User login
Antegrade Femoral Nail Distal Interlocking Screw Causing Rupture of the Medial Patellofemoral Ligament and Patellar Instability
ABSTRACT
Antegrade reamed intramedullary nailing has the advantages of high fracture union and early weight-bearing, making it the gold standard for fixation of diaphyseal femur fractures. However, knowledge of distal femoral anatomy may mitigate the risk of secondary complications.
We present a previously unrecognized complication of antegrade femoral nailing in which a 23-year-old man sustained iatrogenic rupture of the medial patellofemoral ligament (MPFL) caused by the distal interlocking screw of the femoral nail. The patient had a history of antegrade intramedullary nailing that was revised for rotational malalignment, after which he began experiencing recurrent episodes of atraumatic bloody joint effusion and swelling of the right knee with associated patellar instability. Plain radiographs and magnetic resonance imaging revealed a large effusion with a prominent intra-articular distal interlocking screw disrupting the MPFL. The patient underwent a right knee arthroscopic-assisted MPFL reconstruction and removal of the distal interlocking screw. Following surgery, the patient experienced resolution of his effusions, no recurrent patellar instability, and was able to return to his activities.
This case demonstrates that iatrogenic MPFL injury is a potential complication of antegrade femoral nailing and a previously unrecognized cause of patellar instability. Surgeons should be aware of this potential complication and strive to avoid the MPFL origin when placing their distal interlocking screw.
Continue to: Reamed intramedullary nails...
Reamed intramedullary nails are the gold standard for fixation of femoral diaphyseal fractures.1 Antegrade or retrograde nails are effective options, with the choice of technique based on factors including surgeon preference, patient factors, and concomitant injuries.2 Interlocking screws are generally placed to allow control of both rotation and length.1 Advantages of intramedullary treatment of femoral diaphyseal fractures compared with plate fixation include low rates of infection, lower nonunion rate, and faster patient mobilization and weight-bearing.3
Complications of antegrade intramedullary fixation of femoral shaft fractures include infection, nonunion, malunion, anterior cortical perforation, heterotopic ossification, abductor weakness, and soft tissue irritation from interlocking screws.2-4 Femoral intramedullary nails are not routinely removed because the hardware is rarely symptomatic and removing the nail involves additional surgical morbidity with the potential for complications.5 Interlocking screws are removed in select cases due to soft tissue irritation, generally after fracture union. Although hardware removal may help in select cases, removal of intramedullary nails is associated with low rates of symptom resolution.6-8
We present a case of iatrogenic medial patellofemoral ligament (MPFL) disruption by the distal interlocking screw leading to patellar instability, a previously unrecognized complication of antegrade femoral nailing for femoral diaphyseal fractures. The patient provided written informed consent for print and electronic publication of this case report.
CASE REPORT
We present a case of a 23-year-old man whose status was 2 years post antegrade reamed femoral intramedullary nailing at an outside institution for a right diaphyseal femur fracture. This issue was revised for external rotational malalignment, and he presented with right anterior knee pain, recurrent patellar subluxation, and recurrent effusions. The extent of external rotational malalignment and subsequent rotational correction were not evident from the available outside institution records. These symptoms began after his femoral nail revision for malalignment, and he had no subsequent trauma. The femoral fracture healed uneventfully. The patient denied any history of knee pain, swelling, or patellar instability before his femoral nail revision for malalignment. These episodes of effusion, instability, and pain occurred several times per year, generally with activities of daily living (ADL). On one occasion, he presented to a local emergency room where knee aspiration revealed no evidence of crystals or infection. The patient was referred to the senior author (Dr. Nho) for consultation.
Physical examination revealed right knee full extension with flexion to 80°. A moderate right knee effusion was present. The patient was tender over the medial femoral epicondyle and the superomedial aspect of the patella without joint line tenderness. Lateral patellar instability was present with 2 quadrants of translation (compared with 1 on the contralateral side) and patellar apprehension. The patient’s knee was ligamentously stable, and meniscal signs were absent. His lower extremity rotational profile was symmetric to the contralateral uninjured side.
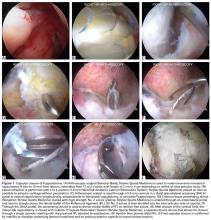
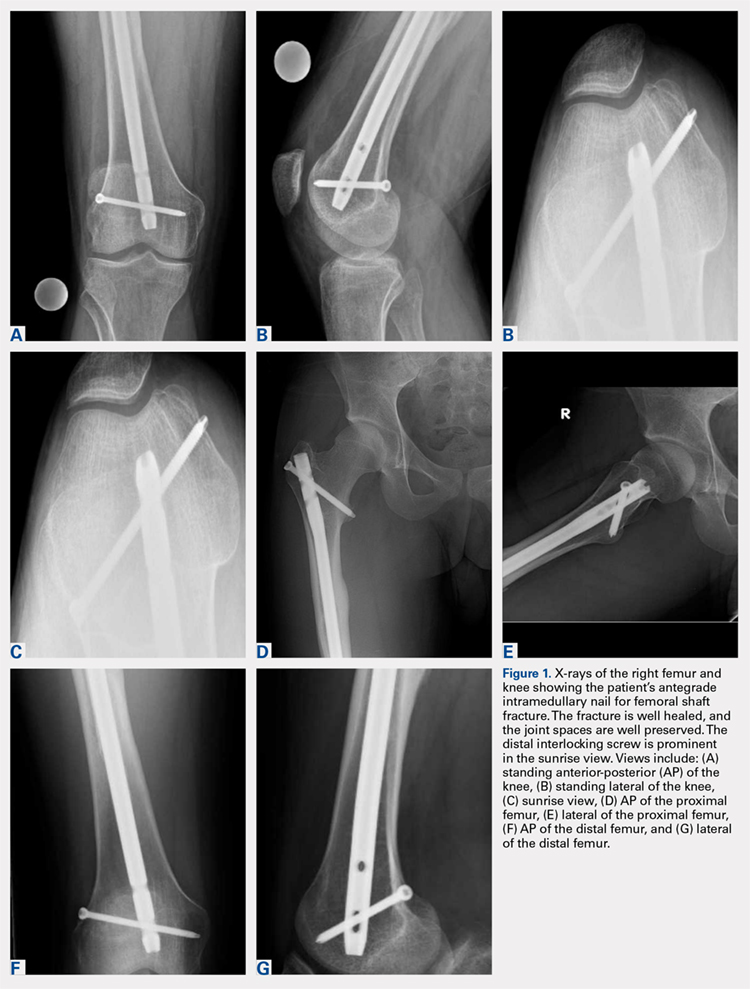
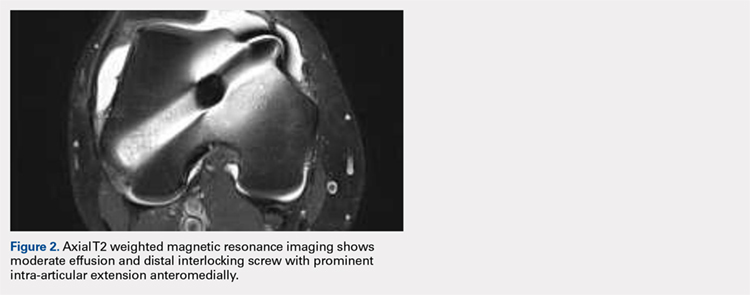
Right femur and knee X-rays showed an antegrade intramedullary nail with a well-healed diaphyseal fracture and a single distal interlocking screw oriented from posterolateral to anteromedial (Figures 1A-1G). The screw tip was prominent on sunrise X-ray view anterior to the medial femoral epicondyle (Figure 1C). Magnetic resonance imaging demonstrated a large effusion and lateral patellar subluxation with a prominent intra-articular distal interlocking screw disrupting the MPFL near the femoral attachment (Figure 2). Patellar height, trochlear morphology, and tibial tubercle-trochlear groove distance were assessed and found to be normal.
Continue to: The patient elected...
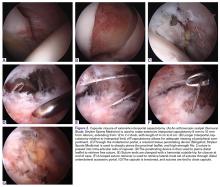
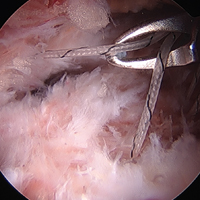
The patient elected to have a right knee arthroscopic-assisted MPFL reconstruction and removal of the distal interlocking screw. Diagnostic arthroscopy revealed the distal interlocking screw to be intra-articular medially, prominent by 3 mm causing attritional disruption of the mid-substance MPFL (Figure 3A). The patella was noted to be subluxated and tracking laterally (Figure 3B). Both the anterior cruciate ligament and posterior cruciate ligament were intact, and menisci and articular cartilage were normal. The distal interlocking screw was removed under fluoroscopic guidance through a small lateral incision (Figure 3C).
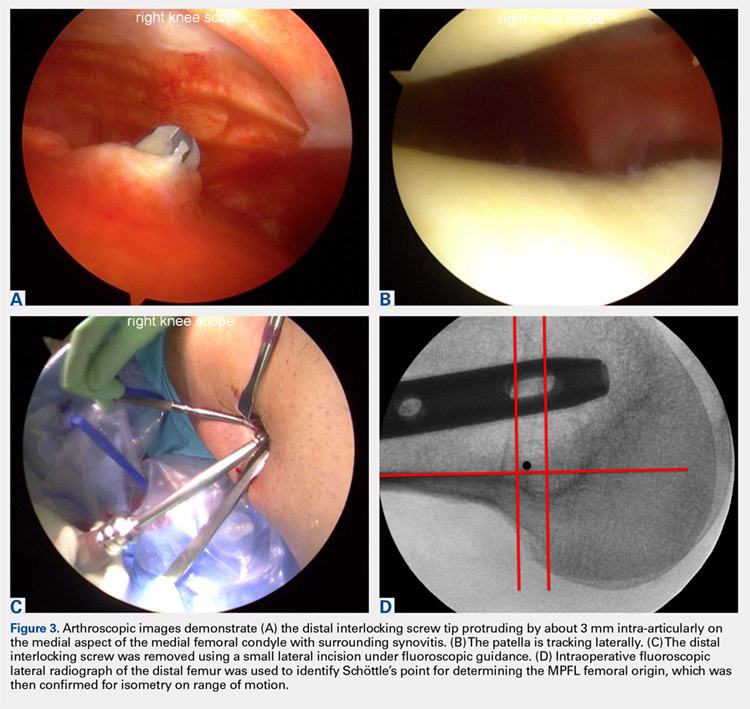
Due to the nature of the longstanding attritional disruption of the MPFL in this case with associated patellar instability over a 2-year period, the decision was made to proceed with formal MPFL reconstruction as opposed to repair. A 2-cm incision was made at the medial aspect of the patella. The proximal half of the patella was decorticated. Guide pins were placed within the proximal half of the patella, ensuring at least a 1-cm bone bridge between them, and two 4.75-mm SwiveLock suture anchors (Arthrex) were inserted. A semitendinosus graft was used for MPFL reconstruction with the 2 ends of the graft secured to 2 suture anchors with a whipstitch. Lateral fluoroscopy was used to identify Schöttle’s point, denoting the femoral origin of the MPFL9 (Figure 3D). A 2-cm incision was made at this location. A guide pin was then placed at Schöttle’s point under fluoroscopic guidance, aimed proximally, and the knee was brought through a range of motion (ROM), to verify graft isometry. Once verified, the guide pin was over-reamed to 8 mm. The layer between the retinaculum and the capsule was carefully dissected, and the graft was passed extra-articularly in the plane between the retinaculum and the capsule, out through the medial incision, and docked into the bone tunnel. An 8-mm BioComposite interference screw (Arthrex) was then placed with the knee flexed to 30°. The knee was then passed through a ROM and an arthroscopic evaluation confirmed that the patella was no longer subluxated laterally. There was normal tracking of the patellofemoral joint on arthroscopic evaluation.
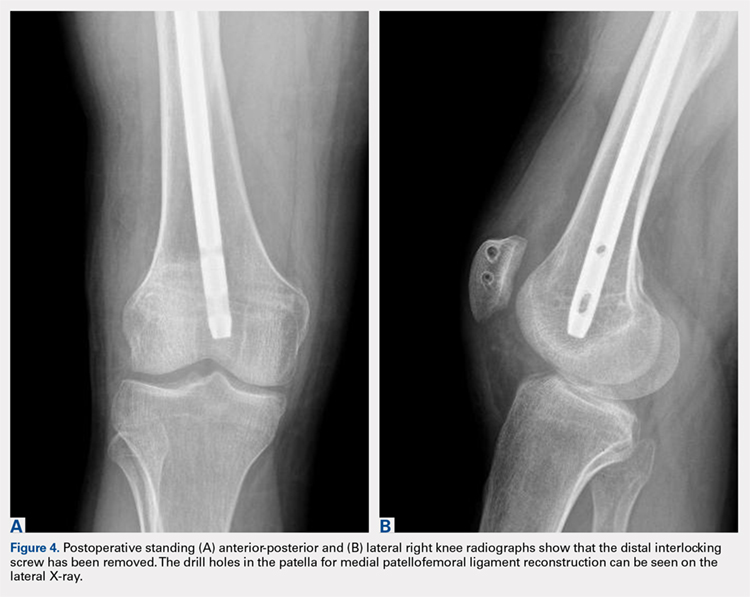
Postoperatively, the patient was maintained in a hinged knee brace for 6 weeks. He was weight-bearing as tolerated when locked in full extension beginning immediately postoperatively, and allowed to unlock the brace to start non-weight-bearing active flexion and extension with therapy on postoperative day 1. Radiographs confirmed removal of the distal interlocking screw (Figures 4A, 4B). Following surgery, the patient experienced resolution of his effusions, no recurrent patellar instability at 1-year postoperative, and was able to return to his ADL and recreational sporting activities (Knee Injury and Osteoarthritis Outcome Score [KOOS] ADL, 100; KOOS sporting and recreational activities, 95; quality of life, 100; Marx Activity Rating Scale, 12).
DISCUSSION
The MPFL connects the superomedial edge of the patella to the medial femur and is injured in nearly 100% of patellar dislocations.6 The femoral origin lies between the adductor tubercle and the medial epicondyle.7 The MPFL prevents lateral subluxation of the patella and acts as the major restraint during the first 20° of knee flexion. Although radiographic parameters for identifying the MPFL femoral origin have been defined by both Schöttle and colleagues9 and Stephen and colleagues10, it is important to check the isometry intraoperatively through a ROM when performing MPFL reconstruction. In this case, the patient’s history and physical examination showed patellar instability, which was determined to be iatrogenically related to the distal interlocking screw rupture of the MPFL. Following screw removal and MPFL reconstruction, the patient had no further symptoms of pain, effusion, or patellar instability and returned to his normal activities.
Femoral malrotation following intramedullary nailing of femoral shaft fractures is a common complication,4 with a 22% incidence of malrotation of at least 15° in 1 series from an academic trauma center.11 There are mixed data as to whether malrotation is more common in complex fracture patterns, in cases performed during night hours, and in cases performed by non-trauma fellowship-trained surgeons.11-13 The natural history of malrotation is not well elucidated, but there is some suggestion that it alters load bearing in the distal joints of the involved leg including the patellofemoral joint. Patients also may not tolerate malrotation due to the abnormal foot progression angle, particularly with malrotation >15°.4 In this case, the patient’s initial femoral nail was placed in an externally rotated position, requiring revision. The result of this was an unusual trajectory of the distal interlocking screw from posterolateral to anteromedial. Combined with the prominent screw tip, the trajectory of this distal interlocking screw likely contributed to the injury to the MPFL observed in this case. This trajectory would also pose potential risk to the common peroneal nerve, which is usually situated posterior to the insertion point for distal femoral interlocking screws. The prominent distal interlock screw is a well-recognized problem with femoral intramedullary nails. This issue results from the tapering of the width of the distal femur from being larger posteriorly to being smaller anteriorly. To avoid placement of a prominent distal interlocking screw, surgeons often will obtain an intraoperative anterior-posterior radiograph with the lower extremity in 30° of internal rotation to account for the angle of the medial aspect of the distal femur.
This practice represents, to our knowledge, a previously unreported cause of patellar instability as well as an unreported complication of antegrade femoral intramedullary nailing. Surgeons treating these conditions should consider this potential complication and pursue advanced imaging if patients present with these complaints after femoral intramedullary nail placement. Knowledge of both MPFL origin and insertional anatomy and avoidance of prominent distal interlocking screws in the region of the MPFL, if possible, would likely prevent this complication.
Limitations of this study include the case report design, which makes it impossible to comment on the incidence of this complication or to make comparisons regarding treatment options. There is, of course, the possibility that the patient had a concurrent MPFL injury from the injury in which he sustained the femur fracture. Nevertheless, the clinical history, examination, imaging, and arthroscopic findings all strongly suggest that the prominent distal interlocking screw was the cause of his MPFL injury and patellar instability. Finally, the point widely defined by Schöttle and colleagues12 was used for MPFL reconstruction in this case based on an intraoperative true lateral radiograph of the distal femur. It should be noted that recent literature has debated the accuracy of this method for determining the femoral origin, the anatomy of the MPFL in relation to the quadriceps, and type of fixation for MPFL reconstruction with some advocating soft tissue only fixation.14-17 For purposes of this case report, we focused on a different cause of MPFL disruption in this patient and our technique for MPFL reconstruction.
CONCLUSION
This case demonstrates that iatrogenic MPFL injury is a potential complication of antegrade femoral nailing and a previously unrecognized cause of patellar instability. Surgeons should be aware of this potential complication and strive to avoid the MPFL origin when placing their distal interlocking screw.
This paper will be judged for the Resident Writer’s Award.
- Brumback RJ, Virkus WW. Intramedullary nailing of the femur: reamed versus nonreamed. J Am Acad Orthop Surg. 2000;8(2):83-90.
- Ricci WM, Bellabarba C, Evanoff B, Herscovici D, DiPasquale T, Sanders R. Retrograde versus antegrade nailing of femoral shaft fractures. J Orthop Trauma 2001;15(3):161-169.
- Ricci WM, Gallagher B, Haidukewych GJ. Intramedullary nailing of femoral shaft fractures: current concepts. J Am Acad Orthop Surg. 2009;17(5):296-305.
- Lindsey JD, Krieg JC. Femoral malrotation following intramedullary nail fixation. J Am Acad Orthop Surg. 2011;19(1):17-26.
- Busam ML, Esther RJ, Obremskey WT. Hardware removal: indications and expectations. J Am Acad Orthop Surg. 2006;14(2):113-120.
- Morshed S, Humphrey M, Corrales LA, Millett M, Hoffinger SA. Retention of flexible intramedullary nails following treatment of pediatric femur fractures. Arch Orthop Trauma Surg. 2007;127(7):509-514.
- Boerger TO, Patel G, Murphy JP. Is routine removal of intramedullary nails justified. Injury. 1999;30(2):79-81.
- Kellan J. Fracture healing: Does hardware removal enhance patient outcomes. Chin J Orthop Trauma (Chin). 2010;12:374-378.
- Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804. doi:10.1177/0363546506296415.
- Stephen JM, Lumpaopong P, Deehan DJ, Kader D, Amis AA. The medial patellofemoral ligament: location of femoral attachment and length change patterns resulting from anatomic and nonanatomic attachments. Am J Sports Med. 2012;40(8):1871-1879. doi:10.1177/0363546512449998.
- Hüfner T, Citak M, Suero EM, et al. Femoral malrotation after unreamed intramedullary nailing: an evaluation of influencing operative factors. J Orthop Trauma. 2011;25(4):224-227. doi:10.1097/BOT.0b013e3181e47e3b.
- Ayalon OB, Patel NM, Yoon RS, Donegan DJ, Koerner JD, Liporace FA. Comparing femoral version after intramedullary nailing performed by trauma-trained and non-trauma trained surgeons: is there a difference? Injury. 2014;45(7):1091-1094. doi:10.1016/j.injury.2014.01.024.
- Patel NM, Yoon RS, Cantlon MB, Koerner JD, Donegan DJ, Liporace FA. Intramedullary nailing of diaphyseal femur fractures secondary to gunshot wounds: predictors of postoperative malrotation. J Orthop Trauma. 2014;28(12):711-714. doi:10.1097/BOT.0000000000000124.
- Ziegler CG, Fulkerson JP, Edgar C. Radiographic reference points are inaccurate with and without a true lateral radiograph: the importance of anatomy in medial patellofemoral ligament reconstruction. Am J Sports Med. 2016;44(1):133-142.
- Fulkerson JP, Edgar C. Medial quadriceps tendon-femoral ligament: surgical anatomy and reconstruction technique to prevent patella instability. Arthrosc Tech. 2013;2(2):e125-e128. doi:10.1016/j.eats.2013.01.002.
- Tanaka MJ, Voss A, Fulkerson JP. The anatomic midpoint of the attachment of the medial patellofemoral complex. J Bone Joint Surg Am. 2016;98(14):1199-1205. doi:10.2106/JBJS.15.01182.
- Mochizuki T, Nimura A, Tateishi T, Yamaguchi K, Muneta T, Akita K. Anatomic study of the attachment of the medial patellofemoral ligament and its characteristic relationships to the vastus intermedius. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):305-310. doi:10.1007/s00167-012-1993-7.
ABSTRACT
Antegrade reamed intramedullary nailing has the advantages of high fracture union and early weight-bearing, making it the gold standard for fixation of diaphyseal femur fractures. However, knowledge of distal femoral anatomy may mitigate the risk of secondary complications.
We present a previously unrecognized complication of antegrade femoral nailing in which a 23-year-old man sustained iatrogenic rupture of the medial patellofemoral ligament (MPFL) caused by the distal interlocking screw of the femoral nail. The patient had a history of antegrade intramedullary nailing that was revised for rotational malalignment, after which he began experiencing recurrent episodes of atraumatic bloody joint effusion and swelling of the right knee with associated patellar instability. Plain radiographs and magnetic resonance imaging revealed a large effusion with a prominent intra-articular distal interlocking screw disrupting the MPFL. The patient underwent a right knee arthroscopic-assisted MPFL reconstruction and removal of the distal interlocking screw. Following surgery, the patient experienced resolution of his effusions, no recurrent patellar instability, and was able to return to his activities.
This case demonstrates that iatrogenic MPFL injury is a potential complication of antegrade femoral nailing and a previously unrecognized cause of patellar instability. Surgeons should be aware of this potential complication and strive to avoid the MPFL origin when placing their distal interlocking screw.
Continue to: Reamed intramedullary nails...
Reamed intramedullary nails are the gold standard for fixation of femoral diaphyseal fractures.1 Antegrade or retrograde nails are effective options, with the choice of technique based on factors including surgeon preference, patient factors, and concomitant injuries.2 Interlocking screws are generally placed to allow control of both rotation and length.1 Advantages of intramedullary treatment of femoral diaphyseal fractures compared with plate fixation include low rates of infection, lower nonunion rate, and faster patient mobilization and weight-bearing.3
Complications of antegrade intramedullary fixation of femoral shaft fractures include infection, nonunion, malunion, anterior cortical perforation, heterotopic ossification, abductor weakness, and soft tissue irritation from interlocking screws.2-4 Femoral intramedullary nails are not routinely removed because the hardware is rarely symptomatic and removing the nail involves additional surgical morbidity with the potential for complications.5 Interlocking screws are removed in select cases due to soft tissue irritation, generally after fracture union. Although hardware removal may help in select cases, removal of intramedullary nails is associated with low rates of symptom resolution.6-8
We present a case of iatrogenic medial patellofemoral ligament (MPFL) disruption by the distal interlocking screw leading to patellar instability, a previously unrecognized complication of antegrade femoral nailing for femoral diaphyseal fractures. The patient provided written informed consent for print and electronic publication of this case report.
CASE REPORT
We present a case of a 23-year-old man whose status was 2 years post antegrade reamed femoral intramedullary nailing at an outside institution for a right diaphyseal femur fracture. This issue was revised for external rotational malalignment, and he presented with right anterior knee pain, recurrent patellar subluxation, and recurrent effusions. The extent of external rotational malalignment and subsequent rotational correction were not evident from the available outside institution records. These symptoms began after his femoral nail revision for malalignment, and he had no subsequent trauma. The femoral fracture healed uneventfully. The patient denied any history of knee pain, swelling, or patellar instability before his femoral nail revision for malalignment. These episodes of effusion, instability, and pain occurred several times per year, generally with activities of daily living (ADL). On one occasion, he presented to a local emergency room where knee aspiration revealed no evidence of crystals or infection. The patient was referred to the senior author (Dr. Nho) for consultation.
Physical examination revealed right knee full extension with flexion to 80°. A moderate right knee effusion was present. The patient was tender over the medial femoral epicondyle and the superomedial aspect of the patella without joint line tenderness. Lateral patellar instability was present with 2 quadrants of translation (compared with 1 on the contralateral side) and patellar apprehension. The patient’s knee was ligamentously stable, and meniscal signs were absent. His lower extremity rotational profile was symmetric to the contralateral uninjured side.
Right femur and knee X-rays showed an antegrade intramedullary nail with a well-healed diaphyseal fracture and a single distal interlocking screw oriented from posterolateral to anteromedial (Figures 1A-1G). The screw tip was prominent on sunrise X-ray view anterior to the medial femoral epicondyle (Figure 1C). Magnetic resonance imaging demonstrated a large effusion and lateral patellar subluxation with a prominent intra-articular distal interlocking screw disrupting the MPFL near the femoral attachment (Figure 2). Patellar height, trochlear morphology, and tibial tubercle-trochlear groove distance were assessed and found to be normal.


Continue to: The patient elected...
The patient elected to have a right knee arthroscopic-assisted MPFL reconstruction and removal of the distal interlocking screw. Diagnostic arthroscopy revealed the distal interlocking screw to be intra-articular medially, prominent by 3 mm causing attritional disruption of the mid-substance MPFL (Figure 3A). The patella was noted to be subluxated and tracking laterally (Figure 3B). Both the anterior cruciate ligament and posterior cruciate ligament were intact, and menisci and articular cartilage were normal. The distal interlocking screw was removed under fluoroscopic guidance through a small lateral incision (Figure 3C).

Due to the nature of the longstanding attritional disruption of the MPFL in this case with associated patellar instability over a 2-year period, the decision was made to proceed with formal MPFL reconstruction as opposed to repair. A 2-cm incision was made at the medial aspect of the patella. The proximal half of the patella was decorticated. Guide pins were placed within the proximal half of the patella, ensuring at least a 1-cm bone bridge between them, and two 4.75-mm SwiveLock suture anchors (Arthrex) were inserted. A semitendinosus graft was used for MPFL reconstruction with the 2 ends of the graft secured to 2 suture anchors with a whipstitch. Lateral fluoroscopy was used to identify Schöttle’s point, denoting the femoral origin of the MPFL9 (Figure 3D). A 2-cm incision was made at this location. A guide pin was then placed at Schöttle’s point under fluoroscopic guidance, aimed proximally, and the knee was brought through a range of motion (ROM), to verify graft isometry. Once verified, the guide pin was over-reamed to 8 mm. The layer between the retinaculum and the capsule was carefully dissected, and the graft was passed extra-articularly in the plane between the retinaculum and the capsule, out through the medial incision, and docked into the bone tunnel. An 8-mm BioComposite interference screw (Arthrex) was then placed with the knee flexed to 30°. The knee was then passed through a ROM and an arthroscopic evaluation confirmed that the patella was no longer subluxated laterally. There was normal tracking of the patellofemoral joint on arthroscopic evaluation.
Postoperatively, the patient was maintained in a hinged knee brace for 6 weeks. He was weight-bearing as tolerated when locked in full extension beginning immediately postoperatively, and allowed to unlock the brace to start non-weight-bearing active flexion and extension with therapy on postoperative day 1. Radiographs confirmed removal of the distal interlocking screw (Figures 4A, 4B). Following surgery, the patient experienced resolution of his effusions, no recurrent patellar instability at 1-year postoperative, and was able to return to his ADL and recreational sporting activities (Knee Injury and Osteoarthritis Outcome Score [KOOS] ADL, 100; KOOS sporting and recreational activities, 95; quality of life, 100; Marx Activity Rating Scale, 12).

DISCUSSION
The MPFL connects the superomedial edge of the patella to the medial femur and is injured in nearly 100% of patellar dislocations.6 The femoral origin lies between the adductor tubercle and the medial epicondyle.7 The MPFL prevents lateral subluxation of the patella and acts as the major restraint during the first 20° of knee flexion. Although radiographic parameters for identifying the MPFL femoral origin have been defined by both Schöttle and colleagues9 and Stephen and colleagues10, it is important to check the isometry intraoperatively through a ROM when performing MPFL reconstruction. In this case, the patient’s history and physical examination showed patellar instability, which was determined to be iatrogenically related to the distal interlocking screw rupture of the MPFL. Following screw removal and MPFL reconstruction, the patient had no further symptoms of pain, effusion, or patellar instability and returned to his normal activities.
Femoral malrotation following intramedullary nailing of femoral shaft fractures is a common complication,4 with a 22% incidence of malrotation of at least 15° in 1 series from an academic trauma center.11 There are mixed data as to whether malrotation is more common in complex fracture patterns, in cases performed during night hours, and in cases performed by non-trauma fellowship-trained surgeons.11-13 The natural history of malrotation is not well elucidated, but there is some suggestion that it alters load bearing in the distal joints of the involved leg including the patellofemoral joint. Patients also may not tolerate malrotation due to the abnormal foot progression angle, particularly with malrotation >15°.4 In this case, the patient’s initial femoral nail was placed in an externally rotated position, requiring revision. The result of this was an unusual trajectory of the distal interlocking screw from posterolateral to anteromedial. Combined with the prominent screw tip, the trajectory of this distal interlocking screw likely contributed to the injury to the MPFL observed in this case. This trajectory would also pose potential risk to the common peroneal nerve, which is usually situated posterior to the insertion point for distal femoral interlocking screws. The prominent distal interlock screw is a well-recognized problem with femoral intramedullary nails. This issue results from the tapering of the width of the distal femur from being larger posteriorly to being smaller anteriorly. To avoid placement of a prominent distal interlocking screw, surgeons often will obtain an intraoperative anterior-posterior radiograph with the lower extremity in 30° of internal rotation to account for the angle of the medial aspect of the distal femur.
This practice represents, to our knowledge, a previously unreported cause of patellar instability as well as an unreported complication of antegrade femoral intramedullary nailing. Surgeons treating these conditions should consider this potential complication and pursue advanced imaging if patients present with these complaints after femoral intramedullary nail placement. Knowledge of both MPFL origin and insertional anatomy and avoidance of prominent distal interlocking screws in the region of the MPFL, if possible, would likely prevent this complication.
Limitations of this study include the case report design, which makes it impossible to comment on the incidence of this complication or to make comparisons regarding treatment options. There is, of course, the possibility that the patient had a concurrent MPFL injury from the injury in which he sustained the femur fracture. Nevertheless, the clinical history, examination, imaging, and arthroscopic findings all strongly suggest that the prominent distal interlocking screw was the cause of his MPFL injury and patellar instability. Finally, the point widely defined by Schöttle and colleagues12 was used for MPFL reconstruction in this case based on an intraoperative true lateral radiograph of the distal femur. It should be noted that recent literature has debated the accuracy of this method for determining the femoral origin, the anatomy of the MPFL in relation to the quadriceps, and type of fixation for MPFL reconstruction with some advocating soft tissue only fixation.14-17 For purposes of this case report, we focused on a different cause of MPFL disruption in this patient and our technique for MPFL reconstruction.
CONCLUSION
This case demonstrates that iatrogenic MPFL injury is a potential complication of antegrade femoral nailing and a previously unrecognized cause of patellar instability. Surgeons should be aware of this potential complication and strive to avoid the MPFL origin when placing their distal interlocking screw.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Antegrade reamed intramedullary nailing has the advantages of high fracture union and early weight-bearing, making it the gold standard for fixation of diaphyseal femur fractures. However, knowledge of distal femoral anatomy may mitigate the risk of secondary complications.
We present a previously unrecognized complication of antegrade femoral nailing in which a 23-year-old man sustained iatrogenic rupture of the medial patellofemoral ligament (MPFL) caused by the distal interlocking screw of the femoral nail. The patient had a history of antegrade intramedullary nailing that was revised for rotational malalignment, after which he began experiencing recurrent episodes of atraumatic bloody joint effusion and swelling of the right knee with associated patellar instability. Plain radiographs and magnetic resonance imaging revealed a large effusion with a prominent intra-articular distal interlocking screw disrupting the MPFL. The patient underwent a right knee arthroscopic-assisted MPFL reconstruction and removal of the distal interlocking screw. Following surgery, the patient experienced resolution of his effusions, no recurrent patellar instability, and was able to return to his activities.
This case demonstrates that iatrogenic MPFL injury is a potential complication of antegrade femoral nailing and a previously unrecognized cause of patellar instability. Surgeons should be aware of this potential complication and strive to avoid the MPFL origin when placing their distal interlocking screw.
Continue to: Reamed intramedullary nails...
Reamed intramedullary nails are the gold standard for fixation of femoral diaphyseal fractures.1 Antegrade or retrograde nails are effective options, with the choice of technique based on factors including surgeon preference, patient factors, and concomitant injuries.2 Interlocking screws are generally placed to allow control of both rotation and length.1 Advantages of intramedullary treatment of femoral diaphyseal fractures compared with plate fixation include low rates of infection, lower nonunion rate, and faster patient mobilization and weight-bearing.3
Complications of antegrade intramedullary fixation of femoral shaft fractures include infection, nonunion, malunion, anterior cortical perforation, heterotopic ossification, abductor weakness, and soft tissue irritation from interlocking screws.2-4 Femoral intramedullary nails are not routinely removed because the hardware is rarely symptomatic and removing the nail involves additional surgical morbidity with the potential for complications.5 Interlocking screws are removed in select cases due to soft tissue irritation, generally after fracture union. Although hardware removal may help in select cases, removal of intramedullary nails is associated with low rates of symptom resolution.6-8
We present a case of iatrogenic medial patellofemoral ligament (MPFL) disruption by the distal interlocking screw leading to patellar instability, a previously unrecognized complication of antegrade femoral nailing for femoral diaphyseal fractures. The patient provided written informed consent for print and electronic publication of this case report.
CASE REPORT
We present a case of a 23-year-old man whose status was 2 years post antegrade reamed femoral intramedullary nailing at an outside institution for a right diaphyseal femur fracture. This issue was revised for external rotational malalignment, and he presented with right anterior knee pain, recurrent patellar subluxation, and recurrent effusions. The extent of external rotational malalignment and subsequent rotational correction were not evident from the available outside institution records. These symptoms began after his femoral nail revision for malalignment, and he had no subsequent trauma. The femoral fracture healed uneventfully. The patient denied any history of knee pain, swelling, or patellar instability before his femoral nail revision for malalignment. These episodes of effusion, instability, and pain occurred several times per year, generally with activities of daily living (ADL). On one occasion, he presented to a local emergency room where knee aspiration revealed no evidence of crystals or infection. The patient was referred to the senior author (Dr. Nho) for consultation.
Physical examination revealed right knee full extension with flexion to 80°. A moderate right knee effusion was present. The patient was tender over the medial femoral epicondyle and the superomedial aspect of the patella without joint line tenderness. Lateral patellar instability was present with 2 quadrants of translation (compared with 1 on the contralateral side) and patellar apprehension. The patient’s knee was ligamentously stable, and meniscal signs were absent. His lower extremity rotational profile was symmetric to the contralateral uninjured side.
Right femur and knee X-rays showed an antegrade intramedullary nail with a well-healed diaphyseal fracture and a single distal interlocking screw oriented from posterolateral to anteromedial (Figures 1A-1G). The screw tip was prominent on sunrise X-ray view anterior to the medial femoral epicondyle (Figure 1C). Magnetic resonance imaging demonstrated a large effusion and lateral patellar subluxation with a prominent intra-articular distal interlocking screw disrupting the MPFL near the femoral attachment (Figure 2). Patellar height, trochlear morphology, and tibial tubercle-trochlear groove distance were assessed and found to be normal.


Continue to: The patient elected...
The patient elected to have a right knee arthroscopic-assisted MPFL reconstruction and removal of the distal interlocking screw. Diagnostic arthroscopy revealed the distal interlocking screw to be intra-articular medially, prominent by 3 mm causing attritional disruption of the mid-substance MPFL (Figure 3A). The patella was noted to be subluxated and tracking laterally (Figure 3B). Both the anterior cruciate ligament and posterior cruciate ligament were intact, and menisci and articular cartilage were normal. The distal interlocking screw was removed under fluoroscopic guidance through a small lateral incision (Figure 3C).

Due to the nature of the longstanding attritional disruption of the MPFL in this case with associated patellar instability over a 2-year period, the decision was made to proceed with formal MPFL reconstruction as opposed to repair. A 2-cm incision was made at the medial aspect of the patella. The proximal half of the patella was decorticated. Guide pins were placed within the proximal half of the patella, ensuring at least a 1-cm bone bridge between them, and two 4.75-mm SwiveLock suture anchors (Arthrex) were inserted. A semitendinosus graft was used for MPFL reconstruction with the 2 ends of the graft secured to 2 suture anchors with a whipstitch. Lateral fluoroscopy was used to identify Schöttle’s point, denoting the femoral origin of the MPFL9 (Figure 3D). A 2-cm incision was made at this location. A guide pin was then placed at Schöttle’s point under fluoroscopic guidance, aimed proximally, and the knee was brought through a range of motion (ROM), to verify graft isometry. Once verified, the guide pin was over-reamed to 8 mm. The layer between the retinaculum and the capsule was carefully dissected, and the graft was passed extra-articularly in the plane between the retinaculum and the capsule, out through the medial incision, and docked into the bone tunnel. An 8-mm BioComposite interference screw (Arthrex) was then placed with the knee flexed to 30°. The knee was then passed through a ROM and an arthroscopic evaluation confirmed that the patella was no longer subluxated laterally. There was normal tracking of the patellofemoral joint on arthroscopic evaluation.
Postoperatively, the patient was maintained in a hinged knee brace for 6 weeks. He was weight-bearing as tolerated when locked in full extension beginning immediately postoperatively, and allowed to unlock the brace to start non-weight-bearing active flexion and extension with therapy on postoperative day 1. Radiographs confirmed removal of the distal interlocking screw (Figures 4A, 4B). Following surgery, the patient experienced resolution of his effusions, no recurrent patellar instability at 1-year postoperative, and was able to return to his ADL and recreational sporting activities (Knee Injury and Osteoarthritis Outcome Score [KOOS] ADL, 100; KOOS sporting and recreational activities, 95; quality of life, 100; Marx Activity Rating Scale, 12).

DISCUSSION
The MPFL connects the superomedial edge of the patella to the medial femur and is injured in nearly 100% of patellar dislocations.6 The femoral origin lies between the adductor tubercle and the medial epicondyle.7 The MPFL prevents lateral subluxation of the patella and acts as the major restraint during the first 20° of knee flexion. Although radiographic parameters for identifying the MPFL femoral origin have been defined by both Schöttle and colleagues9 and Stephen and colleagues10, it is important to check the isometry intraoperatively through a ROM when performing MPFL reconstruction. In this case, the patient’s history and physical examination showed patellar instability, which was determined to be iatrogenically related to the distal interlocking screw rupture of the MPFL. Following screw removal and MPFL reconstruction, the patient had no further symptoms of pain, effusion, or patellar instability and returned to his normal activities.
Femoral malrotation following intramedullary nailing of femoral shaft fractures is a common complication,4 with a 22% incidence of malrotation of at least 15° in 1 series from an academic trauma center.11 There are mixed data as to whether malrotation is more common in complex fracture patterns, in cases performed during night hours, and in cases performed by non-trauma fellowship-trained surgeons.11-13 The natural history of malrotation is not well elucidated, but there is some suggestion that it alters load bearing in the distal joints of the involved leg including the patellofemoral joint. Patients also may not tolerate malrotation due to the abnormal foot progression angle, particularly with malrotation >15°.4 In this case, the patient’s initial femoral nail was placed in an externally rotated position, requiring revision. The result of this was an unusual trajectory of the distal interlocking screw from posterolateral to anteromedial. Combined with the prominent screw tip, the trajectory of this distal interlocking screw likely contributed to the injury to the MPFL observed in this case. This trajectory would also pose potential risk to the common peroneal nerve, which is usually situated posterior to the insertion point for distal femoral interlocking screws. The prominent distal interlock screw is a well-recognized problem with femoral intramedullary nails. This issue results from the tapering of the width of the distal femur from being larger posteriorly to being smaller anteriorly. To avoid placement of a prominent distal interlocking screw, surgeons often will obtain an intraoperative anterior-posterior radiograph with the lower extremity in 30° of internal rotation to account for the angle of the medial aspect of the distal femur.
This practice represents, to our knowledge, a previously unreported cause of patellar instability as well as an unreported complication of antegrade femoral intramedullary nailing. Surgeons treating these conditions should consider this potential complication and pursue advanced imaging if patients present with these complaints after femoral intramedullary nail placement. Knowledge of both MPFL origin and insertional anatomy and avoidance of prominent distal interlocking screws in the region of the MPFL, if possible, would likely prevent this complication.
Limitations of this study include the case report design, which makes it impossible to comment on the incidence of this complication or to make comparisons regarding treatment options. There is, of course, the possibility that the patient had a concurrent MPFL injury from the injury in which he sustained the femur fracture. Nevertheless, the clinical history, examination, imaging, and arthroscopic findings all strongly suggest that the prominent distal interlocking screw was the cause of his MPFL injury and patellar instability. Finally, the point widely defined by Schöttle and colleagues12 was used for MPFL reconstruction in this case based on an intraoperative true lateral radiograph of the distal femur. It should be noted that recent literature has debated the accuracy of this method for determining the femoral origin, the anatomy of the MPFL in relation to the quadriceps, and type of fixation for MPFL reconstruction with some advocating soft tissue only fixation.14-17 For purposes of this case report, we focused on a different cause of MPFL disruption in this patient and our technique for MPFL reconstruction.
CONCLUSION
This case demonstrates that iatrogenic MPFL injury is a potential complication of antegrade femoral nailing and a previously unrecognized cause of patellar instability. Surgeons should be aware of this potential complication and strive to avoid the MPFL origin when placing their distal interlocking screw.
This paper will be judged for the Resident Writer’s Award.
- Brumback RJ, Virkus WW. Intramedullary nailing of the femur: reamed versus nonreamed. J Am Acad Orthop Surg. 2000;8(2):83-90.
- Ricci WM, Bellabarba C, Evanoff B, Herscovici D, DiPasquale T, Sanders R. Retrograde versus antegrade nailing of femoral shaft fractures. J Orthop Trauma 2001;15(3):161-169.
- Ricci WM, Gallagher B, Haidukewych GJ. Intramedullary nailing of femoral shaft fractures: current concepts. J Am Acad Orthop Surg. 2009;17(5):296-305.
- Lindsey JD, Krieg JC. Femoral malrotation following intramedullary nail fixation. J Am Acad Orthop Surg. 2011;19(1):17-26.
- Busam ML, Esther RJ, Obremskey WT. Hardware removal: indications and expectations. J Am Acad Orthop Surg. 2006;14(2):113-120.
- Morshed S, Humphrey M, Corrales LA, Millett M, Hoffinger SA. Retention of flexible intramedullary nails following treatment of pediatric femur fractures. Arch Orthop Trauma Surg. 2007;127(7):509-514.
- Boerger TO, Patel G, Murphy JP. Is routine removal of intramedullary nails justified. Injury. 1999;30(2):79-81.
- Kellan J. Fracture healing: Does hardware removal enhance patient outcomes. Chin J Orthop Trauma (Chin). 2010;12:374-378.
- Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804. doi:10.1177/0363546506296415.
- Stephen JM, Lumpaopong P, Deehan DJ, Kader D, Amis AA. The medial patellofemoral ligament: location of femoral attachment and length change patterns resulting from anatomic and nonanatomic attachments. Am J Sports Med. 2012;40(8):1871-1879. doi:10.1177/0363546512449998.
- Hüfner T, Citak M, Suero EM, et al. Femoral malrotation after unreamed intramedullary nailing: an evaluation of influencing operative factors. J Orthop Trauma. 2011;25(4):224-227. doi:10.1097/BOT.0b013e3181e47e3b.
- Ayalon OB, Patel NM, Yoon RS, Donegan DJ, Koerner JD, Liporace FA. Comparing femoral version after intramedullary nailing performed by trauma-trained and non-trauma trained surgeons: is there a difference? Injury. 2014;45(7):1091-1094. doi:10.1016/j.injury.2014.01.024.
- Patel NM, Yoon RS, Cantlon MB, Koerner JD, Donegan DJ, Liporace FA. Intramedullary nailing of diaphyseal femur fractures secondary to gunshot wounds: predictors of postoperative malrotation. J Orthop Trauma. 2014;28(12):711-714. doi:10.1097/BOT.0000000000000124.
- Ziegler CG, Fulkerson JP, Edgar C. Radiographic reference points are inaccurate with and without a true lateral radiograph: the importance of anatomy in medial patellofemoral ligament reconstruction. Am J Sports Med. 2016;44(1):133-142.
- Fulkerson JP, Edgar C. Medial quadriceps tendon-femoral ligament: surgical anatomy and reconstruction technique to prevent patella instability. Arthrosc Tech. 2013;2(2):e125-e128. doi:10.1016/j.eats.2013.01.002.
- Tanaka MJ, Voss A, Fulkerson JP. The anatomic midpoint of the attachment of the medial patellofemoral complex. J Bone Joint Surg Am. 2016;98(14):1199-1205. doi:10.2106/JBJS.15.01182.
- Mochizuki T, Nimura A, Tateishi T, Yamaguchi K, Muneta T, Akita K. Anatomic study of the attachment of the medial patellofemoral ligament and its characteristic relationships to the vastus intermedius. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):305-310. doi:10.1007/s00167-012-1993-7.
- Brumback RJ, Virkus WW. Intramedullary nailing of the femur: reamed versus nonreamed. J Am Acad Orthop Surg. 2000;8(2):83-90.
- Ricci WM, Bellabarba C, Evanoff B, Herscovici D, DiPasquale T, Sanders R. Retrograde versus antegrade nailing of femoral shaft fractures. J Orthop Trauma 2001;15(3):161-169.
- Ricci WM, Gallagher B, Haidukewych GJ. Intramedullary nailing of femoral shaft fractures: current concepts. J Am Acad Orthop Surg. 2009;17(5):296-305.
- Lindsey JD, Krieg JC. Femoral malrotation following intramedullary nail fixation. J Am Acad Orthop Surg. 2011;19(1):17-26.
- Busam ML, Esther RJ, Obremskey WT. Hardware removal: indications and expectations. J Am Acad Orthop Surg. 2006;14(2):113-120.
- Morshed S, Humphrey M, Corrales LA, Millett M, Hoffinger SA. Retention of flexible intramedullary nails following treatment of pediatric femur fractures. Arch Orthop Trauma Surg. 2007;127(7):509-514.
- Boerger TO, Patel G, Murphy JP. Is routine removal of intramedullary nails justified. Injury. 1999;30(2):79-81.
- Kellan J. Fracture healing: Does hardware removal enhance patient outcomes. Chin J Orthop Trauma (Chin). 2010;12:374-378.
- Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804. doi:10.1177/0363546506296415.
- Stephen JM, Lumpaopong P, Deehan DJ, Kader D, Amis AA. The medial patellofemoral ligament: location of femoral attachment and length change patterns resulting from anatomic and nonanatomic attachments. Am J Sports Med. 2012;40(8):1871-1879. doi:10.1177/0363546512449998.
- Hüfner T, Citak M, Suero EM, et al. Femoral malrotation after unreamed intramedullary nailing: an evaluation of influencing operative factors. J Orthop Trauma. 2011;25(4):224-227. doi:10.1097/BOT.0b013e3181e47e3b.
- Ayalon OB, Patel NM, Yoon RS, Donegan DJ, Koerner JD, Liporace FA. Comparing femoral version after intramedullary nailing performed by trauma-trained and non-trauma trained surgeons: is there a difference? Injury. 2014;45(7):1091-1094. doi:10.1016/j.injury.2014.01.024.
- Patel NM, Yoon RS, Cantlon MB, Koerner JD, Donegan DJ, Liporace FA. Intramedullary nailing of diaphyseal femur fractures secondary to gunshot wounds: predictors of postoperative malrotation. J Orthop Trauma. 2014;28(12):711-714. doi:10.1097/BOT.0000000000000124.
- Ziegler CG, Fulkerson JP, Edgar C. Radiographic reference points are inaccurate with and without a true lateral radiograph: the importance of anatomy in medial patellofemoral ligament reconstruction. Am J Sports Med. 2016;44(1):133-142.
- Fulkerson JP, Edgar C. Medial quadriceps tendon-femoral ligament: surgical anatomy and reconstruction technique to prevent patella instability. Arthrosc Tech. 2013;2(2):e125-e128. doi:10.1016/j.eats.2013.01.002.
- Tanaka MJ, Voss A, Fulkerson JP. The anatomic midpoint of the attachment of the medial patellofemoral complex. J Bone Joint Surg Am. 2016;98(14):1199-1205. doi:10.2106/JBJS.15.01182.
- Mochizuki T, Nimura A, Tateishi T, Yamaguchi K, Muneta T, Akita K. Anatomic study of the attachment of the medial patellofemoral ligament and its characteristic relationships to the vastus intermedius. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):305-310. doi:10.1007/s00167-012-1993-7.
TAKE-HOME POINTS
- Anterograde intramedullary nailing is the gold standard for fixation of diaphyseal femur fractures.
- Damage to the MPFL can be caused by the distal interlocking screw of an anterograde intramedullary nail.
- The trajectory of the distal interlocking screw from posterolateral to anteromedial, and a prominent screw tip, likely contributed to the injury to the MPFL observed in this case.
- Surgeons treating these conditions should pursue advanced imaging if patients present with effusion and patellar instability after femoral intramedullary nail placement.
- Distal interlocking screw removal and arthroscopic MPFL reconstruction can result in successful return of function and normal activities.
Imaging for Nonarthritic Hip Pathology
Take-Home Points
- Be sure to have a well centered AP pelvis without rotation.
- Get at least 3 plain radiographs—AP pelvis, false profile, and lateral hip view.
- Ensure that there is sufficient acetabular coverage, LCEA >20° on AP pelvis and ACEA >20° on false profile view.
- CT scans are helpful for precise hip pathomorphology but must be weighed against risk of radiation exposure.
- MRI or MRA can be helpful to diagnose intra-articular as well as extra-articular hip and pelvis abnormalities.
In the work-up for nonarthritic hip pain, the value of diagnostic imaging is in objective findings, which can support or weaken the leading diagnoses based on subjective complaints, recalled history, and, in some cases, elusive physical examination findings. Morphologic changes alone, however, do not always indicate pathology.1,2 At presentation and at each step in the work-up, it is imperative to evaluate the entire clinical picture. The prudent clinician uses both clinical and radiographic findings to make the diagnosis and direct treatment.
Radiography
The first step in diagnostic imaging is radiography. Although use of plain radiographs is routine, their value cannot be understated. Standard hip radiographs—an anteroposterior (AP) radiograph of the pelvis and AP and frog-leg (cross-table lateral) radiographs of the hip—provide a wealth of information.3-6
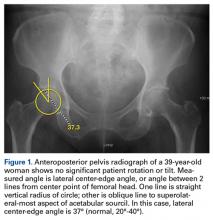
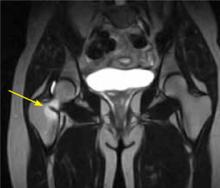
Evaluated first is the radiograph itself. For example, the ideal AP radiograph of the pelvis (Figure 1) is centered on the lower sacrum, and the patient is not rotated.
AP radiographs allow for evaluation of fractures, intraosseous sclerosis, acetabular depth, inclination and version, acetabular overcoverage, joint-space narrowing, femoroacetabular congruency, femoral head sphericity, and femoral head–neck offset.7,8,10 Inspection for labral calcification is important, as it can indicate repetitive damage at the extremes of range of motion.
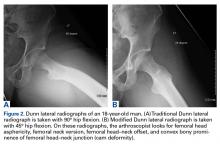
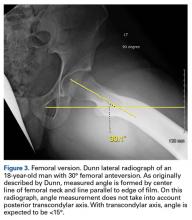
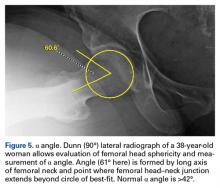
On AP pelvis radiographs, it is important to distinguish coxa profunda from acetabular protrusion. These entities are on the same pathomorphologic spectrum and are similar but distinctively different. Coxa profunda refers to the depth of the acetabulum relative to the ilioischial line, and acetabular protrusion refers to the depth (or medial position) of the femoral head relative to the ilioischial line. Each condition suggests—but is not diagnostic for—pincer-type femoroacetabular impingement (FAI).11Acetabular rotation is another important entity that can be evaluated on well-centered, nontilted AP pelvic radiographs. Acetabular rotation refers to the opening direction of the acetabulum. It may be anterior (anteverted), neutral, or posterior (retroverted). Anteversion is present when the anterior acetabular rim does not traverse the posterior rim shadow4; in other words, the ring formed by the acetabulum is not twisted. When the walls overlap but do not intersect, the cup has neutral version. Retroversion is qualitatively determined by the crossover (figure-of-8) and posterior wall signs12 and is associated with pincer-type FAI and the development of hip osteoarthritis.12Dunn lateral radiographs (Figure 2A), taken with 90° hip flexion, were originally used to measure femoral neck anteversion.13
False-profile radiographs (Figure 6), valuable in evaluating anterior acetabular coverage and femoral head–neck junction morphology,14,15 allow characterization of both cam-type and pincer-type FAI.
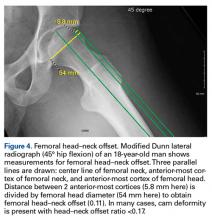
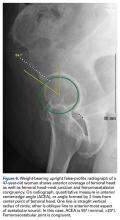
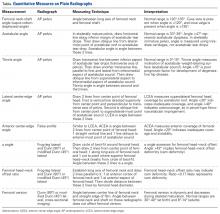
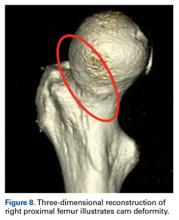
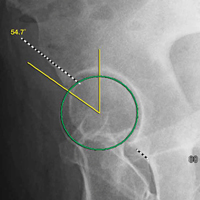
Quantitative measures warrant specific consideration (Table). Femoroacetabular morphology is quantitatively measured by α angle, Tönnis angle (acetabular inclination angle), and lateral center-edge angle (LCEA).7,8,10 The α angle (Figure 4) detects the loss of normal anterosuperior femoral head–neck junction concavity caused by a convex osseous prominence. An α angle >50° represents a cam deformity.16 In a cohort study of 338 patients, Nepple and colleagues17 qualitatively associated increased α angle with severe intra-articular hip disease. Murphy and colleagues18 found a Tönnis angle >15° to be a poor prognostic factor in untreated hip dysplasia. LCEA quantifies superolateral femoral head coverage,19 and its normal range is 20° to 40°.20 LCEA <20° indicates dysplasia of the femoroacetabular joint, and LCEA >40° indicates overcoverage and pincer-type FAI. As with any quantitative radiographic measurement, results should be interpreted within the presenting clinical context.
Radiographic findings, even findings based on these special radiographs, may underestimate the pathologic process.
Computed Tomography
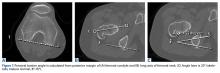
The benefits of computed tomography (CT) outweigh the risk of radiation exposure. CT is most useful in characterizing osseous morphology.21 In FAI cases, CT can distinguish acetabular version abnormalities from femoral torsion (Figures 7A-7C), entities with very different treatment approaches.21
Magnetic Resonance Imaging
MRI is becoming essential in the work-up for nonarthritic hip pain.11,22 It is used for assessment of osseous, chondral, and musculotendinous soft tissues. Further, it affords appreciation of outside-the-hip-joint pathology that may mimic joint-centered pathology.
MRI techniques range from noncontrast to indirect and direct magnetic resonance arthrography (MRA).22 Indirect MRA is performed with contrast medium administered through an intravenous line. Direct MRA has contrast administered intra-articularly and is more sensitive and specific for labral tears and ligamentous injury.23 Excellent detection of intra-articular pathology on noncontrast studies questions the need for MRA.24 Nevertheless, direct MRA can also be used as a therapeutic procedure when lidocaine is included in the injected gadolinium.
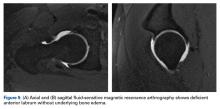
Labral tears, focal chondral defects, and stress or insufficiency fractures are important differentials in the work-up for nonarthritic hip pain. Over the dysplasia-to-FAI spectrum, MRI distinguishes symptomatic pathoanatomy from asymptomatic anatomical variants by revealing underlying bone edema. Capsule findings should also be considered.21The most practical classification of labral tears, proposed by Blankenbaker and colleagues,25 is based on tear type (frayed, unstable, flap), location, and extent. More than half of labral tears occur in the anterosuperior quadrant of the labrum.25
Chondral damage is identified much as labral tears are. With chondral injury, the normal intermediate signal is interrupted by a fluid-intense signal extending to the subchondral bone. A fat-saturated T2or short-tau inversion recovery (STIR) sequence is useful in emphasizing this finding.27
MRI detects osseous pathology from surrounding soft-tissue edema and bone remodeling to stress and fragility fractures. In athletes, the most common fractures are pubic rami, sacral, and apophyseal avulsion fractures.28 In all patients, attention should be given to the lower spine and the proximal femurs. Aside from MRI, nuclear medicine bone scan might also identify active osseous reaction representative of a fracture.
Conclusion
The work-up for nonarthritic hip pain substantiates differential diagnoses. A case’s complexity determines the course of diagnostic imaging. At presentation and at each step in the work-up, it is imperative to evaluate the entire clinical picture. The prudent clinician uses both clinical and radiographic findings to make the diagnosis and direct treatment.
Am J Orthop . 2017;46(1):17-22. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. McCall DA, Safran MR. MRI and arthroscopy correlations of the hip: a case-based approach. Instr Course Lect . 2012;61:327-344.
2. Register B, Pennock AT, Ho CP, Strickland CD, Lawand A, Philippon MJ. Prevalence of abnormal hip findings in asymptomatic participants: a prospective, blinded study. Am J Sports Med . 2012;40(12):2720-2724.
3. Campbell SE. Radiography of the hip: lines, signs, and patterns of disease. Semin Roentgenol . 2005;40(3):290-319.
4. Clohisy JC, Carlisle JC, Beaulé PE, et al. A systematic approach to the plain radiographic evaluation of the young adult hip. J Bone Joint Surg Am . 2008;90(suppl 4):47-66.
5. Malviya A, Raza A, Witt JD. Reliability in the diagnosis of femoroacetabular impingement and dysplasia among hip surgeons: role of surgeon volume and experience. Hip Int . 2016;26(3):284-289.
6. Nepple JJ, Martel JM, Kim YJ, Zaltz I, Clohisy JC, Group AS. Do plain radiographs correlate with CT for imaging of cam-type femoroacetabular impingement? Clin Orthop Relat Res . 2012;470(12):3313-3320.
7. Kosuge D, Cordier T, Solomon LB, Howie DW. Dilemmas in imaging for peri-acetabular osteotomy: the influence of patient position and imaging technique on the radiological features of hip dysplasia. Bone Joint J . 2014;96(9):1155-1160.
8. Tannast M, Fritsch S, Zheng G, Siebenrock KA, Steppacher SD. Which radiographic hip parameters do not have to be corrected for pelvic rotation and tilt? Clin Orthop Relat Res . 2015;473(4):1255-1266.
9. Siebenrock KA, Kalbermatten DF, Ganz R. Effect of pelvic tilt on acetabular retroversion: a study of pelves from cadavers. Clin Orthop Relat Res . 2003;(407):241-248.
10. Griffin JW, Weber AE, Kuhns B, Lewis P, Nho SJ. Imaging in hip arthroscopy for femoroacetabular impingement: a comprehensive approach. Clin Sports Med . 2016;35(3):331-344.
11. Nepple JJ, Lehmann CL, Ross JR, Schoenecker PL, Clohisy JC. Coxa profunda is not a useful radiographic parameter for diagnosing pincer-type femoroacetabular impingement. J Bone Joint Surg Am . 2013;95(5):417-423.
12. Reynolds D, Lucas J, Klaue K. Retroversion of the acetabulum. A cause of hip pain. J Bone Joint Surg Br . 1999;81(2):281-288.
13. Dunn DM. Anteversion of the neck of the femur; a method of measurement. J Bone Joint Surg Br . 1952;34(2):181-186.
14. Meyer DC, Beck M, Ellis T, Ganz R, Leunig M. Comparison of six radiographic projections to assess femoral head/neck asphericity. Clin Orthop Relat Res . 2006;(445):181-185.
15. Hellman MD, Mascarenhas R, Gupta A, et al. The false-profile view may be used to identify cam morphology. Arthroscopy . 2015;31(9):1728-1732.
16. Barton C, Salineros MJ, Rakhra KS, Beaulé PE. Validity of the alpha angle measurement on plain radiographs in the evaluation of cam-type femoroacetabular impingement. Clin Orthop Relat Res . 2011;469(2):464-469.
17. Nepple JJ, Carlisle JC, Nunley RM, Clohisy JC. Clinical and radiographic predictors of intra-articular hip disease in arthroscopy. Am J Sports Med . 2011;39(2):296-303.
18. Murphy SB, Ganz R, Muller ME. The prognosis in untreated dysplasia of the hip. A study of radiographic factors that predict the outcome. J Bone Joint Surg Am . 1995;77(7):985-989.
19. Mast NH, Impellizzeri F, Keller S, Leunig M. Reliability and agreement of measures used in radiographic evaluation of the adult hip. Clin Orthop Relat Res . 2011;469(1):188-199.
20. Monazzam S, Bomar JD, Cidambi K, Kruk P, Hosalkar H. Lateral center-edge angle on conventional radiography and computed tomography. Clin Orthop Relat Res . 2013;471(7):2233-2237.
21. Weber AE, Jacobson JA, Bedi A. A review of imaging modalities for the hip. Curr Rev Musculoskelet Med . 2013;6(3):226-234.
22. Bencardino JT, Palmer WE. Imaging of hip disorders in athletes. Radiol Clin North Am . 2002;40(2):267-287, vi-vii.
23. Byrd JW, Jones KS. Diagnostic accuracy of clinical assessment, magnetic resonance imaging, magnetic resonance arthrography, and intra-articular injection in hip arthroscopy patients. Am J Sports Med . 2004;32(7):1668-1674.
24. Mintz DN, Hooper T, Connell D, Buly R, Padgett DE, Potter HG. Magnetic resonance imaging of the hip: detection of labral and chondral abnormalities using noncontrast imaging. Arthroscopy . 2005;21(4):385-393.
25. Blankenbaker DG, De Smet AA, Keene JS, Fine JP. Classification and localization of acetabular labral tears. Skeletal Radiol . 2007;36(5):391-397.
26. Aydingöz U, Oztürk MH. MR imaging of the acetabular labrum: a comparative study of both hips in 180 asymptomatic volunteers. Eur Radiol . 2001;11(4):567-574.
27. Gold GE, Chen CA, Koo S, Hargreaves BA, Bangerter NK. Recent advances in MRI of articular cartilage. AJR Am J Roentgenol . 2009;193(3):628-638.
28. Liong SY, Whitehouse RW. Lower extremity and pelvic stress fractures in athletes. Br J Radiol . 2012;85(1016):1148-1156.
Take-Home Points
- Be sure to have a well centered AP pelvis without rotation.
- Get at least 3 plain radiographs—AP pelvis, false profile, and lateral hip view.
- Ensure that there is sufficient acetabular coverage, LCEA >20° on AP pelvis and ACEA >20° on false profile view.
- CT scans are helpful for precise hip pathomorphology but must be weighed against risk of radiation exposure.
- MRI or MRA can be helpful to diagnose intra-articular as well as extra-articular hip and pelvis abnormalities.
In the work-up for nonarthritic hip pain, the value of diagnostic imaging is in objective findings, which can support or weaken the leading diagnoses based on subjective complaints, recalled history, and, in some cases, elusive physical examination findings. Morphologic changes alone, however, do not always indicate pathology.1,2 At presentation and at each step in the work-up, it is imperative to evaluate the entire clinical picture. The prudent clinician uses both clinical and radiographic findings to make the diagnosis and direct treatment.
Radiography
The first step in diagnostic imaging is radiography. Although use of plain radiographs is routine, their value cannot be understated. Standard hip radiographs—an anteroposterior (AP) radiograph of the pelvis and AP and frog-leg (cross-table lateral) radiographs of the hip—provide a wealth of information.3-6
Evaluated first is the radiograph itself. For example, the ideal AP radiograph of the pelvis (Figure 1) is centered on the lower sacrum, and the patient is not rotated.
AP radiographs allow for evaluation of fractures, intraosseous sclerosis, acetabular depth, inclination and version, acetabular overcoverage, joint-space narrowing, femoroacetabular congruency, femoral head sphericity, and femoral head–neck offset.7,8,10 Inspection for labral calcification is important, as it can indicate repetitive damage at the extremes of range of motion.
On AP pelvis radiographs, it is important to distinguish coxa profunda from acetabular protrusion. These entities are on the same pathomorphologic spectrum and are similar but distinctively different. Coxa profunda refers to the depth of the acetabulum relative to the ilioischial line, and acetabular protrusion refers to the depth (or medial position) of the femoral head relative to the ilioischial line. Each condition suggests—but is not diagnostic for—pincer-type femoroacetabular impingement (FAI).11Acetabular rotation is another important entity that can be evaluated on well-centered, nontilted AP pelvic radiographs. Acetabular rotation refers to the opening direction of the acetabulum. It may be anterior (anteverted), neutral, or posterior (retroverted). Anteversion is present when the anterior acetabular rim does not traverse the posterior rim shadow4; in other words, the ring formed by the acetabulum is not twisted. When the walls overlap but do not intersect, the cup has neutral version. Retroversion is qualitatively determined by the crossover (figure-of-8) and posterior wall signs12 and is associated with pincer-type FAI and the development of hip osteoarthritis.12Dunn lateral radiographs (Figure 2A), taken with 90° hip flexion, were originally used to measure femoral neck anteversion.13
False-profile radiographs (Figure 6), valuable in evaluating anterior acetabular coverage and femoral head–neck junction morphology,14,15 allow characterization of both cam-type and pincer-type FAI.
Quantitative measures warrant specific consideration (Table). Femoroacetabular morphology is quantitatively measured by α angle, Tönnis angle (acetabular inclination angle), and lateral center-edge angle (LCEA).7,8,10 The α angle (Figure 4) detects the loss of normal anterosuperior femoral head–neck junction concavity caused by a convex osseous prominence. An α angle >50° represents a cam deformity.16 In a cohort study of 338 patients, Nepple and colleagues17 qualitatively associated increased α angle with severe intra-articular hip disease. Murphy and colleagues18 found a Tönnis angle >15° to be a poor prognostic factor in untreated hip dysplasia. LCEA quantifies superolateral femoral head coverage,19 and its normal range is 20° to 40°.20 LCEA <20° indicates dysplasia of the femoroacetabular joint, and LCEA >40° indicates overcoverage and pincer-type FAI. As with any quantitative radiographic measurement, results should be interpreted within the presenting clinical context.
Radiographic findings, even findings based on these special radiographs, may underestimate the pathologic process.
Computed Tomography
The benefits of computed tomography (CT) outweigh the risk of radiation exposure. CT is most useful in characterizing osseous morphology.21 In FAI cases, CT can distinguish acetabular version abnormalities from femoral torsion (Figures 7A-7C), entities with very different treatment approaches.21
Magnetic Resonance Imaging
MRI is becoming essential in the work-up for nonarthritic hip pain.11,22 It is used for assessment of osseous, chondral, and musculotendinous soft tissues. Further, it affords appreciation of outside-the-hip-joint pathology that may mimic joint-centered pathology.
MRI techniques range from noncontrast to indirect and direct magnetic resonance arthrography (MRA).22 Indirect MRA is performed with contrast medium administered through an intravenous line. Direct MRA has contrast administered intra-articularly and is more sensitive and specific for labral tears and ligamentous injury.23 Excellent detection of intra-articular pathology on noncontrast studies questions the need for MRA.24 Nevertheless, direct MRA can also be used as a therapeutic procedure when lidocaine is included in the injected gadolinium.
Labral tears, focal chondral defects, and stress or insufficiency fractures are important differentials in the work-up for nonarthritic hip pain. Over the dysplasia-to-FAI spectrum, MRI distinguishes symptomatic pathoanatomy from asymptomatic anatomical variants by revealing underlying bone edema. Capsule findings should also be considered.21The most practical classification of labral tears, proposed by Blankenbaker and colleagues,25 is based on tear type (frayed, unstable, flap), location, and extent. More than half of labral tears occur in the anterosuperior quadrant of the labrum.25
Chondral damage is identified much as labral tears are. With chondral injury, the normal intermediate signal is interrupted by a fluid-intense signal extending to the subchondral bone. A fat-saturated T2or short-tau inversion recovery (STIR) sequence is useful in emphasizing this finding.27
MRI detects osseous pathology from surrounding soft-tissue edema and bone remodeling to stress and fragility fractures. In athletes, the most common fractures are pubic rami, sacral, and apophyseal avulsion fractures.28 In all patients, attention should be given to the lower spine and the proximal femurs. Aside from MRI, nuclear medicine bone scan might also identify active osseous reaction representative of a fracture.
Conclusion
The work-up for nonarthritic hip pain substantiates differential diagnoses. A case’s complexity determines the course of diagnostic imaging. At presentation and at each step in the work-up, it is imperative to evaluate the entire clinical picture. The prudent clinician uses both clinical and radiographic findings to make the diagnosis and direct treatment.
Am J Orthop . 2017;46(1):17-22. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Be sure to have a well centered AP pelvis without rotation.
- Get at least 3 plain radiographs—AP pelvis, false profile, and lateral hip view.
- Ensure that there is sufficient acetabular coverage, LCEA >20° on AP pelvis and ACEA >20° on false profile view.
- CT scans are helpful for precise hip pathomorphology but must be weighed against risk of radiation exposure.
- MRI or MRA can be helpful to diagnose intra-articular as well as extra-articular hip and pelvis abnormalities.
In the work-up for nonarthritic hip pain, the value of diagnostic imaging is in objective findings, which can support or weaken the leading diagnoses based on subjective complaints, recalled history, and, in some cases, elusive physical examination findings. Morphologic changes alone, however, do not always indicate pathology.1,2 At presentation and at each step in the work-up, it is imperative to evaluate the entire clinical picture. The prudent clinician uses both clinical and radiographic findings to make the diagnosis and direct treatment.
Radiography
The first step in diagnostic imaging is radiography. Although use of plain radiographs is routine, their value cannot be understated. Standard hip radiographs—an anteroposterior (AP) radiograph of the pelvis and AP and frog-leg (cross-table lateral) radiographs of the hip—provide a wealth of information.3-6
Evaluated first is the radiograph itself. For example, the ideal AP radiograph of the pelvis (Figure 1) is centered on the lower sacrum, and the patient is not rotated.
AP radiographs allow for evaluation of fractures, intraosseous sclerosis, acetabular depth, inclination and version, acetabular overcoverage, joint-space narrowing, femoroacetabular congruency, femoral head sphericity, and femoral head–neck offset.7,8,10 Inspection for labral calcification is important, as it can indicate repetitive damage at the extremes of range of motion.
On AP pelvis radiographs, it is important to distinguish coxa profunda from acetabular protrusion. These entities are on the same pathomorphologic spectrum and are similar but distinctively different. Coxa profunda refers to the depth of the acetabulum relative to the ilioischial line, and acetabular protrusion refers to the depth (or medial position) of the femoral head relative to the ilioischial line. Each condition suggests—but is not diagnostic for—pincer-type femoroacetabular impingement (FAI).11Acetabular rotation is another important entity that can be evaluated on well-centered, nontilted AP pelvic radiographs. Acetabular rotation refers to the opening direction of the acetabulum. It may be anterior (anteverted), neutral, or posterior (retroverted). Anteversion is present when the anterior acetabular rim does not traverse the posterior rim shadow4; in other words, the ring formed by the acetabulum is not twisted. When the walls overlap but do not intersect, the cup has neutral version. Retroversion is qualitatively determined by the crossover (figure-of-8) and posterior wall signs12 and is associated with pincer-type FAI and the development of hip osteoarthritis.12Dunn lateral radiographs (Figure 2A), taken with 90° hip flexion, were originally used to measure femoral neck anteversion.13
False-profile radiographs (Figure 6), valuable in evaluating anterior acetabular coverage and femoral head–neck junction morphology,14,15 allow characterization of both cam-type and pincer-type FAI.
Quantitative measures warrant specific consideration (Table). Femoroacetabular morphology is quantitatively measured by α angle, Tönnis angle (acetabular inclination angle), and lateral center-edge angle (LCEA).7,8,10 The α angle (Figure 4) detects the loss of normal anterosuperior femoral head–neck junction concavity caused by a convex osseous prominence. An α angle >50° represents a cam deformity.16 In a cohort study of 338 patients, Nepple and colleagues17 qualitatively associated increased α angle with severe intra-articular hip disease. Murphy and colleagues18 found a Tönnis angle >15° to be a poor prognostic factor in untreated hip dysplasia. LCEA quantifies superolateral femoral head coverage,19 and its normal range is 20° to 40°.20 LCEA <20° indicates dysplasia of the femoroacetabular joint, and LCEA >40° indicates overcoverage and pincer-type FAI. As with any quantitative radiographic measurement, results should be interpreted within the presenting clinical context.
Radiographic findings, even findings based on these special radiographs, may underestimate the pathologic process.
Computed Tomography
The benefits of computed tomography (CT) outweigh the risk of radiation exposure. CT is most useful in characterizing osseous morphology.21 In FAI cases, CT can distinguish acetabular version abnormalities from femoral torsion (Figures 7A-7C), entities with very different treatment approaches.21
Magnetic Resonance Imaging
MRI is becoming essential in the work-up for nonarthritic hip pain.11,22 It is used for assessment of osseous, chondral, and musculotendinous soft tissues. Further, it affords appreciation of outside-the-hip-joint pathology that may mimic joint-centered pathology.
MRI techniques range from noncontrast to indirect and direct magnetic resonance arthrography (MRA).22 Indirect MRA is performed with contrast medium administered through an intravenous line. Direct MRA has contrast administered intra-articularly and is more sensitive and specific for labral tears and ligamentous injury.23 Excellent detection of intra-articular pathology on noncontrast studies questions the need for MRA.24 Nevertheless, direct MRA can also be used as a therapeutic procedure when lidocaine is included in the injected gadolinium.
Labral tears, focal chondral defects, and stress or insufficiency fractures are important differentials in the work-up for nonarthritic hip pain. Over the dysplasia-to-FAI spectrum, MRI distinguishes symptomatic pathoanatomy from asymptomatic anatomical variants by revealing underlying bone edema. Capsule findings should also be considered.21The most practical classification of labral tears, proposed by Blankenbaker and colleagues,25 is based on tear type (frayed, unstable, flap), location, and extent. More than half of labral tears occur in the anterosuperior quadrant of the labrum.25
Chondral damage is identified much as labral tears are. With chondral injury, the normal intermediate signal is interrupted by a fluid-intense signal extending to the subchondral bone. A fat-saturated T2or short-tau inversion recovery (STIR) sequence is useful in emphasizing this finding.27
MRI detects osseous pathology from surrounding soft-tissue edema and bone remodeling to stress and fragility fractures. In athletes, the most common fractures are pubic rami, sacral, and apophyseal avulsion fractures.28 In all patients, attention should be given to the lower spine and the proximal femurs. Aside from MRI, nuclear medicine bone scan might also identify active osseous reaction representative of a fracture.
Conclusion
The work-up for nonarthritic hip pain substantiates differential diagnoses. A case’s complexity determines the course of diagnostic imaging. At presentation and at each step in the work-up, it is imperative to evaluate the entire clinical picture. The prudent clinician uses both clinical and radiographic findings to make the diagnosis and direct treatment.
Am J Orthop . 2017;46(1):17-22. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. McCall DA, Safran MR. MRI and arthroscopy correlations of the hip: a case-based approach. Instr Course Lect . 2012;61:327-344.
2. Register B, Pennock AT, Ho CP, Strickland CD, Lawand A, Philippon MJ. Prevalence of abnormal hip findings in asymptomatic participants: a prospective, blinded study. Am J Sports Med . 2012;40(12):2720-2724.
3. Campbell SE. Radiography of the hip: lines, signs, and patterns of disease. Semin Roentgenol . 2005;40(3):290-319.
4. Clohisy JC, Carlisle JC, Beaulé PE, et al. A systematic approach to the plain radiographic evaluation of the young adult hip. J Bone Joint Surg Am . 2008;90(suppl 4):47-66.
5. Malviya A, Raza A, Witt JD. Reliability in the diagnosis of femoroacetabular impingement and dysplasia among hip surgeons: role of surgeon volume and experience. Hip Int . 2016;26(3):284-289.
6. Nepple JJ, Martel JM, Kim YJ, Zaltz I, Clohisy JC, Group AS. Do plain radiographs correlate with CT for imaging of cam-type femoroacetabular impingement? Clin Orthop Relat Res . 2012;470(12):3313-3320.
7. Kosuge D, Cordier T, Solomon LB, Howie DW. Dilemmas in imaging for peri-acetabular osteotomy: the influence of patient position and imaging technique on the radiological features of hip dysplasia. Bone Joint J . 2014;96(9):1155-1160.
8. Tannast M, Fritsch S, Zheng G, Siebenrock KA, Steppacher SD. Which radiographic hip parameters do not have to be corrected for pelvic rotation and tilt? Clin Orthop Relat Res . 2015;473(4):1255-1266.
9. Siebenrock KA, Kalbermatten DF, Ganz R. Effect of pelvic tilt on acetabular retroversion: a study of pelves from cadavers. Clin Orthop Relat Res . 2003;(407):241-248.
10. Griffin JW, Weber AE, Kuhns B, Lewis P, Nho SJ. Imaging in hip arthroscopy for femoroacetabular impingement: a comprehensive approach. Clin Sports Med . 2016;35(3):331-344.
11. Nepple JJ, Lehmann CL, Ross JR, Schoenecker PL, Clohisy JC. Coxa profunda is not a useful radiographic parameter for diagnosing pincer-type femoroacetabular impingement. J Bone Joint Surg Am . 2013;95(5):417-423.
12. Reynolds D, Lucas J, Klaue K. Retroversion of the acetabulum. A cause of hip pain. J Bone Joint Surg Br . 1999;81(2):281-288.
13. Dunn DM. Anteversion of the neck of the femur; a method of measurement. J Bone Joint Surg Br . 1952;34(2):181-186.
14. Meyer DC, Beck M, Ellis T, Ganz R, Leunig M. Comparison of six radiographic projections to assess femoral head/neck asphericity. Clin Orthop Relat Res . 2006;(445):181-185.
15. Hellman MD, Mascarenhas R, Gupta A, et al. The false-profile view may be used to identify cam morphology. Arthroscopy . 2015;31(9):1728-1732.
16. Barton C, Salineros MJ, Rakhra KS, Beaulé PE. Validity of the alpha angle measurement on plain radiographs in the evaluation of cam-type femoroacetabular impingement. Clin Orthop Relat Res . 2011;469(2):464-469.
17. Nepple JJ, Carlisle JC, Nunley RM, Clohisy JC. Clinical and radiographic predictors of intra-articular hip disease in arthroscopy. Am J Sports Med . 2011;39(2):296-303.
18. Murphy SB, Ganz R, Muller ME. The prognosis in untreated dysplasia of the hip. A study of radiographic factors that predict the outcome. J Bone Joint Surg Am . 1995;77(7):985-989.
19. Mast NH, Impellizzeri F, Keller S, Leunig M. Reliability and agreement of measures used in radiographic evaluation of the adult hip. Clin Orthop Relat Res . 2011;469(1):188-199.
20. Monazzam S, Bomar JD, Cidambi K, Kruk P, Hosalkar H. Lateral center-edge angle on conventional radiography and computed tomography. Clin Orthop Relat Res . 2013;471(7):2233-2237.
21. Weber AE, Jacobson JA, Bedi A. A review of imaging modalities for the hip. Curr Rev Musculoskelet Med . 2013;6(3):226-234.
22. Bencardino JT, Palmer WE. Imaging of hip disorders in athletes. Radiol Clin North Am . 2002;40(2):267-287, vi-vii.
23. Byrd JW, Jones KS. Diagnostic accuracy of clinical assessment, magnetic resonance imaging, magnetic resonance arthrography, and intra-articular injection in hip arthroscopy patients. Am J Sports Med . 2004;32(7):1668-1674.
24. Mintz DN, Hooper T, Connell D, Buly R, Padgett DE, Potter HG. Magnetic resonance imaging of the hip: detection of labral and chondral abnormalities using noncontrast imaging. Arthroscopy . 2005;21(4):385-393.
25. Blankenbaker DG, De Smet AA, Keene JS, Fine JP. Classification and localization of acetabular labral tears. Skeletal Radiol . 2007;36(5):391-397.
26. Aydingöz U, Oztürk MH. MR imaging of the acetabular labrum: a comparative study of both hips in 180 asymptomatic volunteers. Eur Radiol . 2001;11(4):567-574.
27. Gold GE, Chen CA, Koo S, Hargreaves BA, Bangerter NK. Recent advances in MRI of articular cartilage. AJR Am J Roentgenol . 2009;193(3):628-638.
28. Liong SY, Whitehouse RW. Lower extremity and pelvic stress fractures in athletes. Br J Radiol . 2012;85(1016):1148-1156.
1. McCall DA, Safran MR. MRI and arthroscopy correlations of the hip: a case-based approach. Instr Course Lect . 2012;61:327-344.
2. Register B, Pennock AT, Ho CP, Strickland CD, Lawand A, Philippon MJ. Prevalence of abnormal hip findings in asymptomatic participants: a prospective, blinded study. Am J Sports Med . 2012;40(12):2720-2724.
3. Campbell SE. Radiography of the hip: lines, signs, and patterns of disease. Semin Roentgenol . 2005;40(3):290-319.
4. Clohisy JC, Carlisle JC, Beaulé PE, et al. A systematic approach to the plain radiographic evaluation of the young adult hip. J Bone Joint Surg Am . 2008;90(suppl 4):47-66.
5. Malviya A, Raza A, Witt JD. Reliability in the diagnosis of femoroacetabular impingement and dysplasia among hip surgeons: role of surgeon volume and experience. Hip Int . 2016;26(3):284-289.
6. Nepple JJ, Martel JM, Kim YJ, Zaltz I, Clohisy JC, Group AS. Do plain radiographs correlate with CT for imaging of cam-type femoroacetabular impingement? Clin Orthop Relat Res . 2012;470(12):3313-3320.
7. Kosuge D, Cordier T, Solomon LB, Howie DW. Dilemmas in imaging for peri-acetabular osteotomy: the influence of patient position and imaging technique on the radiological features of hip dysplasia. Bone Joint J . 2014;96(9):1155-1160.
8. Tannast M, Fritsch S, Zheng G, Siebenrock KA, Steppacher SD. Which radiographic hip parameters do not have to be corrected for pelvic rotation and tilt? Clin Orthop Relat Res . 2015;473(4):1255-1266.
9. Siebenrock KA, Kalbermatten DF, Ganz R. Effect of pelvic tilt on acetabular retroversion: a study of pelves from cadavers. Clin Orthop Relat Res . 2003;(407):241-248.
10. Griffin JW, Weber AE, Kuhns B, Lewis P, Nho SJ. Imaging in hip arthroscopy for femoroacetabular impingement: a comprehensive approach. Clin Sports Med . 2016;35(3):331-344.
11. Nepple JJ, Lehmann CL, Ross JR, Schoenecker PL, Clohisy JC. Coxa profunda is not a useful radiographic parameter for diagnosing pincer-type femoroacetabular impingement. J Bone Joint Surg Am . 2013;95(5):417-423.
12. Reynolds D, Lucas J, Klaue K. Retroversion of the acetabulum. A cause of hip pain. J Bone Joint Surg Br . 1999;81(2):281-288.
13. Dunn DM. Anteversion of the neck of the femur; a method of measurement. J Bone Joint Surg Br . 1952;34(2):181-186.
14. Meyer DC, Beck M, Ellis T, Ganz R, Leunig M. Comparison of six radiographic projections to assess femoral head/neck asphericity. Clin Orthop Relat Res . 2006;(445):181-185.
15. Hellman MD, Mascarenhas R, Gupta A, et al. The false-profile view may be used to identify cam morphology. Arthroscopy . 2015;31(9):1728-1732.
16. Barton C, Salineros MJ, Rakhra KS, Beaulé PE. Validity of the alpha angle measurement on plain radiographs in the evaluation of cam-type femoroacetabular impingement. Clin Orthop Relat Res . 2011;469(2):464-469.
17. Nepple JJ, Carlisle JC, Nunley RM, Clohisy JC. Clinical and radiographic predictors of intra-articular hip disease in arthroscopy. Am J Sports Med . 2011;39(2):296-303.
18. Murphy SB, Ganz R, Muller ME. The prognosis in untreated dysplasia of the hip. A study of radiographic factors that predict the outcome. J Bone Joint Surg Am . 1995;77(7):985-989.
19. Mast NH, Impellizzeri F, Keller S, Leunig M. Reliability and agreement of measures used in radiographic evaluation of the adult hip. Clin Orthop Relat Res . 2011;469(1):188-199.
20. Monazzam S, Bomar JD, Cidambi K, Kruk P, Hosalkar H. Lateral center-edge angle on conventional radiography and computed tomography. Clin Orthop Relat Res . 2013;471(7):2233-2237.
21. Weber AE, Jacobson JA, Bedi A. A review of imaging modalities for the hip. Curr Rev Musculoskelet Med . 2013;6(3):226-234.
22. Bencardino JT, Palmer WE. Imaging of hip disorders in athletes. Radiol Clin North Am . 2002;40(2):267-287, vi-vii.
23. Byrd JW, Jones KS. Diagnostic accuracy of clinical assessment, magnetic resonance imaging, magnetic resonance arthrography, and intra-articular injection in hip arthroscopy patients. Am J Sports Med . 2004;32(7):1668-1674.
24. Mintz DN, Hooper T, Connell D, Buly R, Padgett DE, Potter HG. Magnetic resonance imaging of the hip: detection of labral and chondral abnormalities using noncontrast imaging. Arthroscopy . 2005;21(4):385-393.
25. Blankenbaker DG, De Smet AA, Keene JS, Fine JP. Classification and localization of acetabular labral tears. Skeletal Radiol . 2007;36(5):391-397.
26. Aydingöz U, Oztürk MH. MR imaging of the acetabular labrum: a comparative study of both hips in 180 asymptomatic volunteers. Eur Radiol . 2001;11(4):567-574.
27. Gold GE, Chen CA, Koo S, Hargreaves BA, Bangerter NK. Recent advances in MRI of articular cartilage. AJR Am J Roentgenol . 2009;193(3):628-638.
28. Liong SY, Whitehouse RW. Lower extremity and pelvic stress fractures in athletes. Br J Radiol . 2012;85(1016):1148-1156.
Current Techniques in Treating Femoroacetabular Impingement: Capsular Repair and Plication
Take-Home Points
- Hip capsule provides static stabilization for the hip joint.
- Capsular management must weigh visualization to address underlying osseous deformity but also repair/plication of the capsule to maintain biomechanical characteristics.
- T-capsulotomy provides optimal visualization with a small interportal incision with a vertical incision along the femoral neck.
- Extensile interportal capsulotomy is the most widely used capsulotomy and size may vary depending on capsular and patient characteristics.
- Orthopedic surgeons should be equipped to employ either technique depending on the patients individual hip pathomorphology.
Hip arthroscopy has emerged as a common surgical treatment for a number of hip pathologies. Surgical treatment strategies, including management of the hip capsule, have evolved. Whereas earlier hip arthroscopies often involved capsulectomy or capsulotomy without repair, more recently capsular closure has been considered an important step in restoring the anatomy of the hip joint and preventing microinstability or gross macroinstability.
The anatomy of the hip joint includes both static and dynamic stabilizers designed to maintain a functioning articulation. The osseous articulation of the femoral head and acetabulum is the first static stabilizer, with variations in offset, version, and inclination of the acetabulum and the proximal femur. The joint capsule consists of 3 ligaments—iliofemoral, pubofemoral, and ischiofemoral—that converge to form the zona orbicularis. Other soft-tissue structures, such as the articular cartilage, the labrum, the transverse acetabular ligament, the pulvinar, and the ligamentum teres, also provide static constraint.1 The surrounding musculature provides the hip joint with dynamic stability, which contributes to overall maintenance of proper joint kinematics.
Management of the hip capsule has evolved as our understanding of hip pathology and biomechanics has matured. Initial articles on using hip arthroscopy to treat labral tears described improvement in clinical outcomes,2 but the cases involved limited focal capsulotomy. Not until the idea of femoroacetabular impingement (FAI) was introduced were extensive capsulotomies and capsulectomies performed to address the underlying osseous deformities and emulate open techniques. Soon after our ability to access osseous pathomorphology improved with enhanced visualization and comprehensive resection, cases of hip instability after hip arthroscopy surfaced.3-5 Although frank dislocation after hip arthroscopy is rare and largely underreported, it is a catastrophic complication. In addition, focal capsular defects were also described in cases of failed hip arthroscopy and thought to lead to microinstability of the hip.6 Iatrogenic microinstability is thought to be more common, but it is also underrecognized as a cause of failure of hip arthroscopy.7Microinstability is a pathologic condition that can affect hip function. In cases of recurrent pain and unimproved functional status after surgery, microinstability should be considered. In an imaging study of capsule integrity, McCormick and colleagues6 found that 78% of patients who underwent revision arthroscopic surgery after hip arthroscopic surgery for FAI showed evidence of capsular and iliofemoral defects on magnetic resonance angiography. Frank and colleagues8 reported that, though all patients showed preoperative-to-postoperative improvement on outcome measures, those who underwent complete repair of their T-capsulotomy (vs repair of only its longitudinal portion) had superior outcomes, particularly increased sport-specific activity.
For patients undergoing hip arthroscopy, several predisposing factors can increase the risk of postoperative instability. Patient-related hip instability factors include generalized ligamentous laxity, supraphysiologic athletics (eg, dance), and borderline or true hip dysplasia. Surgeon-related factors include overaggressive acetabular rim resection, excessive labral débridement, and lack of capsular repair.5,9 Although there are multiple techniques for accessing the hip joint and addressing capsular closure at the end of surgery,9-14 we think capsular closure is an important aspect of the case.
Surgical Technique
For a demonstration of this technique, click here to see the video that accompanies this article. The patient is moved to a traction table and placed in the supine position. Induction of general anesthesia with muscle relaxation allows for atraumatic axial traction. The anesthetized patient is assessed for passive motion and ligamentous laxity. Well-padded boots are applied, and a well-padded perineal post is used for positioning. Gentle traction is applied to the contralateral limb, and axial traction is applied through the surgical limb with the hip abducted and minimally flexed. The leg is then adducted and neutrally extended, inducing a transverse vector cantilever moment to the proximal femur. The foot is internally rotated to optimize femoral neck length on an anteroposterior radiograph. The circulating nursing staff notes the onset of hip distraction in order to ensure safe traction duration.
Bony landmarks are marked with a sterile marking pen. Under fluoroscopic guidance, an anterolateral (AL) portal is established 1 cm proximal and 1 cm anterior to the AL tip of the greater trochanter. Standard cannulation allows for intra-articular visualization with a 70° arthroscope. A needle is used to localize placement of a modified anterior portal. After cannulation, the arthroscope is placed in the modified anterior portal to confirm safe entry of the portal without labral violation. An arthroscopic scalpel (Samurai Blade; Stryker Sports Medicine) is used to make a transverse interportal capsulotomy 8 mm to 10 mm from the labrum and extending from 12 to 2 o’clock; length is 2 cm to 4 cm, depending on the extent of the intra-articular injury (Figure 1A).
The acetabular rim is trimmed with a 5.0-mm arthroscopic burr. Distal AL accessory (DALA) portal placement (4-6 cm distal to and in line with the AL portal) allows for suture anchor–based labral refixation. Generally, 2 to 4 anchors (1.4-mm NanoTack Anatomic Labrum Restoration System; Stryker Sports Medicine) are placed as near the articular cartilage as possible without penetration (Figure 1B). On completion of labral refixation, traction is released, and the hip is flexed to 20° to 30°.
T-Capsulotomy
Pericapsular fatty tissue is débrided with an arthroscopic shaver to visualize the interval between the iliocapsularis and gluteus minimus muscles. An arthroscopic scalpel is used, through a 5.0-mm cannula in the DALA portal, to extend the capsulotomy longitudinally and perpendicular to the interportal capsulotomy (Figure 1C). The T-capsulotomy is performed along the length of the femoral neck distally to the capsular reflection at the intertrochanteric line. The arthroscopic burr is used to perform a femoral osteochondroplasty between the lateral synovial folds (12 o’clock) and the medial synovial folds (6 o’clock). Dynamic examination and fluoroscopic imaging confirm that the entire cam deformity has been excised and that there is no evidence of impingement.
Although various suture-shuttling or tissue-penetrating/retrieving devices may be used, we recommend whichever device is appropriate for closing the capsule in its entirety. With the arthroscope in the modified anterior portal, an 8.25-mm × 90-mm cannula is placed in the AL portal, and an 8.25-mm × 110-mm cannula in the DALA portal. These portals will facilitate suture passage.
The vertical limb of the T-capsulotomy is closed with 2 to 4 side-to-side sutures, and the interportal capsulotomy limb with 2 or 3 sutures. Capsular closure begins with the distal portion of the longitudinal limb at the base of the iliofemoral ligament (IFL). A crescent tissue penetrating device (Slingshot; Stryker Sports Medicine) is loaded with high-strength No. 2 suture (Zipline; Stryker Sports Medicine) and placed through the AL portal to sharply pierce the lateral leaflet of the IFL (Figure 1D). The No. 2 suture is shuttled into the intra-articular side of the capsule (Figure 1E). Through the DALA portal, the penetrating device is used to pierce the medial leaflet to retrieve the free suture (Figure 1F). Next, the looped suture retriever is used to pull the suture from the AL portal to the DALA portal so the suture can be tied. We prefer to tie each suture individually after it is passed, but all of the sutures can be passed first, and then tied. As successive suture placement and knot tying inherently tighten the capsule, successive visualization requires more precision. Each subsequent suture is similarly passed, about 1 cm proximal to the previous stitch.
After closure of the vertical limb of the T-capsulotomy, we prefer to close the interportal capsulotomy with the InJector II Capsule Restoration System (Stryker Sports Medicine), a device that allows for closure through a single cannula lateral to medial. This device is passed through the AL cannula in order to bring the suture end through the proximal IFL attached to the acetabulum (Figure 1G). The device is removed from the cannula, and the other suture end is placed in the device and passed through the distal IFL (Figure 1H). The stitch is then tensioned and tied. Likewise, closure of the medial IFL involves passing the InJector through the DALA cannula and bringing the first suture end through the proximal IFL attached to the acetabulum. The Injector is removed from the cannula, and the other suture end is placed in the device and passed through the distal IFL. The stitch is then tensioned and tied with the hip in neutral extension. Generally, 2 or 3 stitches are used to close the interportal capsulotomy. Complete capsular closure is confirmed by the inability to visualize the underlying femoral head/neck and by probing the anterior capsule to ensure proper tension (Figure 1I).
Extensile Interportal Capsulotomy
An alternative to T-capsulotomy is interportal capsulotomy. Just as with T-capsulotomy closure, multiple different suture passing devices can be used. Good visualization for accessing the peripheral compartment generally is achieved by making the interportal capsulotomy 4 cm to 6 cm longer than the horizontal limb of the T-capsulotomy (Figures 2A, 2B). Capsular closure usually begins with the medial portion of the interportal capsulotomy. With the arthroscope in the AL portal, the 8.25-mm × 90-mm cannula is placed in the midanterior portal (MAP), and an 8.25-mm × 110-mm cannula is placed in the DALA portal.
Ligamentous laxity determines degree of capsular closure. The capsular leaflets can be closed end to end if there is little concern for laxity and instability. If there is more concern for capsular laxity, a larger bite of the capsular tissue can be taken to allow for a greater degree of plication. Further, the interportal capsule can be tightened by alternately advancing the location where sutures are passed through the capsule. Specifically, the sutures are passed such that larger bites of the distal capsule are taken, increasing the tightness of the capsule in external rotation.9
Rehabilitation
After surgery, hip extension and external rotation are limited to decrease stress on the capsular closure. The patient is placed into a hip orthosis with 0° to 90° of flexion and a night abduction pillow to limit hip external rotation. Crutch-assisted gait with 20 lb of foot-flat weight-bearing is maintained the first 3 weeks. Continuous passive motion and use of a stationary bicycle are recommended for the first 3 weeks, and then the patient slowly progresses to muscle strengthening, including core and proximal motor control. Closed-chain exercises are begun 6 weeks after surgery. Treadmill running may start at 12 weeks, with the goal of returning to sport at 4 to 6 months.
Discussion
Capsular closure during hip arthroscopy restores the normal anatomy of the IFL and therefore restores the biomechanical characteristics of the hip joint. Scientific studies have found that capsular repair or plication after hip arthroscopy restores normal hip translation, rotation, and strain. Clinical studies have also demonstrated a lower revision rate and more rapid return to athletic activity. Capsular closure, however, is technically challenging and increases operative time, but gross instability and microinstability can be avoided with meticulous closure/plication.
Am J Orthop. 2017;46(1):49-54. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Boykin RE, Anz AW, Bushnell BD, Kocher MS, Stubbs AJ, Philippon MJ. Hip instability. J Am Acad Orthop Surg. 2011;19(6):340-349.
2. Byrd JW, Jones KS. Hip arthroscopy for labral pathology: prospective analysis with 10-year follow-up. Arthroscopy. 2009;25(4):365-368.
3. Benali Y, Katthagen BD. Hip subluxation as a complication of arthroscopic debridement. Arthroscopy. 2009;25(4):405-407.
4. Matsuda DK. Acute iatrogenic dislocation following hip impingement arthroscopic surgery. Arthroscopy. 2009;25(4):400-404.
5. Ranawat AS, McClincy M, Sekiya JK. Anterior dislocation of the hip after arthroscopy in a patient with capsular laxity of the hip. A case report. J Bone Joint Surg Am. 2009;91(1):192-197.
6. McCormick F, Slikker W 3rd, Harris JD, et al. Evidence of capsular defect following hip arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):902-905.
7. Wylie JD, Beckmann JT, Maak TG, Aoki SK. Arthroscopic capsular repair for symptomatic hip instability after previous hip arthroscopic surgery. Am J Sports Med. 2016;44(1):39-45.
8. Frank RM, Lee S, Bush-Joseph CA, Kelly BT, Salata MJ, Nho SJ. Improved outcomes after hip arthroscopic surgery in patients undergoing T-capsulotomy with complete repair versus partial repair for femoroacetabular impingement: a comparative matched-pair analysis. Am J Sports Med. 2014;42(11):2634-2642.
9. Domb BG, Philippon MJ, Giordano BD. Arthroscopic capsulotomy, capsular repair, and capsular plication of the hip: relation to atraumatic instability. Arthroscopy. 2013;29(1):162-173.
10. Asopa V, Singh PJ. The intracapsular atraumatic arthroscopic technique for closure of the hip capsule. Arthrosc Tech. 2014;3(2):e245-e247.
11. Camp CL, Reardon PJ, Levy BA, Krych AJ. A simple technique for capsular repair after hip arthroscopy. Arthrosc Tech. 2015;4(6):e737-e740.
12. Chow RM, Engasser WM, Krych AJ, Levy BA. Arthroscopic capsular repair in the treatment of femoroacetabular impingement. Arthrosc Tech. 2014;3(1):e27-e30.
13. Harris JD, Slikker W 3rd, Gupta AK, McCormick FM, Nho SJ. Routine complete capsular closure during hip arthroscopy. Arthrosc Tech. 2013;2(2):e89-e94.
14. Kuhns BD, Weber AE, Levy DM, et al. Capsular management in hip arthroscopy: an anatomic, biomechanical, and technical review. Front Surg. 2016;3:13.
Take-Home Points
- Hip capsule provides static stabilization for the hip joint.
- Capsular management must weigh visualization to address underlying osseous deformity but also repair/plication of the capsule to maintain biomechanical characteristics.
- T-capsulotomy provides optimal visualization with a small interportal incision with a vertical incision along the femoral neck.
- Extensile interportal capsulotomy is the most widely used capsulotomy and size may vary depending on capsular and patient characteristics.
- Orthopedic surgeons should be equipped to employ either technique depending on the patients individual hip pathomorphology.
Hip arthroscopy has emerged as a common surgical treatment for a number of hip pathologies. Surgical treatment strategies, including management of the hip capsule, have evolved. Whereas earlier hip arthroscopies often involved capsulectomy or capsulotomy without repair, more recently capsular closure has been considered an important step in restoring the anatomy of the hip joint and preventing microinstability or gross macroinstability.
The anatomy of the hip joint includes both static and dynamic stabilizers designed to maintain a functioning articulation. The osseous articulation of the femoral head and acetabulum is the first static stabilizer, with variations in offset, version, and inclination of the acetabulum and the proximal femur. The joint capsule consists of 3 ligaments—iliofemoral, pubofemoral, and ischiofemoral—that converge to form the zona orbicularis. Other soft-tissue structures, such as the articular cartilage, the labrum, the transverse acetabular ligament, the pulvinar, and the ligamentum teres, also provide static constraint.1 The surrounding musculature provides the hip joint with dynamic stability, which contributes to overall maintenance of proper joint kinematics.
Management of the hip capsule has evolved as our understanding of hip pathology and biomechanics has matured. Initial articles on using hip arthroscopy to treat labral tears described improvement in clinical outcomes,2 but the cases involved limited focal capsulotomy. Not until the idea of femoroacetabular impingement (FAI) was introduced were extensive capsulotomies and capsulectomies performed to address the underlying osseous deformities and emulate open techniques. Soon after our ability to access osseous pathomorphology improved with enhanced visualization and comprehensive resection, cases of hip instability after hip arthroscopy surfaced.3-5 Although frank dislocation after hip arthroscopy is rare and largely underreported, it is a catastrophic complication. In addition, focal capsular defects were also described in cases of failed hip arthroscopy and thought to lead to microinstability of the hip.6 Iatrogenic microinstability is thought to be more common, but it is also underrecognized as a cause of failure of hip arthroscopy.7Microinstability is a pathologic condition that can affect hip function. In cases of recurrent pain and unimproved functional status after surgery, microinstability should be considered. In an imaging study of capsule integrity, McCormick and colleagues6 found that 78% of patients who underwent revision arthroscopic surgery after hip arthroscopic surgery for FAI showed evidence of capsular and iliofemoral defects on magnetic resonance angiography. Frank and colleagues8 reported that, though all patients showed preoperative-to-postoperative improvement on outcome measures, those who underwent complete repair of their T-capsulotomy (vs repair of only its longitudinal portion) had superior outcomes, particularly increased sport-specific activity.
For patients undergoing hip arthroscopy, several predisposing factors can increase the risk of postoperative instability. Patient-related hip instability factors include generalized ligamentous laxity, supraphysiologic athletics (eg, dance), and borderline or true hip dysplasia. Surgeon-related factors include overaggressive acetabular rim resection, excessive labral débridement, and lack of capsular repair.5,9 Although there are multiple techniques for accessing the hip joint and addressing capsular closure at the end of surgery,9-14 we think capsular closure is an important aspect of the case.
Surgical Technique
For a demonstration of this technique, click here to see the video that accompanies this article. The patient is moved to a traction table and placed in the supine position. Induction of general anesthesia with muscle relaxation allows for atraumatic axial traction. The anesthetized patient is assessed for passive motion and ligamentous laxity. Well-padded boots are applied, and a well-padded perineal post is used for positioning. Gentle traction is applied to the contralateral limb, and axial traction is applied through the surgical limb with the hip abducted and minimally flexed. The leg is then adducted and neutrally extended, inducing a transverse vector cantilever moment to the proximal femur. The foot is internally rotated to optimize femoral neck length on an anteroposterior radiograph. The circulating nursing staff notes the onset of hip distraction in order to ensure safe traction duration.
Bony landmarks are marked with a sterile marking pen. Under fluoroscopic guidance, an anterolateral (AL) portal is established 1 cm proximal and 1 cm anterior to the AL tip of the greater trochanter. Standard cannulation allows for intra-articular visualization with a 70° arthroscope. A needle is used to localize placement of a modified anterior portal. After cannulation, the arthroscope is placed in the modified anterior portal to confirm safe entry of the portal without labral violation. An arthroscopic scalpel (Samurai Blade; Stryker Sports Medicine) is used to make a transverse interportal capsulotomy 8 mm to 10 mm from the labrum and extending from 12 to 2 o’clock; length is 2 cm to 4 cm, depending on the extent of the intra-articular injury (Figure 1A).
The acetabular rim is trimmed with a 5.0-mm arthroscopic burr. Distal AL accessory (DALA) portal placement (4-6 cm distal to and in line with the AL portal) allows for suture anchor–based labral refixation. Generally, 2 to 4 anchors (1.4-mm NanoTack Anatomic Labrum Restoration System; Stryker Sports Medicine) are placed as near the articular cartilage as possible without penetration (Figure 1B). On completion of labral refixation, traction is released, and the hip is flexed to 20° to 30°.
T-Capsulotomy
Pericapsular fatty tissue is débrided with an arthroscopic shaver to visualize the interval between the iliocapsularis and gluteus minimus muscles. An arthroscopic scalpel is used, through a 5.0-mm cannula in the DALA portal, to extend the capsulotomy longitudinally and perpendicular to the interportal capsulotomy (Figure 1C). The T-capsulotomy is performed along the length of the femoral neck distally to the capsular reflection at the intertrochanteric line. The arthroscopic burr is used to perform a femoral osteochondroplasty between the lateral synovial folds (12 o’clock) and the medial synovial folds (6 o’clock). Dynamic examination and fluoroscopic imaging confirm that the entire cam deformity has been excised and that there is no evidence of impingement.
Although various suture-shuttling or tissue-penetrating/retrieving devices may be used, we recommend whichever device is appropriate for closing the capsule in its entirety. With the arthroscope in the modified anterior portal, an 8.25-mm × 90-mm cannula is placed in the AL portal, and an 8.25-mm × 110-mm cannula in the DALA portal. These portals will facilitate suture passage.
The vertical limb of the T-capsulotomy is closed with 2 to 4 side-to-side sutures, and the interportal capsulotomy limb with 2 or 3 sutures. Capsular closure begins with the distal portion of the longitudinal limb at the base of the iliofemoral ligament (IFL). A crescent tissue penetrating device (Slingshot; Stryker Sports Medicine) is loaded with high-strength No. 2 suture (Zipline; Stryker Sports Medicine) and placed through the AL portal to sharply pierce the lateral leaflet of the IFL (Figure 1D). The No. 2 suture is shuttled into the intra-articular side of the capsule (Figure 1E). Through the DALA portal, the penetrating device is used to pierce the medial leaflet to retrieve the free suture (Figure 1F). Next, the looped suture retriever is used to pull the suture from the AL portal to the DALA portal so the suture can be tied. We prefer to tie each suture individually after it is passed, but all of the sutures can be passed first, and then tied. As successive suture placement and knot tying inherently tighten the capsule, successive visualization requires more precision. Each subsequent suture is similarly passed, about 1 cm proximal to the previous stitch.
After closure of the vertical limb of the T-capsulotomy, we prefer to close the interportal capsulotomy with the InJector II Capsule Restoration System (Stryker Sports Medicine), a device that allows for closure through a single cannula lateral to medial. This device is passed through the AL cannula in order to bring the suture end through the proximal IFL attached to the acetabulum (Figure 1G). The device is removed from the cannula, and the other suture end is placed in the device and passed through the distal IFL (Figure 1H). The stitch is then tensioned and tied. Likewise, closure of the medial IFL involves passing the InJector through the DALA cannula and bringing the first suture end through the proximal IFL attached to the acetabulum. The Injector is removed from the cannula, and the other suture end is placed in the device and passed through the distal IFL. The stitch is then tensioned and tied with the hip in neutral extension. Generally, 2 or 3 stitches are used to close the interportal capsulotomy. Complete capsular closure is confirmed by the inability to visualize the underlying femoral head/neck and by probing the anterior capsule to ensure proper tension (Figure 1I).
Extensile Interportal Capsulotomy
An alternative to T-capsulotomy is interportal capsulotomy. Just as with T-capsulotomy closure, multiple different suture passing devices can be used. Good visualization for accessing the peripheral compartment generally is achieved by making the interportal capsulotomy 4 cm to 6 cm longer than the horizontal limb of the T-capsulotomy (Figures 2A, 2B). Capsular closure usually begins with the medial portion of the interportal capsulotomy. With the arthroscope in the AL portal, the 8.25-mm × 90-mm cannula is placed in the midanterior portal (MAP), and an 8.25-mm × 110-mm cannula is placed in the DALA portal.
Ligamentous laxity determines degree of capsular closure. The capsular leaflets can be closed end to end if there is little concern for laxity and instability. If there is more concern for capsular laxity, a larger bite of the capsular tissue can be taken to allow for a greater degree of plication. Further, the interportal capsule can be tightened by alternately advancing the location where sutures are passed through the capsule. Specifically, the sutures are passed such that larger bites of the distal capsule are taken, increasing the tightness of the capsule in external rotation.9
Rehabilitation
After surgery, hip extension and external rotation are limited to decrease stress on the capsular closure. The patient is placed into a hip orthosis with 0° to 90° of flexion and a night abduction pillow to limit hip external rotation. Crutch-assisted gait with 20 lb of foot-flat weight-bearing is maintained the first 3 weeks. Continuous passive motion and use of a stationary bicycle are recommended for the first 3 weeks, and then the patient slowly progresses to muscle strengthening, including core and proximal motor control. Closed-chain exercises are begun 6 weeks after surgery. Treadmill running may start at 12 weeks, with the goal of returning to sport at 4 to 6 months.
Discussion
Capsular closure during hip arthroscopy restores the normal anatomy of the IFL and therefore restores the biomechanical characteristics of the hip joint. Scientific studies have found that capsular repair or plication after hip arthroscopy restores normal hip translation, rotation, and strain. Clinical studies have also demonstrated a lower revision rate and more rapid return to athletic activity. Capsular closure, however, is technically challenging and increases operative time, but gross instability and microinstability can be avoided with meticulous closure/plication.
Am J Orthop. 2017;46(1):49-54. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Hip capsule provides static stabilization for the hip joint.
- Capsular management must weigh visualization to address underlying osseous deformity but also repair/plication of the capsule to maintain biomechanical characteristics.
- T-capsulotomy provides optimal visualization with a small interportal incision with a vertical incision along the femoral neck.
- Extensile interportal capsulotomy is the most widely used capsulotomy and size may vary depending on capsular and patient characteristics.
- Orthopedic surgeons should be equipped to employ either technique depending on the patients individual hip pathomorphology.
Hip arthroscopy has emerged as a common surgical treatment for a number of hip pathologies. Surgical treatment strategies, including management of the hip capsule, have evolved. Whereas earlier hip arthroscopies often involved capsulectomy or capsulotomy without repair, more recently capsular closure has been considered an important step in restoring the anatomy of the hip joint and preventing microinstability or gross macroinstability.
The anatomy of the hip joint includes both static and dynamic stabilizers designed to maintain a functioning articulation. The osseous articulation of the femoral head and acetabulum is the first static stabilizer, with variations in offset, version, and inclination of the acetabulum and the proximal femur. The joint capsule consists of 3 ligaments—iliofemoral, pubofemoral, and ischiofemoral—that converge to form the zona orbicularis. Other soft-tissue structures, such as the articular cartilage, the labrum, the transverse acetabular ligament, the pulvinar, and the ligamentum teres, also provide static constraint.1 The surrounding musculature provides the hip joint with dynamic stability, which contributes to overall maintenance of proper joint kinematics.
Management of the hip capsule has evolved as our understanding of hip pathology and biomechanics has matured. Initial articles on using hip arthroscopy to treat labral tears described improvement in clinical outcomes,2 but the cases involved limited focal capsulotomy. Not until the idea of femoroacetabular impingement (FAI) was introduced were extensive capsulotomies and capsulectomies performed to address the underlying osseous deformities and emulate open techniques. Soon after our ability to access osseous pathomorphology improved with enhanced visualization and comprehensive resection, cases of hip instability after hip arthroscopy surfaced.3-5 Although frank dislocation after hip arthroscopy is rare and largely underreported, it is a catastrophic complication. In addition, focal capsular defects were also described in cases of failed hip arthroscopy and thought to lead to microinstability of the hip.6 Iatrogenic microinstability is thought to be more common, but it is also underrecognized as a cause of failure of hip arthroscopy.7Microinstability is a pathologic condition that can affect hip function. In cases of recurrent pain and unimproved functional status after surgery, microinstability should be considered. In an imaging study of capsule integrity, McCormick and colleagues6 found that 78% of patients who underwent revision arthroscopic surgery after hip arthroscopic surgery for FAI showed evidence of capsular and iliofemoral defects on magnetic resonance angiography. Frank and colleagues8 reported that, though all patients showed preoperative-to-postoperative improvement on outcome measures, those who underwent complete repair of their T-capsulotomy (vs repair of only its longitudinal portion) had superior outcomes, particularly increased sport-specific activity.
For patients undergoing hip arthroscopy, several predisposing factors can increase the risk of postoperative instability. Patient-related hip instability factors include generalized ligamentous laxity, supraphysiologic athletics (eg, dance), and borderline or true hip dysplasia. Surgeon-related factors include overaggressive acetabular rim resection, excessive labral débridement, and lack of capsular repair.5,9 Although there are multiple techniques for accessing the hip joint and addressing capsular closure at the end of surgery,9-14 we think capsular closure is an important aspect of the case.
Surgical Technique
For a demonstration of this technique, click here to see the video that accompanies this article. The patient is moved to a traction table and placed in the supine position. Induction of general anesthesia with muscle relaxation allows for atraumatic axial traction. The anesthetized patient is assessed for passive motion and ligamentous laxity. Well-padded boots are applied, and a well-padded perineal post is used for positioning. Gentle traction is applied to the contralateral limb, and axial traction is applied through the surgical limb with the hip abducted and minimally flexed. The leg is then adducted and neutrally extended, inducing a transverse vector cantilever moment to the proximal femur. The foot is internally rotated to optimize femoral neck length on an anteroposterior radiograph. The circulating nursing staff notes the onset of hip distraction in order to ensure safe traction duration.
Bony landmarks are marked with a sterile marking pen. Under fluoroscopic guidance, an anterolateral (AL) portal is established 1 cm proximal and 1 cm anterior to the AL tip of the greater trochanter. Standard cannulation allows for intra-articular visualization with a 70° arthroscope. A needle is used to localize placement of a modified anterior portal. After cannulation, the arthroscope is placed in the modified anterior portal to confirm safe entry of the portal without labral violation. An arthroscopic scalpel (Samurai Blade; Stryker Sports Medicine) is used to make a transverse interportal capsulotomy 8 mm to 10 mm from the labrum and extending from 12 to 2 o’clock; length is 2 cm to 4 cm, depending on the extent of the intra-articular injury (Figure 1A).
The acetabular rim is trimmed with a 5.0-mm arthroscopic burr. Distal AL accessory (DALA) portal placement (4-6 cm distal to and in line with the AL portal) allows for suture anchor–based labral refixation. Generally, 2 to 4 anchors (1.4-mm NanoTack Anatomic Labrum Restoration System; Stryker Sports Medicine) are placed as near the articular cartilage as possible without penetration (Figure 1B). On completion of labral refixation, traction is released, and the hip is flexed to 20° to 30°.
T-Capsulotomy
Pericapsular fatty tissue is débrided with an arthroscopic shaver to visualize the interval between the iliocapsularis and gluteus minimus muscles. An arthroscopic scalpel is used, through a 5.0-mm cannula in the DALA portal, to extend the capsulotomy longitudinally and perpendicular to the interportal capsulotomy (Figure 1C). The T-capsulotomy is performed along the length of the femoral neck distally to the capsular reflection at the intertrochanteric line. The arthroscopic burr is used to perform a femoral osteochondroplasty between the lateral synovial folds (12 o’clock) and the medial synovial folds (6 o’clock). Dynamic examination and fluoroscopic imaging confirm that the entire cam deformity has been excised and that there is no evidence of impingement.
Although various suture-shuttling or tissue-penetrating/retrieving devices may be used, we recommend whichever device is appropriate for closing the capsule in its entirety. With the arthroscope in the modified anterior portal, an 8.25-mm × 90-mm cannula is placed in the AL portal, and an 8.25-mm × 110-mm cannula in the DALA portal. These portals will facilitate suture passage.
The vertical limb of the T-capsulotomy is closed with 2 to 4 side-to-side sutures, and the interportal capsulotomy limb with 2 or 3 sutures. Capsular closure begins with the distal portion of the longitudinal limb at the base of the iliofemoral ligament (IFL). A crescent tissue penetrating device (Slingshot; Stryker Sports Medicine) is loaded with high-strength No. 2 suture (Zipline; Stryker Sports Medicine) and placed through the AL portal to sharply pierce the lateral leaflet of the IFL (Figure 1D). The No. 2 suture is shuttled into the intra-articular side of the capsule (Figure 1E). Through the DALA portal, the penetrating device is used to pierce the medial leaflet to retrieve the free suture (Figure 1F). Next, the looped suture retriever is used to pull the suture from the AL portal to the DALA portal so the suture can be tied. We prefer to tie each suture individually after it is passed, but all of the sutures can be passed first, and then tied. As successive suture placement and knot tying inherently tighten the capsule, successive visualization requires more precision. Each subsequent suture is similarly passed, about 1 cm proximal to the previous stitch.
After closure of the vertical limb of the T-capsulotomy, we prefer to close the interportal capsulotomy with the InJector II Capsule Restoration System (Stryker Sports Medicine), a device that allows for closure through a single cannula lateral to medial. This device is passed through the AL cannula in order to bring the suture end through the proximal IFL attached to the acetabulum (Figure 1G). The device is removed from the cannula, and the other suture end is placed in the device and passed through the distal IFL (Figure 1H). The stitch is then tensioned and tied. Likewise, closure of the medial IFL involves passing the InJector through the DALA cannula and bringing the first suture end through the proximal IFL attached to the acetabulum. The Injector is removed from the cannula, and the other suture end is placed in the device and passed through the distal IFL. The stitch is then tensioned and tied with the hip in neutral extension. Generally, 2 or 3 stitches are used to close the interportal capsulotomy. Complete capsular closure is confirmed by the inability to visualize the underlying femoral head/neck and by probing the anterior capsule to ensure proper tension (Figure 1I).
Extensile Interportal Capsulotomy
An alternative to T-capsulotomy is interportal capsulotomy. Just as with T-capsulotomy closure, multiple different suture passing devices can be used. Good visualization for accessing the peripheral compartment generally is achieved by making the interportal capsulotomy 4 cm to 6 cm longer than the horizontal limb of the T-capsulotomy (Figures 2A, 2B). Capsular closure usually begins with the medial portion of the interportal capsulotomy. With the arthroscope in the AL portal, the 8.25-mm × 90-mm cannula is placed in the midanterior portal (MAP), and an 8.25-mm × 110-mm cannula is placed in the DALA portal.
Ligamentous laxity determines degree of capsular closure. The capsular leaflets can be closed end to end if there is little concern for laxity and instability. If there is more concern for capsular laxity, a larger bite of the capsular tissue can be taken to allow for a greater degree of plication. Further, the interportal capsule can be tightened by alternately advancing the location where sutures are passed through the capsule. Specifically, the sutures are passed such that larger bites of the distal capsule are taken, increasing the tightness of the capsule in external rotation.9
Rehabilitation
After surgery, hip extension and external rotation are limited to decrease stress on the capsular closure. The patient is placed into a hip orthosis with 0° to 90° of flexion and a night abduction pillow to limit hip external rotation. Crutch-assisted gait with 20 lb of foot-flat weight-bearing is maintained the first 3 weeks. Continuous passive motion and use of a stationary bicycle are recommended for the first 3 weeks, and then the patient slowly progresses to muscle strengthening, including core and proximal motor control. Closed-chain exercises are begun 6 weeks after surgery. Treadmill running may start at 12 weeks, with the goal of returning to sport at 4 to 6 months.
Discussion
Capsular closure during hip arthroscopy restores the normal anatomy of the IFL and therefore restores the biomechanical characteristics of the hip joint. Scientific studies have found that capsular repair or plication after hip arthroscopy restores normal hip translation, rotation, and strain. Clinical studies have also demonstrated a lower revision rate and more rapid return to athletic activity. Capsular closure, however, is technically challenging and increases operative time, but gross instability and microinstability can be avoided with meticulous closure/plication.
Am J Orthop. 2017;46(1):49-54. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Boykin RE, Anz AW, Bushnell BD, Kocher MS, Stubbs AJ, Philippon MJ. Hip instability. J Am Acad Orthop Surg. 2011;19(6):340-349.
2. Byrd JW, Jones KS. Hip arthroscopy for labral pathology: prospective analysis with 10-year follow-up. Arthroscopy. 2009;25(4):365-368.
3. Benali Y, Katthagen BD. Hip subluxation as a complication of arthroscopic debridement. Arthroscopy. 2009;25(4):405-407.
4. Matsuda DK. Acute iatrogenic dislocation following hip impingement arthroscopic surgery. Arthroscopy. 2009;25(4):400-404.
5. Ranawat AS, McClincy M, Sekiya JK. Anterior dislocation of the hip after arthroscopy in a patient with capsular laxity of the hip. A case report. J Bone Joint Surg Am. 2009;91(1):192-197.
6. McCormick F, Slikker W 3rd, Harris JD, et al. Evidence of capsular defect following hip arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):902-905.
7. Wylie JD, Beckmann JT, Maak TG, Aoki SK. Arthroscopic capsular repair for symptomatic hip instability after previous hip arthroscopic surgery. Am J Sports Med. 2016;44(1):39-45.
8. Frank RM, Lee S, Bush-Joseph CA, Kelly BT, Salata MJ, Nho SJ. Improved outcomes after hip arthroscopic surgery in patients undergoing T-capsulotomy with complete repair versus partial repair for femoroacetabular impingement: a comparative matched-pair analysis. Am J Sports Med. 2014;42(11):2634-2642.
9. Domb BG, Philippon MJ, Giordano BD. Arthroscopic capsulotomy, capsular repair, and capsular plication of the hip: relation to atraumatic instability. Arthroscopy. 2013;29(1):162-173.
10. Asopa V, Singh PJ. The intracapsular atraumatic arthroscopic technique for closure of the hip capsule. Arthrosc Tech. 2014;3(2):e245-e247.
11. Camp CL, Reardon PJ, Levy BA, Krych AJ. A simple technique for capsular repair after hip arthroscopy. Arthrosc Tech. 2015;4(6):e737-e740.
12. Chow RM, Engasser WM, Krych AJ, Levy BA. Arthroscopic capsular repair in the treatment of femoroacetabular impingement. Arthrosc Tech. 2014;3(1):e27-e30.
13. Harris JD, Slikker W 3rd, Gupta AK, McCormick FM, Nho SJ. Routine complete capsular closure during hip arthroscopy. Arthrosc Tech. 2013;2(2):e89-e94.
14. Kuhns BD, Weber AE, Levy DM, et al. Capsular management in hip arthroscopy: an anatomic, biomechanical, and technical review. Front Surg. 2016;3:13.
1. Boykin RE, Anz AW, Bushnell BD, Kocher MS, Stubbs AJ, Philippon MJ. Hip instability. J Am Acad Orthop Surg. 2011;19(6):340-349.
2. Byrd JW, Jones KS. Hip arthroscopy for labral pathology: prospective analysis with 10-year follow-up. Arthroscopy. 2009;25(4):365-368.
3. Benali Y, Katthagen BD. Hip subluxation as a complication of arthroscopic debridement. Arthroscopy. 2009;25(4):405-407.
4. Matsuda DK. Acute iatrogenic dislocation following hip impingement arthroscopic surgery. Arthroscopy. 2009;25(4):400-404.
5. Ranawat AS, McClincy M, Sekiya JK. Anterior dislocation of the hip after arthroscopy in a patient with capsular laxity of the hip. A case report. J Bone Joint Surg Am. 2009;91(1):192-197.
6. McCormick F, Slikker W 3rd, Harris JD, et al. Evidence of capsular defect following hip arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):902-905.
7. Wylie JD, Beckmann JT, Maak TG, Aoki SK. Arthroscopic capsular repair for symptomatic hip instability after previous hip arthroscopic surgery. Am J Sports Med. 2016;44(1):39-45.
8. Frank RM, Lee S, Bush-Joseph CA, Kelly BT, Salata MJ, Nho SJ. Improved outcomes after hip arthroscopic surgery in patients undergoing T-capsulotomy with complete repair versus partial repair for femoroacetabular impingement: a comparative matched-pair analysis. Am J Sports Med. 2014;42(11):2634-2642.
9. Domb BG, Philippon MJ, Giordano BD. Arthroscopic capsulotomy, capsular repair, and capsular plication of the hip: relation to atraumatic instability. Arthroscopy. 2013;29(1):162-173.
10. Asopa V, Singh PJ. The intracapsular atraumatic arthroscopic technique for closure of the hip capsule. Arthrosc Tech. 2014;3(2):e245-e247.
11. Camp CL, Reardon PJ, Levy BA, Krych AJ. A simple technique for capsular repair after hip arthroscopy. Arthrosc Tech. 2015;4(6):e737-e740.
12. Chow RM, Engasser WM, Krych AJ, Levy BA. Arthroscopic capsular repair in the treatment of femoroacetabular impingement. Arthrosc Tech. 2014;3(1):e27-e30.
13. Harris JD, Slikker W 3rd, Gupta AK, McCormick FM, Nho SJ. Routine complete capsular closure during hip arthroscopy. Arthrosc Tech. 2013;2(2):e89-e94.
14. Kuhns BD, Weber AE, Levy DM, et al. Capsular management in hip arthroscopy: an anatomic, biomechanical, and technical review. Front Surg. 2016;3:13.
T-Capsulotomy to Improve Visualization of the Peripheral Compartment and Repair
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Hip pain in active patients: What you may be missing
• Consider both musculoskeletal and nonmusculoskeletal causes in patients with vague complaints of hip and groin pain. B
• Use imaging studies to confirm a hip pain diagnosis. B
• Refer patients who fail to respond to nonsurgical treatment to a sports medicine specialist or an orthopedic surgeon. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Hip pain is a common complaint, and commonly misunderstood. Although the pain can be associated with a broad spectrum of conditions, the presentation is often vague and nonspecific.
Thus, hip pain and injury are frequently attributed, often incorrectly, to a “hip pointer”—a contusion of soft tissues against the iliac crest. It’s not unusual for patients who receive this diagnosis to be treated conservatively for prolonged periods, leading some previously active individuals to abandon their favorite sport or self-impose limits on the activities they engage in.1
But it doesn’t have to be this way.
Minimally invasive hip arthroscopy and advances in imaging, instrumentation, and devices have made it easier to identify and address underlying pathology associated with hip pain, helping patients return to their previous level of activity more rapidly.2,3 And, while many conditions associated with hip pain can be treated conservatively, family physicians—whom patients often go to first—should not hesitate to provide a referral when more aggressive treatment or diagnostic confirmation is needed.
We created this guide with family physicians in mind. Our focus here is primarily on anterior hip pain—the most common presentation—in active, or athletic, patients.
When did the pain begin? Where does it hurt?
Before performing a physical examination, find out as much as possible about the onset of pain and when and under what circumstances it occurs. (A review of hip anatomy is provided here.) Did it begin suddenly, after an acute injury or a particular physical maneuver? Or is the pain insidious, as was the case with one of our patients?
Osseous morphology of the hip includes the anterior superior iliac spine, the origin of the sartorius muscle and the ilioinguinal ligament. The anterior inferior iliac spine attaches to the rectus femoris, a major hip flexor and knee extender. The adductors of the hip originate in the anterior pelvic region.
The inguinal canal contains the ilioinguinal nerve, which is responsible for radiation of pain to the anterior hip. The hip joint itself is a spheroid comprising the femoral head and acetabulum, with most of the articular hip innervated by the femoral or obturator nerves.
Most intra-articular conditions radiate to the anterior groin or hip, although there are cases in which the pain is referred to either the lateral aspect of the hip or the buttocks. The iliopsoas muscle is the major hip flexor, and crosses under the ilioinguinal ligament to insert on the lesser tuberosity after crossing over the anterior capsule of the hip. A large bursa surrounds it, helping the tendon glide smoothly over the hip.
CASE Mack Q, a 27-year-old man with an 8-month history of right hip pain, sought care at our medical center for an achy pain in his right groin; he also described an occasional “clicking and popping sensation” in his groin but denied any trauma. The pain worsened with prolonged sitting and certain activities, such as squatting, twisting, and putting on shoes and socks. Our patient had stopped playing soccer because it hurt too much. He had tried physical therapy, oral anti-inflammatories, and a corticosteroid injection, with little relief.
Start with a gait assessment
The physical examination should begin with a gait assessment. Consider the patient’s ability to bear weight and his or her foot angle.
An individual with a stress fracture will have difficulty bearing weight on the affected side, resulting in a limp, or antalgic gait. A patient with femoral acetabular impingement (FAI) will often exhibit greater external rotation of the foot on the affected side compared with the other foot. And a patient with weakened abductor muscles, typically because of severe osteoarthritis, will exhibit the Trendelenburg sign—a pelvic tilt when the weight is shifted to the affected extremity.
Although most individuals with hip pain will not have an obvious gait abnormality, any patient who walks with a limp or needs crutches requires an immediate referral to an orthopedic surgeon.
Include these elements in the physical exam
Examine the hip with the patient sitting on the side of the exam table. Assess range of motion (ROM), comparing the range of flexion, extension, and internal/external rotation on the affected and unaffected sides. Include the following maneuvers:
Impingement testing. In patients with FAI and osteoarthritis, impingement testing—encompassing Flexion, ADDuction, and Internal Rotation (FADDIR)—will elicit pain. The maneuver can be tested starting at 45° of hip flexion, increasing to approximately 120°. Pain with <45° of hip flexion indicates that the impingement is severe.
Such testing can also reveal labral tears, which may be caused by FAI or other structural abnormalities. In a patient with anterior labral tears, FADDIR will produce groin pain; posterior labral tears will produce pain when the patient is sitting with legs hanging off the exam table and the contralateral leg is brought to the chest and the affected limb fully extended.
In patients with hip pain and bursitis, applying downward pressure will elicit a snapping sound as the iliopsoas snaps over the iliopectineal eminence or femoral head. Flexion, ABduction, and External Rotation (FABER) can also be used to diagnose iliopsoas tendonitis: The test is positive if it elicits pain in the affected extremity or in the sacroiliac joint on the opposite side.
Log roll. A painful response to this test, which involves internally and externally rotating the affected hip while it is relaxed and the knee fully extended, is an indication of synovitis of the hip caused by intra-articular pathology. To test hip stability, externally rotate the leg while it is extended. If the hip is stable, the leg will return to a neutral position; microinstability of the hip is likely if the leg remains in the rotated position.
Muscular strength testing. To assess for tendinopathy in the hip area, the patient should be in a seated position and contract the internal and external rotators and the adductor muscles while you apply resistance. To test abductor strength, have the patient assume a lateral position and hold and abduct the leg on the affected side while you apply resistance.
Hip flexion strength should be tested with the patient in both supine and seated positions. A patient with quadriceps tendonitis will have much greater pain with resisted hip flexion in the supine position vs the seated position; the opposite is true for a patient with iliopsoas tendonitis. (See “Did you know…? Hip pain facts and findings” on for additional diagnostic tips.)
- A patient with quadriceps tendonitis will have much greater pain with resisted hip flexion in a supine position vs a seated position. The opposite is true for a patient with iliopsoas tendonitis.
- Patients with femoral neck stress fractures typically present with activity-related anterior groin pain that is relieved by rest. Initially, they may be only mildly affected, but the condition worsens in those who continue to “work through the pain.”
- Plain radiography can confirm a diagnosis of osteonecrosis in patients with advanced disease, but magnetic resonance imaging is useful for evaluating earlier clinical presentations.
- Patients with labral tears often exhibit what has been called the “c-sign”—so named for the shape patients make with their hand as they grip their hip just above the greater trochanter to indicate where it hurts.
- Athletes who experience adductor strains often play sports in which kicking or frequent changes in direction are required, such as football, hockey, and soccer, and are generally able to tell you exactly what they were doing when the injury occurred.
- Unlike other hernias, a sports hernia (athletic pubalgia) does not involve a bulge of tissue protruding through one part of the body into another. Instead, it occurs when the oblique abdominal muscles strain or completely tear away from the pubis.
Perform a neurologic evaluation to rule out a back condition that might radiate pain into the anterior hip; ask the patient to do a sit-up while you apply resistance to test for abdominal wall pathology, as well.
Hip palpation. This aspect of the physical exam is important regardless of the cause of the pain but especially crucial for pediatric and adolescent patients, whose anterior hip pain may be related to apophyseal injury. Palpate the superior iliac spine (and over the inferior iliac spine in thin patients) to determine if the sartorius or rectus femoris has been injured. The area just lateral to the symphysis will be tender to palpation in patients with osteitis pubis.
Refer or treat? Here’s what to consider
While the history and physical should provide ample information for a differential diagnosis, imaging studies are generally required for confirmation. Clinical assessment— including physical exam, imaging, and intra-articular injection—of patients with hip pain is up to 98% accurate in identifying hip abnormalities, with arthroscopy as the gold standard.4
CASE On physical examination, Mr. Q had right hip extension to 0°, flexion to 110°, external rotation to 50°, and internal rotation to neutral; he also had positive impingement and subspine impingement tests, a painful arc of motion from 12 to 4 o’clock, tenderness over the hip adductor, and pain with resisted hip adduction. He did not walk with a limp.
Diagnostic studies included plain radiographs, which demonstrated that the joint space was well preserved. We identified subtle anatomical abnormalities on the femoral head-neck junction, known as a cam deformity. Magnetic resonance imaging (MRI) revealed an anterior superior labral tear with cartilage delamination.
Stress fractures affect runners, military recruits
In addition to long-distance runners who have recently increased the frequency, duration, or intensity of training,5,6 military recruits have a higher incidence of stress fractures due to the rapid onset of intensive training. Stress fractures can also occur in patients who do not have a history of intense activity but have metabolically weakened bone, in some cases as a result of an eating disorder.7
Patients with femoral neck stress fractures typically present with activity-related anterior groin pain that is relieved by rest; initially, they may be only mildly affected, but the condition worsens in those who continue to “work through the pain.” By the time such individuals seek treatment, they almost always have pain with weight bearing and an antalgic gait.
Symptoms consistent with a femoral neck stress fracture can be further evaluated with plain radiographs. However, x-rays are often negative for up to 4 weeks after the onset of pain.8 In cases in which radiographs are negative but the physical exam is suggestive of a stress fracture, MRI—which can detect an abnormality within a day or 2 of injury8,9—should be used to confirm the diagnosis (FIGURE 1).
FIGURE 1
MRI reveals a femoral neck stress fracture
Treatment. A complete femoral neck fracture portends impending displacement and requires emergent evaluation by an orthopedist, and superior neck changes, also known as tension-sided stress fractures, require urgent treatment with percutaneous screw fixation.9 However, compression-sided, or inferior, stress fractures can be treated with restricted weight bearing and activity modification. Gradual resumption of activity is allowed only after the patient has been asymptomatic for 6 weeks; recurrent pain indicates residual stress reaction, and signals that activities should be abated.
Osteonecrosis has many causes
Necrosis of the femoral head is a debilitating and progressive condition primarily affecting patients between the ages of 20 and 50 years.10 It has multiple (and diverse) causes, including trauma, steroids, alcohol, smoking, lupus, sickle cell anemia, and coagulopathies, as well as scuba diving. But about 20% of cases have no apparent cause.11,12
Patients with osteonecrosis of the hip typically present with groin pain, often described as a deep, intermittent ache that interferes with activities of daily living. Exam findings depend on the stage of presentation. Early on, pain will occur only with extreme ROM; in advanced cases, ROM is restricted and pain occurs even with limited motion.
Femoral head collapse due to loss of the structural integrity of the subchondral bone—which occurs in 80% of cases12—is thought to be caused by decreased blood flow. Plain radiography can confirm a diagnosis of osteonecrosis in patients with advanced disease, but MRI is useful for evaluating patients with earlier clinical presentations.
Treatment of osteonecrosis is dictated by the stage of the disease, but remains controversial because no intervention has been shown to prevent progression in all cases.12 All patients should be referred to a specialist. Those without collapse or cartilage damage can be treated surgically with core decompression, possibly with additional vascularized bone grafting,13,14 while those with more advanced disease typically require a total hip replacement at a relatively young age. Results for total hip replacement in patients with osteonecrosis are thought to be inferior to hip replacement in patients with osteoarthritis, although comparison is difficult because of the differences in age and activity levels in these 2 groups.15,16
Femoral acetabular impingement can occur on the cam or pincer side
FAI pathology can exist on either the femoral (cam) or acetabular (pincer) side,17 or both.18 In pure cam impingement, the anterior femoral neck loses its normal concave anatomy and develops a “bump,” which impinges on the anterosuperior labrum during hip flexion, causing labral tears and delamination of the adjacent cartilage.
Pure pincer impingement arises from a prominent acetabular rim, causing overcoverage of the femoral head. Acetabular labral tears result from the repetitive impaction with flexion and internal rotation.
Patients report an insidious onset of groin pain that is exacerbated by flexion-type sports, such as hockey, football, and golf,19 as well as activities of daily living. In patients with cartilage damage, even walking can be painful. Physical examination of patients with FAI reveals findings that are similar to those of patients with acetabular labral tears. Abnormally large cam lesions or acetabular overcoverage will result in restriction of hip ROM, especially internal rotation and flexion due to a mechanical block.
Radiographs (FIGURE 2) are essential to diagnose FAI and to distinguish this condition from an isolated labral tear.20 Cam impingement will be best demonstrated on a cross-table lateral radiograph, which shows an asphericity of the femoral head/neck junction anteriorly, while pincer impingement will show overcoverage of the femoral head on an AP radiograph. MRI or magnetic resonance arthrography (MRA) is frequently obtained to see whether any cartilage deterioration has occurred. Computed tomography, which can provide a 3-dimensional reproduction of the hip morphology, is often used for preoperative planning when surgical intervention is required.
FIGURE 2
Femoral acetabular impingement with a prominent pincer lesion
Treatment. Surgical intervention is often needed to correct or remove the abnormal anatomy, and both arthroscopic and open surgery are recommended.20 Both methods include osteoplasty at the femoral head/neck junction and/or the acetabular rim to allow the proximal femur to articulate with the acetabulum without injury to the labrum with flexion and internal rotation.21
Results of both open and arthroscopic osteoplasty of the femur and acetabulum are still preliminary, with only a few studies reporting mid-term results. Open surgery typically has longer recovery and rehabilitation, but advocates emphasize the improved ability to contour the femur or acetabulum. Both open and arthroscopic procedures have about an 8% to 13% rate of revision in short-term follow-up.17
Labral tears occur with trauma and certain sports
In addition to FAI, causes of labral tears include dysplasia, instability, trauma, and degeneration, as well as sports that require repetitive hip flexion and/or pivoting, such as hockey, soccer, and football.22,23
Patients with labral tears typically present with anterior hip pain radiating to the groin, worsening with twisting motions, running, walking, and sitting for prolonged periods. Clicking or catching may occur, as well. Patients may exhibit what one researcher called the “c-sign”—so named for the shape patients make with their hand as they grip their hip just above the greater trochanter to indicate where it hurts.4 The work-up for labral tears includes radiographs and, often, MRA, which is nearly 100% specific.24
Treatment. Conservative treatment, which may include activity modification or rest and ice, nonsteroidal anti-inflammatory drugs (NSAIDs), and physical therapy, is often effective for labral tears; when such measures fail, surgical intervention is indicated. A systematic review found a 67% satisfaction rate after 3.5 years in patients who had undergone labral debridement, and complete resolution of mechanical symptoms in nearly 50%.25 Another study showed similar results for hip arthroscopy, with symptom relief continuing for 4.8 years after surgery, on average, and 84% of patients able to return to their previous level of activity.26
The long-term results of labral debridement are unknown, however, and the possibility of an association between this procedure and the development of arthritis remains. Most specialists prefer anatomic repair to restore normal hip kinematics and, potentially, long-term hip function,27,28 but structural abnormalities must also be addressed to prevent failure of the repair or recurrent tears.
Iliopsoas tendonitis: You know the snap
Often referred to as internal snapping of the hip or internal coxa saltans, iliopsoas tendonitis/bursitis can be a recalcitrant cause of anterior hip pain. Snapping of the iliopsoas leading to bursitis or tendonitis can occur at the iliopectineal eminence, the femoral head, or the lesser trochanter.29 Runners and ballet dancers are often affected.30,31
Snapping in itself is not an indication of pathology, but chronicity of symptoms is. Patients with relatively acute symptoms typically have only bursitis, while a longer duration of symptoms leads to tendonitis or tendinopathy.32
Treatment. First-line therapy is nonoperative, and includes activity modification, rest, ice, NSAIDs, and physical therapy. Advise patients to refrain from activities causing pain, and to apply ice to the affected every 20 minutes (with a 20- to 30-minute off period) for one to 2 hours. Physical therapy focuses on stretching the iliopsoas and rectus femoris muscles and strengthening the hamstring muscles to relieve the stress on the anterior pelvis. If such treatment is unsuccessful, ultrasound can be used to guide a therapeutic injection of cortisone.33 If this fails to bring relief, fractional lengthening of the iliopsoas tendon can be performed to eliminate snapping and relieve pain.34
Muscular strains/avulsion fractures: Sports and age play a role
Although strains can affect any of the anterior muscles around the hip, in active individuals the adductors are most commonly affected. Skeletally immature patients are an exception: apophyseal fractures at the origin of the sartorius and rectus femoris muscles are more common than muscular strains in this patient population.
Athletes who experience adductor strains often play sports in which kicking or frequent changes in direction are required—eg, football, hockey, and soccer35—and generally are able to tell you exactly what they were doing when the injury occurred. Physical examination can reveal focal findings, with swelling and tenderness confined to the anteromedial aspect of the hip along the adductor muscle group. MRI can help differentiate the site of true pathology.36
Treatment of adductor strains is nonoperative, with rest, ice, and activity modification until the tendon heals. In the rare case in which complete tendon avulsion is found, surgical reattachment is needed.
Apophyseal fracture in skeletally immature patients typically occurs during participation in a sport that requires rapid acceleration and deceleration with the hip in an extended position. In such patients, stretching the affected muscle should reproduce the pain. Radiographs are diagnostic and will often show minimal displacement of the apophysis. Treatment is almost always nonoperative. Surgical intervention is rarely needed, and only indicated with displacement >2 cm.37
Athletic pubalgia: A challenging Dx
Also referred to as sports hernia, athletic pubalgia is an enigmatic cause of anterior hip pain in athletes. Diagnosis can be especially challenging, and patients may have lingering symptoms for years before the cause is discovered.38 A sports hernia, unlike other hernias, does not involve a bulge of tissue protruding through one body part into another. In contrast, a sports hernia occurs when the oblique abdominal muscles strain or completely tear away from the pubis. A recent systematic review found that the underlying etiology involves posterior inguinal wall weakening, which can be a result of poorly balanced hip adductor and abdominal muscle activation.39
Patients with sports hernia will often present with anterior hip and/or groin pain, especially with hip extension, twisting, and turning. In addition, patients can have pain in the lower abdomen and, in males, in the testicles. Physical examination will usually show pubic point tenderness, which is exacerbated by resisted hip adduction.40 MRI and ultrasound are extremely helpful in diagnosing and forming a treatment plan.39
The initial treatment of choice for sports hernias is nonoperative, and the first step is always activity modification or temporary avoidance of symptom-producing activities. Additional modalities include NSAIDs, ice, and physical therapy to strengthen the surrounding muscles. Surgical intervention, if needed, may be done laparoscopically or via an open approach with direct repair.40,41
Less common causes to consider
While the conditions detailed here account for most anterior hip etiologies, there are other less common causes to consider. One such cause is osteitis pubis, an umbrella term for conditions that affect the area surrounding the symphysis pubis. Patients with osteitis pubis present with pain over the anterior aspect of the pelvis that is worse with sit-ups, rising from a chair, or any activity where contraction of the rectus muscles occurs.29 Tenderness is found directly over and just lateral to the pubic symphysis. Radiographs are frequently negative, but occasionally chronic degenerative changes at the symphysis are present in addition to symphyseal narrowing. Additional imaging is often necessary for diagnosis.
Neuropathies. When history, physical examination, and imaging studies have ruled out other causes, neuropathies (ilioinguinal, genitofemoral, and obturator) should be considered, particularly in patients with vague, radiating anterior hip and/or groin pain.42 In pediatric patients, Legg-Calve-Perthes disease and slipped capital femoral epiphysis are possibilities, as well.
Getting patients back on track
Rehabilitation after hip injury resulting in anterior hip pain will be determined by the site, type, and mechanism of injury, as well as the severity. Restrictions in weight bearing and the use of an assistive device may be needed to prevent excessive stress on bone and supporting soft-tissue structures in the early stages of healing. Physical therapy, as needed, should initially focus on early controlled ROM of the hip joint to prevent both intra- and extra-articular adhesions and excessive scar tissue formation.2
For patients who undergo surgery, much of the focus will be on strengthening the supporting musculature—the hip abductor group, anterior and posterior thigh musculature, and core stabilizing muscles. Neuromuscular training may be needed to promote normal biomechanics and minimize compensatory movement patterns. For athletes, cardiovascular training and a return-to-play program should be implemented, as well.2,43,44
CASE Mr. Q was diagnosed with right hip pain due to a labral tear secondary to a cam femoral acetabular impingement. Given that he had failed nonoperative treatment and had long-standing pain, we recommended surgery for this patient. He underwent right hip arthroscopic labral repair, acetabular rim trimming, acetabular microfracture, femoral osteochondroplasty with capsular plication. At 12-month follow-up, he was doing well, with resolution of the presurgical pain and return to all athletic activities.
CORRESPONDENCE Rachel M. Frank, MD, Department of Orthopedic Surgery, Rush University Medical Center, 1611 West Harrison Street, Suite 300, Chicago, IL 60612; [email protected]
1. Margo K, Drezner J, Motzkin D. Evaluation and management of hip pain: an algorithmic approach. J Fam Pract. 2003;52:607-617.
2. Leunig M, Beaule PE, Ganz R. The concept of femoroacetabular impingement: current status and future perspectives. Clin Orthop Relat Res. 2009;467:616-622.
3. Enseki KR, Martin RL, Draovitch P, et al. The hip joint: arthroscopic procedures and postoperative rehabilitation J Orthop Sports Phys Ther. 2006;36:516-525.
4. Byrd JW, Jones KS. Diagnostic accuracy of clinical assessment, magnetic resonance imaging, magnetic resonance arthrography, and intra-articular injection in hip arthroscopy patients. Am J Sports Med. 2004;32:1668-1674.
5. Fredericson M, Jennings F, Beaulieu C, et al. Stress fractures in athletes. Top Magn Reson Imaging. 2006;17:309-325.
6. Matheson GO, Clement DB, McKenzie DC, et al. Stress fractures in athletes. A study of 320 cases. Am J Sports Med. 1987;15:46-58.
7. Stanitski CL, McMaster JH, Scranton PE. On the nature of stress fractures. Am J Sports Med. 1978;6:391-396.
8. Sofka CM. Imaging of stress fractures. Clin Sports Med. 2006;25:53-62, viii.
9. Shin AY, Gillingham BL. Fatigue fractures of the femoral neck in athletes. J Am Acad Orthop Surg. 1997;5:293-302.
10. Mont MA, Hungerford DS. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1995;77:459-474.
11. Lavernia CJ, Sierra RJ, Gomez-Marin O. Smoking and joint replacement: resource consumption and short-term outcome. Clin Orthop Relat Res. 1999;(367):172-180.
12. Lavernia CJ, Sierra RJ, Grieco FR. Osteonecrosis of the femoral head. J Am Acad Orthop Surg. 1999;7:250-261.
13. Smith SW, Fehring TK, Griffin WL, Beaver WB. Core decompression of the osteonecrotic femoral head. J Bone Joint Surg Am. 1995;77:674-680.
14. Fairbank AC, Bhatia D, Jinnah RH, et al. Long-term results of core decompression for ischaemic necrosis of the femoral head. J Bone Joint Surg Br. 1995;77:42-49.
15. Chandler HP, Reineck FT, Wixson RL, et al. Total hip replacement in patients younger than thirty years old. A five-year follow-up study. J Bone Joint Surg Am. 1981;63:1426-1434.
16. Wei SY, Klimkiewicz JJ, Lai M, et al. Revision total hip arthroplasty in patients with avascular necrosis. Orthopedics. 1999;22:747-757.
17. Bedi A, Chen N, Robertson W, et al. The management of labral tears and femoroacetabular impingement of the hip in the young, active patient. Arthroscopy. 2008;24:1135-1145.
18. Guanche CA, Bare AA. Arthroscopic treatment of femoroacetabular impingement. Arthroscopy. 2006;22:95-106.
19. Philippon M, Schenker M, Briggs K, et al. Femoroacetabular impingement in 45 professional athletes: Knee Surg Sports Traumatol Arthrosc. 2007;15:908-914.
20. Sierra RJ, Trousdale RT, Ganz R, et al. Hip disease in the young, active patient: evaluation and nonarthroplasty surgical options. J Am Acad Orthop Surg. 2008;16:689-703.
21. Byrd JW, Jones KS. Prospective analysis of hip arthroscopy with 10-year followup. Clin Orthop Relat Res. 2009;468:741-746.
22. Burnett RS, Della Rocca GJ, Prather H, et al. Clinical presentation of patients with tears of the acetabular labrum. J Bone Joint Surg Am. 2006;88:1448-1457.
23. Bare AA, Guanche CA. Hip impingement: the role of arthroscopy. Orthopedics. 2005;28:266-273.
24. Toomayan GA, Holman WR, Major NM, et al. Sensitivity of MR arthrography in the evaluation of acetabular labral tears. AJR Am J Roentgenol. 2006;186:449-453.
25. Robertson WJ, Kadrmas WR, Kelly BT. Arthroscopic management of labral tears in the hip: a systematic review of the literature. Clin Orthop Relat Res. 2007;455:88-92.
26. Kamath AF, Componovo R, Baldwin K, et al. Hip arthroscopy for labral tears: review of clinical outcomes with 4.8-year mean follow-up. Am J Sports Med. 2009;37:1721-1727.
27. Larson CM, Giveans MR. Arthroscopic debridement versus refixation of the acetabular labrum associated with femoroacetabular impingement. Arthroscopy. 2009;25:369-376.
28. Larson CM, Guanche CA, Kelly BT, et al. Advanced techniques in hip arthroscopy. Instr Course Lect. 2009;58:423-436.
29. Tibor LM, Sekiya JK. Differential diagnosis of pain around the hip joint. Arthroscopy. 2008;24:1407-1421.
30. Holmich P. Long-standing groin pain in sportspeople falls into three primary patterns, a “clinical entity” approach: a prospective study of 207 patients. Br J Sports Med. 2007;41:247-252.
31. Winston P, Awan R, Cassidy JD, et al. Clinical examination and ultrasound of self-reported snapping hip syndrome in elite ballet dancers. Am J Sports Med. 2007;35:118-126.
32. Blankenbaker DG, De Smet AA, Keene JS. Sonography of the iliopsoas tendon and injection of the iliopsoas bursa for diagnosis and management of the painful snapping hip. Skeletal Radiol. 2006;35:565-571.
33. Adler RS, Buly R, Ambrose R, et al. Diagnostic and therapeutic use of sonography-guided iliopsoas peritendinous injections. AJR Am J Roentgenol. 2005;185:940-943.
34. Anderson SA, Keene JS. Results of arthroscopic iliopsoas tendon release in competitive and recreational athletes. Am J Sports Med. 2008;36:2363-2371.
35. Maffey L, Emery C. What are the risk factors for groin strain injury in sport? Sports Med. 2007;37:881-894.
36. Verrall GM, Slavotinek JP, Fon GT, et al. Outcome of conservative management of athletic chronic groin injury diagnosed as pubic bone stress injury. Am J Sports Med. 2007;35:467-474.
37. Pointinger H, Munk P, Poeschl GP. Avulsion fracture of the anterior superior iliac spine following apophysitis. Br J Sports Med. 2003;37:361-362.
38. Unverzagt CA, Schuemann T, Mathisen J. Differential diagnosis of a sports hernia in a high-school athlete. J Orthop Sports Phys Ther. 2008;38:63-70.
39. Caudill P, Nyland J, Smith C, et al. Sports hernias: a systematic literature review. Br J Sports Med. 2008;42:954-964.
40. Ahumada LA, Ashruf S, Espinosa-de-los-Monteros A, et al. Athletic pubalgia: definition and surgical treatment. Ann Plast Surg. 2005;55:393-396.
41. Anderson K, Strickland SM, Warren R. Hip and groin injuries in athletes. Am J Sports Med. 2001;29:521-533.
42. Petchprapa CN, Rosenberg ZS, Sconfienza LM, et al. MR imaging of entrapment neuropathies of the lower extremity. Part I. Radiographs. 2010;30:983-1000.
43. Voight M, Robinson K, Gill L, et al. Postoperative guidelines for hip arthroscopy in the active population. Sports Health. 2010;2:222-230.
44. Stalzer S, Wahoff M, Scanlan M. Rehabilitation following hip arthroscopy. Clin Sports Med. 2006;25:337-357.
• Consider both musculoskeletal and nonmusculoskeletal causes in patients with vague complaints of hip and groin pain. B
• Use imaging studies to confirm a hip pain diagnosis. B
• Refer patients who fail to respond to nonsurgical treatment to a sports medicine specialist or an orthopedic surgeon. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Hip pain is a common complaint, and commonly misunderstood. Although the pain can be associated with a broad spectrum of conditions, the presentation is often vague and nonspecific.
Thus, hip pain and injury are frequently attributed, often incorrectly, to a “hip pointer”—a contusion of soft tissues against the iliac crest. It’s not unusual for patients who receive this diagnosis to be treated conservatively for prolonged periods, leading some previously active individuals to abandon their favorite sport or self-impose limits on the activities they engage in.1
But it doesn’t have to be this way.
Minimally invasive hip arthroscopy and advances in imaging, instrumentation, and devices have made it easier to identify and address underlying pathology associated with hip pain, helping patients return to their previous level of activity more rapidly.2,3 And, while many conditions associated with hip pain can be treated conservatively, family physicians—whom patients often go to first—should not hesitate to provide a referral when more aggressive treatment or diagnostic confirmation is needed.
We created this guide with family physicians in mind. Our focus here is primarily on anterior hip pain—the most common presentation—in active, or athletic, patients.
When did the pain begin? Where does it hurt?
Before performing a physical examination, find out as much as possible about the onset of pain and when and under what circumstances it occurs. (A review of hip anatomy is provided here.) Did it begin suddenly, after an acute injury or a particular physical maneuver? Or is the pain insidious, as was the case with one of our patients?
Osseous morphology of the hip includes the anterior superior iliac spine, the origin of the sartorius muscle and the ilioinguinal ligament. The anterior inferior iliac spine attaches to the rectus femoris, a major hip flexor and knee extender. The adductors of the hip originate in the anterior pelvic region.
The inguinal canal contains the ilioinguinal nerve, which is responsible for radiation of pain to the anterior hip. The hip joint itself is a spheroid comprising the femoral head and acetabulum, with most of the articular hip innervated by the femoral or obturator nerves.
Most intra-articular conditions radiate to the anterior groin or hip, although there are cases in which the pain is referred to either the lateral aspect of the hip or the buttocks. The iliopsoas muscle is the major hip flexor, and crosses under the ilioinguinal ligament to insert on the lesser tuberosity after crossing over the anterior capsule of the hip. A large bursa surrounds it, helping the tendon glide smoothly over the hip.
CASE Mack Q, a 27-year-old man with an 8-month history of right hip pain, sought care at our medical center for an achy pain in his right groin; he also described an occasional “clicking and popping sensation” in his groin but denied any trauma. The pain worsened with prolonged sitting and certain activities, such as squatting, twisting, and putting on shoes and socks. Our patient had stopped playing soccer because it hurt too much. He had tried physical therapy, oral anti-inflammatories, and a corticosteroid injection, with little relief.
Start with a gait assessment
The physical examination should begin with a gait assessment. Consider the patient’s ability to bear weight and his or her foot angle.
An individual with a stress fracture will have difficulty bearing weight on the affected side, resulting in a limp, or antalgic gait. A patient with femoral acetabular impingement (FAI) will often exhibit greater external rotation of the foot on the affected side compared with the other foot. And a patient with weakened abductor muscles, typically because of severe osteoarthritis, will exhibit the Trendelenburg sign—a pelvic tilt when the weight is shifted to the affected extremity.
Although most individuals with hip pain will not have an obvious gait abnormality, any patient who walks with a limp or needs crutches requires an immediate referral to an orthopedic surgeon.
Include these elements in the physical exam
Examine the hip with the patient sitting on the side of the exam table. Assess range of motion (ROM), comparing the range of flexion, extension, and internal/external rotation on the affected and unaffected sides. Include the following maneuvers:
Impingement testing. In patients with FAI and osteoarthritis, impingement testing—encompassing Flexion, ADDuction, and Internal Rotation (FADDIR)—will elicit pain. The maneuver can be tested starting at 45° of hip flexion, increasing to approximately 120°. Pain with <45° of hip flexion indicates that the impingement is severe.
Such testing can also reveal labral tears, which may be caused by FAI or other structural abnormalities. In a patient with anterior labral tears, FADDIR will produce groin pain; posterior labral tears will produce pain when the patient is sitting with legs hanging off the exam table and the contralateral leg is brought to the chest and the affected limb fully extended.
In patients with hip pain and bursitis, applying downward pressure will elicit a snapping sound as the iliopsoas snaps over the iliopectineal eminence or femoral head. Flexion, ABduction, and External Rotation (FABER) can also be used to diagnose iliopsoas tendonitis: The test is positive if it elicits pain in the affected extremity or in the sacroiliac joint on the opposite side.
Log roll. A painful response to this test, which involves internally and externally rotating the affected hip while it is relaxed and the knee fully extended, is an indication of synovitis of the hip caused by intra-articular pathology. To test hip stability, externally rotate the leg while it is extended. If the hip is stable, the leg will return to a neutral position; microinstability of the hip is likely if the leg remains in the rotated position.
Muscular strength testing. To assess for tendinopathy in the hip area, the patient should be in a seated position and contract the internal and external rotators and the adductor muscles while you apply resistance. To test abductor strength, have the patient assume a lateral position and hold and abduct the leg on the affected side while you apply resistance.
Hip flexion strength should be tested with the patient in both supine and seated positions. A patient with quadriceps tendonitis will have much greater pain with resisted hip flexion in the supine position vs the seated position; the opposite is true for a patient with iliopsoas tendonitis. (See “Did you know…? Hip pain facts and findings” on for additional diagnostic tips.)
- A patient with quadriceps tendonitis will have much greater pain with resisted hip flexion in a supine position vs a seated position. The opposite is true for a patient with iliopsoas tendonitis.
- Patients with femoral neck stress fractures typically present with activity-related anterior groin pain that is relieved by rest. Initially, they may be only mildly affected, but the condition worsens in those who continue to “work through the pain.”
- Plain radiography can confirm a diagnosis of osteonecrosis in patients with advanced disease, but magnetic resonance imaging is useful for evaluating earlier clinical presentations.
- Patients with labral tears often exhibit what has been called the “c-sign”—so named for the shape patients make with their hand as they grip their hip just above the greater trochanter to indicate where it hurts.
- Athletes who experience adductor strains often play sports in which kicking or frequent changes in direction are required, such as football, hockey, and soccer, and are generally able to tell you exactly what they were doing when the injury occurred.
- Unlike other hernias, a sports hernia (athletic pubalgia) does not involve a bulge of tissue protruding through one part of the body into another. Instead, it occurs when the oblique abdominal muscles strain or completely tear away from the pubis.
Perform a neurologic evaluation to rule out a back condition that might radiate pain into the anterior hip; ask the patient to do a sit-up while you apply resistance to test for abdominal wall pathology, as well.
Hip palpation. This aspect of the physical exam is important regardless of the cause of the pain but especially crucial for pediatric and adolescent patients, whose anterior hip pain may be related to apophyseal injury. Palpate the superior iliac spine (and over the inferior iliac spine in thin patients) to determine if the sartorius or rectus femoris has been injured. The area just lateral to the symphysis will be tender to palpation in patients with osteitis pubis.
Refer or treat? Here’s what to consider
While the history and physical should provide ample information for a differential diagnosis, imaging studies are generally required for confirmation. Clinical assessment— including physical exam, imaging, and intra-articular injection—of patients with hip pain is up to 98% accurate in identifying hip abnormalities, with arthroscopy as the gold standard.4
CASE On physical examination, Mr. Q had right hip extension to 0°, flexion to 110°, external rotation to 50°, and internal rotation to neutral; he also had positive impingement and subspine impingement tests, a painful arc of motion from 12 to 4 o’clock, tenderness over the hip adductor, and pain with resisted hip adduction. He did not walk with a limp.
Diagnostic studies included plain radiographs, which demonstrated that the joint space was well preserved. We identified subtle anatomical abnormalities on the femoral head-neck junction, known as a cam deformity. Magnetic resonance imaging (MRI) revealed an anterior superior labral tear with cartilage delamination.
Stress fractures affect runners, military recruits
In addition to long-distance runners who have recently increased the frequency, duration, or intensity of training,5,6 military recruits have a higher incidence of stress fractures due to the rapid onset of intensive training. Stress fractures can also occur in patients who do not have a history of intense activity but have metabolically weakened bone, in some cases as a result of an eating disorder.7
Patients with femoral neck stress fractures typically present with activity-related anterior groin pain that is relieved by rest; initially, they may be only mildly affected, but the condition worsens in those who continue to “work through the pain.” By the time such individuals seek treatment, they almost always have pain with weight bearing and an antalgic gait.
Symptoms consistent with a femoral neck stress fracture can be further evaluated with plain radiographs. However, x-rays are often negative for up to 4 weeks after the onset of pain.8 In cases in which radiographs are negative but the physical exam is suggestive of a stress fracture, MRI—which can detect an abnormality within a day or 2 of injury8,9—should be used to confirm the diagnosis (FIGURE 1).
FIGURE 1
MRI reveals a femoral neck stress fracture
Treatment. A complete femoral neck fracture portends impending displacement and requires emergent evaluation by an orthopedist, and superior neck changes, also known as tension-sided stress fractures, require urgent treatment with percutaneous screw fixation.9 However, compression-sided, or inferior, stress fractures can be treated with restricted weight bearing and activity modification. Gradual resumption of activity is allowed only after the patient has been asymptomatic for 6 weeks; recurrent pain indicates residual stress reaction, and signals that activities should be abated.
Osteonecrosis has many causes
Necrosis of the femoral head is a debilitating and progressive condition primarily affecting patients between the ages of 20 and 50 years.10 It has multiple (and diverse) causes, including trauma, steroids, alcohol, smoking, lupus, sickle cell anemia, and coagulopathies, as well as scuba diving. But about 20% of cases have no apparent cause.11,12
Patients with osteonecrosis of the hip typically present with groin pain, often described as a deep, intermittent ache that interferes with activities of daily living. Exam findings depend on the stage of presentation. Early on, pain will occur only with extreme ROM; in advanced cases, ROM is restricted and pain occurs even with limited motion.
Femoral head collapse due to loss of the structural integrity of the subchondral bone—which occurs in 80% of cases12—is thought to be caused by decreased blood flow. Plain radiography can confirm a diagnosis of osteonecrosis in patients with advanced disease, but MRI is useful for evaluating patients with earlier clinical presentations.
Treatment of osteonecrosis is dictated by the stage of the disease, but remains controversial because no intervention has been shown to prevent progression in all cases.12 All patients should be referred to a specialist. Those without collapse or cartilage damage can be treated surgically with core decompression, possibly with additional vascularized bone grafting,13,14 while those with more advanced disease typically require a total hip replacement at a relatively young age. Results for total hip replacement in patients with osteonecrosis are thought to be inferior to hip replacement in patients with osteoarthritis, although comparison is difficult because of the differences in age and activity levels in these 2 groups.15,16
Femoral acetabular impingement can occur on the cam or pincer side
FAI pathology can exist on either the femoral (cam) or acetabular (pincer) side,17 or both.18 In pure cam impingement, the anterior femoral neck loses its normal concave anatomy and develops a “bump,” which impinges on the anterosuperior labrum during hip flexion, causing labral tears and delamination of the adjacent cartilage.
Pure pincer impingement arises from a prominent acetabular rim, causing overcoverage of the femoral head. Acetabular labral tears result from the repetitive impaction with flexion and internal rotation.
Patients report an insidious onset of groin pain that is exacerbated by flexion-type sports, such as hockey, football, and golf,19 as well as activities of daily living. In patients with cartilage damage, even walking can be painful. Physical examination of patients with FAI reveals findings that are similar to those of patients with acetabular labral tears. Abnormally large cam lesions or acetabular overcoverage will result in restriction of hip ROM, especially internal rotation and flexion due to a mechanical block.
Radiographs (FIGURE 2) are essential to diagnose FAI and to distinguish this condition from an isolated labral tear.20 Cam impingement will be best demonstrated on a cross-table lateral radiograph, which shows an asphericity of the femoral head/neck junction anteriorly, while pincer impingement will show overcoverage of the femoral head on an AP radiograph. MRI or magnetic resonance arthrography (MRA) is frequently obtained to see whether any cartilage deterioration has occurred. Computed tomography, which can provide a 3-dimensional reproduction of the hip morphology, is often used for preoperative planning when surgical intervention is required.
FIGURE 2
Femoral acetabular impingement with a prominent pincer lesion
Treatment. Surgical intervention is often needed to correct or remove the abnormal anatomy, and both arthroscopic and open surgery are recommended.20 Both methods include osteoplasty at the femoral head/neck junction and/or the acetabular rim to allow the proximal femur to articulate with the acetabulum without injury to the labrum with flexion and internal rotation.21
Results of both open and arthroscopic osteoplasty of the femur and acetabulum are still preliminary, with only a few studies reporting mid-term results. Open surgery typically has longer recovery and rehabilitation, but advocates emphasize the improved ability to contour the femur or acetabulum. Both open and arthroscopic procedures have about an 8% to 13% rate of revision in short-term follow-up.17
Labral tears occur with trauma and certain sports
In addition to FAI, causes of labral tears include dysplasia, instability, trauma, and degeneration, as well as sports that require repetitive hip flexion and/or pivoting, such as hockey, soccer, and football.22,23
Patients with labral tears typically present with anterior hip pain radiating to the groin, worsening with twisting motions, running, walking, and sitting for prolonged periods. Clicking or catching may occur, as well. Patients may exhibit what one researcher called the “c-sign”—so named for the shape patients make with their hand as they grip their hip just above the greater trochanter to indicate where it hurts.4 The work-up for labral tears includes radiographs and, often, MRA, which is nearly 100% specific.24
Treatment. Conservative treatment, which may include activity modification or rest and ice, nonsteroidal anti-inflammatory drugs (NSAIDs), and physical therapy, is often effective for labral tears; when such measures fail, surgical intervention is indicated. A systematic review found a 67% satisfaction rate after 3.5 years in patients who had undergone labral debridement, and complete resolution of mechanical symptoms in nearly 50%.25 Another study showed similar results for hip arthroscopy, with symptom relief continuing for 4.8 years after surgery, on average, and 84% of patients able to return to their previous level of activity.26
The long-term results of labral debridement are unknown, however, and the possibility of an association between this procedure and the development of arthritis remains. Most specialists prefer anatomic repair to restore normal hip kinematics and, potentially, long-term hip function,27,28 but structural abnormalities must also be addressed to prevent failure of the repair or recurrent tears.
Iliopsoas tendonitis: You know the snap
Often referred to as internal snapping of the hip or internal coxa saltans, iliopsoas tendonitis/bursitis can be a recalcitrant cause of anterior hip pain. Snapping of the iliopsoas leading to bursitis or tendonitis can occur at the iliopectineal eminence, the femoral head, or the lesser trochanter.29 Runners and ballet dancers are often affected.30,31
Snapping in itself is not an indication of pathology, but chronicity of symptoms is. Patients with relatively acute symptoms typically have only bursitis, while a longer duration of symptoms leads to tendonitis or tendinopathy.32
Treatment. First-line therapy is nonoperative, and includes activity modification, rest, ice, NSAIDs, and physical therapy. Advise patients to refrain from activities causing pain, and to apply ice to the affected every 20 minutes (with a 20- to 30-minute off period) for one to 2 hours. Physical therapy focuses on stretching the iliopsoas and rectus femoris muscles and strengthening the hamstring muscles to relieve the stress on the anterior pelvis. If such treatment is unsuccessful, ultrasound can be used to guide a therapeutic injection of cortisone.33 If this fails to bring relief, fractional lengthening of the iliopsoas tendon can be performed to eliminate snapping and relieve pain.34
Muscular strains/avulsion fractures: Sports and age play a role
Although strains can affect any of the anterior muscles around the hip, in active individuals the adductors are most commonly affected. Skeletally immature patients are an exception: apophyseal fractures at the origin of the sartorius and rectus femoris muscles are more common than muscular strains in this patient population.
Athletes who experience adductor strains often play sports in which kicking or frequent changes in direction are required—eg, football, hockey, and soccer35—and generally are able to tell you exactly what they were doing when the injury occurred. Physical examination can reveal focal findings, with swelling and tenderness confined to the anteromedial aspect of the hip along the adductor muscle group. MRI can help differentiate the site of true pathology.36
Treatment of adductor strains is nonoperative, with rest, ice, and activity modification until the tendon heals. In the rare case in which complete tendon avulsion is found, surgical reattachment is needed.
Apophyseal fracture in skeletally immature patients typically occurs during participation in a sport that requires rapid acceleration and deceleration with the hip in an extended position. In such patients, stretching the affected muscle should reproduce the pain. Radiographs are diagnostic and will often show minimal displacement of the apophysis. Treatment is almost always nonoperative. Surgical intervention is rarely needed, and only indicated with displacement >2 cm.37
Athletic pubalgia: A challenging Dx
Also referred to as sports hernia, athletic pubalgia is an enigmatic cause of anterior hip pain in athletes. Diagnosis can be especially challenging, and patients may have lingering symptoms for years before the cause is discovered.38 A sports hernia, unlike other hernias, does not involve a bulge of tissue protruding through one body part into another. In contrast, a sports hernia occurs when the oblique abdominal muscles strain or completely tear away from the pubis. A recent systematic review found that the underlying etiology involves posterior inguinal wall weakening, which can be a result of poorly balanced hip adductor and abdominal muscle activation.39
Patients with sports hernia will often present with anterior hip and/or groin pain, especially with hip extension, twisting, and turning. In addition, patients can have pain in the lower abdomen and, in males, in the testicles. Physical examination will usually show pubic point tenderness, which is exacerbated by resisted hip adduction.40 MRI and ultrasound are extremely helpful in diagnosing and forming a treatment plan.39
The initial treatment of choice for sports hernias is nonoperative, and the first step is always activity modification or temporary avoidance of symptom-producing activities. Additional modalities include NSAIDs, ice, and physical therapy to strengthen the surrounding muscles. Surgical intervention, if needed, may be done laparoscopically or via an open approach with direct repair.40,41
Less common causes to consider
While the conditions detailed here account for most anterior hip etiologies, there are other less common causes to consider. One such cause is osteitis pubis, an umbrella term for conditions that affect the area surrounding the symphysis pubis. Patients with osteitis pubis present with pain over the anterior aspect of the pelvis that is worse with sit-ups, rising from a chair, or any activity where contraction of the rectus muscles occurs.29 Tenderness is found directly over and just lateral to the pubic symphysis. Radiographs are frequently negative, but occasionally chronic degenerative changes at the symphysis are present in addition to symphyseal narrowing. Additional imaging is often necessary for diagnosis.
Neuropathies. When history, physical examination, and imaging studies have ruled out other causes, neuropathies (ilioinguinal, genitofemoral, and obturator) should be considered, particularly in patients with vague, radiating anterior hip and/or groin pain.42 In pediatric patients, Legg-Calve-Perthes disease and slipped capital femoral epiphysis are possibilities, as well.
Getting patients back on track
Rehabilitation after hip injury resulting in anterior hip pain will be determined by the site, type, and mechanism of injury, as well as the severity. Restrictions in weight bearing and the use of an assistive device may be needed to prevent excessive stress on bone and supporting soft-tissue structures in the early stages of healing. Physical therapy, as needed, should initially focus on early controlled ROM of the hip joint to prevent both intra- and extra-articular adhesions and excessive scar tissue formation.2
For patients who undergo surgery, much of the focus will be on strengthening the supporting musculature—the hip abductor group, anterior and posterior thigh musculature, and core stabilizing muscles. Neuromuscular training may be needed to promote normal biomechanics and minimize compensatory movement patterns. For athletes, cardiovascular training and a return-to-play program should be implemented, as well.2,43,44
CASE Mr. Q was diagnosed with right hip pain due to a labral tear secondary to a cam femoral acetabular impingement. Given that he had failed nonoperative treatment and had long-standing pain, we recommended surgery for this patient. He underwent right hip arthroscopic labral repair, acetabular rim trimming, acetabular microfracture, femoral osteochondroplasty with capsular plication. At 12-month follow-up, he was doing well, with resolution of the presurgical pain and return to all athletic activities.
CORRESPONDENCE Rachel M. Frank, MD, Department of Orthopedic Surgery, Rush University Medical Center, 1611 West Harrison Street, Suite 300, Chicago, IL 60612; [email protected]
• Consider both musculoskeletal and nonmusculoskeletal causes in patients with vague complaints of hip and groin pain. B
• Use imaging studies to confirm a hip pain diagnosis. B
• Refer patients who fail to respond to nonsurgical treatment to a sports medicine specialist or an orthopedic surgeon. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Hip pain is a common complaint, and commonly misunderstood. Although the pain can be associated with a broad spectrum of conditions, the presentation is often vague and nonspecific.
Thus, hip pain and injury are frequently attributed, often incorrectly, to a “hip pointer”—a contusion of soft tissues against the iliac crest. It’s not unusual for patients who receive this diagnosis to be treated conservatively for prolonged periods, leading some previously active individuals to abandon their favorite sport or self-impose limits on the activities they engage in.1
But it doesn’t have to be this way.
Minimally invasive hip arthroscopy and advances in imaging, instrumentation, and devices have made it easier to identify and address underlying pathology associated with hip pain, helping patients return to their previous level of activity more rapidly.2,3 And, while many conditions associated with hip pain can be treated conservatively, family physicians—whom patients often go to first—should not hesitate to provide a referral when more aggressive treatment or diagnostic confirmation is needed.
We created this guide with family physicians in mind. Our focus here is primarily on anterior hip pain—the most common presentation—in active, or athletic, patients.
When did the pain begin? Where does it hurt?
Before performing a physical examination, find out as much as possible about the onset of pain and when and under what circumstances it occurs. (A review of hip anatomy is provided here.) Did it begin suddenly, after an acute injury or a particular physical maneuver? Or is the pain insidious, as was the case with one of our patients?
Osseous morphology of the hip includes the anterior superior iliac spine, the origin of the sartorius muscle and the ilioinguinal ligament. The anterior inferior iliac spine attaches to the rectus femoris, a major hip flexor and knee extender. The adductors of the hip originate in the anterior pelvic region.
The inguinal canal contains the ilioinguinal nerve, which is responsible for radiation of pain to the anterior hip. The hip joint itself is a spheroid comprising the femoral head and acetabulum, with most of the articular hip innervated by the femoral or obturator nerves.
Most intra-articular conditions radiate to the anterior groin or hip, although there are cases in which the pain is referred to either the lateral aspect of the hip or the buttocks. The iliopsoas muscle is the major hip flexor, and crosses under the ilioinguinal ligament to insert on the lesser tuberosity after crossing over the anterior capsule of the hip. A large bursa surrounds it, helping the tendon glide smoothly over the hip.
CASE Mack Q, a 27-year-old man with an 8-month history of right hip pain, sought care at our medical center for an achy pain in his right groin; he also described an occasional “clicking and popping sensation” in his groin but denied any trauma. The pain worsened with prolonged sitting and certain activities, such as squatting, twisting, and putting on shoes and socks. Our patient had stopped playing soccer because it hurt too much. He had tried physical therapy, oral anti-inflammatories, and a corticosteroid injection, with little relief.
Start with a gait assessment
The physical examination should begin with a gait assessment. Consider the patient’s ability to bear weight and his or her foot angle.
An individual with a stress fracture will have difficulty bearing weight on the affected side, resulting in a limp, or antalgic gait. A patient with femoral acetabular impingement (FAI) will often exhibit greater external rotation of the foot on the affected side compared with the other foot. And a patient with weakened abductor muscles, typically because of severe osteoarthritis, will exhibit the Trendelenburg sign—a pelvic tilt when the weight is shifted to the affected extremity.
Although most individuals with hip pain will not have an obvious gait abnormality, any patient who walks with a limp or needs crutches requires an immediate referral to an orthopedic surgeon.
Include these elements in the physical exam
Examine the hip with the patient sitting on the side of the exam table. Assess range of motion (ROM), comparing the range of flexion, extension, and internal/external rotation on the affected and unaffected sides. Include the following maneuvers:
Impingement testing. In patients with FAI and osteoarthritis, impingement testing—encompassing Flexion, ADDuction, and Internal Rotation (FADDIR)—will elicit pain. The maneuver can be tested starting at 45° of hip flexion, increasing to approximately 120°. Pain with <45° of hip flexion indicates that the impingement is severe.
Such testing can also reveal labral tears, which may be caused by FAI or other structural abnormalities. In a patient with anterior labral tears, FADDIR will produce groin pain; posterior labral tears will produce pain when the patient is sitting with legs hanging off the exam table and the contralateral leg is brought to the chest and the affected limb fully extended.
In patients with hip pain and bursitis, applying downward pressure will elicit a snapping sound as the iliopsoas snaps over the iliopectineal eminence or femoral head. Flexion, ABduction, and External Rotation (FABER) can also be used to diagnose iliopsoas tendonitis: The test is positive if it elicits pain in the affected extremity or in the sacroiliac joint on the opposite side.
Log roll. A painful response to this test, which involves internally and externally rotating the affected hip while it is relaxed and the knee fully extended, is an indication of synovitis of the hip caused by intra-articular pathology. To test hip stability, externally rotate the leg while it is extended. If the hip is stable, the leg will return to a neutral position; microinstability of the hip is likely if the leg remains in the rotated position.
Muscular strength testing. To assess for tendinopathy in the hip area, the patient should be in a seated position and contract the internal and external rotators and the adductor muscles while you apply resistance. To test abductor strength, have the patient assume a lateral position and hold and abduct the leg on the affected side while you apply resistance.
Hip flexion strength should be tested with the patient in both supine and seated positions. A patient with quadriceps tendonitis will have much greater pain with resisted hip flexion in the supine position vs the seated position; the opposite is true for a patient with iliopsoas tendonitis. (See “Did you know…? Hip pain facts and findings” on for additional diagnostic tips.)
- A patient with quadriceps tendonitis will have much greater pain with resisted hip flexion in a supine position vs a seated position. The opposite is true for a patient with iliopsoas tendonitis.
- Patients with femoral neck stress fractures typically present with activity-related anterior groin pain that is relieved by rest. Initially, they may be only mildly affected, but the condition worsens in those who continue to “work through the pain.”
- Plain radiography can confirm a diagnosis of osteonecrosis in patients with advanced disease, but magnetic resonance imaging is useful for evaluating earlier clinical presentations.
- Patients with labral tears often exhibit what has been called the “c-sign”—so named for the shape patients make with their hand as they grip their hip just above the greater trochanter to indicate where it hurts.
- Athletes who experience adductor strains often play sports in which kicking or frequent changes in direction are required, such as football, hockey, and soccer, and are generally able to tell you exactly what they were doing when the injury occurred.
- Unlike other hernias, a sports hernia (athletic pubalgia) does not involve a bulge of tissue protruding through one part of the body into another. Instead, it occurs when the oblique abdominal muscles strain or completely tear away from the pubis.
Perform a neurologic evaluation to rule out a back condition that might radiate pain into the anterior hip; ask the patient to do a sit-up while you apply resistance to test for abdominal wall pathology, as well.
Hip palpation. This aspect of the physical exam is important regardless of the cause of the pain but especially crucial for pediatric and adolescent patients, whose anterior hip pain may be related to apophyseal injury. Palpate the superior iliac spine (and over the inferior iliac spine in thin patients) to determine if the sartorius or rectus femoris has been injured. The area just lateral to the symphysis will be tender to palpation in patients with osteitis pubis.
Refer or treat? Here’s what to consider
While the history and physical should provide ample information for a differential diagnosis, imaging studies are generally required for confirmation. Clinical assessment— including physical exam, imaging, and intra-articular injection—of patients with hip pain is up to 98% accurate in identifying hip abnormalities, with arthroscopy as the gold standard.4
CASE On physical examination, Mr. Q had right hip extension to 0°, flexion to 110°, external rotation to 50°, and internal rotation to neutral; he also had positive impingement and subspine impingement tests, a painful arc of motion from 12 to 4 o’clock, tenderness over the hip adductor, and pain with resisted hip adduction. He did not walk with a limp.
Diagnostic studies included plain radiographs, which demonstrated that the joint space was well preserved. We identified subtle anatomical abnormalities on the femoral head-neck junction, known as a cam deformity. Magnetic resonance imaging (MRI) revealed an anterior superior labral tear with cartilage delamination.
Stress fractures affect runners, military recruits
In addition to long-distance runners who have recently increased the frequency, duration, or intensity of training,5,6 military recruits have a higher incidence of stress fractures due to the rapid onset of intensive training. Stress fractures can also occur in patients who do not have a history of intense activity but have metabolically weakened bone, in some cases as a result of an eating disorder.7
Patients with femoral neck stress fractures typically present with activity-related anterior groin pain that is relieved by rest; initially, they may be only mildly affected, but the condition worsens in those who continue to “work through the pain.” By the time such individuals seek treatment, they almost always have pain with weight bearing and an antalgic gait.
Symptoms consistent with a femoral neck stress fracture can be further evaluated with plain radiographs. However, x-rays are often negative for up to 4 weeks after the onset of pain.8 In cases in which radiographs are negative but the physical exam is suggestive of a stress fracture, MRI—which can detect an abnormality within a day or 2 of injury8,9—should be used to confirm the diagnosis (FIGURE 1).
FIGURE 1
MRI reveals a femoral neck stress fracture
Treatment. A complete femoral neck fracture portends impending displacement and requires emergent evaluation by an orthopedist, and superior neck changes, also known as tension-sided stress fractures, require urgent treatment with percutaneous screw fixation.9 However, compression-sided, or inferior, stress fractures can be treated with restricted weight bearing and activity modification. Gradual resumption of activity is allowed only after the patient has been asymptomatic for 6 weeks; recurrent pain indicates residual stress reaction, and signals that activities should be abated.
Osteonecrosis has many causes
Necrosis of the femoral head is a debilitating and progressive condition primarily affecting patients between the ages of 20 and 50 years.10 It has multiple (and diverse) causes, including trauma, steroids, alcohol, smoking, lupus, sickle cell anemia, and coagulopathies, as well as scuba diving. But about 20% of cases have no apparent cause.11,12
Patients with osteonecrosis of the hip typically present with groin pain, often described as a deep, intermittent ache that interferes with activities of daily living. Exam findings depend on the stage of presentation. Early on, pain will occur only with extreme ROM; in advanced cases, ROM is restricted and pain occurs even with limited motion.
Femoral head collapse due to loss of the structural integrity of the subchondral bone—which occurs in 80% of cases12—is thought to be caused by decreased blood flow. Plain radiography can confirm a diagnosis of osteonecrosis in patients with advanced disease, but MRI is useful for evaluating patients with earlier clinical presentations.
Treatment of osteonecrosis is dictated by the stage of the disease, but remains controversial because no intervention has been shown to prevent progression in all cases.12 All patients should be referred to a specialist. Those without collapse or cartilage damage can be treated surgically with core decompression, possibly with additional vascularized bone grafting,13,14 while those with more advanced disease typically require a total hip replacement at a relatively young age. Results for total hip replacement in patients with osteonecrosis are thought to be inferior to hip replacement in patients with osteoarthritis, although comparison is difficult because of the differences in age and activity levels in these 2 groups.15,16
Femoral acetabular impingement can occur on the cam or pincer side
FAI pathology can exist on either the femoral (cam) or acetabular (pincer) side,17 or both.18 In pure cam impingement, the anterior femoral neck loses its normal concave anatomy and develops a “bump,” which impinges on the anterosuperior labrum during hip flexion, causing labral tears and delamination of the adjacent cartilage.
Pure pincer impingement arises from a prominent acetabular rim, causing overcoverage of the femoral head. Acetabular labral tears result from the repetitive impaction with flexion and internal rotation.
Patients report an insidious onset of groin pain that is exacerbated by flexion-type sports, such as hockey, football, and golf,19 as well as activities of daily living. In patients with cartilage damage, even walking can be painful. Physical examination of patients with FAI reveals findings that are similar to those of patients with acetabular labral tears. Abnormally large cam lesions or acetabular overcoverage will result in restriction of hip ROM, especially internal rotation and flexion due to a mechanical block.
Radiographs (FIGURE 2) are essential to diagnose FAI and to distinguish this condition from an isolated labral tear.20 Cam impingement will be best demonstrated on a cross-table lateral radiograph, which shows an asphericity of the femoral head/neck junction anteriorly, while pincer impingement will show overcoverage of the femoral head on an AP radiograph. MRI or magnetic resonance arthrography (MRA) is frequently obtained to see whether any cartilage deterioration has occurred. Computed tomography, which can provide a 3-dimensional reproduction of the hip morphology, is often used for preoperative planning when surgical intervention is required.
FIGURE 2
Femoral acetabular impingement with a prominent pincer lesion
Treatment. Surgical intervention is often needed to correct or remove the abnormal anatomy, and both arthroscopic and open surgery are recommended.20 Both methods include osteoplasty at the femoral head/neck junction and/or the acetabular rim to allow the proximal femur to articulate with the acetabulum without injury to the labrum with flexion and internal rotation.21
Results of both open and arthroscopic osteoplasty of the femur and acetabulum are still preliminary, with only a few studies reporting mid-term results. Open surgery typically has longer recovery and rehabilitation, but advocates emphasize the improved ability to contour the femur or acetabulum. Both open and arthroscopic procedures have about an 8% to 13% rate of revision in short-term follow-up.17
Labral tears occur with trauma and certain sports
In addition to FAI, causes of labral tears include dysplasia, instability, trauma, and degeneration, as well as sports that require repetitive hip flexion and/or pivoting, such as hockey, soccer, and football.22,23
Patients with labral tears typically present with anterior hip pain radiating to the groin, worsening with twisting motions, running, walking, and sitting for prolonged periods. Clicking or catching may occur, as well. Patients may exhibit what one researcher called the “c-sign”—so named for the shape patients make with their hand as they grip their hip just above the greater trochanter to indicate where it hurts.4 The work-up for labral tears includes radiographs and, often, MRA, which is nearly 100% specific.24
Treatment. Conservative treatment, which may include activity modification or rest and ice, nonsteroidal anti-inflammatory drugs (NSAIDs), and physical therapy, is often effective for labral tears; when such measures fail, surgical intervention is indicated. A systematic review found a 67% satisfaction rate after 3.5 years in patients who had undergone labral debridement, and complete resolution of mechanical symptoms in nearly 50%.25 Another study showed similar results for hip arthroscopy, with symptom relief continuing for 4.8 years after surgery, on average, and 84% of patients able to return to their previous level of activity.26
The long-term results of labral debridement are unknown, however, and the possibility of an association between this procedure and the development of arthritis remains. Most specialists prefer anatomic repair to restore normal hip kinematics and, potentially, long-term hip function,27,28 but structural abnormalities must also be addressed to prevent failure of the repair or recurrent tears.
Iliopsoas tendonitis: You know the snap
Often referred to as internal snapping of the hip or internal coxa saltans, iliopsoas tendonitis/bursitis can be a recalcitrant cause of anterior hip pain. Snapping of the iliopsoas leading to bursitis or tendonitis can occur at the iliopectineal eminence, the femoral head, or the lesser trochanter.29 Runners and ballet dancers are often affected.30,31
Snapping in itself is not an indication of pathology, but chronicity of symptoms is. Patients with relatively acute symptoms typically have only bursitis, while a longer duration of symptoms leads to tendonitis or tendinopathy.32
Treatment. First-line therapy is nonoperative, and includes activity modification, rest, ice, NSAIDs, and physical therapy. Advise patients to refrain from activities causing pain, and to apply ice to the affected every 20 minutes (with a 20- to 30-minute off period) for one to 2 hours. Physical therapy focuses on stretching the iliopsoas and rectus femoris muscles and strengthening the hamstring muscles to relieve the stress on the anterior pelvis. If such treatment is unsuccessful, ultrasound can be used to guide a therapeutic injection of cortisone.33 If this fails to bring relief, fractional lengthening of the iliopsoas tendon can be performed to eliminate snapping and relieve pain.34
Muscular strains/avulsion fractures: Sports and age play a role
Although strains can affect any of the anterior muscles around the hip, in active individuals the adductors are most commonly affected. Skeletally immature patients are an exception: apophyseal fractures at the origin of the sartorius and rectus femoris muscles are more common than muscular strains in this patient population.
Athletes who experience adductor strains often play sports in which kicking or frequent changes in direction are required—eg, football, hockey, and soccer35—and generally are able to tell you exactly what they were doing when the injury occurred. Physical examination can reveal focal findings, with swelling and tenderness confined to the anteromedial aspect of the hip along the adductor muscle group. MRI can help differentiate the site of true pathology.36
Treatment of adductor strains is nonoperative, with rest, ice, and activity modification until the tendon heals. In the rare case in which complete tendon avulsion is found, surgical reattachment is needed.
Apophyseal fracture in skeletally immature patients typically occurs during participation in a sport that requires rapid acceleration and deceleration with the hip in an extended position. In such patients, stretching the affected muscle should reproduce the pain. Radiographs are diagnostic and will often show minimal displacement of the apophysis. Treatment is almost always nonoperative. Surgical intervention is rarely needed, and only indicated with displacement >2 cm.37
Athletic pubalgia: A challenging Dx
Also referred to as sports hernia, athletic pubalgia is an enigmatic cause of anterior hip pain in athletes. Diagnosis can be especially challenging, and patients may have lingering symptoms for years before the cause is discovered.38 A sports hernia, unlike other hernias, does not involve a bulge of tissue protruding through one body part into another. In contrast, a sports hernia occurs when the oblique abdominal muscles strain or completely tear away from the pubis. A recent systematic review found that the underlying etiology involves posterior inguinal wall weakening, which can be a result of poorly balanced hip adductor and abdominal muscle activation.39
Patients with sports hernia will often present with anterior hip and/or groin pain, especially with hip extension, twisting, and turning. In addition, patients can have pain in the lower abdomen and, in males, in the testicles. Physical examination will usually show pubic point tenderness, which is exacerbated by resisted hip adduction.40 MRI and ultrasound are extremely helpful in diagnosing and forming a treatment plan.39
The initial treatment of choice for sports hernias is nonoperative, and the first step is always activity modification or temporary avoidance of symptom-producing activities. Additional modalities include NSAIDs, ice, and physical therapy to strengthen the surrounding muscles. Surgical intervention, if needed, may be done laparoscopically or via an open approach with direct repair.40,41
Less common causes to consider
While the conditions detailed here account for most anterior hip etiologies, there are other less common causes to consider. One such cause is osteitis pubis, an umbrella term for conditions that affect the area surrounding the symphysis pubis. Patients with osteitis pubis present with pain over the anterior aspect of the pelvis that is worse with sit-ups, rising from a chair, or any activity where contraction of the rectus muscles occurs.29 Tenderness is found directly over and just lateral to the pubic symphysis. Radiographs are frequently negative, but occasionally chronic degenerative changes at the symphysis are present in addition to symphyseal narrowing. Additional imaging is often necessary for diagnosis.
Neuropathies. When history, physical examination, and imaging studies have ruled out other causes, neuropathies (ilioinguinal, genitofemoral, and obturator) should be considered, particularly in patients with vague, radiating anterior hip and/or groin pain.42 In pediatric patients, Legg-Calve-Perthes disease and slipped capital femoral epiphysis are possibilities, as well.
Getting patients back on track
Rehabilitation after hip injury resulting in anterior hip pain will be determined by the site, type, and mechanism of injury, as well as the severity. Restrictions in weight bearing and the use of an assistive device may be needed to prevent excessive stress on bone and supporting soft-tissue structures in the early stages of healing. Physical therapy, as needed, should initially focus on early controlled ROM of the hip joint to prevent both intra- and extra-articular adhesions and excessive scar tissue formation.2
For patients who undergo surgery, much of the focus will be on strengthening the supporting musculature—the hip abductor group, anterior and posterior thigh musculature, and core stabilizing muscles. Neuromuscular training may be needed to promote normal biomechanics and minimize compensatory movement patterns. For athletes, cardiovascular training and a return-to-play program should be implemented, as well.2,43,44
CASE Mr. Q was diagnosed with right hip pain due to a labral tear secondary to a cam femoral acetabular impingement. Given that he had failed nonoperative treatment and had long-standing pain, we recommended surgery for this patient. He underwent right hip arthroscopic labral repair, acetabular rim trimming, acetabular microfracture, femoral osteochondroplasty with capsular plication. At 12-month follow-up, he was doing well, with resolution of the presurgical pain and return to all athletic activities.
CORRESPONDENCE Rachel M. Frank, MD, Department of Orthopedic Surgery, Rush University Medical Center, 1611 West Harrison Street, Suite 300, Chicago, IL 60612; [email protected]
1. Margo K, Drezner J, Motzkin D. Evaluation and management of hip pain: an algorithmic approach. J Fam Pract. 2003;52:607-617.
2. Leunig M, Beaule PE, Ganz R. The concept of femoroacetabular impingement: current status and future perspectives. Clin Orthop Relat Res. 2009;467:616-622.
3. Enseki KR, Martin RL, Draovitch P, et al. The hip joint: arthroscopic procedures and postoperative rehabilitation J Orthop Sports Phys Ther. 2006;36:516-525.
4. Byrd JW, Jones KS. Diagnostic accuracy of clinical assessment, magnetic resonance imaging, magnetic resonance arthrography, and intra-articular injection in hip arthroscopy patients. Am J Sports Med. 2004;32:1668-1674.
5. Fredericson M, Jennings F, Beaulieu C, et al. Stress fractures in athletes. Top Magn Reson Imaging. 2006;17:309-325.
6. Matheson GO, Clement DB, McKenzie DC, et al. Stress fractures in athletes. A study of 320 cases. Am J Sports Med. 1987;15:46-58.
7. Stanitski CL, McMaster JH, Scranton PE. On the nature of stress fractures. Am J Sports Med. 1978;6:391-396.
8. Sofka CM. Imaging of stress fractures. Clin Sports Med. 2006;25:53-62, viii.
9. Shin AY, Gillingham BL. Fatigue fractures of the femoral neck in athletes. J Am Acad Orthop Surg. 1997;5:293-302.
10. Mont MA, Hungerford DS. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1995;77:459-474.
11. Lavernia CJ, Sierra RJ, Gomez-Marin O. Smoking and joint replacement: resource consumption and short-term outcome. Clin Orthop Relat Res. 1999;(367):172-180.
12. Lavernia CJ, Sierra RJ, Grieco FR. Osteonecrosis of the femoral head. J Am Acad Orthop Surg. 1999;7:250-261.
13. Smith SW, Fehring TK, Griffin WL, Beaver WB. Core decompression of the osteonecrotic femoral head. J Bone Joint Surg Am. 1995;77:674-680.
14. Fairbank AC, Bhatia D, Jinnah RH, et al. Long-term results of core decompression for ischaemic necrosis of the femoral head. J Bone Joint Surg Br. 1995;77:42-49.
15. Chandler HP, Reineck FT, Wixson RL, et al. Total hip replacement in patients younger than thirty years old. A five-year follow-up study. J Bone Joint Surg Am. 1981;63:1426-1434.
16. Wei SY, Klimkiewicz JJ, Lai M, et al. Revision total hip arthroplasty in patients with avascular necrosis. Orthopedics. 1999;22:747-757.
17. Bedi A, Chen N, Robertson W, et al. The management of labral tears and femoroacetabular impingement of the hip in the young, active patient. Arthroscopy. 2008;24:1135-1145.
18. Guanche CA, Bare AA. Arthroscopic treatment of femoroacetabular impingement. Arthroscopy. 2006;22:95-106.
19. Philippon M, Schenker M, Briggs K, et al. Femoroacetabular impingement in 45 professional athletes: Knee Surg Sports Traumatol Arthrosc. 2007;15:908-914.
20. Sierra RJ, Trousdale RT, Ganz R, et al. Hip disease in the young, active patient: evaluation and nonarthroplasty surgical options. J Am Acad Orthop Surg. 2008;16:689-703.
21. Byrd JW, Jones KS. Prospective analysis of hip arthroscopy with 10-year followup. Clin Orthop Relat Res. 2009;468:741-746.
22. Burnett RS, Della Rocca GJ, Prather H, et al. Clinical presentation of patients with tears of the acetabular labrum. J Bone Joint Surg Am. 2006;88:1448-1457.
23. Bare AA, Guanche CA. Hip impingement: the role of arthroscopy. Orthopedics. 2005;28:266-273.
24. Toomayan GA, Holman WR, Major NM, et al. Sensitivity of MR arthrography in the evaluation of acetabular labral tears. AJR Am J Roentgenol. 2006;186:449-453.
25. Robertson WJ, Kadrmas WR, Kelly BT. Arthroscopic management of labral tears in the hip: a systematic review of the literature. Clin Orthop Relat Res. 2007;455:88-92.
26. Kamath AF, Componovo R, Baldwin K, et al. Hip arthroscopy for labral tears: review of clinical outcomes with 4.8-year mean follow-up. Am J Sports Med. 2009;37:1721-1727.
27. Larson CM, Giveans MR. Arthroscopic debridement versus refixation of the acetabular labrum associated with femoroacetabular impingement. Arthroscopy. 2009;25:369-376.
28. Larson CM, Guanche CA, Kelly BT, et al. Advanced techniques in hip arthroscopy. Instr Course Lect. 2009;58:423-436.
29. Tibor LM, Sekiya JK. Differential diagnosis of pain around the hip joint. Arthroscopy. 2008;24:1407-1421.
30. Holmich P. Long-standing groin pain in sportspeople falls into three primary patterns, a “clinical entity” approach: a prospective study of 207 patients. Br J Sports Med. 2007;41:247-252.
31. Winston P, Awan R, Cassidy JD, et al. Clinical examination and ultrasound of self-reported snapping hip syndrome in elite ballet dancers. Am J Sports Med. 2007;35:118-126.
32. Blankenbaker DG, De Smet AA, Keene JS. Sonography of the iliopsoas tendon and injection of the iliopsoas bursa for diagnosis and management of the painful snapping hip. Skeletal Radiol. 2006;35:565-571.
33. Adler RS, Buly R, Ambrose R, et al. Diagnostic and therapeutic use of sonography-guided iliopsoas peritendinous injections. AJR Am J Roentgenol. 2005;185:940-943.
34. Anderson SA, Keene JS. Results of arthroscopic iliopsoas tendon release in competitive and recreational athletes. Am J Sports Med. 2008;36:2363-2371.
35. Maffey L, Emery C. What are the risk factors for groin strain injury in sport? Sports Med. 2007;37:881-894.
36. Verrall GM, Slavotinek JP, Fon GT, et al. Outcome of conservative management of athletic chronic groin injury diagnosed as pubic bone stress injury. Am J Sports Med. 2007;35:467-474.
37. Pointinger H, Munk P, Poeschl GP. Avulsion fracture of the anterior superior iliac spine following apophysitis. Br J Sports Med. 2003;37:361-362.
38. Unverzagt CA, Schuemann T, Mathisen J. Differential diagnosis of a sports hernia in a high-school athlete. J Orthop Sports Phys Ther. 2008;38:63-70.
39. Caudill P, Nyland J, Smith C, et al. Sports hernias: a systematic literature review. Br J Sports Med. 2008;42:954-964.
40. Ahumada LA, Ashruf S, Espinosa-de-los-Monteros A, et al. Athletic pubalgia: definition and surgical treatment. Ann Plast Surg. 2005;55:393-396.
41. Anderson K, Strickland SM, Warren R. Hip and groin injuries in athletes. Am J Sports Med. 2001;29:521-533.
42. Petchprapa CN, Rosenberg ZS, Sconfienza LM, et al. MR imaging of entrapment neuropathies of the lower extremity. Part I. Radiographs. 2010;30:983-1000.
43. Voight M, Robinson K, Gill L, et al. Postoperative guidelines for hip arthroscopy in the active population. Sports Health. 2010;2:222-230.
44. Stalzer S, Wahoff M, Scanlan M. Rehabilitation following hip arthroscopy. Clin Sports Med. 2006;25:337-357.
1. Margo K, Drezner J, Motzkin D. Evaluation and management of hip pain: an algorithmic approach. J Fam Pract. 2003;52:607-617.
2. Leunig M, Beaule PE, Ganz R. The concept of femoroacetabular impingement: current status and future perspectives. Clin Orthop Relat Res. 2009;467:616-622.
3. Enseki KR, Martin RL, Draovitch P, et al. The hip joint: arthroscopic procedures and postoperative rehabilitation J Orthop Sports Phys Ther. 2006;36:516-525.
4. Byrd JW, Jones KS. Diagnostic accuracy of clinical assessment, magnetic resonance imaging, magnetic resonance arthrography, and intra-articular injection in hip arthroscopy patients. Am J Sports Med. 2004;32:1668-1674.
5. Fredericson M, Jennings F, Beaulieu C, et al. Stress fractures in athletes. Top Magn Reson Imaging. 2006;17:309-325.
6. Matheson GO, Clement DB, McKenzie DC, et al. Stress fractures in athletes. A study of 320 cases. Am J Sports Med. 1987;15:46-58.
7. Stanitski CL, McMaster JH, Scranton PE. On the nature of stress fractures. Am J Sports Med. 1978;6:391-396.
8. Sofka CM. Imaging of stress fractures. Clin Sports Med. 2006;25:53-62, viii.
9. Shin AY, Gillingham BL. Fatigue fractures of the femoral neck in athletes. J Am Acad Orthop Surg. 1997;5:293-302.
10. Mont MA, Hungerford DS. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1995;77:459-474.
11. Lavernia CJ, Sierra RJ, Gomez-Marin O. Smoking and joint replacement: resource consumption and short-term outcome. Clin Orthop Relat Res. 1999;(367):172-180.
12. Lavernia CJ, Sierra RJ, Grieco FR. Osteonecrosis of the femoral head. J Am Acad Orthop Surg. 1999;7:250-261.
13. Smith SW, Fehring TK, Griffin WL, Beaver WB. Core decompression of the osteonecrotic femoral head. J Bone Joint Surg Am. 1995;77:674-680.
14. Fairbank AC, Bhatia D, Jinnah RH, et al. Long-term results of core decompression for ischaemic necrosis of the femoral head. J Bone Joint Surg Br. 1995;77:42-49.
15. Chandler HP, Reineck FT, Wixson RL, et al. Total hip replacement in patients younger than thirty years old. A five-year follow-up study. J Bone Joint Surg Am. 1981;63:1426-1434.
16. Wei SY, Klimkiewicz JJ, Lai M, et al. Revision total hip arthroplasty in patients with avascular necrosis. Orthopedics. 1999;22:747-757.
17. Bedi A, Chen N, Robertson W, et al. The management of labral tears and femoroacetabular impingement of the hip in the young, active patient. Arthroscopy. 2008;24:1135-1145.
18. Guanche CA, Bare AA. Arthroscopic treatment of femoroacetabular impingement. Arthroscopy. 2006;22:95-106.
19. Philippon M, Schenker M, Briggs K, et al. Femoroacetabular impingement in 45 professional athletes: Knee Surg Sports Traumatol Arthrosc. 2007;15:908-914.
20. Sierra RJ, Trousdale RT, Ganz R, et al. Hip disease in the young, active patient: evaluation and nonarthroplasty surgical options. J Am Acad Orthop Surg. 2008;16:689-703.
21. Byrd JW, Jones KS. Prospective analysis of hip arthroscopy with 10-year followup. Clin Orthop Relat Res. 2009;468:741-746.
22. Burnett RS, Della Rocca GJ, Prather H, et al. Clinical presentation of patients with tears of the acetabular labrum. J Bone Joint Surg Am. 2006;88:1448-1457.
23. Bare AA, Guanche CA. Hip impingement: the role of arthroscopy. Orthopedics. 2005;28:266-273.
24. Toomayan GA, Holman WR, Major NM, et al. Sensitivity of MR arthrography in the evaluation of acetabular labral tears. AJR Am J Roentgenol. 2006;186:449-453.
25. Robertson WJ, Kadrmas WR, Kelly BT. Arthroscopic management of labral tears in the hip: a systematic review of the literature. Clin Orthop Relat Res. 2007;455:88-92.
26. Kamath AF, Componovo R, Baldwin K, et al. Hip arthroscopy for labral tears: review of clinical outcomes with 4.8-year mean follow-up. Am J Sports Med. 2009;37:1721-1727.
27. Larson CM, Giveans MR. Arthroscopic debridement versus refixation of the acetabular labrum associated with femoroacetabular impingement. Arthroscopy. 2009;25:369-376.
28. Larson CM, Guanche CA, Kelly BT, et al. Advanced techniques in hip arthroscopy. Instr Course Lect. 2009;58:423-436.
29. Tibor LM, Sekiya JK. Differential diagnosis of pain around the hip joint. Arthroscopy. 2008;24:1407-1421.
30. Holmich P. Long-standing groin pain in sportspeople falls into three primary patterns, a “clinical entity” approach: a prospective study of 207 patients. Br J Sports Med. 2007;41:247-252.
31. Winston P, Awan R, Cassidy JD, et al. Clinical examination and ultrasound of self-reported snapping hip syndrome in elite ballet dancers. Am J Sports Med. 2007;35:118-126.
32. Blankenbaker DG, De Smet AA, Keene JS. Sonography of the iliopsoas tendon and injection of the iliopsoas bursa for diagnosis and management of the painful snapping hip. Skeletal Radiol. 2006;35:565-571.
33. Adler RS, Buly R, Ambrose R, et al. Diagnostic and therapeutic use of sonography-guided iliopsoas peritendinous injections. AJR Am J Roentgenol. 2005;185:940-943.
34. Anderson SA, Keene JS. Results of arthroscopic iliopsoas tendon release in competitive and recreational athletes. Am J Sports Med. 2008;36:2363-2371.
35. Maffey L, Emery C. What are the risk factors for groin strain injury in sport? Sports Med. 2007;37:881-894.
36. Verrall GM, Slavotinek JP, Fon GT, et al. Outcome of conservative management of athletic chronic groin injury diagnosed as pubic bone stress injury. Am J Sports Med. 2007;35:467-474.
37. Pointinger H, Munk P, Poeschl GP. Avulsion fracture of the anterior superior iliac spine following apophysitis. Br J Sports Med. 2003;37:361-362.
38. Unverzagt CA, Schuemann T, Mathisen J. Differential diagnosis of a sports hernia in a high-school athlete. J Orthop Sports Phys Ther. 2008;38:63-70.
39. Caudill P, Nyland J, Smith C, et al. Sports hernias: a systematic literature review. Br J Sports Med. 2008;42:954-964.
40. Ahumada LA, Ashruf S, Espinosa-de-los-Monteros A, et al. Athletic pubalgia: definition and surgical treatment. Ann Plast Surg. 2005;55:393-396.
41. Anderson K, Strickland SM, Warren R. Hip and groin injuries in athletes. Am J Sports Med. 2001;29:521-533.
42. Petchprapa CN, Rosenberg ZS, Sconfienza LM, et al. MR imaging of entrapment neuropathies of the lower extremity. Part I. Radiographs. 2010;30:983-1000.
43. Voight M, Robinson K, Gill L, et al. Postoperative guidelines for hip arthroscopy in the active population. Sports Health. 2010;2:222-230.
44. Stalzer S, Wahoff M, Scanlan M. Rehabilitation following hip arthroscopy. Clin Sports Med. 2006;25:337-357.