User login
Genitourinary syndrome of menopause in breast cancer survivors: Treatments are available
Many breast cancer survivors and women at high risk of breast cancer suffer from genitourinary syndrome of menopause (GSM), a term that encompasses any urinary, genital, or sexual dysfunction related to a hypoestrogenic state. Although GSM is usually caused by postmenopausal estrogen loss, it can also be caused by cancer treatments such as chemotherapy, radiation, and systemic endocrine therapy (eg, tamoxifen, aromatase inhibitors). These treatments can substantially decrease systemic estrogen levels, causing GSM symptoms that can profoundly worsen quality of life.
Managing GSM in these women poses a dilemma because systemic estrogen-containing therapies can increase the risk of breast cancer, and nonhormonal vaginal lubricants and moisturizers provide only minimal benefit. Fortunately, there are hormonal options, including locally applied estrogen, intravaginal dehydroepiandrosterone (DHEA), and estrogen receptor agonists/antagonists (ospemifene and bazedoxifene).
Here, we review the clinical management of GSM in breast cancer survivors and women at high risk of breast cancer and the efficacy and safety of available treatments, including their impact on breast cancer risk.
DRYNESS, IRRITATION, ATROPHY
The term GSM describes vulvovaginal and genitourinary symptoms associated with estrogen loss after menopause. Common symptoms are vaginal dryness, dyspareunia, irritation of genital skin, and pruritus.
LOCAL ESTROGEN THERAPY
Systemic estrogen therapy is widely used and effective for GSM, but there are concerns that it could increase the risk of breast cancer. After the Women’s Health Initiative in 2002 showed higher rates of cardiovascular disease and breast cancer with systemic estrogen-progestin use,5 the use of this hormone therapy declined by approximately 80%.6 Since then, healthcare providers have turned to local (ie, vaginal) estrogen therapies to manage GSM. These therapies have several advantages over systemic hormone therapy:
- Lower risk of adverse effects on the breast and cardiovascular system
- Greater efficacy in treating GSM
- In general, no need for progesterone when low-dose local estrogen is given to a woman with a uterus.7
Is locally applied estrogen systemically absorbed?
Despite these advantages, concerns remain as to whether vaginal estrogen therapy has adverse consequences associated with systemic absorption, particularly from atrophic vaginal tissues.
Santen,8 in a 2015 review of 33 studies, concluded that systemic absorption from low-dose vaginal estrogen is minimal, which provides some rationale for using it to treat vulvovaginal atrophy in postmenopausal women. This finding also suggests that the US Food and Drug Administration (FDA) “black box” warning of possible toxicities with vaginal estrogen is likely overstated, given that serum estrogen levels remained within normal postmenopausal levels.
Nevertheless, many providers are apprehensive about prescribing vaginal estrogen in women with a history of breast cancer because the threshold for systemic estrogen levels associated with breast cancer recurrence has not been established.
ACOG statement. In 2016, a committee of the American College of Obstetricians and Gynecologists cited data showing that low-dose vaginal estrogens do not result in sustained serum estrogen levels exceeding the normal postmenopausal range, and that the use of vaginal estrogens does not increase the risk of cancer recurrence.9 However, they recommend caution with vaginal estrogen use, especially in women with a history of estrogen-dependent breast cancer, reserving it for patients with GSM symptoms nonresponsive to nonhormonal treatment and specifying that it be used in low doses.
Vaginal estrogen formulations
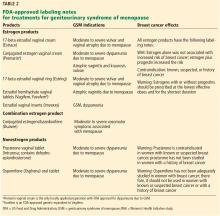
Several types of locally applied estrogens are available, each with different properties and affinity for estrogen receptors. These include conjugated estrogens, 17-beta estradiol, estradiol acetate, and estradiol hemihydrate. Three delivery systems are FDA-approved: creams, rings, and tablets (Table 2).
Vaginal creams. Two vaginal creams are available, one (Estrace) containing 17-beta estradiol and the other (Premarin) containing conjugated estrogens.
The FDA-approved dosage for 17-beta estradiol is 2 to 4 g/day for 1 or 2 weeks, then gradually reduced to half the dose for a similar time. Maintenance dosing is 1 g 1 to 3 times per week. However, the ACOG statement notes that the FDA-approved dosages are higher than those proven to be effective and currently used in clinical practice, eg, 0.5 g twice a week.9
The FDA-approved dosage of conjugated estrogen cream for moderate to severe dyspareunia is 0.5 g daily for 21 days, then off for 7 days, or 0.5 g twice a week.
Vaginal tablets. The vaginal tablet Vagifem and its generic equivalent Yuvafem contain 10 µg of estradiol hemihydrate. The FDA-approved dosage is 10 µg daily for 2 weeks, followed by 10 µg twice a week, inserted into the lower third of the vagina. This dosage is significantly lower than that of estrogen creams.
Vaginal insert. A newly approved vaginal insert (Imvexxy) contains estradiol 4 µg (the lowest dose of vaginal estradiol available) or 10 µg, in a coconut oil vehicle. Its indications are for moderate to severe dyspareunia due to menopause and atrophic vaginitis due to menopause. A study cited in its package insert (www.accessdata.fda.gov/drugsatfda_docs/label/2018/208564s000lbl.pdf) showed that, in patients who used this product, systemic absorption of estradiol remained within the postmenopausal range. Its effects on breast cancer have not yet been studied.
Vaginal rings. Two vaginal rings are marketed. One (Estring) contains 17-beta estradiol, and the other (Femring) contains estradiol acetate. Only the 17-beta estradiol ring delivers a low dose to vaginal tissues, releasing 7.5 µg/day for 90 days. The estradiol acetate ring releases 0.05 mg/day or 0.10 mg/day and is a systemic treatment meant to be used with a progestin, not for local therapy.
VAGINAL ANDROGEN THERAPY: DHEA
After menopause, as the ovaries stop making estrogen from androstenedione, some production continues in other tissues, with DHEA as the primary precursor of androgens that are ultimately converted to estrogen. This has led to the theory that the cause of GSM is not estrogen deficiency but androgen deficiency. Evidence reviewed by Labrie et al11 shows that vulvovaginal atrophy is caused by decreased DHEA availability, which in turn causes sex steroid deficiency-related menopausal symptoms.11 Thus, it is reasonable to conclude that menopausal symptoms can be relieved by giving DHEA.
This theory has been borne out in clinical trials, in which DHEA in a vaginal tablet formulation increased the maturation of vaginal cells and lowered vaginal pH, leading to relief of GSM symptoms.12
The only DHEA product FDA-approved for treating GSM-related symptoms is prasterone (Intrarosa), indicated for moderate to severe dyspareunia due to vulvovaginal atrophy. The recommended dosing is a single 6.5-mg intravaginal tablet (0.5% prasterone) inserted nightly at bedtime. Its efficacy for treating hypoactive sexual desire disorder in postmenopausal women is being investigated.
Breast cancer implications
Because DHEA is converted to estrogen by aromatization, healthcare providers might hesitate to use it in women who have a history of hormone-sensitive cancer. Data on the safety of intravaginal DHEA use in breast cancer survivors are limited. However, studies have found that prasterone has highly beneficial effects on dyspareunia, vaginal dryness, and objective signs of vulvovaginal atrophy without significant drug-related adverse effects.12,13 In these studies, serum estrogen levels in women treated with DHEA were within the values observed in normal postmenopausal women. In addition, there are no aromatase enzymes in the endometrium, so even high doses of vaginal DHEA (in contrast to high doses of vaginal estrogen) will not stimulate the endometrium.
Clinically, this evidence indicates that DHEA exerts both estrogenic and androgenic activity in the vagina without increasing serum estrogen levels, making it a good alternative to topical estrogen therapy.
OSPEMIFENE: AN ESTROGEN RECEPTOR AGONIST/ANTAGONIST
Ospemifene (Osphena) is an estrogen receptor agonist/antagonist, a class of drugs previously called selective estrogen receptor modulators (SERMs). It is FDA-approved to treat moderate to severe dyspareunia secondary to vulvar and vaginal atrophy.
Ospemifene has unique estrogenic effects on the vaginal mucosa, having been shown to increase the number of epithelial cells, lower the vaginal pH, and decrease the percentage of parabasal cells seen on Papanicolaou smears after 12 weeks of use.14
Unlike tamoxifen, another drug of this class, ospemifene does not change the endometrial lining.14 Similarly, ospemifene acts as an estrogenic agonist in bone and, thus, has the potential for use in preventing and managing osteoporosis or for use in women at risk of fractures.
Breast cancer impact
In preclinical trials, ospemifene was found to have antiestrogenic effects on breast tissue, similar to those seen with tamoxifen.
In a model using human tumor grafts, ospemifene decreased tumor growth in mice implanted with estrogen receptor-positive breast cancer cells.15
In a mouse model using breast cancer cells that were biologically and histologically similar to those of humans, ospemifene had strong antiestrogenic effects on the breast tissue.16 The evidence suggests that ospemifene has a favorable effect on vulvar and vaginal atrophy.17
Ospemifene is FDA-approved to treat moderate to severe dyspareunia secondary to menopause. Recommended dosing is 60 mg/day orally with food.
Its antiestrogenic effects on breast tissue make it a promising option for women with a history of estrogen-receptor positive breast cancer. However, further study is needed to fully understand its effects on human breast tissue. According to the manufacturer’s package insert (www.osphena.com/files/pdf/osphena_prescribing_information.pdf), because the drug has not been adequately studied in women with breast cancer, it should not be used in women with known or suspected breast cancer or a history of breast cancer.
CONJUGATED ESTROGENS PLUS BAZEDOXIFENE
The combination of conjugated estrogens and bazedoxifene (Duavee) is a progesterone-free alternative for treating various menopausal symptoms. Bazedoxifene is another estrogen receptor agonist/antagonist, and it was added to counteract estrogen’s effects on the endometrium, thus replacing progesterone. This protective effect has been validated in clinical trials, which also found a favorable safety profile in breast tissue.18,19
SMART trials. The efficacy of this combination was studied in a series of large phase 3 multicenter trials called the SMART (Selective Estrogens, Menopause, and Response to Therapy) trials.20–23 Treated patients had markedly fewer vasomotor symptoms at 1 year, along with an increase in superficial cells and intermediate cells of the vaginal epithelium and a decrease in parabasal cells. They also had a substantial decrease in the incidence of dyspareunia.
Its effects on breast tissue were evaluated in the SMART-5 trial. Therapy had no net impact on breast density, suggesting that it has an estrogen-neutral effect on the breast.23
These results suggest that combined conjugated estrogens and bazedoxifene could be a noteworthy treatment option for GSM in women with a history of estrogen receptor-positive breast cancer, particularly in those with vasomotor symptoms and bone loss. However, the combination has not been studied specifically in breast cancer survivors.
Dosage. The FDA-approved dosing is 20 mg/0.45 mg per day orally to treat vasomotor symptoms, GSM, and osteoporosis in postmenopausal women with a uterus.
LASER THERAPY AND RADIOFREQUENCY HEAT: AN OFF-LABEL OPTION
Low-dose radiofrequency thermal therapy, delivered by carbon dioxide laser or by radiofrequency heat, has been used with some success to treat urinary stress incontinence and vaginal laxity in postpartum women. It may be an option for GSM, although it is not FDA-approved for this indication, and the FDA has recently issued a warning about it.24
Marketing literature promotes laser therapy as an effective option that stimulates vaginal connective tissue to produce new collagen, which then promotes improved blood flow and tissue regeneration for vaginal lubrication and elasticity.
A study comparing fractional carbon dioxide vaginal laser treatment and local estrogen therapy in postmenopausal women with vulvovaginal atrophy found that laser therapy was an effective treatment for vulvovaginal atrophy (dyspareunia, dryness, and burning), both alone and with local estrogen.25
Despite the promising effects of laser therapy for treating vulvovaginal atrophy in GSM, studies have not determined its short-term or long-term safety profile. Furthermore, laser therapy does not improve impaired sexual function, ie, decreased libido, arousal, and sexual satisfaction. Another important consideration is that the cost of laser therapy in 2017 was estimated to be $2,000 to $3,000 per treatment, not covered by healthcare insurance.
CLINICAL APPROACH
Symptoms of GSM are common in breast cancer survivors, both pre- and postmenopausal, especially those treated with tamoxifen or an aromatase inhibitor. Estimates are that 60% of postmenopausal breast cancer survivors and 40% of premenopausal breast cancer survivors suffer from GSM.26 Unfortunately, many women do not seek medical attention for their symptoms.
A variety of hormonal and nonhormonal options are available for these patients. We recommend an interdisciplinary approach to treatment, with the decision to use hormonal options made in collaboration with the patient’s oncologist and the patient herself, in an informed, shared decision-making process that takes into consideration the risks and possible benefits depending on the symptoms.
The first step in selecting a management plan for GSM symptoms in women with breast cancer is to conduct a thorough assessment to provide data for individualizing the care plan. The decision to use a hormonal option should be made in collaboration with a woman’s oncologist and should include an informed decision-making process during which the potential risks and benefits, including the breast cancer impact, are fully disclosed.
Alternatives to systemic estrogen
Vaginal estrogen is an effective and safe option to treat GSM in women with either estrogen receptor-negative or estrogen receptor-positive breast cancer. It often completely cures the symptoms without any noticeable increase in serum estrogen levels.
Vaginal DHEA therapy is a nonestrogen option shown to effectively treat GSM without increasing systemic levels of estrogen or testosterone. This profile makes vaginal DHEA therapy a particularly attractive treatment for symptoms of GSM in women at risk for breast cancer.
Use of an estrogen receptor agonist/antagonist in breast cancer survivors needs careful consideration. Ospemifene has antiestrogenic effects that make it a good option for women with bone loss and those at high risk for breast cancer, but it should not be used concurrently with tamoxifen or raloxifene. Additionally, ospemifene does not cause uterine hyperplasia, so it can be used in women with a uterus.
Although more study is needed, we do have options to improve the overall quality of life in breast cancer survivors with GSM.
- Lester J, Pahouja G, Andersen B, Lustberg M. Atrophic vaginitis in breast cancer survivors: a difficult survivorship issue. J Pers Med 2015; 5(2):50–66. doi:10.3390/jpm5020050
- Chin SN, Trinkaus M, Simmons C, et al. Prevalence and severity of urogenital symptoms in postmenopausal women receiving endocrine therapy for breast cancer. Clin Breast Cancer 2009; 9(2):108–117. doi:10.3816/CBC.2009.n.020
- Fallowfield L, Cella D, Cuzick J, Francis S, Locker G, Howell A. Quality of life of postmenopausal women in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) adjuvant breast cancer trial. J Clin Oncol 2004; 22(21):4261–4271. doi:10.1200/JCO.2004.08.029
- Cella D, Fallowfield LJ. Recognition and management of treatment-related side effects for breast cancer patients receiving adjuvant endocrine therapy. Breast Cancer Res Treat 2008; 107(2):167–180. doi:10.1007/s10549-007-9548-1
- Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002; 288(3):321–333. pmid:12117397
- Tsai SA, Stefanick ML, Stafford RS. Trends in menopausal hormone therapy use of US office-based physicians, 2000–2009. Menopause 2011; 18(4):385–392. doi:10.1097/gme.0b013e3181f43404
- North American Menopause Society. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause 2013; 20(9):888–902. doi:10.1097/GME.0b013e3182a122c2
- Santen RJ. Vaginal administration of estradiol: effects of dose, preparation and timing on plasma estradiol levels. Climacteric 2015; 18(2):121–134. doi:10.3109/13697137.2014.947254
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: the use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol 2016; 127(3):e93–e96. doi:10.1097/AOG.0000000000001351
- Santoro N, Epperson CN, Mathews SB. Menopausal symptoms and their management. Endocrinol Metab Clin North Am 2015; 44(3):497–515. doi:10.1016/j.ecl.2015.05.001
- Labrie F, Archer DF, Martel C, Vaillancourt M, Montesino M. Combined data of intravaginal prasterone against vulvovaginal atrophy of menopause. Menopause 2017; 24(11):1246–1256. doi:10.1097/GME.0000000000000910
- Labrie F, Archer D, Bouchard C, et al. Serum steroid levels during 12-week intravaginal dehydroepiandrosterone administration. Menopause 2009; 16(5):897–906. doi:10.1097/gme.0b013e31819e8930
- Labrie F, Cusan L, Gomez JL, et al. Effect of intravaginal DHEA on serum DHEA and eleven of its metabolites in postmenopausal women. J Steroid Biochem Mol Biol 2008; 111(3-5):178–194. doi:10.1016/j.jsbmb.2008.06.003
- Soe LH, Wurz GT, Kao CJ, Degregorio MW. Ospemifene for the treatment of dyspareunia associated with vulvar and vaginal atrophy: potential benefits in bone and breast. Int J Womens Health 2013; 5:605–611. doi:10.2147/IJWH.S39146
- Taras TL, Wurz GT, DeGregorio MW. In vitro and in vivo biologic effects of ospemifene (FC-1271a) in breast cancer. J Steroid Biochem Mol Biol 2001; 77(4–5):271–279. pmid:11457665
- Wurz GT, Read KC, Marchisano-Karpman C, et al. Ospemifene inhibits the growth of dimethylbenzanthracene-induced mammary tumors in Sencar mice. J Steroid Biochem Mol Biol 2005; 97(3):230–240. doi:10.1016/j.jsbmb.2005.06.027
- Archer DF, Carr BR, Pinkerton JV, Taylor HS, Constantine GD. Effects of ospemifene on the female reproductive and urinary tracts: translation from preclinical models into clinical evidence. Menopause 2015; 22(7):786–796. doi:10.1097/GME.0000000000000365
- Mirkin S, Pickar JH. Management of osteoporosis and menopausal symptoms: focus on bazedoxifene/conjugated estrogen combination. Int J Womens Health 2013; 5:465–475. doi:10.2147/IJWH.S39455
- Kagan R, Goldstein SR, Pickar JH, Komm BS. Patient considerations in the management of menopausal symptoms: role of conjugated estrogens with bazedoxifene. Ther Clin Risk Manag 2016; 12:549–562. doi:10.2147/TCRM.S63833
- Lobo RA, Pinkerton JV, Gass ML, et al. Evaluation of bazedoxifene/conjugated estrogens for the treatment of menopausal symptoms and effects on metabolic parameters and overall safety profile. Fertil Steril 2009; 92(3):1025–1038. doi:10.1016/j.fertnstert.2009.03.113
- Pinkerton JV, Utian WH, Constantine GD, Olivier S, Pickar JH. Relief of vasomotor symptoms with the tissue-selective estrogen complex containing bazedoxifene/conjugated estrogens: a randomized, controlled trial. Menopause 2009; 16(6):1116–1124. doi:10.1097/gme.0b013e3181a7df0d
- Kagan R, Williams RS, Pan K, Mirkin S, Pickar JH. A randomized, placebo- and active-controlled trial of bazedoxifene/conjugated estrogens for treatment of moderate to severe vulvar/vaginal atrophy in postmenopausal women. Menopause 2010; 17(2):281–289. doi:10.1097/GME.0b013e3181b7c65f
- Pinkerton JV, Harvey JA, Pan K, et al. Breast effects of bazedoxifene-conjugated estrogens: a randomized controlled trial. Obstet Gynecol 2013; 121(5):959–968. doi:10.1097/AOG.0b013e31828c5974
- FDA. U.S. Food & Drug Administration. FDA Statement. Statement from FDA Commissioner Scott Gottlieb, M.D., on efforts to safeguard women’s health from deceptive health claims and significant risks related to devices marketed for use in medical procedures for “vaginal rejuvenation.” www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm615130.htm. Accessed August 20, 2018.
- Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause 2018; 25(1):21–28. doi:10.1097/GME.0000000000000955
- Biglia N, Bounous VE, D’Alonzo M, et al. Vaginal atrophy in breast cancer survivors: attitude and approaches among oncologists. Clin Breast Cancer 2017; 17(8):611–617. doi:10.1016/j.clbc.2017.05.008
Many breast cancer survivors and women at high risk of breast cancer suffer from genitourinary syndrome of menopause (GSM), a term that encompasses any urinary, genital, or sexual dysfunction related to a hypoestrogenic state. Although GSM is usually caused by postmenopausal estrogen loss, it can also be caused by cancer treatments such as chemotherapy, radiation, and systemic endocrine therapy (eg, tamoxifen, aromatase inhibitors). These treatments can substantially decrease systemic estrogen levels, causing GSM symptoms that can profoundly worsen quality of life.
Managing GSM in these women poses a dilemma because systemic estrogen-containing therapies can increase the risk of breast cancer, and nonhormonal vaginal lubricants and moisturizers provide only minimal benefit. Fortunately, there are hormonal options, including locally applied estrogen, intravaginal dehydroepiandrosterone (DHEA), and estrogen receptor agonists/antagonists (ospemifene and bazedoxifene).
Here, we review the clinical management of GSM in breast cancer survivors and women at high risk of breast cancer and the efficacy and safety of available treatments, including their impact on breast cancer risk.
DRYNESS, IRRITATION, ATROPHY
The term GSM describes vulvovaginal and genitourinary symptoms associated with estrogen loss after menopause. Common symptoms are vaginal dryness, dyspareunia, irritation of genital skin, and pruritus.
LOCAL ESTROGEN THERAPY
Systemic estrogen therapy is widely used and effective for GSM, but there are concerns that it could increase the risk of breast cancer. After the Women’s Health Initiative in 2002 showed higher rates of cardiovascular disease and breast cancer with systemic estrogen-progestin use,5 the use of this hormone therapy declined by approximately 80%.6 Since then, healthcare providers have turned to local (ie, vaginal) estrogen therapies to manage GSM. These therapies have several advantages over systemic hormone therapy:
- Lower risk of adverse effects on the breast and cardiovascular system
- Greater efficacy in treating GSM
- In general, no need for progesterone when low-dose local estrogen is given to a woman with a uterus.7
Is locally applied estrogen systemically absorbed?
Despite these advantages, concerns remain as to whether vaginal estrogen therapy has adverse consequences associated with systemic absorption, particularly from atrophic vaginal tissues.
Santen,8 in a 2015 review of 33 studies, concluded that systemic absorption from low-dose vaginal estrogen is minimal, which provides some rationale for using it to treat vulvovaginal atrophy in postmenopausal women. This finding also suggests that the US Food and Drug Administration (FDA) “black box” warning of possible toxicities with vaginal estrogen is likely overstated, given that serum estrogen levels remained within normal postmenopausal levels.
Nevertheless, many providers are apprehensive about prescribing vaginal estrogen in women with a history of breast cancer because the threshold for systemic estrogen levels associated with breast cancer recurrence has not been established.
ACOG statement. In 2016, a committee of the American College of Obstetricians and Gynecologists cited data showing that low-dose vaginal estrogens do not result in sustained serum estrogen levels exceeding the normal postmenopausal range, and that the use of vaginal estrogens does not increase the risk of cancer recurrence.9 However, they recommend caution with vaginal estrogen use, especially in women with a history of estrogen-dependent breast cancer, reserving it for patients with GSM symptoms nonresponsive to nonhormonal treatment and specifying that it be used in low doses.
Vaginal estrogen formulations
Several types of locally applied estrogens are available, each with different properties and affinity for estrogen receptors. These include conjugated estrogens, 17-beta estradiol, estradiol acetate, and estradiol hemihydrate. Three delivery systems are FDA-approved: creams, rings, and tablets (Table 2).
Vaginal creams. Two vaginal creams are available, one (Estrace) containing 17-beta estradiol and the other (Premarin) containing conjugated estrogens.
The FDA-approved dosage for 17-beta estradiol is 2 to 4 g/day for 1 or 2 weeks, then gradually reduced to half the dose for a similar time. Maintenance dosing is 1 g 1 to 3 times per week. However, the ACOG statement notes that the FDA-approved dosages are higher than those proven to be effective and currently used in clinical practice, eg, 0.5 g twice a week.9
The FDA-approved dosage of conjugated estrogen cream for moderate to severe dyspareunia is 0.5 g daily for 21 days, then off for 7 days, or 0.5 g twice a week.
Vaginal tablets. The vaginal tablet Vagifem and its generic equivalent Yuvafem contain 10 µg of estradiol hemihydrate. The FDA-approved dosage is 10 µg daily for 2 weeks, followed by 10 µg twice a week, inserted into the lower third of the vagina. This dosage is significantly lower than that of estrogen creams.
Vaginal insert. A newly approved vaginal insert (Imvexxy) contains estradiol 4 µg (the lowest dose of vaginal estradiol available) or 10 µg, in a coconut oil vehicle. Its indications are for moderate to severe dyspareunia due to menopause and atrophic vaginitis due to menopause. A study cited in its package insert (www.accessdata.fda.gov/drugsatfda_docs/label/2018/208564s000lbl.pdf) showed that, in patients who used this product, systemic absorption of estradiol remained within the postmenopausal range. Its effects on breast cancer have not yet been studied.
Vaginal rings. Two vaginal rings are marketed. One (Estring) contains 17-beta estradiol, and the other (Femring) contains estradiol acetate. Only the 17-beta estradiol ring delivers a low dose to vaginal tissues, releasing 7.5 µg/day for 90 days. The estradiol acetate ring releases 0.05 mg/day or 0.10 mg/day and is a systemic treatment meant to be used with a progestin, not for local therapy.
VAGINAL ANDROGEN THERAPY: DHEA
After menopause, as the ovaries stop making estrogen from androstenedione, some production continues in other tissues, with DHEA as the primary precursor of androgens that are ultimately converted to estrogen. This has led to the theory that the cause of GSM is not estrogen deficiency but androgen deficiency. Evidence reviewed by Labrie et al11 shows that vulvovaginal atrophy is caused by decreased DHEA availability, which in turn causes sex steroid deficiency-related menopausal symptoms.11 Thus, it is reasonable to conclude that menopausal symptoms can be relieved by giving DHEA.
This theory has been borne out in clinical trials, in which DHEA in a vaginal tablet formulation increased the maturation of vaginal cells and lowered vaginal pH, leading to relief of GSM symptoms.12
The only DHEA product FDA-approved for treating GSM-related symptoms is prasterone (Intrarosa), indicated for moderate to severe dyspareunia due to vulvovaginal atrophy. The recommended dosing is a single 6.5-mg intravaginal tablet (0.5% prasterone) inserted nightly at bedtime. Its efficacy for treating hypoactive sexual desire disorder in postmenopausal women is being investigated.
Breast cancer implications
Because DHEA is converted to estrogen by aromatization, healthcare providers might hesitate to use it in women who have a history of hormone-sensitive cancer. Data on the safety of intravaginal DHEA use in breast cancer survivors are limited. However, studies have found that prasterone has highly beneficial effects on dyspareunia, vaginal dryness, and objective signs of vulvovaginal atrophy without significant drug-related adverse effects.12,13 In these studies, serum estrogen levels in women treated with DHEA were within the values observed in normal postmenopausal women. In addition, there are no aromatase enzymes in the endometrium, so even high doses of vaginal DHEA (in contrast to high doses of vaginal estrogen) will not stimulate the endometrium.
Clinically, this evidence indicates that DHEA exerts both estrogenic and androgenic activity in the vagina without increasing serum estrogen levels, making it a good alternative to topical estrogen therapy.
OSPEMIFENE: AN ESTROGEN RECEPTOR AGONIST/ANTAGONIST
Ospemifene (Osphena) is an estrogen receptor agonist/antagonist, a class of drugs previously called selective estrogen receptor modulators (SERMs). It is FDA-approved to treat moderate to severe dyspareunia secondary to vulvar and vaginal atrophy.
Ospemifene has unique estrogenic effects on the vaginal mucosa, having been shown to increase the number of epithelial cells, lower the vaginal pH, and decrease the percentage of parabasal cells seen on Papanicolaou smears after 12 weeks of use.14
Unlike tamoxifen, another drug of this class, ospemifene does not change the endometrial lining.14 Similarly, ospemifene acts as an estrogenic agonist in bone and, thus, has the potential for use in preventing and managing osteoporosis or for use in women at risk of fractures.
Breast cancer impact
In preclinical trials, ospemifene was found to have antiestrogenic effects on breast tissue, similar to those seen with tamoxifen.
In a model using human tumor grafts, ospemifene decreased tumor growth in mice implanted with estrogen receptor-positive breast cancer cells.15
In a mouse model using breast cancer cells that were biologically and histologically similar to those of humans, ospemifene had strong antiestrogenic effects on the breast tissue.16 The evidence suggests that ospemifene has a favorable effect on vulvar and vaginal atrophy.17
Ospemifene is FDA-approved to treat moderate to severe dyspareunia secondary to menopause. Recommended dosing is 60 mg/day orally with food.
Its antiestrogenic effects on breast tissue make it a promising option for women with a history of estrogen-receptor positive breast cancer. However, further study is needed to fully understand its effects on human breast tissue. According to the manufacturer’s package insert (www.osphena.com/files/pdf/osphena_prescribing_information.pdf), because the drug has not been adequately studied in women with breast cancer, it should not be used in women with known or suspected breast cancer or a history of breast cancer.
CONJUGATED ESTROGENS PLUS BAZEDOXIFENE
The combination of conjugated estrogens and bazedoxifene (Duavee) is a progesterone-free alternative for treating various menopausal symptoms. Bazedoxifene is another estrogen receptor agonist/antagonist, and it was added to counteract estrogen’s effects on the endometrium, thus replacing progesterone. This protective effect has been validated in clinical trials, which also found a favorable safety profile in breast tissue.18,19
SMART trials. The efficacy of this combination was studied in a series of large phase 3 multicenter trials called the SMART (Selective Estrogens, Menopause, and Response to Therapy) trials.20–23 Treated patients had markedly fewer vasomotor symptoms at 1 year, along with an increase in superficial cells and intermediate cells of the vaginal epithelium and a decrease in parabasal cells. They also had a substantial decrease in the incidence of dyspareunia.
Its effects on breast tissue were evaluated in the SMART-5 trial. Therapy had no net impact on breast density, suggesting that it has an estrogen-neutral effect on the breast.23
These results suggest that combined conjugated estrogens and bazedoxifene could be a noteworthy treatment option for GSM in women with a history of estrogen receptor-positive breast cancer, particularly in those with vasomotor symptoms and bone loss. However, the combination has not been studied specifically in breast cancer survivors.
Dosage. The FDA-approved dosing is 20 mg/0.45 mg per day orally to treat vasomotor symptoms, GSM, and osteoporosis in postmenopausal women with a uterus.
LASER THERAPY AND RADIOFREQUENCY HEAT: AN OFF-LABEL OPTION
Low-dose radiofrequency thermal therapy, delivered by carbon dioxide laser or by radiofrequency heat, has been used with some success to treat urinary stress incontinence and vaginal laxity in postpartum women. It may be an option for GSM, although it is not FDA-approved for this indication, and the FDA has recently issued a warning about it.24
Marketing literature promotes laser therapy as an effective option that stimulates vaginal connective tissue to produce new collagen, which then promotes improved blood flow and tissue regeneration for vaginal lubrication and elasticity.
A study comparing fractional carbon dioxide vaginal laser treatment and local estrogen therapy in postmenopausal women with vulvovaginal atrophy found that laser therapy was an effective treatment for vulvovaginal atrophy (dyspareunia, dryness, and burning), both alone and with local estrogen.25
Despite the promising effects of laser therapy for treating vulvovaginal atrophy in GSM, studies have not determined its short-term or long-term safety profile. Furthermore, laser therapy does not improve impaired sexual function, ie, decreased libido, arousal, and sexual satisfaction. Another important consideration is that the cost of laser therapy in 2017 was estimated to be $2,000 to $3,000 per treatment, not covered by healthcare insurance.
CLINICAL APPROACH
Symptoms of GSM are common in breast cancer survivors, both pre- and postmenopausal, especially those treated with tamoxifen or an aromatase inhibitor. Estimates are that 60% of postmenopausal breast cancer survivors and 40% of premenopausal breast cancer survivors suffer from GSM.26 Unfortunately, many women do not seek medical attention for their symptoms.
A variety of hormonal and nonhormonal options are available for these patients. We recommend an interdisciplinary approach to treatment, with the decision to use hormonal options made in collaboration with the patient’s oncologist and the patient herself, in an informed, shared decision-making process that takes into consideration the risks and possible benefits depending on the symptoms.
The first step in selecting a management plan for GSM symptoms in women with breast cancer is to conduct a thorough assessment to provide data for individualizing the care plan. The decision to use a hormonal option should be made in collaboration with a woman’s oncologist and should include an informed decision-making process during which the potential risks and benefits, including the breast cancer impact, are fully disclosed.
Alternatives to systemic estrogen
Vaginal estrogen is an effective and safe option to treat GSM in women with either estrogen receptor-negative or estrogen receptor-positive breast cancer. It often completely cures the symptoms without any noticeable increase in serum estrogen levels.
Vaginal DHEA therapy is a nonestrogen option shown to effectively treat GSM without increasing systemic levels of estrogen or testosterone. This profile makes vaginal DHEA therapy a particularly attractive treatment for symptoms of GSM in women at risk for breast cancer.
Use of an estrogen receptor agonist/antagonist in breast cancer survivors needs careful consideration. Ospemifene has antiestrogenic effects that make it a good option for women with bone loss and those at high risk for breast cancer, but it should not be used concurrently with tamoxifen or raloxifene. Additionally, ospemifene does not cause uterine hyperplasia, so it can be used in women with a uterus.
Although more study is needed, we do have options to improve the overall quality of life in breast cancer survivors with GSM.
Many breast cancer survivors and women at high risk of breast cancer suffer from genitourinary syndrome of menopause (GSM), a term that encompasses any urinary, genital, or sexual dysfunction related to a hypoestrogenic state. Although GSM is usually caused by postmenopausal estrogen loss, it can also be caused by cancer treatments such as chemotherapy, radiation, and systemic endocrine therapy (eg, tamoxifen, aromatase inhibitors). These treatments can substantially decrease systemic estrogen levels, causing GSM symptoms that can profoundly worsen quality of life.
Managing GSM in these women poses a dilemma because systemic estrogen-containing therapies can increase the risk of breast cancer, and nonhormonal vaginal lubricants and moisturizers provide only minimal benefit. Fortunately, there are hormonal options, including locally applied estrogen, intravaginal dehydroepiandrosterone (DHEA), and estrogen receptor agonists/antagonists (ospemifene and bazedoxifene).
Here, we review the clinical management of GSM in breast cancer survivors and women at high risk of breast cancer and the efficacy and safety of available treatments, including their impact on breast cancer risk.
DRYNESS, IRRITATION, ATROPHY
The term GSM describes vulvovaginal and genitourinary symptoms associated with estrogen loss after menopause. Common symptoms are vaginal dryness, dyspareunia, irritation of genital skin, and pruritus.
LOCAL ESTROGEN THERAPY
Systemic estrogen therapy is widely used and effective for GSM, but there are concerns that it could increase the risk of breast cancer. After the Women’s Health Initiative in 2002 showed higher rates of cardiovascular disease and breast cancer with systemic estrogen-progestin use,5 the use of this hormone therapy declined by approximately 80%.6 Since then, healthcare providers have turned to local (ie, vaginal) estrogen therapies to manage GSM. These therapies have several advantages over systemic hormone therapy:
- Lower risk of adverse effects on the breast and cardiovascular system
- Greater efficacy in treating GSM
- In general, no need for progesterone when low-dose local estrogen is given to a woman with a uterus.7
Is locally applied estrogen systemically absorbed?
Despite these advantages, concerns remain as to whether vaginal estrogen therapy has adverse consequences associated with systemic absorption, particularly from atrophic vaginal tissues.
Santen,8 in a 2015 review of 33 studies, concluded that systemic absorption from low-dose vaginal estrogen is minimal, which provides some rationale for using it to treat vulvovaginal atrophy in postmenopausal women. This finding also suggests that the US Food and Drug Administration (FDA) “black box” warning of possible toxicities with vaginal estrogen is likely overstated, given that serum estrogen levels remained within normal postmenopausal levels.
Nevertheless, many providers are apprehensive about prescribing vaginal estrogen in women with a history of breast cancer because the threshold for systemic estrogen levels associated with breast cancer recurrence has not been established.
ACOG statement. In 2016, a committee of the American College of Obstetricians and Gynecologists cited data showing that low-dose vaginal estrogens do not result in sustained serum estrogen levels exceeding the normal postmenopausal range, and that the use of vaginal estrogens does not increase the risk of cancer recurrence.9 However, they recommend caution with vaginal estrogen use, especially in women with a history of estrogen-dependent breast cancer, reserving it for patients with GSM symptoms nonresponsive to nonhormonal treatment and specifying that it be used in low doses.
Vaginal estrogen formulations
Several types of locally applied estrogens are available, each with different properties and affinity for estrogen receptors. These include conjugated estrogens, 17-beta estradiol, estradiol acetate, and estradiol hemihydrate. Three delivery systems are FDA-approved: creams, rings, and tablets (Table 2).
Vaginal creams. Two vaginal creams are available, one (Estrace) containing 17-beta estradiol and the other (Premarin) containing conjugated estrogens.
The FDA-approved dosage for 17-beta estradiol is 2 to 4 g/day for 1 or 2 weeks, then gradually reduced to half the dose for a similar time. Maintenance dosing is 1 g 1 to 3 times per week. However, the ACOG statement notes that the FDA-approved dosages are higher than those proven to be effective and currently used in clinical practice, eg, 0.5 g twice a week.9
The FDA-approved dosage of conjugated estrogen cream for moderate to severe dyspareunia is 0.5 g daily for 21 days, then off for 7 days, or 0.5 g twice a week.
Vaginal tablets. The vaginal tablet Vagifem and its generic equivalent Yuvafem contain 10 µg of estradiol hemihydrate. The FDA-approved dosage is 10 µg daily for 2 weeks, followed by 10 µg twice a week, inserted into the lower third of the vagina. This dosage is significantly lower than that of estrogen creams.
Vaginal insert. A newly approved vaginal insert (Imvexxy) contains estradiol 4 µg (the lowest dose of vaginal estradiol available) or 10 µg, in a coconut oil vehicle. Its indications are for moderate to severe dyspareunia due to menopause and atrophic vaginitis due to menopause. A study cited in its package insert (www.accessdata.fda.gov/drugsatfda_docs/label/2018/208564s000lbl.pdf) showed that, in patients who used this product, systemic absorption of estradiol remained within the postmenopausal range. Its effects on breast cancer have not yet been studied.
Vaginal rings. Two vaginal rings are marketed. One (Estring) contains 17-beta estradiol, and the other (Femring) contains estradiol acetate. Only the 17-beta estradiol ring delivers a low dose to vaginal tissues, releasing 7.5 µg/day for 90 days. The estradiol acetate ring releases 0.05 mg/day or 0.10 mg/day and is a systemic treatment meant to be used with a progestin, not for local therapy.
VAGINAL ANDROGEN THERAPY: DHEA
After menopause, as the ovaries stop making estrogen from androstenedione, some production continues in other tissues, with DHEA as the primary precursor of androgens that are ultimately converted to estrogen. This has led to the theory that the cause of GSM is not estrogen deficiency but androgen deficiency. Evidence reviewed by Labrie et al11 shows that vulvovaginal atrophy is caused by decreased DHEA availability, which in turn causes sex steroid deficiency-related menopausal symptoms.11 Thus, it is reasonable to conclude that menopausal symptoms can be relieved by giving DHEA.
This theory has been borne out in clinical trials, in which DHEA in a vaginal tablet formulation increased the maturation of vaginal cells and lowered vaginal pH, leading to relief of GSM symptoms.12
The only DHEA product FDA-approved for treating GSM-related symptoms is prasterone (Intrarosa), indicated for moderate to severe dyspareunia due to vulvovaginal atrophy. The recommended dosing is a single 6.5-mg intravaginal tablet (0.5% prasterone) inserted nightly at bedtime. Its efficacy for treating hypoactive sexual desire disorder in postmenopausal women is being investigated.
Breast cancer implications
Because DHEA is converted to estrogen by aromatization, healthcare providers might hesitate to use it in women who have a history of hormone-sensitive cancer. Data on the safety of intravaginal DHEA use in breast cancer survivors are limited. However, studies have found that prasterone has highly beneficial effects on dyspareunia, vaginal dryness, and objective signs of vulvovaginal atrophy without significant drug-related adverse effects.12,13 In these studies, serum estrogen levels in women treated with DHEA were within the values observed in normal postmenopausal women. In addition, there are no aromatase enzymes in the endometrium, so even high doses of vaginal DHEA (in contrast to high doses of vaginal estrogen) will not stimulate the endometrium.
Clinically, this evidence indicates that DHEA exerts both estrogenic and androgenic activity in the vagina without increasing serum estrogen levels, making it a good alternative to topical estrogen therapy.
OSPEMIFENE: AN ESTROGEN RECEPTOR AGONIST/ANTAGONIST
Ospemifene (Osphena) is an estrogen receptor agonist/antagonist, a class of drugs previously called selective estrogen receptor modulators (SERMs). It is FDA-approved to treat moderate to severe dyspareunia secondary to vulvar and vaginal atrophy.
Ospemifene has unique estrogenic effects on the vaginal mucosa, having been shown to increase the number of epithelial cells, lower the vaginal pH, and decrease the percentage of parabasal cells seen on Papanicolaou smears after 12 weeks of use.14
Unlike tamoxifen, another drug of this class, ospemifene does not change the endometrial lining.14 Similarly, ospemifene acts as an estrogenic agonist in bone and, thus, has the potential for use in preventing and managing osteoporosis or for use in women at risk of fractures.
Breast cancer impact
In preclinical trials, ospemifene was found to have antiestrogenic effects on breast tissue, similar to those seen with tamoxifen.
In a model using human tumor grafts, ospemifene decreased tumor growth in mice implanted with estrogen receptor-positive breast cancer cells.15
In a mouse model using breast cancer cells that were biologically and histologically similar to those of humans, ospemifene had strong antiestrogenic effects on the breast tissue.16 The evidence suggests that ospemifene has a favorable effect on vulvar and vaginal atrophy.17
Ospemifene is FDA-approved to treat moderate to severe dyspareunia secondary to menopause. Recommended dosing is 60 mg/day orally with food.
Its antiestrogenic effects on breast tissue make it a promising option for women with a history of estrogen-receptor positive breast cancer. However, further study is needed to fully understand its effects on human breast tissue. According to the manufacturer’s package insert (www.osphena.com/files/pdf/osphena_prescribing_information.pdf), because the drug has not been adequately studied in women with breast cancer, it should not be used in women with known or suspected breast cancer or a history of breast cancer.
CONJUGATED ESTROGENS PLUS BAZEDOXIFENE
The combination of conjugated estrogens and bazedoxifene (Duavee) is a progesterone-free alternative for treating various menopausal symptoms. Bazedoxifene is another estrogen receptor agonist/antagonist, and it was added to counteract estrogen’s effects on the endometrium, thus replacing progesterone. This protective effect has been validated in clinical trials, which also found a favorable safety profile in breast tissue.18,19
SMART trials. The efficacy of this combination was studied in a series of large phase 3 multicenter trials called the SMART (Selective Estrogens, Menopause, and Response to Therapy) trials.20–23 Treated patients had markedly fewer vasomotor symptoms at 1 year, along with an increase in superficial cells and intermediate cells of the vaginal epithelium and a decrease in parabasal cells. They also had a substantial decrease in the incidence of dyspareunia.
Its effects on breast tissue were evaluated in the SMART-5 trial. Therapy had no net impact on breast density, suggesting that it has an estrogen-neutral effect on the breast.23
These results suggest that combined conjugated estrogens and bazedoxifene could be a noteworthy treatment option for GSM in women with a history of estrogen receptor-positive breast cancer, particularly in those with vasomotor symptoms and bone loss. However, the combination has not been studied specifically in breast cancer survivors.
Dosage. The FDA-approved dosing is 20 mg/0.45 mg per day orally to treat vasomotor symptoms, GSM, and osteoporosis in postmenopausal women with a uterus.
LASER THERAPY AND RADIOFREQUENCY HEAT: AN OFF-LABEL OPTION
Low-dose radiofrequency thermal therapy, delivered by carbon dioxide laser or by radiofrequency heat, has been used with some success to treat urinary stress incontinence and vaginal laxity in postpartum women. It may be an option for GSM, although it is not FDA-approved for this indication, and the FDA has recently issued a warning about it.24
Marketing literature promotes laser therapy as an effective option that stimulates vaginal connective tissue to produce new collagen, which then promotes improved blood flow and tissue regeneration for vaginal lubrication and elasticity.
A study comparing fractional carbon dioxide vaginal laser treatment and local estrogen therapy in postmenopausal women with vulvovaginal atrophy found that laser therapy was an effective treatment for vulvovaginal atrophy (dyspareunia, dryness, and burning), both alone and with local estrogen.25
Despite the promising effects of laser therapy for treating vulvovaginal atrophy in GSM, studies have not determined its short-term or long-term safety profile. Furthermore, laser therapy does not improve impaired sexual function, ie, decreased libido, arousal, and sexual satisfaction. Another important consideration is that the cost of laser therapy in 2017 was estimated to be $2,000 to $3,000 per treatment, not covered by healthcare insurance.
CLINICAL APPROACH
Symptoms of GSM are common in breast cancer survivors, both pre- and postmenopausal, especially those treated with tamoxifen or an aromatase inhibitor. Estimates are that 60% of postmenopausal breast cancer survivors and 40% of premenopausal breast cancer survivors suffer from GSM.26 Unfortunately, many women do not seek medical attention for their symptoms.
A variety of hormonal and nonhormonal options are available for these patients. We recommend an interdisciplinary approach to treatment, with the decision to use hormonal options made in collaboration with the patient’s oncologist and the patient herself, in an informed, shared decision-making process that takes into consideration the risks and possible benefits depending on the symptoms.
The first step in selecting a management plan for GSM symptoms in women with breast cancer is to conduct a thorough assessment to provide data for individualizing the care plan. The decision to use a hormonal option should be made in collaboration with a woman’s oncologist and should include an informed decision-making process during which the potential risks and benefits, including the breast cancer impact, are fully disclosed.
Alternatives to systemic estrogen
Vaginal estrogen is an effective and safe option to treat GSM in women with either estrogen receptor-negative or estrogen receptor-positive breast cancer. It often completely cures the symptoms without any noticeable increase in serum estrogen levels.
Vaginal DHEA therapy is a nonestrogen option shown to effectively treat GSM without increasing systemic levels of estrogen or testosterone. This profile makes vaginal DHEA therapy a particularly attractive treatment for symptoms of GSM in women at risk for breast cancer.
Use of an estrogen receptor agonist/antagonist in breast cancer survivors needs careful consideration. Ospemifene has antiestrogenic effects that make it a good option for women with bone loss and those at high risk for breast cancer, but it should not be used concurrently with tamoxifen or raloxifene. Additionally, ospemifene does not cause uterine hyperplasia, so it can be used in women with a uterus.
Although more study is needed, we do have options to improve the overall quality of life in breast cancer survivors with GSM.
- Lester J, Pahouja G, Andersen B, Lustberg M. Atrophic vaginitis in breast cancer survivors: a difficult survivorship issue. J Pers Med 2015; 5(2):50–66. doi:10.3390/jpm5020050
- Chin SN, Trinkaus M, Simmons C, et al. Prevalence and severity of urogenital symptoms in postmenopausal women receiving endocrine therapy for breast cancer. Clin Breast Cancer 2009; 9(2):108–117. doi:10.3816/CBC.2009.n.020
- Fallowfield L, Cella D, Cuzick J, Francis S, Locker G, Howell A. Quality of life of postmenopausal women in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) adjuvant breast cancer trial. J Clin Oncol 2004; 22(21):4261–4271. doi:10.1200/JCO.2004.08.029
- Cella D, Fallowfield LJ. Recognition and management of treatment-related side effects for breast cancer patients receiving adjuvant endocrine therapy. Breast Cancer Res Treat 2008; 107(2):167–180. doi:10.1007/s10549-007-9548-1
- Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002; 288(3):321–333. pmid:12117397
- Tsai SA, Stefanick ML, Stafford RS. Trends in menopausal hormone therapy use of US office-based physicians, 2000–2009. Menopause 2011; 18(4):385–392. doi:10.1097/gme.0b013e3181f43404
- North American Menopause Society. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause 2013; 20(9):888–902. doi:10.1097/GME.0b013e3182a122c2
- Santen RJ. Vaginal administration of estradiol: effects of dose, preparation and timing on plasma estradiol levels. Climacteric 2015; 18(2):121–134. doi:10.3109/13697137.2014.947254
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: the use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol 2016; 127(3):e93–e96. doi:10.1097/AOG.0000000000001351
- Santoro N, Epperson CN, Mathews SB. Menopausal symptoms and their management. Endocrinol Metab Clin North Am 2015; 44(3):497–515. doi:10.1016/j.ecl.2015.05.001
- Labrie F, Archer DF, Martel C, Vaillancourt M, Montesino M. Combined data of intravaginal prasterone against vulvovaginal atrophy of menopause. Menopause 2017; 24(11):1246–1256. doi:10.1097/GME.0000000000000910
- Labrie F, Archer D, Bouchard C, et al. Serum steroid levels during 12-week intravaginal dehydroepiandrosterone administration. Menopause 2009; 16(5):897–906. doi:10.1097/gme.0b013e31819e8930
- Labrie F, Cusan L, Gomez JL, et al. Effect of intravaginal DHEA on serum DHEA and eleven of its metabolites in postmenopausal women. J Steroid Biochem Mol Biol 2008; 111(3-5):178–194. doi:10.1016/j.jsbmb.2008.06.003
- Soe LH, Wurz GT, Kao CJ, Degregorio MW. Ospemifene for the treatment of dyspareunia associated with vulvar and vaginal atrophy: potential benefits in bone and breast. Int J Womens Health 2013; 5:605–611. doi:10.2147/IJWH.S39146
- Taras TL, Wurz GT, DeGregorio MW. In vitro and in vivo biologic effects of ospemifene (FC-1271a) in breast cancer. J Steroid Biochem Mol Biol 2001; 77(4–5):271–279. pmid:11457665
- Wurz GT, Read KC, Marchisano-Karpman C, et al. Ospemifene inhibits the growth of dimethylbenzanthracene-induced mammary tumors in Sencar mice. J Steroid Biochem Mol Biol 2005; 97(3):230–240. doi:10.1016/j.jsbmb.2005.06.027
- Archer DF, Carr BR, Pinkerton JV, Taylor HS, Constantine GD. Effects of ospemifene on the female reproductive and urinary tracts: translation from preclinical models into clinical evidence. Menopause 2015; 22(7):786–796. doi:10.1097/GME.0000000000000365
- Mirkin S, Pickar JH. Management of osteoporosis and menopausal symptoms: focus on bazedoxifene/conjugated estrogen combination. Int J Womens Health 2013; 5:465–475. doi:10.2147/IJWH.S39455
- Kagan R, Goldstein SR, Pickar JH, Komm BS. Patient considerations in the management of menopausal symptoms: role of conjugated estrogens with bazedoxifene. Ther Clin Risk Manag 2016; 12:549–562. doi:10.2147/TCRM.S63833
- Lobo RA, Pinkerton JV, Gass ML, et al. Evaluation of bazedoxifene/conjugated estrogens for the treatment of menopausal symptoms and effects on metabolic parameters and overall safety profile. Fertil Steril 2009; 92(3):1025–1038. doi:10.1016/j.fertnstert.2009.03.113
- Pinkerton JV, Utian WH, Constantine GD, Olivier S, Pickar JH. Relief of vasomotor symptoms with the tissue-selective estrogen complex containing bazedoxifene/conjugated estrogens: a randomized, controlled trial. Menopause 2009; 16(6):1116–1124. doi:10.1097/gme.0b013e3181a7df0d
- Kagan R, Williams RS, Pan K, Mirkin S, Pickar JH. A randomized, placebo- and active-controlled trial of bazedoxifene/conjugated estrogens for treatment of moderate to severe vulvar/vaginal atrophy in postmenopausal women. Menopause 2010; 17(2):281–289. doi:10.1097/GME.0b013e3181b7c65f
- Pinkerton JV, Harvey JA, Pan K, et al. Breast effects of bazedoxifene-conjugated estrogens: a randomized controlled trial. Obstet Gynecol 2013; 121(5):959–968. doi:10.1097/AOG.0b013e31828c5974
- FDA. U.S. Food & Drug Administration. FDA Statement. Statement from FDA Commissioner Scott Gottlieb, M.D., on efforts to safeguard women’s health from deceptive health claims and significant risks related to devices marketed for use in medical procedures for “vaginal rejuvenation.” www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm615130.htm. Accessed August 20, 2018.
- Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause 2018; 25(1):21–28. doi:10.1097/GME.0000000000000955
- Biglia N, Bounous VE, D’Alonzo M, et al. Vaginal atrophy in breast cancer survivors: attitude and approaches among oncologists. Clin Breast Cancer 2017; 17(8):611–617. doi:10.1016/j.clbc.2017.05.008
- Lester J, Pahouja G, Andersen B, Lustberg M. Atrophic vaginitis in breast cancer survivors: a difficult survivorship issue. J Pers Med 2015; 5(2):50–66. doi:10.3390/jpm5020050
- Chin SN, Trinkaus M, Simmons C, et al. Prevalence and severity of urogenital symptoms in postmenopausal women receiving endocrine therapy for breast cancer. Clin Breast Cancer 2009; 9(2):108–117. doi:10.3816/CBC.2009.n.020
- Fallowfield L, Cella D, Cuzick J, Francis S, Locker G, Howell A. Quality of life of postmenopausal women in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) adjuvant breast cancer trial. J Clin Oncol 2004; 22(21):4261–4271. doi:10.1200/JCO.2004.08.029
- Cella D, Fallowfield LJ. Recognition and management of treatment-related side effects for breast cancer patients receiving adjuvant endocrine therapy. Breast Cancer Res Treat 2008; 107(2):167–180. doi:10.1007/s10549-007-9548-1
- Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002; 288(3):321–333. pmid:12117397
- Tsai SA, Stefanick ML, Stafford RS. Trends in menopausal hormone therapy use of US office-based physicians, 2000–2009. Menopause 2011; 18(4):385–392. doi:10.1097/gme.0b013e3181f43404
- North American Menopause Society. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause 2013; 20(9):888–902. doi:10.1097/GME.0b013e3182a122c2
- Santen RJ. Vaginal administration of estradiol: effects of dose, preparation and timing on plasma estradiol levels. Climacteric 2015; 18(2):121–134. doi:10.3109/13697137.2014.947254
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: the use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol 2016; 127(3):e93–e96. doi:10.1097/AOG.0000000000001351
- Santoro N, Epperson CN, Mathews SB. Menopausal symptoms and their management. Endocrinol Metab Clin North Am 2015; 44(3):497–515. doi:10.1016/j.ecl.2015.05.001
- Labrie F, Archer DF, Martel C, Vaillancourt M, Montesino M. Combined data of intravaginal prasterone against vulvovaginal atrophy of menopause. Menopause 2017; 24(11):1246–1256. doi:10.1097/GME.0000000000000910
- Labrie F, Archer D, Bouchard C, et al. Serum steroid levels during 12-week intravaginal dehydroepiandrosterone administration. Menopause 2009; 16(5):897–906. doi:10.1097/gme.0b013e31819e8930
- Labrie F, Cusan L, Gomez JL, et al. Effect of intravaginal DHEA on serum DHEA and eleven of its metabolites in postmenopausal women. J Steroid Biochem Mol Biol 2008; 111(3-5):178–194. doi:10.1016/j.jsbmb.2008.06.003
- Soe LH, Wurz GT, Kao CJ, Degregorio MW. Ospemifene for the treatment of dyspareunia associated with vulvar and vaginal atrophy: potential benefits in bone and breast. Int J Womens Health 2013; 5:605–611. doi:10.2147/IJWH.S39146
- Taras TL, Wurz GT, DeGregorio MW. In vitro and in vivo biologic effects of ospemifene (FC-1271a) in breast cancer. J Steroid Biochem Mol Biol 2001; 77(4–5):271–279. pmid:11457665
- Wurz GT, Read KC, Marchisano-Karpman C, et al. Ospemifene inhibits the growth of dimethylbenzanthracene-induced mammary tumors in Sencar mice. J Steroid Biochem Mol Biol 2005; 97(3):230–240. doi:10.1016/j.jsbmb.2005.06.027
- Archer DF, Carr BR, Pinkerton JV, Taylor HS, Constantine GD. Effects of ospemifene on the female reproductive and urinary tracts: translation from preclinical models into clinical evidence. Menopause 2015; 22(7):786–796. doi:10.1097/GME.0000000000000365
- Mirkin S, Pickar JH. Management of osteoporosis and menopausal symptoms: focus on bazedoxifene/conjugated estrogen combination. Int J Womens Health 2013; 5:465–475. doi:10.2147/IJWH.S39455
- Kagan R, Goldstein SR, Pickar JH, Komm BS. Patient considerations in the management of menopausal symptoms: role of conjugated estrogens with bazedoxifene. Ther Clin Risk Manag 2016; 12:549–562. doi:10.2147/TCRM.S63833
- Lobo RA, Pinkerton JV, Gass ML, et al. Evaluation of bazedoxifene/conjugated estrogens for the treatment of menopausal symptoms and effects on metabolic parameters and overall safety profile. Fertil Steril 2009; 92(3):1025–1038. doi:10.1016/j.fertnstert.2009.03.113
- Pinkerton JV, Utian WH, Constantine GD, Olivier S, Pickar JH. Relief of vasomotor symptoms with the tissue-selective estrogen complex containing bazedoxifene/conjugated estrogens: a randomized, controlled trial. Menopause 2009; 16(6):1116–1124. doi:10.1097/gme.0b013e3181a7df0d
- Kagan R, Williams RS, Pan K, Mirkin S, Pickar JH. A randomized, placebo- and active-controlled trial of bazedoxifene/conjugated estrogens for treatment of moderate to severe vulvar/vaginal atrophy in postmenopausal women. Menopause 2010; 17(2):281–289. doi:10.1097/GME.0b013e3181b7c65f
- Pinkerton JV, Harvey JA, Pan K, et al. Breast effects of bazedoxifene-conjugated estrogens: a randomized controlled trial. Obstet Gynecol 2013; 121(5):959–968. doi:10.1097/AOG.0b013e31828c5974
- FDA. U.S. Food & Drug Administration. FDA Statement. Statement from FDA Commissioner Scott Gottlieb, M.D., on efforts to safeguard women’s health from deceptive health claims and significant risks related to devices marketed for use in medical procedures for “vaginal rejuvenation.” www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm615130.htm. Accessed August 20, 2018.
- Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause 2018; 25(1):21–28. doi:10.1097/GME.0000000000000955
- Biglia N, Bounous VE, D’Alonzo M, et al. Vaginal atrophy in breast cancer survivors: attitude and approaches among oncologists. Clin Breast Cancer 2017; 17(8):611–617. doi:10.1016/j.clbc.2017.05.008
KEY POINTS
- In general, locally applied hormonal therapies relieve GSM symptoms without increasing breast cancer risk.
- DHEA relieves vaginal symptoms without increasing serum estrogen levels.
- Ospemifene has antiestrogenic effects on breast tissue that make it an attractive option for women with breast cancer.
- The combination of conjugated estrogens and bazedoxifene offers a progesterone-free treatment for GSM symptoms in women desiring systemic hormone therapy.
ERAAs for menopause treatment: Welcome the ‘designer estrogens’
Estrogen receptor agonist-antagonists (ERAAs), previously called selective estrogen receptor modulators (SERMs), have extended the options for treating the various conditions that menopausal women suffer from. These drugs act differently on estrogen receptors in different tissues, stimulating receptors in some tissues but inhibiting them in others. This allows selective inhibition or stimulation of estrogen-like action in various target tissues.1
This article highlights the use of ERAAs to treat menopausal vasomotor symptoms (eg, hot flashes, night sweats), genitourinary syndrome of menopause, osteoporosis, breast cancer (and the risk of breast cancer), and other health concerns unique to women at midlife.
SYMPTOMS OF MENOPAUSE: COMMON AND TROUBLESOME
Vasomotor symptoms such as hot flashes and night sweats are common during perimenopause—most women experience them. They are most frequent during the menopause transition but can persist for 10 years or more afterward.2
Genitourinary syndrome of menopause is also common and often worsens with years after menopause.3 It can lead to dyspareunia and vaginal dryness, which may in turn result in lower libido, vaginismus, and hypoactive sexual desire disorder, problems that often arise at the same time as vaginal dryness and atrophy.4
Osteopenia and osteoporosis. A drop in systemic estrogen leads to a decline in bone mineral density, increasing the risk of fractures.5
ESTROGEN-PROGESTIN TREATMENT: THE GOLD STANDARD, BUT NOT IDEAL
The current gold standard for treating moderate to severe hot flashes is estrogen, available in oral, transdermal, and vaginal formulations.6 Estrogen also has antiresorptive effects on bone and is approved for preventing osteoporosis. Systemic estrogen may also be prescribed for genitourinary syndrome of menopause if local vaginal treatment alone is insufficient.
If women who have an intact uterus receive estrogen, they should also receive a progestin to protect against endometrial hyperplasia and reduce the risk of endometrial cancer.
Despite its status as the gold standard, estrogen-progestin therapy presents challenges. In some women, progestins cause side effects such as breast tenderness, bloating, fatigue, and depression.7 Estrogen-progestin therapy often causes vaginal bleeding, which for some women is troublesome or distressing; bleeding may be the reason for repeated evaluations, can increase anxiety, and can lead to poor adherence with hormonal treatment. Women who carry a higher-than-normal risk of developing breast cancer or fear that taking hormones will lead to breast cancer may show decreased adherence to therapy. Women who have estrogen receptor-positive breast cancer cannot take estrogen.
Individualized options are needed for women who have progestin-related side effects, unwanted vaginal bleeding, or a higher risk of breast cancer.
WELCOME THE ERAAs
An ideal treatment for menopause would relieve vasomotor symptoms and genitourinary syndrome of menopause and increase bone mineral density without causing breast tenderness, vaginal bleeding, or endometrial proliferation.
The “designer estrogens,” or ERAAs, have specific positive effects on the bone, heart, and brain with neutral or antagonist effects on estrogen receptors in other tissues such as the breasts and endometrium.8 While not entirely free of adverse effects, these agents have been developed with the aim of minimizing the most common ones related to estrogen and progestin.
Several ERAAs are currently approved by the US Food and Drug Administration (FDA)for various indications, each having a unique profile. Clomifene was the first agent of this class, and it is still used clinically to induce ovulation. This article highlights subsequently approved agents, ie, tamoxifen, raloxifene, ospemifene, and the combination of conjugated estrogens and bazedoxifene (Table 1).
All ERAAs increase the risk of venous thromboembolism, and therefore none of them should be used in women with known venous thromboembolism or at high risk of it.
TAMOXIFEN: CANCER TREATMENT AND PREVENTION
After clomiphene, tamoxifen was the second ERAA on the market. Although researchers were looking for a new contraceptive drug, they found tamoxifen to be useful as a chemotherapeutic agent for breast cancer. First used in 1971, tamoxifen continues to be one of the most commonly prescribed chemotherapeutic medications today.
The FDA has approved tamoxifen to treat breast cancer as well as to prevent breast cancer in pre- and postmenopausal women at risk. It may also have beneficial effects on bone and on cardiovascular risk factors, but these are not approved uses for it.
Trials of tamoxifen for cancer treatment
The Early Breast Cancer Trialists’ Collaborative Group9 performed a meta-analysis and found that 5 years of adjuvant treatment with tamoxifen is associated with a 26% reduction in mortality and a 47% reduction in breast cancer recurrence at 10 years. In absolute terms, we estimate that 21 women would need to be treated to prevent 1 death and 8 would need to be treated to prevent 1 recurrence.
The ATLAS Trial (Adjuvant Tamoxifen Longer Against Shorter)10 and later the UK ATTOM (Adjuvant Tamoxifen Treatment to Offer More)11 trial confirmed an even greater reduction in recurrence and mortality after a total of 10 years of treatment.
Trials of tamoxifen for cancer prevention
Cuzik et al12 performed a meta-analysis of 4 trials of tamoxifen’s effectiveness in preventing breast cancer for women at elevated risk. The incidence of estrogen receptor-positive breast cancer was 48% lower with tamoxifen use, but there was no effect on estrogen-negative breast cancer. From their data, we estimate that 77 women would need to be treated to prevent 1 case of breast cancer.
The IBIS-I trial (International Breast Cancer Intervention Study I)13 found that, in healthy women at high risk of breast cancer, the benefit of taking tamoxifen for 5 years as preventive treatment persisted long afterward. The investigators estimated that at 20 years of follow-up the risk of breast cancer would be 12.3% in placebo recipients and 7.8% in tamoxifen recipients, a 4.5% absolute risk reduction; number needed to treat (NNT) 22.
Data on tamoxifen and osteoporosis
The Breast Cancer Prevention Trial revealed a 19% reduction in the incidence of osteoporotic fractures with tamoxifen, but the difference was not statistically significant.14 The 1-year rates of fracture in women age 50 and older were 0.727% with placebo and 0.567% with tamoxifen, an absolute difference of 0.151%; therefore, if the effect is real, 662 women age 50 or older would need to be treated for 1 year to prevent 1 fracture. Tamoxifen is not FDA-approved to treat osteoporosis.
Data on tamoxifen and cardiovascular risk reduction
Chang et al,15 in a study in women at risk of breast cancer, incidentally found that tamoxifen was associated with a 13% reduction in total cholesterol compared with placebo.
Herrington and Klein,16 in a systematic review, noted similar findings in multiple studies of tamoxifen, with decreases in total cholesterol ranging from 7% to 17% and decreases in low-density lipoprotein cholesterol ranging from 10% to 28%. However, they found no change in high-density lipoprotein cholesterol concentrations or in the cardiovascular mortality rate.
The ATLAS trial10 revealed a relative risk reduction of 0.76 (95% confidence interval [CI] 0.60–0.95, P = .02) in ischemic heart disease for women who took tamoxifen for 10 years compared with 5 years. We calculate that ischemic heart disease occurred in 163 (2.5%) of 6,440 women who took tamoxifen for 5 years compared with 127 (1.9%) of 6,454 women who took it for 10 years, a 0.6% absolute risk reduction, NNT = 167.
Adverse effects of tamoxifen
Uterine neoplasia. Women taking tamoxifen have a 2.5-fold increased risk of endometrial cancer.14 Tamoxifen also increases the risk of benign uterine disease such as endometrial hyperplasia and polyps. As many as 39% of women taking tamoxifen will have evidence of benign uterine changes on pathology.17 Other adverse effects:
Venous thromboembolism (the risk of pulmonary embolism is increased approximately threefold14)
Cataracts (there is a slight increase in cataract diagnosis in tamoxifen users)
Vasomotor symptoms, which limit the use of tamoxifen in many women.
Ideal candidate for tamoxifen
The ideal candidate for tamoxifen is a woman with breast cancer that is estrogen receptor-positive and who has a history of osteopenia or osteoporosis and no risk factors for venous thromboembolism.
RALOXIFENE: FOR OSTEOPOROSIS AND FOR CANCER PREVENTION
Raloxifene, a second-generation ERAA, was first approved for preventing and treating osteoporosis and later for reducing the risk of invasive estrogen receptor-positive breast cancer in postmenopausal women.
Trials of raloxifene for osteoporosis
The MORE trial (Multiple Outcomes of Raloxifene)18 was a large multicenter randomized double-blind study. Raloxifene recipients showed a significant increase in bone mineral density in the lumbar spine and femoral neck at year 3 (P < .001) compared with those receiving placebo. Even after only 1 year of treatment, raloxifene significantly reduced the risk of new fractures, despite only modest gains in bone mineral density. After 3 years of treatment, new clinical vertebral fractures had occurred in 3.5% of the placebo group compared with 2.1% of the group receiving raloxifene 60 mg.19 Relative risk reductions were similar in women who had already had a clinical vertebral fracture at baseline, whose absolute risk is higher. However, no significant effect was seen on the incidence of hip or nonvertebral fractures.
The CORE trial (Continuing Outcomes Relevant to Raloxifene)20 extended the treatment of the women enrolled in the MORE trial another 4 years and found that the benefit of raloxifene with regard to bone mineral density persisted with continued use.
Trials of raloxifene for breast cancer prevention
The MORE trial,21 in postmenopausal women with osteoporosis included breast cancer as a secondary end point, and raloxifene was shown to decrease the incidence of invasive breast cancer. At a median of 40 months, invasive breast cancer had arisen in 13 (0.25%) of the 5,129 women assigned to raloxifene and 27 (1.0%) of the 2,576 women assigned to placebo. The authors calculated that 126 women would need to be treated to prevent 1 case of breast cancer.
The CORE trial,22 as noted, extended the treatment of the women enrolled in the MORE trial another 4 years. The risk of any invasive breast cancer in postmenopausal women with osteoporosis was significantly reduced by 59% after 8 years, and the risk of estrogen receptor-positive invasive breast cancer was reduced by 66%.
There is evidence that raloxifene’s effect on breast cancer risk persists after discontinuation of use.23
Does raloxifene reduce mortality?
Grady et al24 studied the effect of raloxifene on all-cause mortality in a pooled analysis of mortality data from the MORE, CORE, and Raloxifene Use for the Heart (RUTH)25 trials. In older postmenopausal women, the rate of all-cause mortality was 8.65% in those taking placebo compared with 7.88% in those taking raloxifene 60 mg daily—10% lower. The mechanism behind the lower mortality rate is unclear, and Grady et al recommend that the finding be interpreted with caution.
Trials of raloxifene for heart protection
The RUTH trial25 was a 5.6-year study undertaken to study the effects of raloxifene on coronary outcomes and invasive breast cancer in postmenopausal women. Results were mixed. Active treatment:
- Did not significantly affect the risk of coronary artery disease compared with placebo
- Significantly decreased the risk of invasive breast cancer
- Significantly decreased the risk of clinical vertebral fractures
- Increased the risk of fatal stroke (59 vs 39 events, hazard ratio 1.49, 95% CI 1.00–2.24) and venous thromboembolism (103 vs 71 events, hazard ratio 1.44, 95% CI 1.06–1.95).
The STAR trial (Study of Tamoxifen and Raloxifene)26,27 compared raloxifene and tamoxifen in postmenopausal women at increased risk of breast cancer. Women were randomized to receive either tamoxifen 20 mg or raloxifene 60 mg for 5 years. Results:
- No difference in the number of new cases of invasive breast cancer between the groups
- Fewer cases of noninvasive breast cancer in the tamoxifen group, but the difference was not statistically significant
- Fewer cases of uterine cancer in the raloxifene group, annual incidence rates 0.125% vs 0.199%, absolute risk reduction 0.74%, NNT 1,351, relative risk with raloxifene 0.62, 95% CI 0.30–0.50
- Fewer thromboembolic events with raloxifene
- Fewer cataracts with raloxifene.
Adverse effects of raloxifene
Raloxifene increases the risk of venous thromboembolism and stroke in women at high risk of coronary artery disease.19
Ideal candidates for raloxifene
Postmenopausal women with osteopenia or osteoporosis and a higher risk of breast cancer who have minimal to no vasomotor symptoms or genitourinary syndrome of menopause are good candidates for raloxifene. Raloxifene is also a good choice for women who have genitourinary syndrome of menopause treated with local vaginal estrogen. Raloxifene has no effect on vasomotor symptoms or genitourinary syndrome of menopause.
OSPEMIFENE: FOR GENITOURINARY SYNDROME OF MENOPAUSE
Although ospemifene does not have the steroid structure of estrogen, it acts as an estrogen agonist specifically in the vaginal mucosa and an antagonist in other tissues.28 It has been shown on Papanicolaou smears to reduce the number of parabasal cells and increase the number of intermediate and superficial cells after 3 months of treatment.29
Ospemifene 60 mg taken orally with food is approved by the FDA to treat genitourinary syndrome of menopause.
Why ospemifene is needed
First-line treatment options for genitourinary syndrome of menopause include over-the-counter lubricants. However, there is no evidence that these products reverse vaginal atrophy,30 and many women report no relief of symptoms with them.
While various local estrogen preparations positively affect genitourinary syndrome of menopause, some of them can be messy, which can limit-long term adherence.
In one of the largest surveys on genitourinary syndrome of menopause (the REVIVE survey—the Real Women’s View of Treatment Options for Menopausal Vaginal Changes29), 59% of women reported that their vaginal symptoms negatively affected sexual activity. The problem affects not only the patient but also her sexual partner.31 Another large study showed that 38% of women and 39% of male partners reported that it had a worse-than-expected impact on their intimate relationships.31
Genitourinary syndrome of menopause also makes pelvic examinations difficult, may worsen or exacerbate cystitis, and may increase urinary tract infections.
Trials of ospemifene for genitourinary syndrome of menopause
To date, 3 randomized, double-blind clinical trials have demonstrated ospemifene 60 mg to be superior to placebo in treating genitourinary syndrome of menopause. Two were short-term (12-week) and showed significant positive changes in the percent of superficial cells, vaginal pH (lower is better), and number of parabasal cells, along with improvements in the Likert rating of both vaginal dryness and dyspareunia.32,33
A long-term (52-week) randomized placebo-controlled trial compared ospemifene and placebo and showed significant improvement in vaginal maturation index and pH at weeks 12 and 52.34 Other outcome measures included petechiae, pallor, friability, erythema, and dryness, all of which improved from baseline (P < .001). At the end of the trial, 80% of the patients who received ospemifene had no vaginal atrophy.
No serious adverse events were noted in any of the clinical trials to date, and a systemic review and meta-analysis demonstrated ospemifene to be safe and efficacious.35 The most frequently reported reasons for discontinuation were hot flashes, vaginal discharge, muscle spasms, and hyperhidrosis, but the rates of these effects were similar to those with placebo.
Trial of ospemifene’s effect on bone turnover
As an estrogen receptor agonist in bone, ospemifene decreases the levels of bone turnover markers in postmenopausal women.36 A study found ospemifene to be about as effective as raloxifene in suppressing bone turnover,37 but ospemifene does not carry FDA approval for preventing or treating osteoporosis.
Other effects
In experiments in rats, the incidence of breast cancer appears to be lower with ospemifene, and the higher the dose, the lower the incidence.38
Ospemifene also has antagonistic effects on uterine tissue, and no cases of endometrial hyperplasia or carcinoma have been reported in short-term or long-term studies.35
Ospemifene has no effect however on vasomotor symptoms and may in fact worsen vasomotor symptoms in women suffering with hot flashes and night sweats. Further investigation into its long-term safety and effects on breast tissue and bone would provide more insight.
Ideal candidates for ospemifene
Ospemifene could help postmenopausal women with genitourinary syndrome of menopause for whom over-the-counter lubricants fail, who dislike local vaginal estrogen, or who decline systemic hormone therapy, and who do not meet the criteria for treatment with systemic hormone therapy.
CONJUGATED ESTROGENS AND BAZEDOXIFENE COMBINATION
A combination agent consisting of conjugated estrogens 0.45 mg plus bazedoxifene 20 mg has been approved by the FDA for treating moderate to severe vasomotor symptoms associated with menopause and also for preventing postmenopausal osteoporosis in women who have an intact uterus.
Trials of estrogen-bazedoxifene for vasomotor symptoms
The Selective Estrogen Menopause and Response to Therapy (SMART) trials39,40 were a series of randomized, double-blind, placebo-controlled phase 3 studies evaluating the efficacy and safety of the estrogen-bazedoxifene combination in postmenopausal women.
The SMART-2 trial39 evaluated the combination of conjugated estrogens (either 0.45 mg or 0.625) plus bazedoxifene 20 mg and found both dosages significantly reduced the number and severity of hot flashes at weeks 4 and 12 (P < .001). At week 12, the combination with 0.45 mg of estrogen reduced vasomotor symptoms from baseline by 74% (10.3 hot flashes per week at baseline vs 2.8 at week 12); the combination with 0.625 mg of estrogen reduced vasomotor symptoms by 80% (10.4 vs 2.4 flashes); and placebo reduced them by 51% (10.5 vs 5.4 flashes).
For bone density. The SMART-1 trial40 showed that the estrogen-bazedoxifene combination in both estrogen dosages significantly increased mean lumbar spine bone mineral density (P < .001) and total hip bone mineral density (P < .05) from baseline at 12 and 24 months compared with placebo. Increases in density tended to be higher with the higher estrogen dose (0.625 mg), but less with higher doses of bazedoxifene.41 At 24 months, the increase in bone mineral density was even greater than in women treated with raloxifene.42 However, the effect of estrogen-bazedoxifene on the incidence of fractures remains to be studied.
For genitourinary syndrome of menopause. The SMART-3 trial showed that treatment with conjugated estrogens plus bazedoxifene (0.45/20 mg or 0.625/20 mg) was more effective than placebo in increasing the percent of superficial and intermediate cells and decreased the number of parabasal cells at 12 weeks compared with placebo (P < .01).43 Both doses also significantly decreased the mean vaginal pH and improved vaginal dryness.
Patients treated with estrogen-bazedoxifene for a minimum of 12 weeks in a double-blind placebo-controlled study also showed a significant improvement in sexual function and quality-of-life measurements based on 3 well-defined scales, which included ease of lubrication, satisfaction with treatment, control of hot flashes, and sleep parameters.43
Low rates of side effects
To evaluate this regimen’s antagonistic effects on uterine tissue, endometrial hyperplasia was diagnosed by blinded pathologists using endometrial biopsies taken at 6, 12, and 24 months or more if cancer was a suspected diagnosis. At 12 and 24 months of treatment, the incidence of hyperplasia with bazedoxifene 20 or 40 mg at doses of either 0.45 or 0.625 mg of conjugated estrogens was less than 1%, which was similar to placebo rates over the 24 months.44 The lowest dose studied, bazedoxifene 10 mg, did not prevent hyperplasia with conjugated estrogens 0.45 or 0.625 mg, and its use was discontinued.
Rates of amenorrhea with bazedoxifene 20 or 40 mg and conjugated estrogens 0.45 or 0.625 mg were very favorable (83%–93%) and similar to those with placebo.45 For women with continued bleeding on hormone therapy requiring multiple evaluations, or for women who won’t accept the risk of bleeding on hormone therapy, conjugated estrogens and bazedoxifene may be a sustainable option. However, any woman with abnormal bleeding should undergo prompt immediate evaluation.
A typical side effect of estrogen replacement therapy is breast tenderness. For women seeking vasomotor symptom treatment but who experience breast tenderness, this may be a deterrent from continuing hormone therapy. As shown in the SMART-1 and SMART-2 trials,46 conjugated estrogens and bazedoxifene did not cause an increase in breast tenderness, which may enhance medication adherence.
Ideal candidates for conjugated estrogens plus bazedoxifene
This product could help postmenopausal women who have an intact uterus and are suffering with moderate to severe vasomotor symptoms and genitourinary syndrome of menopause who cannot tolerate the side effects of hormone therapy such as bleeding, bloating, or breast tenderness, or who prefer to take an estrogen but without a progestin. It is also ideal for women at higher risk of osteoporosis.
WHO SHOULD GET WHAT?
Not all postmenopausal women have vasomotor symptoms, genitourinary syndrome of menopause, or bone loss. For those who do, standard hormone therapy is an option.
For those who have symptoms and a lower threshold of side effects such as breast tenderness and vaginal bleeding, a combination of an estrogen plus an ERAA (eg, bazedoxifene) is an option.
For women who have no vasomotor symptoms but do have genitourinary syndrome of menopause and don’t want local vaginal treatment, ospemifene is an option.
For women with no vasomotor symptoms but who have bone loss and increased risk of estrogen receptor-positive breast cancer, raloxifene is a good option.
Both premenopausal and postmenopausal women who are at increased risk for breast cancer should be considered for tamoxifen chemoprevention. Postmenopausal women with a uterus at increased risk for breast cancer should be considered for raloxifene, as it has no uterine effect. Raloxifene is not indicated in premenopausal women.
No woman at increased risk of venous thromboembolism is a candidate for ERAA treatment or for oral estrogen. However, the clinician has multiple options to improve quality of life and work productivity and reduce office visits of women at midlife, especially when they are individually assessed and treated.
- Giannini A, Russo E, Mannella P, Simoncini T. Selective steroid receptor modulators in reproductive medicine. Minerva Ginecol 2015; 67:431–455.
- Feldman BM, Voda A, Gronseth E. The prevalence of hot flash and associated variables among perimenopausal women. Res Nurs Health 1985; 8:261–268.
- Versi E, Harvey MA, Cardozo L, Brincat M, Studd JW. Urogenital prolapse and atrophy at menopause: a prevalence study. Int Urogynecol J Pelvic Floor Dysfunct 2001; 12:107–110.
- Hess R, Chang CC, Conigliaro J, McNeil M. Understanding physicians’ attitudes towards hormone therapy. Womens Health Issues 2005; 15:31–38.
- Melton LJ 3rd, Khosla S, Atkinson EJ, O’Fallon WM, Riggs BL. Relationship of bone turnover to bone density and fractures. J Bone Miner Res 1997; 12:1083–1091.
- Sikon A, Thacker HL. Treatment options for menopausal hot flashes. Cleve Clin J Med 2004; 71:578–582.
- Levine JP. Treating menopausal symptoms with a tissue-selective estrogen complex. Gend Med 2011; 8:57–68.
- Pinkerton JV, Thomas S. Use of SERMs for treatment in postmenopausal women. J Steroid Biochem Mol Biol 2014; 142:142–154.
- Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet 1998; 351:1451–1467.
- Davies C, Pan H, Godwin J, et al; Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) Collaborative Group. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 2013; 381:805–816.
- Gray RG, Rea D, Handley K, et al. aTTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J Clin Oncol 2013; (suppl): abstract 5.
- Cuzick J, Powles T, Veronesi U, et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet 2003; 361:296–300.
- Cuzick J, Sestak I, Cawthorn S, et al. Tamoxifen for prevention of breast cancer: extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol 2015; 16:67–75.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998; 90:1371–1388.
- Chang J, Powles TJ, Ashley SE, et al. The effect of tamoxifen and hormone replacement therapy on serum cholesterol, bone mineral density and coagulation factors in healthy postmenopausal women participating in a randomised, controlled tamoxifen prevention study. Ann Oncol 1996; 7:671–675.
- Herrington DM, Klein KP. Effects of SERMs on important indicators of cardiovascular health: lipoproteins, hemostatic factors and endothelial function. Womens Health Issues 2001; 11:95–102.
- Kedar RP, Bourne TH, Powles TJ, et al. Effects of tamoxifen on uterus and ovaries of postmenopausal women in a randomized breast cancer prevention trial. Lancet 1994; 343:1318–1321.
- Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 1999; 282:637–645.
- Maricic M, Adachi JD, Sarkar S, Wu W, Wong M, Harper KD. Early effects of raloxifene on clinical vertebral fractures at 12 months in postmenopausal women with osteoporosis. Arch Intern Med 2002; 162:1140–1143.
- Recker RR, Mitlak BH, Ni X, Krege JH. Long-term raloxifene for postmenopausal osteoporosis. Curr Med Res Opin 2011; 27:1755–1761.
- Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA 1999; 281:2189–2197.
- Martino S, Cauley JA, Barrett-Connor E, et al; CORE Investigators. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst 2004; 96:1751–1761.
- Vogel VG, Qu Y, Wong M, Mitchell B, Mershon JL. Incidence of invasive breast cancer in postmenopausal women after discontinuation of long-term raloxifene administration. Clin Breast Cancer 2009; 9:45–50.
- Grady D, Cauley JA, Stock JL, et al. Effect of raloxifene on all-cause mortality. Am J Med 2010; 123:469.e1–e7.
- Barrett-Connor E, Mosca L, Collins P, et al; Raloxifene Use for The Heart (RUTH) Trial Investigators. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med 2006; 355:125–137.
- Vogel VG. The NSABP Study of Tamoxifen and Raloxifene (STAR) trial. Expert Rev Anticancer Ther 2009; 9:51–60.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 2006; 295:2727–2741.
- Barnes KN, Pearce EF, Yancey AM, Forinash AB. Ospemifene in the treatment of vulvovaginal atrophy. Ann Pharmacother 2014; 48:752–757.
- Rutanen EM, Heikkinen J, Halonen K, Komi J, Lammintausta R, Ylikorkala O. Effects of ospemifene, a novel SERM, on hormones, genital tract, climacteric symptoms, and quality of life in postmenopausal women: a double-blind, randomized trial. Menopause 2003; 10:433–439.
- Constantine G, Graham S, Koltun WD, Kingsberg SA. Assessment of ospemifene or lubricants on clinical signs of VVA. J Sex Med 2014; 11:1033–1041.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE survey. J Sex Med 2013; 10:1790–1799.
- Portman DJ, Bachmann GA, Simon JA; Ospemifene Study Group. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause 2013; 20:623–630.
- Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause 2010; 17:480–486.
- Goldstein SR, Bachmann GA, Koninckx PR, Lin VH, Portman DJ, Ylikorkala O; Ospemifene Study Group. Ospemifene 12-month safety and efficacy in postmenopausal women with vulvar and vaginal atrophy. Climacteric 2014; 17:173–182.
- Cui Y, Zong H, Yan H, Li N, Zhang Y. The efficacy and safety of ospemifene in treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy: a systematic review and meta-analysis. J Sex Med 2014; 11:487–497.
- Komi J, Heikkinen J, Rutanen EM, Halonen K, Lammintausta R, Ylikorkala O. Effects of ospemifene, a novel SERM, on biochemical markers of bone turnover in healthy postmenopausal women. Gynecal Endocrinol 2004; 18:152–158.
- Komi J, Lankinen KS, DeGregorio M, et al. Effects of ospemifene and raloxifene on biochemical markers of bone turnover in postmenopausal women. J Bone Miner Metab 2006; 24:314–318.
- Wurz GT, Read KC, Marchisano-Karpman C, et al. Ospemifene inhibits the growth of dimethylbenzanthracene-induced mammary tumors in Sencar mice. J Steroid Biochem Mol Biol 2005; 97:230–240.
- Pinkerton JV, Utian WH, Constantine GD, Olivier S, Pickar JH. Relief of vasomotor symptoms with the tissue-selective estrogen complex containing bazedoxifene/conjugated estrogens: a randomized, controlled trial. Menopause 2009; 16:1116–1124.
- Pickar JH, Mirkin S. Tissue-selective agents: selective estrogen receptor modulators and the tissue-selective estrogen complex. Menopause Int 2010; 16:121–128.
- Levine JP. Treating menopausal symptoms with a tissue-selective estrogen complex. Gend Med 2011; 8:57–68.
- Lindsay R, Gallagher JC, Kagan R, Pickar JH, Constantine G. Efficacy of tissue-selective estrange complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril 2009; 92:1045–1052.
- Bachmann G, Bobula J, Mirkin S. Effects of bazedoxifene/conjugated estrogens on quality of life in postmenopausal women with symptoms of vulvar/vaginal atrophy. Climacteric 2010; 13:132–140.
- Pickar JH, Yeh IT, Bachmann G, Speroff L. Endometrial effects of a tissue selective estrogen complex containing bazedoxifene/conjugated estrogens as a menopausal therapy. Fertil Steril 2009; 92:1018–1024.
- Archer DF, Lewis V, Carr BR, Olivier S, Pickar JH. Bazedoxifene/conjugated estrogens (BZA/CE): incidence of uterine bleeding in postmenopausal women. Fertil Steril 2009: 92:1039–1044.
- Pinkerton JV, Abraham L, Bushmakin AG, et al. Evaluation of the efficacy and safety of bazedoxifene/conjugated estrogens for secondary outcomes including vasomotor symptoms in postmenopausal women by years since menopause in the Selective estrogens, Menopause and Response to Therapy (SMART) trials. J Womens Health (Larchmt) 2014; 23:18–28.
Estrogen receptor agonist-antagonists (ERAAs), previously called selective estrogen receptor modulators (SERMs), have extended the options for treating the various conditions that menopausal women suffer from. These drugs act differently on estrogen receptors in different tissues, stimulating receptors in some tissues but inhibiting them in others. This allows selective inhibition or stimulation of estrogen-like action in various target tissues.1
This article highlights the use of ERAAs to treat menopausal vasomotor symptoms (eg, hot flashes, night sweats), genitourinary syndrome of menopause, osteoporosis, breast cancer (and the risk of breast cancer), and other health concerns unique to women at midlife.
SYMPTOMS OF MENOPAUSE: COMMON AND TROUBLESOME
Vasomotor symptoms such as hot flashes and night sweats are common during perimenopause—most women experience them. They are most frequent during the menopause transition but can persist for 10 years or more afterward.2
Genitourinary syndrome of menopause is also common and often worsens with years after menopause.3 It can lead to dyspareunia and vaginal dryness, which may in turn result in lower libido, vaginismus, and hypoactive sexual desire disorder, problems that often arise at the same time as vaginal dryness and atrophy.4
Osteopenia and osteoporosis. A drop in systemic estrogen leads to a decline in bone mineral density, increasing the risk of fractures.5
ESTROGEN-PROGESTIN TREATMENT: THE GOLD STANDARD, BUT NOT IDEAL
The current gold standard for treating moderate to severe hot flashes is estrogen, available in oral, transdermal, and vaginal formulations.6 Estrogen also has antiresorptive effects on bone and is approved for preventing osteoporosis. Systemic estrogen may also be prescribed for genitourinary syndrome of menopause if local vaginal treatment alone is insufficient.
If women who have an intact uterus receive estrogen, they should also receive a progestin to protect against endometrial hyperplasia and reduce the risk of endometrial cancer.
Despite its status as the gold standard, estrogen-progestin therapy presents challenges. In some women, progestins cause side effects such as breast tenderness, bloating, fatigue, and depression.7 Estrogen-progestin therapy often causes vaginal bleeding, which for some women is troublesome or distressing; bleeding may be the reason for repeated evaluations, can increase anxiety, and can lead to poor adherence with hormonal treatment. Women who carry a higher-than-normal risk of developing breast cancer or fear that taking hormones will lead to breast cancer may show decreased adherence to therapy. Women who have estrogen receptor-positive breast cancer cannot take estrogen.
Individualized options are needed for women who have progestin-related side effects, unwanted vaginal bleeding, or a higher risk of breast cancer.
WELCOME THE ERAAs
An ideal treatment for menopause would relieve vasomotor symptoms and genitourinary syndrome of menopause and increase bone mineral density without causing breast tenderness, vaginal bleeding, or endometrial proliferation.
The “designer estrogens,” or ERAAs, have specific positive effects on the bone, heart, and brain with neutral or antagonist effects on estrogen receptors in other tissues such as the breasts and endometrium.8 While not entirely free of adverse effects, these agents have been developed with the aim of minimizing the most common ones related to estrogen and progestin.
Several ERAAs are currently approved by the US Food and Drug Administration (FDA)for various indications, each having a unique profile. Clomifene was the first agent of this class, and it is still used clinically to induce ovulation. This article highlights subsequently approved agents, ie, tamoxifen, raloxifene, ospemifene, and the combination of conjugated estrogens and bazedoxifene (Table 1).
All ERAAs increase the risk of venous thromboembolism, and therefore none of them should be used in women with known venous thromboembolism or at high risk of it.
TAMOXIFEN: CANCER TREATMENT AND PREVENTION
After clomiphene, tamoxifen was the second ERAA on the market. Although researchers were looking for a new contraceptive drug, they found tamoxifen to be useful as a chemotherapeutic agent for breast cancer. First used in 1971, tamoxifen continues to be one of the most commonly prescribed chemotherapeutic medications today.
The FDA has approved tamoxifen to treat breast cancer as well as to prevent breast cancer in pre- and postmenopausal women at risk. It may also have beneficial effects on bone and on cardiovascular risk factors, but these are not approved uses for it.
Trials of tamoxifen for cancer treatment
The Early Breast Cancer Trialists’ Collaborative Group9 performed a meta-analysis and found that 5 years of adjuvant treatment with tamoxifen is associated with a 26% reduction in mortality and a 47% reduction in breast cancer recurrence at 10 years. In absolute terms, we estimate that 21 women would need to be treated to prevent 1 death and 8 would need to be treated to prevent 1 recurrence.
The ATLAS Trial (Adjuvant Tamoxifen Longer Against Shorter)10 and later the UK ATTOM (Adjuvant Tamoxifen Treatment to Offer More)11 trial confirmed an even greater reduction in recurrence and mortality after a total of 10 years of treatment.
Trials of tamoxifen for cancer prevention
Cuzik et al12 performed a meta-analysis of 4 trials of tamoxifen’s effectiveness in preventing breast cancer for women at elevated risk. The incidence of estrogen receptor-positive breast cancer was 48% lower with tamoxifen use, but there was no effect on estrogen-negative breast cancer. From their data, we estimate that 77 women would need to be treated to prevent 1 case of breast cancer.
The IBIS-I trial (International Breast Cancer Intervention Study I)13 found that, in healthy women at high risk of breast cancer, the benefit of taking tamoxifen for 5 years as preventive treatment persisted long afterward. The investigators estimated that at 20 years of follow-up the risk of breast cancer would be 12.3% in placebo recipients and 7.8% in tamoxifen recipients, a 4.5% absolute risk reduction; number needed to treat (NNT) 22.
Data on tamoxifen and osteoporosis
The Breast Cancer Prevention Trial revealed a 19% reduction in the incidence of osteoporotic fractures with tamoxifen, but the difference was not statistically significant.14 The 1-year rates of fracture in women age 50 and older were 0.727% with placebo and 0.567% with tamoxifen, an absolute difference of 0.151%; therefore, if the effect is real, 662 women age 50 or older would need to be treated for 1 year to prevent 1 fracture. Tamoxifen is not FDA-approved to treat osteoporosis.
Data on tamoxifen and cardiovascular risk reduction
Chang et al,15 in a study in women at risk of breast cancer, incidentally found that tamoxifen was associated with a 13% reduction in total cholesterol compared with placebo.
Herrington and Klein,16 in a systematic review, noted similar findings in multiple studies of tamoxifen, with decreases in total cholesterol ranging from 7% to 17% and decreases in low-density lipoprotein cholesterol ranging from 10% to 28%. However, they found no change in high-density lipoprotein cholesterol concentrations or in the cardiovascular mortality rate.
The ATLAS trial10 revealed a relative risk reduction of 0.76 (95% confidence interval [CI] 0.60–0.95, P = .02) in ischemic heart disease for women who took tamoxifen for 10 years compared with 5 years. We calculate that ischemic heart disease occurred in 163 (2.5%) of 6,440 women who took tamoxifen for 5 years compared with 127 (1.9%) of 6,454 women who took it for 10 years, a 0.6% absolute risk reduction, NNT = 167.
Adverse effects of tamoxifen
Uterine neoplasia. Women taking tamoxifen have a 2.5-fold increased risk of endometrial cancer.14 Tamoxifen also increases the risk of benign uterine disease such as endometrial hyperplasia and polyps. As many as 39% of women taking tamoxifen will have evidence of benign uterine changes on pathology.17 Other adverse effects:
Venous thromboembolism (the risk of pulmonary embolism is increased approximately threefold14)
Cataracts (there is a slight increase in cataract diagnosis in tamoxifen users)
Vasomotor symptoms, which limit the use of tamoxifen in many women.
Ideal candidate for tamoxifen
The ideal candidate for tamoxifen is a woman with breast cancer that is estrogen receptor-positive and who has a history of osteopenia or osteoporosis and no risk factors for venous thromboembolism.
RALOXIFENE: FOR OSTEOPOROSIS AND FOR CANCER PREVENTION
Raloxifene, a second-generation ERAA, was first approved for preventing and treating osteoporosis and later for reducing the risk of invasive estrogen receptor-positive breast cancer in postmenopausal women.
Trials of raloxifene for osteoporosis
The MORE trial (Multiple Outcomes of Raloxifene)18 was a large multicenter randomized double-blind study. Raloxifene recipients showed a significant increase in bone mineral density in the lumbar spine and femoral neck at year 3 (P < .001) compared with those receiving placebo. Even after only 1 year of treatment, raloxifene significantly reduced the risk of new fractures, despite only modest gains in bone mineral density. After 3 years of treatment, new clinical vertebral fractures had occurred in 3.5% of the placebo group compared with 2.1% of the group receiving raloxifene 60 mg.19 Relative risk reductions were similar in women who had already had a clinical vertebral fracture at baseline, whose absolute risk is higher. However, no significant effect was seen on the incidence of hip or nonvertebral fractures.
The CORE trial (Continuing Outcomes Relevant to Raloxifene)20 extended the treatment of the women enrolled in the MORE trial another 4 years and found that the benefit of raloxifene with regard to bone mineral density persisted with continued use.
Trials of raloxifene for breast cancer prevention
The MORE trial,21 in postmenopausal women with osteoporosis included breast cancer as a secondary end point, and raloxifene was shown to decrease the incidence of invasive breast cancer. At a median of 40 months, invasive breast cancer had arisen in 13 (0.25%) of the 5,129 women assigned to raloxifene and 27 (1.0%) of the 2,576 women assigned to placebo. The authors calculated that 126 women would need to be treated to prevent 1 case of breast cancer.
The CORE trial,22 as noted, extended the treatment of the women enrolled in the MORE trial another 4 years. The risk of any invasive breast cancer in postmenopausal women with osteoporosis was significantly reduced by 59% after 8 years, and the risk of estrogen receptor-positive invasive breast cancer was reduced by 66%.
There is evidence that raloxifene’s effect on breast cancer risk persists after discontinuation of use.23
Does raloxifene reduce mortality?
Grady et al24 studied the effect of raloxifene on all-cause mortality in a pooled analysis of mortality data from the MORE, CORE, and Raloxifene Use for the Heart (RUTH)25 trials. In older postmenopausal women, the rate of all-cause mortality was 8.65% in those taking placebo compared with 7.88% in those taking raloxifene 60 mg daily—10% lower. The mechanism behind the lower mortality rate is unclear, and Grady et al recommend that the finding be interpreted with caution.
Trials of raloxifene for heart protection
The RUTH trial25 was a 5.6-year study undertaken to study the effects of raloxifene on coronary outcomes and invasive breast cancer in postmenopausal women. Results were mixed. Active treatment:
- Did not significantly affect the risk of coronary artery disease compared with placebo
- Significantly decreased the risk of invasive breast cancer
- Significantly decreased the risk of clinical vertebral fractures
- Increased the risk of fatal stroke (59 vs 39 events, hazard ratio 1.49, 95% CI 1.00–2.24) and venous thromboembolism (103 vs 71 events, hazard ratio 1.44, 95% CI 1.06–1.95).
The STAR trial (Study of Tamoxifen and Raloxifene)26,27 compared raloxifene and tamoxifen in postmenopausal women at increased risk of breast cancer. Women were randomized to receive either tamoxifen 20 mg or raloxifene 60 mg for 5 years. Results:
- No difference in the number of new cases of invasive breast cancer between the groups
- Fewer cases of noninvasive breast cancer in the tamoxifen group, but the difference was not statistically significant
- Fewer cases of uterine cancer in the raloxifene group, annual incidence rates 0.125% vs 0.199%, absolute risk reduction 0.74%, NNT 1,351, relative risk with raloxifene 0.62, 95% CI 0.30–0.50
- Fewer thromboembolic events with raloxifene
- Fewer cataracts with raloxifene.
Adverse effects of raloxifene
Raloxifene increases the risk of venous thromboembolism and stroke in women at high risk of coronary artery disease.19
Ideal candidates for raloxifene
Postmenopausal women with osteopenia or osteoporosis and a higher risk of breast cancer who have minimal to no vasomotor symptoms or genitourinary syndrome of menopause are good candidates for raloxifene. Raloxifene is also a good choice for women who have genitourinary syndrome of menopause treated with local vaginal estrogen. Raloxifene has no effect on vasomotor symptoms or genitourinary syndrome of menopause.
OSPEMIFENE: FOR GENITOURINARY SYNDROME OF MENOPAUSE
Although ospemifene does not have the steroid structure of estrogen, it acts as an estrogen agonist specifically in the vaginal mucosa and an antagonist in other tissues.28 It has been shown on Papanicolaou smears to reduce the number of parabasal cells and increase the number of intermediate and superficial cells after 3 months of treatment.29
Ospemifene 60 mg taken orally with food is approved by the FDA to treat genitourinary syndrome of menopause.
Why ospemifene is needed
First-line treatment options for genitourinary syndrome of menopause include over-the-counter lubricants. However, there is no evidence that these products reverse vaginal atrophy,30 and many women report no relief of symptoms with them.
While various local estrogen preparations positively affect genitourinary syndrome of menopause, some of them can be messy, which can limit-long term adherence.
In one of the largest surveys on genitourinary syndrome of menopause (the REVIVE survey—the Real Women’s View of Treatment Options for Menopausal Vaginal Changes29), 59% of women reported that their vaginal symptoms negatively affected sexual activity. The problem affects not only the patient but also her sexual partner.31 Another large study showed that 38% of women and 39% of male partners reported that it had a worse-than-expected impact on their intimate relationships.31
Genitourinary syndrome of menopause also makes pelvic examinations difficult, may worsen or exacerbate cystitis, and may increase urinary tract infections.
Trials of ospemifene for genitourinary syndrome of menopause
To date, 3 randomized, double-blind clinical trials have demonstrated ospemifene 60 mg to be superior to placebo in treating genitourinary syndrome of menopause. Two were short-term (12-week) and showed significant positive changes in the percent of superficial cells, vaginal pH (lower is better), and number of parabasal cells, along with improvements in the Likert rating of both vaginal dryness and dyspareunia.32,33
A long-term (52-week) randomized placebo-controlled trial compared ospemifene and placebo and showed significant improvement in vaginal maturation index and pH at weeks 12 and 52.34 Other outcome measures included petechiae, pallor, friability, erythema, and dryness, all of which improved from baseline (P < .001). At the end of the trial, 80% of the patients who received ospemifene had no vaginal atrophy.
No serious adverse events were noted in any of the clinical trials to date, and a systemic review and meta-analysis demonstrated ospemifene to be safe and efficacious.35 The most frequently reported reasons for discontinuation were hot flashes, vaginal discharge, muscle spasms, and hyperhidrosis, but the rates of these effects were similar to those with placebo.
Trial of ospemifene’s effect on bone turnover
As an estrogen receptor agonist in bone, ospemifene decreases the levels of bone turnover markers in postmenopausal women.36 A study found ospemifene to be about as effective as raloxifene in suppressing bone turnover,37 but ospemifene does not carry FDA approval for preventing or treating osteoporosis.
Other effects
In experiments in rats, the incidence of breast cancer appears to be lower with ospemifene, and the higher the dose, the lower the incidence.38
Ospemifene also has antagonistic effects on uterine tissue, and no cases of endometrial hyperplasia or carcinoma have been reported in short-term or long-term studies.35
Ospemifene has no effect however on vasomotor symptoms and may in fact worsen vasomotor symptoms in women suffering with hot flashes and night sweats. Further investigation into its long-term safety and effects on breast tissue and bone would provide more insight.
Ideal candidates for ospemifene
Ospemifene could help postmenopausal women with genitourinary syndrome of menopause for whom over-the-counter lubricants fail, who dislike local vaginal estrogen, or who decline systemic hormone therapy, and who do not meet the criteria for treatment with systemic hormone therapy.
CONJUGATED ESTROGENS AND BAZEDOXIFENE COMBINATION
A combination agent consisting of conjugated estrogens 0.45 mg plus bazedoxifene 20 mg has been approved by the FDA for treating moderate to severe vasomotor symptoms associated with menopause and also for preventing postmenopausal osteoporosis in women who have an intact uterus.
Trials of estrogen-bazedoxifene for vasomotor symptoms
The Selective Estrogen Menopause and Response to Therapy (SMART) trials39,40 were a series of randomized, double-blind, placebo-controlled phase 3 studies evaluating the efficacy and safety of the estrogen-bazedoxifene combination in postmenopausal women.
The SMART-2 trial39 evaluated the combination of conjugated estrogens (either 0.45 mg or 0.625) plus bazedoxifene 20 mg and found both dosages significantly reduced the number and severity of hot flashes at weeks 4 and 12 (P < .001). At week 12, the combination with 0.45 mg of estrogen reduced vasomotor symptoms from baseline by 74% (10.3 hot flashes per week at baseline vs 2.8 at week 12); the combination with 0.625 mg of estrogen reduced vasomotor symptoms by 80% (10.4 vs 2.4 flashes); and placebo reduced them by 51% (10.5 vs 5.4 flashes).
For bone density. The SMART-1 trial40 showed that the estrogen-bazedoxifene combination in both estrogen dosages significantly increased mean lumbar spine bone mineral density (P < .001) and total hip bone mineral density (P < .05) from baseline at 12 and 24 months compared with placebo. Increases in density tended to be higher with the higher estrogen dose (0.625 mg), but less with higher doses of bazedoxifene.41 At 24 months, the increase in bone mineral density was even greater than in women treated with raloxifene.42 However, the effect of estrogen-bazedoxifene on the incidence of fractures remains to be studied.
For genitourinary syndrome of menopause. The SMART-3 trial showed that treatment with conjugated estrogens plus bazedoxifene (0.45/20 mg or 0.625/20 mg) was more effective than placebo in increasing the percent of superficial and intermediate cells and decreased the number of parabasal cells at 12 weeks compared with placebo (P < .01).43 Both doses also significantly decreased the mean vaginal pH and improved vaginal dryness.
Patients treated with estrogen-bazedoxifene for a minimum of 12 weeks in a double-blind placebo-controlled study also showed a significant improvement in sexual function and quality-of-life measurements based on 3 well-defined scales, which included ease of lubrication, satisfaction with treatment, control of hot flashes, and sleep parameters.43
Low rates of side effects
To evaluate this regimen’s antagonistic effects on uterine tissue, endometrial hyperplasia was diagnosed by blinded pathologists using endometrial biopsies taken at 6, 12, and 24 months or more if cancer was a suspected diagnosis. At 12 and 24 months of treatment, the incidence of hyperplasia with bazedoxifene 20 or 40 mg at doses of either 0.45 or 0.625 mg of conjugated estrogens was less than 1%, which was similar to placebo rates over the 24 months.44 The lowest dose studied, bazedoxifene 10 mg, did not prevent hyperplasia with conjugated estrogens 0.45 or 0.625 mg, and its use was discontinued.
Rates of amenorrhea with bazedoxifene 20 or 40 mg and conjugated estrogens 0.45 or 0.625 mg were very favorable (83%–93%) and similar to those with placebo.45 For women with continued bleeding on hormone therapy requiring multiple evaluations, or for women who won’t accept the risk of bleeding on hormone therapy, conjugated estrogens and bazedoxifene may be a sustainable option. However, any woman with abnormal bleeding should undergo prompt immediate evaluation.
A typical side effect of estrogen replacement therapy is breast tenderness. For women seeking vasomotor symptom treatment but who experience breast tenderness, this may be a deterrent from continuing hormone therapy. As shown in the SMART-1 and SMART-2 trials,46 conjugated estrogens and bazedoxifene did not cause an increase in breast tenderness, which may enhance medication adherence.
Ideal candidates for conjugated estrogens plus bazedoxifene
This product could help postmenopausal women who have an intact uterus and are suffering with moderate to severe vasomotor symptoms and genitourinary syndrome of menopause who cannot tolerate the side effects of hormone therapy such as bleeding, bloating, or breast tenderness, or who prefer to take an estrogen but without a progestin. It is also ideal for women at higher risk of osteoporosis.
WHO SHOULD GET WHAT?
Not all postmenopausal women have vasomotor symptoms, genitourinary syndrome of menopause, or bone loss. For those who do, standard hormone therapy is an option.
For those who have symptoms and a lower threshold of side effects such as breast tenderness and vaginal bleeding, a combination of an estrogen plus an ERAA (eg, bazedoxifene) is an option.
For women who have no vasomotor symptoms but do have genitourinary syndrome of menopause and don’t want local vaginal treatment, ospemifene is an option.
For women with no vasomotor symptoms but who have bone loss and increased risk of estrogen receptor-positive breast cancer, raloxifene is a good option.
Both premenopausal and postmenopausal women who are at increased risk for breast cancer should be considered for tamoxifen chemoprevention. Postmenopausal women with a uterus at increased risk for breast cancer should be considered for raloxifene, as it has no uterine effect. Raloxifene is not indicated in premenopausal women.
No woman at increased risk of venous thromboembolism is a candidate for ERAA treatment or for oral estrogen. However, the clinician has multiple options to improve quality of life and work productivity and reduce office visits of women at midlife, especially when they are individually assessed and treated.
Estrogen receptor agonist-antagonists (ERAAs), previously called selective estrogen receptor modulators (SERMs), have extended the options for treating the various conditions that menopausal women suffer from. These drugs act differently on estrogen receptors in different tissues, stimulating receptors in some tissues but inhibiting them in others. This allows selective inhibition or stimulation of estrogen-like action in various target tissues.1
This article highlights the use of ERAAs to treat menopausal vasomotor symptoms (eg, hot flashes, night sweats), genitourinary syndrome of menopause, osteoporosis, breast cancer (and the risk of breast cancer), and other health concerns unique to women at midlife.
SYMPTOMS OF MENOPAUSE: COMMON AND TROUBLESOME
Vasomotor symptoms such as hot flashes and night sweats are common during perimenopause—most women experience them. They are most frequent during the menopause transition but can persist for 10 years or more afterward.2
Genitourinary syndrome of menopause is also common and often worsens with years after menopause.3 It can lead to dyspareunia and vaginal dryness, which may in turn result in lower libido, vaginismus, and hypoactive sexual desire disorder, problems that often arise at the same time as vaginal dryness and atrophy.4
Osteopenia and osteoporosis. A drop in systemic estrogen leads to a decline in bone mineral density, increasing the risk of fractures.5
ESTROGEN-PROGESTIN TREATMENT: THE GOLD STANDARD, BUT NOT IDEAL
The current gold standard for treating moderate to severe hot flashes is estrogen, available in oral, transdermal, and vaginal formulations.6 Estrogen also has antiresorptive effects on bone and is approved for preventing osteoporosis. Systemic estrogen may also be prescribed for genitourinary syndrome of menopause if local vaginal treatment alone is insufficient.
If women who have an intact uterus receive estrogen, they should also receive a progestin to protect against endometrial hyperplasia and reduce the risk of endometrial cancer.
Despite its status as the gold standard, estrogen-progestin therapy presents challenges. In some women, progestins cause side effects such as breast tenderness, bloating, fatigue, and depression.7 Estrogen-progestin therapy often causes vaginal bleeding, which for some women is troublesome or distressing; bleeding may be the reason for repeated evaluations, can increase anxiety, and can lead to poor adherence with hormonal treatment. Women who carry a higher-than-normal risk of developing breast cancer or fear that taking hormones will lead to breast cancer may show decreased adherence to therapy. Women who have estrogen receptor-positive breast cancer cannot take estrogen.
Individualized options are needed for women who have progestin-related side effects, unwanted vaginal bleeding, or a higher risk of breast cancer.
WELCOME THE ERAAs
An ideal treatment for menopause would relieve vasomotor symptoms and genitourinary syndrome of menopause and increase bone mineral density without causing breast tenderness, vaginal bleeding, or endometrial proliferation.
The “designer estrogens,” or ERAAs, have specific positive effects on the bone, heart, and brain with neutral or antagonist effects on estrogen receptors in other tissues such as the breasts and endometrium.8 While not entirely free of adverse effects, these agents have been developed with the aim of minimizing the most common ones related to estrogen and progestin.
Several ERAAs are currently approved by the US Food and Drug Administration (FDA)for various indications, each having a unique profile. Clomifene was the first agent of this class, and it is still used clinically to induce ovulation. This article highlights subsequently approved agents, ie, tamoxifen, raloxifene, ospemifene, and the combination of conjugated estrogens and bazedoxifene (Table 1).
All ERAAs increase the risk of venous thromboembolism, and therefore none of them should be used in women with known venous thromboembolism or at high risk of it.
TAMOXIFEN: CANCER TREATMENT AND PREVENTION
After clomiphene, tamoxifen was the second ERAA on the market. Although researchers were looking for a new contraceptive drug, they found tamoxifen to be useful as a chemotherapeutic agent for breast cancer. First used in 1971, tamoxifen continues to be one of the most commonly prescribed chemotherapeutic medications today.
The FDA has approved tamoxifen to treat breast cancer as well as to prevent breast cancer in pre- and postmenopausal women at risk. It may also have beneficial effects on bone and on cardiovascular risk factors, but these are not approved uses for it.
Trials of tamoxifen for cancer treatment
The Early Breast Cancer Trialists’ Collaborative Group9 performed a meta-analysis and found that 5 years of adjuvant treatment with tamoxifen is associated with a 26% reduction in mortality and a 47% reduction in breast cancer recurrence at 10 years. In absolute terms, we estimate that 21 women would need to be treated to prevent 1 death and 8 would need to be treated to prevent 1 recurrence.
The ATLAS Trial (Adjuvant Tamoxifen Longer Against Shorter)10 and later the UK ATTOM (Adjuvant Tamoxifen Treatment to Offer More)11 trial confirmed an even greater reduction in recurrence and mortality after a total of 10 years of treatment.
Trials of tamoxifen for cancer prevention
Cuzik et al12 performed a meta-analysis of 4 trials of tamoxifen’s effectiveness in preventing breast cancer for women at elevated risk. The incidence of estrogen receptor-positive breast cancer was 48% lower with tamoxifen use, but there was no effect on estrogen-negative breast cancer. From their data, we estimate that 77 women would need to be treated to prevent 1 case of breast cancer.
The IBIS-I trial (International Breast Cancer Intervention Study I)13 found that, in healthy women at high risk of breast cancer, the benefit of taking tamoxifen for 5 years as preventive treatment persisted long afterward. The investigators estimated that at 20 years of follow-up the risk of breast cancer would be 12.3% in placebo recipients and 7.8% in tamoxifen recipients, a 4.5% absolute risk reduction; number needed to treat (NNT) 22.
Data on tamoxifen and osteoporosis
The Breast Cancer Prevention Trial revealed a 19% reduction in the incidence of osteoporotic fractures with tamoxifen, but the difference was not statistically significant.14 The 1-year rates of fracture in women age 50 and older were 0.727% with placebo and 0.567% with tamoxifen, an absolute difference of 0.151%; therefore, if the effect is real, 662 women age 50 or older would need to be treated for 1 year to prevent 1 fracture. Tamoxifen is not FDA-approved to treat osteoporosis.
Data on tamoxifen and cardiovascular risk reduction
Chang et al,15 in a study in women at risk of breast cancer, incidentally found that tamoxifen was associated with a 13% reduction in total cholesterol compared with placebo.
Herrington and Klein,16 in a systematic review, noted similar findings in multiple studies of tamoxifen, with decreases in total cholesterol ranging from 7% to 17% and decreases in low-density lipoprotein cholesterol ranging from 10% to 28%. However, they found no change in high-density lipoprotein cholesterol concentrations or in the cardiovascular mortality rate.
The ATLAS trial10 revealed a relative risk reduction of 0.76 (95% confidence interval [CI] 0.60–0.95, P = .02) in ischemic heart disease for women who took tamoxifen for 10 years compared with 5 years. We calculate that ischemic heart disease occurred in 163 (2.5%) of 6,440 women who took tamoxifen for 5 years compared with 127 (1.9%) of 6,454 women who took it for 10 years, a 0.6% absolute risk reduction, NNT = 167.
Adverse effects of tamoxifen
Uterine neoplasia. Women taking tamoxifen have a 2.5-fold increased risk of endometrial cancer.14 Tamoxifen also increases the risk of benign uterine disease such as endometrial hyperplasia and polyps. As many as 39% of women taking tamoxifen will have evidence of benign uterine changes on pathology.17 Other adverse effects:
Venous thromboembolism (the risk of pulmonary embolism is increased approximately threefold14)
Cataracts (there is a slight increase in cataract diagnosis in tamoxifen users)
Vasomotor symptoms, which limit the use of tamoxifen in many women.
Ideal candidate for tamoxifen
The ideal candidate for tamoxifen is a woman with breast cancer that is estrogen receptor-positive and who has a history of osteopenia or osteoporosis and no risk factors for venous thromboembolism.
RALOXIFENE: FOR OSTEOPOROSIS AND FOR CANCER PREVENTION
Raloxifene, a second-generation ERAA, was first approved for preventing and treating osteoporosis and later for reducing the risk of invasive estrogen receptor-positive breast cancer in postmenopausal women.
Trials of raloxifene for osteoporosis
The MORE trial (Multiple Outcomes of Raloxifene)18 was a large multicenter randomized double-blind study. Raloxifene recipients showed a significant increase in bone mineral density in the lumbar spine and femoral neck at year 3 (P < .001) compared with those receiving placebo. Even after only 1 year of treatment, raloxifene significantly reduced the risk of new fractures, despite only modest gains in bone mineral density. After 3 years of treatment, new clinical vertebral fractures had occurred in 3.5% of the placebo group compared with 2.1% of the group receiving raloxifene 60 mg.19 Relative risk reductions were similar in women who had already had a clinical vertebral fracture at baseline, whose absolute risk is higher. However, no significant effect was seen on the incidence of hip or nonvertebral fractures.
The CORE trial (Continuing Outcomes Relevant to Raloxifene)20 extended the treatment of the women enrolled in the MORE trial another 4 years and found that the benefit of raloxifene with regard to bone mineral density persisted with continued use.
Trials of raloxifene for breast cancer prevention
The MORE trial,21 in postmenopausal women with osteoporosis included breast cancer as a secondary end point, and raloxifene was shown to decrease the incidence of invasive breast cancer. At a median of 40 months, invasive breast cancer had arisen in 13 (0.25%) of the 5,129 women assigned to raloxifene and 27 (1.0%) of the 2,576 women assigned to placebo. The authors calculated that 126 women would need to be treated to prevent 1 case of breast cancer.
The CORE trial,22 as noted, extended the treatment of the women enrolled in the MORE trial another 4 years. The risk of any invasive breast cancer in postmenopausal women with osteoporosis was significantly reduced by 59% after 8 years, and the risk of estrogen receptor-positive invasive breast cancer was reduced by 66%.
There is evidence that raloxifene’s effect on breast cancer risk persists after discontinuation of use.23
Does raloxifene reduce mortality?
Grady et al24 studied the effect of raloxifene on all-cause mortality in a pooled analysis of mortality data from the MORE, CORE, and Raloxifene Use for the Heart (RUTH)25 trials. In older postmenopausal women, the rate of all-cause mortality was 8.65% in those taking placebo compared with 7.88% in those taking raloxifene 60 mg daily—10% lower. The mechanism behind the lower mortality rate is unclear, and Grady et al recommend that the finding be interpreted with caution.
Trials of raloxifene for heart protection
The RUTH trial25 was a 5.6-year study undertaken to study the effects of raloxifene on coronary outcomes and invasive breast cancer in postmenopausal women. Results were mixed. Active treatment:
- Did not significantly affect the risk of coronary artery disease compared with placebo
- Significantly decreased the risk of invasive breast cancer
- Significantly decreased the risk of clinical vertebral fractures
- Increased the risk of fatal stroke (59 vs 39 events, hazard ratio 1.49, 95% CI 1.00–2.24) and venous thromboembolism (103 vs 71 events, hazard ratio 1.44, 95% CI 1.06–1.95).
The STAR trial (Study of Tamoxifen and Raloxifene)26,27 compared raloxifene and tamoxifen in postmenopausal women at increased risk of breast cancer. Women were randomized to receive either tamoxifen 20 mg or raloxifene 60 mg for 5 years. Results:
- No difference in the number of new cases of invasive breast cancer between the groups
- Fewer cases of noninvasive breast cancer in the tamoxifen group, but the difference was not statistically significant
- Fewer cases of uterine cancer in the raloxifene group, annual incidence rates 0.125% vs 0.199%, absolute risk reduction 0.74%, NNT 1,351, relative risk with raloxifene 0.62, 95% CI 0.30–0.50
- Fewer thromboembolic events with raloxifene
- Fewer cataracts with raloxifene.
Adverse effects of raloxifene
Raloxifene increases the risk of venous thromboembolism and stroke in women at high risk of coronary artery disease.19
Ideal candidates for raloxifene
Postmenopausal women with osteopenia or osteoporosis and a higher risk of breast cancer who have minimal to no vasomotor symptoms or genitourinary syndrome of menopause are good candidates for raloxifene. Raloxifene is also a good choice for women who have genitourinary syndrome of menopause treated with local vaginal estrogen. Raloxifene has no effect on vasomotor symptoms or genitourinary syndrome of menopause.
OSPEMIFENE: FOR GENITOURINARY SYNDROME OF MENOPAUSE
Although ospemifene does not have the steroid structure of estrogen, it acts as an estrogen agonist specifically in the vaginal mucosa and an antagonist in other tissues.28 It has been shown on Papanicolaou smears to reduce the number of parabasal cells and increase the number of intermediate and superficial cells after 3 months of treatment.29
Ospemifene 60 mg taken orally with food is approved by the FDA to treat genitourinary syndrome of menopause.
Why ospemifene is needed
First-line treatment options for genitourinary syndrome of menopause include over-the-counter lubricants. However, there is no evidence that these products reverse vaginal atrophy,30 and many women report no relief of symptoms with them.
While various local estrogen preparations positively affect genitourinary syndrome of menopause, some of them can be messy, which can limit-long term adherence.
In one of the largest surveys on genitourinary syndrome of menopause (the REVIVE survey—the Real Women’s View of Treatment Options for Menopausal Vaginal Changes29), 59% of women reported that their vaginal symptoms negatively affected sexual activity. The problem affects not only the patient but also her sexual partner.31 Another large study showed that 38% of women and 39% of male partners reported that it had a worse-than-expected impact on their intimate relationships.31
Genitourinary syndrome of menopause also makes pelvic examinations difficult, may worsen or exacerbate cystitis, and may increase urinary tract infections.
Trials of ospemifene for genitourinary syndrome of menopause
To date, 3 randomized, double-blind clinical trials have demonstrated ospemifene 60 mg to be superior to placebo in treating genitourinary syndrome of menopause. Two were short-term (12-week) and showed significant positive changes in the percent of superficial cells, vaginal pH (lower is better), and number of parabasal cells, along with improvements in the Likert rating of both vaginal dryness and dyspareunia.32,33
A long-term (52-week) randomized placebo-controlled trial compared ospemifene and placebo and showed significant improvement in vaginal maturation index and pH at weeks 12 and 52.34 Other outcome measures included petechiae, pallor, friability, erythema, and dryness, all of which improved from baseline (P < .001). At the end of the trial, 80% of the patients who received ospemifene had no vaginal atrophy.
No serious adverse events were noted in any of the clinical trials to date, and a systemic review and meta-analysis demonstrated ospemifene to be safe and efficacious.35 The most frequently reported reasons for discontinuation were hot flashes, vaginal discharge, muscle spasms, and hyperhidrosis, but the rates of these effects were similar to those with placebo.
Trial of ospemifene’s effect on bone turnover
As an estrogen receptor agonist in bone, ospemifene decreases the levels of bone turnover markers in postmenopausal women.36 A study found ospemifene to be about as effective as raloxifene in suppressing bone turnover,37 but ospemifene does not carry FDA approval for preventing or treating osteoporosis.
Other effects
In experiments in rats, the incidence of breast cancer appears to be lower with ospemifene, and the higher the dose, the lower the incidence.38
Ospemifene also has antagonistic effects on uterine tissue, and no cases of endometrial hyperplasia or carcinoma have been reported in short-term or long-term studies.35
Ospemifene has no effect however on vasomotor symptoms and may in fact worsen vasomotor symptoms in women suffering with hot flashes and night sweats. Further investigation into its long-term safety and effects on breast tissue and bone would provide more insight.
Ideal candidates for ospemifene
Ospemifene could help postmenopausal women with genitourinary syndrome of menopause for whom over-the-counter lubricants fail, who dislike local vaginal estrogen, or who decline systemic hormone therapy, and who do not meet the criteria for treatment with systemic hormone therapy.
CONJUGATED ESTROGENS AND BAZEDOXIFENE COMBINATION
A combination agent consisting of conjugated estrogens 0.45 mg plus bazedoxifene 20 mg has been approved by the FDA for treating moderate to severe vasomotor symptoms associated with menopause and also for preventing postmenopausal osteoporosis in women who have an intact uterus.
Trials of estrogen-bazedoxifene for vasomotor symptoms
The Selective Estrogen Menopause and Response to Therapy (SMART) trials39,40 were a series of randomized, double-blind, placebo-controlled phase 3 studies evaluating the efficacy and safety of the estrogen-bazedoxifene combination in postmenopausal women.
The SMART-2 trial39 evaluated the combination of conjugated estrogens (either 0.45 mg or 0.625) plus bazedoxifene 20 mg and found both dosages significantly reduced the number and severity of hot flashes at weeks 4 and 12 (P < .001). At week 12, the combination with 0.45 mg of estrogen reduced vasomotor symptoms from baseline by 74% (10.3 hot flashes per week at baseline vs 2.8 at week 12); the combination with 0.625 mg of estrogen reduced vasomotor symptoms by 80% (10.4 vs 2.4 flashes); and placebo reduced them by 51% (10.5 vs 5.4 flashes).
For bone density. The SMART-1 trial40 showed that the estrogen-bazedoxifene combination in both estrogen dosages significantly increased mean lumbar spine bone mineral density (P < .001) and total hip bone mineral density (P < .05) from baseline at 12 and 24 months compared with placebo. Increases in density tended to be higher with the higher estrogen dose (0.625 mg), but less with higher doses of bazedoxifene.41 At 24 months, the increase in bone mineral density was even greater than in women treated with raloxifene.42 However, the effect of estrogen-bazedoxifene on the incidence of fractures remains to be studied.
For genitourinary syndrome of menopause. The SMART-3 trial showed that treatment with conjugated estrogens plus bazedoxifene (0.45/20 mg or 0.625/20 mg) was more effective than placebo in increasing the percent of superficial and intermediate cells and decreased the number of parabasal cells at 12 weeks compared with placebo (P < .01).43 Both doses also significantly decreased the mean vaginal pH and improved vaginal dryness.
Patients treated with estrogen-bazedoxifene for a minimum of 12 weeks in a double-blind placebo-controlled study also showed a significant improvement in sexual function and quality-of-life measurements based on 3 well-defined scales, which included ease of lubrication, satisfaction with treatment, control of hot flashes, and sleep parameters.43
Low rates of side effects
To evaluate this regimen’s antagonistic effects on uterine tissue, endometrial hyperplasia was diagnosed by blinded pathologists using endometrial biopsies taken at 6, 12, and 24 months or more if cancer was a suspected diagnosis. At 12 and 24 months of treatment, the incidence of hyperplasia with bazedoxifene 20 or 40 mg at doses of either 0.45 or 0.625 mg of conjugated estrogens was less than 1%, which was similar to placebo rates over the 24 months.44 The lowest dose studied, bazedoxifene 10 mg, did not prevent hyperplasia with conjugated estrogens 0.45 or 0.625 mg, and its use was discontinued.
Rates of amenorrhea with bazedoxifene 20 or 40 mg and conjugated estrogens 0.45 or 0.625 mg were very favorable (83%–93%) and similar to those with placebo.45 For women with continued bleeding on hormone therapy requiring multiple evaluations, or for women who won’t accept the risk of bleeding on hormone therapy, conjugated estrogens and bazedoxifene may be a sustainable option. However, any woman with abnormal bleeding should undergo prompt immediate evaluation.
A typical side effect of estrogen replacement therapy is breast tenderness. For women seeking vasomotor symptom treatment but who experience breast tenderness, this may be a deterrent from continuing hormone therapy. As shown in the SMART-1 and SMART-2 trials,46 conjugated estrogens and bazedoxifene did not cause an increase in breast tenderness, which may enhance medication adherence.
Ideal candidates for conjugated estrogens plus bazedoxifene
This product could help postmenopausal women who have an intact uterus and are suffering with moderate to severe vasomotor symptoms and genitourinary syndrome of menopause who cannot tolerate the side effects of hormone therapy such as bleeding, bloating, or breast tenderness, or who prefer to take an estrogen but without a progestin. It is also ideal for women at higher risk of osteoporosis.
WHO SHOULD GET WHAT?
Not all postmenopausal women have vasomotor symptoms, genitourinary syndrome of menopause, or bone loss. For those who do, standard hormone therapy is an option.
For those who have symptoms and a lower threshold of side effects such as breast tenderness and vaginal bleeding, a combination of an estrogen plus an ERAA (eg, bazedoxifene) is an option.
For women who have no vasomotor symptoms but do have genitourinary syndrome of menopause and don’t want local vaginal treatment, ospemifene is an option.
For women with no vasomotor symptoms but who have bone loss and increased risk of estrogen receptor-positive breast cancer, raloxifene is a good option.
Both premenopausal and postmenopausal women who are at increased risk for breast cancer should be considered for tamoxifen chemoprevention. Postmenopausal women with a uterus at increased risk for breast cancer should be considered for raloxifene, as it has no uterine effect. Raloxifene is not indicated in premenopausal women.
No woman at increased risk of venous thromboembolism is a candidate for ERAA treatment or for oral estrogen. However, the clinician has multiple options to improve quality of life and work productivity and reduce office visits of women at midlife, especially when they are individually assessed and treated.
- Giannini A, Russo E, Mannella P, Simoncini T. Selective steroid receptor modulators in reproductive medicine. Minerva Ginecol 2015; 67:431–455.
- Feldman BM, Voda A, Gronseth E. The prevalence of hot flash and associated variables among perimenopausal women. Res Nurs Health 1985; 8:261–268.
- Versi E, Harvey MA, Cardozo L, Brincat M, Studd JW. Urogenital prolapse and atrophy at menopause: a prevalence study. Int Urogynecol J Pelvic Floor Dysfunct 2001; 12:107–110.
- Hess R, Chang CC, Conigliaro J, McNeil M. Understanding physicians’ attitudes towards hormone therapy. Womens Health Issues 2005; 15:31–38.
- Melton LJ 3rd, Khosla S, Atkinson EJ, O’Fallon WM, Riggs BL. Relationship of bone turnover to bone density and fractures. J Bone Miner Res 1997; 12:1083–1091.
- Sikon A, Thacker HL. Treatment options for menopausal hot flashes. Cleve Clin J Med 2004; 71:578–582.
- Levine JP. Treating menopausal symptoms with a tissue-selective estrogen complex. Gend Med 2011; 8:57–68.
- Pinkerton JV, Thomas S. Use of SERMs for treatment in postmenopausal women. J Steroid Biochem Mol Biol 2014; 142:142–154.
- Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet 1998; 351:1451–1467.
- Davies C, Pan H, Godwin J, et al; Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) Collaborative Group. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 2013; 381:805–816.
- Gray RG, Rea D, Handley K, et al. aTTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J Clin Oncol 2013; (suppl): abstract 5.
- Cuzick J, Powles T, Veronesi U, et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet 2003; 361:296–300.
- Cuzick J, Sestak I, Cawthorn S, et al. Tamoxifen for prevention of breast cancer: extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol 2015; 16:67–75.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998; 90:1371–1388.
- Chang J, Powles TJ, Ashley SE, et al. The effect of tamoxifen and hormone replacement therapy on serum cholesterol, bone mineral density and coagulation factors in healthy postmenopausal women participating in a randomised, controlled tamoxifen prevention study. Ann Oncol 1996; 7:671–675.
- Herrington DM, Klein KP. Effects of SERMs on important indicators of cardiovascular health: lipoproteins, hemostatic factors and endothelial function. Womens Health Issues 2001; 11:95–102.
- Kedar RP, Bourne TH, Powles TJ, et al. Effects of tamoxifen on uterus and ovaries of postmenopausal women in a randomized breast cancer prevention trial. Lancet 1994; 343:1318–1321.
- Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 1999; 282:637–645.
- Maricic M, Adachi JD, Sarkar S, Wu W, Wong M, Harper KD. Early effects of raloxifene on clinical vertebral fractures at 12 months in postmenopausal women with osteoporosis. Arch Intern Med 2002; 162:1140–1143.
- Recker RR, Mitlak BH, Ni X, Krege JH. Long-term raloxifene for postmenopausal osteoporosis. Curr Med Res Opin 2011; 27:1755–1761.
- Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA 1999; 281:2189–2197.
- Martino S, Cauley JA, Barrett-Connor E, et al; CORE Investigators. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst 2004; 96:1751–1761.
- Vogel VG, Qu Y, Wong M, Mitchell B, Mershon JL. Incidence of invasive breast cancer in postmenopausal women after discontinuation of long-term raloxifene administration. Clin Breast Cancer 2009; 9:45–50.
- Grady D, Cauley JA, Stock JL, et al. Effect of raloxifene on all-cause mortality. Am J Med 2010; 123:469.e1–e7.
- Barrett-Connor E, Mosca L, Collins P, et al; Raloxifene Use for The Heart (RUTH) Trial Investigators. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med 2006; 355:125–137.
- Vogel VG. The NSABP Study of Tamoxifen and Raloxifene (STAR) trial. Expert Rev Anticancer Ther 2009; 9:51–60.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 2006; 295:2727–2741.
- Barnes KN, Pearce EF, Yancey AM, Forinash AB. Ospemifene in the treatment of vulvovaginal atrophy. Ann Pharmacother 2014; 48:752–757.
- Rutanen EM, Heikkinen J, Halonen K, Komi J, Lammintausta R, Ylikorkala O. Effects of ospemifene, a novel SERM, on hormones, genital tract, climacteric symptoms, and quality of life in postmenopausal women: a double-blind, randomized trial. Menopause 2003; 10:433–439.
- Constantine G, Graham S, Koltun WD, Kingsberg SA. Assessment of ospemifene or lubricants on clinical signs of VVA. J Sex Med 2014; 11:1033–1041.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE survey. J Sex Med 2013; 10:1790–1799.
- Portman DJ, Bachmann GA, Simon JA; Ospemifene Study Group. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause 2013; 20:623–630.
- Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause 2010; 17:480–486.
- Goldstein SR, Bachmann GA, Koninckx PR, Lin VH, Portman DJ, Ylikorkala O; Ospemifene Study Group. Ospemifene 12-month safety and efficacy in postmenopausal women with vulvar and vaginal atrophy. Climacteric 2014; 17:173–182.
- Cui Y, Zong H, Yan H, Li N, Zhang Y. The efficacy and safety of ospemifene in treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy: a systematic review and meta-analysis. J Sex Med 2014; 11:487–497.
- Komi J, Heikkinen J, Rutanen EM, Halonen K, Lammintausta R, Ylikorkala O. Effects of ospemifene, a novel SERM, on biochemical markers of bone turnover in healthy postmenopausal women. Gynecal Endocrinol 2004; 18:152–158.
- Komi J, Lankinen KS, DeGregorio M, et al. Effects of ospemifene and raloxifene on biochemical markers of bone turnover in postmenopausal women. J Bone Miner Metab 2006; 24:314–318.
- Wurz GT, Read KC, Marchisano-Karpman C, et al. Ospemifene inhibits the growth of dimethylbenzanthracene-induced mammary tumors in Sencar mice. J Steroid Biochem Mol Biol 2005; 97:230–240.
- Pinkerton JV, Utian WH, Constantine GD, Olivier S, Pickar JH. Relief of vasomotor symptoms with the tissue-selective estrogen complex containing bazedoxifene/conjugated estrogens: a randomized, controlled trial. Menopause 2009; 16:1116–1124.
- Pickar JH, Mirkin S. Tissue-selective agents: selective estrogen receptor modulators and the tissue-selective estrogen complex. Menopause Int 2010; 16:121–128.
- Levine JP. Treating menopausal symptoms with a tissue-selective estrogen complex. Gend Med 2011; 8:57–68.
- Lindsay R, Gallagher JC, Kagan R, Pickar JH, Constantine G. Efficacy of tissue-selective estrange complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril 2009; 92:1045–1052.
- Bachmann G, Bobula J, Mirkin S. Effects of bazedoxifene/conjugated estrogens on quality of life in postmenopausal women with symptoms of vulvar/vaginal atrophy. Climacteric 2010; 13:132–140.
- Pickar JH, Yeh IT, Bachmann G, Speroff L. Endometrial effects of a tissue selective estrogen complex containing bazedoxifene/conjugated estrogens as a menopausal therapy. Fertil Steril 2009; 92:1018–1024.
- Archer DF, Lewis V, Carr BR, Olivier S, Pickar JH. Bazedoxifene/conjugated estrogens (BZA/CE): incidence of uterine bleeding in postmenopausal women. Fertil Steril 2009: 92:1039–1044.
- Pinkerton JV, Abraham L, Bushmakin AG, et al. Evaluation of the efficacy and safety of bazedoxifene/conjugated estrogens for secondary outcomes including vasomotor symptoms in postmenopausal women by years since menopause in the Selective estrogens, Menopause and Response to Therapy (SMART) trials. J Womens Health (Larchmt) 2014; 23:18–28.
- Giannini A, Russo E, Mannella P, Simoncini T. Selective steroid receptor modulators in reproductive medicine. Minerva Ginecol 2015; 67:431–455.
- Feldman BM, Voda A, Gronseth E. The prevalence of hot flash and associated variables among perimenopausal women. Res Nurs Health 1985; 8:261–268.
- Versi E, Harvey MA, Cardozo L, Brincat M, Studd JW. Urogenital prolapse and atrophy at menopause: a prevalence study. Int Urogynecol J Pelvic Floor Dysfunct 2001; 12:107–110.
- Hess R, Chang CC, Conigliaro J, McNeil M. Understanding physicians’ attitudes towards hormone therapy. Womens Health Issues 2005; 15:31–38.
- Melton LJ 3rd, Khosla S, Atkinson EJ, O’Fallon WM, Riggs BL. Relationship of bone turnover to bone density and fractures. J Bone Miner Res 1997; 12:1083–1091.
- Sikon A, Thacker HL. Treatment options for menopausal hot flashes. Cleve Clin J Med 2004; 71:578–582.
- Levine JP. Treating menopausal symptoms with a tissue-selective estrogen complex. Gend Med 2011; 8:57–68.
- Pinkerton JV, Thomas S. Use of SERMs for treatment in postmenopausal women. J Steroid Biochem Mol Biol 2014; 142:142–154.
- Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet 1998; 351:1451–1467.
- Davies C, Pan H, Godwin J, et al; Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) Collaborative Group. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 2013; 381:805–816.
- Gray RG, Rea D, Handley K, et al. aTTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J Clin Oncol 2013; (suppl): abstract 5.
- Cuzick J, Powles T, Veronesi U, et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet 2003; 361:296–300.
- Cuzick J, Sestak I, Cawthorn S, et al. Tamoxifen for prevention of breast cancer: extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol 2015; 16:67–75.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998; 90:1371–1388.
- Chang J, Powles TJ, Ashley SE, et al. The effect of tamoxifen and hormone replacement therapy on serum cholesterol, bone mineral density and coagulation factors in healthy postmenopausal women participating in a randomised, controlled tamoxifen prevention study. Ann Oncol 1996; 7:671–675.
- Herrington DM, Klein KP. Effects of SERMs on important indicators of cardiovascular health: lipoproteins, hemostatic factors and endothelial function. Womens Health Issues 2001; 11:95–102.
- Kedar RP, Bourne TH, Powles TJ, et al. Effects of tamoxifen on uterus and ovaries of postmenopausal women in a randomized breast cancer prevention trial. Lancet 1994; 343:1318–1321.
- Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 1999; 282:637–645.
- Maricic M, Adachi JD, Sarkar S, Wu W, Wong M, Harper KD. Early effects of raloxifene on clinical vertebral fractures at 12 months in postmenopausal women with osteoporosis. Arch Intern Med 2002; 162:1140–1143.
- Recker RR, Mitlak BH, Ni X, Krege JH. Long-term raloxifene for postmenopausal osteoporosis. Curr Med Res Opin 2011; 27:1755–1761.
- Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA 1999; 281:2189–2197.
- Martino S, Cauley JA, Barrett-Connor E, et al; CORE Investigators. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst 2004; 96:1751–1761.
- Vogel VG, Qu Y, Wong M, Mitchell B, Mershon JL. Incidence of invasive breast cancer in postmenopausal women after discontinuation of long-term raloxifene administration. Clin Breast Cancer 2009; 9:45–50.
- Grady D, Cauley JA, Stock JL, et al. Effect of raloxifene on all-cause mortality. Am J Med 2010; 123:469.e1–e7.
- Barrett-Connor E, Mosca L, Collins P, et al; Raloxifene Use for The Heart (RUTH) Trial Investigators. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med 2006; 355:125–137.
- Vogel VG. The NSABP Study of Tamoxifen and Raloxifene (STAR) trial. Expert Rev Anticancer Ther 2009; 9:51–60.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 2006; 295:2727–2741.
- Barnes KN, Pearce EF, Yancey AM, Forinash AB. Ospemifene in the treatment of vulvovaginal atrophy. Ann Pharmacother 2014; 48:752–757.
- Rutanen EM, Heikkinen J, Halonen K, Komi J, Lammintausta R, Ylikorkala O. Effects of ospemifene, a novel SERM, on hormones, genital tract, climacteric symptoms, and quality of life in postmenopausal women: a double-blind, randomized trial. Menopause 2003; 10:433–439.
- Constantine G, Graham S, Koltun WD, Kingsberg SA. Assessment of ospemifene or lubricants on clinical signs of VVA. J Sex Med 2014; 11:1033–1041.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE survey. J Sex Med 2013; 10:1790–1799.
- Portman DJ, Bachmann GA, Simon JA; Ospemifene Study Group. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause 2013; 20:623–630.
- Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause 2010; 17:480–486.
- Goldstein SR, Bachmann GA, Koninckx PR, Lin VH, Portman DJ, Ylikorkala O; Ospemifene Study Group. Ospemifene 12-month safety and efficacy in postmenopausal women with vulvar and vaginal atrophy. Climacteric 2014; 17:173–182.
- Cui Y, Zong H, Yan H, Li N, Zhang Y. The efficacy and safety of ospemifene in treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy: a systematic review and meta-analysis. J Sex Med 2014; 11:487–497.
- Komi J, Heikkinen J, Rutanen EM, Halonen K, Lammintausta R, Ylikorkala O. Effects of ospemifene, a novel SERM, on biochemical markers of bone turnover in healthy postmenopausal women. Gynecal Endocrinol 2004; 18:152–158.
- Komi J, Lankinen KS, DeGregorio M, et al. Effects of ospemifene and raloxifene on biochemical markers of bone turnover in postmenopausal women. J Bone Miner Metab 2006; 24:314–318.
- Wurz GT, Read KC, Marchisano-Karpman C, et al. Ospemifene inhibits the growth of dimethylbenzanthracene-induced mammary tumors in Sencar mice. J Steroid Biochem Mol Biol 2005; 97:230–240.
- Pinkerton JV, Utian WH, Constantine GD, Olivier S, Pickar JH. Relief of vasomotor symptoms with the tissue-selective estrogen complex containing bazedoxifene/conjugated estrogens: a randomized, controlled trial. Menopause 2009; 16:1116–1124.
- Pickar JH, Mirkin S. Tissue-selective agents: selective estrogen receptor modulators and the tissue-selective estrogen complex. Menopause Int 2010; 16:121–128.
- Levine JP. Treating menopausal symptoms with a tissue-selective estrogen complex. Gend Med 2011; 8:57–68.
- Lindsay R, Gallagher JC, Kagan R, Pickar JH, Constantine G. Efficacy of tissue-selective estrange complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril 2009; 92:1045–1052.
- Bachmann G, Bobula J, Mirkin S. Effects of bazedoxifene/conjugated estrogens on quality of life in postmenopausal women with symptoms of vulvar/vaginal atrophy. Climacteric 2010; 13:132–140.
- Pickar JH, Yeh IT, Bachmann G, Speroff L. Endometrial effects of a tissue selective estrogen complex containing bazedoxifene/conjugated estrogens as a menopausal therapy. Fertil Steril 2009; 92:1018–1024.
- Archer DF, Lewis V, Carr BR, Olivier S, Pickar JH. Bazedoxifene/conjugated estrogens (BZA/CE): incidence of uterine bleeding in postmenopausal women. Fertil Steril 2009: 92:1039–1044.
- Pinkerton JV, Abraham L, Bushmakin AG, et al. Evaluation of the efficacy and safety of bazedoxifene/conjugated estrogens for secondary outcomes including vasomotor symptoms in postmenopausal women by years since menopause in the Selective estrogens, Menopause and Response to Therapy (SMART) trials. J Womens Health (Larchmt) 2014; 23:18–28.
KEY POINTS
- Tamoxifen is approved to prevent and treat breast cancer. It may also have beneficial effects on bone and on cardiovascular risk factors, but these are not approved uses.
- Raloxifene, a second-generation ERAA, was initially approved for preventing and treating osteoporosis and later received approval to reduce the risk of invasive estrogen receptor-positive breast cancer in postmenopausal women.
- Ospemifene is approved for treatment of genitourinary syndrome of menopause.
- The combination of conjugated estrogen and bazedoxifene is approved for treating moderate to severe vasomotor symptoms associated with menopause and also for preventing postmenopausal osteoporosis in women with an intact uterus.
Medical management of urinary incontinence in women
Urinary incontinence can lead to a cascade of symptomatic burden on the patient, causing distress, embarrassment, and suffering.
See related patient information
Traditionally, incontinence has been treated by surgeons, and surgery remains an option. However, more patients are now being managed by medical clinicians, who can offer a number of newer therapies. Ideally, a medical physician can initiate the evaluation and treatment and even effectively cure some forms of urinary incontinence.
In 2014, the American College of Physicians (ACP) published recommendations on the medical treatment of urinary incontinence in women (Table 1).1
This review describes the medical management of urinary incontinence in women, emphasizing the ACP recommendations1 and newer over-the-counter options.
COMMON AND UNDERREPORTED
Many women erroneously believe that urinary incontinence is an inevitable consequence of aging and allow it to lessen their quality of life without seeking medical attention.
Indeed, it is common. The 2005–2006 National Health and Nutritional Examination Survey2 found the prevalence of urinary incontinence in US women to be 15.7%. The prevalence increases with age from 6.9% in women ages 20 through 29 to 31.7% in those age 80 and older. A separate analysis of the same data found that 25.0% of women age 20 and older had 1 or more pelvic floor disorders.3 Some estimates are even higher. Wu et al4 reported a prevalence of urinary incontinence of 51.1% in women ages 31 through 54.
Too few of these women are identified and treated, for many reasons, including embarrassment and inadequate screening. Half of women with urinary incontinence do not report their symptoms because of humiliation or anxiety.5
The burden of urinary incontinence extends beyond the individual and into society. The total cost of overactive bladder and urgency urinary incontinence in the United States was estimated to be $65.9 billion in 2007 and is projected to reach $82.6 billion in 2020.6
THREE TYPES
There are 3 types of urinary incontinence: stress, urgency, and mixed.
Stress urinary incontinence is involuntary loss of urine associated with physical exertion or increased abdominal pressure, eg, with coughing or sneezing.
Urgency urinary incontinence is involuntary loss of urine associated with the sudden need to void. Many patients experience these symptoms simultaneously, making the distinction difficult.
Mixed urinary incontinence is loss of urine with both urgency and increased abdominal pressure or physical exertion.
Overactive bladder, a related problem, is defined as urinary urgency, usually accompanied by frequency and nocturia, with or without urgency urinary incontinence, in the absence of a urinary tract infection or other obvious disease.7
Nongenitourinary causes such as neurologic disorders or even malignancy can present with urinary incontinence, and thus it is critical to perform a thorough initial evaluation.
A 2014 study revealed that by age 80, 20% of women may need to undergo surgery for stress urinary incontinence or pelvic organ prolapse. This statistic should motivate healthcare providers to focus on prevention and offer conservative medical management for these conditions first.8
QUESTIONS TO ASK
When doing a pelvic examination, once could inquire about urinary incontinence with questions such as:
Do you leak urine when you cough, sneeze, laugh, or jump or during sexual climax?
Do you have to get up more than once at night to urinate?
Do you feel the urge to urinate frequently?
BEHAVIORAL MODIFICATION AND BLADDER TRAINING
Bladder training is a conservative behavioral treatment for urinary incontinence that primary care physicians can teach. It is primarily used for urgency urinary incontinence but can also be useful in stress urinary incontinence.
After completing a bladder diary and gaining awareness of their daily voiding patterns, patients can learn scheduled voiding to train the bladder, gradually extending the urges to longer intervals.
Clinicians should instruct patients on how to train the bladder, using methods first described by Wyman and Fantl.9 (See Training the bladder.)
There is evidence that bladder training improves urinary incontinence compared with usual care.10,11
The ACP recommends bladder training for women who have urgency urinary incontinence, but grades this recommendation as weak with low-quality evidence.
PELVIC FLOOR MUSCLE TRAINING
Introduced in 1948 by Dr. Arnold Kegel, pelvic floor muscle training has become widely adopted.12
The pelvic floor consists of a group of muscles, resembling a hammock, that support the pelvic viscera. These muscles include the coccygeus and the layers of the levator ani (Figure 1). A weak pelvic floor is one of many risk factors for developing stress urinary incontinence. Like other muscle groups, a weak pelvic floor can be rehabilitated through various techniques, often guided by a physical therapist.
Compared with those who received no treatment, women with stress urinary incontinence who performed pelvic floor muscle training were 8 times more likely to report being cured and 17 times more likely to report cure or improvement.13
To perform a Kegel exercise, a woman consciously contracts her pelvic floor muscles as if stopping the flow of urine.
The Knack maneuver can be used to prevent leakage during anticipated events that increase intra-abdominal pressure. For example, when a cough or sneeze is imminent, patients can preemptively contract their pelvic floor and hold the contraction through the cough or sneeze.
Although many protocols have been compared, no specific pelvic floor exercise strategy has proven superior. A systematic review assessed variations in pelvic floor interventions, exercises, and delivery and found that there was insufficient evidence to make any recommendations about the best approach. However, the benefit was greater with regular supervision during pelvic floor muscle training than with little or no supervision.14
Pelvic floor muscle training strengthens the pelvic floor, which better supports the bladder neck and anatomically compensates for defects in stress urinary incontinence. In urgency urinary incontinence, a strong pelvic floor created by muscle training prevents leaking induced by the involuntary contractions of the detrusor muscle.
Recommendation
The ACP recommends pelvic floor muscle training as first-line treatment for stress urinary incontinence and mixed urinary incontinence, and grades this recommendation as strong with high-quality evidence.
BIOFEEDBACK AND PELVIC STIMULATION
Although pelvic floor exercises are effective in urinary incontinence, 30% of patients perform them incorrectly.15
Biofeedback therapy uses visual, verbal, and acoustic signals to give the patient immediate feedback and a greater awareness of her muscular activity. A commonly used technique employs a vaginal probe to measure and display pressure changes as the patient contracts her levator ani muscles.
Women who received biofeedback in addition to traditional pelvic floor physical therapy had greater improvement in urinary incontinence than those who received pelvic physical therapy alone (risk ratio 0.75, 95% confidence interval 0.66–0.86).16
Pelvic stimulation can be used separately or in conjunction with biofeedback in both urgency and stress urinary incontinence. When pelvic stimulation is used alone, 9 women need to be treated to achieve continence in 1, and 6 women need to be treated to improve continence in 1.16
Traditionally delivered by a pelvic floor physical therapist, pelvic stimulation and biofeedback have also been validated for home use.17,18 Several pelvic stimulation devices have been approved by the US Food and Drug Administration (FDA) for treating stress, urgency, and mixed urinary incontinence. These devices deliver stimulation to the pelvic floor at single or multiple frequencies. Although the mechanisms are not clearly understood, lower frequencies are used to treat urgency incontinence, while higher frequencies are used for stress incontinence. A theory is that higher-frequency stimulation strengthens the pelvic floor in stress urinary incontinence while lower frequency stimulation calms the detrusor muscle in urgency urinary incontinence.
The Apex and Apex M devices are both available over the counter, the former to treat stress urinary incontinence and the latter to treat mixed urinary incontinence, using pelvic stimulation alone. Other available devices, including the InTone and a smaller version, the InTone MV, are available by prescription and combine pelvic stimulation with biofeedback.18
Women who wish to avoid surgery, botulinum toxin injections, and daily oral medications, particularly those who are highly motivated, are ideal candidates for these over-the-counter automatic neuromuscular pelvic exercising devices.
PESSARIES AND OTHER DEVICES
Pessaries are commonly used to treat pelvic organ prolapse but can also be designed to help correct the anatomic defect responsible for stress urinary incontinence. Continence pessaries support the bladder neck so that the urethrovesicular junction is stabilized rather than hypermobile during the increased intra-abdominal pressure that occurs with coughing, sneezing, or physical exertion (Figure 2). In theory, this should decrease leakage.
A systematic review concluded that the value of pessaries in the management of incontinence remains uncertain. However, there are inherent challenges in conducting trials of such devices.19 A pessary needs to be fitted by an appropriately trained healthcare provider. The Ambulatory Treatments for Leakage Associated With Stress Incontinence (ATLAS) trial20 reported that behavioral therapy was more effective than a pessary at 3 months, but the treatments were equivalent at 12 months.
The FDA has approved a disposable, over-the-counter silicone intravaginal device for treating stress urinary incontinence. Patients initially purchase a sizing kit and subsequently insert the nonabsorbent temporary intravaginal bladder supportive device, which is worn for up to 8 hours.
Women may elect to use regular tampons to do the job of a pessary, as they are easy to use and low in cost. No large randomized trials have compared tampons and pessaries, and currently no one device is known to be superior to another.
Overall, these devices are temporizing measures that have few serious adverse effects.
WEIGHT LOSS AND DIETARY CHANGES
Obesity has become a national epidemic, with more than 68% of Americans found to be overweight or obese according to the National Institutes of Health.21
Several studies found obesity to be an independent risk factor for urinary incontinence. As early as 1946, the British Birth Cohort study found that women ages 48 through 54 who were obese earlier in life had a higher risk of urinary incontinence in middle age, and those who were obese by age 20 were more likely to report severe incontinence.22 Likewise, the Nurses’ Health Study showed that women with a body mass index (BMI) more than 30 kg/m2 had 3.1 times the risk of severe incontinence compared with women with a normal BMI. Also, the Study of Women’s Health Across the Nation and the Leicestershire Medical Research Council (MRC) incontinence study both showed that a higher BMI and weight gain are strongly correlated with urinary incontinence.23,24
Increased intra-abdominal pressure may be the causative mechanism of stress urinary incontinence in obesity. The Korean National Health and Nutrition Examination Survey showed that central adiposity correlated with urgency incontinence.25,26
The MRC study was one of the largest to evaluate the effect of diet on urinary symptoms. Consuming a diet dense in vegetables, bread, and chicken was found to reduce the risk of urinary incontinence, while carbonated drinks were associated with a higher risk.25 These studies and others may point to reducing calories, and thus BMI, as a conservative treatment for urinary incontinence.
Newer data show bariatric surgery is associated with a strong reduction in urinary incontinence, demonstrated in a study that followed patients for 3 years after surgery.27 This encouraging result is but one of several positive health outcomes from bariatric surgery.
Recommendation
The ACP recommends both weight loss and exercise for overweight women with urinary incontinence, and grades this as strong with moderate-quality evidence.
DRUG THERAPY
The bladder neck is rich with sympathetic alpha-adrenergic receptors, and the bladder dome is dense with parasympathetic muscarinic receptors and sympathetic beta-adrenergic receptors. When the parasympathetic system is stimulated, the muscarinic receptors are activated, causing detrusor contraction and ultimately bladder emptying.
Agonism of beta-alpha adrenergic receptors and inhibition of parasympathetic receptors are both strategies of drug treatment of urinary incontinence.
Anticholinergic drugs
Anticholinergic medications function by blocking the muscarinic receptor, thereby inhibiting detrusor contraction.
Six oral anticholinergic medications are available: oxybutynin, tolterodine, fesoterodine, solifenacin, trospium, and darifenacin. These drugs have about the same effectiveness in treating urgency urinary incontinence, as measured by achieving continence and improving quality of life.28 Given their similarity in effectiveness, the choice of agent typically relies on the side-effect profile. Extended-release formulations have a more favorable side-effect profile, with fewer cases of dry mouth compared with immediate-release formulations.29
Overall, however, the benefit of these medications is small, with fewer than 200 patients achieving continence per 1,000 treated.28
Other limitations of these medications include their adverse effects and contraindications, and patients’ poor adherence to therapy. The most commonly reported adverse effect is dry mouth, but other common side effects include constipation, abdominal pain, dyspepsia, fatigue, dry eye, and dry skin. Transdermal oxybutynin therapy has been associated with fewer anticholinergic side effects than oral therapy.30
Contraindications to these medications include gastric retention, urinary retention, and angle-closure glaucoma.
Long-term adherence to anticholinergics is low, reported between 14% to 35% after 12 months, with the highest rates of adherence with solifenacin.31 The most commonly cited reason for discontinuation is lack of effect.32
Caution is urged when considering starting anticholinergic medications in older adults because of the central nervous system side effects, including drowsiness, hallucinations, cognitive impairment, and dementia. After 3 weeks, oxybutynin caused a memory decline as measured by delayed recall that was comparable to the decline seen over 10 years in normal aging.33 There is evidence suggesting trospium may cause less cognitive impairment, and therefore may be a better option for older patients.34
Beta-3 adrenoreceptor agonists
Activation of beta-3 adrenergic receptors through the sympathetic nervous system relaxes the detrusor muscle, allowing the bladder to store urine.
Mirabegron is a selective beta-3 adrenoreceptor agonist that effectively relaxes the bladder and increases bladder capacity. It improves continence, treatment satisfaction, and quality of life.35,36 There are fewer reports of dry mouth and constipation with this drug than with the anticholinergics; however, beta-3 adrenoreceptor agonists may be associated with greater risk of hypertension, nasopharyngitis, headache, and urinary tract infection.37
Duloxetine
Duloxetine, an antidepressant, blocks the reuptake of both serotonin and norepinephrine. Consequently, it decreases parasympathetic activity and increases sympathetic and somatic activity in the urinary system.38 While urine is stored, this cascade of neural activity is thought to collectively increase pudendal nerve activity and improve closure of the urethra.
Although duloxetine is approved to treat stress urinary incontinence in Europe, this is an off-label use in the United States.
A meta-analysis39 found that duloxetine improved quality of life in patients with stress urinary incontinence and that subjective cure rates were 10.8% with duloxetine vs 7.7% with placebo (P = .04). However the rate of adverse events is high, with nausea most common. Given its modest benefit and high rate of side effects, physicians may consider starting duloxetine only if there are psychiatric comorbidities such as depression, anxiety, or fibromyalgia.
Recommendations
The ACP recommends against systemic pharmacologic therapy for stress urinary incontinence. For urgency urinary incontinence, pharmacologic therapy is recommended if bladder training fails, and should be individualized based on the patient’s preference and medical comorbidities and the drug’s tolerability, cost, and ease of use.
Hormone therapy
In 2014, the North American Menopause Society recommended replacing the term “vulvovaginal atrophy” with the term genitourinary syndrome of menopause, which better encompasses the postmenopausal changes to the female genital system.40
Estrogen therapy is commercially available in both systemic and local preparations. The effect of exogenous estrogen on urinary incontinence may depend on whether it is given locally or systemically.
A systematic review41 definitively concluded that all commercially prepared local vaginal estrogen preparations can effectively relieve the genitourinary syndrome of menopause, including not only the common complaints of dryness, burning, and irritation but also urinary complaints of frequency, urgency, and urgency urinary incontinence.41 Additionally, the estradiol vaginal ring for vaginal atrophy (Estring) may have dual effects, functioning like an incontinence pessary by supporting the bladder neck while simultaneously providing local estrogen to the atrophied vaginal tissue.
However, in the Women’s Health Initiative,42 continent women who received either systemic estrogen therapy alone or systemic estrogen combined with progestin actually had a higher risk of developing urinary incontinence, and those already experiencing incontinence developed a worsening of their symptoms on systemic hormone therapy. The mechanism by which systemic hormone therapy causes urinary incontinence is unclear; however, previous studies showed that hormone therapy leads to a reduction in periurethral collagen and increased bladder contractility.43,44
TAKE-HOME POINTS
- Half of women with symptomatic urinary incontinence never report their symptoms.
- Bladder training is recommended for urgency incontinence and pelvic floor muscle training for stress incontinence.
- Thirty percent of women perform pelvic floor exercises incorrectly.
- Devices can be considered, including automatic pelvic exercise devices for stress and urgency incontinence and incontinence pessaries and disposable intravaginal bladder support devices for stress incontinence.
- Higher BMIs are strongly correlated with urinary incontinence.
- Anticholinergic medications are recommended for urgency but not stress incontinence.
- Vaginal estrogen cream may help with symptoms of urinary urgency, recurrent bladder infections, and nocturia in addition to incontinence.
- Qaseem A, Dallas P, Forciea MA, Starkey M, Denberg TD, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Nonsurgical management of urinary incontinence in women: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2014; 161:429–440.
- Nygaard I, Barber MD, Burgio KL, et al; Pelvic Floor Disorders Network. Prevalence of symptomatic pelvic floor disorders in US women. JAMA 2008; 300:1311–1316.
- Wu JM, Vaughan CP, Goode PS, et al. Prevalence and trends of symptomatic pelvic floor disorders in US women. Obstet Gynecol 2014; 123:141–148.
- Wu JM, Stinnett S, Jackson RA, Jacoby A, Learman LA, Kuppermann M. Prevalence and incidence of urinary incontinence in a diverse population of women with noncancerous gynecologic conditions. Female Pelvic Med Reconstr Surg 2010; 16:284–289.
- Griffiths AN, Makam A, Edward GJ. Should we actively screen for urinary and anal incontinence in the general gynaecology outpatients setting? A prospective observational study. J Obstet Gynaecol 2006; 26:442–444.
- Coyne KS, Wein A, Nicholson S, Kvasz M, Chen CI, Milsom I. Economic burden of urgency urinary incontinence in the United Stated: a systematic review. J Manag Care Pharm 2014; 20:130–140.
- Haylen BT, Ridder D, Freeman RM, et al; International Urogynecological Association; International Continence Society. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn 2010; 29:4–20.
- Wu JM, Matthews CA, Conover MM, Pate V, Jonsson Funk M. Lifetime risk of stress urinary incontinence or pelvic organ prolapse surgery. Obstet Gynecol 2014; 123:1201–1206.
- Wyman JF, Fantl JA. Bladder training in the ambulatory care management of urinary incontinence. Urol Nurs 1991; 11:11–17.
- Fantl JA, Wyman JF, McClish DK, et al. Efficacy of bladder training in older women with urinary incontinence. JAMA 1991; 265:609–613.
- Subak LL, Quesenberry CP, Posner SF, Cattolica E, Soghikian K. The effect of behavioral therapy on urinary incontinence: a randomized controlled trial. Obstet Gynecol 2002; 100:72–78.
- Kegel AH. Progressive resistance exercise in the functional restoration of the perineal muscles. Am J Obstet Gynecol 1948; 56:238–248.
- Domoulin C, Hay-Smith EJ, Mac Habée-Séguin G. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev 2014; 5:CD005654.
- Hay-Smith EJ, Herderschee R, Dumoulin C, Herbison GP. Comparisons of approaches to pelvic floor muscle training for urinary incontinence in women. Cochrane Database Syst Rev 2011; 12:CD009508.
- Bo K. Pelvic floor muscle strength and response to pelvic floor muscle training for stress urinary incontinence. Neurourol Urodyn 2003; 22:654–658.
- Herderschee R, Hay-Smith EJ, Herbison GP, Roovers JP, Heineman MJ. Feedback or biofeedback to augment pelvic floor muscle training for urinary incontinence in women. Cochrane Database Syst Rev 2011; 7:CD009252.
- Terlikowski R, Dobrzycka B, Kinalski M, Kuryliszyn-Moskal A, Terlikowski SJ. Transvaginal electrical stimulation with surface-EMG biofeedback in managing stress urinary incontinence in women of premenopausal age: a double-blind, placebo-controlled, randomized clinical trial. Int Urogynecol J 2013; 17:1631–1638.
- Guralnick ML, Kelly H, Engelke H, Koduri S, O’Connor RC. InTone: a novel pelvic floor rehabilitation device for urinary incontinence. Int Urogynecol J 2015; 26:99–106.
- Lipp A, Shaw C, Glavind K. Mechanical devices for urinary incontinence in women. Cochrane Database Syst Rev 2014; 12:CD001756.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol 2010; 115:609–617.
- National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Overweight and obesity statistics. www.niddk.nih.gov/health-information/health-statistics/Pages/overweight-obesity-statistics.aspx. Accessed January 6, 2017.
- Mishra GD, Hardy R, Cardozo L, Kuh D. Body weight through adult life and risk of urinary incontinence in middle-aged women. Results from a British prospective cohort. Int J Obes (Lond) 2008; 32:1415–1422.
- Danforth KN, Townsend MK, Lifford K, Curhan GC, Resnick NM, Grodstein F. Risk factors for urinary incontinence among middle age women. Am J Obstet Gynecol 2006; 194:339–345.
- Waetjen LE, Liao S, Johnson WO, et al. Factors associated with prevalence and incident urinary incontinence in a cohort of midlife women: a longitudinal analysis of data: study of women’s health across the nation. Am J Epidemiol 2007; 165:309–318.
- Dallosso HM, McGrother CW, Matthews RJ, Donaldson MM; Leicestershire MRC Incontinence Study Group. The association of diet and other lifestyle factors with overactive bladder and stress incontinence: a longitudinal study in women. BJU Int 2003; 92:69–77.
- Kim IH, Chung H, Kwon JW. Gender differences in the effect of obesity on chronic diseases among the elderly Koreans. J Korean Med Sci. 2011; 26:250–257.
- Subak LL, King WC, Belle SH, et al. Urinary incontinence before and after bariatric surgery. JAMA Intern Med 2015; 175:1378–1387.
- Shamliyan T, Wyman JF, Ramakrishnan R, Sainfort F, Kane RL. Benefits and harms of pharmacologic treatment for urinary incontinence in women: a systematic review. Ann Intern Med 2012; 156:861–874, W301–W310.
- Hay-Smith J, Herbison P, Ellis G, Morris A. Which anticholinergic drug for overactive bladder symptoms in adults. Cochrane Database Syst Rev 2005; 3:CD005429.
- Davila GW, Daugherty CA, Sanders SW; Transdermal Oxybutynin Study Group. A short term, multicenter, randomized double-blind dose titration study of the efficacy and anticholinergic side effects of transdermal compared to immediate release oral oxybutynin treatment of patients with urge urinary incontinence. J Urol 2001; 166:140–145.
- Wagg A, Compion G, Fahey A, Siddiqui E. Persistence with prescribed antimuscarinic therapy for overactive bladder: a UK experience. BJU Int 2012; 110:1767–1774.
- Benner JS, Nichol MB, Rovner ES, et al. Patient-reported reasons for discontinuing overactive bladder medication. BJU Int 2010; 105:1276–1282.
- Kay G, Crook T, Rekeda L, et al. Differential effects of the antimuscarinic agents darifenacin and oxybutynin ER on memory in older subjects. Eur Urol 2006; 50:317–326.
- Staskin D, Kay G, Tannenbaum C, et al. Trospium chloride has no effect on memory testing and is assay undetectable in the central nervous system of older patients with overactive bladder. Int J Clin Pract 2010; 64:1294–1300.
- Chapple CR, Amarenco G, Lopez A, et al; BLOSSOM Investigator Group. A proof of concept study: mirabegron, a new therapy for overactive bladder. Neurourol Urodyn 2013; 32:1116–1122.
- Nitti VB, Khullar V, van Kerrebroeck P, et al. Mirabegron for the treatment of overactive bladder: a prespecified pooled efficacy analysis and pooled safety analysis of three randomised, double-blind, placebo-controlled, phase III studies. Int J Clin Pract 2013; 67:619–632.
- Maman K, Aballea S, Nazir J, et al. Comparative efficacy and safety of medical treatments for the management of overactive bladder: a systematic literature review and mixed treatment comparison. Eur Urol 2014; 65:755–765.
- Katofiasc MA, Nissen J, Audia JE, Thor KB. Comparison of the effects of serotonin selective, norepinephrine, and dual serotonin and norepinephrine reuptake inhibitors on lower urinary tract function in cats. Life Sci 2002; 71:1227–1236.
- Mariappan P, Alhasso A, Ballantyne Z, Grant A, N’Dow J. Duloxetine, a serotonin and noradrenaline reuptake inhibitor for the treatment of stress urinary incontinence: a systematic review. Eur Urol 2007; 51:67–74.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause 2014; 21:1063–1068.
- Rahn DD, Carberry C, Sanses TV, et al; Society of Gynecologic Surgeons Systematic Review Group. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol 2014; 124:1147–1156.
- Hendrix SL, Cochrane BB, Nygaard IE, et al. Effects of estrogen with and without progestin on urinary incontinence. JAMA 2005; 293:935–948.
- Jackson S, James M, Abrams P. The effect of estradiol on vaginal collagen metabolism in postmenopausal women with genuine stress incontinence. BJOG 2002; 109:339–344.
- Lin AD, Levin R, Kogan B, et al. Estrogen induced functional hypertrophy and increased force generation of the female rabbit bladder. Neurourol Urodyn 2006; 25:473–479.
Urinary incontinence can lead to a cascade of symptomatic burden on the patient, causing distress, embarrassment, and suffering.
See related patient information
Traditionally, incontinence has been treated by surgeons, and surgery remains an option. However, more patients are now being managed by medical clinicians, who can offer a number of newer therapies. Ideally, a medical physician can initiate the evaluation and treatment and even effectively cure some forms of urinary incontinence.
In 2014, the American College of Physicians (ACP) published recommendations on the medical treatment of urinary incontinence in women (Table 1).1
This review describes the medical management of urinary incontinence in women, emphasizing the ACP recommendations1 and newer over-the-counter options.
COMMON AND UNDERREPORTED
Many women erroneously believe that urinary incontinence is an inevitable consequence of aging and allow it to lessen their quality of life without seeking medical attention.
Indeed, it is common. The 2005–2006 National Health and Nutritional Examination Survey2 found the prevalence of urinary incontinence in US women to be 15.7%. The prevalence increases with age from 6.9% in women ages 20 through 29 to 31.7% in those age 80 and older. A separate analysis of the same data found that 25.0% of women age 20 and older had 1 or more pelvic floor disorders.3 Some estimates are even higher. Wu et al4 reported a prevalence of urinary incontinence of 51.1% in women ages 31 through 54.
Too few of these women are identified and treated, for many reasons, including embarrassment and inadequate screening. Half of women with urinary incontinence do not report their symptoms because of humiliation or anxiety.5
The burden of urinary incontinence extends beyond the individual and into society. The total cost of overactive bladder and urgency urinary incontinence in the United States was estimated to be $65.9 billion in 2007 and is projected to reach $82.6 billion in 2020.6
THREE TYPES
There are 3 types of urinary incontinence: stress, urgency, and mixed.
Stress urinary incontinence is involuntary loss of urine associated with physical exertion or increased abdominal pressure, eg, with coughing or sneezing.
Urgency urinary incontinence is involuntary loss of urine associated with the sudden need to void. Many patients experience these symptoms simultaneously, making the distinction difficult.
Mixed urinary incontinence is loss of urine with both urgency and increased abdominal pressure or physical exertion.
Overactive bladder, a related problem, is defined as urinary urgency, usually accompanied by frequency and nocturia, with or without urgency urinary incontinence, in the absence of a urinary tract infection or other obvious disease.7
Nongenitourinary causes such as neurologic disorders or even malignancy can present with urinary incontinence, and thus it is critical to perform a thorough initial evaluation.
A 2014 study revealed that by age 80, 20% of women may need to undergo surgery for stress urinary incontinence or pelvic organ prolapse. This statistic should motivate healthcare providers to focus on prevention and offer conservative medical management for these conditions first.8
QUESTIONS TO ASK
When doing a pelvic examination, once could inquire about urinary incontinence with questions such as:
Do you leak urine when you cough, sneeze, laugh, or jump or during sexual climax?
Do you have to get up more than once at night to urinate?
Do you feel the urge to urinate frequently?
BEHAVIORAL MODIFICATION AND BLADDER TRAINING
Bladder training is a conservative behavioral treatment for urinary incontinence that primary care physicians can teach. It is primarily used for urgency urinary incontinence but can also be useful in stress urinary incontinence.
After completing a bladder diary and gaining awareness of their daily voiding patterns, patients can learn scheduled voiding to train the bladder, gradually extending the urges to longer intervals.
Clinicians should instruct patients on how to train the bladder, using methods first described by Wyman and Fantl.9 (See Training the bladder.)
There is evidence that bladder training improves urinary incontinence compared with usual care.10,11
The ACP recommends bladder training for women who have urgency urinary incontinence, but grades this recommendation as weak with low-quality evidence.
PELVIC FLOOR MUSCLE TRAINING
Introduced in 1948 by Dr. Arnold Kegel, pelvic floor muscle training has become widely adopted.12
The pelvic floor consists of a group of muscles, resembling a hammock, that support the pelvic viscera. These muscles include the coccygeus and the layers of the levator ani (Figure 1). A weak pelvic floor is one of many risk factors for developing stress urinary incontinence. Like other muscle groups, a weak pelvic floor can be rehabilitated through various techniques, often guided by a physical therapist.
Compared with those who received no treatment, women with stress urinary incontinence who performed pelvic floor muscle training were 8 times more likely to report being cured and 17 times more likely to report cure or improvement.13
To perform a Kegel exercise, a woman consciously contracts her pelvic floor muscles as if stopping the flow of urine.
The Knack maneuver can be used to prevent leakage during anticipated events that increase intra-abdominal pressure. For example, when a cough or sneeze is imminent, patients can preemptively contract their pelvic floor and hold the contraction through the cough or sneeze.
Although many protocols have been compared, no specific pelvic floor exercise strategy has proven superior. A systematic review assessed variations in pelvic floor interventions, exercises, and delivery and found that there was insufficient evidence to make any recommendations about the best approach. However, the benefit was greater with regular supervision during pelvic floor muscle training than with little or no supervision.14
Pelvic floor muscle training strengthens the pelvic floor, which better supports the bladder neck and anatomically compensates for defects in stress urinary incontinence. In urgency urinary incontinence, a strong pelvic floor created by muscle training prevents leaking induced by the involuntary contractions of the detrusor muscle.
Recommendation
The ACP recommends pelvic floor muscle training as first-line treatment for stress urinary incontinence and mixed urinary incontinence, and grades this recommendation as strong with high-quality evidence.
BIOFEEDBACK AND PELVIC STIMULATION
Although pelvic floor exercises are effective in urinary incontinence, 30% of patients perform them incorrectly.15
Biofeedback therapy uses visual, verbal, and acoustic signals to give the patient immediate feedback and a greater awareness of her muscular activity. A commonly used technique employs a vaginal probe to measure and display pressure changes as the patient contracts her levator ani muscles.
Women who received biofeedback in addition to traditional pelvic floor physical therapy had greater improvement in urinary incontinence than those who received pelvic physical therapy alone (risk ratio 0.75, 95% confidence interval 0.66–0.86).16
Pelvic stimulation can be used separately or in conjunction with biofeedback in both urgency and stress urinary incontinence. When pelvic stimulation is used alone, 9 women need to be treated to achieve continence in 1, and 6 women need to be treated to improve continence in 1.16
Traditionally delivered by a pelvic floor physical therapist, pelvic stimulation and biofeedback have also been validated for home use.17,18 Several pelvic stimulation devices have been approved by the US Food and Drug Administration (FDA) for treating stress, urgency, and mixed urinary incontinence. These devices deliver stimulation to the pelvic floor at single or multiple frequencies. Although the mechanisms are not clearly understood, lower frequencies are used to treat urgency incontinence, while higher frequencies are used for stress incontinence. A theory is that higher-frequency stimulation strengthens the pelvic floor in stress urinary incontinence while lower frequency stimulation calms the detrusor muscle in urgency urinary incontinence.
The Apex and Apex M devices are both available over the counter, the former to treat stress urinary incontinence and the latter to treat mixed urinary incontinence, using pelvic stimulation alone. Other available devices, including the InTone and a smaller version, the InTone MV, are available by prescription and combine pelvic stimulation with biofeedback.18
Women who wish to avoid surgery, botulinum toxin injections, and daily oral medications, particularly those who are highly motivated, are ideal candidates for these over-the-counter automatic neuromuscular pelvic exercising devices.
PESSARIES AND OTHER DEVICES
Pessaries are commonly used to treat pelvic organ prolapse but can also be designed to help correct the anatomic defect responsible for stress urinary incontinence. Continence pessaries support the bladder neck so that the urethrovesicular junction is stabilized rather than hypermobile during the increased intra-abdominal pressure that occurs with coughing, sneezing, or physical exertion (Figure 2). In theory, this should decrease leakage.
A systematic review concluded that the value of pessaries in the management of incontinence remains uncertain. However, there are inherent challenges in conducting trials of such devices.19 A pessary needs to be fitted by an appropriately trained healthcare provider. The Ambulatory Treatments for Leakage Associated With Stress Incontinence (ATLAS) trial20 reported that behavioral therapy was more effective than a pessary at 3 months, but the treatments were equivalent at 12 months.
The FDA has approved a disposable, over-the-counter silicone intravaginal device for treating stress urinary incontinence. Patients initially purchase a sizing kit and subsequently insert the nonabsorbent temporary intravaginal bladder supportive device, which is worn for up to 8 hours.
Women may elect to use regular tampons to do the job of a pessary, as they are easy to use and low in cost. No large randomized trials have compared tampons and pessaries, and currently no one device is known to be superior to another.
Overall, these devices are temporizing measures that have few serious adverse effects.
WEIGHT LOSS AND DIETARY CHANGES
Obesity has become a national epidemic, with more than 68% of Americans found to be overweight or obese according to the National Institutes of Health.21
Several studies found obesity to be an independent risk factor for urinary incontinence. As early as 1946, the British Birth Cohort study found that women ages 48 through 54 who were obese earlier in life had a higher risk of urinary incontinence in middle age, and those who were obese by age 20 were more likely to report severe incontinence.22 Likewise, the Nurses’ Health Study showed that women with a body mass index (BMI) more than 30 kg/m2 had 3.1 times the risk of severe incontinence compared with women with a normal BMI. Also, the Study of Women’s Health Across the Nation and the Leicestershire Medical Research Council (MRC) incontinence study both showed that a higher BMI and weight gain are strongly correlated with urinary incontinence.23,24
Increased intra-abdominal pressure may be the causative mechanism of stress urinary incontinence in obesity. The Korean National Health and Nutrition Examination Survey showed that central adiposity correlated with urgency incontinence.25,26
The MRC study was one of the largest to evaluate the effect of diet on urinary symptoms. Consuming a diet dense in vegetables, bread, and chicken was found to reduce the risk of urinary incontinence, while carbonated drinks were associated with a higher risk.25 These studies and others may point to reducing calories, and thus BMI, as a conservative treatment for urinary incontinence.
Newer data show bariatric surgery is associated with a strong reduction in urinary incontinence, demonstrated in a study that followed patients for 3 years after surgery.27 This encouraging result is but one of several positive health outcomes from bariatric surgery.
Recommendation
The ACP recommends both weight loss and exercise for overweight women with urinary incontinence, and grades this as strong with moderate-quality evidence.
DRUG THERAPY
The bladder neck is rich with sympathetic alpha-adrenergic receptors, and the bladder dome is dense with parasympathetic muscarinic receptors and sympathetic beta-adrenergic receptors. When the parasympathetic system is stimulated, the muscarinic receptors are activated, causing detrusor contraction and ultimately bladder emptying.
Agonism of beta-alpha adrenergic receptors and inhibition of parasympathetic receptors are both strategies of drug treatment of urinary incontinence.
Anticholinergic drugs
Anticholinergic medications function by blocking the muscarinic receptor, thereby inhibiting detrusor contraction.
Six oral anticholinergic medications are available: oxybutynin, tolterodine, fesoterodine, solifenacin, trospium, and darifenacin. These drugs have about the same effectiveness in treating urgency urinary incontinence, as measured by achieving continence and improving quality of life.28 Given their similarity in effectiveness, the choice of agent typically relies on the side-effect profile. Extended-release formulations have a more favorable side-effect profile, with fewer cases of dry mouth compared with immediate-release formulations.29
Overall, however, the benefit of these medications is small, with fewer than 200 patients achieving continence per 1,000 treated.28
Other limitations of these medications include their adverse effects and contraindications, and patients’ poor adherence to therapy. The most commonly reported adverse effect is dry mouth, but other common side effects include constipation, abdominal pain, dyspepsia, fatigue, dry eye, and dry skin. Transdermal oxybutynin therapy has been associated with fewer anticholinergic side effects than oral therapy.30
Contraindications to these medications include gastric retention, urinary retention, and angle-closure glaucoma.
Long-term adherence to anticholinergics is low, reported between 14% to 35% after 12 months, with the highest rates of adherence with solifenacin.31 The most commonly cited reason for discontinuation is lack of effect.32
Caution is urged when considering starting anticholinergic medications in older adults because of the central nervous system side effects, including drowsiness, hallucinations, cognitive impairment, and dementia. After 3 weeks, oxybutynin caused a memory decline as measured by delayed recall that was comparable to the decline seen over 10 years in normal aging.33 There is evidence suggesting trospium may cause less cognitive impairment, and therefore may be a better option for older patients.34
Beta-3 adrenoreceptor agonists
Activation of beta-3 adrenergic receptors through the sympathetic nervous system relaxes the detrusor muscle, allowing the bladder to store urine.
Mirabegron is a selective beta-3 adrenoreceptor agonist that effectively relaxes the bladder and increases bladder capacity. It improves continence, treatment satisfaction, and quality of life.35,36 There are fewer reports of dry mouth and constipation with this drug than with the anticholinergics; however, beta-3 adrenoreceptor agonists may be associated with greater risk of hypertension, nasopharyngitis, headache, and urinary tract infection.37
Duloxetine
Duloxetine, an antidepressant, blocks the reuptake of both serotonin and norepinephrine. Consequently, it decreases parasympathetic activity and increases sympathetic and somatic activity in the urinary system.38 While urine is stored, this cascade of neural activity is thought to collectively increase pudendal nerve activity and improve closure of the urethra.
Although duloxetine is approved to treat stress urinary incontinence in Europe, this is an off-label use in the United States.
A meta-analysis39 found that duloxetine improved quality of life in patients with stress urinary incontinence and that subjective cure rates were 10.8% with duloxetine vs 7.7% with placebo (P = .04). However the rate of adverse events is high, with nausea most common. Given its modest benefit and high rate of side effects, physicians may consider starting duloxetine only if there are psychiatric comorbidities such as depression, anxiety, or fibromyalgia.
Recommendations
The ACP recommends against systemic pharmacologic therapy for stress urinary incontinence. For urgency urinary incontinence, pharmacologic therapy is recommended if bladder training fails, and should be individualized based on the patient’s preference and medical comorbidities and the drug’s tolerability, cost, and ease of use.
Hormone therapy
In 2014, the North American Menopause Society recommended replacing the term “vulvovaginal atrophy” with the term genitourinary syndrome of menopause, which better encompasses the postmenopausal changes to the female genital system.40
Estrogen therapy is commercially available in both systemic and local preparations. The effect of exogenous estrogen on urinary incontinence may depend on whether it is given locally or systemically.
A systematic review41 definitively concluded that all commercially prepared local vaginal estrogen preparations can effectively relieve the genitourinary syndrome of menopause, including not only the common complaints of dryness, burning, and irritation but also urinary complaints of frequency, urgency, and urgency urinary incontinence.41 Additionally, the estradiol vaginal ring for vaginal atrophy (Estring) may have dual effects, functioning like an incontinence pessary by supporting the bladder neck while simultaneously providing local estrogen to the atrophied vaginal tissue.
However, in the Women’s Health Initiative,42 continent women who received either systemic estrogen therapy alone or systemic estrogen combined with progestin actually had a higher risk of developing urinary incontinence, and those already experiencing incontinence developed a worsening of their symptoms on systemic hormone therapy. The mechanism by which systemic hormone therapy causes urinary incontinence is unclear; however, previous studies showed that hormone therapy leads to a reduction in periurethral collagen and increased bladder contractility.43,44
TAKE-HOME POINTS
- Half of women with symptomatic urinary incontinence never report their symptoms.
- Bladder training is recommended for urgency incontinence and pelvic floor muscle training for stress incontinence.
- Thirty percent of women perform pelvic floor exercises incorrectly.
- Devices can be considered, including automatic pelvic exercise devices for stress and urgency incontinence and incontinence pessaries and disposable intravaginal bladder support devices for stress incontinence.
- Higher BMIs are strongly correlated with urinary incontinence.
- Anticholinergic medications are recommended for urgency but not stress incontinence.
- Vaginal estrogen cream may help with symptoms of urinary urgency, recurrent bladder infections, and nocturia in addition to incontinence.
Urinary incontinence can lead to a cascade of symptomatic burden on the patient, causing distress, embarrassment, and suffering.
See related patient information
Traditionally, incontinence has been treated by surgeons, and surgery remains an option. However, more patients are now being managed by medical clinicians, who can offer a number of newer therapies. Ideally, a medical physician can initiate the evaluation and treatment and even effectively cure some forms of urinary incontinence.
In 2014, the American College of Physicians (ACP) published recommendations on the medical treatment of urinary incontinence in women (Table 1).1
This review describes the medical management of urinary incontinence in women, emphasizing the ACP recommendations1 and newer over-the-counter options.
COMMON AND UNDERREPORTED
Many women erroneously believe that urinary incontinence is an inevitable consequence of aging and allow it to lessen their quality of life without seeking medical attention.
Indeed, it is common. The 2005–2006 National Health and Nutritional Examination Survey2 found the prevalence of urinary incontinence in US women to be 15.7%. The prevalence increases with age from 6.9% in women ages 20 through 29 to 31.7% in those age 80 and older. A separate analysis of the same data found that 25.0% of women age 20 and older had 1 or more pelvic floor disorders.3 Some estimates are even higher. Wu et al4 reported a prevalence of urinary incontinence of 51.1% in women ages 31 through 54.
Too few of these women are identified and treated, for many reasons, including embarrassment and inadequate screening. Half of women with urinary incontinence do not report their symptoms because of humiliation or anxiety.5
The burden of urinary incontinence extends beyond the individual and into society. The total cost of overactive bladder and urgency urinary incontinence in the United States was estimated to be $65.9 billion in 2007 and is projected to reach $82.6 billion in 2020.6
THREE TYPES
There are 3 types of urinary incontinence: stress, urgency, and mixed.
Stress urinary incontinence is involuntary loss of urine associated with physical exertion or increased abdominal pressure, eg, with coughing or sneezing.
Urgency urinary incontinence is involuntary loss of urine associated with the sudden need to void. Many patients experience these symptoms simultaneously, making the distinction difficult.
Mixed urinary incontinence is loss of urine with both urgency and increased abdominal pressure or physical exertion.
Overactive bladder, a related problem, is defined as urinary urgency, usually accompanied by frequency and nocturia, with or without urgency urinary incontinence, in the absence of a urinary tract infection or other obvious disease.7
Nongenitourinary causes such as neurologic disorders or even malignancy can present with urinary incontinence, and thus it is critical to perform a thorough initial evaluation.
A 2014 study revealed that by age 80, 20% of women may need to undergo surgery for stress urinary incontinence or pelvic organ prolapse. This statistic should motivate healthcare providers to focus on prevention and offer conservative medical management for these conditions first.8
QUESTIONS TO ASK
When doing a pelvic examination, once could inquire about urinary incontinence with questions such as:
Do you leak urine when you cough, sneeze, laugh, or jump or during sexual climax?
Do you have to get up more than once at night to urinate?
Do you feel the urge to urinate frequently?
BEHAVIORAL MODIFICATION AND BLADDER TRAINING
Bladder training is a conservative behavioral treatment for urinary incontinence that primary care physicians can teach. It is primarily used for urgency urinary incontinence but can also be useful in stress urinary incontinence.
After completing a bladder diary and gaining awareness of their daily voiding patterns, patients can learn scheduled voiding to train the bladder, gradually extending the urges to longer intervals.
Clinicians should instruct patients on how to train the bladder, using methods first described by Wyman and Fantl.9 (See Training the bladder.)
There is evidence that bladder training improves urinary incontinence compared with usual care.10,11
The ACP recommends bladder training for women who have urgency urinary incontinence, but grades this recommendation as weak with low-quality evidence.
PELVIC FLOOR MUSCLE TRAINING
Introduced in 1948 by Dr. Arnold Kegel, pelvic floor muscle training has become widely adopted.12
The pelvic floor consists of a group of muscles, resembling a hammock, that support the pelvic viscera. These muscles include the coccygeus and the layers of the levator ani (Figure 1). A weak pelvic floor is one of many risk factors for developing stress urinary incontinence. Like other muscle groups, a weak pelvic floor can be rehabilitated through various techniques, often guided by a physical therapist.
Compared with those who received no treatment, women with stress urinary incontinence who performed pelvic floor muscle training were 8 times more likely to report being cured and 17 times more likely to report cure or improvement.13
To perform a Kegel exercise, a woman consciously contracts her pelvic floor muscles as if stopping the flow of urine.
The Knack maneuver can be used to prevent leakage during anticipated events that increase intra-abdominal pressure. For example, when a cough or sneeze is imminent, patients can preemptively contract their pelvic floor and hold the contraction through the cough or sneeze.
Although many protocols have been compared, no specific pelvic floor exercise strategy has proven superior. A systematic review assessed variations in pelvic floor interventions, exercises, and delivery and found that there was insufficient evidence to make any recommendations about the best approach. However, the benefit was greater with regular supervision during pelvic floor muscle training than with little or no supervision.14
Pelvic floor muscle training strengthens the pelvic floor, which better supports the bladder neck and anatomically compensates for defects in stress urinary incontinence. In urgency urinary incontinence, a strong pelvic floor created by muscle training prevents leaking induced by the involuntary contractions of the detrusor muscle.
Recommendation
The ACP recommends pelvic floor muscle training as first-line treatment for stress urinary incontinence and mixed urinary incontinence, and grades this recommendation as strong with high-quality evidence.
BIOFEEDBACK AND PELVIC STIMULATION
Although pelvic floor exercises are effective in urinary incontinence, 30% of patients perform them incorrectly.15
Biofeedback therapy uses visual, verbal, and acoustic signals to give the patient immediate feedback and a greater awareness of her muscular activity. A commonly used technique employs a vaginal probe to measure and display pressure changes as the patient contracts her levator ani muscles.
Women who received biofeedback in addition to traditional pelvic floor physical therapy had greater improvement in urinary incontinence than those who received pelvic physical therapy alone (risk ratio 0.75, 95% confidence interval 0.66–0.86).16
Pelvic stimulation can be used separately or in conjunction with biofeedback in both urgency and stress urinary incontinence. When pelvic stimulation is used alone, 9 women need to be treated to achieve continence in 1, and 6 women need to be treated to improve continence in 1.16
Traditionally delivered by a pelvic floor physical therapist, pelvic stimulation and biofeedback have also been validated for home use.17,18 Several pelvic stimulation devices have been approved by the US Food and Drug Administration (FDA) for treating stress, urgency, and mixed urinary incontinence. These devices deliver stimulation to the pelvic floor at single or multiple frequencies. Although the mechanisms are not clearly understood, lower frequencies are used to treat urgency incontinence, while higher frequencies are used for stress incontinence. A theory is that higher-frequency stimulation strengthens the pelvic floor in stress urinary incontinence while lower frequency stimulation calms the detrusor muscle in urgency urinary incontinence.
The Apex and Apex M devices are both available over the counter, the former to treat stress urinary incontinence and the latter to treat mixed urinary incontinence, using pelvic stimulation alone. Other available devices, including the InTone and a smaller version, the InTone MV, are available by prescription and combine pelvic stimulation with biofeedback.18
Women who wish to avoid surgery, botulinum toxin injections, and daily oral medications, particularly those who are highly motivated, are ideal candidates for these over-the-counter automatic neuromuscular pelvic exercising devices.
PESSARIES AND OTHER DEVICES
Pessaries are commonly used to treat pelvic organ prolapse but can also be designed to help correct the anatomic defect responsible for stress urinary incontinence. Continence pessaries support the bladder neck so that the urethrovesicular junction is stabilized rather than hypermobile during the increased intra-abdominal pressure that occurs with coughing, sneezing, or physical exertion (Figure 2). In theory, this should decrease leakage.
A systematic review concluded that the value of pessaries in the management of incontinence remains uncertain. However, there are inherent challenges in conducting trials of such devices.19 A pessary needs to be fitted by an appropriately trained healthcare provider. The Ambulatory Treatments for Leakage Associated With Stress Incontinence (ATLAS) trial20 reported that behavioral therapy was more effective than a pessary at 3 months, but the treatments were equivalent at 12 months.
The FDA has approved a disposable, over-the-counter silicone intravaginal device for treating stress urinary incontinence. Patients initially purchase a sizing kit and subsequently insert the nonabsorbent temporary intravaginal bladder supportive device, which is worn for up to 8 hours.
Women may elect to use regular tampons to do the job of a pessary, as they are easy to use and low in cost. No large randomized trials have compared tampons and pessaries, and currently no one device is known to be superior to another.
Overall, these devices are temporizing measures that have few serious adverse effects.
WEIGHT LOSS AND DIETARY CHANGES
Obesity has become a national epidemic, with more than 68% of Americans found to be overweight or obese according to the National Institutes of Health.21
Several studies found obesity to be an independent risk factor for urinary incontinence. As early as 1946, the British Birth Cohort study found that women ages 48 through 54 who were obese earlier in life had a higher risk of urinary incontinence in middle age, and those who were obese by age 20 were more likely to report severe incontinence.22 Likewise, the Nurses’ Health Study showed that women with a body mass index (BMI) more than 30 kg/m2 had 3.1 times the risk of severe incontinence compared with women with a normal BMI. Also, the Study of Women’s Health Across the Nation and the Leicestershire Medical Research Council (MRC) incontinence study both showed that a higher BMI and weight gain are strongly correlated with urinary incontinence.23,24
Increased intra-abdominal pressure may be the causative mechanism of stress urinary incontinence in obesity. The Korean National Health and Nutrition Examination Survey showed that central adiposity correlated with urgency incontinence.25,26
The MRC study was one of the largest to evaluate the effect of diet on urinary symptoms. Consuming a diet dense in vegetables, bread, and chicken was found to reduce the risk of urinary incontinence, while carbonated drinks were associated with a higher risk.25 These studies and others may point to reducing calories, and thus BMI, as a conservative treatment for urinary incontinence.
Newer data show bariatric surgery is associated with a strong reduction in urinary incontinence, demonstrated in a study that followed patients for 3 years after surgery.27 This encouraging result is but one of several positive health outcomes from bariatric surgery.
Recommendation
The ACP recommends both weight loss and exercise for overweight women with urinary incontinence, and grades this as strong with moderate-quality evidence.
DRUG THERAPY
The bladder neck is rich with sympathetic alpha-adrenergic receptors, and the bladder dome is dense with parasympathetic muscarinic receptors and sympathetic beta-adrenergic receptors. When the parasympathetic system is stimulated, the muscarinic receptors are activated, causing detrusor contraction and ultimately bladder emptying.
Agonism of beta-alpha adrenergic receptors and inhibition of parasympathetic receptors are both strategies of drug treatment of urinary incontinence.
Anticholinergic drugs
Anticholinergic medications function by blocking the muscarinic receptor, thereby inhibiting detrusor contraction.
Six oral anticholinergic medications are available: oxybutynin, tolterodine, fesoterodine, solifenacin, trospium, and darifenacin. These drugs have about the same effectiveness in treating urgency urinary incontinence, as measured by achieving continence and improving quality of life.28 Given their similarity in effectiveness, the choice of agent typically relies on the side-effect profile. Extended-release formulations have a more favorable side-effect profile, with fewer cases of dry mouth compared with immediate-release formulations.29
Overall, however, the benefit of these medications is small, with fewer than 200 patients achieving continence per 1,000 treated.28
Other limitations of these medications include their adverse effects and contraindications, and patients’ poor adherence to therapy. The most commonly reported adverse effect is dry mouth, but other common side effects include constipation, abdominal pain, dyspepsia, fatigue, dry eye, and dry skin. Transdermal oxybutynin therapy has been associated with fewer anticholinergic side effects than oral therapy.30
Contraindications to these medications include gastric retention, urinary retention, and angle-closure glaucoma.
Long-term adherence to anticholinergics is low, reported between 14% to 35% after 12 months, with the highest rates of adherence with solifenacin.31 The most commonly cited reason for discontinuation is lack of effect.32
Caution is urged when considering starting anticholinergic medications in older adults because of the central nervous system side effects, including drowsiness, hallucinations, cognitive impairment, and dementia. After 3 weeks, oxybutynin caused a memory decline as measured by delayed recall that was comparable to the decline seen over 10 years in normal aging.33 There is evidence suggesting trospium may cause less cognitive impairment, and therefore may be a better option for older patients.34
Beta-3 adrenoreceptor agonists
Activation of beta-3 adrenergic receptors through the sympathetic nervous system relaxes the detrusor muscle, allowing the bladder to store urine.
Mirabegron is a selective beta-3 adrenoreceptor agonist that effectively relaxes the bladder and increases bladder capacity. It improves continence, treatment satisfaction, and quality of life.35,36 There are fewer reports of dry mouth and constipation with this drug than with the anticholinergics; however, beta-3 adrenoreceptor agonists may be associated with greater risk of hypertension, nasopharyngitis, headache, and urinary tract infection.37
Duloxetine
Duloxetine, an antidepressant, blocks the reuptake of both serotonin and norepinephrine. Consequently, it decreases parasympathetic activity and increases sympathetic and somatic activity in the urinary system.38 While urine is stored, this cascade of neural activity is thought to collectively increase pudendal nerve activity and improve closure of the urethra.
Although duloxetine is approved to treat stress urinary incontinence in Europe, this is an off-label use in the United States.
A meta-analysis39 found that duloxetine improved quality of life in patients with stress urinary incontinence and that subjective cure rates were 10.8% with duloxetine vs 7.7% with placebo (P = .04). However the rate of adverse events is high, with nausea most common. Given its modest benefit and high rate of side effects, physicians may consider starting duloxetine only if there are psychiatric comorbidities such as depression, anxiety, or fibromyalgia.
Recommendations
The ACP recommends against systemic pharmacologic therapy for stress urinary incontinence. For urgency urinary incontinence, pharmacologic therapy is recommended if bladder training fails, and should be individualized based on the patient’s preference and medical comorbidities and the drug’s tolerability, cost, and ease of use.
Hormone therapy
In 2014, the North American Menopause Society recommended replacing the term “vulvovaginal atrophy” with the term genitourinary syndrome of menopause, which better encompasses the postmenopausal changes to the female genital system.40
Estrogen therapy is commercially available in both systemic and local preparations. The effect of exogenous estrogen on urinary incontinence may depend on whether it is given locally or systemically.
A systematic review41 definitively concluded that all commercially prepared local vaginal estrogen preparations can effectively relieve the genitourinary syndrome of menopause, including not only the common complaints of dryness, burning, and irritation but also urinary complaints of frequency, urgency, and urgency urinary incontinence.41 Additionally, the estradiol vaginal ring for vaginal atrophy (Estring) may have dual effects, functioning like an incontinence pessary by supporting the bladder neck while simultaneously providing local estrogen to the atrophied vaginal tissue.
However, in the Women’s Health Initiative,42 continent women who received either systemic estrogen therapy alone or systemic estrogen combined with progestin actually had a higher risk of developing urinary incontinence, and those already experiencing incontinence developed a worsening of their symptoms on systemic hormone therapy. The mechanism by which systemic hormone therapy causes urinary incontinence is unclear; however, previous studies showed that hormone therapy leads to a reduction in periurethral collagen and increased bladder contractility.43,44
TAKE-HOME POINTS
- Half of women with symptomatic urinary incontinence never report their symptoms.
- Bladder training is recommended for urgency incontinence and pelvic floor muscle training for stress incontinence.
- Thirty percent of women perform pelvic floor exercises incorrectly.
- Devices can be considered, including automatic pelvic exercise devices for stress and urgency incontinence and incontinence pessaries and disposable intravaginal bladder support devices for stress incontinence.
- Higher BMIs are strongly correlated with urinary incontinence.
- Anticholinergic medications are recommended for urgency but not stress incontinence.
- Vaginal estrogen cream may help with symptoms of urinary urgency, recurrent bladder infections, and nocturia in addition to incontinence.
- Qaseem A, Dallas P, Forciea MA, Starkey M, Denberg TD, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Nonsurgical management of urinary incontinence in women: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2014; 161:429–440.
- Nygaard I, Barber MD, Burgio KL, et al; Pelvic Floor Disorders Network. Prevalence of symptomatic pelvic floor disorders in US women. JAMA 2008; 300:1311–1316.
- Wu JM, Vaughan CP, Goode PS, et al. Prevalence and trends of symptomatic pelvic floor disorders in US women. Obstet Gynecol 2014; 123:141–148.
- Wu JM, Stinnett S, Jackson RA, Jacoby A, Learman LA, Kuppermann M. Prevalence and incidence of urinary incontinence in a diverse population of women with noncancerous gynecologic conditions. Female Pelvic Med Reconstr Surg 2010; 16:284–289.
- Griffiths AN, Makam A, Edward GJ. Should we actively screen for urinary and anal incontinence in the general gynaecology outpatients setting? A prospective observational study. J Obstet Gynaecol 2006; 26:442–444.
- Coyne KS, Wein A, Nicholson S, Kvasz M, Chen CI, Milsom I. Economic burden of urgency urinary incontinence in the United Stated: a systematic review. J Manag Care Pharm 2014; 20:130–140.
- Haylen BT, Ridder D, Freeman RM, et al; International Urogynecological Association; International Continence Society. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn 2010; 29:4–20.
- Wu JM, Matthews CA, Conover MM, Pate V, Jonsson Funk M. Lifetime risk of stress urinary incontinence or pelvic organ prolapse surgery. Obstet Gynecol 2014; 123:1201–1206.
- Wyman JF, Fantl JA. Bladder training in the ambulatory care management of urinary incontinence. Urol Nurs 1991; 11:11–17.
- Fantl JA, Wyman JF, McClish DK, et al. Efficacy of bladder training in older women with urinary incontinence. JAMA 1991; 265:609–613.
- Subak LL, Quesenberry CP, Posner SF, Cattolica E, Soghikian K. The effect of behavioral therapy on urinary incontinence: a randomized controlled trial. Obstet Gynecol 2002; 100:72–78.
- Kegel AH. Progressive resistance exercise in the functional restoration of the perineal muscles. Am J Obstet Gynecol 1948; 56:238–248.
- Domoulin C, Hay-Smith EJ, Mac Habée-Séguin G. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev 2014; 5:CD005654.
- Hay-Smith EJ, Herderschee R, Dumoulin C, Herbison GP. Comparisons of approaches to pelvic floor muscle training for urinary incontinence in women. Cochrane Database Syst Rev 2011; 12:CD009508.
- Bo K. Pelvic floor muscle strength and response to pelvic floor muscle training for stress urinary incontinence. Neurourol Urodyn 2003; 22:654–658.
- Herderschee R, Hay-Smith EJ, Herbison GP, Roovers JP, Heineman MJ. Feedback or biofeedback to augment pelvic floor muscle training for urinary incontinence in women. Cochrane Database Syst Rev 2011; 7:CD009252.
- Terlikowski R, Dobrzycka B, Kinalski M, Kuryliszyn-Moskal A, Terlikowski SJ. Transvaginal electrical stimulation with surface-EMG biofeedback in managing stress urinary incontinence in women of premenopausal age: a double-blind, placebo-controlled, randomized clinical trial. Int Urogynecol J 2013; 17:1631–1638.
- Guralnick ML, Kelly H, Engelke H, Koduri S, O’Connor RC. InTone: a novel pelvic floor rehabilitation device for urinary incontinence. Int Urogynecol J 2015; 26:99–106.
- Lipp A, Shaw C, Glavind K. Mechanical devices for urinary incontinence in women. Cochrane Database Syst Rev 2014; 12:CD001756.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol 2010; 115:609–617.
- National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Overweight and obesity statistics. www.niddk.nih.gov/health-information/health-statistics/Pages/overweight-obesity-statistics.aspx. Accessed January 6, 2017.
- Mishra GD, Hardy R, Cardozo L, Kuh D. Body weight through adult life and risk of urinary incontinence in middle-aged women. Results from a British prospective cohort. Int J Obes (Lond) 2008; 32:1415–1422.
- Danforth KN, Townsend MK, Lifford K, Curhan GC, Resnick NM, Grodstein F. Risk factors for urinary incontinence among middle age women. Am J Obstet Gynecol 2006; 194:339–345.
- Waetjen LE, Liao S, Johnson WO, et al. Factors associated with prevalence and incident urinary incontinence in a cohort of midlife women: a longitudinal analysis of data: study of women’s health across the nation. Am J Epidemiol 2007; 165:309–318.
- Dallosso HM, McGrother CW, Matthews RJ, Donaldson MM; Leicestershire MRC Incontinence Study Group. The association of diet and other lifestyle factors with overactive bladder and stress incontinence: a longitudinal study in women. BJU Int 2003; 92:69–77.
- Kim IH, Chung H, Kwon JW. Gender differences in the effect of obesity on chronic diseases among the elderly Koreans. J Korean Med Sci. 2011; 26:250–257.
- Subak LL, King WC, Belle SH, et al. Urinary incontinence before and after bariatric surgery. JAMA Intern Med 2015; 175:1378–1387.
- Shamliyan T, Wyman JF, Ramakrishnan R, Sainfort F, Kane RL. Benefits and harms of pharmacologic treatment for urinary incontinence in women: a systematic review. Ann Intern Med 2012; 156:861–874, W301–W310.
- Hay-Smith J, Herbison P, Ellis G, Morris A. Which anticholinergic drug for overactive bladder symptoms in adults. Cochrane Database Syst Rev 2005; 3:CD005429.
- Davila GW, Daugherty CA, Sanders SW; Transdermal Oxybutynin Study Group. A short term, multicenter, randomized double-blind dose titration study of the efficacy and anticholinergic side effects of transdermal compared to immediate release oral oxybutynin treatment of patients with urge urinary incontinence. J Urol 2001; 166:140–145.
- Wagg A, Compion G, Fahey A, Siddiqui E. Persistence with prescribed antimuscarinic therapy for overactive bladder: a UK experience. BJU Int 2012; 110:1767–1774.
- Benner JS, Nichol MB, Rovner ES, et al. Patient-reported reasons for discontinuing overactive bladder medication. BJU Int 2010; 105:1276–1282.
- Kay G, Crook T, Rekeda L, et al. Differential effects of the antimuscarinic agents darifenacin and oxybutynin ER on memory in older subjects. Eur Urol 2006; 50:317–326.
- Staskin D, Kay G, Tannenbaum C, et al. Trospium chloride has no effect on memory testing and is assay undetectable in the central nervous system of older patients with overactive bladder. Int J Clin Pract 2010; 64:1294–1300.
- Chapple CR, Amarenco G, Lopez A, et al; BLOSSOM Investigator Group. A proof of concept study: mirabegron, a new therapy for overactive bladder. Neurourol Urodyn 2013; 32:1116–1122.
- Nitti VB, Khullar V, van Kerrebroeck P, et al. Mirabegron for the treatment of overactive bladder: a prespecified pooled efficacy analysis and pooled safety analysis of three randomised, double-blind, placebo-controlled, phase III studies. Int J Clin Pract 2013; 67:619–632.
- Maman K, Aballea S, Nazir J, et al. Comparative efficacy and safety of medical treatments for the management of overactive bladder: a systematic literature review and mixed treatment comparison. Eur Urol 2014; 65:755–765.
- Katofiasc MA, Nissen J, Audia JE, Thor KB. Comparison of the effects of serotonin selective, norepinephrine, and dual serotonin and norepinephrine reuptake inhibitors on lower urinary tract function in cats. Life Sci 2002; 71:1227–1236.
- Mariappan P, Alhasso A, Ballantyne Z, Grant A, N’Dow J. Duloxetine, a serotonin and noradrenaline reuptake inhibitor for the treatment of stress urinary incontinence: a systematic review. Eur Urol 2007; 51:67–74.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause 2014; 21:1063–1068.
- Rahn DD, Carberry C, Sanses TV, et al; Society of Gynecologic Surgeons Systematic Review Group. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol 2014; 124:1147–1156.
- Hendrix SL, Cochrane BB, Nygaard IE, et al. Effects of estrogen with and without progestin on urinary incontinence. JAMA 2005; 293:935–948.
- Jackson S, James M, Abrams P. The effect of estradiol on vaginal collagen metabolism in postmenopausal women with genuine stress incontinence. BJOG 2002; 109:339–344.
- Lin AD, Levin R, Kogan B, et al. Estrogen induced functional hypertrophy and increased force generation of the female rabbit bladder. Neurourol Urodyn 2006; 25:473–479.
- Qaseem A, Dallas P, Forciea MA, Starkey M, Denberg TD, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Nonsurgical management of urinary incontinence in women: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2014; 161:429–440.
- Nygaard I, Barber MD, Burgio KL, et al; Pelvic Floor Disorders Network. Prevalence of symptomatic pelvic floor disorders in US women. JAMA 2008; 300:1311–1316.
- Wu JM, Vaughan CP, Goode PS, et al. Prevalence and trends of symptomatic pelvic floor disorders in US women. Obstet Gynecol 2014; 123:141–148.
- Wu JM, Stinnett S, Jackson RA, Jacoby A, Learman LA, Kuppermann M. Prevalence and incidence of urinary incontinence in a diverse population of women with noncancerous gynecologic conditions. Female Pelvic Med Reconstr Surg 2010; 16:284–289.
- Griffiths AN, Makam A, Edward GJ. Should we actively screen for urinary and anal incontinence in the general gynaecology outpatients setting? A prospective observational study. J Obstet Gynaecol 2006; 26:442–444.
- Coyne KS, Wein A, Nicholson S, Kvasz M, Chen CI, Milsom I. Economic burden of urgency urinary incontinence in the United Stated: a systematic review. J Manag Care Pharm 2014; 20:130–140.
- Haylen BT, Ridder D, Freeman RM, et al; International Urogynecological Association; International Continence Society. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn 2010; 29:4–20.
- Wu JM, Matthews CA, Conover MM, Pate V, Jonsson Funk M. Lifetime risk of stress urinary incontinence or pelvic organ prolapse surgery. Obstet Gynecol 2014; 123:1201–1206.
- Wyman JF, Fantl JA. Bladder training in the ambulatory care management of urinary incontinence. Urol Nurs 1991; 11:11–17.
- Fantl JA, Wyman JF, McClish DK, et al. Efficacy of bladder training in older women with urinary incontinence. JAMA 1991; 265:609–613.
- Subak LL, Quesenberry CP, Posner SF, Cattolica E, Soghikian K. The effect of behavioral therapy on urinary incontinence: a randomized controlled trial. Obstet Gynecol 2002; 100:72–78.
- Kegel AH. Progressive resistance exercise in the functional restoration of the perineal muscles. Am J Obstet Gynecol 1948; 56:238–248.
- Domoulin C, Hay-Smith EJ, Mac Habée-Séguin G. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev 2014; 5:CD005654.
- Hay-Smith EJ, Herderschee R, Dumoulin C, Herbison GP. Comparisons of approaches to pelvic floor muscle training for urinary incontinence in women. Cochrane Database Syst Rev 2011; 12:CD009508.
- Bo K. Pelvic floor muscle strength and response to pelvic floor muscle training for stress urinary incontinence. Neurourol Urodyn 2003; 22:654–658.
- Herderschee R, Hay-Smith EJ, Herbison GP, Roovers JP, Heineman MJ. Feedback or biofeedback to augment pelvic floor muscle training for urinary incontinence in women. Cochrane Database Syst Rev 2011; 7:CD009252.
- Terlikowski R, Dobrzycka B, Kinalski M, Kuryliszyn-Moskal A, Terlikowski SJ. Transvaginal electrical stimulation with surface-EMG biofeedback in managing stress urinary incontinence in women of premenopausal age: a double-blind, placebo-controlled, randomized clinical trial. Int Urogynecol J 2013; 17:1631–1638.
- Guralnick ML, Kelly H, Engelke H, Koduri S, O’Connor RC. InTone: a novel pelvic floor rehabilitation device for urinary incontinence. Int Urogynecol J 2015; 26:99–106.
- Lipp A, Shaw C, Glavind K. Mechanical devices for urinary incontinence in women. Cochrane Database Syst Rev 2014; 12:CD001756.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol 2010; 115:609–617.
- National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Overweight and obesity statistics. www.niddk.nih.gov/health-information/health-statistics/Pages/overweight-obesity-statistics.aspx. Accessed January 6, 2017.
- Mishra GD, Hardy R, Cardozo L, Kuh D. Body weight through adult life and risk of urinary incontinence in middle-aged women. Results from a British prospective cohort. Int J Obes (Lond) 2008; 32:1415–1422.
- Danforth KN, Townsend MK, Lifford K, Curhan GC, Resnick NM, Grodstein F. Risk factors for urinary incontinence among middle age women. Am J Obstet Gynecol 2006; 194:339–345.
- Waetjen LE, Liao S, Johnson WO, et al. Factors associated with prevalence and incident urinary incontinence in a cohort of midlife women: a longitudinal analysis of data: study of women’s health across the nation. Am J Epidemiol 2007; 165:309–318.
- Dallosso HM, McGrother CW, Matthews RJ, Donaldson MM; Leicestershire MRC Incontinence Study Group. The association of diet and other lifestyle factors with overactive bladder and stress incontinence: a longitudinal study in women. BJU Int 2003; 92:69–77.
- Kim IH, Chung H, Kwon JW. Gender differences in the effect of obesity on chronic diseases among the elderly Koreans. J Korean Med Sci. 2011; 26:250–257.
- Subak LL, King WC, Belle SH, et al. Urinary incontinence before and after bariatric surgery. JAMA Intern Med 2015; 175:1378–1387.
- Shamliyan T, Wyman JF, Ramakrishnan R, Sainfort F, Kane RL. Benefits and harms of pharmacologic treatment for urinary incontinence in women: a systematic review. Ann Intern Med 2012; 156:861–874, W301–W310.
- Hay-Smith J, Herbison P, Ellis G, Morris A. Which anticholinergic drug for overactive bladder symptoms in adults. Cochrane Database Syst Rev 2005; 3:CD005429.
- Davila GW, Daugherty CA, Sanders SW; Transdermal Oxybutynin Study Group. A short term, multicenter, randomized double-blind dose titration study of the efficacy and anticholinergic side effects of transdermal compared to immediate release oral oxybutynin treatment of patients with urge urinary incontinence. J Urol 2001; 166:140–145.
- Wagg A, Compion G, Fahey A, Siddiqui E. Persistence with prescribed antimuscarinic therapy for overactive bladder: a UK experience. BJU Int 2012; 110:1767–1774.
- Benner JS, Nichol MB, Rovner ES, et al. Patient-reported reasons for discontinuing overactive bladder medication. BJU Int 2010; 105:1276–1282.
- Kay G, Crook T, Rekeda L, et al. Differential effects of the antimuscarinic agents darifenacin and oxybutynin ER on memory in older subjects. Eur Urol 2006; 50:317–326.
- Staskin D, Kay G, Tannenbaum C, et al. Trospium chloride has no effect on memory testing and is assay undetectable in the central nervous system of older patients with overactive bladder. Int J Clin Pract 2010; 64:1294–1300.
- Chapple CR, Amarenco G, Lopez A, et al; BLOSSOM Investigator Group. A proof of concept study: mirabegron, a new therapy for overactive bladder. Neurourol Urodyn 2013; 32:1116–1122.
- Nitti VB, Khullar V, van Kerrebroeck P, et al. Mirabegron for the treatment of overactive bladder: a prespecified pooled efficacy analysis and pooled safety analysis of three randomised, double-blind, placebo-controlled, phase III studies. Int J Clin Pract 2013; 67:619–632.
- Maman K, Aballea S, Nazir J, et al. Comparative efficacy and safety of medical treatments for the management of overactive bladder: a systematic literature review and mixed treatment comparison. Eur Urol 2014; 65:755–765.
- Katofiasc MA, Nissen J, Audia JE, Thor KB. Comparison of the effects of serotonin selective, norepinephrine, and dual serotonin and norepinephrine reuptake inhibitors on lower urinary tract function in cats. Life Sci 2002; 71:1227–1236.
- Mariappan P, Alhasso A, Ballantyne Z, Grant A, N’Dow J. Duloxetine, a serotonin and noradrenaline reuptake inhibitor for the treatment of stress urinary incontinence: a systematic review. Eur Urol 2007; 51:67–74.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause 2014; 21:1063–1068.
- Rahn DD, Carberry C, Sanses TV, et al; Society of Gynecologic Surgeons Systematic Review Group. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol 2014; 124:1147–1156.
- Hendrix SL, Cochrane BB, Nygaard IE, et al. Effects of estrogen with and without progestin on urinary incontinence. JAMA 2005; 293:935–948.
- Jackson S, James M, Abrams P. The effect of estradiol on vaginal collagen metabolism in postmenopausal women with genuine stress incontinence. BJOG 2002; 109:339–344.
- Lin AD, Levin R, Kogan B, et al. Estrogen induced functional hypertrophy and increased force generation of the female rabbit bladder. Neurourol Urodyn 2006; 25:473–479.
KEY POINTS
- The 3 types of urinary incontinence are stress, urgency, and mixed.
- The American College of Physicians (ACP) recommends weight loss and exercise for obese women with any of the 3 types of urinary incontinence.
- Pelvic floor muscle training has a strong ACP recommendation for stress incontinence, bladder training has a weak recommendation for urgency incontinence, and the combination of both has a strong recommendation in mixed incontinence.
- Drug treatment has a strong ACP recommendation for urgency incontinence if bladder training is unsuccessful, whereas the recommendation is against drug treatment for stress incontinence.
Menopausal hormone therapy
To the Editor: I much enjoyed the important article by Drs. Lipold, Batur, and Kagan on whether there is a time limit for systemic menopausal hormone therapy.1 The simple answer is no. The authors did a good job of reviewing the factors to consider in terms of contraindications and precautions when prescribing menopausal hormone therapy.
An important part of the discussion regarding stopping hormone therapy is the recent evidence from Finland that has shown increased risks of myocardial infarction and stroke, especially in women under age 60, when taken off hormone therapy.2 This fact is quite ironic, as many clinicians are trying to rush to get women off hormone therapy in order to protect the heart, when the evidence does not suggest this. Just as with other hormone-deficiency conditions, the status needs to be periodically reviewed, and doses may need to be adjusted. However, after age 60 or 65, women do not automatically start producing the sex hormone that they have been deficient in. While menopause is not a definite endocrinopathy, it is a potential endocrinopathy; and for some women, such as young women who are oophorectomized, it is an absolute endocrinopathy.
The International Menopause Society has published updated guidelines emphasizing that new data and reanalysis of older data show that for most women the benefits of menopausal hormone therapy are much greater than the risks, particularly when started within a few years of menopause.3
- Lipold LD, Batur P, Kagan R. Is there a time limit for systemic menopausal hormone therapy? Cleve Clin J Med 2016; 83:605–612.
- Mikkola TS, Tuomikoski P, Lyytinen H, et al. Increased cardiovascular mortality risk in women discontinuing postmenopausal hormone therapy. J Clin Endocrinol Metab 2015; 100:4588–4594.
- Baber RJ, Panay N, Fenton A; IMS Writing Group. 2016 IMS recommendations on women’s midlife health and menopause hormone therapy. Climacteric 2016; 19:109–150.
To the Editor: I much enjoyed the important article by Drs. Lipold, Batur, and Kagan on whether there is a time limit for systemic menopausal hormone therapy.1 The simple answer is no. The authors did a good job of reviewing the factors to consider in terms of contraindications and precautions when prescribing menopausal hormone therapy.
An important part of the discussion regarding stopping hormone therapy is the recent evidence from Finland that has shown increased risks of myocardial infarction and stroke, especially in women under age 60, when taken off hormone therapy.2 This fact is quite ironic, as many clinicians are trying to rush to get women off hormone therapy in order to protect the heart, when the evidence does not suggest this. Just as with other hormone-deficiency conditions, the status needs to be periodically reviewed, and doses may need to be adjusted. However, after age 60 or 65, women do not automatically start producing the sex hormone that they have been deficient in. While menopause is not a definite endocrinopathy, it is a potential endocrinopathy; and for some women, such as young women who are oophorectomized, it is an absolute endocrinopathy.
The International Menopause Society has published updated guidelines emphasizing that new data and reanalysis of older data show that for most women the benefits of menopausal hormone therapy are much greater than the risks, particularly when started within a few years of menopause.3
To the Editor: I much enjoyed the important article by Drs. Lipold, Batur, and Kagan on whether there is a time limit for systemic menopausal hormone therapy.1 The simple answer is no. The authors did a good job of reviewing the factors to consider in terms of contraindications and precautions when prescribing menopausal hormone therapy.
An important part of the discussion regarding stopping hormone therapy is the recent evidence from Finland that has shown increased risks of myocardial infarction and stroke, especially in women under age 60, when taken off hormone therapy.2 This fact is quite ironic, as many clinicians are trying to rush to get women off hormone therapy in order to protect the heart, when the evidence does not suggest this. Just as with other hormone-deficiency conditions, the status needs to be periodically reviewed, and doses may need to be adjusted. However, after age 60 or 65, women do not automatically start producing the sex hormone that they have been deficient in. While menopause is not a definite endocrinopathy, it is a potential endocrinopathy; and for some women, such as young women who are oophorectomized, it is an absolute endocrinopathy.
The International Menopause Society has published updated guidelines emphasizing that new data and reanalysis of older data show that for most women the benefits of menopausal hormone therapy are much greater than the risks, particularly when started within a few years of menopause.3
- Lipold LD, Batur P, Kagan R. Is there a time limit for systemic menopausal hormone therapy? Cleve Clin J Med 2016; 83:605–612.
- Mikkola TS, Tuomikoski P, Lyytinen H, et al. Increased cardiovascular mortality risk in women discontinuing postmenopausal hormone therapy. J Clin Endocrinol Metab 2015; 100:4588–4594.
- Baber RJ, Panay N, Fenton A; IMS Writing Group. 2016 IMS recommendations on women’s midlife health and menopause hormone therapy. Climacteric 2016; 19:109–150.
- Lipold LD, Batur P, Kagan R. Is there a time limit for systemic menopausal hormone therapy? Cleve Clin J Med 2016; 83:605–612.
- Mikkola TS, Tuomikoski P, Lyytinen H, et al. Increased cardiovascular mortality risk in women discontinuing postmenopausal hormone therapy. J Clin Endocrinol Metab 2015; 100:4588–4594.
- Baber RJ, Panay N, Fenton A; IMS Writing Group. 2016 IMS recommendations on women’s midlife health and menopause hormone therapy. Climacteric 2016; 19:109–150.
Sex, statins, and diabetes
To the Editor: The review article “Statins and diabetes: fact, fiction, and clinical implications” 1 left out one major fact: there are sex-based differences in the statin research results, particularly a higher risk for diabetes in postmenopausal women on statins, with an adjusted hazard ratio of 1.48.2 The article promulgated the fiction that statins should be used for primary prevention in women. The first study the author reviewed when discussing the risk of diabetes in “patients” was WOSCOPS—which was an all male study.3
While statin therapy is an effective intervention for secondary prevention of cardiovascular disease in both sexes, it is important to note there is no benefit in rates of all-cause mortality or stroke in women.4 The use of statins for primary prevention in women rightly remains controversial.
Any review article on statins or any condition or drug used in both sexes should include some discussion about sex-based differences. While it might be advanced that the increased risk for diabetes, depression, cognitive impairment, and musculoskeletal pain can be justified in secondary prevention in both sexes, that argument is much, much weaker for primary prevention in women, especially since we have evidence showing a reduction in all-cause mortality and primary cardiovascular reduction in women given early postmenopausal hormone therapy.5
- Rocco M. Statins and diabetes risk: fact, fiction, and clinical implications. Cleve Clin J Med 2012; 79:883–893.
- Culver AL, Ockene IS, Balasubramanian R, et al. Statin use and risk of diabetes mellitus in postmenopausal women in the Women’s Health Initiative. Arch Intern Med 2012; 172:144–152.
- Freeman DJ, Norrie J, Sattar N, et al. Pravastatin and the development of diabetes mellitus: evience for a protective treatment effect in WOSCOPS. Circulation 2001; 103:357–362.
- Gutierrez J, Ramirez G, Rundek T, et al. Statin therapy in the prevention of recurrent cardiovascular events. A sex-based meta-analysis. Arch Intern Med 2012; 172:909–919.
- Schierbeck LL, Rejnmark L, Tofteng CL, et al. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomized trial. BMJ 2012; 345:e6409.
To the Editor: The review article “Statins and diabetes: fact, fiction, and clinical implications” 1 left out one major fact: there are sex-based differences in the statin research results, particularly a higher risk for diabetes in postmenopausal women on statins, with an adjusted hazard ratio of 1.48.2 The article promulgated the fiction that statins should be used for primary prevention in women. The first study the author reviewed when discussing the risk of diabetes in “patients” was WOSCOPS—which was an all male study.3
While statin therapy is an effective intervention for secondary prevention of cardiovascular disease in both sexes, it is important to note there is no benefit in rates of all-cause mortality or stroke in women.4 The use of statins for primary prevention in women rightly remains controversial.
Any review article on statins or any condition or drug used in both sexes should include some discussion about sex-based differences. While it might be advanced that the increased risk for diabetes, depression, cognitive impairment, and musculoskeletal pain can be justified in secondary prevention in both sexes, that argument is much, much weaker for primary prevention in women, especially since we have evidence showing a reduction in all-cause mortality and primary cardiovascular reduction in women given early postmenopausal hormone therapy.5
To the Editor: The review article “Statins and diabetes: fact, fiction, and clinical implications” 1 left out one major fact: there are sex-based differences in the statin research results, particularly a higher risk for diabetes in postmenopausal women on statins, with an adjusted hazard ratio of 1.48.2 The article promulgated the fiction that statins should be used for primary prevention in women. The first study the author reviewed when discussing the risk of diabetes in “patients” was WOSCOPS—which was an all male study.3
While statin therapy is an effective intervention for secondary prevention of cardiovascular disease in both sexes, it is important to note there is no benefit in rates of all-cause mortality or stroke in women.4 The use of statins for primary prevention in women rightly remains controversial.
Any review article on statins or any condition or drug used in both sexes should include some discussion about sex-based differences. While it might be advanced that the increased risk for diabetes, depression, cognitive impairment, and musculoskeletal pain can be justified in secondary prevention in both sexes, that argument is much, much weaker for primary prevention in women, especially since we have evidence showing a reduction in all-cause mortality and primary cardiovascular reduction in women given early postmenopausal hormone therapy.5
- Rocco M. Statins and diabetes risk: fact, fiction, and clinical implications. Cleve Clin J Med 2012; 79:883–893.
- Culver AL, Ockene IS, Balasubramanian R, et al. Statin use and risk of diabetes mellitus in postmenopausal women in the Women’s Health Initiative. Arch Intern Med 2012; 172:144–152.
- Freeman DJ, Norrie J, Sattar N, et al. Pravastatin and the development of diabetes mellitus: evience for a protective treatment effect in WOSCOPS. Circulation 2001; 103:357–362.
- Gutierrez J, Ramirez G, Rundek T, et al. Statin therapy in the prevention of recurrent cardiovascular events. A sex-based meta-analysis. Arch Intern Med 2012; 172:909–919.
- Schierbeck LL, Rejnmark L, Tofteng CL, et al. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomized trial. BMJ 2012; 345:e6409.
- Rocco M. Statins and diabetes risk: fact, fiction, and clinical implications. Cleve Clin J Med 2012; 79:883–893.
- Culver AL, Ockene IS, Balasubramanian R, et al. Statin use and risk of diabetes mellitus in postmenopausal women in the Women’s Health Initiative. Arch Intern Med 2012; 172:144–152.
- Freeman DJ, Norrie J, Sattar N, et al. Pravastatin and the development of diabetes mellitus: evience for a protective treatment effect in WOSCOPS. Circulation 2001; 103:357–362.
- Gutierrez J, Ramirez G, Rundek T, et al. Statin therapy in the prevention of recurrent cardiovascular events. A sex-based meta-analysis. Arch Intern Med 2012; 172:909–919.
- Schierbeck LL, Rejnmark L, Tofteng CL, et al. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomized trial. BMJ 2012; 345:e6409.
Update on contraceptive options: A case-based discussion
Contraceptive counseling is both an art and a science. The role of the health care provider is to determine the patient’s medical eligibility and match her preferences and lifestyle to an appropriate method for both contraceptive and potentially noncontraceptive benefits, while minimizing the risk of unintended pregnancy.
Women throughout the range of reproductive years need appropriate counseling and education on hormones, the menstrual cycle, and the efficacy of contraception as part of their routine gynecologic evaluation. Issues of access to birth control, cost, possible side effects, and actual effectiveness of methods are important to discuss.
In this paper we will discuss common clinical practice case scenarios to illustrate contraceptive counseling and management, including:
- Perimenopausal women
- Women with thrombophilia
- Women who contemplate becoming pregnant in the future
- Women with psychiatric illness
- Women with hypertension.
HALF OF ALL PREGNANCIES ARE UNPLANNED
Although many contraceptive options are available, 48% of all pregnancies in the United States are unintended.1 In 2009, the national teen birth rate was 39.1 births per 1,000 girls and women age 15 to 19 years, which was 37% lower than in 1991.2 Still, African American and Hispanic teenagers living in southern states have disproportionately higher rates.
The rate of unintended pregnancy is a little lower at the older end of the reproductive age range, but still high: 35% of all pregnancies in women over 40 years old are also unintended.2
To find out why these numbers are so high, in 2007 the US Centers for Disease Control and Prevention (CDC) conducted a survey3 that included 8,000 women reporting unintended pregnancy who had not used contraception. Of these, 39% were married. Surprisingly, more than one-third of women said they did not know they could get pregnant when they did.3
WHAT’S NEW IN CONTRACEPTION?
The “pill” was approved by the US Food and Drug Administration (FDA) more than 50 years ago, and it is still the most commonly used contraceptive method (followed by surgical sterilization). Enovid, the pill formulated by Dr. John Rock and Dr. Gregory Pincus in the 1950s, contained 150 μg of mestranol (equivalent to 90 μg of ethinyl estradiol) and 9.85 mg of norethynodrel, a very potent progestin. Our current oral contraceptive pills contain much lower hormone doses and have fewer androgenic side effects.4
In May 2010, the CDC and the World Health Organization (WHO) updated their safety guidelines for all hormonal contraceptives and the use of these agents in patients with various medical and family histories. They ranked contraceptive methods from those with no restriction to those with unacceptable risk to their use. This document can be accessed at www.cdc.gov/mmwr/preview/mmwrhtml/rr5904a13.htm.5
New developments in oral contraceptives are notably in the 19-nortestosterone derivatives, the family that includes the second-generation progestogens already available such as norgestimate (contained in Ortho-Cyclen) and norethindrone (contained in Loestrin). A newer progestin, dienogest, is available in a preparation that also contains estradiol valerate (Natazia). Drospirenone, which is similar to spironolactone, is contained in Yaz, Yasmin, and newer products that also contain levomefolate calcium (Beyaz, Safyral).
LoLoestrin Fe, which contains active pills containing 10 μg of ethinyl estradiol and 1 mg of norethindrone and placebo pills with 75 mg of ferrous fumarate, was recently approved by the FDA and offers an ultra-low dose of estrogen.
Depot medroxyprogesterone acetate now comes in a 104-mg suspension for subcutaneous injection every 3 months; it is called depo-subQ provera 104. Standard medroxyprogesterone acetate 150 mg for intramuscular injection every 3 months (Depo-Provera) is still available and has gone generic. The newer product offers the advantages of lower dose and less weight-gain. Also, it allows capable and willing patients to self-administer their contraceptives. However, it is more expensive—$ 104 per injection for a patient without insurance at Cleveland Clinic, compared with $46 for Depo-Provera and $10 for the generic intramuscular preparation for a patient with insurance.
A new option for emergency contraception, ulipristal (ella) is a progesterone antagonist-agonist available only by prescription. Taken in a single oral dose of 30 μg, it is effective for up to 120 hours after unprotected intercourse. It joins Plan B (levonorgestrel 1.5 mg in a single dose) and Next Choice (two doses of levonorgestrel 0.75 mg each), which are available over-the-counter for women age 17 years or older, and by prescription for those 16 years and younger, for use up to 72 hours after unprotected intercourse.
CASE 1: CONTRACEPTION IN PERIMENOPAUSE
A 48-year-old attorney who has had two children complains of irregular menstrual cycles and of occasional hot flashes at night that wake her from sleep. She keeps a menstrual calendar; it shows her last menstrual period was 3 months ago. She took oral contraceptives for 15 years before she had her first child. She is using condoms intermittently for contraception. Her body mass index is normal at 24 kg/m2, and she does not smoke. How do you counsel her?
A variety of hormonal options
This healthy perimenopausal woman has a variety of hormonal contraception options that would have the added benefit of regulating her menstrual cycle or suppressing it altogether. These include the levonorgestrel intrauterine system (Mirena IUS), various injectable products (such as Depo-Provera or the newer depo-subQ provera 104), contraceptive pills, the Ortho Evra contraceptive patch, and the vaginal contraceptive ring (NuvaRing). Of these, low-dose birth control pills may be the best option, as they would help with cycle control, offer contraception, and better regulate hormonal fluctuations to reduce her hot flashes.
Hormonal contraception can safely be used in women in their 30s and 40s, and often until menopause if the benefit outweighs the risk.
An estradiol valerate-dienogest oral contraceptive with a quadriphasic dosing schedule (Natazia) has been studied in women up to age 50. Although it was approved in 2010 in the United States, this pill has been used in Europe since the 1990s. The 26 active pills contain tapering doses of the active drugs, with the aim of mimicking the natural menstrual cycle, similar to triphasic pills. Estradiol valerate is a bioidentical estrogen, as it is rapidly metabolized to estradiol (E2), which is identical to 17-beta estradiol and estrone (E3) produced by the ovary. A dose of 2 mg of estradiol valerate is equivalent to 10 μg of ethinyl estradiol, which is the estrogen component in most other oral contraceptives. Low-dose pills by definition contain less than 50 μg of ethinyl estradiol. Dienogest, the progesterone component, has a 17-cyanomethyl group that accounts for its strongly progestogenic and weakly antiandrogenic properties.
All oral hormonal contraceptives can increase triglycerides by inducing the CYP450 system in the liver. However, in clinical trials, estradiol valerate-dienogest also caused other changes in lipid metabolism, such as a nonsignificant increase in high-density lipoprotein cholesterol and a slight reduction in low-density lipoprotein cholesterol and lipoprotein(a) compared with ethinyl estradiol-levonorgestrel preparations.6
It is important to advise patients that, compared with users of other oral contraceptives, estradiol valerate-dienogest users may experience fewer days of menstrual bleeding and more cycles without withdrawal bleeding. This product can therefore be an effective alternative for women with menorrhagia.
All classes of hormonal contraception carry a similar risk of side effects, such as headache, breast tenderness, nausea, irregular bleeding, and mood changes. Some women have no side effects.
CASE 2: THROMBOPHILIA
A 39-year-old woman with a body mass index of 31 kg/m2 (obese) has a history of protein S deficiency with active lower-extremity deep vein thrombosis, for which she is taking warfarin (Coumadin). She experiences menorrhagia and dysmenorrhea due to intramural fibroids and possible adenomyosis seen on transvaginal ultrasonography and confirmed by magnetic resonance imaging. Hysteroscopy reveals no polyps or submucosal fibroids. An endometrial biopsy is negative for malignancy.
She desires contraception. How do you counsel her?
Estrogens are contraindicated—except, perhaps, in select cases
This patient has many reasons for heavy bleeding. She is on warfarin, which effectively inhibits synthesis of vitamin K-dependent coagulation factor. She also has fibroids and adenomyosis. The latter is a difficult condition to control, as the location of the intramuscular glands makes treatments such as ablation, dilation and curettage, and oral agents ineffective.
All estrogen-containing formulations (pills, ring, patch) are contraindicated in women with acute venous thromboembolism (VTE) and known thrombophilia. A newer agent approved for treating menorrhagia (not for contraception), tranexamic acid (Lysteda), also carries a contraindication for patients with thrombophilia or history of VTE; however, the evidence for the latter is controversial.7
The updated CDC guidelines for the use of hormonal contraceptives state that patients who receive anticoagulation for at least 3 months and who have no history of VTE or a low risk of recurrent VTE (no evidence of active cancer, no known thrombophilia) may use estrogen-containing contraceptives in select cases (category 3—theoretical risk outweighs benefits, but not an absolute contraindication).5 Although this is not common clinical practice, select patients may benefit from menstrual cycle control while receiving anticoagulation. However, other contraceptive alternatives are preferred if possible.
Progestin-only treatments such as the Mirena IUS (if the fibroids do not distort the uterine cavity) and the etonogestrel implant (Implanon) are nonsurgical options that may reduce menorrhagia and are safer alternatives for patients with thrombophilia.
The Paragard (copper) intrauterine device would provide nonhormonal contraception without diminishing menorrhagia. Obviously, barrier methods (which are less effective than hormonal contraception) can be suggested for contraception alone. A viable option for women finished with childbearing is hysterectomy, which provides contraceptive benefit and definitive treatment of menorrhagia due to adenomyosis.
Laboratory screening for VTE is not required before starting estrogen-containing contraceptives. However, one should take a detailed history and inquire about VTE events or a family history of recurrent VTE.
VTE rates among reproductive-age women are 4 to 5 per 10,000 women per year.8 The rate of VTE in oral contraceptive users is estimated as 9 to 10 per 10,000 women per year.9 However, rates of VTE associated with pregnancy and postpartum states are exponentially greater. Although recent studies have shown some discrepancy in rates of VTE across different classes of progestins,10,11 the absolute risk of VTE with hormonal contraceptives is very low.
In December 2011, an FDA panel voted 15 to 11 that the benefits of drospirenone-containing contraceptives (eg, Yaz, Yasmin, Beyaz, Safyral), such as preventing pregnancy, outweigh the potential risk. However, product labeling may change in the future to more accurately reflect the risk-benefit ratio. Stay tuned for better-designed trials to further assess VTE risk across progestins.
Health care providers should engage patients in an informed discussion about all risks and benefits of hormonal contraceptives and note this risk of VTE is higher in gravid women.
CASE 3: FUTURE FERTILITY
A 30-year-old surgical resident who has never been pregnant comes for her annual examination. She currently desires birth control but would like to be pregnant 1 to 2 years from now. She has no history of significant medical illness. Her body mass index is 23 kg/m2, and she takes no medications. How do you counsel her?
Many options; also consider folic acid
Effective counseling leads to patient-centered decision-making for all treatments and procedures. Contraceptive counseling should elicit the patient’s perspective about hormonal methods and educate her on efficacy, proper use, and common adverse effects.
Contraception should fit the patient’s lifestyle. Questions as simple as “Are you a good pill-taker?” or “Are you comfortable with injections?” will help you and the patient assess what will work effectively and will maintain good adherence.
Deciding on a contraceptive option that is cost-effective is crucial, particularly for many young women or adolescents. Many oral contraceptives are widely available as generic formulations for less than $10 per month. Although generic drugs are not required to be 100% bioequivalent to their brand-name counterparts, they can provide a more economical option. For a complete guide to different hormonal contraceptive formulations, we suggest Choosing a birth control method, available on the Web site of the Association of Reproductive Health Professionals at www.arhp.org/upload-Docs/choosingqrg.pdf.12
As discussed earlier, half of all pregnancies are unplanned, and so women of childbearing age should be ingesting 400 μg of folic acid daily. Debate exists as to whether Americans who eat a balanced diet need a multivitamin.13 However, there is no debate about folic acid, which is proven to prevent neural tube defects. Newer formulations of ethinyl estradiol-drospirenone (Beyaz, Safyral) now contain an active form of folic acid (levomefolate calcium 451 mg in each pill). For the above patient who needs contraception and is willing to take birth control, the addition of folic acid provides an essential element in preconception counseling.
Regardless of the current contraceptive choice, patients who actively desire pregnancy should take a prenatal vitamin that contains folic acid and iron.
In addition to combined oral contraceptives, other options for this patient include medroxyprogesterone acetate (intramuscular or subcutaneous), NuvaRing, or intrauterine devices. The Ortho Evra patch is also an option for this patient. However, since 2008 the patch has carried an FDA warning that the risk of VTE is twice as high with this product than with oral contraceptives that contain 30 μg of ethinyl estradiol plus levonorgestrel.14 Postmarketing data did not show any higher risk of VTE in patch users compared with oral contraceptive users less than 40 years of age, however.15
CASE 4: PSYCHIATRIC ILLNESS
A 21-year-old woman who has bipolar II disorder comes to your office for her annual gynecologic evaluation. She has one sexual partner and desires oral contraceptive pills. Lithium treatment has failed for her, but her condition is stable on carbamazepine (Tegretol). She asks if it is true that women can still get pregnant while on the birth control pill. How do you counsel her?
Possible interactions with psychiatric drugs
Like the woman in case 3, this patient has many options, including estrogen-containing pills, the vaginal ring, the patch, injectable contraceptives, and intrauterine devices.
Certain antiepileptic, antipsychotic, or headache medications such as carbamezapine, phenytoin (Dilantin), oxcarbazepine (Trileptal), and topiramate (Topamax) decrease levels of hormonal contraceptives by induction of the CYP450 enzymes. Conversely, it is suggested that lamotrigine (Lamictal) levels decrease by up to 49% while patients concomitantly take oral contraceptive pills, which can induce seizure activity.16 Also, antibiotics such as rifampin (Rifadin) and even herbs such as St. John’s Wort can decrease the effectiveness of hormonal contraceptives by increasing their metabolism.
On the positive side, depot medroxyprogesterone acetate raises the seizure threshold by a mechanism attributed to high levels of progestins and is a better option for epileptic patients. A bulletin of the American College of Gynecologists addresses the paucity of data on hormonal treatments in depressed patients. However, some evidence points to slight improvement of depressive symptoms after 1 year in patients who took Depo-Provera compared with those who discontinued the drug.17
The Pearl index, a measure of contraceptive efficacy
We refer to the Pearl index when answering our patients’ questions about contraceptive efficacy. The Pearl index is defined as the number of unintended pregnancies per 100 women per year. The typical (or actual) effectiveness for each contraceptive method is quoted rather than the theoretical (perfect-use) efficacy.
We suggest simplifying this discussion with patients. For example, for every 100 women using male condoms for contraception, 15 women have unintended pregnancies per year. With hormonal contraceptives (pill, patch, or ring), for every 100 women there are 8 per year with unintended pregnancy, 3 of 100 with Depo-Provera, and less than 1 in 100 using intrauterine devices or female or male sterilization.18
Efficacy decreases (and the failure rate increases) with frequency of intercourse, irregular menstrual cycles, missed pills, improper dosing, and drug-drug interactions as described above.
CASE 5: HYPERTENSION
A 33-year-old woman who has been pregnant twice experienced preeclampsia in her last pregnancy, and now her blood pressure is consistently approximately 140/90 mm Hg on multiple office visits and ambulatory monitoring. She desires contraception. How do you counsel her?
Avoid estrogen-containing products
According to the WHO and CDC guidelines,5 women with controlled or uncontrolled hypertension should not be offered combined oral contraceptives, the patch, or the ring (category 3—theoretical or proven risks outweigh the benefits, and category 4 for systolic blood pressure greater than 160 mm Hg or diastolic blood pressure greater than 100 mm Hg).
The progesterone-only pill (“mini pill”), medroxyprogesterone acetate (intramuscular or subcutaneous), Mirena IUS, the copper intrauterine device, and the etonogestrel implant are all safer options.
A small subset of patients develop elevated blood pressure after starting hormonal contraceptives. Estrogen-containing hormones can increase the liver’s output of angiotensinogen, which is a renin substrate that activates the renin-angiotensin-aldosterone system. If this becomes clinically apparent, these patients should refrain from estrogen-containing products and use progestin-only formulations as a safer alternative.
Patients with isolated elevated hypertriglyceridemia should avoid oral contraceptives. However, the patch, the ring, and progestin-only methods may be acceptable.
Diabetic patients with microvascular complications of retinopathy or nephropathy and any patient with macrovascular disease (stroke, cardiovascular disease) should not be offered estrogen-containing contraception.
- Finer LB, Henshaw SK. Disparities in rates of unintended pregnancy in the United States, 1994 and 2001. Perspect Sex Reprod Health 2006; 38:90–96.
- Centers for Disease Control and Prevention. Vital signs: teenage pregnancy—United States 1991–2009. MMWR 2011; 60:414–420. www.cdc.gov/mmwr/preview/mmwrhtml/mm6013a5.htm. Accessed January 10, 2012.
- Nettleman MD, Chung H, Brewer J, Ayoola A, Reed PL. Reasons for unprotected intercourse: analysis of the PRAMS survey. Contraception 2007; 75:361–366.
- Nelson A. New low-dose, extended-cycle pills with levonorgestrel and ethinyl estradiol: an evolutionary step on birth control. Int J Womens Health 2010; 2:99–106.
- US Centers for Disease Control and Prevention. Appendix L. Summary of classifications for hormonal contraceptive methods and intrauterine devices. MMWR 2010; 59( RR04):76–81. www.cdc.gov/mmwr/preview/mmwrhtml/rr5904a13.htm. Accessed January 10, 2012.
- Wiegratz L, Lee JH, Kutchera E, et al. Effect of dienogest-containing oral contraceptives in lipid metabolism. Contraception 2002; 65:223–229.
- Sundström A, Seaman H, Kieler H, Alfredsson L. The risk of venous thromboembolism associated with the use of tranexamic acid and other drugs used to treat menorrhagia: a case-control study using the General Practice Research Database. Br J Obstet Gynecol 2009; 116:91–97.
- Heinemann L, Dinger JC. Range of published estimates of venous thromboembolism incidence in young women. Contraception 2007; 75:328–336.
- Dinger JC, Heinemann LA, Kühl-Habich D. The safety of a drospirenone-containing oral contraceptive: final results from the European Active Surveillance Study on oral contraceptives based on 142,475 women-years of observation. Contraception 2007; 75:344–354.
- Parkin L, Sharples K, Hernandez RK, Jick SS. Risk of venous thromboembolism in users of oral contraceptives containing drospirenone or levonorgestrel: nested case-control study based on UK General Practice Research Database. BMJ 2011; 342:d2139.
- Jick SS, Hernandez RK. Risk of non-fatal venous thromboembolism in women using oral contraceptives containing drospirenone compared with women using oral contraceptives containing levonorgestrel: case-control study using United States claims data. BMJ 2011; 342:d2151.
- Association of Reproductive Health Professionals (ARHP). Choosing a birth control method. www.arhp.org/uploadDocs/choosingqrg.pdf. Accessed January 10, 2012.
- Caballero B. Should healthy people take a multivitamin? Cleve Clin J Med 2010; 77:656–657.
- US Food and Drug Administration. Ortho Evra questions and answers (1/18/2008). www.fda.gov/Drugs/DrugSafety/Postmarket-DrugSafetyInformationforPatientsandProviders/ucm110403.htm. Accessed January 10, 2012.
- Jick SS, Hagberg KW, Hernandez RK, Kaye JA. Postmarketing study of ORTHO EVRA and levonorgestrel oral contraceptives containing hormonal contraceptives with 30 mcg of ethinyl estradiol in relation to nonfatal venous thromboembolism. Contraception 2010; 81:16–21.
- Sabers A, Ohman I, Christensen J, Tomson T. Oral contraceptives reduce lamotrigine plasma levels. Neurology 2003; 61:570–571.
- Westhoff C, Truman C, Kalmuss D, et al; Depressive symptoms and Depo-Provera. Contraception 1998; 57:237–240.
- Trusell Wynn LL. Reducing unintended pregnancy in the United States. Contraception 2008; 77:1–5.
Contraceptive counseling is both an art and a science. The role of the health care provider is to determine the patient’s medical eligibility and match her preferences and lifestyle to an appropriate method for both contraceptive and potentially noncontraceptive benefits, while minimizing the risk of unintended pregnancy.
Women throughout the range of reproductive years need appropriate counseling and education on hormones, the menstrual cycle, and the efficacy of contraception as part of their routine gynecologic evaluation. Issues of access to birth control, cost, possible side effects, and actual effectiveness of methods are important to discuss.
In this paper we will discuss common clinical practice case scenarios to illustrate contraceptive counseling and management, including:
- Perimenopausal women
- Women with thrombophilia
- Women who contemplate becoming pregnant in the future
- Women with psychiatric illness
- Women with hypertension.
HALF OF ALL PREGNANCIES ARE UNPLANNED
Although many contraceptive options are available, 48% of all pregnancies in the United States are unintended.1 In 2009, the national teen birth rate was 39.1 births per 1,000 girls and women age 15 to 19 years, which was 37% lower than in 1991.2 Still, African American and Hispanic teenagers living in southern states have disproportionately higher rates.
The rate of unintended pregnancy is a little lower at the older end of the reproductive age range, but still high: 35% of all pregnancies in women over 40 years old are also unintended.2
To find out why these numbers are so high, in 2007 the US Centers for Disease Control and Prevention (CDC) conducted a survey3 that included 8,000 women reporting unintended pregnancy who had not used contraception. Of these, 39% were married. Surprisingly, more than one-third of women said they did not know they could get pregnant when they did.3
WHAT’S NEW IN CONTRACEPTION?
The “pill” was approved by the US Food and Drug Administration (FDA) more than 50 years ago, and it is still the most commonly used contraceptive method (followed by surgical sterilization). Enovid, the pill formulated by Dr. John Rock and Dr. Gregory Pincus in the 1950s, contained 150 μg of mestranol (equivalent to 90 μg of ethinyl estradiol) and 9.85 mg of norethynodrel, a very potent progestin. Our current oral contraceptive pills contain much lower hormone doses and have fewer androgenic side effects.4
In May 2010, the CDC and the World Health Organization (WHO) updated their safety guidelines for all hormonal contraceptives and the use of these agents in patients with various medical and family histories. They ranked contraceptive methods from those with no restriction to those with unacceptable risk to their use. This document can be accessed at www.cdc.gov/mmwr/preview/mmwrhtml/rr5904a13.htm.5
New developments in oral contraceptives are notably in the 19-nortestosterone derivatives, the family that includes the second-generation progestogens already available such as norgestimate (contained in Ortho-Cyclen) and norethindrone (contained in Loestrin). A newer progestin, dienogest, is available in a preparation that also contains estradiol valerate (Natazia). Drospirenone, which is similar to spironolactone, is contained in Yaz, Yasmin, and newer products that also contain levomefolate calcium (Beyaz, Safyral).
LoLoestrin Fe, which contains active pills containing 10 μg of ethinyl estradiol and 1 mg of norethindrone and placebo pills with 75 mg of ferrous fumarate, was recently approved by the FDA and offers an ultra-low dose of estrogen.
Depot medroxyprogesterone acetate now comes in a 104-mg suspension for subcutaneous injection every 3 months; it is called depo-subQ provera 104. Standard medroxyprogesterone acetate 150 mg for intramuscular injection every 3 months (Depo-Provera) is still available and has gone generic. The newer product offers the advantages of lower dose and less weight-gain. Also, it allows capable and willing patients to self-administer their contraceptives. However, it is more expensive—$ 104 per injection for a patient without insurance at Cleveland Clinic, compared with $46 for Depo-Provera and $10 for the generic intramuscular preparation for a patient with insurance.
A new option for emergency contraception, ulipristal (ella) is a progesterone antagonist-agonist available only by prescription. Taken in a single oral dose of 30 μg, it is effective for up to 120 hours after unprotected intercourse. It joins Plan B (levonorgestrel 1.5 mg in a single dose) and Next Choice (two doses of levonorgestrel 0.75 mg each), which are available over-the-counter for women age 17 years or older, and by prescription for those 16 years and younger, for use up to 72 hours after unprotected intercourse.
CASE 1: CONTRACEPTION IN PERIMENOPAUSE
A 48-year-old attorney who has had two children complains of irregular menstrual cycles and of occasional hot flashes at night that wake her from sleep. She keeps a menstrual calendar; it shows her last menstrual period was 3 months ago. She took oral contraceptives for 15 years before she had her first child. She is using condoms intermittently for contraception. Her body mass index is normal at 24 kg/m2, and she does not smoke. How do you counsel her?
A variety of hormonal options
This healthy perimenopausal woman has a variety of hormonal contraception options that would have the added benefit of regulating her menstrual cycle or suppressing it altogether. These include the levonorgestrel intrauterine system (Mirena IUS), various injectable products (such as Depo-Provera or the newer depo-subQ provera 104), contraceptive pills, the Ortho Evra contraceptive patch, and the vaginal contraceptive ring (NuvaRing). Of these, low-dose birth control pills may be the best option, as they would help with cycle control, offer contraception, and better regulate hormonal fluctuations to reduce her hot flashes.
Hormonal contraception can safely be used in women in their 30s and 40s, and often until menopause if the benefit outweighs the risk.
An estradiol valerate-dienogest oral contraceptive with a quadriphasic dosing schedule (Natazia) has been studied in women up to age 50. Although it was approved in 2010 in the United States, this pill has been used in Europe since the 1990s. The 26 active pills contain tapering doses of the active drugs, with the aim of mimicking the natural menstrual cycle, similar to triphasic pills. Estradiol valerate is a bioidentical estrogen, as it is rapidly metabolized to estradiol (E2), which is identical to 17-beta estradiol and estrone (E3) produced by the ovary. A dose of 2 mg of estradiol valerate is equivalent to 10 μg of ethinyl estradiol, which is the estrogen component in most other oral contraceptives. Low-dose pills by definition contain less than 50 μg of ethinyl estradiol. Dienogest, the progesterone component, has a 17-cyanomethyl group that accounts for its strongly progestogenic and weakly antiandrogenic properties.
All oral hormonal contraceptives can increase triglycerides by inducing the CYP450 system in the liver. However, in clinical trials, estradiol valerate-dienogest also caused other changes in lipid metabolism, such as a nonsignificant increase in high-density lipoprotein cholesterol and a slight reduction in low-density lipoprotein cholesterol and lipoprotein(a) compared with ethinyl estradiol-levonorgestrel preparations.6
It is important to advise patients that, compared with users of other oral contraceptives, estradiol valerate-dienogest users may experience fewer days of menstrual bleeding and more cycles without withdrawal bleeding. This product can therefore be an effective alternative for women with menorrhagia.
All classes of hormonal contraception carry a similar risk of side effects, such as headache, breast tenderness, nausea, irregular bleeding, and mood changes. Some women have no side effects.
CASE 2: THROMBOPHILIA
A 39-year-old woman with a body mass index of 31 kg/m2 (obese) has a history of protein S deficiency with active lower-extremity deep vein thrombosis, for which she is taking warfarin (Coumadin). She experiences menorrhagia and dysmenorrhea due to intramural fibroids and possible adenomyosis seen on transvaginal ultrasonography and confirmed by magnetic resonance imaging. Hysteroscopy reveals no polyps or submucosal fibroids. An endometrial biopsy is negative for malignancy.
She desires contraception. How do you counsel her?
Estrogens are contraindicated—except, perhaps, in select cases
This patient has many reasons for heavy bleeding. She is on warfarin, which effectively inhibits synthesis of vitamin K-dependent coagulation factor. She also has fibroids and adenomyosis. The latter is a difficult condition to control, as the location of the intramuscular glands makes treatments such as ablation, dilation and curettage, and oral agents ineffective.
All estrogen-containing formulations (pills, ring, patch) are contraindicated in women with acute venous thromboembolism (VTE) and known thrombophilia. A newer agent approved for treating menorrhagia (not for contraception), tranexamic acid (Lysteda), also carries a contraindication for patients with thrombophilia or history of VTE; however, the evidence for the latter is controversial.7
The updated CDC guidelines for the use of hormonal contraceptives state that patients who receive anticoagulation for at least 3 months and who have no history of VTE or a low risk of recurrent VTE (no evidence of active cancer, no known thrombophilia) may use estrogen-containing contraceptives in select cases (category 3—theoretical risk outweighs benefits, but not an absolute contraindication).5 Although this is not common clinical practice, select patients may benefit from menstrual cycle control while receiving anticoagulation. However, other contraceptive alternatives are preferred if possible.
Progestin-only treatments such as the Mirena IUS (if the fibroids do not distort the uterine cavity) and the etonogestrel implant (Implanon) are nonsurgical options that may reduce menorrhagia and are safer alternatives for patients with thrombophilia.
The Paragard (copper) intrauterine device would provide nonhormonal contraception without diminishing menorrhagia. Obviously, barrier methods (which are less effective than hormonal contraception) can be suggested for contraception alone. A viable option for women finished with childbearing is hysterectomy, which provides contraceptive benefit and definitive treatment of menorrhagia due to adenomyosis.
Laboratory screening for VTE is not required before starting estrogen-containing contraceptives. However, one should take a detailed history and inquire about VTE events or a family history of recurrent VTE.
VTE rates among reproductive-age women are 4 to 5 per 10,000 women per year.8 The rate of VTE in oral contraceptive users is estimated as 9 to 10 per 10,000 women per year.9 However, rates of VTE associated with pregnancy and postpartum states are exponentially greater. Although recent studies have shown some discrepancy in rates of VTE across different classes of progestins,10,11 the absolute risk of VTE with hormonal contraceptives is very low.
In December 2011, an FDA panel voted 15 to 11 that the benefits of drospirenone-containing contraceptives (eg, Yaz, Yasmin, Beyaz, Safyral), such as preventing pregnancy, outweigh the potential risk. However, product labeling may change in the future to more accurately reflect the risk-benefit ratio. Stay tuned for better-designed trials to further assess VTE risk across progestins.
Health care providers should engage patients in an informed discussion about all risks and benefits of hormonal contraceptives and note this risk of VTE is higher in gravid women.
CASE 3: FUTURE FERTILITY
A 30-year-old surgical resident who has never been pregnant comes for her annual examination. She currently desires birth control but would like to be pregnant 1 to 2 years from now. She has no history of significant medical illness. Her body mass index is 23 kg/m2, and she takes no medications. How do you counsel her?
Many options; also consider folic acid
Effective counseling leads to patient-centered decision-making for all treatments and procedures. Contraceptive counseling should elicit the patient’s perspective about hormonal methods and educate her on efficacy, proper use, and common adverse effects.
Contraception should fit the patient’s lifestyle. Questions as simple as “Are you a good pill-taker?” or “Are you comfortable with injections?” will help you and the patient assess what will work effectively and will maintain good adherence.
Deciding on a contraceptive option that is cost-effective is crucial, particularly for many young women or adolescents. Many oral contraceptives are widely available as generic formulations for less than $10 per month. Although generic drugs are not required to be 100% bioequivalent to their brand-name counterparts, they can provide a more economical option. For a complete guide to different hormonal contraceptive formulations, we suggest Choosing a birth control method, available on the Web site of the Association of Reproductive Health Professionals at www.arhp.org/upload-Docs/choosingqrg.pdf.12
As discussed earlier, half of all pregnancies are unplanned, and so women of childbearing age should be ingesting 400 μg of folic acid daily. Debate exists as to whether Americans who eat a balanced diet need a multivitamin.13 However, there is no debate about folic acid, which is proven to prevent neural tube defects. Newer formulations of ethinyl estradiol-drospirenone (Beyaz, Safyral) now contain an active form of folic acid (levomefolate calcium 451 mg in each pill). For the above patient who needs contraception and is willing to take birth control, the addition of folic acid provides an essential element in preconception counseling.
Regardless of the current contraceptive choice, patients who actively desire pregnancy should take a prenatal vitamin that contains folic acid and iron.
In addition to combined oral contraceptives, other options for this patient include medroxyprogesterone acetate (intramuscular or subcutaneous), NuvaRing, or intrauterine devices. The Ortho Evra patch is also an option for this patient. However, since 2008 the patch has carried an FDA warning that the risk of VTE is twice as high with this product than with oral contraceptives that contain 30 μg of ethinyl estradiol plus levonorgestrel.14 Postmarketing data did not show any higher risk of VTE in patch users compared with oral contraceptive users less than 40 years of age, however.15
CASE 4: PSYCHIATRIC ILLNESS
A 21-year-old woman who has bipolar II disorder comes to your office for her annual gynecologic evaluation. She has one sexual partner and desires oral contraceptive pills. Lithium treatment has failed for her, but her condition is stable on carbamazepine (Tegretol). She asks if it is true that women can still get pregnant while on the birth control pill. How do you counsel her?
Possible interactions with psychiatric drugs
Like the woman in case 3, this patient has many options, including estrogen-containing pills, the vaginal ring, the patch, injectable contraceptives, and intrauterine devices.
Certain antiepileptic, antipsychotic, or headache medications such as carbamezapine, phenytoin (Dilantin), oxcarbazepine (Trileptal), and topiramate (Topamax) decrease levels of hormonal contraceptives by induction of the CYP450 enzymes. Conversely, it is suggested that lamotrigine (Lamictal) levels decrease by up to 49% while patients concomitantly take oral contraceptive pills, which can induce seizure activity.16 Also, antibiotics such as rifampin (Rifadin) and even herbs such as St. John’s Wort can decrease the effectiveness of hormonal contraceptives by increasing their metabolism.
On the positive side, depot medroxyprogesterone acetate raises the seizure threshold by a mechanism attributed to high levels of progestins and is a better option for epileptic patients. A bulletin of the American College of Gynecologists addresses the paucity of data on hormonal treatments in depressed patients. However, some evidence points to slight improvement of depressive symptoms after 1 year in patients who took Depo-Provera compared with those who discontinued the drug.17
The Pearl index, a measure of contraceptive efficacy
We refer to the Pearl index when answering our patients’ questions about contraceptive efficacy. The Pearl index is defined as the number of unintended pregnancies per 100 women per year. The typical (or actual) effectiveness for each contraceptive method is quoted rather than the theoretical (perfect-use) efficacy.
We suggest simplifying this discussion with patients. For example, for every 100 women using male condoms for contraception, 15 women have unintended pregnancies per year. With hormonal contraceptives (pill, patch, or ring), for every 100 women there are 8 per year with unintended pregnancy, 3 of 100 with Depo-Provera, and less than 1 in 100 using intrauterine devices or female or male sterilization.18
Efficacy decreases (and the failure rate increases) with frequency of intercourse, irregular menstrual cycles, missed pills, improper dosing, and drug-drug interactions as described above.
CASE 5: HYPERTENSION
A 33-year-old woman who has been pregnant twice experienced preeclampsia in her last pregnancy, and now her blood pressure is consistently approximately 140/90 mm Hg on multiple office visits and ambulatory monitoring. She desires contraception. How do you counsel her?
Avoid estrogen-containing products
According to the WHO and CDC guidelines,5 women with controlled or uncontrolled hypertension should not be offered combined oral contraceptives, the patch, or the ring (category 3—theoretical or proven risks outweigh the benefits, and category 4 for systolic blood pressure greater than 160 mm Hg or diastolic blood pressure greater than 100 mm Hg).
The progesterone-only pill (“mini pill”), medroxyprogesterone acetate (intramuscular or subcutaneous), Mirena IUS, the copper intrauterine device, and the etonogestrel implant are all safer options.
A small subset of patients develop elevated blood pressure after starting hormonal contraceptives. Estrogen-containing hormones can increase the liver’s output of angiotensinogen, which is a renin substrate that activates the renin-angiotensin-aldosterone system. If this becomes clinically apparent, these patients should refrain from estrogen-containing products and use progestin-only formulations as a safer alternative.
Patients with isolated elevated hypertriglyceridemia should avoid oral contraceptives. However, the patch, the ring, and progestin-only methods may be acceptable.
Diabetic patients with microvascular complications of retinopathy or nephropathy and any patient with macrovascular disease (stroke, cardiovascular disease) should not be offered estrogen-containing contraception.
Contraceptive counseling is both an art and a science. The role of the health care provider is to determine the patient’s medical eligibility and match her preferences and lifestyle to an appropriate method for both contraceptive and potentially noncontraceptive benefits, while minimizing the risk of unintended pregnancy.
Women throughout the range of reproductive years need appropriate counseling and education on hormones, the menstrual cycle, and the efficacy of contraception as part of their routine gynecologic evaluation. Issues of access to birth control, cost, possible side effects, and actual effectiveness of methods are important to discuss.
In this paper we will discuss common clinical practice case scenarios to illustrate contraceptive counseling and management, including:
- Perimenopausal women
- Women with thrombophilia
- Women who contemplate becoming pregnant in the future
- Women with psychiatric illness
- Women with hypertension.
HALF OF ALL PREGNANCIES ARE UNPLANNED
Although many contraceptive options are available, 48% of all pregnancies in the United States are unintended.1 In 2009, the national teen birth rate was 39.1 births per 1,000 girls and women age 15 to 19 years, which was 37% lower than in 1991.2 Still, African American and Hispanic teenagers living in southern states have disproportionately higher rates.
The rate of unintended pregnancy is a little lower at the older end of the reproductive age range, but still high: 35% of all pregnancies in women over 40 years old are also unintended.2
To find out why these numbers are so high, in 2007 the US Centers for Disease Control and Prevention (CDC) conducted a survey3 that included 8,000 women reporting unintended pregnancy who had not used contraception. Of these, 39% were married. Surprisingly, more than one-third of women said they did not know they could get pregnant when they did.3
WHAT’S NEW IN CONTRACEPTION?
The “pill” was approved by the US Food and Drug Administration (FDA) more than 50 years ago, and it is still the most commonly used contraceptive method (followed by surgical sterilization). Enovid, the pill formulated by Dr. John Rock and Dr. Gregory Pincus in the 1950s, contained 150 μg of mestranol (equivalent to 90 μg of ethinyl estradiol) and 9.85 mg of norethynodrel, a very potent progestin. Our current oral contraceptive pills contain much lower hormone doses and have fewer androgenic side effects.4
In May 2010, the CDC and the World Health Organization (WHO) updated their safety guidelines for all hormonal contraceptives and the use of these agents in patients with various medical and family histories. They ranked contraceptive methods from those with no restriction to those with unacceptable risk to their use. This document can be accessed at www.cdc.gov/mmwr/preview/mmwrhtml/rr5904a13.htm.5
New developments in oral contraceptives are notably in the 19-nortestosterone derivatives, the family that includes the second-generation progestogens already available such as norgestimate (contained in Ortho-Cyclen) and norethindrone (contained in Loestrin). A newer progestin, dienogest, is available in a preparation that also contains estradiol valerate (Natazia). Drospirenone, which is similar to spironolactone, is contained in Yaz, Yasmin, and newer products that also contain levomefolate calcium (Beyaz, Safyral).
LoLoestrin Fe, which contains active pills containing 10 μg of ethinyl estradiol and 1 mg of norethindrone and placebo pills with 75 mg of ferrous fumarate, was recently approved by the FDA and offers an ultra-low dose of estrogen.
Depot medroxyprogesterone acetate now comes in a 104-mg suspension for subcutaneous injection every 3 months; it is called depo-subQ provera 104. Standard medroxyprogesterone acetate 150 mg for intramuscular injection every 3 months (Depo-Provera) is still available and has gone generic. The newer product offers the advantages of lower dose and less weight-gain. Also, it allows capable and willing patients to self-administer their contraceptives. However, it is more expensive—$ 104 per injection for a patient without insurance at Cleveland Clinic, compared with $46 for Depo-Provera and $10 for the generic intramuscular preparation for a patient with insurance.
A new option for emergency contraception, ulipristal (ella) is a progesterone antagonist-agonist available only by prescription. Taken in a single oral dose of 30 μg, it is effective for up to 120 hours after unprotected intercourse. It joins Plan B (levonorgestrel 1.5 mg in a single dose) and Next Choice (two doses of levonorgestrel 0.75 mg each), which are available over-the-counter for women age 17 years or older, and by prescription for those 16 years and younger, for use up to 72 hours after unprotected intercourse.
CASE 1: CONTRACEPTION IN PERIMENOPAUSE
A 48-year-old attorney who has had two children complains of irregular menstrual cycles and of occasional hot flashes at night that wake her from sleep. She keeps a menstrual calendar; it shows her last menstrual period was 3 months ago. She took oral contraceptives for 15 years before she had her first child. She is using condoms intermittently for contraception. Her body mass index is normal at 24 kg/m2, and she does not smoke. How do you counsel her?
A variety of hormonal options
This healthy perimenopausal woman has a variety of hormonal contraception options that would have the added benefit of regulating her menstrual cycle or suppressing it altogether. These include the levonorgestrel intrauterine system (Mirena IUS), various injectable products (such as Depo-Provera or the newer depo-subQ provera 104), contraceptive pills, the Ortho Evra contraceptive patch, and the vaginal contraceptive ring (NuvaRing). Of these, low-dose birth control pills may be the best option, as they would help with cycle control, offer contraception, and better regulate hormonal fluctuations to reduce her hot flashes.
Hormonal contraception can safely be used in women in their 30s and 40s, and often until menopause if the benefit outweighs the risk.
An estradiol valerate-dienogest oral contraceptive with a quadriphasic dosing schedule (Natazia) has been studied in women up to age 50. Although it was approved in 2010 in the United States, this pill has been used in Europe since the 1990s. The 26 active pills contain tapering doses of the active drugs, with the aim of mimicking the natural menstrual cycle, similar to triphasic pills. Estradiol valerate is a bioidentical estrogen, as it is rapidly metabolized to estradiol (E2), which is identical to 17-beta estradiol and estrone (E3) produced by the ovary. A dose of 2 mg of estradiol valerate is equivalent to 10 μg of ethinyl estradiol, which is the estrogen component in most other oral contraceptives. Low-dose pills by definition contain less than 50 μg of ethinyl estradiol. Dienogest, the progesterone component, has a 17-cyanomethyl group that accounts for its strongly progestogenic and weakly antiandrogenic properties.
All oral hormonal contraceptives can increase triglycerides by inducing the CYP450 system in the liver. However, in clinical trials, estradiol valerate-dienogest also caused other changes in lipid metabolism, such as a nonsignificant increase in high-density lipoprotein cholesterol and a slight reduction in low-density lipoprotein cholesterol and lipoprotein(a) compared with ethinyl estradiol-levonorgestrel preparations.6
It is important to advise patients that, compared with users of other oral contraceptives, estradiol valerate-dienogest users may experience fewer days of menstrual bleeding and more cycles without withdrawal bleeding. This product can therefore be an effective alternative for women with menorrhagia.
All classes of hormonal contraception carry a similar risk of side effects, such as headache, breast tenderness, nausea, irregular bleeding, and mood changes. Some women have no side effects.
CASE 2: THROMBOPHILIA
A 39-year-old woman with a body mass index of 31 kg/m2 (obese) has a history of protein S deficiency with active lower-extremity deep vein thrombosis, for which she is taking warfarin (Coumadin). She experiences menorrhagia and dysmenorrhea due to intramural fibroids and possible adenomyosis seen on transvaginal ultrasonography and confirmed by magnetic resonance imaging. Hysteroscopy reveals no polyps or submucosal fibroids. An endometrial biopsy is negative for malignancy.
She desires contraception. How do you counsel her?
Estrogens are contraindicated—except, perhaps, in select cases
This patient has many reasons for heavy bleeding. She is on warfarin, which effectively inhibits synthesis of vitamin K-dependent coagulation factor. She also has fibroids and adenomyosis. The latter is a difficult condition to control, as the location of the intramuscular glands makes treatments such as ablation, dilation and curettage, and oral agents ineffective.
All estrogen-containing formulations (pills, ring, patch) are contraindicated in women with acute venous thromboembolism (VTE) and known thrombophilia. A newer agent approved for treating menorrhagia (not for contraception), tranexamic acid (Lysteda), also carries a contraindication for patients with thrombophilia or history of VTE; however, the evidence for the latter is controversial.7
The updated CDC guidelines for the use of hormonal contraceptives state that patients who receive anticoagulation for at least 3 months and who have no history of VTE or a low risk of recurrent VTE (no evidence of active cancer, no known thrombophilia) may use estrogen-containing contraceptives in select cases (category 3—theoretical risk outweighs benefits, but not an absolute contraindication).5 Although this is not common clinical practice, select patients may benefit from menstrual cycle control while receiving anticoagulation. However, other contraceptive alternatives are preferred if possible.
Progestin-only treatments such as the Mirena IUS (if the fibroids do not distort the uterine cavity) and the etonogestrel implant (Implanon) are nonsurgical options that may reduce menorrhagia and are safer alternatives for patients with thrombophilia.
The Paragard (copper) intrauterine device would provide nonhormonal contraception without diminishing menorrhagia. Obviously, barrier methods (which are less effective than hormonal contraception) can be suggested for contraception alone. A viable option for women finished with childbearing is hysterectomy, which provides contraceptive benefit and definitive treatment of menorrhagia due to adenomyosis.
Laboratory screening for VTE is not required before starting estrogen-containing contraceptives. However, one should take a detailed history and inquire about VTE events or a family history of recurrent VTE.
VTE rates among reproductive-age women are 4 to 5 per 10,000 women per year.8 The rate of VTE in oral contraceptive users is estimated as 9 to 10 per 10,000 women per year.9 However, rates of VTE associated with pregnancy and postpartum states are exponentially greater. Although recent studies have shown some discrepancy in rates of VTE across different classes of progestins,10,11 the absolute risk of VTE with hormonal contraceptives is very low.
In December 2011, an FDA panel voted 15 to 11 that the benefits of drospirenone-containing contraceptives (eg, Yaz, Yasmin, Beyaz, Safyral), such as preventing pregnancy, outweigh the potential risk. However, product labeling may change in the future to more accurately reflect the risk-benefit ratio. Stay tuned for better-designed trials to further assess VTE risk across progestins.
Health care providers should engage patients in an informed discussion about all risks and benefits of hormonal contraceptives and note this risk of VTE is higher in gravid women.
CASE 3: FUTURE FERTILITY
A 30-year-old surgical resident who has never been pregnant comes for her annual examination. She currently desires birth control but would like to be pregnant 1 to 2 years from now. She has no history of significant medical illness. Her body mass index is 23 kg/m2, and she takes no medications. How do you counsel her?
Many options; also consider folic acid
Effective counseling leads to patient-centered decision-making for all treatments and procedures. Contraceptive counseling should elicit the patient’s perspective about hormonal methods and educate her on efficacy, proper use, and common adverse effects.
Contraception should fit the patient’s lifestyle. Questions as simple as “Are you a good pill-taker?” or “Are you comfortable with injections?” will help you and the patient assess what will work effectively and will maintain good adherence.
Deciding on a contraceptive option that is cost-effective is crucial, particularly for many young women or adolescents. Many oral contraceptives are widely available as generic formulations for less than $10 per month. Although generic drugs are not required to be 100% bioequivalent to their brand-name counterparts, they can provide a more economical option. For a complete guide to different hormonal contraceptive formulations, we suggest Choosing a birth control method, available on the Web site of the Association of Reproductive Health Professionals at www.arhp.org/upload-Docs/choosingqrg.pdf.12
As discussed earlier, half of all pregnancies are unplanned, and so women of childbearing age should be ingesting 400 μg of folic acid daily. Debate exists as to whether Americans who eat a balanced diet need a multivitamin.13 However, there is no debate about folic acid, which is proven to prevent neural tube defects. Newer formulations of ethinyl estradiol-drospirenone (Beyaz, Safyral) now contain an active form of folic acid (levomefolate calcium 451 mg in each pill). For the above patient who needs contraception and is willing to take birth control, the addition of folic acid provides an essential element in preconception counseling.
Regardless of the current contraceptive choice, patients who actively desire pregnancy should take a prenatal vitamin that contains folic acid and iron.
In addition to combined oral contraceptives, other options for this patient include medroxyprogesterone acetate (intramuscular or subcutaneous), NuvaRing, or intrauterine devices. The Ortho Evra patch is also an option for this patient. However, since 2008 the patch has carried an FDA warning that the risk of VTE is twice as high with this product than with oral contraceptives that contain 30 μg of ethinyl estradiol plus levonorgestrel.14 Postmarketing data did not show any higher risk of VTE in patch users compared with oral contraceptive users less than 40 years of age, however.15
CASE 4: PSYCHIATRIC ILLNESS
A 21-year-old woman who has bipolar II disorder comes to your office for her annual gynecologic evaluation. She has one sexual partner and desires oral contraceptive pills. Lithium treatment has failed for her, but her condition is stable on carbamazepine (Tegretol). She asks if it is true that women can still get pregnant while on the birth control pill. How do you counsel her?
Possible interactions with psychiatric drugs
Like the woman in case 3, this patient has many options, including estrogen-containing pills, the vaginal ring, the patch, injectable contraceptives, and intrauterine devices.
Certain antiepileptic, antipsychotic, or headache medications such as carbamezapine, phenytoin (Dilantin), oxcarbazepine (Trileptal), and topiramate (Topamax) decrease levels of hormonal contraceptives by induction of the CYP450 enzymes. Conversely, it is suggested that lamotrigine (Lamictal) levels decrease by up to 49% while patients concomitantly take oral contraceptive pills, which can induce seizure activity.16 Also, antibiotics such as rifampin (Rifadin) and even herbs such as St. John’s Wort can decrease the effectiveness of hormonal contraceptives by increasing their metabolism.
On the positive side, depot medroxyprogesterone acetate raises the seizure threshold by a mechanism attributed to high levels of progestins and is a better option for epileptic patients. A bulletin of the American College of Gynecologists addresses the paucity of data on hormonal treatments in depressed patients. However, some evidence points to slight improvement of depressive symptoms after 1 year in patients who took Depo-Provera compared with those who discontinued the drug.17
The Pearl index, a measure of contraceptive efficacy
We refer to the Pearl index when answering our patients’ questions about contraceptive efficacy. The Pearl index is defined as the number of unintended pregnancies per 100 women per year. The typical (or actual) effectiveness for each contraceptive method is quoted rather than the theoretical (perfect-use) efficacy.
We suggest simplifying this discussion with patients. For example, for every 100 women using male condoms for contraception, 15 women have unintended pregnancies per year. With hormonal contraceptives (pill, patch, or ring), for every 100 women there are 8 per year with unintended pregnancy, 3 of 100 with Depo-Provera, and less than 1 in 100 using intrauterine devices or female or male sterilization.18
Efficacy decreases (and the failure rate increases) with frequency of intercourse, irregular menstrual cycles, missed pills, improper dosing, and drug-drug interactions as described above.
CASE 5: HYPERTENSION
A 33-year-old woman who has been pregnant twice experienced preeclampsia in her last pregnancy, and now her blood pressure is consistently approximately 140/90 mm Hg on multiple office visits and ambulatory monitoring. She desires contraception. How do you counsel her?
Avoid estrogen-containing products
According to the WHO and CDC guidelines,5 women with controlled or uncontrolled hypertension should not be offered combined oral contraceptives, the patch, or the ring (category 3—theoretical or proven risks outweigh the benefits, and category 4 for systolic blood pressure greater than 160 mm Hg or diastolic blood pressure greater than 100 mm Hg).
The progesterone-only pill (“mini pill”), medroxyprogesterone acetate (intramuscular or subcutaneous), Mirena IUS, the copper intrauterine device, and the etonogestrel implant are all safer options.
A small subset of patients develop elevated blood pressure after starting hormonal contraceptives. Estrogen-containing hormones can increase the liver’s output of angiotensinogen, which is a renin substrate that activates the renin-angiotensin-aldosterone system. If this becomes clinically apparent, these patients should refrain from estrogen-containing products and use progestin-only formulations as a safer alternative.
Patients with isolated elevated hypertriglyceridemia should avoid oral contraceptives. However, the patch, the ring, and progestin-only methods may be acceptable.
Diabetic patients with microvascular complications of retinopathy or nephropathy and any patient with macrovascular disease (stroke, cardiovascular disease) should not be offered estrogen-containing contraception.
- Finer LB, Henshaw SK. Disparities in rates of unintended pregnancy in the United States, 1994 and 2001. Perspect Sex Reprod Health 2006; 38:90–96.
- Centers for Disease Control and Prevention. Vital signs: teenage pregnancy—United States 1991–2009. MMWR 2011; 60:414–420. www.cdc.gov/mmwr/preview/mmwrhtml/mm6013a5.htm. Accessed January 10, 2012.
- Nettleman MD, Chung H, Brewer J, Ayoola A, Reed PL. Reasons for unprotected intercourse: analysis of the PRAMS survey. Contraception 2007; 75:361–366.
- Nelson A. New low-dose, extended-cycle pills with levonorgestrel and ethinyl estradiol: an evolutionary step on birth control. Int J Womens Health 2010; 2:99–106.
- US Centers for Disease Control and Prevention. Appendix L. Summary of classifications for hormonal contraceptive methods and intrauterine devices. MMWR 2010; 59( RR04):76–81. www.cdc.gov/mmwr/preview/mmwrhtml/rr5904a13.htm. Accessed January 10, 2012.
- Wiegratz L, Lee JH, Kutchera E, et al. Effect of dienogest-containing oral contraceptives in lipid metabolism. Contraception 2002; 65:223–229.
- Sundström A, Seaman H, Kieler H, Alfredsson L. The risk of venous thromboembolism associated with the use of tranexamic acid and other drugs used to treat menorrhagia: a case-control study using the General Practice Research Database. Br J Obstet Gynecol 2009; 116:91–97.
- Heinemann L, Dinger JC. Range of published estimates of venous thromboembolism incidence in young women. Contraception 2007; 75:328–336.
- Dinger JC, Heinemann LA, Kühl-Habich D. The safety of a drospirenone-containing oral contraceptive: final results from the European Active Surveillance Study on oral contraceptives based on 142,475 women-years of observation. Contraception 2007; 75:344–354.
- Parkin L, Sharples K, Hernandez RK, Jick SS. Risk of venous thromboembolism in users of oral contraceptives containing drospirenone or levonorgestrel: nested case-control study based on UK General Practice Research Database. BMJ 2011; 342:d2139.
- Jick SS, Hernandez RK. Risk of non-fatal venous thromboembolism in women using oral contraceptives containing drospirenone compared with women using oral contraceptives containing levonorgestrel: case-control study using United States claims data. BMJ 2011; 342:d2151.
- Association of Reproductive Health Professionals (ARHP). Choosing a birth control method. www.arhp.org/uploadDocs/choosingqrg.pdf. Accessed January 10, 2012.
- Caballero B. Should healthy people take a multivitamin? Cleve Clin J Med 2010; 77:656–657.
- US Food and Drug Administration. Ortho Evra questions and answers (1/18/2008). www.fda.gov/Drugs/DrugSafety/Postmarket-DrugSafetyInformationforPatientsandProviders/ucm110403.htm. Accessed January 10, 2012.
- Jick SS, Hagberg KW, Hernandez RK, Kaye JA. Postmarketing study of ORTHO EVRA and levonorgestrel oral contraceptives containing hormonal contraceptives with 30 mcg of ethinyl estradiol in relation to nonfatal venous thromboembolism. Contraception 2010; 81:16–21.
- Sabers A, Ohman I, Christensen J, Tomson T. Oral contraceptives reduce lamotrigine plasma levels. Neurology 2003; 61:570–571.
- Westhoff C, Truman C, Kalmuss D, et al; Depressive symptoms and Depo-Provera. Contraception 1998; 57:237–240.
- Trusell Wynn LL. Reducing unintended pregnancy in the United States. Contraception 2008; 77:1–5.
- Finer LB, Henshaw SK. Disparities in rates of unintended pregnancy in the United States, 1994 and 2001. Perspect Sex Reprod Health 2006; 38:90–96.
- Centers for Disease Control and Prevention. Vital signs: teenage pregnancy—United States 1991–2009. MMWR 2011; 60:414–420. www.cdc.gov/mmwr/preview/mmwrhtml/mm6013a5.htm. Accessed January 10, 2012.
- Nettleman MD, Chung H, Brewer J, Ayoola A, Reed PL. Reasons for unprotected intercourse: analysis of the PRAMS survey. Contraception 2007; 75:361–366.
- Nelson A. New low-dose, extended-cycle pills with levonorgestrel and ethinyl estradiol: an evolutionary step on birth control. Int J Womens Health 2010; 2:99–106.
- US Centers for Disease Control and Prevention. Appendix L. Summary of classifications for hormonal contraceptive methods and intrauterine devices. MMWR 2010; 59( RR04):76–81. www.cdc.gov/mmwr/preview/mmwrhtml/rr5904a13.htm. Accessed January 10, 2012.
- Wiegratz L, Lee JH, Kutchera E, et al. Effect of dienogest-containing oral contraceptives in lipid metabolism. Contraception 2002; 65:223–229.
- Sundström A, Seaman H, Kieler H, Alfredsson L. The risk of venous thromboembolism associated with the use of tranexamic acid and other drugs used to treat menorrhagia: a case-control study using the General Practice Research Database. Br J Obstet Gynecol 2009; 116:91–97.
- Heinemann L, Dinger JC. Range of published estimates of venous thromboembolism incidence in young women. Contraception 2007; 75:328–336.
- Dinger JC, Heinemann LA, Kühl-Habich D. The safety of a drospirenone-containing oral contraceptive: final results from the European Active Surveillance Study on oral contraceptives based on 142,475 women-years of observation. Contraception 2007; 75:344–354.
- Parkin L, Sharples K, Hernandez RK, Jick SS. Risk of venous thromboembolism in users of oral contraceptives containing drospirenone or levonorgestrel: nested case-control study based on UK General Practice Research Database. BMJ 2011; 342:d2139.
- Jick SS, Hernandez RK. Risk of non-fatal venous thromboembolism in women using oral contraceptives containing drospirenone compared with women using oral contraceptives containing levonorgestrel: case-control study using United States claims data. BMJ 2011; 342:d2151.
- Association of Reproductive Health Professionals (ARHP). Choosing a birth control method. www.arhp.org/uploadDocs/choosingqrg.pdf. Accessed January 10, 2012.
- Caballero B. Should healthy people take a multivitamin? Cleve Clin J Med 2010; 77:656–657.
- US Food and Drug Administration. Ortho Evra questions and answers (1/18/2008). www.fda.gov/Drugs/DrugSafety/Postmarket-DrugSafetyInformationforPatientsandProviders/ucm110403.htm. Accessed January 10, 2012.
- Jick SS, Hagberg KW, Hernandez RK, Kaye JA. Postmarketing study of ORTHO EVRA and levonorgestrel oral contraceptives containing hormonal contraceptives with 30 mcg of ethinyl estradiol in relation to nonfatal venous thromboembolism. Contraception 2010; 81:16–21.
- Sabers A, Ohman I, Christensen J, Tomson T. Oral contraceptives reduce lamotrigine plasma levels. Neurology 2003; 61:570–571.
- Westhoff C, Truman C, Kalmuss D, et al; Depressive symptoms and Depo-Provera. Contraception 1998; 57:237–240.
- Trusell Wynn LL. Reducing unintended pregnancy in the United States. Contraception 2008; 77:1–5.
KEY POINTS
- Hormonal contraceptives have a number of noncontraceptive benefits, such as regulating the menstrual cycle.
- The Pearl index is the number of unintended pregnancies per 100 women per year. Rates are 15% using male condoms, 8% with oral contraceptives, 3% with depot medroxyprogesterone acetate (Depo-Provera) injections, and less than 1% with intrauterine devices or female or male sterilization.
- Estrogen-containing products should be avoided in patients with hypertension or who are at risk of venous thromboembolism.
Bioidentical hormone therapy: Clarifying the misconceptions
Recent product endorsements from celebrities on television have brought a new term into the vocabulary of many American women: bioidentical hormone therapy—treatment with hormone products that are identical in molecular structure to those in the human body.
Since 2002, when results of the Women’s Health Initiative1 raised questions about the safety of hormone replacement therapy, women have been inundated by commercials, talk shows, and self-help books that promote bioidentical hormone therapy as a safe and natural way to treat menopausal symptoms—and more.
Although this publicity has helped promote discussion about menopause, it has also perpetuated confusion and misinformation among the lay public and the general medical community concerning menopausal hormone therapy.
Many postmenopausal women suffering from vasomotor symptoms, vaginal dryness, and vaginal atrophy are apprehensive about seeking therapy, owing to concerns resulting from misinterpreted information derived from the Women’s Health Initiative trial.1 (See “What are the known risks of FDA-approved hormone therapy.”2–8) Many others are told to suffer through their symptoms, which may eventually pass. It is not surprising, then, that women turn to unconventional treatments that are claimed to be safer. This unfortunate situation has driven the business of many compounding pharmacies into the multibillion dollar level.
In this paper, we hope to clarify some of the misconceptions surrounding this issue. But first we need to define some terms in what has become a confusing area.

WHAT ARE BIOIDENTICAL HORMONES?
“Bioidentical” means identical in molecular structure to endogenous hormones. However, as we will see, a better distinction should be made between products that are approved and regulated by the US Food and Drug Administration (FDA) and those that are not.
Endogenous reproductive hormones
Women produce various reproductive hormones, including three estrogens—estradiol, estrone, and estriol—as well as progesterone and testosterone.9
17-beta estradiol (E2) is the most bioactive endogenous estrogen. It is primarily produced by the dominant ovarian follicle and the corpus luteum and is synthesized intracellularly through aromatase activity.10,11 The rest of the circulating estradiol is derived from peripheral conversion of estrone to estradiol, and this is the primary source in postmenopausal women not on hormone therapy.11
In postmenopausal women, serum estradiol levels are often below 15 pg/mL. Many physiologic effects of the cellular compartmentalized estradiol contribute to an over-riding force in certain tissues even after menopause.10 With the loss of estradiol, many tissues in postmenopausal women can be affected, particularly resulting in genitourinary atrophy and bone loss.
Estrone (E1), the second dominant human estrogen, is primarily derived from the metabolism of estradiol and from the aromatization of androstenedione in adipose tissue, with a small quantity being secreted directly by the ovary and the adrenal glands.9 In postmenopausal women, mean estrone levels are about 30 pg/mL.11
Estriol (E3), the least active of the endogenous estrogens, is very short-acting.
Progesterone is a 21-carbon steroid secreted by the human ovary.9 It is formed during the transformation of cholesterol to estrogens and androgens and is no longer produced after menopause.9
Testosterone. In premenopausal women, the androgen testosterone is synthesized by the ovary, the adrenal cortex, and the peripheral conversion of circulating androstenedione and dehydroepiandrosterone (DHEA).9 Over a woman’s life span, her androgen levels decline progressively.10 The rate of decline has not been shown to be appreciably affected by the onset of menopause.10
All these hormone therapy products are synthesized
Many nonmedical women’s health books erroneously classify the forms of estrogen used in hormone therapy as either bioidentical or synthetic. In fact, they are all man-made.
Bioidentical hormones are synthesized by chemically extracting diosgenin from plants such as yams and soy.12 Diosgenin is chemically modified to yield the precursor progesterone, which is then used to synthesize bioidentical estrogens and androgens.10
Nonbioidentical estrogen products include conjugated equine estrogens (CEE), which is extracted from the urine of pregnant mares. The two predominant estrogens found in CEE are equilin sulfate (native to horses) and estrone sulfate.10
Other nonbioidentical products include ethinyl estradiol, which is used in most combined oral contraceptives. It is formed after a minor chemical modification of estradiol that makes it one of the most potent estrogens. The ethinyl group at carbon 17 of ring D of the steroid nucleus greatly slows the hepatic and enzymatic degradation of the molecule and, thereby, makes oral ethinyl estradiol 15 to 20 times more active than oral estradiol.
Mestranol is an inactive prodrug that is converted in the body to ethinyl estradiol.
While many women may find the idea of natural bioidentical hormones derived from sweet potatoes or soybeans more acceptable than taking one made from horse’s urine, all the products undergo extensive chemical processing and modification.
Misconception: FDA-regulated products are not bioidentical
WHAT IS CUSTOMIZED COMPOUNDED HORMONAL THERAPY?
There is often confusion between the terms “bioidentical hormones” and “customized compounded therapy,” which are often used interchangeably. Compounded therapy combines ratios of bioidentical hormones into a particular recipe or mixture. Customized compounding can be done by local compounding pharmacies.2
Compounded bioidentical estrogen products
There are several commonly marketed compounded products.
Tri-estrogen (tri-est) is a compounded hormone preparation made up of a mixture of 80% estriol, 10% estrone, and 10% estradiol.12
Bi-estrogen (bi-est) contains estriol and estradiol in a ratio of 8:1 or 9:1.
Although both tri-est and bi-est are largely composed of estriol, given the low potency of estriol, the effects of these products may be solely mediated by their major bioactive component, estradiol.10,12 No large prospective, well-controlled clinical trial has investigated the compounded ratios of these mixtures of estrogens.10
Tri-est and bi-est are frequently promoted as posing less risk of breast or endometrial cancer than FDA-approved agents, although there is no research to back up this claim.12 In fact, estriol may have a stimulatory effect on the breast and endometrium.9
In addition to these “standard” compounded preparations, women can receive more customized compounds.
Valid uses for customized compounded formulations
Some clinical providers use customized compounded formulations when prescribing hormone therapy to women who have allergies to certain ingredients, such as peanut oil (found in the FDA-regulated oral product Prometrium). Customized compounded formulations have also been used when prescribing hormones currently not FDA-approved for women, such as testosterone and DHEA.12 Before oral micronized progesterone was marketed in the United States as Prometrium, it was frequently prescribed as a compounded hormone.
HORMONE THERAPY COMES IN VARIOUS FORMS
Both FDA-regulated hormone therapy and unregulated compounded hormone therapy come in various doses and dosage forms administered by different routes, allowing for individualization for each woman’s specific characteristics.
Estrogens: Oral, transdermal, others
Estrogen therapy can be given orally, transvaginally (as creams, tablets, and rings), transdermally (as patches, gels, and creams), subcutaneously in pellets, intranasally (in Europe), and by injection.11
Most oral contraceptives contain the synthetic estrogen ethinyl estradiol. Ethinyl estradiol is more potent than human estrogens,11 specifically in increasing the production of hepatic proteins (sex-hormone-binding globulin, renin substrate, corticosteroid-binding globulin, and thyroid-binding globulin).11
Bioidentical estradiol, taken orally in tablet form, is first processed through the liver and converted into estrone.12 This stimulates proteins such as C-reactive protein, activated protein C, and clotting factors, which may increase the risk of clotting.12 Estradiol given transdermally by patch or gel or vaginally bypasses the liver and enters the bloodstream as 17-beta estradiol, therefore avoiding stimulation of these proteins.12 Case-control data have shown an associated lower risk of deep venous thromboembolism with transdermal therapy.3
Subcutaneous pellet therapy is a less common, non-FDA-approved method of hormone therapy to relieve postmenopausal symptoms.10 In an outpatient procedure, the pellet is inserted into the subcutaneous fat of the abdomen.10 The crystalline pellet is biodegradable and contains a mixture of testosterone and 17-beta estradiol.10 It is important to remember that endometrial stimulation may be prolonged with this form of therapy and levels may be supraphysiologic.
Progestogens can also be given by different routes
Oral progesterone has poor gastrointestinal absorption and a short half-life.10 Therefore, it is micronized with oil for better absorption. Reported side effects include sedative and anesthetic effects; therefore, it is recommended that oral progesterone be taken at bedtime.9 Medroxyprogesterone acetate may interfere more with estrogen’s positive effects on cholesterol than micronized progesterone does.13
Topical progesterone preparations vary widely in dosage and formulation. Over-the-counter progesterone creams vary in concentration from no active ingredient to 450 mg or more of progesterone per ounce. Application sites for progesterone cream include the inner arm, chest, and inner thigh. No transdermal hormone should be applied to areas of the body that may allow possible contact and transference to others.
Progestogen products
Progestogen products include “natural” progesterone and synthetic progestins. They should be given concurrently with estrogen therapy in women who have an intact uterus to prevent endometrial hyperplasia.9
Bioidentical progesterone is micronized in the laboratory for better absorption in the gut.2
Misconception: Transdermal progesterone protects the endometrium
In general, transdermal progesterone should be avoided, as it does not protect against endometrial cancer.
Many forms of progesterone are available by prescription at compounding pharmacies as lotions, gels, creams, capsules, trochees, and suppositories.9 Transdermal progesterone creams are also available over the counter at health stores. Some of these creams contain only diosgenin, a progesterone precursor derived from wild yams.10 Diosgenin cannot be converted into progesterone within the body and thus does not provide an adequate amount of absorbable progesterone.9 Therefore, progesterone cream that contains only diosgenin is not effective in preventing endometrial hyperplasia and cancer.
To achieve a physiologic response, progesterone levels must be at least in the nanogram range.10 Transdermal progesterone cream has not been shown to reach this level and may not significantly improve vasomotor symptoms.12 Some practitioners prescribe cream that contains more than 400 mg progesterone per ounce. This may achieve physiologic levels of progesterone, but no improvement has been proven for bone mineral density or endometrial protection. In general, no transdermal progesterone cream can be assumed to protect the endometrium against the stimulatory effects of estrogen.
CUSTOM COMPOUNDING AND SALIVA TESTING TO INDIVIDUALIZE THERAPY
Some clinicians who prescribe compounded hormones order saliva tests. They argue the tests help them to establish which hormones are deficient and therefore to customize therapy.12 The basis for this is that saliva is similar to an ultrafiltrate of blood and, theoretically, hormone levels in saliva should represent the bioavailable hormone in serum.10
Unfortunately, this testing is often unreliable due to poor stability of samples in storage and large interassay variability.10 Many factors may alter hormone levels in saliva and make test results unreproducible, including the time of day the sample is collected and dietary habits.10 The FDA states that there is no scientific basis for using salivary testing to adjust hormone levels.2
Levels of drugs with clearance that varies depending on hepatic enzyme activity and plasma binding (capacity-limited metabolism) such as estradiol and testosterone can be monitored with total blood serum concentrations.10 However, many physiologic effects of estrogens are determined intracellularly at the level of tissues.10 Therefore, although levels during therapy with bioidentical estrogens can be monitored more precisely, the FDA states that hormone therapy should be guided by symptom response and findings on physical examination and not by hormone levels alone.2,12 It may be reasonable to order serum levels of estradiol in women being treated with therapeutic doses of bioidentical estrogen but still not achieving symptom relief. If women are being treated with conjugated equine estrogens, serum levels cannot be monitored. Total estrogen can be monitored as a send-out laboratory test.
MISCONCEPTION: HORMONE THERAPY IS A FOUNTAIN OF YOUTH
Customized compounded hormonal therapy is marketed as being able to help with rejuvenation, improve memory, sexual function, and reverse the aging process, essentially promising to be an elixir or fountain of youth.
These claims are not substantiated. However, the actual benefits of hormone therapy in women who have menopausal symptoms include alleviation of moderate to severe vasomotor symptoms and vaginal atrophy that can result in dyspareunia. By alleviating their symptoms, hormone therapy improves women’s quality of life. It also reduces the incidence of postmenopausal osteoporotic fractures.
A research finding that is often overlooked is that postmenopausal women younger than 60 years who started estrogen or estrogenprogestin therapy soon after menopause had a 30% lower rate of death from all causes.2,14 This difference was statistically significant when the estrogen and estrogen-progestin therapy groups were combined. No reduction in the mortality rate was seen if therapy was started after age 60.
MISCONCEPTION: COMPOUNDED THERAPY IS SAFER
Compounded hormone therapy is often marketed as a safer or more effective alternative to government-regulated and approved therapy. Unfortunately, these claims are often false and misleading, and safety information is not consistently provided to patients as is required with FDA-regulated hormone therapy.2
Since these compounds have not been approved by the FDA, there is no guarantee that the ingredients have been tested for purity, potency, and efficacy. There is no batch standardization. These unregulated therapies may use unapproved ingredients, routes of administration, and mixtures with contaminants such as dyes and preservatives.2
Also, custom-compounded prescriptions are considered experimental. Therefore, they are often not covered by insurance, and many women must pay for them out of pocket.11
The North American Menopause Society does not recommend custom-mixed products over well-tested, government-approved commercial products for most women.2 All bioidentical hormone prescriptions should include a patient package insert,11 identical to that required of FDA-approved products.2
- Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002; 288:321–333.
- North American Menopause Society. Estrogen and progestogen use in postmenopausal women: 2010 position statement of the North American Menopause Society. Menopause 2010; 17:242–255.
- Canonico M, Oger E, Plu-Bureau G; Estrogen and Thromboembolism Risk (ESTHER) Study Group. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation 2007; 115:840–845.
- Risks of postmenopausal hormone replacement (letters). JAMA 2002; 288:2819–2825.
- Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007; 297:1465–1477.
- Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med 2000; 133:933–941.
- Shumaker SA, Legault C, Rapp SR, et al; WHIMS Investigators. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA 2003; 289:2651–2662.
- Chlebowski RT, Anderson GL, Gass M, et al; WHI Investigators. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA 2010; 304:1684–1692.
- Lobo RA. Treatment of the Postmenopausal Woman: Basic and Clinical Aspects. 3rd ed. Burlington, MA: Academic Press; 2007.
- Cirigliano M. Bioidentical hormone therapy: a review of the evidence. J Womens Health (Larchmt) 2007; 16:600–631.
- Menopause Practice: A Clinician’s Guide. 4th ed. Cleveland, OH: The North American Menopause Society; 2010.
- What are bioidentical hormones? Natural. Bioidentical. Compounded. Confusion about these terms is only adding to the confusion over hormone therapy. Harv Womens Health Watch 2006; 13:1–3.
- The Writing Group for the PEPI Trial. Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA 1995; 273:199–208.
- Hodis HN, Mack WJ. Postmenopausal hormone therapy in clinical perspective. Menopause 2007; 14:944–957.
Recent product endorsements from celebrities on television have brought a new term into the vocabulary of many American women: bioidentical hormone therapy—treatment with hormone products that are identical in molecular structure to those in the human body.
Since 2002, when results of the Women’s Health Initiative1 raised questions about the safety of hormone replacement therapy, women have been inundated by commercials, talk shows, and self-help books that promote bioidentical hormone therapy as a safe and natural way to treat menopausal symptoms—and more.
Although this publicity has helped promote discussion about menopause, it has also perpetuated confusion and misinformation among the lay public and the general medical community concerning menopausal hormone therapy.
Many postmenopausal women suffering from vasomotor symptoms, vaginal dryness, and vaginal atrophy are apprehensive about seeking therapy, owing to concerns resulting from misinterpreted information derived from the Women’s Health Initiative trial.1 (See “What are the known risks of FDA-approved hormone therapy.”2–8) Many others are told to suffer through their symptoms, which may eventually pass. It is not surprising, then, that women turn to unconventional treatments that are claimed to be safer. This unfortunate situation has driven the business of many compounding pharmacies into the multibillion dollar level.
In this paper, we hope to clarify some of the misconceptions surrounding this issue. But first we need to define some terms in what has become a confusing area.

WHAT ARE BIOIDENTICAL HORMONES?
“Bioidentical” means identical in molecular structure to endogenous hormones. However, as we will see, a better distinction should be made between products that are approved and regulated by the US Food and Drug Administration (FDA) and those that are not.
Endogenous reproductive hormones
Women produce various reproductive hormones, including three estrogens—estradiol, estrone, and estriol—as well as progesterone and testosterone.9
17-beta estradiol (E2) is the most bioactive endogenous estrogen. It is primarily produced by the dominant ovarian follicle and the corpus luteum and is synthesized intracellularly through aromatase activity.10,11 The rest of the circulating estradiol is derived from peripheral conversion of estrone to estradiol, and this is the primary source in postmenopausal women not on hormone therapy.11
In postmenopausal women, serum estradiol levels are often below 15 pg/mL. Many physiologic effects of the cellular compartmentalized estradiol contribute to an over-riding force in certain tissues even after menopause.10 With the loss of estradiol, many tissues in postmenopausal women can be affected, particularly resulting in genitourinary atrophy and bone loss.
Estrone (E1), the second dominant human estrogen, is primarily derived from the metabolism of estradiol and from the aromatization of androstenedione in adipose tissue, with a small quantity being secreted directly by the ovary and the adrenal glands.9 In postmenopausal women, mean estrone levels are about 30 pg/mL.11
Estriol (E3), the least active of the endogenous estrogens, is very short-acting.
Progesterone is a 21-carbon steroid secreted by the human ovary.9 It is formed during the transformation of cholesterol to estrogens and androgens and is no longer produced after menopause.9
Testosterone. In premenopausal women, the androgen testosterone is synthesized by the ovary, the adrenal cortex, and the peripheral conversion of circulating androstenedione and dehydroepiandrosterone (DHEA).9 Over a woman’s life span, her androgen levels decline progressively.10 The rate of decline has not been shown to be appreciably affected by the onset of menopause.10
All these hormone therapy products are synthesized
Many nonmedical women’s health books erroneously classify the forms of estrogen used in hormone therapy as either bioidentical or synthetic. In fact, they are all man-made.
Bioidentical hormones are synthesized by chemically extracting diosgenin from plants such as yams and soy.12 Diosgenin is chemically modified to yield the precursor progesterone, which is then used to synthesize bioidentical estrogens and androgens.10
Nonbioidentical estrogen products include conjugated equine estrogens (CEE), which is extracted from the urine of pregnant mares. The two predominant estrogens found in CEE are equilin sulfate (native to horses) and estrone sulfate.10
Other nonbioidentical products include ethinyl estradiol, which is used in most combined oral contraceptives. It is formed after a minor chemical modification of estradiol that makes it one of the most potent estrogens. The ethinyl group at carbon 17 of ring D of the steroid nucleus greatly slows the hepatic and enzymatic degradation of the molecule and, thereby, makes oral ethinyl estradiol 15 to 20 times more active than oral estradiol.
Mestranol is an inactive prodrug that is converted in the body to ethinyl estradiol.
While many women may find the idea of natural bioidentical hormones derived from sweet potatoes or soybeans more acceptable than taking one made from horse’s urine, all the products undergo extensive chemical processing and modification.
Misconception: FDA-regulated products are not bioidentical
WHAT IS CUSTOMIZED COMPOUNDED HORMONAL THERAPY?
There is often confusion between the terms “bioidentical hormones” and “customized compounded therapy,” which are often used interchangeably. Compounded therapy combines ratios of bioidentical hormones into a particular recipe or mixture. Customized compounding can be done by local compounding pharmacies.2
Compounded bioidentical estrogen products
There are several commonly marketed compounded products.
Tri-estrogen (tri-est) is a compounded hormone preparation made up of a mixture of 80% estriol, 10% estrone, and 10% estradiol.12
Bi-estrogen (bi-est) contains estriol and estradiol in a ratio of 8:1 or 9:1.
Although both tri-est and bi-est are largely composed of estriol, given the low potency of estriol, the effects of these products may be solely mediated by their major bioactive component, estradiol.10,12 No large prospective, well-controlled clinical trial has investigated the compounded ratios of these mixtures of estrogens.10
Tri-est and bi-est are frequently promoted as posing less risk of breast or endometrial cancer than FDA-approved agents, although there is no research to back up this claim.12 In fact, estriol may have a stimulatory effect on the breast and endometrium.9
In addition to these “standard” compounded preparations, women can receive more customized compounds.
Valid uses for customized compounded formulations
Some clinical providers use customized compounded formulations when prescribing hormone therapy to women who have allergies to certain ingredients, such as peanut oil (found in the FDA-regulated oral product Prometrium). Customized compounded formulations have also been used when prescribing hormones currently not FDA-approved for women, such as testosterone and DHEA.12 Before oral micronized progesterone was marketed in the United States as Prometrium, it was frequently prescribed as a compounded hormone.
HORMONE THERAPY COMES IN VARIOUS FORMS
Both FDA-regulated hormone therapy and unregulated compounded hormone therapy come in various doses and dosage forms administered by different routes, allowing for individualization for each woman’s specific characteristics.
Estrogens: Oral, transdermal, others
Estrogen therapy can be given orally, transvaginally (as creams, tablets, and rings), transdermally (as patches, gels, and creams), subcutaneously in pellets, intranasally (in Europe), and by injection.11
Most oral contraceptives contain the synthetic estrogen ethinyl estradiol. Ethinyl estradiol is more potent than human estrogens,11 specifically in increasing the production of hepatic proteins (sex-hormone-binding globulin, renin substrate, corticosteroid-binding globulin, and thyroid-binding globulin).11
Bioidentical estradiol, taken orally in tablet form, is first processed through the liver and converted into estrone.12 This stimulates proteins such as C-reactive protein, activated protein C, and clotting factors, which may increase the risk of clotting.12 Estradiol given transdermally by patch or gel or vaginally bypasses the liver and enters the bloodstream as 17-beta estradiol, therefore avoiding stimulation of these proteins.12 Case-control data have shown an associated lower risk of deep venous thromboembolism with transdermal therapy.3
Subcutaneous pellet therapy is a less common, non-FDA-approved method of hormone therapy to relieve postmenopausal symptoms.10 In an outpatient procedure, the pellet is inserted into the subcutaneous fat of the abdomen.10 The crystalline pellet is biodegradable and contains a mixture of testosterone and 17-beta estradiol.10 It is important to remember that endometrial stimulation may be prolonged with this form of therapy and levels may be supraphysiologic.
Progestogens can also be given by different routes
Oral progesterone has poor gastrointestinal absorption and a short half-life.10 Therefore, it is micronized with oil for better absorption. Reported side effects include sedative and anesthetic effects; therefore, it is recommended that oral progesterone be taken at bedtime.9 Medroxyprogesterone acetate may interfere more with estrogen’s positive effects on cholesterol than micronized progesterone does.13
Topical progesterone preparations vary widely in dosage and formulation. Over-the-counter progesterone creams vary in concentration from no active ingredient to 450 mg or more of progesterone per ounce. Application sites for progesterone cream include the inner arm, chest, and inner thigh. No transdermal hormone should be applied to areas of the body that may allow possible contact and transference to others.
Progestogen products
Progestogen products include “natural” progesterone and synthetic progestins. They should be given concurrently with estrogen therapy in women who have an intact uterus to prevent endometrial hyperplasia.9
Bioidentical progesterone is micronized in the laboratory for better absorption in the gut.2
Misconception: Transdermal progesterone protects the endometrium
In general, transdermal progesterone should be avoided, as it does not protect against endometrial cancer.
Many forms of progesterone are available by prescription at compounding pharmacies as lotions, gels, creams, capsules, trochees, and suppositories.9 Transdermal progesterone creams are also available over the counter at health stores. Some of these creams contain only diosgenin, a progesterone precursor derived from wild yams.10 Diosgenin cannot be converted into progesterone within the body and thus does not provide an adequate amount of absorbable progesterone.9 Therefore, progesterone cream that contains only diosgenin is not effective in preventing endometrial hyperplasia and cancer.
To achieve a physiologic response, progesterone levels must be at least in the nanogram range.10 Transdermal progesterone cream has not been shown to reach this level and may not significantly improve vasomotor symptoms.12 Some practitioners prescribe cream that contains more than 400 mg progesterone per ounce. This may achieve physiologic levels of progesterone, but no improvement has been proven for bone mineral density or endometrial protection. In general, no transdermal progesterone cream can be assumed to protect the endometrium against the stimulatory effects of estrogen.
CUSTOM COMPOUNDING AND SALIVA TESTING TO INDIVIDUALIZE THERAPY
Some clinicians who prescribe compounded hormones order saliva tests. They argue the tests help them to establish which hormones are deficient and therefore to customize therapy.12 The basis for this is that saliva is similar to an ultrafiltrate of blood and, theoretically, hormone levels in saliva should represent the bioavailable hormone in serum.10
Unfortunately, this testing is often unreliable due to poor stability of samples in storage and large interassay variability.10 Many factors may alter hormone levels in saliva and make test results unreproducible, including the time of day the sample is collected and dietary habits.10 The FDA states that there is no scientific basis for using salivary testing to adjust hormone levels.2
Levels of drugs with clearance that varies depending on hepatic enzyme activity and plasma binding (capacity-limited metabolism) such as estradiol and testosterone can be monitored with total blood serum concentrations.10 However, many physiologic effects of estrogens are determined intracellularly at the level of tissues.10 Therefore, although levels during therapy with bioidentical estrogens can be monitored more precisely, the FDA states that hormone therapy should be guided by symptom response and findings on physical examination and not by hormone levels alone.2,12 It may be reasonable to order serum levels of estradiol in women being treated with therapeutic doses of bioidentical estrogen but still not achieving symptom relief. If women are being treated with conjugated equine estrogens, serum levels cannot be monitored. Total estrogen can be monitored as a send-out laboratory test.
MISCONCEPTION: HORMONE THERAPY IS A FOUNTAIN OF YOUTH
Customized compounded hormonal therapy is marketed as being able to help with rejuvenation, improve memory, sexual function, and reverse the aging process, essentially promising to be an elixir or fountain of youth.
These claims are not substantiated. However, the actual benefits of hormone therapy in women who have menopausal symptoms include alleviation of moderate to severe vasomotor symptoms and vaginal atrophy that can result in dyspareunia. By alleviating their symptoms, hormone therapy improves women’s quality of life. It also reduces the incidence of postmenopausal osteoporotic fractures.
A research finding that is often overlooked is that postmenopausal women younger than 60 years who started estrogen or estrogenprogestin therapy soon after menopause had a 30% lower rate of death from all causes.2,14 This difference was statistically significant when the estrogen and estrogen-progestin therapy groups were combined. No reduction in the mortality rate was seen if therapy was started after age 60.
MISCONCEPTION: COMPOUNDED THERAPY IS SAFER
Compounded hormone therapy is often marketed as a safer or more effective alternative to government-regulated and approved therapy. Unfortunately, these claims are often false and misleading, and safety information is not consistently provided to patients as is required with FDA-regulated hormone therapy.2
Since these compounds have not been approved by the FDA, there is no guarantee that the ingredients have been tested for purity, potency, and efficacy. There is no batch standardization. These unregulated therapies may use unapproved ingredients, routes of administration, and mixtures with contaminants such as dyes and preservatives.2
Also, custom-compounded prescriptions are considered experimental. Therefore, they are often not covered by insurance, and many women must pay for them out of pocket.11
The North American Menopause Society does not recommend custom-mixed products over well-tested, government-approved commercial products for most women.2 All bioidentical hormone prescriptions should include a patient package insert,11 identical to that required of FDA-approved products.2
Recent product endorsements from celebrities on television have brought a new term into the vocabulary of many American women: bioidentical hormone therapy—treatment with hormone products that are identical in molecular structure to those in the human body.
Since 2002, when results of the Women’s Health Initiative1 raised questions about the safety of hormone replacement therapy, women have been inundated by commercials, talk shows, and self-help books that promote bioidentical hormone therapy as a safe and natural way to treat menopausal symptoms—and more.
Although this publicity has helped promote discussion about menopause, it has also perpetuated confusion and misinformation among the lay public and the general medical community concerning menopausal hormone therapy.
Many postmenopausal women suffering from vasomotor symptoms, vaginal dryness, and vaginal atrophy are apprehensive about seeking therapy, owing to concerns resulting from misinterpreted information derived from the Women’s Health Initiative trial.1 (See “What are the known risks of FDA-approved hormone therapy.”2–8) Many others are told to suffer through their symptoms, which may eventually pass. It is not surprising, then, that women turn to unconventional treatments that are claimed to be safer. This unfortunate situation has driven the business of many compounding pharmacies into the multibillion dollar level.
In this paper, we hope to clarify some of the misconceptions surrounding this issue. But first we need to define some terms in what has become a confusing area.

WHAT ARE BIOIDENTICAL HORMONES?
“Bioidentical” means identical in molecular structure to endogenous hormones. However, as we will see, a better distinction should be made between products that are approved and regulated by the US Food and Drug Administration (FDA) and those that are not.
Endogenous reproductive hormones
Women produce various reproductive hormones, including three estrogens—estradiol, estrone, and estriol—as well as progesterone and testosterone.9
17-beta estradiol (E2) is the most bioactive endogenous estrogen. It is primarily produced by the dominant ovarian follicle and the corpus luteum and is synthesized intracellularly through aromatase activity.10,11 The rest of the circulating estradiol is derived from peripheral conversion of estrone to estradiol, and this is the primary source in postmenopausal women not on hormone therapy.11
In postmenopausal women, serum estradiol levels are often below 15 pg/mL. Many physiologic effects of the cellular compartmentalized estradiol contribute to an over-riding force in certain tissues even after menopause.10 With the loss of estradiol, many tissues in postmenopausal women can be affected, particularly resulting in genitourinary atrophy and bone loss.
Estrone (E1), the second dominant human estrogen, is primarily derived from the metabolism of estradiol and from the aromatization of androstenedione in adipose tissue, with a small quantity being secreted directly by the ovary and the adrenal glands.9 In postmenopausal women, mean estrone levels are about 30 pg/mL.11
Estriol (E3), the least active of the endogenous estrogens, is very short-acting.
Progesterone is a 21-carbon steroid secreted by the human ovary.9 It is formed during the transformation of cholesterol to estrogens and androgens and is no longer produced after menopause.9
Testosterone. In premenopausal women, the androgen testosterone is synthesized by the ovary, the adrenal cortex, and the peripheral conversion of circulating androstenedione and dehydroepiandrosterone (DHEA).9 Over a woman’s life span, her androgen levels decline progressively.10 The rate of decline has not been shown to be appreciably affected by the onset of menopause.10
All these hormone therapy products are synthesized
Many nonmedical women’s health books erroneously classify the forms of estrogen used in hormone therapy as either bioidentical or synthetic. In fact, they are all man-made.
Bioidentical hormones are synthesized by chemically extracting diosgenin from plants such as yams and soy.12 Diosgenin is chemically modified to yield the precursor progesterone, which is then used to synthesize bioidentical estrogens and androgens.10
Nonbioidentical estrogen products include conjugated equine estrogens (CEE), which is extracted from the urine of pregnant mares. The two predominant estrogens found in CEE are equilin sulfate (native to horses) and estrone sulfate.10
Other nonbioidentical products include ethinyl estradiol, which is used in most combined oral contraceptives. It is formed after a minor chemical modification of estradiol that makes it one of the most potent estrogens. The ethinyl group at carbon 17 of ring D of the steroid nucleus greatly slows the hepatic and enzymatic degradation of the molecule and, thereby, makes oral ethinyl estradiol 15 to 20 times more active than oral estradiol.
Mestranol is an inactive prodrug that is converted in the body to ethinyl estradiol.
While many women may find the idea of natural bioidentical hormones derived from sweet potatoes or soybeans more acceptable than taking one made from horse’s urine, all the products undergo extensive chemical processing and modification.
Misconception: FDA-regulated products are not bioidentical
WHAT IS CUSTOMIZED COMPOUNDED HORMONAL THERAPY?
There is often confusion between the terms “bioidentical hormones” and “customized compounded therapy,” which are often used interchangeably. Compounded therapy combines ratios of bioidentical hormones into a particular recipe or mixture. Customized compounding can be done by local compounding pharmacies.2
Compounded bioidentical estrogen products
There are several commonly marketed compounded products.
Tri-estrogen (tri-est) is a compounded hormone preparation made up of a mixture of 80% estriol, 10% estrone, and 10% estradiol.12
Bi-estrogen (bi-est) contains estriol and estradiol in a ratio of 8:1 or 9:1.
Although both tri-est and bi-est are largely composed of estriol, given the low potency of estriol, the effects of these products may be solely mediated by their major bioactive component, estradiol.10,12 No large prospective, well-controlled clinical trial has investigated the compounded ratios of these mixtures of estrogens.10
Tri-est and bi-est are frequently promoted as posing less risk of breast or endometrial cancer than FDA-approved agents, although there is no research to back up this claim.12 In fact, estriol may have a stimulatory effect on the breast and endometrium.9
In addition to these “standard” compounded preparations, women can receive more customized compounds.
Valid uses for customized compounded formulations
Some clinical providers use customized compounded formulations when prescribing hormone therapy to women who have allergies to certain ingredients, such as peanut oil (found in the FDA-regulated oral product Prometrium). Customized compounded formulations have also been used when prescribing hormones currently not FDA-approved for women, such as testosterone and DHEA.12 Before oral micronized progesterone was marketed in the United States as Prometrium, it was frequently prescribed as a compounded hormone.
HORMONE THERAPY COMES IN VARIOUS FORMS
Both FDA-regulated hormone therapy and unregulated compounded hormone therapy come in various doses and dosage forms administered by different routes, allowing for individualization for each woman’s specific characteristics.
Estrogens: Oral, transdermal, others
Estrogen therapy can be given orally, transvaginally (as creams, tablets, and rings), transdermally (as patches, gels, and creams), subcutaneously in pellets, intranasally (in Europe), and by injection.11
Most oral contraceptives contain the synthetic estrogen ethinyl estradiol. Ethinyl estradiol is more potent than human estrogens,11 specifically in increasing the production of hepatic proteins (sex-hormone-binding globulin, renin substrate, corticosteroid-binding globulin, and thyroid-binding globulin).11
Bioidentical estradiol, taken orally in tablet form, is first processed through the liver and converted into estrone.12 This stimulates proteins such as C-reactive protein, activated protein C, and clotting factors, which may increase the risk of clotting.12 Estradiol given transdermally by patch or gel or vaginally bypasses the liver and enters the bloodstream as 17-beta estradiol, therefore avoiding stimulation of these proteins.12 Case-control data have shown an associated lower risk of deep venous thromboembolism with transdermal therapy.3
Subcutaneous pellet therapy is a less common, non-FDA-approved method of hormone therapy to relieve postmenopausal symptoms.10 In an outpatient procedure, the pellet is inserted into the subcutaneous fat of the abdomen.10 The crystalline pellet is biodegradable and contains a mixture of testosterone and 17-beta estradiol.10 It is important to remember that endometrial stimulation may be prolonged with this form of therapy and levels may be supraphysiologic.
Progestogens can also be given by different routes
Oral progesterone has poor gastrointestinal absorption and a short half-life.10 Therefore, it is micronized with oil for better absorption. Reported side effects include sedative and anesthetic effects; therefore, it is recommended that oral progesterone be taken at bedtime.9 Medroxyprogesterone acetate may interfere more with estrogen’s positive effects on cholesterol than micronized progesterone does.13
Topical progesterone preparations vary widely in dosage and formulation. Over-the-counter progesterone creams vary in concentration from no active ingredient to 450 mg or more of progesterone per ounce. Application sites for progesterone cream include the inner arm, chest, and inner thigh. No transdermal hormone should be applied to areas of the body that may allow possible contact and transference to others.
Progestogen products
Progestogen products include “natural” progesterone and synthetic progestins. They should be given concurrently with estrogen therapy in women who have an intact uterus to prevent endometrial hyperplasia.9
Bioidentical progesterone is micronized in the laboratory for better absorption in the gut.2
Misconception: Transdermal progesterone protects the endometrium
In general, transdermal progesterone should be avoided, as it does not protect against endometrial cancer.
Many forms of progesterone are available by prescription at compounding pharmacies as lotions, gels, creams, capsules, trochees, and suppositories.9 Transdermal progesterone creams are also available over the counter at health stores. Some of these creams contain only diosgenin, a progesterone precursor derived from wild yams.10 Diosgenin cannot be converted into progesterone within the body and thus does not provide an adequate amount of absorbable progesterone.9 Therefore, progesterone cream that contains only diosgenin is not effective in preventing endometrial hyperplasia and cancer.
To achieve a physiologic response, progesterone levels must be at least in the nanogram range.10 Transdermal progesterone cream has not been shown to reach this level and may not significantly improve vasomotor symptoms.12 Some practitioners prescribe cream that contains more than 400 mg progesterone per ounce. This may achieve physiologic levels of progesterone, but no improvement has been proven for bone mineral density or endometrial protection. In general, no transdermal progesterone cream can be assumed to protect the endometrium against the stimulatory effects of estrogen.
CUSTOM COMPOUNDING AND SALIVA TESTING TO INDIVIDUALIZE THERAPY
Some clinicians who prescribe compounded hormones order saliva tests. They argue the tests help them to establish which hormones are deficient and therefore to customize therapy.12 The basis for this is that saliva is similar to an ultrafiltrate of blood and, theoretically, hormone levels in saliva should represent the bioavailable hormone in serum.10
Unfortunately, this testing is often unreliable due to poor stability of samples in storage and large interassay variability.10 Many factors may alter hormone levels in saliva and make test results unreproducible, including the time of day the sample is collected and dietary habits.10 The FDA states that there is no scientific basis for using salivary testing to adjust hormone levels.2
Levels of drugs with clearance that varies depending on hepatic enzyme activity and plasma binding (capacity-limited metabolism) such as estradiol and testosterone can be monitored with total blood serum concentrations.10 However, many physiologic effects of estrogens are determined intracellularly at the level of tissues.10 Therefore, although levels during therapy with bioidentical estrogens can be monitored more precisely, the FDA states that hormone therapy should be guided by symptom response and findings on physical examination and not by hormone levels alone.2,12 It may be reasonable to order serum levels of estradiol in women being treated with therapeutic doses of bioidentical estrogen but still not achieving symptom relief. If women are being treated with conjugated equine estrogens, serum levels cannot be monitored. Total estrogen can be monitored as a send-out laboratory test.
MISCONCEPTION: HORMONE THERAPY IS A FOUNTAIN OF YOUTH
Customized compounded hormonal therapy is marketed as being able to help with rejuvenation, improve memory, sexual function, and reverse the aging process, essentially promising to be an elixir or fountain of youth.
These claims are not substantiated. However, the actual benefits of hormone therapy in women who have menopausal symptoms include alleviation of moderate to severe vasomotor symptoms and vaginal atrophy that can result in dyspareunia. By alleviating their symptoms, hormone therapy improves women’s quality of life. It also reduces the incidence of postmenopausal osteoporotic fractures.
A research finding that is often overlooked is that postmenopausal women younger than 60 years who started estrogen or estrogenprogestin therapy soon after menopause had a 30% lower rate of death from all causes.2,14 This difference was statistically significant when the estrogen and estrogen-progestin therapy groups were combined. No reduction in the mortality rate was seen if therapy was started after age 60.
MISCONCEPTION: COMPOUNDED THERAPY IS SAFER
Compounded hormone therapy is often marketed as a safer or more effective alternative to government-regulated and approved therapy. Unfortunately, these claims are often false and misleading, and safety information is not consistently provided to patients as is required with FDA-regulated hormone therapy.2
Since these compounds have not been approved by the FDA, there is no guarantee that the ingredients have been tested for purity, potency, and efficacy. There is no batch standardization. These unregulated therapies may use unapproved ingredients, routes of administration, and mixtures with contaminants such as dyes and preservatives.2
Also, custom-compounded prescriptions are considered experimental. Therefore, they are often not covered by insurance, and many women must pay for them out of pocket.11
The North American Menopause Society does not recommend custom-mixed products over well-tested, government-approved commercial products for most women.2 All bioidentical hormone prescriptions should include a patient package insert,11 identical to that required of FDA-approved products.2
- Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002; 288:321–333.
- North American Menopause Society. Estrogen and progestogen use in postmenopausal women: 2010 position statement of the North American Menopause Society. Menopause 2010; 17:242–255.
- Canonico M, Oger E, Plu-Bureau G; Estrogen and Thromboembolism Risk (ESTHER) Study Group. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation 2007; 115:840–845.
- Risks of postmenopausal hormone replacement (letters). JAMA 2002; 288:2819–2825.
- Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007; 297:1465–1477.
- Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med 2000; 133:933–941.
- Shumaker SA, Legault C, Rapp SR, et al; WHIMS Investigators. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA 2003; 289:2651–2662.
- Chlebowski RT, Anderson GL, Gass M, et al; WHI Investigators. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA 2010; 304:1684–1692.
- Lobo RA. Treatment of the Postmenopausal Woman: Basic and Clinical Aspects. 3rd ed. Burlington, MA: Academic Press; 2007.
- Cirigliano M. Bioidentical hormone therapy: a review of the evidence. J Womens Health (Larchmt) 2007; 16:600–631.
- Menopause Practice: A Clinician’s Guide. 4th ed. Cleveland, OH: The North American Menopause Society; 2010.
- What are bioidentical hormones? Natural. Bioidentical. Compounded. Confusion about these terms is only adding to the confusion over hormone therapy. Harv Womens Health Watch 2006; 13:1–3.
- The Writing Group for the PEPI Trial. Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA 1995; 273:199–208.
- Hodis HN, Mack WJ. Postmenopausal hormone therapy in clinical perspective. Menopause 2007; 14:944–957.
- Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002; 288:321–333.
- North American Menopause Society. Estrogen and progestogen use in postmenopausal women: 2010 position statement of the North American Menopause Society. Menopause 2010; 17:242–255.
- Canonico M, Oger E, Plu-Bureau G; Estrogen and Thromboembolism Risk (ESTHER) Study Group. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation 2007; 115:840–845.
- Risks of postmenopausal hormone replacement (letters). JAMA 2002; 288:2819–2825.
- Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007; 297:1465–1477.
- Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med 2000; 133:933–941.
- Shumaker SA, Legault C, Rapp SR, et al; WHIMS Investigators. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA 2003; 289:2651–2662.
- Chlebowski RT, Anderson GL, Gass M, et al; WHI Investigators. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA 2010; 304:1684–1692.
- Lobo RA. Treatment of the Postmenopausal Woman: Basic and Clinical Aspects. 3rd ed. Burlington, MA: Academic Press; 2007.
- Cirigliano M. Bioidentical hormone therapy: a review of the evidence. J Womens Health (Larchmt) 2007; 16:600–631.
- Menopause Practice: A Clinician’s Guide. 4th ed. Cleveland, OH: The North American Menopause Society; 2010.
- What are bioidentical hormones? Natural. Bioidentical. Compounded. Confusion about these terms is only adding to the confusion over hormone therapy. Harv Womens Health Watch 2006; 13:1–3.
- The Writing Group for the PEPI Trial. Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA 1995; 273:199–208.
- Hodis HN, Mack WJ. Postmenopausal hormone therapy in clinical perspective. Menopause 2007; 14:944–957.
KEY POINTS
- Hormone therapy is indicated for relief of menopausal symptoms; claims of reversal of the aging process are unsubstantiated.
- Products that are custom-compounded are not regulated by the US Food and Drug Administration and therefore carry no assurance of purity, safety, or efficacy.
- Transdermal progesterone creams do not achieve high enough serum levels to protect the endometrium.
- Hormone therapy is titrated on the basis of symptom response. Measuring hormone levels in saliva is not called for and is probably not reliable.