User login
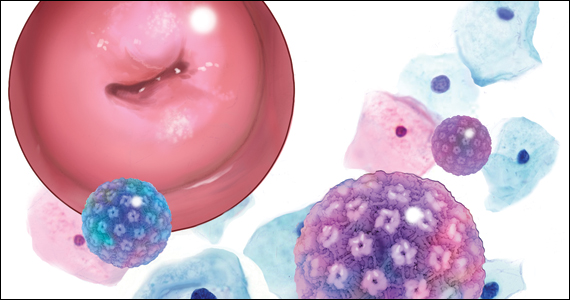
Cervical cancer: A path to eradication
David G. Mutch, MD: The cervical cancer screening guidelines, using Pap testing, have changed significantly since the times of yearly Paps and exams. Coupled with vaccination and new management guidelines (recommending HPV testing, etc), we actually hope that we are on the way to eradicating cervical cancer from our environment.
Screening: Current recommendations
Dr. Mutch: Warner, the American Society of Colposcopy and Cervical Pathology (ASCCP)1 endorses the cervical cancer screening guidelines for several professional organizations, including the American Cancer Society (ACS),2 the US Preventive Services Task Force (USPSTF),3 and the American College of Obstetricians and Gynecologists (ACOG).4 What are the current screening recommendations, as these organizations have disparate views?
Warner Huh, MD: There was a time, around 2012-2013, when for the first time ever, we had significant harmonization of the guidelines between ACOG and the USPSTF and ACS. But in the last 10 years there has been an explosion of data in terms of how to best screen patients.
The move to primary HPV testing. The USPSTF3 initially had recommended looking into primary HPV screening, which is just using HPV testing by itself as the screening modality. But there was a lot of pushback at that time. I think a lot of people thought that we were not prepared to make that leap. Therefore, they endorsed screening with cytology by itself as well as HPV testing by itself, as well as co-testing; but in their recommendations, they made it very clear that they were leaning toward primary HPV screening.
A new patient age to begin screening. In 2020, the ACS put out their new guidelines,2 which are a significant departure from what we are used to—they are recommending that we start screening at 25 years of age. Like you said, Dr. Mutch, it doesn’t seem that long ago when we were screening people at age 18, or within 3 years of sexual intercourse. But the reason for it is that the rate of cervical cancer is extremely low under age 25, and other countries like the United Kingdom already do this.
The other major departure in the ACS guidelines is that they really are asking clinicians and screeners to focus on primary HPV screening. Overall, they have sort of doubled down on why they think primary HPV screening is so important.
ACOG sits sort of in the middle of the other recommendations. ACOG understands the value of primary HPV screening, but I don’t think that they are quite ready to recommend screening at age 25. If you look at their updated guidelines from April 2021,4 they state that we should continue a screening-starting age of 21 years. So there are some disparate views, but I am confident, Dr. Mutch, that in the next 2 to 3 years, there will be greater harmonization of these guidelines and less confusion for our providers. The greatest barrier is understanding the science and the comfort level of clinicians to go with just an HPV test, since for the last 40 years the Pap test has anchored gynecologic care in this country. And it took at least 10 years to get to what I consider to be widespread adoption to co-testing. The other thing that readers should recognize is that the Task Force is actually revisiting their cervical cancer screening guidance now, so expect another major revision.
Reimbursement and access are barriers. Reimbursement is a further real issue. We are now using one less test, but insurance companies may not reimburse when just the HPV test is used. The other issue is access to labs that can do the HPV testing.
Dr. Mutch: We used to see patients yearly and picked up a lot of adjunctive or additional illnesses. Now they are not being seen yearly it could impact negatively their overall health care. We need to understand that cervical cancer screening is simply a test, which should not eliminate other health care.
Dr. Huh: Yes, I think the extended interval between recommended HPV screenings scares people. I have been involved in these screening guidelines (and I can only speak for myself, not for my colleagues), but even I do think we made a leap to a longer interval way too quickly in this country. Screening changes are slow, and sometimes a glacial process. I think it can worry providers when we make rapid changes.
But this is a test that should not anchor the yearly visit. There are plenty of other reasons—and ACOG actually states this4—why patients should come for a wellness exam on a yearly basis. So I think our ObGyns in the United States need to recognize that, but I understand there are underlying concerns that if you extend intervals too long, (a) will patients come back, and (b), as a consequence, is the interval going to miss something in between? Those are real legitimate concerns.
Continue to: Management guidelines...
Management guidelines: The latest
Dr. Mutch: The ASCCP issued new management guidelines in 2019.5 Can you address what you feel are the most important updates?
Dr. Huh: Going back to 2002, we have revised these guidelines every 5 years. For this one, the revision came out a little bit later for various reasons, but the reason we revised it is because we collect new data that we think markedly changes our understanding of the disease process and natural history and the interventions for women that have preinvasive disease of the cervix.
Briefly, I think the biggest changes based on what we were hearing from our providers and users of our apps and algorithms was that our algorithms were becoming way too complicated, and they were. If you look over the last 10 years, the number of branch points on our algorithms basically quadrupled. If we incorporated the new data this time, the algorithms would be unworkable, and you could not use them on your phone because they would be too complicated.
So, we created a system where, in essence, providers have 5 choices for patients:
- treatment
- colposcopy
- follow-up in 1 year
- follow-up in 3 years
- follow-up in 5 years.
Those recommendations are based on what we call “clinically actionable thresholds”—basically, the percent chance of developing immediate CIN3 or worse. That threshold will probably change over time, but what we did is create a system that (a) makes it easier for the provider, (although they have to trust the system—and they can look under the hood and understand how we did this) and (b) allows us to create a foundation where we can add future technologies that use the same rubric or paradigm so that they still wind up getting the same result without having to go to another algorithm.
This new system is probably the most marked change in the history of the ASCCP management guidelines, but we did it to make it ultimately easier for providers going forward for the next 10 to 20 years. There are real opportunities, Dr. Mutch, in terms of how do we integrate this into the electronic medical record (EMR), and how do we pull data so clinicians don’t have to manually enter it.
The other difference is now there is a web-based application. Back in 2012, there were a lot of people that were not using EMRs. Now the majority of the country is, and so they actually are on a browser more than they are on their phone. We actually have an equally robust web platform that allows them to get the information that they need.
Dr. Mutch: I think that is really important—the utility of utilizing a mobile app, if you will, for triaging your patient with a specific test result so that patients are followed up at the proper interval, and that ultimately becomes cost-effective.
Dr. Huh: Yes, the app now is very different than the app that I think people are used to using for the last almost 10 years. You don’t put inputs, pull up the algorithm, and look at the outcome. This is different. You enter the patient’s age. You add their cytology, their HPV results, the clinical scenario that you are in, and then it puts out a recommendation of what to do next. Over time, we want to get away from an algorithm and for our providers to understand what the risk is and how that risk calculation then translates into a clinical recommendation.
Dr. Mutch: I think to utilize an app is almost necessary given the complexity of the triaging process so that it does become, in fact, the most cost-effective way to screen patients.
Dr. Huh: I would agree with that. There is a learning curve for whenever you see new technology. There was a learning curve for even ASCCP leadership as they tried to educate providers. I think people will ultimately see that this is a much better way of managing patients with cervical abnormalities, and I am hoping actually that we will use a similar platform for many other diseases that we manage in women’s health.
Continue to: Chipping away of the yearly exam...
Chipping away of the yearly exam
Dr. Mutch: With this moving away from the yearly exam and Pap test, women may not get yearly examinations. Do you feel that this could affect a stage migration to a higher stage at diagnosis, for instance, of a cervical cancer? Or that it might adversely impact other health issues?
Dr. Huh: I think that’s a good question. I am worried about the interval—I think 5 years is a bit long. I am more worried that patients will miss out on visits because they may think that they need to only come back for their Pap, even though they should be re-educated on that.
COVID-19 has made this a little hard for us to analyze because, clearly, we have had access to care issues. But I am a little concerned that we could see an uptick in invasive cancer rates in this country, including an uptick in the stage and more locally advanced cervical cancer because of the changes in the screening paradigms. But we don’t know that to be the case.
As with all screenings, the bottom line is you have to worry about what the false-negative and false-positive rates of screening are, and that affects everything. I want the readers to know that primary HPV should be used for screenings. It is not perfect, but it is much better than cytology alone. We need to think about how to better adapt screening in the age that we live in.
HPV self-sampling
Dr. Mutch: Could self-sampling for HPV testing, which obviously would be easier for the patient, and certainly useful in terms of screening, address some health care disparities with regard to cervical cancer?
Dr. Huh: The short answer is, yes. Self-sampling is not US Food and Drug Administration-approved in this country. It’s not being widely used without that approval. But there are multiple countries, including the United States, that have done lots of studies on this topic. There are many public health experts and champions for HPV self-sampling. I think we have learned, based on some studies, that the sensitivity is reasonable.6
I live in a part of the country that is woefully underserved; where you are there are pockets in Missouri that are woefully underserved as well. So the issue is, can we reduce these disparities and access to care with something like self-sampling? My personal feeling is I think that we can make a dent in that, and it is never going to fully replace screening, but it at least will allow us to reallocate our resources and attention to those women that are at highest risk for developing cervical cancer or precancer based on the self-sampling result.
I don’t think it will ever replace screening per se, but if we have an abnormal self-sampling test, we might say to that patient, “You really do need to come in to get re-tested or to get re-evaluated.” So it could be a better resource and use of our health care dollars and investments in terms of trying to reduce the incidence of cervical cancer. Of course the verdict is out, but I think there are a lot of people who would love to see this scenario.
If we screen and treat perfectly in this country, we would not even need the HPV vaccine when it comes to cervical cancer. That is how effective screening is. But, up to 50% to 60% of women in this country now still are underscreened or unscreened. We were talking about that number almost 25-30 years ago, Dr. Mutch. So access to screening is a big problem, but the other problem is how do you get patients in to be seen if they have an abnormal screening test? It’s not just about screening. It’s about screening, evaluation, and treatment; all 3 components are really important.
Continue to: Where do we stand with HPV vaccination?...
Where do we stand with HPV vaccination?
Dr. Mutch: Those are great points.
You brought up vaccination. We have a long way to go with regard to that, certainly in the United States, because of the various factions opposed to vaccination and so on. But do you think that vaccination has allowed us to decrease the incidence of cervical cancer?
Dr. Huh: Yes. There is clear evidence from the Nordic countries.7 There is emerging evidence from Australia.8 There is emerging evidence from other industrialized nations that clearly demonstrate vaccination’s positive effect in reducing the incidence of cervical cancer. None of this should be a surprise. Every population-based study that has been published with the HPV vaccine in populations that have a low frequency of vaccination have demonstrated substantial reductions in things like genital warts, abnormal Pap tests, precancer, and now evidence that there is a downward trend in terms of the incidence of cervical cancer.9
I don’t think that there is any debate anymore that vaccination is the way to go. Our challenge is about implementation and getting the vaccine to people. We still have a long way to go with that. There are parts of the world that are so affected by invasive cervical cancers; we need to get the vaccine to those parts of the world.
Dr. Mutch: What are the barriers to vaccination? How can we overcome those barriers?
Dr. Huh: There is a lot of criticism that we are not vaccinating more in the United States. However, the rates of vaccination are going up every single year. The pandemic may have blunted that rise a bit, but if you look at the vaccination curves, they are going up, not down. We need to continue to educate patients, parents, and pediatricians on the importance of vaccination.
Boys still get vaccinated less frequently than girls, so we have some work to do there. I think globally it is the issue of getting the vaccine to people, making sure that vaccine is available. The thing that I think will be the game-changer going forward is whether or not we will have evidence to indicate that 1 dose is as effective as 2 doses or 3 doses. If we can vaccinate boys and girls with just 1 dose, then in the next generation or two, we seriously might eradicate not just cervical cancer but a lot of HPV-related malignancies worldwide.
Educating patients, clinicians is key
Dr. Mutch: So it seems education, education, education, with regard to screening guidelines, with regard to the need for continued examinations, and that HPV testing is only a test, it does not supplant overall care. Finally, education regarding eradication of cervical cancer through vaccination.
Dr. Huh: That summarizes it well. We are still going to screen for cervical cancer. We are still going to vaccinate, and providers are still going to manage abnormal Pap tests. It is confusing because we are changing it up it seems every year or 2, so this conversation you and I are having is particularly important for clinicians to understand the basis of that. There has been an explosion of data that has come out in this area in the last decade.
Dr. Mutch: Thank you, Dr. Huh. I really appreciate your thoughts on this. As you all know, Dr. Huh has been President of the ASCCP and is instrumental in writing and disseminating these guidelines, so we are very grateful that he has consented to agree to come and talk with us today.
Dr. Huh: My pleasure. Thank you for inviting me. This was fun, and I have really enjoyed talking to you and participating. ●
- ASCCP Screening Guidelines. https://www.asccp.org /screening-guidelines. Accessed April 25, 2021.
- Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guidelines update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- US Preventive Services Task Force. Screening for cervical cancer. US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:674-686. doi:10.1001/jama .2018.10897.
- American College of Obstetricians and Gynecologists. Practice Advisory: Updated cervical cancer screening guidelines. April 2022. https://www.acog.org/clinical/clinical-guidance /practice-advisory/articles/2021/04/updated-cervical -cancer-screening-guidelines. Accessed April 25, 2022.
- Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP RiskBased Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10 34 .1097/LGT.0000000000000525.
- Yeh PT, Kennedy CE, de Vuyst H, et al. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Global Health. 2019;4:e001351. doi:10.1136/ bmjgh-2018-001351.
- Kjaer SK, Nygard M, Dillner J, et al. A 12-year follow-up on the long-term effectiveness of the quadrivalent human papillomavirus vaccine in 4 Nordic countries. Clin Infect Dis. 2018;66:339-345. doi: 10.1093/cid/cix797.
- Patel C, Brotherton JM, Pillsbury A, et al. The impact of 10 years of human papillomavirus (HPV) vaccination in Australia: what additional disease burden will a nonvalent vaccine prevent? Euro Surveill. 2018;23:1700737. doi: 10 .2807/1560-7917.
- Falcaro M, Castanon A, Ndlela B, et al. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. 2021;398:2084-2092. doi.org/10.1016/S0140-6736(21) 02178-4.

David G. Mutch, MD: The cervical cancer screening guidelines, using Pap testing, have changed significantly since the times of yearly Paps and exams. Coupled with vaccination and new management guidelines (recommending HPV testing, etc), we actually hope that we are on the way to eradicating cervical cancer from our environment.
Screening: Current recommendations
Dr. Mutch: Warner, the American Society of Colposcopy and Cervical Pathology (ASCCP)1 endorses the cervical cancer screening guidelines for several professional organizations, including the American Cancer Society (ACS),2 the US Preventive Services Task Force (USPSTF),3 and the American College of Obstetricians and Gynecologists (ACOG).4 What are the current screening recommendations, as these organizations have disparate views?
Warner Huh, MD: There was a time, around 2012-2013, when for the first time ever, we had significant harmonization of the guidelines between ACOG and the USPSTF and ACS. But in the last 10 years there has been an explosion of data in terms of how to best screen patients.
The move to primary HPV testing. The USPSTF3 initially had recommended looking into primary HPV screening, which is just using HPV testing by itself as the screening modality. But there was a lot of pushback at that time. I think a lot of people thought that we were not prepared to make that leap. Therefore, they endorsed screening with cytology by itself as well as HPV testing by itself, as well as co-testing; but in their recommendations, they made it very clear that they were leaning toward primary HPV screening.
A new patient age to begin screening. In 2020, the ACS put out their new guidelines,2 which are a significant departure from what we are used to—they are recommending that we start screening at 25 years of age. Like you said, Dr. Mutch, it doesn’t seem that long ago when we were screening people at age 18, or within 3 years of sexual intercourse. But the reason for it is that the rate of cervical cancer is extremely low under age 25, and other countries like the United Kingdom already do this.
The other major departure in the ACS guidelines is that they really are asking clinicians and screeners to focus on primary HPV screening. Overall, they have sort of doubled down on why they think primary HPV screening is so important.
ACOG sits sort of in the middle of the other recommendations. ACOG understands the value of primary HPV screening, but I don’t think that they are quite ready to recommend screening at age 25. If you look at their updated guidelines from April 2021,4 they state that we should continue a screening-starting age of 21 years. So there are some disparate views, but I am confident, Dr. Mutch, that in the next 2 to 3 years, there will be greater harmonization of these guidelines and less confusion for our providers. The greatest barrier is understanding the science and the comfort level of clinicians to go with just an HPV test, since for the last 40 years the Pap test has anchored gynecologic care in this country. And it took at least 10 years to get to what I consider to be widespread adoption to co-testing. The other thing that readers should recognize is that the Task Force is actually revisiting their cervical cancer screening guidance now, so expect another major revision.
Reimbursement and access are barriers. Reimbursement is a further real issue. We are now using one less test, but insurance companies may not reimburse when just the HPV test is used. The other issue is access to labs that can do the HPV testing.
Dr. Mutch: We used to see patients yearly and picked up a lot of adjunctive or additional illnesses. Now they are not being seen yearly it could impact negatively their overall health care. We need to understand that cervical cancer screening is simply a test, which should not eliminate other health care.
Dr. Huh: Yes, I think the extended interval between recommended HPV screenings scares people. I have been involved in these screening guidelines (and I can only speak for myself, not for my colleagues), but even I do think we made a leap to a longer interval way too quickly in this country. Screening changes are slow, and sometimes a glacial process. I think it can worry providers when we make rapid changes.
But this is a test that should not anchor the yearly visit. There are plenty of other reasons—and ACOG actually states this4—why patients should come for a wellness exam on a yearly basis. So I think our ObGyns in the United States need to recognize that, but I understand there are underlying concerns that if you extend intervals too long, (a) will patients come back, and (b), as a consequence, is the interval going to miss something in between? Those are real legitimate concerns.
Continue to: Management guidelines...
Management guidelines: The latest
Dr. Mutch: The ASCCP issued new management guidelines in 2019.5 Can you address what you feel are the most important updates?
Dr. Huh: Going back to 2002, we have revised these guidelines every 5 years. For this one, the revision came out a little bit later for various reasons, but the reason we revised it is because we collect new data that we think markedly changes our understanding of the disease process and natural history and the interventions for women that have preinvasive disease of the cervix.
Briefly, I think the biggest changes based on what we were hearing from our providers and users of our apps and algorithms was that our algorithms were becoming way too complicated, and they were. If you look over the last 10 years, the number of branch points on our algorithms basically quadrupled. If we incorporated the new data this time, the algorithms would be unworkable, and you could not use them on your phone because they would be too complicated.
So, we created a system where, in essence, providers have 5 choices for patients:
- treatment
- colposcopy
- follow-up in 1 year
- follow-up in 3 years
- follow-up in 5 years.
Those recommendations are based on what we call “clinically actionable thresholds”—basically, the percent chance of developing immediate CIN3 or worse. That threshold will probably change over time, but what we did is create a system that (a) makes it easier for the provider, (although they have to trust the system—and they can look under the hood and understand how we did this) and (b) allows us to create a foundation where we can add future technologies that use the same rubric or paradigm so that they still wind up getting the same result without having to go to another algorithm.
This new system is probably the most marked change in the history of the ASCCP management guidelines, but we did it to make it ultimately easier for providers going forward for the next 10 to 20 years. There are real opportunities, Dr. Mutch, in terms of how do we integrate this into the electronic medical record (EMR), and how do we pull data so clinicians don’t have to manually enter it.
The other difference is now there is a web-based application. Back in 2012, there were a lot of people that were not using EMRs. Now the majority of the country is, and so they actually are on a browser more than they are on their phone. We actually have an equally robust web platform that allows them to get the information that they need.
Dr. Mutch: I think that is really important—the utility of utilizing a mobile app, if you will, for triaging your patient with a specific test result so that patients are followed up at the proper interval, and that ultimately becomes cost-effective.
Dr. Huh: Yes, the app now is very different than the app that I think people are used to using for the last almost 10 years. You don’t put inputs, pull up the algorithm, and look at the outcome. This is different. You enter the patient’s age. You add their cytology, their HPV results, the clinical scenario that you are in, and then it puts out a recommendation of what to do next. Over time, we want to get away from an algorithm and for our providers to understand what the risk is and how that risk calculation then translates into a clinical recommendation.
Dr. Mutch: I think to utilize an app is almost necessary given the complexity of the triaging process so that it does become, in fact, the most cost-effective way to screen patients.
Dr. Huh: I would agree with that. There is a learning curve for whenever you see new technology. There was a learning curve for even ASCCP leadership as they tried to educate providers. I think people will ultimately see that this is a much better way of managing patients with cervical abnormalities, and I am hoping actually that we will use a similar platform for many other diseases that we manage in women’s health.
Continue to: Chipping away of the yearly exam...
Chipping away of the yearly exam
Dr. Mutch: With this moving away from the yearly exam and Pap test, women may not get yearly examinations. Do you feel that this could affect a stage migration to a higher stage at diagnosis, for instance, of a cervical cancer? Or that it might adversely impact other health issues?
Dr. Huh: I think that’s a good question. I am worried about the interval—I think 5 years is a bit long. I am more worried that patients will miss out on visits because they may think that they need to only come back for their Pap, even though they should be re-educated on that.
COVID-19 has made this a little hard for us to analyze because, clearly, we have had access to care issues. But I am a little concerned that we could see an uptick in invasive cancer rates in this country, including an uptick in the stage and more locally advanced cervical cancer because of the changes in the screening paradigms. But we don’t know that to be the case.
As with all screenings, the bottom line is you have to worry about what the false-negative and false-positive rates of screening are, and that affects everything. I want the readers to know that primary HPV should be used for screenings. It is not perfect, but it is much better than cytology alone. We need to think about how to better adapt screening in the age that we live in.
HPV self-sampling
Dr. Mutch: Could self-sampling for HPV testing, which obviously would be easier for the patient, and certainly useful in terms of screening, address some health care disparities with regard to cervical cancer?
Dr. Huh: The short answer is, yes. Self-sampling is not US Food and Drug Administration-approved in this country. It’s not being widely used without that approval. But there are multiple countries, including the United States, that have done lots of studies on this topic. There are many public health experts and champions for HPV self-sampling. I think we have learned, based on some studies, that the sensitivity is reasonable.6
I live in a part of the country that is woefully underserved; where you are there are pockets in Missouri that are woefully underserved as well. So the issue is, can we reduce these disparities and access to care with something like self-sampling? My personal feeling is I think that we can make a dent in that, and it is never going to fully replace screening, but it at least will allow us to reallocate our resources and attention to those women that are at highest risk for developing cervical cancer or precancer based on the self-sampling result.
I don’t think it will ever replace screening per se, but if we have an abnormal self-sampling test, we might say to that patient, “You really do need to come in to get re-tested or to get re-evaluated.” So it could be a better resource and use of our health care dollars and investments in terms of trying to reduce the incidence of cervical cancer. Of course the verdict is out, but I think there are a lot of people who would love to see this scenario.
If we screen and treat perfectly in this country, we would not even need the HPV vaccine when it comes to cervical cancer. That is how effective screening is. But, up to 50% to 60% of women in this country now still are underscreened or unscreened. We were talking about that number almost 25-30 years ago, Dr. Mutch. So access to screening is a big problem, but the other problem is how do you get patients in to be seen if they have an abnormal screening test? It’s not just about screening. It’s about screening, evaluation, and treatment; all 3 components are really important.
Continue to: Where do we stand with HPV vaccination?...
Where do we stand with HPV vaccination?
Dr. Mutch: Those are great points.
You brought up vaccination. We have a long way to go with regard to that, certainly in the United States, because of the various factions opposed to vaccination and so on. But do you think that vaccination has allowed us to decrease the incidence of cervical cancer?
Dr. Huh: Yes. There is clear evidence from the Nordic countries.7 There is emerging evidence from Australia.8 There is emerging evidence from other industrialized nations that clearly demonstrate vaccination’s positive effect in reducing the incidence of cervical cancer. None of this should be a surprise. Every population-based study that has been published with the HPV vaccine in populations that have a low frequency of vaccination have demonstrated substantial reductions in things like genital warts, abnormal Pap tests, precancer, and now evidence that there is a downward trend in terms of the incidence of cervical cancer.9
I don’t think that there is any debate anymore that vaccination is the way to go. Our challenge is about implementation and getting the vaccine to people. We still have a long way to go with that. There are parts of the world that are so affected by invasive cervical cancers; we need to get the vaccine to those parts of the world.
Dr. Mutch: What are the barriers to vaccination? How can we overcome those barriers?
Dr. Huh: There is a lot of criticism that we are not vaccinating more in the United States. However, the rates of vaccination are going up every single year. The pandemic may have blunted that rise a bit, but if you look at the vaccination curves, they are going up, not down. We need to continue to educate patients, parents, and pediatricians on the importance of vaccination.
Boys still get vaccinated less frequently than girls, so we have some work to do there. I think globally it is the issue of getting the vaccine to people, making sure that vaccine is available. The thing that I think will be the game-changer going forward is whether or not we will have evidence to indicate that 1 dose is as effective as 2 doses or 3 doses. If we can vaccinate boys and girls with just 1 dose, then in the next generation or two, we seriously might eradicate not just cervical cancer but a lot of HPV-related malignancies worldwide.
Educating patients, clinicians is key
Dr. Mutch: So it seems education, education, education, with regard to screening guidelines, with regard to the need for continued examinations, and that HPV testing is only a test, it does not supplant overall care. Finally, education regarding eradication of cervical cancer through vaccination.
Dr. Huh: That summarizes it well. We are still going to screen for cervical cancer. We are still going to vaccinate, and providers are still going to manage abnormal Pap tests. It is confusing because we are changing it up it seems every year or 2, so this conversation you and I are having is particularly important for clinicians to understand the basis of that. There has been an explosion of data that has come out in this area in the last decade.
Dr. Mutch: Thank you, Dr. Huh. I really appreciate your thoughts on this. As you all know, Dr. Huh has been President of the ASCCP and is instrumental in writing and disseminating these guidelines, so we are very grateful that he has consented to agree to come and talk with us today.
Dr. Huh: My pleasure. Thank you for inviting me. This was fun, and I have really enjoyed talking to you and participating. ●

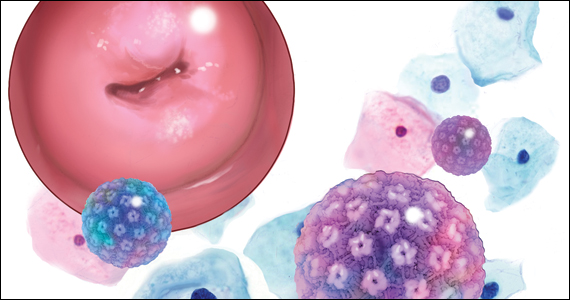
David G. Mutch, MD: The cervical cancer screening guidelines, using Pap testing, have changed significantly since the times of yearly Paps and exams. Coupled with vaccination and new management guidelines (recommending HPV testing, etc), we actually hope that we are on the way to eradicating cervical cancer from our environment.
Screening: Current recommendations
Dr. Mutch: Warner, the American Society of Colposcopy and Cervical Pathology (ASCCP)1 endorses the cervical cancer screening guidelines for several professional organizations, including the American Cancer Society (ACS),2 the US Preventive Services Task Force (USPSTF),3 and the American College of Obstetricians and Gynecologists (ACOG).4 What are the current screening recommendations, as these organizations have disparate views?
Warner Huh, MD: There was a time, around 2012-2013, when for the first time ever, we had significant harmonization of the guidelines between ACOG and the USPSTF and ACS. But in the last 10 years there has been an explosion of data in terms of how to best screen patients.
The move to primary HPV testing. The USPSTF3 initially had recommended looking into primary HPV screening, which is just using HPV testing by itself as the screening modality. But there was a lot of pushback at that time. I think a lot of people thought that we were not prepared to make that leap. Therefore, they endorsed screening with cytology by itself as well as HPV testing by itself, as well as co-testing; but in their recommendations, they made it very clear that they were leaning toward primary HPV screening.
A new patient age to begin screening. In 2020, the ACS put out their new guidelines,2 which are a significant departure from what we are used to—they are recommending that we start screening at 25 years of age. Like you said, Dr. Mutch, it doesn’t seem that long ago when we were screening people at age 18, or within 3 years of sexual intercourse. But the reason for it is that the rate of cervical cancer is extremely low under age 25, and other countries like the United Kingdom already do this.
The other major departure in the ACS guidelines is that they really are asking clinicians and screeners to focus on primary HPV screening. Overall, they have sort of doubled down on why they think primary HPV screening is so important.
ACOG sits sort of in the middle of the other recommendations. ACOG understands the value of primary HPV screening, but I don’t think that they are quite ready to recommend screening at age 25. If you look at their updated guidelines from April 2021,4 they state that we should continue a screening-starting age of 21 years. So there are some disparate views, but I am confident, Dr. Mutch, that in the next 2 to 3 years, there will be greater harmonization of these guidelines and less confusion for our providers. The greatest barrier is understanding the science and the comfort level of clinicians to go with just an HPV test, since for the last 40 years the Pap test has anchored gynecologic care in this country. And it took at least 10 years to get to what I consider to be widespread adoption to co-testing. The other thing that readers should recognize is that the Task Force is actually revisiting their cervical cancer screening guidance now, so expect another major revision.
Reimbursement and access are barriers. Reimbursement is a further real issue. We are now using one less test, but insurance companies may not reimburse when just the HPV test is used. The other issue is access to labs that can do the HPV testing.
Dr. Mutch: We used to see patients yearly and picked up a lot of adjunctive or additional illnesses. Now they are not being seen yearly it could impact negatively their overall health care. We need to understand that cervical cancer screening is simply a test, which should not eliminate other health care.
Dr. Huh: Yes, I think the extended interval between recommended HPV screenings scares people. I have been involved in these screening guidelines (and I can only speak for myself, not for my colleagues), but even I do think we made a leap to a longer interval way too quickly in this country. Screening changes are slow, and sometimes a glacial process. I think it can worry providers when we make rapid changes.
But this is a test that should not anchor the yearly visit. There are plenty of other reasons—and ACOG actually states this4—why patients should come for a wellness exam on a yearly basis. So I think our ObGyns in the United States need to recognize that, but I understand there are underlying concerns that if you extend intervals too long, (a) will patients come back, and (b), as a consequence, is the interval going to miss something in between? Those are real legitimate concerns.
Continue to: Management guidelines...
Management guidelines: The latest
Dr. Mutch: The ASCCP issued new management guidelines in 2019.5 Can you address what you feel are the most important updates?
Dr. Huh: Going back to 2002, we have revised these guidelines every 5 years. For this one, the revision came out a little bit later for various reasons, but the reason we revised it is because we collect new data that we think markedly changes our understanding of the disease process and natural history and the interventions for women that have preinvasive disease of the cervix.
Briefly, I think the biggest changes based on what we were hearing from our providers and users of our apps and algorithms was that our algorithms were becoming way too complicated, and they were. If you look over the last 10 years, the number of branch points on our algorithms basically quadrupled. If we incorporated the new data this time, the algorithms would be unworkable, and you could not use them on your phone because they would be too complicated.
So, we created a system where, in essence, providers have 5 choices for patients:
- treatment
- colposcopy
- follow-up in 1 year
- follow-up in 3 years
- follow-up in 5 years.
Those recommendations are based on what we call “clinically actionable thresholds”—basically, the percent chance of developing immediate CIN3 or worse. That threshold will probably change over time, but what we did is create a system that (a) makes it easier for the provider, (although they have to trust the system—and they can look under the hood and understand how we did this) and (b) allows us to create a foundation where we can add future technologies that use the same rubric or paradigm so that they still wind up getting the same result without having to go to another algorithm.
This new system is probably the most marked change in the history of the ASCCP management guidelines, but we did it to make it ultimately easier for providers going forward for the next 10 to 20 years. There are real opportunities, Dr. Mutch, in terms of how do we integrate this into the electronic medical record (EMR), and how do we pull data so clinicians don’t have to manually enter it.
The other difference is now there is a web-based application. Back in 2012, there were a lot of people that were not using EMRs. Now the majority of the country is, and so they actually are on a browser more than they are on their phone. We actually have an equally robust web platform that allows them to get the information that they need.
Dr. Mutch: I think that is really important—the utility of utilizing a mobile app, if you will, for triaging your patient with a specific test result so that patients are followed up at the proper interval, and that ultimately becomes cost-effective.
Dr. Huh: Yes, the app now is very different than the app that I think people are used to using for the last almost 10 years. You don’t put inputs, pull up the algorithm, and look at the outcome. This is different. You enter the patient’s age. You add their cytology, their HPV results, the clinical scenario that you are in, and then it puts out a recommendation of what to do next. Over time, we want to get away from an algorithm and for our providers to understand what the risk is and how that risk calculation then translates into a clinical recommendation.
Dr. Mutch: I think to utilize an app is almost necessary given the complexity of the triaging process so that it does become, in fact, the most cost-effective way to screen patients.
Dr. Huh: I would agree with that. There is a learning curve for whenever you see new technology. There was a learning curve for even ASCCP leadership as they tried to educate providers. I think people will ultimately see that this is a much better way of managing patients with cervical abnormalities, and I am hoping actually that we will use a similar platform for many other diseases that we manage in women’s health.
Continue to: Chipping away of the yearly exam...
Chipping away of the yearly exam
Dr. Mutch: With this moving away from the yearly exam and Pap test, women may not get yearly examinations. Do you feel that this could affect a stage migration to a higher stage at diagnosis, for instance, of a cervical cancer? Or that it might adversely impact other health issues?
Dr. Huh: I think that’s a good question. I am worried about the interval—I think 5 years is a bit long. I am more worried that patients will miss out on visits because they may think that they need to only come back for their Pap, even though they should be re-educated on that.
COVID-19 has made this a little hard for us to analyze because, clearly, we have had access to care issues. But I am a little concerned that we could see an uptick in invasive cancer rates in this country, including an uptick in the stage and more locally advanced cervical cancer because of the changes in the screening paradigms. But we don’t know that to be the case.
As with all screenings, the bottom line is you have to worry about what the false-negative and false-positive rates of screening are, and that affects everything. I want the readers to know that primary HPV should be used for screenings. It is not perfect, but it is much better than cytology alone. We need to think about how to better adapt screening in the age that we live in.
HPV self-sampling
Dr. Mutch: Could self-sampling for HPV testing, which obviously would be easier for the patient, and certainly useful in terms of screening, address some health care disparities with regard to cervical cancer?
Dr. Huh: The short answer is, yes. Self-sampling is not US Food and Drug Administration-approved in this country. It’s not being widely used without that approval. But there are multiple countries, including the United States, that have done lots of studies on this topic. There are many public health experts and champions for HPV self-sampling. I think we have learned, based on some studies, that the sensitivity is reasonable.6
I live in a part of the country that is woefully underserved; where you are there are pockets in Missouri that are woefully underserved as well. So the issue is, can we reduce these disparities and access to care with something like self-sampling? My personal feeling is I think that we can make a dent in that, and it is never going to fully replace screening, but it at least will allow us to reallocate our resources and attention to those women that are at highest risk for developing cervical cancer or precancer based on the self-sampling result.
I don’t think it will ever replace screening per se, but if we have an abnormal self-sampling test, we might say to that patient, “You really do need to come in to get re-tested or to get re-evaluated.” So it could be a better resource and use of our health care dollars and investments in terms of trying to reduce the incidence of cervical cancer. Of course the verdict is out, but I think there are a lot of people who would love to see this scenario.
If we screen and treat perfectly in this country, we would not even need the HPV vaccine when it comes to cervical cancer. That is how effective screening is. But, up to 50% to 60% of women in this country now still are underscreened or unscreened. We were talking about that number almost 25-30 years ago, Dr. Mutch. So access to screening is a big problem, but the other problem is how do you get patients in to be seen if they have an abnormal screening test? It’s not just about screening. It’s about screening, evaluation, and treatment; all 3 components are really important.
Continue to: Where do we stand with HPV vaccination?...
Where do we stand with HPV vaccination?
Dr. Mutch: Those are great points.
You brought up vaccination. We have a long way to go with regard to that, certainly in the United States, because of the various factions opposed to vaccination and so on. But do you think that vaccination has allowed us to decrease the incidence of cervical cancer?
Dr. Huh: Yes. There is clear evidence from the Nordic countries.7 There is emerging evidence from Australia.8 There is emerging evidence from other industrialized nations that clearly demonstrate vaccination’s positive effect in reducing the incidence of cervical cancer. None of this should be a surprise. Every population-based study that has been published with the HPV vaccine in populations that have a low frequency of vaccination have demonstrated substantial reductions in things like genital warts, abnormal Pap tests, precancer, and now evidence that there is a downward trend in terms of the incidence of cervical cancer.9
I don’t think that there is any debate anymore that vaccination is the way to go. Our challenge is about implementation and getting the vaccine to people. We still have a long way to go with that. There are parts of the world that are so affected by invasive cervical cancers; we need to get the vaccine to those parts of the world.
Dr. Mutch: What are the barriers to vaccination? How can we overcome those barriers?
Dr. Huh: There is a lot of criticism that we are not vaccinating more in the United States. However, the rates of vaccination are going up every single year. The pandemic may have blunted that rise a bit, but if you look at the vaccination curves, they are going up, not down. We need to continue to educate patients, parents, and pediatricians on the importance of vaccination.
Boys still get vaccinated less frequently than girls, so we have some work to do there. I think globally it is the issue of getting the vaccine to people, making sure that vaccine is available. The thing that I think will be the game-changer going forward is whether or not we will have evidence to indicate that 1 dose is as effective as 2 doses or 3 doses. If we can vaccinate boys and girls with just 1 dose, then in the next generation or two, we seriously might eradicate not just cervical cancer but a lot of HPV-related malignancies worldwide.
Educating patients, clinicians is key
Dr. Mutch: So it seems education, education, education, with regard to screening guidelines, with regard to the need for continued examinations, and that HPV testing is only a test, it does not supplant overall care. Finally, education regarding eradication of cervical cancer through vaccination.
Dr. Huh: That summarizes it well. We are still going to screen for cervical cancer. We are still going to vaccinate, and providers are still going to manage abnormal Pap tests. It is confusing because we are changing it up it seems every year or 2, so this conversation you and I are having is particularly important for clinicians to understand the basis of that. There has been an explosion of data that has come out in this area in the last decade.
Dr. Mutch: Thank you, Dr. Huh. I really appreciate your thoughts on this. As you all know, Dr. Huh has been President of the ASCCP and is instrumental in writing and disseminating these guidelines, so we are very grateful that he has consented to agree to come and talk with us today.
Dr. Huh: My pleasure. Thank you for inviting me. This was fun, and I have really enjoyed talking to you and participating. ●
- ASCCP Screening Guidelines. https://www.asccp.org /screening-guidelines. Accessed April 25, 2021.
- Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guidelines update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- US Preventive Services Task Force. Screening for cervical cancer. US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:674-686. doi:10.1001/jama .2018.10897.
- American College of Obstetricians and Gynecologists. Practice Advisory: Updated cervical cancer screening guidelines. April 2022. https://www.acog.org/clinical/clinical-guidance /practice-advisory/articles/2021/04/updated-cervical -cancer-screening-guidelines. Accessed April 25, 2022.
- Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP RiskBased Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10 34 .1097/LGT.0000000000000525.
- Yeh PT, Kennedy CE, de Vuyst H, et al. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Global Health. 2019;4:e001351. doi:10.1136/ bmjgh-2018-001351.
- Kjaer SK, Nygard M, Dillner J, et al. A 12-year follow-up on the long-term effectiveness of the quadrivalent human papillomavirus vaccine in 4 Nordic countries. Clin Infect Dis. 2018;66:339-345. doi: 10.1093/cid/cix797.
- Patel C, Brotherton JM, Pillsbury A, et al. The impact of 10 years of human papillomavirus (HPV) vaccination in Australia: what additional disease burden will a nonvalent vaccine prevent? Euro Surveill. 2018;23:1700737. doi: 10 .2807/1560-7917.
- Falcaro M, Castanon A, Ndlela B, et al. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. 2021;398:2084-2092. doi.org/10.1016/S0140-6736(21) 02178-4.
- ASCCP Screening Guidelines. https://www.asccp.org /screening-guidelines. Accessed April 25, 2021.
- Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guidelines update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- US Preventive Services Task Force. Screening for cervical cancer. US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:674-686. doi:10.1001/jama .2018.10897.
- American College of Obstetricians and Gynecologists. Practice Advisory: Updated cervical cancer screening guidelines. April 2022. https://www.acog.org/clinical/clinical-guidance /practice-advisory/articles/2021/04/updated-cervical -cancer-screening-guidelines. Accessed April 25, 2022.
- Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP RiskBased Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10 34 .1097/LGT.0000000000000525.
- Yeh PT, Kennedy CE, de Vuyst H, et al. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Global Health. 2019;4:e001351. doi:10.1136/ bmjgh-2018-001351.
- Kjaer SK, Nygard M, Dillner J, et al. A 12-year follow-up on the long-term effectiveness of the quadrivalent human papillomavirus vaccine in 4 Nordic countries. Clin Infect Dis. 2018;66:339-345. doi: 10.1093/cid/cix797.
- Patel C, Brotherton JM, Pillsbury A, et al. The impact of 10 years of human papillomavirus (HPV) vaccination in Australia: what additional disease burden will a nonvalent vaccine prevent? Euro Surveill. 2018;23:1700737. doi: 10 .2807/1560-7917.
- Falcaro M, Castanon A, Ndlela B, et al. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. 2021;398:2084-2092. doi.org/10.1016/S0140-6736(21) 02178-4.
Minimally invasive surgery for cervical cancer: Is surgeon volume a factor?
The role of minimally invasive surgery for early-stage cervical cancer has been the subject of heated debate since the presentation of the results of the Laparoscopic Approach to Cervical Cancer (LACC) Trial at the Society of Gynecologic Oncology Annual Meeting on Women’s Cancer in 2018. This was an international, randomized, phase 3 trial comparing minimally invasive radical hysterectomy (MH) to open radical hysterectomy (OH) in the treatment of early-stage cervical cancer. The trial was closed early by the study’s Data and Safety Monitoring Committee due to an imbalance of deaths between the groups, with a higher rate in the minimally invasive arm. The final results, which were largely unexpected by the medical community, showed that the disease-free survival (DFS) at 4.5 years was 86.0% in the MH arm and 96.5% in the OH arm, which was a larger difference than their noninferiority cutoff of -7.2 percentage points.1 Results of an epidemiologic study, which used data from the Surveillance, Epidemiology, and End Results (SEER) program and the National Cancer Database, also were presented at this meeting, and they reinforced the findings of the LACC trial.2
The combined results have caused significant concern and confusion from the medical community regarding the clinical implication that minimally invasive surgery may be an unacceptable approach for radical hysterectomy in cervical cancer. Prior to this study, retrospective data supported similar outcomes between the two approaches.3 Additionally, robotic surgery has made radical hysterectomy an option for those with a higher body mass index, as an open radical hysterectomy can be technically challenging in larger patients and result in a higher rate of adverse outcomes.
LACC trial questioned by US surgeons
Many in the United States have questioned the design and conclusions of the LACC trial. This trial was conducted primarily outside of North America and utilized conventional laparoscopic surgery 85% of the time as opposed to robotic surgery. Additionally, the found difference in DFS between MH and OH may have been driven more by the superior performance of the OH group (compared with historical data) than the poorly performing MH group.4 Other criticisms have touched on the low number of overall survival events, the low bar for surgeon volume or skill assessment, and the inability to make conclusions regarding “low-risk” lesions (<2 cm, no lymphovascular space invasion, <1 cm depth of invasion).
Were requirements for surgical skill adequate? Regarding surgeon skill, the LACC trial required documentation of the perioperative outcomes from 10 laparoscopic or robotic radical hysterectomies, as well as 2 unedited videos of each surgeon participating in the study to verify their technique, which some have considered inadequate to sufficiently vet a surgeon’s ability. Additionally, 14 of the 33 centers enrolled in the study accrued 71% of the patients, and concerns about the surgeon volume of the remaining 19 centers have been raised. Finally, there has been discussion about whether the variance in surgical approach can even be adequately assessed in a trial of this nature, as surgical skill is not a binary variable that is easily amenable to randomization. Unlike other trials, which have clear exposure and control arms, no 2 surgeries are exactly alike, and surgical technique is highly variable between surgeons, institutions, and countries.
Continue to: New data evaluate for surgeon volume
New data evaluate for surgeon volume
In an effort to address the concerns regarding surgical approach and expertise, the recently published study by Cusimano and colleagues uses population-based data from Ontario for all women undergoing radical hysterectomy for cervical cancer over a 10-year period from 2006 through 2016.5 The primary outcome was all-cause death, but the study also sought to address whether surgeon volume has an impact on recurrence rates for patients undergoing MH versus OH. To measure this impact the authors stratified surgeon characteristics by technique-specific volume and cervical cancer volume, splitting these volumes at the 50% percentile for low- and high-volume surgeons. They defined technique-specific volume as the number of simple and radical hysterectomies performed in the prior year using the selected approach (MH or OH). Cervical cancer volume was calculated as the number of hysterectomies of any type for cervical cancer in the previous 2 years. The technique-specific volume variable was subsequently re-categorized into tertiles, examined as a continuous variable, and analyzed at the 50th percentile for each year of the study.
Death and recurrence rates better in the OH group. The final cohort included 958 women that were relatively evenly split between MH and OH procedures. Results from their analysis show no difference in terms of all-cause death, cervical cancer–specific death, or recurrence. However, all 3 of these parameters were significantly different in favor of the OH group in women with Stage IB disease, which comprised over half of the overall cohort. Importantly, neither technique-specific volume nor cervical cancer volume had an effect on death or recurrence in Stage IB patients in any of the investigators’ analyses.
Important limitations. There are several limitations to this study that have to be taken into account before drawing any conclusions. Pathologic data were obtained from the database and did not include some important details about the tumor specimens (including specifying subgroups of Stage IA and IB disease, tumor size, presence of lymphovascular space invasion, and depth of stromal invasion). All of these details have been shown to be important prognostic variables in early-stage cervical cancer. Additionally, the MH group included a predominantly laparoscopic approach with only 10% of cases performed robotically, which again brings into question the generalizability of the data.
However, despite some of these shortcomings, the study authors do make a compelling argument that surgeon volume alone does not seem to play a significant role in cancer outcomes after MH.
With surgical approaches hard to compare, turn to careful patient counseling
Definitive assessment of the impact of surgical skill and experience on cervical cancer outcomes is probably an impossible task, as even a perfectly designed trial cannot entirely account for the intricacies of a complex surgical procedure. Variations in tumor characteristics and patient anatomy that affect operative decision making are not likely to be reflected when a patient’s outcome is plugged into a database. As a result, some surgeons and departments have turned to reporting personal or institutional recurrence rates for MH, which they believe may b
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Wang Y, Deng L, Cao L, et al. The outcome of laparoscopy versus laparotomy for the management of early stage cervical cancer-meta analysis. J Minim Invasive Gynecol. 2015;22:S4-S5.
- Leitao MM Jr. The LACC Trial: has minimally invasive surgery for early-stage cervical cancer been dealt a knockout punch? Int J Gynecol Cancer. 2018;28:1248-1250.
- Cusimano MC, Baxter NN, Gien LT, et al. Impact of surgical approach on oncologic outcomes in women undergoing radical hysterectomy for cervical cancer. Am J Obstet Gynecol. July 6, 2019. doi:10.1016/j.ajog.2019.07.009.
The role of minimally invasive surgery for early-stage cervical cancer has been the subject of heated debate since the presentation of the results of the Laparoscopic Approach to Cervical Cancer (LACC) Trial at the Society of Gynecologic Oncology Annual Meeting on Women’s Cancer in 2018. This was an international, randomized, phase 3 trial comparing minimally invasive radical hysterectomy (MH) to open radical hysterectomy (OH) in the treatment of early-stage cervical cancer. The trial was closed early by the study’s Data and Safety Monitoring Committee due to an imbalance of deaths between the groups, with a higher rate in the minimally invasive arm. The final results, which were largely unexpected by the medical community, showed that the disease-free survival (DFS) at 4.5 years was 86.0% in the MH arm and 96.5% in the OH arm, which was a larger difference than their noninferiority cutoff of -7.2 percentage points.1 Results of an epidemiologic study, which used data from the Surveillance, Epidemiology, and End Results (SEER) program and the National Cancer Database, also were presented at this meeting, and they reinforced the findings of the LACC trial.2
The combined results have caused significant concern and confusion from the medical community regarding the clinical implication that minimally invasive surgery may be an unacceptable approach for radical hysterectomy in cervical cancer. Prior to this study, retrospective data supported similar outcomes between the two approaches.3 Additionally, robotic surgery has made radical hysterectomy an option for those with a higher body mass index, as an open radical hysterectomy can be technically challenging in larger patients and result in a higher rate of adverse outcomes.
LACC trial questioned by US surgeons
Many in the United States have questioned the design and conclusions of the LACC trial. This trial was conducted primarily outside of North America and utilized conventional laparoscopic surgery 85% of the time as opposed to robotic surgery. Additionally, the found difference in DFS between MH and OH may have been driven more by the superior performance of the OH group (compared with historical data) than the poorly performing MH group.4 Other criticisms have touched on the low number of overall survival events, the low bar for surgeon volume or skill assessment, and the inability to make conclusions regarding “low-risk” lesions (<2 cm, no lymphovascular space invasion, <1 cm depth of invasion).
Were requirements for surgical skill adequate? Regarding surgeon skill, the LACC trial required documentation of the perioperative outcomes from 10 laparoscopic or robotic radical hysterectomies, as well as 2 unedited videos of each surgeon participating in the study to verify their technique, which some have considered inadequate to sufficiently vet a surgeon’s ability. Additionally, 14 of the 33 centers enrolled in the study accrued 71% of the patients, and concerns about the surgeon volume of the remaining 19 centers have been raised. Finally, there has been discussion about whether the variance in surgical approach can even be adequately assessed in a trial of this nature, as surgical skill is not a binary variable that is easily amenable to randomization. Unlike other trials, which have clear exposure and control arms, no 2 surgeries are exactly alike, and surgical technique is highly variable between surgeons, institutions, and countries.
Continue to: New data evaluate for surgeon volume
New data evaluate for surgeon volume
In an effort to address the concerns regarding surgical approach and expertise, the recently published study by Cusimano and colleagues uses population-based data from Ontario for all women undergoing radical hysterectomy for cervical cancer over a 10-year period from 2006 through 2016.5 The primary outcome was all-cause death, but the study also sought to address whether surgeon volume has an impact on recurrence rates for patients undergoing MH versus OH. To measure this impact the authors stratified surgeon characteristics by technique-specific volume and cervical cancer volume, splitting these volumes at the 50% percentile for low- and high-volume surgeons. They defined technique-specific volume as the number of simple and radical hysterectomies performed in the prior year using the selected approach (MH or OH). Cervical cancer volume was calculated as the number of hysterectomies of any type for cervical cancer in the previous 2 years. The technique-specific volume variable was subsequently re-categorized into tertiles, examined as a continuous variable, and analyzed at the 50th percentile for each year of the study.
Death and recurrence rates better in the OH group. The final cohort included 958 women that were relatively evenly split between MH and OH procedures. Results from their analysis show no difference in terms of all-cause death, cervical cancer–specific death, or recurrence. However, all 3 of these parameters were significantly different in favor of the OH group in women with Stage IB disease, which comprised over half of the overall cohort. Importantly, neither technique-specific volume nor cervical cancer volume had an effect on death or recurrence in Stage IB patients in any of the investigators’ analyses.
Important limitations. There are several limitations to this study that have to be taken into account before drawing any conclusions. Pathologic data were obtained from the database and did not include some important details about the tumor specimens (including specifying subgroups of Stage IA and IB disease, tumor size, presence of lymphovascular space invasion, and depth of stromal invasion). All of these details have been shown to be important prognostic variables in early-stage cervical cancer. Additionally, the MH group included a predominantly laparoscopic approach with only 10% of cases performed robotically, which again brings into question the generalizability of the data.
However, despite some of these shortcomings, the study authors do make a compelling argument that surgeon volume alone does not seem to play a significant role in cancer outcomes after MH.
With surgical approaches hard to compare, turn to careful patient counseling
Definitive assessment of the impact of surgical skill and experience on cervical cancer outcomes is probably an impossible task, as even a perfectly designed trial cannot entirely account for the intricacies of a complex surgical procedure. Variations in tumor characteristics and patient anatomy that affect operative decision making are not likely to be reflected when a patient’s outcome is plugged into a database. As a result, some surgeons and departments have turned to reporting personal or institutional recurrence rates for MH, which they believe may b
The role of minimally invasive surgery for early-stage cervical cancer has been the subject of heated debate since the presentation of the results of the Laparoscopic Approach to Cervical Cancer (LACC) Trial at the Society of Gynecologic Oncology Annual Meeting on Women’s Cancer in 2018. This was an international, randomized, phase 3 trial comparing minimally invasive radical hysterectomy (MH) to open radical hysterectomy (OH) in the treatment of early-stage cervical cancer. The trial was closed early by the study’s Data and Safety Monitoring Committee due to an imbalance of deaths between the groups, with a higher rate in the minimally invasive arm. The final results, which were largely unexpected by the medical community, showed that the disease-free survival (DFS) at 4.5 years was 86.0% in the MH arm and 96.5% in the OH arm, which was a larger difference than their noninferiority cutoff of -7.2 percentage points.1 Results of an epidemiologic study, which used data from the Surveillance, Epidemiology, and End Results (SEER) program and the National Cancer Database, also were presented at this meeting, and they reinforced the findings of the LACC trial.2
The combined results have caused significant concern and confusion from the medical community regarding the clinical implication that minimally invasive surgery may be an unacceptable approach for radical hysterectomy in cervical cancer. Prior to this study, retrospective data supported similar outcomes between the two approaches.3 Additionally, robotic surgery has made radical hysterectomy an option for those with a higher body mass index, as an open radical hysterectomy can be technically challenging in larger patients and result in a higher rate of adverse outcomes.
LACC trial questioned by US surgeons
Many in the United States have questioned the design and conclusions of the LACC trial. This trial was conducted primarily outside of North America and utilized conventional laparoscopic surgery 85% of the time as opposed to robotic surgery. Additionally, the found difference in DFS between MH and OH may have been driven more by the superior performance of the OH group (compared with historical data) than the poorly performing MH group.4 Other criticisms have touched on the low number of overall survival events, the low bar for surgeon volume or skill assessment, and the inability to make conclusions regarding “low-risk” lesions (<2 cm, no lymphovascular space invasion, <1 cm depth of invasion).
Were requirements for surgical skill adequate? Regarding surgeon skill, the LACC trial required documentation of the perioperative outcomes from 10 laparoscopic or robotic radical hysterectomies, as well as 2 unedited videos of each surgeon participating in the study to verify their technique, which some have considered inadequate to sufficiently vet a surgeon’s ability. Additionally, 14 of the 33 centers enrolled in the study accrued 71% of the patients, and concerns about the surgeon volume of the remaining 19 centers have been raised. Finally, there has been discussion about whether the variance in surgical approach can even be adequately assessed in a trial of this nature, as surgical skill is not a binary variable that is easily amenable to randomization. Unlike other trials, which have clear exposure and control arms, no 2 surgeries are exactly alike, and surgical technique is highly variable between surgeons, institutions, and countries.
Continue to: New data evaluate for surgeon volume
New data evaluate for surgeon volume
In an effort to address the concerns regarding surgical approach and expertise, the recently published study by Cusimano and colleagues uses population-based data from Ontario for all women undergoing radical hysterectomy for cervical cancer over a 10-year period from 2006 through 2016.5 The primary outcome was all-cause death, but the study also sought to address whether surgeon volume has an impact on recurrence rates for patients undergoing MH versus OH. To measure this impact the authors stratified surgeon characteristics by technique-specific volume and cervical cancer volume, splitting these volumes at the 50% percentile for low- and high-volume surgeons. They defined technique-specific volume as the number of simple and radical hysterectomies performed in the prior year using the selected approach (MH or OH). Cervical cancer volume was calculated as the number of hysterectomies of any type for cervical cancer in the previous 2 years. The technique-specific volume variable was subsequently re-categorized into tertiles, examined as a continuous variable, and analyzed at the 50th percentile for each year of the study.
Death and recurrence rates better in the OH group. The final cohort included 958 women that were relatively evenly split between MH and OH procedures. Results from their analysis show no difference in terms of all-cause death, cervical cancer–specific death, or recurrence. However, all 3 of these parameters were significantly different in favor of the OH group in women with Stage IB disease, which comprised over half of the overall cohort. Importantly, neither technique-specific volume nor cervical cancer volume had an effect on death or recurrence in Stage IB patients in any of the investigators’ analyses.
Important limitations. There are several limitations to this study that have to be taken into account before drawing any conclusions. Pathologic data were obtained from the database and did not include some important details about the tumor specimens (including specifying subgroups of Stage IA and IB disease, tumor size, presence of lymphovascular space invasion, and depth of stromal invasion). All of these details have been shown to be important prognostic variables in early-stage cervical cancer. Additionally, the MH group included a predominantly laparoscopic approach with only 10% of cases performed robotically, which again brings into question the generalizability of the data.
However, despite some of these shortcomings, the study authors do make a compelling argument that surgeon volume alone does not seem to play a significant role in cancer outcomes after MH.
With surgical approaches hard to compare, turn to careful patient counseling
Definitive assessment of the impact of surgical skill and experience on cervical cancer outcomes is probably an impossible task, as even a perfectly designed trial cannot entirely account for the intricacies of a complex surgical procedure. Variations in tumor characteristics and patient anatomy that affect operative decision making are not likely to be reflected when a patient’s outcome is plugged into a database. As a result, some surgeons and departments have turned to reporting personal or institutional recurrence rates for MH, which they believe may b
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Wang Y, Deng L, Cao L, et al. The outcome of laparoscopy versus laparotomy for the management of early stage cervical cancer-meta analysis. J Minim Invasive Gynecol. 2015;22:S4-S5.
- Leitao MM Jr. The LACC Trial: has minimally invasive surgery for early-stage cervical cancer been dealt a knockout punch? Int J Gynecol Cancer. 2018;28:1248-1250.
- Cusimano MC, Baxter NN, Gien LT, et al. Impact of surgical approach on oncologic outcomes in women undergoing radical hysterectomy for cervical cancer. Am J Obstet Gynecol. July 6, 2019. doi:10.1016/j.ajog.2019.07.009.
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Wang Y, Deng L, Cao L, et al. The outcome of laparoscopy versus laparotomy for the management of early stage cervical cancer-meta analysis. J Minim Invasive Gynecol. 2015;22:S4-S5.
- Leitao MM Jr. The LACC Trial: has minimally invasive surgery for early-stage cervical cancer been dealt a knockout punch? Int J Gynecol Cancer. 2018;28:1248-1250.
- Cusimano MC, Baxter NN, Gien LT, et al. Impact of surgical approach on oncologic outcomes in women undergoing radical hysterectomy for cervical cancer. Am J Obstet Gynecol. July 6, 2019. doi:10.1016/j.ajog.2019.07.009.
Does HPV testing lead to improved diagnosis of cervical dysplasia for patients with ASC-US cytology?
EXPERT COMMENTARY
The American Society for Colposcopy and Cervical Pathology (ASCCP) has recommended HPV triage for ASC-US cytology for more than 15 years. Since the ALTS trial demonstrated improved detection of CIN2+ in women with ASC-US cytology, HPV testing has become the preferred triage strategy for women with ASC-US cytology, except for women under age 25.1 However, we do not know the long-term outcomes for these women. The study by Cuzick and colleagues uniquely addresses this question.
Details of the study
The retrospective review of data from the New Mexico HPV Pap Registry examined the influence of HPV testing on outcomes in 20,677 women with ASC-US cytology between 2008 and 2012. Of those women, 80.5% had an HPV test, and the authors estimated that 80.6% of those HPV tests were for triage after ASC-US cytology as opposed to co-testing (that is, cytology and HPV testing together). Of note, the majority of these Pap tests were performed prior to the 2012 ASCCP guidelines that recommend HPV co-testing for all women aged 30 to 64 years regardless of cytology. Of the HPV tests performed, 43.1% were positive. The investigators then examined rates of CIN in the interval between ASC-US cytology and biopsy-confirmed CIN, and rates of loop electrosurgical excision procedures (LEEP) and results at 5 years.
The investigators found a non–statistically significant increase in overall detection of CIN3 (relative risk [RR], 1.16; 95% confidence interval [CI], 0.92–1.45) in women who had been triaged with HPV testing, and a significant increase in overall detection of CIN2 (RR, 1.27; 95% CI, 1.06–1.53) and CIN1 (RR, 1.76; 95% CI, 1.56–2.00). CIN1, CIN2, and CIN3 were detected significantly earlier in patients with HPV testing. As expected, the majority of CIN2 and CIN3 was diagnosed in women who were HPV positive.
Related article:
2017 Update on cervical disease
The proportion of women undergoing either endocervical curettage or cervical biopsy was higher in those with HPV testing (32.1% vs 20.6%, P<.001), as were LEEP rates (4.9% vs 4.0%, P = .03). LEEP rates were highest in the year after a positive HPV test and were mostly attributable to CIN1 results. However, the overall ratio of LEEP to CIN3+ diagnosis was similar in women who were tested for HPV compared with those who were not. A larger proportion of patients with HPV testing had follow-up compared with those without HPV testing (84.1% vs 78.9%, P<.001).
The authors concluded that HPV testing in women with ASC-US cytology leads to detecting high-grade disease earlier, but that HPV positivity results in more interventions, largely due to an overdiagnosis of CIN1. They also confirmed that the majority of high-grade lesions are found in women with positive HPV tests.
Related article:
2015 Update on cervical disease: New ammo for HPV prevention and screening
Study strengths and weaknesses
This is the first comprehensive long-term look at women with ASC-US cytology and the impact of HPV testing. The New Mexico HPV Pap Registry is the only US state-based registry with comprehensive follow-up data. This study’s results build on previous data that showed sensitivity is increased with the addition of HPV testing to cervical cytology,1 and they support current ASCCP guidelines that emphasize HPV triage or co-testing for women age 25 or older.
Potential bias. While this study has the benefit of a large cohort, it is limited by biases inherent in retrospective study design. One important potential bias is the differential utilization of HPV testing or procedures by providers. The authors acknowledge preliminary analyses that show that some clinics (rural, federally qualified health centers, public health clinics) serving underserved populations may underutilize or inappropriately utilize HPV testing.
Further, the 2008–2012 study period may make the results less generalizable to current practices since the ASCCP guidelines were adjusted to include more HPV testing in women aged 25 and older in 2012.
Finally, this study examines CIN but does not specifically look at the impact of HPV testing on the ultimate outcome of interest, cervical cancer rates.
The data from the study by Cuzick and colleagues support the importance of continued screening for cervical cancer and its precursors with HPV testing. However, the results also show that we need to improve our strategies for stratifying patients who actually need colposcopy. The authors assert an "enormous predictive value of HPV testing," but this comes at the expense of many unnecessary procedures. Clinicians should continue to use cytology with HPV triage in women aged 25 years and older, but the ASCCP should reconsider guidelines to improve screening specificity. The addition of other screening modalities, such as extended genotyping, methylation testing, and p16/Ki-67 staining, are considerations for ASC-US triage.
-- Sarah Dilley, MD, MPH, and Warner K. Huh, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- ASCUS-LSIL Triage Study (ALTS) Group. Results of a randomized trial on the management of cytology interpretations of atypical squamous cells of undetermined significance. Am J Obstet Gynecol. 2003;188(6):1383–1392.
EXPERT COMMENTARY
The American Society for Colposcopy and Cervical Pathology (ASCCP) has recommended HPV triage for ASC-US cytology for more than 15 years. Since the ALTS trial demonstrated improved detection of CIN2+ in women with ASC-US cytology, HPV testing has become the preferred triage strategy for women with ASC-US cytology, except for women under age 25.1 However, we do not know the long-term outcomes for these women. The study by Cuzick and colleagues uniquely addresses this question.
Details of the study
The retrospective review of data from the New Mexico HPV Pap Registry examined the influence of HPV testing on outcomes in 20,677 women with ASC-US cytology between 2008 and 2012. Of those women, 80.5% had an HPV test, and the authors estimated that 80.6% of those HPV tests were for triage after ASC-US cytology as opposed to co-testing (that is, cytology and HPV testing together). Of note, the majority of these Pap tests were performed prior to the 2012 ASCCP guidelines that recommend HPV co-testing for all women aged 30 to 64 years regardless of cytology. Of the HPV tests performed, 43.1% were positive. The investigators then examined rates of CIN in the interval between ASC-US cytology and biopsy-confirmed CIN, and rates of loop electrosurgical excision procedures (LEEP) and results at 5 years.
The investigators found a non–statistically significant increase in overall detection of CIN3 (relative risk [RR], 1.16; 95% confidence interval [CI], 0.92–1.45) in women who had been triaged with HPV testing, and a significant increase in overall detection of CIN2 (RR, 1.27; 95% CI, 1.06–1.53) and CIN1 (RR, 1.76; 95% CI, 1.56–2.00). CIN1, CIN2, and CIN3 were detected significantly earlier in patients with HPV testing. As expected, the majority of CIN2 and CIN3 was diagnosed in women who were HPV positive.
Related article:
2017 Update on cervical disease
The proportion of women undergoing either endocervical curettage or cervical biopsy was higher in those with HPV testing (32.1% vs 20.6%, P<.001), as were LEEP rates (4.9% vs 4.0%, P = .03). LEEP rates were highest in the year after a positive HPV test and were mostly attributable to CIN1 results. However, the overall ratio of LEEP to CIN3+ diagnosis was similar in women who were tested for HPV compared with those who were not. A larger proportion of patients with HPV testing had follow-up compared with those without HPV testing (84.1% vs 78.9%, P<.001).
The authors concluded that HPV testing in women with ASC-US cytology leads to detecting high-grade disease earlier, but that HPV positivity results in more interventions, largely due to an overdiagnosis of CIN1. They also confirmed that the majority of high-grade lesions are found in women with positive HPV tests.
Related article:
2015 Update on cervical disease: New ammo for HPV prevention and screening
Study strengths and weaknesses
This is the first comprehensive long-term look at women with ASC-US cytology and the impact of HPV testing. The New Mexico HPV Pap Registry is the only US state-based registry with comprehensive follow-up data. This study’s results build on previous data that showed sensitivity is increased with the addition of HPV testing to cervical cytology,1 and they support current ASCCP guidelines that emphasize HPV triage or co-testing for women age 25 or older.
Potential bias. While this study has the benefit of a large cohort, it is limited by biases inherent in retrospective study design. One important potential bias is the differential utilization of HPV testing or procedures by providers. The authors acknowledge preliminary analyses that show that some clinics (rural, federally qualified health centers, public health clinics) serving underserved populations may underutilize or inappropriately utilize HPV testing.
Further, the 2008–2012 study period may make the results less generalizable to current practices since the ASCCP guidelines were adjusted to include more HPV testing in women aged 25 and older in 2012.
Finally, this study examines CIN but does not specifically look at the impact of HPV testing on the ultimate outcome of interest, cervical cancer rates.
The data from the study by Cuzick and colleagues support the importance of continued screening for cervical cancer and its precursors with HPV testing. However, the results also show that we need to improve our strategies for stratifying patients who actually need colposcopy. The authors assert an "enormous predictive value of HPV testing," but this comes at the expense of many unnecessary procedures. Clinicians should continue to use cytology with HPV triage in women aged 25 years and older, but the ASCCP should reconsider guidelines to improve screening specificity. The addition of other screening modalities, such as extended genotyping, methylation testing, and p16/Ki-67 staining, are considerations for ASC-US triage.
-- Sarah Dilley, MD, MPH, and Warner K. Huh, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
The American Society for Colposcopy and Cervical Pathology (ASCCP) has recommended HPV triage for ASC-US cytology for more than 15 years. Since the ALTS trial demonstrated improved detection of CIN2+ in women with ASC-US cytology, HPV testing has become the preferred triage strategy for women with ASC-US cytology, except for women under age 25.1 However, we do not know the long-term outcomes for these women. The study by Cuzick and colleagues uniquely addresses this question.
Details of the study
The retrospective review of data from the New Mexico HPV Pap Registry examined the influence of HPV testing on outcomes in 20,677 women with ASC-US cytology between 2008 and 2012. Of those women, 80.5% had an HPV test, and the authors estimated that 80.6% of those HPV tests were for triage after ASC-US cytology as opposed to co-testing (that is, cytology and HPV testing together). Of note, the majority of these Pap tests were performed prior to the 2012 ASCCP guidelines that recommend HPV co-testing for all women aged 30 to 64 years regardless of cytology. Of the HPV tests performed, 43.1% were positive. The investigators then examined rates of CIN in the interval between ASC-US cytology and biopsy-confirmed CIN, and rates of loop electrosurgical excision procedures (LEEP) and results at 5 years.
The investigators found a non–statistically significant increase in overall detection of CIN3 (relative risk [RR], 1.16; 95% confidence interval [CI], 0.92–1.45) in women who had been triaged with HPV testing, and a significant increase in overall detection of CIN2 (RR, 1.27; 95% CI, 1.06–1.53) and CIN1 (RR, 1.76; 95% CI, 1.56–2.00). CIN1, CIN2, and CIN3 were detected significantly earlier in patients with HPV testing. As expected, the majority of CIN2 and CIN3 was diagnosed in women who were HPV positive.
Related article:
2017 Update on cervical disease
The proportion of women undergoing either endocervical curettage or cervical biopsy was higher in those with HPV testing (32.1% vs 20.6%, P<.001), as were LEEP rates (4.9% vs 4.0%, P = .03). LEEP rates were highest in the year after a positive HPV test and were mostly attributable to CIN1 results. However, the overall ratio of LEEP to CIN3+ diagnosis was similar in women who were tested for HPV compared with those who were not. A larger proportion of patients with HPV testing had follow-up compared with those without HPV testing (84.1% vs 78.9%, P<.001).
The authors concluded that HPV testing in women with ASC-US cytology leads to detecting high-grade disease earlier, but that HPV positivity results in more interventions, largely due to an overdiagnosis of CIN1. They also confirmed that the majority of high-grade lesions are found in women with positive HPV tests.
Related article:
2015 Update on cervical disease: New ammo for HPV prevention and screening
Study strengths and weaknesses
This is the first comprehensive long-term look at women with ASC-US cytology and the impact of HPV testing. The New Mexico HPV Pap Registry is the only US state-based registry with comprehensive follow-up data. This study’s results build on previous data that showed sensitivity is increased with the addition of HPV testing to cervical cytology,1 and they support current ASCCP guidelines that emphasize HPV triage or co-testing for women age 25 or older.
Potential bias. While this study has the benefit of a large cohort, it is limited by biases inherent in retrospective study design. One important potential bias is the differential utilization of HPV testing or procedures by providers. The authors acknowledge preliminary analyses that show that some clinics (rural, federally qualified health centers, public health clinics) serving underserved populations may underutilize or inappropriately utilize HPV testing.
Further, the 2008–2012 study period may make the results less generalizable to current practices since the ASCCP guidelines were adjusted to include more HPV testing in women aged 25 and older in 2012.
Finally, this study examines CIN but does not specifically look at the impact of HPV testing on the ultimate outcome of interest, cervical cancer rates.
The data from the study by Cuzick and colleagues support the importance of continued screening for cervical cancer and its precursors with HPV testing. However, the results also show that we need to improve our strategies for stratifying patients who actually need colposcopy. The authors assert an "enormous predictive value of HPV testing," but this comes at the expense of many unnecessary procedures. Clinicians should continue to use cytology with HPV triage in women aged 25 years and older, but the ASCCP should reconsider guidelines to improve screening specificity. The addition of other screening modalities, such as extended genotyping, methylation testing, and p16/Ki-67 staining, are considerations for ASC-US triage.
-- Sarah Dilley, MD, MPH, and Warner K. Huh, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- ASCUS-LSIL Triage Study (ALTS) Group. Results of a randomized trial on the management of cytology interpretations of atypical squamous cells of undetermined significance. Am J Obstet Gynecol. 2003;188(6):1383–1392.
- ASCUS-LSIL Triage Study (ALTS) Group. Results of a randomized trial on the management of cytology interpretations of atypical squamous cells of undetermined significance. Am J Obstet Gynecol. 2003;188(6):1383–1392.
Fast Tracks
- To assess long-term outcomes of women with ASC-US cytology and HPV triage, researchers examined the interval between ASC-US cytology and biopsy-confirmed CIN, LEEP rates, and results at 5 years
- HPV testing in women with ASC-US cytology leads to earlier detection of high-grade disease, but HPV positivity results in more interventions, largely due to overdiagnosis of CIN1