User login
Petrolatum Is Effective as a Moisturizer, But There Are More Uses for It
Petrolatum recently has received substantial social media attention. In the last year, the number of TikTok and Instagram videos mentioning petrolatum increased by 46% and 93%, respectively. According to Unilever, the company that manufactures Vaseline, mentions of the product have gone up by 327% on social media compared to last year largely due to a trend known as “slugging,” or the practice of slathering on petrolatum overnight to improve skin hydration.1 However, petrolatum has a variety of other uses. Given its increase in popularity, we review the many uses of petrolatum within dermatology.
The main reason for petrolatum’s presence on social media is its effectiveness as a moisturizer, which is due to its occlusive property. Its oil-based nature allows it to seal water in the skin by creating a hydrophobic barrier that decreases transepidermal water loss (TEWL). Among available oil-based moisturizers, petrolatum is the most effective in reducing TEWL by 98%, while others only provide reductions of 20% to 30%,2 which makes it ideal for soothing itch and irritation in several skin conditions, including dry skin, cheilitis, chafing, and diaper rash. Petrolatum is particularly helpful in sensitive areas where the skin is thinner, such as the eyelids or lips, as it is less irritating than lotions.
Petrolatum also may be used to treat dry skin and mild atopic dermatitis with the soak-and-smear technique,3 which entails soaking the affected skin—or the entire body, if needed—in a plain water bath for 20 minutes and then immediately smearing the skin with petrolatum. Soaking hydrates the damaged stratum corneum and enhances desquamation. The moist stratum corneum absorbs topical treatments more effectively, and desquamation leaves a thinner stratum corneum for the product to traverse. Smearing with petrolatum then traps the moisture in the skin and thus has a dual function by both delivering the petrolatum to the skin and trapping the moisture from the soak. The result is decreased TEWL, improved hydration, and increased penetration, thereby enhancing skin barrier repair.3,4
Smearing solely with petrolatum is effective in cases not accompanied by considerable inflammation. In cases involving notable inflammation or severe xerosis, a steroidal ointment may be required.3 This generally is done for several nights to 2 weeks before conversion to maintenance therapy. In these cases, petrolatum may then be used as maintenance therapy or bridge therapy for maintenance with simple moisturizers, which decreases recurrence and flares of dermatitis and also prevents continuous exposure to steroidal agents that can result in atrophy and purpura at application sites. The soak-and-smear technique has been found to be effective, with 90% of patients having 90% to 100% clearance.3
Petrolatum also is particularly useful for wound healing. A study on the molecular responses induced by petrolatum found that it significantly upregulated innate immune genes (P<.01), increased antimicrobial peptides (P<.001), and improved epidermal differentiation.5 Additionally, it keeps wound edges moist, which enhances angiogenesis, improves collagen synthesis, and increases the breakdown of dead tissue and fibrin.6 It also prevents scab formation, which can prolong healing time.7
Petrolatum is superior to antibiotic use after clean cutaneous surgery given its excellent safety profile. In one randomized controlled trial comparing petrolatum to bacitracin, petrolatum was found to be just as effective for wound healing with a similar infection rate. Although 4 patients developed allergic contact dermatitis (ACD) with bacitracin use, no patients who used petrolatum developed ACD.8 There are numerous other reports of bacitracin causing ACD,9,10 with a prevalence as high as 22% in chronic leg ulcer patients.10 There are even multiple reports of bacitracin causing contact urticaria and life-threatening anaphylaxis.11 In the most recent report from the North American Contact Dermatitis Group’s list of top allergens, bacitracin placed 11th with an ACD prevalence of 5.5%. Neomycin, another common postwound emollient, has similar adverse effects and ranked 12th with an ACD prevalence of 5.4%.12 Despite the risk for ACD with antibiotics, one study on wound care handouts from dermatologists (N=169) found that nearly half (43%) still advocated for the use of antibiotics.13 Likewise, another study among nondermatologists found that 40% (10/25) recommended the use of antibiotics for wound care14 despite strong evidence that topical antibiotics in clean dermatologic procedures offer no additional benefit compared with petrolatum. Additionally, topical antibiotics carry a risk of antibiotic resistance, adverse reactions such as ACD and anaphylaxis, and higher health care costs.9 Thus, petrolatum should be used as standard care after clean cutaneous procedures, and the application of antibiotics should be abandoned.
Petrolatum also is an effective treatment for pruritus scroti.15 It is particularly helpful for recalcitrant disease when several topical medications have failed or ACD or irritant contact dermatitis to medications or cleansing products is suspected. Although topical corticosteroids are the mainstay of treatment, severe burning or redness may occur with prolonged use of these medications, thus it often is useful to discontinue topical medications and treat with plain water sitz baths at night followed by petrolatum immediately applied over wet skin. This approach has several benefits, including soothing the area, providing an occlusive barrier, retaining moisture, and eliminating contact with steroids and potential allergens and irritants. This may be followed with patch testing to determine if ACD from cleansing products or medications is the culprit. This treatment also may be used in pruritus ani or pruritus vulvae.15
Finally, petrolatum may even be used to treat parasitic skin infections such as cutaneous furuncular myiasis,16 a condition most commonly caused by the human botfly (Dermatobia hominis) or the African tumbu fly (Cordylobia anthropophaga). The larvae infest the skin by penetrating the dermis and burrowing into the subdermal layer. It is characterized by furuncular nodules with a central black punctum formed by larvae burrowed underneath the skin. An inflammatory reaction occurs in the sites surrounding the larvae with erythematous, edematous, and tender skin. Symptoms range from mild pruritus and a prickly heat sensation to intense cutaneous pain, agitation, and insomnia. Occluding the punctum, or breathing hole, of the infectious organism with petrolatum will asphyxiate the larvae, causing it to emerge within and leading to definitive diagnosis and treatment. This permits rapid removal and avoids extensive incision and extraction.16
The increased social media attention of petrolatum has raised the awareness of its utility as a moisturizer; however, it has many other uses, including soothing itch and irritation, improving wound healing, alleviating scrotal itch, and treating parasitic skin infections. It not only is an effective product but also is a particularly safe one. Petrolatum is well deserving of its positive reputation in dermatology and its current popularity among the general public
- Cramer M. A staple of grandma’s medicine cabinet gets hot on TikTok. New York Times. Published February 11, 2022. Accessed September 15, 2022. https://www.nytimes.com/2022/02/11/business/vaseline-slugging-tiktok.html
- Sethi A, Kaur T, Malhotra SK, et al. Moisturizers: the slippery road. Indian J Dermatol. 2016;61:279-287. doi:10.4103/0019-5154.182427
- Gutman AB, Kligman AM, Sciacca J, et al. Soak and smear: a standard technique revisited. 2005;141:1556-1559. doi:10.1001/archderm.141.12.1556
- Ghadially R, Halkier-Sorensen L, Elias PM. Effects of petrolatum on stratum corneum structure and function. J Am Acad Dermatol. 1992;26:387-396. doi:10.1016/0190-9622(92)70060-S
- Czarnowicki T, Malajian D, Khattri S, et al. Petrolatum: barrier repair and antimicrobial responses underlying this “inert” moisturizer. J Allergy Clin Immunol. 2016;137:1091-1102.e7. doi:10.1016/j.jaci.2015.08.013
- Field CK, Kerstein MD. Overview of wound healing in a moist environment. Am J Surg. 1994;167:2S-6S.
- Winter GD. Some factors affecting skin and wound healing. J Tissue Viability. 2006;16:20-23. doi:10.1016/S0965-206X(06)62006-8
- Smack DP, Harrington AC, Dunn C, et al. Infection and allergy incidence in ambulatory surgery patients using white petrolatum vs bacitracin ointment. a randomized controlled trial. JAMA. 1996;276:972-977.
- Jacob SE, James WD. From road rash to top allergen in a flash: bacitracin. 2004;30(4 pt 1):521-524. doi:10.1111/j.1524-4725.2004.30168.x..
- Zaki I, Shall L, Dalziel KL. Bacitracin: a significant sensitizer in leg ulcer patients? Contact Dermatitis. 1994;31:92-94. doi:10.1111/j.1600-0536.1994.tb01924.x
- Farley M, Pak H, Carregal V, et al. Anaphylaxis to topically applied bacitracin. Am J Contact Dermatitis. 1995;6:28-31. doi:10.1016/1046-199X(95)90066-7
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Nguyen JK, Huang A, Siegel DM, et al. Variability in wound care recommendations following dermatologic procedures. Dermatol Surg. 2020;46:186-191. doi:10.1097/DSS.0000000000001952
- Fathy R, Chu B, Singh P, et al. Variation in topical antibiotics recommendations in wound care instructions by non-dermatologists. J Gen Intern Med. 2021;36:238-239. doi:10.1007/s11606-020-05689-2
- James WD, Elston DM, Treat JR, et al. Andrews’ Diseases of the Skin. 13th ed. Elsevier; 2020.
- Ockenhouse CF, Samlaska CP, Benson PM, et al. Cutaneous myiasis caused by the African tumbu fly (Cordylobia anthropophaga). Arch Dermatol. 1990;126:199-202.
Petrolatum recently has received substantial social media attention. In the last year, the number of TikTok and Instagram videos mentioning petrolatum increased by 46% and 93%, respectively. According to Unilever, the company that manufactures Vaseline, mentions of the product have gone up by 327% on social media compared to last year largely due to a trend known as “slugging,” or the practice of slathering on petrolatum overnight to improve skin hydration.1 However, petrolatum has a variety of other uses. Given its increase in popularity, we review the many uses of petrolatum within dermatology.
The main reason for petrolatum’s presence on social media is its effectiveness as a moisturizer, which is due to its occlusive property. Its oil-based nature allows it to seal water in the skin by creating a hydrophobic barrier that decreases transepidermal water loss (TEWL). Among available oil-based moisturizers, petrolatum is the most effective in reducing TEWL by 98%, while others only provide reductions of 20% to 30%,2 which makes it ideal for soothing itch and irritation in several skin conditions, including dry skin, cheilitis, chafing, and diaper rash. Petrolatum is particularly helpful in sensitive areas where the skin is thinner, such as the eyelids or lips, as it is less irritating than lotions.
Petrolatum also may be used to treat dry skin and mild atopic dermatitis with the soak-and-smear technique,3 which entails soaking the affected skin—or the entire body, if needed—in a plain water bath for 20 minutes and then immediately smearing the skin with petrolatum. Soaking hydrates the damaged stratum corneum and enhances desquamation. The moist stratum corneum absorbs topical treatments more effectively, and desquamation leaves a thinner stratum corneum for the product to traverse. Smearing with petrolatum then traps the moisture in the skin and thus has a dual function by both delivering the petrolatum to the skin and trapping the moisture from the soak. The result is decreased TEWL, improved hydration, and increased penetration, thereby enhancing skin barrier repair.3,4
Smearing solely with petrolatum is effective in cases not accompanied by considerable inflammation. In cases involving notable inflammation or severe xerosis, a steroidal ointment may be required.3 This generally is done for several nights to 2 weeks before conversion to maintenance therapy. In these cases, petrolatum may then be used as maintenance therapy or bridge therapy for maintenance with simple moisturizers, which decreases recurrence and flares of dermatitis and also prevents continuous exposure to steroidal agents that can result in atrophy and purpura at application sites. The soak-and-smear technique has been found to be effective, with 90% of patients having 90% to 100% clearance.3
Petrolatum also is particularly useful for wound healing. A study on the molecular responses induced by petrolatum found that it significantly upregulated innate immune genes (P<.01), increased antimicrobial peptides (P<.001), and improved epidermal differentiation.5 Additionally, it keeps wound edges moist, which enhances angiogenesis, improves collagen synthesis, and increases the breakdown of dead tissue and fibrin.6 It also prevents scab formation, which can prolong healing time.7
Petrolatum is superior to antibiotic use after clean cutaneous surgery given its excellent safety profile. In one randomized controlled trial comparing petrolatum to bacitracin, petrolatum was found to be just as effective for wound healing with a similar infection rate. Although 4 patients developed allergic contact dermatitis (ACD) with bacitracin use, no patients who used petrolatum developed ACD.8 There are numerous other reports of bacitracin causing ACD,9,10 with a prevalence as high as 22% in chronic leg ulcer patients.10 There are even multiple reports of bacitracin causing contact urticaria and life-threatening anaphylaxis.11 In the most recent report from the North American Contact Dermatitis Group’s list of top allergens, bacitracin placed 11th with an ACD prevalence of 5.5%. Neomycin, another common postwound emollient, has similar adverse effects and ranked 12th with an ACD prevalence of 5.4%.12 Despite the risk for ACD with antibiotics, one study on wound care handouts from dermatologists (N=169) found that nearly half (43%) still advocated for the use of antibiotics.13 Likewise, another study among nondermatologists found that 40% (10/25) recommended the use of antibiotics for wound care14 despite strong evidence that topical antibiotics in clean dermatologic procedures offer no additional benefit compared with petrolatum. Additionally, topical antibiotics carry a risk of antibiotic resistance, adverse reactions such as ACD and anaphylaxis, and higher health care costs.9 Thus, petrolatum should be used as standard care after clean cutaneous procedures, and the application of antibiotics should be abandoned.
Petrolatum also is an effective treatment for pruritus scroti.15 It is particularly helpful for recalcitrant disease when several topical medications have failed or ACD or irritant contact dermatitis to medications or cleansing products is suspected. Although topical corticosteroids are the mainstay of treatment, severe burning or redness may occur with prolonged use of these medications, thus it often is useful to discontinue topical medications and treat with plain water sitz baths at night followed by petrolatum immediately applied over wet skin. This approach has several benefits, including soothing the area, providing an occlusive barrier, retaining moisture, and eliminating contact with steroids and potential allergens and irritants. This may be followed with patch testing to determine if ACD from cleansing products or medications is the culprit. This treatment also may be used in pruritus ani or pruritus vulvae.15
Finally, petrolatum may even be used to treat parasitic skin infections such as cutaneous furuncular myiasis,16 a condition most commonly caused by the human botfly (Dermatobia hominis) or the African tumbu fly (Cordylobia anthropophaga). The larvae infest the skin by penetrating the dermis and burrowing into the subdermal layer. It is characterized by furuncular nodules with a central black punctum formed by larvae burrowed underneath the skin. An inflammatory reaction occurs in the sites surrounding the larvae with erythematous, edematous, and tender skin. Symptoms range from mild pruritus and a prickly heat sensation to intense cutaneous pain, agitation, and insomnia. Occluding the punctum, or breathing hole, of the infectious organism with petrolatum will asphyxiate the larvae, causing it to emerge within and leading to definitive diagnosis and treatment. This permits rapid removal and avoids extensive incision and extraction.16
The increased social media attention of petrolatum has raised the awareness of its utility as a moisturizer; however, it has many other uses, including soothing itch and irritation, improving wound healing, alleviating scrotal itch, and treating parasitic skin infections. It not only is an effective product but also is a particularly safe one. Petrolatum is well deserving of its positive reputation in dermatology and its current popularity among the general public
Petrolatum recently has received substantial social media attention. In the last year, the number of TikTok and Instagram videos mentioning petrolatum increased by 46% and 93%, respectively. According to Unilever, the company that manufactures Vaseline, mentions of the product have gone up by 327% on social media compared to last year largely due to a trend known as “slugging,” or the practice of slathering on petrolatum overnight to improve skin hydration.1 However, petrolatum has a variety of other uses. Given its increase in popularity, we review the many uses of petrolatum within dermatology.
The main reason for petrolatum’s presence on social media is its effectiveness as a moisturizer, which is due to its occlusive property. Its oil-based nature allows it to seal water in the skin by creating a hydrophobic barrier that decreases transepidermal water loss (TEWL). Among available oil-based moisturizers, petrolatum is the most effective in reducing TEWL by 98%, while others only provide reductions of 20% to 30%,2 which makes it ideal for soothing itch and irritation in several skin conditions, including dry skin, cheilitis, chafing, and diaper rash. Petrolatum is particularly helpful in sensitive areas where the skin is thinner, such as the eyelids or lips, as it is less irritating than lotions.
Petrolatum also may be used to treat dry skin and mild atopic dermatitis with the soak-and-smear technique,3 which entails soaking the affected skin—or the entire body, if needed—in a plain water bath for 20 minutes and then immediately smearing the skin with petrolatum. Soaking hydrates the damaged stratum corneum and enhances desquamation. The moist stratum corneum absorbs topical treatments more effectively, and desquamation leaves a thinner stratum corneum for the product to traverse. Smearing with petrolatum then traps the moisture in the skin and thus has a dual function by both delivering the petrolatum to the skin and trapping the moisture from the soak. The result is decreased TEWL, improved hydration, and increased penetration, thereby enhancing skin barrier repair.3,4
Smearing solely with petrolatum is effective in cases not accompanied by considerable inflammation. In cases involving notable inflammation or severe xerosis, a steroidal ointment may be required.3 This generally is done for several nights to 2 weeks before conversion to maintenance therapy. In these cases, petrolatum may then be used as maintenance therapy or bridge therapy for maintenance with simple moisturizers, which decreases recurrence and flares of dermatitis and also prevents continuous exposure to steroidal agents that can result in atrophy and purpura at application sites. The soak-and-smear technique has been found to be effective, with 90% of patients having 90% to 100% clearance.3
Petrolatum also is particularly useful for wound healing. A study on the molecular responses induced by petrolatum found that it significantly upregulated innate immune genes (P<.01), increased antimicrobial peptides (P<.001), and improved epidermal differentiation.5 Additionally, it keeps wound edges moist, which enhances angiogenesis, improves collagen synthesis, and increases the breakdown of dead tissue and fibrin.6 It also prevents scab formation, which can prolong healing time.7
Petrolatum is superior to antibiotic use after clean cutaneous surgery given its excellent safety profile. In one randomized controlled trial comparing petrolatum to bacitracin, petrolatum was found to be just as effective for wound healing with a similar infection rate. Although 4 patients developed allergic contact dermatitis (ACD) with bacitracin use, no patients who used petrolatum developed ACD.8 There are numerous other reports of bacitracin causing ACD,9,10 with a prevalence as high as 22% in chronic leg ulcer patients.10 There are even multiple reports of bacitracin causing contact urticaria and life-threatening anaphylaxis.11 In the most recent report from the North American Contact Dermatitis Group’s list of top allergens, bacitracin placed 11th with an ACD prevalence of 5.5%. Neomycin, another common postwound emollient, has similar adverse effects and ranked 12th with an ACD prevalence of 5.4%.12 Despite the risk for ACD with antibiotics, one study on wound care handouts from dermatologists (N=169) found that nearly half (43%) still advocated for the use of antibiotics.13 Likewise, another study among nondermatologists found that 40% (10/25) recommended the use of antibiotics for wound care14 despite strong evidence that topical antibiotics in clean dermatologic procedures offer no additional benefit compared with petrolatum. Additionally, topical antibiotics carry a risk of antibiotic resistance, adverse reactions such as ACD and anaphylaxis, and higher health care costs.9 Thus, petrolatum should be used as standard care after clean cutaneous procedures, and the application of antibiotics should be abandoned.
Petrolatum also is an effective treatment for pruritus scroti.15 It is particularly helpful for recalcitrant disease when several topical medications have failed or ACD or irritant contact dermatitis to medications or cleansing products is suspected. Although topical corticosteroids are the mainstay of treatment, severe burning or redness may occur with prolonged use of these medications, thus it often is useful to discontinue topical medications and treat with plain water sitz baths at night followed by petrolatum immediately applied over wet skin. This approach has several benefits, including soothing the area, providing an occlusive barrier, retaining moisture, and eliminating contact with steroids and potential allergens and irritants. This may be followed with patch testing to determine if ACD from cleansing products or medications is the culprit. This treatment also may be used in pruritus ani or pruritus vulvae.15
Finally, petrolatum may even be used to treat parasitic skin infections such as cutaneous furuncular myiasis,16 a condition most commonly caused by the human botfly (Dermatobia hominis) or the African tumbu fly (Cordylobia anthropophaga). The larvae infest the skin by penetrating the dermis and burrowing into the subdermal layer. It is characterized by furuncular nodules with a central black punctum formed by larvae burrowed underneath the skin. An inflammatory reaction occurs in the sites surrounding the larvae with erythematous, edematous, and tender skin. Symptoms range from mild pruritus and a prickly heat sensation to intense cutaneous pain, agitation, and insomnia. Occluding the punctum, or breathing hole, of the infectious organism with petrolatum will asphyxiate the larvae, causing it to emerge within and leading to definitive diagnosis and treatment. This permits rapid removal and avoids extensive incision and extraction.16
The increased social media attention of petrolatum has raised the awareness of its utility as a moisturizer; however, it has many other uses, including soothing itch and irritation, improving wound healing, alleviating scrotal itch, and treating parasitic skin infections. It not only is an effective product but also is a particularly safe one. Petrolatum is well deserving of its positive reputation in dermatology and its current popularity among the general public
- Cramer M. A staple of grandma’s medicine cabinet gets hot on TikTok. New York Times. Published February 11, 2022. Accessed September 15, 2022. https://www.nytimes.com/2022/02/11/business/vaseline-slugging-tiktok.html
- Sethi A, Kaur T, Malhotra SK, et al. Moisturizers: the slippery road. Indian J Dermatol. 2016;61:279-287. doi:10.4103/0019-5154.182427
- Gutman AB, Kligman AM, Sciacca J, et al. Soak and smear: a standard technique revisited. 2005;141:1556-1559. doi:10.1001/archderm.141.12.1556
- Ghadially R, Halkier-Sorensen L, Elias PM. Effects of petrolatum on stratum corneum structure and function. J Am Acad Dermatol. 1992;26:387-396. doi:10.1016/0190-9622(92)70060-S
- Czarnowicki T, Malajian D, Khattri S, et al. Petrolatum: barrier repair and antimicrobial responses underlying this “inert” moisturizer. J Allergy Clin Immunol. 2016;137:1091-1102.e7. doi:10.1016/j.jaci.2015.08.013
- Field CK, Kerstein MD. Overview of wound healing in a moist environment. Am J Surg. 1994;167:2S-6S.
- Winter GD. Some factors affecting skin and wound healing. J Tissue Viability. 2006;16:20-23. doi:10.1016/S0965-206X(06)62006-8
- Smack DP, Harrington AC, Dunn C, et al. Infection and allergy incidence in ambulatory surgery patients using white petrolatum vs bacitracin ointment. a randomized controlled trial. JAMA. 1996;276:972-977.
- Jacob SE, James WD. From road rash to top allergen in a flash: bacitracin. 2004;30(4 pt 1):521-524. doi:10.1111/j.1524-4725.2004.30168.x..
- Zaki I, Shall L, Dalziel KL. Bacitracin: a significant sensitizer in leg ulcer patients? Contact Dermatitis. 1994;31:92-94. doi:10.1111/j.1600-0536.1994.tb01924.x
- Farley M, Pak H, Carregal V, et al. Anaphylaxis to topically applied bacitracin. Am J Contact Dermatitis. 1995;6:28-31. doi:10.1016/1046-199X(95)90066-7
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Nguyen JK, Huang A, Siegel DM, et al. Variability in wound care recommendations following dermatologic procedures. Dermatol Surg. 2020;46:186-191. doi:10.1097/DSS.0000000000001952
- Fathy R, Chu B, Singh P, et al. Variation in topical antibiotics recommendations in wound care instructions by non-dermatologists. J Gen Intern Med. 2021;36:238-239. doi:10.1007/s11606-020-05689-2
- James WD, Elston DM, Treat JR, et al. Andrews’ Diseases of the Skin. 13th ed. Elsevier; 2020.
- Ockenhouse CF, Samlaska CP, Benson PM, et al. Cutaneous myiasis caused by the African tumbu fly (Cordylobia anthropophaga). Arch Dermatol. 1990;126:199-202.
- Cramer M. A staple of grandma’s medicine cabinet gets hot on TikTok. New York Times. Published February 11, 2022. Accessed September 15, 2022. https://www.nytimes.com/2022/02/11/business/vaseline-slugging-tiktok.html
- Sethi A, Kaur T, Malhotra SK, et al. Moisturizers: the slippery road. Indian J Dermatol. 2016;61:279-287. doi:10.4103/0019-5154.182427
- Gutman AB, Kligman AM, Sciacca J, et al. Soak and smear: a standard technique revisited. 2005;141:1556-1559. doi:10.1001/archderm.141.12.1556
- Ghadially R, Halkier-Sorensen L, Elias PM. Effects of petrolatum on stratum corneum structure and function. J Am Acad Dermatol. 1992;26:387-396. doi:10.1016/0190-9622(92)70060-S
- Czarnowicki T, Malajian D, Khattri S, et al. Petrolatum: barrier repair and antimicrobial responses underlying this “inert” moisturizer. J Allergy Clin Immunol. 2016;137:1091-1102.e7. doi:10.1016/j.jaci.2015.08.013
- Field CK, Kerstein MD. Overview of wound healing in a moist environment. Am J Surg. 1994;167:2S-6S.
- Winter GD. Some factors affecting skin and wound healing. J Tissue Viability. 2006;16:20-23. doi:10.1016/S0965-206X(06)62006-8
- Smack DP, Harrington AC, Dunn C, et al. Infection and allergy incidence in ambulatory surgery patients using white petrolatum vs bacitracin ointment. a randomized controlled trial. JAMA. 1996;276:972-977.
- Jacob SE, James WD. From road rash to top allergen in a flash: bacitracin. 2004;30(4 pt 1):521-524. doi:10.1111/j.1524-4725.2004.30168.x..
- Zaki I, Shall L, Dalziel KL. Bacitracin: a significant sensitizer in leg ulcer patients? Contact Dermatitis. 1994;31:92-94. doi:10.1111/j.1600-0536.1994.tb01924.x
- Farley M, Pak H, Carregal V, et al. Anaphylaxis to topically applied bacitracin. Am J Contact Dermatitis. 1995;6:28-31. doi:10.1016/1046-199X(95)90066-7
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Nguyen JK, Huang A, Siegel DM, et al. Variability in wound care recommendations following dermatologic procedures. Dermatol Surg. 2020;46:186-191. doi:10.1097/DSS.0000000000001952
- Fathy R, Chu B, Singh P, et al. Variation in topical antibiotics recommendations in wound care instructions by non-dermatologists. J Gen Intern Med. 2021;36:238-239. doi:10.1007/s11606-020-05689-2
- James WD, Elston DM, Treat JR, et al. Andrews’ Diseases of the Skin. 13th ed. Elsevier; 2020.
- Ockenhouse CF, Samlaska CP, Benson PM, et al. Cutaneous myiasis caused by the African tumbu fly (Cordylobia anthropophaga). Arch Dermatol. 1990;126:199-202.
Update on Rosacea Classification and Its Controversies
Rosacea is an inflammatory skin condition that affects approximately 5% of the adult population, with the highest prevalence in Europe and North America.1 Despite its prevalence, rosacea remains poorly understood from a pathophysiologic perspective, with no diagnostic laboratory markers.2 Because diagnosis relies on clinical judgment, the nomenclature for describing and characterizing rosacea becomes paramount in ensuring that patients are given an accurate diagnosis and subsequent treatment. We review the shortfalls in the recent history of rosacea classification and discuss their implications.
Subtype to Phenotype Classification
In 2002, the National Rosacea Society (NRS) Expert Committee published a standardized classification schema for rosacea (Table).3 The authors described primary and secondary diagnostic criteria. The presence of 1 or more primary features in a central facial distribution was indicative of rosacea. Primary characteristics included flushing (transient erythema), nontransient erythema, papules and pustules, and telangiectasia. Secondary features, which could occur with or independently of primary features, included burning or stinging of the face, dry appearance, facial edema, ocular manifestations, peripheral (nonfacial) occurrence, phymatous changes, and red facial plaques. Whereas these features often present simultaneously in a characteristic pattern, they were grouped into 4 main subtypes—erythematotelangiectatic (ETR), papulopustular, phymatous, and ocular—and 1 variant, granulomatous rosacea.3
To enhance clinical and research applications of this categorization system as well as offer further standardization, the NRS released a supplementary clinical grading scorecard in 2004 in which each of the primary and secondary characteristics could be assigned a subjective severity score of absent, mild, moderate, or severe. The goal was that the subtype classification and clinical grading system, when used in conjunction with each other, would establish a common language for patients, clinicians, and researchers to describe and further investigate rosacea.4
The 2002 categorization system was certainly an impactful first step in the organization of rosacea. It was not without its critics, however, namely rosacea-oriented dermatologists who were concerned about its lack of specificity.5-7 For instance, the NRS Expert Committee did not address the time frame for flushing, which typically has a long duration in rosacea patients, or for the nontransient erythema; telangiectasia secondary to heliodermatitis; or the often-observed periocular sparing. Additionally, the schema did not account for conditions such as gram-negative folliculitis (pustules characteristically located on the central face) or discuss the need to rule out carcinoid, mastocytosis, or connective-tissue disease, which can lead to nontransient facial erythema. Without strict definitions and exclusions, nonrosacea disorders could be incorrectly labeled as rosacea.
Beyond the lack of specificity, there was additional concern if a subtype system was the ideal way to capture disease presentation and severity. By subtyping, there was unnecessary division of interrelated disease into individual disorders; an individual’s clinical presentation might fall along a spectrum rather than within a discrete box.8
Furthermore, from a research standpoint, subtyping rosacea could hinder or confuse epidemiologic studies. For instance, if patients present with phenotypes from different subtypes, into which subtype would they fall?8-10
The global ROSacea COnsensus (ROSCO) panel, comprising 17 international dermatologists and ophthalmologists, convened in 2016 to address this matter. The panel proposed a new system (published in 2017) based on individual phenotypes.9 In this new system, diagnostic features include persistent centrofacial erythema with periods of increased intensity and phymatous changes. Major features, which are diagnostic when there are at least 2, include flushing (transient erythema), inflammatory papules and pustules, centrofacial telangiectasia, and ocular manifestations. Each feature could then be graded on a severity spectrum independent of concurrent phenotypes (Table).8
The panel concluded that this system would provide a stronger foundation for standardization as new knowledge of rosacea continues to be elucidated.8 In support of their argument, ROSCO also released a treatment algorithm that depended on a phenotype scheme.11 The panel emphasized that by focusing on individual lesions rather than a subtype encompassing many characteristics, treatment could be tailored to the patient. Using this à-la-carte therapy option, physicians could choose those rosacea aspects that are particularly concerning to the patient and manage only those aspects or overlap treatments to improve multiple aspects.11
In 2017, 15 years after the original classification system was proposed, the NRS updated their classification system (published in 2018), taking into consideration some of the criticisms as well as new scientific data on rosacea. Similar to the schema proposed by ROSCO, this system was based on phenotype. Inclusion and exclusion criteria were more robust in this update compared to the original classification in 2002. The criteria provide a timeline for transient flushing—it must occur within seconds or minutes in response to a neurovascular stimulant—and state that it is characteristically prolonged (Table).12
However, the Expert Committee still did not define either the length of time of flushing or nontransient erythema. It also did not specify convex surfaces of the face with periocular sparing as the characteristic pattern or provide additional information on how photoaging fits into the definition. The updated classification stated that centrofacial erythema must not be from cutaneous lupus or seborrheic eczema, and steroid-induced rosacea was still excluded.12 However, there is still the need to exclude other systemic conditions, such as mastocytosis, carcinoid, polycythemia vera, and dermatomyositis. Therefore, the potential for subjective error and inclusion of nonrosacea diseases persists.
A critical change was elimination of the granulomatous rosacea variant. In 2002, this variant was defined by monomorphic, yellow-brown to red papules and nodules that led to scarring. This variant, however, did not share the commonalities of the other subtypes, including persistent facial erythema, limitation to convex surfaces, periocular sparing, and transient flushing.3,13 At the time, Crawford et al6 proposed that the variant be recategorized as granulomatous facial dermatitis. In the updated NRS classification, this variant and phenotypic description was eliminated from the schema.12 It is unclear if it was removed because of these discrepancies or if the NRS panel felt it had a distinct pathogenesis from the proposed rosacea pathophysiology; however, we applaud this change.
Subtype Progression
Both the ROSCO and NRS classification schemes mention progression between the various phenotypes,10,12 suggesting that rosacea phenotypes exist along a continuum, progressing and regressing with disease severity. The main study addressing this point was based on the self-reported retrospective patient memory of disease features in rosacea patients. The authors used a modified criterion of centrofacial erythema alone to define ETR; therefore, a person who began their disease with this finding but then acquired inflammatory lesions or phymas was defined as progressing along a spectrum.14 Given that persistent erythema of convex surfaces of the face is common in all subtypes, we do not find it surprising that the authors found (using their modified criteria) that ETR appeared to progress to papulopustular and phymatous subtypes in a small number of patients. We strongly disagree with their interpretation and conclusion.
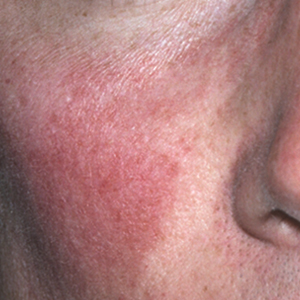
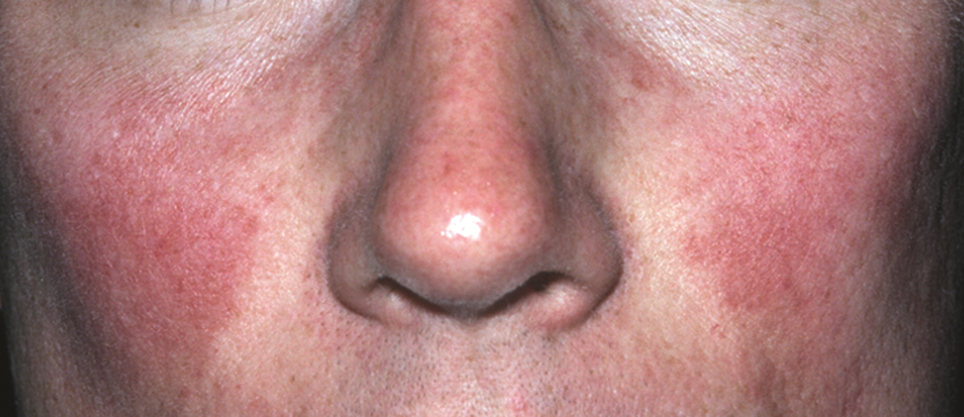
In our experience, ETR patients have fine textured skin without sebaceous quality or a history of extensive acne (Figure 1). Flushing is common and usually lasts 10 minutes to 1 hour. There might be concurrent burning or stinging; however, there is no associated sweating, lightheadedness, palpitations, or diagnostic laboratory findings, which distinguishes ETR from other common causes of flushing. The persistent centrofacial erythema involves convex surfaces, spares periocular skin, and can be best defined as present for longer than 3 months.
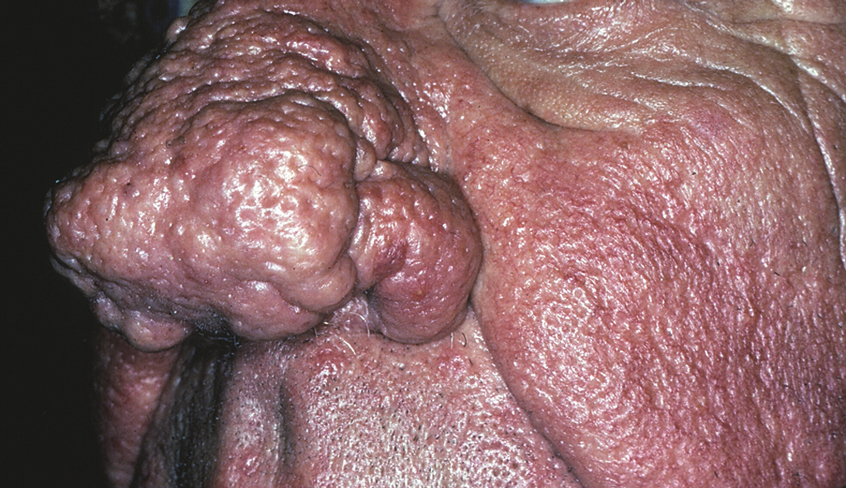
In contrast, phymas occur commonly in patients with thick and sebaceous (glandular) skin (Figure 2).6,15-17 Men are most often affected and usually have a history of moderate to severe acne. It is not uncommon to observe nodules, cysts, and scarring in addition to papules and pustules. These eruptions primarily cluster on the central face and present in areas of nontransient erythema. Flushing, although less prominent than in other phenotypes, also can be seen.
Taken together, we find no convincing evidence from published studies or extensive experience caring for rosacea patients that classic ETR progresses to phymatous rosacea, or the other way around, as displayed in the ROSCO panel report.8 The type of skin seen in Figure 1 will not “progress” to the type seen in Figure 2. Furthermore, treatment will not “reverse” the phymatous skin into thin, ETR-type skin. The implications are important: If a female patient is given a diagnosis of ETR, she will not develop an enlarged phymatous nose. Patients with thick sebaceous skin, as in Figure 2, usually tolerate treatments such as benzoyl peroxide that other rosacea patients do not and frequently respond well to such intervention.
Implications and Future Directions
We present an overview of 2 rosacea classification systems, hoping to stimulate further refinement. Looking forward, there are many directions for further investigation into the pathophysiology of rosacea. From a genetic standpoint, there needs to be continued molecular and epidemiologic data to determine the underlying genetic contributions to disease.
There has been some progress in the realm of understanding the mechanisms of inflammation; we urge further investigation to elucidate how “subclinical neuroinflammation” might lead to glandular hyperplasia.12 We also see value in examining the genetic and hormonal contributions to phymas, as they may be different than those seen in the ETR-type patients. Last, more studies focusing on comorbidities that contribute to or arise from rosacea are welcomed.
The ultimate goal is to develop a classification system that integrates clinical descriptions, pathophysiologic mechanisms, and benchmark indicators of disease. Only then can we have a true gold standard for the diagnosis of rosacea, one that allows for improved personalized treatment and better outcomes.
- Gether L, Overgaard LK, Egeberg A, et al. Incidence and prevalence of rosacea: a systematic review and meta‐analysis. Br J Dermatol. 2018;179:282-289.
- van Zuuren EJ. Rosacea. N Engl J Med. 2017;377:1754-1764.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2004;50:907-912.
- Saleem MD. Revisiting rosacea criteria: where have we been, where are we going, and how will we get there? Dermatol Clin. 2018;36:161-165.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341.
- Tan J, Steinhoff M, Berg M, et al; Rosacea International Study Group. Shortcomings in rosacea diagnosis and classification. Br J Dermatol. 2017;176:197-199.
- Tan J, Almeida LMC, Bewley A, et al. Updating the diagnosis, classification and assessment of rosacea: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176:431-438.
- Wilkin J. Updating the diagnosis, classification and assessment of rosacea by effacement of subtypes. Br J Dermatol. 2017;177:597-598.
- Tan J; ROSCO coauthors. Updating the diagnosis, classification and assessment of rosacea by effacement of subtypes: reply from the author. Br J Dermatol. 2017;177:598-599.
- Schaller M, Almeida LM, Bewley A, et al. Rosacea treatment update: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176:465-471.
- Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148-155.
- Lee GL, Zirwas MJ. Granulomatous rosacea and periorificial dermatitis: controversies and review of management and treatment. Dermatol Clin. 2015;33:447-455.
- Tan J, Blume‐Peytavi U, Ortonne JP, et al. An observational cross‐sectional survey of rosacea: clinical associations and progression between subtypes. Br J Dermatol. 2013;169:555-562.
- James WD, Elston D, Treat JR, et al. Andrews’ Diseases of the Skin: Clinical Dermatology. 13th ed. New York, NY: Elsevier; 2019.
- Reinholz M, Tietze JK, Kilian K, et al. Rosacea - S1 guideline. J Dtsch Dermatol Ges. 2013;11:768-780.
- Reinholz M, Ruzicka T, Steinhoff M, et al. Pathogenesis and clinical presentation of rosacea as a key for a symptom‐oriented therapy. J Dtsch Dermatol Ges. 2016;14(suppl 6):4-15.
Rosacea is an inflammatory skin condition that affects approximately 5% of the adult population, with the highest prevalence in Europe and North America.1 Despite its prevalence, rosacea remains poorly understood from a pathophysiologic perspective, with no diagnostic laboratory markers.2 Because diagnosis relies on clinical judgment, the nomenclature for describing and characterizing rosacea becomes paramount in ensuring that patients are given an accurate diagnosis and subsequent treatment. We review the shortfalls in the recent history of rosacea classification and discuss their implications.
Subtype to Phenotype Classification
In 2002, the National Rosacea Society (NRS) Expert Committee published a standardized classification schema for rosacea (Table).3 The authors described primary and secondary diagnostic criteria. The presence of 1 or more primary features in a central facial distribution was indicative of rosacea. Primary characteristics included flushing (transient erythema), nontransient erythema, papules and pustules, and telangiectasia. Secondary features, which could occur with or independently of primary features, included burning or stinging of the face, dry appearance, facial edema, ocular manifestations, peripheral (nonfacial) occurrence, phymatous changes, and red facial plaques. Whereas these features often present simultaneously in a characteristic pattern, they were grouped into 4 main subtypes—erythematotelangiectatic (ETR), papulopustular, phymatous, and ocular—and 1 variant, granulomatous rosacea.3
To enhance clinical and research applications of this categorization system as well as offer further standardization, the NRS released a supplementary clinical grading scorecard in 2004 in which each of the primary and secondary characteristics could be assigned a subjective severity score of absent, mild, moderate, or severe. The goal was that the subtype classification and clinical grading system, when used in conjunction with each other, would establish a common language for patients, clinicians, and researchers to describe and further investigate rosacea.4
The 2002 categorization system was certainly an impactful first step in the organization of rosacea. It was not without its critics, however, namely rosacea-oriented dermatologists who were concerned about its lack of specificity.5-7 For instance, the NRS Expert Committee did not address the time frame for flushing, which typically has a long duration in rosacea patients, or for the nontransient erythema; telangiectasia secondary to heliodermatitis; or the often-observed periocular sparing. Additionally, the schema did not account for conditions such as gram-negative folliculitis (pustules characteristically located on the central face) or discuss the need to rule out carcinoid, mastocytosis, or connective-tissue disease, which can lead to nontransient facial erythema. Without strict definitions and exclusions, nonrosacea disorders could be incorrectly labeled as rosacea.
Beyond the lack of specificity, there was additional concern if a subtype system was the ideal way to capture disease presentation and severity. By subtyping, there was unnecessary division of interrelated disease into individual disorders; an individual’s clinical presentation might fall along a spectrum rather than within a discrete box.8
Furthermore, from a research standpoint, subtyping rosacea could hinder or confuse epidemiologic studies. For instance, if patients present with phenotypes from different subtypes, into which subtype would they fall?8-10
The global ROSacea COnsensus (ROSCO) panel, comprising 17 international dermatologists and ophthalmologists, convened in 2016 to address this matter. The panel proposed a new system (published in 2017) based on individual phenotypes.9 In this new system, diagnostic features include persistent centrofacial erythema with periods of increased intensity and phymatous changes. Major features, which are diagnostic when there are at least 2, include flushing (transient erythema), inflammatory papules and pustules, centrofacial telangiectasia, and ocular manifestations. Each feature could then be graded on a severity spectrum independent of concurrent phenotypes (Table).8
The panel concluded that this system would provide a stronger foundation for standardization as new knowledge of rosacea continues to be elucidated.8 In support of their argument, ROSCO also released a treatment algorithm that depended on a phenotype scheme.11 The panel emphasized that by focusing on individual lesions rather than a subtype encompassing many characteristics, treatment could be tailored to the patient. Using this à-la-carte therapy option, physicians could choose those rosacea aspects that are particularly concerning to the patient and manage only those aspects or overlap treatments to improve multiple aspects.11
In 2017, 15 years after the original classification system was proposed, the NRS updated their classification system (published in 2018), taking into consideration some of the criticisms as well as new scientific data on rosacea. Similar to the schema proposed by ROSCO, this system was based on phenotype. Inclusion and exclusion criteria were more robust in this update compared to the original classification in 2002. The criteria provide a timeline for transient flushing—it must occur within seconds or minutes in response to a neurovascular stimulant—and state that it is characteristically prolonged (Table).12
However, the Expert Committee still did not define either the length of time of flushing or nontransient erythema. It also did not specify convex surfaces of the face with periocular sparing as the characteristic pattern or provide additional information on how photoaging fits into the definition. The updated classification stated that centrofacial erythema must not be from cutaneous lupus or seborrheic eczema, and steroid-induced rosacea was still excluded.12 However, there is still the need to exclude other systemic conditions, such as mastocytosis, carcinoid, polycythemia vera, and dermatomyositis. Therefore, the potential for subjective error and inclusion of nonrosacea diseases persists.
A critical change was elimination of the granulomatous rosacea variant. In 2002, this variant was defined by monomorphic, yellow-brown to red papules and nodules that led to scarring. This variant, however, did not share the commonalities of the other subtypes, including persistent facial erythema, limitation to convex surfaces, periocular sparing, and transient flushing.3,13 At the time, Crawford et al6 proposed that the variant be recategorized as granulomatous facial dermatitis. In the updated NRS classification, this variant and phenotypic description was eliminated from the schema.12 It is unclear if it was removed because of these discrepancies or if the NRS panel felt it had a distinct pathogenesis from the proposed rosacea pathophysiology; however, we applaud this change.
Subtype Progression
Both the ROSCO and NRS classification schemes mention progression between the various phenotypes,10,12 suggesting that rosacea phenotypes exist along a continuum, progressing and regressing with disease severity. The main study addressing this point was based on the self-reported retrospective patient memory of disease features in rosacea patients. The authors used a modified criterion of centrofacial erythema alone to define ETR; therefore, a person who began their disease with this finding but then acquired inflammatory lesions or phymas was defined as progressing along a spectrum.14 Given that persistent erythema of convex surfaces of the face is common in all subtypes, we do not find it surprising that the authors found (using their modified criteria) that ETR appeared to progress to papulopustular and phymatous subtypes in a small number of patients. We strongly disagree with their interpretation and conclusion.
In our experience, ETR patients have fine textured skin without sebaceous quality or a history of extensive acne (Figure 1). Flushing is common and usually lasts 10 minutes to 1 hour. There might be concurrent burning or stinging; however, there is no associated sweating, lightheadedness, palpitations, or diagnostic laboratory findings, which distinguishes ETR from other common causes of flushing. The persistent centrofacial erythema involves convex surfaces, spares periocular skin, and can be best defined as present for longer than 3 months.

In contrast, phymas occur commonly in patients with thick and sebaceous (glandular) skin (Figure 2).6,15-17 Men are most often affected and usually have a history of moderate to severe acne. It is not uncommon to observe nodules, cysts, and scarring in addition to papules and pustules. These eruptions primarily cluster on the central face and present in areas of nontransient erythema. Flushing, although less prominent than in other phenotypes, also can be seen.

Taken together, we find no convincing evidence from published studies or extensive experience caring for rosacea patients that classic ETR progresses to phymatous rosacea, or the other way around, as displayed in the ROSCO panel report.8 The type of skin seen in Figure 1 will not “progress” to the type seen in Figure 2. Furthermore, treatment will not “reverse” the phymatous skin into thin, ETR-type skin. The implications are important: If a female patient is given a diagnosis of ETR, she will not develop an enlarged phymatous nose. Patients with thick sebaceous skin, as in Figure 2, usually tolerate treatments such as benzoyl peroxide that other rosacea patients do not and frequently respond well to such intervention.
Implications and Future Directions
We present an overview of 2 rosacea classification systems, hoping to stimulate further refinement. Looking forward, there are many directions for further investigation into the pathophysiology of rosacea. From a genetic standpoint, there needs to be continued molecular and epidemiologic data to determine the underlying genetic contributions to disease.
There has been some progress in the realm of understanding the mechanisms of inflammation; we urge further investigation to elucidate how “subclinical neuroinflammation” might lead to glandular hyperplasia.12 We also see value in examining the genetic and hormonal contributions to phymas, as they may be different than those seen in the ETR-type patients. Last, more studies focusing on comorbidities that contribute to or arise from rosacea are welcomed.
The ultimate goal is to develop a classification system that integrates clinical descriptions, pathophysiologic mechanisms, and benchmark indicators of disease. Only then can we have a true gold standard for the diagnosis of rosacea, one that allows for improved personalized treatment and better outcomes.
Rosacea is an inflammatory skin condition that affects approximately 5% of the adult population, with the highest prevalence in Europe and North America.1 Despite its prevalence, rosacea remains poorly understood from a pathophysiologic perspective, with no diagnostic laboratory markers.2 Because diagnosis relies on clinical judgment, the nomenclature for describing and characterizing rosacea becomes paramount in ensuring that patients are given an accurate diagnosis and subsequent treatment. We review the shortfalls in the recent history of rosacea classification and discuss their implications.
Subtype to Phenotype Classification
In 2002, the National Rosacea Society (NRS) Expert Committee published a standardized classification schema for rosacea (Table).3 The authors described primary and secondary diagnostic criteria. The presence of 1 or more primary features in a central facial distribution was indicative of rosacea. Primary characteristics included flushing (transient erythema), nontransient erythema, papules and pustules, and telangiectasia. Secondary features, which could occur with or independently of primary features, included burning or stinging of the face, dry appearance, facial edema, ocular manifestations, peripheral (nonfacial) occurrence, phymatous changes, and red facial plaques. Whereas these features often present simultaneously in a characteristic pattern, they were grouped into 4 main subtypes—erythematotelangiectatic (ETR), papulopustular, phymatous, and ocular—and 1 variant, granulomatous rosacea.3
To enhance clinical and research applications of this categorization system as well as offer further standardization, the NRS released a supplementary clinical grading scorecard in 2004 in which each of the primary and secondary characteristics could be assigned a subjective severity score of absent, mild, moderate, or severe. The goal was that the subtype classification and clinical grading system, when used in conjunction with each other, would establish a common language for patients, clinicians, and researchers to describe and further investigate rosacea.4
The 2002 categorization system was certainly an impactful first step in the organization of rosacea. It was not without its critics, however, namely rosacea-oriented dermatologists who were concerned about its lack of specificity.5-7 For instance, the NRS Expert Committee did not address the time frame for flushing, which typically has a long duration in rosacea patients, or for the nontransient erythema; telangiectasia secondary to heliodermatitis; or the often-observed periocular sparing. Additionally, the schema did not account for conditions such as gram-negative folliculitis (pustules characteristically located on the central face) or discuss the need to rule out carcinoid, mastocytosis, or connective-tissue disease, which can lead to nontransient facial erythema. Without strict definitions and exclusions, nonrosacea disorders could be incorrectly labeled as rosacea.
Beyond the lack of specificity, there was additional concern if a subtype system was the ideal way to capture disease presentation and severity. By subtyping, there was unnecessary division of interrelated disease into individual disorders; an individual’s clinical presentation might fall along a spectrum rather than within a discrete box.8
Furthermore, from a research standpoint, subtyping rosacea could hinder or confuse epidemiologic studies. For instance, if patients present with phenotypes from different subtypes, into which subtype would they fall?8-10
The global ROSacea COnsensus (ROSCO) panel, comprising 17 international dermatologists and ophthalmologists, convened in 2016 to address this matter. The panel proposed a new system (published in 2017) based on individual phenotypes.9 In this new system, diagnostic features include persistent centrofacial erythema with periods of increased intensity and phymatous changes. Major features, which are diagnostic when there are at least 2, include flushing (transient erythema), inflammatory papules and pustules, centrofacial telangiectasia, and ocular manifestations. Each feature could then be graded on a severity spectrum independent of concurrent phenotypes (Table).8
The panel concluded that this system would provide a stronger foundation for standardization as new knowledge of rosacea continues to be elucidated.8 In support of their argument, ROSCO also released a treatment algorithm that depended on a phenotype scheme.11 The panel emphasized that by focusing on individual lesions rather than a subtype encompassing many characteristics, treatment could be tailored to the patient. Using this à-la-carte therapy option, physicians could choose those rosacea aspects that are particularly concerning to the patient and manage only those aspects or overlap treatments to improve multiple aspects.11
In 2017, 15 years after the original classification system was proposed, the NRS updated their classification system (published in 2018), taking into consideration some of the criticisms as well as new scientific data on rosacea. Similar to the schema proposed by ROSCO, this system was based on phenotype. Inclusion and exclusion criteria were more robust in this update compared to the original classification in 2002. The criteria provide a timeline for transient flushing—it must occur within seconds or minutes in response to a neurovascular stimulant—and state that it is characteristically prolonged (Table).12
However, the Expert Committee still did not define either the length of time of flushing or nontransient erythema. It also did not specify convex surfaces of the face with periocular sparing as the characteristic pattern or provide additional information on how photoaging fits into the definition. The updated classification stated that centrofacial erythema must not be from cutaneous lupus or seborrheic eczema, and steroid-induced rosacea was still excluded.12 However, there is still the need to exclude other systemic conditions, such as mastocytosis, carcinoid, polycythemia vera, and dermatomyositis. Therefore, the potential for subjective error and inclusion of nonrosacea diseases persists.
A critical change was elimination of the granulomatous rosacea variant. In 2002, this variant was defined by monomorphic, yellow-brown to red papules and nodules that led to scarring. This variant, however, did not share the commonalities of the other subtypes, including persistent facial erythema, limitation to convex surfaces, periocular sparing, and transient flushing.3,13 At the time, Crawford et al6 proposed that the variant be recategorized as granulomatous facial dermatitis. In the updated NRS classification, this variant and phenotypic description was eliminated from the schema.12 It is unclear if it was removed because of these discrepancies or if the NRS panel felt it had a distinct pathogenesis from the proposed rosacea pathophysiology; however, we applaud this change.
Subtype Progression
Both the ROSCO and NRS classification schemes mention progression between the various phenotypes,10,12 suggesting that rosacea phenotypes exist along a continuum, progressing and regressing with disease severity. The main study addressing this point was based on the self-reported retrospective patient memory of disease features in rosacea patients. The authors used a modified criterion of centrofacial erythema alone to define ETR; therefore, a person who began their disease with this finding but then acquired inflammatory lesions or phymas was defined as progressing along a spectrum.14 Given that persistent erythema of convex surfaces of the face is common in all subtypes, we do not find it surprising that the authors found (using their modified criteria) that ETR appeared to progress to papulopustular and phymatous subtypes in a small number of patients. We strongly disagree with their interpretation and conclusion.
In our experience, ETR patients have fine textured skin without sebaceous quality or a history of extensive acne (Figure 1). Flushing is common and usually lasts 10 minutes to 1 hour. There might be concurrent burning or stinging; however, there is no associated sweating, lightheadedness, palpitations, or diagnostic laboratory findings, which distinguishes ETR from other common causes of flushing. The persistent centrofacial erythema involves convex surfaces, spares periocular skin, and can be best defined as present for longer than 3 months.

In contrast, phymas occur commonly in patients with thick and sebaceous (glandular) skin (Figure 2).6,15-17 Men are most often affected and usually have a history of moderate to severe acne. It is not uncommon to observe nodules, cysts, and scarring in addition to papules and pustules. These eruptions primarily cluster on the central face and present in areas of nontransient erythema. Flushing, although less prominent than in other phenotypes, also can be seen.

Taken together, we find no convincing evidence from published studies or extensive experience caring for rosacea patients that classic ETR progresses to phymatous rosacea, or the other way around, as displayed in the ROSCO panel report.8 The type of skin seen in Figure 1 will not “progress” to the type seen in Figure 2. Furthermore, treatment will not “reverse” the phymatous skin into thin, ETR-type skin. The implications are important: If a female patient is given a diagnosis of ETR, she will not develop an enlarged phymatous nose. Patients with thick sebaceous skin, as in Figure 2, usually tolerate treatments such as benzoyl peroxide that other rosacea patients do not and frequently respond well to such intervention.
Implications and Future Directions
We present an overview of 2 rosacea classification systems, hoping to stimulate further refinement. Looking forward, there are many directions for further investigation into the pathophysiology of rosacea. From a genetic standpoint, there needs to be continued molecular and epidemiologic data to determine the underlying genetic contributions to disease.
There has been some progress in the realm of understanding the mechanisms of inflammation; we urge further investigation to elucidate how “subclinical neuroinflammation” might lead to glandular hyperplasia.12 We also see value in examining the genetic and hormonal contributions to phymas, as they may be different than those seen in the ETR-type patients. Last, more studies focusing on comorbidities that contribute to or arise from rosacea are welcomed.
The ultimate goal is to develop a classification system that integrates clinical descriptions, pathophysiologic mechanisms, and benchmark indicators of disease. Only then can we have a true gold standard for the diagnosis of rosacea, one that allows for improved personalized treatment and better outcomes.
- Gether L, Overgaard LK, Egeberg A, et al. Incidence and prevalence of rosacea: a systematic review and meta‐analysis. Br J Dermatol. 2018;179:282-289.
- van Zuuren EJ. Rosacea. N Engl J Med. 2017;377:1754-1764.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2004;50:907-912.
- Saleem MD. Revisiting rosacea criteria: where have we been, where are we going, and how will we get there? Dermatol Clin. 2018;36:161-165.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341.
- Tan J, Steinhoff M, Berg M, et al; Rosacea International Study Group. Shortcomings in rosacea diagnosis and classification. Br J Dermatol. 2017;176:197-199.
- Tan J, Almeida LMC, Bewley A, et al. Updating the diagnosis, classification and assessment of rosacea: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176:431-438.
- Wilkin J. Updating the diagnosis, classification and assessment of rosacea by effacement of subtypes. Br J Dermatol. 2017;177:597-598.
- Tan J; ROSCO coauthors. Updating the diagnosis, classification and assessment of rosacea by effacement of subtypes: reply from the author. Br J Dermatol. 2017;177:598-599.
- Schaller M, Almeida LM, Bewley A, et al. Rosacea treatment update: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176:465-471.
- Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148-155.
- Lee GL, Zirwas MJ. Granulomatous rosacea and periorificial dermatitis: controversies and review of management and treatment. Dermatol Clin. 2015;33:447-455.
- Tan J, Blume‐Peytavi U, Ortonne JP, et al. An observational cross‐sectional survey of rosacea: clinical associations and progression between subtypes. Br J Dermatol. 2013;169:555-562.
- James WD, Elston D, Treat JR, et al. Andrews’ Diseases of the Skin: Clinical Dermatology. 13th ed. New York, NY: Elsevier; 2019.
- Reinholz M, Tietze JK, Kilian K, et al. Rosacea - S1 guideline. J Dtsch Dermatol Ges. 2013;11:768-780.
- Reinholz M, Ruzicka T, Steinhoff M, et al. Pathogenesis and clinical presentation of rosacea as a key for a symptom‐oriented therapy. J Dtsch Dermatol Ges. 2016;14(suppl 6):4-15.
- Gether L, Overgaard LK, Egeberg A, et al. Incidence and prevalence of rosacea: a systematic review and meta‐analysis. Br J Dermatol. 2018;179:282-289.
- van Zuuren EJ. Rosacea. N Engl J Med. 2017;377:1754-1764.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2004;50:907-912.
- Saleem MD. Revisiting rosacea criteria: where have we been, where are we going, and how will we get there? Dermatol Clin. 2018;36:161-165.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341.
- Tan J, Steinhoff M, Berg M, et al; Rosacea International Study Group. Shortcomings in rosacea diagnosis and classification. Br J Dermatol. 2017;176:197-199.
- Tan J, Almeida LMC, Bewley A, et al. Updating the diagnosis, classification and assessment of rosacea: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176:431-438.
- Wilkin J. Updating the diagnosis, classification and assessment of rosacea by effacement of subtypes. Br J Dermatol. 2017;177:597-598.
- Tan J; ROSCO coauthors. Updating the diagnosis, classification and assessment of rosacea by effacement of subtypes: reply from the author. Br J Dermatol. 2017;177:598-599.
- Schaller M, Almeida LM, Bewley A, et al. Rosacea treatment update: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176:465-471.
- Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148-155.
- Lee GL, Zirwas MJ. Granulomatous rosacea and periorificial dermatitis: controversies and review of management and treatment. Dermatol Clin. 2015;33:447-455.
- Tan J, Blume‐Peytavi U, Ortonne JP, et al. An observational cross‐sectional survey of rosacea: clinical associations and progression between subtypes. Br J Dermatol. 2013;169:555-562.
- James WD, Elston D, Treat JR, et al. Andrews’ Diseases of the Skin: Clinical Dermatology. 13th ed. New York, NY: Elsevier; 2019.
- Reinholz M, Tietze JK, Kilian K, et al. Rosacea - S1 guideline. J Dtsch Dermatol Ges. 2013;11:768-780.
- Reinholz M, Ruzicka T, Steinhoff M, et al. Pathogenesis and clinical presentation of rosacea as a key for a symptom‐oriented therapy. J Dtsch Dermatol Ges. 2016;14(suppl 6):4-15.
Practice Points
- Rosacea therapy is based on a phenotype classification system, in which patients can have major and minor features across all previously denoted subtypes. This system allows for greater flexibility in treatment regimens.
- Despite mention of progression between subtypes, there has not been convincing evidence that patients can progress or regress from one end of the rosacea spectrum (erythematotelangiectatic) to the other (phymatous).