User login
The paranoid business executive
CASE Bipolar-like symptoms
Mr. R, age 48, presents to the psychiatric emergency department (ED) for the third time in 4 days after a change in his behavior over the last 2.5 weeks. He exhibits heightened extroversion, pressured speech, and uncharacteristic irritability. Mr. R’s wife reports that her husband normally is reserved.
Mr. R’s wife first became concerned when she noticed he was not sleeping and spending his nights changing the locks on their home. Mr. R, who is a business executive, occupied his time by taking notes on ways to protect his identity from the senior partners at his company.
Three weeks before his first ED visit, Mr. R had been treated for a neck abscess with incision and drainage. He was sent home with a 10-day course of amoxicillin/clavulanate, 875/125 mg by mouth twice daily. There were no reports of steroid use during or after the procedure. Four days after starting the antibiotic, he stopped taking it because he and his wife felt it was contributing to his mood changes and bizarre behavior.
During his first visit to the ED, Mr. R received a 1-time dose of olanzapine, 5 mg by mouth, which helped temporarily reduce his anxiety; however, he returned the following day with the same anxiety symptoms and was discharged with a 30-day prescription for olanzapine, 5 mg/d, to manage symptoms until he could establish care with an outpatient psychiatrist. Two days later, he returned to the ED yet again convinced people were spying on him and that his coworkers were plotting to have him fired. He was not taking his phone to work due to fears that it would be hacked.
Mr. R’s only home medication is clomiphene citrate, 100 mg/d by mouth, which he’s received for the past 7 months to treat low testosterone. He has no personal or family history of psychiatric illness and no prior signs of mania or hypomania.
At the current ED visit, Mr. R’s testosterone level is checked and is within normal limits. His urine drug screen, head CT, and standard laboratory test results are unremarkable, except for mild transaminitis that does not warrant acute management.
The clinicians in the ED establish a diagnosis of mania, unspecified, and psychotic disorder, unspecified. They recommend that Mr. R be admitted for mood stabilization.
[polldaddy:10485725]
Continue to: The authors' observations
The authors’ observations
Our initial impression was that Mr. R was experiencing a manic episode from undiagnosed bipolar I disorder. The diagnosis was equivocal considering his age, lack of family history, and absence of prior psychiatric symptoms. In most cases, the mean age of onset for mania is late adolescence to early adulthood. It would be less common for a patient to experience a first manic episode at age 48, although mania may emerge at any age. Results from a large British study showed that the incidence of a first manic episode drops from 13.81% in men age 16 to 25 to 2.62% in men age 46 to 55.1 However, some estimates suggest that the prevalence of late-onset mania is much higher than previously expected; medical comorbidities, such as dementia and delirium, may play a significant role in posing as manic-type symptoms in these patients.2
In Mr. R’s case, he remained fully alert and oriented without waxing and waning attentional deficits, which made delirium less likely. His affective symptoms included a reduced need for sleep, anxiety, irritability, rapid speech, and grandiosity lasting at least 2 weeks. He also exhibited psychotic symptoms in the form of paranoia. Altogether, he fit diagnostic criteria for bipolar I disorder well.
At the time of his manic episode, Mr. R was taking clomiphene. Clomiphene-induced mania and psychosis has been reported scarcely in the literature.3 In these cases, behavioral changes occurred within the first month of clomiphene initiation, which is dissimilar from Mr. R’s timeline.4 However, there appeared to be a temporal relationship between Mr. R’s use of amoxicillin/clavulanate and his manic episode.
This led us to consider whether medication-induced bipolar disorder would be a more appropriate diagnosis. There are documented associations between mania and antibiotics5; however, to our knowledge, mania secondary specifically to amoxicillin/clavulanate has not been reported extensively in the American literature. We found 1 case of suspected amoxicillin-induced psychosis,6 as well as a case report from the Netherlands of possible amoxicillin/clavulanate-induced mania.7
EVALUATION Ongoing paranoia
During his psychiatric hospitalization, Mr. R remains cooperative and polite, but exhibits ongoing paranoia, pressured speech, and poor reality testing. He remains convinced that “people are out to get me,” and routinely scans the room for safety during daily evaluations. He reports that he feels safe in the hospital, but does not feel safe to leave. Mr. R does not recall if in the past he had taken any products containing amoxicillin, but he is able to appreciate changes in his mood after being prescribed the antibiotic. He reports that starting the antibiotic made him feel confident in social interactions.
Continue to: During Mr. R's psychiatric hospitalization...
During Mr. R’s psychiatric hospitalization, olanzapine is titrated to 10 mg at bedtime. Clomiphene citrate is discontinued to limit any potential precipitants of mania, and amoxicillin/clavulanate is not restarted.
Mr. R gradually shows improvement in sleep quality and duration and becomes less irritable. His speech returns to a regular rate and rhythm. He eventually begins to question whether his fears were reality-based. After 4 days, Mr. R is ready to be discharged home and return to work.
[polldaddy:10485726]
The authors’ observations
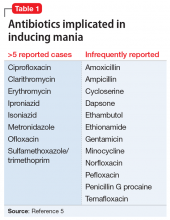
The term “antibiomania” is used to describe manic episodes that coincide with antibiotic usage.8 Clarithromycin and ciprofloxacin are the agents most frequently implicated in antibiomania.9 While numerous reports exist in the literature, antibiomania is still considered a rare or unusual adverse event.
The link between infections and neuropsychiatric symptoms is well documented, which makes it challenging to tease apart the role of the acute infection from the use of antibiotics in precipitating psychiatric symptoms. However, in most reported cases of antibiomania, the onset of manic symptoms typically occurs within the first week of antibiotic initiation and resolves 1 to 3 days after medication discontinuation. The temporal relationship between antibiotic initiation and onset of neuropsychiatric symptoms has been best highlighted in cases where clarithromycin is used to treat a chronic Helicobacter pylori infection.10
While reports of antibiomania date back more than 6 decades, the exact mechanism by which antibiotics cause psychiatric symptoms is mostly unknown, although there are several hypotheses.5 Many hypotheses suggest some antibiotics play a role in reducing gamma-aminobutyric acid (GABA) neurotransmission. Quinolones, for example, have been found to cross the blood–brain barrier and can inhibit GABA from binding to the receptor sites. This can result in hyper-excitability in the CNS. Several quinolones have been implicated in antibiomania (Table 15). Penicillins are also thought to interfere with GABA neurotransmission in a similar fashion; however, amoxicillin-clavulanate has poor CNS penetration in the absence of blood–brain barrier disruption,11 which makes this theory a less plausible explanation for Mr. R’s case.
Continue to: Another possible mechanism...
Another possible mechanism of antibiotic-induced CNS excitability is through the glutamatergic system. Cycloserine, an antitubercular agent, is an N-methyl-D-aspartate receptor (NMDA) partial agonist and has reported neuropsychiatric adverse effects.12 It has been proposed that quinolones may also have NMDA agonist activity.
The prostaglandin hypothesis suggests that a decrease in GABA may increase concentrations of steroid hormones in the rat CNS.13 Steroids have been implicated in the breakdown of prostaglandin E1 (PGE1).13 A disruption in steroid regulation may prevent PGE1 breakdown. Lithium’s antimanic properties are thought to be caused at least in part by limiting prostaglandin production.14 Thus, a shift in PGE1 may lead to mood dysregulation.
Bipolar disorder has been linked with mitochondrial function abnormalities.15 Antibiotics that target ribosomal RNA may disrupt normal mitochondrial function and increase risk for mania precipitation.15 However, amoxicillin exerts its antibiotic effects through binding to penicillin-binding proteins, which leads to inhibition of the cell wall biosynthesis.
Lastly, research into the microbiome has elucidated the gut-brain axis. In animal studies, the microbiome has been found to play a role in immunity, cognitive function, and behavior. Dysbiosis in the microbiome is currently being investigated for its role in schizophrenia and bipolar disorder.16 Both the microbiome and changes in mitochondrial function are thought to develop over time, so while these are plausible explanations, an onset within 4 days of antibiotic initiation is likely too short of an exposure time to produce these changes.
The most likely causes of Mr. R’s manic episode were clomiphene or amoxicillin-clavulanate, and the time course seems to indicate the antibiotic was the most likely culprit. Table 2 lists things to consider if you suspect your patient may be experiencing antibiomania.

Continue to: TREATMENT Stable on olanzapine
TREATMENT Stable on olanzapine
During his first visit to the outpatient clinic 4 weeks after being discharged, Mr. R reports that he has successfully returned to work, and his paranoia has completely resolved. He continues to take olanzapine, 10 mg nightly, and has restarted clomiphene, 100 mg/d.
During this outpatient follow-up visit, Mr. R attributes his manic episode to an adverse reaction to amoxicillin/clavulanate, and requests to be tapered off olanzapine. After he and his psychiatrist discuss the risk of relapse in untreated bipolar disorder, olanzapine is reduced to 7.5 mg at bedtime with a plan to taper to discontinuation.
At his second follow-up visit 1 month later, Mr. R has also stopped clomiphene and is taking a herbal supplement instead, which he reports is helpful for his fatigue.
[polldaddy:10485727]
OUTCOME Lasting euthymic mood
Mr. R agrees to our recommendation of continuing to monitor him every 3 months for at least 1 year. We provide him and his wife with education about early warning signs of mood instability. Eight months after his manic episode, Mr. R no longer receives any psychotropic medications and shows no signs of mood instability. His mood remains euthymic and he is able to function well at work and in his personal life.
Bottom Line
‘Antibiomania’ describes manic episodes that coincide with antibiotic usage. This adverse effect is rare but should be considered in patients who present with unexplained first-episode mania, particularly those with an initial onset of mania after early adulthood.
Continue to: Related Resources
Related Resources
- Rakofsky JJ, Dunlop BW. Nothing to sneeze at: Upper respiratory infections and mood disorders. Current Psychiatry. 2019;18(7):29-34.
- Adiba A, Jackson JC, Torrence CL. Older-age bipolar disorder: A case series. Current Psychiatry. 2019;18(2):24-29
Drug Brand Names
Amoxicillin • Amoxil
Amoxicillin/clavulanate • Augmentin
Ampicillin • Omnipen-N, Polycillin-N
Ciprofloxacin • Cipro
Clarithromycin • Biaxin
Clomiphene • Clomid
Cycloserine • Seromycin
Dapsone • Dapsone
Erythromycin • Erythrocin, Pediamycin
Ethambutol • Myambutol
Ethionamide • Trecator-SC
Gentamicin • Garamycin
Isoniazid • Hyzyd, Nydrazid
Lithium • Eskalith, Lithobid
Metronidazole • Flagyl
Minocycline • Dynacin, Solodyn
Norfloxacin • Noroxin
Ofloxacin • Floxin
Olanzapine • Zyprexa
Penicillin G procaine • Duracillin A-S, Pfizerpen
Sulfamethoxazole/trimethoprim • Bactrim, Septra
1. Kennedy M, Everitt B, Boydell J, et al. Incidence and distribution of first-episode mania by age: results for a 35-year study. Psychol Med. 2005;35(6):855-863.
2. Dols A, Kupka RW, van Lammeren A, et al. The prevalence of late-life mania: a review. Bipolar Disord. 2014;16:113-118.
3. Siedontopf F, Horstkamp B, Stief G, et al. Clomiphene citrate as a possible cause of a psychotic reaction during infertility treatment. Hum Reprod. 1997;12(4):706-707.
4. Oyffe T, Lerner A, Isaacs G, et al. Clomiphene-induced psychosis. Am J Psychiatry. 1997;154(8):1169-1170.
5. Lambrichts S, Van Oudenhove L, Sienaert P. Antibiotics and mania: a systematic review. J Affect Disord. 2017;219:149-156.
6. Beal DM, Hudson B, Zaiac M. Amoxicillin-induced psychosis? Am J Psychiatry. 1986;143(2):255-256.
7. Klain V, Timmerman L. Antibiomania, acute manic psychosis following the use of antibiotics. European Psychiatry. 2013;28(suppl 1):1.
8. Abouesh A, Stone C, Hobbs WR. Antimicrobial-induced mania (antibiomania): a review of spontaneous reports. J Clin Psychopharmacol. 2002;22(1):71-81.
9. Lally L, Mannion L. The potential for antimicrobials to adversely affect mental state. BMJ Case Rep. 2013. pii: bcr2013009659. doi: 10.1136/bcr-2013-009659.
10. Neufeld NH, Mohamed NS, Grujich N, et al. Acute neuropsychiatric symptoms associated with antibiotic treatment of Helicobactor Pylori infections: a review. J Psychiatr Pract. 2017;23(1):25-35.
11. Sutter R, Rüegg S, Tschudin-Sutter S. Seizures as adverse events of antibiotic drugs: a systematic review. Neurology. 2015;85(15):1332-1341.
12. Bakhla A, Gore P, Srivastava S. Cycloserine induced mania. Ind Psychiatry J. 2013;22(1):69-70.
13. Barbaccia ML, Roscetti G, Trabucchi M, et al. Isoniazid-induced inhibition of GABAergic transmission enhances neurosteroid content in the rat brain. Neuropharmacology. 1996;35(9-10):1299-1305.
14. Murphy D, Donnelly C, Moskowitz J. Inhibition by lithium of prostaglandin E1 and norepinephrine effects on cyclic adenosine monophosphate production in human platelets. Clin Pharmacol Ther. 1973;14(5):810-814.
15. Clay H, Sillivan S, Konradi C. Mitochondrial dysfunction and pathology in bipolar disorder and schizophrenia. Int J Dev Neurosci. 2011;29(3):311-324.
16. Dickerson F, Severance E, Yolken R. The microbiome, immunity, and schizophrenia and bipolar disorder. Brain Behav Immun. 2017;62:46-52.
CASE Bipolar-like symptoms
Mr. R, age 48, presents to the psychiatric emergency department (ED) for the third time in 4 days after a change in his behavior over the last 2.5 weeks. He exhibits heightened extroversion, pressured speech, and uncharacteristic irritability. Mr. R’s wife reports that her husband normally is reserved.
Mr. R’s wife first became concerned when she noticed he was not sleeping and spending his nights changing the locks on their home. Mr. R, who is a business executive, occupied his time by taking notes on ways to protect his identity from the senior partners at his company.
Three weeks before his first ED visit, Mr. R had been treated for a neck abscess with incision and drainage. He was sent home with a 10-day course of amoxicillin/clavulanate, 875/125 mg by mouth twice daily. There were no reports of steroid use during or after the procedure. Four days after starting the antibiotic, he stopped taking it because he and his wife felt it was contributing to his mood changes and bizarre behavior.
During his first visit to the ED, Mr. R received a 1-time dose of olanzapine, 5 mg by mouth, which helped temporarily reduce his anxiety; however, he returned the following day with the same anxiety symptoms and was discharged with a 30-day prescription for olanzapine, 5 mg/d, to manage symptoms until he could establish care with an outpatient psychiatrist. Two days later, he returned to the ED yet again convinced people were spying on him and that his coworkers were plotting to have him fired. He was not taking his phone to work due to fears that it would be hacked.
Mr. R’s only home medication is clomiphene citrate, 100 mg/d by mouth, which he’s received for the past 7 months to treat low testosterone. He has no personal or family history of psychiatric illness and no prior signs of mania or hypomania.
At the current ED visit, Mr. R’s testosterone level is checked and is within normal limits. His urine drug screen, head CT, and standard laboratory test results are unremarkable, except for mild transaminitis that does not warrant acute management.
The clinicians in the ED establish a diagnosis of mania, unspecified, and psychotic disorder, unspecified. They recommend that Mr. R be admitted for mood stabilization.
[polldaddy:10485725]
Continue to: The authors' observations
The authors’ observations
Our initial impression was that Mr. R was experiencing a manic episode from undiagnosed bipolar I disorder. The diagnosis was equivocal considering his age, lack of family history, and absence of prior psychiatric symptoms. In most cases, the mean age of onset for mania is late adolescence to early adulthood. It would be less common for a patient to experience a first manic episode at age 48, although mania may emerge at any age. Results from a large British study showed that the incidence of a first manic episode drops from 13.81% in men age 16 to 25 to 2.62% in men age 46 to 55.1 However, some estimates suggest that the prevalence of late-onset mania is much higher than previously expected; medical comorbidities, such as dementia and delirium, may play a significant role in posing as manic-type symptoms in these patients.2
In Mr. R’s case, he remained fully alert and oriented without waxing and waning attentional deficits, which made delirium less likely. His affective symptoms included a reduced need for sleep, anxiety, irritability, rapid speech, and grandiosity lasting at least 2 weeks. He also exhibited psychotic symptoms in the form of paranoia. Altogether, he fit diagnostic criteria for bipolar I disorder well.
At the time of his manic episode, Mr. R was taking clomiphene. Clomiphene-induced mania and psychosis has been reported scarcely in the literature.3 In these cases, behavioral changes occurred within the first month of clomiphene initiation, which is dissimilar from Mr. R’s timeline.4 However, there appeared to be a temporal relationship between Mr. R’s use of amoxicillin/clavulanate and his manic episode.
This led us to consider whether medication-induced bipolar disorder would be a more appropriate diagnosis. There are documented associations between mania and antibiotics5; however, to our knowledge, mania secondary specifically to amoxicillin/clavulanate has not been reported extensively in the American literature. We found 1 case of suspected amoxicillin-induced psychosis,6 as well as a case report from the Netherlands of possible amoxicillin/clavulanate-induced mania.7
EVALUATION Ongoing paranoia
During his psychiatric hospitalization, Mr. R remains cooperative and polite, but exhibits ongoing paranoia, pressured speech, and poor reality testing. He remains convinced that “people are out to get me,” and routinely scans the room for safety during daily evaluations. He reports that he feels safe in the hospital, but does not feel safe to leave. Mr. R does not recall if in the past he had taken any products containing amoxicillin, but he is able to appreciate changes in his mood after being prescribed the antibiotic. He reports that starting the antibiotic made him feel confident in social interactions.
Continue to: During Mr. R's psychiatric hospitalization...
During Mr. R’s psychiatric hospitalization, olanzapine is titrated to 10 mg at bedtime. Clomiphene citrate is discontinued to limit any potential precipitants of mania, and amoxicillin/clavulanate is not restarted.
Mr. R gradually shows improvement in sleep quality and duration and becomes less irritable. His speech returns to a regular rate and rhythm. He eventually begins to question whether his fears were reality-based. After 4 days, Mr. R is ready to be discharged home and return to work.
[polldaddy:10485726]
The authors’ observations
The term “antibiomania” is used to describe manic episodes that coincide with antibiotic usage.8 Clarithromycin and ciprofloxacin are the agents most frequently implicated in antibiomania.9 While numerous reports exist in the literature, antibiomania is still considered a rare or unusual adverse event.
The link between infections and neuropsychiatric symptoms is well documented, which makes it challenging to tease apart the role of the acute infection from the use of antibiotics in precipitating psychiatric symptoms. However, in most reported cases of antibiomania, the onset of manic symptoms typically occurs within the first week of antibiotic initiation and resolves 1 to 3 days after medication discontinuation. The temporal relationship between antibiotic initiation and onset of neuropsychiatric symptoms has been best highlighted in cases where clarithromycin is used to treat a chronic Helicobacter pylori infection.10
While reports of antibiomania date back more than 6 decades, the exact mechanism by which antibiotics cause psychiatric symptoms is mostly unknown, although there are several hypotheses.5 Many hypotheses suggest some antibiotics play a role in reducing gamma-aminobutyric acid (GABA) neurotransmission. Quinolones, for example, have been found to cross the blood–brain barrier and can inhibit GABA from binding to the receptor sites. This can result in hyper-excitability in the CNS. Several quinolones have been implicated in antibiomania (Table 15). Penicillins are also thought to interfere with GABA neurotransmission in a similar fashion; however, amoxicillin-clavulanate has poor CNS penetration in the absence of blood–brain barrier disruption,11 which makes this theory a less plausible explanation for Mr. R’s case.
Continue to: Another possible mechanism...
Another possible mechanism of antibiotic-induced CNS excitability is through the glutamatergic system. Cycloserine, an antitubercular agent, is an N-methyl-D-aspartate receptor (NMDA) partial agonist and has reported neuropsychiatric adverse effects.12 It has been proposed that quinolones may also have NMDA agonist activity.
The prostaglandin hypothesis suggests that a decrease in GABA may increase concentrations of steroid hormones in the rat CNS.13 Steroids have been implicated in the breakdown of prostaglandin E1 (PGE1).13 A disruption in steroid regulation may prevent PGE1 breakdown. Lithium’s antimanic properties are thought to be caused at least in part by limiting prostaglandin production.14 Thus, a shift in PGE1 may lead to mood dysregulation.
Bipolar disorder has been linked with mitochondrial function abnormalities.15 Antibiotics that target ribosomal RNA may disrupt normal mitochondrial function and increase risk for mania precipitation.15 However, amoxicillin exerts its antibiotic effects through binding to penicillin-binding proteins, which leads to inhibition of the cell wall biosynthesis.
Lastly, research into the microbiome has elucidated the gut-brain axis. In animal studies, the microbiome has been found to play a role in immunity, cognitive function, and behavior. Dysbiosis in the microbiome is currently being investigated for its role in schizophrenia and bipolar disorder.16 Both the microbiome and changes in mitochondrial function are thought to develop over time, so while these are plausible explanations, an onset within 4 days of antibiotic initiation is likely too short of an exposure time to produce these changes.
The most likely causes of Mr. R’s manic episode were clomiphene or amoxicillin-clavulanate, and the time course seems to indicate the antibiotic was the most likely culprit. Table 2 lists things to consider if you suspect your patient may be experiencing antibiomania.

Continue to: TREATMENT Stable on olanzapine
TREATMENT Stable on olanzapine
During his first visit to the outpatient clinic 4 weeks after being discharged, Mr. R reports that he has successfully returned to work, and his paranoia has completely resolved. He continues to take olanzapine, 10 mg nightly, and has restarted clomiphene, 100 mg/d.
During this outpatient follow-up visit, Mr. R attributes his manic episode to an adverse reaction to amoxicillin/clavulanate, and requests to be tapered off olanzapine. After he and his psychiatrist discuss the risk of relapse in untreated bipolar disorder, olanzapine is reduced to 7.5 mg at bedtime with a plan to taper to discontinuation.
At his second follow-up visit 1 month later, Mr. R has also stopped clomiphene and is taking a herbal supplement instead, which he reports is helpful for his fatigue.
[polldaddy:10485727]
OUTCOME Lasting euthymic mood
Mr. R agrees to our recommendation of continuing to monitor him every 3 months for at least 1 year. We provide him and his wife with education about early warning signs of mood instability. Eight months after his manic episode, Mr. R no longer receives any psychotropic medications and shows no signs of mood instability. His mood remains euthymic and he is able to function well at work and in his personal life.
Bottom Line
‘Antibiomania’ describes manic episodes that coincide with antibiotic usage. This adverse effect is rare but should be considered in patients who present with unexplained first-episode mania, particularly those with an initial onset of mania after early adulthood.
Continue to: Related Resources
Related Resources
- Rakofsky JJ, Dunlop BW. Nothing to sneeze at: Upper respiratory infections and mood disorders. Current Psychiatry. 2019;18(7):29-34.
- Adiba A, Jackson JC, Torrence CL. Older-age bipolar disorder: A case series. Current Psychiatry. 2019;18(2):24-29
Drug Brand Names
Amoxicillin • Amoxil
Amoxicillin/clavulanate • Augmentin
Ampicillin • Omnipen-N, Polycillin-N
Ciprofloxacin • Cipro
Clarithromycin • Biaxin
Clomiphene • Clomid
Cycloserine • Seromycin
Dapsone • Dapsone
Erythromycin • Erythrocin, Pediamycin
Ethambutol • Myambutol
Ethionamide • Trecator-SC
Gentamicin • Garamycin
Isoniazid • Hyzyd, Nydrazid
Lithium • Eskalith, Lithobid
Metronidazole • Flagyl
Minocycline • Dynacin, Solodyn
Norfloxacin • Noroxin
Ofloxacin • Floxin
Olanzapine • Zyprexa
Penicillin G procaine • Duracillin A-S, Pfizerpen
Sulfamethoxazole/trimethoprim • Bactrim, Septra
CASE Bipolar-like symptoms
Mr. R, age 48, presents to the psychiatric emergency department (ED) for the third time in 4 days after a change in his behavior over the last 2.5 weeks. He exhibits heightened extroversion, pressured speech, and uncharacteristic irritability. Mr. R’s wife reports that her husband normally is reserved.
Mr. R’s wife first became concerned when she noticed he was not sleeping and spending his nights changing the locks on their home. Mr. R, who is a business executive, occupied his time by taking notes on ways to protect his identity from the senior partners at his company.
Three weeks before his first ED visit, Mr. R had been treated for a neck abscess with incision and drainage. He was sent home with a 10-day course of amoxicillin/clavulanate, 875/125 mg by mouth twice daily. There were no reports of steroid use during or after the procedure. Four days after starting the antibiotic, he stopped taking it because he and his wife felt it was contributing to his mood changes and bizarre behavior.
During his first visit to the ED, Mr. R received a 1-time dose of olanzapine, 5 mg by mouth, which helped temporarily reduce his anxiety; however, he returned the following day with the same anxiety symptoms and was discharged with a 30-day prescription for olanzapine, 5 mg/d, to manage symptoms until he could establish care with an outpatient psychiatrist. Two days later, he returned to the ED yet again convinced people were spying on him and that his coworkers were plotting to have him fired. He was not taking his phone to work due to fears that it would be hacked.
Mr. R’s only home medication is clomiphene citrate, 100 mg/d by mouth, which he’s received for the past 7 months to treat low testosterone. He has no personal or family history of psychiatric illness and no prior signs of mania or hypomania.
At the current ED visit, Mr. R’s testosterone level is checked and is within normal limits. His urine drug screen, head CT, and standard laboratory test results are unremarkable, except for mild transaminitis that does not warrant acute management.
The clinicians in the ED establish a diagnosis of mania, unspecified, and psychotic disorder, unspecified. They recommend that Mr. R be admitted for mood stabilization.
[polldaddy:10485725]
Continue to: The authors' observations
The authors’ observations
Our initial impression was that Mr. R was experiencing a manic episode from undiagnosed bipolar I disorder. The diagnosis was equivocal considering his age, lack of family history, and absence of prior psychiatric symptoms. In most cases, the mean age of onset for mania is late adolescence to early adulthood. It would be less common for a patient to experience a first manic episode at age 48, although mania may emerge at any age. Results from a large British study showed that the incidence of a first manic episode drops from 13.81% in men age 16 to 25 to 2.62% in men age 46 to 55.1 However, some estimates suggest that the prevalence of late-onset mania is much higher than previously expected; medical comorbidities, such as dementia and delirium, may play a significant role in posing as manic-type symptoms in these patients.2
In Mr. R’s case, he remained fully alert and oriented without waxing and waning attentional deficits, which made delirium less likely. His affective symptoms included a reduced need for sleep, anxiety, irritability, rapid speech, and grandiosity lasting at least 2 weeks. He also exhibited psychotic symptoms in the form of paranoia. Altogether, he fit diagnostic criteria for bipolar I disorder well.
At the time of his manic episode, Mr. R was taking clomiphene. Clomiphene-induced mania and psychosis has been reported scarcely in the literature.3 In these cases, behavioral changes occurred within the first month of clomiphene initiation, which is dissimilar from Mr. R’s timeline.4 However, there appeared to be a temporal relationship between Mr. R’s use of amoxicillin/clavulanate and his manic episode.
This led us to consider whether medication-induced bipolar disorder would be a more appropriate diagnosis. There are documented associations between mania and antibiotics5; however, to our knowledge, mania secondary specifically to amoxicillin/clavulanate has not been reported extensively in the American literature. We found 1 case of suspected amoxicillin-induced psychosis,6 as well as a case report from the Netherlands of possible amoxicillin/clavulanate-induced mania.7
EVALUATION Ongoing paranoia
During his psychiatric hospitalization, Mr. R remains cooperative and polite, but exhibits ongoing paranoia, pressured speech, and poor reality testing. He remains convinced that “people are out to get me,” and routinely scans the room for safety during daily evaluations. He reports that he feels safe in the hospital, but does not feel safe to leave. Mr. R does not recall if in the past he had taken any products containing amoxicillin, but he is able to appreciate changes in his mood after being prescribed the antibiotic. He reports that starting the antibiotic made him feel confident in social interactions.
Continue to: During Mr. R's psychiatric hospitalization...
During Mr. R’s psychiatric hospitalization, olanzapine is titrated to 10 mg at bedtime. Clomiphene citrate is discontinued to limit any potential precipitants of mania, and amoxicillin/clavulanate is not restarted.
Mr. R gradually shows improvement in sleep quality and duration and becomes less irritable. His speech returns to a regular rate and rhythm. He eventually begins to question whether his fears were reality-based. After 4 days, Mr. R is ready to be discharged home and return to work.
[polldaddy:10485726]
The authors’ observations
The term “antibiomania” is used to describe manic episodes that coincide with antibiotic usage.8 Clarithromycin and ciprofloxacin are the agents most frequently implicated in antibiomania.9 While numerous reports exist in the literature, antibiomania is still considered a rare or unusual adverse event.
The link between infections and neuropsychiatric symptoms is well documented, which makes it challenging to tease apart the role of the acute infection from the use of antibiotics in precipitating psychiatric symptoms. However, in most reported cases of antibiomania, the onset of manic symptoms typically occurs within the first week of antibiotic initiation and resolves 1 to 3 days after medication discontinuation. The temporal relationship between antibiotic initiation and onset of neuropsychiatric symptoms has been best highlighted in cases where clarithromycin is used to treat a chronic Helicobacter pylori infection.10
While reports of antibiomania date back more than 6 decades, the exact mechanism by which antibiotics cause psychiatric symptoms is mostly unknown, although there are several hypotheses.5 Many hypotheses suggest some antibiotics play a role in reducing gamma-aminobutyric acid (GABA) neurotransmission. Quinolones, for example, have been found to cross the blood–brain barrier and can inhibit GABA from binding to the receptor sites. This can result in hyper-excitability in the CNS. Several quinolones have been implicated in antibiomania (Table 15). Penicillins are also thought to interfere with GABA neurotransmission in a similar fashion; however, amoxicillin-clavulanate has poor CNS penetration in the absence of blood–brain barrier disruption,11 which makes this theory a less plausible explanation for Mr. R’s case.
Continue to: Another possible mechanism...
Another possible mechanism of antibiotic-induced CNS excitability is through the glutamatergic system. Cycloserine, an antitubercular agent, is an N-methyl-D-aspartate receptor (NMDA) partial agonist and has reported neuropsychiatric adverse effects.12 It has been proposed that quinolones may also have NMDA agonist activity.
The prostaglandin hypothesis suggests that a decrease in GABA may increase concentrations of steroid hormones in the rat CNS.13 Steroids have been implicated in the breakdown of prostaglandin E1 (PGE1).13 A disruption in steroid regulation may prevent PGE1 breakdown. Lithium’s antimanic properties are thought to be caused at least in part by limiting prostaglandin production.14 Thus, a shift in PGE1 may lead to mood dysregulation.
Bipolar disorder has been linked with mitochondrial function abnormalities.15 Antibiotics that target ribosomal RNA may disrupt normal mitochondrial function and increase risk for mania precipitation.15 However, amoxicillin exerts its antibiotic effects through binding to penicillin-binding proteins, which leads to inhibition of the cell wall biosynthesis.
Lastly, research into the microbiome has elucidated the gut-brain axis. In animal studies, the microbiome has been found to play a role in immunity, cognitive function, and behavior. Dysbiosis in the microbiome is currently being investigated for its role in schizophrenia and bipolar disorder.16 Both the microbiome and changes in mitochondrial function are thought to develop over time, so while these are plausible explanations, an onset within 4 days of antibiotic initiation is likely too short of an exposure time to produce these changes.
The most likely causes of Mr. R’s manic episode were clomiphene or amoxicillin-clavulanate, and the time course seems to indicate the antibiotic was the most likely culprit. Table 2 lists things to consider if you suspect your patient may be experiencing antibiomania.

Continue to: TREATMENT Stable on olanzapine
TREATMENT Stable on olanzapine
During his first visit to the outpatient clinic 4 weeks after being discharged, Mr. R reports that he has successfully returned to work, and his paranoia has completely resolved. He continues to take olanzapine, 10 mg nightly, and has restarted clomiphene, 100 mg/d.
During this outpatient follow-up visit, Mr. R attributes his manic episode to an adverse reaction to amoxicillin/clavulanate, and requests to be tapered off olanzapine. After he and his psychiatrist discuss the risk of relapse in untreated bipolar disorder, olanzapine is reduced to 7.5 mg at bedtime with a plan to taper to discontinuation.
At his second follow-up visit 1 month later, Mr. R has also stopped clomiphene and is taking a herbal supplement instead, which he reports is helpful for his fatigue.
[polldaddy:10485727]
OUTCOME Lasting euthymic mood
Mr. R agrees to our recommendation of continuing to monitor him every 3 months for at least 1 year. We provide him and his wife with education about early warning signs of mood instability. Eight months after his manic episode, Mr. R no longer receives any psychotropic medications and shows no signs of mood instability. His mood remains euthymic and he is able to function well at work and in his personal life.
Bottom Line
‘Antibiomania’ describes manic episodes that coincide with antibiotic usage. This adverse effect is rare but should be considered in patients who present with unexplained first-episode mania, particularly those with an initial onset of mania after early adulthood.
Continue to: Related Resources
Related Resources
- Rakofsky JJ, Dunlop BW. Nothing to sneeze at: Upper respiratory infections and mood disorders. Current Psychiatry. 2019;18(7):29-34.
- Adiba A, Jackson JC, Torrence CL. Older-age bipolar disorder: A case series. Current Psychiatry. 2019;18(2):24-29
Drug Brand Names
Amoxicillin • Amoxil
Amoxicillin/clavulanate • Augmentin
Ampicillin • Omnipen-N, Polycillin-N
Ciprofloxacin • Cipro
Clarithromycin • Biaxin
Clomiphene • Clomid
Cycloserine • Seromycin
Dapsone • Dapsone
Erythromycin • Erythrocin, Pediamycin
Ethambutol • Myambutol
Ethionamide • Trecator-SC
Gentamicin • Garamycin
Isoniazid • Hyzyd, Nydrazid
Lithium • Eskalith, Lithobid
Metronidazole • Flagyl
Minocycline • Dynacin, Solodyn
Norfloxacin • Noroxin
Ofloxacin • Floxin
Olanzapine • Zyprexa
Penicillin G procaine • Duracillin A-S, Pfizerpen
Sulfamethoxazole/trimethoprim • Bactrim, Septra
1. Kennedy M, Everitt B, Boydell J, et al. Incidence and distribution of first-episode mania by age: results for a 35-year study. Psychol Med. 2005;35(6):855-863.
2. Dols A, Kupka RW, van Lammeren A, et al. The prevalence of late-life mania: a review. Bipolar Disord. 2014;16:113-118.
3. Siedontopf F, Horstkamp B, Stief G, et al. Clomiphene citrate as a possible cause of a psychotic reaction during infertility treatment. Hum Reprod. 1997;12(4):706-707.
4. Oyffe T, Lerner A, Isaacs G, et al. Clomiphene-induced psychosis. Am J Psychiatry. 1997;154(8):1169-1170.
5. Lambrichts S, Van Oudenhove L, Sienaert P. Antibiotics and mania: a systematic review. J Affect Disord. 2017;219:149-156.
6. Beal DM, Hudson B, Zaiac M. Amoxicillin-induced psychosis? Am J Psychiatry. 1986;143(2):255-256.
7. Klain V, Timmerman L. Antibiomania, acute manic psychosis following the use of antibiotics. European Psychiatry. 2013;28(suppl 1):1.
8. Abouesh A, Stone C, Hobbs WR. Antimicrobial-induced mania (antibiomania): a review of spontaneous reports. J Clin Psychopharmacol. 2002;22(1):71-81.
9. Lally L, Mannion L. The potential for antimicrobials to adversely affect mental state. BMJ Case Rep. 2013. pii: bcr2013009659. doi: 10.1136/bcr-2013-009659.
10. Neufeld NH, Mohamed NS, Grujich N, et al. Acute neuropsychiatric symptoms associated with antibiotic treatment of Helicobactor Pylori infections: a review. J Psychiatr Pract. 2017;23(1):25-35.
11. Sutter R, Rüegg S, Tschudin-Sutter S. Seizures as adverse events of antibiotic drugs: a systematic review. Neurology. 2015;85(15):1332-1341.
12. Bakhla A, Gore P, Srivastava S. Cycloserine induced mania. Ind Psychiatry J. 2013;22(1):69-70.
13. Barbaccia ML, Roscetti G, Trabucchi M, et al. Isoniazid-induced inhibition of GABAergic transmission enhances neurosteroid content in the rat brain. Neuropharmacology. 1996;35(9-10):1299-1305.
14. Murphy D, Donnelly C, Moskowitz J. Inhibition by lithium of prostaglandin E1 and norepinephrine effects on cyclic adenosine monophosphate production in human platelets. Clin Pharmacol Ther. 1973;14(5):810-814.
15. Clay H, Sillivan S, Konradi C. Mitochondrial dysfunction and pathology in bipolar disorder and schizophrenia. Int J Dev Neurosci. 2011;29(3):311-324.
16. Dickerson F, Severance E, Yolken R. The microbiome, immunity, and schizophrenia and bipolar disorder. Brain Behav Immun. 2017;62:46-52.
1. Kennedy M, Everitt B, Boydell J, et al. Incidence and distribution of first-episode mania by age: results for a 35-year study. Psychol Med. 2005;35(6):855-863.
2. Dols A, Kupka RW, van Lammeren A, et al. The prevalence of late-life mania: a review. Bipolar Disord. 2014;16:113-118.
3. Siedontopf F, Horstkamp B, Stief G, et al. Clomiphene citrate as a possible cause of a psychotic reaction during infertility treatment. Hum Reprod. 1997;12(4):706-707.
4. Oyffe T, Lerner A, Isaacs G, et al. Clomiphene-induced psychosis. Am J Psychiatry. 1997;154(8):1169-1170.
5. Lambrichts S, Van Oudenhove L, Sienaert P. Antibiotics and mania: a systematic review. J Affect Disord. 2017;219:149-156.
6. Beal DM, Hudson B, Zaiac M. Amoxicillin-induced psychosis? Am J Psychiatry. 1986;143(2):255-256.
7. Klain V, Timmerman L. Antibiomania, acute manic psychosis following the use of antibiotics. European Psychiatry. 2013;28(suppl 1):1.
8. Abouesh A, Stone C, Hobbs WR. Antimicrobial-induced mania (antibiomania): a review of spontaneous reports. J Clin Psychopharmacol. 2002;22(1):71-81.
9. Lally L, Mannion L. The potential for antimicrobials to adversely affect mental state. BMJ Case Rep. 2013. pii: bcr2013009659. doi: 10.1136/bcr-2013-009659.
10. Neufeld NH, Mohamed NS, Grujich N, et al. Acute neuropsychiatric symptoms associated with antibiotic treatment of Helicobactor Pylori infections: a review. J Psychiatr Pract. 2017;23(1):25-35.
11. Sutter R, Rüegg S, Tschudin-Sutter S. Seizures as adverse events of antibiotic drugs: a systematic review. Neurology. 2015;85(15):1332-1341.
12. Bakhla A, Gore P, Srivastava S. Cycloserine induced mania. Ind Psychiatry J. 2013;22(1):69-70.
13. Barbaccia ML, Roscetti G, Trabucchi M, et al. Isoniazid-induced inhibition of GABAergic transmission enhances neurosteroid content in the rat brain. Neuropharmacology. 1996;35(9-10):1299-1305.
14. Murphy D, Donnelly C, Moskowitz J. Inhibition by lithium of prostaglandin E1 and norepinephrine effects on cyclic adenosine monophosphate production in human platelets. Clin Pharmacol Ther. 1973;14(5):810-814.
15. Clay H, Sillivan S, Konradi C. Mitochondrial dysfunction and pathology in bipolar disorder and schizophrenia. Int J Dev Neurosci. 2011;29(3):311-324.
16. Dickerson F, Severance E, Yolken R. The microbiome, immunity, and schizophrenia and bipolar disorder. Brain Behav Immun. 2017;62:46-52.
Valproic acid-induced hyperammonemic encephalopathy
Mrs. C, age 75, is transferred to our inpatient medical/surgical hospital from a psychiatric hospital after presenting with shortness of breath and altered mental status.
Eight days earlier, Mrs. C had been admitted to the psychiatric hospital for bipolar mania with psychotic features. While there, Mrs. C received quetiapine, 400 mg nightly, and an initial valproic acid (VPA) dosage of 500 mg 2 times daily. While receiving VPA 500 mg 2 times daily, her VPA total level was 62 µg/mL, which is on the lower end of the therapeutic range (50 to 125 µg/mL). This prompted the team at the psychiatric hospital to increase her VPA dosage to 500 mg 3 times daily the day before she was transferred to our hospital.
At our hospital, she is found to be in hypoxic respiratory failure secondary to pneumonia. Upon admission, her laboratory data show evidence of infection and anemia and she also has an
From hospital Day 3 to Day 6, Mrs. C experiences gradual improvement in her respiratory and mental status. However, on hospital Day 7, she has extreme somnolence and altered mental status without respiratory involvement. Our team suspects VPA toxicity and/or VPA-induced hyperammonemic encephalopathy (VHE).
VPA-induced hyperammonemia
Hyperammonemia can occur in individuals receiving VPA and is most often asymptomatic. However, elevations in ammonia may lead to VHE, which is a rare but serious adverse effect. VHE has been reported early in treatment, in acute VPA overdose, and in chronic VPA use despite normal doses and levels.1 It also can occur in the absence of clinical and laboratory evidence of hepatotoxicity. VHE is associated with significant morbidity and CNS damage. Symptoms of VHE include vomiting, lethargy, and confusion. If left untreated, VHE can lead to coma and death.
Mechanism of VHE. The exact mechanism of VHE is unknown.1-3 Ammonia is a toxic base produced by deamination of amino acids. The liver eliminates ammonia via the urea cycle.2 Valproic acid metabolites, propionate and 4-en-VPA, can directly inhibit N-acetyl glutamate, which can disrupt the urea cycle, leading to elevated ammonia levels.3 Long-term or high-dose VPA can lead to carnitine deficiency, primarily by inhibiting its biosynthesis and depleting stores.4 Carnitine deficiency leads to disturbances in mitochondrial function, causing inhibition of the urea cycle and increasing ammonia. CNS toxicity due to hyperammonemia is thought to be due to activation of glutamate receptors.3
Risk factors. Co-administration of other antiepileptic drugs (AEDs) with VPA is a risk factor for VHE.1,5 This happens because enzyme-inducing AEDs such as phenytoin, phenobarbital, and carbamazepine can increase toxic metabolites of VPA, which can lead to hyperammonemia. Topiramate can also inhibit the urea cycle, leading to increased ammonia levels. Additionally, co-administration of VPA with quetiapine, paliperidone, risperidone, or aripiprazole has been reported to increase the risk of VHE.1,5 Intellectual disability, carnitine deficiency, low albumin, and abnormal liver function have also been reported to increase the risk of VHE.1,5
Continue to: Diagnosis and management
Diagnosis and management. If a patient receiving VPA is experiencing nausea, fatigue, or somnolence, it is important to check the patient’s ammonia level (normal range: 11 to 32 µmol/L) and VPA total levels (therapeutic range: 50 to 125 µg/mL). Consider checking a VPA free level, especially in geriatric patients or patients who have low albumin; the therapeutic range of VPA free is 6 to 22 µg/mL.3 If the ammonia level is elevated, discontinue VPA immediately (Table).1-3 Clinicians may also elect to prescribe lactulose until ammonia levels return to normal range. Adding levocarnitine may also help, although evidence is limited to small case series or retrospective studies.3 Currently, there is no known advantage in combining lactulose and levocarnitine to address VHE. Severe cases of VHE (ammonia levels >400 µmol/L) may require hemodialysis.1

Prevention. Strategies to prevent VHE include avoiding polypharmacy, especially concurrent use of enzyme-inducing AEDs and possibly second-generation antipsychotics. Additionally, VPA should not be used in individuals with urea cycle disorders. It is unknown if levocarnitine supplementation is preventive, but this approach has been suggested.3
CASE CONTINUED
Mrs. C has several possible risk factors for VHE, including co-administration of quetiapine and VPA, and a low albumin level. A further laboratory workup for Mrs. C reveals a VPA free level of 19 µg/mL (21.1% free), a VPA total level of 90 µg/mL, and an ammonia level of 79 µmol/L, confirming our suspicions regarding VHE. We determine that Mrs. C’s altered mental status is likely due her elevated ammonia levels, because the infection had been improving in the days leading up to the sudden, extreme somnolence.
VPA is immediately stopped and Mrs. C receives 1 dose of lactulose. The following day, Mrs. C’s mental status improves, and her ammonia levels return to normal. On hospital Day 9, she is transferred back to the psychiatric facility for management of manic and psychotic symptoms.
Related Resources
- Brown LM, Cupples N, Moore TA. Levocarnitine for valproate-induced hyperammonemia in the psychiatric setting: a case series and literature review. Ment Health Clin. 2018;8(3):148-154.
- Aires CCP, van Cruchten A, Ijlat L, et al. New insights on the mechanisms of valproate-induced hyperammonemia: inhibition of hepatic N-acetylglutamate synthase activity by valproyl-CoA. J Hepatol. 2011;55(2):426-434.
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Lactulose • Enulose
Levocarnitine • Carnitine, Carnitor
Levofloxacin • Levaquin IV
Paliperidone • Invega
Phenobarbital • Luminal
Phenytoin • Dilantin
Quetiapine • Seroquel
Risperidone • Risperdal
Topiramate • Topamax
Valproic acid • Depakene
1. Chopra A, Kolla BP, Mansukhani MP, et al. Valproate-induced hyperammonemic encephalopathy: an update on risk factors, clinical correlates, and management. Gen Hosp Psychiatry. 2012;34(3):290-298.
2. Kowalski PC, Dowben JS, Keltner NL. Ammonium: the deadly toxin you don’t want to miss when using mood stabilizers. Perspect Psychiatr Care. 2013;49(4):221-225.
3. Baddour E, Tewksbury A, Stauner N. Valproic acid-induced hyper ammonemia: incidence, clinical significance, and treatment management. Ment Health Clin. 2018;8(2):73-77.
4. Raskind JY, El-Chaar GM. The role of carnitine supplementation during valproic acid therapy. Ann Pharmacother. 2000;34(5):630-638. 5. Tseng YL, Huang CR, Lin CH, et al. Risk factors of hyperammonemia in patients with epilepsy. Medicine (Baltimore). 2014;93(11):e66. doi: 10.1097/MD.0000000000000066.
Mrs. C, age 75, is transferred to our inpatient medical/surgical hospital from a psychiatric hospital after presenting with shortness of breath and altered mental status.
Eight days earlier, Mrs. C had been admitted to the psychiatric hospital for bipolar mania with psychotic features. While there, Mrs. C received quetiapine, 400 mg nightly, and an initial valproic acid (VPA) dosage of 500 mg 2 times daily. While receiving VPA 500 mg 2 times daily, her VPA total level was 62 µg/mL, which is on the lower end of the therapeutic range (50 to 125 µg/mL). This prompted the team at the psychiatric hospital to increase her VPA dosage to 500 mg 3 times daily the day before she was transferred to our hospital.
At our hospital, she is found to be in hypoxic respiratory failure secondary to pneumonia. Upon admission, her laboratory data show evidence of infection and anemia and she also has an
From hospital Day 3 to Day 6, Mrs. C experiences gradual improvement in her respiratory and mental status. However, on hospital Day 7, she has extreme somnolence and altered mental status without respiratory involvement. Our team suspects VPA toxicity and/or VPA-induced hyperammonemic encephalopathy (VHE).
VPA-induced hyperammonemia
Hyperammonemia can occur in individuals receiving VPA and is most often asymptomatic. However, elevations in ammonia may lead to VHE, which is a rare but serious adverse effect. VHE has been reported early in treatment, in acute VPA overdose, and in chronic VPA use despite normal doses and levels.1 It also can occur in the absence of clinical and laboratory evidence of hepatotoxicity. VHE is associated with significant morbidity and CNS damage. Symptoms of VHE include vomiting, lethargy, and confusion. If left untreated, VHE can lead to coma and death.
Mechanism of VHE. The exact mechanism of VHE is unknown.1-3 Ammonia is a toxic base produced by deamination of amino acids. The liver eliminates ammonia via the urea cycle.2 Valproic acid metabolites, propionate and 4-en-VPA, can directly inhibit N-acetyl glutamate, which can disrupt the urea cycle, leading to elevated ammonia levels.3 Long-term or high-dose VPA can lead to carnitine deficiency, primarily by inhibiting its biosynthesis and depleting stores.4 Carnitine deficiency leads to disturbances in mitochondrial function, causing inhibition of the urea cycle and increasing ammonia. CNS toxicity due to hyperammonemia is thought to be due to activation of glutamate receptors.3
Risk factors. Co-administration of other antiepileptic drugs (AEDs) with VPA is a risk factor for VHE.1,5 This happens because enzyme-inducing AEDs such as phenytoin, phenobarbital, and carbamazepine can increase toxic metabolites of VPA, which can lead to hyperammonemia. Topiramate can also inhibit the urea cycle, leading to increased ammonia levels. Additionally, co-administration of VPA with quetiapine, paliperidone, risperidone, or aripiprazole has been reported to increase the risk of VHE.1,5 Intellectual disability, carnitine deficiency, low albumin, and abnormal liver function have also been reported to increase the risk of VHE.1,5
Continue to: Diagnosis and management
Diagnosis and management. If a patient receiving VPA is experiencing nausea, fatigue, or somnolence, it is important to check the patient’s ammonia level (normal range: 11 to 32 µmol/L) and VPA total levels (therapeutic range: 50 to 125 µg/mL). Consider checking a VPA free level, especially in geriatric patients or patients who have low albumin; the therapeutic range of VPA free is 6 to 22 µg/mL.3 If the ammonia level is elevated, discontinue VPA immediately (Table).1-3 Clinicians may also elect to prescribe lactulose until ammonia levels return to normal range. Adding levocarnitine may also help, although evidence is limited to small case series or retrospective studies.3 Currently, there is no known advantage in combining lactulose and levocarnitine to address VHE. Severe cases of VHE (ammonia levels >400 µmol/L) may require hemodialysis.1

Prevention. Strategies to prevent VHE include avoiding polypharmacy, especially concurrent use of enzyme-inducing AEDs and possibly second-generation antipsychotics. Additionally, VPA should not be used in individuals with urea cycle disorders. It is unknown if levocarnitine supplementation is preventive, but this approach has been suggested.3
CASE CONTINUED
Mrs. C has several possible risk factors for VHE, including co-administration of quetiapine and VPA, and a low albumin level. A further laboratory workup for Mrs. C reveals a VPA free level of 19 µg/mL (21.1% free), a VPA total level of 90 µg/mL, and an ammonia level of 79 µmol/L, confirming our suspicions regarding VHE. We determine that Mrs. C’s altered mental status is likely due her elevated ammonia levels, because the infection had been improving in the days leading up to the sudden, extreme somnolence.
VPA is immediately stopped and Mrs. C receives 1 dose of lactulose. The following day, Mrs. C’s mental status improves, and her ammonia levels return to normal. On hospital Day 9, she is transferred back to the psychiatric facility for management of manic and psychotic symptoms.
Related Resources
- Brown LM, Cupples N, Moore TA. Levocarnitine for valproate-induced hyperammonemia in the psychiatric setting: a case series and literature review. Ment Health Clin. 2018;8(3):148-154.
- Aires CCP, van Cruchten A, Ijlat L, et al. New insights on the mechanisms of valproate-induced hyperammonemia: inhibition of hepatic N-acetylglutamate synthase activity by valproyl-CoA. J Hepatol. 2011;55(2):426-434.
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Lactulose • Enulose
Levocarnitine • Carnitine, Carnitor
Levofloxacin • Levaquin IV
Paliperidone • Invega
Phenobarbital • Luminal
Phenytoin • Dilantin
Quetiapine • Seroquel
Risperidone • Risperdal
Topiramate • Topamax
Valproic acid • Depakene
Mrs. C, age 75, is transferred to our inpatient medical/surgical hospital from a psychiatric hospital after presenting with shortness of breath and altered mental status.
Eight days earlier, Mrs. C had been admitted to the psychiatric hospital for bipolar mania with psychotic features. While there, Mrs. C received quetiapine, 400 mg nightly, and an initial valproic acid (VPA) dosage of 500 mg 2 times daily. While receiving VPA 500 mg 2 times daily, her VPA total level was 62 µg/mL, which is on the lower end of the therapeutic range (50 to 125 µg/mL). This prompted the team at the psychiatric hospital to increase her VPA dosage to 500 mg 3 times daily the day before she was transferred to our hospital.
At our hospital, she is found to be in hypoxic respiratory failure secondary to pneumonia. Upon admission, her laboratory data show evidence of infection and anemia and she also has an
From hospital Day 3 to Day 6, Mrs. C experiences gradual improvement in her respiratory and mental status. However, on hospital Day 7, she has extreme somnolence and altered mental status without respiratory involvement. Our team suspects VPA toxicity and/or VPA-induced hyperammonemic encephalopathy (VHE).
VPA-induced hyperammonemia
Hyperammonemia can occur in individuals receiving VPA and is most often asymptomatic. However, elevations in ammonia may lead to VHE, which is a rare but serious adverse effect. VHE has been reported early in treatment, in acute VPA overdose, and in chronic VPA use despite normal doses and levels.1 It also can occur in the absence of clinical and laboratory evidence of hepatotoxicity. VHE is associated with significant morbidity and CNS damage. Symptoms of VHE include vomiting, lethargy, and confusion. If left untreated, VHE can lead to coma and death.
Mechanism of VHE. The exact mechanism of VHE is unknown.1-3 Ammonia is a toxic base produced by deamination of amino acids. The liver eliminates ammonia via the urea cycle.2 Valproic acid metabolites, propionate and 4-en-VPA, can directly inhibit N-acetyl glutamate, which can disrupt the urea cycle, leading to elevated ammonia levels.3 Long-term or high-dose VPA can lead to carnitine deficiency, primarily by inhibiting its biosynthesis and depleting stores.4 Carnitine deficiency leads to disturbances in mitochondrial function, causing inhibition of the urea cycle and increasing ammonia. CNS toxicity due to hyperammonemia is thought to be due to activation of glutamate receptors.3
Risk factors. Co-administration of other antiepileptic drugs (AEDs) with VPA is a risk factor for VHE.1,5 This happens because enzyme-inducing AEDs such as phenytoin, phenobarbital, and carbamazepine can increase toxic metabolites of VPA, which can lead to hyperammonemia. Topiramate can also inhibit the urea cycle, leading to increased ammonia levels. Additionally, co-administration of VPA with quetiapine, paliperidone, risperidone, or aripiprazole has been reported to increase the risk of VHE.1,5 Intellectual disability, carnitine deficiency, low albumin, and abnormal liver function have also been reported to increase the risk of VHE.1,5
Continue to: Diagnosis and management
Diagnosis and management. If a patient receiving VPA is experiencing nausea, fatigue, or somnolence, it is important to check the patient’s ammonia level (normal range: 11 to 32 µmol/L) and VPA total levels (therapeutic range: 50 to 125 µg/mL). Consider checking a VPA free level, especially in geriatric patients or patients who have low albumin; the therapeutic range of VPA free is 6 to 22 µg/mL.3 If the ammonia level is elevated, discontinue VPA immediately (Table).1-3 Clinicians may also elect to prescribe lactulose until ammonia levels return to normal range. Adding levocarnitine may also help, although evidence is limited to small case series or retrospective studies.3 Currently, there is no known advantage in combining lactulose and levocarnitine to address VHE. Severe cases of VHE (ammonia levels >400 µmol/L) may require hemodialysis.1

Prevention. Strategies to prevent VHE include avoiding polypharmacy, especially concurrent use of enzyme-inducing AEDs and possibly second-generation antipsychotics. Additionally, VPA should not be used in individuals with urea cycle disorders. It is unknown if levocarnitine supplementation is preventive, but this approach has been suggested.3
CASE CONTINUED
Mrs. C has several possible risk factors for VHE, including co-administration of quetiapine and VPA, and a low albumin level. A further laboratory workup for Mrs. C reveals a VPA free level of 19 µg/mL (21.1% free), a VPA total level of 90 µg/mL, and an ammonia level of 79 µmol/L, confirming our suspicions regarding VHE. We determine that Mrs. C’s altered mental status is likely due her elevated ammonia levels, because the infection had been improving in the days leading up to the sudden, extreme somnolence.
VPA is immediately stopped and Mrs. C receives 1 dose of lactulose. The following day, Mrs. C’s mental status improves, and her ammonia levels return to normal. On hospital Day 9, she is transferred back to the psychiatric facility for management of manic and psychotic symptoms.
Related Resources
- Brown LM, Cupples N, Moore TA. Levocarnitine for valproate-induced hyperammonemia in the psychiatric setting: a case series and literature review. Ment Health Clin. 2018;8(3):148-154.
- Aires CCP, van Cruchten A, Ijlat L, et al. New insights on the mechanisms of valproate-induced hyperammonemia: inhibition of hepatic N-acetylglutamate synthase activity by valproyl-CoA. J Hepatol. 2011;55(2):426-434.
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Lactulose • Enulose
Levocarnitine • Carnitine, Carnitor
Levofloxacin • Levaquin IV
Paliperidone • Invega
Phenobarbital • Luminal
Phenytoin • Dilantin
Quetiapine • Seroquel
Risperidone • Risperdal
Topiramate • Topamax
Valproic acid • Depakene
1. Chopra A, Kolla BP, Mansukhani MP, et al. Valproate-induced hyperammonemic encephalopathy: an update on risk factors, clinical correlates, and management. Gen Hosp Psychiatry. 2012;34(3):290-298.
2. Kowalski PC, Dowben JS, Keltner NL. Ammonium: the deadly toxin you don’t want to miss when using mood stabilizers. Perspect Psychiatr Care. 2013;49(4):221-225.
3. Baddour E, Tewksbury A, Stauner N. Valproic acid-induced hyper ammonemia: incidence, clinical significance, and treatment management. Ment Health Clin. 2018;8(2):73-77.
4. Raskind JY, El-Chaar GM. The role of carnitine supplementation during valproic acid therapy. Ann Pharmacother. 2000;34(5):630-638. 5. Tseng YL, Huang CR, Lin CH, et al. Risk factors of hyperammonemia in patients with epilepsy. Medicine (Baltimore). 2014;93(11):e66. doi: 10.1097/MD.0000000000000066.
1. Chopra A, Kolla BP, Mansukhani MP, et al. Valproate-induced hyperammonemic encephalopathy: an update on risk factors, clinical correlates, and management. Gen Hosp Psychiatry. 2012;34(3):290-298.
2. Kowalski PC, Dowben JS, Keltner NL. Ammonium: the deadly toxin you don’t want to miss when using mood stabilizers. Perspect Psychiatr Care. 2013;49(4):221-225.
3. Baddour E, Tewksbury A, Stauner N. Valproic acid-induced hyper ammonemia: incidence, clinical significance, and treatment management. Ment Health Clin. 2018;8(2):73-77.
4. Raskind JY, El-Chaar GM. The role of carnitine supplementation during valproic acid therapy. Ann Pharmacother. 2000;34(5):630-638. 5. Tseng YL, Huang CR, Lin CH, et al. Risk factors of hyperammonemia in patients with epilepsy. Medicine (Baltimore). 2014;93(11):e66. doi: 10.1097/MD.0000000000000066.
What to do when your patient who takes clozapine enters a smoke-free facility
Mr. D, age 30, has a 12-year history of schizophrenia and is experiencing worsening auditory hallucinations despite reported medication adherence. He has been taking clozapine, maintenance dosages 500 to 700 mg/d, for 4 years and smokes 2 packs of cigarettes a day. When Mr. D is admitted to a nonsmoking inpatient psychiatric facility, he receives nicotine transdermal patches, 21 mg/d, for nicotine withdrawal. Mr. D’s most recent outpatient clozapine dosage, 700 mg/d, is resumed. All laboratory tests, including complete blood count with differential, are within normal limits at admission.
Five days later Mr. D is tachycardic with a heart rate of 109 beats per minute. When assessing Mr. D, we notice he has alogia and that, when he does speak, his speech is slowed with a 4 to 5 second delay in response. He also appears sedated. We observe occasional mild jerking of his shoulder and lower legs.
Mr. D reports that his auditory hallucinations have lessened since his admission, but complains of difficulty remembering information and feeling tired during the day. The treatment team suspects clozapine toxicity; his trough clozapine level is 1,350 ng/mL (therapeutic range, 350 to 1,000 ng/mL).
It is well documented that cigarette smoke can induce cytochrome P450 (CYP) isoenzymes, specifically CYP1A1, CYP1A2, and CYP2E1. Because clozapine is primarily metabolized by CYP1A2 (approximately 70%), smoking can induce clozapine metabolism and abruptly stopping smoking can increase clozapine levels.1 The polycyclic aromatic hydrocarbons, not the nicotine, found in cigarettes are thought to be responsible for CYP1A2 induction; therefore, use of a nicotine replacement product did not prevent the increase in Mr. D’s clozapine levels.
Examining the evidence
Meyer1 evaluated clozapine levels before and after implementation of a hospital-wide smoking ban (N = 11). Clozapine dosages were not adjusted at the time of the smoking ban, which resulted in a mean 72% increase in clozapine levels after a minimum of 2 weeks as nonsmokers. Even after eliminating 2 outliers, the mean increase in clozapine levels was 36.1%. Murayama-Sung et al2 reported a statistically significant increase in the level of clozapine (46%, P = .004) and the level of norclozapine (23%, P = .02) after a hospital-wide smoking ban was instituted (N = 14). However, the pre-change and post-change in the ratio of clozapine to norclozapine level was not found to be statistically significant. Haslemo et al3 found that smoking as few as 7 to 12 cigarettes a day was sufficient for maximum induction of CYP1A2. Because Mr. D was smoking 2 packs of cigarettes a day (40 cigarettes) with an clozapine dosage 700 mg/d as an outpatient, he likely experienced significant induction of clozapine metabolism through CYP1A2, which was no longer present when he stopped smoking.
Therapeutic clozapine concentrations are typically above 350 and 420 ng/mL.4 Concentrations >700 ng/mL are associated with increased adverse effects, but generally are not associated with a higher response; levels >900 ng/mL have been associated with toxicity.4 Clozapine-treated patients on a stable dosage who smoke can experience clozapine-related adverse effects after admission to a smoke-free facility secondary to an increase in the clozapine concentration (Table 1).4
Five days after admission to the facility, Mr. D was noted to have myoclonus, somnolence, and tachycardia, with a clozapine level of 1,350 ng/mL. Additional adverse effects that can be seen include orthostatic hypotension, sialorrhea, worsening psychiatric symptoms (eg, hallucinations), and seizures.5 Although there is variability in the timing of the decrease in CYP1A2 activity after smoking cessation, practitioners should begin to monitor for clozapine-related adverse effects 1 or 2 days after smoking cessation.6
Treatment recommendations
Monitoring of the clozapine concentration and adjustment of the dosage might be needed to account for the fluctuation seen with smoking cessation to maintain efficacy and minimize adverse effects. However, a test of the clozapine level may not be available at all facilities, often requiring that the specimen be sent to an outside laboratory, taking 3 to 7 days to receive results.
Faber and Fuhr6 recommended reducing the dosage of a CYP1A2 substrate medication, such as clozapine, olanzapine, or theophylline, by 10% each day until the dosage has been reduced by 40% in patients who stop smoking. Lowe and Ackman5 proposed reducing the clozapine dosage by 30% to 40% to achieve a pre-cessation serum concentration at 1 week. For Mr. D, this would mean decreasing the clozapine dosage to 425 to 500 mg/d.
Assuming that Mr. D’s clozapine dosage is decreased during his hospitalization and that he resumes smoking after discharge, it is likely the dosage will need to be increased. It may take several weeks to see maximal induction, because new CYP enzymes need to be synthesized when the patient resumes smoking.7 One recommendation is to increase the clozapine dosage by a factor of 1.5 over 2 to 4 weeks, with close monitoring of the clozapine concentration and adverse effects because this increase is approximate.7 Depending on when Mr. D’s follow-up appointment is scheduled, the practitioner may need to plan a dosage adjustment to prevent a decrease in his clozapine level caused by smoking to prevent a worsening of symptoms and rehospitalization.
This case emphasizes the importance of asking clozapine-treated patients about their smoking history when they are admitted to a smoke-free facility. For several reasons, >60% of patients with schizophrenia smoke cigarettes8 (Table 2).9-14 Patients who smoke and are on a stable dosage of clozapine might require a dosage reduction when they are admitted to a smoke-free facility to avoid adverse effects. If the dosage is not adjusted, a patient may experience clozapine-induced adverse effects, such as tachycardia, sedation, and seizures. It is likely that patients such as Mr. D will experience fluctuation in the clozapine level and possibly changes in efficacy and tolerability transitioning between inpatient and outpatient settings if the dosage is not adjusted.
Related Resources
• Kroon LA. Drug interactions with smoking. Am J Health Syst Pharm. 2007;64(18):1917-1921.
• Fankhauser MP. Drug interactions with tobacco smoke: Implications for patient care. Current Psychiatry. 2013; 12(1):12-16.
• Greenwood-Smith C, Lubman DI, Castle DJ. Serum clozapine levels: a review of their clinical utility. J Psychopharmacol. 2003;17(2):234-248.
• Olesen OV, Thomsen K, Jensen PN, et al. Clozapine serum levels and side effects during steady state treatment of schizophrenic patients: a cross sectional study. Psychopharmacology (Berl). 1995;117(3):371-378.
Drug Brand Names
Clozapine • Clozaril Theophylline • Theo-Dur
Olanzapine • Zyprexa
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Meyer JM. Individual changes in clozapine levels after smoking cessation: results and a predictive model. J Clin Psychopharmacol. 2001;21(6):569-574.
2. Murayama-Sung L, Ahmed I, Goebert D, et al. The impact of hospital smoking ban on clozapine and norclozapine levels. J Clin Psychopharmacol. 2011;31(1):124-126.
3. Haslemo T, Eikeseth PH, Tanum L, et al. The effect of variable cigarette consumption on the interaction with clozapine and olanzapine. Eur J Clin Psychopharmacol. 2006;62(12): 1049-1053.
4. Nielsen J, Damkier P, Lublin H, et al. Optimizing clozapine treatment. Acta Psychiatr Scand. 2011;123(6):411-422.
5. Lowe EJ, Ackman ML. Impact of tobacco smoking cessation on stable clozapine and olanzapine treatment. Ann Pharmacother. 2010;44(4):727-732.
6. Faber MS, Fuhr U. Time response of cytochrome P450 1A2 activity on cessation of heavy smoking. Clin Pharmacol Ther. 2004;76(2):178-184.
7. de Leon J. Atypical antipsychotic dosing: the effect of smoking and caffeine. Psychiatr Serv. 2004;55(5):491-493.
8. Dickerson F, Stallings CR, Origoni AE, et al. Cigarette smoking among persons with schizophrenia or bipolar disorder in routine clinical settings, 1999-2011. Psychiatr Serv. 2013;64(1):44-50.
9. Esterberg ML, Compton MT. Smoking behavior in persons with a schizophrenia-spectrum disorder: a qualitative investigation of the transtheoretical model. Soc Sci Med. 2005;61(2):293-303.
10. Barr RS, Culhane MA, Jubelt LE, et al. The effects of transdermal nicotine on cognition in nonsmokers with schizophrenia and nonpsychiatric controls. Neuropsychopharmacology. 2008; 33(3):480-490.
11. Adler LE, Hoffer LD, Wiser A, et al. Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am J Psychiatry. 1993;150(12):1856-1861.
12. Sallette J, Pons S, Devillers-Thiery A, et al. Nicotine upregulates its own receptors through enhanced intracellular maturation. Neuron. 2005;46(4):595-607.
13. Breese CR, Lee MJ, Adams CE, et al. Abnormal regulation of high affinity nicotinic receptors in subjects with schizophrenia. Neuropsychopharmacology. 2000;23(4):351-364.
14. Miller DD, Kelly MW, Perry PJ, et al. The influence of cigarette smoking on haloperidol pharmacokinetics. J Clin Psychiatry. 1990;28(6):529-231.
Mr. D, age 30, has a 12-year history of schizophrenia and is experiencing worsening auditory hallucinations despite reported medication adherence. He has been taking clozapine, maintenance dosages 500 to 700 mg/d, for 4 years and smokes 2 packs of cigarettes a day. When Mr. D is admitted to a nonsmoking inpatient psychiatric facility, he receives nicotine transdermal patches, 21 mg/d, for nicotine withdrawal. Mr. D’s most recent outpatient clozapine dosage, 700 mg/d, is resumed. All laboratory tests, including complete blood count with differential, are within normal limits at admission.
Five days later Mr. D is tachycardic with a heart rate of 109 beats per minute. When assessing Mr. D, we notice he has alogia and that, when he does speak, his speech is slowed with a 4 to 5 second delay in response. He also appears sedated. We observe occasional mild jerking of his shoulder and lower legs.
Mr. D reports that his auditory hallucinations have lessened since his admission, but complains of difficulty remembering information and feeling tired during the day. The treatment team suspects clozapine toxicity; his trough clozapine level is 1,350 ng/mL (therapeutic range, 350 to 1,000 ng/mL).
It is well documented that cigarette smoke can induce cytochrome P450 (CYP) isoenzymes, specifically CYP1A1, CYP1A2, and CYP2E1. Because clozapine is primarily metabolized by CYP1A2 (approximately 70%), smoking can induce clozapine metabolism and abruptly stopping smoking can increase clozapine levels.1 The polycyclic aromatic hydrocarbons, not the nicotine, found in cigarettes are thought to be responsible for CYP1A2 induction; therefore, use of a nicotine replacement product did not prevent the increase in Mr. D’s clozapine levels.
Examining the evidence
Meyer1 evaluated clozapine levels before and after implementation of a hospital-wide smoking ban (N = 11). Clozapine dosages were not adjusted at the time of the smoking ban, which resulted in a mean 72% increase in clozapine levels after a minimum of 2 weeks as nonsmokers. Even after eliminating 2 outliers, the mean increase in clozapine levels was 36.1%. Murayama-Sung et al2 reported a statistically significant increase in the level of clozapine (46%, P = .004) and the level of norclozapine (23%, P = .02) after a hospital-wide smoking ban was instituted (N = 14). However, the pre-change and post-change in the ratio of clozapine to norclozapine level was not found to be statistically significant. Haslemo et al3 found that smoking as few as 7 to 12 cigarettes a day was sufficient for maximum induction of CYP1A2. Because Mr. D was smoking 2 packs of cigarettes a day (40 cigarettes) with an clozapine dosage 700 mg/d as an outpatient, he likely experienced significant induction of clozapine metabolism through CYP1A2, which was no longer present when he stopped smoking.
Therapeutic clozapine concentrations are typically above 350 and 420 ng/mL.4 Concentrations >700 ng/mL are associated with increased adverse effects, but generally are not associated with a higher response; levels >900 ng/mL have been associated with toxicity.4 Clozapine-treated patients on a stable dosage who smoke can experience clozapine-related adverse effects after admission to a smoke-free facility secondary to an increase in the clozapine concentration (Table 1).4
Five days after admission to the facility, Mr. D was noted to have myoclonus, somnolence, and tachycardia, with a clozapine level of 1,350 ng/mL. Additional adverse effects that can be seen include orthostatic hypotension, sialorrhea, worsening psychiatric symptoms (eg, hallucinations), and seizures.5 Although there is variability in the timing of the decrease in CYP1A2 activity after smoking cessation, practitioners should begin to monitor for clozapine-related adverse effects 1 or 2 days after smoking cessation.6
Treatment recommendations
Monitoring of the clozapine concentration and adjustment of the dosage might be needed to account for the fluctuation seen with smoking cessation to maintain efficacy and minimize adverse effects. However, a test of the clozapine level may not be available at all facilities, often requiring that the specimen be sent to an outside laboratory, taking 3 to 7 days to receive results.
Faber and Fuhr6 recommended reducing the dosage of a CYP1A2 substrate medication, such as clozapine, olanzapine, or theophylline, by 10% each day until the dosage has been reduced by 40% in patients who stop smoking. Lowe and Ackman5 proposed reducing the clozapine dosage by 30% to 40% to achieve a pre-cessation serum concentration at 1 week. For Mr. D, this would mean decreasing the clozapine dosage to 425 to 500 mg/d.
Assuming that Mr. D’s clozapine dosage is decreased during his hospitalization and that he resumes smoking after discharge, it is likely the dosage will need to be increased. It may take several weeks to see maximal induction, because new CYP enzymes need to be synthesized when the patient resumes smoking.7 One recommendation is to increase the clozapine dosage by a factor of 1.5 over 2 to 4 weeks, with close monitoring of the clozapine concentration and adverse effects because this increase is approximate.7 Depending on when Mr. D’s follow-up appointment is scheduled, the practitioner may need to plan a dosage adjustment to prevent a decrease in his clozapine level caused by smoking to prevent a worsening of symptoms and rehospitalization.
This case emphasizes the importance of asking clozapine-treated patients about their smoking history when they are admitted to a smoke-free facility. For several reasons, >60% of patients with schizophrenia smoke cigarettes8 (Table 2).9-14 Patients who smoke and are on a stable dosage of clozapine might require a dosage reduction when they are admitted to a smoke-free facility to avoid adverse effects. If the dosage is not adjusted, a patient may experience clozapine-induced adverse effects, such as tachycardia, sedation, and seizures. It is likely that patients such as Mr. D will experience fluctuation in the clozapine level and possibly changes in efficacy and tolerability transitioning between inpatient and outpatient settings if the dosage is not adjusted.
Related Resources
• Kroon LA. Drug interactions with smoking. Am J Health Syst Pharm. 2007;64(18):1917-1921.
• Fankhauser MP. Drug interactions with tobacco smoke: Implications for patient care. Current Psychiatry. 2013; 12(1):12-16.
• Greenwood-Smith C, Lubman DI, Castle DJ. Serum clozapine levels: a review of their clinical utility. J Psychopharmacol. 2003;17(2):234-248.
• Olesen OV, Thomsen K, Jensen PN, et al. Clozapine serum levels and side effects during steady state treatment of schizophrenic patients: a cross sectional study. Psychopharmacology (Berl). 1995;117(3):371-378.
Drug Brand Names
Clozapine • Clozaril Theophylline • Theo-Dur
Olanzapine • Zyprexa
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Mr. D, age 30, has a 12-year history of schizophrenia and is experiencing worsening auditory hallucinations despite reported medication adherence. He has been taking clozapine, maintenance dosages 500 to 700 mg/d, for 4 years and smokes 2 packs of cigarettes a day. When Mr. D is admitted to a nonsmoking inpatient psychiatric facility, he receives nicotine transdermal patches, 21 mg/d, for nicotine withdrawal. Mr. D’s most recent outpatient clozapine dosage, 700 mg/d, is resumed. All laboratory tests, including complete blood count with differential, are within normal limits at admission.
Five days later Mr. D is tachycardic with a heart rate of 109 beats per minute. When assessing Mr. D, we notice he has alogia and that, when he does speak, his speech is slowed with a 4 to 5 second delay in response. He also appears sedated. We observe occasional mild jerking of his shoulder and lower legs.
Mr. D reports that his auditory hallucinations have lessened since his admission, but complains of difficulty remembering information and feeling tired during the day. The treatment team suspects clozapine toxicity; his trough clozapine level is 1,350 ng/mL (therapeutic range, 350 to 1,000 ng/mL).
It is well documented that cigarette smoke can induce cytochrome P450 (CYP) isoenzymes, specifically CYP1A1, CYP1A2, and CYP2E1. Because clozapine is primarily metabolized by CYP1A2 (approximately 70%), smoking can induce clozapine metabolism and abruptly stopping smoking can increase clozapine levels.1 The polycyclic aromatic hydrocarbons, not the nicotine, found in cigarettes are thought to be responsible for CYP1A2 induction; therefore, use of a nicotine replacement product did not prevent the increase in Mr. D’s clozapine levels.
Examining the evidence
Meyer1 evaluated clozapine levels before and after implementation of a hospital-wide smoking ban (N = 11). Clozapine dosages were not adjusted at the time of the smoking ban, which resulted in a mean 72% increase in clozapine levels after a minimum of 2 weeks as nonsmokers. Even after eliminating 2 outliers, the mean increase in clozapine levels was 36.1%. Murayama-Sung et al2 reported a statistically significant increase in the level of clozapine (46%, P = .004) and the level of norclozapine (23%, P = .02) after a hospital-wide smoking ban was instituted (N = 14). However, the pre-change and post-change in the ratio of clozapine to norclozapine level was not found to be statistically significant. Haslemo et al3 found that smoking as few as 7 to 12 cigarettes a day was sufficient for maximum induction of CYP1A2. Because Mr. D was smoking 2 packs of cigarettes a day (40 cigarettes) with an clozapine dosage 700 mg/d as an outpatient, he likely experienced significant induction of clozapine metabolism through CYP1A2, which was no longer present when he stopped smoking.
Therapeutic clozapine concentrations are typically above 350 and 420 ng/mL.4 Concentrations >700 ng/mL are associated with increased adverse effects, but generally are not associated with a higher response; levels >900 ng/mL have been associated with toxicity.4 Clozapine-treated patients on a stable dosage who smoke can experience clozapine-related adverse effects after admission to a smoke-free facility secondary to an increase in the clozapine concentration (Table 1).4
Five days after admission to the facility, Mr. D was noted to have myoclonus, somnolence, and tachycardia, with a clozapine level of 1,350 ng/mL. Additional adverse effects that can be seen include orthostatic hypotension, sialorrhea, worsening psychiatric symptoms (eg, hallucinations), and seizures.5 Although there is variability in the timing of the decrease in CYP1A2 activity after smoking cessation, practitioners should begin to monitor for clozapine-related adverse effects 1 or 2 days after smoking cessation.6
Treatment recommendations
Monitoring of the clozapine concentration and adjustment of the dosage might be needed to account for the fluctuation seen with smoking cessation to maintain efficacy and minimize adverse effects. However, a test of the clozapine level may not be available at all facilities, often requiring that the specimen be sent to an outside laboratory, taking 3 to 7 days to receive results.
Faber and Fuhr6 recommended reducing the dosage of a CYP1A2 substrate medication, such as clozapine, olanzapine, or theophylline, by 10% each day until the dosage has been reduced by 40% in patients who stop smoking. Lowe and Ackman5 proposed reducing the clozapine dosage by 30% to 40% to achieve a pre-cessation serum concentration at 1 week. For Mr. D, this would mean decreasing the clozapine dosage to 425 to 500 mg/d.
Assuming that Mr. D’s clozapine dosage is decreased during his hospitalization and that he resumes smoking after discharge, it is likely the dosage will need to be increased. It may take several weeks to see maximal induction, because new CYP enzymes need to be synthesized when the patient resumes smoking.7 One recommendation is to increase the clozapine dosage by a factor of 1.5 over 2 to 4 weeks, with close monitoring of the clozapine concentration and adverse effects because this increase is approximate.7 Depending on when Mr. D’s follow-up appointment is scheduled, the practitioner may need to plan a dosage adjustment to prevent a decrease in his clozapine level caused by smoking to prevent a worsening of symptoms and rehospitalization.
This case emphasizes the importance of asking clozapine-treated patients about their smoking history when they are admitted to a smoke-free facility. For several reasons, >60% of patients with schizophrenia smoke cigarettes8 (Table 2).9-14 Patients who smoke and are on a stable dosage of clozapine might require a dosage reduction when they are admitted to a smoke-free facility to avoid adverse effects. If the dosage is not adjusted, a patient may experience clozapine-induced adverse effects, such as tachycardia, sedation, and seizures. It is likely that patients such as Mr. D will experience fluctuation in the clozapine level and possibly changes in efficacy and tolerability transitioning between inpatient and outpatient settings if the dosage is not adjusted.
Related Resources
• Kroon LA. Drug interactions with smoking. Am J Health Syst Pharm. 2007;64(18):1917-1921.
• Fankhauser MP. Drug interactions with tobacco smoke: Implications for patient care. Current Psychiatry. 2013; 12(1):12-16.
• Greenwood-Smith C, Lubman DI, Castle DJ. Serum clozapine levels: a review of their clinical utility. J Psychopharmacol. 2003;17(2):234-248.
• Olesen OV, Thomsen K, Jensen PN, et al. Clozapine serum levels and side effects during steady state treatment of schizophrenic patients: a cross sectional study. Psychopharmacology (Berl). 1995;117(3):371-378.
Drug Brand Names
Clozapine • Clozaril Theophylline • Theo-Dur
Olanzapine • Zyprexa
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Meyer JM. Individual changes in clozapine levels after smoking cessation: results and a predictive model. J Clin Psychopharmacol. 2001;21(6):569-574.
2. Murayama-Sung L, Ahmed I, Goebert D, et al. The impact of hospital smoking ban on clozapine and norclozapine levels. J Clin Psychopharmacol. 2011;31(1):124-126.
3. Haslemo T, Eikeseth PH, Tanum L, et al. The effect of variable cigarette consumption on the interaction with clozapine and olanzapine. Eur J Clin Psychopharmacol. 2006;62(12): 1049-1053.
4. Nielsen J, Damkier P, Lublin H, et al. Optimizing clozapine treatment. Acta Psychiatr Scand. 2011;123(6):411-422.
5. Lowe EJ, Ackman ML. Impact of tobacco smoking cessation on stable clozapine and olanzapine treatment. Ann Pharmacother. 2010;44(4):727-732.
6. Faber MS, Fuhr U. Time response of cytochrome P450 1A2 activity on cessation of heavy smoking. Clin Pharmacol Ther. 2004;76(2):178-184.
7. de Leon J. Atypical antipsychotic dosing: the effect of smoking and caffeine. Psychiatr Serv. 2004;55(5):491-493.
8. Dickerson F, Stallings CR, Origoni AE, et al. Cigarette smoking among persons with schizophrenia or bipolar disorder in routine clinical settings, 1999-2011. Psychiatr Serv. 2013;64(1):44-50.
9. Esterberg ML, Compton MT. Smoking behavior in persons with a schizophrenia-spectrum disorder: a qualitative investigation of the transtheoretical model. Soc Sci Med. 2005;61(2):293-303.
10. Barr RS, Culhane MA, Jubelt LE, et al. The effects of transdermal nicotine on cognition in nonsmokers with schizophrenia and nonpsychiatric controls. Neuropsychopharmacology. 2008; 33(3):480-490.
11. Adler LE, Hoffer LD, Wiser A, et al. Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am J Psychiatry. 1993;150(12):1856-1861.
12. Sallette J, Pons S, Devillers-Thiery A, et al. Nicotine upregulates its own receptors through enhanced intracellular maturation. Neuron. 2005;46(4):595-607.
13. Breese CR, Lee MJ, Adams CE, et al. Abnormal regulation of high affinity nicotinic receptors in subjects with schizophrenia. Neuropsychopharmacology. 2000;23(4):351-364.
14. Miller DD, Kelly MW, Perry PJ, et al. The influence of cigarette smoking on haloperidol pharmacokinetics. J Clin Psychiatry. 1990;28(6):529-231.
1. Meyer JM. Individual changes in clozapine levels after smoking cessation: results and a predictive model. J Clin Psychopharmacol. 2001;21(6):569-574.
2. Murayama-Sung L, Ahmed I, Goebert D, et al. The impact of hospital smoking ban on clozapine and norclozapine levels. J Clin Psychopharmacol. 2011;31(1):124-126.
3. Haslemo T, Eikeseth PH, Tanum L, et al. The effect of variable cigarette consumption on the interaction with clozapine and olanzapine. Eur J Clin Psychopharmacol. 2006;62(12): 1049-1053.
4. Nielsen J, Damkier P, Lublin H, et al. Optimizing clozapine treatment. Acta Psychiatr Scand. 2011;123(6):411-422.
5. Lowe EJ, Ackman ML. Impact of tobacco smoking cessation on stable clozapine and olanzapine treatment. Ann Pharmacother. 2010;44(4):727-732.
6. Faber MS, Fuhr U. Time response of cytochrome P450 1A2 activity on cessation of heavy smoking. Clin Pharmacol Ther. 2004;76(2):178-184.
7. de Leon J. Atypical antipsychotic dosing: the effect of smoking and caffeine. Psychiatr Serv. 2004;55(5):491-493.
8. Dickerson F, Stallings CR, Origoni AE, et al. Cigarette smoking among persons with schizophrenia or bipolar disorder in routine clinical settings, 1999-2011. Psychiatr Serv. 2013;64(1):44-50.
9. Esterberg ML, Compton MT. Smoking behavior in persons with a schizophrenia-spectrum disorder: a qualitative investigation of the transtheoretical model. Soc Sci Med. 2005;61(2):293-303.
10. Barr RS, Culhane MA, Jubelt LE, et al. The effects of transdermal nicotine on cognition in nonsmokers with schizophrenia and nonpsychiatric controls. Neuropsychopharmacology. 2008; 33(3):480-490.
11. Adler LE, Hoffer LD, Wiser A, et al. Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am J Psychiatry. 1993;150(12):1856-1861.
12. Sallette J, Pons S, Devillers-Thiery A, et al. Nicotine upregulates its own receptors through enhanced intracellular maturation. Neuron. 2005;46(4):595-607.
13. Breese CR, Lee MJ, Adams CE, et al. Abnormal regulation of high affinity nicotinic receptors in subjects with schizophrenia. Neuropsychopharmacology. 2000;23(4):351-364.
14. Miller DD, Kelly MW, Perry PJ, et al. The influence of cigarette smoking on haloperidol pharmacokinetics. J Clin Psychiatry. 1990;28(6):529-231.