User login
Partial, whole breast irradiation 10-year outcomes similar
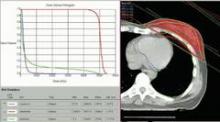
SAN FRANCISCO – Ten years of follow-up showed no significant difference in breast cancer locoregional recurrence, distant metastasis, or survival rates in 274 patients treated with accelerated partial breast irradiation compared with 274 matched patients treated with whole breast irradiation.
The data came from records on 3,009 patients with early-stage breast cancer who were treated with breast-conserving therapy at one institution between 1980 and 2012.
Four percent in each group developed local recurrence, 1% in each group had a regional recurrence, and 6% had distant metastases after partial breast irradiation and 3%, after whole breast irradiation. There was a nonsignificant statistical trend toward a higher rate of contralateral breast failure in the whole breast irradiation group (9%) compared with the partial breast irradiation group (3%, P = .06), Dr. Jessica Wobb reported in a poster presentation at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
Rates of disease-free survival were 91% in the partial breast irradiation group and 93% in the whole breast irradiation group. Cause-specific survival rates were 93% and 94%, respectively, and overall survival rates were 75% and 82%, reported Dr. Wobb of the Beaumont Cancer Institute, Royal Oak, Mich. None of these differences reached statistical significance.
This is one of the first reports on prolonged follow-up after accelerated partial breast irradiation, she noted. Mean follow-up was 7.8 years after partial breast irradiation and 8.1 years after whole breast irradiation, a difference that was statistically significant, but amounted to less than 4 months. All patients were followed for at least 1 year.
Patients in the cohorts were matched by age (within 3 years); T stage (Tis, T1, or T2); and estrogen receptor (ER) status. The mean age was 63 years of age in both groups. Eighty-eight percent in both groups had ER-positive tumors. The stage distribution in both groups consisted of 18% with stage Tis tumors, 71% with T1 tumors, and 11% with T2 tumors.
Significantly fewer patients in the partial breast irradiation group received adjuvant hormonal therapy (54%) compared with those in the whole breast irradiation group (68%). There was a trend toward smaller tumors in patients undergoing partial breast irradiation than in those receiving whole breast irradiation, with mean tumor sizes of 11.4 mm and 13 mm (P = .06).
Other characteristics were similar between the groups, including the proportion with negative lymph nodes (91% of patients undergoing partial breast irradiation and 86% of those who got whole breast irradiation), the proportion with negative final margins (94% and 95%, respectively), and the proportion who received adjuvant chemotherapy (15% and 18%).
Close tumor margins increased the risk for ipsilateral breast tumor recurrence in both groups, and positive margins increased the recurrence risk in the whole breast irradiation group, a univariate analysis found.
Dr. Wobb reported having no relevant financial disclosures.
On Twitter @sherryboschert
In the absence of prospective, randomized trial data on accelerated partial breast irradiation to guide us, we are left with the accumulation of institutional data. The institution that, in my opinion, has contributed most to our knowledge base is the group at William Beaumont Hospital. We’re fortunate to have an update of their experience in that they’ve performed an updated a matched-pair analysis looking at their partial breast irradiation patients (using interstitial catheter or balloon-based brachytherapy two different techniques), compared with their whole breast irradiation patients.
In this matched-pair comparison, the investigators saw no difference in local failure, regional failure, distant metastases, or overall survival.

|
|
Of course, we have to ask, in a matched pair, how good is the match? We do notice that in their group it’s a pretty good match, but we see that for whole breast irradiation, there are slightly larger tumors in that cohort and slightly more positive-node patients. Perhaps the most unsettling aspect is that there is more hormonal therapy in the whole breast irradiation group. This could reflect two things: One is an imbalance in prognostic factors between the two cohorts; the other is an impact of hormonal therapy on local and regional control outcomes.
When we look at their results related to clinical variables and outcome, not surprisingly we find that a negative margin is always better irrespective of whether the patient is getting whole breast irradiation or partial breast irradiation. Interestingly, in the partial breast irradiation group, younger age was associated with a higher risk of local failure.
What’s missing from this analysis? Again, this is not a fault of the investigators; just by virtue of this being a retrospective collection of data, it’s sometimes hard to get all this data. The questions that I think are pertinent in 2013 relate to grade, triple-negative phenotype versus other phenotypes, human epidermal growth factor receptor 2 status, and lymphatic vascular invasion. Unfortunately, none of that information is present in this analysis.
Dr. David E. Wazer is a professor of radiation oncology at Brown University, Providence, R.I. These are excerpts of his remarks as the discussant of Dr. Wobb’s study at the meeting. Dr. Wazer reported financial associations with the American Brachytherapy Society, Advanced Radiation Therapy, and American Journal of Clinical Oncology.
In the absence of prospective, randomized trial data on accelerated partial breast irradiation to guide us, we are left with the accumulation of institutional data. The institution that, in my opinion, has contributed most to our knowledge base is the group at William Beaumont Hospital. We’re fortunate to have an update of their experience in that they’ve performed an updated a matched-pair analysis looking at their partial breast irradiation patients (using interstitial catheter or balloon-based brachytherapy two different techniques), compared with their whole breast irradiation patients.
In this matched-pair comparison, the investigators saw no difference in local failure, regional failure, distant metastases, or overall survival.

|
|
Of course, we have to ask, in a matched pair, how good is the match? We do notice that in their group it’s a pretty good match, but we see that for whole breast irradiation, there are slightly larger tumors in that cohort and slightly more positive-node patients. Perhaps the most unsettling aspect is that there is more hormonal therapy in the whole breast irradiation group. This could reflect two things: One is an imbalance in prognostic factors between the two cohorts; the other is an impact of hormonal therapy on local and regional control outcomes.
When we look at their results related to clinical variables and outcome, not surprisingly we find that a negative margin is always better irrespective of whether the patient is getting whole breast irradiation or partial breast irradiation. Interestingly, in the partial breast irradiation group, younger age was associated with a higher risk of local failure.
What’s missing from this analysis? Again, this is not a fault of the investigators; just by virtue of this being a retrospective collection of data, it’s sometimes hard to get all this data. The questions that I think are pertinent in 2013 relate to grade, triple-negative phenotype versus other phenotypes, human epidermal growth factor receptor 2 status, and lymphatic vascular invasion. Unfortunately, none of that information is present in this analysis.
Dr. David E. Wazer is a professor of radiation oncology at Brown University, Providence, R.I. These are excerpts of his remarks as the discussant of Dr. Wobb’s study at the meeting. Dr. Wazer reported financial associations with the American Brachytherapy Society, Advanced Radiation Therapy, and American Journal of Clinical Oncology.
In the absence of prospective, randomized trial data on accelerated partial breast irradiation to guide us, we are left with the accumulation of institutional data. The institution that, in my opinion, has contributed most to our knowledge base is the group at William Beaumont Hospital. We’re fortunate to have an update of their experience in that they’ve performed an updated a matched-pair analysis looking at their partial breast irradiation patients (using interstitial catheter or balloon-based brachytherapy two different techniques), compared with their whole breast irradiation patients.
In this matched-pair comparison, the investigators saw no difference in local failure, regional failure, distant metastases, or overall survival.

|
|
Of course, we have to ask, in a matched pair, how good is the match? We do notice that in their group it’s a pretty good match, but we see that for whole breast irradiation, there are slightly larger tumors in that cohort and slightly more positive-node patients. Perhaps the most unsettling aspect is that there is more hormonal therapy in the whole breast irradiation group. This could reflect two things: One is an imbalance in prognostic factors between the two cohorts; the other is an impact of hormonal therapy on local and regional control outcomes.
When we look at their results related to clinical variables and outcome, not surprisingly we find that a negative margin is always better irrespective of whether the patient is getting whole breast irradiation or partial breast irradiation. Interestingly, in the partial breast irradiation group, younger age was associated with a higher risk of local failure.
What’s missing from this analysis? Again, this is not a fault of the investigators; just by virtue of this being a retrospective collection of data, it’s sometimes hard to get all this data. The questions that I think are pertinent in 2013 relate to grade, triple-negative phenotype versus other phenotypes, human epidermal growth factor receptor 2 status, and lymphatic vascular invasion. Unfortunately, none of that information is present in this analysis.
Dr. David E. Wazer is a professor of radiation oncology at Brown University, Providence, R.I. These are excerpts of his remarks as the discussant of Dr. Wobb’s study at the meeting. Dr. Wazer reported financial associations with the American Brachytherapy Society, Advanced Radiation Therapy, and American Journal of Clinical Oncology.
SAN FRANCISCO – Ten years of follow-up showed no significant difference in breast cancer locoregional recurrence, distant metastasis, or survival rates in 274 patients treated with accelerated partial breast irradiation compared with 274 matched patients treated with whole breast irradiation.
The data came from records on 3,009 patients with early-stage breast cancer who were treated with breast-conserving therapy at one institution between 1980 and 2012.
Four percent in each group developed local recurrence, 1% in each group had a regional recurrence, and 6% had distant metastases after partial breast irradiation and 3%, after whole breast irradiation. There was a nonsignificant statistical trend toward a higher rate of contralateral breast failure in the whole breast irradiation group (9%) compared with the partial breast irradiation group (3%, P = .06), Dr. Jessica Wobb reported in a poster presentation at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
Rates of disease-free survival were 91% in the partial breast irradiation group and 93% in the whole breast irradiation group. Cause-specific survival rates were 93% and 94%, respectively, and overall survival rates were 75% and 82%, reported Dr. Wobb of the Beaumont Cancer Institute, Royal Oak, Mich. None of these differences reached statistical significance.
This is one of the first reports on prolonged follow-up after accelerated partial breast irradiation, she noted. Mean follow-up was 7.8 years after partial breast irradiation and 8.1 years after whole breast irradiation, a difference that was statistically significant, but amounted to less than 4 months. All patients were followed for at least 1 year.
Patients in the cohorts were matched by age (within 3 years); T stage (Tis, T1, or T2); and estrogen receptor (ER) status. The mean age was 63 years of age in both groups. Eighty-eight percent in both groups had ER-positive tumors. The stage distribution in both groups consisted of 18% with stage Tis tumors, 71% with T1 tumors, and 11% with T2 tumors.
Significantly fewer patients in the partial breast irradiation group received adjuvant hormonal therapy (54%) compared with those in the whole breast irradiation group (68%). There was a trend toward smaller tumors in patients undergoing partial breast irradiation than in those receiving whole breast irradiation, with mean tumor sizes of 11.4 mm and 13 mm (P = .06).
Other characteristics were similar between the groups, including the proportion with negative lymph nodes (91% of patients undergoing partial breast irradiation and 86% of those who got whole breast irradiation), the proportion with negative final margins (94% and 95%, respectively), and the proportion who received adjuvant chemotherapy (15% and 18%).
Close tumor margins increased the risk for ipsilateral breast tumor recurrence in both groups, and positive margins increased the recurrence risk in the whole breast irradiation group, a univariate analysis found.
Dr. Wobb reported having no relevant financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Ten years of follow-up showed no significant difference in breast cancer locoregional recurrence, distant metastasis, or survival rates in 274 patients treated with accelerated partial breast irradiation compared with 274 matched patients treated with whole breast irradiation.
The data came from records on 3,009 patients with early-stage breast cancer who were treated with breast-conserving therapy at one institution between 1980 and 2012.
Four percent in each group developed local recurrence, 1% in each group had a regional recurrence, and 6% had distant metastases after partial breast irradiation and 3%, after whole breast irradiation. There was a nonsignificant statistical trend toward a higher rate of contralateral breast failure in the whole breast irradiation group (9%) compared with the partial breast irradiation group (3%, P = .06), Dr. Jessica Wobb reported in a poster presentation at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
Rates of disease-free survival were 91% in the partial breast irradiation group and 93% in the whole breast irradiation group. Cause-specific survival rates were 93% and 94%, respectively, and overall survival rates were 75% and 82%, reported Dr. Wobb of the Beaumont Cancer Institute, Royal Oak, Mich. None of these differences reached statistical significance.
This is one of the first reports on prolonged follow-up after accelerated partial breast irradiation, she noted. Mean follow-up was 7.8 years after partial breast irradiation and 8.1 years after whole breast irradiation, a difference that was statistically significant, but amounted to less than 4 months. All patients were followed for at least 1 year.
Patients in the cohorts were matched by age (within 3 years); T stage (Tis, T1, or T2); and estrogen receptor (ER) status. The mean age was 63 years of age in both groups. Eighty-eight percent in both groups had ER-positive tumors. The stage distribution in both groups consisted of 18% with stage Tis tumors, 71% with T1 tumors, and 11% with T2 tumors.
Significantly fewer patients in the partial breast irradiation group received adjuvant hormonal therapy (54%) compared with those in the whole breast irradiation group (68%). There was a trend toward smaller tumors in patients undergoing partial breast irradiation than in those receiving whole breast irradiation, with mean tumor sizes of 11.4 mm and 13 mm (P = .06).
Other characteristics were similar between the groups, including the proportion with negative lymph nodes (91% of patients undergoing partial breast irradiation and 86% of those who got whole breast irradiation), the proportion with negative final margins (94% and 95%, respectively), and the proportion who received adjuvant chemotherapy (15% and 18%).
Close tumor margins increased the risk for ipsilateral breast tumor recurrence in both groups, and positive margins increased the recurrence risk in the whole breast irradiation group, a univariate analysis found.
Dr. Wobb reported having no relevant financial disclosures.
On Twitter @sherryboschert
AT THE ASCO BREAST CANCER SYMPOSIUM
Major finding: Four percent developed local recurrence and 1% had regional recurrence in both partial and whole breast irradiation groups. For the partial vs. whole breast irradiation groups, distant metastases developed in 6% and 3%, disease-free survival rates were 91% and 93%, and overall survival rates were 75% and 82.
Data source: A retrospective study of 274 matched pairs of patients with early-stage breast cancer treated with breast-conserving therapy at one institution.
Disclosures: Dr. Wobb reported having no relevant financial disclosures.
Heart irradiation is lower with contemporary breast radiotherapy
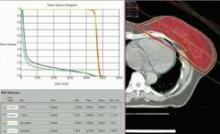
SAN FRANCISCO – The hearts of 100 consecutive patients who underwent adjuvant radiotherapy for left-sided breast cancer in 2011 received an average of 2.9 Gray of radiation, considerably less than the mean cardiac exposure of 4.9-Gy reported in a recent review of 2,168 patients treated from 1958 to 2001 in Sweden and Denmark.
The findings confirm that three-dimensional conformal radiation therapy (3D-CRT) reduces cardiac exposure to radiation, Dr. Federico Lonardi and his associates reported at a breast cancer symposium sponsored by the American Society of Clinical Oncology. But certain areas of the heart still receive high doses when patients have adverse anatomic conditions that are not well suited to 3D-CRT. Because heart structures may differ in radiosensitivity, higher doses to small volumes of the heart, such as the coronary artery, might be associated with more risk, the researchers cautioned.
Most patients received a mean cardiac dose of 2-3 Gy (32%), 21% of patients were exposed to 1.15-1.99 Gy, and 1% got 0.8 Gy in a study of a consecutive series of breast cancer patients treated at Mater Salutis Hospital in Legnago, Italy. Only 17% of patients received a mean cardiac dose of more than 5 Gy, and 13% received 4.16-4.83 Gy.
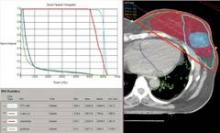
The cardiac dose ranged from 0.8 to 13.05 Gy in Dr. Lonardi’s study, compared with a range of 0.03 to 27.72 Gy in the recently published Scandinavian study (N. Engl. J. Med. 2013;368:987-998). In the published study, the longitudinal risk for major cardiac events increased in a linear fashion, with a 7% increase for cardiac events with every 1 Gy increase in radiation to the heart.
In the Italian study, the median volumes of heart exposed to higher doses of radiation were "consistently low" with 4% of heart volumes exposed to 5 Gy or more, 3% exposed to 10Gy or more, 2% exposed to 15 Gy or more, and 0.7% exposed to 25 Gy or more Dr. Lonardi reported.
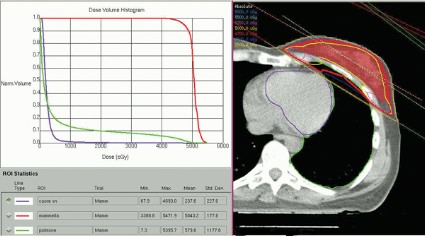
These patients received full-breast 3D-CRT with two to four customized tangential fields after mastectomy (10% of patients) or quadrantectomy (90%). The whole breast (or chest wall) received 50 Gy/25 fractions in 66 patients and 45 Gy/18 fractions in 34 patients. Boost to surgical bed (10 Gy/4-5 fractions) was delivered by photons in 10 patients. Median number of tangential fields was two (range, two to four). Patients were treated while supine on a breast board, without immobilization devices or instructions to hold their breath. They were freely breathing but were asked to minimize respiratory motion during the CT scan used to plan radiation delivery and the treatment itself. No dose constraints were specified for heart structures; a mean heart dose lower than 5 Gy was recommended at the time of treatment.
A preliminary assessment of radiation delivered to the left anterior descending coronary arteries in this series suggests that they received 9-25 Gy, Dr. Lonardi reported.
Based on estimates using previous models, the probability of death from cardiac causes within 15 years after standard fractionated radiotherapy may be less than 1% if less than 10% of the heart is exposed to 25Gy or more, he noted. "In this perspective, our results appear very favorable, though they confirm that the heart may receive high doses to limited volumes despite the use of standard 3D techniques. In such cases, high-conformal, intensity-modulated techniques are helpful" to further reduce the exposure of critical heart structures to radiation.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Dr. Lonardi reported having no financial disclosures.
On Twitter @sherryboschert
This was an interesting study. I really give the authors a lot of credit. They basically looked at 100 consecutive cases of patients treated with adjuvant radiotherapy for left-sided breast cancer and brought forward what they were doing without trying to optimize or minimize the presentation or cardiac dose in the patients they were treating.

|
| Dr. Julia White |
Their mean doses were lower than those reported by Darby et al. in a study that looked at 2,168 patients treated during 1958-2001 in both Sweden and Denmark. The researchers reviewed individual radiotherapy charts, and then did 20 consecutive individual CT-based three-dimensional planning scans to model what the type of radiotherapy looked like many years ago (N. Engl. J. Med. 2013;368:987-98).
This was an interesting study. I really give the authors a lot of credit. They basically looked at 100 consecutive cases of patients treated with adjuvant radiotherapy for left-sided breast cancer and brought forward what they were doing without trying to optimize or minimize the presentation or cardiac dose in the patients they were treating.

|
| Dr. Julia White |
Their mean doses were lower than those reported by Darby et al. in a study that looked at 2,168 patients treated during 1958-2001 in both Sweden and Denmark. The researchers reviewed individual radiotherapy charts, and then did 20 consecutive individual CT-based three-dimensional planning scans to model what the type of radiotherapy looked like many years ago (N. Engl. J. Med. 2013;368:987-98).
This was an interesting study. I really give the authors a lot of credit. They basically looked at 100 consecutive cases of patients treated with adjuvant radiotherapy for left-sided breast cancer and brought forward what they were doing without trying to optimize or minimize the presentation or cardiac dose in the patients they were treating.

|
| Dr. Julia White |
Their mean doses were lower than those reported by Darby et al. in a study that looked at 2,168 patients treated during 1958-2001 in both Sweden and Denmark. The researchers reviewed individual radiotherapy charts, and then did 20 consecutive individual CT-based three-dimensional planning scans to model what the type of radiotherapy looked like many years ago (N. Engl. J. Med. 2013;368:987-98).
SAN FRANCISCO – The hearts of 100 consecutive patients who underwent adjuvant radiotherapy for left-sided breast cancer in 2011 received an average of 2.9 Gray of radiation, considerably less than the mean cardiac exposure of 4.9-Gy reported in a recent review of 2,168 patients treated from 1958 to 2001 in Sweden and Denmark.
The findings confirm that three-dimensional conformal radiation therapy (3D-CRT) reduces cardiac exposure to radiation, Dr. Federico Lonardi and his associates reported at a breast cancer symposium sponsored by the American Society of Clinical Oncology. But certain areas of the heart still receive high doses when patients have adverse anatomic conditions that are not well suited to 3D-CRT. Because heart structures may differ in radiosensitivity, higher doses to small volumes of the heart, such as the coronary artery, might be associated with more risk, the researchers cautioned.
Most patients received a mean cardiac dose of 2-3 Gy (32%), 21% of patients were exposed to 1.15-1.99 Gy, and 1% got 0.8 Gy in a study of a consecutive series of breast cancer patients treated at Mater Salutis Hospital in Legnago, Italy. Only 17% of patients received a mean cardiac dose of more than 5 Gy, and 13% received 4.16-4.83 Gy.
The cardiac dose ranged from 0.8 to 13.05 Gy in Dr. Lonardi’s study, compared with a range of 0.03 to 27.72 Gy in the recently published Scandinavian study (N. Engl. J. Med. 2013;368:987-998). In the published study, the longitudinal risk for major cardiac events increased in a linear fashion, with a 7% increase for cardiac events with every 1 Gy increase in radiation to the heart.
In the Italian study, the median volumes of heart exposed to higher doses of radiation were "consistently low" with 4% of heart volumes exposed to 5 Gy or more, 3% exposed to 10Gy or more, 2% exposed to 15 Gy or more, and 0.7% exposed to 25 Gy or more Dr. Lonardi reported.
These patients received full-breast 3D-CRT with two to four customized tangential fields after mastectomy (10% of patients) or quadrantectomy (90%). The whole breast (or chest wall) received 50 Gy/25 fractions in 66 patients and 45 Gy/18 fractions in 34 patients. Boost to surgical bed (10 Gy/4-5 fractions) was delivered by photons in 10 patients. Median number of tangential fields was two (range, two to four). Patients were treated while supine on a breast board, without immobilization devices or instructions to hold their breath. They were freely breathing but were asked to minimize respiratory motion during the CT scan used to plan radiation delivery and the treatment itself. No dose constraints were specified for heart structures; a mean heart dose lower than 5 Gy was recommended at the time of treatment.
A preliminary assessment of radiation delivered to the left anterior descending coronary arteries in this series suggests that they received 9-25 Gy, Dr. Lonardi reported.
Based on estimates using previous models, the probability of death from cardiac causes within 15 years after standard fractionated radiotherapy may be less than 1% if less than 10% of the heart is exposed to 25Gy or more, he noted. "In this perspective, our results appear very favorable, though they confirm that the heart may receive high doses to limited volumes despite the use of standard 3D techniques. In such cases, high-conformal, intensity-modulated techniques are helpful" to further reduce the exposure of critical heart structures to radiation.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Dr. Lonardi reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – The hearts of 100 consecutive patients who underwent adjuvant radiotherapy for left-sided breast cancer in 2011 received an average of 2.9 Gray of radiation, considerably less than the mean cardiac exposure of 4.9-Gy reported in a recent review of 2,168 patients treated from 1958 to 2001 in Sweden and Denmark.
The findings confirm that three-dimensional conformal radiation therapy (3D-CRT) reduces cardiac exposure to radiation, Dr. Federico Lonardi and his associates reported at a breast cancer symposium sponsored by the American Society of Clinical Oncology. But certain areas of the heart still receive high doses when patients have adverse anatomic conditions that are not well suited to 3D-CRT. Because heart structures may differ in radiosensitivity, higher doses to small volumes of the heart, such as the coronary artery, might be associated with more risk, the researchers cautioned.
Most patients received a mean cardiac dose of 2-3 Gy (32%), 21% of patients were exposed to 1.15-1.99 Gy, and 1% got 0.8 Gy in a study of a consecutive series of breast cancer patients treated at Mater Salutis Hospital in Legnago, Italy. Only 17% of patients received a mean cardiac dose of more than 5 Gy, and 13% received 4.16-4.83 Gy.
The cardiac dose ranged from 0.8 to 13.05 Gy in Dr. Lonardi’s study, compared with a range of 0.03 to 27.72 Gy in the recently published Scandinavian study (N. Engl. J. Med. 2013;368:987-998). In the published study, the longitudinal risk for major cardiac events increased in a linear fashion, with a 7% increase for cardiac events with every 1 Gy increase in radiation to the heart.
In the Italian study, the median volumes of heart exposed to higher doses of radiation were "consistently low" with 4% of heart volumes exposed to 5 Gy or more, 3% exposed to 10Gy or more, 2% exposed to 15 Gy or more, and 0.7% exposed to 25 Gy or more Dr. Lonardi reported.
These patients received full-breast 3D-CRT with two to four customized tangential fields after mastectomy (10% of patients) or quadrantectomy (90%). The whole breast (or chest wall) received 50 Gy/25 fractions in 66 patients and 45 Gy/18 fractions in 34 patients. Boost to surgical bed (10 Gy/4-5 fractions) was delivered by photons in 10 patients. Median number of tangential fields was two (range, two to four). Patients were treated while supine on a breast board, without immobilization devices or instructions to hold their breath. They were freely breathing but were asked to minimize respiratory motion during the CT scan used to plan radiation delivery and the treatment itself. No dose constraints were specified for heart structures; a mean heart dose lower than 5 Gy was recommended at the time of treatment.
A preliminary assessment of radiation delivered to the left anterior descending coronary arteries in this series suggests that they received 9-25 Gy, Dr. Lonardi reported.
Based on estimates using previous models, the probability of death from cardiac causes within 15 years after standard fractionated radiotherapy may be less than 1% if less than 10% of the heart is exposed to 25Gy or more, he noted. "In this perspective, our results appear very favorable, though they confirm that the heart may receive high doses to limited volumes despite the use of standard 3D techniques. In such cases, high-conformal, intensity-modulated techniques are helpful" to further reduce the exposure of critical heart structures to radiation.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Dr. Lonardi reported having no financial disclosures.
On Twitter @sherryboschert
AT THE ASCO BREAST CANCER SYMPOSIUM
Major finding: In an Italian study, the median volumes of heart exposed to higher doses of radiation were "consistently low" with 4% of heart volumes exposed to 5 Gy or more, 3% exposed to 10Gy or more, 2% exposed to 15 Gy or more, and 0.7% exposed to 25 Gy or more.
Data source: Retrospective review of 100 consecutive patients treated with radiotherapy for left-sided breast cancer at one institution in 2011.
Disclosures: Dr. Lonardi reported having no financial disclosures.
Inadequate neuromuscular blockade common during surgery
SAN FRANCISCO – Patients under general anesthesia may be getting insufficient neuromuscular blockade in 1%-45% of operations, depending on the definition, according to several studies presented in a joint session at the annual meeting of the American Society of Anesthesiologists.
Regardless of the exact definition, the findings suggest that the problem of insufficient blockade is considerably more common than expected, the anesthesiologists in attendance agreed.
A lack of clinical guidelines for neuromuscular blockade probably contributes to the problem, some speakers suggested. There is no established definition of insufficient neuromuscular blockade, which has been associated in prior studies with compromised surgical visualization, impaired ventilation leading to barotraumas, direct injury through unexpected movement, and other complications.
Investigators presented their results in posters and in a joint discussion session at the meeting. All studies were sponsored by Merck, which markets a neuromuscular blocking agent (rocuronium bromide, or Zemuron) and is seeking U.S. approval for a drug that rapidly reverses neuromuscular blockade (sugammadex, or Bridion).
One percent of 129,209 adults who underwent general anesthesia and received a nondepolarizing neuromuscular blockade agent in 2005-2013 experienced insufficient blockade in a way that interrupted surgery, either through undesired patient movement (0.3%) or an explicit request from the surgeon for additional muscle relaxation and administration of more neuromuscular blockade (0.7%), Dr. Timur Dubovoy and his associates reported.
They also found indirect evidence of insufficient neuromuscular blockade through two other criteria that were much more common, said Dr. Dubovoy of the University of Michigan, Ann Arbor. Anesthesiologists gave more neuromuscular blockade after documenting twitches on peripheral nerve stimulation (train-of-four monitoring) in 39% of patients, indicative of unintended recovery from neuromuscular blockade. Large or even "excessive" maintenance doses were given to 45% of patients, consistent with insufficient neuromuscular blockade, he said.
Those kinds of events typically don’t interrupt a procedure but can lead to residual neuromuscular blockade due to excessive dosing, potentially increasing complications and delaying recovery after anesthesia. The study looked only at the incidence of insufficient neuromuscular blockade, however, not outcomes.
"Current use of nondepolarizing neuromuscular blockade agents and subjective tactile train-of-four monitoring frequently exposes patients to inadequate neuromuscular blockade," Dr. Dubovoy said.
In a separate study, insufficient neuromuscular blockade affected 21%-28% of 48,315 adults undergoing abdominal, laparoscopic, and interventional neurovascular procedures at the Cleveland Clinic in 2005-2013, Dr. Brian D. Hesler and his associates reported.
"Our results suggest that insufficient block is relatively common, even in operations that are generally thought to require muscle relaxation," said Dr. Hesler of the Cleveland Clinic. "It is difficult to separate inadequate anesthesia from inadequate neuromuscular block, and both probably contributed in many cases."
He and his associates formed a panel of seven experienced anesthesiologists to identify anesthesiology actions that are indicative of episodes of insufficient neuromuscular block and searched for those criteria in patient records, with a three-person adjudication committee approving the search criteria through a random sample of at least 50 charts for each criterion.
Overall, 28% of operations had evidence of insufficient neuromuscular blockade, or 21% if the investigators excluded cases identified solely by electromyogram criteria.
In a separate analysis of the same cohort, Dr. Hesler and his associates searched for comments in the anesthetic records and found that insufficient blockade usually was identified more than 30 minutes before emergence, defined as the time when maintenance anesthesia was discontinued (106 cases), but 18% of the time it occurred 15-30 minutes before emergence (9 cases) or less than 15 minutes before emergence (14 cases).
The closer to the end of surgery, the more likely the anesthesiologist was to respond by deepening anesthesia instead of redosing the neuromuscular blocking agent, with other sedatives (opioids) used at a consistent rate in each time period.
A separate prospective, observational study of 448 patients undergoing elective laparoscopic or open abdominal surgical procedures at eight Canadian centers in 2011-2012 stratified residual neuromuscular blockade by train-of-four (TOF) ratios.
Lower TOF ratios at tracheal extubation and at arrival in the postanesthesia care unit (PACU) were associated with greater risk for complications and greater use of perioperative resources, Dr. Dolores McKeen and her associates reported.
Every 0.1-increment increase in the TOF ratio at tracheal extubation was associated with a 30% reduction in the odds of needing placement of an oral or nasal airway due to upper airway obstruction from the time of patient extubation to PACU discharge. Each 0.1-increment increase in the TOF ratio at tracheal extubation also was associated with 3% fewer bed visits by nurses, said Dr. McKeen of Dalhousie University, Halifax, N.S. Similar results were seen for TOF ratios upon arrival at the PACU.
This suggests that "more effective strategies to prevent and/or manage residual neuromuscular blockade are required to minimize the impact on the patient and health care provider," she said.
The incidence of postoperative residual neuromuscular blockade was 19% for patients with a TOF ratio less than 0.6 at tracheal extubation, 12% with a ratio of 0.6-0.7, 9% with a ratio of 0.7-0.8, 16% with a ratio of 0.8-0.9, and 44% with a ratio of 0.9 or greater. The incidence of residual blockade was 8% for patients with a TOF ratio less than 0.6 upon arrival to the PACU, 7% with a ratio of 0.6-0.7, 14% with a ratio of 0.7-0.8, 16% with a ratio of 0.8-0.9, and 56% with a ratio of 0.9 or greater.
In the United States, neostigmine, an acetylcholinesterase inhibitor, is the most common means of reversing neuromuscular blocking agents, according to Scott Devine, Ph.D., of Merck. Giving neostigmine too early can be ineffective, and giving it too late might induce skeletal muscle weakness.
He and his associates analyzed data from the Anesthesia Quality Institute’s National Anesthesia Clinical Outcomes Registry (NACOR) on 113,276 procedures utilizing rocuronium or vecuronium that were reversed with neostigmine in 2010-2012. The reversal agent was given a mean of 63 minutes after the last dose of a neuromuscular blocking agent, 7 minutes before surgical site closure, 14 minutes prior to emergence, and 29 minutes before the end of anesthesia time, though each administration time had a wide range, he reported.
A substantial number of patients would have spontaneously recovered from the effects of the neuromuscular blockers after 63 minutes, suggesting that neostigmine often may be given later than needed, he said. If neostigmine is given 7 minutes before surgical site closure, reversal of neuromuscular blockade could be well underway, resulting in increased muscle tension during surgical site closure, which could increase the risk of complications such as dehiscence or postsurgical hernias, he added.
The large variability in practice may be due to multiple factors and deserves further research, he said.
Merck, which markets a neuromuscular blockade agent, sponsored the studies and supplied at least one investigator for each study.
[email protected] On Twitter @sherryboschert
One part of what we anesthesiologists do, other than keeping patients asleep and pain free, is to have them in a condition so that surgeons can do their operations optimally. If patients are not relaxed, and if muscles are not relaxed, that makes the surgical conditions more difficult, which could potentially lead to longer duration of surgery, more complications, and things like that. So this topic is actually very important to surgeons.

|
|
In these studies, the neuromuscular blockade frequently seems to be insufficient, which is a little bit of a surprise. We hear from our surgery colleagues fairly often during surgery, "Give more muscle relaxants," because they have their hands in the field and they feel what’s going on. But anesthesiologists haven’t always agreed that it’s necessary to give more muscle relaxants, because we thought surgeons overestimated the conditions. It does seem that insufficient muscle relaxation is much more common than we had thought.
Many of these studies come from the Cleveland Clinic, so we’ve gone through their data in detail. We probably will be a little bit more liberal with muscle relaxation, which is difficult. If you overrelax patients, then it takes us longer to extubate them, which means we don’t get out of the OR before the surgeon comes to start the next case. So it’s a give and take.
That’s why to a certain extent it’s important that there are drugs available that promptly, within seconds, reverse our muscle relaxants. But they are not available in the United States yet. We are still waiting. Europe, South America – everybody is already using them.
It’s important for anesthesia and surgery to work together and see whether any of this actually does improve intermediate- or long-term outcomes of patients. That’s ultimately the goal.
Dr. Andrea Kurz is a professor and vice-chair of the Anesthesiology Institute at the Cleveland Clinic. She gave these remarks in an interview. Dr. Kurz reported having no financial disclosures.
One part of what we anesthesiologists do, other than keeping patients asleep and pain free, is to have them in a condition so that surgeons can do their operations optimally. If patients are not relaxed, and if muscles are not relaxed, that makes the surgical conditions more difficult, which could potentially lead to longer duration of surgery, more complications, and things like that. So this topic is actually very important to surgeons.

|
|
In these studies, the neuromuscular blockade frequently seems to be insufficient, which is a little bit of a surprise. We hear from our surgery colleagues fairly often during surgery, "Give more muscle relaxants," because they have their hands in the field and they feel what’s going on. But anesthesiologists haven’t always agreed that it’s necessary to give more muscle relaxants, because we thought surgeons overestimated the conditions. It does seem that insufficient muscle relaxation is much more common than we had thought.
Many of these studies come from the Cleveland Clinic, so we’ve gone through their data in detail. We probably will be a little bit more liberal with muscle relaxation, which is difficult. If you overrelax patients, then it takes us longer to extubate them, which means we don’t get out of the OR before the surgeon comes to start the next case. So it’s a give and take.
That’s why to a certain extent it’s important that there are drugs available that promptly, within seconds, reverse our muscle relaxants. But they are not available in the United States yet. We are still waiting. Europe, South America – everybody is already using them.
It’s important for anesthesia and surgery to work together and see whether any of this actually does improve intermediate- or long-term outcomes of patients. That’s ultimately the goal.
Dr. Andrea Kurz is a professor and vice-chair of the Anesthesiology Institute at the Cleveland Clinic. She gave these remarks in an interview. Dr. Kurz reported having no financial disclosures.
One part of what we anesthesiologists do, other than keeping patients asleep and pain free, is to have them in a condition so that surgeons can do their operations optimally. If patients are not relaxed, and if muscles are not relaxed, that makes the surgical conditions more difficult, which could potentially lead to longer duration of surgery, more complications, and things like that. So this topic is actually very important to surgeons.

|
|
In these studies, the neuromuscular blockade frequently seems to be insufficient, which is a little bit of a surprise. We hear from our surgery colleagues fairly often during surgery, "Give more muscle relaxants," because they have their hands in the field and they feel what’s going on. But anesthesiologists haven’t always agreed that it’s necessary to give more muscle relaxants, because we thought surgeons overestimated the conditions. It does seem that insufficient muscle relaxation is much more common than we had thought.
Many of these studies come from the Cleveland Clinic, so we’ve gone through their data in detail. We probably will be a little bit more liberal with muscle relaxation, which is difficult. If you overrelax patients, then it takes us longer to extubate them, which means we don’t get out of the OR before the surgeon comes to start the next case. So it’s a give and take.
That’s why to a certain extent it’s important that there are drugs available that promptly, within seconds, reverse our muscle relaxants. But they are not available in the United States yet. We are still waiting. Europe, South America – everybody is already using them.
It’s important for anesthesia and surgery to work together and see whether any of this actually does improve intermediate- or long-term outcomes of patients. That’s ultimately the goal.
Dr. Andrea Kurz is a professor and vice-chair of the Anesthesiology Institute at the Cleveland Clinic. She gave these remarks in an interview. Dr. Kurz reported having no financial disclosures.
SAN FRANCISCO – Patients under general anesthesia may be getting insufficient neuromuscular blockade in 1%-45% of operations, depending on the definition, according to several studies presented in a joint session at the annual meeting of the American Society of Anesthesiologists.
Regardless of the exact definition, the findings suggest that the problem of insufficient blockade is considerably more common than expected, the anesthesiologists in attendance agreed.
A lack of clinical guidelines for neuromuscular blockade probably contributes to the problem, some speakers suggested. There is no established definition of insufficient neuromuscular blockade, which has been associated in prior studies with compromised surgical visualization, impaired ventilation leading to barotraumas, direct injury through unexpected movement, and other complications.
Investigators presented their results in posters and in a joint discussion session at the meeting. All studies were sponsored by Merck, which markets a neuromuscular blocking agent (rocuronium bromide, or Zemuron) and is seeking U.S. approval for a drug that rapidly reverses neuromuscular blockade (sugammadex, or Bridion).
One percent of 129,209 adults who underwent general anesthesia and received a nondepolarizing neuromuscular blockade agent in 2005-2013 experienced insufficient blockade in a way that interrupted surgery, either through undesired patient movement (0.3%) or an explicit request from the surgeon for additional muscle relaxation and administration of more neuromuscular blockade (0.7%), Dr. Timur Dubovoy and his associates reported.
They also found indirect evidence of insufficient neuromuscular blockade through two other criteria that were much more common, said Dr. Dubovoy of the University of Michigan, Ann Arbor. Anesthesiologists gave more neuromuscular blockade after documenting twitches on peripheral nerve stimulation (train-of-four monitoring) in 39% of patients, indicative of unintended recovery from neuromuscular blockade. Large or even "excessive" maintenance doses were given to 45% of patients, consistent with insufficient neuromuscular blockade, he said.
Those kinds of events typically don’t interrupt a procedure but can lead to residual neuromuscular blockade due to excessive dosing, potentially increasing complications and delaying recovery after anesthesia. The study looked only at the incidence of insufficient neuromuscular blockade, however, not outcomes.
"Current use of nondepolarizing neuromuscular blockade agents and subjective tactile train-of-four monitoring frequently exposes patients to inadequate neuromuscular blockade," Dr. Dubovoy said.
In a separate study, insufficient neuromuscular blockade affected 21%-28% of 48,315 adults undergoing abdominal, laparoscopic, and interventional neurovascular procedures at the Cleveland Clinic in 2005-2013, Dr. Brian D. Hesler and his associates reported.
"Our results suggest that insufficient block is relatively common, even in operations that are generally thought to require muscle relaxation," said Dr. Hesler of the Cleveland Clinic. "It is difficult to separate inadequate anesthesia from inadequate neuromuscular block, and both probably contributed in many cases."
He and his associates formed a panel of seven experienced anesthesiologists to identify anesthesiology actions that are indicative of episodes of insufficient neuromuscular block and searched for those criteria in patient records, with a three-person adjudication committee approving the search criteria through a random sample of at least 50 charts for each criterion.
Overall, 28% of operations had evidence of insufficient neuromuscular blockade, or 21% if the investigators excluded cases identified solely by electromyogram criteria.
In a separate analysis of the same cohort, Dr. Hesler and his associates searched for comments in the anesthetic records and found that insufficient blockade usually was identified more than 30 minutes before emergence, defined as the time when maintenance anesthesia was discontinued (106 cases), but 18% of the time it occurred 15-30 minutes before emergence (9 cases) or less than 15 minutes before emergence (14 cases).
The closer to the end of surgery, the more likely the anesthesiologist was to respond by deepening anesthesia instead of redosing the neuromuscular blocking agent, with other sedatives (opioids) used at a consistent rate in each time period.
A separate prospective, observational study of 448 patients undergoing elective laparoscopic or open abdominal surgical procedures at eight Canadian centers in 2011-2012 stratified residual neuromuscular blockade by train-of-four (TOF) ratios.
Lower TOF ratios at tracheal extubation and at arrival in the postanesthesia care unit (PACU) were associated with greater risk for complications and greater use of perioperative resources, Dr. Dolores McKeen and her associates reported.
Every 0.1-increment increase in the TOF ratio at tracheal extubation was associated with a 30% reduction in the odds of needing placement of an oral or nasal airway due to upper airway obstruction from the time of patient extubation to PACU discharge. Each 0.1-increment increase in the TOF ratio at tracheal extubation also was associated with 3% fewer bed visits by nurses, said Dr. McKeen of Dalhousie University, Halifax, N.S. Similar results were seen for TOF ratios upon arrival at the PACU.
This suggests that "more effective strategies to prevent and/or manage residual neuromuscular blockade are required to minimize the impact on the patient and health care provider," she said.
The incidence of postoperative residual neuromuscular blockade was 19% for patients with a TOF ratio less than 0.6 at tracheal extubation, 12% with a ratio of 0.6-0.7, 9% with a ratio of 0.7-0.8, 16% with a ratio of 0.8-0.9, and 44% with a ratio of 0.9 or greater. The incidence of residual blockade was 8% for patients with a TOF ratio less than 0.6 upon arrival to the PACU, 7% with a ratio of 0.6-0.7, 14% with a ratio of 0.7-0.8, 16% with a ratio of 0.8-0.9, and 56% with a ratio of 0.9 or greater.
In the United States, neostigmine, an acetylcholinesterase inhibitor, is the most common means of reversing neuromuscular blocking agents, according to Scott Devine, Ph.D., of Merck. Giving neostigmine too early can be ineffective, and giving it too late might induce skeletal muscle weakness.
He and his associates analyzed data from the Anesthesia Quality Institute’s National Anesthesia Clinical Outcomes Registry (NACOR) on 113,276 procedures utilizing rocuronium or vecuronium that were reversed with neostigmine in 2010-2012. The reversal agent was given a mean of 63 minutes after the last dose of a neuromuscular blocking agent, 7 minutes before surgical site closure, 14 minutes prior to emergence, and 29 minutes before the end of anesthesia time, though each administration time had a wide range, he reported.
A substantial number of patients would have spontaneously recovered from the effects of the neuromuscular blockers after 63 minutes, suggesting that neostigmine often may be given later than needed, he said. If neostigmine is given 7 minutes before surgical site closure, reversal of neuromuscular blockade could be well underway, resulting in increased muscle tension during surgical site closure, which could increase the risk of complications such as dehiscence or postsurgical hernias, he added.
The large variability in practice may be due to multiple factors and deserves further research, he said.
Merck, which markets a neuromuscular blockade agent, sponsored the studies and supplied at least one investigator for each study.
[email protected] On Twitter @sherryboschert
SAN FRANCISCO – Patients under general anesthesia may be getting insufficient neuromuscular blockade in 1%-45% of operations, depending on the definition, according to several studies presented in a joint session at the annual meeting of the American Society of Anesthesiologists.
Regardless of the exact definition, the findings suggest that the problem of insufficient blockade is considerably more common than expected, the anesthesiologists in attendance agreed.
A lack of clinical guidelines for neuromuscular blockade probably contributes to the problem, some speakers suggested. There is no established definition of insufficient neuromuscular blockade, which has been associated in prior studies with compromised surgical visualization, impaired ventilation leading to barotraumas, direct injury through unexpected movement, and other complications.
Investigators presented their results in posters and in a joint discussion session at the meeting. All studies were sponsored by Merck, which markets a neuromuscular blocking agent (rocuronium bromide, or Zemuron) and is seeking U.S. approval for a drug that rapidly reverses neuromuscular blockade (sugammadex, or Bridion).
One percent of 129,209 adults who underwent general anesthesia and received a nondepolarizing neuromuscular blockade agent in 2005-2013 experienced insufficient blockade in a way that interrupted surgery, either through undesired patient movement (0.3%) or an explicit request from the surgeon for additional muscle relaxation and administration of more neuromuscular blockade (0.7%), Dr. Timur Dubovoy and his associates reported.
They also found indirect evidence of insufficient neuromuscular blockade through two other criteria that were much more common, said Dr. Dubovoy of the University of Michigan, Ann Arbor. Anesthesiologists gave more neuromuscular blockade after documenting twitches on peripheral nerve stimulation (train-of-four monitoring) in 39% of patients, indicative of unintended recovery from neuromuscular blockade. Large or even "excessive" maintenance doses were given to 45% of patients, consistent with insufficient neuromuscular blockade, he said.
Those kinds of events typically don’t interrupt a procedure but can lead to residual neuromuscular blockade due to excessive dosing, potentially increasing complications and delaying recovery after anesthesia. The study looked only at the incidence of insufficient neuromuscular blockade, however, not outcomes.
"Current use of nondepolarizing neuromuscular blockade agents and subjective tactile train-of-four monitoring frequently exposes patients to inadequate neuromuscular blockade," Dr. Dubovoy said.
In a separate study, insufficient neuromuscular blockade affected 21%-28% of 48,315 adults undergoing abdominal, laparoscopic, and interventional neurovascular procedures at the Cleveland Clinic in 2005-2013, Dr. Brian D. Hesler and his associates reported.
"Our results suggest that insufficient block is relatively common, even in operations that are generally thought to require muscle relaxation," said Dr. Hesler of the Cleveland Clinic. "It is difficult to separate inadequate anesthesia from inadequate neuromuscular block, and both probably contributed in many cases."
He and his associates formed a panel of seven experienced anesthesiologists to identify anesthesiology actions that are indicative of episodes of insufficient neuromuscular block and searched for those criteria in patient records, with a three-person adjudication committee approving the search criteria through a random sample of at least 50 charts for each criterion.
Overall, 28% of operations had evidence of insufficient neuromuscular blockade, or 21% if the investigators excluded cases identified solely by electromyogram criteria.
In a separate analysis of the same cohort, Dr. Hesler and his associates searched for comments in the anesthetic records and found that insufficient blockade usually was identified more than 30 minutes before emergence, defined as the time when maintenance anesthesia was discontinued (106 cases), but 18% of the time it occurred 15-30 minutes before emergence (9 cases) or less than 15 minutes before emergence (14 cases).
The closer to the end of surgery, the more likely the anesthesiologist was to respond by deepening anesthesia instead of redosing the neuromuscular blocking agent, with other sedatives (opioids) used at a consistent rate in each time period.
A separate prospective, observational study of 448 patients undergoing elective laparoscopic or open abdominal surgical procedures at eight Canadian centers in 2011-2012 stratified residual neuromuscular blockade by train-of-four (TOF) ratios.
Lower TOF ratios at tracheal extubation and at arrival in the postanesthesia care unit (PACU) were associated with greater risk for complications and greater use of perioperative resources, Dr. Dolores McKeen and her associates reported.
Every 0.1-increment increase in the TOF ratio at tracheal extubation was associated with a 30% reduction in the odds of needing placement of an oral or nasal airway due to upper airway obstruction from the time of patient extubation to PACU discharge. Each 0.1-increment increase in the TOF ratio at tracheal extubation also was associated with 3% fewer bed visits by nurses, said Dr. McKeen of Dalhousie University, Halifax, N.S. Similar results were seen for TOF ratios upon arrival at the PACU.
This suggests that "more effective strategies to prevent and/or manage residual neuromuscular blockade are required to minimize the impact on the patient and health care provider," she said.
The incidence of postoperative residual neuromuscular blockade was 19% for patients with a TOF ratio less than 0.6 at tracheal extubation, 12% with a ratio of 0.6-0.7, 9% with a ratio of 0.7-0.8, 16% with a ratio of 0.8-0.9, and 44% with a ratio of 0.9 or greater. The incidence of residual blockade was 8% for patients with a TOF ratio less than 0.6 upon arrival to the PACU, 7% with a ratio of 0.6-0.7, 14% with a ratio of 0.7-0.8, 16% with a ratio of 0.8-0.9, and 56% with a ratio of 0.9 or greater.
In the United States, neostigmine, an acetylcholinesterase inhibitor, is the most common means of reversing neuromuscular blocking agents, according to Scott Devine, Ph.D., of Merck. Giving neostigmine too early can be ineffective, and giving it too late might induce skeletal muscle weakness.
He and his associates analyzed data from the Anesthesia Quality Institute’s National Anesthesia Clinical Outcomes Registry (NACOR) on 113,276 procedures utilizing rocuronium or vecuronium that were reversed with neostigmine in 2010-2012. The reversal agent was given a mean of 63 minutes after the last dose of a neuromuscular blocking agent, 7 minutes before surgical site closure, 14 minutes prior to emergence, and 29 minutes before the end of anesthesia time, though each administration time had a wide range, he reported.
A substantial number of patients would have spontaneously recovered from the effects of the neuromuscular blockers after 63 minutes, suggesting that neostigmine often may be given later than needed, he said. If neostigmine is given 7 minutes before surgical site closure, reversal of neuromuscular blockade could be well underway, resulting in increased muscle tension during surgical site closure, which could increase the risk of complications such as dehiscence or postsurgical hernias, he added.
The large variability in practice may be due to multiple factors and deserves further research, he said.
Merck, which markets a neuromuscular blockade agent, sponsored the studies and supplied at least one investigator for each study.
[email protected] On Twitter @sherryboschert
AT THE ASA ANNUAL MEETING
Major finding: The incidence of inadequate neuromuscular blockade during surgery ranged from 1% to 45%, depending on the definition.
Data source: Multiple retrospective studies of adults undergoing surgery with general anesthesia who received neuromuscular blocking agents.
Disclosures: Merck, which markets a neuromuscular blockade reversal agent, sponsored the studies and supplied at least one investigator for each study.
Medical conferences going digital
The medical conferences of the future made a preview appearance at this year’s Transcatheter Cardiovascular Therapeutics annual meeting in San Francisco. Paperless, electronic, interactive, and definitely high tech it was.
Every paid attendee was offered a new Samsung tablet computer, preloaded with pertinent apps and information, to personalize and keep if they wanted or return at the end of the meeting. If attendees preferred to download the apps to their own devices, that was fine too, and many of them did. (I got a loaner through the press room, and found it easy to use.)
Rather than tack the cost of the tablets onto registration fees, the organizers shifted funds from the no-longer-needed bulky printed programs and other materials to pay for the tablets, according to the Cardiovascular Research Foundation, cosponsor of the TCT meeting with the American College of Cardiology. No funds from industry were solicited for the tablets, no advertising appeared on the home screens, and the tablets were not being used to mine for user data of any kind, but the preloaded apps did contain some advertisements.
Paperless medical conferences are not new – many conferences eschew pulp these days, providing materials on zip drives instead of printed programs that attendees can load onto their computers. And apps for the larger medical conferences now are commonplace, too, for those who have their own smartphones or tablets. But this is the first time I’ve seen a conference give out tablets and include interactive social media features, convenient continuing medical education mechanisms, and more.
Through the apps, attendees could navigate the convention center; view abstracts; download speaker slides and disclosures; watch live cases; take notes; contact some faculty; find shuttle buses, hotels, and restaurants; and access exhibition materials. After attending a session, they could log their hours, write a review, and apply for CME credits through the apps. If they were willing to enable certain settings, they could see who else at the meeting was in their vicinity, and communicate with them.
Each of the major sessions I covered included a "digital moderator" in addition to the regular moderator. Instead of standing in line at microphones to ask questions, members of the audience texted comments and questions that appeared on a screen to the side of the main screen showing the presenter’s slides, so everyone could see them in real time. This feature wasn’t as much used as one might fear – doctors were still paying attention to the speaker, not staring down at their devices, for the most part. From what I could see, the digital moderators provided most of the texted comments and questions, though at one session the live moderator noted that audience texts were asking the speaker to comment about stroke risk, so he raised the question.
Keep in mind, the TCT always has been one of the most high-tech conferences happening in a very high-tech specialty, interventional cardiology. The typical setup in their main forum was similar to that in past meetings, a multitasking-palooza featuring a long dais of speakers and multiple video screens, with individual headsets that let you tune into whichever "channel" interests you most at the moment. Screens with live cases flank either end, with the presenter and his or her slides in the middle and screens promoting upcoming sessions and showing the audience texts in between the other screens.
TCT comes to San Francisco regularly because the city has the infrastructure to support these technologic demands, a spokeswoman in their press room told me. Some other locations haven’t been able to handle their needs.
I wondered if the technology will be so appealing that attendees might prefer virtual attendance rather than having to be there. It’s possible, she said, but unlikely. Like most people, these doctors value their face time.
On Twitter @sherryboschert
The medical conferences of the future made a preview appearance at this year’s Transcatheter Cardiovascular Therapeutics annual meeting in San Francisco. Paperless, electronic, interactive, and definitely high tech it was.
Every paid attendee was offered a new Samsung tablet computer, preloaded with pertinent apps and information, to personalize and keep if they wanted or return at the end of the meeting. If attendees preferred to download the apps to their own devices, that was fine too, and many of them did. (I got a loaner through the press room, and found it easy to use.)
Rather than tack the cost of the tablets onto registration fees, the organizers shifted funds from the no-longer-needed bulky printed programs and other materials to pay for the tablets, according to the Cardiovascular Research Foundation, cosponsor of the TCT meeting with the American College of Cardiology. No funds from industry were solicited for the tablets, no advertising appeared on the home screens, and the tablets were not being used to mine for user data of any kind, but the preloaded apps did contain some advertisements.
Paperless medical conferences are not new – many conferences eschew pulp these days, providing materials on zip drives instead of printed programs that attendees can load onto their computers. And apps for the larger medical conferences now are commonplace, too, for those who have their own smartphones or tablets. But this is the first time I’ve seen a conference give out tablets and include interactive social media features, convenient continuing medical education mechanisms, and more.
Through the apps, attendees could navigate the convention center; view abstracts; download speaker slides and disclosures; watch live cases; take notes; contact some faculty; find shuttle buses, hotels, and restaurants; and access exhibition materials. After attending a session, they could log their hours, write a review, and apply for CME credits through the apps. If they were willing to enable certain settings, they could see who else at the meeting was in their vicinity, and communicate with them.
Each of the major sessions I covered included a "digital moderator" in addition to the regular moderator. Instead of standing in line at microphones to ask questions, members of the audience texted comments and questions that appeared on a screen to the side of the main screen showing the presenter’s slides, so everyone could see them in real time. This feature wasn’t as much used as one might fear – doctors were still paying attention to the speaker, not staring down at their devices, for the most part. From what I could see, the digital moderators provided most of the texted comments and questions, though at one session the live moderator noted that audience texts were asking the speaker to comment about stroke risk, so he raised the question.
Keep in mind, the TCT always has been one of the most high-tech conferences happening in a very high-tech specialty, interventional cardiology. The typical setup in their main forum was similar to that in past meetings, a multitasking-palooza featuring a long dais of speakers and multiple video screens, with individual headsets that let you tune into whichever "channel" interests you most at the moment. Screens with live cases flank either end, with the presenter and his or her slides in the middle and screens promoting upcoming sessions and showing the audience texts in between the other screens.
TCT comes to San Francisco regularly because the city has the infrastructure to support these technologic demands, a spokeswoman in their press room told me. Some other locations haven’t been able to handle their needs.
I wondered if the technology will be so appealing that attendees might prefer virtual attendance rather than having to be there. It’s possible, she said, but unlikely. Like most people, these doctors value their face time.
On Twitter @sherryboschert
The medical conferences of the future made a preview appearance at this year’s Transcatheter Cardiovascular Therapeutics annual meeting in San Francisco. Paperless, electronic, interactive, and definitely high tech it was.
Every paid attendee was offered a new Samsung tablet computer, preloaded with pertinent apps and information, to personalize and keep if they wanted or return at the end of the meeting. If attendees preferred to download the apps to their own devices, that was fine too, and many of them did. (I got a loaner through the press room, and found it easy to use.)
Rather than tack the cost of the tablets onto registration fees, the organizers shifted funds from the no-longer-needed bulky printed programs and other materials to pay for the tablets, according to the Cardiovascular Research Foundation, cosponsor of the TCT meeting with the American College of Cardiology. No funds from industry were solicited for the tablets, no advertising appeared on the home screens, and the tablets were not being used to mine for user data of any kind, but the preloaded apps did contain some advertisements.
Paperless medical conferences are not new – many conferences eschew pulp these days, providing materials on zip drives instead of printed programs that attendees can load onto their computers. And apps for the larger medical conferences now are commonplace, too, for those who have their own smartphones or tablets. But this is the first time I’ve seen a conference give out tablets and include interactive social media features, convenient continuing medical education mechanisms, and more.
Through the apps, attendees could navigate the convention center; view abstracts; download speaker slides and disclosures; watch live cases; take notes; contact some faculty; find shuttle buses, hotels, and restaurants; and access exhibition materials. After attending a session, they could log their hours, write a review, and apply for CME credits through the apps. If they were willing to enable certain settings, they could see who else at the meeting was in their vicinity, and communicate with them.
Each of the major sessions I covered included a "digital moderator" in addition to the regular moderator. Instead of standing in line at microphones to ask questions, members of the audience texted comments and questions that appeared on a screen to the side of the main screen showing the presenter’s slides, so everyone could see them in real time. This feature wasn’t as much used as one might fear – doctors were still paying attention to the speaker, not staring down at their devices, for the most part. From what I could see, the digital moderators provided most of the texted comments and questions, though at one session the live moderator noted that audience texts were asking the speaker to comment about stroke risk, so he raised the question.
Keep in mind, the TCT always has been one of the most high-tech conferences happening in a very high-tech specialty, interventional cardiology. The typical setup in their main forum was similar to that in past meetings, a multitasking-palooza featuring a long dais of speakers and multiple video screens, with individual headsets that let you tune into whichever "channel" interests you most at the moment. Screens with live cases flank either end, with the presenter and his or her slides in the middle and screens promoting upcoming sessions and showing the audience texts in between the other screens.
TCT comes to San Francisco regularly because the city has the infrastructure to support these technologic demands, a spokeswoman in their press room told me. Some other locations haven’t been able to handle their needs.
I wondered if the technology will be so appealing that attendees might prefer virtual attendance rather than having to be there. It’s possible, she said, but unlikely. Like most people, these doctors value their face time.
On Twitter @sherryboschert
Adding melatonin to alprazolam boosts preoperative anxiolysis
SAN FRANCISCO – Adding melatonin to alprazolam significantly decreased preoperative anxiety, compared with either medication alone or with placebo, in a randomized, double-blind trial of 80 patients.
Adult patients undergoing laparoscopic cholecystectomy who reported a preoperative anxiety level of at least 3 cm on a 10-cm Visual Analog Scale (VAS) had average anxiety scores of 5 cm before being randomized to preoperative medication with alprazolam 0.5 mg, melatonin 3 mg, both drugs, or placebo (with 20 patients in each group).
After 1 hour spent in a quiet room following the premedication, VAS scores had fallen by an average of 3 cm in the two-drug group, significantly more than average 2-cm reductions with either drug alone, or a 1-cm decline on placebo, Dr. Krishna Pokharel and her associates reported.
Adding melatonin did not seem to worsen the sedative or amnesiac effects of alprazolam, she reported in a poster presentation at the annual meeting of the American Society of Anesthesiologists.
In the past, some of her patients who had been premedicated with a benzodiazepine before general anesthesia and surgery sometimes became aroused during the procedure, perhaps because benzodiazepines suppress endogenous melatonin levels, Dr. Pokharel said. She hypothesized that adding melatonin might help, and the study results have convinced her institution to routinely add melatonin to alprazolam for surgical premedication in anxious patients, said Dr. Pokharel of B.P. Koirala Institute of Health Sciences, Dharan, Nepal.
Patients were shown different pictures during assessments of anxiety and sedation at various time points before surgery. At 24 hours after surgery, 10 patients on alprazolam plus melatonin could recall the picture they saw 1 hour after taking the presurgical medication, compared with 9 patients on alprazolam alone, 18 patients on melatonin alone, and 16 patients on placebo, the poster reported.
In other results, average scores on a 5-point scale for sedation at 1 hour were 0.5 with melatonin, 1 for each group using alprazolam, and 0 with placebo, among other secondary outcomes. At 24 hours after surgery, five patients in the two-drug group could not remember being transferred to the OR, compared with four patients on alprazolam, one patient on melatonin, and none of the patients on placebo.
All groups scored 2 on a 3-point scale for orientation 1 hour after taking the premedication. The amount of propofol needed to achieve a loss of response to verbal commands at the time of general anesthesia induction averaged 66 mg in the alprazolam plus melatonin group, 59 mg after alprazolam alone, 79 mg after melatonin alone, and 76 mg on placebo.
No patients developed serious adverse events.
Dr. Pokharel reported having no financial disclosures.
On Twitter @sherryboschert
laparoscopic cholecystectomy, Visual Analog Scale, VAS, anxiety, Dr. Krishna Pokharel, the American Society of Anesthesiologists, benzodiazepines,
SAN FRANCISCO – Adding melatonin to alprazolam significantly decreased preoperative anxiety, compared with either medication alone or with placebo, in a randomized, double-blind trial of 80 patients.
Adult patients undergoing laparoscopic cholecystectomy who reported a preoperative anxiety level of at least 3 cm on a 10-cm Visual Analog Scale (VAS) had average anxiety scores of 5 cm before being randomized to preoperative medication with alprazolam 0.5 mg, melatonin 3 mg, both drugs, or placebo (with 20 patients in each group).
After 1 hour spent in a quiet room following the premedication, VAS scores had fallen by an average of 3 cm in the two-drug group, significantly more than average 2-cm reductions with either drug alone, or a 1-cm decline on placebo, Dr. Krishna Pokharel and her associates reported.
Adding melatonin did not seem to worsen the sedative or amnesiac effects of alprazolam, she reported in a poster presentation at the annual meeting of the American Society of Anesthesiologists.
In the past, some of her patients who had been premedicated with a benzodiazepine before general anesthesia and surgery sometimes became aroused during the procedure, perhaps because benzodiazepines suppress endogenous melatonin levels, Dr. Pokharel said. She hypothesized that adding melatonin might help, and the study results have convinced her institution to routinely add melatonin to alprazolam for surgical premedication in anxious patients, said Dr. Pokharel of B.P. Koirala Institute of Health Sciences, Dharan, Nepal.
Patients were shown different pictures during assessments of anxiety and sedation at various time points before surgery. At 24 hours after surgery, 10 patients on alprazolam plus melatonin could recall the picture they saw 1 hour after taking the presurgical medication, compared with 9 patients on alprazolam alone, 18 patients on melatonin alone, and 16 patients on placebo, the poster reported.
In other results, average scores on a 5-point scale for sedation at 1 hour were 0.5 with melatonin, 1 for each group using alprazolam, and 0 with placebo, among other secondary outcomes. At 24 hours after surgery, five patients in the two-drug group could not remember being transferred to the OR, compared with four patients on alprazolam, one patient on melatonin, and none of the patients on placebo.
All groups scored 2 on a 3-point scale for orientation 1 hour after taking the premedication. The amount of propofol needed to achieve a loss of response to verbal commands at the time of general anesthesia induction averaged 66 mg in the alprazolam plus melatonin group, 59 mg after alprazolam alone, 79 mg after melatonin alone, and 76 mg on placebo.
No patients developed serious adverse events.
Dr. Pokharel reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Adding melatonin to alprazolam significantly decreased preoperative anxiety, compared with either medication alone or with placebo, in a randomized, double-blind trial of 80 patients.
Adult patients undergoing laparoscopic cholecystectomy who reported a preoperative anxiety level of at least 3 cm on a 10-cm Visual Analog Scale (VAS) had average anxiety scores of 5 cm before being randomized to preoperative medication with alprazolam 0.5 mg, melatonin 3 mg, both drugs, or placebo (with 20 patients in each group).
After 1 hour spent in a quiet room following the premedication, VAS scores had fallen by an average of 3 cm in the two-drug group, significantly more than average 2-cm reductions with either drug alone, or a 1-cm decline on placebo, Dr. Krishna Pokharel and her associates reported.
Adding melatonin did not seem to worsen the sedative or amnesiac effects of alprazolam, she reported in a poster presentation at the annual meeting of the American Society of Anesthesiologists.
In the past, some of her patients who had been premedicated with a benzodiazepine before general anesthesia and surgery sometimes became aroused during the procedure, perhaps because benzodiazepines suppress endogenous melatonin levels, Dr. Pokharel said. She hypothesized that adding melatonin might help, and the study results have convinced her institution to routinely add melatonin to alprazolam for surgical premedication in anxious patients, said Dr. Pokharel of B.P. Koirala Institute of Health Sciences, Dharan, Nepal.
Patients were shown different pictures during assessments of anxiety and sedation at various time points before surgery. At 24 hours after surgery, 10 patients on alprazolam plus melatonin could recall the picture they saw 1 hour after taking the presurgical medication, compared with 9 patients on alprazolam alone, 18 patients on melatonin alone, and 16 patients on placebo, the poster reported.
In other results, average scores on a 5-point scale for sedation at 1 hour were 0.5 with melatonin, 1 for each group using alprazolam, and 0 with placebo, among other secondary outcomes. At 24 hours after surgery, five patients in the two-drug group could not remember being transferred to the OR, compared with four patients on alprazolam, one patient on melatonin, and none of the patients on placebo.
All groups scored 2 on a 3-point scale for orientation 1 hour after taking the premedication. The amount of propofol needed to achieve a loss of response to verbal commands at the time of general anesthesia induction averaged 66 mg in the alprazolam plus melatonin group, 59 mg after alprazolam alone, 79 mg after melatonin alone, and 76 mg on placebo.
No patients developed serious adverse events.
Dr. Pokharel reported having no financial disclosures.
On Twitter @sherryboschert
laparoscopic cholecystectomy, Visual Analog Scale, VAS, anxiety, Dr. Krishna Pokharel, the American Society of Anesthesiologists, benzodiazepines,
laparoscopic cholecystectomy, Visual Analog Scale, VAS, anxiety, Dr. Krishna Pokharel, the American Society of Anesthesiologists, benzodiazepines,
AT THE ASA ANNUAL MEETING
Major finding: Anxiety VAS scores at 60 minutes, compared with baseline, fell by 3 cm with melatonin plus alprazolam, 2 cm with either drug alone, or 1 cm with placebo.
Data source: A prospective, randomized, controlled trial of 80 adults undergoing laparoscopic cholecystectomy at one hospital.
Disclosures: Dr. Pokharel reported having no financial disclosures.
Shorter antiplatelet therapy after stenting found noninferior
SAN FRANCISCO – Adverse event rates 1 year after implantation of a second-generation zotarolimus-eluting coronary stent were similar for 3,120 patients regardless of whether they took 3 months or 12 months of dual-antiplatelet therapy in a prospective trial that randomized 3,120 patients in real-world settings.
Six percent in the 3-month group and 5.8% in the 12-month group developed one or more adverse events that were included in a composite primary endpoint: death from any cause; myocardial infarction; stroke; or major bleeding, Dr. Fausto Feres and his associates reported at the Transcatheter Cardiovascular Therapeutics annual meeting. The results were significant in a noninferiority analysis.
The results should comfort clinicians who think they have to stop dual-antiplatelet therapy (DAPT) earlier than recommended in some patients who are at higher risk for bleeding complications, such as the elderly and patients with a history of hemorrhagic events, said Dr. Feres, an interventional cardiologist at the Instituto Dante Pazzanese de Cardiologia, São Paulo, Brazil, an institution known for pioneering stent procedures.
The study tapped 33 clinical sites to enroll Brazilian patients who were undergoing percutaneous coronary intervention (PCI) with the second-generation Endeavor zotarolimus-eluting stent. All patients had stable or unstable angina or a recent MI, at least one coronary lesion suitable for PCI with the Endeavor stent, and a native vessel diameter of at least 2.5 mm with stenosis greater than 50%. The cohort consisted mainly of patients with stable coronary artery disease and a low risk of acute coronary syndrome.
Equal numbers were randomized to 3 or 12 months of DAPT with aspirin and 75 mg/day of clopidogrel to reduce the risk of thrombotic events. Current guidelines call for 12 months of DAPT after stent implantation. One-year follow-up data were available for 98% in each group.
Known as the OPTIMIZE trial (Optimized Duration of Clopidogrel Therapy Following Treatment With the Endeavor Zotarolimus-Eluting Stent in Real-World Clinical Practice), the study found that more than 99% of patients in each group completed 3 months of DAPT. One year after stent implantation, 6% who had been randomized to the 3-month group were still on DAPT, as were 97% of those randomized to the 12-month group.
The individual components of the combined endpoint did not differ significantly at 1-year follow-up. All-cause mortality was seen in 1.9% and 1.7% of the 3- and 12-month therapy groups; MI rates were 0.8% and 0.6%, respectively; stroke rates were 0.3% and 0.1%; and major bleeding occurred in 0.2% and 0.4%. Landmark analyses also found no significant differences between groups for these endpoints at 3 months.
Rates of definite or probable stent thrombosis at 1 year were 0.3% in the 3-month therapy group and 0.1% in the 12-month group, which was not a significant difference. There was a trend toward higher risk of bleeding at 1 year in patients on 12 months of DAPT, seen in 1% compared with 0.4% of those on 3 months of therapy, Dr. Feres reported at the meeting, cosponsored by the American College of Cardiology.
Secondary outcomes at 1 year showed that rates of other adverse clinical events also did not differ significantly between the two groups. Eight percent in the 3-month group and 7% in the 12-month group developed one or more major adverse coronary events: death, MI, emergent coronary artery bypass grafting, or target lesion revascularization. Three percent in each group died, 3% in each developed an MI, 4% in each had either cardiac death or an MI, and 0.3% in each developed a stroke. Major bleeding occurred in 0.6% of the 3-month group and 0.9% of the 12-month group. Target lesion revascularization rates were 3.5% in the 3-month group and 3.2% in the 12-month group.
Results did not differ by subgroups.
Rates for the primary outcome were lower than expected in both arms of the trial. Investigators expected a 9% rate, not the roughly 6% rate seen in both arms. The rate of major adverse coronary events at 1 year, however, was 8.4% in the 3-month group and 7.5% in the 12-month group.
Two previous studies in Italy and Korea of shortened-duration DAPT for patients receiving drug-eluting stents compared 6 months of therapy with 12 months of therapy. One other previous trial compared 3 months with 12 months of DAPT but compared two different stents, he said. All suggested that 12 months of DAPT may not always be needed, he said in an interview.
The current study excluded patients with primary or rescue PCI for ST-segment elevation MI, lesions located in a saphenous vein graft, patients with a previous PCI with a drug-eluting stent, and other less common exclusion criteria.
The findings from OPTIMIZE were published online concurrently with Dr. Feres’ presentation (JAMA 2013 Oct. 31 [doi: 10.1001/jama.2013.282183]).
Dr. Feres reported having financial relationships with BioSensors, Eli Lilly, and Medtronic, which markets the Endeavor stent and funded the study.
[email protected]
On Twitter @sherryboschert
My concern with this study lies with a noninferiority comparison using a composite primary endpoint that combines both efficacy (ischemic event) and safety (bleeding event) measures. Although this type of endpoint is selected to enhance power, it is problematic because of a lack of actual or expected concordance among its components. When concordant differences are present, the individual components lack power to determine if clinically meaningful differences exist.
In OPTIMIZE, a 10%-14% relative increase in ischemic events with 3 months of DAPT is counterbalanced by a 12% relative decrease in major bleeding. A counterbalance in relationship is graphically depicted in the analysis of stent thrombosis and major bleeding, with a 90-day landmark. Beyond 90 days, the absolute increase in events is 0.2% for both, which represents a fourfold relative increase in stent thrombosis with 3 months of DAPT and a twofold relative increase in major bleeding with longer therapy.
Indeed, the upper boundaries of the confidence intervals allow for a 35-fold increase in stent thrombosis with short treatment and an eightfold increase in major bleeding with longer therapy. This observation must be viewed in the context that OPTIMIZE excluded biomarker-positive acute coronary syndrome (ACS) within 30 days – those patients at greatest risk for stent thrombosis – and included subjects with an average age of 61 years – patients with a lower risk of bleeding events.
The exclusion of biomarker-positive ACS represents a significant portion of contemporary clinical practice, particularly in the context of relative efficacy for PCI and ACS vs. stable ischemic heart disease. Although the choice of clopidogrel vs. novel agents may be justified by exclusion of biomarker-positive ACS, the loading dose was not standardized by protocol. Finally, the Endeavor stent platform is problematic and difficult to extrapolate to other drug-eluting stents. Endeavor has largely been replaced by Resolute, which has a different polymer and drug-release kinetics.
More definitive conclusions about optimal DAPT duration still await the results of adequately powered, more inclusive, and contemporary randomized controlled trials.
Dean J. Kereiakes, M.D., is an interventional cardiologist at the Ohio Heart and Vascular Center, Cincinnati. These are excerpts of his remarks as discussant of the study at the meeting. Dr. Kereiakes reported financial associations with Medpace, HCRI, and other companies.
My concern with this study lies with a noninferiority comparison using a composite primary endpoint that combines both efficacy (ischemic event) and safety (bleeding event) measures. Although this type of endpoint is selected to enhance power, it is problematic because of a lack of actual or expected concordance among its components. When concordant differences are present, the individual components lack power to determine if clinically meaningful differences exist.
In OPTIMIZE, a 10%-14% relative increase in ischemic events with 3 months of DAPT is counterbalanced by a 12% relative decrease in major bleeding. A counterbalance in relationship is graphically depicted in the analysis of stent thrombosis and major bleeding, with a 90-day landmark. Beyond 90 days, the absolute increase in events is 0.2% for both, which represents a fourfold relative increase in stent thrombosis with 3 months of DAPT and a twofold relative increase in major bleeding with longer therapy.
Indeed, the upper boundaries of the confidence intervals allow for a 35-fold increase in stent thrombosis with short treatment and an eightfold increase in major bleeding with longer therapy. This observation must be viewed in the context that OPTIMIZE excluded biomarker-positive acute coronary syndrome (ACS) within 30 days – those patients at greatest risk for stent thrombosis – and included subjects with an average age of 61 years – patients with a lower risk of bleeding events.
The exclusion of biomarker-positive ACS represents a significant portion of contemporary clinical practice, particularly in the context of relative efficacy for PCI and ACS vs. stable ischemic heart disease. Although the choice of clopidogrel vs. novel agents may be justified by exclusion of biomarker-positive ACS, the loading dose was not standardized by protocol. Finally, the Endeavor stent platform is problematic and difficult to extrapolate to other drug-eluting stents. Endeavor has largely been replaced by Resolute, which has a different polymer and drug-release kinetics.
More definitive conclusions about optimal DAPT duration still await the results of adequately powered, more inclusive, and contemporary randomized controlled trials.
Dean J. Kereiakes, M.D., is an interventional cardiologist at the Ohio Heart and Vascular Center, Cincinnati. These are excerpts of his remarks as discussant of the study at the meeting. Dr. Kereiakes reported financial associations with Medpace, HCRI, and other companies.
My concern with this study lies with a noninferiority comparison using a composite primary endpoint that combines both efficacy (ischemic event) and safety (bleeding event) measures. Although this type of endpoint is selected to enhance power, it is problematic because of a lack of actual or expected concordance among its components. When concordant differences are present, the individual components lack power to determine if clinically meaningful differences exist.
In OPTIMIZE, a 10%-14% relative increase in ischemic events with 3 months of DAPT is counterbalanced by a 12% relative decrease in major bleeding. A counterbalance in relationship is graphically depicted in the analysis of stent thrombosis and major bleeding, with a 90-day landmark. Beyond 90 days, the absolute increase in events is 0.2% for both, which represents a fourfold relative increase in stent thrombosis with 3 months of DAPT and a twofold relative increase in major bleeding with longer therapy.
Indeed, the upper boundaries of the confidence intervals allow for a 35-fold increase in stent thrombosis with short treatment and an eightfold increase in major bleeding with longer therapy. This observation must be viewed in the context that OPTIMIZE excluded biomarker-positive acute coronary syndrome (ACS) within 30 days – those patients at greatest risk for stent thrombosis – and included subjects with an average age of 61 years – patients with a lower risk of bleeding events.
The exclusion of biomarker-positive ACS represents a significant portion of contemporary clinical practice, particularly in the context of relative efficacy for PCI and ACS vs. stable ischemic heart disease. Although the choice of clopidogrel vs. novel agents may be justified by exclusion of biomarker-positive ACS, the loading dose was not standardized by protocol. Finally, the Endeavor stent platform is problematic and difficult to extrapolate to other drug-eluting stents. Endeavor has largely been replaced by Resolute, which has a different polymer and drug-release kinetics.
More definitive conclusions about optimal DAPT duration still await the results of adequately powered, more inclusive, and contemporary randomized controlled trials.
Dean J. Kereiakes, M.D., is an interventional cardiologist at the Ohio Heart and Vascular Center, Cincinnati. These are excerpts of his remarks as discussant of the study at the meeting. Dr. Kereiakes reported financial associations with Medpace, HCRI, and other companies.
SAN FRANCISCO – Adverse event rates 1 year after implantation of a second-generation zotarolimus-eluting coronary stent were similar for 3,120 patients regardless of whether they took 3 months or 12 months of dual-antiplatelet therapy in a prospective trial that randomized 3,120 patients in real-world settings.
Six percent in the 3-month group and 5.8% in the 12-month group developed one or more adverse events that were included in a composite primary endpoint: death from any cause; myocardial infarction; stroke; or major bleeding, Dr. Fausto Feres and his associates reported at the Transcatheter Cardiovascular Therapeutics annual meeting. The results were significant in a noninferiority analysis.
The results should comfort clinicians who think they have to stop dual-antiplatelet therapy (DAPT) earlier than recommended in some patients who are at higher risk for bleeding complications, such as the elderly and patients with a history of hemorrhagic events, said Dr. Feres, an interventional cardiologist at the Instituto Dante Pazzanese de Cardiologia, São Paulo, Brazil, an institution known for pioneering stent procedures.
The study tapped 33 clinical sites to enroll Brazilian patients who were undergoing percutaneous coronary intervention (PCI) with the second-generation Endeavor zotarolimus-eluting stent. All patients had stable or unstable angina or a recent MI, at least one coronary lesion suitable for PCI with the Endeavor stent, and a native vessel diameter of at least 2.5 mm with stenosis greater than 50%. The cohort consisted mainly of patients with stable coronary artery disease and a low risk of acute coronary syndrome.
Equal numbers were randomized to 3 or 12 months of DAPT with aspirin and 75 mg/day of clopidogrel to reduce the risk of thrombotic events. Current guidelines call for 12 months of DAPT after stent implantation. One-year follow-up data were available for 98% in each group.
Known as the OPTIMIZE trial (Optimized Duration of Clopidogrel Therapy Following Treatment With the Endeavor Zotarolimus-Eluting Stent in Real-World Clinical Practice), the study found that more than 99% of patients in each group completed 3 months of DAPT. One year after stent implantation, 6% who had been randomized to the 3-month group were still on DAPT, as were 97% of those randomized to the 12-month group.
The individual components of the combined endpoint did not differ significantly at 1-year follow-up. All-cause mortality was seen in 1.9% and 1.7% of the 3- and 12-month therapy groups; MI rates were 0.8% and 0.6%, respectively; stroke rates were 0.3% and 0.1%; and major bleeding occurred in 0.2% and 0.4%. Landmark analyses also found no significant differences between groups for these endpoints at 3 months.
Rates of definite or probable stent thrombosis at 1 year were 0.3% in the 3-month therapy group and 0.1% in the 12-month group, which was not a significant difference. There was a trend toward higher risk of bleeding at 1 year in patients on 12 months of DAPT, seen in 1% compared with 0.4% of those on 3 months of therapy, Dr. Feres reported at the meeting, cosponsored by the American College of Cardiology.
Secondary outcomes at 1 year showed that rates of other adverse clinical events also did not differ significantly between the two groups. Eight percent in the 3-month group and 7% in the 12-month group developed one or more major adverse coronary events: death, MI, emergent coronary artery bypass grafting, or target lesion revascularization. Three percent in each group died, 3% in each developed an MI, 4% in each had either cardiac death or an MI, and 0.3% in each developed a stroke. Major bleeding occurred in 0.6% of the 3-month group and 0.9% of the 12-month group. Target lesion revascularization rates were 3.5% in the 3-month group and 3.2% in the 12-month group.
Results did not differ by subgroups.
Rates for the primary outcome were lower than expected in both arms of the trial. Investigators expected a 9% rate, not the roughly 6% rate seen in both arms. The rate of major adverse coronary events at 1 year, however, was 8.4% in the 3-month group and 7.5% in the 12-month group.
Two previous studies in Italy and Korea of shortened-duration DAPT for patients receiving drug-eluting stents compared 6 months of therapy with 12 months of therapy. One other previous trial compared 3 months with 12 months of DAPT but compared two different stents, he said. All suggested that 12 months of DAPT may not always be needed, he said in an interview.
The current study excluded patients with primary or rescue PCI for ST-segment elevation MI, lesions located in a saphenous vein graft, patients with a previous PCI with a drug-eluting stent, and other less common exclusion criteria.
The findings from OPTIMIZE were published online concurrently with Dr. Feres’ presentation (JAMA 2013 Oct. 31 [doi: 10.1001/jama.2013.282183]).
Dr. Feres reported having financial relationships with BioSensors, Eli Lilly, and Medtronic, which markets the Endeavor stent and funded the study.
[email protected]
On Twitter @sherryboschert
SAN FRANCISCO – Adverse event rates 1 year after implantation of a second-generation zotarolimus-eluting coronary stent were similar for 3,120 patients regardless of whether they took 3 months or 12 months of dual-antiplatelet therapy in a prospective trial that randomized 3,120 patients in real-world settings.
Six percent in the 3-month group and 5.8% in the 12-month group developed one or more adverse events that were included in a composite primary endpoint: death from any cause; myocardial infarction; stroke; or major bleeding, Dr. Fausto Feres and his associates reported at the Transcatheter Cardiovascular Therapeutics annual meeting. The results were significant in a noninferiority analysis.
The results should comfort clinicians who think they have to stop dual-antiplatelet therapy (DAPT) earlier than recommended in some patients who are at higher risk for bleeding complications, such as the elderly and patients with a history of hemorrhagic events, said Dr. Feres, an interventional cardiologist at the Instituto Dante Pazzanese de Cardiologia, São Paulo, Brazil, an institution known for pioneering stent procedures.
The study tapped 33 clinical sites to enroll Brazilian patients who were undergoing percutaneous coronary intervention (PCI) with the second-generation Endeavor zotarolimus-eluting stent. All patients had stable or unstable angina or a recent MI, at least one coronary lesion suitable for PCI with the Endeavor stent, and a native vessel diameter of at least 2.5 mm with stenosis greater than 50%. The cohort consisted mainly of patients with stable coronary artery disease and a low risk of acute coronary syndrome.
Equal numbers were randomized to 3 or 12 months of DAPT with aspirin and 75 mg/day of clopidogrel to reduce the risk of thrombotic events. Current guidelines call for 12 months of DAPT after stent implantation. One-year follow-up data were available for 98% in each group.
Known as the OPTIMIZE trial (Optimized Duration of Clopidogrel Therapy Following Treatment With the Endeavor Zotarolimus-Eluting Stent in Real-World Clinical Practice), the study found that more than 99% of patients in each group completed 3 months of DAPT. One year after stent implantation, 6% who had been randomized to the 3-month group were still on DAPT, as were 97% of those randomized to the 12-month group.
The individual components of the combined endpoint did not differ significantly at 1-year follow-up. All-cause mortality was seen in 1.9% and 1.7% of the 3- and 12-month therapy groups; MI rates were 0.8% and 0.6%, respectively; stroke rates were 0.3% and 0.1%; and major bleeding occurred in 0.2% and 0.4%. Landmark analyses also found no significant differences between groups for these endpoints at 3 months.
Rates of definite or probable stent thrombosis at 1 year were 0.3% in the 3-month therapy group and 0.1% in the 12-month group, which was not a significant difference. There was a trend toward higher risk of bleeding at 1 year in patients on 12 months of DAPT, seen in 1% compared with 0.4% of those on 3 months of therapy, Dr. Feres reported at the meeting, cosponsored by the American College of Cardiology.
Secondary outcomes at 1 year showed that rates of other adverse clinical events also did not differ significantly between the two groups. Eight percent in the 3-month group and 7% in the 12-month group developed one or more major adverse coronary events: death, MI, emergent coronary artery bypass grafting, or target lesion revascularization. Three percent in each group died, 3% in each developed an MI, 4% in each had either cardiac death or an MI, and 0.3% in each developed a stroke. Major bleeding occurred in 0.6% of the 3-month group and 0.9% of the 12-month group. Target lesion revascularization rates were 3.5% in the 3-month group and 3.2% in the 12-month group.
Results did not differ by subgroups.
Rates for the primary outcome were lower than expected in both arms of the trial. Investigators expected a 9% rate, not the roughly 6% rate seen in both arms. The rate of major adverse coronary events at 1 year, however, was 8.4% in the 3-month group and 7.5% in the 12-month group.
Two previous studies in Italy and Korea of shortened-duration DAPT for patients receiving drug-eluting stents compared 6 months of therapy with 12 months of therapy. One other previous trial compared 3 months with 12 months of DAPT but compared two different stents, he said. All suggested that 12 months of DAPT may not always be needed, he said in an interview.
The current study excluded patients with primary or rescue PCI for ST-segment elevation MI, lesions located in a saphenous vein graft, patients with a previous PCI with a drug-eluting stent, and other less common exclusion criteria.
The findings from OPTIMIZE were published online concurrently with Dr. Feres’ presentation (JAMA 2013 Oct. 31 [doi: 10.1001/jama.2013.282183]).
Dr. Feres reported having financial relationships with BioSensors, Eli Lilly, and Medtronic, which markets the Endeavor stent and funded the study.
[email protected]
On Twitter @sherryboschert
AT TCT 2013
Major finding: The incidence at 12 months of a composite of adverse events was 6% in patients randomized to 3 months of DAPT and 5.8% in those randomized to 12 months of therapy.
Data source: A prospective, randomized trial in 3,120 adult Brazilians receiving the Endeavor zotarolimus-eluting stent.
Disclosures: Dr. Feres reported having financial relationships with Biosensors, Eli Lilly and Co., and Medtronic, which markets the Endeavor stent and funded the study.
Prehospital bivalirudin reduced bleeding with PCI
SAN FRANCISCO – Giving bivalirudin in the ambulance to patients with ST-segment elevation MI before primary percutaneous coronary intervention significantly improved 30-day bleeding outcomes in a randomized controlled trial in 2,218 patients, compared with giving unfractionated or low-molecular-weight heparin and optional glycoprotein IIb/IIIa inhibitors.
The bivalirudin group showed nearly a 40% decrease in the primary outcome, a composite of death or major bleeding not associated with coronary artery bypass grafting (CABG), compared with the control group. Rates for the primary outcome were 5.1% in the bivalirudin group and 8.4% in the control group at 30 days, Dr. Philippe Gabriel Steg and his associates reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
The 30-day rate of acute stent thrombosis, however, was approximately sixfold higher in the bivalirudin group (1.1%) than in the control group (0.2%) in the EUROMAX (European Ambulance Acute Coronary Syndrome Angiography) trial. That did not translate into an increased risk of infarction, which was similar in the bivalirudin group (1.7%) and the control group (0.9%), Dr. Steg and his colleagues said.
The rates of the main secondary outcome – a composite of death, reinfarction, or non-CABG major bleeding at 30 days – also were significantly lower in the bivalirudin group (6.7%) than in the control group (9.1%), said Dr. Steg, professor of cardiology at Université Paris-Diderot and director of the coronary care unit at Hôpital Bichat, Paris.
The study was published online simultaneously with the presentation (N. Engl. J. Med. 2013 Oct. 30 [doi: 10.1056/NEJMoa1311096]).
The benefit from bivalirudin came mainly from reduced bleeding, not reduced mortality. The risk of major bleeding not associated with CABG was 2.7% in the bivalirudin group and 6.1% in the control group, a significant 57% reduction.
Rates of cardiac or noncardiac death at 30 days did not differ significantly between groups, with cardiac death in 2.4% with bivalirudin and 3% in the control group, and noncardiac death in 0.5% and 0.1%, respectively.
The study was underpowered to assess mortality, Dr. Steg said in an interview. He hopes to assess mortality risk at 1 year by analyzing combined data from EUROMAX and a previous major trial that showed bivalirudin’s utility before percutaneous coronary intervention, the HORIZONS-AMI (Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction) trial (N. Engl. J. Med. 2008;358:2218-30).
Dr. Steg and his associates conducted the EUROMAX study to see if these benefits were still true in the modern era of prehospital treatment and evolving use of glycoprotein IIb/IIIa inhibitors, platelet P2Y12 receptor inhibitors, and radial access for PCI.
The EUROMAX findings were consistent across subgroups of patients, including subgroups defined by PCI access site or by the choice of P2Y12 inhibitor, Dr. Steg reported at the meeting, cosponsored by the American College of Cardiology.
He believes the findings will change practices and convince emergency crews to choose bivalirudin in Europe, where anticoagulation commonly is started in ambulances before arrival at the hospital, a practice that has not yet caught on in most of the United States.
Dr. Gregg W. Stone, primary investigator of the HORIZONS-AMI trial and a discussant of EUROMAX at the meeting, put together a preliminary meta-analysis of the two studies, he reported. Preliminary results suggest significant benefits from bivalirudin in 30-day rates of major bleeding, transfusion, thrombocytopenia, mortality, and subacute stent thrombosis, with a roughly fivefold increase in the risk of acute stent thrombosis. Studies are warranted to determine whether antiplatelet therapy with cangrelor might be a solution to the acute thrombosis risk, said Dr. Stone, professor of medicine and director of cardiovascular research and education at Columbia University, New York.
Dr. Steg received fees from The Medicines Company, which sponsored the study and markets bivalirudin, and he reported financial associations with 16 other companies. Four of his colleagues in the study were employees of The Medicines Company, and 12 other colleagues reported financial associations with that company and/or multiple other companies. Dr. Stone reported financial associations with Boston Scientific, Eli Lilly, and other companies.
[email protected]
On Twitter @sherryboschert

|
Sherry Boschert/IMNG Medical Media
|
This is an important trial that will change clinical practice, Dr. Bernard J. Gersh said in a panel discussion at a press briefing.
Dr. James B. Hermiller Jr. cautioned that it remains to be seen how the large difference between groups in major bleeding events translates into mortality over time.
Dr. Philippe Généreux said the results especially are important for a vast country like Canada, where transport to PCI often takes 45-60 minutes.
Asked what changes could get the United States to adopt prehospital initiation of anticoagulation, as is common in Europe, none of the panelists had a solution.

|
Sherry Boschert/IMNG Medical Media
|
"We have a different competitive system" in Canada, said Dr. Généreux of New York–Presbyterian Hospital and Hôpital Sacré Coeur, Montreal. "I’ve never seen anything that suggests that prehospital administration and diagnosis are not beneficial, but how we are going to achieve that in the United States, I don’t know."
"It’s difficult to just get ECGs in the field, let alone administer anticoagulants, but we need to get there because this is very important," said Dr. Hermiller of St. Vincent Heart Center of Indiana, Indianapolis.
Dr. Gersh, professor of medicine at the Mayo Clinic, Rochester, Minn., said that it’s possible to change systemic practices in a regional system such as his that spans rural areas, but it’s much more difficult in big cities with multiple competing medical systems.

|
Sherry Boschert/IMNG Medical Media
|
"I think there are real benefits to a national health care system as opposed to pure and simple competition. With a national or even a regional health care system, you can develop protocols and mandate them. This is what’s happened in many countries in Europe," he said. "Our current system, where you may have a city of a million people and four hospitals competing and four different ambulance systems, is not conducive to this. Unless people are prepared to sit down and say, ‘We’ll share the burden,’ I don’t have any idea" how the U.S. system will change.
Dr. Généreux reported financial associations with Cardiovascular Systems Inc. and Abbott Vascular. Dr. Gersh reported financial associations with Pharmaceutical Product Development, InspireMD, and multiple other companies.

|
Sherry Boschert/IMNG Medical Media
|
This is an important trial that will change clinical practice, Dr. Bernard J. Gersh said in a panel discussion at a press briefing.
Dr. James B. Hermiller Jr. cautioned that it remains to be seen how the large difference between groups in major bleeding events translates into mortality over time.
Dr. Philippe Généreux said the results especially are important for a vast country like Canada, where transport to PCI often takes 45-60 minutes.
Asked what changes could get the United States to adopt prehospital initiation of anticoagulation, as is common in Europe, none of the panelists had a solution.

|
Sherry Boschert/IMNG Medical Media
|
"We have a different competitive system" in Canada, said Dr. Généreux of New York–Presbyterian Hospital and Hôpital Sacré Coeur, Montreal. "I’ve never seen anything that suggests that prehospital administration and diagnosis are not beneficial, but how we are going to achieve that in the United States, I don’t know."
"It’s difficult to just get ECGs in the field, let alone administer anticoagulants, but we need to get there because this is very important," said Dr. Hermiller of St. Vincent Heart Center of Indiana, Indianapolis.
Dr. Gersh, professor of medicine at the Mayo Clinic, Rochester, Minn., said that it’s possible to change systemic practices in a regional system such as his that spans rural areas, but it’s much more difficult in big cities with multiple competing medical systems.

|
Sherry Boschert/IMNG Medical Media
|
"I think there are real benefits to a national health care system as opposed to pure and simple competition. With a national or even a regional health care system, you can develop protocols and mandate them. This is what’s happened in many countries in Europe," he said. "Our current system, where you may have a city of a million people and four hospitals competing and four different ambulance systems, is not conducive to this. Unless people are prepared to sit down and say, ‘We’ll share the burden,’ I don’t have any idea" how the U.S. system will change.
Dr. Généreux reported financial associations with Cardiovascular Systems Inc. and Abbott Vascular. Dr. Gersh reported financial associations with Pharmaceutical Product Development, InspireMD, and multiple other companies.

|
Sherry Boschert/IMNG Medical Media
|
This is an important trial that will change clinical practice, Dr. Bernard J. Gersh said in a panel discussion at a press briefing.
Dr. James B. Hermiller Jr. cautioned that it remains to be seen how the large difference between groups in major bleeding events translates into mortality over time.
Dr. Philippe Généreux said the results especially are important for a vast country like Canada, where transport to PCI often takes 45-60 minutes.
Asked what changes could get the United States to adopt prehospital initiation of anticoagulation, as is common in Europe, none of the panelists had a solution.

|
Sherry Boschert/IMNG Medical Media
|
"We have a different competitive system" in Canada, said Dr. Généreux of New York–Presbyterian Hospital and Hôpital Sacré Coeur, Montreal. "I’ve never seen anything that suggests that prehospital administration and diagnosis are not beneficial, but how we are going to achieve that in the United States, I don’t know."
"It’s difficult to just get ECGs in the field, let alone administer anticoagulants, but we need to get there because this is very important," said Dr. Hermiller of St. Vincent Heart Center of Indiana, Indianapolis.
Dr. Gersh, professor of medicine at the Mayo Clinic, Rochester, Minn., said that it’s possible to change systemic practices in a regional system such as his that spans rural areas, but it’s much more difficult in big cities with multiple competing medical systems.

|
Sherry Boschert/IMNG Medical Media
|
"I think there are real benefits to a national health care system as opposed to pure and simple competition. With a national or even a regional health care system, you can develop protocols and mandate them. This is what’s happened in many countries in Europe," he said. "Our current system, where you may have a city of a million people and four hospitals competing and four different ambulance systems, is not conducive to this. Unless people are prepared to sit down and say, ‘We’ll share the burden,’ I don’t have any idea" how the U.S. system will change.
Dr. Généreux reported financial associations with Cardiovascular Systems Inc. and Abbott Vascular. Dr. Gersh reported financial associations with Pharmaceutical Product Development, InspireMD, and multiple other companies.
SAN FRANCISCO – Giving bivalirudin in the ambulance to patients with ST-segment elevation MI before primary percutaneous coronary intervention significantly improved 30-day bleeding outcomes in a randomized controlled trial in 2,218 patients, compared with giving unfractionated or low-molecular-weight heparin and optional glycoprotein IIb/IIIa inhibitors.
The bivalirudin group showed nearly a 40% decrease in the primary outcome, a composite of death or major bleeding not associated with coronary artery bypass grafting (CABG), compared with the control group. Rates for the primary outcome were 5.1% in the bivalirudin group and 8.4% in the control group at 30 days, Dr. Philippe Gabriel Steg and his associates reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
The 30-day rate of acute stent thrombosis, however, was approximately sixfold higher in the bivalirudin group (1.1%) than in the control group (0.2%) in the EUROMAX (European Ambulance Acute Coronary Syndrome Angiography) trial. That did not translate into an increased risk of infarction, which was similar in the bivalirudin group (1.7%) and the control group (0.9%), Dr. Steg and his colleagues said.
The rates of the main secondary outcome – a composite of death, reinfarction, or non-CABG major bleeding at 30 days – also were significantly lower in the bivalirudin group (6.7%) than in the control group (9.1%), said Dr. Steg, professor of cardiology at Université Paris-Diderot and director of the coronary care unit at Hôpital Bichat, Paris.
The study was published online simultaneously with the presentation (N. Engl. J. Med. 2013 Oct. 30 [doi: 10.1056/NEJMoa1311096]).
The benefit from bivalirudin came mainly from reduced bleeding, not reduced mortality. The risk of major bleeding not associated with CABG was 2.7% in the bivalirudin group and 6.1% in the control group, a significant 57% reduction.
Rates of cardiac or noncardiac death at 30 days did not differ significantly between groups, with cardiac death in 2.4% with bivalirudin and 3% in the control group, and noncardiac death in 0.5% and 0.1%, respectively.
The study was underpowered to assess mortality, Dr. Steg said in an interview. He hopes to assess mortality risk at 1 year by analyzing combined data from EUROMAX and a previous major trial that showed bivalirudin’s utility before percutaneous coronary intervention, the HORIZONS-AMI (Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction) trial (N. Engl. J. Med. 2008;358:2218-30).
Dr. Steg and his associates conducted the EUROMAX study to see if these benefits were still true in the modern era of prehospital treatment and evolving use of glycoprotein IIb/IIIa inhibitors, platelet P2Y12 receptor inhibitors, and radial access for PCI.
The EUROMAX findings were consistent across subgroups of patients, including subgroups defined by PCI access site or by the choice of P2Y12 inhibitor, Dr. Steg reported at the meeting, cosponsored by the American College of Cardiology.
He believes the findings will change practices and convince emergency crews to choose bivalirudin in Europe, where anticoagulation commonly is started in ambulances before arrival at the hospital, a practice that has not yet caught on in most of the United States.
Dr. Gregg W. Stone, primary investigator of the HORIZONS-AMI trial and a discussant of EUROMAX at the meeting, put together a preliminary meta-analysis of the two studies, he reported. Preliminary results suggest significant benefits from bivalirudin in 30-day rates of major bleeding, transfusion, thrombocytopenia, mortality, and subacute stent thrombosis, with a roughly fivefold increase in the risk of acute stent thrombosis. Studies are warranted to determine whether antiplatelet therapy with cangrelor might be a solution to the acute thrombosis risk, said Dr. Stone, professor of medicine and director of cardiovascular research and education at Columbia University, New York.
Dr. Steg received fees from The Medicines Company, which sponsored the study and markets bivalirudin, and he reported financial associations with 16 other companies. Four of his colleagues in the study were employees of The Medicines Company, and 12 other colleagues reported financial associations with that company and/or multiple other companies. Dr. Stone reported financial associations with Boston Scientific, Eli Lilly, and other companies.
[email protected]
On Twitter @sherryboschert
SAN FRANCISCO – Giving bivalirudin in the ambulance to patients with ST-segment elevation MI before primary percutaneous coronary intervention significantly improved 30-day bleeding outcomes in a randomized controlled trial in 2,218 patients, compared with giving unfractionated or low-molecular-weight heparin and optional glycoprotein IIb/IIIa inhibitors.
The bivalirudin group showed nearly a 40% decrease in the primary outcome, a composite of death or major bleeding not associated with coronary artery bypass grafting (CABG), compared with the control group. Rates for the primary outcome were 5.1% in the bivalirudin group and 8.4% in the control group at 30 days, Dr. Philippe Gabriel Steg and his associates reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
The 30-day rate of acute stent thrombosis, however, was approximately sixfold higher in the bivalirudin group (1.1%) than in the control group (0.2%) in the EUROMAX (European Ambulance Acute Coronary Syndrome Angiography) trial. That did not translate into an increased risk of infarction, which was similar in the bivalirudin group (1.7%) and the control group (0.9%), Dr. Steg and his colleagues said.
The rates of the main secondary outcome – a composite of death, reinfarction, or non-CABG major bleeding at 30 days – also were significantly lower in the bivalirudin group (6.7%) than in the control group (9.1%), said Dr. Steg, professor of cardiology at Université Paris-Diderot and director of the coronary care unit at Hôpital Bichat, Paris.
The study was published online simultaneously with the presentation (N. Engl. J. Med. 2013 Oct. 30 [doi: 10.1056/NEJMoa1311096]).
The benefit from bivalirudin came mainly from reduced bleeding, not reduced mortality. The risk of major bleeding not associated with CABG was 2.7% in the bivalirudin group and 6.1% in the control group, a significant 57% reduction.
Rates of cardiac or noncardiac death at 30 days did not differ significantly between groups, with cardiac death in 2.4% with bivalirudin and 3% in the control group, and noncardiac death in 0.5% and 0.1%, respectively.
The study was underpowered to assess mortality, Dr. Steg said in an interview. He hopes to assess mortality risk at 1 year by analyzing combined data from EUROMAX and a previous major trial that showed bivalirudin’s utility before percutaneous coronary intervention, the HORIZONS-AMI (Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction) trial (N. Engl. J. Med. 2008;358:2218-30).
Dr. Steg and his associates conducted the EUROMAX study to see if these benefits were still true in the modern era of prehospital treatment and evolving use of glycoprotein IIb/IIIa inhibitors, platelet P2Y12 receptor inhibitors, and radial access for PCI.
The EUROMAX findings were consistent across subgroups of patients, including subgroups defined by PCI access site or by the choice of P2Y12 inhibitor, Dr. Steg reported at the meeting, cosponsored by the American College of Cardiology.
He believes the findings will change practices and convince emergency crews to choose bivalirudin in Europe, where anticoagulation commonly is started in ambulances before arrival at the hospital, a practice that has not yet caught on in most of the United States.
Dr. Gregg W. Stone, primary investigator of the HORIZONS-AMI trial and a discussant of EUROMAX at the meeting, put together a preliminary meta-analysis of the two studies, he reported. Preliminary results suggest significant benefits from bivalirudin in 30-day rates of major bleeding, transfusion, thrombocytopenia, mortality, and subacute stent thrombosis, with a roughly fivefold increase in the risk of acute stent thrombosis. Studies are warranted to determine whether antiplatelet therapy with cangrelor might be a solution to the acute thrombosis risk, said Dr. Stone, professor of medicine and director of cardiovascular research and education at Columbia University, New York.
Dr. Steg received fees from The Medicines Company, which sponsored the study and markets bivalirudin, and he reported financial associations with 16 other companies. Four of his colleagues in the study were employees of The Medicines Company, and 12 other colleagues reported financial associations with that company and/or multiple other companies. Dr. Stone reported financial associations with Boston Scientific, Eli Lilly, and other companies.
[email protected]
On Twitter @sherryboschert
AT TCT 2013
Major finding: Rates of death or major bleeding not associated with CABG were 5.1% in the bivalirudin group at 30 days and 8.4% in the control group.
Data source: A prospective, randomized study of 2,218 adults with ST-segment elevation myocardial infarction who received either bivalirudin or unfractionated or low-molecular-weight heparin with optional glycoprotein IIb/IIIa inhibitors while being transported for primary PCI.
Disclosures: Dr. Steg received fees from The Medicines Company, which sponsored the study and markets bivalirudin, and he reported financial associations with 16 other companies. Four of his colleagues in the study were employees of The Medicines Company, and 12 other colleagues reported financial associations with that company and/or multiple other companies. Dr. Stone reported financial associations with Boston Scientific, Eli Lilly, and other companies.
Transradial PCI in women appears safe, feasible
SAN FRANCISCO – Rates of bleeding or vascular complications in women undergoing percutaneous coronary intervention were 59% lower using radial access, compared with femoral access, a difference that did not reach statistical significance in a randomized study of 1,787 patients.
Bleeding or vascular complications within 72 hours or at hospital discharge were seen in 1.2% of 345 women who had transradial percutaneous coronary intervention (PCI) and 2.9% of 345 women who had transfemoral PCI, Dr. Sunil V. Rao reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
Although that difference was not statistically significant in this prespecified analysis of patients who actually underwent PCI, the rate of bleeding and vascular complications was significantly lower for the group randomized to radial access in an analysis of the whole cohort, regardless of whether they had PCI or just diagnostic catheterization. Bleeding and vascular complication rates were 0.6% in those randomized to radial access and 1.7% in those randomized to femoral access,
In both the PCI cohort and the total cohort, significantly more women in the radial group needed to cross over to femoral access for PCI compared with the crossover rate in the femoral group. In the PCI cohort, 6.1% of the radial group crossed over, as did 1.7% of the femoral group. In the total cohort, crossover was needed in 6.7% of the radial group and 1.9% of the femoral group, said Dr. Rao of Duke University, Durham, N.C. The main reason for crossover from radial to femoral access was radial artery spasm, in 43% of cases.
The Study of Access Site for Enhancement of PCI for Women (SAFE-PCI) leveraged data from the National Cardiovascular Data Registry’s CathPCI Registry from 60 institutions on adult women undergoing elective or urgent PCI or undergoing diagnostic angiography to evaluate ischemic symptoms with the possibility of PCI. The primary outcome measure was Bleeding Academic Research Consortium (BARC) type 2, 3, or 5 bleeding events and vascular complications requiring intervention.
The investigators had planned to randomize 3,000 women to obtain 1,800 who underwent PCI, but a routine review after randomizing 1,120 patients suggested that the trial would be too small to show a difference because of lower-than-expected bleeding rates. Because no harm was noted in either the radial or femoral group, the investigators continued until they had enough patients for a quality-of-life substudy, then prematurely discontinued the trial.
The reduction in bleeding with the radial approach was similar to reductions seen in previous studies, Dr. Rao said at the meeting, cosponsored by the American College of Cardiology. The conversion rate from radial to femoral access was similar to the 7.6% rate reported in a prior trial (Lancet 2011;377:1409-20).
Compared with men, women have an increased risk for bleeding from antithrombotic therapy and from femoral access for PCI. Radial access can decrease bleeding risk, but transradial PCI has been less common in women, in part because they have smaller radial arteries.
The current trial’s results suggest that "an initial strategy of radial access is reasonable and may be preferred by some operators for women undergoing cardiac catheterization or PCI, with the recognition that a proportion of patients will require conversion to femoral access," he said.
Women preferred the radial approach over femoral access in the study, he added.
The results could be looked at as a glass half empty or half full, Dr. Roxana Mehran said as the discussant of the study at the meeting. She served on the executive committee of the SAFE-PCI trial.
In the eyes of a purist statistician, the results show no significant evidence that radial access prevents bleeding or vascular complications, the primary endpoint, said Dr. Mehran, professor of medicine and director of interventional cardiovascular research at Mount Sinai School of Medicine, New York.
From a clinician’s viewpoint, however, the estimates of benefit from radial access in the overall cohort and the PCI cohort were similar, yielding approximately a 60% reduction in bleeding with radial access, she noted. "The study provides evidence, albeit not conclusive, for greater efficacy with radial access in women," she said.
The crossover rates suggest that for every bleeding event or vascular complication prevented in the radial access group, three patients would cross over. "While there’s a higher crossover from [the] radial to the femoral approach, it’s reasonable and intuitive to begin with the radial approach in women," she said, "especially in those women at high risk for bleeding."
Dr. Rao has been a consultant for the Medicines Co., which helped fund the trial, and for AstraZeneca. Dr. Mehran reported financial associations with these companies and with Abbott Vascular and Daiichi-Sankyo/Eli Lilly & Co., which also funded the trial. Other funders of the study included Terumo Medical, Medtronic, ACIST Medical Systems, and Guerbet. Dr. Mehran also reported financial associations with nine other medical companies.
On Twitter @sherryboschert
I don’t think we have the data to say that this is a positive study or that we should change our practice because of these results. It’s an incomplete study, so we really don’t have the data. We need to think about why we didn’t have the anticipated enrollment and why the bleeding rate was lower than expected in the femoral access group. Those are the things that I think led to the futility. But it’s not really a negative trial. We simply don’t know whether radial access will be better in high-risk women undergoing PCI. It’s promising, and it makes all the sense in the world because of the bleeding risk in women.
Alice K. Jacobs, M.D., is a professor of medicine and director of the cardiac catheterization laboratory and interventional cardiology at Boston University. She gave these remarks at a press briefing. Dr. Jacobs reported having no relevant financial disclosures.
I don’t think we have the data to say that this is a positive study or that we should change our practice because of these results. It’s an incomplete study, so we really don’t have the data. We need to think about why we didn’t have the anticipated enrollment and why the bleeding rate was lower than expected in the femoral access group. Those are the things that I think led to the futility. But it’s not really a negative trial. We simply don’t know whether radial access will be better in high-risk women undergoing PCI. It’s promising, and it makes all the sense in the world because of the bleeding risk in women.
Alice K. Jacobs, M.D., is a professor of medicine and director of the cardiac catheterization laboratory and interventional cardiology at Boston University. She gave these remarks at a press briefing. Dr. Jacobs reported having no relevant financial disclosures.
I don’t think we have the data to say that this is a positive study or that we should change our practice because of these results. It’s an incomplete study, so we really don’t have the data. We need to think about why we didn’t have the anticipated enrollment and why the bleeding rate was lower than expected in the femoral access group. Those are the things that I think led to the futility. But it’s not really a negative trial. We simply don’t know whether radial access will be better in high-risk women undergoing PCI. It’s promising, and it makes all the sense in the world because of the bleeding risk in women.
Alice K. Jacobs, M.D., is a professor of medicine and director of the cardiac catheterization laboratory and interventional cardiology at Boston University. She gave these remarks at a press briefing. Dr. Jacobs reported having no relevant financial disclosures.
SAN FRANCISCO – Rates of bleeding or vascular complications in women undergoing percutaneous coronary intervention were 59% lower using radial access, compared with femoral access, a difference that did not reach statistical significance in a randomized study of 1,787 patients.
Bleeding or vascular complications within 72 hours or at hospital discharge were seen in 1.2% of 345 women who had transradial percutaneous coronary intervention (PCI) and 2.9% of 345 women who had transfemoral PCI, Dr. Sunil V. Rao reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
Although that difference was not statistically significant in this prespecified analysis of patients who actually underwent PCI, the rate of bleeding and vascular complications was significantly lower for the group randomized to radial access in an analysis of the whole cohort, regardless of whether they had PCI or just diagnostic catheterization. Bleeding and vascular complication rates were 0.6% in those randomized to radial access and 1.7% in those randomized to femoral access,
In both the PCI cohort and the total cohort, significantly more women in the radial group needed to cross over to femoral access for PCI compared with the crossover rate in the femoral group. In the PCI cohort, 6.1% of the radial group crossed over, as did 1.7% of the femoral group. In the total cohort, crossover was needed in 6.7% of the radial group and 1.9% of the femoral group, said Dr. Rao of Duke University, Durham, N.C. The main reason for crossover from radial to femoral access was radial artery spasm, in 43% of cases.
The Study of Access Site for Enhancement of PCI for Women (SAFE-PCI) leveraged data from the National Cardiovascular Data Registry’s CathPCI Registry from 60 institutions on adult women undergoing elective or urgent PCI or undergoing diagnostic angiography to evaluate ischemic symptoms with the possibility of PCI. The primary outcome measure was Bleeding Academic Research Consortium (BARC) type 2, 3, or 5 bleeding events and vascular complications requiring intervention.
The investigators had planned to randomize 3,000 women to obtain 1,800 who underwent PCI, but a routine review after randomizing 1,120 patients suggested that the trial would be too small to show a difference because of lower-than-expected bleeding rates. Because no harm was noted in either the radial or femoral group, the investigators continued until they had enough patients for a quality-of-life substudy, then prematurely discontinued the trial.
The reduction in bleeding with the radial approach was similar to reductions seen in previous studies, Dr. Rao said at the meeting, cosponsored by the American College of Cardiology. The conversion rate from radial to femoral access was similar to the 7.6% rate reported in a prior trial (Lancet 2011;377:1409-20).
Compared with men, women have an increased risk for bleeding from antithrombotic therapy and from femoral access for PCI. Radial access can decrease bleeding risk, but transradial PCI has been less common in women, in part because they have smaller radial arteries.
The current trial’s results suggest that "an initial strategy of radial access is reasonable and may be preferred by some operators for women undergoing cardiac catheterization or PCI, with the recognition that a proportion of patients will require conversion to femoral access," he said.
Women preferred the radial approach over femoral access in the study, he added.
The results could be looked at as a glass half empty or half full, Dr. Roxana Mehran said as the discussant of the study at the meeting. She served on the executive committee of the SAFE-PCI trial.
In the eyes of a purist statistician, the results show no significant evidence that radial access prevents bleeding or vascular complications, the primary endpoint, said Dr. Mehran, professor of medicine and director of interventional cardiovascular research at Mount Sinai School of Medicine, New York.
From a clinician’s viewpoint, however, the estimates of benefit from radial access in the overall cohort and the PCI cohort were similar, yielding approximately a 60% reduction in bleeding with radial access, she noted. "The study provides evidence, albeit not conclusive, for greater efficacy with radial access in women," she said.
The crossover rates suggest that for every bleeding event or vascular complication prevented in the radial access group, three patients would cross over. "While there’s a higher crossover from [the] radial to the femoral approach, it’s reasonable and intuitive to begin with the radial approach in women," she said, "especially in those women at high risk for bleeding."
Dr. Rao has been a consultant for the Medicines Co., which helped fund the trial, and for AstraZeneca. Dr. Mehran reported financial associations with these companies and with Abbott Vascular and Daiichi-Sankyo/Eli Lilly & Co., which also funded the trial. Other funders of the study included Terumo Medical, Medtronic, ACIST Medical Systems, and Guerbet. Dr. Mehran also reported financial associations with nine other medical companies.
On Twitter @sherryboschert
SAN FRANCISCO – Rates of bleeding or vascular complications in women undergoing percutaneous coronary intervention were 59% lower using radial access, compared with femoral access, a difference that did not reach statistical significance in a randomized study of 1,787 patients.
Bleeding or vascular complications within 72 hours or at hospital discharge were seen in 1.2% of 345 women who had transradial percutaneous coronary intervention (PCI) and 2.9% of 345 women who had transfemoral PCI, Dr. Sunil V. Rao reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
Although that difference was not statistically significant in this prespecified analysis of patients who actually underwent PCI, the rate of bleeding and vascular complications was significantly lower for the group randomized to radial access in an analysis of the whole cohort, regardless of whether they had PCI or just diagnostic catheterization. Bleeding and vascular complication rates were 0.6% in those randomized to radial access and 1.7% in those randomized to femoral access,
In both the PCI cohort and the total cohort, significantly more women in the radial group needed to cross over to femoral access for PCI compared with the crossover rate in the femoral group. In the PCI cohort, 6.1% of the radial group crossed over, as did 1.7% of the femoral group. In the total cohort, crossover was needed in 6.7% of the radial group and 1.9% of the femoral group, said Dr. Rao of Duke University, Durham, N.C. The main reason for crossover from radial to femoral access was radial artery spasm, in 43% of cases.
The Study of Access Site for Enhancement of PCI for Women (SAFE-PCI) leveraged data from the National Cardiovascular Data Registry’s CathPCI Registry from 60 institutions on adult women undergoing elective or urgent PCI or undergoing diagnostic angiography to evaluate ischemic symptoms with the possibility of PCI. The primary outcome measure was Bleeding Academic Research Consortium (BARC) type 2, 3, or 5 bleeding events and vascular complications requiring intervention.
The investigators had planned to randomize 3,000 women to obtain 1,800 who underwent PCI, but a routine review after randomizing 1,120 patients suggested that the trial would be too small to show a difference because of lower-than-expected bleeding rates. Because no harm was noted in either the radial or femoral group, the investigators continued until they had enough patients for a quality-of-life substudy, then prematurely discontinued the trial.
The reduction in bleeding with the radial approach was similar to reductions seen in previous studies, Dr. Rao said at the meeting, cosponsored by the American College of Cardiology. The conversion rate from radial to femoral access was similar to the 7.6% rate reported in a prior trial (Lancet 2011;377:1409-20).
Compared with men, women have an increased risk for bleeding from antithrombotic therapy and from femoral access for PCI. Radial access can decrease bleeding risk, but transradial PCI has been less common in women, in part because they have smaller radial arteries.
The current trial’s results suggest that "an initial strategy of radial access is reasonable and may be preferred by some operators for women undergoing cardiac catheterization or PCI, with the recognition that a proportion of patients will require conversion to femoral access," he said.
Women preferred the radial approach over femoral access in the study, he added.
The results could be looked at as a glass half empty or half full, Dr. Roxana Mehran said as the discussant of the study at the meeting. She served on the executive committee of the SAFE-PCI trial.
In the eyes of a purist statistician, the results show no significant evidence that radial access prevents bleeding or vascular complications, the primary endpoint, said Dr. Mehran, professor of medicine and director of interventional cardiovascular research at Mount Sinai School of Medicine, New York.
From a clinician’s viewpoint, however, the estimates of benefit from radial access in the overall cohort and the PCI cohort were similar, yielding approximately a 60% reduction in bleeding with radial access, she noted. "The study provides evidence, albeit not conclusive, for greater efficacy with radial access in women," she said.
The crossover rates suggest that for every bleeding event or vascular complication prevented in the radial access group, three patients would cross over. "While there’s a higher crossover from [the] radial to the femoral approach, it’s reasonable and intuitive to begin with the radial approach in women," she said, "especially in those women at high risk for bleeding."
Dr. Rao has been a consultant for the Medicines Co., which helped fund the trial, and for AstraZeneca. Dr. Mehran reported financial associations with these companies and with Abbott Vascular and Daiichi-Sankyo/Eli Lilly & Co., which also funded the trial. Other funders of the study included Terumo Medical, Medtronic, ACIST Medical Systems, and Guerbet. Dr. Mehran also reported financial associations with nine other medical companies.
On Twitter @sherryboschert
AT TCT 2013
Major finding: Rates of bleeding or vascular complications were 1.2% using radial access and 2.9% using femoral access in the PCI subgroup and 6.7% and 1.9%, respectively, in the entire cohort.
Data source: A randomized study of 1,787 women undergoing definite or possible PCI at 60 institutions.
Disclosures: Dr. Rao has been a consultant for the Medicines Co., which helped fund the trial, and for AstraZeneca. Dr. Mehran reported financial associations with these companies and with Abbott Vascular and Daiichi-Sankyo/Eli Lilly & Co., which also funded the trial. Other funders of the study included Abbott Vascular, Daiichi-Sankyo/Eli Lilly & Co., Terumo Medical, Medtronic, ACIST Medical Systems, and Guerbet. Dr. Mehran also reported financial associations with nine other medical companies.
Trauma center rankings differ by mortality, morbidity
SAN FRANCISCO – Trauma centers are ranked on the basis of in-hospital mortality rates, but pay-for-performance programs will benchmark them based on in-hospital complications – and there’s not good concordance between the two measures, a study of data from 248 trauma centers suggests.
Investigators used data on 449,743 patients aged 16 years or older who had blunt/penetrating injuries and an Injury Severity Score of 9 or higher to generate risk-adjusted, observed-to-expected mortality rates for each trauma center They ranked each facility based on mortality rate as a high-performing, average, or low-performing center and used complication rates to rank them again based on observed-to-expected morbidity ratios.
Only 40% of centers received the same benchmark using these two measures, Dr. Zain G. Hashmi and his associates reported at the annual meeting of the American Association for the Surgery of Trauma.
Dividing each performance ranking into quintiles, the two rankings diverged by at least one quintile for 79% of trauma centers. Only 21% were assigned the same quintile rank in the mortality benchmarking as in the morbidity benchmarking. A two-quintile divergence in rankings was noted in 21%, and a three-quintile difference in 23%, said Dr. Hashmi, a research fellow at Johns Hopkins University, Baltimore.
Overall, the unadjusted mortality rate was 7% and the morbidity rate was 10%. The most frequent complications were pneumonia in 4%, acute respiratory distress syndrome in 2%, and deep venous thrombosis in 2%.
The complications used for the morbidity benchmarking included pneumonia, deep venous thrombosis, acute respiratory distress syndrome, acute renal failure, sepsis, pulmonary embolism, decubitus ulcer, surgical site infection, myocardial infarction, cardiac arrest, unplanned intubation, and stroke.
The Centers for Medicare and Medicaid Services is implementing pay-for-performance programs in the public health sector nationwide under the Affordable Care Act to incentivize high quality of care and penalize low quality of care. The programs may soon be extended to trauma care, which could incorrectly penalize centers that are the best performers based on mortality benchmarks, he said.
"We need to develop more appropriate measures of trauma quality before pay-for-performance" programs come to trauma centers, perhaps using multiple quality indicators such as mortality, length of stay, complications, and failure to rescue, he said.
Data for the study came from the National Trauma Data Bank for 2007-2010.
Dr. Hashmi reported having no financial disclosures.
On Twitter @sherryboschert
The authors reached the very predictable conclusion that the two benchmarking approaches have no correlation whatsoever. They did not come quite as close to embracing the other obvious conclusion that, in fact, neither benchmark encompasses, or perhaps even approximates, the quality of care given at an individual center. And they don’t really offer us an alternative.
We’ve been seeking the best way to measure the quality of care for the injured patient for decades, long before the concepts "pay for performance" or "value-based purchasing" became something of our daily lives. One thing we certainly learned is that quality is a complex, nuanced, and maybe even an elusive concept, sort of like one of Plato’s forms – we can’t see it directly, and we have to figure out what it is by the shadows it casts.

|
|
Unfortunately, before you can really measure something, you do have to know a little bit about what it is you’re trying to measure. Otherwise, you’re likely to pick the wrong tool. For reasons of obvious practicality, the approach that is most commonly taken, just like the approach in this paper, is to measure the things we can, perhaps in very, very sophisticated ways, and then try somehow to take that result and connect it in some way to that elusive concept, quality.
If nothing else, this paper illustrates the weakness inherent in that approach. Without going into the potential methodological flaws, I would submit that the hypothesis is poorly focused. There was no observed concordance between mortality and morbidity because there is no reason to expect that there should be. They measure entirely different things, and neither one of those things is necessarily very much connected to quality, which is really what we’d like to get a handle on.
The better approach, I’d suggest, is to postulate, a priori, a definition of what quality might be or at least a set of characteristics that might represent quality, and then set about to measure against that model.
Dr. Robert Winchell is a surgeon at Maine Medical Center in Portland. These are excerpts of his remarks as discussant of the study at the meeting. He reported having no financial disclosures.
The authors reached the very predictable conclusion that the two benchmarking approaches have no correlation whatsoever. They did not come quite as close to embracing the other obvious conclusion that, in fact, neither benchmark encompasses, or perhaps even approximates, the quality of care given at an individual center. And they don’t really offer us an alternative.
We’ve been seeking the best way to measure the quality of care for the injured patient for decades, long before the concepts "pay for performance" or "value-based purchasing" became something of our daily lives. One thing we certainly learned is that quality is a complex, nuanced, and maybe even an elusive concept, sort of like one of Plato’s forms – we can’t see it directly, and we have to figure out what it is by the shadows it casts.

|
|
Unfortunately, before you can really measure something, you do have to know a little bit about what it is you’re trying to measure. Otherwise, you’re likely to pick the wrong tool. For reasons of obvious practicality, the approach that is most commonly taken, just like the approach in this paper, is to measure the things we can, perhaps in very, very sophisticated ways, and then try somehow to take that result and connect it in some way to that elusive concept, quality.
If nothing else, this paper illustrates the weakness inherent in that approach. Without going into the potential methodological flaws, I would submit that the hypothesis is poorly focused. There was no observed concordance between mortality and morbidity because there is no reason to expect that there should be. They measure entirely different things, and neither one of those things is necessarily very much connected to quality, which is really what we’d like to get a handle on.
The better approach, I’d suggest, is to postulate, a priori, a definition of what quality might be or at least a set of characteristics that might represent quality, and then set about to measure against that model.
Dr. Robert Winchell is a surgeon at Maine Medical Center in Portland. These are excerpts of his remarks as discussant of the study at the meeting. He reported having no financial disclosures.
The authors reached the very predictable conclusion that the two benchmarking approaches have no correlation whatsoever. They did not come quite as close to embracing the other obvious conclusion that, in fact, neither benchmark encompasses, or perhaps even approximates, the quality of care given at an individual center. And they don’t really offer us an alternative.
We’ve been seeking the best way to measure the quality of care for the injured patient for decades, long before the concepts "pay for performance" or "value-based purchasing" became something of our daily lives. One thing we certainly learned is that quality is a complex, nuanced, and maybe even an elusive concept, sort of like one of Plato’s forms – we can’t see it directly, and we have to figure out what it is by the shadows it casts.

|
|
Unfortunately, before you can really measure something, you do have to know a little bit about what it is you’re trying to measure. Otherwise, you’re likely to pick the wrong tool. For reasons of obvious practicality, the approach that is most commonly taken, just like the approach in this paper, is to measure the things we can, perhaps in very, very sophisticated ways, and then try somehow to take that result and connect it in some way to that elusive concept, quality.
If nothing else, this paper illustrates the weakness inherent in that approach. Without going into the potential methodological flaws, I would submit that the hypothesis is poorly focused. There was no observed concordance between mortality and morbidity because there is no reason to expect that there should be. They measure entirely different things, and neither one of those things is necessarily very much connected to quality, which is really what we’d like to get a handle on.
The better approach, I’d suggest, is to postulate, a priori, a definition of what quality might be or at least a set of characteristics that might represent quality, and then set about to measure against that model.
Dr. Robert Winchell is a surgeon at Maine Medical Center in Portland. These are excerpts of his remarks as discussant of the study at the meeting. He reported having no financial disclosures.
SAN FRANCISCO – Trauma centers are ranked on the basis of in-hospital mortality rates, but pay-for-performance programs will benchmark them based on in-hospital complications – and there’s not good concordance between the two measures, a study of data from 248 trauma centers suggests.
Investigators used data on 449,743 patients aged 16 years or older who had blunt/penetrating injuries and an Injury Severity Score of 9 or higher to generate risk-adjusted, observed-to-expected mortality rates for each trauma center They ranked each facility based on mortality rate as a high-performing, average, or low-performing center and used complication rates to rank them again based on observed-to-expected morbidity ratios.
Only 40% of centers received the same benchmark using these two measures, Dr. Zain G. Hashmi and his associates reported at the annual meeting of the American Association for the Surgery of Trauma.
Dividing each performance ranking into quintiles, the two rankings diverged by at least one quintile for 79% of trauma centers. Only 21% were assigned the same quintile rank in the mortality benchmarking as in the morbidity benchmarking. A two-quintile divergence in rankings was noted in 21%, and a three-quintile difference in 23%, said Dr. Hashmi, a research fellow at Johns Hopkins University, Baltimore.
Overall, the unadjusted mortality rate was 7% and the morbidity rate was 10%. The most frequent complications were pneumonia in 4%, acute respiratory distress syndrome in 2%, and deep venous thrombosis in 2%.
The complications used for the morbidity benchmarking included pneumonia, deep venous thrombosis, acute respiratory distress syndrome, acute renal failure, sepsis, pulmonary embolism, decubitus ulcer, surgical site infection, myocardial infarction, cardiac arrest, unplanned intubation, and stroke.
The Centers for Medicare and Medicaid Services is implementing pay-for-performance programs in the public health sector nationwide under the Affordable Care Act to incentivize high quality of care and penalize low quality of care. The programs may soon be extended to trauma care, which could incorrectly penalize centers that are the best performers based on mortality benchmarks, he said.
"We need to develop more appropriate measures of trauma quality before pay-for-performance" programs come to trauma centers, perhaps using multiple quality indicators such as mortality, length of stay, complications, and failure to rescue, he said.
Data for the study came from the National Trauma Data Bank for 2007-2010.
Dr. Hashmi reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Trauma centers are ranked on the basis of in-hospital mortality rates, but pay-for-performance programs will benchmark them based on in-hospital complications – and there’s not good concordance between the two measures, a study of data from 248 trauma centers suggests.
Investigators used data on 449,743 patients aged 16 years or older who had blunt/penetrating injuries and an Injury Severity Score of 9 or higher to generate risk-adjusted, observed-to-expected mortality rates for each trauma center They ranked each facility based on mortality rate as a high-performing, average, or low-performing center and used complication rates to rank them again based on observed-to-expected morbidity ratios.
Only 40% of centers received the same benchmark using these two measures, Dr. Zain G. Hashmi and his associates reported at the annual meeting of the American Association for the Surgery of Trauma.
Dividing each performance ranking into quintiles, the two rankings diverged by at least one quintile for 79% of trauma centers. Only 21% were assigned the same quintile rank in the mortality benchmarking as in the morbidity benchmarking. A two-quintile divergence in rankings was noted in 21%, and a three-quintile difference in 23%, said Dr. Hashmi, a research fellow at Johns Hopkins University, Baltimore.
Overall, the unadjusted mortality rate was 7% and the morbidity rate was 10%. The most frequent complications were pneumonia in 4%, acute respiratory distress syndrome in 2%, and deep venous thrombosis in 2%.
The complications used for the morbidity benchmarking included pneumonia, deep venous thrombosis, acute respiratory distress syndrome, acute renal failure, sepsis, pulmonary embolism, decubitus ulcer, surgical site infection, myocardial infarction, cardiac arrest, unplanned intubation, and stroke.
The Centers for Medicare and Medicaid Services is implementing pay-for-performance programs in the public health sector nationwide under the Affordable Care Act to incentivize high quality of care and penalize low quality of care. The programs may soon be extended to trauma care, which could incorrectly penalize centers that are the best performers based on mortality benchmarks, he said.
"We need to develop more appropriate measures of trauma quality before pay-for-performance" programs come to trauma centers, perhaps using multiple quality indicators such as mortality, length of stay, complications, and failure to rescue, he said.
Data for the study came from the National Trauma Data Bank for 2007-2010.
Dr. Hashmi reported having no financial disclosures.
On Twitter @sherryboschert
AT THE AAST ANNUAL MEETING
Major finding: Only 40% of trauma centers received the same ranking when judged by mortality or morbidity rates.
Data source: Retrospective analysis that ranked 238 centers as high, average, or low performing, based on data on 449,743 patients with blunt/penetrating injuries and an Injury Severity Score of 9 or higher.
Disclosures: Dr. Hashmi reported having no financial disclosures.
Blunt trauma outcomes improved by early transfusion
SAN FRANCISCO – Giving patients severely injured by blunt trauma a blood transfusion before they arrived at a trauma center was associated with a 95% reduction in deaths within 24 hours and a 64% reduction in deaths within 30 days, a retrospective study of 1,415 patients found.
Transfusion before arrival at the trauma center also was associated with an 88% reduction in the incidence of trauma-induced coagulopathy, Dr. Joshua B. Brown and his associates reported at the annual meeting of the American Association for the Surgery of Trauma.
They analyzed data from the prospective Inflammation and Host Response to Injury cohort study for patients with blunt injury in hemorrhagic shock who arrived at a trauma center within 2 hours of injury, 50 of whom received a blood transfusion before arrival. The investigators found that prearrival blood transfusion was associated with better outcomes after controlling for the effects of demographics, time to the trauma center, the severity of injury and shock, early resuscitation, and other confounders.
These preliminary data are compelling but require prospective validation, said Dr. Brown of the University of Pittsburgh.
Prehospital resuscitation of patients severely injured by blunt trauma has focused on use of crystalloids, and the next logical step is to bring blood-based resuscitation to prehospital settings, he said. Hemorrhage and coagulopathy have been major causes of death in blunt trauma patients.
In the study, patients who got a transfusion before arrival at the trauma center received a median of 1.3 units of blood prearrival. They were more likely to be hypotensive and to have a lower base deficit compared with patients who were not transfused before arrival, suggesting a higher severity of injury and shock in the transfusion group, he said. The groups did not differ significantly in age, gender, or Injury Severity Score.
Patients who received a transfusion before arrival at a trauma center showed a 95% lower 24-hour mortality rate, a 64% lower 30-day mortality rate, and an 88% lower risk of trauma-induced coagulopathy.
In a subsequent matched cohort analysis of 113 patients from the study, those receiving a pre–trauma center transfusion (35 patients) had a 98% reduction in mortality at 24 hours, an 88% reduction in 30-day mortality, and a 99% reduction in the risk of trauma-induced coagulopathy, Dr. Brown reported.
Dr. Brown reported having no financial disclosures.
[email protected]
On Twitter @sherryboschert
The role of blood-based resuscitation in salvaging the severely injured patient remains an area of intense scrutiny. We spend a lot of time at meetings talking about this very subject.
The authors note that there are multiple studies that have looked at blood-based resuscitation, including things like blood to plasma and platelet ratios, the timing of blood product transfusion, and the use of institutional transfusion protocols. Sometimes they give completely different answers.
Despite these variabilities, I think what we are seeing is that early use of blood products in the correct patient population does result in better survival. It’s a logical question for the trauma researcher to then ask, how early should blood be given? How early can blood be given?
The authors identified over 1,400 patients who met inclusion criteria. Ultimately, they demonstrated a statistically significant benefit to prehospital transfusion in terms of both 24-hour and 30-day mortality as well as trauma-induced coagulopathy. These are very intriguing results.
They state that the median volume of blood transfused prior to reaching the trauma center was 1.3 units, ranging from 1-2.3 units. I wonder how they can explain the fact that 1 unit of prehospital blood resulted in such a significant difference in mortality and coagulopathy. That is a lot of bang for your buck.
The low numbers in the prehospital transfusion group raise some questions, but I do agree that this is interesting research and it is worthy of prospective study.
Dr. Stephanie Savage is a surgeon at the University of Tennessee, Memphis. These are excerpts of her remarks as discussant of the study at the meeting. She reported having no financial disclosures.
The role of blood-based resuscitation in salvaging the severely injured patient remains an area of intense scrutiny. We spend a lot of time at meetings talking about this very subject.
The authors note that there are multiple studies that have looked at blood-based resuscitation, including things like blood to plasma and platelet ratios, the timing of blood product transfusion, and the use of institutional transfusion protocols. Sometimes they give completely different answers.
Despite these variabilities, I think what we are seeing is that early use of blood products in the correct patient population does result in better survival. It’s a logical question for the trauma researcher to then ask, how early should blood be given? How early can blood be given?
The authors identified over 1,400 patients who met inclusion criteria. Ultimately, they demonstrated a statistically significant benefit to prehospital transfusion in terms of both 24-hour and 30-day mortality as well as trauma-induced coagulopathy. These are very intriguing results.
They state that the median volume of blood transfused prior to reaching the trauma center was 1.3 units, ranging from 1-2.3 units. I wonder how they can explain the fact that 1 unit of prehospital blood resulted in such a significant difference in mortality and coagulopathy. That is a lot of bang for your buck.
The low numbers in the prehospital transfusion group raise some questions, but I do agree that this is interesting research and it is worthy of prospective study.
Dr. Stephanie Savage is a surgeon at the University of Tennessee, Memphis. These are excerpts of her remarks as discussant of the study at the meeting. She reported having no financial disclosures.
The role of blood-based resuscitation in salvaging the severely injured patient remains an area of intense scrutiny. We spend a lot of time at meetings talking about this very subject.
The authors note that there are multiple studies that have looked at blood-based resuscitation, including things like blood to plasma and platelet ratios, the timing of blood product transfusion, and the use of institutional transfusion protocols. Sometimes they give completely different answers.
Despite these variabilities, I think what we are seeing is that early use of blood products in the correct patient population does result in better survival. It’s a logical question for the trauma researcher to then ask, how early should blood be given? How early can blood be given?
The authors identified over 1,400 patients who met inclusion criteria. Ultimately, they demonstrated a statistically significant benefit to prehospital transfusion in terms of both 24-hour and 30-day mortality as well as trauma-induced coagulopathy. These are very intriguing results.
They state that the median volume of blood transfused prior to reaching the trauma center was 1.3 units, ranging from 1-2.3 units. I wonder how they can explain the fact that 1 unit of prehospital blood resulted in such a significant difference in mortality and coagulopathy. That is a lot of bang for your buck.
The low numbers in the prehospital transfusion group raise some questions, but I do agree that this is interesting research and it is worthy of prospective study.
Dr. Stephanie Savage is a surgeon at the University of Tennessee, Memphis. These are excerpts of her remarks as discussant of the study at the meeting. She reported having no financial disclosures.
SAN FRANCISCO – Giving patients severely injured by blunt trauma a blood transfusion before they arrived at a trauma center was associated with a 95% reduction in deaths within 24 hours and a 64% reduction in deaths within 30 days, a retrospective study of 1,415 patients found.
Transfusion before arrival at the trauma center also was associated with an 88% reduction in the incidence of trauma-induced coagulopathy, Dr. Joshua B. Brown and his associates reported at the annual meeting of the American Association for the Surgery of Trauma.
They analyzed data from the prospective Inflammation and Host Response to Injury cohort study for patients with blunt injury in hemorrhagic shock who arrived at a trauma center within 2 hours of injury, 50 of whom received a blood transfusion before arrival. The investigators found that prearrival blood transfusion was associated with better outcomes after controlling for the effects of demographics, time to the trauma center, the severity of injury and shock, early resuscitation, and other confounders.
These preliminary data are compelling but require prospective validation, said Dr. Brown of the University of Pittsburgh.
Prehospital resuscitation of patients severely injured by blunt trauma has focused on use of crystalloids, and the next logical step is to bring blood-based resuscitation to prehospital settings, he said. Hemorrhage and coagulopathy have been major causes of death in blunt trauma patients.
In the study, patients who got a transfusion before arrival at the trauma center received a median of 1.3 units of blood prearrival. They were more likely to be hypotensive and to have a lower base deficit compared with patients who were not transfused before arrival, suggesting a higher severity of injury and shock in the transfusion group, he said. The groups did not differ significantly in age, gender, or Injury Severity Score.
Patients who received a transfusion before arrival at a trauma center showed a 95% lower 24-hour mortality rate, a 64% lower 30-day mortality rate, and an 88% lower risk of trauma-induced coagulopathy.
In a subsequent matched cohort analysis of 113 patients from the study, those receiving a pre–trauma center transfusion (35 patients) had a 98% reduction in mortality at 24 hours, an 88% reduction in 30-day mortality, and a 99% reduction in the risk of trauma-induced coagulopathy, Dr. Brown reported.
Dr. Brown reported having no financial disclosures.
[email protected]
On Twitter @sherryboschert
SAN FRANCISCO – Giving patients severely injured by blunt trauma a blood transfusion before they arrived at a trauma center was associated with a 95% reduction in deaths within 24 hours and a 64% reduction in deaths within 30 days, a retrospective study of 1,415 patients found.
Transfusion before arrival at the trauma center also was associated with an 88% reduction in the incidence of trauma-induced coagulopathy, Dr. Joshua B. Brown and his associates reported at the annual meeting of the American Association for the Surgery of Trauma.
They analyzed data from the prospective Inflammation and Host Response to Injury cohort study for patients with blunt injury in hemorrhagic shock who arrived at a trauma center within 2 hours of injury, 50 of whom received a blood transfusion before arrival. The investigators found that prearrival blood transfusion was associated with better outcomes after controlling for the effects of demographics, time to the trauma center, the severity of injury and shock, early resuscitation, and other confounders.
These preliminary data are compelling but require prospective validation, said Dr. Brown of the University of Pittsburgh.
Prehospital resuscitation of patients severely injured by blunt trauma has focused on use of crystalloids, and the next logical step is to bring blood-based resuscitation to prehospital settings, he said. Hemorrhage and coagulopathy have been major causes of death in blunt trauma patients.
In the study, patients who got a transfusion before arrival at the trauma center received a median of 1.3 units of blood prearrival. They were more likely to be hypotensive and to have a lower base deficit compared with patients who were not transfused before arrival, suggesting a higher severity of injury and shock in the transfusion group, he said. The groups did not differ significantly in age, gender, or Injury Severity Score.
Patients who received a transfusion before arrival at a trauma center showed a 95% lower 24-hour mortality rate, a 64% lower 30-day mortality rate, and an 88% lower risk of trauma-induced coagulopathy.
In a subsequent matched cohort analysis of 113 patients from the study, those receiving a pre–trauma center transfusion (35 patients) had a 98% reduction in mortality at 24 hours, an 88% reduction in 30-day mortality, and a 99% reduction in the risk of trauma-induced coagulopathy, Dr. Brown reported.
Dr. Brown reported having no financial disclosures.
[email protected]
On Twitter @sherryboschert
AT THE AAST ANNUAL MEETING
Major finding: Patients transfused before arrival at a trauma center showed a 95% lower 24-hour mortality rate, a 64% lower 30-day mortality rate, and an 88% lower risk of trauma-induced coagulopathy.
Data source: Secondary retrospective analysis of data from a prospective cohort study on 1,415 patients with blunt injury and hemorrhagic shock who arrived at a trauma center within 2 hours of injury.
Disclosures: Dr. Brown reported having no financial disclosures.