User login
Perioperative cardiovascular medicine: 5 questions for 2018
A plethora of studies are under way in the field of perioperative medicine. As a result, evidence-based care of surgical patients is evolving at an exponential rate.
We performed a literature search and, using consensus, identified recent articles we believe will have a great impact on perioperative cardiovascular medicine. These articles report studies that were presented at national meetings in 2018, including the Perioperative Medicine Summit, Society of General Internal Medicine, and Society of Hospital Medicine. These articles are grouped under 5 questions that will help guide clinical practice in perioperative cardiovascular medicine.
SHOULD ASPIRIN BE CONTINUED PERIOPERATIVELY IN PATIENTS WITH A CORONARY STENT?
The Perioperative Ischemic Evaluation 2 (POISE-2) trial1 found that giving aspirin before surgery and throughout the early postoperative period had no significant effect on the rate of a composite of death or nonfatal myocardial infarction; moreover, aspirin increased the risk of major bleeding. However, many experts felt uncomfortable stopping aspirin preoperatively in patients taking it for secondary prophylaxis, particularly patients with a coronary stent.
[Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018; 168(4):237–244.]
This post hoc subgroup analysis2 of POISE-2 evaluated the benefit and harm of perioperative aspirin in patients who had previously undergone percutaneous coronary intervention, more than 90% of whom had received a stent. Patients were age 45 or older with atherosclerotic heart disease or risk factors for it who had previously undergone percutaneous coronary intervention and were now undergoing noncardiac surgery.
Patients who had received a bare-metal stent within the previous 6 weeks or a drug-eluting stent within 12 months before surgery were excluded because guidelines at that time said to continue dual antiplatelet therapy for that long. Recommendations have since changed; the optimal duration for dual antiplatelet therapy with drug-eluting stents is now 6 months. Second-generation drug-eluting stents pose a lower risk of stent thrombosis and require a shorter duration of dual antiplatelet therapy than first-generation drug-eluting stents. Approximately 25% of the percutaneous coronary intervention subgroup had a drug-eluting stent, but the authors did not specify the type of drug-eluting stent.
The post hoc analysis2 included a subgroup of 234 of 4,998 patients receiving aspirin and 236 of 5,012 patients receiving placebo initiated within 4 hours before surgery and continued postoperatively. The primary outcome measured was the rate of death or nonfatal myocardial infarction within 30 days after surgery, and bleeding was a secondary outcome.
Findings. Although the overall POISE-2 study found no benefit from aspirin, in the subgroup who had previously undergone percutaneous coronary intervention, aspirin significantly reduced the risk of the primary outcome, which occurred in 6% vs 11.5% of the patients:
- Absolute risk reduction 5.5% (95% confidence interval 0.4%–10.5%)
- Hazard ratio 0.50 (0.26–0.95).
The reduction was primarily due to fewer myocardial infarctions:
- Absolute risk reduction 5.9% (1.0%–10.8%)
- Hazard ratio 0.44 (0.22–0.87).
The type of stent had no effect on the primary outcome, although this subgroup analysis had limited power. In the nonpercutaneous coronary intervention subgroup, there was no significant difference in outcomes between the aspirin and placebo groups. This subgroup analysis was underpowered to evaluate the effect of aspirin on the composite of major and life-threatening bleeding in patients with prior percutaneous coronary intervention, which was reported as “uncertain” due to wide confidence intervals (absolute risk increase 1.3%, 95% confidence interval –2.6% to 5.2%), but the increased risk of major or life-threatening bleeding with aspirin demonstrated in the overall POISE-2 study population likely applies:
- Absolute risk increase 0.8% (0.1%–1.6%)
- Hazard ratio 1.22 (1.01–1.48).
Limitations. This was a nonspecified subgroup analysis that was underpowered and had a relatively small sample size with few events.
Conclusion. In the absence of a very high bleeding risk, continuing aspirin perioperatively in patients with prior percutaneous coronary intervention undergoing noncardiac surgery is more likely to result in benefit than harm. This finding is in agreement with current recommendations from the American College Cardiology and American Heart Association (class I; level of evidence C).3
WHAT IS THE INCIDENCE OF MINS? IS MEASURING TROPONIN USEFUL?
Despite advances in anesthesia and surgical techniques, about 1% of patients over age 45 die within 30 days of noncardiac surgery.4 Studies have demonstrated a high mortality rate in patients who experience myocardial injury after noncardiac surgery (MINS), defined as elevations of troponin T with or without ischemic symptoms or electrocardiographic changes.5 Most of these studies used earlier, “non-high-sensitivity” troponin T assays. Fifth-generation, highly sensitive troponin T assays are now available that can detect troponin T at lower concentrations, but their utility in predicting postoperative outcomes remains uncertain. Two recent studies provide further insight into these issues.
[Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, Sigamani A, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2017; 317(16):1642–1651.]
The Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) study5 was an international, prospective cohort study that initially evaluated the association between MINS and the 30-day mortality rate using a non-high-sensitivity troponin T assay (Roche fourth-generation Elecsys TnT assay) in patients age 45 or older undergoing noncardiac surgery and requiring hospital admission for at least 1 night. After the first 15,000 patients, the study switched to the Roche fifth-generation assay, with measurements at 6 to 12 hours after surgery and on postoperative days 1, 2, and 3.
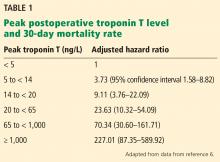
A 2017 analysis by Devereaux et al6 included only these later-enrolled patients and correlated their high-sensitivity troponin T levels with 30-day mortality rates. Patients with a level 14 ng/L or higher, the upper limit of normal in this study, were also assessed for ischemic symptoms and electrocardiographic changes. Although not required by the study, more than 7,800 patients had their troponin T levels measured before surgery, and the absolute change was also analyzed for an association with the 30-day mortality rate.
Findings. Of the 21,842 patients, about two-thirds underwent some form of major surgery; some of them had more than 1 type. A total of 1.2% of the patients died within 30 days of surgery.
Based on their analysis, the authors proposed that MINS be defined as:
- A postoperative troponin T level of 65 ng/L or higher, or
- A level in the range of 20 ng/L to less than 65 ng/L with an absolute increase from the preoperative level at least 5 ng/L, not attributable to a nonischemic cause.
Seventeen percent of the study patients met these criteria, and of these, 21.7% met the universal definition of myocardial infarction, although only 6.9% had symptoms of it.
Limitations. Only 40.4% of the patients had a preoperative high-sensitivity troponin T measurement for comparison, and in 13.8% of patients who had an elevated perioperative measurement, their preoperative value was the same or higher than their postoperative one. Thus, the incidence of MINS may have been overestimated if patients were otherwise not known to have troponin T elevations before surgery.
[Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation 2018; 137(12):1221–1232.]
Puelacher et al7 investigated the prevalence of MINS in 2,018 patients at increased cardiovascular risk (age ≥ 65, or age ≥ 45 with a history of coronary artery disease, peripheral vascular disease, or stroke) who underwent major noncardiac surgery (planned overnight stay ≥ 24 hours) at a university hospital in Switzerland. Patients had their troponin T measured with a high-sensitivity assay within 30 days before surgery and on postoperative days 1 and 2.
Instead of MINS, the investigators used the term “perioperative myocardial injury” (PMI), defined as an absolute increase in troponin T of at least 14 ng/L from before surgery to the peak postoperative reading. Similar to MINS, PMI did not require ischemic features, but in this study, noncardiac triggers (sepsis, stroke, or pulmonary embolus) were not excluded.
Findings. PMI occurred in 16% of surgeries, and of the patients with PMI, 6% had typical chest pain and 18% had any ischemic symptoms. Unlike in the POISE-2 study discussed above, PMI triggered an automatic referral to a cardiologist.
The unadjusted 30-day mortality rate was 8.9% among patients with PMI and 1.5% in those without. Multivariable logistic regression analysis showed an adjusted hazard ratio for 30-day mortality of 2.7 (95% CI 1.5–4.8) for those with PMI vs without, and this difference persisted for at least 1 year.
In patients with PMI, the authors compared the 30-day mortality rate of those with no ischemic signs or symptoms (71% of the patients) with those who met the criteria for myocardial infarction and found no difference. Patients with PMI triggered by a noncardiac event had a worse prognosis than those with a presumed cardiac etiology.
Limitations. Despite the multivariate analysis that included adjustment for age, nonelective surgery, and Revised Cardiac Risk Index (RCRI), the increased risk associated with PMI could simply reflect higher risk at baseline. Although PMI resulted in automatic referral to a cardiologist, only 10% of patients eventually underwent coronary angiography; a similar percentage were discharged with additional medical therapy such as aspirin, a statin, or a beta-blocker. The effect of these interventions is not known.
Conclusions. MINS is common and has a strong association with mortality risk proportional to the degree of troponin T elevation using high-sensitivity assays, consistent with data from previous studies of earlier assays. Because the mechanism of MINS may differ from that of myocardial infarction, its prevention and treatment may differ, and it remains unclear how serial measurement in postoperative patients should change clinical practice.
The recently published Dabigatran in Patients With Myocardial Injury After Non-cardiac Surgery (MANAGE) trial8 suggests that dabigatran may reduce arterial and venous complications in patients with MINS, but the study had a number of limitations that may restrict the clinical applicability of this finding.
While awaiting further clinical outcomes data, pre- and postoperative troponin T measurement may be beneficial in higher-risk patients (such as those with cardiovascular disease or multiple RCRI risk factors) if the information will change perioperative management.
WHAT IS THE ROLE OF HYPOTENSION OR BLOOD PRESSURE CONTROL?
Intraoperative hypotension is associated with organ ischemia, which may cause postoperative myocardial infarction, myocardial injury, and acute kidney injury.9 Traditional anesthesia practice is to maintain intraoperative blood pressure within 20% of the preoperative baseline, based on the notion that hypertensive patients require higher perfusion pressures.
[Futier E, Lefrant J-Y, Guinot P-G, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA 2017; 318(14):1346–1357.]
Futier et al10 sought to address uncertainty in intraoperative and immediate postoperative management of systolic blood pressure. In this multicenter, randomized, parallel-group trial, 298 patients at increased risk of postoperative renal complications were randomized to blood pressure management that was either “individualized” (within 10% of resting systolic pressure) or “standard” (≥ 80 mm Hg or ≥ 40% of resting systolic pressure) from induction to 4 hours postoperatively.
Blood pressure was monitored using radial arterial lines and maintained using a combination of intravenous fluids, norepinephrine (the first-line agent for the individualized group), and ephedrine (in the standard treatment group only). The primary outcome was a composite of systemic inflammatory response syndrome (SIRS) and organ dysfunction affecting at least 1 organ system (cardiovascular, respiratory, renal, hematologic, or neurologic).
Findings. Data on the primary outcome were available for 292 of 298 patients enrolled. The mean age was 70 years, 15% were women, and 82% had previously diagnosed hypertension. Despite the requirement for an elevated risk of acute kidney injury, only 13% of the patients had a baseline estimated glomerular filtration rate of less than 60 mL/min/1.73 m2, and the median was 88 mL/min/1.73 m2. Ninety-five percent of patients underwent abdominal surgery, and 50% of the surgeries were elective.
The mean systolic blood pressure was 123 mm Hg in the individualized treatment group compared with 116 mm Hg in the standard treatment group. Despite this small difference, 96% of individualized treatment patients received norepinephrine, compared with 26% in the standard treatment group.
The primary outcome of SIRS with organ dysfunction occurred in 38.1% of patients in the individualized treatment group and 51.7% of those in the standard treatment group. After adjusting for center, surgical urgency, surgical site, and acute kidney injury risk index, the relative risk of developing SIRS in those receiving individualized management was 0.73 (P = .02). Renal dysfunction (based on Acute Dialysis Quality Initiative criteria11) occurred in 32.7% of individualized treatment patients and 49% of standardized treatment patients.
Limitations of this study included differences in pharmacologic approach to maintain blood pressure in the 2 protocols (ephedrine and fluids vs norepinephrine) and a modest sample size.
Conclusions. Despite this, the difference in organ dysfunction was striking, with a number needed to treat of only 7 patients. This intervention extended 4 hours postoperatively, a time when many of these patients have left the postanesthesia care unit and have returned to hospitalist care on inpatient wards.
While optimal management of intraoperative and immediate postoperative blood pressure may not be settled, this study suggests that even mild relative hypotension may justify immediate action. Further studies may be useful to delineate high- and low-risk populations, the timing of greatest risk, and indications for intraarterial blood pressure monitoring.
[Salmasi V, Maheswari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology 2017; 126(1):47–65.]
This retrospective cohort study12 assessed the association between myocardial or kidney injury and absolute or relative thresholds of intraoperative mean arterial pressure. It included 57,315 adults who underwent inpatient noncardiac surgery, had a preoperative and at least 1 postoperative serum creatinine measurement within 7 days, and had blood pressure recorded in preoperative appointments within 6 months. Patients with chronic kidney disease (glomerular filtration rate < 60 mL/min/1.73 m2) and those on dialysis were excluded. The outcomes were MINS5 and acute kidney injury as defined by the Acute Kidney Injury Network.9
Findings. A mean arterial pressure below an absolute threshold of 65 mm Hg or a relative threshold of 20% lower than baseline value was associated with myocardial and kidney injury. At each threshold, prolonged periods of hypotension were associated with progressively increased risk.
An important conclusion of the study was that relative thresholds of mean arterial pressure were not any more predictive than absolute thresholds. Absolute thresholds are easier to use intraoperatively, especially when baseline values are not available. The authors did not find a clinically significant interaction between baseline blood pressure and the association of hypotension and myocardial and kidney injury.
Limitations included use of cardiac enzymes postoperatively to define MINS. Since these were not routinely collected, clinically silent myocardial injury may have been missed. Baseline blood pressure may have important implications in other forms of organ injury (ie, cerebral ischemia) that were not studied.
Summary. The lowest absolute mean arterial pressure is as predictive of postoperative myocardial and kidney injury as the relative pressure reduction, at least in patients with normal renal function. Limiting exposure to intraoperative hypotension is important. Baseline blood pressure values may have limited utility for intraoperative management.
In combination, these studies confirm that intraoperative hypotension is a predictor of postoperative organ dysfunction, but the definition and management remain unclear. While aggressive intraoperative management is likely beneficial, how to manage the antihypertensive therapy the patient has been taking as an outpatient when he or she comes into the hospital for surgery remains uncertain.
DOES PATENT FORAMEN OVALE INCREASE THE RISK OF STROKE?
Perioperative stroke is an uncommon, severe complication of noncardiac surgery. The pathophysiology has been better defined in cardiac than in noncardiac surgeries. In nonsurgical patients, patent foramen ovale (PFO) is associated with stroke, even in patients considered to be at low risk.13 Perioperative patients have additional risk for venous thromboembolism and may have periprocedural antithrombotic medications altered, increasing their risk of paradoxical embolism through the PFO.
[Ng PY, Ng AK, Subramaniam B, et al. Association of preoperatively diagnosed patent foramen ovale with perioperative ischemic stroke. JAMA 2018; 319(5):452–462.]
This retrospective cohort study of noncardiac surgery patients at 3 hospitals14 sought to determine the association of preoperatively diagnosed PFO with the risk of perioperative ischemic stroke identified by International Classification of Diseases diagnoses.
Of 150,198 patients, 1.0% had a preoperative diagnosis of PFO, and at baseline, those with PFO had significantly more comorbidities than those without PFO. Stroke occurred in 3.2% of patients with PFO vs 0.5% of those without. Patients known to have a PFO were much more likely to have cardiovascular and thromboembolic risk factors for stroke. In the adjusted analysis, the absolute risk difference between groups was 0.4% (95% CI 0.2–0.6%), with an estimated perioperative stroke risk of 5.9 per 1,000 in patients with known patent foramen ovale and 2.2 per 1,000 in those without. A diagnosis of PFO was also associated with increased risk of large-vessel-territory stroke and more severe neurologic deficit.
Further attempts to adjust for baseline risk factors and other potential bias, including a propensity score-matched cohort analysis and an analysis limited to patients who had echocardiography performed in the same healthcare system, still showed a higher risk of perioperative stroke among patients with preoperatively detected patent foramen ovale.
Limitations. The study was retrospective and observational, used administrative data, and had a low rate of PFO diagnosis (1%), compared with about 25% in population-based studies.15 Indications for preoperative echocardiography are unknown. In addition, the study specifically examined preoperatively diagnosed PFO, rather than including those diagnosed in the postoperative period.
Discussion. How does this study affect clinical practice? The absolute stroke risk was increased by 0.4% in patients with PFO compared with those without. Although this is a relatively small increase, millions of patients undergo noncardiac surgery annually. The risks of therapeutic anticoagulation or PFO closure are likely too high in this context; however, clinicians may approach the perioperative management of antiplatelet agents and venous thromboembolism prophylaxis in patients with known PFO with additional caution.
HOW DOES TIMING OF EMERGENCY SURGERY AFTER PRIOR STROKE AFFECT OUTCOMES?
A history of stroke or transient ischemic attack is a known risk factor for perioperative vascular complications. A recent large cohort study demonstrated that a history of stroke within 9 months of elective surgery was associated with increased adverse outcomes.16 Little is known, however, of the perioperative risk in patients with a history of stroke who undergo emergency surgery.
[Christiansen MN, Andersson C, Gislason GH, et al. Risks of cardiovascular adverse events and death in patients with previous stroke undergoing emergency noncardiac, nonintracranial surgery: the importance of operative timing. Anesthesiology 2017; 127(1):9–19.]
In this study,17 all emergency noncardiac and nonintracranial surgeries from 2005 to 2011 were analyzed using multiple national patient registries in Denmark according to time elapsed between previous stroke and surgery. Primary outcomes were 30-day all-cause mortality and 30-day major adverse cardiac events (MACE), defined as nonfatal ischemic stroke, nonfatal myocardial infarction, and cardiovascular death. Statistical analysis to assess the risk of adverse outcomes included logistic regression models, spline analyses, and propensity-score matching.
Findings. The authors identified 146,694 emergency surgeries, with 7,861 patients (5.4%) having had a previous stroke (transient ischemic attacks and hemorrhagic strokes were not included). Rates of postoperative stroke were as follows:
- 9.9% in patents with a history of ischemic stroke within 3 months of surgery
- 2.8% in patients with a history of stroke 3 to 9 months before surgery
- 0.3% in patients with no previous stroke.
The risk plateaued when the time between stroke and surgery exceeded 4 to 5 months.15
Interestingly, in patients who underwent emergency surgery within 14 days of stroke, the risk of MACE was significantly lower immediately after surgery (1–3 days after stroke) compared with surgery that took place 4 to 14 days after stroke. The authors hypothesized that because cerebral autoregulation does not become compromised until approximately 5 days after a stroke, the risk was lower 1 to 3 days after surgery and increased thereafter.
Limitations of this study included the possibility of residual confounding, given its retrospective design using administrative data, not accounting for preoperative antithrombotic and anticoagulation therapy, and lack of information regarding the etiology of recurrent stroke (eg, thromboembolic, atherothrombotic, hypoperfusion).
Conclusions. Although it would be impractical to postpone emergency surgery in a patient who recently had a stroke, this study shows that the incidence rates of postoperative recurrent stroke and MACE are high. Therefore, it is important that the patient and perioperative team be aware of the risk. Further research is needed to confirm these estimates of postoperative adverse events in more diverse patient populations.
- Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018; 168(4):237–244. doi:10.7326/M17-2341
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 130(24):2215–2245. doi:10.1161/CIR.0000000000000105
- Smilowitz NR, Gupta N, Ramakrishna H, Guo Y, Berger JS, Bangalore S. Perioperative major adverse cardiovascular and cerebrovascular events associated with noncardiac surgery. JAMA Cardiol 2017; 2(2):181–187. doi:10.1001/jamacardio.2016.4792
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120(3):564–578. doi:10.1097/ALN.0000000000000113
- Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, Sigamani A, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2017; 317(16):1642–1651. doi:10.1001/jama.2017.4360
- Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation 2018; 137(12):1221–1232. doi:10.1161/CIRCULATIONAHA.117.030114
- Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8
- Walsh M, Devereaux PJ, Garg AX, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology 2013; 119(3):507–515. doi:10.1097/ALN.0b013e3182a10e26
- Futier E, Lefrant JY, Guinot PG, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA 2017; 318(14):1346–1357. doi:10.1001/jama.2017.14172
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) group. Crit Care 2004; 8:R204. doi:10.1186/cc2872
- Salmasi V, Maheswari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology 2017; 126(1):47–65. doi:10.1097/ALN.0000000000001432
- Lechat P, Mas JL, Lascault G, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med 1988; 318(18):1148–1152. doi:10.1056/NEJM198805053181802
- Ng PY, Ng AK, Subramaniam B, et al. Association of preoperatively diagnosed patent foramen ovale with perioperative ischemic stroke. JAMA 2018; 319(5):452–462. doi:10.1001/jama.2017.21899
- Meissner I, Whisnant JP, Khandheria BK, et al. Prevalence of potential risk factors for stroke assessed by transesophageal echocardiography and carotid ultrasonography: the SPARC study. Stroke Prevention: Assessment of Risk in a Community. Mayo Clin Proc 1999; 74(9):862–869. pmid:10488786
- Jørgensen ME, Torp-Pedersen C, Gislason GH, et al. Time elapsed after ischemic stroke and risk of adverse cardiovascular events and mortality following elective noncardiac surgery. JAMA 2014; 312:269–277. doi:10.1001/jama.2014.8165
- Christiansen MN, Andersson C, Gislason GH, et al. Risks of cardiovascular adverse events and death in patients with previous stroke undergoing emergency noncardiac, nonintracranial surgery: the importance of operative timing. Anesthesiology 2017; 127(1):9–19. doi:10.1097/ALN.0000000000001685
A plethora of studies are under way in the field of perioperative medicine. As a result, evidence-based care of surgical patients is evolving at an exponential rate.
We performed a literature search and, using consensus, identified recent articles we believe will have a great impact on perioperative cardiovascular medicine. These articles report studies that were presented at national meetings in 2018, including the Perioperative Medicine Summit, Society of General Internal Medicine, and Society of Hospital Medicine. These articles are grouped under 5 questions that will help guide clinical practice in perioperative cardiovascular medicine.
SHOULD ASPIRIN BE CONTINUED PERIOPERATIVELY IN PATIENTS WITH A CORONARY STENT?
The Perioperative Ischemic Evaluation 2 (POISE-2) trial1 found that giving aspirin before surgery and throughout the early postoperative period had no significant effect on the rate of a composite of death or nonfatal myocardial infarction; moreover, aspirin increased the risk of major bleeding. However, many experts felt uncomfortable stopping aspirin preoperatively in patients taking it for secondary prophylaxis, particularly patients with a coronary stent.
[Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018; 168(4):237–244.]
This post hoc subgroup analysis2 of POISE-2 evaluated the benefit and harm of perioperative aspirin in patients who had previously undergone percutaneous coronary intervention, more than 90% of whom had received a stent. Patients were age 45 or older with atherosclerotic heart disease or risk factors for it who had previously undergone percutaneous coronary intervention and were now undergoing noncardiac surgery.
Patients who had received a bare-metal stent within the previous 6 weeks or a drug-eluting stent within 12 months before surgery were excluded because guidelines at that time said to continue dual antiplatelet therapy for that long. Recommendations have since changed; the optimal duration for dual antiplatelet therapy with drug-eluting stents is now 6 months. Second-generation drug-eluting stents pose a lower risk of stent thrombosis and require a shorter duration of dual antiplatelet therapy than first-generation drug-eluting stents. Approximately 25% of the percutaneous coronary intervention subgroup had a drug-eluting stent, but the authors did not specify the type of drug-eluting stent.
The post hoc analysis2 included a subgroup of 234 of 4,998 patients receiving aspirin and 236 of 5,012 patients receiving placebo initiated within 4 hours before surgery and continued postoperatively. The primary outcome measured was the rate of death or nonfatal myocardial infarction within 30 days after surgery, and bleeding was a secondary outcome.
Findings. Although the overall POISE-2 study found no benefit from aspirin, in the subgroup who had previously undergone percutaneous coronary intervention, aspirin significantly reduced the risk of the primary outcome, which occurred in 6% vs 11.5% of the patients:
- Absolute risk reduction 5.5% (95% confidence interval 0.4%–10.5%)
- Hazard ratio 0.50 (0.26–0.95).
The reduction was primarily due to fewer myocardial infarctions:
- Absolute risk reduction 5.9% (1.0%–10.8%)
- Hazard ratio 0.44 (0.22–0.87).
The type of stent had no effect on the primary outcome, although this subgroup analysis had limited power. In the nonpercutaneous coronary intervention subgroup, there was no significant difference in outcomes between the aspirin and placebo groups. This subgroup analysis was underpowered to evaluate the effect of aspirin on the composite of major and life-threatening bleeding in patients with prior percutaneous coronary intervention, which was reported as “uncertain” due to wide confidence intervals (absolute risk increase 1.3%, 95% confidence interval –2.6% to 5.2%), but the increased risk of major or life-threatening bleeding with aspirin demonstrated in the overall POISE-2 study population likely applies:
- Absolute risk increase 0.8% (0.1%–1.6%)
- Hazard ratio 1.22 (1.01–1.48).
Limitations. This was a nonspecified subgroup analysis that was underpowered and had a relatively small sample size with few events.
Conclusion. In the absence of a very high bleeding risk, continuing aspirin perioperatively in patients with prior percutaneous coronary intervention undergoing noncardiac surgery is more likely to result in benefit than harm. This finding is in agreement with current recommendations from the American College Cardiology and American Heart Association (class I; level of evidence C).3
WHAT IS THE INCIDENCE OF MINS? IS MEASURING TROPONIN USEFUL?
Despite advances in anesthesia and surgical techniques, about 1% of patients over age 45 die within 30 days of noncardiac surgery.4 Studies have demonstrated a high mortality rate in patients who experience myocardial injury after noncardiac surgery (MINS), defined as elevations of troponin T with or without ischemic symptoms or electrocardiographic changes.5 Most of these studies used earlier, “non-high-sensitivity” troponin T assays. Fifth-generation, highly sensitive troponin T assays are now available that can detect troponin T at lower concentrations, but their utility in predicting postoperative outcomes remains uncertain. Two recent studies provide further insight into these issues.
[Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, Sigamani A, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2017; 317(16):1642–1651.]
The Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) study5 was an international, prospective cohort study that initially evaluated the association between MINS and the 30-day mortality rate using a non-high-sensitivity troponin T assay (Roche fourth-generation Elecsys TnT assay) in patients age 45 or older undergoing noncardiac surgery and requiring hospital admission for at least 1 night. After the first 15,000 patients, the study switched to the Roche fifth-generation assay, with measurements at 6 to 12 hours after surgery and on postoperative days 1, 2, and 3.
A 2017 analysis by Devereaux et al6 included only these later-enrolled patients and correlated their high-sensitivity troponin T levels with 30-day mortality rates. Patients with a level 14 ng/L or higher, the upper limit of normal in this study, were also assessed for ischemic symptoms and electrocardiographic changes. Although not required by the study, more than 7,800 patients had their troponin T levels measured before surgery, and the absolute change was also analyzed for an association with the 30-day mortality rate.
Findings. Of the 21,842 patients, about two-thirds underwent some form of major surgery; some of them had more than 1 type. A total of 1.2% of the patients died within 30 days of surgery.
Based on their analysis, the authors proposed that MINS be defined as:
- A postoperative troponin T level of 65 ng/L or higher, or
- A level in the range of 20 ng/L to less than 65 ng/L with an absolute increase from the preoperative level at least 5 ng/L, not attributable to a nonischemic cause.
Seventeen percent of the study patients met these criteria, and of these, 21.7% met the universal definition of myocardial infarction, although only 6.9% had symptoms of it.
Limitations. Only 40.4% of the patients had a preoperative high-sensitivity troponin T measurement for comparison, and in 13.8% of patients who had an elevated perioperative measurement, their preoperative value was the same or higher than their postoperative one. Thus, the incidence of MINS may have been overestimated if patients were otherwise not known to have troponin T elevations before surgery.
[Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation 2018; 137(12):1221–1232.]
Puelacher et al7 investigated the prevalence of MINS in 2,018 patients at increased cardiovascular risk (age ≥ 65, or age ≥ 45 with a history of coronary artery disease, peripheral vascular disease, or stroke) who underwent major noncardiac surgery (planned overnight stay ≥ 24 hours) at a university hospital in Switzerland. Patients had their troponin T measured with a high-sensitivity assay within 30 days before surgery and on postoperative days 1 and 2.
Instead of MINS, the investigators used the term “perioperative myocardial injury” (PMI), defined as an absolute increase in troponin T of at least 14 ng/L from before surgery to the peak postoperative reading. Similar to MINS, PMI did not require ischemic features, but in this study, noncardiac triggers (sepsis, stroke, or pulmonary embolus) were not excluded.
Findings. PMI occurred in 16% of surgeries, and of the patients with PMI, 6% had typical chest pain and 18% had any ischemic symptoms. Unlike in the POISE-2 study discussed above, PMI triggered an automatic referral to a cardiologist.
The unadjusted 30-day mortality rate was 8.9% among patients with PMI and 1.5% in those without. Multivariable logistic regression analysis showed an adjusted hazard ratio for 30-day mortality of 2.7 (95% CI 1.5–4.8) for those with PMI vs without, and this difference persisted for at least 1 year.
In patients with PMI, the authors compared the 30-day mortality rate of those with no ischemic signs or symptoms (71% of the patients) with those who met the criteria for myocardial infarction and found no difference. Patients with PMI triggered by a noncardiac event had a worse prognosis than those with a presumed cardiac etiology.
Limitations. Despite the multivariate analysis that included adjustment for age, nonelective surgery, and Revised Cardiac Risk Index (RCRI), the increased risk associated with PMI could simply reflect higher risk at baseline. Although PMI resulted in automatic referral to a cardiologist, only 10% of patients eventually underwent coronary angiography; a similar percentage were discharged with additional medical therapy such as aspirin, a statin, or a beta-blocker. The effect of these interventions is not known.
Conclusions. MINS is common and has a strong association with mortality risk proportional to the degree of troponin T elevation using high-sensitivity assays, consistent with data from previous studies of earlier assays. Because the mechanism of MINS may differ from that of myocardial infarction, its prevention and treatment may differ, and it remains unclear how serial measurement in postoperative patients should change clinical practice.
The recently published Dabigatran in Patients With Myocardial Injury After Non-cardiac Surgery (MANAGE) trial8 suggests that dabigatran may reduce arterial and venous complications in patients with MINS, but the study had a number of limitations that may restrict the clinical applicability of this finding.
While awaiting further clinical outcomes data, pre- and postoperative troponin T measurement may be beneficial in higher-risk patients (such as those with cardiovascular disease or multiple RCRI risk factors) if the information will change perioperative management.
WHAT IS THE ROLE OF HYPOTENSION OR BLOOD PRESSURE CONTROL?
Intraoperative hypotension is associated with organ ischemia, which may cause postoperative myocardial infarction, myocardial injury, and acute kidney injury.9 Traditional anesthesia practice is to maintain intraoperative blood pressure within 20% of the preoperative baseline, based on the notion that hypertensive patients require higher perfusion pressures.
[Futier E, Lefrant J-Y, Guinot P-G, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA 2017; 318(14):1346–1357.]
Futier et al10 sought to address uncertainty in intraoperative and immediate postoperative management of systolic blood pressure. In this multicenter, randomized, parallel-group trial, 298 patients at increased risk of postoperative renal complications were randomized to blood pressure management that was either “individualized” (within 10% of resting systolic pressure) or “standard” (≥ 80 mm Hg or ≥ 40% of resting systolic pressure) from induction to 4 hours postoperatively.
Blood pressure was monitored using radial arterial lines and maintained using a combination of intravenous fluids, norepinephrine (the first-line agent for the individualized group), and ephedrine (in the standard treatment group only). The primary outcome was a composite of systemic inflammatory response syndrome (SIRS) and organ dysfunction affecting at least 1 organ system (cardiovascular, respiratory, renal, hematologic, or neurologic).
Findings. Data on the primary outcome were available for 292 of 298 patients enrolled. The mean age was 70 years, 15% were women, and 82% had previously diagnosed hypertension. Despite the requirement for an elevated risk of acute kidney injury, only 13% of the patients had a baseline estimated glomerular filtration rate of less than 60 mL/min/1.73 m2, and the median was 88 mL/min/1.73 m2. Ninety-five percent of patients underwent abdominal surgery, and 50% of the surgeries were elective.
The mean systolic blood pressure was 123 mm Hg in the individualized treatment group compared with 116 mm Hg in the standard treatment group. Despite this small difference, 96% of individualized treatment patients received norepinephrine, compared with 26% in the standard treatment group.
The primary outcome of SIRS with organ dysfunction occurred in 38.1% of patients in the individualized treatment group and 51.7% of those in the standard treatment group. After adjusting for center, surgical urgency, surgical site, and acute kidney injury risk index, the relative risk of developing SIRS in those receiving individualized management was 0.73 (P = .02). Renal dysfunction (based on Acute Dialysis Quality Initiative criteria11) occurred in 32.7% of individualized treatment patients and 49% of standardized treatment patients.
Limitations of this study included differences in pharmacologic approach to maintain blood pressure in the 2 protocols (ephedrine and fluids vs norepinephrine) and a modest sample size.
Conclusions. Despite this, the difference in organ dysfunction was striking, with a number needed to treat of only 7 patients. This intervention extended 4 hours postoperatively, a time when many of these patients have left the postanesthesia care unit and have returned to hospitalist care on inpatient wards.
While optimal management of intraoperative and immediate postoperative blood pressure may not be settled, this study suggests that even mild relative hypotension may justify immediate action. Further studies may be useful to delineate high- and low-risk populations, the timing of greatest risk, and indications for intraarterial blood pressure monitoring.
[Salmasi V, Maheswari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology 2017; 126(1):47–65.]
This retrospective cohort study12 assessed the association between myocardial or kidney injury and absolute or relative thresholds of intraoperative mean arterial pressure. It included 57,315 adults who underwent inpatient noncardiac surgery, had a preoperative and at least 1 postoperative serum creatinine measurement within 7 days, and had blood pressure recorded in preoperative appointments within 6 months. Patients with chronic kidney disease (glomerular filtration rate < 60 mL/min/1.73 m2) and those on dialysis were excluded. The outcomes were MINS5 and acute kidney injury as defined by the Acute Kidney Injury Network.9
Findings. A mean arterial pressure below an absolute threshold of 65 mm Hg or a relative threshold of 20% lower than baseline value was associated with myocardial and kidney injury. At each threshold, prolonged periods of hypotension were associated with progressively increased risk.
An important conclusion of the study was that relative thresholds of mean arterial pressure were not any more predictive than absolute thresholds. Absolute thresholds are easier to use intraoperatively, especially when baseline values are not available. The authors did not find a clinically significant interaction between baseline blood pressure and the association of hypotension and myocardial and kidney injury.
Limitations included use of cardiac enzymes postoperatively to define MINS. Since these were not routinely collected, clinically silent myocardial injury may have been missed. Baseline blood pressure may have important implications in other forms of organ injury (ie, cerebral ischemia) that were not studied.
Summary. The lowest absolute mean arterial pressure is as predictive of postoperative myocardial and kidney injury as the relative pressure reduction, at least in patients with normal renal function. Limiting exposure to intraoperative hypotension is important. Baseline blood pressure values may have limited utility for intraoperative management.
In combination, these studies confirm that intraoperative hypotension is a predictor of postoperative organ dysfunction, but the definition and management remain unclear. While aggressive intraoperative management is likely beneficial, how to manage the antihypertensive therapy the patient has been taking as an outpatient when he or she comes into the hospital for surgery remains uncertain.
DOES PATENT FORAMEN OVALE INCREASE THE RISK OF STROKE?
Perioperative stroke is an uncommon, severe complication of noncardiac surgery. The pathophysiology has been better defined in cardiac than in noncardiac surgeries. In nonsurgical patients, patent foramen ovale (PFO) is associated with stroke, even in patients considered to be at low risk.13 Perioperative patients have additional risk for venous thromboembolism and may have periprocedural antithrombotic medications altered, increasing their risk of paradoxical embolism through the PFO.
[Ng PY, Ng AK, Subramaniam B, et al. Association of preoperatively diagnosed patent foramen ovale with perioperative ischemic stroke. JAMA 2018; 319(5):452–462.]
This retrospective cohort study of noncardiac surgery patients at 3 hospitals14 sought to determine the association of preoperatively diagnosed PFO with the risk of perioperative ischemic stroke identified by International Classification of Diseases diagnoses.
Of 150,198 patients, 1.0% had a preoperative diagnosis of PFO, and at baseline, those with PFO had significantly more comorbidities than those without PFO. Stroke occurred in 3.2% of patients with PFO vs 0.5% of those without. Patients known to have a PFO were much more likely to have cardiovascular and thromboembolic risk factors for stroke. In the adjusted analysis, the absolute risk difference between groups was 0.4% (95% CI 0.2–0.6%), with an estimated perioperative stroke risk of 5.9 per 1,000 in patients with known patent foramen ovale and 2.2 per 1,000 in those without. A diagnosis of PFO was also associated with increased risk of large-vessel-territory stroke and more severe neurologic deficit.
Further attempts to adjust for baseline risk factors and other potential bias, including a propensity score-matched cohort analysis and an analysis limited to patients who had echocardiography performed in the same healthcare system, still showed a higher risk of perioperative stroke among patients with preoperatively detected patent foramen ovale.
Limitations. The study was retrospective and observational, used administrative data, and had a low rate of PFO diagnosis (1%), compared with about 25% in population-based studies.15 Indications for preoperative echocardiography are unknown. In addition, the study specifically examined preoperatively diagnosed PFO, rather than including those diagnosed in the postoperative period.
Discussion. How does this study affect clinical practice? The absolute stroke risk was increased by 0.4% in patients with PFO compared with those without. Although this is a relatively small increase, millions of patients undergo noncardiac surgery annually. The risks of therapeutic anticoagulation or PFO closure are likely too high in this context; however, clinicians may approach the perioperative management of antiplatelet agents and venous thromboembolism prophylaxis in patients with known PFO with additional caution.
HOW DOES TIMING OF EMERGENCY SURGERY AFTER PRIOR STROKE AFFECT OUTCOMES?
A history of stroke or transient ischemic attack is a known risk factor for perioperative vascular complications. A recent large cohort study demonstrated that a history of stroke within 9 months of elective surgery was associated with increased adverse outcomes.16 Little is known, however, of the perioperative risk in patients with a history of stroke who undergo emergency surgery.
[Christiansen MN, Andersson C, Gislason GH, et al. Risks of cardiovascular adverse events and death in patients with previous stroke undergoing emergency noncardiac, nonintracranial surgery: the importance of operative timing. Anesthesiology 2017; 127(1):9–19.]
In this study,17 all emergency noncardiac and nonintracranial surgeries from 2005 to 2011 were analyzed using multiple national patient registries in Denmark according to time elapsed between previous stroke and surgery. Primary outcomes were 30-day all-cause mortality and 30-day major adverse cardiac events (MACE), defined as nonfatal ischemic stroke, nonfatal myocardial infarction, and cardiovascular death. Statistical analysis to assess the risk of adverse outcomes included logistic regression models, spline analyses, and propensity-score matching.
Findings. The authors identified 146,694 emergency surgeries, with 7,861 patients (5.4%) having had a previous stroke (transient ischemic attacks and hemorrhagic strokes were not included). Rates of postoperative stroke were as follows:
- 9.9% in patents with a history of ischemic stroke within 3 months of surgery
- 2.8% in patients with a history of stroke 3 to 9 months before surgery
- 0.3% in patients with no previous stroke.
The risk plateaued when the time between stroke and surgery exceeded 4 to 5 months.15
Interestingly, in patients who underwent emergency surgery within 14 days of stroke, the risk of MACE was significantly lower immediately after surgery (1–3 days after stroke) compared with surgery that took place 4 to 14 days after stroke. The authors hypothesized that because cerebral autoregulation does not become compromised until approximately 5 days after a stroke, the risk was lower 1 to 3 days after surgery and increased thereafter.
Limitations of this study included the possibility of residual confounding, given its retrospective design using administrative data, not accounting for preoperative antithrombotic and anticoagulation therapy, and lack of information regarding the etiology of recurrent stroke (eg, thromboembolic, atherothrombotic, hypoperfusion).
Conclusions. Although it would be impractical to postpone emergency surgery in a patient who recently had a stroke, this study shows that the incidence rates of postoperative recurrent stroke and MACE are high. Therefore, it is important that the patient and perioperative team be aware of the risk. Further research is needed to confirm these estimates of postoperative adverse events in more diverse patient populations.
A plethora of studies are under way in the field of perioperative medicine. As a result, evidence-based care of surgical patients is evolving at an exponential rate.
We performed a literature search and, using consensus, identified recent articles we believe will have a great impact on perioperative cardiovascular medicine. These articles report studies that were presented at national meetings in 2018, including the Perioperative Medicine Summit, Society of General Internal Medicine, and Society of Hospital Medicine. These articles are grouped under 5 questions that will help guide clinical practice in perioperative cardiovascular medicine.
SHOULD ASPIRIN BE CONTINUED PERIOPERATIVELY IN PATIENTS WITH A CORONARY STENT?
The Perioperative Ischemic Evaluation 2 (POISE-2) trial1 found that giving aspirin before surgery and throughout the early postoperative period had no significant effect on the rate of a composite of death or nonfatal myocardial infarction; moreover, aspirin increased the risk of major bleeding. However, many experts felt uncomfortable stopping aspirin preoperatively in patients taking it for secondary prophylaxis, particularly patients with a coronary stent.
[Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018; 168(4):237–244.]
This post hoc subgroup analysis2 of POISE-2 evaluated the benefit and harm of perioperative aspirin in patients who had previously undergone percutaneous coronary intervention, more than 90% of whom had received a stent. Patients were age 45 or older with atherosclerotic heart disease or risk factors for it who had previously undergone percutaneous coronary intervention and were now undergoing noncardiac surgery.
Patients who had received a bare-metal stent within the previous 6 weeks or a drug-eluting stent within 12 months before surgery were excluded because guidelines at that time said to continue dual antiplatelet therapy for that long. Recommendations have since changed; the optimal duration for dual antiplatelet therapy with drug-eluting stents is now 6 months. Second-generation drug-eluting stents pose a lower risk of stent thrombosis and require a shorter duration of dual antiplatelet therapy than first-generation drug-eluting stents. Approximately 25% of the percutaneous coronary intervention subgroup had a drug-eluting stent, but the authors did not specify the type of drug-eluting stent.
The post hoc analysis2 included a subgroup of 234 of 4,998 patients receiving aspirin and 236 of 5,012 patients receiving placebo initiated within 4 hours before surgery and continued postoperatively. The primary outcome measured was the rate of death or nonfatal myocardial infarction within 30 days after surgery, and bleeding was a secondary outcome.
Findings. Although the overall POISE-2 study found no benefit from aspirin, in the subgroup who had previously undergone percutaneous coronary intervention, aspirin significantly reduced the risk of the primary outcome, which occurred in 6% vs 11.5% of the patients:
- Absolute risk reduction 5.5% (95% confidence interval 0.4%–10.5%)
- Hazard ratio 0.50 (0.26–0.95).
The reduction was primarily due to fewer myocardial infarctions:
- Absolute risk reduction 5.9% (1.0%–10.8%)
- Hazard ratio 0.44 (0.22–0.87).
The type of stent had no effect on the primary outcome, although this subgroup analysis had limited power. In the nonpercutaneous coronary intervention subgroup, there was no significant difference in outcomes between the aspirin and placebo groups. This subgroup analysis was underpowered to evaluate the effect of aspirin on the composite of major and life-threatening bleeding in patients with prior percutaneous coronary intervention, which was reported as “uncertain” due to wide confidence intervals (absolute risk increase 1.3%, 95% confidence interval –2.6% to 5.2%), but the increased risk of major or life-threatening bleeding with aspirin demonstrated in the overall POISE-2 study population likely applies:
- Absolute risk increase 0.8% (0.1%–1.6%)
- Hazard ratio 1.22 (1.01–1.48).
Limitations. This was a nonspecified subgroup analysis that was underpowered and had a relatively small sample size with few events.
Conclusion. In the absence of a very high bleeding risk, continuing aspirin perioperatively in patients with prior percutaneous coronary intervention undergoing noncardiac surgery is more likely to result in benefit than harm. This finding is in agreement with current recommendations from the American College Cardiology and American Heart Association (class I; level of evidence C).3
WHAT IS THE INCIDENCE OF MINS? IS MEASURING TROPONIN USEFUL?
Despite advances in anesthesia and surgical techniques, about 1% of patients over age 45 die within 30 days of noncardiac surgery.4 Studies have demonstrated a high mortality rate in patients who experience myocardial injury after noncardiac surgery (MINS), defined as elevations of troponin T with or without ischemic symptoms or electrocardiographic changes.5 Most of these studies used earlier, “non-high-sensitivity” troponin T assays. Fifth-generation, highly sensitive troponin T assays are now available that can detect troponin T at lower concentrations, but their utility in predicting postoperative outcomes remains uncertain. Two recent studies provide further insight into these issues.
[Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, Sigamani A, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2017; 317(16):1642–1651.]
The Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) study5 was an international, prospective cohort study that initially evaluated the association between MINS and the 30-day mortality rate using a non-high-sensitivity troponin T assay (Roche fourth-generation Elecsys TnT assay) in patients age 45 or older undergoing noncardiac surgery and requiring hospital admission for at least 1 night. After the first 15,000 patients, the study switched to the Roche fifth-generation assay, with measurements at 6 to 12 hours after surgery and on postoperative days 1, 2, and 3.
A 2017 analysis by Devereaux et al6 included only these later-enrolled patients and correlated their high-sensitivity troponin T levels with 30-day mortality rates. Patients with a level 14 ng/L or higher, the upper limit of normal in this study, were also assessed for ischemic symptoms and electrocardiographic changes. Although not required by the study, more than 7,800 patients had their troponin T levels measured before surgery, and the absolute change was also analyzed for an association with the 30-day mortality rate.
Findings. Of the 21,842 patients, about two-thirds underwent some form of major surgery; some of them had more than 1 type. A total of 1.2% of the patients died within 30 days of surgery.
Based on their analysis, the authors proposed that MINS be defined as:
- A postoperative troponin T level of 65 ng/L or higher, or
- A level in the range of 20 ng/L to less than 65 ng/L with an absolute increase from the preoperative level at least 5 ng/L, not attributable to a nonischemic cause.
Seventeen percent of the study patients met these criteria, and of these, 21.7% met the universal definition of myocardial infarction, although only 6.9% had symptoms of it.
Limitations. Only 40.4% of the patients had a preoperative high-sensitivity troponin T measurement for comparison, and in 13.8% of patients who had an elevated perioperative measurement, their preoperative value was the same or higher than their postoperative one. Thus, the incidence of MINS may have been overestimated if patients were otherwise not known to have troponin T elevations before surgery.
[Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation 2018; 137(12):1221–1232.]
Puelacher et al7 investigated the prevalence of MINS in 2,018 patients at increased cardiovascular risk (age ≥ 65, or age ≥ 45 with a history of coronary artery disease, peripheral vascular disease, or stroke) who underwent major noncardiac surgery (planned overnight stay ≥ 24 hours) at a university hospital in Switzerland. Patients had their troponin T measured with a high-sensitivity assay within 30 days before surgery and on postoperative days 1 and 2.
Instead of MINS, the investigators used the term “perioperative myocardial injury” (PMI), defined as an absolute increase in troponin T of at least 14 ng/L from before surgery to the peak postoperative reading. Similar to MINS, PMI did not require ischemic features, but in this study, noncardiac triggers (sepsis, stroke, or pulmonary embolus) were not excluded.
Findings. PMI occurred in 16% of surgeries, and of the patients with PMI, 6% had typical chest pain and 18% had any ischemic symptoms. Unlike in the POISE-2 study discussed above, PMI triggered an automatic referral to a cardiologist.
The unadjusted 30-day mortality rate was 8.9% among patients with PMI and 1.5% in those without. Multivariable logistic regression analysis showed an adjusted hazard ratio for 30-day mortality of 2.7 (95% CI 1.5–4.8) for those with PMI vs without, and this difference persisted for at least 1 year.
In patients with PMI, the authors compared the 30-day mortality rate of those with no ischemic signs or symptoms (71% of the patients) with those who met the criteria for myocardial infarction and found no difference. Patients with PMI triggered by a noncardiac event had a worse prognosis than those with a presumed cardiac etiology.
Limitations. Despite the multivariate analysis that included adjustment for age, nonelective surgery, and Revised Cardiac Risk Index (RCRI), the increased risk associated with PMI could simply reflect higher risk at baseline. Although PMI resulted in automatic referral to a cardiologist, only 10% of patients eventually underwent coronary angiography; a similar percentage were discharged with additional medical therapy such as aspirin, a statin, or a beta-blocker. The effect of these interventions is not known.
Conclusions. MINS is common and has a strong association with mortality risk proportional to the degree of troponin T elevation using high-sensitivity assays, consistent with data from previous studies of earlier assays. Because the mechanism of MINS may differ from that of myocardial infarction, its prevention and treatment may differ, and it remains unclear how serial measurement in postoperative patients should change clinical practice.
The recently published Dabigatran in Patients With Myocardial Injury After Non-cardiac Surgery (MANAGE) trial8 suggests that dabigatran may reduce arterial and venous complications in patients with MINS, but the study had a number of limitations that may restrict the clinical applicability of this finding.
While awaiting further clinical outcomes data, pre- and postoperative troponin T measurement may be beneficial in higher-risk patients (such as those with cardiovascular disease or multiple RCRI risk factors) if the information will change perioperative management.
WHAT IS THE ROLE OF HYPOTENSION OR BLOOD PRESSURE CONTROL?
Intraoperative hypotension is associated with organ ischemia, which may cause postoperative myocardial infarction, myocardial injury, and acute kidney injury.9 Traditional anesthesia practice is to maintain intraoperative blood pressure within 20% of the preoperative baseline, based on the notion that hypertensive patients require higher perfusion pressures.
[Futier E, Lefrant J-Y, Guinot P-G, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA 2017; 318(14):1346–1357.]
Futier et al10 sought to address uncertainty in intraoperative and immediate postoperative management of systolic blood pressure. In this multicenter, randomized, parallel-group trial, 298 patients at increased risk of postoperative renal complications were randomized to blood pressure management that was either “individualized” (within 10% of resting systolic pressure) or “standard” (≥ 80 mm Hg or ≥ 40% of resting systolic pressure) from induction to 4 hours postoperatively.
Blood pressure was monitored using radial arterial lines and maintained using a combination of intravenous fluids, norepinephrine (the first-line agent for the individualized group), and ephedrine (in the standard treatment group only). The primary outcome was a composite of systemic inflammatory response syndrome (SIRS) and organ dysfunction affecting at least 1 organ system (cardiovascular, respiratory, renal, hematologic, or neurologic).
Findings. Data on the primary outcome were available for 292 of 298 patients enrolled. The mean age was 70 years, 15% were women, and 82% had previously diagnosed hypertension. Despite the requirement for an elevated risk of acute kidney injury, only 13% of the patients had a baseline estimated glomerular filtration rate of less than 60 mL/min/1.73 m2, and the median was 88 mL/min/1.73 m2. Ninety-five percent of patients underwent abdominal surgery, and 50% of the surgeries were elective.
The mean systolic blood pressure was 123 mm Hg in the individualized treatment group compared with 116 mm Hg in the standard treatment group. Despite this small difference, 96% of individualized treatment patients received norepinephrine, compared with 26% in the standard treatment group.
The primary outcome of SIRS with organ dysfunction occurred in 38.1% of patients in the individualized treatment group and 51.7% of those in the standard treatment group. After adjusting for center, surgical urgency, surgical site, and acute kidney injury risk index, the relative risk of developing SIRS in those receiving individualized management was 0.73 (P = .02). Renal dysfunction (based on Acute Dialysis Quality Initiative criteria11) occurred in 32.7% of individualized treatment patients and 49% of standardized treatment patients.
Limitations of this study included differences in pharmacologic approach to maintain blood pressure in the 2 protocols (ephedrine and fluids vs norepinephrine) and a modest sample size.
Conclusions. Despite this, the difference in organ dysfunction was striking, with a number needed to treat of only 7 patients. This intervention extended 4 hours postoperatively, a time when many of these patients have left the postanesthesia care unit and have returned to hospitalist care on inpatient wards.
While optimal management of intraoperative and immediate postoperative blood pressure may not be settled, this study suggests that even mild relative hypotension may justify immediate action. Further studies may be useful to delineate high- and low-risk populations, the timing of greatest risk, and indications for intraarterial blood pressure monitoring.
[Salmasi V, Maheswari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology 2017; 126(1):47–65.]
This retrospective cohort study12 assessed the association between myocardial or kidney injury and absolute or relative thresholds of intraoperative mean arterial pressure. It included 57,315 adults who underwent inpatient noncardiac surgery, had a preoperative and at least 1 postoperative serum creatinine measurement within 7 days, and had blood pressure recorded in preoperative appointments within 6 months. Patients with chronic kidney disease (glomerular filtration rate < 60 mL/min/1.73 m2) and those on dialysis were excluded. The outcomes were MINS5 and acute kidney injury as defined by the Acute Kidney Injury Network.9
Findings. A mean arterial pressure below an absolute threshold of 65 mm Hg or a relative threshold of 20% lower than baseline value was associated with myocardial and kidney injury. At each threshold, prolonged periods of hypotension were associated with progressively increased risk.
An important conclusion of the study was that relative thresholds of mean arterial pressure were not any more predictive than absolute thresholds. Absolute thresholds are easier to use intraoperatively, especially when baseline values are not available. The authors did not find a clinically significant interaction between baseline blood pressure and the association of hypotension and myocardial and kidney injury.
Limitations included use of cardiac enzymes postoperatively to define MINS. Since these were not routinely collected, clinically silent myocardial injury may have been missed. Baseline blood pressure may have important implications in other forms of organ injury (ie, cerebral ischemia) that were not studied.
Summary. The lowest absolute mean arterial pressure is as predictive of postoperative myocardial and kidney injury as the relative pressure reduction, at least in patients with normal renal function. Limiting exposure to intraoperative hypotension is important. Baseline blood pressure values may have limited utility for intraoperative management.
In combination, these studies confirm that intraoperative hypotension is a predictor of postoperative organ dysfunction, but the definition and management remain unclear. While aggressive intraoperative management is likely beneficial, how to manage the antihypertensive therapy the patient has been taking as an outpatient when he or she comes into the hospital for surgery remains uncertain.
DOES PATENT FORAMEN OVALE INCREASE THE RISK OF STROKE?
Perioperative stroke is an uncommon, severe complication of noncardiac surgery. The pathophysiology has been better defined in cardiac than in noncardiac surgeries. In nonsurgical patients, patent foramen ovale (PFO) is associated with stroke, even in patients considered to be at low risk.13 Perioperative patients have additional risk for venous thromboembolism and may have periprocedural antithrombotic medications altered, increasing their risk of paradoxical embolism through the PFO.
[Ng PY, Ng AK, Subramaniam B, et al. Association of preoperatively diagnosed patent foramen ovale with perioperative ischemic stroke. JAMA 2018; 319(5):452–462.]
This retrospective cohort study of noncardiac surgery patients at 3 hospitals14 sought to determine the association of preoperatively diagnosed PFO with the risk of perioperative ischemic stroke identified by International Classification of Diseases diagnoses.
Of 150,198 patients, 1.0% had a preoperative diagnosis of PFO, and at baseline, those with PFO had significantly more comorbidities than those without PFO. Stroke occurred in 3.2% of patients with PFO vs 0.5% of those without. Patients known to have a PFO were much more likely to have cardiovascular and thromboembolic risk factors for stroke. In the adjusted analysis, the absolute risk difference between groups was 0.4% (95% CI 0.2–0.6%), with an estimated perioperative stroke risk of 5.9 per 1,000 in patients with known patent foramen ovale and 2.2 per 1,000 in those without. A diagnosis of PFO was also associated with increased risk of large-vessel-territory stroke and more severe neurologic deficit.
Further attempts to adjust for baseline risk factors and other potential bias, including a propensity score-matched cohort analysis and an analysis limited to patients who had echocardiography performed in the same healthcare system, still showed a higher risk of perioperative stroke among patients with preoperatively detected patent foramen ovale.
Limitations. The study was retrospective and observational, used administrative data, and had a low rate of PFO diagnosis (1%), compared with about 25% in population-based studies.15 Indications for preoperative echocardiography are unknown. In addition, the study specifically examined preoperatively diagnosed PFO, rather than including those diagnosed in the postoperative period.
Discussion. How does this study affect clinical practice? The absolute stroke risk was increased by 0.4% in patients with PFO compared with those without. Although this is a relatively small increase, millions of patients undergo noncardiac surgery annually. The risks of therapeutic anticoagulation or PFO closure are likely too high in this context; however, clinicians may approach the perioperative management of antiplatelet agents and venous thromboembolism prophylaxis in patients with known PFO with additional caution.
HOW DOES TIMING OF EMERGENCY SURGERY AFTER PRIOR STROKE AFFECT OUTCOMES?
A history of stroke or transient ischemic attack is a known risk factor for perioperative vascular complications. A recent large cohort study demonstrated that a history of stroke within 9 months of elective surgery was associated with increased adverse outcomes.16 Little is known, however, of the perioperative risk in patients with a history of stroke who undergo emergency surgery.
[Christiansen MN, Andersson C, Gislason GH, et al. Risks of cardiovascular adverse events and death in patients with previous stroke undergoing emergency noncardiac, nonintracranial surgery: the importance of operative timing. Anesthesiology 2017; 127(1):9–19.]
In this study,17 all emergency noncardiac and nonintracranial surgeries from 2005 to 2011 were analyzed using multiple national patient registries in Denmark according to time elapsed between previous stroke and surgery. Primary outcomes were 30-day all-cause mortality and 30-day major adverse cardiac events (MACE), defined as nonfatal ischemic stroke, nonfatal myocardial infarction, and cardiovascular death. Statistical analysis to assess the risk of adverse outcomes included logistic regression models, spline analyses, and propensity-score matching.
Findings. The authors identified 146,694 emergency surgeries, with 7,861 patients (5.4%) having had a previous stroke (transient ischemic attacks and hemorrhagic strokes were not included). Rates of postoperative stroke were as follows:
- 9.9% in patents with a history of ischemic stroke within 3 months of surgery
- 2.8% in patients with a history of stroke 3 to 9 months before surgery
- 0.3% in patients with no previous stroke.
The risk plateaued when the time between stroke and surgery exceeded 4 to 5 months.15
Interestingly, in patients who underwent emergency surgery within 14 days of stroke, the risk of MACE was significantly lower immediately after surgery (1–3 days after stroke) compared with surgery that took place 4 to 14 days after stroke. The authors hypothesized that because cerebral autoregulation does not become compromised until approximately 5 days after a stroke, the risk was lower 1 to 3 days after surgery and increased thereafter.
Limitations of this study included the possibility of residual confounding, given its retrospective design using administrative data, not accounting for preoperative antithrombotic and anticoagulation therapy, and lack of information regarding the etiology of recurrent stroke (eg, thromboembolic, atherothrombotic, hypoperfusion).
Conclusions. Although it would be impractical to postpone emergency surgery in a patient who recently had a stroke, this study shows that the incidence rates of postoperative recurrent stroke and MACE are high. Therefore, it is important that the patient and perioperative team be aware of the risk. Further research is needed to confirm these estimates of postoperative adverse events in more diverse patient populations.
- Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018; 168(4):237–244. doi:10.7326/M17-2341
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 130(24):2215–2245. doi:10.1161/CIR.0000000000000105
- Smilowitz NR, Gupta N, Ramakrishna H, Guo Y, Berger JS, Bangalore S. Perioperative major adverse cardiovascular and cerebrovascular events associated with noncardiac surgery. JAMA Cardiol 2017; 2(2):181–187. doi:10.1001/jamacardio.2016.4792
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120(3):564–578. doi:10.1097/ALN.0000000000000113
- Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, Sigamani A, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2017; 317(16):1642–1651. doi:10.1001/jama.2017.4360
- Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation 2018; 137(12):1221–1232. doi:10.1161/CIRCULATIONAHA.117.030114
- Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8
- Walsh M, Devereaux PJ, Garg AX, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology 2013; 119(3):507–515. doi:10.1097/ALN.0b013e3182a10e26
- Futier E, Lefrant JY, Guinot PG, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA 2017; 318(14):1346–1357. doi:10.1001/jama.2017.14172
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) group. Crit Care 2004; 8:R204. doi:10.1186/cc2872
- Salmasi V, Maheswari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology 2017; 126(1):47–65. doi:10.1097/ALN.0000000000001432
- Lechat P, Mas JL, Lascault G, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med 1988; 318(18):1148–1152. doi:10.1056/NEJM198805053181802
- Ng PY, Ng AK, Subramaniam B, et al. Association of preoperatively diagnosed patent foramen ovale with perioperative ischemic stroke. JAMA 2018; 319(5):452–462. doi:10.1001/jama.2017.21899
- Meissner I, Whisnant JP, Khandheria BK, et al. Prevalence of potential risk factors for stroke assessed by transesophageal echocardiography and carotid ultrasonography: the SPARC study. Stroke Prevention: Assessment of Risk in a Community. Mayo Clin Proc 1999; 74(9):862–869. pmid:10488786
- Jørgensen ME, Torp-Pedersen C, Gislason GH, et al. Time elapsed after ischemic stroke and risk of adverse cardiovascular events and mortality following elective noncardiac surgery. JAMA 2014; 312:269–277. doi:10.1001/jama.2014.8165
- Christiansen MN, Andersson C, Gislason GH, et al. Risks of cardiovascular adverse events and death in patients with previous stroke undergoing emergency noncardiac, nonintracranial surgery: the importance of operative timing. Anesthesiology 2017; 127(1):9–19. doi:10.1097/ALN.0000000000001685
- Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018; 168(4):237–244. doi:10.7326/M17-2341
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 130(24):2215–2245. doi:10.1161/CIR.0000000000000105
- Smilowitz NR, Gupta N, Ramakrishna H, Guo Y, Berger JS, Bangalore S. Perioperative major adverse cardiovascular and cerebrovascular events associated with noncardiac surgery. JAMA Cardiol 2017; 2(2):181–187. doi:10.1001/jamacardio.2016.4792
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120(3):564–578. doi:10.1097/ALN.0000000000000113
- Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, Sigamani A, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2017; 317(16):1642–1651. doi:10.1001/jama.2017.4360
- Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation 2018; 137(12):1221–1232. doi:10.1161/CIRCULATIONAHA.117.030114
- Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8
- Walsh M, Devereaux PJ, Garg AX, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology 2013; 119(3):507–515. doi:10.1097/ALN.0b013e3182a10e26
- Futier E, Lefrant JY, Guinot PG, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA 2017; 318(14):1346–1357. doi:10.1001/jama.2017.14172
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) group. Crit Care 2004; 8:R204. doi:10.1186/cc2872
- Salmasi V, Maheswari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology 2017; 126(1):47–65. doi:10.1097/ALN.0000000000001432
- Lechat P, Mas JL, Lascault G, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med 1988; 318(18):1148–1152. doi:10.1056/NEJM198805053181802
- Ng PY, Ng AK, Subramaniam B, et al. Association of preoperatively diagnosed patent foramen ovale with perioperative ischemic stroke. JAMA 2018; 319(5):452–462. doi:10.1001/jama.2017.21899
- Meissner I, Whisnant JP, Khandheria BK, et al. Prevalence of potential risk factors for stroke assessed by transesophageal echocardiography and carotid ultrasonography: the SPARC study. Stroke Prevention: Assessment of Risk in a Community. Mayo Clin Proc 1999; 74(9):862–869. pmid:10488786
- Jørgensen ME, Torp-Pedersen C, Gislason GH, et al. Time elapsed after ischemic stroke and risk of adverse cardiovascular events and mortality following elective noncardiac surgery. JAMA 2014; 312:269–277. doi:10.1001/jama.2014.8165
- Christiansen MN, Andersson C, Gislason GH, et al. Risks of cardiovascular adverse events and death in patients with previous stroke undergoing emergency noncardiac, nonintracranial surgery: the importance of operative timing. Anesthesiology 2017; 127(1):9–19. doi:10.1097/ALN.0000000000001685
KEY POINTS
- Patients undergoing noncardiac surgery who have a history of percutaneous coronary intervention will benefit from continuing aspirin perioperatively if they are not at very high risk of bleeding.
- Myocardial injury after noncardiac surgery is strongly associated with a risk of death, and the higher the troponin level, the higher the risk. Measuring troponin T before and after surgery may be beneficial in patients at high risk if the information leads to a change in management.
- Perioperative hypotension can lead to end-organ dysfunction postoperatively. There is conflicting evidence whether the absolute or relative reduction in blood pressure is more predictive.
- Perioperative risk of stroke is higher in patients with patent foramen ovale than in those without.
- Many patients who recently had a stroke suffer recurrent stroke and major adverse cardiac events if they undergo emergency surgery.
In reply: Perioperative interruption of dual antiplatelet therapy
In Reply: We reported on publications from 2016–2017 and, unfortunately, at the time we were writing our paper, the European Society of Cardiology (ESC) update on dual antiplatelet therapy1 had not yet been published. We presented the recommendations from the American College of Cardiology (ACC) and American Heart Association (AHA),2 which differ from the recently published ESC guidelines. The ESC suggests that the minimum waiting period after drug-eluting stent placement before noncardiac surgery should be 1 month rather than 3 months but acknowledges that in the setting of complex stenting or recent acute coronary syndrome, 6 months is preferred. The recommendation in this latter scenario is a class IIb C recommendation—essentially expert consensus opinion.
Further, in the study by Egholm et al,3 the event rates in patients undergoing noncardiac surgery in the 1- to 2-month period were numerically higher than in the control group, and no adjusted odds ratios were given. The numbers of events were very low, and a change of only 1 or 2 events in the other direction in the groups would likely make it statistically significant.
All of these recommendations are based on observational studies and registry data, as there are no randomized controlled trials to address this issue. There are many complexities to be accounted for including the type of stent, timing, circumstances surrounding stenting, anatomy, number of stents, patient comorbidities (particularly age, diabetes mellitus, cardiac disease), type of surgery and anesthesia, and perioperative management of antiplatelet therapy. While we acknowledge the ESC recommendation, we would urge caution in the recommendation to wait only 1 month, and in the United States most would prefer to wait 3 months if possible.
- Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018; 39(3):213–260. doi:10.1093/eurheartj/ehx419
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016; 134(10):e123–e155. doi:10.1161/CIR.0000000000000404
- Egholm G, Kristensen SD, Thim T, et al. Risk associated with surgery within 12 months after coronary drug-eluting stent implantation. J Am Coll Cardiol 2016; 68(24):2622–2632. doi:10.1016/j.jacc.2016.09.967
In Reply: We reported on publications from 2016–2017 and, unfortunately, at the time we were writing our paper, the European Society of Cardiology (ESC) update on dual antiplatelet therapy1 had not yet been published. We presented the recommendations from the American College of Cardiology (ACC) and American Heart Association (AHA),2 which differ from the recently published ESC guidelines. The ESC suggests that the minimum waiting period after drug-eluting stent placement before noncardiac surgery should be 1 month rather than 3 months but acknowledges that in the setting of complex stenting or recent acute coronary syndrome, 6 months is preferred. The recommendation in this latter scenario is a class IIb C recommendation—essentially expert consensus opinion.
Further, in the study by Egholm et al,3 the event rates in patients undergoing noncardiac surgery in the 1- to 2-month period were numerically higher than in the control group, and no adjusted odds ratios were given. The numbers of events were very low, and a change of only 1 or 2 events in the other direction in the groups would likely make it statistically significant.
All of these recommendations are based on observational studies and registry data, as there are no randomized controlled trials to address this issue. There are many complexities to be accounted for including the type of stent, timing, circumstances surrounding stenting, anatomy, number of stents, patient comorbidities (particularly age, diabetes mellitus, cardiac disease), type of surgery and anesthesia, and perioperative management of antiplatelet therapy. While we acknowledge the ESC recommendation, we would urge caution in the recommendation to wait only 1 month, and in the United States most would prefer to wait 3 months if possible.
In Reply: We reported on publications from 2016–2017 and, unfortunately, at the time we were writing our paper, the European Society of Cardiology (ESC) update on dual antiplatelet therapy1 had not yet been published. We presented the recommendations from the American College of Cardiology (ACC) and American Heart Association (AHA),2 which differ from the recently published ESC guidelines. The ESC suggests that the minimum waiting period after drug-eluting stent placement before noncardiac surgery should be 1 month rather than 3 months but acknowledges that in the setting of complex stenting or recent acute coronary syndrome, 6 months is preferred. The recommendation in this latter scenario is a class IIb C recommendation—essentially expert consensus opinion.
Further, in the study by Egholm et al,3 the event rates in patients undergoing noncardiac surgery in the 1- to 2-month period were numerically higher than in the control group, and no adjusted odds ratios were given. The numbers of events were very low, and a change of only 1 or 2 events in the other direction in the groups would likely make it statistically significant.
All of these recommendations are based on observational studies and registry data, as there are no randomized controlled trials to address this issue. There are many complexities to be accounted for including the type of stent, timing, circumstances surrounding stenting, anatomy, number of stents, patient comorbidities (particularly age, diabetes mellitus, cardiac disease), type of surgery and anesthesia, and perioperative management of antiplatelet therapy. While we acknowledge the ESC recommendation, we would urge caution in the recommendation to wait only 1 month, and in the United States most would prefer to wait 3 months if possible.
- Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018; 39(3):213–260. doi:10.1093/eurheartj/ehx419
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016; 134(10):e123–e155. doi:10.1161/CIR.0000000000000404
- Egholm G, Kristensen SD, Thim T, et al. Risk associated with surgery within 12 months after coronary drug-eluting stent implantation. J Am Coll Cardiol 2016; 68(24):2622–2632. doi:10.1016/j.jacc.2016.09.967
- Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018; 39(3):213–260. doi:10.1093/eurheartj/ehx419
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016; 134(10):e123–e155. doi:10.1161/CIR.0000000000000404
- Egholm G, Kristensen SD, Thim T, et al. Risk associated with surgery within 12 months after coronary drug-eluting stent implantation. J Am Coll Cardiol 2016; 68(24):2622–2632. doi:10.1016/j.jacc.2016.09.967
2017 Update in perioperative medicine: 6 questions answered
Perioperative care is increasingly complex, and the rapid evolution of literature in this field makes it a challenge for clinicians to stay up-to-date. To help meet this challenge, we used a systematic approach to identify appropriate articles in the medical literature and then, by consensus, to develop a list of 6 clinical questions based on their novelty and potential to change perioperative medical practice:
- How should we screen for cardiac risk in patients undergoing noncardiac surgery?
- What is the appropriate timing for surgery after coronary intervention?
- Can we use statin therapy to reduce perioperative cardiac risk?
- How should we manage sleep apnea risk perioperatively?
- Which patients with atrial fibrillation should receive perioperative bridging anticoagulation?
- Is frailty screening beneficial for elderly patients before noncardiac surgery?
The summaries in this article are a composite of perioperative medicine updates presented at the Perioperative Medicine Summit and the annual meetings of the Society for General Internal Medicine and the Society of Hospital Medicine. “Perioperative care is complex and changing”1–10 (page 864) offers a brief overview.
HOW TO SCREEN FOR CARDIAC RISK BEFORE NONCARDIAC SURGERY
Perioperative cardiac risk can be estimated by clinical risk indexes (based on history, physical examination, common blood tests, and electrocardiography), cardiac biomarkers (natriuretic peptide or troponin levels), and noninvasive cardiac tests.

American and European guidelines
In 2014, the American College of Cardiology/American Heart Association2 and the European Society of Cardiology11 published guidelines on perioperative cardiovascular evaluation and management. They recommended several tools to calculate the risk of postoperative cardiac complications but did not specify a preference. These tools include:
- The Revised Cardiac Risk Index (RCRI)12 (www.mdcalc.com/revised-cardiac-risk-index-pre-operative-risk), which has been externally validated in multiple studies and is the most widely used
- The American College of Surgeons surgical risk calculator13 (www.riskcalculator.facs.org), derived from the National Surgery Quality Improvement Program (NSQIP) database
- The myocardial infarction or cardiac arrest (MICA) calculator14 (www.surgicalriskcalculator.com/miorcardiacarrest), also derived from the NSQIP database.
2017 Canadian guidelines differ
In 2017, the Canadian Cardiovascular Society published its own guidelines on perioperative risk assessment and management.1 These differ from the American and European guidelines on several points.
RCRI recommended. The Canadian guidelines suggested using the RCRI over the other risk predictors, which despite superior discrimination lacked external validation (conditional recommendation; low-quality evidence). Additionally, the Canadians believed that the NSQIP risk indexes underestimated cardiac risk because patients did not undergo routine biomarker screening.
Biomarker measurement. The Canadian guidelines went a step further in their algorithm (Figure 1) and recommended measuring N-terminal-pro B-type natriuretic peptide (NT-proBNP) or BNP preoperatively to improve risk prediction in 3 groups (strong recommendation; moderate-quality evidence):
- Patients ages 65 and older
- Patients ages 45 to 64 with significant cardiovascular disease
- Patients with an RCRI score of 1 or more.
This differs from the American guidelines, which did not recommend measuring preoperative biomarkers but did acknowledge that they may provide incremental value. The American College of Cardiology/American Heart Association authors felt that there were no data to suggest that targeting these biomarkers for treatment and intervention would reduce postoperative risk. The European guidelines did not recommend routinely using biomarkers, but stated that they may be considered in high-risk patients (who have a functional capacity ≤ 4 metabolic equivalents or an RCRI score > 1 undergoing vascular surgery, or > 2 undergoing nonvascular surgery).
Stress testing deemphasized. The Canadian guidelines recommended biomarker testing rather than noninvasive tests to enhance risk assessment based on cost, potential delays in surgery, and absence of evidence of an overall absolute net improvement in risk reclassification. This contrasts with the American and European guidelines and algorithms, which recommended pharmacologic stress testing in patients at elevated risk with poor functional capacity undergoing intermediate- to high-risk surgery if the results would change how they are managed.
Postoperative monitoring. The Canadian guidelines recommended that if patients have an NT-proBNP level higher than 300 mg/L or a BNP level higher than 92 mg/L, they should receive postoperative monitoring with electrocardiography in the postanesthesia care unit and daily troponin measurements for 48 to 72 hours. The American guidelines recommended postoperative electrocardiography and troponin measurement only for patients suspected of having myocardial ischemia, and the European guidelines said postoperative biomarkers may be considered in patients at high risk.
Physician judgment needed
While guidelines and risk calculators are potentially helpful in risk assessment, the lack of consensus and the conflicting recommendations force the physician to weigh the evidence and make individual decisions based on his or her interpretation of the data.
Until there are studies directly comparing the various risk calculators, physicians will most likely use the RCRI, which is simple and has been externally validated, in conjunction with the American guidelines.
At this time, it is unclear how biomarkers should be used—preoperatively, postoperatively, or both—because there are no studies demonstrating that management strategies based on the results lead to better outcomes. We do not believe that biomarker testing will be accepted in lieu of stress testing by our surgery, anesthesiology, or cardiology colleagues, but going forward, it will probably be used more frequently postoperatively, particularly in patients at moderate to high risk.
WHAT IS THE APPROPRIATE TIMING FOR SURGERY AFTER PCI?
A 2014 American College of Cardiology/American Heart Association guideline recommended delaying noncardiac surgery for 1 month after percutaneous coronary intervention (PCI) with bare-metal stents and 1 year after PCI with drug-eluting stents.15 The guideline suggested that surgery may be performed 6 months after drug-eluting stent placement if the risks of delaying surgery outweigh the risk of thrombosis.15
The primary rationale behind these timeframes was to provide dual antiplatelet therapy for a minimally acceptable duration before temporary interruption for a procedure. These recommendations were influenced largely by observational studies of first-generation devices, which are no longer used. Studies of newer-generation stents have suggested that the risk of stent thrombosis reaches a plateau considerably earlier than 6 to 12 months after PCI.
2016 Revised guideline on dual antiplatelet therapy
Although not separately delineated in the recommendations, risk factors for stent thrombosis that should influence the decision include smoking, multivessel coronary artery disease, and suboptimally controlled diabetes mellitus or hyperlipidemia.17 The presence of such stent thrombosis risk factors should be factored into the decision about proceeding with surgery within 3 to 6 months after drug-eluting stent placement.
Holcomb et al: Higher postoperative risk after PCI for myocardial infarction
Another important consideration is the indication for which PCI was performed. In a recent study, Holcomb et al16 found an association between postoperative major adverse cardiac events and PCI for myocardial infarction (MI) that was independent of stent type.
Compared with patients who underwent PCI not associated with acute coronary syndrome, the odds ratios and 95% confidence intervals (CIs) for major adverse cardiac events in those who underwent PCI for MI were:
- 5.25 (4.08–6.75) in the first 3 months
- 2.45 (1.80–3.35) in months 3 to 6
- 2.50 (1.90–3.28) in months 6 to 12.
In absolute terms, patients with stenting performed for an MI had an incidence of major adverse cardiac events of:
- 22.2% in the first 3 months
- 9.4% in months 3 to 6
- 5.8% in months 6 to 12
- 4.4% in months 12 to 24.
The perioperative risks were reduced after 12 months but still remained greater in patients whose PCI was performed for MI rather than another indication.16
The authors of this study suggested delaying noncardiac surgery for up to 6 months after PCI for MI, regardless of stent type.16
A careful, individualized approach
Optimal timing of noncardiac surgery PCI requires a careful, individualized approach and should always be coordinated with the patient’s cardiologist, surgeon, and anesthesiologist.3,15 For most patients, surgery should be delayed for 30 days after bare-metal stent placement and 6 months after drug-eluting stent placement.3 However, for those with greater surgical need and less thrombotic risk, noncardiac surgery can be considered 3 to 6 months after drug-eluting stent placement.3
Additional discussion of the prolonged increased risk of postoperative major adverse cardiac events is warranted in patients whose PCI was performed for MI, in whom delaying noncardiac surgery for up to 6 months (irrespective of stent type) should be considered.16
CAN WE USE STATINS TO REDUCE PERIOPERATIVE RISK?
Current recommendations from the American College of Cardiology/American Heart Association support continuing statins in the perioperative period, but the evidence supporting starting statins in this period has yet to be fully determined. In 2013, a Cochrane review18 found insufficient evidence to conclude that statins reduced perioperative adverse cardiac events, though several large studies were excluded due to controversial methods and data.
In contrast, the Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) study,4 a multicenter, prospective, cohort-matched study of approximately 7,200 patients, found a lower risk of a composite primary outcome of all-cause mortality, myocardial injury after noncardiac surgery, or stroke at 30 days for patients exposed to statin therapy (relative risk [RR] 0.83, 95% CI 0.73–0.95, P = .007).4
London et al retrospective study: 30-day mortality rate is lower with statins
In 2017, London et al5 published the results of a very large retrospective, observational cohort study of approximately 96,000 elective or emergency surgery patients in Department of Veterans Affairs hospitals. The patients were propensity-matched and evaluated for exposure to statins on the day of or the day after surgery, for a total of approximately 48,000 pairs.
The primary outcome was death at 30 days, and statin exposure was associated with a significant reduction (RR 0.82; 95% CI 0.75–0.89; P < .001). Significant risk reductions were demonstrated in nearly all secondary end points as well, except for stroke or coma and thrombosis (pulmonary embolism, deep vein thrombosis, or graft failure). Overall, the number needed to treat to prevent any complication was 67. Statin therapy did not show significant harm, though on subgroup analysis, those who received high-intensity statin therapy had a slightly higher risk of renal injury (odds ratio 1.18, 95% CI 1.02–1.37, P = .03). Also on subgroup analysis, after propensity matching, patients on long-term moderate- or high-intensity statin therapy for 6 to 12 months before surgery had a small risk reduction for many of the outcomes, including death.
The authors also noted that only 62% of the patients who were prescribed statins as outpatients received them in the hospital, which suggests that improvement is necessary in educating perioperative physicians about the benefits and widespread support for continuing statins perioperatively.5
LOAD trial: No benefit from starting statins
Both London et al5 and the VISION investigators4 called for a large randomized controlled trial of perioperative statin initiation. The Lowering the Risk of Operative Complications Using Atorvastatin Loading Dose (LOAD) trial attempted to answer this call.6
This trial randomized 648 statin-naïve Brazilian patients at high risk of perioperative cardiac events to receive either atorvastatin or placebo before surgery and then continuously for another 7 days. The primary outcomes were the rates of death, nonfatal myocardial injury after noncardiac surgery, and cerebrovascular accident at 30 days.6
The investigators found no significant difference in outcomes between the two groups and estimated that the sample size would need to be approximately 7,000 patients to demonstrate a significant benefit. Nonetheless, this trial established that a prospective perioperative statin trial is feasible.
When to continue or start statins
Although we cannot recommend starting statins for all perioperative patients, perioperative statins clearly can carry significant benefit and should be continued in all patients who have been taking them. It is also likely beneficial to initiate statins in those patients who would otherwise warrant therapy based on the American College of Cardiology/American Heart Association Pooled Cohort Equations Risk calculator.19
HOW SHOULD WE MANAGE SLEEP APNEA RISK PERIOPERATIVELY?
From 20% to 30% of US men and 10% to 15% of US women have obstructive sleep apnea, and many are undiagnosed. Obstructive sleep apnea increases the risk of perioperative respiratory failure, unplanned reintubation, unplanned transfer to the intensive care unit, and death.20 Sentinel events (unexpected respiratory arrest after surgery on general surgical wards) have prompted the development of guidelines that aim to identify patients with previously undiagnosed obstructive sleep apnea before surgery and to develop approaches to reduce perioperative morbidity and mortality.
Kaw et al: Beware obesity hypoventilation syndrome
A 2016 study suggested that patients with obstructive sleep apnea and obesity hypoventilation syndrome may be at particularly high risk of perioperative complications.21
Kaw et al21 queried a database of patients with obstructive sleep apnea undergoing elective noncardiac surgery at Cleveland Clinic. All patients (N = 519) had obstructive sleep apnea confirmed by polysomnography, and a body mass index greater than 30 kg/m2. The authors considered a patient to have obesity hypoventilation syndrome (n = 194) if he or she also had hypercapnia (Paco2 ≥ 45 mm Hg) on at least 2 occasions before or after surgery.
In an adjusted analysis, the odds ratios and 95% CIs for adverse outcomes in patients with obesity hypoventilation syndrome were:
- 10.9 (3.7–32.3) for respiratory failure
- 5.4 (1.9–15.7) for heart failure
- 10.9 (3.7–32.3) for intensive care unit transfer.
The absolute increases in risk in the presence of obesity hypoventilation syndrome were:
- 19% (21% vs 2%) for respiratory failure
- 8% (8% vs 0) for heart failure
- 15% (21% vs 6%) for intensive care unit transfer.
There was no difference in rates of perioperative mortality.21
The authors proposed an algorithm to identify patients with possible obesity hypoventilation syndrome before surgery that included prior sleep study results, STOP-BANG score (Table 2),22 and serum bicarbonate level.
Important limitations of the study were that most patients with obesity hypoventilation syndrome were undiagnosed at the time of surgery. Still, the study does offer a tool to potentially identify patients at high risk for perioperative morbidity due to obesity hypoventilation syndrome. Clinicians could then choose to cancel nonessential surgery, propose a lower-risk alternative procedure, or maximize the use of strategies known to reduce perioperative risk for patients with obstructive sleep apnea in general.
Two guidelines on obstructive sleep apnea
Two professional societies have issued guidelines aiming to improve detection of previously undiagnosed obstructive sleep apnea and perioperative outcomes in patients known to have it or suspected of having it:
- The American Society of Anesthesiologists in 201423
- The Society of Anesthesia and Sleep Medicine in 2016.7
Both guidelines recommend that each institution develop a local protocol to screen patients for possible obstructive sleep apnea before elective surgery. The American Society of Anesthesiologists does not recommend any particular tool, but does recommend taking a history and performing a focused examination that includes evaluation of the airway, nasopharyngeal characteristics, neck circumference, and tonsil and tongue size. The Society of Anesthesia and Sleep Medicine recommends using a validated tool such as the STOP-BANG score to estimate the risk of obstructive sleep apnea.
If this screening suggests that a patient has obstructive sleep apnea, should surgery be delayed until a formal sleep study can be done? Or should the patient be treated empirically as if he or she has obstructive sleep apnea? Both professional societies recommend shared decision-making with the patient in this situation, with the Society of Anesthesia and Sleep Medicine recommending additional cardiopulmonary evaluation for patients with hypoventilation, severe pulmonary hypertension, or resting hypoxemia.
Both recommend using continuous positive airway pressure (CPAP) after surgery in patients with known obstructive sleep apnea, although there is not enough evidence to determine if empiric CPAP for screening-positive patients (without polysomnography-diagnosed obstructive sleep apnea) is beneficial. The Society of Anesthesia and Sleep Medicine advises that it is safe to proceed to surgery if obstructive sleep apnea is suspected as long as monitoring and risk-reduction strategies are implemented after surgery to reduce complication rates.
During surgery, the American Society of Anesthesiologists advises peripheral nerve blocks when appropriate, general anesthesia with a secure airway rather than deep sedation, capnography when using moderate sedation, awake extubation, and full reversal of neuromuscular blockade before extubation. After surgery, they recommend reducing opioid use, minimizing postoperative sedatives, supplemental oxygen, and continuous pulse oximetry. The Society of Anesthesia and Sleep Medicine guideline addresses preoperative assessment and therefore makes no recommendations regarding postoperative care.
In conclusion, use of pertinent findings from the history and physical examination and a validated obstructive sleep apnea screening tool such as STOP-BANG before surgery are recommended, with joint decision-making as to proceeding with surgery with empiric CPAP vs a formal sleep study for patients who screen as high risk. The Society of Anesthesia and Sleep Medicine recommends further cardiopulmonary evaluation if there is evidence of hypoventilation, hypoxemia, or pulmonary hypertension in addition to likely obstructive sleep apnea.
WHICH ATRIAL FIBRILLATION PATIENTS NEED BRIDGING ANTICOAGULATION?
When patients receiving anticoagulation need surgery, we need to carefully assess the risks of thromboembolism without anticoagulation vs bleeding with anticoagulation.
Historically, we tended to worry more about thromboembolism24; however, recent studies have revealed a significant risk of bleeding when long-term anticoagulant therapy is bridged (ie, interrupted and replaced with a shorter-acting agent in the perioperative period), with minimal to no decrease in thromboembolic events.25–27
American College of Cardiology guideline
In 2017, the American College of Cardiology8 published a guideline on periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation. The guideline includes a series of decision algorithms on whether and when to interrupt anticoagulation, whether and how to provide bridging anticoagulation, and how to restart postprocedural anticoagulation.
When deciding whether to interrupt anticoagulation, we need to consider the risk of bleeding posed both by patient-specific factors and by the type of surgery. Bridging anticoagulation is not indicated when direct oral anticoagulants (eg, dabigatran, apixaban, edoxaban, rivaroxaban) are interrupted for procedures.
Unlike an earlier guideline statement by the American College of Chest Physicians,24 this consensus statement emphasizes using the CHA2DS2-VASc score as a predictor of thromboembolic events rather than the CHADS2 core.
Table 3 summarizes the key points in the guidance statement about which patients should receive periprocedural bridging anticoagulation.
As evidence continues to evolve in this complicated area of perioperative medicine, it will remain important to continue to create patient management plans that take individual patient and procedural risks into account.
IS FRAILTY SCREENING BENEFICIAL BEFORE NONCARDIAC SURGERY?
Frailty, defined as a composite score of a patient’s age and comorbidities, has great potential to become an obligatory factor in perioperative risk assessment. However, it remains difficult to incorporate frailty scoring into clinical practice due to variations among scoring systems,28 uncertain outcome data, and the imprecise role of socioeconomic factors. In particular, the effect of frailty on perioperative mortality over longer periods of time is uncertain.
McIsaac et al: Higher risk in frail patients
McIsaac and colleagues at the University of Ottawa used a frailty scoring system developed at Johns Hopkins University to evaluate the effect of frailty on all-cause postoperative mortality in approximately 202,000 patients over a 10-year period.9 Although this scoring system is proprietary, it is based on factors such as malnutrition, dementia, impaired vision, decubitus ulcers, urinary incontinence, weight loss, poverty, barriers to access of care, difficulty in walking, and falls.
After adjusting for the procedure risk, patient age, sex, and neighborhood income quintile, the 1-year mortality risk was significantly higher in the frail group (absolute risk 13.6% vs 4.8%; adjusted hazard ratio 2.23; 95% CI 2.08–2.40). The risk of death in the first 3 days was much higher in frail than in nonfrail patients (hazard ratio 35.58; 95% CI 29.78–40.1), but the hazard ratio decreased to approximately 2.4 by day 90.
The authors emphasize that the elevated risk for frail patients warrants particular perioperative planning, though it is not yet clear what frailty-specific interventions should be performed. Further study is needed into the benefit of “prehabilitation” (ie, exercise training to “build up” a patient before surgery) for perioperative risk reduction.
Hall et al: Better care for frail patients
Hall et al10 instituted a quality improvement initiative for perioperative care of patients at the Omaha Veterans Affairs Hospital. Frail patients were identified using the Risk Analysis Index, a 14-question screening tool previously developed and validated over several years using Veterans Administration databases.29 Questions in the Risk Analysis Index cover living situation, any diagnosis of cancer, ability to perform activities of daily living, and others.
To maximize compliance, a Risk Analysis Index score was required to schedule a surgery. Patients with high scores underwent further review by a designated team of physicians who initiated informal and formal consultations with anesthesiologists, critical care physicians, surgeons, and palliative care providers, with the goals of minimizing risk, clarifying patient goals or resuscitation wishes, and developing comprehensive perioperative planning.10
Approximately 9,100 patients were included in the cohort. The authors demonstrated a significant improvement in mortality for frail patients at 30, 180, and 365 days, but noted an improvement in postoperative mortality for the nonfrail patients as well, perhaps due to increased focus on geriatric patient care. In particular, the mortality rate at 365 days dropped from 34.5% to 11.7% for frail patients who underwent this intervention.
While this quality improvement initiative was unable to examine how surgical rates changed in frail patients, it is highly likely that very high-risk patients opted out of surgery or had their surgical plan change, though the authors point out that the overall surgical volume at the institution did not change significantly. As well, it remains unclear which particular interventions may have had the most effect in improving survival, as the perioperative plans were individualized and continually adjusted throughout the study period.
Nonetheless, this article highlights how higher vigilance, individualized planning and appreciation of the high risks of frail patients is associated with improved patient survival postoperatively. Although frailty screening is still in its early stages and further work is needed, it is likely that performing frailty screening in elderly patients and utilizing interdisciplinary collaboration for comprehensive management of frail patients can improve their postoperative course.
- Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol 2017; 33:17–32.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64:2373–2405.
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016; 134:e123–e155.
- Berwanger O, Le Manach Y, Suzumura EA, et al. Association between pre-operative statin use and major cardiovascular complications among patients undergoing non-cardiac surgery: the VISION study. Eur Heart J 2016; 37:177–185.
- London MJ, Schwartz GG, Hur K, Henderson WG. Association of perioperative statin use with mortality and morbidity after major noncardiac surgery. JAMA Intern Med 2017; 177:231–242.
- Berwanger O, de Barros E Silva PG, Barbosa RR, et al. Atorvastatin for high-risk statin-naïve patients undergoing noncardiac surgery: the Lowering the Risk of Operative Complications Using Atorvastatin Loading Dose (LOAD) randomized trial. Am Heart J 2017; 184:88–96.
- Chung F, Memtsoudis SG, Ramachandran SK, et al. Society of Anesthesia and Sleep Medicine guidelines on preoperative screening and assessment of adult patients with obstructive sleep apnea. Anesth Analg 2016; 123:452–473.
- Doherty JU, Gluckman TJ, Hucker W, et al. 2017 ACC expert consensus decision pathway for periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation: a report of the American College of Cardiology Clinical Expert Consensus Document Task Force. J Am Coll Cardiol 2017; 69:871–898.
- McIsaac DI, Bryson GL, van Walraven C. Association of frailty and 1-year postoperative mortality following major elective noncardiac surgery: a population-based cohort study. JAMA Surg 2016; 151:538–545.
- Hall DE, Arya S, Schmid KK, et al. Association of a frailty screening initiative with postoperative survival at 30, 180, and 365 days. JAMA Surg 2017; 152:233–240.
- Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014; 35:2383–2431.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Bilimoria KY, Liu Y, Paruch JL, Zhou L, Kmiecik TE, Ko CY, Cohen ME. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013; 217:833–842.
- Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation 2011; 124:381–387.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 64:e77–e137.
- Holcomb CN, Hollis RH, Graham LA, et al. Association of coronary stent indication with postoperative outcomes following noncardiac surgery. JAMA Surg 2016; 151:462–469.
- Lemesle G, Tricot O, Meurice T, et al. Incident myocardial infarction and very late stent thrombosis in outpatients with stable coronary artery disease. J Am Coll Cardiol 2017; 69:2149–2156.
- Sanders RD, Nicholson A, Lewis SR, Smith AF, Alderson P. Perioperative statin therapy for improving outcomes during and after noncardiac vascular surgery. Cochrane Database Syst Rev 2013; 7:CD009971.
- Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:2935–2959.
- Kaw R, Pasupuleti V, Walker E, et al. Postoperative complications in patients with obstructive sleep apnea. Chest 2012; 141:436–441.
- Kaw R, Bhateja P, Mar HP, et al. Postoperative complications in patients with unrecognized obesity hypoventilation syndrome undergoing elective noncardiac surgery. Chest 2016; 149:84–91.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Gross JB, Apfelbaum JL, Caplan RA, et al. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Obstructive Sleep Apnea. Anesthesiology 2014; 120:268–286.
- Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(2 suppl):e326S–e350S.
- Siegal D, Yudin J, Kaatz S, Douketis JD, Lim W, Spyropoulos AC. Periprocedural heparin bridging in patients receiving vitamin K antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates. Circulation 2012; 126:1630–1639.
- Clark NP, Witt DM, Davies LE, et al. Bleeding, recurrent venous thromboembolism, and mortality risks during warfarin interruption for invasive procedures. JAMA Intern Med 2015; 175:1163–1168.
- Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015; 373:823–833.
- Theou O, Brothers TD, Mitnitski A, Rockwood K. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc 2013; 61:1537–1551.
- Hall DE, Arya S, Schmid KK, et al. Development and initial validation of the risk analysis index for measuring frailty in surgical populations. JAMA Surg 2017; 152:175–182.
Perioperative care is increasingly complex, and the rapid evolution of literature in this field makes it a challenge for clinicians to stay up-to-date. To help meet this challenge, we used a systematic approach to identify appropriate articles in the medical literature and then, by consensus, to develop a list of 6 clinical questions based on their novelty and potential to change perioperative medical practice:
- How should we screen for cardiac risk in patients undergoing noncardiac surgery?
- What is the appropriate timing for surgery after coronary intervention?
- Can we use statin therapy to reduce perioperative cardiac risk?
- How should we manage sleep apnea risk perioperatively?
- Which patients with atrial fibrillation should receive perioperative bridging anticoagulation?
- Is frailty screening beneficial for elderly patients before noncardiac surgery?
The summaries in this article are a composite of perioperative medicine updates presented at the Perioperative Medicine Summit and the annual meetings of the Society for General Internal Medicine and the Society of Hospital Medicine. “Perioperative care is complex and changing”1–10 (page 864) offers a brief overview.
HOW TO SCREEN FOR CARDIAC RISK BEFORE NONCARDIAC SURGERY
Perioperative cardiac risk can be estimated by clinical risk indexes (based on history, physical examination, common blood tests, and electrocardiography), cardiac biomarkers (natriuretic peptide or troponin levels), and noninvasive cardiac tests.

American and European guidelines
In 2014, the American College of Cardiology/American Heart Association2 and the European Society of Cardiology11 published guidelines on perioperative cardiovascular evaluation and management. They recommended several tools to calculate the risk of postoperative cardiac complications but did not specify a preference. These tools include:
- The Revised Cardiac Risk Index (RCRI)12 (www.mdcalc.com/revised-cardiac-risk-index-pre-operative-risk), which has been externally validated in multiple studies and is the most widely used
- The American College of Surgeons surgical risk calculator13 (www.riskcalculator.facs.org), derived from the National Surgery Quality Improvement Program (NSQIP) database
- The myocardial infarction or cardiac arrest (MICA) calculator14 (www.surgicalriskcalculator.com/miorcardiacarrest), also derived from the NSQIP database.
2017 Canadian guidelines differ
In 2017, the Canadian Cardiovascular Society published its own guidelines on perioperative risk assessment and management.1 These differ from the American and European guidelines on several points.
RCRI recommended. The Canadian guidelines suggested using the RCRI over the other risk predictors, which despite superior discrimination lacked external validation (conditional recommendation; low-quality evidence). Additionally, the Canadians believed that the NSQIP risk indexes underestimated cardiac risk because patients did not undergo routine biomarker screening.
Biomarker measurement. The Canadian guidelines went a step further in their algorithm (Figure 1) and recommended measuring N-terminal-pro B-type natriuretic peptide (NT-proBNP) or BNP preoperatively to improve risk prediction in 3 groups (strong recommendation; moderate-quality evidence):
- Patients ages 65 and older
- Patients ages 45 to 64 with significant cardiovascular disease
- Patients with an RCRI score of 1 or more.
This differs from the American guidelines, which did not recommend measuring preoperative biomarkers but did acknowledge that they may provide incremental value. The American College of Cardiology/American Heart Association authors felt that there were no data to suggest that targeting these biomarkers for treatment and intervention would reduce postoperative risk. The European guidelines did not recommend routinely using biomarkers, but stated that they may be considered in high-risk patients (who have a functional capacity ≤ 4 metabolic equivalents or an RCRI score > 1 undergoing vascular surgery, or > 2 undergoing nonvascular surgery).
Stress testing deemphasized. The Canadian guidelines recommended biomarker testing rather than noninvasive tests to enhance risk assessment based on cost, potential delays in surgery, and absence of evidence of an overall absolute net improvement in risk reclassification. This contrasts with the American and European guidelines and algorithms, which recommended pharmacologic stress testing in patients at elevated risk with poor functional capacity undergoing intermediate- to high-risk surgery if the results would change how they are managed.
Postoperative monitoring. The Canadian guidelines recommended that if patients have an NT-proBNP level higher than 300 mg/L or a BNP level higher than 92 mg/L, they should receive postoperative monitoring with electrocardiography in the postanesthesia care unit and daily troponin measurements for 48 to 72 hours. The American guidelines recommended postoperative electrocardiography and troponin measurement only for patients suspected of having myocardial ischemia, and the European guidelines said postoperative biomarkers may be considered in patients at high risk.
Physician judgment needed
While guidelines and risk calculators are potentially helpful in risk assessment, the lack of consensus and the conflicting recommendations force the physician to weigh the evidence and make individual decisions based on his or her interpretation of the data.
Until there are studies directly comparing the various risk calculators, physicians will most likely use the RCRI, which is simple and has been externally validated, in conjunction with the American guidelines.
At this time, it is unclear how biomarkers should be used—preoperatively, postoperatively, or both—because there are no studies demonstrating that management strategies based on the results lead to better outcomes. We do not believe that biomarker testing will be accepted in lieu of stress testing by our surgery, anesthesiology, or cardiology colleagues, but going forward, it will probably be used more frequently postoperatively, particularly in patients at moderate to high risk.
WHAT IS THE APPROPRIATE TIMING FOR SURGERY AFTER PCI?
A 2014 American College of Cardiology/American Heart Association guideline recommended delaying noncardiac surgery for 1 month after percutaneous coronary intervention (PCI) with bare-metal stents and 1 year after PCI with drug-eluting stents.15 The guideline suggested that surgery may be performed 6 months after drug-eluting stent placement if the risks of delaying surgery outweigh the risk of thrombosis.15
The primary rationale behind these timeframes was to provide dual antiplatelet therapy for a minimally acceptable duration before temporary interruption for a procedure. These recommendations were influenced largely by observational studies of first-generation devices, which are no longer used. Studies of newer-generation stents have suggested that the risk of stent thrombosis reaches a plateau considerably earlier than 6 to 12 months after PCI.
2016 Revised guideline on dual antiplatelet therapy
Although not separately delineated in the recommendations, risk factors for stent thrombosis that should influence the decision include smoking, multivessel coronary artery disease, and suboptimally controlled diabetes mellitus or hyperlipidemia.17 The presence of such stent thrombosis risk factors should be factored into the decision about proceeding with surgery within 3 to 6 months after drug-eluting stent placement.
Holcomb et al: Higher postoperative risk after PCI for myocardial infarction
Another important consideration is the indication for which PCI was performed. In a recent study, Holcomb et al16 found an association between postoperative major adverse cardiac events and PCI for myocardial infarction (MI) that was independent of stent type.
Compared with patients who underwent PCI not associated with acute coronary syndrome, the odds ratios and 95% confidence intervals (CIs) for major adverse cardiac events in those who underwent PCI for MI were:
- 5.25 (4.08–6.75) in the first 3 months
- 2.45 (1.80–3.35) in months 3 to 6
- 2.50 (1.90–3.28) in months 6 to 12.
In absolute terms, patients with stenting performed for an MI had an incidence of major adverse cardiac events of:
- 22.2% in the first 3 months
- 9.4% in months 3 to 6
- 5.8% in months 6 to 12
- 4.4% in months 12 to 24.
The perioperative risks were reduced after 12 months but still remained greater in patients whose PCI was performed for MI rather than another indication.16
The authors of this study suggested delaying noncardiac surgery for up to 6 months after PCI for MI, regardless of stent type.16
A careful, individualized approach
Optimal timing of noncardiac surgery PCI requires a careful, individualized approach and should always be coordinated with the patient’s cardiologist, surgeon, and anesthesiologist.3,15 For most patients, surgery should be delayed for 30 days after bare-metal stent placement and 6 months after drug-eluting stent placement.3 However, for those with greater surgical need and less thrombotic risk, noncardiac surgery can be considered 3 to 6 months after drug-eluting stent placement.3
Additional discussion of the prolonged increased risk of postoperative major adverse cardiac events is warranted in patients whose PCI was performed for MI, in whom delaying noncardiac surgery for up to 6 months (irrespective of stent type) should be considered.16
CAN WE USE STATINS TO REDUCE PERIOPERATIVE RISK?
Current recommendations from the American College of Cardiology/American Heart Association support continuing statins in the perioperative period, but the evidence supporting starting statins in this period has yet to be fully determined. In 2013, a Cochrane review18 found insufficient evidence to conclude that statins reduced perioperative adverse cardiac events, though several large studies were excluded due to controversial methods and data.
In contrast, the Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) study,4 a multicenter, prospective, cohort-matched study of approximately 7,200 patients, found a lower risk of a composite primary outcome of all-cause mortality, myocardial injury after noncardiac surgery, or stroke at 30 days for patients exposed to statin therapy (relative risk [RR] 0.83, 95% CI 0.73–0.95, P = .007).4
London et al retrospective study: 30-day mortality rate is lower with statins
In 2017, London et al5 published the results of a very large retrospective, observational cohort study of approximately 96,000 elective or emergency surgery patients in Department of Veterans Affairs hospitals. The patients were propensity-matched and evaluated for exposure to statins on the day of or the day after surgery, for a total of approximately 48,000 pairs.
The primary outcome was death at 30 days, and statin exposure was associated with a significant reduction (RR 0.82; 95% CI 0.75–0.89; P < .001). Significant risk reductions were demonstrated in nearly all secondary end points as well, except for stroke or coma and thrombosis (pulmonary embolism, deep vein thrombosis, or graft failure). Overall, the number needed to treat to prevent any complication was 67. Statin therapy did not show significant harm, though on subgroup analysis, those who received high-intensity statin therapy had a slightly higher risk of renal injury (odds ratio 1.18, 95% CI 1.02–1.37, P = .03). Also on subgroup analysis, after propensity matching, patients on long-term moderate- or high-intensity statin therapy for 6 to 12 months before surgery had a small risk reduction for many of the outcomes, including death.
The authors also noted that only 62% of the patients who were prescribed statins as outpatients received them in the hospital, which suggests that improvement is necessary in educating perioperative physicians about the benefits and widespread support for continuing statins perioperatively.5
LOAD trial: No benefit from starting statins
Both London et al5 and the VISION investigators4 called for a large randomized controlled trial of perioperative statin initiation. The Lowering the Risk of Operative Complications Using Atorvastatin Loading Dose (LOAD) trial attempted to answer this call.6
This trial randomized 648 statin-naïve Brazilian patients at high risk of perioperative cardiac events to receive either atorvastatin or placebo before surgery and then continuously for another 7 days. The primary outcomes were the rates of death, nonfatal myocardial injury after noncardiac surgery, and cerebrovascular accident at 30 days.6
The investigators found no significant difference in outcomes between the two groups and estimated that the sample size would need to be approximately 7,000 patients to demonstrate a significant benefit. Nonetheless, this trial established that a prospective perioperative statin trial is feasible.
When to continue or start statins
Although we cannot recommend starting statins for all perioperative patients, perioperative statins clearly can carry significant benefit and should be continued in all patients who have been taking them. It is also likely beneficial to initiate statins in those patients who would otherwise warrant therapy based on the American College of Cardiology/American Heart Association Pooled Cohort Equations Risk calculator.19
HOW SHOULD WE MANAGE SLEEP APNEA RISK PERIOPERATIVELY?
From 20% to 30% of US men and 10% to 15% of US women have obstructive sleep apnea, and many are undiagnosed. Obstructive sleep apnea increases the risk of perioperative respiratory failure, unplanned reintubation, unplanned transfer to the intensive care unit, and death.20 Sentinel events (unexpected respiratory arrest after surgery on general surgical wards) have prompted the development of guidelines that aim to identify patients with previously undiagnosed obstructive sleep apnea before surgery and to develop approaches to reduce perioperative morbidity and mortality.
Kaw et al: Beware obesity hypoventilation syndrome
A 2016 study suggested that patients with obstructive sleep apnea and obesity hypoventilation syndrome may be at particularly high risk of perioperative complications.21
Kaw et al21 queried a database of patients with obstructive sleep apnea undergoing elective noncardiac surgery at Cleveland Clinic. All patients (N = 519) had obstructive sleep apnea confirmed by polysomnography, and a body mass index greater than 30 kg/m2. The authors considered a patient to have obesity hypoventilation syndrome (n = 194) if he or she also had hypercapnia (Paco2 ≥ 45 mm Hg) on at least 2 occasions before or after surgery.
In an adjusted analysis, the odds ratios and 95% CIs for adverse outcomes in patients with obesity hypoventilation syndrome were:
- 10.9 (3.7–32.3) for respiratory failure
- 5.4 (1.9–15.7) for heart failure
- 10.9 (3.7–32.3) for intensive care unit transfer.
The absolute increases in risk in the presence of obesity hypoventilation syndrome were:
- 19% (21% vs 2%) for respiratory failure
- 8% (8% vs 0) for heart failure
- 15% (21% vs 6%) for intensive care unit transfer.
There was no difference in rates of perioperative mortality.21
The authors proposed an algorithm to identify patients with possible obesity hypoventilation syndrome before surgery that included prior sleep study results, STOP-BANG score (Table 2),22 and serum bicarbonate level.
Important limitations of the study were that most patients with obesity hypoventilation syndrome were undiagnosed at the time of surgery. Still, the study does offer a tool to potentially identify patients at high risk for perioperative morbidity due to obesity hypoventilation syndrome. Clinicians could then choose to cancel nonessential surgery, propose a lower-risk alternative procedure, or maximize the use of strategies known to reduce perioperative risk for patients with obstructive sleep apnea in general.
Two guidelines on obstructive sleep apnea
Two professional societies have issued guidelines aiming to improve detection of previously undiagnosed obstructive sleep apnea and perioperative outcomes in patients known to have it or suspected of having it:
- The American Society of Anesthesiologists in 201423
- The Society of Anesthesia and Sleep Medicine in 2016.7
Both guidelines recommend that each institution develop a local protocol to screen patients for possible obstructive sleep apnea before elective surgery. The American Society of Anesthesiologists does not recommend any particular tool, but does recommend taking a history and performing a focused examination that includes evaluation of the airway, nasopharyngeal characteristics, neck circumference, and tonsil and tongue size. The Society of Anesthesia and Sleep Medicine recommends using a validated tool such as the STOP-BANG score to estimate the risk of obstructive sleep apnea.
If this screening suggests that a patient has obstructive sleep apnea, should surgery be delayed until a formal sleep study can be done? Or should the patient be treated empirically as if he or she has obstructive sleep apnea? Both professional societies recommend shared decision-making with the patient in this situation, with the Society of Anesthesia and Sleep Medicine recommending additional cardiopulmonary evaluation for patients with hypoventilation, severe pulmonary hypertension, or resting hypoxemia.
Both recommend using continuous positive airway pressure (CPAP) after surgery in patients with known obstructive sleep apnea, although there is not enough evidence to determine if empiric CPAP for screening-positive patients (without polysomnography-diagnosed obstructive sleep apnea) is beneficial. The Society of Anesthesia and Sleep Medicine advises that it is safe to proceed to surgery if obstructive sleep apnea is suspected as long as monitoring and risk-reduction strategies are implemented after surgery to reduce complication rates.
During surgery, the American Society of Anesthesiologists advises peripheral nerve blocks when appropriate, general anesthesia with a secure airway rather than deep sedation, capnography when using moderate sedation, awake extubation, and full reversal of neuromuscular blockade before extubation. After surgery, they recommend reducing opioid use, minimizing postoperative sedatives, supplemental oxygen, and continuous pulse oximetry. The Society of Anesthesia and Sleep Medicine guideline addresses preoperative assessment and therefore makes no recommendations regarding postoperative care.
In conclusion, use of pertinent findings from the history and physical examination and a validated obstructive sleep apnea screening tool such as STOP-BANG before surgery are recommended, with joint decision-making as to proceeding with surgery with empiric CPAP vs a formal sleep study for patients who screen as high risk. The Society of Anesthesia and Sleep Medicine recommends further cardiopulmonary evaluation if there is evidence of hypoventilation, hypoxemia, or pulmonary hypertension in addition to likely obstructive sleep apnea.
WHICH ATRIAL FIBRILLATION PATIENTS NEED BRIDGING ANTICOAGULATION?
When patients receiving anticoagulation need surgery, we need to carefully assess the risks of thromboembolism without anticoagulation vs bleeding with anticoagulation.
Historically, we tended to worry more about thromboembolism24; however, recent studies have revealed a significant risk of bleeding when long-term anticoagulant therapy is bridged (ie, interrupted and replaced with a shorter-acting agent in the perioperative period), with minimal to no decrease in thromboembolic events.25–27
American College of Cardiology guideline
In 2017, the American College of Cardiology8 published a guideline on periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation. The guideline includes a series of decision algorithms on whether and when to interrupt anticoagulation, whether and how to provide bridging anticoagulation, and how to restart postprocedural anticoagulation.
When deciding whether to interrupt anticoagulation, we need to consider the risk of bleeding posed both by patient-specific factors and by the type of surgery. Bridging anticoagulation is not indicated when direct oral anticoagulants (eg, dabigatran, apixaban, edoxaban, rivaroxaban) are interrupted for procedures.
Unlike an earlier guideline statement by the American College of Chest Physicians,24 this consensus statement emphasizes using the CHA2DS2-VASc score as a predictor of thromboembolic events rather than the CHADS2 core.
Table 3 summarizes the key points in the guidance statement about which patients should receive periprocedural bridging anticoagulation.
As evidence continues to evolve in this complicated area of perioperative medicine, it will remain important to continue to create patient management plans that take individual patient and procedural risks into account.
IS FRAILTY SCREENING BENEFICIAL BEFORE NONCARDIAC SURGERY?
Frailty, defined as a composite score of a patient’s age and comorbidities, has great potential to become an obligatory factor in perioperative risk assessment. However, it remains difficult to incorporate frailty scoring into clinical practice due to variations among scoring systems,28 uncertain outcome data, and the imprecise role of socioeconomic factors. In particular, the effect of frailty on perioperative mortality over longer periods of time is uncertain.
McIsaac et al: Higher risk in frail patients
McIsaac and colleagues at the University of Ottawa used a frailty scoring system developed at Johns Hopkins University to evaluate the effect of frailty on all-cause postoperative mortality in approximately 202,000 patients over a 10-year period.9 Although this scoring system is proprietary, it is based on factors such as malnutrition, dementia, impaired vision, decubitus ulcers, urinary incontinence, weight loss, poverty, barriers to access of care, difficulty in walking, and falls.
After adjusting for the procedure risk, patient age, sex, and neighborhood income quintile, the 1-year mortality risk was significantly higher in the frail group (absolute risk 13.6% vs 4.8%; adjusted hazard ratio 2.23; 95% CI 2.08–2.40). The risk of death in the first 3 days was much higher in frail than in nonfrail patients (hazard ratio 35.58; 95% CI 29.78–40.1), but the hazard ratio decreased to approximately 2.4 by day 90.
The authors emphasize that the elevated risk for frail patients warrants particular perioperative planning, though it is not yet clear what frailty-specific interventions should be performed. Further study is needed into the benefit of “prehabilitation” (ie, exercise training to “build up” a patient before surgery) for perioperative risk reduction.
Hall et al: Better care for frail patients
Hall et al10 instituted a quality improvement initiative for perioperative care of patients at the Omaha Veterans Affairs Hospital. Frail patients were identified using the Risk Analysis Index, a 14-question screening tool previously developed and validated over several years using Veterans Administration databases.29 Questions in the Risk Analysis Index cover living situation, any diagnosis of cancer, ability to perform activities of daily living, and others.
To maximize compliance, a Risk Analysis Index score was required to schedule a surgery. Patients with high scores underwent further review by a designated team of physicians who initiated informal and formal consultations with anesthesiologists, critical care physicians, surgeons, and palliative care providers, with the goals of minimizing risk, clarifying patient goals or resuscitation wishes, and developing comprehensive perioperative planning.10
Approximately 9,100 patients were included in the cohort. The authors demonstrated a significant improvement in mortality for frail patients at 30, 180, and 365 days, but noted an improvement in postoperative mortality for the nonfrail patients as well, perhaps due to increased focus on geriatric patient care. In particular, the mortality rate at 365 days dropped from 34.5% to 11.7% for frail patients who underwent this intervention.
While this quality improvement initiative was unable to examine how surgical rates changed in frail patients, it is highly likely that very high-risk patients opted out of surgery or had their surgical plan change, though the authors point out that the overall surgical volume at the institution did not change significantly. As well, it remains unclear which particular interventions may have had the most effect in improving survival, as the perioperative plans were individualized and continually adjusted throughout the study period.
Nonetheless, this article highlights how higher vigilance, individualized planning and appreciation of the high risks of frail patients is associated with improved patient survival postoperatively. Although frailty screening is still in its early stages and further work is needed, it is likely that performing frailty screening in elderly patients and utilizing interdisciplinary collaboration for comprehensive management of frail patients can improve their postoperative course.
Perioperative care is increasingly complex, and the rapid evolution of literature in this field makes it a challenge for clinicians to stay up-to-date. To help meet this challenge, we used a systematic approach to identify appropriate articles in the medical literature and then, by consensus, to develop a list of 6 clinical questions based on their novelty and potential to change perioperative medical practice:
- How should we screen for cardiac risk in patients undergoing noncardiac surgery?
- What is the appropriate timing for surgery after coronary intervention?
- Can we use statin therapy to reduce perioperative cardiac risk?
- How should we manage sleep apnea risk perioperatively?
- Which patients with atrial fibrillation should receive perioperative bridging anticoagulation?
- Is frailty screening beneficial for elderly patients before noncardiac surgery?
The summaries in this article are a composite of perioperative medicine updates presented at the Perioperative Medicine Summit and the annual meetings of the Society for General Internal Medicine and the Society of Hospital Medicine. “Perioperative care is complex and changing”1–10 (page 864) offers a brief overview.
HOW TO SCREEN FOR CARDIAC RISK BEFORE NONCARDIAC SURGERY
Perioperative cardiac risk can be estimated by clinical risk indexes (based on history, physical examination, common blood tests, and electrocardiography), cardiac biomarkers (natriuretic peptide or troponin levels), and noninvasive cardiac tests.

American and European guidelines
In 2014, the American College of Cardiology/American Heart Association2 and the European Society of Cardiology11 published guidelines on perioperative cardiovascular evaluation and management. They recommended several tools to calculate the risk of postoperative cardiac complications but did not specify a preference. These tools include:
- The Revised Cardiac Risk Index (RCRI)12 (www.mdcalc.com/revised-cardiac-risk-index-pre-operative-risk), which has been externally validated in multiple studies and is the most widely used
- The American College of Surgeons surgical risk calculator13 (www.riskcalculator.facs.org), derived from the National Surgery Quality Improvement Program (NSQIP) database
- The myocardial infarction or cardiac arrest (MICA) calculator14 (www.surgicalriskcalculator.com/miorcardiacarrest), also derived from the NSQIP database.
2017 Canadian guidelines differ
In 2017, the Canadian Cardiovascular Society published its own guidelines on perioperative risk assessment and management.1 These differ from the American and European guidelines on several points.
RCRI recommended. The Canadian guidelines suggested using the RCRI over the other risk predictors, which despite superior discrimination lacked external validation (conditional recommendation; low-quality evidence). Additionally, the Canadians believed that the NSQIP risk indexes underestimated cardiac risk because patients did not undergo routine biomarker screening.
Biomarker measurement. The Canadian guidelines went a step further in their algorithm (Figure 1) and recommended measuring N-terminal-pro B-type natriuretic peptide (NT-proBNP) or BNP preoperatively to improve risk prediction in 3 groups (strong recommendation; moderate-quality evidence):
- Patients ages 65 and older
- Patients ages 45 to 64 with significant cardiovascular disease
- Patients with an RCRI score of 1 or more.
This differs from the American guidelines, which did not recommend measuring preoperative biomarkers but did acknowledge that they may provide incremental value. The American College of Cardiology/American Heart Association authors felt that there were no data to suggest that targeting these biomarkers for treatment and intervention would reduce postoperative risk. The European guidelines did not recommend routinely using biomarkers, but stated that they may be considered in high-risk patients (who have a functional capacity ≤ 4 metabolic equivalents or an RCRI score > 1 undergoing vascular surgery, or > 2 undergoing nonvascular surgery).
Stress testing deemphasized. The Canadian guidelines recommended biomarker testing rather than noninvasive tests to enhance risk assessment based on cost, potential delays in surgery, and absence of evidence of an overall absolute net improvement in risk reclassification. This contrasts with the American and European guidelines and algorithms, which recommended pharmacologic stress testing in patients at elevated risk with poor functional capacity undergoing intermediate- to high-risk surgery if the results would change how they are managed.
Postoperative monitoring. The Canadian guidelines recommended that if patients have an NT-proBNP level higher than 300 mg/L or a BNP level higher than 92 mg/L, they should receive postoperative monitoring with electrocardiography in the postanesthesia care unit and daily troponin measurements for 48 to 72 hours. The American guidelines recommended postoperative electrocardiography and troponin measurement only for patients suspected of having myocardial ischemia, and the European guidelines said postoperative biomarkers may be considered in patients at high risk.
Physician judgment needed
While guidelines and risk calculators are potentially helpful in risk assessment, the lack of consensus and the conflicting recommendations force the physician to weigh the evidence and make individual decisions based on his or her interpretation of the data.
Until there are studies directly comparing the various risk calculators, physicians will most likely use the RCRI, which is simple and has been externally validated, in conjunction with the American guidelines.
At this time, it is unclear how biomarkers should be used—preoperatively, postoperatively, or both—because there are no studies demonstrating that management strategies based on the results lead to better outcomes. We do not believe that biomarker testing will be accepted in lieu of stress testing by our surgery, anesthesiology, or cardiology colleagues, but going forward, it will probably be used more frequently postoperatively, particularly in patients at moderate to high risk.
WHAT IS THE APPROPRIATE TIMING FOR SURGERY AFTER PCI?
A 2014 American College of Cardiology/American Heart Association guideline recommended delaying noncardiac surgery for 1 month after percutaneous coronary intervention (PCI) with bare-metal stents and 1 year after PCI with drug-eluting stents.15 The guideline suggested that surgery may be performed 6 months after drug-eluting stent placement if the risks of delaying surgery outweigh the risk of thrombosis.15
The primary rationale behind these timeframes was to provide dual antiplatelet therapy for a minimally acceptable duration before temporary interruption for a procedure. These recommendations were influenced largely by observational studies of first-generation devices, which are no longer used. Studies of newer-generation stents have suggested that the risk of stent thrombosis reaches a plateau considerably earlier than 6 to 12 months after PCI.
2016 Revised guideline on dual antiplatelet therapy
Although not separately delineated in the recommendations, risk factors for stent thrombosis that should influence the decision include smoking, multivessel coronary artery disease, and suboptimally controlled diabetes mellitus or hyperlipidemia.17 The presence of such stent thrombosis risk factors should be factored into the decision about proceeding with surgery within 3 to 6 months after drug-eluting stent placement.
Holcomb et al: Higher postoperative risk after PCI for myocardial infarction
Another important consideration is the indication for which PCI was performed. In a recent study, Holcomb et al16 found an association between postoperative major adverse cardiac events and PCI for myocardial infarction (MI) that was independent of stent type.
Compared with patients who underwent PCI not associated with acute coronary syndrome, the odds ratios and 95% confidence intervals (CIs) for major adverse cardiac events in those who underwent PCI for MI were:
- 5.25 (4.08–6.75) in the first 3 months
- 2.45 (1.80–3.35) in months 3 to 6
- 2.50 (1.90–3.28) in months 6 to 12.
In absolute terms, patients with stenting performed for an MI had an incidence of major adverse cardiac events of:
- 22.2% in the first 3 months
- 9.4% in months 3 to 6
- 5.8% in months 6 to 12
- 4.4% in months 12 to 24.
The perioperative risks were reduced after 12 months but still remained greater in patients whose PCI was performed for MI rather than another indication.16
The authors of this study suggested delaying noncardiac surgery for up to 6 months after PCI for MI, regardless of stent type.16
A careful, individualized approach
Optimal timing of noncardiac surgery PCI requires a careful, individualized approach and should always be coordinated with the patient’s cardiologist, surgeon, and anesthesiologist.3,15 For most patients, surgery should be delayed for 30 days after bare-metal stent placement and 6 months after drug-eluting stent placement.3 However, for those with greater surgical need and less thrombotic risk, noncardiac surgery can be considered 3 to 6 months after drug-eluting stent placement.3
Additional discussion of the prolonged increased risk of postoperative major adverse cardiac events is warranted in patients whose PCI was performed for MI, in whom delaying noncardiac surgery for up to 6 months (irrespective of stent type) should be considered.16
CAN WE USE STATINS TO REDUCE PERIOPERATIVE RISK?
Current recommendations from the American College of Cardiology/American Heart Association support continuing statins in the perioperative period, but the evidence supporting starting statins in this period has yet to be fully determined. In 2013, a Cochrane review18 found insufficient evidence to conclude that statins reduced perioperative adverse cardiac events, though several large studies were excluded due to controversial methods and data.
In contrast, the Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) study,4 a multicenter, prospective, cohort-matched study of approximately 7,200 patients, found a lower risk of a composite primary outcome of all-cause mortality, myocardial injury after noncardiac surgery, or stroke at 30 days for patients exposed to statin therapy (relative risk [RR] 0.83, 95% CI 0.73–0.95, P = .007).4
London et al retrospective study: 30-day mortality rate is lower with statins
In 2017, London et al5 published the results of a very large retrospective, observational cohort study of approximately 96,000 elective or emergency surgery patients in Department of Veterans Affairs hospitals. The patients were propensity-matched and evaluated for exposure to statins on the day of or the day after surgery, for a total of approximately 48,000 pairs.
The primary outcome was death at 30 days, and statin exposure was associated with a significant reduction (RR 0.82; 95% CI 0.75–0.89; P < .001). Significant risk reductions were demonstrated in nearly all secondary end points as well, except for stroke or coma and thrombosis (pulmonary embolism, deep vein thrombosis, or graft failure). Overall, the number needed to treat to prevent any complication was 67. Statin therapy did not show significant harm, though on subgroup analysis, those who received high-intensity statin therapy had a slightly higher risk of renal injury (odds ratio 1.18, 95% CI 1.02–1.37, P = .03). Also on subgroup analysis, after propensity matching, patients on long-term moderate- or high-intensity statin therapy for 6 to 12 months before surgery had a small risk reduction for many of the outcomes, including death.
The authors also noted that only 62% of the patients who were prescribed statins as outpatients received them in the hospital, which suggests that improvement is necessary in educating perioperative physicians about the benefits and widespread support for continuing statins perioperatively.5
LOAD trial: No benefit from starting statins
Both London et al5 and the VISION investigators4 called for a large randomized controlled trial of perioperative statin initiation. The Lowering the Risk of Operative Complications Using Atorvastatin Loading Dose (LOAD) trial attempted to answer this call.6
This trial randomized 648 statin-naïve Brazilian patients at high risk of perioperative cardiac events to receive either atorvastatin or placebo before surgery and then continuously for another 7 days. The primary outcomes were the rates of death, nonfatal myocardial injury after noncardiac surgery, and cerebrovascular accident at 30 days.6
The investigators found no significant difference in outcomes between the two groups and estimated that the sample size would need to be approximately 7,000 patients to demonstrate a significant benefit. Nonetheless, this trial established that a prospective perioperative statin trial is feasible.
When to continue or start statins
Although we cannot recommend starting statins for all perioperative patients, perioperative statins clearly can carry significant benefit and should be continued in all patients who have been taking them. It is also likely beneficial to initiate statins in those patients who would otherwise warrant therapy based on the American College of Cardiology/American Heart Association Pooled Cohort Equations Risk calculator.19
HOW SHOULD WE MANAGE SLEEP APNEA RISK PERIOPERATIVELY?
From 20% to 30% of US men and 10% to 15% of US women have obstructive sleep apnea, and many are undiagnosed. Obstructive sleep apnea increases the risk of perioperative respiratory failure, unplanned reintubation, unplanned transfer to the intensive care unit, and death.20 Sentinel events (unexpected respiratory arrest after surgery on general surgical wards) have prompted the development of guidelines that aim to identify patients with previously undiagnosed obstructive sleep apnea before surgery and to develop approaches to reduce perioperative morbidity and mortality.
Kaw et al: Beware obesity hypoventilation syndrome
A 2016 study suggested that patients with obstructive sleep apnea and obesity hypoventilation syndrome may be at particularly high risk of perioperative complications.21
Kaw et al21 queried a database of patients with obstructive sleep apnea undergoing elective noncardiac surgery at Cleveland Clinic. All patients (N = 519) had obstructive sleep apnea confirmed by polysomnography, and a body mass index greater than 30 kg/m2. The authors considered a patient to have obesity hypoventilation syndrome (n = 194) if he or she also had hypercapnia (Paco2 ≥ 45 mm Hg) on at least 2 occasions before or after surgery.
In an adjusted analysis, the odds ratios and 95% CIs for adverse outcomes in patients with obesity hypoventilation syndrome were:
- 10.9 (3.7–32.3) for respiratory failure
- 5.4 (1.9–15.7) for heart failure
- 10.9 (3.7–32.3) for intensive care unit transfer.
The absolute increases in risk in the presence of obesity hypoventilation syndrome were:
- 19% (21% vs 2%) for respiratory failure
- 8% (8% vs 0) for heart failure
- 15% (21% vs 6%) for intensive care unit transfer.
There was no difference in rates of perioperative mortality.21
The authors proposed an algorithm to identify patients with possible obesity hypoventilation syndrome before surgery that included prior sleep study results, STOP-BANG score (Table 2),22 and serum bicarbonate level.
Important limitations of the study were that most patients with obesity hypoventilation syndrome were undiagnosed at the time of surgery. Still, the study does offer a tool to potentially identify patients at high risk for perioperative morbidity due to obesity hypoventilation syndrome. Clinicians could then choose to cancel nonessential surgery, propose a lower-risk alternative procedure, or maximize the use of strategies known to reduce perioperative risk for patients with obstructive sleep apnea in general.
Two guidelines on obstructive sleep apnea
Two professional societies have issued guidelines aiming to improve detection of previously undiagnosed obstructive sleep apnea and perioperative outcomes in patients known to have it or suspected of having it:
- The American Society of Anesthesiologists in 201423
- The Society of Anesthesia and Sleep Medicine in 2016.7
Both guidelines recommend that each institution develop a local protocol to screen patients for possible obstructive sleep apnea before elective surgery. The American Society of Anesthesiologists does not recommend any particular tool, but does recommend taking a history and performing a focused examination that includes evaluation of the airway, nasopharyngeal characteristics, neck circumference, and tonsil and tongue size. The Society of Anesthesia and Sleep Medicine recommends using a validated tool such as the STOP-BANG score to estimate the risk of obstructive sleep apnea.
If this screening suggests that a patient has obstructive sleep apnea, should surgery be delayed until a formal sleep study can be done? Or should the patient be treated empirically as if he or she has obstructive sleep apnea? Both professional societies recommend shared decision-making with the patient in this situation, with the Society of Anesthesia and Sleep Medicine recommending additional cardiopulmonary evaluation for patients with hypoventilation, severe pulmonary hypertension, or resting hypoxemia.
Both recommend using continuous positive airway pressure (CPAP) after surgery in patients with known obstructive sleep apnea, although there is not enough evidence to determine if empiric CPAP for screening-positive patients (without polysomnography-diagnosed obstructive sleep apnea) is beneficial. The Society of Anesthesia and Sleep Medicine advises that it is safe to proceed to surgery if obstructive sleep apnea is suspected as long as monitoring and risk-reduction strategies are implemented after surgery to reduce complication rates.
During surgery, the American Society of Anesthesiologists advises peripheral nerve blocks when appropriate, general anesthesia with a secure airway rather than deep sedation, capnography when using moderate sedation, awake extubation, and full reversal of neuromuscular blockade before extubation. After surgery, they recommend reducing opioid use, minimizing postoperative sedatives, supplemental oxygen, and continuous pulse oximetry. The Society of Anesthesia and Sleep Medicine guideline addresses preoperative assessment and therefore makes no recommendations regarding postoperative care.
In conclusion, use of pertinent findings from the history and physical examination and a validated obstructive sleep apnea screening tool such as STOP-BANG before surgery are recommended, with joint decision-making as to proceeding with surgery with empiric CPAP vs a formal sleep study for patients who screen as high risk. The Society of Anesthesia and Sleep Medicine recommends further cardiopulmonary evaluation if there is evidence of hypoventilation, hypoxemia, or pulmonary hypertension in addition to likely obstructive sleep apnea.
WHICH ATRIAL FIBRILLATION PATIENTS NEED BRIDGING ANTICOAGULATION?
When patients receiving anticoagulation need surgery, we need to carefully assess the risks of thromboembolism without anticoagulation vs bleeding with anticoagulation.
Historically, we tended to worry more about thromboembolism24; however, recent studies have revealed a significant risk of bleeding when long-term anticoagulant therapy is bridged (ie, interrupted and replaced with a shorter-acting agent in the perioperative period), with minimal to no decrease in thromboembolic events.25–27
American College of Cardiology guideline
In 2017, the American College of Cardiology8 published a guideline on periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation. The guideline includes a series of decision algorithms on whether and when to interrupt anticoagulation, whether and how to provide bridging anticoagulation, and how to restart postprocedural anticoagulation.
When deciding whether to interrupt anticoagulation, we need to consider the risk of bleeding posed both by patient-specific factors and by the type of surgery. Bridging anticoagulation is not indicated when direct oral anticoagulants (eg, dabigatran, apixaban, edoxaban, rivaroxaban) are interrupted for procedures.
Unlike an earlier guideline statement by the American College of Chest Physicians,24 this consensus statement emphasizes using the CHA2DS2-VASc score as a predictor of thromboembolic events rather than the CHADS2 core.
Table 3 summarizes the key points in the guidance statement about which patients should receive periprocedural bridging anticoagulation.
As evidence continues to evolve in this complicated area of perioperative medicine, it will remain important to continue to create patient management plans that take individual patient and procedural risks into account.
IS FRAILTY SCREENING BENEFICIAL BEFORE NONCARDIAC SURGERY?
Frailty, defined as a composite score of a patient’s age and comorbidities, has great potential to become an obligatory factor in perioperative risk assessment. However, it remains difficult to incorporate frailty scoring into clinical practice due to variations among scoring systems,28 uncertain outcome data, and the imprecise role of socioeconomic factors. In particular, the effect of frailty on perioperative mortality over longer periods of time is uncertain.
McIsaac et al: Higher risk in frail patients
McIsaac and colleagues at the University of Ottawa used a frailty scoring system developed at Johns Hopkins University to evaluate the effect of frailty on all-cause postoperative mortality in approximately 202,000 patients over a 10-year period.9 Although this scoring system is proprietary, it is based on factors such as malnutrition, dementia, impaired vision, decubitus ulcers, urinary incontinence, weight loss, poverty, barriers to access of care, difficulty in walking, and falls.
After adjusting for the procedure risk, patient age, sex, and neighborhood income quintile, the 1-year mortality risk was significantly higher in the frail group (absolute risk 13.6% vs 4.8%; adjusted hazard ratio 2.23; 95% CI 2.08–2.40). The risk of death in the first 3 days was much higher in frail than in nonfrail patients (hazard ratio 35.58; 95% CI 29.78–40.1), but the hazard ratio decreased to approximately 2.4 by day 90.
The authors emphasize that the elevated risk for frail patients warrants particular perioperative planning, though it is not yet clear what frailty-specific interventions should be performed. Further study is needed into the benefit of “prehabilitation” (ie, exercise training to “build up” a patient before surgery) for perioperative risk reduction.
Hall et al: Better care for frail patients
Hall et al10 instituted a quality improvement initiative for perioperative care of patients at the Omaha Veterans Affairs Hospital. Frail patients were identified using the Risk Analysis Index, a 14-question screening tool previously developed and validated over several years using Veterans Administration databases.29 Questions in the Risk Analysis Index cover living situation, any diagnosis of cancer, ability to perform activities of daily living, and others.
To maximize compliance, a Risk Analysis Index score was required to schedule a surgery. Patients with high scores underwent further review by a designated team of physicians who initiated informal and formal consultations with anesthesiologists, critical care physicians, surgeons, and palliative care providers, with the goals of minimizing risk, clarifying patient goals or resuscitation wishes, and developing comprehensive perioperative planning.10
Approximately 9,100 patients were included in the cohort. The authors demonstrated a significant improvement in mortality for frail patients at 30, 180, and 365 days, but noted an improvement in postoperative mortality for the nonfrail patients as well, perhaps due to increased focus on geriatric patient care. In particular, the mortality rate at 365 days dropped from 34.5% to 11.7% for frail patients who underwent this intervention.
While this quality improvement initiative was unable to examine how surgical rates changed in frail patients, it is highly likely that very high-risk patients opted out of surgery or had their surgical plan change, though the authors point out that the overall surgical volume at the institution did not change significantly. As well, it remains unclear which particular interventions may have had the most effect in improving survival, as the perioperative plans were individualized and continually adjusted throughout the study period.
Nonetheless, this article highlights how higher vigilance, individualized planning and appreciation of the high risks of frail patients is associated with improved patient survival postoperatively. Although frailty screening is still in its early stages and further work is needed, it is likely that performing frailty screening in elderly patients and utilizing interdisciplinary collaboration for comprehensive management of frail patients can improve their postoperative course.
- Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol 2017; 33:17–32.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64:2373–2405.
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016; 134:e123–e155.
- Berwanger O, Le Manach Y, Suzumura EA, et al. Association between pre-operative statin use and major cardiovascular complications among patients undergoing non-cardiac surgery: the VISION study. Eur Heart J 2016; 37:177–185.
- London MJ, Schwartz GG, Hur K, Henderson WG. Association of perioperative statin use with mortality and morbidity after major noncardiac surgery. JAMA Intern Med 2017; 177:231–242.
- Berwanger O, de Barros E Silva PG, Barbosa RR, et al. Atorvastatin for high-risk statin-naïve patients undergoing noncardiac surgery: the Lowering the Risk of Operative Complications Using Atorvastatin Loading Dose (LOAD) randomized trial. Am Heart J 2017; 184:88–96.
- Chung F, Memtsoudis SG, Ramachandran SK, et al. Society of Anesthesia and Sleep Medicine guidelines on preoperative screening and assessment of adult patients with obstructive sleep apnea. Anesth Analg 2016; 123:452–473.
- Doherty JU, Gluckman TJ, Hucker W, et al. 2017 ACC expert consensus decision pathway for periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation: a report of the American College of Cardiology Clinical Expert Consensus Document Task Force. J Am Coll Cardiol 2017; 69:871–898.
- McIsaac DI, Bryson GL, van Walraven C. Association of frailty and 1-year postoperative mortality following major elective noncardiac surgery: a population-based cohort study. JAMA Surg 2016; 151:538–545.
- Hall DE, Arya S, Schmid KK, et al. Association of a frailty screening initiative with postoperative survival at 30, 180, and 365 days. JAMA Surg 2017; 152:233–240.
- Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014; 35:2383–2431.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Bilimoria KY, Liu Y, Paruch JL, Zhou L, Kmiecik TE, Ko CY, Cohen ME. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013; 217:833–842.
- Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation 2011; 124:381–387.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 64:e77–e137.
- Holcomb CN, Hollis RH, Graham LA, et al. Association of coronary stent indication with postoperative outcomes following noncardiac surgery. JAMA Surg 2016; 151:462–469.
- Lemesle G, Tricot O, Meurice T, et al. Incident myocardial infarction and very late stent thrombosis in outpatients with stable coronary artery disease. J Am Coll Cardiol 2017; 69:2149–2156.
- Sanders RD, Nicholson A, Lewis SR, Smith AF, Alderson P. Perioperative statin therapy for improving outcomes during and after noncardiac vascular surgery. Cochrane Database Syst Rev 2013; 7:CD009971.
- Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:2935–2959.
- Kaw R, Pasupuleti V, Walker E, et al. Postoperative complications in patients with obstructive sleep apnea. Chest 2012; 141:436–441.
- Kaw R, Bhateja P, Mar HP, et al. Postoperative complications in patients with unrecognized obesity hypoventilation syndrome undergoing elective noncardiac surgery. Chest 2016; 149:84–91.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Gross JB, Apfelbaum JL, Caplan RA, et al. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Obstructive Sleep Apnea. Anesthesiology 2014; 120:268–286.
- Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(2 suppl):e326S–e350S.
- Siegal D, Yudin J, Kaatz S, Douketis JD, Lim W, Spyropoulos AC. Periprocedural heparin bridging in patients receiving vitamin K antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates. Circulation 2012; 126:1630–1639.
- Clark NP, Witt DM, Davies LE, et al. Bleeding, recurrent venous thromboembolism, and mortality risks during warfarin interruption for invasive procedures. JAMA Intern Med 2015; 175:1163–1168.
- Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015; 373:823–833.
- Theou O, Brothers TD, Mitnitski A, Rockwood K. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc 2013; 61:1537–1551.
- Hall DE, Arya S, Schmid KK, et al. Development and initial validation of the risk analysis index for measuring frailty in surgical populations. JAMA Surg 2017; 152:175–182.
- Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol 2017; 33:17–32.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64:2373–2405.
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016; 134:e123–e155.
- Berwanger O, Le Manach Y, Suzumura EA, et al. Association between pre-operative statin use and major cardiovascular complications among patients undergoing non-cardiac surgery: the VISION study. Eur Heart J 2016; 37:177–185.
- London MJ, Schwartz GG, Hur K, Henderson WG. Association of perioperative statin use with mortality and morbidity after major noncardiac surgery. JAMA Intern Med 2017; 177:231–242.
- Berwanger O, de Barros E Silva PG, Barbosa RR, et al. Atorvastatin for high-risk statin-naïve patients undergoing noncardiac surgery: the Lowering the Risk of Operative Complications Using Atorvastatin Loading Dose (LOAD) randomized trial. Am Heart J 2017; 184:88–96.
- Chung F, Memtsoudis SG, Ramachandran SK, et al. Society of Anesthesia and Sleep Medicine guidelines on preoperative screening and assessment of adult patients with obstructive sleep apnea. Anesth Analg 2016; 123:452–473.
- Doherty JU, Gluckman TJ, Hucker W, et al. 2017 ACC expert consensus decision pathway for periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation: a report of the American College of Cardiology Clinical Expert Consensus Document Task Force. J Am Coll Cardiol 2017; 69:871–898.
- McIsaac DI, Bryson GL, van Walraven C. Association of frailty and 1-year postoperative mortality following major elective noncardiac surgery: a population-based cohort study. JAMA Surg 2016; 151:538–545.
- Hall DE, Arya S, Schmid KK, et al. Association of a frailty screening initiative with postoperative survival at 30, 180, and 365 days. JAMA Surg 2017; 152:233–240.
- Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014; 35:2383–2431.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Bilimoria KY, Liu Y, Paruch JL, Zhou L, Kmiecik TE, Ko CY, Cohen ME. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013; 217:833–842.
- Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation 2011; 124:381–387.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 64:e77–e137.
- Holcomb CN, Hollis RH, Graham LA, et al. Association of coronary stent indication with postoperative outcomes following noncardiac surgery. JAMA Surg 2016; 151:462–469.
- Lemesle G, Tricot O, Meurice T, et al. Incident myocardial infarction and very late stent thrombosis in outpatients with stable coronary artery disease. J Am Coll Cardiol 2017; 69:2149–2156.
- Sanders RD, Nicholson A, Lewis SR, Smith AF, Alderson P. Perioperative statin therapy for improving outcomes during and after noncardiac vascular surgery. Cochrane Database Syst Rev 2013; 7:CD009971.
- Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:2935–2959.
- Kaw R, Pasupuleti V, Walker E, et al. Postoperative complications in patients with obstructive sleep apnea. Chest 2012; 141:436–441.
- Kaw R, Bhateja P, Mar HP, et al. Postoperative complications in patients with unrecognized obesity hypoventilation syndrome undergoing elective noncardiac surgery. Chest 2016; 149:84–91.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Gross JB, Apfelbaum JL, Caplan RA, et al. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Obstructive Sleep Apnea. Anesthesiology 2014; 120:268–286.
- Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(2 suppl):e326S–e350S.
- Siegal D, Yudin J, Kaatz S, Douketis JD, Lim W, Spyropoulos AC. Periprocedural heparin bridging in patients receiving vitamin K antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates. Circulation 2012; 126:1630–1639.
- Clark NP, Witt DM, Davies LE, et al. Bleeding, recurrent venous thromboembolism, and mortality risks during warfarin interruption for invasive procedures. JAMA Intern Med 2015; 175:1163–1168.
- Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015; 373:823–833.
- Theou O, Brothers TD, Mitnitski A, Rockwood K. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc 2013; 61:1537–1551.
- Hall DE, Arya S, Schmid KK, et al. Development and initial validation of the risk analysis index for measuring frailty in surgical populations. JAMA Surg 2017; 152:175–182.
KEY POINTS
- Noncardiac surgery after drug-eluting stent placement can be considered after 3 to 6 months for those with greater surgical need and lower risk of stent thrombosis.
- Perioperative statin use continues to show benefits with minimal risk in large cohort studies, but significant randomized controlled trial data are lacking.
- Patients should be screened for obstructive sleep apnea before surgery, and further cardiopulmonary testing should be performed if the patient has evidence of significant sequelae from obstructive sleep apnea.
- For patients with atrial fibrillation on vitamin K antagonists, bridging can be considered for those with a CHA2DS2-VASc score of 5 or 6 and a history of stroke, transient ischemic attack, or systemic thromboembolism. Direct oral anticoagulation should not be bridged.
- Frailty carries significant perioperative mortality risk; systems-based changes to minimize these patients’ risks can be beneficial and warrant further study.
Updated guidelines on cardiovascular evaluation before noncardiac surgery: A view from the trenches
Guidelines jointly issued by the American College of Cardiology and American Heart Association (ACC/AHA)1 provide a framework for evaluating and managing perioperative cardiac risk in noncardiac surgery. An overriding theme in successive documents from these organizations through the years has been that preoperative intervention, coronary artery bypass grafting, or percutaneous coronary intervention is rarely necessary just to get the patient through surgery, unless it is otherwise indicated independent of the need for surgery.
This article highlights some of the key recommendations in the 2014 updates to these guidelines,1–3 how they differ from previous guidelines,4 and the ongoing challenges and unresolved issues facing physicians involved in perioperative care.
Of note, while these guidelines were being updated, Erasmus University5 expressed concern about the scientific integrity of some of the Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography (DECREASE) trials. As a result, the evidence review committee included these trials in its analysis but not in a systematic review of beta-blockers.2 These trials were not included in the clinical practice guideline supplements and tables but were cited in the text if relevant.
The European Society of Cardiology and European Society of Anesthesiology6 revised their guidelines concurrently with but independently of the ACC/AHA, and although they discussed and aligned some recommendations, many differences remain between the two sets of guidelines. Readers should consult the full guidelines for more detailed information.1
THE ROLE OF THE PREOPERATIVE CARDIAC EVALUATION
The purpose of preoperative medical evaluation is not to "get medical clearance" but rather to evaluate the patient’s medical status and risk of complications. The process includes:
- Identifying risk factors and assessing their severity and stability
- Establishing a clinical risk profile for informed and shared decision-making
- Recommending needed changes in management, further testing, or specialty consultation.
The updated guidelines emphasize the importance of communication among the perioperative team and with the patient. They reiterate the focus on appropriateness of care and cost containment—one should order a test only if the result may change the patient’s management.
HOW URGENT IS SURGERY? HOW RISKY?
The new guidelines classify the urgency of surgery as follows:
- Emergency (necessary within 6 hours)
- Urgent (necessary within 6–24 hours)
- Time-sensitive (can delay 1–6 weeks)
- Elective (can delay up to 1 year).
Surgical risk is now classified as either low (< 1% risk of major adverse cardiac events) or elevated (≥ 1%) on the basis of surgical and patient characteristics. Previous schemas included an intermediate-risk category. Low-risk procedures include endoscopic procedures, superficial procedures, cataract surgery, breast surgery, and ambulatory surgery. Elevated-risk procedures include vascular surgery, intraperitoneal and intrathoracic surgery, head and neck surgery, orthopedic surgery, and prostate surgery.
Risk calculators and biomarkers
To estimate the perioperative risk of major adverse cardiac events, the guidelines suggest incorporating the Revised Cardiac Risk Index (RCRI)7 with an estimation of surgical risk or using a newer surgical risk calculator derived from a database of the American College of Surgeons’ National Surgical Quality Improvement Project (ACS NSQIP).
The RCRI is based on six risk factors, each worth 1 point:
- High-risk surgery
- Ischemic heart disease
- Heart failure
- Stroke or transient ischemic attack
- Diabetes requiring insulin
- Renal insufficiency (serum creatinine > 2.0 mg/dL).7
MICA. The Myocardial Infarction or Cardiac Arrest (MICA) calculator8 has a narrower focus and was validated in only one center.
ACS NSQIP. The recommended newer ACS NSQIP surgical risk calculator9 provides an estimate of procedure-specific risk based on Current Procedural Terminology code and includes 21 patient-specific variables to predict death, major adverse cardiac events, and eight other outcomes. While more comprehensive, this risk calculator has yet to be validated outside of the ACS NSQIP database.
Reconstructed RCRI. The RCRI has been externally validated, but it underestimates risk in major vascular surgery and was outperformed by the MICA calculator. Although not discussed in the new guidelines, a recently published "reconstructed RCRI,"10 in which a serum creatinine level greater than 2 mg/dL in the original RCRI is replaced by a glomerular filtration rate less than 30 mL/min and diabetes is eliminated, may outperform the standard RCRI. A patient with either an RCRI score or a reconstructed RCRI score of 0 or 1 would be considered to be at low risk, whereas patients with two or more risk factors would have an elevated risk.
Cardiac biomarkers, primarily B-type natriuretic peptide (BNP) and N-terminal (NT) proBNP, are independent predictors of cardiac risk, and their addition to preoperative risk indices may provide incremental predictive value. However, how to use these biomarkers and whether any treatment aimed at them will reduce risk is unclear, and the new guidelines did not recommend their routine use.
CLINICAL RISK FACTORS
Coronary artery disease
Ischemic symptoms, a history of myocardial infarction, and elevated cardiac biomarkers are individually associated with perioperative risk of morbidity and death. The risk is modified by how long ago the infarction occurred, whether the patient underwent coronary revascularization, and if so, what type (bypass grafting or percutaneous coronary intervention). A patient with acute coronary syndrome (currently or in the recent past) is at higher risk, and should have elective surgery delayed and be referred for cardiac evaluation and management according to guidelines.
Heart failure
In terms of posing a risk for major adverse cardiac events, heart failure is at least equal to coronary artery disease, and is possibly worse. Its impact depends on its stability, its symptoms, and the patient’s left ventricular function. Symptomatic decompensated heart failure and depressed left ventricular function (ejection fraction < 30% or 40%) confer higher risk than asymptomatic heart failure and preserved left ventricular function. However, evidence is limited with respect to asymptomatic left ventricular dysfunction and diastolic dysfunction. Patients with stable heart failure treated according to guidelines may have better perioperative outcomes.
Valvular heart disease
Significant valvular heart disease is associated with increased risk of postoperative cardiac complications. This risk depends on the type and severity of the valvular lesion and type of noncardiac surgery, but can be minimized by clinical and echocardiographic assessment, choosing appropriate anesthesia, and closer perioperative monitoring. Aortic and mitral stenosis are associated with greater risk of perioperative adverse cardiac events than regurgitant valvular disease.
Echocardiography is recommended in patients suspected of having moderate to severe stenotic or regurgitant lesions if it has not been done within the past year or if the patient’s clinical condition has worsened.
If indicated, valvular intervention can reduce perioperative risk in these patients. Even if the planned noncardiac surgery is high-risk, it may be reasonable to proceed with it (using appropriate perioperative hemodynamic monitoring, which is not specified but typically would be with an arterial line, central line, and possibly a pulmonary arterial catheter) in patients who have asymptomatic severe aortic or mitral regurgitation or aortic stenosis. Surgery may also be reasonable in patients with asymptomatic severe mitral stenosis who are not candidates for repair.
Arrhythmias
Cardiac arrhythmias and conduction defects are often seen in the perioperative period, but there is only limited evidence as to how they affect surgical risk. In addition to their hemodynamic effects, certain arrhythmias (atrial fibrillation, ventricular tachycardia) often indicate underlying structural heart disease, which requires further evaluation before surgery.
The new guidelines refer the reader to previously published clinical practice guidelines for atrial fibrillation,11 supraventricular arrhythmias,12 and device-based therapy.13
ALGORITHM FOR PREOPERATIVE CARDIAC ASSESSMENT
The new algorithm for evaluating a patient who is known to have coronary artery disease or risk factors for it has seven steps (Figure 1).1,11,12,14–17 It differs from the previous algorithm in several details:
- Instead of listing the four active cardiac conditions for which elective surgery should be delayed while the patient is being evaluated and treated (unstable coronary syndrome, decompensated heart failure, significant arrhythmias, severe valvular heart disease), the new version specifically asks about acute coronary syndrome and recommends cardiac evaluation and treatment according to guidelines. A footnote directs readers to other clinical practice guidelines for symptomatic heart failure,14 valvular heart disease,15 and arrhythmias.11,12
- Instead of asking if the procedure is low-risk, the guidelines recommend estimating risk of major adverse cardiac events on the basis of combined clinical and surgical risk and define only two categories: low or elevated. Patients at low risk proceed to surgery with no further testing, as in the earlier algorithm.
- "Excellent" exercise capacity (> 10 metabolic equivalents of task [METs]) is separated from "moderate/good" (4–10 METs), presumably to indicate a stronger recommendation, but patients in both categories proceed to surgery as before.
- If the patient cannot exercise to at least 4 METs, the new algorithm asks whether further testing will affect decision-making or perioperative care (an addition to the previous algorithm). This entails discussing with the patient and perioperative team whether the original surgery will be performed and whether the patient is willing to undergo revascularization if indicated. If so, pharmacologic stress testing is recommended. Previously, this decision also included the number of RCRI factors as well as the type of surgery (vascular or nonvascular).
- If testing will not affect the decision or if the stress test is normal, in addition to recommending proceeding to surgery according to guidelines the new algorithm also lists an option for alternative strategies, including palliation.
- If the stress test is abnormal, especially with left main disease, it recommends coronary revascularization according to the 2011 clinical practice guidelines.18,19
TESTING FOR LEFT VENTRICULAR DYSFUNCTION OR ISCHEMIA
In patients with dyspnea of unexplained cause or worsening dyspnea, assessment of left ventricular function is reasonable, but this is not part of a routine preoperative evaluation.
Pharmacologic stress testing is reasonable for patients at elevated risk with poor functional capacity if the results will change their management, but it is not useful for patients undergoing low-risk surgery. Although dobutamine stress echocardiography may be slightly superior to pharmacologic myocardial perfusion imaging, there are no head-to-head randomized controlled trials, and the guidelines suggest considering local expertise in deciding which test to use.
The presence of moderate to large areas of ischemia (reversible perfusion defects or new wall-motion abnormalities) is associated with risk of perioperative myocardial infarction or death, whereas evidence of an old infarction is associated with long-term but not short-term risk. The negative predictive value of these tests in predicting postoperative cardiac events is high (> 90%), but the positive predictive value is low.
CORONARY REVASCULARIZATION
Coronary artery bypass grafting and percutaneous coronary intervention
The guidelines recommend coronary revascularization before noncardiac surgery only when it is indicated anyway, on the basis of existing clinical practice guidelines.
Whether performing percutaneous coronary intervention before surgery will reduce perioperative cardiac complications is uncertain, and coronary revascularization should not be routinely performed solely to reduce perioperative cardiac events. The only two randomized controlled trials, Coronary Artery Revascularization Prophylaxis (CARP)20 and DECREASE V21 evaluating prophylactic coronary revascularization before noncardiac surgery found no difference in either short-term or long-term outcomes, although subgroup analysis found a survival benefit in patients with left main disease who underwent bypass grafting. Preoperative percutaneous coronary intervention should be limited to patients with left main disease in whom comorbidities preclude bypass surgery and those with unstable coronary disease who may benefit from early invasive management.
The urgency and timing of the noncardiac surgery needs to be taken into account if percutaneous coronary intervention is being considered because of the need for antiplatelet therapy after the procedure, and the potential risks of bleeding and stent thrombosis. If the planned surgery is deemed time-sensitive, then balloon angioplasty or bare-metal stenting is preferred over placement of a drug-eluting stent.
The new guidelines continue to recommend that elective noncardiac surgery be delayed at least 14 days after balloon angioplasty, 30 days after bare-metal stent implantation, and ideally 365 days after drug-eluting stent placement, and reiterate that it is potentially harmful to perform elective surgery within these time frames without any antiplatelet therapy. However, a new class IIb recommendation (benefit ≥ risk) states that "elective noncardiac surgery after [drug-eluting stent] implantation may be considered after 180 days if the risk of further delay is greater than the expected risks of ischemia and stent thrombosis."
This is an important addition to the guidelines because we are often faced with patients needing to undergo surgery in the 6 to 12 months after placement of a drug-eluting stent. Based on previous guidelines, whether it was safe to proceed in this setting created controversy among the perioperative team caring for the patient, and surgery was often delayed unnecessarily. Recent studies22,23 suggest that the newer drug-eluting stents may require a shorter duration of dual antiplatelet therapy, at least in the nonsurgical setting.
MEDICAL THERAPY
Antiplatelet therapy: Stop or continue?
The risk of perioperative bleeding if antiplatelet drugs are continued must be weighed against the risk of stent thrombosis and ischemia if they are stopped before the recommended duration of therapy. Ideally, some antiplatelet therapy should be continued perioperatively in these situations, but the guidelines recommend that a consensus decision among the treating physicians should be made regarding the relative risks of surgery and discontinuation or continuation of antiplatelet therapy. Whenever possible, aspirin should be continued in these patients.
Although the Perioperative Ischemic Evaluation (POISE)-2 trial24 found that perioperative aspirin use was not associated with lower rates of postoperative myocardial infarction or death, it increased bleeding. Patients with stents who had not completed the recommended duration of antiplatelet therapy were excluded from the trial. Additionally, only 5% of the study patients had undergone percutaneous coronary intervention.
According to the guidelines and package inserts, if antiplatelet agents need to be discontinued before surgery, aspirin can be stopped 3 to 7 days before, clopidogrel and ticagrelor 5 days before, and prasugrel 7 days before. In patients without stents, it may be reasonable to continue aspirin perioperatively if the risk of cardiac events outweighs the risk of bleeding, but starting aspirin is not beneficial for patients undergoing elective noncardiac noncarotid surgery unless the risk of ischemic events outweighs the risk of bleeding.
Beta-blockers
In view of the issue of scientific integrity of the DECREASE trials, a separately commissioned systematic review2 of perioperative beta-blocker therapy was performed. This review suggested that giving beta-blockers before surgery was associated with fewer postoperative cardiac events, primarily ischemia and nonfatal myocardial infarction, but few data supported their use to reduce postoperative mortality. Beta-blocker use was associated with adverse outcomes that included bradycardia and stroke. These findings were similar with the inclusion or exclusion of the DECREASE trials in question or of the POISE trial.25
In addition to recommending continuing beta-blockers in patients already on them (class I—the highest recommendation), the guidelines say that it may be reasonable to start them in patients with intermediate- or high-risk ischemia on stress tests as well as in patients with three or more RCRI risk factors (class IIb). In the absence of these indications, initiating beta-blockers preoperatively to reduce risk even in patients with long-term indications is of uncertain benefit. They also recommended starting beta-blockers more than 1 day preoperatively, preferably at least 2 to 7 days before, and note that it was harmful to start them on the day of surgery, particularly at high doses, and with long-acting formulations.
Additionally, there is evidence of differences in outcome within the class of beta-blockers, with the more cardioselective drugs bisoprolol and atenolol being associated with more favorable outcomes than metoprolol in observational studies.
Statins
Multiple observational trials have reported that statins are associated with decreased perioperative morbidity and mortality. Limited evidence from three randomized controlled trials (including two from the discredited DECREASE group) suggests that there is a benefit in patients undergoing vascular surgery, but it is unclear for nonvascular surgery.26–30
The ACC/AHA guidelines again give a class I recommendation to continue statin therapy perioperatively in patients already taking statins and undergoing noncardiac surgery, as there is some evidence that statin withdrawal is associated with increased risk. The guidelines comment that starting statin therapy perioperatively is reasonable for patients undergoing vascular surgery (class IIa) and may be considered in patients with other clinical guideline indications who are undergoing elevated-risk surgery (class IIb).
The mechanism of this benefit is unclear and may relate to the pleotropic as well as the lipid-lowering effects of the statins. Statins may also have beneficial effects in reducing the incidence of acute kidney injury and postoperative atrial fibrillation.
Whether a particular statin, dose, or time of initiation before surgery affects risk is also unknown at this time. The European guidelines6 recommend starting a longer-acting statin ideally at least 2 weeks before surgery for maximal plaque-stabilizing effects.
The risk of statin-induced myopathy, rhabdomyolysis, and hepatic injury appears to be minimal.
Other medications
Of note, the new guidelines do not recommend starting alpha-2 agonists for preventing cardiac events in patients undergoing noncardiac surgery. Despite previous evidence from smaller studies suggesting a benefit, the POISE-2 trial31 demonstrated that perioperative use of clonidine did not reduce cardiac events and was associated with a significant increase in hypotension and nonfatal cardiac arrest. However, clonidine should be continued in patients already taking it.
A somewhat surprising recommendation is that it is reasonable to continue angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), and if they are held before surgery, to restart them as soon as possible postoperatively (class IIa). The guidelines note reports of increased hypotension associated with induction of anesthesia in patients taking these drugs but also note that there was no change in important postoperative cardiac and other outcomes. Although evidence of harm if these drugs are temporarily discontinued before surgery is sparse, the guidelines advocate continuing them in patients with heart failure or hypertension.
ANESTHESIA AND INTRAOPERATIVE MANAGEMENT
The classes of anesthesia include local, regional (nerve block or neuraxial), monitored anesthesia care (ie, intravenous sedation), and general (volatile agent, total intravenous, or a combination). The guideline committee found no evidence to support the use of neuraxial over general anesthesia, volatile over total intravenous anesthesia, or monitored anesthesia care over general anesthesia. Neuraxial anesthesia for postoperative pain relief in patients undergoing abdominal aortic surgery did reduce the incidence of myocardial infarction.
The guidelines do not recommend routinely using intraoperative transesophageal echocardiography during noncardiac surgery to screen for cardiac abnormalities or to monitor for myocardial ischemia in patients without risk factors or procedural risks for significant hemodynamic, pulmonary, or neurologic compromise. Only in emergency settings do they deem perioperative transesophageal echocardiography reasonable to determine the cause of hemodynamic instability when it persists despite attempted corrective therapy.
Maintenance of normothermia is reasonable, as studies evaluating hypothermia or use of warmed air did not find a lower rate of cardiac events.32,33
POSTOPERATIVE SURVEILLANCE
In observational studies, elevated troponin levels, and even detectable levels within the normal range, have been associated with adverse outcomes and predict mortality after noncardiac surgery—the higher the level, the higher the mortality rate.34 Elevated troponins have many potential causes, both cardiac and noncardiac.
An entity termed myocardial injury after noncardiac surgery (MINS)35 was described as prognostically relevant myocardial injury with a troponin T level higher than 0.03 ng/mL in the absence of a nonischemic etiology but not requiring the presence of ischemic features. Patients who had MINS had a higher 30-day mortality rate (9.8% vs 1.1%) and were also at higher risk of nonfatal cardiac arrest, heart failure, and stroke compared with patients who did not.
The guidelines recommend obtaining an electrocardiogram and troponin levels if there are signs or symptoms suggesting myocardial ischemia or infarction. However, despite the association between troponin and mortality, the guidelines state that "the usefulness of postoperative screening with troponin levels (and electrocardiograms) in patients at high risk for perioperative myocardial infarction, but without signs or symptoms suggestive of myocardial ischemia or infarction, is uncertain in the absence of established risks and benefits of a defined management strategy." They also recommend against routinely measuring postoperative troponins in unselected patients without signs or symptoms suggestive of myocardial ischemia or infarction, stating it is not useful for guiding perioperative management.
Although there was a suggestion that patients in the POISE trial36 who suffered postoperative myocardial infarction had better outcomes if they had received aspirin and statins, and another study37 showed that intensification of cardiac therapy in patients with elevated postoperative troponin levels after vascular surgery led to better 1-year outcomes, there are no randomized controlled trials at this time to support any specific plan or intervention.
IMPACT ON CLINICAL PRACTICE: A PERIOPERATIVE HOSPITALIST'S VIEW
Regarding testing
Although the updated guidelines provide some novel concepts in risk stratification, the new algorithm still leaves many patients in a gray zone with respect to noninvasive testing. Patients with heart failure, valvular heart disease, and arrhythmias appear to be somewhat disconnected from the algorithm in this version, and management according to clinical practice guidelines is recommended.
Patients with acute coronary syndrome remain embedded in the algorithm, with recommendations for cardiology evaluation and management according to standard guidelines before proceeding to elective surgery.
The concept of a combined risk based on clinical factors along with the surgical procedure is important, and an alternative to the RCRI factors is offered. However, while this new NSQIP surgical risk calculator is more comprehensive, it may be too time-consuming for routine clinical use and still needs to be externally validated.
The concept of shared decision-making and team communication is stressed, but the physician may still have difficulty deciding when further testing may influence management. The guidelines remain somewhat vague, and many physicians may be uncomfortable and will continue to look for further guidance in this area.
Without more specific recommendations, this uncertainty may result in more stress tests being ordered—often inappropriately, as they rarely change management. Future prospective studies using biomarkers in conjunction with risk calculators may shed some light on this decision.
The new perioperative guidelines incorporate other ACC/AHA guidelines for valvular heart disease15 and heart failure.14 Some of their recommendations, in my opinion, may lead to excessive testing (eg, repeat echocardiograms) that will not change perioperative management.
Regarding revascularization
The ACC/AHA guidelines continue to emphasize the important concept that coronary revascularization is rarely indicated just to get the patient through surgery.
The new guidelines give physicians some leeway in allowing patients with drug-eluting stents to undergo surgery after 6 rather than 12 months of dual antiplatelet therapy if they believe that delaying surgery would place the patient at more risk than that of stent thrombosis. There is evidence in the nonsurgical setting that the newer stents currently being used may require no more than 6 months of therapy. In my opinion it was never clear that there was a statistically significant benefit in delaying surgery more than 6 months after placement of a drug-eluting stent, so this is a welcome addition.
Regarding beta-blockers
The systematic review of beta-blockers reinforces the importance of continuing them preoperatively while downgrading recommendations for their prophylactic use in patients who are not at increased risk.
Although the debate continues, there is no doubt that beta-blockers are associated with a decrease in myocardial ischemia and infarction but an increase in bradycardia and hypotension. They probably are associated with some increased risk of stroke, although this may be related to the specific beta-blocker used as well as the time of initiation before surgery. Evidence of a possible effect on mortality depends on whether the DECREASE and POISE trials are included or excluded in the analysis.
In the absence of new large-scale randomized controlled trials, we are forced to rely on observational trials and expert opinion in the meantime. I think that if a beta-blocker is to be started preoperatively, it should be done at least 1 week before surgery, and a more cardioselective beta-blocker should be used.
Regarding other drugs and tests
I agree with the recommendation to continue ACE inhibitors and ARBs preoperatively in patients with heart failure and poorly controlled hypertension. Although somewhat contrary to current practice, continuance of these drugs has not been associated with an increase in myocardial infarction or death despite concern about intraoperative hypotension.
Data from randomized controlled trials of perioperative statins are limited, but the information from observational studies is favorable, and I see little downside to initiating statins preoperatively in patients who otherwise have indications for their use, particularly if undergoing vascular or other high-risk noncardiac surgery. It is not known whether the specific drug, dose, or timing of initiation of statins influences outcome.
Although multiple studies of biomarkers suggest that there is an association with outcome, there are no randomized controlled trials or specific interventions shown to improve outcome.
Some of the recommended interventions have included various cardiac medications, stress testing, possible coronary angiography, and revascularization, which are not without risk. In the absence of data and following the directive to "first do no harm," the ACC/AHA has been appropriately cautious in not recommending them for routine use at this time.
The updated guidelines have summarized the new evidence in perioperative cardiac evaluation and management. Many of their recommendations were reinforced by this information and remain essentially unchanged. Several new recommendations will lead to changes in management going forward. Unfortunately, we lack the evidence to answer many questions that arise in routine practice and are therefore forced to rely on expert opinion and our clinical judgment in these cases. The ACC/AHA guidelines do provide a framework for our evaluation and management and help keep clinicians up-to-date with the latest evidence.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; Jul 29. pii: S0735-1097(14)05536-3. doi: 10.1016/j.jacc.2014.07.944. [Epub ahead of print].
- Wijeysundera DN, Duncan D, Nkonde-Price C, et al. Perioperative beta blockade in noncardiac surgery: a systematic review for the 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; Jul 29. pii: S0735-1097(14)05528-4. doi: 10.1016/j.jacc.2014.07.939. [Epub ahead of print].
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; Jul 29. pii: S0735-1097(14)05537-5. doi: 10.1016/j.jacc.2014.07.945. [Epub ahead of print].
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. J Am Coll Cardiol 2007; 50:e159–e242.
- Erasmus MC Follow-up Investigation Committee. Report on the 2012 follow-up investigation of possible breaches of academic integrity. September 30, 2012. http://cardiobrief.files.wordpress.com/2012/10/integrity-report-2012-10-english-translation.pdf. Accessed October 30, 2014.
- Anderson JL, Antman EM, Harold JG, et al. Clinical practice guidelines on perioperative cardiovascular evaluation: collaborative efforts among the ACC, AHA, and ESC. J Am Coll Cardiol 2014 Jul 29. pii: S0735-1097(14)05527-2. doi: 10.1016/j.jacc.2014.07.938. [Epub ahead of print].
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation 2011; 124:381–387.
- Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013; 217:833–842. e1-3.
- Davis C, Tait G, Carroll J, Wijeysundera DN, Beattie WS. The Revised Cardiac Risk Index in the new millennium: a single-centre prospective cohort re-evaluation of the original variables in 9,519 consecutive elective surgical patients. Can J Anaesth 2013; 60:855–863.
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; e-pub before print. doi:10.1016/j.jacc.2014.03.022.
- Aliot EM, Alpert JS, Calkins H, et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias. http://www.escardio.org/guidelines-surveys/esc-guidelines/GuidelinesDocuments/guidelines-SVA-FT.pdf. Accessed October 30,2014.
- Crossley GH, Poole JE, Rozner MA, et al. The Heart Rhythm Society (HRS)/American Society of Anesthesiologists (ASA) Expert Consensus Statement on the perioperative management of patients with implantable defibrillators, pacemakers and arrhythmia monitors: facilities and patient management. Developed as a joint project with the American Society of Anesthesiologists (ASA), and in collaboration with the American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Heart Rhythm 2011; 8:1114–1154.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62:e147–e239.
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:e57–e185.
- Jneid H, Anderson JL, Wright RS, et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2012; 60:645-681.
- O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61:e78–e140.
- Hillis LD, Smith PK, Anderson JL, et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. J Am Coll Cardiol 2011; 58:e123–e210.
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011; 58:e44–e122.
- McFalls EO, Ward HB, Krupski WC, et al. Prophylactic coronary artery revascularization for elective vascular surgery: study design. Veterans Affairs Cooperative Study Group on Coronary Artery Revascularization Prophylaxis for Elective Vascular Surgery. Control Clin Trials 1999; 20:297–308.
- Schouten O, van Kuijk JP, Flu WJ, et al. Long-term outcome of prophylactic coronary revascularization in cardiac high-risk patients undergoing major vascular surgery (from the randomized DECREASE-V Pilot Study). Am J Cardiol 2009; 103:897–901.
- Wijeysundera DN, Wijeysundera HC, Yun L, et al. risk of elective major noncardiac surgery after coronary stent insertion: a population-based study. Circulation 2012; 126:1355-1362.
- Hawn MT, Graham LA, Richman JS, et al. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA 2013; 310:1462–1472.
- Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370:1494–1503.
- Group PS, Devereaux PJ, Yang H, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008; 371:1839–1847.
- Lindenauer PK, Pekow P, Wang K, et al. Lipid-lowering therapy and in-hospital mortality following major noncardiac surgery. JAMA 2004; 291:2092–2099.
- Kennedy J, Quan H, Buchan AM, et al. Statins are associated with better outcomes after carotid endarterectomy in symptomatic patients. Stroke 2005; 36:2072–2076.
- Raju MG, Pachika A, Punnam SR, et al. Statin therapy in the reduction of cardiovascular events in patients undergoing intermediate-risk noncardiac, nonvascular surgery. Clin Cardiol 2013; 36:456–461.
- Desai H, Aronow WS, Ahn C, et al. Incidence of perioperative myocardial infarction and of 2-year mortality in 577 elderly patients undergoing noncardiac vascular surgery treated with and without statins. Arch Gerontol Geriatr 2010; 51:149–151.
- Durazzo AES, Machado FS, Ikeoka DT, et al. Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial. J Vasc Surg 2004; 39:967–975.
- Devereaux PJ, Sessler DI, Leslie K, et al. Clonidine in patients undergoing noncardiac surgery. N Engl J Med 2014; 370:1504–1513.
- Nguyen HP, Zaroff JG, Bayman EO, et al. Perioperative hypothermia (33 degrees C) does not increase the occurrence of cardiovascular events in patients undergoing cerebral aneurysm surgery: findings from the Intraoperative Hypothermia for Aneurysm Surgery Trial. Anesthesiology 2010; 113:327–342.
- Frank SM, Fleisher LA, Breslow MJ, et al. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events. A randomized clinical trial. JAMA 1997; 277:1127–1134.
- Vascular Events In Noncardiac Surgery Patients Cohort Evaluation Study I, Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307:2295–2304.
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120:564–578.
- Devereaux PJ, Xavier D, Pogue J, et al. Characteristics and short-term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med 2011; 154:523–528.
- Foucrier A, Rodseth R, Aissaoui M, Ibanes C, et al. The long-term impact of early cardiovascular therapy intensification for postoperative troponin elevation after major vascular surgery. Anesth Analg 2014; 119:1053–1063.
Guidelines jointly issued by the American College of Cardiology and American Heart Association (ACC/AHA)1 provide a framework for evaluating and managing perioperative cardiac risk in noncardiac surgery. An overriding theme in successive documents from these organizations through the years has been that preoperative intervention, coronary artery bypass grafting, or percutaneous coronary intervention is rarely necessary just to get the patient through surgery, unless it is otherwise indicated independent of the need for surgery.
This article highlights some of the key recommendations in the 2014 updates to these guidelines,1–3 how they differ from previous guidelines,4 and the ongoing challenges and unresolved issues facing physicians involved in perioperative care.
Of note, while these guidelines were being updated, Erasmus University5 expressed concern about the scientific integrity of some of the Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography (DECREASE) trials. As a result, the evidence review committee included these trials in its analysis but not in a systematic review of beta-blockers.2 These trials were not included in the clinical practice guideline supplements and tables but were cited in the text if relevant.
The European Society of Cardiology and European Society of Anesthesiology6 revised their guidelines concurrently with but independently of the ACC/AHA, and although they discussed and aligned some recommendations, many differences remain between the two sets of guidelines. Readers should consult the full guidelines for more detailed information.1
THE ROLE OF THE PREOPERATIVE CARDIAC EVALUATION
The purpose of preoperative medical evaluation is not to "get medical clearance" but rather to evaluate the patient’s medical status and risk of complications. The process includes:
- Identifying risk factors and assessing their severity and stability
- Establishing a clinical risk profile for informed and shared decision-making
- Recommending needed changes in management, further testing, or specialty consultation.
The updated guidelines emphasize the importance of communication among the perioperative team and with the patient. They reiterate the focus on appropriateness of care and cost containment—one should order a test only if the result may change the patient’s management.
HOW URGENT IS SURGERY? HOW RISKY?
The new guidelines classify the urgency of surgery as follows:
- Emergency (necessary within 6 hours)
- Urgent (necessary within 6–24 hours)
- Time-sensitive (can delay 1–6 weeks)
- Elective (can delay up to 1 year).
Surgical risk is now classified as either low (< 1% risk of major adverse cardiac events) or elevated (≥ 1%) on the basis of surgical and patient characteristics. Previous schemas included an intermediate-risk category. Low-risk procedures include endoscopic procedures, superficial procedures, cataract surgery, breast surgery, and ambulatory surgery. Elevated-risk procedures include vascular surgery, intraperitoneal and intrathoracic surgery, head and neck surgery, orthopedic surgery, and prostate surgery.
Risk calculators and biomarkers
To estimate the perioperative risk of major adverse cardiac events, the guidelines suggest incorporating the Revised Cardiac Risk Index (RCRI)7 with an estimation of surgical risk or using a newer surgical risk calculator derived from a database of the American College of Surgeons’ National Surgical Quality Improvement Project (ACS NSQIP).
The RCRI is based on six risk factors, each worth 1 point:
- High-risk surgery
- Ischemic heart disease
- Heart failure
- Stroke or transient ischemic attack
- Diabetes requiring insulin
- Renal insufficiency (serum creatinine > 2.0 mg/dL).7
MICA. The Myocardial Infarction or Cardiac Arrest (MICA) calculator8 has a narrower focus and was validated in only one center.
ACS NSQIP. The recommended newer ACS NSQIP surgical risk calculator9 provides an estimate of procedure-specific risk based on Current Procedural Terminology code and includes 21 patient-specific variables to predict death, major adverse cardiac events, and eight other outcomes. While more comprehensive, this risk calculator has yet to be validated outside of the ACS NSQIP database.
Reconstructed RCRI. The RCRI has been externally validated, but it underestimates risk in major vascular surgery and was outperformed by the MICA calculator. Although not discussed in the new guidelines, a recently published "reconstructed RCRI,"10 in which a serum creatinine level greater than 2 mg/dL in the original RCRI is replaced by a glomerular filtration rate less than 30 mL/min and diabetes is eliminated, may outperform the standard RCRI. A patient with either an RCRI score or a reconstructed RCRI score of 0 or 1 would be considered to be at low risk, whereas patients with two or more risk factors would have an elevated risk.
Cardiac biomarkers, primarily B-type natriuretic peptide (BNP) and N-terminal (NT) proBNP, are independent predictors of cardiac risk, and their addition to preoperative risk indices may provide incremental predictive value. However, how to use these biomarkers and whether any treatment aimed at them will reduce risk is unclear, and the new guidelines did not recommend their routine use.
CLINICAL RISK FACTORS
Coronary artery disease
Ischemic symptoms, a history of myocardial infarction, and elevated cardiac biomarkers are individually associated with perioperative risk of morbidity and death. The risk is modified by how long ago the infarction occurred, whether the patient underwent coronary revascularization, and if so, what type (bypass grafting or percutaneous coronary intervention). A patient with acute coronary syndrome (currently or in the recent past) is at higher risk, and should have elective surgery delayed and be referred for cardiac evaluation and management according to guidelines.
Heart failure
In terms of posing a risk for major adverse cardiac events, heart failure is at least equal to coronary artery disease, and is possibly worse. Its impact depends on its stability, its symptoms, and the patient’s left ventricular function. Symptomatic decompensated heart failure and depressed left ventricular function (ejection fraction < 30% or 40%) confer higher risk than asymptomatic heart failure and preserved left ventricular function. However, evidence is limited with respect to asymptomatic left ventricular dysfunction and diastolic dysfunction. Patients with stable heart failure treated according to guidelines may have better perioperative outcomes.
Valvular heart disease
Significant valvular heart disease is associated with increased risk of postoperative cardiac complications. This risk depends on the type and severity of the valvular lesion and type of noncardiac surgery, but can be minimized by clinical and echocardiographic assessment, choosing appropriate anesthesia, and closer perioperative monitoring. Aortic and mitral stenosis are associated with greater risk of perioperative adverse cardiac events than regurgitant valvular disease.
Echocardiography is recommended in patients suspected of having moderate to severe stenotic or regurgitant lesions if it has not been done within the past year or if the patient’s clinical condition has worsened.
If indicated, valvular intervention can reduce perioperative risk in these patients. Even if the planned noncardiac surgery is high-risk, it may be reasonable to proceed with it (using appropriate perioperative hemodynamic monitoring, which is not specified but typically would be with an arterial line, central line, and possibly a pulmonary arterial catheter) in patients who have asymptomatic severe aortic or mitral regurgitation or aortic stenosis. Surgery may also be reasonable in patients with asymptomatic severe mitral stenosis who are not candidates for repair.
Arrhythmias
Cardiac arrhythmias and conduction defects are often seen in the perioperative period, but there is only limited evidence as to how they affect surgical risk. In addition to their hemodynamic effects, certain arrhythmias (atrial fibrillation, ventricular tachycardia) often indicate underlying structural heart disease, which requires further evaluation before surgery.
The new guidelines refer the reader to previously published clinical practice guidelines for atrial fibrillation,11 supraventricular arrhythmias,12 and device-based therapy.13
ALGORITHM FOR PREOPERATIVE CARDIAC ASSESSMENT
The new algorithm for evaluating a patient who is known to have coronary artery disease or risk factors for it has seven steps (Figure 1).1,11,12,14–17 It differs from the previous algorithm in several details:
- Instead of listing the four active cardiac conditions for which elective surgery should be delayed while the patient is being evaluated and treated (unstable coronary syndrome, decompensated heart failure, significant arrhythmias, severe valvular heart disease), the new version specifically asks about acute coronary syndrome and recommends cardiac evaluation and treatment according to guidelines. A footnote directs readers to other clinical practice guidelines for symptomatic heart failure,14 valvular heart disease,15 and arrhythmias.11,12
- Instead of asking if the procedure is low-risk, the guidelines recommend estimating risk of major adverse cardiac events on the basis of combined clinical and surgical risk and define only two categories: low or elevated. Patients at low risk proceed to surgery with no further testing, as in the earlier algorithm.
- "Excellent" exercise capacity (> 10 metabolic equivalents of task [METs]) is separated from "moderate/good" (4–10 METs), presumably to indicate a stronger recommendation, but patients in both categories proceed to surgery as before.
- If the patient cannot exercise to at least 4 METs, the new algorithm asks whether further testing will affect decision-making or perioperative care (an addition to the previous algorithm). This entails discussing with the patient and perioperative team whether the original surgery will be performed and whether the patient is willing to undergo revascularization if indicated. If so, pharmacologic stress testing is recommended. Previously, this decision also included the number of RCRI factors as well as the type of surgery (vascular or nonvascular).
- If testing will not affect the decision or if the stress test is normal, in addition to recommending proceeding to surgery according to guidelines the new algorithm also lists an option for alternative strategies, including palliation.
- If the stress test is abnormal, especially with left main disease, it recommends coronary revascularization according to the 2011 clinical practice guidelines.18,19
TESTING FOR LEFT VENTRICULAR DYSFUNCTION OR ISCHEMIA
In patients with dyspnea of unexplained cause or worsening dyspnea, assessment of left ventricular function is reasonable, but this is not part of a routine preoperative evaluation.
Pharmacologic stress testing is reasonable for patients at elevated risk with poor functional capacity if the results will change their management, but it is not useful for patients undergoing low-risk surgery. Although dobutamine stress echocardiography may be slightly superior to pharmacologic myocardial perfusion imaging, there are no head-to-head randomized controlled trials, and the guidelines suggest considering local expertise in deciding which test to use.
The presence of moderate to large areas of ischemia (reversible perfusion defects or new wall-motion abnormalities) is associated with risk of perioperative myocardial infarction or death, whereas evidence of an old infarction is associated with long-term but not short-term risk. The negative predictive value of these tests in predicting postoperative cardiac events is high (> 90%), but the positive predictive value is low.
CORONARY REVASCULARIZATION
Coronary artery bypass grafting and percutaneous coronary intervention
The guidelines recommend coronary revascularization before noncardiac surgery only when it is indicated anyway, on the basis of existing clinical practice guidelines.
Whether performing percutaneous coronary intervention before surgery will reduce perioperative cardiac complications is uncertain, and coronary revascularization should not be routinely performed solely to reduce perioperative cardiac events. The only two randomized controlled trials, Coronary Artery Revascularization Prophylaxis (CARP)20 and DECREASE V21 evaluating prophylactic coronary revascularization before noncardiac surgery found no difference in either short-term or long-term outcomes, although subgroup analysis found a survival benefit in patients with left main disease who underwent bypass grafting. Preoperative percutaneous coronary intervention should be limited to patients with left main disease in whom comorbidities preclude bypass surgery and those with unstable coronary disease who may benefit from early invasive management.
The urgency and timing of the noncardiac surgery needs to be taken into account if percutaneous coronary intervention is being considered because of the need for antiplatelet therapy after the procedure, and the potential risks of bleeding and stent thrombosis. If the planned surgery is deemed time-sensitive, then balloon angioplasty or bare-metal stenting is preferred over placement of a drug-eluting stent.
The new guidelines continue to recommend that elective noncardiac surgery be delayed at least 14 days after balloon angioplasty, 30 days after bare-metal stent implantation, and ideally 365 days after drug-eluting stent placement, and reiterate that it is potentially harmful to perform elective surgery within these time frames without any antiplatelet therapy. However, a new class IIb recommendation (benefit ≥ risk) states that "elective noncardiac surgery after [drug-eluting stent] implantation may be considered after 180 days if the risk of further delay is greater than the expected risks of ischemia and stent thrombosis."
This is an important addition to the guidelines because we are often faced with patients needing to undergo surgery in the 6 to 12 months after placement of a drug-eluting stent. Based on previous guidelines, whether it was safe to proceed in this setting created controversy among the perioperative team caring for the patient, and surgery was often delayed unnecessarily. Recent studies22,23 suggest that the newer drug-eluting stents may require a shorter duration of dual antiplatelet therapy, at least in the nonsurgical setting.
MEDICAL THERAPY
Antiplatelet therapy: Stop or continue?
The risk of perioperative bleeding if antiplatelet drugs are continued must be weighed against the risk of stent thrombosis and ischemia if they are stopped before the recommended duration of therapy. Ideally, some antiplatelet therapy should be continued perioperatively in these situations, but the guidelines recommend that a consensus decision among the treating physicians should be made regarding the relative risks of surgery and discontinuation or continuation of antiplatelet therapy. Whenever possible, aspirin should be continued in these patients.
Although the Perioperative Ischemic Evaluation (POISE)-2 trial24 found that perioperative aspirin use was not associated with lower rates of postoperative myocardial infarction or death, it increased bleeding. Patients with stents who had not completed the recommended duration of antiplatelet therapy were excluded from the trial. Additionally, only 5% of the study patients had undergone percutaneous coronary intervention.
According to the guidelines and package inserts, if antiplatelet agents need to be discontinued before surgery, aspirin can be stopped 3 to 7 days before, clopidogrel and ticagrelor 5 days before, and prasugrel 7 days before. In patients without stents, it may be reasonable to continue aspirin perioperatively if the risk of cardiac events outweighs the risk of bleeding, but starting aspirin is not beneficial for patients undergoing elective noncardiac noncarotid surgery unless the risk of ischemic events outweighs the risk of bleeding.
Beta-blockers
In view of the issue of scientific integrity of the DECREASE trials, a separately commissioned systematic review2 of perioperative beta-blocker therapy was performed. This review suggested that giving beta-blockers before surgery was associated with fewer postoperative cardiac events, primarily ischemia and nonfatal myocardial infarction, but few data supported their use to reduce postoperative mortality. Beta-blocker use was associated with adverse outcomes that included bradycardia and stroke. These findings were similar with the inclusion or exclusion of the DECREASE trials in question or of the POISE trial.25
In addition to recommending continuing beta-blockers in patients already on them (class I—the highest recommendation), the guidelines say that it may be reasonable to start them in patients with intermediate- or high-risk ischemia on stress tests as well as in patients with three or more RCRI risk factors (class IIb). In the absence of these indications, initiating beta-blockers preoperatively to reduce risk even in patients with long-term indications is of uncertain benefit. They also recommended starting beta-blockers more than 1 day preoperatively, preferably at least 2 to 7 days before, and note that it was harmful to start them on the day of surgery, particularly at high doses, and with long-acting formulations.
Additionally, there is evidence of differences in outcome within the class of beta-blockers, with the more cardioselective drugs bisoprolol and atenolol being associated with more favorable outcomes than metoprolol in observational studies.
Statins
Multiple observational trials have reported that statins are associated with decreased perioperative morbidity and mortality. Limited evidence from three randomized controlled trials (including two from the discredited DECREASE group) suggests that there is a benefit in patients undergoing vascular surgery, but it is unclear for nonvascular surgery.26–30
The ACC/AHA guidelines again give a class I recommendation to continue statin therapy perioperatively in patients already taking statins and undergoing noncardiac surgery, as there is some evidence that statin withdrawal is associated with increased risk. The guidelines comment that starting statin therapy perioperatively is reasonable for patients undergoing vascular surgery (class IIa) and may be considered in patients with other clinical guideline indications who are undergoing elevated-risk surgery (class IIb).
The mechanism of this benefit is unclear and may relate to the pleotropic as well as the lipid-lowering effects of the statins. Statins may also have beneficial effects in reducing the incidence of acute kidney injury and postoperative atrial fibrillation.
Whether a particular statin, dose, or time of initiation before surgery affects risk is also unknown at this time. The European guidelines6 recommend starting a longer-acting statin ideally at least 2 weeks before surgery for maximal plaque-stabilizing effects.
The risk of statin-induced myopathy, rhabdomyolysis, and hepatic injury appears to be minimal.
Other medications
Of note, the new guidelines do not recommend starting alpha-2 agonists for preventing cardiac events in patients undergoing noncardiac surgery. Despite previous evidence from smaller studies suggesting a benefit, the POISE-2 trial31 demonstrated that perioperative use of clonidine did not reduce cardiac events and was associated with a significant increase in hypotension and nonfatal cardiac arrest. However, clonidine should be continued in patients already taking it.
A somewhat surprising recommendation is that it is reasonable to continue angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), and if they are held before surgery, to restart them as soon as possible postoperatively (class IIa). The guidelines note reports of increased hypotension associated with induction of anesthesia in patients taking these drugs but also note that there was no change in important postoperative cardiac and other outcomes. Although evidence of harm if these drugs are temporarily discontinued before surgery is sparse, the guidelines advocate continuing them in patients with heart failure or hypertension.
ANESTHESIA AND INTRAOPERATIVE MANAGEMENT
The classes of anesthesia include local, regional (nerve block or neuraxial), monitored anesthesia care (ie, intravenous sedation), and general (volatile agent, total intravenous, or a combination). The guideline committee found no evidence to support the use of neuraxial over general anesthesia, volatile over total intravenous anesthesia, or monitored anesthesia care over general anesthesia. Neuraxial anesthesia for postoperative pain relief in patients undergoing abdominal aortic surgery did reduce the incidence of myocardial infarction.
The guidelines do not recommend routinely using intraoperative transesophageal echocardiography during noncardiac surgery to screen for cardiac abnormalities or to monitor for myocardial ischemia in patients without risk factors or procedural risks for significant hemodynamic, pulmonary, or neurologic compromise. Only in emergency settings do they deem perioperative transesophageal echocardiography reasonable to determine the cause of hemodynamic instability when it persists despite attempted corrective therapy.
Maintenance of normothermia is reasonable, as studies evaluating hypothermia or use of warmed air did not find a lower rate of cardiac events.32,33
POSTOPERATIVE SURVEILLANCE
In observational studies, elevated troponin levels, and even detectable levels within the normal range, have been associated with adverse outcomes and predict mortality after noncardiac surgery—the higher the level, the higher the mortality rate.34 Elevated troponins have many potential causes, both cardiac and noncardiac.
An entity termed myocardial injury after noncardiac surgery (MINS)35 was described as prognostically relevant myocardial injury with a troponin T level higher than 0.03 ng/mL in the absence of a nonischemic etiology but not requiring the presence of ischemic features. Patients who had MINS had a higher 30-day mortality rate (9.8% vs 1.1%) and were also at higher risk of nonfatal cardiac arrest, heart failure, and stroke compared with patients who did not.
The guidelines recommend obtaining an electrocardiogram and troponin levels if there are signs or symptoms suggesting myocardial ischemia or infarction. However, despite the association between troponin and mortality, the guidelines state that "the usefulness of postoperative screening with troponin levels (and electrocardiograms) in patients at high risk for perioperative myocardial infarction, but without signs or symptoms suggestive of myocardial ischemia or infarction, is uncertain in the absence of established risks and benefits of a defined management strategy." They also recommend against routinely measuring postoperative troponins in unselected patients without signs or symptoms suggestive of myocardial ischemia or infarction, stating it is not useful for guiding perioperative management.
Although there was a suggestion that patients in the POISE trial36 who suffered postoperative myocardial infarction had better outcomes if they had received aspirin and statins, and another study37 showed that intensification of cardiac therapy in patients with elevated postoperative troponin levels after vascular surgery led to better 1-year outcomes, there are no randomized controlled trials at this time to support any specific plan or intervention.
IMPACT ON CLINICAL PRACTICE: A PERIOPERATIVE HOSPITALIST'S VIEW
Regarding testing
Although the updated guidelines provide some novel concepts in risk stratification, the new algorithm still leaves many patients in a gray zone with respect to noninvasive testing. Patients with heart failure, valvular heart disease, and arrhythmias appear to be somewhat disconnected from the algorithm in this version, and management according to clinical practice guidelines is recommended.
Patients with acute coronary syndrome remain embedded in the algorithm, with recommendations for cardiology evaluation and management according to standard guidelines before proceeding to elective surgery.
The concept of a combined risk based on clinical factors along with the surgical procedure is important, and an alternative to the RCRI factors is offered. However, while this new NSQIP surgical risk calculator is more comprehensive, it may be too time-consuming for routine clinical use and still needs to be externally validated.
The concept of shared decision-making and team communication is stressed, but the physician may still have difficulty deciding when further testing may influence management. The guidelines remain somewhat vague, and many physicians may be uncomfortable and will continue to look for further guidance in this area.
Without more specific recommendations, this uncertainty may result in more stress tests being ordered—often inappropriately, as they rarely change management. Future prospective studies using biomarkers in conjunction with risk calculators may shed some light on this decision.
The new perioperative guidelines incorporate other ACC/AHA guidelines for valvular heart disease15 and heart failure.14 Some of their recommendations, in my opinion, may lead to excessive testing (eg, repeat echocardiograms) that will not change perioperative management.
Regarding revascularization
The ACC/AHA guidelines continue to emphasize the important concept that coronary revascularization is rarely indicated just to get the patient through surgery.
The new guidelines give physicians some leeway in allowing patients with drug-eluting stents to undergo surgery after 6 rather than 12 months of dual antiplatelet therapy if they believe that delaying surgery would place the patient at more risk than that of stent thrombosis. There is evidence in the nonsurgical setting that the newer stents currently being used may require no more than 6 months of therapy. In my opinion it was never clear that there was a statistically significant benefit in delaying surgery more than 6 months after placement of a drug-eluting stent, so this is a welcome addition.
Regarding beta-blockers
The systematic review of beta-blockers reinforces the importance of continuing them preoperatively while downgrading recommendations for their prophylactic use in patients who are not at increased risk.
Although the debate continues, there is no doubt that beta-blockers are associated with a decrease in myocardial ischemia and infarction but an increase in bradycardia and hypotension. They probably are associated with some increased risk of stroke, although this may be related to the specific beta-blocker used as well as the time of initiation before surgery. Evidence of a possible effect on mortality depends on whether the DECREASE and POISE trials are included or excluded in the analysis.
In the absence of new large-scale randomized controlled trials, we are forced to rely on observational trials and expert opinion in the meantime. I think that if a beta-blocker is to be started preoperatively, it should be done at least 1 week before surgery, and a more cardioselective beta-blocker should be used.
Regarding other drugs and tests
I agree with the recommendation to continue ACE inhibitors and ARBs preoperatively in patients with heart failure and poorly controlled hypertension. Although somewhat contrary to current practice, continuance of these drugs has not been associated with an increase in myocardial infarction or death despite concern about intraoperative hypotension.
Data from randomized controlled trials of perioperative statins are limited, but the information from observational studies is favorable, and I see little downside to initiating statins preoperatively in patients who otherwise have indications for their use, particularly if undergoing vascular or other high-risk noncardiac surgery. It is not known whether the specific drug, dose, or timing of initiation of statins influences outcome.
Although multiple studies of biomarkers suggest that there is an association with outcome, there are no randomized controlled trials or specific interventions shown to improve outcome.
Some of the recommended interventions have included various cardiac medications, stress testing, possible coronary angiography, and revascularization, which are not without risk. In the absence of data and following the directive to "first do no harm," the ACC/AHA has been appropriately cautious in not recommending them for routine use at this time.
The updated guidelines have summarized the new evidence in perioperative cardiac evaluation and management. Many of their recommendations were reinforced by this information and remain essentially unchanged. Several new recommendations will lead to changes in management going forward. Unfortunately, we lack the evidence to answer many questions that arise in routine practice and are therefore forced to rely on expert opinion and our clinical judgment in these cases. The ACC/AHA guidelines do provide a framework for our evaluation and management and help keep clinicians up-to-date with the latest evidence.
Guidelines jointly issued by the American College of Cardiology and American Heart Association (ACC/AHA)1 provide a framework for evaluating and managing perioperative cardiac risk in noncardiac surgery. An overriding theme in successive documents from these organizations through the years has been that preoperative intervention, coronary artery bypass grafting, or percutaneous coronary intervention is rarely necessary just to get the patient through surgery, unless it is otherwise indicated independent of the need for surgery.
This article highlights some of the key recommendations in the 2014 updates to these guidelines,1–3 how they differ from previous guidelines,4 and the ongoing challenges and unresolved issues facing physicians involved in perioperative care.
Of note, while these guidelines were being updated, Erasmus University5 expressed concern about the scientific integrity of some of the Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography (DECREASE) trials. As a result, the evidence review committee included these trials in its analysis but not in a systematic review of beta-blockers.2 These trials were not included in the clinical practice guideline supplements and tables but were cited in the text if relevant.
The European Society of Cardiology and European Society of Anesthesiology6 revised their guidelines concurrently with but independently of the ACC/AHA, and although they discussed and aligned some recommendations, many differences remain between the two sets of guidelines. Readers should consult the full guidelines for more detailed information.1
THE ROLE OF THE PREOPERATIVE CARDIAC EVALUATION
The purpose of preoperative medical evaluation is not to "get medical clearance" but rather to evaluate the patient’s medical status and risk of complications. The process includes:
- Identifying risk factors and assessing their severity and stability
- Establishing a clinical risk profile for informed and shared decision-making
- Recommending needed changes in management, further testing, or specialty consultation.
The updated guidelines emphasize the importance of communication among the perioperative team and with the patient. They reiterate the focus on appropriateness of care and cost containment—one should order a test only if the result may change the patient’s management.
HOW URGENT IS SURGERY? HOW RISKY?
The new guidelines classify the urgency of surgery as follows:
- Emergency (necessary within 6 hours)
- Urgent (necessary within 6–24 hours)
- Time-sensitive (can delay 1–6 weeks)
- Elective (can delay up to 1 year).
Surgical risk is now classified as either low (< 1% risk of major adverse cardiac events) or elevated (≥ 1%) on the basis of surgical and patient characteristics. Previous schemas included an intermediate-risk category. Low-risk procedures include endoscopic procedures, superficial procedures, cataract surgery, breast surgery, and ambulatory surgery. Elevated-risk procedures include vascular surgery, intraperitoneal and intrathoracic surgery, head and neck surgery, orthopedic surgery, and prostate surgery.
Risk calculators and biomarkers
To estimate the perioperative risk of major adverse cardiac events, the guidelines suggest incorporating the Revised Cardiac Risk Index (RCRI)7 with an estimation of surgical risk or using a newer surgical risk calculator derived from a database of the American College of Surgeons’ National Surgical Quality Improvement Project (ACS NSQIP).
The RCRI is based on six risk factors, each worth 1 point:
- High-risk surgery
- Ischemic heart disease
- Heart failure
- Stroke or transient ischemic attack
- Diabetes requiring insulin
- Renal insufficiency (serum creatinine > 2.0 mg/dL).7
MICA. The Myocardial Infarction or Cardiac Arrest (MICA) calculator8 has a narrower focus and was validated in only one center.
ACS NSQIP. The recommended newer ACS NSQIP surgical risk calculator9 provides an estimate of procedure-specific risk based on Current Procedural Terminology code and includes 21 patient-specific variables to predict death, major adverse cardiac events, and eight other outcomes. While more comprehensive, this risk calculator has yet to be validated outside of the ACS NSQIP database.
Reconstructed RCRI. The RCRI has been externally validated, but it underestimates risk in major vascular surgery and was outperformed by the MICA calculator. Although not discussed in the new guidelines, a recently published "reconstructed RCRI,"10 in which a serum creatinine level greater than 2 mg/dL in the original RCRI is replaced by a glomerular filtration rate less than 30 mL/min and diabetes is eliminated, may outperform the standard RCRI. A patient with either an RCRI score or a reconstructed RCRI score of 0 or 1 would be considered to be at low risk, whereas patients with two or more risk factors would have an elevated risk.
Cardiac biomarkers, primarily B-type natriuretic peptide (BNP) and N-terminal (NT) proBNP, are independent predictors of cardiac risk, and their addition to preoperative risk indices may provide incremental predictive value. However, how to use these biomarkers and whether any treatment aimed at them will reduce risk is unclear, and the new guidelines did not recommend their routine use.
CLINICAL RISK FACTORS
Coronary artery disease
Ischemic symptoms, a history of myocardial infarction, and elevated cardiac biomarkers are individually associated with perioperative risk of morbidity and death. The risk is modified by how long ago the infarction occurred, whether the patient underwent coronary revascularization, and if so, what type (bypass grafting or percutaneous coronary intervention). A patient with acute coronary syndrome (currently or in the recent past) is at higher risk, and should have elective surgery delayed and be referred for cardiac evaluation and management according to guidelines.
Heart failure
In terms of posing a risk for major adverse cardiac events, heart failure is at least equal to coronary artery disease, and is possibly worse. Its impact depends on its stability, its symptoms, and the patient’s left ventricular function. Symptomatic decompensated heart failure and depressed left ventricular function (ejection fraction < 30% or 40%) confer higher risk than asymptomatic heart failure and preserved left ventricular function. However, evidence is limited with respect to asymptomatic left ventricular dysfunction and diastolic dysfunction. Patients with stable heart failure treated according to guidelines may have better perioperative outcomes.
Valvular heart disease
Significant valvular heart disease is associated with increased risk of postoperative cardiac complications. This risk depends on the type and severity of the valvular lesion and type of noncardiac surgery, but can be minimized by clinical and echocardiographic assessment, choosing appropriate anesthesia, and closer perioperative monitoring. Aortic and mitral stenosis are associated with greater risk of perioperative adverse cardiac events than regurgitant valvular disease.
Echocardiography is recommended in patients suspected of having moderate to severe stenotic or regurgitant lesions if it has not been done within the past year or if the patient’s clinical condition has worsened.
If indicated, valvular intervention can reduce perioperative risk in these patients. Even if the planned noncardiac surgery is high-risk, it may be reasonable to proceed with it (using appropriate perioperative hemodynamic monitoring, which is not specified but typically would be with an arterial line, central line, and possibly a pulmonary arterial catheter) in patients who have asymptomatic severe aortic or mitral regurgitation or aortic stenosis. Surgery may also be reasonable in patients with asymptomatic severe mitral stenosis who are not candidates for repair.
Arrhythmias
Cardiac arrhythmias and conduction defects are often seen in the perioperative period, but there is only limited evidence as to how they affect surgical risk. In addition to their hemodynamic effects, certain arrhythmias (atrial fibrillation, ventricular tachycardia) often indicate underlying structural heart disease, which requires further evaluation before surgery.
The new guidelines refer the reader to previously published clinical practice guidelines for atrial fibrillation,11 supraventricular arrhythmias,12 and device-based therapy.13
ALGORITHM FOR PREOPERATIVE CARDIAC ASSESSMENT
The new algorithm for evaluating a patient who is known to have coronary artery disease or risk factors for it has seven steps (Figure 1).1,11,12,14–17 It differs from the previous algorithm in several details:
- Instead of listing the four active cardiac conditions for which elective surgery should be delayed while the patient is being evaluated and treated (unstable coronary syndrome, decompensated heart failure, significant arrhythmias, severe valvular heart disease), the new version specifically asks about acute coronary syndrome and recommends cardiac evaluation and treatment according to guidelines. A footnote directs readers to other clinical practice guidelines for symptomatic heart failure,14 valvular heart disease,15 and arrhythmias.11,12
- Instead of asking if the procedure is low-risk, the guidelines recommend estimating risk of major adverse cardiac events on the basis of combined clinical and surgical risk and define only two categories: low or elevated. Patients at low risk proceed to surgery with no further testing, as in the earlier algorithm.
- "Excellent" exercise capacity (> 10 metabolic equivalents of task [METs]) is separated from "moderate/good" (4–10 METs), presumably to indicate a stronger recommendation, but patients in both categories proceed to surgery as before.
- If the patient cannot exercise to at least 4 METs, the new algorithm asks whether further testing will affect decision-making or perioperative care (an addition to the previous algorithm). This entails discussing with the patient and perioperative team whether the original surgery will be performed and whether the patient is willing to undergo revascularization if indicated. If so, pharmacologic stress testing is recommended. Previously, this decision also included the number of RCRI factors as well as the type of surgery (vascular or nonvascular).
- If testing will not affect the decision or if the stress test is normal, in addition to recommending proceeding to surgery according to guidelines the new algorithm also lists an option for alternative strategies, including palliation.
- If the stress test is abnormal, especially with left main disease, it recommends coronary revascularization according to the 2011 clinical practice guidelines.18,19
TESTING FOR LEFT VENTRICULAR DYSFUNCTION OR ISCHEMIA
In patients with dyspnea of unexplained cause or worsening dyspnea, assessment of left ventricular function is reasonable, but this is not part of a routine preoperative evaluation.
Pharmacologic stress testing is reasonable for patients at elevated risk with poor functional capacity if the results will change their management, but it is not useful for patients undergoing low-risk surgery. Although dobutamine stress echocardiography may be slightly superior to pharmacologic myocardial perfusion imaging, there are no head-to-head randomized controlled trials, and the guidelines suggest considering local expertise in deciding which test to use.
The presence of moderate to large areas of ischemia (reversible perfusion defects or new wall-motion abnormalities) is associated with risk of perioperative myocardial infarction or death, whereas evidence of an old infarction is associated with long-term but not short-term risk. The negative predictive value of these tests in predicting postoperative cardiac events is high (> 90%), but the positive predictive value is low.
CORONARY REVASCULARIZATION
Coronary artery bypass grafting and percutaneous coronary intervention
The guidelines recommend coronary revascularization before noncardiac surgery only when it is indicated anyway, on the basis of existing clinical practice guidelines.
Whether performing percutaneous coronary intervention before surgery will reduce perioperative cardiac complications is uncertain, and coronary revascularization should not be routinely performed solely to reduce perioperative cardiac events. The only two randomized controlled trials, Coronary Artery Revascularization Prophylaxis (CARP)20 and DECREASE V21 evaluating prophylactic coronary revascularization before noncardiac surgery found no difference in either short-term or long-term outcomes, although subgroup analysis found a survival benefit in patients with left main disease who underwent bypass grafting. Preoperative percutaneous coronary intervention should be limited to patients with left main disease in whom comorbidities preclude bypass surgery and those with unstable coronary disease who may benefit from early invasive management.
The urgency and timing of the noncardiac surgery needs to be taken into account if percutaneous coronary intervention is being considered because of the need for antiplatelet therapy after the procedure, and the potential risks of bleeding and stent thrombosis. If the planned surgery is deemed time-sensitive, then balloon angioplasty or bare-metal stenting is preferred over placement of a drug-eluting stent.
The new guidelines continue to recommend that elective noncardiac surgery be delayed at least 14 days after balloon angioplasty, 30 days after bare-metal stent implantation, and ideally 365 days after drug-eluting stent placement, and reiterate that it is potentially harmful to perform elective surgery within these time frames without any antiplatelet therapy. However, a new class IIb recommendation (benefit ≥ risk) states that "elective noncardiac surgery after [drug-eluting stent] implantation may be considered after 180 days if the risk of further delay is greater than the expected risks of ischemia and stent thrombosis."
This is an important addition to the guidelines because we are often faced with patients needing to undergo surgery in the 6 to 12 months after placement of a drug-eluting stent. Based on previous guidelines, whether it was safe to proceed in this setting created controversy among the perioperative team caring for the patient, and surgery was often delayed unnecessarily. Recent studies22,23 suggest that the newer drug-eluting stents may require a shorter duration of dual antiplatelet therapy, at least in the nonsurgical setting.
MEDICAL THERAPY
Antiplatelet therapy: Stop or continue?
The risk of perioperative bleeding if antiplatelet drugs are continued must be weighed against the risk of stent thrombosis and ischemia if they are stopped before the recommended duration of therapy. Ideally, some antiplatelet therapy should be continued perioperatively in these situations, but the guidelines recommend that a consensus decision among the treating physicians should be made regarding the relative risks of surgery and discontinuation or continuation of antiplatelet therapy. Whenever possible, aspirin should be continued in these patients.
Although the Perioperative Ischemic Evaluation (POISE)-2 trial24 found that perioperative aspirin use was not associated with lower rates of postoperative myocardial infarction or death, it increased bleeding. Patients with stents who had not completed the recommended duration of antiplatelet therapy were excluded from the trial. Additionally, only 5% of the study patients had undergone percutaneous coronary intervention.
According to the guidelines and package inserts, if antiplatelet agents need to be discontinued before surgery, aspirin can be stopped 3 to 7 days before, clopidogrel and ticagrelor 5 days before, and prasugrel 7 days before. In patients without stents, it may be reasonable to continue aspirin perioperatively if the risk of cardiac events outweighs the risk of bleeding, but starting aspirin is not beneficial for patients undergoing elective noncardiac noncarotid surgery unless the risk of ischemic events outweighs the risk of bleeding.
Beta-blockers
In view of the issue of scientific integrity of the DECREASE trials, a separately commissioned systematic review2 of perioperative beta-blocker therapy was performed. This review suggested that giving beta-blockers before surgery was associated with fewer postoperative cardiac events, primarily ischemia and nonfatal myocardial infarction, but few data supported their use to reduce postoperative mortality. Beta-blocker use was associated with adverse outcomes that included bradycardia and stroke. These findings were similar with the inclusion or exclusion of the DECREASE trials in question or of the POISE trial.25
In addition to recommending continuing beta-blockers in patients already on them (class I—the highest recommendation), the guidelines say that it may be reasonable to start them in patients with intermediate- or high-risk ischemia on stress tests as well as in patients with three or more RCRI risk factors (class IIb). In the absence of these indications, initiating beta-blockers preoperatively to reduce risk even in patients with long-term indications is of uncertain benefit. They also recommended starting beta-blockers more than 1 day preoperatively, preferably at least 2 to 7 days before, and note that it was harmful to start them on the day of surgery, particularly at high doses, and with long-acting formulations.
Additionally, there is evidence of differences in outcome within the class of beta-blockers, with the more cardioselective drugs bisoprolol and atenolol being associated with more favorable outcomes than metoprolol in observational studies.
Statins
Multiple observational trials have reported that statins are associated with decreased perioperative morbidity and mortality. Limited evidence from three randomized controlled trials (including two from the discredited DECREASE group) suggests that there is a benefit in patients undergoing vascular surgery, but it is unclear for nonvascular surgery.26–30
The ACC/AHA guidelines again give a class I recommendation to continue statin therapy perioperatively in patients already taking statins and undergoing noncardiac surgery, as there is some evidence that statin withdrawal is associated with increased risk. The guidelines comment that starting statin therapy perioperatively is reasonable for patients undergoing vascular surgery (class IIa) and may be considered in patients with other clinical guideline indications who are undergoing elevated-risk surgery (class IIb).
The mechanism of this benefit is unclear and may relate to the pleotropic as well as the lipid-lowering effects of the statins. Statins may also have beneficial effects in reducing the incidence of acute kidney injury and postoperative atrial fibrillation.
Whether a particular statin, dose, or time of initiation before surgery affects risk is also unknown at this time. The European guidelines6 recommend starting a longer-acting statin ideally at least 2 weeks before surgery for maximal plaque-stabilizing effects.
The risk of statin-induced myopathy, rhabdomyolysis, and hepatic injury appears to be minimal.
Other medications
Of note, the new guidelines do not recommend starting alpha-2 agonists for preventing cardiac events in patients undergoing noncardiac surgery. Despite previous evidence from smaller studies suggesting a benefit, the POISE-2 trial31 demonstrated that perioperative use of clonidine did not reduce cardiac events and was associated with a significant increase in hypotension and nonfatal cardiac arrest. However, clonidine should be continued in patients already taking it.
A somewhat surprising recommendation is that it is reasonable to continue angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), and if they are held before surgery, to restart them as soon as possible postoperatively (class IIa). The guidelines note reports of increased hypotension associated with induction of anesthesia in patients taking these drugs but also note that there was no change in important postoperative cardiac and other outcomes. Although evidence of harm if these drugs are temporarily discontinued before surgery is sparse, the guidelines advocate continuing them in patients with heart failure or hypertension.
ANESTHESIA AND INTRAOPERATIVE MANAGEMENT
The classes of anesthesia include local, regional (nerve block or neuraxial), monitored anesthesia care (ie, intravenous sedation), and general (volatile agent, total intravenous, or a combination). The guideline committee found no evidence to support the use of neuraxial over general anesthesia, volatile over total intravenous anesthesia, or monitored anesthesia care over general anesthesia. Neuraxial anesthesia for postoperative pain relief in patients undergoing abdominal aortic surgery did reduce the incidence of myocardial infarction.
The guidelines do not recommend routinely using intraoperative transesophageal echocardiography during noncardiac surgery to screen for cardiac abnormalities or to monitor for myocardial ischemia in patients without risk factors or procedural risks for significant hemodynamic, pulmonary, or neurologic compromise. Only in emergency settings do they deem perioperative transesophageal echocardiography reasonable to determine the cause of hemodynamic instability when it persists despite attempted corrective therapy.
Maintenance of normothermia is reasonable, as studies evaluating hypothermia or use of warmed air did not find a lower rate of cardiac events.32,33
POSTOPERATIVE SURVEILLANCE
In observational studies, elevated troponin levels, and even detectable levels within the normal range, have been associated with adverse outcomes and predict mortality after noncardiac surgery—the higher the level, the higher the mortality rate.34 Elevated troponins have many potential causes, both cardiac and noncardiac.
An entity termed myocardial injury after noncardiac surgery (MINS)35 was described as prognostically relevant myocardial injury with a troponin T level higher than 0.03 ng/mL in the absence of a nonischemic etiology but not requiring the presence of ischemic features. Patients who had MINS had a higher 30-day mortality rate (9.8% vs 1.1%) and were also at higher risk of nonfatal cardiac arrest, heart failure, and stroke compared with patients who did not.
The guidelines recommend obtaining an electrocardiogram and troponin levels if there are signs or symptoms suggesting myocardial ischemia or infarction. However, despite the association between troponin and mortality, the guidelines state that "the usefulness of postoperative screening with troponin levels (and electrocardiograms) in patients at high risk for perioperative myocardial infarction, but without signs or symptoms suggestive of myocardial ischemia or infarction, is uncertain in the absence of established risks and benefits of a defined management strategy." They also recommend against routinely measuring postoperative troponins in unselected patients without signs or symptoms suggestive of myocardial ischemia or infarction, stating it is not useful for guiding perioperative management.
Although there was a suggestion that patients in the POISE trial36 who suffered postoperative myocardial infarction had better outcomes if they had received aspirin and statins, and another study37 showed that intensification of cardiac therapy in patients with elevated postoperative troponin levels after vascular surgery led to better 1-year outcomes, there are no randomized controlled trials at this time to support any specific plan or intervention.
IMPACT ON CLINICAL PRACTICE: A PERIOPERATIVE HOSPITALIST'S VIEW
Regarding testing
Although the updated guidelines provide some novel concepts in risk stratification, the new algorithm still leaves many patients in a gray zone with respect to noninvasive testing. Patients with heart failure, valvular heart disease, and arrhythmias appear to be somewhat disconnected from the algorithm in this version, and management according to clinical practice guidelines is recommended.
Patients with acute coronary syndrome remain embedded in the algorithm, with recommendations for cardiology evaluation and management according to standard guidelines before proceeding to elective surgery.
The concept of a combined risk based on clinical factors along with the surgical procedure is important, and an alternative to the RCRI factors is offered. However, while this new NSQIP surgical risk calculator is more comprehensive, it may be too time-consuming for routine clinical use and still needs to be externally validated.
The concept of shared decision-making and team communication is stressed, but the physician may still have difficulty deciding when further testing may influence management. The guidelines remain somewhat vague, and many physicians may be uncomfortable and will continue to look for further guidance in this area.
Without more specific recommendations, this uncertainty may result in more stress tests being ordered—often inappropriately, as they rarely change management. Future prospective studies using biomarkers in conjunction with risk calculators may shed some light on this decision.
The new perioperative guidelines incorporate other ACC/AHA guidelines for valvular heart disease15 and heart failure.14 Some of their recommendations, in my opinion, may lead to excessive testing (eg, repeat echocardiograms) that will not change perioperative management.
Regarding revascularization
The ACC/AHA guidelines continue to emphasize the important concept that coronary revascularization is rarely indicated just to get the patient through surgery.
The new guidelines give physicians some leeway in allowing patients with drug-eluting stents to undergo surgery after 6 rather than 12 months of dual antiplatelet therapy if they believe that delaying surgery would place the patient at more risk than that of stent thrombosis. There is evidence in the nonsurgical setting that the newer stents currently being used may require no more than 6 months of therapy. In my opinion it was never clear that there was a statistically significant benefit in delaying surgery more than 6 months after placement of a drug-eluting stent, so this is a welcome addition.
Regarding beta-blockers
The systematic review of beta-blockers reinforces the importance of continuing them preoperatively while downgrading recommendations for their prophylactic use in patients who are not at increased risk.
Although the debate continues, there is no doubt that beta-blockers are associated with a decrease in myocardial ischemia and infarction but an increase in bradycardia and hypotension. They probably are associated with some increased risk of stroke, although this may be related to the specific beta-blocker used as well as the time of initiation before surgery. Evidence of a possible effect on mortality depends on whether the DECREASE and POISE trials are included or excluded in the analysis.
In the absence of new large-scale randomized controlled trials, we are forced to rely on observational trials and expert opinion in the meantime. I think that if a beta-blocker is to be started preoperatively, it should be done at least 1 week before surgery, and a more cardioselective beta-blocker should be used.
Regarding other drugs and tests
I agree with the recommendation to continue ACE inhibitors and ARBs preoperatively in patients with heart failure and poorly controlled hypertension. Although somewhat contrary to current practice, continuance of these drugs has not been associated with an increase in myocardial infarction or death despite concern about intraoperative hypotension.
Data from randomized controlled trials of perioperative statins are limited, but the information from observational studies is favorable, and I see little downside to initiating statins preoperatively in patients who otherwise have indications for their use, particularly if undergoing vascular or other high-risk noncardiac surgery. It is not known whether the specific drug, dose, or timing of initiation of statins influences outcome.
Although multiple studies of biomarkers suggest that there is an association with outcome, there are no randomized controlled trials or specific interventions shown to improve outcome.
Some of the recommended interventions have included various cardiac medications, stress testing, possible coronary angiography, and revascularization, which are not without risk. In the absence of data and following the directive to "first do no harm," the ACC/AHA has been appropriately cautious in not recommending them for routine use at this time.
The updated guidelines have summarized the new evidence in perioperative cardiac evaluation and management. Many of their recommendations were reinforced by this information and remain essentially unchanged. Several new recommendations will lead to changes in management going forward. Unfortunately, we lack the evidence to answer many questions that arise in routine practice and are therefore forced to rely on expert opinion and our clinical judgment in these cases. The ACC/AHA guidelines do provide a framework for our evaluation and management and help keep clinicians up-to-date with the latest evidence.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; Jul 29. pii: S0735-1097(14)05536-3. doi: 10.1016/j.jacc.2014.07.944. [Epub ahead of print].
- Wijeysundera DN, Duncan D, Nkonde-Price C, et al. Perioperative beta blockade in noncardiac surgery: a systematic review for the 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; Jul 29. pii: S0735-1097(14)05528-4. doi: 10.1016/j.jacc.2014.07.939. [Epub ahead of print].
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; Jul 29. pii: S0735-1097(14)05537-5. doi: 10.1016/j.jacc.2014.07.945. [Epub ahead of print].
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. J Am Coll Cardiol 2007; 50:e159–e242.
- Erasmus MC Follow-up Investigation Committee. Report on the 2012 follow-up investigation of possible breaches of academic integrity. September 30, 2012. http://cardiobrief.files.wordpress.com/2012/10/integrity-report-2012-10-english-translation.pdf. Accessed October 30, 2014.
- Anderson JL, Antman EM, Harold JG, et al. Clinical practice guidelines on perioperative cardiovascular evaluation: collaborative efforts among the ACC, AHA, and ESC. J Am Coll Cardiol 2014 Jul 29. pii: S0735-1097(14)05527-2. doi: 10.1016/j.jacc.2014.07.938. [Epub ahead of print].
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation 2011; 124:381–387.
- Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013; 217:833–842. e1-3.
- Davis C, Tait G, Carroll J, Wijeysundera DN, Beattie WS. The Revised Cardiac Risk Index in the new millennium: a single-centre prospective cohort re-evaluation of the original variables in 9,519 consecutive elective surgical patients. Can J Anaesth 2013; 60:855–863.
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; e-pub before print. doi:10.1016/j.jacc.2014.03.022.
- Aliot EM, Alpert JS, Calkins H, et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias. http://www.escardio.org/guidelines-surveys/esc-guidelines/GuidelinesDocuments/guidelines-SVA-FT.pdf. Accessed October 30,2014.
- Crossley GH, Poole JE, Rozner MA, et al. The Heart Rhythm Society (HRS)/American Society of Anesthesiologists (ASA) Expert Consensus Statement on the perioperative management of patients with implantable defibrillators, pacemakers and arrhythmia monitors: facilities and patient management. Developed as a joint project with the American Society of Anesthesiologists (ASA), and in collaboration with the American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Heart Rhythm 2011; 8:1114–1154.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62:e147–e239.
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:e57–e185.
- Jneid H, Anderson JL, Wright RS, et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2012; 60:645-681.
- O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61:e78–e140.
- Hillis LD, Smith PK, Anderson JL, et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. J Am Coll Cardiol 2011; 58:e123–e210.
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011; 58:e44–e122.
- McFalls EO, Ward HB, Krupski WC, et al. Prophylactic coronary artery revascularization for elective vascular surgery: study design. Veterans Affairs Cooperative Study Group on Coronary Artery Revascularization Prophylaxis for Elective Vascular Surgery. Control Clin Trials 1999; 20:297–308.
- Schouten O, van Kuijk JP, Flu WJ, et al. Long-term outcome of prophylactic coronary revascularization in cardiac high-risk patients undergoing major vascular surgery (from the randomized DECREASE-V Pilot Study). Am J Cardiol 2009; 103:897–901.
- Wijeysundera DN, Wijeysundera HC, Yun L, et al. risk of elective major noncardiac surgery after coronary stent insertion: a population-based study. Circulation 2012; 126:1355-1362.
- Hawn MT, Graham LA, Richman JS, et al. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA 2013; 310:1462–1472.
- Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370:1494–1503.
- Group PS, Devereaux PJ, Yang H, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008; 371:1839–1847.
- Lindenauer PK, Pekow P, Wang K, et al. Lipid-lowering therapy and in-hospital mortality following major noncardiac surgery. JAMA 2004; 291:2092–2099.
- Kennedy J, Quan H, Buchan AM, et al. Statins are associated with better outcomes after carotid endarterectomy in symptomatic patients. Stroke 2005; 36:2072–2076.
- Raju MG, Pachika A, Punnam SR, et al. Statin therapy in the reduction of cardiovascular events in patients undergoing intermediate-risk noncardiac, nonvascular surgery. Clin Cardiol 2013; 36:456–461.
- Desai H, Aronow WS, Ahn C, et al. Incidence of perioperative myocardial infarction and of 2-year mortality in 577 elderly patients undergoing noncardiac vascular surgery treated with and without statins. Arch Gerontol Geriatr 2010; 51:149–151.
- Durazzo AES, Machado FS, Ikeoka DT, et al. Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial. J Vasc Surg 2004; 39:967–975.
- Devereaux PJ, Sessler DI, Leslie K, et al. Clonidine in patients undergoing noncardiac surgery. N Engl J Med 2014; 370:1504–1513.
- Nguyen HP, Zaroff JG, Bayman EO, et al. Perioperative hypothermia (33 degrees C) does not increase the occurrence of cardiovascular events in patients undergoing cerebral aneurysm surgery: findings from the Intraoperative Hypothermia for Aneurysm Surgery Trial. Anesthesiology 2010; 113:327–342.
- Frank SM, Fleisher LA, Breslow MJ, et al. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events. A randomized clinical trial. JAMA 1997; 277:1127–1134.
- Vascular Events In Noncardiac Surgery Patients Cohort Evaluation Study I, Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307:2295–2304.
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120:564–578.
- Devereaux PJ, Xavier D, Pogue J, et al. Characteristics and short-term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med 2011; 154:523–528.
- Foucrier A, Rodseth R, Aissaoui M, Ibanes C, et al. The long-term impact of early cardiovascular therapy intensification for postoperative troponin elevation after major vascular surgery. Anesth Analg 2014; 119:1053–1063.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; Jul 29. pii: S0735-1097(14)05536-3. doi: 10.1016/j.jacc.2014.07.944. [Epub ahead of print].
- Wijeysundera DN, Duncan D, Nkonde-Price C, et al. Perioperative beta blockade in noncardiac surgery: a systematic review for the 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; Jul 29. pii: S0735-1097(14)05528-4. doi: 10.1016/j.jacc.2014.07.939. [Epub ahead of print].
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; Jul 29. pii: S0735-1097(14)05537-5. doi: 10.1016/j.jacc.2014.07.945. [Epub ahead of print].
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. J Am Coll Cardiol 2007; 50:e159–e242.
- Erasmus MC Follow-up Investigation Committee. Report on the 2012 follow-up investigation of possible breaches of academic integrity. September 30, 2012. http://cardiobrief.files.wordpress.com/2012/10/integrity-report-2012-10-english-translation.pdf. Accessed October 30, 2014.
- Anderson JL, Antman EM, Harold JG, et al. Clinical practice guidelines on perioperative cardiovascular evaluation: collaborative efforts among the ACC, AHA, and ESC. J Am Coll Cardiol 2014 Jul 29. pii: S0735-1097(14)05527-2. doi: 10.1016/j.jacc.2014.07.938. [Epub ahead of print].
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation 2011; 124:381–387.
- Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013; 217:833–842. e1-3.
- Davis C, Tait G, Carroll J, Wijeysundera DN, Beattie WS. The Revised Cardiac Risk Index in the new millennium: a single-centre prospective cohort re-evaluation of the original variables in 9,519 consecutive elective surgical patients. Can J Anaesth 2013; 60:855–863.
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; e-pub before print. doi:10.1016/j.jacc.2014.03.022.
- Aliot EM, Alpert JS, Calkins H, et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias. http://www.escardio.org/guidelines-surveys/esc-guidelines/GuidelinesDocuments/guidelines-SVA-FT.pdf. Accessed October 30,2014.
- Crossley GH, Poole JE, Rozner MA, et al. The Heart Rhythm Society (HRS)/American Society of Anesthesiologists (ASA) Expert Consensus Statement on the perioperative management of patients with implantable defibrillators, pacemakers and arrhythmia monitors: facilities and patient management. Developed as a joint project with the American Society of Anesthesiologists (ASA), and in collaboration with the American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Heart Rhythm 2011; 8:1114–1154.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62:e147–e239.
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:e57–e185.
- Jneid H, Anderson JL, Wright RS, et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2012; 60:645-681.
- O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61:e78–e140.
- Hillis LD, Smith PK, Anderson JL, et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. J Am Coll Cardiol 2011; 58:e123–e210.
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011; 58:e44–e122.
- McFalls EO, Ward HB, Krupski WC, et al. Prophylactic coronary artery revascularization for elective vascular surgery: study design. Veterans Affairs Cooperative Study Group on Coronary Artery Revascularization Prophylaxis for Elective Vascular Surgery. Control Clin Trials 1999; 20:297–308.
- Schouten O, van Kuijk JP, Flu WJ, et al. Long-term outcome of prophylactic coronary revascularization in cardiac high-risk patients undergoing major vascular surgery (from the randomized DECREASE-V Pilot Study). Am J Cardiol 2009; 103:897–901.
- Wijeysundera DN, Wijeysundera HC, Yun L, et al. risk of elective major noncardiac surgery after coronary stent insertion: a population-based study. Circulation 2012; 126:1355-1362.
- Hawn MT, Graham LA, Richman JS, et al. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA 2013; 310:1462–1472.
- Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370:1494–1503.
- Group PS, Devereaux PJ, Yang H, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008; 371:1839–1847.
- Lindenauer PK, Pekow P, Wang K, et al. Lipid-lowering therapy and in-hospital mortality following major noncardiac surgery. JAMA 2004; 291:2092–2099.
- Kennedy J, Quan H, Buchan AM, et al. Statins are associated with better outcomes after carotid endarterectomy in symptomatic patients. Stroke 2005; 36:2072–2076.
- Raju MG, Pachika A, Punnam SR, et al. Statin therapy in the reduction of cardiovascular events in patients undergoing intermediate-risk noncardiac, nonvascular surgery. Clin Cardiol 2013; 36:456–461.
- Desai H, Aronow WS, Ahn C, et al. Incidence of perioperative myocardial infarction and of 2-year mortality in 577 elderly patients undergoing noncardiac vascular surgery treated with and without statins. Arch Gerontol Geriatr 2010; 51:149–151.
- Durazzo AES, Machado FS, Ikeoka DT, et al. Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial. J Vasc Surg 2004; 39:967–975.
- Devereaux PJ, Sessler DI, Leslie K, et al. Clonidine in patients undergoing noncardiac surgery. N Engl J Med 2014; 370:1504–1513.
- Nguyen HP, Zaroff JG, Bayman EO, et al. Perioperative hypothermia (33 degrees C) does not increase the occurrence of cardiovascular events in patients undergoing cerebral aneurysm surgery: findings from the Intraoperative Hypothermia for Aneurysm Surgery Trial. Anesthesiology 2010; 113:327–342.
- Frank SM, Fleisher LA, Breslow MJ, et al. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events. A randomized clinical trial. JAMA 1997; 277:1127–1134.
- Vascular Events In Noncardiac Surgery Patients Cohort Evaluation Study I, Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307:2295–2304.
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120:564–578.
- Devereaux PJ, Xavier D, Pogue J, et al. Characteristics and short-term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med 2011; 154:523–528.
- Foucrier A, Rodseth R, Aissaoui M, Ibanes C, et al. The long-term impact of early cardiovascular therapy intensification for postoperative troponin elevation after major vascular surgery. Anesth Analg 2014; 119:1053–1063.
KEY POINTS
- Like earlier guidelines, the update recommends preoperative cardiac testing only when the results may influence the patient’s management.
- Preoperative intervention is rarely necessary just to get the patient through surgery, unless it is otherwise indicated independent of the need for surgery.
- The update proposes a modified algorithm for preoperative risk assessment and management and suggests using a new calculator of surgical risk.
- The report also updates information on the timing of surgery after percutaneous coronary intervention, as well as on antiplatelet therapy, other medical therapy, and biomarkers.