User login
Genitourinary syndrome of menopause in breast cancer survivors: Treatments are available
Many breast cancer survivors and women at high risk of breast cancer suffer from genitourinary syndrome of menopause (GSM), a term that encompasses any urinary, genital, or sexual dysfunction related to a hypoestrogenic state. Although GSM is usually caused by postmenopausal estrogen loss, it can also be caused by cancer treatments such as chemotherapy, radiation, and systemic endocrine therapy (eg, tamoxifen, aromatase inhibitors). These treatments can substantially decrease systemic estrogen levels, causing GSM symptoms that can profoundly worsen quality of life.
Managing GSM in these women poses a dilemma because systemic estrogen-containing therapies can increase the risk of breast cancer, and nonhormonal vaginal lubricants and moisturizers provide only minimal benefit. Fortunately, there are hormonal options, including locally applied estrogen, intravaginal dehydroepiandrosterone (DHEA), and estrogen receptor agonists/antagonists (ospemifene and bazedoxifene).
Here, we review the clinical management of GSM in breast cancer survivors and women at high risk of breast cancer and the efficacy and safety of available treatments, including their impact on breast cancer risk.
DRYNESS, IRRITATION, ATROPHY
The term GSM describes vulvovaginal and genitourinary symptoms associated with estrogen loss after menopause. Common symptoms are vaginal dryness, dyspareunia, irritation of genital skin, and pruritus.
LOCAL ESTROGEN THERAPY
Systemic estrogen therapy is widely used and effective for GSM, but there are concerns that it could increase the risk of breast cancer. After the Women’s Health Initiative in 2002 showed higher rates of cardiovascular disease and breast cancer with systemic estrogen-progestin use,5 the use of this hormone therapy declined by approximately 80%.6 Since then, healthcare providers have turned to local (ie, vaginal) estrogen therapies to manage GSM. These therapies have several advantages over systemic hormone therapy:
- Lower risk of adverse effects on the breast and cardiovascular system
- Greater efficacy in treating GSM
- In general, no need for progesterone when low-dose local estrogen is given to a woman with a uterus.7
Is locally applied estrogen systemically absorbed?
Despite these advantages, concerns remain as to whether vaginal estrogen therapy has adverse consequences associated with systemic absorption, particularly from atrophic vaginal tissues.
Santen,8 in a 2015 review of 33 studies, concluded that systemic absorption from low-dose vaginal estrogen is minimal, which provides some rationale for using it to treat vulvovaginal atrophy in postmenopausal women. This finding also suggests that the US Food and Drug Administration (FDA) “black box” warning of possible toxicities with vaginal estrogen is likely overstated, given that serum estrogen levels remained within normal postmenopausal levels.
Nevertheless, many providers are apprehensive about prescribing vaginal estrogen in women with a history of breast cancer because the threshold for systemic estrogen levels associated with breast cancer recurrence has not been established.
ACOG statement. In 2016, a committee of the American College of Obstetricians and Gynecologists cited data showing that low-dose vaginal estrogens do not result in sustained serum estrogen levels exceeding the normal postmenopausal range, and that the use of vaginal estrogens does not increase the risk of cancer recurrence.9 However, they recommend caution with vaginal estrogen use, especially in women with a history of estrogen-dependent breast cancer, reserving it for patients with GSM symptoms nonresponsive to nonhormonal treatment and specifying that it be used in low doses.
Vaginal estrogen formulations
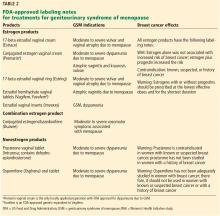
Several types of locally applied estrogens are available, each with different properties and affinity for estrogen receptors. These include conjugated estrogens, 17-beta estradiol, estradiol acetate, and estradiol hemihydrate. Three delivery systems are FDA-approved: creams, rings, and tablets (Table 2).
Vaginal creams. Two vaginal creams are available, one (Estrace) containing 17-beta estradiol and the other (Premarin) containing conjugated estrogens.
The FDA-approved dosage for 17-beta estradiol is 2 to 4 g/day for 1 or 2 weeks, then gradually reduced to half the dose for a similar time. Maintenance dosing is 1 g 1 to 3 times per week. However, the ACOG statement notes that the FDA-approved dosages are higher than those proven to be effective and currently used in clinical practice, eg, 0.5 g twice a week.9
The FDA-approved dosage of conjugated estrogen cream for moderate to severe dyspareunia is 0.5 g daily for 21 days, then off for 7 days, or 0.5 g twice a week.
Vaginal tablets. The vaginal tablet Vagifem and its generic equivalent Yuvafem contain 10 µg of estradiol hemihydrate. The FDA-approved dosage is 10 µg daily for 2 weeks, followed by 10 µg twice a week, inserted into the lower third of the vagina. This dosage is significantly lower than that of estrogen creams.
Vaginal insert. A newly approved vaginal insert (Imvexxy) contains estradiol 4 µg (the lowest dose of vaginal estradiol available) or 10 µg, in a coconut oil vehicle. Its indications are for moderate to severe dyspareunia due to menopause and atrophic vaginitis due to menopause. A study cited in its package insert (www.accessdata.fda.gov/drugsatfda_docs/label/2018/208564s000lbl.pdf) showed that, in patients who used this product, systemic absorption of estradiol remained within the postmenopausal range. Its effects on breast cancer have not yet been studied.
Vaginal rings. Two vaginal rings are marketed. One (Estring) contains 17-beta estradiol, and the other (Femring) contains estradiol acetate. Only the 17-beta estradiol ring delivers a low dose to vaginal tissues, releasing 7.5 µg/day for 90 days. The estradiol acetate ring releases 0.05 mg/day or 0.10 mg/day and is a systemic treatment meant to be used with a progestin, not for local therapy.
VAGINAL ANDROGEN THERAPY: DHEA
After menopause, as the ovaries stop making estrogen from androstenedione, some production continues in other tissues, with DHEA as the primary precursor of androgens that are ultimately converted to estrogen. This has led to the theory that the cause of GSM is not estrogen deficiency but androgen deficiency. Evidence reviewed by Labrie et al11 shows that vulvovaginal atrophy is caused by decreased DHEA availability, which in turn causes sex steroid deficiency-related menopausal symptoms.11 Thus, it is reasonable to conclude that menopausal symptoms can be relieved by giving DHEA.
This theory has been borne out in clinical trials, in which DHEA in a vaginal tablet formulation increased the maturation of vaginal cells and lowered vaginal pH, leading to relief of GSM symptoms.12
The only DHEA product FDA-approved for treating GSM-related symptoms is prasterone (Intrarosa), indicated for moderate to severe dyspareunia due to vulvovaginal atrophy. The recommended dosing is a single 6.5-mg intravaginal tablet (0.5% prasterone) inserted nightly at bedtime. Its efficacy for treating hypoactive sexual desire disorder in postmenopausal women is being investigated.
Breast cancer implications
Because DHEA is converted to estrogen by aromatization, healthcare providers might hesitate to use it in women who have a history of hormone-sensitive cancer. Data on the safety of intravaginal DHEA use in breast cancer survivors are limited. However, studies have found that prasterone has highly beneficial effects on dyspareunia, vaginal dryness, and objective signs of vulvovaginal atrophy without significant drug-related adverse effects.12,13 In these studies, serum estrogen levels in women treated with DHEA were within the values observed in normal postmenopausal women. In addition, there are no aromatase enzymes in the endometrium, so even high doses of vaginal DHEA (in contrast to high doses of vaginal estrogen) will not stimulate the endometrium.
Clinically, this evidence indicates that DHEA exerts both estrogenic and androgenic activity in the vagina without increasing serum estrogen levels, making it a good alternative to topical estrogen therapy.
OSPEMIFENE: AN ESTROGEN RECEPTOR AGONIST/ANTAGONIST
Ospemifene (Osphena) is an estrogen receptor agonist/antagonist, a class of drugs previously called selective estrogen receptor modulators (SERMs). It is FDA-approved to treat moderate to severe dyspareunia secondary to vulvar and vaginal atrophy.
Ospemifene has unique estrogenic effects on the vaginal mucosa, having been shown to increase the number of epithelial cells, lower the vaginal pH, and decrease the percentage of parabasal cells seen on Papanicolaou smears after 12 weeks of use.14
Unlike tamoxifen, another drug of this class, ospemifene does not change the endometrial lining.14 Similarly, ospemifene acts as an estrogenic agonist in bone and, thus, has the potential for use in preventing and managing osteoporosis or for use in women at risk of fractures.
Breast cancer impact
In preclinical trials, ospemifene was found to have antiestrogenic effects on breast tissue, similar to those seen with tamoxifen.
In a model using human tumor grafts, ospemifene decreased tumor growth in mice implanted with estrogen receptor-positive breast cancer cells.15
In a mouse model using breast cancer cells that were biologically and histologically similar to those of humans, ospemifene had strong antiestrogenic effects on the breast tissue.16 The evidence suggests that ospemifene has a favorable effect on vulvar and vaginal atrophy.17
Ospemifene is FDA-approved to treat moderate to severe dyspareunia secondary to menopause. Recommended dosing is 60 mg/day orally with food.
Its antiestrogenic effects on breast tissue make it a promising option for women with a history of estrogen-receptor positive breast cancer. However, further study is needed to fully understand its effects on human breast tissue. According to the manufacturer’s package insert (www.osphena.com/files/pdf/osphena_prescribing_information.pdf), because the drug has not been adequately studied in women with breast cancer, it should not be used in women with known or suspected breast cancer or a history of breast cancer.
CONJUGATED ESTROGENS PLUS BAZEDOXIFENE
The combination of conjugated estrogens and bazedoxifene (Duavee) is a progesterone-free alternative for treating various menopausal symptoms. Bazedoxifene is another estrogen receptor agonist/antagonist, and it was added to counteract estrogen’s effects on the endometrium, thus replacing progesterone. This protective effect has been validated in clinical trials, which also found a favorable safety profile in breast tissue.18,19
SMART trials. The efficacy of this combination was studied in a series of large phase 3 multicenter trials called the SMART (Selective Estrogens, Menopause, and Response to Therapy) trials.20–23 Treated patients had markedly fewer vasomotor symptoms at 1 year, along with an increase in superficial cells and intermediate cells of the vaginal epithelium and a decrease in parabasal cells. They also had a substantial decrease in the incidence of dyspareunia.
Its effects on breast tissue were evaluated in the SMART-5 trial. Therapy had no net impact on breast density, suggesting that it has an estrogen-neutral effect on the breast.23
These results suggest that combined conjugated estrogens and bazedoxifene could be a noteworthy treatment option for GSM in women with a history of estrogen receptor-positive breast cancer, particularly in those with vasomotor symptoms and bone loss. However, the combination has not been studied specifically in breast cancer survivors.
Dosage. The FDA-approved dosing is 20 mg/0.45 mg per day orally to treat vasomotor symptoms, GSM, and osteoporosis in postmenopausal women with a uterus.
LASER THERAPY AND RADIOFREQUENCY HEAT: AN OFF-LABEL OPTION
Low-dose radiofrequency thermal therapy, delivered by carbon dioxide laser or by radiofrequency heat, has been used with some success to treat urinary stress incontinence and vaginal laxity in postpartum women. It may be an option for GSM, although it is not FDA-approved for this indication, and the FDA has recently issued a warning about it.24
Marketing literature promotes laser therapy as an effective option that stimulates vaginal connective tissue to produce new collagen, which then promotes improved blood flow and tissue regeneration for vaginal lubrication and elasticity.
A study comparing fractional carbon dioxide vaginal laser treatment and local estrogen therapy in postmenopausal women with vulvovaginal atrophy found that laser therapy was an effective treatment for vulvovaginal atrophy (dyspareunia, dryness, and burning), both alone and with local estrogen.25
Despite the promising effects of laser therapy for treating vulvovaginal atrophy in GSM, studies have not determined its short-term or long-term safety profile. Furthermore, laser therapy does not improve impaired sexual function, ie, decreased libido, arousal, and sexual satisfaction. Another important consideration is that the cost of laser therapy in 2017 was estimated to be $2,000 to $3,000 per treatment, not covered by healthcare insurance.
CLINICAL APPROACH
Symptoms of GSM are common in breast cancer survivors, both pre- and postmenopausal, especially those treated with tamoxifen or an aromatase inhibitor. Estimates are that 60% of postmenopausal breast cancer survivors and 40% of premenopausal breast cancer survivors suffer from GSM.26 Unfortunately, many women do not seek medical attention for their symptoms.
A variety of hormonal and nonhormonal options are available for these patients. We recommend an interdisciplinary approach to treatment, with the decision to use hormonal options made in collaboration with the patient’s oncologist and the patient herself, in an informed, shared decision-making process that takes into consideration the risks and possible benefits depending on the symptoms.
The first step in selecting a management plan for GSM symptoms in women with breast cancer is to conduct a thorough assessment to provide data for individualizing the care plan. The decision to use a hormonal option should be made in collaboration with a woman’s oncologist and should include an informed decision-making process during which the potential risks and benefits, including the breast cancer impact, are fully disclosed.
Alternatives to systemic estrogen
Vaginal estrogen is an effective and safe option to treat GSM in women with either estrogen receptor-negative or estrogen receptor-positive breast cancer. It often completely cures the symptoms without any noticeable increase in serum estrogen levels.
Vaginal DHEA therapy is a nonestrogen option shown to effectively treat GSM without increasing systemic levels of estrogen or testosterone. This profile makes vaginal DHEA therapy a particularly attractive treatment for symptoms of GSM in women at risk for breast cancer.
Use of an estrogen receptor agonist/antagonist in breast cancer survivors needs careful consideration. Ospemifene has antiestrogenic effects that make it a good option for women with bone loss and those at high risk for breast cancer, but it should not be used concurrently with tamoxifen or raloxifene. Additionally, ospemifene does not cause uterine hyperplasia, so it can be used in women with a uterus.
Although more study is needed, we do have options to improve the overall quality of life in breast cancer survivors with GSM.
- Lester J, Pahouja G, Andersen B, Lustberg M. Atrophic vaginitis in breast cancer survivors: a difficult survivorship issue. J Pers Med 2015; 5(2):50–66. doi:10.3390/jpm5020050
- Chin SN, Trinkaus M, Simmons C, et al. Prevalence and severity of urogenital symptoms in postmenopausal women receiving endocrine therapy for breast cancer. Clin Breast Cancer 2009; 9(2):108–117. doi:10.3816/CBC.2009.n.020
- Fallowfield L, Cella D, Cuzick J, Francis S, Locker G, Howell A. Quality of life of postmenopausal women in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) adjuvant breast cancer trial. J Clin Oncol 2004; 22(21):4261–4271. doi:10.1200/JCO.2004.08.029
- Cella D, Fallowfield LJ. Recognition and management of treatment-related side effects for breast cancer patients receiving adjuvant endocrine therapy. Breast Cancer Res Treat 2008; 107(2):167–180. doi:10.1007/s10549-007-9548-1
- Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002; 288(3):321–333. pmid:12117397
- Tsai SA, Stefanick ML, Stafford RS. Trends in menopausal hormone therapy use of US office-based physicians, 2000–2009. Menopause 2011; 18(4):385–392. doi:10.1097/gme.0b013e3181f43404
- North American Menopause Society. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause 2013; 20(9):888–902. doi:10.1097/GME.0b013e3182a122c2
- Santen RJ. Vaginal administration of estradiol: effects of dose, preparation and timing on plasma estradiol levels. Climacteric 2015; 18(2):121–134. doi:10.3109/13697137.2014.947254
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: the use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol 2016; 127(3):e93–e96. doi:10.1097/AOG.0000000000001351
- Santoro N, Epperson CN, Mathews SB. Menopausal symptoms and their management. Endocrinol Metab Clin North Am 2015; 44(3):497–515. doi:10.1016/j.ecl.2015.05.001
- Labrie F, Archer DF, Martel C, Vaillancourt M, Montesino M. Combined data of intravaginal prasterone against vulvovaginal atrophy of menopause. Menopause 2017; 24(11):1246–1256. doi:10.1097/GME.0000000000000910
- Labrie F, Archer D, Bouchard C, et al. Serum steroid levels during 12-week intravaginal dehydroepiandrosterone administration. Menopause 2009; 16(5):897–906. doi:10.1097/gme.0b013e31819e8930
- Labrie F, Cusan L, Gomez JL, et al. Effect of intravaginal DHEA on serum DHEA and eleven of its metabolites in postmenopausal women. J Steroid Biochem Mol Biol 2008; 111(3-5):178–194. doi:10.1016/j.jsbmb.2008.06.003
- Soe LH, Wurz GT, Kao CJ, Degregorio MW. Ospemifene for the treatment of dyspareunia associated with vulvar and vaginal atrophy: potential benefits in bone and breast. Int J Womens Health 2013; 5:605–611. doi:10.2147/IJWH.S39146
- Taras TL, Wurz GT, DeGregorio MW. In vitro and in vivo biologic effects of ospemifene (FC-1271a) in breast cancer. J Steroid Biochem Mol Biol 2001; 77(4–5):271–279. pmid:11457665
- Wurz GT, Read KC, Marchisano-Karpman C, et al. Ospemifene inhibits the growth of dimethylbenzanthracene-induced mammary tumors in Sencar mice. J Steroid Biochem Mol Biol 2005; 97(3):230–240. doi:10.1016/j.jsbmb.2005.06.027
- Archer DF, Carr BR, Pinkerton JV, Taylor HS, Constantine GD. Effects of ospemifene on the female reproductive and urinary tracts: translation from preclinical models into clinical evidence. Menopause 2015; 22(7):786–796. doi:10.1097/GME.0000000000000365
- Mirkin S, Pickar JH. Management of osteoporosis and menopausal symptoms: focus on bazedoxifene/conjugated estrogen combination. Int J Womens Health 2013; 5:465–475. doi:10.2147/IJWH.S39455
- Kagan R, Goldstein SR, Pickar JH, Komm BS. Patient considerations in the management of menopausal symptoms: role of conjugated estrogens with bazedoxifene. Ther Clin Risk Manag 2016; 12:549–562. doi:10.2147/TCRM.S63833
- Lobo RA, Pinkerton JV, Gass ML, et al. Evaluation of bazedoxifene/conjugated estrogens for the treatment of menopausal symptoms and effects on metabolic parameters and overall safety profile. Fertil Steril 2009; 92(3):1025–1038. doi:10.1016/j.fertnstert.2009.03.113
- Pinkerton JV, Utian WH, Constantine GD, Olivier S, Pickar JH. Relief of vasomotor symptoms with the tissue-selective estrogen complex containing bazedoxifene/conjugated estrogens: a randomized, controlled trial. Menopause 2009; 16(6):1116–1124. doi:10.1097/gme.0b013e3181a7df0d
- Kagan R, Williams RS, Pan K, Mirkin S, Pickar JH. A randomized, placebo- and active-controlled trial of bazedoxifene/conjugated estrogens for treatment of moderate to severe vulvar/vaginal atrophy in postmenopausal women. Menopause 2010; 17(2):281–289. doi:10.1097/GME.0b013e3181b7c65f
- Pinkerton JV, Harvey JA, Pan K, et al. Breast effects of bazedoxifene-conjugated estrogens: a randomized controlled trial. Obstet Gynecol 2013; 121(5):959–968. doi:10.1097/AOG.0b013e31828c5974
- FDA. U.S. Food & Drug Administration. FDA Statement. Statement from FDA Commissioner Scott Gottlieb, M.D., on efforts to safeguard women’s health from deceptive health claims and significant risks related to devices marketed for use in medical procedures for “vaginal rejuvenation.” www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm615130.htm. Accessed August 20, 2018.
- Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause 2018; 25(1):21–28. doi:10.1097/GME.0000000000000955
- Biglia N, Bounous VE, D’Alonzo M, et al. Vaginal atrophy in breast cancer survivors: attitude and approaches among oncologists. Clin Breast Cancer 2017; 17(8):611–617. doi:10.1016/j.clbc.2017.05.008
Many breast cancer survivors and women at high risk of breast cancer suffer from genitourinary syndrome of menopause (GSM), a term that encompasses any urinary, genital, or sexual dysfunction related to a hypoestrogenic state. Although GSM is usually caused by postmenopausal estrogen loss, it can also be caused by cancer treatments such as chemotherapy, radiation, and systemic endocrine therapy (eg, tamoxifen, aromatase inhibitors). These treatments can substantially decrease systemic estrogen levels, causing GSM symptoms that can profoundly worsen quality of life.
Managing GSM in these women poses a dilemma because systemic estrogen-containing therapies can increase the risk of breast cancer, and nonhormonal vaginal lubricants and moisturizers provide only minimal benefit. Fortunately, there are hormonal options, including locally applied estrogen, intravaginal dehydroepiandrosterone (DHEA), and estrogen receptor agonists/antagonists (ospemifene and bazedoxifene).
Here, we review the clinical management of GSM in breast cancer survivors and women at high risk of breast cancer and the efficacy and safety of available treatments, including their impact on breast cancer risk.
DRYNESS, IRRITATION, ATROPHY
The term GSM describes vulvovaginal and genitourinary symptoms associated with estrogen loss after menopause. Common symptoms are vaginal dryness, dyspareunia, irritation of genital skin, and pruritus.
LOCAL ESTROGEN THERAPY
Systemic estrogen therapy is widely used and effective for GSM, but there are concerns that it could increase the risk of breast cancer. After the Women’s Health Initiative in 2002 showed higher rates of cardiovascular disease and breast cancer with systemic estrogen-progestin use,5 the use of this hormone therapy declined by approximately 80%.6 Since then, healthcare providers have turned to local (ie, vaginal) estrogen therapies to manage GSM. These therapies have several advantages over systemic hormone therapy:
- Lower risk of adverse effects on the breast and cardiovascular system
- Greater efficacy in treating GSM
- In general, no need for progesterone when low-dose local estrogen is given to a woman with a uterus.7
Is locally applied estrogen systemically absorbed?
Despite these advantages, concerns remain as to whether vaginal estrogen therapy has adverse consequences associated with systemic absorption, particularly from atrophic vaginal tissues.
Santen,8 in a 2015 review of 33 studies, concluded that systemic absorption from low-dose vaginal estrogen is minimal, which provides some rationale for using it to treat vulvovaginal atrophy in postmenopausal women. This finding also suggests that the US Food and Drug Administration (FDA) “black box” warning of possible toxicities with vaginal estrogen is likely overstated, given that serum estrogen levels remained within normal postmenopausal levels.
Nevertheless, many providers are apprehensive about prescribing vaginal estrogen in women with a history of breast cancer because the threshold for systemic estrogen levels associated with breast cancer recurrence has not been established.
ACOG statement. In 2016, a committee of the American College of Obstetricians and Gynecologists cited data showing that low-dose vaginal estrogens do not result in sustained serum estrogen levels exceeding the normal postmenopausal range, and that the use of vaginal estrogens does not increase the risk of cancer recurrence.9 However, they recommend caution with vaginal estrogen use, especially in women with a history of estrogen-dependent breast cancer, reserving it for patients with GSM symptoms nonresponsive to nonhormonal treatment and specifying that it be used in low doses.
Vaginal estrogen formulations
Several types of locally applied estrogens are available, each with different properties and affinity for estrogen receptors. These include conjugated estrogens, 17-beta estradiol, estradiol acetate, and estradiol hemihydrate. Three delivery systems are FDA-approved: creams, rings, and tablets (Table 2).
Vaginal creams. Two vaginal creams are available, one (Estrace) containing 17-beta estradiol and the other (Premarin) containing conjugated estrogens.
The FDA-approved dosage for 17-beta estradiol is 2 to 4 g/day for 1 or 2 weeks, then gradually reduced to half the dose for a similar time. Maintenance dosing is 1 g 1 to 3 times per week. However, the ACOG statement notes that the FDA-approved dosages are higher than those proven to be effective and currently used in clinical practice, eg, 0.5 g twice a week.9
The FDA-approved dosage of conjugated estrogen cream for moderate to severe dyspareunia is 0.5 g daily for 21 days, then off for 7 days, or 0.5 g twice a week.
Vaginal tablets. The vaginal tablet Vagifem and its generic equivalent Yuvafem contain 10 µg of estradiol hemihydrate. The FDA-approved dosage is 10 µg daily for 2 weeks, followed by 10 µg twice a week, inserted into the lower third of the vagina. This dosage is significantly lower than that of estrogen creams.
Vaginal insert. A newly approved vaginal insert (Imvexxy) contains estradiol 4 µg (the lowest dose of vaginal estradiol available) or 10 µg, in a coconut oil vehicle. Its indications are for moderate to severe dyspareunia due to menopause and atrophic vaginitis due to menopause. A study cited in its package insert (www.accessdata.fda.gov/drugsatfda_docs/label/2018/208564s000lbl.pdf) showed that, in patients who used this product, systemic absorption of estradiol remained within the postmenopausal range. Its effects on breast cancer have not yet been studied.
Vaginal rings. Two vaginal rings are marketed. One (Estring) contains 17-beta estradiol, and the other (Femring) contains estradiol acetate. Only the 17-beta estradiol ring delivers a low dose to vaginal tissues, releasing 7.5 µg/day for 90 days. The estradiol acetate ring releases 0.05 mg/day or 0.10 mg/day and is a systemic treatment meant to be used with a progestin, not for local therapy.
VAGINAL ANDROGEN THERAPY: DHEA
After menopause, as the ovaries stop making estrogen from androstenedione, some production continues in other tissues, with DHEA as the primary precursor of androgens that are ultimately converted to estrogen. This has led to the theory that the cause of GSM is not estrogen deficiency but androgen deficiency. Evidence reviewed by Labrie et al11 shows that vulvovaginal atrophy is caused by decreased DHEA availability, which in turn causes sex steroid deficiency-related menopausal symptoms.11 Thus, it is reasonable to conclude that menopausal symptoms can be relieved by giving DHEA.
This theory has been borne out in clinical trials, in which DHEA in a vaginal tablet formulation increased the maturation of vaginal cells and lowered vaginal pH, leading to relief of GSM symptoms.12
The only DHEA product FDA-approved for treating GSM-related symptoms is prasterone (Intrarosa), indicated for moderate to severe dyspareunia due to vulvovaginal atrophy. The recommended dosing is a single 6.5-mg intravaginal tablet (0.5% prasterone) inserted nightly at bedtime. Its efficacy for treating hypoactive sexual desire disorder in postmenopausal women is being investigated.
Breast cancer implications
Because DHEA is converted to estrogen by aromatization, healthcare providers might hesitate to use it in women who have a history of hormone-sensitive cancer. Data on the safety of intravaginal DHEA use in breast cancer survivors are limited. However, studies have found that prasterone has highly beneficial effects on dyspareunia, vaginal dryness, and objective signs of vulvovaginal atrophy without significant drug-related adverse effects.12,13 In these studies, serum estrogen levels in women treated with DHEA were within the values observed in normal postmenopausal women. In addition, there are no aromatase enzymes in the endometrium, so even high doses of vaginal DHEA (in contrast to high doses of vaginal estrogen) will not stimulate the endometrium.
Clinically, this evidence indicates that DHEA exerts both estrogenic and androgenic activity in the vagina without increasing serum estrogen levels, making it a good alternative to topical estrogen therapy.
OSPEMIFENE: AN ESTROGEN RECEPTOR AGONIST/ANTAGONIST
Ospemifene (Osphena) is an estrogen receptor agonist/antagonist, a class of drugs previously called selective estrogen receptor modulators (SERMs). It is FDA-approved to treat moderate to severe dyspareunia secondary to vulvar and vaginal atrophy.
Ospemifene has unique estrogenic effects on the vaginal mucosa, having been shown to increase the number of epithelial cells, lower the vaginal pH, and decrease the percentage of parabasal cells seen on Papanicolaou smears after 12 weeks of use.14
Unlike tamoxifen, another drug of this class, ospemifene does not change the endometrial lining.14 Similarly, ospemifene acts as an estrogenic agonist in bone and, thus, has the potential for use in preventing and managing osteoporosis or for use in women at risk of fractures.
Breast cancer impact
In preclinical trials, ospemifene was found to have antiestrogenic effects on breast tissue, similar to those seen with tamoxifen.
In a model using human tumor grafts, ospemifene decreased tumor growth in mice implanted with estrogen receptor-positive breast cancer cells.15
In a mouse model using breast cancer cells that were biologically and histologically similar to those of humans, ospemifene had strong antiestrogenic effects on the breast tissue.16 The evidence suggests that ospemifene has a favorable effect on vulvar and vaginal atrophy.17
Ospemifene is FDA-approved to treat moderate to severe dyspareunia secondary to menopause. Recommended dosing is 60 mg/day orally with food.
Its antiestrogenic effects on breast tissue make it a promising option for women with a history of estrogen-receptor positive breast cancer. However, further study is needed to fully understand its effects on human breast tissue. According to the manufacturer’s package insert (www.osphena.com/files/pdf/osphena_prescribing_information.pdf), because the drug has not been adequately studied in women with breast cancer, it should not be used in women with known or suspected breast cancer or a history of breast cancer.
CONJUGATED ESTROGENS PLUS BAZEDOXIFENE
The combination of conjugated estrogens and bazedoxifene (Duavee) is a progesterone-free alternative for treating various menopausal symptoms. Bazedoxifene is another estrogen receptor agonist/antagonist, and it was added to counteract estrogen’s effects on the endometrium, thus replacing progesterone. This protective effect has been validated in clinical trials, which also found a favorable safety profile in breast tissue.18,19
SMART trials. The efficacy of this combination was studied in a series of large phase 3 multicenter trials called the SMART (Selective Estrogens, Menopause, and Response to Therapy) trials.20–23 Treated patients had markedly fewer vasomotor symptoms at 1 year, along with an increase in superficial cells and intermediate cells of the vaginal epithelium and a decrease in parabasal cells. They also had a substantial decrease in the incidence of dyspareunia.
Its effects on breast tissue were evaluated in the SMART-5 trial. Therapy had no net impact on breast density, suggesting that it has an estrogen-neutral effect on the breast.23
These results suggest that combined conjugated estrogens and bazedoxifene could be a noteworthy treatment option for GSM in women with a history of estrogen receptor-positive breast cancer, particularly in those with vasomotor symptoms and bone loss. However, the combination has not been studied specifically in breast cancer survivors.
Dosage. The FDA-approved dosing is 20 mg/0.45 mg per day orally to treat vasomotor symptoms, GSM, and osteoporosis in postmenopausal women with a uterus.
LASER THERAPY AND RADIOFREQUENCY HEAT: AN OFF-LABEL OPTION
Low-dose radiofrequency thermal therapy, delivered by carbon dioxide laser or by radiofrequency heat, has been used with some success to treat urinary stress incontinence and vaginal laxity in postpartum women. It may be an option for GSM, although it is not FDA-approved for this indication, and the FDA has recently issued a warning about it.24
Marketing literature promotes laser therapy as an effective option that stimulates vaginal connective tissue to produce new collagen, which then promotes improved blood flow and tissue regeneration for vaginal lubrication and elasticity.
A study comparing fractional carbon dioxide vaginal laser treatment and local estrogen therapy in postmenopausal women with vulvovaginal atrophy found that laser therapy was an effective treatment for vulvovaginal atrophy (dyspareunia, dryness, and burning), both alone and with local estrogen.25
Despite the promising effects of laser therapy for treating vulvovaginal atrophy in GSM, studies have not determined its short-term or long-term safety profile. Furthermore, laser therapy does not improve impaired sexual function, ie, decreased libido, arousal, and sexual satisfaction. Another important consideration is that the cost of laser therapy in 2017 was estimated to be $2,000 to $3,000 per treatment, not covered by healthcare insurance.
CLINICAL APPROACH
Symptoms of GSM are common in breast cancer survivors, both pre- and postmenopausal, especially those treated with tamoxifen or an aromatase inhibitor. Estimates are that 60% of postmenopausal breast cancer survivors and 40% of premenopausal breast cancer survivors suffer from GSM.26 Unfortunately, many women do not seek medical attention for their symptoms.
A variety of hormonal and nonhormonal options are available for these patients. We recommend an interdisciplinary approach to treatment, with the decision to use hormonal options made in collaboration with the patient’s oncologist and the patient herself, in an informed, shared decision-making process that takes into consideration the risks and possible benefits depending on the symptoms.
The first step in selecting a management plan for GSM symptoms in women with breast cancer is to conduct a thorough assessment to provide data for individualizing the care plan. The decision to use a hormonal option should be made in collaboration with a woman’s oncologist and should include an informed decision-making process during which the potential risks and benefits, including the breast cancer impact, are fully disclosed.
Alternatives to systemic estrogen
Vaginal estrogen is an effective and safe option to treat GSM in women with either estrogen receptor-negative or estrogen receptor-positive breast cancer. It often completely cures the symptoms without any noticeable increase in serum estrogen levels.
Vaginal DHEA therapy is a nonestrogen option shown to effectively treat GSM without increasing systemic levels of estrogen or testosterone. This profile makes vaginal DHEA therapy a particularly attractive treatment for symptoms of GSM in women at risk for breast cancer.
Use of an estrogen receptor agonist/antagonist in breast cancer survivors needs careful consideration. Ospemifene has antiestrogenic effects that make it a good option for women with bone loss and those at high risk for breast cancer, but it should not be used concurrently with tamoxifen or raloxifene. Additionally, ospemifene does not cause uterine hyperplasia, so it can be used in women with a uterus.
Although more study is needed, we do have options to improve the overall quality of life in breast cancer survivors with GSM.
Many breast cancer survivors and women at high risk of breast cancer suffer from genitourinary syndrome of menopause (GSM), a term that encompasses any urinary, genital, or sexual dysfunction related to a hypoestrogenic state. Although GSM is usually caused by postmenopausal estrogen loss, it can also be caused by cancer treatments such as chemotherapy, radiation, and systemic endocrine therapy (eg, tamoxifen, aromatase inhibitors). These treatments can substantially decrease systemic estrogen levels, causing GSM symptoms that can profoundly worsen quality of life.
Managing GSM in these women poses a dilemma because systemic estrogen-containing therapies can increase the risk of breast cancer, and nonhormonal vaginal lubricants and moisturizers provide only minimal benefit. Fortunately, there are hormonal options, including locally applied estrogen, intravaginal dehydroepiandrosterone (DHEA), and estrogen receptor agonists/antagonists (ospemifene and bazedoxifene).
Here, we review the clinical management of GSM in breast cancer survivors and women at high risk of breast cancer and the efficacy and safety of available treatments, including their impact on breast cancer risk.
DRYNESS, IRRITATION, ATROPHY
The term GSM describes vulvovaginal and genitourinary symptoms associated with estrogen loss after menopause. Common symptoms are vaginal dryness, dyspareunia, irritation of genital skin, and pruritus.
LOCAL ESTROGEN THERAPY
Systemic estrogen therapy is widely used and effective for GSM, but there are concerns that it could increase the risk of breast cancer. After the Women’s Health Initiative in 2002 showed higher rates of cardiovascular disease and breast cancer with systemic estrogen-progestin use,5 the use of this hormone therapy declined by approximately 80%.6 Since then, healthcare providers have turned to local (ie, vaginal) estrogen therapies to manage GSM. These therapies have several advantages over systemic hormone therapy:
- Lower risk of adverse effects on the breast and cardiovascular system
- Greater efficacy in treating GSM
- In general, no need for progesterone when low-dose local estrogen is given to a woman with a uterus.7
Is locally applied estrogen systemically absorbed?
Despite these advantages, concerns remain as to whether vaginal estrogen therapy has adverse consequences associated with systemic absorption, particularly from atrophic vaginal tissues.
Santen,8 in a 2015 review of 33 studies, concluded that systemic absorption from low-dose vaginal estrogen is minimal, which provides some rationale for using it to treat vulvovaginal atrophy in postmenopausal women. This finding also suggests that the US Food and Drug Administration (FDA) “black box” warning of possible toxicities with vaginal estrogen is likely overstated, given that serum estrogen levels remained within normal postmenopausal levels.
Nevertheless, many providers are apprehensive about prescribing vaginal estrogen in women with a history of breast cancer because the threshold for systemic estrogen levels associated with breast cancer recurrence has not been established.
ACOG statement. In 2016, a committee of the American College of Obstetricians and Gynecologists cited data showing that low-dose vaginal estrogens do not result in sustained serum estrogen levels exceeding the normal postmenopausal range, and that the use of vaginal estrogens does not increase the risk of cancer recurrence.9 However, they recommend caution with vaginal estrogen use, especially in women with a history of estrogen-dependent breast cancer, reserving it for patients with GSM symptoms nonresponsive to nonhormonal treatment and specifying that it be used in low doses.
Vaginal estrogen formulations
Several types of locally applied estrogens are available, each with different properties and affinity for estrogen receptors. These include conjugated estrogens, 17-beta estradiol, estradiol acetate, and estradiol hemihydrate. Three delivery systems are FDA-approved: creams, rings, and tablets (Table 2).
Vaginal creams. Two vaginal creams are available, one (Estrace) containing 17-beta estradiol and the other (Premarin) containing conjugated estrogens.
The FDA-approved dosage for 17-beta estradiol is 2 to 4 g/day for 1 or 2 weeks, then gradually reduced to half the dose for a similar time. Maintenance dosing is 1 g 1 to 3 times per week. However, the ACOG statement notes that the FDA-approved dosages are higher than those proven to be effective and currently used in clinical practice, eg, 0.5 g twice a week.9
The FDA-approved dosage of conjugated estrogen cream for moderate to severe dyspareunia is 0.5 g daily for 21 days, then off for 7 days, or 0.5 g twice a week.
Vaginal tablets. The vaginal tablet Vagifem and its generic equivalent Yuvafem contain 10 µg of estradiol hemihydrate. The FDA-approved dosage is 10 µg daily for 2 weeks, followed by 10 µg twice a week, inserted into the lower third of the vagina. This dosage is significantly lower than that of estrogen creams.
Vaginal insert. A newly approved vaginal insert (Imvexxy) contains estradiol 4 µg (the lowest dose of vaginal estradiol available) or 10 µg, in a coconut oil vehicle. Its indications are for moderate to severe dyspareunia due to menopause and atrophic vaginitis due to menopause. A study cited in its package insert (www.accessdata.fda.gov/drugsatfda_docs/label/2018/208564s000lbl.pdf) showed that, in patients who used this product, systemic absorption of estradiol remained within the postmenopausal range. Its effects on breast cancer have not yet been studied.
Vaginal rings. Two vaginal rings are marketed. One (Estring) contains 17-beta estradiol, and the other (Femring) contains estradiol acetate. Only the 17-beta estradiol ring delivers a low dose to vaginal tissues, releasing 7.5 µg/day for 90 days. The estradiol acetate ring releases 0.05 mg/day or 0.10 mg/day and is a systemic treatment meant to be used with a progestin, not for local therapy.
VAGINAL ANDROGEN THERAPY: DHEA
After menopause, as the ovaries stop making estrogen from androstenedione, some production continues in other tissues, with DHEA as the primary precursor of androgens that are ultimately converted to estrogen. This has led to the theory that the cause of GSM is not estrogen deficiency but androgen deficiency. Evidence reviewed by Labrie et al11 shows that vulvovaginal atrophy is caused by decreased DHEA availability, which in turn causes sex steroid deficiency-related menopausal symptoms.11 Thus, it is reasonable to conclude that menopausal symptoms can be relieved by giving DHEA.
This theory has been borne out in clinical trials, in which DHEA in a vaginal tablet formulation increased the maturation of vaginal cells and lowered vaginal pH, leading to relief of GSM symptoms.12
The only DHEA product FDA-approved for treating GSM-related symptoms is prasterone (Intrarosa), indicated for moderate to severe dyspareunia due to vulvovaginal atrophy. The recommended dosing is a single 6.5-mg intravaginal tablet (0.5% prasterone) inserted nightly at bedtime. Its efficacy for treating hypoactive sexual desire disorder in postmenopausal women is being investigated.
Breast cancer implications
Because DHEA is converted to estrogen by aromatization, healthcare providers might hesitate to use it in women who have a history of hormone-sensitive cancer. Data on the safety of intravaginal DHEA use in breast cancer survivors are limited. However, studies have found that prasterone has highly beneficial effects on dyspareunia, vaginal dryness, and objective signs of vulvovaginal atrophy without significant drug-related adverse effects.12,13 In these studies, serum estrogen levels in women treated with DHEA were within the values observed in normal postmenopausal women. In addition, there are no aromatase enzymes in the endometrium, so even high doses of vaginal DHEA (in contrast to high doses of vaginal estrogen) will not stimulate the endometrium.
Clinically, this evidence indicates that DHEA exerts both estrogenic and androgenic activity in the vagina without increasing serum estrogen levels, making it a good alternative to topical estrogen therapy.
OSPEMIFENE: AN ESTROGEN RECEPTOR AGONIST/ANTAGONIST
Ospemifene (Osphena) is an estrogen receptor agonist/antagonist, a class of drugs previously called selective estrogen receptor modulators (SERMs). It is FDA-approved to treat moderate to severe dyspareunia secondary to vulvar and vaginal atrophy.
Ospemifene has unique estrogenic effects on the vaginal mucosa, having been shown to increase the number of epithelial cells, lower the vaginal pH, and decrease the percentage of parabasal cells seen on Papanicolaou smears after 12 weeks of use.14
Unlike tamoxifen, another drug of this class, ospemifene does not change the endometrial lining.14 Similarly, ospemifene acts as an estrogenic agonist in bone and, thus, has the potential for use in preventing and managing osteoporosis or for use in women at risk of fractures.
Breast cancer impact
In preclinical trials, ospemifene was found to have antiestrogenic effects on breast tissue, similar to those seen with tamoxifen.
In a model using human tumor grafts, ospemifene decreased tumor growth in mice implanted with estrogen receptor-positive breast cancer cells.15
In a mouse model using breast cancer cells that were biologically and histologically similar to those of humans, ospemifene had strong antiestrogenic effects on the breast tissue.16 The evidence suggests that ospemifene has a favorable effect on vulvar and vaginal atrophy.17
Ospemifene is FDA-approved to treat moderate to severe dyspareunia secondary to menopause. Recommended dosing is 60 mg/day orally with food.
Its antiestrogenic effects on breast tissue make it a promising option for women with a history of estrogen-receptor positive breast cancer. However, further study is needed to fully understand its effects on human breast tissue. According to the manufacturer’s package insert (www.osphena.com/files/pdf/osphena_prescribing_information.pdf), because the drug has not been adequately studied in women with breast cancer, it should not be used in women with known or suspected breast cancer or a history of breast cancer.
CONJUGATED ESTROGENS PLUS BAZEDOXIFENE
The combination of conjugated estrogens and bazedoxifene (Duavee) is a progesterone-free alternative for treating various menopausal symptoms. Bazedoxifene is another estrogen receptor agonist/antagonist, and it was added to counteract estrogen’s effects on the endometrium, thus replacing progesterone. This protective effect has been validated in clinical trials, which also found a favorable safety profile in breast tissue.18,19
SMART trials. The efficacy of this combination was studied in a series of large phase 3 multicenter trials called the SMART (Selective Estrogens, Menopause, and Response to Therapy) trials.20–23 Treated patients had markedly fewer vasomotor symptoms at 1 year, along with an increase in superficial cells and intermediate cells of the vaginal epithelium and a decrease in parabasal cells. They also had a substantial decrease in the incidence of dyspareunia.
Its effects on breast tissue were evaluated in the SMART-5 trial. Therapy had no net impact on breast density, suggesting that it has an estrogen-neutral effect on the breast.23
These results suggest that combined conjugated estrogens and bazedoxifene could be a noteworthy treatment option for GSM in women with a history of estrogen receptor-positive breast cancer, particularly in those with vasomotor symptoms and bone loss. However, the combination has not been studied specifically in breast cancer survivors.
Dosage. The FDA-approved dosing is 20 mg/0.45 mg per day orally to treat vasomotor symptoms, GSM, and osteoporosis in postmenopausal women with a uterus.
LASER THERAPY AND RADIOFREQUENCY HEAT: AN OFF-LABEL OPTION
Low-dose radiofrequency thermal therapy, delivered by carbon dioxide laser or by radiofrequency heat, has been used with some success to treat urinary stress incontinence and vaginal laxity in postpartum women. It may be an option for GSM, although it is not FDA-approved for this indication, and the FDA has recently issued a warning about it.24
Marketing literature promotes laser therapy as an effective option that stimulates vaginal connective tissue to produce new collagen, which then promotes improved blood flow and tissue regeneration for vaginal lubrication and elasticity.
A study comparing fractional carbon dioxide vaginal laser treatment and local estrogen therapy in postmenopausal women with vulvovaginal atrophy found that laser therapy was an effective treatment for vulvovaginal atrophy (dyspareunia, dryness, and burning), both alone and with local estrogen.25
Despite the promising effects of laser therapy for treating vulvovaginal atrophy in GSM, studies have not determined its short-term or long-term safety profile. Furthermore, laser therapy does not improve impaired sexual function, ie, decreased libido, arousal, and sexual satisfaction. Another important consideration is that the cost of laser therapy in 2017 was estimated to be $2,000 to $3,000 per treatment, not covered by healthcare insurance.
CLINICAL APPROACH
Symptoms of GSM are common in breast cancer survivors, both pre- and postmenopausal, especially those treated with tamoxifen or an aromatase inhibitor. Estimates are that 60% of postmenopausal breast cancer survivors and 40% of premenopausal breast cancer survivors suffer from GSM.26 Unfortunately, many women do not seek medical attention for their symptoms.
A variety of hormonal and nonhormonal options are available for these patients. We recommend an interdisciplinary approach to treatment, with the decision to use hormonal options made in collaboration with the patient’s oncologist and the patient herself, in an informed, shared decision-making process that takes into consideration the risks and possible benefits depending on the symptoms.
The first step in selecting a management plan for GSM symptoms in women with breast cancer is to conduct a thorough assessment to provide data for individualizing the care plan. The decision to use a hormonal option should be made in collaboration with a woman’s oncologist and should include an informed decision-making process during which the potential risks and benefits, including the breast cancer impact, are fully disclosed.
Alternatives to systemic estrogen
Vaginal estrogen is an effective and safe option to treat GSM in women with either estrogen receptor-negative or estrogen receptor-positive breast cancer. It often completely cures the symptoms without any noticeable increase in serum estrogen levels.
Vaginal DHEA therapy is a nonestrogen option shown to effectively treat GSM without increasing systemic levels of estrogen or testosterone. This profile makes vaginal DHEA therapy a particularly attractive treatment for symptoms of GSM in women at risk for breast cancer.
Use of an estrogen receptor agonist/antagonist in breast cancer survivors needs careful consideration. Ospemifene has antiestrogenic effects that make it a good option for women with bone loss and those at high risk for breast cancer, but it should not be used concurrently with tamoxifen or raloxifene. Additionally, ospemifene does not cause uterine hyperplasia, so it can be used in women with a uterus.
Although more study is needed, we do have options to improve the overall quality of life in breast cancer survivors with GSM.
- Lester J, Pahouja G, Andersen B, Lustberg M. Atrophic vaginitis in breast cancer survivors: a difficult survivorship issue. J Pers Med 2015; 5(2):50–66. doi:10.3390/jpm5020050
- Chin SN, Trinkaus M, Simmons C, et al. Prevalence and severity of urogenital symptoms in postmenopausal women receiving endocrine therapy for breast cancer. Clin Breast Cancer 2009; 9(2):108–117. doi:10.3816/CBC.2009.n.020
- Fallowfield L, Cella D, Cuzick J, Francis S, Locker G, Howell A. Quality of life of postmenopausal women in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) adjuvant breast cancer trial. J Clin Oncol 2004; 22(21):4261–4271. doi:10.1200/JCO.2004.08.029
- Cella D, Fallowfield LJ. Recognition and management of treatment-related side effects for breast cancer patients receiving adjuvant endocrine therapy. Breast Cancer Res Treat 2008; 107(2):167–180. doi:10.1007/s10549-007-9548-1
- Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002; 288(3):321–333. pmid:12117397
- Tsai SA, Stefanick ML, Stafford RS. Trends in menopausal hormone therapy use of US office-based physicians, 2000–2009. Menopause 2011; 18(4):385–392. doi:10.1097/gme.0b013e3181f43404
- North American Menopause Society. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause 2013; 20(9):888–902. doi:10.1097/GME.0b013e3182a122c2
- Santen RJ. Vaginal administration of estradiol: effects of dose, preparation and timing on plasma estradiol levels. Climacteric 2015; 18(2):121–134. doi:10.3109/13697137.2014.947254
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: the use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol 2016; 127(3):e93–e96. doi:10.1097/AOG.0000000000001351
- Santoro N, Epperson CN, Mathews SB. Menopausal symptoms and their management. Endocrinol Metab Clin North Am 2015; 44(3):497–515. doi:10.1016/j.ecl.2015.05.001
- Labrie F, Archer DF, Martel C, Vaillancourt M, Montesino M. Combined data of intravaginal prasterone against vulvovaginal atrophy of menopause. Menopause 2017; 24(11):1246–1256. doi:10.1097/GME.0000000000000910
- Labrie F, Archer D, Bouchard C, et al. Serum steroid levels during 12-week intravaginal dehydroepiandrosterone administration. Menopause 2009; 16(5):897–906. doi:10.1097/gme.0b013e31819e8930
- Labrie F, Cusan L, Gomez JL, et al. Effect of intravaginal DHEA on serum DHEA and eleven of its metabolites in postmenopausal women. J Steroid Biochem Mol Biol 2008; 111(3-5):178–194. doi:10.1016/j.jsbmb.2008.06.003
- Soe LH, Wurz GT, Kao CJ, Degregorio MW. Ospemifene for the treatment of dyspareunia associated with vulvar and vaginal atrophy: potential benefits in bone and breast. Int J Womens Health 2013; 5:605–611. doi:10.2147/IJWH.S39146
- Taras TL, Wurz GT, DeGregorio MW. In vitro and in vivo biologic effects of ospemifene (FC-1271a) in breast cancer. J Steroid Biochem Mol Biol 2001; 77(4–5):271–279. pmid:11457665
- Wurz GT, Read KC, Marchisano-Karpman C, et al. Ospemifene inhibits the growth of dimethylbenzanthracene-induced mammary tumors in Sencar mice. J Steroid Biochem Mol Biol 2005; 97(3):230–240. doi:10.1016/j.jsbmb.2005.06.027
- Archer DF, Carr BR, Pinkerton JV, Taylor HS, Constantine GD. Effects of ospemifene on the female reproductive and urinary tracts: translation from preclinical models into clinical evidence. Menopause 2015; 22(7):786–796. doi:10.1097/GME.0000000000000365
- Mirkin S, Pickar JH. Management of osteoporosis and menopausal symptoms: focus on bazedoxifene/conjugated estrogen combination. Int J Womens Health 2013; 5:465–475. doi:10.2147/IJWH.S39455
- Kagan R, Goldstein SR, Pickar JH, Komm BS. Patient considerations in the management of menopausal symptoms: role of conjugated estrogens with bazedoxifene. Ther Clin Risk Manag 2016; 12:549–562. doi:10.2147/TCRM.S63833
- Lobo RA, Pinkerton JV, Gass ML, et al. Evaluation of bazedoxifene/conjugated estrogens for the treatment of menopausal symptoms and effects on metabolic parameters and overall safety profile. Fertil Steril 2009; 92(3):1025–1038. doi:10.1016/j.fertnstert.2009.03.113
- Pinkerton JV, Utian WH, Constantine GD, Olivier S, Pickar JH. Relief of vasomotor symptoms with the tissue-selective estrogen complex containing bazedoxifene/conjugated estrogens: a randomized, controlled trial. Menopause 2009; 16(6):1116–1124. doi:10.1097/gme.0b013e3181a7df0d
- Kagan R, Williams RS, Pan K, Mirkin S, Pickar JH. A randomized, placebo- and active-controlled trial of bazedoxifene/conjugated estrogens for treatment of moderate to severe vulvar/vaginal atrophy in postmenopausal women. Menopause 2010; 17(2):281–289. doi:10.1097/GME.0b013e3181b7c65f
- Pinkerton JV, Harvey JA, Pan K, et al. Breast effects of bazedoxifene-conjugated estrogens: a randomized controlled trial. Obstet Gynecol 2013; 121(5):959–968. doi:10.1097/AOG.0b013e31828c5974
- FDA. U.S. Food & Drug Administration. FDA Statement. Statement from FDA Commissioner Scott Gottlieb, M.D., on efforts to safeguard women’s health from deceptive health claims and significant risks related to devices marketed for use in medical procedures for “vaginal rejuvenation.” www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm615130.htm. Accessed August 20, 2018.
- Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause 2018; 25(1):21–28. doi:10.1097/GME.0000000000000955
- Biglia N, Bounous VE, D’Alonzo M, et al. Vaginal atrophy in breast cancer survivors: attitude and approaches among oncologists. Clin Breast Cancer 2017; 17(8):611–617. doi:10.1016/j.clbc.2017.05.008
- Lester J, Pahouja G, Andersen B, Lustberg M. Atrophic vaginitis in breast cancer survivors: a difficult survivorship issue. J Pers Med 2015; 5(2):50–66. doi:10.3390/jpm5020050
- Chin SN, Trinkaus M, Simmons C, et al. Prevalence and severity of urogenital symptoms in postmenopausal women receiving endocrine therapy for breast cancer. Clin Breast Cancer 2009; 9(2):108–117. doi:10.3816/CBC.2009.n.020
- Fallowfield L, Cella D, Cuzick J, Francis S, Locker G, Howell A. Quality of life of postmenopausal women in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) adjuvant breast cancer trial. J Clin Oncol 2004; 22(21):4261–4271. doi:10.1200/JCO.2004.08.029
- Cella D, Fallowfield LJ. Recognition and management of treatment-related side effects for breast cancer patients receiving adjuvant endocrine therapy. Breast Cancer Res Treat 2008; 107(2):167–180. doi:10.1007/s10549-007-9548-1
- Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002; 288(3):321–333. pmid:12117397
- Tsai SA, Stefanick ML, Stafford RS. Trends in menopausal hormone therapy use of US office-based physicians, 2000–2009. Menopause 2011; 18(4):385–392. doi:10.1097/gme.0b013e3181f43404
- North American Menopause Society. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause 2013; 20(9):888–902. doi:10.1097/GME.0b013e3182a122c2
- Santen RJ. Vaginal administration of estradiol: effects of dose, preparation and timing on plasma estradiol levels. Climacteric 2015; 18(2):121–134. doi:10.3109/13697137.2014.947254
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: the use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol 2016; 127(3):e93–e96. doi:10.1097/AOG.0000000000001351
- Santoro N, Epperson CN, Mathews SB. Menopausal symptoms and their management. Endocrinol Metab Clin North Am 2015; 44(3):497–515. doi:10.1016/j.ecl.2015.05.001
- Labrie F, Archer DF, Martel C, Vaillancourt M, Montesino M. Combined data of intravaginal prasterone against vulvovaginal atrophy of menopause. Menopause 2017; 24(11):1246–1256. doi:10.1097/GME.0000000000000910
- Labrie F, Archer D, Bouchard C, et al. Serum steroid levels during 12-week intravaginal dehydroepiandrosterone administration. Menopause 2009; 16(5):897–906. doi:10.1097/gme.0b013e31819e8930
- Labrie F, Cusan L, Gomez JL, et al. Effect of intravaginal DHEA on serum DHEA and eleven of its metabolites in postmenopausal women. J Steroid Biochem Mol Biol 2008; 111(3-5):178–194. doi:10.1016/j.jsbmb.2008.06.003
- Soe LH, Wurz GT, Kao CJ, Degregorio MW. Ospemifene for the treatment of dyspareunia associated with vulvar and vaginal atrophy: potential benefits in bone and breast. Int J Womens Health 2013; 5:605–611. doi:10.2147/IJWH.S39146
- Taras TL, Wurz GT, DeGregorio MW. In vitro and in vivo biologic effects of ospemifene (FC-1271a) in breast cancer. J Steroid Biochem Mol Biol 2001; 77(4–5):271–279. pmid:11457665
- Wurz GT, Read KC, Marchisano-Karpman C, et al. Ospemifene inhibits the growth of dimethylbenzanthracene-induced mammary tumors in Sencar mice. J Steroid Biochem Mol Biol 2005; 97(3):230–240. doi:10.1016/j.jsbmb.2005.06.027
- Archer DF, Carr BR, Pinkerton JV, Taylor HS, Constantine GD. Effects of ospemifene on the female reproductive and urinary tracts: translation from preclinical models into clinical evidence. Menopause 2015; 22(7):786–796. doi:10.1097/GME.0000000000000365
- Mirkin S, Pickar JH. Management of osteoporosis and menopausal symptoms: focus on bazedoxifene/conjugated estrogen combination. Int J Womens Health 2013; 5:465–475. doi:10.2147/IJWH.S39455
- Kagan R, Goldstein SR, Pickar JH, Komm BS. Patient considerations in the management of menopausal symptoms: role of conjugated estrogens with bazedoxifene. Ther Clin Risk Manag 2016; 12:549–562. doi:10.2147/TCRM.S63833
- Lobo RA, Pinkerton JV, Gass ML, et al. Evaluation of bazedoxifene/conjugated estrogens for the treatment of menopausal symptoms and effects on metabolic parameters and overall safety profile. Fertil Steril 2009; 92(3):1025–1038. doi:10.1016/j.fertnstert.2009.03.113
- Pinkerton JV, Utian WH, Constantine GD, Olivier S, Pickar JH. Relief of vasomotor symptoms with the tissue-selective estrogen complex containing bazedoxifene/conjugated estrogens: a randomized, controlled trial. Menopause 2009; 16(6):1116–1124. doi:10.1097/gme.0b013e3181a7df0d
- Kagan R, Williams RS, Pan K, Mirkin S, Pickar JH. A randomized, placebo- and active-controlled trial of bazedoxifene/conjugated estrogens for treatment of moderate to severe vulvar/vaginal atrophy in postmenopausal women. Menopause 2010; 17(2):281–289. doi:10.1097/GME.0b013e3181b7c65f
- Pinkerton JV, Harvey JA, Pan K, et al. Breast effects of bazedoxifene-conjugated estrogens: a randomized controlled trial. Obstet Gynecol 2013; 121(5):959–968. doi:10.1097/AOG.0b013e31828c5974
- FDA. U.S. Food & Drug Administration. FDA Statement. Statement from FDA Commissioner Scott Gottlieb, M.D., on efforts to safeguard women’s health from deceptive health claims and significant risks related to devices marketed for use in medical procedures for “vaginal rejuvenation.” www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm615130.htm. Accessed August 20, 2018.
- Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause 2018; 25(1):21–28. doi:10.1097/GME.0000000000000955
- Biglia N, Bounous VE, D’Alonzo M, et al. Vaginal atrophy in breast cancer survivors: attitude and approaches among oncologists. Clin Breast Cancer 2017; 17(8):611–617. doi:10.1016/j.clbc.2017.05.008
KEY POINTS
- In general, locally applied hormonal therapies relieve GSM symptoms without increasing breast cancer risk.
- DHEA relieves vaginal symptoms without increasing serum estrogen levels.
- Ospemifene has antiestrogenic effects on breast tissue that make it an attractive option for women with breast cancer.
- The combination of conjugated estrogens and bazedoxifene offers a progesterone-free treatment for GSM symptoms in women desiring systemic hormone therapy.