User login
Trends in cardiovascular risk profiles
Many clinical improvements in treating patients with acute ST-elevation myocardial infarction (STEMI) have been realized in the past 20 years, including angiotensin-converting enzyme inhibitors, antiplatelet agents, and reduced time to cardiac cauterization procedures for acute myocardial infaction.1 Presumably, primary and secondary prevention measures have also resulted in changes in coronary artery disease (CAD) risk factors over the past 20 years. We sought to quantify mortality outcomes for patients treated in our catherization laboratory and to investigate trends in cardiovascular risk factors in patients during the same period.2
STEMI OUTCOMES
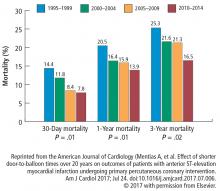
Data from our catherization laboratory database of 3,913 patients treated for STEMI at our tertiary care center from 1995 through 2014 were analyzed. To evaluate outcomes over time, patients were grouped based on years treated in 5-year increments resulting in 4 groups spanning 20 years.2
CARDIOVASCULAR RISK FACTORS
A reduction in mortality rates in patients treated for STEMI is to be expected over time, given the improvements in clinical practices and procedures and novel medications developed since 1996. But it is also possible that patients presenting with STEMI are healthier than in the past as a result of primary prevention efforts to minimize CAD risk factors and changes in CAD risk factors over time.
To determine whether CAD risk factors have changed over time, we analyzed the risk factors in the 3,913 patients treated for STEMI in our database. Risk factors included in the analysis were:
- Age
- Sex
- Diabetes mellitus
- Hypertension
- Smoking
- Hyperlipidemia
- Chronic renal impairment (serum creatinine greater than 1.5 mg/dL)
- Obesity (body mass index greater than 30 kg/m2).2
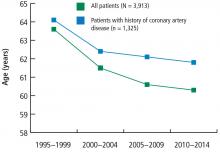
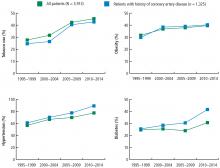
The prevalence of risk factors was determined in the entire cohort as well as in the 34% (n = 1,325) of patients previously diagnosed with CAD. The trend in risk factors in patients previously diagnosed with CAD could indicate the effectiveness of secondary prevention efforts compared with primary prevention in the broader patient population.
These data suggest that despite a better understanding of cardiovascular risk factors, the cardiovascular risk profiles of patients with acute STEMI have deteriorated over the past 20 years: patients are younger at presentation and more likely to be obese, to smoke, and to have hypertension and diabetes. These trends hold true in patients with and without a history of CAD, suggesting primary and secondary prevention efforts are ineffective.
TRENDS IN THE UNITED STATES
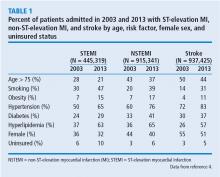
To evaluate whether geographic or patient population characteristics could have biased our results, we analyzed mortality and risk factor data from the National (Nationwide) Inpatient Sample (NIS) for patients presenting with STEMI (N = 445,319), non-STEMI (N = 915,341), and stroke (N = 937,425) from 2003 to 2013.4,5
Mortality rates
Consistent with the trend in our data, the 10-year NIS data showed a lower mortality rate in 2003 compared with 2013 in patients admitted with extreme-severity STEMI (22% vs 18%), non-STEMI (13% vs 8%), and stroke (15% vs 10%), as well as in patients with moderate-severity disease.4
Risk factors
Unfortunately, the prevalence of these relatively preventable CAD risk factors is moving in the wrong direction. The prevalence of smoking in patients presenting with non-STEMI, STEMI, or acute stroke is higher than in the past, contrary to the nationwide trend of decreasing rates of smoking.6 The increased rate of obesity evident in our data and the NSI data is consistent with rising obesity rates in the United States, which went from 30% to 37% in adults and from 14% to 17% in youth from 2000 to 2014.7 The percentage of adults with diabetes has increased tremendously in the United States, from 4.4% of adults in 1994 to 9.1% of adults in 2015.8 The rise in diabetes has led to increased rates of CAD, heart disease, and stroke in patients with diabetes.9
OPPORTUNITIES AHEAD
Despite improved STEMI outcomes, trends in cardiovascular risk profiles are deteriorating, emphasizing the critical need to educate people about primary and secondary prevention. Folsom et al10 conducted an analysis of a community-based sample to determine the prevalence of ideal cardiovascular health based on 4 ideal health behaviors (nonsmoking, low body mass index, adequate physical activity, healthy diet) and 3 ideal risk health factors (total cholesterol, blood pressure, and moderate glucose control).10 Each of the 7 behavior and risk factors was defined by ideal, intermediate, and poor characteristics. Very few study participants (0.1%) had ideal levels for all 7 healthy cardiovascular behaviors and risk factors, and over 82% had poor levels for all 7 behaviors and characteristics. The need to educate and improve cardiovascular health exists for both adults and youth. Measures of cardiovascular health in the United States indicate that 18% of adults age 50 or older and 46% of youth (ages 12 to 19) have 5 or more of the 7 health cardiovascular behaviors and risk factors at ideal levels.11
Improvement in primary and secondary prevention measures may also present opportunities to contain or reduce the cost of care. Thus far, according to NIS registry data from 2003 to 2013, the mean adjusted cost of hospitalization for patients with STEMI increased about 14%, remained about the same for patients with non-STEMI, and increased about 3% for patients with stroke.4
CONCLUSION
Advances in clinical care have improved outcomes for patients with CAD during the past 2 decades. These gains have come despite a higher prevalence of CAD risk factors in patients. More emphasis on primary and secondary prevention to reduce CAD risk factors may further improve outcomes and possibly lower the cost of care. Aggressive encouragement of risk factor modification is necessary and should go beyond cardiologists to include primary care physicians, preventive clinics, secondary cardiovascular prevention, and population-based efforts.
- Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 2004; 129:e28–e292.
- Mentias A, Hill E, Barakat AF, et al. An alarming trend: change in the risk profile of patients with ST elevation myocardial infarction over the last two decades. Int J Cardiol 2017; doi:10.1016/j.ijcard.2017.05.011. [Epub ahead of print]
- Mentias A, Raza MQ, Barakat AF, et al. Effect of shorter door-to-balloon times over 20 years on outcomes of patients with anterior ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol 2017; Jul 24. doi:10.1016/j.amjcard.2017.07.006. [Epub ahead of print].
- Agarwal S, Sud K, Thakkar B, Menon V, Jaber WA, Kapadia SR. Changing trends of atherosclerotic risk factors among patients with acute myocardial infarction and acute ischemic stroke. Am J Cardiol 2017; 119:1532–1541.
- HCUP NIS Database Documentation. Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality, Rockville, MD. https://www.hcup-us.ahrq.gov/db/nation/nis/nisdbdocumentation.jsp. March 2017. Accessed September 11 2017.
- Centers for Disease Control and Prevention. Trends in current cigarette smoking among high school students and adults, United States, 1965–2014. https://www.cdc.gov/tobacco/data_statistics/tables/trends/cig_smoking. Updated March 30, 2016. Accessed September 11, 2017.
- Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS data brief, no 219. Hyattsville, MD: National Center for Health Statistics. 2015. Available at https://www.cdc.gov/nchs/data/databriefs/db219.htm. Accessed September 11, 2017.
- Centers for Disease Control and Prevention. Diabetes data and statistics. https://gis.cdc.gov/grasp/diabetes/DiabetesAtlas.html. Updated July 17, 2017. Accessed September 11, 2017.
- Centers for Disease Control and Prevention. Diabetes, heart disease, and you. https://www.cdc.gov/features/diabetes-heart-disease/index.html. Updated November 19, 2016. Accessed September 11, 2017.
- Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD; for the ARIC Study Investigators. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol 2011; 57:1690–1696.
- Mozaffarian D, Benjamin EJ, Go AS, et al; on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016; 133:e38–e360.
Many clinical improvements in treating patients with acute ST-elevation myocardial infarction (STEMI) have been realized in the past 20 years, including angiotensin-converting enzyme inhibitors, antiplatelet agents, and reduced time to cardiac cauterization procedures for acute myocardial infaction.1 Presumably, primary and secondary prevention measures have also resulted in changes in coronary artery disease (CAD) risk factors over the past 20 years. We sought to quantify mortality outcomes for patients treated in our catherization laboratory and to investigate trends in cardiovascular risk factors in patients during the same period.2
STEMI OUTCOMES
Data from our catherization laboratory database of 3,913 patients treated for STEMI at our tertiary care center from 1995 through 2014 were analyzed. To evaluate outcomes over time, patients were grouped based on years treated in 5-year increments resulting in 4 groups spanning 20 years.2
CARDIOVASCULAR RISK FACTORS
A reduction in mortality rates in patients treated for STEMI is to be expected over time, given the improvements in clinical practices and procedures and novel medications developed since 1996. But it is also possible that patients presenting with STEMI are healthier than in the past as a result of primary prevention efforts to minimize CAD risk factors and changes in CAD risk factors over time.
To determine whether CAD risk factors have changed over time, we analyzed the risk factors in the 3,913 patients treated for STEMI in our database. Risk factors included in the analysis were:
- Age
- Sex
- Diabetes mellitus
- Hypertension
- Smoking
- Hyperlipidemia
- Chronic renal impairment (serum creatinine greater than 1.5 mg/dL)
- Obesity (body mass index greater than 30 kg/m2).2
The prevalence of risk factors was determined in the entire cohort as well as in the 34% (n = 1,325) of patients previously diagnosed with CAD. The trend in risk factors in patients previously diagnosed with CAD could indicate the effectiveness of secondary prevention efforts compared with primary prevention in the broader patient population.
These data suggest that despite a better understanding of cardiovascular risk factors, the cardiovascular risk profiles of patients with acute STEMI have deteriorated over the past 20 years: patients are younger at presentation and more likely to be obese, to smoke, and to have hypertension and diabetes. These trends hold true in patients with and without a history of CAD, suggesting primary and secondary prevention efforts are ineffective.
TRENDS IN THE UNITED STATES
To evaluate whether geographic or patient population characteristics could have biased our results, we analyzed mortality and risk factor data from the National (Nationwide) Inpatient Sample (NIS) for patients presenting with STEMI (N = 445,319), non-STEMI (N = 915,341), and stroke (N = 937,425) from 2003 to 2013.4,5
Mortality rates
Consistent with the trend in our data, the 10-year NIS data showed a lower mortality rate in 2003 compared with 2013 in patients admitted with extreme-severity STEMI (22% vs 18%), non-STEMI (13% vs 8%), and stroke (15% vs 10%), as well as in patients with moderate-severity disease.4
Risk factors
Unfortunately, the prevalence of these relatively preventable CAD risk factors is moving in the wrong direction. The prevalence of smoking in patients presenting with non-STEMI, STEMI, or acute stroke is higher than in the past, contrary to the nationwide trend of decreasing rates of smoking.6 The increased rate of obesity evident in our data and the NSI data is consistent with rising obesity rates in the United States, which went from 30% to 37% in adults and from 14% to 17% in youth from 2000 to 2014.7 The percentage of adults with diabetes has increased tremendously in the United States, from 4.4% of adults in 1994 to 9.1% of adults in 2015.8 The rise in diabetes has led to increased rates of CAD, heart disease, and stroke in patients with diabetes.9
OPPORTUNITIES AHEAD
Despite improved STEMI outcomes, trends in cardiovascular risk profiles are deteriorating, emphasizing the critical need to educate people about primary and secondary prevention. Folsom et al10 conducted an analysis of a community-based sample to determine the prevalence of ideal cardiovascular health based on 4 ideal health behaviors (nonsmoking, low body mass index, adequate physical activity, healthy diet) and 3 ideal risk health factors (total cholesterol, blood pressure, and moderate glucose control).10 Each of the 7 behavior and risk factors was defined by ideal, intermediate, and poor characteristics. Very few study participants (0.1%) had ideal levels for all 7 healthy cardiovascular behaviors and risk factors, and over 82% had poor levels for all 7 behaviors and characteristics. The need to educate and improve cardiovascular health exists for both adults and youth. Measures of cardiovascular health in the United States indicate that 18% of adults age 50 or older and 46% of youth (ages 12 to 19) have 5 or more of the 7 health cardiovascular behaviors and risk factors at ideal levels.11
Improvement in primary and secondary prevention measures may also present opportunities to contain or reduce the cost of care. Thus far, according to NIS registry data from 2003 to 2013, the mean adjusted cost of hospitalization for patients with STEMI increased about 14%, remained about the same for patients with non-STEMI, and increased about 3% for patients with stroke.4
CONCLUSION
Advances in clinical care have improved outcomes for patients with CAD during the past 2 decades. These gains have come despite a higher prevalence of CAD risk factors in patients. More emphasis on primary and secondary prevention to reduce CAD risk factors may further improve outcomes and possibly lower the cost of care. Aggressive encouragement of risk factor modification is necessary and should go beyond cardiologists to include primary care physicians, preventive clinics, secondary cardiovascular prevention, and population-based efforts.
Many clinical improvements in treating patients with acute ST-elevation myocardial infarction (STEMI) have been realized in the past 20 years, including angiotensin-converting enzyme inhibitors, antiplatelet agents, and reduced time to cardiac cauterization procedures for acute myocardial infaction.1 Presumably, primary and secondary prevention measures have also resulted in changes in coronary artery disease (CAD) risk factors over the past 20 years. We sought to quantify mortality outcomes for patients treated in our catherization laboratory and to investigate trends in cardiovascular risk factors in patients during the same period.2
STEMI OUTCOMES
Data from our catherization laboratory database of 3,913 patients treated for STEMI at our tertiary care center from 1995 through 2014 were analyzed. To evaluate outcomes over time, patients were grouped based on years treated in 5-year increments resulting in 4 groups spanning 20 years.2
CARDIOVASCULAR RISK FACTORS
A reduction in mortality rates in patients treated for STEMI is to be expected over time, given the improvements in clinical practices and procedures and novel medications developed since 1996. But it is also possible that patients presenting with STEMI are healthier than in the past as a result of primary prevention efforts to minimize CAD risk factors and changes in CAD risk factors over time.
To determine whether CAD risk factors have changed over time, we analyzed the risk factors in the 3,913 patients treated for STEMI in our database. Risk factors included in the analysis were:
- Age
- Sex
- Diabetes mellitus
- Hypertension
- Smoking
- Hyperlipidemia
- Chronic renal impairment (serum creatinine greater than 1.5 mg/dL)
- Obesity (body mass index greater than 30 kg/m2).2
The prevalence of risk factors was determined in the entire cohort as well as in the 34% (n = 1,325) of patients previously diagnosed with CAD. The trend in risk factors in patients previously diagnosed with CAD could indicate the effectiveness of secondary prevention efforts compared with primary prevention in the broader patient population.
These data suggest that despite a better understanding of cardiovascular risk factors, the cardiovascular risk profiles of patients with acute STEMI have deteriorated over the past 20 years: patients are younger at presentation and more likely to be obese, to smoke, and to have hypertension and diabetes. These trends hold true in patients with and without a history of CAD, suggesting primary and secondary prevention efforts are ineffective.
TRENDS IN THE UNITED STATES
To evaluate whether geographic or patient population characteristics could have biased our results, we analyzed mortality and risk factor data from the National (Nationwide) Inpatient Sample (NIS) for patients presenting with STEMI (N = 445,319), non-STEMI (N = 915,341), and stroke (N = 937,425) from 2003 to 2013.4,5
Mortality rates
Consistent with the trend in our data, the 10-year NIS data showed a lower mortality rate in 2003 compared with 2013 in patients admitted with extreme-severity STEMI (22% vs 18%), non-STEMI (13% vs 8%), and stroke (15% vs 10%), as well as in patients with moderate-severity disease.4
Risk factors
Unfortunately, the prevalence of these relatively preventable CAD risk factors is moving in the wrong direction. The prevalence of smoking in patients presenting with non-STEMI, STEMI, or acute stroke is higher than in the past, contrary to the nationwide trend of decreasing rates of smoking.6 The increased rate of obesity evident in our data and the NSI data is consistent with rising obesity rates in the United States, which went from 30% to 37% in adults and from 14% to 17% in youth from 2000 to 2014.7 The percentage of adults with diabetes has increased tremendously in the United States, from 4.4% of adults in 1994 to 9.1% of adults in 2015.8 The rise in diabetes has led to increased rates of CAD, heart disease, and stroke in patients with diabetes.9
OPPORTUNITIES AHEAD
Despite improved STEMI outcomes, trends in cardiovascular risk profiles are deteriorating, emphasizing the critical need to educate people about primary and secondary prevention. Folsom et al10 conducted an analysis of a community-based sample to determine the prevalence of ideal cardiovascular health based on 4 ideal health behaviors (nonsmoking, low body mass index, adequate physical activity, healthy diet) and 3 ideal risk health factors (total cholesterol, blood pressure, and moderate glucose control).10 Each of the 7 behavior and risk factors was defined by ideal, intermediate, and poor characteristics. Very few study participants (0.1%) had ideal levels for all 7 healthy cardiovascular behaviors and risk factors, and over 82% had poor levels for all 7 behaviors and characteristics. The need to educate and improve cardiovascular health exists for both adults and youth. Measures of cardiovascular health in the United States indicate that 18% of adults age 50 or older and 46% of youth (ages 12 to 19) have 5 or more of the 7 health cardiovascular behaviors and risk factors at ideal levels.11
Improvement in primary and secondary prevention measures may also present opportunities to contain or reduce the cost of care. Thus far, according to NIS registry data from 2003 to 2013, the mean adjusted cost of hospitalization for patients with STEMI increased about 14%, remained about the same for patients with non-STEMI, and increased about 3% for patients with stroke.4
CONCLUSION
Advances in clinical care have improved outcomes for patients with CAD during the past 2 decades. These gains have come despite a higher prevalence of CAD risk factors in patients. More emphasis on primary and secondary prevention to reduce CAD risk factors may further improve outcomes and possibly lower the cost of care. Aggressive encouragement of risk factor modification is necessary and should go beyond cardiologists to include primary care physicians, preventive clinics, secondary cardiovascular prevention, and population-based efforts.
- Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 2004; 129:e28–e292.
- Mentias A, Hill E, Barakat AF, et al. An alarming trend: change in the risk profile of patients with ST elevation myocardial infarction over the last two decades. Int J Cardiol 2017; doi:10.1016/j.ijcard.2017.05.011. [Epub ahead of print]
- Mentias A, Raza MQ, Barakat AF, et al. Effect of shorter door-to-balloon times over 20 years on outcomes of patients with anterior ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol 2017; Jul 24. doi:10.1016/j.amjcard.2017.07.006. [Epub ahead of print].
- Agarwal S, Sud K, Thakkar B, Menon V, Jaber WA, Kapadia SR. Changing trends of atherosclerotic risk factors among patients with acute myocardial infarction and acute ischemic stroke. Am J Cardiol 2017; 119:1532–1541.
- HCUP NIS Database Documentation. Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality, Rockville, MD. https://www.hcup-us.ahrq.gov/db/nation/nis/nisdbdocumentation.jsp. March 2017. Accessed September 11 2017.
- Centers for Disease Control and Prevention. Trends in current cigarette smoking among high school students and adults, United States, 1965–2014. https://www.cdc.gov/tobacco/data_statistics/tables/trends/cig_smoking. Updated March 30, 2016. Accessed September 11, 2017.
- Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS data brief, no 219. Hyattsville, MD: National Center for Health Statistics. 2015. Available at https://www.cdc.gov/nchs/data/databriefs/db219.htm. Accessed September 11, 2017.
- Centers for Disease Control and Prevention. Diabetes data and statistics. https://gis.cdc.gov/grasp/diabetes/DiabetesAtlas.html. Updated July 17, 2017. Accessed September 11, 2017.
- Centers for Disease Control and Prevention. Diabetes, heart disease, and you. https://www.cdc.gov/features/diabetes-heart-disease/index.html. Updated November 19, 2016. Accessed September 11, 2017.
- Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD; for the ARIC Study Investigators. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol 2011; 57:1690–1696.
- Mozaffarian D, Benjamin EJ, Go AS, et al; on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016; 133:e38–e360.
- Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 2004; 129:e28–e292.
- Mentias A, Hill E, Barakat AF, et al. An alarming trend: change in the risk profile of patients with ST elevation myocardial infarction over the last two decades. Int J Cardiol 2017; doi:10.1016/j.ijcard.2017.05.011. [Epub ahead of print]
- Mentias A, Raza MQ, Barakat AF, et al. Effect of shorter door-to-balloon times over 20 years on outcomes of patients with anterior ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol 2017; Jul 24. doi:10.1016/j.amjcard.2017.07.006. [Epub ahead of print].
- Agarwal S, Sud K, Thakkar B, Menon V, Jaber WA, Kapadia SR. Changing trends of atherosclerotic risk factors among patients with acute myocardial infarction and acute ischemic stroke. Am J Cardiol 2017; 119:1532–1541.
- HCUP NIS Database Documentation. Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality, Rockville, MD. https://www.hcup-us.ahrq.gov/db/nation/nis/nisdbdocumentation.jsp. March 2017. Accessed September 11 2017.
- Centers for Disease Control and Prevention. Trends in current cigarette smoking among high school students and adults, United States, 1965–2014. https://www.cdc.gov/tobacco/data_statistics/tables/trends/cig_smoking. Updated March 30, 2016. Accessed September 11, 2017.
- Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS data brief, no 219. Hyattsville, MD: National Center for Health Statistics. 2015. Available at https://www.cdc.gov/nchs/data/databriefs/db219.htm. Accessed September 11, 2017.
- Centers for Disease Control and Prevention. Diabetes data and statistics. https://gis.cdc.gov/grasp/diabetes/DiabetesAtlas.html. Updated July 17, 2017. Accessed September 11, 2017.
- Centers for Disease Control and Prevention. Diabetes, heart disease, and you. https://www.cdc.gov/features/diabetes-heart-disease/index.html. Updated November 19, 2016. Accessed September 11, 2017.
- Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD; for the ARIC Study Investigators. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol 2011; 57:1690–1696.
- Mozaffarian D, Benjamin EJ, Go AS, et al; on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016; 133:e38–e360.
KEY POINTS
- Advances in treatment of CAD have improved patient outcomes over the past 20 years.
- Prevalence of risk factors for CAD has increased over the past 20 years in patients presenting with STEMI with patients now more likely to be younger and with higher prevalence of smoking, obesity, hypertension, and diabetes.
- Emphasis on primary and secondary prevention to reduce CAD risk factors is needed to improve outcomes and reduce the cost of care.
Transcatheter aortic valve replacement: History and current indications
Transcatheter aortic valve replacement (TAVR) has established itself as an effective way of treating high-risk patients with severe aortic valve stenosis. With new generations of existing valves and newer alternative devices, the procedure promises to become increasingly safer. The field is evolving rapidly and it will be important for interventional cardiologists and cardiac surgeons alike to stay abreast of developments. This article reviews the history of this promising procedure and examines its use in current practice.
HISTORICAL PERSPECTIVE
In 1980, Danish researcher H. R. Anderson reported developing and testing a balloon-expandable valve in animals.1 The technology was eventually acquired and further developed by Edwards Life Sciences (Irvine, California).
Alain Cribier started early work in humans in 2002 in France.2 He used a transfemoral arterial access to approach the aortic valve transseptally, but this procedure was associated with high rates of mortality and stroke.3 At the same time, in the United States, animal studies were being carried out by Lars G. Svensson, Todd Dewey, and Michael Mack to develop a transapical method of implantation,4,5 while John Webb and colleagues were also developing a transapical aortic valve implantation technique,6,7 and later went on to develop a retrograde transfemoral technique. This latter technique became feasible once Edwards developed a catheter that could be flexed to get around the aortic arch and across the aortic valve.
As the Edwards balloon-expandable valve (Sapien) was being developed, a nitinol-based self-expandable valve system was introduced by Medtronic: the CoreValve. Following feasibility studies,5,8 the safety and efficacy of these valves were established thorough the Placement of Aortic Transcatheter Valves (PARTNER) trial and the US Core Valve Pivotal Trial. These valves are currently approved by the US Food and Drug Administration (FDA) for patients for whom conventional surgery would pose an extreme or high risk.9–11
CLINICAL TRIALS OF TAVR
The two landmark prospective randomized trials of TAVR were the PARTNER trial and CoreValve Pivotal Trial.
The PARTNER trial consisted of two parts: PARTNER A, which compared the Sapien balloon-expandable transcatheter valve with surgical aortic valve replacement in patients at high surgical risk (Society of Thoracic Surgeons [STS] score > 10%), and PARTNER B, which compared TAVR with medical therapy in patients who could not undergo surgery (combined risk of serious morbidity or death of 50% or more, and two surgeons agreeing that the patient was inoperable).
Similarly, the CoreValve Pivotal Trial compared the self-expandable transcatheter valve with conventional medical and surgical treatment.
TAVR is comparable to surgery in outcomes, with caveats
In the PARTNER A trial, mortality rates were similar between patients who underwent Sapien TAVR and those who underwent surgical valve replacement at 30 days (3.4% and 6.5%, P = .07), 1 year (24.2% and 26.8%), and 2 years (33.9% and 35.0%). The patients in this group were randomized to either Sapien TAVR or surgery (Table 1).10,12
The combined rate of stroke and transient ischemic attack was higher in the patients assigned to TAVR at 30 days (5.5% with TAVR vs 2.4% with surgery, P = .04) and at 1 year (8.3% with TAVR vs 4.3% with surgery, P = .04). The difference was of small significance at 2 years (11.2% vs 6.5%, P = .05). At 30 days, the rate of major vascular complications was higher with TAVR (11.0% vs 3.2%), while surgery was associated with more frequent major bleeding episodes (19.5% vs 9.3%) and new-onset atrial fibrillation (16.0% vs 8.6%). The rate of new pacemaker requirement at 30 days was similar between the TAVR and surgical groups (3.8% vs 3.6%). Moderate or severe paravalvular aortic regurgitation was more common after TAVR at 30 days, 1 year, and 2 years. This aortic insufficiency was associated with increased late mortality.10,12
In the US CoreValve High Risk Study, no difference was found in the 30-day mortality rate in patients at high surgical risk randomized to CoreValve TAVR or surgery (3.3% and 4.5%) (Table 1). Surprisingly, the 1-year mortality rate was lower in the TAVR group than in the surgical group (14.1% vs 18.9%, respectively), a finding sustained at 2 years in data presented at the American College of Cardiology conference in March 2015.13–16
TAVR is superior to medical management, but the risk of stroke is higher
In the PARTNER B trial, inoperable patients were randomly assigned to undergo TAVR with a Sapien valve or medical management. TAVR resulted in lower mortality rates at 1 year (30.7% vs 50.7%) and 2 years (43.4% vs 68.0%) compared with medical management (Table 1).17 Of note, medical management included balloon valvuloplasty. The rate of the composite end point of death or repeat hospitalization was also lower with TAVR compared with medical therapy (44.1% vs 71.6%, respectively, at 1 year and 56.7% and 87.9%, respectively, at 2 years).17 The TAVR group had a higher stroke rate than the medical therapy group at 30 days (11.2% vs 5.5%, respectively) and at 2 years (13.8% vs 5.5%).17 Survival improved with TAVR in patients with an STS score of less than 15% but not in those with an STS score of 15% or higher.9
The very favorable results from the PARTNER trial rendered a randomized trial comparing self-expanding (CoreValve) TAVR and medical therapy unethical. Instead, a prospective single-arm study, the CoreValve Extreme Risk US Pivotal Trial, was used to compare the 12-month rate of death or major stroke with CoreValve TAVR vs a prespecified estimate of this rate with medical therapy.14 In about 500 patients who had a CoreValve attempt, the rate of all-cause mortality or major stroke at 1 year was significantly lower than the prespecified expected rate (26% vs 43%), reinforcing the results from the PARTNER Trial.14
Five-year outcomes
The 5-year PARTNER clinical and valve performance outcomes were published recently18 and continued to demonstrate equivalent outcomes for high-risk patients who underwent surgical aortic valve replacement or TAVR; there were no significant differences in all-cause mortality, cardiovascular mortality, stroke, or need for readmission to the hospital. The functional outcomes were similar as well, and no differences were demonstrated between surgical and TAVR valve performance.
Of note, moderate or severe aortic regurgitation occurred in 14% of patients in the TAVR group compared with 1% in the surgical aortic valve replacement group (P < .0001). This was associated with increased 5-year risk of death in the TAVR group (72.4% in those with moderate or severe aortic regurgitation vs 56.6% in those with mild aortic regurgitation or less; P = .003).
If the available randomized data are combined with observational reports, overall mortality and stroke rates are comparable between surgical aortic valve replacement and balloon-expandable or self-expandable TAVR in high-risk surgical candidates. Vascular complications, aortic regurgitation and permanent pacemaker insertion occur more frequently after TAVR, while major bleeding is more likely to occur after surgery.19 As newer generations of valves are developed, it is expected that aortic regurgitation and pacemaker rates will decrease over time. Indeed, trial data presented at the American College of Cardiology meeting in March 2015 for the third-generation Sapien valve (Sapien S3) showed only a 3.0% to 4.2% rate of significant paravalvular leak.
Contemporary valve comparison data
The valve used in the original PARTNER data was the first-generation Sapien valve. Since then, the second generation of this valve, the Sapien XT, has been introduced and is the model currently used in the United States (with the third-generation valve mentioned above, the Sapien S3, still available only through clinical trials). Thus, the two contemporary valves available for commercial use in the United States are the Edwards Sapien XT and Medtronic CoreValve. There are limited data comparing these valves head-to-head, but one recent trial attempted to do just that.
The Comparison of Transcatheter Heart Valves in High Risk Patients with Severe Aortic Stenosis: Medtronic CoreValve vs Edwards Sapien XT (CHOICE) trial compared the Edwards Sapien XT and CoreValve devices. Two hundred and forty-one patients were randomized. The primary end point of this trial was “device success” (a composite end point of four components: successful vascular access and deployment of the device with retrieval of the delivery system, correct position of the device, intended performance of the valve without moderate or severe insufficiency, and only one valve implanted in the correct anatomical location).
In this trial, the balloon-expandable Sapien XT valve showed a significantly higher device success rate than the self-expanding CoreValve, due to a significantly lower rate of aortic regurgitation (4.1% vs 18.3%, P < .001) and the less frequent need for implantation of more than one valve (0.8% vs 5.8%, P = .03). Placement of a permanent pacemaker was considerably less frequent in the balloon-expandable valve group (17.3% vs 37.6%, P = .001).20
PREOPERATIVE CONSIDERATIONS AND EVALUATION CRITERIA
Currently, TAVR is indicated for patients with symptomatic severe native aortic valve stenosis who are deemed at high risk or inoperable by a heart team including interventional cardiologists and cardiac surgeons. The CoreValve was also recently approved for valve-in-valve insertion in high-risk or inoperable patients with a prosthetic aortic valve in place.
The STS risk score is a reasonable preliminary risk assessment tool and is applicable to most patients being evaluated for aortic valve replacement. The STS risk score represents the percentage risk of unfavorable outcomes based on certain clinical variables. A calculator is available at riskcalc.sts.org. Patients considered at high risk are those with an STS operative risk score of 8% or higher or a postoperative 30-day risk of death of 15% or higher.
It is important to remember, though, that the STS score does not account for certain severe surgical risk factors. These include the presence of a "porcelain aorta" (heavy circumferential calcification of the ascending aorta precluding cross-clamping), history of mediastinal radiation, “hostile chest” (kyphoscoliosis, other deformities, previous coronary artery bypass grafting with adhesion of internal mammary artery to the back of sternum), severely compromised respiratory function (forced expiratory volume in 1 second < 1 L or < 40% predicted, diffusing capacity for carbon monoxide < 30%), severe pulmonary hypertension, severe liver disease (Model for End-stage Liver Disease score 8–20), severe dementia, severe cerebrovascular disease, and frailty.
With regard to this last risk factor, frailty is not simply old age but rather a measurable characteristic akin to weakness or disability. Several tests exist to measure frailty, including the “eyeball test” (the physician’s subjective assessment), Mini-Mental State Examination, gait speed/15-foot walk test, hand grip strength, serum albumin, and assessment of activities of daily living. Formal frailty testing is recommended during the course of a TAVR workup.
Risk assessment and patient suitability for TAVR is ultimately determined by the combined judgment of the heart valve team using both the STS score and consideration of these other factors.
Implantation approaches
Today, TAVR could be performed by several approaches: transfemoral arterial, transapical, transaortic via partial sternotomy or right anterior thoracotomy,21,22 transcarotid,23–25 and transaxillary or subclavian.26,27 Less commonly, transfemoral-venous routes have been performed utilizing either transseptal28 or caval-aortic puncture.29
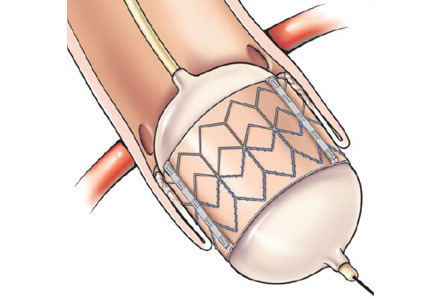
The transfemoral approach is used most commonly by most institutions, including Cleveland Clinic. It allows for a completely percutaneous insertion and, in select cases, without endotracheal intubation and general anesthesia (Figure 1).
In patients with difficult femoral access due to severe calcification, extreme tortuosity, or small diameter, alternative access routes become a consideration. In this situation, at our institution, we favor the transaortic approach in patients who have not undergone cardiac surgery in the past, while the transapical approach is used in patients who had previous cardiac surgery. With the transapical approach, we have found the outcomes similar to those of transfemoral TAVR after propensity matching.30,31 Although there is a learning curve,32 transapical TAVR can be performed with very limited mortality and morbidity. In a recent series at Cleveland Clinic, the mortality rate with the transapical approach was 1.2%, renal failure occurred in 4.7%, and a pacemaker was placed in 5.9% of patients; there were no strokes.33 This approach can be utilized for simultaneous additional procedures like transcatheter mitral valve reimplantation and percutaneous coronary interventions.34–36
- Andersen HR, Knudsen LL, Hasenkam JM. Transluminal implantation of artificial heart valves. Description of a new expandable aortic valve and initial results with implantation by catheter technique in closed chest pigs. Eur Heart J 1992; 13:704– 708.
- Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case descrip- tion. Circulation 2002; 106:3006–3008.
- Cribier A, Eltchaninoff H, Tron C, et al. Early experience with percutaneous transcatheter implantation of heart valve prosthesis for the treatment of end-stage inoperable patients with calcific aortic stenosis. J Am Coll Cardiol 2004; 43:698– 703.
- Dewey TM, Walther T, Doss M, et al. Transapical aortic valve implantation: an animal feasibility study. Ann Thorac Surg 2006; 82:110–116.
- Svensson LG, Dewey T, Kapadia S, et al. United States feasibility study of trans- catheter insertion of a stented aortic valve by the left ventricular apex. Ann Thorac Surg 2008; 86:46–54.
- Lichtenstein SV, Cheung A, Ye J, et al. Transapical transcatheter aortic valve im- plantation in humans: initial clinical experience. Circulation 2006; 114:591–596.
- Webb JG, Pasupati S, Hyumphries K, et al. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation 2007; 116:755–763.
- Leon MB, Kodali S, Williams M, et al. Transcatheter aortic valve replacement in patients with critical aortic stenosis: rationale, device descriptions, early clinical experiences, and perspectives. Semin Thorac Cardiovasc Surg 2006; 18:165–174.
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo sur- gery. N Engl J Med 2010; 363:1597–1607.
- Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187–2198.
- Adams DH, Popma JJ, Reardon MJ, et al; U.S. CoreValve Clinical Investigators. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014; 370:1790–1798.
- Kodali SK, Williams MR, Smith CR, et al; PARTNER Trial Investigators. Two- year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012; 366:1686–1695.
- Reardon M, et al. A randomized comparison of self-expanding
- Popma JJ, Adams DH, Reardon MJ, et al; CoreValve United States Clinical In- vestigators. Transcatheter aortic valve replacement using a self-expanding biopros- thesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol 2014; 63:1972–1981.
- Adams DH, Popma JJ, Reardon MJ. Transcatheter aortic-valve replacement with a self-expanding prosthesis (letter). N Engl J Med 2014; 371:967–968.
- Kaul S. Transcatheter aortic-valve replacement with a self-expanding prosthesis (letter). N Engl J Med 2014; 371:967.
- Makkar RR, Fontana GP, Jilaihawi H, et al. Transcathether aortic-valve re- placement for inoperable severe aortic stenosis. N Engl J Med 2012; 366: 1696–704.
- Mack MJ, Leon MB, Smith CR, et al; PARTNER 1 trial investigators. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve re- placement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015; 385:2477–2484.
- Cao C, Ang SC, Indraratna P, et al. Systematic review and meta-analysis of trans- catheter aortic valve implantation versus surgical aortic valve replacement for severe aortic stenosis. Ann Cardiothorac Surg 2013; 2:10–23.
- Abdel-Wahab M, Mehilli J, Frerker C, et al; CHOICE investigators. Comparison of balloon-expandable vs self-expandable valves in patients undergoing transcath- eter aortic valve replacement: the CHOICE randomized clinical trial. JAMA 2014; 311:1503–1514.
- Okuyama K, Jilaihawi H, Mirocha J, et al. Alternative access for balloon-ex- pandable transcatheter aortic valve replacement: comparison of the transaortic approach using right anterior thoracotomy to partial J-sternotomy. J Thorac Car- diovasc Surg 2014; 149:789–797.
- Lardizabal JA, O’Neill BP, Desai HV, et al. The transaortic approach for transcath- eter aortic valve replacement: initial clinical experience in the United States. J Am Coll Cardiol 2013; 61:2341–2345.
- Thourani VH, Gunter RL, Neravetla S, et al. Use of transaortic, transapical, and transcarotid transcatheter aortic valve replacement in inoperable patients. Ann Thorac Surg 2013; 96:1349–1357.
- Azmoun A, Amabile N, Ramadan R, et al. Transcatheter aortic valve implantation through carotid artery access under local anaesthesia. Eur J Cardiothorac Surg 2014; 46: 693–698.
- Rajagopal R, More RS, Roberts DH. Transcatheter aortic valve implantation through a transcarotid approach under local anesthesia. Catheter Cardiovasc In- terv 2014; 84:903–907.
- Fraccaro C, Napodano M, Tarantini G, et al. Expanding the eligibility for trans- catheter aortic valve implantation the trans-subclavian retrograde approach using: the III generation CoreValve revalving system. JACC Cardiovasc Interv 2009; 2:828–333.
- Petronio AS, De Carlo M, Bedogni F, et al. Safety and efficacy of the subclavian approach for transcatheter aortic valve implantation with the CoreValve revalving system. Circ Cardiovasc Interv 2010; 3:359–366.
- Cohen MG, Singh V, Martinez CA, et al. Transseptal antegrade transcatheter aor- tic valve replacement for patients with no other access approach—a contemporary experience. Catheter Cardiovasc Interv 2013; 82:987–993.
- Greenbaum AB, O’Neill WW, Paone G, et al. Caval-aortic access to allow trans- catheter aortic valve replacement in otherwise ineligible patients: initial human experience. J Am Coll Cardiol 2014; 63:2795–2804.
- D’Onofrio A, Salizzoni S, Agrifoglio M, et al. Medium term outcomes of trans- apical aortic valve implantation: results from the Italian Registry of Trans-Apical Aortic Valve Implantation. Ann Thorac Surg 2013; 96:830–835.
- Johansson M, Nozohoor S, Kimblad PO, Harnek J, Olivecrona GK, Sjögren J. Transapical versus transfemoral aortic valve implantation: a comparison of survival and safety. Ann Thorac Surg 2011; 91:57–63.
- Kempfert J, Rastan A, Holzhey D, et al. Transapical aortic valve implantation: analysis of risk factors and learning experience in 299 patients. Circulation 2011; 124(suppl):S124–S129.
- Aguirre J, Waskowski R, Poddar K, et al. Transcatheter aortic valve replacement: experience with the transapical approach, alternate access sites, and concomitant cardiac repairs. J Thorac Cardiovasc Surg 2014; 148:1417–1422.
- Al Kindi AH, Salhab KF, Roselli EE, Kapadia S, Tuzcu EM, Svensson LG. Alternative access options for transcatheter aortic valve replacement in patients with no conventional access and chest pathology. J Thorac Cardiovasc Surg 2014; 147:644–651.
- Salhab KF, Al Kindi AH, Lane JH, et al. Concomitant percutaneous coronary intervention and transcatheter aortic valve replacement: safe and feasible replace- ment alternative approaches in high-risk patients with severe aortic stenosis and coronary artery disease. J Card Surg 2013; 28:481–483.
- Al Kindi AH, Salhab KF, Kapadia S, et al. Simultaneous transapical transcatheter aortic and mitral valve replacement in a high-risk patient with a previous mitral bioprosthesis. J Thorac Cardiovasc Surg 2012; 144:e90–e91.
Transcatheter aortic valve replacement (TAVR) has established itself as an effective way of treating high-risk patients with severe aortic valve stenosis. With new generations of existing valves and newer alternative devices, the procedure promises to become increasingly safer. The field is evolving rapidly and it will be important for interventional cardiologists and cardiac surgeons alike to stay abreast of developments. This article reviews the history of this promising procedure and examines its use in current practice.
HISTORICAL PERSPECTIVE
In 1980, Danish researcher H. R. Anderson reported developing and testing a balloon-expandable valve in animals.1 The technology was eventually acquired and further developed by Edwards Life Sciences (Irvine, California).
Alain Cribier started early work in humans in 2002 in France.2 He used a transfemoral arterial access to approach the aortic valve transseptally, but this procedure was associated with high rates of mortality and stroke.3 At the same time, in the United States, animal studies were being carried out by Lars G. Svensson, Todd Dewey, and Michael Mack to develop a transapical method of implantation,4,5 while John Webb and colleagues were also developing a transapical aortic valve implantation technique,6,7 and later went on to develop a retrograde transfemoral technique. This latter technique became feasible once Edwards developed a catheter that could be flexed to get around the aortic arch and across the aortic valve.
As the Edwards balloon-expandable valve (Sapien) was being developed, a nitinol-based self-expandable valve system was introduced by Medtronic: the CoreValve. Following feasibility studies,5,8 the safety and efficacy of these valves were established thorough the Placement of Aortic Transcatheter Valves (PARTNER) trial and the US Core Valve Pivotal Trial. These valves are currently approved by the US Food and Drug Administration (FDA) for patients for whom conventional surgery would pose an extreme or high risk.9–11
CLINICAL TRIALS OF TAVR
The two landmark prospective randomized trials of TAVR were the PARTNER trial and CoreValve Pivotal Trial.
The PARTNER trial consisted of two parts: PARTNER A, which compared the Sapien balloon-expandable transcatheter valve with surgical aortic valve replacement in patients at high surgical risk (Society of Thoracic Surgeons [STS] score > 10%), and PARTNER B, which compared TAVR with medical therapy in patients who could not undergo surgery (combined risk of serious morbidity or death of 50% or more, and two surgeons agreeing that the patient was inoperable).
Similarly, the CoreValve Pivotal Trial compared the self-expandable transcatheter valve with conventional medical and surgical treatment.
TAVR is comparable to surgery in outcomes, with caveats
In the PARTNER A trial, mortality rates were similar between patients who underwent Sapien TAVR and those who underwent surgical valve replacement at 30 days (3.4% and 6.5%, P = .07), 1 year (24.2% and 26.8%), and 2 years (33.9% and 35.0%). The patients in this group were randomized to either Sapien TAVR or surgery (Table 1).10,12
The combined rate of stroke and transient ischemic attack was higher in the patients assigned to TAVR at 30 days (5.5% with TAVR vs 2.4% with surgery, P = .04) and at 1 year (8.3% with TAVR vs 4.3% with surgery, P = .04). The difference was of small significance at 2 years (11.2% vs 6.5%, P = .05). At 30 days, the rate of major vascular complications was higher with TAVR (11.0% vs 3.2%), while surgery was associated with more frequent major bleeding episodes (19.5% vs 9.3%) and new-onset atrial fibrillation (16.0% vs 8.6%). The rate of new pacemaker requirement at 30 days was similar between the TAVR and surgical groups (3.8% vs 3.6%). Moderate or severe paravalvular aortic regurgitation was more common after TAVR at 30 days, 1 year, and 2 years. This aortic insufficiency was associated with increased late mortality.10,12
In the US CoreValve High Risk Study, no difference was found in the 30-day mortality rate in patients at high surgical risk randomized to CoreValve TAVR or surgery (3.3% and 4.5%) (Table 1). Surprisingly, the 1-year mortality rate was lower in the TAVR group than in the surgical group (14.1% vs 18.9%, respectively), a finding sustained at 2 years in data presented at the American College of Cardiology conference in March 2015.13–16
TAVR is superior to medical management, but the risk of stroke is higher
In the PARTNER B trial, inoperable patients were randomly assigned to undergo TAVR with a Sapien valve or medical management. TAVR resulted in lower mortality rates at 1 year (30.7% vs 50.7%) and 2 years (43.4% vs 68.0%) compared with medical management (Table 1).17 Of note, medical management included balloon valvuloplasty. The rate of the composite end point of death or repeat hospitalization was also lower with TAVR compared with medical therapy (44.1% vs 71.6%, respectively, at 1 year and 56.7% and 87.9%, respectively, at 2 years).17 The TAVR group had a higher stroke rate than the medical therapy group at 30 days (11.2% vs 5.5%, respectively) and at 2 years (13.8% vs 5.5%).17 Survival improved with TAVR in patients with an STS score of less than 15% but not in those with an STS score of 15% or higher.9
The very favorable results from the PARTNER trial rendered a randomized trial comparing self-expanding (CoreValve) TAVR and medical therapy unethical. Instead, a prospective single-arm study, the CoreValve Extreme Risk US Pivotal Trial, was used to compare the 12-month rate of death or major stroke with CoreValve TAVR vs a prespecified estimate of this rate with medical therapy.14 In about 500 patients who had a CoreValve attempt, the rate of all-cause mortality or major stroke at 1 year was significantly lower than the prespecified expected rate (26% vs 43%), reinforcing the results from the PARTNER Trial.14
Five-year outcomes
The 5-year PARTNER clinical and valve performance outcomes were published recently18 and continued to demonstrate equivalent outcomes for high-risk patients who underwent surgical aortic valve replacement or TAVR; there were no significant differences in all-cause mortality, cardiovascular mortality, stroke, or need for readmission to the hospital. The functional outcomes were similar as well, and no differences were demonstrated between surgical and TAVR valve performance.
Of note, moderate or severe aortic regurgitation occurred in 14% of patients in the TAVR group compared with 1% in the surgical aortic valve replacement group (P < .0001). This was associated with increased 5-year risk of death in the TAVR group (72.4% in those with moderate or severe aortic regurgitation vs 56.6% in those with mild aortic regurgitation or less; P = .003).
If the available randomized data are combined with observational reports, overall mortality and stroke rates are comparable between surgical aortic valve replacement and balloon-expandable or self-expandable TAVR in high-risk surgical candidates. Vascular complications, aortic regurgitation and permanent pacemaker insertion occur more frequently after TAVR, while major bleeding is more likely to occur after surgery.19 As newer generations of valves are developed, it is expected that aortic regurgitation and pacemaker rates will decrease over time. Indeed, trial data presented at the American College of Cardiology meeting in March 2015 for the third-generation Sapien valve (Sapien S3) showed only a 3.0% to 4.2% rate of significant paravalvular leak.
Contemporary valve comparison data
The valve used in the original PARTNER data was the first-generation Sapien valve. Since then, the second generation of this valve, the Sapien XT, has been introduced and is the model currently used in the United States (with the third-generation valve mentioned above, the Sapien S3, still available only through clinical trials). Thus, the two contemporary valves available for commercial use in the United States are the Edwards Sapien XT and Medtronic CoreValve. There are limited data comparing these valves head-to-head, but one recent trial attempted to do just that.
The Comparison of Transcatheter Heart Valves in High Risk Patients with Severe Aortic Stenosis: Medtronic CoreValve vs Edwards Sapien XT (CHOICE) trial compared the Edwards Sapien XT and CoreValve devices. Two hundred and forty-one patients were randomized. The primary end point of this trial was “device success” (a composite end point of four components: successful vascular access and deployment of the device with retrieval of the delivery system, correct position of the device, intended performance of the valve without moderate or severe insufficiency, and only one valve implanted in the correct anatomical location).
In this trial, the balloon-expandable Sapien XT valve showed a significantly higher device success rate than the self-expanding CoreValve, due to a significantly lower rate of aortic regurgitation (4.1% vs 18.3%, P < .001) and the less frequent need for implantation of more than one valve (0.8% vs 5.8%, P = .03). Placement of a permanent pacemaker was considerably less frequent in the balloon-expandable valve group (17.3% vs 37.6%, P = .001).20
PREOPERATIVE CONSIDERATIONS AND EVALUATION CRITERIA
Currently, TAVR is indicated for patients with symptomatic severe native aortic valve stenosis who are deemed at high risk or inoperable by a heart team including interventional cardiologists and cardiac surgeons. The CoreValve was also recently approved for valve-in-valve insertion in high-risk or inoperable patients with a prosthetic aortic valve in place.
The STS risk score is a reasonable preliminary risk assessment tool and is applicable to most patients being evaluated for aortic valve replacement. The STS risk score represents the percentage risk of unfavorable outcomes based on certain clinical variables. A calculator is available at riskcalc.sts.org. Patients considered at high risk are those with an STS operative risk score of 8% or higher or a postoperative 30-day risk of death of 15% or higher.
It is important to remember, though, that the STS score does not account for certain severe surgical risk factors. These include the presence of a "porcelain aorta" (heavy circumferential calcification of the ascending aorta precluding cross-clamping), history of mediastinal radiation, “hostile chest” (kyphoscoliosis, other deformities, previous coronary artery bypass grafting with adhesion of internal mammary artery to the back of sternum), severely compromised respiratory function (forced expiratory volume in 1 second < 1 L or < 40% predicted, diffusing capacity for carbon monoxide < 30%), severe pulmonary hypertension, severe liver disease (Model for End-stage Liver Disease score 8–20), severe dementia, severe cerebrovascular disease, and frailty.
With regard to this last risk factor, frailty is not simply old age but rather a measurable characteristic akin to weakness or disability. Several tests exist to measure frailty, including the “eyeball test” (the physician’s subjective assessment), Mini-Mental State Examination, gait speed/15-foot walk test, hand grip strength, serum albumin, and assessment of activities of daily living. Formal frailty testing is recommended during the course of a TAVR workup.
Risk assessment and patient suitability for TAVR is ultimately determined by the combined judgment of the heart valve team using both the STS score and consideration of these other factors.
Implantation approaches
Today, TAVR could be performed by several approaches: transfemoral arterial, transapical, transaortic via partial sternotomy or right anterior thoracotomy,21,22 transcarotid,23–25 and transaxillary or subclavian.26,27 Less commonly, transfemoral-venous routes have been performed utilizing either transseptal28 or caval-aortic puncture.29
The transfemoral approach is used most commonly by most institutions, including Cleveland Clinic. It allows for a completely percutaneous insertion and, in select cases, without endotracheal intubation and general anesthesia (Figure 1).
In patients with difficult femoral access due to severe calcification, extreme tortuosity, or small diameter, alternative access routes become a consideration. In this situation, at our institution, we favor the transaortic approach in patients who have not undergone cardiac surgery in the past, while the transapical approach is used in patients who had previous cardiac surgery. With the transapical approach, we have found the outcomes similar to those of transfemoral TAVR after propensity matching.30,31 Although there is a learning curve,32 transapical TAVR can be performed with very limited mortality and morbidity. In a recent series at Cleveland Clinic, the mortality rate with the transapical approach was 1.2%, renal failure occurred in 4.7%, and a pacemaker was placed in 5.9% of patients; there were no strokes.33 This approach can be utilized for simultaneous additional procedures like transcatheter mitral valve reimplantation and percutaneous coronary interventions.34–36
Transcatheter aortic valve replacement (TAVR) has established itself as an effective way of treating high-risk patients with severe aortic valve stenosis. With new generations of existing valves and newer alternative devices, the procedure promises to become increasingly safer. The field is evolving rapidly and it will be important for interventional cardiologists and cardiac surgeons alike to stay abreast of developments. This article reviews the history of this promising procedure and examines its use in current practice.
HISTORICAL PERSPECTIVE
In 1980, Danish researcher H. R. Anderson reported developing and testing a balloon-expandable valve in animals.1 The technology was eventually acquired and further developed by Edwards Life Sciences (Irvine, California).
Alain Cribier started early work in humans in 2002 in France.2 He used a transfemoral arterial access to approach the aortic valve transseptally, but this procedure was associated with high rates of mortality and stroke.3 At the same time, in the United States, animal studies were being carried out by Lars G. Svensson, Todd Dewey, and Michael Mack to develop a transapical method of implantation,4,5 while John Webb and colleagues were also developing a transapical aortic valve implantation technique,6,7 and later went on to develop a retrograde transfemoral technique. This latter technique became feasible once Edwards developed a catheter that could be flexed to get around the aortic arch and across the aortic valve.
As the Edwards balloon-expandable valve (Sapien) was being developed, a nitinol-based self-expandable valve system was introduced by Medtronic: the CoreValve. Following feasibility studies,5,8 the safety and efficacy of these valves were established thorough the Placement of Aortic Transcatheter Valves (PARTNER) trial and the US Core Valve Pivotal Trial. These valves are currently approved by the US Food and Drug Administration (FDA) for patients for whom conventional surgery would pose an extreme or high risk.9–11
CLINICAL TRIALS OF TAVR
The two landmark prospective randomized trials of TAVR were the PARTNER trial and CoreValve Pivotal Trial.
The PARTNER trial consisted of two parts: PARTNER A, which compared the Sapien balloon-expandable transcatheter valve with surgical aortic valve replacement in patients at high surgical risk (Society of Thoracic Surgeons [STS] score > 10%), and PARTNER B, which compared TAVR with medical therapy in patients who could not undergo surgery (combined risk of serious morbidity or death of 50% or more, and two surgeons agreeing that the patient was inoperable).
Similarly, the CoreValve Pivotal Trial compared the self-expandable transcatheter valve with conventional medical and surgical treatment.
TAVR is comparable to surgery in outcomes, with caveats
In the PARTNER A trial, mortality rates were similar between patients who underwent Sapien TAVR and those who underwent surgical valve replacement at 30 days (3.4% and 6.5%, P = .07), 1 year (24.2% and 26.8%), and 2 years (33.9% and 35.0%). The patients in this group were randomized to either Sapien TAVR or surgery (Table 1).10,12
The combined rate of stroke and transient ischemic attack was higher in the patients assigned to TAVR at 30 days (5.5% with TAVR vs 2.4% with surgery, P = .04) and at 1 year (8.3% with TAVR vs 4.3% with surgery, P = .04). The difference was of small significance at 2 years (11.2% vs 6.5%, P = .05). At 30 days, the rate of major vascular complications was higher with TAVR (11.0% vs 3.2%), while surgery was associated with more frequent major bleeding episodes (19.5% vs 9.3%) and new-onset atrial fibrillation (16.0% vs 8.6%). The rate of new pacemaker requirement at 30 days was similar between the TAVR and surgical groups (3.8% vs 3.6%). Moderate or severe paravalvular aortic regurgitation was more common after TAVR at 30 days, 1 year, and 2 years. This aortic insufficiency was associated with increased late mortality.10,12
In the US CoreValve High Risk Study, no difference was found in the 30-day mortality rate in patients at high surgical risk randomized to CoreValve TAVR or surgery (3.3% and 4.5%) (Table 1). Surprisingly, the 1-year mortality rate was lower in the TAVR group than in the surgical group (14.1% vs 18.9%, respectively), a finding sustained at 2 years in data presented at the American College of Cardiology conference in March 2015.13–16
TAVR is superior to medical management, but the risk of stroke is higher
In the PARTNER B trial, inoperable patients were randomly assigned to undergo TAVR with a Sapien valve or medical management. TAVR resulted in lower mortality rates at 1 year (30.7% vs 50.7%) and 2 years (43.4% vs 68.0%) compared with medical management (Table 1).17 Of note, medical management included balloon valvuloplasty. The rate of the composite end point of death or repeat hospitalization was also lower with TAVR compared with medical therapy (44.1% vs 71.6%, respectively, at 1 year and 56.7% and 87.9%, respectively, at 2 years).17 The TAVR group had a higher stroke rate than the medical therapy group at 30 days (11.2% vs 5.5%, respectively) and at 2 years (13.8% vs 5.5%).17 Survival improved with TAVR in patients with an STS score of less than 15% but not in those with an STS score of 15% or higher.9
The very favorable results from the PARTNER trial rendered a randomized trial comparing self-expanding (CoreValve) TAVR and medical therapy unethical. Instead, a prospective single-arm study, the CoreValve Extreme Risk US Pivotal Trial, was used to compare the 12-month rate of death or major stroke with CoreValve TAVR vs a prespecified estimate of this rate with medical therapy.14 In about 500 patients who had a CoreValve attempt, the rate of all-cause mortality or major stroke at 1 year was significantly lower than the prespecified expected rate (26% vs 43%), reinforcing the results from the PARTNER Trial.14
Five-year outcomes
The 5-year PARTNER clinical and valve performance outcomes were published recently18 and continued to demonstrate equivalent outcomes for high-risk patients who underwent surgical aortic valve replacement or TAVR; there were no significant differences in all-cause mortality, cardiovascular mortality, stroke, or need for readmission to the hospital. The functional outcomes were similar as well, and no differences were demonstrated between surgical and TAVR valve performance.
Of note, moderate or severe aortic regurgitation occurred in 14% of patients in the TAVR group compared with 1% in the surgical aortic valve replacement group (P < .0001). This was associated with increased 5-year risk of death in the TAVR group (72.4% in those with moderate or severe aortic regurgitation vs 56.6% in those with mild aortic regurgitation or less; P = .003).
If the available randomized data are combined with observational reports, overall mortality and stroke rates are comparable between surgical aortic valve replacement and balloon-expandable or self-expandable TAVR in high-risk surgical candidates. Vascular complications, aortic regurgitation and permanent pacemaker insertion occur more frequently after TAVR, while major bleeding is more likely to occur after surgery.19 As newer generations of valves are developed, it is expected that aortic regurgitation and pacemaker rates will decrease over time. Indeed, trial data presented at the American College of Cardiology meeting in March 2015 for the third-generation Sapien valve (Sapien S3) showed only a 3.0% to 4.2% rate of significant paravalvular leak.
Contemporary valve comparison data
The valve used in the original PARTNER data was the first-generation Sapien valve. Since then, the second generation of this valve, the Sapien XT, has been introduced and is the model currently used in the United States (with the third-generation valve mentioned above, the Sapien S3, still available only through clinical trials). Thus, the two contemporary valves available for commercial use in the United States are the Edwards Sapien XT and Medtronic CoreValve. There are limited data comparing these valves head-to-head, but one recent trial attempted to do just that.
The Comparison of Transcatheter Heart Valves in High Risk Patients with Severe Aortic Stenosis: Medtronic CoreValve vs Edwards Sapien XT (CHOICE) trial compared the Edwards Sapien XT and CoreValve devices. Two hundred and forty-one patients were randomized. The primary end point of this trial was “device success” (a composite end point of four components: successful vascular access and deployment of the device with retrieval of the delivery system, correct position of the device, intended performance of the valve without moderate or severe insufficiency, and only one valve implanted in the correct anatomical location).
In this trial, the balloon-expandable Sapien XT valve showed a significantly higher device success rate than the self-expanding CoreValve, due to a significantly lower rate of aortic regurgitation (4.1% vs 18.3%, P < .001) and the less frequent need for implantation of more than one valve (0.8% vs 5.8%, P = .03). Placement of a permanent pacemaker was considerably less frequent in the balloon-expandable valve group (17.3% vs 37.6%, P = .001).20
PREOPERATIVE CONSIDERATIONS AND EVALUATION CRITERIA
Currently, TAVR is indicated for patients with symptomatic severe native aortic valve stenosis who are deemed at high risk or inoperable by a heart team including interventional cardiologists and cardiac surgeons. The CoreValve was also recently approved for valve-in-valve insertion in high-risk or inoperable patients with a prosthetic aortic valve in place.
The STS risk score is a reasonable preliminary risk assessment tool and is applicable to most patients being evaluated for aortic valve replacement. The STS risk score represents the percentage risk of unfavorable outcomes based on certain clinical variables. A calculator is available at riskcalc.sts.org. Patients considered at high risk are those with an STS operative risk score of 8% or higher or a postoperative 30-day risk of death of 15% or higher.
It is important to remember, though, that the STS score does not account for certain severe surgical risk factors. These include the presence of a "porcelain aorta" (heavy circumferential calcification of the ascending aorta precluding cross-clamping), history of mediastinal radiation, “hostile chest” (kyphoscoliosis, other deformities, previous coronary artery bypass grafting with adhesion of internal mammary artery to the back of sternum), severely compromised respiratory function (forced expiratory volume in 1 second < 1 L or < 40% predicted, diffusing capacity for carbon monoxide < 30%), severe pulmonary hypertension, severe liver disease (Model for End-stage Liver Disease score 8–20), severe dementia, severe cerebrovascular disease, and frailty.
With regard to this last risk factor, frailty is not simply old age but rather a measurable characteristic akin to weakness or disability. Several tests exist to measure frailty, including the “eyeball test” (the physician’s subjective assessment), Mini-Mental State Examination, gait speed/15-foot walk test, hand grip strength, serum albumin, and assessment of activities of daily living. Formal frailty testing is recommended during the course of a TAVR workup.
Risk assessment and patient suitability for TAVR is ultimately determined by the combined judgment of the heart valve team using both the STS score and consideration of these other factors.
Implantation approaches
Today, TAVR could be performed by several approaches: transfemoral arterial, transapical, transaortic via partial sternotomy or right anterior thoracotomy,21,22 transcarotid,23–25 and transaxillary or subclavian.26,27 Less commonly, transfemoral-venous routes have been performed utilizing either transseptal28 or caval-aortic puncture.29
The transfemoral approach is used most commonly by most institutions, including Cleveland Clinic. It allows for a completely percutaneous insertion and, in select cases, without endotracheal intubation and general anesthesia (Figure 1).
In patients with difficult femoral access due to severe calcification, extreme tortuosity, or small diameter, alternative access routes become a consideration. In this situation, at our institution, we favor the transaortic approach in patients who have not undergone cardiac surgery in the past, while the transapical approach is used in patients who had previous cardiac surgery. With the transapical approach, we have found the outcomes similar to those of transfemoral TAVR after propensity matching.30,31 Although there is a learning curve,32 transapical TAVR can be performed with very limited mortality and morbidity. In a recent series at Cleveland Clinic, the mortality rate with the transapical approach was 1.2%, renal failure occurred in 4.7%, and a pacemaker was placed in 5.9% of patients; there were no strokes.33 This approach can be utilized for simultaneous additional procedures like transcatheter mitral valve reimplantation and percutaneous coronary interventions.34–36
- Andersen HR, Knudsen LL, Hasenkam JM. Transluminal implantation of artificial heart valves. Description of a new expandable aortic valve and initial results with implantation by catheter technique in closed chest pigs. Eur Heart J 1992; 13:704– 708.
- Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case descrip- tion. Circulation 2002; 106:3006–3008.
- Cribier A, Eltchaninoff H, Tron C, et al. Early experience with percutaneous transcatheter implantation of heart valve prosthesis for the treatment of end-stage inoperable patients with calcific aortic stenosis. J Am Coll Cardiol 2004; 43:698– 703.
- Dewey TM, Walther T, Doss M, et al. Transapical aortic valve implantation: an animal feasibility study. Ann Thorac Surg 2006; 82:110–116.
- Svensson LG, Dewey T, Kapadia S, et al. United States feasibility study of trans- catheter insertion of a stented aortic valve by the left ventricular apex. Ann Thorac Surg 2008; 86:46–54.
- Lichtenstein SV, Cheung A, Ye J, et al. Transapical transcatheter aortic valve im- plantation in humans: initial clinical experience. Circulation 2006; 114:591–596.
- Webb JG, Pasupati S, Hyumphries K, et al. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation 2007; 116:755–763.
- Leon MB, Kodali S, Williams M, et al. Transcatheter aortic valve replacement in patients with critical aortic stenosis: rationale, device descriptions, early clinical experiences, and perspectives. Semin Thorac Cardiovasc Surg 2006; 18:165–174.
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo sur- gery. N Engl J Med 2010; 363:1597–1607.
- Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187–2198.
- Adams DH, Popma JJ, Reardon MJ, et al; U.S. CoreValve Clinical Investigators. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014; 370:1790–1798.
- Kodali SK, Williams MR, Smith CR, et al; PARTNER Trial Investigators. Two- year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012; 366:1686–1695.
- Reardon M, et al. A randomized comparison of self-expanding
- Popma JJ, Adams DH, Reardon MJ, et al; CoreValve United States Clinical In- vestigators. Transcatheter aortic valve replacement using a self-expanding biopros- thesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol 2014; 63:1972–1981.
- Adams DH, Popma JJ, Reardon MJ. Transcatheter aortic-valve replacement with a self-expanding prosthesis (letter). N Engl J Med 2014; 371:967–968.
- Kaul S. Transcatheter aortic-valve replacement with a self-expanding prosthesis (letter). N Engl J Med 2014; 371:967.
- Makkar RR, Fontana GP, Jilaihawi H, et al. Transcathether aortic-valve re- placement for inoperable severe aortic stenosis. N Engl J Med 2012; 366: 1696–704.
- Mack MJ, Leon MB, Smith CR, et al; PARTNER 1 trial investigators. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve re- placement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015; 385:2477–2484.
- Cao C, Ang SC, Indraratna P, et al. Systematic review and meta-analysis of trans- catheter aortic valve implantation versus surgical aortic valve replacement for severe aortic stenosis. Ann Cardiothorac Surg 2013; 2:10–23.
- Abdel-Wahab M, Mehilli J, Frerker C, et al; CHOICE investigators. Comparison of balloon-expandable vs self-expandable valves in patients undergoing transcath- eter aortic valve replacement: the CHOICE randomized clinical trial. JAMA 2014; 311:1503–1514.
- Okuyama K, Jilaihawi H, Mirocha J, et al. Alternative access for balloon-ex- pandable transcatheter aortic valve replacement: comparison of the transaortic approach using right anterior thoracotomy to partial J-sternotomy. J Thorac Car- diovasc Surg 2014; 149:789–797.
- Lardizabal JA, O’Neill BP, Desai HV, et al. The transaortic approach for transcath- eter aortic valve replacement: initial clinical experience in the United States. J Am Coll Cardiol 2013; 61:2341–2345.
- Thourani VH, Gunter RL, Neravetla S, et al. Use of transaortic, transapical, and transcarotid transcatheter aortic valve replacement in inoperable patients. Ann Thorac Surg 2013; 96:1349–1357.
- Azmoun A, Amabile N, Ramadan R, et al. Transcatheter aortic valve implantation through carotid artery access under local anaesthesia. Eur J Cardiothorac Surg 2014; 46: 693–698.
- Rajagopal R, More RS, Roberts DH. Transcatheter aortic valve implantation through a transcarotid approach under local anesthesia. Catheter Cardiovasc In- terv 2014; 84:903–907.
- Fraccaro C, Napodano M, Tarantini G, et al. Expanding the eligibility for trans- catheter aortic valve implantation the trans-subclavian retrograde approach using: the III generation CoreValve revalving system. JACC Cardiovasc Interv 2009; 2:828–333.
- Petronio AS, De Carlo M, Bedogni F, et al. Safety and efficacy of the subclavian approach for transcatheter aortic valve implantation with the CoreValve revalving system. Circ Cardiovasc Interv 2010; 3:359–366.
- Cohen MG, Singh V, Martinez CA, et al. Transseptal antegrade transcatheter aor- tic valve replacement for patients with no other access approach—a contemporary experience. Catheter Cardiovasc Interv 2013; 82:987–993.
- Greenbaum AB, O’Neill WW, Paone G, et al. Caval-aortic access to allow trans- catheter aortic valve replacement in otherwise ineligible patients: initial human experience. J Am Coll Cardiol 2014; 63:2795–2804.
- D’Onofrio A, Salizzoni S, Agrifoglio M, et al. Medium term outcomes of trans- apical aortic valve implantation: results from the Italian Registry of Trans-Apical Aortic Valve Implantation. Ann Thorac Surg 2013; 96:830–835.
- Johansson M, Nozohoor S, Kimblad PO, Harnek J, Olivecrona GK, Sjögren J. Transapical versus transfemoral aortic valve implantation: a comparison of survival and safety. Ann Thorac Surg 2011; 91:57–63.
- Kempfert J, Rastan A, Holzhey D, et al. Transapical aortic valve implantation: analysis of risk factors and learning experience in 299 patients. Circulation 2011; 124(suppl):S124–S129.
- Aguirre J, Waskowski R, Poddar K, et al. Transcatheter aortic valve replacement: experience with the transapical approach, alternate access sites, and concomitant cardiac repairs. J Thorac Cardiovasc Surg 2014; 148:1417–1422.
- Al Kindi AH, Salhab KF, Roselli EE, Kapadia S, Tuzcu EM, Svensson LG. Alternative access options for transcatheter aortic valve replacement in patients with no conventional access and chest pathology. J Thorac Cardiovasc Surg 2014; 147:644–651.
- Salhab KF, Al Kindi AH, Lane JH, et al. Concomitant percutaneous coronary intervention and transcatheter aortic valve replacement: safe and feasible replace- ment alternative approaches in high-risk patients with severe aortic stenosis and coronary artery disease. J Card Surg 2013; 28:481–483.
- Al Kindi AH, Salhab KF, Kapadia S, et al. Simultaneous transapical transcatheter aortic and mitral valve replacement in a high-risk patient with a previous mitral bioprosthesis. J Thorac Cardiovasc Surg 2012; 144:e90–e91.
- Andersen HR, Knudsen LL, Hasenkam JM. Transluminal implantation of artificial heart valves. Description of a new expandable aortic valve and initial results with implantation by catheter technique in closed chest pigs. Eur Heart J 1992; 13:704– 708.
- Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case descrip- tion. Circulation 2002; 106:3006–3008.
- Cribier A, Eltchaninoff H, Tron C, et al. Early experience with percutaneous transcatheter implantation of heart valve prosthesis for the treatment of end-stage inoperable patients with calcific aortic stenosis. J Am Coll Cardiol 2004; 43:698– 703.
- Dewey TM, Walther T, Doss M, et al. Transapical aortic valve implantation: an animal feasibility study. Ann Thorac Surg 2006; 82:110–116.
- Svensson LG, Dewey T, Kapadia S, et al. United States feasibility study of trans- catheter insertion of a stented aortic valve by the left ventricular apex. Ann Thorac Surg 2008; 86:46–54.
- Lichtenstein SV, Cheung A, Ye J, et al. Transapical transcatheter aortic valve im- plantation in humans: initial clinical experience. Circulation 2006; 114:591–596.
- Webb JG, Pasupati S, Hyumphries K, et al. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation 2007; 116:755–763.
- Leon MB, Kodali S, Williams M, et al. Transcatheter aortic valve replacement in patients with critical aortic stenosis: rationale, device descriptions, early clinical experiences, and perspectives. Semin Thorac Cardiovasc Surg 2006; 18:165–174.
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo sur- gery. N Engl J Med 2010; 363:1597–1607.
- Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187–2198.
- Adams DH, Popma JJ, Reardon MJ, et al; U.S. CoreValve Clinical Investigators. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014; 370:1790–1798.
- Kodali SK, Williams MR, Smith CR, et al; PARTNER Trial Investigators. Two- year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012; 366:1686–1695.
- Reardon M, et al. A randomized comparison of self-expanding
- Popma JJ, Adams DH, Reardon MJ, et al; CoreValve United States Clinical In- vestigators. Transcatheter aortic valve replacement using a self-expanding biopros- thesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol 2014; 63:1972–1981.
- Adams DH, Popma JJ, Reardon MJ. Transcatheter aortic-valve replacement with a self-expanding prosthesis (letter). N Engl J Med 2014; 371:967–968.
- Kaul S. Transcatheter aortic-valve replacement with a self-expanding prosthesis (letter). N Engl J Med 2014; 371:967.
- Makkar RR, Fontana GP, Jilaihawi H, et al. Transcathether aortic-valve re- placement for inoperable severe aortic stenosis. N Engl J Med 2012; 366: 1696–704.
- Mack MJ, Leon MB, Smith CR, et al; PARTNER 1 trial investigators. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve re- placement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015; 385:2477–2484.
- Cao C, Ang SC, Indraratna P, et al. Systematic review and meta-analysis of trans- catheter aortic valve implantation versus surgical aortic valve replacement for severe aortic stenosis. Ann Cardiothorac Surg 2013; 2:10–23.
- Abdel-Wahab M, Mehilli J, Frerker C, et al; CHOICE investigators. Comparison of balloon-expandable vs self-expandable valves in patients undergoing transcath- eter aortic valve replacement: the CHOICE randomized clinical trial. JAMA 2014; 311:1503–1514.
- Okuyama K, Jilaihawi H, Mirocha J, et al. Alternative access for balloon-ex- pandable transcatheter aortic valve replacement: comparison of the transaortic approach using right anterior thoracotomy to partial J-sternotomy. J Thorac Car- diovasc Surg 2014; 149:789–797.
- Lardizabal JA, O’Neill BP, Desai HV, et al. The transaortic approach for transcath- eter aortic valve replacement: initial clinical experience in the United States. J Am Coll Cardiol 2013; 61:2341–2345.
- Thourani VH, Gunter RL, Neravetla S, et al. Use of transaortic, transapical, and transcarotid transcatheter aortic valve replacement in inoperable patients. Ann Thorac Surg 2013; 96:1349–1357.
- Azmoun A, Amabile N, Ramadan R, et al. Transcatheter aortic valve implantation through carotid artery access under local anaesthesia. Eur J Cardiothorac Surg 2014; 46: 693–698.
- Rajagopal R, More RS, Roberts DH. Transcatheter aortic valve implantation through a transcarotid approach under local anesthesia. Catheter Cardiovasc In- terv 2014; 84:903–907.
- Fraccaro C, Napodano M, Tarantini G, et al. Expanding the eligibility for trans- catheter aortic valve implantation the trans-subclavian retrograde approach using: the III generation CoreValve revalving system. JACC Cardiovasc Interv 2009; 2:828–333.
- Petronio AS, De Carlo M, Bedogni F, et al. Safety and efficacy of the subclavian approach for transcatheter aortic valve implantation with the CoreValve revalving system. Circ Cardiovasc Interv 2010; 3:359–366.
- Cohen MG, Singh V, Martinez CA, et al. Transseptal antegrade transcatheter aor- tic valve replacement for patients with no other access approach—a contemporary experience. Catheter Cardiovasc Interv 2013; 82:987–993.
- Greenbaum AB, O’Neill WW, Paone G, et al. Caval-aortic access to allow trans- catheter aortic valve replacement in otherwise ineligible patients: initial human experience. J Am Coll Cardiol 2014; 63:2795–2804.
- D’Onofrio A, Salizzoni S, Agrifoglio M, et al. Medium term outcomes of trans- apical aortic valve implantation: results from the Italian Registry of Trans-Apical Aortic Valve Implantation. Ann Thorac Surg 2013; 96:830–835.
- Johansson M, Nozohoor S, Kimblad PO, Harnek J, Olivecrona GK, Sjögren J. Transapical versus transfemoral aortic valve implantation: a comparison of survival and safety. Ann Thorac Surg 2011; 91:57–63.
- Kempfert J, Rastan A, Holzhey D, et al. Transapical aortic valve implantation: analysis of risk factors and learning experience in 299 patients. Circulation 2011; 124(suppl):S124–S129.
- Aguirre J, Waskowski R, Poddar K, et al. Transcatheter aortic valve replacement: experience with the transapical approach, alternate access sites, and concomitant cardiac repairs. J Thorac Cardiovasc Surg 2014; 148:1417–1422.
- Al Kindi AH, Salhab KF, Roselli EE, Kapadia S, Tuzcu EM, Svensson LG. Alternative access options for transcatheter aortic valve replacement in patients with no conventional access and chest pathology. J Thorac Cardiovasc Surg 2014; 147:644–651.
- Salhab KF, Al Kindi AH, Lane JH, et al. Concomitant percutaneous coronary intervention and transcatheter aortic valve replacement: safe and feasible replace- ment alternative approaches in high-risk patients with severe aortic stenosis and coronary artery disease. J Card Surg 2013; 28:481–483.
- Al Kindi AH, Salhab KF, Kapadia S, et al. Simultaneous transapical transcatheter aortic and mitral valve replacement in a high-risk patient with a previous mitral bioprosthesis. J Thorac Cardiovasc Surg 2012; 144:e90–e91.
KEY POINTS
- In randomized trials, transcatheter aortic valve replacement (TAVR) has produced results that are comparable to surgical aortic valve replacement in high-risk patients. TAVR is superior to medical management in patients who cannot undergo surgery, although it is associated with higher rates of stroke.
- Risk assessment and suitability for TAVR is determined by a heart team composed of interventional cardiologists and cardiac surgeons. Society of Thoracic Surgeons Score and a number of other criteria mentioned below are considered during this process.
- The transfemoral arterial approach is the most common approach used by most institutions, but other approaches such as transaortic, transapical, transaxillary, and transcarotid are utilized if suitable in patients who have difficult femoral access.