib for osteoarthritis. Cochrane Database Syst Rev. 2017;(5):CD009865.
41. Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;9:571-576.
42. Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354:795-808.
43. Singh JA, Noorbaloochi S, MacDonald R, et al. Chondroitin for osteoarthritis. Cochrane Database Syst Rev. 2015;(1):CD005614.
44. Yang S, Eaton CB, McAlindon TE, et al. Effects of glucosamine and chondroitin on treating knee osteoarthritis: an analysis with marginal structural models. Arthritis Rheumatol. 2015;67:714-723.
45. Ogata T, Yuki Ideno Y, Masami Akai M,et al. Effects of glucosamine in patients with osteoarthritis of the knee: a systematic review and meta-analysis. Clin Rheumatol. 2018;37:2479-2487.
46. Towheed TE, Maxwell L, Anastassiades TP, et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev. 2009;(2):CD002946.
47. Bruyèreetal O, Cooper C, Pelletier JP, et al. A consensus statement on the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) algorithm for the management of knee osteoarthritis—from evidence-based medicine to the real-life setting. Semin Arthritis Rheum. 2016;45(4 suppl):S3-S11.
48. Ishijima M, Nakamura T, Shimizu K, et al. Intra-articular hyaluronic acid injection versus oral non-steroidal anti-inflammatory drug for the treatment of knee osteoarthritis: a multi-center, randomized, open-label, non-inferiority trial. Arthritis Res Ther. 2014;16:R18.
49. Juni P, Hari R, Rutjes AW, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev. 2015;(10):CD005328.
50. McAlindon TE, LaValley MP, Harvey FW, et al. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: a randomized clinical trial. JAMA. 2017;317:1967-1975.
51. Derry S, Conaghan P, Da Silva JA, et al. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2016;(4):CD007400.
52. Tugwell PS, Wells GA, Shainhouse JZ. Equivalence study of a topical diclofenac solution (pennsaid) compared with oral diclofenac in symptomatic treatment of osteoarthritis of the knee: a randomized controlled trial. J Rheumatol. 2004;31:2002-2012.
53. Wadsworth LT, Kent JD, Holt RJ. Efficacy and safety of diclofenac sodium 2% topical solution for osteoarthritis of the knee: a randomized, double-blind, vehicle-controlled, 4 week study. Curr Med Res Opin. 2016;32:241-250.
54. Roth SH, Shainhouse JZ. Efficacy and safety of a topical diclofenac solution (pennsaid) in the treatment of primary osteoarthritis of the knee: a randomized, double-blind, vehicle-controlled clinical trial. Arch Intern Med. 2004;164:2017-2023.
55. Baer PA, Thomas LM, Shainhouse Z. Treatment of osteoarthritis of the knee with a topical diclofenac solution: a randomised controlled, 6-week trial. BMC Musculoskelet Disord. 2005;6:44.
56. Zeng C, Wei J, Persson MSM, et al. Relative efficacy and safety of topical non-steroidal anti-inflammatory drugs for osteoarthritis: a systematic review and network meta-analysis of randomised controlled trials and observational studies. Br J Sports Med. 2018;52:642-650.
57. Guedes V, Castro JP, Brito I. Topical capsaicin for pain in osteoarthritis: a literature review. Reumatol Clin. 2018;14:40-45.
58. Persson MSM, Stocks J, Walsh DA, et al. The relative efficacy of topical non-steroidal anti-inflammatory drugs and capsaicin in osteoarthritis: a network meta-analysis of randomised controlled trials. Osteoarthritis Cartilage. 2018;26:1575-1582.
59. Cole BJ, Karas V, Hussey K, et al. Hyaluronic acid versus platelet-rich plasma: a prospective, double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am J Sports Med. 2017;45:339-346.
60. Laudy AB, Bakker EW, Rekers M, et al. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med. 2015;49:657-672.
61. Han Y, Huang H, Pan J, et al. Meta-analysis comparing platelet-rich plasma vs hyaluronic acid injection in patients with knee osteoarthritis. Pain Med. 2019;20:1418-1429.
62. Filardo G, Di Matteo B, Di Martino A, et al. Platelet-rich plasma intra-articular knee injections show no superiority versus viscosupplementation: a randomized controlled trial. Am J Sports Med. 2015;43:1575-1582.
63. Di Martino A, Di Matteo B, Papio T, et al. Platelet-rich plasma versus hyaluronic acid injections for the treatment of knee osteoarthritis: results at 5 years of a double-blind, randomized controlled trial. Am J Sports Med. 2019;47:347-354.
64. Bodick N, Lufkin J, Willwerth C, et al. An intra-articular, extended-release formulation of triamcinolone acetonide prolongs and amplifies analgesic effect in patients with osteoarthritis of the knee: a randomized clinical trial. J Bone Joint Surg Am. 2015;97:877-888.
65. Conaghan PG, Cohen SB, Berenbaum F, et al. Brief report: a phase IIb trial of a novel extended-release microsphere formulation of triamcinolone acetonide for intraarticular injection in knee osteoarthritis. Arthritis Rheumatol. 2018;70:204-211.
66. Conaghan PG, Hunter DJ, Cohen SB, et al. Effects of a single intra-articular injection of a microsphere formulation of triamcinolone acetonide on knee osteoarthritis pain: a double-blinded, randomized, placebo-controlled, multinational study. J Bone Jt Surg Am. 2018;100:666–677.
67. Thorlund JB, Juhl CB, Roos EM, et al. Arthroscopic surgery for degenerative knee: systematic review and meta-analysis of benefits and harms. BMJ. 2015;350:h2747.
CASE A 73-year-old woman presents to your clinic with 1 year of gradual-onset left knee pain. The pain is worse at the medial knee and at the beginning and end of the day, with some mild improvement after activity in the morning. The patient has already tried oral acetaminophen, an over-the-counter menthol cream, and a soft elastic knee brace, but these interventions have helped only minimally.
On physical exam, there is no obvious deformity of the knee. There is a bit of small joint effusion without redness or warmth. There is mild tenderness to palpation of the medial joint line. Radiographic findings include osteophytes of the medial and lateral tibial plateaus and medial and lateral femoral condyles with mild joint-space narrowing of the medial compartment, consistent with mild osteoarthritis.
How would you manage this patient’s care?
The knee is the most common joint to be affected by osteoarthritis (OA) and accounts for the majority of the disease’s total burden.1 More than 19% of American adults ages ≥ 45 years have knee OA,1,2 and more than half of the people with symptomatic knee OA in the United States are younger than 65 years of age.3 Longer lifespan and increasing rates of obesity are thought to be driving the increasing prevalence of knee OA, although this remains debated.1 Risk factors for knee OA are outlined in TABLE.1,4-8

Diagnosis: Radiographs are helpful, not essential
The diagnosis of knee OA is relatively straightforward. Gradual onset of knee joint pain is present most days, with pain worse after activity and better with rest. Patients are usually middle-aged or older and/or have a distant history of knee joint injury. Other signs, symptoms, and physical exam findings associated with knee OA include: morning stiffness < 30 minutes, crepitus, instability, range-of-motion deficit, varus or valgus deformity, bony exostosis, joint-line tenderness, joint swelling/effusion, and the absence of erythema/warmth.1,9,10
Although radiographs are not necessary to diagnose knee OA, they can be helpful in confirming the diagnosis by assessing the degree and location of OA and ruling out other pathology. Standing, weight-bearing radiographs are particularly helpful for assessing the degree of joint-space narrowing. In addition to joint-space narrowing, radiographic findings indicative of knee OA include marginal osteophytes, subchondral sclerosis, and subchondral cysts. (See FIGURE 1.)

Keep in mind that radiographs are less sensitive for early OA, that the degree of OA seen on radiographs does not correlate well with symptoms, and that radiographic evidence of OA is a common incidental finding—especially in elderly individuals.11 Although not routinely utilized for knee OA diagnosis, magnetic resonance imaging (MRI) can be used to assess for earlier stages of the disease and to rule out pathology associated with the soft tissue and cartilage that is not directly associated with OA.
Continue to: Management
Management: Decrease pain, improve function, slow progression
Because there is no cure for OA, the primary goals of treatment are to decrease pain, improve function of the joint, and slow progression of the disease. As a result, a multifaceted treatment approach is usually undertaken that includes weight reduction and exercise therapy and may include pharmacotherapy, depending on the degree of symptoms. FIGURE 2 contains a summary of the stepwise management of knee OA.

Weight management can slow progression of the disease
Obesity is a causative factor in knee OA.12,13 Patients with knee OA who achieve and maintain an appropriate body weight can potentially slow progression of the disease.13,14 One pound of weight loss can lead to a 4-fold reduction in the load exerted on the knee per step.15
Specific methods of weight reduction are beyond the scope of this article; however, one randomized controlled trial (RCT) involving 399 overweight and obese adults with knee OA found that individuals who participated in a dietary intervention or a combined diet and exercise intervention achieved more weight loss than those who undertook exercise alone.16 Additionally, the diet group had greater reductions in knee compression forces compared to the exercise group, and the combined diet and exercise group had less pain and better function than both the diet group and the exercise group.16 This would suggest that both diet and exercise interventions should be employed in the treatment of knee OA, not only for weight management, but also for knee joint health.
What kind of exercise? Evidence exists to support the utilization of various forms of exercise. In general, land-based therapeutic exercise improves knee pain, physical function, and quality of life, but these benefits often last less than 1 year because people often fail to maintain exercise programs for the long term.17
Specific therapies such as yoga, Tai Chi, balance training, and aquatic exercise have shown some minor improvement in symptoms related to knee OA.18-22 Weight-bearing strength training, non–weight-bearing strength training, and aerobic exercise have all been shown to be effective for short-term pain relief in knee OA, with non–weight-bearing strength training being the most effective.23
Continue to: Strengthening of the upper leg muscles...
Strengthening of the upper leg muscles is thought to be one of the factors involved in reducing pain associated with knee OA.24 Strength training, Tai Chi, and aerobic exercise have also been shown to decrease fall risk in the elderly with knee OA.25 In general, lower impact activities (eg, walking, swimming, biking, yoga) are preferred over higher impact activities (eg, running, jumping) in order to lessen pain with exercise.26-28
Knee orthoses: Many forms and mixed findings
Knee braces come in many forms, including soft braces (eg, elastic sleeves, simple hinged braces) and unloading braces. Many of these braces have been purported to help with knee OA although the evidence remains mixed, with a lack of high-quality trials. A systematic review of RCTs comparing various knee braces, foot orthotics, and conservative treatment for the management of medial compartment OA concluded that the optimal choice for orthosis remains unclear, and long-term evidence is lacking.29
The medial unloading (valgus) knee brace is often used to treat medial compartment OA and varus malalignment of the knee by applying a valgus force, thereby reducing the load on the medial compartment. One recent systematic review concluded that medial unloading braces improve pain from medial compartment OA, but whether they improve function and stiffness is unclear.30 Another study showed that compared to conservative treatment alone, valgus knee bracing has some benefit in decreasing pain and improving knee function.31 Additionally, an 8-year prospective study found that the valgus unloading brace can delay the time before patients need to undergo knee arthroplasty.32 However, another prospective study examining the efficacy of valgus bracing at 2.7 years and 11.2 years showed short-term but not long-term benefit.33
Soft knee braces include a variety of elastic sleeves and simple hinged knee braces. These braces are available commercially at most pharmacies and athletic retail stores. Soft braces are thought to improve pain by a thermal and compressive effect, and to provide stability to the knee joint. One systematic review concluded that soft knee braces have a moderate effect on pain and a small-to-moderate effect on self-reported physical function.34 A small trial showed that soft knee braces reduced pain and dynamic instability in individuals with knee OA.35
In summary, many types of soft knee braces exist, but the evidence for recommending them individually or collectively is limited, as high-quality trials are lacking. However, the available evidence does suggest some mild benefit with regard to pain and function with no concern for adverse effects.
Continue to: Pharmacotherapy
Pharmacotherapy: Oral agents
Acetaminophen. Although people commonly use this over-the-counter analgesic for knee OA pain, recent meta-analyses have shown that acetaminophen provides little to no benefit.36,37 Furthermore, although many believe acetaminophen causes fewer adverse effects than oral nonsteroidal anti-inflammatory drugs (NSAIDs), liver, gastrointestinal, and renal complications are not uncommon with long-term acetaminophen use. Nevertheless, a trial of acetaminophen may be beneficial in patients with cardiovascular disease or who are taking oral anticoagulants.
Oral NSAIDs. Many studies have concluded that NSAIDs are more effective at controlling pain from knee OA than acetaminophen.37,38 They are among the most commonly prescribed treatments for knee OA, but patients and their physicians should be cautious about long-term use because of potential cardiac, renal, gastrointestinal, and other adverse effects. Although evidence regarding optimal frequency of use is scarce, oral NSAIDs should be used intermittently and at the minimal effective dose in order to decrease the risk of adverse events.
One recent meta-analysis of RCTs concluded that diclofenac at a dose of 150 mg/d is the most effective NSAID for improving pain and function associated with knee OA.37 Another recent systematic review and meta-analysis analyzing multiple pharmacologic treatments found an association between celecoxib and decreased pain from knee OA.39 However, this study also concluded that uncertainty surrounded all of the estimates of effect size for change in pain compared to placebo for all of the pharmacologic treatments included in the study.39
A meta-analysis of RCTs comparing celecoxib to no treatment, placebo, naproxen, and diclofenac concluded that celecoxib is slightly better than placebo and the aforementioned NSAIDs in reducing pain and improving function in general OA. However, the authors had reservations regarding pharmaceutical industry involvement in the studies and overall limited data.40
With all of that said, the American Academy of Orthopaedic Surgeons (AAOS) recommends strongly for the use of oral NSAIDs in the management of knee OA.41
Continue to: Glucosamine and chondroitin
Glucosamine and chondroitin. Glucosamine and chondroitin are supplements that have gained popularity in the treatment of knee OA. These constituents are found naturally in articular cartilage, which explains the rationale for their use. Glucosamine and chondroitin (or a combination of the 2) are associated with few adverse effects, but the evidence to support their use in knee OA management is mixed.
One large double-blind RCT (the Glucosamine/Chondroitin Arthritis Intervention Trial [GAIT]) concluded that glucosamine, chondroitin, or the combination of the 2 did not have a significant effect on reducing pain from knee OA compared to placebo and did not slow structural joint disease.42 However, this same study found that in a subset of patients with moderate-to-severe knee OA, the combination of glucosamine and chondroitin was mildly effective in reducing pain.42
Multiple studies have shown either no benefit, inconsistent results, or limited benefit of glucosamine and chondroitin in the treatment of knee OA, with the patented crystalline form of glucosamine showing the most efficacy.43-47 The AAOS and the American College of Rheumatology (ACR) do not recommend glucosamine and chondroitin for knee OA management.10,41
In summary, the evidence for glucosamine, chondroitin, or a combination of the 2 for knee OA is mixed with likely limited benefit, but because they are associated with few adverse effects, patients may be offered a 3- to 6-month trial of these supplements if other effective options are exhausted.
Injections
Limited-quality evidence suggests that oral NSAIDs and intra-articular (IA) hyaluronic acid (HA) injections are equally efficacious for knee OA pain.38,48 There is insufficient evidence directly comparing oral NSAIDs with IA corticosteroid (CS) injections.
Continue to: HA is found naturally...
HA is found naturally in articular cartilage, which explains the rationale behind its use. A network meta-analysis performed by the American Medical Society for Sports Medicine concluded that knee OA is more likely to respond to IAHA than to IACS or IA placebo, leading the society to recommend the use of IAHA in knee OA management, especially for patients > 60 years with mild-to-moderate knee OA.9 Conversely, the AAOS does not recommend the use of IAHA, and the ACR does not recommend for or against the use of IAHA.10,41
IACSs are commonly used to provide pain relief in those with moderate-to-severe knee OA. There is evidence that a single IACS injection provides mild pain relief for up to 6 weeks.49 However, there is some concern that repetitive IACS injections may speed cartilage loss. A 2-year randomized double-blind placebo-controlled trial comparing the effectiveness of repetitive IA triamcinolone vs saline in knee OA found no difference in pain severity and concluded that there was greater cartilage volume loss in the triamcinolone group.50
AAOS does not recommend for or against the use of IACSs, whereas the ACR does recommend for the use of IACSs.10,41 Given the available evidence, conservative use of IACS injections remains an option for patients with refractory moderate-to-severe knee OA.
Topicals
Topical analgesics are often utilized for knee OA because of their efficacy, tolerability, low risk of adverse effects, and ease of use. They are generally recommended over oral NSAIDs in the elderly and in individuals at risk for cardiac, renal, and gastrointestinal complications from oral NSAIDs.
One review found that topical diclofenac and topical ketoprofen were comparable to the oral forms of these medications.51 One RCT concluded that topical and oral diclofenac were equally efficacious in treating knee OA symptoms, although topical diclofenac was associated with significantly fewer gastrointestinal adverse effects.52 In multiple randomized trials, topical diclofenac has shown efficacy compared to placebo.53-55 A recent systematic review and meta-analysis of RCTs concluded that topical NSAIDs were safe and effective for treating general OA compared to placebo, with diclofenac patches most effective for pain relief and piroxicam most effective for functional improvement.56
Continue to: Topical capsaicin has shown...
Topical capsaicin has shown some efficacy in treating pain associated with knee OA.57 One meta-analysis of RCTs concluded that topical NSAIDs and capsaicin may be equally efficacious for OA-associated pain relief, although none of the RCTs directly compared the two.58 The major limitation of capsaicin is a patient-reported mild-to-moderate burning sensation with application that may decrease compliance.
Emerging treatments: IA PRP & extended-release IA triamcinolone acetonide
IA platelet-rich plasma (PRP) has been investigated for efficacy in treating knee OA. PRP is thought to decrease inflammation in the joint, although its exact mechanism remains unknown.59 Multiple studies have shown some benefit of PRP in reducing pain and improving function in individuals with knee OA, but nearly all of these studies have failed to show a clear benefit of PRP over HA injections.59-63 Additionally, the authors of most of these studies mention a high risk of bias. PRP therapy is expensive and generally is not covered by insurance companies, which precludes its use for many people.
Extended-release (ER) IA triamcinolone acetonide (Zilretta) has shown some superiority to standard IA triamcinolone acetonide in both degree and duration of pain relief for knee OA.64-66 The ER version tolerability did not differ from placebo and also showed prolonged synovial presence, lower systemic absorption, and lower blood glucose elevations compared with standard triamcinolone.64-66
Surgical intervention: A last resort
Select patients with severe pain and disability from knee OA that is refractory to conservative management options should be referred for consideration of knee arthroplasty. Age, weight, OA location, and degree of OA are all considered with respect to knee arthroplasty timing and technique.
There is good evidence that arthroscopy with debridement, on the other hand, is no more effective than conservative management.67
Continue to: Unicompartmental or "partial"...
Unicompartmental or “partial” knee replacements are reserved for select cases when 1 knee compartment has a significantly higher degree of degenerative change.
CASE After reviewing the therapeutic options with your patient, you agree that she will undergo a course of physical therapy and try using topical diclofenac along with a hinged knee brace. Because of the patient’s age and co-morbidities of cardiovascular disease and mild chronic kidney disease, oral NSAIDs are avoided at this time.
The patient returns to the office in 2 months reporting mild improvement in her pain. To provide additional pain relief, an ultrasound-guided IA steroid injection is attempted. The patient also continues home physical therapy, activity modification, topical diclofenac, and use of a hinged knee brace.
She returns to the office 2 months later, reporting continued improvement in her pain. No further intervention is undertaken at this time.
CORRESPONDENCE
Ryan A. Sprouse, MD, CAQSM, West Virginia University School of Medicine–Eastern Campus, WVU Medicine Orthopaedics and Sports Medicine, 912 Somerset Boulevard, Charles Town, WV 25414; [email protected].
CASE A 73-year-old woman presents to your clinic with 1 year of gradual-onset left knee pain. The pain is worse at the medial knee and at the beginning and end of the day, with some mild improvement after activity in the morning. The patient has already tried oral acetaminophen, an over-the-counter menthol cream, and a soft elastic knee brace, but these interventions have helped only minimally.
On physical exam, there is no obvious deformity of the knee. There is a bit of small joint effusion without redness or warmth. There is mild tenderness to palpation of the medial joint line. Radiographic findings include osteophytes of the medial and lateral tibial plateaus and medial and lateral femoral condyles with mild joint-space narrowing of the medial compartment, consistent with mild osteoarthritis.
How would you manage this patient’s care?
The knee is the most common joint to be affected by osteoarthritis (OA) and accounts for the majority of the disease’s total burden.1 More than 19% of American adults ages ≥ 45 years have knee OA,1,2 and more than half of the people with symptomatic knee OA in the United States are younger than 65 years of age.3 Longer lifespan and increasing rates of obesity are thought to be driving the increasing prevalence of knee OA, although this remains debated.1 Risk factors for knee OA are outlined in TABLE.1,4-8

Diagnosis: Radiographs are helpful, not essential
The diagnosis of knee OA is relatively straightforward. Gradual onset of knee joint pain is present most days, with pain worse after activity and better with rest. Patients are usually middle-aged or older and/or have a distant history of knee joint injury. Other signs, symptoms, and physical exam findings associated with knee OA include: morning stiffness < 30 minutes, crepitus, instability, range-of-motion deficit, varus or valgus deformity, bony exostosis, joint-line tenderness, joint swelling/effusion, and the absence of erythema/warmth.1,9,10
Although radiographs are not necessary to diagnose knee OA, they can be helpful in confirming the diagnosis by assessing the degree and location of OA and ruling out other pathology. Standing, weight-bearing radiographs are particularly helpful for assessing the degree of joint-space narrowing. In addition to joint-space narrowing, radiographic findings indicative of knee OA include marginal osteophytes, subchondral sclerosis, and subchondral cysts. (See FIGURE 1.)

Keep in mind that radiographs are less sensitive for early OA, that the degree of OA seen on radiographs does not correlate well with symptoms, and that radiographic evidence of OA is a common incidental finding—especially in elderly individuals.11 Although not routinely utilized for knee OA diagnosis, magnetic resonance imaging (MRI) can be used to assess for earlier stages of the disease and to rule out pathology associated with the soft tissue and cartilage that is not directly associated with OA.
Continue to: Management
Management: Decrease pain, improve function, slow progression
Because there is no cure for OA, the primary goals of treatment are to decrease pain, improve function of the joint, and slow progression of the disease. As a result, a multifaceted treatment approach is usually undertaken that includes weight reduction and exercise therapy and may include pharmacotherapy, depending on the degree of symptoms. FIGURE 2 contains a summary of the stepwise management of knee OA.

Weight management can slow progression of the disease
Obesity is a causative factor in knee OA.12,13 Patients with knee OA who achieve and maintain an appropriate body weight can potentially slow progression of the disease.13,14 One pound of weight loss can lead to a 4-fold reduction in the load exerted on the knee per step.15
Specific methods of weight reduction are beyond the scope of this article; however, one randomized controlled trial (RCT) involving 399 overweight and obese adults with knee OA found that individuals who participated in a dietary intervention or a combined diet and exercise intervention achieved more weight loss than those who undertook exercise alone.16 Additionally, the diet group had greater reductions in knee compression forces compared to the exercise group, and the combined diet and exercise group had less pain and better function than both the diet group and the exercise group.16 This would suggest that both diet and exercise interventions should be employed in the treatment of knee OA, not only for weight management, but also for knee joint health.
What kind of exercise? Evidence exists to support the utilization of various forms of exercise. In general, land-based therapeutic exercise improves knee pain, physical function, and quality of life, but these benefits often last less than 1 year because people often fail to maintain exercise programs for the long term.17
Specific therapies such as yoga, Tai Chi, balance training, and aquatic exercise have shown some minor improvement in symptoms related to knee OA.18-22 Weight-bearing strength training, non–weight-bearing strength training, and aerobic exercise have all been shown to be effective for short-term pain relief in knee OA, with non–weight-bearing strength training being the most effective.23
Continue to: Strengthening of the upper leg muscles...
Strengthening of the upper leg muscles is thought to be one of the factors involved in reducing pain associated with knee OA.24 Strength training, Tai Chi, and aerobic exercise have also been shown to decrease fall risk in the elderly with knee OA.25 In general, lower impact activities (eg, walking, swimming, biking, yoga) are preferred over higher impact activities (eg, running, jumping) in order to lessen pain with exercise.26-28
Knee orthoses: Many forms and mixed findings
Knee braces come in many forms, including soft braces (eg, elastic sleeves, simple hinged braces) and unloading braces. Many of these braces have been purported to help with knee OA although the evidence remains mixed, with a lack of high-quality trials. A systematic review of RCTs comparing various knee braces, foot orthotics, and conservative treatment for the management of medial compartment OA concluded that the optimal choice for orthosis remains unclear, and long-term evidence is lacking.29
The medial unloading (valgus) knee brace is often used to treat medial compartment OA and varus malalignment of the knee by applying a valgus force, thereby reducing the load on the medial compartment. One recent systematic review concluded that medial unloading braces improve pain from medial compartment OA, but whether they improve function and stiffness is unclear.30 Another study showed that compared to conservative treatment alone, valgus knee bracing has some benefit in decreasing pain and improving knee function.31 Additionally, an 8-year prospective study found that the valgus unloading brace can delay the time before patients need to undergo knee arthroplasty.32 However, another prospective study examining the efficacy of valgus bracing at 2.7 years and 11.2 years showed short-term but not long-term benefit.33
Soft knee braces include a variety of elastic sleeves and simple hinged knee braces. These braces are available commercially at most pharmacies and athletic retail stores. Soft braces are thought to improve pain by a thermal and compressive effect, and to provide stability to the knee joint. One systematic review concluded that soft knee braces have a moderate effect on pain and a small-to-moderate effect on self-reported physical function.34 A small trial showed that soft knee braces reduced pain and dynamic instability in individuals with knee OA.35
In summary, many types of soft knee braces exist, but the evidence for recommending them individually or collectively is limited, as high-quality trials are lacking. However, the available evidence does suggest some mild benefit with regard to pain and function with no concern for adverse effects.
Continue to: Pharmacotherapy
Pharmacotherapy: Oral agents
Acetaminophen. Although people commonly use this over-the-counter analgesic for knee OA pain, recent meta-analyses have shown that acetaminophen provides little to no benefit.36,37 Furthermore, although many believe acetaminophen causes fewer adverse effects than oral nonsteroidal anti-inflammatory drugs (NSAIDs), liver, gastrointestinal, and renal complications are not uncommon with long-term acetaminophen use. Nevertheless, a trial of acetaminophen may be beneficial in patients with cardiovascular disease or who are taking oral anticoagulants.
Oral NSAIDs. Many studies have concluded that NSAIDs are more effective at controlling pain from knee OA than acetaminophen.37,38 They are among the most commonly prescribed treatments for knee OA, but patients and their physicians should be cautious about long-term use because of potential cardiac, renal, gastrointestinal, and other adverse effects. Although evidence regarding optimal frequency of use is scarce, oral NSAIDs should be used intermittently and at the minimal effective dose in order to decrease the risk of adverse events.
One recent meta-analysis of RCTs concluded that diclofenac at a dose of 150 mg/d is the most effective NSAID for improving pain and function associated with knee OA.37 Another recent systematic review and meta-analysis analyzing multiple pharmacologic treatments found an association between celecoxib and decreased pain from knee OA.39 However, this study also concluded that uncertainty surrounded all of the estimates of effect size for change in pain compared to placebo for all of the pharmacologic treatments included in the study.39
A meta-analysis of RCTs comparing celecoxib to no treatment, placebo, naproxen, and diclofenac concluded that celecoxib is slightly better than placebo and the aforementioned NSAIDs in reducing pain and improving function in general OA. However, the authors had reservations regarding pharmaceutical industry involvement in the studies and overall limited data.40
With all of that said, the American Academy of Orthopaedic Surgeons (AAOS) recommends strongly for the use of oral NSAIDs in the management of knee OA.41
Continue to: Glucosamine and chondroitin
Glucosamine and chondroitin. Glucosamine and chondroitin are supplements that have gained popularity in the treatment of knee OA. These constituents are found naturally in articular cartilage, which explains the rationale for their use. Glucosamine and chondroitin (or a combination of the 2) are associated with few adverse effects, but the evidence to support their use in knee OA management is mixed.
One large double-blind RCT (the Glucosamine/Chondroitin Arthritis Intervention Trial [GAIT]) concluded that glucosamine, chondroitin, or the combination of the 2 did not have a significant effect on reducing pain from knee OA compared to placebo and did not slow structural joint disease.42 However, this same study found that in a subset of patients with moderate-to-severe knee OA, the combination of glucosamine and chondroitin was mildly effective in reducing pain.42
Multiple studies have shown either no benefit, inconsistent results, or limited benefit of glucosamine and chondroitin in the treatment of knee OA, with the patented crystalline form of glucosamine showing the most efficacy.43-47 The AAOS and the American College of Rheumatology (ACR) do not recommend glucosamine and chondroitin for knee OA management.10,41
In summary, the evidence for glucosamine, chondroitin, or a combination of the 2 for knee OA is mixed with likely limited benefit, but because they are associated with few adverse effects, patients may be offered a 3- to 6-month trial of these supplements if other effective options are exhausted.
Injections
Limited-quality evidence suggests that oral NSAIDs and intra-articular (IA) hyaluronic acid (HA) injections are equally efficacious for knee OA pain.38,48 There is insufficient evidence directly comparing oral NSAIDs with IA corticosteroid (CS) injections.
Continue to: HA is found naturally...
HA is found naturally in articular cartilage, which explains the rationale behind its use. A network meta-analysis performed by the American Medical Society for Sports Medicine concluded that knee OA is more likely to respond to IAHA than to IACS or IA placebo, leading the society to recommend the use of IAHA in knee OA management, especially for patients > 60 years with mild-to-moderate knee OA.9 Conversely, the AAOS does not recommend the use of IAHA, and the ACR does not recommend for or against the use of IAHA.10,41
IACSs are commonly used to provide pain relief in those with moderate-to-severe knee OA. There is evidence that a single IACS injection provides mild pain relief for up to 6 weeks.49 However, there is some concern that repetitive IACS injections may speed cartilage loss. A 2-year randomized double-blind placebo-controlled trial comparing the effectiveness of repetitive IA triamcinolone vs saline in knee OA found no difference in pain severity and concluded that there was greater cartilage volume loss in the triamcinolone group.50
AAOS does not recommend for or against the use of IACSs, whereas the ACR does recommend for the use of IACSs.10,41 Given the available evidence, conservative use of IACS injections remains an option for patients with refractory moderate-to-severe knee OA.
Topicals
Topical analgesics are often utilized for knee OA because of their efficacy, tolerability, low risk of adverse effects, and ease of use. They are generally recommended over oral NSAIDs in the elderly and in individuals at risk for cardiac, renal, and gastrointestinal complications from oral NSAIDs.
One review found that topical diclofenac and topical ketoprofen were comparable to the oral forms of these medications.51 One RCT concluded that topical and oral diclofenac were equally efficacious in treating knee OA symptoms, although topical diclofenac was associated with significantly fewer gastrointestinal adverse effects.52 In multiple randomized trials, topical diclofenac has shown efficacy compared to placebo.53-55 A recent systematic review and meta-analysis of RCTs concluded that topical NSAIDs were safe and effective for treating general OA compared to placebo, with diclofenac patches most effective for pain relief and piroxicam most effective for functional improvement.56
Continue to: Topical capsaicin has shown...
Topical capsaicin has shown some efficacy in treating pain associated with knee OA.57 One meta-analysis of RCTs concluded that topical NSAIDs and capsaicin may be equally efficacious for OA-associated pain relief, although none of the RCTs directly compared the two.58 The major limitation of capsaicin is a patient-reported mild-to-moderate burning sensation with application that may decrease compliance.
Emerging treatments: IA PRP & extended-release IA triamcinolone acetonide
IA platelet-rich plasma (PRP) has been investigated for efficacy in treating knee OA. PRP is thought to decrease inflammation in the joint, although its exact mechanism remains unknown.59 Multiple studies have shown some benefit of PRP in reducing pain and improving function in individuals with knee OA, but nearly all of these studies have failed to show a clear benefit of PRP over HA injections.59-63 Additionally, the authors of most of these studies mention a high risk of bias. PRP therapy is expensive and generally is not covered by insurance companies, which precludes its use for many people.
Extended-release (ER) IA triamcinolone acetonide (Zilretta) has shown some superiority to standard IA triamcinolone acetonide in both degree and duration of pain relief for knee OA.64-66 The ER version tolerability did not differ from placebo and also showed prolonged synovial presence, lower systemic absorption, and lower blood glucose elevations compared with standard triamcinolone.64-66
Surgical intervention: A last resort
Select patients with severe pain and disability from knee OA that is refractory to conservative management options should be referred for consideration of knee arthroplasty. Age, weight, OA location, and degree of OA are all considered with respect to knee arthroplasty timing and technique.
There is good evidence that arthroscopy with debridement, on the other hand, is no more effective than conservative management.67
Continue to: Unicompartmental or "partial"...
Unicompartmental or “partial” knee replacements are reserved for select cases when 1 knee compartment has a significantly higher degree of degenerative change.
CASE After reviewing the therapeutic options with your patient, you agree that she will undergo a course of physical therapy and try using topical diclofenac along with a hinged knee brace. Because of the patient’s age and co-morbidities of cardiovascular disease and mild chronic kidney disease, oral NSAIDs are avoided at this time.
The patient returns to the office in 2 months reporting mild improvement in her pain. To provide additional pain relief, an ultrasound-guided IA steroid injection is attempted. The patient also continues home physical therapy, activity modification, topical diclofenac, and use of a hinged knee brace.
She returns to the office 2 months later, reporting continued improvement in her pain. No further intervention is undertaken at this time.
CORRESPONDENCE
Ryan A. Sprouse, MD, CAQSM, West Virginia University School of Medicine–Eastern Campus, WVU Medicine Orthopaedics and Sports Medicine, 912 Somerset Boulevard, Charles Town, WV 25414; [email protected].
1. Wallace IJ, Worthington S,Felson DT, et al. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc Natl Acad Sci. 2017;114:9332-9336.
2. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35.
3. Vina ER, Kwoh CK. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol. 2018;30:160-167.
4. Warner SC, Valdes AM. Genetic association studies in osteoarthritis: is it fairytale? Curr Opin Rheumatol. 2017;29:103-109.
5. Srikanth VK, Fryer JL, Zhai G, et al. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. 2005;13:769-781.
6. Palazzo C, Nguyen C, Lefevre-Colau MM, et al. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med. 2016;59:134-138.
7. Tanamas S, Hanna FS, Cicuttini FM, et al. Does knee malalignment increase the risk of development and progression of knee osteoarthritis? A systematic review. Arthritis Rheum. 2009;61:459-467.
8. Yucesoy B, Charles LE, Baker B, et al. Occupational and genetic risk factors for osteoarthritis: a review. Work. 2015;50:261-273.
9. Trojian TH, Concoff AL, Joy SM, et al. AMSSM scientific statement concerning viscosupplementation injections for knee osteoarthritis: importance for individual patient outcomes. Br J Sports Med. 2016;50:84-92.
10. Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 Recommendations for the Use of Nonpharmacologic and Pharmacologic Therapies in Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2012;64:465-474.
11. Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord. 2008;9:116.
12. Felson DT, Anderson JJ, Naimark A, et al. Obesity and knee osteoarthritis. The Framingham Study. Ann Intern Med. 1988;109:18-24.
13. Yusuf E, Bijsterbosch J, Slagboom PE, et al. Body mass index and alignment and their interaction as risk factors for progression of knees with radiographic signs of osteoarthritis. Osteoarthritis Cartilage. 2011;19:1117-1122.
14. Niu J, Zhang YQ, Torner J, et al. Is obesity a risk factor for progressive radiographic knee osteoarthritis? Arthritis Rheum. 2009;61:329-335.
15. Messier SP, Gutekunst DJ, Davis C, et al. Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis Rheum. 2005;52:2026-2032.
16. Messier SP, Mihalko SL, Legault C, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310:1263-1273.
17. Fransen M, McConnell S, Harmer AR, et al. Exercise for osteoarthritis of the knee: a Cochrane systematic review. Br J Sports Med.
18. Kan L, Zhang J, Yang Y, et al. The effects of yoga on pain, mobility, and quality of life in patients with knee osteoarthritis: a systematic review. Evid Based Complement Alternat Med. 2016;2016:6016532.
19. Chang WD, Chen S, Lee CL, et al. The effects of tai chi chuan on improving mind-body health for knee osteoarthritis patients: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2016;2016:1813979.
20. Takacs J, Krowchuk NM, Garland SJ, et al. Dynamic balance training improves physical function in individuals with knee osteoarthritis: a pilot randomized controlled trial. Arch Phys Med Rehabil. 2017;98:1586-1593.
21. Bartels EM, Juhl CB, Christensen R, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016;(3):CD005523.
22. Hinman RS, Heywood SE, Day AR. Aquatic physical therapy for hip and knee osteoarthritis: results of a single-blind randomized controlled trial. Phys Ther. 2007;87:32-43.
23. Tanaka R, Ozawa J, Kito N, et al. Efficacy of strengthening or aerobic exercise on pain relief in people with knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Clin Rehabil. 2013;27:1059-1071.
24. Knoop J, Steultjens MP, Roorda LD, et al. Improvement in upper leg muscle strength underlies beneficial effects of exercise therapy in knee osteoarthritis: secondary analysis from a randomised controlled trial. Physiotherapy. 2015;101:171-177.
25. Mat S, Tan MP, Kamaruzzaman SB, et al. Physical therapies for improving balance and reducing falls risk in osteoarthritis of the knee: a systematic review. Age Ageing. 2015;44:16-24.
26. Peeler J, Christian M, Cooper J, et al. Managing knee osteoarthritis: the effects of body weight supported physical activity on joint pain, function, and thigh muscle strength. Clin J Sport Med. 2015;25:518-523.
27. Peeler J, Ripat J. The effect of low-load exercise on joint pain, function, and activities of daily living in patients with knee osteoarthritis. Knee. 2018;25:135-145.
28. Takacs J, Anderson JE, Leiter JR, et al. Lower body positive pressure: an emerging technology in the battle against knee osteoarthritis? Clin Interv Aging. 2013;8:983-991.
29. Duivenvoorden T, Brouwer RW, van Raaij TM, et al. Braces and orthoses for treating osteoarthritis of the knee. Cochrane Database Syst Rev. 2015;(3):CD004020.
30. Gohal C, Shanmugaraj A, Tate P, et al. Effectiveness of valgus offloading knee braces in the treatment of medial compartment knee osteoarthritis: a systematic review. Sports Health. 2018;10:500-514.
31. Brouwer RW, van Raaij TM, Verhaar JA, et al. Brace treatment for osteoarthritis of the knee: a prospective randomized multi-centre trial. Osteoarthritis Cartilage. 2006;14:777-783.
32. Lee PY, Winfield TG, Harris SR, et al. Unloading knee brace is a cost-effective method to bridge and delay surgery in unicompartmental knee arthritis. BMJ Open Sport Exerc Med. 2017;2:e000195.
33. Wilson B, Rankin H, Barnes CL. Long-term results of an unloader brace in patients with unicompartmental knee osteoarthritis. Orthopedics. 2011;34:334-347.
34. Cudejko T, van der Esch M, van der Leeden M, et al. Effect of soft braces on pain and physical function in patients with knee osteoarthritis: systematic review with meta-analyses. Arch Phys Med Rehabil. 2018;99:153-163.
35. Cudejko T, van der Esch M, van den Noort JC. Decreased pain and improved dynamic knee instability mediate the beneficial effect of wearing a soft knee brace on activity limitations in persons with knee osteoarthritis. Arthritis Care Res (Hoboken). 2019;71:1036-1043.
36. Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225.
37. da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2017;390:e21-e33.
38. Bannuru RR, Schmid CH, Kent DM, et al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162:46-54.
39. Gregori D, Giacovelli G, Minto C, et al. Association of pharmacological treatments with long-term pain control in patients with knee osteoarthritis: a systematic review and meta-analysis. JAMA. 2018;320:2564-2579.
40. Puljak L, Marin A, Vrdoljak D, et al. Celecoxib for osteoarthritis. Cochrane Database Syst Rev. 2017;(5):CD009865.
41. Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;9:571-576.
42. Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354:795-808.
43. Singh JA, Noorbaloochi S, MacDonald R, et al. Chondroitin for osteoarthritis. Cochrane Database Syst Rev. 2015;(1):CD005614.
44. Yang S, Eaton CB, McAlindon TE, et al. Effects of glucosamine and chondroitin on treating knee osteoarthritis: an analysis with marginal structural models. Arthritis Rheumatol. 2015;67:714-723.
45. Ogata T, Yuki Ideno Y, Masami Akai M,et al. Effects of glucosamine in patients with osteoarthritis of the knee: a systematic review and meta-analysis. Clin Rheumatol. 2018;37:2479-2487.
46. Towheed TE, Maxwell L, Anastassiades TP, et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev. 2009;(2):CD002946.
47. Bruyèreetal O, Cooper C, Pelletier JP, et al. A consensus statement on the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) algorithm for the management of knee osteoarthritis—from evidence-based medicine to the real-life setting. Semin Arthritis Rheum. 2016;45(4 suppl):S3-S11.
48. Ishijima M, Nakamura T, Shimizu K, et al. Intra-articular hyaluronic acid injection versus oral non-steroidal anti-inflammatory drug for the treatment of knee osteoarthritis: a multi-center, randomized, open-label, non-inferiority trial. Arthritis Res Ther. 2014;16:R18.
49. Juni P, Hari R, Rutjes AW, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev. 2015;(10):CD005328.
50. McAlindon TE, LaValley MP, Harvey FW, et al. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: a randomized clinical trial. JAMA. 2017;317:1967-1975.
51. Derry S, Conaghan P, Da Silva JA, et al. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2016;(4):CD007400.
52. Tugwell PS, Wells GA, Shainhouse JZ. Equivalence study of a topical diclofenac solution (pennsaid) compared with oral diclofenac in symptomatic treatment of osteoarthritis of the knee: a randomized controlled trial. J Rheumatol. 2004;31:2002-2012.
53. Wadsworth LT, Kent JD, Holt RJ. Efficacy and safety of diclofenac sodium 2% topical solution for osteoarthritis of the knee: a randomized, double-blind, vehicle-controlled, 4 week study. Curr Med Res Opin. 2016;32:241-250.
54. Roth SH, Shainhouse JZ. Efficacy and safety of a topical diclofenac solution (pennsaid) in the treatment of primary osteoarthritis of the knee: a randomized, double-blind, vehicle-controlled clinical trial. Arch Intern Med. 2004;164:2017-2023.
55. Baer PA, Thomas LM, Shainhouse Z. Treatment of osteoarthritis of the knee with a topical diclofenac solution: a randomised controlled, 6-week trial. BMC Musculoskelet Disord. 2005;6:44.
56. Zeng C, Wei J, Persson MSM, et al. Relative efficacy and safety of topical non-steroidal anti-inflammatory drugs for osteoarthritis: a systematic review and network meta-analysis of randomised controlled trials and observational studies. Br J Sports Med. 2018;52:642-650.
57. Guedes V, Castro JP, Brito I. Topical capsaicin for pain in osteoarthritis: a literature review. Reumatol Clin. 2018;14:40-45.
58. Persson MSM, Stocks J, Walsh DA, et al. The relative efficacy of topical non-steroidal anti-inflammatory drugs and capsaicin in osteoarthritis: a network meta-analysis of randomised controlled trials. Osteoarthritis Cartilage. 2018;26:1575-1582.
59. Cole BJ, Karas V, Hussey K, et al. Hyaluronic acid versus platelet-rich plasma: a prospective, double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am J Sports Med. 2017;45:339-346.
60. Laudy AB, Bakker EW, Rekers M, et al. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med. 2015;49:657-672.
61. Han Y, Huang H, Pan J, et al. Meta-analysis comparing platelet-rich plasma vs hyaluronic acid injection in patients with knee osteoarthritis. Pain Med. 2019;20:1418-1429.
62. Filardo G, Di Matteo B, Di Martino A, et al. Platelet-rich plasma intra-articular knee injections show no superiority versus viscosupplementation: a randomized controlled trial. Am J Sports Med. 2015;43:1575-1582.
63. Di Martino A, Di Matteo B, Papio T, et al. Platelet-rich plasma versus hyaluronic acid injections for the treatment of knee osteoarthritis: results at 5 years of a double-blind, randomized controlled trial. Am J Sports Med. 2019;47:347-354.
64. Bodick N, Lufkin J, Willwerth C, et al. An intra-articular, extended-release formulation of triamcinolone acetonide prolongs and amplifies analgesic effect in patients with osteoarthritis of the knee: a randomized clinical trial. J Bone Joint Surg Am. 2015;97:877-888.
65. Conaghan PG, Cohen SB, Berenbaum F, et al. Brief report: a phase IIb trial of a novel extended-release microsphere formulation of triamcinolone acetonide for intraarticular injection in knee osteoarthritis. Arthritis Rheumatol. 2018;70:204-211.
66. Conaghan PG, Hunter DJ, Cohen SB, et al. Effects of a single intra-articular injection of a microsphere formulation of triamcinolone acetonide on knee osteoarthritis pain: a double-blinded, randomized, placebo-controlled, multinational study. J Bone Jt Surg Am. 2018;100:666–677.
67. Thorlund JB, Juhl CB, Roos EM, et al. Arthroscopic surgery for degenerative knee: systematic review and meta-analysis of benefits and harms. BMJ. 2015;350:h2747.
1. Wallace IJ, Worthington S,Felson DT, et al. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc Natl Acad Sci. 2017;114:9332-9336.
2. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35.
3. Vina ER, Kwoh CK. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol. 2018;30:160-167.
4. Warner SC, Valdes AM. Genetic association studies in osteoarthritis: is it fairytale? Curr Opin Rheumatol. 2017;29:103-109.
5. Srikanth VK, Fryer JL, Zhai G, et al. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. 2005;13:769-781.
6. Palazzo C, Nguyen C, Lefevre-Colau MM, et al. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med. 2016;59:134-138.
7. Tanamas S, Hanna FS, Cicuttini FM, et al. Does knee malalignment increase the risk of development and progression of knee osteoarthritis? A systematic review. Arthritis Rheum. 2009;61:459-467.
8. Yucesoy B, Charles LE, Baker B, et al. Occupational and genetic risk factors for osteoarthritis: a review. Work. 2015;50:261-273.
9. Trojian TH, Concoff AL, Joy SM, et al. AMSSM scientific statement concerning viscosupplementation injections for knee osteoarthritis: importance for individual patient outcomes. Br J Sports Med. 2016;50:84-92.
10. Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 Recommendations for the Use of Nonpharmacologic and Pharmacologic Therapies in Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2012;64:465-474.
11. Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord. 2008;9:116.
12. Felson DT, Anderson JJ, Naimark A, et al. Obesity and knee osteoarthritis. The Framingham Study. Ann Intern Med. 1988;109:18-24.
13. Yusuf E, Bijsterbosch J, Slagboom PE, et al. Body mass index and alignment and their interaction as risk factors for progression of knees with radiographic signs of osteoarthritis. Osteoarthritis Cartilage. 2011;19:1117-1122.
14. Niu J, Zhang YQ, Torner J, et al. Is obesity a risk factor for progressive radiographic knee osteoarthritis? Arthritis Rheum. 2009;61:329-335.
15. Messier SP, Gutekunst DJ, Davis C, et al. Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis Rheum. 2005;52:2026-2032.
16. Messier SP, Mihalko SL, Legault C, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310:1263-1273.
17. Fransen M, McConnell S, Harmer AR, et al. Exercise for osteoarthritis of the knee: a Cochrane systematic review. Br J Sports Med.
18. Kan L, Zhang J, Yang Y, et al. The effects of yoga on pain, mobility, and quality of life in patients with knee osteoarthritis: a systematic review. Evid Based Complement Alternat Med. 2016;2016:6016532.
19. Chang WD, Chen S, Lee CL, et al. The effects of tai chi chuan on improving mind-body health for knee osteoarthritis patients: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2016;2016:1813979.
20. Takacs J, Krowchuk NM, Garland SJ, et al. Dynamic balance training improves physical function in individuals with knee osteoarthritis: a pilot randomized controlled trial. Arch Phys Med Rehabil. 2017;98:1586-1593.
21. Bartels EM, Juhl CB, Christensen R, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016;(3):CD005523.
22. Hinman RS, Heywood SE, Day AR. Aquatic physical therapy for hip and knee osteoarthritis: results of a single-blind randomized controlled trial. Phys Ther. 2007;87:32-43.
23. Tanaka R, Ozawa J, Kito N, et al. Efficacy of strengthening or aerobic exercise on pain relief in people with knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Clin Rehabil. 2013;27:1059-1071.
24. Knoop J, Steultjens MP, Roorda LD, et al. Improvement in upper leg muscle strength underlies beneficial effects of exercise therapy in knee osteoarthritis: secondary analysis from a randomised controlled trial. Physiotherapy. 2015;101:171-177.
25. Mat S, Tan MP, Kamaruzzaman SB, et al. Physical therapies for improving balance and reducing falls risk in osteoarthritis of the knee: a systematic review. Age Ageing. 2015;44:16-24.
26. Peeler J, Christian M, Cooper J, et al. Managing knee osteoarthritis: the effects of body weight supported physical activity on joint pain, function, and thigh muscle strength. Clin J Sport Med. 2015;25:518-523.
27. Peeler J, Ripat J. The effect of low-load exercise on joint pain, function, and activities of daily living in patients with knee osteoarthritis. Knee. 2018;25:135-145.
28. Takacs J, Anderson JE, Leiter JR, et al. Lower body positive pressure: an emerging technology in the battle against knee osteoarthritis? Clin Interv Aging. 2013;8:983-991.
29. Duivenvoorden T, Brouwer RW, van Raaij TM, et al. Braces and orthoses for treating osteoarthritis of the knee. Cochrane Database Syst Rev. 2015;(3):CD004020.
30. Gohal C, Shanmugaraj A, Tate P, et al. Effectiveness of valgus offloading knee braces in the treatment of medial compartment knee osteoarthritis: a systematic review. Sports Health. 2018;10:500-514.
31. Brouwer RW, van Raaij TM, Verhaar JA, et al. Brace treatment for osteoarthritis of the knee: a prospective randomized multi-centre trial. Osteoarthritis Cartilage. 2006;14:777-783.
32. Lee PY, Winfield TG, Harris SR, et al. Unloading knee brace is a cost-effective method to bridge and delay surgery in unicompartmental knee arthritis. BMJ Open Sport Exerc Med. 2017;2:e000195.
33. Wilson B, Rankin H, Barnes CL. Long-term results of an unloader brace in patients with unicompartmental knee osteoarthritis. Orthopedics. 2011;34:334-347.
34. Cudejko T, van der Esch M, van der Leeden M, et al. Effect of soft braces on pain and physical function in patients with knee osteoarthritis: systematic review with meta-analyses. Arch Phys Med Rehabil. 2018;99:153-163.
35. Cudejko T, van der Esch M, van den Noort JC. Decreased pain and improved dynamic knee instability mediate the beneficial effect of wearing a soft knee brace on activity limitations in persons with knee osteoarthritis. Arthritis Care Res (Hoboken). 2019;71:1036-1043.
36. Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225.
37. da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2017;390:e21-e33.
38. Bannuru RR, Schmid CH, Kent DM, et al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162:46-54.
39. Gregori D, Giacovelli G, Minto C, et al. Association of pharmacological treatments with long-term pain control in patients with knee osteoarthritis: a systematic review and meta-analysis. JAMA. 2018;320:2564-2579.
40. Puljak L, Marin A, Vrdoljak D, et al. Celecoxib for osteoarthritis. Cochrane Database Syst Rev. 2017;(5):CD009865.
41. Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;9:571-576.
42. Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354:795-808.
43. Singh JA, Noorbaloochi S, MacDonald R, et al. Chondroitin for osteoarthritis. Cochrane Database Syst Rev. 2015;(1):CD005614.
44. Yang S, Eaton CB, McAlindon TE, et al. Effects of glucosamine and chondroitin on treating knee osteoarthritis: an analysis with marginal structural models. Arthritis Rheumatol. 2015;67:714-723.
45. Ogata T, Yuki Ideno Y, Masami Akai M,et al. Effects of glucosamine in patients with osteoarthritis of the knee: a systematic review and meta-analysis. Clin Rheumatol. 2018;37:2479-2487.
46. Towheed TE, Maxwell L, Anastassiades TP, et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev. 2009;(2):CD002946.
47. Bruyèreetal O, Cooper C, Pelletier JP, et al. A consensus statement on the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) algorithm for the management of knee osteoarthritis—from evidence-based medicine to the real-life setting. Semin Arthritis Rheum. 2016;45(4 suppl):S3-S11.
48. Ishijima M, Nakamura T, Shimizu K, et al. Intra-articular hyaluronic acid injection versus oral non-steroidal anti-inflammatory drug for the treatment of knee osteoarthritis: a multi-center, randomized, open-label, non-inferiority trial. Arthritis Res Ther. 2014;16:R18.
49. Juni P, Hari R, Rutjes AW, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev. 2015;(10):CD005328.
50. McAlindon TE, LaValley MP, Harvey FW, et al. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: a randomized clinical trial. JAMA. 2017;317:1967-1975.
51. Derry S, Conaghan P, Da Silva JA, et al. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2016;(4):CD007400.
52. Tugwell PS, Wells GA, Shainhouse JZ. Equivalence study of a topical diclofenac solution (pennsaid) compared with oral diclofenac in symptomatic treatment of osteoarthritis of the knee: a randomized controlled trial. J Rheumatol. 2004;31:2002-2012.
53. Wadsworth LT, Kent JD, Holt RJ. Efficacy and safety of diclofenac sodium 2% topical solution for osteoarthritis of the knee: a randomized, double-blind, vehicle-controlled, 4 week study. Curr Med Res Opin. 2016;32:241-250.
54. Roth SH, Shainhouse JZ. Efficacy and safety of a topical diclofenac solution (pennsaid) in the treatment of primary osteoarthritis of the knee: a randomized, double-blind, vehicle-controlled clinical trial. Arch Intern Med. 2004;164:2017-2023.
55. Baer PA, Thomas LM, Shainhouse Z. Treatment of osteoarthritis of the knee with a topical diclofenac solution: a randomised controlled, 6-week trial. BMC Musculoskelet Disord. 2005;6:44.
56. Zeng C, Wei J, Persson MSM, et al. Relative efficacy and safety of topical non-steroidal anti-inflammatory drugs for osteoarthritis: a systematic review and network meta-analysis of randomised controlled trials and observational studies. Br J Sports Med. 2018;52:642-650.
57. Guedes V, Castro JP, Brito I. Topical capsaicin for pain in osteoarthritis: a literature review. Reumatol Clin. 2018;14:40-45.
58. Persson MSM, Stocks J, Walsh DA, et al. The relative efficacy of topical non-steroidal anti-inflammatory drugs and capsaicin in osteoarthritis: a network meta-analysis of randomised controlled trials. Osteoarthritis Cartilage. 2018;26:1575-1582.
59. Cole BJ, Karas V, Hussey K, et al. Hyaluronic acid versus platelet-rich plasma: a prospective, double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am J Sports Med. 2017;45:339-346.
60. Laudy AB, Bakker EW, Rekers M, et al. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med. 2015;49:657-672.
61. Han Y, Huang H, Pan J, et al. Meta-analysis comparing platelet-rich plasma vs hyaluronic acid injection in patients with knee osteoarthritis. Pain Med. 2019;20:1418-1429.
62. Filardo G, Di Matteo B, Di Martino A, et al. Platelet-rich plasma intra-articular knee injections show no superiority versus viscosupplementation: a randomized controlled trial. Am J Sports Med. 2015;43:1575-1582.
63. Di Martino A, Di Matteo B, Papio T, et al. Platelet-rich plasma versus hyaluronic acid injections for the treatment of knee osteoarthritis: results at 5 years of a double-blind, randomized controlled trial. Am J Sports Med. 2019;47:347-354.
64. Bodick N, Lufkin J, Willwerth C, et al. An intra-articular, extended-release formulation of triamcinolone acetonide prolongs and amplifies analgesic effect in patients with osteoarthritis of the knee: a randomized clinical trial. J Bone Joint Surg Am. 2015;97:877-888.
65. Conaghan PG, Cohen SB, Berenbaum F, et al. Brief report: a phase IIb trial of a novel extended-release microsphere formulation of triamcinolone acetonide for intraarticular injection in knee osteoarthritis. Arthritis Rheumatol. 2018;70:204-211.
66. Conaghan PG, Hunter DJ, Cohen SB, et al. Effects of a single intra-articular injection of a microsphere formulation of triamcinolone acetonide on knee osteoarthritis pain: a double-blinded, randomized, placebo-controlled, multinational study. J Bone Jt Surg Am. 2018;100:666–677.
67. Thorlund JB, Juhl CB, Roos EM, et al. Arthroscopic surgery for degenerative knee: systematic review and meta-analysis of benefits and harms. BMJ. 2015;350:h2747.
PRACTICE RECOMMENDATIONS
› Treat pain from knee osteoarthritis (OA) with weight management and low-impact exercise to decrease the risk of disease progression. A
› Prescribe oral or topical nonsteroidal anti-inflammatory drugs to relieve pain from knee OA, as both forms are equally effective. B
› Recommend a medial unloading (valgus) knee brace for short-term relief of medial knee OA. B
› Consider a trial of intra-articular corticosteroids or intra-articular hyaluronic acid derivatives for short-term relief of knee OA pain. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Sport-related concussion: How best to help young athletes
› Require athletes who sustain a concussion to wait a minimum of 7 to 10 days before returning to full unrestricted activity. C
› Ensure that any player diagnosed with concussion follows a guided return-to-play progression, supervised by an athletic trainer or physical therapist experienced in post-concussion care. C
› Advise patients who are old enough to drive not to do so for at least 24 hours after a concussion. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Each year in the United States, more than 44 million young people participate in sports activities.1 Yet the number of concussions incurred annually by children and adolescents engaged in sports and recreational play has been underestimated for years, and largely unknown.1,2
Some estimates were based solely on the number of young athletes treated in emergency departments or sports concussion clinics. Others focused only on team players of middle school or high school age, excluding younger children who were hit in the head on playgrounds or during other recreational activities. What’s more, large numbers of concussions—as many as 4 in 10 incurred by high school athletes—were never reported to a coach or medical professional.3
In a new study published in the journal Pediatrics in June, researchers used national databases and current literature to provide what they believe to be “the most accurate and precise estimate of youth concussion” thus far: Between 1.1 and 1.9 million sports- and recreation-related concussions occur among US youth ages 18 or younger annually.1
Among young people playing team sports, concussions are between 2 and 7 times more likely to occur during competitive games than in practice sessions.4-7 Boys on football and ice hockey teams have the highest rates of concussion in young athletes.For overall number of concussions, however, girls on soccer teams are second only to football players.4 Female soccer players are more likely than male soccer players to sustain concussions during equal number of hours of play.4,7
An increase in incidence. The incidence of concussion among young athletes appears to have increased in the past decade, a likely result of greater involvement in team sports, an increasing focus on safeguarding young people from the potential dangers associated with a blow to the brain, and better diagnostic techniques.4,8-10 And a recent study based on data from electronic medical records at a large regional pediatric health care network found that more than three-quarters of young people with sports-related concussions were first seen in a primary care setting.2
With this in mind, we present a comprehensive update of the evidence regarding the diagnosis and management of sport-related concussion. The recommendations we include are consistent with professional association guidelines.8-10 Although we focus on concussion in children and adolescents involved in athletic activities, the principles generally apply to patients of all ages and to concussions that may not be sports related.
Removal from play: A vital first step
Whenever you conduct a physical exam for a young athlete, remind him or her—and the patient’s parents—that after a blow to the head, immediate removal from play is critical. Concussion is caused by a direct or indirect force to the brain that results in a transient disturbance in brain function,8-10 manifested by alterations in neurocognitive and motor function. While the signs and symptoms (TABLE 1)8-10 resolve within 10 days of injury in about 90% of cases, those who incur additional head impact within 24 hours have a higher symptom burden and prolonged recovery period.11 Even without repetitive impact, younger athletes may take longer to recover.8-10
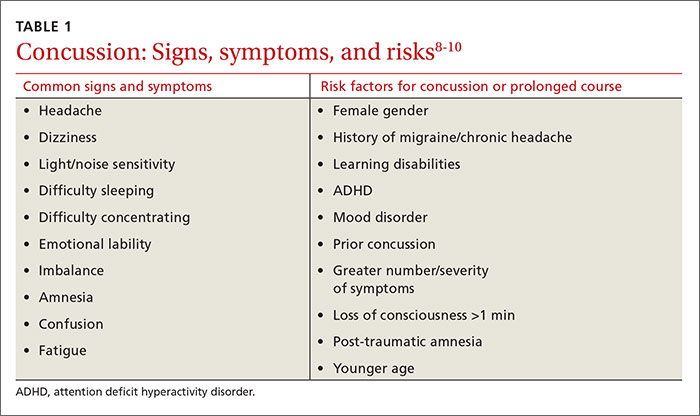
The initial assessment
A child or adolescent who sustains a suspected concussion should be seen by a physician within 24 to 48 hours. Whether the initial assessment occurs in your office or on the sidelines of a game, it is important to confirm the time the incident occurred and the mechanism of injury.
Concussion is diagnosed by a combination of history, physical exam, and objective testing when symptoms or exam findings associated with mild brain trauma—headache, dizziness, light and/or noise sensitivity, among others—closely follow a head injury.8-10 Certain maneuvers—assessing eye movements by asking the athlete to look in various directions, for instance, then to follow a pen or finger as you move it closer to his or her face—may provoke dizziness, headache, or other symptoms of concussion that were not apparent initially.
The differential diagnosis includes cervical musculoskeletal injury, craniofacial injury, epidural and subdural hematoma, heat-related illness, uncomplicated headache and migraine, upper respiratory infection, and vertigo.8-10
Tools aid in diagnosis
Many clinical assessment tools exist to aid in the diagnosis of concussion (TABLE 2).8-10,12-14 Any one of these tools, many of which use combinations of symptom checklists, balance exams, and cognitive assessments, may be included in your evaluation. No single tool has been found to be superior to any other.8-10 A combination of tools may improve diagnostic accuracy, but assessment tools should not be the sole basis used to diagnose or rule out concussion.
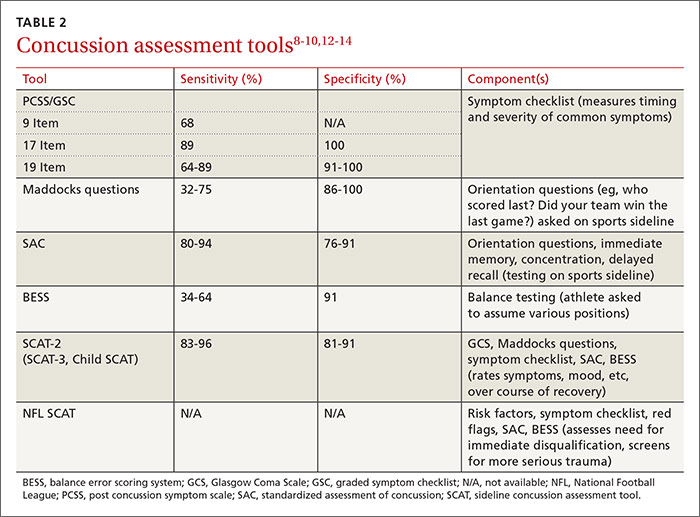
Any child or adolescent who had a blow to the head and at least one sign or symptom of concussion should be evaluated as soon as possible and assessed again later that day or the next day if any reason for concern remains.
Neuropsychological (NP) testing may involve computerized tests developed specifically for athletes. Patients may be required to react to objects that appear on a screen, for example, in a way that tests memory, performance, and reaction time. Because cognitive recovery often lags behind symptom resolution, NP testing may identify subtle brain deficits even in athletes who are asymptomatic at rest or with exercise. In general, NP testing has a sensitivity of 71% to 88% for athletes with concussion,10 but it is most beneficial when baseline test results are available. Interpretation of NP testing should be done only by qualified clinicians.
While NP testing may provide additional prognostic information, it should not alter the management of athletes who are symptomatic either at rest or with exercise.15 Nor is NP testing vital, as concussion can be accurately diagnosed and adequately managed without it.
Neuroimaging, including computed tomography (CT) and magnetic resonance imaging (MRI), is often used unnecessarily in the initial assessment of a patient who sustained a possible concussion.8-10 In fact, neuroimaging should be reserved for cases in which it is necessary to rule out more serious pathology: intracranial or subdural hematoma or a craniofacial injury, for example, in patients with clinical findings that are red flags. These red flags include focal neurologic deficits, continuing nausea/vomiting, or persistent disorientation (TABLE 3),8-10 or symptoms that worsen or persist beyond a few weeks. In such cases, further evaluation—with MRI of the brain, formal NP testing, and/or referral to a neurologist, physiatrist, or other physician who specializes in concussion care—is indicated.

Concussion management: Rest is key
While there is a dearth of high-quality studies on the management of sport-related concussion across all age groups, standardized protocols for both children and adults have been adopted in most clinical settings.8-10,16,17 The protocols provide a framework for an individualized treatment plan. Yet their use among primary care physicians is inconsistent.18-20
Traditionally, concussion management begins with relative physical and cognitive rest to allow the brain time to recover.8-10 Recent randomized controlled trials have challenged this premise by suggesting that mild to moderate physical activity for post-concussion patients who are mildly symptomatic does not adversely affect recovery.21,22 These studies have significant limitations, however, and further research is needed to provide specific guidance on this aspect of concussion management before it is adopted.
Physical restrictions include organized sports, recreational activity, recess, and physical education classes. Walking is permitted unless it exacerbates symptoms. These restrictions should continue until the patient is symptom-free.
Cognitive restrictions include modifications at school and at home. Once an athlete is able to concentrate and tolerate visual and auditory stimuli, he or she may return to school. But classroom modifications should be considered, possibly including shortened school days, extra time for testing and homework, help with note taking, and restrictions from classes likely to provoke symptoms, such as computer science or music. Limiting use of mobile devices, television viewing, noisy environments, and other possible provocations may help speed symptom resolution. These restrictions, too, should remain in place until the patient is symptom-free.
Driving is often not addressed by physicians managing the care of athletes with concussion, but evidence suggests it should be. A study of patients presenting to the emergency department found that within 24 hours of a concussion diagnosis, individuals had an impaired response to traffic hazards.23,24 And Canadian clinical practice guidelines recommend that athletes with mild traumatic brain injury (TBI) avoid driving within the first 24 hours.25
While American guidelines are silent on the question of driving for this patient population, we recommend that athletes with concussion be restricted from driving and engaging in other risky complex tasks, such as welding or shop class, for at least 24 hours. For many athletes diagnosed with concussion, driving restrictions of longer duration may be necessary based on their symptom profile and neurocognitive test results. Continued dizziness or visual deficits would pose a greater risk than fatigue or short-term memory loss, for example.
Overseeing the return to play
Return-to-activity progression follows a stepwise protocol, with 6 steps that the injured athlete must complete before resuming full activity (FIGURE 1A).8-10 This stepwise progression begins only when athletes are symptom free, even during provocative maneuvers; have had a normal neurologic exam, are back to school full time with no restriction; are off any medications prescribed for concussion symptoms (TABLE 4),8-10 and when neurocognitive testing, if performed, is back to baseline. If an athlete develops symptoms at any stage of the progression, rest is required until he or she remains asymptomatic for at least 24 hours. The progression is then restarted at the last stage at which the patient was symptom free.
Some individualization, of course, is recommended here, too. Younger athletes and those with a prior history of concussion may require 10 days or more to complete all the steps, allowing an extra day at various steps. Neurologic maturation affects recovery time, and for younger individuals, a more conservative return-to-play protocol based on initial concussion symptom duration has been proposed (FIGURE 1B).16
Return to activity is often supervised by a certified athletic trainer at the athlete’s school. In the event that no athletic trainer is available, patients may be referred to physical therapists with experience in monitoring injured athletes.26 Anyone involved in the patient’s care, including the athlete himself, may use a symptom checklist to monitor recovery.
Although there is no evidence that the ongoing use of a symptom checklist affects the course of recovery, its use is often helpful in identifying specific symptoms that can be managed by means other than physical and cognitive rest—a sleep hygiene program for an individual with lingering difficulty sleeping, for example, or the continued application of ice, heat, and massage for persistent neck pain.
Checklist monitoring may be especially helpful for athletes whose symptoms extend beyond 10 days or who have multiple symptoms. Final clearance once all the steps have been completed requires follow-up with a health care provider.
Is a symptom-free waiting period necessary?
There is no evidence suggesting a need for a symptom-free waiting period before starting the return-to-play protocol.10,27 Because a repeat concussion is most likely within 7 to 10 days of the initial injury,8,9 however, most athletes should not return to contact play during that time frame, regardless of symptom resolution.
It is helpful to have asymptomatic athletes participate in non-contact activity before the 7 to 10 days are up, however. Doing so can help prevent deconditioning and injury upon return to contact sport, as there is evidence of increased risk of lower-extremity injury in the 90 days after concussion.28
What to tell athletes—and parents—about repetitive head trauma
There is growing concern about the long-term risks of concussion and repetitive head impact that may manifest as chronic traumatic encephalopathy (CTE) and chronic neurocognitive impairment (CNI) later in life. Indeed, some data strongly suggest—but do not definitively prove—a relationship between repetitive head injury and chronic neurodegenerative disease.8-10 You can tell worried patients or parents, however, that the majority of research on CTE and CNI has been based on professional football players.
Studies of long-term effects of soccer heading have shown conflicting results, with some finding cognitive impairment, altered postural control, and anatomic changes of the brain, while others found no effect on encephalopathy, concussion symptoms, or neurocognitive performance.29-36Here, too, most studies showing negative effects of soccer heading involved professional athletes.
Repetitive sub-concussive impact in high school football athletes has been found to induce biochemical changes to the brain,37 but the long-term effects are unknown. And, while concussion in high school athletes has been associated with short-term cognitive impairment, altered neurochemistry, and evidence of increased symptoms on baseline neurocognitive testing,8-10,38 no studies have linked concussion during middle school or high school with CNI. What’s more, a long-term (50-year) follow-up study of individuals who played football in high school found no difference in rates of neurodegenerative disease compared with age-matched controls.39
A new study of high school and college football players (mean age: 17.4 years) presented at the American Academy of Neurology 2016 Sports Concussion Conference in Chicago in July, however, found significant alterations in white matter 6 months post injury.40 The researchers compared 17 athletes with sport-related concussion with matched controls, using diffusion tensor imaging and diffusion kurtosis tensor imaging as biomarkers of brain recovery. The concussed athletes underwent MRI and symptom assessment at 24 hours, 8 days, and 6 months. The controls followed identical protocols.
At the 6-month assessment, there were no differences between the concussed group and the controls in terms of self-reported concussion symptoms, cognition, or balance. However, the concussed athletes had widespread decreased mean diffusivity compared with the controls. Despite the lack of clinical symptoms, the concussed athletes showed significant alterations in white matter “that were related to initial symptom severity ratings,” the authors concluded. These findings have implications both for determination of recovery from concussion and concussion management, they added.40
Although there is no way to eliminate all concussions, limited evidence suggests that improving athletic technique, limiting contact at practices, better enforcement of game rules, and rule changes regarding physical contact may decrease concussion risk.41-43 Many youth sports organizations have developed policies placing restrictions on head impact during practices and games. Studies are ongoing, too, to see if better headgear—or requiring helmets for soccer players—makes a difference.
CORRESPONDENCE
Ryan A. Sprouse, MD, CAQSM, 203 East Fourth Avenue, Ranson, WV 25438; [email protected].
1. Bryan MA, Rowhani-Rahbar A, Comstock RD, et al. Sports- and recreation-related concussions in US youth. Pediatrics. 2016; June 20 [Epub ahead of print].
2. Arbogast KB, Curry AE, Pfeiffer MR, et al. Point of health care entry for youth with concussion within a large pediatric care network. JAMA Pediatr. 2016; May 31 [Epub ahead of print].
3. Mihalik JK, Guskiewicz KM, Valovich McLeod TC, et al. Knowledge, attitude, and concussion-reporting behaviors among high school athletes: a preliminary study. J Ath Tr. 2013;48:645-653.
4. Marar M, McIlvain NM, Fields SK, et al. Epidemiology of concussions among United States high school athletes in 20 sports. Am J Sports Med. 2012;40:747.
5. Kontos AP, Elbin RJ, Fazio-Sumrock VC. Incidence of sports-related concussion among youth football players aged 8-12 years. J Pediatr. 2013;163:717-720.
6. Dompier TP, Kerr ZY, Marshall SW, et al. Incidence of concussion during practice and games in youth, high school, and collegiate American football players. JAMA Pediatr. 2015;169:659-665.
7. Comstock RD, Currie DW, Pierpont LA, et al. An evidence-based discussion of heading the ball and concussions in high school soccer. JAMA Pediatr. 2015;169:830-837.
8. Harmon KG, Drezner JA, Gammons M, et al. American Medical Society for Sports Medicine position statement: concussion in sport. Br J Sports Med. 2013;47:15-26.
9. McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47:250-258.
10. Giza CC, Kutcher JS, Ashwal S, et al. Summary of the evidence-based guideline update: evaluation and management of concussion in sports: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80:2250-2257.
11. Terwilliger VK, Pratson L, Vaughan CG, et al. Additional post-concussion impact exposure may affect recovery in adolescent athletes. J Neurotrauma. 2016;33:761-765.
12. Putukian M, Echemendia R, Dettwiler-Danspeckgruber A. Prospective clinical assessment using Sideline Concussion Assessment Tool-2 testing in the evaluation of sport-related concussion in college athletes. Clin J Sport Med. 2015;25:36-42.
13. Broglio SP, Macciocchi SN, Ferrara MS. Sensitivity of the concussion assessment battery. Neurosurgery. 2007;60:1050-1057.
14. Randolph C, McCrea M, Barr WB. Is neuropsychological testing useful in the management of sport-related concussion? J Athl Train. 2005;40:139-152.
15. Shrier I. Neuropsychological testing and concussions: a reasoned approach. Clin J Sport Med. 2012;22:211-213.
16. DeMatteo C, Stazyk K, Singh SK, et al. Development of a conservative protocol to return children and youth to activity following concussive injury. Clin Pediatr (Phila). 2015;54:152-163.
17. Broglio SP, Cantu RC, Gioia GA, et al. National Athletic Trainers Association position statement: management of sport concussion. J Athl Train. 2014;49:245-265.
18. Stoller J, Carson JD, Garel A, et al. Do family physicians, emergency department physicians, and pediatricians give consistent sport-related concussion management advice? Can Fam Physician. 2014;60:548, 550-552.
19. Lebrun CM, Mrazik M, Prasad AS, et al. Sport concussion knowledge base, clinical practices and needs for continuing medical education: a survey of family physicians and cross-border comparison. Br J Sports Med. 2013;47:54-59.
20. Zemek R, Eady K, Moreau K, et al. Knowledge of paediatric concussion among front-line primary care providers. Paediatr Child Health. 2014;19:475-480.
21. Maerlender A, Rieman W, Lichtenstein J, et al. Programmed physical exertion in recovery from sports-related concussion: a randomized pilot study. Dev Neuropsychol. 2015;40:273-278.
22. Buckley TA, Munkasy BA, Clouse BP. Acute cognitive and physical rest may not improve concussion recovery time. J Head Trauma Rehabil. 2015; July 24 [Epub ahead of print].
23. Preece MH, Horswill MS, Langlois JA, et al. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21:375-378.
24. Baker A, Unsworth CA, Lannin NA. Fitness-to-drive after mild traumatic brain injury: mapping the time trajectory of recovery in the acute stages post injury. Accid Anal Prev. 2015;79:50-55.
25. Marshall S, Bayley M, McCullagh S, et al. Clinical practice guidelines for mild traumatic brain injury and persistent symptoms. Can Fam Physician. 2012;58:257-267.
26. Yorke AM, Littleton S, Alsalaheen BA. Concussion attitudes and beliefs, knowledge, and clinical practice: a survey of physical therapists. Phys Ther. Available at: http://dx.doi.org/10.2522/ptj.20140598. Accessed January 21, 2016.
27. McCrea M, Guskiewicz K, Randolph C, et al. Effects of a symptom-free waiting period on clinical outcome and risk of reinjury after sport-related concussion. Neurosurgery. 2009;65:876-883.
28. Brooks MA, Peterson K, Biese K, et al. Concussion increases odds of sustaining a lower extremity musculoskeletal injury after return to play among collegiate athletes. Am J Sports Med. 2016;44:742-747.
29. Witol AD, Webbe FM. Soccer heading frequency predicts neuropsychological deficits. Arch Clin Neuropsychol. 2003;18:397-417.
30. Haran FJ, Tierney R, Wright WG, et al. Acute changes in postural control after soccer heading. Int J Sports Med. 2013;34:350-354.
31. Lipton ML, Kim N, Zimmerman ME, et al. Soccer heading is associated with white matter microstructural and cognitive abnormalities. Radiology. 2013;268:850-857.
32. Jordan SE, Green GA, Galanty HL, et al. Acute and chronic brain injury in United States national team soccer players. Am J Sports Med. 1996;24:205-210.
33. Kontos AP, Dolese A, Elbin RJ, et al. Relationship of soccer heading to computerized neurocognitive performance and symptoms among female and male youth soccer players. Brain Inj. 2011;25:1234-1241.
34. Straume-Naesheim TM, Andersen TE, Dvorak J, et al. Effects of heading exposure and previous concussions on neuropsychological performance among Norwegian elite footballers. Br J Sports Med. 2005;39:70-77.
35. Stephens R, Rutherford A, Potter D, et al. Neuropsychological impairment as a consequence of football (soccer) play and football heading: a preliminary analysis and report on school students (13-16 years). Child Neuropsychol. 2005;11:513-526.
36. Stephens R, Rutherford A, Potter D, et al. Neuropsychological consequence of soccer play in adolescent UK school team soccer players. J Neuropsychiatry Clin Neurosci. 2010;22:295-303.
37. Poole VN, Breedlove EL, Shenk TE, et al. Sub-concussive hit characteristics predict deviant brain metabolism in football athletes. Dev Neuropsychol. 2015;40:12-17.
38. Mannix R, Iverson GL, Maxwell B, et al. Multiple prior concussions are associated with symptoms in high school athletes. Ann Clin Trans Neurol. 2014;1:433-438.
39. Savica R, Parisi JE, Wold LE, et al. High school football and risk of neurodegeneration: a community-based study. Mayo Clin Proc. 2012;87:335-340.
40. Lancaster M, Muftuler T, Olson D, et al. Chronic white matter changes following sport-related concussion measured by diffusion tensor and diffusion kurtosis imaging. Paper presented at: American Academy of Neurology 2016 Sports Concussion Conference; July 8-10, 2016; Chicago, Ill.
41. Kerr ZY, Yeargin SW, Valovich McLeod TC, et al. Comprehensive coach education reduces head impact exposures in American youth football. Orthop J Sports Med. 2015;3(ecollection):e232596711561545.
42. Black AM, Macpherson AK, Hagel BE, et al. Policy change eliminating body checking in non-elite ice hockey leads to a threefold reduction in injury and concussion risk in 11- and 12-year-old players. Br J Sports Med. 2016;50:55-61.
43. Council on Sports Medicine and Fitness. Tackling in youth football. Policy Statement of the American Academy of Pediatrics. Pediatrics. 2015;136:e1419-e1430.
› Require athletes who sustain a concussion to wait a minimum of 7 to 10 days before returning to full unrestricted activity. C
› Ensure that any player diagnosed with concussion follows a guided return-to-play progression, supervised by an athletic trainer or physical therapist experienced in post-concussion care. C
› Advise patients who are old enough to drive not to do so for at least 24 hours after a concussion. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Each year in the United States, more than 44 million young people participate in sports activities.1 Yet the number of concussions incurred annually by children and adolescents engaged in sports and recreational play has been underestimated for years, and largely unknown.1,2
Some estimates were based solely on the number of young athletes treated in emergency departments or sports concussion clinics. Others focused only on team players of middle school or high school age, excluding younger children who were hit in the head on playgrounds or during other recreational activities. What’s more, large numbers of concussions—as many as 4 in 10 incurred by high school athletes—were never reported to a coach or medical professional.3
In a new study published in the journal Pediatrics in June, researchers used national databases and current literature to provide what they believe to be “the most accurate and precise estimate of youth concussion” thus far: Between 1.1 and 1.9 million sports- and recreation-related concussions occur among US youth ages 18 or younger annually.1
Among young people playing team sports, concussions are between 2 and 7 times more likely to occur during competitive games than in practice sessions.4-7 Boys on football and ice hockey teams have the highest rates of concussion in young athletes.For overall number of concussions, however, girls on soccer teams are second only to football players.4 Female soccer players are more likely than male soccer players to sustain concussions during equal number of hours of play.4,7
An increase in incidence. The incidence of concussion among young athletes appears to have increased in the past decade, a likely result of greater involvement in team sports, an increasing focus on safeguarding young people from the potential dangers associated with a blow to the brain, and better diagnostic techniques.4,8-10 And a recent study based on data from electronic medical records at a large regional pediatric health care network found that more than three-quarters of young people with sports-related concussions were first seen in a primary care setting.2
With this in mind, we present a comprehensive update of the evidence regarding the diagnosis and management of sport-related concussion. The recommendations we include are consistent with professional association guidelines.8-10 Although we focus on concussion in children and adolescents involved in athletic activities, the principles generally apply to patients of all ages and to concussions that may not be sports related.
Removal from play: A vital first step
Whenever you conduct a physical exam for a young athlete, remind him or her—and the patient’s parents—that after a blow to the head, immediate removal from play is critical. Concussion is caused by a direct or indirect force to the brain that results in a transient disturbance in brain function,8-10 manifested by alterations in neurocognitive and motor function. While the signs and symptoms (TABLE 1)8-10 resolve within 10 days of injury in about 90% of cases, those who incur additional head impact within 24 hours have a higher symptom burden and prolonged recovery period.11 Even without repetitive impact, younger athletes may take longer to recover.8-10

The initial assessment
A child or adolescent who sustains a suspected concussion should be seen by a physician within 24 to 48 hours. Whether the initial assessment occurs in your office or on the sidelines of a game, it is important to confirm the time the incident occurred and the mechanism of injury.
Concussion is diagnosed by a combination of history, physical exam, and objective testing when symptoms or exam findings associated with mild brain trauma—headache, dizziness, light and/or noise sensitivity, among others—closely follow a head injury.8-10 Certain maneuvers—assessing eye movements by asking the athlete to look in various directions, for instance, then to follow a pen or finger as you move it closer to his or her face—may provoke dizziness, headache, or other symptoms of concussion that were not apparent initially.
The differential diagnosis includes cervical musculoskeletal injury, craniofacial injury, epidural and subdural hematoma, heat-related illness, uncomplicated headache and migraine, upper respiratory infection, and vertigo.8-10
Tools aid in diagnosis
Many clinical assessment tools exist to aid in the diagnosis of concussion (TABLE 2).8-10,12-14 Any one of these tools, many of which use combinations of symptom checklists, balance exams, and cognitive assessments, may be included in your evaluation. No single tool has been found to be superior to any other.8-10 A combination of tools may improve diagnostic accuracy, but assessment tools should not be the sole basis used to diagnose or rule out concussion.

Any child or adolescent who had a blow to the head and at least one sign or symptom of concussion should be evaluated as soon as possible and assessed again later that day or the next day if any reason for concern remains.
Neuropsychological (NP) testing may involve computerized tests developed specifically for athletes. Patients may be required to react to objects that appear on a screen, for example, in a way that tests memory, performance, and reaction time. Because cognitive recovery often lags behind symptom resolution, NP testing may identify subtle brain deficits even in athletes who are asymptomatic at rest or with exercise. In general, NP testing has a sensitivity of 71% to 88% for athletes with concussion,10 but it is most beneficial when baseline test results are available. Interpretation of NP testing should be done only by qualified clinicians.
While NP testing may provide additional prognostic information, it should not alter the management of athletes who are symptomatic either at rest or with exercise.15 Nor is NP testing vital, as concussion can be accurately diagnosed and adequately managed without it.
Neuroimaging, including computed tomography (CT) and magnetic resonance imaging (MRI), is often used unnecessarily in the initial assessment of a patient who sustained a possible concussion.8-10 In fact, neuroimaging should be reserved for cases in which it is necessary to rule out more serious pathology: intracranial or subdural hematoma or a craniofacial injury, for example, in patients with clinical findings that are red flags. These red flags include focal neurologic deficits, continuing nausea/vomiting, or persistent disorientation (TABLE 3),8-10 or symptoms that worsen or persist beyond a few weeks. In such cases, further evaluation—with MRI of the brain, formal NP testing, and/or referral to a neurologist, physiatrist, or other physician who specializes in concussion care—is indicated.

Concussion management: Rest is key
While there is a dearth of high-quality studies on the management of sport-related concussion across all age groups, standardized protocols for both children and adults have been adopted in most clinical settings.8-10,16,17 The protocols provide a framework for an individualized treatment plan. Yet their use among primary care physicians is inconsistent.18-20
Traditionally, concussion management begins with relative physical and cognitive rest to allow the brain time to recover.8-10 Recent randomized controlled trials have challenged this premise by suggesting that mild to moderate physical activity for post-concussion patients who are mildly symptomatic does not adversely affect recovery.21,22 These studies have significant limitations, however, and further research is needed to provide specific guidance on this aspect of concussion management before it is adopted.
Physical restrictions include organized sports, recreational activity, recess, and physical education classes. Walking is permitted unless it exacerbates symptoms. These restrictions should continue until the patient is symptom-free.
Cognitive restrictions include modifications at school and at home. Once an athlete is able to concentrate and tolerate visual and auditory stimuli, he or she may return to school. But classroom modifications should be considered, possibly including shortened school days, extra time for testing and homework, help with note taking, and restrictions from classes likely to provoke symptoms, such as computer science or music. Limiting use of mobile devices, television viewing, noisy environments, and other possible provocations may help speed symptom resolution. These restrictions, too, should remain in place until the patient is symptom-free.
Driving is often not addressed by physicians managing the care of athletes with concussion, but evidence suggests it should be. A study of patients presenting to the emergency department found that within 24 hours of a concussion diagnosis, individuals had an impaired response to traffic hazards.23,24 And Canadian clinical practice guidelines recommend that athletes with mild traumatic brain injury (TBI) avoid driving within the first 24 hours.25
While American guidelines are silent on the question of driving for this patient population, we recommend that athletes with concussion be restricted from driving and engaging in other risky complex tasks, such as welding or shop class, for at least 24 hours. For many athletes diagnosed with concussion, driving restrictions of longer duration may be necessary based on their symptom profile and neurocognitive test results. Continued dizziness or visual deficits would pose a greater risk than fatigue or short-term memory loss, for example.

Overseeing the return to play
Return-to-activity progression follows a stepwise protocol, with 6 steps that the injured athlete must complete before resuming full activity (FIGURE 1A).8-10 This stepwise progression begins only when athletes are symptom free, even during provocative maneuvers; have had a normal neurologic exam, are back to school full time with no restriction; are off any medications prescribed for concussion symptoms (TABLE 4),8-10 and when neurocognitive testing, if performed, is back to baseline. If an athlete develops symptoms at any stage of the progression, rest is required until he or she remains asymptomatic for at least 24 hours. The progression is then restarted at the last stage at which the patient was symptom free.

Some individualization, of course, is recommended here, too. Younger athletes and those with a prior history of concussion may require 10 days or more to complete all the steps, allowing an extra day at various steps. Neurologic maturation affects recovery time, and for younger individuals, a more conservative return-to-play protocol based on initial concussion symptom duration has been proposed (FIGURE 1B).16

Return to activity is often supervised by a certified athletic trainer at the athlete’s school. In the event that no athletic trainer is available, patients may be referred to physical therapists with experience in monitoring injured athletes.26 Anyone involved in the patient’s care, including the athlete himself, may use a symptom checklist to monitor recovery.
Although there is no evidence that the ongoing use of a symptom checklist affects the course of recovery, its use is often helpful in identifying specific symptoms that can be managed by means other than physical and cognitive rest—a sleep hygiene program for an individual with lingering difficulty sleeping, for example, or the continued application of ice, heat, and massage for persistent neck pain.
Checklist monitoring may be especially helpful for athletes whose symptoms extend beyond 10 days or who have multiple symptoms. Final clearance once all the steps have been completed requires follow-up with a health care provider.
Is a symptom-free waiting period necessary?
There is no evidence suggesting a need for a symptom-free waiting period before starting the return-to-play protocol.10,27 Because a repeat concussion is most likely within 7 to 10 days of the initial injury,8,9 however, most athletes should not return to contact play during that time frame, regardless of symptom resolution.
It is helpful to have asymptomatic athletes participate in non-contact activity before the 7 to 10 days are up, however. Doing so can help prevent deconditioning and injury upon return to contact sport, as there is evidence of increased risk of lower-extremity injury in the 90 days after concussion.28
What to tell athletes—and parents—about repetitive head trauma
There is growing concern about the long-term risks of concussion and repetitive head impact that may manifest as chronic traumatic encephalopathy (CTE) and chronic neurocognitive impairment (CNI) later in life. Indeed, some data strongly suggest—but do not definitively prove—a relationship between repetitive head injury and chronic neurodegenerative disease.8-10 You can tell worried patients or parents, however, that the majority of research on CTE and CNI has been based on professional football players.
Studies of long-term effects of soccer heading have shown conflicting results, with some finding cognitive impairment, altered postural control, and anatomic changes of the brain, while others found no effect on encephalopathy, concussion symptoms, or neurocognitive performance.29-36Here, too, most studies showing negative effects of soccer heading involved professional athletes.
Repetitive sub-concussive impact in high school football athletes has been found to induce biochemical changes to the brain,37 but the long-term effects are unknown. And, while concussion in high school athletes has been associated with short-term cognitive impairment, altered neurochemistry, and evidence of increased symptoms on baseline neurocognitive testing,8-10,38 no studies have linked concussion during middle school or high school with CNI. What’s more, a long-term (50-year) follow-up study of individuals who played football in high school found no difference in rates of neurodegenerative disease compared with age-matched controls.39
A new study of high school and college football players (mean age: 17.4 years) presented at the American Academy of Neurology 2016 Sports Concussion Conference in Chicago in July, however, found significant alterations in white matter 6 months post injury.40 The researchers compared 17 athletes with sport-related concussion with matched controls, using diffusion tensor imaging and diffusion kurtosis tensor imaging as biomarkers of brain recovery. The concussed athletes underwent MRI and symptom assessment at 24 hours, 8 days, and 6 months. The controls followed identical protocols.
At the 6-month assessment, there were no differences between the concussed group and the controls in terms of self-reported concussion symptoms, cognition, or balance. However, the concussed athletes had widespread decreased mean diffusivity compared with the controls. Despite the lack of clinical symptoms, the concussed athletes showed significant alterations in white matter “that were related to initial symptom severity ratings,” the authors concluded. These findings have implications both for determination of recovery from concussion and concussion management, they added.40
Although there is no way to eliminate all concussions, limited evidence suggests that improving athletic technique, limiting contact at practices, better enforcement of game rules, and rule changes regarding physical contact may decrease concussion risk.41-43 Many youth sports organizations have developed policies placing restrictions on head impact during practices and games. Studies are ongoing, too, to see if better headgear—or requiring helmets for soccer players—makes a difference.
CORRESPONDENCE
Ryan A. Sprouse, MD, CAQSM, 203 East Fourth Avenue, Ranson, WV 25438; [email protected].
› Require athletes who sustain a concussion to wait a minimum of 7 to 10 days before returning to full unrestricted activity. C
› Ensure that any player diagnosed with concussion follows a guided return-to-play progression, supervised by an athletic trainer or physical therapist experienced in post-concussion care. C
› Advise patients who are old enough to drive not to do so for at least 24 hours after a concussion. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Each year in the United States, more than 44 million young people participate in sports activities.1 Yet the number of concussions incurred annually by children and adolescents engaged in sports and recreational play has been underestimated for years, and largely unknown.1,2
Some estimates were based solely on the number of young athletes treated in emergency departments or sports concussion clinics. Others focused only on team players of middle school or high school age, excluding younger children who were hit in the head on playgrounds or during other recreational activities. What’s more, large numbers of concussions—as many as 4 in 10 incurred by high school athletes—were never reported to a coach or medical professional.3
In a new study published in the journal Pediatrics in June, researchers used national databases and current literature to provide what they believe to be “the most accurate and precise estimate of youth concussion” thus far: Between 1.1 and 1.9 million sports- and recreation-related concussions occur among US youth ages 18 or younger annually.1
Among young people playing team sports, concussions are between 2 and 7 times more likely to occur during competitive games than in practice sessions.4-7 Boys on football and ice hockey teams have the highest rates of concussion in young athletes.For overall number of concussions, however, girls on soccer teams are second only to football players.4 Female soccer players are more likely than male soccer players to sustain concussions during equal number of hours of play.4,7
An increase in incidence. The incidence of concussion among young athletes appears to have increased in the past decade, a likely result of greater involvement in team sports, an increasing focus on safeguarding young people from the potential dangers associated with a blow to the brain, and better diagnostic techniques.4,8-10 And a recent study based on data from electronic medical records at a large regional pediatric health care network found that more than three-quarters of young people with sports-related concussions were first seen in a primary care setting.2
With this in mind, we present a comprehensive update of the evidence regarding the diagnosis and management of sport-related concussion. The recommendations we include are consistent with professional association guidelines.8-10 Although we focus on concussion in children and adolescents involved in athletic activities, the principles generally apply to patients of all ages and to concussions that may not be sports related.
Removal from play: A vital first step
Whenever you conduct a physical exam for a young athlete, remind him or her—and the patient’s parents—that after a blow to the head, immediate removal from play is critical. Concussion is caused by a direct or indirect force to the brain that results in a transient disturbance in brain function,8-10 manifested by alterations in neurocognitive and motor function. While the signs and symptoms (TABLE 1)8-10 resolve within 10 days of injury in about 90% of cases, those who incur additional head impact within 24 hours have a higher symptom burden and prolonged recovery period.11 Even without repetitive impact, younger athletes may take longer to recover.8-10

The initial assessment
A child or adolescent who sustains a suspected concussion should be seen by a physician within 24 to 48 hours. Whether the initial assessment occurs in your office or on the sidelines of a game, it is important to confirm the time the incident occurred and the mechanism of injury.
Concussion is diagnosed by a combination of history, physical exam, and objective testing when symptoms or exam findings associated with mild brain trauma—headache, dizziness, light and/or noise sensitivity, among others—closely follow a head injury.8-10 Certain maneuvers—assessing eye movements by asking the athlete to look in various directions, for instance, then to follow a pen or finger as you move it closer to his or her face—may provoke dizziness, headache, or other symptoms of concussion that were not apparent initially.
The differential diagnosis includes cervical musculoskeletal injury, craniofacial injury, epidural and subdural hematoma, heat-related illness, uncomplicated headache and migraine, upper respiratory infection, and vertigo.8-10
Tools aid in diagnosis
Many clinical assessment tools exist to aid in the diagnosis of concussion (TABLE 2).8-10,12-14 Any one of these tools, many of which use combinations of symptom checklists, balance exams, and cognitive assessments, may be included in your evaluation. No single tool has been found to be superior to any other.8-10 A combination of tools may improve diagnostic accuracy, but assessment tools should not be the sole basis used to diagnose or rule out concussion.

Any child or adolescent who had a blow to the head and at least one sign or symptom of concussion should be evaluated as soon as possible and assessed again later that day or the next day if any reason for concern remains.
Neuropsychological (NP) testing may involve computerized tests developed specifically for athletes. Patients may be required to react to objects that appear on a screen, for example, in a way that tests memory, performance, and reaction time. Because cognitive recovery often lags behind symptom resolution, NP testing may identify subtle brain deficits even in athletes who are asymptomatic at rest or with exercise. In general, NP testing has a sensitivity of 71% to 88% for athletes with concussion,10 but it is most beneficial when baseline test results are available. Interpretation of NP testing should be done only by qualified clinicians.
While NP testing may provide additional prognostic information, it should not alter the management of athletes who are symptomatic either at rest or with exercise.15 Nor is NP testing vital, as concussion can be accurately diagnosed and adequately managed without it.
Neuroimaging, including computed tomography (CT) and magnetic resonance imaging (MRI), is often used unnecessarily in the initial assessment of a patient who sustained a possible concussion.8-10 In fact, neuroimaging should be reserved for cases in which it is necessary to rule out more serious pathology: intracranial or subdural hematoma or a craniofacial injury, for example, in patients with clinical findings that are red flags. These red flags include focal neurologic deficits, continuing nausea/vomiting, or persistent disorientation (TABLE 3),8-10 or symptoms that worsen or persist beyond a few weeks. In such cases, further evaluation—with MRI of the brain, formal NP testing, and/or referral to a neurologist, physiatrist, or other physician who specializes in concussion care—is indicated.

Concussion management: Rest is key
While there is a dearth of high-quality studies on the management of sport-related concussion across all age groups, standardized protocols for both children and adults have been adopted in most clinical settings.8-10,16,17 The protocols provide a framework for an individualized treatment plan. Yet their use among primary care physicians is inconsistent.18-20
Traditionally, concussion management begins with relative physical and cognitive rest to allow the brain time to recover.8-10 Recent randomized controlled trials have challenged this premise by suggesting that mild to moderate physical activity for post-concussion patients who are mildly symptomatic does not adversely affect recovery.21,22 These studies have significant limitations, however, and further research is needed to provide specific guidance on this aspect of concussion management before it is adopted.
Physical restrictions include organized sports, recreational activity, recess, and physical education classes. Walking is permitted unless it exacerbates symptoms. These restrictions should continue until the patient is symptom-free.
Cognitive restrictions include modifications at school and at home. Once an athlete is able to concentrate and tolerate visual and auditory stimuli, he or she may return to school. But classroom modifications should be considered, possibly including shortened school days, extra time for testing and homework, help with note taking, and restrictions from classes likely to provoke symptoms, such as computer science or music. Limiting use of mobile devices, television viewing, noisy environments, and other possible provocations may help speed symptom resolution. These restrictions, too, should remain in place until the patient is symptom-free.
Driving is often not addressed by physicians managing the care of athletes with concussion, but evidence suggests it should be. A study of patients presenting to the emergency department found that within 24 hours of a concussion diagnosis, individuals had an impaired response to traffic hazards.23,24 And Canadian clinical practice guidelines recommend that athletes with mild traumatic brain injury (TBI) avoid driving within the first 24 hours.25
While American guidelines are silent on the question of driving for this patient population, we recommend that athletes with concussion be restricted from driving and engaging in other risky complex tasks, such as welding or shop class, for at least 24 hours. For many athletes diagnosed with concussion, driving restrictions of longer duration may be necessary based on their symptom profile and neurocognitive test results. Continued dizziness or visual deficits would pose a greater risk than fatigue or short-term memory loss, for example.

Overseeing the return to play
Return-to-activity progression follows a stepwise protocol, with 6 steps that the injured athlete must complete before resuming full activity (FIGURE 1A).8-10 This stepwise progression begins only when athletes are symptom free, even during provocative maneuvers; have had a normal neurologic exam, are back to school full time with no restriction; are off any medications prescribed for concussion symptoms (TABLE 4),8-10 and when neurocognitive testing, if performed, is back to baseline. If an athlete develops symptoms at any stage of the progression, rest is required until he or she remains asymptomatic for at least 24 hours. The progression is then restarted at the last stage at which the patient was symptom free.

Some individualization, of course, is recommended here, too. Younger athletes and those with a prior history of concussion may require 10 days or more to complete all the steps, allowing an extra day at various steps. Neurologic maturation affects recovery time, and for younger individuals, a more conservative return-to-play protocol based on initial concussion symptom duration has been proposed (FIGURE 1B).16

Return to activity is often supervised by a certified athletic trainer at the athlete’s school. In the event that no athletic trainer is available, patients may be referred to physical therapists with experience in monitoring injured athletes.26 Anyone involved in the patient’s care, including the athlete himself, may use a symptom checklist to monitor recovery.
Although there is no evidence that the ongoing use of a symptom checklist affects the course of recovery, its use is often helpful in identifying specific symptoms that can be managed by means other than physical and cognitive rest—a sleep hygiene program for an individual with lingering difficulty sleeping, for example, or the continued application of ice, heat, and massage for persistent neck pain.
Checklist monitoring may be especially helpful for athletes whose symptoms extend beyond 10 days or who have multiple symptoms. Final clearance once all the steps have been completed requires follow-up with a health care provider.
Is a symptom-free waiting period necessary?
There is no evidence suggesting a need for a symptom-free waiting period before starting the return-to-play protocol.10,27 Because a repeat concussion is most likely within 7 to 10 days of the initial injury,8,9 however, most athletes should not return to contact play during that time frame, regardless of symptom resolution.
It is helpful to have asymptomatic athletes participate in non-contact activity before the 7 to 10 days are up, however. Doing so can help prevent deconditioning and injury upon return to contact sport, as there is evidence of increased risk of lower-extremity injury in the 90 days after concussion.28
What to tell athletes—and parents—about repetitive head trauma
There is growing concern about the long-term risks of concussion and repetitive head impact that may manifest as chronic traumatic encephalopathy (CTE) and chronic neurocognitive impairment (CNI) later in life. Indeed, some data strongly suggest—but do not definitively prove—a relationship between repetitive head injury and chronic neurodegenerative disease.8-10 You can tell worried patients or parents, however, that the majority of research on CTE and CNI has been based on professional football players.
Studies of long-term effects of soccer heading have shown conflicting results, with some finding cognitive impairment, altered postural control, and anatomic changes of the brain, while others found no effect on encephalopathy, concussion symptoms, or neurocognitive performance.29-36Here, too, most studies showing negative effects of soccer heading involved professional athletes.
Repetitive sub-concussive impact in high school football athletes has been found to induce biochemical changes to the brain,37 but the long-term effects are unknown. And, while concussion in high school athletes has been associated with short-term cognitive impairment, altered neurochemistry, and evidence of increased symptoms on baseline neurocognitive testing,8-10,38 no studies have linked concussion during middle school or high school with CNI. What’s more, a long-term (50-year) follow-up study of individuals who played football in high school found no difference in rates of neurodegenerative disease compared with age-matched controls.39
A new study of high school and college football players (mean age: 17.4 years) presented at the American Academy of Neurology 2016 Sports Concussion Conference in Chicago in July, however, found significant alterations in white matter 6 months post injury.40 The researchers compared 17 athletes with sport-related concussion with matched controls, using diffusion tensor imaging and diffusion kurtosis tensor imaging as biomarkers of brain recovery. The concussed athletes underwent MRI and symptom assessment at 24 hours, 8 days, and 6 months. The controls followed identical protocols.
At the 6-month assessment, there were no differences between the concussed group and the controls in terms of self-reported concussion symptoms, cognition, or balance. However, the concussed athletes had widespread decreased mean diffusivity compared with the controls. Despite the lack of clinical symptoms, the concussed athletes showed significant alterations in white matter “that were related to initial symptom severity ratings,” the authors concluded. These findings have implications both for determination of recovery from concussion and concussion management, they added.40
Although there is no way to eliminate all concussions, limited evidence suggests that improving athletic technique, limiting contact at practices, better enforcement of game rules, and rule changes regarding physical contact may decrease concussion risk.41-43 Many youth sports organizations have developed policies placing restrictions on head impact during practices and games. Studies are ongoing, too, to see if better headgear—or requiring helmets for soccer players—makes a difference.
CORRESPONDENCE
Ryan A. Sprouse, MD, CAQSM, 203 East Fourth Avenue, Ranson, WV 25438; [email protected].
1. Bryan MA, Rowhani-Rahbar A, Comstock RD, et al. Sports- and recreation-related concussions in US youth. Pediatrics. 2016; June 20 [Epub ahead of print].
2. Arbogast KB, Curry AE, Pfeiffer MR, et al. Point of health care entry for youth with concussion within a large pediatric care network. JAMA Pediatr. 2016; May 31 [Epub ahead of print].
3. Mihalik JK, Guskiewicz KM, Valovich McLeod TC, et al. Knowledge, attitude, and concussion-reporting behaviors among high school athletes: a preliminary study. J Ath Tr. 2013;48:645-653.
4. Marar M, McIlvain NM, Fields SK, et al. Epidemiology of concussions among United States high school athletes in 20 sports. Am J Sports Med. 2012;40:747.
5. Kontos AP, Elbin RJ, Fazio-Sumrock VC. Incidence of sports-related concussion among youth football players aged 8-12 years. J Pediatr. 2013;163:717-720.
6. Dompier TP, Kerr ZY, Marshall SW, et al. Incidence of concussion during practice and games in youth, high school, and collegiate American football players. JAMA Pediatr. 2015;169:659-665.
7. Comstock RD, Currie DW, Pierpont LA, et al. An evidence-based discussion of heading the ball and concussions in high school soccer. JAMA Pediatr. 2015;169:830-837.
8. Harmon KG, Drezner JA, Gammons M, et al. American Medical Society for Sports Medicine position statement: concussion in sport. Br J Sports Med. 2013;47:15-26.
9. McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47:250-258.
10. Giza CC, Kutcher JS, Ashwal S, et al. Summary of the evidence-based guideline update: evaluation and management of concussion in sports: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80:2250-2257.
11. Terwilliger VK, Pratson L, Vaughan CG, et al. Additional post-concussion impact exposure may affect recovery in adolescent athletes. J Neurotrauma. 2016;33:761-765.
12. Putukian M, Echemendia R, Dettwiler-Danspeckgruber A. Prospective clinical assessment using Sideline Concussion Assessment Tool-2 testing in the evaluation of sport-related concussion in college athletes. Clin J Sport Med. 2015;25:36-42.
13. Broglio SP, Macciocchi SN, Ferrara MS. Sensitivity of the concussion assessment battery. Neurosurgery. 2007;60:1050-1057.
14. Randolph C, McCrea M, Barr WB. Is neuropsychological testing useful in the management of sport-related concussion? J Athl Train. 2005;40:139-152.
15. Shrier I. Neuropsychological testing and concussions: a reasoned approach. Clin J Sport Med. 2012;22:211-213.
16. DeMatteo C, Stazyk K, Singh SK, et al. Development of a conservative protocol to return children and youth to activity following concussive injury. Clin Pediatr (Phila). 2015;54:152-163.
17. Broglio SP, Cantu RC, Gioia GA, et al. National Athletic Trainers Association position statement: management of sport concussion. J Athl Train. 2014;49:245-265.
18. Stoller J, Carson JD, Garel A, et al. Do family physicians, emergency department physicians, and pediatricians give consistent sport-related concussion management advice? Can Fam Physician. 2014;60:548, 550-552.
19. Lebrun CM, Mrazik M, Prasad AS, et al. Sport concussion knowledge base, clinical practices and needs for continuing medical education: a survey of family physicians and cross-border comparison. Br J Sports Med. 2013;47:54-59.
20. Zemek R, Eady K, Moreau K, et al. Knowledge of paediatric concussion among front-line primary care providers. Paediatr Child Health. 2014;19:475-480.
21. Maerlender A, Rieman W, Lichtenstein J, et al. Programmed physical exertion in recovery from sports-related concussion: a randomized pilot study. Dev Neuropsychol. 2015;40:273-278.
22. Buckley TA, Munkasy BA, Clouse BP. Acute cognitive and physical rest may not improve concussion recovery time. J Head Trauma Rehabil. 2015; July 24 [Epub ahead of print].
23. Preece MH, Horswill MS, Langlois JA, et al. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21:375-378.
24. Baker A, Unsworth CA, Lannin NA. Fitness-to-drive after mild traumatic brain injury: mapping the time trajectory of recovery in the acute stages post injury. Accid Anal Prev. 2015;79:50-55.
25. Marshall S, Bayley M, McCullagh S, et al. Clinical practice guidelines for mild traumatic brain injury and persistent symptoms. Can Fam Physician. 2012;58:257-267.
26. Yorke AM, Littleton S, Alsalaheen BA. Concussion attitudes and beliefs, knowledge, and clinical practice: a survey of physical therapists. Phys Ther. Available at: http://dx.doi.org/10.2522/ptj.20140598. Accessed January 21, 2016.
27. McCrea M, Guskiewicz K, Randolph C, et al. Effects of a symptom-free waiting period on clinical outcome and risk of reinjury after sport-related concussion. Neurosurgery. 2009;65:876-883.
28. Brooks MA, Peterson K, Biese K, et al. Concussion increases odds of sustaining a lower extremity musculoskeletal injury after return to play among collegiate athletes. Am J Sports Med. 2016;44:742-747.
29. Witol AD, Webbe FM. Soccer heading frequency predicts neuropsychological deficits. Arch Clin Neuropsychol. 2003;18:397-417.
30. Haran FJ, Tierney R, Wright WG, et al. Acute changes in postural control after soccer heading. Int J Sports Med. 2013;34:350-354.
31. Lipton ML, Kim N, Zimmerman ME, et al. Soccer heading is associated with white matter microstructural and cognitive abnormalities. Radiology. 2013;268:850-857.
32. Jordan SE, Green GA, Galanty HL, et al. Acute and chronic brain injury in United States national team soccer players. Am J Sports Med. 1996;24:205-210.
33. Kontos AP, Dolese A, Elbin RJ, et al. Relationship of soccer heading to computerized neurocognitive performance and symptoms among female and male youth soccer players. Brain Inj. 2011;25:1234-1241.
34. Straume-Naesheim TM, Andersen TE, Dvorak J, et al. Effects of heading exposure and previous concussions on neuropsychological performance among Norwegian elite footballers. Br J Sports Med. 2005;39:70-77.
35. Stephens R, Rutherford A, Potter D, et al. Neuropsychological impairment as a consequence of football (soccer) play and football heading: a preliminary analysis and report on school students (13-16 years). Child Neuropsychol. 2005;11:513-526.
36. Stephens R, Rutherford A, Potter D, et al. Neuropsychological consequence of soccer play in adolescent UK school team soccer players. J Neuropsychiatry Clin Neurosci. 2010;22:295-303.
37. Poole VN, Breedlove EL, Shenk TE, et al. Sub-concussive hit characteristics predict deviant brain metabolism in football athletes. Dev Neuropsychol. 2015;40:12-17.
38. Mannix R, Iverson GL, Maxwell B, et al. Multiple prior concussions are associated with symptoms in high school athletes. Ann Clin Trans Neurol. 2014;1:433-438.
39. Savica R, Parisi JE, Wold LE, et al. High school football and risk of neurodegeneration: a community-based study. Mayo Clin Proc. 2012;87:335-340.
40. Lancaster M, Muftuler T, Olson D, et al. Chronic white matter changes following sport-related concussion measured by diffusion tensor and diffusion kurtosis imaging. Paper presented at: American Academy of Neurology 2016 Sports Concussion Conference; July 8-10, 2016; Chicago, Ill.
41. Kerr ZY, Yeargin SW, Valovich McLeod TC, et al. Comprehensive coach education reduces head impact exposures in American youth football. Orthop J Sports Med. 2015;3(ecollection):e232596711561545.
42. Black AM, Macpherson AK, Hagel BE, et al. Policy change eliminating body checking in non-elite ice hockey leads to a threefold reduction in injury and concussion risk in 11- and 12-year-old players. Br J Sports Med. 2016;50:55-61.
43. Council on Sports Medicine and Fitness. Tackling in youth football. Policy Statement of the American Academy of Pediatrics. Pediatrics. 2015;136:e1419-e1430.
1. Bryan MA, Rowhani-Rahbar A, Comstock RD, et al. Sports- and recreation-related concussions in US youth. Pediatrics. 2016; June 20 [Epub ahead of print].
2. Arbogast KB, Curry AE, Pfeiffer MR, et al. Point of health care entry for youth with concussion within a large pediatric care network. JAMA Pediatr. 2016; May 31 [Epub ahead of print].
3. Mihalik JK, Guskiewicz KM, Valovich McLeod TC, et al. Knowledge, attitude, and concussion-reporting behaviors among high school athletes: a preliminary study. J Ath Tr. 2013;48:645-653.
4. Marar M, McIlvain NM, Fields SK, et al. Epidemiology of concussions among United States high school athletes in 20 sports. Am J Sports Med. 2012;40:747.
5. Kontos AP, Elbin RJ, Fazio-Sumrock VC. Incidence of sports-related concussion among youth football players aged 8-12 years. J Pediatr. 2013;163:717-720.
6. Dompier TP, Kerr ZY, Marshall SW, et al. Incidence of concussion during practice and games in youth, high school, and collegiate American football players. JAMA Pediatr. 2015;169:659-665.
7. Comstock RD, Currie DW, Pierpont LA, et al. An evidence-based discussion of heading the ball and concussions in high school soccer. JAMA Pediatr. 2015;169:830-837.
8. Harmon KG, Drezner JA, Gammons M, et al. American Medical Society for Sports Medicine position statement: concussion in sport. Br J Sports Med. 2013;47:15-26.
9. McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47:250-258.
10. Giza CC, Kutcher JS, Ashwal S, et al. Summary of the evidence-based guideline update: evaluation and management of concussion in sports: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80:2250-2257.
11. Terwilliger VK, Pratson L, Vaughan CG, et al. Additional post-concussion impact exposure may affect recovery in adolescent athletes. J Neurotrauma. 2016;33:761-765.
12. Putukian M, Echemendia R, Dettwiler-Danspeckgruber A. Prospective clinical assessment using Sideline Concussion Assessment Tool-2 testing in the evaluation of sport-related concussion in college athletes. Clin J Sport Med. 2015;25:36-42.
13. Broglio SP, Macciocchi SN, Ferrara MS. Sensitivity of the concussion assessment battery. Neurosurgery. 2007;60:1050-1057.
14. Randolph C, McCrea M, Barr WB. Is neuropsychological testing useful in the management of sport-related concussion? J Athl Train. 2005;40:139-152.
15. Shrier I. Neuropsychological testing and concussions: a reasoned approach. Clin J Sport Med. 2012;22:211-213.
16. DeMatteo C, Stazyk K, Singh SK, et al. Development of a conservative protocol to return children and youth to activity following concussive injury. Clin Pediatr (Phila). 2015;54:152-163.
17. Broglio SP, Cantu RC, Gioia GA, et al. National Athletic Trainers Association position statement: management of sport concussion. J Athl Train. 2014;49:245-265.
18. Stoller J, Carson JD, Garel A, et al. Do family physicians, emergency department physicians, and pediatricians give consistent sport-related concussion management advice? Can Fam Physician. 2014;60:548, 550-552.
19. Lebrun CM, Mrazik M, Prasad AS, et al. Sport concussion knowledge base, clinical practices and needs for continuing medical education: a survey of family physicians and cross-border comparison. Br J Sports Med. 2013;47:54-59.
20. Zemek R, Eady K, Moreau K, et al. Knowledge of paediatric concussion among front-line primary care providers. Paediatr Child Health. 2014;19:475-480.
21. Maerlender A, Rieman W, Lichtenstein J, et al. Programmed physical exertion in recovery from sports-related concussion: a randomized pilot study. Dev Neuropsychol. 2015;40:273-278.
22. Buckley TA, Munkasy BA, Clouse BP. Acute cognitive and physical rest may not improve concussion recovery time. J Head Trauma Rehabil. 2015; July 24 [Epub ahead of print].
23. Preece MH, Horswill MS, Langlois JA, et al. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21:375-378.
24. Baker A, Unsworth CA, Lannin NA. Fitness-to-drive after mild traumatic brain injury: mapping the time trajectory of recovery in the acute stages post injury. Accid Anal Prev. 2015;79:50-55.
25. Marshall S, Bayley M, McCullagh S, et al. Clinical practice guidelines for mild traumatic brain injury and persistent symptoms. Can Fam Physician. 2012;58:257-267.
26. Yorke AM, Littleton S, Alsalaheen BA. Concussion attitudes and beliefs, knowledge, and clinical practice: a survey of physical therapists. Phys Ther. Available at: http://dx.doi.org/10.2522/ptj.20140598. Accessed January 21, 2016.
27. McCrea M, Guskiewicz K, Randolph C, et al. Effects of a symptom-free waiting period on clinical outcome and risk of reinjury after sport-related concussion. Neurosurgery. 2009;65:876-883.
28. Brooks MA, Peterson K, Biese K, et al. Concussion increases odds of sustaining a lower extremity musculoskeletal injury after return to play among collegiate athletes. Am J Sports Med. 2016;44:742-747.
29. Witol AD, Webbe FM. Soccer heading frequency predicts neuropsychological deficits. Arch Clin Neuropsychol. 2003;18:397-417.
30. Haran FJ, Tierney R, Wright WG, et al. Acute changes in postural control after soccer heading. Int J Sports Med. 2013;34:350-354.
31. Lipton ML, Kim N, Zimmerman ME, et al. Soccer heading is associated with white matter microstructural and cognitive abnormalities. Radiology. 2013;268:850-857.
32. Jordan SE, Green GA, Galanty HL, et al. Acute and chronic brain injury in United States national team soccer players. Am J Sports Med. 1996;24:205-210.
33. Kontos AP, Dolese A, Elbin RJ, et al. Relationship of soccer heading to computerized neurocognitive performance and symptoms among female and male youth soccer players. Brain Inj. 2011;25:1234-1241.
34. Straume-Naesheim TM, Andersen TE, Dvorak J, et al. Effects of heading exposure and previous concussions on neuropsychological performance among Norwegian elite footballers. Br J Sports Med. 2005;39:70-77.
35. Stephens R, Rutherford A, Potter D, et al. Neuropsychological impairment as a consequence of football (soccer) play and football heading: a preliminary analysis and report on school students (13-16 years). Child Neuropsychol. 2005;11:513-526.
36. Stephens R, Rutherford A, Potter D, et al. Neuropsychological consequence of soccer play in adolescent UK school team soccer players. J Neuropsychiatry Clin Neurosci. 2010;22:295-303.
37. Poole VN, Breedlove EL, Shenk TE, et al. Sub-concussive hit characteristics predict deviant brain metabolism in football athletes. Dev Neuropsychol. 2015;40:12-17.
38. Mannix R, Iverson GL, Maxwell B, et al. Multiple prior concussions are associated with symptoms in high school athletes. Ann Clin Trans Neurol. 2014;1:433-438.
39. Savica R, Parisi JE, Wold LE, et al. High school football and risk of neurodegeneration: a community-based study. Mayo Clin Proc. 2012;87:335-340.
40. Lancaster M, Muftuler T, Olson D, et al. Chronic white matter changes following sport-related concussion measured by diffusion tensor and diffusion kurtosis imaging. Paper presented at: American Academy of Neurology 2016 Sports Concussion Conference; July 8-10, 2016; Chicago, Ill.
41. Kerr ZY, Yeargin SW, Valovich McLeod TC, et al. Comprehensive coach education reduces head impact exposures in American youth football. Orthop J Sports Med. 2015;3(ecollection):e232596711561545.
42. Black AM, Macpherson AK, Hagel BE, et al. Policy change eliminating body checking in non-elite ice hockey leads to a threefold reduction in injury and concussion risk in 11- and 12-year-old players. Br J Sports Med. 2016;50:55-61.
43. Council on Sports Medicine and Fitness. Tackling in youth football. Policy Statement of the American Academy of Pediatrics. Pediatrics. 2015;136:e1419-e1430.
From The Journal of Family Practice | 2016;65(8):538-544,546.